Ospemifene is an efficacious, well-tolerated, and safe therapeutic option for postmenopausal women with moderate to severe symptoms of vulvovaginal atrophy (VVA). Efficacy and safety outcomes with ospemifene are similar to other VVA therapies in North America and Europe
Key Words: Endometrium, Meta-analysis, Ospemifene, Postmenopausal, Systematic review, Vaginal atrophy
Abstract
Importance
Ospemifene is a novel selective estrogen receptor modulator developed for the treatment of moderate to severe postmenopausal vulvovaginal atrophy (VVA).
Objective
The aim of the study is to perform a systematic literature review (SLR) and network meta-analysis (NMA) to assess the efficacy and safety of ospemifene compared with other therapies used in the treatment of VVA in North America and Europe.
Evidence Review
Electronic database searches were conducted in November 2021 in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Randomized or nonrandomized controlled trials targeting postmenopausal women with moderate to severe dyspareunia and/or vaginal dryness and involving ospemifene or at least one VVA local treatment were considered. Efficacy data included changes from baseline in superficial and parabasal cells, vaginal pH, and the most bothersome symptom of vaginal dryness or dyspareunia, as required for regulatory approval. Endometrial outcomes were endometrial thickness and histologic classifications, including endometrial polyp, hyperplasia, and cancer. For efficacy and safety outcomes, a Bayesian NMA was performed. Endometrial outcomes were compared in descriptive analyses.
Findings
A total of 44 controlled trials met the eligibility criteria (N = 12,637 participants). Network meta-analysis results showed that ospemifene was not statistically different from other active therapies in most efficacy and safety results. For all treatments, including ospemifene, the posttreatment endometrial thickness values (up to 52 wk of treatment) were under the recognized clinical threshold value of 4 mm for significant risk of endometrial pathology. Specifically, for women treated with ospemifene, endometrial thickness ranged between 2.1 and 2.3 mm at baseline and 2.5 and 3.2 mm after treatment. No cases of endometrial carcinoma or hyperplasia were observed in ospemifene trials, nor polyps with atypical hyperplasia or cancer after up to 52 weeks of treatment.
Conclusions and Relevance
Ospemifene is an efficacious, well-tolerated, and safe therapeutic option for postmenopausal women with moderate to severe symptoms of VVA. Efficacy and safety outcomes with ospemifene are similar to other VVA therapies in North America and Europe.
Key points
Objective: To assess the efficacy, tolerability, and safety of ospemifene compared with other therapies currently used for the treatment of vulvovaginal atrophy (VVA).
Findings: Network meta-analysis results showed that in most efficacy and safety endpoints, ospemifene did not differ statistically compared with other active therapies. The analysis revealed that ospemifene and other treatments were not associated with clinically significant increases in endometrial thickness nor clinically relevant endometrial pathology.
Meaning: Ospemifene is an efficacious and safe therapeutic option for postmenopausal women with moderate to severe symptoms of VVA, similar to current therapies used in North America and Europe.
Genitourinary syndrome of menopause (GSM) is caused by hypoestrogenism during menopause.1 A wholly contained subgroup within GSM, vulvovaginal atrophy (VVA) describes the changes in appearance and physiological functions of the genital tissues that may cause vulvovaginal symptoms of GSM, including vaginal dryness, burning, irritation, and sexual symptoms such as lack of lubrication, discomfort, pain, and impaired sexual function.2 Vulvovaginal symptoms occur in 39% to 51% of women, with 55% to 62% of symptoms categorized as moderate or severe, the most frequent being vaginal dryness and dyspareunia (ie, pain during intercourse).3
Treatment goals include symptom relief and restoration of the vaginal environment to a healthy state.4 Nonhormone lubricants and moisturizers provide short-term relief but do not treat nor reverse the underlying condition.5 Considering the pathophysiology of VVA (ie, hypoestrogenism), hormonal therapy is common, which includes local estrogen therapy (ET) and dehydroepiandrosterone (DHEA) when systemic therapy is unnecessary.6
Ospemifene is a selective estrogen receptor modulator or estrogen agonist/antagonist indicated for treating moderate to severe dyspareunia and/or vaginal dryness.7 Unlike other selective estrogen receptor modulators (eg, tamoxifen, raloxifene, bazedoxifene), which have no reproducible estrogenic effects on the vagina, ospemifene binds to estrogen receptors in the vulvovaginal squamous epithelium resulting in activation of their estrogenic pathways.7 Ospemifene, which is approved by Health Canada, the Food and Drug Administration and the European Medicines Agency, increases superficial cells, while decreasing parabasal cells, vaginal pH, and the severity of vaginal dryness and dyspareunia, all hallmarks of an estrogenic effect.8-13 The Society of Obstetricians and Gynecologists of Canada and The North American Menopause Society guidelines recommend lubricants and moisturizers as first-line and local ET, DHEA, and ospemifene as second-line therapies.6,14 The objective of this study was to perform a systematic literature review (SLR) and network meta-analysis (NMA) to evaluate ospemifene for VVA due to menopause.
METHODS
Systematic literature review
Literature search
This SLR was conducted according to the Preferred Items for Systematic Reviews and Meta-Analyses.15 Electronic databases (Embase, MEDLINE, and PubMed) were searched in November 2021 (see Supplemental Digital Content 1, http://links.lww.com/MENO/B141). Additional publications were identified by hand searching reference lists of the retrieved publications and prior SLRs. Two reviewers (A.C. and F.D.) screened titles and abstracts and full-text articles, with discrepancies resolved by consensus or a third reviewer.
Study eligibility
Randomized or nonrandomized controlled phase II or III clinical trials of women with VVA were eligible. Outcomes included efficacy (ie, superficial and parabasal cell changes, vaginal pH, and most bothersome symptom [MBS] of vaginal dryness or dyspareunia), safety (ie, treatment-emergent adverse events [TEAE], serious TEAE, urinary tract infection [UTI], headaches, hot flashes, and discontinuation due to AEs), endometrial thickness by ultrasound and current histologic classifications.
Data extraction
Two reviewers extracted data using a predefined extraction form and a third reviewer validated all extracted data. Data extracted included baseline participants' characteristics, sample size, study designs, drugs compared and relevant efficacy, safety, and endometrial outcomes. The risk of bias (ROB) was assessed by two reviewers using the Cochrane tool.16
Network meta-analysis
The NMA combines direct and indirect comparisons, which contribute to the total evidence. It includes multiple pairwise comparisons for a range of interventions and it provides estimates of relative treatment effects.17 Fixed-effect (FE) and random-effect (RE) Bayesian NMA were performed using R (getmc version 1.0-1). The main difference between FE and RE models is that RE models assume heterogeneity between studies. Markov Chain Monte Carlo sampling with four chains, with a “burn-in” period until convergence and another 50,000 samples from the posterior distribution, was conducted to estimate treatment effects and 95% credible intervals (Crl). Model selection (FE or RE model) was based on the deviance information criterion (DIC), as per NICE technical support document for pairwise and NMA.18 More specifically, the model with the best measure of models fit (ie, lower DIC) was preferred for each outcome. Node splitting was performed to assess consistency between direct and indirect evidence. Pairwise meta-analyses were conducted using R (meta version 5.2–0). Statistical heterogeneity (I2) was interpreted according to Cochrane.16,19 Comparisons with an I2 ≥ 50% were investigated. The mean difference (MD) was reported for continuous outcomes and the risk ratio (RR) was reported for binary outcomes. Results were considered statistically significant when the 95% CrI did not cross the line of equality (MD = 0 or RR = 1).
RESULTS
Systematic literature review
Search results
A total of 636 individual phase II or III controlled trials were independently screened by two reviewers during title and abstract screening (Fig. 1). Of these, 530 records were excluded, and 106 full-text articles were reviewed to confirm their eligibility. At this stage, 66 records were excluded, leading to 40 retained studies. Main reasons for exclusion were no treatment or outcome of interest and inappropriate study designs (observational studies, cohort, and cross-sectional studies). In addition, four articles were identified through the review of reference lists, resulting in a total of 44 unique controlled trials (N = 12,637 participants) included in the SLR.5,8,10-13,20-57
FIG. 1.
