Abstract
Background
Integrated treatments for comorbid depression (often with anxiety) and obesity are lacking; mechanisms are poorly investigated.
Methods
In a mechanistic pilot trial, adults with body mass index ≥30 and Patient Health Questionnaire-9 scores ≥10 were randomized to usual care (n = 35) or an integrated behavioral intervention (n = 71). Changes at 6 months in body mass index and Depression Symptom Checklist-20 scores were co-primary outcomes, and Generalized Anxiety Disorder Scale-7 score was a secondary outcome. Changes at 2 months in the activation and functional connectivity of regions of interest in the negative affect circuit were primary neural targets, and secondary targets were in the cognitive control, default mode, and positive affect circuits.
Results
Participants were 47.0 years (SD = 11.9 years), 76% women, 55% Black, and 20% Latino. Depression Symptom Checklist-20 (between-group difference, −0.3 [95% CI: −0.6 to −0.1]) and Generalized Anxiety Disorder Scale-7 (−2.9 [−4.7 to −1.1]) scores, but not body mass index, decreased significantly at 6 months in the intervention versus usual care groups. Only Generalized Anxiety Disorder Scale-7 score changes at 6 months significantly correlated with neural target changes at 2 months in the negative affect (anterior insula, subgenual/pregenual anterior cingulate cortex, amygdala) and cognitive control circuits (dorsal lateral prefrontal cortex, dorsal anterior cingulate cortex). Effects were medium to large (0.41–1.18 SDs). Neural target changes at 2 months in the cognitive control circuit only differed by treatment group. Effects were medium (0.58–0.79 SDs).
Conclusions
Compared with usual care, the study intervention led to significantly improved depression but not weight loss, and the results on neural targets were null for both outcomes. The significant intervention effect on anxiety might be mediated through changes in the cognitive control circuit, but this warrants replication.
Keywords: Anxiety, Cognitive control, Depression, Functional neuroimaging, Negative affect, Obesity
Multimorbidity, such as depression and obesity, is a pressing public health concern (1), severely exacerbated by the COVID-19 pandemic (2, 3, 4, 5). Effective integrated treatments for comorbid depression and obesity are lacking, and mechanisms are poorly investigated.
A recent randomized clinical trial (RCT) demonstrated the effectiveness of an integrated collaborative care intervention, I-CARE (Integrated Coaching for Better Mood and Weight), grounded in behavior change theories (6,7), in improving comorbid depression (often with associated anxiety symptoms) and obesity among 409 adults with these conditions (8). The trial was of high methodological rigor and the largest among the RCTs of comorbid depression and obesity treatment trials based on a recent review (9). Using an experimental medicine approach (10), an ancillary study of that trial, ENGAGE (Engaging self-regulation targets to understand the mechanisms of behavior change and improve mood and weight outcomes) (11), was conducted to explore possible neural mechanisms underlying the integrated behavioral treatment of depression and obesity based on the premise that behavior change could be better understood and optimized when assessed in relation to its underlying brain functions. Specifically, the construct of self-regulation offered a framework for understanding how brain functions related to behavior change in people experiencing depression and obesity. It was posited that self-regulation required ongoing adjustment of emotional reactions, the contents of cognition, and self-directed reflection to maximize adaptive—and minimize maladaptive—outcomes (11). The results of the ENGAGE study suggested that large-scale neural circuits, particularly the negative affect circuit, predicted or mediated treatment effects on problem-solving ability, physical activity, and depressive symptoms (12,13).
As the first exploration of potential neural mechanisms underlying the integrated behavioral treatment of depression and obesity, the ENGAGE study findings were promising but exploratory, and study limitations included a relatively homogenous sample. To advance this line of discovery research, a follow-on clinical trial was conducted in an independent, racially and ethnically diverse sample. ENGAGE-2 was a mechanistic pilot RCT with several methodological enhancements (e.g., diverse sample, refined intervention and neural targets, improved outcome measures) (14). The primary aim was to test the degree to which engaging prespecified neural circuits produces desired changes in clinical outcomes in an independent sample of primarily underrepresented minority participants (14). Accordingly, this mechanistic trial investigated 1) whether changes in clinical outcomes differed by treatment group at 6 months (i.e., treatment effect), 2) whether early changes (2 months) in neural targets predicted subsequent changes (6 months) in clinical outcomes differentially by intervention versus usual care (i.e., treatment-dependent temporal relationship), and 3) whether early changes in neural targets differed by treatment group (i.e., causal effect).
Methods and Materials
The Institutional Review Boards for the University of Illinois (UI) at Chicago and Stanford University approved the study. All participants provided written consent. The trial protocol was previously published (14).
Study Design and Participants
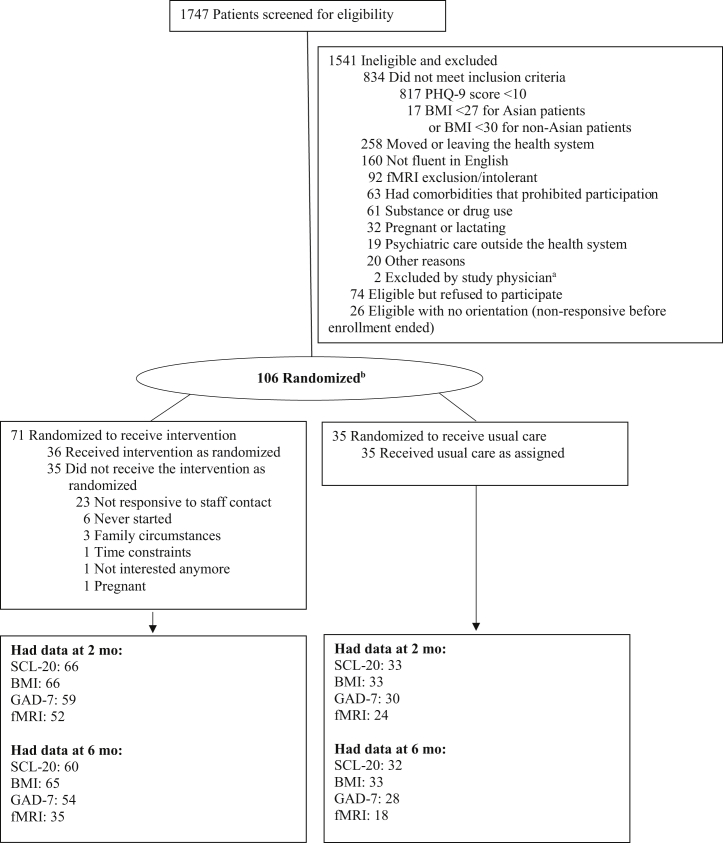
Enrollment followed a multistep process (Figure 1). Participants were recruited between March 1, 2019, and March 19, 2020, from the internal medicine outpatient care clinics at UI Health, a minority-serving, tertiary care academic health system. Adults were eligible if they had a body mass index (BMI) ≥30 (≥27, if Asian) and a Patient Health Questionnaire-9 (PHQ-9) score ≥10, without serious medical or psychiatric comorbidities or other exclusions (Table S1).
Figure 1.
Enrollment, randomization, and follow-up of the study patients. aOne patient was newly diagnosed with diabetes, and the other patient was recently hospitalized after loss of consciousness. bThe ENGAGE-2 trial uses 2:1 randomization allocation. In operation, participants are randomized in a 1:1:1 ratio to 1 of 3 groups to protect blinding and preserve the allocation ratio at every allocation based on covariate-adaptive minimization. BMI, body mass index; fMRI, functional magnetic resonance imaging; GAD-7, Generalized Anxiety Disorder Scale-7; PHQ-9, Patient Health Questionnaire-9; SCL-20, Depression Symptom Checklist-20.
Randomization and Masking
Participants (N = 106) were randomly assigned in a 2:1 ratio to receive the I-CARE2 intervention or usual care using a validated online system (15) based on covariate-adaptive minimization (16). The 2:1 allocation allowed more participants to receive the study intervention (8) without substantially reducing statistical power (17). The minimization method was used to achieve better-than-chance marginal balance across multiple baseline characteristics: age, sex, race/ethnicity, education, BMI, Depression Symptom Checklist-20 (SCL-20) score, and current use of antidepressant medication (yes/no). An imbalance in these covariates between the treatment groups could bias treatment effect estimates. Investigators, the Data and Safety Monitoring Board, outcome assessors, and the data analyst were blinded to participants’ treatment assignment until after completing the primary data lock.
Intervention
I-CARE2, an updated version of the I-CARE intervention (14), combined problem-solving therapy (PST), involving 7-step problem-solving and behavioral activation strategies as first-line, with antidepressant medications as needed for depression management (18,19), and the Group Lifestyle Balance video program (20) for weight loss. There were 6 one-on-one in-person PST sessions in the first 2 months, 3 additional PST sessions, and 11 home-viewed Group Lifestyle Balance videos over the next 4 months. Participants were expected to self-monitor their weight and diet and synchronize their activity tracker data via the Fitbit application throughout the intervention period.
Usual Care
Participants in both the intervention and usual care control groups were advised to continue routine medical care and were provided with a summary of behavioral health and weight management services at UI Health. Control participants also received Alta HR activity trackers (Fitbit [Google LLC]) but not any other intervention materials.
Clinical Outcome Measures
Assessments of depression, BMI, and anxiety occurred at baseline, 2 months, and 6 months. Depression was measured by SCL-20, with scores between 0 (best) and 4 (worst) (21,22). BMI was based on measured height (baseline only) and weight using standardized protocols (23). Anxiety was measured by the Generalized Anxiety Disorder Scale-7 (GAD-7), with scores between 0 (best) and 21 (worst) (24). Changes in SCL-20 scores and BMI at 6 months were the co-primary outcomes, and change in GAD-7 scores was secondary. Other secondary outcomes included depression treatment response (i.e., ≥50% improvement in SCL-20 scores from baseline) (18,19), depression remission (i.e., SCL-20 scores < 0.5) (18,19), and 5% weight loss. Post hoc outcomes included 3% weight loss, anxiety treatment response (i.e., ≥50% improvement in GAD-7 scores from baseline) (25), and anxiety remission (i.e., GAD-7 scores < 0.5) (25).
