Abstract
Background
The brain and muscle Arnt-like protein-1 (BMAL1) gene is an important circadian clock gene and previous studies have found that certain polymorphisms are associated with type 2 diabetes in adults. However, it remains unknown if such polymorphisms can affect fasting glucose in children and if other factors modify the associations.
Methods
A school-based cross-sectional study with 947 Chinese children was conducted. A multivariable linear regression model was used to analyze the association between BMAL1 gene polymorphisms and fasting glucose level.
Results
After adjusting for age, sex, body mass index (BMI), physical activity, and unhealthy diet, GG genotype carriers of BMAL1 rs3789327 had higher fasting glucose than AA/GA genotype carriers (b = 0.101, SE = 0.050, P = 0.045). Adjusting for the same confounders, rs3816358 was shown to be significantly associated with fasting glucose (b = 0.060, SE = 0.028, P = 0.032). Furthermore, a significant interaction between rs3789327 and nutritional status on fasting glucose was identified (Pinteraction = 0.009); rs3789327 was associated with fasting glucose in the overweight/obese subgroup (b = 0.353, SE = 0.126, P = 0.006), but not in non-overweight/non-obese children.
Conclusions
BMAL1 polymorphisms were significantly associated with the fasting glucose level in children. Additionally, the observed interaction between nutritional status and BMAL1 supports promoting an optimal BMI in children genetically predisposed to higher glucose level.
Impact
Polymorphisms in the essential circadian clock gene BMAL1 were associated with fasting blood glucose levels in children. Additionally, there was a significant interaction between nutritional status and BMAL1 affecting fasting glucose levels.
BMAL1 rs3789327 was associated with fasting glucose only in overweight/obese children.
This finding could bring novel insights into mechanisms by which nutritional status influences fasting glucose in children.
Introduction
It is estimated that the prevalence of diabetes in the global adult population in 2021 is approximately 10.5% (536.6 million), which is projected to rise to 12.2% (783.2 million) by 2045.1 A similar rising trend can be observed regarding the prevalence of impaired fasting glucose in children or adolescents.2,3 In China, the prevalence of impaired/abnormal fasting glucose and diabetes in children aged 6–17 was 1.89 and 0.1% in 2014, respectively.4 In addition, the earlier the onset of diabetes, the longer the patient’s lifetime exposure to hyperglycemia, the more destructive the disease is and the more likely it is going to lead to the earlier development of diabetes related complications reducing the overall quality of life.5 This indicates that it is necessary to explore the causes of hyperglycemia in children and adolescents, which will be beneficial to an early-life prevention and control of diabetes at childhood. A Chinese and Danish cross-population twin study demonstrated a high genetic influence on fasting glucose levels, with heritability ranging from 55 to 71%.6 However, blood glucose levels are influenced by both environmental and genetic factors, and the gene–environment interaction is also very important.7,8
Many aspects of physiology and behavior, such as the daily rhythms of food intake, metabolism, and, more specifically, glucose metabolism are regulated by the circadian clock system.9 Brain and muscle Arnt-like protein-1 (BMAL1) is an important component of the circadian clock in mammals and controls the oscillations of the circadian rhythm. It has been shown that disruptions of circadian rhythm oscillations in glucose metabolism are involved in the pathogenesis of type 2 diabetes.10 Sadacca et al. demonstrated that BMAL1 is essential for normal insulin secretion and glucose homeostasis in pancreatic beta cells.11 Additionally, an experimental study suggested that obesity and diabetes may reduce the rhythmic expression of clock genes in the liver and adipose tissue.12 Population studies about BMAL1 gene polymorphisms and risk of type 2 diabetes were controversial and all conducted in adults.13–15 Until now, the relation between BMAL1 gene polymorphisms and fasting glucose levels in children is not clear. Therefore, the purpose of this study was to investigate the relationship between BMAL1 gene polymorphisms and fasting glucose levels in Chinese children.
In addition, whether BMAL1 polymorphisms could interact with nutritional status affecting fasting glucose has not been demonstrated before. Thus, we also investigated the interaction between BMAL1 gene polymorphisms and nutritional status on fasting glucose levels in Chinese children.
Subjects and methods
Subjects
Using a cluster sampling method, a total of 1019 children aged 10–15 years from 3 middle schools in Changsha city, Hunan Province, China were included. The study was conducted in 2019. Seven participants were excluded for invalid questionnaire data. Sixty-five participants were excluded for absence of glucose phenotype data or genotype data. Therefore, a total of 947 participants were included in the present study. The study was approved by the Medical Ethic Committee of Hunan Normal University. Written informed consents was obtained from all participants and their parents.
