ABSTRACT
Obesity remains a major public health problem, affecting almost half of adults in the United States. Increased risk of cardiovascular disease (CVD) and CVD mortality are major obesity‐related complications, and management guidelines now recommend weight loss as a key strategy for the primary prevention of CVD in patients with overweight or obesity. The recently demonstrated efficacy of some pharmacologic therapies for chronic weight management may encourage health care professionals to recognize obesity as a treatable serious chronic disease and motivate patients to re‐engage with weight loss when previous attempts have been ineffective or unsustainable. This review article summarizes the benefits and challenges associated with lifestyle changes, bariatric surgery, and historical pharmacologic interventions in the treatment of obesity, and focuses on the current evidence for the efficacy and safety of the newer glucagon‐like peptide‐1 receptor agonist medications in the management of obesity and potential reduction of CVD risk. We conclude that the available evidence demonstrates glucagon‐like peptide‐1 receptor agonists should be strongly considered in clinical practice for the treatment of obesity and reduction of CVD risk in people with type 2 diabetes. If ongoing research proves glucagon‐like peptide‐1 receptor agonists to be effective in reducing the risk of CVD onset in patients with obesity, irrespective of type 2 diabetes status, it will herald a new treatment paradigm in this setting, and now is the time for health care professionals to better recognize the benefits of these agents.
Keywords: GLP‐1 RA, obesity, overweight, weight loss
Subject Categories: Obesity, Primary Prevention
Nonstandard Abbreviations and Acronyms
- FDA
Food and Drug Administration
- GLP‐1R
GLP‐1 receptor
- GLP‐1 RA
glucagon‐like peptide‐1 receptor agonist
- HCP
health care professional
- MACE
major adverse cardiovascular event
- T2D
type 2 diabetes
Obesity remains a major public health problem, with ≈42% of the US adult population having obesity (body mass index [BMI] ≥30 kg/m2), and a further 31% considered to have overweight (BMI 25–29.9 kg/m2). 1 , 2 Recent estimates for 2030 predict that, if left unaddressed, the prevalence of adult obesity in the United States will reach 48.9%, and nearly 1 in 4 US adults will have class 2 obesity (BMI ≥35 kg/m2). 3
Obesity is associated with a multitude of comorbidities, including type 2 diabetes (T2D), 4 cardiovascular disease (CVD), 4 , 5 dyslipidemia, hypertension, several forms of cancer, 4 obstructive sleep apnea, 5 and many more. 6 Increased risk of CVD and CVD mortality are major obesity‐related complications. 5 , 7 Globally, 41% of BMI‐related deaths are attributed to CVD in people with obesity. 8 Weight loss has been associated with moderate improvements in cardiometabolic measures, such as blood pressure, glucose control, high‐density lipoprotein cholesterol, and triglycerides, in adults with overweight or obesity. 9 , 10 Evidence for the benefits of clinically meaningful weight loss (defined as ≥5% of initial body weight 11 ) on CVD has been well documented. 5 For instance, in patients with heart failure with preserved ejection fraction and atrial fibrillation who also had obesity, weight loss has been shown to improve clinical outcomes in terms of quality of life and exercise capacity, 12 and a reduction in the burden of atrial fibrillation. 13 , 14 Data demonstrating the cardiometabolic benefits of weight loss have led to recommendations that weight loss should be a key strategy for the primary prevention of CVD in patients with overweight or obesity by The Obesity Society in 2014 and the 2019 American College of Cardiology/American Heart Association guideline. 11 , 15
An additional burden for people with obesity is the stigma they often receive from health care professionals (HCPs), who may consider obesity to be a lifestyle problem as opposed to a serious chronic disease. 16 This can contribute to undertreatment. The recently demonstrated efficacy of some pharmacologic therapies for chronic weight management may encourage recognition of obesity as a treatable serious chronic disease and remotivate HCPs to attain weight loss in their patients with obesity. 16 Based on our clinical experience, we believe that the availability of pharmacologic therapy may motivate patients to re‐engage with losing weight when previous weight‐loss methods have been ineffective or unsustainable.
In this review article, we summarize the benefits and challenges associated with lifestyle, bariatric surgery, and historical pharmacologic interventions in the treatment of obesity, before focusing on the available evidence for the newer glucagon‐like peptide‐1 receptor agonist (GLP‐1 RA) medications in the management of obesity and potential implications for reducing CVD risk.
Lifestyle Interventions, Bariatric Surgery, and Historical Pharmacologic Treatments for Obesity
Clinical practice guidelines recommend lifestyle modifications, including following a healthy diet and optimizing physical activity, for people with overweight or obesity to achieve weight loss and reduce the risk of future CVD events. 15 This approach is supported by data from a secondary analysis of the Look AHEAD (Action for Health in Diabetes) study, which included adults with overweight or obesity and T2D. 17 Adults in the study were enrolled in an intensive weight loss intervention program aiming to achieve sustained weight loss and increased physical activity. 17 Achievement of a 10% weight loss or a substantial increase in fitness (assessed in terms of metabolic equivalents) in the first year was associated with an ≈20% reduction in CVD risk. 17 However, maintaining weight reduction is one of the most challenging aspects of obesity care. This is because weight is highly regulated by hormonal, metabolic, and neural factors, and various hormonal adaptations take place in response to weight loss that drive weight regain, with these continuing for at least 1 year after the initial weight reduction. 18 Weight loss results in compensatory mechanisms relating to reduced resting energy expenditure and increased food preoccupation, whereas neural factors increase appetite. 19 Unsurprisingly, therefore, data from 14 studies assessing reduced‐calorie diets demonstrated that although initial weight loss was achieved (−4.5 kg to −30 kg), most individuals regained a large proportion of their initial weight loss within a few years. 20 These findings suggest the need for additional interventions to prevent regaining weight, including surgery or pharmaceutical treatments, to manage the serious chronic disease of obesity.
Bariatric surgery is a suitable option to reduce weight, mortality, and incidence of CVD in people with severe obesity. 21 Surgical intervention provides substantial weight loss and may attenuate obesity‐associated comorbidities. 22 Recent trials reported reductions in body weight at 5 years following a Roux‐en‐Y gastric bypass (≈25%), 23 sleeve gastrectomy (≈16%), 24 and laparoscopic‐adjustable gastric banding (≈13%). 23 A recent systematic review and meta‐analysis concluded that bariatric surgery was associated with reductions in all‐cause and cardiovascular mortality, and lowered the incidence of several cardiovascular outcomes in patients with obesity. 21 However, historically only patients with class 3 obesity (BMI ≥40 kg/m2) or with class 2 obesity (BMI ≥35 to <40 kg/m2) and an associated comorbidity would qualify for bariatric surgery. 25 Additionally, like most surgical procedures, bariatric interventions are associated with potential complications. Postoperative complications vary depending on the type of procedure and risk factors of individual patients, and include fistulae, strictures, obstruction, hemorrhage, and gastroesophageal reflux. 26 , 27 However, improved surgical techniques (eg, laparoscopic procedures) have reduced the incidence of serious complications, resulting in current perioperative mortality rates of <0.2%. 21 , 27 , 28 Overall, Roux‐en‐Y gastric bypass is the most effective bariatric procedure for weight management, although this surgical method is associated with a higher rate of reoperation and associated complications compared with sleeve gastrectomy and laparoscopic‐adjustable gastric banding. 22 , 24
Before the introduction of incretin‐based therapies in recent years, only a few pharmaceutical treatments for the management of overweight and obesity were available. Some of these treatments were associated with reductions in cardiovascular risk factors, 29 but they were not tested for the ability to reduce actual cardiovascular events in large‐scale outcome trials. Historically, antiobesity medications included anorectics such as rimonabant, sibutramine, and phentermine‐fenfluramine. Despite their effectiveness for short‐term weight management, these drugs were withdrawn from the market for various reasons, such as serious adverse psychiatric effects, 30 increased risk of myocardial infarction and stroke, 31 and valvular heart disease. 32 Lorcaserin, a selective 5‐hydroxytryptamine 2C receptor agonist, was shown to achieve ≥5% weight loss after 1 year in 38.7% of participants in the CAMELLIA–TIMI 61 (Cardiovascular and Metabolic Effects of Lorcaserin in Overweight and Obese Patients–Thrombolysis in Myocardial Infarction 61) trial 33 ; however, it was withdrawn from the market due to concerns over an increased risk of cancer among patients taking the drug. 34
Orlistat, a lipase inhibitor that reduces absorption of dietary fats, was approved for the treatment of obesity by the Food and Drug Administration (FDA) in 1999. A systematic review of placebo‐controlled studies showed orlistat to be consistent in producing weight loss, but only to a modest extent (≈2.8 kg overall versus placebo over 1 year). 35 Furthermore, lipase inhibitors can produce unfavorable gastrointestinal side effects, 36 limiting their usefulness in clinical practice.
Current antiobesity medications approved by the FDA for chronic weight management include naltrexone‐bupropion, a combination of opioid antagonist and aminoketone antidepressant, 37 and phentermine‐topiramate, which suppresses appetite, reduces food cravings, and enhances weight loss in patients with obesity. 38 Although the effect of phentermine‐topiramate on CV outcomes has not been fully established, in the SEQUEL extension trial (A Phase 3, Double‐Blind, Placebo‐Controlled, Multicenter Extension Study (From Study OB‐303 [NCT00553787]) to Determine the Safety and Efficacy Of VI‐0521 for the Long‐Term Treatment Of Obesity in Adults With Obesity‐Related Co‐Morbid Conditions), 2‐year treatment with phentermine‐topiramate (15 mg/92 mg) resulted in a mean weight loss of 10.5% and improved CV risk factors in patients with overweight and obesity, and select cardiometabolic diseases such as hypertension, diabetes or associated disorders. 39 However, phentermine has been associated with elevated heart rate and blood pressure, 38 , 40 , 41 and further investigation is warranted to assess its long‐term efficacy and safety in patients at elevated cardiovascular risk.
