Abstract
Abnormally prolonged labor, or labor dystocia, is a common complication of parturition. It is the indication for about half of unplanned cesarean deliveries in low-risk nulliparous women. Reducing the rate of unplanned cesarean birth in the USA has been a public health priority over the last two decades with limited success. Labor dystocia is a complex disorder due to multiple causes with a common clinical outcome of slow cervical dilation and fetal descent. A better understanding of the pathophysiologic mechanisms of labor dystocia could lead to new clinical opportunities to increase the rate of normal vaginal delivery, reduce cesarean birth rates, and improve maternal and neonatal health. We conducted a literature review of the causes and pathophysiologic mechanisms of labor dystocia. We summarize known mechanisms supported by clinical and experimental data and newer hypotheses with less supporting evidence. We review recent data on uterine preparation for labor, uterine contractility, cervical preparation for labor, maternal obesity, cephalopelvic disproportion, fetal malposition, intrauterine infection, and maternal stress. We also describe current clinical approaches to preventing and managing labor dystocia. The variation in pathophysiologic causes of labor dystocia probably limits the utility of current general treatment options. However, treatments targeting specific underlying etiologies could be more effective. We found that the pathophysiologic basis of labor dystocia is under-researched, offering wide opportunities for translational investigation of individualized labor management, particularly regarding uterine metabolism and fetal position. More precise diagnostic tools and individualized therapies for labor dystocia might lead to better outcomes. We conclude that additional knowledge of parturition physiology coupled with rigorous clinical evaluation of novel biologically directed treatments could improve obstetric quality of care.
Keywords: Amniotic fluid, Cesarean section, Contraction, Intrapartum, Dystocia, Labor, Lactate, Myometrium, Obesity, Parturition, Pathophysiology, Physiologic birth, Pregnancy, Uterus
Summary sentence:
Labor dystocia leading to unplanned cesarean delivery is mediated by distinct mechanisms which we summarize here to facilitate translational research and new clinical approaches to increase the rate of successful vaginal birth.
Background: Current Diagnosis and Management of Labor Dystocia
Cesarean surgery is a life-saving procedure that can dramatically improve obstetric safety [1]. However, cesarean surgery is a major abdominal operation with significant risks, while vaginal birth affords important physiologic signals that contribute to maternal and neonatal health and wellbeing [2]. Benefits of vaginal birth include reduced postpartum pain [3], improved maternal/neonatal bonding [4], increased breastfeeding rates [5], and reduced rates of childhood asthma, type I diabetes, allergies, and obesity [6–8]. Additionally, cesarean surgery increases risks for surgical complications, postoperative infection, and future pregnancy complications such as uterine rupture, abnormal placentation, ectopic pregnancy, and stillbirth [2, 6, 8]. Cesarean birth can be overused, especially in the USA where cesarean deliveries are more frequent than recommended by consensus opinion to balance the risks and benefits for patients and society [1, 9, 10]. In the USA [9, 11], multidisciplinary campaigns have been implemented through the American College of Nurse Midwives (ACNM) [10], the American College of Obstetricians and Gynecologist (ACOG) [1], and the Society for Maternal–Fetal Medicine (SMFM)[1] to increase vaginal births, but with little apparent change in the national cesarean rate [12].
Labor dystocia is a broad clinical diagnosis defined by slow progress during labor. It is the most common indication for unplanned cesarean, accounting for about one-third of unplanned cesarean deliveries [13, 14]. The diagnosis of labor dystocia depends on clinical assessment (e.g., slow cervical dilation, slow fetal descent). Labor management algorithms for labor dystocia are imprecise which contributes to overuse of unplanned cesarean birth. Typically, labor dystocia is established by comparing the rate of cervical dilation or fetal descent with population norms. Labor curves that define normal dilation and descent were first introduced by Friedman in 1954 [15]. More recently, Zhang et al. published a larger contemporary cohort with slower average cervical dilation rates for successful vaginal deliveries than seen in Friedman’s cohort [16]. Partly due to these newer labor curves, ACOG/SMFM and ACNM adjusted clinical guidelines for labor augmentation and indications for cesarean birth, recognizing that allowing for longer labor leads to fewer cesarean births without increasing adverse outcomes [17–19].
Treatment options for labor dystocia are limited, primarily involving the administration of exogenous synthetic oxytocin to augment uterine contractions [1]. While oxytocin augmentation is known to shorten the overall duration of labor, a systematic review found no difference in the unplanned cesarean delivery rate with augmentation compared to placebo-treated patients [20]. The response to oxytocin augmentation is variable, and there are no clear indicators associated with oxytocin response or mode of delivery. Additionally, contemporary labor dystocia studies are confounded by frequent oxytocin augmentation in women with statistically normal labor progress [16, 21, 22]. To date, we do not have a deep understanding of the pathophysiologic causes of labor dystocia which could improve diagnostic accuracy, clinical management, and careful individualization of labor treatment plans. In this article, we review the literature on mechanisms of labor dystocia and its pathophysiologic etiologies. We outline opportunities for future basic and translational research to decrease the rate of unplanned cesarean due to labor dystocia and thereby improve maternal and neonatal health.
Methods
We conducted a literature search to explore the clinical and experimental evidence to identify varied pathophysiologic causes of labor dystocia especially leading to unplanned cesarean birth. Our primary question was: What are the causes and physiologic mechanisms of labor dystocia? In June 2020 and September 2021, we performed a comprehensive literature review using PubMed, Google Scholar, and MEDLINE databases with the primary medical subject heading (MeSH) search terms: dystocia, obstetric labor complications, and physiopathology. Additional search terms included: cephalopelvic disproportion, fetal malposition, obesity, myometrial dysfunction, and uterine contractility. We reviewed all original research published in English with no date restrictions. We also considered relevant articles that were cited in these publications. We reviewed the full text of relevant articles to summarize content, quality, and relevance for identifying pathophysiologic causes of labor dystocia.
The Pathophysiology of Labor Dystocia
For a common problem, we know surprisingly little about the molecular and physiologic causes of labor dystocia. Despite decades of clinical information, we lack data on the underlying biology and potential for mechanism-driven interventions. Some labor dystocia is due to poorly stimulated contractions which might be resolved with oxytocin augmentation, but this does not explain all dystocia diagnoses. In Table 1, we summarize pathophysiologic causes of labor dystocia. We propose three general categories of active phase labor dystocia:
Table 1.
Labor dystocia and unplanned cesarean delivery
| Unplanned cesarean indication | Clinical presentation | Potential pathophysiology |
|---|---|---|
| Failure to achieve active labor[22] | Does not meet active labor criteria1 despite oxytocin induction |
|
| Arrest of dilation | Stalled cervical dilation during active labor1 [1, 22] | |
| Arrest of descent | Failure of fetal presenting part to descend past 0 to + 1 station |
|
Active labor is defined as ≥ 6-cm cervical dilation or 4–5 cm with ≥ 1-cm cervical change over ≤ 2 h[1]
Cephalopelvic disproportion can also cause arrest of dilation
Adequate uterine contraction force without cervical dilation;
Inadequate uterine contractions that become adequate with pharmacologic augmentation leading to cervical dilation; and
Inadequate uterine contractions that are unresponsive to oxytocin augmentation.
We will review the biological basis of labor dystocia, especially dystocia associated with inadequate uterine contractions unresponsive to oxytocin, an important area for therapeutic innovation.
Uterine Molecular Preparation for Labor
Prior to the onset of labor, the myometrium undergoes molecular activation which supports strong, regular uterine smooth muscle contraction. The overall balance between contractile and quiescence factors influences preparation for uterine contractility [23, 24]. For example, expression of oxytocin receptors, gap junction subunits, calcium channels, and pro-labor signaling enzymes (e.g., cyclooxygenase) are increased in uterine smooth muscle cells during term labor [25, 26]. However, if quiescence genes associated with maintenance of pregnancy predominate, the uterine muscle may not be able to display the labor phenotype, possibly leading to prolonged or ineffective labor. Therefore, adequate myocyte preparation is necessary for normal vaginal delivery [27]. Table 2 describes mechanisms of uterine contractility that might increase the risk for labor dystocia.
Table 2.
