Abstract
Background
Even after qualified detoxification, alcohol-dependent (AD) patients may relapse to drinking alcohol despite their decision to abstain. Two mechanisms may play important roles. First, the impact of environmental cues on instrumental behavior (i.e., Pavlovian-to-instrumental transfer [PIT] effect), which was found to be stronger in prospectively relapsing AD patients than in abstaining patients. Second, an automatic approach bias toward alcohol stimuli was observed in AD patients, and interventions targeting this bias reduced the relapse risk in some studies. Previous findings suggest a potential behavioral and neurobiological overlap between these two mechanisms.
Methods
In this study, we examined the association between alcohol approach bias and both behavioral and neural non–drug-related PIT effects in AD patients after detoxification. A total of 100 AD patients (17 females) performed a PIT task and an alcohol approach/avoidance task. Patients were followed for 6 months.
Results
A stronger alcohol approach bias was associated with both a more pronounced behavioral PIT effect and stronger PIT-related neural activity in the right nucleus accumbens. Moreover, the association between alcohol approach bias and behavioral PIT increased with the severity of alcohol dependence and trait impulsivity and was stronger in patients who relapsed during follow-up in the exploratory analysis.
Conclusions
These findings indicate partial behavioral and neurobiological overlap between alcohol approach bias and the PIT effect assessed with our tasks. The association was stronger in patients with more severe alcohol dependence.
Keywords: Alcohol approach bias, Alcohol use disorder, fMRI, Pavlovian-to-instrumental transfer, Relapse, Trait impulsivity
SEE COMMENTARY ON PAGE 317
Alcohol-dependent (AD) patients frequently relapse after detoxification despite their intention to remain abstinent (1). Pavlovian conditioning has been hypothesized to contribute to relapse, because environmental cues associated with alcohol intake can become conditioned stimuli (CS) that elicit drug craving and may bias instrumental behavior toward drug seeking [e.g., (2, 3, 4)]. This phenomenon—the impact of environmental cues on instrumental behavior (i.e., Pavlovian-to-instrumental transfer [PIT] effect)—has been investigated in animal and human studies. Ethanol-associated cues can promote seeking behavior for not only ethanol but also non–ethanol-related reward in ethanol-treated rats (5). Repeated drug intake can further enhance non–drug-related reward-seeking elicited by nondrug cues in rats (6, 7, 8, 9, 10). Comparable alterations in motivational processes have also been observed in humans: enhanced non–drug-related PIT effects have been observed in AD patients compared with healthy control subjects and in prospectively relapsing patients compared with abstaining patients (11, 12, 13). Functional magnetic resonance imaging (fMRI) studies revealed that PIT effects can induce activation in the nucleus accumbens (NAcc) (14, 15, 16, 17). This brain area has long been associated with reinforcement learning (18,19), processing of alcohol cues, and craving (20).
It has been suggested that relapse can be triggered by an automatic approach tendency to alcohol stimuli, which was observed to be stronger in AD patients and heavy drinkers in some studies [e.g., (21, 22, 23)]. An alcohol approach bias in a laboratory can be operationalized as a shorter response latency to approach alcohol cues than to avoid them, even though the content of alcohol cues is task irrelevant. Applying an alcohol approach/avoidance task (aAAT), there is evidence that alcohol approach bias was positively associated with past hazardous drinking and future drinking (24,25). Inconsistently, there are studies using a stimulus-response compatibility task that found no approach bias or even an avoidance bias toward alcohol in AD patients (26,27) and a predictive role of the avoidance bias in future drinking or relapse (27,28). The discrepant findings could partly be explained by differences in the tasks (29). Cognitive bias modification (CBM) intervention adapted from the aAAT to retrain the approach bias has shown promising effects on decreasing relapse risk in AD patients [e.g., (30, 31, 32, 33)].
The previous findings indicate that both alcohol approach bias and PIT effect are closely associated with alcohol dependence. Although the association between alcohol approach bias and PIT effect in AD patients has so far not been directly investigated, previous theories and findings indicated a potential overlap between these two phenomena. Theoretically, in view of a dual-process model, an automatic approach bias was suggested to occur when the appetitive stimulus activates an impulsive or automatic system, which cannot be overridden by cognitive control processes because this reflective system is weakened (34). Automatic or impulsive approach biases may then drive addictive behavior (35), promoting drug seeking despite long-term harm (36). The strength of a non–drug-related PIT effect in AD patients has also been associated with impulsivity as assessed by a delay-discounting task (12). Moreover, impairments in inhibiting automatic approach biases to appetitive Pavlovian stimuli in a non–drug-related PIT task predicted relapse risk in AD patients (13). Based on those findings, we hypothesize that the impulsivity process could contribute to linking alcohol approach bias with PIT effects. On the neural level, neuroimaging studies also suggest that the underlying neurobiological mechanisms of the two effects overlap at least partly, because functional NAcc activation relates to both alcohol approach bias (37) and PIT effects (14, 15, 16, 17).
This study tested the hypothesis that alcohol approach bias is associated with behavioral and neural correlates of the non–drug-related PIT effect in recently detoxified AD patients. We hypothesized that patients with a stronger alcohol approach bias would show a more pronounced PIT effect behaviorally and in the NAcc. Furthermore, we hypothesized that the association between alcohol approach bias and PIT effect increases with the severity of alcohol dependence and trait impulsivity. In addition, we explored if the association between PIT and alcohol approach bias differed between prospective relapsers and abstainers with a 6-month follow-up.
Methods and Materials
Participants
AD patients were assessed in a bicentric research project conducted in Berlin and Dresden, Germany (ClinicalTrials.gov identifier: NCT02615977). The study was approved by local ethics committees of Charité Universitätsmedizin Berlin (EA1/268/14) and Technische Universität Dresden (EK 300082014). All participants gave written informed consent before participation.
