Abstract
The gut microbiome provides the host access to otherwise indigestible nutrients, which are often further metabolized by the microbiome into bioactive components. The gut microbiome can also shift the balance of host-produced compounds, which may alter host health. One precursor to bioactive metabolites is the essential aromatic amino acid tryptophan. Tryptophan is mostly shunted into the kynurenine pathway but is also the primary metabolite for serotonin production and the bacterial indole pathway. Balance between tryptophan-derived bioactive metabolites is crucial for neurological homeostasis and metabolic imbalance can trigger or exacerbate neurological diseases. Alzheimer’s, depression, and schizophrenia have been linked to diverging levels of tryptophan-derived anthranilic, kynurenic, and quinolinic acid. Anthranilic acid from collective microbiome metabolism plays a complex but important role in systemic host health. Although anthranilic acid and its metabolic products are of great importance for host–microbe interaction in neurological health, literature examining the mechanistic relationships between microbial production, host regulation, and neurological diseases is scarce and at times conflicting. This narrative review provides an overview of the current understanding of anthranilic acid’s role in neurological health and disease, with particular focus on the contribution of the gut microbiome, the gut–brain axis, and the involvement of the three major tryptophan pathways.
Keywords: tryptophan, kynurenine, anthranilic acid, microbiome, gut–brain axis, metabolites, neuroactive compounds
1. Introduction
The metabolome, small molecules produced by the collective metabolism of an organism and its microbiome, is difficult to comprehensively examine, in large part due to the sheer complexity of the interaction between the microbiome and the host metabolic state. This difficulty is especially pronounced in the human gut, where extensive overlap between small molecule metabolism of the host and microbiome exists [1]. Despite this complexity, determining causation and correlation with host health requires distinguishing which molecules are derived from the host’s diet, produced by the microbiome, or produced and secreted into the gastrointestinal tract (GT) from the host metabolism. In the broader context of host health, the importance of the host GT activity and resident microbiome contributions cannot be overstated, specifically with the extensive reach of small molecules to distant tissues in the body [2]. For example, the gut and brain are inextricably linked, exerting bi-directional control, with the gut microbiome playing the part of the complementary brain in this feedback loop [3]. The microbiome density, diversity, and composition differ across the GT. The human GT consists of many specific sections that have differential metabolic capacity and microbiome members, but for the purpose of this review will be broadly discussed in terms of the small intestine, which has low-density microbial consortia and the contrasting microbially dense large intestine [4].
The small intestine utilizes digestive juices and enzymes that are secreted by the pancreas and gallbladder to breakdown carbohydrates, lipids, and proteins. The small intestine also contains microbes, such as Bacteroides and Prevotella, that aid in the synthesis of micronutrients, like vitamin K2 and B12 [4,5]. The microbiome of the small intestine is exposed to transient conditions that include periodic pH fluctuation from the influx of stomach contents and the microbiome must adapt to the ever-shifting local environment from stomach proximity [6]. This instability may explain why the small intestine harbors only ~105 microbial cells/gram, whereas the large intestine supports ~1012 microbial cells/gram [4]. Despite the relatively lower abundance, the small intestine microbiome is an important contributor to host digestion as the small intestine is the primary site of macronutrient digestion and absorption.
Although the small intestine accounts for most macronutrient digestion, the large intestine is an essential contributor to host metabolism and has a differing role in digestion. Host cells of the large intestine, goblet cells and enterocytes, produce mucosa and digestive enzymes for the further breakdown of food matter from the small intestine [7]. The large intestine is also responsible for the absorption of any remaining water and salts not absorbed during transit through the small intestine. In addition to host cell activity, microbes expand the digestive and metabolic functionality of the large intestine by an estimated multiplier of ~150,000 [8], which expands the host’s metabolism beyond its genome capacity.
The microbiome of the large intestine, especially of the colon, is arguably more well studied than that of the small intestine, likely due in large part to the more robust study material, typically feces via non-invasive methods. The diverse microbiome of the colon adds metabolic breadth to the GT, providing the host with access to otherwise inaccessible small molecules and nutrients, like the short-chain fatty acid (SCFA) butyrate, which is a key contributor to healthy gut epithelial function (e.g., increasing tight junction integrity) [9,10] and has shown to alter immune function (for details see Kim [11]). In addition to synthesizing nutrients, the gut microbiome can also produce metabolites, ultimately shifting the balance and activity of overall metabolism.
Amino acids are one substrate set that both host and microbe break down and divert to key and central metabolic pathways. Glutamine and asparagine, for instance, are utilized by host intestinal cells as an energy source [12]. In bacteria, glutamine is also of central importance and serves as a precursor for the synthesis of key nitrogen-compounds and glutamine supplementation has shown to promote and support populations of fiber-degrading bacteria [13,14,15]. The consequential role of the microbiota in amino acid homeostasis has further been highlighted by studies that showed germ-free mice display altered amino acid distribution along the intestinal tract when compared to mice that possessed their natural microbiome [13]. While amino acids are centrally important to protein synthesis and other basic host–microbe functions, amino acids can also regulate host neurological health by serving as precursors to bioactive molecules that circulate throughout the host.
Tryptophan (Trp) is one such amino acid involved in bioactive metabolite production and Trp metabolism is a central pathway shared by the host and its microbiome (Figure 1). Trp is derived from the digestion of dietary protein in the small intestine, where free Trp is absorbed through the intestinal epithelium and enters systemic circulation. Any unabsorbed Trp is transported through the intestinal tract until it reaches the colon where it is utilized by the colonic microbiome [16]. In the colon, Trp plays a major role as a primary substrate for microbial metabolism [17], since easily digested carbohydrates have been utilized early on after indigestion and are in short supply in the distal portion of the digestive system. Trp metabolism is not limited to a single genus of microorganism but is found across many gut microbes, including multiple Lactobacillus, Bifidobacterium, Bacteroides, and Clostridium species [18]. Some of the microbes known to utilize Trp are often associated with host health and are part of the gut–brain axis.
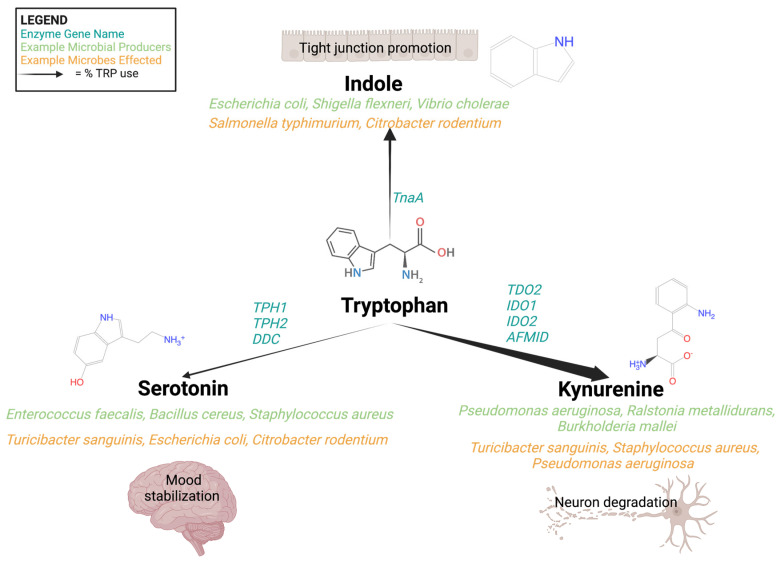
Figure 1.
Tryptophan is a central amino acid and is channeled into three pathways: indole, kynurenine, and serotonin. Serotonin and kynurenine are produced by both microbes and mammals via multiple enzymes, listed in teal above. Indole is a tryptophan derivative produced solely by microbes. The majority of tryptophan is moved into the production of kynurenine and related metabolites, with the remaining portion split between indoles and serotonin. Examples of microbial producers of these small molecules are in green while examples of microbes affected by these metabolites are in orange. The broader host effects are graphically represented above.
Digestion of Trp results in the well-known neuroactive compounds, serotonin and melatonin, and is a starting point for the NAD+-producing kynurenine pathway in humans. In addition to the ability to perform parts of the serotonin and kynurenine pathways, microbes have the additional option of producing indoles from Trp catabolism. Humans lack this indole pathway and instead rely on exogenous indoles, either from dietary sources or microbial metabolism, which is metabolically straightforward but the resulting bioactivity in the microbiome is complex and can alter biofilm formation, virulence, and host interaction [19,20]. The kynurenine pathway, one of three main Trp metabolic pathways, has garnered increasing interest for its connection to neurological diseases through the production of the bioactive compounds quinolinic, picolinic, kynurenic, and anthranilic acid in this pathway [21,22,23,24,25,26,27,28]. The three primary Trp pathways are critically interconnected. Shunting Trp into the kynurenine pathway removes the substrate from the production of indole by microbes or serotonin (in microbes and humans). This ‘push–pull’ nature of Trp metabolism, along with the notable bioactivity of the many related metabolic products, make understanding the equilibrium of these interconnected pathways important from the perspective of host health.
The role of microbes as drivers of, and not just responders to, bioactive metabolites (and metabolism) is quickly emerging as an important component of host wellbeing that is modulated by the microbiome co-metabolism. At the same time, this newly elucidated role for microbial metabolites is not well understood or defined. This is in part due to the complex set of in flux metabolites that, combined with microbiome membership changes, leads to altered metabolic potential. Additionally, host diet, the source of substrates for the microbiome, may also be in flux [29]. Many studies examining the gut microbiome focus primarily on taxonomic diversity and do not make the link to metabolism and metabolite changes that are inevitably from diet and host metabolism [30,31]. While a necessary part of the puzzle, bacterial identity alone does not indicate microbiome functionality, which changes the collective metabolism and metabolome [32,33,34,35]. A companion focus on the resultant metabolome is imperative for a holistic understanding of microbiome function, and ultimately, for an understanding of its role in host health. Although there are hundreds of known bioactive metabolites stemming from many dietary substrates, this review will focus first on Trp and then more specifically delve into Trp-derived anthranilic acid and its direct derivatives.
Anthranilate and its derivatives are only one part of the broader Trp puzzle; however, they are an understudied piece with important influence on holistic host health. As will be discussed in more detail later in this review, anthranilate is not often the target metabolite of metabolome/microbiome/host health studies, but a pattern of anthranilate and derivatives is emerging alongside neurological findings. And specifically, as a shared metabolic substrate between host and microbe, a closer look at anthranilate and its derivatives may help fill in some missing pieces around the enigmatic gut–brain and bioactive metabolites connection.
The production, circulation, and interactions of Trp-derived anthranilic acid and subsequent derivatives have profound impacts on host health. Despite the important role of the human microbiome in anthranilic acid production from Trp, no comprehensive review that summarizes the role of microbially derived anthranilic acid in neurodegenerative diseases exists to date. To place anthranilate in the wider framework of this family of bioactive metabolites, this review also contains a brief discussion of the activity of other more widely known neuroactive Trp metabolites. The objective of this review is to highlight the importance of anthranilic acid and its derivatives by outlining the current understanding of anthranilic acid’s role in health and disease, followed by a discussion of the microbes that may affect the production and circulation of anthranilic acid.
2. A Gut Feeling: The Microbiome as a Complementary Brain
Microbes in the gastrointestinal (GI) tract work synergistically to break down substrates and survive as a complex consortium with expansive metabolic capability that exceeds human metabolism at least 10-fold [36]. Microbes provide nutrients to the host and it is increasingly clear that the microbiome contributes to host health and behavior through the production of neurologically active compounds, like serotonin, that move from the gut to distant locations throughout the body [37]. Within the gut, these metabolites are often beneficial, supporting immune function and promoting tight junctions, but they can also be detrimental, stimulating inflammation and dysbiosis (Table 1) [38,39,40,41].
Table 1.
Tryptophan-derived bioactive metabolites and their functions.
| Substrate | Metabolite | Known Microbial Producer (s) | Bioactive Function | Cofactors |
|---|---|---|---|---|
| Tryptophan | Kynurenine | Pseudomonas aeruginosa [42], Ralstonia metallidurans [43] | Neuronal damage mediator | INF-gamma [44] |
| Indole | Escherichia coli, Proteus vulgaris, Clostridium sp., Bacteroides sp. [45] | Anti-inflammatory, epithelial tight-junction regulation | Iron [46] | |
| Serotonin | Pseudomonas putida KT2440 [47] | Mood regulation | Vitamin B2 [48] | |
| Kynurenine | Anthranilic acid |
Pseudomonas aeruginosa [49], Burkholderia fungorum, Bacillus cereus, Ralstonia solanacearum, and Ralstonia metallidurans [43] |
Inflammation activation | Iron [50] |
| 3-Hydroxyanthranilic acid | Proteobacteria sp., Actinobacteria sp., Firmicutes sp. Bacteroidetes sp., Chloroflexi sp., Cyanobacteria sp., Euryarchaeota sp. [51] | Free radical generation, apoptotic regulation | Pyridoxal-5′-phosphate [52] | |
| Kynurenic acid | Escherichia coli [53] | NMDA agonist, anticonvulsant | ||
| 3-hydroxykynurenine | Proteobacteria sp., Actinobacteria sp., Firmicutes sp. Bacteroidetes sp., Chloroflexi sp., Cyanobacteria sp., Euryarchaeota sp. [51] | Oxidative damage, apoptotic regulation | ||
| Quinolinic acid | Streptomyces antibioticus, Cyanidium caldarium, Karlingia rosea [54] | NMDA activator | Anthranilic Acid [55] | |
| Picolinic acid | Burkholderia xenovorans, [56] | Iron and zinc chelator, antiviral, antifungal | Anthranilic Acid [55] | |
| Indole | Indole-3-acetamide | Acidobacteria sp., Bacteroidetes sp., Firmicutes sp. [57] | Antioxidant, anti-hyperglycemic | |
| Tryptamine | Ruminococcus gnavus and Clostridium sporogenes [58] | Neuroinflammatory modulation | ||
| Indole-3-acetic acid | Clostridium sp., Bacteroides sp., Bifidobacterium sp. [59] | Anti-angiogenic | ||
| Indole-3-pyruvic acid | Pseudomonas fluorescens and Candida tropicalis [60] | Anti-inflammatory, anti-angiogenic | ||
| Indole-3-acetate | Clostridium sporogenes [61] | Anti-inflammatory | ||
| Indole-3-aldehyde | Lactobacillus sp. [62] | Epithelial cell cycle regulation, goblet cell differentiation regulation | ||
| Indole-3-propionic acid | Lactobacillus reuteri, Akkermansia sp., Clostridium sp., Peptostreptococi sp. [63] | Anti-inflammatory, neuronal apoptosis regulation | ||
| Skatole | Clostridium sp. and Bacteroides sp. [64] | Induces colonocyte apoptosis | ||
| Serotonin | Melatonin | Roseburia hominis [65] | Circadian rhythm regulation, antioxidant | Tetrahydrobiop-terin and O2 [66] |
Microbial metabolites find their way out of the gastrointestinal tract and into circulation via both passive and active transport [16], and once in circulation bioactive compounds are free to travel to any number of body locations, including the liver, kidneys, lymph nodes, reproductive tract, and even the brain [37,67,68].
Crossing through the gut epithelium into systemic circulation is not always necessary for these microbial metabolites to exert control over host neurology. Interactions between luminal gut metabolites and the host nervous system can be local in nature but with wide-reaching effects [69]. The enteric nervous system, containing sensory afferent and motor efferent nerve fibers, provides a direct link between the brain and physical/chemical gut activity [70]. The Vagus nerve, a part of the enteric nervous system, is central to the basic physiological processes and innervates, among others, the cardiovascular system and respiratory system [71]. The bi-directional (with both afferent and efferent fibers) Vagus nerve is a big part of the gut–brain connection and has recently been shown to exert direct control from the gut to the brain via physical contact with enteroendocrine cells in the gut epithelium [72]. In addition to direct interactions with host organs through active circulation, microbial metabolites can signal to the host via the Vagus nerve and the broader enteric nervous system [73]. Beyond the simple function of digesting food for energy production, the gastrointestinal tract is intimately linked with systemic host physiology through both the uptake and transit of metabolites through the circulatory system and the conveyance of signals through the fibers of the nervous system. This inextricable connection between the host brain and the microbial brain of the gut highlights the importance of metabolic control in host health.