Neural Target Measures
Functional magnetic resonance imaging data were collected at baseline and 2 months, using previously established functional magnetic resonance imaging sequences and parameters (11,26) (see Supplemental Functional Neuroimaging Methods).
The negative affect circuit was engaged by viewing threat and sad faces. The regions of interest (ROIs) were defined in a prior systematic procedure (27) validated with the same facial emotion task as used in the ENGAGE and ENGAGE-2 trials (11, 12, 13, 14). Consistent with these previous findings, the primary target ROIs in this study were the subgenual anterior cingulate cortex (ACC) and amygdala (bilaterally) for threat faces in the nonconscious viewing condition and the pregenual ACC, amygdala (bilaterally), and anterior insula (bilaterally) for sad faces in the conscious viewing condition. A priori secondary neural targets included functional connectivity between these ROIs and global circuit dysfunction scores for the negative affect circuit engaged by nonconscious threat and conscious sad face viewing tasks. A priori secondary neural targets also included ROI and global circuit dysfunction scores for the cognitive control circuit using a go/no-go task, the default mode circuit, the negative affect circuit engaged by conscious threat face viewing task, and the positive affect circuit engaged by conscious happy face viewing task.
Patient-level activation of the ROIs for each contrast of interest for each task (e.g., threat vs. neutral, sad vs. neutral, happy vs. neutral, no-go vs. go) was derived in a manner consistent with the methods used for a healthy reference sample (26). Similarly, psychophysiological interaction and intrinsic functional connectivity analyses were used to quantify functional connectivity between ROIs (26). These activation and connectivity values were expressed in standard deviation units relative to the healthy reference sample and then winsorized using ± 3 SD (26). A global circuit score was computed by averaging the constituent activation and connectivity values for each circuit by task; higher global circuit scores indicate greater dysfunction according to Williams’ theoretical framework (27,28). This standard imaging quantification approach has been applied in our prior ENGAGE trial outcome study (12,13).
Statistical Analysis
To evaluate the intervention effects on clinical outcomes, between-group differences in the co-primary (SCL-20, BMI) and secondary (GAD-7) outcomes were tested in separate linear mixed models. Per protocol, the fixed effects of each model included baseline value of the outcome, randomization covariates, group (intervention or control), time point (2 or 6 months), and group-by-time interaction. Also, an indicator of whether a participant’s outcome was assessed before or after the COVID-19 lockdown date on March 16, 2020, in Illinois (Supplemental Methods and Figure S1) was added as a fixed effect. However, changes in the outcomes at 2 and 6 months did not differ significantly among participants assessed before versus after this date (Table S2). The random effects accounted for repeated measures with an unstructured covariance matrix. Each model included all participants with follow-up data on the outcome at 2 and/or 6 months, and participants were analyzed based on the group to which they were assigned. Missing data were handled through maximum-likelihood estimation using mixed modeling. Per protocol, in the case of missing study-measured weight, the closest clinical weight documented in the electronic health record within 3 months of the due date of a missed study visit or the self-reported weight (if no clinical weight) was used. Model-based adjusted mean differences with 95% CIs were reported. Moderation analysis was performed using marginal models with repeated measures that included the same fixed effects as above plus the main effect of each potential effect modifier (e.g., sex) and its interaction with group; the latter, if significant, rejected the null hypothesis of no moderation. χ2 tests were used for comparing the percentages of participants achieving clinically significant outcomes (depression response and remission, 3% and 5% weight loss, and anxiety response and remission) at 6 months between the intervention and control groups.
To evaluate the mediation effects of early change in neural targets on subsequent change in clinical outcomes, we applied the approach by Kraemer et al. (29) in 2 sets of analyses to test 1) whether changes in neural targets at 2 months (M) correlated with changes in clinical outcomes at 6 months (Y) and 2) whether changes in neural targets at 2 months differed between the intervention and control groups (X). According to Kraemer et al. (29), potential causal mediation would be indicated if both 1) a neural target change at 2 months correlated significantly with a clinical outcome change at 6 months either in the usual care group or by interaction with the intervention (M → Y) and 2) the intervention effect on the neural target change at 2 months was significant compared with usual care (X → M) (Figure S2). The correlation of change in a neural target at 2 months with change in a clinical outcome at 6 months was tested using ordinary least square regression, adjusting for the baseline value of the clinical outcome and the COVID-19 lockdown indicator for the 6-month outcome measure (all baseline and 2-month functional magnetic resonance imaging scans occurred before the lockdown). Participants with complete data on the neural target and the outcome of interest in a model were included. To obtain standardized coefficients, outcome measures at baseline and 6 months were standardized using the baseline standard deviation. Regression coefficients with 95% CIs for the usual care group and the interaction (i.e., the difference between the intervention and usual care groups) were reported. Regression coefficients in the usual care group show the correlations of changes in neural targets at 2 months with changes in clinical outcomes at 6 months within the usual care group. Regression coefficients for the interaction show the differences in the correlations between the intervention and usual care groups. Changes in neural targets at 2 months were compared between the intervention and control groups using t tests.
All analyses were conducted using SAS, version 9.4 (SAS Institute Inc.). Due to the pilot nature of this mechanistic trial, we focused on reporting standardized mean estimates with 95% CIs as per recommendations (30,31). To aid transparent interpretation, we also presented p values unadjusted and adjusted (padj) using the false discovery rate (FDR) procedure (32) for defined families of tests (Table S3).
Results
Sample Characteristics
Of 106 participants, the mean age was 47.0 years (SD = 11.9 years), 76% were women, 55% were Black, 20% were Latino, 54% had a high school or some college education, and 57% reported an annual family income <$55,000 (Table 1). On average, participants had moderately severe obesity (mean BMI = 37.1 [SD = 6.0]), moderate depression (mean PHQ-9 score = 12.8 [SD = 2.8]; mean SCL-20 score = 1.2 [SD = 0.7]), with 18% reporting taking antidepressant medications, and mild anxiety (mean GAD-7 score = 6.9 [SD = 4.8]). A total of 92 participants (86.8%) completed 6-month follow-up.
Table 1.
Baseline Characteristics by Treatment Group
| Characteristic | Intervention, n = 71 | Usual Care, n = 35 |
|---|---|---|
| Age, Yearsa | 46.7 (11.7) | 47.4 (12.5) |
| Sex, Femalea | 55 (77%) | 26 (74%) |
| Race/Ethnicitya | ||
| African American | 41 (58%) | 17 (49%) |
| Asian/Pacific Islander | 2 (3%) | 0 (0%) |
| Hispanic | 10 (14%) | 11 (31%) |
| Non-Hispanic White | 13 (18%) | 6 (17%) |
| Other (e.g., decline to state, multirace) | 5 (7%) | 1 (3%) |
| Educationa | ||
| High school/GED or less | 7 (10%) | 7 (20%) |
| College, 1 year to 3 years | 31 (44%) | 12 (34%) |
| College, 4 years or more | 19 (27%) | 10 (29%) |
| Post college | 14 (20%) | 6 (17%) |
| Income | ||
| <$35,000 | 22 (31%) | 12 (34%) |
| $35,000 to <$55,000 | 16 (23%) | 10 (29%) |
| $55,000 to <$75,000 | 12 (17%) | 3 (9%) |
| ≥$75,000 | 21 (30%) | 10 (29%) |
| BMI, kg/m2a | 37.0 (6.0) | 37.2 (6.3) |
| Weight, kg | 101.9 (15.4) | 100.5 (15.0) |
| Waist Circumference, cm | 111.9 (11.7) | 114.5 (14.3) |
| PHQ-9 Score | 12.9 (2.9) | 12.8 (2.4) |
| SCL-20 Scorea | 1.2 (0.7) | 1.1 (0.6) |
| Antidepressant Medication Use by Patient Report, n (%)a | 12 (17%) | 7 (20%) |
| GAD-7 Score | 7.0 (5.0) | 6.8 (4.4) |
Values are presented as mean (SD) or n (%).
BMI, body mass index; GAD-7, Generalized Anxiety Disorder Scale-7; GED, general educational development; PHQ-9, Patient Health Questionnaire-9; SCL-20, Depression Symptom Checklist-20.
Prognostic factors for randomization: age, sex, race/ethnicity, education, BMI, SCL-20 score, and current use of antidepressant medication.
Intervention Effect on Clinical Outcomes
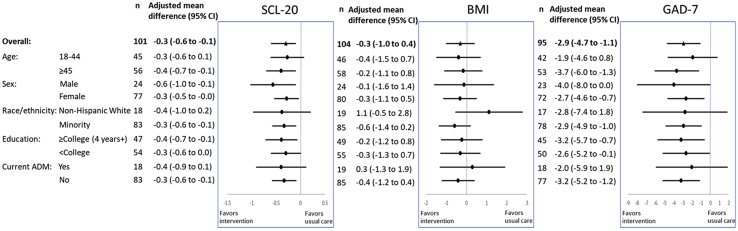
At 6 months, intervention participants had significantly greater improvements in SCL-20 and GAD-7 scores, but not BMI, than usual care participants (Figure 2). The between-group mean difference was −0.3 (95% CI = −0.6 to −0.1, p = .002) for SCL-20 and −2.9 (95% CI = −4.7 to −1.1, p = .002) for GAD-7. The mean differences in SCL-20 and GAD-7 were consistently in favor of the intervention group, with no evidence for effect modification by age, sex, race/ethnicity, education, or antidepressant medication use. The between-group mean difference for BMI was −0.3 (95% CI = −1.0 to 0.4, p = .45). A significantly higher percentage of participants in the intervention compared with usual care achieved remission of depressive symptoms (43% vs. 22%, p = .04) and anxiety symptoms (63% vs. 39%, p = .04) at 6 months (Figure S3). A higher percentage of participants in the intervention compared with usual care were also defined as responders for anxiety symptoms (56% vs. 25%, p = .008), but not for depression. Percentages of participants achieving 3% or 5% weight loss at 6 months did not differ significantly by group. At 2 months, the mean differences were also in favor of the intervention group overall for SCL-20 (−0.2 [95% CI = −0.4 to 0.0], p = .09) and GAD-7 (−3.1 [95% CI = −4.6 to −1.6], p < .001) scores and by subgroups (Figure S4). The between-group mean difference for BMI was null (0.1 [95% CI = −0.3 to 0.4], p = .65). Post hoc analysis suggested a trend of greater improvements in SCL-20 and GAD-7 scores, but less weight loss, in the intervention versus usual care after the COVID-19 lockdown (Table S2).
