Abstract
BACKGROUND:
Cystic fibrosis (CF) is a chronic, progressive genetic disease caused by mutations in the CF transmembrane conductance regulator (CFTR) gene resulting in a dysfunctional CFTR protein. Elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) is a triple combination oral drug therapy with an annual cost greater than $300,000 and available to nearly 90% of the CF population based on age and genotype. Limited real-world direct medical cost offset data are available for ELX/TEZ/IVA among commercially insured individuals.
OBJECTIVE:
To describe and compare total cost of care and health care resource utilization (HRU) 180 days before and 180 days after first ELX/TEZ/IVA drug claim among CFTR modulator treatment-naive, commercially insured members.
METHODS:
This study was a retrospective analysis of integrated pharmacy and medical claims data from 17.9 million commercially insured members. A 180-day prestudy and 180-day poststudy design was used to compare outcomes prior to and following ELX/TEZ/IVA initiation. Study inclusion was limited to members with first ELX/TEZ/IVA claim (index date) between October 21, 2019, and December 31, 2021, continuously enrolled 180 days before and 180 days after index date, and no CFTR-modulator drug claim 180 days prior to index date. Total paid amounts from medical and pharmacy claims after network discounts (defined as total cost of care), HRU, and pulmonary exacerbation events were summarized using descriptive statistics and compared using Wilcoxon signed rank test.
RESULTS:
494 members newly initiating ELX/TEZ/IVA met inclusion criteria. Prestudy to poststudy mean member total cost of care increased from $58,180 to $198,815 (difference: $140,635; P < 0.001). Mean member medical benefit costs decreased from $28,764 to $12,484 (difference: -$16,280; P < 0.001), whereas mean member pharmacy benefit costs increased from $29,416 to $186,331 (difference: $156,915; P < 0.001). Mean member inpatient hospitalizations (62% absolute reduction; P < 0.001), emergency department visits (43% absolute reduction; P < 0.01), and pulmonary exacerbation events (44% absolute reduction; P < 0.001) were significantly lower in the postperiod compared with the preperiod.
CONCLUSIONS:
Among members with CF newly initiating CFTR modulator with ELX/TEZ/IVA, mean member total cost of care increased 3-fold despite significant and meaningful reductions in pulmonary exacerbation events, HRU, and medical benefit spend. Pharmacy benefit spend outpaced medical benefit spend at a rate of $9.64 to $1 in the 180 days following ELX/TEZ/IVA initiation. Real-world data should be used to objectively measure the clinical and economic benefits of costly medications, such as CFTR modulators, to align price with value.
Plain language summary
Among 494 commercially insured members diagnosed with cystic fibrosis, a meaningful decrease in hospitalizations, emergency department visits, and respiratory exacerbation events were observed following the start of elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA) drug therapy. Average member total cost of care was 3 times higher in the first 6 months following ELX/TEZ/IVA therapy start compared with the previous 6 months. ELX/TEX/IVA therapy improved key clinical outcomes, despite concerns regarding ELX/TEX/IVA affordability.
Implications for managed care pharmacy
Across the prestudy to poststudy 180-day periods, clinically meaningful reductions in inpatient hospitalizations, emergency department visits, and pulmonary exacerbations were observed. However, mean member total cost of care increased by $140,635 across the same period. Plans and manufactures should use real-world data to better align price with value and mitigate growing spend trends in cystic fibrosis care.
