Key Points
Question
What are the associated expenses of clinical research and what factors underly the translational failure of inhibitors of the insulin-like growth factor-1 receptor (IGF-1R) in oncology?
Findings
In this cross-sectional study, 16 inhibitors of IGF-1R underwent 183 clinical trials in more than 12 000 patients; none of the agents was approved for clinical use in oncology practice and the trials were estimated to have had expenses of greater than $1.6 billion. Half of the published in vivo preclinical data analyzed showed less than a 50% inhibition of tumor growth by IGF-1R inhibitors.
Meaning
With high attrition rates for oncology drugs, the fruitless and expensive clinical trials of 16 IGF-1R inhibitors draw attention to the need for improved preclinical models and better decision-making before trials are launched, reducing substantial financial losses and avoiding exposure of patients to potential toxic effects.
Abstract
Importance
The development of oncology drugs is expensive and beset by a high attrition rate. Analysis of the costs and causes of translational failure may help to reduce attrition and permit the more appropriate use of resources to reduce mortality from cancer.
Objective
To analyze the causes of failure and expenses incurred in clinical trials of novel oncology drugs, with the example of insulin-like growth factor-1 receptor (IGF-1R) inhibitors, none of which was approved for use in oncology practice.
Design, Setting, and Participants
In this cross-sectional study, inhibitors of the IGF-1R and their clinical trials for use in oncology practice between January 1, 2000, and July 31, 2021, were identified by searching PubMed and ClinicalTrials.gov. A proprietary commercial database was interrogated to provide expenses incurred in these trials. If data were not available, estimates were made of expenses using mean values from the proprietary database. A search revealed studies of the effects of IGF-1R inhibitors in preclinical in vivo assays, permitting calculation of the percentage of tumor growth inhibition. Archival data on the clinical trials of IGF-1R inhibitors and proprietary estimates of their expenses were examined, together with an analysis of preclinical data on IGF-1R inhibitors obtained from the published literature.
Main Outcomes and Measures
Expenses associated with research and development of IGF-1R inhibitors.
Results
Sixteen inhibitors of IGF-1R studied in 183 clinical trials were found. None of the trials, in a wide range of tumor types, showed efficacy permitting drug approval. More than 12 000 patients entered trials of IGF-1R inhibitors in oncology indications in 2003 to 2021. These trials incurred aggregate research and development expenses estimated at between $1.6 billion and $2.3 billion. Analysis of the results of preclinical in vivo assays of IGF-1R inhibitors that supported subsequent clinical investigations showed mixed activity and protocols that poorly reflected the treatment of advanced metastatic tumors in humans.
Conclusions and Relevance
Failed drug development in oncology incurs substantial expense. At an industry level, an estimated $50 billion to $60 billion is spent annually on failed oncology trials. Improved target validation and more appropriate preclinical models are required to reduce attrition, with more attention to decision-making before launching clinical trials. A more appropriate use of resources may better reduce cancer mortality.
This cross-sectional study examines the costs generated in clinical trials, using the research and development studies of 16 insulin-like receptor-1 inhibitors in cancer, none of which was approved for use in the oncology practice setting, as an example.
Introduction
Over the past 2 decades, the pharmaceutical industry has become heavily focused on the discovery of therapeutic agents for the treatment of metastatic cancer.1 These agents largely either target genetic changes implicated in cancer pathology to provide targeted therapies2 or they modulate the immune system.3 However, development of oncology drugs has been reported to have a persistent attrition rate of greater than 95%,4,5,6 highlighting the disparity between positively assessed preclinical drug activity and subsequent inactivity in patients. The costs of clinical trials in oncology have been estimated to exceed those of other therapeutic areas so that failure is likely to be expensive.7 Failure is not only expensive in time and money but is disappointing for the scientists and clinicians dedicated to projects that flounder. Failure is ultimately most disappointing for patients, some of whom may be exposed in clinical trials to therapeutically inactive drugs that carry toxicity.
To our knowledge, estimates of the costs of clinical drug attrition in oncology have not been published. We have chosen to estimate the development expenses associated with the search for clinically active, targeted inhibitors of the insulin-like growth factor-1 receptor (IGF-1R).8,9,10,11,12 Insulin-like growth factor-1 receptor inhibitors (ie, small molecules, antibodies, and cell therapy), were chosen as an example as we were able to capture the results for 16 IGF-1R inhibitors evaluated in trials against a broad range of tumor types by different pharmaceutical and biotechnology companies, many of which had published preclinical data. All the inhibitors, alone or in drug combinations, failed to demonstrate clinical activity deemed sufficient for approval in oncology practice.
We have estimated the expense of the clinical phases of IGF-1R inhibitor development programs, collating details of treatments, including the numbers of patients entered in trials. We discuss the quality of the preclinical data leading to the launch of IGF-1R inhibitors into clinical trials and address more broadly the financial consequences of failure. We attempt to place the failure of the IGF-1R inhibitor programs into a temporal scientific and clinical context.
Methods
Creation of Databases of Clinical Trials of IGF-1R Inhibitors
This study of published research data did not involve personal medical records and does not constitute human participant research. We searched public databases (Google Scholar, PubMed, SCOPUS, and Web of Science) to find details on inhibitors of IGF-1R. Specifically, the key words used were molecular IGF-1R, therap*, targeted therap*, cancer, oncology, clinical trial, and drug development.
A database of clinical trials of IGF-1R inhibitors was then created by searching the ClinicalTrials.gov registry (NCT)13 for the key terms IGF-1R and the individual drug names or codes, with the date range from January 1, 2000, to July 31, 2021. Clinical trials not in the field of oncology, identified by pathology, were removed from the database. Trials were annotated for trial title, drug code, trial sponsor (industry or other), cancer indication, date of trial, patient numbers, trial phase, and program costs. Consideration of the study lead organizations allowed an insight into the primary sources of financing for each clinical trial. As such, organizations were considered to belong to the pharmaceutical or biotechnology industry, academia, the US National Cancer Institute, or other.
Estimate of Patient Numbers
We estimated patient numbers from reports in the NCT database13 for the key terms IGF-1R and the individual drug names or codes, again with the date range January 1, 2000, to July 31, 2021. Where patient numbers were not reported (14 of 183 entries), we assumed that the trial had recruited the same number of patients as the mean of other IGF-1R trials of the same phase.
Estimate of Clinical Development Expenses
The first research and development (R&D) expense estimates were from a proprietary database made accessible to us by Evaluate Ltd,14 which provided data for 129 of the total of 183 trials found. United States publicly traded companies are required by the Securities and Exchange Commission to file a detailed annual report, known as a 10-K. For many small and medium-sized biotechnology and pharmaceutical companies, the 10-K, and sometimes other publicly available documents, contains R&D expense information at the level of individual drugs. Evaluate Ltd collates this drug-level expense information on a nominal basis (ie, not inflated or deflated to a reference year) and combines it with other data in the public domain (eg, from ClinicalTrials.gov and other company disclosures in the 10-K) to derive per-patient benchmarks. The empirical phase 2 and 3 trial benchmarks consider a fixed trial expense plus a per-patient expense. There are further adjustments for geographic location and trial duration vs the technology-by-EPHMRA benchmark. The benchmark estimates are checked by comparing forecast R&D expenses with company-reported R&D expenses for the top 20 global biopharmaceutical firms. Evaluate Ltd method centers on what can be thought of as an accounting view of R&D expenses since they are derived from accounting-based reports (eg, 10-K filings). The Evaluate Ltd figures do not include the cost of capital that one would see in an investment view of R&D costs. However, the figures in the 10-K filings will generally include program expenses above the narrow cost of clinical trials (eg, manufacture of drugs for trials, any ongoing nonhuman toxicology studies, data processing, and preparing for regulatory submissions). Furthermore, companies will vary in what they exclude and include in when they report drug-level R&D expenses in their 10-Ks. We note that the estimates of Evaluate Ltd are used extensively in the drug industry by companies that know their own R&D costs. Details and a general primer on R&D cost estimates are provided in the eMethods in Supplement 1.
