Abstract
Modern agriculture is facing the challenges of salinity and heat stresses, which pose a serious threat to crop productivity and global food security. Thus, it is necessary to develop the appropriate measures to minimize the impacts of these serious stresses on field crops. Silicon (Si) is the second most abundant element on earth and has been recognized as an important substance to mitigate the adverse effects of abiotic stresses. Thus, the present study determined the role of Si in mitigating adverse impacts of salinity stress (SS) and heat stress (HS) on wheat crop. This study examined response of different wheat genotypes, namely Akbar-2019, Subhani-2021, and Faisalabad-2008, under different treatments: control, SS (8 dSm−1), HS, SS + HS, control + Si, SS + Si, HS+ Si, and SS + HS+ Si. This study’s findings reveal that HS and SS caused a significant decrease in the growth and yield of wheat by increasing electrolyte leakage (EL), malondialdehyde (MDA), and hydrogen peroxide (H2O2) production; sodium (Na+) and chloride (Cl−) accumulation; and decreasing relative water content (RWC), chlorophyll and carotenoid content, total soluble proteins (TSP), and free amino acids (FAA), as well as nutrient uptake (potassium, K; calcium, Ca; and magnesium, Mg). However, Si application offsets the negative effects of both salinity and HS and improved the growth and yield of wheat by increasing chlorophyll and carotenoid contents, RWC, antioxidant activity, TSP, FAA accumulation, and nutrient uptake (Ca, K, and Mg); decreasing EL, electrolyte leakage, MDA, and H2O2; and restricting the uptake of Na+ and Cl−. Thus, the application of Si could be an important approach to improve wheat growth and yield under normal and combined saline and HS conditions by improving plant physiological functioning, antioxidant activities, nutrient homeostasis, and osmolyte accumulation.
Keywords: antioxidants, chlorophyll, growth, nutrient homeostasis, reactive oxygen species, yield
1. Introduction
Salinity stress (SS) is a serious threat to crop production and global food security. The extent of soil salinity is continuously increasing, and every year, 4 × 104 hectares around the globe lose their ability to produce crops, owing to SS [1,2]. Globally, 6% of lands are salt-affected, which accounts for 20% of total cultivated lands, and out of which 50% of lands are irrigated [3,4]. The excessive salts in the rhizosphere induce a deleterious impact on crop growth and development [5]. Soil salinity has adverse impacts on wheat crops and causes water shortage, reduces photosynthetic rates and nutrient uptake, and increases the uptake of toxic ions, which in turn decreases wheat growth and yield [6]. Further, SS also reduces the leaf area and root and shoot growth, and induces the excessive production of reactive oxygen species (ROS), which cause significant damage to the wheat crop [7,8]. Moreover, SS also disturbs the photosynthetic process in wheat by decreasing chlorophyll contents, denaturing enzymes, and damaging photosynthetic apparatus and creating negative water potential [9,10]. However, wheat plants have developed an excellent enzymatic defense system, and they also accumulate the potential osmolytes and hormones to mitigate the adverse impacts of SS [11].
The global climate is continuously warming with the corresponding increase in atmospheric temperature and carbon dioxide (CO2) concentration; as a result, plants are adversely affected by these changes [12]. Heat stress (HS) refers to an increased atmospheric temperature that affects plants’ physiological and biochemical processes. Wheat crops are very sensitive to HS and this stress negatively affects wheat growth and yield [13,14,15]. Heat stress also decreases chlorophyll synthesis and electron transport and damages the photosynthetic apparatus, which results in reduced assimilate production, thereby resulting in significant yield losses in wheat [16]. Moreover, HS also induced ROS production, which damages enzymes, proteins, and nucleic acid; induces stomata closing; and increases leaf temperature, which causes a significant reduction in final productivity [17]. Additionally, HS also causes substantial yield losses in wheat by impairing pollen vitality, pollen tubes, and seed settings and inducing flower abortion [18].
The research on combined stresses indicates that plants act differently and evoke distinct integrated signaling networks, which result in different responses, as compared to single stresses [19]. The results of combined stresses could be neutral, additive, or synergistic [20]. Nonetheless, in plants, common pathways and responses are also shared by stress combinations, and thus, diverse physiological and molecular reactions occur in plants. For instance, HS impairs plant enzymatic activities, which create a metabolic imbalance that affects plant growth as a result [21,22]. On the other hand, SS-induced Na+ toxicity reduces nutrient uptake, causes deleterious impacts on plant genes and enzymes, and causes various metabolic deficiencies [23]. Moreover, combined heat and saline stress can inhibit the root and shoot growth and RWC and can increase the MDA and EL, as well as Na+ to a significant extent [24,25].
Silicon (Si) is the second most common element found in the Earth’s crust (27.2%) after oxygen, and it is considered a beneficial element for plants [26]. Silicon is widely used to alleviate abiotic stress in wheat and the application of Si improves tolerance against abiotic stress by increasing water uptake, antioxidant activities, osmolyte accumulation, and nutrient uptake [27,28]. Si has been recognized to improve antioxidant activities, maintain ionic balance and the K⁺/Na⁺ ratio, and reduce ROS, which improves SS tolerance in wheat [29,30]. Si nutrition has been found to improve plant growth, biomass production, and the root and shoot growth of wheat by reducing Na+ and Cl− uptake and partitioning Na+, Cl−, and mineral ions [31]. Furthermore, Si also improves K+ uptake, chlorophyll synthesis, osmolyte and hormone accumulation, and antioxidant activities, which improve wheat growth under saline conditions [32]. Further, Si also triggers signaling molecules and stress-associated genes and improves antioxidant activities and osmolyte accumulation, which in turn alleviate the adverse impact of HS on wheat [33]. Additionally, Si prevents the degradation of cellular proteins, improves osmolyte accumulation, prolongs the stay-green trait, improves photosynthetic activities, and reduces MDA and ROS accumulation, which ensures better wheat growth under HS [34].
Wheat is an important food crop and a staple food of many nations; however, it is considered to be sensitive to salinity and heat stress. Salinity and heat stresses can cause a significant reduction in the growth and yield of wheat [35,36]. Here, in this study, we evaluated the response of SS and HS, both alone and in combination on f diverse wheat genotypes under Si application. We hypothesized that wheat genotypes may vary in their ability to tolerate SS and HS. We also hypothesized that the application of Si would be able to alleviate the deleterious impacts of SS and HS by improving physiological functions, antioxidant activities, osmolyte accumulation, and nutrient homeostasis. Therefore, the present study aimed to determine the effect of Si on growth, physiological and antioxidant activities, nutrient homeostasis, and the grain yield of diverse wheat cultivars growing under SS and HS and a combination of both stresses.
2. Material and Methods
2.1. Growth Conditions and Plant Materials
A pot experiment was conducted in the greenhouse of University of Agriculture Faisalabad to determine the effect of exogenously applied silicon in mitigating heat and salt stress in different wheat cultivars. The soil used for filling the pots (5 kg capacity) was collected from an agronomic field (31.8 °N, 73.8 °E, 184 m asl) previously sown with a rice crop. The soil was collected from the upper 0–20 cm layer and had a 7.88 pH, organic matter 1.22%, EC 0.99 d ms−1, total nitrogen (N) 0.63%, and available phosphorus (P) and potassium (K) at 6.91 and 158 ppm, respectively. The concentration of sodium chloride (NaCl) required for achieving the 8 dS m−1 EC value was calculated using the following formula.
TSS indicates total soluble salts, and these were calculated by multiplying EC differences (required soil EC–initial soil EC) with 10. NaCl was added at a rate of 8.24 g/kg to obtain the required EC (8 dSm−1). To determine the soil saturation percentage (SSP), 250 g of soil was utilized, and soil paste was prepared by adding the distilled water. Soil paste was kept at room temperature for 2 h, and extract was obtained and SSP was determined via the following equation:
The sodium silicate was used as a source of Si and was applied at a rate of 500 mg kg−1 of soil [37]. Si was applied at the time of the filling of pots and was thoroughly mixed with the soil. Furthermore, heat stress was applied to pots by placing them in a greenhouse at the reproductive stage for two weeks.
2.2. Experimental Treatments
This study encompasses different wheat genotypes, namely Akbar-2019, Subhani-2021, and Faisalabad-2008, as well as different treatments: control, salt stress (8 dSm−1), heat stress, salt stress + heat stress, control + Si, salt stress + Si, heat stress+ Si, and salt stress + heat stress + Si. The present study was carried out in a completely randomized design under a factorial arrangement (3 × 8 × 3) with three replications. The pots were examined regularly, and plants were watered based on visual observation. Moreover, fertilizers urea (1.46 g), di-ammonium phosphate (DAP) (1.56 g), and sulfate of potassium (1.10 g) were applied together to fulfill NPK requirements. P and K were applied as a basal dose by mixing fertilizers in the soil, while N was applied to two splits at the sowing and tillering stages.
2.3. Measurement of Growth Traits
Three plants were randomly chosen from each pot, and their heights were measured and averages were taken. Likewise, three plants were selected and their root and shoot lengths and dry weights were measured.
2.4. Determination of Photosynthetic Pigments, Leaf Relative Water Content, and Electrolyte Leakage
Chlorophyll and carotenoid contents were determined using the method of Arnon [38]. Fresh samples (0.5 g) of plants were homogenized in 80% methanol solution to obtain the extract, and then the extract was centrifuged and filtered, and absorbance was noted at 645, 480, and 663 nm wavelengths to determine the chlorophyll a and b and carotenoid contents. To determine relative water content (RWC), fresh leaf samples were taken from the plant and weighed to determine fresh weight (FW), then the sample leaves were submerged in water for 24 h, and after removal from water, the turgid weight (TW) was taken. Thereafter, turgid leaf samples were oven-dried (70 °C) until constant weight was reached in order to determine dry weight (DW), and RWC was determined via the following procedure: RWC (%) = FW − DR/TW − DR × 100. For the determination of electrolyte leakage (EL), 0.5 g of fresh leaf samples were submerged in water for 30 min, after which EC1 was measured, and then the samples were heated for 50 min and EC2 was measured. The final electrolyte (EL) values were determined through the following equation: EL = EC1/EC2 × 100.
2.5. Determination of Osmolytes and Oxidative Stress Markers
For the determination of total soluble protein (TSP), frozen leaf samples were ground in 5 mL phosphate buffer and then centrifuged at 14,000 rpm for 15 min at 4 °C. Then, the plant samples were treated with 2 mL Bradford reagent, and this mixture was allowed to sit for 15–20 min and absorbance was noted at 595 nm [39]. For total free amino acid (FAA) determination: 1 mL of crude extract was homogenized with a buffer, poured into test tubes, and 1 mL pyridine with 1 mL ninhydrin was added. Then, these test tubes were placed in a water bath for 30 min at 90 °C at a volume of 25 mL, and absorbance was noted at 570 nm to determine FAA [40]. In order to determine H2O2 concentration, 0.5 g of samples was ground with trichloroacetic acid (TCA: 0.5 mL) to obtain the supernatant, and then 1 M potassium iodide (KI) and potassium phosphate buffer (PPB: 1 mL) was added into the supernatant and allowed to sit for 30 min, and then the absorbance was noted at 390 nm. For the determination of MDA contents, plant leaf samples were homogenized by adding TCA (5 mL) and centrifuged at 12,000 rpm for 15 min. Afterward, the supernatant was added to 5 mL of thiobarbituric acid (TBA) and boiled at 100 °C for 30 min, then cooled quickly, and absorbance was measured at 532 nm to determine MDA concentration.
