Abstract
Aging skin, wrinkles, pigmentation, and dryness are problems that plague people, and researchers are working to solve them. Recent studies have shown that intestinal microbiota homeostasis can influence skin health, demonstrating the existence of a gut–skin axis. Recently, improving skin health through probiotic interventions has been proposed, and micro-ecological skin care is becoming a popular concept. By regulating skin health and gut–skin axis interactions, probiotics can be used as potential management tools to suppress and improve skin diseases in multiple ways, including decreasing oxidative stress, suppressing inflammatory responses, and keeping immune effects. The purpose of this paper is to provide a comprehensive review of the application and mechanisms of probiotic-mediated gut microbiota homeostasis in skin care and to offer a theoretical basis for the application of probiotics in skin care.
Keywords: skin, probiotics, intestinal microbiota, gut–skin axis
1. Introduction
Skin accounts for about 15 percent of the total body weight of adults, with an average surface area of 1.5–2 m2 [1]. One of the main functions of the skin is its use as a mechanical barrier to disease-causing microorganisms and harmful substances; in fact, it could be viewed as one of the host’s vital defenses against infections, as well as the innate and adaptive immune system [2]. Other important features include inhibition of transcutaneous water loss (TEWL), thermoregulation, structural support, and vitamin synthesis, all of which assist in maintaining a healthy host [2,3,4].
The quest for beauty never ends. It is often hard to know whether skin issues, including skin pigmentation, skin wrinkles, skin aging, and skin dehydration, occur due to external elements or internal changes. Skin issues have various causes, and investigators are continuously researching safe and efficient skin treatment products to address skin issues. Today, various cosmetic products contain chemicals, including titanium dioxide, which are more or less toxic and may be harmful to an individual’s health [5]. There are also various researchers who use raw materials extracted from herbal medicines as important components in skin treatment products, while they show certain results due to the complexity of herbal ingredients, their influences sometimes fail to meet expectations and their quality still needs to be improved [6,7]. Thus, there is an urgent need to explore safe and efficient ingredients for skin treatment products that can effectively address skin issues. Recently, researchers have suggested that probiotics can be used as an efficient ingredient in cosmetics to address the above-mentioned skin issues to a better effect. In addition, experimental research have indicated that probiotics have no or less toxic influences on hosts and can be better used in the development of skin treatments.
Recently, we have become increasingly aware of the powerful effect of probiotics in many fields, and the effects of probiotics in skin care have been increasingly studied and argued by researchers. Probiotics can act through a variety of mechanisms (Figure 1).
Figure 1.
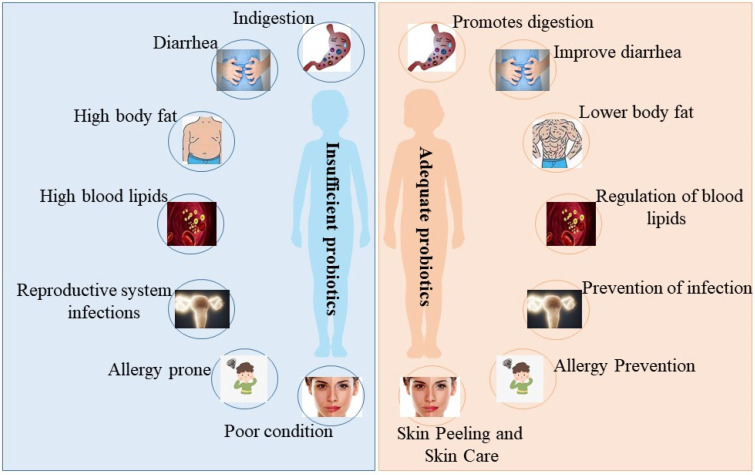
The beneficial effects of probiotics on the organism. When the abundance of probiotics in the organism is insufficient, the organism suffers from the following issues: indigestion, diarrhea, high body fat, high blood lipids, reproductive system infections, allergy prone, and poor skin condition (left picture). When the abundance of probiotics in the organism is sufficient, the organism behaves as follows: promotes digestion, improves diarrhea, lowers body fat, regulates blood lipids, prevents reproductive system infections, prevents allergies, and skin peeling and skin care (right picture).
This paper reviews the applications of probiotics in skin care, such as skin whitening, skin moisturizing, skin anti-aging, skin anti-wrinkle, and body odor removal, and their mechanisms, which offers a theory basis for further applications of probiotics in skin care in the future.
Although the applications of topical biologic therapies date back to 1912, when topical use of Lactobacillus bulgaricus ameliorated skin problems, including acne and seborrhea, the skin care industry has recently seen a proliferation of topical preparations containing microorganisms [8]. Table 1 lists skin care products containing probiotics sold worldwide.
Table 1.
Skin care products with added probiotics and their effects.
| Product | Probiotics | Efficacy |
|---|---|---|
| Okana |
Bacillus bacterial ferment extract |
Helps skin retain its firmness and elasticity and keeps it feeling smooth and plump. |
| Amperna | Unique probiotic complex |
Soothes irritated skin and calms redness. Tested on eczema, dermatitis, perioral dermatitis, rosacea, and acne-prone skin. |
| Cream | 1. Lactobacillus acidophilus
2. Lactobacillus rhamnosus |
Anti-photoaging |
| Elissah Bio P2 Laviol Skin Care |
16 types and 35 strains of bacteria including 14 Bifidobacterium and Lactobacilli. |
Strengthens the skin’s barrier against environmental threats and reduces the factors that trigger skin sensitivities, redness, and irritation. |
| Probiotic Skin Cream Melvory |
lactobacilli probiotic (Lactobacillus ferment filtrate) |
Cleans away the bad bacteria on the skin. For acne-prone or teenage skin. |
| Andalou Brightening Probiotic + C Renewal Cream |
Bacillus coagulans | Skin-friendly vegan probiotic microflora enzymatically supports dermal vitality, targeting over-exposed surface cells for a lighter, tighter, brighter looking appearance, and a luminous complexion. |
| Biossance Squalane + Probiotic Gel |
Lactococcus ferment lysate | Helps restore the skin’s balance and renew the skin barrier |
| Neogen Dermalogy Probiotics Double Action |
The patented complex of Bifida ferment lysate, Lactobacillus, and Streptococcus thermophilus ferment |
Protects the skin barrier |
| Elemis Dynamic Resurfacing Facial Pads |
Lactococcus ferment lysate | Stimulates skin-cell renewal and reinforce the skin barrier |
| Manyo Factory Bifida Complex Ampoule |
Bifida ferment lysate, Bifida ferment filtrate, Lactobacillus ferment lysate, and Lactococcus ferment lysate |
Encourages self-repair of skin, hydrates, replenishes moisture and prevents aging |
| LaFlore Probiotic Serum Concentrate |
Lactococcus ferment lysate and live kefir Probiotics (Hansenula/Kloeckera/ Lactobacillus/Lactococcus/ Leuconostoc/Pediococcus/ Saccharomyces) |
Helps calm and smooth fine lines and wrinkles and boosts the skin’s natural defense system. |
| Elizabeth Arden Superstart Probiotic Boost Skin Renewal Biocellulose Mask |
Lactococcus ferment lysate; inactivated strains of Lactobacillus casei and Lactobacillus acidophilus |
Optimizes skin’s microflora and natural defense. Moisturizes and smoothens skin |
| Dot and Key 72 h hydrating gel and Probiotics |
Saccharomyces black tea ferment, Lactobacillus |
Provides hours long moisturization and restores microbiome balance |
However, there are many problems with topical probiotics that have not yet been solved. External products cannot be manufactured under sterile status and, therefore, do not require sterility testing. These manufacturers usually include antiseptics to regulate the microorganism’s growth. These antiseptics may influence the viability of probiotic strains and also inadvertently change the microbiota of the receptor [8]. Topical formulations containing probiotics have not yet moved outside the personal care manufacture category; because they assess a high load of colony-forming units, such formulations have difficulty passing the US Food and Drug Administration’s (USFDA) modulatory provisions for microbiota load. Antiseptic effect testing is a vital barrier to measuring these applications. According to the United States Pharmacopeia (USP), topical probiotic preparations for the treatment of acne were tested for microbiota counts. Studies have shown that topical products do not contain “undesirable” amounts of live microorganisms and, therefore, according to the USP [9], do not require less than 1000 colony-forming units (CFU). Meanwhile, since the stratum corneum maintains the skin’s strict natural and protective barrier function, it regulates the absorption of effective substances into the deeper layers of the skin, thus also limiting the choice of treatments [10]. The preparation requirements for topical applications, including live microorganisms, are significantly different from those for products containing only smaller molecules resulting from the need to maintain microbial stabilization. Key factors that are required for microbial control are pH and osmolarity contents, as well as temperature and humidity levels of the storage environment [11].
Topical probiotics have been used to maintain skin health since the beginning of the 20th century, and the last decade has seen a dramatic rise in commercially available topical probiotics [12]. With the increasing popularity of these topical products and the dearth of clinical trials or efficacy studies to establish their clinical efficiency, we are gradually focusing on internal probiotics in treating skin disorders. Since internal probiotics first enter the intestinal tract and inevitably interfere with skin conditions by affecting intestinal homeostasis, this article elaborates on the relationship between probiotics, the intestine and skin, in an attempt to provide potential solutions and clinical value for finding appropriate skin interventions.
2. The Different Effects of Probiotics on the Skin (Figure 2)
2.1. Skin Whiting
Recently, there has been an increasing interest in skin lightening, and the focus of brightening products is to decrease melanin content and suppress overproduction pigmentation [13]. Melanin is photoprotective and protects the skin from ultraviolet (UV) radiation, but the overexpression of pigmentation can affect skin tone and even induce various skin disorders, including freckles and melasma [14,15,16]. The process of melanin production involves various enzymes and chemical catalytic reactions [17,18]. There are three main enzymes associated with melanogenesis, containing tyrosinase, tyrosinase-related protein 1 (TYRP-1), and tyrosinase-related protein 2 (TYRP-2), with tyrosinase being the indispensable primary enzyme [19]. A lot of whitening cosmetics can precisely suppress the tyrosinase activity, thus reducing melanin content and achieving a brightening effect. Recently, probiotics have been increasingly used in brightening products, which is tightly associated with their great antagonistic influence on tyrosinase (Figure 2).
Figure 2.
