Abstract
Background
Atezolizumab plus bevacizumab (Atezo/Bev) is first line-treatment for unresectable hepatocellular carcinoma (HCC). Body mass index (BMI) has demonstrated predictive value for response to immunotherapy in non-HCC cancer types. Our study investigated the effect of BMI on safety and efficacy of real-life use of Atezo/Bev for unresectable HCC.
Methods
191 consecutive patients from seven centres receiving Atezo/Bev were included in the retrospective study. Overall survival (OS), progression-free survival (PFS), overall response rate (ORR) and disease control rate (DCR) defined by RECIST v1.1 were measured in overweight (BMI ≥ 25) and non-overweight (BMI < 25) patients. Treatment-related adverse events (trAEs) were evaluated.
Results
Patients in the overweight cohort (n = 94) had higher rates of non-alcoholic fatty liver disease (NAFLD) and lower rates of Hepatitis B compared to non-overweight cohort (n = 97). Baseline Child–Pugh class and Barcelona Clinic Liver Cancer stage were similar between cohorts, with lower rates of extrahepatic spread in the overweight group. Overweight patients had similar OS compared to non-overweight (median OS 15.1 vs. 14.9 months; p = 0.99). BMI did not influence median PFS (7.1 vs. 6.1 months; p = 0.42), ORR (27.2% vs. 22.0%; p = 0.44) and DCR (74.1% vs. 71.9%; p = 0.46). There were higher rates of atezolizumab-related fatigue (22.3% vs. 10.3%; p = 0.02) and bevacizumab-related thrombosis (8.5% vs. 2.1%; p = 0.045) in the overweight patients, but overall trAEs and treatment discontinuation were comparable between cohorts.
Conclusion
Atezo/Bev has comparable efficacy in overweight HCC patients, with an increase in treatment-related fatigue and thrombosis. Combination therapy is safe and efficacious to use in overweight patients, including those with underlying NAFLD.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12072-023-10491-3.
Keywords: Immunotherapy, Anti-programmed death-ligand, Anti-vascular endothelial growth factor, Checkpoint inhibitor, Obesity, Cirrhosis, Overall survival, Progression-free survival, Overweight, Non-alcoholic fatty liver disease
Background
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide, and the fourth leading cause of cancer-related mortality [1]. The mainstay of advanced, unresectable HCC has been systemic therapy; previously sorafenib [2] and most recently lenvatinib [3]. However, the advent of immunotherapy has transformed the treatment landscape of advanced unresectable HCC [4]. Atezolizumab, an anti-programmed death-ligand (PD-L1) monoclonal antibody, and bevacizumab, an anti-vascular endothelial growth factor (VEGF) monoclonal antibody, have been used in combination for unresectable HCC. The IMbrave150 study demonstrated atezolizumab and bevacizumab (Atezo/Bev) combination therapy superiority over sorafenib for overall survival (OS) and progression-free survival (PFS) [5, 6]. Combination therapy extended median OS to 19.2 vs 13.4 months (hazard ratio (HR) 0.66, 95% confidence interval (CI) 0.52–0.85) and median PFS to 6.9 vs 4.3 months (HR 0.65, 95% CI 0.53–0.81) compared with sorafenib. In view of these findings Atezo/Bev now represents a first-line treatment option, along with single tremelimumab regular interval durvalumab (STRIDE) [7], for unresectable HCC [8].
Elevated body mass index (BMI) is an established risk factor for development HCC [9, 10]. However, the role of BMI in predicting HCC survival is less clear. Retrospective studies have varied in demonstrating increased BMI (overweight or obese defined as BMI ≥ 25) is associated with reduced [11–14], increased [15] or no change [16, 17] in OS in HCC patients undergoing treatments including surgical resection, trans-arterial chemoembolisation (TACE) and systemic therapy.
The effect of BMI on immunotherapy has been evaluated in multiple cancer sites. Overweight/obese patients receiving immune checkpoint inhibition have favourable OS and PFS across multiple cancer sites [18, 19] including melanoma [20], renal cell carcinoma [18, 19] and non-small cell lung cancer [21] (NSCLC); the largest study being in 1434 patients with NSCLC demonstrating a survival benefit of atezolizumab in overweight/obese individuals [21]. The effect of BMI on bevacizumab response has similarly been evaluated in multiple cancer types, with positive [22], negative [23] and no associations [24] seen across multiple cancer sites. The association of BMI with immunotherapy in HCC is less well studied, with a single study demonstrating that BMI ≥ 25 is associated with improved OS in patients receiving PD-1 antibody-based regimens (17.5 vs. 5.0 months; p = 0.034). No difference in PFS was observed (2.7 vs. 2.9 months; p = 0.74) [25]. In this study, over 70% of patients had previous systemic treatment, and the effects of BMI on treatment-naïve patients receiving combination immunotherapy is not known.
To-date no study has evaluated the association of BMI on efficacy and safety outcomes of atezolizumab plus bevacizumab used for the first-line treatment of HCC in routine clinical practice. We conducted a retrospective analysis of patients receiving atezolizumab plus bevacizumab for unresectable HCC across seven tertiary centres, evaluating the effect of BMI on efficacy and safety.
Methods and materials
Study participants and design
Patients previously undergoing systemic therapy, including oral multikinase inhibitors and immune checkpoint inhibitors, were excluded from the study. Consecutive patients with unresectable HCC receiving atezolizumab plus bevacizumab across eight tertiary centres in Germany (n = 30), Japan (n = 51), Austria (n = 12), United Kingdom (n = 15), Italy (n = 12), Taiwan (n = 11) and United States of America (n = 60) were recruited in the study. Inclusion criteria included the following : age at least 18 years old; histological or radiological diagnosis of HCC in accordance with the American Association for the Study of Liver Diseases (AASLD) criteria [26]; diagnosis of advanced disease as per Barcelona Clinic Liver Cancer (BCLC) criteria [27]—BCLC C or BCLC B not amenable to locoregional therapy.
Treatment protocol
Combination atezolizumab plus bevacizumab were administered according to the IMbrave150 protocol [5]: atezolizumab 1200 mg and bevacizumab 15 mg/kg intravenously every 3 weeks. Toxicity management and dose modifications were managed by local institutions as per summary of product characteristics (SmPC). Treatment was continued until disease progression or unacceptable toxicity as per local multidisciplinary assessment.
Patient outcomes
Patients’ baseline demographics were collected retrospectively, and clinical outcomes were prospectively maintained at each participating site. Radiological response following atezolizumab plus bevacizumab therapy was assessed as per RECIST criteria v1.1 [28] on CT or MRI performed at 9–12 week intervals. Overall response rate was defined as all patients having complete response (CR) or partial response (PR). Disease control rate included all patients with CR, PR and stable disease (SD).Treatment-related adverse effects (trAEs) were assessed at every point of patient contact and graded as per the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v. 5.0 [29]. Atezolizumab-related and bevacizumab-related adverse events were defined by the treating physician at each treatment centre as per the SmPC for each drug.
