Background:
Various treatment options have been introduced for the management of primary tumors of the brachial plexus (BP), ranging from conservative therapy to wide local excision with/without postoperative chemoradiotherapy. However, no consensus exists regarding optimal treatment strategies based on collated and published data.
Objective:
The aim of this study was to investigate the clinicopathological characteristics and outcome of patients with primary tumors of the BP who underwent surgical treatment.
Data sources:
A systematic search of the four main online databases, including Web of Science (WOS), PubMed, Scopus, and Google Scholar, was conducted.
Study selection:
All related articles addressing the clinical outcome and role of surgical interventions for management of primary tumors of the BP.
Intervention:
Optimal surgical and radiotherapeutic interventions for benign and malignant lesions based on the pathologic characteristics and location of primary BP tumors.
Results:
A total of 687 patients (693 tumors) with a mean age of 41.7±8.7 years old were evaluated. In total, 629 (90.8%) tumors were benign, and 64 (9.2%) were malignant, with a mean tumor size of 5.4±3.1 cm. The location of the tumor was reported for 639 patients. For these tumors, 444 (69.5%) originated from the supraclavicular region, and 195 (30.5%) were infraclavicular. The trunks were the most common location for tumor involvement, followed by the roots, cords, and terminal branches. Gross total resection was achieved in 432 patients and subtotal resection (STR) was performed in 109 patients. With neurofibromas, STR still resulted in good outcomes. The outcomes following treatment of malignant peripheral nerve sheath tumors were poor regardless of the type of resection. In general, symptoms related to pain and sensory issues resolved rapidly postoperatively. However, the resolution of motor deficits was often incomplete. Local tumor recurrence occurred in 15 (2.2%), patients and distant metastasis was observed in only eight (1.2%) cases. The overall mortality was 21 (3.1%) patients among the study population.
Limitations:
The main limitation was the lack of level I and II evidence.
Conclusions:
The ideal management strategy for primary BP tumors is complete surgical resection. However, in some cases, particularly for neurofibromas, STR may be preferable to preserve maximal neurological function. The degree of surgical excision (total or subtotal) mainly depends on the pathological characteristics and primary location of the tumor.
Keywords: brachial plexus, malignant peripheral nerve sheath tumor, nerve tumor, neurofibroma, schwannoma
Introduction
Highlights
The degree of tumor resection depends on the pathological characteristics, location, and extent of the tumor.
Schwannomas have a strong predilection for the trunks and roots in the supraclavicular brachial plexus.
Gross total resection is favored for schwannomas. However, subtotal resection for neurofibromas also results in good outcomes.
Surgery is effective at treating pain and sensory deficits. However, recovery of motor deficits is often incomplete after surgery.
Primary tumors of the brachial plexus (BP) are rare, challenging-to-treat entities that mainly originate from the neuroectodermal cells. These constitute nearly 5% of all upper extremity tumors, causing pain, and disability in affected patients1. BP tumors vary widely depending on their anatomical origin, pathological characteristics, and extension to the adjacent tissues. They are classified as benign or malignant, and arising from neural or non-neuronal structures. Benign nerve sheath tumors (BNSTs) (constituting ~80% of all primary nerve tumors) include schwannomas and neurofibromas (NFs)2. Schwannomas are benign, well-encapsulated tumors arising from Schwann cells, with occasional cystic degeneration or hemorrhage. Characteristically, they occur within the endoneurium and are encapsulated by the fibrous perineurium3. NFs in contrast, originate from neural sheath perineurial cells and present as multiple nonencapsulated tumors3.
The association of nerve sheath tumors with type 1 neurofibromatosis (NF-1) has been well investigated in the literature. They are strongly associated with autosomal dominant NF-1 and the majority of NF-1-associated NFs occur throughout the first to second decades of life2,4,5. Similar to NF, patients with a background of NF-1 have a higher than expected chance of progression to malignant peripheral nerve sheath tumors (MPNSTs), especially at younger ages6,7. These comprise only 3% of all soft tissue sarcomas. Malignant PNSTs are also thought to be associated with a history of irradiation and may occur within 10–15 years following exposure8,9. The clinical presentation of BP tumors can differ depending on their location and size. Most often, they present with a painful palpable mass, with or without neurologic deficits. This is the most common symptom in patients with BP tumors10. As the tumor progresses, painful paresthesia at the distribution of the affected nerve occurs. Ultimately, progressive loss of motor function may occur11.
One of the potential difficulties concerning optimal management of BP tumors relates to the risk of preoperative biopsy due to the proximity of the lesions to or involvement of adjacent nerves, which can lead to neurological deficits12. Thus, the pathologic characteristics of the tumor are not commonly available prior to surgery. In addition, there are numerous variations in BP anatomy, which may increase the risk of intraoperative complications1. In the existing literature, various treatment strategies have been introduced for the management of BP tumors, ranging from conservative therapy to wide local excision with/without postoperative chemoradiotherapy. However, each of these surgical interventions has been the subject of significant debate. A paper by Jia et al.13 reported that for some patients with malignant tumors, an extended resection was not possible due to extension into the intervertebral foramen, hence a subtotal resection (STR) was performed instead13. In another study, Soltani et al. 14 showed satisfactory outcomes in some patients with supraclavicular (SC) schwannomas who underwent a nonoperative treatment option. Therefore, conservative treatment strategies remain an alternative for some asymptomatic patients with benign tumors to avoid the potential morbidity associated with excision.
However, there is a discrepancy about the optimal treatment strategies for benign and malignant lesions based on the pathologic characteristics and location of the BP tumors. Also, no consensus has been established to describe the optimal operative approach for tumors of the BP.
Hence, in the current study, a systematic review of BP tumors was performed to determine whether the location of the primary BP tumors differs based on the type of tumor; evaluate the optimal surgical interventions according to the pathologic characteristics of the tumor; describe overall patient outcomes, survival rates, and possible prognostic factors; and identify the impact of radiotherapeutic interventions on the patient’s final outcome.
Methods
Search strategy
The literature search was conducted by the two reviewers using the four main online databases, including Web of Science (WOS), PubMed, Scopus, and Google Scholar, with the aim of assessing all related articles addressing the clinical outcome and role of surgical interventions for the management of primary tumors of the BP. All articles from 2000 until July 2022 were included in the study. The study was conducted in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses), Supplemental Digital Content 1, http://links.lww.com/JS9/A120 and AMSTAR 2 guidelines15,16), Supplemental Digital Content 2, http://links.lww.com/JS9/A121. The reviewers conducted the search using the following MeSH terms for this review, as well as all possible similar combinations: ‘Brachial Plexus’, and ‘Neoplasm’.