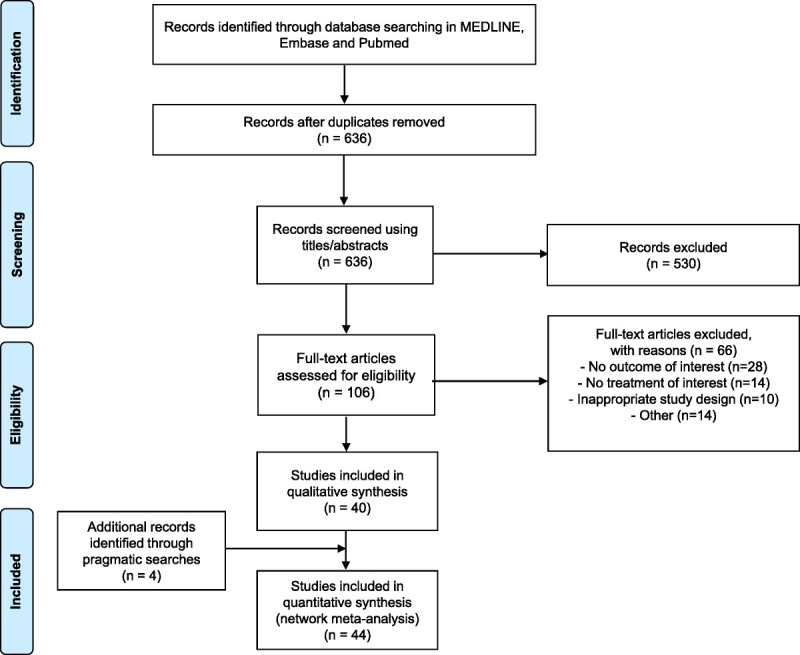
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart.
Studies characteristics
Table 1 summarizes the baseline characteristics of included studies, which were published between 1994 and 2021, with sample sizes ranging from 21 to 1,612 participants.33,53 All studies included were randomized controlled trials (RCT), except for one study that was a non-RCT.35 Durations of studies varied between 2 and 52 weeks. The mean age of the women comprising the study samples ranged from 51.4 to 62.9 years.11,49 Ospemifene was evaluated in six RCT.8-13 Remaining studies evaluated treatments such as conjugated equine estrogens (CEE) vaginal cream, E2 vaginal insert, E2 softgel vaginal insert, E2 vaginal ring, prasterone vaginal ovule (DHEA), lubricants, and/or moisturizers. Inclusion and exclusion criteria were relatively homogeneous and, hence, study populations were considered equivalent across studies. The complete inclusion and exclusion criteria of each included study are provided in Supplemental Digital Content 2, http://links.lww.com/MENO/B142. In summary, patients were postmenopausal women (hysterectomized or nonhysterectomized) with moderate to severe VVA and with symptoms of vaginal dryness and/or dyspareunia. Some studies were restricted to women with 5% or lesser superficial cells on their vaginal wall smear or a vaginal pH more than 5.0 at inclusion. As for exclusion criteria, the use of hormonal therapy (systemic or local) other than evaluated drugs during study was prohibited, with substantially similar washout periods within trials. In addition, patients having an abnormal endometrial histology other than atrophy based on baseline biopsies were excluded, as well as those having uterine bleeding of unknown origin or clinically significant abnormal gynecological finding.
TABLE 1.
Characteristics and reported outcomes of included studies
| Geographic region | Study design | Duration | Sample size, n | Age, mean | Interventions | Efficacy outcomes reported | Endometrial outcomes reported | |
|---|---|---|---|---|---|---|---|---|
| Archer56 (2015) | North America | RCT | 12 wk | 253 | 58.5 | DHEA 3.25/6.5 mg: 1 ovule daily (n = 86/n = 87) Placebo: 1 ovule daily (n = 80) |
MBS dyspareunia and vaginal dryness, parabasal and superficial cells, vaginal pH | Endometrial histology |
| Archer57 (2018) | N/A | RCT | 12 wk | 573 | 59 | E2 cream 0.015 mg: twice weekly (n = 286) Placebo cream: twice weekly (n = 287) |
MBS dyspareunia and vaginal dryness, parabasal and superficial cells, vaginal pH | — |
| Archer13 (2019) | North America | RCT | 12 wk | 627 | 59.8 | Ospemifene 60 mg: 1 tablet daily (n = 313) Placebo: 1 tablet daily (n = 314) |
MBS dyspareunia and vaginal dryness, parabasal and superficial cells, vaginal pH | Endometrial histology and endometrial thickness |
| Ayton20 (1996) | Australia | RCT | 12 wk | 194 | 59.5 | E2 ring: in situ (for 12 wk) (n = 131) CEE cream 0.625 mg/g: 21 d on/ 7 d off (n = 63) |
Vaginal pH | Endometrial histology |
| Bachmann22 (2008) | North America | RCT | 12 wk | 230 | 57.9 | E2 insert 10/25 μg: twice weekly (n = 92/n = 91) Placebo insert: twice weekly (n = 47) |
— | Vaginal pH |
| Bachmann21 (2009) | North America | RCT | 52 wk | 423 | 57.9 | CEE cream 0.3 mg/g: 21 d on/ 7 d off (n = 143) CEE cream 0.3 mg/g: twice weekly (n = 140) Placebo cream: 21 d on/ 7 d off (n = 72) Placebo cream: twice weekly (n = 68) |
MBS dyspareunia and vaginal dryness, parabasal and superficial cells, vaginal pH | Endometrial histology |
| Bachmann8 (2010) | North America | RCT | 12 wk | 826 | 58.6 | Ospemifene 30/60 mg: 1 oral tablet daily (n = 282/n = 276) Placebo: 1 oral tablet daily (n = 268) |
MBS dyspareunia and vaginal dryness, parabasal and superficial cells, vaginal pH | Endometrial thickness |
| Barentsen23 (1997) | Europe | RCT | 12 wk | 165 | 58.2 | E2 ring: in situ (for 12 wk) (n = 83) Estriol cream: three times weekly (n = 82) |
Vaginal pH | — |
| Barton24 (2018) | North America | RCT | 12 wk | 443 | 57.4 | Moisturizer: daily (n = 147) DHEA 3.25/6.5 mg: 1 ovule daily (n = 147/n = 149) |
MBS dyspareunia and vaginal dryness | — |
| Botsis25 (1997) | Europe | RCT | 24 wk | 72 | NA | CEE cream 0.625 mg/g (n = 36) Tibolone oral tablet: daily (n = 36) |
— | Endometrial thickness |
| Bouchard26 (2015) | North America | RCT | 12 wk | 441 | 58.1 | DHEA 3.25/6.5 mg: 1 ovule twice weekly (n = 143/n = 148) Placebo: 1 ovule twice weekly (n = 150) |
MBS dyspareunia and vaginal dryness, parabasal and superficial cells, vaginal pH | Endometrial histology |
| Bygdeman5 (1996) | N/A | RCT | 12 wk | 39 | 58.3 | Dionestrol cream: daily for 2 wk then 3 times weekly (n = 19) Replens gel: 3 times weekly (n = 20) |
Vaginal pH | — |
| Casper27 (1999) | Europe | RCT | 24 wk | 67 | NA | E2 ring: in situ (for 12 wk) (n = 33) Placebo ring: in situ (for 12 wk) (n = 34) |
Vaginal pH | Endometrial thickness |
| Chompootaweep28 (1998) | Asia | RCT | 8 wk | 40 | 54.5 | Levonorgestrel/ethinyl E2 tablet: weekly (n = 20) CEE cream 0.625 mg/g: twice weekly (n = 20) |
Vaginal pH | — |
| Constantine29 (2017) | North America | RCT | 12 wk | 747 | 59.0 | E2 softgel insert 4/10/25 μg: twice weekly (n = 186/n = 188/n = 186) Placebo insert: twice weekly (n = 187) |
MBS dyspareunia, parabasal and superficial cells, vaginal pH | — |
| Dugal30 (2000) | Europe | RCT | 24 wk | 96 | 58.8 | E2 insert: twice weekly (n = 48) Estriol vagitories: 0.5 mg twice weekly (n = 48) |
— | Endometrial thickness |
| Parnan Emamverdikhan45 (2016) | Asia | RCT | 12 wk | 52 | 52.5 | Vitamin E cream: twice weekly (n = 26) CE vaginal 0.625 mg/g: twice weekly (n = 26) |
Parabasal and superficial cells | — |
| Fernandes31 (2018) | South America | RCT | 12 wk | 60 | 56.8 | Testosterone cream: 3 times weekly (n = 20) CE vaginal 0.625 mg/g: 3 times weekly (n = 20) Lubricant cream: 3 times weekly (n = 20) |
— | Endometrial thickness |
| Freedman32 (2009) | North America | RCT | 12 wk | 305 | 60.0 | CEE cream 0.625 mg/g: twice weekly (n = 150) Placebo cream: twice weekly (n = 155) |
Parabasal and superficial cells and vaginal pH | — |
