Abstract
Proposals for the use of live attenuated human immunodeficiency virus (HIV) type 1 (HIV-1) as a vaccine candidate in humans have been based on the protection afforded by attenuated simian immunodeficiency virus in the macaque model. Although it is not yet known if this strategy could succeed in humans, a study of the Sydney Blood Bank Cohort (SBBC), infected with an attenuated HIV-1 quasispecies with natural nef and nef/long terminal repeat deletions for up to 17 years, could provide insights into the long-term immunological consequences of living with an attenuated HIV-1 infection. In this study, HIV-specific cytoxic T-lymphocyte (CTL) responses in an SBBC donor and six recipients were examined over a 3-year period with enzyme-linked immunospot, tetrameric complex binding, direct CTL lysis, and CTL precursor level techniques. Strong HIV-specific CTL responses were detected in four of seven patients, including one patient with an undetectable viral load. Two of seven patients had weak CTL responses, and in one recipient, no HIV-specific CTLs were detected. High levels of circulating effector and memory HIV-specific CTLs can be maintained for prolonged periods in these patients despite very low viral loads.
Live attenuated vaccines are regarded as the most efficient means for inducing sustained protective cytotoxic T-lymphocyte (CTL) responses against human immunodeficiency virus type 1 (HIV) type 1 (HIV-1) (13, 19). Despite serious concerns raised in a study demonstrating the pathogenicity of attenuated simian immunodeficiency virus (SIV) infection in neonatal macaques (2), an extensive array of SIV mutants with levels of attenuation ranging from partial to complete has been developed (14, 15). Strong and persistent CTL responses in macaques infected with two of these attenuated mutants for more than 6 years have been demonstrated (19), and infection with either of these strains has resulted in protection against challenge with pathogenic SIV (11, 49). Furthermore, it has been demonstrated that these attenuated SIV strains are not vertically transmitted and that pathogenic infection in neonatal macaques was restricted (two of three macaques) to a high oral dosage in neonates born to unvaccinated macaques (50). Although the use of this approach in humans has not been attempted for HIV infection, various studies have demonstrated an association between infection with nef-defective viral strains and the apparent lack of disease progression (12, 20, 31, 43). In particular, a study of individuals infected with an attenuated nef and nef/long terminal repeat deletion strain of HIV-1 through blood transfusion from a single donor (the Sydney Blood Bank Cohort [SBBC]) (12, 27, 28) could provide insights into the long-term immunological consequences of living with an attenuated HIV-1 infection. The findings of this study would be of crucial interest to the growing number of physicians currently prepared to volunteer in a trial of live attenuated HIV as a vaccine candidate (9).
There is substantial evidence supporting a role for HIV-specific CTLs in the containment of HIV replication and for stimulating memory CTLs as part of a prophylactic vaccine strategy. The appearance of CTLs during primary HIV-1 infection has been associated with the initial control of viremia and the resolution of symptoms (5, 25), and it is evident that CTLs exert strong selective pressure on the virus at an early stage of infection (6). High levels of CTLs have also been associated with the ongoing control of viremia and the lack of disease progression in asymptomatic individuals (7, 22, 39), and although CTLs continue to exert strong selective pressure, their ultimate failure to respond to escape mutants is associated with symptomatic disease progression (17, 24). Recently, we used highly sensitive effector CTL-binding tetrameric complexes to demonstrate a significant inverse correlation between the level of circulating effector CTLs and the plasma load of viral RNA (36).
It was demonstrated recently that CD8+ T lymphocytes from recipients in the SBBC are less activated than those in other long-term HIV-1-infected individuals and that the slight elevation in the level of these activation markers was associated with individuals in the cohort with a detectable plasma viral load (52). We now report the levels of HIV-1-specific memory and effector CTLs in members of the SBBC studied over a 1.5- to 3-year period. We measured levels of CTL precursors (CTLp) by using limiting-dilution analysis (LDA) and direct lysis by freshly isolated peripheral blood mononuclear cells (PBMC) of recombinant vaccinia virus-infected targets. We measured levels of effector and memory CTLs against various HIV-1 determinants in freshly isolated PBMC by using HLA-specific tetramer-binding assays and enzyme-linked immunospot (ELISPOT) assays. Despite 13 to 17 years of infection with an attenuated strain of HIV-1, strong CTL responses were detected in four of seven SBBC patients; two of these four individuals had very low and undetectable viral loads, respectively.
MATERIALS AND METHODS
Study subjects.
Stored PBMC from the donor (D36) and six recipients in the SBBC were studied from samples collected between March 1994 and December 1997. Clinical data taken from these time periods and HLA typing are shown in Tables 1 and 2, respectively. The SBBC has been described elsewhere (12, 27, 28). An update of the current clinical status of the SBBC was recently compiled (29). For the purposes of this study, we divided the SBBC on the basis of the level of viremia in plasma (<200 or >200 viral copies/ml). Although viral load levels were generally low in the viremia-positive subgroup (mean, 1,980 copies/ml), this subgroup was associated with the ability to isolate virus, increased activation of CD8+ T lymphocytes (52), and a gradual decline in absolute numbers or percentages of CD4+ T lymphocytes (29).
TABLE 1.
Analysis of T-cell counts and viral load in the SBBC during the study perioda
| Study subject (yr of age at infection) | Sexb | Count (cells/μl) of:
|
CD4/CD8 ratio | Viral load (copies/ml)
|
||||
|---|---|---|---|---|---|---|---|---|
| CD4
|
CD8
|
Mean | Slope | |||||
| Mean | Slope | Mean | Slope | |||||
| D36 (22) | M | 466 | −61 | 1,024 | +21 | 0.46 | 3,320 | +1,500 |
| C18 (70) | M | 732 | −138 | 1,511 | +19 | 0.48 | 1,810 | +90 |
| C54 (56) | M | 1,205 | −197 | 1,757 | −295 | 0.69 | 2,148 | +232 |
| C98 (44) | M | 561 | −57 | 818 | −50 | 0.69 | 653 | −77 |
| C49 (30) | F | 971 | −6 | 468 | +16 | 2.07 | <200 | 0 |
| C64 (56) | F | 895 | +11 | 905 | −40 | 0.99 | <200 | 0 |
| C135 (35) | M | 496 | −66 | 498 | −46 | 1.00 | <200 | 0 |
Means were calculated from all available data taken during the study period. Slopes represent the yearly change during the study period.
