Abstract
The cis-acting genomic RNA requirements for the assembly of vesicular stomatitis virus (VSV) ribonucleocapsids into infectious particles were investigated. Using a biological assay based on particle infectivity, we demonstrated that subgenomic replicons that contained all four possible combinations of the natural genomic termini, the 3′ leader (Le) and 5′ trailer (Tr) regions, were replication competent; however, a 3′ copyback replicon (3′CB), containing the natural 3′ terminus but having the 5′ Tr replaced by a sequence complementary to the 3′ Le for 46 nucleotides, was unable to assemble infectious particles, despite efficient replication. When a copy of Tr was inserted 51 nucleotides from the 5′ end of 3′CB, infectious particles were produced. However, analysis of the replication products of these particles showed that the 51 nucleotides which corresponded to the Le complement sequences at the 5′ terminus were removed during RNA replication, thus restoring the wild-type 5′ Tr to the exact 5′ terminus. These data showed that a cis-acting signal was necessary for assembly of VSV RNAs into infectious particles and that this signal was supplied by Tr when located at the 5′ end. The regions within Tr required for assembly were analyzed by a series of deletions and exchanges for Le complement sequences, which demonstrated that the 5′ terminal 29 nucleotides of Tr allowed assembly of infectious particles but that the 5′ terminal 22 nucleotides functioned poorly. Deletions in Tr also altered the balance between negative- and positive-strand genomic RNA and affected levels of replication. RNAs that retained fewer than 45 but at least 22 nucleotides of the 5′ terminus could replicate but were impaired in RNA replication, and RNAs that retained only 14 nucleotides of the 5′ terminus were severely reduced in ability to replicate. These data define the VSV Tr as a position-dependent, cis-acting element for the assembly of RNAs into infectious particles, and they delineate RNA sequences that are essential for negative-strand RNA synthesis. These observations are consistent with, and offer an explanation for, the absence of 3′ copyback defective interfering particles in nature.
Generation of infectious particles of vesicular stomatitis virus (VSV), an enveloped nonsegmented negative-strand RNA virus, requires first the interaction of the viral genome with the nucleocapsid (N) protein to form the ribonucleocapsid. This process, called encapsidation, occurs concomitant with genome replication (31), to produce the tightly encapsidated full-length genomes and antigenomes. The subsequent assembly of the ribonucleocapsids into infectious particles requires the interaction of the ribonucleocapsid complex with the matrix (M) protein and the plasma membrane which has been altered by the insertion of the transmembrane viral glycoprotein (G). This latter process, referred to here as assembly, results in the budding of infectious particles. Thus, assembly represents a key step in the viral replication cycle at which a variety of protein-protein, protein-lipid, and protein-nucleic acid interactions must occur.
VSV has been used as a model system to investigate several steps in the process of assembly of an enveloped virus. Consequently, the overall structure of the virus and its composition are known (25, 40). The requirements for incorporation of glycoproteins into the plasma membrane (37) and also certain aspects of matrix protein interaction with the viral nucleocapsid (18, 26), the glycoprotein (22), and the host cell membrane (8) have been characterized. It is expected that specific cis-acting signals are required for encapsidation and assembly of VSV RNAs. These signals result in the selective encapsidation of the replication products compared with other viral and cellular RNAs and in the assembly of predominantly negative-sense genomic ribonucleoprotein (RNP) complexes into mature particles (4, 33, 35; for a review, see reference 2).
The signals required for the encapsidation process are poorly defined, but in vitro analyses have indicated that two short RNAs, the Le+, produced from the 3′ Le of the genomic RNA, and the Le−, made from the 3′ end of the antigenomic RNA, can be encapsidated by N protein (5, 6). While the evidence for Le− encapsidation remains controversial (41, 47), it seems likely that the leader RNAs contain essential encapsidation signals because RNAs that lack these sequences are not encapsidated. Available evidence suggests that the 5′ terminal 15 to 19 nucleotides (nt) of the leader RNAs are crucial for encapsidation (24, 38). It also has been postulated that A residues, repeated at every third nucleotide of the first 15 nt, of both Le+ and Le− may function as the nucleation sites for encapsidation (5, 6).
Current understanding of the genomic RNA signals required for assembly and budding of encapsidated VSV RNAs is based on a deletion analysis of a cDNA clone of a naturally occurring 5′ copyback defective interfering (DI) particle, DI-T (28). This analysis demonstrated that the 5′ terminal 51 nt together with its complement at the 3′ terminus flanking a heterologous RNA were sufficient to signal the encapsidation, replication, assembly, and budding of infectious particles. This study, however, was unable to demonstrate whether a specific cis-acting element was required for assembly, as all RNAs that were competent templates for replication also assembled and budded infectious particles.
In the work described here, we used a biological assay to measure the ability of subgenomic RNAs containing altered terminal sequences to assemble and bud infectious particles. This biological assay, which relied on the ability of correctly assembled particles to be infectious, was chosen to circumvent problems such as liposomes released from cells expressing M protein (19) and virus particles assembled in the absence of G protein (23). In contrast, the assay used here depends on the presence of all five VSV proteins to assemble infectious particles (29, 39). In this study, the assay was used to demonstrate that a specific RNA signal is required for assembly of VSV RNAs into infectious particles and that the 5′ terminal Tr of the genome is an essential component of this signal. This requirement cannot be provided by the 3′ terminus or its complement, a finding that provides an explanation for the absence of 3′ copyback DI RNAs in nature.
MATERIALS AND METHODS
Plasmid construction.
Previously we described the construction of cDNA clones of VSV DI-T, called pDI 2,0 (29), and a subgenomic replicon that contained the wild-type leader and trailer regions of the VSV genome, called p8(−) (42). The subgenomic replicon, p8(−), referred to here as the WT (wild-type) replicon, comprised the 3′ terminal 210 nt of the wild-type VSV genome fused to the 5′ terminal 265 nt of the genome. The VSV sequences of DI-T are derived from the 5′ terminal 2.2 kb of the genome, but the 3′ terminus is a complementary copy of the 5′ terminus for 45 nt. These VSV sequences were inserted in plasmids between a copy of the promoter for bacteriophage T7 RNA polymerase and a cDNA copy of the self-cleaving ribozyme from the antigenomic strand of hepatitis delta virus (HDV), such that the resultant plasmids directed transcription of negative-sense VSV RNA. T7 transcripts contained two non-VSV nucleotides (GG) at their 5′ ends and were cleaved by the HDV ribozyme to generate a 3′ terminus that corresponded precisely to the 3′ end of the VSV sequence, which is an essential requirement for RNA replication (29). Alterations were engineered into the termini of the WT replicon and pDI-T, using standard techniques (34), to generate a panel of engineered cDNAs. The terminal sequences of each of the replicons are given in the figures where they are first represented and were confirmed by sequence analysis through the altered regions.
Analysis of RNA synthesis.
