Abstract
Transduction by murine leukemia virus-based retrovirus vectors is limited in certain cell types, particularly in nondividing cells. But transduction can be inefficient even in cells that divide rapidly. For example, exposure of 208F rat embryo fibroblasts to an excess of an amphotropic retrovirus vector encoding alkaline phosphatase results in a transduction efficiency of only about 10%, even though these cells divide rapidly. Here we show that transduction of 208F cells is limited by cell surface retrovirus receptor levels; overexpression of the amphotropic retrovirus receptor Pit2 markedly improved the transduction efficiency to 50%. To characterize receptor levels and binding affinity, we synthesized a fusion protein that joins the amino terminus of the amphotropic envelope protein to the Fc region of a human immunoglobulin G1 molecule for use in binding assays. In comparison to the parental cell line, the modified cell line showed an order of magnitude increase in binding sites of from 18,000 to 150,000 per cell. Thus, efficient transduction by an amphotropic retrovirus vector requires high-level expression of the retrovirus receptor Pit2. These results provide the rationale for further examination of the role of receptor levels in inefficient transduction, especially with regard to target cells for gene therapy, where a high transduction rate is often crucial.
The clinical application of retrovirus-based gene therapy is limited by the poor efficiency of transduction of target cells such as hematopoietic stem cells. This cannot be resolved by simply increasing the amount of vector added. It has been well documented that this is in part due to the quiescent nature of these cells (14, 17), and evidence is emerging that lentiviral vectors may help overcome this block to transduction (29, 32, 34). Resistance of cells to retroviral infection may also be the result of inhibitory factors in the medium of cell cultures (19, 25), or it can be limited at the receptor level (5, 28, 30, 35). Cells express multiple retrovirus receptors, the abundance of which may vary from tissue to tissue (15), and a correlation of receptor mRNA expression and the susceptibility to infection has been proposed (16, 35).
We have previously found that 208F rat embryo fibroblasts are inefficiently transduced by amphotropic retrovirus vectors, showing a transduction rate between 10 and 20% (24). In contrast, primary human diploid fibroblasts exhibit rates of 50% or more (31). This result is puzzling because 208F cells divide rapidly, while the human fibroblasts divide slowly and ultimately undergo senescence and death with continued cultivation, indicating that some block to transduction other than division rate is operative in the 208F cells. Thus, we have studied the basis for inefficient transduction of 208F cells as a model for the resistance of other cells that are inefficiently transduced by retrovirus vectors.
In initial experiments we confirmed the low rate of 208F cell transduction with an amphotropic vector encoding alkaline phosphatase, and we showed that inefficient transduction of 208F cells was not due to the production of a soluble inhibitory factor by the cells. Next, modified 208F cell lines that overexpress the rat amphotropic receptor Pit2 were used to study effects of high-level receptor expression on transduction efficiency, and the results showed an increased susceptibility to infection compared to the parental cell line. In further studies a fusion protein joining the amphotropic envelope SU portion with the human immunoglobulin G1 (IgG) Fc domain (ASU-hFc) was designed to study receptor expression and binding kinetics by Scatchard analysis. The current study provides evidence in a fibroblast cell culture model that efficient transduction of target cells requires high-level receptor expression.
MATERIALS AND METHODS
Cell lines and retrovirus vectors.
The 208F rat embryo fibroblasts (33) and 293 human kidney fibroblasts (ATCC CRL-1573) were maintained in Dulbecco’s modified Eagle medium with a high glucose concentration (4.5 g/liter) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Hyclone, Logan, Utah), 100 U of penicillin G per ml, and 100 μg of streptomycin sulfate per ml at 37°C in a 5% CO2-air atmosphere.
The LAPSN vector contains human placental alkaline phosphatase (AP) and neomycin phosphotransferase (neo) genes under the transcriptional control of Moloney murine leukemia virus (MoMLV) and simian virus 40 (SV40) promoters, respectively (26). The LAPSN vector was produced by using PE501 ecotropic (22), PA317 amphotropic (21), and PG13 GALV-based (23) packaging cell lines. Vector-containing medium was harvested 24 h after the feeding of confluent layers of packaging cells and was filtered through a 0.45-μm (pore size) cellulose acetate filter and stored at −70°C.
Clones of 208F cells that overexpressed the rat amphotropic retrovirus receptor Pit2 (previously called Ram-1) were generated by the transduction of 208F cells with a retrovirus vector LPit2SN (previously called LrRAMSN) that contains rat pit2 and bacterial neo genes driven by MoMLV and SV40 promoters, respectively (208F/LPit2SN cells), as previously described (15). Clones of 208F cells transduced with the parental LXSN vector that lacks the pit2 gene (208F/LXSN cells) were generated by the same method. G418 selection (geneticin, 900 μg/ml [active concentration]) was maintained for the culture of 208F/LPit2SN and 208F/LXSN clonal cell lines.
