Abstract
With the prevalence of obesity and associated comorbidities, studies aimed at revealing mechanisms that regulate energy homeostasis have gained increasing interest. In 1994, the cloning of leptin was a milestone in metabolic research. As an adipocytokine, leptin governs food intake and energy homeostasis through leptin receptors (LepR) in the brain. The failure of increased leptin levels to suppress feeding and elevate energy expenditure is referred to as leptin resistance, which encompasses complex pathophysiological processes. Within the brain, LepR-expressing neurons are distributed in hypothalamus and other brain areas, and each population of the LepR-expressing neurons may mediate particular aspects of leptin effects. In LepR-expressing neurons, the binding of leptin to LepR initiates multiple signaling cascades including janus kinase (JAK)–signal transducers and activators of transcription (STAT) phosphatidylinositol 3-kinase (PI3K)-protein kinase B (AKT), extracellular regulated protein kinase (ERK), and AMP-activated protein kinase (AMPK) signaling, etc., mediating leptin actions. These findings place leptin at the intersection of metabolic and neuroendocrine regulations, and render leptin a key target for treating obesity and associated comorbidities. This review highlights the main discoveries that shaped the field of leptin for better understanding of the mechanism governing metabolic homeostasis, and guides the development of safe and effective interventions to treat obesity and associated diseases.
Keywords: leptin, leptin cellular pathways, leptin neural pathways, leptin resistance
Introduction
The World Health Organization (WHO) estimates that over 1.9 billion of adults were overweight or obese in 2016. Even worse, over 340 million adolescents and children aged 5–19 years, and over 41 million children under 5 years of age were overweight or obese in 2016. To date, obesity and obesity-associated comorbidities have been major burdens to the health-care systems worldwide.
Obesity is caused by the imbalance between food intake and energy expenditure. Leptin is a hormone produced mainly by adipocytes, which involves in a wide variety of physiological functions, especially in the regulation of energy balance. In 1969, Dr. Coleman and his colleagues performed a series of parabiosis studies on the naturally obese ob/ob and db/db mice, and found an unknown blood-borne circulating factor, which might be prominently involved in the regulation of body weight [1]. The ob/ob mice were missing this circulating factor, while db/db mice were unresponsive to it, resulting in their obesity [1]. In 1994, Dr. Friedman and his colleagues in the Rockefeller University first identified this circulating factor, and named it as leptin [2].
Leptin is a 167 amino acid protein with a molecular weight of 16 kDa. Leptin, encoded by the ob gene, is produced by and released from adipose tissues [3]. The ob gene is widely expressed in mammals, amphibians, reptiles, fish, etc. The amino acid sequence of leptin is highly conservative across species [4]. Six leptin receptor (LepR) isoforms (LepRa-f) are generated via alternative splicing of the ob gene, and are expressed in both central nervous system (CNS) and peripheral organs [5, 6]. Among these receptors, LepRb plays a critical role in mediating the effects of leptin on energy homeostasis [7]. In the CNS, leptin mainly targets on LepR-expressing neurons and transmits signals through multiple neural and cellular circuits to maintain energy balance and metabolic homeostasis by suppressing appetite and enhancing energy expenditure [7, 8]. In addition, leptin also participates in the regulation of cognition, neuroendocrine and immunity [8].
Leptin resistance is defined by a reduced sensitivity or a failure in response to leptin, showing a decrease in the ability of leptin to suppress appetite or enhance energy expenditure, which ultimately leads to overweight and obesity [9]. The impairment of leptin signaling is also closely associated with obesity-related diseases, including hyperlipidemia, diabetes mellitus, metabolic syndrome, etc. [10, 11].
Nowadays, with the rapid upsurge of global obesity epidemic, obesity is becoming a challenging public health problem. Thus, further studies of leptin signaling and leptin resistance is essential for understanding of the pathogenesis of obesity.
Neural mechanism underlying leptin function
In the brain, leptin transmits the peripheral lipid and glucose metabolic information, activates leptin signaling pathways and elicits a cascade of reactions, leading to reduced feeding, decreased appetite, enhanced mitochondrial oxidation and elevated thermogenesis [12]. The function of leptin depends on the interaction of leptin with LepRb in specific neurons located in multiple neural nuclei or brain regions [13]. To date, a series of LepR-expressing nuclei have been unveiled, which play critical roles in metabolic regulation. In recent years, with the advent of cutting-edge biotechnology, new functions of leptin in some nuclei have also been revealed, which are greatly expanding the understanding of leptin physiology.
Classic brain nuclei related to leptin function
Arcuate nucleus (ARC)
The hypothalamic ARC lies in close proximity to the median eminence with a permeable vasculature, which permits rapid access of ARC to circulating factors. Generally, the ARC is responsible for transmitting leptin signals to the hypothalamus and other brain regions for regulating the energy balance and metabolic homeostasis [14]. There are multiple cell types in the ARC, among which the agouti-related peptide/neuropeptide Y (AgRP/NPY) neurons and the proopiomelanocortin/cocaine-and amphetamine-regulated transcript (POMC/CART) neurons mainly participate in the reception and transmission of peripheral leptin signals [15]. These two types of neurons are named as their secretion of the orexigenic AgRP and NPY or the anorexigenic POMC and CART, respectively [15].
Under physiological conditions, leptin activates POMC/CART neurons, whereas inhibits AgRP/NPY neurons [15]. The leptin signals from the POMC/CART neurons project to the paraventricular hypothalamic nucleus (PVN), the lateral hypothalamic area (LHA), and other brain regions through the melanocortin system [16]. In general, POMC neuron-derived peptides, such as α-MSH, can activate the melanocortin-4 receptor (MC4R) in the PVN, which can be inhibited by AgRP released from the AgRP/NPY neurons [16, 17]. Notably, leptin control of the melanocortin system can be indirectly mediated by GABA- or nitric oxide synthase 1-expressing neurons [18, 19].
Intriguingly, the inhibition of AgRP neurons by leptin mainly induces transient effects on feeding and energy expenditure, whereas the activation of POMC neurons usually causes long-term changes in energy homeostasis [20]. The cooperation of leptin signaling in the POMC and AgRP neurons leads to suppressed food intake, increased energy expenditure, and inhibited liver glucose production [21].
Dorsomedial hypothalamus (DMH) and preoptic area (POA)
The DMH and POA are a functional complex of nuclei in the hypothalamus, which are involved in the regulation of feeding, drinking, body-weight, and circadian rhythms. The POA is also responsible for the central thermoregulation. Physiologically, leptin activates neurons in the POA to activate the downstream effector neurons in the nucleus raphe pallidus (RPa) via a projection from POA to DMH, enhancing the sympathetic activity of brown adipose tissue (BAT) and thermogenesis [22], [23], [24]. This process is independent of food intake [24]. In addition, DMH contains neurons expressing NPY, which also play a role in elevating energy expenditure through activating the sympathetic nervous system [25, 26].
As reported recently, silencing the neurotransmission in DMH LepR-expressing neurons reduces energy expenditure without affecting food intake, increases body weight and adiposity; and phase-advances diurnal rhythms of feeding and metabolism into the light cycle, and abolishes the normal increase in dark-cycle locomotor activity [27]. Interestingly, chronic chemo-genetic stimulation of POA LepR-expressing neurons decreases both food intake and energy expenditure, and results in body weight loss, indicating that the reduced food intake surpasses the decreased energy expenditure [28]. Taken together, the DMH and POA leptin signaling contributes to body weight homeostasis by modulating energy expenditure and/or food intake in interaction with environmental information.
LHA
The LHA contains both glutamatergic and GABAergic neurons, although these classical neurotransmitters do not fully explain the roles of LHA in feeding and metabolic regulation [29]. The discovery of the sub-populations of neurons expressing distinct neuropeptides within the LHA considerably advances our understanding of the roles of LHA. These sub-populations of LHA neurons mainly include hypocretin/orexin-expressing neurons and melanin concentrating hormone (MCH)-releasing neurons [30].
Functionally, the orexin, also known as hypocretin, is a neuropeptide that regulates arousal, sleep, appetite and motivated behavior [31]. In addition, intracisternal injection of orexin antibody reduces food intake, suggesting that orexin regulates feeding [32]. Leptin treatment inhibits orexin expression and neuronal activity, while silencing of leptin signals increases orexin expression and elevates the excitatory synaptic inputs onto orexin neurons [33]. Leptin reduces orexin synthesis and secretion through LepR in LHA neurons or through the dopaminergic projection from the ventral tegmental area (VTA) to LHA to suppress food intake [33, 34]. Surprisingly, orexin over-expression prevents diet-induced obesity through improving leptin sensitivity and enhancing energy expenditure [35]. Overall, leptin control of orexin neurons is complex but required for appropriate energy balance.
MCH is characterized as an orexigenic neuropeptide. Treatment with MCH increases food intake and body weight. The mice over-expressing MCH are obese [36, 37]. Treatment with leptin induces the release of melanocortin in ARC POMC neurons, which further inhibits the expression of MCH in LHA neurons [38]. The inhibition of MCH expression reduces neuronal firing in the nucleus accumbens (NAc) [39]. MCH neurons send inhibitory project to orexin neurons, resulting in reduced food intake [40].
The LHA neurotensin (Nts) neurons co-express LepRb [41]. These neurons project to the VTA and substantia nigra compacta to coordinate feeding and drinking [41]. Specific modulation of these neurons might be useful to specialize the treatment of polydipsia or obesity.
PVN
The PVN is a major sympathetic output originating from the hypothalamus. The PVN receives input from the hypothalamic sites, such as ARC, DMH, ventromedial hypothalamus (VMH), etc. [42]. The PVN also receives innervation from forebrain regions, such as POA, and brainstem sites, such as parabrachial nucleus (PBN), contributing to the control of feeding, anxiety and stress response [42]. AgRP neuron-stimulated feeding requires inhibition of PVN neurons [43]. Activation of PVN neurons acutely increases energy expenditure and decreases food intake [44, 45]. The PVN responds to peripheral signals of leptin, and is critical for leptin action and the overall control of energy balance.
Generally, the PVN integrates the leptin signals from the ARC and other brain regions, and subsequently acts in the following ways. First, PVN projects to autonomic centers for regulating body temperature, cardiovascular activity, locomotor activity, and stress response [46, 47]. Second, PVN innervates the pituitary (PIT) portal system to achieve neuroendocrine regulatory effects [47, 48]. Third, PVN projects to the nucleus tractus solitaries (NTS) in the brainstem to induce satiety and reduce food intake [42, 49]. Fourth, MC4R neurons in the PVN project to the PBN to modulate satiety [45, 50]. The nitric oxide synthase 1 (Nos1)-expressing neurons in the PVN control feeding and energy expenditure via the PBN as well as the intermediolateral nucleus (IML) [44].
Medial nucleus tractus solitaries (mNTS) and area postrema (AP)
The NTS is a series of sensory nuclei in the mammalian brainstem. NTS receives viscerosensory information carried by vagal afferent fibers from gastrointestinal, cardiovascular, and respiratory systems [51]. Leptin receptors are expressed in vagal afferent nerves and a population of NTS neurons, and leptin signaling within the NTS contributes to the reduction of food intake and body weight [52, 53]. Selective ablation of LepR in the NTS neurons causes hyperphagia, weight gain, and weakened responses to satiety signals, such as cholecystokinin (CCK) [54]. The AP is a circumventricular organ and plays important roles in central autonomic regulation. The LepR-expressing neurons in the AP modulate metabolic and energy status [55].
Leptin regulates gastrointestinal satiation signals, such as gastric distension, CCK or glucagon-like-peptide-1 (GLP-1) by acting on LepR in mNTS/AP neurons [56], [57], [58], [59]. Knockdown of LepR in the mNTS and AP neurons dampens the leptin action on gastrointestinal satiation signaling, hyperphagia, thereby increasing body weight and adiposity [56]. Thus, the leptin signals in mNTS and AP are essential for energy balance control.
Ventral tegmental area (VTA)
The VTA is located close to the midline on the floor of the midbrain, participating in the regulation of reward, motivation, and learning [60]. The ARC within the forebrain, the VTA within the midbrain, and the NTS within the hindbrain are three main regions responsive to direct leptin stimulation [51, 61].
Leptin directly inhibits the dopaminergic neurons in the VTA, or indirectly inhibits these neurons by reducing orexin levels in the LHA [62, 63]. The inhibition of the dopaminergic neurons in the VTA further prevents sucrose-induced dopamine release into the NAc, thereby down-regulating feeding motivation, inhibiting food reward, and suppressing appetite [64].
LepRb-specific anterograde tracing shows that LepR-expressing neurons in the VTA mainly project to the extended central nucleus of the amygdala (extCeA) [65]. These LepRb neurons in the VTA innervate the extCeA to control the cocaine and amphetamine regulated transcript (CART) neurons in the extCeA, contributing to the reward functions [65].
Newly-discovered brain nuclei related to leptin function
Recently, multiple studies identified a series of clusters of neurons expressing LepR in certain nuclei as novel mediators involved in metabolic regulation.
PBN
The PBN integrates visceral, oral, and other sensory information, and plays an integral role in the neural control of feeding and body weight. There is a GABAergic inhibitory projection from AgRP neurons to the PBN neurons, which is critical for the leptin effects on food intake reduction and energy expenditure enhancement [66, 67].
The PBN neurons also receive signals from the NTS [68, 69]. These NTS neurons respond to visceral signals from the vagus nerve, or anorexic serotonin signals from the raphe obscurus (ROb) and raphe magnus (RMg) [70]. The PBN integrates glutamatergic information from the NTS to reduce food intake through projecting into the central amygdala nucleus (CeA) [68, 71]. CCK-expressing neurons in the NTS activate calcitonin gene-related protein (CGRP)-expressing neurons in the PBN to reduce food intake [69]. However, several studies also point out that PBN LepR neurons are not essential for the NTS→PBN→CeA anorexia circuit [72].
Hypoglycemia directly promotes CCK release in the PBN neurons in the state of negative energy balance [73]. These activated PBN neurons project to VMH neurons and cause counterregulatory response (CRR), thereby stimulating glucose production and inhibiting glucose uptake to restore normal blood glucose levels in the hypoglycemic state [73, 74]. In this process, leptin inactivates PBN neurons and inhibits CRR to down-regulate blood glucose levels and maintain glucose metabolism balance [72].
Central nucleus of the amygdala (CeA)
The CeA is a major output nucleus of the amygdala, which receives and processes the pain information. Physiologically and pharmacologically, the CeA plays a role in mediating the leptin function to suppress appetite and food intake. In these processes, leptin signaling from the hypothalamus and other brain regions activates the PBN-CeA circuit to suppress feeding [71]. This leptin signaling pathway also affects the extCeA to suppress the CeA CART expression, and thus reduces food intake [65].
Hippocampus
The hippocampus is a major component of the brain for learning, memory and space exploring. The hippocampal-dependent mnemonic function mediates the feeding behavior [75]. Leptin interacts with LepR in the hippocampal neurons to inhibit food-related memory processing and thus reduce food intake [76].
Additionally, leptin facilitates the cellular actions underlying hippocampal-dependent learning and memory, including glutamate receptor trafficking, neuronal morphology and activity-dependent synaptic plasticity [77, 78]. These effects of leptin may be beneficial for preventing the neurodegenerative disorders such as Alzheimer’s disease (AD) [77, 78].
Periaqueductal gray (PAG)
The PAG, an area of the gray matter in the midbrain, is essential for regulating analgesia, defensive behavior and reproductive behavior. In the brainstem, the PAG LepRb-expressing neurons are the largest population of LepRb neurons [79]. Noxious stimuli, such as pain, affect the PAG LepRb neurons to activate the PBN→VMH circuit, increasing sympathetic activity and blood glucose concentrations [72, 80]. Ablation of LepRb in PAG neurons augments the activation of PAG→PBN→VMN circuit and the hyperglycemic response to noxious stimuli [80]. Thus, the diminished PAG leptin signaling ensures adequate sympathetic activation and glucose mobilization to meet the metabolic needs associated with noxious stimuli or negative energy balance.
