Abstract
Severe congenital neutropenia caused by jagunal homolog 1 (JAGN1) mutation is a rare condition resulting from maturation arrest secondary to endoplasmic reticulum stress response from impaired neutrophil protein glycosylation. Here, we report a case of a 4-year-old boy who presented with a history of recurrent infections and manifestations, including recurrent intracranial hemorrhage. A review of similar cases reported in the literature indicates that a bleeding diathesis has not been previously described in these patients. We hypothesize that this newly described association of bleeding complications in this patient with JAGN1 mutation is secondary to defective glycosylation in the normal functioning of platelets or clotting factors. Recurrent infections with intracranial hemorrhage, new focal neurologic defects, or altered mental status in a child should warrant a suspicion for this immunodeficiency for the prompt initiation of treatment and prophylaxis for life-threatening infections or trauma.
Keywords: JAGN1, congenital, neutropenia, children, severe congenital neutropenia (SCN)
Background
Neutrophils are key effectors of innate immunity that contribute to defense against bacterial and fungal infections (1). Neutropenia is usually acquired, resulting from increased destruction, granulocyte apoptosis, or decreased granulocyte production. Severe congenital neutropenia (SCN) is a rare primary immunodeficiency resulting in recurrent infections (2). Patients with congenital neutropenia are at an increased risk of acquiring myelodysplastic syndrome or acute myeloblastic leukemia (3). Jagunal homolog 1 (JAGN1) is a protein important for neutrophil maturation and survival (4). Mutations in the gene encoding JAGN1 are a rare and recently identified cause of SCN characterized by recurrent infections along with bony abnormalities and a heterogeneous clinical presentation (3).
Here, we report a case of a patient with autosomal recessive JAGN1 deficiency leading to SCN, with recurrent infections associated with bleeding manifestations. To our knowledge, bleeding manifestations associated with JAGN1 deficiency have not been previously reported, and this may be the first case of JAGN1 neutropenia related to biallelic pathogenic heterozygous mutations. We also review the literature for other cases of congenital neutropenia resulting from JAGN1 deficiency.
Case presentation
A 4-year-old boy presented with high-grade fever and recurrent right periorbital swelling following the recent treatment of right periorbital cellulitis and right ethmoidal sinusitis with broad-spectrum antibiotics for 2 weeks. His medical history was notable for the following: at birth, bilateral temporoparietal intraparenchymal hemorrhagic infarcts were noted with associated residual facial weakness. An extensive evaluation did not reveal any bleeding disorder or any underlying anatomic cause for his intracranial hemorrhage. He had an absolute neutrophil count (ANC) of 40 cells/µl, initially ascribed to acute illness. At 2 weeks of age, he developed a spontaneously draining abscess in his right groin. Abscess drainage culture revealed methicillin-resistant Staphylococcus aureus (MRSA). At that time, his ANC was 10 cells/µl. Bone marrow aspirate demonstrated myeloid maturation arrest with a few mature myeloid forms. Erythroid elements were of limited quantity and exhibited full-spectrum maturation. Megakaryocytes exhibited normal morphology. Genetic analysis was performed using targeted capture for genes associated with SCN (the University of Washington Department of Laboratory Medicine, MarrowSeq Panel, which includes ABCB7, ADA, AK2, ANKRD26, ATM, ATR, ATRX, BLM, BRCA1, BRCA2, BRIP1, C150RF41, CBL, CDAN1, CEBPA, CSF3R, CTC1, CXCR4, DKC1, ELANE, ERCC4, ETV6, FANCA, FANCB, FANCC, FANCD2, FANCE, FANCF, FANCG, FANCI, FANCL, FANCM, G6PC3, GATA1, GATA2, GFI1, HAX1, IL2RG, JAGN1, JAK2, KIF23, KLF1, LIG4, LYST, MPL, MRE11A, NBN, NHP2, NOP10, PALB2, PAX5, RAB27A, RAD50, RAD51C, RBM8A, RMRP, RNF168, RPL10, RPL11, RPL26, RPL35A, RPL5, RPS10, RPS14, RPS17, RPS19, RPS24, RPS26, RPS7, RTEL1, RUNX1, SBDS, SEC23B, SLX4, SRP72, TAZ, TCIRG1, TERC, TERT, TINF2, TP53, USB1, VPS45, WAS, and WRAP53), followed by next-generation sequencing with Illumina technology, which revealed biallelic pathogenic heterozygous mutations (p.S64X, NM_032492.3:c.191C > G and p.Q127X, NM_03492.3:c.379C > T) in the JAGN1 gene.