To analyze the effects of alterations to pWT or pDI on RNA synthesis, cDNA clones designed to produce altered VSV RNAs were transfected into vTF7-3 (15)-infected baby hamster kidney (BHK-21) cells, together with plasmids encoding the VSV N, P (phosphoprotein), and L (large polymerase subunit) proteins, as described elsewhere (29, 42). Following transfection, cells were incubated at 37°C for 15 h and then were exposed for 5 h to [3H]uridine (33 μCi/ml) in the presence of actinomycin D-mannitol (ActD) (10 μg/ml) or, for analysis of immunoprecipitable RNAs cells, were exposed to [3H]uridine (33 μCi/ml) for 8 h as described previously (29). Cells were harvested, cytoplasmic extracts were prepared, and either total RNA or N-protein-encapsidated RNA was analyzed by electrophoresis on 0.025 M citrate (pH 3.0)–1.75% agarose–6 M urea gels as described previously (29). Autoradiographs of fluorogrammed gels were scanned and quantitated with a PDI densitometer 320i. To confirm that deletions or insertions engineered into the 5′ Tr were maintained on passage, the 5′ ends of the minus-sense replication products generated from infectious particles were analyzed by primer extension using Moloney murine leukemia virus reverse transcriptase (GIBCO/BRL) and a primer that annealed to negative-sense RNA in the L gene at positions 10908 to 10925 of the complete VSV genome sequence.
Infectivity assay.
An infectivity assay was used to determine whether VSV RNAs were assembled and budded into infectious particles. In this assay, (outlined schematically in Fig. 1), cDNAs encoding the VSV RNA of interest were transfected into cells together with plasmids encoding the VSV N, P, M, G, and L proteins (29). At 36 h posttransfection, the supernatant fluids from 106 transfected cells (1.5 ml in total) were harvested and cellular debris was removed by centrifugation (14,000 × g, 5 min). The presence of budded infectious VSV particles was monitored by passage of 0.5 ml of these supernatants onto fresh BHK-21 cells that had been transfected 5 h previously with plasmids encoding the VSV N, P, and L proteins, required to support replication. Following a 45-min adsorption, the inoculum was removed, 1.5 ml of culture medium was added, and the cells were incubated for 7 to 13 h, at which time VSV-specific RNAs were analyzed as described above. During evaluation of the infectivity assay, qualitatively similar results were obtained in assays using a variety of support plasmid concentrations and labeling times. Consequently, each infectivity assay was performed with the same conditions (8.0 μg of VSV replicon; 6.0 μg of N, 3.3 μg of P, 2.0 μg of L, 5.0 of μg M, and 5.0 μg of G plasmids).
FIG. 1.
Diagram of the infectivity assay. Details of the assay are described in Materials and Methods. Plasmids are represented by circles with an arrow to highlight the promoter for T7 RNA polymerase (T7 pro). vTF7-3, is a recombinant vaccinia virus expressing T7 RNA polymerase.
RESULTS
Examination of the role of the VSV genomic termini in assembly.
To assess the role of the 3′ Le and 5′ Tr of VSV in assembly of infectious particles, the subgenomic replicon WT, with wild-type termini 3′ Le-Tr 5′, was altered to have either 5′ copyback termini 3′ TrC-Tr 5′ (5′CB), where TrC indicates the complement of Tr, 3′ copyback termini 3′ Le-LeC 5′ (3′CB), or switched termini 3′ TrC-LeC 5′ (Inv), as shown in Fig. 2A. The effects of these exchanges on RNA synthesis was examined by transfection of cDNA clones of each replicon into BHK-21 cells along with plasmids encoding the VSV N, P, and L proteins and was monitored by metabolic labeling with [3H]uridine in the presence of ActD. Cytoplasmic extracts were prepared, and the total RNA was analyzed by electrophoresis on agarose-urea gels as described in Materials and Methods. The products of RNA replication and transcription directed by each of these replicons are identified in the accompanying report (45) and are shown here (Fig. 2B) as essential controls for the infectivity assay.
FIG. 2.
Role of the VSV genomic termini in RNA synthesis and assembly of infectious particles. (A) Arrangements of the termini. A region of the T7 transcription plasmid, pWT, is shown to illustrate the sections of the VSV genome, the T7 promoter, the HDV ribozyme (δ), and the T7 terminator (TΦ). The negative-sense primary T7 transcripts transcribed from pWT are shown to illustrate the sequence of the wild-type 3′ Le and 5′ Tr. Numbers indicate nucleotides from the exact VSV genomic 3′ or 5′ terminus and do not include the two G residues (gg) present at the 5′ end of the primary transcripts. pWT was altered to generate three additional plasmids designed to generate RNAs with 5′ copyback (5′CB), 3′ copyback (3′CB), or inverted (Inv) termini. Substitution of the 3′ Le for 46 nt of TrC was performed such that the sequence and position of the nontranscribed Le-N junction (boxed) and N gene start site (underlined), which are important requirements for transcriptional initiation, remained as in the WT replicon. Substitution of the 5′ Tr by 46 nt of LeC was performed so that the size of Tr was unaltered. Sequences of these modified termini are shown to illustrate the nucleotide differences from WT. (B) RNA synthesis. Cells were infected with vTF7-3, transfected with cDNAs for the VSV genomic analog WT, 5′CB, 3′CB, or Inv, as indicated, and with plasmids encoding the VSV N, P, and L proteins, and exposed to [3H]uridine (33 μCi/ml) in the presence of ActD (10 μg/ml) as described in Materials and Methods. Cytoplasmic extracts were prepared, and total RNA was analyzed by electrophoresis on agarose-urea gels and visualized by fluorography, as described in Materials and Methods. (C) Infectivity assay. Shown is agarose-urea gel analysis of VSV-specific RNAs synthesized in BHK-21 cells that were transfected with the VSV N, P, and L support plasmids and infected with 0.5 ml of clarified supernatants from cells that were transfected 36 h previously with cDNAs encoding a VSV genomic analog (WT, 5′CB, 3′CB, or Inv) and the VSV N, P, L, M, and G support plasmids as described in Materials and Methods. Genomic analogs that produced RNAs that were assembled and budded infectious particles into the culture media were detected by the ability to initiate VSV-specific RNA synthesis in these infected cells. Rep and Tx represent replication and transcription (N/L mRNA) products; respectively.
Since each replicon was competent to replicate, we used the infectivity assay to examine whether they could assemble and bud infectious particles. Plasmids encoding each of the replicons were first transfected into BHK-21 cells together with plasmids encoding the VSV N, P, L, M, and G proteins. After 36 h, supernatant fluids were harvested, clarified, and assayed for the presence of budded infectious particles by testing their ability to initiate an infection of fresh BHK-21 cells. Since these particles were derived from subgenomic replicons, the trans-acting factors required for RNA synthesis, the N, P, and L proteins, were supplied by transfection of the appropriate plasmids into the fresh cells. Virus-specific RNA synthesis was analyzed following infection of BHK-21 cells with supernatant fluids obtained from transfections of each of the above four replicons. WT, 5′CB, and Inv were competent to assemble into infectious particles and initiate an infection of fresh BHK-21 cells (Fig. 2C, lanes 1, 2, and 4). In contrast, despite the high level of replication directed by 3′CB in the primary cDNA transfection, RNA synthesis was not observed in the infectivity assay (Fig. 2C, lane 3). These data demonstrated that high levels of RNA replication alone were not sufficient to mediate assembly of ribonucleocapsids into infectious particles. Since replicon 3′CB lacked the 5′ trailer region of the genome, these data demonstrated that the trailer contained an essential cis-acting element for assembly.