Substantia nigra (SN)
The SN, a basal ganglia structure in the midbrain, involves in the regulation of reward and movement. LepR is expressed in the SN neurons [79]. Intriguingly, the LepR neurons in the SN and VTA exhibit diverse functions [81]. Activation of the VTA LepR neurons mainly decreases motivation for feeding and food reward, whereas activation of the SN LepR neurons mainly reduces locomotor activity [81].
Other brain structures
There are some other brain structures expressing leptin receptors, such as certain areas of the cerebral cortex, etc. [79]. The potential roles of leptin in these brain regions remain to be elucidated.
Neural pathways related to leptin function
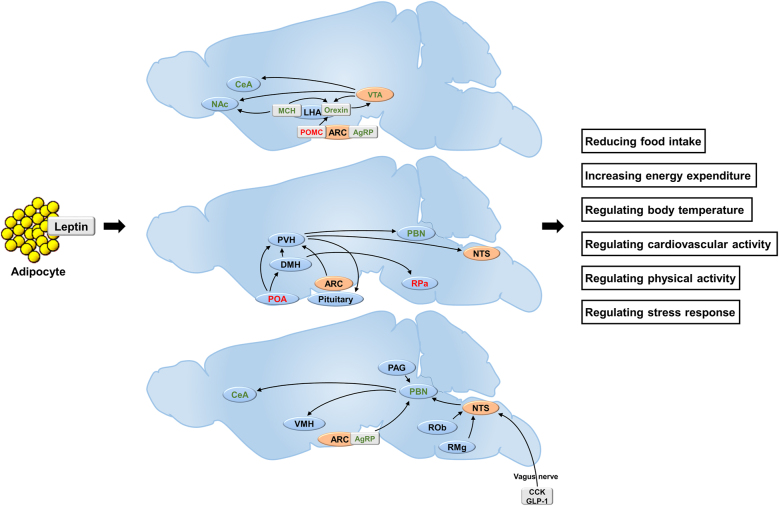
The classical neural pathways underlying the leptin function include the ARC/VMH/DMH→PVN circuit, the ARC→LHA circuit, and the ARC→PBN circuit. Recently, several new neural circuits have been discovered, such as the VTA→CeA circuit, the NTS→PBN→CeA circuit, and the PAG-PBN circuit. The classical and novel neural pathways underlying leptin function are summarized as Figure 1.
Figure 1:
Brain nucleus and neural pathways underlying leptin function. Orange background: regions of neurons responsive to direct leptin stimulation; red color text: regions of neurons activated by leptin; green color text: regions of neurons inactivated by leptin. ARC, arcuate nucleus; AgRP, agouti-related peptide; CCK, cholecystokinin; CeA, central nucleus of amygdala; DMH, dorsomedial hypothalamus; GLP-1, glucagon-like peptide-1; LHA, lateral hypothalamic area; MCH, melanin concentrating hormone; NAc, nucleus accumbens; NTS, nucleus tractus solitaries; POMC, proopiomelanocortin; PBN, parabrachial nucleus; POA, preoptic area; PVH, paraventricular nucleus; PAG, periaqueductal grey matter; RPa, raphe pallidus; ROb, raphe obscurus; RMg, raphe magnus; VMH, ventromedial hypothalamic nucleus; VTA, ventral tegmental area.
Cellular mechanism underlying leptin function
Leptin acts in the brain to govern metabolic homeostasis. Cellular leptin signaling pathways mediate these processes. Once leptin binds to the LepR, a series of intracellular reactions are triggered, leading to reduced food intake and enhanced energy expenditure.
Classic leptin signaling pathways
LepRb-JAK2-STAT3/5
Numerous evidence demonstrates that the janus kinase (JAK)–signal transducers and activators of transcription (STAT) signaling is the major signaling pathway, through which leptin controls energy homeostasis and neuroendocrine function [82]. This pathway is also linked to immunity, cell division, cell death, and tumor formation [83]. This pathway conveys the extracellular information to the nucleus, resulting in transcription of certain genes.
LepRb belongs to the type I family of cytokine receptors and lacks an intrinsic catalytic activity [84]. Instead, binding of leptin to LepRb initiates a signaling cascade beginning with activation of the tyrosine kinase of the Jak kinase family (JAK2) [84, 85]. The phosphorylated JAKs, especially JAK2, in turn stimulates the phosphorylation of three residues on the intracellular domain of LepRb (Tyr985, Tyr1077 and Tyr1138), and recruits distinct downstream signaling molecules to a leptin-specific signaling pathway to exhibit diverse physiological functions [84, 86].
The STATs are cytoplasmic proteins activated by various cytokines, growth factors and hormones, including leptin. STAT3 is widely expressed in the CNS. The neuron-specific deletion of STAT3 causes hyperphagia, obesity, diabetes, and hyperleptinemia [87, 88]. In response to leptin, JAK2 phosphorylates LepRb on Tyr1138 and this phospho-Tyr1138 binds to the Src homology 2 (SH2) domain of STAT3 to trigger STAT3 phosphorylation [89, 90]. Phosphorylation promotes STAT3 dimerization and its translocation from the cytoplasm into the nucleus, where STAT3 acts as a transcription factor to modulate the transcription of target genes, such as neuropeptides POMC, AgRP, and NPY [91], [92], [93]. In this process, the expression of orexigenic POMC is upregulated, whereas the anorexigenic AgRP and NPY are repressed, leading to reduced food intake and increased energy expenditure [91], [92], [93]. The phosphorylated STAT3 also enhances the transcription of the suppressor of cytokine signaling 3 (SOCS3), which in turn creates a negative feedback loop and counterbalances the leptin signaling [94].
STAT5 involves in the leptin signaling pathways. STAT5 deletion in the brain causes hyperphagia and obesity [95]. In response to leptin, JAK2 activates LepRb on Tyr1077 to phosphorylate STAT5 [96]. PhosphoTyr1138 also is partially involved in STAT5 activation [97]. LepRb Tyr1138 and STAT3 activation attenuate STAT5-dependent transcription over the long term [97].
The leptin-induced tyrosine phosphorylation of JAK2 can be inhibited by SOCS3, which terminates the leptin signaling cascade [98]. SOCS3 mRNA expression in the hypothalamus is specifically induced by leptin. The neuron-specific deletion of SOCS3 increases leptin sensitivity through STAT3 activation, indicating that SOCS3 is an important negative feedback regulator of leptin signaling [99, 100]. Protein tyrosine phosphatase 1B (PTP1B) is also a well-recognized negative regulator of the leptin signaling, which attenuates the leptin-induced JAK2/STAT3 signaling by dephosphorylating JAK2 [101].
LepRb-JAK2-IRS-PI3K-AKT-mTOR-S6K1
Leptin activates certain components of the insulin-signaling cascade to achieve its functions, including reducing food intake and increasing energy expenditure [102]. Leptin enhances the insulin-receptor substrates 1/2 (IRS1/2) phosphorylation via activation of the JAK2 [103, 104]. Phosphorylation of both IRS1 and IRS2 activates the phosphatidylinositol 3-kinase (PI3K) [105]. SH2B adaptor protein 1 (SH2B1) recruits IRS to JAK2, and IRS proteins are phosphorylated by JAK2 and activate the PI3K pathway [106, 107]. PTP1B suppresses the IRS1/2 to inhibit the IRS-PI3K axis [108, 109].
In leptin-sensitive neurons, PI3K catalyzes the phosphatidylinositol-4, 5-bisphosphate (PIP2) to phosphatidylinositol-3, 4, 5-trisphosphate (PIP3). Increased PIP3 level leads to activation of the phosphoinositide-dependent kinase 1 (PDK1), and activates the v-akt murine thymoma viral oncogene homolog 1 (AKT) through the PH domain of AKT [110, 111]. This process enhances the downstream signaling that depends on AKT, leading to a reduced body fat mass [110].
AKT, also known as protein kinase B (PKB), is a serine/threonine-specific protein kinase that plays critical roles in metabolism, apoptosis, cell proliferation, and cell migration [112]. AKT activates the mammalian target of rapamycin (mTOR), and also activates the cAMP response element-binding protein (CREB), and localizes the forkhead box protein O1 (FoxO1) in the cytoplasm [113].
In the CNS, mTOR is a downstream target of AKT in NPY/AgRP neurons and POMC neurons [114]. The activated mTOR phosphorylates ribosomal protein S6 kinase beta-1 (S6K1) at Thr389 in these neurons to reduce food intake, elevate the renal sympathetic nerve outflow, increase energy expenditure and protect against obesity [114, 115].
LepRb-IRS-PI3K-AKT-FoxO1
FoxO1 is a transcription factor involved in regulation of gluconeogenesis, glycogenolysis as well as adipogenesis. FoxO1 is a phosphorylation target of the PI3K-AKT axis, mediating the anorectic effects of leptin through transcriptional regulation of POMC and AgRP [116]. In the LepR neurons in the ARC, VMH and DMH, activated FoxO1 translocates from the cytoplasm to the nucleus, where it upregulates the orexigenic NPY/AgRP and downregulates the anorexigenic POMC to promote feeding [117, 118]. Leptin suppresses the FoxO1-mediated transcriptional regulation of POMC, NPY and AgRP through the PI3K-AKT signaling pathway to reduce food intake [119]. Conversely, excessive activation of FoxO1 antagonizes the effects of STAT3, dampens the leptin ability to stimulate POMC transcription, and leads to a decreased leptin sensitivity [120]. FoxO1 inhibition in POMC neurons elevates expression of the carboxypeptidase E (Cpe), decreasing food intake without altering energy expenditure [121].
LepRb-IRS-PI3K-PDE3B-cAMP
Cyclic nucleotide phosphodiesterases (PDEs) are enzymes that regulate the cellular levels of cAMP and cGMP by controlling their degradation rates. PDE3B is a downstream regulator of PI3K in neurons in the hypothalamus and other brain regions [122]. In the hypothalamic neurons, the IRS-PI3K signaling activates PDE3B to decrease cAMP levels and inhibit CREB activity, which suppresses the expression of NPY to induce anorexic effects of leptin [123, 124].
Leptin administration induces PDE3B activity and reduces cAMP levels in the hypothalamus, while PDE3 inhibition by cilostamide weakens the anorectic and body-weight-reducing effects of leptin [86, 125]. Cilostamide also dampens the leptin-induced STAT3 activation in the hypothalamus, suggesting a crosstalk between the PDE3B-cAMP and JAK2-STAT3 pathways [86].
LepRb-AMPK-ACC
The AMP-activated protein kinase (AMPK) is an evolutionarily conserved enzyme that senses the energy status of the cell and regulates fuel availability [126, 127]. AMPK consists of catalytic α subunit and regulatory β and γ subunits; phosphorylation of Thr172 on the catalytic α subunit of AMPK leads to ATP depletion [126, 127]. AMPK is involved in the regulation of energy homeostasis by integrating hormonal and nutritional signals in both the periphery and the CNS [128].
Leptin has tissue-specific effects on AMPK. In the ARC and PVN, leptin inhibits AMPK activity to reduce appetite with consequent reduction of body weight [128, 129]. In the skeletal muscle, leptin stimulates AMPK activity, inactivates the AMP downstream target Acetyl-CoA carboxylase (ACC), and decreases the malonyl CoA levels, hence enhancing the mitochondrial fatty acid oxidation [130, 131]. Leptin induces anorexia, reduces hepatic glucose production, and promotes sympathetic nerve outflows to kidney, brown adipose tissue (BAT) and white adipose tissue (WAT) [132, 133]. mTOR-S6K1 signaling serves as an upstream pathway of AMPK in the hypothalamic leptin signaling cascades [134]. The activated S6K1 phosphorylates AMPK-α2 subunit at Ser491, leading to a reduced α2-AMPK activity in the hypothalamus [134].
LepRb-SHP2-MAPKs
The mitogen-activated protein kinases (MAPKs) are a series of protein kinases, and play important roles in mediating cell proliferation, differentiation, survival and apoptosis. MAPKs, particularly the extracellular regulated protein kinase 1/2 (ERK1/2), are associated with the leptin signaling [135, 136]. In response to leptin, JAK2 stimulates the phosphorylation of Tyr985 in the LepR intracellular domain, which provides a docking site for the SH2-containing protein tyrosine phosphatase 2 (SHP2) [137]. Subsequently, the phosphorylated SHP2 together with its adapter molecule growth factor receptor-bound protein 2 (Grb2) activates the downstream ERK signaling cascades, inducing thermogenesis by controlling sympathetic activity on BAT, and promoting the anorectic effects of leptin [136, 137]. The SHP2-ERK1/2 pathway also exhibits a neurotrophic function during the development of hypothalamic feeding circuits; disrupted ERK signaling impairs development of these neuronal circuits [138].
New regulators of leptin signaling pathways
Rho-kinase1 (ROCK1)
ROCK1 is a protein serine/threonine kinase, serving as a key regulator of actin-myosin contraction and cell polarity. ROCK1 is also a regulator of leptin action, it involves in the leptin-induced activation of JAK2 [139]. JAK2 is an initial trigger of leptin receptor signaling. Leptin promotes the physical interaction of JAK2 and ROCK1 to enhance the phosphorylation of JAK2 and the activation of the downstream STAT3 [139]. Deletion of ROCK1 in either POMC or AgRP neurons induces impaired leptin sensitivity, increased food intake, decreased energy expenditure, and severe obesity [139].
The activation of ROCK1 by leptin is dependent on the JAK2 signaling. For example, leptin activates Ras homolog gene family member A (RhoA)/ROCK in colon cancer cell lines, which can be inhibited by the JAK2 inhibitor AG490 [140]. Leptin activates ROCK to alter actin dynamics in cardiomyocytes in JAK2/PI3K axis dependent way [141].
Transient receptor potential C (TRPC)
TRPC is a family of transient receptor potential cation channels in the mammalian cells. Leptin activates TRPC channels to depolarize steroidogenic factor 1 (SF1) neurons in the VMH, and thus maintains energy balance and glucose homeostasis [142]. Leptin activates TRPC channels to depolarize LepR-expressing neurons in the ventral premammillary nucleus (PMV) and POMC neurons in the ARC [143, 144]. Leptin participates in normal hippocampal dendritic spine formation through activation of TRPC channels in hippocampal neurons [145]. Notably, TRPC channels exist in a macromolecular complex with T-type channels, and these T-type channels are considered to be essential for the leptin-induced activation of TRPC channels [146].
Sirtuin 1 (SIRT1)
SIRT1 is an enzyme located primarily in the cell nucleus that deacetylates transcription factors to regulate cellular processes including longevity and stress response. Under condition of insulin resistance, SIRT1 expression is downregulated; while elevation of SIRT1 expression enhances insulin sensitivity [147]. The decreased SIRT1 expression is linked to leptin resistance and adiposity [148], [149], [150].
Deletion of SIRT1 in the anorexigenic POMC neurons attenuates leptin sensitivity and reduces energy expenditure, whereas over-expression of SIRT1 in POMC neurons sensitizes leptin signals and elevates energy expenditure [151, 152]. Ablation of SIRT1 in the orexigenic AgRP neurons reduces the firing rate of AgRP neurons, suppresses food intake, and decreases body weight [153]. Surprisingly, increased SIRT1 level in AgRP neurons suppresses food intake, and reduces body weight owing to the improved nutrient/hormone sensing [154]. In VMH SF1 neurons, raised SIRT1 level elevates the leptin and orexin sensitivity of these neurons, and thus prevents diet-induced obesity [155]. Taken together, SIRT1 potentiates leptin signaling in POMC neurons, AgRP neurons, and SF1-positive neurons to reduce food intake, enhance energy expenditure, and maintain glucose homeostasis.
SIRT1 downregulates negative regulator of leptin signaling, such as PTP1B, T cell protein-tyrosine phosphatase (TCPTP) and SOCS3, and thus improves the hypothalamic leptin sensitivity [154]. SIRT1 downregulates the nuclear factor kappa-B (NF-κB) signaling to improve central leptin/insulin sensitivity [156]. SIRT1 regulates autophagy, and attenuates endoplasmic reticulum (ER) stress to prevent hypothalamic leptin resistance [157], [158], [159].