Statistical analysis
For BMI analysis, patients were divided into two cohorts. BMI was defined as height (in metres) divided by weight squared (in kilograms). Patients were divided into those with a BMI of 25 or greater (overweight) and those with a BMI less than 25 (non-overweight). Baseline characteristics were compared within the divided BMI (overweight vs. non-overweight). χ2 test was used to compare categorical data, and unpaired student t test for continuous data. Treatment-related adverse events and ORR/DCR were compared between BMI cohorts using χ2 test. The distribution of BMI with patient characteristics were determined using Pearson’s correlation coefficient for continuous variables and unpaired student t tests for categorical variables.
Time-to-event analysis was performed using Kaplan–Meier method. OS was defined as the time in months from the date of first administration of atezolizumab plus bevacizumab to date of death or last follow-up. PFS was defined as time in months from the date of first treatment to date of death or date of progression on radiological imaging. OS and PFS were compared between overweight and non-overweight cohorts using log-rank. A p value of less than 0.05 was defined as statistically significant. Univariate and multivariate Cox regression models for overweight/non-overweight cohorts and established prognostic factors were conducted for OS and PFS. BCLC stage (C vs. A or B), Child-Turcotte-Pugh class (B vs. A), tumour size (7 cm vs. ≤ 7 cm), macrovascular invasion (MVI), metastatic disease and alpha-fetoprotein (AFP) (> 400 ng/dL vs. ≤ 400 ng/dL) are all prognostic factors previously shown to correlate with HCC survival [30] and were included in Cox regression models. We conducted further analysis assessing the impact of different BMI classes on patient survival. BMI classes were defined as underweight (BMI < 18.5), normal (18.5 ≤ BMI < 25), overweight (25 ≤ BMI < 30) and obese (BMI ≥ 30). Kaplan–Meier analysis and Cox regression models were performed for the different BMI classes.
Results
Baseline characteristics
191 patients received atezolizumab plus bevacizumab consecutively across eight tertiary referral centres. The baseline characteristics of patients are shown in Table 1. Viral hepatitis was the leading cause of chronic liver disease, with 23.0% of patients with Child-Turcotte-Pugh class B. Prior to immunotherapy, 60.1% of patients were BCLC stage C and 37.7% of patients had extra-hepatic disease. Correlation of BMI with patient characteristics is shown in Supplementary Fig. 1. Mean BMI was significantly elevated in patients with underlying non-alcoholic fatty liver disease (NAFLD) (28.7 vs. 25.3; p = 0.001). Patients with viral hepatitis had a lower BMI (24.9 vs. 26.7; p = 0.01). Ninety-four patients (49.2%) had a BMI of 25 or greater. Average BMI was 29.6 ± 3.4 vs. 21.9 ± 2.2 (p < 0.0001) in the overweight group compared to the non-overweight group. In the overweight group 43 (45.7%) patients were obese. A higher proportion of NAFLD was present in the overweight patients (19.2% vs. 7.2%; p = 0.01), with lower rates of chronic hepatitis B seen (12.8% vs. 25.8%; p = 0.02). There was a lower rate of extrahepatic disease in the overweight cohort (29.8% vs. 45.4%; p = 0.03) and a higher rate of macrovascular invasion in the overweight group compared to the non-overweight group (4.3% vs. 5.4%; p = 0.05). A lower proportion of overweight patients had a previous resection (14.9% vs. 30.9%; p = 0.02), with other previous treatments comparable between the two groups.
Table 1.
Baseline characteristics of study population stratified by BMI
| All patients (n = 191) | BMI < 25 (n = 97) | BMI 25 + (n = 94) | p value | |
|---|---|---|---|---|
| Centre | ||||
| Germany | 30 (15.7) | 12 (12.4) | 18 (19.2) | 0.06 |
| Austria | 121 (6.3) | 3 (3.1) | 9 (9.6) | |
| United Kingdom | 15 (7.9) | 6 (6.2) | 9 (9.6) | |
| Italy | 12 (6.3) | 6 (6.2) | 6 (6.4) | |
| United States of America | 60 (31.4) | 29 (29.9) | 31 (33.0) | |
| Japan | 51 (26.7) | 35 (36.1) | 16 (17.0) | |
| Taiwan | 11 (5.8) | 6 (6.2) | 5 (5.3) | |
| Median Age (IQR) | 68.4 (61.8–75.2) | 68.2 (60.4–75.2) | 69.3 (62.2–75.0) | 0.76 |
| Male Sex | 161 (84.3) | 83 (85.6) | 78 (83.0) | 0.78 |
| Risk factors for chronic liver disease | ||||
| Non-alcoholic fatty liver disease | 25 (13.1) | 7 (7.2) | 18 (19.2) | 0.01 |
| Alcohol related | 73 (38.2) | 36 (37.1) | 37 (39.4) | 0.75 |
| Hepatitis B infection | 37 (19.4) | 25 (25.8) | 12 (12.8) | 0.02 |
| Hepatitis C infection | 72 (37.7) | 40 (41.2) | 32 (34.0) | 0.31 |
| Other | 12 (8.6) | 6 (9.7) | 6 (7.7) | 0.68 |
| Child-Turcotte-Pugh class | ||||
| A | 147 (77.0) | 77 (79.4) | 70 (74.5) | 0.42 |
| B | 44 (23.0) | 20 (20.6) | 24 (25.5) | |
| Varices present | 39 (20.4) | 15 (15.5) | 24 (25.5) | 0.08 |
| Maximum tumor diameter (cm) | 6.8 (4.9) | 6.8 (5.4) | 6.9 (4.3) | 0.92 |
| Macrovascular invasion (MVI) | 78 (40.8) | 33 (34.0) | 45 (47.9) | 0.05 |
| AFP (ng/dL) | ||||
| ≤ 400 | 126 (66.0) | 68 (66.0) | 58 (61.7) | 0.22 |
| > 400 | 65 (34.0) | 33 (34.0) | 36 (38.3) | |
| Extrahepatic spread (EHS) | 72 (37.7) | 44 (45.4) | 28 (29.8) | 0.03 |
| ECOG-PS | ||||
| 0 | 119 (63.0) | 64 (66.7) | 55 (59.1) | 0.09 |
| 1 | 64 (33.9) | 27 (28.1) | 37 (39.8) | |
| 2 | 6 (3.2) | 5 (5.2) | 1 (1.1) | |
| Barcelona clinic liver cancer stage | ||||
| A | 7 (3.7) | 4 (4.2) | 3 (3.3) | 0.24 |
| B | 68 (36.2) | 40 (41.7) | 28 (30.4) | |
| C | 113 (60.1) | 52 (54.2) | 61 (66.3) | |
| Previous locoregional treatment | ||||
| Resection | 44 (23.0) | 30 (30.9) | 14 (14.9) | 0.02 |
| Radiofrequency ablation | 38 (19.9) | 20 (20.6) | 18 (19.2) | 0.80 |
| Transarterial chemoembolization | 57 (29.8) | 31 (32.0) | 26 (27.7) | 0.52 |
| Y90 | 21 (11.0) | 7 (7.2) | 14 (14.9) | 0.09 |
| External Beam Radiotherapy | 6 (3.1) | 4 (4.1) | 2 (2.1) | 0.43 |
n (%) for discrete variables; mean ± standard deviation for continuous variables
AFP alpha-fetoprotein, ECOG-PS Eastern cooperative oncology group performance status
Efficacy
Median overall survival for the entire cohort was 14.9 months (95% CI 13.6–23.9 months). Seventy-four patients died during the study observation period. Median progression-free survival was 6.7 months (95% CI 4.9–7.9 months). Overweight patients had a similar survival compared to non-overweight patients (median OS: 15.1 vs. 14.9 months; p = 0.99) (Fig. 1). Similarly, median PFS was comparable between the overweight and non-overweight cohorts (median PFS: 7.1 vs. 6.1 months; p = 0.42) (Fig. 2). Overweight BMI did not impact overall survival in univariate (HR 1.00, 95% CI 0.60–1.64); p = 0.98) and multivariate (HR 0.72, 95% CI 0.42–1.23); p = 0.23) analysis (Table 2). Similarly, no effect was observed for progression-free survival (HR 0.70, 95% CI 0.41–1.20; p = 0.20) (Table 3).