2.6. Determination of Antioxidant Activities
The method of Aebi [41] was used to determine the catalase (CAT) activity. We used 0.5 g of wheat leaf sample and blended it with 5 mL of PPB, then centrifuged the mixture for 15 min at 1000 rpm, and absorbance was measured at 240 nm to determine CAT activity. In the case of ascorbate peroxidase (APX), 0.5 g leaf sample was taken and homogenized in 5 mL potassium phosphate buffer (PPB: PH 7.8) using a mortar and pestle. Afterward, the prepared solution was centrifuged for 15 min at 10,000 rpm and absorbance was recorded at 290 nm to determine APX activity [42]. To determine peroxidase (POD) activity, 0.5 g leaf sample was homogenized in PPB (5 mL) with mortar and pestle, after which solution was centrifuged for 15 min at 10,000 rpm and the absorbance was recorded at 470 nm to determine POD activity [43]. In the case of ascorbic acid activity (AsA), 0.5 g plant samples were homogenized in 5 mL of trichloro-acetic acid (TCA) and centrifuged for 10 min to obtain the supernatant, and then absorbance was noted to determine AsA activity [43]. Lastly, to determine SOD activity, a reaction mixture of 400 µL H2O2, 25 mL buffer, 100 µL Triton, 50 µL sample, and 50 µL riboflavin was prepared and absorbance was measured at 560 for the determination of SOD activity [44].
2.7. Ionic Concentration
The wheat plant samples oven-dried (65 °C) and ground into powder. Afterward, 0.5% of ground sample was digested at 180 °C in a mixture containing two acids (hydrochloric acid, HCl, and nitric acid, HNO3) at a 1:2 ratio. Then, samples were filtered and diluted by adding the water. The concentration of Na, Ca, Mg, and K was determined using a flame photometer, and the concentration of Cl was determined using a chloride analyzer.
2.8. Determination of Yield Traits
The spike-bearing tillers were counted on every plant and the average calculated. Similarly, spikes per plant were counted on each plant and an average was calculated, while 10 spikes from each pot were used to determine the spike length and grains per spike. Lastly, the plants in each pot were harvested and biological yield was measured, and later the plants were threshed to determine grain yield.
2.9. Data Analysis
The data obtained from the collected traits were analyzed using a two-way analysis of variance (ANOVA) for wheat cultivars, stress treatments, and their interactions, and the least significant difference (LSD) was used at a 5% probability level to determine significance among treatments [45]. The results were achieved using a sigma plot, and principal component analysis was performed using R-studio.
3. Results
3.1. Growth and Morphological Traits
The results indicate that different stress treatments negatively affected growth parameters; similarly, cultivars also behaved differently in terms of growth and morphological traits (Table 1). Taller plants (54.77 cm) with more leaves per plant (LPP: 11.75) were recorded after treatment with Si, and shorter plants with low LPP were observed in case of combined salinity and heat stress (Table 1). In terms of cultivars, taller plants with more leaves were recorded in Akbar-2019, while shorter plants with the lowest number of leaves were recorded in Faisalabad-2008 (Table 1). Regarding interactions, the tallest plants with the most leaves were recorded in Akbar-2019 after Si application, and shorter plants with fewer leaves were recorded in Faisalabad-2008 with the combined imposition of heat and salinity stress (Table 1). The highest root fresh weight (RFW), shoot fresh weight (SFW), root dry weight (RDW), and shoot dry weight (SDW) were recorded in plants treated with Si without any stress, and the lowest values for these parameters were recorded in plants growing under combined heat and salinity stress (Table 1). Regarding interactions, the cultivar Akbar-2019 performed well with the highest RFW, SFW, RDW, and SDW parameters, and Faisalabad-2008 performed poorly, with the lowest RFW, SFW, RDW, and SDW parameters (Table 1).
Table 1.
Effect of Si application on growth traits of different wheat cultivars grown under salinity and heat stress conditions.
| Wheat Cultivars | Treatments | PH (cm) | LPP | RFW (g) | SFW (g) | RDW (g) | SDW (g) |
|---|---|---|---|---|---|---|---|
| Akbar-2019 | Control | 53.00 ± 0.47 c | 10.93 ± 0.32 df | 9.90 ± 0.12 d | 14.30 ± 0.19 d | 8.43 ± 0.09 c | 6.58 ± 0.037 d |
| Salt stress (8 dS m−1) | 43.66 ± 0.55 gh | 10.50 ± 0.29 ef | 9.46 ± 0.21 c | 14.26 ± 0.21 d | 8.26 ± 0.12 d | 6.44 ± 0.019 f | |
| Heat stress | 41.33 ± 0.29 hi | 9.23 ± 0.20 g | 8.83 ± 0.15 f | 13.23 ± 0.13 e | 7.93 ± 0.021 e | 6.35 ± 0.020 f | |
| Salt + Heat | 38.66 ± 0.48 ij | 8.50 ± 0.22 hi | 8.26 ± 0.20 g | 13.13 ± 0.21 e | 7.81 ± 0.14 f | 6.21 ± 0.022 g | |
| Si (500 mg kg−1) | 58.00 ± 0.72 a | 14.50 ± 0.12 a | 12.93 ± 0.7 a | 16.40 ± 0.25 a | 8.84 ± 0.10 a | 6.95 ± 0.015 a | |
| Salt + Si | 57.00 ± 0.63 ab | 14.16 ± 0.29 ab | 11.36 ± 0.12 b | 15.43 ± 0.15 b | 8.75 ± 0.08 a | 6.84 ± 0.029 b | |
| Heat + Si | 53.33 ± 0.55 c | 11.50 ± 0.28 cd | 10.83 ± 0.14 c | 14.43 ± 0.19 cd | 8.56 b ± 0.10 b | 6.62 ± 0.031 d | |
| Salt + Heat + Si | 54.66 b ± 0.49 bc | 13.83 ± 0.42 b | 11.00 ± 0.19 bc | 14.80 ± 0.32 c | 8.64 ± 0.12 b | 6.71 ± 0.03 c | |
| Subhani-2021 | Control | 45.33 ± 0.33 fg | 10.23 ± 0.12 f | 7.66 ± 0.20 ij | 10.86 ± 0.12 hi | 7.34 ± 0.024 i | 5.62 ± 0.049 k |
| Salt stress (8 dS m−1) | 45.00 ± 0.66 fg | 8.60 ± 0.22 hi | 7.30 ± 0.22 k | 10.43 ± 0.09 ij | 6.80 ± 0.10 j | 5.38 ± 0.018 l | |
| Heat stress | 40.00 ± 0.82 ij | 8.16 ± 0.24 i-k | 6.60 ± 0.18 l | 10.06 ± 0.18 gh | 6.78 ± 0.09 j | 5.16 ± 0.026 m | |
| Salt + Heat | 37.00 ± 0.33 j | 7.10 ± 0.29 m | 6.26 ± 0.15 m | 9.70 ± 0.06 jk | 6.64 ± 0.014 k | 6.96 ± 0.012 n | |
| Si (500 mg kg−1) | 54.66 ± 0.22 bc | 11.93 ± 0.32 c | 8.80 ± 0.09 gh | 12.93 ± 0.32 e | 7.82 ± 0.019 f | 6.12 ± 0.040 h | |
| Salt + Si | 53.33 ± 0.19 c | 10.66 ± 0.13 ef | 7.40 ± 0.12 jk | 12.06 ± 0.38 f | 7.62 ± 0.020 g | 5.87 ± 0.020 i | |
| Heat + Si | 51.66 ± 0.38 cd | 10.26 ± 0.42 f | 7.33 ± 0.05 k | 11.06 ± 0.12 gh | 7.46 ± 0.12 h | 5.64 ± 0.019 k | |
| Salt + Heat + Si | 52.66 ± 0.42 c | 10.63 ± 0.21 ef | 7.93 ± 0.12 hi | 11.46 ± 0.14 g | 7.53 ± 0.10 gh | 5.73 ± 0.064 j | |
| Faisalabad-2008 | Control | 47.00 ± 0.26 ef | 7.60 ± 0.34 k-m | 4.96 ± 0.22 p | 6.73 ± 0.06 o | 5.77 ± 0.05 op | 4.28 ± 0.042 r |
| Salt stress (8 dS m−1) | 46.00 ± 0.48 fg | 7.33 ± 0.42 lm | 4.76 ± 0.24 pq | 5.96 ± 0.10 p | 5.69 ± 0.07 pq | 3.91 ± 0.032 s | |
| Heat stress | 41.66 ± 0.55 hi | 7.23 ± 0.37 lm | 4.53 ± 0.15 q | 4.83 ± 0.05 q | 5.64 ± 0.08 q | 3.61 ± 0.074 t | |
| Salt + Heat | 31.00 ± 0.41 k | 7.20 ± 0.22 m | 3.86 ± 0.11 r | 3.56 ± 0.06 r | 5.38 ± 0.012 r | 3.43 ± 0.09 u | |
| Si (500 mg kg−1) | 51.66 ± 0.56 cd | 8.83 ± 0.19 gh | 6.13 ± 0.07 m | 9.16 ± 0.14 l | 6.45 ± 0.029 l | 4.77 ± 0.11 o | |
| Salt + Si | 49.33 ± 0.43 df | 8.30 ± 0.28 h-j | 5.80 ± 0.08 n | 8.56 ± 0.12 m | 6.30 ± 0.026 m | 4.61 ± 0.025 p | |
| Heat + Si | 47.33 ± 0.40 ef | 7.80 ± 0.22 j-l | 5.46 ± 0.04 o | 7.90 ± 0.10 n | 5.84 ± 0.041 o | 4.33 ± 0.013 r | |
| Salt + Heat + Si | 47.66 ± 0.59 ef | 8.03 ± 0.20 i-k | 5.70 ± 0.11 no | 8.20 ± 0.16 mn | 6.12 ± 0.033 n | 4.43 ± 0.021 p |
PH: plant height; LPP: leaves per plant; RFW: root fresh weight; SFW: shoot fresh weight; RDW: root dry weight; SDW: shoot dry weight. The values given in the table are the mean of three replicates with ± SD (standard deviation), and different letters indicate significance at p < 0.05.
3.2. Photosynthetic Pigments, Relative Water Contents, and Electrolyte Leakage
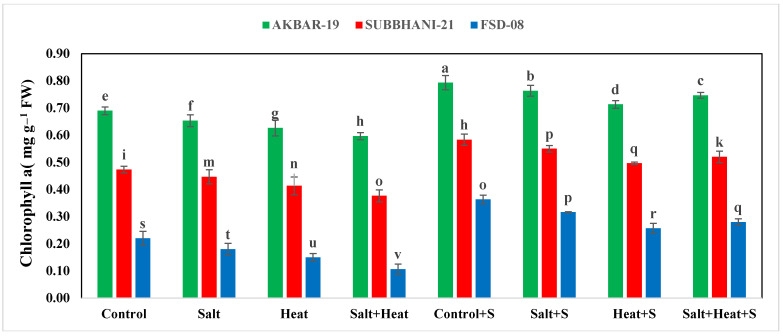
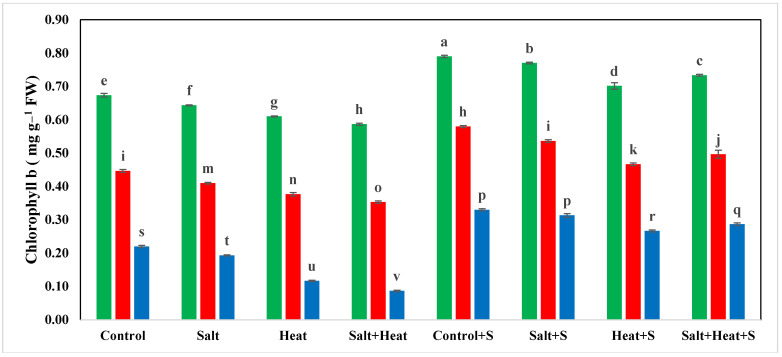
The results indicate that heat and saline conditions significantly reduced the photosynthetic pigments and RWC of wheat plants (Table 1). The maximum chlorophyll-a (0.79 mg g−1 FW), chlorophyll-b (0.79 mg g−1 FW), and carotenoid (4.83 mg g−1 FW) values were recorded after Si application without any stresses, and the lowest values for chlorophyll-a (0.10 mg g−1 FW), chlorophyll-b (0.08 mg g−1 FW), and carotenoid (1.23 mg g−1 FW) were recorded in combined heat and salinity stress conditions without Si application. In terms of cultivars, the highest chlorophyll and carotenoid concentrations were identified in Akbar-2019 and the lowest was observed in Faisalabad-2008. The lowest chlorophyll-a content was recorded in Faisalabad-2008 (Figure 1).