Crucially, among the historical and current antiobesity medications, there is limited evidence on long‐term safety, efficacy, and cardiovascular outcomes, with only a few long‐term randomized controlled trials conducted and none demonstrating reduction in cardiovascular events or mortality. 30 , 31 , 33 , 40 , 41 , 42 , 43 , 44
Emerging Role for GLP‐1 Receptor Agonists in Obesity
GLP‐1 is an incretin‐peptide hormone secreted from the small intestine following food ingestion. It signals via the GLP‐1R (GLP‐1 receptor), which is present in various organs, including the brain, pancreas, and gastrointestinal tract. 45 , 46 GLP‐1 RAs are a class of drugs that mimic the naturally occurring GLP‐1 hormone and act glucose‐dependently on GLP‐1Rs in the pancreas to stimulate insulin secretion and inhibit glucagon release. 47 Due to this mechanism, they are widely used for the treatment of T2D to help regulate glucose levels. 48 In addition to glucose regulation, GLP‐1 RAs act on GLP‐1Rs found in the gastrointestinal tract, reducing the rate of gastric emptying, and in the brain. 49 Obesity‐related benefits resulting from GLP‐1 RA actions in the brain include reduction in body weight, appetite, food cravings, and energy intake, along with increased satiety and improved eating control. 49 Emerging evidence also suggests potential CVD risk reduction (based on improvement in key cardiovascular risk factors; see Table 1) with GLP‐1 RAs in people with overweight or obesity. 50
Table 1.
Trial Data Investigating the Use of Glucagon‐Like Peptide‐1 Receptor Agonists for the Treatment of Obesity
| Study | Study design and population | Treatment arms | Baseline data, mean, overall | Changes in end points during the randomization period, active trial drug vs placebo |
|---|---|---|---|---|
| SCALE Obesity and Prediabetes 68 | 56‐week, double‐blind, randomized placebo‐controlled trial in people with obesity/overweight and without T2D | Once‐daily subcutaneous liraglutide 3.0 mg (n=2487) vs placebo (n=1244) |
Body weight, 106.2 kg BMI, 38.3 mg/kg2 WC, ≈115 cm Systolic BP, ≈123 mm Hg Diastolic BP, ≈78.8 mm Hg Total cholesterol, ≈194 mg/dL Triglycerides, ≈127 mg/dL |
Body weight, % change: −8.0 vs −2.6 (ETD: −5.4 [95% CI, –5.8 to −5.0]), P<0.001 BMI, mg/kg2: −3.0% vs −1.0% (ETD: −2.0 [95% CI, −2.2 to −1.9], P<0.001) WC, cm: −8.2 vs −3.9 (ETD: −4.2 [95% C, −4.7 to −3.7], P<0.001) Systolic BP, mm Hg: −4.2 vs −1.5 (ETD: −2.8 [95% CI, −3.56 to −2.09], P<0.001) Diastolic BP, mm Hg: −2.6 vs −1.9 (ETD: −0.9 [95% CI, −1.41 to −0.37], P<0.001) Total cholesterol, mg/dL: −3.1% vs −1.0% (ETD: −2.3 [95% CI, −3.3 to −1.3], P<0.001) Triglycerides, mg/dL: −13.3% vs −5.5% (ETD: −9.3 [95% CI, −11.5 to −7.0], P<0.001) |
| SCALE Diabetes 67 | 56‐week, double‐blind, randomized placebo‐controlled trial in people with obesity/overweight and T2D | Once‐daily subcutaneous liraglutide 3.0 mg (n=423) vs liraglutide 1.8 mg (n=211) vs placebo (n=212) |
Body weight, ≈106 kg BMI, ≈37 mg/kg2 WC, ≈118 cm Systolic BP, ≈129 mm Hg Diastolic BP, ≈79 mm Hg Total cholesterol, ≈169–≈178 mg/dL Triglycerides, 158–170 mg/dL |
Body weight, % change: −6.0 vs −2.0 (ETD for liraglutide 3.0 mg vs placebo: −4.00 [95% CI, –5.10 to −2.90], P<0.001) BMI, mg/kg2: −2.2% vs −0.8% (ETD for liraglutide 3.0 mg vs placebo: −1.50 [95% CI, –1.83 to −1.18], P<0.001) WC, cm: −6.1 vs −2.7 (ETD for liraglutide 3.0 mg vs placebo: −3.22 [95% CI, –4.20 to −2.23], P<0.001) Systolic BP, mm Hg: −2.8 vs −0.4 (ETD for liraglutide 3.0 mg vs placebo: −2.59 [95% CI, –4.56 to −0.62], P=0.01) Diastolic BP, mm Hg: −0.9 vs −0.5 (ETD for liraglutide 3.0 mg vs placebo: −0.36 [95% CI, –1.69 to 0.96], P=0.59) Total cholesterol, geometric mean (CoV), %: −1.46 vs 3.8 (ETD for liraglutide 3.0 mg vs placebo: 0.96 [95% CI, 0.94 to 0.99], P=0.01) Triglycerides, geometric mean (CoV), %: −14.68 vs 0.41 (ETD for liraglutide 3.0 mg vs placebo: 0.86 [95% CI, 0.80 to 0.92], P<0.001) |
| SCALE Maintenance 69 | 56‐week, double‐blind, randomized placebo‐controlled trial in people with obesity or overweight (with comorbidities), without T2D who lost ≥5% of initial weight during a low‐calorie diet run‐in | Once‐daily subcutaneous liraglutide 3.0 mg (n=212) vs placebo (n=210) |
Body weight, ≈106 kg BMI, ≈38 mg/kg2 WC, ≈113.5 cm Systolic BP, ≈123 mm Hg Diastolic BP, 78.5 mm Hg Total cholesterol, ≈5.0 mmol/L Triglycerides, ≈1.55 mmol/L |
Body weight, % change: −6.2 vs −0.2 (ETD: −6.1 [95% CI, −7.5 to −4.6], P<0.0001) BMI, mg/kg2: −2.1% vs −0.0% (ETD: −2.1 [95% CI, –2.5 to −1.6], P<0.0001) WC, cm: −4.7 vs −1.2 (ETD: −3.5 [95% CI, –4.8 to −2.2], P<0.0001) Systolic BP, mm Hg: 0.2 vs 2.8 (ETD: −2.7 [95% CI, –4.7 to −0.8], P=0.007) Diastolic BP, mm Hg: 1.4 vs 1.2 (ETD: −0.3 [95% CI –1.7 to 1.1], P=0.64) Total cholesterol, mg/dL: 0.2% vs 0.3% (ETD: −0.1 [95% CI, –0.2 to 0.03], P=0.11) Triglycerides, mg/dL: 0% vs 0.1% (ETD: −0.11 [95% CI, –0.20 to −0.01], P=0.03) |
| STEP 1 71 | 68‐week double‐blind, randomized placebo‐controlled trial in people with overweight (plus at least 1 untreated weight‐related comorbidity) or obesity, without T2D | Once‐weekly subcutaneous semaglutide 2.4 mg (n=1306) vs placebo (n=655) |
Body weight, ≈105 kg BMI, ≈38 mg/kg2 WC, ≈115 cm Systolic BP, ≈126 mm Hg Diastolic BP, 80 mm Hg Total cholesterol, ≈190–≈192 mg/dL Triglycerides, 126–128 mg/dL |
Body weight, % change: −14.9% vs −2.4% (ETD: −12.4%‐points [95% CI, –13.4 to −11.5], P<0.001) BMI, mg/kg2: −5.5 vs −0.9 (ETD: −4.6 [95% CI, –5.0 to −4.3]) WC, cm: −13.54 vs −4.13 (ETD: −9.42 [95% CI, –10.30 to −8.53], P<0.001) Systolic BP, mm Hg: −6.16 vs −1.06, (ETD: −5.10 [95% CI, –6.34 to −3.87], P<0.001) Diastolic BP, mm Hg: −2.83 vs −0.42 (ETD: −2.41 [95% CI, –3.25 to −1.57]) Total cholesterol, ratio of week 68 to baseline: 0.97 vs 1.00 (ETD: 0.97 [95% CI, 0.95 to 0.98]) Triglycerides, ratio of week 68 to baseline: 0.78 vs 0.93 (ETD: 0.84 [95% CI, 0.81 to 0.87]) |
| STEP 2 72 | 68‐week double‐blind, randomized placebo‐controlled trial in people with obesity/overweight and T2D | Once‐weekly subcutaneous semaglutide 2.4 mg (n=404) vs semaglutide 1.0 mg (n=403) vs placebo (n=403) |
Body weight, 99.8 kg BMI, 35.7 mg/kg2 WC, 114.6 cm Systolic BP, 130 mm Hg Diastolic BP, 80 mm Hg Total cholesterol, 4.4 mmol/L Triglycerides, 1.8 mmol/L |
Body weight, % change: −9.64% vs −6.99% vs −3.42% (placebo) (ETD for semaglutide 2.4 mg vs placebo: −6.21 [95% CI, −7.28 to −5.15], P<0.0001) BMI, mg/kg2: −3.5 vs −2.5 vs −1.3 (ETD for semaglutide 2.4 mg vs placebo: −2.3 [95% CI, −2.6 to −1.9]) WC, cm: −9.4 vs −6.7 vs −4.5 (ETD for semaglutide 2.4 mg vs placebo: −4.9 [95% CI, −6.0 to −3.8]; P<0.0001) Systolic BP, mm Hg: −3.9 vs −2.9 vs −0.5 (ETD for semaglutide 2.4 mg vs placebo: −3.4 [95% CI, –5.6 to −1.3], P=0.0016) Diastolic BP, mm Hg: −1.6 vs −0.6 vs −0.9 (ETD for semaglutide 2.4 mg vs placebo: −0.7 [95% CI, −2.0 to 0.6]) Total cholesterol, ratio of week 68 to baseline: 0.99 vs 0.98 vs 0.99 (ETR for semaglutide 2.4 mg vs placebo: 0.99 [95% CI, 0.96 to 1.02]) Triglycerides, ratio of week 68 to baseline: 0.78 vs 0.83 vs 0.91 (ETR for semaglutide 2.4 mg vs placebo: 0.86 [95% CI, 0.81 to 0.92]) |