Patients fulfilled the criteria of alcohol dependence according to the DSM-IV-TR, assessed by the Munich Composite International Diagnostic Instrument (38,39). After data cleaning (Supplement), 100 AD patients (age [mean ± SD] = 46.86 ± 10.30 years; 17 females; abstinence before study participation [mean ± SD]: 22.44 ± 12.77 days) were included for behavioral analyses and a subcohort of 72 patients (age [mean ± SD] = 44.97 ± 9.64 years; 10 females; abstinence before study participation [mean ± SD]: 23.33 ± 12.54 days) for imaging analyses.
The severity of alcohol dependence was measured by the Alcohol Dependence Scale (ADS) (40). Trait impulsivity was assessed by the Barratt Impulsiveness Scale-15 (BIS-15) (41). This study was conducted within a research consortium (DFG FOR 1617 and CRC-TRR 265), which also applied other tasks not reported in this paper. In addition, a subset of the participants underwent CBM training after the task assessments (nonsignificant training effects will be reported elsewhere). Patients had follow-ups 6 months (at weeks 6, 10, 14, 18, 22, and 26) after study participation and retrospectively reported their alcohol consumption since the last follow-up interview using the Timeline Follow-Back (42). We applied an intention-to-treat analysis (43) and classified all patients who relapsed to heavy drinking (i.e., ≥5 standard drinks [e.g., one standard drink = 0.33 L beer] for males and ≥4 standard drinks for females consumed on one drinking occasion), did not respond, or had incomplete follow-up information as belonging to the relapser group [as in (30,31,44)], while the rest of the patients were categorized as abstainers. We additionally conducted explorative analyses that included only patients with clear relapse status and reported results in the Supplement.
The aAAT
In this task, images of drink (alcohol drink or soft drink) were randomly presented inclined to the left or the right, and participants pulled or pushed a joystick (approach or avoid) according to the inclination of the image (see detailed description in the Supplement).
PIT Task
Participants performed an instrumental task (pressing the button to collect shells) while monetary CS learned from Pavlovian training were presented in the background [see the Supplement and (11, 12, 13) for a detailed description].
MRI Acquisition
Functional imaging was performed on Siemens Trio 3T MRI scanners at both study centers using echo-planar imaging sequences (repetition time: 2410 ms; echo time: 25 ms; flip angle: 80°; field of view: 192 × 192 mm2; voxel size: 3 × 3 × 2 mm3) comprising 42 slices approximately −25° to the bicommissural plane. We acquired a three-dimensional magnetization-prepared rapid gradient echo image (repetition time: 1900 ms; echo time: 5.25 ms; flip angle: 9°; field of view: 256 × 256 mm2; 192 sagittal slices; voxel size: 1 × 1 × 1 mm3) for coregistration and normalization during fMRI data preprocessing. A field map was collected before functional scanning to account for individual homogeneity differences of the magnetic field.
Data Analysis
Data were analyzed using MATLAB R2020b (MATLAB version 9.9, 2020; The MathWorks, Inc.) and the R System for Statistical Computing version 4.0.3 (R Development Core Team, 2020). SPM12 software package (http://www.fil.ion.ucl.ac.uk/spm/; Wellcome Centre for Human Neuroimaging) was used for fMRI data analyses.
Behavioral Analyses
For the aAAT, 6 patients were excluded because of excessive errors (>35%) (30,31). To exclude extreme outlier response times, the 1% fastest and 1% slowest responses were excluded in the overall response time distribution, consistent with the method used in previous studies (33,45, 46, 47). Trials with incorrect responses on the first try were also discarded. In line with Wiers et al. (31), D scores were calculated to reflect the approach bias to each stimulus category (see below). We further calculated a D-diff score to reflect an approach bias to alcohol relative to soft drink:
where RT is the response time and personal SD is the standard deviation of all response times including alcohol trials and soft drink trials, per participant.
For the PIT task, 10 patients who did not successfully learn the correlation between Pavlovian CS and unconditioned stimuli (i.e., performance in the forced choice task was not above chance) were excluded from analyses. A generalized linear mixed-effects model (GLMM) [R package lme4 (48)] with Poisson distribution was used to predict the number of button presses in each trial in the transfer part. The number of button presses depending on Pavlovian CS value was used to assess the behavioral PIT effect to be consistent with the imaging analyses (see Imaging Analyses). Parameters of Pavlovian CS value (i.e., the monetary value of Pavlovian CS in the background: +2, +1, 0, −1, −2), trial type of the instrumental condition (go and no-go; coded as +0.5 vs. −0.5), the individual alcohol approach bias (i.e., D-diff score in the aAAT), the interaction of Pavlovian CS value and D-diff score, and the interaction of instrumental condition and D-diff score were included as fixed effects in the GLMM. Subject IDs, instrumental stimuli (shells), and Pavlovian CS (fractal combined with pure tone) were treated as random effects to be controlled.
In addition, we further established GLMMs with additional parameters of ADS score and BIS-15 score separately and their interaction with other predictors (i.e., Pavlovian CS value and D-diff score) to examine if the association between alcohol approach bias and PIT effect interacts with those factors. We applied another GLMM to explore if this association differed between patients who abstained from alcohol and those who relapsed in follow-up.