Figure 2.
Intervention effects on outcomes at 6 months, overall and by subgroup. ADM, antidepressant medication; BMI, body mass index; GAD-7, Generalized Anxiety Disorder Scale-7; SCL-20, Depression Symptom Checklist-20.
Association of Neural Targets With Clinical Outcomes
Changes in neural targets at 2 months were not significantly associated with changes in SCL-20 scores or BMI at 6 months.
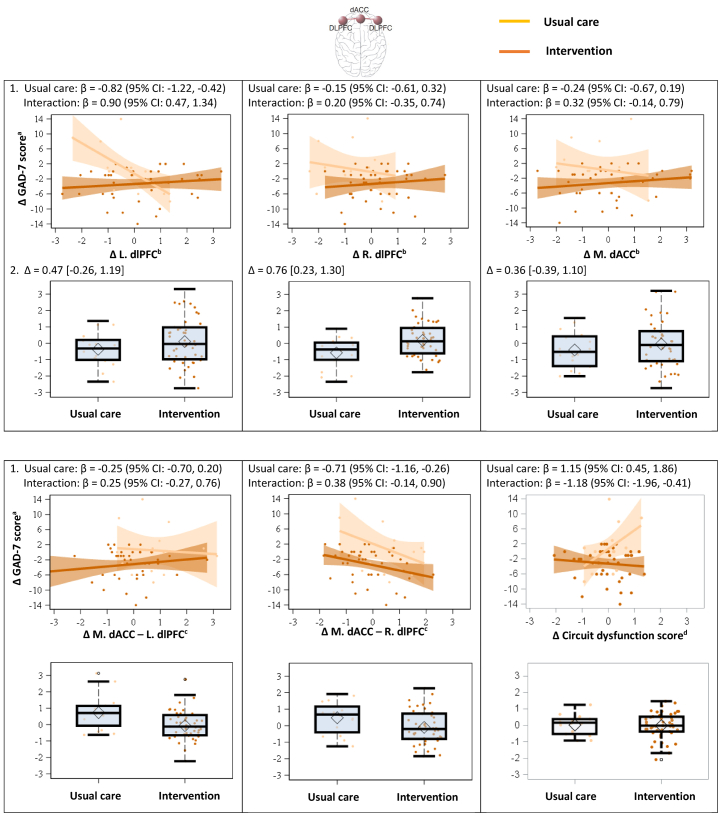
Changes in multiple neural targets in the negative affect and cognitive control circuits at 2 months were associated with changes in GAD-7 scores at 6 months. This was found both in the usual care group and by interaction with the intervention versus usual care (Table 2 and Figure 3), suggesting a treatment-dependent temporal relationship. In the negative affect circuit, increased activation of the left anterior insula for sad stimuli at 2 months was associated with increased GAD-7 scores at 6 months in the usual care group (0.41 [95% CI = 0.10 to 0.73], p = .01, padj = .11), but this relationship was reversed in the intervention group (interaction, −0.43 [95% CI = −0.82 to −0.05], p = .03, padj = .14). In the negative affect circuit, decreased connectivity of multiple ROIs at 2 months were associated with increased GAD-7 scores at 6 months in the usual care group, but the relationship was tempered or reversed in the intervention group. These include connectivity between the subgenual ACC and right amygdala engaged by nonconscious threat stimuli (usual care, −0.72 [95% CI = −1.16 to −0.29], p = .002, padj = .03; interaction, 0.72 [95% CI = 0.25 to 1.19], p = .003, padj = .03), between the pregenual ACC and right amygdala engaged by sad stimuli (usual care, −0.45 [95% CI = −0.78 to −0.12], p = .01, padj = .07; interaction, 0.52 [95% CI = 0.11 to 0.92], p = .02, padj = .09), and between the dorsal ACC (dACC) and right amygdala engaged by conscious threat stimuli (usual care, −0.89 [95% CI = −1.58 to −0.20], p = .01, padj = .09; interaction, 0.88 [95% CI = 0.13 to 1.63], p = .02, padj = .12). In the cognitive control circuit, decreased activation of the left dorsal lateral prefrontal cortex (dlPFC) at 2 months was associated with increased GAD-7 scores at 6 months in the usual care group (−0.82 [95% CI = −1.22 to −0.42], p < .001, padj = .01), which was reversed in the intervention group (interaction, 0.90 [95% CI = 0.47 to 1.34], p < .001, padj = .01). Decreased connectivity between the dACC and right dlPFC was associated with increased GAD-7 scores in both groups (usual care, −0.71 [95% CI = −1.16 to −0.26], p = .003, padj = .03; interaction, 0.38 [95% CI = −0.14 to 0.90], p = .15, padj = .35). In the cognitive control circuit, increased global circuit dysfunction scores at 2 months were associated with increased GAD-7 scores at 6 months in the usual care group (1.15 [95% CI = 0.45 to 1.86], p = .002, padj = .03), which was reversed in the intervention group (interaction, −1.18 [95% CI = −1.96 to −0.41], p = .004, padj = .03). While the results suggest medium to large effects, some of the padj values were >.05.
Table 2.
Association of Changes in Neural Targets at 2 Months and Changes in Clinical Outcomes at 6 Months
| Neural Target | Hemi.a | SCL-20, Primary Clinical Outcome |
BMI, Primary Clinical Outcome |
GAD-7, Secondary Clinical Outcome |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Usual Careb |
Interactionb |
Usual Careb |
Interactionb |
Usual Careb |
Interactionb |
||||||||||||||
| Mean (95% CI) | p | padjc | Mean (95% CI) | p | padjc | Mean (95% CI) | p | padjc | Mean (95% CI) | p | padjc | Mean (95% CI) | p | padjc | Mean (95% CI) | p | padjc | ||
| Primary Neural Targets | |||||||||||||||||||
| Negative Affect Circuit—Engaged by Threat (Nonconscious) | |||||||||||||||||||
| Amygdala | L | 0.18 (−0.15 to 0.50) | .29 | .90 | −0.16 (−0.55 to 0.22) | .40 | .90 | 0.01 (−0.07 to 0.09) | .79 | .95 | −0.00 (−0.09 to 0.09) | .99 | .99 | 0.39 (−0.04 to 0.82) | .07 | .36 | −0.37 (−0.85 to 0.10) | .12 | .36 |
| R | 0.05 (−0.28 to 0.38) | .78 | .90 | 0.10 (−0.31 to 0.51) | .62 | .90 | 0.03 (−0.05 to 0.11) | .49 | .86 | −0.03 (−0.12 to 0.07) | .57 | .86 | −0.02 (−0.47 to 0.44) | .94 | .94 | 0.20 (−0.33 to 0.74) | .45 | .54 | |
| sgACC | M | 0.01 (−0.17 to 0.19) | .90 | .90 | −0.02 (−0.23 to 0.20) | .89 | .90 | −0.01 (−0.06 to 0.03) | .57 | .86 | 0.02 (−0.03 to 0.07) | .36 | .86 | 0.09 (−0.09 to 0.27) | .32 | .54 | −0.09 (−0.30 to 0.12) | .37 | .54 |
| Negative Affect Circuit—Engaged by Sad (Conscious) | |||||||||||||||||||
| Amygdala | L | 0.25 (−0.13 to 0.62) | .19 | .48 | −0.44 (−0.86 to −0.02) | .04 | .39 | −0.01 (−0.10 to 0.08) | .83 | .83 | −0.02 (−0.12 to 0.08) | .69 | .78 | 0.23 (−0.19 to 0.65) | .27 | .39 | −0.28 (−0.75 to 0.18) | .23 | .38 |
| R | 0.22 (−0.25 to 0.69) | .35 | .50 | −0.39 (−0.93 to 0.14) | .15 | .48 | −0.04 (−0.15 to 0.07) | .45 | .68 | 0.02 (−0.10 to 0.14) | .70 | .78 | 0.06 (−0.46 to 0.58) | .83 | .85 | −0.05 (−0.65 to 0.54) | .85 | .85 | |
| Anterior insula | L | 0.25 (−0.05 to 0.56) | .10 | .48 | −0.18 (−0.56 to 0.19) | .33 | .50 | −0.03 (−0.09 to 0.04) | .42 | .68 | 0.06 (−0.02 to 0.15) | .16 | .66 | 0.41 (0.10 to 0.73)d | .01 | .11 | −0.43 (−0.82 to −0.05)d | .03 | .14 |
| R | 0.19 (−0.17 to 0.55) | .30 | .50 | −0.08 (−0.51 to 0.35) | .71 | .81 | −0.03 (−0.11 to 0.05) | .48 | .68 | 0.05 (−0.04 to 0.15) | .27 | .66 | 0.28 (−0.10 to 0.66) | .15 | .37 | −0.15 (−0.60 to 0.30) | .51 | .63 | |
| pgACC | M | 0.03 (−0.27 to 0.33) | .85 | .85 | −0.06 (−0.42 to 0.29) | .73 | .81 | −0.04 (−0.10 to 0.02) | .20 | .66 | 0.08 (0.01 to 0.16) | .03 | .27 | 0.21 (−0.11 to 0.53) | .19 | .37 | −0.31 (−0.68 to 0.06) | .10 | .34 |
| Secondary Neural Targets | |||||||||||||||||||
| Negative Affect Circuit—Engaged by Threat (Nonconscious) | |||||||||||||||||||
| sgACC to amygdala | M-L | −0.05 (−0.53 to 0.43) | .83 | >.99 | −0.10 (−0.62 to 0.42) | .71 | .95 | −0.03 (−0.15 to 0.08) | .57 | .98 | 0.00 (−0.12 to 0.13) | .96 | .98 | −0.41 (−0.88 to 0.07) | .09 | .31 | 0.39 (−0.13 to 0.90) | .14 | .35 |
| M-R | −0.33 (−0.8 to 0.13) | .15 | .80 | 0.25 (−0.26 to 0.75) | .33 | .80 | −0.00 (−0.11 to 0.11) | .98 | .98 | −0.01 (−0.13 to 0.10) | .81 | .98 | −0.72 (−1.16 to −0.29)d | .002 | .03 | 0.72 (0.25 to 1.19)d | .003 | .03 | |
| Circuite | – | 0.24 (−0.34 to 0.82) | .40 | .80 | −0.03 (−0.7 to 0.63) | .92 | >.99 | 0.06 (−0.08 to 0.20) | .40 | .98 | −0.05 (−0.21 to 0.11) | .55 | .98 | 0.45 (−0.29 to 1.18) | .23 | .41 | −0.36 (−1.16 to 0.44) | .37 | .52 |
| Negative Affect Circuit—Engaged by Sad (Conscious) | |||||||||||||||||||