The gut microbiome and metabolome have been correlated with, and in some cases suggested to be, the causative agent in common disorders, such as irritable bowel disease (IBD). IBD is a broad inflammatory disease of the gastrointestinal tract and one of the first conditions that was cited as a link between the gastrointestinal tract and host neurological state [74]. Patients diagnosed with IBD in a Canadian cohort were twice as likely to have mood disorders, such as depression or anxiety when compared to the control group [74]. The directionality of the link between IBD and mood disorders remained ambiguous for many years, as 80% of the IBD cohort were diagnosed with anxiety two or more years before the IBD diagnosis and so directionality nor causation between the mood disorders and IBD was clearly established [74]. A 2016 review re-evaluated this IBD observation in conjunction with other studies to tease apart the gut–brain connection, ultimately finding that mood disorders such as anxiety, depression, and stress all have tight links to increased inflammatory states, potentially contributing to a physiological state that favors a long-term inflammatory disease [75]. Since this initial work in 2008, additional research has emerged on the connection between different gut disease and depression, as well as on the more specific routes and signals of communication between the physically separate body locales of the gut and brain via microbially derived metabolic products [76]. One example is the bacterial-derived metabolite, p-cresol, a compound that has been connected to exacerbation of autism spectrum disorders in an expanding body of literature [77,78,79,80,81,82]. p-Cresol is an aromatic derivative of tyrosine produced as a catabolite by many members of the gut microbiota, including some Bifidobacteriaceae, Enterobacteriaceae, Clostridiaceae, Lactobacillaceae, Coriobacteriaceae, and Bacteroidaceae [77]. Clostridia difficile has displayed a particular growth advantage on p-cresol and people with autism spectrum disorders often have a gut microbiome that is enriched in Clostridia, suggesting a direct relationship between microbiome membership, microbial metabolism, and neurological symptoms [77]. More systematic studies in mice support this suggestion of a gut–brain axis link for symptoms that fall into the autism spectrum [77,79], further suggesting that microbial metabolism might result in disorders beyond the gut barrier.
Microbially derived metabolic products play a dual function, serving both as substrates for the microbes and as interkingdom signaling molecules between the gut microbiome and peripheral host systems [39,83]. Perhaps one of the most widely recognized examples of these is the mood-modulating serotonin which is a Trp catabolite that acts on multiple brain regions via its specific receptors that are found throughout the circulatory system, but also in the brain [84]. Though known primarily for its activity in the brain, over 90% of serotonin is found in the GT lumen [85] (Figure 1). Other small molecules, such as SCFAs are imperative for healthy gut function, but they are also able to cross the gut epithelium and have been reported to breach the blood–brain barrier in small concentrations [86]. SCFAs that make the journey to the blood–brain barrier help to restore the barrier’s integrity [86]. These small molecules can serve as sources of energy (e.g., succinic acid) for the host and multiple members of the microbiome, but their role as signaling molecules with an array of effects should not be ignored due to their wide array of functional activities [87,88]. Propionic acid, an SCFA produced through fiber and amino acid fermentation by gut microbes in the colon, is transported through the portal vein to the liver, where it can inhibit liver damage and [89] gluconeogenesis [90]. Propionic acid is a microbially derived metabolite with broad implications for host health given its ability to move to the liver and into circulation, where the compound can exert effects across a range of distant sites to the GT. Microbial metabolites that cross the epithelial barrier or interact with the host nervous system from the GT have the potential to act upon several tissue systems ranging from the circulatory system to the brain [91]. The system-wide health effects of microbial metabolites in the host makes the modulation of these pathways a relevant strategy that might alter the outcome of many human diseases. Microbial metabolism can drive the production of bioactive metabolites that have either direct roles in the development and progression of diseases, or that exert more indirect effects on the disease progression. Modulating these microbial metabolite–host system interactions, like those seen with metabolites from Trp catabolism, through microbial engineering, gut microbiome remodeling, or dietary interventions could produce treatment for diseases for which effective treatments are currently not available or are instead extremely invasive.
3. The Tryptophan Pathway: Microbial-Derived Metabolic Mind Control
Trp is a significant contributor to the crosstalk between the gut microbiome and the brain. As the precursor for many important products, like indole, serotonin, and NAD+, Trp is crucial to basic microbial and human physiology. Trp is an essential amino acid that is introduced to the host primarily through diet, though some gut microbes can synthesize Trp from indole and serine via the Trp synthase enzyme complex (TrpAB) [83,92]. A study looking at the distribution of amino acid biosynthesis pathways across the publicly accessible genomes of 2856 human GT microbiome members from over 800 distinct microbial species found Trp biosynthesis was highly variable and individual microbes lacked a complete enzymatic repertoire to produce Trp [92]. There was a group of 38 genomes that predicted Trp auxotrophs making them unable to produce anthranilate, but interestingly possessed the genes for synthesis of Trp from anthranilate [92]. The authors posited that Trp biosynthesis pathways may show high variation across human gut microbes due to the high energetic cost of producing Trp in comparison to other amino acids, because Trp is encoded by only a single codon, UGG, and is a rarely incorporated amino acid with high molecular weight (204.23 g/mol) [92]. The high variation in Trp biosynthesis across gut microbes pulls the focus from basic host use towards the microbial use of this central amino acid and the metabolites resulting from such use. Trp, regardless of the source, is central to many cellular functions and the general path from Trp to bioactive derivatives is an important study for host health and disease impacts.
Trp in the host intestinal tract is freed during protein digestion within the small intestine or via microbial digestion and biosynthesis in the large intestine. Free Trp in the small intestine is absorbed into systemic circulation by the transmembrane broad spectrum neutral amino acid transporter (B0AT1) that is encoded by the SLC6A19 gene in intestinal epithelial cells [93]. Interestingly, 19 SLC6A19 paralogues that encode transporters in the GT have been reported. All paralogues are part of the SLC6 transporter family, a group that is implicated in human diseases and are actively being investigated as therapeutic targets for diseases that include Parkinson’s, depression, anxiety, hyperekplexia, and Tourette Syndrome [93,94]. Once in systemic circulation, albumin-bound Trp is carried to the liver where it is digested via the kynurenine pathway, or to the brain, where Trp crosses the blood–brain barrier with the help of amino acid transporters and is shunted into both the serotonin and kynurenine pathways [95,96]. Neutral amino acid transporters able to facilitate Trp-derivative absorption exist in the small intestine, but most of the gut’s Trp metabolism occurs in the large intestine and is mediated by microbial metabolism [97]. There is some evidence that disease states, such as small intestinal bacterial overgrowth (SIBO), can shift Trp metabolism in the small intestine, driving an increase in locally produced kynurenine [98], but the basal level of kynurenine production in the small intestine appears to be low under normal in vivo conditions [53]. The fraction of Trp that is not digested and absorbed in the small intestine is instead digested by the gut microbiome of the large intestine via the kynurenine, serotonin, and indole pathways [16]. Trp metabolites produced via microbial metabolism in the large intestine are utilized locally or cross the GT epithelium via passive diffusion (kynurenine and indole) or with the help of transporters (serotonin) [99,100]. Bioactive molecules stemming from Trp digestion, whether from digestion in the small intestine or from microbial production in the large intestine, can act on the local gut tissue or exert control over distant host tissues via circulation.
The bioactive products of these pathways, like kynurenic acid, have been associated with neurological conditions. Alzheimer’s, schizophrenia, and depression have been linked to the serotonin, kynurenine, and indole pathways, and intermediates of these pathways have been identified as potential causative agents or as potential biomarkers for disease states [21,23]. The microbiome, alongside the host, contributes to fluctuating intestine luminal and circulating serum levels of Trp-derived bioactive compounds, which are critical to the regulation of the gut–brain axis. A more mechanistic understanding of the function of these compounds in disease and of the microbial control of their production is imperative for improved neurology therapeutics. The three major pathways of Trp digestion, microbial metabolism, and the link to neurological diseases will be discussed briefly below and are summarized in Figure 2.
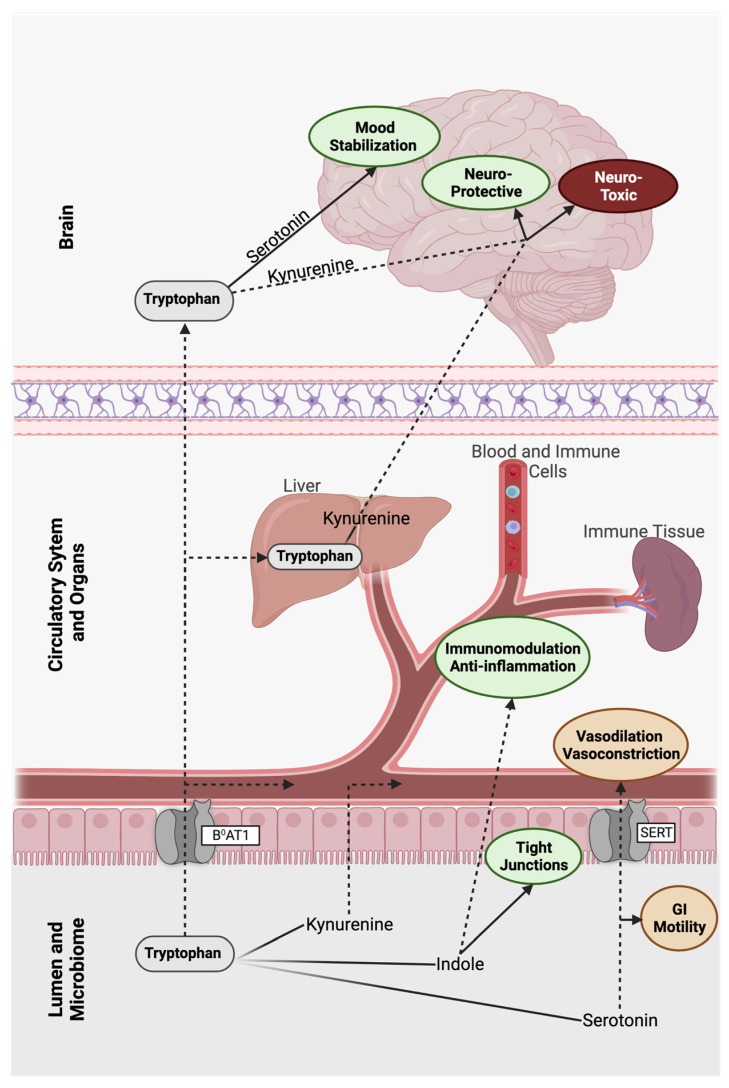
Figure 2.
The three major routes of tryptophan after ingestion, metabolism, and absorption in the human gut. Tryptophan can be absorbed into the blood stream and metabolized via the kynurenine pathway in the liver or via the serotonin or kynurenine pathway in the brain. Within the gut, tryptophan can also be funneled towards the kynurenine, indole, or serotonin pathway. These three metabolic pathways contain neuroactive metabolites that affect host physiology and neurology. Fading lines represent metabolism to a primary compound, dotted lines represent metabolism to secondary or downstream metabolites, green text ovals represent positive effects, red ovals represent negative effects, and orange ovals represent neutral outcomes.
3.1. Indole
Exogeneous Trp that makes it through the small intestine and into the large intestine is primarily digested by gut microbes, as colonic epithelial cells do not absorb amino acids in great quantity [101]. Whereas gut microbes can convert Trp into indole via a one-step reaction catalyzed by tryptophanase (TnaA), humans lack the ability to synthesize indole and depend instead on the microbially derived indole. Indole and its directly related compounds, collectively called indoles, include indole-3-acetamine, tryptamine, indole-3-acetylaldehyde, and inole-3-pyruvate, and are produced by members of the Proteobacteria, Firmicutes, Fusobacteria, and Bacteroidetes [102]. Further transformation of indoles results in the production of indole-3-acetate, indole-3-aldehyde, and skatole [102]. Not all microbes can produce indole. Fungi and bacteria that are unable to synthesize indole or its derivatives still have mechanisms for sensing and utilizing indoles as metabolic precursors or ligands for signaling cascades [103,104]. Candida albicans, Staphylococcus aureus, and Pseudomonas aeruginosa all use indoles to induce biofilm formation, and Salmonella enterica serovar Typhimurium relies on indole signaling to activate an oxidative stress response to resist antibiotic activity [104]. Indoles that are not utilized by other gut microbes and instead absorbed into circulation can be used in the liver to produce indoxyl sulfate, a toxic metabolite known for its ability to affect drug clearance in the liver through the modulation of drug transporter expression [105]. Indoles also act as interkingdom signaling molecules between microbes and their host, promoting tight epithelial junctions and regulating inflammation [39,106]. Indoles can directly induce interferon and interleukin 22, two immune system products that mediate repair of the gut barrier and inflammation, but this induction appears dependent on acute stressors in the gut and is thought to be a spatially regulated response [106]. Indoles regulate a variety of bacterial responses within the gut microbiome itself, including biofilm formation and virulence [19,107,108,109,110].
Humans may not be able to produce indoles, but they do interact with indoles via the gut epithelium and through systemic circulation. Indole concentrations in feces of healthy individuals range widely, and studies suggest there are many factors contributing to this variance, including microbiome diversity, free exogenous Trp, and the availability of other substrates [103,111]. Dietary Trp is also a significant driver of luminal indole concentration [112]. Once dietary Trp is microbially converted to indoles, it can travel through the lumen and gut mucosa via the host’s aryl hydrocarbon receptors (AhR) on the gut epithelial cell surface [113]. AhRs are not limited to the gastrointestinal tract, instead this cytosolic receptor is broadly distributed in the host [105,114,115]. AhRs are most highly expressed in the nervous system, especially the tibial nerve, and in the lungs, but are also expressed across much of the cardiovascular system and in reproductive tissues as well [116]. The breadth of tissues expressing these receptors is due to the crucial role of AhRs throughout the human body. AhRs serve as a mediating signal between environmental conditions and appropriate host response, allowing for dynamic interplay between internal host physiology and fluctuating environmental cues [117]. As a signaling mechanism, AhRs are a key link between the gut microbiota and host via their binding to numerous ligands [118], but these receptors and indole together display a unique affinity for one another [113]. Interestingly, though also found in mice, AhRs and indole in the mouse gut do not display the same unique binding activity as has been observed in human cells [113]. In humans, once bound with indole, AhRs move intracellularly and, through modifications within the cell, regulate the associated target genes [119].
Though indole is now understood to be a central player in the metabolic crosstalk between host and microbiome, current research primarily focuses on the localized effects in the GT system, neglecting potential effects of indole on distant tissues. Indole can affect host neurology from the gut lumen via the enteric nervous system, or through direct contact with host tissues via transit in the circulatory system. In the lumen, indole can activate enteroendocrine L-cells, which are specialized and electrically excitable epithelial cells that interface with the gut lumen, secrete peptide hormone glucagon-like peptide1, and are directly innervated by enteric afferents [120]. In addition to stimulating nerve-connected L-cells, indole can also cross the blood–brain barrier, granting microbially derived metabolites direct access to host neural circuitry inside the brain [121]. In a recent study using a rat model, the effect of indole derivatives oxindole and isatin on depressive and anxiety-related actions was examined [122]. Rats injected with an acute high dose of these indole derivatives in the cecum displayed lower motor activity and increased anxious behaviors [122]. To test the effect of a less extreme condition, gnotobiotic rats were inoculated with indole-producing E. coli to generate more constant and medial indole levels [122]. Rats with indole-producing bacteria were found to display more anxiety behaviors as compared to their uninoculated counterparts [122], suggesting that even modest concentrations of elevated indole promote anxiety.
Somewhat contrasting to the behavioral effects observed by Jaglin et al. (2018), Wei et al. (2021) reported that indole promoted neurogenesis of adult brain tissue in mice [123]. Mice treated with indole, both through systemic inoculation and via monocolonization with E. coli, showed increased neuronal growth and greater circuitry development than germ-free mice without access to indole [123]. Indole’s part in reviving neurogenesis in the adult mouse brain, where neural growth is typically on the decline, is linked back to AhR-mediated signaling and the upregulation of β-catenin, Neurog2, and VEGF-alpha in the hippocampus [123]. The results of this study, which investigated both mechanistic and holistic actions, underscore the importance of microbial metabolites in regulating host neurology.
3.2. Serotonin
In contrast to indole, serotonin can be synthesized both by gut microbes and by the host in various tissues. Serotonin (5-hydroxytryptamine) and melatonin (N-acetyl-5-methoxytryptamine) are well known as mood- and sleep-regulating neurotransmitters. Though a key element in neural activity, only 5% of the body’s serotonin is found in the brain, the remaining 95% of serotonin can be found instead in the gastrointestinal tract, primarily in serotonin-producing enterochromaffin cells of the gut barrier [85]. Serotonin is also the precursor of melatonin, which is synthesized in both humans and microbes via a two-step acetylation and methylation process [124]. Studies with gnotobiotic mice indicated that although gut serotonin is produced mostly by host cells, the microbiome still plays a crucial role in the regulation of serotonin production, both via the consumption or production of metabolic precursors and through control of host activity by signaling cascades [125].