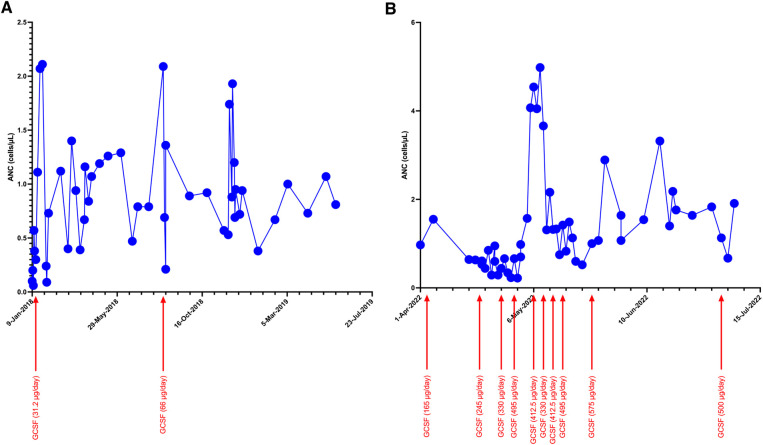
The patient was treated with granulocyte colony-stimulating factor (GCSF). However, his response to GCSF was inconsistent even with higher doses of 10 mcg/kg/day, with widely fluctuating ANC values (Figure 1). Additional screening revealed hypogammaglobulinemia with an IgG level of 53 mg/dl at 5 months of age (normal range: 200–1,200 mg/dl). Therefore, he was started on immunoglobulin replacement therapy as well. Despite these interventions, he continued to develop recurrent infections, including skin and soft tissue infections as well as chronic nasal congestion. At 1 year of age, after sustaining minor head trauma following a fall, he developed a venous hemorrhagic cerebral infarct and a retro-clival and cerebellopontine angle cistern hematoma. At 2 years of age, he experienced acute respiratory failure and bradycardic cardiac arrest attributed to an aspiration event while receiving non-invasive positive pressure ventilation after undergoing adenotonsillectomy for obstructive sleep apnea. Until the performance of the tonsillectomy, he was on continuous positive airway pressure (CPAP) for obstructive sleep apnea. In addition, he developed right parasagittal watershed infarcts between the anterior and the middle cerebral arteries with subsequent left hemiparesis. Several months later, he experienced a focal seizure and Todd's paralysis and was treated with levetiracetam for seizure prevention.
Figure 1.
Trends of ANC during initial (A) and current (B) hospitalization with increasing doses of GCSF.
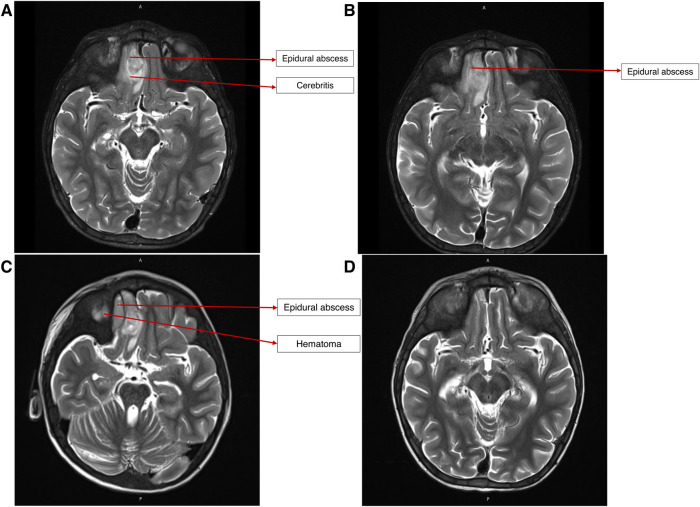
On presentation, he was alert, febrile, tachycardic, and tachypneic. He had mild right periorbital edema and erythema. Neurologic examination was significant for mild left lower facial weakness and mild left upper-extremity weakness, with slightly exaggerated reflexes on the left side globally. No dysmorphic features were observed. His height-for-age was under the 5th percentile, and his weight-for-age was between the 15th and the 25th percentile. A partially treated bacterial infection following his recent hospitalization was suspected. A brain computed tomography (CT) revealed complicated acute sinusitis with an epidural abscess. Magnetic resonance imaging (MRI) confirmed an epidural abscess with adjacent cerebritis, which was thought to be reactive in nature (Figure 2). He was hospitalized for further care.
Figure 2.
(A) MRI brain on presentation, demonstrating epidural abscess and reactive cerebritis. (B) MRI brain after endoscopic drainage procedure, showing persistence of epidural abscess and cerebritis. (C) MRI brain after craniotomy and debridement, showing persistence of epidural abscess and new adjacent hematoma. (D) MRI brain after ∼6 weeks of broad-spectrum antibiotic therapy, showing no abscess or residual parenchymal changes.
During hospitalization, hematologic studies revealed anemia, a normal leukocyte count with neutropenia and monocytosis, thrombocytopenia, and elevated C-reactive protein (CRP) (Table 1). Initial blood cultures, cerebrospinal fluid (CSF) analysis, and CSF culture did not reveal anything significant. He was treated empirically with vancomycin, metronidazole, and cefepime. He underwent endoscopic drainage of the abscess, which demonstrated growth of Pseudomonas aeruginosa on culture, which was sensitive to cefepime. Nevertheless, a broad antibiotic coverage was maintained, given the inability to exclude polymicrobial infection in the context of recent antibiotic use. Achieving therapeutic vancomycin trough levels was challenging, prompting a change to daptomycin. An interval imaging demonstrated an increased size of the abscess after endoscopic surgery, and he subsequently underwent frontal craniotomy.