M, male; F, female.
TABLE 2.
HLA restriction of CTLp and peptide specificity in ELISPOT and tetramer assays
| Study subject | HLA type
|
HLA restriction determined by bulk CTL lysisa | Peptide specificity for ELISPOT and tetramer-binding assays | Reference | |
|---|---|---|---|---|---|
| A | B | ||||
| D36 | 1,23 | 8,18 | B8 Gag, A23 Pol | B8 p17 Gag (24–32): GGKKKYKLK | 35 |
| B8 Nef (90–97): FLKEKGGL | 33 | ||||
| C18 | 2,11 | 44,60 | B60 Pol | A2 Gag (77–85): SLYNTVATL | 37 |
| A2 Pol (476–484): ILKEPVHGV | 46 | ||||
| C98 | 2,28 | 7,60 | A2 and A28 Gag | A2 Gag (77–85): SLYNTVATL | 37 |
| A2 and A28 Pol | A2 Pol (476–484): ILKEPVHGV | 46 | |||
| C49 | 2,11 | 7,60 | A11 Env, A11 Gag | A2 Gag (77–85): SLYNTVATL | 37 |
| A2 Pol (476–484): ILKEPVHGV | 46 | ||||
| C54 | 25,32 | 18,35 | Low activityb | B35 Env (77–85): DPNPQEVVL | 45 |
| B35 Pol (587–595): EPIVGAETF | 45 | ||||
| C64 | 3,32 | 7,44 | Low activity | NAc | |
| C135 | 1,33 | 50,57 | Low activity | NA | |
Lysis of vaccinia virus-infected HLA-matched BLCL panel; peptide specificity was not detected.
Low activity was less than 10% specific lysis.
NA, peptides not available.
Tetramer-binding assays.
The assay for tetramer-binding cells in freshly isolated PBMC was performed as previously described (1, 36). Specific peptides (33, 35, 37, 45, 46) used in the construction of the tetrameric complexes or in the ELISPOT assay are listed in Table 2. Briefly, PBMC were centrifuged at 300 × g for 5 min and resuspended in 50 μl of cold phosphate-buffered saline. PBMC were incubated with tetramers and antibodies on ice for 30 to 60 min and then washed twice before formaldehyde fixation. Triple-color analysis was performed with tetramer-bound phycoerythrin, anti-CD8–Tricolor (Caltag, Burlingame, Calif.), and anti-CD38–fluorescein isothiocyanate (Dako, Carpinteria, Calif.). PBMC were then analyzed for the expression of cell surface markers (CD8 and CD38) by use of a FACScan with CellQuest software (Becton Dickinson, San Jose, Calif.). Gates were applied to contain >99.98% control samples. Controls for the tetramers included both A*0201-negative individuals and A*0201-positive HIV-uninfected donors. The limit of detection was 0.02% CD8+ T cells.
ELISPOT assays.
ELISPOT assays were performed as previously described (26). Briefly, polyvinylidene difluoride-backed 96-well plates (Millipore, Bedford, Mass.) were coated with anti-human gamma interferon (IFN-γ) antibody overnight (Mabtech, Stockholm, Sweden). Plates were washed and blocked for 1 h with RPMI 1640 and 5% human serum. Target cells (T2 cells for HLA-A2-positive individuals or HLA-matched B-lymphocyte cell lines [BLCL] for HLA-B8- or HLA-B35-positive individuals) prepulsed with 1 μM epitope peptide (Table 2) were plated at 104 cells/well. Effector cells (freshly isolated PBMC, up to 2.5 × 105/well) were added and incubated for 16 h at 37°C in 5% CO2. The cells were removed, and the plates were incubated with a biotinylated monoclonal antibody to human IFN-γ (Mabtech) followed by streptavidin-alkaline phosphatase. Spots were developed by incubation with a chromogenic alkaline phosphatase substrate (Bio-Rad Laboratories, Hercules, Calif.). Results are expressed as spot-forming cells/106 PBMC. The limit of detection per well was 100/106 PBMC.
Freshly isolated CTL assays.
Fresh uncultured PBMC (effectors) were added to round-bottom 96-well culture plates at various concentrations to produce effector/target ratios ranging from 100 to 12.5. Targets expressing cell-processed HIV-1-derived antigens were autologous Epstein-Barr virus-transformed BLCL infected with 5 PFU of either a vaccinia virus control (vac-lacZ) or recombinant vaccinia virus expressing HIV-1IIIB-derived env, gag, pol, and nef (kindly provided by Therion Biologics, Cambridge, Mass.) per cell and incubated overnight. This step was followed by incubation with Na251CrO4 (Amersham) for 2 h and three washes before 2,500 cells were added per well. The plates were incubated at 37°C for 4 h before supernatants were harvested for β-particle counting. Percent lysis was calculated as follows: (experimental counts − medium control counts) × 100/(detergent counts − medium control counts). Lysis of control vaccinia virus-infected BLCL was subtracted to yield specific lysis. Medium control counts were between 10 and 15% detergent control counts. Low-level CTL results (<10% specific lysis) were included, since this level of activity has been shown (36) to correlate with substantial tetramer binding.
LDA of CTLp.