Inhibition of uterine contractility
| Factor | Mechanism |
|---|---|
| Endogenous | |
| Reduced oxytocin receptors | Decreased uterine response to oxytocin (maternal/endogenous or pharmacologic/exogenous) during labor[57] |
| Reduced gap junctions | Decreased propagation of uterine depolarization leading to disrupted or inefficient contraction coordination[28, 136] |
| Reduced COX-2 | Inhibited labor signaling via decreased prostaglandin production[137] |
| Acidification | Nonspecific blockade of calcium ion influx, disrupted calcium/MLCK coupling, and dysregulated uterine contraction coordination[23, 57] |
| Exogenous | |
| Calcium channel blocker (e.g., nifedipine) | Decreased intracellular calcium levels[138] |
| Oxytocin receptor antagonist (e.g., atosiban) | Inhibited oxytocin and vasopressin receptor stimulation of ER calcium release[138] |
| Beta-adrenergic receptor agonist (e.g., terbutaline) | Increased adenylyl cyclase production of cAMP to activate PKA which decreases MLCK activity[138] |
| COX-2 inhibitor (e.g., indomethacin) | Blocked labor-stimulating prostaglandin production[137, 138] |
COX-2 cyclooxygenase 2, MLCK myosin light chain kinase, PKA protein kinase A, cAMP cyclic adenosine monophosphate, ER endoplasmic reticulum
Uterine Contraction Force
In addition to uterine molecular preparation, the force of uterine contractions must also be adequate to sustain cervical dilation and fetal descent. Contraction of uterine smooth muscle cells is initiated by receptor stimulation and by intercellular ionic transmission. When the myocyte depolarizes, plasma membrane voltage-gated calcium channels (e.g., L-type) open and calcium ions enter the cytoplasm [28]. Receptor-stimulated (e.g., oxytocin receptor) calcium release from the sarcoplasmic reticulum can also increase cytoplasmic calcium levels [29]. Intracellular calcium binds to calmodulin and activates myosin light chain kinase to phosphorylate myosin and stimulate myosin-actin ATP-dependent cross-bridge cycling to produce uterine contraction force. The uterine action potential spreads from a uterine pacemaker to neighboring myocytes via plasma membrane gap junctions formed by connexin-43 [30]. Following a contraction, calcium is extruded from the cell or restored to the endoplasmic stores by calcium-ATPase and sodium/calcium exchange [30]. The resting membrane potential is restored by potassium channels to repolarize the plasma membrane[26] in preparation for further calciumstimulated contractions.
Exogenous oxytocin administration is often used to stimulate stronger uterine contractions, but sometimes weak or uncoordinated contractions are not corrected by oxytocin. Skeletal muscle biologists have described metabolic fatigue due to hypoxia [31, 32], decreased glycogen availability [33], and reduced calcium ion release [34, 35]; lactic acidosis is a biomarker of metabolic fatigue [32, 36]. Recent work suggests that similar processes could contribute to dysfunctional uterine smooth muscle contraction during labor [23, 29]. For example, elevated amniotic fluid lactate is proposed as a marker of myometrial lactic acidosis possibly associated with labor dystocia and unplanned cesarean birth [37–40]. One group theorizes that increased amniotic fluid lactate is the result of myometrial hypoxia, reduced energy substrate availability, decreased lactate clearance from the utero-fetal unit due to low blood flow, or reduced uterine buffering capacity [37–40]. It is not clear whether the elevated amniotic fluid lactate is a cause or an outcome of dysfunctional uterine contractility, although elevated lactate has been associated with cellular acidosis, blockade of calcium channels, and inhibition of uterine oxytocin receptor stimulation [23].
It is unclear whether endogenous or pharmacologic treatment with oxytocin could overcome these metabolic processes [41], and women with uterine “metabolic fatigue” might require different care strategies to optimize the chance for successful vaginal delivery. Amniotic fluid lactate has poor predictive value for mode of delivery or maternal/fetal morbidity, and importantly there is no evidence to support early cesarean delivery when amniotic fluid lactate is high. Further work is needed to clarify a role for uterine metabolic fatigue and its association with dysfunctional uterine contractility. Other work suggests that estrogen-mediated redox changes[42] and altered uterine adiponectin receptor activity[43] could be other metabolic mechanisms regulating uterine smooth muscle contractility.
Cervical Preparation for Labor
In addition to uterine preparation, the cervix must also make structural, biochemical, and mechanical changes in order to permit effective labor [44–46]. In pregnancy, the uterine cervix is the fetal conduit and must ripen (i.e., soften) and dilate during labor to allow fetal passage. The cervical tissue softens through collagen breakdown, extracellular hydration, and sterile inflammatory signaling [44, 47]. Prior to labor, there are increased leukocytes particularly resident macrophages, decreased cell nuclei density, and increased matrix metalloproteinase expression in the cervix [44–46]. Similarly, proximity to fetal membranes increases inflammation through amniochorion membrane senescence signaling [45, 46]. Increased intrauterine pressure in term pregnancy or multiple gestation can decrease blood flow to the cervix, leading to hypoxia-stimulated inflammatory processes [45, 46]. If uterine contractions occur without sufficient cervical ripening, the cervix may not dilate regardless of contraction strength. The discrete impact of cervical ripening on labor dystocia has not been well described.
Recent research also indicates that the smooth muscle component of the cervix could participate in labor preparation. Histologically, the tissue surrounding the outer cervical os consists primarily of collagen (80–90%), with minimal cellularity, while the tissue surrounding the internal cervical os contains up to 50–60% contractile smooth muscle organized circumferentially around the endocervical canal (Fig. 1) [44]. The muscular tissue surrounding the internal cervical os exhibits regular contractility immediately before labor [48]. Cervical muscle activity has been detected through electromyography and ultrasonography during labor, suggesting that the cervix could have a role in delaying or facilitating labor [48, 49].
Fig. 1.
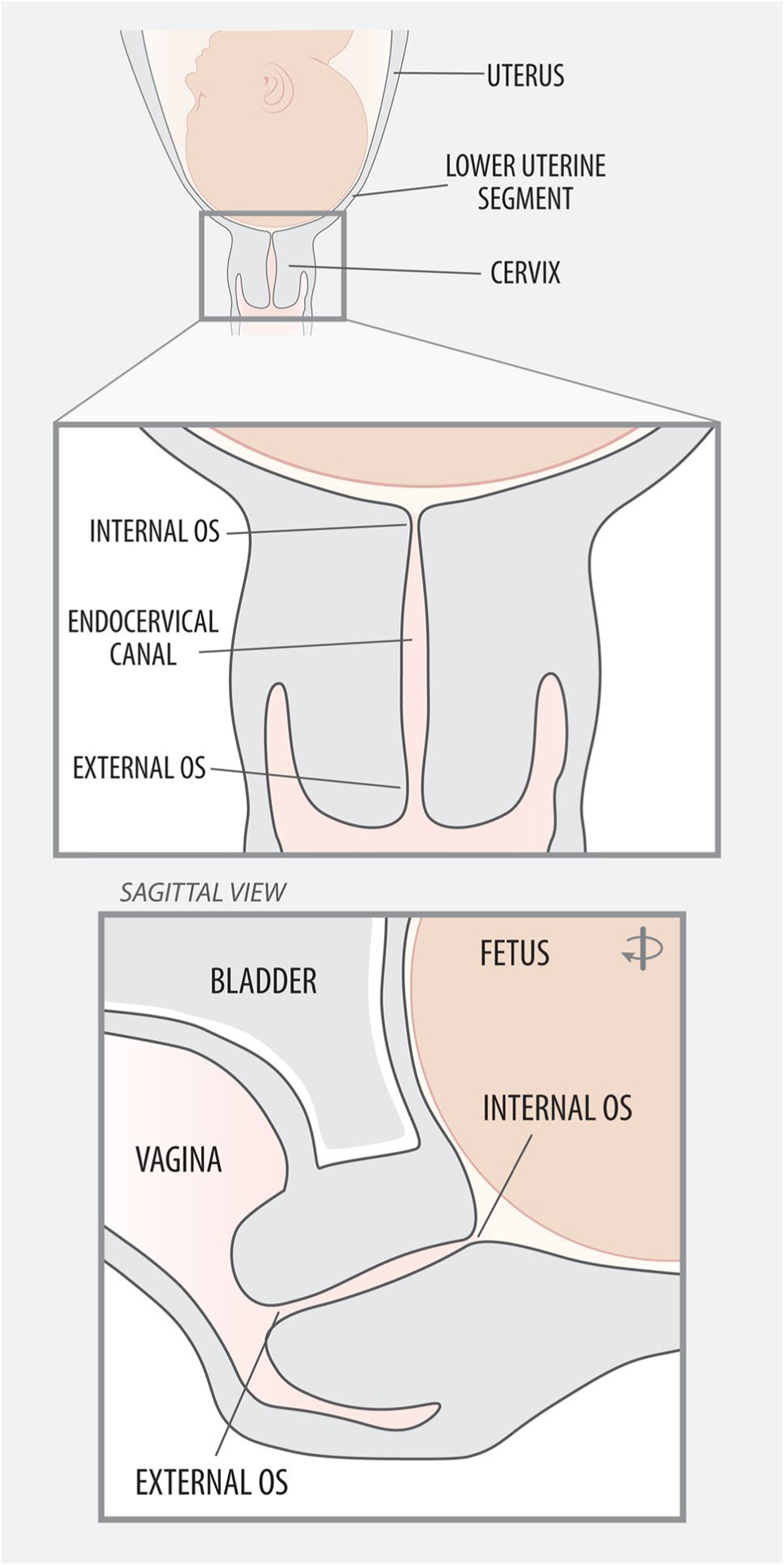
Cervical anatomy. There is growing evidence that cervical bio-mechanical properties and cellular composition (e.g., smooth muscle at the internal os) contribute to labor onset and progress[44]
Several processes might contribute to cervix-related labor dystocia. Cervical scarring with stenosis, typically following prior procedures (e.g., LEEP), can physically disrupt cervical dilation [50–52], especially in early labor even with strong uterine contractions. Later in the first stage of labor, persistent anterior cervical lip can delay labor progress and progression to the second stage [53, 54]. Persistent anterior lip is often caused by fetal malposition or asymmetry; the cervix may become edematous and ultimately impede progress with pushing in the second stage [51, 53, 54]. The specific role of cervical preparation leading to labor dystocia has not been well appreciated. Identifying associations between cervical preparation and unplanned cesarean delivery could suggest new targets for labor intervention and prevention of labor dystocia.