| STEP 3 73 | 68‐week double‐blind, randomized placebo‐controlled trial in people with overweight (plus at least 1 weight‐related comorbidity) or obesity, without diabetes, combined with intensive behavioral therapy | Once‐weekly subcutaneous semaglutide 2.4 mg (n=407) vs placebo (n=204) |
Body weight, ≈107–≈104 kg BMI, ≈38 mg/kg2 WC, ≈113 cm Systolic BP, ≈124 mm Hg Diastolic BP, ≈80 mm Hg Total cholesterol, ≈185 – ≈189 mg/dL Triglycerides, 108–111 mg/dL |
Body weight, % change: −16.0 vs −5.7 (ETD: −10.3 [95% CI, –12.0 to −8.6], P<0.001) BMI, mg/kg2: −6.0 vs −2.2 (ETD: −3.8 [95% CI, –4.4 to −3.1], P<0.001) WC, cm: −14.6 vs −6.3 (ETD: −8.3 [95% CI, –10.1 to −6.6], P<0.001) Systolic BP, mm Hg: −5.6 vs −1.6 (ETD: −3.9 [95% CI, –6.4 to −1.5], P=0.001) Diastolic BP, mm Hg: −3.0 vs −0.8 (ETD: −2.2 [95% CI, –3.9 to −0.6], P=0.008) Total cholesterol, % change at week 68: −3.8 vs 2.1 (ETD: −5.8 [95% CI, –8.4 to −3.2], P<0.001) Triglycerides, % change at week 68: −22.5 vs −6.5 (ETD: −17.0 [95% CI, –22.8 to −10.8], P<0.001) |
| STEP 4 74 | 68‐wk double‐blind, randomized placebo‐controlled trial evaluating sustained weight loss in people with overweight (plus at least 1 weight‐related comorbidity) or obesity, without diabetes | 20‐wk run‐in: once‐weekly subcutaneous semaglutide 2.4 mg (n=902); 48‐week randomized period: once‐weekly subcutaneous semaglutide 2.4 mg (n=535) vs placebo (n=268) |
Body weight, 107.2 kg BMI, 38.4 mg/kg2 WC, 115.3 cm Systolic BP, 127 mm Hg Diastolic BP, 81 mm Hg Total cholesterol, 194.6 mg/dL Triglycerides, 117.5 mg/dL |
Body weight, % change: −7.9 vs 6.9 (ETD: −14.8 [95% CI, −16.0 to −13.5], P<0.001) BMI, mg/kg2: −2.6 vs 2.2 (ETD: −4.7 [95% CI, −5.2 to −4.3], P<0.001) WC, cm: −6.4 vs 3.3 (ETD: −9.7 [95% CI, −10.9 to −8.5], P<0.001) Systolic BP, mm Hg: 0.5 vs 4.4 (ETD: −3.9 [95% CI, −5.8 to −2.0], P<0.001) Diastolic BP, mm Hg 0.3 vs 0.9 (ETD: −0.6 [95% CI, −2.0 to 0.9], P=0.46) Total cholesterol, % change: 5 vs 11 (ETD: −6 [95% CI, −8 to −4], P<0.001) Triglycerides, % change: −6 vs 15, (ETD: −18 [95% CI, −24 to −11], P<0.001) |
| SURMOUNT‐1 78 | 72‐wk double‐blind, randomized placebo‐controlled trial in people with overweight (plus at least 1 weight‐related comorbidity) or obesity, without diabetes | Once‐weekly subcutaneous tirzepatide 5 mg (n=630) vs tirzepatide 10 mg (n=636) vs tirzepatide 15 mg (n=630) vs placebo (n=643) |
Body weight, 104.8 kg BMI, 38 mg/kg2 WC, 114.1 cm Systolic BP, 123.3 mm Hg Diastolic BP, 79.5 mm Hg Total cholesterol, 187.9 mg/dL Triglycerides, 128.4 mg/dL |
Body weight, % change: −15.0 (5 mg; ETD: −11.9% points [95% CI, −13.4 to −10.4]) vs −19.5 (10 mg; ETD: −16.4% points [95% CI, −17.9 to −14.8]) vs −20.9 (15 mg; ETD: −17.8% points [95% CI, −19.3 to −16.3]) vs −3.1 (placebo) WC, cm: −14.0 (5 mg; ETD: −10.1 [95% CI, −1.6 to −8.6]) vs −17.7 (10 mg; ETD: −13.8 [95% CI, −15.2 to −12.3]) vs −18.5 (15 mg; ETD: −14.5 [95% CI −15.9 to −13.0]) vs −4.0 (placebo) Systolic BP, mm Hg: † −7.2 vs −1.0 (ETD: −6.2 [95% CI, −7.7 to −4.8]) Diastolic BP, mm Hg: † −4.8 vs −0.8 (ETD: −4.0 [95% CI, −4.9 to −3.1]) Total cholesterol, mg/dL: † −4.8 vs −1.8 (ETD: −3.1 [95% CI, −5.2 to −1.0]) Triglycerides, mg/dL: † −24.8 vs −5.6 (ETD: −20.3 [95% CI, −24.3 to −16.1]) All comparisons for tirzepatide vs placebo were significant at P<0.001 |
BP indicates blood pressure; BMI, body mass index; CoV, coefficient of variation; ETD, estimated treatment difference; ETR, estimated treatment ratio; SCALE, Satiety and Clinical Adiposity – Liraglutide Evidence in individuals with and without diabetes; STEP 1, Research Study Investigating How Well Semaglutide Works in People Suffering From Overweight or Obesity; STEP 2, Research Study Investigating How Well Semaglutide Works in People With Type 2 Diabetes Suffering From Overweight or Obesity; STEP 3, Research Study to Look at How Well Semaglutide is at Lowering Weight When Taken Together With an Intensive Lifestyle Program; SURMOUNT‐1, Research Study Investigating How Well Semaglutide Works in People Suffering From Overweight or Obesity (STEP 4); A Study of Tirzepatide (LY3298176) in Participants With Obesity or Overweight; T2D, type 2 diabetes; and WC, waist circumference.
Where pooled data for all study groups were not given in the original publication, the data have been rounded/approximated for convenience so as to be illustrative of the overall study cohorts.
Pooled data for the 5‐mg, 10‐mg, and 15‐mg tirzepatide groups vs placebo.
Trial Data Supporting a Reduction in CVD Risk With the Use of GLP‐1 RAs in People With T2D
Because T2D is associated with an increased risk of CVD, regulators recommended in 2008 that clinical trial programs for all new T2D therapies should demonstrate that the therapy will not result in an unacceptable increase in cardiovascular risk. 51 Following this guidance, numerous trials were conducted in people with T2D to evaluate the effect of GLP‐1 RAs on cardiovascular outcomes (Table 2). 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 These phase 3 clinical trials all included a large population, ranging from 3183 participants Peptide Innovation for Early Diabetes Treatment (PIONEER)‐6 55 to 14 752 participants Exenatide Study of Cardiovascular Event Lowering Trial (EXSCEL). 56 The median follow‐up times ranged from 15.9 months (PIONEER‐6) 55 to 5.4 years Researching Cardiovascular Events With a Weekly Incretin in Diabetes (REWIND) 59 (Table 2). In these trials, the GLP‐1 RAs under investigation were all shown to be noninferior to placebo for the primary composite cardiovascular end point, time to first major adverse cardiovascular event (MACE; which included cardiovascular death, along with other cardiovascular end points such as myocardial infarction, stroke, and hospitalization for unstable angina), confirming cardiovascular safety. Furthermore, of the long‐acting GLP‐1 RAs, Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER), semaglutide (SUSTAIN‐6), albiglutide (Harmony Outcomes), dulaglutide (REWIND), and Effect of Efpeglenatide on Cardiovascular Outcomes (AMPLITUDE‐O) 60 were all shown to be superior to placebo in reduction of the primary MACE end point (Table 2), suggesting significant prevention of CVD. These studies were included in a recent meta‐analysis, which found that GLP‐1 RAs reduced the risk of MACEs by 14%, with a hazard ratio (HR) of 0.86 (95% CI, 0.80–0.93; P<0.0001), all‐cause mortality by 12% (HR, 0.88 [95% CI, 0.82–0.94]; P=0.0001), and hospital admission for heart failure by 11% (HR, 0.89 [95% CI, 0.82–0.98]; P=0.013). 61 No significant heterogeneity was found between the effect of a GLP‐1 RA in the prevention of MACEs in patients at risk (without CVD) and those with CVD, or between trials when ranked low, intermediate, or high risk based on MACE rates in the placebo group. 61 Overall, these studies demonstrate that GLP‐1 RAs have a significant favorable effect on cardiovascular outcomes in patients with T2D. Once‐daily subcutaneous liraglutide and once‐weekly subcutaneous semaglutide and dulaglutide have been approved by the FDA for risk reduction of MACEs in adults with T2D and established CVD. 62 , 63 , 64
Table 2.