Fig. 1.
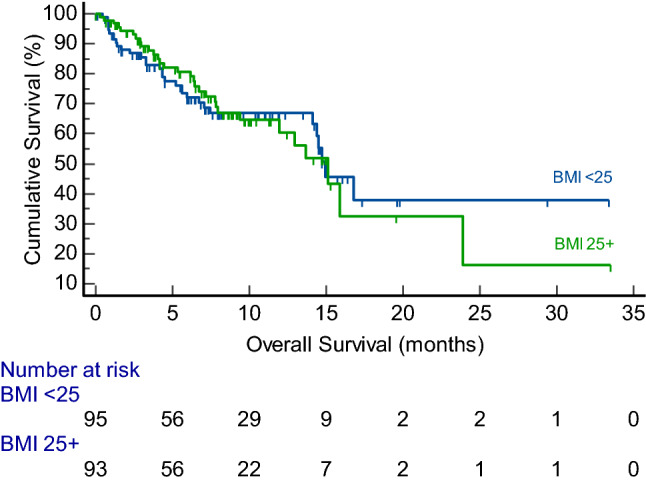
Kaplan–Meier curve showing overall survival (months) for overweight (BMI ≥ 25) and non-overweight (BMI < 25) patients with unresectable hepatocellular carcinoma patients after atezolizumab plus bevacizumab administration
Fig. 2.
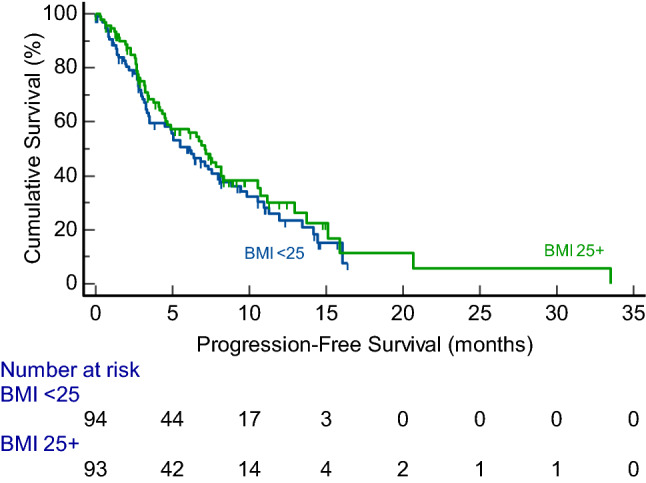
Kaplan–Meier curve showing progression-free survival (months) for overweight (BMI ≥ 25) and non-overweight (BMI < 25) patients with unresectable hepatocellular carcinoma patients after atezolizumab plus bevacizumab administration
Table 2.
Effects of BMI and prognostic factors on overall survival after atezolizumab and bevacizumab in univariate and multivariate Cox regression models
| Univariate models | Multivariable models | |||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value | |
| BMI 25+ | 1.00 (0.60–1.64) | 0.98 | 0.72 (0.42–1.23) | 0.23 |
| BCLC stage (C vs A or B) | 1.50 (0.89–2.52) | 0.13 | 1.06 (0.57–1.97) | 0.85 |
| CTP class (B vs A) | 3.01 (1.77–5.13) | < 0.001 | 2.44 (1.35–4.42) | 0.003 |
| Tumour size > 7 cm | 1.30 (0.77–2.20) | 0.32 | 1.04 (0.61–1.78) | 0.89 |
| MVI | 2.51 (1.15–4.18) | < 0.001 | 1.87 (0.99–3.55) | 0.05 |
| Metastatic disease | 0.80 (0.47–1.36) | 0.41 | 0.88 (0.49–1.57) | 0.66 |
| AFP > 400 ng/dL | 1.32 (0.79–2.19) | 0.29 | 1.21 (0.71–2.05) | 0.49 |
95% CI 95% confidence interval, BCLC Barcelona clinic liver cancer, CTP Child-Turcotte-Pugh, MVI Macrovascular invasion, AFP alpha-fetoprotein
Table 3.
Effects of BMI and prognostic factors on progression-free survival after atezolizumab and bevacizumab in univariate and multivariate Cox regression models
| Univariate models | Multivariable models | |||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value | |
| BMI 25 + | 0.90 (0.55–1.49) | 0.69 | 0.70 (0.41–1.20) | 0.20 |
| BCLC stage (C vs A or B) | 1.56 (0.92–2.64) | 0.10 | 1.03 (0.54–1.94) | 0.94 |
| CTP class (B vs A) | 2.29 (1.35–3.87) | 0.002 | 1.88 (1.04–3.40) | 0.04 |
| Tumour size > 7 cm | 1.28 (0.76–2.15) | 0.36 | 1.04 (0.61–1.79) | 0.88 |
| MVI | 2.30 (1.38–3.82) | 0.001 | 1.92 (0.99–3.73) | 0.06 |
| Metastatic disease | 1.03 (0.60–1.75) | 0.92 | 1.11 (0.62–1.98) | 0.74 |
| AFP > 400 ng/dL | 1.35 (0.81–2.26) | 0.24 | 1.19 (0.69–2.04) | 0.53 |
95% CI 95% confidence interval, BCLC Barcelona clinic liver cancer, CTP Child-Turcotte-Pugh, MVI Macrovascular invasion, AFP alpha-fetoprotein
Treatment response using the RECIST criteria was available in 163 patients. ORR and DCR for the entire cohort were 24.5% and 73.0%, respectively (Supplementary Table 1). Progressive disease was present in 27.0% of patients. Overweight patients had similar ORR (27.2% vs. 22.0%; p = 0.44) and DCR (74.1% vs. 71.9%; p = 0.76) compared to non-overweight patients.
We further assessed the impact of different BMI classes on survival after therapy. Baseline characteristics were comparable between the classes (Supplementary Table 2). There was no difference in median OS (underweight 11.4 vs. normal 18.8 vs. overweight 19.2 vs. obese 11.5; p = 0.67) and PFS (underweight 7.4 vs. normal 7.4 vs. overweight 9.9 vs. obese 8.7; p = 0.83) between BMI classes (Supplementary Figs. 2 and 3). There was no impact of BMI class on survival in univariate and multivariate Cox regression models (Supplementary Tables 3 and 4).