Vector titers were determined by limiting dilution assay of the transfer of G418 resistance to 208F cells. Staining of G418 resistant colonies was performed with Coomassie brilliant blue G (1.5 g/liter in 30% methanol–10% glacial acetic acid) 10 to 12 days after virus infection. Titers ranged from 2 × 105 to 9 × 105 CFU/ml.
Retroviral infections.
All retroviral infections were performed in the presence of 4 μg of Polybrene (Sigma, St. Louis, Mo.) per ml. Target cells were plated at 5 × 104 cells per 6-cm-diameter dish 1 day prior to infection. Vectors were added at a multiplicities of infection (MOIs) of 4 for LAPSN(PA317), 4 for LAPSN(PE501), and 18 for LAPSN(PG13) unless otherwise noted.
Flow cytometry.
Cells were analyzed for surface AP expression 2 days after vector exposure. The cells were trypsinized and incubated at 4°C for 30 min with a primary mouse anti-human AP monoclonal antibody (MAb) of the IgG2a isotype (DAKO, Carpenteria, Calif.) or an irrelevant mouse IgG2a MAb (DAKO) as a negative isotype control. Secondary antibody staining was performed by incubating the cells with fluorescein-isothiocyanate (FITC)-labeled anti-mouse IgG2a MAb (PharMingen, San Diego, Calif.) at 4°C for 30 min. Cells were washed twice with phosphate-buffered saline (PBS) containing 2% FBS, suspended in 2 μg of propidium iodide per ml, and analyzed by using a FACScan flow cytometer and Cellquest software (Becton Dickinson, San Jose, Calif.) with appropriate gating for cell size and viability.
For flow cytometric analysis of amphotropic SU-human Fc portion fusion protein (ASU-hFc) binding, cells were suspended by treatment with trypsin-EDTA or 0.5 mM EDTA only and then were incubated with either the amphotropic SU fusion protein or an irrelevant polyclonal human IgG antibody (Dako) for 60 min at 4°C. The cells were washed twice, incubated with an FITC-conjugated F(ab)2 fragment from a rabbit antibody directed against human Fc (Dako) for 30 min at 4°C, washed twice, and suspended in PBS containing 2 μg of propidium iodide per ml. Fluorescence-activated cell sorter analysis was carried out as described above.
Fusion protein construction, purification, and analysis.
A DNA fragment was constructed that encoded the SU portion of the amphotropic 4070A Env protein, minus the carboxy-terminal 9 amino acids, and was linked to a human IgG-Fc protein fragment lacking the amino-terminal 3 amino acids after the Fc cleavage site (kindly provided by David Cosman, Immunex, Seattle, Wash.). This DNA was cloned in place of β-galactosidase cDNA in the plasmid pCMV–β-gal (Clontech, Palo Alto, Calif.), which contains a cytomegalovirus promoter upstream of the ASU-hFc gene, an SV40 intron, and an SV40 polyadenylation signal. The ASU-hFc expression plasmid was transfected by CaPO4 coprecipitation (8) into 293 cells plated at an ∼70% confluence in 15-cm dishes 1 day earlier. The fusion protein was then harvested in culture medium containing low-IgG serum (Gemini Bioproducts, Inc., Calabasas, Calif.) at 24 and 48 h after transfection and filtered (0.45-μm pore size) to remove particles and debris. Subsequent purification was carried out by affinity chromatography with a protein A column, elution in citrate-phosphate buffer at a pH of 2.8, and neutralization in 10× PBS (pH 8.5). Stock solutions of concentrated fusion protein were stored at −20°C in PBS until used.
125I labeling of ASU-hFc and analysis of binding.
Radioactive labeling was performed according to the method of Bolton and Hunter (6). Briefly, Na125I was converted to its active form by oxidation with chloramine T and bound to an N-hydroxysuccinimide ester of 3-(4-hydroxyphenyl) propionic acid to generate the Bolton-Hunter (B-H) reagent. Next, the fusion protein was incubated with the B-H reagent for 2 h on ice, leading to covalent binding of the labeled B-H reagent to lysine residues in the fusion protein. The labeled fusion protein was purified by chromatography by using a Sephadex G10 column (Boehringer Mannheim, Mannheim, Germany) to remove unincorporated isotope and yielded specific activities of labeled protein of 0.15 to 0.24 mCi/mg. Dilutions of this labeled fusion protein (0.045 to 4.5 nM) were prepared in RPMI 1640 containing 1% BSA.
Cells were grown to <50% confluence and were suspended by trypsinization, and 1 × 105 to 10 × 105 cells were incubated with labeled fusion protein at room temperature on a tube rotator revolving at ∼30 rpm. After a thorough washing in RPMI 1640 containing 1% BSA, cell-associated (bound) radioactivity was measured by scintillation counting.