Trial Data Investigating Change in CVD Risk With the Use of Glucagon‐Like Peptide‐1 Receptor Agonists in Patients With T2D
| Study | Study design and population | Treatment arms | Median follow‐up period | Primary composite end point, time to first occurrence | Primary outcome, active trial drug vs placebo, % of patients |
|---|---|---|---|---|---|
| ELIXA 52 | Double‐blind, randomized placebo‐controlled trial in people with T2D who had a recent acute coronary syndrome | Once‐daily subcutaneous lixisenatide 20 μg (n=3034) vs placebo (n=3034) | 25 mo | Cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for unstable angina | 13.4% vs 13.2% (HR, 1.02 [95% CI, 0.89–1.17]; P<0.001 for noninferiority; P=0.81 for superiority) |
| LEADER 53 | Double‐blind, randomized placebo‐controlled trial in people with T2D and high cardiovascular risk | Once‐daily subcutaneous liraglutide 1.8 mg (n=4668) vs placebo (n=4672) | 3.8 y | Death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke | 13.0% vs 14.9% (HR, 0.87 [95% CI, 0.78–0.97]; P<0.001 for noninferiority; P=0.01 for superiority) |
| SUSTAIN 6 54 | Double‐blind, randomized placebo‐controlled trial in people with T2D at high cardiovascular risk | Once‐weekly subcutaneous semaglutide 0.5–1.0 mg (n=1648) vs placebo (n=1649) | 2.1 y | Cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke | 6.6% vs 8.9% (HR, 0.74 [95% CI, 0.58–0.95]; P<0.001 for noninferiority, P=0.02 for superiority) |
| PIONEER‐6 55 | Double‐blind, randomized placebo‐controlled trial in people with T2D with high cardiovascular risk | Once‐daily oral semaglutide 14 mg (n=1591) vs placebo (n=1592) | 15.9 mo | Major adverse cardiovascular event (death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke) | 3.8% vs 4.8% (HR, 0.79 [95% CI, 0.57–1.11]; P<0.001 for noninferiority, P=0.17 for superiority) |
| EXSCEL 56 | Double‐blind, randomized placebo‐controlled trial in people with T2D with or without previous CVD | Once‐weekly subcutaneous exenatide 2 mg (n=7356) vs placebo (n=7396) | 3.2 y | Death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke | 11.4% vs 12.2% (HR, 0.91 [95% CI, 0.83–1.00]; P<0.001 for noninferiority; P=0.06 for superiority) |
| Harmony Outcomes 58 | Double‐blind, randomized placebo‐controlled trial in people with T2D and CVD | Once‐weekly subcutaneous albiglutide 30–50 mg (n=4731) vs placebo (n=4732) | 1.6 y | Cardiovascular death, myocardial infarction, or stroke | 7% vs 9% (HR, 0.78 [95% CI, 0.68–0.90]; P<0.0001 for noninferiority; P=0.0006 for superiority) |
| REWIND 59 | Double‐blind, randomized placebo‐controlled trial in people with T2D with previous CVD or cardiovascular risk factors | Once‐weekly subcutaneous dulaglutide 1.5 mg (n=4949) vs placebo (n=4952) | 5.4 y | Nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes (including unknown causes) | 12.0% vs 13.4% (HR, 0.88 [95% CI, 0.79–0.99]; P=0.026) |
| AMPLITUDE‐O 60 | Blinded, randomized placebo‐controlled trial in people with T2D and a history of CVD or current kidney disease with at least 1 additional cardiovascular risk factor | Once‐weekly subcutaneous efpeglenatide 4 or 6 mg (n=2717) vs placebo (n=1359) | 1.8 y | Major adverse cardiovascular event (nonfatal myocardial) infarction, nonfatal stroke, or death from cardiovascular or undetermined causes | 7.0% vs 9.2% (HR, 0.73 [95% CI, 0.58–0.92]; P<0.001 for noninferiority; P=0.007 for superiority) |
AMPLITUDE‐O indicates Effect of Efpeglenatide on Cardiovascular Outcomes; CVD, cardiovascular disease; ELIXA, Evaluation of Lixisenatide in Acute Coronary Syndrome; EXSCEL, Exenatide Study of Cardiovascular Event Lowering Trial; HR, hazard ratio; LEADER, Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results; mo, months PIONEER‐6, Peptide Innovation for Early Diabetes Treatment 6; REWIND, Researching Cardiovascular Events With a Weekly Incretin in Diabetes; SUSTAIN 6, Trial to Evaluate Cardiovascular and Other Long‐term Outcomes With Semaglutide in Subjects With Type 2 Diabetes; T2D, type 2 diabetes; and y, years.
In addition to the primary outcome, it was noted in the trials described in Table 2 that there were significant reductions from baseline for several cardiovascular risk factors, including body weight, glycated hemoglobin, and systolic blood pressure, in the GLP‐1 RA treatment groups compared with placebo. The PIONEER‐6 trial demonstrated a difference of −3.4 kg weight loss between oral semaglutide 14 mg and placebo. Greater weight loss was achieved with higher doses of semaglutide (1.0 mg subcutaneous formulation and 14 mg oral formulation) 54 , 55 compared with the lower dose (0.5 mg subcutaneous). 54 Similarly, in the SUSTAIN‐6 trial, there were mean changes of −4.9 kg in the group receiving 1.0 mg subcutaneous semaglutide and −3.6 kg in those receiving 0.5 mg, versus placebo. 54 There are several factors that may contribute to the cardioprotective effects of GLP‐1 RAs. Although the exact mechanism of cardiovascular risk reduction from GLP‐1 RAs is unknown, similar cardiovascular benefits have not been demonstrated when using other effective glucose‐lowering therapies, such as insulin, for the treatment of T2D. 65 , 66 The cardiovascular benefits of GLP‐1 RAs seem to be independent of their glycated hemoglobin‐lowering effects. This indicates that the improved cardiovascular outcomes following GLP‐1 RA treatment may occur through a unique glucose‐independent mechanism, and thus could be beneficial to populations beyond patients with T2D.
Trial Data Supporting the Use of GLP‐1 RAs in Obesity
GLP‐1 RAs have also been shown to be effective for weight management and maintenance of weight loss in the treatment of people with overweight or obesity (Table 1). Because absolute weight losses are greater for individuals without diabetes, trials included individuals with obesity, both with and without T2D. In the Phase 3a Satiety and Clinical Adiposity – Liraglutide Evidence in individuals with and without diabetes (SCALE) program, treatment with once‐daily subcutaneous liraglutide 3.0 mg was associated with significantly greater and sustained weight loss compared with placebo in individuals with overweight and obesity with and without diabetes. Additionally, improvements in various cardiometabolic risk factors were also seen (Table 1). 67 , 68 , 69 Similarly, in a post hoc analysis of the SCALE trials, it was determined that liraglutide 3.0 mg was not associated with excess cardiovascular risk. 70
In the Phase 3 Semaglutide Treatment Effect in People with Obesity (STEP) program, in people with overweight or obesity with and without diabetes, once‐weekly subcutaneous semaglutide 2.4 mg was associated with a sustained, clinically relevant reduction in body weight and a greater improvement in cardiometabolic risk factors, versus placebo (Table 1). 71 , 72 , 73 , 74 STEP 1 was a randomized, placebo‐controlled trial designed to compare the effect of once‐weekly subcutaneous semaglutide 2.4 mg versus placebo, as an adjunct to lifestyle intervention (reduced‐calorie diet and increased physical activity), on body weight in adults with overweight or obesity without T2D. 71 In STEP 1, the change in body weight from baseline (105.4±22.1 kg) to week 68 was −15.3 kg in the semaglutide group compared with −2.6 kg in the placebo group (baseline weight of 105.2±21.5 kg); estimated treatment difference of −12.7 kg (95% CI, –13.7 to −11.7). 71 The reduction in BMI from baseline (average BMI 37.8±6.7 kg/m2) to week 68 was −5.54 kg/m2 with semaglutide 2.4 mg versus −0.92 kg/m2 with placebo (baseline BMI of 38.0±6.5 kg/m2; estimated treatment difference −4.61 kg/m2 [95% CI, −4.96 to −4.27]). 71 In comparison, a previous meta‐analysis of randomized controlled trials investigating the effectiveness of bariatric surgery found that the overall estimated change in BMI after 1 year following surgery was −13.53 kg/m2 (95% CI, –15.51 to −11.55). 22 The results of a recent meta‐analysis support that treatment with GLP‐1 RAs is associated with cardiovascular risk reduction in adults with obesity but without diabetes. 75 However, a dedicated Semaglutide Effects on Cardiovascular Outcomes in People With Overweight or Obesity (SELECT; NCT03574597) assessing use of weekly subcutaneous semaglutide 2.4 mg in people with obesity and overweight at high cardiovascular risk but without T2D is still ongoing.
This demonstrates that GLP‐1 RAs, in particular liraglutide and semaglutide, can be used as part of a comprehensive, holistic approach to substantially improve weight loss outcomes in populations with overweight and obesity. Both liraglutide (3.0 mg once daily) and semaglutide (2.4 mg once weekly) have been approved by the FDA for chronic weight management in patients with obesity or overweight in the setting of having 1 weight‐related comorbidity (ie, T2D, hypertension, or dyslipidemia). 76 , 77 Additionally, tirzepatide, a GLP‐1 and glucose‐dependent insulinotropic peptide dual agonist, has been evaluated for the treatment of obesity. 78 In the A Study of Tirzepatide (LY3298176) in Participants With Obesity or Overweight (SURMOUNT‐1) trial, weekly subcutaneous tirzepatide conferred dose‐dependent reductions in weight compared with placebo, with the highest tirzepatide dose (15 mg) demonstrating a reduction of 23.6 kg, versus 2.4 kg with placebo (baseline weight 105.6±22.92 kg and 104.8±21.37 kg, respectively) at 72 weeks. 78 Tirzepatide is approved for the treatment of T2D, but has not yet received FDA approval for chronic weight management at the time of this review. However, tirzepatide has received fast‐track designation by the FDA for use in those with obesity or overweight with weight‐related comorbidities.
Limitations of the aforementioned trials were that they enrolled participants who were predominantly of female sex and White race, with less data from other racial and ethnic groups, and the trials were of relatively short duration (<72 weeks). More data on the efficacy and safety of GLP‐1 RAs in other populations with overweight and obesity and trials of longer duration are needed.
Side Effects and Safety of GLP‐1 RA Therapy
Safety analyses performed in the phase 3 trials of GLP‐1 RAs demonstrate that gastrointestinal side effects represent the most frequently reported side effects. 70 , 71 , 72 , 73 , 74 , 78 In the Phase 3 STEP program, 81.3% to 95.8% of participants with overweight or obesity with and without T2D experienced ≥1 adverse event following once‐weekly subcutaneous use of semaglutide 2.4 mg. 71 , 72 , 73 , 74 Gastrointestinal adverse events were the most common and included nausea, diarrhea, vomiting, and constipation, most of which were transient and of mild‐to‐moderate severity. 71 , 72 , 73 , 74 The majority (78.9%–81.8%, versus 72.0% for placebo) of participants treated with tirzepatide in the SURMOUNT‐1 trial reported ≥1 adverse event, with the most common being mild‐to‐moderate gastrointestinal events. 78 Gastrointestinal side effects, such as nausea, are commonly reported upon initiation of GLP‐1 RA treatment; however, these often diminish within the first month of treatment and can be mitigated through a gradual dose‐escalation period and temporary dietary adjustments. 79 The commonly reported side effects associated with GLP‐1 RA use, along with clinical advice to manage and ameliorate these, are documented in Table 3.
Table 3.