| pgACC to anterior insula | M-L | 0.31 (−0.10 to 0.72) | .13 | .80 | −0.18 (−0.67 to 0.31) | .46 | .80 | 0.05 (−0.04 to 0.15) | .25 | .98 | −0.05 (−0.16 to 0.06) | .39 | .98 | 0.41 (−0.02 to 0.83) | .06 | .28 | −0.36 (−0.87 to 0.15) | .16 | .36 |
| M-R | 0.19 (−0.25 to 0.63) | .39 | .80 | 0.03 (−0.46 to 0.51) | .91 | >.99 | −0.03 (−0.13 to 0.08) | .62 | .98 | 0.03 (−0.08 to 0.15) | .55 | .98 | 0.34 (−0.11 to 0.80) | .14 | .35 | −0.28 (−0.79 to 0.23) | .27 | .43 | |
| pgACC to amygdala | M-L | 0.06 (−0.35 to 0.46) | .78 | >.99 | 0.04 (−0.45 to 0.52) | .88 | >.99 | −0.01 (−0.10 to 0.09) | .92 | .98 | 0.00 (−0.11 to 0.11) | .98 | .98 | −0.40 (−0.81 to 0.02) | .06 | .28 | 0.46 (−0.03 to 0.95) | .07 | .28 |
| M-R | −0.23 (−0.57 to 0.11) | .18 | .80 | 0.24 (−0.18 to 0.66) | .26 | .80 | −0.02 (−0.09 to 0.06) | .65 | .98 | −0.01 (−0.10 to 0.09) | .90 | .98 | −0.45 (−0.78 to −0.12)d | .01 | .07 | 0.52 (0.11 to 0.92)d | .02 | .09 | |
| Circuite | – | 0.20 (−0.61 to 1.01) | .63 | .89 | −0.55 (−1.52 to 0.43) | .27 | .80 | −0.11 (−0.30 to 0.06) | .19 | .98 | 0.14 (−0.08 to 0.36) | .20 | .98 | −0.03 (−0.97 to 0.90) | .94 | .97 | −0.03 (−1.13 to 1.06) | .95 | .97 |
| Cognitive Control Circuit | |||||||||||||||||||
| dlPFC | L | −0.16 (−0.60 to 0.29) | .48 | .80 | 0.23 (−0.26 to 0.72) | .35 | .80 | 0.00 (−0.10 to 0.11) | .97 | .98 | 0.02 (−0.10 to 0.13) | .77 | .98 | −0.82 (−1.22 to −0.42)d | <.001 | .01 | 0.90 (0.47 to 1.34)d | <.001 | .01 |
| R | −0.11 (−0.59 to 0.37) | .65 | .89 | 0.22 (−0.34 to 0.78) | .44 | .80 | −0.05 (−0.16 to 0.06) | .35 | .98 | 0.08 (−0.05 to 0.22) | .21 | .98 | −0.15 (−0.61 to 0.32) | .53 | .64 | 0.20 (−0.35 to 0.74) | .47 | .62 | |
| dACC | M | −0.12 (−0.54 to 0.29) | .55 | .83 | 0.26 (−0.19 to 0.72) | .25 | .80 | −0.01 (−0.11 to 0.08) | .76 | .98 | 0.06 (−0.05 to 0.16) | .28 | .98 | −0.24 (−0.67 to 0.19) | .27 | .43 | 0.32 (−0.14 to 0.79) | .17 | .37 |
| dACC to dlPFC | M-L | −0.28 (−0.73 to 0.17) | .21 | .80 | 0.29 (−0.24 to 0.81) | .27 | .80 | −0.02 (−0.12 to 0.09) | .71 | .98 | 0.02 (−0.10 to 0.14) | .70 | .98 | −0.25 (−0.70 to 0.20) | .27 | .43 | 0.25 (−0.27 to 0.76) | .35 | .50 |
| M-R | −0.29 (−0.74 to 0.17) | .21 | .80 | 0.02 (−0.52 to 0.56) | .95 | >.99 | 0.02 (−0.09 to 0.12) | .77 | .98 | 0.02 (−0.10 to 0.15) | .71 | .98 | −0.71 (−1.16 to −0.26)d | .003 | .03 | 0.38 (−0.14 to 0.90) | .15 | .35 | |
| Circuite | – | 0.58 (−0.20 to 1.35) | .14 | .80 | −0.68 (−1.53 to 0.17) | .11 | .80 | 0.04 (−0.14 to 0.22) | .64 | .98 | −0.11 (−0.31 to 0.09) | .28 | .98 | 1.15 (0.45 to 1.86)d | .002 | .03 | −1.18 (−1.96 to −0.41)d | .004 | .03 |
| Default Mode Circuit | |||||||||||||||||||
| amPFC to AG | M-L | 0.15 (−0.24 to 0.54) | .44 | .80 | −0.00 (−0.52 to 0.52) | >.99 | >.99 | −0.02 (−0.11 to 0.07) | .66 | .98 | −0.02 (−0.14 to 0.10) | .72 | .98 | 0.13 (−0.32 to 0.59) | .56 | .65 | 0.06 (−0.51 to 0.63) | .84 | .90 |
| M-R | −0.20 (−0.8 to 0.40) | .51 | .80 | 0.43 (−0.28 to 1.14) | .23 | .80 | 0.01 (−0.14 to 0.15) | .95 | .98 | −0.05 (−0.22 to 0.11) | .51 | .98 | −0.56 (−1.23 to 0.12) | .10 | .33 | 0.71 (−0.07 to 1.48) | .07 | .28 | |
| PCC to amPFC | M-M | −0.01 (−0.45 to 0.43) | .96 | >.99 | 0.07 (−0.49 to 0.63) | .80 | >.99 | −0.01 (−0.11 to 0.09) | .88 | .98 | 0.06 (−0.06 to 0.19) | .30 | .98 | 0.01 (−0.49 to 0.50) | .97 | .97 | 0.06 (−0.54 to 0.66) | .84 | .90 |
| PCC to AG | M-L | 0.59 (−0.06 to 1.24) | .07 | .80 | −0.68 (−1.41 to 0.05) | .07 | .80 | 0.04 (−0.11 to 0.19) | .59 | .98 | −0.12 (−0.29 to 0.06) | .18 | .98 | 0.50 (−0.21 to 1.21) | .16 | .36 | −0.58 (−1.38 to 0.21) | .15 | .35 |
| M-R | 0.05 (−0.66 to 0.77) | .88 | >.99 | 0.03 (−0.76 to 0.81) | .95 | >.99 | 0.07 (−0.08 to 0.23) | .34 | .98 | −0.17 (−0.34 to 0.00) | .05 | .98 | −0.20 (−0.93 to 0.53) | .58 | .67 | 0.28 (−0.53 to 1.09) | .49 | .62 | |
| Circuite | – | 0.17 (−0.58 to 0.91) | .65 | .89 | −0.01 (−0.90 to 0.88) | .98 | >.99 | 0.01 (−0.16 to 0.19) | .89 | .98 | −0.09 (−0.29 to 0.12) | .39 | .98 | −0.05 (−0.85 to 0.76) | .91 | .95 | 0.20 (−0.75 to 1.16) | .67 | .76 |
| Negative Affect Circuit—Engaged by Threat (Conscious) | |||||||||||||||||||
| Amygdala | L | 0.31 (0.00 to 0.62) | .05 | .80 | −0.33 (−0.71 to 0.06) | .10 | .80 | −0.01 (−0.08 to 0.06) | .85 | .98 | −0.03 (−0.12 to 0.05) | .45 | .98 | 0.41 (−0.04 to 0.86) | .07 | .28 | −0.38 (−0.89 to 0.14) | .15 | .35 |
| R | 0.27 (−0.07 to 0.60) | .11 | .80 | −0.41 (−0.88 to 0.06) | .09 | .80 | −0.03 (−0.11 to 0.04) | .36 | .98 | −0.02 (−0.12 to 0.09) | .74 | .98 | 0.19 (−0.20 to 0.57) | .33 | .48 | −0.08 (−0.61 to 0.44) | .75 | .83 | |
| dACC | M | 0.21 (−0.13 to 0.55) | .22 | .80 | −0.36 (−0.75 to 0.04) | .08 | .80 | −0.01 (−0.09 to 0.06) | .74 | .98 | 0.02 (−0.07 to 0.11) | .67 | .98 | 0.20 (−0.17 to 0.57) | .28 | .43 | −0.28 (−0.71 to 0.14) | .19 | .38 |
| dACC to amygdala | M-L | 0.18 (−0.27 to 0.63) | .44 | .80 | −0.08 (−0.64 to 0.48) | .78 | >.99 | 0.05 (−0.05 to 0.15) | .30 | .98 | 0.00 (−0.12 to 0.12) | .95 | .98 | −0.26 (−0.80 to 0.26) | .31 | .47 | 0.39 (−0.24 to 1.01) | .22 | .41 |
| M-R | 0.01 (−0.62 to 0.64) | .97 | >.99 | 0.02 (−0.68 to 0.72) | .96 | >.99 | 0.06 (−0.08 to 0.2) | .37 | .98 | −0.05 (−0.20 to 0.10) | .52 | .98 | −0.89 (−1.58 to −0.20)d | .01 | .09 | 0.88 (0.13 to 1.63)d | .02 | .12 | |
| Circuite | – | 0.40 (−0.47 to 1.26) | .36 | .80 | −0.37 (−1.40 to 0.66) | .47 | .80 | −0.10 (−0.28 to 0.09) | .29 | .98 | −0.02 (−0.24 to 0.20) | .85 | .98 | 0.81 (−0.14 to 1.76) | .09 | .31 | −0.67 (−1.78 to 0.44) | .23 | .41 |
| Positive Affect Circuit—Engaged by Happy (Conscious) | |||||||||||||||||||
| vMPFC | M | 0.14 (−0.34 to 0.62) | .56 | .83 | −0.14 (−0.65 to 0.38) | .60 | .88 | −0.06 (−0.17 to 0.04) | .25 | .98 | 0.07 (−0.04 to 0.18) | .22 | .98 | 0.21 (−0.30 to 0.72) | .42 | .56 | −0.18 (−0.72 to 0.37) | .52 | .64 |
| vStriatum | L | 0.14 (−0.24 to 0.51) | .48 | .80 | −0.13 (−0.54 to 0.27) | .51 | .80 | −0.04 (−0.12 to 0.05) | .38 | .98 | 0.03 (−0.06 to 0.12) | .44 | .98 | 0.17 (−0.22 to 0.56) | .39 | .53 | −0.13 (−0.56 to 0.29) | .53 | .64 |
| R | 0.16 (−0.20 to 0.52) | .37 | .80 | −0.14 (−0.53 to 0.25) | .47 | .80 | −0.02 (−0.10 to 0.06) | .63 | .98 | 0.03 (−0.06 to 0.11) | .53 | .98 | 0.29 (−0.09 to 0.66) | .13 | .35 | −0.25 (−0.65 to 0.16) | .23 | .41 | |
| Circuite | – | −0.21 (−0.67 to 0.26) | .38 | .80 | 0.20 (−0.30 to 0.69) | .43 | .80 | 0.05 (−0.05 to 0.15) | .35 | .98 | −0.06 (−0.16 to 0.06) | .32 | .98 | −0.31 (−0.80 to 0.18) | .20 | .41 | 0.27 (−0.25 to 0.79) | .30 | .46 |
p = p value at an uncorrected threshold of 0.05 before adjustment for FDR; padj = p value adjusted for FDR.