Imaging Analyses
Nipype (49) was used for preprocessing the PIT fMRI data. First, correction for differences in slice time acquisition was performed to the middle slice as reference. Based on acquired field maps, voxel displacement maps were estimated. Images were realigned to correct for head motion, distortion, and their interaction. Coregistration of the individual structural T1 image to the individual mean echo-planar imaging was conducted. Then, the structural image was spatially normalized with a resampling solution of 2 × 2 × 2 mm3, and the normalization parameters were applied to all echo-planar imaging images. Finally, images were partially smoothed with a Gaussian kernel of 8-mm full width at half maximum. Before statistical analysis, data were high-pass filtered with a cutoff of 128 seconds to remove low-frequency fluctuation in the blood oxygen level–dependent signal.
After preprocessing, individual general linear models were established in SPM12. Non–drug-related PIT trials were modeled as one condition with three parametric modulators: the Pavlovian CS value, the transformed number of button presses [calculated as In(the original number of button presses + e)], and the PIT parameter, which is the product of the Pavlovian CS value and the transformed number of button presses. We added (Euler’s number) to the log transformation function for the number of button presses so that 0 button presses would be transformed to 1, resulting in different numerical values in the PIT parametric modulator after weighing by different Pavlovian CS values. In the end, a higher number of button presses to a higher Pavlovian CS value leads to a higher numerical value in the PIT parametric modulator. To account for variance caused by motor responses associated with button pressing, button presses of all trials were modeled in an additional regressor as stick functions. Drug-related PIT trials with similar parametric modulators as a separate condition and the realignment parameters with derivatives were included as regressors of no interest. The individual neural PIT effect was measured with a contrast in which the non–drug-related PIT parametric modulator was weighted with 1 and other regressors weighted with 0.
At the second-level analysis, a one-sample t test was established with individual contrast images. Individual alcohol approach bias (i.e., D-diff score) was treated as a covariate of interest in the model. In addition, participants’ age, sex, and study center were taken as additional covariates to control their potential impact on the results. Consistent with Garbusow et al. (11), a region of interest analysis was conducted with an a priori–defined compound region of interest in the left and right NAcc (NAccL, NAccR) (derived from the Wake Forest University PickAtlas software; http://www.fmri.wfubmc.edu/download.htm). Moreover, we performed an explorative whole-brain analysis for the main PIT effect on a significance level of uncorrected p < .001 and with k ≥ 20 activated voxels per cluster (Supplement). In addition, similar to the behavioral analysis, we also explored if retrospective relapsers and abstainers differ in the association of alcohol approach bias and neural PIT effect.
Results
Behavioral Results
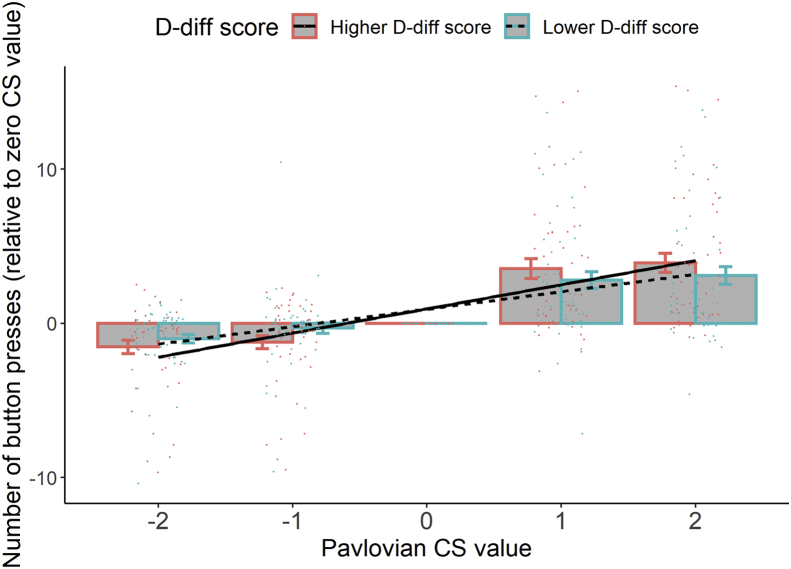
Patients showed a significant behavioral PIT effect—more button presses in the presence of higher monetary value–associated Pavlovian CS (main effect of Pavlovian CS value: estimate = 0.28, z = 77.81, p < .001) (Table 1). Moreover, there was a significant association of PIT effect elicited by the Pavlovian CS value with aAAT D-diff score (Pavlovian CS value × D-diff score: estimate = 0.14, z = 11.34, p < .001). Patients with a stronger alcohol approach bias in the aAAT task showed a more pronounced PIT effect (Figure 1). For a visual inspection of the raw D-diff scores and individual PIT slopes, see the Supplement.
Table 1.
Results of the Generalized Linear Mixed-Effects Model Regarding Effects of the Different Variables (Pavlovian CS Value and Instrumental Condition) and Association of Alcohol Approach Bias With Number of Button Presses in the PIT Task
| Parameter | Estimate | SE | z | p |
|---|---|---|---|---|
| Intercept | 1.44 | 0.05 | 28.80 | <.001 |
| D-Diff Score | 0.01 | 0.13 | 0.09 | .93 |
| Pavlovian CS Value | 0.28 | 0.004 | 77.81 | <.001 |
| Instrumental Condition (Go vs. No-Go) | 0.56 | 0.04 | 15.93 | <.001 |
| D-Diff Score × Pavlovian CS Value | 0.14 | 0.01 | 11.34 | <.001 |
| D-Diff Score × Instrumental Condition | −0.06 | 0.04 | −1.74 | .083 |
CS, conditioned stimulus; PIT, Pavlovian-to-instrumental transfer.
Figure 1.
Patients who displayed a stronger alcohol approach bias (a higher D-diff score in the alcohol approach/avoidance task) showed a higher Pavlovian-to-instrumental transfer effect (a steeper slope) than patients who had a lower alcohol approach bias (a lower D-diff score). The continuous D-diff score was transferred to a factor with two levels with a median split in this figure for illustration. Group means and SEMs are shown with bars and error bars. Individual values (mean number of button presses) are represented by colored dots. CS, conditioned stimulus.