General Guidance to Alleviate and Manage Side Effects Associated With GLP‐1 RA Therapy*
| Potential common side effects | Clinical guidance to alleviate and manage side effects 79 |
|---|---|
| Nausea | For gastrointestinal side effects:
|
| Diarrhea | |
| Vomiting | |
| Constipation | |
| Dyspepsia | |
| Decreased appetite | |
| Gastroesophageal reflux | |
| Gallbladder disorders |
|
| Increased heart rate or cardiac arrhythmias |
|
GLP‐1 RA indicates glucagon‐like peptide‐1 receptor agonist.
This commentary and advice are the opinions of the authors. Practical guidance is provided only on an advisory basis.
Preclinical studies found evidence of GLP‐1 RAs causing dose‐dependent and treatment duration‐dependent thyroid C‐cell tumors, 80 which are rare in humans. The potential risk of developing these tumors was included as a warning in the FDA approvals for all long‐acting GLP‐1 RAs. 62 , 63 , 64 , 76 , 77 , 81 , 82 However, since these approvals, clinical trials have found no evidence of an increased risk of any cancer with GLP‐1 RA therapy. 56 , 60 , 67 , 70 , 71 , 72 , 73 , 74 Additionally, there is a lack of evidence suggesting any psychiatric, metabolic, or cardiovascular complications associated with GLP‐1 RA therapy. In the aforementioned large meta‐analysis of 8 trials including >60 000 people with T2D, the incidence of severe hypoglycemia, retinopathy, pancreatitis, and pancreatic cancer did not differ significantly between the GLP‐1 RA and placebo treatment groups. 61 Overall, despite the mild‐to‐moderate gastrointestinal side effects associated with therapy initiation and dose escalation, GLP‐1 RAs have a well characterized and tolerable safety profile.
Although the cardiovascular outcome trials in people with T2D have reassuring safety data up to ≥5 years (Table 2), the length of trial follow‐up in people with overweight or obesity was of shorter duration, up to 72 weeks (Table 1). The ongoing SELECT cardiovascular outcome trial (NCT03574597) will provide additional insights into longer‐term safety data in this population, as well as data from continued postmarketing surveillance.
Generally, after initiation, GLP‐1 RA therapy is intended to be long term. Data from the STEP 1 trial showed that 1 year after stopping their GLP‐1 RA therapy, participants regained approximately two‐thirds of their prior weight loss. 83 It should be noted that weight regain has been commonly reported with cessation of other pharmacotherapies such as orlistat and locaserin, 84 , 85 as well as with lifestyle interventions. 86 , 87
Future of GLP‐1 RA Therapy in the Management of Obesity and CVD, and Current Barriers to Treatment
Until recently, GLP‐1 RAs were only available via injectable administration. Lixisenatide and liraglutide are available as once‐daily subcutaneous injections, 62 , 88 whereas exenatide extended release, 82 albiglutide, 64 and dulaglutide 64 are available as once‐weekly subcutaneous injections. Additionally, exenatide immediate release is available as a twice‐daily injection. 89 Semaglutide is available as both once‐weekly subcutaneous injection and once‐daily oral administration for the treatment of T2D. 63 , 81 The introduction of orally administered GLP‐1 RAs such as semaglutide could be especially beneficial for patients with T2D who have CVD risk factors (including overweight and obesity) and comorbidities for which they are using polypharmacy. Although the oral semaglutide formulation has not yet been demonstrated to reduce the risk of cardiovascular events, trial results showed a favorable trend in cardiovascular risk reduction and effectively improved some cardiovascular risk factors. 55 A dedicated cardiovascular outcome trial investigating oral semaglutide in T2D, A Heart Disease Study of Semaglutide in Patients With Type 2 Diabetes (SOUL) (NCT03914326), is, however, ongoing. 90 At this time, only subcutaneous semaglutide 2.4 mg and subcutaneous liraglutide 3.0 mg have an FDA indication specifically for chronic weight management.
Future Directions of GLP‐1 RAs
As mentioned above, the ongoing SELECT trial is investigating the effects of once‐weekly semaglutide 2.4 mg on risk of heart disease and stroke in people with overweight or obesity and established CVD but without T2D, and is expected to be completed in 2023. 91 Although more research is needed to determine the extent of weight loss and cardiovascular benefits of GLP‐1 RAs in populations without T2D, these drugs present a promising therapy option for patients with overweight or obesity, particularly among those at high risk for CVD.
In addition to the weight management benefits in people with overweight or obesity described above, GLP‐1 RAs may have benefits in people with nonalcoholic fatty liver disease and nonalcoholic steatohepatitis, which are diseases of the liver strongly associated with obesity. A meta‐analysis found that treatment of nonalcoholic fatty liver disease or nonalcoholic steatohepatitis with GLP‐1 RAs resulted in significant reductions in the percentage of liver fat content (mean difference, − 3.92% [95% CI, –6.27% to −1.56%]). 92 This evidence suggests that GLP‐1 RAs have the potential to treat a wider range of obesity‐related conditions in the future. Studies have also shown that GLP‐1 RAs may be beneficial for the treatment of other insulin‐resistant states, such as polycystic ovary syndrome, 93 or for patients with a history of gestational diabetes. 94
Current Barriers to Treatment
As alluded to earlier, some HCPs perceive obesity to be a lifestyle choice rather than a treatable serious chronic disease. This contributes to an insufficient rate of formal diagnosis and undertreatment by HCPs, major barriers in the successful treatment of obesity and CVD. In a survey of people with obesity in the US, 71% had discussed their weight with a HCP in the past 5 years, and 55% were diagnosed with obesity, of whom only 24% had a scheduled weight‐related follow‐up appointment. 95 Furthermore, in US patients who had recent myocardial infarction, just 9% of patients with obesity had weight management described as part of their goals or plans at discharge, 96 and in people with CVD and obesity, only 62% reported they had been informed that they had excess adiposity by a physician. Further analysis showed that physician‐diagnosed overweight or obesity was a significant predictor of weight loss (odds ratio, 2.70 [95% CI, 1.40–5.19]; P=0.001), 97 demonstrating the importance of clinician involvement in treating obesity in individuals with CVD.
Exacerbating the problems of underdiagnosis and undertreatment of obesity is the fact that, despite recent FDA‐approved medications (eg, semaglutide for weight management in adults with BMI ≥27 kg/m2 with at least 1 weight‐related comorbidity and patients with BMI ≥30 kg/m2), Medicare and most health insurers in the US will not cover antiobesity medications, meaning that patients are required to pay for these. 98 For many, these treatment costs are unaffordable and create an access barrier. Exclusion of antiobesity medications from insurance policies is likely a false economy, because the costs of treating the comorbidities associated with obesity may exceed the cost of the medications. 98
GLP‐1 RAs are generally covered by insurance policies when obesity is associated with T2D, but the evidence suggests that prescription rates and use of GLP‐1 RAs in US patients with T2D nevertheless remain low, particularly among those at high risk of CVD. 98 , 99 , 100 In addition, there are disparities in access to GLP‐1 RAs associated with racial and socioeconomic factors; patients with T2D who were of Asian, Black, or Hispanic race and ethnicity, or from lower income households, were found to have lower uptake of GLP‐1 RAs. 100 These health inequities prevent patients at high risk of T2D‐related cardiovascular morbidity from receiving adequate care. 100 Furthermore, American Diabetes Association guidelines for CVD and risk management recommend GLP‐1 RA use for patients with T2D at risk of CVD, 101 and American Heart Association/American College of Cardiology guidelines recommend GLP‐1 RA therapy in addition to metformin in patients with established atherosclerotic CVD (including ischemic stroke) and T2D in the prevention of further cardiovascular events. 102 Despite this, cardiologists account for a low proportion of GLP‐1 RA prescriptions, being responsible for just 0.4% of GLP‐1 RA prescriptions in the US in 2020. 103 Because there are far more cardiologists than endocrinologists or obesity medicine specialists in the US, patients with CVD risk factors are more likely to engage with cardiologists than obesity medicine specialists or endocrinologists. 100 , 104 This is supported by findings from a study in US patients with T2D that found that the ratio of cardiology‐to‐endocrinology outpatient encounters was 2:1 for all patients with T2D, and 4:1 for those with T2D and CVD. 104 Because obesity is known to increase cardiovascular risk in people with and without T2D, cardiologists should take an active role in obesity management to support weight‐loss goals and manage the risk of cardiovascular events in these patient populations.
Conclusions
The evidence discussed in this article demonstrates that GLP‐1 RAs should be strongly considered as an option for use in clinical practice for the treatment of obesity and reduction of CVD risk in people with T2D (Figure). Because cardiologists frequently see patients with overweight or obesity and T2D, they have a crucial role in initiating and managing weight loss in these patients to reduce their risk of CVD. If ongoing and future research prove GLP‐1 RAs to be effective in reducing the risk of CVD in patients with obesity, irrespective of T2D status, it will herald a new treatment paradigm in this setting, and now is the time for cardiologists to better recognize the benefits of these agents.
Figure 1. The role of GLP‐1 RAs in achieving weight loss and improving cardiovascular outcomes in people with overweight and obesity. 1 , 2 , 4 , 16 , 49 , 53 , 54 , 59 , 62 , 63 , 64 , 68 , 70 , 71 , 72 , 73 , 74 , 76 , 77 , 78 , 89 , 99 , 100 , 105 , 106 , 107 , 108 , 109 , 110 .
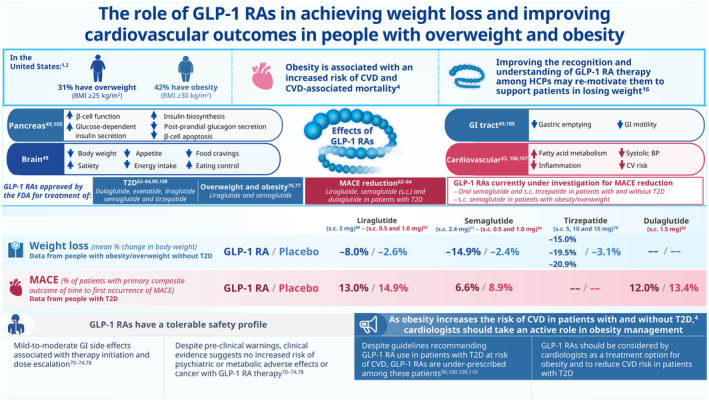
BP indicates blood pressure; BMI, body mass index; CV, cardiovascular; CVD, cardiovascular disease; FDA, Food and Drug Administration; GI, gastrointestinal; GLP‐1 RA, glucagon‐like peptide‐1 receptor agonist; HCP, health care professional; MACE, major adverse cardiovascular event; s.c., subcutaneous; and T2D, type 2 diabetes.