AG, angular gyrus; amPFC, anterior medial prefrontal cortex; BMI, body mass index; dACC, dorsal anterior cingulate cortex; dlPFC, dorsal lateral prefrontal cortex; FDR, false discovery rate; GAD-7, Generalized Anxiety Disorder Scale-7; Hemi., hemisphere; L, left; M, medial; PCC, posterior cingulate cortex; pgACC, pregenual anterior cingulate cortex; R, right; SCL-20, Depression Symptom Checklist-20; sgACC, subgenual anterior cingulate cortex; vMPFC, ventral medial prefrontal cortex; vStriatum, ventral striatum.
Single letter indicates task activation; paired letters indicate task-related connectivity.
Ordinary least square regression model including baseline of outcome, indicator of the outcome data collected before or after COVID-19 shut down at study site (March 16, 2020), biotype, treatment, and interaction of biotype and treatment. Regression coefficients in the usual care group show the correlations of changes in neural targets at 2 months with changes in clinical outcomes at 6 months within the usual care group. Regression coefficients for the interaction show the differences in the correlations between the intervention and usual care groups.
FDR adjustment was conducted for each clinical outcome within each family of neural targets (see Table S3 for definitions of the families of neural targets).
95% CIs do not include null.
Global circuit dysfunction score, composite of primary and secondary neural targets.
Figure 3.
Association of changes in neural targets in the cognitive control circuit at 2 months and changes in Generalized Anxiety Disorder Scale-7 (GAD-7) score at 6 months by treatment group (scatter plots) and differences in changes in neural targets at 2 months between the usual care and intervention groups (box plots). 1) Scatter plots with regression lines show the associations of changes in neural targets in the cognitive control circuit at 2 months with changes in GAD-7 scores at 6 months, and standardized β coefficients with 95% confidence intervals (CIs) are provided for the usual care group and the interaction (i.e., the difference between the intervention and usual care groups). Regression coefficients in the usual care group show the correlations of changes in neural targets at 2 months with changes in clinical outcomes at 6 months within the usual care group. Regression coefficients for the interaction show the differences in the correlations between the intervention and usual care groups. 2) Box plots show changes in neural targets in the cognitive control circuit at 2 months in the usual care and intervention groups, and between-group mean differences (Δ) with 95% CIs are provided. All changes are relative to baseline. For the box plots, the central thick black bar represents the median, the diamond represents the mean, the boxes represent the lower and upper quartiles (Q1 and Q3), and the whiskers represent the minimum and maximum, excluding outliers. aAnxiety symptoms were assessed using GAD-7, scores ranging from 0 (best) to 21 (worst). bBlood oxygen level–dependent activation vs. neutral cue, z-scores. cPsychophysiological interaction connectivity, z-scores. dOverall score from blood oxygen level–dependent derived activation and connectivity within the cognitive control circuit. dACC, dorsal anterior cingulate cortex; dlPFC, dorsal lateral prefrontal cortex; L, left; M, medial; R, right.
Several of the neural targets, including left anterior insula activation engaged by sad stimuli, connectivity between the subgenual ACC and right amygdala engaged by nonconscious threat stimuli, and left dlPFC activation in the cognitive control circuit, were also associated with changes in GAD-7 scores at 2 months (Table S4).
Intervention Effect on Neural Targets
The intervention and usual care groups did not differ significantly in changes in any of the primary ROIs engaged by nonconscious threat stimuli or conscious sad stimuli from baseline to 2 months (Table 3 and Figure 3).
Table 3.
Intervention Effect on Changes in Neural Targets at 2 Months
| Neural Target | Hemi.a | Unadjusted Mean (SD) |
Between-Group Difference |
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline |
Δ 2 Months |
Mean (95% CI) | p | padjc | ||||
| Interventionb | Control | Interventionb | Control | |||||
| Primary Neural Targets | ||||||||
| Negative Affect Circuit—Engaged by Threat (Nonconscious) (Baseline: n = 67, 30; 2 Months: n = 45, 18)d | ||||||||
| Amygdala | L | 0.24 (1.08) | −0.08 (0.70) | −0.20 (1.36) | −0.06 (1.34) | −0.14 (−0.89 to 0.62) | .72 | .96 |
| R | 0.34 (0.99) | 0.18 (0.69) | −0.47 (1.17) | −0.45 (1.32) | −0.02 (−0.7 to 0.65) | .95 | .96 | |
| sgACC | M | 0.44 (1.90) | 0.40 (1.50) | −0.01 (2.67) | −0.05 (2.44) | 0.04 (−1.42 to 1.5) | .96 | .96 |
| Negative Affect Circuit—Engaged by Sad (Conscious) (Baseline: n = 65, 27; 2 Months: n = 41, 18)d | ||||||||
| Amygdala | L | −0.08 (1.12) | −0.31 (0.81) | 0.21 (1.45) | 0.76 (1.13) | −0.55 (−1.32 to 0.22) | .16 | .65 |
| R | 0.08 (0.87) | −0.16 (0.63) | 0.15 (1.10) | 0.32 (0.93) | −0.17 (−0.77 to 0.43) | .57 | .72 | |
| Anterior insula | L | 0.17 (0.83) | −0.09 (0.91) | −0.01 (1.27) | 0.4 (1.42) | −0.41 (−1.15 to 0.34) | .28 | .65 |
| R | 0.11 (0.77) | 0.17 (0.73) | −0.00 (1.21) | 0.11 (1.22) | −0.12 (−0.8 to 0.57) | .74 | .74 | |
| pgACC | M | −0.40 (1.40) | −0.43 (0.86) | −0.35 (1.57) | 0.03 (1.49) | −0.38 (−1.25 to 0.49) | .39 | .65 |
| Secondary Neural Targets | ||||||||
| Negative Affect Circuit—Engaged by Threat (Nonconscious) (Baseline: n = 67, 30; 2 Months: n = 45, 18)d | ||||||||
| sgACC to amygdala | M-L | −0.23 (1.01) | −0.16 (0.78) | 0.29 (1.53) | 0.02 (0.90) | 0.27 (−0.36 to 0.90) | .39 | .61 |
| M-R | −0.00 (1.00) | −0.21 (0.78) | 0.17 (1.66) | 0.10 (0.96) | 0.07 (−0.61 to 0.74) | .84 | .91 | |
| Circuite | – | 0.07 (0.61) | 0.02 (0.43) | −0.23 (0.88) | −0.12 (0.75) | −0.11 (−0.58 to 0.36) | .65 | .82 |
| Negative Affect Circuit—Engaged by Sad (Conscious) (Baseline: n = 65, 27; 2 Months: n = 41, 18)d | ||||||||
| pgACC to anterior insula | M-L | 0.06 (1.01) | −0.12 (0.86) | 0.00 (1.06) | −0.14 (1.05) | 0.14 (−0.46 to 0.74) | .64 | .82 |
| M-R | −0.12 (0.97) | −0.50 (0.75) | 0.04 (1.30) | 0.23 (0.97) | −0.19 (−0.88 to 0.50) | .58 | .79 | |
| pgACC to amygdala | M-L | 0.11 (0.84) | −0.14 (0.87) | 0.05 (1.11) | 0.31 (1.10) | −0.26 (−0.89 to 0.37) | .41 | .61 |
| M-R | 0.22 (0.86) | −0.09 (0.73) | −0.09 (1.18) | 0.34 (1.29) | −0.43 (−1.12 to 0.26) | .21 | .49 | |
| Circuite | – | 0.03 (0.44) | −0.05 (0.39) | −0.00 (0.52) | 0.24 (0.54) | −0.24 (−0.54 to 0.06) | .11 | .41 |
| Cognitive Control Circuit (Baseline: n = 67, 27; 2 Months: n = 47, 18)d | ||||||||
| dlPFC | L | 0.20 (1.09) | 0.50 (0.89) | 0.10 (1.40) | −0.37 (1.02) | 0.47 (−0.26 to 1.19) | .20 | .49 |
| R | −0.24 (0.74) | −0.06 (0.90) | 0.19 (0.97) | −0.57 (0.93) | 0.76 (0.23 to 1.30)f | .01 | .11 | |
| dACC | M | 0.46 (1.17) | 0.49 (1.28) | −0.05 (1.43) | −0.40 (1.07) | 0.36 (−0.39 to 1.10) | .34 | .60 |
| dACC to dlPFC | M-L | 0.29 (0.88) | −0.22 (0.86) | −0.06 (1.03) | 0.73 (1.00) | −0.79 (−1.36 to −0.23)f | .01 | .11 |
| M-R | 0.18 (0.81) | −0.08 (0.99) | −0.10 (1.00) | 0.48 (0.95) | −0.58 (−1.13 to −0.04)f | .04 | .28 | |
| Circuite | – | −0.18 (0.57) | −0.12 (0.51) | −0.02 (0.77) | 0.03 (0.58) | −0.04 (−0.44 to 0.36) | .83 | .91 |
| Default Mode Circuit (Baseline: n = 63, 22; 2 Months: n = 39, 16)d | ||||||||
| amPFC to AG | M-L | −0.54 (0.94) | −0.07 (1.19) | 0.31 (0.93) | 0.00 (1.21) | 0.31 (−0.30, 0.91) | .31 | .58 |
| M-R | −0.62 (0.92) | −0.20 (0.99) | 0.16 (0.82) | −0.23 (0.78) | 0.39 (−0.09 to 0.87) | .11 | .41 | |
| PCC to amPFC | M-M | −1.14 (0.96) | −0.62 (1.27) | 0.25 (0.96) | −0.26 (1.08) | 0.51 (−0.08 to 1.11) | .09 | .41 |