Initially, two independent reviewers conducted a title and abstract screening using the following MeSH terms, as well as all possible similar combinations of ‘Brachial Plexus’, and ‘Neoplasm’. This included [brachial plexus disease, brachial plexus diseases, brachial plexus disorder, brachial plexus disorders, brachial plexus neuritis, brachial plexus neuritides, brachial plexus neuropathy, brachial plexus neuralgia, brachial plexus palsy, brachial plexus paresis, and brachial plexus plexopathy] AND [tumor, cancer, cancers, mass, neoplasm, neoplasms, neoplasia, malignancy, malignancies, malignant neoplasms, benign neoplasms, benign neoplasm, schwannoma, neurofibroma, benign nerve sheath, malignant nerve sheath, and sarcoma]. In order to find any additional studies, the reviewers used a reference list of relevant articles. Eventually, a full-text screening of the remaining articles was conducted by the reviewers based on the inclusion and exclusion criteria.
The inclusion criteria for study selection were human studies of all age groups describing the clinical outcomes and optimal management of primary BP neoplasms (regardless of the tumor type), which were published in the English literature. Additionally, manuscripts containing nonprimary neoplasms (metastatic), cadaveric, nonhuman studies, and studies without quantitative data (including case reports and technical notes) were excluded. Furthermore, the study protocol was registered in Prospero with a Unique Identification Number (UIN) of CRD42022347024 (Link).
Data abstraction
The reviewers evaluated study quality and methodology in order to meet the inclusion criteria. For risk of bias (ROB) assessment, authors used the National Institutes of Health ROB Tool for case-series studies17. Data including the baseline study characteristics, descriptive and clinical data such as preoperative and postoperative symptoms, pathologic characteristics of the tumors, types of therapeutic interventions, and final outcomes of participants were extracted and recorded in Excel 2019. Where the data distributions were unavailable, means of ranges were reported.
Statistical analysis
A quantitative synthesis (meta-analysis) was not performed due to high levels of methodological heterogeneity among the included study. Thus, the study findings were summarized descriptively and presented as means and SD. Also, all data reporting as median were converted to mean and SD using the formula by Hozo et al.18.
Results
Study characteristics
The initial search yielded a total of 2748 articles. After excluding 329 duplicates, a systematic screening process evaluating the titles and abstracts of 2419 articles was initiated. During this phase, 2190 entries that did not meet the inclusion criteria, were excluded. A full-text review was conducted on the remaining 229 articles, and an additional 203 articles were also excluded. The reasons for exclusion are provided in the Prisma flow diagram of literature search and study selection (Fig. 1), Supplemental Digital Content 3, http://links.lww.com/JS9/A122. Out of the remaining 26 studies, four articles from the Louisiana State University Health Sciences Center were excluded due to cohort duplication19–21 or a lack of qualitative data22. Ultimately, a total of 22 articles were included in the qualitative synthesis of this systematic review1–3,12–14,23–38.
Figure 1.
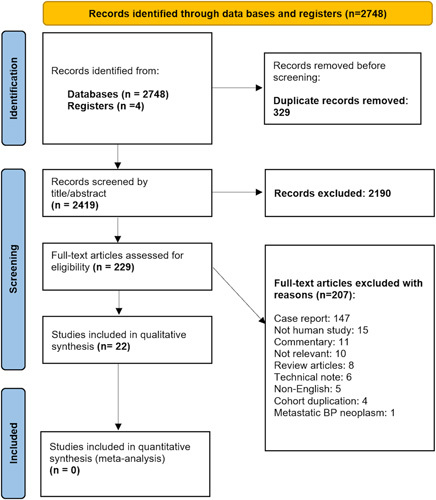
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart of included studies.
Patient and tumor characteristics
The overall features of the included studies are shown in Table 1. A total of 687 patients and 693 tumors were evaluated; the mean age of the participants was 41.7±8.7 years (range: 1–92 years). Information on gender was available for 645 patients, of whom 298 (46.2%) were male and 347 (53.8%) female. Of the 687 patients analyzed, 79 (11.5%) had a history of neurofibromatosis, and only one (0.1%) had a history of schwannomatosis. The most common clinical symptoms were swelling (447, 65.1%), pain (320, 46.6%), sensory (327, 47.6%), and motor deficits (129, 18.8%). The mean duration of symptoms before medical intervention was 25.2±12.3 months. The symptomatic presentations of patients are summarized in Table 1.
Table 1.
Preoperative symptoms, therapeutic interventions, and clinical outcome of included studies.
| Preoperative symptoms, n (%) | Surgical intervention, n (%) | ||||||
|---|---|---|---|---|---|---|---|
| References | Swelling | Pain | Sensory deficit | Motor weakness | GTR | STR | Adjuvant/neoadjuvant RT |
| Maiuri et al.29 | 4 (100.0) | 2 (50.0) | –– | –– | 4 (100) | –– | No |
| Ganju et al.26 | 55 (514) | 65 (60.7) | 21 (19.6) | 35 (32.7) | 77 (71.9) | 34 (31.7) | No |
| Huang et al.1 | 13 (29.5) | 31 (70.4) | 27 (61.3) | 23 (52.3) | NA | NA | NA |
| Binder et al.23 | 15 (60.0) | 15 (60.0) | 11 (44.0) | 3 (12.0) | 16 (64.0) | 9 (36.0) | No |
| Rawal et al.34 | 3 (100.0) | 2 (66.6) | 1 (33.3) | 1 (33.3) | 3 (100.0) | –– | One patient received adjuvant RT |
| Seinfeld et al.35 | 4 (100.0) | 3 (75.0) | 1 (25.0) | 1 (25.0) | 4 (100.0) | –– | Three patients underwent adjuvant RT (54 Gy in 30 fractions) |
| Ranalli et al. 33 | 3 (60.0) | 5 (100.0) | 4 (80.0) | 1 (20.0) | 4 (80.0) | 1 (20.0) | No |
| Siqueira et al.36 | 15 (83.3) | 12 (66.6) | 5 (27.7) | 6 (33.3) | 14 (77.7) | 4 (22.2) | No |
| Desai et al. 3 | 102 (88.6) | 115 (100.0) | 115 (100.0) | 14 (12.1) | 74 (64.3) | 41 (35.6) | No |
| Tubbs et al.37 | 4 (100.0) | 1 (25.0) | – | 1 (25.0) | 3 (75.0) | 1 (25.0) | No |
| Go et al.2 | 20 (95.2) | 11 (52.4) | 12 (57.1) | 9 (42.8) | 16 (72.7) | 6 (27.3) | No |
| Soltani et al.14 | 10 (76.9) | 10 (76.9) | 7 (53.8) | 3 (23.1) | 10 (76.9) | – | One of the nonoperative patients underwent adjuvant RT |
| Lee et al.28 | 19 (100.0) | 2 (10.6) | 10 (52.6) | 1 (5.3) | 19 (100.0) | – | No |
| Millan et al.30 | 2 (40.0) | 1 (20.0) | 2 (40.0) | 2 (40.0) | 5 (100.0) | – | No |
| Jia et al.13 | 129 (90.1) | – | 68 (47.5) | 12 (8.3) | 128 (89.5) | 10 (6.9) | Seven patients underwent adjuvant RT |
| Graf et al. (2017) | 6 (54.5) | 3 (27.2) | 3 (27.2) | 2 (18.2) | – | – | No |
| Jung et al. 27 | 17 (94.4) | 9 (50.0) | 8 (44.4) | 4 (22.2) | 17 (94.4) | 1 (5.6) | No |
| Bourque et al.24 | – | 1 (33.3) | 1 (33.3) | 1 (33.3) | – | – | Two patients (40 Gy in 15 fractions) |
| Yuce et al.38 | NA | 5 (45.4) | 10 (90.1) | 6 (54.5) | 11 (100.0) | – | No |
| Pressney et al. 32 | NA | NA | NA | NA | NA | NA | NA |
| Dubuisson et al.12 | 12 (70.5) | 15 (88.2) | 10 (58.8) | – | 14 (82.3) | 1 (5.9) | No |
| Gaba et al.25 | 14 (100.0) | 12 (85.7) | 11 (78.5) | 4 (25.6) | 13 (92.8) | 1 (7.2) | No |
| Total (22 articles) | 447 (65.1) | 320 (46.6) | 327 (47.6) | 129 (18.8) | 432 (79.9) | 109 (20.1) | 14 (2.0) |
GTR, gross total resection; NA, not applicable; RT, radiotherapy; STR, subtotal resection.