Sources of Funding
The authors of this article received no monetary or financial compensation. Novo Nordisk funded the medical writing support for this review.
Disclosures
E.D.M. has served on advisory boards for Amgen, Amarin, AstraZeneca, Bayer, Boehringer Ingelheim, Esperion, Novartis, Novo Nordisk, and Pfizer. F.L.‐J. has served on the advisory board for Novo Nordisk. M.G. has served on advisory boards for Novartis.
Acknowledgments
Medical writing support for the development of this article, under the direction of the authors, was provided by Carrie Fielden, MSc, of Ashfield MedComms, an Inizio company, and funded by Novo Nordisk, which also reviewed the article for medical accuracy. Author contributions: the concept for the article was agreed on by all authors and the sponsor. Drafts of the article were prepared by a medical writer, funded by the sponsor, and circulated to all authors for review. The sponsor commented on all drafts. All authors critically reviewed the article and approved the final version for submission.
This article was sent to Tiffany M. Powell‐Wiley, MD, MPH, Guest Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 11.
REFERENCES
- 1. Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960–1962 through 2017–2018. NCHS Health E‐Stats. Centers for Disease Control and Prevention. 2020. Accessed April 1, 2023. www.cdc.gov/nchs/data/hestat/obesity‐adult‐17‐18/obesity‐adult.htm [Google Scholar]
- 2. Stierman B, Afful J, Carroll MD, Chen TC, Davy O, Fink S, Fryar CD, Gu Q, Hales CM, Hughes JP, et al. National Health and Nutrition Examination Survey 2017–March 2020 prepandemic data files—development of files and prevalence estimates for selected health outcomes [published correction appears in NHSR No. 158]. National Health Statistics Reports. Centers for Disease Control and Prevention. 2021. Accessed April 1, 2023. https://www.cdc.gov/nchs/data/nhsr/nhsr158‐508.pdf.
- 3. Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, Long MW, Gortmaker SL. Projected U.S. state‐level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381:2440–2450. doi: 10.1056/NEJMsa1909301 [DOI] [PubMed] [Google Scholar]
- 4. Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co‐morbidities related to obesity and overweight: a systematic review and meta‐analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lopez‐Jimenez F, Almahmeed W, Bays H, Cuevas A, Di Angelantonio E, le Roux CW, Sattar N, Sun MC, Wittert G, Pinto FJ, et al. Obesity and cardiovascular disease: mechanistic insights and management strategies. A joint position paper by the World Heart Federation and World Obesity Federation. Eur J Prev Cardiol. 2022;29:2218–2237. doi: 10.1093/eurjpc/zwac187 [DOI] [PubMed] [Google Scholar]
- 6. Klop B, Elte JW, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013;5:1218–1240. doi: 10.3390/nu5041218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Powell‐Wiley TM, Poirier P, Burke LE, Després JP, Gordon‐Larsen P, Lavie CJ, Lear SA, Ndumele CE, Neeland IJ, Sanders P, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143:e984–e1010. doi: 10.1161/CIR.0000000000000973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi‐Lakeh M, Naghavi M, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haase CL, Lopes S, Olsen AH, Satylganova A, Schnecke V, McEwan P. Weight loss and risk reduction of obesity‐related outcomes in 0.5 million people: evidence from a UK primary care database. Int J Obes. 2021;45:1249–1258. doi: 10.1038/s41366-021-00788-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, Hill JO, Brancati FL, Peters A, Wagenknecht L, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–1486. doi: 10.2337/dc10-2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. El Hajj EC, El Hajj MC, Sykes B, Lamicq M, Zile MR, Malcolm R, O'Neil PM, Litwin SE. Pragmatic weight management program for patients with obesity and heart failure with preserved ejection fraction. J Am Heart Assoc. 2021;10:e022930. doi: 10.1161/JAHA.121.022930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX, Twomey D, Elliott AD, Kalman JM, Abhayaratna WP, et al. Long‐term Effect of Goal‐directed Weight Management in an Atrial Fibrillation Cohort: a Long‐term Follow‐up Study (LEGACY). J Am Coll Cardiol. 2015;65:2159–2169. doi: 10.1016/j.jacc.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 14. Middeldorp ME, Pathak RK, Meredith M, Mehta AB, Elliott AD, Mahajan R, Twomey D, Gallagher C, Hendriks JML, Linz D, et al. PREVEntion and regReSsive effect of weight‐loss and risk factor modification on atrial fibrillation: the REVERSE‐AF study. Europace. 2018;20:1929–1935. doi: 10.1093/europace/euy117 [DOI] [PubMed] [Google Scholar]
- 15. Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. Circulation. 2014;129:S102–S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shahed Q, Baranowska K, Marije C, Galavazi MC, Cao Y, van Nieuwenhoven MA. Doctors and patients' perspectives on obesity. A Q‐Methodology Study. Fam Pract. 2022;39:694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Look ARG, Gregg EW, Jakicic JM, Blackburn G, Bloomquist P, Bray GA, Clark JM, Coday M, Curtis JM, Egan C, et al. Association of the magnitude of weight loss and changes in physical fitness with long‐term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post‐hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016;4:913–921. doi: 10.1016/S2213-8587(16)30162-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Evert AB, Franz MJ. Why weight loss maintenance is difficult. Diabetes Spectr. 2017;30:153–156. doi: 10.2337/ds017-0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376:254–266. doi: 10.1056/NEJMra1514009 [DOI] [PubMed] [Google Scholar]
- 20. Mann T, Tomiyama AJ, Westling E, Lew AM, Samuels B, Chatman J. Medicare's search for effective obesity treatments: diets are not the answer. Am Psychol. 2007;62:220–233. doi: 10.1037/0003-066X.62.3.220 [DOI] [PubMed] [Google Scholar]
- 21. van Veldhuisen SL, Gorter TM, van Woerden G, de Boer RA, Rienstra M, Hazebroek EJ, van Veldhuisen DJ. Bariatric surgery and cardiovascular disease: a systematic review and meta‐analysis. Eur Heart J. 2022;43:1955–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta‐analysis, 2003‐2012. JAMA Surg. 2014;149:275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Courcoulas AP, Gallagher JW, Jakicic JM. Bariatric surgery vs lifestyle intervention for diabetes treatment: 5‐year outcomes from a randomized trial. J Clin Endocrinol Metab. 2020;105:866–876. doi: 10.1210/clinem/dgaa006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McTigue KM, Wellman R, Arterburn D. Comparing the 5‐year diabetes outcomes of sleeve gastrectomy and gastric bypass. JAMA Surg. 2020;155:e200087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, Nadolsky K, Pessah‐Pollack R, Plodkowski R. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22:1–203. doi: 10.4158/EP161365.GL [DOI] [PubMed] [Google Scholar]
- 26. Contival N, Menahem B, Gautier T, Le Roux Y, Alves A. Guiding the non‐bariatric surgeon through complications of bariatric surgery. J Visc Surg. 2018;155:27–40. [DOI] [PubMed] [Google Scholar]
- 27. Eisenberg D, Shikora SA, Aarts E, Aminian A, Angrisani L, Cohen RV, De Luca M, Faria SL, Goodpaster KPS, Haddad A, et al. 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of obesity and metabolic disorders (IFSO): indications for metabolic and bariatric surgery. Surg Obes Relat Dis. 2022;33:3–14. doi: 10.1007/s11695-022-06332-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Robertson AGN, Wiggins T, Robertson FP, Doleman B, Harrison EM, Hollyman M, Welbourn R. Perioperative mortality in bariatric surgery: meta‐analysis. BJS. 2021;108:892–897. doi: 10.1093/bjs/znab245 [DOI] [PubMed] [Google Scholar]
- 29. Ebbert JO, Elrashidi MY, Jensen MD. Managing overweight and obesity in adults to reduce cardiovascular disease risk. Curr Atheroscler Rep. 2014;16:445. doi: 10.1007/s11883-014-0445-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Topol EJ, Bousser MG, Fox KA, Creager MA, Despres JP, Easton JD, Hamm CW, Montalescot G, Steg PG, Pearson TA, et al. Rimonabant for prevention of cardiovascular events (CRESCENDO): a randomised, multicentre, placebo‐controlled trial. Lancet. 2010;376:517–523. doi: 10.1016/S0140-6736(10)60935-X [DOI] [PubMed] [Google Scholar]
- 31. James WPT, Caterson ID, Coutinho W, Finer N, Van Gaal L, Maggioni A, Torp‐Pedersen C, Sharma AM, Shepherd GM, Rode RA, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363:905–917. doi: 10.1056/NEJMoa1003114 [DOI] [PubMed] [Google Scholar]
- 32. Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, Schaff HV. Valvular heart disease associated with fenfluramine‐phentermine. N Engl J Med. 1997;337:581–588. doi: 10.1056/NEJM199708283370901 [DOI] [PubMed] [Google Scholar]
- 33. Bohula EA, Wiviott SD, McGuire DK, Inzucchi SE, Kuder J, Im K, Fanola CL, Qamar A, Brown C, Budaj A, et al. Cardiovascular safety of lorcaserin in overweight or obese patients. N Engl J Med. 2018;379:1107–1117. [DOI] [PubMed] [Google Scholar]
- 34. FDA requests the withdrawal of the weight‐loss drug Belviq, Belviq XR (lorcaserin) from the market. U.S. Food and Drug Administration. 2020. Accessed April 1, 2023. https://www.fda.gov/drugs/drug‐safety‐and‐availability/fda‐requests‐withdrawal‐weight‐loss‐drug‐belviq‐belviq‐xr‐lorcaserin‐market.