Estimate of Expenditure
We found 54 clinical trials of IGF-1R inhibitors for which Evaluate Ltd did not provide an expense estimate. To estimate these expenses, the mean cost per patient per trial phase was calculated from the Evaluate Ltd data given in eTable 1 in Supplement 2 and eTable 3 in Supplement 2) and multiplied by the number of patients described in the NCT database13 for each of these 54 trials. Eleven of these trials were performed by industry or biotechnology companies and we assumed their costs at the mean (per phase) of trial expenses estimated by Evaluate Ltd. Since estimates of the costs of academic and other trials, such as those by the US National Cancer Institute, are inaccessible, we estimated a range of possible expenses at 1 × the Evaluate Ltd mean, 0.5 × the Evaluate Ltd mean, and 0.2 × the Evaluate Ltd mean (eMethods in Supplement 1).
Basket Trials
When the IGF-1R inhibitors were in used in basket trials (ie, IGF-1R inhibitors were only one of several different interventions), we first took the expense of the complete basket trial and divided this by the total number of patients enrolled to determine the expense per patient. We then investigated the numbers of patients enrolled in the IGF-1R inhibitor arm from available publications and estimated the expense of that arm alone by multiplying the cost per patient by the number of patients in the IGF-1R inhibitor arm.
Analysis of Selected In Vivo Preclinical Data
A search for published articles describing preclinical data in vivo for each inhibitor was made using PubMed, using the code number or drug name reported in Table 1 (with one drug for oncology only, subsequently developed for Graves disease by Horizon15). The percentage tumor growth inhibition was calculated as described in Carboni et al16 by using values shown in the published graphic representations of the results of in vivo assays of the effects of single IGF-1R inhibitors.
Table 1. The 16 IGF-1R Inhibitors and the Estimated Number of Patients Entered Into Clinical Trialsa.
| Drug name | IGF-1R inhibitor type | Company | Estimated No. of patients |
|---|---|---|---|
| AMG479 (ganitumab) | Antibody | Amgen/NantCell | 2864 |
| AVE1642 | Antibody | Sanofi-Aventis | 57 |
| AXL1717 | Small molecule | Axelar AB | 204 |
| BIIB022 | Antibody | Biogen Idec | 98 |
| BMS-754807 | Small molecule | Bristol-Myers Squibb | 296 |
| CP-751 871 (figitumumab) | Antibody | Pfizer | 2029 |
| IGV-001 | Antisense/cell therapy | Imvax | 93 |
| IMCA12 (cixutumumab) | Antibody | Eli Lilly and Company/NCI | 2791 |
| KW-2450 | Small molecule | Kyowa Hakko Kirin Pharma Inc | 83 |
| MK7454 (robatumumab) | Antibody | Merck & Co/Schering Plough | 305 |
| MK0646 (dalotuzumab) | Antibody | Merck, Sharpe & Dohme Corp | 1436 |
| MM141 (istiratumab) | Antibody | Merrimack Pharmaceuticals | 135 |
| OSI906 (linsitinib) | Small molecule | Oncogene Sciences/Astellas Pharma Inc | 1277 |
| PL225B | Small molecule | Piramal Enterprises Ltd | 70 |
| RG1507 (teprotumumab) | Antibody | Hoffmann-La Roche | 525b |
| XL228 | Small molecule | Exelixis | 133 |
Abbreviations: IGF-1R, insulin-like growth factor-1 receptor; NCI, National Cancer Institute.
The estimated number of patients in trial programs with data taken from eTables 1 and 2 in Supplement 2.
Oncology only, subsequently developed for Graves disease by Horizon.15
Results
Clinical Trial Data and Program Expenditures
We found 16 IGF-1R inhibitors, small molecules, a mixed antisense cell therapy, and antibodies that entered oncology clinical trials between 2003 and 2021 (Table 1). Table 1 also presents our estimate of the number of patients who were entered into trial programs as found in the NCT database13 and, if not, the mean patient number was used for each phase. We estimate that a total of 12 396 patients were entered into 183 trial programs. Details are presented in eTable 1 and eTable 2 in Supplement 2, with basket trials reported separately.
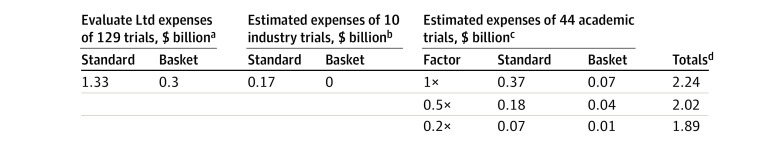
Figure 1 presents the estimated R&D expenses of the 129 clinical trials that were costed by Evaluate Ltd ($1.63 billion; eTable 1 in Supplement 2) for trials initiated by industry. It also reports our estimates (eTable 2 and eTable 3 in Supplement 2 and the eMethods in Supplement 1) for the remaining 54 trials not costed by Evaluate Ltd using factors explained in the Methods section to estimate expenditure.
Figure 1. Estimates of Expenses for the 183 IGF-1R Inhibitor Programs.
IGF-1R indicates insulin-like growth factor-1 receptor.
aExpenses incurred provided by Evaluate Ltd for 129 programs performed by industry and biotechnology companies.
bEstimated expenses of 10 industry programs not provided by Evaluate Ltd (Methods section).
cEstimated expenses of 44 nonindustry and biotechnology programs not provided by Evaluate Ltd (Methods section; eTables 1-3 in Supplement 2).
dEstimates of expenses for 183 IGF-1R programs.