Figure 1.
Effect of Si application on chlorophyll−a and chlorophyll−b contents of different wheat cultivars grown under salinity and heat stress conditions. The values given the figures are the means of three replicates with ± SD, and different letters indicate significance at p < 0.05.
Regarding the interactive effects, the highest values for photosynthetic pigments were recorded in Akbar-2019 after Si application, and the lowest were observed in Faisalabad-2008 under combined heat and salinity stress (Table 2). The variable stress treatments and cultivars also had a significant impact on RWC and EL. The highest RWC was recorded in Akbar-2019 after silicon application and the minimum was recorded in Faisalabad-2008 under combined heat and salinity stress conditions (Table 2). Conversely, maximum EL was recorded in Faisalabad-2008 under combined heat and salinity stresses, and the lowest EL was recorded for Akbar-2019 after the application of Si (Table 2).
Table 2.
Effect of Si application on photosynthetic pigments and physiological traits of different wheat cultivars grown under salinity and heat stress conditions.
| Wheat Cultivars | Treatments | Carotenoids (mg g−1 FW) | Electrolyte Leakage (%) | Relative Water Contents (%) |
|---|---|---|---|---|
| Akbar-2019 | Control | 4.34 ± 0.019 c | 54.45 ± 1.26 c | 79.94 ± 0.34 d |
| Salt stress (8 dS m−1) | 4.18 ± 0.035 cd | 56.11 ± 1.15 b | 78.78 ± 1.16 df | |
| Heat stress | 4.08 ± 0.060 df | 57.17 ± 1.29 b | 77.89 ± 1.38 e–g | |
| Salt + Heat | 3.99 ± 0.087 ef | 58.58 ± 0.78 a | 77.14 ± 0.89 fg | |
| Si (500 mg kg−1) | 4.83 ± 0.015 a | 50.39 ± 0.98 f | 86.48 ± 0.38 a | |
| Salt + Si | 4.60 ± 0.021 b | 54.27 ± 0.56 cd | 84.26 ± 0.37 b | |
| Heat + Si | 4.36 ± 0.012 c | 53.10 ± 1.10 de | 82.36 ± 0.46 c | |
| Salt + Heat + Si | 4.69 ± 0.020 ab | 51.90 ± 0.78 e | 84.54 ± 0.66 b | |
| Subhani-2021 | Control | 3.26 ± 0.016 h | 47.33 ± 0.97 hi | 70.16 ± 0.56 j |
| Salt stress (8 dS m−1) | 3.15 ± 0.11 h | 48.10 ± 0.42 gh | 68.89 ± 0.78 j | |
| Heat stress | 2.82 ± 0.062 i | 48.97 ± 1.10 g | 65.42 ± 0.89 k | |
| Salt + Heat | 2.68 ± 0.014 ij | 50.40 ± 0.92 f | 63.37 ± 0.33 l | |
| Si (500 mg kg−1) | 3.83 ± 0.029 f | 42.57 ± 0.42 l | 78.59 ± 0.35 df | |
| Salt + Si | 3.59 ± 0.022 g | 46.24 ± 0.31 ij | 76.48 ± 1.11 g | |
| Heat + Si | 3.25 ± 0.040 h | 45.42 ± 0.98 j | 72.3 ± 0.88 i | |
| Salt + Heat + Si | 3.48 ± 0.034 g | 43.99 ± 1.14 k | 74.39 ± 0.78 h | |
| Faisalabad-2008 | Control | 1.90 ± 0.022 n | 37.98 ± 1.29 no | 49.43 ± 0.36 p |
| Salt stress (8 dS m−1) | 1.55 ± 0.031 o | 39.04 ± 1.41 n | 46.17 ± 0.99 q | |
| Heat stress | 1.35 ± 0.025 p | 40.90 ± 1.56 m | 44.94 ± 0.89 q | |
| Salt + Heat | 1.23 ± 0.02 p | 42.72 ± 1.12 l | 41.36 ± 1.12 r | |
| Si (500 mg kg−1) | 2.56 ± 0.022 jk | 34.30 ± 1.14 q | 59.48 ± 0.88 m | |
| Salt + Si | 2.40 ± 0.016 kl | 36.92 ± 1.38 op | 57.99 ± 0.99 m | |
| Heat + Si | 2.16 ± 0.011 m | 36.29 ± 1.42 p | 52.46 ± 0.99 o | |
| Salt + Heat + Si | 2.25 ± 0.012 lm | 35.33 ± 1.56 pq | 54.72 ± 1.12 n |
The values given in the table are the means of three replicates with ± SD, and different letters indicate significance at p < 0.05.
3.3. Osmo-Protectants, Antioxidant Activities, and Oxidative Stress Causes
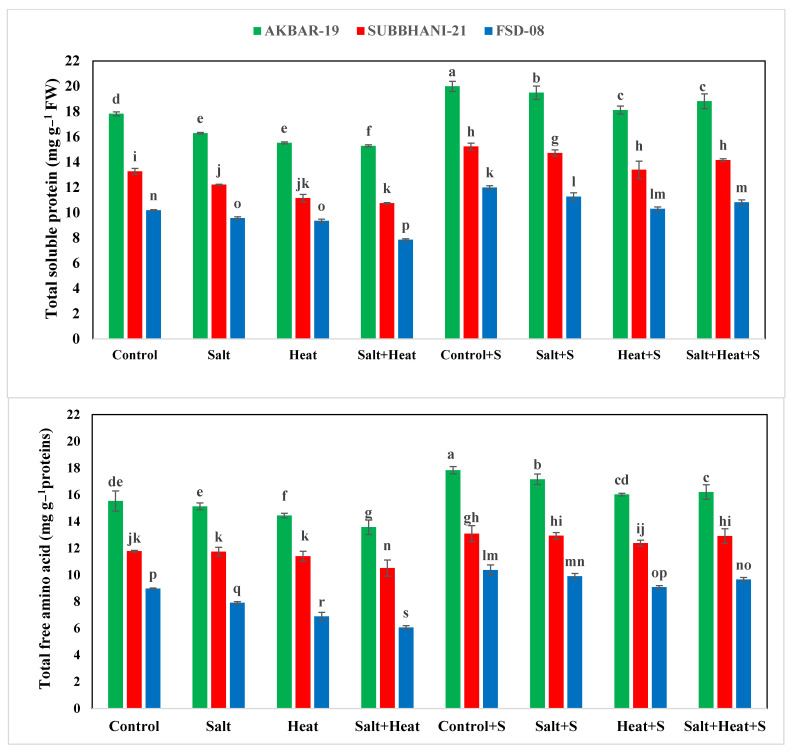
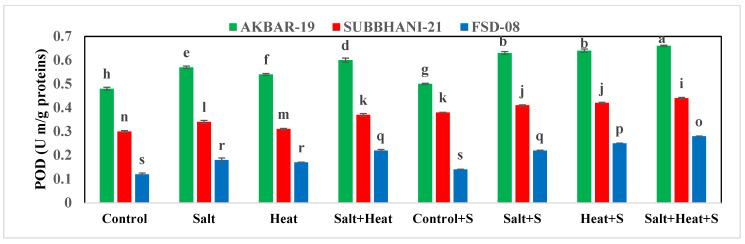
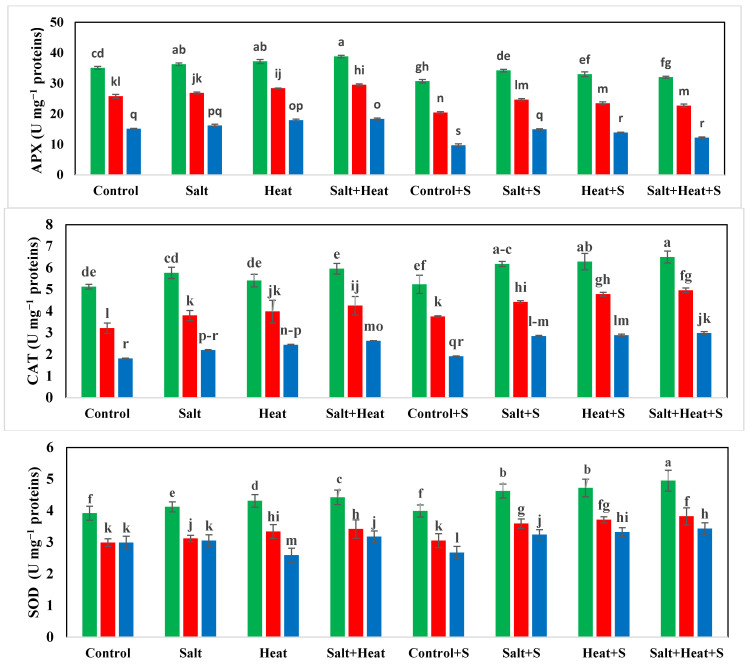
The highest TSP (15.73 mg g−1 protein) and FAA (19.99 mg g−1 protein) were recorded with Si treatment, and the lowest TSP and FAA were recorded when plants were subjected to heat and saline conditions (Figure 2). For the interaction effect, the highest TSP and FAA were observed in Akbar-2019 after Si supplementation, and the lowest TSP and FAA were recorded in Faisalabad-2008 under saline and heat conditions without the use of Si Figure 2). These results indicate that different treatments had significant impacts on all tested antioxidant activities. The activities of APX, POD, CAT, and SOD were significantly increased under stress conditions, and the highest activities of all these antioxidants (APX, POD, CAT, and SOD) were recorded under heat and saline conditions. After the application of Si under control conditions, the lowest antioxidant activities (APX, POD, CAT, and SOD) were recorded (Figure 3).
Figure 2.
Effect of Si application on total soluble protein and free amino acids of different wheat cultivars grown under salinity and heat stress conditions. The values given in the figures are the means of three replicates with ± SD, and different letters indicate significance at p < 0.05.
Figure 3.
Effect of Si application on antioxidant activities of different wheat cultivars grown under salinity and heat stress conditions. APX: ascorbate peroxidase; CAT: catalase; POD: peroxidase; SOD: superoxide dismutase. The values given in the figures are the means of three replicates with ± SD, and different letters indicate significance at p < 0.05.
Similarly, oxidative stress markers (MDA and H2O2) also revealed a substantial increase in saline, heat stress, and combined saline and heat stress conditions; however, Si offset MDA and H2O2 accumulation by increasing antioxidant activities. In terms of cultivars, Akbar-2019 displayed the highest APX, POD, CAT, and SOD activities, which resulted in a substantial decrease in MDA and H2O2 production, while Faisalabad-2008 displayed the highest MDA and H2O2 accumulation (Table 3).
Table 3.
