Abstract
The contractile state of resistance arteries and arterioles is a crucial determinant of blood pressure and blood flow. Physiological regulation of arterial contractility requires constant communication between endothelial and smooth muscle cells. Various Ca2+ signals and Ca2+-sensitive targets ensure dynamic control of intercellular communications in the vascular wall. The functional effect of a Ca2+ signal on arterial contractility depends on the type of Ca2+-sensitive target engaged by that signal. Recent studies using advanced imaging methods have identified the spatiotemporal signatures of individual Ca2+ signals that control arterial and arteriolar contractility. Broadly speaking, intracellular Ca2+ is increased by ion channels and transporters on the plasma membrane and endoplasmic reticular membrane. Physiological roles for many vascular Ca2+ signals have already been confirmed, while further investigation is needed for other Ca2+ signals. This article focuses on endothelial and smooth muscle Ca2+ signaling mechanisms in resistance arteries and arterioles. We discuss the Ca2+ entry pathways at the plasma membrane, Ca2+ release signals from the intracellular stores, the functional and physiological relevance of Ca2+ signals, and their regulatory mechanisms. Finally, we describe the contribution of abnormal endothelial and smooth muscle Ca2+ signals to the pathogenesis of vascular disorders.
Introduction
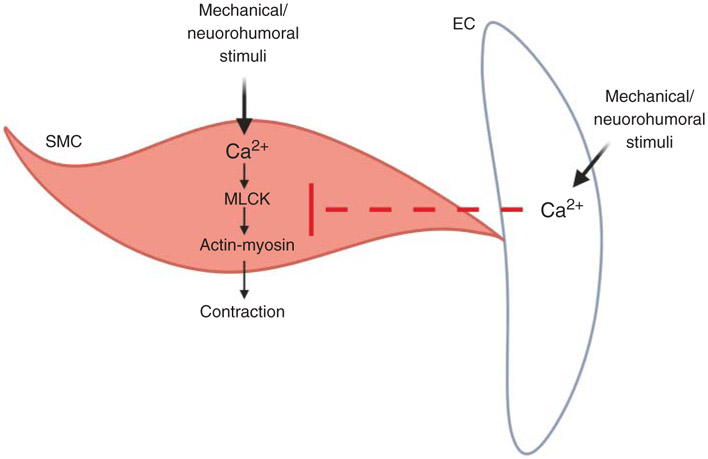
Vascular resistance is a crucial determinant of blood pressure and blood flow to target organs. The contractile state of small arteries and arterioles determines vascular resistance. Smooth muscle cells (SMCs) and endothelial cells (ECs) are the two main cell-types involved in the dynamic regulation of vascular contractility. Both SMCs and ECs recruit various Ca2+ signaling mechanisms to regulate vascular contractility (Figure 1) (344). The canonical view is that endothelial and SMC Ca2+ have opposite effects on vascular diameter. While increases in endothelial Ca2+ cause vasodilation, increases in SMC Ca2+ have mostly been linked to vasoconstriction, except for Ca2+ sparks (Table 1), which can cause vasodilation. Moreover, intracellular Ca2+ plays a central role in EC-SMC communication, which is pivotal for physiological regulation of vascular contractility.
Figure 1. The contrasting effects of smooth muscle cell (SMC) and endothelial cell (EC) Ca2+ on vascular contractility.
Mechanical and neurohumoral stimuli can increase intracellular Ca2+ in SMCs and ECs. Intracellular Ca2+ in SMCs and ECs, in general, has opposite effects on vascular resistance. Increase in SMC Ca2+ activates the contractile machinery in SMCs (myosin light chain kinase or MLCK/Actin-Myosin). In contrast, an increase in EC Ca2+ inhibits SMC contractile mechanisms. The dotted red line indicates inhibition of SMC contractility.
Table 1.
Individual Ca2+ Signals in smooth muscle cells (SMCs) and endothelial cells (ECs)
| Type of Ca2+ signal | Source | Physiological effect | References |
|---|---|---|---|
| VDCC Ca2+ sparklets (SMC) | Unitary Ca2+ influx events occurring through single VDCCs |
|
(16, 314-317, 323, 324, 464) |
| TRPV4 sparklets (SMC, and EC) | Unitary Ca2+ influx events occurring through single TRPV4 channels |
|
(166, 178, 282, 292, 342, 432, 433, 459) |
| Ca2+ sparks (SMC) | Unitary Ca2+ release events occurring through RyRs |
|
(226, 318, 353) |
| Ca2+ blips, puff, and waves (SMC, and EC) | Local (blips and puff) or whole cell propagating (waves) Ca2+ release events occurring through IP3Rs |
|
(44, 109, 137, 202, 212, 287, 468) |
| Ca2+ pulsars and wavelets (EC) | Local Ca2+ release events occurring at MEPs via IP3Rs |
|
(242, 488) |
| JCaTs (SMC) | Local Ca2+ influx events occurring through P2X1R activated by ATP release from perivascular nerves |
|
(238, 239, 369) |
| TRPA1 sparklets (EC) | Unitary Ca2+ influx events occurring through TRPA1 channels |
|
(359, 448) |
| TRPV3 sparklets (EC) | Unitary Ca2+ influx events occurring through TRPV3 channels |
|
(99, 360) |
Cytosolic Ca2+ levels can increase via the influx of extracellular Ca2+ or release of Ca2+ from intracellular stores, including endoplasmic reticulum (ER) or sarcoplasmic reticulum (SR) and lysosomes. The majority of Ca2+ signals in the arteriolar walls occur in a spatially restricted manner, with the diffusion of Ca2+ limited by numerous Ca2+-binding proteins and high viscosity of the cytosol. The spatially restricted nature of Ca2+ signals confers the specificity of targets/functional effects and limits toxicity to the cell. Moreover, signaling microdomains that localize Ca2+ signals with their signaling targets ensure specific activation of the targets. Such signaling microdomains also provide efficient Ca2+ signal-target coupling, whereby smaller increases in Ca2+ can activate a small number of nearby target molecules to achieve physiological effects. EC projections to SMCs, or myoendothelial projections (MEPs), are prime examples of signaling microdomains enabled by specialized microstructures. The majority of endothelial Ca2+ signals occur at MEPs, and Ca2+-sensitive targets also localize to MEPs. The Ca2+ signal-target proximity at MEPs facilitates efficient and precise communication between ECs and SMCs. Similarly, signaling nanodomains involving localization of proteins inside the caveolae have been shown in ECs and SMCs. In SMCs, signaling microdomains are enabled by structural features (e.g., proximity of the SR to the membrane) or co-localization of Ca2+ channels with other ion channels or anchoring proteins. In this article, we discuss the Ca2+ signal-target linkages in arteries and arterioles, regulatory mechanisms, and abnormalities in Ca2+ signaling that contribute to the pathogenesis of vascular disorders.
SMC Ca2+ Signals in Small Arteries and Arterioles
SMCs are the contractile cells in vascular walls. Several physiological stimuli, including intravascular pressure, G-protein coupled receptors (GPCRs), and neurohumoral mediators, contract SMCs from resistance-sized arteries. Under resting conditions, arterial contractility is mainly determined by intraluminal pressure-induced constriction (myogenic constriction) and nerve-induced (neurogenic) constriction. Myogenic vasoconstriction is an inherent feature of SMCs from resistance-sized arteries (27). It is also a crucial contributor to vascular resistance and autoregulation of blood flow (83).
Both myogenic and neurogenic vasoconstrictions are accomplished predominantly through an increase in SMC Ca2+. Moreover, neurohumoral mediators can activate GPCRs on SMC membranes to increase SMC Ca2+. The importance of distinguishing global versus local increases in SMC Ca2+ is well documented (reviewed in Ref. 344). Whole-cell increases in SMCs Ca2+ result in vasoconstriction. On the contrary, some localized increases in Ca2+ (Ca2+ sparks, Table 1) can cause vasodilation (305, 318, 352). The canonical pathway for SMC contraction involves Ca2+-calmodulin (CaM)-dependent activation of myosin light chain kinase (MLCK). MLCK phosphorylates the myosin regulatory light chain (RLC20), initiating actin-myosin cross-bridge formation that results in SMC contraction (429). A parallel GPCR-mediated pathway activates RhoA-dependent kinase (ROCK), which phosphorylates and inhibits myosin light chain phosphatase (MLCP). MLCP inhibition results in reduced RLC20 dephosphorylation and sustained SMC contraction. Additionally, the p90 Ribosomal S6 Kinase 2 (RSK2) has been recently proposed as an upstream mediator of Ca2+-dependent and Ca2+-independent SMC contraction (19). Intraluminal pressure and GPCR activation facilitated RSK2 activation via extracellular signal-regulated kinase (ERK1/2) and phosphoinositide-dependent kinase (PKD) signaling pathway. RSK2, in turn, directly phosphorylated RLC20 and activated the Na+/H+ exchanger causing alkalization-dependent Ca2+ release and SMC contraction.
Ca2+ influx from extracellular compartment
The cytosolic concentration of free Ca2+ is maintained low (~100 nM) by the presence of Ca2+-binding proteins and intracellular organelles that act as storages of Ca2+ (72). A negatively charged intracellular environment, coupled with high extracellular Ca2+ concentration (~1–2 mM), accounts for an electrochemical gradient favorable for Ca2+ influx into the cells. The opening of Ca2+ permeable ion channels on the SMC membranes allows extracellular Ca2+ to move into the cytosol along the electrochemical gradient. The Ca2+ entry mechanisms on SMC membranes can be broadly divided into voltage-gated and non-voltage-gated. The voltage-gated Ca2+ entry pathways on SMC membranes include L-type and T-type Ca2+ channels and the non-voltage-gated Ca2+ entry mechanisms include transient receptor potential (TRP) channels, PIEZO channels, store-operated Ca2+ entry (SOCE), purinergic receptors, and Na+/Ca2+ exchangers (NCXs).
Voltage-gated Ca2+ entry pathways (L-type and T-type Ca2+ channels)
Whole-cell patch-clamp studies on SMCs isolated from rat mesenteric arteries identified two types of Ca2+ currents—transient (T-type) and long-lasting (L-type) (29). The ion channels underlying T-type and L-type Ca2+ currents were voltage-gated, implying that a structural feature (voltage sensor) enables channel opening in response to membrane depolarization. T-type or transient Ca2+ channel (TTCC) currents exhibited faster inactivation properties when compared to L-type or long-lasting Ca2+ channel (LTCC) currents. However, this distinction can be misleading since the observed channel inactivation properties depend heavily on the experimental conditions (28). A more meaningful distinction between the two channel types can be derived from comparisons of their voltage-gating properties. TTCCs are activated at more negative voltages (−60 mV) compared to LTCCs, which are activated at more depolarized voltages (−30 mV) (331, 533). The current-voltage relationship for TTCCs shows a peak current at −15 mV whereas LTCCs show peak currents at +20 mV (108). LTCC and TTCC expression varies among different vascular beds and different-sized arteries within the same vascular bed. Western blotting experiments showed similar expression of TTCCs and LTCCs in the aorta (24). On the contrary, TTCC expression was found to be higher than LTCCs in mesenteric arteries, arterioles (24), and cerebral arteries (1, 150, 154). A definitive assessment of the relative abundance of arterial LTCCs and TTCCs may require more precise quantitative techniques such as mass spectrometry.
Structure of voltage-gated Ca2+ channels.
Three different families of voltage-gated Ca2+ channels (CaV1, CaV2, and CaV3) share a similar structure (107, 427). The amino acid sequence of the large pore-forming α1 subunit (~190 kDa, ~2000 amino acids) determines the gating properties and sensitivity to Ca2+ channel blockers (55, 460). Ten α1 subunits, encoded by ten different genes, have been identified. The α1 subunit is organized in four (I-IV) homologous domains, each composed of six transmembrane segments (TM 1-6). A membrane-associated loop (loop P) between TM5-6 from each domain forms the channel pore. TM4 is enriched with positively charged amino acids (lysine or glycine) and has been described as the voltage-sensing domain (VSD) (180). In response to membrane depolarization, TM4 rotates and opens the channel pore. Glutamate residues on loop P confer Ca2+ selectivity to the channel. TM6, which lines the inner pore, is the binding site for phenylalkylamines and dihydropyridines (Ca2+ channel blockers). Therefore, the amino acid sequence of TM6 determines the selectivity of the Ca2+ channel blockers against different Ca2+ channel subtypes (176).
Four additional accessory subunits have been identified for LTCCs: a dimer α2δ of 170 kDa, an intracellular β subunit of approximately 55 kDa, and a TM γ subunit of 33 kDa (460). α2δ and γ subunits are type-I TM proteins. α2 subunit is localized extracellularly and bound to δ subunit by a disulfide bond (55). β subunit binds with a high affinity to the intracellular I-II linker of the α1 subunit (370). The presence of Ca2+ currents in a cell line overexpressing α1 subunit alone revealed that α1 subunit is sufficient to form a functional Ca2+ channel, albeit with altered gating properties (354). Co-expression of α1 and β subunits increased channel expression and normalized the gating properties (237), indicating an essential role for the regulatory subunits in controlling channel expression and function. Among the accessory β subunits, β3 was the principal isoform in SMCs (221). Current evidence suggests that unlike LTCCs, TTCCs are not associated with any auxiliary subunits (55).
L-type Ca2+ channels (LTCC).
CaV1.1-1.3 gene family encodes the α1 subunits of LTCCs. CaV1.2, encoding for α1C subunit, has been regarded as the primary voltage-gated Ca2+ influx pathway in SMCs (Figure 2). CaV1.2 channels are the primary mediators of myogenic vasoconstriction and vasoconstriction induced by the activation of α1-adrenergic receptors and angiotensin II receptors (128, 300). CaV1.2 channel displays a unitary conductance of approximately 25 pS with Ba2+ as a charge carrier (71). Ca2+ profoundly influences the open-state probability and inactivation kinetics of the CaV1.2 channel. The C-terminal tail of the α1 subunit contains a CaM-binding isoleucine-glutamine (IQ) domain (385). Ca2+-CaM binding to the IQ domain results in the modulation of channel activity. Consistent with this property, the substitution of isoleucine with alanine in the IQ motif impaired Ca2+-dependent inactivation and revealed the Ca2+-dependent activation of the channel. However, the substitution of the same isoleucine with glutamate resulted in the loss of Ca2+-dependent activation and inactivation of the channel (142, 357, 565). Ca2+ -dependent inactivation limit Ca2+ entry through the channel during sustained membrane depolarization and prevents Ca2+ overload and cytotoxicity.
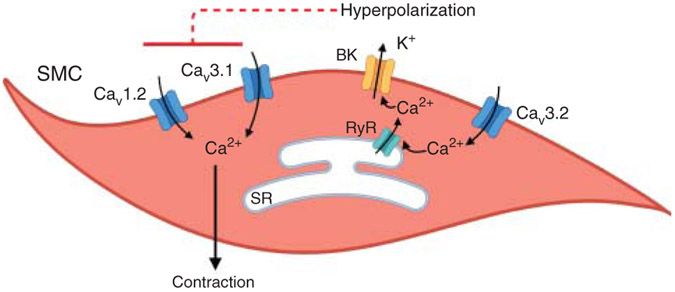
Figure 2. Regulation of vascular smooth muscle cell (SMC) contractility by voltage-gated Ca2+ channels.
Ca2+ entry through CaV1.2 and CaV3.1 channels promotes SMC contraction. Ca2+ influx through CaV3.2 channels activates ryanodine receptors (RyRs), triggering Ca2+ release from the sarcoplasmic reticulum (SR) in the vicinity of large conductance, Ca2+-activated K+ (BK) channels. BK channel activation results in SMC hyperpolarization and vasodilation. The dotted line indicates the deactivation of CaV1.2 and CaV3.1 channels.
Protein kinases (PKA, PKC, and PKG) are among the most important regulators of LTCC activity in SMCs (289). Nitric oxide (NO) induced cyclic guanosine monophosphate (cGMP)-PKG activation to reduce LTCC currents in SMCs, partly accounting for NO-dependent vasodilation (9, 39). Furthermore, inhibition of protein kinase G (PKG) increased LTCC activity, further supporting the inhibitory role of NO-cGMP-PKG signaling on LTCC activity in SMCs (393, 473). There are conflicting reports on PKA-modulation of LTCC activity in SMCs (218). Protein kinase A (PKA) phosphorylated Ser1928 on the C-tail of α1C subunit of LTCC, potentiating channel activity (125). In SMCs from cerebral arteries, exposure to high extracellular glucose increased LTTC currents. Interestingly, glucose-induced increase in LTTC activity was mediated by PKA activation and its anchoring close to LTCCs by A-kinase anchoring protein 150 (AKAP150) (317). β Adrenergic receptor-mediated activation of PKA, however, has concentration-dependent effects on LTCC activity. Low concentrations of isoproterenol (ISO, β adrenergic receptor agonist) or forskolin (PKA activator) increased LTCC currents, whereas high concentrations of ISO or forskolin had a biphasic effect—an immediate increase in LTCC currents followed by a decrease in currents (197). Compartmentalization of cAMP/PKA signaling in AKAP150-enriched plasma membrane microdomains could explain the biphasic effects of cAMP/PKA signaling in SMCs. In this regard, PKA-dependent activation of Ca2+-sensitive K+ channels hyperpolarized the plasma membrane, thereby deactivating LTCCs (348, 366). Protein kinase C (PKC) regulates LTCC via multiple modes of action. Inhibition of PKC impaired the development of myogenic constriction in cremaster arteries (173). Still, it did not affect myogenic constriction in ophthalmic arteries (198), indicating heterogeneous effects of PKC on LTCC-dependent myogenic vasoconstriction. Among the four canonical PKC isoforms (α, βI, βII, and γ) (439), PKCα appears to be the mediator of myogenic constriction in coronary arteries (89). PKCα also increased the open-state probability of LTTCs in AKAP150-enriched microdomains on SMC membranes in cerebral arteries (314, 315). Navedo and colleagues (316) indicated that AKAP150 recruits PKCα close to LTCCs and allows spatially restricted activation of LTCCs (Ca2+ sparklets, Table 1). Thus, protein kinases play a crucial role in fine-tuning the activity of SMC LTCCs.
T-type Ca2+ channels (TTCC).
CaV3 (3.1–3.3) genes encode for α1G, α1H, and α1I subunits that mediate TTCC currents. TTCCs show a single-channel conductance of 7.5 to 9 pS, and similar conductance with Ba2+ or Ca2+ as a charge carrier (56). A critical structural distinction between LTCCs and TTCCs is that the TTCCs have not been associated with any auxiliary subunits (55). CaV3.1-3.3 RNA levels and expression were detected in cremaster, renal, mesenteric, and cerebral arteries (47, 141, 148, 236, 495). The majority of the studies on vascular TTCCs have used mibefradil, a non-specific TTCC inhibitor. Therefore, the importance of SMC TTCC in the development of myogenic vasoconstriction remains unclear (236, 495). In rat cerebral main basilar arteries, myogenic constriction was mostly mediated by LTCCs, whereas TTCCs were important for myogenic constriction in large and small side branches (236). Pressure myography studies in mesenteric arteries from CaV3.1−/− mice suggested a predominant role for TTCCs in the development of myogenic constriction at lower intravascular pressures (40 mmHg) and a more important role for LTCCs at higher intravascular pressures (100 mmHg) (38). Similarly, in cerebral arteries, TTCCs contributed to myogenic constriction at lower pressures (20 mmHg) and hyperpolarized membrane potential (−60 mV) (Figure 2). Computational modeling predicts that TTCCs might be playing a predominant role in facilitating myogenic vasoconstriction under resting conditions, although further studies are needed to confirm this hypothesis (1). In this regard, Harraz and colleagues (150, 152) recently linked the Ca2+ influx through CaV3.2 channels to the dilation of cerebral arteries (Figure 2). The authors showed that CaV3.2 channels activate ryanodine receptors (RyRs) on the SR membrane, triggering Ca2+ sparks (Ca2+ release signals from the SR, Table 1). Ca2+ sparks activate large-conductance, Ca2+ activated potassium (BK) channels, thus initiating a negative feedback mechanism that counteracts myogenic vasoconstriction. Notably, TTCCs lack Ca2+-dependent inactivation, making them an ideal source of Ca2+ for RyR activation and initiation of Ca2+ sparks via Ca2+-induced Ca2+ release. In a recent study, this research team reported that CaV3.2 and RyRs co-localize in caveolar nanodomains, and genetic deletion of caveolin-1 disrupts CaV3.2-RyR interaction (161).
Regulation of TTCCs occurs through different cellular mechanisms. NO-dependent activation of cGMP/PKG signaling inhibited TTCC currents and TTCC-induced myogenic constriction in rat cerebral arteries (151). PKA also inhibited TTCC currents, particularly the currents through CaV3.2 isoform (155). Reactive oxygen species (ROS) have diverse effects on TTCC activity. Superoxide radicals enhanced the expression of CaV3.1 and CaV3.2 channels and their contribution to myogenic vasoconstriction in cremaster and mesenteric arteries (185). In contrast, hydrogen peroxide (H2O2) inhibited CaV3.2 channel currents (340). Furthermore, Ang II-dependent activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase enzyme suppressed CaV3.2 currents, thereby impairing CaV3.2-RyR-BK channel signaling and promoting vasoconstriction in response to Ang II (163).
Non-voltage-gated Ca2+ entry pathways (TRP channels, PIEZO channel, Purinergic Receptor, and Na+/Ca2+ exchanger)
TRP channels are the primary non-voltage-gated Ca2+ influx pathways on SMC membranes. TRP channels participate in the regulation of SMC contractility and proliferation. These functions are achieved either by promoting global or localized increases in intracellular Ca2+ or by inducing the activation of ion channels that cause membrane depolarization. The mammalian family of TRP channels can be divided into six subfamilies: TRPC (Canonical), TRPM (Melastatin), TRPML (Mucolipins), TRPV (Vanilloid), TRPP (Polycystic), and TRPA (Ankyrin-rich protein). All TRP channels share the same general structure. Functional TRP channels are composed of four subunits, each subunit with six TM domains (S1-S6) and intracellular C- and N-terminal tails of variable lengths. A 25-amino acids domain named “TRP domain”, located immediately after S6 toward the C-terminal is conserved among TRPV, TRPM, and TRPC subfamilies but is not found in TRPA1, TRPP, and TRPML channels (378). TRP domain is the binding site for phosphatidylinositol 4,5-bisphosphate (PIP2) (456). PIP2 modulation of TRP channels is complex and may result in channel inhibition or activation depending on the channel and experimental conditions (388). TRPV, TRPA, and TRPC channels exhibit multiple Ankyrin repeat domains (ARDs) on the N-terminal tail that contribute to channel regulation via protein-protein interactions (98). ARD3 is essential for the physical assembly of the functional tetrameric structure of TRPV5 and TRPV6 channels (106). While TRPV1 and TRPV4 channel activity is enhanced by adenosine triphosphate (ATP) binding to the concave surface located between ARD1-3 (260), TRPV3 channels sensitization is prevented following ATP binding to ARD1-3 (358). Ca2+-CaM binding site at the C-terminal tail is responsible for the modulation of TRP channel activity by cytosolic Ca2+, an essential regulator of TRP channel activity (159, 560, 562). Additionally, a Ca2+-CaM binding site within the TRP domain has also been shown for the TRPV family (135).
TRPV (TRPV1 and TRPV4) channels.
High unitary conductance and permeability for Ca2+ are characteristic properties of TRPV channels. TRPV channels show a range of selectivity for Ca2+, although most TRPV channels are more selective for Ca2+ over Na+. Among the TRPV subfamily members, only TRPV1 and TRPV4 channels have been shown to be expressed in native SMCs from resistance arteries (Figure 3A) (95). TRPV1 channel has a unitary conductance of 35 to 70 pS and higher permeability for divalent over monovalent cations (PCa2+/PNa+ = 10) (54, 397). TRPV1 channel agonist, capsaicin, constricted canine denervated mesenteric arteries, supporting a contractile role of TRPV1 channels (365). Studies by Kark and colleagues (214) further demonstrated a vasoconstrictor role for TRPV1 channels in skeletal muscle arteries, although the cell-type containing TRPV1 channels in the vascular wall was not clear. Studies by Cavanaugh and colleagues (57) in TRPV1-LacZ reporter mouse confirmed a robust expression of TRPV1 channels in SMCs from cerebral arteries. Moreover, TRPV1 channel activation increased intracellular Ca2+ in SMCs, an effect that was blunted in the arteries from TRPV1−/− mice (57). Although the ex vivo findings suggest that SMC TRPV1 channels are contractile, their potential physiological role in influencing vascular resistance remains unknown. Indeed, resting blood pressure is unaltered in TRPV1−/− mice (280, 561). Therefore, studies in SMC specific TRPV1−/− mice are needed to address the physiological role of SMC TRPV1 channel.
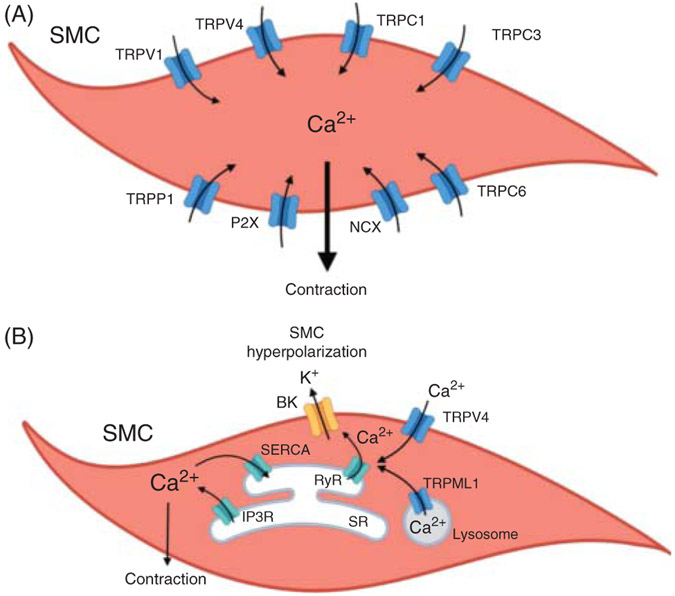
Figure 3. Regulation of vascular smooth muscle cell (SMC) contractility by non-voltage-gated Ca2+ entry mechanisms.
(A) Activation of purinergic P2X receptor, TRPV4, TRPV1, TRPP1, TRPC3, and TRPC6 channels, and NCX in reverse mode increases SMC intracellular Ca2+, leading to vasoconstriction. (B) Ca2+ release from endolysosome via TRPML1 channel, or Ca2+ entry through TRPV4 channel at the plasma membrane activates ryanodine receptors (RyRs), triggering Ca2+ release signals (Ca2+ sparks) from the sarcoplasmic reticulum (SR). Ca2+ sparks activate large-conductance Ca2+-activated potassium (BK) channels. BK channels hyperpolarize the SMC membrane and cause vasodilation. Ca2+ release through IP3R induces SMC contraction. Sarco-endoplasmic reticulum Ca2+-ATPase (SERCA), by sequestering cytoplasmic Ca2+ back into the SR, maintains low cytosolic Ca2+ concentration. TRPV, TRPP, TRPC, TRPML, members of transient receptor potential channel family; NCX, Na+/Ca2+ exchanger.
Cytosolic Ca2+, PKC, and calcineurin are the main endogenous regulators of TRPV1 channel activity (361). TRPV1 channel current is inhibited by physiological Ca2+ concentrations (397). Ca2+-CaM dependent decrease in TRPV1 channel currents was prevented by deleting a 35-amino acids sequence (Glu767-Thr801) on the C-terminal tail of the TRPV1 channel (333). PKC-dependent phosphorylation of Ser502, Thr704, and Ser800 activated TRPV1 channels (334, 507), whereas calcineurin-dependent dephosphorylation inhibited channel activity (265).
SMC TRPV4 channels have been variously reported to cause dilation or constriction depending on the signaling targets they activate and the vascular bed under consideration. The unitary conductances of TRPV4 channels are 50 to 60 pS at −60 mV, and 90 to 100 pS at +60 mV (441, 511, 512). TRPV4 channels display higher permeability for Ca2+ over Na+ (PCa2+/PNa+ = 6–10) (85, 501), and can be activated by temperature, mechanical stimuli, and neurohumoral mediators. In SMCs from cerebral arteries, Ca2+ influx through TRPV4 channels (Table 1) promoted vasodilation. Ca2+ influx through TRPV4 channels increased the activity of RyR-BK channel signaling, hyperpolarizing SMC by approximately 10 mV and causing vasodilation (Figure 3B) (96). A similar mechanism was described in resistance mesenteric arteries and was shown to be impaired in TRPV4−/− mice (101). SMC TRPV4 channels appear to play a pivotal role in counteracting Ang II-induced vasoconstriction in cerebral arteries. Mercado and colleagues (292) indicated that Ang II enhances SMC TRPV4 channel activity in cerebral arteries via AKAP150 anchoring of PKCα close to TRPV4 channels and subsequent channel phosphorylation. Indeed, Ang II signaling increased the proximity between AKAP150 and TRPV4 channels. Superresolution nanoscopic studies showed that TRPV4 channel activation by Ang II decreases exponentially with the distance between AKAP150 and TRPV4 channel. TRPV4 channel activity was undetectable if AKAP150 and TRPV4 channel are more than 200 nm apart. Interestingly, the distance between AKAP150 and TRPV4 channel, and the role of TRPV4 channel in counteracting Ang II-dependent vasoconstriction were variable among different vascular beds (459). TRPV4 channels are also expressed in SMCs from pulmonary arteries (281). In chronic hypoxia, TRPV4 channel is upregulated in mice pulmonary arteries resulting in higher contractility and a pulmonary hypertensive phenotype (528, 538). Thus, SMC TRPV4 channels appear to have distinct effects on vascular diameter in systemic and pulmonary arteries.
Several endogenous regulators of TRPV4 channel activity have been identified. Cytosolic Ca2+ has a biphasic effect on TRPV4 channel activity. Low concentrations of intracellular Ca2+ facilitate TRPV4 channel opening, whereas high concentrations limit the channel activity. Ca2+-dependent activation/inactivation of TRPV4 channels occurs via Ca2+-CaM binding on the C-terminal tail (C-CaMB) of the channel (362, 442, 510). Half-maximal CaM binding affinity at C-CaMB was observed at nanomolar concentrations of Ca2+ (150 nM). Mutations in C-CaMB resulted in the impairment of Ca2+-dependent potentiation of TRPV4 channel activity (442). Notably, Ca2+-dependent inhibition of the TRPV4 channel at a higher concentration of Ca2+ (IC50 of 406 nM) was maintained in the mutants (442, 510). These findings indicated that Ca2+-dependent inhibition of TRPV4 channel does not rely on Ca2+-CaM binding to C-CaMB. Strotmann and colleagues (443) proposed a mechanism whereby an interaction between the N- and C-terminal tails prevents channel activation. An increase in cytosolic Ca2+ enabled CaM binding to the C-CaMB, which resulted in the displacement of N-terminal from the C-terminal tail and TRPV4 channel activation. Recently, a Ca2+-CaM binding site was identified on the N-terminal tail (aa 132–383, N-CaMB) (358). Phelps and colleagues (358) demonstrated that ATP interaction with N-CaMB increases TRPV4 channel currents. It could be speculated that at higher cytosolic Ca2+ concentrations, Ca2+-CaM competes with ATP for binding to N-CaMB, resulting in TRPV4 channel inhibition. Such a mechanism has been proposed for Ca2+-CaM-dependent TRPV1 channel inhibition (260, 389). Unfortunately, studies on Ca2+-induced inactivation of the TRPV4 channel are scarce.
Protein kinases are another important endogenous regulators of TRPV4 channel activity. In expression systems, PKC augmented TRPV4 channel activity by phosphorylating Ser162, Thr175, and Ser189 on the N-terminal tail; whereas PKA increased TRPV4 channel activity by phosphorylating Ser824 on the C-terminal tail. Moreover, AKAP150 enhanced TRPV4 channel phosphorylation by PKA and PKC (51, 111). Epoxyeicosatrienoic acids (EETs), formed from arachidonic acid (AA) by phospholipase A2, are also known to activate TRPV4 channels (502, 511). Overall, the presence of TRPV4 channels in SMCs and their role in controlling vascular contractility are well established. However, addressing the significance of SMC TRPV4 channels at the whole-animal level awaits the development of SMC-specific TRPV4−/− mice.
TRPC (TRPC1/TRPC3/TRPC6) channels.
TRPC1 is a non-selective cation channel with similar permeability to Ca2+ and monovalent cations (PNa+/PCa2+ = 0.95). The unitary conductance of TRPC1 channels is approximately 5 pS (444). It is unclear whether TRPC1 monomers form a functional channel or form heteromeric structures with other TRP channels, thereby influencing their properties. TRPC1 channels have been proposed to mediate SOCE into SMCs, although the SOCE through TRPC1 channels remains controversial (10, 30, 235, 322, 462). In SMCs from rabbit cerebral arteries, inhibiting TRPC1 channel with a specific antibody impaired thapsigargin (sarco-endoplasmic reticulum Ca2+-ATPase or SERCA inhibitor)-induced increase in intracellular Ca2+ by approximately 20%. This result might suggest that the Ca2+ influx following the depletion of intracellular Ca2+ stores is mediated, in part, by TRPC1 channel (532). Additionally, SOCE was impaired in SMCs from mesenteric arteries of TRPC1−/− mice. Later studies showed that TRPC1 channel activation by depletion of Ca2+ stores occurs through PKC-phosphorylation of the channel, facilitating PIP2 binding and channel activation (415, 416, 504). The depletion of intracellular Ca2+ stores enhanced PKC activity via Gαq-dependent activation of phospholipase Cβ1 (PLCβ1) (417). However, studies in cerebral arteries by Dietrich et al. (91) suggested that TRPC1 channel is not a required element for SOCE. Furthermore, TRPC1 channels did not contribute to myogenic vasoconstriction. Saleh et al. (395) documented that in freshly isolated SMC from rabbit mesenteric artery, a high concentration of Ang II (100 nM) evokes currents inhibited by TRPC1 antibodies. In a vascular injury model, TRPC1 channel was upregulated in SMCs, resulting in enhanced Ca2+ entry. TRPC1 channel upregulation in this model was prevented by arresting the cell cycle (G1-S phase), indicating that TRPC1 channel may be involved in cell proliferation (234). Despite several studies on SMC TRPC1 channels, the physiological role of SMC TRPC1 channels remains a matter of debate.
The functional expression of TRPC3 channels in SMCs has also been supported by multiple studies (Figure 3A) (497). The unitary conductance for TRPC3 channel is approximately 68 pS (367). As with TRPC1 channel, the permeability of TRPC3 channel is similar for monovalent and divalent cations (PCa2+/PNa+ = 1.62) (210). Diacylglycerol (DAG) is a direct activator of TRPC3 channels and was found to activate the channels in a PKC-independent manner. Unlike TRPV and TRPC1 channels, PKC seemed to inhibit TRPC3 channel activity (177, 489). Conditional SMC-specific TRPC3−/− mice were protected against sustained seizure activity in a mouse model (81). The authors proposed that SMC TRPC3 channels mediated the seizure-induced neurovascular uncoupling and subsequent reduction in cerebral blood flow (81). In cerebral arteries, C-terminal tail of TRPC3 channel interacted with N-terminal tail of nearby inositol triphosphate receptor 1 (IP3R1) channels, resulting in TRPC3 channel activation and membrane depolarization. In turn, SMC membrane depolarization activated voltage-gated Ca2+ channels and caused vasoconstriction (5, 525). Future studies may use the newly generated SMC-specific TRPC3−/− mice (81) to unravel the physiological roles of SMC TRPC3 channel.