Localization of the sequences in Tr required for assembly of infectious particles: effects of exchanges between the 5′ termini of WT and 3′CB.
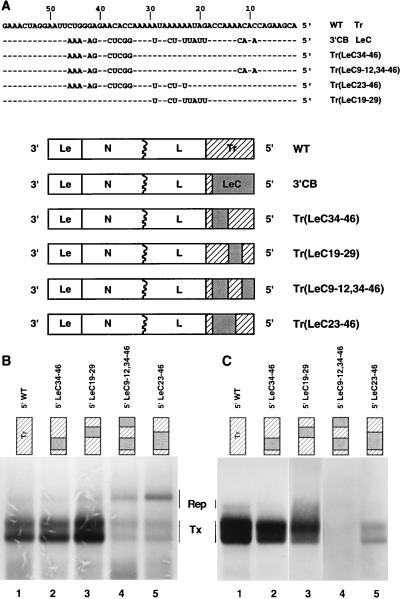
The sequences of the 5′ termini of replicons WT (3′ Le-Tr 5′), which generated infectious particles, and 3′CB (3′ Le-LeC 5′), which did not, differ at 21 nucleotide positions over a region of 46 nt (Fig. 3A). As these were the only sequence changes between WT and 3′CB, we concluded that they were responsible for the failure of replicated 3′CB RNAs to assemble into infectious particles. To localize the assembly signal to a specific region of Tr, it was not practical to make all possible permutations of these 21 nucleotide differences. Therefore, groups of nucleotides that contained these differences were exchanged between LeC and Tr to generate chimeric 5′ termini. Four plasmids were constructed, and sequences of the altered regions are shown in Fig. 3A. These plasmids were named to reflect the nucleotides of authentic Tr that were replaced by the complement of the genomic 3′ Le, (LeC), i.e., Tr(LeCX-X), where X indicates the nucleotide residues that are LeC sequence.
FIG. 3.
Exchanges between the 5′ termini of WT and 3′CB. (A) Sequences of altered Tr regions. Four subgenomic replicons, Tr(LeC34-46), Tr(LeC23-46), Tr(LeC9-12,34-46), and Tr(LeC19-29), that contained 5′ terminal sequences derived from both LeC and the natural Tr are shown. To illustrate the sequence differences between Tr and LeC, the 5′ termini of WT and 3′CB are shown. (B) RNA synthesis, determined by agarose-urea gel analysis of VSV-specific RNAs generated from genomic analogs with altered Tr sequences. RNAs were labeled with [3H]uridine in the presence of ActD and analyzed as described in Materials and Methods. The products of replication and transcription by the VSV polymerase are indicated by Rep and Tx, respectively. (C) Infectivity assay. Shown is agarose-urea gel analysis of VSV-specific RNAs generated by infectious particles recovered from genomic analogs containing altered Tr sequences. Supernatants were generated and tested for infectious particles by the infectivity assay described in Materials and Methods.
The effect of each of these alterations on RNA synthesis and assembly of infectious particles was then examined. RNA synthesis was affected in a complex manner that reflected the fact that levels of transcription and replication are affected by both the sequence and the extent of complementarity between the termini (45). All four of the replicons replicated at least as well as WT (Fig. 3B, lanes 1 to 5); thus, any potential effects on the assembly of infectious particles could not be attributed to a defect in RNA replication. The effect of these exchanges on assembly of infectious particles was examined in the infectivity assay. Replicon Tr(LeC34-46), in which the 5′ terminal 33 nt were wild-type Tr, produced infectious particles (Fig. 3C, lane 2). However, when in addition positions 9, 11, and 12 were LeC sequence, as found for replicon Tr(LeC9-12,34-46), infectious particles were not recovered (Fig. 3C, lane 4). This demonstrated that the 5′ terminal 33 nt of Tr were able to mediate efficient assembly of infectious particles and further that the sequences at positions 9, 11, and 12 were important for assembly. While positions 9 to 12 of Tr were clearly important, replicon Tr(LeC23-46), which had the 5′ terminal 22 nt of Tr, functioned only poorly in the infectivity assay (Fig. 3C, lane 5), thus demonstrating that additional sequences were required for efficient assembly of infectious particles. That these additional sequences could also be derived from nt 34 to 46 of trailer was shown by Tr(LeC19-29), which assembled infectious particles (Fig. 3C, lane 3). While the assay used here does not measure assembly quantitatively, the abundance of the RNA species in the infectivity assay suggested that none of the replicons having mutations in Tr assembled into infectious particles as readily as WT (compare Fig. 3B and C). Taken together, these data confirmed that genomic Tr contained an essential signal for assembly and demonstrated that nt 9 to 12 were important but other Tr sequences also were required for assembly. These additional requirements were provided by the unique sequences present between nt 19 and 29 or 34 and 46 but not by those present between nt 19 and 22.
Effect of insertion of Tr at an internal site of 3′CB.
The above data confirmed that Tr contained an essential signal for assembly of RNA into infectious particles. However, the pleiotropic effects of alterations engineered into the genomic Tr provided a significant challenge in mapping the assembly signal. This is because, in addition to its role in assembly, the 5′ end of the genome contains signals for N protein encapsidation, and its complement, the 3′ end of the positive strand, acts as the promoter for negative-strand synthesis. In an attempt to dissect out the assembly signal without affecting the signals for encapsidation and replication, we inserted a copy of the 5′ terminal 51 nt of Tr at an internal site of replicon 3′CB. We postulated that the encapsidation and replication functions might be provided by LeC present at the 5′ end, leaving the internal Tr sequence available for a mutational analysis of the assembly signal.
The 5′-most 51 nt of the trailer region were introduced in either orientation at a naturally occurring AflII site, 51 nt from the genomic 5′ end of 3′CB. This created two replicons, 3′CB/Tr and 3′CB/TrC, that were named to reflect the orientation of the inserted trailer (Fig. 4A). The effects of these insertions on RNA synthesis and assembly of infectious particles were determined. Both 3′CB/Tr and 3′CB/TrC replicated well in comparison to WT, but 3′CB/TrC replicated less well than its parent 3′CB, whereas 3′CB/Tr replicated at even higher levels than 3′CB (Fig. 4B). The greatest differences in RNA synthesis were in the levels of mRNA transcribed. 3′CB/Tr transcribed at levels higher than the parent 3′CB levels and just slightly less than levels for WT, whereas 3′CB/TrC transcribed similarly to 3′CB (Fig. 4B).
FIG. 4.
