Abstract
Our gut microbiome is constituted by trillions of microorganisms including bacteria, archaea and eukaryotic microbes. Nowadays, gut microbiome has been gradually recognized as a new organ system that systemically and biochemically interact with the host. Accumulating evidence suggests that the imbalanced gut microbiome contributes to the dysregulation of immune system and the disruption of cardiovascular homeostasis. Specific microbiome profiles and altered intestinal permeability are often observed in the pathophysiology of cardiovascular diseases. Gut-derived metabolites, toxins, peptides and immune cell-derived cytokines play pivotal roles in the induction of inflammation and the pathogenesis of dysfunction of heart and vasculature. Impaired crosstalk between gut microbiome and multiple organ systems, such as gut-vascular, heart-gut, gut-liver and brain-gut axes, are associated with higher cardiovascular risks. Medications and strategies that restore healthy gut microbiome might therefore represent novel therapeutic options to lower the incidence of cardiovascular and metabolic disorders.
Keywords: cardiovascular diseases, dysbiosis, endothelium, endotoxin, fecal microbiota transplantation, gut microbiome
Introduction
During the lengthy evolutionary history of life, a delicate balance, usually a symbiotic relationship, develops between host organisms and microbial communities [1]. Our gastrointestinal tract harbors trillions of residents, of which some behave like a ‘friendly neighborhood’ while some act as a ‘potential threat’ to host homeostasis [2]. The residential microbes in the digestive tract, collectively termed as gut microbiome, are constituted by diverse microorganisms, including archaea, bacteria and eukaryotic microbes [3]. These tiny residents intimately interact with the host to profoundly impact essential aspects of host fitness, such as lifespan, metabolism, development and fecundity [4]. Any imbalance between the commensal and pathogenic microbes, termed as dysbiosis, poses threat to host health [5]. With the emergence of basic research and clinical findings on gut microbiome-host crosstalk, we increasingly consider the gut microbiome as a new organ system [6]. Of noted, this newly recognized organ system affects host homeostasis and disease pathophysiology through close communication with multiple host organs. This review article primarily focuses on addressing the tight linkage between gut microbiome and the cardiovascular system (gut-cardiovascular connection) and providing novel therapeutic insights to the treatment of cardiovascular complications.
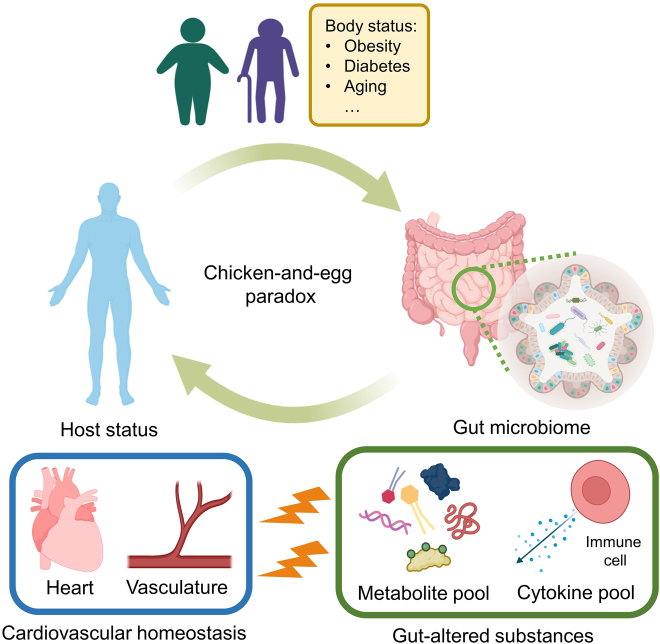
Cardiovascular disease is the leading cause of mortality in China, accounting for approximately 40% of overall deaths in the Chinese population [7]. The rising morbidity and mortality of cardiovascular diseases indicate the urgency for improving pre-existing and developing new therapeutic strategies. The gut-cardiovascular connection has been recently proposed to represent one of the newest druggable targets for the prevention and treatment of cardiovascular disorders [8]. Across the intestinal barrier, the systemic circulation of the cardiovascular system is probably the closest neighbor to the gastrointestinal tract and its tiny residents. The close distance correlates to higher sensitivity and vulnerability of the cardiovascular system to gut microbiome imbalance. In other words, dysbiosis increases cardiovascular risks by exerting harmful effects on the heart and vasculature [9]. However, it is not a one-way action that gut microbiome profiles unidirectionally influence host health status to alter disease pathogenesis. The host health status can also reversely shape gut microbiome [10]. For instance, host aging is associated with the dynamic shift of gut microbiome profiles, which predisposes host to aging-associated diseases (e.g. cardiovascular complications) [11]. Meanwhile, many murine studies supported the casual role of dysbiosis in the development of obesity and diabetes, two critical risk factors in cardiovascular diseases [12]. Due to limited evidence on the causality in human subjects, it therefore remains a chicken-and-egg debate on the exact pathological sequence of gut microbiome and host status in the disease development (Figure 1).
Figure 1:
Interplay between the host status and gut microbiome. Host statuses such as obesity, diabetes, and aging, may alter gut microbiome profile of the host. On the other hand, imbalance of gut microbiome alters metabolite pool and cytokine pool of the body, which consequently impair the host homeostasis (e.g. cardiovascular homeostasis).
During the development of dysbiosis-accelerated cardiovascular pathogenesis, multiple key players or crucial contributors are sequentially present, including the gut microbiome, intestinal permeability, gut-derived substances (e.g. metabolites, toxins, and peptides), immune system and the cellular components of the cardiovascular system. Across the intestinal barrier, the imbalanced gut microbiome contributes to the altered metabolite and cytokine pool of the host [13], which consequently cause stress on the cellular components of heart and vasculature, namely endothelial cells, vascular smooth muscle cells (VSMCs) and cardiomyocytes, to elevate cardiovascular risks (Figure 1). This general pattern could be observed in various cardiovascular complications, including but not limited to atherosclerosis, hypertension, myocardial infarction (MI), coronary heart disease (CHD) and stroke. In addition to the gut-vascular and heart-gut axes, the gut microbiome could possibly impair cardiovascular homeostasis via the gut-liver and brain-gut axes [14]. Medications and therapeutic strategies that could restore or reshape the dysregulated gut microbiome of the host would therefore reduce cardiovascular risks. Fecal microbiota transplantation (FMT), the transfer of microbes from healthy donors to high risk individuals or patients [15], might represent novel preventive and therapeutic opportunities for the treatment of cardiovascular diseases. This review article provides a brief overview on some potential therapeutic options for cardiovascular disorders in related to gut microbiology.
Gut microbiome and cardiovascular diseases
Host microbiome and cardiovascular risks
Different cardiovascular complications are associated with specialized alterations in gut microbiome composition. Extensive efforts in both basic and clinical studies have been made to link a particular cardiovascular disease to the outgrowth and the decline in certain microbial strains. In these studies, some critical metabolites were highlighted for their potential roles in the development of cardiovascular complications (Table 1). Such gut microbiome alterations, in terms of diversity and abundance, might serve as either a predictor or index on the onset or the severity of some major cardiovascular diseases.
Table 1:
Cardiovascular disease and host microbiome.
| Cardiovascular disease | Sample | Microbiome profile | Key substance(s) | Mechanism | Ref. |
|---|---|---|---|---|---|
| Atherosclerosis | ApoE−/− mouse | Bacteroidetes ↓, Firmicutes ↑ Proteobacteria ↑ | TMAO | Choline diet → TMAO↑ → lesion↑ | [19] |
| Ldlr−/− mouse | Akkermansia↓, Christensenellaceae ↓ Odoribacter ↓ | SCFA | Systemic inflammation↑ | [21] | |
| Human | Bacteroidetes ↓ Actinobacteria ↑ | Unknown | Endotoxin ↑, Cholesterol metabolism↓ | [23] | |
| Human | Enterobacteriaceae ↑ Streptococcus↑ | Unknown | Inflammatory response↑ | [24] | |
| Hypertension | C57BL/6J mouse | Unknown | Cytokine (IL-17) | Inflammatory response↑ endothelial dysfunction↑, blood pressure↑ | [28] |
| C57BL/6J mouse | Lactobacillus ↓. | Unknown | High salt → T helper 17 cells↑ inflammatory response↑ | [29] | |
| Human | Anaerovorax ↑, Clostridium IV ↑ Oscillibacter ↑, Sporobacter ↑ | Unknown | Unknown | [30] | |
| Human | Lactobacillus ↓ | Unknown | Sodium uptake↑ | [31] | |
| SHR | Bifidobacterium ↓ Streptococcus ↑ | SCFA | Inflammatory response↑ | [32] | |
| Human | Prevotella ↑, Klebsiella ↑, Porphyromonas ↑, Actinomyces ↑ Faecalibacterium ↓, Roseburia↓ Bifidobacterium ↓, Butyrivibrio ↓ | LPS SCFA | Inflammatory response↑ | [33] | |
| Stroke | C57BL/6J mouse | Firmicutes ↑, Bacteroidetes ↑ | Cytokines | Pro-inflammatory T cell polarization↑ | [36] |
| Rag1−/−mouse | Actinobacteria ↑ | (IL-17 and IFN-γ) | Infarct volume↑, post-stroke outcome↓ | ||
| C57BL/6 mouse | Bifidobacterium ↓, Clostridium ↓, Faecalibacterium↓, Lactobacillus ↓ | SCFA | Inflammatory response↑ | [37] | |
| Human | Lactobacillaceae↑, Akkermansia ↑ Enterobacteriaceae ↑, Porphyromonadaceae ↑ | SCFA | Inflammatory response↑ | [38] | |
| Human C57BL/6J mouse | Unknown | TMAO | TMAO↑ adverse cardiovascular event↑ | [39] | |
| Human C57BL/6J mouse | Coriobacteriaceae ↑ Erysipelotrichaceae↑, Allobaculum ↑ | TMAO | TMAO↑ thrombosis potential↑ | [40] | |
| C57B/6 mouse Tlr2−/− mouse | Unknown | von Willebrand factor | von Willebrand factor↑ thrombosis potential↑ | [41] | |
| Coronary heart disease | Human | Bacteroidetes ↓, Firmicutes ↑ LactobaciNales ↑ | Unknown | Unknown | [44] |
| Human | Clostridium ↑ | GlcNAc-6-P | Sugar metabolism | [45] | |
| Streptococcus ↑ | Mannitol | → Cardiovascular activity | |||
| C57BL/6J mouse | Cbstridium symbiosum ↑ Eggerthella ↑ | Bile acids Cytokine (IL-17) | Bile acids↓ → Circulatory cholesterol↑ inflammatory response↑ | [46] | |
| Human | Unknown | TMAO | TMAO ↑ adverse cardiovascular event↑ | [47] | |
| Perpheral artery disease | Human | Unknown | TMAO | TMAO↑ adverse cardiovascular event↑ | [50] |
| Myocardial infarction | Human | LactobaciHus↑, Bacteroides ↑ Streptococcus ↑ | LPS d-lactate | Systemic inflammation↑ | [53] |
| Dahl S rats | Unknown | Aromatic amino acids | Activation of survival pathways in heart | [54] | |
| Human | Lachnospiraceae ↓, Aerococcaceae ↑, Ruminococcaceae ↑ | TMAO | TMAO↑ adverse cardiovascular event↑ | [55] | |
| C57BL/6J mouse | Unknown | Unknown | Presence of microbiota → ejection fraction↑ myocardial infarction↓ | [56] | |
| Aneurysm | C57BL ApoE−/− mouse | Akkermansia↓, Odoribacter↑ Helicobacter↑, Ruminococcus ↑ | Unknown | Inflammatory response↑ | [58] |
| C57BL/6J mouse | Unknown | Cytokines (IL-1β. IL-6. MCP-1) | Inflammatory response↑ | [60] | |
| Human C57BL/6N mouse | H. hathewayi↓ | Taurine | Taurine↓ →Inflammatory response↑ | [61] |
IL: interleukin; LPS: lipopolysaccharide; MCP: monocyte chemoattractant protein; SCFA: short-chain fatty acid; TMAO: trimethylamine N-oxide.
Atherosclerosis and its devastating complications, especially CHD, peripheral artery disease and MI, represent one of the leading causes of global mortality [16]. Characterized by plaque formation that is the accumulation of fatty substances, cellular components, and fibrous elements within the arterial intima [17], atherosclerosis is a chronic inflammatory disorder of the arterial wall [18]. Atherosclerosis is the primary cause for the forementioned fatal cardiovascular diseases, where extensive basic research and clinical study are still seeking to uncover its comprehensive molecular signature. In recent years, the rapid development of gut microbiome research has facilitated the identification of novel biomarkers for atherosclerotic vascular diseases. Disrupted gut microbiome was shown to favor the conversion of dietary choline to trimethylamine N-oxide (TMAO) [19], a circulating toxic biomarker which positively correlates with atherosclerotic lesions [20]. In addition, gut microbiome imbalance could aggravate atherosclerotic progression by lowering the abundance of short-chain fatty acids (SCFAs; e.g. acetate, proprionate and butyrate) [21], which normally serve as the suppressors of vascular inflammation [22]. In patients with atherosclerotic vascular diseases, their dysregulated fecal microbiome profiles were correlated with impaired cholesterol metabolism and enhanced inflammatory response [23, 24]. Although previous literature suggested that microbial regulation of bile acids shall play a role in the development of atherosclerosis [25], consolidated findings are still lacking. Further efforts are needed to explore and verify the alterations in the metabolite pool of human subjects.
Hypertension, the most significant risk for global morbidity and mortality, serves as a major risk factor causing cardiovascular diseases. Due to the silent nature of hypertension in its early stages, many un-diagnosed patients are usually unaware of the need for early and adequate treatment [26]. Recently, the gut microbiome has been suggested to participate in the regulation of blood pressure (BP) and hence the development of hypertension [27]. Colonization of germ-free mice with conventional gut microbiome aggravated angiotensin II-induced endothelial dysfunction and arterial hypertension [28], highlighting the contribution of gut microbiome in hypertensive cardiovascular diseases. By contrast, colonization of germ-free mice with L. murinus significantly inhibited autoimmunity and salt-induced hypertension [29]. These important findings imply the opposite effects of pathogenic and commensal microbes in the development of hypertension. A microbiome study on 529 middle-aged participants showed a positive correlation between hypertension and certain bacterial genera (i.e. Anaerovorax, Clostridium IV, Oscillibacter, and Sporobacter) [30]. Another cohort study on human subjects reported that the Lactobacillus species was negatively correlated with both arterial BP and sodium uptake [31]. A previous study on spontaneously hypertensive rats (SHRs) indicated that dysbiosis was pro-inflammatory during hypertension, probably by mediating microbial release of SCFAs [32]. Moreover, the increased level of lipopolysaccharide (LPS) may underlie dysbiosis-associated hypertension [33].
Stroke, a sudden focal injury to the area within the central nervous system (CNS) because of brain arterial occlusion, remains the second leading cause of mortality and third leading cause of disability globally, affecting nearly 25% of adults during their lifetime [34]. Of noted, cerebral infarction during stroke and post-stroke recovery might be related to the altered gut microbiome profile [35]. Remarkably, transplantation of dysbiotic microbiome from brain-injured donor mice resulted in larger infarct size and poorer post-stroke outcome in mice [36]. By contrast, a healthy gut microbiome, characterized by higher abundance of SCFA-producing bacteria (e.g. Bifidobacterium longum, Clostridium symbiosum, and Lactobacillus fermentum) are found to accelerate post-stroke recovery [37]. A previous clinical study also implied that a reduced level of gut-derived SCFA could serve as a potential prognostic marker for acute ischemic stroke [38]. Importantly, the elevated content of gut microbiome-dependent TMAO correlates with a higher incidence of adverse cardiovascular events (e.g. recurrent stroke, MI and cardiovascular mortality) [39]. In addition, the existing experimental findings suggest a contributory role of gut microbiome in arterial thrombosis, the precursor event of ischemic stroke. The gut microbiome-dependent TMAO production mechanistically links to a higher thrombosis potential [40]. In a toll-like receptor-2 (TLR2)-dependent mechanism, gut microbiome regulates the synthesis of von Willebrand factor from hepatic endothelial cells to promote thrombosis formation [41]. Therefore, a dysbiotic microbiome are more likely to serve as a prognostic factor for ischemic stroke, while restoration of a healthy microbiome might enhance the post-stroke recovery of the host.