Measurement
Height and weight were measured according to standard protocols. Peripheral venous blood sample were collected under an overnight fasting condition. Then the fasting plasma blood glucose was measured using GOD-PAP method with the auto analyzer OLYMPUS AU400 with a standard protocol. For the fasting requirement, parents of the included children were informed one day before the blood collection by text message, and also during the blood collection, the nurses confirmed the fasting status of the participants. Covariates, including demographic characteristics and lifestyle factors, such as physical activity and dietary behaviors, were investigated by questionnaire.16,17 Dietary behaviors included fried chips/cakes/cookies and soft drinks. Participants were given the option of “eating/drinking in the past 7 days” (Yes), or “not eating/drinking in the past 7 days” (No). According to the “Dietary Guidelines for Chinese School-Age Children (2022)”, the physical activity was divided into “<1 h/day” and “≥1 h/day”.16,18
According to fasting blood glucose, the definition of prediabetes was 5.6–6.9 mmol/L and diabetes was ≥7.0 mmol/L in Chinese children.19 Also, the stratified association between BMAL1 gene polymorphisms and blood glucose level were analyzed with multiple linear regression models in children with different glucose status (normal blood glucose and prediabetes/diabetes).
Body mass Index (BMI) was calculated by weight divided by square of height (kg/m2). Nutritional status of children was defined according to Working Group of Obesity in China (WGOC) criteria, of which the age and sex-specific cut-off points are the 85th and 95th percentiles of BMI (Overweight: BMI ≥ 85th but <90th percentile; Obese: BMI ≥ 90th percentile).20
Genetic polymorphisms selection and genotyping
Selection of the BMAL1 gene polymorphisms was based on previous literature regarding population studies on the BMAL1 gene and cardiometabolic risk factors. Polymorphisms with significant association with cardiometabolic risk factors were selected. Then, we used the 1000 genome database to identify the MAF of the SNPs in East Asians and only SNPs with MAF > 0.05 were included in the present study. Finally, four SNPs from the BMAL1 gene were selected (rs10832020,21 rs3789327,13 rs7950226,14,15 and rs381635822).
Genomic DNA of peripheral blood leukocytes were extracted from participants’ fasting venous blood employing a salt extraction method.16,23 Matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS, Agena) was used for the genotyping of BMALI polymorphisms.16 Genotyping was performed by investigators who were blind to the participants’ phenotypes. All the call rates of the genotyping were above 98% (Table S1). In addition, the genotyping was conducted with 1% randomly selected duplicated DNA samples and the consistent rate of genotyping results were 100%.
Statistical analyses
Hardy–Weinberg equilibrium was checked with the Chi-square test. F-statistics (FST) was calculated using the formula FST = (P1 − P2)2/[(P1 + P2) × (2 − (P1 + P2))].24 P1 is the effect allele frequency of a gene polymorphism in the 1000 Genomes Project database of European ancestry and P2 indicates the gene frequency of the effect allele in this study. FST reflects the ancestral differences in the same gene polymorphism between the two populations. FST between 0 and 0.05 indicates a small ancestral difference; A range of 0.05–0.15 indicates a moderate ancestral difference; 0.15–0.25 indicates a large ancestral differences; a FST > 0.25 indicates very large ancestral difference.25 Descriptive statistical analysis was used to analyze the general demographic characteristics, genotypes and allele frequencies of the participants. T tests were used for continuous variables and Chi-square test was used for categorical variables. The best genetic models were selected according to the Akaike Information Criterion (AIC).26 Multivariable linear regression analysis was conducted to analyze the association between gene polymorphism and fasting glucose, and the regression coefficients (b) and standard error (SE) were presented. Model 1 is the crude model; for Model 2, we added sex, age, and BMI as covariates; and Model 3 included sex, age, BMI, physical activity, and unhealthy diet (including soft drink and consumption of fried chips/cakes/cookies) as covariates. Considering 4 SNPs of BMAL1 were selected in the present study, we adjusted multiple testing for Bonferroni correction (P < 0.05/4 = 0.0125). SPSS for Windows (version 22.0, SPSS Inc., Chicago, IL) was used for statistical analysis.
Results
General characteristics
The general characteristics of the participants are shown in Table 1. A total of 947 children were included in the present study. The average age of the participants was 11.69 years, including 476 girls and 471 boys, respectively. Boys had significantly higher fasting glucose level and a higher percentage of being active (physical activity ≥2 h/day) than girls (P < 0.05). The prevalence of overweight and obesity in boys (24.6%) was significantly higher than that in girls (15.9%) (P = 0.001). For age, BMI, glucose status, unhealthy diet (including soft drink, fried chips/cakes/cookies consumption), and genotype frequency of BMAL1 polymorphisms, no significant sex differences were found (P > 0.05). The ancestral differences in BMAL1 gene polymorphisms between European population in the 1000 Genomes Project database and our population are all small (all FST values <0.05, Table S1).
Table 1.