Scatchard analysis.
Analysis of ASU-hFc fusion protein binding to cells was performed as described by Badger et al. (3). In that study the authors modeled antibody binding to cell surface antigens, and for our analysis the virus receptor is the equivalent of the cell surface antigen, and the fusion protein is the equivalent of the antibody. Importantly, we have included the correction for the amount of fusion protein that is still able to bind receptors after radiolabeling of the fusion protein (3, 20), which is the equivalent of the immunoreactive antibody in the analysis by Badger et al. The mean value for the percentage of reactive fusion protein was 15% in several experiments, and this value was used for the calculations in all experiments.
RESULTS
Transduction of 208F cells with an amphotropic vector is inefficient.
We exposed 208F cells to increasing amounts of an amphotropic vector encoding AP (LAPSN) in an attempt to maximize the transduction rate. The highest rate of transduction as measured by flow cytometry was 12% (Fig. 1A). No significant increase in transduction was seen at vector doses above 1 ml, which corresponds to an MOI of 4.
FIG. 1.
Efficiency of 208F cell transduction following exposure to the LAPSN(PA317) vector. 208F cells were seeded at 5 × 104 cells per 6-cm dish and exposed to LAPSN(PA317) vector beginning the day after seeding. Flow cytometric analysis of alkaline phosphatase expression was performed 2 days after the last exposure to vector. (A) Percentage transduction after a single exposure to various amounts of vector. (B) Percentage transduction after one to six daily exposures to a constant volume of 1 ml of vector. Values represent means ± standard deviations of three independent experiments.
Next we attempted to maximize the transduction rate by repeated exposure of the cells to equal 1-ml volumes of vector at 12-h intervals. There was an increase in the percentage of AP+ cells with each virus exposure, reaching a maximum of 34% after six exposures (Fig. 1B). Thus, transduction of 208F cells with an amphotropic vector remains inefficient even after repeated exposures.
208F fibroblasts divide rapidly.
Given the requirement of cells to divide for successful transduction by murine leukemia virus-based retrovirus vectors, we calculated the cell division rate by plating a constant number of cells into multiple dishes and performing trypsinization at various time points for serial cell counts. The results confirmed that 208F cells are rapidly dividing, with a doubling time of ∼18 h in their log phase of growth (data not shown). For comparison, human diploid fibroblasts have a doubling time of between 24 and 32 h (7, 12), and mouse fibroblasts have a doubling time between 15 and 16 h (13, 18).
Overexpression of the amphotropic retrovirus receptor in 208F cells promotes efficient transduction.
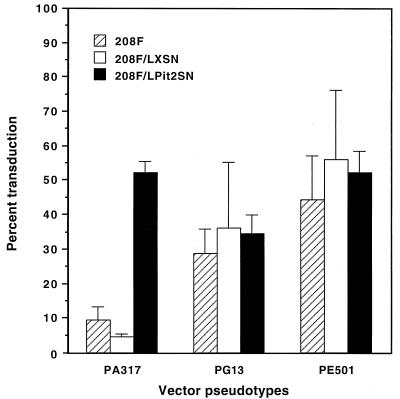
We transduced 208F cells with the retrovirus vector LPit2SN, which contains the rat Pit2 cDNA under transcriptional control of an MoMLV long terminal repeat promoter and enhancer, and isolated several clonal lines that overexpressed Pit2 (26). We also transduced 208F cells with the empty parental vector LXSN and isolated several clonal lines that contained this vector. Exposure of two clonal lines of 208F/LPit2SN cells to 1 ml of LAPSN(PA317) virus gave a mean transduction rate of 52% as measured by flow cytometry, while infection of three 208F/LXSN clones gave a mean transduction rate of only 5.6% (Fig. 2). Exposure of 208F, 208F/LXSN, and 208F/LPit2SN cells to the LAPSN vector with a GALV (PG13) pseudotype resulted in similar transduction rates in all of these cell lines, as did exposure of the cell lines to the LAPSN vector with an ecotropic (PE501) pseudotype (Fig. 2). Thus, overexpression of Pit2 increased transduction specifically for an amphotropic vector and not for vectors with other pseudotypes.
FIG. 2.
The effect of rat Pit2 overexpression on transduction by various pseudotypes of the LAPSN vector. Cells were transduced by a single exposure of cells plated the day before at 5 × 104 per 6-cm dish to LAPSN vector at an MOI of 4 for amphotropic and ecotropic pseudotypes and at an MOI of 18 for the GALV pseudotype. The results are means ± standard deviations of three independent experiments for the parental 208F cells, two clones of 208F/LPit2SN cells, and three clones of 208F/LXSN cells.