CHD, or coronary artery disease, refers to the narrowing or even blockage of coronary arteries. The build-up of plaques underneath the wall of coronary arteries highlights the atherosclerotic and inflammatory nature of CHD [42]. Notably, the incidence of CHD also correlates with the gut microbiome profile [43]. Clinically, CHD patients were associated with elevated Firmicutes/Bacteroidetes ratio and increased abundance of the order Lactobacillales in their intestinal microbiome, which were often detected in obese and diabetic individuals [44]. Another clinical study suggested that gut microbiome-derived N-acetylglucosamine-6-phosphate(GlcNAc-6-P) and mannitol could be metabolic biomarkers for CHD [45]. Recently, Liu et al. [46] proposed that CHD-associated dysbiosis potentially leads to bile acid imbalance, and hence impaired cholesterol homeostasis. As for an atherosclerotic cardiovascular disease, gut microbiome-derived TMAO can also be considered as a risk biomarker for CHD [47]. Sharing major risk factors and pathophysiologic mechanisms with CHD [48], peripheral artery disease (PAD), a chronic disorder characterized by atherosclerosis of the abdominal aorta and lower extremity arteries affects more than 200 million people globally [49]. Similar to CHD, TMAO level could be a risk predictor for PAD [50]. However, very few studies have solely explored the relationship between intestinal microbiome and PAD.
MI is pathologically defined as the death of myocardial cells upon prolonged ischemia in myocardium [51]. By 2030, the number of MI patients in China is expected to be approximately 23 million [52]. Recent studies attempt to address the poor prognosis and recovery of MI on a microbiome perspective. In 2018, Zhou et al. [53] were among the first to indicate that the post-MI cardiovascular outcomes were affected by the translocation of gut microbiome into systemic circulation (i.e. endotoxin). Another group suggested that the severity of MI was related to the metabolites (e.g. amino acids) derived by intestinal microbiome, hinting the diagnostic potential [54]. Thus the gut microbiome-derived TMAO could serve as a predictive marker for adverse cardiovascular events post-MI [55]. By contrast, the presence of certain commensal microbes may confer a cardioprotective role. For instance, conventionally raised mice were associated with higher ejection fraction when compared to germ-free mice upon ligation of left anterior descending artery [56]. This in vivo finding implied the presence of certain commensal microbes in eliciting the anti-myocardial infarction effects.
Aneurysm is an irreversible and permanent change of aortic wall structure, resulting from the degradation of extracellular matrix and loss of VSMCs. The mortality rate of abdominal aortic aneurysm (AAA) rupture strikingly reaches 50% [57]. In mouse model of AAA, a positive correlation between AAA diameter and the dysbiotic gut microbiome was reported [58]. Strongly associated with aortic aneurysm [59], intracranial aneurysm could be worsened by dysbiosis. Gut microbiome depletion by antibiotics alleviated intracranial aneurysm in mice [60]. In 2020, Li et al. [61] demonstrated that H. hathewayi-associated depletion of taurine promotes the progression of intracranial aneurysm. More interrogations on the gut microbiome composition of other types of aneurysm, such as AAA and thoracic aortic aneurysm, are urged.
Maternal microbiome and cardiovascular risks
The transmission of maternal microbiome to infants was clinically proven as infants can acquire microbes from intestine, skin, vagina and oral cavity of their mothers [62]. More importantly, the maternal microbiome profile and the resultant metabolite pool contribute to the programming of gut microbiome and immunity of fetus. Briefly, there is a translocation of microbes from the maternal intestine to the placenta, resulting in the microbial colonization in fetus. Moreover, the gut-derived metabolites from mother, particularly SCFAs, could cause epigenetic alterations and subsequent immune programming in fetus [63]. Therefore, it is possible that maternal dysbiosis may predispose a child to higher cardiovascular risk (Table 2).
Table 2:
Cardiovascular risk and maternal microbiome.
| Condition(s) | Sample | Microbiome profile | Key substance(s) | Mechanism | Ref. |
|---|---|---|---|---|---|
| Metabolic syndrome | Germ-free ICR mouse C57BL/6J mouse | Unknown | SCFA (e.g. Proprionate) | Maternal SCFA↓ →Embryonic GPR41 and GPR43 →Insulin resistance, obesity →Cardiovascular risk↑ |
[64] |
| Gestational diabetes mellitus | Human | Prevotella ↑, Streptococcus ↑ Bacteroides↑, Lactobacillus ↑ | Viruses (e.g. herpesvirus, mastadenovirus) | Viral infection↑ | [65] |
| Metabolic abnormalities + exercise | C57BL/6 mouse | Odoribacter ↑ Helicobacter↑ Clostridium XIVb↑ | SCFA | Maternal SCFA↑ →Insulin sensitivity, body weight↓ →Cardiovascular risk↓ |
[66] |
| Hypertension + N-acetylcysteine | SHR rat | Verrucomicrobia ↓, Actinobacteria ↑ Turicibacter ↓, Akkermansia↓ Bifidobacterium ↑, Allobaculum ↑ | Hydrogen sulfide (H2S) | H2St ↑ →Hypertension↓ |
[67] |
| Hypertension + captopril | SHR rat | Erysipelotrichia ↑, Erysipelotrichaceae ↑ Clostridials ↑, Clostridia ↑ Allobaculum ↑ | LPS Cytokines (e.g. TNF-α GlcNAc-6-P: N-acetylglucosamine-6-phosphate, IL-1β) | Gut inflammation and permeability↓ Neuroinflammation↓ sympathetic activity↓ | [68] |
| Hypertension + probiotic | SHR rat | Lactobacillus casei ↑ | SCFA | Maternal SCFA↑ →BP↓ →Cardiovascular risk↓ |
[69] |
BP: blood pressure; H2S: hydrogen sulfide; IL: interleukin; LPS: lipopolysaccharide; SCFA: short-chain fatty acid; SHR: spontaneously hypertensive rat; TNF: tumour necrosis factor.
In 2020, Kimura et al. [64] provided clues that maternal gut microbiome was crucial to the development of metabolic syndrome (e.g. obesity and insulin resistance) in offspring, which is a multiplex cardiovascular risk factor. An intergenerational concordance of dysbiotic gut microbiome was observed in pregnant women and neonates suffering from gestational diabetes mellitus [65], potentially implying a higher risk of type 2 diabetes mellitus, obesity and cardiovascular complications in offspring. Interestingly, maternal exercise could boost SCFA-producing microbes in maternal microbiome to improve insulin sensitivity and metabolic disorders in offspring [66], conferring a lower cardiovascular risk. Offspring hypertension risk appears to be modulated by the maternal gut microbiome. Maternal supplementation of N-acetylcysteine could markedly ameliorate hypertension in SHR offspring through increasing the production of hydrogen sulfide (H2S) [67]. Meanwhile, maternal treatment of captopril could lower BP in SHR offspring via a gut-brain axis [68]. Maternal administration of probiotic also protects SHR offspring against hypertension [69]. These important findings highlight the transfer of cardiovascular risks between generations from a microbial perspective. Notably, long-term follow-up studies on the effect of maternal microbiome on offspring cardiovascular health in human subjects are at present lacking. Whether transmission of dysbiotic microbiome could aggravate the progression of atherosclerotic cardiovascular diseases in offspring requires further investigation.
Intestinal permeability
Loss of balance between commensal and pathogenic microbes in our gut microbiome causes damage on intestinal lining, in which the pathogenic microbes generate ‘holes’ and ‘pores’ in the intestinal barrier [70]. The increased intestinal permeability, termed as ‘leaky’, is characterized by loose tight junctions between adjacent epithelial cells and a damaged intestinal mucus layer [71]. When the intestine becomes leaky, antigens, bacteria, endotoxins, and toxins from the lumen would readily enter the systemic circulation [72]. Importantly, the leaky gut is often observed along with various diseases, including cardiovascular complications, diabetes mellitus, carcinoma and neurological disorders [73]. Improper lifestyles favored by a sedentary pattern, disrupted circadian rhythm and imbalanced diet (e.g. high fat, high glucose, low fiber) prompt the loss of intestinal integrity. Aging is generally accompanied by a gradual decline of intestinal permeability and low-grade inflammation [74]. Such lifestyles and aging, as a results, increase the host vulnerability to dysbiosis-related chronic diseases.
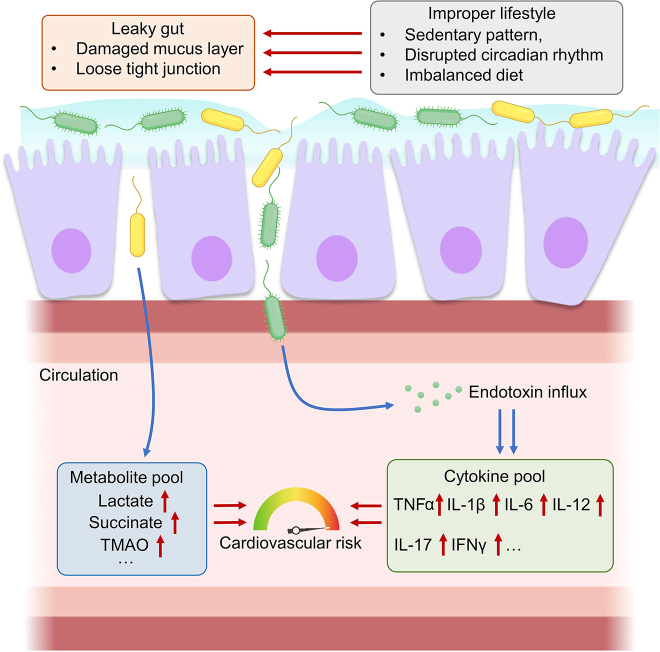
Higher cardiovascular risk is correlated with the impaired intestinal permeability. The leaky gut accelerates the progression of dysbiosis-related cardiovascular diseases by facilitating endotoxin influx, resulting in the alterations in the metabolite and cytokine pools in the host systemic circulation (Figure 2). Overgrowth of certain pathogenic microbes (e.g. Helicobacter pylori [75], Clostridium spp [24], Enterococcus faecalis [76], and Staphylococcus spp [77]) were often recognized upon leaky gut and cardiovascular diseases. The influx of endotoxin (i.e. LPS) from Gram-negative bacteria induce host systemic inflammation, causing dysfunction of the cardiovascular system [78]. Recognition of endotoxins by sensors, such as TLRs and receptor for advanced glycation end products (RAGE) [79], remarkably boosts the production of cytokines (e.g. TNFα, interleukin(IL)-1β, IL-6 and IFNγ) [80]. Elevated cytokine levels trigger arterial inflammation, the hallmark of many cardiovascular complications (e.g. atherosclerosis, hypertension and CHD) [81].
Figure 2:
Intestinal permeability and cardiovascular risk. Improper lifestyles contribute to the increased intestinal permeability (leaky gut), which leads to the influx of endotoxin and microbiome-derived metabolites into the systemic circulation. Endotoxin influx induces the immune cells to secrete pro-inflammatory cytokines. The altered metabolite pool and cytokine pool cause damage on cellular components of the cardiovascular system to increase cardiovascular risks. IFN: interferon; IL: interleukin; TMAO: trimethylamine N-oxide; TNF: tumor necrosis factor.
The leakage of gut-derived metabolites into the host circulation may pose significant threat to the cardiovascular health. High level of gut-derived lactate in the host circulation is predictive to major adverse cardiovascular events [53]. Lactate accumulation in circulation causes lactic acidosis, which flushes the cellular components of cardiovascular system with acidic microenvironment and hence directly hinders cardiovascular function [82]. Besides, leakage of gut-derived succinate also impairs cardiovascular homeostasis. The oxidation of succinate causes oxidative damage to cardiovascular tissues, especially during myocardial ischemia-reperfusion [83]. As aforementioned, TMAO level serves as an established risk factor for cardiovascular diseases. A leaky gut increases the circulatory level of gut-derived trimethylamine (TMA), which is further metabolized to TMAO for igniting endothelial activation and atherogenesis [84]. Healthy lifestyles (e.g. regular exercise), certain medications and probiotics that improve intestinal integrity may therefore partially confer protection to the cardiovascular system.
Endothelium: the first-line barrier
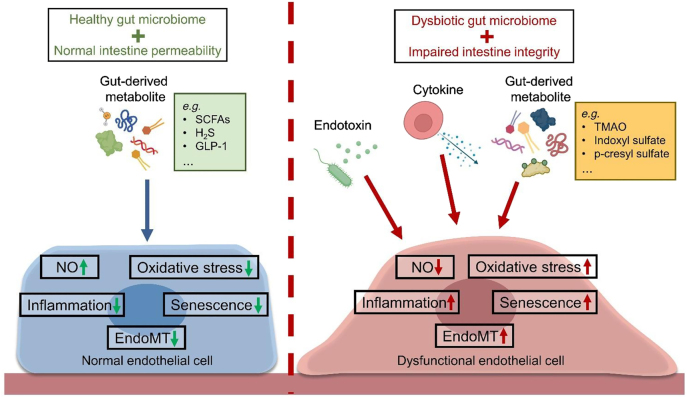
The inner lining of blood vessels is formed with a monolayer of endothelial cells, which continually experience biomechanical and biochemical stimuli in the circulatory system [85]. Endothelial cells play pivotal roles in the modulation of vascular tone, and the trafficking of substances between circulation and underlying vascular cells [86]. Dysfunction of endothelial cells is an early event predisposing the development of both cardiac and vascular complications, particularly atherosclerotic cardiovascular disease [87]. The molecular features of endothelial dysfunction include diminished NO bioavailability, augmented oxidative stress, enhanced inflammation and increased cellular senescence [88]. Endothelial dysfunction results in the dysregulated communication between endothelial cells and proximal cellular components of the cardiovascular system (e.g. VSMCs and cardiomyocytes), and accelerates disease progression [89]. Upon a damaged intestinal barrier, the endothelial cells are exposed to endotoxin, cytokines and gut-derived metabolites, and are therefore prone to endothelial dysfunction (Figure 3).
Figure 3:
Gut-derived substances and endothelial homeostasis. Healthy gut microbiome and normal intestinal permeability protect endothelial function. On the other hand, dysbiosis and leaky gut trigger endothelial dysfunction via causing entry of endotoxin, cytokines, and metabolites into the bloodstream. GLP-1: Glucagon-like peptide 1; H2S: hydrogen sulfide; MT: endothelial-to mesenchymal-transition; NO: nitric oxide; SCFAs: short-chain fatty acids; TMAO: trimethylamine N-oxide.
Exposure to endotoxin directly injures endothelial cells and induces endothelial dysfunction. In addition to oxidative damage and inflammatory response [90], endotoxin even triggers endothelial-to-mesenchymal transition (EndoMT) [91], the loss of normal endothelial phenotype alongside the gain of mesenchymal markers in endothelial cells. The morphological change in endothelial cells signifies the loss of normal endothelial function. The endotoxemia-induced cytokine production also impairs endothelial function. The association between inflammatory cytokines and endothelial dysfunction highlight the underlying mechanisms of many cardiovascular diseases. Such endotoxin-associated link is common in endothelial cells during atherosclerotic cardiovascular diseases [92]. Cytokine-induced EndoMT remarkably injuries endothelial function and correlates with the incidence of multiple cardiovascular diseases, such as atherosclerosis and pulmonary hypertension [93]. Prevention of endotoxemia represents one of the therapeutic perspectives for maintaining endothelial homeostasis.
Gut-derived metabolites modulate endothelial homeostasis. Certain metabolites that serve as biomarkers for dysbiosis-related cardiovascular diseases often cause damage to endothelial cells. TMAO causes endothelial dysfunction by lowering nitric oxide (NO) production [94]. The gut microbiome can metabolize the aromatic amino acids to form uremic toxins (e.g. indoxyl sulfate and p-cresyl sulfate). Leakage of uremic toxins is hazardous to endothelial health. Both indoxyl sulfate and p-cresyl sulfate were shown to be pro-inflammatory in endothelial cells [95, 96]. In addition, indoxyl sulfate lowers endothelial viability and induces endothelial dysfunction, as revealed by increased generation of reactive oxygen species (ROS) and decreased NO bioavailability [97]. Although acidosis results in the apoptosis of coronary endothelial cells [98], whether dysbiosis-associated lactic acidosis disrupts endothelial homeostasis remains elusive. However, gut-derived metabolites are not always harmful to endothelial health.
Gut-derived SCFAs and gaseous metabolites can be beneficial to endothelial function. Multiple groups have provided clues to elucidate the direct beneficial effects of SCFAs on endothelial cells. In 2019, Bartolomaeus et al. [99] demonstrated the antihypertensive effects of propionate in murine, via the improvement on endothelial function. Furthermore, acetate and butyrate were described to protect endothelial function against angiotensin II insult by facilitating NO production [100]. Our intestine is one of the biological sources of the gaseous molecule H2S, which is also beneficial to the endothelial health. The various benefits of H2S in the cardiovascular function, such as its vasodilatory, anti-inflammatory, antioxidant and antihypertensive effects, have been well-established [101]. Administration of sulfate prebiotic was shown to increase colonic H2S content, which subsequently stimulates glucagon-like peptide-1 (GLP-1) production from intestinal L-cells [102]. Importantly, endothelial cells can sense GLP-1 by GLP-1 receptor to mediate vasodilatory, antioxidant and anti-inflammatory effects [103]. The endothelial cells serve as the first-line interface for signal transduction from gut-derived metabolites to the cardiovascular system.