Cystic fibrosis (CF) is a life-threatening, autosomal recessive disease affecting nearly 40,000 individuals in the United States resulting from CF transmembrane conductance regulator (CFTR) gene mutation responsible for coding the CFTR protein.1 More than 2,000 unique CFTR gene mutations have been identified, with the deletion of phenylalanine at the location 508 allele (F508del) being the most prevalent.2-4 The CFTR protein functions as an ion channel responsible for regulating chloride and fluid flow across epithelium cells in the respiratory tract, pancreas, gastrointestinal organs, sweat glands, and other organ systems. Impaired CFTR protein function leads to pathogenic viscous secretions.1,5
Despite CF being a multiorgan disease, pulmonary manifestations remain the leading cause of morbidity and mortality.5,6 Viscous airway secretions lead to respiratory tract inflammation and bacterial infections, contributing to a progressive decline in lung function over time with acute episodes of worsening pulmonary symptoms. These acute episodes, known as pulmonary exacerbations, can vary based on the severity of the exacerbation and are costly, decrease quality of life, cause irreversible damage to lung tissue leading to accelerated loss of lung function, and decrease overall survival.5-8
In 2012, the first of a new CF treatment medication class came to market: the CFTR modulators, designed to increase or potentially restore the function of the CFTR protein by targeting specific disease-causing CFTR mutations.9 Currently, there are 4 CFTR modulators available for patients with select mutations. CFTR modulators are classified as either potentiators (ivacaftor) or correctors (lumacaftor, tezacaftor, and elexacaftor).10 Potentiators improve the opening of the CFTR channel while correctors improve CFTR protein folding. Previously, the available CFTR modulators (ivacaftor, lumacaftor/ivacaftor, and tezacaftor/ivacaftor) allowed approximately 50% of the CF population to be eligible for this type of therapy based on targeted CFTR mutations.11 With the most recent approval of elexacaftor/tezacaftor/ivacaftor (ELX/TEZ/IVA), approximately 90% of the CF population is now eligible to receive CFTR modulator therapy. ELX/TEZ/IVA is indicated for patients with CF aged 6 years and older with at least 1 copy of the F508del mutation or a mutation in the CFTR gene that is responsive based on in vitro data.12 Following ELX/TEZ/IVA approval, Blue Cross Blue Shield North Carolina found 88% of commercially insured patients with CF were being treated with a CFTR modulator at an average $1.96 per member per month expense.13
In the clinical trial setting, ELX/TEZ/IVA has demonstrated clinically meaningful improvements in lung function and reduced pulmonary exacerbations, compared with the standard of care with a favorable safety profile.14,15 Data from the real-world setting show a doubling in CF per-person expenditures for privately insured patients from 2010 (pre-CFTR modulator availability) to 2016 (post the first 2 CFTR modulator products availability) creating cost concerns for insurers and patients using CFTR modulatory therapy.16 A cost-effectiveness assessment by the Institute of Clinical and Economic Review concluded CFTR modulator therapy is substantially overpriced to the value delivered.17 With an annual ELX/TEZ/IVA list price of $311,741 and a near doubling of patients with CF eligible for CFTR modulator therapy the cost concerns identified in 2016 are further accentuated and in many countries the CFTR modulators are so expensive they are essentially unavailable.18
Limited real-world direct medical cost offset data are available for ELX/TEZ/IVA among commercially insured individuals. The purpose of this analysis is to assess the short term real-world direct medical cost offsets of ELX/TEZ/IVA among commercially insured patients with CF to provide insights for patients, payers, providers, and other stakeholders when discussing medication affordability, access, and value.
Methods
STUDY DESIGN
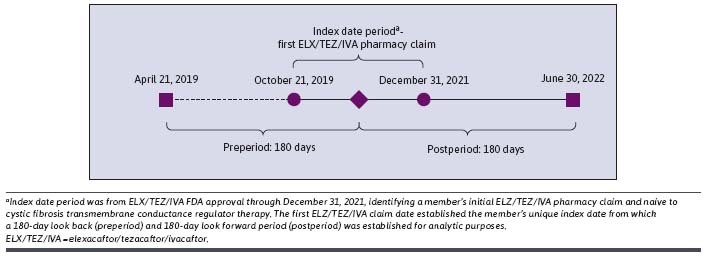
This was a retrospective, observational cohort study using a 180-day prestudy and 180-day poststudy of all-cause cost of care and health resource utilization among CFTR modulator–naive members newly initiating ELX/TEZ/IVA. The study index date was defined as the first prescription claim date for ELX/TEZ/IVA between October 21, 2019 (date ELX/TEZ/IVA was approved), and December 31, 2021 (Figure 1).
FIGURE 1.