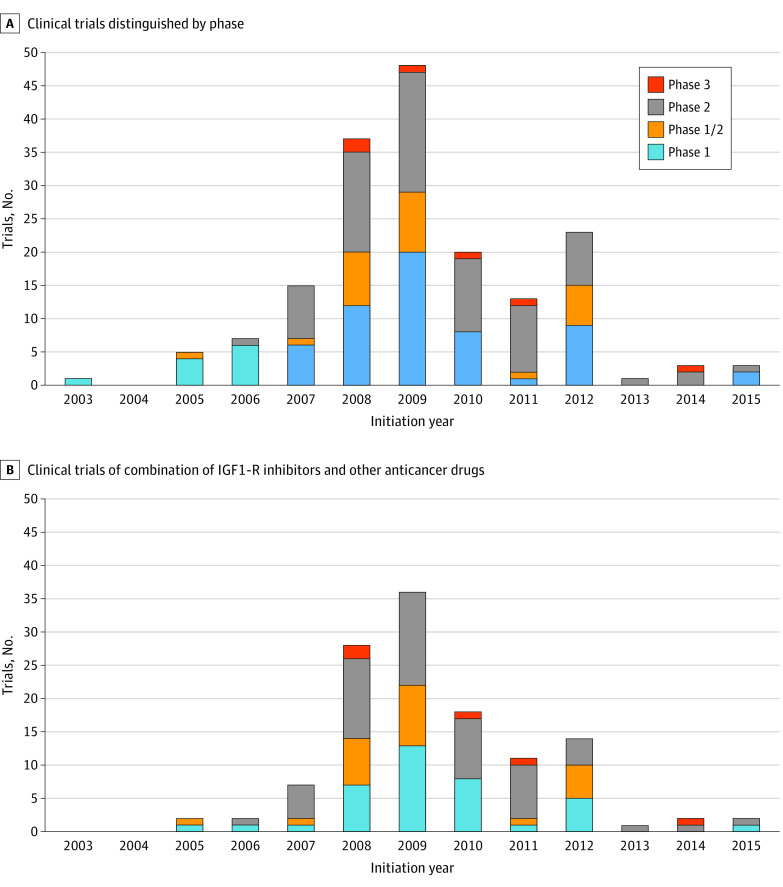
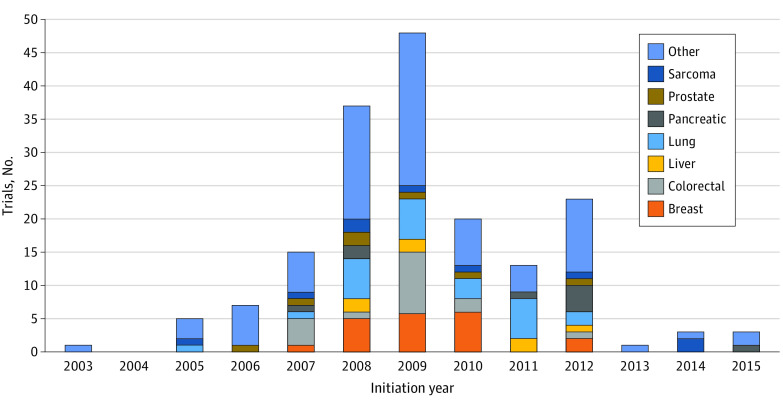
Figure 2A shows the number of clinical trials by trial phase (1-3) that were initiated from 2000 to 2015. The first clinical phase 1 trials, using a single IGF-1R inhibitor figitumumab, began in 2003, with phase 3 trials starting in 2008. The number of trials initiated each year increased from 2003 to 2012 and then decreased substantially from 2013 onward. The data for Figure 2A are taken from eTables 1 and 2 in Supplement 2 and are cutoff at 2015 for clarity. Figure 2B shows, for each year, the number of clinical trials using combinations of an IGF-1R inhibitor with other anticancer drugs, broken down by trial phase. Details of these combinations can be found in eTables 1 and 2 in Supplement 2. Figure 3 shows, year by year, trials in which the cancer (eg, breast, colon, and prostate) being treated could be extracted from publicly available data or the proprietary data from the Evaluate Ltd and our database eTables 1 and 2 in Supplement 2).
Figure 2. Numbers of Clinical Trials of Insulin-Like Growth Factor-1 Receptor (IGF-1R) Inhibitors.
A, Number of clinical trials of IGF-1R inhibitors distinguished by phase, initiated in the period 2003 to 2015. B, Number of clinical trials started using combinations of an IGF-1R inhibitor with other anticancer drugs. Details on these trials are reported in eTables 1 and 2 in Supplement 2.
Figure 3. The Principal Cancers Under Investigation in Clinical Trials of Insulin-like Growth Factor-1 Receptor Inhibitors From 2003 to 2021.
Specification of the category of others is included in eTables 1 and 2 in Supplement 2 included adrenocortical tumors, brain tumors, carcinomas (not specified), chronic myeloid leukemia, gastroesophageal tumors, head and neck tumors, melanoma, mesothelioma, multiple indications, multiple myeloma, neoplasms (not specified), neuroendocrine, nonhematologic, ovarian, solid tumors (not specified), thymus, and not specified.
Analysis of a Selection of In Vivo Preclinical Data
Table 2 presents a survey of the published preclinical in vivo data testing IGF-1R inhibitors, detailing the inhibitor used, the publication details, the xenografted tumor type, brief observations on the assay methods, and results, with estimates of percentage tumor growth inhibition for 9 inhibitors tested as a single agent against a broad range of tumor xenografts.16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50 Of the 62 cell line results annotated, 31 had a percentage of tumor growth inhibition of less than 50%.
Table 2. Activity of Single Agent IGF-1R Inhibitors on a Selection of Tumor Xenograft Models.
| Drug code (name) and source | Tumor typea | End point | Comments | Cell line and estimated %TGIb |
|---|---|---|---|---|
| AMG479 (ganitumab) | ||||
| Fahrenholtz et al,17 2013 | Prostate (VACaP) | Regression and cytostasis | Twice weekly for 3 wk; tumor regrowth following cessation of treatment | VACaP, 92% |
| Beltran et al,18 2009 | Pancreatic (BxPC3 and MiaCaPa2) | Growth delay | Palpable tumors remaining | BxPCR, 82%; MiaCaPa2, 83% |
| Beltran et al,19 2014 | Ovarian (OV90, OVCAR3, and TOV −21G) | Stasis (OV90); growth delay (OVCAR3); no effect (TOV-21G) | Twice weekly to end point | OV90, 100%; OVCAR3 57%; TOV-21G, 0% |
| Tabernero et al,20 2015 | Colon (colon-205) | Modest growth delay, leaving palpable tumors | Twice weekly to end point (3 wk) | Colon-205, 35% |
| Beltran et al,21 2011 | Ewing sarcoma (SK-ES-1 and A673) and osteosarcoma (SJSA-1) | Growth delay leaving palpable tumors | Twice weekly to end point (3 wk) | SK-ES-1, 50%; A673, 43%; SJSA-1, 33% |
| AVE1642 | ||||
| Geoerger et al,22 2010 | Neuroblastoma (IGR-N91 and SK-N-AS) | Modest growth delay leaving palpable tumors | Twice weekly to end point (21 or 28 d) | IGR-N91, 22%; SKN-AS, 19% |
| BMS-754807 | ||||
| Litzenburger et al,23 2011 | Triple negative breast cancer tumor graft | Growth delay leaving palpable tumors | Daily until end point (28 d) | 61% |
| Kolb et al,24 2011 | Pediatric tumors: KT-5 (Wilms), KT-14 (rhabdoid), Rh28 (rhabdomyosarcoma), and OS-1 (osteosarcoma) | Variable growth delay; most effective in rhabdomyosarcoma | Twice daily for 6 d/wk for 6 consecutive wk to end point (6 wk) | KT-5, 88%; KT-14, 83%; Rh28, 0% (regrowth); OS-1, 97% |
| Awasthi et al,25 2012 | Pancreas (PDAC) | Modest growth delay | 25 mg/kg 5 times weekly to end point (12 d) | PDAC, 80% |
| Lee et al,26 2013 | Non-small cell lung cancer (H292) | Ineffective | 50 mg/kg/d from day 0 to day 11 | H292, 0% |
| Halvorson et al,27 2015 | Glioma, genetically engineered mouse model | Ineffective | 50 mg/kg/d for 21 d | No increase n survival |
| Carboni et al,16 2009 | Colon carcinoma (GEO) | Modest growth delay | 25 mg/kg twice daily for 17 d; a list of other xenograft results is reported | GEO, 25% |
| CP-751, 871 (figitu-mumab) | ||||
| Cohen et al,28 2005 | Colon (colon 205); breast (MCF7) | Modest growth delay | Single dose (antibody) | COLO 205, 56%; MCF7, 20% |
| Iwasa et al,29 2009 | Non–small cell lung (H460 and H1299) | Negligible effect | Single dose (antibody) | H460, 22%; H1299, 17% |
| Chakraborty et al,30 2015 | Breast cancer (BT474 and MCF7) | Ineffective | Weekly for 8 wk | BT474 0%; MCF7 0% |
| IMCA12 (cixutu-mumab) | ||||
| Barnes et al,31 2007 | Head and neck cancer (TU159) | Cytostasis | Highly variable growth patterns between individual mice with some showing no response | TU159, 100% |