- 35. Hutton B, Fergusson D. Changes in body weight and serum lipid profile in obese patients treated with orlistat in addition to a hypocaloric diet: a systematic review of randomized clinical trials. Am J Clin Nutr. 2004;80:1461–1468. doi: 10.1093/ajcn/80.6.1461 [DOI] [PubMed] [Google Scholar]
- 36. May M, Schindler C, Engeli S. Modern pharmacological treatment of obese patients. Ther Adv Endocrinol Metab. 2020;11:2042018819897527. doi: 10.1177/2042018819897527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sherman MM, Ungureanu S, Rey JA. Naltrexone/bupropion ER (Contrave): newly approved treatment option for chronic weight management in obese adults. PT. 2016;41:164–172. [PMC free article] [PubMed] [Google Scholar]
- 38. QYSMIA (Phentermine and Topiramate Extended‐Release) Capsules. Prescribing Information. VIVUS Inc; 2012. Accessed April 1, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022580s000lbl.pdf [Google Scholar]
- 39. Garvey WT, Ryan DH, Look M, Gadde KM, Allison DB, Peterson CA, Schwiers ML, Day WW, Bowden CH. Two‐year sustained weight loss and metabolic benefits with controlled‐release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo‐controlled, phase 3 extension study. Am J Clin Nutr. 2012;95:297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Allison DB, Gadde KM, Garvey WT, Peterson CA, Schwiers ML, Najarian T, Tam PY, Troupin B, Day WW. Controlled‐release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity. 2012;20:330–342. doi: 10.1038/oby.2011.330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML, Day WW. Effects of low‐dose, controlled‐release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo‐controlled, phase 3 trial. Lancet. 2011;377:1341–1132. doi: 10.1016/S0140-6736(11)60205-5 [DOI] [PubMed] [Google Scholar]
- 42. Fujioka K, Plodkowski R, O'Neil PM, Gilder K, Walsh B, Greenway FL. The relationship between early weight loss and weight loss at 1 year with naltrexone ER/bupropion ER combination therapy. Int J Obes. 2016;40:1369–1375. doi: 10.1038/ijo.2016.67 [DOI] [PubMed] [Google Scholar]
- 43. Nissen SE, Wolski KE, Prcela L, Wadden TA, Buse JB, Bakris G, Perez A, Smith SR. Effect of naltrexone‐bupropion on major adverse cardiovascular events in overweight and obese patients with cardiovascular risk factors. JAMA. 2016;315:990–1004. [DOI] [PubMed] [Google Scholar]
- 44. Naltrexone/Bupropion Cardiovascular Outcomes Study [NCT02638129]. ClinicalTrials.gov. Accessed April 1, 2023. https://clinicaltrials.gov/ct2/show/NCT02638129.
- 45. Merchenthaler I, Lane M, Shughrue P. Distribution of pre‐pro‐glucagon and glucagon‐like peptide‐1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–280. [DOI] [PubMed] [Google Scholar]
- 46. Pyke C, Heller RS, Kirk RK, Ørskov C, Reedtz‐Runge S, Kaastrup P, Hvelplund A, Bardram L, Calatayud D, Knudsen LB. GLP‐1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014;155:1280–1290. doi: 10.1210/en.2013-1934 [DOI] [PubMed] [Google Scholar]
- 47. Nauck MA, Heimesaat MM, Behle K, Holst JJ, Nauck MS, Ritzel R, Hüfner M, Schmiegel WH. Effects of glucagon‐like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab. 2002;87:1239–1246. doi: 10.1210/jcem.87.3.8355 [DOI] [PubMed] [Google Scholar]
- 48. Meier JJ. GLP‐1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8:728–742. doi: 10.1038/nrendo.2012.140 [DOI] [PubMed] [Google Scholar]
- 49. Ard JD, Fitch A, Fruh S, Herman L. Weight loss and maintenance related to the mechanism of action of glucagon‐like peptide 1 receptor agonists. Adv Ther. 2021;38:2821–2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Iqbal J, Wu HX, Hu N, Zhou YH, Li L, Xiao F, Wang T, Jiang HL, Xu SN, Huang BL, et al. Effect of glucagon‐like peptide‐1 receptor agonists on body weight in adults with obesity without diabetes mellitus‐a systematic review and meta‐analysis of randomized control trials. Obes Rev. 2022;23:e13435. [DOI] [PubMed] [Google Scholar]
- 51. Diabetes mellitus: evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes. U.S. Food and Drug Administration. 2020. Accessed April 1, 2023. https://www.fda.gov/media/135936/download.
- 52. Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, Lawson FC, Ping L, Wei X, Lewis EF, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–2257. doi: 10.1056/NEJMoa1509225 [DOI] [PubMed] [Google Scholar]
- 53. Marso SP, Daniels GH, Brown‐Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141 [DOI] [PubMed] [Google Scholar]
- 55. Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, Jeppesen OK, Lingvay I, Mosenzon O, Pedersen SD, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841–851. doi: 10.1056/NEJMoa1901118 [DOI] [PubMed] [Google Scholar]
- 56. Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, Iqbal N, et al. Effects of once‐weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228–1239. doi: 10.1056/NEJMoa1612917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mentz RJ, Thompson VP, Aguilar D, Choi J, Gustavson SM, Iqbal N, Kong AP, Öhman P, Sattar N, Scott RS, et al. Effects of once‐weekly exenatide on clinical outcomes in patients with preexisting cardiovascular disease. Circulation. 2018;138:2576–2578. doi: 10.1161/CIRCULATIONAHA.118.036811 [DOI] [PubMed] [Google Scholar]
- 58. Hernandez AF, Green JB, Janmohamed S, D'Agostino RB Sr, Granger CB, Jones NP, Leiter LA, Rosenberg AE, Sigmon KN, Somerville MC, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double‐blind, randomised placebo‐controlled trial. Lancet. 2018;392:1519–1529. doi: 10.1016/S0140-6736(18)32261-X [DOI] [PubMed] [Google Scholar]
- 59. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Rydén L, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double‐blind, randomised placebo‐controlled trial. Lancet. 2019;394:121–130. doi: 10.1016/S0140-6736(19)31149-3 [DOI] [PubMed] [Google Scholar]
- 60. Gerstein HC, Sattar N, Rosenstock J, Ramasundarahettige C, Pratley R, Lopes RD, Lam CSP, Khurmi NS, Heenan L, Del Prato S, et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med. 2021;385:896–907. doi: 10.1056/NEJMoa2108269 [DOI] [PubMed] [Google Scholar]
- 61. Sattar N, Lee MMY, Kristensen SL, Branch KRH, Del Prato S, Khurmi NS, Lam CSP, Lopes RD, McMurray JJV, Pratley RE, et al. Cardiovascular, mortality, and kidney outcomes with GLP‐1 receptor agonists in patients with type 2 diabetes: a systematic review and meta‐analysis of randomised trials. Lancet Diabetes Endocrinol. 2021;9:653–662. doi: 10.1016/S2213-8587(21)00203-5 [DOI] [PubMed] [Google Scholar]
- 62. Victoza® (liraglutide) Injection. Prescribing Information. Novo Nordisk A/S; 2010. Accessed April 1, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/022341s027lbl.pdf [Google Scholar]
- 63. OZEMPIC (Semaglutide) Injection. Prescribing Information. Novo Nordisk A/S; 2017. Accessed April 1, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209637s003lbl.pdf [Google Scholar]
- 64. Trulicity (Dulaglutide) Injection. Prescribing Information. Eli Lilly and Company; 2014. Accessed April 1, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125469s007s008lbl.pdf [Google Scholar]
- 65. Gerstein HC, Bosch J, Dagenais GR, Díaz R, Jung H, Maggioni AP, Pogue J, Probstfield J, Ramachandran A, Riddle MC, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367:319–328. doi: 10.1056/NEJMoa1203858 [DOI] [PubMed] [Google Scholar]
- 66. Herman ME, O'Keefe JH, Bell DSH, Schwartz SS. Insulin therapy increases cardiovascular risk in type 2 diabetes. Prog Cardiovasc Dis. 2017;60:422–434. [DOI] [PubMed] [Google Scholar]
- 67. Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjøth TV, Andreasen AH, Jensen CB, DeFronzo RA. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;314:687–699. doi: 10.1001/jama.2015.9676 [DOI] [PubMed] [Google Scholar]
- 68. Pi‐Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, Lau DC, le Roux CW, Violante Ortiz R, Jensen CB, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11–22. doi: 10.1056/NEJMoa1411892 [DOI] [PubMed] [Google Scholar]
- 69. Wadden TA, Hollander P, Klein S, Niswender K, Woo V, Hale PM, Aronne L. Weight maintenance and additional weight loss with liraglutide after low‐calorie‐diet‐induced weight loss: the SCALE Maintenance randomized study. Int J Obes. 2013;37:1443–1451. doi: 10.1038/ijo.2013.120 [DOI] [PubMed] [Google Scholar]
- 70. Davies MJ, Aronne LJ, Caterson ID, Thomsen AB, Jacobsen PB, Marso SP. Liraglutide and cardiovascular outcomes in adults with overweight or obesity: a post hoc analysis from SCALE randomized controlled trials. Diabetes Obes Metab. 2018;20:734–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, McGowan BM, Rosenstock J, Tran MTD, Wadden TA, et al. Once‐weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989–1002. doi: 10.1056/NEJMoa2032183 [DOI] [PubMed] [Google Scholar]
- 72. Davies M, Færch L, Jeppesen OK, Pakseresht A, Pedersen SD, Perreault L, Rosenstock J, Shimomura I, Viljoen A, Wadden TA, et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double‐blind, double‐dummy, placebo‐controlled, phase 3 trial. Lancet. 2021;397:971–984. doi: 10.1016/S0140-6736(21)00213-0 [DOI] [PubMed] [Google Scholar]
- 73. Wadden TA, Bailey TS, Billings LK, Davies M, Frias JP, Koroleva A, Lingvay I, O'Neil PM, Rubino DM, Skovgaard D, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 2021;325:1403–1413. doi: 10.1001/jama.2021.1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rubino D, Abrahamsson N, Davies M, Hesse D, Greenway FL, Jensen C, Lingvay I, Mosenzon O, Rosenstock J, Rubio MA, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA. 2021;325:1414–1425. doi: 10.1001/jama.2021.3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Leite AR, Angélico‐Gonçalves A, Vasques‐Nóvoa F, Borges‐Canha M, Leite‐Moreira A, Neves JS, Ferreira JP. Effect of glucagon‐like peptide‐1 receptor agonists on cardiovascular events in overweight or obese adults without diabetes: a meta‐analysis of placebo‐controlled randomized trials. Diabetes Obes Metab. 2022;24:1676–1680. doi: 10.1111/dom.14707 [DOI] [PubMed] [Google Scholar]