Table 1.
Investigations on presentation.
| Investigation | Result | Normal range |
|---|---|---|
| Blood profile | ||
| Hemoglobin (g/dl) | 9.1 | 11–13.7 |
| Hematocrit (%) | 29.7 | 34–44 |
| Total WBC count (cells/µl) | 7,180 | 4,500–13,500 |
| Differential WBC count (%) | ||
| Neutrophil/band Lymphocyte Eosinophil Basophil Monocyte | 8.8 44.2 0.9 1.8 44.2 | 55–70 20–40 1–4 0.5–1 2–8 |
| Absolute neutrophil count (cells/µl) | 630 | 1,800–8,000 |
| Platelet count (x109 cells/L) | 128 | 150–450 |
| PT (sec) | 18.1 | 12.1–14.6 |
| PTT (sec) | 31.9 | 25.0–37.0 |
| INR | 1.45 | 0.92–1.14 |
| Fibrinogen (mg/dl) | 398 | 200–400 |
| Functional antithrombin (%) | 71 | 80–120 |
| ESR (mm/h) | 23 | 0–10 |
| Urine analysis | ||
| Glucose | Normal | |
| Blood | Negative | |
| Protein | Negative | |
| Ketone | Negative | |
| Bilirubin | Negative | |
| Liver function tests | ||
| Total bilirubin (mg/dl) | 0.2 | 0.1–1.2 |
| Direct bilirubin (mg/dl) | <0.2 | <0.3 |
| Aspartate transaminase (U/L) | 29 | 10–40 |
| Alanine transaminase (U/L) | 10 | 10–40 |
| Alkaline phosphatase (U/L) | 225 | 130–260 |
| Renal function tests | ||
| Creatinine (mg/dl) | 0.23 | 0.44–0.65 |
| BUN (mg/dl) | 8 | 5–18 |
| Inflammatory markers | ||
| C-reactive protein (mg/dl) | 4.51 | <0.9 |
| Microbiology/virology | ||
| Blood cultures | No growth | |
| MRSA surveillance cultures | Negative | |
| Respiratory pathogen testsa | Negative | |
| Coagulation profile from 2018b | ||
| Platelets (x103 cells/µl) | 108 | 223–461 |
| PT (sec) | 15.4 | 12.1–14.6 |
| INR | 1.19 | |
| PTT (sec) | 37.5 | 25.0–37.0 |
| Thrombin time (sec) | 15.7 | 14.4–18.0 |
| Fibrinogen (mg/dl) | 267 | 200–400 |
| Factor II activity (%) | 50 | 50–150 |
| Factor V activity (%) | 77 | 50–150 |
| Factor VII activity (%) | 66 | 50–150 |
| Factor XII activity (%) | 98 | 69–143 |
| PTT inhibitor screen | Negative | |
| PT inhibitor screen | Negative | |
| Lupus anticoagulant screen | Negative | |
| vWF Risto Cofactor (%) | 63 | 50–150 |
| vWF Antigen (%) | 83 | 50–160 |
| vWF VIII (%) | 79 | 70–170 |
WBC, white blood cells; PT, prothrombin time; PTT, partial thromboplastin time; INR, international normalized ratio; ESR, erythrocyte sedimentation rate; BUN, blood urea nitrogen; vWF, von Willebrand Factor.
Values outside the normal range are in bold.
Negative respiratory pathogen PCR tests for adenovirus, SARS-CoV-2, influenza A, influenza B, human metapneumovirus, parainfluenza 1, parainfluenza 2, parainfluenza 3, parainfluenza 4, respiratory syncytial virus, and rhinovirus.
After the second bleeding event.
After craniotomy, an interval MRI demonstrated a new epidural collection lateral to the epidural abscess, which was thought to represent a postoperative hematoma. A final interval MRI obtained 5 weeks after craniotomy showed a resolution of the abscess and hematoma.
Because of the nature of his surgeries, an alternative to CPAP was preferred, ultimately leading to the performance of a tracheostomy. Early during his course of stay, ANC dropped to 0, and GCSF dosing increased to ∼40 μg/kg/day to maintain an ANC >1,000 cells/µl. Because of his bleeding history, sequential compression device boots were employed while he was immobile, instead of using systemic anticoagulation.
During his hospital stay, the patient developed a febrile illness, and a blood culture taken from his central venous catheter grew Candida lusitaniae (Clavispora lusitaniae). Fungal staging examinations showed no evidence of fungal infection in CSF culture, ocular exam, or abdominal ultrasonography. His central venous catheter was removed, and he was treated with micafungin for 2 weeks.