Effect of Si application on oxidative stress markers of different wheat cultivars grown under salinity and heat stress conditions.
| Wheat Cultivars | Treatments | MDA (µ mol g−1 FW) | H2O2 µ mol g−1 FW) |
|---|---|---|---|
| Akbar-2019 | Control | 3.92 ± 0.12 n | 2.62 ± 0.26 j |
| Salt stress (8 dS m−1) | 4.81 ± 0.41 ij | 3.49 ± 0.21 f | |
| Heat stress | 4.92 ± 0.10 j | 3.61 ± 0.09 ef | |
| Salt + Heat | 5.11 ± 0.22 i | 3.72 ± 0.10 e | |
| Si (500 mg kg−1) | 3.70 ± 0.31 p | 2.50 ± 0.19 k | |
| Salt+ Si | 4.52 ± 0.15 l | 3.39 ± 0.10 g | |
| Heat + Si | 4.60 ± 0.30 k | 3.41 ± 0.12 g | |
| Salt + Heat + Si | 4.72 ± 0.21 jk | 3.52 ± 0.29 f | |
| Subhani-2021 | Control | 4.02 ± 0.10 o | 2.83 ± 0.22 i |
| Salt stress (8 dS m−1) | 5.59 ± 0.14 g | 4.13 ± 0.10 v | |
| Heat stress | 5.70 ± 0.11 g | 4.19 ± 0.15 v | |
| Salt + Heat | 5.88 ± 0.40 f | 4.32 ± 0.12 b | |
| Si (500 mg kg−1) | 3.81 ± 0.19 p | 2.72 ± 0.21 i | |
| Salt + Si | 5.20 ± 0.12 i | 3.86 ± 0.18 ef | |
| Heat + Si | 5.34 ± 0.33 h | 3.92 ± 0.15 e | |
| Salt + Heat + Si | 5.42 ± 0.22 h | 4.04 ± 0.20 d | |
| Faisalabad-2008 | Control | 4.22 ± 0.12 m | 2.99 ± 0.31 h |
| Salt stress (8 dS m−1) | 6.92 ± 0.19 b | 4.62 ± 0.19 a | |
| Heat stress | 7.02 ± 0.31 b | 4.70 ± 0.22 a | |
| Salt + Heat | 7.14 ± 0.22 a | 4.72 ± 0.15 a | |
| Si (500 mg kg−1) | 3.98 ± 0.18 n | 2.80 ± 0.11 i | |
| Salt + Si | 6.51 ± 0.26 e | 4.33 ± 0.20 b | |
| Heat + Si | 6.65 ± 0.14 d | 4.41 ± 0.14 b | |
| Salt + Heat + Si | 6.80 ± 0.10 c | 4.52 ± 0.19 ab |
MDA: malondialdehyde; H2O2: hydrogen peroxide. The values given in the table are the mean of three replicates with ± SD, and different letters indicate significance at p < 0.05.
3.4. Yield Traits
The highest number of tillers per plant (TPP: 13.76), grains per spike (GPS: 58.91), and spike lengths (SL: 9.84 cm) were recorded after Si application under normal conditions and the lowest number of TPP, GPS, and SL were recorded in conditions where salt and heat stress were applied to plants without Si application (Table 4). In terms of cultivars, the highest TPP, GPS, and SL were recorded in Akbar-2019, followed by Subhani-2021, and the lowest TPP, GPS, and SL were recorded in Faisalabad-2008 (Table 4). Regarding interactions, Akbar-2019 again exhibited the highest TPP, GPS, and SL after Si application, and Faisalabad-2008 displayed the lowest TPP, GPS, and SL under combined heat and saline conditions without Si application (Table 4). The application of Si alone resulted in the highest 100-grain weight (100-GW: 8.82 g), grain yield per plant (GYPP: 9.79 g), and biological yield per plant (BYPP: 29.50 g) without any stresses, and the lowest 100-GW (5.45 g), GYPP (5.33 g), and BYPP (17.60 g) were recorded under conditions of combined heat and salinity stress (Table 4). In terms of cultivars, Akbar-2019 displayed the highest 100-GW, GYPP, and BYPP, and Faisalabad-2008 exhibited the lowest 100-GW, GYPP, and BYPP (Table 4).
Table 4.
Effect of Si application on the yield traits of different wheat cultivars grown under salinity and heat stress conditions.
| Wheat Cultivars | Treatments | TPP | GPS | SL (cm) | 100 GW (g) | GYPP (g) | BYPP (g) |
|---|---|---|---|---|---|---|---|
| Akbar-2019 | Control | 11.63 ± 0.04 d | 50.97 ± 0.69 e | 9.57 ± 0.018 d | 8.22 ± 0.035 d | 9.17 ± 0.10 c | 27.60 ± 0.23 e |
| Salt stress (8 dS m−1) | 11.16 ± 0.03 e | 50.20 ± 0.21 ef | 9.51 ± 0.012 df | 8.15 ± 0.023 d | 8.81 ± 0.23 d | 27.41 ± 0.10 f | |
| Heat stress | 10.26 ± 0.09 f | 50.09 ± 0.54 ef | 9.44 ± 0.024 e | 7.95 ± 0.062 e | 8.56 ± 0.14 e | 26.63 ± 0.11 g | |
| Salt + Heat | 10.06 ± 0.02 fg | 49.88 ± 0.29 f | 9.36 ± 0.046 f | 7.77 ± 0.090 f | 8.31 ± 0.22 f | 26.35 ± 0.11 h | |
| Si (500 mg kg−1) | 13.76 ± 0.10 a | 58.91 ± 0.34 a | 9.84 ± 0.052 a | 8.82 ± 0.054 a | 9.79 ± 0.15 a | 29.50 ± 0.17 a | |
| Salt + Si | 13.23 ± 0.09 b | 56.53 ± 0.41 b | 9.81 ± 0.11 ab | 8.66 ± 0.042 b | 9.69 ± 0.075 a | 29.35 ± 0.22 b | |
| Heat + Si | 11.83 ± 0.12 d | 52.68 ± 0.65 d | 9.67 ± 0.098 c | 8.47 ± 0.061 c | 9.29 ± 0.038 bc | 28.42 ± 0.23 d | |
| Salt + Heat + Si | 12.90 ± 0.14 c | 47.83 ± 0.56 g | 9.75 ± 0.012 b | 8.50 ± 0.050 c | 9.45 b ± 0.18 b | 28.67 ± 0.17 e | |
| Subhani-2021 | Control | 8.93 ± 0.10 j | 39.55 ± 0.49 j | 8.42 ± 0.033 jk | 6.89 ± 0.038 j | 7.29 ± 0.042 i | 22.61 ± 0.056 m |
| Salt stress (8 dS m−1) | 8.46 ± 0.09 k | 39.21 ± 0.62 jk | 8.38 ± 0.11 k | 6.72 ± 0.027 k | 6.89 ± 0.015 j | 21.50 ± 0.22 n | |
| Heat stress | 8.26 ± 0.19 k | 38.96 ± 0.54 jk | 8.30 ± 0.012 l | 6.46 ± 0.035 l | 6.43 ± 0.12 k | 21.22 ± 0.21 o | |
| Salt + Heat | 7.80 ± 0.18 l | 38.23 ± 0.48 k | 8.14 ± 0.029 m | 6.22 ± 0.10 m | 6.21 ± 0.044 l | 20.71 ± 0.18 p | |
| Si (500 mg kg−1) | 9.83 ± 0.20 g | 47.83 ± 0.50 g | 9.22 ± 0.034 g | 7.62 ± 0.078 g | 7.90 ± 0.015 g | 25.53 ± 0.32 i | |
| Salt+ Si | 9.43 ± 0.14 h | 46.43 ± 0.26 h | 8.84 ± 0.012 h | 7.35 ± 0.053 h | 7.54 ± 0.044 h | 24.66 ± 0.098 j | |
| Heat + Si | 9.13 ± 0.010 ij | 39.94 ± 0.39 j | 8.46 ± 0.10 j | 7.09 ± 0.042 i | 7.42 ± 0.078 hi | 23.57 ± 0.11 l | |
| Salt + Heat+ Si | 9.36 ± 0.012 hi | 44.90 ± 0.42 j | 8.54 ± 0.018 i | 7.13 ± 0.054 i | 7.49 ± 0.065 h | 24.35 ± 0.12 k | |
| Faisalabad-2008 | Control | 5.26 ± 0.014 p | 24.22 ± 0.31 p | 7.20 ± 0.019 r | 5.24 ± 0.029 q | 4.89 ± 0.053 p | 16.59 ± 0.13 u |
| Salt stress (8 dS m−1) | 4.80 ± 0.024 q | 21.77 ± 0.46 q | 7.14 ± 0.069 r | 4.87 ± 0.49 r | 4.42 ± 0.046 q | 15.74 ± 0.29 v | |
| Heat stress | 4.26 ± 0.039 r | 19.73 ± 0.12 r | 6.93 ± 0.12 s | 4.52 ± 0.54 s | 4.36 ± 0.035 q | 14.59 ± 0.24 w | |
| Salt + Heat | 3.83 ± 0.043 s | 18.29 ± 0.19 s | 6.74 ± 0.043 t | 3.87 ± 0.042 t | 3.93 ± 0.052 r | 13.26 ± 0.12 x | |
| Si (500 mg kg−1) | 6.66 ± 0.053 l | 35.53 ± 0.29 l | 7.94 ± 0.023 n | 5.82 ± 0.031 m | 5.87 ± 0.016 m | 19.70 ± 0.13 q | |
| Salt + Si | 6.23 ± 0.044 n | 33.27 ± 0.25 m | 7.82 ± 0.017 o | 5.65 ± 0.064 n | 5.62 ± 0.029 n | 18.66 ± 0.22 r | |
| Heat + Si | 5.06 ± 0.029 pq | 29.83 ± 0.21 o | 7.35 ± 0.015 q | 5.38 ± 0.05 pq | 5.26 ± 0.043 o | 17.47 ± 0.24 t | |
| Salt + Heat + Si | 5.66 ± 0.11 o | 31.92 ± 0.14 n | 7.49 ± 0.025 p | 5.45 ± 0.039 p | 5.33 ± 0.040 o | 17.60 ± 0.36 s |
TPP: total productive tillers; GPS: grains per spike; SL: spike length; GW: grain weight; GYPP: grain yield per plant; BYPP: biological yield per plant. The values given in the table are the means of three replicates with ± SD, and different letters indicate significance at p < 0.05.
