Abstract
Graphical abstract
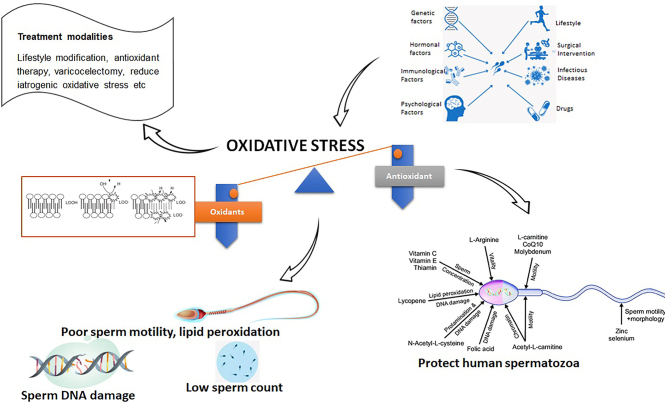
Abstract
Infertility affects millions of couples worldwide. Oxidative stress (OS) causes peroxidation of lipids and damage to spermatozoa, thus, reducing the quality of seminal parameters. In addition, the differences in the levels of antioxidants and reactive oxygen species (ROS) caused by intrinsic and extrinsic variables linked to lifestyle, diet, genetics, and OS also contribute to male infertility. High levels of ROS result in sperm damage of sperm parameters due to lipid peroxidation and oxidation of proteins. Other significant causes of ROS include changes in sex hormone levels, sperm DNA damage, including mutations, and immature spermatozoa. Treating the root causes of OS, by changing one’s lifestyle, as well as antioxidant therapy, may be helpful strategies to fight OS-related infertility. However, the determination of male infertility induced by OS is currently a challenge in the field of reproductive health research. This review intends to describe the role of oxidative stress on male infertility and the current understanding of its management.
Lay summary
The inability to conceive affects many couples globally. Oxidative stress refers to imbalances between different oxygen species which can lead to male fertility problems by damaging sperm and semen. Oxidative stress may be caused by several factors, including diets high in fats, sugars and processed foods, lifestyle (including smoking, alcohol consumption and having a sedentary lifestyle), and genetics. Treatment that focuses on the root cause may help combat male infertility. However, there is currently no consensus on the best way to treat male fertility problems, particularly those associated with oxidative stress. This paper describes the role of oxidative stress on male infertility and discusses the current techniques employed in treating male fertility issues.
Keywords: oxidative stress, reactive oxygen species, antioxidant, male infertility, oxidation–reduction potential, sperm cells
Introduction
Infertility is defined as the failure to achieve pregnancy conceive following after 12 months of regular, unprotected sexual intercourse without contraceptives (Larsen 2005). About 40–50% of male infertility is caused by ‘male factor’ infertility, with 2% of these exhibiting suboptimal sperm parameters (Kumar & Singh 2015). Infertility has been associated with emotional, sociocultural, and physical difficulties (Larsen 2005). Infertility is also associated with a high prevalence of sexually transmitted infections, underdeveloped or undeveloped testes, and hypothalamic and pituitary abnormalities (Agarwal et al. 2021).
Furthermore, about 186 million people and 48 million couples globally are infertile (Boivin et al. 2007, Mascarenhas et al. 2012). In addition, the prevalence of infertility is highest in South Asia, sub-Saharan Africa, North Africa/Middle East, Central/Eastern Europe, and Central Asia (Mascarenhas et al. 2012). Although not accurately representing the global statistics, males are found to be solely responsible for 20–30% of infertility cases and contribute to 50% of overall cases (Vander Borght & Wyns 2018). Indeed, as many as 2% of all men will exhibit suboptimal sperm parameters (Kumar & Singh 2015). Roughly 50% of cases associated with infertility are influenced by poor seminal parameters referred to as male factor infertility; this however, varies from region to region (Agarwal et al. 2015).
Infertility is classified as primary and secondary (Larsen 2000). Primary infertility refers to a couple who have not achieved pregnancy after 1 year of regular sexual intercourse within their childbearing age (Larsen 2000). Secondary infertility is identified after 6–12 months of unsuccessful attempts to get pregnant. The inability to conceive or bring a pregnancy to term after giving birth is referred to as secondary infertility (Larsen 2000). Also, prior pregnancy must have occurred naturally, without using fertility drugs or procedures like in vitro fertilization, for it to be classified as secondary infertility (Katib et al. 2014). A study on the longitudinal trend between 1993 and 2017 demonstrated that the primary and secondary infertility prevalence rate globally was lower among men than women and also decreased in high-income countries (Borumandnia et al. 2022).
The World Health Organization reports that half of the incidence of infertility is caused by male factor infertility (World Health Organization 2018), which is characterized by poor sperm quantity, poor sperm motility, and morphological defects in at least one sample of two semen analyses and collected in an interval of 1 to 4 weeks apart (Patel et al. 2017). Roughly a quarter of men experiencing infertility present cases of teratozoospermia, asthenozoospermia, oligozoospermia, or a combination of all these anomalies, termed oligoasthenoteratozoospermia (Gatimel et al. 2017, Alahmar 2019). Idiopathic male infertility is diagnosed when there are unexplained sperm abnormalities, with no female factor infertility, in contrast to unexplained male infertility, where there are normal sperm parameters (Table 1) (Agarwal et al. 2019). Oxidative stress has been identified as one of the mechanisms for idiopathic male infertility. Previous studies have reported that spermatozoa with morphological defects are prone to producing excessive reactive oxygen species (ROS) and have reduced antioxidant capacity (Agarwal et al. 2019). Furthermore, oxidative stress is commonly detected in the male population with idiopathic infertility having an imbalanced level of ROS and antioxidant capacity compared to fertile male counterparts (Ayad et al. 2022).
Table 1.
WHO 2010 (5th edition) and WHO 2021 (6th edition) report on male semen parameters.
| Semen parameter | WHO 2010 | WHO 2O22 |
|---|---|---|
| Semen volume (mL) | 1.5 (1.4–1.7) | 1.4 (1.3–1.5) |
| Total sperm count (106 per ejaculate) | 39 (33–46) | 39 (35–40) |
| Overall motility (%) | 40 (38–42) | 42 (40–43) |
| Progressive motility (%) | 32 (31–34) | 30 (29–31) |
| Non-progressive motility (%) | 1 | 1 (1–1) |
| Immotile sperm (%) | 22 | 20 (19–20) |
| Vitality (%) | 58 (55–63) | 54 (50–560 |
| Normal forms (%) | 4 (3–4) | 4 (3.9–4) |
WHO, World Health Organization.
Despite the association between oxidative stress and idiopathic infertility, there is still a lack in its definite treatment (Ayad et al. 2022). For instance, there are no conclusions about which patients should be screened for oxidative stress or what tests should be performed to measure the amount of ROS in the semen sample. Also, controversy exists regarding the type, dose, and duration of antioxidant treatment for patients with excessive ROS levels (Wagner et al. 2018). This review intends to describe the role of oxidative stress on male infertility, and the current understanding of its management.
Seminal ROS and male infertility
Human spermatozoa are highly vulnerable to oxidative stress due to the extraordinarily high content of polyunsaturated fatty acids (PUFA) in the plasma membrane and the absence of cytoplasmic antioxidant enzymes (Henkel 2011). Excessive ROS and its metabolites damage lipids, proteins, and DNA, causing apoptosis, altering enzyme activity, and affect sperm parameters which are a prerequisite for fertilization (Alahmar 2019).
An imbalance between ROS production and the body’s antioxidant defense mechanisms results in oxidative stress, resulting in a disturbance in cellular functions. ROS are defined as oxygen-containing species (Li et al. 2016), which includes (O2▪−), hydrogen peroxide (H2O2), hydroxyl radical (OH▪), singlet oxygen (1O2), peroxyl radical (LOO▪), alkoxyl radical (LO▪), lipid hydroperoxide (LOOH), peroxynitrite (ONOO−), hypochlorous acid (HOCl), and ozone (O3) (Li et al. 2016).
The human semen sample contains a variety of cells, including immature and mature spermatozoa, round-shaped cells of different phases of spermatogenesis, epithelial cells, and leukocytes (Long & Kenworthy 2022). The leukocytes (particularly macrophages and neutrophils are usually activated in response to stimuli during infection and inflammation) and immature, morphologically abnormal spermatozoa are the primary sources of ROS (Agarwal et al. 2014). However, the rate of production of ROS is up to 1000 times more in the leukocytes (extrinsic source) compared to the spermatozoa (intrinsic source) (Alahmar 2019). The mitochondrial oxidoreductase and the oxidase in the sperm plasma membrane, both dependent on sperm-specific NADPH, have been proposed to be responsible for ROS production (Gavella & Lipovac 1992, Aitken 1999). In addition, unhealthy lifestyle-related factors such as cigarette smoking, excessive alcohol consumption, unhealthy diet, psychological stress, sedentary lifestyle, and environmental factors (e.g. radiation, metals, and environmental toxicants) are shown to increase the level of ROS in spermatozoa, thereby contributing to the risk of male infertility (Agarwal et al. 2014, Durairajanayagam 2019).On the other hand, low levels of ROS generated by spermatozoa play a crucial role in the optimal functioning of spermatozoa. ROS are also involved in physiological processes, such as tyrosine phosphorylation, capacitation, hyperactivation, acrosome reaction, and sperm-oocyte fusion (Castellini et al. 2021). For example, during capacitation, there is an increased ROS level, intracellular calcium, and tyrosine kinase, which result in increased cyclic AMP and, subsequently, hyperactivation (de Lamirande et al. 1997).