General characteristics of the present study.
| Variables | Total (n = 947) | Boys (n = 471) | Girls (n = 476) | P |
|---|---|---|---|---|
| Age (years) | 11.69 ± 0.66 | 11.74 ± 0.65 | 11.64 ± 0.67 | 0.150 |
| Glucose (mmol/L) | 4.88 ± 0.42 | 4.92 ± 0.40 | 4.84 ± 0.44 | 0.007 |
| BMI (kg/m2) | 17.69 (16.05, 20.21) | 17.84 (16.07, 20.52) | 17.60 (16.03, 19.81) | 0.369 |
| Nutritional status | 0.001 | |||
| Non-overweight/obesity | 742 (79.8%) | 350 (75.4%) | 392 (84.1%) | |
| Overweight/obesity | 188 (20.2%) | 114 (24.6%) | 74 (15.9%) | |
| Glucose status | 0.053 | |||
| Normal blood glucose | 912 (96.3%) | 449 (95.3%) | 463 (97.3%) | |
| 5.6–6.9 mmol/L (prediabetes) | 33 (3.5%) | 22 (4.7%) | 11 (2.3%) | |
| ≥7.0 mmol/L (diabetes) | 2 (0.2%) | 0 | 2 (0.4%) | |
| Physical activity | <0.001 | |||
| ≥1 h/day | 344 (38.9%) | 205 (45.8%) | 139 (31.8%) | |
| <1 h/day | 541 (61.1%) | 243 (54.2%) | 298 (68.2%) | |
| Soft drink | 0.160 | |||
| Yes | 580 (61.2%) | 299 (63.5%) | 281 (59.0%) | |
| No | 367 (38.8%) | 172 (36.5%) | 195 (41.0%) | |
| Fried chips/cakes/cookies | 0.350 | |||
| Yes | 193 (21.8%) | 103 (23.0%) | 90 (20.5%) | |
| No | 694 (78.2%) | 344 (77.0%) | 350 (79.5%) | |
| rs10832020 | 0.672 | |||
| Genotyping [n (%)] | ||||
| TT | 473 (50.1%) | 241 (51.3%) | 232 (48.9%) | |
| TC | 375 (39.7%) | 180 (38.3%) | 195 (41.1%) | |
| CC | 96 (10.2%) | 49 (10.4%) | 47 (9.9%) | |
| rs3789327 | 0.159 | |||
| Genotyping [n (%)] | ||||
| AA | 438 (46.4%) | 226 (48.1%) | 212 (44.7%) | |
| AG | 417 (44.2%) | 208 (44.3%) | 209 (44.1%) | |
| GG | 89 (9.4%) | 36 (7.7%) | 53 (11.2%) | |
| rs7950226 | 0.102 | |||
| Genotyping [n (%)] | ||||
| AA | 333 (35.8%) | 169 (36.7%) | 164 (35.0%) | |
| GA | 458 (49.3%) | 213 (46.3%) | 245 (52.2%) | |
| GG | 138 (14.9%) | 78 (17%) | 60 (12.8%) | |
| rs3816358 | 0.150 | |||
| Genotyping [n (%)] | ||||
| GG | 658 (69.9%) | 342 (72.8%) | 316 (66.9%) | |
| GT | 260 (27.6%) | 117 (24.9%) | 143 (30.3%) | |
| TT | 24 (2.5%) | 11 (2.3%) | 13 (2.8%) | |
BMI body mass index.
P values <0.05 are set in bold.
Association between BMAL1 gene polymorphisms and fasting glucose levels
According to AIC criterion, a dominant genetic model was the best model for rs10832020, a recessive genetic model for rs3789327, and an additive genetic model for rs7950226 and rs3816358 (Table S2). Table 2 shows the associations between BMAL1 polymorphisms and fasting glucose. In the recessive genetic model, using sex, age, BMI, physical activity, and unhealthy diet as covariates, a significant association between rs3789327 and fasting glucose was found. For the rs3789327 polymorphism, GG genotype carriers had higher blood glucose levels than GA/AA genotype carriers (b = 0.101, SE = 0.050, P = 0.045). In the additive genetic model, we found that rs3816358 polymorphism A allele was significantly associated with fasting glucose level after adjustment for sex, age, BMI, physical activity, and unhealthy diet (b = 0.060, SE = 0.028, P = 0.032). No significant associations between rs10832020 or rs7950226 and fasting glucose levels were found (P > 0.05). However, none of the polymorphisms were significantly associated with glucose level after correction for multiple comparison (P < 0.05/4 = 0.0125). We also analyzed the association between BMAL1 gene polymorphisms and blood glucose level in children with different glucose status (normal blood glucose and prediabetes/diabetes), and neither in normal blood glucose group nor in children with prediabetes/diabetes participants significant association between the four SNPs of BMAL1 and blood glucose level was found (P > 0.05, Table S3).
Table 2.
Association of BMAL1 gene polymorphism with fasting glucose level.
| Models | SNP | b | SE | P |
|---|---|---|---|---|
| Model 1 | rs10832020 | −0.041 | 0.027 | 0.130 |
| rs3789327 | 0.101 | 0.047 | 0.031 | |
| rs7950226 | 0.006 | 0.020 | 0.780 | |
| rs3816358 | 0.052 | 0.026 | 0.046 | |
| Model 2 | rs10832020 | −0.045 | 0.027 | 0.102 |
| rs3789327 | 0.100 | 0.047 | 0.033 | |
| rs7950226 | 0.006 | 0.020 | 0.782 | |
| rs3816358 | 0.059 | 0.026 | 0.026 | |
| Model 3 | rs10832020 | −0.039 | 0.029 | 0.175 |
| rs3789327 | 0.101 | 0.050 | 0.045 | |
| rs7950226 | 0.002 | 0.021 | 0.932 | |
| rs3816358 | 0.060 | 0.028 | 0.032 |
Model 1 is the crude model; for Model 2, we add sex, age and BMI as covariates; and Model 3 with sex, age, BMI, physical activity, soft drink and fried chips/cakes/cookies as covariates.