Information on tumor type was available for all 687 patients. Of the 693 tumors, 629 (90.8%) tumors were benign and 64 (9.2%) were malignant, with a mean tumor size of 5.4 3.1 cm. Of all tumors treated, benign tumors included 421 (60.8%) schwannomas, 126 (18.2%) NFs, 17 (2.5%) desmoid tumors, and 14 (2.0%) lipomas, as well as smaller numbers of ganglioneuromas, cystic hygroma, hemangiomas, neurogenic cysts, and aneurysmal bone cysts. Malignant lesions included 48 (6.9%) malignant nerve sheath tumors, as well as 4 (0.6%) malignant granular cell tumors, three lymphomas (0.4%), two (0.3%) synovial sarcomas, and smaller numbers of peripheral primitive neuroectodermal tumor, papillary carcinomas, and other sarcomas of the BP (Table 2).
Table 2.
Study characteristics, type of tumors (benign/malignant), and risk of bias in included articles.
| Tumor type, n (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| References | Study design | Number of patients (female; male) | Mean age (years) | Diagnostic work-upa | Sch | NF | MPNST | Lipoma | Desmoid tumor | Others | ROB assessment |
| Maiuri et al., 20011 | Case-series | 4 (2;2) | 24.0 | US/MRI/CT | 4 (100) | – | – | – | – | – | Good (7/9) |
| Ganju et al., 20012 | Case-series | 107 (55;52) | 41.06 | CT/MRI/EMG | 35 (32.7) | 31 (28.9) | 12 (11.2) | 1 (0.9) | 6 (5.6) | 26 (23.4) | Good (9/9) |
| Huang et al., 20043 | Case-series | 42 (NA) | 43.4 | MRI | 11 (25.0) | 9 (20.4) | 4 (9.1) | – | 4 (9.1) | 14 (33.3) | Fair (6/9) |
| Binder et al., 20044 | Case-series | 25 (17;8) | 47.0 | MRI | 15 (60.0) | 5 (20.0) | 4 (16.0) | – | 1 (4.0) | – | Good (8/9) |
| Rawal et al., 20055 | Case-series | 3 (1; 2) | 35 | MRI/CT | – | – | 3 (100.0) | – | – | – | Good (8/9) |
| Seinfeld et al., 20066 | Case-series | 4 (3; 1) | 49.25 | NA | – | – | – | – | 4 (100.0) | – | Fair (6/9) |
| Ranalli et al., 20087 | Case-series | 5 (5; 0) | 36.8 | Radiography/MRI/CT/EMG | – | – | – | – | – | 5 (100.0) | Good (9/9) |
| Siqueira et al.; 20098 | Case-series | 18 (11;7) | 31.11 | 7 (38.8) | 1 (5.5) | 1 (5.5) | – | 2 (11.1) | 7 (38.9) | Fair (6/9) | |
| Desai et al., 20119 | Case-series | 115 (78;37) | 29.0 | Contrast-enhanced MRI/biopsy | 70 (60.8) | 45 (39.1) | – | – | – | – | Good (9/9) |
| Tubbs et al., 201110 | Case-series | 4 (2; 2) | 6 | MRI | – | – | – | – | – | 4 (100.0) | Good (9/9) |
| Go et al., 201211 | Case-series | 21 (10;11) | 39.0 | CT/MRI/biopsy | 15 (68.2) | 4 (18.2) | 1 (4.5) | – | – | 2 (9.1) | Fair (6/9) |
| Soltani et al., 201312 | Case-series | 13 (6;7) | 52.5 | US/MRI | 12 (92.3) | 1(7.7) | – | – | – | – | Good (8/9) |
| Lee et al., 201413 | Case-series | 19 (11; 8) | 50.2 | MRI/EMG | 19 (100.0) | – | – | – | – | – | Good (9/9) |
| Millan et al., 201514 | Case-series | 5 (1;4) | 40.9 | CT/MRI | 4 (80.0) | 1 (20.0) | – | – | – | – | Good (9/9) |
| Jia et al., 201615 | Case-series | 143 (65;78) | 48.17 | US/MRI/CT/biopsy | 119 (83.2) | 12 (8.4) | 8 (5.6) | – | – | 4 (2.8) | Good (8/9) |
| Graf et al., 201716 | Case-series | 11 (4;7) | 49.0 | MRI | – | – | – | 11 (100.0) | – | – | Good (8/9) |
| Jung et al., 201717 | Case-series | 18 (10;8) | 51.3 | MRI | 11 (61.2) | 4 (22.2) | 1 (5.6) | – | – | 2 (11.1) | Good (9/9) |
| Bourque et al., 201839 | Case-series | 3 (1; 2) | 61.3 | MR neurography | – | – | – | – | – | 3 (100.0) | Good (8/9) |
| Yuce et al., 201919 | Case-series | 11 (7; 4) | 45 | US/MRI/biopsy | 11 (100.0) | – | – | – | – | – | Good (9/9) |
| Pressney et al., 202020 | Case-series | 85 (38;47) | 46.7 | MRI | 66 (77.6) | 7 (8.2) | 12 (14.2) | – | – | NA | Fair (6/9) |
| Dubuisson et al., 202121 | Case-series | 17 (10;7) | 44.4 | MRI/PET scan | 14 (82.3) | 3 (17.7) | – | – | – | – | Good (9/9) |
| Gaba et al., 202122 | Case-series | 14 (10;4) | 37.8 | CT/MRI/FNA | 8 (53.3) | 3 (20.0) | 2 (13.3) | 2 (13.3) | – | – | Good (9/9) |
| Total (22 articles) | 687 (347; 298) | 41.7±8.7 | 421 (60.8) | 126 (18.2) | 48 (6.9) | 14 (2.0) | 17 (2.5) | 67 (9.6) | |||
CT indicates computerized tomography scan; EMG, electromyography; FNA, fine needle aspiration; MPNST, malignant peripheral nerve sheet tumor; NF, neurofibroma; PET, positron emission tomography; ROB, risk of bias; Sch, schwannoma.