Shortly after the patient completed a 6-week course of cefepime, daptomycin, and metronidazole for epidural abscess, his hospital course was complicated by a new requirement for supplemental oxygen and by a new retrocardiac opacity on chest x-ray, and he was diagnosed with pneumonia, which was treated with cefepime for 7 days.
His hospital stay is summarized in Table 2. On the day of discharge, his ANC was 1,910 cells/µl, and he was hemodynamically stable.
Table 2.
Course during the hospital stay.
| Day | Events |
|---|---|
| 1 | Presentation: pyrexia of unknown origin, moderate neutropenia. Ceftriaxone started empirically. GCSF and immune globulin therapy continued for primary immunodeficiency. |
| 2–4 | Continued fever with chronic nasal congestion and residual right eye discoloration. Partially treated periorbital cellulitis and sinusitis were suspected. Ceftriaxone was replaced with ampicillin-sulbactam. Maxillofacial CT revealed complicated acute sinusitis with frontobasal epidural abscess and adjacent cerebritis. Brain MRI confirmed epidural abscess with focal leptomeningeal enhancement. Antibiotics changed to vancomycin, metronidazole, and cefepime. |
| 5 | Source reduction—endoscopic endonasal drainage of the abscess. Pus culture: Pseudomonas aeruginosa. Procedural complication: CSF leak—managed by the insertion of a lumbar drain. P. aeruginosa sensitive to cefepime. Difficulty in achieving therapeutic vancomycin levels; vancomycin switched to daptomycin. Antibiotic regimen: daptomycin, metronidazole, and cefepime for 6 weeks. |
| 11 | Frontal craniotomy was done to manage the increasing size of the abscess. Debrided tissue culture from craniotomy: P. aeruginosa (sensitive to cefepime). |
| 19 | Tracheostomy was performed because of the inability to use CPAP in the context of recent maxillofacial surgeries. |
| 21–26 | New fevers. Central venous line (CVL) culture grew Candida lusitaniae (Clavispora lusitaniae)—CVL removed. LP was performed to evaluate for CNS involvement of fungal infection: no fungal elements or pleocytosis noted. Ophthalmologic exam reassuring. Antifungal added: micafungin for 2 weeks. |
| 45–46 | Transferred out of ICU in stable condition. Remains with tracheostomy in place. Interval brain MRI shows a resolution of intracranial infections. Broad-spectrum antibiotic course terminated. |
| 58–70 | Recrudescence of fever with worsening supplemental oxygen requirement and worsening abdominal distention. Chest x-ray: new retrocardiac opacity. Abdominal ultrasound showed acute appendicitis. Tracheostomy tube aspirate culture: P. aeruginosa. Received 7 days of cefepime for pneumonia, and was transitioned to ceftriaxone and metronidazole for a 14-day course because of the finding of appendicitis. Resolution of fever, with the persistence of tachycardia. Cardiology consultation: likely benign tachycardia. Pneumocystis jirovecii prophylaxis with trimethoprim-sulfamethoxazole initiated. |
| 82 | Discharged home. |
He was readmitted approximately 2 months after discharge for planned sibling donor allogeneic hematopoietic stem cell transplantation.
Discussion
Herein, we describe a rare case of a patient with SCN associated with biallelic JAGN1 mutations. SCN is uncommon, with associated genetic mutations occurring in >20 genes, including ELANE, GFI1, HAX1, G6PC3, WAS, and VPS45, and diverse inheritance patterns that can be autosomal-dominant, autosomal-recessive, or X-linked (5, 6). SCN caused by JAGN1 deficiency is extremely rare, accounting for ∼10% of cases (5).
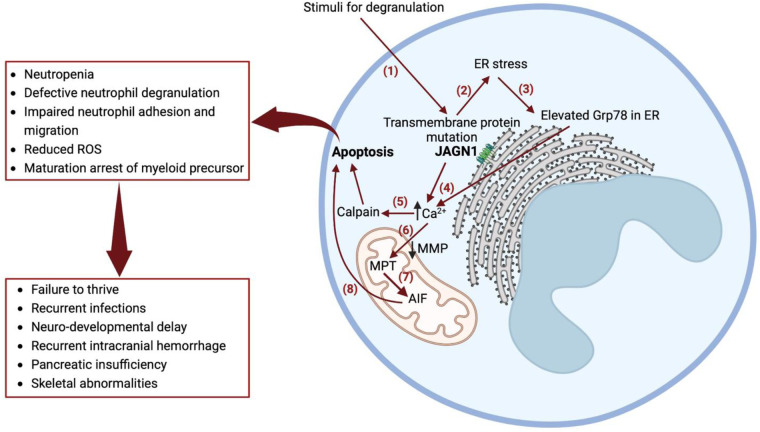
JAGN1 is expressed in the endoplasmic reticulum (ER), which contributes to the early secretory pathway in the ER and is a critical regulator of neutrophil differentiation and survival via GCSF receptor-mediated signaling (4). Biallelic mutations in JAGN1 lead to several defects in the granulocyte structure and cellular function and also affect longevity (4). A knockdown of JAGN1 expression in HeLa cells interferes with STAT3 phosphorylation upon recombinant human GCSF treatment, suggesting that decreased GCSF receptor signaling may contribute to defective granulocytes (4). Granulocyte-macrophage colony–stimulating factor (GM-CSF) treatment of bone marrow granulocytes in patients with JAGN1 deficiency restores the phosphorylation of STAT5 and cytotoxicity in response to Candida albicans (7). JAGN1 deficiency is also associated with a decreased expression of myeloperoxidase (MPO) in neutrophils, contributing to an ineffective killing of C. albicans via neutrophil extracellular traps—a phenotype reversible via GM-CSF administration (8). Mutations in JAGN1 can result in ER stress and intracellular calcium activation of calpain, leading to myeloid cell apoptosis (Figure 3) (9).