Insertion of Tr at an internal site of replicon 3′CB. (A) 3′CB genomic analogs with Tr insertions. The 5′ terminal 51 nt of the genomic Tr were introduced in both orientations at a unique AflII site 51 nt from the 5′ end of mutant 3′CB, to yield two subgenomic replicons, 3′CB/Tr and 3′CB/TrC. The structures of 3′CB/Tr and 3′CB/TrC are shown, together with the sequence of the inserted nucleotides. The nucleotides that correspond to the AflII compatible ends are underlined. (B) RNA synthesis, determined by agarose-urea gel analysis of VSV-specific RNAs generated from 3′CB replicons with inserted Tr sequences. RNAs were labeled with [3H]uridine in the presence of ActD and analyzed as described in Materials and Methods. The products of replication and transcription by the VSV polymerase are indicated by Rep and Tx, respectively. (C) Infectivity assay. Shown is agarose-urea gel analysis of RNAs generated by infectious particles recovered from genomic analogs containing inserted Tr sequences. Supernatants were generated and assayed for infectious particles by using the infectivity assay described in Materials and Methods. WT and 3′CB replicons are shown as positive and negative controls, respectively.
As both 3′CB/Tr and 3′CB/TrC replicated, we next examined their ability to assemble and bud infectious particles in the infectivity assay. RNA synthesis was not detected for replicon 3′CB/TrC, suggesting that like 3′CB, it lacked a functional assembly signal (Fig. 4C, lanes 2 and 3). RNA synthesis was observed for 3′CB/Tr in the infectivity assay, indicating that nucleocapsids from 3′CB/Tr were assembled into infectious particles (Fig. 4C, lane 4). However, the pattern of RNA synthesis for 3′CB/Tr now differed from that observed during the primary cDNA transfection, in that levels of replicated RNA were reduced and transcription of the N/L mRNA was increased (compare lanes 4 in Fig. 4B and C). In fact, the observed pattern of RNA synthesis resembled that of WT (Fig. 4C, lane 1). This prompted a further investigation of these RNA species.
Extensions to the authentic VSV 5′ termini are removed during RNA replication.
Previously we demonstrated that small (2- and 4-nt) extensions at the 5′ terminus of VSV genomic RNAs generated by T7 RNA polymerase in the primary transfection were removed by the VSV polymerase during subsequent RNA replication (29, 42). Therefore, we tested whether LeC, which was now a 51-nt extension beyond the end of Tr at the 5′ end of 3′CB/Tr (Fig. 4A), was removed during RNA replication. Primer extension analyses were performed with an oligonucleotide primer that annealed to residues 10908 to 10925 of the complete VSV genome sequence (Fig. 5). Following extension by Moloney murine leukemia virus MMLV reverse transcriptase, this primer should yield a product of 136 nt from extension on the negative-sense replication product of WT and 3′CB or a product of 187 nt from extension on the replication product of 3′CB/TrC and 3′CB/Tr. In addition, products that corresponded to the primary negative-sense T7 RNA polymerase transcripts were expected. These should be readily distinguished from the negative-sense replication product because as a consequence of the plasmid construction, the primary transcripts contained two additional G residues at their 5′ termini. In addition, products of the VSV polymerase were distinguished from those of T7 RNA polymerase, by primer extension analysis of RNAs purified from cDNA transfections that lacked the plasmid (L) that encodes the large subunit of the viral polymerase (Fig. 5A, lanes 5 to 8), and hence could not be replicated. Primer extension products of the predicted sizes were detected for WT, 3′CB, 3′CB/TrC, and 3′CB/Tr when RNAs extracted from cDNA transfections were analyzed (Fig. 5A). The most abundant products corresponded to the T7 transcripts. The 187-nt product corresponded to the minus-sense replication product of 3′CB/Tr in which the T7-transcribed two G residues were removed. An additional product of 136 nt which comigrated with the primer extension product obtained from the WT replicon (Fig. 5A, lane 4) was observed for replicon 3′CB/Tr (Fig. 5A, lane 1), suggesting that the 51 nt that corresponded to LeC were removed during replication.
FIG. 5.
Primer extension analysis on the negative-sense RNAs generated in transfections of 3′CB replicons that contained inserted Tr sequences. (A) Polyacrylamide gel analysis of the primer extension products obtained from total cytoplasmic RNAs extracted from BHK-21 cells that were transfected 23 h earlier with cDNAs of either the 3′CB, 3′CB/Tr, 3′CB/TrC, or WT replicon together with the VSV N and P support plasmids in the presence (+Pol) or absence (−Pol) of the L support plasmid. The oligonucleotide primer (large arrow) annealed to negative-sense RNA at residues 10925 to 10908 of the complete VSV genome sequence. The schematic of the genome of 3′CB/Tr depicts the positions at which the polymerase-dependent products map. (B) Primer extension analysis of the products of replication from WT and 3′CB/Tr in the infectivity assay. A sequence ladder of 3′CB/TrC sequenced with the same primer is shown for reference.
When RNAs obtained from the infectivity assay were analyzed, the 136-nt product was detected for both WT and 3′CB/Tr (Fig. 5B, lanes 1 and 2). In contrast, the 187-nt product observed for 3′CB/Tr in the primary cDNA transfection was not detected. These data indicated that the 51 nt of leader complement were removed from 3′CB/Tr during RNA replication, as RNAs which contained these 51 nt were not observed in the infectivity assay. A series of products approximately 170 nt in size were also observed for both WT and 3′CB/Tr. These latter products were attributed to nonspecific background, as any product larger than 136 nt would correspond to extension of the primer beyond the wild-type genomic 5′ terminus. Taken together, these findings suggested that the assembly signal cannot function internally and showed that a 51-nt extension was removed during replication of 3′CB/Tr. Whether this was achieved by internal initiation or premature termination of either replication or encapsidation is not known.
Effect on assembly of deletions within the genomic 5′ trailer.
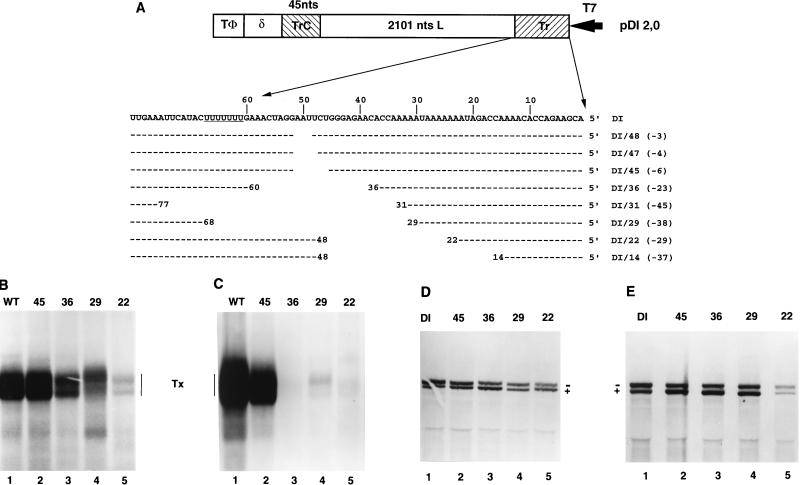
The above data demonstrated that the VSV genomic Tr contained a signal for assembly and budding of infectious particles. To further define this requirement, we made a series of deletions at the 5′ genomic terminus of cDNAs encoding the WT replicon or a second replicon that encoded the naturally occurring DI RNA, DI-T. These deletions were generated from the naturally occurring AflII site located 51 nt from the 5′ end of both the WT replicon and DI-T, using either exonuclease III or site-directed mutagenesis to yield plasmids designed to generate WT or DI-T RNAs in which the authentic VSV genomic 5′ termini were incrementally reduced from 51 to 22 nt. These were named to reflect the number of authentic nucleotides remaining at their 5′ termini, and their sequences are shown in Fig. 6A.