| Lu et al,32 2005 | Pancreatic and colon cancers (BxPC3 & HT29) | Growth delay | Twice weekly for 6 wk | BxPC3, 63%; HT29, 57% |
| Tonra et al,33 2009 | Colon carcinoma (HCT-8 &HT29-LP) | Modest growth delay | 3 times weekly to end point | HCT-8, 15%; HT29-LP, 33% |
| SCH717454 (robatu-mumab) | ||||
| Wang et al,34 2010 | Pediatric tumors: (SK-N-FI neuroblastoma, SJSA-1 osteosarcoma, RD rhabdomyosarcoma) | Tumor regression | Treatments started in unestablished tumors | SK-N-F1, 100%; SJSA-1, 49%; RD, 57% |
| Kolb et al,35 2008 | Pediatric tumor xenografts: (EW5 and CHLA-258, Ewing sarcomas; NB-SD neuroblastoma; OS-1 and OS9, osteosarcomas) | CHLA: regression and regrowth; NB-SD: regression and regrowth | Individual tumor growth curves presented with regrowth after initial inhibition | EW5, 100%; CHLA-258, 0%; NB-SD, 0%; OS-1, 100%; OS-9, 100% |
| MK0646 (dalotuzumab) | ||||
| Fagan et al,36 2012 | Breast cancer (MCF7L) | Modest growth delay with regrowth | Twice weekly to end point | MCF-7L 0% |
| Di Cosimo et al,37 2015 | Lung adenocarcinoma LXFA629 | Left palpable tumors after growth inhibition | Once weekly until 28-d end point (reported TGI 70%) | LXFA629 67% |
| Lamhamedi-Cherradi et al,38 2016 | Ewing sarcoma (EW5 and TC71) | Ineffective as a single agent | Growth delay with regrowth | EW5 0%; TC71 0% |
| MM141 (istiratumab) | ||||
| Fitzgerald et al,39 2014 | Pancreas (BxPC-3) | Growth delay | Bispecific antibody to IGF-1R and ERBB3 | BxPC-3 97% |
| Camblin et al,40 2018 | Pancreas (CFPAC-1, HPAF-11) | Initial growth delay and regrowth | Every 3 d to end point | CFPAC-1 98%; HPAF-11 0% |
| OSI906 (linsitinib) (dual IGF-1R and InsR antagonist) | ||||
| Pitts et al,41 2010 | Colon (CUCRC007 and CUCRC026) | Growth delay reported 50% CUCRC007) and approximately 75% CUCRC026) | Once daily until end point (25 d) | CUCRC007 61%; CUCRC026 92% |
| Zeng et al,42 2012 | Breast (LCC6) | Modest growth delay | Daily to end point (30 d) | LCC6 56% |
| Kuhn et al,43 2012 | Multiple myeloma (8226.BR) | No growth delay | Stimulation of growth at 20 mg/kg twice weekly | 8226.BR −44% |
| Flanigan et al,44 2010 | Colon (HCT15 and CUCRC006) | Growth delay (HCT15) or no effect (CUCRC006) | Dosing to end point (20 or 62 d) | HCT15 48%; CUCRC006 7% |
| Kim et al,45 2012 | Lung (H226B–K-Ras) | No effect | Daily to end point (8 d) | H226B-K-ras 0% |
| Zinn et al,46 2013 | Small cell lung cancer (NCI-H187 and PDXs LX 33, LX36) | Modest growth delay | Daily to end point | H187 64%; LX33 39%; LX36 50% |
| Ma et al,47 2016 | Glioblastoma (GBN76 and 39) | Modest growth delay (GBN76) and stimulation of growth (GBN 39) | Daily to end point | GBM76 44%; GBM39 − 53% |
| Ramcharan et al,48 2015 | Melanoma (A375M) | Growth delay | 3 times week to end point (approximately 48 d) | A375M 61% |
| Min et al,49 2015 | Non–small cell lung cancer (H1975) | Modest growth delay | Daily to end point | H1975 17% |
| Murakami et al,50 2016 | Ewing sarcoma (PDX) | Cytostasis | Daily to 14 d; end point 21 d | PDOX 80% |
Abbreviations: IGF-1R, insulin-like growth factor-1 receptor; %TGI, percentage of tumor growth inhibition.
Cell lines used for xenografts.
Calculated as in Carboni et al.16 Estimations of %TGI of single-agent IGF-1R inhibitors on a selection of tumor xenograft models as published in the literature.
Discussion
From 2003 onward, preclinical data generated by competing pharmaceutical and biotechnology companies propelled 16 IGFIR inhibitor candidates into 183 clinical trials against a broad range of cancers and in a wide array of drug combinations. Details of single-agent trials and of combination trials of IGF-1R with other oncology drugs are detailed in eTables 1 and 2 in Supplement 2. None of these IGF-1R inhibitors received approval for use in oncology practice as single agents or in combinations, although 1 inhibitor (Tepezza, teprotumumab; Roche) was subsequently approved for treatment of Graves disease.15 More than 12 000 patients were estimated to have entered these futile trials. Used as single agents, the drugs had an acceptable toxicity profile, although some drug combinations were toxic.51,52 For example, in the study of ganitumab with hormonal treatment of receptor-positive breast cancer, serious adverse events developed in 25% of the patients in the ganitumab group compared with a placebo, with the most common grade 3 or higher adverse event being neutropenia.51
Publications analyzing these trials addressed important issues that could have explained the lack of clinical efficacy of the IGF-1R inhibitors.53,54,55,56,57,58,59 They included redundancy within the IGF-1R–stimulated signaling pathways, with compensation of signaling by alternative pathways—an occurrence common to most signaling inhibitors60—and the lack of or failure to use predictive biomarkers for the selection of groups of patients who might have benefited from IGF-1R inhibitor therapy. In a 2017 retrospective analysis of failed late-stage clinical trials, across all of oncology, Jardim and colleagues61 concluded that the lack of a biomarker-driven strategy was commonly associated with drug attrition. However, Allison,54 in reviewing the IGF-1R inhibitor clinical trials, suggested that most used biomarkers. For example, a preclinical study identified potential biomarkers (IRS2 copy number gain, KRAS and BRAF mutation status) for the treatment of colon cancer62 with the IGF-1R inhibitor BMS-754807; its clinical trial in colon cancer (NCT00908024) was nevertheless a failure (eTable 1 in Supplement 2).