Heat shock protein 60 (HSP60)
HSP60 is responsible for the mitochondrial protein import and the macromolecular assembly. HSP60 is a mitochondrial chaperone induced by leptin [160], and leptin-induced HSP60 improves hypothalamic mitochondrial function and insulin sensitivity [160, 161]. In hypothalamic neurons, the leptin-activated STAT3 interacts with HSP60 gene promoter to enhance the HSP60 expression, improving insulin sensitivity in these neurons [160]. Knockdown of HSP60 in hypothalamic neurons raises ROS production, impairs mitochondrial function, and results in diabetes and obesity [160].
Melanoma antigen-like gene 2 (MAGEL2)
Mutation of MAGEL2 leads to the development of Prader-Willi Syndrome (PWS), a genetic disease that causes obesity and mental retardation [162]. Knockout of MAGEL2 reduces leptin sensitivity and inhibits POMC neurons activity, causing disruption of hypothalamic feeding circuits, hyperphagia and obesity [163].
Physiologically, MAGEL2 increases LepR abundance in cell surface, while decreases LepR degradation [164]. Ablation of MAGEL2 reduces LepR distribution in the hypothalamus [164]. In hypothalamic neurons, LepR is internalized by endocytosis for transport to lysosomes or cell membrane. The deubiquitinase USP8 stabilizes the E3 ligase RNF41, and RNF41 in turn ubiquitinates and destabilizes USP8 to regulate LepR degradation [165]. MAGEL2 links LepR to the USP8-RNF41 ubiquitination complex, while ablation of MAGEL2 suppresses RNF41 stabilization and prevents the MAGEL2-related increase of cell surface LepR [164].
Steroidogenic factor 1 (SF1)
SF1, a member of the nuclear receptor family, plays important roles in the sexual development. SF1 is a direct transcriptional target of FoxO1 in the VMH, which regulates leptin signaling to control glucose homeostasis [118, 166]. In the VMH SF1 neurons, leptin upregulates SF1 expression by inhibiting FoxO1 [166, 167]. These leptin-activated SF1 neurons enhance insulin sensitivity in peripheral tissues, and stimulate the whole-body glucose utilization [166, 168].
G protein-coupled receptor 17 (Gpr17)
Gpr17, a G protein coupled receptor, is linked to the Gi alpha subunit or the Gq alpha subunit to achieve its function. Gpr17 is an inhibitory factor of leptin signaling [169], [170], [171]. In the ARC AgRP neurons, leptin inhibits FoxO1 through the LepRb/IRS/PI3K/AKT axis to suppress Gpr17 activation, and thus decreases firing rate of the AgRP neurons to reduce food intake and improve glucose homeostasis [169, 170]. In the POMC neurons, Gpr17 deficiency contributes to maintenance of metabolic homeostasis during dietary and aging challenges [171].
Protein tyrosine phosphatases (PTPs)
PTPs are a series of enzymes removing phosphate groups from phosphorylated tyrosine residues. A series of PTPs are implicated in leptin signaling, including SHP2, PTP1B, PTP epsilon (PTPε), TCPTP, phosphatase and tensin homolog (PTEN), CD45, etc.
SHP2 in the hypothalamic neuron links LepR signal to MAPK, inducing thermogenesis by controlling sympathetic activity in BAT, and promoting the anorectic effects of leptin [136, 137]. SHP2 dephosphorylates and inactivates STAT3 by binding to the phosphorylation site of STAT3 [172]. PTP1B and PTPε downregulate leptin/STAT3 signaling by inactivating JAK2 [173], [174], [175]. PTP1B suppresses IRS1/2 signaling to inhibit the IRS-PI3K axis [176]. TCPTP is an inhibitor of the JAK2/STAT3 axis, which can block the Tyr705 phosphorylation site of STAT3, and thus prevents STAT3 dimerization and translocation to the nucleus [177]. PTEN is an inhibitory modulator of PI3K signaling. PTEN downregulates PIP3 and upregulates FoxO1 [178]. CD45, a transmembrane PTP, inhibits STAT3 activity through dephosphorylating JAKs [179].
Taken together, PTPs are activated for downregulation of leptin signaling. Genetic knockout of PTPs in the brain potentiates leptin signaling, and prevents diet-induced obesity, type 2 diabetes and non-alcoholic fatty liver disease (NAFLD) [180].
Angiotensin AT1A receptors
Leptin contributes to the control of resting metabolic rate (RMR) through targeting the ARC. AT1A receptors colocalize with leptin receptors in the AgRP neurons [181]. Ablation of AT1A receptors specifically in the LepR-expressing neurons diminishes the RMR in response to a high-fat diet or deoxycorticosterone acetate-salt treatments [181, 182]. Thus, angiotensin interacts with leptin in the brain to regulate RMR.
Brain-derived neurotrophic factor (BDNF)
BDNF is a member of the neurotrophin family. In the brain, BDNF is active in the cortex, hippocampus, and basal forebrain, contributing to the growth and differentiation of neurons [183]. Central BDNF involves in the neuro-regulation of energy balance, which mediates appetite suppression and food intake reduction [184, 185]. Leptin stimulates translation of the long 3′ UTR (untranslated regions) BDNF mRNA in dendrites of hypothalamic neurons to regulate body weight [186]. Truncation of the long 3′ UTR of BDNF mRNA inhibits the ability of leptin to reduce food intake and causes severe hyperphagic obesity, which can be reversed by overexpression of BDNF mRNA [186].
Histone deacetylase 5 (HDAC5)
HDAC5 plays a critical role in the histone deacetylation to alter chromosome structure and regulate expression of transcription factors. HDAC5 is involved in mediating the hypothalamic leptin signaling [187]. Ablation of HDAC5 in the mediobasal hypothalamus leads to impaired leptin sensitivity, increased food intake and severe obesity [187]. Overexpression of hypothalamic HDAC5 protects against high-fat-diet induced leptin resistance and obesity [187]. In these processes, HDAC5 deacetylates STAT3 at Lys685 and phosphorylates STAT3 at Tyr705, and thus regulates STAT3 localization and transcriptional activity to accelerate the leptin signaling [187].
Cilia
Neuronal primary cilia are associated with the hyperphagia-induced obesity and the elevated serum leptin, insulin, and glucose levels [188]. Mice with short hypothalamic cilia exhibit attenuated anorectic responses to leptin, insulin and glucose, suggesting that the hypothalamic cilia are essential for the satiety signal sensing [189]. Leptin increases the length of neural cilia to improve neuronal leptin sensitivity [189]. This process is mediated by the hypothalamic LepRb/JAK2/PI3K axis and can be downregulated by the leptin signaling inhibitors such as PTEN and glycogen synthase kinase 3β (GSK3β) [189]. Primary cilia regulate the developing hypothalamus, and are critical in the early life programming of adiposity [190]. Mutation of the cilia relevant protein retinitis pigmentosa GTPase regulator-interacting protein-1 like protein (RPGRIP1L) diminishes the cilia numbers in hypothalamic neurons, and inactivates STAT3 to dampen leptin sensitivity [191].
Astrocytes
Astrocytes are glial cells involved in homeostatic control and neuroprotection. LepR is expressed in hypothalamic astrocytes [192, 193], and hypothalamic astrocytes participate in the mediation of leptin signaling [194, 195]. Absence of LepR in astrocytes weakens pSTAT3 signaling and increases TCPTP level, leading to altered glial morphology, reduced astrocytic coverage of melanocortin cells, and augmented synaptic inputs onto hypothalamic neurons [195, 196]. These alterations of astrocytes promote the development of diet-induced obesity [194, 195].
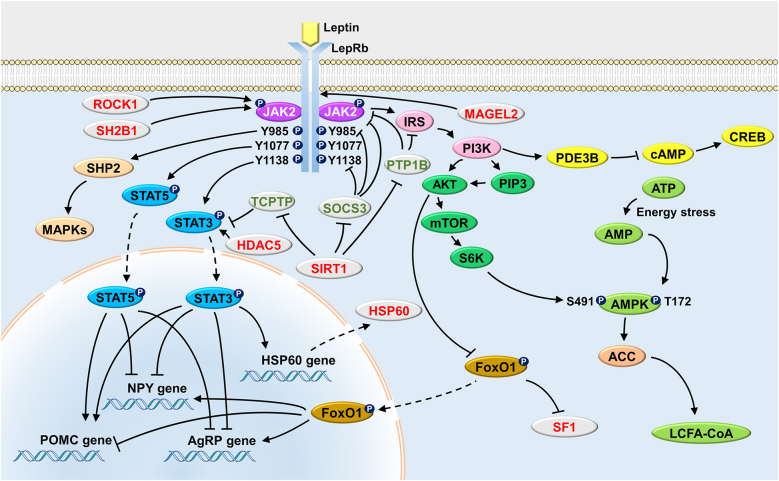
We summarize the classic and novel cellular pathways of leptin signaling in Figure 2.
Figure 2:
Cellular pathways underlying leptin function.  : Activating;
: Activating;  : Inhibiting;
: Inhibiting;  : Translocation. ACC, acetyl-CoA carboxylase; AgRP, agouti-related peptide; AKT, protein kinase B; AMP, adenosine monophosphate; AMPK, adenosine monophosphate activated protein kinase; ATP, adenosine-5′-triphosphate; CREB, cAMP response element-binding protein; FoxO1, forkhead box protein O1; HDAC5, histone deacetylases 5; HSP60, heat shock protein: 60; IRS, insuline receptor substrate; JAK2, janus kinases 2; LCFA-CoA, long chain fatty acid-coenzyme A; LepR, leptin receptor; MAGEL2, melanoma antigen-like gene 2; MAPKs, mitogen-activated protein kinases; mTOR, mammalian target of rapamycin; NPY, neuropeptide Y; PDE3B, phosphodiesterase 3B; PI3K, phosphatidylinositol 3-OH kinase; PIP3, phosphatidylinositol-3,4,5-trisphosphate; POMC, proopiomelanocortin; PTP1B, protein tyrosine phosphatase 1B; ROCK1, Rho-kinasel; S6K, S6 kinase; SF1, steroidogenic factor 1; SH2B1, Src-homology-2 B adaptor protein-1; SHP2, SH2-containing protein tyrosine phosphatase 2; SIRT1, NAD+-dependent deacetylase sirtuin 1; SOCS3, suppressor of cytokine signaling 3; STAT3, signal transducer and activator of transcription 3; STAT5, signal transducer and activator of transcription 5; TCPTP, T cell protein tyrosine phosphatase.
: Translocation. ACC, acetyl-CoA carboxylase; AgRP, agouti-related peptide; AKT, protein kinase B; AMP, adenosine monophosphate; AMPK, adenosine monophosphate activated protein kinase; ATP, adenosine-5′-triphosphate; CREB, cAMP response element-binding protein; FoxO1, forkhead box protein O1; HDAC5, histone deacetylases 5; HSP60, heat shock protein: 60; IRS, insuline receptor substrate; JAK2, janus kinases 2; LCFA-CoA, long chain fatty acid-coenzyme A; LepR, leptin receptor; MAGEL2, melanoma antigen-like gene 2; MAPKs, mitogen-activated protein kinases; mTOR, mammalian target of rapamycin; NPY, neuropeptide Y; PDE3B, phosphodiesterase 3B; PI3K, phosphatidylinositol 3-OH kinase; PIP3, phosphatidylinositol-3,4,5-trisphosphate; POMC, proopiomelanocortin; PTP1B, protein tyrosine phosphatase 1B; ROCK1, Rho-kinasel; S6K, S6 kinase; SF1, steroidogenic factor 1; SH2B1, Src-homology-2 B adaptor protein-1; SHP2, SH2-containing protein tyrosine phosphatase 2; SIRT1, NAD+-dependent deacetylase sirtuin 1; SOCS3, suppressor of cytokine signaling 3; STAT3, signal transducer and activator of transcription 3; STAT5, signal transducer and activator of transcription 5; TCPTP, T cell protein tyrosine phosphatase.
Leptin resistance
Leptin resistance is defined by reduced sensitivity or failure in response to leptin [9]. Leptin resistance weakens the leptin ability to suppress appetite or enhance energy expenditure, and causes overweight and obesity [9]. Leptin resistance is involved in the development of metabolic diseases [11].
Several studies have reported the quantification of the serum leptin levels in healthy and obese population. These studies demonstrate that the serum leptin levels vary in different sex, age and body mass index (BMI) [197], [198], [199], [200], [201], [202]. For instance, a study on healthy children and adolescents (6–18 years) conducted in Danish showed that girls had a higher median value of serum leptin levels compared to boys (9.554 vs. 3.255 ng/mL, P < 0.001) [197]. This study also suggests that there may be an upward trend in the serum leptin levels over time in girls [197]. A study on underweight, normal weight, overweight and obese adolescents from European cities shows that leptin exhibits a significant progressive and linear increase according to BMI [200]. These studies suggest that the reference levels of serum leptin may contribute to the understanding of leptin resistance, although it is still difficult to delineate the definition of leptin resistance in a universal and quantifiable manner.
Main mechanisms underlying leptin resistance
Leptin resistance can be induced by multiple conditions. Largely, leptin resistance can be classified into HFD induced leptin resistance, leptin induced leptin resistance, inflammation induced leptin resistance, seasonal leptin resistance, pregnancy/lactation induced leptin resistance, ER stress induced leptin resistance, etc. In fact, it is difficult to define leptin resistance in a universal and quantifiable manner, but understanding of the mechanisms that attenuated leptin action could provide new insights to decipher the myth of leptin resistance and obesity. Until recently, the essential mechanisms relative to the leptin resistance are as follows.
Disruption of the blood-brain barrier (BBB) transport
Leptin is secreted by adipose tissues, and transported across the BBB to achieve its function. In this process, LepRa in the BBB is required [203]. Pathologically, the excessively high leptin level in the plasma causes a saturation of LepRa, and thereby reduces the ratio of leptin transport across the BBB, leading to leptin resistance [203, 204].
Recent studies point towards a direct involvement of endothelial cells of the BBB as one of major mechanism of leptin transport [205]. Specific deletion of LepR in endothelial cells of the BBB impairs the transport of leptin into cerebrospinal fluid (CSF) and LepR-positive brain regions, leading to aggravated obesity [205]. The choroid plexus (CP) plays a critical role in controlling the leptin transport into the CSF and target areas such as the hypothalamus [206].
Competitive inhibition of leptin
Circulating leptin-binding proteins, such as the plasma soluble LepR and C-reactive protein, competitively bind to leptin and promote the development of leptin resistance [207, 208]. The binding of leptin to circulating leptin-binding proteins inhibits the leptin transport to the CNS, suppresses the interaction between leptin and LepR neurons, and induces leptin resistance-related phenotypes [209].
Destruction of LepR
Certain mutations of the ob gene shorten the length of the intracellular signaling domain of LepRb, which impair the ability of LepRb to mediate leptin signaling [210]. In these states, binding of leptin to LepRb cannot achieve its function, leading to severe leptin resistance [210].
Impairment of the leptin cellular signaling
Mechanisms of the leptin resistance mainly include two points with regard to the leptin cellular signaling. First, neurons expressing LepR are not sensitive enough to measure the circulating leptin level, decreasing the efficacy of leptin binding to LepR. Second, the LepR-expressing cells have an impaired signaling ability.
A number of factors and certain mechanisms underlying development of leptin resistance are linked to the leptin cellular signaling. For example, increased IL-6 elevates the levels of the leptin signaling inhibitory regulators SOCS3 and PTP1B to promote leptin resistance [211]. Myeloid differentiation factor 88 (MyD88) activates the inhibitor of NF-κB kinase to upregulate NF-κB and inactivate STAT3, and thus reduces leptin sensitivity [212]. Exchange factor directly activated by cAMP (Epac) upregulates expression of the negative leptin signaling modulators SOCS3 and PTP1B, whereas inhibits the positive modulators STAT3 and S6K1, impairing the leptin signaling cascades in hypothalamic neurons [213, 214]. Deletion of the methyl-CpG-binding protein 2 (MeCP2) increases DNA methylation of the POMC promoter and suppresses POMC expression in POMC neurons, exacerbating the degree of leptin resistance [215]. PTP receptor type J (PTPRJ) inhibits JAK2 activation through dephosphorylation of Tyr813 and Tyr868 in JAK2 autophosphorylation sites, and thus incurs an increased food intake and body weight [216]. Specific deletion of the activating transcription factor 4 (ATF4) in the AgRP neurons or POMC neurons inhibits FoxO1 expression, and thus elevates leptin sensitivity to reduce body weight [217], [218], [219].