| Goldstein11 (2014) | Europe | RCT | 52 wk | 426 | 61.9 | Ospemifene 60 mg: 1 oral tablet daily (n = 363) Placebo: 1 oral tablet daily (63) |
Parabasal and superficial cells and vaginal pH | Endometrial histology and endometrial thickness |
| Gupta33 (2008) | North America | RCT | 12 wk | 21 | 57.1 | E2 ring: in situ (for 12 wk) (n = 11) E2 transdermal patch: continuously for 12 wk (n = 10) |
Parabasal and superficial cells and vaginal pH | — |
| Henriksson34 (1994) | Europe | RCT | 12 wk | 165 | 59.8 | E2 ring: in situ (for 12 wk) (n = 112) Estriol 0.5 mg: 1 pessary twice weekly (n = 53) |
Vaginal pH | Endometrial histology |
| Ilhan35 (2021) | Europe | Non-RCT | 12 wk | 91 | 54.1 | Sodium hyaluronate: ovule every other day (n = 31) E2 insert 10 μg: twice weekly (n = 30) Promestriene: 1 ovule every other day (n = 30) |
Vaginal pH | Endometrial thickness |
| Kroll36 (2018) | North America | RCT | 12 wk | 548 | 58.0 | E2 cream 0.015 mg: 3 times weekly (n = 277) Placebo cream: 3 times weekly (n = 271) |
MBS dyspareunia and vaginal dryness, parabasal and superficial cells, vaginal pH | — |
| Labrie37 (2009) | North America | RCT | 12 wk | 216 | 59.0 | DHEA 3.25/6.5/13 mg: 1 ovule daily (n = 53/n = 56/n = 54) Placebo: 1 ovule daily (n = 53) |
MBS dyspareunia and vaginal dryness, parabasal and superficial cells, vaginal pH | — |
| Labrie38 (2011) | North America | RCT | 12 wk | 216 | NA | DHEA 3.25/6.5/13 mg: 1 ovule daily (n = 29/n = 30/n = 29) Placebo: 1 ovule daily (n = 26) |
MBS dyspareunia, parabasal and superficial cells, vaginal pH | — |
| Labrie39 (2018) | North America | RCT | 12 wk | 482 | 59.5 | DHEA 6.5 mg: 1 ovule daily (n = 325) Placebo: 1 ovule daily (n = 157) |
MBS dyspareunia and vaginal dryness, parabasal and superficial cells, vaginal pH | — |
| Manonai41 (2001) | Asia | RCT | 12 wk | 53 | 55.4 | E2 insert 25 μg: twice weekly (n = 27) CE cream 0.625 mg/g: twice weekly (n = 26) |
— | Endometrial thickness |
| Mitchell42 (2018) | North America | RCT | 12 wk | 302 | 61.0 | E2 insert 10 μg + placebo gel: twice weekly + 1 application every 3 d (n = 102) Placebo insert + Replens gel: twice weekly + 1 application every 3 d (n = 100) Dual placebo: twice weekly + 1 application every 3 d (n = 100) |
MBS dyspareunia and vaginal dryness | — |
| Nachtigall43 (1994) | N/A | RCT | 12 wk | 30 | NA | Replens gel: 3 times per week (n = 15) Estrogens cream: 1 application daily (n- = 15) |
Vaginal pH | — |
| Palacios44 (2021) | Europe | RCT | 12 wk | 120 | 56.3 | E2 insert (n = 60) Promestriene cream (n = 60) |
Parabasal and superficial cells | — |
| Pickar46 (2016) | North America | RCT | 2 wk | 50 | 62.5 | E2 softgel insert 10 μg: daily (n = 24) Placebo insert: daily (n = 26) |
Parabasal and superficial cells and vaginal pH | — |
| Politano47 (2019) | South America | RCT | 14 wk | 72 | 57.3 | Fractional CO2: 3 sessions at 30-d intervals (n = 24) Promestriene cream: 10 mg 3 times weekly (n = 24) Lubricant: as needed with sexual activity (n = 24) |
Superficial cells and vaginal pH | — |
| Portman48 (2014) | North America | RCT | 12 wk | 314 | 59.7 | Ospemifene 60 mg: 1 oral tablet daily (n = 160) Placebo: 1 oral tablet daily (n = 154) |
MBS vaginal dryness, parabasal and superficial cells, vaginal pH | Endometrial histology and endometrial thickness |
| Portman12 (2013) | North America | RCT | 12 wk | 605 | 58.0 | Ospemifene 60 mg: 1 oral tablet daily (n = 303) Placebo: 1 oral tablet daily (n = 302) |
MBS dyspareunia, parabasal and superficial cells, vaginal pH | Endometrial histology and endometrial thickness |
| Raghunandan49 (2010) | Asia | RCT | 12 wk | 75 | 51.7 | CEE cream 0.625 mg/g: twice weekly (n = 25) CEE/testosterone cream 0.625 mg per g/1 mg: twice weekly (n = 25) Lubricant: twice weekly (n = 25) |
— | Endometrial thickness |
| Rioux50 (2018) | North America | RCT | 24 wk | 159 | 57.3 | E2 insert 25 μg: twice weekly (n = 80) CEE 0.625 mg/g: 21 d on/ 7 d off (n = 79) |
— | Endometrial histology |
| Lima40 (2013) | South America | RCT | 12 wk | 75 | 59.4 | Isoflavone gel: daily (n = 30) CEE cream 0.3 mg: daily (n = 20) Placebo gel: daily (n = 25) |
— | Endometrial thickness |
| Simon51 (2008) | North America | RCT | 52 wk | 309 | 57.6 | E2 insert 10 μg: twice weekly (n = 205) Placebo insert: twice weekly (n = 104) |
Parabasal and superficial cells | — |
| Simon52 (2010) | North America | RCT | 52 wk | 644 | 58.6 | E2 insert 10 μg: twice weekly (n = 541) Placebo insert: twice weekly (n = 103) |
— | Endometrial histology and endometrial thickness |
| Simon10 (2013) | North America | RCT | 52 wk | 180 | 58.1 | Ospemifene 30/60 mg: 1 oral tablet daily (n = 62/n = 69) Placebo: 1 oral tablet daily (n = 49) |
— | Endometrial histology and endometrial thickness |
| Simunic53 (2003) | Europe | RCT | 52 wk | 1,612 | 58.8 | E2 insert 25 μg: twice weekly (n = 828) Placebo insert: twice weekly (n = 784) |
— | Endometrial thickness |
| Suwanvesh54 (2017) | Asia | RCT | 52 wk | 82 | 56.1 |
Pueraria mirifica gel: 3 times weekly (n = 41) CEE cream 0.625 mg/g: 3 times weekly (n = 41) |
— | Endometrial thickness |
| Weisberg55 (2005) | Australia | RCT | 48 wk | 185 | 58.0 | E2 ring: in situ (n = 126) E2 insert 25 μg: twice weekly (n = 59) |
— | Endometrial histology and endometrial thickness |
CEE, conjugated equine estrogens; DHEA, dehydroepiandrosterone; E2, estradiol; MBS, most bothersome symptom; NRCT, non-randomized controlled trial; RCT, randomized controlled trials.
Risk of Bias
The methodological quality of each study was evaluated using the Cochrane ROB tool. A total of 15 of the included studies were deemed to have a high ROB for the blinding of participants and personnel. Given the different modes of administration of VVA treatments (vaginal cream, insert, ring, and oral), blinding of participants and researchers is not always possible. Indeed, participants and clinicians are frequently aware of treatment allocation in open-label studies and study extensions where individuals using placebo in a randomized portion of a trial are offered participation in the active treatment arm of the extension. Otherwise, most included trials were RCT with appropriate blinding and were classified as low ROB.