Figure 3.
Mechanism of severe congenital neutropenia caused by JAGN1 deficiency. (1) Stimuli for degranulation in a wild-type JAGN1 transmembrane protein produces degranulation. (2) Mutant JAGN1 produces ER stress. (3) ER stress results in increased Grp78 protein. (4) Elevated Grp78 causes N-glycosylation. (5) Altered N-glycosylation results in elevated calcium in the cytoplasm, causing activation of calpain, which leads to apoptosis. (6) Reduced MMP leads to Ca2+ entry to mitochondria, causing MPT. (7) MPT causes stimulation of AIF, which is cleaved by calpain. (8) AIF released in cytoplasm activates programmed cell death (apoptosis). Grp78—immunoglobulin heavy-chain binding protein of heat shock protein 70 family, a regulator of unfolded protein response, which is induced in cells with endoplasmic reticulum stress. They act as a molecular chaperone and a key regulator of Ca2+ homeostasis; GM-CSF improves N-glycosylation and is therefore used as a treatment for congenital neutropenia in JAGN1 mutation. MMP, mitochondrial membrane potential; AIF, apoptosis-inducing factor; JAGN1, jagunal homolog 1 membrane protein; MPT, mitochondrial permeability transition; Ca2+, calcium ion; ROS, reactive oxygen species.
Similar to the case of our patient, who had significantly low IgG levels by 5 months of age, at least two other patients with JAGN1 deficiency have been described as having antibody deficiency (10). In a murine model, JAGN1-deficient B cells had defective antibody production and secretion, altered immunoglobulin glycosylation, and defects in the differentiation and maintenance of plasma cells (11). These findings have been attributed to increased ER stress and dysregulation of the unfolded protein response. Notably, even JAGN1-deficient patients with normal serum immunoglobulin levels demonstrate an altered immunoglobulin glycoprofile when compared with healthy donors, suggesting the need for a very low threshold for immunoglobulin replacement in all patients with JAGN1 deficiency, regardless of the IgG levels (11).
SCN caused by JAGN1 deficiency may be associated with recurrent intracranial hemorrhage, pancreatic insufficiency, failure to thrive, developmental delay, skeletal abnormalities, and recurrent infections (including fungal infections) in the context of erratic neutrophil counts despite treatment with GCSF. In such cases, hematopoietic stem cell transplantation may be considered and is curative (4).
A summary of published case reports with SCN caused by JAGN1 deficiency is presented in Table 3. The case of an index family in Northern Africa was reported, but few details were provided (5). Of the other reported cases, the majority were females either from Turkey or Algeria. The mean age was 10 years and the mean ANC at the time of presentation was 100 cells/µl. Common clinical features associated with the mutation were sepsis, abscess, pneumonia, failure to thrive, and neurodevelopmental delay, and genetic defects were homozygous mutations in exons 1 and 2 of JAGN1 in addition to the alteration of proteins.
Table 3.
Case reports of severe congenital neutropenia caused by the JAGN1 mutation.