Obesity and Maternal Metabolism
Obese women more often experience prolonged pregnancy [55], prolonged labor [56], and unplanned cesarean birth. Obesity appears to influence the physiology of parturition via altered uterine contractility [57], metabolic fatigue [58], and cervical ripening [59]. Clinically, obese women are more likely to undergo labor induction, artificial rupture of amniotic membranes, and augmentation with higher doses of exogenous oxytocin [60]. The physiologic mechanisms for these clinical observations are not well known. Obesity in pregnancy is associated with, decreased placental corticotropin releasing hormone (pCRH) at term [55], elevated leptin [61, 62], increased placental reactive oxygen species [58, 63, 64], and decreased ATP production in the myometrium [58, 63, 64]. Obese women frequently have elevated cholesterol, which inhibits uterine contractions [65, 66]. Table 3 describes effects of obesity on labor physiology, clinical observations for laboring obese women, and possible pathophysiologic mechanisms linking obesity and disordered labor [58, 67]. Because abnormal maternal metabolism due to obesity influences normal parturition biology, it could be the cause of some slow labor [58]. Before proceeding with usual labor dystocia routines, recent work suggests that additional time is needed for obese parturients to achieve adequate cervical dilation and fetal descent leading to vaginal birth [56, 60].
Table 3.
Physiologic and clinical effects of obesity on labor
| Effect of obesity on labor physiology | Clinical observation | Possible physiologic mechanism |
|---|---|---|
| Disruption of endocrine signaling initiating labor onset | Delayed onset of parturition | Decreased CRH leading to inhibited functional progesterone withdrawal[55] |
| Decreased cervical ripening | Elevated leptin disrupts collagen degradation and cervical cell apoptosis[61, 62] | |
| Decreased spontaneous rupture of membranes | Elevated leptin inhibits membrane apoptosis[61, 62] | |
| Disruption of uterine contractility | Inadequate uterine contractions leading to increased risk for active phase cesarean | Elevated leptin, visfatin, and apelin inhibit spontaneous and oxytocin augmented uterine contractility in rats in vitro[43, 61, 62] |
| Cholesterol inhibits spontaneous and oxytocin augmented uterine contractility in vitro[65, 66] | ||
| Hypercholesterolemia activates potassium channels and may have effects on estrogen receptors and oxytocin receptors[139, 140] | ||
| Increased required oxytocin doses | Mechanism is unknown, however BMI is not associated with oxytocin receptor gene expres- sion[141, 142] | |
| Disruption of contraction synchronization | Irregular uterine contractions or decreased intrauterine pressure | Uterine biopsies show decreasing connexin-43 (gap junctions) mRNA in dystocia compared to normal labor[136] |
| Rats with high cholesterol diet showed lower con- nexin-43 expression although contractility was not examined[66, 143] | ||
| Metabolic fatigue | Inadequate uterine contractions with poor response to oxytocin augmentation | Increased BMI is associated with excess placental ROS and decreased ATP production[58, 63, 64] |
CRH corticotropin releasing hormone, BMI body mass index, mRNA messenger RNA, ATP adenosine triphosphate
Cephalopelvic Disproportion
Labor arrest is often attributed to cephalopelvic disproportion (CPD), a maternal–fetal mismatch in which the fetal head is too large for the maternal pelvis. There is no reliable way to objectively assess CPD prior to labor [68]. CPD differs from fetal malposition in which the presenting fetal part has an increased diameter that impedes labor progress and sometimes prevents vaginal birth [69]. True CPD during labor, although difficult to diagnose clinically, is not modifiable and requires cesarean birth. Some instances of fetal malposition (particularly occiput posterior, suboptimal fetal flexion and rotation, and asynclitism) can resolve allowing for vaginal birth.
Maternal risks for CPD include short maternal stature, maternal diabetes, increased maternal age, increased weight gain in pregnancy, personal history of CPD, and maternal pelvic deformity [68]. Studies to objectively assess maternal pelvic size using manual exam, ultrasonography, and radiographic pelvimetry have all failed to provide clinically actionable data. In the absence of a restrictive pelvic deformity or extreme variation in pelvic size or shape, there are no reliable tools for diagnosing inadequate pelvic outlet before labor or for predicting neonatal birth injury or maternal morbidities due to CPD [70, 71]. Additionally, CPD can only be diagnosed by comparing the maternal pelvis with the size of the fetus.
Fetal macrosomia (variously described as 4000–4500 g estimated fetal weight)[72] and fetal anomalies that increase the size of the fetal head are risks for CPD and associated complications such as shoulder dystocia, birth injury, perineal laceration, and postpartum hemorrhage [68]. Fundal height and sonographic fetal biometry in the third trimester are inexact measures of fetal size [68, 72]. At term, sonographic estimated fetal weight overdiagnoses macrosomia up to 70% of the time; in 20% of term pregnancies, the birth-weight is overestimated by more than 500 g [73]. Over 80% of neonates delivered by cesarean for suspected macrosomia weigh less than 4500 g, suggesting that some cesarean operations were unnecessary or the indication of macrosomia was incorrect. [73].
In the absence of other more effective predictors of vaginal delivery success, ACOG recommends planned cesarean only for estimated fetal weight over 5000 g (or 4500 g in diabetic patients). The benefit of planned cesarean delivery for fetal macrosomia is based on expert opinion and requires careful discussion of the risks and benefits of vaginal or cesarean delivery with the patient [72]. Based on currently available evidence, the best test of feto-pelvic adequacy is an adequate trial of labor [68, 72].
Fetal Malposition
Successful labor and vaginal birth require appropriate fetal positioning to navigate the maternal pelvic inlet and outlet. The fetal cardinal movements of labor permit a term fetus to negotiate the maternal pelvis with the smallest fetal dimensions and greatest opportunity for vaginal delivery. Fetal malposition means the presenting fetal part enters the pelvis at larger than optimal diameter. It can be caused by asymmetry of maternal pelvic soft tissue, irregular maternal pelvic shape, or fetal anomalies [74]. Fetal malposition frequently leads to increased pain in labor, increased labor duration, and increased risk for unplanned cesarean delivery [51, 69, 75].
The ideal position for delivery is occiput anterior when the back of the fetal head is oriented to the maternal abdomen. The most common fetal malposition is occiput posterior when the back of the fetal head is toward the maternal back and is a topic of study for interventions to increase vaginal delivery. Women with risks such as African American race, nulliparity, advanced maternal age, macrosomia, and post-term pregnancy are more likely to have a term singleton cephalic fetus in the occiput posterior position [76]. Persistent occiput posterior position is associated with increased epidural administration, artificial rupture of membranes, chorioamnionitis, operative vaginal birth, perineal injury, and unplanned cesarean birth [76]. Resolution of occiput posterior fetal position can sometimes be facilitated by maternal position changes or manual rotation of the fetal head [69]. Irregular contractions and contraction coupling have been described in association with fetal malposition; [51] however, in a small nested case–control study, intrauterine pressure (mm Hg) was not lower in the presence of persistent occiput posterior positioning, indicating that reduced uterine contractility probably does not contribute to labor dystocia due to fetal malposition [77].
Brow and face presentations each account for just 0.1–0.2% of births and are associated with prolonged labor, increased need for cesarean birth, and increased risk for neonatal injury [74, 78]. Brow and face presentations necessitate close monitoring and management especially when labor is prolonged [74, 78, 79].
Intrauterine Infection
Intrauterine infection, or chorioamnionitis, is associated with labor dystocia, unplanned cesarean delivery, and postpartum uterine atony [80–83]. Two hours after chorioamnionitis diagnosis, laboring women demonstrated decreased uterine contractile force as measured by an intrauterine pressure catheter [84]. Diminished uterine contractility was directly associated with risk for unplanned cesarean, establishing links between intrauterine infection, labor dystocia, and vaginal delivery success [84]. Intrauterine infection influences contractility via inflammation signals, especially inflammatory cytokines such as IL-1β and TNF-α which can inhibit oxytocin receptor expression [84–87]. Additionally, chorioamnionitis increases uterine expression of matrix metalloproteinases causing myometrial extracellular matrix remodeling and altered contractility [84, 88, 89]. Long labors are also associated with increased risk for chorioamnionitis, perhaps due to increased cervical exams and extended rupture of membranes [90, 91], suggesting that labor management can influence the risk for labor dystocia.
Maternal Stress
Fear of childbirth is associated with longer labor duration and increased risk of augmentation, assisted vaginal birth, and cesarean [92]. Maternal stress with elevated epinephrine levels inhibits uterine contractility and lengthens labor overall [93]. Immigrants, younger and older women (< 20 years or > 32 years at the time of first birth), and women with low social support are at higher risk for fear and stress during childbirth [94]. A history of trauma or abuse especially within the health care system or during a previous labor/birth exacerbates the stress/labor association [94]. In addition, apprehension about fetal descent through the birth canal, perhaps related to maternal concern about perineal lacerations, inhibits maternal pushing efforts during the second stage. The role of maternal stress in regulating parturition physiology is not well explained, but might contribute to women’s overall experience of prolonged labor [95].