The difference in rates of MVI and extrahepatic spread observed in the overweight and non-overweight groups may impact survival outcomes. We conducted subgroup analysis assessing survival in overweight and non-overweight patients without MVI or extrahepatic spread. Overweight and non-overweight patients had comparable OS (median OS 23.8 vs. 14.9 months; p = 0.26) and PFS (median PFS 20.7 vs. 14.2 months; p = 0.27). Similarly, overweight BMI did not impact OS or PFS in patients with MVI and/or extrahepatic spread.
Safety
In total, 127 patients experienced treatment-related adverse events. Similar number of patients experienced atezolizumab-related (43.5%) and bevacizumab-related (43.5%) adverse events (Table 4). Thirty-nine patients (20.4%) experience at least one grade 3 or greater adverse event. Twelve patients (6.3%) discontinued combination therapy due to treatment-related adverse events. Atezolizumab-related fatigue was higher in the overweight group compared to the non-overweight group (22.3% vs. 10.3%; p = 0.02). Atezolizumab-related thyroid dysfunction was lower in the overweight group compared to the non-overweight group (1.1% vs. 8.3%; p = 0.02). Bevacizumab-related thrombosis was higher in overweight patients (8.5% vs. 2.1%; p = 0.045). However, trAEs requiring drug discontinuation were similar between the two cohorts (7.4% vs. 5.2%; p = 0.51). When reviewing BMI class, atezolizumab-related thyroid dysfunction was significantly higher in underweight (20.0%) and normal BMI (6.9%) patients compared to overweight and obese patients (Supplementary Table 5). Rates of bevacizumab-related thrombosis were significantly higher in obese patients (14.0%).
Table 4.
Atezolizumab and bevacizumab treatment-related adverse events stratified by BMI
| All patients (n = 191) | BMI < 25 (n = 97) | BMI 25+ (n = 94) | p value | |
|---|---|---|---|---|
| Any grade trAEs | 127 (66.5) | 62 (63.9) | 65 (69.2) | 0.44 |
| Grade ≥ 3a trAEs | 39 (20.4) | 21 (21.7) | 18 (19.2) | 0.67 |
| Atezolizumab-related | 15 (7.9) | 11 (11.3) | 4 (4.3) | 0.07 |
| Bevacizumab-related | 26 (13.6) | 12 (12.4) | 14 (14.9) | 0.61 |
| trAEs requiring drug discontinuation | 12 (6.3) | 5 (5.2) | 7 (7.4) | 0.51 |
| Atezolizumab trAEs | ||||
| Overall | 83 (43.5) | 42 (43.3) | 41 (43.6) | 0.96 |
| Fatigue | 31 (16.2) | 10 (10.3) | 21 (22.3) | 0.02 |
| Hepatotoxicity | 28 (14.7) | 16 (16.5) | 12 (12.8) | 0.47 |
| Skin toxicity | 9 (4.7) | 3 (3.1) | 6 (6.4) | 0.28 |
| Colitis | 24 (12.6) | 12 (12.4) | 12 (12.8) | 0.93 |
| Thyroid dysfunction | 9 (4.7) | 8 (8.3) | 1 (1.1) | 0.02 |
| Pneumonitis | 4 (2.1) | 1 (1.0) | 3 (3.2) | 0.30 |
| Bevacizumab trAEs | ||||
| Overall | 83 (43.5) | 40 (41.2) | 43 (45.7) | 0.53 |
| Bleeding | 20 (10.5) | 10 (10.3) | 10 (10.6) | 0.94 |
| Hypertension | 44 (23.0) | 23 (23.7) | 21 (22.3) | 0.82 |
| Proteinuria | 38 (19.9) | 23 (23.7) | 15 (16.0) | 0.18 |
| Thrombosis | 10 (5.2) | 2 (2.1) | 8 (8.5) | 0.045 |
agraded as per the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE)
trAE treatment-related adverse event
Discussion
This multi-centre study is the first to evaluate the effect of BMI on treatment efficacy and safety of Atezo/Bev for advanced/unresectable HCC. We show a BMI of 25 and above is associated with similar OS and PFS compared to patients with normal or underweight BMI. We observe higher rates of atezolizumab-related fatigue and bevacizumab-related thrombosis in overweight patients, without an increase in overall trAEs or treatment discontinuation.
Studies evaluating the effect of BMI on HCC survival have demonstrated varied results. Elevated BMI does not influence survival in patients undergoing curative resection [16, 17] and TACE [15]. In contrast, a positive association between BMI and survival for patients undergoing systemic chemotherapy has been reported [14, 31]. Secondary analysis of a phase III study demonstrated a BMI 25 or above was associated with an increased OS but not PFS in 544 patients receiving sorafenib for unresectable HCC [14]. These findings were replicated in a Japanese cohort of 234 patients receiving sorafenib [31]. However, there is a paucity of studies investigating the impact of BMI on outcomes secondary to immunotherapy in HCC. In a large meta-analysis of 4900 patients from 16 studies that investigated the prognostic role of BMI in patients receiving immunotherapy for different cancer sites (not including HCC) [18], overweight/obese patients showed reduced mortality compared to normal/underweight BMI patients in advanced melanoma (HR 0.69; 95% CI 0.51–0.95), NSCLC (HR 0.82; 95% CI 0.66–1.01) but not renal cell or other cancer types. High BMI was associated with improved PFS across all cancer types (HR 0.87; 95% CI 0.48–0.95), though not replicated in single cancer-site analysis. The authors reported that adverse events, assessed from four studies, were higher in the overweight/obese cohort. A retrospective study specifically evaluating anti-PD-1/PD-L1 in NSCLC, melanoma and renal cell carcinoma patients also showed OS, PFS and ORR were significantly longer in overweight/obese patients [19]. We did not observe this increased survival overweight patients with HCC. There may be several reasons for this observation. In the meta-analysis, only 35 (3.6%) patients underwent PD-L1 inhibition with atezolizumab, with over 96% of patients receiving PD-1 inhibitors nivolumab and pembrolizumab. Inter-class variation in efficacy of immunotherapy agents has been previously reported [32], and elevated BMI may affect anti-PD-1 and anti-PD-L1 response differentially. Additionally, we observed higher rates of NAFLD in patients with the overweight cohort. Previous studies have demonstrated a lack of efficacy of immunotherapy in patients with non-viral HCC [6, 33, 34]. This may be due to aberrant T-cell activation within hepatic tissue impairing immune response to checkpoint inhibition [33]. Therefore, underlying NAFLD and changes in the hepatic tissue immune environment in overweight HCC patients may attenuate the survival benefit from immunotherapy observed in other cancer sites.