Human production of serotonin in the gut primarily takes place in the epithelial enterochromaffin cells via the activity of the rate-limiting Trp hydroxylase enzyme (TPH1). Although the serotonin-producing enterochromaffin cells of germ-free mice are normally developed, these germ-free mice are deficient in serotonin and display a Trp buildup in the large intestine [126]. In conjunction with decreased serotonin production, the expression of serotonin transporter SLC6A4 is increased, likely to compensate for the diminished levels. This same trend of decreased serotonin and increased Trp is not observed in the inherently microbially sparse small intestine [126]. To explain this microbial-induced serotonin deficit, one study inoculated gnotobiotic mice with altered Schaedler flora, a defined consortia of gut microbes that is widely used with mice models. Interestingly this study found that the deficit remained even in these minimally colonized mice [126]. Colonic serotonin levels did increase back to normal levels after inoculation with spore-forming members of the specific pathogen-free microbiota, mostly clostridial species, via stimulation of TPH1 expression in enterochromaffin cells [126]. Gnotobiotic mice studies have demonstrated that the microbiome is responsible for modulating around 64% of the colonic serotonin concentration and affected almost half (49%) of the circulating serotonin [126,127]. It appears that the microbial control of serotonin production by the host is not a one-way mechanism, as gut bacteria have also been shown to modulate their activity in response to colonic serotonin concentrations. Increased luminal serotonin levels stimulate bacterial synthesis of serotonin [126] and serotonin produced in the GT improves the local integrity of the enteric nervous system, which then regulates gut motility and nutrient absorption [128]. Circulating serotonin has been reported to be excluded from crossing the blood–brain barrier (BBB) due to its slight polarity and is instead produced in the brain by the raphe nuclei from free Trp, which does cross the BBB [129]. It is noteworthy that BBB permeability is altered by many factors including age, temperature, and pharmaceuticals as extensively reviewed by Zhao, Gan [130]. Pathogens can also alter BBB permeability or take advantage of decreased barrier integrity, as seen with uropathogenic Escherichia coli reducing activity in the TGFBRII/Gli-2 signaling pathway that normally works to maintain barrier integrity [131]. Changes in the BBB integrity open the door for normally blocked substrates, like serotonin and other small molecules, to cross this barrier and gain access to the brain. The reported inability of serotonin to cross the BBB suggests that high levels of serotonin produced in the GT has a function outside of regulating behavior via direct interaction with receptors located in the brain. Indeed, serotonin is a potent and ubiquitous neurotransmitter involved in the pulmonary, enteric, immune, and circulatory systems that remains to be studied for the exact linkage from the GT to the brain and the possible behavior changes that this compound can impart [132,133,134].
Melatonin, like serotonin, can be produced by the host and when secreted by the pineal gland in the brain, melatonin exhibits behavioral control. Host melatonin production in the brain typically follows an exogenous light cycle to sync host behavior to light exposure [135]. Interestingly, it is also produced by enterochromaffin cells in the gut at concentrations 400 times higher than what has been observed for pineal gland production [136]. In the gut, melatonin contributes to the relaxation of gut tissue and lower overall motility, effects which oppose those of serotonin [137]. Exogenous melatonin administration alleviated some irritable bowel (IBS) symptoms, potentially due to the relaxation regulation by melatonin [138], but further studies are needed to pinpoint a specific mode of action. Additionally, melatonin supplementation was shown to reduce obesity-related bacterial taxa in mice on a high-fat diet, restoring the microbiota to that resembling the one associated with normal chow mice [139]. Melatonin may also be a potential therapeutic agent in neurological disorders like multiple sclerosis and Huntington’s disease [140], but more work in this area is needed to understand the mechanics of this potential metabolite as a therapeutic. The regulation of host and microbe activity by gut-derived serotonin and melatonin is a crucial aspect of the gut–brain connection. Though these bioactive Trp metabolites may be well known, they are not necessarily well understood. Further evaluation of how serotonin and melatonin regulation may play into the control of neurological disease will be critical for the development of advanced disease treatments.
One aspect of melatonin that also requires further study is its role as a signaling molecule amongst gut microbes. Multiple studies have reported the use of indole as a mechanism for bacterial communication, allowing both for symbiotic coordination amongst gut bacteria and conveying the existence of competitive environmental conditions [19,141,142]. Gut-derived melatonin may also support chrono-related bacterial changes. Klebsiella (formerly Enterobacter) aerogenes, a human commensal organism, shows increased motility with increased luminal melatonin levels, indicating that the host secretion may be regulating the microbial activity in a circadian-dependent manner [143]. In vitro work provided evidence that K. aerogenes regulates key physiological pathways in response to melatonin exposure, including pathways involved in stress response, carbohydrate transport, and metal ion homeostasis [144]. This same study also showed circadian rhythm-dependent synchronization of gene expression across K. aerogenes cultures in response to melatonin concentration [144]. Though this same depth of study regarding the gene expression and growth response of a single gut commensal to melatonin has not been conducted in many other microbes, it stands to reason that the circadian clock and sensitivity to melatonin is not unique to K. aerogenes. The exploration of circadian rhythmicity in gut bacteria may provide an even more direct connection between host homeostatic rhythms and microbiome activity, further tightening the link between hosts and their microbes.
3.3. Kynurenine
Utilizing approximately 90% of Trp, the kynurenine pathway is a robust multistep metabolic pathway with numerous bioactive intermediates and the terminal product of NAD+, as well as other small molecules [145]. Kynurenine metabolism produces both neuroactive and neurotoxic products which creates a ‘push–pull’ network that depends on other aromatic compound intermediates in the overall pathway. This complex metabolic pathway has garnered interest for its many bioactive metabolites and for its role in neurological diseases [146,147].
The production of kynurenic acid, a single-step metabolite of kynurenine, pulls the pathway flux from the production of neurotoxic quinolinic acid; thereby detoxifying the local niche. Due in part to this metabolic trade-off, kynurenic acid is thought to have some neuroprotective properties [148]. In a study looking at metabolic markers of fibromyalgia and chronic fatigue syndrome, the kynurenic acid/quinolinic acid ratio decreased in patients with chronic fatigue syndrome [148] and kynurenic acid/3-hydroxykynurenine ratios decreased in patients with fibromyalgia compared to healthy patients [148]. These findings suggest these ratios could serve as potential biomarkers to evaluate the overall metabolite balance of patients. A different study, investigating kynurenine levels in the blood of dementia patients over a duration of 5 years, found that both unusually high and low levels of circulating kynurenine correlated to negative cognition prognoses [149]. During this work, the investigators looked more holistically at the pathway and related metabolic ratios and found that mean kynurenine serum levels did not appear to correlate with progressing dementia [149]. Instead, a non-linear relationship was detected where deviation from midline kynurenine concentrations in either direction was associated with more extreme neurological and dementia symptoms [149]. Kynurenine metabolites have also been linked to bipolar disorder, which is a complex neurological disorder that affects approximately 2% of the population [150,151]. Notably, recent meta-analyses have shown there is a connection between metabolites of the kynurenine pathway and bipolar disorder, but the mechanistic and possibly causative relationship remains less well understood [150,151]. Though the causative relationship may not yet be fully elucidated, it is nevertheless clear that metabolites stemming from the kynurenine pathway play key roles as markers of disease or perhaps even causative agents.
Though current scientific literature has abundant coverage of the widespread implications and potential systemic results of the dysregulation of the kynurenine pathway, a deep dive into the many branches of this pathway is outside the scope of this review. Fortunately, there are many excellent reviews on this subject [152,153,154,155,156]. Instead, the remainder of this review will focus solely on one kynurenine metabolite that may hold the key to understanding a subset of neurological disease in the host–microbe context: anthranilic acid.
4. Anthranilic Acid and Beyond
Anthranilic acid, a kynurenine metabolite produced by host and microbe alike, is a bioactive compound with potential systemic neurological effects. Though in the past much research has focused on the kynurenine pathway at large, the role of anthranilic acid in the gut–brain axis is emerging as an important piece of the puzzle. Anthranilic acid is a direct metabolic product of kynurenine digestion in humans and microbes and represents an alternate branch to two other immediate products (i.e., 3-hydroxykynurenine and kynurenic acid) [157]. Kynurenine is hydrolyzed to anthranilic acid and L-alanine with the help of kynureninase. With the transfer of an amino group, kynurenine becomes the alternate product kynurenic acid, and the addition of an oxygen by kynurenine-3-monoxygenase turns kynurenine into 3-hydroxykynurenine. Anthranilic acid, through non-specific hydroxylation, becomes 3-hydroxyanthranilic acid, which is the precursor for quinolinic acid, picolinic acid, and the terminal product NAD+ [157]. The ratio of anthranilic acid to the downstream 3-hydroxyanthranilic acid has been suggested as one potential biomarker for both neurological and physiological disorders [55]. Given its pivotal position as a diverging branch of the kynurenine pathway, its bioactive properties, and the dual production by host and microbes, anthranilic acid is a key Trp metabolite to study in the context of the gut–brain axis.
Pharmaceutical research has built on the inherent production and utilization of anthranilic acid metabolites in vivo for the creation of novel drugs and therapeutics. A 2021 review of anthranilic acid medicinal research highlights the bioactivity of anthranilic acid derivatives [158]. In this review, anthranilic acid is discussed as a pharmacore, which is a basic compound that is biologically active and that provides a starting point for more complex pharmaceuticals. Specific substitutions and additions on the basic anthranilic structure, 2-amino benzoic acid, yielded active therapeutics with a wide array of targets, and anthranilic acid analogues are currently in use for the treatment of multiple metabolic disorders and as anti-inflammatories. Analogues created in pharmaceutical research have exhibited anti-viral properties, activity on drug-resistant cancer cells, and some varied effects on basic cellular processes like the hedgehog signaling pathway [158]. Other reviews and research in this area have likewise supported the notion of anthranilic acid as a key contributor to pharmaceutical research, due in large part to the highly active role anthranilic acid already has in vivo [159,160].
As noted above in the context of pharmaceuticals and further outlined in the sections below, anthranilic acid and chemically similar compounds are important in host physiology beyond the localized intestinal tract. Dual production of these compounds by the host and the microbiome increases the complexity of this host–metabolite interaction. A more mechanistic understanding of anthranilic acid as an agent of neurological disease is imperative.
4.1. Human Production of Anthranilic Acid and Derivatives
The production of anthranilic acid by humans is integral to this review since it supports the concept of co-production between the host and the microbiome of a bioactive compound. Though the gut microbiome contributes many important and biologically relevant metabolites, humans make their own contributions to systemic metabolism. Many host tissues and cell types, including neurons and macrophages, have the enzymatic repertoire to support most or all of the kynurenine pathway [161,162,163]. There are various drivers of the kynurenine pathway in vivo and many factors that exert control over the divergence into the many branches and resultant metabolites.
In vitro studies have shown the kynurenine pathway in multiple human cell types can be induced and specifically altered by interferon-gamma [164]. Macrophages in particular were driven towards the production of 3-hydroxyanthranilic acid from L-tryptophan via interferon-gamma activation [164]. A recent review has further highlighted the interplay of immune markers and regulation of the kynurenine pathway, stating that key to this relationship is the indoleamine 2,3-dioxygenase enzyme (IDO) [165]. IDO appears to participate in crosstalk with markers of inflammation and though IDO plays a secondary role in pushing TRP towards kynurenine and further down, kynurenine-metabolites under homeostatic conditions, the onset of inflammation drives IDO activity [165]. Pro-inflammatory cytokines, IL6, TNF-alpha, and IL-4, drive the expression of IDO through shared signaling pathways, like through the activation of AHRs [166]. IDO expression creates an environment that suppresses the immune response through the production of neurotoxic metabolites and depletion of the central immune-mediating amino acid Trp [167]. Multiple studies have also revealed that IDO is highly induced in colonic tissue and mucosa of patients with IBD and Crohn’s diseases, both diseases that are marked by chronic inflammatory states in the gut and potential microbial interference, but for which the underlying taxa and mechanisms are currently unknown [168,169]. Inflammation is often correlated, if not implicated, with neurological disorders and declining health. The tight partnership between IDO activity and inflammatory markers may provide one piece of the metabolic–neurologic activity relationship puzzle. Tryptophan 2,3-dioxygenase (TDO), like IDO, is a rate-limiting enzyme that controls conversion of Trp into the early metabolites of the kynurenine pathway [18]. Whereas IDO can be found throughout host tissues, it is primarily expressed in the liver, where 90% of kynurenine metabolites are produced [170]. Hormonal cascades in the host, such as those induced by stress, have been shown to drive TDO activity in the liver and cause a resultant increase in circulating kynurenine metabolites [170].
Hepatic circulation and utilization of Trp metabolites are not limited to the kynurenine pathway. Tryptamine, produced from the decarboxylation of Trp by gut microbes, attenuates pro-inflammatory cytokine production in the liver [171]. Skatole is another Trp derivative, specifically a product of the indole pathway, that is produced in the gut by microbes but potentially bioactive in the liver. One study found that patients without liver disease (hepatic encephalopathy) had no detectable serum skatole, but those with signs of liver disease had detectable skatole levels between 0 and 442 nmol/L [172]. This study did not test the directionality of skatole levels and so did not postulate whether skatole was a causative agent of disease or a byproduct [172]. Skatole may be toxic at high concentrations in the body, but a recent study showed skatole may attenuate hyperlipidemic conditions of the liver [173]. Trp metabolites produced by microbes in the gut, like skatole and tryptamine, find their way to the liver through hepatic circulation and once localized, can either attenuate or exacerbate disease conditions. p-Cresol, a product of amino acid fermentation and [2] mentioned previously in relation to autism spectrum disorders, is another metabolite that is derived from microbial activity that enters hepatic circulation. Modification of p-cresol in the liver gives rise to uremic toxins and can intensify renal disease as well as promote systemic inflammation [2]. Microbial compounds in the gut that make their way to the liver through hepatic circulation can then be further modified by the host, altering catabolite activity. Though liver activity is integral to understanding the interaction of Trp metabolites and host health, the gut–liver axis is not the direct focus of this review. More in-depth coverage of the gut–liver axis and the contributions of gut microbes can be found in other reviews [2,174,175].
There are internal controls of metabolic-related enzymatic activity in the liver, and the original limiting factor for the kynurenine pathway is ultimately the availability of the primary substrate, Trp. As Trp is an essential amino acid only acquired from diet and microbial metabolism, the availability of Trp in the gut is a necessary starting point for the estimation of circulating secondary metabolites. An 8-week-long high-fat diet in mice resulted in a measurable perturbation of gut taxa and subsequently significantly altered the gut, serum, and liver metabolome [171]. The gut microbial community has been shown to be a key driver of metabolism and so it cannot be discounted in the discussion around circulating kynurenine metabolites like anthranilic acid. Despite our understanding of the human contribution to circulating anthranilic acid compounds, it is not always possible to disentangle host and microbe metabolisms from one another. The inability to fully dissociate the production of anthranilic acid complicates the already challenging problem of tracing this metabolite’s biological activity in the host. Just as is it important to contextualize anthranilic acid research with the potential metabolic contributions of the humans being studied, so too is it important to understand microbial contributions to the shared metabolic pool.
4.2. Bacterial Production of Anthranilic Acid and Derivatives
The gut microbiome has the functional capacity to produce anthranilic acid and downstream metabolites from the breakdown of kynurenine, and ultimately from the original source of Trp. Research on microbial production of anthranilic acid and its derivatives mostly focuses on industrially applicable microbes, which produce anthranilic acid in large quantities Anthranilic acid, and its derivatives, are used in the pharmaceutical industry for a myriad of applications, including as antimicrobials and as a potential regulator of diabetic symptoms [158]. In the food industry, the anthranilic acid derivative, methyl-anthranilate, is used as a flavor compound to impart a grape essence to candies and other food items [176]. Many microbes, though it may vary by species, have the enzymatic repertoire to perform either all or part of the kynurenine pathway [177]. A search using the OrthoDB database revealed six major bacterial phyla contain organisms with the kynureninase gene (KYNU), which catalyzes the conversion of kynurenine to anthranilic acid (Figure 3) [178]. Anthranilic acid to 3-hydroxyanthranilic acid is a non-specific hydroxylation step, but the conversion of 3-hydroxyanthranilic acid to subsequent quinolinic acid relies on the expression of 3-hydroxyanthranilate 3,4-diozygenase (HAAO). Five major phyla contain organisms with the HAAO gene, including the gut-dominating Firmicutes and Bacteroidetes (Figure 3) [178]. The microbial production of anthranilic compounds in the gut is an important, though understudied, area of research on Trp-related neuroactive compounds.
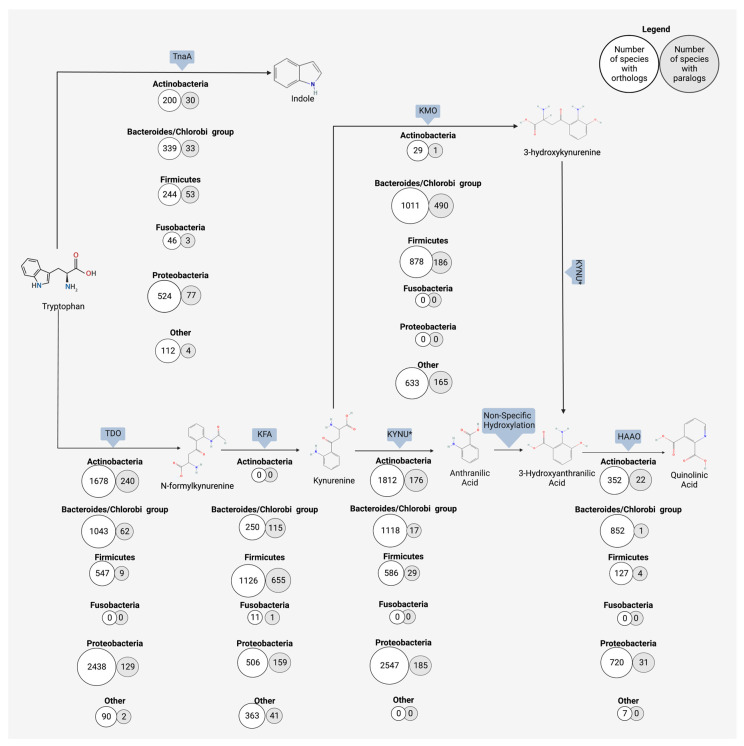
Figure 3.