Network meta-analysis
Treatments of interest at their commercialized dosages in Canada, United States, and Europe were considered for the efficacy analyses, while all dosages were considered for the safety analyses.
Efficacy outcomes
All approved strengths and dosing regimens were combined for each included treatment, except for CEE vaginal cream, for which dosages were separated as low (twice weekly, 0.3–0.625 mg) and high doses (daily for 21 d, 7 d off, 0.3–1.25 mg), according to the product monograph. Based on the model fit statistics, the RE model was used for every outcome except the MBS score of vaginal dryness, for which the FE model was used.
Table 2 presents the relative effects of ospemifene compared with other treatments for all efficacy outcomes. For MBS score of vaginal dryness and dyspareunia, percentage of parabasal cells, and vaginal pH, a greater mean reduction was favorable for ospemifene, while a greater mean increase of the percentage of superficial cells was favorable for ospemifene. Statistically significant results are highlighted (green when in favor of ospemifene and orange when not in favor of ospemifene). Results in gray are not statistically significant and, thus, they were not interpreted.
TABLE 2.
Summary of the relative effects from the NMA results (MD with 95% CrI) of ospemifene compared with placebo and other active treatments (efficacy outcomes)
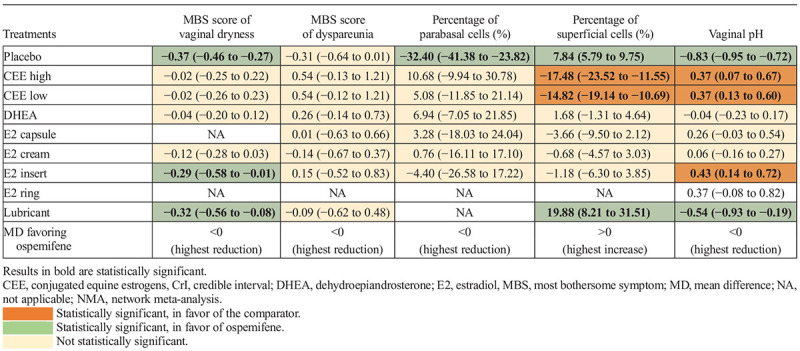
Ospemifene showed a meaningful improvement versus E2 insert 10 μg, placebo and lubricant for the MBS score of vaginal dryness, demonstrating its superiority against this treatment for this outcome. An improvement was also observed for this endpoint compared with placebo and lubricant.
For the percentage of parabasal and superficial cells, ospemifene demonstrated a statistically significant improvement versus placebo. In addition, for superficial cells, ospemifene demonstrated an improvement versus lubricant, while being less favorable compared with both CEE low and high dose. For vaginal pH, ospemifene was favorable versus placebo and lubricant, while being less favorable compared with CEE low dose, CEE high dose, and E2 insert. Other results of ospemifene compared with other treatments were not statistically significant.
Finally, for the MBS score of dyspareunia, while not statistically significant, it is worth mentioning that relative effects were in favor of ospemifene compared with placebo, to E2 cream and to lubricant. Although ospemifene was statistically significantly superior to placebo in the three clinical trials evaluated for this outcome, the choice of RE model led to a nonsignificant result versus placebo in the present NMA.8,12,13 Indeed, the relative effect using the FE model for this outcome was statistically significant for ospemifene (−0.31 [−0.41 to −0.22]). However, for the present outcome, the RE model demonstrated a better measure of model fit, characterized by a lower DIC, compared with the FE model.18
Safety outcomes
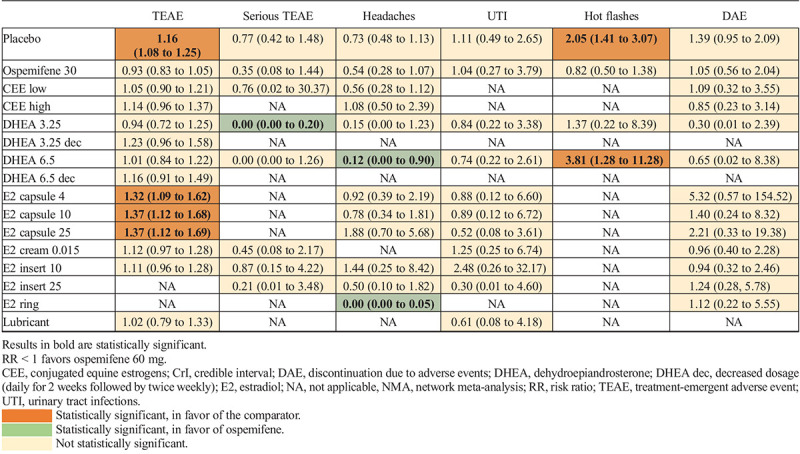
Conjugated equine estrogen was separated between low and high dose according to its product monograph.58 Doses of other comparators with multiple dosages were combined. Based on the model fit statistics, the FE model was used for every outcome except UTI, for which the RE model was used.
Table 3 presents the relative effects of ospemifene compared with other treatments for all safety outcomes. As previously explained, only statistically significant results were highlighted and are discussed. Ospemifene 60 mg demonstrated an increase in the risk of TEAE (eg, urinary tract infection and hot flashes) versus placebo, E2 capsule 4 μg, E2 capsule 10 μg, and E2 capsule 25 μg, while being associated with a decrease in the risk of serious TEAE compared with DHEA 3.25 mg. Ospemifene 60 mg showed a decrease in the risk of headaches relative to DHEA 6.5 mg and E2 vaginal ring, while being associated with an increase in the risk of hot flashes versus placebo and DHEA 6.5 mg. Other results of ospemifene 60 mg compared with other treatments were not statistically meaningful.
TABLE 3.
Summary of the relative effects for the NMA results (RR With 95% CrI) of ospemifene 60 mg compared with placebo and other active treatments (safety outcomes)
Endometrial safety outcomes
A total of 13 studies reported baseline and post-treatment endometrial histology outcomes. Of these, seven reported on endometrial thickness as well, including five studies evaluating ospemifene. Posttreatment endometrial results were measured after 12 to 52 weeks. Endometrial biopsies were read by two or three community type or expert gynecological pathologists blinded to each other's readings and to clinical data in most clinical trials. The rate of inadequate samples for histologic interpretation ranged from 8% to 47%.
Figure 2 represents the endometrial thickness reported at baseline and posttreatment in ospemifene studies (Fig. 2A) and in other treatment studies (Fig. 2B), in relation to the acceptable endometrial thickness clinical threshold of 4 mm in postmenopausal women.59 Seventeen studies reported on endometrial thickness at different time points (from baseline to week 52). Of these, there were six trials evaluating ospemifene (Fig. 2A). Baseline endometrial thickness ranged between 0.37 and 3.0 mm, while posttreatment endometrial thickness ranged between 0.46 and 3.6 mm. Specifically for ospemifene 60 mg, baseline endometrial thickness ranged between 2.1 and 2.3 mm, and from 2.5 to 3.2 mm after treatment. These results show that the endometrial thickness reported in these trials was under the clinical threshold of 4 mm at baseline and posttreatment.
FIG. 2.
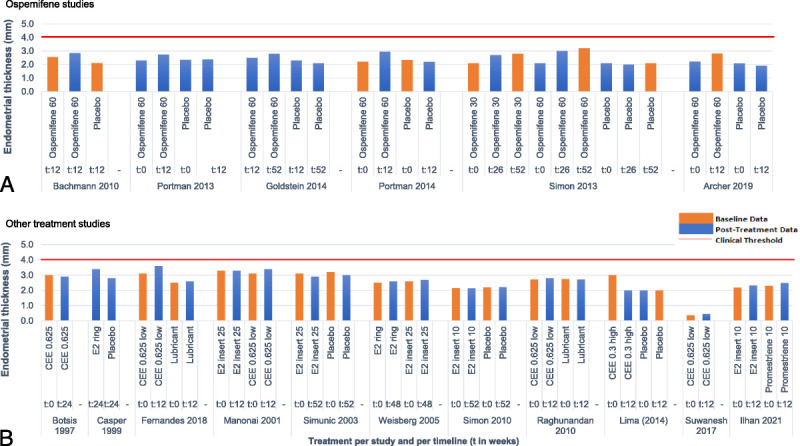
Endometrial thickness at different time points for each ospemifene studies (A) and each other treatment studies (B) in relation to the acceptable endometrial thickness clinical threshold of 4 mm (red line). “t,” time in weeks.