The method for quantifying HIV-1-specific CTLp levels has been described elsewhere (25). Briefly, two LDA plates were set up with PBMC (0, 250, 500, 1,000, 3,000, 6,000, 12,000, and 16,000 per well; 24 replicates) in RPMI 1640 supplemented with 15% fetal calf serum and 100 IU of interleukin-2 per ml. Gamma-irradiated allogeneic PBMC (2.5 × 104) and 0.1 μg of anti-CD3 antibody (clone 12F6; from J. T. Wong, Massachusetts General Hospital, Boston) per ml were added to each well. The medium was changed twice a week by removing half of the supernatant and replacing it with an equal volume of fresh medium, and cultures were maintained for 14 days prior to assays. Standard 51Cr release assays were performed, with the effectors from the LDA plates split six ways. Effectors were assayed against autologous BLCL targets produced as described above. The assays were performed with a BIOMEK-2000 robotic machine controlled by programs written with BIOWORKS software (Beckman Instruments, Fullerton, Calif.).
In the split-well analysis, a well was regarded as positive for antigen-specific CTL activity when the percent specific lysis of autologous target cells exceeded 10% and was 10% more than that of the corresponding control target cells. After the wells were classified as either positive or negative for CTL activity, the number of replicates and the number of negative wells were entered into programs written in Microsoft Excel according to the maximum-likelihood method (kindly provided by S. Kalams, Boston, Mass.) to calculate the CTLp levels and their corresponding 95% confidence intervals. All results are presented as HIV-specific CTLp levels, with background CTL activity against control vaccinia virus subtracted, and are expressed as the number of CTLp per 106 input PBMC. The operational limit of detection in these assays was 10 CTLp/106 PBMC.
HLA restriction of CTLp responses.
PBMC were stimulated and cultured under the same growth conditions as those used for the CTLp assays and tested for specific lytic activity against vaccinia virus-infected targets. Cultures with CTL activity were further tested against HLA-A and HLA-B single-allele matched target sets to restrict each CTL response. Killing of BLCL homozygous for the HLA-A and HLA-B loci was compared with killing of autologous BLCL targets.
Viral load assessment.
Plasma viral load (RNA copies per milliliter) was assessed by PCR with an Amplicor HIV Monitor kit (Roche, Branchburg, N.J.) in accordance with the methods specified by the manufacturer.
T-lymphocyte counts.
Whole blood counts and flow cytometric analysis of T-cell subsets were performed by standard methods (52). CD4+ and CD8+ lymphocytes were determined by direct immunofluorescence with monoclonal antibodies against CD4 and CD8 (Ortho Diagnostics, Inc., Raritan, N.J., and Coulter Electronics, Inc., Hialeah, Fla.). The samples were analyzed on an Epics V flow cytometer (Coulter) and expressed as numbers of CD4+ or CD8+ cells per microliter of whole blood.
RESULTS
HIV-1-specific CTL responses from individuals in the SBBC were studied over a 1.5- to 3-year period up to the end of 1997, with the exception of recipient C18, discussed below. The mean and yearly change (slope) in T-cell counts and plasma viral RNA load are summarized in Table 1. Since these data apply only to the time period of this study, there are minor differences between the current assessment of long-term trends in the data and an assessment reported elsewhere (29). In this study, the individuals were sorted according to the presence of detectable plasma viral RNA (Table 1). Samples from individuals without detectable viremia were furthermore below the detection limit (<20 copies/ml) when the Roche ultrasensitive PCR assay was performed (results not shown).
HIV-1-specific CTL responses are detected in the donor and recipients with detectable plasma viremia.
Results from the four individuals (the donor and three of the recipients, C18, C54, and C98) with detectable plasma viremia are shown in Fig. 1. The presence and quantity of circulating memory and activated effector CTLs were assessed by IFN-γ ELISPOT assays, HLA-A2-specific tetramer binding, direct lysis of autologous B-lymphocyte cell lines expressing HIV-1-derived epitopes from recombinant vaccinia virus by cryopreserved PBMC, and LDA.
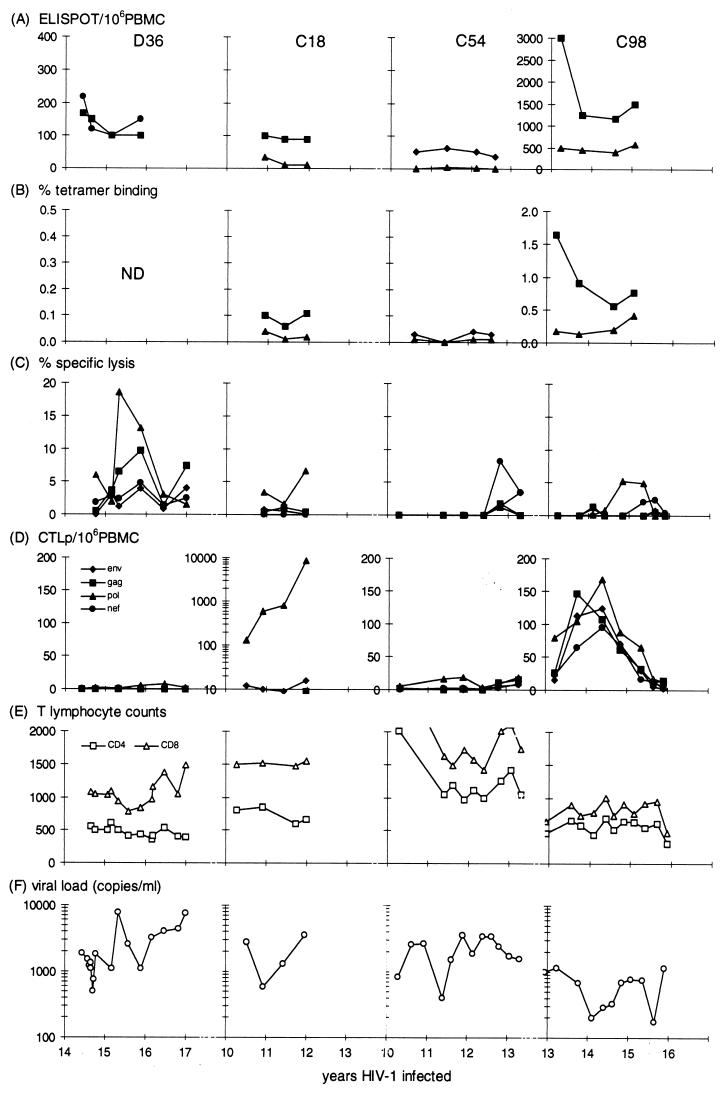
FIG. 1.