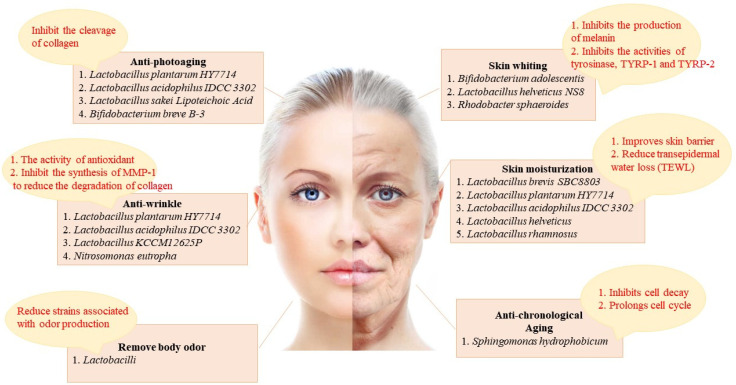
The skin improvement effect of probiotics and its related mechanism. The skin improving effects of probiotics include: anti-photoaging (inhibit the cleavage of collagen), skin whiting (inhibits the production of melanin and inhibits the activities of tyrosinase, TYRP-1 and TYRP-2), anti-wrinkle (the activity of antioxidant and inhibition of the synthesis of matrix metalloproteinase-1 (MMP-1) to reduce the degradation of collagen), skin moisturization (improves skin barrier and reduces TEWL), body odor removal (reduce strains associated with odor production), and anti-chronological aging (inhibits cell decay and prolongs cell cycle).
A study suggested that the antagonistic influence of Bifidobacterium adolescentis culture filtrate on mushroom tyrosinase and tyrosinase activity strengthened with increasing content, thereby reducing the melanin levels in B16F10 cells [20]. According to these studies, Bifidobacterium adolescentis culture filtrate could modulate tyrosinase activity via its antioxidant effect, thereby decreasing melanin content and achieving a whitening purpose. Moreover, they also uncovered that lactic acid in Lactobacillus can suppress melanin synthesis directly by down-regulating tyrosinase activity and also regulate melanin synthesis by affecting tyrosinase expression or tyrosinase, tyrosine 1 and tyrp-2 to exhibit a brightening effect [21].
Probiotics can decrease melanin content not only by regulating tyrosinase activity but also by other means to achieve a whitening effect. Jingjing Rong et al. indicated the brightening effect of Lactobacillus helveticus NS8 fermented milk supernatant (NS8-FS) [22]. Data demonstrated that NS8-FS decreased melanin levels in B16F10 cells by suppressing the activity of tyrosinase and proteins associated with tyrosinase expression. Furthermore, a UV radiation-induced pigmentation model was established in guinea pigs. Masson-Fontana staining and tyrosinase staining tests confirmed that NS8-FS improved skin pigmentation. The potential mechanism by which NS8-FS improved skin pigmentation is the modulation of the Nrf2 activity, which promotes melanogenesis in melanocytes under UV-mediated oxidative stress [23]. Liu et al. explored the inhibitory effect of Rhodobacter spheroides (Lysogen™) on melanin synthesis [24]. Research demonstrated that melanin content in B16F10 cells up-regulated after α-MSH supplementation and down-regulated in a dose-dependent manner after Lysogen™ treatment.
2.2. Skin Moisturization
There are various reasons for dry skin, such as seasonal alterations, skin barrier damage, and disorderly flaking [25]. Skin hydration is important for health and beauty, and it exerts vital effects on maintaining proper body activity and beauty. Thus, we are continuously searching for molecules that are beneficial for maintain skin moisture. Probiotics can decrease TEWL and improve skin dryness, which can be used to modulate dry skin. Moreover, probiotics can also decrease skin water-loss by modulating skin–barrier function and are good skin moisturizers [26].
A study confirmed that the oral supplementation of Lactobacillus plantarum HY7714 increased ceramide levels by elevating serine palmitoyltransferase (SPT) mRNA expression and decreasing ceramidase mRNA expression [27]. Ceramides exert an important effect on keeping the structural support of the epidermal barrier and the epidermal hydration [28,29]. Elevated ceramide contents lead to lower TEWL values and up-regulated hydration. Studies used ELISA to detect hyaluronic acid (HA) content and found that the use of acidophilic lactic acid IDCC 3302 exerts a beneficial effect on skin hydration [30,31]. In conclusion, the treatment of Lactobacillus acidophilus IDCC 3302 resulted in improved skin dryness and decreased TWEL, thereby up-regulating skin hydration. Hidoko BABA et al. indicated that the administration of Lactobacillus helveticus-fermented milk whey (LHMW) led to an obvious reduction in TWEL of intact skin and an increase in skin water content, proving that LHMW milk has a moisturizing effect and is used in cosmetics [32].
2.3. Skin Barrier Integrity
Damage to the skin barrier function can adversely affect the skin by disrupting the moisture balance on the skin surface [33]. Ye-On Jung et al. demonstrated experimentally that Lactobacillus rhamnosus (LR) can effectively improve the skin barrier and can be regarded as a moisturizing skin care product [34]. They used immunofluorescence staining to identify up-regulated expression levels of Claudin-1 and Occludin, two tightly bound molecules and indicated that the stratum corneum of tissues treated with LR lysate was tighter and more organized. Moreover, qPCR results suggested elevated expression levels of loricrin and filaggrin which exert a vital effect on the restoration of skin barrier function [35]. Furthermore, the strengthened skin barrier function was further suggested by reducing sodium dodecyl sulfate (SLS)-induced cytotoxicity and decreasing skin permeability.
2.4. Anti-Aging
Chronological aging and photoaging are two primary forms of skin aging [36]. Chronological aging is mainly influenced by internal elements, whereas photoaging is mainly influenced by external elements [37]. These influences are different but have similar regulatory mechanisms, but probiotics have a beneficial effect on both forms of skin aging.
2.4.1. Anti-Chronological Aging
Chronological aging is mainly associated with genetic elements and is a regular physiological process in the human body. As we age, the body ages, and so does the skin, which is characterized by thinning and dryness [38]. Probiotics achieve anti-aging mainly by suppressing cell decay and prolonging the cell cycle. Sandie Gervason et al. indicated that the exclusion of Sphingomonas hydrophobicum (SH) could suppress the production of proteins associated with aging, such as P16 and P21, using an immunohistological experiment. P16 and P21 are cell cycle antagonists, which suppress the cell cycle and result in cell aging [39,40]. The production level of P16 and P21 in the experimental group were obviously down-regulated versus the control group without SH extraction. SH extraction was also demonstrated to suppress the SA-β-galactosidase level, which is linked to aging, to improve cell senescence. Moreover, the level of fibrillin-1 and Versican was up-regulated after SH extraction supplementation. Previous research indicated that fibrillin-1 participates in the production of elastic skin fibers [41] and that an up-regulated Versican level can inhibit the apoptosis response of fibroblasts [42], both of which can slow down cell senescence. In the case of SH extraction, it can be used as an anti-aging skin-care product.
2.4.2. Anti-Photoaging
Photoaging is primarily influenced by external environmental elements, such as UV radiation and toxins. These external elements will induce injury to the skin, causing it to lose elasticity, lose moisture, thicken, and become rough and sluggish [43]. Probiotics have a significant influence on the treatment of photoaging, which is primarily achieved by suppressing collagen division.
Research indicated that patients taking Lactobacillus plantarum HY7714 had decreased epidermal moisture loss, decreased wrinkle depth, and ameliorated skin gloss and elasticity [44]. Research demonstrated that tyndallized Lactobacillus acidophilus IDCC 3302 could restore the reduction in collagen expression after UV irradiation via Western blot analysis [45]. Meanwhile, it was shown that tyndallized Lactobacillus acidophilus IDCC 3302 could obviously decrease the contents of MMP-1, MMP-2, and MMP-9 in HaCaT, which were up-regulated due to exposure to UV rays, primarily by suppressing the MAPK signaling pathway. Moreover, tyndallized Lactobacillus acidophilus IDCC 3302 can improve the inflammation response by reducing the levels of proinflammatory cytokines, including IL-1β, IL-8, and TNF-α. The above data showed that tyndallized Lactobacillus acidophilus IDCC 3302 can inhibit photoaging and ameliorate inflammation responses induced by UV irradiation. You et al. suggested that Lactobacillus sakei Lipoteichoic Acid (sLTA) could suppress the phosphorylation of MAPK and further block the MMP-1 synthesis when hosts are exposed to UV rays [46].
2.5. Anti-Wrinkle
Wrinkles are induced by atrophy of the skin and repeated contractions of facial muscles underneath [47]. The use of probiotics has been proven to regulate facial wrinkles. The antioxidant activity of probiotics is closely associated with their anti-wrinkle properties. Moreover, MMP-1 synthesis activates the degradation of collagen produced by fibroblasts, resulting in wrinkles on the surface of human skin [48]. Probiotics could suppress MMP-1 synthesis and decrease collagen degradation, resulting in anti-wrinkle properties.
Researchers found that tyndallized Lactobacillus KCCM12625P (AL) can suppress the MMP-1 synthesis, thus preventing wrinkle formation. AL can effectively inhibit the formation of facial wrinkles and act as an anti-wrinkle mainly through the above two aspects.
Hyun Mee Kim et al. found that Lactobacillus plantarum HY7714 had a powerful blocking function on UV-induced MMP-1 according to Western blot [49]. Moreover, Lactobacillus plantarum HY7714 inhibited the MMP-1 expression and the MMP-2 and MMP-9 activity, which effectively improved the area and depth of wrinkles and exerted a vital effect on wrinkle elimination. A study indicated that tyndallized Lactobacillus acidophilus IDCC 3302 could effectively decrease MMP-1, MMP-2, and MMP-9 contents in UV-exposed HaCaT cells measured by ELISA. Therefore, tyndallized Lactobacillus acidophilus IDCC 3302 could decrease wrinkles by suppressing MMPs.
3. Presentation of the Gut–Skin Axis
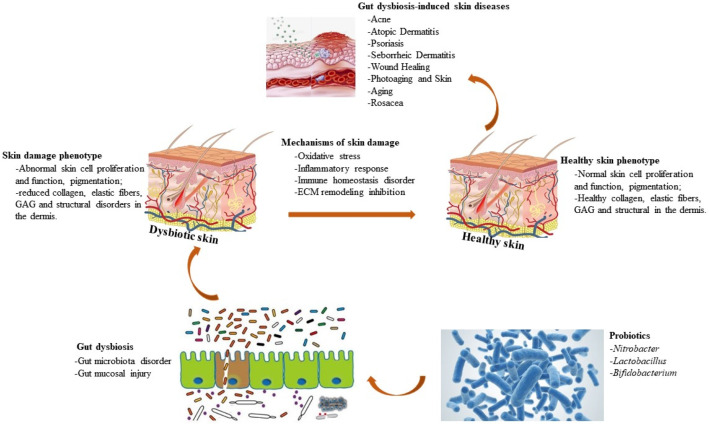
The microbiota of the gut is similar to that of the skin. Various studies have linked inflammatory skin status to intestinal microbiota disorder. The intestinal microbiota affects the body’s immunological function. The immune system defends itself from external pathogenic bacteria. Once the intestinal microbiota is imbalanced, the changed intestinal microbiota may lead to autoimmune and inflammation status not only in the intestines but also in remote organs, including the skin [50]. Various research prove the notion that disorders in the intestinal microbiota could contribute to skin diseases, including acne [51,52], atopic dermatitis [53], psoriasis [54], and rosacea [55]. The immune system appears to mediate the connection between the skin and the intestine. Microbial interactions with the host immune system are important for maintaining skin homeostasis [56]. Thus, a balance between skin and intestines is a logical way to cure many skin disorders. Probiotics exert a crucial effect in improving the microbiota and are a vital therapeutic modality in the cure of inflammatory skin disorders [50].