The retrospective commentaries on the failure of the clinical trials of IGF-1R inhibitors53,54,55,56,57,58,59 did not question the predictive value of preclinical data deemed sufficient to launch investigational studies in humans. Some of us have contributed to critical comments on preclinical models that fail to capture the key features of a number of diseases, including cancer, and of the decision-making that advances drug candidates to clinical trial.63 Those criticisms are pertinent to the models used to select IGF-1R inhibitors for clinical trials. Although it is beyond the scope of this study to review in depth all the preclinical data on IGF-1R inhibitors, we have surveyed the published literature reporting the single-drug activity in vivo in xenografts generated from a wide variety of cancers (Table 2). The results present a mixed picture, with half of these studies resulting in less than a 50% tumor growth inhibition. Many of the in vivo studies sampled reported tumor growth inhibition after immediate and then prolonged treatments of tumors implanted as fragments, treatments often administered before growth was fully established. Most studies also made no estimation of tumor regrowth after treatment cessation. The study of ganitumab (AMG479) by Fahrenholtz et al17 is an example of a study in which strong regrowth of the VACaP prostate xenograft was observed immediately on cessation of treatment. This suggests that the cytotoxic (apoptosis-inducing) effects of inhibiting IGF-1 signaling observed in vitro were limited in vivo, leaving viable clones capable of regrowth. Modest inhibition of tumor growth (percentage tumor growth inhibition <50%) by IGF-1R inhibitors in a variety of tumor types, together with tumor regrowth in some assays, should have sent warning signals regarding the status of IGF-1R as a validated drug target, a question raised in a 2009 study of a transgenic mouse model of IGF-1R–induced tumors.64
In his 2013 review of the clinical failure of IGF-1R inhibitors, Renato Baserga56 referred to “the problem of how cancer cures obtained in mice can be transferred to human beings.” There was no evidence of prolonged cures by IGF-1R inhibitors in tumor-bearing mice (Table 2), despite overall preclinical in vivo data having been described as compelling.11
Xenograft data considered to be predictive of the successful clinical activity of IGF-1R inhibitors, all of which subsequently failed to show clinical efficacy, can either be considered to invalidate the models themselves (when there was 100% tumor growth inhibition but no clinical activity) or, at the least, the interpretation of them. In 2014, drug researchers at AstraZeneca articulated a 5-dimensional framework that reduced their drug attrition in clinical trials: more rigorous target validation and improved preclinical models were key aspects that required attention.65 This study of IGF-1R inhibitors supports that recommendation, as does the recent suggestion by some of us to use more effective and stringent decision tools during preclinical development.63
Published estimates of drug development costs generally focus on successful drug registrations, whereas herein we estimate the cost of failure (Figure 1). The 183 IGF-1R trials had cumulative R&D expenses estimated to be greater than $1.63 billion over the period of our survey. Looking at the drug industry, cancer R&D that is failing or destined to fail will currently incur an annual expense that is in the order of $50 billion to $60 billion (eMethods in Supplement 1).
Moser and Verdin66 in 2018 questioned the crowded space of oncology trials, with1405 new molecular entities under investigation in 3158 different indications, many of which are me-too programs. Fojo and colleagues67 have also criticized the futility of multiple me-too programs of anticancer drug discovery. What drove 16 companies to dash competitively toward clinical trials of IGF-1R inhibitors, all of which failed? In the early years of the 2000s, the success of trastuzumab may have been a force creating strong expectations.68,69 It is also possible that company management, with these high expectations, faced with strong competition and the need to fill their drug pipelines, proceeded in a case of herd instinct, as discussed recently.70 To quote Borup et al71 in an analysis of expectations in scientific research, “behavior is not only based on rational risk-return considerations, but also influenced by expectations and perceptions of other’s behavior.” It will be interesting in the future to analyze the costs and outcomes of the current intense competition in oncology, with many me-too programs.
What might be learned from the failure of the IGF-1R inhibitor programs? We see at least 3 lessons. The first relates to the evidential hurdles that cancer drugs should pass before moving into the clinic; specifically, the predictive validity of the preclinical models and performance standards that drug candidates should meet in those models.63 The second relates to technical diversification to avoid overinvestment in some mechanisms (eg, IGF-1R) and underinvestment in others.66,67 The third relates to a lack of rigorous analyses of major translational failures.63
Limitations
This study has limitations. The limitations in estimating drug development costs and failure are elaborated in the eMethods in Supplement 1. In 129 of the trials we found of IGF-1R inhibitors, the Evaluate Ltd algorithm (eMethods in Supplement 1) was used to estimate expenditure on these trials. This algorithm could be challenged, although many international pharmaceutical companies use data generated by it. When we lacked data from Evaluate Ltd, we used their mean expenditure per patient per phase of clinical trial. It is not based on real data that were gleaned from company reports and is therefore questionable. Trials performed by academic centers or institutions, such as the US National Cancer Institute, do not have transparent sources to permit estimations of trial expenditure and so had to be estimated.
We have discussed herein clinical trials and preclinical data that date from the first 2 decades of 2000. It is possible that discovery and improved use of biomarkers for IGF-1R inhibitors could reduce these examples of clinical trial failure, although we are not aware of recent data to substantiate this.
We may have overlooked some of the publications of in vivo preclinical data that allowed us to derive the percentage inhibition of tumor growth by IGF-1R inhibitors. It is also likely that more data, especially from pharmaceutical companies, were not published and that only representative data or optimal data on in vivo assays were published. However, we were able to capture data from 35 publications in which a wide range of cancers xenografted in mice were used. We have not analyzed the data from in vitro tests of IGF-1R inhibitors on cell lines as it is likely that the in vivo data were most important in the decision to progress a candidate drug to clinical trial.
Conclusions
We do not dismiss the challenges of drug discovery in cancer. During the period that IGF-1R inhibitor projects were launched (1999-2009), it was reported that 83% of the claims that cancer biology could be successfully translated into treatments proved futile, and of drugs that offered an overall survival benefit, it was for a mean of only 6 months.72 This limited impact of many systemic therapies to improve overall survival from cancer has continued.73,74 The biology of cancer is complex and understanding of cancer mechanisms continues to evolve. In the early years of the 2000s, when IGF-1R inhibitor programs were being launched, 6 hallmarks of cancer were identified; in 2022 this had grown to 14.75 Our growing understanding of the complexity and heterogeneity of cancer should provide pause for thought about the human and financial resources involved in the enterprise of drug discovery and to where effort and resources may be better focused to reduce cancer mortality.