3.5. Nutrient Concentration
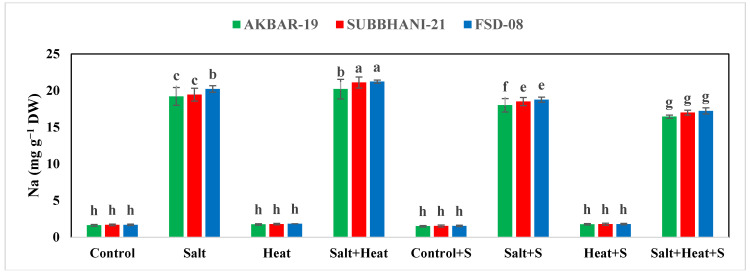
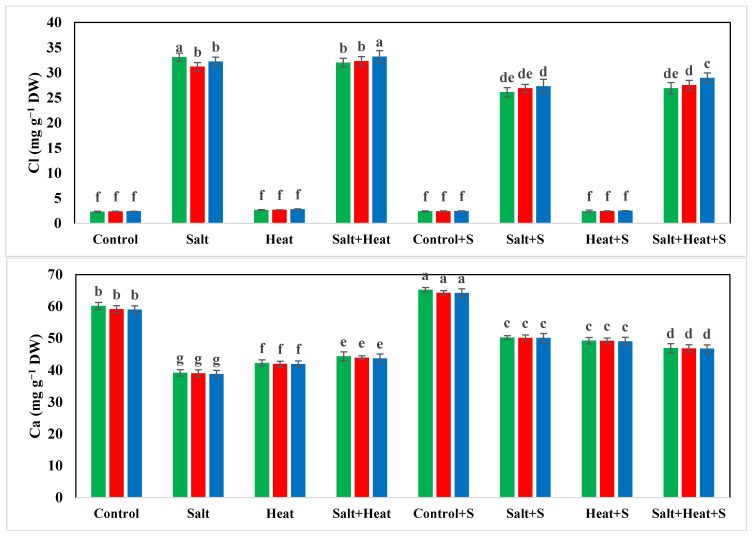
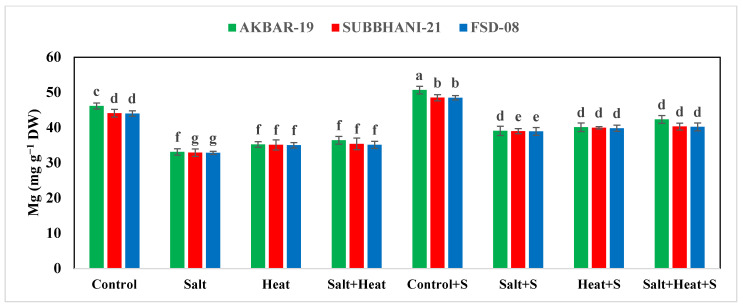
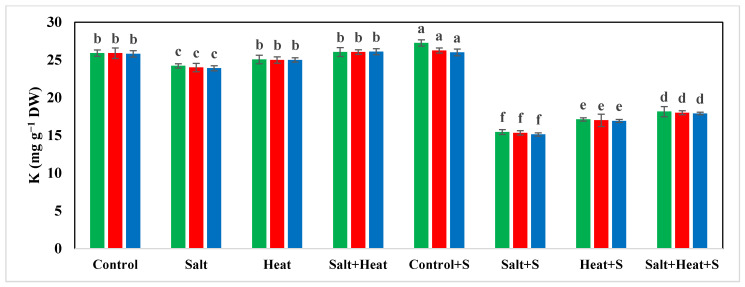
The results indicate a substantial reduction in the concentration of Ca, Mg, and K, while an increase in the concentration of Na+ and Cl− under salinity and heat stress conditions (Figure 4). The highest Ca, Mg, and K concentrations were recorded in plants grown under normal conditions with the application of Si, and the lowest Ca, Mg, and K concentrations were recorded in plants grown under saline and heat stress conditions (Figure 4 and Figure 5). The application of Si offset the negative effects of saline and heat stress and considerably increased the uptake of Ca, Mg, and K under both saline and heat conditions (Table 5). The cultivar type had a non-significant impact on Ca, Mg, and K concentration; however, Akbar-2019 displayed slightly higher nutrient concentrations in plant parts (Figure 4 and Figure 5). The highest Na+ and Cl− concentrations were recorded under combined saline and heat stress conditions and were comparable with saline stress alone, and the lowest Na+ and Cl− concentrations were recorded in control conditions with Si application (Figure 4 and Figure 5).
Figure 4.
Effect of Si application on the Na, Cl, and Ca concentrations of different wheat cultivars grown under salinity and heat stress conditions. Na: sodium; Cl: chloride; Ca: calcium; DW: dry weight. The values given in the figures are the means of three replicates with ± SD, and different letters indicate significance at p < 0.05.
Figure 5.
Effect of Si application on Mg and K concentrations of different wheat cultivars grown under salinity and heat stress conditions. Mg: magnesium; K: potassium; DW: dry weight. The values given in the figures are the means of three replicates with ± SD, and different letters indicate significance at p < 0.05.
Table 5.
Effect of Si application on growth traits of different wheat cultivars grown under salinity and heat stress conditions cultivars.
| Wheat Cultivars | Treatments | Na (mg g−1 DW) | Cl (mg g−1 DW) | Ca (mg g−1 DW) | Mg (mg g−1 DW) | K (mg g−1 DW) |
|---|---|---|---|---|---|---|
| Akbar-19 | Control | 1.64 ± 0.12 h | 2.32 ± 0.12 f | 60.12 ± 1.12 b | 46.12 ± 0.85 c | 25.90 ± 0.42 b |
| Salt stress (8 dS m−1) | 19.20 ± 1.22 c | 33.10 ± 0.78 a | 39.21 ± 1.00 g | 33.10 ± 0.92 f | 24.22 ± 0.29 c | |
| Heat stress | 1.76 ± 0.10 h | 2.65 ± 0.10 f | 42.23 ± 0.99 f | 35.22 ± 0.78 f | 25.07 ± 0.56 b | |
| Salt + Heat | 20.20 ± 1.34 b | 32.00 ± 0.89 b | 44.33 ± 1.42 e | 36.41 ± 1.11 f | 26.05 ± 0.60 b | |
| Si (500 mg kg−1) | 1.52 ± 0.10 h | 2.40 ± 0.10 f | 65.23 ± 0.68 a | 50.67 ± 1.09 a | 27.25 ± 0.42 a | |
| Salt+ Si | 18.00 ± 0.92 f | 26.12 ± 0.92 f | 50.24 ± 0.56 c | 39.10 ± 1.32 d | 15.45 ± 0.33 f | |
| Heat + Si | 1.78 ± 0.10 h | 2.42 ± 0.022 f | 49.23 ± 0.99 c | 40.11 ± 1.21 d | 17.12 ± 0.22 e | |
| Salt+ Heat+ Si | 16.44 ± 0.22 h | 26.91 ± 1.11 de | 46.88 ± 1.43 d | 42.33 ± 1.11 d | 18.15 ± 0.67 d | |
| Subhani-21 | Control | 1.70 ± 0.49 h | 2.38 ± 0.029 f | 59.12 ± 1.14 b | 44.10 ± 1.09 d | 25.90 ± 0.70 b |
| Salt stress (8 dS m−1) | 19.44 ± 0.89 c | 31.20 ± 0.82 b | 39.00 ± 1.10 g | 32.91 ± 1.05 g | 24.00 ± 0.55 c | |
| Heat stress | 1.80 ± 0.10 h | 2.70 ± 0.002 f | 41.91 ± 0.86 f | 35.10 ± 1.22 f | 25.00 ± 0.42 b | |
| Salt + Heat | 21.10 ± 0.75 a | 32.30 ± 0.89 b | 43.90 ± 0.58 e | 35.40 ± 1.10 f | 26.05 ± 0.29 b | |
| Si (500 mg kg−1) | 1.55 ± 0.40 h | 2.41 ± 0.10 f | 64.23 ± 0.72 a | 48.50 ± 0.87 b | 26.25 ± 0.33 a | |
| Salt+ Si | 18.50 ± 0.56 e | 26.88 ± 0.78 de | 50.12 ± 0.92 c | 38.99 ± 0.72 e | 15.33 ± 0.29 f | |
| Heat + Si | 1.80 ± 0.29 h | 2.46 ± 0.022 f | 49.20 ± 0.88 c | 39.98 ± 0.33 d | 17.00 ± 0.82 e | |
| Salt+ Heat+ Si | 16.99 ± 0.33 g | 27.50 ± 0.98 d | 46.80 ± 1.13 d | 40.30 ± 0.92 d | 18.00 ± 0.26 d | |
| Faisalabad-08 | Control | 1.70 ± 0.10 h | 2.40 ± 0.020 f | 59.00 ± 1.19 b | 44.00 ± 0.76 d | 25.80 ± 0.42 b |
| Salt stress (8 dS m−1) | 20.22 ± 0.45 b | 32.20 ± 0.89 b | 38.78 ± 1.21 g | 32.85 ± 0.42 g | 23.90 ± 0.33 c | |
| Heat stress | 1.85 ± 0.013 h | 2.79 ± 0.10 f | 41.90 ± 0.99 f | 35.00 ± 0.78 f | 25.00 ± 0.29 b | |
| Salt + Heat | 21.22 ± 0.22 a | 33.20 ± 1.20 a | 43.65 ± 1.40 e | 35.11 ± 0.99 f | 26.10 ± 0.40 b | |
| Si (500 mg kg−1) | 1.55 ± 0.10 h | 2.45 ± 0.033 f | 64.20 ± 1.32 a | 48.49 ± 0.72 b | 26.00 ± 0.44 a | |
| Salt+ Si | 18.76 ± 0.34 e | 27.20 ± 1.41 d | 50.10 ± 1.33 c | 38.92 ± 0.88 e | 15.12 ± 0.22 f | |
| Heat + Si | 1.80 ± 0.10 h | 2.50 ± 0.020 d | 49.01 ± 1.29 c | 39.83 ± 0.89 d | 16.92 ± 0.20 e | |
| Salt+ Heat+ Si | 17.22 ± 0.42 g | 28.96 ± 0.99 c | 46.72 ± 1.20 d | 40.22 ± 1.10 d | 17.90 ± 0.17 d |
Na: sodium, Cl: chloride, Ca: calcium, Mg: magnesium, K: potassium, DW: dry weight. The values give in tables are mean of three replicates with ± and different letters indicating significant at p < 0.05.
3.6. Principle Component Analysis
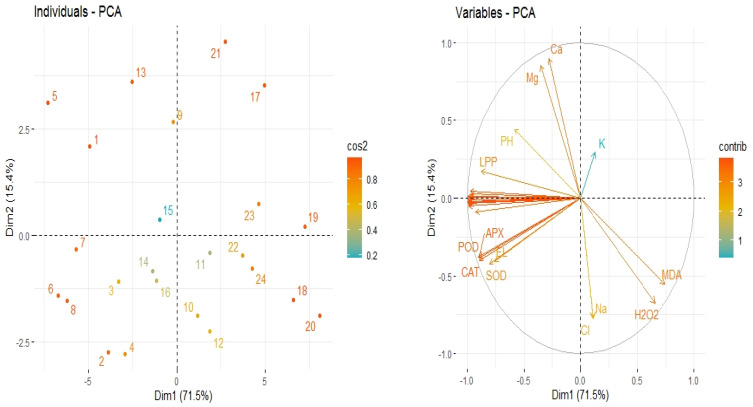
The collected data set on different traits were subjected to principal component analysis (PCA) in order to determine the relationships of the studied traits. The analysis given in Figure 6 indicates that two components (PC1 and PC2) showed 86.9% total variance, where PC1 comprised 71.5%, and PC2 comprised 15.4% (Figure 6). PCA findings indicated that salinity and heat stress induced negative impacts on the growth and yield traits; however, Si application mitigated the adverse effects of both these stresses. There was a negative relationship between stress treatments and growth and yield traits; RWC; chlorophyll concentration; and Mg, Ca, and K uptake and there was positive association between the stress treatments and antioxidant activities; MDA; H2O2; EL; and Na+ and Cl− accumulation (Figure 6).
Figure 6.
The scores on left and loading plots on right of the principal component analysis (PCA) show the effect of diverse treatments on the studied traits.
4. Discussion
Heat and salinity stresses are serious co-occurring abiotic stresses that negatively affect plant growth and development. Thus, it is essential to understand the response of plants against both these abiotic stresses [46,47]. In the current study, SS significantly reduced the growth and biomass of all the cultivars, but the most adverse impacts of SS were reported for Faisalabad-2008. This indicates that salinity adversely affects the growth and biomass production in the salt-sensitive cultivar Faisalabad-2008, as compared to salt-tolerant cultivars, and it has been well documented that SS causes a significant reduction in the growth of salt-sensitive cultivars [48,49]. The salinity-induced reduction in wheat cultivars was linked to Na+-induced ionic toxicity [50] and increased MDA and H2O2 production (Table 1). The salt stress in the growing medium significantly increased Na+ accumulation; however, salt-tolerant cultivars accumulated less Na+, which might be an important reason for the marked improvement in growth and biomass of this cultivar.