Prevention, Diagnosis, and Management of Labor Dystocia
Prevention of Labor Dystocia
Targeting common causes of labor dystocia has been somewhat successful in preventing unplanned cesarean. The following are suggested prevention measures:
Clinical tolerance for labor dystocia in the presence of maternal and fetal well-being [1, 18, 19] and
Continuous labor support [100]
To reduce fetal macrosomia, careful management of maternal diabetes [101, 102], maternal exercise throughout pregnancy [96, 103], and induction of labor at 39 weeks[97, 104] have been studied. Early identification of pregestational and gestational diabetes and appropriate maternal glucose [101, 105] and lipid [102] control can decrease the rate of fetal macrosomia in women with diabetes. Lifestyle interventions to prevent macrosomia such as prenatal exercise can reduce maternal gestational weight gain, but meta-analysis showed no difference in fetal macrosomia, birth weight, or cesarean birth rate for obese women on a prenatal diet or exercise intervention compared with usual care; exercise may be effective, however, in normal weight women [96, 103]. Induction of labor at 39 weeks appears to reduce the risk of unplanned cesarean in nulliparous women, possibly due to lower fetal growth/size [97, 104]. However, induction of labor increases labor interventions, including increased length of labor and operative vaginal birth, and may not be preferred by some patients [97, 104]. A targeted decision about labor induction based on individual risks, benefits, and patient values is recommended.
“Active management of labor” was proposed in Ireland in the 1970s as a measure to reduce labor dystocia and decrease cesarean rates [99]. The original model of active management included objective diagnosis of labor, early amniotomy (artificial rupture of membranes), oxytocin augmentation when the cervix is dilating less than 1 cm per hour, one-to-one nursing or midwifery care, and cesarean 12 h after the onset of active labor unless birth is imminent [99]. Among nulliparous women with actively managed labor with a term singleton vertex fetus in Dublin, the cesarean rate was 5.2% (about half for arrest of labor and half for fetal intolerance of labor), and the rate of assisted vaginal births (forceps and/or vacuum) was 19.5% [98, 99]. In the USA, active management protocols reduced the length of labor but not the cesarean delivery rate [106, 107]. The overall impact of active management is therefore unclear and depends on population, medical resources, and clinical setting.
Current labor management recommendations in the USA contrast with active management protocols, emphasizing tolerance of slow labor as long as maternal and fetal monitoring are reassuring [1, 10]. ACOG and SMFM recommend waiting on cesarean birth for women without cervical dilation until the cervix is at least 6 cm dilated, membranes are ruptured, and there has been 4 h of adequate uterine activity (200 Montevideo units) or 6 h of oxytocin administration with inadequate uterine activity [1]. Extending the previously recommended 2 hour augmentation of labor with arrest to 4–6 h before moving to unplanned cesarean can increase the proportion of safe successful vaginal births [18, 19]. While not adequately powered to detect differences in fetal outcomes, studies of these wait-and-see recommendations do not indicate worse fetal outcomes [1, 18, 19]. The optimal time before moving to cesarean birth using tolerant labor curves is unknown, and the outer acceptable limits of labor progress in the context of modern, high-resource maternity care has not been determined. Integrated midwifery care reduces the rate of unplanned cesarean birth [108–111]. Cesarean rates among patients cared for by certified nurse midwives or in an integrated physician-midwifery model are lower than for patients cared for by physician obstetricians alone [109, 112] perhaps due to differences in labor management or by allowing for longer labor curves.
Continuous labor support (e.g., doula attendance) shows some promise in reducing unplanned cesarean delivery due to labor dystocia. Continuous labor support was part of the original active management of labor protocols [98, 99, 106]. In a systematic review, continuous labor support resulted in shorter labor length and lower risk of unplanned cesarean although the evidence quality was low and mechanisms are not well explained [100]. Support is hypothesized to affect labor through maternal stress reduction [51, 92, 94, 113]. Race-concordant doula care has also been proposed to reduce racial disparities in birth outcomes by promoting trust, agency, and culturally relevant social support [114].
Diagnosis of Labor Dystocia
Labor dystocia is often diagnosed based on the rate of cervical dilation. Cervical change less than 1 cm every 1 or 2 h is a common clinical threshold for initiating oxytocin augmentation. However, use of a labor partograph can improve dystocia diagnostic accuracy. Labor partographs clinically document labor progress over time[115, 116] and identify individual labor curves that are within the 95th percentile for cervical dilation and fetal descent [16, 115]. Clinically, a partograph can help to identify women for whom no additional treatment is immediately indicated and to prevent the overdiagnosis of labor dystocia [115], potentially reducing unplanned cesareans [117]. BMI-specific partographs have also been created, recognizing slower labor progression in obese women [56]. The diagnosis of labor dystocia focusses on the outcome (slow dilation or descent) and not on the underlying cause of the altered labor trajectory, which has implications for the identification and investigation of dystocia treatments.
Managing Labor Dystocia
Once labor dystocia has been identified, augmentation of uterine contractions with exogenous oxytocin infusion is the primary clinical management. There is poorly understood variation in the maternal response to oxytocin augmentation and no strong predictors of individual response. Uterine activity measured via intrauterine pressure catheter guides titration of oxytocin augmentation [1]. While sometimes debated, 200 Montevideo units (calculated as the sum of the change in uterine pressure for each contraction occurring in a 10-min segment) is the most common threshold for adequate uterine contractions sufficient for cervical progress in augmented labor [1, 118, 119].
Multiple oxytocin dosing regimens have been evaluated, but none have emerged as clearly superior in preventing cesarean birth [120]. High-dose oxytocin regimens (starting at 4 mu/min and increasing incrementally by 4 mu/min every 30 min) reduce labor by 1.5 to 2 h compared to low-dose regimens (starting at 1–2 mu/min and increasing incrementally by 1–2 mu/min every 30 min), but the unplanned cesarean rate is unaffected [20, 121, 122]. Systematic review of three small randomized trials comparing treatment of slow labor with oxytocin augmentation versus no treatment found no difference in the cesarean rate [20].
Amniotomy combined with oxytocin augmentation is frequently used to shorten the length of labor [99]. A systematic review found that compared to routine care, earlier use of preventive oxytocin augmentation with amniotomy, regardless of labor progress, modestly reduced cesarean rates [122]. However, it did not reduce cesareans when used after diagnosis of labor dystocia [122].
In addition to augmenting uterine contraction frequency and strength, oxytocin can sometimes resolve dystocia from other causes. For example, stimulating strong, regular contractions can potentially resolve malposition by encouraging flexion of the fetal head [123]. Oxytocin can also facilitate contractility caused by myometrial acidification [124]. In other cases, oxytocin appears to have the opposite effect and exacerbate dystocia by promoting uterine fatigue [41, 57]. Variation in response to oxytocin augmentation might be elucidated by better understanding of the underlying pathophysiologic cause of dystocia. Expected actions of oxytocin for different causes of dystocia are listed in Table 4.
Table 4.
Effects of exogenous oxytocin by cause of labor dystocia
| Phenotype | Anticipated response to oxytocin augmentation | Possible negative effects of oxytocin ugmentation |
|---|---|---|
| Signaling issues/reduced uterine contraction preparation | Expected to increase contraction force, frequency, duration, and coordination Increases gap junction formation |
No response from a molecularly unprepared uterus |
| Relative CPD/fetal malposition | Expected to increase contraction force and coordination Increased contractions encourages fetal rotation and flexion of the fetal head |
Overtime if the malposition does not resolve and in the case of true CPD, uterus may fatigue from ineffective contractions despite augmentation |
| Infection and metabolic fatigue | In the presence of mild fatigue processes, oxytocin augmentation increases contractility Depending on the level of fatigue, cervical dilation may progress sufficiently to allow for vaginal birth |
If infection or fatigue is unresolved, oxytocin augmentation may exacerbate fatigue processes and decrease contractility Increased risk of postpartum hemorrhage |
CPD cephalopelvic disproportion
Other treatments to improve uterine contractility and labor progress include intravenous hydration [125], glucose administration [126], and oral consumption of food and fluids [127, 128]. Higher intravenous fluid infusion (250 ml/h vs 150 ml/h) reduces unplanned cesarean birth and shortens labor especially in women with oral intake restrictions [125]. Intravenous fluids with dextrose are associated with shorter first stage of labor, but no change in total labor duration or cesarean rate [126]. Oral intake of food, water, and carbohydrate drinks reduces labor duration moderately but did not change rates of cesarean birth in two large systematic reviews [127, 128]. Oral sodium bicarbonate supplementation reduces amniotic fluid lactate in women with dystocia [129], but without significant change in mode of delivery [130]. Although data on intravenous and oral supplementation are heterogenous with small effect sizes for labor outcomes, differences could be more apparent if the interventions were targeted toward women with uterine metabolic fatigue rather than implemented for all women in labor.
Addressing Current Knowledge Gaps
In high-resource settings such as the USA, the rate of cesarean birth surpasses the World Health Organization cesarean goal of 10–15% above which there is not convincing evidence of maternal or fetal benefit [9, 131, 132]. Despite efforts to reduce the rate of cesarean birth in the USA, the overall cesarean rate was 31.8% in 2020, and the low-risk cesarean birth rate for nulliparous, term, singleton, vertex labors was 25.9% [12], well above the goal set by Healthy People 2020 to reduce low-risk cesareans [11]. As emphasized by both ACOG and SMFM, improving labor dystocia management is an opportunity to improve vaginal delivery success.