Vascular smooth muscle and gut microbiome
VSMCs are the stromal components in the medial layer of vascular wall, playing critical role in arterial pathophysiology. In response to different vasoactive substances (e.g. neurotransmitters, metabolites and hormones), VSMCs dynamically contract and relax to modulate vascular tone, local blood flow and BP [104]. Importantly, the tight endothelial cell-VSMC communication is crucial to the vascular homeostasis, contributing to the vasoreactivity and the structural integrity of the vascular wall. Dysregulated endothelial cell-VSMC interaction restricts vasodilation, increases oxidative stress, and promotes inflammation in the vascular wall [105]. Plasticity of mature VSMCs allows their phenotypic alterations in response to stimuli. Physiologically, mature VSMCs exhibit limited proliferation, restricted migration and enhanced expression of contractile proteins. Notably, VSMCs can de-differentiate to a ‘synthetic’ phenotype, characterized by hyperactive proliferation and migration, upon biochemical stimulation and vascular injury [106]. Such synthetic phenotype is constructive to vascular repair at the injured site, but excessive synthetic activity causes undesirable remodeling of vascular structure. Dysregulated endothelial cell-VSMC interaction and dysfunctional VSMCs elevate the risk of hypertensive and atherosclerotic cardiovascular diseases [107].
Overgrowth of pathogenic microbes and damaged intestinal integrity can impair VSMC homeostasis and disrupt the communication between endothelial cells and VSMCs (Figure 4). As forementioned, endotoxemia and gut-derived metabolites can cause endothelial dysfunction with less NO diffusing to the underlying VSMCs, encouraging VSMC contractility and proliferation [88]. In a ROS-dependent mechanism, dysfunctional endothelial cells could stimulate inflammation, ROS overproduction and calcification of VSMCs [108]. At the injured site of vasculature, dysfunctional endothelial cells also drive VSMC inflammation by enhancing the recruitment of and permeability to immune cells (e.g. leukocytes) [109]. In a paracrine manner (e.g. miRNAs and extracellular vesicles), dysfunctional endothelial cells are able to induce an inflammatory and senescent phenotype of VSMCs [110]. The close proximity and interaction between endothelial cells and VSMCs shall not be negligible during endotoxemia-associated cardiovascular diseases.
Figure 4:
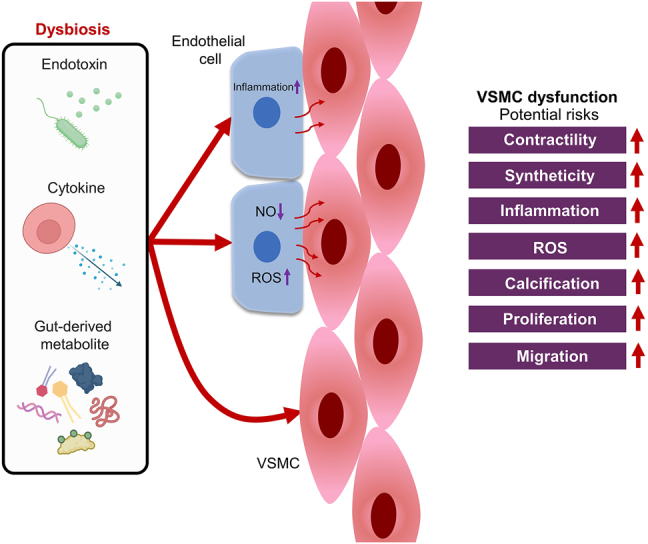
Dysbiosis-associated VSMC dysfunction. Dysbiosis may cause VSMC dysfunction by directly impairing VSMC homeostasis, and by disrupting the communication between endothelial cells and VSMCs. Reduced NO bioavailability, increased oxidative stress, and pronounced inflammation in endothelial cells would drive the dysfunction of VSMCs. Dysfunctional VSMCs are associated with increased contractility, syntheticity, inflammation, ROS overproduction, calcification, proliferation, and migration. NO: nitric oxide; ROS: reactive oxygen species; VSMC: vascular smooth muscle cell.
Similar to endothelial cells, exposure of VSMCs to the substances derived by pathogenic microbes also corresponds to a higher cardiovascular risk. Endotoxin could directly trigger vascular inflammation by upregulating TLR4 expression in human aortic smooth muscle cells, highlighting the risk of atherosclerosis and restenosis [111]. Through a Toll-like receptor (TLR4)/Akt pathway, LPS could stimulate the proliferation of VSMCs [112]. As a sensor for endotoxin, activation of TLR2 promotes the de-differentiation of VSMCs to the synthetic phenotype, associated with inflammation and calcification [113]. Additionally, endotoxemia-associated cytokine production may further aggravate the inflammation and phenotypic shift in VSMCs [114]. Endotoxemia and its associated cytokine production shall be responsible for the loss of stability and functionality of VSMCs during the development of dysbiosis-related cardiovascular diseases.
Metabolites, such as uremic toxins, derived from the gut microbiome also impair the homeostasis of VSMCs. Indoxyl sulfate was shown to induce VSMC proliferation, migration, and ROS overproduction through platelet-derived growth factor-β receptors [115]. P-cresyl sulfate could aggravate the formation of atherosclerotic plaques in ApoE−/− mice by enhancing the proliferation and migration of VSMCs [116]. Higher level of TMAO, the gut microbiome-dependent product, also triggers inflammation and calcification of VSMCs by activating both nuclear factor-κB (NF-κB) and nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 (NLRP3) inflammasome [117]. Remarkably, a microenvironment rich in lactate can promote the synthetic phenotype of VSMCs [118]. It is therefore reasonable to postulate that the abnormal outgrowth of lactic acid-producing bacteria would be a potential hazard to VSMC homeostasis. In short, a dysbiotic gut microbiome and impaired intestinal integrity are risky to health and functionality of both endothelium and vascular smooth muscle.
Gut-heart connection: gut microbiome and cardiac homeostasis
Human heart can be segregated into a compendium of 5 major cell types, namely cardiomyocytes, endothelial cells, fibroblasts, smooth muscle cells (SMCs) and macrophages [119]. Dysbiosis has been clinically shown to be associated with the severity or outcomes of cardiac complications, such as CHD and MI [44, 53]. The relatively novel concept of the ‘gut-heart axis’ has been proposed to describe the connection between the gut microbiome profile and cardiac homeostasis. Upon intestinal absorption, gut-derived substances flow through the heart before entering the arterial circulation. Theoretically, the hearts shall be more vulnerable or sensitive to the higher concentrations of endotoxin and metabolites than the peripheral vascular system. Substances derived from the gut microbiome reach the heart to bring about stressful challenges, such as inflammatory injury and oxidative damage, on different cardiac cells, including the cardiomyocytes, fibroblasts, endothelial cells, and SMCs.
The endothelial cells and SMCs of the coronary vascular system are the frontline cells that first encounter the stream of gut-derived substances. Dysfunction of these cells underlie the progressive deterioration in the functionality and blockage of coronary arteries, leading to ischemia of cardiac tissue (i.e. CHD). Mechanistically, endotoxin and gut-derived toxic substances could induce the dysfunction of these cells of the coronary arteries, as illustrated in Figures 3 and 4. In addition, these harmful substances could also potentially impair the functionality of cardiomyocytes, the fundamental unit of cardiac muscles, and hence the cardiac homeostasis (Figure 5). As a hallmark of cardiac complications (e.g. MI, heart failure and ischemia/reperfusion), cardiomyocyte dysfunction generally describes the decreased active relaxation and undesirable remodeling (i.e. hypertrophy and fibrosis) of cardiomyocytes [120]. A healthier gut microbiome and intestinal barrier function shall reduce the burden on coronary arteries and cardiac muscles, implying a lower risk of CHD and heart failure.
Figure 5:
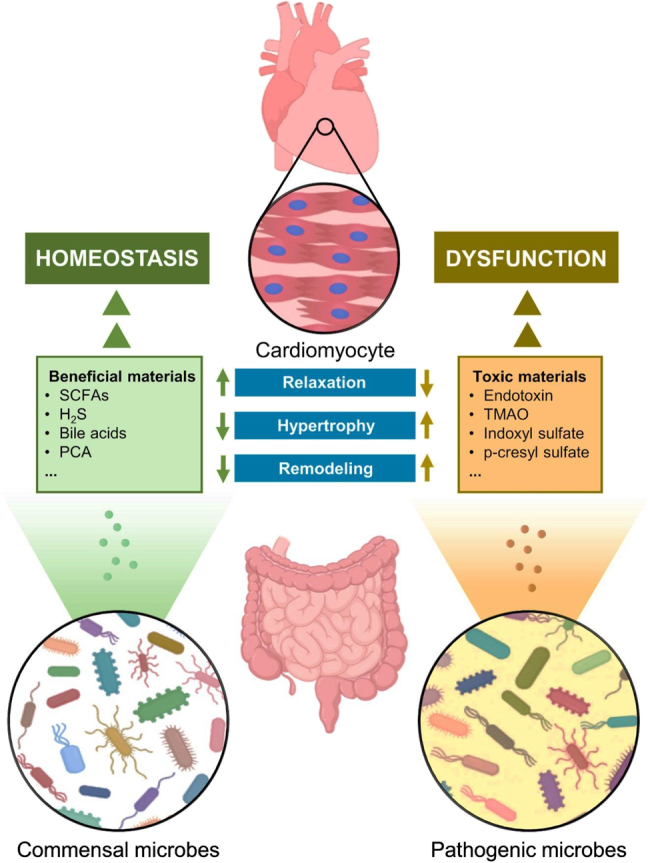
Gut-heart axis and cardiomyocyte homeostasis.
Commensal and pathogenic microbes resident in the intestine can generate both beneficial and toxic substances and release them into the circulation. The beneficial substances sustain cardiomyocyte homeostasis, while the toxic agents promote cardiomyocyte dysfunction. H2S: hydrogen sulfide; PCA: protocatechuic acid; SCFAs: short-chain fatty acids; TMAO: trimethylamine N-oxide.
Endotoxin exposure causes dysfunction and apoptosis in cardiomyocytes in vivo [121], in which a lower number of functional cardiomyocytes corresponds to a higher risk of heart failure. Furthermore, endotoxin augments total and mitochondrial ROS production to disrupt redox homeostasis in cardiomyocytes [122], a prerequisite to fibrosis and hypertrophy of cardiomyocytes [123]. Endotoxemia also stimulates the autocrine secretion of pro-inflammatory cytokines (e.g. IL-1β, IL-6 and TNFα) by cardiomyocytes [121]. Attracted by the injured cardiomyocytes, infiltrated immune cells (e.g. monocytes and macrophages) also contribute to the cytokine production [124]. Collectively, the autocrine and paracrine production of cytokines provide an inflammatory microenvironment favorable to cardiomyocyte remodeling [125]. Notably, disrupted endothelial cell-cardiomyocyte and fibroblast-cardiomyocyte interactions might also participate in the development of cardiomyocyte dysfunction [126], although the comprehensive mechanism still remains elusive.
Exposure to the elevated concentration of gut-derived toxic substances poses threat to the cardiomyocytes. Gut-derived TMAO impairs cardiomyocyte function by inducing fibrosis and hypertrophy [127], possibly via the activation of NLRP3 inflammasome [128]. On the other hand, pharmacological inhibition of the microbial synthesis of TMAO by iodomethylcholine, the choline trimethylamine lyase inhibitor, could repair cardiac remodeling and function in murine [129]. Indoxyl sulfate, the uremic toxin, has been demonstrated to elicit pro-inflammatory, pro-hypertrophic and pro-fibrotic effects in cardiomyocytes [130]. In vitro findings also suggest that indoxyl sulfate triggers apoptosis of human cardiomyocytes by increasing endoplasmic reticulum (ER) stress [131]. p-cresyl sulfate, another uremic toxin, was also found to be pro-apoptotic to cardiomyocytes in a ROS-dependent manner [132]. The tight linkage between the build-up of intracellular oxidative stress and ER stress might imply a similar mechanism of these gut-derived uremic toxins. High levels of these toxic substances in the circulation might serve as a predictive value for cardiac disorders.
Production of compounds or molecules by commensal microbes could confer cardioprotection against cardiac disorders. SCFAs, major gut-derived metabolites from dietary fibers, are generally beneficial to cardiomyocytes. Butyrate treatment can prevent the enlargement of human cardiomyocytes against angiotensin II insult [133]. In murine, propionate administration significantly alleviated cardiac fibrosis and hypertrophy [99]. Generated by both gut microbiome and intestinal epithelial cells from cysteine, the gaseous compound H2S is associated with various cardioprotective benefits. The anti-hypertrophic, anti-apoptotic, anti-fibrotic, and anti-inflammatory effects of H2S have been well documented [134]. Fluctuation in the levels of gut-derived bile acids can disrupt the homeostasis of the cardiovascular system [135]. For instance, excessive bile acids can cause cardiac hypertrophy and dysfunction by hindering the fatty acid oxidation by cardiomyocytes [136]. Further efforts are needed to define the threshold concentration between the beneficial and harmful roles of bile acids on cardiovascular homeostasis. In addition, the gut microbiome can convert the dietary anthocyanin to protocatechuic acid (PCA) [137], a phenolic compound shown to suppress oxidative stress and apoptosis in cardiomyocytes [138]. These findings highlight the importance of balance between commensal and pathogenic microbes in maintaining cardiac homeostasis.
Gut-liver axis and cardiovascular homeostasis
After intestinal absorption, gut-derived substances are transferred to liver via portal vein, prior to the exportation to heart for systemic circulation. Liver functions as an organic ‘factory’ to modify or detoxify the absorbed substances before they are transferred to other organs. The concept ‘gut-liver axis’ describes the bidirectional interplay between gut, in conjunction with gut microbiome, and liver [139]. The gut-liver axis is believed to be involved in the pathogenesis of multiple hepatic complications, including alcoholic liver disease (ALD), nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), cirrhosis and hepatocellular carcinoma [140]. In addition to hepatic disorders, the gut-liver axis could also participate in the pathogenesis of cardiovascular complications (Figure 6). By metabolizing the gut-derived substances, the liver eventually generates a spectrum of metabolic products that could be harmful to the cellular components of the cardiovascular system. Moreover, dysbiosis-related hepatic diseases could also serve as a potential risk factor to cardiovascular diseases.
Figure 6:
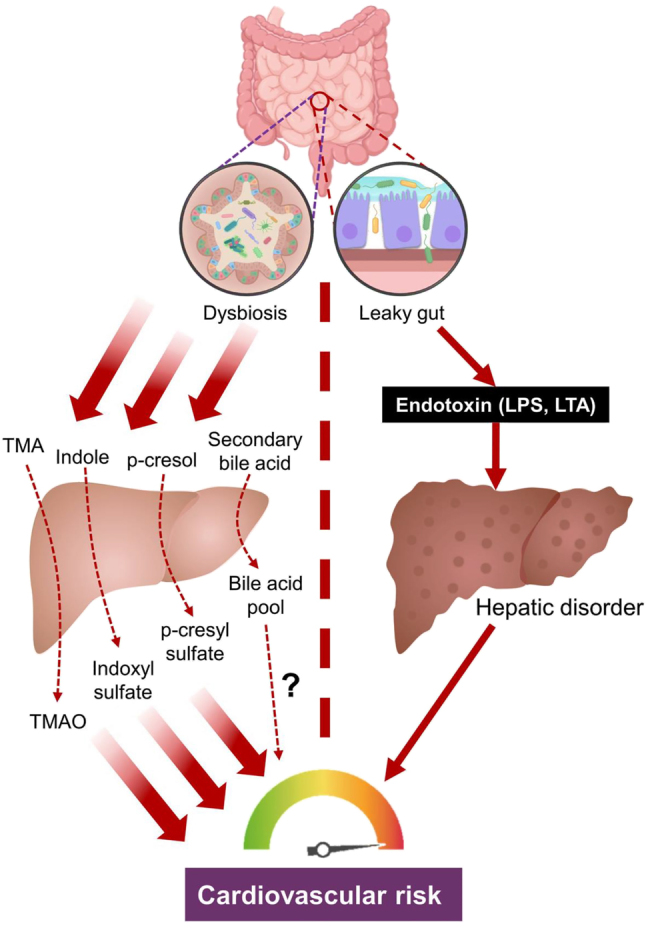
Gut-liver axis and cardiovascular risk. Dysbiosis leads to the influx of more gut-derived precursor metabolites, which are further metabolized by the liver to form cytotoxic chemicals to impair the cardiovascular function. In addition, leaky gut causes the leakage of endotoxin into the portal vein, damaging the liver function. The occurrence of hepatic disorders increase the cardiovascular risk. LPS: lipopolysaccharide; LTA: lipoteichoic acid; MA: trimethylamine; TMAO: trimethylamine N-oxide.