Orthologous and paralogous genes encoding enzymes for the microbial metabolism of tryptophan towards the kynurenine and indole pathways. The number of phyla containing orthologs of the microbial enzyme responsible for each reaction was determined through a search using the OrthoDB database. Enzymes or non-specific reaction types are indicated by rectangular shapes above each phyla list. White circles indicate the number of species in the taxa containing orthologs and gray circles represent the number of species containing paralogs in that phyla. Six chosen phyla are shown, and all other phyla with hits are grouped into “other”. Indole is derived from tryptophan digestion by tryptophanase (TnaA). Down an alternate route from indole, kynurenine can be produced from tryptophan with a two-step process involving tyrptophan 2,3-dioxygenase (TDO) and kynurenine formamidase (KFA). The breakdown of the resulting kynurenine to anthranilic acid by microbes is catalyzed by kynureninase (KNYU). KYNU also catalyzes the reaction of 3-hydroxykynurenine to 3-hydroxyanthranilic acid; this overlap in activity is denoted by an asterisk (*). Anthranilic acid is converted to 3-hydroxyanthranilic acid via non-specific hydroxylation. 3-hydroxyanthranilic acid can then be converted to multiple downstream metabolites, such as picolinic acid (not shown) or quinolinic acid (shown here). The production of quinolinic acid from 3-hydroxyanthranilic acid is catalyzed by 3-hydroxyanthranilate 3,4-dioxygenase (HAAO).
Studies that focus on the production of anthranilic acid by gut microbiota many times rely on a more universal approach to this analysis, highlighting the production in the gut by broad functional groupings and not by a specific taxon. The difficulty of teasing apart microbes and their metabolic products in the gut is in large part due to the complex interactions between microbes and the present diversity [177], which is coupled to a very high genomic diversity of microbiome members [179,180]. One point that is indisputable, however, is that the gut microbiota plays a major role in shifting the availability of free Trp within the host. Germ-free mice have altered free Trp levels compared to inoculated mice and show increased kynurenine pathway metabolites [181,182]. The gut microbiota at large has the capacity to support the kynurenine pathway in the gut. Previous work demonstrated that the genomes of gut bacteria contain the necessary enzyme homologs to produce anthranilic acid and kynureninase, as well as other enzymes responsible for producing neuroactive metabolites in this pathway [97,177]. The strain or species that have the higher or lower metabolic capacity for Trp turnover are largely missing from the literature in this area.
The ability to produce anthranilic acid or other Trp-related neuroactive metabolites may be species specific. Metabolic production is also context specific, as the host dietary intake and the resulting concentrations of these shared metabolic products shifts the equilibrium for both microbe and host. Human pathogen Psuedomonas can produce multiple components of the kynurenine pathway [49]. More relevant to this review, however, is that P. aeruginosa produces anthranilate and then uses it as a precursor to several virulence factors [49]. Kurnasov and colleagues characterized the anthranilate pathway in prokaryotes by conducting a genome comparison and searching for three enzymes, tryptophan 2,3-dioxygenase, kynurenine formamidase, and kynureninase, which together produce a eukaryotic-like anthranilate pathway [43]. Among others, Burkholderia fungorum, Bacillus cereus, Ralstonia solanacearum, and Ralstonia metallidurans were found to encode for all three anthranilate-related enzymes [43]. Another study looking at cold stress in pigs and its effect on microbiome diversity and activity identified anthranilic acid as one of the few metabolites that increased under cold stress conditions [183]. Prevotella UCG-003 was revealed to be the most strongly correlated microbe with this cold stress-induced increase in anthranilic acid [183]. It is well defined that the gut microbiota is an important contributor to the production of neuroactive compounds from Trp.
4.3. Neurological Outcomes of Anthranilic Acid
Research on the mechanistic role of anthranilic acid in neurological diseases has been limited up until now. Many studies focus primarily on the use of anthranilic acid and its derivatives as biomarkers [184], but do not necessarily drill down to determine the causative or protective effects of these compounds on host systems. While there is a place for studying these bioactive metabolites from the perspective of diagnostic potential, future therapeutic development will inevitably rest squarely on a mechanistic understanding of metabolic bioactivity. Current literature suggests that anthranilic acid leads a double life; the metabolite appears to exert neuroprotective effects in some cases while displaying neurotoxic effects in others [185,186]. The potential neurological activity of anthranilic acid in vivo is complex and at times conflicting. Anthranilic acid resides at the increasingly explored intersection of diet, microbe, and host neuroactivity, and a more thorough understanding of anthranilic acid’s bioactivity is important for future use as a biomarker or therapeutic target.
Anthranilic acid is often seen as part of the neurotoxic branch of the kynurenine pathway, a branch that includes quinolinic acid and hydroxykynurenine [187,188]. Elevated serum levels of anthranilic acid have been measured in people with schizophrenia and in those diagnosed with Parkinson’s disease [184,189,190]. Oxenkrug and colleagues showed circulating anthranilic acid levels in patients with schizophrenia were two times higher than those of the control population, confirming the results of a previous rat model study [184]. Though the study could not attribute the elevated anthranilic acid to anything specific, such as increased enzymatic activity in the pathway, the authors did affirm that the increase was not correlated with anti-psychotic drug intake [184]. Adding another layer of complexity to this puzzle is the difference seen between the sexes. One study found that increased circulating anthranilic acid in schizophrenic subjects was not only related to neurological diagnosis, but also correlated with being female [189]. Females in the study had plasma anthranilic acid levels 27% greater than those of their male counterparts, all of whom had a schizophrenic diagnosis [189]. It is possible this difference could be explained by sex hormones. For example, estrogen can inhibit some enzymes of the kynurenine pathway, or potentially by the deficiency of these enzymes resulting from other multifaceted signaling cascades that differ between biologically male and female subjects [191,192]. The impact of estrogen on other signaling pathways has already been shown with another host and microbe-derived neurotransmitter, dopamine. Previous work established estrogen affects dopamine activity, typically boosting synthesis and release, in both animal models and follow-up human studies [193]. Gut bacteria, including E. coli, some Bacillus species, Klebsiella pneuomoniae, and Staphylococcus aureus, have been reported to produce dopamine from tyrosine [194]. Though primarily thought of as a mammal’s molecule, dopamine may be produced by bacteria as a quorum-sensing molecule, increasing motility and biofilm formation, or as a growth-enhancing factor, through improved iron regulation [194]. The interplay between the hormone estrogen, neurotransmitter dopamine, and microbial production of these bioactive compounds is already established in the literature, so it is not surprising to see emerging literature suggesting a hormone–metabolite crosstalk in the context of other microbially produced bioactive metabolites like kynurenine.
Beyond schizophrenia, other neurological conditions (e.g., chronic migraines) have been correlated to the levels of circulating anthranilic acid. A study on chronic migraines in humans tracked the serum levels of multiple kynurenine metabolites and found a 339% increase in the concentration of anthranilic acid in those suffering from chronic migraines [195]. In tandem with that increase, the same study showed a 63% decrease in the downstream metabolite 3-hydroxyanthranilic acid [195]. Importantly, the study excluded subjects with neurological comorbidities, suggesting that this marked an increase in anthranilic acid was specific to migraines and not due to a known underlying cause. It is possible that the raised anthranilic acid level is a response to neural activity and simply marks the diagnosis of chronic migraines. More interestingly, the authors of this study propose a combination of N-methyl D-aspartate (NMDA) receptor overactivity in migraines and the reduction of neuroprotectant kynurenic acid, which correlates with an increase in anthranilic acid, which together contribute to migraine symptoms [195]. This same research group also demonstrated concurring results in patients with a similar diagnosis of repeated cluster headaches. Those with cluster headaches had a 54% increase in serum anthranilic acid and a 54% decrease in 3-hydroxyanthranilic acid as compared to the healthy control patients [196]. Taken together, these studies indicate anthranilic acid is certainly a part of headache-related diagnoses. It remains to be determined if this metabolite is a causative or a responsive biomarker.
Evaluation of depression’s metabolic roots has indicated that there are correlating Trp metabolite concentrations. In one study on circulating anthranilic acid in patients diagnosed with depression, anthranilic acid was found to be inversely correlated to the severity of depression, though this finding was dependent on sex [197]. As was indicated in the case of schizophrenia and biological sex, anthranilic acid levels associated with depression appeared more pronounced in women as compared to men in the same study [197]. This study was careful to exclude medication as a factor. Previous work has noted a difference in circulating kynurenine metabolites between medicated and non-medicated subjects [198], making medication an additional confounding factor for gut–brain axis research. A study detailing the onset of depression in patients undergoing treatment for Hepatitis C showed a decrease in Trp availability with a corresponding increase in anthranilic acid circulation [199]. During this 24-week longitudinal study, subjects with increased anthranilic acid levels were significantly more likely to be diagnosed with major depressive disorder [199]. The role of anthranilic acid as a biomarker, or even as a cause, of depression disorders is an ongoing area of research with promising findings for the development of potentially more targeted treatments. There are, however, conflicting studies which show no correlation or weak correlation between depressive symptoms and anthranilic acid serum concentrations [200]. As human studies are often rife with confounding factors, this conflict amongst studies comes as no surprise. Biological sex [189,197] and medication [198] can affect the availability of Trp metabolites and are examples of factors that are not always accounted for but can play significant roles in determining the outcomes of these studies. To obtain meaningful and reproducible results, it is imperative that mechanistic animal model approaches are employed for a more concrete resolution to the involvement of anthranilic acid in depressive disorders.
Contrasting to predicted neurotoxic properties, studies have suggested that anthranilic acid derivatives may have neuroprotective bioactivity [55,185]. Decreased 3-hydroxyanthranilic acid levels in stroke patients, along with the corresponding increase in precursor anthranilic acid, had a negative effect on health outcomes for recovering patients [201]. The inverse, increased 3-hydroxyanthranilic acid levels, is correlated with more signs of recovery in patients that suffered from a stroke [201]. 3-hydroxyanthranilic acid is a redox-active Trp metabolite. The increased levels of this compound in circulation may have a potent anti-inflammatory effect, stemming from both this redox activity and through the redirecting of anthranilic acid towards a more beneficial chemical structure [185,201]. In vitro work with 3-hydroxyanthranilic acid has shown this compound to modulate the pro-inflammatory activities of macrophages, ultimately reducing generalized inflammation [202]. Inflammation plays a known part in many neurological disorders, as highlighted by a recent review [203]. Altogether, this indicates the immunomodulatory activities, and specifically inflammation-regulating activities, of anthranilic acid derivatives which are key parts of this metabolite–host neurological link and a specific disease or etiology.
Research on Trp metabolites and their relation to neurological outcomes is ongoing and has resulted in an abundance of broad descriptive studies for Trp compound relationships in patients with neurological symptoms. Though examples have been identified for strong correlations between anthranilic acid and multiple disease states, not all neurological conditions are showing the same trend. Studies on multiple sclerosis in children and Huntington’s disease showed no or weak correlations with anthranilic acid [204,205]. A recent meta-analysis investigating the presence of Trp-associated compounds across multiple neurological studies showed specific diseases have a weak correlation with anthranilic acid [206]. Results from human–microbiome studies can sometimes be conflicting, underscoring the complexity of host health and metabolic activity research. However, the observation of the Trp and kynurenine metabolic pathway dysregulation as related to host health is well-supported in the literature, but the lack of correlation in cases means this neurological relationship is more complex than a single molecule or single enzymatic reaction. Instead, the relationship for a suite of metabolites to host neurology is likely owed to something more specific regarding the branching and balance (i.e., ‘push/pull’) of these metabolic pathways that are interconnected between the Trp concentration and the co-metabolism of the microbiome and the host.
The key to understanding this apparent paradox between neuroprotective and neurotoxic activity may be in part due to those metabolic pathways which are not linear. These pathways are dynamic networks with many forces directing the movement of metabolism between the arms of complex interconnecting routes and shunts. Anthranilic acid alters the availability of its own primary precursor, kynurenine, and in so doing changes the compound equilibrium. Anthranilic acid is an inhibitor of 3-hydroxyanthranilic acid oxidase (3-HAO) enzymatic activity, the inhibition of which results in decreased production of quinolinic and picolinic acid from 3-hydroxyantranilic acid [55]. Iron is likewise able to inhibit 2-HAO enzymatic activity [50]. Interestingly anthranilic acid can readily chelate this circulating iron, resulting in a complex interaction where iron may indirectly increase anthranilic acid concentrations through 3-HAO inhibition and the increased concentration may then sequester that very same iron [50,55].
In pharmaceutical applications, anthranilic acid and related compounds have exhibited anti-inflammatory properties [158,207]. In a study on rats modeling rheumatoid arthritis, a positive dose-dependent response to synthetic anthranilic acid derivative N-(3′,4′-dimethoxycinnamonyl) anthranilic acid (3,4-DAA) was observed [207]. 3,4-DAA is structurally highly similar with 3-hydroxyanthranilic acid and 3-hydroxykynurenic acid. Arthritic rats received a daily dose of 200, 300, or 400 mg/kg, and all dose groups displayed reduced inflammation via immunomodulatory activity [207]. Endogenous anthranilic acid is also suggested to have these same anti-inflammatory properties [55,208]. 3-hydroxyanthranilic acid also has illustrated anti-inflammatory and antioxidant properties. Such properties are partially derived from the metabolite’s induction of hemooxygenase-1, which is involved in the control of inflammation [185]. As metabolomics work is never black and white, focusing on a single metabolite alone does not provide a clear picture of potential bioactivity. Instead, it is necessary to examine the interplay of network flux, protein regulation, and gene expression, all in combination with an understanding of the host–microbe interplay.
Anthranilic acid, along with its related compounds, cannot be considered singularly neuroprotective or neurotoxic. Perhaps these Trp-related metabolites should be considered more broadly as neuroactive, leaving space for the host context as a driver of ultimate effect relative to the local niche conditions. In the context of human studies, the directionality of metabolic levels cannot be fully assessed. One study reported that increased 3-hydroxyanthranilic acid levels, along with decreased anthranilic acid, were correlated with more beneficial clinic outcomes in stroke patients [201]. Measured serum concentrations post disease diagnosis allows for the theory that many of these measured compounds are the clean-up or response crew rather than the instigators of disease conditions. There is also the additional component of host genetics and the predisposition to certain disease states to consider. Host genetics have been shown to predispose certain individuals to the onset of disease, as seen in some stroke cases [209] and with depressive symptoms [210]. Previous studies have also established that genetic variation across hosts is at least partially responsible, in conjunction with host diet and environment, for driving the composition of the gut microbiota [211,212]. As host genetics play a role in both disease state and microbial composition, it is important to also consider the host genotype in the study of metabolic drivers of disease. Some studies with conflicting metabolic findings may look to the host genotype as one possible explanation for subject-specific differences. To tease apart correlation and causality, along with host-specific findings, future research necessitates the use of tightly controlled studies and the additional use of in vitro models that utilize a time series design to understand the flux and interconversion between the Trp neuroactive catabolites.
5. Balancing the Microbes and Metabolites
Metabolic activity is a balancing act between different metabolic routes rather than a singular linear continuation to a compound in a static situation. Much of the current literature discussing bioactive compounds within the same metabolic network nods to this complexity by using ratios rather than absolute concentrations. The concentration of a single compound within a complex metabolic cascade does not often provide the necessary holistic perspective to determine potential health outcomes. Likewise, a singular focus on microorganisms via taxonomic analysis in this metabolic context is likely inadequate to describe a complete picture of such complex microbial interactions and relative abundance changes, even within the gut. Utilizing metabolic ratios, which highlight the push and pull nature of metabolism, in place of static concentrations is one key component for an improved study design. This change enables many complexities to be captured in a dynamic niche that leads considering functional rather than taxonomic location to analyzing microbial consortia. This approach could also be very helpful when considering the microbiome changes to understand what microbes, but also what pathways, are enriching or depleting within the community of metabolism. These combined approaches also enable a quantitative assessment to determine the ranked importance of metabolic routes as well as taxonomic groups as they change in the population relative abundance.