Analysis of direct CTL activity and CTLp levels, T-cell counts, and viral load measurements from the SBBC members with detectable plasma viremia: D36, C18, C54, and C98. (A to D) Responses to env, gag, pol, and nef determinants are shown. Peptide determinants in panels A and B are listed in Table 2. Direct CTL activity was measured by a peptide-induced ELISPOT assay (IFN-γ-releasing cells/106 PBMC) (A), a tetramer-binding assay (percent CD8+ T cells) (B), and direct lysis of recombinant vaccinia virus-infected BLCL (effector/target cell ratio, 50:1) (C). CTLp levels were measured against recombinant vaccinia virus-infected BLCL (D). (E and F) CD4 and CD8 counts (cells per microliter) (E) and plasma RNA viral load measurements (PCR) (F) determined during the study period. ND, tests not done.
Donor 36 had the highest viral load of the individuals studied and, over the time course of the CTL measurements, had one spike of plasma viremia. This spike was associated with an increase in the level of direct effectors against pol determinants (Fig. 1C). Based on bulk CTL cultures, this pol-specific response was HLA-A23 restricted (Table 2). Interestingly, D36 showed a nef-specific effector response, as indicated by the HLA-B8 Nef peptide reactivity in ELISPOT assays. However, since the Pol peptide was not included in this analysis, the ELISPOT assay-positive population, (reactive against HLA-B8 gag and nef peptides) did not change in response to increasing viremia. The HIV-specific CTLp level, which measures purely a proliferating memory cell population, was negative over the time course of the study; this fact may have contributed to the failure to adequately contain viremia (Fig. 1D and F).
Recipient C18 showed a dramatic increase in the level of HIV-1 pol-specific memory CTLs, which increased 70-fold during the course of the study. This pol-specific memory level, nearly 1% of the total PBMC level, is remarkable not only for its rate of increase in the absence of significant changes in viral load (<3,000 copies/ml) but also because of the age (83 years old) of the individual. The level of in vivo-activated CTLs (lysis of pol targets) was low but increasing; however, this fact was not reflected in HLA-A2 pol-specific ELISPOT assay or tetramer-binding responses, since the pol-specific response was HLA-B60 specific (Table 2). This sample was the last available sample from C18, who died 3 months later, in November 1995. The official cause of death was bacterial pneumonia; however, a full autopsy found no evidence to suggest HIV-1-related disease progression (29).
HIV-specific CTL responses in C54 were very weak, with low ELISPOT assay reactivity to the HLA-B35 env peptide and a transient low gag-specific response on direct lysis. In contrast, the CTL responses in C98 were strong and broad, including responses to gag, pol, env, and nef. The high levels in the gag-specific ELISPOT assay were paralleled by high levels of gag tetramer-staining cells.
Variable HIV-1-specific CTL responses in individuals without detectable plasma viremia.
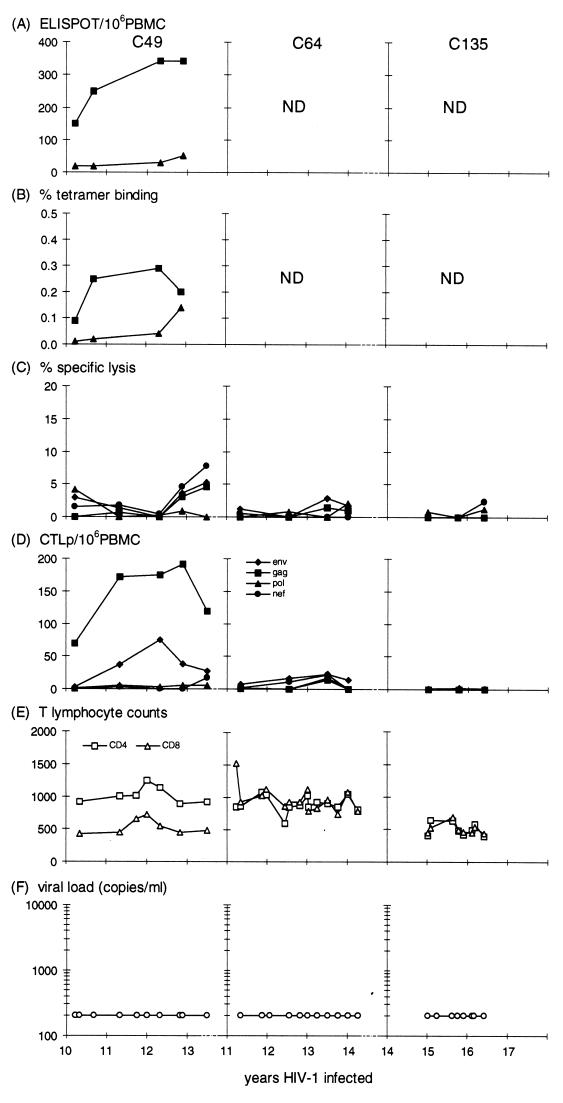
Two of the three individuals (C49 and C64) with consistently undetectable plasma viremia had detectable HIV-1-specific CTL responses, although CTLp levels in C64 were very low (Fig. 2). In C49 the increases in HLA-A2 gag-specific ELISPOT assay and tetramer-binding reactivities were comparable with the gag-specific CTLp levels. The CTL response was broadly reactive, with bulk culture assays showing HLA-A11 env and gag specificities (Table 2) and ELISPOT assay and tetramer-binding results showing HLA-A2-restricted responses. While the CTL responses in C49 could suggest active viral suppression, CTL responses were either weak (as in C64) or absent (as in C135). We did not have the opportunity to measure CTL activity in earlier samples from these individuals, so we cannot rule out the possibility that they showed a stronger CTL response at an earlier stage. However, the weak or absent responses would suggest that there was insufficient viral replication to maintain a memory CTL response after long-term infection with nef-defective HIV-1 in these two individuals.