Only a single previous study has evaluated effect of BMI on response to immunotherapy in unresectable HCC [25]. The effect of BMI and sarcopenia was investigated in 57 patients receiving anti-PD-1 antibody combination therapy in a single centre. Median OS was significantly longer in the BMI ≥ 25 group compared to BMI < 25 (17.5 vs. 5.0 months; p = 0.034), with similar PFS (2.7 vs. 2.9 months). The author’s observed sarcopenia was associated with a non-significant reduced OS (5.0 vs. 14.3 months; p = 0.054), which may correlate with lower BMI. The majority of patients in this study had received previous lines of systemic therapy, and 41% had a PS of 2–3, factors which may have adversely impacted on both BMI and sarcopenia, as it is likely that patients with poor PS will have a degree of cachexia [35]. Furthermore, no information is given by the authors regarding the type of immunotherapy administered. We did not observe a correlation between BMI and either OS or PFS in our larger cohort of patients receiving first-line treatment with anti-PD-L1 antibody and anti-VEGF therapy. The differences observed between the studies may be attributed to mechanistic differences of pharmacotherapy and differences in patient demographics as described.
Underlying NAFLD is highly represented in our cohort of patients with elevated BMI. This is expected as elevated BMI is associated with NAFLD as part of the metabolic syndrome spectrum [36]. The efficacy of immunotherapy in patients with NAFLD remains debated. A subgroup analysis of IMbrave150 showed no survival benefit with Atezo/Bev compared to sorafenib in patients with non-viral HCC [6]. This lack of efficacy of immunotherapy in non-viral related HCC was further demonstrated in two meta-analyses [33, 34]. However, these studies did not distinguish between alcohol-related and NAFLD-related HCC. We observe a higher rate of NAFLD-related HCC in our overweight cohort, with no difference in survival compared to the non-overweight group. These results suggest Atezo/Bev is efficacious in NAFLD patients, but further prospective study is needed. We found a higher rate of macrovascular invasion with overweight patients, which has been shown to be associated with NAFLD [37] as have larger sized tumors [38]. The higher rates of MVI in overweight patients may arise due to the effect of adipose tissue on the tumors microenvironment. Increased adipose tissue is associated with higher rates of hypoxia, causing release of pro-inflammatory cytokines including monocyte chemoattractant protein-1, interleukin-1β and tumors necrosis factor-α [34, 35]. Higher levels of Interleukin-6 are seen in obesity, with increased secretion from hepatic stellate cells and Kupffer cells within the liver [41]. Cytokine release drives chronic inflammation through macrophage and lymphocyte infiltration [42], promoting angiogenesis and alterations in the extracellular matrix leading to tumors growth [43]. In liver tissue, excessive saturated fatty acids can result in alterations in glucose metabolism and production of reactive oxidative species leading to progression of hepatocellular carcinoma [44, 45]. Diabetes mellitus is associated with higher rates of MVI in HCC patients [46], consistent with our observations with overweight and NAFLD patients, as part of the metabolic syndrome spectrum. Though the higher rates of MVI in the elevated BMI cohort did impact survival after immunotherapy, it may have implications for other treatments in overweight patients, including curative therapy such as hepatectomy and liver transplantation.
The impact of BMI on the safety of immunotherapy in patients has been evaluated. Cortellini et al., showed higher BMI was associated with higher rates of trAEs and subsequent treatment discontinuation in a cohort of 1070 patients receiving PD-1/PD-L1 inhibition for multiple primary cancer sites [47]. The authors observed BMI as an independent predictor for trAE in multivariate analysis. The authors speculate the higher rate of trAEs observed with higher efficacy may represent an immunogenic phenotype observed in higher BMI patients [48]. Similarly, higher BMI was associated with increase in overall trAEs in a meta-analysis of 4090 patients across multiple cancer sites receiving immunotherapy [18]. In our study, we did not report an overall increase in overall trAEs in overweight patients. We observed higher rates of atezolizumab-related fatigue and bevacizumab-related thrombosis in the overweight cohort. The mechanism for bevacizumab-related thrombosis is unclear, though may be mediated by increased vascular damage and inflammation [49]. Elevated BMI is an established risk factor for thrombosis through promotion of chronic inflammation and impaired fibrinolysis [50]. This is consistent with the higher rates of macrovascular invasion, encompassing portal vein thrombosis, observed in our overweight cohort. Sparks et al., observed BMI did not impact on rates of thrombosis in colorectal, ovarian, lung and gliblastoma multiforme cancer patients receiving bevacizumab [51]. The higher rates of thrombosis observed in our overweight cohort of HCC patients may be due to underlying liver dysfunction. Patients with cirrhosis are at increased risk of both bleeding and thrombosis [52], due to the liver’s role in synthesising both anticoagulant and coagulant factors. Obesity is an independent risk factor for thrombosis in pre-transplant cirrhosis patients [53] and may be a key driver in the increased risk of bevacizumab-related thrombosis in HCC patients. Atezolizumab-related thyroiditis has been seen in up to 10% of cases [54]. We observe a lower rate of atezolizumab-related thyroid dysfunction in the overweight cohort. The relationship between elevated BMI and thyroid dysfunction is complex, and mediated by adipocytes, cytokines and iodine uptake in thyroid cells [55]. This interplay may influence the rates of immunotherapy-related thyroid dysfunction in overweight patients. The difference in trAEs observed between the overweight and non-overweight cohorts may also be due to unmeasured confounding factors between the two groups. Further large studies will be required to assess trAEs with increased use of atezolizumab plus bevacizumab specific for HCC.
Our study has limitations. Though follow-up was prospectively collected, this is a retrospective study which is subject to selection and collection bias. Baseline characteristics influencing survival such as underlying NAFLD and extrahepatic spread were higher in the overweight cohorts and may have an impact on patient survival. Additionally, there may be unmeasured confounding factors. Though patients received treatment from tertiary centres, inter-site variation in treatment protocols, follow-up and efficacy and safety assessments cannot be excluded. Lower BMI may represent poor global nutritional status and sarcopenia. Clinically and radiologically measured sarcopenia is associated with increased mortality in patients with cirrhosis [56] and HCC [57] and, therefore, may confound our findings. Further studies assessing sarcopenia and other body composition measures such as subcutaneous adipose tissue, visceral adipose tissue and muscle volume would allow understanding of the impact of muscle mass and adipose tissue on immunotherapy response in HCC. In survival analysis we report all-cause mortality rather than liver-specific mortality. This may be influenced by unreported medical comorbidities. Despite these limitations, this study represents the largest cohort of post-registration real-time use of atezolizumab plus bevacizumab for HCC in overweight patients. As an increasing number of immunotherapy agents emerge for use in HCC [58], further studies evaluating the efficacy and safety of these agents in overweight patients will guide future clinical practice.
Conclusion
Our study demonstrates atezolizumab plus bevacizumab therapy is associated with comparable efficacy in overweight HCC patients with higher rates of NALFD. Increased body mass index is associated with higher rates of treatment-related fatigue and thrombosis, but no increase in overall treatment-related adverse events. Combination therapy is safe and efficacious to use in overweight patients, including those with underlying NAFLD.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
All authors are thankful to all the clinical teams across the tertiary centres providing care for the patient cohort.