Salinity toxicity also reduced photosynthetic pigments and RWC and increased MDA and H2O2 accumulation. Higher concentrations of salts induced oxidative stress, which damaged chlorophyll enzymes and increased the activity of chlorophyll-degrading enzymes, thus resulting in less chlorophyll synthesis [50]. Moreover, SS also reduced RWC due to its detrimental effects on water absorption and water availability from soil, which affects the overall water status of plants [50]. Likewise, SS also increased the EL of leaves due to the overproduction of ROS, which disrupts membrane integrity and results in an increase in EL under high-saline conditions [28]. The results indicate that antioxidant activities were increased in high-saline conditions, and Akbar-2019 displayed the most antioxidant activities, while Faisalabad-2008 exhibited fewer antioxidant activities. These results are same as the outcomes of different authors, who also found a marked increase in the antioxidant activities of salt-tolerant cultivars, as compared to salt-sensitive cultivars [51,52,53,54,55].
HS hampered the growth of wheat cultivars; nonetheless, the severity of HS depends on the intensity of HS and the genotypes [56,57,58]. In this study, HS induced more negative effects on the cultivar Faisalabad-2008 as compared to Akbar-2019. HS reduced the chlorophyll synthesis of wheat cultivars, possibly due to excessive MDA and H2O2 production and the increase in the activity of chlorophyll-degrading enzyme activities [59,60]. The generation of ROS is considered to play a key role in the activation of the mechanism leading to a plant’s adaptation to stress conditions [61], and the activation of antioxidants is considered an important response to scavenging ROS. However, other factors, such as osmolyte accumulation, play an important role in heat tolerance. In the present study, the accumulation of TSP and FAA increased under HS, and the cultivar Akbar-2019 showed the highest TSP and FAA concentrations. The increased concentrations of TSP and FAA maintained a cell redox balance by increasing antioxidant activities, which ensured better chlorophyll synthesis and the production of assimilates and resulted in a significant increase in plant growth [28]. Moreover, HS also caused a marked reduction in yield traits; however, less reduction was found in Akbar-2019. The cultivar Akbar-2019 was heat tolerant, and it displayed better antioxidant activities, osmolyte accumulation, and nutrient uptake (Ca, Mg, and K), which resulted in better yields and yield traits in this cultivar. Our results are same as the findings of different authors, who also found that salt-tolerant cultivars displayed a better performance when compared to salt-sensitive cultivars [62,63].
Salinity induces a marked reduction in the growth of wheat cultivars via the excessive build-up of Na+ and Cl− and reducing nutrient uptake and photosynthetic performance [64,65,66]. However, the application of Si significantly improved wheat growth and yield under saline conditions. The exogenous application of Si increases the endogenous Si level, which protect the plants from the damages of salt stress by reducing Na+ uptake, ROS production, and EL and increasing water and nutrient uptake, thus ensuring better plant growth [29,67,68]. Si substantially increased antioxidant activity and osmolyte accumulation (TSP and FAA), which neutralize the effects of oxidative stress in plants by protecting them from cell outbursts, thus resulting in better plant performance [28,67]. The increase in antioxidant activity and osmolyte accumulation indicates that Si-induced cellular signaling boosts the plant’s endogenous defense system to counter the toxic effects of SS. The possible mechanism of the Si-induced increase in antioxidant activities is due to increased K+ uptake, reduced Na+ uptake, and increased water and nutrient uptake [69,70,71,72]. However, a knowledge gap still exists in terms of clarifying the interaction of exogenously applied Si and the antioxidants in wheat plants growing under SS conditions.
The results indicate that HS caused a marked reduction in wheat growth; however, exogenous Si mitigated the adverse impacts of HS. The application of Si increases chlorophyll synthesis, maintains plant water stats, reduces ROS production and EL, triggers antioxidant activities, and stimulates osmolyte accumulation, which counters the toxic effects of HS and ensures better plant growth under stress conditions [73,74,75]. In this study, the activity of all antioxidants and osmolytes was significantly increased in wheat cultivars under HS. This indicates that Si triggers cell signaling, which activates the antioxidant defense system and increases the accumulation of potential osmolytes to counter the toxic effects of HS, which has been well documented in the literature by various authors [76,77,78].
We also found that combined heat and SS markedly reduced the growth of wheat cultivars. The combined imposition of heat and saline conditions markedly reduced chlorophyll contents by increasing H2O2 production and enzyme activity involved in chlorophyll degradation [79,80,81,82,83]. However, Si application enabled the better chlorophyll and carotenoid synthesis due to less ROS production, more antioxidant activities, better stay-green traits, and higher leaf area [80,81]. The results indicate that saline and heat conditions increase the TSP and FAA of wheat cultivars. Furthermore, Si application increased TSP and FAA, which could be ascribed to increased protein kinase synthesis and improved cellular signaling, which stimulated TSP and FAA accumulation to counter the effects of heat and SS. Si application is considered to be very useful in normalizing osmolytes synthesis, which ensures proper plant functioning and better growth under stress conditions [84,85]. Higher antioxidant activities are important means for a plant to tolerate abiotic stresses. In the present study, Si application significantly increased all antioxidants in wheat cultivars. These results are the same as the findings of many studies where authors found that Si application increased antioxidant activities and neutralized oxidative stress in plants under high heat and saline conditions [86]. However, the mechanisms by which Si increases antioxidants under combined heat and saline conditions are still unknown. Therefore, in-depth studies are needed to underpin how Si affects the mechanism of the antioxidant system to induce combined heat and salinity tolerance in plants.
We noted that the accumulation of Na+ and Cl− was significantly increased under combined heat and saline conditions (Figure 5 and 6), while the uptake of K+ was significantly decreased under HS and SS, which could be due to an increase in K+ efflux [87]. However, this mechanism remains to be confirmed, although this efflux of K+ can be reduced via the application of osmo-protectants and nutrients application [88]. In the present study, the application Si improved the K+ uptake under combined heat and saline conditions, which could be due to a substantial decrease in the K+ efflux. The results also indicate that saline and heat conditions cause a significant reduction in Ca, Mg, and K uptake and application, and Si significantly increases the uptake of the aforementioned nutrients (Table 5). However, it is still necessary to explore the mechanism through which Si increases the uptake and accumulation of these aforementioned nutrients in wheat under combined heat and saline conditions. The findings of the present study indicate that combined heat and salinity stress conditions significantly decreased the growth and yield of wheat plants (Table 1 and Table 5). Nonetheless, Si application significantly increased the yield of wheat under combined heat and saline conditions, which can be ascribed to improved plant physiological functioning, leaf water status, antioxidant activities, metabolic activities, nutrient absorption, osmolyte accumulation, and the restricted uptake of Na+ and Cl− [89].
5. Conclusions
In conclusion, combined heat and saline conditions significantly reduce wheat growth and final yield by decreasing leaf water status, photosynthetic pigments, osmolyte accumulation, and nutrient uptake. Nonetheless, exogenous Si supplementation mitigated the deleterious impacts of both saline and heat stress and improves the yield of wheat by increasing nutrient uptake, leaf water status, photosynthetic pigments, osmolyte accumulation, and antioxidant activities. Thus, the application of Si could be an effective approach for minimizing the toxic effects of combined heat and salinity stress in wheat. However, more field studies under a wide range of climate conditions are urgently needed to optimize the rate of exogenous Si application for wheat crops. In addition, more metabolomic, proteomic, and transcriptomic studies are required to discover the mechanism behind the mitigation of heat and salinity stress in wheat via silicon application. Additionally, in-depth studies are also needed to explore how silicon effects the mechanism of antioxidant systems and ion absorption to induce heat and salinity-tolerance in plants.
Acknowledgments
The authors are thankful to Muhammad Aamer for his suggestions for improving the quality of our work. The authors also extend their appreciation to the Deanship of Scientific Research, King Khalid University, for supporting this work through a research groups program under grant number R.G.P. 2/205/44.
Author Contributions
Conceptualization, I.K. and M.U.C.; investigation, A.A. (Ansa Aouz); resources, I.K. and M.U.C.; writing—original draft preparation, I.K., M.U.C. and M.U.H.; writing—review and editing, M.B.C., S.A., M.A., I.A., A.A. (Abid Ali), F.M.A., M.H., T.S.A. and S.H.Q. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The authors also extend their appreciation to the Deanship of Scientific Research, King Khalid University, for supporting this work through a research groups program under grant number R.G.P. 2/205/44.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Shrivastava P., Kumar R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015;22:123–131. doi: 10.1016/j.sjbs.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dustgeer Z.M., Seleiman M.F., Khan I., Chattha M.U., Ali E.F., Alhammad B.A., Jalal R.S., Refay Y., Hassan M.U. Glycine-betaine induced salinity tolerance in maize by regulating the physiological attributes, antioxidant defense system and ionic homeostasis. Not. Bot. Horti Agrobot. Cluj Napoca. 2021;49:12248. doi: 10.15835/nbha49112248. [DOI] [Google Scholar]
- 3.Bhattarai S., Biswas D., Fu Y.B., Biligetu B. Morphological, physiological, and genetic responses to salt stress in alfalfa: A Review. Agronomy. 2020;10:577. doi: 10.3390/agronomy10040577. [DOI] [Google Scholar]
- 4.Zhao S., Zhang Q., Liu M., Zhou H., Ma C., Wang P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021;22:4609. doi: 10.3390/ijms22094609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hussain S., Shaukat M., Ashraf M., Zhu C., Jin Q., Zhang J. Salinity stress in arid and semi-arid climates: Effects and management in field crops. J. Clim. Change Agric. 2019;13:197–222. [Google Scholar]
- 6.Pour-Aboughadareh A., Mehrvar M.R., Sanjani S., Amini A., Nikkhah-Chamanabad H., Asadi A. Effects of salinity stress on seedling biomass, physiochemical properties, and grain yield in different breeding wheat genotypes. Acta Physiol. Plantarum. 2021;43:1–14. doi: 10.1007/s11738-021-03265-7. [DOI] [Google Scholar]