Study Design
DATA SOURCE
Integrated medical and pharmacy claims data from April 21, 2019, to June 30, 2022, across 16 commercial health plans covering all regions of the United States were obtained for the study. During the study index period, the database contained an average of 17.9 million members per year with at least 1 month of eligibility. Data obtained for this study included medical claims (date of service, diagnoses received, procedures performed, place of service, and claim paid amounts), pharmacy claims (fill dates, National Drug Code numbers, and claim paid amounts), and eligibility information (patient demographics and enrollment history). This study was conducted to address the objectives stated for health insurance business purposes and, therefore, is IRB exempt. All authors are employees of Prime Therapeutics, a pharmacy benefits manager. Prime Therapeutics adjudicates and pays pharmacy claims as well as and stores all data according to HIPAA regulations from which a limited dataset was created to answer the study objectives.
SAMPLE SELECTION
Members with their first ELX/TEZ/IVA pharmacy claim (study index date) between October 21, 2019, and December 31, 2021 (study index period), were included. Additionally, members were required to be continuously enrolled 180-days prior to and 180-days after study index date, and no history of any CFTR modulator drug claims in the 180-day pre-index study period.
STUDY OUTCOMES
The primary outcome was 180-day, all-cause direct total cost of care obtained from total paid amounts summed across medical and pharmacy benefits during the measure period. All-cause medical benefit and pharmacy benefit costs were obtained from paid, finalized claims by summing the total paid amounts inclusive of plan paid, member paid, and any other third-party payment, if applicable. The total paid to a provider are insurer allowed amounts after network discounts. The pharmaceutical manufacturer did not offer ELX/TEZ/IVA rebates. Pharmaceutical manufacturer patient assistance program discounts, provided to patients, are not included. Total cost of care was calculated using paid claims costs across the medical and pharmacy benefit.
Other study outcomes included baseline demographics and clinical characteristics at the time of first ELX/TEZ/IVA pharmacy claim, CF treatment patterns, all-cause health care resource utilization (HRU), and pulmonary exacerbation events. All-cause HRU included inpatient hospitalizations and emergency department visits. Treatment patterns were characterized according to ELX/TEZ/IVA average cost and claim count and pancreatic enzyme replacement therapy claim count. All study outcomes were assessed in the 180-day pre-index and 180-day post-index study periods.
Pulmonary exacerbation was operationalized based on Tesell et al6 and defined as any of the following events: (1) Any inpatient hospitalization with a medical diagnosis code in any position indicating CF pulmonary exacerbation or respiratory infection; (2) any emergency department visit with a medical diagnosis code in any position indicating CF pulmonary exacerbation or respiratory infection; and (3) respiratory antibiotic (excluding oral macrolides and inhaled antibiotics) use identified from paid medical or pharmacy benefit claims.
A pulmonary exacerbation was considered new when a gap of 7 or more days occurred between the end of a previous event and the start of a subsequent event.
STATISTICAL ANALYSIS
Descriptive statistics were used to summarize member demographics and clinical characteristics, direct total cost of care, all-cause HRU, treatment characteristics, and pulmonary exacerbation events. Counts and percentages were reported for dichotomous and categorical variables, and measures of centrality (mean, median) and spread (SD, interquartile range, as appropriate) were reported for continuous variables. Skewness and normality across continuous variables were inspected graphically and quantitatively assessed using the Shapiro-Wilks test for normality.
Differences across normally distributed costs and HRU endpoints were analyzed using parametric paired test (Student’s t-test), whereas nonnormally distributed outcomes were analyzed using nonparametric (Wilcoxon signed rank test) paired test to compare the mean difference across each study endpoint over the 180-day preperiod vs the 180-day postperiod. An a priori α level of 0.05 was used to determine statistically significance.
Results
DEMOGRAPHIC AND CLINICAL CHARACTERISTICS
Among 1,894 ELX/TEZ/IVA users during the study index period, 494 (26.1%) met full study inclusion and exclusion criteria (Supplementary Figure 1 (152.5KB, pdf) , available in online article). A majority were male (52.6%) with a mean age of 26.2 (SD = 13.4) at time of ELX/TEZ/IVA initiation (Table 1). Half (50.2%) of the final analytical cohort was in the southern US region with 34.0%, 9.9%, and 5.9% from the Midwest, West, and Northeast, respectively. Pancreatic enzyme replacement therapy utilization was observed in 70.2% of members during the 180-day preperiod. Average days’ supply for all ELX/TEZ/IVA claims was 28.2 days (SD = 3.4) with an average of 6.5 ELX/TEZ/IVA claims (SD = 1.7) per member observed during the 180-day postperiod (Table 1).