- 76. WEGOVY (Semaglutide) Injection. Prescribing Information. Novo Nordisk A/S; 2017. Accessed April 1, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215256s000lbl.pdf [Google Scholar]
- 77. Saxenda (Liraglutide) Injection. Prescribing information. Novo Nordisk A/S; 2014. Accessed April 1, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206321Orig1s000lbl.pdf [Google Scholar]
- 78. Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, Kiyosue A, Zhang S, Liu B, Bunck MC, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387:205–216. [DOI] [PubMed] [Google Scholar]
- 79. Wharton S, Davies M, Dicker D, Lingvay I, Mosenzon O, Rubino DM, Pedersen SD. Managing the gastrointestinal side effects of GLP‐1 receptor agonists in obesity: recommendations for clinical practice. Postgrad Med. 2022;134:14–19. doi: 10.1080/00325481.2021.2002616 [DOI] [PubMed] [Google Scholar]
- 80. Knudsen LB, Madsen LW, Andersen S, Almholt K, de Boer AS, Drucker DJ, Gotfredsen C, Egerod FL. Glucagon‐like peptide‐1 receptor agonists activate rodent thyroid C‐cells causing calcitonin release and C‐cell proliferation. Endocrinology. 2010;151:1473–1486. doi: 10.1210/en.2009-1272 [DOI] [PubMed] [Google Scholar]
- 81. RYBELSUS (Semaglutide) Tablets. Prescribing Information. Novo Nordisk A/S; 2017. Accessed April 1, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/213051s000lbl.pdf [Google Scholar]
- 82. BYDUREON® (Exenatide) Injection. Prescribing Information. AstraZeneca Pharmaceuticals LP; 2005. Accessed April 1, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/022200s026lbl.pdf [Google Scholar]
- 83. Wilding JPH, Batterham RL, Davies M, Van Gaal LF, Kandler K, Konakli K, Lingvay I, McGowan BM, Oral TK, Rosenstock J, et al. Weight regain and cardiometabolic effects after withdrawal of semaglutide: the STEP 1 trial extension. Diabetes Obes Metab. 2022;24:1553–1564. doi: 10.1111/dom.14725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sjostrom L, Rissanen A, Andersen T, Boldrin M, Golay A, Koppeschaar HP, Krempf M. Randomised placebo‐controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352:167–172. [DOI] [PubMed] [Google Scholar]
- 85. Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, Bays H, Shanahan WR; Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) Study Group . Multicenter, placebo‐controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245–256. [DOI] [PubMed] [Google Scholar]
- 86. Look ARG, Chao AM, Wadden TA, Berkowitz RI, Blackburn G, Bolin P, Clark JM, Coday M, Curtis JM, Delahanty LM, et al. Weight change 2 years after termination of the intensive lifestyle intervention in the Look AHEAD study. Obesity. 2020;28:893–901. doi: 10.1002/oby.22769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Machado AM, Guimaraes NS, Bocardi VB, da Silva TPR, Carmo ASD, Menezes MC, Duarte CK. Understanding weight regain after a nutritional weight loss intervention: systematic review and meta‐analysis. Clin Nutr ESPEN. 2022;49:138–153. doi: 10.1016/j.clnesp.2022.03.020 [DOI] [PubMed] [Google Scholar]
- 88. ADLYXIN (Lixisenatide) Injection. Prescribing Information. Sanofi‐Aventis US; 2016. Accessed April 1, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208471orig1s000lbl.pdf [Google Scholar]
- 89. BYETTA® (Exenatide) Injection. Prescribing Information. Amylin Pharmaceuticals, Inc.; 2005. Accessed April 1, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021773s9s11s18s22s25lbl.pdf [Google Scholar]
- 90. A Heart Disease Study of Semaglutide in Patients With Type 2 Diabetes (SOUL) [NCT03914326]. ClinicalTrials.gov. Accessed April 1, 2023. https://clinicaltrials.gov/ct2/show/NCT03914326.
- 91. Ryan DH, Lingvay I, Colhoun HM, Deanfield J, Emerson SS, Kahn SE, Kushner RF, Marso S, Plutzky J, Brown‐Frandsen K, et al. Semaglutide Effects on Cardiovascular Outcomes in People With Overweight or Obesity (SELECT) rationale and design. Am Heart J. 2020;229:61–69. doi: 10.1016/j.ahj.2020.07.008 [DOI] [PubMed] [Google Scholar]
- 92. Mantovani A, Petracca G, Beatrice G, Csermely A, Lonardo A, Targher G. Glucagon‐like peptide‐1 receptor agonists for treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: an updated meta‐analysis of randomized controlled trials. Metabolites. 2021;11:73. doi: 10.3390/metabo11020073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Siamashvili M, Davis SN. Update on the effects of GLP‐1 receptor agonists for the treatment of polycystic ovary syndrome. Expert Rev Clin Pharmacol. 2021;14:1081–1089. [DOI] [PubMed] [Google Scholar]
- 94. Elkind‐Hirsch KE, Shaler D, Harris R. Postpartum treatment with liraglutide in combination with metformin versus metformin monotherapy to improve metabolic status and reduce body weight in overweight/obese women with recent gestational diabetes: a double‐blind, randomized, placebo‐controlled study. J Diabetes Complications. 2020;34:107548. doi: 10.1016/j.jdiacomp.2020.107548 [DOI] [PubMed] [Google Scholar]
- 95. Kaplan LM, Golden A, Jinnett K, Kolotkin RL, Kyle TK, Look M, Nadglowski J, O'Neil PM, Parry T, Tomaszewski KJ, et al. Perceptions of barriers to effective obesity care: results from the national ACTION study. Obesity. 2018;26:61–69. doi: 10.1002/oby.22054 [DOI] [PubMed] [Google Scholar]
- 96. Lopez‐Jimenez F, Malinski M, Gutt M, Sierra‐Johnson J, Wady Aude Y, Rimawi AA, Mego PA, Thomas RJ, Allison TG, Kirby B, et al. Recognition, diagnosis and management of obesity after myocardial infarction. Int J Obes. 2005;29:137–141. [DOI] [PubMed] [Google Scholar]
- 97. Singh S, Somers VK, Clark MM, Vickers K, Hensrud DD, Korenfeld Y, Lopez‐Jimenez F. Physician diagnosis of overweight status predicts attempted and successful weight loss in patients with cardiovascular disease and central obesity. Am Heart J. 2010;160:934–942. doi: 10.1016/j.ahj.2010.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Roser P, Bajaj SS, Stanford FC. International lack of equity in modern obesity therapy: the critical need for change in health policy. Int J Obes. 2022;46:1571–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. McCoy RG, Van Houten HK, Karaca‐Mandic P, Ross JS, Montori VM, Shah ND. Second‐line therapy for type 2 diabetes management: the treatment/benefit paradox of cardiovascular and kidney comorbidities. Diabetes Care. 2021;44:2302–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Eberly LA, Yang L, Essien UR, Eneanya ND, Julien HM, Luo J, Nathan AS, Khatana SAM, Dayoub EJ, Fanaroff AC, et al. Racial, ethnic, and socioeconomic inequities in glucagon‐like peptide‐1 receptor agonist use among patients with diabetes in the US. JAMA Health Forum. 2021;2:e214182. doi: 10.1001/jamahealthforum.2021.4182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. American Diabetes Association . Chapter 10. Cardiovascular disease and risk management: standards of medical care in diabetes–2020. Diabetes Care. 2020;43:S111–S134. [DOI] [PubMed] [Google Scholar]
- 102. Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi‐Hill D. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021;52:e364–e467. doi: 10.1161/STR.0000000000000375 [DOI] [PubMed] [Google Scholar]
- 103. Adhikari R, Jha K, Dardari Z, Heyward J, Blumenthal RS, Eckel RH, Alexander GC, Blaha MJ. National Trends in use of sodium‐glucose cotransporter‐2 inhibitors and glucagon‐like peptide‐1 receptor agonists by cardiologists and other specialties, 2015 to 2020. J Am Heart Assoc. 2022;11:e023811. doi: 10.1161/JAHA.121.023811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Gunawan F, Nassif ME, Partridge C, Ahmad T, Kosiborod M, Inzucchi SE. Relative frequency of cardiology vs. endocrinology visits by type 2 diabetes patients with cardiovascular disease in the USA: implications for implementing evidence‐based use of glucose‐lowering medications. Cardiovasc Endocrinol Metab. 2020;9:56–59. doi: 10.1097/XCE.0000000000000195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819–837. [DOI] [PubMed] [Google Scholar]
- 106. Ryan D, Acosta A. GLP‐1 receptor agonists: Nonglycemic clinical effects in weight loss and beyond. Obesity. 2015;23:1119–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hogan AE, Gaoatswe G, Lynch L, Corrigan MA, Woods C, O'Connell J, O'Shea D. Glucagon‐like peptide 1 analogue therapy directly modulates innate immune‐mediated inflammation in individuals with type 2 diabetes mellitus. Diabetologia. 2014;57:781–784. [DOI] [PubMed] [Google Scholar]
- 108. FDA . MOUNJAROTM (tirzepatide) injection, for subcutaneous use prescribing information. Accessed April 20, 2023. https://pi.lilly.com/us/mounjaro‐uspi.pdf?s=pi.
- 109. Nargesi AA, Jeyashanmugaraja GP, Desai N, Lipska K, Krumholz H, Khera R. Contemporary national patterns of eligibility and use of novel cardioprotective antihyperglycemic agents in type 2 diabetes mellitus. JAMA. 2021;10:e021084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hamid A, Vaduganathan M, Oshunbade AA, Ayyalasomayajula KK, Kalogeropoulos AP, Lien LF, Shafi T, Hall ME, Butler J. Antihyperglycemic therapies with expansions of US Food and Drug Administration indications to reduce cardiovascular events: prescribing patterns within an academic medical center. J Cardiovasc Pharmacol. 2020;76:313–320. [DOI] [PubMed] [Google Scholar]