| Patient | Sex | Country | Clinical features | Investigations | Genetic defect | Treatment and Outcome | |
|---|---|---|---|---|---|---|---|
| 1. | 10-year-old (Y) (12) | M | Turkey | Facial dysmorphism; neutropenia; cough; rhinorrhea; neonatal sepsis; recurrent skin ulcers and abscesses; recurrent pneumonia, otitis media, sinusitis; cavernous lesions; asthma; allergic conjunctivitis | ANC: 100/mm3; leukocyte count: 5,120/mm3; Hb: 10.9 g/dl; platelet count: 357,000/mm3; bone marrow: maturation arrest of neutrophils | Homozygous mutation c.130 c > T in JAGN1 gene; p.His44Tyr protein alteration | GCSF (5 µg/kg) |
| 2. | 5 children from index family (5) | — | Northern Africa | Failure to thrive, developmental delay, skeletal abnormalities | — | Homozygous mutation c.3G > A in JAGN1 gene | — |
| 3. | 23-Y (4) | F | Algeria | ENT infections, aphthosis, perianal cellulitis, skin abscesses | ANC: 830/µl; bone marrow: maturation arrest | Homozygous mutation c.3G > A in JAGN1 gene; p.Met1lle protein alteration | Poor response to GCSF; alive |
| 4. | 17-Y (4) | F | Algeria | ENT infections, short stature | ANC: 800/µl | Homozygous mutation c.3G > A in JAGN1 gene; p.Met1lle protein alteration | Poor response to GCSF; alive |
| 5. | 19-Y (4) | M | Algeria | Aphthosis, skin abscesses, balanitis, pneumonitis, lung abscess, osteitis, perianal cellulitis, pyloric stenosis | ANC: 570/µl; bone marrow: maturation arrest (intermittent) | Homozygous mutation c.3G > A in JAGN1 gene; p.Met1lle protein alteration | Poor response to GCSF; alive |
| 6. | 17-Y (4) | F | Algeria | Otitis, paraodontopathy, scoliosis, dental malformations | ANC: 501/µl; bone marrow: maturation arrest (intermittent) | Homozygous mutation c.3G > A in JAGN1 gene; p.Met1lle protein alteration | Poor response to GCSF; alive |
| 7. | 5-Y (4) | M | Algeria | ENT infections, aphthosis, skin abscesses, pneumonitis, lung abscess, perianal cellulitis | ANC: 165/µl; bone marrow: maturation arrest | Homozygous mutation c.3G > A in JAGN1 gene; p.Met1lle protein alteration | Poor response to GCSF; alive |
| 8. | 12-Y (4) | F | Iran | Upper respiratory tract infections, pneumonia, skin abscesses, febrile convulsion, focal epilepsy | ANC: 892/µl; bone marrow: maturation arrest | Homozygous mutation c.59G > A in JAGN1 gene; p.Arg20Glu protein alteration | Poor response to GCSF; alive |
| 9. | 10-Y (4) | M | Turkey | Upper respiratory tract infections, pneumonia, skin and perianal abscesses, sepsis (Haemophilus influenzae), extramedullary hematopoiesis with a thickening of the skull bones | ANC: 191/µl; bone marrow: maturation arrest | Homozygous mutation c.130C > T in JAGN1 gene; p.His44Tyr protein alteration | Poor response to GCSF; alive |
| 10. | 7-Y (4) | F | Turkey | Upper respiratory tract infections, skin abscesses, B/L hip dysplasia, extramedullary hematopoiesis with a thickening of the skull bones | ANC: 3,587/µl; bone marrow: maturation arrest | Homozygous mutation c.130C > T in JAGN1 gene; p.His44Tyr protein alteration | Poor response to GCSF; alive |
| 11. | 28-Y (4) | F | Iran | Skin abscesses, onycholysis | ANC: 920/µl; bone marrow: maturation arrest | Homozygous mutation c.40G > A in JAGN1 gene; p.Gly14Ser protein alteration | Poor response to GCSF; alive |
| 12. | 13-Y (4) | M | Israel | Aspergillosis, severe osteoporosis, repeated bone fractures | ANC: 130/µl; bone marrow: maturation arrest | Homozygous mutation c.297C > G in JAGN1 gene; p.Tyr99* protein alteration | HSCT; alive |
| 13. | 5-Y (4) | F | Morocco | Skin abscesses, omphalitis, pancolitis, lipomatosis, pancreatic insufficiency, bone abnormalities, dental malformations | ANC: 70/µl; bone marrow: maturation arrest (intermittent) | Homozygous mutation c.485A > G in JAGN1 gene; p.Gln162Arg protein alteration | Died (pancolitis and septicemia) |
| 14. | 16-Y (4) | F | Albania | Skin abscess, upper respiratory tract infections, pneumonia, short stature, amelogenesis imperfecta, neurodevelopmental delay | ANC: 408/µl; bone marrow: maturation arrest | Homozygous mutation c.63G > T in JAGN1 gene; p.Glu21Asp protein alteration | Poor response to GCSF; alive |
| 15. | <1-month-old (4) | F | Pakistan | ENT infections, upper respiratory tract infections, pneumonia, sepsis (Escherichia coli), failure to thrive, coarctation of the aorta, mild developmental delay | ANC: 290/µl; bone marrow: maturation arrest, slight dyserythropoiesis | Homozygous mutation c.485A > G in JAGN1 gene; p.Gln162Arg protein alteration | HSCT; alive |
| 16. | 25-Y (4) | F | Germany | Pneumonia, bronchiectasis | ANC: 128/µl; bone marrow: maturation arrest | Homozygous mutation c.35_43delCCGACGGCA in JAGN1 gene; p.Thr12_Gly14del protein alteration | HSCT; alive |