The etiology of male infertility is heavily influenced by oxidative stress (Agarwal et al. 2019). High seminal ROS levels exist in 30% to 80% of infertile men (Agarwal et al. 2014). Therefore, the seminal oxidative stress must be considered to measure male reproductive potential correctly. Formerly referred to as idiopathic male infertility, male oxidative stress infertility (MOSI) is a new term for infertile males with abnormal semen features and oxidative stress (Agarwal et al. 2014, 2019).
Measurement techniques for oxidative stress in human semen
ROS can be measured using direct and indirect assays. The indirect assays (such as myeloperoxidase, 8-hydroxy-2-deoxyguanosine, thiobarbituric acid reactive substances test, and total antioxidant capacity (TAC)) measure the extent of ROS-induced adverse effect, while the direct assays quantify the level of ROS directly (Katerji et al. 2019). Chemiluminescence, dihydroethidium probe, nitroblue tetrazolium test (NBT), electron spin resonance, and cytochrome c reduction analysis are some of the methods used in the direct measurement of ROS in semen samples (Agarwal et al. 2004). Tables 2 and 3 summarize the indirect and direct testing methods for oxidative stress and ROS in human semen.
Table 2.
Pros and cons of direct tests used to measure oxidative stress levels in the seminal plasma.
| Assay | Description | Pros | Cons | Reference |
|---|---|---|---|---|
| Chemiluminescence assay | The emission of electromagnetic radiation brought on by a chemical process that results in the production of light is known as chemiluminescence (CL). Chemiluminescence immunoassay (CLIA) is a test that combines immunochemical responses with the chemiluminescence method. CLIA uses chemical probes that can label the antibody by a chemical reaction, much as other labeled immunoassays (RIA, FIA, and ELISA). |
|
|
Agarwal et al. (2015), Li et al. (2016) |
| Cytochrome c reduction test | This test gauges how much cytochrome c is reduced by NADPH-cytochrome c reductase when NADPH is present. Cytochrome C’s oxidation/reduction status affects its absorption spectrum. At 550 nm, a significant absorption peak is seen after reduction. |
|
|
Melendez-Ferro et al. (2013) |
| Nitroblue tetrazolium (NBT) | The nitroblue tetrazolium measures intracellular ROS level as well as give insight to the potential source of OS with the aid of a light microscope. NBT is converted into a blue pigment (diformazin) following its interaction with the superoxide released from the leukocytes or spermatozoa. The concentration of diformazin is positively correlated with the concentration of intracellular ROS. |
|
|
Tvrdá et al. (2011), Agarwal & Majzoub (2017) |
| Oxidation–reduction potential (ORP) | The interaction between oxidants and antioxidants is measured by ORP, which offers a thorough assessment of oxidative stress. Higher ORP readings distinguish the level of oxidative stress caused by an imbalance in the activity of oxidants and antioxidants. reproductive issues caused by men |
|
|
Agarwal et al. (2017) |
| Electron spin resonance | A spectroscopic method called electron spin resonance (ESR) uses a static magnetic field to detect transitions between the energy levels of electron spins caused by electromagnetic radiation. |
|
|
Kopáni et al. (2006) |
Table 3.
The advantages and disadvantages of indirect tests used to measure oxidative stress levels in the seminal plasma.
| Assay | Description | Advantages | Disadvantages | Reference |
|---|---|---|---|---|
| Myeloperoxidase | Myeloperoxidase, which is found in the leukocyte granules, converts substrates from an insoluble blue/brown derivative to a colorless form in the presence of hydrogen peroxide. As substrates, benzidine, 3,3′-diaminobenzidine, or p-phenylenediamine dihydrochloride are used. |
|
|
Shekarriz et al. (1995) |
| Total antioxidant capacity | Antioxidant capacity (AC) provides an integrated metric rather than the mere total of measured antioxidants by taking into account the cumulative activity of all antioxidants present in plasma and bodily fluids. |
|
|
Robert et al. (2021) |
| HNE-HIS adduct ELISA | The protein-HNE adduct ELISA is a technique for detecting HNE bound to proteins, which is thought to be the type of HNE occurrence in biological systems |
|
|
Agarwal et al. (2017) |
| Malondialdehyde assay |
This assay is based on the formation of the MDA-TBA2 adduct, a strong absorber at 532 nm, by the interaction of malondialdehyde (MDA) with thiobarbituric acid (TBA). The most widely used technique for calculating MDA in biological samples is this reaction. |
|
|
Khoubnasabjafari et al. (2015) |
| DNA fragmentation | TUNEL is a technique for identifying apoptotic DNA fragmentation that is frequently used to recognize and measure apoptotic cells or to find cells that have excessively broken DNA. The test depends on the enzyme terminal deoxynucleotidyl transferase (TdT), which is used to attach deoxynucleotides that have been dyed or otherwise marked to the 3'-hydroxyl termini of DNA double-strand breaks. Additionally, it may identify cells whose DNA has been damaged in ways beyond apoptosis. |
|
|
Homa et al. (2019) |
| Protein alterations ASSA | Determine the quantity or concentration of a particular protein or a variety of distinct proteins in a sample using a protein test. Many clinical and scientific procedures include the isolation and detection of proteins. |
|
|
Agarwal et al. (2017) |
8-OHdG, 8-hydroxy-2-deoxyguanosine; ELISA, enzyme linked immunosorbent assay; MDA, malondialdehyde; OS, oxidative stress; PMN, polymorphonuclear neutrophils; ROS, reactive oxygen species; SCD, sperm chromatin dispersion; SCSA, sperm chromatin structure assay; TBA, thiobarbituric acid; TUNEL, terminal deoxynucleotidyl transferase dUTP nick-end labeling; WBC, white blood cell; WHO, World Health Organization.
In addition, intracellular ROS levels are recognized as a crucial method for detecting changes in redox status and oxidative stress because of its susceptibility to ROS-induced oxidation by peroxynitrite, hydrogen peroxide, hydroxyl radicals, peroxide ions, and H2DCF-DA (Schieber & Chandel 2014). Therefore, cytochrome c centers are being used to grow cells with the fluorescent probe H2DCF-DA (2.5 M) to study intracellular ROS generation in spermatozoa, leukocytes, and blood cells other cellular components (McCarthy et al. 2010).
The thiobarbituric acid reactive substance assay (spectrophotometry or fluorometry probe) is frequently used to measure malondialdehyde and 4-hydroxyalkenals as an indication of lipid peroxidation (Seljeskog et al. 2006). Sensitive high-pressured liquid chemigraphy is recommended for low malondialdehyde levels, while mass spectrometry signifies another method to investigate lipid peroxidation product as isoprostanes (Ito et al. 2019). Sperm MDA levels positively correlate with ROS production in the semen of infertile men. Chemiluminescence quantifies seminal ROS levels and involves using a luminometer and a chemiluminescent probe, such as luminol (5-amino-2,3-dihydro-1,4-phthalazinedione) (Dutta et al. 2019). This assay directly measures intracellular and extracellular ROS (Agarwal et al. 2015). Light signal is emitted after free radicals in the semen samples react with luminol, which is converted to an electrical signal by the luminometer. The ROS level in the sample is represented as relative light units (RLU) per second per 106 spermatozoa per milliliter (RLU/s/106 sperm/mL) (Dutta et al. 2019, Dias 2021). The usual range of ROS levels in washed sperm suspensions is 0.10–1.03 × 106 counted photons per minute per 20 × 106 sperm (Agarwal & Majzoub 2017). The chemiluminescence assay is reported to be a reproducible and reliable assay for measuring seminal ROS and used for diagnosing male infertility (Dutta et al. 2019). More so, it is extremely sensitive and reacts with various ROS at neutral pH and should be measured within 4 h of sample collection (Kobayashi et al. 2001). A significant limitation of this assay is that luminol can act as a source of O2 − in the presence of other univalent oxidants(Khan et al. 2014).
TAC measures the total amount of antioxidants in seminal plasma to inhibit ABTS 2,29-azinobis-(3-ethyl benzothiazoline-6-sulphonic acid) oxidation to ABTS+ after incubation with metmyoglobin and hydrogen peroxide (Miller et al. 1993). The TAC may be examined by using improved chemiluminescence or colorimetric methods. ROS–TAC scores were suggested as a new approach to assessing the impact of redox status on infertility (Sharma et al. 1999). In particular, the ROS–TAC score might be used to identify oxidative damage in semen samples from asthenozoospermic men (Sharma et al. 1999). A continued focus on a particular global index that can easily distinguish between fertile and infertile males rather than using ROS or TAC alone is sought (Robert et al. 2021).