Production of an ASU-hFc fusion protein for receptor binding studies.
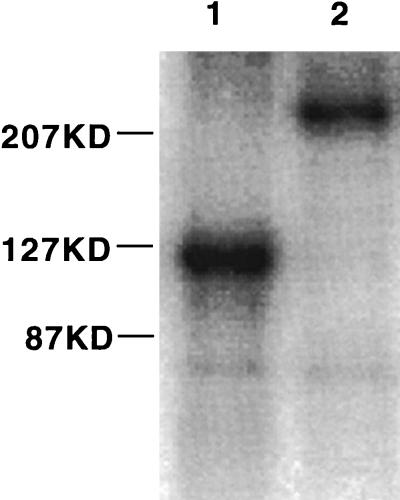
For analysis of Pit2 levels and Pit2/Env binding properties, we used an envelope-antibody fusion protein (ASU-hFc), which consisted of the SU portion of the amphotropic envelope glycoprotein fused to the Fc domain of a human IgG heavy-chain molecule. The addition of the antibody constant region fragment allows simple purification of the fusion protein on a protein A column and provides an epitope for fusion protein detection that is recognized by commonly available antibodies. This protein was transiently expressed in 293 cells, harvested, and purified as a homodimer that separated in an acrylamide gel at ∼220 kDa under nonreducing conditions and as a monomer of ∼110 kDa under reducing conditions (Fig. 3).
FIG. 3.
Acrylamide gel (6%) analysis of the 125I-labeled ASU-hFc fusion protein. Lane 1, protein incubated with β-mercaptoethanol at 100°C for 5 min; lane 2, untreated.
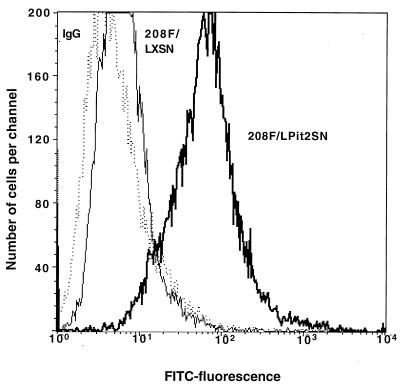
In a preliminary experiment we preincubated 208F/LPit2SN cells with the fusion protein prior to exposure to vector and were able to effect a significant decrease in the efficiency of transduction with amphotropic retrovirus, but not with ecotropic or GALV pseudotype (data not shown), indicating that the fusion protein specifically bound to the amphotropic receptor. We then analyzed fusion protein binding to cells directly by flow cytometry with an FITC-conjugated secondary antibody against the human Fc region. Fusion protein binding to 208F or 208F/LXSN cells was similar and was significantly higher than that observed when the fusion protein was replaced with an irrelevant polyclonal human IgG antibody as a negative control (Fig. 4). The mean level of fusion protein binding to 208F/LPit2SN cells was fivefold higher than that to 208F or 208F/LXSN cells (Fig. 4), indicating a higher level of Pit2 expression on these cells.
FIG. 4.
Flow cytometric analysis of ASU-hFc fusion protein binding to 208F, 208F/LXSN, and 208F/LPit2SN cells. Cells were incubated with ASU-hFc at 4°C for 1 h, washed, incubated with FITC-conjugated rabbit anti-human Fc antibody for 30 min at 4°C, washed, and analyzed by flow cytometry. As a control for nonspecific binding, the fusion protein was replaced with an irrelevant polyclonal human IgG antibody for analysis of 208F cells (IgG isotype control). Mean fluorescence units were calculated from two independent experiments: IgG isotype (dotted line), 5.4 ± 2.1 U; 208F/LXSN (thin line [208F was identical {data not shown}]), 13.2 ± 3.8 U; and 208F/LPit2SN (heavy line), 74 ± 12 U.
Amphotropic receptor quantitation and SU binding properties.
We used Scatchard analysis of iodinated ASU-hFc binding to cells to quantitate receptor levels and SU binding affinity. In preliminary experiments, we found that fusion protein binding to 208F/LPit2SN cells stabilized after an hour at room temperature (Fig. 5A), and further binding studies were performed by incubation with the fusion protein for 2 h. Measurement of [125I]ASU-hFc binding to cells after incubation of an identical number of cells with increasing concentrations of the fusion protein revealed a binding curve with a shape consistent with specific binding in the left-hand part of the curve at lower concentrations of fusion protein and nonsaturable, nonspecific binding in the right-hand part of the curve at higher concentrations of the fusion protein (Fig. 5B). Addition of a 100-fold excess of unlabeled fusion protein to the incubations performed with 0.045, 0.45, and 2.3 nM labeled fusion protein reduced the bound radioactivity to ≤5% of the binding measured in the absence of unlabeled competitor, showing that nonspecific binding at these concentrations of labeled ASU-hFc was a minor fraction of the total (data not shown).