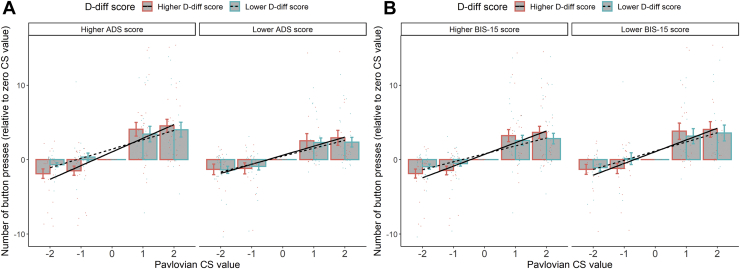
When including alcohol dependence severity (i.e., ADS score) in the GLMM, the results showed a significant interaction effect (Pavlovian CS value × D-diff score × ADS score: estimate = 0.02, z = 12.51, p < .001). Specifically, the more severe the alcohol dependence of the patient, the stronger the association between aAAT score and PIT effect (Figure 2A). Similar results also showed in the model with trait impulsivity (i.e., BIS-15 score) (Pavlovian CS value × D-diff score × BIS-15 score: estimate = 0.04, z = 14.58, p < .001) (Figure 2B). The association between alcohol approach bias and PIT effect increased with trait impulsivity. It should be noted that ADS score was positively correlated with BIS-15 score (rho = 0.24, p = .026, Spearman rank correlation) (see the Supplement for a visual inspection of the raw data).
Figure 2.
(A) Patients with higher Alcohol Dependence Scale (ADS) scores had a stronger association between alcohol approach bias (i.e., D-diff score) and the Pavlovian-to-instrumental transfer effect. (B) Patients with higher Barratt Impulsiveness Scale-15 (BIS-15) scores showed a stronger association between alcohol approach bias and the Pavlovian-to-instrumental transfer effect. Alcohol approach bias, ADS score, and BIS-15 score in this figure were all transferred to factors with two levels with a median split for illustration. Group means and SEMs are shown with bars and error bars. Individual values (mean number of button presses) are represented by colored dots.
For the exploratory analysis regarding aAAT D-diff score and PIT association between abstainers (n = 21) and relapsers (n = 79) using the intention-to-treat analysis approach, results yielded a significant interaction of Pavlovian CS value, aAAT D-diff score, and relapse group (estimate = 0.08, z = 2.34, p = .020). Follow-up analyses examined the Pavlovian CS value × D-diff score interaction in abstainers and relapsers separately and showed a higher parameter estimate of Pavlovian CS value × D-diff score interaction in relapsers (estimate = 0.15, z = 10.57, p < .001) compared with abstainers (estimate = 0.07, z = 2.43, p = .015).
Functional MRI Results
We observed a significant activation elicited by PIT in NAccL (x = −10, y = 8, z = −10; t67 = 2.92, small volume–corrected [SVC] and familywise error–corrected [FWE] pSVC-FWE = .035; voxel-based analysis) and NAccR (x = 8, y = 8, z = −12, t67 = 3.29, pSVC-FWE = .014).
More importantly, we observed a significant effect of aAAT D-diff score on PIT-related blood oxygen level–dependent signals in NAccR (x = 16, y = 14, z = −12; t67 = 3.40, pSVC-FWE = .010) (Figure 3) and a trendwise effect in NAccL (x = −14, y = 12, z = −12; t67 = 2.74, pSVC-FWE = .053).
Figure 3.
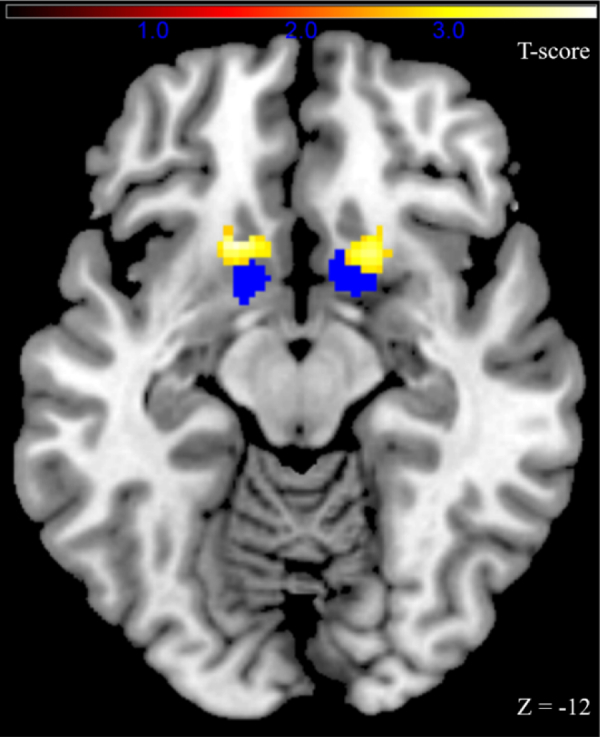
Strength of the alcohol approach bias was associated with Pavlovian-to-instrumental transfer–related neural activation in the nucleus accumbens. The bilateral nucleus accumbens region of interest is marked in blue, and functional Pavlovian-to-instrumental transfer activation associated with alcohol approach bias is marked in yellow (uncorrected p < .005 for illustration).