| PCC to AG | M-L | −0.33 (0.93) | −0.00 (1.00) | 0.12 (0.89) | −0.26 (0.71) | 0.38 (−0.12 to 0.88) | .14 | .41 |
| M-R | −0.23 (1.22) | −0.07 (1.35) | −0.04 (0.98) | −0.46 (0.67) | 0.42 (−0.12 to 0.96) | .12 | .41 | |
| Circuite | – | −0.58 (0.70) | −0.19 (0.94) | 0.16 (0.66) | −0.25 (0.64) | 0.42 (0.03 to 0.80)f | .04 | .28 |
| Negative Affect Circuit—Engaged by Threat (Conscious) (Baseline: n = 65, 27; 2 Months: n = 41, 18)d | ||||||||
| Amygdala | L | −0.11 (1.06) | 0.04 (0.77) | 0.13 (1.29) | 0.26 (1.38) | −0.13 (−0.88 to 0.62) | .73 | .88 |
| R | 0.13 (0.91) | 0.10 (0.69) | 0.01 (0.91) | 0.28 (1.29) | −0.27 (−0.85 to 0.32) | .37 | .61 | |
| dACC | M | 0.27 (1.14) | 0.32 (0.81) | −0.16 (1.46) | −0.16 (1.27) | −0.00 (−0.80 to 0.80) | >.99 | >.99 |
| dACC to amygdala | M-L | −0.35 (0.72) | −0.20 (0.64) | 0.30 (0.91) | −0.16 (0.98) | 0.46 (−0.07 to 0.99) | .09 | .41 |
| M-R | −0.31 (0.68) | −0.18 (0.64) | 0.22 (0.99) | −0.06 (0.72) | 0.28 (−0.24 to 0.79) | .29 | .58 | |
| Circuite | – | 0.08 (0.44) | 0.04 (0.40) | −0.04 (0.58) | 0.18 (0.51) | −0.22 (−0.54 to 0.10) | .17 | .46 |
| Positive Affect Circuit—Engaged by Happy (Conscious) (Baseline: n = 65, 27; 2 Months: n = 41, 18)d | ||||||||
| vMPFC | M | 0.03 (1.06) | 0.19 (0.82) | 0.08 (1.55) | −0.27 (0.93) | 0.34 (−0.32, 1.00) | .30 | .58 |
| vStriatum | L | −0.22 (1.33) | −0.01 (0.95) | 0.20 (1.92) | 0.12 (1.17) | 0.08 (−0.74 to 0.90) | .85 | .91 |
| R | −0.09 (1.31) | −0.02 (0.95) | 0.04 (1.97) | 0.37 (1.23) | −0.33 (−1.18 to 0.52) | .44 | .63 | |
| Circuite | – | 0.09 (1.03) | −0.05 (0.80) | −0.09 (1.66) | −0.07 (0.95) | −0.02 (−0.71 to 0.67) | .96 | .99 |
p = p value at an uncorrected threshold of 0.05 before adjustment for FDR; padj = p value adjusted for FDR.
AG, angular gyrus; amPFC, anterior medial prefrontal cortex; dACC, dorsal anterior cingulate cortex; dlPFC, dorsal lateral prefrontal cortex; FDR, false discovery rate; Hemi., hemisphere; L, left; M, medial; PCC, posterior cingulate cortex; pgACC, pregenual anterior cingulate cortex; R, right; sgACC, subgenual anterior cingulate cortex; vMPFC, ventral medial prefrontal cortex; vStriatum, ventral striatum.
Single letter indicates task activation; paired letters indicate task-related connectivity.
Represents the initial 2-month intervention phase of the I-CARE2 program that implemented a 7-step problem-solving process as its core component.
FDR adjustment was conducted within each family of neural targets (see Table S3 for definitions of the families of neural targets).
Baseline and 2-month n values are for intervention and control groups separately.
Global circuit dysfunction score, composite of primary and secondary neural targets.
95% CIs do not include null.
Among changes in secondary neural targets from baseline to 2 months, the intervention led to increased right dlPFC activation (0.76 [95% CI = 0.23 to 1.30], p = .01, padj = .11), decreased connectivity between the dACC and left dlPFC (−0.79 [95% CI = −1.36 to −0.23], p = .01, padj = .11) and between the dACC and right dlPFC (−0.58 [95% CI = −1.13 to −0.04], p = .04, padj = .28) in the cognitive control circuit, and an increased global circuit dysfunction score of the default mode (0.42 [95% CI = 0.03 to 0.80], p = .04, padj = .28) (Table 3 and Figure 3). While these results suggest medium effects, the padj values are all >.05.
Intervention Adherence
Of the 71 intervention participants, 65 (91.5%) completed at least 2 sessions, 56 (78.9%) completed at least 5 sessions, and 36 (50.7%) completed all 9 sessions. Excluding those who completed no sessions (n = 6), the mean (SD) number of sessions completed was 6.9 (2.6), with a median (interquartile range) of 9 (4).
Adverse Events
Seven serious adverse events (6 in the intervention group vs. 1 in the control group) occurred in 7 different participants over 6 months, all requiring hospitalizations, but none were unanticipated or related to the study. A total of 50 nonserious adverse events (37 in the intervention group vs. 13 in the control group) occurred, mostly involving minor musculoskeletal injuries, non–COVID-19 pulmonary/respiratory symptoms, and gastrointestinal symptoms; all were unrelated to the study. There were no deaths.
Discussion
This mechanistic pilot RCT found that 1) the integrated collaborative care intervention led to significantly improved depressive and anxiety symptoms, but not weight loss, at 6 months compared with usual care; 2) changes in the activation or functional connectivity of neural targets in both the negative affect circuit and the cognitive control circuit at 2 months correlated with changes in anxiety symptoms, but not depressive symptoms or weight loss, at 6 months in a treatment-dependent manner; and 3) changes in the neural targets of the cognitive control circuit, but not the negative affect circuit, and the global circuit dysfunction score of the default mode at 2 months differed by treatment group.
The study intervention had robust effects on depressive and anxiety symptoms. In this study, the between-group mean difference at 6 months for SCL-20 was equivalent to the effect observed in the RAINBOW (Research Aimed at Improving Both Mood and Weight) efficacy trial (8) (−0.3 [95% CI = −0.4 to −0.1]) and comparable to the effects (0.3–0.4) in prior trials that targeted depression using similar treatment approaches (18,19,33). SCL-20 is a valid and reliable measure of depression severity (21,22) where a mean (SD) difference of 0.165 (0.5) is considered clinically meaningful (33,34). For GAD-7, the between-group mean difference at 6 months in this study was 2.4 times the effect in RAINBOW (−1.2 [95% CI = −2.1 to −0.3]). The intervention effects on depression and anxiety were consistently favorable across the prespecified sociodemographic subgroups. These effects persisted, and even improved slightly, in GAD-7 after the COVID-19 lockdown in March 2020. For BMI, the between-group mean difference at 6 months was only half of that observed in RAINBOW (−0.6 [95% CI = −0.9 to −0.3]). This effect decreased after the lockdown, reflecting the rampant disruptions to people’s lifestyle routines [e.g., detriments in physical activity, diet, and sleep (35,36)] and deleterious secondary health effects [e.g., worsened mental health (5,35) and prevalent weight gain (3,37,38)] disproportionally in under-resourced populations (36) during the pandemic. The effects of the pandemic may have contributed to a diluted intervention effect for weight loss.
The primary focus of this mechanistic trial was on establishing the relationship between neural target engagement with the outcomes of interest to uncover a potential mechanism of action. The findings indicate a treatment-dependent temporal relationship specifically for GAD-7 scores at 6 months and neural target changes at 2 months (following early PST) in both the negative affect and cognitive control circuits. These treatment-dependent effects are consistent with previous studies demonstrating neural correlates of psychotherapy treatment response (39,40). The absolute values of the standardized regression coefficients for these GAD-7 and neural target associations ranged from 0.41 to 1.18, suggesting medium to large effects. Moreover, compared with usual care, the intervention resulted in medium effects ranging from 0.58 to 0.79 in absolute values for increased activation of the right dlPFC, but decreased connectivity between the dACC and both the left and right dlPFC in the cognitive control circuit at 2 months. These effect sizes are in the range of the estimates used in the study design, although some of the results did not survive FDR adjustment.