The liver is responsible for the metabolism of gut-derived materials. In the intestine, the microbes convert dietary choline to TMA, which is further metabolized to TMAO in the liver [141]. As aforementioned, TMAO is a predictive biomarker of cardiovascular complications, promoting the dysfunction of endothelial cells, VSMCs and cardiomyocytes. The uremic toxins follow a similar metabolic fate as to TMAO. The intestinal microbes first degrade the dietary tryptophan into indole, which is further metabolized to indoxyl sulfate by liver [142]. Meanwhile, dietary tyrosine is transformed to p-cresol, which is then converted to p-cresyl sulfate by sulfotransferase in the liver [143]. These two uremic toxins, as previously described, are pro-inflammatory and pro-atherosclerotic, and are positively correlated with cardiovascular mortality in patients with chronic kidney disease [132]. By contrast, the SCFAs derived by microbe-mediated carbohydrate fermentation can reduce hepatic lipogenesis [144], exerting anti-atherogenic effects on vasculature. Limited dietary intake of choline and aromatic amino acid may be preventive to reduce cardiovascular diseases, through the gut-liver axis.
Bile acids are involved in the bidirectional communication between liver and intestine [140]. In the liver, primary bile acids, cholic acid and chenodeoxycholic acid, are synthesized from cholesterol. The conjugated primary bile acids are released to the intestine through the bile duct for microbe-mediated deconjugation and dehydroxylation to form secondary bile acids, deoxycholic acid (DCA) and lithocholic acid (LCA) [145]. Most secondary bile acids are reabsorbed into liver via portal vein to constitute the bile acid pool, along with primary bile acids. A small proportion (5%–10%) of the total bile acid pool enters the systemic circulation to act on different organs by agonistic activation of corresponding receptors [e.g. farnesoid X receptor (FXR) and membrane Takeda G protein-coupled receptor 5 (TGR5)] [146]. The role of bile acids in the cardiovascular system remains debatable. Doubtful findings are present to show that circulatory bile acids promote cardiac dysfunction [145], but they improve endothelial function [147]. Uncertain ratios between primary and secondary bile acids, and differential expression of bile acid receptors in different cells may underlie the controversial roles of bile acids. Clinically, a higher ratio of secondary to primary bile acids correlated to higher mortality rate in patients with chronic heart failure [148]. More pre-clinical and clinical studies are required to resolve the uncertainty in the cardiovascular role of bile acids.
Endotoxin from the leaky gut can lead to the pathogenesis of hepatic diseases. Both LPS, the component on the outer membrane of Gram-negative bacteria, and lipoteichoic acid (LTA), the component on the cell wall of Gram-positive bacteria, can be recognized by TLR2 and TLR4 [149]. Docking to TLRs ignite innate immunity and trigger fibrosis in liver, promoting the development of hepatic disorders, like NAFLD, NASH and cirrhosis [150]. Endotoxin exposure also enhances the progression of hepatocellular carcinoma [151]. Epidemiologically, these hepatic diseases are the risk factors for cardiovascular diseases. Patients with these hepatic disorders are more vulnerable to endothelial dysfunction, arterial calcification, and carotid thickening [152]. Although the detailed molecular mechanisms on the causality of hepatic disorders on cardiovascular diseases are not fully elucidated, hepatic dysfunction-associated systemic inflammation and oxidative stress shall increase the burden on the cardiovascular system [153]. Recently, another concept ‘liver-heart axis’ has been postulated to describe the potential molecular crosstalk between liver and the cardiovascular system [154].
In brief, the dysbiotic gut microbiome can impair the homeostasis of cardiovascular system through liver-mediated metabolic modification, and potentially through sequential impairment on the liver and then on the cardiovascular function (Figure 6). Comparative study on the gut microbiome profiles of patients with hepatic disorders, and those with cardiovascular diseases may imply some mechanistic commonality between these two distinct types of disorders. In other words, the dysbiotic microbiome profile observed in patients with hepatic diseases might be partially predictive to reduce the risk of cardiovascular diseases.
Brain-gut axis and cardiovascular homeostasis
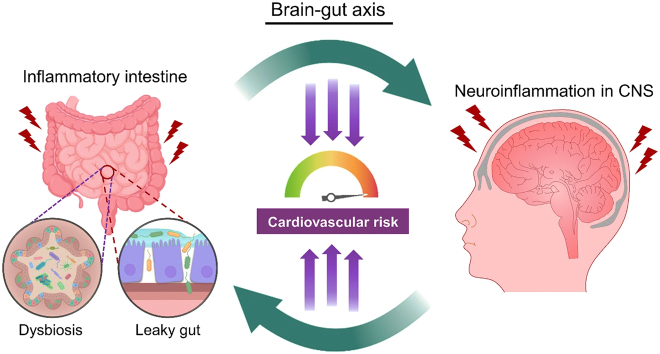
The ‘brain-gut axis’ describes the bidirectional connections between the gut microbiome and CNS. Chemical transmitters, neural pathways and the immune system are believed to be the pivotal contributors to the brain-gut axis. This axis is particularly important to the homeostasis of both intestinal and nervous systems of the host [155]. Since the signal transducers participating in the brain-gut axis and the immune cells of the immune system are transported via the systemic circulation, it is reasonable to postulate that the brain-gut axis somehow would affect the cardiovascular function. As observed in some novel findings, the brain-gut axis eventually influence some cardiovascular parameters of the host. The bidirectional interplay between the intestine and the brain may incidentally affect the cardiovascular system (Figure 7). The ‘brain-gut-heart’ and ‘brain-gut-vascular’ axes may potentially participate in the development of cardiovascular diseases.
Figure 7:
Bidirectionality in brain-gut axis and cardiovascular risk. The vicious cycle between the inflammatory intestine and the inflammatory CNS. Chemical transmitters, neural pathways and the immune system may collectively participate in the pathogenesis of cardiovascular dysfunction. CNS: central nervous system.
The dysbiotic intestine may be associated with the morbidity of neurological disorders. Subsequently, dysfunction of the CNS affects the cardiovascular function via the circulatory system. One possible example is the Crohn’s disease, a type of inflammatory bowel disease. Notably, the chronic inflammation of the intestine is often associated with dysbiosis and impaired intestinal barrier [156]. Patients with Crohn’s disease are more vulnerable to anxiety and depression disorders [157]. Endotoxemia is believed to arouse peripheral inflammation in the host, which then spreads to the CNS to cause neuroinflammation, a prerequisite of neurological dysfunction [158]. Moreover, in patients with Crohn’s disease, anxiety was clinically found positively correlated with blood pressure and aortic stiffness [14]. The stress- or anxiety-related secretion of hormones, such as cortisol and insulin [159], into the bloodstream directly cause burdens on cellular components of cardiovascular system (e.g. endothelial cells and cardiomyocytes) [160, 161]. These interesting observations imply a complex network between gut microbiome and the cardiovascular system.
In an opposite fashion, the stressed CNS may elicit detrimental effects on the intestine, which eventually increases the cardiovascular risks via the gut-cardiovascular communication. Psychological stress on the CNS could raise the permeability of intestinal barrier through hormonal control and sympathetic nerve stimulation, leading to endotoxemia and systemic low-grade inflammation [162]. Importantly, mental disorder (e.g. depression) could reversely raise the risk of inflammatory bowel disease [163], which further exaggerates neuroinflammation [164]. In other words, a vicious cycle may exist between the diseased CNS and the inflammatory intestine (Figure 7). In spite of the sequential order, unhealthy states of both the CNS and intestine increase the risk of cardiovascular diseases. In recent years, a healthier dietary intervention has been shown to improve mental health through favorable modulation of the host gut microbiome [165]. Thus, a healthier dietary pattern is beneficial not only to the cardiovascular function, but also to the CNS health. However, extensive future studies are needed to deepen our understanding of the molecular basis involving the brain-gut axis during the development of cardiovascular complications.
Drug–microbiome interaction and cardiovascular health
In the past decades, tremendous amount of efforts have been devoted to develop medications and supplements to improve cardiovascular health. The molecular mechanisms and pathways of many cardiovascular drugs have been extensively investigated and elucidated. In recent years, the importance of gut microbiome has been raised that such microbial communities might serve as a new organ system in human body [6]. Dietary nutrients can interact with the gut microbiome [166], so the ingested medications and supplements do. In a bidirectional manner, the drugs can alter the gut microbiome profile of the host, while the intestinal microbes are also involved in the metabolism of drugs to become pharmacologically active [167]. Such drug–microbiome interaction might provide novel mechanistic insights into the pharmacological properties of cardiovascular drugs. Herein we aimed to revisit the mechanisms of cardiovascular benefits of certain drugs, which has been previously investigated, from the perspective of drug–microbiome interface (Table 3).
Table 3:
Drug–microbiome interaction and host cardiovascular health.
| Drug | Sample | Microbiome profile | Key substance(s) | Implication | Ref. |
|---|---|---|---|---|---|
| Metformin | Wistar rat | Allobaculum↑, Bacteriodes↑, Blautia↑ | SCFA | SCFA↑ | [171] |
| Butyricoccus↑, Phascolarctobacterium↑ | Cardiovascular health↑ | ||||
| Human with | Intestinibacter↓, Escherichia↑, A. muciniphila↑ | SCFA | SCFA↑ | [173] | |
| Type 2 diabetes | B. adolescentis↑, Bifidobacterium↑ | Bile acids | |||
| C57BL/6J mouse | Klebsiella↓, Escherichia↓, Sarcina↓ | Cholesterol | Cholesterol↓→Atherosclerosis↓ | [174] | |
| Lactobacillus↑, Enterococcus↓, Achromobacter↓ | Glucose | ||||
| C57BL/6 mouse | Unknown | Endotoxin | Intestinal integrity↑ | [175] | |
| GLP-1 analogues | Wistar rat | Firmicutes↓, Bacteroidetes↑ | Lipid | Lipogenesis↓→Atherosclerosis↓ | [180] |
| (e.g. liraglutide) | Goto-Kakizaki rat | Bacteroidia↓, Clostridia↑ | |||
| C57BL/6 mouse | Helicobacter↓, Proteobacteria↑ | SCFA | Hepatic disorder→ | [181] | |
| Ob/ob mouse | Akkermansia↑, Oscillospira↑, Thermotogae↑ | Cardiovascular risk↓ | |||
| Human with | Sutterella↓ | Endotoxin | Intestinal integrity↑ | [182] | |
| Type 2 diabetes | Akkermansia↑, Christensenellaceae↑ | ||||
| DPP-4 inhibitors | Zucker diabetic fatty rat | Verrucomicrobia↓, Proteobacteria↓, Bacteroidetes↓ | SCFA | SCFA↑ | [184] |
| (e.g. sitagliptin) | Lactobacillus↑, Firmicutes↑ | Cardiovascular health↑ | |||
| Human | Firmicutes↓, Ruminococcaceae↓ | SCFA | SCFA↑ | [185] | |
| Bacteroidetes↑, Streptococcaceae↓ | Bile acids | Cardiovascular health↑ | |||
| Resveratrol | db/db mouse | Bacteroides↑, Alistipes↑, Rikenella↑ | Endotoxin | Intestinal integrity↑ | [187] |
| Odoribacter↑, Parabacteroides↑, Alloprevotella↑ | Cytokines | Intestinal inflammation↓ | |||
| ApoE−/− mouse | Firmicutes↓, Lactobacillus↑ | TMAO | TMAO↓ | [188] | |
| Bacteroidetes↑, Bifidobacterium↑, Akkermansia↑ | Bile acids | Cardiovascular risk↓ | |||
| Berberine | ApoE−/− mouse | Roseburia↑, Blautia↑, Allobaculum↑ | TMAO | TMAO↓ | [192] |
| Alistipes↑, Turicibacter↑ | SCFA | Cardiovascular risk↓ | |||
| Human with | Roseburia spp.↓, Ruminococcus bromii↓ | Bile acids | Cardiovascular risk↓ | [193] | |
| Type 2 diabetes | Faecalibacterium prausnitzii↓, Bifidobacterium spp.↓ | ||||
| Bacteroides spp.↑, γ-Proteobacteria↑ | |||||
| Vitamin D | Aged human | Unknown | SCFA | SCFA↑ | [196] |
| (e.g. butyrate) | Cardiovascular health↑ | ||||
| Intestinal | Bacteroides fragilis↑ | Endotoxin | Intestinal inflammation↑ | [197] | |
| Vitmain D receptor | Butyrivibrio fibrisolvens↑ | (e.g. LPS) | Dysbiosis↑ | ||
| Conditional knockout mouse | Firmicutes peptostreptococcus↑ | Bile acids | Cardiovascular risk↑ | ||
| Statin | Obese human | Eggerthella↓, Bacteroides↑ | SCFA | Dysbiosis↓ | [200] |
| Akkermansia↑, Faecalibacterium↑ | (e.g. butyrate) | Systemic inflammation↓ | |||
| C57BL/6N mouse | Bacteroides↑, Butyricimonas↑ | Cytokines | Intestinal inflammation↓ | [201] | |
| Mucispirillum↑ | (e.g. TGFβ1, IL-1β) | Cardiovascular health↑ | |||
| C57BL/6 mouse | Mucispirillum↓, Acinetobacter↓, Christensenella↓ | Endotoxin | Intestinal integrity↑ | [202] | |
| Weissella↓, Streptophyta↓, Anaerotruncus↑ | SCFA | Systemic inflammation↓ | |||
| Sporobacter↑, Collinsella↑, Rubrobacter↑, Microlunatus↑ | Cytokines | ||||
| Telmisartan | ApoE−/− mouse | Firmicutes↓ | Endotoxin | Intestinal integrity↑ | [205] |
| Bacteroidetes↑, Helicobacter↑ | (e.g. LPS) |
IL: interleukin; LPS: lipopolysaccharide; SCFA: short-chain fatty acid; TGFβ: transforming growth factor β; TMAO: trimethylamine N-oxide.
Metformin, the antidiabetic drug, is clinically used to reduce the cardiovascular morbidity and mortality in patients with type 2 diabetes mellitus [168]. Mechanistically, Cheang et al. [169] demonstrated that metformin can improve endothelial function by alleviating ER stress through activation of 5′ adenosine monophosphate-activated protein kinase (AMPK). Prolonged treatment of metformin also protects the cardiomyocytes against apoptosis and remodeling [170]. The cardiovascular benefits of metformin can be interpreted in terms of drug–microbiome interaction. In obese rats, metformin increased the abundance of SCFA-producing microbes (e.g. Allobaculum, Bacteriodes, and Butyricoccus) [171], in which SCFAs can activate AMPK [172]. Consistently, the abundance of SCFA-producing genera was shown to be greater in human trials on metformin [173]. Moreover, metformin can lower circulatory cholesterol levels via the modulation of gut microbiome [174], correlating with a lower atherosclerotic risk. In addition, metformin treatment improves the intestinal integrity in vivo [175]. These intestinal benefits provide new mechanistic insights into the cardiovascular benefits of metformin.
GLP-1 analogues (e.g. exendin-4, semaglutide and liraglutide), another group of well-established antidiabetic drugs, can mitigate cardiovascular risks [176]. These analogues can improve endothelial homeostasis by docking to GLP-1 receptors on endothelial cells [177]. Particularly, GLP-1 analogues can attenuate endothelial dysfunction by AMPK activation and ER stress alleviation [178]. The GLP-1 analogue liraglutide elicits anti-atherogenic effects by suppressing VSMC proliferation [179]. Of noted, GLP-1 analogues can also modulate the gut microbiome profile. In rats, liraglutide was shown to restrain hepatic lipogenesis through the modulation of gut microbiome [180]. Moreover, liraglutide also reduces NAFLD by modulating gut microbiome [181], hinting that the GLP-1 analogues may confer cardiovascular protection via the gut-liver axis. In human subjects, liraglutide administration can both alter gut microbiome profile and improve intestinal integrity [182]. Nevertheless, investigation into intestinal effects of other GLP-1 analogues is required.