The third TRPC channel expressed in SMCs, TRPC6 channel (Figure 3A), shows a unitary conductance of approximately 35 pS. Importantly, TRPC6 channel is several times more permeable to bivalent cations over monovalent cations (PCa2+/PNa+ = 5). Intracellular Ca2+ has a biphasic effect of potentiation followed by inhibition on TRPC6 channels (177, 193). Similar to TRPC3 channels, TRPC6 channels are directly activated by DAG in a PKC-independent manner (177). In a recent cryo-electron microscopy (EM) study, the region between segment 6 (S6) and the pore-helix formed by adjacent subunits was proposed as the binding site for DAG (22). In a study by Cayouette and colleagues (58) in human embryonic kidney cells, Gq-protein coupled receptor (GqPCR) signaling induced the trafficking of TRPC6 channels to the plasma membrane, resulting in increased Ca2+ influx. In SMCs from rat mesenteric arteries, stimulation of α1 adrenergic receptor (α1AR) and consequent PLC-DAG signaling increased TRPC6 channel currents (22). Furthermore, Ca2+ influx through TRPC6 channel enhanced the channel activity via Ca2+-CaM-dependent protein kinase II (CaMKII)-phosphorylation of Thr487 on TRPC6 channel. On the contrary, chronic increases in intracellular Ca2+ inhibited TRPC6 channels via PKC activation (418).
Current evidence suggests that TRPC6 channels are not directly mechanosensitive, although GqPCR-activation of TRPC6 channels was shown to prime the channel for mechanosensation (192). In a separate study, Spassova et al. (435) indicated that TRPC6 channels could sense the membrane stretch, which may explain the contribution of TRPC6 channels to myogenic vasoconstriction. In SMCs from cerebral arteries of TRPC6−/− mice, TRPC3 channels were upregulated and myogenic vasoconstriction was shifted toward lower pressure values. The increase in vasoreactivity in TRPC6−/− mice raises the possibility of a heteromeric TRPC6/TRPC3 channel complex in which TRPC6 channel inhibits TRPC3 channel activity (435). On the contrary, in an earlier study, Welsh and colleagues (515) showed that acute TRPC6 channel knockdown in SMCs from cerebral arteries impaired myogenic vasoconstriction. The discrepancy between the two studies could be explained by potential compensatory upregulation of other Ca2+ entry pathways in the global TRPC6−/− mice (e.g., TRPC3 channel upregulation). A recent study in cerebral arteries suggested that intraluminal pressure-induced Ca2+ influx via TRPC6 channels enhances inositol triphosphate receptor (IP3R) activity. Ca2+ release through IP3Rs then activates nearby TRPM4 channels. The role of TRPM4 channels in initiating SMC membrane depolarization and vasoconstriction is well known (133). TRPC6 channels have also been shown to limit SMC proliferation via inhibition of phosphoinositide 3-kinase (PIP3)-protein kinase B (PKB) (Akt) pathway. Along similar lines, transforming growth factor (TGF-β) was shown to reduce TRPC6 channel activity, thereby enabling the Akt pathway and SMC proliferation (332). Convincing evidence in the literature supports the concept that TRPC6 channels enhance vascular tone. However, further research is needed to confirm the roles of TRPC6 channels in regulating blood pressure and SMC proliferation.
TRPM4 channel.
TRPM4 channels have emerged as an essential ion channel for pressure-induced depolarization of SMC membranes. TRPM4 channel is a Ca2+-activated, Ca2+-impermeable, non-selective cation channel (256). The unitary conductance of TRPM4 channel is approximately 24 pS. TRPM4 channel mostly conducts monovalent cations and shows minimal conductance for divalent cations (PCa2+/PNa+ = 0.09). Intracellular Ca2+, via CaM binding, interacts with the C-terminal tail of TRPM4 channel and increases channel activity (EC50 = 300 nM) (241, 328). In SMCs, TRPM4 channel currents induce membrane depolarization and LTCC activation. TRPM4 channel knockdown with antisense oligonucleotides resulted in SMC membrane hyperpolarization and attenuated myogenic vasoconstriction (103). Moreover, PKC enhanced the Ca2+ sensitivity of TRPM4 channels, thereby facilitating myogenic vasoconstriction (102). Inhibition of IP3R Ca2+ release from the SR attenuated SMC TRPM4 channel activity, confirming the importance of IP3R Ca2+ signals for TRPM4 channel activity (132). A recent study showed that spatial coupling between TRPM4 and TRPC6 channels, brought about by their nanometer proximity, facilitates myogenic constriction of cerebral arteries (133). Additionally, pressure-induced mechanical stretch resulted in PLCγ-dependent formation of inositol triphosphate (IP3). IP3, in turn, sensitized IP3Rs to TRPC6-mediated Ca2+ influx, thus creating microdomains of high Ca2+ that activated nearby TRPM4 channels (133). The physiological relevance of vascular TRPM4 channels was demonstrated in a study by Reading and Brayden (380). In this study, the authors showed that acute deletion of TRPM4 channels, accomplished by infusing antisense oligonucleotides into the cerebrospinal fluid, elevated cerebral blood flow. Moreover, myogenic vasoconstriction was reduced in cerebral arteries from the mice treated with TRPM4 antisense oligonucleotides (380). Surprisingly, TRPM4−/− mice are hypertensive, possibly due to increased catecholamine secretion (283). The discrepancies in the role of TRPM4 channels in controlling vasoconstriction and blood pressure could be resolved by using SMC-specific TRPM4−/− mice. Floxed TRPM4 mice have already been generated (217) and will prove useful in future investigations of SMC TRPM4 channels.
TRPP1/TRPP2 channels.
Ion channels of TRPP subfamily, TRPP1 and TRPP2 channels, have also been linked to the regulation of vascular function (Figure 3A). Stretch-activated cation channels (SACs) are thought to be key contributors to myogenic vasoconstriction (172, 394, 500). However, SACs remained poorly characterized. Studies by Sharif-Naeini showed that SMC-specific TRPP1 deletion decreased SAC currents in SMCs and attenuated myogenic vasoconstriction. Moreover, siRNA-induced TRPP2 knockdown in TRPP1-deficient arteries rescued SAC currents and myogenic vasoconstriction (411), suggesting an inhibitory effect of TRPP2 channels on SAC currents. In a recent study, mesenteric arteries from inducible, SMC-TRPP1−/− mice showed unaltered myogenic constriction but attenuated phenylephrine-evoked constriction (49). Moreover, SMC-TRPP1−/− mice had lower resting blood pressure. Additionally, SMC-TRPP1−/− mice were partially protected against Ang II-induced hypertension and vascular remodeling (49). The different effects of SMC-specific TRPP1 deletion on myogenic vasoconstriction in the two studies could be explained by inducible versus constitutive deletion of TRPP1 channels. Regardless, the SMC TRPP1 channel appears to be a vital controller of arterial contractility and could be a promising target for lowering the blood pressure in hypertension.
PIEZO1 channel.
PIEZO channels in vascular cells have been a topic of intense research in recent times. PIEZO proteins are mechanosensitive, non-selective cation channels that show a slight preference for Ca2+ over monovalent cations. Two PIEZO channel isoforms have been identified: PIEZO1 and PIEZO2 (75). Mammalian PIEZO1 channel shows a unitary conductance of approximately 30 pS, about 10 times higher than the Drosophila PIEZO1 channel (76). Mammalian PIEZO1 channel is a large protein composed of 2547 amino acids. Cryo-EM at 4.8 Å resolution revealed a trimeric three-bladed propeller-like structure of approximately 900 kDa for PIEZO1 channel. Each subunit has 14 TM α-helices. The channel pore is formed by two helices, outer (OH) and inner (IH) helix, located close to the C-terminal intracellular tail. The remaining 12 peripheral TM helices (PH) of each subunit contain the N-terminal tail, and function as mechanosensor units (127, 557). PIEZO1 channel is expressed at low levels in conduit arteries but is highly expressed in resistance arteries (93, 381). Although SMC-specific PIEZO1 deletion did not alter myogenic constriction of caudal and cerebral arteries, it was protective against SMC remodeling in two different hypertension models. It was proposed that PIEZO1 channel induces the activation of Ca2+-sensitive enzyme transglutaminase, which protects against SMC remodeling in hypertension (381). Studies of PIEZO1 channel in the vasculature are still in the early stages, and further research is needed to address the role of SMC PIEZO1 channels in vasoconstriction and blood pressure regulation.
Purinergic P2X receptor ion channels (P2XR).
Purinergic signaling is considered to be a crucial controller of vascular resistance and remodeling (Figure 3A). Endogenous purinergic receptor agonist, ATP, can be released by perivascular nerve terminals at the neuromuscular junctions (50) or by the opening of Pannexin-1 channels on SMC and EC membranes (35, 84, 205, 412). Amongst all known purinergic receptors, only P2X receptors (P2XRs) are ionotropic receptors. There are seven different P2XR subtypes (P2XR1-7) (330). Three subunits form functional P2XR. Each subunit is composed of intracellular N- and C-terminal tails linked to two α helix TM domains (TM1 and TM2, respectively), both connected to an extracellular ATP-binding domain (216). P2XRs are non-selective cation channels with similar permeability for monovalent and divalent cations and a single-channel conductance of 10 to 30 pS (398). ATP-P2XR signaling increased intracellular Ca2+ levels and contractility of SMCs from glomerular afferent arterioles, a response that was boosted by the AA metabolite 20-hydroxyeicosatetraenoic acid (20-HETE) (558). Moreover, inhibition of 20-HETE impaired ATP-induced constriction of glomerular afferent artery (559). Both P2X1R and P2X4R are expressed in arterial SMCs (149). Mesenteric arteries isolated from P2X1−/− mice showed impaired ATP- and nerve-induced-constriction (499).
Perivascular nerve stimulation causes spatially restricted Ca2+ influx signals through P2XRs in SMCs, described as junctional Ca2+ Transients (jCaTs) (Table 1). jCaTs can be easily distinguished from Ca2+ sparks from their wider spatial propagation (5 μm) and longer duration (t1/2 = 145 ms) (239). Nerve stimulation-induced vasoconstriction has two components, an initial brief vasoconstriction mediated by jCaTs, followed by the α1AR-dependent prolonged vasoconstriction (238). jCaT-induced local membrane depolarization activates voltage-dependent Ca2+ channels (VDCCs) and stimulates Ca2+ release from the SR through IP3Rs (369). The role of P2X1R in mediating SMC contraction to ATP during sympathetic neurotransmission is well established. However, future studies are needed to address the functional roles of SMC P2X4Rs.
Na+/Ca2+ exchanger (NCX).
NCX is another important regulator of SMC Ca2+ levels (Figure 3A). NCX is an antiporter system that moves Ca2+ in exchange for Na+ across the plasma membrane (stoichiometric ratio 1Ca2+ :3Na+). The driving force and directionality of Na+/Ca2+ exchange depend upon the chemical gradient of Na+/Ca2+ ions across the plasma membrane and the membrane potential (41). Three different genes encode the three NCX isoforms (NCX1-3), amongst which NCX1 is the most abundant in SMCs (247). Crystal structure (1.9 Å resolution) revealed that NCX is a monomer composed of 10 TM helices (TM1-10). TM2-3 and TM7-8 form the core binding domains for Na+/Ca2+ (254). Upon Na+/Ca2+ binding, NCX undergoes a conformational change that alternatively exposes the ligand-binding domain to the extra- or intracellular compartment and allows Na+/Ca2+ trafficking across the plasma membrane (171, 220, 326). Early studies suggested that ATP increases the affinity of NCX to intracellular Ca2+ and extracellular Na+. However, ATP was unable to influence NCX activity in the presence of saturating intracellular Ca2+ concentrations (42).
The first evidence of NCX in SMCs was reported in 1973 (382). Later studies suggested that NCX distribution on the plasma membrane is not random but is instead restricted to the regions underlying junctional SR (207). This localization pattern may suggest a role for NCX in regulating SR Ca2+ levels (40). Pharmacological inhibition (377) or SMC-specific deletion of NCX1 (509, 550) reduced cytosolic Ca2+ levels, impaired vasoconstriction, and lowered resting blood pressure. In a recent study by Zhang et al. (551), pressurized femoral arteries isolated from SMC-specific NCX1 overexpressing mouse (SM-NCX1-TG) showed increased SMC Ca2+ levels and higher myogenic constriction. SM-NCX1-TG mice also showed higher resting blood pressures. These findings support the idea that NCX1 mediates net Ca2+ influx into SMCs (known as a reverse mode) in resistance arteries. TRPM4 channels are known to mediate SMC depolarization by facilitating Na+ influx (133). Therefore, Na+ influx through TRPM4 channels may generate the driving force necessary for NCX to function in the reverse mode. However, further investigation is needed into the potential coupling of TRP channels with NCX protein.
NCX is modulated mainly by two intrinsic mechanisms. Na+-dependent inactivation occurs when intracellular Na+ concentration reaches 100 nM (170). The other intrinsic modulation is by intracellular Ca2+. By binding to a high affinity region on NCX, intracellular Ca2+ alleviates Na+-dependent inactivation and augments NCX activity in both the forward and reverse modes. Regulatory Ca2+ does not get transported by NCX (249, 286). Li and colleagues (253) identified a specific region of 20 amino acids (XIP) on the intracellular N-tail of TM5 that showed a high binding affinity for Ca2+-CaM. A synthetic exchanger inhibitory peptide (XIP) inhibited NCX activity by competing with the endogenous sequence. Single-site modifications of the XIP sequence drastically impaired Na+-dependent inactivation and diminished Ca2+-modulation of NCX (285). Additionally, high-affinity PIP2 biding to XIP eliminated the Na+-dependent inactivation of NCX (165). In rabbit renal arterioles, PKC was also found to enhance NCX activity, although the precise site of action for PKC remains unclear (118).
Store-operated Ca2+ entry (SOCE) channels.
Several studies have focused on SOCE channels in SMCs; however, the functionality of SOCE in controlling vascular resistance remains controversial (98). SOCE is defined as Ca2+ influx activated by the depletion of ER/SR Ca2+ stores. The ionic currents recorded through SOCE are called Ca2+ release-activated Ca2+ current (CRAC). The two main proteins involved in mediating SOCE are stromal interaction molecule (STIM) and Orai. STIM is a single TM protein located on the ER membrane. The N-terminal tail contains the Ca2+-sensitive domain (CSD) facing the ER lumen and comprises two sub-domains: EF-hand domain and sterile α-motif (SAM) domain. EF-domain is the Ca2+-binding site that senses the decrease in ER Ca2+ levels. The C-terminal tail faces the cytosol and contains a 100-amino acid sequence called STIM-Orai activating region/CRAC activating domain (SOAR/CAD), which is pivotal for physical interaction with Orai. Orai is a 33 kDa protein located on the plasma membrane. It is a Ca2+-selective pore formed by four TM segments and N- and C-terminal tails facing the cytosol. Orai shows a very small unitary conductance (1 pS) and a high selectivity for Ca2+ (182). The depletion of ER Ca2+ leads to disassociation of Ca2+ from EF-hand domain, thereby promoting a conformational change that causes STIM monomer/dimers to oligomerize. CAD domain is required for store-dependent STIM oligomerization (78, 437). STIM oligomers tether with Orai channels through electrostatic interaction between SOAR/CAD and C-terminal tail of Orai channels. The STIM-Orai interaction creates the functional SOCE channel complex for replenishing the ER Ca2+ stores. It has also been suggested that STIM oligomers are tetramers with each monomer interacting with each of the four TM segments of Orai (78, 345). Two STIM (STIM1-2) and three Orai (Orai1-3) isoforms have been identified to date (87, 428).
The role of STIM and Orai in influencing SMC contractility is considered negligible. Indeed, Bisaillon and colleagues (36) reported that STIM and Orai expression is low in native SMCs. Orai- and STIM-deficient mice did not show impairment in vascular contractility (113). Studies over the past decade suggest that SOCE channels mostly control SMC proliferation and migration. Proliferative SMC culture showed higher Orai1 and STIM1 expression (368). In aortic SMC culture, knockdown of STIM1 and Orai1 impaired platelet-derived growth factor (PDGF)-induced migration. Furthermore, STIM1 and Orai1 were upregulated in SMCs from injured carotid arteries (36), and in vivo knockdown of STIM1 and Orai1 lowered neointima formation in injured carotid arteries (554). Overall, SOCE pathway in SMCs appears to be more important for SMC migration and proliferation than for the regulation of vascular contractility.
Ca2+ mobilization from intracellular organelles
Inositol trisphosphate receptors (IP3Rs)
The SR is an intracellular organelle characterized by high concentrations of both bound and free Ca2+ (100–700 μM) (410). A high concentration gradient for ER Ca2+ against cytosolic Ca2+ is created by Ca2+-ATPase on the SR membrane (13, 299). Activation of IP3Rs on the SR membrane is one of the mechanisms for the release of SR Ca2+ into the cytosol (Table 1). Several mechanical and neurohumoral mediators, including pressure, Ang II, norepinephrine, endothelin-1, and serotonin, activate GqPCR-PLC signaling to increase IP3 formation and IP3R Ca2+ release. Out of the three IP3R isoforms (IP3R1-3) identified to date (117), IP3R1 and IP3R3 are expressed in arterial SMCs (Figure 3B) (312). IP3Rs are organized in micro-clusters of 2 μm diameter (277). Functional IP3R is a tetramer with each monomer consisting of six TM segments (TM1-6). N- and C-terminal tails face the cytosol and are linked to TM1 and TM6, respectively. The channel pore is lined by four TM6 segments (23). TM1-4 are located at the periphery and are connected to the pore unit TM5-6 by a lateral TM4-5 linker helix. Patch-clamp studies of the nuclear membrane reveal single-channel conductances of 113 pS at 0 mV and 300 pS at +60 mV for IP3Rs. IP3R is a bivalent cation-selective ion channel (Ca2+/K+ = 8). IP3 relieves Ca2+-inhibition of IP3R and enables Ca2+-activation of the channel. The IP3-binding domain (IBC) is localized on the N-terminal tail of each subunit (117, 145, 541). Recent evidence suggests that the reversal of Ca2+-inhibition of IP3R can occur when IP3 is bound to all four binding sites (14). However, prolonged exposure to IP3 (>2 s) causes IP3R to transition to an inactivated state that can only be recovered when IP3 is removed.
Ca2+ has a concentration-dependent, biphasic effect on IP3R activity. Ca2+ acts as pure agonist of IP3Rs at lower concentrations (0–300 nM Ca2+) and turns into an inhibitor at higher concentrations (>300 nM Ca2+) (190). Multiple Ca2+-binding sites have been identified on different regions of IP3Rs (347, 422). The Ca2+-regulation of IP3R activity is also dependent on IP3 levels. Increasing the concentration of IP3 reduces the affinity of Ca2+ for the inhibitory sites (278). Low levels of IP3 (10–30 nM) activate only one IP3R resulting in spatially restricted Ca2+ signals named “blips.” The amplitude of blips can reach approximately 30 nM above the baseline Ca2+ levels. Progressively higher IP3 concentrations (30–60 nM) recruit more IP3Rs within the same cluster, resulting in a larger Ca2+ release that potentiates the signal via Ca2+-induced Ca2+-release. These larger IP3R-mediated Ca2+ events are called “puffs.” The occurrence of puffs requires activation of approximately five IP3Rs in a cluster, and puff amplitude was recorded to be 170 nM above the basal Ca2+ levels. Modeling studies of IP3R kinetics and spatial spread of Ca2+ indicate that cooperative activation of two IP3Rs can occur with high probability only if they are approximately 12 nm apart. Increasing the distance up to 50 nm reduced the cooperative activation to 50% (449, 454, 539). Even higher IP3 levels relieve Ca2+-dependent IP3R inhibition and facilitate Ca2+ release from multiple IP3R clusters, triggering the formation of Ca2+ waves that can propagate across the cell. While Ca2+ puffs are terminated by Ca2+ binding to the inhibitory sites on IP3Rs, Ca2+ waves seem to dissipate upon IP3-unbinding from IP3R (391).
In cremaster arteries, IP3R inhibition impaired Ca2+ waves and myogenic vasoconstriction (516). However, IP3R1 knockdown did not alter myogenic vasoconstriction in mesenteric arteries (556), suggesting heterogeneity in the role of IP3Rs among different vascular beds. In a recent study, Gabani and colleagues (122) showed that the small noncoding RNA (MiR-204) lowers IP3R1 expression in mesenteric arteries. MiR-204−/− mice showed higher expression of IP3R1, increased Ang II-induced vasoconstriction, and a higher increase in blood pressure in response to Ang II. Inhibition of IP3R also impaired SMC proliferation in cerebral arteries (518). Thus, IP3R Ca2+ signaling plays a pivotal role in regulating SMC contractility and proliferation.
Ryanodine receptors (RyRs)
RyR is a Ca2+-permeable ion channel located on the SR membrane. Recent high-resolution Cryo-EM (4.8 Å) studies show that the functional channel comprises four subunits, 560 kDa each. Each subunit has six TM helices. The pore is formed by TM5-6 and segment P, which acts as a selectivity filter. Under physiological conditions, segment P and TM6 have many negatively charged amino acid residues, thereby facilitating high unitary conductance of 103 pS (338, 545). The N-terminal tail linked to TM1 is a large structure (2217 amino acids) facing the cytosol. It encompasses different binding sites essential for RyR channel regulation, including the CaM-like domain (EF-hands) that constitutes the conserved Ca2+-binding domain (CBD) (529). RyR is activated upon Ca2+ binding to EF-hands (33, 545). Three RyR isoforms have been identified-RyR1-3 (467, 564), and all three have been shown to be expressed in SMCs (321).
Ca2+ release from RyRs in SMCs was, for the first time, described in cerebral arteries (318). The individual Ca2+ release signals through RyRs were termed “Ca2+ sparks” (Table 1), akin to the previously described Ca2+ sparks in cardiac myocytes (66). Ca2+ sparks peak in approximately 20 ms, decay in approximately 200 ms, and are mediated by the activation of four to six RyRs. They produce highly localized increases in Ca2+ (10–100 μM) within 20 nm diameter from the site of initiation (67, 203). RyRs can be activated by Ca2+ influx from the extracellular environment or Ca2+ release from nearby IP3Rs or RyRs. SMC membrane potential is a well-known regulator of RyR activity. Membrane depolarization-induced increase in the activity of Ca2+ sparks was linked to Ca2+ entry via LTCCs (226) and TTCCs (150). Notably, membrane depolarization, per se, did not activate Ca2+ sparks when extracellular Ca2+ was replaced with Ba2+ as a charge carrier (420). Jaggar et al. (204) reported that SMC membrane depolarization from −70 to −30 mV increases Ca2+ spark activity. Moreover, CaV3.2 channels were essential for triggering Ca2+ sparks under physiological membrane potentials (−40 mV), whereas CaV1.2 channels were the predominant source of Ca2+ for Ca2+ spark activity at depolarized membrane potentials (−20 mV). Interestingly, NCX, activated in the reverse mode, was found to be partially responsible for Ca2+ spark initiation at −20 mV (162).
SMC RyRs are a crucial negative regulatory mechanism for myogenic vasoconstriction. Myogenic vasoconstriction involves pressure-induced SMC membrane depolarization followed by VDCC activation. Ca2+ influx through VDCCs activates RyR Ca2+ sparks, which stimulate the activity of nearby BK channels, causing membrane hyperpolarization and vasodilation (226, 318). In partially depolarized SMCs (Em = −40 mV), most Ca2+ sparks are associated with spontaneous transient outward current (STOC) that represent activation of roughly 18 BK channels. The probability of STOCs increases to 104 when associated with Ca2+ sparks, suggesting a strong spatial interaction between RyRs and BK channels. Since BK channels exhibit a low Ca2+ affinity (100–200 μM), their activation requires high levels of Ca2+. Spatial coupling between RyRs and BK channels ensures high local Ca2+ concentrations (10–100 μM) near (~20 nm) the Ca2+ spark foci (353, 537). Intracellular microtubule structures are essential for ensuring spatial proximity between RyRs and BK channels. Indeed, disruption of microtubules uncoupled RyRs from BK channels and increased myogenic vasoconstriction in cerebral arteries (373). While SMC RyRs are known for their vasodilatory role, Krishnamoorthy et al. (233) demonstrated that a high-level activation of RyRs could contribute to whole-cell increases in Ca2+ and vasoconstriction in response to nerve stimulation.
Studies on the role of individual RyR isoforms in SMCs have been challenging as RyR1−/− (465) and RyR2−/− (466) mice are lethal. Lohn et al. (266) addressed the role of RyR3 in Ca2+ spark singling. Interestingly, RyR3−/− mice showed increased Ca2+ spark frequency and reduced myogenic vasoconstriction. These findings suggest that RyR3 may negatively regulate RyR1 and RyR2 activity. In a recent study, Kassmann and colleagues (215) reported higher myogenic vasoconstriction and elevated blood pressure in SMC-specific, tamoxifen-inducible RyR2−/− mice. The generation of SMC-specific RyR2−/− may be the necessary first step in understanding the relative contribution of specific RyR isoforms to Ca2+ spark activity in SMCs and their role in blood pressure regulation.
Ca2+-ATPase (SERCA)
Cytosolic Ca2+ levels in SMCs are lowered mainly by Ca2+ uptake into the SR via the SERCA on the SR membrane (Figure 3B). SERCA is encoded by three different genes, SERCA1-3 (355), and different splice variants of these genes have been documented. In SMCs, the predominant isoform is SERCA2b, followed by SERCA2a and SERCA3 (523). SERCA is a P-type ATPase that was discovered by Nobel laureate Jens Skou in the year 1957. A common feature of P-type pumps is to undergo two main conformational changes (E1 and E2), with the formation of a phosphorylated (P) aspartyl intermediate (E1-E2), which gives the family its name. E1 state has a high affinity for Ca2+, and E2 state has a low affinity for Ca2+ (376). The transition from E1 to E2 is ATP-dependent (Figure 4). In each cycle, SERCA uses one molecule of ATP to pump two Ca2+ into the SR in exchange for two to three H+ released into the cytosol (523).
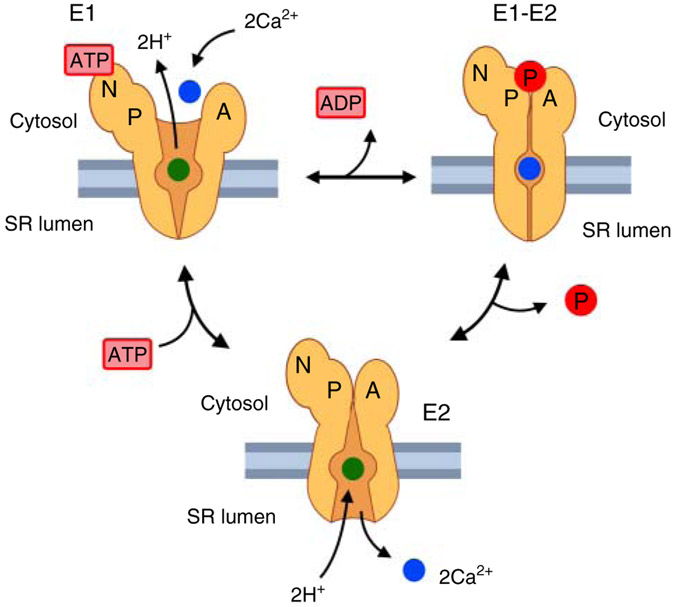
Figure 4. Sarco-endoplasmic reticulum Ca2+-ATPase (SERCA) transporting cycle.
E1 indicates SERCA conformation characterized by a high affinity for Ca2+. E1-E2 represents a transient high energy state. E2 represents SERCA conformation characterized by a low affinity for Ca2+. Two cytosolic Ca2+ ions bind to SERCA in E1 conformation. ATP tethers to the nucleotide (N) domain and phosphorylates the (P) domain. The phosphorylated (P) domain interacts with the (A) domain resulting in two sequential conformational changes (E1-E2, and E2). SERCA in E2 conformation releases Ca2+ into the SR lumen. Pi, inorganic phosphate; ADP, adenosine diphosphate; H+, proton.
X-ray crystallography studies showed that SERCA comprises three cytoplasmic domains (A, N, and P) and a TM domain. The TM domain is characterized by 10 TM helices (TM1-10). TM4 and TM5 are longer and protrude from the SR membrane to the cytosol. Two putative Ca2+-binding sites have been identified on the TM domains-site I between TM5 and TM6, and site II on TM4. The N (nucleotide) domain is essential for ATP binding, and ATP-dependent phosphorylation of domain P. Asp351 found in domain P is highly conserved across species and is pivotal for the formation of high energy phosphorylated-aspartyl intermediate. The A domain transduces the conformational change of domain P to TM domain (337, 487) (Figure 4). At low cytosolic Ca2+ concentrations, SERCA is inhibited by phospholamban. Phospholamban is a 52-amino acids membrane integral protein that binds to the low Ca2+ affinity E2 state and inhibits the activity of the pump. Inhibition is relieved either by an increase in cytosolic Ca2+ or by phosphorylation of phospholamban by PKA (513) or Ca2+-CaMKII (275).
Wellman and colleagues (513) reported higher Ca2+ spark and STOC frequency in arteries from phospholamban−/− mice due to increased SR Ca2+ loading. These findings implied that SERCA might influence IP3R and RyR Ca2+ signaling by altering SR Ca2+ loading. Schneider and colleagues (404) showed that phospholamban phosphorylation by 5′-AMP-activated protein kinase (AMPK) disinhibits SERCA, promotes Ca2+ sequestration into the SR, and causes vasodilation. Moreover, the elevation of extracellular K+ from 3 to 6mM dilated cerebral arteries, an effect that was prevented by SERCA inhibition (179, 288). SERCA is also known to be a potent inhibitor of SMC proliferation (518). SERCA2a was shown to impair injury-induced SMC proliferation via inhibition of calcineurin-nuclear factor of activated T-cells (NFAT) signaling (259). SERCA2 deletion is embryonic lethal (356); therefore, generating SMC-specific SERCA2−/− mice may be desirable for obtaining precise insights into the physiological roles of SMC SERCA2.
TRPML1 channel
Ion channels of the TRPML subfamily (TRPML1-3) are encoded by Mcoln1, Mcoln2, and Mcoln3 genes. TRPML channels are mainly localized in the membranes of late endosomes (LEL) (68). Thakore and colleagues (474) recently demonstrated the importance of TRPML1 channels in regulating SMC contractility and blood pressure (Figure 3B). Endosomal TRPML1 channels colocalized with RyR2 channels. Ca2+ release from LEL through TRPML1 channels activated RyRs and lowered vascular resistance. Moreover, TRPML1-deficient (Mcoln1−/−) mice showed elevated blood pressure and increased vasoconstriction. Thus, SMC TRPML1 channels appear to be important regulators of vascular resistance and blood pressure.
Endothelial Cell Ca2+ Signals in Small Arteries and Arterioles
The endothelium is a single cell layer of cells that lines the inner walls of all the blood vessels. ECs are constantly exposed to the mediators in the blood and mechanical forces exerted by the bloodstream. Endothelial function in arteries and arterioles is also modulated by stimuli from SMCs (126, 178, 313, 488). In this section, we will focus on the physiological Ca2+ signaling mechanisms that alter EC function. ECs in resistance-sized arteries send out projections, across the internal elastic lamina, to the SMC layer. The sites of contact between ECs and SMCs are enriched with connexin proteins (Cx37, Cx40, and Cx43) that form myoendothelial gap junctions (MEGJs) (143, 196, 400). MEGJs are characterized by two hemichannels, one each on the EC and SMC membranes. Each hemichannel is a hexamer composed of six connexins (32). MEGJs allow the passage of second messengers and electrical signals (92, 104, 178, 284), and serve as a crucial communication site for ECs and SMCs. ECs can influence the contractile state of the adjacent SMCs via endothelium-derived hyperpolarization (EDH) or by releasing substances that activate vasodilatory signaling in SMCs in a paracrine manner. The preferential activation of one pathway over another may be determined by the vascular bed under consideration (341) and the size of the artery (419, 492). Recent studies show that neighboring ECs are heterogeneous with respect to Ca2+ signaling mechanisms (246). Indeed, McCarron and colleagues demonstrated that neighboring ECs are organized into Ca2+ signaling clusters, and communication amongst these clusters is essential for normal vascular function (246). Here, we elaborate on the Ca2+ signaling pathways that initiate EC to SMC, SMC to EC, and EC to EC communications in the vascular wall, and the target proteins that transduce the Ca2+ signals into a physiological response.
Ca2+ influx from extracellular compartment
Non-voltage-gated Ca2+ entry pathways (TRP channels, PIEZO channel, P2X receptor, SoCE channels, and Na+/Ca2+ exchanger)
TRPA1 channel.
In the past decade, TRPA1 channels have emerged as a crucial Ca2+ influx pathway in ECs from specialized vascular beds. TRPA1 channels show a unitary conductance of approximately 96 pS at −60 mV (309), and a higher permeability to Ca2+ than Na+ (PCa2+/PNa+ = 7.9) (213). TRPA1 channels are activated by several pungent natural compounds in food such as allicin (garlic) (276), allyl isothiocyanate (mustard), and cinnamon (cinnamaldehyde) (200). TRPA1 channels are gated by extracellular Ca2+ in a voltage-dependent manner. In patch-clamp studies, TRPA1 channels displayed slow activation at a holding potential of −80 mV and in the absence of extracellular Ca2+. However, in the presence of extracellular Ca2+ and ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA)-buffered intracellular Ca2+, holding potential of −80 mV caused fast channel activation followed by fast inactivation. Notably, fast channel inactivation did not occur at a more depolarized membrane potential (−20 mV) (309).
Earley and colleagues (99) provided the first evidence for vasodilatory effects of endothelial TRPA1 channel activation in cerebral arteries. TRPA1 channels co-localized with intermediate-conductance Ca2+-activated K+ (IK) channels at MEPs (Figure 5). Ca2+ influx through TRPA1 channels (Table 1) activated nearby IK channels, resulting in EC membrane hyperpolarization and vasodilation. Moreover, the vasodilatory effect of TRPA1-IK channels was boosted by inward-rectifier potassium (Kir) channels on SMC membranes. In a subsequent study, TRPA1 channels were shown to promote IP3R Ca2+ release from the ER. Furthermore, endothelial TRPA1 channel activation inhibited the formation of Ca2+ waves in SMCs, providing additional evidence supporting the inhibitory effect of endothelial TRPA1 channels on SMC contraction (375).
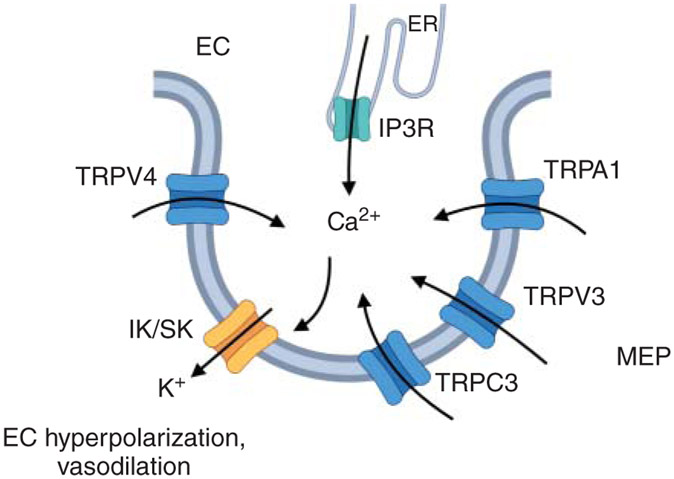
Figure 5. Ca2+ signaling networks at myoendothelial projections (MEPs).