FIG. 2.
Direct CTL and CTLp levels measured in the SBBC recipients with a plasma viral load consistently below detection (<200 copies/ml): C49, C64, and C135. See the legend to Fig. 1 for details.
DISCUSSION
The proposal to use live attenuated HIV-1 as a candidate vaccine is a controversial issue due in part to the lack of direct comparison between the macaque SIV model and human HIV-1 infection. While recent progress has been made in understanding pathogenesis during infection with different experimental strains of SIV exhibiting various levels of attenuation (15) and the immunological correlates of protection during challenge with pathogenic SIV (19, 49), our study provides the first detailed insight into the long-term immunological consequences of infection with attenuated HIV-1 in a group of humans. The SBBC provides a unique model for live attenuated HIV-1 vaccination in humans, in that all members were infected with a natural attenuated strain of HIV-1 and have low levels of plasma viremia 13 to 17 years after infection. We demonstrated that strong CTL responses (CTLp and/or effector CTL) were detectable in four of seven individuals and that these were comparable to strong responses reported elsewhere with identical methods for other cohorts infected with HIV-1 (wild-type nef) (36, 39) or for levels of CTLs in response to experimental vaccination with live attenuated SIV in macaques (19). Although unable to demonstrate protection in the SBBC against challenge with wild-type HIV-1, we showed that these CTL responses were durable and were associated with low levels of viremia. Since protection against challenge with pathogenic SIV in macaques has been attributed to a robust SIV-specific CTL response to the vaccine strain (19, 30), our observations may suggest that a similar outcome may be anticipated if this vaccine strategy is attempted in humans.
The lack of an effective HIV vaccine is the major impediment in containing the rising incidence of new infections seen in poorer nations, and although many individuals have responded to this need by volunteering to test a live attenuated HIV vaccine (9), some efficacy and safety concerns must first be addressed. The primary concern is that attenuated SIV is pathogenic when given to neonatal macaques (2), suggesting that attenuated HIV-1 may be pathogenic under certain conditions (neonates and immunosuppressed individuals). This concern has been counteracted to some extent by a recent study in which attenuated SIV did not cause vertical infection and that the pathogenicity of SIV mutants was restricted to only some neonates born to unvaccinated mothers (50). In a different study, protective immunity in macaques vaccinated with partially attenuated SIV was demonstrated only with a vaccine strain that retained pathogenicity (30). Conflicting results in which protection against challenge was observed in transiently infected animals in one study whereas ongoing viral replication was necessary for protection in another study have been attributed to differences in study design (8, 49). While a unified approach to the design of SIV vaccine studies may resolve some of these differences, there are also concerns that reversion of attenuation or recombination with wild-type virus could occur in vivo (47, 48). However, reversion to pathogenic SIV has not occurred in macaques infected with sufficiently attenuated SIV (Δnef and Δnef vpr), even after 9 years of nonprogressive infection (14).
Studies of the SBBC have addressed some of these concerns. No cases of sexual transmission of the SBBC virus have been established, and because of the ages of the SBBC individuals, vertical transmission was not an issue. We have demonstrated that attenuated HIV-1 does not have any increased pathogenicity in elderly recipients, who retain strong immune responses to this virus. Furthermore, virological and serological studies of the SBBC have demonstrated that the original genomic deletions have in fact increased in size since infection (12, 18). This phenomenon has also been reported in another long-term nonprogressor with nef deletion size increasing over time (20) as well as in macaques infected with SIV Δnef (21). The Nef-specific antibody reactivity for each member of the SBBC recognized all overlapping Nef peptides except one (amino acids 162 to 177), which corresponds to the consensus nef deletion, suggesting that all other deletions occurred subsequent to the establishment of the antibody response (18). Antibody responses to the remaining Nef peptides were of a magnitude similar to those in cohorts of individuals with wild-type nef, including one peptide (amino acids 89 to 97) similar to the HLA-B8 Nef peptide used in this study, and the wild-type nef sequence in D36 was conserved in the region that encodes this peptide (18).
This study has shown that attenuated HIV-1 elicits strong CTL responses that may be associated with low viral loads in certain individuals, as suggested by the responses in recipients C49 and C98. Although CTLp were not detected in D36, the response to viral antigen in D36 (transient viral load peak at 15.5 years postinfection) was effective, as seen by the close association between the rapid disappearance of the viral load peak and the persistence for 6 months of pol-specific direct lysis. The ability to generate a bulk CTL response in D36 despite absent CTLp may be due to the low cell input number (maximum concentration, 16,000/well) in the CTLp assay; in contrast, approximately 5 × 106 PBMC were expanded for bulk cultures, a fact which may explain the presence of effector CTLs found by direct lysis and ELISPOT assays. In addition, CTL activity persisted in other members of the SBBC, despite advancing age (C18). However, some individuals in the SBBC tended to have lower viral loads with reduced tetramer binding when compared with other long-term asymptomatic individuals (36). Either CTLs are more efficient at controlling nef-defective HIV-1 strains and are therefore not required at comparatively high levels in the SBBC or, alternatively, the lack of viral replication in the SBBC elicits lower levels of CTL activity in comparison with long-term infection with wild-type HIV-1.