The gut–skin axis suggests a relationship in which the immune properties of the gut microbiota can also influence skin health. The positive modulation of the skin or intestinal microbiota via oral probiotics is regarded as a potential clinical approach to prevent photoaging of the skin. Oral probiotics are a kind of live microbiota that modify the intestinal microbe and can have direct photoprotective influences on special skin cells via regulating immune responses and inflammation factors. In addition, they can increase the serum contents of short-chain fatty acids (SCFAs), which induce a range of immune and inflammatory responses. Oral probiotics have been investigated as a means to directly improve the intestinal microbiota to suppress and cure skin photoaging. In addition, oral probiotics have been used in the treatment of other skin diseases, including atopic dermatitis, acne, rosacea, and psoriasis, by regulating the skin microbiota and gut–skin axis [57,58,59,60,61]. This section discusses the effect of oral probiotics in photoaging and their associated mechanisms.
3.1. Improvement of Intestinal Homeostasis by Probiotics
3.1.1. Enhancement of Barrier Function
Probiotics ameliorate gut barrier dysfunction through a variety of potential mechanisms. These mechanisms include upregulation of the expression of mucin proteins, including mucin-type glycoproteins (MUC)1, MUC2, and MUC3, which in turn restrict the movement of bacteria in the mucus, and upregulation of the secretion and expression of antimicrobial peptides and tight junction proteins, including α-defensins, β-defensins and occlusion bands (ZO), to prevent cell proliferation [62].
3.1.2. Suppression of Pathogens
Probiotics compete with pathogenic bacteria or commensals to combine with mucins or epithelial cells and prevent the overgrowth of potentially pathogenic bacteria. Moreover, probiotics offer anti-microbial ingredients, including anti-microbial peptides, SCFA, and bacteriocins, which are associated with inhibiting or killing pathogenic microorganisms. In addition, SCFA, including butyrate, for example, help regulate the expression of occludin and ZO, both of which are associated with the improvement of the epithelial barrier integrity [63].
Probiotics also up-regulate the production of IgA in the host gastrointestinal (GI) tract. Secretory IgA defends the intestinal epithelium from colonization and/or invasion via ligation of pathogenic or commensal antigens, induces reverse transcriptional transport of antigens to dendritic cells (DCs), and reduces pro-inflammatory factors [64].
3.2. The Pathway of Probiotic-Mediated Intestinal Microbiota Regulating Skin Status (Figure 3)
3.2.1. Immunologic Pathway
In terms of immunity to Staphylococcus aureus, the most popular bacterial strain influencing atopic dermatitis (AD), an association was indicated between the intestines and skin. Staphylococcus aureus is the most common pathogenic bacteria in the skin of AD patients. By contrast, a new birth cohort research showed that colonization with Staphylococcus aureus strains exerted a vital effect on preventing the development of AD in infancy, as early responses to Staphylococcus aureus, similar to other skin strains, promoted the maturation of the infant’s immune system. Staphylococcus aureus strains on the mucosa can play a protective role through immune stimulation [65]. This research supports the speculation that the intestines and skin control the immune environment through the gut microbiota (Figure 3).
Figure 3.
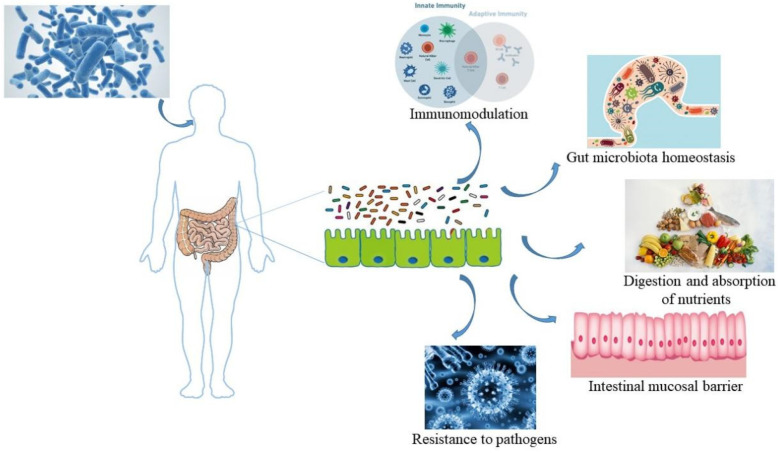
Oral probiotics mediate the beneficial effects of intestinal homeostasis on the organism. After the host ingests probiotics through the oral route, the probiotics enter the intestinal tract and play a role in improving intestinal homeostasis, mainly manifested as: immunomodulation, gut microbiota homeostasis, digestion and absorption of nutrients, and intestinal mucosal barrier.
Specific intestinal microbiota and their metabolites, including retinoic acid and polysaccharides A separated from Bacteroides fragilis, Facecalibacterium prausnitzii, and bacterial species attributing to Clostridium groups IV and XI, facilitate the accumulation of Tregs and lymphocytes that activate anti-inflammatory responses. In addition, some SCFAs, particularly butyrate, can modulate immune cell activation and apoptosis [66].
The gut microbiome has been studied as an important contributing factor to the immunologic pathway of skin disorders via probiotics. Orally administered probiotics could interact with gastrointestinal mucosa and gut-associated lymphoid tissue (GALT), where more than 70% of immune cells are located [67]. Probiotics interact with epithelial cells, mucosal DC, and macrophages in diverse ways. Depending on the probiotic strain, they can either induce immune activation signaling by producing IL-12, IL-18, and TNF-α or trigger tolerance signaling by stimulating anti-inflammatory cytokines, such as IL-10 and TGF-β [68,69]. In the IL-10/TGF-β-enriched cytokine milieu, DCs and macrophages can enhance the generation of the induced Treg cells that play key roles in maintaining peripheral immune tolerance by balancing the ratio of effector and Treg cells. Apart from probiotics, alterations in the gut microbiome might affect the development of host immune cell function through differences in gut microbiome genes, particularly in infants with AD [70].
3.2.2. Metabolite Pathway
Metabolites originating from intestinal microbiota, including SCFAs, or further supplemented by oral administration, also resolve the link between the intestines and the skin through the microbiota. SCFAs produced by intestinal microbiota including Akkermansia muciniphila exert an important effect on the pathology and etiology of AD, which could account for its correlation with the cutaneous immune system. A study showed that linoleic acid and 10-hydroxy-cis-12-octadecenoic acid alleviated AD diseases and controlled the intestinal microbiota in mice [71]. Further, three differential subgroups of the neonatal intestinal microbiota (NGM1–3) and its metabolites have been shown to play a role in early allergic sensitization [72]. Among these three subgroups, NGM3 is associated with multiple allergic sensitizations and was found to be relatively low in content in Bifidobacterium, Ackermannia and Fasciola [65]. For instance, 12,13-dihydroxy-9Zoctadecenoic acid (12,13-DiHome), a metabolite with inflammatory actions on in vitro immune control, was abundant in NGM3. In addition, 12,13-DiHome was up-regulated in the protective layer of casein, the white waxy coating on newborn host skin. These results may suggest the presence of a metabolite pathway in the gut–skin axis [65].
3.2.3. Neuroendocrine Pathway
Similarly to the skin, the lining of the GI is in direct contact with the outside environment, including food and microbes. One of the important roles of the skin and intestine is to suppress the entry of any pathogenic bacteria, and the microorganisms on both organs can be beneficial for the removal of these pathogenic bacteria through immune function, so it is essential to establish a balanced intestinal microbiota in both tissues and keep the appropriate balance for good health. In addition, these two microbiomes can interact with each other via neuroendocrine signaling. This influence can occur through two pathways: direct and indirect [65]. Tryptophan production by gut microbes leading to itchy skin in AD patients is an example of a direct signal. Conversely, γ-aminobutyric acid produced from Lactobacillus and Bifidobacterium in the intestines inhibits skin itching [65,73].
By indirect route, gut microbes control the content of cytokines such as IL-10 and IFN-γ in the blood, which can result in unusual alterations in brain function, leading to anxiety and stress [65]. Cortisol is a representative stress hormone in hosts that can change the permeability and barrier function of the intestinal epithelium by altering the composition of the intestinal microbe [74]. Cortisol can also alter the contents of circulating neuroendocrine molecules, including tryptamine, trimethylamine, and 5-hydroxytryptamine, further enhancing the skin barrier and immunological function [75].
4. Probiotics-Mediate Intestinal Microbiota to Improve Skin Disorders (Figure 4)
4.1. Acne
Acne patients have a special skin microbe. Available treatments for acne have various challenges because it injures the mechanical barrier of the skin, thereby drying it out and stimulating it. Research examining the skin–gut axis relationship in acne have shown that treatment with probiotics can improve the immune response beyond the intestines and extend it to the skin [61]. There is increasing evidence that topical probiotics also modulate the skin’s mechanical barrier and generate a secondary up-regulation in antimicrobial peptides. For instance, the lactic acid bacterium Streptococcus thermophiles promoted ceramide synthesis when used as a cream, lasting one week in vitro and in vivo [76,77,78]. Ceramides can confine water to the skin, and certain ceramide sphingolipids, including sphingomyelin, have antibacterial activity against Cutibacterium acnes, further restoring acne. Through the generation of ceramides, probiotics are used to enhance the skin’s mechanical barrier, which is helpful for acne-affected skin, as ceramides soothe irritated skin [79].
Thus, probiotics may be used to enhance protective barriers, suppress acne-causing bacteria, decrease pustules, and offer relief from skin irritation in acne patients (Figure 4).
Figure 4.