Seven studies reported endometrial polyp data and the percentage posttreatment ranged from 0.0% to 1.6%. For women treated with ospemifene 60 mg, the percentage of women with polyps ranged between 0.0% and 1.1% posttreatment. Specifically, the percentage of polyps was 0.3% after 52 weeks, while being of 1.1% after 12 weeks on ospemifene. No polyps with hyperplasia or carcinoma were reported.
Twelve studies reported endometrial carcinoma or hyperplasia data. The percentage of women with endometrial carcinoma or hyperplasia after treatment ranged from 0.0% to 2.5%. No mesenchymal malignancy (sarcoma) was reported. Specifically for ospemifene, one case of nonatypical (simple) endometrial hyperplasia was reported at 52 weeks in the study by Goldstein et al.11 Additional endometrial outcome results (atrophic endometrium, inactive endometrium, and proliferation) are provided in Supplemental Digital Content 3, http://links.lww.com/MENO/B143.
DISCUSSION
Ospemifene demonstrated a statistically significant improvement compared with placebo in all efficacy outcomes except the reduction in the MBS score of dyspareunia, although this result was numerically in favor of ospemifene (MD [95% CrI] vs placebo = −0.31 [−0.64 to 0.01]). Of note, for the reduction in the MBS score of dyspareunia, ospemifene was statistically significantly superior to placebo in the three pivotal studies included in the NMA for this comparison, based on which it was approved in the United States and Canada for postmenopausal dyspareunia and vaginal dryness.8,12,13 However, when using the RE model, in which a distribution of true effect sizes is assumed, the relative effect of ospemifene compared with placebo becomes not statistically significant, while being statistically significant using the FE model.
Among safety results, no result was statistically significant except for TEAE and hot flashes, favoring placebo, as expected. Compared with other active treatments, the NMA results showed that ospemifene was not statistically different compared with these therapies for most efficacy and safety outcomes. It was also observed in studies that compared ospemifene to placebo that lubricants were provided to participants to be used as needed in both groups. Despite this factor, ospemifene was superior to placebo in all pivotal trials. Vulvovaginal atrophy therapies may also differ in terms of compliance and adherence, which are likely to be better for ospemifene compared with vaginal treatments, as ospemifene is a daily oral therapy.60,61
The analysis of endometrial outcomes included endometrial thickness and histology. The endometrial thickness slightly increased with all treatments including ospemifene, without being clinically significant.59,62 Indeed, the descriptive analysis of endometrial thickness results showed that endometrial thickness ranged between 0.37 and 3.0 mm at baseline and 0.46 to 3.6 mm after treatment. For ospemifene, endometrial thickness ranged between 2.1 and 2.3 mm at baseline and from 2.5 to 3.2 mm after treatment, with maximal treatment duration being 52 weeks. These rates are below the recognized postmenopausal cut-off value of 4 mm endometrial double thickness, even after 52 weeks of treatment.59,62
Endometrial thickness data for the predominant findings included atrophic, inactive, and weak/weakly proliferative endometrium both pretreatment and posttreatment. According to the Canadian Association of Pathologists Consensus Guidelines for Endometrial Biopsy, the term “weakly proliferative endometrium” is an inactive appearing endometrium, which at high power examination contains rare gland and/or stromal cell mitoses.63
Unfortunately, what should be the lowest threshold of the number of mitoses (1 or more) is not included in the definition, nor is the obligation to examine otherwise inactive endometria at high magnification. The maximum endometrial thickness did not exceed 3.6 and 3.2 mm after treatment overall and for ospemifene, respectively. This suggests that weak proliferation, at least short term, in the participant group with “weakly proliferative endometrium,” is likely to correspond to early phases of endometrial senescence. However, postmenopausal atrophic, inactive, and presumably weakly proliferative endometria are sex hormone positive and, therefore, retain their potential to respond to estrogenic exposure. Nevertheless, according to our review, endometrial thickness remained in the range of 3 mm regardless of treatment regimens used including ET. This fact lends credence to the concept of declining rather than resurging estrogenic environment. Admittedly, long-term, longitudinal studies are needed to gain further insight into the growth potential of postmenopausal, weakly proliferative endometrium. It is comforting to observe that the estrogenic response of the postmenopausal endometrium to ospemifene is negligible as per the results of this NMA.
The percentage of women with endometrial hyperplasia with or without atypia or carcinoma posttreatment in all groups ranged between 0.0% and 2.5%: one case of simple hyperplasia without atypia (0.3%) was reported in the ospemifene group.11 As for the incidence of polyps after treatment, the percentage ranged between 0.0% and 2.9%. Specifically, for women treated with ospemifene 60 mg, the percentage with posttreatment polyps ranged from 0.0% to 1.1%. One polyp was reported after 12 weeks by Archer et al13 in a woman in the ospemifene 60-mg group. In the 52-week study by Goldstein et al,11 one woman in each treatment group (placebo and ospemifene 60 mg) had an endometrial polyp. On the other hand, no cases of polyps were reported in the studies by Bachmann et al8 and Portman et al.12,48 As for other VVA treatments, Freedman et al32 reported one participant in the placebo group who was found to have an atrophic polyp. In the study by Simon et al,51 one case of endometrial adenocarcinoma, Federation of Gynecology and Obstetrics stage II was reported in the E2 insert 10-μg group. Finally, in the 52-week study by Simon et al,52 one case of complex hyperplasia without atypia was reported in a woman exposed to E2 insert 10 μg and 5 women were found to have endometrial polyps in this treatment group. Overall, it seems that ospemifene triggers negligible endometrial stimulatory response.
The findings demonstrate that ospemifene is an efficacious, well-tolerated, and safe treatment option for postmenopausal women with moderate to severe VVA. Ospemifene significantly improved outcomes relative to placebo in terms of vaginal dryness and lowered percentage of parabasal versus superficial squamous epithelial cells and vaginal pH, although TEAE and hot flashes may be more likely to occur. However, the majority of hot flushes usually waned after 4 weeks of ospemifene treatment.64 The results are consistent with previous meta-analyses, which demonstrated superior efficacy and no major safety concerns with ospemifene compared with placebo, except for the prior NMA by Lee et al,65 which failed to find statistically significant differences in outcomes.66-68 However, the network used was different than that of the present study, as different comparators were included and, thus, different results are expected. Nevertheless, results of the study by Lee et al65 suggest that ospemifene was effective against dyspareunia, vaginal dryness, endometrial thickness, and percentage changes in superficial and parabasal cells. Real-world evidence also confirms the short- and long-term therapeutic value of ospemifene in routine clinical practice.69,70 In addition, limited experience has shown that ospemifene may also have positive effects on bone health.71
The strength of this study is that it was a systematic review and synthesis of the highest-quality evidence (ie, mostly RCT). The eligibility criteria and population characteristics were similar between studies, limiting the presence of both methodological and clinical heterogeneity. The results harvested are consistent with the vast majority of the previously published meta-analyses on the topic and include additional, up-to-date data from a recently completed trial.19 The outcomes evaluated in the present NMA include clinically important and patient-reported measures, highlighting its relevance to informing real-world treatment decisions. The limitations of this study include the fact that a limited number of databases were searched, although databases used are among those with the most optimal literature coverage.72 The lack of trials directly comparing ospemifene to other active (ie, nonplacebo) treatments and some active treatment comparisons were not even measurable for a number of outcomes. Thus, direct evidence comparing ospemifene and other active treatment would be needed to confirm the findings of this NMA. Information on both endometrial thickness and histology was available in only 7 of 44 studies evaluated and lacked direct comparisons between these two parameters. In addition, the rate of inadequate endometrial samples varied between 8% and 47% in the present experience but is consistent with previous reports.73 The fact that endometrial thickness in cases reported as “active” proliferative endometrium did not exceed 3.6 mm either pretreatment or posttreatment is difficult to reconcile with “active proliferation” because such endometria contain numerous gland and stromal cell mitoses resulting in endometrial thickness well over 4 mm. Additional head-to-head trials with expert histologic ascertainment are needed before making any definitive conclusions on the comparative safety of ospemifene relative to other VVA treatments. High-quality studies evaluating the long-term (ie, ≥12 mo) value of ospemifene are also required. Finally, all studies were conducted in well-selected population of women that is more representative of candidates for ospemifene, while ensuring the safety of participants and, thus, generalizability of the findings to other populations could be limited.