Dipeptidyl peptidase-4 (DPP-4) inhibitors, another group of antidiabetic medications, improve glucose homeostasis by inhibiting GLP-1 cleavage. DPP-4 inhibitors, such as sitagliptin, were previously shown to be beneficial to the homeostasis of endothelial cells [183]. Sitagliptin can promote SCFA production through modulating the gut microbiome [184]. Sitagliptin treatment in human subjects increases the production of SCFAs [185], although the shift in microbiome profile somehow contradictory to that in rodents. Resveratrol, the natural phenolic compound in red wine, is a cardioprotective supplement which improves both cardiac and vascular functions [186]. Resveratrol remarkably attenuates intestinal damage and inflammation in diabetic mice [187], implying that resveratrol may confer cardioprotection through prohibiting systemic inflammation. More importantly, resveratrol could limit TMAO production by altering the gut microbiome profile to exert its anti-atherogenic effect [188]. These findings highlight the dietary benefits of resveratrol in counteracting cardiovascular complications.
Berberine, the major active component of the Chinese herb Coptis chinensis, elicits antidiabetic and cardioprotective effects [189]. Berberine can protect endothelial function by ameliorating oxidative stress through downregulating cyclooxygenase-2 [190]. Berberine is also involved in the mitochondrial homeostasis in cardiomyocytes [191]. Recent microbiological studies extend our mechanistic understanding of the cardiovascular benefits of berberine. The anti-atherogenic effect of berberine can be partially explained by the TMAO-lowering effect through altering microbial composition [192]. Furthermore, berberine may mediate its beneficial effects by altering microbial metabolism of bile acids, and hence the circulatory bile acid pool [193]. Calcitriol, the vitamin D analogue, is the commercially available supplement to boost cardiovascular health. In renal arteries, calcitriol improves endothelial function by suppressing oxidative damage [194]. The anti-hypertrophic effect of calcitriol has been elucidated in patients with hyperparathyroidism [195]. There is a bidirectional interaction between vitamin D and its homologues, and gut microbiome. In particular, a higher body level of vitamin D would enrich the butyrate-producing microbes [196]. Intestinal deletion of vitamin D receptor aggravates intestinal inflammation and dysbiosis [197], conditions that correlate with higher cardiovascular risk. These findings imply the importance of dietary supplementation of vitamin D in providing cardiovascular benefits.
Statins, the lipid-lowering drugs, are commonly used to prevent and treat CHD [198]. The anti-atherogenic property of statins attributes to their cholesterol lowering effect [199]. The recent novel findings on microbial involvement significantly add new therapeutic insights into the use of statins against cardiovascular complications. Clinically, statin therapy decreases the prevalence of dysbiosis [200]. Administration of atorvastatin and rosuvastatin in murine can suppress intestinal inflammation by lowering the level of pro-inflammatory cytokines (e.g. IL-1β) [201]. Atorvastatin administration facilitates post-stroke recovery by inhibiting endotoxemia, increasing SCFA production and suppressing inflammatory response [202]. Gut-liver and brain-gut axes may be also involved in statin-mediated cardiovascular benefits. Telmisartan, a widely used antihypertensive drug, is a highly selective antagonist for angiotensin II type 1 receptor [203]. Telmisartan increases NO production in endothelial cells and restrains vasoconstriction [204]. Telmisartan treatment can decrease atherosclerotic lesions in ApoE−/− mice, partially through altering gut microbiome and limiting endotoxemia [205].
In short, most of the aforementioned drugs confer cardioprotection by modulating SCFA, intestinal permeability and inflammatory response. However, fewer studies focus on how the gut microbiome reversely modulate the metabolism of these drugs. Since the gut microbiome profile varies among individuals, which would cause variation in the therapeutic efficacy of these drugs among individuals. Potential preconditioning or ‘tuning’ of gut microbiome prior to drug therapy is worthwhile consideration in order to amplify the therapeutic efficacy and cardiovascular benefits. In addition, extensive efforts are still required to comprehensively explore the underlying network among these drugs, the gut microbiome, the cardiovascular system and other organs potentially involved.
Fecal microbiota transplantation and cardiovascular health
FMT refers to the strategy which transfers the intestinal microbial content from a healthy donor to the gastrointestinal tract of an unhealthy recipient [206]. The therapeutic potential of FMT has been evaluated by a number of pre-clinical and clinical studies in recent years. In murine studies, FMT can be simply achieved by oral gavage of the microbial content into the recipient mice [207]. However, more rigorous considerations shall be given to optimize the transfer approaches in human subjects. In human recipients, FMT is often delivered to the colon by using colonoscopy, or to the upper gastrointestinal tract by capsules [208]. FMT may represent a novel therapeutic strategy in cardiovascular therapy by improving the metabolic profile and systemic inflammation of the recipients. In particular, some investigations have provided therapeutic insights against cardiovascular diseases (Table 4), hinting that FMT may become a new therapeutic option in addition to traditional classical medications.
Table 4:
Fecal microbiota transplantation and cardiovascular implications.
| Study design | Microbiome profile | Phenotypic alterations | Implication | Ref. |
|---|---|---|---|---|
| Donor: BALB/c mouse | Firmicutes↓ | Dysbiosis↓ | Myocarditis↓ | [209] |
| ↓ | ||||
| Recipient: BALB/c mouse with | Bacteroidetes↑ | Inflammatory infiltration↓ | Cardiomyocyte health↑ | |
| Experimental autoimmune myocarditis | ||||
| Donor: Lean vegan | Lachnospiraceae↑ | TMAO↓ | Vascular inflammation↓ | [210] |
| ↓ | Bryantella formatexigens↑ | |||
| Recipient: patient with | Megamonas hypermegale↑ | Oxidized low‐density lipoprotein↓ | Atherosclerotic risk↓ | |
| Metabolic syndrome | L bovis↑ | |||
| Donor: Wistar-Kyoto rat | Blautia↑, Odoribacter↑ | Systolic BP↓, diastolic BP↓ | Endotoxin↓ | [211] |
| ↓ | Pro-inflammatory cytokines↓ | Vascular inflammation↓ | ||
| Recipient: SHR rat | Intestinal integrity↑ | Hypertension↓ | ||
| Donor: Sprague-Dawley rat | Lactobacillus↑ | SCFAs↑ | Endotoxin↓ | [212] |
| ↓ | Butyricicoccus↑ | Infarct volume↓ | Cardiovascular benefit↑ | |
| Recipient: Sprague-Dawley rat | Meganonas↑ | Intestinal integrity↑ | Ischemic stroke↓ | |
| Donor: pre-eclampsia patient | Faecalibacterium↓ | pregestational BP↑, proteinuria↑ | Endotoxin↑ | [213] |
| ↓ | Akkermansia↓ | Intestinal integrity↓ | Systemic inflammation↑ | |
| Recipient: C57BL/6 mouse | Fusobacterium↑ | Pro-inflammatory cytokines↑ | Hypertension↑ | |
| Donor: CHD patient | Clostridium symbiosum↑ | Bile acid balance↓ | Systemic inflammation↑ | [46] |
| ↓ | Eggerthella↑ | Inflammatory response↑ | Endothelial dysfunction↑ | |
| Recipient: C57BL/6J mouse | Butyricimonas↑ | Vascular stiffness↑ | CHD risk↑ |
BP, blood pressure; CHD, coronary heart disease; SCFA, short-chain fatty acid; SHR, spontaneously hypertensive rat; TMAO, trimethylamine N-oxide.
The transfer of intestinal content from a healthy individual to improve the gut microbiome profile, and hence the symptoms of diseased recipient is the general gist. In a pre-clinical study, FMT could retard myocarditis in recipient mice by repairing dysbiosis and cardiac inflammation [209], implying the anti-inflammatory potential of FMT. Levels of hazardous biomarker (e.g. TMAO) can be reduced by FMT. In patients with metabolic syndrome, FMT from lean vegan-donors lowered the plasma level of TMAO [210], implying reduced vascular inflammation and atherosclerotic risk. FMT can also elicit anti-hypertensive effects by modulating the gut microbiome and improving the intestinal integrity [211]. Transfer of intestinal content rich in SCFA can improve intestinal integrity and alleviate the progression of ischemic stroke in rats [212].
On the other hand, FMT from diseased donors would result in the onset of disorders. FMT from patients to mouse models were commonly applied to interrogate the pathological mechanisms of cardiovascular diseases. For instance, FMT from pre-eclampsia patients trigger pre-eclampsia phenotypes in recipient mice [213]. Additionally, FMT from CHD patients impaired vascular function and affected bile acid metabolism in recipient mice [46]. These important findings confirm the therapeutic potential of FMT in cardiovascular diseases. Of noted, similar preconditioning or ‘tuning’ of gut microbiome profile might be needed prior to the FMT regimen. Importantly, young healthy donor with regular physical activity might serve as the ‘ultimate’ donor of intestinal microbial content for FMT. In future decades, it is reasonable to anticipate that ‘stool donation’ would be as common as ‘blood donation’ for wider therapeutic opportunities to target multiple disorders, including cardiovascular diseases.
Future perspectives and conclusive statement
In this review, we discuss the clinical relevance of the gut microbiome to cardiovascular diseases, possible interactions between gut microbiome and the cellular components of the cardiovascular system, and therapeutic opportunities on cardiovascular complications by targeting gut microbiome. During the discussion, some unsolved questions underlying the gut-cardiovascular connection are identified. Further efforts are still required to extend our understanding towards addressing these questions. For instance, relatively fewer studies are available to examine the role of gut microbiome in the development of PAD. Besides, the complex network interconnecting the brain-gut axis and cardiovascular system requires further extensive investigation. In terms of drug–microbiome interaction, more focus needs to be directed on how gut microbiome modulate the metabolism of different drugs. Procedures and efficacy of FMT regimen in human subjects still require further optimization. Future efforts to unify the preconditioning agent prior to FMT regimen, and to identify ‘ultimate’ donor to optimize therapeutic efficacy, might be also needed.
Extensive efforts have been directed at evaluating the profiles of gut microbiome in rodents and human during the development of cardiovascular diseases. Certain critical metabolites identified may serve as potentially useful prognostic factors for multiple cardiovascular diseases. However, the high level of those metabolites (e.g. TMAO) could not specifically predict the risk of a particular cardiovascular disorder. Further investigation is needed to confirm the linkage between the unique set(s) of gut microbiome profiles among individuals and the risk of a particular cardio-metabolic disease, before applying microbiome sequencing for future diagnostic purposes. To conclude, the extensive pre-clinical and clinical studies have deepened our understanding towards our ‘friendly neighborhood’, opening up new therapeutic opportunities for different disorders, such as cardiovascular diseases. We need to ‘love’ and ‘care’ them on a daily basis, preventing them from turning into ‘potential threats’ to our cardiovascular system.
Acknowledgements
The authors thank members of the Y.H. group for constructive discussions. Figures were created with BioRender.com.
Footnotes
Research funding: This work was supported by Hong Kong RGC-Senior Research Fellow Scheme (SRFS2021-4S04) and National Natural Science Foundation of China (91939302).
Author contributions: Conceptualization, C.K.C., and Y.H.; Discussion, C.K.C., and Y.H.; Original draft, C.K.C., and Y.H.; Review & Editing, C.K.C., and Y.H.; Funding Acquisition, Y.H.; Resources, Y.H.; Supervision, Y.H.
Conflict of interest: The authors declare that there is no conflict of interest.
Ethical approval: Not applicable.
Informed consent: Not applicable.
References
- 1.Zilber-Rosenberg I, Rosenberg E. Role of microorganisms in the evolution of animals and plants: the hologenome theory of evolution. FEMS Microbiol Rev. 2008;32:723–35. doi: 10.1111/j.1574-6976.2008.00123.x. [DOI] [PubMed] [Google Scholar]
- 2.Woyke T. Beyond the census of human gut dwellers. Nat Rev Microbiol. 2019;17:401. doi: 10.1038/s41579-019-0220-7. [DOI] [PubMed] [Google Scholar]
- 3.Tremlett H, Bauer KC, Appel-Cresswell S, Finlay BB, Waubant E. The gut microbiome in human neurological disease: a review. Ann Neurol. 2017;81:369–82. doi: 10.1002/ana.24901. [DOI] [PubMed] [Google Scholar]
- 4.Gould AL, Zhang V, Lamberti L, Jones EW, Obadia B, Korasidis N, et al. Microbiome interactions shape host fitness. Proc Natl Acad Sci USA. 2018;115:E11951–60. doi: 10.1073/pnas.1809349115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamada N, Chen GY, Inohara N, Núñez G. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol. 2013;14:685–90. doi: 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marchesi JR, Adams DH, Fava F, Hermes GDA, Hirschfield GM, Hold G, et al. The gut microbiota and host health: a new clinical Frontier. Gut. 2016;65:330–9. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao D, Liu J, Wang M, Zhang X, Zhou M. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol. 2019;16:203–12. doi: 10.1038/s41569-018-0119-4. [DOI] [PubMed] [Google Scholar]
- 8.Kazemian N, Mahmoudi M, Halperin F, Wu JC, Pakpour S. Gut microbiota and cardiovascular disease: opportunities and challenges. Microbiome. 2020;8:1–17. doi: 10.1186/s40168-020-00821-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witkowski M, Weeks TL, Hazen SL. Gut microbiota and cardiovascular disease. Circ Res. 2020;127:553–70. doi: 10.1161/circresaha.120.316242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang CS, Kao CY. Current understanding of the gut microbiota shaping mechanisms. J Biomed Sci. 2019;26:1–11. doi: 10.1186/s12929-019-0554-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dejong EN, Surette MG, Bowdish DME. The gut microbiota and unhealthy aging: disentangling cause from consequence. Cell Host Microbe. 2020;28:180–9. doi: 10.1016/j.chom.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Meijnikman AS, Gerdes VE, Nieuwdorp M, Herrema H. Evaluating causality of gut microbiota in obesity and diabetes in humans. Endocr Rev. 2018;39:133–53. doi: 10.1210/er.2017-00192. [DOI] [PubMed] [Google Scholar]
- 13.Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341–52. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zanoli L, Tuttolomondo A, Inserra G, Cappello M, Granata A, Malatino L, et al. Anxiety, depression, chronic inflammation and aortic stiffness in Crohn’s disease: the brain-gut-vascular axis. J Hypertens. 2020;38:2008–17. doi: 10.1097/hjh.0000000000002517. [DOI] [PubMed] [Google Scholar]
- 15.Giles EM, D’Adamo GL, Forster SC. The future of faecal transplants. Nat Rev Microbiol. 2019;17:719. doi: 10.1038/s41579-019-0271-9. [DOI] [PubMed] [Google Scholar]
- 16.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 17.Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Atherosclerosis. Nat Rev Dis Prim. 2019;5:1–18. doi: 10.1038/s41572-019-0106-z. [DOI] [PubMed] [Google Scholar]
- 18.Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res. 2019;124:315–27. doi: 10.1161/circresaha.118.313591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonsson AL, Caesar R, Akrami R, Reinhardt C, Hållenius FF, Borén J, et al. Impact of gut microbiota and diet on the development of atherosclerosis in ApoE−/− mice. Arterioscler Thromb Vasc Biol. 2018;38:2318–26. doi: 10.1161/atvbaha.118.311233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–85. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brandsma E, Kloosterhuis NJ, Koster M, Dekker DC, Gijbels MJJ, Van DerVelden S, et al. A proinflammatory gut microbiota increases systemic inflammation and accelerates atherosclerosis. Circ Res. 2019;124:94–100. doi: 10.1161/circresaha.118.313234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dekker Nitert M. Knights in shining armor: short chain fatty acid producers to prevent atherosclerotic plaques? Circ Res. 2019;124:12–4. doi: 10.1161/circresaha.118.314246. [DOI] [PubMed] [Google Scholar]