TABLE 1.
Member Characteristics
| Member characteristics | N = 494a |
|---|---|
| Mean age at first ELX/TEZ/IVA claim, years (SD) | 26.2 (13.4) |
| Sex, n (%) | |
| Female | 234 (47.4) |
| Male | 260 (52.6) |
| Geographic region, n (%) | |
| South | 248 (50.2) |
| Midwest | 168 (34.0) |
| West | 49 (9.9) |
| Northeast | 29 (5.9) |
| Pancreatic enzyme replacement therapy use during 180-day preperiod, n (%) | |
| Yes | 347 (70.2) |
| No | 147 (29.8) |
| Average ELX/TEZ/IVA claims during 180-day postperiod,a per member (SD) | 6.5 (1.7) |
| Average day supply across ELX/TEZ/IVA claims during 180-day postperioda (SD) | 28.2 (3.4) |
aAll members naive to cystic fibrosis transmembrane conductance regulator therapy and newly initiating ELX/TEZ/IVA.
ELX/TEZ/IVA = elexacaftor/tezacaftor/ivacaftor.
Pulmonary Exacerbations and HRU. A statistically significant 44.1% reduction in pulmonary exacerbation events was observed during the study period (Table 2). The total number of pulmonary exacerbation events significantly decreased from the prestudy to poststudy periods (preperiod: 726 compared with postperiod: 406; P < 0.001) with fewer members in the postperiod experiencing one or more pulmonary exacerbation events (preperiod: 364 compared with post period: 258). Similarly, significant reductions in total number of inpatient hospitalizations (61.9% reduction; preperiod: 202 compared with postperiod: 77; P < 0.001) and total number of emergency department visits (42.8% reduction; preperiod: 152 compared with postperiod: 87; P < 0.001) were observed. Overall, 76 fewer (preperiod: 124; postperiod: 48) members had an inpatient hospitalization and 28 fewer (preperiod: 84; postperiod: 56) members experienced an emergency department visit following ELX/TEZ/IVA initiation.
TABLE 2.
Pulmonary Exacerbation Events and Health Care Resource Utilization 180 Days Before and After ELX/TEZ/IVA New Initiation
| 180-day preperiod | 180-day postperiod | Difference (% change) | P value | |||
|---|---|---|---|---|---|---|
| Pulmonary exacerbation events | ||||||
| Total number of pulmonary exacerbation events | 726 | — | 406 | — | -44.1 | < 0.001 |
| Mean (SD) | 1.47 | (1.29) | 0.82 | (1.0) | ||
| Number of pulmonary exacerbation events (categorical) per member, n (%) | ||||||
| 0 | 130 | (26.3) | 236 | (47.8) | ||
| ≥1 | 364 | (73.7) | 258 | (52.2) | ||
| All-cause health care resource utilization | ||||||
| Inpatient hospitalizations | ||||||
| Total number of inpatient hospitalizations | 202 | — | 77 | — | -61.9 | < 0.001 |
| Mean (SD) | 0.41 | (0.89) | 0.16 | (0.59) | ||
| Number of inpatient hospitalizations (categorical) per member, n (%) | ||||||
| 0 | 370 | (74.9) | 446 | (90.3) | ||
| ≥1 | 124 | (25.1) | 48 | (9.7) | ||
| Emergency department visits | ||||||
| Total number of emergency department visits | 152 | — | 87 | — | -42.8 | < 0.001 |
| Mean (SD) | 0.30 | (1.08) | 0.18 | (0.66) | ||
| Number of emergency department visits (categorical) per member, n (%) | ||||||
| 0 | 410 | (83.0) | 438 | (88.7) | ||
| ≥1 | 84 | (17.0) | 56 | (11.3) | ||
All members naive to cystic fibrosis transmembrane conductance regulator therapy and newly initiating ELX/TEZ/IVA.
ELX/TEZ/IVA = elexacaftor/tezacaftor/ivacaftor.