This column represents the initial preoperative modality that was used for planning of the operation and final diagnosis.
A considerable discrepancy was noted among the included studies when reporting the anatomical location of the tumors. Table 3 demonstrates the locations of BP tumors according to the included studies. Information on the tumor location the of tumor was available for 639 patients. The majority of the lesions (444, 69.5%) originated from the SC region, and 195 (30.5%) of the lesions were infraclavicular (IC).
Table 3.
Locations of brachial plexus tumors (n=639) according to included studies.
| Supraclavicular, 444 (69.5%) | Infraclavicular, 195 (30.5%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Roots (n=202) | Trunks (n=210) | Cords (n=124) | |||||||||||
| References | C4 | C5 | C6 | C7 | C8 | T1 | Upper | Middle | Lower | Lateral | Medial | Posterior | Terminal branches (n=17) |
| Maiuri et al. 29 | 4 (100.0) | – | – | – | – | ||||||||
| Ganju et al. 26 | – | 9 (8.4) | 13 (12.1) | 13 (12.1) | 10 (9.3) | 11 (10.3) | 18 (16.8) | 8 (7.5) | 12 (11.2) | 6 (5.6) | 16 (14.9) | 10 (9.3) | – |
| Huang et al. 1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Binder et al. 23 | – | 3 (12.0) | 2 (8.0) | 1 (4.0) | 1 (4.0) | 1 (4.0) | 9 (36.0) | 2 (8.0) | 4 (16.0) | – | – | – | 3 (12.0) |
| Rawal et al. 34 | – | – | – | 1 (33.30) | – | – | – | – | – | – | – | 1 (33.3) | 1 (33.3) |
| Seinfeld et al. 35 | 1 (25.0) | – | – | – | – | – | 2 (50.0) | – | – | – | – | – | – |
| Ranalli et al. 33 | – | – | – | 1 (20.0) | 1 (20.0) | 1 (20.0) | 1 (20.0) | 1 (20.0) | 1 (20.0) | – | – | – | 1 (20.0) |
| Siqueira et al.36 | 14 (66.6) | 7 (33.3) | |||||||||||
| Desai et al. 3 | – | 14 (12.2) | 9 (7.8) | 21 (18.3) | – | – | 28 (24.3) | 13 (11.3) | – | 6 (5.2) | 27 (23.4) | 11 (9.5) | – |
| Tubbs et al.37 | – | – | – | – | – | – | 3 (75.0) | – | – | – | – | – | 1 (25.0) |
| Go et al. 2 | 19 (73.1) | 7 (26.9) | |||||||||||
| Soltani et al. 14 | 1 (7.7) | 1 (7.7) | 1 (7.7) | 5 (38.4) | 1 (7.7) | 1 (7.7) | – | – | – | 1 (7.7) | 1 (7.7) | 2 (1.5) | – |
| Lee et al. 28 | 1 (5.3) | 2 (10.6) | 1 (5.3) | 1 (5.3) | 1 (5.3) | 2 (10.6) | 5 (26.3) | 2 (10.6) | – | 2 (10.6) | – | 5 (26.3) | |
| Millan et al. 30 | – | – | – | – | – | – | – | – | – | – | – | 1 (20.0) | 4 (80.0) |
| Jia et al.13 | 47 (32.9) | 68 (47.5) | 22 (15.4) | ||||||||||
| Graf et al. (2017) | – | – | – | – | – | – | 6 (54.5) | – | 1 (9.1) | 1 (9.1) | – | 3 (27.3 | – |
| Jung et al. 27 | – | 1 (5.5) | 1 (5.5) | 2 (11.1) | 1 (5.5) | 1 (5.5) | 5 (27.7) | 4 (22.2) | 2 (11.1) | 1 (5.5) | 1 (5.5) | 2 (11.1) | – |
| Bourque et al. 24 | – | – | – | – | 1 (33.3) | – | – | – | – | – | – | – | 2 (66.6) |
| Yuce et al. 38 | 8 (72.7) | 3 (27.3) | |||||||||||
| Pressney et al. 32 | 69 (81.2) | 16 (18.8) | |||||||||||
| Dubuisson et al. 12 | – | 2 (11.7) | 2 (11.7) | 2 (11.7) | 1 (5.8) | 1 (5.8) | 2 (11.7) | – | 3 (17.6) | 2 (11.7) | – | 2 (11.7) | – |
| Gaba et al. 25 | – | 5 (35.7) | 3 (21.4) | 1 (7.1) | 1 (7.1) | 1 (7.1) | 3 (21.4) | 3 (21.4) | 2 (14.2) | – | 6 (42.8) | – | – |
| Total | 2 (0.5) | 36 (8.7) | 33 (7.9) | 48 (11.5) | 18 (4.3) | 18 (4.3) | 79 (19.0) | 36 (8.7) | 27 (6.5) | 17 (4.1) | 53 (12.7) | 32 (7.7) | 17 (4.1) |
NA, not applicable.
Information was available for some patients (n=553) regarding the specific location of the tumor within the SC and IC BP. The trunks were the most common location for tumor involvement, with 210 (37.9%) cases, followed by 202 (36.5%) involving the nerve roots, 124 (22.4%) tumors involving the cords, and 17 (3.1%) involving the terminal branches.
For tumors with trunk involvement for which further detailed information was available (n=142) (Table 3), the upper trunk (79, 55.6%) was the most common location followed by the middle (36, 25.4%) and lower trunks (27, 19.0%). For patients with nerve root involvement for whom detailed information was available (n=155) (Table 3), most patients had tumors affecting the C7 nerve root (48, 31.0%), followed by the C5 (36, 23.2%) and C6 (33, 21.3%) roots. For patients with cord involvement for whom further detailed information was available (n=102) (Table 3), the medial cord (53, 52.0%) was most involved, followed by the posterior (32, 31.4%) and lateral (17, 16.7%) cords. Our review showed that terminal branches of the BP were less involved in BP tumors. Out of the 17 cases with tumors affecting the terminal branches, eight (47.1%) patients had involvement of the median nerve, five (29.4%) involved the ulnar nerve, and four (23.5%) involved the radial nerve.
The location of the tumor was further classified according to tumor type where information was available. We found that 89 (76.7%) of NFs occurred in the SC BP, and only 27 (23.3%) of NFs were of IC origin (Fig. 2). A lesser predilection for SC BP was found for schwannomas. Accordingly, 125 (59.5%) of BP schwannomas originated from the SC region, and 85 (40.5%) of these tumors were IC in location (Fig. 2).
Figure 2.
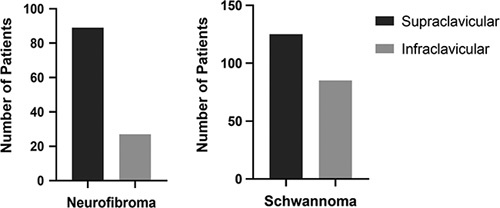
Anatomical distribution of neurofibromas and schwannomas involving the brachial plexus.