CONCLUSIONS
The present NMA provides confirmatory evidence to previous data that ospemifene is efficacious, well-tolerated, and a safe treatment option for postmenopausal women with moderate to severe VVA. Ospemifene is statistically superior to placebo for most efficacy parameters and does not seem to be statistically different from other active treatments. To establish more definitive conclusions on the comparative effects between the various treatments available for this population, direct head-to-head and long-term studies comparing ospemifene against other active VVA therapies are required.
Supplementary Material
Acknowledgments
We thank Fatema Dodat, employee at PeriPharm, Inc, at the time of the study, for participating in the systematic literature review search, study selection, and data extraction.
Footnotes
Funding/support: This study was funded by Duchesnay, Inc.
Financial disclosure/conflicts of interest: C.B. is a partner at PeriPharm, Inc, and A.C. and C.R. are employees at PeriPharm, Inc, a company that has served as a consultant to Duchesnay, Inc, and has received funding from Duchesnay, Inc. A.F. and D.B. have served as consultants for Duchesnay, Inc. D.B. currently receives funding from AbbVie, Bayer, BioSyent, Duchesnay, Merck, Organon, Pfizer, and Searchlight. R.M. is an employee at Duchesnay, Inc. J.A.S. receives grant/research support from the following: AbbVie, Bayer Healthcare, Dare Bioscience, Enteris BioPharma, Mylan/Viatris, Myovant Sciences, ObsEva, and Viveve Medical; is on consulting/advisory boards of the following: Bayer Healthcare, Besins Healthcare, California Institute of Integral Studies (CIIS), Dare Bioscience, DEKA M.E.L.A S.r.l., Duchesnay, Inc, Femasys, KaNDy/NeRRe Therapeutics, Khyria, Madorra, Mitsubishi Tanabe Pharma Development America, QUE Oncology, Scynexis, Inc, Sprout Pharmaceuticals, Vella Bioscience; serves on the speaker's bureaus of the following: Mayne Pharma, Myovant Sciences, Pfizer, Pharmavite, Scynexis, and TherapeuticsMD; and is a stockholder (direct purchase) in Sermonix Pharmaceuticals.
This study was presented as a poster (P-78) at the Annual Meeting of The North American Menopause Society; October 12–15, 2022; Atlanta, GA.
Supplemental digital content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s Website (www.menopause.org).
Contributor Information
James A. Simon, Email: jsimon@intimmedicine.com.
Alex Ferenczy, Email: alex.ferenczy@mcgill.ca.
Denise Black, Email: drdsblack@gmail.com.
Catherine Royer, Email: catherine.royer@peripharm.com.
Rafik Marouf, Email: rmarouf@duchesnay.com.
Catherine Beauchemin, Email: catherine.beauchemin@peripharm.com.
REFERENCES
- 1.Angelou K, Grigoriadis T, Diakosavvas M, Zacharakis D, Athanasiou S. The genitourinary syndrome of menopause: an overview of the recent data. Cureus 2020;12:e7586. doi: 10.7759/cureus.7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shifren JL. Genitourinary syndrome of menopause. Clin Obstet Gynecol 2018;61:508–516. doi: 10.1097/GRF.0000000000000380 [DOI] [PubMed] [Google Scholar]
- 3.Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women's VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med 2013;10:1790–1799. doi: 10.1111/jsm.12190 [DOI] [PubMed] [Google Scholar]
- 4.Naumova I, Castelo-Branco C. Current treatment options for postmenopausal vaginal atrophy. Int J Womens Health 2018;10:387–395. doi: 10.2147/IJWH.S158913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas 1996;23:259–263. doi: 10.1016/0378-5122(95)00955-8 [DOI] [PubMed] [Google Scholar]
- 6.Johnston S, Bouchard C, Fortier M, Wolfman W. Guideline no. 422b: menopause and genitourinary health. J Obstet Gynaecol Can 2021;43:1301–1307.e1. doi: 10.1016/j.jogc.2021.09.001 [DOI] [PubMed] [Google Scholar]
- 7.Duchesnay, Inc . OSPHENA (ospemifene), tablets, 60 mg, oral (Selective Estrogen Receptor Modulator (SERM)) - Product monograph including patient medication information. 2021.
- 8.Bachmann GA Komi JO, Ospemifene Study Group . Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause 2010;17:480–486. doi: 10.1097/gme.0b013e3181c1ac01 [DOI] [PubMed] [Google Scholar]
- 9.Simon J Portman D Mabey RG Jr., Ospemifene Study Group . Long-term safety of ospemifene (52-week extension) in the treatment of vulvar and vaginal atrophy in hysterectomized postmenopausal women. Maturitas 2014;77:274–281. doi: 10.1016/j.maturitas.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 10.Simon JA Lin VH Radovich C Bachmann GA, Ospemifene Study Group . One-year long-term safety extension study of ospemifene for the treatment of vulvar and vaginal atrophy in postmenopausal women with a uterus. Menopause 2013;20:418–427. doi: 10.1097/gme.0b013e31826d36ba [DOI] [PubMed] [Google Scholar]
- 11.Goldstein SR Bachmann GA Koninckx PR, et al. Ospemifene 12-month safety and efficacy in postmenopausal women with vulvar and vaginal atrophy. Climacteric 2014;17:173–182. doi: 10.3109/13697137.2013.834493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Portman DJ Bachmann GA Simon JA, Ospemifene Study Group . Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause 2013;20:623–630. doi: 10.1097/gme.0b013e318279ba64 [DOI] [PubMed] [Google Scholar]
- 13.Archer DF Goldstein SR Simon JA, et al. Efficacy and safety of ospemifene in postmenopausal women with moderate-to-severe vaginal dryness: a phase 3, randomized, double-blind, placebo-controlled, multicenter trial. Menopause 2019;26:611–621. doi: 10.1097/GME.0000000000001292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The NAMS 2020 GSM Position Statement Editorial Panel . The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause 2020;27:976–992. doi: 10.1097/GME.0000000000001609 [DOI] [PubMed] [Google Scholar]
- 15.Moher D Liberati A Tetzlaff J Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT Altman DG Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jansen JP Fleurence R Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health 2011;14:417–428. [DOI] [PubMed] [Google Scholar]
- 18.Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 2: A Generalised Linear Modelling Framework for Pairwise and Network Meta-Analysis of Randomised Controlled Trials. London: National Institute for Health and Care Excellence (NICE); 2014. [PubMed]
- 19.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 20.Ayton RA Darling GM Murkies AL, et al. A comparative study of safety and efficacy of continuous low dose oestradiol released from a vaginal ring compared with conjugated equine oestrogen vaginal cream in the treatment of postmenopausal urogenital atrophy. Br J Obstet Gynaecol 1996;103:351–358. doi: 10.1111/j.1471-0528.1996.tb09741.x [DOI] [PubMed] [Google Scholar]
- 21.Bachmann G Bouchard C Hoppe D, et al. Efficacy and safety of low-dose regimens of conjugated estrogens cream administered vaginally. Menopause 2009;16:719–727. doi: 10.1097/gme.0b013e3181a48c4e [DOI] [PubMed] [Google Scholar]
- 22.Bachmann G, Lobo RA, Gut R, Nachtigall L, Notelovitz M. Efficacy of low-dose estradiol vaginal tablets in the treatment of atrophic vaginitis: a randomized controlled trial. Obstet Gynecol 2008;111:67–76. doi: 10.1097/01.AOG.0000296714.12226.0f [DOI] [PubMed] [Google Scholar]