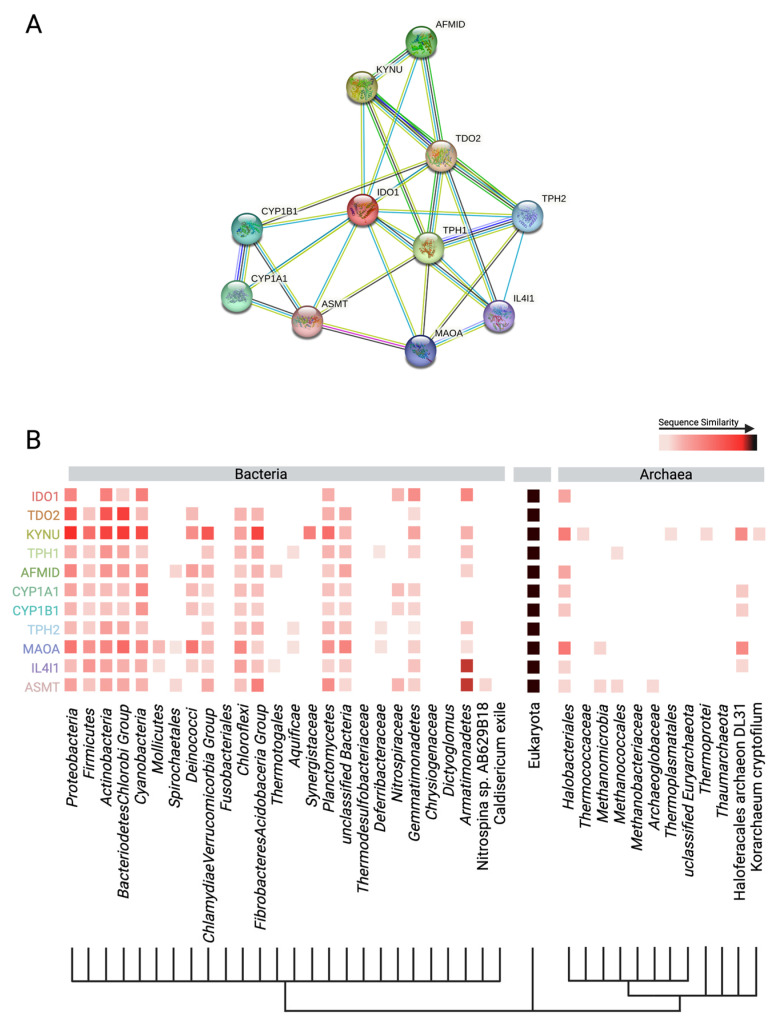
One part of a functional approach to host–microbe–metabolite interactions is looking at the population distribution of Trp-related metabolic genes in bacteria. Considering the push–pull method to regulate this pathway, it is very likely that specific steps in the pathway will be increased or decreased to change the intermediate concentrations as a flux balance. For example, when shunted towards the kynurenine pathway, Trp is first converted to kynurenine via tryptophan indoleamine 2,3-dioxygenase 1 or 2 (IDO1, IDO2) or via the liver enzyme tryptophan 2,3-dioxygenase (TDO) [177]. Kynurenine can then become kynurenic acid, 3-hydroxykynurenine, or anthranilic acid, which pulls the pathway towards other downstream activities [177]. 3-hydroxykynurenine and anthranilic acid both then feed into the production of 3-hydroxyanthranilic acid, the precursor for picolinic acid, quinolinic acid, and NAD+ [177]. A functional protein association network of human IDO1 (Figure 4) [213] confirms the interconnectedness and push–pull nature of the three Trp pathways. A central node of IDO1 shows kynureninase (KYNU), tryptophan 5-monoxygenase (TPH1), and kynurenine formamidase (AFMID) are all in a shared genetic neighborhood (Figure 4A). Gene co-occurrence, as determined using the STRING database [213], reveals a broad distribution of these kynurenine-related enzyme encoding genes across the bacterial kingdom, making the concept of a taxon ratio useful (Figure 4B). Higher resolution down to specific strains will be required to obtain more meaningful insights. Two dominant gut phyla, Firmicutes and Proteobacteria, both contain species with genes encoding KYNU, TPH1, AFMID. Some Proteobacteria also encode IDO1 but no Firmicutes exhibit this ability. A deeper look at Firmicutes reveals the distribution of these genes is skewed towards the Psuedomonadales order and, interestingly, Escherichia lack these four genes. The five Proteobacteria orders display a more even distribution of kynurenine enzyme-encoding genes, with Betaproteobacteria the only order lacking species encoding IDO1. Though the analysis here covers only a small piece of the larger Trp metabolic network, the broad distribution shown here of kynurenine metabolism genes amongst common gut phyla both highlights the complexity of metabolic-related research but also the necessity of a functional approach to such work.
Figure 4.
STRING database output from the search for Escherichia coli K12 MG1655 indole amine 2,3-dioxygenase 1 (IDO1), one enzyme responsible for the gut conversion of tryptophan to kynurenine. Full protein names are tryptophan 2,3-dioxygenase (TDO2), kynureninase (KYNU), tryptophan hydroxylase (TPH1), kynurenine formamidase (AFMID), human cytochromes P450 1A1 (CYP1A1) P450 1B1 (CYP1B1), tryptophan hydroxylase 2 (TPH2), monoamine oxidase A (MAOA), IL4-induced gene 1 (IL4I1), and acetylserotonin O-methyltransferase (ASMT). (A) Protein interaction network with IDO1 as the central node. Light blue connections indicate known interaction from curated databases; pink lines indicate the interaction has been experimentally validated. Light green connections indicate the encoding genes are in the same gene neighborhood; red-orange lines indicate gene fusions, and dark blue indicate gene co-occurrence. Black lines indicate co-expression and light blue indicate protein homology. (B) Gene co-occurrence across Bacteria, Eukarya, and Archaea of IDO1- encoding genes and related protein-encoding genes. Color indicates highest sequence similarity within the taxa, with darker reds and black indicating higher similarity.
6. Conclusions
Host neurology and microbial activity can work in union or conflict with each other, and thus the tight regulation of both is essential for optimal host health. A symbiotic partnership between the gut microbiota and host is a beneficial exchange of substrates that results in increased access to nutrients for the host and readily accessible dietary precursors for the microbes. When the balance shifts, however, the host can experience detrimental neurological effects that are either a result of or exacerbated by microbially produced metabolic products.
Trp is one metabolite whose balance must be carefully regulated. Trp is funneled into the indole, kynurenine, or serotonin pathway, all of which contain other bioactive molecules in their ranks. Some Trp-derived compounds can have positive effects on systemic host functions, like serotonin and the immune and circulatory systems. Other Trp-derived compounds, like quinolinic acid, have detrimental effects on host function and have connections to neurological disorders such as dementia. Another kynurenine-derived bioactive compound produced by both the host and microbe is anthranilic acid. As detailed above, anthranilic acid can be useful as a biomarker for disorders like Alzheimer’s but anthranilic acid may also have a promising future use as a potential therapeutic target for the treatment of devastating and currently incurable neurological diseases. The gut microbiome has the functional capacity to shift the balance of anthranilic acid and subsequent metabolites to the point of disease exacerbation or onset. Controlling this finely tuned metabolic network, especially in the context of the human microbiome, is a challenge that needs additional investigation before being used more practically as biomarkers or therapeutic targets.
Data Availability Statement
The STRING database can be accessed at https://string-db.org and Ortho DB can be accessed at https://orthodb.org.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Visconti A., Le Roy C.I., Rosa F., Rossi N., Martin T.C., Mohney R.P., Li W., de Rinaldis E., Bell J.T., Venter J.C., et al. Interplay between the human gut microbiome and host metabolism. Nat. Commun. 2019;10:4505. doi: 10.1038/s41467-019-12476-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anand S., Mande S.S. Host-microbiome interactions: Gut-Liver axis and its connection with other organs. NPJ Biofilms Microbiomes. 2022;8:89. doi: 10.1038/s41522-022-00352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ochoa-Reparaz J., Kasper L.H. The Second Brain: Is the Gut Microbiota a Link between Obesity and Central Nervous System Disorders? Curr. Obes. Rep. 2016;5:51–64. doi: 10.1007/s13679-016-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kastl A.J., Jr., Terry N.A., Wu G.D., Albenberg L.G. The Structure and Function of the Human Small Intestinal Microbiota: Current Understanding and Future Directions. Cell Mol. Gastroenterol. Hepatol. 2020;9:33–45. doi: 10.1016/j.jcmgh.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morowitz M.J., Carlisle E.M., Alverdy J.C. Contributions of intestinal bacteria to nutrition and metabolism in the critically ill. Surg. Clin. N. Am. 2011;91:771–785. doi: 10.1016/j.suc.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zoetendal E.G., Raes J., van den Bogert B., Arumugam M., Booijink C.C., Troost F.J., Bork P., Wels M., de Vos W.M., Kleerebezem M. The human small intestinal microbiota is driven by rapid uptake and conversion of simple carbohydrates. ISME J. 2012;6:1415–1426. doi: 10.1038/ismej.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez-Guryn K., Hubert N., Frazier K., Urlass S., Musch M.W., Ojeda P., Pierre J.F., Miyoshi J., Sontag T.J., Cham C.M., et al. Small Intestine Microbiota Regulate Host Digestive and Absorptive Adaptive Responses to Dietary Lipids. Cell Host Microbe. 2018;23:458–469.e5. doi: 10.1016/j.chom.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin J., Li R., Raes J., Arumugam M., Burgdorf K.S., Manichanh C., Nielsen T., Pons N., Levenez F., Yamada T., et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng L., He Z., Chen W., Holzman I.R., Lin J. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatr. Res. 2007;61:37–41. doi: 10.1203/01.pdr.0000250014.92242.f3. [DOI] [PubMed] [Google Scholar]
- 10.Riviere A., Selak M., Lantin D., Leroy F., De Vuyst L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016;7:979. doi: 10.3389/fmicb.2016.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim C.H. Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids. Cell Mol. Immunol. 2021;18:1161–1171. doi: 10.1038/s41423-020-00625-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neis E.P., Dejong C.H., Rensen S.S. The role of microbial amino acid metabolism in host metabolism. Nutrients. 2015;7:2930–2946. doi: 10.3390/nu7042930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macfarlane G.T., Allison C., Gibson S.A., Cummings J.H. Contribution of the microflora to proteolysis in the human large intestine. J. Appl. Bacteriol. 1988;64:37–46. doi: 10.1111/j.1365-2672.1988.tb02427.x. [DOI] [PubMed] [Google Scholar]
- 14.Yan Q., Rogan C.J., Pang Y.Y., Davis E.W., 2nd, Anderson J.C. Ancient co-option of an amino acid ABC transporter locus in Pseudomonas syringae for host signal-dependent virulence gene regulation. PLoS Pathog. 2020;16:e1008680. doi: 10.1371/journal.ppat.1008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma J., Zhu Y., Wang Z., Yu X., Hu R., Wang X., Cao G., Zou H., Shah A.M., Peng Q., et al. Glutamine supplementation affected the gut bacterial community and fermentation leading to improved nutrient digestibility in growth-retarded yaks. FEMS Microbiol. Ecol. 2021;97:fiab084. doi: 10.1093/femsec/fiab084. [DOI] [PubMed] [Google Scholar]
- 16.Roth W., Zadeh K., Vekariya R., Ge Y., Mohamadzadeh M. Tryptophan Metabolism and Gut-Brain Homeostasis. Int. J. Mol. Sci. 2021;22:2973. doi: 10.3390/ijms22062973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliphant K., Allen-Vercoe E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome. 2019;7:91. doi: 10.1186/s40168-019-0704-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao K., Mu C.L., Farzi A., Zhu W.Y. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. 2020;11:709–723. doi: 10.1093/advances/nmz127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar A., Sperandio V. Indole Signaling at the Host-Microbiota-Pathogen Interface. mBio. 2019;10:e01031-19. doi: 10.1128/mBio.01031-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boon N., Kaur M., Aziz A., Bradnick M., Shibayama K., Eguchi Y., Lund P.A. The Signaling Molecule Indole Inhibits Induction of the AR2 Acid Resistance System in Escherichia coli. Front. Microbiol. 2020;11:474. doi: 10.3389/fmicb.2020.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y., Xu J., Chen Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients. 2021;13:2099. doi: 10.3390/nu13062099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cryan J.F., O’Riordan K.J., Cowan C.S.M., Sandhu K.V., Bastiaanssen T.F.S., Boehme M., Codagnone M.G., Cussotto S., Fulling C., Golubeva A.V., et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019;99:1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 23.Guillemin G.J., Brew B.J. Implications of the kynurenine pathway and quinolinic acid in Alzheimer’s disease. Redox Rep. 2002;7:199–206. doi: 10.1179/135100002125000550. [DOI] [PubMed] [Google Scholar]
- 24.Heyes M.P., Saito K., Crowley J.S., Davis L.E., Demitrack M.A., Der M., Dilling L.A., Elia J., Kruesi M.J., Lackner A., et al. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Pt 5Brain. 1992;115:1249–1273. doi: 10.1093/brain/115.5.1249. [DOI] [PubMed] [Google Scholar]
- 25.Pappolla M.A., Perry G., Fang X., Zagorski M., Sambamurti K., Poeggeler B. Indoles as essential mediators in the gut-brain axis. Their role in Alzheimer’s disease. Neurobiol. Dis. 2021;156:105403. doi: 10.1016/j.nbd.2021.105403. [DOI] [PubMed] [Google Scholar]
- 26.Ting K.K., Brew B.J., Guillemin G.J. Effect of quinolinic acid on human astrocytes morphology and functions: Implications in Alzheimer’s disease. J. Neuroinflamm. 2009;6:36. doi: 10.1186/1742-2094-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiedlocha M., Marcinowicz P., Janoska-Jazdzik M., Szulc A. Gut microbiota, kynurenine pathway and mental disorders—Review. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2021;106:110145. doi: 10.1016/j.pnpbp.2020.110145. [DOI] [PubMed] [Google Scholar]
- 28.Schwarcz R., Bruno J.P., Muchowski P.J., Wu H.Q. Kynurenines in the mammalian brain: When physiology meets pathology. Nat. Rev. Neurosci. 2012;13:465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasan N., Yang H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ. 2019;7:e7502. doi: 10.7717/peerj.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart C.J., Ajami N.J., O’Brien J.L., Hutchinson D.S., Smith D.P., Wong M.C., Ross M.C., Lloyd R.E., Doddapaneni H., Metcalf G.A., et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. 2018;562:583–588. doi: 10.1038/s41586-018-0617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma Z.S., Li W., Shi P. Microbiome-host-phylogeny relationships in animal gastrointestinal tract microbiomes. FEMS Microbiol. Ecol. 2022;98:fiac021. doi: 10.1093/femsec/fiac021. [DOI] [PubMed] [Google Scholar]
- 32.Fontana F., Longhi G., Tarracchini C., Mancabelli L., Lugli G.A., Alessandri G., Turroni F., Milani C., Ventura M. The human gut microbiome of athletes: Metagenomic and metabolic insights. Microbiome. 2023;11:27. doi: 10.1186/s40168-023-01470-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franzosa E.A., Morgan X.C., Segata N., Waldron L., Reyes J., Earl A.M., Giannoukos G., Boylan M.R., Ciulla D., Gevers D., et al. Relating the metatranscriptome and metagenome of the human gut. Proc. Natl. Acad. Sci. USA. 2014;111:E2329–E2338. doi: 10.1073/pnas.1319284111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madi N., Chen D., Wolff R., Shapiro B.J., Garud N.R. Community diversity is associated with intra-species genetic diversity and gene loss in the human gut microbiome. eLife. 2023;12:e78530. doi: 10.7554/eLife.78530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersen L.M., Bautista E.J., Nguyen H., Hanson B.M., Chen L., Lek S.H., Sodergren E., Weinstock G.M. Community characteristics of the gut microbiomes of competitive cyclists. Microbiome. 2017;5:98. doi: 10.1186/s40168-017-0320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tremaroli V., Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 37.Braidy N., Guillemin G.J., Mansour H., Chan-Ling T., Grant R. Changes in kynurenine pathway metabolism in the brain, liver and kidney of aged female Wistar rats. FEBS J. 2011;278:4425–4434. doi: 10.1111/j.1742-4658.2011.08366.x. [DOI] [PubMed] [Google Scholar]
- 38.Gourbeyre P., Desbuards N., Gremy G., Tranquet O., Champ M., Denery-Papini S., Bodinier M. Perinatal and postweaning exposure to galactooligosaccharides/inulin prebiotics induced biomarkers linked to tolerance mechanism in a mouse model of strong allergic sensitization. J. Agric. Food Chem. 2013;61:6311–6320. doi: 10.1021/jf305315g. [DOI] [PubMed] [Google Scholar]
- 39.Bansal T., Alaniz R.C., Wood T.K., Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc. Natl. Acad. Sci. USA. 2010;107:228–233. doi: 10.1073/pnas.0906112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kountouras J., Chatzopoulos D., Zavos C. Reactive oxygen metabolites and upper gastrointestinal diseases. Hepatogastroenterology. 2001;48:743–751. [PubMed] [Google Scholar]
- 41.Xu M., Cen M., Shen Y., Zhu Y., Cheng F., Tang L., Hu W., Dai N. Deoxycholic Acid-Induced Gut Dysbiosis Disrupts Bile Acid Enterohepatic Circulation and Promotes Intestinal Inflammation. Dig. Dis. Sci. 2021;66:568–576. doi: 10.1007/s10620-020-06208-3. [DOI] [PubMed] [Google Scholar]
- 42.Genestet C., Le Gouellec A., Chaker H., Polack B., Guery B., Toussaint B., Stasia M.J. Scavenging of reactive oxygen species by tryptophan metabolites helps Pseudomonas aeruginosa escape neutrophil killing. Free Radic. Biol. Med. 2014;73:400–410. doi: 10.1016/j.freeradbiomed.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Kurnasov O., Jablonski L., Polanuyer B., Dorrestein P., Begley T., Osterman A. Aerobic tryptophan degradation pathway in bacteria: Novel kynurenine formamidase. FEMS Microbiol. Lett. 2003;227:219–227. doi: 10.1016/S0378-1097(03)00684-0. [DOI] [PubMed] [Google Scholar]