We have highlighted the existing research on the varied causes of labor dystocia, including incomplete uterine preparation, inadequate uterine force generation, incomplete cervical ripening, obesity, cephalopelvic disproportion, fetal malposition, infection, and maternal stress. Additional study in each of these etiologies is needed to better understand the physiologic mechanisms of labor dystocia and to develop new clinical approaches. Areas of particular interest could include:
Identify pathophysiology-based care to prevent labor dystocia. Can we better diagnose labor dystocia and use that information to individualize labor care? Are there unidentified mechanisms of labor dystocia due to cervical, uterine, placental, or fetal abnormalities?
Understand the relationship between fetal malposition and uterine contractility. Does fetal position influence uterine or cervical force generation? Does poor contractility cause malposition as a side effect of inadequate or disorganized uterine tone?
Determine the causal association between elevated amniotic fluid lactate and labor dystocia. Does extracellular amniotic fluid lactate represent myometrial hypoxia and metabolic dysfunction? In what situations does myometrial and/or amniotic fluid lactate accumulate? Can amniotic fluid lactate (or other metabolic markers) be used to diagnose specific types of labor dystocia?
Investigate preventive prenatal measures to enhance vaginal delivery success. Are there specific exercises for pregnancy and labor that can encourage optimal fetal positioning [51, 69, 133]?
Study labor and delivery approaches to optimize labor progress. Does treatment with sodium bicarbonate, intravenous fluid, or glucose for specific women with labor dystocia reduce unplanned cesarean rates [129]? Can those approaches be personalized depending on the underlying mechanism of labor dystocia?
Develop improved diagnostic criteria for labor arrest and indications for unplanned cesarean delivery. Can we build on known clinical approaches to improve delivery outcomes and vaginal delivery rates [134]? Is watchful patience the best approach and how can we encourage this behavior from the labor and delivery attendant?
Pathophysiologic Variations and Personalized Therapy
Individualizing treatment based on pathophysiologic mechanisms may reduce risks, optimize care, and reduce unplanned cesareans. There are multiple treatment options for labor dystocia, including watchful waiting, symptom and pain management, and augmentation [97, 134]; however, gaps in the biological/translational literature and limited assessment technologies limit current opportunities for clinical individualization. Clinical and translational research that could alter diagnosis and therapy of labor dystocia should be prioritized as part of programs to increase the rate of successful vaginal birth. Targeted therapeutic strategies might optimize outcomes while reducing unnecessary risk.
Given the limited evidence on personalized care of labor, clinical decisions should weigh patient preference and risk tolerance against clinical safety and maternal/neonatal outcomes. Based on current evidence, watchful waiting is probably our best intervention for labor dystocia[1], consistent with current US national guidelines at least until alternate pathophysiology-based therapies are developed [1]. With careful monitoring of maternal and fetal well-being, support of maternal coping, appropriate pain management, and shared decision making, time and patience may be the best chance to improve the chance for a vaginal birth.
Funding
This work was supported by an F31 Ruth L. Kirschstein Pre-Doctoral Individual National Research Service Award from the National Institute of Nursing Research of the National Institutes of Health under award number FN31NR018582-01 (to KK).
Abbreviations
- ACNM
American College of Nurse Midwives
- ACOG
American College of Obstetricians and Gynecologists
- SMFM
Society for Maternal–Fetal Medicine
- CPD
Cephalopelvic disproportion
- ATP
Adenosine triphosophate
- pCRH
Placental corticotropin releasing hormone
Footnotes
Consent for Publication All authors consent to the publication of this work. No other consent is required.
Conflict of Interest The authors declare no competing interests.
Data Availability
All data and material are available upon request to the corresponding author.
References
- 1.Caughey AB, Cahill AG, Guise JM, Rouse DJ. Safe prevention of the primary cesarean delivery. Am J Obstet Gynecol. 2014;210(3):179–93. [DOI] [PubMed] [Google Scholar]
- 2.Gregory KD, Jackson S, Korst L, Fridman M. Cesarean versus vaginal delivery: whose risks? Whose benefits? Am J Perinatol. 2012;29(1):7–18. [DOI] [PubMed] [Google Scholar]
- 3.Declercq ER, Sakala C, Corry MP, Applebaum S, Herrlich A. Major survey findings of listening to mothers III: new mothers speak out: report of national surveys of women’s childbearing experiences. J Perinat Educ. 2014;23(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tichelman E, Westerneng M, Witteveen AB, van Baar AL, van der Horst HE, de Jonge A, et al. Correlates of prenatal and postnatal mother-to-infant bonding quality: a systematic review. PLoS ONE. 2019;14(9):e0222998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prior E, Santhakumaran S, Gale C, Philipps LH, Modi N, Hyde MJ. Breastfeeding after cesarean delivery: a systematic review and meta-analysis of world literature. Am J Clin Nutr. 2012;95(5):1113–35. [DOI] [PubMed] [Google Scholar]
- 6.Keag OE, Norman JE, Stock SJ. Long-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: systematic review and meta-analysis. PLoS Med. 2018;15(1):e1002494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 2014;63(4):559–66. [DOI] [PubMed] [Google Scholar]
- 8.Sandall J, Tribe RM, Avery L, Mola G, Visser GH, Homer CS, et al. Short-term and long-term effects of caesarean section on the health of women and children. Lancet. 2018;392(10155):1349–57. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. WHO Statement on Caesarean Section Rates. World Health Organization; 2015. Report No.: WHO/ RHR/15.02. Available at: https://apps.who.int/iris/bitstream/handle/10665/161442/WHO_RHR_15.02_eng.pdf. Accessed 11/10/2021 [Google Scholar]
- 10.Gams B, Neerland C, Kennedy S. Reducing primary cesareans: an innovative multipronged approach to supporting physiologic labor and vaginal birth. J Perinat Neonatal Nurs. 2019;33(1):52–60. [DOI] [PubMed] [Google Scholar]
- 11.Office of Disease Prevention and Health Promotion. Healthy people 2020: Maternal infant and child health. 2017. Available at: https://www.healthypeople.gov/2020/topics-objectives/topic/maternal-infant-and-child-health. Accessed 11/10/2021.
- 12.Hamilton BE, Martin JA, Osterman MJ, Office of Disease Prevention and Health Promotion. Births: Provisional Data for 2020. Vital Statistics Rapid Release; no 12. Hyattsville, MD: National Center for Health Statistics. 2021. [Google Scholar]
- 13.Barber EL, Lundsberg L, Belanger K, Pettker CM, Funai EF, Illuzzi JL. Contributing indications to the rising cesarean delivery rate. Obstet Gynecol. 2011;118(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Troendle J, Reddy UM, Laughon SK, Branch DW, Burkman R, et al. Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol. 2010;203(4):326 e1–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman E The graphic analysis of labor. Am J Obstet Gynecol. 1954;68(6):1568–75. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Landy HJ, Branch DW, Burkman R, Haberman S, Gregory KD, et al. Contemporary patterns of spontaneous labor with normal neonatal outcomes. Obstet Gynecol. 2010;116(6):1281–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lundborg L, Aberg K, Sandstrom A, Discacciati A, Tilden EL, Stephansson O, et al. First stage progression in women with spontaneous onset of labor: a large population-based cohort study. PLoS ONE. 2020;15(9):e0239724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rouse D Active-phase labor arrest: oxytocin augmentation for at least 4 hours. Obstet Gynecol. 1999;93(3):323–8. [DOI] [PubMed] [Google Scholar]
- 19.Rouse D Active phase labor arrest: revisiting the 2-hour minimum. Obstet Gynecol. 2001;98(4):550–4. [DOI] [PubMed] [Google Scholar]
- 20.Bugg GJ, Siddiqui F, Thornton JG. Oxytocin versus no treatment or delayed treatment for slow progress in the first stage of spontaneous labour. Cochrane Database Syst Rev. 2013;6:CD007123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neal JL, Lamp JM, Buck JS, Lowe NK, Gillespie SL, Ryan SL. Outcomes of nulliparous women with spontaneous labor onset admitted to hospitals in preactive versus active labor. J Midwifery Womens Health. 2014;59(1):28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neal JL, Lowe NK, Schorn MN, Holley SL, Ryan SL, Buxton M, et al. Labor dystocia: a common approach to diagnosis. J Midwifery Womens Health. 2015;60(5):499–509. [DOI] [PubMed] [Google Scholar]
- 23.Quenby S, Pierce SJ, Brigham S, Wray S. Dysfunctional labor and myometrial lactic acidosis. Obstet Gynecol. 2004;103(4):718–23. [DOI] [PubMed] [Google Scholar]
- 24.Parturition SR. N Engl J Med. 2007;356(3):271–83. [DOI] [PubMed] [Google Scholar]
- 25.Vannuccini S, Bocchi C, Severi FM, Challis JR, Petraglia F. Endocrinology of human parturition. Ann Endocrinol (Paris). 2016;77(2):105–13. [DOI] [PubMed] [Google Scholar]
- 26.Arrowsmith S, Kendrick A, Hanley JA, Noble K, Wray S. Myometrial physiology—time to translate? Exp Physiol. 2014;99(3):495–502. [DOI] [PubMed] [Google Scholar]
- 27.Tilden EL, Phillippi JC, Carlson N, Dissanayake M, Lee CS, Caughey AB, et al. The association between longer durations of the latent phase of labor and subsequent perinatal processes and outcomes among midwifery patients. Birth. 2020;47(4):418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aguilar HN, Mitchell BF. Physiological pathways and molecular mechanisms regulating uterine contractility. Hum Reprod Update. 2010;16(6):725–44. [DOI] [PubMed] [Google Scholar]