Abbreviations
- HCC
Hepatocellular carcinoma
- PD-L1
Programmed death ligand 1
- VEGF
Vascular endothelial growth factor
- Atezo/Bev
Atezolizumab and bevacizumab
- OS
Overall survival
- PFS
Progression-free survival
- HR
Hazard ratio
- CI
Confidence interval
- STRIDE
Single tremelimumab regular interval durvalumab
- BMI
Body mass index
- TACE
Trans-arterial chemoembolization
- NSCLC
Non-small cell lung cancer
- AASLD
American Association for the Study of Liver Diseases
- BCLC
Barcelona clinic liver cancer
- SmPC
Summary of product characteristics
- CR
Complete response
- PR
Partial response
- SD
Stable disease
- trAE
Treatment-related adverse event
- CTCAE
Common terminology criteria for adverse events
- MVI
Macrovascular invasion
- AFP
Alpha-fetoprotein
- NAFLD
Non-alcoholic fatty liver disease
Author contributions
RS is the guarantor of the study. DP and RS: conceptualized the study. Investigators in the study were AW, PG, DB, BB, AV, LB, BS, MM, SA, AM, AN, NP, TP, AC, MP, LR, DP and RS. Data was collected and curated by ADA, CA, MF, PF, AC and DP. MV and RS conducted formal analysis. MV wrote the manuscript with supervision from RS and DP. All authors critically reviewed the final manuscript and approved for submission.
Funding
MV is supported by the National Institute of Health Research. AD is supported by grant funding from the European Association for the Study of the Liver (Andrew Burroughs Fellowship). DJP is supported by grant funding from the Wellcome Trust Strategic Fund (PS3416) and acknowledges grant support from the Cancer Treatment and Research Trust (CTRT) and infrastructural support by the Cancer Research UK Imperial Centre and the NIHR Imperial Biomedical Research Centre. AC is supported by the NIHR Imperial Biomedical Research Centre.
Data availability
All data is available upon request from the corresponding author Dr Rohini Sharma, email: r.sharma@imperial.ac.uk.
Declarations
Conflict of interest
AD received educational support for congress attendance from Roche. JvF received advisory board fees from Roche. HW received lecture fees and advisory board honoraria from Roche, Bayer, Ipsen, Eisai, BMS. AS received research grants (to institution) from AstraZeneca, Merck, Bristol Myers Squibb, Exelixis, Clovis, KAHR medical, Actuate therapeutics, Incyte Corp. and Advisory board fees from AstraZeneca, Bristol Myers Squibb, Merck, Exelixis, and Pfizer. PRG reports a consulting or advisory role and received honoraria from AdaptImmune, AstraZeneca, Bayer, Bristol Myers Squibb, Eisai, Ipsen, Lilly, Merck Sharp & Dohme, Roche, and Sirtex; has been on a speakers bureau for AstraZeneca, Bayer, Bristol Myers Squibb, Eisai, Ipsen, Lilly, Merck Sharp & Dohme, Roche, and Sirtex; has received research funding from Bayer and Roche; has provided expert testimony for Lilly; and has received travel or accommodation expenses from AstraZeneca, Bayer, Bristol Myers Squibb, Eisai, Ipsen, Lilly, and Roche. DB has received lecture and speaker fees from Bayer Healthcare, the Falk Foundation Germany and consulting fees from Boston Scientific. AV reports honoraria for speaker, consultancy and advisory role from Roche, AstraZeneca, EISAI, Bayer, Merck, Bristol Myers Squibb, Merck Sharp & Dohme, Incyte, PierreFabre, Ipsen, and Sanofi. BS received travel support from Gilead, Ipsen and AbbVie. NP received consulting fees from Amgen, Merck Serono, Servier; lectures fees from AbbVie, Gilead, Lilly, Sanofi; travel expenses from Amgen, ArQule; and institutional research funding from Basilea, Merck Serono, Servier. TP received consulting fees from Bayer; and institutional research funding from Bayer, Lilly, Roche. RS received consulting fees for EISAI, Roche, Bayer, SIRTEX, Novartis; research funding (to institution) from Incyte, Novartis, Astex Pharmaceuticals, Bayer and Boston Scientific. MP is an investigator for Bayer, BMS, Ipsen, Lilly, and Roche; he received speaker honoraria fromBayer, BMS, Eisai, Lilly, MSD, and Roche; he is a consultant for Bayer, BMS, Eisai, Ipsen, Lilly, MSD, and Roche; he received travel support from Bayer and BMS. AC received consulting fees from MSD, BMS, AstraZeneca, Roche; speakers’ fee from AstraZeneca, MSD, Novartis and Astellas. LR has received consulting fees from Amgen, ArQule, AstraZeneca, Basilea, Bayer, BMS, Celgene, Eisai, Exelixis, Genenta, Hengrui, Incyte, Ipsen, IQVIA, Lilly, MSD, Nerviano Medical Sciences, Roche, Sanofi, Servier, Taiho Oncology, Zymeworks; lecture fees from AbbVie, Amgen, Bayer, Eisai, Gilead, Incyte, Ipsen, Lilly, Merck Serono, Roche, Sanofi, Servier; travel expenses from AstraZeneca; and institutional research funding from Agios, ARMO BioSciences, AstraZeneca, BeiGene, Eisai, Exelixis, Fibrogen, Incyte, Ipsen, Lilly, MSD, Nerviano Medical Sciences, Roche, Zymeworks. DJP received lecture fees from ViiV Healthcare, Bayer Healthcare, BMS, Roche, Eisai, Falk Foundation, travel expenses from BMS and Bayer Healthcare; consulting fees for Mina Therapeutics, EISAI, Roche, DaVolterra, Mursla, Exact Sciences and Astra Zeneca; research funding (to institution) from MSD and BMS. All remaining authors have declared no conflicts of interest. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Ethics approval
The study was approved by the Imperial College Tissue Bank (R16008) and local ethics committees at each participating site.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J-F, de Oliveira AC, Santoro A, Raoul J-L, Forner A, Schwartz M, Porta C, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 3.Kudo M, Finn RS, Qin S, Han K-H, Ikeda K, Piscaglia F, Baron A, Park J-W, Han G, Jassem J, Blanc JF, Vogel A, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet (London, England) 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 4.Fulgenzi CAM, Talbot T, Murray SM, Silletta M, Vincenzi B, Cortellini A, Pinato DJ. Immunotherapy in hepatocellular carcinoma. Curr Treat Options Oncol. 2021;22:87. doi: 10.1007/s11864-021-00886-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 6.Cheng A-L, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, Lim HY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862–873. doi: 10.1016/j.jhep.2021.11.030. [DOI] [PubMed] [Google Scholar]
- 7.Abou-Alfa GK, Chan SL, Kudo M, Lau G, Kelley RK, Furuse J, Sukeepaisarnjaroen W, Kang Y-K, Dao TV, De Toni EN, Rimassa L, Breder VV, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40:379–379. doi: 10.1200/JCO.2022.40.4_suppl.379. [DOI] [Google Scholar]