- 44.Ulvik A., Theofylaktopoulou D., Midttun O., Nygard O., Eussen S.J., Ueland P.M. Substrate product ratios of enzymes in the kynurenine pathway measured in plasma as indicators of functional vitamin B-6 status. Am. J. Clin. Nutr. 2013;98:934–940. doi: 10.3945/ajcn.113.064998. [DOI] [PubMed] [Google Scholar]
- 45.Tennoune N., Andriamihaja M., Blachier F. Production of Indole and Indole-Related Compounds by the Intestinal Microbiota and Consequences for the Host: The Good, the Bad, and the Ugly. Microorganisms. 2022;10:930. doi: 10.3390/microorganisms10050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nelp M.T., Kates P.A., Hunt J.T., Newitt J.A., Balog A., Maley D., Zhu X., Abell L., Allentoff A., Borzilleri R., et al. Immune-modulating enzyme indoleamine 2,3-dioxygenase is effectively inhibited by targeting its apo-form. Proc. Natl. Acad. Sci. USA. 2018;115:3249–3254. doi: 10.1073/pnas.1719190115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goncalves S., Nunes-Costa D., Cardoso S.M., Empadinhas N., Marugg J.D. Enzyme Promiscuity in Serotonin Biosynthesis, From Bacteria to Plants and Humans. Front. Microbiol. 2022;13:873555. doi: 10.3389/fmicb.2022.873555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu J., Hua X., Yang T., Guo M., Li Q., Xiao L., Li L., Chen J., Li T. Alterations in Gut Vitamin and Amino Acid Metabolism are Associated with Symptoms and Neurodevelopment in Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2022;52:3116–3128. doi: 10.1007/s10803-021-05066-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bortolotti P., Hennart B., Thieffry C., Jausions G., Faure E., Grandjean T., Thepaut M., Dessein R., Allorge D., Guery B.P., et al. Tryptophan catabolism in Pseudomonas aeruginosa and potential for inter-kingdom relationship. BMC Microbiol. 2016;16:137. doi: 10.1186/s12866-016-0756-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chobot V., Hadacek F., Weckwerth W., Kubicova L. Iron chelation and redox chemistry of anthranilic acid and 3-hydroxyanthranilic acid: A comparison of two structurally related kynurenine pathway metabolites to obtain improved insights into their potential role in neurological disease development. J. Organomet. Chem. 2015;782:103–110. doi: 10.1016/j.jorganchem.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vujkovic-Cvijin I., Dunham R.M., Iwai S., Maher M.C., Albright R.G., Broadhurst M.J., Hernandez R.D., Lederman M.M., Huang Y., Somsouk M., et al. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci. Transl. Med. 2013;5:193ra191. doi: 10.1126/scitranslmed.3006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phillips R.S. Structure and mechanism of kynureninase. Arch. Biochem. Biophys. 2014;544:69–74. doi: 10.1016/j.abb.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 53.Kuc D., Zgrajka W., Parada-Turska J., Urbanik-Sypniewska T., Turski W.A. Micromolar concentration of kynurenic acid in rat small intestine. Amino Acids. 2008;35:503–505. doi: 10.1007/s00726-007-0631-z. [DOI] [PubMed] [Google Scholar]
- 54.Kurnasov O., Goral V., Colabroy K., Gerdes S., Anantha S., Osterman A., Begley T.P. NAD biosynthesis: Identification of the tryptophan to quinolinate pathway in bacteria. Chem. Biol. 2003;10:1195–1204. doi: 10.1016/j.chembiol.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 55.Darlington L.G., Forrest C.M., Mackay G.M., Smith R.A., Smith A.J., Stoy N., Stone T.W. On the Biological Importance of the 3-hydroxyanthranilic Acid: Anthranilic Acid Ratio. Int. J. Tryptophan Res. 2010;3:51–59. doi: 10.4137/IJTR.S4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chirino B., Strahsburger E., Agullo L., Gonzalez M., Seeger M. Genomic and functional analyses of the 2-aminophenol catabolic pathway and partial conversion of its substrate into picolinic acid in Burkholderia xenovorans LB400. PLoS ONE. 2013;8:e75746. doi: 10.1371/journal.pone.0075746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang P., Jin T., Kumar Sahu S., Xu J., Shi Q., Liu H., Wang Y. The Distribution of Tryptophan-Dependent Indole-3-Acetic Acid Synthesis Pathways in Bacteria Unraveled by Large-Scale Genomic Analysis. Molecules. 2019;24:1411. doi: 10.3390/molecules24071411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams B.B., Van Benschoten A.H., Cimermancic P., Donia M.S., Zimmermann M., Taketani M., Ishihara A., Kashyap P.C., Fraser J.S., Fischbach M.A. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe. 2014;16:495–503. doi: 10.1016/j.chom.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jin U.H., Lee S.O., Sridharan G., Lee K., Davidson L.A., Jayaraman A., Chapkin R.S., Alaniz R., Safe S. Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol. Pharmacol. 2014;85:777–788. doi: 10.1124/mol.113.091165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miyagi M., Wilson R., Saigusa D., Umeda K., Saijo R., Hager C.L., Li Y., McCormick T., Ghannoum M.A. Indole-3-acetic acid synthesized through the indole-3-pyruvate pathway promotes Candida tropicalis biofilm formation. PLoS ONE. 2020;15:e0244246. doi: 10.1371/journal.pone.0244246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Y., Xu W., Zhang F., Zhong S., Sun Y., Huo J., Zhu J., Wu C. The Gut Microbiota-Produced Indole-3-Propionic Acid Confers the Antihyperlipidemic Effect of Mulberry-Derived 1-Deoxynojirimycin. mSystems. 2020;5:e00313-20. doi: 10.1128/mSystems.00313-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Montgomery T.L., Eckstrom K., Lile K.H., Caldwell S., Heney E.R., Lahue K.G., D’Alessandro A., Wargo M.J., Krementsov D.N. Lactobacillus reuteri tryptophan metabolism promotes host susceptibility to CNS autoimmunity. Microbiome. 2022;10:198. doi: 10.1186/s40168-022-01408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Konopelski P., Mogilnicka I. Biological Effects of Indole-3-Propionic Acid, a Gut Microbiota-Derived Metabolite, and Its Precursor Tryptophan in Mammals’ Health and Disease. Int. J. Mol. Sci. 2022;23:1222. doi: 10.3390/ijms23031222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cook K.L., Rothrock M.J., Jr., Loughrin J.H., Doerner K.C. Characterization of skatole-producing microbial populations in enriched swine lagoon slurry. FEMS Microbiol. Ecol. 2007;60:329–340. doi: 10.1111/j.1574-6941.2007.00299.x. [DOI] [PubMed] [Google Scholar]
- 65.Song L., He M., Sun Q., Wang Y., Zhang J., Fang Y., Liu S., Duan L. Roseburia hominis Increases Intestinal Melatonin Level by Activating p-CREB-AANAT Pathway. Nutrients. 2021;14:117. doi: 10.3390/nu14010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xie X., Ding D., Bai D., Zhu Y., Sun W., Sun Y., Zhang D. Melatonin biosynthesis pathways in nature and its production in engineered microorganisms. Synth. Syst. Biotechnol. 2022;7:544–553. doi: 10.1016/j.synbio.2021.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Broekhuizen M., Danser A.H.J., Reiss I.K.M., Merkus D. The Function of the Kynurenine Pathway in the Placenta: A Novel Pharmacotherapeutic Target? Int. J. Environ. Res. Public Health. 2021;18:11545. doi: 10.3390/ijerph182111545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mayer E.A., Knight R., Mazmanian S.K., Cryan J.F., Tillisch K. Gut microbes and the brain: Paradigm shift in neuroscience. J. Neurosci. 2014;34:15490–15496. doi: 10.1523/JNEUROSCI.3299-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ye L., Bae M., Cassilly C.D., Jabba S.V., Thorpe D.W., Martin A.M., Lu H.Y., Wang J., Thompson J.D., Lickwar C.R., et al. Enteroendocrine cells sense bacterial tryptophan catabolites to activate enteric and vagal neuronal pathways. Cell Host Microbe. 2021;29:179–196.e9. doi: 10.1016/j.chom.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu C.D., Xu Q.J., Chang R.B. Vagal sensory neurons and gut-brain signaling. Curr. Opin. Neurobiol. 2020;62:133–140. doi: 10.1016/j.conb.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prescott S.L., Liberles S.D. Internal senses of the vagus nerve. Neuron. 2022;110:579–599. doi: 10.1016/j.neuron.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaelberer M.M., Buchanan K.L., Klein M.E., Barth B.B., Montoya M.M., Shen X., Bohorquez D.V. A gut-brain neural circuit for nutrient sensory transduction. Science. 2018;361:eaat5236. doi: 10.1126/science.aat5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jacobson A., Yang D., Vella M., Chiu I.M. The intestinal neuro-immune axis: Crosstalk between neurons, immune cells, and microbes. Mucosal Immunol. 2021;14:555–565. doi: 10.1038/s41385-020-00368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Walker J.R., Ediger J.P., Graff L.A., Greenfeld J.M., Clara I., Lix L., Rawsthorne P., Miller N., Rogala L., McPhail C.M., et al. The Manitoba IBD cohort study: A population-based study of the prevalence of lifetime and 12-month anxiety and mood disorders. Am. J. Gastroenterol. 2008;103:1989–1997. doi: 10.1111/j.1572-0241.2008.01980.x. [DOI] [PubMed] [Google Scholar]
- 75.Bernstein C.N. Psychological Stress and Depression: Risk Factors for IBD? Dig. Dis. 2016;34:58–63. doi: 10.1159/000442929. [DOI] [PubMed] [Google Scholar]
- 76.Limbana T., Khan F., Eskander N. Gut Microbiome and Depression: How Microbes Affect the Way We Think. Cureus. 2020;12:e9966. doi: 10.7759/cureus.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bermudez-Martin P., Becker J.A.J., Caramello N., Fernandez S.P., Costa-Campos R., Canaguier J., Barbosa S., Martinez-Gili L., Myridakis A., Dumas M.E., et al. The microbial metabolite p-Cresol induces autistic-like behaviors in mice by remodeling the gut microbiota. Microbiome. 2021;9:157. doi: 10.1186/s40168-021-01103-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zheng Y., Prince N.Z., Peralta Marzal L.N., Ahmed S., Garssen J., Perez Pardo P., Kraneveld A.D. The Autism Spectrum Disorder-Associated Bacterial Metabolite p-Cresol Derails the Neuroimmune Response of Microglial Cells Partially via Reduction of ADAM17 and ADAM10. Int. J. Mol. Sci. 2022;23:11013. doi: 10.3390/ijms231911013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pascucci T., Colamartino M., Fiori E., Sacco R., Coviello A., Ventura R., Puglisi-Allegra S., Turriziani L., Persico A.M. P-cresol Alters Brain Dopamine Metabolism and Exacerbates Autism-Like Behaviors in the BTBR Mouse. Brain Sci. 2020;10:233. doi: 10.3390/brainsci10040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guzman-Salas S., Weber A., Malci A., Lin X., Herrera-Molina R., Cerpa W., Dorador C., Signorelli J., Zamorano P. The metabolite p-cresol impairs dendritic development, synaptogenesis, and synapse function in hippocampal neurons: Implications for autism spectrum disorder. J. Neurochem. 2022;161:335–349. doi: 10.1111/jnc.15604. [DOI] [PubMed] [Google Scholar]
- 81.Altieri L., Neri C., Sacco R., Curatolo P., Benvenuto A., Muratori F., Santocchi E., Bravaccio C., Lenti C., Saccani M., et al. Urinary p-cresol is elevated in small children with severe autism spectrum disorder. Biomarkers. 2011;16:252–260. doi: 10.3109/1354750X.2010.548010. [DOI] [PubMed] [Google Scholar]
- 82.Gabriele S., Sacco R., Cerullo S., Neri C., Urbani A., Tripi G., Malvy J., Barthelemy C., Bonnet-Brihault F., Persico A.M. Urinary p-cresol is elevated in young French children with autism spectrum disorder: A replication study. Biomarkers. 2014;19:463–470. doi: 10.3109/1354750X.2014.936911. [DOI] [PubMed] [Google Scholar]
- 83.Bosi A., Banfi D., Bistoletti M., Giaroni C., Baj A. Tryptophan Metabolites Along the Microbiota-Gut-Brain Axis: An Interkingdom Communication System Influencing the Gut in Health and Disease. Int. J. Tryptophan Res. 2020;13:1178646920928984. doi: 10.1177/1178646920928984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Karayol R., Medrihan L., Warner-Schmidt J.L., Fait B.W., Rao M.N., Holzner E.B., Greengard P., Heintz N., Schmidt E.F. Serotonin receptor 4 in the hippocampus modulates mood and anxiety. Mol. Psychiatry. 2021;26:2334–2349. doi: 10.1038/s41380-020-00994-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Banskota S., Ghia J.E., Khan W.I. Serotonin in the gut: Blessing or a curse. Biochimie. 2019;161:56–64. doi: 10.1016/j.biochi.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 86.Braniste V., Al-Asmakh M., Kowal C., Anuar F., Abbaspour A., Toth M., Korecka A., Bakocevic N., Ng L.G., Kundu P., et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Banerjee A., Herring C.A., Chen B., Kim H., Simmons A.J., Southard-Smith A.N., Allaman M.M., White J.R., Macedonia M.C., McKinley E.T., et al. Succinate Produced by Intestinal Microbes Promotes Specification of Tuft Cells to Suppress Ileal Inflammation. Gastroenterology. 2020;159:2101–2115.e5. doi: 10.1053/j.gastro.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De Vadder F., Kovatcheva-Datchary P., Zitoun C., Duchampt A., Backhed F., Mithieux G. Microbiota-Produced Succinate Improves Glucose Homeostasis via Intestinal Gluconeogenesis. Cell Metab. 2016;24:151–157. doi: 10.1016/j.cmet.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 89.Kawasoe J., Uchida Y., Kawamoto H., Miyauchi T., Watanabe T., Saga K., Tanaka K., Ueda S., Terajima H., Taura K., et al. Propionic Acid, Induced in Gut by an Inulin Diet, Suppresses Inflammation and Ameliorates Liver Ischemia and Reperfusion Injury in Mice. Front. Immunol. 2022;13:862503. doi: 10.3389/fimmu.2022.862503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yoshida H., Ishii M., Akagawa M. Propionate suppresses hepatic gluconeogenesis via GPR43/AMPK signaling pathway. Arch. Biochem. Biophys. 2019;672:108057. doi: 10.1016/j.abb.2019.07.022. [DOI] [PubMed] [Google Scholar]
- 91.Bellono N.W., Bayrer J.R., Leitch D.B., Castro J., Zhang C., O’Donnell T.A., Brierley S.M., Ingraham H.A., Julius D. Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways. Cell. 2017;170:185–198.e16. doi: 10.1016/j.cell.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ashniev G.A., Petrov S.N., Iablokov S.N., Rodionov D.A. Genomics-Based Reconstruction and Predictive Profiling of Amino Acid Biosynthesis in the Human Gut Microbiome. Microorganisms. 2022;10:740. doi: 10.3390/microorganisms10040740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Romeo E., Dave M.H., Bacic D., Ristic Z., Camargo S.M., Loffing J., Wagner C.A., Verrey F. Luminal kidney and intestine SLC6 amino acid transporters of B0AT-cluster and their tissue distribution in Mus musculus. Am. J. Physiol. Renal Physiol. 2006;290:F376–F383. doi: 10.1152/ajprenal.00286.2005. [DOI] [PubMed] [Google Scholar]
- 94.Pramod A.B., Foster J., Carvelli L., Henry L.K. SLC6 transporters: Structure, function, regulation, disease association and therapeutics. Mol. Aspects Med. 2013;34:197–219. doi: 10.1016/j.mam.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li Y., Liu N., Ge Y., Yang Y., Ren F., Wu Z. Tryptophan and the innate intestinal immunity: Crosstalk between metabolites, host innate immune cells, and microbiota. Eur. J. Immunol. 2022;52:856–868. doi: 10.1002/eji.202149401. [DOI] [PubMed] [Google Scholar]
- 96.Pardridge W.M., Fierer G. Transport of tryptophan into brain from the circulating, albumin-bound pool in rats and in rabbits. J. Neurochem. 1990;54:971–976. doi: 10.1111/j.1471-4159.1990.tb02345.x. [DOI] [PubMed] [Google Scholar]