Lower activated CTL measurements could be associated with increased efficiency in controlling a nef-attenuated virus. It has been shown that nef down-regulates HLA class 1 surface expression (38, 44), and this mechanism has been shown to protect infected cells against CTL killing (10). However, despite potential down-regulation of class 1 antigens by nef-positive HIV-1, it has been demonstrated that CTLs are in fact capable of killing infected cells before the release of new virions (51), and it has been recognized that even relatively weak CTL activity can eliminate a large fraction of infected cells (23). Therefore, it is possible that CTL activity is responsible for keeping plasma viremia below detection in C49 and below 1,000 copies/ml in C98 due to enhanced efficiency of CTLs in controlling nef-defective HIV-1.
On the other hand, failure to elicit strong CTL responses in C64 and C135 may be related to the lack of detectable viral replication, and further attenuation of the virus could further abrogate CTL responses. This idea may seem at odds with observations that high-risk exposed but seronegative individuals have significant HIV-specific CTL responses (41, 42) which demonstrated memory responses without ongoing infection. However, C64 and C135 were infected 15 and 17 years ago, respectively, and since virus cannot be isolated by conventional techniques, we suspect that the lack of ongoing exposure to replicating virus has caused CTL levels to decline. A similar situation has been described for an individual who was infected with defective HIV-1 for a similar length of time without detectable plasma viremia and who showed a decline in CTL activity over time while retaining HIV-1-specific antibody responses (3). Additionally, we have found that individuals who commence combination antiretroviral therapy have declining activated CTL and CTLp levels in association with the drop in plasma viremia (32, 34, 36). Therefore, it is likely that HIV-1 infection in C64 and C135 is predominantly latent and does not provide sufficient stimulation to activate strong CTL responses. In fact, C135 has always displayed an indeterminate Western blot pattern (4). In addition, C64 and C135 have the strongest proliferative responses in the SBBC (stimulation index and proliferative precursor cell levels) to p24 (unpublished results). A strong HIV-1-specific helper T-cell response in the absence of virus-driven immune activation (52) may be an alternative mechanism for containing viral replication (40) and may signify protective immunity in highly attenuated HIV-1 infections.
As a final word of caution, while we previously demonstrated comparable immune function in the SBBC and in matched uninfected individuals (16), our recent follow-up of these individuals suggests that slow disease progression may be occurring in some members with detectable viral replication (29). Also, declining CTLp levels in C98 suggest that CTLs may fail to adequately control viral replication in the future. We suggest that a potential vaccine candidate would require further attenuation than that in the natural SBBC viral strain. Regardless of whether a prophylactic HIV vaccine contains a live attenuated or otherwise modified strain of HIV-1, this study emphasizes that an attenuated strain of HIV-1 is capable of inducing sustained CTL responses, but whether such responses are capable of protecting against a wild-type HIV-1 challenge remains to be determined.
ACKNOWLEDGMENTS
Experimental work was contributed equally by W.B.D. and G.S.O.
We thank Peter Kuebler, Bill Kakimoto, and Elizabeth DeFalcon, Aaron Diamond AIDS Research Center, and Harmjan Kuipers and Marcel Coolen, Australian Red Cross Blood Service-NSW, for technical assistance; John Zaunders, Centre for Immunology, St. Vincents Hospital, Darlinghurst, New South Wales, Australia, for immunophenotyping; Jenny Learmont and Camille Raynes-Greenow, Australian Red Cross Blood Service-NSW, for arranging blood sample collection and compiling patient details; and the members of the SBBC for participation in this study.
This work was supported by the Macfarlane-Burnet Research Syndicate, the Medical Research Council (United Kingdom), and Aaron Diamond AIDS Research Center funds and in part by NIH grant U01 A141534.
REFERENCES
- 1.Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 2.Baba T W, Jeong Y S, Pennick D, Bronson R, Greene M F, Ruprecht R M. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science. 1995;267:1820–1825. doi: 10.1126/science.7892606. [DOI] [PubMed] [Google Scholar]
- 3.Binley J M, Jin X, Huang Y, Zhang L, Cao Y, Ho D D, Moore J P. Persistent antibody responses but declining cytotoxic T-lymphocyte responses to multiple human immunodeficiency virus type 1 antigens in a long-term nonprogressing individual with a defective p17 proviral sequence and no detectable viral RNA expression. J Virol. 1998;72:3472–3474. doi: 10.1128/jvi.72.4.3472-3474.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolton, W. Personal communication.