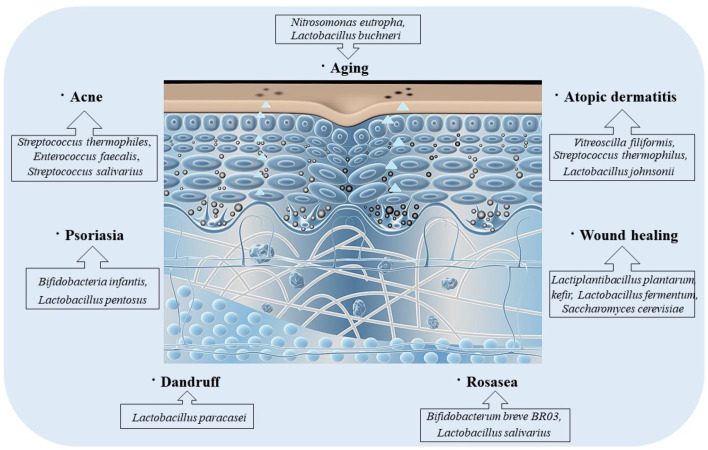
Probiotics can treat skin diseases. Different probiotics can treat different skin diseases, for example, Nitrosomonas eutropha and Lactobacillus buchneri can improve skin aging; Streptococcus thermophiles, Enterococcus faecalis and Streptococcus salivarius can improve acne; Vitreoscilla filiformis, Streptococcus thermophilus and Lactobacillus johnsonii can improve atopic dermatitis, Bifidobacteria infantis and Lactobacillus pentosus can improve psoriasia; Lactiplantibacillus plantarum kefir, Lactobacillus fermentum and Saccharomyces cerevisiae can improve wound healing; Lactobacillus paracasei can improve dandruff; Bifidobacterum breve BR03 and Lactobacillus salivarius can improve rosasea.
4.2. Atopic Dermatitis
AD is mainly caused by a decrease in microbial diversity; as mentioned above, the main microorganism in AD patients is Staphylococcus aureus. Various research suggest that oral probiotics may be used as a better option for its treatment [80]. One research showed that Streptococcus thermophilus obviously decreased eczema linked to AD and also decreased the severity of symptoms [78]. Another research suggested the validity of the lactic acid bacterium Streptococcus thermophilus on the stratum corneum by increasing ceramide levels in the skin [77].
In a randomized, double-blind experiment with patients with atopic dermatitis, researchers compared the use of emollients produced by Lactobaciilus with the use of regular emollients. Emollients containing Lactobacillus suppressed the extensions of Staphylococcus aureus, offered a mechanical barrier, and restored symptoms in patients with AD [81]. An experiment exploring the influence of lotions, including the heat-treated probiotic strain Lactobacillus johnsonii NCC on Staphylococcus aureus colonization, exhibited a useful effect in clinical symptoms in patients with atopic dermatitis.
Likewise, other experiments studying the treatment of Roseomonas mucosa via supplementation demonstrated an obvious decrease in disease severity, topical steroid needs, and Staphylococcus aureus burden. No adverse reactions or complications were reported in this trial [82]. Most of the trials conducted so far have shown that probiotics have a positive effect on patients with atopic dermatitis.
4.3. Psoriasis
Psoriasis is an autoimmune chronic skin disorder that is usually treated with topical emollients and oral immunosuppressants. Few research have included topical probiotics as a treatment for psoriasis. While research has shown that changes in the skin microbiota may help control psoriasis symptoms, oral probiotics have demonstrated therapeutic effects in clinical symptoms in some individuals. However, studies on the validity of oral probiotics in patients with psoriasis are needed to clinically demonstrate the advantages of oral probiotics [60].
4.4. Seborrheic Dermatitis
Yeast overgrowth on the scalp and decreased diversity of microbiota leads to dandruff and seborrheic dermatitis. Much research has been conducted to assess the treatment of probiotics in this context. A study of 60 patients exhibited a decrease in erythema, desquamation, and pruritus after the topical application of filamentous Staphylococci [78,83]. Another study revealed that Vitreoscilla filiformis lysate induced Treg activity through IL-10 production by dendritic cells [84]. Dandruff, seborrheic dermatitis, and scalp-associated disorders showed beneficial effects after oral supplementation of Lactobacillus paracasei. More research is necessary on the local efficacy of probiotics in treating this disease [85].
4.5. Rosacea
Overexpression of TLR2 receptors-induced rosacea arises, resulting in an inflammatory response and changed skin microbiota [86,87]. In addition to doxycycline as an antibiotic, oral probiotics are used to treat scalp rosacea; however, the use of topical probiotics for rosacea treatment has not been explored [88].
5. Probiotics That Regulate Skin Physiology (Figure 5)
5.1. Nitrobacter
Nitrobacter is a nitrifying bacterium that generates nitrate, a molecule that may have positive influences on the host, contained on the skin. Research has shown that dietary nitrates depletion, such as green leafy vegetables, has positive effects, such as increased blood flow to exercising skeletal muscles, decreased exercise oxygen demand, increased exercise tolerance in patients with peripheral arterial disorders, and lower blood pressure. Research has shown that these influences of nitrate depletion are largely the result of NOS-independent increases in NO synthesis and have been indicated to increase cutaneous reflex vasodilation through NOS-independent mechanisms in healthy hosts [89]. Antifungal activity of Nitrobacter spp. has been found to defend the skin from dermatophytes [90] and Staphylococcus aureus [91], a microbiota that can induce many dermatological infections. The ability of Nitrobacter to generate nitrate could also offer nitrate to the skin, which has been proven to conserve the skin’s progenitor cells from UV injury [92,93].
Figure 5.
The mechanism of probiotics to improve skin diseases. Probiotics, including Nitrobacter, Lactobacillus and Bifidobacterium, can restore intestinal homeostasis by improving intestinal microbiota disorders and repairing intestinal mucosal damage, and then treat skin damage phenotype, including abnormal skin cell proliferation and function, pigmentation, reduced collagen, elastic fibers, glycosaminoglycan (GAG), and structural disorders in the dermis by inhibiting oxidative stress, inflammation response, immune homeostasis, and extracellular matrix (ECM) remodeling inhibition, ultimately treating skin diseases (acne, atopic dermatitis, psoriasis, seborrheic dermatitis, wound healing, photoaging and aging skin, and rosacea).
5.2. Lactobacillus
Lactobacillus is the most abundant and diverse genus of lactic acid bacteria [94]. Lactobacillus strains have anti-inflammation activity on host keratinocytes and have specific irritation effects on the growth of Staphylococcus epidermidis in vitro [95]. Lactobacillus also suppresses substance P-caused skin inflammatory responses and promotes the restoration of skin barrier function [96]. A clinical experiment suggested that a six-week oral Lactobacillus johnsonii intervention regimen promoted the restoration of skin immune function in UV-caused immunosuppression [97]. In a randomized, double-blind, placebo-controlled experiment program with 20 adults, Fabroccini et al. found that a liquid intervention, including Lactobacillus rhamnosus, normalized skin expression of insulin signaling-related genes and improved adult acne [98]. The study showed that tyndalized Lactobacillus acidophilus, an oral probiotic, suppressed wrinkle formation resulting from UV irradiation in mice [99].
These beneficial influences were due to the reduction in MMPs. Furthermore, Park and Bae indicated in an in vitro study that combined the fermentation of Lactobacillus and Bifidobacterium to ferment A. koreanum extract inhibited the senescence phenotype of skin fibroblasts [100]. The senescence status is driven by UV- or hydrogen peroxide, while the protection of senescence is partially mediated by the inference of MMP-1 (Figure 5).
5.3. Bifidobacterium
Results showed that Bifidobacterium breve B-3, as an oral supplementation, obviously inhibited TEWL, skin dryness, changes in epidermal thickening, and improved injury to tight junction structures and basement membranes under excess UV irradiation in mice. Bifidobacterium breve B-3 supplementation also inhibited UV-caused production of IL-1β in the skin [101].
Bifidobacterium and Lactobacillus as lyophilized powders in capsules suppress atopic sensitization to general food allergens and decrease the prevalence of atopic eczema in early childhood [102]. In adult patients with atopic dermatitis, Bifidobacterium bifidum as an oral supplementation has antipruritic influences linked to up-regulated levels of antipruritic and analgesic metabolite acetonide [103]. In a double-blind, placebo-controlled, randomized trial, ingestion of fermented milk, including Bifidobacterium breve and galactooligosaccharides, inhibited reduced levels of stratum corneum hydration, inhibited increased histone l-like protease activity, and decreased serum and urinary phenol contents in healthy adult female volunteers [104].
6. Potential Mechanisms of Probiotic-Mediated Regulation of Skin Conditions by the Gut–Skin Axis
6.1. Oxidative Stress Level Decreases
The pathological physiology of skin photoaging is tightly related to ROS-caused injury, containing activation of MAPK and NF-κB loop, decrease in MMPs level, and collagen content, resulting in skin photoaging. A study suggested that the partial fermentation of Agastache rugosa-fermented extract (ARE-F) with a probiotic Lactobacillus promoted UV-caused concentrations of total glutathione and superoxide dismutase activity while down-regulating UV-caused ROS and MMPs levels in UV-treated human keratinocytes cells (HaCaT) keratinocytes [105]. A study indicated that partial Lactobacillus acidophilus IDCC 3302 defended against UV-caused photodamage in the skin epidermis by promoting the antioxidant capacity of the skin, hydrating cytokines, and inhibiting MMPs synthesis via suppression of MAPK loop [45].
Another study suggested that Lactobacillus acidophilus KCCM12625 had great antioxidant functions and obviously decreased up-regulated ROS contents in vitro after UV irradiation and improved photodamage induced by oxidative injury [22]. A study suggested that the oral supplementation of Bifidobacterium breve Yakult suppressed ROS content and improved UV-caused skin mechanical barrier injury and oxidative stress in vivo [106]. Research showed that a plant extract fermented with Lactobacillus buchneri improved the influence of oxidative injury in UV-caused skin photoaging in vitro by up-regulating the type I procollagen content, suppressing elastase synthesis, and up-regulating the level of UV-caused MMPs on HaCaT keratinocytes and dermal fibroblasts [107]. A study suggested that Limosilactobacillus fermentum XJC60 could enhance mitochondrial capabilities, decrease ROS content in UV-damaged skin cells, and, therefore, keep the skin status [108]. In addition, new research has indicated antioxidant effect as the primary pathway by which Lacticaseibacillus rhamnosus GG (ATCC 53103, LGG) [109] and Lacticaseibacillus casei strain Shirota improve skin photoaging [110].
6.2. Inflammatory Response Suppression
Up-regulated skin inflammation elements lead to the dysfunction of barrier function, TEWL, incremental permeability of the epidermis, and rapid skin photoaging. Research suggested that oral supplementation of Bifidobacterium breve B-3 was effective in decreasing UV-caused IL-1β content in the skin in UV-irradiated mice. As a result, TEWL, skin dryness, and epidermal thickening were inhibited [111,112]. Lactobacillus acidophilus IDCC3302, in addition to its antioxidant effects, suppressed MAPK signaling pathway-mediated production of pro-inflammatory factors and decreased UV-radiation-induced skin inflammation [45]. To improve skin photoaging, a study indicated that the oral application of Lactobacillus reuteri DSM 17938 showed an anti-inflammation effect which resisted UV-induced IL-6 and IL-8 [113].
