Abstract
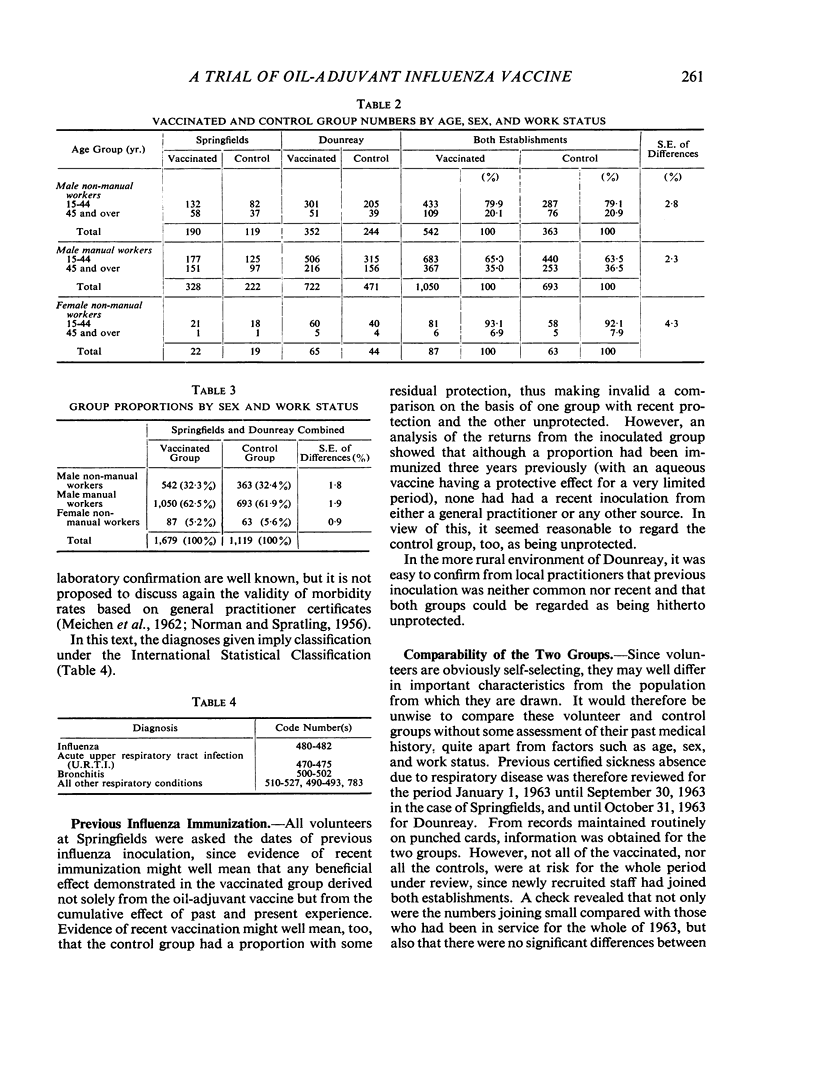
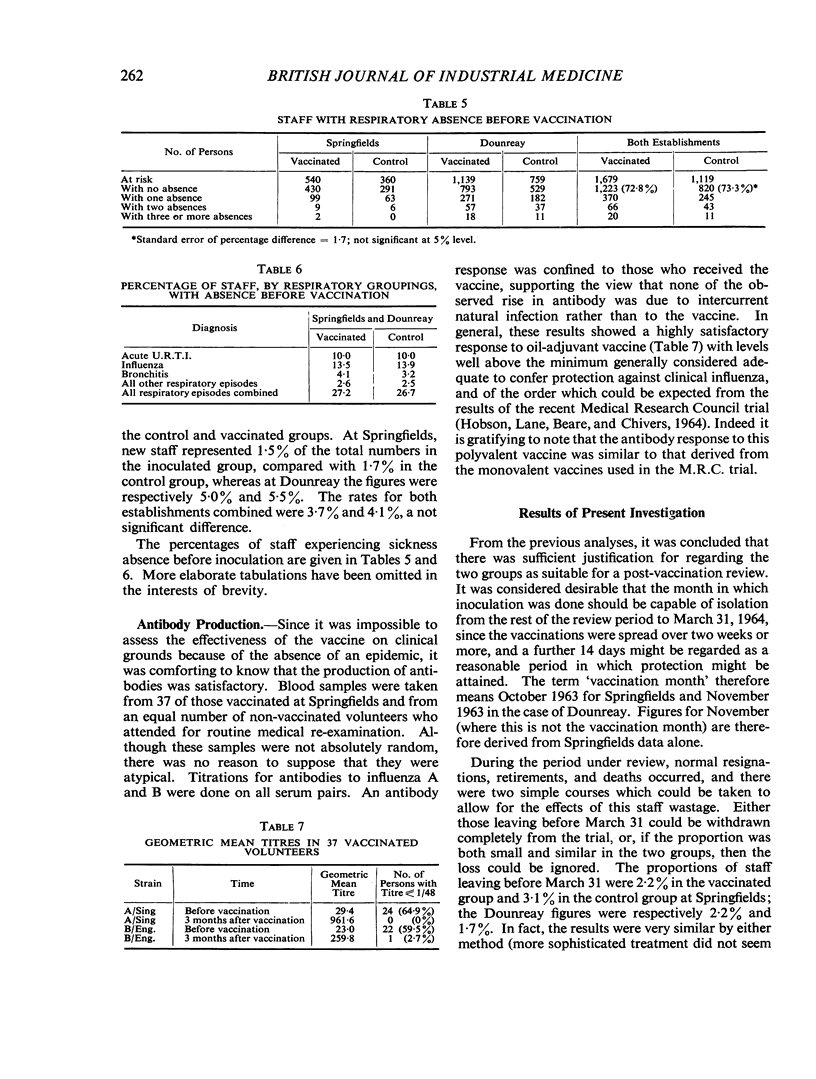
In the autumn of 1963, 1,679 employees at two U.K.A.E.A. establishments were inoculated with a single dose (0·25 ml.) of polyvalent oil-adjuvant influenza vaccine. In a non-epidemic winter, no significant improvement in certified sickness absence could be demonstrated in the vaccinated group when compared with a randomly selected uninoculated group. In particular, no real protection was shown, in those vaccinated, against influenza and acute upper respiratory tract infections. Once respiratory sickness had occurred the vaccine showed no significant effect on the length of sickness absence.
In this trial, unpleasant reactions to the vaccine occurred in about a quarter of the inoculated, and, in particular, a large number of participants complained of general malaise which persisted for more than two weeks. These reactions were thought to be severe enough to induce consumer resistance to routine annual immunization, which is thought to be neither justified nor acceptable in a healthy industrial population at the present stage of vaccine development. This in no way detracts from the suitability or acceptability of the vaccine to high-risk patients.
Further research and testing, which is being carried out by pharmaceutical firms, is essential, and so, too, is the need for more controlled trials to evaluate changing vaccines in different population groups.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HIMMELWEIT F. IMMUNIZATION AGAINST INFLUENZA. Br J Clin Pract. 1963 Nov;17:661–671. [PubMed] [Google Scholar]