- 97.Agus A., Planchais J., Sokol H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe. 2018;23:716–724. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 98.Chojnacki C., Konrad P., Blonska A., Medrek-Socha M., Przybylowska-Sygut K., Chojnacki J., Poplawski T. Altered Tryptophan Metabolism on the Kynurenine Pathway in Depressive Patients with Small Intestinal Bacterial Overgrowth. Nutrients. 2022;14:3217. doi: 10.3390/nu14153217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Singhal M., Turturice B.A., Manzella C.R., Ranjan R., Metwally A.A., Theorell J., Huang Y., Alrefai W.A., Dudeja P.K., Finn P.W., et al. Serotonin Transporter Deficiency is Associated with Dysbiosis and Changes in Metabolic Function of the Mouse Intestinal Microbiome. Sci. Rep. 2019;9:2138. doi: 10.1038/s41598-019-38489-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lai Y., Liu C.W., Yang Y., Hsiao Y.C., Ru H., Lu K. High-coverage metabolomics uncovers microbiota-driven biochemical landscape of interorgan transport and gut-brain communication in mice. Nat. Commun. 2021;12:6000. doi: 10.1038/s41467-021-26209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Van der Wielen N., Moughan P.J., Mensink M. Amino Acid Absorption in the Large Intestine of Humans and Porcine Models. J. Nutr. 2017;147:1493–1498. doi: 10.3945/jn.117.248187. [DOI] [PubMed] [Google Scholar]
- 102.Ye X., Li H., Anjum K., Zhong X., Miao S., Zheng G., Liu W., Li L. Dual Role of Indoles Derived from Intestinal Microbiota on Human Health. Front. Immunol. 2022;13:903526. doi: 10.3389/fimmu.2022.903526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee J.H., Lee J. Indole as an intercellular signal in microbial communities. FEMS Microbiol. Rev. 2010;34:426–444. doi: 10.1111/j.1574-6976.2009.00204.x. [DOI] [PubMed] [Google Scholar]
- 104.Lee J.H., Wood T.K., Lee J. Roles of indole as an interspecies and interkingdom signaling molecule. Trends Microbiol. 2015;23:707–718. doi: 10.1016/j.tim.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 105.Santana Machado T., Poitevin S., Paul P., McKay N., Jourde-Chiche N., Legris T., Mouly-Bandini A., Dignat-George F., Brunet P., Masereeuw R., et al. Indoxyl Sulfate Upregulates Liver P-Glycoprotein Expression and Activity through Aryl Hydrocarbon Receptor Signaling. J. Am. Soc. Nephrol. 2018;29:906–918. doi: 10.1681/ASN.2017030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Powell D.N., Swimm A., Sonowal R., Bretin A., Gewirtz A.T., Jones R.M., Kalman D. Indoles from the commensal microbiota act via the AHR and IL-10 to tune the cellular composition of the colonic epithelium during aging. Proc. Natl. Acad. Sci. USA. 2020;117:21519–21526. doi: 10.1073/pnas.2003004117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rattanaphan P., Mittraparp-Arthorn P., Srinoun K., Vuddhakul V., Tansila N. Indole signaling decreases biofilm formation and related virulence of Listeria monocytogenes. FEMS Microbiol. Lett. 2020;367:fnaa116. doi: 10.1093/femsle/fnaa116. [DOI] [PubMed] [Google Scholar]
- 108.Domka J., Lee J., Wood T.K. YliH (BssR) and YceP (BssS) regulate Escherichia coli K-12 biofilm formation by influencing cell signaling. Appl. Environ. Microbiol. 2006;72:2449–2459. doi: 10.1128/AEM.72.4.2449-2459.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hirakawa H., Kodama T., Takumi-Kobayashi A., Honda T., Yamaguchi A. Secreted indole serves as a signal for expression of type III secretion system translocators in enterohaemorrhagic Escherichia coli O157:H7. Microbiology (Reading) 2009;155 Pt 2:541–550. doi: 10.1099/mic.0.020420-0. [DOI] [PubMed] [Google Scholar]
- 110.Vega N.M., Allison K.R., Samuels A.N., Klempner M.S., Collins J.J. Salmonella typhimurium intercepts Escherichia coli signaling to enhance antibiotic tolerance. Proc. Natl. Acad. Sci. USA. 2013;110:14420–14425. doi: 10.1073/pnas.1308085110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Darkoh C., Chappell C., Gonzales C., Okhuysen P. A rapid and specific method for the detection of indole in complex biological samples. Appl. Environ. Microbiol. 2015;81:8093–8097. doi: 10.1128/AEM.02787-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhu C., Sawrey-Kubicek L., Beals E., Rhodes C.H., Houts H.E., Sacchi R., Zivkovic A.M. Human gut microbiome composition and tryptophan metabolites were changed differently by fast food and Mediterranean diet in 4 days: A pilot study. Nutr. Res. 2020;77:62–72. doi: 10.1016/j.nutres.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 113.Hubbard T.D., Murray I.A., Bisson W.H., Lahoti T.S., Gowda K., Amin S.G., Patterson A.D., Perdew G.H. Adaptation of the human aryl hydrocarbon receptor to sense microbiota-derived indoles. Sci. Rep. 2015;5:12689. doi: 10.1038/srep12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Petersen S.L., Curran M.A., Marconi S.A., Carpenter C.D., Lubbers L.S., McAbee M.D. Distribution of mRNAs encoding the arylhydrocarbon receptor, arylhydrocarbon receptor nuclear translocator, and arylhydrocarbon receptor nuclear translocator-2 in the rat brain and brainstem. J. Comp. Neurol. 2000;427:428–439. doi: 10.1002/1096-9861(20001120)427:3<428::AID-CNE9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 115.Sheu M.L., Pan L.Y., Sheehan J., Yang M.Y., Pan H.C. Modulation of Aryl Hydrocarbon Receptor Expression Alleviated Neuropathic Pain in a Chronic Constriction Nerve Injury Animal Model. Int. J. Mol. Sci. 2022;23:1255. doi: 10.3390/ijms231911255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kou Z., Dai W. Aryl hydrocarbon receptor: Its roles in physiology. Biochem. Pharmacol. 2021;185:114428. doi: 10.1016/j.bcp.2021.114428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Denison M.S., Faber S.C. And Now for Something Completely Different: Diversity in Ligand-Dependent Activation of Ah Receptor Responses. Curr. Opin. Toxicol. 2017;2:124–131. doi: 10.1016/j.cotox.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dong F., Perdew G.H. The aryl hydrocarbon receptor as a mediator of host-microbiota interplay. Gut Microbes. 2020;12:1859812. doi: 10.1080/19490976.2020.1859812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stockinger B., Di Meglio P., Gialitakis M., Duarte J.H. The aryl hydrocarbon receptor: Multitasking in the immune system. Annu. Rev. Immunol. 2014;32:403–432. doi: 10.1146/annurev-immunol-032713-120245. [DOI] [PubMed] [Google Scholar]
- 120.Buckley M.M., O’Brien R., Brosnan E., Ross R.P., Stanton C., Buckley J.M., O’Malley D. Glucagon-Like Peptide-1 Secreting L-Cells Coupled to Sensory Nerves Translate Microbial Signals to the Host Rat Nervous System. Front. Cell Neurosci. 2020;14:95. doi: 10.3389/fncel.2020.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Young S.N., Anderson G.M., Gauthier S., Purdy W.C. The origin of indoleacetic acid and indolepropionic acid in rat and human cerebrospinal fluid. J. Neurochem. 1980;34:1087–1092. doi: 10.1111/j.1471-4159.1980.tb09944.x. [DOI] [PubMed] [Google Scholar]
- 122.Jaglin M., Rhimi M., Philippe C., Pons N., Bruneau A., Goustard B., Dauge V., Maguin E., Naudon L., Rabot S. Indole, a Signaling Molecule Produced by the Gut Microbiota, Negatively Impacts Emotional Behaviors in Rats. Front. Neurosci. 2018;12:216. doi: 10.3389/fnins.2018.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wei G.Z., Martin K.A., Xing P.Y., Agrawal R., Whiley L., Wood T.K., Hejndorf S., Ng Y.Z., Low J.Z.Y., Rossant J., et al. Tryptophan-metabolizing gut microbes regulate adult neurogenesis via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA. 2021;118:e2021091118. doi: 10.1073/pnas.2021091118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Slominski A., Semak I., Pisarchik A., Sweatman T., Szczesniewski A., Wortsman J. Conversion of L-tryptophan to serotonin and melatonin in human melanoma cells. FEBS Lett. 2002;511:102–106. doi: 10.1016/S0014-5793(01)03319-1. [DOI] [PubMed] [Google Scholar]
- 125.Hata T., Asano Y., Yoshihara K., Kimura-Todani T., Miyata N., Zhang X.T., Takakura S., Aiba Y., Koga Y., Sudo N. Regulation of gut luminal serotonin by commensal microbiota in mice. PLoS ONE. 2017;12:e0180745. doi: 10.1371/journal.pone.0180745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yano J.M., Yu K., Donaldson G.P., Shastri G.G., Ann P., Ma L., Nagler C.R., Ismagilov R.F., Mazmanian S.K., Hsiao E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ge X., Ding C., Zhao W., Xu L., Tian H., Gong J., Zhu M., Li J., Li N. Antibiotics-induced depletion of mice microbiota induces changes in host serotonin biosynthesis and intestinal motility. J. Transl. Med. 2017;15:13. doi: 10.1186/s12967-016-1105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.De Vadder F., Grasset E., Manneras Holm L., Karsenty G., Macpherson A.J., Olofsson L.E., Backhed F. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc. Natl. Acad. Sci. USA. 2018;115:6458–6463. doi: 10.1073/pnas.1720017115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shah P.A., Park C.J., Shaughnessy M.P., Cowles R.A. Serotonin as a Mitogen in the Gastrointestinal Tract: Revisiting a Familiar Molecule in a New Role. Cell Mol. Gastroenterol. Hepatol. 2021;12:1093–1104. doi: 10.1016/j.jcmgh.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhao Y., Gan L., Ren L., Lin Y., Ma C., Lin X. Factors influencing the blood-brain barrier permeability. Brain Res. 2022;1788:147937. doi: 10.1016/j.brainres.2022.147937. [DOI] [PubMed] [Google Scholar]
- 131.Fu J., Li L., Huo D., Yang R., Yang B., Xu B., Yang X., Dai M., Tan C., Chen H., et al. Meningitic Escherichia coli alpha-hemolysin aggravates blood-brain barrier disruption via targeting TGFbeta1-triggered hedgehog signaling. Mol. Brain. 2021;14:116. doi: 10.1186/s13041-021-00826-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pirina P., Zinellu E., Paliogiannis P., Fois A.G., Marras V., Sotgia S., Carru C., Zinellu A. Circulating serotonin levels in COPD patients: A pilot study. BMC Pulm. Med. 2018;18:167. doi: 10.1186/s12890-018-0730-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Herr N., Bode C., Duerschmied D. The Effects of Serotonin in Immune Cells. Front. Cardiovasc. Med. 2017;4:48. doi: 10.3389/fcvm.2017.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mann D.A., Oakley F. Serotonin paracrine signaling in tissue fibrosis. Biochim. Biophys. Acta. 2013;1832:905–910. doi: 10.1016/j.bbadis.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Brzezinski A. Melatonin in humans. N. Engl. J. Med. 1997;336:186–195. doi: 10.1056/NEJM199701163360306. [DOI] [PubMed] [Google Scholar]
- 136.Huether G. Melatonin synthesis in the gastrointestinal tract and the impact of nutritional factors on circulating melatonin. Ann. N. Y. Acad. Sci. 1994;719:146–158. doi: 10.1111/j.1749-6632.1994.tb56826.x. [DOI] [PubMed] [Google Scholar]
- 137.Velarde E., Alonso-Gomez A.L., Azpeleta C., Isorna E., De Pedro N., Delgado M.J. Melatonin effects on gut motility are independent of the relaxation mediated by the nitrergic system in the goldfish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011;159:367–371. doi: 10.1016/j.cbpa.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 138.Song G.H., Leng P.H., Gwee K.A., Moochhala S.M., Ho K.Y. Melatonin improves abdominal pain in irritable bowel syndrome patients who have sleep disturbances: A randomised, double blind, placebo controlled study. Gut. 2005;54:1402–1407. doi: 10.1136/gut.2004.062034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xu P., Wang J., Hong F., Wang S., Jin X., Xue T., Jia L., Zhai Y. Melatonin prevents obesity through modulation of gut microbiota in mice. J. Pineal Res. 2017;62:e12399. doi: 10.1111/jpi.12399. [DOI] [PubMed] [Google Scholar]
- 140.Escribano B.M., Colin-Gonzalez A.L., Santamaria A., Tunez I. The role of melatonin in multiple sclerosis, Huntington’s disease and cerebral ischemia. CNS Neurol. Disord. Drug Targets. 2014;13:1096–1119. doi: 10.2174/1871527313666140806160400. [DOI] [PubMed] [Google Scholar]
- 141.Bentley G.E. Unraveling the enigma: The role of melatonin in seasonal processes in birds. Microsc. Res. Tech. 2001;53:63–71. doi: 10.1002/jemt.1069. [DOI] [PubMed] [Google Scholar]
- 142.Filadelfi A.M., Castrucci A.M. Comparative aspects of the pineal/melatonin system of poikilothermic vertebrates. J. Pineal Res. 1996;20:175–186. doi: 10.1111/j.1600-079X.1996.tb00256.x. [DOI] [PubMed] [Google Scholar]
- 143.Paulose J.K., Wright J.M., Patel A.G., Cassone V.M. Human Gut Bacteria Are Sensitive to Melatonin and Express Endogenous Circadian Rhythmicity. PLoS ONE. 2016;11:e0146643. doi: 10.1371/journal.pone.0146643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Graniczkowska K.B., Shaffer C.L., Cassone V.M. Transcriptional effects of melatonin on the gut commensal bacterium Klebsiella aerogenes. Genomics. 2022;114:110321. doi: 10.1016/j.ygeno.2022.110321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen L.M., Bao C.H., Wu Y., Liang S.H., Wang D., Wu L.Y., Huang Y., Liu H.R., Wu H.G. Tryptophan-kynurenine metabolism: A link between the gut and brain for depression in inflammatory bowel disease. J. Neuroinflamm. 2021;18:135. doi: 10.1186/s12974-021-02175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tan V.X., Guillemin G.J. Kynurenine Pathway Metabolites as Biomarkers for Amyotrophic Lateral Sclerosis. Front. Neurosci. 2019;13:1013. doi: 10.3389/fnins.2019.01013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Venkatesan D., Iyer M., Narayanasamy A., Siva K., Vellingiri B. Kynurenine pathway in Parkinson’s disease-An update. eNeurologicalSci. 2020;21:100270. doi: 10.1016/j.ensci.2020.100270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Groven N., Reitan S.K., Fors E.A., Guzey I.C. Kynurenine metabolites and ratios differ between Chronic Fatigue Syndrome, Fibromyalgia, and healthy controls. Psychoneuroendocrinology. 2021;131:105287. doi: 10.1016/j.psyneuen.2021.105287. [DOI] [PubMed] [Google Scholar]
- 149.Solvang S.H., Nordrehaug J.E., Aarsland D., Lange J., Ueland P.M., McCann A., Midttun O., Tell G.S., Giil L.M. Kynurenines, Neuropsychiatric Symptoms, and Cognitive Prognosis in Patients with Mild Dementia. Int. J. Tryptophan Res. 2019;12:1178646919877883. doi: 10.1177/1178646919877883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bartoli F., Cioni R.M., Cavaleri D., Callovini T., Crocamo C., Misiak B., Savitz J.B., Carra G. The association of kynurenine pathway metabolites with symptom severity and clinical features of bipolar disorder: An overview. Eur. Psychiatry. 2022;65:e82. doi: 10.1192/j.eurpsy.2022.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bartoli F., Misiak B., Callovini T., Cavaleri D., Cioni R.M., Crocamo C., Savitz J.B., Carra G. The kynurenine pathway in bipolar disorder: A meta-analysis on the peripheral blood levels of tryptophan and related metabolites. Mol. Psychiatry. 2021;26:3419–3429. doi: 10.1038/s41380-020-00913-1. [DOI] [PubMed] [Google Scholar]
- 152.Kennedy P.J., Cryan J.F., Dinan T.G., Clarke G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology. 2017;112 Pt B:399–412. doi: 10.1016/j.neuropharm.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 153.Savitz J. The kynurenine pathway: A finger in every pie. Mol. Psychiatry. 2020;25:131–147. doi: 10.1038/s41380-019-0414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Brown S.J., Huang X.F., Newell K.A. The kynurenine pathway in major depression: What we know and where to next. Neurosci. Biobehav. Rev. 2021;127:917–927. doi: 10.1016/j.neubiorev.2021.05.018. [DOI] [PubMed] [Google Scholar]
- 155.Wang Q., Liu D., Song P., Zou M.H. Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune activation. Front. Biosci. (Landmark Ed.) 2015;20:1116–1143. doi: 10.2741/4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Joisten N., Ruas J.L., Braidy N., Guillemin G.J., Zimmer P. The kynurenine pathway in chronic diseases: A compensatory mechanism or a driving force? Trends Mol. Med. 2021;27:946–954. doi: 10.1016/j.molmed.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 157.Huang Y.S., Ogbechi J., Clanchy F.I., Williams R.O., Stone T.W. IDO and Kynurenine Metabolites in Peripheral and CNS Disorders. Front. Immunol. 2020;11:388. doi: 10.3389/fimmu.2020.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Prasher P., Sharma M. Medicinal chemistry of anthranilic acid derivatives: A mini review. Drug Dev. Res. 2021;82:945–958. doi: 10.1002/ddr.21842. [DOI] [PubMed] [Google Scholar]