FIG. 5.
Binding of 125I-labeled ASU-hFc fusion protein to 208F/LPit2SN cells. Cell-associated (bound) radioactivity was measured after incubation of 105 cells with labeled fusion protein in a volume of 1 ml at room temperature. (A) Binding as a function of time for different fusion protein concentrations. (B) Binding after a 120-min incubation as a function of fusion protein concentration. The experiment was repeated three times with similar results.
Scatchard analysis of [125I]ASU-hFc binding to 208F, 208F/LXSN, and 208F/LPit2SN cells gave results typified by those shown in Fig. 6. Assuming a 1:1 binding ratio between receptor and fusion protein dimer, the x intercept of the trendline is equal to the number of receptors per cell, and the Kd for ASU-hFc binding to the receptor is equal to −1/(slope of the line). Mean values for the receptor number and Kd for four independent experiments per cell line are shown in Table 1. The number of amphotropic SU binding sites detected on 208F/LXSN cells was equal to that for 208F cells, showing that transduction with the control vector did not affect the receptor level. In contrast, 208F/LPit2SN cells showed an almost eightfold higher receptor level, which correlates well with the 5- to 10-fold improvement in transduction efficiency observed for 208F/LPit2SN cells compared to that observed for 208F or 208F/LXSN cells (Fig. 2).
FIG. 6.
Scatchard analysis of ASU-hFc binding to 208F, 208F/LXSN, and 208F/LPit2SN cells. The bound/free ratio is plotted against the amount of bound fusion protein, where the bound protein is expressed in molecules per cell and the free protein is expressed in picomolar units. Calculation of the x intercept allows enumeration of binding sites, assuming a binding pattern of one fusion protein homodimer per binding site. Data from a representative experiment are shown.
TABLE 1.
Pit2 receptor number and fusion protein binding affinity for parental 208F cells and 208F cells transduced by a Pit2 expression vectora
| Cell line | Receptor properties
|
|
|---|---|---|
| Binding sites/cell | Kd (pM) | |
| 208F | 18,000 ± 2,700 | 21 ± 5 |
| 208F/LXSN | 20,000 ± 3,900 | 35 ± 23 |
| 208F/LPit2SN | 150,000 ± 11,000 | 92 ± 67 |
The average number of ASU-hFc fusion protein binding sites per cell and the Kd of binding were determined by Scatchard analysis. The results are means ± standard deviations of four independent experiments for the parental 208F cells and two clones each of the 208F/LXSN and the 208F/LPit2SN cells.
The results suggest that the amphotropic SU has a lower affinity for the receptor when it is overexpressed. However, the Kd values for a given cell line showed a relatively high variation between experiments compared to the receptor number measurements (Table 1). This is due to the strong influence that the estimate of the fraction of active [125I]ASU-hFc has on the Kd calculation. In contrast, the receptor number calculation is not affected by this variable.
DISCUSSION
Several groups have found that the ability of a retrovirus with a particular pseudotype to infect target cells correlates with the relative abundance of receptor RNA message (5, 16, 35). In contrast to the present study, these data provide only indirect evidence that the number of surface receptors expressed is crucial to the efficiency of retroviral infection.
Others have investigated receptor expression levels by using a sandwich assay involving antibody to the virus envelope glycoprotein gp70 (9, 27, 37). In this assay the binding of virus can be studied by the detection of antibody bound to the virions that in turn are bound to receptors on the cell surface. Studies show that gp70 binds in a saturable pattern that is dependent on the cell number and thus the receptor (11). However, the analysis of gp70 binding is not without pitfalls in that gp70 is shed from viral particles and from producer cell lines and is thus present in vector preparations (2). Free gp70 can therefore occupy receptors and compete with functional virus for binding sites.
Yu et al. (37) have systematically analyzed the binding kinetics of ecotropic pseudotype retrovirus to NIH 3T3 cells. In contrast to our study, they utilized an anti-gp70 antibody flow cytometric assay that did not allow the quantification of receptors. They found significant discrepancies between the dissociation constants measured directly in their experiments and those calculated from association and dissociation rates. Contamination of their virus preparations with free gp70, receptor-ligand internalization, and surface dissociation of the cell-bound SU portion from the virions were thought to account for these problems. This illustrates the shortcomings of this indirect, antibody-based assay.
Ganguly et al. (11) used radiolabeled gp70 antibody and estimated the number of ecotropic receptors on the surface of an NIH 3T3 cell to be about 5 × 105. While vectors of ecotropic pseudotype efficiently infect NIH 3T3 cells at up to 80% after one vector exposure (data not shown), the lower susceptibility of 208F fibroblasts to infection (9.5%) correlates with the lower number of amphotropic receptors (1.8 × 104) found in our study.