Flow cytometry is a technology that analyzes single cells in solution in real time using multiple parameters. Lasers are used as light sources in flow cytometers, producing both scattered and fluorescent light signals that are read by detectors such as photodiodes or photomultiplier tubes (McKinnon 2018). Flow cytometry measures intracellular ROS by quantifying a cell’s fluorescence amount (Agarwal & Majzoub 2017).
The NBT is colorimetric marker of superoxide and is based on the use of NBT, a yellow, water-soluble, nitro substituted aromatic tetrazolium salt (2,20-bis(4-nitrophenyl)-5,50-diphenyl-3,30-(3,30-dimethoxy-4,40-diphenyl) ditetrazolium chloride), with the ability to interact with intracellular superoxide to produce formazan (purple), which can then be measured spectrophotometrically or microscopically (Armstrong et al. 2002, Esfandiari et al. 2003). The NBT test measures intracellular ROS levels and gives insight into the potential source of oxidative stress with the aid of a light microscope (Agarwal & Majzoub 2017).
Measurement of oxidation–reduction potential
Oxidation–reduction potential (ORP, redox potential) is a practical and straightforward direct assay for measuring oxidative stress. It measures the balance between oxidants and antioxidants (Agarwal et al. 2016). The ORP measures the levels of antioxidants and oxidants in several biological fluids (Okouchi et al. 2002). In addition, ORP could supplement semen analysis due to its strong association with impaired sperm function (Agarwal et al. 2019).
The Male Infertility Oxidative System (MiOXSYS) is a novel, user-friendly, and less expensive system that is used to evaluate the ORP in human semen. It expresses the ORP as the static ORP (sORP; mV). The reading is then normalized to the sperm concentration and expressed as mV/106 sperm/mL (Agarwal et al. 2018a ). The MiOXSYS analyzer consists of an ultrahigh impedance electrometer and a sensor with working electrodes and references (Agarwal & Bui 2017). The test is done by adding a small volume (30 µL) of the liquefied neat semen on a pre-inserted sensor. The electrochemical circuit is completed when the sample fills the sensor's reference terminal, and the test begins. The principle of this methodology is based on comparing the electrical conductance of an internal reference standard to the Nernst equation:
E(ORP) = E0 − RT∕nF
E is the redox potential or ORP.
E0 is the standard potential of a redox system measured concerning hydrogen electrons, which is arbitrarily assigned an E0 of 0 volts.
R= gas is constant.
T= absolute temperature measured in degrees Kelvin.
n= number of moles of electrons transferred in the balanced equation for the reaction occurring in the cell.
F= Faraday’s constant.
A cutoff value of 1.34 mV/106 sperm/mL for sORP is set to demonstrate the quality of seminal parameters (Agarwal et al. 2016, 2017). A higher sORP indicates an imbalance between oxidants and antioxidants in favor of the oxidants, thus indicating oxidative stress (Agarwal et al. 2017).
A good correlation has been found between the ORP and male semen parameters such as sperm motility and morphology, indicating ORP as a marker of seminal oxidative stress (Agarwal et al. 2019). Semen from the male partners of fertile couples tends to have lower sORP than those from infertile male partners (Agarwal et al. 2016). These findings suggest that ORP measurement may be the most effective method for predicting and managing sperm infertility (Aitken 2022, Castleton et al. 2022, Joao et al. 2022). Additionally, research confirmed an association between DNA fragmentation and seminal ORP (Panner Selvam et al. 2022). Furthermore, it was also noted that ORP is a more precise method compared to chemiluminescent ROS assessment for determining the redox status in male infertility (Vassiliou et al. 2021).
The available assays for the measurement of seminal ORP are a reliable marker for assessing the overall redox state is seminal ORP. These assays are trustworthy and user-friendly with great potential of being used in clinical andrology settings (Panner Selvam et al. 2022). In addition, they usually provide one single marker of oxidative stress, that is, oxidant level, antioxidant level, or posthoc damage (Agarwal et al. 2018b ). Furthermore, conventional semen analysis is criticized for its poor reproducibility, subjectivity, and fertility prediction. Consequently, ORP is recommended as a clinical biomarker for MOSI in men with abnormal semen analysis and male infertility based on the limitation of conventional semen analysis, pathological consequences, and ubiquity of oxidative stress among the subfertility male population (Nago et al. 2021, Henkel et al. 2022, Kavoussi et al. 2022, Niu et al. 2023).
Management of MOSI
Despite the progress made to measure oxidative stress in semen analysis, well-defined procedures for treatment associated with male infertility oxidative stress as a result of undefined aetiologies of male infertility are still lacking (Ayad et al. 2022). Therefore, this section will review the literature regarding treatment options, including empirical treatment for men with elevated ROS, specific treatments, and procedures for reducing iatrogenic oxidative stress.
Empirical medical treatment for men with elevated ROS: evidence for antioxidants
Empirical medical treatment (EMT) is widely used in men with idiopathic infertility. Based on the mode of action, EMT is categorized into two groups: hormonal therapy and antioxidant supplementation (Jung & Seo 2014). The agents of the hormonal treatment target the hypothalamic–pituitary–gonadal axis to correct subclinical endocrinopathy and includes aromatase inhibitors, gonadotropins, androgens, and selective estrogen receptor modulators (Dabaja & Schlegel 2014, Jung & Seo 2014). Although hormonal therapy in male counterparts with detectable aberrations like hypogonadotropic hypogonadism is well articulated, 10% of infertility cases in men are culminated by an imbalance in the endocrine system (Ko et al. 2012). Therefore, studies recommend using EMT to combat idiopathic infertility; however, there is a lack of evidence to validate successful birth outcomes in in-vitro (Thaker et al. 2020, Ayad et al. 2022). On the other hand, it is suggested for males with no genetic aberration, bacterial infections, and imbalanced endocrine system, identification of the primary source of MOSI rather than EMT should be employed to treat male infertility (Thaker et al. 2020).
Antioxidant therapy
An imbalance between ROS production and antioxidant level is implicated as the primary cause of idiopathic male infertility. Scavenging enzymes in the cellular cytoplasm and antioxidants in the seminal fluid play a crucial role in the antioxidant defense mechanism to counteract the adverse effects of ROS (Agarwal et al. 2014). However, the relatively low concentration of the cytoplasmic scavenging enzymes and the high amount of PUFA within the plasma membrane of human spermatozoa make them vulnerable to ROS from lipid peroxidation (Sanocka & Kurpisz 2004). Antioxidant defense mechanisms against ROS may be enzymatic or non-enzymatic (Sies 1997).
Several studies have been undertaken to determine the effectiveness of various antioxidants such as vitamins C and E, zinc, selenium, L-carnitine, folic acid, or coenzyme Q10 on seminal fluid oxidative stress as well as sperm parameters (Alahmar & Sengupta 2021). A few of these reported improved semen parameters such as DNA fragmentation, sperm motility, morphology, and concentration (Alahmar & Sengupta 2021, Gupta et al. 2021). Furthermore, significant improvements in sperm redox status as well as a good correlation with pregnancy outcomes were demonstrated (Gharagozloo & Aitken 2011). In addition, oral administration of antioxidants for 3 months significantly improved sperm health and count in idiopathic infertile male counterparts (Agarwal et al. 2019). This improvement might increase the likelihood of conceiving naturally (Ko et al. 2012). Other studies also support antioxidants’ efficacy in increasing live birth rates and semen parameters (Showell et al. 2020). Although antioxidant therapy in male infertility is still debated, once a patient has been diagnosed with infertility caused by oxidative stress, treatment should focus on amelioration and classification of the leading cause before resorting to antioxidant therapy (Lanzafame et al. 2009). The combination of MOSI diagnosis with ORP monitoring may be a reliable strategy in antioxidant therapy (Agarwal et al. 2019). Compared with hormonal EMT and assisted reproductive technology (ART), antioxidants are generally safe, affordable, and widely accessible (Agarwal et al. 2019). Table 4 summarizes a wide range of antioxidants used in a clinical trial to combat male infertility.
Table 4.