Surgical and chemoradiotherapeutic interventions
Information regarding the surgical intervention was available for 541 patients (Table 1). Gross total resection (GTR) was performed for the majority of the cases (432, 79.9%) and STR was performed in 109 (20.1%) of patients. The type of resection was further analyzed by tumor type. Accordingly, for patients with schwannomas (n=344), GTR was successfully achieved in 341 (99.1%) and only three patients (0.9%) underwent STR. In contrast, for patients with NFs (n=109), GTR was performed for only 38 (34.9%) and 71 (65.1%) underwent STR (Fig. 3). Out of the 48 patients with the diagnosis of MPNSTs data about the surgical interventions was available for 32 of them. Accordingly, 16 (50.0%) patients with MPNST underwent extended surgical excision with wide margins, and 16 (50.0%) underwent STR.
Figure 3.
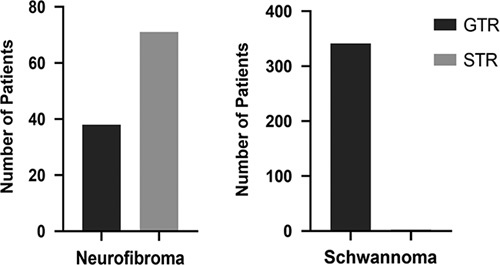
Type of surgical interventions (total/subtotal) for neurofibromas and schwannomas. GTR, gross total resection; STR, subtotal resection.
For malignant tumors, a total of 14 (2.0%) patients underwent adjuvant radiotherapy (RT) following the surgery. In the study by Seinfeld et al. 35 three cases of aggressive desmoid tumors underwent adjuvant radiation therapy (receiving a total of 54 Gy in 30 fractions). In addition, one of these patients underwent tamoxifen hormone therapy after her surgery. In the study by Jia et al. 13 postoperative RT was administered to five patients with MPNST, one with synovial sarcoma, and one with pPNET. Both adjuvant and neoadjuvant chemotherapy were also applied for the patient with pPNET. No studies were available to compare the role of adjuvant RT with neoadjuvant radiation and to evaluate their roles in improving the outcome of patients with plexal tumors.
Postoperative clinical outcome, tumor recurrence, and distant metastasis
A descriptive report of patient outcomes following surgical intervention is available in Table 4. Accordingly, the mean duration of follow-up was 31.1 months. Out of the 320 patients who presented initially with pain (Table 1), 43 (13.4%) patients complained of remaining symptoms following the surgery (Table 4). Also, 295 (90.2%) of the patients with sensory deficits were symptom-free at the last follow-up. However, motor weakness remained for 79 (61.2%) of patients following the surgical interventions (Fig. 4). Reviewing long-term outcomes (n=687), local tumor recurrence occurred in 15 (2.2%) patients, and distant metastasis was observed in only eight (1.2%) cases. The overall mortality was 21 (3.1%) patients among the study population.
Table 4.
Postoperative symptoms, complications, and clinical outcome in included studies.
| Symptoms after interventions, n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| References | Pain | Sensory deficits | Motor weakness | Complications | Mortality, n (%) | Recurrence | Metastasis | Follow-up duration (mean) |
| Maiuri et al. 29 | – | – | – | Transient deltoid muscle weakness | None | None | None | 7 years |
| Ganju et al. 26 | 16 (14.9) | NA | 31 (28.9) | Major vessel injury, pneumothorax | 10 (9.3) | Yes | NA | 38.2 months |
| Huang et al. 1 | 15 (35.7) | NA | 17 (40.5) | – | NA | NA | NA | NA |
| Binder et al. 23 | – | – | – | Intraoperative avulsion of an artery | None | None | None | NA |
| Rawal et al. 34 | 1 (33.3) | 1 (33.3) | 2 (66.6) | NA | 2 (66.6) | Yes | Yes | 2 years |
| Seinfeld et al. 35 | 1 (25.0) | 2 (50.0) | – | Hemidiaphragmatic paralysis | None | Yes | None | NA |
| Ranalli et al.33 | 1 (20.0) | – | – | – | None | None | None | 2 months |
| Siqueira et al. 36 | – | 2 (11.1) | 3 (16.6) | Iatrogenic injury to the C5 root and hematoma formation | 3 (16.6) | Yes | Yes | 24 months |
| Desai et al. 3 | 6 (5.2) | 6 (5.2) | 2 (1.7) | Premature loosening of the clavicle plate | None | None | None | 31 months |
| Tubbs et al. 37 | – | – | – | – | None | None | None | 3.2 years |
| Go et al. 2 | 2 (9.5) | 2 (9.5) | 4 (19.1) | – | None | None | None | 13.7 months |
| Soltani et al. 14 | 1 (7.6) | 4 (30.7) | 2 (15.4) | – | None | None | None | 7.4 months |
| Lee et al. 28 | – | – | 1 (5.2) | – | None | None | None | None |
| Millan et al. 30 | – | 2 (40.0) | 1 (20.0) | – | None | None | None | 24 months |
| Jia et al. 13 | NA | 6 (4.2) | 6 (4.2) | NA | 6 (4.2) | Yes | Yes | 36 months |
| Graf et al. (2017) | – | 1 (9.1) | 1 (9.1) | – | None | None | None | NA |
| Jung et al. 1 | – | 1 (5.5) | 1 (5.5) | – | None | Yes | None | 19.8 months |
| Bourque et al. 24 | – | 1 (33.3) | 3 (100.0) | NA | None | None | None | NA |
| Yuce et al. 38 | – | – | – | – | None | None | None | 7.9 months |
| Pressney et al.32 | – | – | – | – | NA | NA | NA | NA |
| Dubuisson et al. 12 | – | 4 (23.5) | 4 (23.5) | Fibroinflammatory reaction of the scalene muscles | None | None | None | 5.3 years |
| Gaba et al. 25 | – | – | 1 (7.1) | Horner’s syndrome | None | None | Yes | 24 months |
| Total (22 articles) | 43 (6.3) | 32 (4.7) | 79 (11.5) | 21 (3.1) | 15 (2.1) | 8 (1.1) | 31.1 months | |
NA, not applicable.
Figure 4.
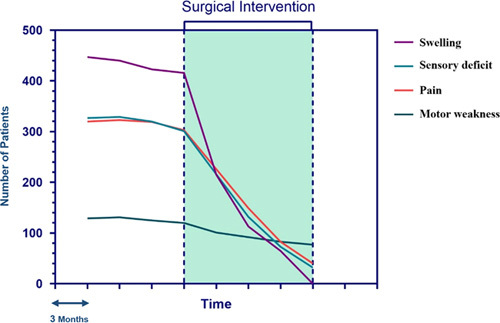
Changes in clinical symptoms of patients over time following surgical intervention.
Risk of bias assessment
The National Institutes of Health Quality Assessment Tool for Case-Series was used for the evaluation of the ROB for all the included studies17. Accordingly, out of the 22 articles included, 17 had ‘good’ quality, and the other five managed to achieve a ‘fair’ level of quality. A summary of ROB evaluations is available in Supplementary Table 1, Supplemental Digital Content 4, http://links.lww.com/JS9/A123.