Another approach has been to directly label gp70 purified from virus preparations. DeLarco and Todaro (10) used this approach to study retrovirus receptor expression on NIH 3T3 cells. While they do not provide data on binding kinetics, they were able to calculate the number of receptors to be approximately 5.3 × 105 per cell, a finding similar to the results Ganguly et al. (11). Interestingly, DeLarco and Todaro encountered some of the same problems we did with their experimental design that were related to the variation in the amount of labeled protein participating in binding. They report a range of active protein from 7 to 41% between experiments and also note some deterioration of the labeled preparation over the time of storage prior to the individual experiment. This parallels our observation regarding the variability in equilibrium dissociation constants (Kd) between experiments. The Kd is affected by the fraction of labeled fusion protein actually participating in binding (3), which averaged 15% (range, 10 to 22%) in our series of experiments. This average was applied to all calculations, but the reactive fraction of fusion protein was not assessed prior to each avidity experiment. Some variation over time due to degradation of the labeled protein is conceivable, and this likely accounts for the magnitude of the standard deviation for Kd in Table 1. DeLarco and Todaro (10) also performed cold competition experiments and found 1.8% residual binding of labeled protein in the presence of 500-fold excess of unlabeled protein. This is in agreement with the ≤5% nonspecific binding of labeled protein we observed in our experiments.
Less data is available on the binding characteristics of amphotropic pseudotype retrovirus to its cellular receptor. Battini et al. (4) have purified a fragment containing the N-terminal binding domain of the amphotropic envelope SU portion. After radioactive labeling of the fragment they studied its binding characteristics to cells, performed Scatchard analysis, and estimated the number of binding sites to be 7 × 104 per cell for NIH 3T3 mouse fibroblasts. This again correlates well with the relatively lower number of amphotropic fusion protein binding sites (1.8 × 104) and more inefficient transduction (9.5% versus 20% on 3T3 cells [data not shown]) in 208F cells. A number of other points distinguish the present study from the one by Battini et al. First, the ability of our fusion protein to be labeled radioactively or by fluorescent secondary antibody permits us to perform Scatchard analysis or alternatively to measure the relative receptor expression levels on individual cells within a population. Second, we correlated the efficiency of transduction and receptor number by studying the effect of receptor overexpression on both parameters in the same cell line. Third, Battini et al. do not provide a correction for the reactive fraction of labeled protein (personal communication), resulting in an overestimate of free fusion protein in the calculations. Fourth, Battini et al. found a downward concave shape of the Scatchard, which contrasts with our more typical linear plots.
A question remains concerning the precise stoichiometry of binding of our fusion protein. The above results are based on the assumption that a fusion protein homodimer will bind to a single binding site, i.e., the receptor. If a single homodimer binds two receptors, this may lead to a two-fold underestimate of binding sites and might affect the Kd values.
Finally, the use of hematopoietic reconstituting cells in clinical gene therapy, with their apparently decreased propensity to enter cell cycle (1, 14, 17), will make it all the more important to find retrovirus vectors targeting an appropriately abundant receptor. We have previously provided evidence in the baboon competitive repopulation assay that transduction by a vector pseudotyped for the more abundantly available GALV receptor on hematopoietic CD34+ cells is preferentially detected over amphotropic pseudotype vector following reconstitution of the lethally irradiated animal (16). This data confirmed similar observations made in vitro (36). The fusion protein used in the present study will allow the direct quantification of retrovirus binding sites on the surface of such target cells.
In summary, we have systematically analyzed a 208F rat fibroblast culture model in which the block to efficient retrovirus transduction is primarily at the level of target cell surface receptor expression. Using this model, we provide direct evidence that efficient retrovirus transduction requires high-level expression of surface receptor as measured by fusion protein binding sites. These results have important implications for the maximization of retroviral infection in animal gene transfer studies and human gene therapy.
ACKNOWLEDGMENTS
We acknowledge the contribution of David Cosman and Immunex Corporation in providing the human IgG Fc portion of the fusion protein and the technical assistance of Jennifer Smith.
This work was supported by grants DK47754, HL54881, and HL36444 from the National Institutes of Health. H.-P.K. is a Markey Molecular Medicine Investigator.