- 5.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B A. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borrow P, Lewicki H, Wei X, Horwitz M S, Peffer N, Meyers H, Nelson J A, Gairin J E, Hahn B H, Oldstone M B A, Shaw G M. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 7.Carmichael A, Jin X, Sissons P, Borysiewicz L. Quantitative analysis of the human immunodeficiency type 1 (HIV-1) specific cytotoxic T lymphocyte (CTL) response at different stages of HIV-1 infection: differential CTL response to HIV-1 and Epstein-Barr virus in late disease. J Exp Med. 1993;177:249–256. doi: 10.1084/jem.177.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clerici M, Clark E A, Polacino P, Axberg I, Kuller L, Casey N I, Morton W R, Shearer G M, Benveniste R E. T-cell proliferation to subinfectious SIV correlates with lack of infection after challenge of macaques. AIDS. 1994;8:1391–1395. doi: 10.1097/00002030-199410000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Cohen J. Novel campaign to test live HIV vaccine. Science. 1997;277:1035. doi: 10.1126/science.277.5329.1035. [DOI] [PubMed] [Google Scholar]
- 10.Collins K L, Chen B K, Kalams S A, Walker B D, Baltimore D. HIV-1 nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 11.Daniel M D, Kirchhoff F, Czajak S C, Sehgal P K, Desrosiers R C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 12.Deacon N J, Tsykin A, Solomon A, Smith K, Ludford-Menting M, Hooker D J, McPhee D A, Greenway A L, Ellet A, Chatfield C, Lawson V A, Crowe S, Maerz A, Sonza S, Learmont J, Sullivan J S, Cunningham A, Dwyer D, Dowton D, Mills J. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 13.Desrosiers R C. HIV with multiple gene deletions as a live attenuated vaccine for AIDS. AIDS Res Hum Retroviruses. 1992;8:411–421. doi: 10.1089/aid.1992.8.411. [DOI] [PubMed] [Google Scholar]
- 14.Desrosiers R C. Prospects for live attenuated HIV. Nat Med. 1998;4:982. doi: 10.1038/1949. [DOI] [PubMed] [Google Scholar]
- 15.Desrosiers R C, Lifson J D, Gibbs J S, Czajak S C, Howe A Y, Arthur L O, Johnson R P. Identification of highly attenuated mutants of simian immunodeficiency virus. J Virol. 1998;72:1431–1437. doi: 10.1128/jvi.72.2.1431-1437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dyer W B, Geczy A F, Kent S J, McIntyre L B, Blasdall S A, Learmont J C, Sullivan J S. Lymphoproliferative immune function in the Sydney Blood Bank Cohort, infected with natural nef/long terminal repeat mutants, and in other long-term survivors of transfusion-acquired HIV-1 infection. AIDS. 1997;11:1565–1574. doi: 10.1097/00002030-199713000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Goulder P J R, Phillips R E, Colbert R A, McAdam S, Ogg G, Nowak M A, Giangrande P, Luzzi G, Morgan B, Edwards A, McMichael A J, Rowland-Jones S. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat Med. 1997;3:212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- 18.Greenway A L, Mills J, Rhodes D, Deacon N J, McPhee D A. Serological detection of attenuated HIV-1 variants with nef gene deletions. AIDS. 1998;12:555–561. doi: 10.1097/00002030-199806000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Johnson R P, Glickman R L, Yang J Q, Kaur A, Dion J T, Mulligan M J, Desrosiers R C. Induction of vigorous cytotoxic T-lymphocyte responses by live attenuated simian immunodeficiency virus. J Virol. 1997;71:7711–7718. doi: 10.1128/jvi.71.10.7711-7718.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirchhoff F, Greenough T C, Brettler D B, Sullivan J L, Desrosiers R C. Absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med. 1995;332:228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 21.Kirchhoff F, Kestler H W, Desrosiers R C. Upstream U3 sequences in simian immunodeficiency virus are selectively deleted in vivo in the absence of an intact nef gene. J Virol. 1994;68:2031–2037. doi: 10.1128/jvi.68.3.2031-2037.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein M R, Van Baalen C A, Holwerda A M, Kerkhof Garde S R K, Bende R J, Keet I P M, Eeftinck-Schattenkerk J-K M, Osterhaus A D M E, Schuitemaker H, Miedema F. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long term asymptomatics. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klenerman P, Phillips R E, Rinaldo C R, Wahl L M, Ogg G, May R M, McMichael A, Nowak M A. Cytotoxic T lymphocytes and viral turnover in HIV type 1 infection. Proc Natl Acad Sci USA. 1996;93:15323–15328. doi: 10.1073/pnas.93.26.15323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koenig S, Conley A, Brewah Y A, Jones G M, Leath S, Boots L J, Davey V, Pantaleo G, Demarest J F, Carter C. Transfer of HIV-1 specific cytotoxic T lymphocytes to an AIDS patient leads to selection of mutant HIV variants and subsequent disease progression. Nat Med. 1995;1:330–336. doi: 10.1038/nm0495-330. [DOI] [PubMed] [Google Scholar]
- 25.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lalvani A, Brookes R, Hambleton S, Britton W J, Hill A V S, McMichael A J. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Learmont J, Cook L, Dunckley H, Sullivan J S. Update on long-term symptomless HIV type 1 infection in recipients of blood products from a single donor. AIDS Res Hum Retroviruses. 1995;11:1. doi: 10.1089/aid.1995.11.1. [DOI] [PubMed] [Google Scholar]
- 28.Learmont J, Tindall B, Evans L, Cunningham A, Cunningham P, Wells J, Penny R, Kaldor J, Cooper D A. Long-term symptomless HIV-1 infection in recipients of blood products from a single donor. Lancet. 1992;340:863–867. doi: 10.1016/0140-6736(92)93281-q. [DOI] [PubMed] [Google Scholar]
- 29.Learmont, J. C., A. F. Geczy, L. J. Ashton, C. H. Raynes-Greenow, R. J. Garsia, W. B. Dyer, L. McIntyre, R. B. Oelrichs, D. I. Rhodes, A. E. Solomon, J. Mills, and J. S. Sullivan. The Sydney Blood Bank Cohort (SBBC) of long-term nonprogressors infected with an attenuated quasispecies of HIV-1: immunological and virological status, 13 to 17 years after infection. Submitted for publication.
- 30.Lohman B L, McChesney M B, Miller C J, McGowan E, Joye S M, Van Rompay K K, Reay E, Antipa L, Pedersen N C, Marthas M L. A partially attenuated simian immunodeficiency virus induces host immunity that correlates with resistance to pathogenic virus challenge. J Virol. 1994;68:7021–7029. doi: 10.1128/jvi.68.11.7021-7029.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mariani R, Kirchhoff F, Greenough T C, Sullivan J L, Desrosiers R C, Skowronski J. High frequency of defective nef alleles in a long-term survivor with nonprogressive human immunodeficiency virus type 1 infection. J Virol. 1996;70:7752–7764. doi: 10.1128/jvi.70.11.7752-7764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Markowitz, M., M. Vesanen, K. Tenner-Racz, Y. Cao, J. Binley, A. Talal, A. Hurley, X. Jin, M. Heath-Chiozzi, J. M. Leonard, J. P. Moore, P. Racz, D. F. Nixon, and D. D. Ho. The impact of combination antiretroviral therapy commenced soon after infection on HIV-1 replication and antiviral immune responses. Submitted for publication. [DOI] [PubMed]
- 33.McMichael, A. J., and B. D. Walker. 1994. Cytotoxic T lymphocyte epitopes: implications for HIV vaccines. AIDS 8(Suppl.):S155–S173.