Discussion
The majority of evidence for management of BP tumors is sourced from single institutional case-series, and to date there is not good, collated data with information on patient and tumor characteristics as well as efficacy of different surgical interventions for primary BP tumors. To address this, we conducted a systematic review to investigate the clinicopathological characteristics and outcomes of patients with primary tumors of BP undergoing operative management.
The clinical presentations of BP tumors vary widely depending on the location and pathologic characteristics of the tumor. Our data show that BNSTs of the BP occur more commonly in the SC plexus than the IC plexus. This trend was observed particularly among patients with NF, with the predilection for a SC location seen in all of the included studies. For schwannomas, the same propensity was observed to a weaker extent.
The most common initial clinical symptom of BP tumors was found to be a palpable SC mass followed by pain, sensory deficits, and motor weaknesses. Notably, the postsurgical symptoms mainly depend on the pathological characteristics of the tumor rather than the type of surgery performed. Our data indicated that BNSTs, especially schwannomas, were associated with significantly fewer remaining symptoms following surgical interventions. This correlates with the pathology of schwannomas, which tend to be encapsulated and separate from the adjacent nerves. In addition, the nerve fascicles are usually displaced to the periphery by schwannomas, with a few nonfunctioning nerve fascicles entering and exiting the tumor; these can be easily sacrificed without any major neurological deficits40. Therefore, complete resection (GTR) is achievable in most patients, which leads to better symptom improvement and a lower rate of recurrence following the surgery.
In line with our statements, Jia et al. 13 reported that GTR was successfully achieved in all 119 patients with schwannoma of the BP without any reported recurrences. Also, the study by Desai et al. 3 reported the same results for all 70 patients with schwannoma of the BP, in which GTR was successfully introduced. To the best of our knowledge, there were only three cases of schwannoma in the existing literature where GTR was not achieved, in which one of the patients had schwannomatosis with multiple tumors involving both the IC and SC regions12. Thus, GTR with preservation of all surrounding neural structures is optimal for schwannomas of the BP.
An opposite trend could potentially be observed for NFs. NFs are more infiltrative compared with schwannomas41,42. Unlike schwannomas, the NF tumor margins are often not discrete from the nerve fascicles. Hence, STR might be considered a better option to preserve nerve integrity and neurological function compared to GTR, which may not be achievable without significant postoperative neurological deficits. Although GTR is not feasible in some cases of NF, existing literature suggests that there is no difference in terms of recurrence and overall survival between the two. For instance, in the study of Jia et al. 13, 83.3% of patients with NF underwent STR with satisfactory outcomes. Another case-series by Desai et al. 3 reported that out of 45 patients with NF, only four patients were able to undergo GTR. Since NFs are rarely associated with tumor recurrence, STR is accompanied by favorable clinical outcomes. It should be highlighted that in patients with NF-1, surgical treatment of benign BP tumors was only performed in cases with a rapidly growing mass causing severe neurological dysfunction.
MPNST, on the other hand, is associated with an extremely poor outcome. Most MPNSTs tend to grow rapidly, leading to sudden loss of both motor and sensory function43. Accordingly, the overall estimated 5-year survival rate ranges from 15 to 50%44. Major poor prognostic factors for patient survival include the history of NF-1, larger tumor sizes (>5 cm), and the degree of tumor infiltration involving adjacent tissues9,45. It is speculated that mutations in the NF-1 tumor suppressor gene result in a lack of neurofibromin synthesis, which ultimately increases the risk of malignant degeneration of BNSTs, particularly plexiform NFs7.
There are controversies regarding the optimal management of MPNST in the existing literature. Surgical excision with wide margins is considered the ideal therapeutic intervention. However, total resection is not possible in some cases of MPNST due to the involvement of surrounding structures and would lead to unacceptable neurological deficits if attempted. In all cases managed surgically, postoperative neurological deficits were relatively common. This systematic review suggests that complete resection with wide margins should be the first approach for treatment of MPNSTs. However, the degree of resection depends upon the tumor location and involvement of surrounding structures. In cases of incomplete resection (STR), adjuvant RT could be beneficial to reduce the rate of local or distant recurrence and improve the clinical symptoms of patients9,44,46.
The role of neoadjuvant RT is not fully understood in the literature. It can be used to reduce the size of tumors prior to surgical intervention. However, RT may lead to severe tissue inflammation and fibrosis. Accordingly, operating on a surgical field in the BP where previous radiation therapy has been administered is very challenging. Similarly, the role of adjuvant RT for the management of MPNSTs has been a subject of significant debate. Numerous studies have suggested the use of postsurgical RT to be beneficial in overall patient survival, especially when clear surgical margins were not achievable47–49. In contrast, many studies showed the opposite, that RT has a negligible role for local control or survival50–53. One of the most robust studies reported by Stucky et al.50 concluded that postoperation RT should only be administered for patients with highly aggressive MPNST and a surgical margin status of R1 or R2. Notably, due to the retrospective nature of these studies, an inherent selection bias should be taken into consideration. The majority of patients selected for RT in the context of MPNST had locally aggressive tumors with positive margins after the surgery. Thus, it is possible that the results of these studies were biased toward not showing satisfactory outcomes following radiation therapy. Lastly, MPNSTs are generally classified as chemotherapy-resistant tumors and their use remains optional. However, a few studies have reported favorable outcomes following chemotherapy. Kroep et al.54 observed that postoperative doxorubicin–ifosfamide combination therapy was associated with better outcomes in patients with advanced MPNST. To the best of our knowledge, no studies have evaluated the safety and efficacy of different chemotherapeutic regimens in the management of primary MPNST of BP, and hence its role remains unclear.
The role of preoperative biopsy is not fully established in the literature. Generally, preoperative biopsy is not indicated for benign or malignant BP tumors. Even when a malignancy is suspected, percutaneous or needle biopsy is not a risk-free procedure and may lead to further neurological deficits. However, imaging studies, for example MRI, provide a good provisional diagnosis for treatment planning and prognostication.
In terms of surgical treatment, tumor size and, most importantly, the primary location of the tumor should be noted for determining the optimal approach. The anterior SC surgical approach is the most common method of surgical resection for BP tumors13. This approach allows easy access to the roots, trunks, and divisions (with the aid of clavicle retraction or osteotomy). The main complications associated with the anterior approach are unilateral phrenic nerve injury, new-onset or increased motor dysfunction due to iatrogenic injury to plexus elements, and a lower incidence of pleural injury54.
The posterior/dorsal approach is mainly used for the treatment of recurrent BP tumors following RT. The main downside of the dorsal subscapular approach is the extensive amount of muscle dissection required56. Overall, the anterior SC approach is preferred for most cases12,57.