- HOBSON D., LANE C. A., BEARE A. S., CHIVERS C. P. SEROLOGICAL STUDIES ON ADULT VOLUNTEERS INOCULATED WITH OIL-ADJUVANT ASIAN INFLUENZA VACCINE. REPORT TO THE M.R.C. COMMITTEE ON INFLUENZA AND OTHER RESPIRATORY VIRUS VACCINES. Br Med J. 1964 Aug 1;2(5404):271–274. doi: 10.1136/bmj.2.5404.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEICHEN F. W., ROGAN E., HOWELL R. W. A study of the effectiveness of influenza vaccination in an industrial population. Br J Ind Med. 1962 Jul;19:203–210. doi: 10.1136/oem.19.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEIKLEJOHN G. Adjuvant influenza adenovirus vaccine. JAMA. 1962 Feb 24;179:594–597. doi: 10.1001/jama.1962.03050080006002. [DOI] [PubMed] [Google Scholar]
- SALK J. E., BAILEY M. L., LAURENT A. M. The use of adjuvants in studies on influenza immunization. II. Increased antibody formation in human subjects inoculated with influenza virus vaccine in a water in-oil emulsion. Am J Hyg. 1952 May;55(3):439–456. doi: 10.1093/oxfordjournals.aje.a119534. [DOI] [PubMed] [Google Scholar]
- SALK J. E., CONTAKOS M., LAURENT A. M., SORENSEN M., RAPALSKI A. J., SIMMONS I. H., SANDBERG H. Use of adjuvants in studies on influenza immunization. III. Degree of persistence of antibody in human subjects two years after vaccination. J Am Med Assoc. 1953 Apr 4;151(14):1169–1175. doi: 10.1001/jama.1953.02940140013005. [DOI] [PubMed] [Google Scholar]