- 23.Koren O, Spor A, Felin J, Fåk F, Stombaugh J, Tremaroli V, et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci USA. 2011;108:4592–8. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jie Z, Xia H, Zhong SL, Feng Q, Li S, Liang S, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8:1–12. doi: 10.1038/s41467-017-00900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jonsson AL, Bäckhed F. Role of gut microbiota in atherosclerosis. Nat Rev Cardiol. 2017;14:79–87. doi: 10.1038/nrcardio.2016.183. [DOI] [PubMed] [Google Scholar]
- 26.Oparil S, Acelajado MC, Bakris GL, Berlowitz DR, Cífková R, Dominiczak AF, et al. Hypertension. Nat Rev Dis Prim. 2018;4:18014. doi: 10.1038/nrdp.2018.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma J, Li H. The role of gut microbiota in atherosclerosis and hypertension. Front Pharmacol. 2018;9:1082. doi: 10.3389/fphar.2018.01082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karbach SH, Schönfelder T, Brandão I, Wilms E, Hörmann N, Jäckel S, et al. Gut microbiota promote angiotensin II-induced arterial hypertension and vascular dysfunction. J Am Heart Assoc. 2016;5:e003698. doi: 10.1161/JAHA.116.003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature. 2017;551:585–9. doi: 10.1038/nature24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun S, Lulla A, Sioda M, Winglee K, Wu MC, Jacobs DR, et al. Gut microbiota composition and blood pressure: the CARDIA study. Hypertension. 2019;73:998–1006. doi: 10.1161/hypertensionaha.118.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmu J, Salosensaari A, Havulinna AS, Cheng S, Inouye M, Jain M, et al. Association between the gut microbiota and blood pressure in a population cohort of 6953 individuals. J Am Heart Assoc. 2020;9:e016641. doi: 10.1161/jaha.120.016641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santisteban MM, Qi Y, Zubcevic J, Kim S, Yang T, Shenoy V, et al. Hypertension-linked pathophysiological alterations in the gut. Circ Res. 2017;120:312–23. doi: 10.1161/circresaha.116.309006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5:14. doi: 10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campbell BCV, Khatri P. Stroke. Lancet. 2020;396:129–42. doi: 10.1016/s0140-6736(20)31179-x. [DOI] [PubMed] [Google Scholar]
- 35.Durgan DJ, Lee J, McCullough LD, Bryan RM. Examining the role of the microbiota-gut-brain Axis in stroke. Stroke. 2019;50:2270–7. doi: 10.1161/strokeaha.119.025140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh V, Roth S, Llovera G, Sadler R, Garzetti D, Stecher B, et al. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J Neurosci. 2016;36:7428–40. doi: 10.1523/jneurosci.1114-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J, D’Aigle J, Atadja L, Quaicoe V, Honarpisheh P, Ganesh BP, et al. Gut microbiota-derived short-chain fatty acids promote poststroke recovery in aged mice. Circ Res. 2020;127:453–65. doi: 10.1161/circresaha.119.316448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan C, Wu Q, Wang H, Gao X, Xu R, Cui Z, et al. Dysbiosis of gut microbiota and short‐chain fatty acids in acute ischemic stroke and the subsequent risk for poor functional outcomes. J Parenter Enteral Nutr. 2021;45:518–29. doi: 10.1002/jpen.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haghikia A, Li XS, Liman TG, Bledau N, Schmidt D, Zimmermann F, et al. Gut microbiota-dependent trimethylamine N-oxide predicts risk of cardiovascular events in patients with stroke and is related to proinflammatory monocytes. Arterioscler Thromb Vasc Biol. 2018;38:2225–35. doi: 10.1161/atvbaha.118.311023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165:111–24. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jäckel S, Kiouptsi K, Lillich M, Hendrikx T, Khandagale A, Kollar B, et al. Gut microbiota regulate hepatic von Willebrand factor synthesis and arterial thrombus formation via Toll-like receptor-2. Blood. 2017;130:542–53. doi: 10.1182/blood-2016-11-754416. [DOI] [PubMed] [Google Scholar]
- 42.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481–8. doi: 10.1161/circulationaha.105.537878. [DOI] [PubMed] [Google Scholar]
- 43.Trøseid M, Andersen GØ, Broch K, Hov JR. The gut microbiome in coronary artery disease and heart failure: current knowledge and future directions. EBioMedicine. 2020;52:102649. doi: 10.1016/j.ebiom.2020.102649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Emoto T, Yamashita T, Sasaki N, Hirota Y, Hayashi T, So A, et al. Analysis of gut microbiota in coronary artery disease patients: a possible link between gut microbiota and coronary artery disease. J Atherosclerosis Thromb. 2016;23:908–21. doi: 10.5551/jat.32672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng Q, Liu Z, Zhong S, Li R, Xia H, Jie Z, et al. Integrated metabolomics and metagenomics analysis of plasma and urine identified microbial metabolites associated with coronary heart disease. Sci Rep. 2016;6:11. doi: 10.1038/srep22525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu H, Tian R, Wang H, Feng S, Li H, Xiao Y, et al. Gut microbiota from coronary artery disease patients contributes to vascular dysfunction in mice by regulating bile acid metabolism and immune activation. J Transl Med. 2020;18:382. doi: 10.1186/s12967-020-02539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heianza Y, Ma W, DiDonato JA, Sun Q, Rimm EB, Hu FB, et al. Long-term changes in gut microbial metabolite trimethylamine N-oxide and coronary heart disease risk. J Am Coll Cardiol. 2020;75:763–72. doi: 10.1016/j.jacc.2019.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116:1509–26. doi: 10.1161/circresaha.116.303849. [DOI] [PubMed] [Google Scholar]
- 49.Song P, Rudan D, Zhu Y, Fowkes FJI, Rahimi K, Fowkes FGR, et al. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Heal. 2019;7:e1020–30. doi: 10.1016/s2214-109x(19)30255-4. [DOI] [PubMed] [Google Scholar]
- 50.Senthong V, Wang Z, Fan Y, Wu Y, Hazen SL, Tang WHW. Trimethylamine N-oxide and mortality risk in patients with peripheral artery disease. J Am Heart Assoc. 2016;5:e004237. doi: 10.1161/JAHA.116.004237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeFilippis AP, Chapman AR, Mills NL, DeLemos JA, Arbab-Zadeh A, Newby LK, et al. Assessment and treatment of patients with type 2 myocardial infarction and acute nonischemic myocardial injury. Circulation. 2019;140:1661–78. doi: 10.1161/circulationaha.119.040631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li J, Li X, Wang Q, Hu S, Wang Y, Masoudi FA, et al. ST-segment elevation myocardial infarction in China from 2001 to 2011 (the China PEACE-Retrospective Acute Myocardial Infarction Study): a retrospective analysis of hospital data. Lancet. 2015;385:441–51. doi: 10.1016/s0140-6736(14)60921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou X, Li J, Guo J, Geng B, Ji W, Zhao Q, et al. Gut-dependent microbial translocation induces inflammation and cardiovascular events after ST-elevation myocardial infarction. Microbiome. 2018;6:66. doi: 10.1186/s40168-018-0441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lam V, Su J, Hsu A, Gross GJ, Salzman NH, Baker JE. Intestinal microbial metabolites are linked to severity of myocardial infarction in rats. PLoS One. 2016;11:e0160840. doi: 10.1371/journal.pone.0160840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao J, Yan KT, Wang JX, Dou J, Wang J, Ren M, et al. Gut microbial taxa as potential predictive biomarkers for acute coronary syndrome and post-STEMI cardiovascular events. Sci Rep. 2020;10:1–11. doi: 10.1038/s41598-020-59235-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kiouptsi K, Finger S, Garlapati VS, Knorr M, Brandt M, Walter U, et al. Hypoxia evokes increased PDI and PDIA6 expression in the infarcted myocardium of ex-germ-free and conventionally raised mice. Biol Open. 2019;8:bio038851. doi: 10.1242/bio.038851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sakalihasan N, Michel JB, Katsargyris A, Kuivaniemi H, Defraigne JO, Nchimi A, et al. Abdominal aortic aneurysms. Nat Rev Dis Prim. 2018;4:1–22. doi: 10.1038/s41572-018-0030-7. [DOI] [PubMed] [Google Scholar]
- 58.Xie J, Lu W, Zhong L, Hu Y, Li Q, Ding R, et al. Alterations in gut microbiota of abdominal aortic aneurysm mice. BMC Cardiovasc Disord. 2020;20:32. doi: 10.1186/s12872-020-01334-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kurtelius A, Väntti N, Rezai Jahromi B, Tähtinen O, Manninen H, Koskenvuo J, et al. Association of intracranial aneurysms with aortic aneurysms in 125 patients with fusiform and 4253 patients with saccular intracranial aneurysms and their family members and population controls. J Am Heart Assoc. 2019;8:e013277. doi: 10.1161/JAHA.119.013277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shikata F, Shimada K, Sato H, Ikedo T, Kuwabara A, Furukawa H, et al. Potential influences of gut microbiota on the formation of intracranial aneurysm. Hypertension. 2019;73:491–6. doi: 10.1161/hypertensionaha.118.11804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li H, Xu H, Li Y, Jiang Y, Hu Y, Liu T, et al. Alterations of gut microbiota contribute to the progression of unruptured intracranial aneurysms. Nat Commun. 2020;11:1–15. doi: 10.1038/s41467-020-16990-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferretti P, Pasolli E, Tett A, Asnicar F, Gorfer V, Fedi S, et al. Mother-to-Infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe. 2018;24:133–45.e5. doi: 10.1016/j.chom.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nyangahu DD, Jaspan HB. Influence of maternal microbiota during pregnancy on infant immunity. Clin Exp Immunol. 2019;198:47–56. doi: 10.1111/cei.13331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kimura I, Miyamoto J, Ohue-Kitano R, Watanabe K, Yamada T, Onuki M, et al. Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science. 2020;367:eaaw8429. doi: 10.1126/science.aaw8429. [DOI] [PubMed] [Google Scholar]
- 65.Wang J, Zheng J, Shi W, Du N, Xu X, Zhang Y, et al. Dysbiosis of maternal and neonatal microbiota associated with gestational diabetes mellitus. Gut. 2018;67:1614–25. doi: 10.1136/gutjnl-2018-315988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou L, Xiao X, Li M, Zhang Q, Yu M, Zheng J, et al. Maternal exercise improves high-fat diet-induced metabolic abnormalities and gut microbiota profiles in mouse dams and offspring. Front Cell Infect Microbiol. 2020;10:292. doi: 10.3389/fcimb.2020.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hsu CN, Hou CY, Chang-Chien GP, Lin S, Tain YL. Maternal n-acetylcysteine therapy prevents hypertension in spontaneously hypertensive rat offspring: implications of hydrogen sulfide-generating pathway and gut microbiota. Antioxidants. 2020;9:1–15. doi: 10.3390/antiox9090856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li HB, Yang T, Richards EM, Pepine CJ, Raizada MK. Maternal treatment with captopril persistently alters gut-brain communication and attenuates hypertension of male offspring. Hypertension. 2020;75:1315–24. doi: 10.1161/hypertensionaha.120.14736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hsu CN, Lin YJ, Hou CY, Tain YL. Maternal administration of probiotic or prebiotic prevents male adult rat offspring against developmental programming of hypertension induced by high fructose consumption in pregnancy and lactation. Nutrients. 2018;10:1229. doi: 10.3390/nu10091229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. 2019;68:1516–26. doi: 10.1136/gutjnl-2019-318427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mu Q, Kirby J, Reilly CM, Luo XM. Leaky gut as a danger signal for autoimmune diseases. Front Immunol. 2017;8:598. doi: 10.3389/fimmu.2017.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen YY, Chen DQ, Chen L, Liu JR, Vaziri ND, Guo Y, et al. Microbiome–metabolome reveals the contribution of gut-kidney axis on kidney disease 11 medical and health sciences 1103 clinical sciences. J Transl Med. 2019;17:5. doi: 10.1186/s12967-018-1756-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chakaroun RM, Massier L, Kovacs P. Gut microbiome, intestinal permeability, and tissue bacteria in metabolic disease: perpetrators or bystanders? Nutrients. 2020;12:1082. doi: 10.3390/nu12041082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe. 2017;21:455–66.e4. doi: 10.1016/j.chom.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang B, Yu M, Zhang R, Chen S, Xi Y, Duan G. A meta‐analysis of the association between Helicobacter pylori infection and risk of atherosclerotic cardiovascular disease. Helicobacter. 2020;25:e12761. doi: 10.1111/hel.12761. [DOI] [PubMed] [Google Scholar]
- 76.Dahl A, Rasmussen RV, Bundgaard H, Hassager C, Bruun LE, Lauridsen TK, et al. Enterococcus faecalis infective endocarditis a pilot study of the relationship between duration of gentamicin treatment and outcome. Circulation. 2013;127:1810–7. doi: 10.1161/circulationaha.112.001170. [DOI] [PubMed] [Google Scholar]
- 77.Knuefermann P, Sakata Y, Baker JS, Huang CH, Sekiguchi K, Hardarson HS, et al. Toll-like receptor 2 mediates Staphylococcus aureus-induced myocardial dysfunction and cytokine production in the heart. Circulation. 2004;110:3693–8. doi: 10.1161/01.cir.0000143081.13042.04. [DOI] [PubMed] [Google Scholar]
- 78.Ramana KV, Willis MS, White MD, Horton JW, Dimaio JM, Srivastava D, et al. Endotoxin-induced cardiomyopathy and systemic inflammation in mice is prevented by aldose reductase inhibition. Circulation. 2006;114:1838–46. doi: 10.1161/circulationaha.106.630830. [DOI] [PubMed] [Google Scholar]
- 79.Deng M, Tang Y, Li W, Wang X, Zhang R, Zhang X, et al. The endotoxin delivery protein HMGB1 mediates caspase-11-dependent lethality in sepsis. Immunity. 2018;49:740–53.e7. doi: 10.1016/j.immuni.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cavaillon JM. Exotoxins and endotoxins: inducers of inflammatory cytokines. Toxicon. 2018;149:45–53. doi: 10.1016/j.toxicon.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 81.Williams JW, Huang L, Randolph GJ. Cytokine circuits in cardiovascular disease. Immunity. 2019;50:941–54. doi: 10.1016/j.immuni.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kimmoun A, Novy E, Auchet T, Ducrocq N, Levy B. Hemodynamic consequences of severe lactic acidosis in shock states: from bench to bedside. Crit Care. 2015;19:1–13. doi: 10.1186/s13054-015-0896-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang J, Wang YT, Miller JH, Day MM, Munger JC, Brookes PS. Accumulation of succinate in cardiac ischemia primarily occurs via canonical krebs cycle activity. Cell Rep. 2018;23:2617–28. doi: 10.1016/j.celrep.2018.04.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tang WHW, Li DY, Hazen SL. Dietary metabolism, the gut microbiome, and heart failure. Nat Rev Cardiol. 2019;16:137–54. doi: 10.1038/s41569-018-0108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baeyens N, Bandyopadhyay C, Coon BG, Yun S, Schwartz MA. Endothelial fluid shear stress sensing in vascular health and disease. J Clin Invest. 2016;126:821–8. doi: 10.1172/jci83083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McCarron JG, Lee MD, Wilson C. The endothelium solves problems that endothelial cells do not know exist. Trends Pharmacol Sci. 2017;38:322–38. doi: 10.1016/j.tips.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Widlansky ME, Gokce N, Keaney JF, Vita JA, Boston F. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–60. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 88.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–95. doi: 10.1161/circulationaha.106.652859. [DOI] [PubMed] [Google Scholar]