Clinical trials conducted using different antioxidants to combat male infertility.
| Type of antioxidant or ingredient | Evaluated semen parameter/fertility condition | Dosage | Outcome | Reference |
|---|---|---|---|---|
| Vitamin C–E | Human sperm DNA fragmentation, sperm motility, morphology, | One gram of vitamin E and 1 g of vitamin C. 200 μg vitamin E | The intervention group had less DNA damage after 2 months. However, major semen parameters like motility and concentration were found to have no significant relationship with vitamin E or C intake. According to Greco and colleagues, a 2-month course of treatment with 1 g of vitamin E and C reduced the amount of DNA damage and improved ICSI success in patients with sperm DNA damage. | Greco et al. (2005), Moslem & Tavanbakhsh (2011), Lobascio et al. (2015) |
| 690 infertile men with idiopathic asthenoteratospermia who received daily supplement of selenium (200 μg) in combination with vitamin E (400 IU) for at least 100 days. They reported 52.6% (362 cases) total improvement in sperm motility, morphology, or both, and 10.8% (75 cases) spontaneous pregnancy in comparison with no treatment. | ||||
| Vitamin E | Motility and viability in asthenoteratozoospermic men | 2, 4, and 6 h. that the test group was incubated with VE (2 mM) | By maintaining normal antioxidant processes, in vitro vitamin E supplementation may shield spermatozoa from the negative effects of oxidative stress during sperm preparation. | Ghafarizadeh et al. (2021) |
| Vitamin C | Sperm parameters of teratozoospermic semen samples | 600 µm ascorbic acid | Vitamin C improves sperm motility, progressive motility, and acrosome reaction | Fanaei et al. (2014) |
| Zinc | Asthenoteratospermia | 220 mg per capsule/ per day for 3 months | Zinc supplementation increased the volume of sperm, progressive sperm motility percentage, and total normal sperm count. | Hadwan et al. (2012) |
| Folic acid | Subfertility parameter | 5 mg/day | Treatment significantly increased sperm concentration in sub fertile males. Other semen and endocrine parameters were not affected by intervention treatment. | Ebisch et al. (2006) |
| CoQ10 | Semen parameters in infertile men | 150 mg CoQ10/day | Because CoQ10 increases total antioxidant capacity, it was found to be associated with important sperm parameters like concentration, motility, and morphology. Thakur suggested that infertile men’s sperm parameters could be improved by taking 150 mg of CoQ10 daily. | Thakur et al. (2015) |
| Selenium and NAC | Idiopathic oligo-asthenoteratospermia | 100 μg selenium orally daily, 600 mg NAC orally daily | The parameters of the sperm and the concentrations of selenium and NAC in the seminal plasma showed a strong positive correlation. The sum of the selenium and NAC levels was found to have a strong correlation with the mean sperm concentration (r = 0.67, P = 0.01), sperm motility (r = 0.64, P = 0.01), and percentage of normal morphology (r = 0.66, P = 0.01). | Safarinejad & Safarinejad (2009) |
CoQ10,Coenzyme Q10; NAC, N-acetyl-cysteine.
Non-enzymatic antioxidants such as (vitamin C and vitamin E complex, GSH, coenzyme Q10, carnitine, and minerals such as zinc, copper, selenium, and chromium) have been reported to be crucial in maintaining sperm physiology (Collins & Rossi 2015, Ahmad et al. 2017). GSH is a tripeptide thiol that is derived from cysteine, glutamine, and glycine and has many biological functions such as maintaining the redox state and detoxifying endogenous and exogenous compounds (Aquilano et al. 2014). Vitamin C is considered the most important water-soluble antioxidant in the extracellular fluid as it inhibits the formation of ROS (Aquilano et al. 2014) and protects sperm from DNA damage by inhibiting the harmful effects of ROS before activating LPO. This is accomplished by lowering oxidized tocopherol and reversing the hydroxyl effect, which protects human germ cells from oxidative damage (Aquilano et al. 2014). A study demonstrated that spermatozoa with excessive ROS had lower levels of vitamin C (Rahimlou et al. 2019). Tocopherols and tocotrienols are the components of vitamin E (Rahimlou et al. 2019). Vitamin E can be found in wheat germ, avocados, palm oil, and veggie oils. Tocopherol and ROS interact during LPO to produce lipid radicals that stop the degradation of cell membranes (Bartolini et al. 2022). Vitamin C and E taken together have been shown to protect sperm from peroxide damage and DNA damage (Rahimlou et al. 2019). Zinc, the is the second most prevalent in the human body, is involved in protein synthesis during DNA transcription, which is crucial for reproduction (Fallah et al. 2018). Zinc plays a role in several biological reproduction processes, including germ cell development and the synthesis of luteinizing, follicular, and testicular hormones (Fallah et al. 2018). Zinc in combination with other antioxidant enzymes helps men with sperm deficiencies increase their fertility and sperm health (Fallah et al. 2018). Another essential micronutrient that can end up in sperm and testosterone production is selenium (Fallah et al. 2018). About 25 selenoproteins have been found in animals and humans, significantly affecting sperm integrity (Rahimlou et al. 2019).
Enzymatic antioxidants (SOD, GPx, and CAT) are another category of antioxidants known to scavenge ROS from the gonads and seminal fluids (Ahmad et al. 2017). Glutathione peroxidase is a selenium-containing antioxidant enzyme found in the cell's mitochondria and cytoplasm (Lubos et al. 2011) and exists in two forms, selenium-dependent and selenium-independent enzymes (Lubos et al. 2011). Superoxide dismutase in the prostate gland and seminal vesicles is a first-line defense against oxidative stress in reproductive cells and a regulator of ROS production (Lubos et al. 2011). Additionally, SOD works well with CAT and GPx due to its scavenging property (Lubos et al. 2011). Catalase is an antioxidant enzymatic found in (Ohta et al. 1996, Rubio-Riquelme et al. 2020).
The B-type vitamins folate, vitamin B6, and vitamin B12 enhance the enzymatic activity of the methylenetetrahydrofolate reductase (Serapinas et al. 2017). Compounds of the cystathionine b-synthase family are responsible for eliminating homocysteine from the plasma (Jhee & Kruger 2005). It is suggested that any male with hyperhomocysteinemia and oxidative stress should take a B vitamin supplement (5 mg folate, 100 mg vitamin B6, and 100 mg vitamin B12) since it is affordable and has no significant adverse effects (Kaye et al. 2020).
Several studies have been published to date examining the effects of various antioxidant therapies on sperm parameters and pregnancy outcomes (Sies 1997, Smits et al. 2018, Agarwal et al. 2023). Conclusions regarding the clinical viability of oral antioxidant therapy on sperm functionality and pregnancy outcomes should be readily available, given the abundance of research references (Arafa et al. 2020, Scaruffi et al. 2021). However, this is not the case due to heterogeneous data such as differences in dosage of the antioxidants, absence of appropriate viewpoint placebo-controlled research design, and small scale-sample size (Martin-Hidalgo et al. 2019). A preliminary report revealed a significant improvement in sperm quantity, motility, and sperm morphology following antioxidant therapy (Wang et al. 2019). Antioxidants such as astaxanthin, carnitine, or a blend of antioxidants, for example, acetylcysteine, β-carotene, vitamin E, and unsaturated fatty acids have all been shown to decrease ROS levels (Tremellen 2008, Kefer et al. 2009, Zhaku et al. 2021). In a placebo-controlled study, a combination of 225 mg of selenium, 400 mg of vitamin E, or 300 mg of vitamin E significantly reduced malondialdehyde levels in spermatozoa (Ammar-Keskes et al. 2003). A randomized clinical trial revealed a positive effect toward improvement in the health of the sperm DNA following 2 months of treatment with 1 g of vitamins C and E (Ammar-Keskes et al. 2003). Another study also reported on the reduction of sperm DNA aberration after treatment with a variety of vitamins E and C (400 mg each), β-carotene (18 mg), zinc selenium, or a mixture of 30 mg β-carotene, 180 mg of vitamin E and fatty acids (Punjabi et al. 2022). Furthermore, several studies have revealed antioxidant therapy to improve sperm quantity and quality (Büyükleblebici et al. 2014, Moichela et al. 2021, Takalani et al. 2021, Setumo et al. 2022).
However, the most reported parameter which seems to have been improved through antioxidant supplementation and reported frequently is sperm motility (Agarwal & Said 2004). For instance, significant positive effects on the motility of sperm were observed following antioxidant therapy using carnitine, selenium, vitamin E, glutathione, and a combination of selenium and vitamin E supplements (Pehlivan 2017). On the other hand, randomized clinical trials assessing vitamin C and E therapy with placebo revealed antioxidants to have no capacity for sperm improvement (Bartolini et al. 2022).
Matorras et al. (2020) reported that treatment with vitamin E brought about a considerable decrease in the damaging effect of ROS on sperm and an improvement in unconstrained pregnancy rates during the preceding 6 months (21% pregnancy rate in the vitamin E group vs 0% placebo). On the other hand, Rolf et al. (1999) detailed no improvement in spontaneous gestation resulting from 2 months of treatment with a blend of vitamin C and E. However, a new randomized clinical trial contrasting the antioxidant formulation Menevit and placebo treatment detailed a significant rise in clinical pregnancy in circumstances where the antioxidant treatment was administered for 3 months before IVF-ICSI therapy (Tremellen et al. 2020). Menevit, a nutraceutical hypothesized to improve sperm health through three correlative systems, contains antioxidant agents like vitamins C and E, selenium, and lycopene to shield sperm from previously generated ROS (Tremellen et al. 2020). Lastly, it contains zinc, selenium, and folate, which play a significant role in enlarging the protamine bundling of sperm DNA (Arafa et al. 2020) and protecting the sperm against the damaging effect of ROS. While it is yet to be demonstrated that combinational treatment like Menevit has a positive impact on DNA structure, it is suggested that the use of different antioxidants with a variety of modes of action together with an agent to limit the production of leukocytes ROS is probably going to bring about a beneficial impact (Beltrán et al. 2018).