Ca2+ influx via TRPV4/TRPV3/TRPA1/TRPC3 channels or Ca2+ release from the ER via IP3Rs at MEPs activates nearby small (SK) and intermediate (IK) conductance Ca2+-activated K+ channels. IK/SK channel activation hyperpolarizes endothelial cells (EC) membrane and results in vasodilation. TRPV/TRPA/TRPC, members of transient receptor potential channel family.
ROS and products formed by lipid peroxidation, including 4-hydroxynonenal (4-HNE), are the main endogenous modulators of endothelial TRPA1 channel activity (97, 490). ROS generating enzyme NADPH oxidase 2 (NOX2) was present in nanometer proximity with TRPA1 channel in cerebral arteries. NOX2-generated ROS induced membrane lipid peroxidation and 4-HNE formation, thereby increasing TRPA1 channel activity and causing vasodilation. This effect was blunted in the arteries from endothelium-specific TRPA1−/− mice (448). Studies by Pires and colleagues (359) suggested that endothelial TRPA1 channels are neuroprotective under hypoxic conditions. Under hypoxic conditions, mitochondrial ROS production enhanced TRPA1 channel-mediated dilation of cerebral arteries. In support of this concept, endothelium-specific TRPA1−/− mice showed larger cerebral damage following stroke-induced hypoxia. Overall, the current evidence suggests a central role for endothelial TRPA1 channels in mediating Ca2+ influx in the cerebral vasculature.
TRPV4 channel.
TRPV4 is one of the most studied Ca2+ influx pathways in the intact endothelium. Until recently, the physiological roles of endothelial TRPV4 channels were not known (reviewed in Ref. 63). Systemic administration of a potent and selective TRPV4 channel agonist evoked a dose-dependent drop in blood pressure in dogs, rats, and mice (519). Moreover, acetylcholine-induced decrease in blood pressure was attenuated in global TRPV4−/− mice (549). However, global TRPV4−/− mice showed unaltered resting blood pressure (178, 549), possibly due to a compensatory upregulation of other ion channels or the absence of TRPV4 channels from multiple cell types in these mice. Ottolini and colleagues (342), in a recent study, demonstrated the importance of endothelial TRPV4 channels and its regulation by AKAP150 in lowering the resting blood pressure. In this study, tamoxifen-inducible, endothelium-specific TRPV4−/− or AKAP150−/− mice showed higher resting blood pressures, confirming the pivotal role of endothelial AKAP150-TRPV4 signaling in blood pressure regulation.
Multimodal physiological stimuli can activate endothelial TRPV4 channels. Early studies supported a mechanosensory role of endothelial TRPV4 channels, although recent evidence suggests that TRPV4 channels are not direct mechanosensors (327). An alternative explanation for mechanoactivation of TRPV4 channels is that the channels can be activated by mechanical stimuli via signaling pathways involving the activation of cytochrome P450 (CYP) epoxygenases and EET production (101, 268). Kohler and colleagues (228) demonstrated that sheer stress-induced vasodilation in rat gracilis arteries is reduced by ruthenium red (RuR), a non-selective TRPV4 channel blocker. Flow-induced, TRPV4 channel-mediated vasodilation was also reported in carotid arteries (158) and mesenteric arteries (291) (Figure 8). Inhibiting AA metabolism eliminated sheer stress-induced vasodilation, suggesting that AA metabolites are necessary for mechanotransduction by TRPV4 channels. In cremaster arteries, sheer stress increased the functional coupling of M3 muscarinic receptors with endothelial TRPV4 channels for vasodilation (82). Bagher and colleagues (20) showed that low intravascular pressure (5–50 mmHg) enhances the activity of endothelial TRPV4 channels, further supporting the activation of endothelial TRPV4 channels by mechanical stimuli. Studies by Saliez et al. (396) in EC culture demonstrated that TRPV4 channels co-immunoprecipitate with caveolin-1. Moreover, endothelial Ca2+ influx was impaired in the absence of caveolin-1. Although a direct interaction between caveolin-1 and TRPV4 channel appears likely, the functional evidence on caveolin-1 regulation of TRPV4 channel activity is lacking. Studies using EC-specific caveolin-1 knockout mice will be crucial for unraveling the functional and physiological significance of caveolin-1-TRPV4 channel interaction in the endothelium.
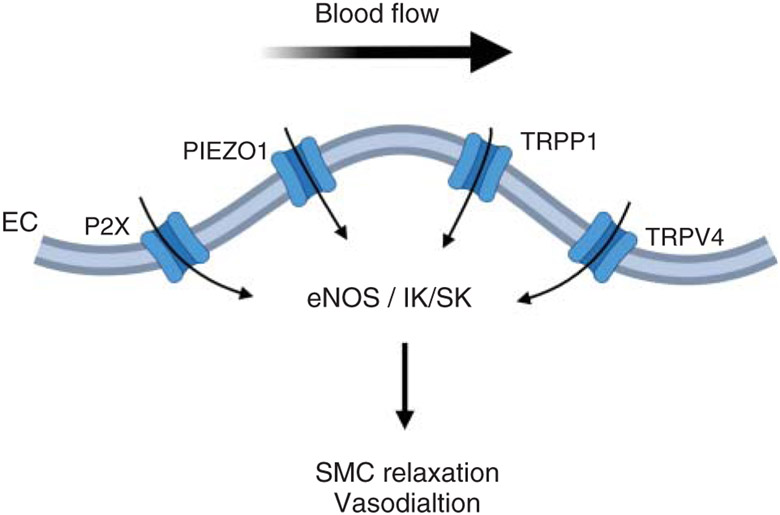
Figure 8. The contribution of endothelial P2X purinergic receptor, PIEZO1, TRPP1, and TRPV4 channels to flow-induced vasodilation.
Sheer stress-dependent activation of P2X, PIEZO1, TRPP1, and TRPV4 channels increases endothelial Ca2+. Shear stress-induced increase in endothelial Ca2+ can cause vasodilation via one of the two pathways (i) activation of endothelial nitric oxide synthase (eNOS) and nitric oxide (NO)-mediated vasodilation; and (ii) activation of IK/SK channels, leading to endothelium-dependent hyperpolarization and vasodilation. TRPP1, transient receptor potential polycystic 1 channel; TRPV4, transient receptor potential vanilloid 4 channel.
Multiple endogenous modulators of endothelial TRPV4 channels have been identified. TRPV4 channel activity is heavily influenced by GqPCR-PLC signaling in both arterial and capillary endothelium (153, 432, 433). PLC-DAG-activated PKC can phosphorylate TRPV4 channels and potentiate their activity (111). Moreover, PLC-mediated decrease in PIP2, a negative modulator of TRPV4 channels, increases TRPV4 channel activity (153, 461). Furthermore, IP3 was shown to bind to TRPV4 channels and increase their activity (178, 461). As described with SMC TRPV4 channels, Ca2+ itself has a biphasic effect on TRPV4 channel activity. In ECs, Ca2+ influx through TRPV4 channels potentiated the activity of the neighboring TRPV4 channels in a cluster, resulting in cooperative channel openings (432, 433). On the contrary, NO impaired the cooperative openings of TRPV4 channels via activation of endothelial guanylyl cyclase (GC)-PKG pathway (282, 540) and reduced channel activity. Hong and colleagues (178) described the presence of a myoendothelial feedback mechanism whereby α1AR stimulation-induced vasoconstriction was limited by endothelial TRPV4 channels (Figure 6). Phenylephrine (PE) activated SMC α1ARs and increased the levels of IP3, which diffused across the MEGJs to ECs and activated TRPV4 channels at MEPs. H2S, a gasotransmitter molecule produced by ECs, was shown to activate endothelial TRPV4 channels in a study by Naik and colleagues (310). H2S-activation of TRPV4 channels increased endothelial BK channel currents. The authors also showed that H2S induces sulfhydration of endothelial TRPV4 channels. Further studies to identify the precise site of action for H2S on the TRPV4 channel are awaited.
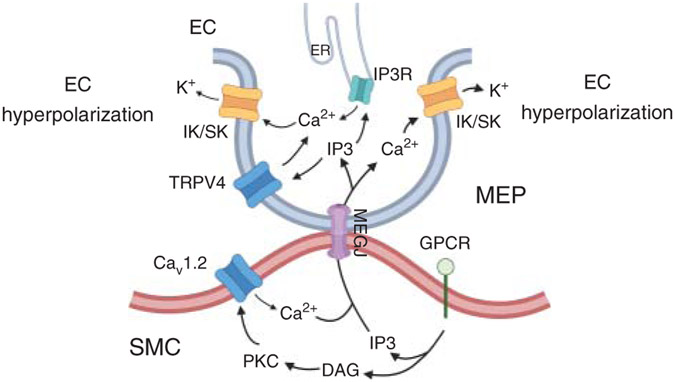
Figure 6. Signaling mechanisms at myoendothelial projections (MEPs) that control the communication between endothelial cells (ECs) and smooth muscle cells (SMCs) and SMC contractility.
Stimulation of Gq-protein coupled receptors (GqPCRs) on SMC membrane leads to the formation of inositol triphosphate (IP3) and diacylglycerol (DAG). DAG activates protein kinase C (PKC), which phosphorylates voltage-gated Ca2+ (CaV1.2) channel, leading to an increase in SMC Ca2+ and vasoconstriction. IP3 and Ca2+ can diffuse to ECs through myoendothelial gap junctions (MEGJ). Elevation of IP3 and Ca2+ at MEPs limits vasoconstriction by activating TRPV4-IK/SK channel and IP3R-IK/SK channel signaling. TRPV4, transient receptor potential vanilloid channel 4 (TRPV4); SK and IK, small (SK) and intermediate (IK) conductance Ca2+-activated K+ channels.
The detrimental effects of excessive TRPV4 channel activity in pulmonary endothelium are well known (12, 451, 478, 540), although the physiological roles of pulmonary endothelial TRPV4 channels have not been resolved. Marziano et al. (282) showed that ATP activates endothelial TRPV4 channels via P2 purinergic receptor signaling in resistance pulmonary arteries. However, global TRPV4−/− mice showed unaltered mean pulmonary arterial pressure (PAP) (528). In this regard, TRPV4 channels are also expressed in SMCs from pulmonary arteries (281), where they promote vasoconstriction (430). Therefore, lack of a PAP phenotype in TRPV4−/− mice could be due to the activation of compensatory mechanisms in SMCs and ECs. Future studies in EC-specific TRPV4−/− (342) and SMC-specific TRPV4−/− are warranted to separate the contributions of endothelial and SMC TRPV4 channels to the regulation of PAP.
Unitary Ca2+ influx signals through TRPV4 channels, called TRPV4 Ca2+ sparklets (Table 1), have been recorded in the intact endothelium from resistance arteries and in EC culture (432, 447). Notably, TRPV4 sparklets are not randomly distributed throughout the EC membrane. Instead, the majority of TRPV4 sparklet activity was observed at MEPs (Figure 5) (178, 432, 433). It was later proposed that MEP-localized AKAP150 anchors PKC in the vicinity of TRPV4 channels and facilitates the coupling among TRPV4 channels (433). IK and SK channels also localize to MEPs (20, 244, 400), explaining the preferential activation of IK/SK channels by TRPV4 sparklets in systemic resistance arteries (341). Contrary to the systemic arteries, TRPV4 sparklets selectively activated endothelial nitric oxide synthase (eNOS) to dilate resistance pulmonary arteries (282). Very recently, Ottolini et al. (341) provided evidence that spatial coupling determines the TRPV4 sparklets-target linkage in different vascular beds (Figure 7). In this study, the authors showed that TRPV4 channels co-localize with IK/SK channels at MEPs in resistance mesenteric arteries. MEPs in this vascular bed are also enriched with hemoglobin α (Hbα) (440), a protein that limits NO release and diffusion (440). TRPV4 channels also localize at MEPs in resistance pulmonary arteries. However, Hbα is absent from MEPs in resistance pulmonary arteries. Additionally, IK/SK channels do not localize at MEPs in this vascular bed. These differences in spatial coupling favor TRPV4-IK/SK channel signaling in resistance mesenteric arteries and TRPV4-eNOS signaling in resistance pulmonary arteries.
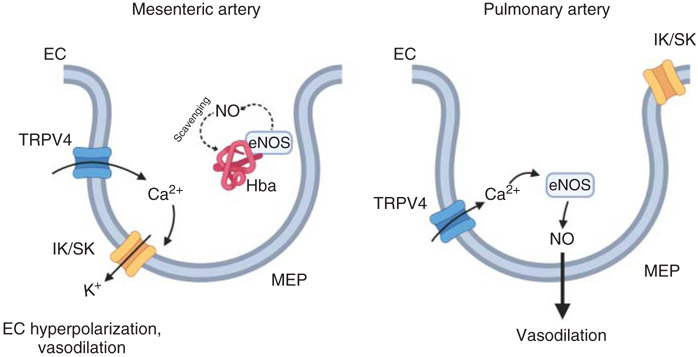
Figure 7. The molecular mechanism underlying selective activation of IK/SK channels in mesenteric arteries versus eNOS in pulmonary arteries.
In mesenteric arteries, Ca2+ entry through the TRPV4 channel at the myoendothelial projections (MEPs) determines vasodilation via activation of nearby small (SK) and intermediate (IK) conductance Ca2+-activated K+ channels. Co-localization of endothelial nitric oxide synthase (eNOS) with hemoglobin alpha (Hbα), a nitric oxide (NO) scavenging protein, prevents TRPV4-eNOS signaling. On the contrary, in pulmonary arteries, IK/SK channels and Hbα do not localize at MEPs. Therefore, Ca2+ influx via TRPV4 channel activates eNOS causing NO-dependent vasodilation. EC, endothelial cell.
TRPV3 channel.
Consistent with other ion channels of TRPV subfamily, TRPV3 channels show high unitary conductance (~170 pS at +60 mV) and Ca2+ permeability (PCa2+/PNa+ = 12). An increase in temperature from 25 to 37 °C increases the outward currents through TRPV3 channels nearly fourfold (531). TRPV3 channels are also activated by dietary monoterpenes, including carvacrol, thymol, vanillin, and ethyl-vanillin (530). Earley and colleagues (100) provided the first evidence for the functional expression of TRPV3 channels in ECs from cerebral arteries (Figure 5). Endothelial TRPV3 channel activation by carvacrol dilated cerebral arteries through IK/SK channels. In a more recent study, Pires et al. (360) recorded unitary Ca2+ influx events through TRPV3 channels (TRPV3 sparklets, Table 1) in ECs from cerebral parenchymal arterioles. The authors reported that TRPV3 sparklets show higher single-channel amplitudes when compared to TRPA1 sparklets, results that are consistent with a higher unitary conductance of TRPV3 channels. Endothelium-specific knockout for TRPV3 channels has not been generated; therefore, the physiological roles of endothelial TRPV3 channels remain unclear.
TRPV1 channel.
The expression and function of TRPV1 channels in ECs are controversial. Several studies have relied upon TRPV1 channel antibodies to assess its endothelial expression, although the specificity of these antibodies has not been verified using knockout tissue (484). In two recent studies on transgenic TRPV1-LacZ and TRPV1-Cre:tdTomato mice, TRPV1 channels were expressed in the SMC layer but not in the endothelial layer (57). Nevertheless, some studies have proposed an important role for TRPV1 channels in endothelium-dependent vasodilation. Yang and colleagues (535) suggested that TRPV1 channel agonist capsaicin activated eNOS and dilated mesenteric arteries, effects that were absent in the arteries from TRPV1−/− mice. TRPV1-eNOS signaling in the vasculature has also been proposed by other studies (46, 483). It should be noted that capsaicin can activate TRPV1 channels in sensory nerves to release calcitonin gene-related peptide (CGRP) and substance P (SP) (279), which could affect the endothelium. Therefore, a definitive assessment of the role of endothelial TRPV1 channels in vasodilation awaits the development of endothelium-specific TRPV1−/− mice.
TRPC channel.
Multiple studies have reported significant roles for endothelial TRPC channels as Ca2+ influx pathways in resistance arteries. TRPC1 channels formed heteromeric complexes with TRPV4 channels in freshly dissociated ECs from rabbit mesenteric arteries. Furthermore, TRPC1-TRPV4 complex activated eNOS and caused vasodilation (138). Ma and colleagues (273) indicated that the TRPC1-TRPV4 channel complex plays a crucial role in sheer stress-induced increase in endothelial Ca2+ and vasodilation. In a study by Senadheera and colleagues (409), TRPC3 channels were found to be localized with IK/SK channels at MEPs of rat mesenteric arteries (Figure 5). Moreover, TRPC3-IK/SK channel signaling mediated acetylcholine-induced dilation in these arteries (409). Co-localization of TRPC3 channels with SK/IK channels at MEPs was also observed in rat popliteal arteries. Additionally, rat mesenteric arteries treated with TRPC3 antisense oligonucleotides showed impaired relaxation to bradykinin (261), further supporting a role for TRPC3 channels in endothelium-dependent vasodilation. In a recent study, ATP-induced EC hyperpolarization was shown to have two components: an early hyperpolarization through IK channels; and a sustained hyperpolarization through TRPC3-SK channel signaling, revealing a central role for TRPC3 channels in ATP-induced endothelial hyperpolarization (227).
TRPM2 channel.
The unitary conductances of TRPM2 channel are 58 and 76 pS at negative and positive voltages, respectively. TRPM2 channel is equally permeable to divalent and monovalent cations (401). The activity of TRPM2 channels was shown to be increased by H2O2, an important redox signaling molecule with a vasodilatory activity (232, 270). A recent study in cremaster arteries showed that H2O2-induced vasodilation is impaired by an antibody against TRPM2 channels, and by inhibiting SK/IK channels (70). Therefore, endothelial TRPM2-SK/IK signaling may underlie H2O2-dependent vasodilation, although further studies are required to confirm the role of TRPM2 channels in controlling endothelial function.
TRPP1 channel.
TRPP1 channel is encoded by PKD2, a gene mutated in patients with autosomal-dominant polycystic kidney disease (296). The outward unitary conductances of TRPP1 channel for Ca2+, Na+, and K+ are 90, 99, and 117 pS, and inward conductances are 4, 89, and 144 pS, respectively. TRPP1 channels conduct ionic currents with permeability ratios of PK+: PNa+:PCa2+ of 1:0.4:0.025 (264). In a recent study, MacKay and colleagues (274) demonstrated the importance of endothelial TRPP1 channel in mediating flow-induced dilation of mesenteric arteries (Figure 8). Pressurized mesenteric arteries from endothelium specific TRPP1−/− mice showed impaired dilation to shear stress. Moreover, endothelium-specific TRPP1−/− mice had higher diastolic and systolic blood pressures. Thus, endothelial TRPP1 channels appear to be a key element in EC mechanosensing and vasodilation.
Purinergic P2X receptor ion channels (P2XR).
Evidence in the literature supports a functional role for P2X1, P2X3, and P2X4 purinergic receptors in ECs. P2X1 receptor was found to be expressed on the endothelium of mesenteric arteries from rats (157) and mice (156) (Figure 8). Endogenous purinergic receptor agonist ATP induced a dilation of mesenteric arteries; an effect that was blunted by inhibition of SK/IK channels (157). Importantly, ATP failed to dilate mesenteric arteries from P2X1−/− mice, supporting the idea that ATP activates P2X1 receptors in this vascular bed (156). Immunohistochemistry analysis by Glass and colleagues (130) showed that P2X3 receptor is expressed in the endothelium from thymus arteries. Moreover, Yamamoto et al. (534) found that ATP- and flow-induced vasodilation is markedly reduced in cremaster and mesenteric arteries from P2X4−/− mice. Furthermore, P2X4−/− mice had a hypertensive phenotype accompanied by a reduction in nitrite and nitrate production. These results led the authors to postulate that impaired eNOS activity and NO production may be partially responsible for increasing blood pressure in the P2X4−/− mice.
PIEZO1 channel.
Endothelium-dependent vasodilation in response to blood flow/shear stress is well established; however, the exact endothelial mechanosensor underlying this effect has remained elusive. PIEZO1 channel on EC membrane has recently emerged as the mechanosensor for flow/shear stress-induced changes in vascular resistance (Figure 8). Wang and colleagues (506) showed that PIEZO1 channels mediate the dilation to flow/shear stress via eNOS activation in U46619 pre-constricted third- and fourth-order mesenteric arteries. Mesenteric arteries from endothelial PIEZO1−/− mice (EC-PIEZO1−/−) showed impaired flow-induced vasodilation. Furthermore, PIEZO1 channel promoted extracellular ATP release via Pannexin1/2 channels in response to flow. ATP, in turn, activated eNOS via P2Y2 purinergic receptor signaling. Consistent with impaired vasodilation, EC-PIEZO1−/− mice showed higher resting systolic blood pressures (506). On the contrary, Rode and colleagues reported that endothelial PIEZO1 channels cause flow-induced vasoconstriction of second-order mesenteric arteries during whole-body exercise. EC-PIEZO1−/− mice on a running wheel showed lower systolic and diastolic blood pressures. Moreover, endothelial PIEZO1 deletion did not affect the reactivity of larger arteries (saphenous and carotid arteries). The discrepancy in results between the two studies could be explained by blood pressure recordings during exercise versus resting conditions, and studies of vascular function using pressure myography versus wire myography. In a recent study, Lhomme and colleagues (250) demonstrated the importance of endothelial PIEZO1 channels in triggering relaxation of pulmonary arteries via NO production. Furthermore, endothelial PIEZO1 channel-induced vasorelaxation was not impaired in a mouse model of pulmonary hypertension. Thus, regardless of the conflicting reports, strong evidence supports the functional significance of endothelial PIEZO1 channels.
Na+/Ca2+ exchanger (NCX).
A recent study demonstrated that NCX contributes to acetylcholine-induced dilation of mesenteric resistance arteries (255). Lillo et al. (255) showed that during acetylcholine activation of endothelial muscarinic receptor signaling, NCX works in reverse mode (NCXrm), enhancing Ca2+ entry in the endothelium and facilitating endothelium-dependent vasodilation. Moreover, NCX was concentrated within the caveolae, in nanometer proximity to eNOS. This result may suggest NCXrm-eNOS signaling during acetylcholine-induced vasodilation. Most other studies have used cell culture systems or conduit arteries (129, 136, 405). Overall, the involvement of NXC in influencing endothelial Ca2+ signaling in resistance arteries and arterioles remains poorly understood.
Ca2+ release from intracellular compartments
Inositol trisphosphate receptors (IP3Rs)
All three isoforms of IP3Rs (IP3R1-3) have been reported in ECs. Ledoux and colleagues (244) described spatially restricted IP3R Ca2+ release from the ER at MEPs. These events were termed “Ca2+ pulsars” (Table 1). The kinetic properties of Ca2+ pulsars allow a clear distinction between Ca2+ puffs and Ca2+ waves. When compared to Ca2+ sparks, Ca2+ pulsars showed slower rise times and longer durations. Consistent with the occurrence of Ca2+ pulsars at MEPs, IP3Rs were also shown to localize at MEPs. Ca2+ pulsars signaled through IK channels to induce EC hyperpolarization and vasodilation (Figure 5). In a recent study, conditional endothelial IP3R1 deletion did not alter vascular dynamics. However, endothelium-specific triple IP3R−/− mice (ECTKO) resulted in higher resting blood pressures. Second-order mesenteric arteries from ECTKO mice also showed impaired acetylcholine-induced dilation (257).
As described for SMCs, EC IP3R activity is also influenced by Ca2+ influx pathways. Heathcote and colleagues (166) explained that Ca2+ influx through endothelial TRPV4 channels triggers IP3R opening. Ca2+ release via IP3Rs amplifies the initial increase in Ca2+ through TRPV4 channel activation, resulting in long and sustained Ca2+ waves that lower vascular contractility. The focal application of acetylcholine on cremasteric arteries resulted in the formation of Ca2+ waves that propagated for over 1 mm with a velocity of 116 μm/s. Interestingly, the initial vasodilatory responses to acetylcholine preceded the propagation of Ca2+ waves. The authors described two temporally distinct vasodilatory phases—an early “electrically conducted vasodilation” occurring through the transmission of hyperpolarizing signal and a later “Ca2+ wave-dependent vasodilation”(21, 468). Communication through gap junctions was found to be pivotal for the propagation of Ca2+ waves between neighboring ECs (105).
IP3 and Ca2+ play crucial roles in the myoendothelial feedback mechanism that limits α1AR-induced SMC contraction (Figure 6). Garland and colleagues recently demonstrated phenylephrine-induced increase in SMC Ca2+ and diffusion of SMC Ca2+ to MEPs via MEGJs, giving rise to the signals called VECTors (VDCC-dependent Endothelial cell Ca2+ Transients). VECTors activated endothelial IP3Rs and facilitated the formation of Ca2+ puffs and Ca2+ waves in the endothelium. In summary, the study by Garland and colleagues (126) suggested that the diffusion of Ca2+ from SMCs to ECs across MEGJ can counteract α1AR-induced vasoconstriction. In another study, endothelial TRPV4 sparklets were implicated as an essential element of the myoendothelial feedback mechanism. Phenylephrine-induced vasoconstriction was counterbalanced by the diffusion of IP3 from SMCs to ECs across MEGJs and subsequent activation of TRPV4 channels at MEPs. Interestingly, the authors observed that endothelial TRPV4 channel activity is influenced only by diffusion of IP3 and not Ca2+ from SMCs to ECs (178). Tran and colleagues (488) provided further evidence for IP3 diffusion from SMCs to ECs during phenylephrine-induced vasoconstriction. α1AR signaling resulted in IP3R activation at MEPs and formation of distinct Ca2+ events termed “Ca2+ wavelets” (Table 1). Ca2+ wavelets could be distinguished from Ca2+ puffs based on their longer duration and larger spatial spread. Ca2+ wavelets activated nearby IK/SK channels at MEPs to limit α1AR-induced vasoconstriction. Similarly, Nausch et al. (313) suggested that sympathetic nerve stimulation activates IP3R Ca2+ pulsars at MEPs and limits sympathetic vasoconstriction. Finally, the Ca2+-binding chaperon protein calreticulin (Calr) was also shown to play an important role in myoendothelial feedback mechanism. Calr was highly localized at MEPs in mesenteric arteries. α1AR-induced increase in endothelial Ca2+ signals at MEPs was absent in the arteries from endothelial Calr−/− mice, which resulted in higher α1AR-induced vasoconstriction. Moreover, endothelial Calr−/− mice showed higher blood pressure, further supporting the role of endothelial Calr in blood pressure regulation (37).
Ca2+-ATPase (SERCA)
Two isoforms of SERCA (SERCA2 and 3) were shown to be expressed in freshly dissociated ECs from coronary arteries and aorta (219), with SERCA3 described as the predominant isoform (304). In an early study, Liu et al. (263) generated SERCA3−/− mice and showed that endothelium-dependent vasodilation is impaired in these mice, although blood pressure was not affected. S100A1 is an intracellular Ca2+-binding protein known to regulate SERCA activity (302, 303). S100A1−/− mice showed reduced NO production, impaired endothelium-dependent vasodilation, and higher blood pressure (363), suggesting that SERCA may be an essential regulator of eNOS activity and blood pressure. Consistent with this postulate, adenovirus-mediated SERCA2 delivery into coronary arteries of Yorkshire-Landrace swine increased eNOS activity. Similarly, an increase in eNOS activity was observed in cultured ECs from human coronary arteries infected with SERCA2-adenovirus (144). Li and colleagues (251) demonstrated that epicardial and endocardial ECs from mice treated with SERCA2-adenovirus are protected against Ca2+ overload-induced necroptosis known to occur during cardiac ischemia/reperfusion injury. Interestingly, a recent study by Zhang et al. (553) revealed a physical interaction between SERCA2 and PIEZO1 channel that results in suppression of PIEZO1 mechanosensation. The disruption of SERCA2-PIEZO1 interaction resulted in increased EC migration. It should be noted that the studies described above have been performed either in conduit arteries or in cell culture systems. Therefore, further investigation is needed to confirm the potential roles of different SERCA isoforms in regulating EC function in resistance arteries. In this regard, possible interactions between SERCA and other sources of Ca2+ in the endothelium will be particularly interesting.
Signaling Targets of Ca2+ in SMCs and ECs
Ca2+-activated potassium channels
Large conductance Ca2+-activated potassium (BK) channels in SMCs
BK channels have mostly been reported in SMCs, although a recent study suggested that functional BK channels are also present in native ECs (311). The unitary conductance of BK channels is 100 to 300 pS. BK channels are gated by membrane depolarization and intracellular binding to Ca2+ and Mg2+. Yuan et al. (543) reported a complex structure of BK channels containing a functional pore formed by four α-subunits that represent multiple splice variants of the Slo1 gene. Four accessory β subunits modulate channel activity. β1-subunit is the predominant β subunit in SMCs. Each α-subunit is composed of 11 hydrophobic segments (S0-S10) that form 3 main functional domains: the VSD, the cytosolic CSD, and the pore gate domain (PGD). The VSD is formed by S0-S4 TM segments and PGD is formed by TM S5-S6 and the P-loop, which confers selectivity for K+. The remaining S7-S10 segments form the CSD and are located intracellularly. S4 helix is enriched with positively charged amino acids that constitute the channel voltage sensor. S0 helix is essential for bridging the α subunit with the β subunit, allowing β subunit-dependent channel modulation. The CSD comprises two potassium regulatory domains (RCK1 and RCK2), both containing putative high-affinity Ca2+-binding sites. RCK2 Ca2+ biding site is called “Ca2+-bowl” and includes a series of Asp residues. Finally, the binding site for Mg2+ is located at the interface between the VSD and CSD. The voltage sensitivity of BK channels is Ca2+- and Mg2+-independent. However, the interaction with Ca2+ and Mg2+ shifts the voltage-dependence of BK channels toward more negative potentials, allowing the channel to function under physiological condition (79, 469, 536). The Ca2+ bowl facilitates channel opening at low intracellular Ca2+ concentrations (<10 μM Ca2+), whereas the RKC1 binding site influences activation over a broader range of Ca2+ concentrations (10–300 μM) (546).
BK channels are activated by RyR Ca2+ sparks in SMCs, resulting in SMC hyperpolarization and vasodilation (226). Therefore, BK channels are exposed to high local concentrations of intracellular Ca2+ (Ca2+ sparks 1–100 μM Ca2+) (353). In this regard, β1 subunit is critical in enhancing the Ca2+ sensitivity of BK channels. Indeed, BK channels were uncoupled from Ca2+ sparks in SMCs from cerebral arteries of β1−/− mice. Consequently, myogenic vasoconstriction and blood pressure were significantly elevated in β1−/− mice (48, 267, 364). Isacson and colleagues (195) showed that the scaffolding protein receptor for activated C kinase 1 (RACK1) co-localizes with BK channels and slows BK channel activation in response to membrane depolarization. Further studies are needed to elucidate the physiological relevance of the RACK1-BK channel interaction.
Around 95% of α-subunits are found at the plasma membrane, whereas only a small percentage (10%) of β1 subunits are located at the plasma membrane. PKG and PKA enhance the trafficking of β1 subunits from the SR to the plasma membrane, increasing the open-state probability of BK channels and promoting vasodilation (248). Furthermore, PKG increases the open-state probability of BK channels via direct channel phosphorylation (387). In a recent study, Khavandi and colleagues (222) proposed a novel mechanism for indirect activation of BK channels by PKG. The authors reported that myogenic vasoconstriction generates H2O2, which activates PKG. PKG, in turn, enhances the activity of Ca2+ sparks and BK channels. PKA also increases BK channel activity through an indirect mechanism involving phosphorylation of phospholamban, causing SERCA disinhibition. Consequently, the increased SR Ca2+ load increases Ca2+ spark activity. Indeed, the effect of PKA on Ca2+ sparks and BK channels was impaired in phospholamban−/− mice (513). PKC, on the contrary, has an inhibitory effect on BK channel activity. Patch-clamp studies on freshly dissociated SMCs showed that PKC could directly inhibit BK channels independently of Ca2+ sparks (406). In guinea pig basilar arteries, β-adrenergic receptor stimulation activated BK channels via cAMP-dependent PKA activation, in a PKG-independent manner (431). On the contrary, a separate study showed that β-adrenergic receptor stimulation of coronary arteries activates BK channels via PKG activation (517). Thus, the effects of protein kinases on BK channel activity are diverse and vary from one vascular bed to another.
GPCRs also alter BK channel activity via G protein-dependent and independent effects. Early studies showed that Ang II receptor 1 (AT1R) inhibited BK channel currents through a G protein-independent mechanism in coronary arteries (482, 555). Membrane-bound PIP2, which is regulated by GqPCR signaling, was shown to directly activate BK channels, thereby promoting the vasodilation of cerebral arteries (493). Fatty acids have also been documented to modulate BK channel activity. Ahn and colleagues (7) demonstrated that AA and long-chain fatty acids directly facilitate BK channel opening in rabbit coronary arteries. The BK channel opening effect was similar between saturated and unsaturated fatty acids. Docosahexaenoic acid (DHA), a polyunsaturated fatty acid (PUFA), evoked BK channel currents and vasodilation indirectly via its CYP metabolite 16,17-epoxydocosapentaenoic acid (505). In support of these findings, tail vein injection of a bolus of DHA lowered blood pressure, an effect that was absent in Slo1 deficient mice (181). In summary, the activity of BK channels is regulated by a plethora of endogenous mechanisms in vascular SMCs.
Intermediate and small conductance Ca2+ activated K+ channels (IK/SK)
Endothelial cells (EC).
IK and SK channels have mostly been described in ECs. The unitary conductance of SK channels is approximately 10 pS, and that of IK channels is 20 to 30 pS. SK channel family is composed of four isoforms. SK1 (KCa2.1), SK2 (KCa2.2), and SK3 (KCa2.3) are encoded by KCNN1-3 genes, respectively. The fourth isoform, SK4 (IK or KCa3.1), shows higher conductance and is encoded by KCNN4 (6, 245). Functional SK/IK channel consists of four homologous subunits, each containing six TM segments (S1-6). Segments 5 and 6 line the pore-forming unit of the channel. S1 and S6 are connected to intracellular N- and C-terminal domains, respectively. SK/IK channels share a similar structure to voltage-gated K+ channels (Kv) but do not exhibit voltage-dependence. This difference appears to be due to the diverse amino acid sequence of the S4 segment. Ca2+-dependent activation of SK/IK channels does not rely on Ca2+-binding directly onto the channel. Xia and colleagues (527) demonstrated that Ca2+ sensitivity of the channel is imparted by interaction with Ca2+-CaM. The C-terminal lobe (C-lobe) of CaM associates with the C-terminal tail of SK channels in a Ca2+-independent manner. The channel gate opens upon Ca2+ binding to the CaM N-lobe (407). Moreover, CaM binding to SK/IK channels is essential for channel assembly and trafficking to the plasma membrane (206). SK/IK channels show half-maximal activation response at similar Ca2+ concentrations (EC50 = 300–500 nM). Moreover, SK/IK channels display a fast time constant for activation (5–15 ms) (175, 527). This feature makes SK/IK channels an ideal target for coupling with distinct Ca2+ signals. Indeed, numerous studies have demonstrated that IK/SK channels are concentrated at MEPs in ECs, where the majority of localized Ca2+ signals also occur (20, 341, 342, 432, 433). Spatial proximity between SK/IK channels and Ca2+ signals at MEPs ensures selective activation of SK/IK channels by Ca2+ signals. SK/IK channel activation, in turn, results in EC membrane hyperpolarization, which is transmitted to SMC via MEGJs, causing vasodilation. SK and IK channel currents have been recorded in freshly isolated ECs from mesenteric (432), cerebral (147), and pulmonary resistance (341) arteries. Taylor and colleagues (472) reported that SK3 channel deletion depolarizes EC membrane in mesenteric arteries and increases vasoconstriction and blood pressure. A subsequent study showed that inhibition of SK or IK channels depolarizes ECs from mesenteric arteries by approximately 8 and 3 mV, respectively (242). These results suggest that SK/IK channels might be constitutively activated by spontaneously occurring Ca2+ signals in ECs.