Cost of Care. Mean member all-cause direct health care costs are reported in Table 3. Mean member total cost of care (241.7% increase; preperiod: $58,180 [SD = $66,478] compared with postperiod: $198,815 [SD = $68,220]; P < 0.001) was significantly greater in the 180-day postperiod compared with the 180-day preperiod. Similarly, mean member pharmacy benefit cost (533.4% increase; preperiod: $29,416 [SD = $25,860] compared with postperiod: $186,331 [SD = $49,466]; P < 0.001) was significantly greater in the 180-day postperiod compared with the 180-day preperiod. Mean member 180-day ELX/TEZ/IVA cost was $156,629 (SD = $39,832). Across the prestudy and poststudy periods, pharmacy benefit spend was responsible for 50.5% and 93.7% of total cost of care, respectively (Figure 2). Significantly lower spend was observed across the medical benefit. Mean member medical benefit cost decreased by 56.6% (preperiod: $28,764 [SD = $59,703] compared with postperiod: $12,484 [SD = $51,826]; P < 0.001). Similarly, mean member inpatient hospitalization cost (preperiod: $16,771 [SD = $50,590] compared with postperiod: $4,573 [SD = $39,470]; P < 0.001) and mean member emergency department cost (preperiod: $424 [SD = $2,356] compared with postperiod: $248 [$1,441]; P = 0.01) were reduced by 72.7% and 41.4%, respectively.
TABLE 3.
Prestudy/Poststudy Change in All-Cause Direct Health Care Cost 180 Days Before and After ELX/TEZ/IVA New Initiation
| All-cause direct health care cost | 180-day preperiod | 180-day postperiod | Difference (% change) | P value |
|---|---|---|---|---|
| Total cost of care, mean (SD) | 58,180 (66,478) | 198,815 (68,220) | 241.7 | < 0.001 |
| Total medical benefit cost, mean (SD) | 28,764 (59,703) | 12,484 (51,826) | -56.6 | < 0.001 |
| Total pharmacy benefit cost, mean (SD) | 29,416 (25,860) | 186,331 (49,466) | 533.4 | < 0.001 |
| Total ELX/TEZ/IVA, cost mean (SD) | — | 156,629 (39,832) | — | — |
| Total inpatient hospitalization cost, mean (SD) | 16,771 (50,590) | 4,573 (39,470) | -72.7 | < 0.001 |
| Total emergency department cost, mean (SD) | 424 (2,356) | 248 (1,441) | -41.4 | 0.01 |
All members naive to cystic fibrosis transmembrane conductance regulator therapy and newly initiating ELX/TEZ/IVA. Costs are insurer allowed amounts, including network discounts and member share. Manufacturer did not offer ELX/TEZ/IVA rebates. Manufacturer patient assistance program discounts not included. All costs are shown in US dollars.
ELX/TEZ/IVA = elexacaftor/tezacaftor/ivacaftor.
FIGURE 2.
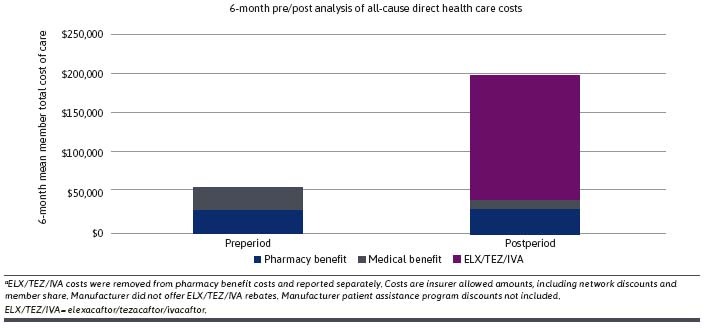
Breakdown of 180-Day Prestudy/Poststudy All-Cause Direct Total Health Care Costs
Discussion
This study reports on the real-world use, clinical effectiveness, and costs associated with ELX/TEZ/IVA therapy in a large CFTR modulator–naive commercially insured cohort. Our findings highlight the real-world clinical effectiveness of ELX/TEZ/IVA demonstrating a substantial reduction in the total number of pulmonary exacerbation events, hospitalizations, and emergency department visits. Additionally, fewer members had one or more HRU and clinical outcome of interest following ELX/TEZ/IVA initiation compared with the 180-day period prior to therapy start.