Procedures for reducing iatrogenic oxidative stress
In andrology, centrifugation of sperm samples before use can exacerbate oxidative stress (Marzano et al. 2020); this can be mitigated by reducing centrifugation time, using non-centrifuge partitioning procedures such as ‘swim-up’ or glass wool filtration, and reducing the time interval in which human sperm are cultured in a media separate from seminal plasma (Martí et al. 2006). Furthermore, cultivating spermatozoa in low oxygen tension settings has been shown to improve sperm health by decreasing seminal leukocytes during ROS formation (Agarwal et al. 2022). Furthermore, avoiding cryopreserved sperm for fertilization is advised because ROS are produced during sperm banking, lowering the quality of the sperm cells (Tafuri et al. 2015, Agarwal et al. 2022). Also, throughout the sperm preparation process, the media used may be supplemented with various antioxidants to protect the cells from oxidative damage. For example, supplementing sperm medium with vitamin C, vitamin E, catalase, ferric acid, EDTA, albumin, and glutathione has been shown to protect against oxidative damage (Fanaei et al. 2014). However, aside from amino acids and albumin, currently employed sperm preparation media do not contain any type of antioxidant supplement (Bui et al. 2018). Unfortunately, better sperm media still lack features of the complicated sequential media produced for embryos, which needs extensive research (Bui et al. 2018).
Oxidative pathology direct treatment
Varicocele surgery has been shown to effectively reduce oxidative stress and enhance sperm DNA integrity (Kavoussi et al. 2022). Even though the most recent meta-analysis examining the effect of varicocelectomy on spontaneous conception found a significant benefit, the Cochrane Database claims no benefit. Randomized trials evaluating oxidative stress (sperm lipid peroxidation and oxidative DNA damage) and pregnancy rates must be carried out before varicocelectomy may be considered in males with oxidative stress.
Conclusions
The physiological level of ROS has been reported to be essential in the fertilizing capacity of spermatozoa, including capacitation and acrosome reaction. However, a high level of ROS results in oxidative stress, resulting in sperm membrane lipid peroxidation, damage of seminal parameters (sperm motility, viability, morphology), and poor pregnancy and artificial reproduction outcomes. Nevertheless, non-diagnostic and therapeutic methods have been developed to combat infertility and oxidative stress. However, profound evidence to recommend a suitable oxidative stress test is still lacking. Further research is required to overcome the current limitations.
Declaration of interest
RH is employed at LogixX Pharma. The other authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This study did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author contribution statement
N B Takalani contributed to conceptualization, drafted the manuscript, and created the tables; C S Opuwari contributed to conceptualization, manuscript writing, and editing and provided supervision. T K Monsees, R Henkel, E Monaneng, and K Mohlala contributed to manuscript editing.
References
- Adewoyin M Ibrahim M Roszaman R Isa MLM Alewi NAM Rafa AAA & Anuar MNN. 2017Male infertility: the effect of natural antioxidants and phytocompounds on seminal oxidative stress. Diseases 5 9. ( 10.3390/diseases5010009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A & Said TM. 2004Carnitines and male infertility. Reproductive Biomedicine Online 8376–384. ( 10.1016/s1472-6483(1060920-0) [DOI] [PubMed] [Google Scholar]
- Agarwal A & Majzoub A. 2017Laboratory tests for oxidative stress. Indian Journal of Urology 33199–206. ( 10.4103/iju.IJU_9_17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A & Bui AD. 2017. Oxidation-reduction potential as a new marker for oxidative stress: Correlation to male infertility. Investigative and Clinical Urology 58385. ( 10.4111/icu.2017.58.6.385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A Allamaneni SS & Said TM. 2004Chemiluminescence technique for measuring reactive oxygen species. Reproductive Biomedicine Online 9466–468. ( 10.1016/S1472-6483(1061284-9) [DOI] [PubMed] [Google Scholar]
- Agarwal A Virk G Ong C & du Plessis SS. 2014Effect of oxidative stress on male reproduction. World J. Mens Health 321–17. ( 10.5534/wjmh.2014.32.1.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A Mulgund A Hamada A & Chyatte MR. 2015A unique view on male infertility around the globe. Reproductive Biology and Endocrinology: RB&E 13 37. ( 10.1186/s12958-015-0032-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A Roychoudhury S Bjugstad KB & Cho CL. 2016Oxidation-reduction potential of semen: what is its role in the treatment of male infertility? Therapeutic Advances in Urology 8302–318. ( 10.1177/1756287216652779) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A Roychoudhury S Sharma R Gupta S Majzoub A & Sabanegh E. 2017Diagnostic application of oxidation-reduction potential assay for measurement of oxidative stress: clinical utility in male factor infertility. Reproductive Biomedicine Online 3448–57. ( 10.1016/j.rbmo.2016.10.008) [DOI] [PubMed] [Google Scholar]
- Agarwal A Henkel R Sharma R Tadros NN & Sabanegh E. 2018aDetermination of seminal oxidation-reduction potential (ORP) as an easy and cost-effective clinical marker of male infertility. Andrologia 50. ( 10.1111/and.12914) [DOI] [PubMed] [Google Scholar]
- Agarwal A Qiu E & Sharma R. 2018bLaboratory assessment of oxidative stress in semen. Arab Journal of Urology 1677–86. ( 10.1016/j.aju.2017.11.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A, Parekh N, Panner Selvam MK, Henkel R, Shah R, Homa ST, Ramasamy R, Ko E, Tremellen K, Esteves S, et al. 2019Male oxidative stress infertility (MOSI): proposed terminology and clinical practice guidelines for management of idiopathic male infertility. World J. Mens Health 37 296–312. ( 10.5534/wjmh.190055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A Baskaran S Parekh N Cho C-L Henkel R Vij S Arafa M Panner Selvam MK & Shah R. 2021Male infertility. Lancet 397319–333. ( 10.1016/S0140-6736(2032667-2) [DOI] [PubMed] [Google Scholar]
- Agarwal A Maldonado Rosas I Anagnostopoulou C Cannarella R Boitrelle F Munoz LV Finelli R Durairajanayagam D Henkel R & Saleh R. 2022Oxidative stress and assisted reproduction: a comprehensive review of its pathophysiological role and strategies for optimizing embryo culture environment. Antioxidants 11 477. ( 10.3390/antiox11030477) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A, Cannarella R, Saleh R, Harraz AM, Kandil H, Salvio G, Boitrelle F, Kuroda S, Farkouh A, Rambhatla A, et al. 2023Impact of antioxidant therapy on natural pregnancy outcomes and semen parameters in infertile men: a systematic review and meta-analysis of randomized controlled trials. World J. Mens Health 4114–48. ( 10.5534/wjmh.220067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad KA Yuan Yuan D Nawaz W Ze H Zhuo CX Talal B Taleb A Mais E & Qilong D. 2017Antioxidant therapy for management of oxidative stress induced hypertension. Free Radical Research 51428–438. ( 10.1080/10715762.2017.1322205) [DOI] [PubMed] [Google Scholar]
- Aitken RJ.1999The amoroso lecture. The human spermatozoon–a cell in crisis? Journal of Reproduction and Fertility 1151–7. ( 10.1530/jrf.0.1150001) [DOI] [PubMed] [Google Scholar]
- Aitken RJ.2022The changing tide of human fertility. Human Reproduction 37629–638. ( 10.1093/humrep/deac011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alahmar AT.2019Role of oxidative stress in male infertility: an updated review. Journal of Human Reproductive Sciences 124–18. ( 10.4103/jhrs.JHRS_150_18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alahmar AT & Sengupta P. 2021. Impact of Coenzyme Q10 and Selenium on Seminal Fluid Parameters and Antioxidant Status in Men with Idiopathic Infertility. Biological Trace Element Research 1991246–1252. ( 10.1007/s12011-020-02251-3) [DOI] [PubMed] [Google Scholar]
- Ammar-Keskes L Feki N Rebai T Sahnoun Z Ghozzi Z Hammami S Zeghal K Fki H Damak J & Bahloul A. 2003. Sperm oxidative stress and the effect of an oral vitamin E and selenium supplement on semen quality in infertile men. Archives of Andrology 4983–94. ( 10.1080/713828100) [DOI] [PubMed] [Google Scholar]