Peripheral leptin resistance
Leptin resistance develops not only in the brain, but also in the peripheral tissues including skeletal muscle, adipose tissue and liver [220]. The loss of leptin’s physiological actions that occurs in peripheral tissues is referred to as peripheral leptin resistance. Recently, peripheral leptin resistance especially leptin resistance in muscle, has drawn more attention, which may provide new insights into the treatment of obesity [220].
In the skeletal muscle, leptin increases muscle mass and enhances fatty acid oxidation through activating the JAK2-STAT3, AMPK and insulin-like growth factor I (IGF-1) signaling [130, 221], [222], [223]. Leptin also enhances both basal and insulin-stimulated glucose uptake in the skeletal muscle by promoting the transport of the intracellular glucose transporter 4 (GLUT4) [224, 225]. Obese humans and animals exhibit leptin resistance in skeletal muscle, decreased muscle mass, reduced fatty acid oxidation and diminished glucose uptake [226], [227], [228]. These processes are thought to be associated with the increased muscular SOCS3 and PTP1B and the weakened JAK2-STAT3, AMPK, IGF-1 and GLUT4 signaling [226, 229], [230], [231], [232].
The relationship between insulin resistance and leptin resistance
Under physiological conditions, insulin promotes the secretion of leptin [233, 234]. Leptin inhibits the secretion of insulin, increases insulin sensitivity, reduces the synthesis and storage of fatty acids and triglycerides, and contributes to the maintenance of energy homeostasis [235], [236], [237]. Under pathological conditions, the disturbances in the balance of leptin and insulin signaling may lead to metabolic disorders, such as obesity, type 2 diabetes, and non-alcoholic fatty liver disease, accompanied with insulin resistance and leptin resistance [238], [239], [240]. Overall, leptin and insulin are important hormones involving in the regulation of glucose and lipid metabolism, and leptin resistance and insulin resistance may underlie the pathogenesis of metabolic disorders.
Diseases related to leptin and leptin resistance
Cardiovascular diseases (CVDs)
CVDs are a class of diseases that involve the heart or blood vessels, including coronary artery diseases, stroke, heart failure, hypertensive heart disease, etc. Most CVDs involve atherosclerosis. LepR is detected in the atherosclerotic lesions, and ob/ob mice are resistant to atherosclerosis, suggesting leptin may act as a direct atherogenic modulator [241, 242]. The reciprocal modulation of leptin and inflammatory pathways is also closely associated with the cardiovascular risk [243, 244].
Diabetes
Diabetes is characterized by the high blood glucose levels over a prolonged period. Obesity and diabetes are two important public health concerns throughout the world. Obese individuals have an increased morbidity of related disorders, including diabetes. Leptin is known as a key adipokine, which regulates appetite, energy expenditure, behavior, and glucose metabolism. Leptin may normalize hyperglycemia and hyperinsulinemia, and increase insulin sensitivity [235]. The leptin deficient ob/ob mice exhibits phenotypes of diabetes [245], and these diabetic features are improved by leptin administration [246]. Leptin treatment can relieve insulin resistance in the MKR mice, a specific mouse model of type 2 diabetes [247]. Physiologically, the leptin and insulin signaling pathways are coordinately involved in the regulation of energy balance and glucose homeostasis [248, 249].
Insulin stimulates leptin production and secretion in adipocytes [250]. In contrast, leptin suppresses insulin secretion in insulin-producing beta cells of the pancreas [236]. These processes are relative to the IRS-PI3K-PDE3B-cAMP axis [251]. Leptin also directly mediates the glucose metabolism in the CNS. For example, in POMC and AgRP neurons in ARC, leptin activates the PI3K signaling to increase insulin sensitivity [252, 253]. Leptin inactivates the hypothalamus-pituitary-adrenal (HPA) axis to suppress glucocorticoid releasing, attenuate ketogenesis, and relieve diabetic phenotypes [254].
Hypertension
Hypertension is a long-term medical condition in which the blood pressure is persistently raised. Obesity is closely associated with the hypertension development [255]. Leptin contributes to the regulation of increased blood pressure (BP) in obese population [256, 257].
The development of hypertension is not seen in the leptin- or LepR-deficiency animals [256, 258]. In contrast, loss-of-function mutations of leptin or LepR lead to low BP despite severe obesity [256, 258]. Thus, the leptin signaling plays critical roles in stimulating hypertension. In ARC POMC neurons, leptin activates α-MSH to agonize MC4R and MC3R, increases sympathetic nervous outflow to the kidney and the heart, and exacerbates hypertension [259]. The NTS participates in controlling hypertension in response to leptin, and leptin administration into the NTS increases the renal sympathetic nervous activity [260]. The AgRP/NPY neurons, DMH and VMH also involve in the leptin’s effects on BP [261, 262].
In contrast, a number of patients with lipodystrophy show hypertension in the leptin-deficient state [263, 264]. There is no increase in either systolic or diastolic blood pressure in these patients after leptin treatment [263, 264]. Thus, there may be mechanisms independent of leptin signals for hypertension development.
Cancer
Epidemiological studies have demonstrated the association between obesity and cancer [265, 266]. Leptin level is linked to the development of cancer [267, 268]. In the state of obesity, the increased size of adipocytes enhances leptin secretion. The excessive leptin acts as a growth factor through the JAK2-STAT3, PI3K-AKT and ERK signaling, which stimulates the growth of cancer cells and promotes cancer progression [269], [270], [271]. Leptin also induces epithelial-mesenchymal transition (EMT) to accelerate tumor invasion [272].
Immunological diseases
Leptin is a key regulator of the immune system in peripheral tissues, serving as a link between metabolism and immunological diseases, such as the rheumatoid arthritis, the systemic lupus erythematosus and the multiple sclerosis [273], [274], [275], [276]. Leptin activates immune cells including monocytes, granulocytes, natural killer (NK) cells and T cells, and promotes the release of pro-inflammatory cytokines. These processes stimulate the innate and adaptive immunity, and cause inflammation [277], [278], [279].
Neurodegenerative diseases
Leptin plays regulatory roles in the neuroendocrine system [280]. Leptin alters hippocampal synaptic plasticity, improves learning and memory, and protects against several neurodegenerative diseases, including AD and Parkinson’s disease (PD) [280].
AD is one of the most common chronic neurodegenerative diseases. Amyloid-β is the main component of amyloid plaques, which is highly expressed in the brain of AD patients [281]. Leptin treatment decreases the amyloid-β levels in brain and blood plasma, and alleviates spatial memory impairment [282, 283]. In this process, the phosphorylation of JAK2, STAT3, AKT and the activation of AMPK signaling are involved [284].
PD, another common neurodegenerative disease, is characterized by classical motor function deficits due to loss of dopaminergic neurons in the substantia nigra [285]. Leptin administration can reduce dopaminergic cell death and rescue behavioral abnormalities in the 6-hydroxydopamine (6-OHDA)-induced PD models [286]. In this process, pERK1/2 and BDNF serve as key survival factors of dopaminergic neurons [286].
Other diseases related to leptin
Abnormal leptin level is associated with the development of several other diseases, such as the frontotemporal dementia (FTD) [287, 288]. The role of leptin in these diseases and the mechanism underlying these processes remain to be further elucidated.
Leptin relative therapies for obesity
Leptin therapy has been found to relieve hyperglycemia and to prevent mortality in several rodent models of type 1 diabetes [289], [290], [291]. Liraglutide and metformin, as anti-obesity and diabetes agents, may enhance the leptin sensitivity [292, 293]. Celastrol and Withaferin A, as naturally occurring compounds, may act as leptin sensitizers to mitigate the leptin resistance and promote the weight loss [294, 295]. The physical exercise can relieve central and peripheral leptin resistance in humans and animals [296], [297], [298], [299]. Recently, the partial leptin reduction is considered a therapeutic strategy for leptin sensitization and weight loss [300]. In the context of obesity, partial reduction in plasma leptin levels by means of cell-specific leptin elimination or neutralization with anti-leptin antibodies can restore hypothalamic leptin sensitivity, reduce weight gain, and enhance insulin sensitivity [300].
Conclusions
Over the last 30 years, since Dr. Friedman discovered the leptin, numerous studies have been focused on leptin signaling and leptin resistance. These studies reveal that leptin neural and cellular signaling are critical in the regulation of metabolic homeostasis, and that leptin resistance is a pathogenic factor causing obesity and associated comorbidities. Novel therapies for obesity and associated comorbidities are invented based on these findings. Due to safety issues as well as leptin resistance, the development of leptin-relative agents is disappointing. Further studies are essential to solve these problems, which may lead to new strategies for obesity treatment.
Footnotes
Research funding: This work was supported by grants from the National Natural Science Foundation of China (No. 82170864. No. 81471064. No. 81670779 and No. 81870590 to R.Z), the Beijing Municipal Natural Science Foundation (No. 7162097 and No. H2018206641 to R.Z), and the Peking University Research Foundation (No. BMU20140366 to R.Z), and the National Key Research and Development Program of China (2017YFC1700402 to R.Z.).
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Competing interests: Authors state no conflict of interest.
Informed consent: Informed consent was obtained from all individuals included in this study.
Ethical approval: The local Institutional Review Board deemed the study exempt from review.
Contributor Information
Jiarui Liu, Email: ljr123@pku.edu.cn.
Futing Lai, Email: 1810305209@pku.edu.cn.
Yujia Hou, Email: 1710305331@pku.edu.cn.
Ruimao Zheng, Email: rmzheng@pku.edu.cn.
References
- 1.Coleman DL. A historical perspective on leptin. Nat Med. 2010;16:1097–9. doi: 10.1038/nm1010-1097. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 3.Munzberg H, Morrison CD. Structure, production and signaling of leptin. Metabolism. 2015;64:13–23. doi: 10.1016/j.metabol.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denver RJ, Bonett RM, Boorse GC. Evolution of leptin structure and function. Neuroendocrinology. 2011;94:21–38. doi: 10.1159/000328435. [DOI] [PubMed] [Google Scholar]
- 5.Tartaglia LA. The leptin receptor. J Biol Chem. 1997;272:6093–6. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 6.Chua SC, Koutras IK, Han L, Liu SM, Kay J, Young SJ, et al. Fine structure of the murine leptin receptor gene: splice site suppression is required to form two alternatively spliced transcripts. Genomics. 1997;45:264–70. doi: 10.1006/geno.1997.4962. [DOI] [PubMed] [Google Scholar]
- 7.Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–56. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 8.Dalamaga M, Chou SH, Shields K, Papageorgiou P, Polyzos SA, Mantzoros CS. Leptin at the intersection of neuroendocrinology and metabolism: current evidence and therapeutic perspectives. Cell Metabol. 2013;18:29–42. doi: 10.1016/j.cmet.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Myers MG, Heymsfield SB, Haft C, Kahn BB, Laughlin M, Leibel RL, et al. Challenges and opportunities of defining clinical leptin resistance. Cell Metabol. 2012;15:150–6. doi: 10.1016/j.cmet.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genchi VA, D’Oria R, Palma G, Caccioppoli C, Cignarelli A, Natalicchio A, et al. Impaired leptin signalling in obesity: is leptin a new thermolipokine? Int J Mol Sci. 2021;22:6445. doi: 10.3390/ijms22126445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gruzdeva O, Borodkina D, Uchasova E, Dyleva Y, Barbarash O. Leptin resistance: underlying mechanisms and diagnosis. Diabetes, Metab Syndrome Obes Targets Ther. 2019;12:191–8. doi: 10.2147/dmso.s182406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramos-Lobo AM, Donato J. The role of leptin in health and disease. Temperature. 2017;4:258–91. doi: 10.1080/23328940.2017.1327003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myers MG, Munzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metabol. 2009;9:117–23. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 15.Timper K, Bruning JC. Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis Model Mech. 2017;10:679–89. doi: 10.1242/dmm.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morton GJ. Hypothalamic leptin regulation of energy homeostasis and glucose metabolism. J Physiol. 2007;583:437–43. doi: 10.1113/jphysiol.2007.135590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krashes MJ, Lowell BB, Garfield AS. Melanocortin-4 receptor-regulated energy homeostasis. Nat Neurosci. 2016;19:206–19. doi: 10.1038/nn.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vong L, Ye C, Yang Z, Choi B, Chua S, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–54. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leshan RL, Greenwald-Yarnell M, Patterson CM, Gonzalez IE, Myers MG. Leptin action through hypothalamic nitric oxide synthase-1-expressing neurons controls energy balance. Nat Med. 2012;18:820–3. doi: 10.1038/nm.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–5. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myers MG, Olson DP. SnapShot: neural pathways that control feeding. Cell Metabol. 2014;19:732–e1. doi: 10.1016/j.cmet.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol. 2008;93:773–97. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi A, Kishi E, Ishimaru H, Ikarashi Y, Maruyama Y. Role of preoptic and anterior hypothalamic cholinergic input on water intake and body temperature. Brain Res. 2001;889:191–9. doi: 10.1016/s0006-8993(00)03132-2. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Kerman IA, Laque A, Nguyen P, Faouzi M, Louis GW, et al. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J Neurosci. 2011;31:1873–84. doi: 10.1523/jneurosci.3223-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chao PT, Yang L, Aja S, Moran TH, Bi S. Knockdown of NPY expression in the dorsomedial hypothalamus promotes development of brown adipocytes and prevents diet-induced obesity. Cell Metabol. 2011;13:573–83. doi: 10.1016/j.cmet.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bi S, Kim YJ, Zheng F. Dorsomedial hypothalamic NPY and energy balance control. Neuropeptides. 2012;46:309–14. doi: 10.1016/j.npep.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faber CL, Deem JD, Phan BA, Doan TP, Ogimoto K, Mirzadeh Z, et al. Leptin receptor neurons in the dorsomedial hypothalamus regulate diurnal patterns of feeding, locomotion, and metabolism. Elife. 2021;10:e63671. doi: 10.7554/elife.63671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu S, Cheng H, Francois M, Qualls-Creekmore E, Huesing C, He Y, et al. Preoptic leptin signaling modulates energy balance independent of body temperature regulation. Elife. 2018;7:e33505. doi: 10.7554/elife.33505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stuber GD, Wise RA. Lateral hypothalamic circuits for feeding and reward. Nat Neurosci. 2016;19:198–205. doi: 10.1038/nn.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonnavion P, Mickelsen LE, Fujita A, de Lecea L, Jackson AC. Hubs and spokes of the lateral hypothalamus: cell types, circuits and behaviour. J Physiol. 2016;594:6443–62. doi: 10.1113/jp271946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsujino N, Sakurai T. Role of orexin in modulating arousal, feeding, and motivation. Front Behav Neurosci. 2013;7:28. doi: 10.3389/fnbeh.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamada H, Okumura T, Motomura W, Kobayashi Y, Kohgo Y. Inhibition of food intake by central injection of anti-orexin antibody in fasted rats. Biochem Biophys Res Commun. 2000;267:527–31. doi: 10.1006/bbrc.1999.1998. [DOI] [PubMed] [Google Scholar]