There are several limitations to this study. The first limitation was the lack of level I and II evidence. Thus, the evidence used in this study has a significant risk of selection and reporting biases. Moreover, our analysis may not reflect the entire distribution of all BP tumors managed surgically in the literature because some studies were excluded for not meeting the inclusion criteria for this study. Finally, there was a relatively high degree of heterogeneity within studies in variables including tumor size and location, as well as type of surgical intervention. Despite these limitations, this study is the first and most comprehensive systematic review describing patient and tumor characteristics as well as outcomes following surgical management of primary tumors of the BP.
Conclusions
This review suggests that the degree of surgical excision (total or subtotal) for primary BP tumors mainly depends on the pathological characteristics, location, and extent of the tumor. Even with STR, patients with NFs have excellent clinical outcomes with a very low rate of recurrence. In contrast, MPNSTs are highly aggressive tumors and are associated with an extremely poor outcome, even when complete resection is achieved. Furthermore, the role of chemoradiotherapeutic interventions on patients’ outcomes requires further investigation. In general, our findings suggest that pain and sensory deficits resolve in the majority of patients following surgery. However, motor deficits are slower to recover.
Ethical approval
Not applicable.
Sources of funding
No funding was obtained for this study.
Author contribution
R.S.: data screening/extraction, data analysis, manuscript writing and editing. H.C.: data screening/extraction, manuscript writing and critical editing, supervision.
Conflicts of interest disclosure
The authors declare that they have no competing interests.
Research registration unique identifying number (UIN)
Name of the registry: Prospero.
Unique identifying number or registration ID:CRD42022347024.
Hyperlink to your specific registration (must be publicly accessible and will be checked):https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=347024
Guarantor
Harvey Chim, MD, is the guarantor for this study.
Availability of data and materials
All the data generated or analyzed during this study are included in this published article. The SPSS data of the participant can be requested from the authors. Please write to the corresponding author if you are interested in such data.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Acknowledgments
Not applicable.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.journal-surgery.net.
Published online 14 March 2023
Contributor Information
Ramin Shekouhi, Email: rshekouhi@ufl.edu.
Harvey Chim, Email: harveychim@yahoo.com.
References
- 1. Huang JH, Zaghloul K, Zager EL. Surgical management of brachial plexus region tumors. Surg Neurol 2004;61:372–378. [DOI] [PubMed] [Google Scholar]
- 2. Go MH, Kim SH, Cho KH. Brachial plexus tumors in a consecutive series of twenty one patients. J Korean Neurosurg Soc 2012;52:138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Desai KI. Primary benign brachial plexus tumors: an experience of 115 operated cases. Neurosurgery 2012;70:220–233. [DOI] [PubMed] [Google Scholar]
- 4. Levi AD, Ross AL, Cuartas E, et al. The surgical management of symptomatic peripheral nerve sheath tumors. Neurosurgery 2010;66:833–840. [DOI] [PubMed] [Google Scholar]
- 5. Adani R, Tarallo L, Mugnai R, et al. Schwannomas of the upper extremity: analysis of 34 cases. Acta Neurochir (Wien) 2014;156:2325–2330. [DOI] [PubMed] [Google Scholar]
- 6. Gachiani J, Kim D, Nelson A, et al. Surgical management of malignant peripheral nerve sheath tumors. Neurosurg Focus 2007;22:1–8. [PubMed] [Google Scholar]
- 7. Koshy S. Soft-tissue considerations in mandibular setback. Am J Orthod Dentofac Orthop 2010;137:447–448. [DOI] [PubMed] [Google Scholar]
- 8. Baehring JM, Betensky RA, Batchelor TT. Malignant peripheral nerve sheath tumor: the clinical spectrum and outcome of treatment. Neurology 2003;61:696–698. [DOI] [PubMed] [Google Scholar]
- 9. Cashen DV, Parisien RC, Raskin K, et al. Survival data for patients with malignant schwannoma. Clin Orthop Relat Res 2004;426:69–73. [DOI] [PubMed] [Google Scholar]
- 10. Jaeckle KA. Neurologic manifestations of neoplastic and radiation-induced plexopathies. Semin Neurol 2010;30:254–262. [DOI] [PubMed] [Google Scholar]
- 11. Lee MH, Park HK, Park HR, et al. Brachial plexus neurofibroma: a case report. Nerve 2020;6:12–14. [Google Scholar]
- 12. Dubuisson A, Reuter G, Kaschten B, et al. Management of benign nerve sheath tumors of the brachial plexus: relevant diagnostic and surgical features. About a series of 17 patients (19 tumors) and review of the literature. Acta Neurol Belg 2021;121:125–131. [DOI] [PubMed] [Google Scholar]
- 13. Jia X, Yang J, Chen L, et al. Primary brachial plexus tumors: clinical experiences of 143 cases. Clin Neurol Neurosurg 2016;148:91–95. [DOI] [PubMed] [Google Scholar]
- 14. Soltani AM, Francis CS, Kane JT, et al. Neural sheath tumors of the brachial plexus: a multidisciplinary team-based approach. Ann Plast Surg 2013;71:80–83. [DOI] [PubMed] [Google Scholar]
- 15. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg, 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 16. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. National Institutes of Health. Quality Assessment Tool for Case Series Studies. National Heart, Lung, and Blood Institute; 2017. [Google Scholar]
- 18. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC med res methodol 2005;5:1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim DH, Murovic JA, Tiel RL, et al. A series of 146 peripheral non-neural sheath nerve tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg 2005;102:256–266. [DOI] [PubMed] [Google Scholar]
- 20. Kim DH, Cho YJ, Tiel RL, et al. Outcomes of surgery in 1019 brachial plexus lesions treated at Louisiana State University Health Sciences Center. J Neurosurg 2003;98:1005–1016. [DOI] [PubMed] [Google Scholar]
- 21. Kim DH, Murovic JA, Tiel RL, et al. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg 2005;102:246–255. [DOI] [PubMed] [Google Scholar]
- 22. Das S, Ganju A, Tiel RL, et al. Tumors of the brachial plexus. Neurosurg Focus 2007;22:1–6. [DOI] [PubMed] [Google Scholar]
- 23. Binder DK, Smith JS, Barbaro NM. Primary brachial plexus tumors: imaging, surgical, and pathological findings in 25 patients. Neurosurg focus 2004;16:1–6. [DOI] [PubMed] [Google Scholar]
- 24. Bourque PR, Chardon JW, Bryanton M, et al. Neurolymphomatosis of the brachial plexus and its branches: case series and literature review. Can J Neurol Sci 2018;45:137–143. [DOI] [PubMed] [Google Scholar]