- 89.Talman V, Kivelä R. Cardiomyocyte—endothelial cell interactions in cardiac remodeling and regeneration. Front Cardiovasc Med. 2018;5:101. doi: 10.3389/fcvm.2018.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu H, Wang Y, Zhang Y, Xu F, Chen J, Duan L, et al. Breaking the vicious loop between inflammation, oxidative stress and coagulation, a novel anti-thrombus insight of nattokinase by inhibiting LPS-induced inflammation and oxidative stress. Redox Biol. 2020;32:101500. doi: 10.1016/j.redox.2020.101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pérez L, Muñoz-Durango N, Riedel CA, Echeverría C, Kalergis AM, Cabello-Verrugio C, et al. Endothelial-to-mesenchymal transition: cytokine-mediated pathways that determine endothelial fibrosis under inflammatory conditions. Cytokine Growth Factor Rev. 2017;33:41–54. doi: 10.1016/j.cytogfr.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 92.Stoll LL, Denning GM, Weintraub NL. Potential role of endotoxin as a proinflammatory mediator of atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:2227–36. doi: 10.1161/01.atv.0000147534.69062.dc. [DOI] [PubMed] [Google Scholar]
- 93.Xiong J, Kawagishi H, Yan Y, Liu J, Wells QS, Edmunds LR, et al. A metabolic basis for endothelial-to-mesenchymal transition. Mol Cell. 2018;69:689–98.e7. doi: 10.1016/j.molcel.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brunt VE, Gioscia-Ryan RA, Casso AG, Vandongen NS, Ziemba BP, Sapinsley ZJ, et al. Trimethylamine-N-oxide promotes age-related vascular oxidative stress and endothelial dysfunction in mice and healthy humans. Hypertension. 2020;76:101–12. doi: 10.1161/hypertensionaha.120.14759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tumur Z, Shimizu H, Enomoto A, Miyazaki H, Niwa T. Indoxyl sulfate upregulates expression of ICAM-1 and MCP-1 by oxidative stress-induced NF-κB activation. Am J Nephrol. 2010;31:435–41. doi: 10.1159/000299798. [DOI] [PubMed] [Google Scholar]
- 96.Jing YJ, Ni JW, Ding FH, Fang YH, Wang XQ, Wang HB, et al. p-Cresyl sulfate is associated with carotid arteriosclerosis in hemodialysis patients and promotes atherogenesis in ApoE−/− mice. Kidney Int. 2016;89:439–49. doi: 10.1038/ki.2015.287. [DOI] [PubMed] [Google Scholar]
- 97.Tumur Z, Niwa T. Indoxyl sulfate inhibits nitric oxide production and cell viability by inducing oxidative stress in vascular endothelial cells. Am J Nephrol. 2009;29:551–7. doi: 10.1159/000191468. [DOI] [PubMed] [Google Scholar]
- 98.Kumar S, Kasseckert S, Kostin S, Abdallah Y, Schafer C, Kaminski A, et al. Ischemic acidosis causes apoptosis in coronary endothelial cells through activation of caspase-12. Cardiovasc Res. 2007;73:172–80. doi: 10.1016/j.cardiores.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 99.Bartolomaeus H, Balogh A, Yakoub M, Homann S, Markó L, Höges S, et al. Short-chain fatty acid propionate protects from hypertensive cardiovascular damage. Circulation. 2019;139:1407–21. doi: 10.1161/circulationaha.118.036652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Robles-Vera I, Toral M, dela Visitación N, Aguilera-Sánchez N, Redondo JM, Duarte J. Protective effects of short-chain fatty acids on endothelial dysfunction induced by angiotensin II. Front Physiol. 2020;11:277. doi: 10.3389/fphys.2020.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li Z, Polhemus DJ, Lefer DJ. Evolution of hydrogen sulfide therapeutics to treat cardiovascular disease. Circ Res. 2018;123:590–600. doi: 10.1161/circresaha.118.311134. [DOI] [PubMed] [Google Scholar]
- 102.Pichette J, Fynn-Sackey N, Gagnon J. Hydrogen sulfide and sulfate prebiotic stimulates the secretion of GLP-1 and improves glycemia in male mice. Endocrinology. 2017;158:3416–25. doi: 10.1210/en.2017-00391. [DOI] [PubMed] [Google Scholar]
- 103.Helmstädter J, Frenis K, Filippou K, Grill A, Dib M, Kalinovic S, et al. Endothelial GLP-1 (Glucagon-Like peptide-1) receptor mediates cardiovascular protection by liraglutide in mice with experimental arterial hypertension. Arterioscler Thromb Vasc Biol. 2020;40:145–58. doi: 10.1161/atv.0000615456.97862.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shi J, Yang Y, Cheng A, Xu G, He F. Metabolism of vascular smooth muscle cells in vascular diseases. Am J Physiol Heart Circ Physiol. 2020;319:H613–31. doi: 10.1152/ajpheart.00220.2020. [DOI] [PubMed] [Google Scholar]
- 105.Li M, Qian M, Kyler K, Xu J. Endothelial–vascular smooth muscle cells interactions in atherosclerosis. Front Cardiovasc Med. 2018;5:151. doi: 10.3389/fcvm.2018.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Man AWC, Li H, Xia N. Resveratrol and the interaction between gut microbiota and arterial remodelling. Nutrients. 2020;12:119. doi: 10.3390/nu12010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Michel J-B, Li Z, Lacolley P. Smooth muscle cells and vascular diseases. Cardiovasc Res. 2012;95:135–7. doi: 10.1093/cvr/cvs172. [DOI] [PubMed] [Google Scholar]
- 108.Byon CH, Heath JM, Chen Y. Redox signaling in cardiovascular pathophysiology: a focus on hydrogen peroxide and vascular smooth muscle cells. Redox Biol. 2016;9:244–53. doi: 10.1016/j.redox.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ren J, Zhou T, Sekhar Pilli VS, Phan N, Wang Q, Gupta K, et al. Novel paracrine functions of smooth muscle cells in supporting endothelial regeneration following arterial injury. Circ Res. 2019;124:1253–65. doi: 10.1161/circresaha.118.314567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boyer MJ, Kimura Y, Akiyama T, Baggett AY, Preston KJ, Scalia R, et al. Endothelial cell-derived extracellular vesicles alter vascular smooth muscle cell phenotype through high-mobility group box proteins. J Extracell Vesicles. 2020;9:1781427. doi: 10.1080/20013078.2020.1781427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lin FY, Chen YH, Tasi JS, Chen JW, Yang TL, Wang HJ, et al. Endotoxin induces toll-like receptor 4 expression in vascular smooth muscle cells via NADPH oxidase activation and mitogen-activated protein kinase signaling pathways. Arterioscler Thromb Vasc Biol. 2006;26:2630–7. doi: 10.1161/01.atv.0000247259.01257.b3. [DOI] [PubMed] [Google Scholar]
- 112.Yin Q, Jiang D, Li L, Yang Y, Wu P, Luo Y, et al. LPS promotes vascular smooth muscle cells proliferation through the TLR4/Rac1/akt signalling pathway. Cell Physiol Biochem. 2017;44:2189–200. doi: 10.1159/000486024. [DOI] [PubMed] [Google Scholar]
- 113.Lee GL, Yeh CC, Wu JY, Lin HC, Wang YF, Kuo YY, et al. TLR2 promotes vascular smooth muscle cell chondrogenic differentiation and consequent calcification via the concerted actions of osteoprotegerin suppression and IL-6-mediated RANKL induction. Arterioscler Thromb Vasc Biol. 2019;39:432–45. doi: 10.1161/atvbaha.118.311874. [DOI] [PubMed] [Google Scholar]
- 114.Choi S, Park M, Kim J, Park W, Kim S, Lee DK, et al. TNF-α elicits phenotypic and functional alterations of vascular smooth muscle cells by miR-155-5p-dependent down-regulation of cGMP-dependent kinase 1. J Biol Chem. 2018;293:14812–22. doi: 10.1074/jbc.ra118.004220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shimizu H, Hirose Y, Nishijima F, Tsubakihara Y, Miyazaki H. ROS and PDFG-β receptors are critically involved in indoxyl sulfate actions that promote vascular smooth muscle cell proliferation and migration. Am J Physiol Physiol. 2009;297:C389–96. doi: 10.1152/ajpcell.00206.2009. [DOI] [PubMed] [Google Scholar]
- 116.Han H, Chen Y, Zhu Z, Su X, Ni J, Du R, et al. p-Cresyl sulfate promotes the formation of atherosclerotic lesions and induces plaque instability by targeting vascular smooth muscle cells. Front Med. 2016;10:320–9. doi: 10.1007/s11684-016-0463-x. [DOI] [PubMed] [Google Scholar]
- 117.Zhang X, Li Y, Yang P, Liu X, Lu L, Chen Y, et al. Trimethylamine-N-oxide promotes vascular calcification through activation of NLRP3 (nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3) inflammasome and NF-κB (nuclear factor κb) signals. Arterioscler Thromb Vasc Biol. 2020;40:751–65. doi: 10.1161/atvbaha.119.313414. [DOI] [PubMed] [Google Scholar]
- 118.Yang L, Gao L, Nickel T, Yang J, Zhou J, Gilbertsen A, et al. Lactate promotes synthetic phenotype in vascular smooth muscle cells. Circ Res. 2017;121:1251–62. doi: 10.1161/circresaha.117.311819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang L, Yu P, Zhou B, Song J, Li Z, Zhang M, et al. Single-cell reconstruction of the adult human heart during heart failure and recovery reveals the cellular landscape underlying cardiac function. Nat Cell Biol. 2020;22:108–19. doi: 10.1038/s41556-019-0446-7. [DOI] [PubMed] [Google Scholar]
- 120.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–71. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 121.Chagnon F, Metz CN, Bucala R, Lesur O. Endotoxin-induced myocardial dysfunction: effects of macrophage migration inhibitory factor neutralization. Circ Res. 2005;96:1095–102. doi: 10.1161/01.res.0000168327.22888.4d. [DOI] [PubMed] [Google Scholar]
- 122.Joseph LC, Kokkinaki D, Valenti MC, Kim GJ, Barca E, Tomar D, et al. Inhibition of NADPH oxidase 2 (NOX2) prevents sepsis-induced cardiomyopathy by improving calcium handling and mitochondrial function. JCI insight. 2017;2:e94248. doi: 10.1172/jci.insight.94248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Takimoto E, Kass DA. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension. 2007;49:241–8. doi: 10.1161/01.hyp.0000254415.31362.a7. [DOI] [PubMed] [Google Scholar]
- 124.Tourki B, Halade G. Leukocyte diversity in resolving and nonresolving mechanisms of cardiac remodeling. FASEB J. 2017;31:4226–39. doi: 10.1096/fj.201700109r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Frati G, Schirone L, Chimenti I, Yee D, Biondi-Zoccai G, Volpe M, et al. An overview of the inflammatory signalling mechanisms in the myocardium underlying the development of diabetic cardiomyopathy. Cardiovasc Res. 2017;113:378–88. doi: 10.1093/cvr/cvx011. [DOI] [PubMed] [Google Scholar]
- 126.Zhang W, Xu X, Kao R, Mele T, Kvietys P, Martin CM, et al. Cardiac fibroblasts contribute to myocardial dysfunction in mice with sepsis: the role of NLRP3 inflammasome activation. PLoS One. 2014;9:e107639. doi: 10.1371/journal.pone.0107639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li Z, Wu Z, Yan J, Liu H, Liu Q, Deng Y, et al. Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab Invest. 2019;99:346–57. doi: 10.1038/s41374-018-0091-y. [DOI] [PubMed] [Google Scholar]
- 128.Li X, Geng J, Zhao J, Ni Q, Zhao C, Zheng Y, et al. Trimethylamine N-oxide exacerbates cardiac fibrosis via activating the NLRP3 inflammasome. Front Physiol. 2019;10:866. doi: 10.3389/fphys.2019.00866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Organ CL, Li Z, Sharp TE, Polhemus DJ, Gupta N, Goodchild TT, et al. Nonlethal inhibition of gut microbial trimethylamine N-oxide production improves cardiac function and remodeling in a murine model of heart failure. J Am Heart Assoc. 2020;9:e016223. doi: 10.1161/JAHA.119.016223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lekawanvijit S, Adrahtas A, Kelly DJ, Kompa AR, Wang BH, Krum H. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur Heart J. 2010;31:1771–9. doi: 10.1093/eurheartj/ehp574. [DOI] [PubMed] [Google Scholar]
- 131.Tan X, CaoXS, Zhang P, Xiang FF, Teng J, Zou JZ, et al. Endoplasmic reticulum stress associated apoptosis as a novel mechanism in indoxyl sulfate-induced cardiomyocyte toxicity. Mol Med Rep. 2018;18:5117–22. doi: 10.3892/mmr.2018.9496. [DOI] [PubMed] [Google Scholar]
- 132.Han H, Zhu J, Zhu Z, Ni J, Du R, Dai Y, et al. p-Cresyl sulfate aggravates cardiac dysfunction associated with chronic kidney disease by enhancing apoptosis of cardiomyocytes. J Am Heart Assoc. 2015;4:e001852. doi: 10.1161/JAHA.115.001852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang L, Deng M, Lu A, Chen Y, Chen Y, Wu C, et al. Sodium butyrate attenuates angiotensin II-induced cardiac hypertrophy by inhibiting COX2/PGE2 pathway via a HDAC5/HDAC6-dependent mechanism. J Cell Mol Med. 2019;23:8139–50. doi: 10.1111/jcmm.14684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shen Y, Shen Z, Luo S, Guo W, Zhu YZ. The cardioprotective effects of hydrogen sulfide in heart diseases: from molecular mechanisms to therapeutic potential. Oxid Med Cell Longev. 2015;2015:925167. doi: 10.1155/2015/925167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ryan PM, Stanton C, Caplice NM. Bile acids at the cross-roads of gut microbiome-host cardiometabolic interactions. Diabetol Metab Syndrome. 2017;9:102. doi: 10.1186/s13098-017-0299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Desai MS, Mathur B, Eblimit Z, Vasquez H, Taegtmeyer H, Karpen SJ, et al. Bile acid excess induces cardiomyopathy and metabolic dysfunctions in the heart. Hepatology. 2017;65:189–201. doi: 10.1002/hep.28890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang Z, Zhao Y. Gut microbiota derived metabolites in cardiovascular health and disease. Protein Cell. 2018;9:416–31. doi: 10.1007/s13238-018-0549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Semaming Y, Kumfu S, Pannangpetch P, Chattipakorn SC, Chattipakorn N. Protocatechuic acid exerts a cardioprotective effect in type 1 diabetic rats. J Endocrinol. 2014;223:13–23. doi: 10.1530/joe-14-0273. [DOI] [PubMed] [Google Scholar]
- 139.Albillos A, deGottardi A, Rescigno M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol. 2020;72:558–77. doi: 10.1016/j.jhep.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 140.Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, et al. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15:397–411. doi: 10.1038/s41575-018-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zeisel SH, Warrier M. Trimethylamine N-oxide, the microbiome, and heart and kidney disease. Annu Rev Nutr. 2017;37:157–81. doi: 10.1146/annurev-nutr-071816-064732. [DOI] [PubMed] [Google Scholar]
- 142.Jourde-Chiche N, Burtey S. Accumulation of protein-bound uremic toxins: the kidney remains the leading culprit in the gut-liver-kidney axis. Kidney Int. 2020;97:1102–4. doi: 10.1016/j.kint.2020.02.026. [DOI] [PubMed] [Google Scholar]
- 143.Paroni R, Casati S, Dei Cas M, Bignotto M, Rubino FM, Ciuffreda P. Unambiguous characterization of p-cresyl sulfate, a protein-bound uremic toxin, as biomarker of heart and kidney disease. Molecules. 2019;24:3704. doi: 10.3390/molecules24203704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tilg H, Cani PD, Mayer EA. Gut microbiome and liver diseases. Gut. 2016;65:2035–44. doi: 10.1136/gutjnl-2016-312729. [DOI] [PubMed] [Google Scholar]
- 145.Vasavan T, Ferraro E, Ibrahim E, Dixon P, Gorelik J, Williamson C. Heart and bile acids – clinical consequences of altered bile acid metabolism. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1345–55. doi: 10.1016/j.bbadis.2017.12.039. [DOI] [PubMed] [Google Scholar]
- 146.Chiang JYL. Bile acid metabolism and signaling. Comp Physiol. 2013;3:1191–212. doi: 10.1002/cphy.c120023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kida T, Tsubosaka Y, Hori M, Ozaki H, Murata T. Bile acid receptor tgr5 agonism induces no production and reduces monocyte adhesion in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33:1663–9. doi: 10.1161/atvbaha.113.301565. [DOI] [PubMed] [Google Scholar]
- 148.Mayerhofer CCK, Ueland T, Broch K, Vincent RP, Cross GF, Dahl CP, et al. Increased secondary/primary bile acid ratio in chronic heart failure. J Card Fail. 2017;23:666–71. doi: 10.1016/j.cardfail.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 149.Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol. 2010;16:1321–9. doi: 10.3748/wjg.v16.i11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology. 2020;158:1611–25.e12. doi: 10.1053/j.gastro.2020.01.043. [DOI] [PubMed] [Google Scholar]
- 151.Yu L-X, Yan H-X, Liu Q, Yang W, Wu H-P, Dong W, et al. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology. 2010;52:1322–33. doi: 10.1002/hep.23845. [DOI] [PubMed] [Google Scholar]
- 152.Francque SM, van derGraaff D, Kwanten WJ. Non-alcoholic fatty liver disease and cardiovascular risk: pathophysiological mechanisms and implications. J Hepatol. 2016;65:425–43. doi: 10.1016/j.jhep.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 153.Matyas C, Haskó G, Liaudet L, Trojnar E, Pacher P. Interplay of cardiovascular mediators, oxidative stress and inflammation in liver disease and its complications. Nat Rev Cardiol. 2021;18:117–35. doi: 10.1038/s41569-020-0433-5. [DOI] [PubMed] [Google Scholar]