| 17. | 9-months-old (10) | M | Turkey | Gluteal abscess, hepatomegaly, cervical lymphadenopathies, dysmorphic face, failure to thrive, developmental delay, hypospadias, left undescended testis, pneumonia, diarrhea, otitis, gingivitis, B/L bronchiectasis | ANC: 136/mm3; leukocyte count: 10,528/mm3; bone marrow: maturation arrest at the promyelocyte/myelocyte stage, reduced mature neutrophils | Homozygous mutation c.130C > T in JAGN1 gene; p.His44Tyr protein alteration | IVIG (0.5 g/kg), GCSF (5 µg/kg); alive |
| 18. | 4 months old (10) | F | Turkey | Dysmorphic face, amelogenesis imperfecta, gingival hypertrophy, short stature, recurrent skin abscesses, otitis, pneumonia, mild learning disability | ANC: 300/mm3; leukocyte count: 2,000/mm3; monocytes: 1,500; bone marrow: maturation arrest at the promyelocyte/myelocyte stage with mild nuclear dysplasia | Homozygous mutation c.130C > T in JAGN1 gene; p.His44Tyr protein alteration | IVIG, GCSF; alive |
| 19. | 2-Y (13) | M | Romania | Dysmorphic face, convergent monocular strabismus (syndrome of Stilling–Turk–Duane type 1), moderate growth, 2/6 systolic murmur, multiple B/L inguinal abscesses, recurrent respiratory infections, pneumonia, otitis, oral and genital candidiasis, recurrent skin abscesses | ANC: 2,400/mm3; CRP: 1.04 mg/dl; wound swab positive for multiresistant Pseudomonas aeruginosa and Klebsiella pneumoniae; bone marrow: absence of mature myeloid cells | Homozygous mutation c.G63T in JAGN1 gene; p.Glu21Asp protein alteration | GCSF; intravenous broad-spectrum antibiotics; oral and topical antifungals; surgical curettage |
| 20. | 2-Y (9) | M | Iraq | Umbilical infection, recurrent bronchitis, pneumonia, neutropenia, severe periodontitis, secondary diabetes mellitus, liver GVHD | ANC: < 0.5 × 109/L; bone marrow: maturation arrest of myelopoiesis; pro-LL-37 absent in plasma | Homozygous mutation c.40G > A in JAGN1 gene; p.Gly14Ser protein alteration | Recombinant GCSF |
| 21. | 6-Y (9) | M | Roma origin | Neutropenia, recurrent bacterial infections, nephrolithiasis | ANC: < 0.5 × 109/L; bone marrow: maturation defect (granulocytic series left-shifted to metamyelocytes); antigranulocytic antibodies absent | Homozygous mutation in JAGN1 gene; p.Glu21Asp protein alteration | GCSF |
| 22. | 2-Y (9) | M | Roma origin | Neutropenia, monocytosis, recurrent and severe bacterial infections, chronic gingivitis, mouth ulcers, short stature, failure to thrive, severe periodontitis, loss of teeth | ANC: 0.06 to 0.6 × 109/L; bone marrow: maturation arrest at the myelocyte stage | Homozygous mutation in JAGN1 gene; p.Glu21Asp protein alteration | GCSF (poor tolerance), antibiotic prophylaxis |
M, male; F, female; Hb, hemoglobin; JAGN1, jagunal homolog 1; ENT, ear nose throat; B/L, bilateral; HSCT, hematopoietic stem cell transplant; IVIG, intravenous immunoglobulin; GVHD, graft vs. host disease.
Our patient shared some common clinical features with cases reported in the literature, such as recurrent infections during hospital stay. To the best of our knowledge, this is the first report of recurrent intracranial hemorrhage occurring after minimal trauma among patients with JAGN1 deficiency. Our patient was subjected to a thorough evaluation for discovering possible etiologies for his bleeding diathesis. This involved an extensive evaluation for any underlying coagulopathy that was negative (Table 1) as well as platelet function assays. In addition, imaging studies did not reveal any arteriovenous malformations. Despite this thorough outpatient investigation, the etiology of bleeding diathesis remains unknown. It is possible that JAGN1, which enhances glycosylation of neutrophil proteins, has as-yet unknown effects on platelet or coagulation factor function as well.
Conclusion
In this study Here, we report that an invasive epidural abscess caused by P. aeruginosa occurred in a patient with SCN secondary to JAGN1 mutation, who initially presented with periorbital cellulitis. Given the variability seen in the clinical presentation of patients with SCN secondary to JAGN1 deficiency, a high index of suspicion for invasive pyogenic infection should be maintained in those with this immunodeficiency. This should lead to prompt initiation of treatment and prophylaxis for life-threatening infections, especially when patients with SCN secondary to JAGN1 mutation present with new focal neurologic defects, altered mental status, or have new abnormalities noted during physical examination. We also report a history of recurrent intracranial hemorrhage in our patient, which is considered unusual. Bleeding diatheses can be considered a contributor to any new symptom or finding in patients with this condition, and clinicians should consider providing anticipatory guidance and precautions to such patients, which will help achieve the goal of trauma prevention.