Taken together, these results imply the potential for causal mediation involving the cognitive control circuit for an anxiolytic effect of the intervention. Participants showing target engagement (a meaningful change in activation of the dlPFC and its connectivity with the dACC from baseline) early in the treatment may be more likely to experience improvement in anxiety symptoms at the end of treatment. Increased cognitive control activation has been found to be associated with anxiety in people with major depressive disorder (41). The cognitive control circuit is also heavily involved in emotion regulation (42). The amygdala and insula, and their interaction with medial cortical regions such as the ACC, are key regions of the negative affect circuit (27,28). Dysfunction in these regions is a hallmark of depression and anxiety, thought to reflect a heightened reactivity to negative emotion that accompanies the negative mood features of these disorders (27,28). This study also suggests that the interaction between the cognitive control and negative affect circuits (such as connectivity between subregions of the ACC and amygdala and anterior insula activation) may mediate the changes in anxiety symptoms, even though the intervention did not result in differential target engagement in the negative affect circuit compared with usual care.
In this study, no relationships were found for SCL-20 or BMI changes at 6 months and any of the neural target changes at 2 months. Results in the proof-of-mechanism ENGAGE study showed that amygdala activation engaged by nonconscious threat stimuli decreased by magnitude of medium to large size (standardized effect estimates of −0.63 to −0.75) from baseline to 2 months in the intervention vs. usual care, which mediated the intervention effects on SCL-20 at 6 months (12). Multiple reasons may explain the discrepant results between the studies. First, compared with the ENGAGE study, this study sample was younger, more racially and ethnically diverse, of lower socioeconomic status, more obese, and less depressed (Table S5). Second, this study sample had significantly higher baseline activation of the left dlPFC and dACC in the cognitive control circuit and a lower global dysfunction score of this circuit. Finally, this study sample had a markedly better response to the intervention for reduced anxiety symptoms, an equivalent response for reduced depressive symptoms, and a diminished response for weight loss.
Despite some divergent findings, both ENGAGE and ENGAGE-2 studies reveal that the ability of PST to engage neural circuits involved in the regulation of negative affect and cognition may be a causal mechanism underlying the intervention effect on depression and anxiety. The heterogeneity in sociodemographic, neural, and clinical characteristics and the mixed study findings may be indicative of the complexity and challenges in transdisciplinary research aimed to uncover the mechanism of action within the context of pragmatic behavioral interventions for diverse patient populations. It is possible that given the same behavioral intervention, different mechanistic pathways dominate in people with different clinical and neural profiles for different outcomes. Capitalizing on the discoveries from these early studies, future research is needed on finer-grained analyses and to design mechanism-targeted precision clinical trials (43,44). Our current approach employed a priori selected brain circuits. Data-driven whole-brain analyses can be used to probe the interaction between neural circuits (e.g., negative affect and cognitive control) or discover novel circuits in the mechanistic pathway. Further subgroup analysis or moderated mediation analysis can help understand how and why the intervention worked for whom and under what conditions. These results could inform personalized management of depression and anxiety. For example, future trials may test neural target-driven enhancements of the integrated collaborative care intervention with noninvasive brain stimulation [e.g., transcranial magnetic stimulation (45,46) or transcranial direct current stimulation (47, 48, 49)] to specifically engage the prefrontal cortex as a way to augment the intervention for those who fail to show target engagement in the cognitive control circuit. Such neural target-driven experimental designs can also test hypotheses of causal mediation where treatment effect on a neural target is hypothesized to be causally related to treatment effect on a clinical outcome. Although confirmation of causal mediation was beyond the scope of this pilot trial by design, we examined the relationship of changes in neural targets at 2 months with subsequent changes in clinical outcomes at 6 months and, additionally, with concurrent changes in clinical outcomes at 2 months. For the neural target changes at 2 months that correlated significantly with GAD-7 score changes at 6 months, it is encouraging that the magnitude of the standardized β coefficients was consistently larger than their correlations with GAD-7 score changes at 2 months, suggesting increased strength of association.
Several limitations are worth noting. First, this mechanistic trial was a pilot study with a small sample size and short duration. Second, the control group received usual care only. Future research is needed to compare the effects of different active treatments on the same neural targets to further elucidate underlying mechanisms. Third, task-based neuroimaging measures have shown varying levels of within-subject reliability, which could have impacted our ability to detect changes in some targets. Finally, several changes had to be made in the conduct of the study due to the pandemic (see Supplemental Methods), which may have confounded the results.
In conclusion, compared with usual care, the study intervention led to significantly improved depression but not weight loss, and the results on neural targets were null for both of these primary outcomes. The significant intervention effect on the secondary anxiety outcome might be mediated through changes in the cognitive control circuit, but this warrants replication in primary research on interventions targeting the cognitive control circuit for anxiety. This study highlights possible neural mechanisms underlying the integrated collaborative care intervention and a fruitful direction for the development of neural target-driven treatment strategies that may enhance the intervention effect and can be subject to focused hypothesis testing of causal mediation in future RCTs.
Acknowledgments and Disclosures
This research was supported by the National Institutes of Health Science of Behavior Change Common Fund Program through an award administered by the National Heart, Lung, and Blood Institute (Grant Nos. UH2HL132368 and UH3HL132368 [to JM and LMW]). The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
JM had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: JM, LMW, PWL; acquisition, analysis, or interpretation of data: all authors; drafting of the manuscript: NL, OAA, JM, LX, PS; critical revision of the manuscript for important intellectual content: all authors; statistical analysis: LX, PWL; obtained funding: JM, LMW; administrative, technical, or material support: NL, OAA, EMV, MBS, PS, ANG-P; supervision: JM, LMW, OAA.
We thank our Data and Safety Monitoring Board members who received compensation for their time: Manisha Desai (chair), Sandra Tsai, Mickey Trockel, and Manpreet Singh (all from Stanford University). The Data and Safety Monitoring Board reviewed the study protocol initially and data quality and safety monitoring reports semiannually thereafter. We extend special thanks to the participants and their families who made this study possible.
A previous version of this article was published as a preprint on the preprint server SSRN: https://doi.org/10.2139/ssrn.3887444
The data and associated documentation are available to users only under a formal data sharing and use agreement that provides for a commitment to the following: 1) using the data only for research purposes and not to identify any individual participant, 2) securing the data using appropriate computer technology, 3) destroying or returning the data after analyses are completed, 4) accepting reporting responsibilities, 5) abiding by restrictions on redistribution of the data for commercial purposes or to third parties, and 6) properly acknowledging the data resource. Appropriate fees may be assessed upon mutual agreement on requests for information in a format other than that we intend to provide. We will not be responsible for providing any analytical support.
JM is a paid scientific consultant for Health Mentor, Inc. LMW is on the Scientific Advisory Board for One Mind Psyberguide and the External Advisory Board for the Laureate Institute for Brain Research. OAA is the cofounder of Keywise AI and serves on the advisory boards of Blueprint Health and Embodied Labs. TK is a paid consultant for Pfizer, Inc., outside of this work. All other authors report no biomedical financial interests or potential conflicts of interest.
ClinicalTrials.gov: Engaging Self-regulation Targets to Improve Mood and Weight and Understand Mechanism in Depressed and Obese Adults; https://clinicaltrials.gov/ct2/show/NCT03841682; NCT03841682.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2022.03.012.