- 154.ElHadi H, DiVincenzo A, Vettor R, Rossato M. Relationship between heart disease and liver disease: a two-way street. Cells. 2020;9:567. doi: 10.3390/cells9030567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Morais LH, Schreiber HL, Mazmanian SK. The gut microbiota–brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2021;19:241–55. doi: 10.1038/s41579-020-00460-0. [DOI] [PubMed] [Google Scholar]
- 156.Yu LCH. Microbiota dysbiosis and barrier dysfunction in inflammatory bowel disease and colorectal cancers: exploring a common ground hypothesis. J Biomed Sci. 2018;25:1–14. doi: 10.1186/s12929-018-0483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wynne B, McHugh L, Gao W, Keegan D, Byrne K, Rowan C, et al. Acceptance and commitment therapy reduces psychological stress in patients with inflammatory bowel diseases. Gastroenterology. 2019;156:935–45.e1. doi: 10.1053/j.gastro.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 158.Peirce JM, Alviña K. The role of inflammation and the gut microbiome in depression and anxiety. J Neurosci Res. 2019;97:1223–41. doi: 10.1002/jnr.24476. [DOI] [PubMed] [Google Scholar]
- 159.Chauvet-Gelinier JC, Bonin B. Stress, anxiety and depression in heart disease patients: a major challenge for cardiac rehabilitation. Ann Phys Rehabil Med. 2017;60:6–12. doi: 10.1016/j.rehab.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 160.Whitworth JA, Williamson PM, Mangos G, Kelly JJ. Cardiovascular consequences of cortisol excess. Vasc Health Risk Manag. 2005;1:291–9. doi: 10.2147/vhrm.2005.1.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Arcaro G, Cretti A, Balzano S, Lechi A, Muggeo M, Bonora E, et al. Insulin causes endothelial dysfunction in humans: sites and mechanisms. Circulation. 2002;105:576–82. doi: 10.1161/hc0502.103333. [DOI] [PubMed] [Google Scholar]
- 162.DePunder K, Pruimboom L. Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability. Front Immunol. 2015;6:223. doi: 10.3389/fimmu.2015.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Frolkis AD, Vallerand IA, Shaheen AA, Lowerison MW, Swain MG, Barnabe C, et al. Depression increases the risk of inflammatory bowel disease, which may be mitigated by the use of antidepressants in the treatment of depression. Gut. 2019;68:1606–12. doi: 10.1136/gutjnl-2018-317182. [DOI] [PubMed] [Google Scholar]
- 164.Lakhan SE, Kirchgessner A. Neuroinflammation in inflammatory bowel disease. J Neuroinflammation. 2010;7:37. doi: 10.1186/1742-2094-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bear TLK, Dalziel JE, Coad J, Roy NC, Butts CA, Gopal PK. The role of the gut microbiota in dietary interventions for depression and anxiety. Adv Nutr. 2020;11:890–907. doi: 10.1093/advances/nmaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kolodziejczyk AA, Zheng D, Elinav E. Diet–microbiota interactions and personalized nutrition. Nat Rev Microbiol. 2019;17:742–53. doi: 10.1038/s41579-019-0256-8. [DOI] [PubMed] [Google Scholar]
- 167.Weersma RK, Zhernakova A, Fu J. Interaction between drugs and the gut microbiome. Gut. 2020;69:1510–9. doi: 10.1136/gutjnl-2019-320204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rena G, Lang CC. Repurposing metformin for cardiovascular disease. Circulation. 2018;137:422–4. doi: 10.1161/circulationaha.117.031735. [DOI] [PubMed] [Google Scholar]
- 169.Cheang WS, Tian XY, Wong WT, Lau CW, Lee SST, Chen ZY, et al. Metformin protects endothelial function in diet-induced obese mice by inhibition of endoplasmic reticulum stress through 5′ adenosine monophosphate-activated protein kinase-peroxisome proliferator-activated receptor δ pathway. Arterioscler Thromb Vasc Biol. 2014;34:830–6. doi: 10.1161/atvbaha.113.301938. [DOI] [PubMed] [Google Scholar]
- 170.Kanamori H, Naruse G, Yoshida A, Minatoguchi S, Watanabe T, Kawaguchi T, et al. Metformin enhances autophagy and provides cardioprotection in δ-sarcoglycan deficiency-induced dilated cardiomyopathy. Circ Hear Fail. 2019;12:e005418. doi: 10.1161/circheartfailure.118.005418. [DOI] [PubMed] [Google Scholar]
- 171.Zhang X, Zhao Y, Xu J, Xue Z, Zhang M, Pang X, et al. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci Rep. 2015;5:14405. doi: 10.1038/srep14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Elamin EE, Masclee AA, Dekker J, Pieters H-J, Jonkers DM. Short-chain fatty acids activate AMP-activated protein kinase and ameliorate ethanol-induced intestinal barrier dysfunction in CaCo-2 cell monolayers. J Nutr. 2013;143:1872–81. doi: 10.3945/jn.113.179549. [DOI] [PubMed] [Google Scholar]
- 173.Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23:850–8. doi: 10.1038/nm.4345. [DOI] [PubMed] [Google Scholar]
- 174.Bauer PV, Duca FA, Waise TMZ, Rasmussen BA, Abraham MA, Dranse HJ, et al. Metformin alters upper small intestinal microbiota that impact a glucose-SGLT1-sensing glucoregulatory pathway. Cell Metabol. 2018;27:101–17.e5. doi: 10.1016/j.cmet.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 175.Deng J, Zeng L, Lai X, Li J, Liu L, Lin Q, et al. Metformin protects against intestinal barrier dysfunction via AMPKα1-dependent inhibition of JNK signalling activation. J Cell Mol Med. 2018;22:546–57. doi: 10.1111/jcmm.13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zweck E, Roden M. GLP-1 receptor agonists and cardiovascular disease: drug-specific or class effects? Lancet Diabetes Endocrinol. 2019;7:89–90. doi: 10.1016/s2213-8587(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 177.Liu L, Liu J, Huang Y. Protective effects of glucagon-like peptide 1 on endothelial function in hypertension. J Cardiovasc Pharmacol. 2015;65:399–405. doi: 10.1097/fjc.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 178.Cheng CK, Luo JY, Lau CW, Cho WCS, Ng CF, Ma RCW, et al. A GLP-1 analog lowers ER stress and enhances protein folding to ameliorate homocysteine-induced endothelial dysfunction. Acta Pharmacol Sin. 2021;42:1598–1609. doi: 10.1038/s41401-020-00589-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jojima T, Uchida K, Akimoto K, Tomotsune T, Yanagi K, Iijima T, et al. Liraglutide, a GLP-1 receptor agonist, inhibits vascular smooth muscle cell proliferation by enhancing AMP-activated protein kinase and cell cycle regulation, and delays atherosclerosis in ApoE deficient mice. Atherosclerosis. 2017;261:44–51. doi: 10.1016/j.atherosclerosis.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 180.Zhao L, Chen Y, Xia F, Abudukerimu B, Zhang W, Guo Y, et al. A glucagon-like peptide-1 receptor agonist lowers weight by modulating the structure of gut microbiota. Front Endocrinol (Lausanne) 2018;9:233. doi: 10.3389/fendo.2018.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Moreira GV, Azevedo FF, Ribeiro LM, Santos A, Guadagnini D, Gama P, et al. Liraglutide modulates gut microbiota and reduces NAFLD in obese mice. J Nutr Biochem. 2018;62:143–54. doi: 10.1016/j.jnutbio.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 182.Wang Z, Saha S, VanHorn S, Thomas E, Traini C, Sathe G, et al. Gut microbiome differences between metformin- and liraglutide-treated T2DM subjects. Endocrinol Diabetes Metab. 2018;1:e00009. doi: 10.1002/edm2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Liu L, Liu J, Wong WT, Tian XY, Lau CW, Wang YX, et al. Dipeptidyl peptidase 4 inhibitor sitagliptin protects endothelial function in hypertension through a glucagon-like peptide 1-dependent mechanism. Hypertension. 2012;60:833–41. doi: 10.1161/hypertensionaha.112.195115. [DOI] [PubMed] [Google Scholar]
- 184.Zhang M, Feng R, Yang M, Qian C, Wang Z, Liu W, et al. Effects of metformin, acarbose, and sitagliptin monotherapy on gut microbiota in Zucker diabetic fatty rats. BMJ Open Diabetes Res Care. 2019;7:717. doi: 10.1136/bmjdrc-2019-000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Liao X, Song L, Zeng B, Liu B, Qiu Y, Qu H, et al. Alteration of gut microbiota induced by DPP-4i treatment improves glucose homeostasis. EBioMedicine. 2019;44:665–74. doi: 10.1016/j.ebiom.2019.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cheng CK, Luo JY, Lau CW, Chen ZY, Tian XY, Huang Y. Pharmacological basis and new insights of resveratrol action in the cardiovascular system. Br J Pharmacol. 2020;177:1258–77. doi: 10.1111/bph.14801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cai T-T, Ye X-L, Li R-R, Chen H, Wang Y-Y, Yong H-J, et al. Resveratrol modulates the gut microbiota and inflammation to protect against diabetic nephropathy in mice. Front Pharmacol. 2020;11:1249. doi: 10.3389/fphar.2020.01249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chen ML, Yi L, Zhang Y, Zhou X, Ran L, Yang J, et al. Resveratrol attenuates trimethylamine-N-oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. MBio. 2016;7:e02210–15. doi: 10.1128/mBio.02210-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Feng X, Sureda A, Jafari S, Memariani Z, Tewari D, Annunziata G, et al. Berberine in cardiovascular and metabolic diseases: from mechanisms to therapeutics. Theranostics. 2019;9:1923–51. doi: 10.7150/thno.30787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Liu L, Liu J, Huang Z, Yu X, Zhang X, Dou D, et al. Berberine improves endothelial function by inhibiting endoplasmic reticulum stress in the carotid arteries of spontaneously hypertensive rats. Biochem Biophys Res Commun. 2015;458:796–801. doi: 10.1016/j.bbrc.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 191.Hang W, He B, Chen J, Xia L, Wen B, Liang T, et al. Berberine ameliorates high glucose-induced cardiomyocyte injury via AMPK signaling activation to stimulate mitochondrial biogenesis and restore autophagic flux. Front Pharmacol. 2018;9:1121. doi: 10.3389/fphar.2018.01121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wu M, Yang S, Wang S, Cao Y, Zhao R, Li X, et al. Effect of berberine on atherosclerosis and gut microbiota modulation and their correlation in high-fat diet-fed ApoE−/− mice. Front Pharmacol. 2020;11:223. doi: 10.3389/fphar.2020.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang Y, Gu Y, Ren H, Wang S, Zhong H, Zhao X, et al. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study) Nat Commun. 2020;11:1–12. doi: 10.1038/s41467-020-18414-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dong J, Wong SL, Lau CW, Lee HK, Ng CF, Zhang L, et al. Calcitriol protects renovascular function in hypertension by down-regulating angiotensin II type 1 receptors and reducing oxidative stress. Eur Heart J. 2012;33:2980–90. doi: 10.1093/eurheartj/ehr459. [DOI] [PubMed] [Google Scholar]
- 195.Park CW, Oh YS, Shin YS, Kim CM, Kim YS, Kim SY, et al. Intravenous calcitriol regresses myocardial hypertrophy in hemodialysis patients with secondary hyperparathyroidism. Am J Kidney Dis. 1999;33:73–81. doi: 10.1016/s0272-6386(99)70260-x. [DOI] [PubMed] [Google Scholar]
- 196.Thomas RL, Jiang L, Adams JS, Xu ZZ, Shen J, Janssen S, et al. Vitamin D metabolites and the gut microbiome in older men. Nat Commun. 2020;11:1–10. doi: 10.1038/s41467-020-19793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhang YG, Lu R, Wu S, Chatterjee I, Zhou D, Xia Y, et al. Vitamin D receptor protects against dysbiosis and tumorigenesis via the JAK/STAT pathway in intestine. CMGH. 2020;10:729–46. doi: 10.1016/j.jcmgh.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Dorresteijn JAN, Matthijs Boekholdt S, Van DerGraaf Y, Kastelein JJP, Larosa JC, Pedersen TR, et al. High-dose statin therapy in patients with stable coronary artery disease: treating the right patients based on individualized prediction of treatment effect. Circulation. 2013;127:2485–93. doi: 10.1161/circulationaha.112.000712. [DOI] [PubMed] [Google Scholar]
- 199.Lima JAC, Desai MY, Steen H, Warren WP, Gautam S, Lai S. Statin-induced cholesterol lowering and plaque regression after 6 months of magnetic resonance imaging-monitored therapy. Circulation. 2004;110:2336–41. doi: 10.1161/01.cir.0000145170.22652.51. [DOI] [PubMed] [Google Scholar]
- 200.Vieira-Silva S, Falony G, Belda E, Nielsen T, Aron-Wisnewsky J, Chakaroun R, et al. Statin therapy is associated with lower prevalence of gut microbiota dysbiosis. Nature. 2020;581:310–5. doi: 10.1038/s41586-020-2269-x. [DOI] [PubMed] [Google Scholar]
- 201.Kim J, Lee H, An J, Song Y, Lee C-K, Kim K, et al. Alterations in gut microbiota by statin therapy and possible intermediate effects on hyperglycemia and hyperlipidemia. Front Microbiol. 2019;10:1947. doi: 10.3389/fmicb.2019.01947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhang P, Zhang X, Huang Y, Chen J, Shang W, Shi G, et al. Atorvastatin alleviates microglia-mediated neuroinflammation via modulating the microbial composition and the intestinal barrier function in ischemic stroke mice. Free Radic Biol Med. 2021;162:104–17. doi: 10.1016/j.freeradbiomed.2020.11.032. [DOI] [PubMed] [Google Scholar]
- 203.Battershill AJ, Scott LJ. Telmisartan: a review of its use in the management of hypertension. Drugs. 2006;66:51–83. doi: 10.2165/00003495-200666010-00004. [DOI] [PubMed] [Google Scholar]
- 204.Yuen CY, Wong WT, Tian XY, Wong SL, Lau CW, Yu J, et al. Telmisartan inhibits vasoconstriction via PPARγ-dependent expression and activation of endothelial nitric oxide synthase. Cardiovasc Res. 2011;90:122–9. doi: 10.1093/cvr/cvq392. [DOI] [PubMed] [Google Scholar]
- 205.Chan YK, Brar MS, Kirjavainen PV, Chen Y, Pen GJ, Li D, et al. High fat diet induced atherosclerosis is accompanied with low colonic bacterial diversity and altered abundances that correlates with plaque size, plasma A-FABP and cholesterol: a pilot study of high fat diet and its intervention with Lactobacillus rhamno. BMC Microbiol. 2016;16:1–13. doi: 10.1186/s12866-016-0883-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ooijevaar RE, Terveer EM, Verspaget HW, Kuijper EJ, Keller JJ. Clinical application and potential of fecal microbiota transplantation. Annu Rev Med. 2019;70:335–51. doi: 10.1146/annurev-med-111717-122956. [DOI] [PubMed] [Google Scholar]
- 207.Stebegg M, Silva-Cayetano A, Innocentin S, Jenkins TP, Cantacessi C, Gilbert C, et al. Heterochronic faecal transplantation boosts gut germinal centres in aged mice. Nat Commun. 2019;10:1–13. doi: 10.1038/s41467-019-10430-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Allegretti JR, Kassam Z, Osman M, Budree S, Fischer M, Kelly CR. The 5D framework: a clinical primer for fecal microbiota transplantation to treat Clostridium difficile infection. Gastrointest Endosc. 2018;87:18–29. doi: 10.1016/j.gie.2017.05.036. [DOI] [PubMed] [Google Scholar]
- 209.Hu XF, Zhang WY, Wen Q, Chen WJ, Wang ZM, Chen J, et al. Fecal microbiota transplantation alleviates myocardial damage in myocarditis by restoring the microbiota composition. Pharmacol Res. 2019;139:412–21. doi: 10.1016/j.phrs.2018.11.042. [DOI] [PubMed] [Google Scholar]
- 210.Smits LP, Kootte RS, Levin E, Prodan A, Fuentes S, Zoetendal EG, et al. Effect of vegan fecal microbiota transplantation on carnitine- and choline-derived trimethylamine-N-oxide production and vascular inflammation in patients with metabolic syndrome. J Am Heart Assoc. 2018;7:e008342. doi: 10.1161/JAHA.117.008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Toral M, Robles-Vera I, dela Visitación N, Romero M, Yang T, Sánchez M, et al. Critical role of the interaction gut microbiota – sympathetic nervous system in the regulation of blood pressure. Front Physiol. 2019;10:231. doi: 10.3389/fphys.2019.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chen R, Xu Y, Wu P, Zhou H, Lasanajak Y, Fang Y, et al. Transplantation of fecal microbiota rich in short chain fatty acids and butyric acid treat cerebral ischemic stroke by regulating gut microbiota. Pharmacol Res. 2019;148:104403. doi: 10.1016/j.phrs.2019.104403. [DOI] [PubMed] [Google Scholar]
- 213.Chen X, Li P, Liu M, Zheng H, He Y, Chen MX, et al. Gut dysbiosis induces the development of pre-eclampsia through bacterial translocation. Gut. 2020;69:513–22. doi: 10.1136/gutjnl-2019-319101. [DOI] [PubMed] [Google Scholar]