- 159.Nittoli T., Curran K., Insaf S., DiGrandi M., Orlowski M., Chopra R., Agarwal A., Howe A.Y., Prashad A., Floyd M.B., et al. Identification of anthranilic acid derivatives as a novel class of allosteric inhibitors of hepatitis C NS5B polymerase. J. Med. Chem. 2007;50:2108–2116. doi: 10.1021/jm061428x. [DOI] [PubMed] [Google Scholar]
- 160.Jahan H., Choudhary M.I., Atta A., Khan K.M., Ur-Rahman A. Anthranilic Acid Derivatives: Novel Inhibitors of Protein Glycation and the Associated Oxidative Stress in the Hepatocytes. Med. Chem. 2018;14:516–523. doi: 10.2174/1573406413666171020120528. [DOI] [PubMed] [Google Scholar]
- 161.Guillemin G.J., Cullen K.M., Lim C.K., Smythe G.A., Garner B., Kapoor V., Takikawa O., Brew B.J. Characterization of the kynurenine pathway in human neurons. J. Neurosci. 2007;27:12884–12892. doi: 10.1523/JNEUROSCI.4101-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Guillemin G.J., Kerr S.J., Smythe G.A., Smith D.G., Kapoor V., Armati P.J., Croitoru J., Brew B.J. Kynurenine pathway metabolism in human astrocytes: A paradox for neuronal protection. J. Neurochem. 2001;78:842–853. doi: 10.1046/j.1471-4159.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- 163.Guillemin G.J., Smith D.G., Smythe G.A., Armati P.J., Brew B.J. Expression of the kynurenine pathway enzymes in human microglia and macrophages. Adv. Exp. Med. Biol. 2003;527:105–112. doi: 10.1007/978-1-4615-0135-0_12. [DOI] [PubMed] [Google Scholar]
- 164.Werner-Felmayer G., Werner E.R., Fuchs D., Hausen A., Reibnegger G., Wachter H. Characteristics of interferon induced tryptophan metabolism in human cells in vitro. Biochim. Biophys. Acta. 1989;1012:140–147. doi: 10.1016/0167-4889(89)90087-6. [DOI] [PubMed] [Google Scholar]
- 165.Sorgdrager F.J.H., Naude P.J.W., Kema I.P., Nollen E.A., Deyn P.P. Tryptophan Metabolism in Inflammaging: From Biomarker to Therapeutic Target. Front. Immunol. 2019;10:2565. doi: 10.3389/fimmu.2019.02565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang Y., Shi H., Yang G., Yang Y., Li W., Song M., Shao M., Su X., Lv L. Associations between expression of indoleamine 2, 3-dioxygenase enzyme and inflammatory cytokines in patients with first-episode drug-naive Schizophrenia. Transl. Psychiatry. 2021;11:595. doi: 10.1038/s41398-021-01688-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Litzenburger U.M., Opitz C.A., Sahm F., Rauschenbach K.J., Trump S., Winter M., Ott M., Ochs K., Lutz C., Liu X., et al. Constitutive IDO expression in human cancer is sustained by an autocrine signaling loop involving IL-6, STAT3 and the AHR. Oncotarget. 2014;5:1038–1051. doi: 10.18632/oncotarget.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wolf A.M., Wolf D., Rumpold H., Moschen A.R., Kaser A., Obrist P., Fuchs D., Brandacher G., Winkler C., Geboes K., et al. Overexpression of indoleamine 2,3-dioxygenase in human inflammatory bowel disease. Clin. Immunol. 2004;113:47–55. doi: 10.1016/j.clim.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 169.Zhou L., Chen H., Wen Q., Zhang Y. Indoleamine 2,3-dioxygenase expression in human inflammatory bowel disease. Eur. J. Gastroenterol. Hepatol. 2012;24:695–701. doi: 10.1097/MEG.0b013e328351c1c2. [DOI] [PubMed] [Google Scholar]
- 170.Gibney S.M., Fagan E.M., Waldron A.M., O’Byrne J., Connor T.J., Harkin A. Inhibition of stress-induced hepatic tryptophan 2,3-dioxygenase exhibits antidepressant activity in an animal model of depressive behaviour. Int. J. Neuropsychopharmacol. 2014;17:917–928. doi: 10.1017/S1461145713001673. [DOI] [PubMed] [Google Scholar]
- 171.Krishnan S., Ding Y., Saedi N., Choi M., Sridharan G.V., Sherr D.H., Yarmush M.L., Alaniz R.C., Jayaraman A., Lee K. Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Rep. 2018;23:1099–1111. doi: 10.1016/j.celrep.2018.03.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Suyama Y., Hirayama C. Serum indole and skatole in patients with various liver diseases. Clin. Chim. Acta. 1988;176:203–206. doi: 10.1016/0009-8981(88)90208-2. [DOI] [PubMed] [Google Scholar]
- 173.Hong S.H., Hong Y., Lee M., Keum B.R., Kim G.H. Natural Product Skatole Ameliorates Lipotoxicity-Induced Multiple Hepatic Damage under Hyperlipidemic Conditions in Hepatocytes. Nutrients. 2023;15:1490. doi: 10.3390/nu15061490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Albillos A., de Gottardi A., Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020;72:558–577. doi: 10.1016/j.jhep.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 175.Teunis C., Nieuwdorp M., Hanssen N. Interactions between Tryptophan Metabolism, the Gut Microbiome and the Immune System as Potential Drivers of Non-Alcoholic Fatty Liver Disease (NAFLD) and Metabolic Diseases. Metabolites. 2022;12:514. doi: 10.3390/metabo12060514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Luo Z.W., Cho J.S., Lee S.Y. Microbial production of methyl anthranilate, a grape flavor compound. Proc. Natl. Acad. Sci. USA. 2019;116:10749–10756. doi: 10.1073/pnas.1903875116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dehhaghi M., Kazemi Shariat Panahi H., Guillemin G.J. Microorganisms, Tryptophan Metabolism, and Kynurenine Pathway: A Complex Interconnected Loop Influencing Human Health Status. Int. J. Tryptophan Res. 2019;12:1178646919852996. doi: 10.1177/1178646919852996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zdobnov E.M., Kuznetsov D., Tegenfeldt F., Manni M., Berkeley M., Kriventseva E.V. OrthoDB in 2020: Evolutionary and functional annotations of orthologs. Nucleic Acids Res. 2021;49:D389–D393. doi: 10.1093/nar/gkaa1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Karcher N., Nigro E., Puncochar M., Blanco-Miguez A., Ciciani M., Manghi P., Zolfo M., Cumbo F., Manara S., Golzato D., et al. Genomic diversity and ecology of human-associated Akkermansia species in the gut microbiome revealed by extensive metagenomic assembly. Genome Biol. 2021;22:209. doi: 10.1186/s13059-021-02427-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tierney B.T., Yang Z., Luber J.M., Beaudin M., Wibowo M.C., Baek C., Mehlenbacher E., Patel C.J., Kostic A.D. The Landscape of Genetic Content in the Gut and Oral Human Microbiome. Cell Host Microbe. 2019;26:283–295.e8. doi: 10.1016/j.chom.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Xiao L., Yan J., Yang T., Zhu J., Li T., Wei H., Chen J. Fecal Microbiome Transplantation from Children with Autism Spectrum Disorder Modulates Tryptophan and Serotonergic Synapse Metabolism and Induces Altered Behaviors in Germ-Free Mice. mSystems. 2021;6:e01343-20. doi: 10.1128/mSystems.01343-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Clarke G., Grenham S., Scully P., Fitzgerald P., Moloney R.D., Shanahan F., Dinan T.G., Cryan J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry. 2013;18:666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 183.Zhang Y., Sun L., Zhu R., Zhang S., Liu S., Wang Y., Wu Y., Xing S., Liao X., Mi J. Porcine gut microbiota in mediating host metabolic adaptation to cold stress. NPJ Biofilms Microbiomes. 2022;8:18. doi: 10.1038/s41522-022-00283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Oxenkrug G., van der Hart M., Roeser J., Summergrad P. Anthranilic Acid: A Potential Biomarker and Treatment Target for Schizophrenia. Ann. Psychiatry Ment. Health. 2016;4:1059. [PMC free article] [PubMed] [Google Scholar]
- 185.Krause D., Suh H.S., Tarassishin L., Cui Q.L., Durafourt B.A., Choi N., Bauman A., Cosenza-Nashat M., Antel J.P., Zhao M.L., et al. The tryptophan metabolite 3-hydroxyanthranilic acid plays anti-inflammatory and neuroprotective roles during inflammation: Role of hemeoxygenase-1. Am. J. Pathol. 2011;179:1360–1372. doi: 10.1016/j.ajpath.2011.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Coplan J.D., George R., Syed S.A., Rozenboym A.V., Tang J.E., Fulton S.L., Perera T.D. Early Life Stress and the Fate of Kynurenine Pathway Metabolites. Front. Hum. Neurosci. 2021;15:636144. doi: 10.3389/fnhum.2021.636144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Parrott J.M., Redus L., Santana-Coelho D., Morales J., Gao X., O’Connor J.C. Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl. Psychiatry. 2016;6:e918. doi: 10.1038/tp.2016.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Birner A., Platzer M., Bengesser S.A., Dalkner N., Fellendorf F.T., Queissner R., Pilz R., Rauch P., Maget A., Hamm C., et al. Increased breakdown of kynurenine towards its neurotoxic branch in bipolar disorder. PLoS ONE. 2017;12:e0172699. doi: 10.1371/journal.pone.0172699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Steiner J., Dobrowolny H., Guest P.C., Bernstein H.G., Fuchs D., Roeser J., Summergrad P., Oxenkrug G. Gender-specific elevation of plasma anthranilic acid in schizophrenia: Protection against glutamatergic hypofunction? Schizophr. Res. 2022;243:483–485. doi: 10.1016/j.schres.2022.01.048. [DOI] [PubMed] [Google Scholar]
- 190.Oxenkrug G., van der Hart M., Roeser J., Summergrad P. Peripheral Tryptophan—Kynurenine Metabolism Associated with Metabolic Syndrome is Different in Parkinson’s and Alzheimer’s Diseases. Endocrinol. Diabetes Metab. J. 2017;1 doi: 10.31038/EDMJ.2017141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Giorgini F., Huang S.Y., Sathyasaikumar K.V., Notarangelo F.M., Thomas M.A., Tararina M., Wu H.Q., Schwarcz R., Muchowski P.J. Targeted deletion of kynurenine 3-monooxygenase in mice: A new tool for studying kynurenine pathway metabolism in periphery and brain. J. Biol. Chem. 2013;288:36554–36566. doi: 10.1074/jbc.M113.503813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jayawickrama G.S., Nematollahi A., Sun G., Gorrell M.D., Church W.B. Inhibition of human kynurenine aminotransferase isozymes by estrogen and its derivatives. Sci. Rep. 2017;7:17559. doi: 10.1038/s41598-017-17979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Jacobs E., D’Esposito M. Estrogen shapes dopamine-dependent cognitive processes: Implications for women’s health. J. Neurosci. 2011;31:5286–5293. doi: 10.1523/JNEUROSCI.6394-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018;1693 Pt B:128–133. doi: 10.1016/j.brainres.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Curto M., Lionetto L., Negro A., Capi M., Fazio F., Giamberardino M.A., Simmaco M., Nicoletti F., Martelletti P. Altered kynurenine pathway metabolites in serum of chronic migraine patients. J. Headache Pain. 2015;17:47. doi: 10.1186/s10194-016-0638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Curto M., Lionetto L., Negro A., Capi M., Perugino F., Fazio F., Giamberardino M.A., Simmaco M., Nicoletti F., Martelletti P. Altered serum levels of kynurenine metabolites in patients affected by cluster headache. J. Headache Pain. 2015;17:27. doi: 10.1186/s10194-016-0620-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Steiner J., Dobrowolny H., Guest P.C., Bernstein H.G., Fuchs D., Roeser J., Summergrad P., Oxenkrug G.F. Plasma Anthranilic Acid and Leptin Levels Predict HAM-D Scores in Depressed Women. Int. J. Tryptophan Res. 2021;14:11786469211016474. doi: 10.1177/11786469211016474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Colle R., Masson P., Verstuyft C., Feve B., Werner E., Boursier-Neyret C., Walther B., David D.J., Boniface B., Falissard B., et al. Peripheral tryptophan, serotonin, kynurenine, and their metabolites in major depression: A case-control study. Psychiatry Clin. Neurosci. 2020;74:112–117. doi: 10.1111/pcn.12944. [DOI] [PubMed] [Google Scholar]
- 199.Pawlowski T., Pawlak D., Inglot M., Zalewska M., Marciniak D., Bugajska J., Janocha-Litwin J., Malyszczak K. The role of anthranilic acid in the increase of depressive symptoms and major depressive disorder during treatment for hepatitis C with pegylated interferon-alpha2a and oral ribavirin. J. Psychiatry Neurosci. 2021;46:E166–E175. doi: 10.1503/jpn.190139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Pompili M., Lionetto L., Curto M., Forte A., Erbuto D., Montebovi F., Seretti M.E., Berardelli I., Serafini G., Innamorati M., et al. Tryptophan and Kynurenine Metabolites: Are They Related to Depression? Neuropsychobiology. 2019;77:23–28. doi: 10.1159/000491604. [DOI] [PubMed] [Google Scholar]
- 201.Darlington L.G., Mackay G.M., Forrest C.M., Stoy N., George C., Stone T.W. Altered kynurenine metabolism correlates with infarct volume in stroke. Eur. J. Neurosci. 2007;26:2211–2221. doi: 10.1111/j.1460-9568.2007.05838.x. [DOI] [PubMed] [Google Scholar]
- 202.Lee K., Kwak J.H., Pyo S. Inhibition of LPS-induced inflammatory mediators by 3-hydroxyanthranilic acid in macrophages through suppression of PI3K/NF-kappaB signaling pathways. Food Funct. 2016;7:3073–3082. doi: 10.1039/C6FO00187D. [DOI] [PubMed] [Google Scholar]
- 203.Cervellati C., Trentini A., Pecorelli A., Valacchi G. Inflammation in Neurological Disorders: The Thin Boundary between Brain and Periphery. Antioxid. Redox Signal. 2020;33:191–210. doi: 10.1089/ars.2020.8076. [DOI] [PubMed] [Google Scholar]
- 204.Nourbakhsh B., Bhargava P., Tremlett H., Hart J., Graves J., Waubant E. Altered tryptophan metabolism is associated with pediatric multiple sclerosis risk and course. Ann. Clin. Transl. Neurol. 2018;5:1211–1221. doi: 10.1002/acn3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Rodrigues F.B., Byrne L.M., Lowe A.J., Tortelli R., Heins M., Flik G., Johnson E.B., De Vita E., Scahill R.I., Giorgini F., et al. Kynurenine pathway metabolites in cerebrospinal fluid and blood as potential biomarkers in Huntington’s disease. J. Neurochem. 2021;158:539–553. doi: 10.1111/jnc.15360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Fathi M., Vakili K., Yaghoobpoor S., Tavasol A., Jazi K., Hajibeygi R., Shool S., Sodeifian F., Klegeris A., McElhinney A., et al. Dynamic changes in metabolites of the kynurenine pathway in Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease: A systematic Review and meta-analysis. Front. Immunol. 2022;13:997240. doi: 10.3389/fimmu.2022.997240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Inglis J.J., Criado G., Andrews M., Feldmann M., Williams R.O., Selley M.L. The anti-allergic drug, N-(3′,4′-dimethoxycinnamonyl) anthranilic acid, exhibits potent anti-inflammatory and analgesic properties in arthritis. Rheumatology. 2007;46:1428–1432. doi: 10.1093/rheumatology/kem160. [DOI] [PubMed] [Google Scholar]
- 208.Maes M., Leonard B.E., Myint A.M., Kubera M., Verkerk R. The new ‘5-HT’ hypothesis of depression: Cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35:702–721. doi: 10.1016/j.pnpbp.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 209.Traylor M., Rutten-Jacobs L.C., Holliday E.G., Malik R., Sudlow C., Rothwell P.M., Maguire J.M., Koblar S.A., Bevan S., Boncoraglio G., et al. Differences in Common Genetic Predisposition to Ischemic Stroke by Age and Sex. Stroke. 2015;46:3042–3047. doi: 10.1161/STROKEAHA.115.009816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Caspi A., Sugden K., Moffitt T.E., Taylor A., Craig I.W., Harrington H., McClay J., Mill J., Martin J., Braithwaite A., et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 211.Qin Y., Havulinna A.S., Liu Y., Jousilahti P., Ritchie S.C., Tokolyi A., Sanders J.G., Valsta L., Brozynska M., Zhu Q., et al. Combined effects of host genetics and diet on human gut microbiota and incident disease in a single population cohort. Nat. Genet. 2022;54:134–142. doi: 10.1038/s41588-021-00991-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Campbell J.H., Foster C.M., Vishnivetskaya T., Campbell A.G., Yang Z.K., Wymore A., Palumbo A.V., Chesler E.J., Podar M. Host genetic and environmental effects on mouse intestinal microbiota. ISME J. 2012;6:2033–2044. doi: 10.1038/ismej.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Szklarczyk D., Franceschini A., Wyder S., Forslund K., Heller D., Huerta-Cepas J., Simonovic M., Roth A., Santos A., Tsafou K.P., et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The STRING database can be accessed at https://string-db.org and Ortho DB can be accessed at https://orthodb.org.