Keshari et al. suggested that butyrate from a new production of probiotic Staphylococcus epidermidis could reduce UV-caused pro-inflammation factor IL-6 factors using SCFA receptors [114]. A study showed that oral oligosaccharides regulated UV-induced inflammatory immune responses and reduced TEWL and sunburn erythema, therefore inhibiting skin photoaging [115].
6.3. Immune Homeostasis Maintaining
Many specified probiotics, such as Lactobacillus paracasei, regulate immune response to inhibit pathogens [116]. In addition, it inhibits unwanted immunological effects to maintain immune homeostasis against chronic inflammation diseases. It may be due to the modulation of the Tregs number by probiotics. Treg exerts a vital effect on the immunosuppression caused by skin photoaging. Lactobacillus johnsonii suppressed UV-caused decrease in epidermal Langerhans cell density and promoted the restoration of skin immune homeostasis after UV-caused immunosuppression. Moreover, probiotics exert different effects under different immune statuses. Under a physiological status, probiotics decrease cytotoxic T cell attack on the skin, up-regulate induction of functional damage to CD8+ T cells, and cause the activation of quiescent dendritic cells and the activation and function of all regulatory T cell subsets. A study performed three clinical experiments to analyze the influences of dietary supplements (DS), including Lactobacillus johnsonii and nutritional carotenoids, on early UV-caused skin injury [117]. These results suggest that the ingestion of degenerative vertebral slippage has useful influences on the long-term and repetitive influences of UV exposure and is more specific to photoaging. Data showed that oral interventions, including Bifidobacterium longum and galacto-oligosaccharides, defend the skin from UV-caused photoaging, resulting in their anti-inflammation and anti-oxidative effects [118]. In addition, they up-regulated serum levels of SCFAs and acetates, which have been shown to up-regulate and activate histone acetylation-dependent skin resident treg.
6.4. ECM Remodeling Suppression
ROS contents were up-regulateed after UV exposure, leading to increased MMPs contents, skin collagen protein and elastin protein degradation, and rough, dry and sagging skin. Probiotics can not only directly decrease ROS content but also indirectly regulate MMP levels in the skin, decreasing collagen and elastin protein degradation following UV exposure [119]. Oral Lactobacillus acidophilus KCCM12625 could decrease the mRNA level of MMPs after skin photoaging by damaging the AP-1 loop of skin while up-regulating procollagen level and down-regulating collagen protein losses in the dermis [22]. A study indicated that oral Lactobacillus plantarum HY7714 decreased the overproduction of MMP-13 content and the activities of MMP-2 and MMP-9 in UV-induced cell injuries by suppressing the activation of the JNK/AP-1 loop [49]. Research suggested that oral Lactobacillus sakei could suppress theAP-1 expression by inhibiting the MAPK loop to up-regulate collagen in the dermis and improve skin photoaging [46]. Another study demonstrated that extracellular Lactobacilli exopolysaccharides (LEPS) can decrease the MMPs level and increase TIMPs [120]. Data showed that LEPS of B9-1 from Lactobacillus casei can strengthen the anti-collagenase and anti-elastase functions of the skin and efficiently decrease the breakdown of collagen after UV radiation. A study demonstrated that localized extracts originating Lactobacillus brucei fermented plants in kimchi can significantly suppress UV-induced elastase activity and expression of MMPs and enhance the synthesis of type I procollagen. Negari et al. showed that the metabolites from the topical probiotic Staphylococcus epidermidis of Cetearyl isononanoate (CIN) as a potential carbon source could restore damaged collagen and cause the synthesis of collagen through phosphorylated extracellular signal-regulated kinase (p-ERK) activation, thereby inhibiting skin photoaging [121].
7. Concluding Remarks
In recent years, significant advances have been made in understanding the composition of skin probiotics and how dysbiosis affects skin health. Topical and internal probiotics in the form of various dermatological formulations are an important part of the treatment of skin conditions. While topical probiotics’ functions and protective nature maintain the skin’s homeostasis, their shortcomings and limitations result in inflammatory skin conditions that are difficult to completely cure via topical probiotics. Several clinical trials are being carried out in order to study the efficacy as well as the adverse effects of internal probiotics formulations for the treatment of conditions such as atopic dermatitis, acne, psoriasis, wound healing, and many other skin problems. We hope that this review forms a contribution to promoting enhanced research activities in the field of internal probiotics as a novel therapeutic approach for the treatment of skin disorders.
Abbreviations
TEWL: transcutaneous water loss; MMP-1: matrix metalloproteinase-1; UV: ultraviolet; TYRP-1: tyrosinase-related protein 1; TYRP-2: tyrosinase-related protein 2; SPT: serine palmitoyltransferase; HA: hyaluronic acid; SLS: sodium dodecyl sulfate; SH: Sphingomonas hydrophobicum; sLTA: Lactobacillus sakei Lipoteichoic Acid; AL: Lactobacillus KCCM12625P; SCFAs: short-chain fatty acids; MUC: mucin-type glycoproteins; TJ: tight junction; GI: gastrointestinal; DCs: dendritic cells; AD: matitis; NGM1–3: neonatal intestinal microbiota; GAG: glycosaminoglycan; ECM: extracellular matrix; ARE-F: Agastache rugosa-fermented extract; HaCaT: human keratinocytes cells; DS: dietary supplements; LEPS: Lactobacilli exopolysaccharides; CIN: Cetearyl isononanoate; CFU: colony forming units; GALT: gut-associated lymphoid tissue.
Author Contributions
T.G.: Conceptualization, Methodology, Formal analysis, Writing—original draft, Visualization; Y.L.: Resources, Supervision, Project administration, Funding acquisition; X.W.: Methodology; F.R.: Conceptualization, Formal analysis, Validation, Writing—review and editing, Project administration, Funding acquisition, Supervision. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Funding Statement
This work was supported by the 111 project of the Education Ministry of China (No. B18053) and the National Natural Science Foundation (32130081).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sender R., Fuchs S., Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14:e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grice E.A., Segre J.A. The skin microbiome. Nat. Rev. Microbiol. 2011;9:244–253. doi: 10.1038/nrmicro2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh J., Byrd A.L., Park M., Kong H.H., Segre J.A. Temporal stability of the human skin microbiome. Cell. 2016;165:854–866. doi: 10.1016/j.cell.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schommer N.N., Gallo R.L. Structure and function of the human skin microbiome. Trends Microbiol. 2013;21:660–668. doi: 10.1016/j.tim.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dréno B., Alexis A., Chuberre B., Marinovich M. Safety of titanium dioxide nanoparticles in cosmetics. J. Eur. Acad. Dermatol. Venereol. 2019;33:34–46. doi: 10.1111/jdv.15943. [DOI] [PubMed] [Google Scholar]
- 6.Hu Y., Zeng H., Huang J., Jiang L., Chen J., Zeng Q. Traditional Asian aerbs in skin whitening: The current development and limitations. Front. Pharmacol. 2020;11:982. doi: 10.3389/fphar.2020.00982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu J., Ma X., Wang X., Cui X., Ding K., Wang S., Han C. Application and mechanism of probiotics in skin care: A review. J. Cosmet. Dermatol. 2022;21:886–894. doi: 10.1111/jocd.14734. [DOI] [PubMed] [Google Scholar]
- 8.Puebla-Barragan S., Reid G. Probiotics in cosmetic and personal care products: Trends and challenges. Molecules. 2021;26:1249. doi: 10.3390/molecules26051249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osborne D., Tan P., Varma Y., Carbol J. Formulating topical products containing live microorganisms as the active ingredient. Pharm. Technol. 2018;42:32–36. [Google Scholar]
- 10.Marto J., Ascenso A., Simoes S., Almeida A.J., Ribeiro H.M. Pickering emulsions: Challenges and opportunities in topical delivery. Expert. Opin. Drug Deliv. 2016;13:1093–1107. doi: 10.1080/17425247.2016.1182489. [DOI] [PubMed] [Google Scholar]
- 11.Sreeja V., Prajapati J.B. Probiotic formulations: Application and status as pharmaceuticals-a review. Probiotics Antimicrob. Proteins. 2013;5:81–91. doi: 10.1007/s12602-013-9126-2. [DOI] [PubMed] [Google Scholar]
- 12.Lee G.R., Maarouf M., Hendricks A.J., Lee D.E., Shi V.Y. Topical probiotics: The unknowns behind their rising popularity. Dermatol. Online J. 2019;25:15–21. doi: 10.5070/D3255044062. [DOI] [PubMed] [Google Scholar]
- 13.Pillaiyar T., Manickam M., Jung S.H. Recent development of signaling pathways inhibitors of melanogenesis. Cell. Signal. 2017;40:99–115. doi: 10.1016/j.cellsig.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Brenner M., Hearing V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008;84:539–549. doi: 10.1111/j.1751-1097.2007.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iozumi K., Hoganson G.E., Pennella R., Everett M.A., Fuller B.B. Role of tyrosinase as the determinant of pigmentation in cultured human melanocytes. J. Investig. Dermatol. 1993;100:806–811. doi: 10.1111/1523-1747.ep12476630. [DOI] [PubMed] [Google Scholar]