- Aquilano K Baldelli S & Ciriolo MR. 2014Glutathione: new roles in redox signaling for an old antioxidant. Frontiers in Pharmacology 5 196. ( 10.3389/fphar.2014.00196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arafa M Agarwal A Majzoub A Panner Selvam MK Baskaran S Henkel R & Elbardisi H. 2020Efficacy of antioxidant supplementation on conventional and advanced sperm function tests in patients with idiopathic male infertility. Antioxidants 9 219. ( 10.3390/antiox9030219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong JS Bivalacqua TJ Chamulitrat W Sikka S & Hellstrom WJG. 2002A comparison of the NADPH oxidase in human sperm and white blood cells. International Journal of Andrology 25223–229. ( 10.1046/j.1365-2605.2002.00351.x) [DOI] [PubMed] [Google Scholar]
- Ayad B Omolaoye TS Louw N Ramsunder Y Skosana BT Oyeipo PI & Du Plessis SS. 2022Oxidative stress and male infertility: evidence from a research perspective. Frontiers in Reproductive Health 4 822257. ( 10.3389/frph.2022.822257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolini D, Marinelli R, Stabile AM, Frammartino T, Guerrini A, Garetto S, Lucci J, Migni A, Zatini L, Marcantonini G, et al. 2022Wheat germ oil vitamin E cytoprotective effect and its nutrigenomics signature in human hepatocyte lipotoxicity. Heliyon 8 e10748. ( 10.1016/j.heliyon.2022.e10748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrán JMG Espinosa C Guardiola FA & Esteban MÁ. 2018In vitro effects of Origanum vulgare leaf extracts on gilthead seabream (Sparus aurata L.) leucocytes, cytotoxic, bactericidal and antioxidant activities. Fish and Shellfish Immunology 791–10. ( 10.1016/j.fsi.2018.05.005) [DOI] [PubMed] [Google Scholar]
- Boivin J Bunting L Collins JA & Nygren KG. 2007International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Human Reproduction 221506–1512. ( 10.1093/humrep/dem046) [DOI] [PubMed] [Google Scholar]
- Borumandnia N Alavi Majd H Khadembashi N & Alaii H. 2022Worldwide trend analysis of primary and secondary infertility rates over past decades: a cross-sectional study. International Journal of Reproductive Biomedicine 2037–46. ( 10.18502/ijrm.v20i1.10407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui AD Sharma R Henkel R & Agarwal A. 2018Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia 50 e13012. ( 10.1111/and.13012) [DOI] [PubMed] [Google Scholar]
- Büyükleblebici S Tuncer PB Bucak MN Eken A Sarıözkan S Taşdemir U & Endirlik BÜ. 2014Cryopreservation of bull sperm: effects of extender supplemented with different cryoprotectants and antioxidants on sperm motility, antioxidant capacity and fertility results. Animal Reproduction Science 15077–83. ( 10.1016/j.anireprosci.2014.09.006) [DOI] [PubMed] [Google Scholar]
- Castellini C D’Andrea S Cordeschi G Totaro M Parisi A Di Emidio G Tatone C Francavilla S & Barbonetti A. 2021Pathophysiology of mitochondrial dysfunction in human spermatozoa: focus on energetic metabolism, oxidative stress and apoptosis. Antioxidants 10 695. ( 10.3390/antiox10050695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castleton P Gyawali P Mathews N Mutuku SM Sharkey DJ & McPherson NO. 2022MiOXSYS® and OxiSperm® II assays appear to provide no clinical utility for determining oxidative stress in human sperm-results from repeated semen collections. Andrology. ( 10.1111/andr.13356) [DOI] [PubMed] [Google Scholar]
- Collins GG & Rossi BV. 2015The impact of lifestyle modifications, diet, and vitamin supplementation on natural fertility. Fertil Res Pract 1 11. ( 10.1186/s40738-015-0003-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabaja AA & Schlegel PN. 2014Medical treatment of male infertility. Translational Andrology and Urology 39–16. ( 10.3978/j.issn.2223-4683.2014.01.06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lamirande E Leclerc P & Gagnon C. 1997Capacitation as a regulatory event that primes spermatozoa for the acrosome reaction and fertilization. Molecular Human Reproduction 3175–194. ( 10.1093/molehr/3.3.175) [DOI] [PubMed] [Google Scholar]
- Dias TR.person-group>2021Measurement of reactive oxygen species in semen samples using chemiluminescence. In Reactive Oxygen Species: Methods and Protocols, Methods in Molecular Biology Espada J. Ed. New York, NY: Springer; 103–109. ( 10.1007/978-1-0716-0896-8_9) [DOI] [PubMed] [Google Scholar]
- Durairajanayagam D.2019. Chapter 1.8 - Physiological role of reactive oxygen species in male reproduction. In Oxidants, Antioxidants and Impact of the Oxidative Status in Male Reproduction. Henkel R Samanta L & Agarwal A Eds. Academic Press, pp. 65–78. ( 10.1016/B978-0-12-812501-4.00008-0) [DOI] [Google Scholar]
- Dutta S Majzoub A & Agarwal A. 2019Oxidative stress and sperm function: a systematic review on evaluation and management. Arab Journal of Urology 1787–97. ( 10.1080/2090598X.2019.1599624) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisch IMW, Peters WHM, Thomas CMG, Wetzel AMM, Peer PGM & Steegers-Theunissen RPM 2006. Homocysteine, glutathione and related thiols affect fertility parameters in the (sub)fertile couple. Human Reproduction 211725–1733. ( 10.1093/humrep/del081) [DOI] [PubMed] [Google Scholar]
- Esfandiari N Sharma RK Saleh RA Thomas AJ & Agarwal A. 2003Utility of the nitroblue tetrazolium reduction test for assessment of reactive oxygen species production by seminal leukocytes and spermatozoa. Journal of Andrology 24862–870. ( 10.1002/j.1939-4640.2003.tb03137.x) [DOI] [PubMed] [Google Scholar]
- Fallah A Mohammad-Hasani A & Colagar AH. 2018Zinc is an essential element for male fertility: a review of Zn roles in Men’s health, germination, sperm quality, and fertilization. Journal of Reproduction and Infertility 1969–81. [PMC free article] [PubMed] [Google Scholar]
- Fanaei H Khayat S Halvaei I Ramezani V Azizi Y Kasaeian A Mardaneh J Parvizi MR & Akrami M. 2014Effects of ascorbic acid on sperm motility, viability, acrosome reaction and DNA integrity in teratozoospermic samples. Iranian Journal of Reproductive Medicine 12103–110. [PMC free article] [PubMed] [Google Scholar]
- Gatimel N Moreau J Parinaud J & Léandri RD. 2017. Sperm morphology: assessment, pathophysiology, clinical relevance, and state of the art in 2017. Andrology 5845–862. ( 10.1111/andr.12389) [DOI] [PubMed] [Google Scholar]
- Gatimel N Moreau J Parinaud J & Léandri RD. 2017. Sperm morphology: assessment, pathophysiology, clinical relevance, and state of the art in 2017. Andrology 5845–862. ( 10.1111/andr.12389) [DOI] [PubMed] [Google Scholar]
- Gavella M & Lipovac V. 1992NADH-dependent oxidoreductase (diaphorase) activity and isozyme pattern of sperm in infertile men. Archives of Andrology 28135–141. ( 10.3109/01485019208987691) [DOI] [PubMed] [Google Scholar]
- Ghafarizadeh A Malmir M Naderi Noreini S & Faraji T. 2021. Antioxidant effects of N-acetylcysteine on the male reproductive system: A systematic review. Andrologia 53e13898. ( 10.1111/and.13898) [DOI] [PubMed] [Google Scholar]
- Gharagozloo P & Aitken RJ. 2011. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Human Reproduction 261628–1640. ( 10.1093/humrep/der132) [DOI] [PubMed] [Google Scholar]
- Greco E Iacobelli M Rienzi L Ubaldi F Ferrero S & Tesarik J. 2005. Reduction of the Incidence of Sperm DNA Fragmentation by Oral Antioxidant Treatment. Journal of Andrology 26349–353. ( 10.2164/jandrol.04146) [DOI] [PubMed] [Google Scholar]
- Gupta SK.2021. Human Zona Pellucida Glycoproteins: Binding Characteristics With Human Spermatozoa and Induction of Acrosome Reaction. Frontiers in Cell and Developmental Biology 9. ( 10.3389/fcell.2021.619868) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwan MH Almashhedy LA & Alsalman ARS. 2012. Oral zinc supplementation restore high molecular weight seminal zinc binding protein to normal value in Iraqi infertile men. BMC Urology 1232. ( 10.1186/1471-2490-12-32) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel RR.2011Leukocytes and oxidative stress: dilemma for sperm function and male fertility. Asian Journal of Andrology 1343–52. ( 10.1038/aja.2010.76) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel R, Morris A, Vogiatzi P, Saleh R, Sallam H, Boitrelle F, Garrido N, Arafa M, Gül M, Rambhatla A, et al. 2022Predictive value of seminal oxidation-reduction potential analysis for reproductive outcomes of ICSI. Reproductive Biomedicine Online 451007–1020. ( 10.1016/j.rbmo.2022.05.010) [DOI] [PubMed] [Google Scholar]
- Homa ST Vassiliou AM Stone J Killeen AP Dawkins A Xie J Gould F & Ramsay JWA. 2019. A Comparison Between Two Assays for Measuring Seminal Oxidative Stress and their Relationship with Sperm DNA Fragmentation and Semen Parameters. Genes 10236. ( 10.3390/genes10030236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito F Sono Y & Ito T. 2019Measurement and clinical significance of lipid peroxidation as a biomarker of oxidative stress: oxidative stress in diabetes, atherosclerosis, and chronic inflammation. Antioxidants 8 72. ( 10.3390/antiox8030072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhee KH & Kruger WD. 2005The role of cystathionine beta-synthase in homocysteine metabolism. Antioxidants and Redox Signaling 7813–822. ( 10.1089/ars.2005.7.813) [DOI] [PubMed] [Google Scholar]