FIG. 6.
Deletions in Tr. (A) Sequences of altered Tr regions of WT and DI-T 5′ deletions. Deletions were generated by using exonuclease III at a unique AflII site located 51 nt in from the genomic 5′ terminus or by site-specific mutagenesis. Each construct is designated WT/X or DI/X, where X is the number of nucleotides at the genomic 5′ terminus that remain. Numbers shown in parentheses are total numbers of nucleotides removed, and positions of nucleotide identity are indicated by dashes. The structure of the WT replicon is shown in Fig. 2; for comparison, the structure of the DI-T replicon is shown here. Note that the deletions were identical for both WT and DI-T. T7, T7 promoter; δ, HDV ribozyme; TΦ, T7 terminator. (B and D) RNA synthesis, determined by agarose-urea gel analysis of VSV-specific RNAs synthesized in cells transfected with the indicated WT (B) or DI-T (D) variants and the VSV N, P, and L support plasmids. RNAs were labeled, harvested, and analyzed as described in the text. Note that the positive (+) and negative (−) strands of DI-T separate under these gel conditions. (C and E) Infectivity assay, determined by agarose-urea gel analysis of VSV-specific RNAs synthesized in cells transfected with the VSV N, P, and L support plasmids and infected with 0.5 ml of the clarified supernatant fluid harvested from transfections that received the VSV N, P, L, M, and G support plasmids and either a WT (C) or DI-T (E) replicon that contained deletions in Tr. Transfections, infections, and RNA analysis were performed as described in Materials and Methods. Tx, products of transcription.
In the subgenomic WT replicon, RNA synthesis was suppressed by deletions in Tr that reduced the 5′ terminus below 45 nt, and larger deletions decreased levels of RNA synthesis further (Fig. 6B). Analysis of the ability of these deleted RNAs to assemble showed that infectious particles were readily detected only from WT and WT/45 (Fig. 6C); however, on a longer exposure of the autoradiograph shown here, a faint signal was observed for WT/29. These analyses confirmed that the genomic Tr was essential for assembly of RNAs into infectious particles, but as a consequence of the reduction of RNA synthesis, these studies were unable to further define the region of trailer required for assembly of infectious particles.
Analyses of the same deletions at the 5′ terminus of DI-T, which replicates to higher levels than the WT replicon, revealed that each was an effective template for RNA replication, although levels decreased as the extent of the deletion increased (Fig. 6D). As each of the deletions was an effective template for replication, we examined their ability to assemble infectious particles. Consistent with the exchanges shown in Fig. 3, the DI-T deletion mutants were efficiently assembled into infectious particles if they retained at least 29 nt of the 5′ Tr (Fig. 6E, lanes 1 to 4). Primer extension analysis of the negative-sense replication products generated in the infectivity assay confirmed that these deletions were maintained (data not shown). RNAs that retained less than the 5′ terminal 29 nt were severely impaired in the ability to assemble into infectious particles. On the exposure of the autoradiograph shown, a small amount of RNA synthesis was observed for DI/22 in the infectivity assay, again confirming that 22 nt of the genomic 5′ trailer functioned poorly in assembly (Fig. 6E, lane 5). These data confirm that the genomic 5′ terminus provided an essential cis-acting element for the budding of infectious particles and that for DI-T this was effectively provided by the 5′ terminal 29 nt.
While each of the deleted DI RNAs that retained at least 22 nt of the 5′ terminus was an effective template for RNA replication, a deleted RNA that retained only 14 nt replicated inefficiently (45). In addition, it was evident that the overall levels of replicated RNA were reduced as the extent of the deletion increased. The effect of these deletions on RNA replication was quantitated following a separate analysis of immunoprecipitated RNAs (Table 1). Immunoprecipitation separated the encapsidated RNAs that were replicated by the VSV polymerase from any T7 transcripts. Quantitation of the total amount of replicated RNA revealed that as the deletions reduced the 5′ terminus below 45 nt, the level of replication was progressively reduced. In addition, the ratios of positive- and negative-strand RNA synthesized by these deleted DI RNAs altered. For the parent wild-type DI RNA, a ratio of 56% negative-strand RNA to 44% positive-strand RNA was observed in infected cells (Table 1), whereas the ratio for DI/48, which lacked 3 nt within the trailer, was 44:56. This bias was even more pronounced for the larger deletions, resulting in an observed ratio of 31:69 for DI/29 (Table 1). These data demonstrated that RNAs that retained as few as the 5′ terminal 22 nt of the genome functioned as templates for negative-strand RNA synthesis, but that 45 nt were required for maximal efficiency. In addition, they demonstrated that the genomic Tr played a crucial role in controlling the balance of positive- and negative-strand RNA synthesis. However, whether these effects were mediated by alterations to essential signals for encapsidation that presumably lie in the Tr at the 5′ end of the negative strand or to the promoter for negative-strand RNA synthesis, the TrC found at the 3′ end of the positive strand, could not be distinguished by these experiments.
TABLE 1.
Effects on RNA synthesis and assembly of deletions at the 5′ terminus of DI-T
| DI | Replication (% DI-T)a | Minus strand (%)a | Plus strand (%)a | Assembly |
|---|---|---|---|---|
| DI-T | 100 | 56 | 44 | Yes |
| DI/48 | 125 | 44 | 56 | Yes |
| DI/47 | 125 | 46 | 54 | Yes |
| DI/45 | 125 | 40 | 60 | Yes |
| DI/36 | 36 | 37 | 63 | Yes |
| DI/31 | 44 | 35 | 65 | Yes |
| DI/29 | 46 | 31 | 69 | Yes |
| DI/22 | ND | ND | ND | Low |
| DI/14 | tr | ND | ND | No |
Average of two to four independent experiments using immunoprecipitated RNAs. Standard deviations ranged from 1.0 to 3.0. Not all of these DIs are represented in the autoradiograph shown in Fig. 6. ND, not determined.