BMI body mass index.
P values <0.05 are set in bold.
Interaction between BMAL1 gene polymorphisms and nutritional status on fasting glucose levels
Associations between BMAL1 gene polymorphisms and fasting glucose stratified according to nutritional status are illustrated in Table 3.
Table 3.
Interaction between nutritional status and BMAL1 gene polymorphism in fasting glucose level.
| SNP | Category | Genotype | N | Mean | SD | b | SE | P | Pinteraction |
|---|---|---|---|---|---|---|---|---|---|
| rs10832020 | Non-overweight/obesity | TT | 372 | 4.92 | 0.39 | 0.237 | |||
| TC + CC | 369 | 4.86 | 0.43 | −0.053 | 0.031 | 0.091 | |||
| Overweight/obesity | TT | 90 | 4.82 | 0.38 | |||||
| TC + CC | 96 | 4.84 | 0.52 | 0.015 | 0.072 | 0.834 | |||
| rs3789327 | Non-overweight/obesity | AA + AG | 674 | 4.88 | 0.41 | 0.009 | |||
| GG | 65 | 4.92 | 0.38 | 0.041 | 0.055 | 0.456 | |||
| Overweight/obesity | AA + AG | 170 | 4.80 | 0.37 | |||||
| GG | 18 | 5.13 | 0.88 | 0.353 | 0.126 | 0.006 | |||
| rs7950226 | Non-overweight/obesity | AA/GA/GG | 269/359/98 | 4.88/4.88/4.91 | 0.37/0.39/0.50 | −0.001 | 0.023 | 0.965 | 0.407 |
| Overweight/obesity | AA/GA/GG | 60/91/35 | 4.80/4.87/4.82 | 0.40/0.51/0.39 | 0.011 | 0.052 | 0.831 | ||
| rs3816358 | Non-overweight/obesity | CC/CA/AA | 514/204/20 | 4.88/4.90/5.09 | 039/0.45/0.37 | 0.048 | 0.03 | 0.114 | 0.592 |
| Overweight/obesity | CC/CA/AA | 136/48/3 | 4.81/4.90/5.11 | 0.39/0.61/0.33 | 0.099 | 0.074 | 0.183 |
SD standard deviation, SE standard error.
P and Pinteraction was adjusted for age, gender, physical activity, soft drink, and fried chips/cakes/cookies; P values <0.05 are set in bold.
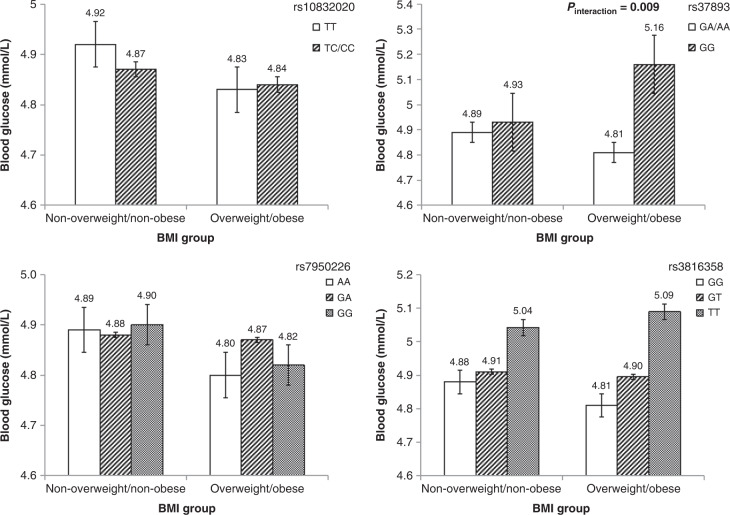
A significant interaction between rs3789327 and nutritional status on fasting glucose was found (Pinteraction = 0.009). In the overweight/obese subgroup, rs3789327 polymorphism GG genotype carriers had significantly higher fasting glucose than the AG/AA genotype carriers (b = 0.353, SE = 0.126, P = 0.006), but no significant association was examined in the subgroup who were not-overweight/non-obese. No significant interactions were observed for the rs10832020, rs7950226, and rs3816358 polymorphisms of BMAL1. The adjusted fasting glucose levels stratified by different genotypes and nutritional status are shown in Fig. 1.
Fig. 1. Adjusted means and standard errors of fasting glucose stratified by brain and muscle Arnt-like protein-1 (BMAL1) gene polymorphism phenotypes and nutritional status.
BMI body mass index. Adjusted mean and standard errors were estimated under a general linear regression model that adjusted for age, sex, physical activity, and unhealthy diet.