- Joao F Duval C Bélanger MC Lamoureux J Xiao CW Ates S Benkhalifa M & Miron P. 2022Reassessing the interpretation of oxidation–reduction potential in male infertility. Reproduction and Fertility 367–76. ( 10.1530/RAF-21-0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JH & Seo JT. 2014Empirical medical therapy in idiopathic male infertility: promise or panacea? Clinical and Experimental Reproductive Medicine 41108–114. ( 10.5653/cerm.2014.41.3.108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katerji M Filippova M & Duerksen-Hughes P. 2019Approaches and methods to measure oxidative stress in clinical samples: research applications in the cancer field. Oxidative Medicine and Cellular Longevity 2019 1279250. ( 10.1155/2019/1279250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katib AA Al–Hawsawi K Motair W & Bawa AM. 2014Secondary infertility and the aging male, overview. Central European Journal of Urology 67184–188. ( 10.5173/ceju.2014.02.art13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavoussi PK Gilkey MS Machen GL Kavoussi SK & Dorsey C. 2022Varicocele repair improves static oxidation reduction potential as a measure of seminal oxidative stress levels in infertile men: a prospective clinical trial using the MiOXSYS system. Urology 165193–197. ( 10.1016/j.urology.2022.04.007) [DOI] [PubMed] [Google Scholar]
- Kaye AD Jeha GM Pham AD Fuller MC Lerner ZI Sibley GT Cornett EM Urits I Viswanath O & Kevil CG. 2020Folic acid supplementation in patients with elevated homocysteine levels. Advances in Therapy 374149–4164. ( 10.1007/s12325-020-01474-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefer JC Agarwal A & Sabanegh E. 2009Role of antioxidants in the treatment of male infertility. International Journal of Urology 16449–457. ( 10.1111/j.1442-2042.2009.02280.x) [DOI] [PubMed] [Google Scholar]
- Khan P Idrees D Moxley MA Corbett JA Ahmad F von Figura G Sly WS Waheed A & Hassan MdI. 2014Luminol-based chemiluminescent signals: clinical and non-clinical application and future uses. Applied Biochemistry and Biotechnology 173333–355. ( 10.1007/s12010-014-0850-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoubnasabjafari M Ansarin K & Jouyban A. 2015. Reliability of malondialdehyde as a biomarker of oxidative stress in psychological disorders. BioImpacts 5123–127. ( 10.15171/bi.2015.20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko EY Siddiqi K Brannigan RE & Sabanegh ES. 2012Empirical medical therapy for idiopathic male infertility: a survey of the American Urological Association. Journal of Urology 187973–978. ( 10.1016/j.juro.2011.10.137) [DOI] [PubMed] [Google Scholar]
- Kobayashi H Gil-Guzman E Mahran AM Rakesh Nelson DR Thomas AJ & Agarwa A. 2001Quality control of reactive oxygen species measurement by luminol-dependent chemiluminescence assay. Journal of Andrology 22568–574. [PubMed] [Google Scholar]
- Kopáni M Celec P Danišovič L Michalka P & Biró C. 2006. Oxidative stress and electron spin resonance. Clinica Chimica Acta 36461–66. ( 10.1016/j.cca.2005.05.016) [DOI] [PubMed] [Google Scholar]
- Kumar N & Singh AK. 2015Trends of male factor infertility, an important cause of infertility: a review of literature. Journal of Human Reproductive Sciences 8191–196. ( 10.4103/0974-1208.170370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzafame FM La Vignera S Vicari E & Calogero AE. 2009Oxidative stress and medical antioxidant treatment in male infertility. Reproductive Biomedicine Online 19638–659. ( 10.1016/j.rbmo.2009.09.014) [DOI] [PubMed] [Google Scholar]
- Larsen U.2000Primary and secondary infertility in sub-Saharan Africa. International Journal of Epidemiology 29285–291. ( 10.1093/ije/29.2.285) [DOI] [PubMed] [Google Scholar]
- Larsen U.2005Research on infertility: which definition should we use? Fertility and Sterility 83846–852. ( 10.1016/j.fertnstert.2004.11.033) [DOI] [PubMed] [Google Scholar]
- Li R Jia Z & Trush MA. 2016Defining ROS in biology and Medicine. Reactive Oxygen Species 19–21. ( 10.20455/ros.2016.803) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobascio AM Felici MD Anibaldi M Greco P Minasi MG & Greco E. 2015. Involvement of seminal leukocytes, reactive oxygen species, and sperm mitochondrial membrane potential in the DNA damage of the human spermatozoa. Andrology 3265–270. ( 10.1111/andr.302) [DOI] [PubMed] [Google Scholar]
- Long S & Kenworthy S. 2022Round cells in diagnostic semen analysis: a guide for laboratories and clinicians. British Journal of Biomedical Science 79 10129. ( 10.3389/bjbs.2021.10129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubos E Loscalzo J & Handy DE. 2011Glutathione Peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxidants and Redox Signaling 151957–1997. ( 10.1089/ars.2010.3586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martí E Pérez-Pé R Muiño-Blanco T & Cebrián-Pérez JA. 2006Comparative study of four different sperm washing methods using apoptotic markers in ram spermatozoa. Journal of Andrology 27746–753. ( 10.2164/jandrol.106.000109) [DOI] [PubMed] [Google Scholar]
- Martin-Hidalgo D Bragado MJ Batista AR Oliveira PF & Alves MG. 2019Antioxidants and male fertility: from molecular studies to clinical evidence. Antioxidants 8. ( 10.3390/antiox8040089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzano G, Moscatelli N, Di Giacomo M, Martino NA, Lacalandra GM, Dell’Aquila ME, Maruccio G, Primiceri E, Chiriacò MS, Zara V, et al. 2020Centrifugation force and time alter CASA parameters and oxidative status of cryopreserved stallion sperm. Biology 9 22. ( 10.3390/biology9020022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas MN Flaxman SR Boerma T Vanderpoel S & Stevens GA. 2012National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLOS Medicine 9e1001356. ( 10.1371/journal.pmed.1001356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matorras R, Pérez-Sanz J, Corcóstegui B, Pérez-Ruiz I, Malaina I, Quevedo S, Aspichueta F, Crisol L, Martinez-Indart L, Prieto B, et al. 2020Effect of vitamin E administered to men in infertile couples on sperm and assisted reproduction outcomes: a double-blind randomized study. F&S Reports 1219–226. ( 10.1016/j.xfre.2020.09.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MJ Baumber J Kass PH & Meyers SA. 2010Osmotic stress induces oxidative cell damage to rhesus macaque spermatozoa. Biology of Reproduction 82644–651. ( 10.1095/biolreprod.109.080507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon KM.2018Flow cytometry: an overview. Current Protocols in Immunology 1205.1.1–5.1.11. ( 10.1002/cpim.40) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez-Ferro M Rice MW Roberts RC & Perez-Costas E. 2013. An accurate method for the quantification of cytochrome C oxidase in tissue sections. Journal of Neuroscience Methods 214156–162. ( 10.1016/j.jneumeth.2013.01.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NJ Rice-Evans C Davies MJ Gopinathan V & Milner A. 1993A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clinical Science 84407–412. ( 10.1042/cs0840407) [DOI] [PubMed] [Google Scholar]
- Moichela FT Adefolaju GA Henkel RR & Opuwari CS. 2021Aqueous leaf extract of Moringa oleifera reduced intracellular ROS production, DNA fragmentation and acrosome reaction in Human spermatozoa in vitro. Andrologia 53e13903. ( 10.1111/and.13903) [DOI] [PubMed] [Google Scholar]
- Moslemi MK & Tavanbakhsh S. 2011. Selenium–vitamin E supplementation in infertile men: effects on semen parameters and pregnancy rate. International Journal of General Medicine 499–104. ( 10.2147/IJGM.S16275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nago M Arichi A Omura N Iwashita Y Kawamura T & Yumura Y. 2021Aging increases oxidative stress in semen. Investigative and Clinical Urology 62233–238. ( 10.4111/icu.20200066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J Chang Q Xu J Li J Liu W Chen Z Jiao X Guo Y & Teng X. 2023Relationship of the levels of reactive oxygen species in the fertilization medium with the outcome of in vitro fertilization following brief incubation. Frontiers in Endocrinology 141133566. ( 10.3389/fendo.2023.1133566) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Y Yamasaki T Niwa T Niimi K Majima Y & Ishiguro I. 1996Role of catalase in retinal antioxidant defence system: its comparative study among rabbits, guinea pigs, and rats. Ophthalmic Research 28336–342. ( 10.1159/000267925) [DOI] [PubMed] [Google Scholar]