- 16.Li G., Ju H.K., Chang H.W., Jahng Y., Lee S.H., Son J.K. Melanin biosynthesis inhibitors from the bark of Machilus thunbergii. Biol. Pharm. Bull. 2003;26:1039–1041. doi: 10.1248/bpb.26.1039. [DOI] [PubMed] [Google Scholar]
- 17.Pillaiyar T., Manickam M., Jung S.H. Inhibitors of melanogenesis: A patent review (2009–2014) Expert. Opin. Ther. Pat. 2015;25:775–788. doi: 10.1517/13543776.2015.1039985. [DOI] [PubMed] [Google Scholar]
- 18.Schiaffino M.V. Signaling pathways in melanosome biogenesis and pathology. Int. J. Biochem. Cell Biol. 2010;42:1094–1104. doi: 10.1016/j.biocel.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pillaiyar T., Manickam M., Namasivayam V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017;32:403–425. doi: 10.1080/14756366.2016.1256882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang H.C., Chang T.M. Antioxidative properties and inhibitory effect of Bifidobacterium adolescentis on melanogenesis. World J. Microbiol. Biotechnol. 2012;28:2903–2912. doi: 10.1007/s11274-012-1096-0. [DOI] [PubMed] [Google Scholar]
- 21.Huang H.C., Lee I.J., Huang C., Chang T.M. Lactic acid bacteria and lactic acid for skin health and melanogenesis inhibition. Curr. Pharm. Biotechnol. 2020;21:566–577. doi: 10.2174/1389201021666200109104701. [DOI] [PubMed] [Google Scholar]
- 22.Lim H.Y., Jeong D., Park S.H., Shin K.K., Hong Y.H., Kim E., Yu Y.G., Kim T.R., Kim H., Lee J., et al. Antiwrinkle and antimelanogenesis effects of tyndallized Lactobacillus acidophilus KCCM12625P. Int. J. Mol. Sci. 2020;21:1620. doi: 10.3390/ijms21051620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaiprasongsuk A., Onkoksoong T., Pluemsamran T., Limsaengurai S., Panich U. Photoprotection by dietary phenolics against melanogenesis induced by UVA through Nrf2-dependent antioxidant responses. Redox Biol. 2016;8:79–90. doi: 10.1016/j.redox.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu W.S., Kuan Y.D., Chiu K.H., Wang W.K., Chang F.H., Liu C.H., Lee C.H. The extract of Rhodobacter sphaeroides inhibits melanogenesis through the MEK/ERK signaling pathway. Mar. Drugs. 2013;11:1899–1908. doi: 10.3390/md11061899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim H., Kim J.T., Barua S., Yoo S.Y., Hong S.C., Lee K.B., Lee J. Seeking better topical delivery technologies of moisturizing agents for enhanced skin moisturization. Expert. Opin. Drug Deliv. 2018;15:17–31. doi: 10.1080/17425247.2017.1306054. [DOI] [PubMed] [Google Scholar]
- 26.Harding C.R., Watkinson A., Rawlings A.V., Scott I.R. Dry skin, moisturization and corneodesmolysis. Int. J. Cosmet. Sci. 2000;22:21–52. doi: 10.1046/j.1467-2494.2000.00001.x. [DOI] [PubMed] [Google Scholar]
- 27.Ra J., Lee D.E., Kim S.H., Jeong J.W., Ku H.K., Kim T.Y., Choi I.D., Jeung W., Sim J.H., Ahn Y.T. Effect of oral administration of Lactobacillus plantarum HY7714 on epidermal hydration in ultraviolet B-irradiated hairless mice. J. Microbiol. Biotechnol. 2014;24:1736–1743. doi: 10.4014/jmb.1408.08023. [DOI] [PubMed] [Google Scholar]
- 28.Elias P.M., Menon G.K. Structural and lipid biochemical correlates of the epidermal permeability barrier. Adv. Lipid Res. 1991;24:1–26. doi: 10.1016/b978-0-12-024924-4.50005-5. [DOI] [PubMed] [Google Scholar]
- 29.Holleran W.M., Uchida Y., Halkier-Sorensen L., Haratake A., Hara M., Epstein J.H., Elias P.M. Structural and biochemical basis for the UVB-induced alterations in epidermal barrier function. Photodermatol. Photoimmunol. Photomed. 1997;13:117–128. doi: 10.1111/j.1600-0781.1997.tb00214.x. [DOI] [PubMed] [Google Scholar]
- 30.Im A.R., Lee B., Kang D.J., Chae S. Skin moisturizing and antiphotodamage effects of tyndallized Lactobacillus acidophilus IDCC 3302. J. Med. Food. 2018;21:1016–1023. doi: 10.1089/jmf.2017.4100. [DOI] [PubMed] [Google Scholar]
- 31.Papakonstantinou E., Roth M., Karakiulakis G. Hyaluronic acid: A key molecule in skin aging. Derm.-Endocrinol. 2012;4:253–258. doi: 10.4161/derm.21923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baba H., Masuyama A., Yoshimura C., Aoyama Y., Takano T., Ohki K. Oral intake of Lactobacillus helveticus-fermented milk whey decreased transepidermal water loss and prevented the onset of sodium dodecylsulfate-induced dermatitis in mice. Biosci. Biotechnol. Biochem. 2010;74:18–23. doi: 10.1271/bbb.90370. [DOI] [PubMed] [Google Scholar]
- 33.Segre J.A. Epidermal barrier formation and recovery in skin disorders. J. Clin. Investig. 2006;116:1150–1158. doi: 10.1172/JCI28521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung Y.O., Jeong H., Cho Y., Lee E.O., Jang H.W., Kim J., Nam K., Lim K.M. Lysates of a probiotic, Lactobacillus rhamnosus, can improve skin barrier function in a reconstructed human epidermis model. Int. J. Mol. Sci. 2019;20:4289. doi: 10.3390/ijms20174289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Draelos Z.D. New treatments for restoring impaired epidermal barrier permeability: Skin barrier repair creams. Clin. Dermatol. 2012;30:345–348. doi: 10.1016/j.clindermatol.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 36.Cho S. The role of functional foods in cutaneous anti-aging. J. Lifestyle Med. 2014;4:8–16. doi: 10.15280/jlm.2014.4.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trojahn C., Dobos G., Lichterfeld A., Blume-Peytavi U., Kottner J. Characterizing facial skin ageing in humans: Disentangling extrinsic from intrinsic biological phenomena. Biomed Res. Int. 2015;2015:318586. doi: 10.1155/2015/318586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mora Huertas A.C., Schmelzer C.E., Hoehenwarter W., Heyroth F., Heinz A. Molecular-level insights into aging processes of skin elastin. Biochimie. 2016;128–129:163–173. doi: 10.1016/j.biochi.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 39.Gervason S., Napoli M., Dreux-Zhiga A., Lazzarelli C., Garcier S., Briand A., Albouy M., Thepot A., Berthon J.Y., Filaire E. Attenuation of negative effects of senescence in human skin using an extract from Sphingomonas hydrophobicum: Development of new skin care solution. Int. J. Cosmet. Sci. 2019;41:391–397. doi: 10.1111/ics.12534. [DOI] [PubMed] [Google Scholar]
- 40.Dolan D.W., Zupanic A., Nelson G., Hall P., Miwa S., Kirkwood T.B., Shanley D.P. Integrated stochastic model of DNA damage repair by non-homologous end joining and p53/p21-mediated early senescence signalling. PLoS Comput. Biol. 2015;11:e1004246. doi: 10.1371/journal.pcbi.1004246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Penner A.S., Rock M.J., Kielty C.M., Shipley J.M. Microfibril-associated glycoprotein-2 interacts with fibrillin-1 and fibrillin-2 suggesting a role for MAGP-2 in elastic fiber assembly. J. Biol. Chem. 2002;277:35044–35049. doi: 10.1074/jbc.M206363200. [DOI] [PubMed] [Google Scholar]
- 42.Sheng W., Wang G., Wang Y., Liang J., Wen J., Zheng P.S., Wu Y., Lee V., Slingerland J., Dumont D., et al. The roles of versican V1 and V2 isoforms in cell proliferation and apoptosis. Mol. Biol. Cell. 2005;16:1330–1340. doi: 10.1091/mbc.e04-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fisher G.J., Kang S., Varani J., Bata-Csorgo Z., Wan Y., Datta S., Voorhees J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002;138:1462–1470. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- 44.Lee D.E., Huh C.S., Ra J., Choi I.D., Jeong J.W., Kim S.H., Ryu J.H., Seo Y.K., Koh J.S., Lee J.H., et al. Clinical evidence of effects of Lactobacillus plantarum HY7714 on skin aging: A randomized, double blind, placebo-controlled study. J. Microbiol. Biotechnol. 2015;25:2160–2168. doi: 10.4014/jmb.1509.09021. [DOI] [PubMed] [Google Scholar]
- 45.Im A.R., Lee B., Kang D.J., Chae S. Protective effects of tyndallized Lactobacillus acidophilus IDCC 3302 against UVB-induced photodamage to epidermal keratinocytes cells. Int. J. Mol. Med. 2019;43:2499–2506. doi: 10.3892/ijmm.2019.4161. [DOI] [PubMed] [Google Scholar]
- 46.You G.E., Jung B.J., Kim H.R., Kim H.G., Kim T.R., Chung D.K. Lactobacillus sakeilipoteichoic acid inhibits MMP-1 induced by UVA in normal dermal fibroblasts of human. J. Microbiol. Biotechnol. 2013;23:1357–1364. doi: 10.4014/jmb.1306.06026. [DOI] [PubMed] [Google Scholar]
- 47.Small R. Botulinum toxin injection for facial wrinkles. Am. Fam. Physician. 2014;90:168–175. [PubMed] [Google Scholar]
- 48.Park S.H., Lee K.H., Han C.S., Kim K.H., Kim Y.H. Inhibitory effects of carex humilis extract on elastase activity and matrix metalloproteinase-1 expression. J. Soc. Cosmet. Sci. Korea. 2010;36:129–136. [Google Scholar]
- 49.Kim H.M., Lee D.E., Park S.D., Kim Y.T., Kim Y.J., Jeong J.W., Jang S.S., Ahn Y.T., Sim J.H., Huh C.S., et al. Oral administration of Lactobacillus plantarum HY7714 protects hairless mouse against ultraviolet B induced photoaging. J. Microbiol. Biotechnol. 2014;24:1583–1591. doi: 10.4014/jmb.1406.06038. [DOI] [PubMed] [Google Scholar]
- 50.Szántó M., Dózsa A., Antal D., Szabó K., Kemény L., Bai P. Targeting the gut-skin axis-Probiotics as new tools for skin disorder management? Exp. Dermatol. 2019;28:1210–1218. doi: 10.1111/exd.14016. [DOI] [PubMed] [Google Scholar]
- 51.Bowe W.P., Filip J.C., DiRienzo J.M., Volgina A., Margolis D.J. Inhibition of propionibacterium acnes by bacteriocin-like inhibitory substances (BLIS) produced by Streptococcus salivarius. J. Drugs Dermatol. 2006;5:868–870. [PMC free article] [PubMed] [Google Scholar]