Discussion
To the best of our knowledge, this study is the first to demonstrate a significant association between the BMAL1 rs3789327 and rs3816358 polymorphisms and fasting glucose levels in children. In addition, we found that there was a significant interaction between BMAL1 rs3789327 polymorphism and nutritional status regarding fasting glucose levels. More specifically, the association between the rs3789327 polymorphism and fasting glucose levels only existed in overweight/obese children, not in children who were not overweight or obese. Our results suggest that BMAL1 and nutritional status have an interacting effect on fasting blood glucose in children.
The finding of an association between the BMAL1 rs3816358 polymorphism and fasting blood glucose in Chinese children is new. Although this SNP has not been reported in relation to fasting blood glucose or risk of diabetes, Leu et al. identified that the T allele of the rs3816358 polymorphism is associated with an increased risk of non-dipper hypertension (OR = 1.50, 95% CI: 1.04–2.18) in adults.22 In addition, Evans et al. found that the T allele of rs3816358 is significantly related with later sleep onset time (b = 0.13, SE = 0.05) objectively measured actigraphy in the Elderly in the United States.27 Previous studies reported that sleep onset time is significantly related with glucose-related phenotypes, such as diabetes, obesity, and fasting blood glucose, since later bedtime is likely to be associated with shorter sleep duration, leading to disruption of glycemic control or glycemic rhythmicity.28,29 But in the present study, the sleep traits of children were not investigated, which limited us to explore the potential mediation effect of sleep onset time in the association between rs3816358 and fasting blood glucose. Future studies could be conducted to further characterize this mediation effect.
In addition, our study shows that the GG genotype of rs3789327 might be a risk genotype for high fasting glucose levels in children. Previous studies have focused on different readouts, demonstrating associations with myocardial infarction, multiple sclerosis, and depression.30–32 This is the first study detecting association between rs3789327 polymorphisms and glycemia related phenotypes. However, the significant association was not significant after correcting for multiple testing. The findings should be interpreted with caution, and larger sample size studies and functional studies are needed to verify these results.
The current study did not detect significant associations between rs7950226/rs10832020 and fasting glucose. In addition, we also analyzed the association between BMAL1 gene polymorphisms and blood glucose levels in normal blood glucose and prediabetes/diabetes participants, no significant results were found. Several previous studies have shown that BMAL1 gene polymorphisms are associated with the risk of developing type 2 diabetes in adults.13–15 However, studies have noted that BMAL1 gene polymorphisms are not associated with the occurrence of type 2 diabetes in Japanese33 and African-American34 cohorts. In contrast to the findings of our study, Pappa et al. showed that rs7950226 polymorphism of BMAL1 gene is associated with susceptibility to gestational diabetes in Greek pregnant women.14 However, this might be due to the different age, ethnic diversity and varying environmental conditions of the study population. Furthermore, different genetic risk factors might exist in different population with different ethnicity.
Regarding potential mechanism underlying the associations between BMAL1 gene polymorphisms and fasting glucose, it is noteworthy that BMAL1 acts an essential component of the circadian oscillation, which drives the daily rhythms of physiology and behavior.35 In pancreatic β cells, BMAL1 plays a key role in mediating insulin secretion, exocytosis and metabolism.36 According to previous functional studies about clock genes and glycemic phenotypes, the possible link between BMAL1 and fasting glucose levels might be mitochondrial dysfunction.37–39Mitochondrial dysfunction is increasingly considered to be the main driver of pancreatic β cell failure in the pathogenesis of diabetes mellitus, and the loss of normal β cell function is the core factor of impaired insulin secretion in diabetes mellitus.40
Furthermore, results of the current study demonstrated a significant interacting effect between nutritional status and rs3789327 on fasting blood glucose levels. In overweight/obese individuals, GG genotype carriers had significantly higher levels of fasting blood glucose than AA/AG genotype carriers, which was not detected in children without overweight/obesity. To the best of our knowledge, no gene–nutritional status interaction of BMAL1 gene was reported before. However, recent studies have found an association between BMAL1 and indicators of obesity and obesity is closely associated with elevated fasting glucose in children.41,42 Being overweight or obese might amplify the genetic susceptibility of unfavorable glucose level for specific genotype carriers of BMAL1 gene polymorphism. Previous studies have reported that glucose-related phenotypes (Type 2 diabetes) which are attributed to genetic predisposition can be significantly different in people with different nutritional status.43,44 The identified interaction of rs3789327 with nutritional status on glucose in our study is of clinical interest, considering that mounting evidence have shown that fasting glucose levels in childhood are significant predictors for diabetes and other related cardiometabolic risk factors in adulthood.45,46 Mechanistically, studies have demonstrated that BMAL1 can activate CRY gene expression in conjunction with the CLOCK gene when BMAL1 levels are high.47 The degradation of CRY1 induces gluconeogenesis and maintains blood glucose levels, but high fat intake accelerates the degradation of autophagy CRY1 and contributes to the development of obesity-related hyperglycemia. Furthermore, a recent study observed that BMAL1 overexpression can enhance circadian clock function and β cell function, thus enhancing GSIS and systemic glucose metabolism in the context of diet-induced obesity.48 In short, the above-mentioned findings could imply that BMAL1 gene polymorphisms and nutritional status have an essential role in affecting fasting blood glucose levels in children.