Exploratory analyses with 17 abstainers and 55 relapsers did not find a significant difference between the two subgroups in the association between aAAT D-diff score and neural PIT effect in either NAccR (relapsers > abstainers: x = 14, y = 4, z = −14, t65 = 0.98, pSVC-FWE = .667; abstainers > relapsers: x = 16, y = 14, z = −12, t65 = 0.42, pSVC-FWE = .815) or NAccL (relapsers > abstainers: x = −14, y = 8, z = −8, t65 = −0.04, pSVC-FWE = .870; abstainers > relapsers: x = −12, y = 8, z = −12, t65 = 1.33, pSVC-FWE = .523).
Discussion
This study examined the association between a non–drug-related PIT effect and automatic alcohol approach bias in AD patients. These two paradigms were chosen because both may reflect an impulsive approach bias (12,34), one alcohol cue–related and one reflecting an effect of nondrug Pavlovian cues, and because approach effects assessed in both paradigms have been associated with poor treatment outcomes or greater future drinking (11,13,25). Our key finding is that detoxified AD patients who had a stronger alcohol approach bias (relative to soft drinks) displayed a higher behavioral PIT effect (i.e., a stronger effect of Pavlovian CS in the background on unrelated instrumental behavior, indicated by more button presses) and a stronger PIT-related functional activation of NAccR. Furthermore, as expected, the association between alcohol approach bias and behavioral PIT effect increased with the severity of alcohol dependence and trait impulsivity. These findings link two well-established paradigms in alcohol research and indicate at least partially shared underlying mechanisms between alcohol approach bias and behavioral PIT effect.
From the perspective of dual-process accounts, alcohol approach bias is mainly driven by a system associated with impulsive and automatic decision making (34), and PIT effect has also been associated with choice impulsivity in AD patients (12). In this study, the association between the two effects was indeed larger in patients who reported higher trait impulsivity. Conditioned cues in the PIT paradigm were associated with monetary reward, while conditioned cues in the aAAT task reflect drug versus nondrug cues. These findings suggest that impulsive decision making can be triggered by the impact of drug-related and drug-unrelated cues on approach behavior in AD patients.
The severity of alcohol dependence might modulate the association of these two effects, because stronger associations between PIT and aAAT effects were found among patients with more severe alcohol dependence. Correlations are not causations, and potential explanations for these observations indicate two directions of further research. First, a stronger effect of nondrug Pavlovian background cues on approach behavior was already observed in young adults with higher versus lower levels of alcohol intake (50) and may reflect a risk factor for excessive consumption. Second, higher levels of alcohol intake can impact monoaminergic neurotransmission and promote associative learning of drug-related and contextual cues (51,52), thus potentially modifying cue-induced approach biases. With more severe alcohol dependence and higher levels of drug intake, the impact of conditioned cues on fast and impulsive decision making can increase, which may then lead to the observed, stronger association between cue effects assessed with both aAAT and the PIT paradigm. In this study, we observed a positive correlation between the severity of alcohol dependence (i.e., ADS score) and trait impulsivity (i.e., BIS-15 score), which emphasizes the role of impulsive decision making in more severe forms of alcohol dependence. Again, impulsive decision making can be both a cause and a consequence of excessive alcohol intake, because alcohol is known to impact not only monoaminergic systems but also prefrontal cortical brain areas associated with impulse control (53). Future studies in nonclinical high risky drinkers are needed to longitudinally assess the development of associations between impulsive decision making, conditioned cues responses, and alcohol intake.
On the neural level, functional NAcc activation has been associated with both PIT effect (14, 15, 16, 17) and alcohol approach bias (54). In this study, the strength of the behavioral alcohol approach bias was associated with the PIT-related functional activation of NAccR. Previous literature suggested a lateralized dopamine function in the NAcc, with dopamine release in NAccR reflecting the impact of drink-related CS (i.e., beer flavor) (55). In this study, NAccR was related to the association between alcohol approach bias and neural PIT effect, which underlies the role of this brain area in mediating the effects of Pavlovian conditioned cues as assessed in both paradigms. In the aAAT, Pavlovian conditioning to alcohol stimuli has been established during prolonged alcohol consumption, while in the PIT task, the Pavlovian conditioning was drug-unrelated and had been established in a laboratory setting. The correlation of the two effects is likely to reflect a more general alteration in the Pavlovian learning processes in alcohol dependence. Future longitudinal research can help assess changes in Pavlovian conditioning across the addiction cycle.
Some research has shown the predictive role of alcohol approach bias in drinking behavior (24,25). The CBM intervention targeted on retraining alcohol approach bias showed evidence of reducing the relapse risk in AD patients [e.g., (30,31)]. In contrast, the instrumental go/no-go responses in PIT can also be understood as an approach/no-approach behavior. The stronger impact of environmental cues on approach/no-approach behavior in the PIT task was particularly pronounced in prospective relapsers compared with abstainers (13). Our exploratory analysis compared subsequent relapsers with abstainers and observed a stronger association between alcohol approach bias and PIT effect in relapsers, indicating a potential role of the association of two approach behaviors in predicting treatment outcome.
Several limitations should be addressed. First, we lost track of a substantial number of patients during follow-up, which limits the interpretation of our exploratory analysis regarding the treatment outcome. Our study categorized patients with missing follow-up information as relapsers, in accordance with the method used in previous studies under the assumption that missing data is indicative of relapse [e.g., (30,31,44)]. When excluding patients who had unclear relapse status (n = 49) from the analysis, there was no more significant group difference between relapsers and abstainers in the association between alcohol approach bias and behavioral PIT (Supplement). We suspect that this null effect could be due to the insufficient statistical power of the small sample size. Future studies are warranted to elucidate the predictive role of the association between alcohol approach bias and PIT regarding relapse. Second, abstaining in our study was defined as not relapsing to heavy drinking. Other studies with different abstaining definitions (e.g., no alcohol consumption at all) might have different results. Third, most of the participants in this study underwent a CBM training procedure after conducting the aAAT and PIT, which we expected to reduce the relapse risk in AD patients. However, the relapse ratio did not differ between the training and placebo groups (results will be reported elsewhere). Considering that the null effect of training on relapse status could be due to insufficient statistical power (56), we included the training condition as a covariate in additional analyses. By doing that, we still observed a statistically significant interaction of treatment outcome (relapsers versus abstainers, categorizing patients lost to follow-up as relapsers) with the association between D-diff score and behavioral PIT and no difference between relapsers and abstainers in the association between D-diff score and neural PIT in either NAccR or NAccL. There is no indication that the training impacted findings regarding relapse in this study.