- Okouchi S Suzuki M Sugano K Kagamimori S & Ikeda S. 2002Water desirable for the human body in terms of oxidation-reduction potential (ORP) to pH relationship. Journal of Food Science 671594–1598. ( 10.1111/j.1365-2621.2002.tb08689.x) [DOI] [Google Scholar]
- Panner Selvam MK Baskaran S O’Connell S Almajed W Hellstrom WJG & Sikka SC. 2022Association between seminal oxidation-reduction potential and sperm DNA fragmentation—A meta-analysis. Antioxidants 11 1563. ( 10.3390/antiox11081563) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AS Leong JY & Ramasamy R. 2017. Prediction of male infertility by the World Health Organization laboratory manual for assessment of semen analysis: A systematic review. Arab Journal of Urology 1696–102. ( 10.1016/j.aju.2017.10.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehlivan FE.2017Vitamin C: an Antioxidant Agent, Vitamin C. IntechOpen. ( 10.5772/intechopen.69660) [DOI] [Google Scholar]
- Punjabi U Goovaerts I Peeters K Van Mulders H & De Neubourg D. 2022Sperm as a carrier of genome instability in relation to paternal lifestyle and nutritional conditions. Nutrients 14 3155. ( 10.3390/nu14153155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimlou M Sohaei S Nasr-Esfahani M & Nouri M. 2019Dietary antioxidant intake in relation to semen quality parameters in infertile men: a cross-sectional study. Clinical Nutrition Research 8229–237. ( 10.7762/cnr.2019.8.3.229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert KA Sharma R Henkel R & Agarwal A. 2021An update on the techniques used to measure oxidative stress in seminal plasma. Andrologia 53e13726. ( 10.1111/and.13726) [DOI] [PubMed] [Google Scholar]
- Rolf C Cooper TG Yeung CH & Nieschlag E. 1999Antioxidant treatment of patients with asthenozoospermia or moderate oligoasthenozoospermia with high-dose vitamin C and vitamin E: a randomized, placebo-controlled, double-blind study. Human Reproduction 141028–1033. ( 10.1093/humrep/14.4.1028) [DOI] [PubMed] [Google Scholar]
- Rubio-Riquelme N Huerta-Retamal N Gómez-Torres MJ & Martínez-Espinosa RM. 2020Catalase as a molecular target for male infertility diagnosis and monitoring: an overview. Antioxidants 9 78. ( 10.3390/antiox9010078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safarinejad MR & Safarinejad S. 2009. Efficacy of selenium and/or N-acetyl-cysteine for improving semen parameters in infertile men: a double-blind, placebo controlled, randomized study. Journal of Urology 181741–751. ( 10.1016/j.juro.2008.10.015) [DOI] [PubMed] [Google Scholar]
- Sanocka D & Kurpisz M. 2004Reactive oxygen species and sperm cells. Reproductive Biology and Endocrinology: RB&E 2 12. ( 10.1186/1477-7827-2-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaruffi P, Licata E, Maccarini E, Massarotti C, Bovis F, Sozzi F, Stigliani S, Dal Lago A, Casciano I, Rago R, et al. 2021Oral antioxidant treatment of men significantly improves the reproductive outcome of IVF cycles. Journal of Clinical Medicine 10 3254. ( 10.3390/jcm10153254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber M & Chandel NS. 2014ROS Function in redox signaling and oxidative stress. Current Biology: CB 24R453–R462. ( 10.1016/j.cub.2014.03.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seljeskog E Hervig T & Mansoor MA. 2006A novel HPLC method for the measurement of thiobarbituric acid reactive substances (TBARS). A comparison with a commercially available kit. Clinical Biochemistry 39947–954. ( 10.1016/j.clinbiochem.2006.03.012) [DOI] [PubMed] [Google Scholar]
- Serapinas D Boreikaite E Bartkeviciute A Bandzeviciene R Silkunas M & Bartkeviciene D. 2017The importance of folate, vitamins B6 and B12 for the lowering of homocysteine concentrations for patients with recurrent pregnancy loss and MTHFR mutations. Reproductive Toxicology Elmsford 72159–163. ( 10.1016/j.reprotox.2017.07.001) [DOI] [PubMed] [Google Scholar]
- Setumo MA Choma SS Henkel R & Opuwari CS. 2022Green tea (Camellia sinensis) aqueous extract improved human spermatozoa functions in vitro. Journal of Medicinal Plants for Economic Development 6 8. ( 10.4102/jomped.v6i1.166) [DOI] [Google Scholar]
- Sharma RK Pasqualotto FF Nelson DR Thomas AJ & Agarwal A. 1999The reactive oxygen species—total antioxidant capacity score is a new measure of oxidative stress to predict male infertility. Human Reproduction 142801–2807. ( 10.1093/humrep/14.11.2801) [DOI] [PubMed] [Google Scholar]
- Shekarriz M Sharma RK Thomas AJ & Agarwal A. 1995. Positive myeloperoxidase staining (Endtz test) as an indicator of excessive reactive oxygen species formation in semen. Journal of Assisted Reproduction and Genetics 1270–74. ( 10.1007/BF02211372) [DOI] [PubMed] [Google Scholar]
- Showell MG Mackenzie-Proctor R Jordan V & Hart RJ. 2020Antioxidants for female subfertility. Cochrane Database of Systematic Reviews 8CD007807 doi:10.1002/14651858.CD007807.pub4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H.1997Oxidative stress: oxidants and antioxidants. Experimental Physiology 82291–295. ( 10.1113/expphysiol.1997.sp004024) [DOI] [PubMed] [Google Scholar]
- Smits RM Mackenzie-Proctor R Fleischer K & Showell MG. 2018Antioxidants in fertility: impact on male and female reproductive outcomes. Fertility and Sterility 110578–580. ( 10.1016/j.fertnstert.2018.05.028) [DOI] [PubMed] [Google Scholar]
- Tafuri S Ciani F Iorio EL Esposito L & Cocchia N. 2015Reactive Oxygen Species (ROS) and Male Fertility, New Discoveries in Embryology. IntechOpen. ( 10.5772/60632) [DOI] [Google Scholar]
- Takalani NB Adefolaju GA Henkel R & Opuwari CS. 2021In vitro effects of aqueous extract of fermented rooibos (Aspalathus linearis) on human sperm function. Andrologia 53 e14114. ( 10.1111/and.14114) [DOI] [PubMed] [Google Scholar]
- Thaker H Ko EY Sabanegh ES Brannigan RE Alukal JP & Samplaski MK. 2020Empirical medical therapy for idiopathic male infertility. F&S Reports 115–20. ( 10.1016/j.xfre.2020.04.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur AS Littarru GP Funahashi I Painkara US Dange NS & Chauhan P. 2015. Effect of Ubiquinol Therapy on Sperm Parameters and Serum Testosterone Levels in Oligoasthenozoospermic Infertile Men. Journal of Clinical and Diagnostic Research 9BC01–BC03. ( 10.7860/JCDR/2015/13617.6424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremellen K.2008Oxidative stress and male infertility—a clinical perspective. Human Reproduction Update 14243–258. ( 10.1093/humupd/dmn004) [DOI] [PubMed] [Google Scholar]
- Tremellen K Woodman R Hill A Shehadeh H Lane M & Zander-Fox D. 2020Use of a male antioxidant nutraceutical is associated with superior live birth rates during IVF treatment. Asian Journal of Andrology 2316–23. ( 10.4103/aja.aja_41_20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tvrdá E Kňažická Z Bárdos L Massányi P & Lukáč N. 2011. Impact of oxidative stress on male fertility — A review. Acta Veterinaria Hungarica 59465–484. ( 10.1556/avet.2011.034) [DOI] [PubMed] [Google Scholar]
- Vander Borght M & Wyns C. 2018Fertility and infertility: definition and epidemiology. Clinical Biochemistry 622–10. ( 10.1016/j.clinbiochem.2018.03.012) [DOI] [PubMed] [Google Scholar]
- Vassiliou A Martin CH Homa ST Stone J Dawkins A Genkova MN Skyla Dela Roca H Parikh S Patel J Yap T & Killeen AP. 2021. Redox potential in human semen: Validation and qualification of the MiOXsys assay. Andrologia 53e13938. ( 10.1111/and.13938) [DOI] [PubMed] [Google Scholar]
- Wagner H Cheng JW & Ko EY. 2018Role of reactive oxygen species in male infertility: an updated review of literature. Arab Journal of Urology 1635–43. ( 10.1016/j.aju.2017.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C & Swerdloff RS. 2014Limitations of semen analysis as a test of male fertility and anticipated needs from newer tests. Fertility and Sterility 1021502–1507. ( 10.1016/j.fertnstert.2014.10.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J Wang T Ding W Wu J Wu G Wang Y Zhou Z Xu L & Cui Y. 2019Efficacy of antioxidant therapy on sperm quality measurements after varicocelectomy: a systematic review and meta-analysis. Andrologia 51 e13396. ( 10.1111/and.13396) [DOI] [PubMed] [Google Scholar]
- World Health Organization 2018Infertility [WWW Document]. World Health Organ. Available at: https://www.who.int/news-room/fact-sheets/detail/infertility. [Google Scholar]
- Zhaku V, Agarwal A, Beadini S, Henkel R, Finelli R, Beadini N, Micic S, Zhaku V, Agarwal A, Beadini S et al. 2021Male Infertility, Oxidative Stress and Antioxidants, Vitamin E in Health and Disease - Interactions, Diseases and Health Aspects. IntechOpen. ( 10.5772/intechopen.98204) [DOI] [Google Scholar]



 This work is licensed under a
This work is licensed under a