Acknowledgments
We would like to thank the child's family for providing their consent to publish this case report. We are also grateful to Sauradeep Sarkar, MCh., at the Department of Neurosurgery, Brigham and Women's Hospital, for his help with the diagnostic imaging presented in this case report.
Data availability statement
All relevant clinical data are included in the article. Further inquires can be directed to the corresponding author.
Ethics statement
Ethical review and approval were not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent was provided by the patient's legal guardian/next of kin for the publication of this case report.
Author contributions
ST: writing, first and corresponding author, review/proofreading. GG: clinical input, review/proofreading. JR: clinical input, review/proofreading. CP: clinical input, review/proofreading. AS: clinical input, review/proofreading. OL: clinical input, review/proofreading. LG: clinical input, review/proofreading, senior author. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Levy O. Antimicrobial proteins and peptides of blood: templates for novel antimicrobial agents. Blood. (2000) 96(8):2664–72. 10.1182/blood.V96.8.2664 [DOI] [PubMed] [Google Scholar]
- 2.Gong R-L, Wu J, Chen T-X. Clinical, laboratory, and molecular characteristics and remission status in children with severe congenital and non-congenital neutropenia. Front Pediatr. (2018) 6:305. 10.3389/fped.2018.00305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skokowa J, Dale DC, Touw IP, Zeidler C, Welte K. Severe congenital neutropenias. Nat Rev Dis Primers. (2017) 3:17032. 10.1038/nrdp.2017.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boztug K, Järvinen PM, Salzer E, Racek T, Mönch S, Garncarz W, et al. JAGN1 deficiency causes aberrant myeloid cell homeostasis and congenital neutropenia. Nat Genet. (2014) 46:1021–7. 10.1038/ng.3069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boztug K, Järvinen PM, Salzer E, Racek T, Mönch S, Garncarz W, et al. Deficiency of JAGN1 causes severe congenital neutropenia associated with defective secretory pathway and aberrant myeloid cell homeostasis. Blood. (2013) 122:439–439. 10.1182/blood.V122.21.439.439 [DOI] [Google Scholar]
- 6.Donadieu J, Beaupain B, Fenneteau O, Bellanné-Chantelot C. Congenital neutropenia in the era of genomics: classification, diagnosis, and natural history. Brit J Haematol. (2017) 179:557–74. 10.1111/bjh.14887 [DOI] [PubMed] [Google Scholar]
- 7.Wirnsberger G, Zwolanek F, Stadlmann J, Tortola L, Liu SW, Perlot T, et al. Jagunal homolog 1 is a critical regulator of neutrophil function in fungal host defense. Nat Genet. (2014) 46:1028–33. 10.1038/ng.3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khandagale A, Lazzaretto B, Carlsson G, Sundin M, Shafeeq S, Römling U, et al. JAGN1 Is required for fungal killing in neutrophil extracellular traps: implications for severe congenital neutropenia. J Leukocyte Biol. (2018) 104:1199–213. 10.1002/JLB.4A0118-030RR [DOI] [PubMed] [Google Scholar]
- 9.Khandagale A, Holmlund T, Entesarian M, Nilsson D, Kalwak K, Klaudel-Dreszler M, et al. Severe congenital neutropenia-associated JAGN1 mutations unleash a calpain-dependent cell death programme in myeloid cells. Brit J Haematol. (2021) 192:200–11. 10.1111/bjh.17137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baris S, Karakoc–Aydiner E, Ozen A, Delil K, Kiykim A, Ogulur I, et al. JAGN1 deficient severe congenital neutropenia: two cases from the same family. J Clin Immunol. (2015) 35:339–43. 10.1007/s10875-015-0156-2 [DOI] [PubMed] [Google Scholar]
- 11.Hagelkruys A, Wirnsberger G, Stadlmann J, Wöhner M, Horrer M, Vilagos B, et al. A crucial role for Jagunal homolog 1 in humoral immunity and antibody glycosylation in mice and humans. J Exp Med. (2020) 218:e20200559. 10.1084/jem.20200559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Çipe FE, Aydoğmuş Ç, Baskın K, Keskindemirci G, Garncarz W, Boztuğ K. A rare case of syndromic severe congenital neutropenia: jagn1 mutation. Turk J Pediatr. (2020) 62:326. 10.24953/turkjped.2020.02.022 [DOI] [PubMed] [Google Scholar]
- 13.Cifaldi C, Serafinelli J, Petricone D, Brigida I, Di Cesare S, Di Matteo G, et al. Next-generation sequencing reveals A JAGN1 mutation in a syndromic child with intermittent neutropenia. J Pediatr Hematol Oncol. (2018) 41:e266–9. 10.1097/MPH.0000000000001256 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant clinical data are included in the article. Further inquires can be directed to the corresponding author.