Smooth muscle cells (SMC).
IK channels have been shown to be involved in SMC dedifferentiation and proliferation (131, 229, 320). Tharp et al. (475) showed that platelet-derived growth factor-BB (PDGF-BB) increased functional IK channels in SMCs from swine coronary arteries. Moreover, increased IK channel activity reduced the expression of SMC differentiation markers. Overexpression of IK channels in non-proliferating SMCs promoted dedifferentiation and proliferation by creating the electrochemical gradient favoring Ca2+ influx. Increased intracellular Ca2+ activated cAMP response element-binding protein (CREB), a mitogen-induced transcriptional factor, thereby driving SMC proliferation (34). Augmented IK channel transcription in proliferating SMCs was attributed to reduced expression of repressor element 1-silencing transcription factor (REST) (69). IK channel inhibition decreased SMC proliferation in response to growth factors (131, 475), further supporting the role of IK channels in SMC proliferation. Gole and colleagues (131) proposed that fibroblast growth factor induces IK channel upregulation via NOX5-dependent ROS production in SMCs from porcine coronary arteries. Of particular clinical importance is the finding that IK channel inhibition was protective against restenosis in the rat (229) and swine models (476). Moreover, genetic deletion and pharmacological inhibition of IK channels was protective against atherosclerosis in a mouse model (486). The substantial evidence supporting a crucial role of IK channels in SMC proliferation suggests that IK channels could be therapeutically targeted in vascular lesions linked to SMC proliferation. However, the central role of IK channels in endothelium-dependent vasodilation may prove problematic with this approach.
Protein kinases
Ca2+-CaM-dependent protein kinase II (CaMKII)
Endothelial cells (EC).
Ser or Thr residues are the target sites of phosphorylation by Ca2+-CaM kinase family (CaMK); therefore, CaMKs are also known as Ser/Thr kinases. CaMKs share a conserved structure with three characteristic domains: catalytic, autoinhibitory, and CaM-binding domains. CaMK family is divided into two main groups—multifunctional CaMKs (CaMKI, CaMKII, and CaMKIV), which have multiple downstream intracellular targets; and substrate-specific CaMKIII, which has only one downstream target (350). CaMKII has recently emerged as an essential vasoregulatory mechanism. CaMKII is expressed as one of the four isoforms (α, β, δ, and γ), with a and β isoforms occurring primarily in the brain and δ and γ isoforms expressed in blood vessels (481). CaMKII, unlike other CaMKs, does not exist in a monomeric state. Indeed, CaMKII has a peculiar association domain that allows the multimeric association of 6-12 monomers, which is partly responsible for the ability of CaMKII to respond to diverse intracellular Ca2+ oscillations (455). Activation of CaMKII occurs in multiple steps. The first step (Ca2+-CaM-dependent) involves Ca2+-CaM binding to CaMKII and disrupts the interaction between the autoinhibitory and catalytic domains. In the second step (Ca2+-CaM independent), the Ca2+-CaM activated subunit phosphorylates the catalytic subunit and activates it (77).
The significance of CaMKII in orchestrating the functional responses to intracellular Ca2+ is unclear, mainly due to two reasons (i) the majority of studies on CaMKII have been performed in cell culture systems and evidence from intact tissue is scarce (485); and (ii) numerous studies have used KN-93, a non-specific CaMKII inhibitor (351). Therefore, caution must be used while interpreting the currently available literature on CaMKII. In a recent development, Murthy and colleagues (307) investigated the role of CaMKII in vivo using a transgenic mouse model, where AC3-I (CaMKII inhibitory peptide) was conditionally expressed in the endothelium. AC3-I mice showed unaltered circulating NO levels and blood pressure compared to control mice. Furthermore, endothelium-dependent vasodilation of mesenteric arteries was also unchanged in AC3-I mice. However, in culture systems, AC3-I-dependent CaMKII inhibition prevented the increase in intracellular Ca2+ in response to bradykinin. Therefore, further evidence is needed for a definitive assessment of the functional roles of CaMKs in ECs.
Smooth muscle cells (SMC).
The involvement of CaMKII in SMC proliferation and migration has mainly been studied in cell culture systems and conduit arteries, and therefore, will not be discussed in this article. Ledoux and colleagues (243) recently postulated that CaMKII facilitates LTCC activity by exerting rapid positive feedback on LTCC currents in SMCs. As acknowledged by the authors, this study used non-specific CaMKII inhibitor KN-93 (124, 243, 384). Prasad and colleagues (372) developed a novel mouse model that conditionally expresses specific CaMKII peptide inhibitor CaM-KIIN (TG SM-CaMKIIN mice) in SMCs. TG SM-CaMKIIN mice showed reduced CaMKII-dependent phosphorylation of LTCC channel β3 subunit and phospholamban, resulting in lower LTCC channel currents and reduced SERCA activity. Furthermore, Ang II- and phenylephrine-induced increase in intracellular Ca2+ was attenuated in TG SM-CaMKIIN mice, although Ang II- and phenylephrine-evoked vasoconstriction was not altered. Interestingly, TG SM-CaMKIIN mice showed reduced CaMKII-dependent inhibition of MLCK, which was proposed as a compensatory mechanism that normalized Ang II- and phenylephrine-evoked vasoconstriction. In a subsequent study, Prasad et al. (371) demonstrated that chronic Ang II infusion in TG SM-CaMKIIN mice leads to higher mesenteric artery remodeling. These findings propose an exciting paradigm where CaMKII, per se, does not alter vasoconstriction, but by limiting vascular hypertrophy, may prevent the damaging effects of long-term hypertension.
Protein kinase C (PKC)
This large family of serine/threonine protein kinases has been divided into four functionally diverse subfamilies according to their enzymatic properties. PKCα, PKCβ, and PKCγ belong to the conventional family (cPKC). This article will specifically focus on cPKCs as they are activated in a Ca2+-dependent manner (290). The remaining families are novel PKCs (nPKCs) composed of the δ, η, ε, and θ isotypes; atypical PKCs (aPKCs) λ and ζ; and the protein kinase C-related kinase (PRK) family. The enzymatic activity of nPKCs and aPKCS is Ca2+ independent (390). PKC is activated by DAG (463), phosphatidylserine (PS) (426), and by phorbol 12-myristate 13-acetate (PMA) (53).
The N-terminal regulatory (36 kDa) and C-terminal catalytic domains (42 kDa) of PKC are separated by a hinge region (224). The regulatory domain contains (i) a cysteine-rich sequence that coordinates two Zn2+ ions (439) and is essential for DAG, PMA (339, 424, 439), and PS binding (223, 319); (ii) a conserved autoinhibitory sequence (183) that maintains PKC in an inactive state in the cytosol (184); and (iii) a CBD (230). Ca2+ binding is a pivotal step for PKC activation and its membrane association. Binding of cytosolic PKC with two Ca2+ ions results in a weak association with the plasma membrane, whereas interaction of PKC with the third Ca2+ ion enables its strong association with the plasma membrane (230). The Ca2+-bound form of PKC is also required for its interaction with PIP2 (399). The catalytic domain comprises ATP binding sequence and a region important for PKC-substrate interaction (329). PKC activators, including Ca2+, DAG, PS, and PMA, promote complete allosteric activation and translocation to the plasma membrane (386).
Endothelial cells (EC).
PKC has been shown to promote EC proliferation by participating in the vascular endothelial growth factor (VEGF) signaling cascade (524). PKCβ is thought to be the main isoform associated with the mitogenic effects of VEGF (8, 526), although PKCα has also been associated with VEGF-induced angiogenesis (514). Suzuma et al. (453) demonstrated that PKCβII promotes retinal neovascularization in a mouse model of oxygen-induced retinal neovascularization. Indeed, retinal neovascularization was diminished in PKCβ−/− mice, whereas a more extensive network of neovascularization was observed in a mouse model overexpressing PKCβII (PKCβII Tg) (453). On the contrary, a separate study by Spyridopoulos and colleagues (436) reported that specific PKCα and PKCβ inhibition increases VEGF-induced angiogenesis. In this study, PKCα and PKCβ inhibition also enhanced the VEGF-dependent increase in vascular permeability via NOS activation. The reasons for the conflicting results in the two studies (436, 453) remain unclear.
Adapala et al. (3) demonstrated that PKCα is necessary for acetylcholine-induced Ca2+ entry via TRPV4 channels. Further studies by Sonkusare and colleagues demonstrated that PKC anchoring by AKAP150 is necessary for enhancing Ca2+ influx through TRPV4 channels at MEPs. In this regard, Fan et al. (111) showed that PKC phosphorylates TRPV4 channels, an effect that is enhanced by the presence of AKAP150. Along similar lines, Ottolini et al. (343) demonstrated that acetylcholine- and PKC-activation of TRPV4 channels were absent in endothelium-specific TRPV4−/− mice. However, cell-specific knockout mice are needed to obtain an accurate understanding of the relative contributions of different PKC isoforms to vascular function.
Smooth muscle cells (SMC).
DAG- or Ca2+-activation of PKC promotes SMC contractility via a multitude of mechanisms (17). SMC contractility was enhanced following PKC-dependent inhibition of myosin light chain phosphatase (MLCP) activity (225, 521). Moreover, PKC activation had an inhibitory effect on BK channel currents in SMCs from rat mesenteric arteries (457), rat pulmonary arteries (26), and cat cerebral arteries (240). In SMC isolated from rabbit coronary arteries, Ang II-induced inhibition of the inward rectifier K+ (Kir) channel current was also mediated by PKCα (346). Kanashiro and colleagues (211) showed that PKCα mediated eicosanoid-induced vasoconstriction of porcine coronary arteries. In pressurized rat cerebral arteries, PKC inhibition impaired myogenic vasoconstriction (425), suggesting an essential role for PKC in the development of myogenic vasoconstriction. It was suggested that PKC might favor myogenic vasoconstriction through its actions on the cytoskeletal organization. Specifically, by phosphorylating the heat shock protein 27 (HSP27), PKC reduced SMC G-actin content and favored actin polymerization, promoting force generation during myogenic vasoconstriction (301). Consistent with these ex vivo findings, Wynne et al. showed that global PKCα−/− mice have lower resting blood pressure (301).
Myosin light-chain kinase (MLCK)
MLCK is a Ca2+/CaM-dependent kinase that promotes actin-myosin cross-bridge formation and vasoconstriction (429) (Figure 1). PKA-dependent phosphorylation on Ser512 negatively modulates the Ca2+/CaM-sensitivity of MLCK (191, 271) and reduces its affinity for Ca2+/CaM (Ca2+ desensitization) (74). CaMKII also appears to be involved in MLCK phosphorylation and Ca2+ desensitization (470, 471). Two different genes encode for MLCKs: smooth muscle MLCK (mylkl (123) and skeletal muscle MLCK (mylk2) (169, 209). For this article, we will focus on SMC MLCK and will refer to it as MLCK. Two known isoforms of MLCK are: short MLCK (130–150 kDa), which is expressed in mature SMCs (43); and long MLCK (208–214 kDa), which is expressed mainly in embryonic SMCs (209) and mature ECs (498).
Endothelial cells (EC).
The phosphorylation status of myosin light chain (MLC) is an essential regulator of EC permeability. MLCK enhances actomyosin contractility and weakens EC-EC adhesion by phosphorylating MLC (94, 479, 480). Thus, MLCK-dependent MLC phosphorylation regulates endothelial barrier function by maintaining a basal degree of permeability. Indeed, MLCK inhibition resulted in reduced vascular permeability (544). Huang et al. (187) showed that MLCK inhibition lowers microvascular hyperpermeability in thermal injury. Genetic deletion of the long MLKC isoform (MLCK-210−/−) confirmed the importance of long MLKC in increasing the pulmonary microvascular permeability in response to lung injury (503). Moreover, MLCK-210−/− mice are protected against microvascular hyperpermeability induced by burn injury (383). Moitra and colleagues (297) generated genetically engineered mice overexpressing the long MLKC isoform in ECs (VE-MLCK-210). VE-MLCK-210 mice showed increased pulmonary microvascular permeability under resting conditions and augmented hyperpermeability following lung injury (297). These findings established a pivotal role for long MLKC in increasing microvascular permeability. Therefore, targeting long MLKC isoform may be a promising therapeutic intervention in pathological conditions characterized by increased microvascular leakage. In this regard, further research on developing isoform-specific MLKC inhibitors is needed to reduce possible side effects with inhibition of long MLCK (413).
Smooth muscle cells (SMC).
MLCK initiates SMC contraction by phosphorylating Ser19 of the myosin II RLC. Phosphorylation of RLC activates actomyosin ATPase, which turns on cross-bridge cycling and promotes force development (208, 445). Indeed, SMC-specific MLCK deletion resulted in reduced vasoconstriction and lower blood pressure (164). In a recent study, Chen et al. (62) created a mouse model with SMC-specific deletion of short MLCK. These mice showed reduced contractility of gastrointestinal SMCs, suggesting that short MLCK is essential for gastrointestinal smooth muscle contractility (62). To the best of our knowledge, vascular contractility studies have not been performed in this mouse model.
Endothelial nitric oxide synthase (eNOS)
NO was first identified as the major endothelium-derived relaxing factor nearly 30 years ago (120, 121, 188, 189, 306). To this date, NO-dependent vasodilation remains the most studied mechanism for endothelium-dependent vasodilation under physiological and pathological conditions. Three different nitric oxide synthases (NOSs) catalyze the production of NO from l-arginine—neuronal or nNOS; inducible or iNOS; and endothelial or eNOS (116). Although other NOS can be present in the vascular wall, endothelial NO mainly comes from the activation of eNOS or NOS3 (11, 298, 496). Synthesis of NO by eNOS requires the precursor l-arginine, cofactors tetrahydrobiopterin (BH4), flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), CaM, and iron protoporphyrin IX (Heme Fe). eNOS protein is synthesized as monomers but needs to form homodimers to produce NO. As monomers, the oxygenase domain of eNOS produces superoxide; a phenomenon commonly referred to as eNOS uncoupling that contributes to oxidative stress in diseases (496).
eNOS is localized in the caveolae, which are membrane invaginations rich in caveolin-1 (116, 434). eNOS can be activated in a Ca2+-dependent manner or through posttranslational modifications. Ca2+ can activate eNOS by inducing CaM binding to the CaM-binding domain on eNOS. Increased Ca2+-CaM displaces eNOS from caveolin-1 and relieves the inhibitory effect of caveolin-1 on eNOS activity (88). Moreover, the displacement of eNOS from caveolin-1 promotes its translocation to the cytosol, where eNOS can undergo posttranslational modifications that activate it. Association of eNOS with caveolin-1 also localizes it with several other signaling molecules, including ion channels, protein kinases, GPCRs, and tyrosine kinases, thus potentially increasing the chances of eNOS activation by these signaling elements (496).
Phosphorylation can have variable effects on eNOS activity depending on the site of phosphorylation. Phosphorylation at Ser1177 activates, whereas phosphorylation at Thr495 inhibits the enzyme (115, 116, 167, 293). PKA, PKB, AMPK, CaMK, and ERKs 1 or 2 can phosphorylate eNOS at Ser1177 (167). On the contrary, Rho kinase and PKC phosphorylate eNOS at Thr495 and thus inhibit it (61, 446). Moreover, tyrosine kinase can also phosphorylate eNOS on Tyr657 and lower eNOS activity (114). Phosphorylation of eNOS at Tyr81 by Src kinase facilitates NO production (119). In addition to phosphorylation, other posttranslational modifications that modify eNOS activity include cysteine palmitoylation, which localizes eNOS to the caveolae and brings it closer to regulatory signaling elements (262); S-nitrosylation at Cys94 and Cys99 that inhibits enzyme activity (379); acetylation at Lys610 and deacetylation at Lys497 and Lys507, which increase the enzyme activity (167); glycosylation at Ser1177, which decreases eNOS activity (308); S-glutathionylation at Cys689 and Cys908, which promotes eNOS uncoupling (59, 60).
NO released as a result of eNOS activation can passively diffuse to the SMC layer, where it activates a soluble GC-cGMP-PKG signaling pathway to cause vasodilation (86). NO can also activate the endothelial GC-PKG pathway that limits Ca2+ influx in ECs and endothelium-dependent vasodilation (282). Two main K+ channels that regulate SMC membrane potential are BK and Kv channels (201). While it is well established that NO activates both BK and Kv channels, the mechanism of activation appears to be twofold. Some studies show GC-cGMP-dependent activation of BK channels by NO (18, 387), whereas other studies indicate that NO can activate both BK and Kv channels in a GC-PKG-independent manner (45, 295). The latter mechanism possibly involves post-translational modification of the channel by NO, such as S-nitrosylation, or cAMP-PKA signaling (194). Activation of SMC K+ channels by NO results in membrane hyperpolarization, which deactivates LTCCs to relax SMCs. PKG also promotes the uptake of Ca2+ by the SR by phosphorylating phospholamban, which increases SERCA activity (52, 496). IP3R Ca2+ release from the SR in SMCs is crucial for myogenic vasoconstriction (132, 516). PKG has also been shown to phosphorylate IP3R-associated PKG substrate (IRAG), inhibiting Ca2+ release from the SR (402, 403) and myogenic vasoconstriction. The NO-GC-cGMP-PKG-I pathway also activates MLCP in SMCs. PKG-I binds to and phosphorylates the regulatory subunit of MLCP, MYPT1, and prevents the inhibition of MLCP. This MLCP de-inhibition lowers cross-bridge cycling and SMC contraction (258, 452, 522).
Intriguingly, both IK/SK channels and eNOS can be activated by Ca2+, and yet either eNOS or IK/SK channels play a predominant role in endothelium-dependent vasodilation in each vascular bed. A generally accepted distinction is that eNOS regulates endothelium-dependent vasodilation in larger (conduit) arteries, whereas IK/SK channel-mediated hyperpolarization plays a major role in smaller, resistance-sized arteries (419, 492). Moreover, lower level, localized increases in Ca2+ seem to preferentially couple with IK/SK channels (20, 178, 432, 433) except in the case of small pulmonary arteries where they couple with eNOS (282). Ottolini and colleagues (341) recently showed that Ca2+ signal-IK/SK channel co-localization and NO scavenging protein Hbα favor Ca2+-IK/SK channel signaling in systemic arteries. On the contrary, Ca2+ signal-eNOS co-localization and absence of Hbα facilitated Ca2+ signal-eNOS signaling in the pulmonary circulation (Figure 7). It should be noted that the blood pressure is elevated in eNOS global knockout mice (414, 438, 494). eNOS is also expressed in cell types other than ECs. Therefore, investigations in endothelium-specific eNOS−/− are likely to result in interesting findings on endothelium-dependent vasodilation and blood pressure regulation.
Arterial and Arteriolar Ca2+ Signaling in Vascular Disorders
The role of abnormal Ca2+ signaling mechanisms in the pathogenesis of vascular disorders is well established, particularly in resistance-sized arteries and arterioles. Both endothelial and SMC Ca2+ signaling mechanisms are impaired in various disorders. Ca2+ signals in SMCs have mostly been associated with increased in activity, resulting in vasoconstriction and higher vascular resistance. RyR-mediated Ca2+ sparks are an exception to this rule as they are vasodilatory signals, and their activity is decreased in vascular disorders. Impairment of endothelial Ca2+ signaling mechanisms attenuates endothelium-dependent vasodilation, contributing to increased vasoconstriction in vascular disorders. Increased activity of SMC and endothelial Ca2+ signals has also been associated with hyperproliferation, migration, and hyperpermeability in vascular disorders. In this section, we will discuss the studies on Ca2+ signaling mechanisms in resistance arteries and arterioles in vascular disorders.
Abnormal Ca2+ signaling in SMCs
SMCs from small mesenteric arteries of hypertensive rats showed higher LTCC current density and a leftward shift in the voltage-dependence of LTCC activation. Furthermore, a linear correlation was observed between systolic blood pressure and LTCC current density (269). Notably, treatment of hypertensive rats with angiotensin-converting enzyme (ACE) inhibitor normalized LTCC currents and reduced blood pressure (80). Bannister and colleagues (25) demonstrated that increased expression of auxiliary LTCC subunits α2δ-1 in cerebral arteries from spontaneously hypertensive rats (SHRs) promotes LTCC channel trafficking to the membrane and increases LTCC currents. An increase in SMC LTCC density has also been reported in non-genetic models of hypertension (423). In an Ang II-induced hypertension model, the increase in LTCC currents was associated with the upregulation of β3 regulatory subunit and increased translocation of pore-forming α1C subunit to the membrane (221). Intriguingly, Tajada and colleagues (458) reported lower number of LTCCs, but highly active subpopulations of LTCCs in a mouse model of essential hypertension. These findings support the idea that localized, as opposed to wholecell, changes in intracellular Ca2+ may be a more accurate readout of pathological outcomes. In this regard, Nieves-Cintron and colleagues showed that AKAP150 anchoring of PKCα in the vicinity of LTCCs created microdomains of increased LTCC activity. Indeed, AKAP150−/− and PKCα−/− mice were protected against Ang II-induced hypertension (316). AKAP150 also anchors calcineurin (323), which is excessively activated by Ca2+ influx through LTCC in hypertension and diabetes (16, 335). LTCC-mediated activation of calcineurin dephosphorylated NFATc3, resulting in its translocation to the nucleus, where it downregulated BK channel β1 subunit (324). Reduced β1 subunit expression impaired the activity of BK channels and elevated blood pressure (335, 364). Excessive LTCC activation has also been demonstrated in cerebral arteries from a mouse model of type 2 diabetes (317) and a rat model of subarachnoid hemorrhage (336). Thus, evidence in the literature strongly supports the concept that increased LTCC activity in SMCs contributes to elevated vasoconstriction in vascular disorders.
Abnormalities of SMC Ca2+ signaling mechanisms have also been reported in pulmonary SMCs. The upregulation of SMC LTCCs was shown to contribute to hypoxia-induced pulmonary hypertension (174). Recent studies by Jernigan and colleagues identified acid-sensing ion channel-1 (ASIC1) overexpression as a novel mechanism for elevated SMC Ca2+ in hypoxic pulmonary hypertension (134). In a subsequent study, Jernigan and colleagues showed that RhoA increases the plasma membrane localization of ASIC1 in SMCs. In this regard, the scaffolding protein interacting with C-kinase-1 (PICK1) (252, 508) interacts with ASIC1 (186). Furthermore, Herbert et al. (168) showed that PICK1 anchors calcineurin close to ASIC1, promoting dephosphorylation of and Ca2+ entry through ASIC1 in SMCs from pulmonary arteries. Based on these results, PICK1-calcineurin-ASIC1 interaction may represent a novel signaling pathway that augments SMC Ca2+ in pulmonary hypertension.
In a separate study, Yuan and colleagues demonstrated that TRPC1 channels increase pulmonary vasoconstriction through capacitative Ca2+ entry (235) and are upregulated in pulmonary arterial hypertension (542). In a chronic hypoxic mouse model of pulmonary hypertension, Sham and colleagues showed that the SMC TRPV4 channel is upregulated and contributes to increased pulmonary artery vasoconstriction (528, 538). Thus, abnormalities in voltage-gated and non-voltage-gated Ca2+ channels on SMC membranes have been shown to contribute to pulmonary vasoconstriction in pulmonary hypertension.
Abnormal Ca2+ sparks-BK channel coupling has been proposed as a contributor to vascular dysfunction. Angiotensin II-induced hypertension caused a pathological downregulation of β1-subunit of the BK channel, resulting in the uncoupling of BK channels from Ca2+ sparks (15). Consistent with these findings, a gain-of-function point mutation on KCNMB1 (gene encoding for β1 subunit) was protective against diastolic hypertension (112). Pritchard and colleagues (374) identified the effects of Duchenne muscular dystrophy (DMD) on Ca2+ events in SMCs from cerebral arteries. In this study, the cluster size of RyRs, which co-localize with BK channels at the plasma membrane, was significantly larger in DMD mice. A higher number of RyRs within a cluster increased “Ca2+-induced Ca2+-release” and elevated the probability of Ca2+ spark occurrence. The abnormally high Ca2+ spark-BK channel signaling resulted in impaired myogenic vasoconstriction. On the contrary, the expression of RyRs was reduced in cerebral arteries from db/db mice, resulting in impaired Ca2+ sparks-BK channel signaling (392). In a recent study, Nieves-Cintrón and colleagues (325) observed that reduced functional coupling between α and β1 subunits of BK channels contributed to higher vasoconstriction in patients with type 2 diabetes.
SMC NCX1 appears to be a substantial contributor to the pathogenesis of salt-sensitive hypertension. A high-salt diet augments the production of cardiotonic steroids (CTS). CTS inhibited Na+/K+ ATPase and led to a pathological increase in intracellular Na+ (146, 160). Under these conditions, NCX1 worked in the reverse mode and caused SMC Ca2+ overload. Consistent with these findings, SMC-specific NCX1−/− mice were protected against salt-induced hypertension (509). Moreover, SMC-specific NCX1 overexpression worsened high-salt diet-induced hypertension (199). Therefore, NCX1-dependent signaling could be therapeutically targeted in salt-sensitive hypertension.
SMC IP3Rs were involved in increased phenylephrine (α1AR agonist)-induced vasoconstriction of femoral arteries from rats with heart failure (231). Moreover, Ang II-induced NFAT activation increased IP3R expression in SMCs, resulting in exaggerated vasoconstriction (2). Along similar lines, SMCs from mesenteric arteries of hypertensive rats showed increased ET-1 induced contraction due to enhanced spatial coupling between IP3R1 and TRPC3 channels (4). Preglomerular microvascular smooth muscle cells (PGVMSCs) from SHRs showed augmented Ang II- and neuropeptide Y1-induced proliferation due to excessive IP3R activation (65, 563). This effect was attributed to increased plasma membrane localization of RACK1. RACK1 facilitated the proximity of PLC to AT1R and neuropeptide Y1-receptor, thereby facilitating PLC-IP3R signaling (65, 563). RACK1 can also directly activate IP3Rs, resulting in PGVMSC proliferation (64, 349). Use of SMC-specific RACK1 knockout mice will provide a definitive answer on the role of RACK1 in influencing SMC function. In summary, multiple studies support a pathological role for SMC IP3Rs in vascular disorders.
Abnormal Ca2+ signaling in ECs
In the endothelium, AKAP150 and TRPV4 channels are highly localized at MEPs, where AKAP150 anchors PKC in the vicinity of TRPV4 channels and increases the coupling among TRPV4 channels (433). Ca2+ influx through endothelial TRPV4 channels causes vasodilation via IK/SK channel activation (20, 432). Sonkusare and colleagues (433) demonstrated that Ang II-induced hypertension disrupts endothelial AKAP150-TRPV4 channel signaling by lowering the expression of AKAP150 at MEPs. A recent study by Ottolini et al. (342) proposed the concept of pathological signaling microdomains in obesity, whereby pathological elements localized at MEPs impaired endothelial Ca2+ signaling. The authors first demonstrated the importance of endothelial AKAP150-TRPV4 channel signaling in lowering systemic blood pressure under normal conditions. In obesity, increased expression of inducible nitric oxide synthase (iNOS) and NADPH oxidase 1 (NOX1) at MEPs correlated with elevated levels of NO and superoxide radicals (O2−), respectively. Interaction of NO and O2−. resulted in localized formation of the oxidant molecule peroxynitrite. Peroxynitrite, in turn, disrupted AKAP150-PKC interaction, thereby reducing TRPV4 channel activity at MEPs. The loss of endothelium-dependent vasodilation, as a result of this pathological signaling, led to increased blood pressure in obesity. In a separate study, Wilson and colleagues (520) indicated that altered Ca2+ signaling networks of ECs contribute to the loss of endothelial function in obesity. Ca2+ signaling networks of ECs are critical for determining overall vascular resistance (246). While the loss of endothelial function in obesity is well documented (342, 520), a study by Greenstein and colleagues (139) reported that obesity is not associated with a loss of endothelium-dependent vasodilation. Differences in diet regimens and analytical techniques may be responsible for the divergent findings. Regardless, it should also be noted that obesity-induced vascular dysfunction is multifactorial and likely caused by both endothelial and smooth muscle impairments.
Alterations in Ca2+ signals through endothelial TRPV4 channels have been demonstrated in multiple disease models. In cerebral arteries from a mouse model of Alzheimer’s disease, endothelial TRPV4 channel activity was impaired with a consequent decrease in acetylcholine-evoked vasodilation. The impairment of TRPV4 channel activity was linked to the excessive formation of ROS in this model. Indeed, reducing H2O2 and O2− levels rescued the vasodilation to acetylcholine (552). Similarly, Ma et al. (272) observed decreased acetylcholine-induced vasodilation in mesenteric arteries from diabetic mice that was attributed to impaired TRPV4-SK channel signaling. Stroke-prone SHRs also showed a downregulation of endothelial TRPV4 and SK channels, resulting in impaired acetylcholine-induced vasodilation (408).
Recent discoveries suggest a detrimental role for excessive Ca2+ in ECs from arteries or capillaries in the pathogenesis of some vascular disorders. Excessive endothelial Ca2+ activity in systemic arteries was correlated with elevated histone levels after traumatic brain injury (73). Collier and colleagues (73) found that high levels of histones in the plasma from trauma patients evoked an exaggerated increase in endothelial Ca2+. Prolonged exposure to histones led to endothelial Ca2+ overload, EC death, and subsequent loss of endothelium-dependent vasodilation. Another study by Suresh et al. (450) suggested that higher endothelial Ca2+ levels, resulting from increased TRPV4 channel activity, underlies capillary endothelial migration in pulmonary hypertension. Contrary to these results, a recent study presented an exciting idea that reduced membrane cholesterol content lowers Ca2+ entry in ECs in pulmonary hypertension (548).
Methodologies for Recording Ca2+ signals in SMCs and ECs
Ca2+ signals within the cells have unique spatiotemporal properties that translate into numerous cellular functions (31). Therefore, the diversity of Ca2+ signals cannot be captured with one technique. However, over the last two decades, the use of sensitive fluorescent Ca2+ indicators and fast, high-resolution imaging techniques (confocal, total internal reflection fluorescence or TIRF, and multi-photon imaging) have enabled effective spatiotemporal resolution of individual Ca2+ signals. Fluorescent indicators are synthesized as membrane-permeable acetoxymethyl (AM) esters. Cytosolic esterases release the anionic cell impermeable form, which remains in the cytosol (491). Fluorescent Ca2+ indicators can be classified into ratiometric (dual-wavelength) and non-ratiometric (single wavelength) indicators. Ratiometric indicators show a shift in their excitation (Fura-2) or emission (Indo-1) spectrum upon Ca2+ binding. A classic example of ratiometric indicators is Fura-2, which presents two excitation wavelengths (Ca2+-free form/Ca2+-bound form = 380/340 nm). Elevation in cytosolic Ca2+ levels increases Fura-2 fluorescence at 340 nm (Ca2+-bound form) and decreases the fluorescence at 380 nm (Ca2+-free form). Therefore, the ratio of the emission intensity at 340 nm to that at 380 nm is proportional to cytosolic Ca2+ concentration. Ratiometric dyes have mostly been used for quantification of whole-cell changes in Ca2+ concentration. Non-ratiometric indicators (fluo-indicators), on the contrary, do not show a shift in excitation/emission wavelengths upon Ca2+ binding. Instead, the fluorescence intensity increases upon Ca2+ binding (140). Usually, fluorescence values of non-ratiometric indicators are presented as a ratio between the fluorescent value during the occurrence of a Ca2+ signal (F) and that at the baseline (F0, no Ca2+ activity) (294). The use of ratiometric indicators is not associated with loading and photobleaching issues that are commonly experienced with non-ratiometric indicators. The drawbacks of ratiometric indicators are (i) they are not suitable for capturing fast, spatially restricted Ca2+ signals; (ii) they require excitation with ultraviolet light, which is harmful to biological samples; and (iii) they cannot be used for studies of individual fast Ca2+ signals. Moreover, ultraviolet light is not effectively transmitted through most high numerical aperture objectives. Therefore, non-ratiometric indicators are the “gold standard” for studying fast, spatially restricted Ca2+ signals (sparks, sparklets, etc.). The main drawbacks of these indicators are uneven sample loading, photobleaching, and leakage issues. Photobleaching occurs when the indicator, in the excited form, undergoes oxidation by interacting with molecular oxygen. Therefore, lowering the laser intensity can prevent photobleaching (477). Non-ratiometric indicators can compartmentalize to specific intracellular organelles. Blocking of anionic-organic cellular transporters has been used as a strategy to reduce this phenomenon (90). Moreover, determining the optimum duration for dye-loading can lower the risk of uneven loading or overloading of the sample.
The selection criteria for Ca2+ indicators should include the concentration range of Ca2+ signals being recorded. In an ideal scenario, the mid-point of Ca2+ concentration range is close to the dissociation constant (Kd) of the indicator. Indicators with high Kd (low Ca2+ affinity) are suitable for detecting large increases in Ca2+. In contrast, indicators with low Kd (high Ca2+ affinity) are better suited for detecting lower levels of Ca2+ and become saturated at high Ca2+ concentrations. Indeed, some localization studies of Ca2+ entry events have used a combination of low- and high-affinity Ca2+ indicators. A combination of Fluo-5 (Kd = 2.3 μM) and an excess of non-fluorescent EGTA (Kd = 150 nM) was used to study the initiation sites of LTCC sparklets (315). The Ca2+-binding rate (Kon = Koff/Kd) of Fluo-5 is 100-times faster than that of EGTA. Therefore, Fluo-5 can detect the more recent Ca2+ ions that have entered into the cell before ceding them to EGTA. The combination of Fluo-5 and EGTA allowed the identification of Ca2+ entry sites, which are expected to be within 50 and a few hundred nanometers from the fluorescence signal (110, 292, 315, 547). The combination of Fluo-4 (Kd = 335 nM) and EGTA was used by Sonkusare and colleagues (433) to decipher Ca2+-dependent potentiation of endothelial TRPV4 channels in a cluster. EGTA chelates intracellular Ca2+ and was found to disrupt Ca2+-dependent channel to channel communication.
Genetically encoded Ca2+ indicators (GECIs) have proved immensely helpful for studying Ca2+ signals in biological systems. GECI are encoded by engineered DNA sequences that can be incorporated into the cell genome. Indeed, combining GECI with cell-specific promoters results in cell-specific expression of the Ca2+ biosensor proteins, which constitutes an enormous advantage over the use of fluorescent indicators. Ca2+ indicators can show undesirable compartmentalization to intracellular organelles and get pumped out from the cell by ATPases at the cell membrane. GECIs are devoid of these disadvantages. The most popular GECI is GCaMP. Briefly, GCaMP protein is formed by a circularly permutated version of GFP (cpGFP) fused with CaM and CaM-interacting MLCK M13 peptide. Ca2+ biding with CaM causes a conformational change in cpGFP and results in a substantial increase in fluorescence. Point mutations on the GCaMP amino acid sequence have yielded different GCaMP generations with improved dynamic range, kinetic properties, and Ca2+ sensitivity. In the vascular field, mice expressing GCaMP under the connexin40 (Cx40) promoter or acta2 promoter have been engineered to study Ca2+ events restricted to endothelium (244, 432, 468) or SMCs (421), respectively.