- 25. Gaba S, Mohsina S, John JR, et al. Clinical outcomes of surgical management of primary brachial plexus tumors. Indian J Plast Surg 2021;54:124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ganju A, Roosen N, Kline DG, et al. Outcomes in a consecutive series of 111 surgically treated plexal tumors: a review of the experience at the Louisiana State University Health Sciences Center. J Neurosurg 2001;95:51–60. [DOI] [PubMed] [Google Scholar]
- 27. Jung IH, Yoon KW, Kim YJ, et al. Analysis according to characteristics of 18 cases of brachial plexus tumors: a review of surgical treatment experience. J Korean Neurosurg Soc 2018;61:625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee HJ, Kim JH, Rhee SH, et al. Is surgery for brachial plexus schwannomas safe and effective? Clin Orthop Relat Res 2014;472:1893–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maiuri F, Donzelli R, Benvenuti D, et al. Schwannomas of the brachial plexus – diagnostic and surgical problems. Zentralbl Neurochir 2001;62:93–97. [DOI] [PubMed] [Google Scholar]
- 30. Millan G, Casal D. Brachial plexus tumors in a tertiary referral center: a case series and literature review. Acta Reumatol Port 2015;2015:372–377. [PubMed] [Google Scholar]
- 31. Noonan AM, McCaffrey J. Frequency and outcome of neoplastic brachial plexopathy: Single institution experience institution experience. Ir Med J 2011;104:76. [PubMed] [Google Scholar]
- 32. Pressney I, Khoo M, Khan R, et al. Morphology of the entering and exiting nerve as a differentiating feature of benign from malignant peripheral nerve sheath tumours of the brachial plexus. Skeletal Radiol 2021;50:1557–1565. [DOI] [PubMed] [Google Scholar]
- 33. Ranalli NJ, Huang JH, Lee EB, et al. Hemangiomas of the brachial plexus: a case series. Neurosurgery 2009;65(Suppl 4):A181–A188. [DOI] [PubMed] [Google Scholar]
- 34. Rawal A, Yin Q, Roebuck M, et al. Atypical and malignant peripheral nerve‐sheath tumors of the brachial plexus: report of three cases and review of the literature. Microsurgery 2006;26:80–86. [DOI] [PubMed] [Google Scholar]
- 35. Seinfeld J, Kleinschmidt-Demasters BK, Tayal S, et al. Desmoid-type fibromatoses involving the brachial plexus: treatment options and assessment of c-KIT mutational status. J neurosurg 2006;104:749–756. [DOI] [PubMed] [Google Scholar]
- 36. Siqueira MG, Martins RS, Teixeira MJ. Management of brachial plexus region tumours and tumour-like conditions: relevant diagnostic and surgical features in a consecutive series of eighteen patients. Acta Neurochir (Wien) 2009;151:1089–1098. [DOI] [PubMed] [Google Scholar]
- 37. Tubbs RS, Bradley N, Harmon D, et al. Involvement of the brachial plexus and its branches by cystic hygromas: report of 4 cases. J Neurosurg Pediatr 2011;7:282–285. [DOI] [PubMed] [Google Scholar]
- 38. Yuce I, Kahyaoglu O, Mertan P, et al. Ultrasound-guided microsurgical excision for brachial plexus schwannomas: short-term clinical study. Turk Neurosurg 2019;29:594–597. [DOI] [PubMed] [Google Scholar]
- 39. Sterne JA, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366. [DOI] [PubMed] [Google Scholar]
- 40. Russell SM. Preserve the nerve: microsurgical resection of peripheral nerve sheath tumors. Neurosurgery 2007;61(Suppl):113–117; discussion 117. [DOI] [PubMed] [Google Scholar]
- 41. Aboh IV, Chisci G, Cascino F, et al. Giant palatal schwannoma. J Craniofac Surg 2014;25:e418–e420. [DOI] [PubMed] [Google Scholar]
- 42. Gennaro P, Nastro Siniscalchi E, Gabriele G, et al. Trigeminal and facial schwannoma: a case load and review of the literature. Eur Rev Med Pharmacol Sci 2012;16(Suppl 4):8–12. [PubMed] [Google Scholar]
- 43. Fiani B, El-Farra MH, Dahan A, et al. Brachial plexus tumors extending into the cervicothoracic spine: a review with operative nuances and outcomes. Clin Transl Oncol 2021;23:1263–1271. [DOI] [PubMed] [Google Scholar]
- 44. Farid M, Demicco EG, Garcia R, et al. Malignant peripheral nerve sheath tumors. Oncologist 2014;19:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dunn GP, Spiliopoulos K, Plotkin SR, et al. Role of resection of malignant peripheral nerve sheath tumors in patients with neurofibromatosis type 1. J Neurosurg 2013;118:142–148. [DOI] [PubMed] [Google Scholar]
- 46. Kar M, Deo SV, Shukla NK, et al. Malignant peripheral nerve sheath tumors (MPNST) – clinicopathological study and treatment outcome of twenty-four cases. World J Surg Oncol 2006;4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Carli M, Ferrari A, Mattke A, et al. Pediatric malignant peripheral nerve sheath tumor: the Italian and German soft tissue sarcoma cooperative grou. J Clin Oncol 2005;23:8422–8430. [DOI] [PubMed] [Google Scholar]
- 48. Wanebo JE, Malik JM, Vandenberg SR, et al. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 28 cases. Cancer 1993;71:1247–1253. [DOI] [PubMed] [Google Scholar]
- 49. Anghileri M, Miceli R, Fiore M, et al. Malignant peripheral nerve sheath tumors: prognostic factors and survival in a series of patients treated at a single institution. Cancer 2006;107:1065–1074. [DOI] [PubMed] [Google Scholar]
- 50. Stucky CC, Johnson KN, Gray RJ, et al. Malignant peripheral nerve sheath tumors (MPNST): the Mayo Clinic experience. Ann Surg Oncol 2012;19:878–885. [DOI] [PubMed] [Google Scholar]
- 51. Evans DG, Baser ME, McGaughran J, et al. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet 2002;39:311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Moretti VM, Crawford EA, Staddon AP, et al. Early outcomes for malignant peripheral nerve sheath tumor treated with chemotherapy. Am J Clin Oncol 2011;34:417–421. [DOI] [PubMed] [Google Scholar]
- 53. Kahn J, Gillespie A, Tsokos M, et al. Radiation therapy in management of sporadic and neurofibromatosis type 1-associated malignant peripheral nerve sheath tumors. Front Oncol 2014;4:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kroep JR, Ouali M, Gelderblom H, et al. First-line chemotherapy for malignant peripheral nerve sheath tumor (MPNST) versus other histological soft tissue sarcoma subtypes and as a prognostic factor for MPNST: an EORTC soft tissue and bone sarcoma group study. Ann Oncol 2011;22:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vandermaesen J, Horemans B, Bers K, et al. Application of biodegradation in mitigating and remediating pesticide contamination of freshwater resources: state of the art and challenges for optimization. Appl Microbiol Biotechnol 2016;100:7361–7376. [DOI] [PubMed] [Google Scholar]
- 56. Crutcher CL, Kline DG, Tender GC. A modified, less invasive posterior subscapular approach to the brachial plexus: case report and technical note. Neurosurg Focus 2017;42:E7. [DOI] [PubMed] [Google Scholar]
- 57. Dubuisson AS, Kline DG, Weinshel SS. Posterior subscapular approach to the brachial plexus: report of 102 patients. J Neurosurg 1993;79:319–330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data generated or analyzed during this study are included in this published article. The SPSS data of the participant can be requested from the authors. Please write to the corresponding author if you are interested in such data.