These findings further describe a notable shift in spend across both the medical and pharmacy benefits. The 180-day prestudy to poststudy periods found a 56% reduction in medical benefit and a 5.3-fold increase in pharmacy benefit spend. The results are consistent with previous analyses showing that CFTR modulator therapy direct costs exceed direct medical cost offset.13,17 Recently, in a 10-month pre/post study design consisting of 51 commercially insured member with CF newly initiating ELX/TEZ/IVA regardless of prior CFTR modulator use, Smith and Borchardt reported significant increases in average member total cost of care (pre: $216,318; post: $329,583; P < 0.001) and average member pharmacy spend (pre: $182,783; post: $316,635; P < 0.001).13 Our analysis among 494 members with CF, initiating ELX/TEZ/IVA as their first CFTR modulator, average pharmacy benefit spend per member increased by $156,915 prestudy to poststudy 180 days, whereas average medical benefit cost was reduced by an average of $16,280 per member across the same study periods resulting in a pharmacy benefit increase in spend of $9.64 to save $1 of medical benefit spend. If we were to account for the current manufacturer patient assistance program maximal annual benefit of $20,000 ($10,000 benefit over 180 days),18 our results would show a pharmacy benefit spend of $9.02 to save $1 of medical benefit spend.
The value concerns of ELX/TEZ/IVA are consistent with other therapies in the CFTR modulator class. For instance, Tessel and colleagues measured pulmonary exacerbation events using real-world data and reported inconsistent clinical benefits in the real-world setting vs trial setting for patients initiating TEZ/IVA therapy.6 Additionally, the Institute for Clinical and Economic Review concluded in their CFTR modulator value assessment that a fair price for ELX/TEZ/IVA is at an annual price of $67,900-$79,900, 4-fold lower than the list price.17 In our study, the average claim cost for a single 28-day supply of ELX/TEZ/IVA was $23,805. As commercial insurance groups are responsible for significant share of CFTR modulator therapies, we found 3-fold increases in direct total cost of CF care attributed to low baseline medical benefit spend relative to high ELX/TEZ/IVA drug spend over 180 days. Interestingly, when the clinical benefits of ELX/TEZ/IVA therapy are sustained long term (ie, 12 months, 18 months, etc.), pharmacy benefit spend, and subsequently total cost of care would grow, whereas the potential for medical cost savings shrinks.
When considering the substantial annual CFTR treatment cost, cost of drug production should be considered. It is estimated ELX/TEZ/IVA annual per patient treatment production cost is $5,676, more than 90% lower than the list price.19 When developing their actual ELX/TEZ/IVA solid dosage form oral formulation drug production cost estimate, the academic authors from the United States and United Kingdom used “Methodologies utilising prices of active pharmaceutical ingredient (API) described in previous research to reliably estimate minimum costs of production for standard oral formulations” and production occurring in India.19 The high ELX/TEZ/IVA costs are perpetuating international disparities in CF care.20 In the United States, the CFTR modulator annual costs exist within a growing subset of the drug categories that have an annual cost of more than $250,000. Individuals with drug therapy costs more than $250,000 annual has doubled from 2016 to 2019, and 2019 accounted for 0.032% of members who were responsible for 9.6% of all drug spend among 17.9 million commercially insured members.21 The rising drug costs ultimately drive-up premiums for all covered individuals, not just those with CF. Value-based performance contracts are potential options that should be explored to align CFTR modulator prices with the value they deliver. This study demonstrates that claims data can be used to objectively measure endpoints, such as hospitalizations and pulmonary exacerbations events, that can be the foundation to current outcomes-based contracts and facilitate payer/manufacturer discussions around future enhancements to standard outcomes-based agreements.
LIMITATIONS
There are study limitations to note. First, this study was retrospective in nature and data were sourced from administrative health care claims data. Retrospective administrative claim studies are dependent on data collected for purposes other than the study’s intent. However, such data are commonly stored in a format that enable cost-effective and timely assessment of relatively large cohorts of interest. Payer claims data offer unique insights into the real-world use of drug therapies and cost resulting from health resource consumption but do not infer causality unless advanced methodologies to infer causality are deployed.