In conclusion, our study observed a significant association between alcohol approach bias and behavioral and neurobiological non–drug-related PIT effect in AD patients, and the behavioral association was correlated with the severity of alcohol dependence and trait impulsivity. These findings indicate at least a partial overlap of the underlying mechanisms of learning and decision making assessed in both paradigms and emphasize their relevance for severe alcohol use disorders.
Acknowledgments and Disclosures
The study was supported by the Deutsche Forschungsgemeinschaft (German Research Foundation) under Germany’s Excellence Strategy—EXC-2049 (Project No. 390688087), TRR SFB 265 (Project No. 402170461), and FOR 1617 (Project No. 186318919), as well as the China Scholarship Council (Grant No. 201806750014 [to KC]).
QJMH has received consultancy fees and options from Aya Health and a research grant from Koa Health. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2022.03.014.
Contributor Information
Ke Chen, Email: ke.chen@charite.de.
Andreas Heinz, Email: andreas.heinz@charite.de.
Supplementary Material
References
- 1.Bottlender M., Spanagel R., Soyka M. One drink, one drunk - Ist kontrolliertes Trinken möglich? Neue Ergebnisse aus Grundlagen- und Therapieforschung [One drink, one drunk—Controlled drinking by alcoholics? 3-year-outcome after intensive outpatient treatment] Psychother Psychosom Med Psychol. 2007;57:32–38. doi: 10.1055/s-2006-951918. [DOI] [PubMed] [Google Scholar]
- 2.Heinz A., Deserno L., Zimmermann U.S., Smolka M.N., Beck A., Schlagenhauf F. Targeted intervention: Computational approaches to elucidate and predict relapse in alcoholism. Neuroimage. 2017;151:33–44. doi: 10.1016/j.neuroimage.2016.07.055. [DOI] [PubMed] [Google Scholar]
- 3.Valyear M.D., Villaruel F.R., Chaudhri N. Alcohol-seeking and relapse: A focus on incentive salience and contextual conditioning. Behav Processes. 2017;141:26–32. doi: 10.1016/j.beproc.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 4.Perry C.J., Zbukvic I., Kim J.H., Lawrence A.J. Role of cues and contexts on drug-seeking behaviour. Br J Pharmacol. 2014;171:4636–4672. doi: 10.1111/bph.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corbit L.H., Janak P.H. Ethanol-associated cues produce general Pavlovian-instrumental transfer. Alcohol Clin Exp Res. 2007;31:766–774. doi: 10.1111/j.1530-0277.2007.00359.x. [DOI] [PubMed] [Google Scholar]
- 6.LeBlanc K.H., Maidment N.T., Ostlund S.B. Repeated cocaine exposure facilitates the expression of incentive motivation and induces habitual control in rats. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LeBlanc K.H., Maidment N.T., Ostlund S.B. Impact of repeated intravenous cocaine administration on incentive motivation depends on mode of drug delivery. Addict Biol. 2014;19:965–971. doi: 10.1111/adb.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostlund S.B., LeBlanc K.H., Kosheleff A.R., Wassum K.M., Maidment N.T. Phasic mesolimbic dopamine signaling encodes the facilitation of incentive motivation produced by repeated cocaine exposure. Neuropsychopharmacology. 2014;39:2441–2449. doi: 10.1038/npp.2014.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saddoris M.P., Stamatakis A., Carelli R.M. Neural correlates of Pavlovian-to-instrumental transfer in the nucleus accumbens shell are selectively potentiated following cocaine self-administration. Eur J Neurosci. 2011;33:2274–2287. doi: 10.1111/j.1460-9568.2011.07683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shields C.N., Gremel C.M. Prior chronic alcohol exposure enhances Pavlovian-to-instrumental transfer. Alcohol. 2021;96:83–92. doi: 10.1016/j.alcohol.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garbusow M., Schad D.J., Sebold M., Friedel E., Bernhardt N., Koch S.P., et al. Pavlovian-to-instrumental transfer effects in the nucleus accumbens relate to relapse in alcohol dependence. Addict Biol. 2016;21:719–731. doi: 10.1111/adb.12243. [DOI] [PubMed] [Google Scholar]
- 12.Sommer C., Garbusow M., Jünger E., Pooseh S., Bernhardt N., Birkenstock J., et al. Strong seduction: Impulsivity and the impact of contextual cues on instrumental behavior in alcohol dependence. Transl Psychiatry. 2017;7:e1183. doi: 10.1038/tp.2017.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sommer C., Birkenstock J., Garbusow M., Obst E., Schad D.J., Bernhardt N., et al. Dysfunctional approach behavior triggered by alcohol-unrelated Pavlovian cues predicts long-term relapse in alcohol dependence. Addict Biol. 2020;25 doi: 10.1111/adb.12703. [DOI] [PubMed] [Google Scholar]
- 14.Bray S., Rangel A., Shimojo S., Balleine B., O’Doherty J.P. The neural mechanisms underlying the influence of Pavlovian cues on human decision making. J Neurosci. 2008;28:5861–5866. doi: 10.1523/JNEUROSCI.0897-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geurts D.E.M., Huys Q.J.M., den Ouden H.E.M., Cools R. Aversive Pavlovian control of instrumental behavior in humans. J Cogn Neurosci. 2013;25:1428–1441. doi: 10.1162/jocn_a_00425. [DOI] [PubMed] [Google Scholar]