DISCUSSION
The work described here demonstrated that a specific cis-acting signal was required for assembly of VSV RNAs into infectious particles and that an essential component of this signal was supplied by the genomic 5′ Tr. Alterations to the Tr sequence mapped both essential and dispensable regions for assembly, and attempts to locate Tr at a position 51 nt internal to the 5′ end indicated that the assembly function could not be provided from an internal site. In addition, deletions engineered into Tr affected RNA replication, in particular the control of minus-strand RNA synthesis. Whether these effects were mediated by modification of the signals required for N protein encapsidation or by changes to the negative-strand promoter were not determined. These findings define cis-acting requirements for assembly of VSV RNAs into infectious particles, delineate the requirements at the genomic 5′ terminus for replication, have implications for DI particle formation, and emphasize that mutations engineered into the genomic Tr affect multiple processes.
Assembly.
Specific cis-acting genomic signals have been identified for assembly of some classes of RNA viruses (reviewed in references 1, 13, and 36). For some enveloped RNA viruses, cis-acting signals for encapsidation of the genome with the nucleocapsid protein have been addressed, but the subsequent assembly and release of infectious particles have been attributed to specific protein-protein interactions in, for example, retroviruses (reviewed in reference 17) and alphaviruses (20). For the negative-strand RNA viruses, the requirements for encapsidation of the genome with nucleocapsid protein and assembly of infectious particles have been implicated as being located at the termini (3, 7, 9–11, 21, 27). However, a requirement for specific cis-acting signals for assembly has not been described in studies using these systems.
The demonstration here that a VSV subgenomic replicon, 3′CB, replicated to high levels but was unable to generate infectious particles showed that a specific signal for assembly and budding of infectious VSV particles exists and that high levels of RNA replication alone did not mediate nonspecific assembly of infectious particles. In contrast, an alphavirus-based vector that encoded the Semliki Forest virus polymerase (nsP1-4) and the VSV G protein spread throughout a monolayer of BHK-21 cells and thus represented a simple enveloped infectious agent (32). However, this nonspecific packaging of RNA into budding vesicles was a consequence of the amount of G protein and RNA produced in the cell and may have been facilitated by the localization of Semliki Forest virus replication complexes in membranous compartments at the cell surface (14). In contrast, the assembly process investigated in this study depended on the presence of all five VSV proteins for the budding of infectious particles (30, 39).
Further evidence that the genomic Tr contained an essential assembly signal was provided by replicons with chimeric 5′ termini that contained regions of LeC and natural Tr. That the entire sequence of Tr was not required for assembly of infectious particles was shown by replicon Tr(LeC34-46), which produced infectious particles but has only the 5′ terminal 33 nt of the natural Tr. This finding was further substantiated by the deletions at the 5′ end of DI-T, which demonstrated that the 5′ terminal 29 nt allowed assembly and budding of infectious particles, whereas in both the subgenomic replicon exchanges and the DI-T deletions, the 5′ terminal 22 nt functioned poorly. An examination of the sequences required within the 5′ terminal 29 nt showed that the signal for assembly was complex. Replicon Tr(LeC 9-12,34-46) showed that nt 13 to 33 of natural Tr were unable to mediate assembly and thus demonstrated that the three nucleotide differences found at positions 9, 11, and 12 of trailer were important. In addition, Tr(LeC19-29) demonstrated that the unique sequences found between nt 19 and 29 of the natural trailer were not essential for assembly. However, the unique sequences present between nt 23 and 29 of the natural Tr could influence the efficiency of assembly, as shown by Tr(LeC23-46), which assembled only poorly. One interpretation of these data is that each of these mutations in Tr reduced the efficiency of assembly. In addition, these data may indicate that the assembly signal is multipartite. However, in the absence of a quantitative assay, the extent to which these mutations down-regulated assembly could not be determined.
Insertion of the 5′ Tr 51 nt internal to the 5′ end of 3′CB resulted in the generation of infectious particles, confirmed that Tr contained an essential signal for assembly of infectious particles, and suggested that the signal must be located at the 5′ terminus. Further evidence in support of this was provided by replicon 3′CB/TrC, which had a copy of Tr in the positive strand but was unable to assemble and bud infectious particles. Analysis of the 3′CB/Tr replication products during the cDNA transfection demonstrated that a 51-nt extension that corresponded in size to the complementary copy of Le was removed during RNA replication, and only these RNAs were detected in the infectivity assay. Combined with the observation that RNA synthesis directed by these particles was indistinguishable from that of the WT replicon, these observations suggested that 3′CB/Tr had restored the wild-type termini and that only RNAs with a 5′ trailer were capable of budding into infectious particles. Whether the 51-nt extension was removed by internal initiation or premature termination of either N protein encapsidation or RNA replication is unknown, but irrespective of the mechanism, these data show that in contrast to the requirement for Tr to be at the 5′ terminus for assembly, it can function internally during RNA replication. In other cases where terminal extensions were removed during RNA replication, these were short (usually 2 to 4 nt) and of non-VSV origin (29, 42). In contrast, the extension removed here was a VSV sequence (LeC) that enhanced RNA replication (compare the levels of replicated RNA for 3′CB/Tr and WT in Fig. 4B). What drives this removal is unknown, but the subsequent selective advantage that the corrected RNAs have in assembly appears to overcome any apparent replicative disadvantage.
In contrast to our findings that the genomic Tr contains an essential signal for assembly, a rabies virus with 3′CB termini exhibited indiscriminate packaging of positive- and negative-sense RNPs into particles (12), demonstrating that the 5′ Tr was not required for assembly. However, such a terminal arrangement abrogated recovery of virus from an infectious cDNA clone of VSV (43), presumably as a consequence of the inability of 3′CB RNAs to assemble and the down-regulation of transcription observed for 3′CB (45).
DI particle formation.
During high-multiplicity infection, VSV readily generates DI RNAs which out compete viral genome-length RNAs for essential trans-acting factors. During RNA replication, there are two permissible initiation sites for the polymerase: the 3′ Le, and the 3′ TrC. Consequently, a total of four arrangements of the termini should allow replication of any RNA molecule by the VSV polymerase: 3′ Le-Tr 5′, 3′ TrC-Tr 5′, 3′ Le-LeC 5′, and 3′ TrC-LeC 5′. However, among the naturally occurring DI RNAs, none that have the terminal structure 3′ Le-LeC 5′ have been reported (for reviews, see reference 16 and 44). In the work reported here, we constructed subgenomic replicons that contained each of these possible terminal organizations and showed that while all could replicate, the 3′ Le-LeC 5′ (3′CB) arrangement was unable to assemble into infectious particles. Thus, as evidenced by replicon 3′CB, VSV RNAs of such a structure replicate with high efficiency but are unable to assemble infectious particles. These observations provide an explanation for the absence of 3′ CB DI RNAs in nature.
Replication.