Conclusions
Ca2+ signals in SMCs and ECs arise from different sources and couple with disparate targets. Various Ca2+ signals combined with multiple Ca2+-sensitive targets and physiological stimuli result in numerous stimulus-Ca2+ signal-target linkages and functional effects. Ca2+ signals in ECs and SMCs occur in a spatially restricted manner; however, excessive activation of individual signals can result in a whole-cell increase in Ca2+. Ca2+ influx is mediated by voltage-gated and non-voltage-gated (both SMCs and ECs) Ca2+ entry pathways. Voltage-gated ion channels play a crucial role in SMCs, but their functional expression has not been shown in the intact endothelium. Recent developments in image acquisition speed, combined with confocal, TIRF, and multi-photon imaging, have advanced our understanding of spatial and kinetic properties of the individual Ca2+ signals in the vasculature. Recent functional studies have also provided the understanding that Ca2+ signaling pathways are indispensable for intercellular communications that regulate vascular contractility. Moreover, the individual Ca2+ signaling elements can interact with one another to achieve a finer control of vascular function.
There is remarkable heterogeneity in the Ca2+ signaling mechanisms among different vascular beds, contributing to functional heterogeneity of SMCs or ECs. The Ca2+ signaling elements, their regulatory proteins, and signaling targets vary from large arteries to small arteries and from one vascular bed to another. While the functional effects of most Ca2+ signaling pathways are well established at the level of resistance arteries or arterioles, the physiological roles of many pathways at the whole-animal level remain unknown. In some cases, data interpretation is confounded by the presence of an ion channel in both SMCs and ECs. Cell-specific knockout mice will provide a definitive answer to the physiological significance of such pathways in either ECs or SMCs. The Ca2+ signaling mechanisms in SMCs and ECs can be activated by mechanical (pressure or flow) or neurohumoral (GPCR signaling, nerve-stimulation) stimuli. Although significant progress has been made in understanding the mechanisms for myogenic vasoconstriction and flow-mediated vasodilation, the precise mechanosensor proteins remain unclear. Recent discoveries suggest that TRPP1, TRPML1, and PIEZO1 channels may represent new Ca2+ signaling pathways contributing to mechanosensation in the vasculature. It is anticipated that future studies will reveal newer Ca2+ signaling elements, their physiological roles, and abnormalities in pathological conditions. Ca2+ signaling mechanisms and signaling organizations of cells have been shown to be abnormal in vascular disorders. Therefore, a major objective of future studies will be to identify “targetable” abnormalities in Ca2+ signaling mechanisms in vascular disorders.
Didactic Synopsis.
Major Teaching Points
Contractile state of small arteries and arterioles determines vascular resistance.
Vasoconstriction increases vascular resistance and blood pressure, whereas vasodilation reduces vascular resistance and blood pressure.
Two main cell types in the vascular wall control the contractile state of the small arteries and arterioles: smooth muscle cells (SMC) and endothelial cells (EC).
Intracellular Ca2+ in SMCs and ECs is a crucial controller of vascular contractility.
Increase in SMC Ca2+ mostly causes vasoconstriction, whereas increase in EC Ca2+ results in vasodilation.
Increase in intracellular Ca2+ in SMCs and ECs occurs via the influx of extracellular Ca2+ or release of Ca2+ from intracellular stores.
Physiological Ca2+ signals in SMCs and ECs have unique spatiotemporal properties that enable the activation of specific targets and limit Ca2+ toxicity.
Abnormal Ca2+ signaling mechanisms in SMCs and EC contribute to pathogenesis of vascular disorders.
Acknowledgement
This work was supported by grants from the National Institutes of Health to SKS (HL142808, HL146914, HL138496) and American Heart Association predoctoral Fellowship to MO (20PRE35210176).
References
- 1.Abd El-Rahman RR, Harraz OF, Brett SE, Anfinogenova Y, Mufti RE, Goldman D, Welsh DG. Identification of L- and T-type Ca2+ channels in rat cerebral arteries: Role in myogenic tone development. Am J Physiol Heart Circ Physiol 304: H58–H71, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abou-Saleh H, Pathan AR, Daalis A, Hubrack S, Abou-Jassoum H, Al-Naeimi H, Rusch NJ, Machaca K. Inositol 1,4,5-trisphosphate (IP3) receptor up-regulation in hypertension is associated with sensitization of Ca2+ release and vascular smooth muscle contractility. J Biol Chem 288: 32941–32951, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adapala RK, Talasila PK, Bratz IN, Zhang DX, Suzuki M, Meszaros JG, Thodeti CK. PKCalpha mediates acetylcholine-induced activation of TRPV4-dependent calcium influx in endothelial cells. Am J Physiol Heart Circ Physiol 301: H757–H765, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adebiyi A, Thomas-Gatewood CM, Leo MD, Kidd MW, Neeb ZP, Jaggar JH. An elevation in physical coupling of type 1 inositol 1,4,5-trisphosphate (IP3) receptors to transient receptor potential 3 (TRPC3) channels constricts mesenteric arteries in genetic hypertension. Hypertension 60: 1213–1219, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adebiyi A, Zhao G, Narayanan D, Thomas-Gatewood CM, Bannister JP, Jaggar JH. Isoform-selective physical coupling of TRPC3 channels to IP3 receptors in smooth muscle cells regulates arterial contractility. Circ Res 106: 1603–1612, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adelman JP, Maylie J, Sah P. Small-conductance Ca2+-activated K+ channels: Form and function. Annu Rev Physiol 74: 245–269, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Ahn DS, Kim YB, Lee YH, Kang BS, Kang DH. Fatty acids directly increase the activity of Ca(2+)-activated K+ channels in rabbit coronary smooth muscle cells. Yonsei Med J 35: 10–24, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Aiello LP, Bursell SE, Clermont A, Duh E, Ishii H, Takagi C, Mori F, Ciulla TA, Ways K, Jirousek M, Smith LE, King GL. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes 46: 1473–1480, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Akbarali HI, Goyal RK. Effect of sodium nitroprusside on Ca2+ currents in opossum esophageal circular muscle cells. Am J Physiol 266: G1036–G1042, 1994. [DOI] [PubMed] [Google Scholar]
- 10.Albert AP, Large WA. Store-operated Ca2+-permeable non-selective cation channels in smooth muscle cells. Cell Calcium 33: 345–356, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: Structure, function and inhibition. Biochem J 357: 593–615, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvarez DF, King JA, Weber D, Addison E, Liedtke W, Townsley MI. Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: A novel mechanism of acute lung injury. Circ Res 99: 988–995, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvarez J, Montero M. Measuring [Ca2+] in the endoplasmic reticulum with aequorin. Cell Calcium 32: 251–260, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Alzayady KJ, Wang L, Chandrasekhar R, Wagner LE 2nd, Van Petegem F, Yule DI. Defining the stoichiometry of inositol 1,4,5-trisphosphate binding required to initiate Ca2+ release. Sci Signal 9: ra35, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amberg GC, Bonev AD, Rossow CF, Nelson MT, Santana LF. Modulation of the molecular composition of large conductance, Ca(2+) activated K(+) channels in vascular smooth muscle during hypertension. J Clin Invest 112: 717–724, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amberg GC, Rossow CF, Navedo MF, Santana LF. NFATc3 regulates Kv2.1 expression in arterial smooth muscle. J Biol Chem 279: 47326–47334, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Andrea JE, Walsh MP. Protein kinase C of smooth muscle. Hypertension 20: 585–595, 1992. [DOI] [PubMed] [Google Scholar]
- 18.Archer SL, Huang JM, Hampl V, Nelson DP, Shultz PJ, Weir EK. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc Natl Acad Sci USA 91: 7583–7587, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Artamonov M, Momotani K, Utepbergenov D, Franke A, Khromov A, Derewenda ZS, Somlyo AV. The p90 ribosomal S6 kinase (RSK) is a mediator of smooth muscle contractility. PLoS One 8: e58703, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bagher P, Beleznai T, Kansui Y, Mitchell R, Garland CJ, Dora KA. Low intravascular pressure activates endothelial cell TRPV4 channels, local Ca2+ events, and IKCa channels, reducing arteriolar tone. Proc Natl Acad Sci USA 109: 18174–18179, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bagher P, Davis MJ, Segal SS. Visualizing calcium responses to acetylcholine convection along endothelium of arteriolar networks in Cx40BAC-GCaMP2 transgenic mice. Am J Physiol Heart Circ Physiol 301: H794–H802, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bai Y, Yu X, Chen H, Horne D, White R, Wu X, Lee P, Gu Y, Ghimire-Rijal S, Lin DC, Huang X. Structural basis for pharmacological modulation of the TRPC6 channel. Elife 9: e53311, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker MR, Fan G, Serysheva II. Structure of IP3R channel: High-resolution insights from cryo-EM. Curr Opin Struct Biol 46: 38–47, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ball CJ, Wilson DP, Turner SP, Saint DA, Beltrame JF. Heterogeneity of L- and T-channels in the vasculature: Rationale for the efficacy of combined L- and T-blockade. Hypertension 53: 654–660, 2009. [DOI] [PubMed] [Google Scholar]
- 25.Bannister JP, Bulley S, Narayanan D, Thomas-Gatewood C, Luzny P, Pachuau J, Jaggar JH. Transcriptional upregulation of alpha2delta-1 elevates arterial smooth muscle cell voltage-dependent Ca2+ channel surface expression and cerebrovascular constriction in genetic hypertension. Hypertension 60: 1006–1015, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barman SA, Zhu S, White RE. Protein kinase C inhibits BKCa channel activity in pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 286: L149–L155, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Bayliss WM. On the local reactions of the arterial wall to changes of internal pressure. J Physiol 28: 220–231, 1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bean BP. Classes of calcium channels in vertebrate cells. Annu Rev Physiol 51: 367–384, 1989. [DOI] [PubMed] [Google Scholar]
- 29.Bean BP, Sturek M, Puga A, Hermsmeyer K. Calcium channels in muscle cells isolated from rat mesenteric arteries: Modulation by dihydropyridine drugs. Circ Res 59: 229–235, 1986. [DOI] [PubMed] [Google Scholar]
- 30.Bergdahl A, Gomez MF, Dreja K, Xu SZ, Adner M, Beech DJ, Broman J, Hellstrand P, Sward K. Cholesterol depletion impairs vascular reactivity to endothelin-1 by reducing store-operated Ca2+ entry dependent on TRPC1. Circ Res 93: 839–847, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1: 11–21, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Beyer EC, Berthoud VM. Gap junction structure: Unraveled, but not fully revealed. F1000Res 6: 568, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature 351:751–754, 1991. [DOI] [PubMed] [Google Scholar]
- 34.Bi D, Toyama K, Lemaitre V, Takai J, Fan F, Jenkins DP, Wulff H, Gutterman DD, Park F, Miura H. The intermediate conductance calcium-activated potassium channel KCa3.1 regulates vascular smooth muscle cell proliferation via controlling calcium-dependent signaling. J Biol Chem 288:15843–15853, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Billaud M, Chiu YH, Lohman AW, Parpaite T, Butcher JT, Mutchler SM, DeLalio LJ, Artamonov MV, Sandilos JK, Best AK, Somlyo AV, Thompson RJ, Le TH, Ravichandran KS, Bayliss DA, Isakson BE. A molecular signature in the pannexin1 intracellular loop confers channel activation by the alpha1 adrenoreceptor in smooth muscle cells. Sci Signal 8: ra17, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bisaillon JM, Motiani RK, Gonzalez-Cobos JC, Potier M, Halligan KE, Alzawahra WF, Barroso M, Singer HA, Jourd’heuil D, Trebak M. Essential role for STIM1/Orai1-mediated calcium influx in PDGF-induced smooth muscle migration. Am J Physiol Cell Physiol 298: C993–C1005, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biwer LA, Good ME, Hong K, Patel RK, Agrawal N, Looft-Wilson R, Sonkusare SK, Isakson BE. Non-endoplasmic reticulum-based Calr (calreticulin) can coordinate heterocellular calcium signaling and vascular function. Arterioscler Thromb Vasc Biol 38: 120–130, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bjorling K, Morita H, Olsen MF, Prodan A, Hansen PB, Lory P, Holstein-Rathlou NH, Jensen LJ. Myogenic tone is impaired at low arterial pressure in mice deficient in the low-voltage-activated CaV 3.1 T-type Ca(2+) channel. Acta Physiol (Oxf) 207: 709–720, 2013. [DOI] [PubMed] [Google Scholar]
- 39.Blatter LA, Wier WG. Nitric oxide decreases [Ca2+]i in vascular smooth muscle by inhibition of the calcium current. Cell Calcium 15: 122–131, 1994. [DOI] [PubMed] [Google Scholar]
- 40.Blaustein MP. Physiological effects of endogenous ouabain: Control of intracellular Ca2+ stores and cell responsiveness. Am J Physiol 264: C1367–C1387, 1993. [DOI] [PubMed] [Google Scholar]
- 41.Blaustein MP, Lederer WJ. Sodium/calcium exchange: Its physiological implications. Physiol Rev 79: 763–854, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Blaustein MP, Santiago EM. Effects of internal and external cations and of ATP on sodium-calcium and calcium-calcium exchange in squid axons. Biophys J 20: 79–111, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blue EK, Goeckeler ZM, Jin Y, Hou L, Dixon SA, Herring BP, Wysolmerski RB, Gallagher PJ. 220- and 130-kDa MLCKs have distinct tissue distributions and intracellular localization patterns. Am J Physiol Cell Physiol 282: C451–C460, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boittin FX, Macrez N, Halet G, Mironneau J. Norepinephrine-induced Ca(2+) waves depend on InsP(3) and ryanodine receptor activation in vascular myocytes. Am J Physiol 277: C139–C151, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature 368: 850–853, 1994. [DOI] [PubMed] [Google Scholar]
- 46.Bratz IN, Dick GM, Tune JD, Edwards JM, Neeb ZP, Dincer UD, Sturek M. Impaired capsaicin-induced relaxation of coronary arteries in a porcine model of the metabolic syndrome. Am J Physiol Heart Circ Physiol 294: H2489–H2496, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Braunstein TH, Inoue R, Cribbs L, Oike M, Ito Y, Holstein-Rathlou NH, Jensen LJ. The role of L- and T-type calcium channels in local and remote calcium responses in rat mesenteric terminal arterioles. J Vasc Res 46: 138–151, 2009. [DOI] [PubMed] [Google Scholar]
- 48.Brenner R, Perez GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature 407: 870–876, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Bulley S, Fernandez-Pena C, Hasan R, Leo MD, Muralidharan P, Mackay CE, Evanson KW, Moreira-Junior L, Mata-Daboin A, Burris SK, Wang Q, Kuruvilla KP, Jaggar JH. Arterial smooth muscle cell PKD2 (TRPP1) channels regulate systemic blood pressure. Elife 7: e42628, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burnstock G, Ralevic V. Purinergic signaling and blood vessels in health and disease. Pharmacol Rev 66: 102–192, 2014. [DOI] [PubMed] [Google Scholar]
- 51.Cao DS, Yu SQ, Premkumar LS. Modulation of transient receptor potential Vanilloid 4-mediated membrane currents and synaptic transmission by protein kinase C. Mol Pain 5: 5, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carvajal JA, Germain AM, Huidobro-Toro JP, Weiner CP. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J Cell Physiol 184: 409–420, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Castagna M, Takai Y, Kaibuchi K, Sano K, Kikkawa U, Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem 257: 7847–7851, 1982. [PubMed] [Google Scholar]
- 54.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997. [DOI] [PubMed] [Google Scholar]
- 55.Catterall WA. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol 3: a003947, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev 57: 411–425, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O’Donnell D, Nicoll RA, Shah NM, Julius D, Basbaum AI. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci 31:5067–5077, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cayouette S, Lussier MP, Mathieu EL, Bousquet SM, Boulay G. Exocytotic insertion of TRPC6 channel into the plasma membrane upon Gq protein-coupled receptor activation. J Biol Chem 279: 7241–7246, 2004. [DOI] [PubMed] [Google Scholar]
- 59.Chen CA, De Pascali F, Basye A, Hemann C, Zweier JL. Redox modulation of endothelial nitric oxide synthase by glutaredoxin-1 through reversible oxidative post-translational modification. Biochemistry 52: 6712–6723, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen CA, Wang TY, Varadharaj S, Reyes LA, Hemann C, Talukder MA, Chen YR, Druhan LJ, Zweier JL. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature 468: 1115–1118, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen F, Kumar S, Yu Y, Aggarwal S, Gross C, Wang Y, Chakraborty T, Verin AD, Catravas JD, Lucas R, Black SM, Fulton DJ. PKC-dependent phosphorylation of eNOS at T495 regulates eNOS coupling and endothelial barrier function in response to G+-toxins. PLoS One 9: e99823, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen M, Zhang W, Lu X, Hoggatt AM, Gunst SJ, Kassab GS, Tune JD, Herring BP. Regulation of 130-kDa smooth muscle myosin light chain kinase expression by an intronic CArG element. J Biol Chem 288: 34647–34657, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen YL, Sonkusare SK. Endothelial TRPV4 channels and vasodilator reactivity. Curr Top Membr 85: 89–117, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng D, Zhu X, Barchiesi F, Gillespie DG, Dubey RK, Jackson EK. Receptor for activated protein kinase C1 regulates cell proliferation by modulating calcium signaling. Hypertension 58: 689–695, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheng D, Zhu X, Gillespie DG, Jackson EK. Role of RACK1 in the differential proliferative effects of neuropeptide Y(1-36) and peptide YY(1-36) in SHR vs. WKY preglomerular vascular smooth muscle cells. Am J Physiol Renal Physiol 304: F770–F780, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng H, Lederer MR, Lederer WJ, Cannell MB. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am J Physiol 270: C148–C159, 1996. [DOI] [PubMed] [Google Scholar]
- 67.Cheng H, Lederer WJ. Calcium sparks. Physiol Rev 88: 1491–1545, 2008. [DOI] [PubMed] [Google Scholar]
- 68.Cheng X, Shen D, Samie M, Xu H. Mucolipins: Intracellular TRPML1-3 channels. FEBS Lett 584: 2013–2021, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheong A, Bingham AJ, Li J, Kumar B, Sukumar P, Munsch C, Buckley NJ, Neylon CB, Porter KE, Beech DJ, Wood IC. Downregulated REST transcription factor is a switch enabling critical potassium channel expression and cell proliferation. Mol Cell 20: 45–52, 2005. [DOI] [PubMed] [Google Scholar]
- 70.Chidgey J, Fraser PA, Aaronson PI. Reactive oxygen species facilitate the EDH response in arterioles by potentiating intracellular endothelial Ca(2+) release. Free Radic Biol Med 97: 274–284, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Church PJ, Stanley EF. Single L-type calcium channel conductance with physiological levels of calcium in chick ciliary ganglion neurons. J Physiol 496 (Pt 1): 59–68, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clapham DE. Calcium signaling. Cell 131: 1047–1058, 2007. [DOI] [PubMed] [Google Scholar]
- 73.Collier DM, Villalba N, Sackheim A, Bonev AD, Miller ZD, Moore JS, Shui B, Lee JC, Lee FK, Reining S, Kotlikoff MI, Nelson MT, Freeman K. Extracellular histones induce calcium signals in the endothelium of resistance-sized mesenteric arteries and cause loss of endothelium-dependent dilation. Am J Physiol Heart Circ Physiol 316: H1309–H1322, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Conti MA, Adelstein RS. The relationship between calmodulin binding and phosphorylation of smooth muscle myosin kinase by the catalytic subunit of 3’:5’ cAMP-dependent protein kinase. J Biol Chem 256: 3178–3181, 1981. [PubMed] [Google Scholar]
- 75.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330: 55–60, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, Kim SE, Schmidt M, Mathur J, Dubin AE, Montal M, Patapoutian A. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483: 176–181, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Couchonnal LF, Anderson ME. The role of calmodulin kinase II in myocardial physiology and disease. Physiology (Bethesda) 23: 151–159, 2008. [DOI] [PubMed] [Google Scholar]
- 78.Covington ED, Wu MM, Lewis RS. Essential role for the CRAC activation domain in store-dependent oligomerization of STIM1. Mol Biol Cell 21: 1897–1907, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cox DH, Cui J, Aldrich RW. Allosteric gating of a large conductance Ca-activated K+ channel. J Gen Physiol 110: 257–281, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cox RH, Lozinskaya I, Matsuda K, Dietz NJ. Ramipril treatment alters Ca(2+) and K(+) channels in small mesenteric arteries from Wistar-Kyoto and spontaneously hypertensive rats. Am J Hypertens 15: 879–890, 2002. [PubMed] [Google Scholar]
- 81.Cozart MA, Phelan KD, Wu H, Mu S, Birnbaumer L, Rusch NJ, Zheng F. Vascular smooth muscle TRPC3 channels facilitate the inverse hemodynamic response during status epilepticus. Sci Rep 10: 812, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Darby WG, Potocnik S, Ramachandran R, Hollenberg MD, Woodman OL, McIntyre P. Shear stress sensitizes TRPV4 in endothelium-dependent vasodilatation. Pharmacol Res 133: 152–159, 2018. [DOI] [PubMed] [Google Scholar]
- 83.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423, 1999. [DOI] [PubMed] [Google Scholar]
- 84.DeLalio LJ, Keller AS, Chen J, Boyce AKJ, Artamonov MV, Askew-Page HR, Stevenson Keller TC, Johnstone SR, Weaver RB, Good ME, Murphy SA, Best AK, Mintz EL, Penuela S, Greenwood IA, Machado RF, Somlyo AV, Swayne LA, Minshall RD, Isakson BE. Interaction between pannexin 1 and caveolin-1 in smooth muscle can regulate blood pressure. Arterioscler Thromb Vasc Biol 38: 2065–2078, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Deng Z, Paknejad N, Maksaev G, Sala-Rabanal M, Nichols CG, Hite RK, Yuan P. Cryo-EM and X-ray structures of TRPV4 reveal insight into ion permeation and gating mechanisms. Nat Struct Mol Biol 25: 252–260, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Denninger JW, Marletta MA. Guanylate cyclase and the .NO/cGMP signaling pathway. Biochim Biophys Acta 1411: 334–350, 1999. [DOI] [PubMed] [Google Scholar]
- 87.Derler I, Jardin I, Romanin C. Molecular mechanisms of STIM/Orai communication. Am J Physiol Cell Physiol 310: C643–C662, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dessy C, Feron O, Balligand JL. The regulation of endothelial nitric oxide synthase by caveolin: A paradigm validated in vivo and shared by the ‘endothelium-derived hyperpolarizing factor’. Pflugers Arch 459: 817–827, 2010. [DOI] [PubMed] [Google Scholar]
- 89.Dessy C, Matsuda N, Hulvershorn J, Sougnez CL, Sellke FW, Morgan KG. Evidence for involvement of the PKC-alpha isoform in myogenic contractions of the coronary microcirculation. Am J Physiol Heart Circ Physiol 279: H916–H923, 2000. [DOI] [PubMed] [Google Scholar]
- 90.Di Virgilio F, Steinberg TH, Silverstein SC. Organic-anion transport inhibitors to facilitate measurement of cytosolic free Ca2+ with fura-2. Methods Cell Biol 31: 453–462, 1989. [DOI] [PubMed] [Google Scholar]
- 91.Dietrich A, Kalwa H, Storch U, Mederos y Schnitzler M, Salanova B, Pinkenburg O, Dubrovska G, Essin K, Gollasch M, Birnbaumer L, Gudermann T. Pressure-induced and store-operated cation influx in vascular smooth muscle cells is independent of TRPC1. Pflugers Arch 455: 465–477, 2007. [DOI] [PubMed] [Google Scholar]
- 92.Dora KA, Doyle MP, Duling BR. Elevation of intracellular calcium in smooth muscle causes endothelial cell generation of NO in arterioles. Proc Natl Acad Sci USA 94: 6529–6534, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Douguet D, Patel A, Xu A, Vanhoutte PM, Honore E. Piezo ion channels in cardiovascular mechanobiology. Trends Pharmacol Sci 40: 956–970, 2019. [DOI] [PubMed] [Google Scholar]
- 94.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol (1985) 91: 1487–1500, 2001. [DOI] [PubMed] [Google Scholar]
- 95.Earley S. Vanilloid and melastatin transient receptor potential channels in vascular smooth muscle. Microcirculation 17: 237–249, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Earley S. Endothelium-dependent cerebral artery dilation mediated by transient receptor potential and Ca2+-activated K+ channels. J Cardiovasc Pharmacol 57: 148–153, 2011. [DOI] [PubMed] [Google Scholar]
- 97.Earley S. TRPA1 channels in the vasculature. Br J Pharmacol 167: 13–22, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Earley S, Brayden JE. Transient receptor potential channels in the vasculature. Physiol Rev 95: 645–690, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Earley S, Gonzales AL, Crnich R. Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-activated K+ channels. Circ Res 104: 987–994, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Earley S, Gonzales AL, Garcia ZI. A dietary agonist of transient receptor potential cation channel V3 elicits endothelium-dependent vasodilation. Mol Pharmacol 77: 612–620, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Earley S, Pauyo T, Drapp R, Tavares MJ, Liedtke W, Brayden JE. TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am J Physiol Heart Circ Physiol 297: H1096–H1102, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Earley S, Straub SV, Brayden JE. Protein kinase C regulates vascular myogenic tone through activation of TRPM4. Am J Physiol Heart Circ Physiol 292: H2613–H2622, 2007. [DOI] [PubMed] [Google Scholar]
- 103.Earley S, Waldron BJ, Brayden JE. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res 95: 922–929, 2004. [DOI] [PubMed] [Google Scholar]
- 104.Emerson GG, Segal SS. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: Role in vasomotor control. Circ Res 87: 474–479, 2000. [DOI] [PubMed] [Google Scholar]
- 105.Emerson GG, Segal SS. Endothelial cell pathway for conduction of hyperpolarization and vasodilation along hamster feed artery. Circ Res 86:94–100, 2000. [DOI] [PubMed] [Google Scholar]
- 106.Erler I, Hirnet D, Wissenbach U, Flockerzi V, Niemeyer BA. Ca2+-selective transient receptor potential V channel architecture and function require a specific ankyrin repeat. J Biol Chem 279: 34456–34463, 2004. [DOI] [PubMed] [Google Scholar]
- 107.Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe T, Birnbaumer L, Tsien RW, Catterall WA. Nomenclature of voltage-gated calcium channels. Neuron 25: 533–535, 2000. [DOI] [PubMed] [Google Scholar]
- 108.Ertel SI, Ertel EA, Clozel JP. T-type Ca2+ channels and pharmacological blockade: Potential pathophysiological relevance. Cardiovasc Drugs Ther 11: 723–739, 1997. [DOI] [PubMed] [Google Scholar]
- 109.Esfandiarei M, Fameli N, Choi YY, Tehrani AY, Hoskins JG, van Breemen C. Waves of calcium depletion in the sarcoplasmic reticulum of vascular smooth muscle cells: An inside view of spatiotemporal Ca2+ regulation. PLoS One 8: e55333, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fakler B, Adelman JP. Control of K(Ca) channels by calcium nano/microdomains. Neuron 59: 873–881, 2008. [DOI] [PubMed] [Google Scholar]
- 111.Fan HC, Zhang X, McNaughton PA. Activation of the TRPV4 ion channel is enhanced by phosphorylation. J Biol Chem 284: 27884–27891, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fernandez-Fernandez JM, Tomas M, Vazquez E, Orio P, Latorre R, Senti M, Marrugat J, Valverde MA. Gain-of-function mutation in the KCNMB1 potassium channel subunit is associated with low prevalence of diastolic hypertension. J Clin Invest 113: 1032–1039, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Feske S. CRAC channelopathies. Pflugers Arch 460: 417–435, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fisslthaler B, Loot AE, Mohamed A, Busse R, Fleming I. Inhibition of endothelial nitric oxide synthase activity by proline-rich tyrosine kinase 2 in response to fluid shear stress and insulin. Circ Res 102: 1520–1528, 2008. [DOI] [PubMed] [Google Scholar]
- 115.Fleming I. Molecular mechanisms underlying the activation of eNOS. Pflugers Arch 459: 793–806, 2010. [DOI] [PubMed] [Google Scholar]
- 116.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J 33: 829–837, 837a-837d, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev 87: 593–658, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fowler BC, Carmines PK, Nelson LD, Bell PD. Characterization of sodium-calcium exchange in rabbit renal arterioles. Kidney Int 50: 1856–1862, 1996. [DOI] [PubMed] [Google Scholar]
- 119.Fulton D, Church JE, Ruan L, Li C, Sood SG, Kemp BE, Jennings IG, Venema RC. Src kinase activates endothelial nitric-oxide synthase by phosphorylating Tyr-83. J Biol Chem 280: 35943–35952, 2005. [DOI] [PubMed] [Google Scholar]
- 120.Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J 3: 2007–2018, 1989. [PubMed] [Google Scholar]
- 121.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980. [DOI] [PubMed] [Google Scholar]
- 122.Gabani M, Liu J, Ait-Aissa K, Koval O, Kim YR, Castaneda D, Vikram A, Jacobs JS, Grumbach I, Trebak M, Irani K, Kassan M. MiR-204 regulates type 1 IP3R to control vascular smooth muscle cell contractility and blood pressure. Cell Calcium 80: 18–24, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gallagher PJ, Herring BP. The carboxyl terminus of the smooth muscle myosin light chain kinase is expressed as an independent protein, telokin. J Biol Chem 266: 23945–23952, 1991. [PMC free article] [PubMed] [Google Scholar]
- 124.Gao L, Blair LA, Marshall J. CaMKII-independent effects of KN93 and its inactive analog KN92: Reversible inhibition of L-type calcium channels. Biochem Biophys Res Commun 345: 1606–1610, 2006. [DOI] [PubMed] [Google Scholar]
- 125.Gao T, Yatani A, Dell’Acqua ML, Sako H, Green SA, Dascal N, Scott JD, Hosey MM. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channelsubunits. Neuron 19: 185–196, 1997. [DOI] [PubMed] [Google Scholar]
- 126.Garland CJ, Bagher P, Powell C, Ye X, Lemmey HAL, Borysova L, Dora KA. Voltage-dependent Ca(2+) entry into smooth muscle during contraction promotes endothelium-mediated feedback vasodilation in arterioles. Sci Signal 10: eaal3806, 2017. [DOI] [PubMed] [Google Scholar]
- 127.Ge J, Li W, Zhao Q, Li N, Chen M, Zhi P, Li R, Gao N, Xiao B, Yang M. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature 527: 64–69, 2015. [DOI] [PubMed] [Google Scholar]
- 128.Ghosh D, Syed AU, Prada MP, Nystoriak MA, Santana LF, Nieves-Cintron M, Navedo MF. Calcium channels in vascular smooth muscle. Adv Pharmacol 78: 49–87, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Girardin NC, Antigny F, Frieden M. Electrophysiological characterization of store-operated and agonist-induced Ca2+ entry pathways in endothelial cells. Pflugers Arch 460: 109–120, 2010. [DOI] [PubMed] [Google Scholar]
- 130.Glass R, Townsend-Nicholson A, Burnstock G. P2 receptors in the thymus: Expression of P2X and P2Y receptors in adult rats, an immunohistochemical and in situ hybridisation study. Cell Tissue Res 300: 295–306, 2000. [DOI] [PubMed] [Google Scholar]
- 131.Gole HK, Tharp DL, Bowles DK. Upregulation of intermediate-conductance Ca2+-activated K+ channels (KCNN4) in porcine coronary smooth muscle requires NADPH oxidase 5 (NOX5). PLoS One 9: e105337, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gonzales AL, Amberg GC, Earley S. Ca2+ release from the sarcoplasmic reticulum is required for sustained TRPM4 activity in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol 299: C279–C288, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gonzales AL, Yang Y, Sullivan MN, Sanders L, Dabertrand F, Hill-Eubanks DC, Nelson MT, Earley S. A PLCgamma1-dependent, force-sensitive signaling network in the myogenic constriction of cerebral arteries. Sci Signal 7: ra49, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gonzalez Bosc LV, Plomaritas DR, Herbert LM, Giermakowska W, Browning C, Jernigan NL. ASIC1-mediated calcium entry stimulates NFATc3 nuclear translocation via PICK1 coupling in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 311: L48–L58, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gordon-Shaag A, Zagotta WN, Gordon SE. Mechanism of Ca(2+)-dependent desensitization in TRP channels. Channels (Austin) 2: 125–129, 2008. [DOI] [PubMed] [Google Scholar]
- 136.Goto Y, Miura M, Iijima T. Extrusion mechanisms of intracellular Ca2+ in human aortic endothelial cells. Eur J Pharmacol 314: 185–192, 1996. [DOI] [PubMed] [Google Scholar]
- 137.Grayson TH, Haddock RE, Murray TP, Wojcikiewicz RJ, Hill CE. Inositol 1,4,5-trisphosphate receptor subtypes are differentially distributed between smooth muscle and endothelial layers of rat arteries. Cell Calcium 36: 447–458, 2004. [DOI] [PubMed] [Google Scholar]