- 16.Lewis A.H., Niznikiewicz M.A., Delamater A.R., Delgado M.R. Avoidance-based human Pavlovian-to-instrumental transfer. Eur J Neurosci. 2013;38:3740–3748. doi: 10.1111/ejn.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talmi D., Seymour B., Dayan P., Dolan R.J. Human Pavlovian-instrumental transfer. J Neurosci. 2008;28:360–368. doi: 10.1523/JNEUROSCI.4028-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flagel S.B., Clark J.J., Robinson T.E., Mayo L., Czuj A., Willuhn I., et al. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lesaint F., Sigaud O., Clark J.J., Flagel S.B., Khamassi M. Experimental predictions drawn from a computational model of sign-trackers and goal-trackers. J Physiol Paris. 2015;109:78–86. doi: 10.1016/j.jphysparis.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinz A., Siessmeier T., Wrase J., Hermann D., Klein S., Grüsser S.M., et al. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving [published correction appears in Am J Psychiatry 2004; 161:2344] Am J Psychiatry. 2004;161:1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- 21.Field M., Kiernan A., Eastwood B., Child R. Rapid approach responses to alcohol cues in heavy drinkers. J Behav Ther Exp Psychiatry. 2008;39:209–218. doi: 10.1016/j.jbtep.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Wiers R.W., Rinck M., Dictus M., van den Wildenberg E. Relatively strong automatic appetitive action-tendencies in male carriers of the OPRM1 G-allele. Genes Brain Behav. 2009;8:101–106. doi: 10.1111/j.1601-183X.2008.00454.x. [DOI] [PubMed] [Google Scholar]
- 23.Wiers R.W., Rinck M., Kordts R., Houben K., Strack F. Retraining automatic action-tendencies to approach alcohol in hazardous drinkers. Addiction. 2010;105:279–287. doi: 10.1111/j.1360-0443.2009.02775.x. [DOI] [PubMed] [Google Scholar]
- 24.Kersbergen I., Woud M.L., Field M. The validity of different measures of automatic alcohol action tendencies [published correction appears in Psychol Addict Behave 2015; 29:337] Psychol Addict Behav. 2015;29:225–230. doi: 10.1037/adb0000009. [DOI] [PubMed] [Google Scholar]
- 25.Martin Braunstein L., Kuerbis A., Ochsner K., Morgenstern J. Implicit alcohol approach and avoidance tendencies predict future drinking in problem drinkers. Alcohol Clin Exp Res. 2016;40:1945–1952. doi: 10.1111/acer.13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barkby H., Dickson J.M., Roper L., Field M. To approach or avoid alcohol? Automatic and self-reported motivational tendencies in alcohol dependence. Alcohol Clin Exp Res. 2012;36:361–368. doi: 10.1111/j.1530-0277.2011.01620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spruyt A., De Houwer J., Tibboel H., Verschuere B., Crombez G., Verbanck P., et al. On the predictive validity of automatically activated approach/avoidance tendencies in abstaining alcohol-dependent patients. Drug Alcohol Depend. 2013;127:81–86. doi: 10.1016/j.drugalcdep.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 28.Field M., Di Lemma L., Christiansen P., Dickson J. Automatic avoidance tendencies for alcohol cues predict drinking after detoxification treatment in alcohol dependence. Psychol Addict Behav. 2017;31:171–179. doi: 10.1037/adb0000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiers R.W., Gladwin T.E., Rinck M. Should we train alcohol-dependent patients to avoid alcohol? Front Psychiatry. 2013;4:33. doi: 10.3389/fpsyt.2013.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eberl C., Wiers R.W., Pawelczack S., Rinck M., Becker E.S., Lindenmeyer J. Approach bias modification in alcohol dependence: Do clinical effects replicate and for whom does it work best? Dev Cogn Neurosci. 2013;4:38–51. doi: 10.1016/j.dcn.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiers R.W., Eberl C., Rinck M., Becker E.S., Lindenmeyer J. Retraining automatic action tendencies changes alcoholic patients’ approach bias for alcohol and improves treatment outcome. Psychol Sci. 2011;22:490–497. doi: 10.1177/0956797611400615. [DOI] [PubMed] [Google Scholar]
- 32.Salemink E., Rinck M., Becker E., Wiers R.W., Lindenmeyer J. Does comorbid anxiety or depression moderate effects of approach bias modification in the treatment of alcohol use disorders? Psychol Addict Behav. 2022;36:547–554. doi: 10.1037/adb0000642. [DOI] [PubMed] [Google Scholar]
- 33.Rinck M., Wiers R.W., Becker E.S., Lindenmeyer J. Relapse prevention in abstinent alcoholics by cognitive bias modification: Clinical effects of combining approach bias modification and attention bias modification. J Consult Clin Psychol. 2018;86:1005–1016. doi: 10.1037/ccp0000321. [DOI] [PubMed] [Google Scholar]
- 34.Fleming K.A., Bartholow B.D. Alcohol cues, approach bias, and inhibitory control: Applying a dual process model of addiction to alcohol sensitivity. Psychol Addict Behav. 2014;28:85–96. doi: 10.1037/a0031565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiers R.W., Stacy A.W., editors. Handbook of Implicit Cognition and Addiction. Sage Publications; Thousand Oaks, CA: 2006. [Google Scholar]
- 36.Wiers R.W., Stacy A.W. Implicit cognition and addiction. Curr Dir Psychol Sci. 2006;15:292–296. [Google Scholar]