Differences observed across the prestudy to poststudy periods may be attributed to factors other than ELX/TEZ/IVA therapy. The 180-day observation windows were selected as a trade-off to mitigate the impact of extraneous factors while providing useful insight into short-term cost and clinical benefit immediately following ELX/TEZ/IVA initiation. Also, manufacturer patient assistant program discounts were not included in our analysis of ELX/TEZ/IVA costs; therefore, we may have overestimated ELX/TEZ/IVA costs, although current maximal copay assistance for individuals with insurance is $20,000 annually.18
Additionally, this analysis is limited to direct medical cost offsets and drug therapy cost-effectiveness should not only be measured by direct medical use and cost offsets. In addition, impacts on quality of life, caregiving, independence, disease progression, and productivity are also important and valuable outcomes. Because of the life-extension associated with CFTR modulators and the difficulty in financially quantifying all CFTR modulator impacts, in an alternative model, CFTR modulators were found to be priced fairly.22
The postperiod in our study overlaps with the timing of the COVID-19 pandemic. Although the overall impacts of the pandemic on our study results are unknown, it is suspected that severe pulmonary exacerbations likely require management or intervention by a health care provider or encounter at a health care facility (ie, hospital admission or emergency department visit) and would not significantly change the findings of our study.
Lastly, all findings are limited to commercially insured members and may not extrapolate to patients with CF receiving benefits from government sponsored programs.
Conclusions
This real-world study demonstrates the clinical effectiveness and cost impacts of ELX/TEZ/IVA in a large commercially insured CF cohort. Meaningful reductions in HRU occurred with ELX/TEZ/IVA therapy, including reductions of 62% in inpatient hospitalizations, 43% in emergency department visits, and 44% in pulmonary exacerbations. However, these improved outcomes come at substantial drug therapy investment with mean member total cost of care increasing by $140,635 over 180 days, with pharmacy benefit spend accounting for $9.64 for every $1 of medical benefit spend. These increased costs are straining health care affordability. It is essential for therapies to be priced based on value. One method to ensure fair pricing is to implement a value-based contract with the manufacturer to obtain remuneration if the therapy does not deliver expected outcomes.23 This study demonstrates that claims data can be used to objectively measure endpoints such as hospitalizations and pulmonary exacerbations events that can be the foundation to outcomes-based arrangements.
REFERENCES
- 1.Patient Registry 2021 Annual Data Report. Cystic Fibrosis Foundation. Accessed December 5, 2022. https://www.cff.org/sites/default/files/2021-11/Patient-Registry-Annual-Data-Report.pdf
- 2.Zaher A, ElSaygh J, Elsori D, ElSaygh H, Sanni A.. A review of Trikafta: Triple cystic fibrosis transmembrane conductance regulator (CFTR) modulator therapy. Cureus. 2021;13(7):e16144. doi:10.7759/cureus.16144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fajac I, Sermet I. Therapeutic approaches for patients with cystic fibrosis not eligible for current CFTR modulators. Cells. 2021;10(10):2793. doi:10.3390/cells10102793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US CF Foundation, Johns Hopkins University, The Hospital for Sick Children. The Clinical and Functional Translation of CFTR (CFTR2). Accessed October 5, 2022. http://cftr2.org/ [Google Scholar]
- 5.Sheppard MN, Nicholson AG. The pathology of cystic fibrosis. Current Diagnostic Pathology. 2002;8(1):50-9. doi:10.1054/cdip.2001.0088 [Google Scholar]