During RNA replication, the genomic Tr is copied to become the 3′ end of the positive strand, which subsequently is recognized by the polymerase to promote minus-strand RNA synthesis. Therefore, in addition to effects on assembly, modifications of Tr may also influence encapsidation, polymerase binding, and RNA replication. Consistent with this possibility, deletions in pDI-T that retained the 5′ terminal 45 nt exhibited either elevated or normal replication levels but altered ratios of genomic negative-strand to antigenomic positive-strand RNAs, and those that retained fewer than 45 nt showed progressively reduced levels of replication as well as altered ratios. Thus, these DI RNAs identified a region of the negative-strand promoter (nt 46 to 51) that was not essential for RNA replication but which regulated the balance between positive- and negative-strand synthesis. In addition, these DI RNAs demonstrated that as few as 22 nt could promote negative-strand synthesis, though 45 nt were required for full activity; 14 nt allowed inefficient replication. As the termini of DI-T are complementary for 45 nt, these findings agree with our previous work which showed that increased terminal complementarity offered a replicative advantage (42). Whether the observed effects on replication were caused by alterations to the extent of terminal complementarity, N encapsidation signals, polymerase binding sites, or synthesis of the 45-nt Le− RNA was not determined. However, based on earlier in vitro observations that localized an N protein encapsidation site to the first 14 to 19 nt of the genome (5, 24), it seems unlikely that encapsidation is the primary defect for these deletions. Repeated passage of one of these DI-T deletions resulted in the generation of a series of DI particles which had restored the balance between positive- and negative-strand RNA synthesis (46). Analysis of the terminal sequences of these DI RNAs may further illuminate the function of TrC in regulating minus-strand synthesis.
Implications and future work.
The work described here identified the genomic Tr as a position-dependent assembly signal and showed that the 5′ terminal 29 nucleotides were able to efficiently provide this requirement. In future work, it would be of interest to determine what trans-acting factor discriminates the 5′ Tr during assembly. As the matrix protein is a major structural component of the virion that has been shown to bind both nucleocapsids (18, 26) and the membrane (8, 22), it represents a suitable candidate. The demonstration that the trailer region of the genome is an essential assembly signal creates an apparent paradox, especially for DI-T. Previously it was shown for DI-T that RNAs assembled into infectious particles are almost exclusively negative sense (41), and as a consequence of the 5′ copyback structure of DI-T, the positive strand has 45 nucleotides of trailer at its 5′ end. It seems likely that a sequence element outside the trailer region functions as part of the assembly signal either by providing a specific sequence or contributing to an RNA structure. In any event, further experiments will be needed to determine what regulates the polarity of budding in DI-T.
ACKNOWLEDGMENTS
We acknowledge Brett Skinner for the generation of some deletions at the 5′ terminus of DI-T. We thank the members of the G. W. Wertz and L. A. Ball laboratories both past and present for helpful comments throughout the course of the project and for a critical review of the manuscript.
This work was supported by Public Health grants R37AI12464 and AI20181 from the National Institute of Allergy and Infectious Disease to G.W.W.
REFERENCES
- 1.Bamford D H, Wickner R B. Assembly of double-stranded RNA viruses: bacteriophage Ø6 and yeast virus L-A. Semin Virol. 1984;5:61–69. [Google Scholar]
- 2.Banerjee A K, Barik S. Gene expression of vesicular stomatitis virus genome RNA. Virology. 1992;188:417–428. doi: 10.1016/0042-6822(92)90495-b. [DOI] [PubMed] [Google Scholar]
- 3.Barclay W S, Palese P. Influenza B viruses with site-specific mutations introduced into the HA gene. J Virol. 1995;69:1275–1279. doi: 10.1128/jvi.69.2.1275-1279.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishop D H, Roy P. Properties of the product synthesized by vesicular stomatitis virus particles. J Mol Biol. 1971;58:799–814. doi: 10.1016/0022-2836(71)90041-6. [DOI] [PubMed] [Google Scholar]
- 5.Blumberg B M, Giorgi C, Kolakofsky D. N protein of vesicular stomatitis virus selectively encapsidates leader RNA in vitro. Cell. 1983;32:559–567. doi: 10.1016/0092-8674(83)90475-0. [DOI] [PubMed] [Google Scholar]
- 6.Blumberg B M, Leppert M, Kolakofsky D. Interaction of VSV leader RNA and nucleocapsid protein may control VSV genome replication. Cell. 1981;23:837–845. doi: 10.1016/0092-8674(81)90448-7. [DOI] [PubMed] [Google Scholar]
- 7.Bridgen A, Elliott R M. Rescue of a segmented negative-strand RNA virus entirely from cloned complementary DNAs. Proc Natl Acad Sci USA. 1996;93:15400–15404. doi: 10.1073/pnas.93.26.15400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chong L D, Rose J K. Membrane association of functional vesicular stomatitis virus matrix protein in vivo. J Virol. 1993;67:407–414. doi: 10.1128/jvi.67.1.407-414.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins P L, Mink M A, Stec D S. Rescue of synthetic analogs of respiratory syncytial virus genomic RNA and effect of truncations and mutations on the expression of a foreign reporter gene. Proc Natl Acad Sci USA. 1991;88:9663–9667. doi: 10.1073/pnas.88.21.9663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De B P, Banerjee A K. Rescue of synthetic analogs of genome RNA of human parainfluenza virus type 3. Virology. 1993;196:344–348. doi: 10.1006/viro.1993.1486. [DOI] [PubMed] [Google Scholar]
- 11.Dimock K, Collins P L. Rescue of synthetic analogs of genomic RNA and replicative-intermediate RNA of human parainfluenza virus type 3. J Virol. 1993;67:2772–2778. doi: 10.1128/jvi.67.5.2772-2778.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finke S, Conzelmann K K. Ambisense gene expression from recombinant rabies virus: random packaging of positive- and negative-strand ribonucleoprotein complexes into rabies virions. J Virol. 1997;71:7281–7288. doi: 10.1128/jvi.71.10.7281-7288.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox J M, Johnson J J, Young M J. RNA/protein interactions in icosahedral virus assembly. Semin Virol. 1994;5:51–60. [Google Scholar]
- 14.Froshauer S, Kartrenbeck J, Helenius A. Alphavirus RNA replicase is located on the cytoplasmic surface of endosomes and lysosomes. J Cell Biol. 1988;107:2075–2086. doi: 10.1083/jcb.107.6.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuerst T R, Niles E G, Studier F W, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holland J J. Defective interfering rhabdoviruses. In: Wagner R R, editor. The rhabdoviruses. New York, N.Y: Plenum Press; 1987. pp. 297–360. [Google Scholar]
- 17.Hunter E. Macromolecular interactions in the assembly of HIV and other retroviruses. Semin Virol. 1994;5:71–83. [Google Scholar]