- 33.Goforth PB, Leinninger GM, Patterson CM, Satin LS, Myers MG. Leptin acts via lateral hypothalamic area neurotensin neurons to inhibit orexin neurons by multiple GABA-independent mechanisms. J Neurosci. 2014;34:11405–15. doi: 10.1523/jneurosci.5167-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27:11075–82. doi: 10.1523/jneurosci.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Funato H, Tsai AL, Willie JT, Kisanuki Y, Williams SC, Sakurai T, et al. Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell Metabol. 2009;9:64–76. doi: 10.1016/j.cmet.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, et al. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–7. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 37.Ludwig DS, Tritos NA, Mastaitis JW, Kulkarni R, Kokkotou E, Elmquist J, et al. Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J Clin Invest. 2001;107:379–86. doi: 10.1172/jci10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sahu A. Leptin decreases food intake induced by melanin-concentrating hormone (MCH), galanin (GAL) and neuropeptide Y (NPY) in the rat. Endocrinology. 1998;139:4739–42. doi: 10.1210/endo.139.11.6432. [DOI] [PubMed] [Google Scholar]
- 39.Sears RM, Liu RJ, Narayanan NS, Sharf R, Yeckel MF, Laubach M, et al. Regulation of nucleus accumbens activity by the hypothalamic neuropeptide melanin-concentrating hormone. J Neurosci. 2010;30:8263–73. doi: 10.1523/jneurosci.5858-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rao Y, Lu M, Ge F, Marsh DJ, Qian S, Wang AH, et al. Regulation of synaptic efficacy in hypocretin/orexin-containing neurons by melanin concentrating hormone in the lateral hypothalamus. J Neurosci. 2008;28:9101–10. doi: 10.1523/jneurosci.1766-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown JA, Wright A, Bugescu R, Christensen L, Olson DP, Leinninger GM. Distinct subsets of lateral hypothalamic neurotensin neurons are activated by leptin or dehydration. Sci Rep. 2019;9:1873. doi: 10.1038/s41598-018-38143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sutton AK, Myers MG, Olson DP. The role of PVH circuits in leptin action and energy balance. Annu Rev Physiol. 2016;78:207–21. doi: 10.1146/annurev-physiol-021115-105347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–7. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sutton AK, Pei H, Burnett KH, Myers MG, Rhodes CJ, Olson DP. Control of food intake and energy expenditure by Nos1 neurons of the paraventricular hypothalamus. J Neurosci. 2014;34:15306–18. doi: 10.1523/jneurosci.0226-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garfield AS, Li C, Madara JC, Shah BP, Webber E, Steger JS, et al. A neural basis for melanocortin-4 receptor-regulated appetite. Nat Neurosci. 2015;18:863–71. doi: 10.1038/nn.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sawchenko PE, Brown ER, Chan RK, Ericsson A, Li HY, Roland BL, et al. The paraventricular nucleus of the hypothalamus and the functional neuroanatomy of visceromotor responses to stress. Prog Brain Res. 1996;107:201–22. doi: 10.1016/s0079-6123(08)61866-x. [DOI] [PubMed] [Google Scholar]
- 47.Geerling JC, Shin JW, Chimenti PC, Loewy AD. Paraventricular hypothalamic nucleus: axonal projections to the brainstem. J Comp Neurol. 2010;518:1460–99. doi: 10.1002/cne.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qin C, Li J, Tang K. The paraventricular nucleus of the hypothalamus: development, function, and human diseases. Endocrinology. 2018;159:3458–72. doi: 10.1210/en.2018-00453. [DOI] [PubMed] [Google Scholar]
- 49.Perello M, Raingo J. Leptin activates oxytocin neurons of the hypothalamic paraventricular nucleus in both control and diet-induced obese rodents. PLoS One. 2013;8:e59625. doi: 10.1371/journal.pone.0059625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah BP, Vong L, Olson DP, Koda S, Krashes MJ, Ye C, et al. MC4R-expressing glutamatergic neurons in the paraventricular hypothalamus regulate feeding and are synaptically connected to the parabrachial nucleus. Proc Natl Acad Sci U S A. 2014;111:13193–8. doi: 10.1073/pnas.1407843111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metabol. 2012;16:296–309. doi: 10.1016/j.cmet.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology. 2002;143:239–46. doi: 10.1210/endo.143.1.8589. [DOI] [PubMed] [Google Scholar]
- 53.Matheny M, Strehler KY, King M, Tumer N, Scarpace PJ. Targeted leptin receptor blockade: role of ventral tegmental area and nucleus of the solitary tract leptin receptors in body weight homeostasis. J Endocrinol. 2014;222:27–41. doi: 10.1530/joe-13-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neyens D, Zhao H, Huston NJ, Wayman GA, Ritter RC, Appleyard SM. Leptin sensitizes NTS neurons to vagal input by increasing postsynaptic NMDA receptor currents. J Neurosci. 2020;40:7054–64. doi: 10.1523/jneurosci.1865-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith PM, Brzezinska P, Hubert F, Mimee A, Maurice DH, Ferguson AV. Leptin influences the excitability of area postrema neurons. Am J Physiol Regul Integr Comp Physiol. 2016;310:R440–8. doi: 10.1152/ajpregu.00326.2015. [DOI] [PubMed] [Google Scholar]
- 56.Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, DiLeone RJ, Bence KK, et al. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metabol. 2010;11:77–83. doi: 10.1016/j.cmet.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huo L, Maeng L, Bjorbaek C, Grill HJ. Leptin and the control of food intake: neurons in the nucleus of the solitary tract are activated by both gastric distension and leptin. Endocrinology. 2007;148:2189–97. doi: 10.1210/en.2006-1572. [DOI] [PubMed] [Google Scholar]
- 58.Fan W, Ellacott KL, Halatchev IG, Takahashi K, Yu P, Cone RD. Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat Neurosci. 2004;7:335–6. doi: 10.1038/nn1214. [DOI] [PubMed] [Google Scholar]
- 59.Williams DL, Baskin DG, Schwartz MW. Leptin regulation of the anorexic response to glucagon-like peptide-1 receptor stimulation. Diabetes. 2006;55:3387–93. doi: 10.2337/db06-0558. [DOI] [PubMed] [Google Scholar]
- 60.Morales M, Margolis EB. Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci. 2017;18:73–85. doi: 10.1038/nrn.2016.165. [DOI] [PubMed] [Google Scholar]
- 61.Bruijnzeel AW, Corrie LW, Rogers JA, Yamada H. Effects of insulin and leptin in the ventral tegmental area and arcuate hypothalamic nucleus on food intake and brain reward function in female rats. Behav Brain Res. 2011;219:254–64. doi: 10.1016/j.bbr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–10. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 63.Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metabol. 2009;10:89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leshan RL, Opland DM, Louis GW, Leinninger GM, Patterson CM, Rhodes CJ, et al. Ventral tegmental area leptin receptor neurons specifically project to and regulate cocaine- and amphetamine-regulated transcript neurons of the extended central amygdala. J Neurosci. 2010;30:5713–23. doi: 10.1523/jneurosci.1001-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–34. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Essner RA, Smith AG, Jamnik AA, Ryba AR, Trutner ZD, Carter ME. AgRP neurons can increase food intake during conditions of appetite suppression and inhibit anorexigenic parabrachial neurons. J Neurosci. 2017;37:8678–87. doi: 10.1523/jneurosci.0798-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu Q, Clark MS, Palmiter RD. Deciphering a neuronal circuit that mediates appetite. Nature. 2012;483:594–7. doi: 10.1038/nature10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roman CW, Derkach VA, Palmiter RD. Genetically and functionally defined NTS to PBN brain circuits mediating anorexia. Nat Commun. 2016;7:11905. doi: 10.1038/ncomms11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morton GJ, Meek TH, Schwartz MW. Neurobiology of food intake in health and disease. Nat Rev Neurosci. 2014;15:367–78. doi: 10.1038/nrn3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature. 2013;503:111–4. doi: 10.1038/nature12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Flak JN, Patterson CM, Garfield AS, D’Agostino G, Goforth PB, Sutton AK, et al. Leptin-inhibited PBN neurons enhance responses to hypoglycemia in negative energy balance. Nat Neurosci. 2014;17:1744–50. doi: 10.1038/nn.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garfield AS, Shah BP, Madara JC, Burke LK, Patterson CM, Flak J, et al. A parabrachial-hypothalamic cholecystokinin neurocircuit controls counterregulatory responses to hypoglycemia. Cell Metabol. 2014;20:1030–7. doi: 10.1016/j.cmet.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marty N, Dallaporta M, Thorens B. Brain glucose sensing, counterregulation, and energy homeostasis. Physiology. 2007;22:241–51. doi: 10.1152/physiol.00010.2007. [DOI] [PubMed] [Google Scholar]
- 75.Kanoski SE, Grill HJ. Hippocampus contributions to food intake control: mnemonic, neuroanatomical, and endocrine mechanisms. Biol Psychiatr. 2017;81:748–56. doi: 10.1016/j.biopsych.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kanoski SE, Hayes MR, Greenwald HS, Fortin SM, Gianessi CA, Gilbert JR, et al. Hippocampal leptin signaling reduces food intake and modulates food-related memory processing. Neuropsychopharmacology. 2011;36:1859–70. doi: 10.1038/npp.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harvey J. Leptin regulation of neuronal morphology and hippocampal synaptic function. Front Synaptic Neurosci. 2013;5:3. doi: 10.3389/fnsyn.2013.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McGregor G, Harvey J. Leptin regulation of synaptic function at hippocampal TA-CA1 and SC-CA1 synapses: implications for health and disease. Neurochem Res. 2019;44:650–60. doi: 10.1007/s11064-017-2362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Patterson CM, Leshan RL, Jones JC, Myers MG. Molecular mapping of mouse brain regions innervated by leptin receptor-expressing cells. Brain Res. 2011;1378:18–28. doi: 10.1016/j.brainres.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Flak JN, Arble D, Pan W, Patterson C, Lanigan T, Goforth PB, et al. A leptin-regulated circuit controls glucose mobilization during noxious stimuli. J Clin Invest. 2017;127:3103–13. doi: 10.1172/jci90147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.de Vrind VAJ, van ’t Sant LJ, Rozeboom A, Luijendijk-Berg MCM, Omrani A, Adan RAH. Leptin receptor expressing neurons in the substantia nigra regulate locomotion, and in the ventral tegmental area motivation and feeding. Front Endocrinol. 2021;12:680494. doi: 10.3389/fendo.2021.680494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park HK, Ahima RS. Leptin signaling. F1000Prime Rep. 2014;6:73. doi: 10.12703/p6-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hu X, Li J, Fu M, Zhao X, Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Targeted Ther. 2021;6:402. doi: 10.1038/s41392-021-00791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu H, Du T, Li C, Yang G. STAT3 phosphorylation in central leptin resistance. Nutr Metab. 2021;18:39. doi: 10.1186/s12986-021-00569-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kloek C, Haq AK, Dunn SL, Lavery HJ, Banks AS, Myers MG. Regulation of Jak kinases by intracellular leptin receptor sequences. J Biol Chem. 2002;277:41547–55. doi: 10.1074/jbc.m205148200. [DOI] [PubMed] [Google Scholar]
- 86.Sahu A. Intracellular leptin-signaling pathways in hypothalamic neurons: the emerging role of phosphatidylinositol-3 kinase-phosphodiesterase-3B-cAMP pathway. Neuroendocrinology. 2011;93:201–10. doi: 10.1159/000326785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ladyman SR, Grattan DR. JAK-STAT and feeding. JAK-STAT. 2013;2:e23675. doi: 10.4161/jkst.23675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI, et al. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci U S A. 2004;101:4661–6. doi: 10.1073/pnas.0303992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vaisse C, Halaas JL, Horvath CM, Darnell JE, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet. 1996;14:95–7. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 90.White DW, Kuropatwinski KK, Devos R, Baumann H, Tartaglia LA. Leptin receptor (OB-R) signaling. Cytoplasmic domain mutational analysis and evidence for receptor homo-oligomerization. J Biol Chem. 1997;272:4065–71. doi: 10.1016/s0021-9258(18)40585-6. [DOI] [PubMed] [Google Scholar]
- 91.Munzberg H, Huo L, Nillni EA, Hollenberg AN, Bjorbaek C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology. 2003;144:2121–31. doi: 10.1210/en.2002-221037. [DOI] [PubMed] [Google Scholar]
- 92.Mesaros A, Koralov SB, Rother E, Wunderlich FT, Ernst MB, Barsh GS, et al. Activation of Stat3 signaling in AgRP neurons promotes locomotor activity. Cell Metabol. 2008;7:236–48. doi: 10.1016/j.cmet.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 93.Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–9. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 94.Banks AS, Davis SM, Bates SH, Myers MG. Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275:14563–72. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- 95.Lee JY, Muenzberg H, Gavrilova O, Reed JA, Berryman D, Villanueva EC, et al. Loss of cytokine-STAT5 signaling in the CNS and pituitary gland alters energy balance and leads to obesity. PLoS One. 2008;3:e1639. doi: 10.1371/journal.pone.0001639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mutze J, Roth J, Gerstberger R, Hubschle T. Nuclear translocation of the transcription factor STAT5 in the rat brain after systemic leptin administration. Neurosci Lett. 2007;417:286–91. doi: 10.1016/j.neulet.2007.02.074. [DOI] [PubMed] [Google Scholar]
- 97.Gong Y, Ishida-Takahashi R, Villanueva EC, Fingar DC, Munzberg H, Myers MG. The long form of the leptin receptor regulates STAT5 and ribosomal protein S6 via alternate mechanisms. J Biol Chem. 2007;282:31019–27. doi: 10.1074/jbc.m702838200. [DOI] [PubMed] [Google Scholar]
- 98.Dunn SL, Bjornholm M, Bates SH, Chen Z, Seifert M, Myers MG. Feedback inhibition of leptin receptor/Jak2 signaling via Tyr1138 of the leptin receptor and suppressor of cytokine signaling 3. Mol Endocrinol. 2005;19:925–38. doi: 10.1210/me.2004-0353. [DOI] [PubMed] [Google Scholar]
- 99.Wunderlich CM, Hovelmeyer N, Wunderlich FT. Mechanisms of chronic JAK-STAT3-SOCS3 signaling in obesity. JAK-STAT. 2013;2:e23878. doi: 10.4161/jkst.23878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pedroso JA, Silveira MA, Lima LB, Furigo IC, Zampieri TT, Ramos-Lobo AM, et al. Changes in leptin signaling by SOCS3 modulate fasting-induced hyperphagia and weight regain in mice. Endocrinology. 2016;157:3901–14. doi: 10.1210/en.2016-1038. [DOI] [PubMed] [Google Scholar]
- 101.Kaszubska W, Falls HD, Schaefer VG, Haasch D, Frost L, Hessler P, et al. Protein tyrosine phosphatase 1B negatively regulates leptin signaling in a hypothalamic cell line. Mol Cell Endocrinol. 2002;195:109–18. doi: 10.1016/s0303-7207(02)00178-8. [DOI] [PubMed] [Google Scholar]