Supplementary Material
References
- 1.Walker E.R., Druss B.G. A public health perspective on mental and medical comorbidity. JAMA. 2016;316:1104–1105. doi: 10.1001/jama.2016.10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ettman C.K., Abdalla S.M., Cohen G.H., Sampson L., Vivier P.M., Galea S. Prevalence of depression symptoms in US adults before and during the COVID-19 pandemic. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.19686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeigler Z., Forbes B., Lopez B., Pedersen G., Welty J., Deyo A., Kerekes M. Self-quarantine and weight gain related risk factors during the COVID-19 pandemic. Obes Res Clin Pract. 2020;14:210–216. doi: 10.1016/j.orcp.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czeisler M.É., Lane R.I., Petrosky E., Wiley J.F., Christensen A., Njai R., et al. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic — United States, June 24–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1049–1057. doi: 10.15585/mmwr.mm6932a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiong J., Lipsitz O., Nasri F., Lui L.M.W., Gill H., Phan L., et al. Impact of COVID-19 pandemic on mental health in the general population: A systematic review. J Affect Disord. 2020;277:55–64. doi: 10.1016/j.jad.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bandura A. Human agency in social cognitive theory. Am Psychol. 1989;44:1175–1184. doi: 10.1037/0003-066x.44.9.1175. [DOI] [PubMed] [Google Scholar]
- 7.D’Zurilla T.J. Springer; New York: 1986. Problem-Solving Therapy: A Social Competence Approach to Clinical Interventions. [Google Scholar]
- 8.Ma J., Rosas L.G., Lv N., Xiao L., Snowden M.B., Venditti E.M., et al. Effect of integrated behavioral weight loss treatment and problem-solving therapy on body mass index and depressive symptoms among patients with obesity and depression: The RAINBOW randomized clinical trial. JAMA. 2019;321:869–879. doi: 10.1001/jama.2019.0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lv N., Kringle E.A., Ma J. Integrated behavioral interventions for adults with comorbid obesity and depression: A systematic review. Curr Diab Rep. 2022;22:157–168. doi: 10.1007/s11892-022-01458-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nielsen L., Riddle M., King J.W., NIH Science of Behavior Change Implementation Team. Aklin W.M., et al. The NIH Science of Behavior Change Program: Transforming the science through a focus on mechanisms of change. Behav Res Ther. 2018;101:3–11. doi: 10.1016/j.brat.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams L.M., Pines A., Goldstein-Piekarski A.N., Rosas L.G., Kullar M., Sacchet M.D., et al. The ENGAGE study: Integrating neuroimaging, virtual reality and smartphone sensing to understand self-regulation for managing depression and obesity in a precision medicine model. Behav Res Ther. 2018;101:58–70. doi: 10.1016/j.brat.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein-Piekarski A.N., Wielgosz J., Xiao L., Stetz P., Correa C.G., Chang S.E., et al. Early changes in neural circuit function engaged by negative emotion and modified by behavioural intervention are associated with depression and problem-solving outcomes: A report from the ENGAGE randomized controlled trial. EBioMedicine. 2021;67:103387. doi: 10.1016/j.ebiom.2021.103387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lv N., Lefferts W.K., Xiao L., Goldstein-Piekarski A.N., Wielgosz J., Lavori P.W., et al. Problem-solving therapy-induced amygdala engagement mediates lifestyle behavior change in obesity with comorbid depression: A randomized proof-of-mechanism trial. Am J Clin Nutr. 2021;114:2060–2073. doi: 10.1093/ajcn/nqab280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lv N., Ajilore O.A., Ronneberg C.R., Venditti E.M., Snowden M.B., Lavori P.W., et al. The ENGAGE-2 study: Engaging self-regulation targets to understand the mechanisms of behavior change and improve mood and weight outcomes in a randomized controlled trial (Phase 2) Contemp Clin Trials. 2020;95:106072. doi: 10.1016/j.cct.2020.106072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao L., Huang Q., Yank V., Ma J. An easily accessible Web-based minimization random allocation system for clinical trials. J Med Internet Res. 2013;15:e139. doi: 10.2196/jmir.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott N.W., McPherson G.C., Ramsay C.R., Campbell M.K. The method of minimization for allocation to clinical trials. A review. Control Clin Trials. 2002;23:662–674. doi: 10.1016/s0197-2456(02)00242-8. [DOI] [PubMed] [Google Scholar]
- 17.Meinert C.L. Oxford University Press; New York: 1986. Clinical Trials: Design, Conduct, and Analysis. [Google Scholar]
- 18.Ciechanowski P., Wagner E., Schmaling K., Schwartz S., Williams B., Diehr P., et al. Community-integrated home-based depression treatment in older adults: A randomized controlled trial. JAMA. 2004;291:1569–1577. doi: 10.1001/jama.291.13.1569. [DOI] [PubMed] [Google Scholar]
- 19.Ciechanowski P., Chaytor N., Miller J., Fraser R., Russo J., Unutzer J., Gilliam F. PEARLS depression treatment for individuals with epilepsy: A randomized controlled trial. Epilepsy Behav. 2010;19:225–231. doi: 10.1016/j.yebeh.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Kramer M.K., Kriska A.M., Venditti E.M., Miller R.G., Brooks M.M., Burke L.E., et al. Translating the Diabetes Prevention Program: A comprehensive model for prevention training and program delivery. Am J Prev Med. 2009;37:505–511. doi: 10.1016/j.amepre.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 21.Glass R.M., Allan A.T., Uhlenhuth E.H., Kimball C.P., Borinstein D.I. Psychiatric screening in a medical clinic. An evaluation of a self-report inventory. Arch Gen Psychiatry. 1978;35:1189–1195. doi: 10.1001/archpsyc.1978.01770340039003. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg D.P., Rickels K., Downing R., Hesbacher P. A comparison of two psychiatric screening tests. Br J Psychiatry. 1976;129:61–67. doi: 10.1192/bjp.129.1.61. [DOI] [PubMed] [Google Scholar]
- 23.PhenX Toolkit, ver 4.2. 2011. www.phenxtoolkit.org Available at: [Google Scholar]
- 24.Spitzer R.L., Kroenke K., Williams J.B.W., Löwe B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 25.Tuinstra D., Percifield C., Stilwell K., Plattner A., Edwards E., Sanders W., Koval M. Treatment of anxiety symptoms in patients receiving rTMS for treatment resistant depression. Psychiatry Res Commun. 2022;2:100014. [Google Scholar]
- 26.Goldstein-Piekarski A.N., Ball T.M., Samara Z., Staveland B.R., Keller A.S., Fleming S.L., et al. Mapping neural circuit biotypes to symptoms and behavioral dimensions of depression and anxiety. Biol Psychiatry. 2022;91:561–571. doi: 10.1016/j.biopsych.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams L.M. Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: A theoretical review of the evidence and future directions for clinical translation. Depress Anxiety. 2017;34:9–24. doi: 10.1002/da.22556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams L.M. Precision psychiatry: A neural circuit taxonomy for depression and anxiety. Lancet Psychiatry. 2016;3:472–480. doi: 10.1016/S2215-0366(15)00579-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraemer H.C., Wilson G.T., Fairburn C.G., Agras W.S. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002;59:877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- 30.Kraemer H.C. Is it time to ban the P value? JAMA Psychiatry. 2019;76:1219–1220. doi: 10.1001/jamapsychiatry.2019.1965. [DOI] [PubMed] [Google Scholar]
- 31.Harrington D., D’Agostino R.B., Sr, Gatsonis C., Hogan J.W., Hunter D.J., Normand S.T., et al. New guidelines for statistical reporting in the Journal. N Engl J Med. 2019;381:285–286. doi: 10.1056/NEJMe1906559. [DOI] [PubMed] [Google Scholar]
- 32.Benjamini Y., Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Statist. 2001;29:1165–1188. [Google Scholar]
- 33.Katon W.J., Lin E.H.B., Von Korff M., Ciechanowski P., Ludman E.J., Young B., et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363:2611–2620. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katon W., Lin E.H.B., Von Korff M., Ciechanowski P., Ludman E., Young B., et al. Integrating depression and chronic disease care among patients with diabetes and/or coronary heart disease: The design of the TEAMcare study. Contemp Clin Trials. 2010;31:312–322. doi: 10.1016/j.cct.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flanagan E.W., Beyl R.A., Fearnbach S.N., Altazan A.D., Martin C.K., Redman L.M. The impact of COVID-19 stay-at-home orders on health behaviors in adults. Obesity (Silver Spring) 2021;29:438–445. doi: 10.1002/oby.23066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin A.L., Vittinghoff E., Olgin J.E., Pletcher M.J., Marcus G.M. Body weight changes during pandemic-related shelter-in-place in a longitudinal cohort study. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson E., Gillespie S., Jones A. Weight-related lifestyle behaviours and the COVID-19 crisis: An online survey study of UK adults during social lockdown. Obes Sci Pract. 2020;6:735–740. doi: 10.1002/osp4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pellegrini M., Ponzo V., Rosato R., Scumaci E., Goitre I., Benso A., et al. Changes in weight and nutritional habits in adults with obesity during the “lockdown” period caused by the COVID-19 virus emergency. Nutrients. 2020;12:2016. doi: 10.3390/nu12072016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gorka S.M., Young C.B., Klumpp H., Kennedy A.E., Francis J., Ajilore O., et al. Emotion-based brain mechanisms and predictors for SSRI and CBT treatment of anxiety and depression: A randomized trial. Neuropsychopharmacology. 2019;44:1639–1648. doi: 10.1038/s41386-019-0407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brehl A.K., Kohn N., Schene A.H., Fernández G. A mechanistic model for individualised treatment of anxiety disorders based on predictive neural biomarkers. Psychol Med. 2020;50:727–736. doi: 10.1017/S0033291720000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crane N.A., Jenkins L.M., Dion C., Meyers K.K., Weldon A.L., Gabriel L.B., et al. Comorbid anxiety increases cognitive control activation in major depressive disorder. Depress Anxiety. 2016;33:967–977. doi: 10.1002/da.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langenecker S.A., Jacobs R.H., Passarotti A.M. Current neural and behavioral dimensional constructs across mood disorders. Curr Behav Neurosci Rep. 2014;1:144–153. doi: 10.1007/s40473-014-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lenze E.J., Rodebaugh T.L., Nicol G.E. A framework for advancing precision medicine in clinical trials for mental disorders [published correction appears in JAMA Psychiatry 2020; 77:768] JAMA Psychiatry. 2020;77:663–664. doi: 10.1001/jamapsychiatry.2020.0114. [DOI] [PubMed] [Google Scholar]
- 44.Lenze E.J., Nicol G.E., Barbour D.L., Kannampallil T., Wong A.W.K., Piccirillo J., et al. Precision clinical trials: A framework for getting to precision medicine for neurobehavioural disorders. J Psychiatry Neurosci. 2021;46:E97–E110. doi: 10.1503/jpn.200042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voigt J., Carpenter L., Leuchter A. A systematic literature review of the clinical efficacy of repetitive transcranial magnetic stimulation (rTMS) in non-treatment resistant patients with major depressive disorder. BMC Psychiatry. 2019;19:13. doi: 10.1186/s12888-018-1989-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cirillo P., Gold A.K., Nardi A.E., Ornelas A.C., Nierenberg A.A., Camprodon J., Kinrys G. Transcranial magnetic stimulation in anxiety and trauma-related disorders: A systematic review and meta-analysis. Brain Behav. 2019;9 doi: 10.1002/brb3.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nord C.L., Halahakoon D.C., Limbachya T., Charpentier C., Lally N., Walsh V., et al. Neural predictors of treatment response to brain stimulation and psychological therapy in depression: A double-blind randomized controlled trial. Neuropsychopharmacology. 2019;44:1613–1622. doi: 10.1038/s41386-019-0401-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goerigk S.A., Padberg F., Bühner M., Sarubin N., Kaster T.S., Daskalakis Z.J., et al. Distinct trajectories of response to prefrontal tDCS in major depression: Results from a 3-arm randomized controlled trial. Neuropsychopharmacology. 2021;46:774–782. doi: 10.1038/s41386-020-00935-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chalah M.A., Ayache S.S. Noninvasive brain stimulation and psychotherapy in anxiety and depressive disorders: A viewpoint. Brain Sci. 2019;9:82. doi: 10.3390/brainsci9040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.