- 23.Barentsen R, van de Weijer PH, Schram JH. Continuous low dose estradiol released from a vaginal ring versus estriol vaginal cream for urogenital atrophy. Eur J Obstet Gynecol Reprod Biol 1997;71:73–80. doi: 10.1016/s0301-2115(96)02612-7 [DOI] [PubMed] [Google Scholar]
- 24.Barton DL Sloan JA Shuster LT, et al. Evaluating the efficacy of vaginal dehydroepiandosterone for vaginal symptoms in postmenopausal cancer survivors: NCCTG N10C1 (Alliance). Support Care Cancer 2018;26:643–650. doi: 10.1007/s00520-017-3878-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Botsis D, Kassanos D, Kalogirou D, Antoniou G, Vitoratos N, Karakitsos P. Vaginal ultrasound of the endometrium in postmenopausal women with symptoms of urogenital atrophy on low-dose estrogen or tibolone treatment: a comparison. Maturitas 1997;26:57–62. doi: 10.1016/s0378-5122(96)01070-5 [DOI] [PubMed] [Google Scholar]
- 26.Bouchard C Labrie F Archer DF, et al. Decreased efficacy of twice-weekly intravaginal dehydroepiandrosterone on vulvovaginal atrophy. Climacteric 2015;18:590–607. doi: 10.3109/13697137.2014.992012 [DOI] [PubMed] [Google Scholar]
- 27.Casper F, Petri E. Local treatment of urogenital atrophy with an estradiol-releasing vaginal ring: a comparative and a placebo-controlled multicenter study. Vaginal Ring Study Group. Int Urogynecol J Pelvic Floor Dysfunct 1999;10:171–176. doi: 10.1007/s001920050040 [DOI] [PubMed] [Google Scholar]
- 28.Chompootaweep S, Nunthapisud P, Trivijitsilp P, Sentrakul P, Dusitsin N. The use of two estrogen preparations (a combined contraceptive pill versus conjugated estrogen cream) intravaginally to treat urogenital symptoms in postmenopausal Thai women: a comparative study. Clin Pharmacol Ther 1998;64:204–210. doi: 10.1016/S0009-9236(98)90154-0 [DOI] [PubMed] [Google Scholar]
- 29.Constantine GD Simon JA Pickar JH, et al. The REJOICE trial: a phase 3 randomized, controlled trial evaluating the safety and efficacy of a novel vaginal estradiol soft-gel capsule for symptomatic vulvar and vaginal atrophy. Menopause 2017;24:409–416. doi: 10.1097/GME.0000000000000786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dugal R, Hesla K, Sordal T, Aase KH, Lilleeidet O, Wickstrom E. Comparison of usefulness of estradiol vaginal tablets and estriol vagitories for treatment of vaginal atrophy. Acta Obstet Gynecol Scand 2000;79:293–297. [PubMed] [Google Scholar]
- 31.Fernandes T, Pedro AO, Baccaro LF, Costa-Paiva LH. Hormonal, metabolic, and endometrial safety of testosterone vaginal cream versus estrogens for the treatment of vulvovaginal atrophy in postmenopausal women: a randomized, placebo-controlled study. Menopause 2018;25:641–647. doi: 10.1097/GME.0000000000001059 [DOI] [PubMed] [Google Scholar]
- 32.Freedman M, Kaunitz AM, Reape KZ, Hait H, Shu H. Twice-weekly synthetic conjugated estrogens vaginal cream for the treatment of vaginal atrophy. Menopause 2009;16:735–741. doi: 10.1097/gme.0b013e318199e734 [DOI] [PubMed] [Google Scholar]
- 33.Gupta P, Ozel B, Stanczyk FZ, Felix JC, Mishell DR, Jr. The effect of transdermal and vaginal estrogen therapy on markers of postmenopausal estrogen status. Menopause 2008;15:94–97. doi: 10.1097/gme.0b013e318148b98b [DOI] [PubMed] [Google Scholar]
- 34.Henriksson L, Stjernquist M, Boquist L, Alander U, Selinus I. A comparative multicenter study of the effects of continuous low-dose estradiol released from a new vaginal ring versus estriol vaginal pessaries in postmenopausal women with symptoms and signs of urogenital atrophy. Am J Obstet Gynecol 1994;171:624–632. doi: 10.1016/0002-9378(94)90074-4 [DOI] [PubMed] [Google Scholar]
- 35.Ilhan G, Aslan MM, Cevrioglu AS, Yildirim M, Erkorkmaz U. Clinical efficacy of hormonal and nonhormonal agents in the treatment of vulvovaginal atrophy. J Menopausal Med 2021;27:15–23. doi: 10.6118/jmm.20026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kroll R, Archer DF, Lin Y, Sniukiene V, Liu JH. A randomized, multicenter, double-blind study to evaluate the safety and efficacy of estradiol vaginal cream 0.003% in postmenopausal women with dyspareunia as the most bothersome symptom. Menopause 2018;25:133–138. doi: 10.1097/GME.0000000000000985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Labrie F Archer D Bouchard C, et al. Intravaginal dehydroepiandrosterone (Prasterone), a physiological and highly efficient treatment of vaginal atrophy. Menopause 2009;16:907–922. doi: 10.1097/gme.0b013e31819e8e2d [DOI] [PubMed] [Google Scholar]
- 38.Labrie F Archer DF Bouchard C, et al. Intravaginal dehydroepiandrosterone (prasterone), a highly efficient treatment of dyspareunia. Climacteric 2011;14:282–288. doi: 10.3109/13697137.2010.535226 [DOI] [PubMed] [Google Scholar]
- 39.Labrie F Archer DF Koltun W, et al. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause 2018;25:1339–1353. doi: 10.1097/GME.0000000000001238 [DOI] [PubMed] [Google Scholar]
- 40.Lima SM, Yamada SS, Reis BF, Postigo S, Galvao da Silva MA, Aoki T. Effective treatment of vaginal atrophy with isoflavone vaginal gel. Maturitas 2013;74:252–258. doi: 10.1016/j.maturitas.2012.11.012 [DOI] [PubMed] [Google Scholar]
- 41.Manonai J, Theppisai U, Suthutvoravut S, Udomsubpayakul U, Chittacharoen A. The effect of estradiol vaginal tablet and conjugated estrogen cream on urogenital symptoms in postmenopausal women: a comparative study. J Obstet Gynaecol Res 2001;27:255–260. doi: 10.1111/j.1447-0756.2001.tb01266.x [DOI] [PubMed] [Google Scholar]
- 42.Mitchell CM Reed SD Diem S, et al. Efficacy of vaginal estradiol or vaginal moisturizer vs placebo for treating postmenopausal vulvovaginal symptoms: a randomized clinical trial. JAMA Intern Med 2018;178:681–690. doi: 10.1001/jamainternmed.2018.0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nachtigall LE. Comparative study: replens versus local estrogen in menopausal women. Fertil Steril 1994;61:178–180. doi: 10.1016/s0015-0282(16)56474-7 [DOI] [PubMed] [Google Scholar]
- 44.Palacios S, Ramirez M, Lilue M. Efficacy of low-dose vaginal 17β-estradiol versus vaginal promestriene for vulvovaginal atrophy. Climacteric 2021;25:383–387. doi: 10.1080/13697137.2021.1998436 [DOI] [PubMed] [Google Scholar]
- 45.Parnan Emamverdikhan A, Golmakani N, Tabassi SA, Hassanzadeh M, Sharifi N, Shakeri MT. A survey of the therapeutic effects of vitamin E suppositories on vaginal atrophy in postmenopausal women. Iran J Nurs Midwifery Res 2016;21:475–481. doi: 10.4103/1735-9066.193393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pickar JH, Amadio JM, Hill JM, Bernick BA, Mirkin S. A randomized, double-blind, placebo-controlled phase 2 pilot trial evaluating a novel, vaginal softgel capsule containing solubilized estradiol. Menopause 2016;23:506–510. doi: 10.1097/GME.0000000000000592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Politano CA, Costa-Paiva L, Aguiar LB, Machado HC, Baccaro LF. Fractional CO2 laser versus promestriene and lubricant in genitourinary syndrome of menopause: a randomized clinical trial. Menopause 2019;26:833–840. doi: 10.1097/GME.0000000000001333 [DOI] [PubMed] [Google Scholar]