- 6.Tesell MA, Alper CJ, Bacon R, et al. . Effect of lumacaftor/ivacaftor on pulmonary exacerbation rates in members with cystic fibrosis in a Medicaid population. J Manag Care Spec Pharm. 2019;25(9):1021-5. doi:10.18553/jmcp.2019.25.9.1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ren CL, Morgan RL, Oermann C, et al. . Cystic fibrosis pulmonary guidelines: Use of CFTR modulator therapy in patients with cystic fibrosis. Ann Am Thorac Soc. 2018;15(3):271-80. doi:10.1513/AnnalsATS.201707-539OT [DOI] [PubMed] [Google Scholar]
- 8.Stanford GE, Dave K, Simmonds NJ. Pulmonary exacerbations in adults with cystic fibrosis: A grown-up issue in a changing cystic fibrosis landscape. Chest. 2021;159(1):93-102. doi:10.1016/j.chest.2020.09.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clancy JP. Rapid therapeutic advances in CFTR modulator science. Pediatr Pulmonol. 2018;53(S3):S4-11. doi:10.1002/ppul.24157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solomon GM, Marshall SG, Ramsey BW, Rowe SM. Breakthrough therapies: Cystic fibrosis (CF) potentiators and correctors. Pediatr Pulmonol. 2015;50 Suppl 40(0 40):S3-13. doi:10.1002/ppul.23240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ridley K, Condren M. Elexacaftor-tezacaftor-ivacaftor: The first triple-combination cystic fibrosis transmembrane conductance regulator modulating therapy. J Pediatr Pharmacol Ther. 2020;25(3):192-7. doi:10.5863/1551-6776-25.3.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trikafta. Package insert. Vertex Pharmaceuticals Inc; 2021. [Google Scholar]
- 13.Smith S, Borchardt M. Analyzing the use and impact of elexacaftor/tezacaftor/ivacaftor on total cost of care and other health care resource utilization in a commercially insured population. J Manag Care Spec Pharm. 2022;28(7):721-31. doi:10.18553/jmcp.2022.28.7.721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heijerman HG, McKone EF, Downey DG, et al. . Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: A double-blind, randomised, phase 3 trial. Lancet. 2019;394(10212):1940-48. doi:10.1016/S0140-6736(19)32597-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Middleton PG, Mall MA, Dřevínek P, et al. . Elexacaftor–tezacaftor–ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. 2019;381(19):1809-19. doi:10.1056/NEJMoa1908639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grosse SD, Do TQ, Vu M, Feng LB, Berry JG, Sawicki GS. Healthcare expenditures for privately insured US patients with cystic fibrosis, 2010-2016. Pediatr Pulmonol. 2018;53(12):1611-8. doi:10.1002/ppul.24178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tice JA, Kuntz KM, Wherry K, et al. . Modulator treatments for cystic fibrosis: Effectiveness and value: Final evidence report and meeting summary. Institute for Clinical and Economic Review. Accessed November 18, 2022. https://icer.org/wp-content/uploads/2020/08/ICER_CF_Final_Report_092320.pdf [Google Scholar]
- 18.Grant J. Vertex copay assistance: Tips from a CF pharmacist. Cystic Fibrosis Foundation. Accessed March 2, 2023. https://www.cff.org/community-posts/2022-11/vertex-copay-assistance-tips-cf-pharmacist [Google Scholar]
- 19.Guo J, Wang J, Zhang J, Fortunak J, Hill A. Current prices versus minimum costs of production for CFTR modulators. J Cyst Fibros. 2022;21(5):866-72. doi:10.1016/j.jcf.2022.04.007 [DOI] [PubMed] [Google Scholar]
- 20.Guo J, Garratt A, Hill A. Worldwide rates of diagnosis and effective treatment for cystic fibrosis. J Cyst Fibros. 2022;21(3):456-62. doi:10.1016/j.jcf.2022.01.009 [DOI] [PubMed] [Google Scholar]
- 21.Starner CI, Bowen K, Gleason PP. Drug super spender tsunami: An integrated medical and pharmacy benefits assessment. J Manag Care Spec Pharm. 2022;28(11):1200-6. doi:10.18553/jmcp.2022.28.11.1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubin JL, Lopez A, Booth J, Gunther P, Jena AB. Limitations of standard cost-effectiveness methods for health technology assessment of treatments for rare, chronic diseases: A case study of treatment for cystic fibrosis. J Med Econ. 2022;25(1):783-91. doi:10.1080/13696998.2022.2077550 [DOI] [PubMed] [Google Scholar]
- 23.Academy of Managed Care Pharmacy. Value based contract resources. August 1, 2022. Accessed March 2, 2023. https://www.amcp.org/resource-center/value-based-contracts/value-based-contracts-resources