One strength of our study is that it focused on children. Previous studies have generally set their focus on the association of cardiovascular metabolic risk factors in adults with BMAL1 gene polymorphisms and results have been controversial in different ethnic populations. Genetic studies during childhood, a period when environmental risk factors (alcohol drinking, smoking, etc.) are relatively minimal, could increase the possibility to detect a genetic risk factor that might otherwise be masked, therefore contribute to elucidate the etiology of abnormal glucose and early onset diabetes. Fasting blood glucose levels in children are closely related to genetic factors and an early onset of diabetes also dictates the development and severity of chronic complications. Children are also the ideal population for genetic association study of blood glucose.
However, there are also some limitations in our study. We only selected 4 representative SNPs of the BMAL1 gene but there are far more known SNPs in this gene. Secondly, studies in adults have observed controversial results regarding the association between BMAL1 gene and risk of diabetes in different ethnic groups. Therefore, further studies on children of other ethnic groups are needed. Finally, we did not investigate information about puberty among the study participants, so we were unable to explore the potential effect of puberty on fasting blood glucose levels.
In conclusion, our study demonstrates that BMAL1 rs3789327 and rs3816358 polymorphisms are significantly associated with fasting blood glucose levels in Chinese children. We also found that rs3789327 was associated with fasting blood glucose in overweight/obese children, but not in non-overweight/non-obese children. In addition, nutritional status and rs3789327 had an interacting effect on fasting blood glucose levels. Our finding highlights the importance of promoting healthy nutrition, especially in children with a genetic susceptibility to higher fasting glucose. Thus, these findings might also contribute to the development of early prevention strategies for elevated blood glucose in children.
Supplementary Information
Acknowledgements
We want to thank all the children and their parents, as well as the doctors and nurses who had assisted with physical examination in our study.
Author contributions
Y.-D.Y., Y.Z., C.-J.Z., and B.H. conceptualized and designed the study, carried out the data analysis, drafted the initial manuscript, and reviewed and revised the manuscript. Y.-D.Y. and Y.Z. performed the data analysis, drafted the initial manuscript, and reviewed and revised the manuscript; Y.-D.Y., Y.Z., C.-J.Z., and B.H. assisted with the data processing, statistical analyses and the interpretation of results; Y.-D.Y., J.L., J.-H.Z., Q.-Y.H., C.R., and B.K.K. contributed to the conceptualization and design of the study, supervised the data collection, the statistical analyses and initial drafting of the manuscript, and reviewed and revised the manuscript; Y.-D.Y., J.L., and C.-J.Z. coordinated and supervised data collection and critically reviewed the manuscript. Y.-D.Y. and B.H. polished the language and reviewed and revised the manuscript. All authors reviewed and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (81903336, Y.D.Y., http://www.nsfc.gov.cn/), the Hunan Provincial Natural Science Foundation of China (2019JJ50376, Y.D.Y.), the Hunan Provincial Natural Science Foundation of China (2020JJ5386, C.J.Z.), and Scientific Research Project of Hunan Provincial Health Commission (202112031516, Y.D.Y.). Open Access funding enabled and organized by Projekt DEAL.
Data availability
The datasets analyzed in our study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Our research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The study was approved by Hunan Normal University Ethics Committee (2019-88), and written informed consent was obtained from all participants in the present study.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yi-De Yang, Yuan Zeng.
Supplementary information
The online version contains supplementary material available at 10.1038/s41390-023-02467-8.