- 29.Noble K, Matthew A, Burdyga T, Wray S. A review of recent insights into the role of the sarcoplasmic reticulum and Ca entry in uterine smooth muscle. Eur J Obstet Gynecol Reprod Biol. 2009;144(Suppl 1):S11–9. [DOI] [PubMed] [Google Scholar]
- 30.Wray S, Arrowsmith S. Uterine excitability and ion channels and their changes with gestation and hormonal environment. Annu Rev Physiol. 2021;83:331–57. [DOI] [PubMed] [Google Scholar]
- 31.Tachi M, Kouzaki M, Kanehisa H, Fukunaga T. The influence of circulatory difference on muscle oxygenation and fatigue during intermittent static dorsiflexion. Eur J Appl Physiol. 2004;91(5–6):682–8. [DOI] [PubMed] [Google Scholar]
- 32.Wan JJ, Qin Z, Wang PY, Sun Y, Liu X. Muscle fatigue: general understanding and treatment. Exp Mol Med. 2017;49(10):e384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ortenblad N, Westerblad H, Nielsen J. Muscle glycogen stores and fatigue. J Physiol. 2013;591(18):4405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen DG, Lamb GD, Westerblad H. Impaired calcium release during fatigue. J Appl Physiol (1985). 2008;104(1):296–305. [DOI] [PubMed] [Google Scholar]
- 35.MacIntosh BR, Holash RJ, Renaud JM. Skeletal muscle fatigue—regulation of excitation-contraction coupling to avoid metabolic catastrophe. J Cell Sci. 2012;125(Pt 9):2105–14. [DOI] [PubMed] [Google Scholar]
- 36.Westerblad H, Bruton JD, Katz A. Skeletal muscle: energy metabolism, fiber types, fatigue and adaptability. Exp Cell Res. 2010;316(18):3093–9. [DOI] [PubMed] [Google Scholar]
- 37.Wiberg-Itzel E, Pembe AB, Jarnbert-Pettersson H, Norman M, Wihlback AC, Hoesli I, et al. Lactate in amniotic fluid: predictor of labor outcome in oxytocin-augmented primiparas’ deliveries. PLoS ONE. 2016;11(10):e0161546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiberg-Itzel E, Pettersson H, Andolf E, Hansson A, Winbladh B, Akerud H. Lactate concentration in amniotic fluid: a good predictor of labor outcome. Eur J Obstet Gynecol Reprod Biol. 2010;152(1):34–8. [DOI] [PubMed] [Google Scholar]
- 39.Wiberg-Itzel E, Pettersson H, Cnattingius S, Nordstrom L. Association between lactate concentration in amniotic fluid and dysfunctional labor. Acta Obstet Gynecol Scand. 2008;87(9):924–8. [DOI] [PubMed] [Google Scholar]
- 40.Murphy M, Butler M, Coughlan B, Brennan D, O’Herlihy C, Robson M. Elevated amniotic fluid lactate predicts labor disorders and cesarean delivery in nulliparous women at term. Am J Obstet Gynecol. 2015;213(5):673 e1–8. [DOI] [PubMed] [Google Scholar]
- 41.Wiberg-Itzel E, Pembe AB, Wray S, Wihlback AC, Darj E, Hoesli I, et al. Level of lactate in amniotic fluid and its relation to the use of oxytocin and adverse neonatal outcome. Acta Obstet Gynecol Scand. 2014;93(1):80–5. [DOI] [PubMed] [Google Scholar]
- 42.Guerra DD, Bok R, Breen K, Vyas V, Jiang H, MacLean KN, et al. Estrogen regulates local cysteine metabolism in mouse myometrium. Reprod Sci. 2021;28(1):79–90. [DOI] [PubMed] [Google Scholar]
- 43.Vyas V, Guerra DD, Bok R, Powell T, Jansson T, Hurt KJ. Adiponectin links maternal metabolism to uterine contractility. FASEB J. 2019;33(12):14588–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vink J, Myers K. Cervical alterations in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2018;52:88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yellon SM. Contributions to the dynamics of cervix remodeling prior to term and preterm birth. Biol Reprod. 2017;96(1):13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Menon R, Bonney EA, Condon J, Mesiano S, Taylor RN. Novel concepts on pregnancy clocks and alarms: redundancy and synergy in human parturition. Hum Reprod Update. 2016;22(5):535–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Timmons B, Akins M, Mahendroo M. Cervical remodeling during pregnancy and parturition. Trends Endocrinol Metab. 2010;21(6):353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vrhovec J Evaluating the progress of the labour with sample entropy calculated from the uterine EMG activity. Elektrotehniski vestnik: Ljubljana, Slovenia. 2009;76(4):165–70. [Google Scholar]
- 49.Santoso AP, Vink JY, Gallos G, Feltovich H, Hall TJ. Quantitative ultrasound detects smooth muscle activity at the cervical internal os in vitro. Ultrasound Med Biol. 2020;46(1):149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hay R, Patterson J. Effects of cervical excisional procedures for cervical intraepithelial neoplasia on pregnancy and birth: a literature review. N Z Coll Midwives J. 2018;54:22–9. [Google Scholar]
- 51.Simkin P, Hanson L, Ancheta R. The labor progress handbook: early interventions to prevent and treat dystocia: John Wiley & Sons, Hoboken, NJ; 2017;978 1119170464 [Google Scholar]
- 52.Houlard S, Perrotin F, Fourquet F, Marret H, Lansac J, Body G. Risk factors for cervical stenosis after laser cone biopsy. Eur J Obstet Gynecol Reprod Biol. 2002;104(2):144–7. [DOI] [PubMed] [Google Scholar]
- 53.Hofmeyr GJ, Singata-Madliki M. The second stage of labor. Best Pract Res Clin Obstet Gynaecol. 2020;67:53–64. [DOI] [PubMed] [Google Scholar]
- 54.Hanson L Second-stage labor care: challenges in spontaneous bearing down. J Perinat Neonatal Nurs. 2009;23(1):31–9; quiz 40–1. [DOI] [PubMed] [Google Scholar]
- 55.Denison FC, Price J, Graham C, Wild S, Liston WA. Maternal obesity, length of gestation, risk of postdates pregnancy and spontaneous onset of labour at term. BJOG. 2008;115(6):720–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kominiarek MA, Zhang J, Vanveldhuisen P, Troendle J, Beaver J, Hibbard JU. Contemporary labor patterns: the impact of maternal body mass index. Am J Obstet Gynecol. 2011;205(3):244 e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wray S Insights from physiology into myometrial function and dysfunction. Exp Physiol. 2015;100(12):1468–76. [DOI] [PubMed] [Google Scholar]
- 58.Carlson NS, Hernandez TL, Hurt KJ. Parturition dysfunction in obesity: Time to target the pathobiology. Reprod Biol Endocrinol. 2015;13:135–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zelig CM, Nichols SF, Dolinsky BM, Hecht MW, Napolitano PG. Interaction between maternal obesity and Bishop score in predicting successful induction of labor in term, nulliparous patients. Am J Perinatol. 2013;30(1):75–80. [DOI] [PubMed] [Google Scholar]
- 60.Carlson NS, Lowe NK. Intrapartum management associated with obesity in nulliparous women. J Midwifery Womens Health. 2014;59(1):43–53. [DOI] [PubMed] [Google Scholar]
- 61.Moynihan AT, Hehir MP, Glavey SV, Smith TJ, Morrison JJ. Inhibitory effect of leptin on human uterine contractility in vitro. Am J Obstet Gynecol. 2006;195(2):504–9. [DOI] [PubMed] [Google Scholar]
- 62.Mumtaz S, AlSaif S, Wray S, Noble K. Inhibitory effect of visfatin and leptin on human and rat myometrial contractility. Life Sci. 2015;125:57–62. [DOI] [PubMed] [Google Scholar]
- 63.Frias AE, Morgan TK, Evans AE, Rasanen J, Oh KY, Thornburg KL, et al. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology. 2011;152(6):2456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mele J, Muralimanoharan S, Maloyan A, Myatt L. Impaired mitochondrial function in human placenta with increased maternal adiposity. Am J Physiol Endocrinol Metab. 2014;307(5):E419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shmygol A, Noble K, Wray S. Depletion of membrane cholesterol eliminates the Ca2+-activated component of outward potassium current and decreases membrane capacitance in rat uterine myocytes. J Physiol. 2007;581(Pt 2):445–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang J, Kendrick A, Quenby S, Wray S. Contractility and calcium signaling of human myometrium are profoundly affected by cholesterol manipulation: implications for labor? Reprod Sci. 2007;14(5):456–66. [DOI] [PubMed] [Google Scholar]
- 67.Poobalan AS, Aucott LS, Gurung T, Smith WC, Bhattacharya S. Obesity as an independent risk factor for elective and emergency caesarean delivery in nulliparous women—systematic review and meta-analysis of cohort studies. Obes Rev. 2009;10(1):28–35. [DOI] [PubMed] [Google Scholar]
- 68.Rossi AC, Mullin P, Prefumo F. Prevention, management, and outcomes of macrosomia: a systematic review of literature and meta-analysis. Obstet Gynecol Surv. 2013;68(10):702–9. [DOI] [PubMed] [Google Scholar]
- 69.Simkin P The fetal occiput posterior position: state of the science and a new perspective. Birth. 2010;37(1):61–71. [DOI] [PubMed] [Google Scholar]
- 70.Lenhard MS, Johnson TR, Weckbach S, Nikolaou K, Friese K, Hasbargen U. Pelvimetry revisited: analyzing cephalopelvic disproportion. Eur J Radiol. 2010;74(3):e107–11. [DOI] [PubMed] [Google Scholar]
- 71.Rizzo G, Aiello E, Bosi C, D’Antonio F, Arduini D. Fetal head circumference and subpubic angle are independent risk factors for unplanned cesarean and operative delivery. Acta Obstet Gynecol Scand. 2017;96(8):1006–11. [DOI] [PubMed] [Google Scholar]
- 72.Committee on Practice B-O. Macrosomia: ACOG Practice Bulletin, Number 216. Obstet Gynecol. 2020;135(1):e18–35. [DOI] [PubMed] [Google Scholar]
- 73.Zafman KB, Bergh E, Fox NS. Accuracy of sonographic estimated fetal weight in suspected macrosomia: the likelihood of overestimating and underestimating the true birthweight. J Matern Fetal Neonatal Med. 2020;33(6):967–72. [DOI] [PubMed] [Google Scholar]
- 74.Pilliod RA, Caughey AB. Fetal malpresentation and malposition: diagnosis and management. Obstet Gynecol Clin North Am. 2017;44(4):631–43. [DOI] [PubMed] [Google Scholar]
- 75.Tully G Understanding Labor Patterns: Spinning Babies; 2022. Available from: https://www.spinningbabies.com/pregnancy-birth/labor/understanding-labor-patterns/. Accessed 11/10/2021.