- 7.Seleiman M.F., Aslam M.T., Alhammad B.A., Hassan M.U., Maqbool R., Chattha M.U., Khan I., Gitari H.I., Uslu O.S., Rana R., et al. Salinity stress in wheat: Effects, mechanisms and management strategies. Phyton. 2022;91:667. doi: 10.32604/phyton.2022.017365. [DOI] [Google Scholar]
- 8.Shaheen S., Baber M., Aslam S., Aslam S., Shaheen M., Waheed R., Seo H., Azhar M.T. Effect of nacl on morphophysiological and biochemical responses in Gossypium hirsutum L. Agronomy. 2023;13:1012. doi: 10.3390/agronomy13041012. [DOI] [Google Scholar]
- 9.Abdelaal K.A., EL-Maghraby L.M., Elansary H., Hafez Y.M., Ibrahim E.I., El-Banna M., El-Esawi M., Elkelish A. Treatment of sweet pepper with stress tolerance-inducing compounds alleviates salinity stress oxidative damage by mediating the physio-biochemical activities and antioxidant systems. Agronomy. 2019;10:26. doi: 10.3390/agronomy10010026. [DOI] [Google Scholar]
- 10.Alnusairi G.S., Mazrou Y.S., Qari S.H., Elkelish A.A., Soliman M.H., Eweis M., Abdelaal K., El-Samad G.A., Ibrahim M.F., ElNahhas N. Exogenous nitric oxide reinforces photosynthetic efficiency, osmolyte, mineral uptake, antioxidant, expression of stress-responsive genes and ameliorates the effects of salinity stress in wheat. Plants. 2021;10:1693. doi: 10.3390/plants10081693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.EL-Sabagh A., Islam M.S., Skalicky M., Ali Raza M., Singh K., Anwar H.M., Hossain A., Mahboob W., Iqbal M.A., Ratnasekera D., et al. Salinity stress in wheat (Triticum aestivum L.) in the changing climate: Adaptation and management strategies. Front. Agron. 2021;3:661932. doi: 10.3389/fagro.2021.661932. [DOI] [Google Scholar]
- 12.Chen W.L., Yang W.J., Lo H.F., Yeh D.M. Physiology, anatomy, and cell membrane thermostability selection of leafy radish (Raphanus sativus var. Oleiformis Pers.) with different tolerance under heat stress. Sci. Hortic. 2014;179:367–375. doi: 10.1016/j.scienta.2014.10.003. [DOI] [Google Scholar]
- 13.Hassan M.U., Chattha M.U., Khan I., Chattha M.B., Barbanti L., Aamer M., Iqbal M.M., Nawaz M., Mahmood A., Ali A., et al. Heat stress in cultivated plants: Nature, impact, mechanisms, and mitigation strategies—A review. Plant Biosyst. 2021;155:211–234. doi: 10.1080/11263504.2020.1727987. [DOI] [Google Scholar]
- 14.Zha Q., Xi X., He Y., Jiang A. Transcriptomic analysis of the leaves of two grapevine cultivars under high-temperature stress. Sci. Hortic. 2020;265:109265. doi: 10.1016/j.scienta.2020.109265. [DOI] [Google Scholar]
- 15.Ullah A., Nadeem F., Nawaz A., Siddique K.H., Farooq M. Heat stress effects on the reproductive physiology and yield of wheat. J. Agron. Crop Sci. 2022;208:1–17. doi: 10.1111/jac.12572. [DOI] [Google Scholar]
- 16.Iqbal N., Fatma M., Gautam H., Umar S., Sofo A., D’ippolito I., Khan N.A. The crosstalk of melatonin and hydrogen sulfide determines photosynthetic performance by regulation of carbohydrate metabolism in wheat under heat stress. Plants. 2021;10:1778. doi: 10.3390/plants10091778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu J., Krishna J.S.V., Bowden R.L. Effects of post-flowering heat stress on chlorophyll content and yield components of a spring wheat diversity panel. Crop Sci. 2022;62:1926–1936. doi: 10.1002/csc2.20778. [DOI] [Google Scholar]
- 18.Li M., Feng J., Zhou H., Najeeb U., Li J., Song Y., Zhu Y. Overcoming reproductive compromise under heat stress in wheat: Physiological and genetic regulation, and breeding strategy. Front. Plant Sci. 2022;13:881813. doi: 10.3389/fpls.2022.881813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan I., Wu J., Sajjad M. Pollen viability-based heat susceptibility index (HSIpv): A useful selection criterion for heat-tolerant genotypes in wheat. Front. Plant Sci. 2022;13:1064569. doi: 10.3389/fpls.2022.1064569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandey P., Ramegowda V., Senthil-Kumar M. Shared and unique responses of plants to multiple individual stresses and stress combinations: Physiological and molecular mechanisms. Front. Plant Sci. 2015;6:723. doi: 10.3389/fpls.2015.00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumarathunge D.P., Medlyn B.E., Drake J.E., Tjoelker M.G., Aspinwall M.J., Battaglia M., Cano F.J., Carter K.R., Cavaleri M.A., Cernusak L.A. Acclimation and adaptation components of the temperature dependence of plant photosynthesis at the global scale. New Phytol. 2019;222:768–784. doi: 10.1111/nph.15668. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y., Lin-Wang K., Espley R.V., Wang L., Li Y., Liu Z., Zhou P., Zeng L., Zhang X., Zhang J. StMYB44 negatively regulates anthocyanin biosynthesis at high temperatures in tuber flesh of potato. J. Exp. Bot. 2019;70:3809–3824. doi: 10.1093/jxb/erz194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petretto G.L., Urgeghe P.P., Massa D., Melito S. Effect of salinity (NaCl)on plant growth, nutrient content, and glucosinolate hydrolysis products trends in rocket genotypes. Plant Physiol. Biochem. 2019;141:30–39. doi: 10.1016/j.plaphy.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Luyckx M., Hausman J.F., Lutts S., Guerriero G. Silicon and plants: Current knowledge and technological perspectives. Front. Plant Sci. 2017;8:411. doi: 10.3389/fpls.2017.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shunkao S., Thitisaksakul M., Pongdontri P., Theerakulpisut P. Additive effects of combined heat and salt stress is manifested in an enhanced sodium ions accumulation and increased membrane damage in wheat seedlings. Chilean J. Agri. Res. 2022;82:552–563. doi: 10.4067/S0718-58392022000400552. [DOI] [Google Scholar]
- 26.Malhotra C., Kapoor R.T. Plant Abiotic Stress Tolerance: Agronomic. Springer; Berlin/Heidelberg, Germany: 2019. Silicon: A sustainable tool in abiotic stress tolerance in plants; pp. 333–356. [Google Scholar]
- 27.Mushtaq A., Khan Z., Khan S., Rizwan S., Jabeen U., Bashir F., Ismail T., Anjum S., Masood A. Effect of silicon on antioxidant enzymes of wheat (Triticum aestivum L.) grown under salt stress. Silicon. 2020;12:2783–2788. doi: 10.1007/s12633-020-00524-z. [DOI] [Google Scholar]
- 28.Singh P., Kumar V., Sharma J., Saini S., Sharma P., Kumar S., Sinhmar Y., Kumar D., Sharma A. Silicon supplementation alleviates the salinity stress in wheat plants by enhancing the plant water status, photosynthetic pigments, proline content and antioxidant enzyme activities. Plants. 2022;1:252. doi: 10.3390/plants11192525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Javaid T., Farooq M.A., Akhtar J., Saqib Z.A., Anwar-ul-Haq M. Silicon nutrition improves growth of salt-stressed wheat by modulating flows and partitioning of Na+, Cl− and mineral ions. Plant Physiol. Biochem. 2019;141:291–299. doi: 10.1016/j.plaphy.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Daoud A.M., Hemada M.M., Saber N., Moussa E.A. Effect of silicon on the tolerance of wheat (Triticum aestivum L.) to salt stress at different growth stages: Case study for the management of irrigation water. Plants. 2018;7:29. doi: 10.3390/plants7020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ali A., Mahmood R., Jaan M., Abbas M.N. Stimulating the anti-oxidative role and wheat growth improvement through silicon under salt stress. Silicon. 2019;11:2403–2406. doi: 10.1007/s12633-015-9378-4. [DOI] [Google Scholar]
- 32.Farouk S., Elhindi K.M., Alotaibi M.A. Silicon supplementation mitigates salinity stress on Ocimum basilicum L. via improving water balance, ion homeostasis, and antioxidant defense system. Ecotoxicol. Environ. Saf. 2020;206:111396. doi: 10.1016/j.ecoenv.2020.111396. [DOI] [PubMed] [Google Scholar]
- 33.Mushtaq A., Khan Z., Khan S., Rizwan S., Jabeen U., Bashir F., Ismail T., Anjum S., Cheraghi M., Motesharezadeh B., et al. Silicon (Si): A regulator nutrient for optimum growth of wheat under salinity and drought stresses—A review. J. Plant Growth Reg. 2023:1–25. [Google Scholar]
- 34.Abdelrahman M., El-Sayed M., Jogaiah S., Burritt D.J., Tran L.S.P. The “stay-green” trait and phytohormone signaling networks in plants under heat stress. Plant Cell Rep. 2017;36:1009–1025. doi: 10.1007/s00299-017-2119-y. [DOI] [PubMed] [Google Scholar]
- 35.Saddiq M.S., Iqbal S., Hafeez M.B., Ibrahim A.M., Raza A., Fatima E.M., Baloch H., Woodrow P., Ciarmiello L.F. Effect of salinity stress on physiological changes in winter and spring wheat. Agron. 2021;11:1193. doi: 10.3390/agronomy11061193. [DOI] [Google Scholar]
- 36.Choudhary M., Yan G., Siddique K.H., Cowling W.A. Heat stress during meiosis has lasting impacts on plant growth and reproduction in wheat (Triticum aestivum L.) Agronomy. 2022;12:987. doi: 10.3390/agronomy12050987. [DOI] [Google Scholar]
- 37.Ahmed A.H., Harb E., Higazy M., Morgan S.H. Effect of silicon and boron foliar applications on wheat plants grown under saline soil conditions. Intern. J. Agric. Res. 2008;3:1–26. doi: 10.3923/ijar.2008.1.26. [DOI] [Google Scholar]
- 38.Arnon D.I. Copper enzymes in isolated chloroplasts. Polyphe-noloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 40.Hamilton P.B., Van-Slyke D.D. Amino acid determination with ninhydrin. J. Biol. Chem. 1943;150:231–250. doi: 10.1016/S0021-9258(18)51268-0. [DOI] [Google Scholar]
- 41.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 42.Nakano Y., Asada K. Purification of ascorbate peroxidase in spinach chloroplasts; its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. 1987;28:131–140. [Google Scholar]
- 43.Zhang X. Research Methodology of Crop Physiology. Agriculture Press; Beijing, China: 1992. The measurement and mechanism of lipid peroxidation and SOD, POD and CAT activities in biological system; pp. 208–211. [Google Scholar]
- 44.Mukherjee S., Choudhuri M. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983;58:166–170. doi: 10.1111/j.1399-3054.1983.tb04162.x. [DOI] [Google Scholar]
- 45.Steel R.G., Torrie J.H. Principles and Procedures of Statistics: A Biometrical Approach. McGraw-Hill; New York, NY, USA: 1980. [Google Scholar]
- 46.Kakar N., Jumaa S.H., Redoña E.D., Warburton M.L., Reddy K.R. Evaluating rice for salinity using pot-culture provides a systematic tolerance assessment at the seedling stage. Rice. 2019;12:1–14. doi: 10.1186/s12284-019-0317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu C., Cui K., Li Q., Li L., Wang W., Hu Q., Ding Y., Li G., Fahad S., Huang J. Estimating the yield stability of heat-tolerant rice genotypes under various heat conditions across reproductive stages: A 5-year case study. Sci. Rep. 2021;11:1–11. doi: 10.1038/s41598-021-93079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farooq M., Park J.R., Jang Y.H., Kim E.G., Kim K.M. Rice cultivars under salt stress Show differential expression of genes related to the regulation of Na+/K+ balance. Front. Plant Sci. 2021;12:680131. doi: 10.3389/fpls.2021.680131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abdi N., Van Biljon A., Steyn C., Labuschagne M.T. Salicylic acid improves growth and physiological attributes and salt tolerance differentially in two bread wheat cultivars. Plants. 2022;11:1853. doi: 10.3390/plants11141853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan I., Muhammad A., Chattha M.U., Skalicky M., Bilal C.M., Ahsin A.M., Rizwan A.M., Soufan W., Hassan M.U., Rahman M.A., et al. Mitigation of salinity-induced oxidative damage, growth, and yield reduction in fine rice by sugarcane press mud application. Front. Plant Sci. 2022;13:840900. doi: 10.3389/fpls.2022.840900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaya C., Higgs D., Ashraf M., Alyemeni M.N., Ahmad P. Integrative roles of nitric oxide and hydrogen sulfide in melatonin-induced tolerance of pepper (Capsicum annuum L.) plants to iron deficiency and salt stress alone or in combination. Physio Planta. 2020;168:256–277. doi: 10.1111/ppl.12976. [DOI] [PubMed] [Google Scholar]