- 8.Vogel A, Rimassa L, Sun H-C, Abou-Alfa GK, El-Khoueiry A, Pinato DJ, Sanchez Alvarez J, Daigl M, Orfanos P, Leibfried M, Blanchet Zumofen M-H, Gaillard VE, et al. Comparative efficacy of atezolizumab plus bevacizumab and other treatment options for patients with unresectable hepatocellular carcinoma: a network meta-analysis. Liver cancer. 2021;10:240–248. doi: 10.1159/000515302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao K-F, Ma M, Ding G-Y, Li Z-M, Chen H-L, Han B, Chen Q, Jiang X-Q, Wang L-S. Meta-analysis reveals gender difference in the association of liver cancer incidence and excess BMI. Oncotarget. 2017;8:72959. doi: 10.18632/oncotarget.20127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vithayathil M, Carter P, Kar S, Mason AM, Burgess S, Larsson SC, Karx S, Mason AM, Burgess S, Larsson SC. Body size and composition and risk of site-specific cancers in the UK Biobank and large international consortia: a mendelian randomisation study. PLoS Med. 2021;18:e1003706. doi: 10.1371/journal.pmed.1003706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q, Xing H, Liu D, Li H. Negative impact of low body mass index on liver cirrhosis patients with hepatocellular carcinoma. World J Surg Oncol. 2015;13:294. doi: 10.1186/s12957-015-0713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cha B, Yu JH, Jin Y-J, Suh YJ, Lee J-W. Survival outcomes according to body mass index in hepatocellular carcinoma patient: analysis of nationwide cancer registry database. Sci Rep. 2020;10:8347. doi: 10.1038/s41598-020-65460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Utsunomiya T, Okamoto M, Kameyama T, Matsuyama A, Yamamoto M, Fujiwara M, Mori M, Aimitsu S, Ishida T. Impact of obesity on the surgical outcome following repeat hepatic resection in Japanese patients with recurrent hepatocellular carcinoma. World J Gastroenterol. 2008;14:1553–1558. doi: 10.3748/wjg.14.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdel-Rahman O. Impact of baseline characteristics on outcomes of advanced HCC patients treated with sorafenib: a secondary analysis of a phase III study. J Cancer Res Clin Oncol. 2018;144:901–908. doi: 10.1007/s00432-018-2610-z. [DOI] [PubMed] [Google Scholar]
- 15.Wu SE, Charles HW, Park JS, Goldenberg AS, Deipolyi AR. Obesity conveys poor outcome in patients with hepatocellular carcinoma treated by transarterial chemoembolization. Diagn Interv Imaging. 2017;98:37–42. doi: 10.1016/j.diii.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Nishikawa H, Arimoto A, Wakasa T, Kita R, Kimura T, Osaki Y. The relation between obesity and survival after surgical resection of hepatitis C virus-related hepatocellular carcinoma. Gastroenterol Res Pract. 2013;2013:430438. doi: 10.1155/2013/430438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishikawa H, Osaki Y, Takeda H, Sakamoto A, Saito S, Nishijima N, Nasu A, Arimoto A, Kita R, Kimura T. Effect of body mass index on survival after curative therapy for non-B non-C hepatocellular carcinoma. J Gastrointestin Liver Dis. 2013;22:173–181. [PubMed] [Google Scholar]
- 18.Xu H, Cao D, He A, Ge W. The prognostic role of obesity is independent of sex in cancer patients treated with immune checkpoint inhibitors: a pooled analysis of 4090 cancer patients. Int Immunopharmacol. 2019;74:105745. doi: 10.1016/j.intimp.2019.105745. [DOI] [PubMed] [Google Scholar]
- 19.Cortellini A, Bersanelli M, Buti S, Cannita K, Santini D, Perrone F, Giusti R, Tiseo M, Michiara M, Di Marino P, Tinari N, De Tursi M, et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer. 2019;7:57. doi: 10.1186/s40425-019-0527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McQuade JL, Daniel CR, Hess KR, Mak C, Wang DY, Rai RR, Park JJ, Haydu LE, Spencer C, Wongchenko M, Lane S, Lee D-YY, et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018;19:310–322. doi: 10.1016/S1470-2045(18)30078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Hopkins AM, Sorich MJ. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non-small cell lung cancer. JAMA Oncol. 2020;6:512–518. doi: 10.1001/jamaoncol.2019.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pizzuti L, Sergi D, Sperduti I, Di LL, Mazzotta M, Botti C, Izzo F, Marchetti L, Tomao S, Marchetti P, Natoli C, Grassadonia A, et al. Body mass index in HER2-negative metastatic breast cancer treated with first-line paclitaxel and bevacizumab. Cancer Biol Ther. 2018;19:328–334. doi: 10.1080/15384047.2017.1416938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faruk Aykan N, Yildiz I, Sen F, Kilic L, Keskin S, Ciftci R, Karabulut S, Sakar B, Disci R. Effect of increased body mass index (BMI) on time to tumour progression (TTP) in unresectable metastatic colorectal cancer (mCRC) patients treated with bevacizumab-based therapy. Med Oncol. 2013;30:679. doi: 10.1007/s12032-013-0679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shukla S, Babcock Z, Pizzi L, Brunetti L. Impact of body mass index on survival and serious adverse events in advanced non-small cell lung cancer treated with bevacizumab: a meta-analysis of randomized clinical trials. Curr Med Res Opin. 2021;37:811–817. doi: 10.1080/03007995.2021.1900091. [DOI] [PubMed] [Google Scholar]
- 25.Akce M, Liu Y, Zakka K, Martini DJ, Draper A, Alese OB, Shaib WL, Wu C, Wedd JP, Sellers MT, Bilen MA, El-Rayes BF. Impact of sarcopenia, BMI, and inflammatory biomarkers on survival in advanced hepatocellular carcinoma treated with anti-PD-1 antibody. Am J Clin Oncol. 2021;44:74–81. doi: 10.1097/COC.0000000000000787. [DOI] [PubMed] [Google Scholar]
- 26.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 27.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 28.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 29.US Department of Health and Human Services, National Institutes of Health NCI. Common Terminology Criteria for Adverse Events (CTCAE) Version 5. 2017
- 30.Hajiev S, Allara E, Motedayen Aval L, Arizumi T, Bettinger D, Pirisi M, Rimassa L, Pressiani T, Personeni N, Giordano L, Kudo M, Thimme R, et al. Impact of age on sorafenib outcomes in hepatocellular carcinoma: an international cohort study. Br J Cancer. 2021;124:407–413. doi: 10.1038/s41416-020-01116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishikawa H, Nishijima N, Enomoto H, Sakamoto A, Nasu A, Komekado H, Nishimura T, Kita R, Kimura T, Iijima H, Nishiguchi S, Osaki Y. Predictive factors in patients with hepatocellular carcinoma receiving sorafenib therapy using time-dependent receiver operating characteristic analysis. J Cancer. 2017;8:378–387. doi: 10.7150/jca.16786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banna GL, Cantale O, Bersanelli M, Del Re M, Friedlaender A, Cortellini A, Addeo A. Are anti-PD1 and anti-PD-L1 alike? The non-small-cell lung cancer paradigm. Oncol Rev. 2020;14:490. doi: 10.4081/oncol.2020.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfister D, Núñez NG, Pinyol R, Govaere O, Pinter M, Szydlowska M, Gupta R, Qiu M, Deczkowska A, Weiner A, Müller F, Sinha A, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592:450–456. doi: 10.1038/s41586-021-03362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haber PK, Puigvehí M, Castet F, Lourdusamy V, Montal R, Tabrizian P, Buckstein M, Kim E, Villanueva A, Schwartz M, Llovet JM. Evidence-based management of hepatocellular carcinoma: systematic review and meta-analysis of randomized controlled trials (2002–2020) Gastroenterology. 2021;161:879–898. doi: 10.1053/j.gastro.2021.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, Cohen MH, Douglass HO, Engstrom PF, Ezdinli EZ, Horton J, Johnson GJ, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. 1980;69:491–497. doi: 10.1016/S0149-2918(05)80001-3. [DOI] [PubMed] [Google Scholar]