- 52.Bowe W.P., Logan A.C. Acne vulgaris, probiotics and the gut-brain-skin axis-back to the future? Gut Pathog. 2011;3:1. doi: 10.1186/1757-4749-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim B.J., Lee S.Y., Kim H.B., Lee E., Hong S.J. Environmental changes, microbiota, and allergic diseases. Allergy Asthma Immunol. Res. 2014;6:389–400. doi: 10.4168/aair.2014.6.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hidalgo-Cantabrana C., Gómez J., Delgado S., Requena-López S., Queiro-Silva R., Margolles A., Coto E., Sánchez B., Coto-Segura P. Gut microbiota dysbiosis in a cohort of patients with psoriasis. Br. J. Dermatol. 2019;181:1287–1295. doi: 10.1111/bjd.17931. [DOI] [PubMed] [Google Scholar]
- 55.Nam J.H., Yun Y., Kim H.S., Kim H.N., Jung H.J., Chang Y., Ryu S., Shin H., Kim H.L., Kim W.S. Rosacea and its association with enteral microbiota in Korean females. Exp. Dermatol. 2018;27:37–42. doi: 10.1111/exd.13398. [DOI] [PubMed] [Google Scholar]
- 56.De Pessemier B., Grine L., Debaere M., Maes A., Paetzold B., Callewaert C. Gut-skin axis: Current knowledge of the interrelationship between microbial dysbiosis and skin conditions. Microorganisms. 2021;9:353. doi: 10.3390/microorganisms9020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.D’Elios S., Trambusti I., Verduci E., Ferrante G., Rosati S., Marseglia G.L., Drago L., Peroni D.G. Probiotics in the prevention and treatment of atopic dermatitis. Pediatr. Allergy Immunol. 2020;31:43–45. doi: 10.1111/pai.13364. [DOI] [PubMed] [Google Scholar]
- 58.Habeebuddin M., Karnati R.K., Shiroorkar P.N., Nagaraja S., Asdaq S.M.B., Khalid Anwer M., Fattepur S. Topical probiotics: More than a skin deep. Pharmaceutics. 2022;14:557. doi: 10.3390/pharmaceutics14030557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Atabati H., Esmaeili S.A., Saburi E., Akhlaghi M., Raoofi A., Rezaei N., Momtazi-Borojeni A.A. Probiotics with ameliorating effects on the severity of skin inflammation in psoriasis: Evidence from experimental and clinical studies. J. Cell. Physiol. 2020;235:8925–8937. doi: 10.1002/jcp.29737. [DOI] [PubMed] [Google Scholar]
- 60.Yu Y., Dunaway S., Champer J., Kim J., Alikhan A. Changing our microbiome: Probiotics in dermatology. Br. J. Dermatol. 2020;182:39–46. doi: 10.1111/bjd.18659. [DOI] [PubMed] [Google Scholar]
- 61.França K. Topical probiotics in dermatological therapy and skincare: A concise review. Dermatol. Ther. 2021;11:71–77. doi: 10.1007/s13555-020-00476-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ohland C.L., Macnaughton W.K. Probiotic bacteria and intestinal epithelial barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;298:G807–G819. doi: 10.1152/ajpgi.00243.2009. [DOI] [PubMed] [Google Scholar]
- 63.Eslami M., Bahar A., Keikha M., Karbalaei M., Kobyliak N., Yousefi B. Probiotics function and modulation of the immune system in allergic diseases. Allergol. Immunopathol. 2020;48:771–788. doi: 10.1016/j.aller.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 64.Mantis N.J., Rol N., Corthésy B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal. Immunol. 2011;4:603–611. doi: 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yokoyama S., Hiramoto K., Koyama M., Ooi K. Impairment of skin barrier function via cholinergic signal transduction in a dextran sulphate sodium-induced colitis mouse model. Exp. Dermatol. 2015;24:779–784. doi: 10.1111/exd.12775. [DOI] [PubMed] [Google Scholar]
- 66.Salem I., Ramser A., Isham N., Ghannoum M.A. The gut microbiome as a major regulator of the gut-skin axis. Front. Microbiol. 2018;9:1459. doi: 10.3389/fmicb.2018.01459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lebeer S., Vanderleyden J., De Keersmaecker S.C. Host interactions of probiotic bacterial surface molecules: Comparison with commensals and pathogens. Nat. Rev. Microbiol. 2010;8:171–184. doi: 10.1038/nrmicro2297. [DOI] [PubMed] [Google Scholar]
- 68.Ueno N., Fujiya M., Segawa S., Nata T., Moriichi K., Tanabe H., Mizukami Y., Kobayashi N., Ito K., Kohgo Y. Heat-killed body of lactobacillus brevis SBC8803 ameliorates intestinal injury in a murine model of colitis by enhancing the intestinal barrier function. Inflamm. Bowel Dis. 2011;17:2235–2250. doi: 10.1002/ibd.21597. [DOI] [PubMed] [Google Scholar]
- 69.Smits H.H., Engering A., van der Kleij D., de Jong E.C., Schipper K., van Capel T.M., Zaat B.A., Yazdanbakhsh M., Wierenga E.A., van Kooyk Y., et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J. Allergy Clin. Immunol. 2005;115:1260–1267. doi: 10.1016/j.jaci.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 70.Lee M.J., Kang M.J., Lee S.Y., Lee E., Kim K., Won S., Suh D.I., Kim K.W., Sheen Y.H., Ahn K., et al. Perturbations of gut microbiome genes in infants with atopic dermatitis according to feeding type. J. Allergy Clin. Immunol. 2018;141:1310–1319. doi: 10.1016/j.jaci.2017.11.045. [DOI] [PubMed] [Google Scholar]
- 71.Kaikiri H., Miyamoto J., Kawakami T., Park S.B., Kitamura N., Kishino S., Yonejima Y., Hisa K., Watanabe J., Ogita T., et al. Supplemental feeding of a gut microbial metabolite of linoleic acid, 10-Hydroxy-Cis-12-Octadecenoic acid, alleviates spontaneous atopic dermatitis and modulates intestinal microbiota in NC/Nga mice. Int. J. Food Sci. Nutr. 2017;68:941–951. doi: 10.1080/09637486.2017.1318116. [DOI] [PubMed] [Google Scholar]
- 72.Johnson A.M.F., DePaolo R.W. Window-of-opportunity: Neonatal gut microbiota and atopy. Hepatobiliary Surg. Nutr. 2017;6:190–192. doi: 10.21037/hbsn.2017.03.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jin U.H., Lee S.O., Sridharan G., Lee K., Davidson L.A., Jayaraman A., Chapkin R.S., Alaniz R., Safe S. Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol. Pharmacol. 2014;85:777–788. doi: 10.1124/mol.113.091165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cryan J.F., Dinan T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 75.Zipperer A., Konnerth M.C., Laux C., Berscheid A., Janek D., Weidenmaier C., Burian M., Schilling N.A., Slavetinsky C., Marschal M., et al. Human commensals producing a novel antibiotic impair pathogen colonization. Nature. 2016;535:511–516. doi: 10.1038/nature18634. [DOI] [PubMed] [Google Scholar]
- 76.Di Marzio L., Cinque B., De Simone C., Cifone M.G. Effect of the lactic acid bacterium Streptococcus thermophilus on ceramide levels in human keratinocytes in vitro and stratum corneum in vivo. J. Investig. Dermatol. 1999;113:98–106. doi: 10.1046/j.1523-1747.1999.00633.x. [DOI] [PubMed] [Google Scholar]
- 77.Di Marzio L., Cinque B., Cupelli F., De Simone C., Cifone M.G., Giuliani M. Increase of skin-ceramide levels in aged subjects following a short-term topical application of bacterial sphingomyelinase from Streptococcus thermophilus. Int. J. Immunopathol. Pharmacol. 2008;21:137–143. doi: 10.1177/039463200802100115. [DOI] [PubMed] [Google Scholar]
- 78.Di Marzio L., Centi C., Cinque B., Masci S., Giuliani M., Arcieri A., Zicari L., De Simone C., Cifone M.G. Effect of the lactic acid bacterium Streptococcus thermophilus on stratum corneum ceramide levels and signs and symptoms of atopic dermatitis patients. Exp. Dermatol. 2003;12:615–620. doi: 10.1034/j.1600-0625.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 79.Lee Y.B., Byun E.J., Kim H.S. Potential role of the microbiome in acne: A comprehensive review. J. Clin. Med. 2019;8:987. doi: 10.3390/jcm8070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ambrożej D., Kunkiel K., Dumycz K., Feleszko W. The use of probiotics and bacteria-derived preparations in topical treatment of atopic dermatitis—A systematic review. J. Allergy Clin. Immunol. Pract. 2021;9:570–575. doi: 10.1016/j.jaip.2020.07.051. [DOI] [PubMed] [Google Scholar]
- 81.Park S.B., Im M., Lee Y., Lee J.H., Lim J., Park Y.H., Seo Y.J. Effect of emollients containing vegetable-derived lactobacillus in the treatment of atopic dermatitis symptoms: Split-body clinical trial. Ann. Dermatol. 2014;26:150–155. doi: 10.5021/ad.2014.26.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Myles I.A., Castillo C.R., Barbian K.D., Kanakabandi K., Virtaneva K., Fitzmeyer E., Paneru M., Otaizo-Carrasquero F., Myers T.G., Markowitz T.E., et al. Therapeutic responses to Roseomonas mucosa in atopic dermatitis may involve lipid-mediated TNF-related epithelial repair. Sci. Transl. Med. 2020;12:eaaz8631. doi: 10.1126/scitranslmed.aaz8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guéniche A., Hennino A., Goujon C., Dahel K., Bastien P., Martin R., Jourdain R., Breton L. Improvement of atopic dermatitis skin symptoms by Vitreoscilla filiformis bacterial extract. Eur. J. Dermatol. EJD. 2006;16:380–384. [PubMed] [Google Scholar]
- 84.Volz T., Skabytska Y., Guenova E., Chen K.M., Frick J.S., Kirschning C.J., Kaesler S., Röcken M., Biedermann T. Nonpathogenic bacteria alleviating atopic dermatitis inflammation induce IL-10-producing dendritic cells and regulatory Tr1 cells. J. Investig. Dermatol. 2014;134:96–104. doi: 10.1038/jid.2013.291. [DOI] [PubMed] [Google Scholar]
- 85.Reygagne P., Bastien P., Couavoux M.P., Philippe D., Renouf M., Castiel-Higounenc I., Gueniche A. The positive benefit of Lactobacillus paracasei NCC2461 ST11 in healthy volunteers with moderate to severe dandruff. Benef. Microbes. 2017;8:671–680. doi: 10.3920/BM2016.0144. [DOI] [PubMed] [Google Scholar]
- 86.Knackstedt R., Knackstedt T., Gatherwright J. The role of topical probiotics in skin conditions: A systematic review of animal and human studies and implications for future therapies. Exp. Dermatol. 2020;29:15–21. doi: 10.1111/exd.14032. [DOI] [PubMed] [Google Scholar]