eMethods. Further Details
eTable 1. Calculation of Trial Expenses in $s
eTable 2. Basket Trials Not in the Evaluate Ltd Database With the Estimation of Trial Expenses for the IGF-1R Inhibitor Arm Only
eTable 3. Calculations to Establish the Mean Expenses per Patient per Phase
Data Sharing Statement
References
- 1.EvaluatePharma . World Preview 2021. Outlook to 2026. 14th edition. July 2021. Accessed July 31, 2022. https://info.evaluate.com/rs/607-YGS-364/images/WorldPreviewReport_Final_2021.pdf
- 2.Bedard PL, Hyman DM, Davids MS, Siu LL. Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet. 2020;395(10229):1078-1088. doi: 10.1016/S0140-6736(20)30164-1 [DOI] [PubMed] [Google Scholar]
- 3.Morad G, Helmink BA, Sharma P, Wargo JA. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. 2021;184(21):5309-5337. doi: 10.1016/j.cell.2021.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong CH, Siah KW, Lo AW. Estimation of clinical trial success rates and related parameters. Biostatistics. 2019;20(2):273-286. doi: 10.1093/biostatistics/kxx069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowden H, Munro J. Trends in clinical success rates and therapeutic focus. Nat Rev Drug Discov. 2019;18(7):495-496. doi: 10.1038/d41573-019-00074-z [DOI] [PubMed] [Google Scholar]
- 6.Mullard A. R&D re-balancing act. Nat Rev Drug Discov. 2023;22:258. [DOI] [PubMed] [Google Scholar]
- 7.Schlander M, Hernandez-Villafuerte K, Cheng C-Y, Mestre-Ferrandiz J, Baumann M. How much does it cost to research and develop a new drug? a systematic review and assessment. Pharmacoeconomics. 2021;39(11):1243-1269. doi: 10.1007/s40273-021-01065-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baserga R, Peruzzi F, Reiss K. The IGF-1 receptor in cancer biology. Int J Cancer. 2003;107(6):873-877. doi: 10.1002/ijc.11487 [DOI] [PubMed] [Google Scholar]
- 9.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4(7):505-518. doi: 10.1038/nrc1387 [DOI] [PubMed] [Google Scholar]
- 10.Gualberto A, Pollak M. Emerging role of insulin-like growth factor receptor inhibitors in oncology: early clinical trial results and future directions. Oncogene. 2009;28(34):3009-3021. doi: 10.1038/onc.2009.172 [DOI] [PubMed] [Google Scholar]
- 11.Osher E, Macaulay VM. Therapeutic targeting of the IGF axis. Cells. 2019;8(8):895. doi: 10.3390/cells8080895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neal JW, Sequist LV. Exciting new targets in lung cancer therapy: ALK, IGF-1R, HDAC, and Hh. Curr Treat Options Oncol. 2010;11(1-2):36-44. doi: 10.1007/s11864-010-0120-6 [DOI] [PubMed] [Google Scholar]
- 13.ClinicalTrials.gov. Find trials. Accessed June 28, 2021. https://clinicaltrials.gov/ct2/search
- 14.Evaluate. Evaluate Ltd website. Accessed September 21, 2022. https://www.evaluate.com/
- 15.Dolgin E. IGF-1R drugs travel from cancer cradle to Graves’. Nat Biotechnol. 2020;38(4):385-388. doi: 10.1038/s41587-020-0481-8 [DOI] [PubMed] [Google Scholar]
- 16.Carboni JM, Wittman M, Yang Z, et al. BMS-754807, a small molecule inhibitor of insulin-like growth factor-1R/IR. Mol Cancer Ther. 2009;8(12):3341-3349. doi: 10.1158/1535-7163.MCT-09-0499 [DOI] [PubMed] [Google Scholar]
- 17.Fahrenholtz CD, Beltran PJ, Burnstein KL. Targeting IGF-IR with ganitumab inhibits tumorigenesis and increases durability of response to androgen-deprivation therapy in VCaP prostate cancer xenografts. Mol Cancer Ther. 2013;12(4):394-404. doi: 10.1158/1535-7163.MCT-12-0648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beltran PJ, Mitchell P, Chung YA, et al. AMG 479, a fully human anti-insulin-like growth factor receptor type I monoclonal antibody, inhibits the growth and survival of pancreatic carcinoma cells. Mol Cancer Ther. 2009;8(5):1095-1105. doi: 10.1158/1535-7163.MCT-08-1171 [DOI] [PubMed] [Google Scholar]
- 19.Beltran PJ, Calzone FJ, Mitchell P, et al. Ganitumab (AMG 479) inhibits IGF-II-dependent ovarian cancer growth and potentiates platinum-based chemotherapy. Clin Cancer Res. 2014;20(11):2947-2958. doi: 10.1158/1078-0432.CCR-13-3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabernero J, Chawla SP, Kindler H, et al. Anticancer activity of the type I insulin-like growth factor receptor antagonist, ganitumab, in combination with the death receptor 5 agonist, conatumumab. Target Oncol. 2015;10(1):65-76. doi: 10.1007/s11523-014-0315-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beltran PJ, Chung YA, Moody G, et al. Efficacy of ganitumab (AMG 479), alone and in combination with rapamycin, in Ewing’s and osteogenic sarcoma models. J Pharmacol Exp Ther. 2011;337(3):644-654. doi: 10.1124/jpet.110.178400 [DOI] [PubMed] [Google Scholar]
- 22.Geoerger B, Brasme JF, Daudigeos-Dubus E, et al. Anti-insulin-like growth factor 1 receptor antibody EM164 (murine AVE1642) exhibits anti-tumour activity alone and in combination with temozolomide against neuroblastoma. Eur J Cancer. 2010;46(18):3251-3262. doi: 10.1016/j.ejca.2010.06.005 [DOI] [PubMed] [Google Scholar]
- 23.Litzenburger BC, Creighton CJ, Tsimelzon A, et al. High IGF-IR activity in triple-negative breast cancer cell lines and tumorgrafts correlates with sensitivity to anti-IGF-IR therapy. Clin Cancer Res. 2011;17(8):2314-2327. doi: 10.1158/1078-0432.CCR-10-1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolb EA, Gorlick R, Lock R, et al. Initial testing (stage 1) of the IGF-1 receptor inhibitor BMS-754807 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2011;56(4):595-603. doi: 10.1002/pbc.22741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Awasthi N, Zhang C, Ruan W, Schwarz MA, Schwarz RE. BMS-754807, a small-molecule inhibitor of insulin-like growth factor-1 receptor/insulin receptor, enhances gemcitabine response in pancreatic cancer. Mol Cancer Ther. 2012;11(12):2644-2653. doi: 10.1158/1535-7163.MCT-12-0447 [DOI] [PubMed] [Google Scholar]
- 26.Lee SJ, Kim EJ, Lee HJ, et al. A pilot study for the early assessment of the effects of BMS-754807 plus gefitinib in an H292 tumor model by [(18)F]fluorothymidine-positron emission tomography. Invest New Drugs. 2013;31(3):506-515. doi: 10.1007/s10637-012-9874-y [DOI] [PubMed] [Google Scholar]