- 102.Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413:794–5. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- 103.Bjorbaek C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem. 1997;272:32686–95. doi: 10.1074/jbc.272.51.32686. [DOI] [PubMed] [Google Scholar]
- 104.Kim YB, Uotani S, Pierroz DD, Flier JS, Kahn BB. In vivo administration of leptin activates signal transduction directly in insulin-sensitive tissues: overlapping but distinct pathways from insulin. Endocrinology. 2000;141:2328–39. doi: 10.1210/endo.141.7.7536. [DOI] [PubMed] [Google Scholar]
- 105.White MF. The IRS-signalling system: a network of docking proteins that mediate insulin action. Mol Cell Biochem. 1998;182:3–11. [PubMed] [Google Scholar]
- 106.Li Z, Zhou Y, Carter-Su C, Myers MG, Rui L. SH2B1 enhances leptin signaling by both Janus kinase 2 Tyr813 phosphorylation-dependent and -independent mechanisms. Mol Endocrinol. 2007;21:2270–81. doi: 10.1210/me.2007-0111. [DOI] [PubMed] [Google Scholar]
- 107.Duan C, Li M, Rui L. SH2-B promotes insulin receptor substrate 1 (IRS1)- and IRS2-mediated activation of the phosphatidylinositol 3-kinase pathway in response to leptin. J Biol Chem. 2004;279:43684–91. doi: 10.1074/jbc.m408495200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Galic S, Hauser C, Kahn BB, Haj FG, Neel BG, Tonks NK, et al. Coordinated regulation of insulin signaling by the protein tyrosine phosphatases PTP1B and TCPTP. Mol Cell Biol. 2005;25:819–29. doi: 10.1128/mcb.25.2.819-829.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gonzalez-Rodriguez A, Mas Gutierrez JA, Sanz-Gonzalez S, Ros M, Burks DJ, Valverde AM. Inhibition of PTP1B restores IRS1-mediated hepatic insulin signaling in IRS2-deficient mice. Diabetes. 2010;59:588–99. doi: 10.2337/db09-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schultze SM, Hemmings BA, Niessen M, Tschopp O. PI3K/AKT, MAPK and AMPK signalling: protein kinases in glucose homeostasis. Expet Rev Mol Med. 2012;14:e1. doi: 10.1017/s1462399411002109. [DOI] [PubMed] [Google Scholar]
- 111.Parikh C, Janakiraman V, Wu WI, Foo CK, Kljavin NM, Chaudhuri S, et al. Disruption of PH-kinase domain interactions leads to oncogenic activation of AKT in human cancers. Proc Natl Acad Sci U S A. 2012;109:19368–73. doi: 10.1073/pnas.1204384109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vanhaesebroeck B, Stephens L, Hawkins P. PI3K signalling: the path to discovery and understanding. Nat Rev Mol Cell Biol. 2012;13:195–203. doi: 10.1038/nrm3290. [DOI] [PubMed] [Google Scholar]
- 113.Sun EJ, Wankell M, Palamuthusingam P, McFarlane C, Hebbard L. Targeting the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Biomedicines. 2021;9:1639. doi: 10.3390/biomedicines9111639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–30. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 115.Cota D, Matter EK, Woods SC, Seeley RJ. The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity. J Neurosci. 2008;28:7202–8. doi: 10.1523/jneurosci.1389-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kwon O, Kim KW, Kim MS. Leptin signalling pathways in hypothalamic neurons. Cell Mol Life Sci. 2016;73:1457–77. doi: 10.1007/s00018-016-2133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kitamura T, Feng Y, Kitamura YI, Chua SC, Xu AW, Barsh GS, et al. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med. 2006;12:534–40. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- 118.Kim MS, Pak YK, Jang PG, Namkoong C, Choi YS, Won JC, et al. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat Neurosci. 2006;9:901–6. doi: 10.1038/nn1731. [DOI] [PubMed] [Google Scholar]
- 119.Varela L, Horvath TL. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO Rep. 2012;13:1079–86. doi: 10.1038/embor.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ma W, Fuentes G, Shi X, Verma C, Radda GK, Han W. FoxO1 negatively regulates leptin-induced POMC transcription through its direct interaction with STAT3. Biochem J. 2015;466:291–8. doi: 10.1042/bj20141109. [DOI] [PubMed] [Google Scholar]
- 121.Plum L, Lin HV, Dutia R, Tanaka J, Aizawa KS, Matsumoto M, et al. The obesity susceptibility gene Cpe links FoxO1 signaling in hypothalamic pro-opiomelanocortin neurons with regulation of food intake. Nat Med. 2009;15:1195–201. doi: 10.1038/nm.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sahu A, Koshinaka K, Sahu M. Phosphatidylinositol 3-kinase is an upstream regulator of the phosphodiesterase 3B pathway of leptin signalling that may not involve activation of Akt in the rat hypothalamus. J Neuroendocrinol. 2013;25:168–79. doi: 10.1111/j.1365-2826.2012.02386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhao AZ, Shinohara MM, Huang D, Shimizu M, Eldar-Finkelman H, Krebs EG, et al. Leptin induces insulin-like signaling that antagonizes cAMP elevation by glucagon in hepatocytes. J Biol Chem. 2000;275:11348–54. doi: 10.1074/jbc.275.15.11348. [DOI] [PubMed] [Google Scholar]
- 124.Zhao AZ, Huan JN, Gupta S, Pal R, Sahu A. A phosphatidylinositol 3-kinase phosphodiesterase 3B-cyclic AMP pathway in hypothalamic action of leptin on feeding. Nat Neurosci. 2002;5:727–8. doi: 10.1038/nn885. [DOI] [PubMed] [Google Scholar]
- 125.Sahu M, Anamthathmakula P, Sahu A. Hypothalamic phosphodiesterase 3B pathway mediates anorectic and body weight-reducing effects of insulin in male mice. Neuroendocrinology. 2017;104:145–56. doi: 10.1159/000445523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–23. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–62. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–74. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 129.Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, et al. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem. 2004;279:12005–8. doi: 10.1074/jbc.c300557200. [DOI] [PubMed] [Google Scholar]
- 130.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–43. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 131.Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol. 1997;273:E1107–12. doi: 10.1152/ajpendo.1997.273.6.e1107. [DOI] [PubMed] [Google Scholar]
- 132.Obici S, Feng Z, Arduini A, Conti R, Rossetti L. Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production. Nat Med. 2003;9:756–61. doi: 10.1038/nm873. [DOI] [PubMed] [Google Scholar]
- 133.Tanida M, Yamamoto N, Shibamoto T, Rahmouni K. Involvement of hypothalamic AMP-activated protein kinase in leptin-induced sympathetic nerve activation. PLoS One. 2013;8:e56660. doi: 10.1371/journal.pone.0056660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dagon Y, Hur E, Zheng B, Wellenstein K, Cantley LC, Kahn BB. p70S6 kinase phosphorylates AMPK on serine 491 to mediate leptin’s effect on food intake. Cell Metabol. 2012;16:104–12. doi: 10.1016/j.cmet.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ueyama E, Morikawa Y, Yasuda T, Senba E. Attenuation of fasting-induced phosphorylation of mitogen-activated protein kinases (ERK/p38) in the mouse hypothalamus in response to refeeding. Neurosci Lett. 2004;371:40–4. doi: 10.1016/j.neulet.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 136.Rahmouni K, Sigmund CD, Haynes WG, Mark AL. Hypothalamic ERK mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes. 2009;58:536–42. doi: 10.2337/db08-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Myers MG. Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res. 2004;59:287–304. doi: 10.1210/rp.59.1.287. [DOI] [PubMed] [Google Scholar]
- 138.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–10. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- 139.Huang H, Kong D, Byun KH, Ye C, Koda S, Lee DH, et al. Rho-kinase regulates energy balance by targeting hypothalamic leptin receptor signaling. Nat Neurosci. 2012;15:1391–8. doi: 10.1038/nn.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jaffe T, Schwartz B. Leptin promotes motility and invasiveness in human colon cancer cells by activating multiple signal-transduction pathways. Int J Cancer. 2008;123:2543–56. doi: 10.1002/ijc.23821. [DOI] [PubMed] [Google Scholar]
- 141.Zeidan A, Hunter JC, Javadov S, Karmazyn M. mTOR mediates RhoA-dependent leptin-induced cardiomyocyte hypertrophy. Mol Cell Biochem. 2011;352:99–108. doi: 10.1007/s11010-011-0744-2. [DOI] [PubMed] [Google Scholar]
- 142.Sohn JW, Oh Y, Kim KW, Lee S, Williams KW, Elmquist JK. Leptin and insulin engage specific PI3K subunits in hypothalamic SF1 neurons. Mol Metabol. 2016;5:669–79. doi: 10.1016/j.molmet.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Williams KW, Sohn JW, Donato J, Lee CE, Zhao JJ, Elmquist JK, et al. The acute effects of leptin require PI3K signaling in the hypothalamic ventral premammillary nucleus. J Neurosci. 2011;31:13147–56. doi: 10.1523/jneurosci.2602-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Qiu J, Fang Y, Ronnekleiv OK, Kelly MJ. Leptin excites proopiomelanocortin neurons via activation of TRPC channels. J Neurosci. 2010;30:1560–5. doi: 10.1523/jneurosci.4816-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dhar M, Wayman GA, Zhu M, Lambert TJ, Davare MA, Appleyard SM. Leptin-induced spine formation requires TrpC channels and the CaM kinase cascade in the hippocampus. J Neurosci. 2014;34:10022–33. doi: 10.1523/jneurosci.2868-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Perissinotti PP, Martinez-Hernandez E, Piedras-Renteria ES. TRPC1/5-Ca V 3 complex mediates leptin-induced excitability in hypothalamic neurons. Front Neurosci. 2021;15:679078. doi: 10.3389/fnins.2021.679078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, et al. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metabol. 2007;6:307–19. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 148.Mariani S, Di Giorgio MR, Rossi E, Tozzi R, Contini S, Bauleo L, et al. Blood SIRT1 shows a coherent association with leptin and adiponectin in relation to the degree and distribution of adiposity: a study in obesity, normal weight and Anorexia Nervosa. Nutrients. 2020;12:3506. doi: 10.3390/nu12113506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sasaki T. Age-associated weight gain, leptin, and SIRT1: a possible role for hypothalamic SIRT1 in the prevention of weight gain and aging through modulation of leptin sensitivity. Front Endocrinol. 2015;6:109. doi: 10.3389/fendo.2015.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ramis MR, Esteban S, Miralles A, Tan DX, Reiter RJ. Caloric restriction, resveratrol and melatonin: role of SIRT1 and implications for aging and related-diseases. Mech Ageing Dev. 2015;146–148:28–41. doi: 10.1016/j.mad.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 151.Ramadori G, Fujikawa T, Fukuda M, Anderson J, Morgan DA, Mostoslavsky R, et al. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metabol. 2010;12:78–87. doi: 10.1016/j.cmet.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Susanti VY, Sasaki T, Yokota-Hashimoto H, Matsui S, Lee YS, Kikuchi O, et al. Sirt1 rescues the obesity induced by insulin-resistant constitutively-nuclear FoxO1 in POMC neurons of male mice. Obesity. 2014;22:2115–9. doi: 10.1002/oby.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dietrich MO, Antunes C, Geliang G, Liu ZW, Borok E, Nie Y, et al. Agrp neurons mediate Sirt1’s action on the melanocortin system and energy balance: roles for Sirt1 in neuronal firing and synaptic plasticity. J Neurosci. 2010;30:11815–25. doi: 10.1523/jneurosci.2234-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sasaki T, Kikuchi O, Shimpuku M, Susanti VY, Yokota-Hashimoto H, Taguchi R, et al. Hypothalamic SIRT1 prevents age-associated weight gain by improving leptin sensitivity in mice. Diabetologia. 2014;57:819–31. doi: 10.1007/s00125-013-3140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ramadori G, Fujikawa T, Anderson J, Berglund ED, Frazao R, Michan S, et al. SIRT1 deacetylase in SF1 neurons protects against metabolic imbalance. Cell Metabol. 2011;14:301–12. doi: 10.1016/j.cmet.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–80. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Quan W, Kim HK, Moon EY, Kim SS, Choi CS, Komatsu M, et al. Role of hypothalamic proopiomelanocortin neuron autophagy in the control of appetite and leptin response. Endocrinology. 2012;153:1817–26. doi: 10.1210/en.2011-1882. [DOI] [PubMed] [Google Scholar]
- 158.Coupe B, Ishii Y, Dietrich MO, Komatsu M, Horvath TL, Bouret SG. Loss of autophagy in pro-opiomelanocortin neurons perturbs axon growth and causes metabolic dysregulation. Cell Metabol. 2012;15:247–55. doi: 10.1016/j.cmet.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metabol. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 160.Kleinridders A, Lauritzen HP, Ussar S, Christensen JH, Mori MA, Bross P, et al. Leptin regulation of Hsp60 impacts hypothalamic insulin signaling. J Clin Invest. 2013;123:4667–80. doi: 10.1172/jci67615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Marker T, Sell H, Zillessen P, Glode A, Kriebel J, Ouwens DM, et al. Heat shock protein 60 as a mediator of adipose tissue inflammation and insulin resistance. Diabetes. 2012;61:615–25. doi: 10.2337/db10-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Patak J, Gilfert J, Byler M, Neerukonda V, Thiffault I, Cross L, et al. MAGEL2-related disorders: a study and case series. Clin Genet. 2019;96:493–505. doi: 10.1111/cge.13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mercer RE, Michaelson SD, Chee MJ, Atallah TA, Wevrick R, Colmers WF. Magel2 is required for leptin-mediated depolarization of POMC neurons in the hypothalamic arcuate nucleus in mice. PLoS Genet. 2013;9:e1003207. doi: 10.1371/journal.pgen.1003207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wijesuriya TM, De Ceuninck L, Masschaele D, Sanderson MR, Carias KV, Tavernier J, et al. The Prader-Willi syndrome proteins MAGEL2 and necdin regulate leptin receptor cell surface abundance through ubiquitination pathways. Hum Mol Genet. 2017;26:4215–30. doi: 10.1093/hmg/ddx311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.De Ceuninck L, Wauman J, Masschaele D, Peelman F, Tavernier J. Reciprocal cross-regulation between RNF41 and USP8 controls cytokine receptor sorting and processing. J Cell Sci. 2013;126:3770–81. doi: 10.1242/jcs.131250. [DOI] [PubMed] [Google Scholar]
- 166.Kim KW, Donato J, Berglund ED, Choi YH, Kohno D, Elias CF, et al. FOXO1 in the ventromedial hypothalamus regulates energy balance. J Clin Invest. 2012;122:2578–89. doi: 10.1172/jci62848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 168.Coutinho EA, Okamoto S, Ishikawa AW, Yokota S, Wada N, Hirabayashi T, et al. Activation of SF1 neurons in the ventromedial hypothalamus by DREADD technology increases insulin sensitivity in peripheral tissues. Diabetes. 2017;66:2372–86. doi: 10.2337/db16-1344. [DOI] [PubMed] [Google Scholar]
- 169.Ren H, Orozco IJ, Su Y, Suyama S, Gutierrez-Juarez R, Horvath TL, et al. FoxO1 target Gpr17 activates AgRP neurons to regulate food intake. Cell. 2012;149:1314–26. doi: 10.1016/j.cell.2012.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ren H, Cook JR, Kon N, Accili D. Gpr17 in AgRP neurons regulates feeding and sensitivity to insulin and leptin. Diabetes. 2015;64:3670–9. doi: 10.2337/db15-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Reilly AM, Zhou S, Panigrahi SK, Yan S, Conley JM, Sheets PL, et al. Gpr17 deficiency in POMC neurons ameliorates the metabolic derangements caused by long-term high-fat diet feeding. Nutr Diabetes. 2019;9:29. doi: 10.1038/s41387-019-0096-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li C, Friedman JM. Leptin receptor activation of SH2 domain containing protein tyrosine phosphatase 2 modulates Ob receptor signal transduction. Proc Natl Acad Sci U S A. 1999;96:9677–82. doi: 10.1073/pnas.96.17.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, Haj F, Wang Y, Minokoshi Y, et al. PTP1B regulates leptin signal transduction in vivo. Dev Cell. 2002;2:489–95. doi: 10.1016/s1534-5807(02)00148-x. [DOI] [PubMed] [Google Scholar]