- 87.Knackstedt R., Knackstedt T., Gatherwright J. The role of topical probiotics on wound healing: A review of animal and human studies. Int. Wound J. 2020;17:1687–1694. doi: 10.1111/iwj.13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fortuna M.C., Garelli V., Pranteda G., Romaniello F., Cardone M., Carlesimo M., Rossi A. A case of Scalp Rosacea treated with low dose doxycycline and probiotic therapy and literature review on therapeutic options. Dermatol. Ther. 2016;29:249–251. doi: 10.1111/dth.12355. [DOI] [PubMed] [Google Scholar]
- 89.Levitt E.L., Keen J.T., Wong B.J. Augmented reflex cutaneous vasodilatation following short-term dietary nitrate supplementation in humans. Exp. Physiol. 2015;100:708–718. doi: 10.1113/EP085061. [DOI] [PubMed] [Google Scholar]
- 90.Mishra M., Kumar A., Satsangi G.P., Bhatnagar A.K., Shrivastava J.N. Inhibitory effects of antibiotics from Nitrobacter spp. against Tinea capitis. Allelopath. J. 2007;19:535–542. [Google Scholar]
- 91.Shrivastava J.N., Shukla J.P., Kumar V. Antibacterial Potential of Nitrobacter species against Staphylococcus aureus. VEGETOS. 2011;24:26–28. [Google Scholar]
- 92.Opländer C., Suschek C.V. New aspects of nitrite homeostasis in human skin. J. Investig. Dermatol. 2009;129:820–822. doi: 10.1038/jid.2009.11. [DOI] [PubMed] [Google Scholar]
- 93.Opländer C., Suschek C.V. The role of photolabile dermal nitric oxide derivates in ultraviolet radiation (UVR)-induced cell death. Int. J. Mol. Sci. 2012;14:191–204. doi: 10.3390/ijms14010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sun Z., Harris H.M.B., McCann A., Guo C., Argimo´n S., Zhang W., Yang X., Jeffery I.B., Cooney J.C., Kagawa T.F., et al. Expanding the biotechnology potential of lactobacilli through comparative genomics of 213 strains and associated genera. Nat. Commun. 2015;6:8322. doi: 10.1038/ncomms9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Holz C., Benning J., Schaudt M., Heilmann A., Schultchen J., Goelling D., Lang C. Novel bioactive from Lactobacillus brevis DSM17250 to stimulate the growth of Staphylococcus epidermidis: A pilot study. Benef. Microbes. 2017;8:121–131. doi: 10.3920/BM2016.0073. [DOI] [PubMed] [Google Scholar]
- 96.Gueniche A., Benyacoub J., Philippe D., Bastien P., Kusy N., Breton L., Blum S., Castiel-Higounenc I. Lactobacillus paracasei CNCM I-2116 (ST11) inhibits substance P-induced skin inflammation and accelerates skin barrier function recovery in vitro. Eur. J. Dermatol. 2010;20:731–737. doi: 10.1684/ejd.2010.1108. [DOI] [PubMed] [Google Scholar]
- 97.Patra V., Byrne S.N., Wolf P. The skin microbiome: Is it affected by UV-induced immune suppression? Front. Microbiol. 2016;7:1235. doi: 10.3389/fmicb.2016.01235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fabbrocini G., Bertona M., Picazo O., Pareja-Galeano H., Emanuele E. Supplementation with Lactobacillus rhamnosus SP1 normalises skin expression of genes implicated in insulin signalling and improves adult acne. Benef. Microbes. 2016;7:625–630. doi: 10.3920/BM2016.0089. [DOI] [PubMed] [Google Scholar]
- 99.Im A.R., Kim H.S., Hyun J.W., Chae S. Potential for tyndalized Lactobacillus acidophilus as an effective component in moisturizing skin and anti-wrinkle products. Exp. Ther. Med. 2016;12:759–764. doi: 10.3892/etm.2016.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Park M.J., Bae Y.S. Fermented acanthopanax koreanum root extract reduces UVB- and H2O2-induced senescence in human skin fibroblast cells. J. Microbiol. Biotechnol. 2016;26:1224–1233. doi: 10.4014/jmb.1602.02049. [DOI] [PubMed] [Google Scholar]
- 101.Satoh T., Murata M., Iwabuchi N., Odamaki T., Wakabayashi H., Yamauchi K., Abe F., Xiao J.Z. Effect of Bifidobacterium breve B-3 on skin photoaging induced by chronic UV irradiation in mice. Benef. Microbes. 2015;6:497–504. doi: 10.3920/BM2014.0134. [DOI] [PubMed] [Google Scholar]
- 102.Allen S.J., Jordan S., Storey M., Thornton C.A., Gravenor M.B., Garaiova I., Plummer S.F., Wang D., Morgan G. Probiotics in the prevention of eczema: A randomised controlled trial. Arch. Dis. Child. 2014;99:1014–1019. doi: 10.1136/archdischild-2013-305799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Makarova K.S., Grishin N.V., Shabalina S.A., Wolf Y.I., Koonin E.V. A putative RNA-interference-based immune system in prokaryotes: Computational analysis of the predicted enzymatic machinery, functional analogies with eukaryotic RNAi, and hypothetical mechanisms of action. Biol. Direct Mar. 2006;16:7. doi: 10.1186/1745-6150-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kano M., Masauoka N., Kaga C., Sugimoto S., Iizuka R., Manabe K., Sone T., Oeda K., Nonaka C., Miazaki K., et al. Consecutive intake of fermented milk containing Bifidobacterium breve strain yakult and galacto-oligosaccharides benefits skin condition in healthy adult women. Biosci. Microbiota Food Health. 2013;32:33–39. doi: 10.12938/bmfh.32.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shin D., Lee Y., Huang Y.H., Lim H.W., Jang K., Kim D.D., Lim C.J. Probiotic fermentation augments the skin anti-photoaging properties of Agastache rugosa through up-regulating antioxidant components in UV-B-irradiated HaCaT keratinocytes. BMC Complement. Altern. Med. 2018;18:196. doi: 10.1186/s12906-018-2194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ishii Y., Sugimoto S., Izawa N., Sone T., Chiba K., Miyazaki K. Oral administration of Bifidobacterium breve attenuates UV-induced barrier perturbation and oxidative stress in hairless mice skin. Arch. Dermatol. Res. 2014;306:467–473. doi: 10.1007/s00403-014-1441-2. [DOI] [PubMed] [Google Scholar]
- 107.Kang Y.M., Hong C.H., Kang S.H., Seo D.S., Kim S.O., Lee H.Y., Sim H.J., An H.J. Anti-photoaging effect of plant extract fermented with lactobacillus buchneri on CCD-986sk fibroblasts and HaCaT keratinocytes. J. Funct. Biomater. 2020;11:3. doi: 10.3390/jfb11010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen H., Li Y., Xie X., Chen M., Xue L., Wang J., Ye Q., Wu S., Yang R., Zhao H., et al. Exploration of the molecular mechanisms underlying the anti-photoaging effect of limosilactobacillus fermentum XJC60. Front. Cell. Infect. Microbiol. 2022;12:838060. doi: 10.3389/fcimb.2022.838060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yau Y.F., El-Nezami H., Galano J.M., Kundi Z.M., Durand T., Lee J.C. Lactobacillus rhamnosus GG and oat beta-glucan regulated fatty acid profiles along the gut-liver-brain axis of mice fed with high fat diet and demonstrated antioxidant and anti-inflammatory potentials. Mol. Nutr. Food Res. 2020;64:e2000566. doi: 10.1002/mnfr.202000566. [DOI] [PubMed] [Google Scholar]
- 110.Mai C., Qiu L., Zeng Y., Tan X. Lactobacillus casei strain shirota enhances the ability of geniposide to activate SIRT1 and decrease inflammation and oxidative stress in septic mice. Front. Physiol. 2021;12:678838. doi: 10.3389/fphys.2021.678838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ansel J.C., Luger T.A., Green I. The effect of in vitro and in vivo UV irradiation on the production of ETAF activity by human and murine keratinocytes. J. Investig. Dermatol. 1983;81:519–523. doi: 10.1111/1523-1747.ep12522862. [DOI] [PubMed] [Google Scholar]
- 112.Kupper T.S., Groves R.W. The interleukin-1 axis and cutaneous inflammation. J. Investig. Dermatol. 1995;105:62s–66s. doi: 10.1038/jid.1995.13. [DOI] [PubMed] [Google Scholar]
- 113.Khmaladze I., Butler É., Fabre S., Gillbro J.M. Lactobacillus reuteri DSM 17938-A comparative study on the effect of probiotics and lysates on human skin. Exp. Dermatol. 2019;28:822–828. doi: 10.1111/exd.13950. [DOI] [PubMed] [Google Scholar]
- 114.Keshari S., Balasubramaniam A., Myagmardoloonjin B., Herr D.R., Negari I.P., Huang C.M. Butyric acid from probiotic Staphylococcus epidermidis in the skin microbiome down-regulates the ultraviolet-induced pro-inflammatory IL-6 cytokine via short-chain fatty acid receptor. Int. J. Mol. Sci. 2019;20:4477. doi: 10.3390/ijms20184477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hong K.B., Jeong M., Han K.S., Hwan Kim J., Park Y., Suh H.J. Photoprotective effects of galacto-oligosaccharide and/or Bifidobacterium longum supplementation against skin damage induced by ultraviolet irradiation in hairless mice. Int. J. Food Sci. Nutr. 2015;66:923–930. doi: 10.3109/09637486.2015.1088823. [DOI] [PubMed] [Google Scholar]
- 116.Kober M.M., Bowe W.P. The effect of probiotics on immune regulation, acne, and photoaging. Int. J. Womens Dermatol. 2015;1:85–89. doi: 10.1016/j.ijwd.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Goodarzi A., Mozafarpoor S., Bodaghabadi M., Mohamadi M. The potential of probiotics for treating acne vulgaris: A review of literature on acne and microbiota. Dermatol. Ther. 2020;33:e13279. doi: 10.1111/dth.13279. [DOI] [PubMed] [Google Scholar]
- 118.Kim D., Lee K.R., Kim N.R., Park S.J., Lee M., Kim O.K. Combination of bifidobacterium longum and galacto-oligosaccharide protects the skin from photoaging. J. Med. Food. 2021;24:606–616. doi: 10.1089/jmf.2021.K.0032. [DOI] [PubMed] [Google Scholar]
- 119.Lavker R.M., Zheng P., Dong G. Morphology of aged skin. Clin. Geriatr. Med. 1989;5:53–67. doi: 10.1016/S0749-0690(18)30695-5. [DOI] [PubMed] [Google Scholar]
- 120.Shirzad M., Hamedi J., Motevaseli E., Modarressi M.H. Anti-elastase and anti-collagenase potential of Lactobacilli exopolysaccharides on human fibroblast. Artif. Cells Nanomed. Biotechnol. 2018;46:1051–1061. doi: 10.1080/21691401.2018.1443274. [DOI] [PubMed] [Google Scholar]
- 121.Negari I.P., Keshari S., Huang C.M. Probiotic activity of Staphylococcus epidermidis induces collagen type i production through FFaR2/p-ERK signaling. Int. J. Mol. Sci. 2021;22:1414. doi: 10.3390/ijms22031414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.