- 27.Halvorson KG, Barton KL, Schroeder K, et al. A high-throughput in vitro drug screen in a genetically engineered mouse model of diffuse intrinsic pontine glioma identifies BMS-754807 as a promising therapeutic agent. PLoS One. 2015;10(3):e0118926. doi: 10.1371/journal.pone.0118926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen BD, Baker DA, Soderstrom C, et al. Combination therapy enhances the inhibition of tumor growth with the fully human anti-type 1 insulin-like growth factor receptor monoclonal antibody CP-751,871. Clin Cancer Res. 2005;11(5):2063-2073. doi: 10.1158/1078-0432.CCR-04-1070 [DOI] [PubMed] [Google Scholar]
- 29.Iwasa T, Okamoto I, Suzuki M, et al. Inhibition of insulin-like growth factor 1 receptor by CP-751,871 radiosensitizes non-small cell lung cancer cells. Clin Cancer Res. 2009;15(16):5117-5125. doi: 10.1158/1078-0432.CCR-09-0478 [DOI] [PubMed] [Google Scholar]
- 30.Chakraborty AK, Zerillo C, DiGiovanna MP. In vitro and in vivo studies of the combination of IGF1R inhibitor figitumumab (CP-751,871) with HER2 inhibitors trastuzumab and neratinib. Breast Cancer Res Treat. 2015;152(3):533-544. doi: 10.1007/s10549-015-3504-2 [DOI] [PubMed] [Google Scholar]
- 31.Barnes CJ, Ohshiro K, Rayala SK, El-Naggar AK, Kumar R. Insulin-like growth factor receptor as a therapeutic target in head and neck cancer. Clin Cancer Res. 2007;13(14):4291-4299. doi: 10.1158/1078-0432.CCR-06-2040 [DOI] [PubMed] [Google Scholar]
- 32.Lu D, Zhang H, Koo H, et al. A fully human recombinant IgG-like bispecific antibody to both the epidermal growth factor receptor and the insulin-like growth factor receptor for enhanced antitumor activity. J Biol Chem. 2005;280(20):19665-19672. doi: 10.1074/jbc.M500815200 [DOI] [PubMed] [Google Scholar]
- 33.Tonra JR, Corcoran E, Deevi DS, et al. Prioritization of EGFR/IGF-IR/VEGFR2 combination targeted therapies utilizing cancer models. Anticancer Res. 2009;29(6):1999-2007. [PubMed] [Google Scholar]
- 34.Wang Y, Lipari P, Wang X, et al. A fully human insulin-like growth factor-I receptor antibody SCH 717454 (Robatumumab) has antitumor activity as a single agent and in combination with cytotoxics in pediatric tumor xenografts. Mol Cancer Ther. 2010;9(2):410-418. doi: 10.1158/1535-7163.MCT-09-0555 [DOI] [PubMed] [Google Scholar]
- 35.Kolb EA, Gorlick R, Houghton PJ, et al. Initial testing (stage 1) of a monoclonal antibody (SCH 717454) against the IGF-1 receptor by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50(6):1190-1197. doi: 10.1002/pbc.21450 [DOI] [PubMed] [Google Scholar]
- 36.Fagan DH, Uselman RR, Sachdev D, Yee D. Acquired resistance to tamoxifen is associated with loss of the type I insulin-like growth factor receptor: implications for breast cancer treatment. Cancer Res. 2012;72(13):3372-3380. doi: 10.1158/0008-5472.CAN-12-0684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Cosimo S, Sathyanarayanan S, Bendell JC, et al. Combination of the mTOR inhibitor ridaforolimus and the anti-IGF1R monoclonal antibody dalotuzumab: preclinical characterization and phase I clinical trial. Clin Cancer Res. 2015;21(1):49-59. doi: 10.1158/1078-0432.CCR-14-0940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamhamedi-Cherradi SE, Menegaz BA, Ramamoorthy V, et al. IGF-1R and mTOR Blockade: Novel Resistance Mechanisms and Synergistic Drug Combinations for Ewing Sarcoma. J Natl Cancer Inst. 2016;108(12):djw182. doi: 10.1093/jnci/djw182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fitzgerald JB, Johnson BW, Baum J, et al. MM-141, an IGF-IR- and ErbB3-directed bispecific antibody, overcomes network adaptations that limit activity of IGF-IR inhibitors. Mol Cancer Ther. 2014;13(2):410-425. doi: 10.1158/1535-7163.MCT-13-0255 [DOI] [PubMed] [Google Scholar]
- 40.Camblin AJ, Pace EA, Adams S, et al. Dual inhibition of IGF-1R and ErbB3 enhances the activity of gemcitabine and nab-paclitaxel in preclinical models of pancreatic cancer. Clin Cancer Res. 2018;24(12):2873-2885. doi: 10.1158/1078-0432.CCR-17-2262 [DOI] [PubMed] [Google Scholar]
- 41.Pitts TM, Tan AC, Kulikowski GN, et al. Development of an integrated genomic classifier for a novel agent in colorectal cancer: approach to individualized therapy in early development. Clin Cancer Res. 2010;16(12):3193-3204. doi: 10.1158/1078-0432.CCR-09-3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng X, Zhang H, Oh A, Zhang Y, Yee D. Enhancement of doxorubicin cytotoxicity of human cancer cells by tyrosine kinase inhibition of insulin receptor and type I IGF receptor. Breast Cancer Res Treat. 2012;133(1):117-126. doi: 10.1007/s10549-011-1713-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuhn DJ, Berkova Z, Jones RJ, et al. Targeting the insulin-like growth factor-1 receptor to overcome bortezomib resistance in preclinical models of multiple myeloma. Blood. 2012;120(16):3260-3270. doi: 10.1182/blood-2011-10-386789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flanigan SA, Pitts TM, Eckhardt SG, et al. The insulin-like growth factor I receptor/insulin receptor tyrosine kinase inhibitor PQIP exhibits enhanced antitumor effects in combination with chemotherapy against colorectal cancer models. Clin Cancer Res. 2010;16(22):5436-5446. doi: 10.1158/1078-0432.CCR-10-2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim WY, Prudkin L, Feng L, et al. Epidermal growth factor receptor and K-Ras mutations and resistance of lung cancer to insulin-like growth factor 1 receptor tyrosine kinase inhibitors. Cancer. 2012;118(16):3993-4003. doi: 10.1002/cncr.26656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zinn RL, Gardner EE, Marchionni L, et al. ERK phosphorylation is predictive of resistance to IGF-1R inhibition in small cell lung cancer. Mol Cancer Ther. 2013;12(6):1131-1139. doi: 10.1158/1535-7163.MCT-12-0618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma Y, Tang N, Thompson RC, et al. InsR/IGF1R Pathway Mediates Resistance to EGFR Inhibitors in Glioblastoma. Clin Cancer Res. 2016;22(7):1767-1776. doi: 10.1158/1078-0432.CCR-15-1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramcharan R, Aleksic T, Kamdoum WP, et al. IGF-1R inhibition induces schedule-dependent sensitization of human melanoma to temozolomide. Oncotarget. 2015;6(37):39877-39890. doi: 10.18632/oncotarget.5631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Min HY, Yun HJ, Lee JS, et al. Targeting the insulin-like growth factor receptor and Src signaling network for the treatment of non-small cell lung cancer. Mol Cancer. 2015;14:113. doi: 10.1186/s12943-015-0392-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murakami T, Singh AS, Kiyuna T, et al. Effective molecular targeting of CDK4/6 and IGF-1R in a rare FUS-ERG fusion CDKN2A-deletion doxorubicin-resistant Ewing’s sarcoma patient-derived orthotopic xenograft (PDOX) nude-mouse model. Oncotarget. 2016;7(30):47556-47564. doi: 10.18632/oncotarget.9879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robertson JF, Ferrero JM, Bourgeois H, et al. Ganitumab with either exemestane or fulvestrant for postmenopausal women with advanced, hormone-receptor–positive breast cancer: a randomised, controlled, double-blind, phase 2 trial. Lancet Oncol. 2013;14(3):228-235. doi: 10.1016/S1470-2045(13)70026-3 [DOI] [PubMed] [Google Scholar]