- 76.Cheng YW, Shaffer BL, Caughey AB. Associated factors and outcomes of persistent occiput posterior position: a retrospective cohort study from 1976 to 2001. J Matern Fetal Neonatal Med. 2006;19(9):563–8. [DOI] [PubMed] [Google Scholar]
- 77.Buhimschi CS, Buhimschi IA, Malinow AM, Weiner CP. Uterine contractility in women whose fetus is delivered in the occipitoposterior position. Am J Obstet Gynecol. 2003;188(3):734–9. [DOI] [PubMed] [Google Scholar]
- 78.Gardberg M, Leonova Y, Laakkonen E. Malpresentations—impact on mode of delivery. Acta Obstet Gynecol Scand. 2011;90(5):540–2. [DOI] [PubMed] [Google Scholar]
- 79.Makajeva J, Ashraf M. Delivery, Face and Brow Presentation: StatPearls Publishing; 2022. [PubMed]
- 80.Bommarito KM, Gross GA, Willers DM, Fraser VJ, Olsen MA. The effect of clinical chorioamnionitis on cesarean delivery in the United States. Health Serv Res. 2016;51(5):1879–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fan S-R, Liu P, Yan S-M, Peng J-Y, Liu X-P. Diagnosis and management of intraamniotic infection. Maternal-Fetal Medicine. 2020;2(4):223–30. [Google Scholar]
- 82.Peng CC, Chang JH, Lin HY, Cheng PJ, Su BH. Intrauterine inflammation, infection, or both (Triple I): a new concept for chorioamnionitis. Pediatr Neonatol. 2018;59(3):231–7. [DOI] [PubMed] [Google Scholar]
- 83.Conde-Agudelo A, Romero R, Jung EJ, Garcia Sanchez AJ. Management of clinical chorioamnionitis: an evidence-based approach. Am J Obstet Gynecol. 2020;223(6):848–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zackler A, Flood P, Dajao R, Maramara L, Goetzl L. Suspected chorioamnionitis and myometrial contractility: mechanisms for increased risk of cesarean delivery and postpartum hemorrhage. Reprod Sci. 2019;26(2):178–83. [DOI] [PubMed] [Google Scholar]
- 85.Rauk PN, Friebe-Hoffmann U. Interleukin-1 beta down-regulates the oxytocin receptor in cultured uterine smooth muscle cells. Am J Reprod Immunol. 2000;43(2):85–91. [DOI] [PubMed] [Google Scholar]
- 86.Leroy MJ, Dallot E, Czerkiewicz I, Schmitz T, Breuiller-Fouche M. Inflammation of choriodecidua induces tumor necrosis factor alpha-mediated apoptosis of human myometrial cells. Biol Reprod. 2007;76(5):769–76. [DOI] [PubMed] [Google Scholar]
- 87.Gomez-Lopez N, Romero R, Xu Y, Garcia-Flores V, Leng Y, Panaitescu B, et al. Inflammasome assembly in the chorioamniotic membranes during spontaneous labor at term. Am J Reprod Immunol. 2017;77(5):e.12648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lirussi F, O’Brien M, Wendremaire M, Goirand F, Sagot P, Dumas M, et al. SAR150640, a selective beta3-adrenoceptor agonist, prevents human myometrial remodelling and activation of matrix metalloproteinase in an in vitro model of chorioamnionitis. Br J Pharmacol. 2010;159(6):1354–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oner C, Schatz F, Kizilay G, Murk W, Buchwalder LF, Kayisli UA, et al. Progestin-inflammatory cytokine interactions affect matrix metalloproteinase-1 and −3 expression in term decidual cells: implications for treatment of chorioamnionitis-induced preterm delivery. J Clin Endocrinol Metab. 2008;93(1):252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Seaward PG, Hannah ME, Myhr TL, Farine D, Ohlsson A, Wang EE, et al. International Multicentre Term Prelabor Rupture of Membranes Study: evaluation of predictors of clinical chorioamnionitis and postpartum fever in patients with prelabor rupture of membranes at term. Am J Obstet Gynecol. 1997;177(5):1024–9. [DOI] [PubMed] [Google Scholar]
- 91.Imseis HM, Trout WC, Gabbe SG. The microbiologic effect of digital cervical examination. Am J Obstet Gynecol. 1999;180(3):578–80. [DOI] [PubMed] [Google Scholar]
- 92.Adams SS, Eberhard-Gran M, Eskild A. Fear of childbirth and duration of labour: a study of 2206 women with intended vaginal delivery. BJOG. 2012;119(10):1238–46. [DOI] [PubMed] [Google Scholar]
- 93.Lederman RP, Lederman E, Work BA Jr, McCann DS. The relationship of maternal anxiety, plasma catecholamines, and plasma cortisol to progress in labor. Am J Obstet Gynecol. 1978;132(5):495–500. [DOI] [PubMed] [Google Scholar]
- 94.Dencker A, Nilsson C, Begley C, Jangsten E, Mollberg M, Patel H, et al. Causes and outcomes in studies of fear of childbirth: a systematic review. Women Birth. 2019;32(2):99–111. [DOI] [PubMed] [Google Scholar]
- 95.Kissler K, Jones J, McFarland AK, Luchsinger J. A qualitative meta-synthesis of women’s experiences of labor dystocia. Women Birth. 2020;33(4):e332–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oteng-Ntim E, Varma R, Croker H, Poston L, Doyle P. Lifestyle interventions for overweight and obese pregnant women to improve pregnancy outcome: systematic review and meta-analysis. BMC Med. 2012;10:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grobman WA, Rice MM, Reddy UM, Tita ATN, Silver RM, Mallett G, et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379(6):513–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bohra U, Donnelly J, O’Connell MP, Geary MP, MacQuillan K, Keane DP. Active management of labour revisited: the first 1000 primiparous labours in 2000. J Obstet Gynaecol. 2003;23(2):118–20. [DOI] [PubMed] [Google Scholar]
- 99.O’Driscoll K, Stronge JM, Minogue M. Active management of labour. Br Med J. 1973;3(5872):135–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bohren MA, Hofmeyr GJ, Sakala C, Fukuzawa RK, Cuthbert A. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2017;7:CD003766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bastidas K, Romero XC, Uriel M, De la Hoz JA. Perinatal outcomes associated with the diagnosis of gestational diabetes: systematic review and meta-analysis. Diabetes Metab Syndr. 2021;15(5):102262. [DOI] [PubMed] [Google Scholar]
- 102.Barbour LA, Hernandez TL. Maternal non-glycemic contributors to fetal growth in obesity and gestational diabetes: spotlight on lipids. Curr Diab Rep. 2018;18(6):37. [DOI] [PubMed] [Google Scholar]
- 103.Barakat R, Franco E, Perales M, Lopez C, Mottola MF. Exercise during pregnancy is associated with a shorter duration of labor. A randomized clinical trial. Eur J Obstet Gynecol Reprod Biol. 2018;224:33–40. [DOI] [PubMed] [Google Scholar]
- 104.Souter V, Painter I, Sitcov K, Caughey AB. Maternal and new-born outcomes with elective induction of labor at term. Am J Obstet Gynecol. 2019;220(3):273 e1–11. [DOI] [PubMed] [Google Scholar]
- 105.Sweeting A, Mijatovic J, Brinkworth GD, Markovic TP, Ross GP, Brand-Miller J, et al. The Carbohydrate Threshold in Pregnancy and Gestational Diabetes: How Low Can We Go? Nutrients. 2021;13(8):2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Frigoletto FD Jr, Lieberman E, Lang JM, Cohen A, Barss V, Ringer S, et al. A clinical trial of active management of labor. N Engl J Med. 1995;333(12):745–50. [DOI] [PubMed] [Google Scholar]
- 107.Lopez-Zeno JA, Peaceman AM, Adashek JA, Socol ML. A controlled trial of a program for the active management of labor. N Engl J Med. 1992;326(7):450–4. [DOI] [PubMed] [Google Scholar]
- 108.Carlson NS, Corwin EJ, Hernandez TL, Holt E, Lowe NK, Hurt KJ. Association between provider type and cesarean birth in healthy nulliparous laboring women: a retrospective cohort study. Birth. 2018;45(2):159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Souter V, Nethery E, Kopas ML, Wurz H, Sitcov K, Caughey AB. Comparison of midwifery and obstetric care in low-risk hospital births. Obstet Gynecol. 2019;134(5):1056–65. [DOI] [PubMed] [Google Scholar]