- 18.Kaptur P E, Rhodes R B, Lyles D S. Sequences of vesicular stomatitis virus matrix protein involved in binding to nucleocapsids. J Virol. 1991;65:1057–1065. doi: 10.1128/jvi.65.3.1057-1065.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Luo L, Schubert M, Wagner R R, Kang C Y. Viral liposomes released from insect cells infected with recombinant baculovirus expressing the matrix protein in vesicular stomatitis virus. J Virol. 1993;67:4415–4420. doi: 10.1128/jvi.67.7.4415-4420.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez S, Yao J-S, Kuhn R J, Strauss E G, Strauss J H. Nucleocapsid-glycoprotein interactions required for assembly of alphaviruses. J Virol. 1994;68:1316–1323. doi: 10.1128/jvi.68.3.1316-1323.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luytjes W, Krystal M, Enami M, Parvin J D, Palese P. Amplification, expression, and packaging of a foreign gene by influenza virus. Cell. 1989;59:1107–1113. doi: 10.1016/0092-8674(89)90766-6. [DOI] [PubMed] [Google Scholar]
- 22.Lyles D S, McKenzie M, Wallace Parce J. Subunit interactions of vesicular stomatitis virus envelope glycoprotein stabilized by binding to viral matrix protein. J Virol. 1992;66:349–358. doi: 10.1128/jvi.66.1.349-358.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mebatsion T, Konig M, Conzelmann K K. Budding of rabies virus particles in the absence of the spike glycoprotein. Cell. 1996;84:941–51. doi: 10.1016/s0092-8674(00)81072-7. [DOI] [PubMed] [Google Scholar]
- 24.Moyer S A, Smallwood-Kentro S, Haddad A, Prevec L. Assembly and transcription of synthetic vesicular stomatitis virus nucleocapsids. J Virol. 1991;65:2170–2178. doi: 10.1128/jvi.65.5.2170-2178.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Odenwald W F, Arnheiter H, Dubois-Dalcq M, Lazzarini R A. Stereo images of vesicular stomatitis virus assembly. J Virol. 1986;57:922–932. doi: 10.1128/jvi.57.3.922-932.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogden J R, Pal R, Wagner R R. Mapping regions of the matrix protein of vesicular stomatitis virus which bind to ribonucleocapsids, liposomes, and monoclonal antibodies. J Virol. 1986;58:860–868. doi: 10.1128/jvi.58.3.860-868.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park K H, Huang T, Correia F F, Krystal M. Rescue of a foreign gene by Sendai virus. Proc Natl Acad Sci USA. 1991;88:5537–5541. doi: 10.1073/pnas.88.13.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pattnaik A K, Ball L A, LeGrone A, Wertz G W. The termini of VSV DI particle RNAs are sufficient to signal RNA encapsidation, replication, and budding to generate infectious particles. Virology. 1995;206:760–764. doi: 10.1016/S0042-6822(95)80005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pattnaik A K, Ball L A, LeGrone A W, Wertz G W. Infectious defective interfering particles of VSV from transcripts of a cDNA clone. Cell. 1992;69:1011–1020. doi: 10.1016/0092-8674(92)90619-n. [DOI] [PubMed] [Google Scholar]
- 30.Pattnaik A K, Wertz G W. Cells that express all five proteins of vesicular stomatitis virus from cloned cDNAs support replication, assembly, and budding of defective interfering particles. Proc Natl Acad Sci USA. 1991;88:1379–1383. doi: 10.1073/pnas.88.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patton J T, Davis N L, Wertz G W. N protein alone satisfies the requirement for protein synthesis during RNA replication of vesicular stomatitis virus. J Virol. 1984;49:303–309. doi: 10.1128/jvi.49.2.303-309.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rolls M M, Webster P, Balba N H, Rose J K. Novel infectious particles generated by expression of the vesicular stomatitis glycoprotein from a self-replicating RNA. Cell. 1994;79:497–506. doi: 10.1016/0092-8674(94)90258-5. [DOI] [PubMed] [Google Scholar]
- 33.Roy P, Repik P, Hefti E, Bishop D H. Complementary RNA species isolated from vesicular stomatitis (HR strain) defective virions. J Virol. 1973;11:915–925. doi: 10.1128/jvi.11.6.915-925.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 35.Schaffer F L, Hackett A J, Soergel M E. Vesicular stomatitis virus RNA: complementarity between infected cell RNA and RNA’s from infectious and autointerfering viral fractions. Biochem Biophys Res Commun. 1968;31:685–692. doi: 10.1016/0006-291x(68)90616-5. [DOI] [PubMed] [Google Scholar]
- 36.Schlesinger S, Makino S, Linial M L. Cis-acting genomic elements and trans-acting proteins involved in the assembly of RNA viruses. Semin Virol. 1994;5:39–49. doi: 10.1006/smvy.1994.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schnell M J, Buonocore L, Boritz E, Ghosh H P, Chernish R, Rose J K. Requirement for a non-specific glycoprotein cytoplasmic domain sequence to drive efficient budding of vesicular stomatitis virus. EMBO J. 1998;17:1289–1296. doi: 10.1093/emboj/17.5.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smallwood S, Moyer S A. Promoter analysis of the vesicular stomatitis virus RNA polymerase. Virology. 1993;192:254–263. doi: 10.1006/viro.1993.1028. [DOI] [PubMed] [Google Scholar]
- 39.Stillman E A, Rose J K, Whitt M A. Replication and amplification of novel vesicular stomatitis virus minigenomes encoding viral structural proteins. J Virol. 1995;69:2946–2953. doi: 10.1128/jvi.69.5.2946-2953.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas D, Newcomb W W, Brown J C, Wall J S, Hainfeld J F, Trus B L, Steven A C. Mass and molecular composition of vesicular stomatitis virus: a scanning transmission electron microscopy analysis. J Virol. 1985;54:598–607. doi: 10.1128/jvi.54.2.598-607.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wertz G W. Replication of vesicular stomatitis virus defective interfering particle RNA in vitro: transition from synthesis of defective interfering leader RNA to synthesis of full-length defective interfering RNA. J Virol. 1983;46:513–522. doi: 10.1128/jvi.46.2.513-522.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wertz G W, Whelan S, LeGrone A, Ball L A. Extent of terminal complementarity modulates the balance between transcription and replication of vesicular stomatitis virus RNA. Proc Natl Acad Sci USA. 1994;91:8587–8591. doi: 10.1073/pnas.91.18.8587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whelan S P, Ball L A, Barr J N, Wertz G T. Efficient recovery of infectious vesicular stomatitis virus entirely from cDNA clones. Proc Natl Acad Sci USA. 1995;92:8388–8392. doi: 10.1073/pnas.92.18.8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whelan S P, Wertz G W. Defective interfering particles of vesicular stomatitis virus: functions of the genomic termini. Semin Virol. 1997;8:131–139. [Google Scholar]
- 45.Whelan S P J, Wertz G W. Regulation of RNA synthesis by the genomic termini of vesicular stomatitis virus: identification of distinct sequences essential for transcription but not replication. J Virol. 1999;73:297–306. doi: 10.1128/jvi.73.1.297-306.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whelan, S. P., and G. W. Wertz. Unpublished observations.
- 47.Wilusz J, Kurilla M G, Keene J D. A host protein (La) binds to a unique species of minus-sense leader RNA during replication of vesicular stomatitis virus. Proc Natl Acad Sci USA. 1983;80:5827–5831. doi: 10.1073/pnas.80.19.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]