- 52.Ma H, Zhang T, Shen H, Cao H, Du J. The adverse events profile of anti-IGF-1R monoclonal antibodies in cancer therapy. Br J Clin Pharmacol. 2014;77(6):917-928. doi: 10.1111/bcp.12228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yee D. Insulin-like growth factor receptor inhibitors: baby or the bathwater? J Natl Cancer Inst. 2012;104(13):975-981. doi: 10.1093/jnci/djs258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allison M. Clinical setbacks reduce IGF-1 inhibitors to cocktail mixers. Nat Biotechnol. 2012;30(10):906-907. doi: 10.1038/nbt1012-906c [DOI] [PubMed] [Google Scholar]
- 55.Guha M. Anticancer IGF1R classes take more knocks. Nat Rev Drug Discov. 2013;12(4):250. doi: 10.1038/nrd3992 [DOI] [PubMed] [Google Scholar]
- 56.Baserga R. The decline and fall of the IGF-I receptor. J Cell Physiol. 2013;228(4):675-679. doi: 10.1002/jcp.24217 [DOI] [PubMed] [Google Scholar]
- 57.Wilson S, Chia SK. IGF-1R inhibition: right direction, wrong pathway? Lancet Oncol. 2013;14(3):182-183. doi: 10.1016/S1470-2045(13)70019-6 [DOI] [PubMed] [Google Scholar]
- 58.Janssen JAMJL, Varewijck AJ. IGF-IR targeted therapy: past, present and future. Front Endocrinol (Lausanne). 2014;5:224. doi: 10.3389/fendo.2014.00224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beckwith H, Yee D. Minireview: were the IGF signaling inhibitors all bad? Mol Endocrinol. 2015;29(11):1549-1557. doi: 10.1210/me.2015-1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chandarlapaty S. Negative feedback and adaptive resistance to the targeted therapy of cancer. Cancer Discov. 2012;2(4):311-319. doi: 10.1158/2159-8290.CD-12-0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jardim DL, Groves ES, Breitfeld PP, Kurzrock R. Factors associated with failure of oncology drugs in late-stage clinical development: a systematic review. Cancer Treat Rev. 2017;52:12-21. doi: 10.1016/j.ctrv.2016.10.009 [DOI] [PubMed] [Google Scholar]
- 62.Huang F, Chang H, Greer A, et al. IRS2 copy number gain, KRAS and BRAF mutation status as predictive biomarkers for response to the IGF-1R/IR inhibitor BMS-754807 in colorectal cancer cell lines. Mol Cancer Ther. 2015;14(2):620-630. doi: 10.1158/1535-7163.MCT-14-0794-T [DOI] [PubMed] [Google Scholar]
- 63.Scannell JW, Bosley J, Hickman JA, et al. Predictive validity in drug discovery: what it is, why it matters and how to improve it. Nat Rev Drug Discov. 2022;21(12):915-931. doi: 10.1038/s41573-022-00552-x [DOI] [PubMed] [Google Scholar]
- 64.Jones RA, Campbell CI, Wood GA, Petrik JJ, Moorehead RA. Reversibility and recurrence of IGF-IR–induced mammary tumors. Oncogene. 2009;28(21):2152-2162. doi: 10.1038/onc.2009.79 [DOI] [PubMed] [Google Scholar]
- 65.Cook D, Brown D, Alexander R, et al. Lessons learned from the fate of AstraZeneca’s drug pipeline: a five-dimensional framework. Nat Rev Drug Discov. 2014;13(6):419-431. doi: 10.1038/nrd4309 [DOI] [PubMed] [Google Scholar]
- 66.Moser J, Verdin P. Trial watch: burgeoning oncology pipeline raises questions about sustainability. Nat Rev Drug Discov. 2018;17(10):698-699. doi: 10.1038/nrd.2018.165 [DOI] [PubMed] [Google Scholar]
- 67.Fojo T, Mailankody S, Lo A. Unintended consequences of expensive cancer therapeutics—the pursuit of marginal indications and a me-too mentality that stifles innovation and creativity: the John Conley Lecture. JAMA Otolaryngol Head Neck Surg. 2014;140(12):1225-1236. doi: 10.1001/jamaoto.2014.1570 [DOI] [PubMed] [Google Scholar]
- 68.Pegram MD, Konecny G, Slamon DJ. The molecular and cellular biology of HER2/neu gene amplification/overexpression and the clinical development of Herceptin (trastuzumab) therapy for breast cancer. In: Gradishar WJ, Wood WC, eds. Cancer Treatment and Research. Springer US; 2000:57-75. Advances in Breast Cancer Management. doi: 10.1007/978-1-4757-3147-7_4 [DOI] [PubMed] [Google Scholar]
- 69.Shawver LK, Slamon D, Ullrich A. Smart drugs: tyrosine kinase inhibitors in cancer therapy. Cancer Cell. 2002;1(2):117-123. doi: 10.1016/S1535-6108(02)00039-9 [DOI] [PubMed] [Google Scholar]
- 70.Fougner C, Cannon J, The L, Smith JF, Leclerc O. Herding in the drug development pipeline. Nat Rev Drug Discov. Published online April 28, 2023. doi: 10.1038/d41573-023-00063-3 [DOI] [PubMed] [Google Scholar]
- 71.Borup M, Brown N, Konrad K, Van Lente H. The sociology of expectations in science and technology. Technol Anal Strateg Manage. 2006;18:285-298. doi: 10.1080/09537320600777002 [DOI] [Google Scholar]
- 72.Waters RS, Prasad V. How often do highly promising cancer biology discoveries translate into effective treatments? Ann Oncol. 2021;32(2):136-138. doi: 10.1016/j.annonc.2020.10.484 [DOI] [PubMed] [Google Scholar]
- 73.Schilsky RL, Schnipper LE. Hans Christian Andersen and the value of new cancer treatments. J Natl Cancer Inst. 2018;110(5):441-442. doi: 10.1093/jnci/djx261 [DOI] [PubMed] [Google Scholar]
- 74.Cherny NI. An appraisal of FDA approvals for adult solid tumours in 2017-2021: has the eagle landed? Nat Rev Clin Oncol. 2022;19(7):486-492. doi: 10.1038/s41571-022-00636-y [DOI] [PubMed] [Google Scholar]
- 75.Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31-46. doi: 10.1158/2159-8290.CD-21-1059 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Further Details
eTable 1. Calculation of Trial Expenses in $s
eTable 2. Basket Trials Not in the Evaluate Ltd Database With the Estimation of Trial Expenses for the IGF-1R Inhibitor Arm Only
eTable 3. Calculations to Establish the Mean Expenses per Patient per Phase
Data Sharing Statement