- 138.Greenberg HZE, Carlton-Carew SRE, Khan DM, Zargaran AK, Jahan KS, Vanessa Ho WS, Albert AP. Heteromeric TRPV4/TRPC1 channels mediate calcium-sensing receptor-induced nitric oxide production and vasorelaxation in rabbit mesenteric arteries. Vascul Pharmacol 96-98: 53–62, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Greenstein AS, Kadir S, Csato V, Sugden SA, Baylie RA, Eisner DA, Nelson MT. Disruption of pressure-induced Ca(2+) spark vasoregulation of resistance arteries, rather than endothelial dysfunction, underlies obesity-related hypertension. Hypertension 75: 539–548, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985. [PubMed] [Google Scholar]
- 141.Gustafsson F, Andreasen D, Salomonsson M, Jensen BL, Holstein-Rathlou N. Conducted vasoconstriction in rat mesenteric arterioles: Role for dihydropyridine-insensitive Ca(2+) channels. Am J Physiol Heart Circ Physiol 280: H582–H590, 2001. [DOI] [PubMed] [Google Scholar]
- 142.Haack JA, Rosenberg RL. Calcium-dependent inactivation of L-type calcium channels in planar lipid bilayers. Biophys J 66: 1051–1060, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Haddock RE, Grayson TH, Brackenbury TD, Meaney KR, Neylon CB, Sandow SL, Hill CE. Endothelial coordination of cerebral vasomotion via myoendothelial gap junctions containing connexins 37 and 40. Am J Physiol Heart Circ Physiol 291: H2047–H2056, 2006. [DOI] [PubMed] [Google Scholar]
- 144.Hadri L, Bobe R, Kawase Y, Ladage D, Ishikawa K, Atassi F, Lebeche D, Kranias EG, Leopold JA, Lompre AM, Lipskaia L, Hajjar RJ. SERCA2a gene transfer enhances eNOS expression and activity in endothelial cells. Mol Ther 18: 1284–1292, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hamada K, Miyatake H, Terauchi A, Mikoshiba K. IP3-mediated gating mechanism of the IP3 receptor revealed by mutagenesis and X-ray crystallography. Proc Natl Acad Sci USA 114: 4661–4666, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hamlyn JM, Hamilton BP, Manunta P. Endogenous ouabain, sodium balance and blood pressure: A review and a hypothesis. J Hypertens 14: 151–167, 1996. [DOI] [PubMed] [Google Scholar]
- 147.Hannah RM, Dunn KM, Bonev AD, Nelson MT. Endothelial SK(Ca) and IK(Ca) channels regulate brain parenchymal arteriolar diameter and cortical cerebral blood flow. J Cereb Blood Flow Metab 31: 1175–1186, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hansen PB, Jensen BL, Andreasen D, Skott O. Differential expression of T- and L-type voltage-dependent calcium channels in renal resistance vessels. Circ Res 89: 630–638, 2001. [DOI] [PubMed] [Google Scholar]
- 149.Harhun MI, Povstyan OV, Gordienko DV. Purinoreceptor-mediated current in myocytes from renal resistance arteries. Br J Pharmacol 160: 987–997, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Harraz OF, Abd El-Rahman RR, Bigdely-Shamloo K, Wilson SM, Brett SE, Romero M, Gonzales AL, Earley S, Vigmond EJ, Nygren A, Menon BK, Mufti RE, Watson T, Starreveld Y, Furstenhaupt T, Muellerleile PR, Kurjiaka DT, Kyle BD, Braun AP, Welsh DG. Ca(V)3.2 channels and the induction of negative feedback in cerebral arteries. Circ Res 115: 650–661, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Harraz OF, Brett SE, Welsh DG. Nitric oxide suppresses vascular voltage-gated T-type Ca2+ channels through cGMP/PKG signaling. Am J Physiol Heart Circ Physiol 306: H279–H285, 2014. [DOI] [PubMed] [Google Scholar]
- 152.Harraz OF, Brett SE, Zechariah A, Romero M, Puglisi JL, Wilson SM, Welsh DG. Genetic ablation of CaV3.2 channels enhances the arterial myogenic response by modulating the RyR-BKCa axis. Arterioscler Thromb Vasc Biol 35: 1843–1851, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Harraz OF, Longden TA, Hill-Eubanks D, Nelson MT. PIP2 depletion promotes TRPV4 channel activity in mouse brain capillary endothelial cells. Elife 7: e38689, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Harraz OF, Visser F, Brett SE, Goldman D, Zechariah A, Hashad AM, Menon BK, Watson T, Starreveld Y, Welsh DG. CaV1.2/CaV3.x channels mediate divergent vasomotor responses in human cerebral arteries. J Gen Physiol 145: 405–418, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Harraz OF, Welsh DG. Protein kinase A regulation of T-type Ca2+ channels in rat cerebral arterial smooth muscle. J Cell Sci 126: 2944–2954, 2013. [DOI] [PubMed] [Google Scholar]
- 156.Harrington LS, Evans RJ, Wray J, Norling L, Swales KE, Vial C, Ali F, Carrier MJ, Mitchell JA. Purinergic 2X1 receptors mediate endothelial dependent vasodilation to ATP. Mol Pharmacol 72: 1132–1136, 2007. [DOI] [PubMed] [Google Scholar]
- 157.Harrington LS, Mitchell JA. Novel role for P2X receptor activation in endothelium-dependent vasodilation. Br J Pharmacol 143: 611–617, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hartmannsgruber V, Heyken WT, Kacik M, Kaistha A, Grgic I, Harteneck C, Liedtke W, Hoyer J, Kohler R. Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLoS One 2: e827, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hasan R, Zhang X. Ca(2+) regulation of TRP ion channels. Int J Mol Sci 19: 1256, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hasegawa T, Masugi F, Ogihara T, Kumahara Y. Increase in plasma ouabainlike inhibitor of Na+, K+-ATPase with high sodium intake in patients with essential hypertension. J Clin Hypertens 3: 419–429, 1987. [PubMed] [Google Scholar]
- 161.Hashad AM, Harraz OF, Brett SE, Romero M, Kassmann M, Puglisi JL, Wilson SM, Gollasch M, Welsh DG. Caveolae link CaV3.2 channels to BKCa-mediated feedback in vascular smooth muscle. Arterioscler Thromb Vasc Biol 38: 2371–2381, 2018. [DOI] [PubMed] [Google Scholar]
- 162.Hashad AM, Mazumdar N, Romero M, Nygren A, Bigdely-Shamloo K, Harraz OF, Puglisi JL, Vigmond EJ, Wilson SM, Welsh DG. Interplay among distinct Ca(2+) conductances drives Ca(2+) sparks/spontaneous transient outward currents in rat cerebral arteries. J Physiol 595: 1111–1126, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hashad AM, Sancho M, Brett SE, Welsh DG. Reactive oxygen species mediate the suppression of arterial smooth muscle T-type Ca(2+) channels by angiotensin II. Sci Rep 8: 3445, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.He WQ, Qiao YN, Zhang CH, Peng YJ, Chen C, Wang P, Gao YQ, Chen C, Chen X, Tao T, Su XH, Li CJ, Kamm KE, Stull JT, Zhu MS. Role of myosin light chain kinase in regulation of basal blood pressure and maintenance of salt-induced hypertension. Am J Physiol Heart Circ Physiol 301: H584–H591, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.He Z, Feng S, Tong Q, Hilgemann DW, Philipson KD. Interaction of PIP(2) with the XIP region of the cardiac Na/Ca exchanger. Am J Physiol Cell Physiol 278: C661–C666, 2000. [DOI] [PubMed] [Google Scholar]
- 166.Heathcote HR, Lee MD, Zhang X, Saunter CD, Wilson C, McCarron JG. Endothelial TRPV4 channels modulate vascular tone by Ca(2+)-induced Ca(2+) release at inositol 1,4,5-trisphosphate receptors. Br J Pharmacol 176: 3297–3317, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Heiss EH, Dirsch VM. Regulation of eNOS enzyme activity by post-translational modification. Curr Pharm Des 20: 3503–3513, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Herbert LM, Nitta CH, Yellowhair TR, Browning C, Gonzalez Bosc LV, Resta TC, Jernigan NL. PICK1/calcineurin suppress ASIC1-mediated Ca2+ entry in rat pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol 310: C390–C400, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Herring BP, El-Mounayri O, Gallagher PJ, Yin F, Zhou J. Regulation of myosin light chain kinase and telokin expression in smooth muscle tissues. Am J Physiol Cell Physiol 291: C817–C827, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hilgemann DW. Regulation and deregulation of cardiac Na(+)-Ca2+ exchange in giant excised sarcolemmal membrane patches. Nature 344: 242–245, 1990. [DOI] [PubMed] [Google Scholar]
- 171.Hilgemann DW, Nicoll DA, Philipson KD. Charge movement during Na+ translocation by native and cloned cardiac Na+/Ca2+ exchanger. Nature 352:715–718, 1991. [DOI] [PubMed] [Google Scholar]
- 172.Hill MA, Davis MJ, Meininger GA, Potocnik SJ, Murphy TV. Arteriolar myogenic signalling mechanisms: Implications for local vascular function. Clin Hemorheol Microcirc 34: 67–79, 2006. [PubMed] [Google Scholar]
- 173.Hill MA, Falcone JC, Meininger GA. Evidence for protein kinase C involvement in arteriolar myogenic reactivity. Am J Physiol 259: H1586–H1594, 1990. [DOI] [PubMed] [Google Scholar]
- 174.Hirenallur SD, Haworth ST, Leming JT, Chang J, Hernandez G, Gordon JB, Rusch NJ. Upregulation of vascular calcium channels in neonatal piglets with hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 295: L915–L924, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hirschberg B, Maylie J, Adelman JP, Marrion NV. Gating of recombinant small-conductance Ca-activated K+ channels by calcium. J Gen Physiol 111: 565–581, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hockerman GH, Peterson BZ, Johnson BD, Catterall WA. Molecular determinants of drug binding and action on L-type calcium channels. Annu Rev Pharmacol Toxicol 37: 361–396, 1997. [DOI] [PubMed] [Google Scholar]
- 177.Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397: 259–263, 1999. [DOI] [PubMed] [Google Scholar]
- 178.Hong K, Cope EL, DeLalio LJ, Marziano C, Isakson BE, Sonkusare SK. TRPV4 (transient receptor potential vanilloid 4) channel-dependent negative feedback mechanism regulates Gq protein-coupled receptor-induced vasoconstriction. Arterioscler Thromb Vasc Biol 38: 542–554, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Horiuchi T, Dietrich HH, Hongo K, Dacey RG Jr. Mechanism of extracellular K+-induced local and conducted responses in cerebral penetrating arterioles. Stroke 33: 2692–2699, 2002. [DOI] [PubMed] [Google Scholar]
- 180.Horn R. How S4 segments move charge. Let me count the ways. J Gen Physiol 123: 1–4, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hoshi T, Wissuwa B, Tian Y, Tajima N, Xu R, Bauer M, Heinemann SH, Hou S. Omega-3 fatty acids lower blood pressure by directly activating large-conductance Ca(2)(+)-dependent K(+) channels. Proc Natl Acad Sci USA 110: 4816–4821, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol 465: 359–386, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.House C, Kemp BE. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science 238: 1726–1728, 1987. [DOI] [PubMed] [Google Scholar]
- 184.House C, Kemp BE. Protein kinase C pseudosubstrate prototope: Structure-function relationships. Cell Signal 2: 187–190, 1990. [DOI] [PubMed] [Google Scholar]
- 185.Howitt L, Kuo IY, Ellis A, Chaston DJ, Shin HS, Hansen PB, Hill CE. Chronic deficit in nitric oxide elicits oxidative stress and augments T-type calcium-channel contribution to vascular tone of rodent arteries and arterioles. Cardiovasc Res 98: 449–457, 2013. [DOI] [PubMed] [Google Scholar]
- 186.Hruska-Hageman AM, Wemmie JA, Price MP, Welsh MJ. Interaction of the synaptic protein PICK1 (protein interacting with C kinase 1) with the non-voltage gated sodium channels BNC1 (brain Na+ channel 1) and ASIC (acid-sensing ion channel). Biochem J 361: 443–450, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Huang Q, Xu W, Ustinova E, Wu M, Childs E, Hunter F, Yuan S. Myosin light chain kinase-dependent microvascular hyperpermeability in thermal injury. Shock 20: 363–368, 2003. [DOI] [PubMed] [Google Scholar]
- 188.Ignarro LJ. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol 30: 535–560, 1990. [DOI] [PubMed] [Google Scholar]
- 189.Ignarro LJ. Signal transduction mechanisms involving nitric oxide. Biochem Pharmacol 41: 485–490, 1991. [DOI] [PubMed] [Google Scholar]
- 190.Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J Gen Physiol 95: 1103–1122, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ikebe M, Maruta S, Reardon S. Location of the inhibitory region of smooth muscle myosin light chain kinase. J Biol Chem 264: 6967–6971, 1989. [PubMed] [Google Scholar]
- 192.Inoue R, Jensen LJ, Jian Z, Shi J, Hai L, Lurie AI, Henriksen FH, Salomonsson M, Morita H, Kawarabayashi Y, Mori M, Mori Y, Ito Y. Synergistic activation of vascular TRPC6 channel by receptor and mechanical stimulation via phospholipase C/diacylglycerol and phospholipase A2/omega-hydroxylase/20-HETE pathways. Circ Res 104: 1399–1409, 2009. [DOI] [PubMed] [Google Scholar]
- 193.Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, Ito Y, Mori Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha(1)-adrenoceptor-activated Ca(2+)-permeable cation channel. Circ Res 88: 325–332, 2001. [DOI] [PubMed] [Google Scholar]
- 194.Irvine JC, Favaloro JL, Kemp-Harper BK. NO- activates soluble guanylate cyclase and Kv channels to vasodilate resistance arteries. Hypertension 41: 1301–1307, 2003. [DOI] [PubMed] [Google Scholar]
- 195.Isacson CK, Lu Q, Karas RH, Cox DH. RACK1 is a BKCa channel binding protein. Am J Physiol Cell Physiol 292: C1459–C1466, 2007. [DOI] [PubMed] [Google Scholar]
- 196.Isakson Be, Best AK, Duling BR. Incidence of protein on actin bridges between endothelium and smooth muscle in arterioles demonstrates heterogeneous connexin expression and phosphorylation. Am J Physiol Heart Circ Physiol 294: H2898–H2904, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ishikawa T, Hume JR, Keef KD. Regulation of Ca2+ channels by cAMP and cGMP in vascular smooth muscle cells. Circ Res 73: 1128–1137, 1993. [DOI] [PubMed] [Google Scholar]
- 198.Ito I, Jarajapu YP, Grant MB, Knot HJ. Characteristics of myogenic tone in the rat ophthalmic artery. Am J Physiol Heart Circ Physiol 292: H360–H368, 2007. [DOI] [PubMed] [Google Scholar]
- 199.Iwamoto T, Kita S, Zhang J, Blaustein MP, Arai Y, Yoshida S, Wakimoto K, Komuro I, Katsuragi T. Salt-sensitive hypertension is triggered by Ca2+ entry via Na+/Ca2+ exchanger type-1 in vascular smooth muscle. Nat Med 10: 1193–1199, 2004. [DOI] [PubMed] [Google Scholar]
- 200.Iwasaki Y, Tanabe M, Kobata K, Watanabe T. TRPA1 agonists--allyl isothiocyanate and cinnamaldehyde--induce adrenaline secretion. Biosci Biotechnol Biochem 72: 2608–2614, 2008. [DOI] [PubMed] [Google Scholar]
- 201.Jackson WF. Potassium channels in regulation of vascular smooth muscle contraction and growth. Adv Pharmacol 78: 89–144, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jaggar JH, Nelson MT. Differential regulation of Ca(2+) sparks and Ca(2+) waves by UTP in rat cerebral artery smooth muscle cells. Am J Physiol Cell Physiol 279: C1528–C1539, 2000. [DOI] [PubMed] [Google Scholar]
- 203.Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol 278: C235–C256, 2000. [DOI] [PubMed] [Google Scholar]
- 204.Jaggar JH, Stevenson AS, Nelson MT. Voltage dependence of Ca2+ sparks in intact cerebral arteries. Am J Physiol 274: C1755–C1761, 1998. [DOI] [PubMed] [Google Scholar]
- 205.Jankowski J, Perry HM, Medina CB, Huang L, Yao J, Bajwa A, Lorenz UM, Rosin DL, Ravichandran KS, Isakson BE, Okusa MD. Epithelial and endothelial pannexin1 channels mediate AKI. J Am Soc Nephrol 29: 1887–1899, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Joiner WJ, Khanna R, Schlichter LC, Kaczmarek LK. Calmodulin regulates assembly and trafficking of SK4/IK1 Ca2+-activated K+ channels. J Biol Chem 276: 37980–37985, 2001. [DOI] [PubMed] [Google Scholar]
- 207.Juhaszova M, Ambesi A, Lindenmayer GE, Bloch RJ, Blaustein MP. Na(+)-Ca2+ exchanger in arteries: Identification by immunoblotting and immunofluorescence microscopy. Am J Physiol 266: C234–C242, 1994. [DOI] [PubMed] [Google Scholar]
- 208.Kamm KE, Stull JT. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol 25: 593–620, 1985. [DOI] [PubMed] [Google Scholar]
- 209.Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem 276: 4527–4530, 2001. [DOI] [PubMed] [Google Scholar]
- 210.Kamouchi M, Philipp S, Flockerzi V, Wissenbach U, Mamin A, Raeymaekers L, Eggermont J, Droogmans G, Nilius B. Properties of heterologously expressed hTRP3 channels in bovine pulmonary artery endothelial cells. J Physiol 518(Pt 2): 345–358, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kanashiro CA, Khalil RA. Isoform-specific protein kinase C activity at variable Ca2+ entry during coronary artery contraction by vasoactive eicosanoids. Can J Physiol Pharmacol 76: 1110–1119, 1998. [DOI] [PubMed] [Google Scholar]
- 212.Kansui Y, Garland CJ, Dora KA. Enhanced spontaneous Ca2+ events in endothelial cells reflect signalling through myoendothelial gap junctions in pressurized mesenteric arteries. Cell Calcium 44: 135–146, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Karashima Y, Prenen J, Talavera K, Janssens A, Voets T, Nilius B. Agonist-induced changes in Ca(2+) permeation through the nociceptor cation channel TRPA1. Biophys J 98: 773–783, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kark T, Bagi Z, Lizanecz E, Pasztor ET, Erdei N, Czikora A, Papp Z, Edes I, Porszasz R, Toth A. Tissue-specific regulation of microvascular diameter: opposite functional roles of neuronal and smooth muscle located vanilloid receptor-1. Mol Pharmacol 73: 1405–1412, 2008. [DOI] [PubMed] [Google Scholar]
- 215.Kassmann M, Szijarto IA, Garcia-Prieto CF, Fan G, Schleifenbaum J, Anistan YM, Tabeling C, Shi Y, le Noble F, Witzenrath M, Huang Y, Marko L, Nelson MT, Gollasch M. Role of ryanodine type 2 receptors in elementary Ca(2+) signaling in arteries and vascular adaptive responses. J Am Heart Assoc 8: e010090, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kawate T, Michel JC, Birdsong WT, Gouaux E. Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature 460: 592–598, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kecskes M, Jacobs G, Kerselaers S, Syam N, Menigoz A, Vangheluwe P, Freichel M, Flockerzi V, Voets T, Vennekens R. The Ca(2+)-activated cation channel TRPM4 is a negative regulator of angiotensin II-induced cardiac hypertrophy. Basic Res Cardiol 110: 43, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Keef KD, Hume JR, Zhong J. Regulation of cardiac and smooth muscle Ca(2+) channels (Ca(V)1.2a,b) by protein kinases. Am J Physiol Cell Physiol 281: C1743–C1756, 2001. [DOI] [PubMed] [Google Scholar]
- 219.Khan I, Sandhu V, Misquitta CM, Grover AK. SERCA pump isoform expression in endothelium of veins and arteries: Every endothelium is not the same. Mol Cell Biochem 203: 11–15, 2000. [DOI] [PubMed] [Google Scholar]
- 220.Khananshvili D. Distinction between the two basic mechanisms of cation transport in the cardiac Na(+)-Ca2+ exchange system. Biochemistry 29: 2437–2442, 1990. [DOI] [PubMed] [Google Scholar]
- 221.Kharade SV, Sonkusare SK, Srivastava AK, Thakali KM, Fletcher TW, Rhee SW, Rusch NJ. The beta3 subunit contributes to vascular calcium channel upregulation and hypertension in angiotensin II-infused C57BL/6 mice. Hypertension 61: 137–142, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Khavandi K, Baylie RA, Sugden SA, Ahmed M, Csato V, Eaton P, Hill-Eubanks DC, Bonev AD, Nelson MT, Greenstein AS. Pressure-induced oxidative activation of PKG enables vasoregulation by Ca2+ sparks and BK channels. Sci Signal 9: ra100, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kim HR, Gallant C, Morgan KG. Regulation of PKC autophosphorylation by calponin in contractile vascular smooth muscle tissue. Biomed Res Int 2013: 358643, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kishimoto A, Mikawa K, Hashimoto K, Yasuda I, Tanaka S, Tominaga M, Kuroda T, Nishizuka Y. Limited proteolysis of protein kinase C subspecies by calcium-dependent neutral protease (calpain). J Biol Chem 264: 4088–4092, 1989. [PubMed] [Google Scholar]
- 225.Kitazawa T, Takizawa N, Ikebe M, Eto M. Reconstitution of protein kinase C-induced contractile Ca2+ sensitization in triton X-100-demembranated rabbit arterial smooth muscle. J Physiol 520 (Pt 1): 139–152, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Knot HJ, Standen NB, Nelson MT. Ryanodine receptors regulate arterial diameter and wall [Ca2+] in cerebral arteries of rat via Ca2+-dependent K+ channels. J Physiol 508 (Pt 1): 211–221, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kochukov MY, Balasubramanian A, Abramowitz J, Birnbaumer L, Marrelli SP. Activation of endothelial transient receptor potential C3 channel is required for small conductance calcium-activated potassium channel activation and sustained endothelial hyperpolarization and vasodilation of cerebral artery. J Am Heart Assoc 3: e000913, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kohler R, Heyken WT, Heinau P, Schubert R, Si H, Kacik M, Busch C, Grgic I, Maier T, Hoyer J. Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol 26: 1495–1502, 2006. [DOI] [PubMed] [Google Scholar]
- 229.Kohler R, Wulff H, Eichler I, Kneifel M, Neumann D, Knorr A, Grgic I, Kampfe D, Si H, Wibawa J, Real R, Borner K, Brakemeier S, Orzechowski HD, Reusch HP, Paul M, Chandy KG, Hoyer J. Blockade of the intermediate-conductance calcium-activated potassium channel as a new therapeutic strategy for restenosis. Circulation 108: 1119–1125, 2003. [DOI] [PubMed] [Google Scholar]
- 230.Kohout SC, Corbalan-Garcia S, Torrecillas A, Gomez-Fernandez JC, Falke JJ. C2 domains of protein kinase C isoforms alpha, beta, and gamma: Activation parameters and calcium stoichiometries of the membrane-bound state. Biochemistry 41: 11411–11424, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Koida S, Ohyanagi M, Ueda A, Mori Y, Iwasaka T. Mechanism of increased alpha-adrenoceptor-mediated contraction in small resistance arteries of rats with heart failure. Clin Exp Pharmacol Physiol 33: 47–52, 2006. [DOI] [PubMed] [Google Scholar]
- 232.Kolisek M, Beck A, Fleig A, Penner R. Cyclic ADP-ribose and hydrogen peroxide synergize with ADP-ribose in the activation of TRPM2 channels. Mol Cell 18: 61–69, 2005. [DOI] [PubMed] [Google Scholar]
- 233.Krishnamoorthy G, Sonkusare SK, Heppner TJ, Nelson MT. Opposing roles of smooth muscle BK channels and ryanodine receptors in the regulation of nerve-evoked constriction of mesenteric resistance arteries. Am J Physiol Heart Circ Physiol 306: H981–H988, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kumar B, Dreja K, Shah SS, Cheong A, Xu SZ, Sukumar P, Naylor J, Forte A, Cipollaro M, McHugh D, Kingston PA, Heagerty AM, Munsch CM, Bergdahl A, Hultgardh-Nilsson A, Gomez MF, Porter KE, Hellstrand P, Beech DJ. Upregulated TRPC1 channel in vascular injury in vivo and its role in human neointimal hyperplasia. Circ Res 98: 557–563, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kunichika N, Yu Y, Remillard CV, Platoshyn O, Zhang S, Yuan JX. Overexpression of TRPC1 enhances pulmonary vasoconstriction induced by capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 287: L962–L969, 2004. [DOI] [PubMed] [Google Scholar]
- 236.Kuo IY, Ellis A, Seymour VA, Sandow SL, Hill CE. Dihydropyridine-insensitive calcium currents contribute to function of small cerebral arteries. J Cereb Blood Flow Metab 30: 1226–1239, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lacerda AE, Kim HS, Ruth P, Perez-Reyes E, Flockerzi V, Hofmann F, Birnbaumer L, Brown AM. Normalization of current kinetics by interaction between the alpha 1 and beta subunits of the skeletal muscle dihydropyridine-sensitive Ca2+ channel. Nature 352: 527–530, 1991. [DOI] [PubMed] [Google Scholar]
- 238.Lamont C, Vainorius E, Wier WG. Purinergic and adrenergic Ca2+ transients during neurogenic contractions of rat mesenteric small arteries. J Physiol 549: 801–808, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lamont C, Wier WG. Evoked and spontaneous purinergic junctional Ca2+ transients (jCaTs) in rat small arteries. Circ Res 91: 454–456, 2002. [DOI] [PubMed] [Google Scholar]
- 240.Lange A, Gebremedhin D, Narayanan J, Harder D. 20-Hydroxyeicosatetraenoic acid-induced vasoconstriction and inhibition of potassium current in cerebral vascular smooth muscle is dependent on activation of protein kinase C. J Biol Chem 272: 27345–27352, 1997. [DOI] [PubMed] [Google Scholar]
- 241.Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 109: 397–407, 2002. [DOI] [PubMed] [Google Scholar]
- 242.Ledoux J, Bonev AD, Nelson MT. Ca2+-activated K+ channels in murine endothelial cells: Block by intracellular calcium and magnesium. J Gen Physiol 131: 125–135, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ledoux J, Chartier D, Leblanc N. Inhibitors of calmodulin-dependent protein kinase are nonspecific blockers of voltage-dependent K+ channels in vascular myocytes. J Pharmacol Exp Ther 290: 1165–1174, 1999. [PubMed] [Google Scholar]
- 244.Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci USA 105: 9627–9632, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 21: 69–78, 2006. [DOI] [PubMed] [Google Scholar]
- 246.Lee MD, Wilson C, Saunter CD, Kennedy C, Girkin JM, McCarron JG. Spatially structured cell populations process multiple sensory signals in parallel in intact vascular endothelium. Sci Signal 11: eaar4411, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Lee SL, Yu AS, Lytton J. Tissue-specific expression of Na(+)-Ca2+ exchanger isoforms. J Biol Chem 269: 14849–14852, 1994. [PubMed] [Google Scholar]
- 248.Leo MD, Bannister JP, Narayanan D, Nair A, Grubbs JE, Gabrick KS, Boop FA, Jaggar JH. Dynamic regulation of beta1 subunit trafficking controls vascular contractility. Proc Natl Acad Sci USA 111: 2361–2366, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Levitsky DO, Nicoll DA, Philipson KD. Identification of the high affinity Ca(2+)-binding domain of the cardiac Na(+)-Ca2+ exchanger. J Biol Chem 269: 22847–22852, 1994. [PubMed] [Google Scholar]
- 250.Lhomme A, Gilbert G, Pele T, Deweirdt J, Henrion D, Baudrimont I, Campagnac M, Marthan R, Guibert C, Ducret T, Savineau JP, Quignard JF. Stretch-activated piezo1 channel in endothelial cells relaxes mouse intrapulmonary arteries. Am J Respir Cell Mol Biol 60: 650–658, 2019. [DOI] [PubMed] [Google Scholar]
- 251.Li C, Ma Q, Toan S, Wang J, Zhou H, Liang J. SERCA overexpression reduces reperfusion-mediated cardiac microvascular damage through inhibition of the calcium/MCU/mPTP/necroptosis signaling pathways. Redox Biol 36: 101659, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Li YH, Zhang N, Wang YN, Shen Y, Wang Y. Multiple faces of protein interacting with C kinase 1 (PICK1): Structure, function, and diseases. Neurochem Int 98: 115–121, 2016. [DOI] [PubMed] [Google Scholar]
- 253.Li Z, Nicoll DA, Collins A, Hilgemann DW, Filoteo AG, Penniston JT, Weiss JN, Tomich JM, Philipson KD. Identification of a peptide inhibitor of the cardiac sarcolemmal Na(+)-Ca2+ exchanger. J Biol Chem 266: 1014–1020, 1991. [PubMed] [Google Scholar]
- 254.Liao J, Li H, Zeng W, Sauer DB, Belmares R, Jiang Y. Structural insight into the ion-exchange mechanism of the sodium/calcium exchanger. Science 335: 686–690, 2012. [DOI] [PubMed] [Google Scholar]
- 255.Lillo MA, Gaete PS, Puebla M, Ardiles NM, Poblete I, Becerra A, Simon F, Figueroa XF. Critical contribution of Na(+)-Ca(2+) exchanger to the Ca(2+)-mediated vasodilation activated in endothelial cells of resistance arteries. FASEB J 32: 2137–2147, 2018. [DOI] [PubMed] [Google Scholar]
- 256.Liman ER. The Ca(2+)-activated TRP channels: TRPM4 and TRPM5. In: Liedtke WB, Heller S, editors. Frontiers in Neuroscience. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades, Chapter 15. Boca Raton, FL: Taylor & Francis Group, LLC, 2007, p. 203. [Google Scholar]
- 257.Lin Q, Zhao L, Jing R, Trexler C, Wang H, Li Y, Tang H, Huang F, Zhang F, Fang X, Liu J, Jia N, Chen J, Ouyang K. Inositol 1,4,5-trisphosphate receptors in endothelial cells play an essential role in vasodilation and blood pressure regulation. J Am Heart Assoc 8:e011704, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Lincoln TM. Myosin phosphatase regulatory pathways: Different functions or redundant functions? Circ Res 100: 10–12, 2007. [DOI] [PubMed] [Google Scholar]
- 259.Lipskaia L, del Monte F, Capiod T, Yacoubi S, Hadri L, Hours M, Hajjar RJ, Lompre AM. Sarco/endoplasmic reticulum Ca2+-ATPase gene transfer reduces vascular smooth muscle cell proliferation and neointima formation in the rat. Circ Res 97: 488–495, 2005. [DOI] [PubMed] [Google Scholar]
- 260.Lishko PV, Procko E, Jin X, Phelps CB, Gaudet R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron 54: 905–918, 2007. [DOI] [PubMed] [Google Scholar]
- 261.Liu CL, Huang Y, Ngai CY, Leung YK, Yao XQ. TRPC3 is involved in flow- and bradykinin-induced vasodilation in rat small mesenteric arteries. Acta Pharmacol Sin 27: 981–990, 2006. [DOI] [PubMed] [Google Scholar]
- 262.Liu J, Garcia-Cardena G, Sessa WC. Palmitoylation of endothelial nitric oxide synthase is necessary for optimal stimulated release of nitric oxide: Implications for caveolae localization. Biochemistry 35: 13277–13281, 1996. [DOI] [PubMed] [Google Scholar]
- 263.Liu LH, Paul RJ, Sutliff RL, Miller ML, Lorenz JN, Pun RY, Duffy JJ, Doetschman T, Kimura Y, MacLennan DH, Hoying JB, Shull GE. Defective endothelium-dependent relaxation of vascular smooth muscle and endothelial cell Ca2+ signaling in mice lacking sarco(endo)plasmic reticulum Ca2+-ATPase isoform 3. J Biol Chem 272: 30538–30545, 1997. [DOI] [PubMed] [Google Scholar]
- 264.Liu X, Vien T, Duan J, Sheu SH, DeCaen PG, Clapham DE. Polycystin-2 is an essential ion channel subunit in the primary cilium of the renal collecting duct epithelium. Elife 7: e33183, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Lizanecz E, Bagi Z, Pasztor ET, Papp Z, Edes I, Kedei N, Blumberg PM, Toth A. Phosphorylation-dependent desensitization by anandamide of vanilloid receptor-1 (TRPV1) function in rat skeletal muscle arterioles and in Chinese hamster ovary cells expressing TRPV1. Mol Pharmacol 69: 1015–1023, 2006. [DOI] [PubMed] [Google Scholar]
- 266.Lohn M, Jessner W, Furstenau M, Wellner M, Sorrentino V, Haller H, Luft FC, Gollasch M. Regulation of calcium sparks and spontaneous transient outward currents by RyR3 in arterial vascular smooth muscle cells. Circ Res 89: 1051–1057, 2001. [DOI] [PubMed] [Google Scholar]
- 267.Lohn M, Lauterbach B, Haller H, Pongs O, Luft FC, Gollasch M. beta(1)-Subunit of BK channels regulates arterial wall[Ca(2+)] and diameter in mouse cerebral arteries. J Appl Physiol (1985) 91: 1350–1354, 2001. [DOI] [PubMed] [Google Scholar]
- 268.Loot AE, Popp R, Fisslthaler B, Vriens J, Nilius B, Fleming I. Role of cytochrome P450-dependent transient receptor potential V4 activation in flow-induced vasodilatation. Cardiovasc Res 80: 445–452, 2008. [DOI] [PubMed] [Google Scholar]
- 269.Lozinskaya IM, Cox RH. Effects of age on Ca2+ currents in small mesenteric artery myocytes from Wistar–Kyoto and spontaneously hypertensive rats. Hypertension 29: 1329–1336, 1997. [DOI] [PubMed] [Google Scholar]
- 270.Lucchesi PA, Belmadani S, Matrougui K. Hydrogen peroxide acts as both vasodilator and vasoconstrictor in the control of perfused mouse mesenteric resistance arteries. J Hypertens 23: 571–579, 2005. [DOI] [PubMed] [Google Scholar]
- 271.Lukas TJ, Burgess WH, Prendergast FG, Lau W, Watterson DM. Calmodulin binding domains: Characterization of a phosphorylation and calmodulin binding site from myosin light chain kinase. Biochemistry 25: 1458–1464, 1986. [DOI] [PubMed] [Google Scholar]
- 272.Ma X, Du J, Zhang P, Deng J, Liu J, Lam FF, Li RA, Huang Y, Jin J, Yao X. Functional role of TRPV4-KCa2.3 signaling in vascular endothelial cells in normal and streptozotocin-induced diabetic rats. Hypertension 62: 134–139, 2013. [DOI] [PubMed] [Google Scholar]
- 273.Ma X, Qiu S, Luo J, Ma Y, Ngai CY, Shen B, Wong CO, Huang Y, Yao X. Functional role of vanilloid transient receptor potential 4-canonical transient receptor potential 1 complex in flow-induced Ca2+ influx. Arterioscler Thromb Vasc Biol 30: 851–858, 2010. [DOI] [PubMed] [Google Scholar]
- 274.MacKay CE, Leo MD, Fernandez-Pena C, Hasan R, Yin W, Mata-Daboin A, Bulley S, Gammons J, Mancarella S, Jaggar JH. Intravascular flow stimulates PKD2 (polycystin-2) channels in endothelial cells to reduce blood pressure. Elife 9: e56655, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.MacLennan DH, Kranias EG. Phospholamban: A crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol 4: 566–577, 2003. [DOI] [PubMed] [Google Scholar]
- 276.Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, Patapoutian A. The pungency of garlic: Activation of TRPA1 and TRPV1 in response to allicin. Curr Biol 15: 929–934, 2005. [DOI] [PubMed] [Google Scholar]
- 277.Mak DO, Foskett JK. Single-channel kinetics, inactivation, and spatial distribution of inositol trisphosphate (IP3) receptors in Xenopus oocyte nucleus. J Gen Physiol 109: 571–587, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Mak DO, McBride S, Foskett JK. Inositol 1,4,5-trisphosphate [correction of tris-phosphate] activation of inositol trisphosphate [correction of tris-phosphate] receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc Natl Acad Sci USA 95: 15821–15825, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Mapp PI, McWilliams DF, Turley MJ, Hargin E, Walsh DA. A role for the sensory neuropeptide calcitonin gene-related peptide in endothelial cell proliferation in vivo. Br J Pharmacol 166: 1261–1271, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Marshall NJ, Liang L, Bodkin J, Dessapt-Baradez C, Nandi M, Collot-Teixeira S, Smillie SJ, Lalgi K, Fernandes ES, Gnudi L, Brain SD. A role for TRPV1 in influencing the onset of cardiovascular disease in obesity. Hypertension 61: 246–252, 2013. [DOI] [PubMed] [Google Scholar]
- 281.Martin E, Dahan D, Cardouat G, Gillibert-Duplantier J, Marthan R, Savineau JP, Ducret T. Involvement of TRPV1 and TRPV4 channels in migration of rat pulmonary arterial smooth muscle cells. Pflugers Arch 464: 261–272, 2012. [DOI] [PubMed] [Google Scholar]