References
- 1.Sun H, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kloppenborg JT, et al. Impaired fasting glucose and the metabolic profile in Danish children and adolescents with normal weight, overweight, or obesity. Pediatr. Diabetes. 2018;19:356–365. doi: 10.1111/pedi.12604. [DOI] [PubMed] [Google Scholar]
- 3.Smetanina N, Valickas R, Vitkauskiene A, Albertsson-Wikland K, Verkauskienė R. Prevalence of metabolic syndrome and impaired glucose metabolism among 10- to 17-year-old overweight and obese Lithuanian children and adolescents. Obesity Facts. 2021;14:271–282. doi: 10.1159/000514720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z, et al. Prevalence and risk factors of impaired fasting glucose and diabetes among Chinese children and adolescents: a national observational study. Br. J. Nutr. 2018;120:813–819. doi: 10.1017/S0007114518002040. [DOI] [PubMed] [Google Scholar]
- 5.Copeland KC, et al. Management of newly diagnosed type 2 diabetes mellitus (T2DM) in children and adolescents. Pediatrics. 2013;131:364–382. doi: 10.1542/peds.2012-3494. [DOI] [PubMed] [Google Scholar]
- 6.Li S, et al. Heritability of eleven metabolic phenotypes in Danish and Chinese twins: a cross-population comparison. Obesity. 2013;21:1908–1914. doi: 10.1002/oby.20217. [DOI] [PubMed] [Google Scholar]
- 7.Jabbour G, Bragazzi NL. Continuous blood glucose monitoring increases vigorous physical activity levels and is associated with reduced hypoglycemia avoidance behavior in youth with type 1 diabetes. Front. Endocrinol. 2021;12:722123. doi: 10.3389/fendo.2021.722123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molyneaux L, Constantino M, Yue D. Strong family history predicts a younger age of onset for subjects diagnosed with type 2 diabetes. Diabetes Obes. Metab. 2004;6:187–194. doi: 10.1111/j.1462-8902.2004.00330.x. [DOI] [PubMed] [Google Scholar]
- 9.Lowrey PL, Takahashi JS. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu. Rev. Genomics Hum. Genet. 2004;5:407–441. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stamenkovic JA, et al. Regulation of core clock genes in human islets. Metabolism. 2012;61:978–985. doi: 10.1016/j.metabol.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Sadacca LA, Lamia KA, deLemos AS, Blum B, Weitz CJ. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia. 2011;54:120–124. doi: 10.1007/s00125-010-1920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ando H, et al. Rhythmic messenger ribonucleic acid expression of clock genes and adipocytokines in mouse visceral adipose tissue. Endocrinology. 2005;146:5631–5636. doi: 10.1210/en.2005-0771. [DOI] [PubMed] [Google Scholar]
- 13.Woon PY, et al. Aryl hydrocarbon receptor nuclear translocator-like (Bmal1) is associated with susceptibility to hypertension and type 2 diabetes. Proc. Natl Acad. Sci. USA. 2007;104:14412–14417. doi: 10.1073/pnas.0703247104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pappa KI, et al. The major circadian pacemaker arnt-like protein-1 (Bmal1) is associated with susceptibility to gestational diabetes mellitus. Diabetes Res. Clin. Pract. 2013;99:151–157. doi: 10.1016/j.diabres.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 15.Li GY, Wang H, Chen H. Association of insulin resistance with polymorphic variants of clock and Bmal1 genes: a case-control study. Clin. Exp. Hypertens. 2020;42:371–375. doi: 10.1080/10641963.2019.1676769. [DOI] [PubMed] [Google Scholar]
- 16.Yang YD, et al. Interaction between lifestyle behaviors and genetic polymorphism in SCAP gene on blood pressure among Chinese children. Pediatr. Res. 2019;86:389–395. doi: 10.1038/s41390-019-0402-z. [DOI] [PubMed] [Google Scholar]
- 17.Yang YD, et al. Combined effects of the rs9810888 polymorphism in calcium voltage-gated channel subunit alpha1 D (Cacna1d) and lifestyle behaviors on blood pressure level among Chinese children. PLoS ONE. 2019;14:e0216950. doi: 10.1371/journal.pone.0216950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chinese Nutrition Society. Chinese Dietary Guidelines for School-Age Children (2022) (People’s Medical Publishing House, 2022).
- 19.Expert consensus on standardized diagnosis and treatment of type 1 diabetes in children in China (2020 Version). Chin. J. Pediatr.58, 447–454 (2020). [DOI] [PubMed]
- 20.Ji CY. Report on childhood obesity in China (1)–body mass index reference for screening overweight and obesity in Chinese school-age children. Biomed. Environ. Sci. 2005;18:390–400. [PubMed] [Google Scholar]
- 21.Lin E, et al. Effects of circadian clock genes and health-related behavior on metabolic syndrome in a Taiwanese population: evidence from association and interaction analysis. PLoS ONE. 2017;12:e0173861. doi: 10.1371/journal.pone.0173861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leu HB, et al. Association of circadian genes with diurnal blood pressure changes and non-dipper essential hypertension: a genetic association with young-onset hypertension. Hypertens. Res. 2015;38:155–162. doi: 10.1038/hr.2014.152. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, et al. Associations of genetic variants of lysophosphatidylcholine metabolic enzymes with levels of serum lipids. Pediatr. Res. 2021;91:1595–1599. doi: 10.1038/s41390-021-01549-9. [DOI] [PubMed] [Google Scholar]
- 24.Duan S, Zhang W, Cox NJ, Dolan ME. FstSNP-HapMap3: a database of SNPs with high population differentiation for HapMap3. Bioinformation. 2008;3:139–141. doi: 10.6026/97320630003139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balloux F, Lugon-Moulin N. The estimation of population differentiation with microsatellite markers. Mol. Ecol. 2002;11:155–165. doi: 10.1046/j.0962-1083.2001.01436.x. [DOI] [PubMed] [Google Scholar]
- 26.Quinn, G. P., Yin K, Keough M. J., Ao J & Yan Z. Biological Experiment Design and Data Analysis (Higher Education Press, 2003).