- 174.Cheng A, Uetani N, Simoncic PD, Chaubey VP, Lee-Loy A, McGlade CJ, et al. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev Cell. 2002;2:497–503. doi: 10.1016/s1534-5807(02)00149-1. [DOI] [PubMed] [Google Scholar]
- 175.Rousso-Noori L, Knobler H, Levy-Apter E, Kuperman Y, Neufeld-Cohen A, Keshet Y, et al. Protein tyrosine phosphatase epsilon affects body weight by downregulating leptin signaling in a phosphorylation-dependent manner. Cell Metabol. 2011;13:562–72. doi: 10.1016/j.cmet.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 176.Nieto-Vazquez I, Fernandez-Veledo S, de Alvaro C, Rondinone CM, Valverde AM, Lorenzo M. Protein-tyrosine phosphatase 1B-deficient myocytes show increased insulin sensitivity and protection against tumor necrosis factor-alpha-induced insulin resistance. Diabetes. 2007;56:404–13. doi: 10.2337/db06-0989. [DOI] [PubMed] [Google Scholar]
- 177.Loh K, Fukushima A, Zhang X, Galic S, Briggs D, Enriori PJ, et al. Elevated hypothalamic TCPTP in obesity contributes to cellular leptin resistance. Cell Metabol. 2011;14:684–99. doi: 10.1016/j.cmet.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Plum L, Ma X, Hampel B, Balthasar N, Coppari R, Munzberg H, et al. Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. J Clin Invest. 2006;116:1886–901. doi: 10.1172/jci27123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Irie-Sasaki J, Sasaki T, Matsumoto W, Opavsky A, Cheng M, Welstead G, et al. CD45 is a JAK phosphatase and negatively regulates cytokine receptor signalling. Nature. 2001;409:349–54. doi: 10.1038/35053086. [DOI] [PubMed] [Google Scholar]
- 180.Zhang ZY, Dodd GT, Tiganis T. Protein tyrosine phosphatases in hypothalamic insulin and leptin signaling. Trends Pharmacol Sci. 2015;36:661–74. doi: 10.1016/j.tips.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 181.Claflin KE, Sandgren JA, Lambertz AM, Weidemann BJ, Littlejohn NK, Burnett CM, et al. Angiotensin AT1A receptors on leptin receptor-expressing cells control resting metabolism. J Clin Invest. 2017;127:1414–24. doi: 10.1172/jci88641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Deng Y, Deng G, Grobe JL, Cui H. Hypothalamic GPCR signaling pathways in cardiometabolic control. Front Physiol. 2021;12:691226. doi: 10.3389/fphys.2021.691226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bathina S, Das UN. Brain-derived neurotrophic factor and its clinical implications. Arch Med Sci. 2015;11:1164–78. doi: 10.5114/aoms.2015.56342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pelleymounter MA, Cullen MJ, Wellman CL. Characteristics of BDNF-induced weight loss. Exp Neurol. 1995;131:229–38. doi: 10.1016/0014-4886(95)90045-4. [DOI] [PubMed] [Google Scholar]
- 185.Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19:1290–300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Liao GY, An JJ, Gharami K, Waterhouse EG, Vanevski F, Jones KR, et al. Dendritically targeted Bdnf mRNA is essential for energy balance and response to leptin. Nat Med. 2012;18:564–71. doi: 10.1038/nm.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kabra DG, Pfuhlmann K, Garcia-Caceres C, Schriever SC, Casquero Garcia V, Kebede AF, et al. Hypothalamic leptin action is mediated by histone deacetylase 5. Nat Commun. 2016;7:10782. doi: 10.1038/ncomms10782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kuyk JK, Walton RE. Comparison of the radiographic appearance of root canal size to its actual diameter. J Endod. 1990;16:528–33. doi: 10.1016/s0099-2399(07)80215-9. [DOI] [PubMed] [Google Scholar]
- 189.Han YM, Kang GM, Byun K, Ko HW, Kim J, Shin MS, et al. Leptin-promoted cilia assembly is critical for normal energy balance. J Clin Invest. 2014;124:2193–7. doi: 10.1172/jci69395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lee CH, Song DK, Park CB, Choi J, Kang GM, Shin SH, et al. Primary cilia mediate early life programming of adiposity through lysosomal regulation in the developing mouse hypothalamus. Nat Commun. 2020;11:5772. doi: 10.1038/s41467-020-19638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Stratigopoulos G, Martin Carli JF, O’Day DR, Wang L, Leduc CA, Lanzano P, et al. Hypomorphism for RPGRIP1L, a ciliary gene vicinal to the FTO locus, causes increased adiposity in mice. Cell Metabol. 2014;19:767–79. doi: 10.1016/j.cmet.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Verkhratsky A, Parpura V, Vardjan N, Zorec R. Physiology of astroglia. Adv Exp Med Biol. 2019;1175:45–91. doi: 10.1007/978-981-13-9913-8_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hsuchou H, He Y, Kastin AJ, Tu H, Markadakis EN, Rogers RC, et al. Obesity induces functional astrocytic leptin receptors in hypothalamus. Brain. 2009;132:889–902. doi: 10.1093/brain/awp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kim JG, Suyama S, Koch M, Jin S, Argente-Arizon P, Argente J, et al. Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat Neurosci. 2014;17:908–10. doi: 10.1038/nn.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang Y, Hsuchou H, He Y, Kastin AJ, Pan W. Role of astrocytes in leptin signaling. J Mol Neurosci. 2015;56:829–39. doi: 10.1007/s12031-015-0518-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Debarba LK, Vechiato FMV, Veida-Silva H, Borges BC, Jamur MC, Antunes-Rodrigues J, et al. The role of TCPTP on leptin effects on astrocyte morphology. Mol Cell Endocrinol. 2019;482:62–9. doi: 10.1016/j.mce.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 197.Lausten-Thomsen U, Lund MAV, Frithioff-Bojsoe C, Hedley PL, Pedersen O, Hansen T, et al. Reference values for leptin/adiponectin ratio in healthy children and adolescents. Clin Chim Acta. 2019;493:123–8. doi: 10.1016/j.cca.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 198.Hamann TV, Mork FCB, Jensen AK, Jorgensen NR, Heidemann MS, Schou AJ, et al. Reference serum percentile values of adiponectin, leptin, and adiponectin/leptin ratio in healthy Danish children and adolescents. Scand J Clin Lab Invest. 2022:1–10. doi: 10.1080/00365513.2022.2073911. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 199.Lausten-Thomsen U, Christiansen M, Louise Hedley P, Esmann Fonvig C, Stjernholm T, Pedersen O, et al. Reference values for serum leptin in healthy non-obese children and adolescents. Scand J Clin Lab Invest. 2016;76:561–7. doi: 10.1080/00365513.2016.1210226. [DOI] [PubMed] [Google Scholar]
- 200.Koester-Weber T, Valtuena J, Breidenassel C, Beghin L, Plada M, Moreno S, et al. Reference values for leptin, cortisol, insulin and glucose, among European adolescents and their association with adiposity: the HELENA study. Nutr Hosp. 2014;30:1181–90. doi: 10.3305/nh.2014.30.5.7982. [DOI] [PubMed] [Google Scholar]
- 201.Karakosta P, Georgiou V, Fthenou E, Margioris A, Castanas E, Kogevinas M, et al. Gender-specific reference intervals for cord blood leptin in Crete, Greece. Eur J Pediatr. 2012;171:1563–6. doi: 10.1007/s00431-012-1804-7. [DOI] [PubMed] [Google Scholar]
- 202.Wu XY, Wu XP, Xie H, Zhang H, Luo XH, Liu SP, et al. Relationship between age-related reference values of serum osteoprotegerin and leptin in native Chinese women and compared with those in women of other races. Clin Chim Acta. 2008;389:72–8. doi: 10.1016/j.cca.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 203.Bjorbaek C, Elmquist JK, Michl P, Ahima RS, van Bueren A, McCall AL, et al. Expression of leptin receptor isoforms in rat brain microvessels. Endocrinology. 1998;139:3485–91. doi: 10.1210/endo.139.8.6154. [DOI] [PubMed] [Google Scholar]
- 204.Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–11. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 205.Di Spiezio A, Sandin ES, Dore R, Muller-Fielitz H, Storck SE, Bernau M, et al. The LepR-mediated leptin transport across brain barriers controls food reward. Mol Metabol. 2018;8:13–22. doi: 10.1016/j.molmet.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Harrison L, Schriever SC, Feuchtinger A, Kyriakou E, Baumann P, Pfuhlmann K, et al. Fluorescent blood-brain barrier tracing shows intact leptin transport in obese mice. Int J Obes. 2019;43:1305–18. doi: 10.1038/s41366-018-0221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lammert A, Kiess W, Bottner A, Glasow A, Kratzsch J. Soluble leptin receptor represents the main leptin binding activity in human blood. Biochem Biophys Res Commun. 2001;283:982–8. doi: 10.1006/bbrc.2001.4885. [DOI] [PubMed] [Google Scholar]
- 208.Sudhakar M, Silambanan S, Chandran AS, Prabhakaran AA, Ramakrishnan R. C-reactive protein (CRP) and leptin receptor in obesity: binding of monomeric CRP to leptin receptor. Front Immunol. 2018;9:1167. doi: 10.3389/fimmu.2018.01167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Elinav E, Ali M, Bruck R, Brazowski E, Phillips A, Shapira Y, et al. Competitive inhibition of leptin signaling results in amelioration of liver fibrosis through modulation of stellate cell function. Hepatology. 2009;49:278–86. doi: 10.1002/hep.22584. [DOI] [PubMed] [Google Scholar]
- 210.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–5. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 211.Ropelle ER, Flores MB, Cintra DE, Rocha GZ, Pauli JR, Morari J, et al. IL-6 and IL-10 anti-inflammatory activity links exercise to hypothalamic insulin and leptin sensitivity through IKKbeta and ER stress inhibition. PLoS Biol. 2010;8:e1000465. doi: 10.1371/journal.pbio.1000465. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 212.Kleinridders A, Schenten D, Konner AC, Belgardt BF, Mauer J, Okamura T, et al. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metabol. 2009;10:249–59. doi: 10.1016/j.cmet.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Fukuda M, Williams KW, Gautron L, Elmquist JK. Induction of leptin resistance by activation of cAMP-Epac signaling. Cell Metabol. 2011;13:331–9. doi: 10.1016/j.cmet.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yan J, Mei FC, Cheng H, Lao DH, Hu Y, Wei J, et al. Enhanced leptin sensitivity, reduced adiposity, and improved glucose homeostasis in mice lacking exchange protein directly activated by cyclic AMP isoform 1. Mol Cell Biol. 2013;33:918–26. doi: 10.1128/mcb.01227-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wang X, Lacza Z, Sun YE, Han W. Leptin resistance and obesity in mice with deletion of methyl-CpG-binding protein 2 (MeCP2) in hypothalamic pro-opiomelanocortin (POMC) neurons. Diabetologia. 2014;57:236–45. doi: 10.1007/s00125-013-3072-0. [DOI] [PubMed] [Google Scholar]
- 216.Shintani T, Higashi S, Suzuki R, Takeuchi Y, Ikaga R, Yamazaki T, et al. PTPRJ inhibits leptin signaling, and induction of PTPRJ in the hypothalamus is a cause of the development of leptin resistance. Sci Rep. 2017;7:11627. doi: 10.1038/s41598-017-12070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Seo J, Fortuno ES, Suh JM, Stenesen D, Tang W, Parks EJ, et al. Atf4 regulates obesity, glucose homeostasis, and energy expenditure. Diabetes. 2009;58:2565–73. doi: 10.2337/db09-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Deng J, Yuan F, Guo Y, Xiao Y, Niu Y, Deng Y, et al. Deletion of ATF4 in AgRP neurons promotes fat loss mainly via increasing energy expenditure. Diabetes. 2017;66:640–50. doi: 10.2337/db16-0954. [DOI] [PubMed] [Google Scholar]
- 219.Xiao Y, Deng Y, Yuan F, Xia T, Liu H, Li Z, et al. ATF4/ATG5 signaling in hypothalamic proopiomelanocortin neurons regulates fat mass via affecting energy expenditure. Diabetes. 2017;66:1146–58. doi: 10.2337/db16-1546. [DOI] [PubMed] [Google Scholar]
- 220.Peng J, Yin L, Wang X. Central and peripheral leptin resistance in obesity and improvements of exercise. Horm Behav. 2021;133:105006. doi: 10.1016/j.yhbeh.2021.105006. [DOI] [PubMed] [Google Scholar]
- 221.Bartell SM, Rayalam S, Ambati S, Gaddam DR, Hartzell DL, Hamrick M, et al. Central (ICV) leptin injection increases bone formation, bone mineral density, muscle mass, serum IGF-1, and the expression of osteogenic genes in leptin-deficient ob/ob mice. J Bone Miner Res. 2011;26:1710–20. doi: 10.1002/jbmr.406. [DOI] [PubMed] [Google Scholar]
- 222.Hamrick MW, Dukes A, Arounleut P, Davis C, Periyasamy-Thandavan S, Mork S, et al. The adipokine leptin mediates muscle- and liver-derived IGF-1 in aged mice. Exp Gerontol. 2015;70:92–6. doi: 10.1016/j.exger.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Koo YD, Lee JS, Lee SA, Quaresma PGF, Bhat R, Haynes WG, et al. SUMO-specific protease 2 mediates leptin-induced fatty acid oxidation in skeletal muscle. Metabolism. 2019;95:27–35. doi: 10.1016/j.metabol.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ceddia RB, William WN, Curi R. Comparing effects of leptin and insulin on glucose metabolism in skeletal muscle: evidence for an effect of leptin on glucose uptake and decarboxylation. Int J Obes Relat Metab Disord. 1999;23:75–82. doi: 10.1038/sj.ijo.0800762. [DOI] [PubMed] [Google Scholar]
- 225.Sainz N, Rodriguez A, Catalan V, Becerril S, Ramirez B, Lancha A, et al. Leptin reduces the expression and increases the phosphorylation of the negative regulators of GLUT4 traffic TBC1D1 and TBC1D4 in muscle of ob/ob mice. PLoS One. 2012;7:e29389. doi: 10.1371/journal.pone.0029389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Pardina E, Ferrer R, Baena-Fustegueras JA, Lecube A, Fort JM, Vargas V, et al. The relationships between IGF-1 and CRP, NO, leptin, and adiponectin during weight loss in the morbidly obese. Obes Surg. 2010;20:623–32. doi: 10.1007/s11695-010-0103-5. [DOI] [PubMed] [Google Scholar]
- 227.Gonzalez-Gil AM, Peschard-Franco M, Castillo EC, Gutierrez-DelBosque G, Trevino V, Silva-Platas C, et al. Myokine-adipokine cross-talk: potential mechanisms for the association between plasma irisin and adipokines and cardiometabolic risk factors in Mexican children with obesity and the metabolic syndrome. Diabetol Metab Syndrome. 2019;11:63. doi: 10.1186/s13098-019-0458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Steinberg GR, Parolin ML, Heigenhauser GJ, Dyck DJ. Leptin increases FA oxidation in lean but not obese human skeletal muscle: evidence of peripheral leptin resistance. Am J Physiol Endocrinol Metab. 2002;283:E187–92. doi: 10.1152/ajpendo.00542.2001. [DOI] [PubMed] [Google Scholar]
- 229.Guerra B, Ponce-Gonzalez JG, Morales-Alamo D, Guadalupe-Grau A, Kiilerich K, Fuentes T, et al. Leptin signaling in skeletal muscle after bed rest in healthy humans. Eur J Appl Physiol. 2014;114:345–57. doi: 10.1007/s00421-013-2779-4. [DOI] [PubMed] [Google Scholar]
- 230.Ritchie IR, Gulli RA, Stefanyk LE, Harasim E, Chabowski A, Dyck DJ. Restoration of skeletal muscle leptin response does not precede the exercise-induced recovery of insulin-stimulated glucose uptake in high-fat-fed rats. Am J Physiol Regul Integr Comp Physiol. 2011;300:R492–500. doi: 10.1152/ajpregu.00602.2010. [DOI] [PubMed] [Google Scholar]
- 231.Fuentes T, Ara I, Guadalupe-Grau A, Larsen S, Stallknecht B, Olmedillas H, et al. Leptin receptor 170 kDa (OB-R170) protein expression is reduced in obese human skeletal muscle: a potential mechanism of leptin resistance. Exp Physiol. 2010;95:160–71. doi: 10.1113/expphysiol.2009.049270. [DOI] [PubMed] [Google Scholar]
- 232.Zabolotny JM, Kim YB, Welsh LA, Kershaw EE, Neel BG, Kahn BB. Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. J Biol Chem. 2008;283:14230–41. doi: 10.1074/jbc.m800061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lee MJ, Fried SK. Multilevel regulation of leptin storage, turnover, and secretion by feeding and insulin in rat adipose tissue. J Lipid Res. 2006;47:1984–93. doi: 10.1194/jlr.m600065-jlr200. [DOI] [PubMed] [Google Scholar]