- 282.Marziano C, Hong K, Cope EL, Kotlikoff MI, Isakson BE, Sonkusare SK. Nitric oxide-dependent feedback loop regulates transient receptor potential vanilloid 4 (TRPV4) channel cooperativity and endothelial function in small pulmonary arteries. J Am Heart Assoc 6: e007157, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Mathar I, Vennekens R, Meissner M, Kees F, Van der Mieren G, Camacho Londono JE, Uhl S, Voets T, Hummel B, van den Bergh A, Herijgers P, Nilius B, Flockerzi V, Schweda F, Freichel M. Increased catecholamine secretion contributes to hypertension in TRPM4-deficient mice. J Clin Invest 120: 3267–3279, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Mather S, Dora KA, Sandow SL, Winter P, Garland CJ. Rapid endothelial cell-selective loading of connexin 40 antibody blocks endothelium-derived hyperpolarizing factor dilation in rat small mesenteric arteries. Circ Res 97: 399–407, 2005. [DOI] [PubMed] [Google Scholar]
- 285.Matsuoka S, Nicoll DA, He Z, Philipson KD. Regulation of cardiac Na(+)-Ca2+ exchanger by the endogenous XIP region. J Gen Physiol 109:273–286, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Matsuoka S, Nicoll DA, Hryshko LV, Levitsky DO, Weiss JN, Philipson KD. Regulation of the cardiac Na(+)-Ca2+ exchanger by Ca2+. Mutational analysis of the Ca(2+)-binding domain. J Gen Physiol 105: 403–420, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.McCarron JG, Chalmers S, MacMillan D, Olson ML. Agonist-evoked Ca(2+) wave progression requires Ca(2+) and IP(3). J Cell Physiol 224: 334–344, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.McCarron JG, Halpern W. Potassium dilates rat cerebral arteries by two independent mechanisms. Am J Physiol 259: H902–H908, 1990. [DOI] [PubMed] [Google Scholar]
- 289.McDonald TF, Pelzer S, Trautwein W, Pelzer DJ. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev 74: 365–507, 1994. [DOI] [PubMed] [Google Scholar]
- 290.Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J 332 (Pt 2): 281–292, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, Suzuki M, Zhang DX. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol 298: H466–H476, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Mercado J, Baylie R, Navedo MF, Yuan C, Scott JD,Nelson MT, Brayden JE, Santana LF. Local control of TRPV4 channels by AKAP150-targeted PKC in arterial smooth muscle. J Gen Physiol 143: 559–575, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Michel T, Vanhoutte PM. Cellular signaling and NO production. Pflugers Arch 459: 807–816, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Minta A, Kao JP, Tsien RY. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem 264: 8171–8178, 1989. [PubMed] [Google Scholar]
- 295.Mistry DK, Garland CJ. Nitric oxide (NO)-induced activation of large conductance Ca2+-dependent K+ channels (BK(Ca)) in smooth muscle cells isolated from the rat mesenteric artery. Br J Pharmacol 124: 1131–1140, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJ, Somlo S. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272: 1339–1342, 1996. [DOI] [PubMed] [Google Scholar]
- 297.Moitra J, Evenoski C, Sammani S, Wadgaonkar R, Turner JR, Ma SF, Garcia JG. A transgenic mouse with vascular endothelial overexpression of the non-muscle myosin light chain kinase-2 isoform is susceptible to inflammatory lung injury: Role of sexual dimorphism and age. Transl Res 151: 141–153, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Moncada S Nitric oxide in the vasculature: Physiology and pathophysiology. Ann N Y Acad Sci 811: 60–67; discussion 67-69, 1997. [DOI] [PubMed] [Google Scholar]
- 299.Montero M, Brini M, Marsault R, Alvarez J, Sitia R, Pozzan T, Rizzuto R. Monitoring dynamic changes in free Ca2+ concentration in the endoplasmic reticulum of intact cells. EMBO J 14: 5467–5475, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Moosmang S, Schulla V, Welling A, Feil R, Feil S, Wegener JW, Hofmann F, Klugbauer N. Dominant role of smooth muscle L-type calcium channel Cav1.2 for blood pressure regulation. EMBO J 22: 6027–6034, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Moreno-Dominguez A, El-Yazbi AF, Zhu HL, Colinas O, Zhong XZ, Walsh EJ, Cole DM, Kargacin GJ, Walsh MP, Cole WC. Cytoskeletal reorganization evoked by Rho-associated kinase- and protein kinase C-catalyzed phosphorylation of cofilin and heat shock protein 27, respectively, contributes to myogenic constriction of rat cerebral arteries. J Biol Chem 289: 20939–20952, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Most P, Koch WJ. S100A1: A calcium-modulating inotropic prototype for future clinical heart failure therapy. Future Cardiol 3: 5–11, 2007. [DOI] [PubMed] [Google Scholar]
- 303.Most P, Remppis A, Pleger ST, Katus HA, Koch WJ. S100A1: A novel inotropic regulator of cardiac performance. Transition from molecular physiology to pathophysiological relevance. Am J Physiol Regul Integr Comp Physiol 293: R568–R577, 2007. [DOI] [PubMed] [Google Scholar]
- 304.Mountian II, Baba-Aissa F, Jonas JC, De Humbert S, Wuytack F, Parys JB. Expression of Ca(2+) transport genes in platelets and endothelial cells in hypertension. Hypertension 37: 135–141, 2001. [DOI] [PubMed] [Google Scholar]
- 305.Mufti RE, Brett SE, Tran CH, Abd El-Rahman R, Anfinogenova Y, El-Yazbi A, Cole WC, Jones PP, Chen SR, Welsh DG. Intravascular pressure augments cerebral arterial constriction by inducing voltage-insensitive Ca2+ waves. J Physiol 588: 3983–4005, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Murad F. Discovery of some of the biological effects of nitric oxide and its role in cell signaling. Biosci Rep 24: 452–474, 2004. [DOI] [PubMed] [Google Scholar]
- 307.Murthy S, Koval OM, Ramiro Diaz JM, Kumar S, Nuno D, Scott JA, Allamargot C, Zhu LJ, Broadhurst K, Santhana V, Kutschke WJ, Irani K, Lamping KG, Grumbach IM. Endothelial CaMKII as a regulator of eNOS activity and NO-mediated vasoreactivity. PLoS One 12: e0186311, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Musicki B, Kramer MF, Becker RE, Burnett AL. Inactivation of phosphorylated endothelial nitric oxide synthase (Ser-1177) by O-GlcNAc in diabetes-associated erectile dysfunction. Proc Natl Acad Sci USA 102: 11870–11875, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Nagata K, Duggan A, Kumar G, Garcia-Anoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci 25: 4052–4061, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Naik JS, Osmond JM, Walker BR, Kanagy NL. Hydrogen sulfide-induced vasodilation mediated by endothelial TRPV4 channels. Am J Physiol Heart Circ Physiol 311: H1437–H1444, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Naik JS, Walker BR. Endothelial-dependent dilation following chronic hypoxia involves TRPV4-mediated activation of endothelial BK channels. Pflugers Arch 470: 633–648, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Narayanan D, Adebiyi A, Jaggar JH. Inositol trisphosphate receptors in smooth muscle cells. Am J Physiol Heart Circ Physiol 302: H2190–H2210, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Nausch LW, Bonev AD, Heppner TJ, Tallini Y, Kotlikoff MI, Nelson MT. Sympathetic nerve stimulation induces local endothelial Ca2+ signals to oppose vasoconstriction of mouse mesenteric arteries. Am J Physiol Heart Circ Physiol 302: H594–H602, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Navedo MF, Amberg GC, Nieves M, Molkentin JD, Santana LF. Mechanisms underlying heterogeneous Ca2+ sparklet activity in arterial smooth muscle. J Gen Physiol 127: 611–622, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Navedo MF, Amberg GC, Votaw VS, Santana LF. Constitutively active L-type Ca2+ channels. Proc Natl Acad Sci USA 102: 11112–11117, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Navedo MF, Nieves-Cintron M, Amberg GC, Yuan C, Votaw VS, Lederer WJ, McKnight GS, Santana LF. AKAP150 is required for stuttering persistent Ca2+ sparklets and angiotensin II-induced hypertension. Circ Res 102: e1–e11, 2008. [DOI] [PubMed] [Google Scholar]
- 317.Navedo MF, Takeda Y, Nieves-Cintron M, Molkentin JD, Santana LF. Elevated Ca2+ sparklet activity during acute hyperglycemia and diabetes in cerebral arterial smooth muscle cells. Am J Physiol Cell Physiol 298: C211–C220, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science 270: 633–637, 1995. [DOI] [PubMed] [Google Scholar]
- 319.Newton AC, Keranen LM. Phosphatidyl-L-serine is necessary for protein kinase C’s high-affinity interaction with diacylglycerol-containing membranes. Biochemistry 33: 6651–6658, 1994. [DOI] [PubMed] [Google Scholar]
- 320.Neylon CB. Potassium channels and vascular proliferation. Vascul Pharmacol 38: 35–41, 2002. [DOI] [PubMed] [Google Scholar]
- 321.Neylon CB, Richards SM, Larsen MA, Agrotis A, Bobik A. Multiple types of ryanodine receptor/Ca2+ release channels are expressed in vascular smooth muscle. Biochem Biophys Res Commun 215: 814–821, 1995. [DOI] [PubMed] [Google Scholar]
- 322.Ng LC, McCormack MD, Airey JA, Singer CA, Keller PS, Shen XM, Hume JR. TRPC1 and STIM1 mediate capacitative Ca2+ entry in mouse pulmonary arterial smooth muscle cells. J Physiol 587: 2429–2442, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Nieves-Cintron M, Amberg GC, Navedo MF, Molkentin JD, Santana LF. The control of Ca2+ influx and NFATc3 signaling in arterial smooth muscle during hypertension. Proc Natl Acad Sci USA 105: 15623–15628, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Nieves-Cintron M, Amberg GC, Nichols CB, Molkentin JD, Santana LF. Activation of NFATc3 down-regulates the beta1 subunit of large conductance, calcium-activated K+ channels in arterial smooth muscle and contributes to hypertension. J Biol Chem 282: 3231–3240, 2007. [DOI] [PubMed] [Google Scholar]
- 325.Nieves-Cintron M, Syed AU, Buonarati OR, Rigor RR, Nystoriak MA, Ghosh D, Sasse KC, Ward SM, Santana LF, Hell JW, Navedo MF. Impaired BKCa channel function in native vascular smooth muscle from humans with type 2 diabetes. Sci Rep 7: 14058, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Niggli E, Lederer WJ. Molecular operations of the sodium-calcium exchanger revealed by conformation currents. Nature 349: 621–624, 1991. [DOI] [PubMed] [Google Scholar]
- 327.Nikolaev YA, Cox CD, Ridone P, Rohde PR, Cordero-Morales JF, Vasquez V, Laver DR, Martinac B. Mammalian TRP ion channels are insensitive to membrane stretch. J Cell Sci 132: jcs238360, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Nilius B, Prenen J, Tang J, Wang C, Owsianik G, Janssens A, Voets T, Zhu MX. Regulation of the Ca2+ sensitivity of the nonselective cation channel TRPM4. J Biol Chem 280: 6423–6433, 2005. [DOI] [PubMed] [Google Scholar]
- 329.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 258: 607–614, 1992. [DOI] [PubMed] [Google Scholar]
- 330.North RA. P2X receptors. Philos Trans R Soc Lond B Biol Sci 371: 20150427, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Nowycky MC, Fox AP, Tsien RW. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature 316: 440–443, 1985. [DOI] [PubMed] [Google Scholar]
- 332.Numaga-Tomita T, Shimauchi T, Oda S, Tanaka T, Nishiyama K, Nishimura A, Birnbaumer L, Mori Y, Nishida M. TRPC6 regulates phenotypic switching of vascular smooth muscle cells through plasma membrane potential-dependent coupling with PTEN. FASEB J 33: 9785–9796, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Numazaki M, Tominaga T, Takeuchi K, Murayama N, Toyooka H, Tominaga M. Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc Natl Acad Sci USA 100: 8002–8006, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J Biol Chem 277: 13375–13378, 2002. [DOI] [PubMed] [Google Scholar]
- 335.Nystoriak MA, Nieves-Cintron M, Nygren PJ, Hinke SA, Nichols CB, Chen CY, Puglisi JL, Izu LT, Bers DM, Dell’acqua ML, Scott JD, Santana LF, Navedo MF. AKAP150 contributes to enhanced vascular tone by facilitating large-conductance Ca2+-activated K+ channel remodeling in hyperglycemia and diabetes mellitus. Circ Res 114: 607–615, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Nystoriak MA, O’Connor KP, Sonkusare SK, Brayden JE, Nelson MT, Wellman GC. Fundamental increase in pressure-dependent constriction of brain parenchymal arterioles from subarachnoid hemorrhage model rats due to membrane depolarization. Am J Physiol Heart Circ Physiol 300: H803–H812, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Olesen C, Picard M, Winther AM, Gyrup C, Morth JP, Oxvig C, Moller JV, Nissen P. The structural basis of calcium transport by the calcium pump. Nature 450: 1036–1042, 2007. [DOI] [PubMed] [Google Scholar]
- 338.Ondrias K, Brillantes AM, Scott A, Ehrlich BE, Marks AR. Single channel properties and calcium conductance of the cloned expressed ryanodine receptor/calcium-release channel. Soc Gen Physiol Ser 51: 29–45, 1996. [PubMed] [Google Scholar]
- 339.Ono Y, Fujii T, Igarashi K, Kuno T, Tanaka C, Kikkawa U, Nishizuka Y. Phorbol ester binding to protein kinase C requires a cysteine-rich zinc-finger-like sequence. Proc Natl Acad Sci USA 86: 4868–4871, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Orestes P, Bojadzic D, Lee J, Leach E, Salajegheh R, Digruccio MR, Nelson MT, Todorovic SM. Free radical signalling underlies inhibition of CaV3.2 T-type calcium channels by nitrous oxide in the pain pathway. J Physiol 589: 135–148, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Ottolini M, Daneva Z, Chen YL, Cope EL, Kasetti RB, Zode GS, Sonkusare SK. Mechanisms underlying selective coupling of endothelial Ca(2+) signals with eNOS vs. IK/SK channels in systemic and pulmonary arteries. J Physiol 598 (17): 3577–3596, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Ottolini M, Hong K, Cope EL, Daneva Z, DeLalio LJ, Sokolowski JD, Marziano C, Nguyen NY, Altschmied J, Haendeler J, Johnstone SR, Kalani MY, Park MS, Patel RP, Liedtke W, Isakson BE, Sonkusare SK. Local peroxynitrite impairs endothelial transient receptor potential vanilloid 4 channels and elevates blood pressure in obesity. Circulation 141: 1318–1333, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Ottolini M, Hong K, Cope EL, Daneva Z, DeLalio LJ, Sokolowski JD, Marziano C, Nguyen NY, Altschmied J, Haendeler J, Johnstone SR, Kalani MY, Park MS, Patel RP, Liedtke W, Isakson BE, Sonkusare SK. Local peroxynitrite impairs endothelial TRPV4 channels and elevates blood pessure in obesity. Circulation 141 (16): 1318–1333, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Ottolini M, Hong K, Sonkusare SK. Calcium signals that determine vascular resistance. Wiley Interdiscip Rev Syst Biol Med 11: e1448, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 136: 876–890, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Park WS, Kim N, Youm JB, Warda M, Ko JH, Kim SJ, Earm YE, Han J. Angiotensin II inhibits inward rectifier K+ channels in rabbit coronary arterial smooth muscle cells through protein kinase Calpha. Biochem Biophys Res Commun 341: 728–735, 2006. [DOI] [PubMed] [Google Scholar]
- 347.Patel S, Joseph SK, Thomas AP. Molecular properties of inositol 1,4,5-trisphosphate receptors. Cell Calcium 25: 247–264, 1999. [DOI] [PubMed] [Google Scholar]
- 348.Paterno R, Faraci FM, Heistad DD. Role of Ca(2+)-dependent K+ channels in cerebral vasodilatation induced by increases in cyclic GMP and cyclic AMP in the rat. Stroke 27: 1603–1607; discussion 1607-1608, 1996. [DOI] [PubMed] [Google Scholar]
- 349.Patterson RL, van Rossum DB, Barrow RK, Snyder SH. RACK1 binds to inositol 1,4,5-trisphosphate receptors and mediates Ca2+ release. Proc Natl Acad Sci USA 101: 2328–2332, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Pearson RB, Kemp BE. Protein kinase phosphorylation site sequences and consensus specificity motifs: Tabulations. Methods Enzymol 200: 62–81, 1991. [DOI] [PubMed] [Google Scholar]
- 351.Pellicena P, Schulman H. CaMKII inhibitors: From research tools to therapeutic agents. Front Pharmacol 5: 21, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Perez GJ, Bonev AD, Nelson MT. Micromolar Ca(2+) from sparks activates Ca(2+)-sensitive K(+) channels in rat cerebral artery smooth muscle. Am J Physiol Cell Physiol 281: C1769–C1775, 2001. [DOI] [PubMed] [Google Scholar]
- 353.Perez GJ, Bonev AD, Patlak JB, Nelson MT. Functional coupling of ryanodine receptors to KCa channels in smooth muscle cells from rat cerebral arteries. J Gen Physiol 113: 229–238, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Perez-Reyes E, Kim HS, Lacerda AE, Horne W, Wei XY, Rampe D, Campbell KP, Brown AM, Birnbaumer L. Induction of calcium currents by the expression of the alpha 1-subunit of the dihydropyridine receptor from skeletal muscle. Nature 340: 233–236, 1989. [DOI] [PubMed] [Google Scholar]
- 355.Periasamy M, Kalyanasundaram A. SERCA pump isoforms: Their role in calcium transport and disease. Muscle Nerve 35:430–442, 2007. [DOI] [PubMed] [Google Scholar]
- 356.Periasamy M, Reed TD, Liu LH, Ji Y, Loukianov E, Paul RJ, Nieman ML, Riddle T, Duffy JJ, Doetschman T, Lorenz JN, Shull GE. Impaired cardiac performance in heterozygous mice with a null mutation in the sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2 (SERCA2) gene. J Biol Chem 274: 2556–2562, 1999. [DOI] [PubMed] [Google Scholar]
- 357.Peterson BZ, DeMaria CD, Adelman JP, Yue DT. Calmodulin is the Ca2+ sensor for Ca2+ -dependent inactivation of L-type calcium channels. Neuron 22: 549–558, 1999. [DOI] [PubMed] [Google Scholar]
- 358.Phelps CB, Wang RR, Choo SS, Gaudet R. Differential regulation of TRPV1, TRPV3, and TRPV4 sensitivity through a conserved binding site on the ankyrin repeat domain. J Biol Chem 285: 731–740, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Pires PW, Earley S. Neuroprotective effects of TRPA1 channels in the cerebral endothelium following ischemic stroke. Elife 7: e35316, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Pires PW, Sullivan MN, Pritchard HA, Robinson JJ, Earley S. Unitary TRPV3 channel Ca2+ influx events elicit endothelium-dependent dilation of cerebral parenchymal arterioles. Am J Physiol Heart Circ Physiol 309: H2031–H2041, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Planells-Cases R, Valente P, Ferrer-Montiel A, Qin F, Szallasi A. Complex regulation of TRPV1 and related thermo-TRPs: Implications for therapeutic intervention. Adv Exp Med Biol 704: 491–515, 2011. [DOI] [PubMed] [Google Scholar]
- 362.Plant TD, Strotmann R. TRPV4: A multifunctional nonselective cation channel with complex regulation. In: Liedtke WB, Heller S, editors. Frontiers in Neuroscience. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades, Chapter 9. Boca Raton, FL: Taylor & Francis Group, LLC, 2007, p. 125. [PubMed] [Google Scholar]
- 363.Pleger ST, Harris DM, Shan C, Vinge LE, Chuprun JK, Berzins B, Pleger W, Druckman C, Volkers M, Heierhorst J, Oie E, Remppis A, Katus HA, Scalia R, Eckhart AD, Koch WJ, Most P. Endothelial S100A1 modulates vascular function via nitric oxide. Circ Res 102: 786–794, 2008. [DOI] [PubMed] [Google Scholar]
- 364.Pluger S, Faulhaber J, Furstenau M, Lohn M, Waldschutz R, Gollasch M, Haller H, Luft FC, Ehmke H, Pongs O. Mice with disrupted BK channel beta1 subunit gene feature abnormal Ca(2+) spark/STOC coupling and elevated blood pressure. Circ Res 87: E53–E60, 2000. [DOI] [PubMed] [Google Scholar]
- 365.Porszasz R, Porkolab A, Ferencz A, Pataki T, Szilvassy Z, Szolcsanyi J. Capsaicin-induced nonneural vasoconstriction in canine mesenteric arteries. Eur J Pharmacol 441: 173–175, 2002. [DOI] [PubMed] [Google Scholar]
- 366.Porter VA, Bonev AD, Knot HJ, Heppner TJ, Stevenson AS, Kleppisch T, Lederer WJ, Nelson MT. Frequency modulation of Ca2+ sparks is involved in regulation of arterial diameter by cyclic nucleotides. Am J Physiol 274: C1346–C1355, 1998. [DOI] [PubMed] [Google Scholar]
- 367.Poteser M, Graziani A, Rosker C, Eder P, Derler I, Kahr H, Zhu MX, Romanin C, Groschner K. TRPC3 and TRPC4 associate to form a redox-sensitive cation channel. Evidence for expression of native TRPC3-TRPC4 heteromeric channels in endothelial cells. J Biol Chem 281: 13588–13595, 2006. [DOI] [PubMed] [Google Scholar]
- 368.Potier M, Gonzalez JC, Motiani RK, Abdullaev IF, Bisaillon JM, Singer HA, Trebak M. Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: Role in proliferation and migration. FASEB J 23: 2425–2437, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Povstyan OV, Harhun MI, Gordienko DV. Ca2+ entry following P2X receptor activation induces IP3 receptor-mediated Ca2+ release in myocytes from small renal arteries. Br J Pharmacol 162: 1618–1638, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Pragnell M, De Waard M, Mori Y, Tanabe T, Snutch TP, Campbell KP. Calcium channel beta-subunit binds to a conserved motif in the I-II cytoplasmic linker of the alpha 1-subunit. Nature 368: 67–70, 1994. [DOI] [PubMed] [Google Scholar]
- 371.Prasad AM, Ketsawatsomkron P, Nuno DW, Koval OM, Dibbern ME, Venema AN, Sigmund CD, Lamping KG, Grumbach IM. Role of CaMKII in Ang-II-dependent small artery remodeling. Vascul Pharmacol 87: 172–179, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Prasad AM, Nuno DW, Koval OM, Ketsawatsomkron P, Li W, Li H, Shen FY, Joiner ML, Kutschke W, Weiss RM, Sigmund CD, Anderson ME, Lamping KG, Grumbach IM. Differential control of calcium homeostasis and vascular reactivity by Ca2+/calmodulin-dependent kinase II. Hypertension 62:434–441, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Pritchard HAT, Gonzales AL, Pires PW, Drumm BT, Ko EA, Sanders KM, Hennig GW, Earley S. Microtubule structures underlying the sarcoplasmic reticulum support peripheral coupling sites to regulate smooth muscle contractility. Sci Signal 10: eaan2694, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Pritchard HAT, Pires PW, Yamasaki E, Thakore P, Earley S. Nanoscale remodeling of ryanodine receptor cluster size underlies cerebral microvascular dysfunction in Duchenne muscular dystrophy. Proc Natl Acad Sci USA 115: E9745–E9752, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Qian X, Francis M, Solodushko V, Earley S, Taylor MS. Recruitment of dynamic endothelial Ca2+ signals by the TRPA1 channel activator AITC in rat cerebral arteries. Microcirculation 20: 138–148, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Raguimova ON, Smolin N, Bovo E, Bhayani S, Autry JM, Zima AV, Robia SL. Redistribution of SERCA calcium pump conformers during intracellular calcium signaling. J Biol Chem 293: 10843–10856, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Raina H, Ella SR, Hill MA. Decreased activity of the smooth muscle Na+/Ca2+ exchanger impairs arteriolar myogenic reactivity. J Physiol 586: 1669–1681, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol 68: 619–647, 2006. [DOI] [PubMed] [Google Scholar]
- 379.Ravi K, Brennan LA, Levic S, Ross PA, Black SM. S-nitrosylation of endothelial nitric oxide synthase is associated with monomerization and decreased enzyme activity. Proc Natl Acad Sci USA 101: 2619–2624, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Reading SA, Brayden JE. Central role of TRPM4 channels in cerebral blood flow regulation. Stroke 38: 2322–2328, 2007. [DOI] [PubMed] [Google Scholar]
- 381.Retailleau K, Duprat F, Arhatte M, Ranade SS, Peyronnet R, Martins JR, Jodar M, Moro C, Offermanns S, Feng Y, Demolombe S, Patel A, Honore E. Piezo1 in smooth muscle cells is involved in hypertension-dependent arterial remodeling. Cell Rep 13: 1161–1171, 2015. [DOI] [PubMed] [Google Scholar]
- 382.Reuter H, Blaustein MP, Haeusler G. Na-Ca exchange and tension development in arterial smooth muscle. Philos Trans R Soc Lond B Biol Sci 265: 87–94, 1973. [DOI] [PubMed] [Google Scholar]
- 383.Reynoso R, Perrin RM, Breslin JW, Daines DA, Watson KD, Watterson DM, Wu MH, Yuan S. A role for long chain myosin light chain kinase (MLCK-210) in microvascular hyperpermeability during severe burns. Shock 28:589–595, 2007. [DOI] [PubMed] [Google Scholar]
- 384.Rezazadeh S, Claydon TW, Fedida D. KN-93 (2-[N-(2-hydroxyethyl)]-N-(4-methoxybenzenesulfonyl)amino-N-(4-chlorocinnamyl)-N-methylbenzylamine), a calcium/calmodulin-dependent protein kinase II inhibitor, is a direct extracellular blocker of voltage-gated potassium channels. J Pharmacol Exp Ther 317: 292–299, 2006. [DOI] [PubMed] [Google Scholar]
- 385.Rhoads AR, Friedberg F. Sequence motifs for calmodulin recognition. FASEB J 11: 331–340, 1997. [DOI] [PubMed] [Google Scholar]
- 386.Ringvold HC, Khalil RA. Protein kinase C as regulator of vascular smooth muscle function and potential target in vascular disorders. Adv Pharmacol 78: 203–301, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Robertson BE, Schubert R, Hescheler J, Nelson MT. cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am J Physiol 265: C299–C303, 1993. [DOI] [PubMed] [Google Scholar]
- 388.Rohacs T. Phosphoinositide regulation of TRP channels. Handb Exp Pharmacol 223: 1143–1176, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Rosenbaum T, Gordon-Shaag A, Munari M, Gordon SE. Ca2+/calmodulin modulates TRPV1 activation by capsaicin. J Gen Physiol 123: 53–62, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Rosse C, Linch M, Kermorgant S, Cameron AJ, Boeckeler K, Parker PJ. PKC and the control of localized signal dynamics. Nat Rev Mol Cell Biol 11: 103–112, 2010. [DOI] [PubMed] [Google Scholar]
- 391.Rudiger S, Jung P, Shuai JW. Termination of Ca(2)+ release for clustered IP(3)r channels. PLoS Comput Biol 8: e1002485, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Rueda A, Fernandez-Velasco M, Benitah JP, Gomez AM. Abnormal Ca2+ spark/STOC coupling in cerebral artery smooth muscle cells of obese type 2 diabetic mice. PLoS One 8: e53321, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Ruiz-Velasco V, Zhong J, Hume JR, Keef KD. Modulation of Ca2+ channels by cyclic nucleotide cross activation of opposing protein kinases in rabbit portal vein. Circ Res 82: 557–565, 1998. [DOI] [PubMed] [Google Scholar]
- 394.Sachs F. Stretch-activated ion channels: What are they? Physiology (Bethesda) 25: 50–56, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Saleh SN, Albert AP, Peppiatt CM, Large WA. Angiotensin II activates two cation conductances with distinct TRPC1 and TRPC6 channel properties in rabbit mesenteric artery myocytes. J Physiol 577: 479–495, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Saliez J, Bouzin C, Rath G, Ghisdal P, Desjardins F, Rezzani R, Rodella LF, Vriens J, Nilius B, Feron O, Balligand JL, Dessy C. Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation 117: 1065–1074, 2008. [DOI] [PubMed] [Google Scholar]
- 397.Samways DS, Egan TM. Calcium-dependent decrease in the single-channel conductance of TRPV1. Pflugers Arch 462: 681–691, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Samways DS, Li Z, Egan TM. Principles and properties of ion flow in P2X receptors. Front Cell Neurosci 8: 6, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Sanchez-Bautista S, Marin-Vicente C, Gomez-Fernandez JC, Corbalan-Garcia S. The C2 domain of PKCalpha is a Ca2+ - dependent PtdIns(4,5)P2 sensing domain: A new insight into an old pathway. J Mol Biol 362: 901–914, 2006. [DOI] [PubMed] [Google Scholar]
- 400.Sandow SL, Neylon CB, Chen MX, Garland CJ. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (K(Ca)) and connexins: Possible relationship to vasodilator function? J Anat 209: 689–698, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Sano Y, Inamura K, Miyake A, Mochizuki S, Yokoi H, Matsushime H, Furuichi K. Immunocyte Ca2+ influx system mediated by LTRPC2. Science 293: 1327–1330, 2001. [DOI] [PubMed] [Google Scholar]
- 402.Schlossmann J, Ammendola A, Ashman K, Zong X, Huber A, Neubauer G, Wang GX, Allescher HD, Korth M, Wilm M, Hofmann F, Ruth P. Regulation of intracellular calcium by a signalling complex of IRAG, IP3 receptor and cGMP kinase Ibeta. Nature 404: 197–201, 2000. [DOI] [PubMed] [Google Scholar]
- 403.Schlossmann J, Desch M. IRAG and novel PKG targeting in the cardiovascular system. Am J Physiol Heart Circ Physiol 301: H672–H682, 2011. [DOI] [PubMed] [Google Scholar]
- 404.Schneider H, Schubert KM, Blodow S, Kreutz CP, Erdogmus S, Wiedenmann M, Qiu J, Fey T, Ruth P, Lubomirov LT, Pfitzer G, Mederos YSM, Hardie DG, Gudermann T, Pohl U. AMPK dilates resistance arteries via activation of SERCA and BKCa channels in smooth muscle. Hypertension 66: 108–116, 2015. [DOI] [PubMed] [Google Scholar]
- 405.Schneider JC, El Kebir D, Chereau C, Mercier JC, Dall’Ava-Santucci J, Dinh-Xuan AT. Involvement of Na(+)/Ca(2+) exchanger in endothelial NO production and endothelium-dependent relaxation. Am J Physiol Heart Circ Physiol 283: H837–H844, 2002. [DOI] [PubMed] [Google Scholar]
- 406.Schubert R, Noack T, Serebryakov VN. Protein kinase C reduces the KCa current of rat tail artery smooth muscle cells. Am J Physiol 276: C648–C658, 1999. [DOI] [PubMed] [Google Scholar]
- 407.Schumacher MA, Rivard AF, Bachinger HP, Adelman JP. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature 410: 1120–1124, 2001. [DOI] [PubMed] [Google Scholar]
- 408.Seki T, Goto K, Kiyohara K, Kansui Y, Murakami N, Haga Y, Ohtsubo T, Matsumura K, Kitazono T. Downregulation of endothelial transient receptor potential vanilloid type 4 channel and small-conductance of Ca2+-activated K+ channels underpins impaired endothelium-dependent hyperpolarization in hypertension. Hypertension 69: 143–153, 2017. [DOI] [PubMed] [Google Scholar]
- 409.Senadheera S, Kim Y, Grayson TH, Toemoe S, Kochukov MY, Abramowitz J, Housley GD, Bertrand RL, Chadha PS, Bertrand PP, Murphy TV, Tare M, Birnbaumer L, Marrelli SP, Sandow SL. Transient receptor potential canonical type 3 channels facilitate endothelium-derived hyperpolarization-mediated resistance artery vasodilator activity. Cardiovasc Res 95: 439–447, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Shannon TR, Bers DM. Assessment of intra-SR free [Ca] and buffering in rat heart. Biophys J 73: 1524–1531, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Sharif-Naeini R, Folgering JH, Bichet D, Duprat F, Lauritzen I, Arhatte M, Jodar M, Dedman A, Chatelain FC, Schulte U, Retailleau K, Loufrani L, Patel A, Sachs F, Delmas P, Peters DJ, Honore E. Polycystin-1 and −2 dosage regulates pressure sensing. Cell 139: 587–596, 2009. [DOI] [PubMed] [Google Scholar]
- 412.Sharma AK, Charles EJ, Zhao Y, Narahari AK, Baderdinni PK, Good ME, Lorenz UM, Kron IL, Bayliss DA, Ravichandran KS, Isakson BE, Laubach VE. Pannexin-1 channels on endothelial cells mediate vascular inflammation during lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol 315: L301–L312, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Shen Q, Rigor RR, Pivetti CD, Wu MH, Yuan SY. Myosin light chain kinase in microvascular endothelial barrier function. Cardiovasc Res 87: 272–280, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci USA 93: 13176–13181, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Shi J, Birnbaumer L, Large WA, Albert AP. Myristoylated alanine-rich C kinase substrate coordinates native TRPC1 channel activation by phosphatidylinositol 4,5-bisphosphate and protein kinase C in vascular smooth muscle. FASEB J 28: 244–255, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Shi J, Ju M, Abramowitz J, Large WA, Birnbaumer L, Albert AP. TRPC1 proteins confer PKC and phosphoinositol activation on native heteromeric TRPC1/C5 channels in vascular smooth muscle: comparative study of wild-type and TRPC1−/− mice. FASEB J 26: 409–419, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Shi J, Miralles F, Birnbaumer L, Large WA, Albert AP. Store depletion induces Galphaq-mediated PLCbeta1 activity to stimulate TRPC1 channels in vascular smooth muscle cells. FASEB J 30:702–715, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Shi J, Mori E, Mori Y, Mori M, Li J, Ito Y, Inoue R. Multiple regulation by calcium of murine homologues of transient receptor potential proteins TRPC6 and TRPC7 expressed in HEK293 cells. J Physiol 561: 415–432, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, Takayanagi T, Nagao T, Egashira K, Fujishima M, Takeshita A. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol 28: 703–711, 1996. [DOI] [PubMed] [Google Scholar]
- 420.Shorofsky SR, Izu L, Wier WG, Balke CW. Ca2+ sparks triggered by patch depolarization in rat heart cells. Circ Res 82: 424–429, 1998. [DOI] [PubMed] [Google Scholar]
- 421.Shui B, Lee JC, Reining S, Lee FK, Kotlikoff MI. Optogenetic sensors and effectors: CHROMus-the Cornell Heart Lung Blood Institute Resource for Optogenetic Mouse Signaling. Front Physiol 5: 428, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Sienaert I, Missiaen L, De Smedt H, Parys JB, Sipma H, Casteels R. Molecular and functional evidence for multiple Ca2+-binding domains in the type 1 inositol 1,4,5-trisphosphate receptor. J Biol Chem 272: 25899–25906, 1997. [DOI] [PubMed] [Google Scholar]
- 423.Simard JM, Li X, Tewari K. Increase in functional Ca2+ channels in cerebral smooth muscle with renal hypertension. Circ Res 82: 1330–1337, 1998. [DOI] [PubMed] [Google Scholar]
- 424.Slater SJ, Ho C, Kelly MB, Larkin JD, Taddeo FJ, Yeager MD, Stubbs CD. Protein kinase Calpha contains two activator binding sites that bind phorbol esters and diacylglycerols with opposite affinities. J Biol Chem 271: 4627–4631, 1996. [DOI] [PubMed] [Google Scholar]
- 425.Slish DF, Welsh DG, Brayden JE. Diacylglycerol and protein kinase C activate cation channels involved in myogenic tone. Am J Physiol Heart Circ Physiol 283: H2196–H2201, 2002. [DOI] [PubMed] [Google Scholar]