- 27.Evans DS, et al. Common genetic variants in Arntl and Npas2 and at chromosome 12p13 are associated with objectively measured sleep traits in the elderly. Sleep. 2013;36:431–446. doi: 10.5665/sleep.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reutrakul S, et al. Relationships among sleep timing, sleep duration and glycemic control in type 2 diabetes in Thailand. Chronobiol. Int. 2015;32:1469–1476. doi: 10.3109/07420528.2015.1105812. [DOI] [PubMed] [Google Scholar]
- 29.Knutson KL, et al. Association between sleep timing, obesity, diabetes: The Hispanic Community Health Study/Study of Latinos (HCHS/SOL) Cohort Study. Sleep. 2017;40:zsx014. doi: 10.1093/sleep/zsx014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavtar P, et al. Association of circadian rhythm genes Arntl/Bmal1 and clock with multiple sclerosis. PLoS ONE. 2018;13:e0190601. doi: 10.1371/journal.pone.0190601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Škrlec I, Milić J, Steiner R. The impact of the circadian genes clock and Arntl on myocardial infarction. J. Clin. Med. 2020;9:484. doi: 10.3390/jcm9020484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maciukiewicz M, et al. Analysis of genetic association and epistasis interactions between circadian clock genes and symptom dimensions of bipolar affective disorder. Chronobiol. Int. 2014;31:770–778. doi: 10.3109/07420528.2014.899244. [DOI] [PubMed] [Google Scholar]
- 33.Yamaguchi M, et al. Association between brain-muscle-Arnt-like protein-2 (Bmal2) gene polymorphism and type 2 diabetes mellitus in obese Japanese individuals: a cross-sectional analysis of the Japan Multi-Institutional Collaborative Cohort Study. Diabetes Res. Clin. Pract. 2015;110:301–308. doi: 10.1016/j.diabres.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Salazar P, et al. Common genetic variation in circadian clock genes are associated with cardiovascular risk factors in an African American and Hispanic/Latino Cohort. Int. J. Cardiol. Heart Vasc. 2021;34:100808. doi: 10.1016/j.ijcha.2021.100808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.la Fleur SE, Kalsbeek A, Wortel J, Fekkes ML, Buijs RM. A daily rhythm in glucose tolerance: a role for the suprachiasmatic nucleus. Diabetes. 2001;50:1237–1243. doi: 10.2337/diabetes.50.6.1237. [DOI] [PubMed] [Google Scholar]
- 36.Perelis M, et al. Pancreatic Β cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science. 2015;350:aac4250. doi: 10.1126/science.aac4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eisner V, et al. Mitochondrial fusion dynamics is robust in the heart and depends on calcium oscillations and contractile activity. Proc. Natl Acad. Sci. USA. 2017;114:E859–e868. doi: 10.1073/pnas.1617288114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye L, Wu H, Xu W. Deletion of Bmal1 impairs pancreatic Β-cell function via mitochondrial signaling pathway. Biomed. Res. Int. 2020;2020:9803024. doi: 10.1155/2020/9803024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu R, Jin SB, Lendahl U, Nistér M, Zhao J. Human Fis1 regulates mitochondrial dynamics through inhibition of the fusion machinery. EMBO J. 2019;38:e99748. doi: 10.15252/embj.201899748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerber PA, Rutter GA. The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antioxid. Redox Signal. 2017;26:501–518. doi: 10.1089/ars.2016.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shimba S, et al. Deficient of a clock gene, brain and muscle Arnt-like protein-1 (Bmal1), induces dyslipidemia and ectopic fat formation. PLoS ONE. 2011;6:e25231. doi: 10.1371/journal.pone.0025231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turek FW, et al. Obesity and metabolic syndrome in circadian clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lv X, et al. Interaction between peroxisome proliferator-activated receptor gamma polymorphism and obesity on type 2 diabetes in a Chinese Han population. Diabetol. Metab. Syndr. 2017;9:7. doi: 10.1186/s13098-017-0205-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu D, Pan JM, Pei X, Li JS. Interaction between apolipoprotein M gene single-nucleotide polymorphisms and obesity and its effect on type 2 diabetes mellitus susceptibility. Sci. Rep. 2020;10:7859. doi: 10.1038/s41598-020-64467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen QM, et al. Utility of childhood glucose homeostasis variables in predicting adult diabetes and related cardiometabolic risk factors: The Bogalusa Heart Study. Diabetes Care. 2010;33:670–675. doi: 10.2337/dc09-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kivimäki M, et al. Neighbourhood socioeconomic disadvantage, risk factors, and diabetes from childhood to middle age in the Young Finns Study: a cohort study. Lancet Public Health. 2018;3:e365–e373. doi: 10.1016/S2468-2667(18)30111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu W, Nomura M, Ikeda M. Interactivating feedback loops within the mammalian clock: Bmal1 is negatively autoregulated and upregulated by Cry1, Cry2, and Per2. Biochem. Biophys. Res. Commun. 2002;290:933–941. doi: 10.1006/bbrc.2001.6300. [DOI] [PubMed] [Google Scholar]
- 48.Rakshit K, Matveyenko AV. Induction of core circadian clock transcription factor Bmal1 enhances Β-cell function and protects against obesity-induced glucose intolerance. Diabetes. 2021;70:143–154. doi: 10.2337/db20-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed in our study are available from the corresponding author on reasonable request.