- 48.Portman D, Palacios S, Nappi RE, Mueck AO. Ospemifene, a non-oestrogen selective oestrogen receptor modulator for the treatment of vaginal dryness associated with postmenopausal vulvar and vaginal atrophy: a randomised, placebo-controlled, phase III trial. Maturitas 2014;78:91–98. doi: 10.1016/j.maturitas.2014.02.015 [DOI] [PubMed] [Google Scholar]
- 49.Raghunandan C, Agrawal S, Dubey P, Choudhury M, Jain A. A comparative study of the effects of local estrogen with or without local testosterone on vulvovaginal and sexual dysfunction in postmenopausal women. J Sex Med 2010;7:1284–1290. doi: 10.1111/j.1743-6109.2009.01667.x [DOI] [PubMed] [Google Scholar]
- 50.Rioux JE, Devlin MC, Gelfand MM, Steinberg WM, Hepburn DS. 17β-Eestradiol vaginal tablet versus conjugated equine estrogen vaginal cream to relieve menopausal atrophic vaginitis. Menopause 2018;25:1208–1213. doi: 10.1097/GME.0000000000001220 [DOI] [PubMed] [Google Scholar]
- 51.Simon J, Nachtigall L, Gut R, Lang E, Archer DF, Utian W. Effective treatment of vaginal atrophy with an ultra-low-dose estradiol vaginal tablet. Obstet Gynecol 2008;112:1053–1060. doi: 10.1097/AOG.0b013e31818aa7c3 [DOI] [PubMed] [Google Scholar]
- 52.Simon J, Nachtigall L, Ulrich LG, Eugster-Hausmann M, Gut R. Endometrial safety of ultra-low-dose estradiol vaginal tablets. Obstet Gynecol 2010;116:876–883. doi: 10.1097/AOG.0b013e3181f386bb [DOI] [PubMed] [Google Scholar]
- 53.Simunic V, Banovic I, Ciglar S, Jeren L, Pavicic Baldani D, Sprem M. Local estrogen treatment in patients with urogenital symptoms. Int J Gynaecol Obstet 2003;82:187–197. doi: 10.1016/s0020-7292(03)00200-5 [DOI] [PubMed] [Google Scholar]
- 54.Suwanvesh N, Manonai J, Sophonsritsuk A, Cherdshewasart W. Comparison of Pueraria mirifica gel and conjugated equine estrogen cream effects on vaginal health in postmenopausal women. Menopause 2017;24:210–215. doi: 10.1097/GME.0000000000000742 [DOI] [PubMed] [Google Scholar]
- 55.Weisberg E Ayton R Darling G, et al. Endometrial and vaginal effects of low-dose estradiol delivered by vaginal ring or vaginal tablet. Climacteric 2005;8:83–92. doi: 10.1080/13697130500087016 [DOI] [PubMed] [Google Scholar]
- 56.Archer DF Labrie F Bouchard C, et al. Treatment of pain at sexual activity (dyspareunia) with intravaginal dehydroepiandrosterone (prasterone). Menopause 2015;22:950–963. doi: 10.1097/GME.0000000000000428 [DOI] [PubMed] [Google Scholar]
- 57.Archer DF, Kimble TD, Lin FDY, Battucci S, Sniukiene V, Liu JH. A randomized, multicenter, double-blind, study to evaluate the safety and efficacy of estradiol vaginal cream 0.003% in postmenopausal women with vaginal dryness as the most bothersome symptom. J Womens Health (Larchmt) 2018;27:231–237. doi: 10.1089/jwh.2017.6515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pfizer Canada, Inc . Complete Prescribing Information - Pr Premarin® Vaginal Cream (conjugated estrogens CSD, 0.625 mg/g) — Estrogenic Hormones. 2018. Available at: https://www.pfizer.ca/files/Premarin-VC_PM_E_12-May-2020_OSIP_N.pdf. Accessed June 8, 2023.
- 59.ACOG committee opinion no. 734: the role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol 2018;131:e124–e129. doi: 10.1097/AOG.0000000000002631 [DOI] [PubMed] [Google Scholar]
- 60.Faught BM Soulban G Yeaw J, et al. Ospemifene versus local estrogen: adherence and costs in postmenopausal dyspareunia. J Comp Eff Res 2019;8:1111–1123. doi: 10.2217/cer-2019-0091 [DOI] [PubMed] [Google Scholar]
- 61.Cagnacci A, Xholli A, Venier M. Ospemifene in the management of vulvar and vaginal atrophy: focus on the assessment of patient acceptability and ease of use. Patient Prefer Adherence 2020;14:55–62. doi: 10.2147/PPA.S203614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sahdev A. Imaging the endometrium in postmenopausal bleeding. BMJ 2007;334:635–636. doi: 10.1136/bmj.39126.628924.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parra-Herran C Cesari M Djordjevic B, et al. Canadian consensus-based and evidence-based guidelines for benign endometrial pathology reporting in biopsy material. Int J Gynecol Pathol 2019;38:119–127. doi: 10.1097/PGP.0000000000000481 [DOI] [PubMed] [Google Scholar]
- 64.Constantine GD, Archer DF, Pollycove R, Jiang W, Altomare C, Pinkerton JV. Ospemifene's effect on vasomotor symptoms: a post hoc analysis of phase 2 and 3 clinical data. Menopause 2016;23:957–964. doi: 10.1097/GME.0000000000000656 [DOI] [PubMed] [Google Scholar]
- 65.Lee A Kim TH Lee HH, et al. Therapeutic approaches to atrophic vaginitis in postmenopausal women: a systematic review with a network meta-analysis of randomized controlled trials. J Menopausal Med 2018;24:1–10. doi: 10.6118/jmm.2018.24.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cui Y, Zong H, Yan H, Li N, Zhang Y. The efficacy and safety of ospemifene in treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy: a systematic review and meta-analysis. J Sex Med 2014;11:487–497. doi: 10.1111/jsm.12377 [DOI] [PubMed] [Google Scholar]
- 67.Di Donato V Schiavi MC Iacobelli V, et al. Ospemifene for the treatment of vulvar and vaginal atrophy: a meta-analysis of randomized trials. Part I: evaluation of efficacy. Maturitas 2019;121:86–92. doi: 10.1016/j.maturitas.2018.11.016 [DOI] [PubMed] [Google Scholar]
- 68.Di Donato V Schiavi MC Iacobelli V, et al. Ospemifene for the treatment of vulvar and vaginal atrophy: a meta-analysis of randomized trials. Part II: evaluation of tolerability and safety. Maturitas 2019;121:93–100. doi: 10.1016/j.maturitas.2018.11.017 [DOI] [PubMed] [Google Scholar]
- 69.Pingarrón C, Lafuente P, Poyo Torcal S, López Verdú H, Martínez García MS, Palacios S. Vaginal health, endometrial thickness and changes in bone markers in postmenopausal women after 6 months of treatment with ospemifene in real clinical practice. Gynecol Endocrinol 2022;38:78–82. doi: 10.1080/09513590.2021.1970740 [DOI] [PubMed] [Google Scholar]
- 70.Pingarrón Santofímia C Lafuente González P Guitiérrez Vélez MDC, et al. Long-term use of ospemifene in clinical practice for vulvo-vaginal atrophy: end results at 12 months of follow-up. Gynecol Endocrinol 2022;38:577–582. doi: 10.1080/09513590.2022.2083103 [DOI] [PubMed] [Google Scholar]
- 71.de Villiers TJ, Altomare C, Particco M, Gambacciani M. Effects of ospemifene on bone in postmenopausal women. Climacteric 2019;22:442–447. doi: 10.1080/13697137.2019.1631789 [DOI] [PubMed] [Google Scholar]
- 72.Bramer WM, Rethlefsen ML, Kleijnen J, Franco OH. Optimal database combinations for literature searches in systematic reviews: a prospective exploratory study. Syst Rev 2017;6:245. doi: 10.1186/s13643-017-0644-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dermawan JKT Hur C Uberti MG, et al. Thickened endometrium in postmenopausal women with an initial biopsy of limited, benign, surface endometrium: clinical outcome and subsequent pathologic diagnosis. Int J Gynecol Pathol 2019;38:310–317. doi: 10.1097/PGP.0000000000000525 [DOI] [PubMed] [Google Scholar]