- 234.Tsai M, Asakawa A, Amitani H, Inui A. Stimulation of leptin secretion by insulin. Indian J Endocrinol Metabol. 2012;16:S543–8. doi: 10.4103/2230-8210.105570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kamohara S, Burcelin R, Halaas JL, Friedman JM, Charron MJ. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature. 1997;389:374–7. doi: 10.1038/38717. [DOI] [PubMed] [Google Scholar]
- 236.Seufert J, Kieffer TJ, Leech CA, Holz GG, Moritz W, Ricordi C, et al. Leptin suppression of insulin secretion and gene expression in human pancreatic islets: implications for the development of adipogenic diabetes mellitus. J Clin Endocrinol Metab. 1999;84:670–6. doi: 10.1210/jc.84.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Park HK, Ahima RS. Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism. 2015;64:24–34. doi: 10.1016/j.metabol.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yang R, Barouch LA. Leptin signaling and obesity: cardiovascular consequences. Circ Res. 2007;101:545–59. doi: 10.1161/circresaha.107.156596. [DOI] [PubMed] [Google Scholar]
- 239.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–71. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Konner AC, Bruning JC. Selective insulin and leptin resistance in metabolic disorders. Cell Metabol. 2012;16:144–52. doi: 10.1016/j.cmet.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 241.Kang SM, Kwon HM, Hong BK, Kim D, Kim IJ, Choi EY, et al. Expression of leptin receptor (Ob-R) in human atherosclerotic lesions: potential role in intimal neovascularization. Yonsei Med J. 2000;41:68–75. doi: 10.3349/ymj.2000.41.1.68. [DOI] [PubMed] [Google Scholar]
- 242.Beltowski J. Leptin and atherosclerosis. Atherosclerosis. 2006;189:47–60. doi: 10.1016/j.atherosclerosis.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 243.Koh KK, Park SM, Quon MJ. Leptin and cardiovascular disease: response to therapeutic interventions. Circulation. 2008;117:3238–49. doi: 10.1161/circulationaha.107.741645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Martin SS, Qasim A, Reilly MP. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol. 2008;52:1201–10. doi: 10.1016/j.jacc.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–3. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 246.Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341:879–84. doi: 10.1056/nejm199909163411204. [DOI] [PubMed] [Google Scholar]
- 247.Toyoshima Y, Gavrilova O, Yakar S, Jou W, Pack S, Asghar Z, et al. Leptin improves insulin resistance and hyperglycemia in a mouse model of type 2 diabetes. Endocrinology. 2005;146:4024–35. doi: 10.1210/en.2005-0087. [DOI] [PubMed] [Google Scholar]
- 248.Paz-Filho G, Mastronardi C, Wong ML, Licinio J. Leptin therapy, insulin sensitivity, and glucose homeostasis. Indian J Endocrinol Metabol. 2012;16:S549–55. doi: 10.4103/2230-8210.105571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Amitani M, Asakawa A, Amitani H, Inui A. The role of leptin in the control of insulin-glucose axis. Front Neurosci. 2013;7:51. doi: 10.3389/fnins.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Barr VA, Malide D, Zarnowski MJ, Taylor SI, Cushman SW. Insulin stimulates both leptin secretion and production by rat white adipose tissue. Endocrinology. 1997;138:4463–72. doi: 10.1210/endo.138.10.5451. [DOI] [PubMed] [Google Scholar]
- 251.Zhao AZ, Bornfeldt KE, Beavo JA. Leptin inhibits insulin secretion by activation of phosphodiesterase 3B. J Clin Invest. 1998;102:869–73. doi: 10.1172/jci3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Hill JW, Xu Y, Preitner F, Fukuda M, Cho YR, Luo J, et al. Phosphatidyl inositol 3-kinase signaling in hypothalamic proopiomelanocortin neurons contributes to the regulation of glucose homeostasis. Endocrinology. 2009;150:4874–82. doi: 10.1210/en.2009-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Goncalves GH, Li W, Garcia AV, Figueiredo MS, Bjorbaek C. Hypothalamic agouti-related peptide neurons and the central melanocortin system are crucial mediators of leptin’s antidiabetic actions. Cell Rep. 2014;7:1093–103. doi: 10.1016/j.celrep.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Perry RJ, Zhang XM, Zhang D, Kumashiro N, Camporez JP, Cline GW, et al. Leptin reverses diabetes by suppression of the hypothalamic-pituitary-adrenal axis. Nat Med. 2014;20:759–63. doi: 10.1038/nm.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Jiang SZ, Lu W, Zong XF, Ruan HY, Liu Y. Obesity and hypertension. Exp Ther Med. 2016;12:2395–9. doi: 10.3892/etm.2016.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Simonds SE, Pryor JT, Ravussin E, Greenway FL, Dileone R, Allen AM, et al. Leptin mediates the increase in blood pressure associated with obesity. Cell. 2014;159:1404–16. doi: 10.1016/j.cell.2014.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Bravo PE, Morse S, Borne DM, Aguilar EA, Reisin E. Leptin and hypertension in obesity. Vasc Health Risk Manag. 2006;2:163–9. doi: 10.2147/vhrm.2006.2.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Mark AL, Shaffer RA, Correia ML, Morgan DA, Sigmund CD, Haynes WG. Contrasting blood pressure effects of obesity in leptin-deficient ob/ob mice and agouti yellow obese mice. J Hypertens. 1999;17:1949–53. doi: 10.1097/00004872-199917121-00026. [DOI] [PubMed] [Google Scholar]
- 259.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci. 2003;23:5998–6004. doi: 10.1523/jneurosci.23-14-05998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Mark AL, Agassandian K, Morgan DA, Liu X, Cassell MD, Rahmouni K. Leptin signaling in the nucleus tractus solitarii increases sympathetic nerve activity to the kidney. Hypertension. 2009;53:375–80. doi: 10.1161/hypertensionaha.108.124255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Patel HR, Qi Y, Hawkins EJ, Hileman SM, Elmquist JK, Imai Y, et al. Neuropeptide Y deficiency attenuates responses to fasting and high-fat diet in obesity-prone mice. Diabetes. 2006;55:3091–8. doi: 10.2337/db05-0624. [DOI] [PubMed] [Google Scholar]
- 262.Marsh AJ, Fontes MA, Killinger S, Pawlak DB, Polson JW, Dampney RA. Cardiovascular responses evoked by leptin acting on neurons in the ventromedial and dorsomedial hypothalamus. Hypertension. 2003;42:488–93. doi: 10.1161/01.hyp.0000090097.22678.0a. [DOI] [PubMed] [Google Scholar]
- 263.von Schnurbein J, Manzoor J, Brandt S, Denzer F, Kohlsdorf K, Fischer-Posovszky P, et al. Leptin is not essential for obesity-associated hypertension. Obes Facts. 2019;12:460–75. doi: 10.1159/000501319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Brown RJ, Meehan CA, Gorden P. Leptin does not mediate hypertension associated with human obesity. Cell. 2015;162:465–6. doi: 10.1016/j.cell.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/s0140-6736(08)60269-x. [DOI] [PubMed] [Google Scholar]
- 266.Li D, Morris JS, Liu J, Hassan MM, Day RS, Bondy ML, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA. 2009;301:2553–62. doi: 10.1001/jama.2009.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Stattin P, Soderberg S, Hallmans G, Bylund A, Kaaks R, Stenman UH, et al. Leptin is associated with increased prostate cancer risk: a nested case-referent study. J Clin Endocrinol Metab. 2001;86:1341–5. doi: 10.1210/jc.86.3.1341. [DOI] [PubMed] [Google Scholar]
- 268.Tamakoshi K, Toyoshima H, Wakai K, Kojima M, Suzuki K, Watanabe Y, et al. Leptin is associated with an increased female colorectal cancer risk: a nested case-control study in Japan. Oncology. 2005;68:454–61. doi: 10.1159/000086988. [DOI] [PubMed] [Google Scholar]
- 269.Shida D, Kitayama J, Mori K, Watanabe T, Nagawa H. Transactivation of epidermal growth factor receptor is involved in leptin-induced activation of janus-activated kinase 2 and extracellular signal-regulated kinase 1/2 in human gastric cancer cells. Cancer Res. 2005;65:9159–63. doi: 10.1158/0008-5472.can-05-0598. [DOI] [PubMed] [Google Scholar]
- 270.Huang XF, Chen JZ. Obesity, the PI3K/Akt signal pathway and colon cancer. Obes Rev. 2009;10:610–6. doi: 10.1111/j.1467-789x.2009.00607.x. [DOI] [PubMed] [Google Scholar]
- 271.Saxena NK, Sharma D, Ding X, Lin S, Marra F, Merlin D, et al. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007;67:2497–507. doi: 10.1158/0008-5472.can-06-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Nath AK, Brown RM, Michaud M, Sierra-Honigmann MR, Snyder M, Madri JA. Leptin affects endocardial cushion formation by modulating EMT and migration via Akt signaling cascades. J Cell Biol. 2008;181:367–80. doi: 10.1083/jcb.200708197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Hersoug LG, Linneberg A. The link between the epidemics of obesity and allergic diseases: does obesity induce decreased immune tolerance? Allergy. 2007;62:1205–13. doi: 10.1111/j.1398-9995.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 274.Otero M, Lago R, Gomez R, Lago F, Dieguez C, Gomez-Reino JJ, et al. Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65:1198–201. doi: 10.1136/ard.2005.046540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Lourenco EV, Liu A, Matarese G, La Cava A. Leptin promotes systemic lupus erythematosus by increasing autoantibody production and inhibiting immune regulation. Proc Natl Acad Sci U S A. 2016;113:10637–42. doi: 10.1073/pnas.1607101113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Matarese G, Carrieri PB, Montella S, De Rosa V, La Cava A. Leptin as a metabolic link to multiple sclerosis. Nat Rev Neurol. 2010;6:455–61. doi: 10.1038/nrneurol.2010.89. [DOI] [PubMed] [Google Scholar]
- 277.Sanchez-Margalet V, Martin-Romero C, Santos-Alvarez J, Goberna R, Najib S, Gonzalez-Yanes C. Role of leptin as an immunomodulator of blood mononuclear cells: mechanisms of action. Clin Exp Immunol. 2003;133:11–9. doi: 10.1046/j.1365-2249.2003.02190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Tian Z, Sun R, Wei H, Gao B. Impaired natural killer (NK) cell activity in leptin receptor deficient mice: leptin as a critical regulator in NK cell development and activation. Biochem Biophys Res Commun. 2002;298:297–302. doi: 10.1016/s0006-291x(02)02462-2. [DOI] [PubMed] [Google Scholar]
- 279.Procaccini C, De Rosa V, Galgani M, Carbone F, La Rocca C, Formisano L, et al. Role of adipokines signaling in the modulation of T cells function. Front Immunol. 2013;4:332. doi: 10.3389/fimmu.2013.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Zou X, Zhong L, Zhu C, Zhao H, Zhao F, Cui R, et al. Role of leptin in mood disorder and neurodegenerative disease. Front Neurosci. 2019;13:378. doi: 10.3389/fnins.2019.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Murphy MP, LeVine H. Alzheimer’s disease and the amyloid-beta peptide. J Alzheim Dis. 2010;19:311–23. doi: 10.3233/jad-2010-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Perez-Gonzalez R, Antequera D, Vargas T, Spuch C, Bolos M, Carro E. Leptin induces proliferation of neuronal progenitors and neuroprotection in a mouse model of Alzheimer’s disease. J Alzheim Dis. 2011;24(2 Suppl):17–25. doi: 10.3233/jad-2011-102070. [DOI] [PubMed] [Google Scholar]
- 283.Xing Y, Liu J, Xu J, Yin L, Wang L, Li J, et al. Association between plasma leptin and estrogen in female patients of amnestic mild cognitive impairment. Dis Markers. 2015;2015:450237. doi: 10.1155/2015/450237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Liu Z, Zhang Y, Liu J, Yin F. Geniposide attenuates the level of Abeta1-42 via enhancing leptin signaling in cellular and APP/PS1 transgenic mice. Arch Pharm Res. 2017;40:571–8. doi: 10.1007/s12272-016-0875-9. [DOI] [PubMed] [Google Scholar]
- 285.Mhyre TR, Boyd JT, Hamill RW, Maguire-Zeiss KA. Parkinson’s disease. Subcell Biochem. 2012;65:389–455. doi: 10.1007/978-94-007-5416-4_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Weng Z, Signore AP, Gao Y, Wang S, Zhang F, Hastings T, et al. Leptin protects against 6-hydroxydopamine-induced dopaminergic cell death via mitogen-activated protein kinase signaling. J Biol Chem. 2007;282:34479–91. doi: 10.1074/jbc.m705426200. [DOI] [PubMed] [Google Scholar]
- 287.Alberici A, Bocchio L, Geroldi C, Zanardini R, Bonomini C, Bugari G, et al. Serum leptin levels are higher in females affected by frontotemporal lobar degeneration than Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2008;79:712–5. doi: 10.1136/jnnp.2007.137026. [DOI] [PubMed] [Google Scholar]
- 288.Woolley JD, Khan BK, Natesan A, Karydas A, Dallman M, Havel P, et al. Satiety-related hormonal dysregulation in behavioral variant frontotemporal dementia. Neurology. 2014;82:512–20. doi: 10.1212/wnl.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Chinookoswong N, Wang JL, Shi ZQ. Leptin restores euglycemia and normalizes glucose turnover in insulin-deficient diabetes in the rat. Diabetes. 1999;48:1487–92. doi: 10.2337/diabetes.48.7.1487. [DOI] [PubMed] [Google Scholar]
- 290.Fujikawa T, Chuang JC, Sakata I, Ramadori G, Coppari R. Leptin therapy improves insulin-deficient type 1 diabetes by CNS-dependent mechanisms in mice. Proc Natl Acad Sci U S A. 2010;107:17391–6. doi: 10.1073/pnas.1008025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Denroche HC, Levi J, Wideman RD, Sequeira RM, Huynh FK, Covey SD, et al. Leptin therapy reverses hyperglycemia in mice with streptozotocin-induced diabetes, independent of hepatic leptin signaling. Diabetes. 2011;60:1414–23. doi: 10.2337/db10-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Lyu X, Yan K, Wang X, Xu H, Guo X, Zhu H, et al. A novel anti-obesity mechanism for liraglutide by improving adipose tissue leptin resistance in high-fat diet-fed obese mice. Endocr J. 2022 doi: 10.1507/endocrj.EJ21-0802. [DOI] [PubMed] [Google Scholar]
- 293.Kim J, Lee N, Suh SB, Jang S, Kim S, Kim DG, et al. Metformin ameliorates olanzapine-induced disturbances in POMC neuron number, axonal projection, and hypothalamic leptin resistance. BMB Rep. 2022;55:293–8. doi: 10.5483/bmbrep.2022.55.6.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Liu J, Lee J, Salazar Hernandez MA, Mazitschek R, Ozcan U. Treatment of obesity with celastrol. Cell. 2015;161:999–1011. doi: 10.1016/j.cell.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Lee J, Liu J, Feng X, Salazar Hernandez MA, Mucka P, Ibi D, et al. Withaferin A is a leptin sensitizer with strong antidiabetic properties in mice. Nat Med. 2016;22:1023–32. doi: 10.1038/nm.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Bharath LP, Choi WW, Cho JM, Skobodzinski AA, Wong A, Sweeney TE, et al. Combined resistance and aerobic exercise training reduces insulin resistance and central adiposity in adolescent girls who are obese: randomized clinical trial. Eur J Appl Physiol. 2018;118:1653–60. doi: 10.1007/s00421-018-3898-8. [DOI] [PubMed] [Google Scholar]
- 297.Kim SW, Jung WS, Park W, Park HY. Twelve weeks of combined resistance and aerobic exercise improves cardiometabolic biomarkers and enhances red blood cell hemorheological function in obese older men: a randomized controlled trial. Int J Environ Res Publ Health. 2019;16:5020. doi: 10.3390/ijerph16245020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Zheng X, Niu S. Leptin-induced basal Akt phosphorylation and its implication in exercise-mediated improvement of insulin sensitivity. Biochem Biophys Res Commun. 2018;496:37–43. doi: 10.1016/j.bbrc.2017.12.161. [DOI] [PubMed] [Google Scholar]
- 299.Steinberg GR, Smith AC, Wormald S, Malenfant P, Collier C, Dyck DJ. Endurance training partially reverses dietary-induced leptin resistance in rodent skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E57–63. doi: 10.1152/ajpendo.00302.2003. [DOI] [PubMed] [Google Scholar]
- 300.Zhao S, Zhu Y, Schultz RD, Li N, He Z, Zhang Z, et al. Partial leptin reduction as an insulin sensitization and weight loss strategy. Cell Metabol. 2019;30:706–19 e6. doi: 10.1016/j.cmet.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]