- 426.Snoek GT, Rosenberg I, de Laat SW, Gitler C. The interaction of protein kinase C and other specific cytoplasmic proteins with phospholipid bilayers. Biochim Biophys Acta 860: 336–344, 1986. [DOI] [PubMed] [Google Scholar]
- 427.Snutch TP, Reiner PB. Ca2+ channels: Diversity of form and function. Curr Opin Neurobiol 2: 247–253, 1992. [DOI] [PubMed] [Google Scholar]
- 428.Soboloff J, Rothberg BS, Madesh M, Gill DL. STIM proteins: Dynamic calcium signal transducers. Nat Rev Mol Cell Biol 13: 549–565, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and non-muscle myosin II: Modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003. [DOI] [PubMed] [Google Scholar]
- 430.Song S, Yamamura A, Yamamura H, Ayon RJ, Smith KA, Tang H, Makino A, Yuan JX. Flow shear stress enhances intracellular Ca2+ signaling in pulmonary artery smooth muscle cells from patients with pulmonary arterial hypertension. Am J Physiol Cell Physiol 307: C373–C383, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Song Y, Simard JM. beta-Adrenoceptor stimulation activates large-conductance Ca2+-activated K+ channels in smooth muscle cells from basilar artery of guinea pig. Pflugers Arch 430: 984–993, 1995. [DOI] [PubMed] [Google Scholar]
- 432.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 336: 597–601, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Sonkusare SK, Dalsgaard T, Bonev AD, Hill-Eubanks DC, Kotlikoff MI, Scott JD, Santana LF, Nelson MT. AKAP150-dependent cooperative TRPV4 channel gating is central to endothelium-dependent vasodilation and is disrupted in hypertension. Sci Signal 7: ra66, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Sowa G, Pypaert M, Sessa WC. Distinction between signaling mechanisms in lipid rafts vs. caveolae. Proc Natl Acad Sci USA 98: 14072–14077, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci USA 103: 16586–16591, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Spyridopoulos I, Luedemann C, Chen D, Kearney M, Chen D, Murohara T, Principe N, Isner JM, Losordo DW. Divergence of angiogenic and vascular permeability signaling by VEGF: Inhibition of protein kinase C suppresses VEGF-induced angiogenesis, but promotes VEGF-induced, NO-dependent vascular permeability. Arterioscler Thromb Vasc Biol 22: 901–906, 2002. [DOI] [PubMed] [Google Scholar]
- 437.Stathopulos PB, Zheng L, Li GY, Plevin MJ, Ikura M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell 135: 110–122, 2008. [DOI] [PubMed] [Google Scholar]
- 438.Stauss HM, Godecke A, Mrowka R, Schrader J, Persson PB. Enhanced blood pressure variability in eNOS knockout mice. Hypertension 33: 1359–1363, 1999. [DOI] [PubMed] [Google Scholar]
- 439.Steinberg SF. Structural basis of protein kinase C isoform function. Physiol Rev 88: 1341–1378, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Straub AC, Lohman AW, Billaud M, Johnstone SR, Dwyer ST, Lee MY, Bortz PS, Best AK, Columbus L, Gaston B, Isakson BE. Endothelial cell expression of haemoglobin alpha regulates nitric oxide signalling. Nature 491: 473–477, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol 2: 695–702, 2000. [DOI] [PubMed] [Google Scholar]
- 442.Strotmann R, Schultz G, Plant TD. Ca2+-dependent potentiation of the nonselective cation channel TRPV4 is mediated by a C-terminal calmodulin binding site. J Biol Chem 278: 26541–26549, 2003. [DOI] [PubMed] [Google Scholar]
- 443.Strotmann R, Semtner M, Kepura F, Plant TD, Schoneberg T. Interdomain interactions control Ca2+-dependent potentiation in the cation channel TRPV4. PLoS One 5: e10580, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron 29: 645–655, 2001. [DOI] [PubMed] [Google Scholar]
- 445.Stull JT, Lin PJ, Krueger JK, Trewhella J, Zhi G. Myosin light chain kinase: Functional domains and structural motifs. Acta Physiol Scand 164: 471–482, 1998. [DOI] [PubMed] [Google Scholar]
- 446.Sugimoto M, Nakayama M, Goto TM, Amano M, Komori K, Kaibuchi K. Rho-kinase phosphorylates eNOS at threonine 495 in endothelial cells. Biochem Biophys Res Commun 361: 462–467, 2007. [DOI] [PubMed] [Google Scholar]
- 447.Sullivan MN, Francis M, Pitts NL, Taylor MS, Earley S. Optical recording reveals novel properties of GSK1016790A-induced vanilloid transient receptor potential channel TRPV4 activity in primary human endothelial cells. Mol Pharmacol 82: 464–472, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Sullivan MN, Gonzales AL, Pires PW, Bruhl A, Leo MD, Li W, Oulidi A, Boop FA, Feng Y, Jaggar JH, Welsh DG, Earley S. Localized TRPA1 channel Ca2+ signals stimulated by reactive oxygen species promote cerebral artery dilation. Sci Signal 8: ra2, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Sun XP, Callamaras N, Marchant JS, Parker I. A continuum of InsP3-mediated elementary Ca2+ signalling events in Xenopus oocytes. J Physiol 509 (Pt 1): 67–80, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Suresh K, Servinsky L, Jiang H, Bigham Z, Yun X, Kliment C, Huetsch J, Damarla M, Shimoda LA. Reactive oxygen species induced Ca(2+) influx via TRPV4 and microvascular endothelial dysfunction in the SU5416/hypoxia model of pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 314: L893–L907, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Suresh K, Servinsky L, Reyes J, Baksh S, Undem C, Caterina M, Pearse DB, Shimoda LA. Hydrogen peroxide-induced calcium influx in lung microvascular endothelial cells involves TRPV4. Am J Physiol Lung Cell Mol Physiol 309: L1467–L1477, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Surks HK, Mochizuki N, Kasai Y, Georgescu SP, Tang KM, Ito M, Lincoln TM, Mendelsohn ME. Regulation of myosin phosphatase by a specific interaction with cGMP- dependent protein kinase Ialpha. Science 286: 1583–1587, 1999. [DOI] [PubMed] [Google Scholar]
- 453.Suzuma K, Takahara N, Suzuma I, Isshiki K, Ueki K, Leitges M, Aiello LP, King GL. Characterization of protein kinase C beta isoform’s action on retinoblastoma protein phosphorylation, vascular endothelial growth factor-induced endothelial cell proliferation, and retinal neovascularization. Proc Natl Acad Sci USA 99: 721–726, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Swillens S, Dupont G, Combettes L, Champeil P. From calcium blips to calcium puffs: Theoretical analysis of the requirements for interchannel communication. Proc Natl Acad Sci USA 96: 13750–13755, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Swulius MT, Waxham MN. Ca(2+)/calmodulin-dependent protein kinases. Cell Mol Life Sci 65: 2637–2657, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Taberner FJ, Fernandez-Ballester G, Fernandez-Carvajal A, Ferrer-Montiel A. TRP channels interaction with lipids and its implications in disease. Biochim Biophys Acta 1848: 1818–1827, 2015. [DOI] [PubMed] [Google Scholar]
- 457.Taguchi K, Kaneko K, Kubo T. Protein kinase C modulates Ca2+-activated K+ channels in cultured rat mesenteric artery smooth muscle cells. Biol Pharm Bull 23: 1450–1454, 2000. [DOI] [PubMed] [Google Scholar]
- 458.Tajada S, Cidad P, Colinas O, Santana LF, Lopez-Lopez JR, Perez-Garcia MT. Down-regulation of CaV1.2 channels during hypertension: How fewer CaV1.2 channels allow more Ca(2+) into hypertensive arterial smooth muscle. J Physiol 591: 6175–6191, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Tajada S, Moreno CM, O’Dwyer S, Woods S, Sato D, Navedo MF, Santana LF. Distance constraints on activation of TRPV4 channels by AKAP150-bound PKCalpha in arterial myocytes. J Gen Physiol 149: 639–659, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Takahashi M, Seagar MJ, Jones JF, Reber BF, Catterall WA. Subunit structure of dihydropyridine-sensitive calcium channels from skeletal muscle. Proc Natl Acad Sci USA 84: 5478–5482, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Takahashi N, Hamada-Nakahara S, Itoh Y, Takemura K, Shimada A, Ueda Y, Kitamata M, Matsuoka R, Hanawa-Suetsugu K, Senju Y, Mori MX, Kiyonaka S, Kohda D, Kitao A, Mori Y, Suetsugu S. TRPV4 channel activity is modulated by direct interaction of the ankyrin domain to PI(4,5)P(2). Nat Commun 5: 4994, 2014. [DOI] [PubMed] [Google Scholar]
- 462.Takahashi Y, Watanabe H, Murakami M, Ohba T, Radovanovic M, Ono K, Iijima T, Ito H. Involvement of transient receptor potential canonical 1 (TRPC1) in angiotensin II-induced vascular smooth muscle cell hypertrophy. Atherosclerosis 195: 287–296, 2007. [DOI] [PubMed] [Google Scholar]
- 463.Takai Y, Kishimoto A, Kikkawa U, Mori T, Nishizuka Y. Unsaturated diacylglycerol as a possible messenger for the activation of calcium-activated, phospholipid-dependent protein kinase system. Biochem Biophys Res Commun 91: 1218–1224, 1979. [DOI] [PubMed] [Google Scholar]
- 464.Takeda Y, Nystoriak MA, Nieves-Cintron M, Santana LF, Navedo MF. Relationship between Ca2+ sparklets and sarcoplasmic reticulum Ca2+ load and release in rat cerebral arterial smooth muscle. Am J Physiol Heart Circ Physiol 301: H2285–H2294, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Takeshima H, Iino M, Takekura H, Nishi M, Kuno J, Minowa O, Takano H, Noda T. Excitation-contraction uncoupling and muscular degeneration in mice lacking functional skeletal muscle ryanodine-receptor gene. Nature 369: 556–559, 1994. [DOI] [PubMed] [Google Scholar]
- 466.Takeshima H, Komazaki S, Hirose K, Nishi M, Noda T, Iino M. Embryonic lethality and abnormal cardiac myocytes in mice lacking ryanodine receptor type 2. EMBO J 17: 3309–3316, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Takeshima H, Nishimura S, Matsumoto T, Ishida H, Kangawa K, Minamino N, Matsuo H, Ueda M, Hanaoka M, Hirose T, Takeshima H, Nishimura S, Matsumoto T, Ishida H, Kangawa K, Minamino N, Matsuo H, Ueda M, Hanaoka M, Hirose T, Numa S. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature 339: 439–445, 1989. [DOI] [PubMed] [Google Scholar]
- 468.Tallini YN, Brekke JF, Shui B, Doran R, Hwang SM, Nakai J, Salama G, Segal SS, Kotlikoff MI. Propagated endothelial Ca2+ waves and arteriolar dilation in vivo: Measurements in Cx40BAC GCaMP2 transgenic mice. Circ Res 101: 1300–1309, 2007. [DOI] [PubMed] [Google Scholar]
- 469.Talukder G, Aldrich RW. Complex voltage-dependent behavior of single unliganded calcium-sensitive potassium channels. Biophys J 78: 761–772, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Tansey MG, Luby-Phelps K, Kamm KE, Stull JT. Ca(2+)-dependent phosphorylation of myosin light chain kinase decreases the Ca2+ sensitivity of light chain phosphorylation within smooth muscle cells. J Biol Chem 269: 9912–9920, 1994. [PubMed] [Google Scholar]
- 471.Tansey MG, Word RA, Hidaka H, Singer HA, Schworer CM, Kamm KE, Stull JT. Phosphorylation of myosin light chain kinase by the multifunctional calmodulin-dependent protein kinase II in smooth muscle cells. J Biol Chem 267: 12511–12516, 1992. [PubMed] [Google Scholar]
- 472.Taylor MS, Bonev AD, Gross TP, Eckman DM, Brayden JE, Bond CT, Adelman JP, Nelson MT. Altered expression of small-conductance Ca2+-activated K+ (SK3) channels modulates arterial tone and blood pressure. Circ Res 93: 124–131, 2003. [DOI] [PubMed] [Google Scholar]
- 473.Tewari K, Simard JM. Sodium nitroprusside and cGMP decrease Ca2+ channel availability in basilar artery smooth muscle cells. Pflugers Arch 433: 304–311, 1997. [DOI] [PubMed] [Google Scholar]
- 474.Thakore P, Pritchard HAT, Griffin CS, Yamasaki E, Drumm BT, Lane C, Sanders KM, Feng Earley Y, Earley S. TRPML1 channels initiate Ca(2+) sparks in vascular smooth muscle cells. Sci Signal 13: eaba1015, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Tharp DL, Wamhoff BR, Turk JR, Bowles DK. Upregulation of intermediate-conductance Ca2+-activated K+ channel (IKCa1) mediates phenotypic modulation of coronary smooth muscle. Am J Physiol Heart Circ Physiol 291: H2493–H2503, 2006. [DOI] [PubMed] [Google Scholar]
- 476.Tharp DL, Wamhoff BR, Wulff H, Raman G, Cheong A, Bowles DK. Local delivery of the KCa3.1 blocker, TRAM-34, prevents acute angioplasty-induced coronary smooth muscle phenotypic modulation and limits stenosis. Arterioscler Thromb Vasc Biol 28: 1084–1089, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Thomas D, Tovey SC, Collins TJ, Bootman MD, Berridge MJ, Lipp P. A comparison of fluorescent Ca2+ indicator properties and their use in measuring elementary and global Ca2+ signals. Cell Calcium 28: 213–223, 2000. [DOI] [PubMed] [Google Scholar]
- 478.Thorneloe KS, Cheung M, Bao W, Alsaid H, Lenhard S, Jian MY, Costell M, Maniscalco-Hauk K, Krawiec JA, Olzinski A, Gordon E, Lozinskaya I, Elefante L, Qin P, Matasic DS, James C, Tunstead J, Donovan B, Kallal L, Waszkiewicz A, Vaidya K, Davenport EA, Larkin J, Burgert M, Casillas LN, Marquis RW, Ye G, Eidam HS, Goodman KB, Toomey JR, Roethke TJ, Jucker BM, Schnackenberg CG, Townsley MI, Lepore JJ, Willette RN. An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Sci Transl Med 4: 159ra148, 2012. [DOI] [PubMed] [Google Scholar]
- 479.Tinsley JH, De Lanerolle P, Wilson E, Ma W, Yuan SY. Myosin light chain kinase transference induces myosin light chain activation and endothelial hyperpermeability. Am J Physiol Cell Physiol 279: C1285–C1289, 2000. [DOI] [PubMed] [Google Scholar]
- 480.Tinsley JH, Yuan SY, Wilson E. Isoform-specific knockout of endothelial myosin light chain kinase: Closing the gap on inflammatory lung disease. Trends Pharmacol Sci 25: 64–66, 2004. [DOI] [PubMed] [Google Scholar]
- 481.Tobimatsu T, Fujisawa H. Tissue-specific expression of four types of rat calmodulin-dependent protein kinase II mRNAs. J Biol Chem 264: 17907–17912, 1989. [PubMed] [Google Scholar]
- 482.Toro L, Amador M, Stefani E. ANG II inhibits calcium-activated potassium channels from coronary smooth muscle in lipid bilayers. Am J Physiol 258: H912–H915, 1990. [DOI] [PubMed] [Google Scholar]
- 483.Torres-Narvaez JC, Mondragon Ldel V, Varela Lopez E, Perez-Torres I, Diaz Juarez JA, Suarez J, Hernandez GP. Role of the transient receptor potential vanilloid type 1 receptor and stretch-activated ion channels in nitric oxide release from endothelial cells of the aorta and heart in rats. Exp Clin Cardiol 17: 89–94, 2012. [PMC free article] [PubMed] [Google Scholar]
- 484.Toth A, Czikora A, Pasztor ET, Dienes B, Bai P, Csernoch L, Rutkai I, Csato V, Manyine IS, Porszasz R, Edes I, Papp Z, Boczan J. Vanilloid receptor-1 (TRPV1) expression and function in the vasculature of the rat. J Histochem Cytochem 62: 129–144, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Toussaint F, Charbel C, Allen BG, Ledoux J. Vascular CaMKII: Heart and brain in your arteries. Am J Physiol Cell Physiol 311: C462–C478, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Toyama K, Wulff H, Chandy KG, Azam P, Raman G, Saito T, Fujiwara Y, Mattson DL, Das S, Melvin Je, Pratt PF, Hatoum OA, Gutterman DD, Harder DR, Miura H. The intermediate-conductance calcium-activated potassium channel KCa3.1 contributes to atherogenesis in mice and humans. J Clin Invest 118: 3025–3037, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Toyoshima C, Nakasako M, Nomura H, Ogawa H. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 A resolution. Nature 405: 647–655, 2000. [DOI] [PubMed] [Google Scholar]
- 488.Tran CH, Taylor MS, Plane F, Nagaraja S, Tsoukias NM, Solodushko V, Vigmond EJ, Furstenhaupt T, Brigdan M, Welsh DG. Endothelial Ca2+ wavelets and the induction of myoendothelial feedback. Am J Physiol Cell Physiol 302: C1226–C1242, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Trebak M, St JBG, McKay RR, Birnbaumer L, Putney JW Jr. Signaling mechanism for receptor-activated canonical transient receptor potential 3 (TRPC3) channels. J Biol Chem 278: 16244–16252, 2003. [DOI] [PubMed] [Google Scholar]
- 490.Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andre E, Patacchini R, Cottrell GS, Gatti R, Basbaum AI, Bunnett NW, Julius D, Geppetti P. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA 104: 13519–13524, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Tsien RY, Pozzan T, Rink TJ. Calcium homeostasis in intact lymphocytes: Cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol 94: 325–334, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Urakami-Harasawa L, Shimokawa H, Nakashima M, Egashira K, Takeshita A. Importance of endothelium-derived hyperpolarizing factor in human arteries. J Clin Invest 100:2793–2799, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Vaithianathan T, Bukiya A, Liu J, Liu P, Asuncion-Chin M, Fan Z, Dopico A. Direct regulation of BK channels by phosphatidylinositol 4,5-bisphosphate as a novel signaling pathway. J Gen Physiol 132: 13–28, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Van Vliet BN, Chafe LL, Montani JP. Characteristics of 24 h telemetered blood pressure in eNOS-knockout and C57Bl/6J control mice. J Physiol 549: 313–325, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.VanBavel E, Sorop O, Andreasen D, Pfaffendorf M, Jensen BL. Role of T-type calcium channels in myogenic tone of skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol 283: H2239–H2243, 2002. [DOI] [PubMed] [Google Scholar]
- 496.Vanhoutte PM, Zhao Y, Xu A, Leung SW. Thirty years of saying no: Sources, fate, actions, and misfortunes of the endothelium-derived vasodilator mediator. Circ Res 119: 375–396, 2016. [DOI] [PubMed] [Google Scholar]
- 497.Vazquez G, Wedel BJ, Aziz O, Trebak M, Putney JW Jr. The mammalian TRPC cation channels. Biochim Biophys Acta 1742: 21–36, 2004. [DOI] [PubMed] [Google Scholar]
- 498.Verin AD, Lazar V, Torry RJ, Labarrere CA, Patterson CE, Garcia JG. Expression of a novel high molecular-weight myosin light chain kinase in endothelium. Am J Respir Cell Mol Biol 19: 758–766, 1998. [DOI] [PubMed] [Google Scholar]
- 499.Vial C, Evans RJ. P2X(1) receptor-deficient mice establish the native P2X receptor and a P2Y6-like receptor in arteries. Mol Pharmacol 62: 1438–1445, 2002. [DOI] [PubMed] [Google Scholar]
- 500.Voets T, Nilius B. TRPCs, GPCRs and the Bayliss effect. EMBO J 28: 4–5, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Voets T, Prenen J, Vriens J, Watanabe H, Janssens A, Wissenbach U, Bodding M, Droogmans G, Nilius B. Molecular determinants of permeation through the cation channel TRPV4. J Biol Chem 277: 33704–33710, 2002. [DOI] [PubMed] [Google Scholar]
- 502.Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci USA 101:396–401, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Wainwright MS, Rossi J, Schavocky J, Crawford S, Steinhorn D, Velentza AV, Zasadzki M, Shirinsky V, Jia Y, Haiech J, Van Eldik LJ, Watterson DM. Protein kinase involved in lung injury susceptibility: Evidence from enzyme isoform genetic knockout and in vivo inhibitor treatment. Proc Natl Acad Sci USA 100: 6233–6238, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Wang H, Cheng X, Tian J, Xiao Y, Tian T, Xu F, Hong X, Zhu MX. TRPC channels: Structure, function, regulation and recent advances in small molecular probes. Pharmacol Ther 209: 107497, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Wang RX, Chai Q, Lu T, Lee HC. Activation of vascular BK channels by docosahexaenoic acid is dependent on cytochrome P450 epoxygenase activity. Cardiovasc Res 90: 344–352, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Wang S, Chennupati R, Kaur H, Iring A, Wettschureck N, Offermanns S. Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J Clin Invest 126: 4527–4536, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Wang S, Joseph J, Ro JY, Chung MK. Modality-specific mechanisms of protein kinase C-induced hypersensitivity of TRPV1: S800 is a polymodal sensitization site. Pain 156: 931–941, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Wang WL, Yeh SF, Chang YI, Hsiao SF, Lian WN, Lin CH, Huang CY, Lin WJ. PICK1, an anchoring protein that specifically targets protein kinase Calpha to mitochondria selectively upon serum stimulation in NIH 3T3 cells. J Biol Chem 278: 37705–37712, 2003. [DOI] [PubMed] [Google Scholar]
- 509.Wang Y, Chen L, Li M, Cha H, Iwamoto T, Zhang J. Conditional knockout of smooth muscle sodium calcium exchanger type-1 lowers blood pressure and attenuates Angiotensin II-salt hypertension. Physiol Rep 3: e12273, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, Flockerzi V, Droogmans G, Benham CD, Nilius B. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem 277: 13569–13577, 2002. [DOI] [PubMed] [Google Scholar]
- 511.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424: 434–438, 2003. [DOI] [PubMed] [Google Scholar]
- 512.Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem 277: 47044–47051, 2002. [DOI] [PubMed] [Google Scholar]
- 513.Wellman GC, Santana LF, Bonev AD, Nelson MT. Role of phospholamban in the modulation of arterial Ca(2+) sparks and Ca(2+)-activated K(+) channels by cAMP. Am J Physiol Cell Physiol 281: C1029–C1037, 2001. [DOI] [PubMed] [Google Scholar]
- 514.Wellner M, Maasch C, Kupprion C, Lindschau C, Luft FC, Haller H. The proliferative effect of vascular endothelial growth factor requires protein kinase C-alpha and protein kinase C-zeta. Arterioscler Thromb Vasc Biol 19: 178–185, 1999. [DOI] [PubMed] [Google Scholar]
- 515.Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res 90: 248–250, 2002. [DOI] [PubMed] [Google Scholar]
- 516.Westcott EB, Jackson WF. Heterogeneous function of ryanodine receptors, but not IP3 receptors, in hamster cremaster muscle feed arteries and arterioles. Am J Physiol Heart Circ Physiol 300: H1616–H1630, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.White RE, Kryman JP, El-Mowafy AM, Han G, Carrier GO. cAMP-dependent vasodilators cross-activate the cGMP-dependent protein kinase to stimulate BK(Ca) channel activity in coronary artery smooth muscle cells. Circ Res 86: 897–905, 2000. [DOI] [PubMed] [Google Scholar]
- 518.Wilkerson MK, Heppner TJ, Bonev AD, Nelson MT. Inositol trisphosphate receptor calcium release is required for cerebral artery smooth muscle cell proliferation. Am J Physiol Heart Circ Physiol 290: H240–H247, 2006. [DOI] [PubMed] [Google Scholar]
- 519.Willette RN, Bao W, Nerurkar S, Yue TL, Doe CP, Stankus G, Turner GH, Ju H, Thomas H, Fishman CE, Sulpizio A, Behm DJ, Hoffman S, Lin Z, Lozinskaya I, Casillas LN, Lin M, Trout RE, Votta BJ, Thorneloe K, Lashinger ES, Figueroa DJ, Marquis R, Xu X. Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory J Pharmacol Exp Ther 326: 443–452, 2008. [DOI] [PubMed] [Google Scholar]
- 520.Wilson C, Zhang X, Lee MD, MacDonald M, Heathcote HR, Alorfi NMN, Buckley C, Dolan S, McCarron JG. Disrupted endothelial cell heterogeneity and network organization impair vascular function in prediabetic obesity. Metabolism 111: 154340, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Woodsome TP, Eto M, Everett A, Brautigan DL, Kitazawa T. Expression of CPI-17 and myosin phosphatase correlates with Ca(2+) sensitivity of protein kinase C-induced contraction in rabbit smooth muscle. J Physiol 535: 553–564, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Wooldridge AA, MacDonald JA, Erdodi F, Ma C, Borman MA, Hartshorne DJ, Haystead TA. Smooth muscle phosphatase is regulated in vivo by exclusion of phosphorylation of threonine 696 of MYPT1 by phosphorylation of Serine 695 in response to cyclic nucleotides. J Biol Chem 279: 34496–34504, 2004. [DOI] [PubMed] [Google Scholar]
- 523.Wu KD, Bungard D, Lytton J. Regulation of SERCA Ca2+ pump expression by cytoplasmic Ca2+ in vascular smooth muscle cells. Am J Physiol Cell Physiol 280: C843–C851, 2001. [DOI] [PubMed] [Google Scholar]
- 524.Wu LW, Mayo LD, Dunbar JD, Kessler KM, Baerwald MR, Jaffe EA, Wang D, Warren RS, Donner DB. Utilization of distinct signaling pathways by receptors for vascular endothelial cell growth factor and other mitogens in the induction of endothelial cell proliferation. J Biol Chem 275: 5096–5103, 2000. [DOI] [PubMed] [Google Scholar]
- 525.Xi Q, Adebiyi A, Zhao G, Chapman KE, Waters CM, Hassid A, Jaggar JH. IP3 constricts cerebral arteries via IP3 receptor-mediated TRPC3 channel activation and independently of sarcoplasmic reticulum Ca2+ release. Circ Res 102: 1118–1126, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Xia P, Aiello LP, Ishii H, Jiang ZY, Park DJ, Robinson GS, Takagi H, Newsome WP, Jirousek MR, King GL. Characterization of vascular endothelial growth factor’s effect on the activation of protein kinase C, its isoforms, and endothelial cell growth. J Clin Invest 98: 2018–2026, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, Ishii T, Hirschberg B, Bond CT, Lutsenko S, Maylie J, Adelman JP. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature 395: 503–507, 1998. [DOI] [PubMed] [Google Scholar]
- 528.Xia Y, Fu Z, Hu J, Huang C, Paudel O, Cai S, Liedtke W, Sham JS. TRPV4 channel contributes to serotonin-induced pulmonary vasoconstriction and the enhanced vascular reactivity in chronic hypoxic pulmonary hypertension. Am J Physiol Cell Physiol 305: C704–C715, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Xiong L, Zhang JZ, He R, Hamilton SL. A Ca2+-binding domain in RyR1 that interacts with the calmodulin binding site and modulates channel activity. Biophys J 90: 173–182, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci 9: 628–635, 2006. [DOI] [PubMed] [Google Scholar]
- 531.Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, DiStefano PS, Curtis R, Clapham DE. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 418: 181–186, 2002. [DOI] [PubMed] [Google Scholar]
- 532.Xu SZ, Beech DJ. TrpC1 is a membrane-spanning subunit of store-operated Ca(2+) channels in native vascular smooth muscle cells. Circ Res 88: 84–87, 2001. [DOI] [PubMed] [Google Scholar]
- 533.Xu X, Best PM. Postnatal changes in T-type calcium current density in rat atrial myocytes. J Physiol 454: 657–672, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Yamamoto K, Sokabe T, Matsumoto T, Yoshimura K, Shibata M, Ohura N, Fukuda T, Sato T, Sekine K, Kato S, Isshiki M, Fujita T, Kobayashi M, Kawamura K, Masuda H, Kamiya A, Ando J. Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat Med 12: 133–137, 2006. [DOI] [PubMed] [Google Scholar]
- 535.Yang D, Luo Z, Ma S, Wong WT, Ma L, Zhong J, He H, Zhao Z, Cao T, Yan Z, Liu D, Arendshorst WJ, Huang Y, Tepel M, Zhu Z. Activation of TRPV1 by dietary capsaicin improves endothelium-dependent vasorelaxation and prevents hypertension. Cell Metab 12: 130–141, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Yang H, Hu L, Shi J, Delaloye K, Horrigan FT, Cui J. Mg2+ mediates interaction between the voltage sensor and cytosolic domain to activate BK channels. Proc Natl Acad Sci USA 104: 18270–18275, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Yang H, Zhang G, Cui J. BK channels: Multiple sensors, one activation gate. Front Physiol 6: 29, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Yang XR, Lin AH, Hughes JM, Flavahan NA, Cao YN, Liedtke W, Sham JS. Upregulation of osmo-mechanosensitive TRPV4 channel facilitates chronic hypoxia-induced myogenic tone and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 302: L555–L568, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Yao Y, Choi J, Parker I. Quantal puffs of intracellular Ca2+ evoked by inositol trisphosphate in Xenopus oocytes. J Physiol 482 (Pt 3): 533–553, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Yin J, Hoffmann J, Kaestle SM, Neye N, Wang L, Baeurle J, Liedtke W, Wu S, Kuppe H, Pries AR, Kuebler WM. Negative-feedback loop attenuates hydrostatic lung edema via a cGMP-dependent regulation of transient receptor potential vanilloid 4. Circ Res 102: 966–974, 2008. [DOI] [PubMed] [Google Scholar]
- 541.Yoshikawa F, Morita M, Monkawa T, Michikawa T, Furuichi T, Mikoshiba K. Mutational analysis of the ligand binding site of the inositol 1,4,5-trisphosphate receptor. J Biol Chem 271: 18277–18284, 1996. [DOI] [PubMed] [Google Scholar]
- 542.Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, Tigno DD, Thistlethwaite PA, Rubin LJ, Yuan JX. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci USA 101: 13861–13866, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Yuan P, Leonetti MD, Pico AR, Hsiung Y, MacKinnon R. Structure of the human BK channel Ca2+-activation apparatus at 3.0 A resolution. Science 329: 182–186, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Yuan Y, Huang Q, Wu HM. Myosin light chain phosphorylation: Modulation of basal and agonist-stimulated venular permeability. Am J Physiol 272: H1437–H1443, 1997. [DOI] [PubMed] [Google Scholar]
- 545.Zalk R, Clarke OB, des Georges A, Grassucci RA, Reiken S, Mancia F, Hendrickson WA, Frank J, Marks AR. Structure of a mammalian ryanodine receptor. Nature 517: 44–49, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Zeng XH, Xia XM, Lingle CJ. Divalent cation sensitivity of BK channel activation supports the existence of three distinct binding sites. J Gen Physiol 125: 273–286, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Zenisek D, Davila V, Wan L, Almers W. Imaging calcium entry sites and ribbon structures in two presynaptic cells. J Neurosci 23: 2538–2548,2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Zhang B, Naik JS, Jernigan NL, Walker BR, Resta TC. Reduced membrane cholesterol limits pulmonary endothelial Ca(2+) entry after chronic hypoxia. Am J Physiol Heart Circ Physiol 312: H1176–H1184, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Zhang DX, Mendoza SA, Bubolz AH, Mizuno A, Ge ZD, Li R, Warltier DC, Suzuki M, Gutterman DD. Transient receptor potential vanilloid type 4-deficient mice exhibit impaired endothelium-dependent relaxation induced by acetylcholine in vitro and in vivo. Hypertension 53: 532–538, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Zhang J, Ren C, Chen L, Navedo MF, Antos LK, Kinsey SP, Iwamoto T, Philipson KD, Kotlikoff MI, Santana LF, Wier WG, Matteson DR, Blaustein MP. Knockout of Na+/Ca2+ exchanger in smooth muscle attenuates vasoconstriction and L-type Ca2+ channel current and lowers blood pressure. Am J Physiol Heart Circ Physiol 298: H1472–H1483, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Zhang J, Wang Y, Chen L, Wier WG, Blaustein MP. Na(+)/Ca(2+) exchanger overexpression in smooth muscle augments cytosolic Ca(2+) in femoral arteries of living mice. Am J Physiol Heart Circ Physiol 316: H298–H310, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Zhang L, Papadopoulos P, Hamel E. Endothelial TRPV4 channels mediate dilation of cerebral arteries: Impairment and recovery in cerebrovascular pathologies related to Alzheimer’s disease. Br J Pharmacol 170: 661–670, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Zhang T, Chi S, Jiang F, Zhao Q, Xiao B. A protein interaction mechanism for suppressing the mechanosensitive Piezo channels. Nat Commun 8: 1797, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Zhang W, Halligan KE, Zhang X, Bisaillon JM, Gonzalez-Cobos JC, Motiani RK, Hu G, Vincent PA, Zhou J, Barroso M, Singer HA, Matrougui K, Trebak M. Orai1-mediated I (CRAC) is essential for neointima formation after vascular injury. Circ Res 109: 534–542, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Zhang Z, Li M, Lu R, Alioua A, Stefani E, Toro L. The angiotensin II type 1 receptor (AT1R) closely interacts with large conductance voltage- and Ca2+-activated K+ (BK) channels and inhibits their activity independent of G-protein activation. J Biol Chem 289: 25678–25689, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Zhao G, Adebiyi A, Blaskova E, Xi Q, Jaggar JH. Type 1 inositol 1,4,5-trisphosphate receptors mediate UTP-induced cation currents, Ca2+ signals, and vasoconstriction in cerebral arteries. Am J Physiol Cell Physiol 295: C1376–C1384, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Zhao Q, Wu K, Geng J, Chi S, Wang Y, Zhi P, Zhang M, Xiao B. Ion permeation and mechanotransduction mechanisms of mechanosensitive piezo channels. Neuron 89: 1248–1263, 2016. [DOI] [PubMed] [Google Scholar]
- 558.Zhao X, Falck JR, Gopal VR, Inscho EW, Imig JD. P2X receptor-stimulated calcium responses in preglomerular vascular smooth muscle cells involves 20-hydroxyeicosatetraenoic acid. J Pharmacol Exp Ther 311:1211–1217, 2004. [DOI] [PubMed] [Google Scholar]
- 559.Zhao X, Inscho EW, Bondlela M, Falck JR, Imig JD. The CYP450 hydroxylase pathway contributes to P2X receptor-mediated afferent arteriolar vasoconstriction. Am J Physiol Heart Circ Physiol 281: H2089–H2096, 2001. [DOI] [PubMed] [Google Scholar]
- 560.Zheng J. Molecular mechanism of TRP channels. Compr Physiol 3: 221–242, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Zhong B, Ma S, Wang DH. Ablation of TRPV1 elevates nocturnal blood pressure in western diet-fed mice. Curr Hypertens Rev 15: 144–153, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Zhu MX. Multiple roles of calmodulin and other Ca(2+)-binding proteins in the functional regulation of TRP channels. Pflugers Arch 451: 105–115, 2005. [DOI] [PubMed] [Google Scholar]
- 563.Zhu X, Jackson EK. RACK1 regulates angiotensin II-induced contractions of SHR preglomerular vascular smooth muscle cells. Am J Physiol Renal Physiol 312: F565–F576, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Zorzato F, Fujii J, Otsu K, Phillips M, Green NM, Lai FA, Meissner G, MacLennan DH. Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J Biol Chem 265: 2244–2256, 1990. [PubMed] [Google Scholar]
- 565.Zuhlke RD, Pitt GS, Deisseroth K, Tsien RW, Reuter H. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature 399: 159–162, 1999. [DOI] [PubMed] [Google Scholar]