REFERENCES
- 1.Agrawal Y P, Agrawal R S, Sinclair A M, Young D, Maruyama M, Levine F, Ho A D. Cell-cycle kinetics in VSV-G pseudotyped retrovirus-mediated gene transfer in blood-derived CD34+ cells. Exp Hematol. 1996;24:738–747. [PubMed] [Google Scholar]
- 2.Andersen K B. A domain of murine retrovirus surface protein gp70 mediates cell fusion, as shown in a novel SC-1 cell fusion system. J Virol. 1994;68:3175–3182. doi: 10.1128/jvi.68.5.3175-3182.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badger C C, Krohn K A, Bernstein I D. In vitro measurement of avidity of radioiodinated antibodies. Nucl Med Biol. 1987;6:605–610. doi: 10.1016/0883-2897(87)90033-x. [DOI] [PubMed] [Google Scholar]
- 4.Battini J L, Rodrigues P, Mueller R, Danos O, Heard J M. Receptor-binding properties of a purified fragment of the 4070A amphotropic murine leukemia virus envelope glycoprotein. J Virol. 1996;70:4387–4393. doi: 10.1128/jvi.70.7.4387-4393.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer T R, Jr, Miller A D, Hickstein D D. Improved transfer of the leucocyte integrin CD18 subunit into hematopoietic cell lines by using retroviral vectors having a gibbon ape leukemia virus envelope. Blood. 1995;86:2379–2387. [PubMed] [Google Scholar]
- 6.Bolton A E, Hunter W M. The labeling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973;133:529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coelho A M. Cell cycle analysis. In: Kruse P F, Patterson M K, editors. Tissue culture: methods and applications. New York, N.Y: Raven Press; 1973. pp. 412–422. [Google Scholar]
- 8.Corsaro C M, Pearson M L. Enhancing the efficiency of DNA-mediated gene transfer in mammalian cells. Somatic Cell Mol Genet. 1981;7:603–616. doi: 10.1007/BF01549662. [DOI] [PubMed] [Google Scholar]
- 9.Crooks G M, Kohn D B. Growth factors increase amphotropic retrovirus binding to human CD34+ bone marrow progenitor cells. Blood. 1993;82:3290–3297. [PubMed] [Google Scholar]
- 10.DeLarco J, Todaro G J. Membrane receptors for murine leukemia viruses: characterization using the purified viral envelope glycoprotein, gp71. Cell. 1976;8:365–371. doi: 10.1016/0092-8674(76)90148-3. [DOI] [PubMed] [Google Scholar]
- 11.Ganguly K, Kalyanaraman V S, Samgadharan M G. Analysis of the interaction between Rauscher murine leukemia virus and murine cell membrane receptor by in vitro binding assay. Cancer Lett. 1983;18:79–86. doi: 10.1016/0304-3835(83)90120-9. [DOI] [PubMed] [Google Scholar]
- 12.Hayflick L, Moorhead P S. The serial cultivation of human diploid cell strains. In: Pollack R, editor. Readings in mammalian cell culture. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1981. pp. 27–63. [Google Scholar]
- 13.Holley R W, Kiernan J A. Contact inhibition of cell division in 3T3 cells. In: Pollack R, editor. Readings in mammalian cell culture. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1981. pp. 261–265. [Google Scholar]
- 14.Jordan C T, Yamasaki G, Minamoto D. High-resolution cell cycle analysis of defined phenotypic subsets within primitive human hematopoietic cell populations. Exp Hematol. 1996;24:1347–1355. [PubMed] [Google Scholar]
- 15.Kavanaugh M P, Miller D G, Zhang W, Law W, Kozak S L, Kabat D, Miller A D. Cell-surface receptors for gibbon ape leukemia virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc Natl Acad Sci USA. 1994;91:7071–7075. doi: 10.1073/pnas.91.15.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiem H P, Heyward S, Winkler A, Potter J, Allen J M, Miller A D, Andrews R G. Gene transfer into marrow repopulating cells: comparison between amphotropic and gibbon ape leukemia virus pseudotyped retroviral vectors in a competitive repopulation assay in baboons. Blood. 1997;90:4638–4645. [PubMed] [Google Scholar]
- 17.Knaan-Shanzer F, Valerio D, van Beusechem V W. Cell cycle state, response to hemopoietic growth factors and retroviral vector-mediated transduction of human hemopoietic stem cells. Hum Gene Ther. 1996;3:323–333. [PubMed] [Google Scholar]
- 18.Kuchler R J. Development of animal cell populations in vitro. In: Kuchler R J, editor. Biochemical methods in cell culture and virology. Stroudsburg, Pa: Dowden, Hutchinson & Ross, Inc.; 1977. pp. 90–113. [Google Scholar]
- 19.LeDoux J M, Morgan J R, Snow R G, Yarmush M L. Proteoglycans secreted by packaging cell lines inhibit retrovirus infection. J Virol. 1996;70:6468–6473. doi: 10.1128/jvi.70.9.6468-6473.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindmo T, Boven E, Cuttitta T, Fedorko J, Bunn P A., Jr Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Res. 1984;72:77–89. doi: 10.1016/0022-1759(84)90435-6. [DOI] [PubMed] [Google Scholar]