- 36.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ, American Gastroenterological Association, American Association for the Study of Liver Diseases, American College of Gastroenterologyh The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Stine JG, Shah NL, Argo CK, Pelletier SJ, Caldwell SH, Northup PG. Increased risk of portal vein thrombosis in patients with cirrhosis due to nonalcoholic steatohepatitis. Liver Transpl. 2015;21:1016–1021. doi: 10.1002/lt.24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akkiz H, Carr BI, Kuran S, Karaoğullarından Ü, Üsküdar O, Tokmak S, Arslan B, Doran F, Balli HT, Ülkü A, Akçam TA, Bahçeci Hİ, et al. Macroscopic portal vein thrombosis in HCC patients. Can J Gastroenterol Hepatol. 2018;2018:3120185. doi: 10.1155/2018/3120185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park EJ, Lee JH, Yu G-Y, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brocco D, Florio R, De Lellis L, Veschi S, Grassadonia A, Tinari N, Cama A. The role of dysfunctional adipose tissue in pancreatic cancer: a molecular perspective. Cancers (Basel) 2020;12:1849. doi: 10.3390/cancers12071849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J Clin Oncol. 2016;34:4270–4276. doi: 10.1200/JCO.2016.67.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seo BR, Bhardwaj P, Choi S, Gonzalez J, Andresen Eguiluz RC, Wang K, Mohanan S, Morris PG, Du B, Zhou XK, Vahdat LT, Verma A, et al. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci Transl Med. 2015;7:301ra130. doi: 10.1126/scitranslmed.3010467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Broadfield LA, Duarte JAG, Schmieder R, Broekaert D, Veys K, Planque M, Vriens K, Karasawa Y, Napolitano F, Fujita S, Fujii M, Eto M, et al. Fat induces glucose metabolism in nontransformed liver cells and promotes liver tumorigenesis. Cancer Res. 2021;81:1988–2001. doi: 10.1158/0008-5472.CAN-20-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chong L-W, Tsai C-L, Yang K-C, Liao C-C, Hsu Y-C. Targeting protein palmitoylation decreases palmitate-induced sphere formation of human liver cancer cells. Mol Med Rep. 2020;22:939–947. doi: 10.3892/mmr.2020.11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Connolly GC, Safadjou S, Kashyap R, Chen R, Orloff MS, Hezel AF. Diabetes mellitus impacts risk of macrovascular invasion in patients undergoing transplantation for hepatocellular carcinoma. BMC Gastroenterol. 2013;13:9. doi: 10.1186/1471-230X-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cortellini A, Bersanelli M, Santini D, Buti S, Tiseo M, Cannita K, Perrone F, Giusti R, De Tursi M, Zoratto F, Marconcini R, Russano M, et al. Another side of the association between body mass index (BMI) and clinical outcomes of cancer patients receiving programmed cell death protein-1 (PD-1)/Programmed cell death-ligand 1 (PD-L1) checkpoint inhibitors: a multicentre analysis of immune-related. Eur J Cancer. 2020;128:17–26. doi: 10.1016/j.ejca.2019.12.031. [DOI] [PubMed] [Google Scholar]
- 48.Davar D, Kirkwood JM. PD-1 immune checkpoint inhibitors and immune-related adverse events. JAMA Oncol. 2019;5:942. doi: 10.1001/jamaoncol.2019.0413. [DOI] [PubMed] [Google Scholar]
- 49.Alahmari AK, Almalki ZS, Alahmari AK, Guo JJ. Thromboembolic events associated with bevacizumab plus chemotherapy for patients with colorectal cancer: a meta-analysis of randomized controlled trials. Am Heal drug benefits. 2016;9:221–232. [PMC free article] [PubMed] [Google Scholar]
- 50.Blokhin IO, Lentz SR. Mechanisms of thrombosis in obesity. Curr Opin Hematol. 2013;20:437–444. doi: 10.1097/MOH.0b013e3283634443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sparks J, Wu X, Knable MK, Rai SN, Sharma V. Predictors of thrombosis in patients treated with bevacizumab. Thromb Updat. 2022;6:100095. doi: 10.1016/j.tru.2021.100095. [DOI] [Google Scholar]
- 52.Ha NB, Regal RE. Anticoagulation in patients with cirrhosis: caught between a rock-liver and a hard place. Ann Pharmacother. 2016;50:402–409. doi: 10.1177/1060028016631760. [DOI] [PubMed] [Google Scholar]
- 53.Ayala R, Grande S, Bustelos R, Ribera C, García-Sesma A, Jimenez C, Moreno E, Martínez-López J. Obesity is an independent risk factor for pre-transplant portal vein thrombosis in liver recipients. BMC Gastroenterol. 2012;12:114. doi: 10.1186/1471-230X-12-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McDermott DF, Sosman JA, Sznol M, Massard C, Gordon MS, Hamid O, Powderly JD, Infante JR, Fassò M, Wang YV, Zou W, Hegde PS, et al. Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase Ia study. J Clin Oncol. 2016;34:833–842. doi: 10.1200/JCO.2015.63.7421. [DOI] [PubMed] [Google Scholar]
- 55.Song R-H, Wang B, Yao Q-M, Li Q, Jia X, Zhang J-A. The Impact of Obesity on thyroid autoimmunity and dysfunction: a systematic review and meta-analysis. Front Immunol. 2019;10:2349. doi: 10.3389/fimmu.2019.02349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kikuchi N, Uojima H, Hidaka H, Iwasaki S, Wada N, Kubota K, Nakazawa T, Shibuya A, Fujikawa T, Kako M, Sato T, Koizumi W. Prospective study for an independent predictor of prognosis in liver cirrhosis based on the new sarcopenia criteria produced by the Japan Society of Hepatology. Hepatol Res. 2021;51:968–978. doi: 10.1111/hepr.13698. [DOI] [PubMed] [Google Scholar]
- 57.Qayyum A, Bhosale P, Aslam R, Avritscher R, Ma J, Pagel MD, Sun J, Mohamed Y, Rashid A, Beretta L, Kaseb AO. Effect of sarcopenia on systemic targeted therapy response in patients with advanced hepatocellular carcinoma. Abdom Radiol (New York) 2021;46:1008–1015. doi: 10.1007/s00261-020-02751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Foerster F, Gairing SJ, Ilyas SI, Galle PR. Emerging immunotherapy for HCC: a guide for hepatologists. Hepatology. 2022;75:1604–1626. doi: 10.1002/hep.32447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data is available upon request from the corresponding author Dr Rohini Sharma, email: r.sharma@imperial.ac.uk.