- 37.Wiers C.E., Stelzel C., Gladwin T.E., Park S.Q., Pawelczack S., Gawron C.K., et al. Effects of cognitive bias modification training on neural alcohol cue reactivity in alcohol dependence. Am J Psychiatry. 2015;172:335–343. doi: 10.1176/appi.ajp.2014.13111495. [DOI] [PubMed] [Google Scholar]
- 38.Wittchen H.U., Pfister H. Swets & Zeitlinger Publishers; 1997. DIA-X-Interviews: Manual für Screening-Verfahren und Interview; Interviewheft. (DIA-X-Interviews: Manual for Screening Procedures and Interview; Interview Booklet.) Frankfurt, Germany. [Google Scholar]
- 39.Jacobi F., Mack S., Gerschler A., Scholl L., Höfler M., Siegert J., et al. The design and methods of the mental health module in the German Health Interview and Examination Survey for Adults (DEGS1-MH) Int J Methods Psychiatr Res. 2013;22:83–99. doi: 10.1002/mpr.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skinner H.A., Horn J.L. Addiction Research Foundation of Ontario; Toronto: 1984. Alcohol Dependence Scale (ADS): User’s Guide. [Google Scholar]
- 41.Meule A., Vögele C., Kübler A. Psychometrische evaluation der Deutschen Barratt impulsiveness scale–Kurzversion (BIS-15) [Psychometric evaluation of the German Barratt Impulsiveness Scale - Short Version (BIS-15).] Diagnostica. 2011;57:126–133. [Google Scholar]
- 42.Sobell L.C., Sobell M.B. In: Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Litten R.Z., Allen J.P., editors. Humana Press; Totowa, NJ: 1992. Timeline follow-back: A technique for assessing self-reported alcohol consumption; pp. 41–72. [Google Scholar]
- 43.Tripepi G., Chesnaye N.C., Dekker F.W., Zoccali C., Jager K.J. Intention to treat and per protocol analysis in clinical trials. Nephrology (Carlton) 2020;25:513–517. doi: 10.1111/nep.13709. [DOI] [PubMed] [Google Scholar]
- 44.Falk D., Wang X.Q., Liu L., Fertig J., Mattson M., Ryan M., et al. Percentage of subjects with no heavy drinking days: Evaluation as an efficacy endpoint for alcohol clinical trials. Alcohol Clin Exp Res. 2010;34:2022–2034. doi: 10.1111/j.1530-0277.2010.01290.x. [DOI] [PubMed] [Google Scholar]
- 45.Becker E.S., Barth A., Smits J.A.J., Beisel S., Lindenmeyer J., Rinck M. Positivity-approach training for depressive symptoms: A randomized controlled trial. J Affect Disord. 2019;245:297–304. doi: 10.1016/j.jad.2018.11.042. [DOI] [PubMed] [Google Scholar]
- 46.Nasrin F., Rimes K., Reinecke A., Rinck M., Barnhofer T. Effects of brief behavioural activation on approach and avoidance tendencies in acute depression: Preliminary findings. Behav Cogn Psychother. 2017;45:58–72. doi: 10.1017/S1352465816000394. [DOI] [PubMed] [Google Scholar]
- 47.Swinkels L.M.J., Gramser H., Becker E.S., Rinck M. Self-approach tendencies: Relations with explicit and implicit self-evaluations. Front Psychol. 2019;10:309. doi: 10.3389/fpsyg.2019.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bates D., Mächler M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 49.Gorgolewski K., Burns C.D., Madison C., Clark D., Halchenko Y.O., Waskom M.L., Ghosh S.S. Nipype: A flexible, lightweight and extensible neuroimaging data processing framework in python. Front Neuroinform. 2011;5:13. doi: 10.3389/fninf.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garbusow M., Nebe S., Sommer C., Kuitunen-Paul S., Sebold M., Schad D.J., et al. Pavlovian-to-instrumental transfer and alcohol consumption in young male social drinkers: Behavioral, neural and polygenic correlates. J Clin Med. 2019;8:1188. doi: 10.3390/jcm8081188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heinz A., Schlagenhauf F., Beck A., Wackerhagen C. Dimensional psychiatry: Mental disorders as dysfunctions of basic learning mechanisms. J Neural Transm (Vienna) 2016;123:809–821. doi: 10.1007/s00702-016-1561-2. [DOI] [PubMed] [Google Scholar]
- 52.Saunders B.T., Richard J.M., Margolis E.B., Janak P.H. Dopamine neurons create Pavlovian conditioned stimuli with circuit-defined motivational properties. Nat Neurosci. 2018;21:1072–1083. doi: 10.1038/s41593-018-0191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Volkow N.D., Michaelides M., Baler R. The neuroscience of drug reward and addiction. Physiol Rev. 2019;99:2115–2140. doi: 10.1152/physrev.00014.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiers C.E., Stelzel C., Park S.Q., Gawron C.K., Ludwig V.U., Gutwinski S., et al. Neural correlates of alcohol-approach bias in alcohol addiction: The spirit is willing but the flesh is weak for spirits. Neuropsychopharmacology. 2014;39:688–697. doi: 10.1038/npp.2013.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oberlin B.G., Dzemidzic M., Tran S.M., Soeurt C.M., O’Connor S.J., Yoder K.K., Kareken D.A. Beer self-administration provokes lateralized nucleus accumbens dopamine release in male heavy drinkers. Psychopharmacology (Berl) 2015;232:861–870. doi: 10.1007/s00213-014-3720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rinck M. CBM research needs more power: Commentary on the special issue on cognitive bias modification. J Behav Ther Exp Psychiatry. 2017;57:215. doi: 10.1016/j.jbtep.2016.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.