- 52.Dugasa M.T., Cao F.B., Ibrahim W., Wu F.B. Genotypic difference in physiological and biochemical characteristics in response to single and combined stresses of drought and salinity between the two wheat genotypes (Triticum aestivum) differing in salt tolerance. Physio. Planta. 2019;165:134–143. doi: 10.1111/ppl.12743. [DOI] [PubMed] [Google Scholar]
- 53.Hannachi S., Van Labeke M.-C. Salt stress affects germination, seedling growth and physiological responses differentially in eggplant cultivars (Solanum melongena L.) Sci. Hortic. 2018;228:56–65. doi: 10.1016/j.scienta.2017.10.002. [DOI] [Google Scholar]
- 54.Farooq M., Ahmad R., Shahzad M., Sajjad Y., Hassan A., Shah M.M., Naz S., Khan S.A. Differential variations in total flavonoid content and antioxidant enzymes activities in pea under different salt and drought stresses. Sci. Hortic. 2021;287:110258. doi: 10.1016/j.scienta.2021.110258. [DOI] [Google Scholar]
- 55.Guo X., Ahmad N., Zhao S., Zhao C., Zhong W., Wang X., Li G. Effect of salt stress on growth and physiological properties of Asparagus seedlings. Plants. 2022;11:2836. doi: 10.3390/plants11212836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Djanaguiraman M., Narayanan S., Erdayani E., Prasad P.V.V. Effects of high temperature stress during anthesis and grain filling periods on photosynthesis, lipids and grain yield in wheat. BMC Plant Biol. 2020;20:268. doi: 10.1186/s12870-020-02479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumar R.R., Ahuja S., Rai G.K., Kumar S., Mishra D., Kumar S.N., Rai A., Singh B., Chinnusamy V., Praveen S. Silicon triggers the signalling molecules and stress-associated genes for alleviating the adverse effect of terminal heat stress in wheat with improved grain quality. Acta Physiol. Plant. 2022;44:30. doi: 10.1007/s11738-022-03365-y. [DOI] [Google Scholar]
- 58.Langridge P., Reynolds M. Breeding for drought and heat tolerance in wheat. Theor. Appl. Genet. 2021;134:1753–1769. doi: 10.1007/s00122-021-03795-1. [DOI] [PubMed] [Google Scholar]
- 59.Rehman H.U., Tariq A., Ashraf I., Ahmed M., Muscolo A., Basra S.M.A., Reynolds M. Evaluation of physiological and morphological traits for improving spring wheat adaptation to terminal heat stress. Plants. 2021;10:455. doi: 10.3390/plants10030455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yadav M.R., Choudhary M., Singh J., Lal M.K., Jha P.K., Udawat P., Gupta N.K., Rajput V.D., Garg N.K., Maheshwari C. Impacts, tolerance, adaptation, and mitigation of heat stress on wheat under changing climates. Int. J. Mol. Sci. 2022;23:2838. doi: 10.3390/ijms23052838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choudhary A., Kumar A., Kaur N. ROS and oxidative burst: Roots in plant development. Plant Divers. 2020;42:33–43. doi: 10.1016/j.pld.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iqbal N., Sehar Z., Fatma M., Umar S., Sofo A., Khan N.A. Nitric oxide and abscisic acid mediate heat stress tolerance through regulation of osmolytes and antioxidants to protect photosynthesis and growth in wheat plants. Antioxidants. 2022;11:372. doi: 10.3390/antiox11020372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Irshad A., Ahmed R.I., Ur Rehman S., Sun G., Ahmad F., Sher M.A., Aslam M.Z., Hassan M.M., Qari S.H., Aziz M.K., et al. Characterization of salt tolerant wheat genotypes by using morpho-physiological, biochemical, and molecular analysis. Front. Plant Sci. 2022;13:956298. doi: 10.3389/fpls.2022.956298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sharma V., Singh C.M., Chugh V., Prajapati P.K., Mishra A., Kaushik P., Dhanda P.S., Yadav A. Morpho-physiological and biochemical responses of field pea genotypes under terminal heat stress. Plants. 2023;12:256. doi: 10.3390/plants12020256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tao R., Ding J., Li C., Zhu X., Guo W., Zhu M. Evaluating and screening of agro-physiological indices for salinity stress tolerance in wheat at the seedling stage. Front. Plant Sci. 2021;12:646175. doi: 10.3389/fpls.2021.646175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zeeshan M., Lu M., Sehar S., Holford P., Wu F. Comparison of biochemical, anatomical, morphological, and physiological responses to salinity stress in wheat and barley genotypes deferring in salinity tolerance. Agronomy. 2020;10:127. doi: 10.3390/agronomy10010127. [DOI] [Google Scholar]
- 67.Thorne S.J., Stirnberg P.M., Hartley S.E., Maathuis F.J. The ability of silicon fertilisation to alleviate salinity stress in rice is critically dependent on cultivar. Rice. 2022;15:1–10. doi: 10.1186/s12284-022-00555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Conceição S.S., Oliveira Neto C.F.d., Marques E.C., Barbosa A.V.C., Galvão J.R., Oliveira T.B.d., Okumura R.S., Martins J.T.d.S., Costa T.C., Gomes-Filho E. Silicon modulates the activity of antioxidant enzymes and nitrogen compounds in sunflower plants under salt stress. Archiv. Agron. Soil Sci. 2019;65:1237–1247. doi: 10.1080/03650340.2018.1562272. [DOI] [Google Scholar]
- 69.Ahmad P., Ahanger M.A., Alam P., Alyemeni M.N., Wijaya L., Ali S., Ashraf M. Silicon (Si) supplementation alleviates NaCl toxicity in mung bean [Vigna radiata (L.) Wilczek] through the modifications of physio-biochemical attributes and key antioxidant enzymes. J. Plant Growth Regul. 2019;38:70–82. doi: 10.1007/s00344-018-9810-2. [DOI] [Google Scholar]
- 70.Akhter M.S., Noreen S., Ummara U., Aqeel M., Saleem N., Ahmed M.M., Mahmood S., Athar H.U.R., Alyemeni M.N., Kaushik P., et al. Silicon-induced mitigation of NaCl stress in barley (Hordeum vulgare L.), associated with enhanced enzymatic and non-enzymatic antioxidant activities. Plants. 2022;11:2379. doi: 10.3390/plants11182379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mahmoud A.W.M., Abdeldaym E.A., Abdelaziz S.M., El-Sawy M.B., Mottaleb S.A. Synergetic effects of zinc, boron, silicon, and zeolite nanoparticles on confer tolerance in potato plants subjected to salinity. Agronomy. 2020;10:19. doi: 10.3390/agronomy10010019. [DOI] [Google Scholar]
- 72.Kim Y.H., Khan A.L., Waqas M., Lee I.J. Silicon regulates antioxidant activities of crop plants under abiotic-induced oxidative stress: A review. Front. Plant Sci. 2017;8:510. doi: 10.3389/fpls.2017.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mustafa T., Sattar A., Sher A., Ul-Allah S., Ijaz M., Irfan M., Butt M., Cheema M. Exogenous application of silicon improves the performance of wheat under terminal heat stress by triggering physio-biochemical mechanisms. Sci. Rep. 2021;11:23170. doi: 10.1038/s41598-021-02594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Naz N., Durrani F., Shah Z., Khan N., Ullah I. Influence of heat stress on growth and physiological activities of potato (Solanum tuberosum L.) Phyton. Int. J. Exp. Bot. 2018;87:225–230. [Google Scholar]
- 75.Chalanika D.H.C., Asaeda T. effects of heat stress on growth, photosynthetic pigments, oxidative damage and competitive capacity of three submerged macrophytes. J. Plant Interact. 2017;12:228–236. doi: 10.1080/17429145.2017.1322153. [DOI] [Google Scholar]
- 76.Li X., Wei J.P., Scott E., Liu J.W., Guo S., Li Y., Zhang L., Han W.Y. Exogenous melatonin alleviates cold stress by promoting antioxidant defense and redox homeostasis in Camellia sinensis L. Molecules. 2018;23:165. doi: 10.3390/molecules23010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khan A., Khan A.L., Imran M., Asaf S., Kim Y.H., Bilal S., Numan M., Al-Harrasi A., Al-Rawahi A., Lee I.J. Silicon-induced thermotolerance in Solanum lycopersicum L. via activation of antioxidant system, heat shock proteins, and endogenous phytohormones. BMC Plant Biol. 2022;20:1–18. doi: 10.1186/s12870-020-02456-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ashfaq W., Fuentes S., Brodie G., Gupta D. The role of silicon in regulating physiological and biochemical mechanisms of contrasting bread wheat cultivars under terminal drought and heat stress environments. Front. Plant Sci. 2022;13:955490. doi: 10.3389/fpls.2022.955490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nahar L., Aycan M., Hanamata S., Baslam M., Mitsui T. Impact of single and combined salinity and high-temperature stresses on agro-physiological, biochemical, and transcriptional responses in rice and stress-release. Plants. 2022;11:501. doi: 10.3390/plants11040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khan A., Khan A.L., Muneer S., Kim Y.H., Al-Rawahi A., Al-Harrasi A. Silicon and salinity: Crosstalk in crop-mediated stress tolerance mechanisms. Front. Plant Sci. 2019;10:1429. doi: 10.3389/fpls.2019.01429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ismail L.M., Soliman M.I., Abd El-Aziz M.H., Abdel-Aziz H.M. Impact of silica ions and nano silica on growth and productivity of pea plants under salinity stress. Plants. 2022;11:494. doi: 10.3390/plants11040494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morales F., Ancín M., Fakhet D., González-Torralba J., Gámez A.L., Seminario A., Soba D., Ben Mariem S., Garriga M., Aranjuelo I. Photosynthetic metabolism under stressful growth conditions as a bases for crop breeding and yield improvement. Plants. 2020;9:88. doi: 10.3390/plants9010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sharma A., Kumar V., Shahzad B., Ramakrishnan M., Singh-Sidhu G.P., Bali A.S., Handa N., Kapoor D., Yadav P., Khanna K., et al. Photosynthetic response of plants under different abiotic stresses: A review. J. Plant Growth Regul. 2020;39:509–531. doi: 10.1007/s00344-019-10018-x. [DOI] [Google Scholar]
- 84.Alsaeedi A., El-Ramady H., Alshaal T., El-Garawany M., Elhawat N., Al-Otaibi A. Silica nanoparticles boost growth and productivity of cucumber under water deficit and salinity stresses by balancing nutrients uptake. Plant Physiol. Biochem. 2019;139:1–10. doi: 10.1016/j.plaphy.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 85.Sehar Z., Jahan B., Masood A., Anjum N.A., Khan N.A. Hydrogen peroxide potentiates defense system in presence of sulfur to protect chloroplast damage and photosynthesis of wheat under drought stress. Physiol Plant. 2021;172:922–934. doi: 10.1111/ppl.13225. [DOI] [PubMed] [Google Scholar]
- 86.Khan A., Bilal S., Khan A.L., Imran M., Al-Harrasi A., Al-Rawahi A., Lee I.J. Silicon-mediated alleviation of combined salinity and cadmium stress in date palm (Phoenix dactylifera L.) by regulating physio-hormonal alteration. Ecotoxicol. Environ. Saf. 2020;188:109885. doi: 10.1016/j.ecoenv.2019.109885. [DOI] [PubMed] [Google Scholar]
- 87.Hryvusevich P., Navaselsky I., Talkachova Y., Straltsova D., Keisham M., Viatoshkin A., Samokhina V., Smolich I., Sokolik A., Huang X., et al. Sodium influx and potassium efflux currents in sunflower root cells under high salinity. Front. Plant Sci. 2021;11:613936. doi: 10.3389/fpls.2020.613936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Otie V., Udo I., Shao Y., Itam M.O., Okamoto H., An P. Salinity effects on morpho-physiological and yield traits of soybean (Glycine max L.) as mediated by foliar spray with brassinolide. Plants. 2021;10:541. doi: 10.3390/plants10030541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Taha R.S., Seleiman M.F., Shami A., Alhammad B.A., Mahdi A.H. Integrated application of selenium and silicon enhances growth and anatomical structure, antioxidant defense system and yield of wheat grown in salt-stressed soil. Plants. 2021;10:1040. doi: 10.3390/plants10061040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.