- 110.Carlson NS, Breman R, Neal JL, Phillippi JC. Preventing cesarean birth in women with obesity: influence of unit-level midwifery presence on use of cesarean among women in the consortium on safe labor data set. J Midwifery Womens Health. 2020;65(1):22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fore MS, Allshouse AA, Carlson NS, Hurt KJ. Outcomes of trial of labor after cesarean birth by provider type in low-risk women. Birth. 2020;47(1):123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Smith DC, Phillippi JC, Lowe NK, Breman RB, Carlson NS, Neal JL, et al. Using the Robson 10-group classification system to compare cesarean birth utilization between US centers with and without midwives. J Midwifery Womens Health. 2020;65(1):10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dweik D, Girasek E, Meszaros G, Toreki A, Kereszturi A, Pal A. Non-medical determinants of cesarean section in a medically dominated maternity system. Acta Obstet Gynecol Scand. 2014;93(10):1025–33. [DOI] [PubMed] [Google Scholar]
- 114.Kozhimannil KB, Vogelsang CA, Hardeman RR, Prasad S. Disrupting the pathways of social determinants of health: doula support during pregnancy and childbirth. J Am Board Fam Med. 2016;29(3):308–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Neal JL, Lowe NK. Physiologic partograph to improve birth safety and outcomes among low-risk, nulliparous women with spontaneous labor onset. Med Hypotheses. 2012;78(2):319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.WHO. World Health Organization partograph in management of labour. Lancet. 1994;343(8910):1399–404. [PubMed] [Google Scholar]
- 117.Neal JL, Lowe NK, Nacht AS, Koschoreck K, Anderson J. Pilot study of physiologic partograph use among low-risk, nulliparous women with spontaneous labor onset. J Midwifery Womens Health. 2016;61(2):235–41. [DOI] [PubMed] [Google Scholar]
- 118.Hauth JC, Hankins GD, Gilstrap LC 3rd, Strickland DM, Vance P. Uterine contraction pressures with oxytocin induction/augmentation. Obstet Gynecol. 1986;68(3):305–9. [DOI] [PubMed] [Google Scholar]
- 119.Frey H, Tuuli M, Roehl K, Odibo A, Macones G, Cahill A. Montevideo units: A poor predictor of second stage outcomes. Am J Obstet Gynecol. 2013;208(1):S141. [Google Scholar]
- 120.Kernberg A, Caughey AB. Augmentation of labor: a review of oxytocin augmentation and active management of labor. Obstet Gynecol Clin North Am. 2017;44(4):593–600. [DOI] [PubMed] [Google Scholar]
- 121.Zhang J, Branch DW, Ramirez MM, Laughon SK, Reddy U, Hoffman M, et al. Oxytocin regimen for labor augmentation, labor progression, and perinatal outcomes. Obstet Gynecol. 2011;118(2 Pt 1):249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wei S, Wo BL, Qi HP, Xu H, Luo ZC, Roy C, et al. Early amniotomy and early oxytocin for prevention of, or therapy for, delay in first stage spontaneous labour compared with routine care. Cochrane Database Syst Rev. 2013;8:CD006794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Blanc-Petitjean P, Le Ray C, Lepleux F, De La Calle A, Dreyfus M, Chantry AA. Factors affecting rotation of occiput posterior position during the first stage of labor. J Gynecol Obstet Hum Reprod. 2018;47(3):119–25. [DOI] [PubMed] [Google Scholar]
- 124.Hanley JA, Weeks A, Wray S. Physiological increases in lactate inhibit intracellular calcium transients, acidify myocytes and decrease force in term pregnant rat myometrium. J Physiol. 2015;593(20):4603–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dawood F, Dowswell T, Quenby S. Intravenous fluids for reducing the duration of labour in low risk nulliparous women. The Cochrane Library. 2013;208(3):226–e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Riegel M, Quist-Nelson J, Saccone G, Locci M, Shrivastava VK, Salim R, et al. Dextrose intravenous fluid therapy in labor reduces the length of the first stage of labor. Eur J Obstet Gynecol Reprod Biol. 2018;228:284–94. [DOI] [PubMed] [Google Scholar]
- 127.Singata M, Tranmer J, Gyte GM. Restricting oral fluid and food intake during labour. Cochrane Database Syst Rev. 2010;1:CD003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ciardulli A, Saccone G, Anastasio H, Berghella V. Less-restrictive food intake during labor in low-risk singleton pregnancies: a systematic review and meta-analysis. Obstet Gynecol. 2017;129(3):473–80. [DOI] [PubMed] [Google Scholar]
- 129.Wiberg-Itzel E, Wray S, Akerud H. A randomized controlled trial of a new treatment for labor dystocia. J Matern Fetal Neonatal Med. 2018;31(17):2237–44. [DOI] [PubMed] [Google Scholar]
- 130.Seyedi M, Ghorashi Z, Sedighi DT. Randomized controlled trial of oral bicarbonate treatment for labor stagnation. J Obstet Gynaecol Res. 2021;47(1):114–8. [DOI] [PubMed] [Google Scholar]
- 131.Betran AP, Torloni MR, Zhang J, Ye J, Mikolajczyk R, Deneux-Tharaux C, et al. What is the optimal rate of caesarean section at population level? A systematic review of ecologic studies. Reprod Health. 2015;12:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ye J, Zhang J, Mikolajczyk R, Torloni MR, Gulmezoglu AM, Betran AP. Association between rates of caesarean section and maternal and neonatal mortality in the 21st century: a worldwide population-based ecological study with longitudinal data. BJOG. 2016;123(5):745–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cypher RL. Cesarean birth: a journey in historical trends. J Perinat Neonatal Nurs. 2016;30(3):259–64. [DOI] [PubMed] [Google Scholar]
- 134.Carlson NS, Lowe NK. A concept analysis of watchful waiting among providers caring for women in labour. J Adv Nurs. 2014;70(3):511–22. [DOI] [PubMed] [Google Scholar]
- 135.Lowe NK, Corwin EJ. Proposed biological linkages between obesity, stress, and inefficient uterine contractility during labor in humans. Med Hypotheses. 2011;76(5):755–60. [DOI] [PubMed] [Google Scholar]
- 136.Cluff AH, Bystrom B, Klimaviciute A, Dahlqvist C, Cebers G, Malmstrom A, et al. Prolonged labour associated with lower expression of syndecan 3 and connexin 43 in human uterine tissue. Reprod Biol Endocrinol. 2006;4:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Reinebrant HE, Pileggi-Castro C, Romero CL, Dos Santos RA, Kumar S, Souza JP, et al. Cyclo-oxygenase (COX) inhibitors for treating preterm labour. Cochrane Database Syst Rev. 2015;6:CD001992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Arrowsmith S, Kendrick A, Wray S. Drugs acting on the pregnant uterus. Obstet Gynaecol Reprod Med. 2010;20(8):241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Turi A, Kiss AL, Mullner N. Estrogen downregulates the number of caveolae and the level of caveolin in uterine smooth muscle. Cell Biol Int. 2001;25(8):785–94. [DOI] [PubMed] [Google Scholar]
- 140.Klein U, Gimpl G, Fahrenholz F. Alteration of the myometrial plasma membrane cholesterol content with beta-cyclodextrin modulates the binding affinity of the oxytocin receptor. Biochemistry. 1995;34(42):13784–93. [DOI] [PubMed] [Google Scholar]
- 141.Grotegut CA, Gunatilake RP, Feng L, Heine RP, Murtha AP. The influence of maternal body mass index on myometrial oxytocin receptor expression in pregnancy. Reprod Sci. 2013;20(12):1471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Crankshaw DJ, O’Brien YM, Crosby DA, Morrison JJ. Maternal body mass index and spontaneous contractility of human myometrium in pregnancy. J Perinatol. 2017;37(5):492–7. [DOI] [PubMed] [Google Scholar]
- 143.Elmes MJ, Tan DS, Cheng Z, Wathes DC, McMullen S. The effects of a high-fat, high-cholesterol diet on markers of uterine contractility during parturition in the rat. Reproduction. 2011;141(2):283–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and material are available upon request to the corresponding author.