- 34.Nixon, D. F., D. Douek, P. J. Kuebler, X. Jin, M. Vesanen, S. Bonhoeffer, Y. Cao, R. A. Koup, D. D. Ho, and M. Markowitz. Molecular tracking of an HIV nef specific CTL clone shows persistence of clone-specific TCR DNA but not mRNA following early combination antiretroviral therapy. Immunol. Lett., in press. [DOI] [PubMed]
- 35.Nixon D F, McMichael A J. Cytotoxic T-cell recognition of HIV proteins and peptides. AIDS. 1991;5:1049–1059. [PubMed] [Google Scholar]
- 36.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Cerundolo V, Hurley A, Markowitz M, Ho D D, Nixon D F, McMichael A J. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 37.Parker K C, Bednarek M A, Hull L K, Utz U, Cunningham B, Zweerink H J, Biddison W E, Coligan J E. Sequence motifs important for peptide binding to the human MHC class I molecule, HLA-A2. J Immunol. 1992;149:3580–3587. [PubMed] [Google Scholar]
- 38.Puppo F, Brenci S, Bosco O, Lanza L, Barocci S, Nocera A, Ghio M, Contini P, Setti M, Scudeletti M, Indiveri F. Downregulation of HLA class I antigen expression in CD4+ T lymphocytes from HIV type 1-infected individuals. AIDS Res Hum Retroviruses. 1997;13:1509–1516. doi: 10.1089/aid.1997.13.1509. [DOI] [PubMed] [Google Scholar]
- 39.Rinaldo C, Huang X-L, Fan Z, Ding M, Beltz L, Logar A, Panicali D, Mazzara G, Liebmann J, Cottrill M, Gupta P. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J Virol. 1995;69:5838–5842. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 41.Rowland-Jones S, Sutton J, Ariyoshi K, Dong T, Gotch F, McAdam S, Whitby D, Sabally S, Gallimore A, Corrah T, Takiguchi M, Schultz T, McMichael A, Whittle H. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 42.Rowland-Jones S L, Nixon D F, Aldhous M C, Gotch F, Ariyoshi K, Hallam N, Kroll J S, Froebel K, McMichael A. HIV-specific cytotoxic T-cell activity in an HIV-exposed but uninfected infant. Lancet. 1993;341:860–861. doi: 10.1016/0140-6736(93)93063-7. [DOI] [PubMed] [Google Scholar]
- 43.Salvi R, Garbuglia A R, Di Caro A, Pulciani S, Montella F, Benedetto A. Grossly defective nef gene sequences in a human immunodeficiency virus type 1-seropositive long-term nonprogressor. J Virol. 1998;72:3646–3657. doi: 10.1128/jvi.72.5.3646-3657.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard J-M. Endocytosis of major histocompatibility complex class 1 molecules is induced by the HIV-1 nef protein. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 45.Shiga H, Shioda T, Tomiyama H, Takamiya Y, Oka S, Kimura S, Yamaguchi Y, Gojoubori T, Rammensee H G, Miwa K, Takiguchi M. Identification of multiple HIV-1 cytotoxic T-cell epitopes presented by human leukocyte antigen B35 molecules. AIDS. 1996;10:1075–1083. [PubMed] [Google Scholar]
- 46.Tsomides T J, Walker B D, Eisen H N. An optimal viral peptide recognized by CD8+ T cells binds very tightly to the restricting class I major histocompatibility complex protein on intact cells but not to the purified class I protein. Proc Natl Acad Sci USA. 1991;88:11276–11280. doi: 10.1073/pnas.88.24.11276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whatmore A M, Cook N, Hall G A, Sharpe S, Rud E W, Cranage M P. Repair and evolution of nef in vivo modulates simian immunodeficiency virus virulence. J Virol. 1995;69:5117–5123. doi: 10.1128/jvi.69.8.5117-5123.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wooley D P, Smith R A, Czajak S, Desrosiers R C. Direct demonstration of retroviral recombination in a rhesus monkey. J Virol. 1997;71:9650–9653. doi: 10.1128/jvi.71.12.9650-9653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wyand M S, Manson K H, Garcia-Moll M, Montefiori D, Desrosiers R C. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J Virol. 1996;70:3724–3733. doi: 10.1128/jvi.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wyand M S, Manson K H, Lackner A A, Desrosiers R C. Resistance of neonatal monkeys to live attenuated vaccine strains of simian immunodeficiency virus. Nat Med. 1997;3:32–36. doi: 10.1038/nm0197-32. [DOI] [PubMed] [Google Scholar]
- 51.Yang O O, Kalams S, Rosenzwieg M, Trocha A, Koziel M, Walker B D, Johnson R P. Efficient lysis of human immunodeficiency virus type 1-infected cells by cytotoxic T lymphocytes. J Virol. 1996;70:5799–5806. doi: 10.1128/jvi.70.9.5799-5806.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zaunders, J. J., A. F. Geczy, W. B. Dyer, L. B. McIntyre, M. A. Cooley, L. J. Ashton, C. H. Raynes-Greenow, J. Learmont, D. A. Cooper, and J. S. Sullivan. Evidence for ongoing CD4+CD45RO+ T lymphocyte responses and minimal activation of CD8+ T lymphocytes in the Sydney Blood Bank Cohort (SBBC) infected with natural nef/LTR mutant HIV-1. Submitted for publication.