- 21.Miller A D, Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986;6:2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller A D, Rosman G J. Improved retroviral vectors for gene transfer and expression. BioTechniques. 1989;7:980–990. [PMC free article] [PubMed] [Google Scholar]
- 23.Miller A D, Garcia J V, von Suhr N, Lynch C M, Wilson C, Eiden M V. Construction and properties of retrovirus packaging cells based on gibbon ape leukemia virus. J Virol. 1991;65:2220–2224. doi: 10.1128/jvi.65.5.2220-2224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller D G, Adam M A, Miller A D. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol Cell Biol. 1990;10:4239–4242. doi: 10.1128/mcb.10.8.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller D G, Miller A D. Tunicamycin treatment of CHO cells abrogates multiple blocks to infection, one of which is due to a secreted inhibitor. J Virol. 1992;66:78–84. doi: 10.1128/jvi.66.1.78-84.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller D G, Edwards R H, Miller A D. Cloning of the cellular receptor for amphotropic murine retrovirus reveals homology to that for gibbon ape leukemia virus. Proc Natl Acad Sci USA. 1994;91:78–82. doi: 10.1073/pnas.91.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan J R, LeDoux J M, Snow R G, Thompkins R G, Yarmush M L. Retrovirus infection: effect of time and target cell number. J Virol. 1995;69:6994–7000. doi: 10.1128/jvi.69.11.6994-7000.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Movassagh M, Desmyter C, Baillou C, Chapel-Fernandes S, Guigon M, Klatzmann D, Lemoine F M. High level gene transfer to cord blood progenitors using gibbon ape leukemia virus pseudotype retroviral vectors and an improved clinically applicable protocol. Hum Gene Ther. 1998;9:225–234. doi: 10.1089/hum.1998.9.2-225. [DOI] [PubMed] [Google Scholar]
- 29.Miyake K, Suzuki N, Matsuoka H, Tohyama T, Shimada T. Stable integration of human immunodeficiency virus-based retroviral vectors into the chromosomes of nondividing cells. Hum Gene Ther. 1998;9:467–475. doi: 10.1089/hum.1998.9.4-467. [DOI] [PubMed] [Google Scholar]
- 30.Orlic D, Girard L J, Jordan C T, Anderson S M, Cline A P, Bodine D M. The level of mRNA encoding the amphotropic retrovirus receptor in mouse and human hematopoietic stem cells is low and correlates with the efficiency of retrovirus transduction. Proc Natl Acad Sci USA. 1996;93:11097–11102. doi: 10.1073/pnas.93.20.11097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmer T D, Hock R A, Osborne W R A, Miller A D. Efficient retrovirus-mediated transfer and expression of a human adenosine deaminase gene in diploid skin fibroblasts from an adenosine deaminase-deficient human. Proc Natl Acad Sci USA. 1987;84:1055–1059. doi: 10.1073/pnas.84.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poeschla E M, Wong-Staal F, Looney D J. Efficient transduction of nondividing human cells by feline immunodeficiency virus lentiviral vectors. Nat Med. 1998;4:354–357. doi: 10.1038/nm0398-354. [DOI] [PubMed] [Google Scholar]
- 33.Quade K. Transformation of mammalian cells by avian myelocytomatosis virus and avian erythroblastosis virus. Virology. 1979;98:461–465. doi: 10.1016/0042-6822(79)90569-5. [DOI] [PubMed] [Google Scholar]
- 34.Reiser J, Harmison G, Kluepfel-Stahl S, Brady R O, Karlsson S, Schubert M. Transduction of nondividing cells using pseudotyped defective high-titer HIV type 1 particles. Proc Natl Acad Sci USA. 1996;93:15266–15271. doi: 10.1073/pnas.93.26.15266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabatino D E, Do B Q, Pyle L C, Seidel N E, Girard L J, Spratt S K, Orlic D, Bodine D M. Amphotropic or gibbon ape leukemia virus (GALV) retrovirus binding and transduction correlates with the level of receptor mRNA in human hematopoietic cell lines. Blood Cells Mol Dis. 1997;23:422–433. doi: 10.1006/bcmd.1997.0161. [DOI] [PubMed] [Google Scholar]
- 36.von Kalle C, Kiem H P, Goehle S, Darovsky B, Heimfeld S, Torok-Storb B, Storb R, Schuening F G. Increased gene transfer into human hematopoietic progenitor cells by extended in vitro exposure to a pseudotyped retroviral vector. Blood. 1994;9:2890–2897. [PubMed] [Google Scholar]
- 37.Yu H, Soong N, Anderson W F. Binding kinetics of ecotropic (Moloney) murine leukemia retrovirus with NIH 3T3 cells. J Virol. 1995;69:6557–6562. doi: 10.1128/jvi.69.10.6557-6562.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]