Introduction:
Cervical spine fractures with severe spinal cord injury (SCI) are common following cervical spine trauma and are associated with a high mortality rate. Understanding the mortality patterns of patients with cervical spine fractures and severe SCI can offer valuable evidence to surgeons and family members who are required to make critical healthcare decisions. The authors aimed to evaluate the instantaneous death risk and conditional survival (CS) of such patients and developed conditional nomograms to account for different periods of survivors and predict the survival rates.
Methods:
Their instantaneous death risks were calculated using the hazard function, and the Kaplan–Meier method was used to evaluate the survival rates. Cox regression was used to choose the variables for the construction of the nomograms. The area under the receiver operating characteristic curve and calibration plots were used to validate the performance of the nomograms.
Results:
The authors finally included 450 patients with cervical spine fractures and severe SCI using propensity score matching. The instantaneous death risk was the highest during the first 12 months after injury. Surgical treatment can help decrease the instantaneous death risk quickly, especially in early-term surgery. The 5-year CS increased constantly from 73.3% at baseline to 88.0% after 2 years of survival. Conditional nomograms were constructed at baseline and in those who survived for 6 and 12 months. The area under the receiver operating characteristic curve and calibration curves indicated that the nomograms had a good performance.
Conclusion:
Their results improve our understanding of the instantaneous death risk of patients in different periods following injury. CS demonstrated the exact survival rate among medium-term and long-term survivors. Conditional nomograms are suitable for different survival periods in predicting the probability of survival. Conditional nomograms help in understanding the prognosis and improve the shared decision-making approaches.
Keywords: cervical spine fracture, conditional nomogram, conditional survival, instantaneous death risk, severe spinal cord injury
Introduction
HIGHLIGHTS
We provided the instantaneous death risk data of these populations, which adds to our knowledge regarding the instantaneous death risk faced by patients in different time periods and has not been reported previously.
We showed the conditional survival rates of these patients, which helps us understand the exact survival rate among medium-term and long-term survivors.
We developed conditional nomograms suitable for different survival periods to accurately predict the survival rates and to fulfill our drive toward personalized medicine.
We created nomograms with favorable discrimination and predictive abilities with which to predict survival rates.
Cervical spine fractures are very common, and their incidence is rising1. The incidence of cervical spine fractures is ∼0.29 per 1000 person-years in the U.S.2. The common causes of cervical spine fractures include motor vehicle accidents, falls, violence, and sports activities3,4. Spinal cord injury (SCI) is the most common complication following cervical spine fractures. The annual incidence of SCI is ∼0.93 million globally5. The prevalence of SCI in patients with cervical spine fractures is ∼10–51%2,6–8. Cervical spine fractures with severe SCI can lead to high mortality, severe disability, central neuropathic pain, and a large socioeconomic burden9–12.
The primary management approach for cervical spine fractures with severe SCI is early surgical decompression, which can significantly reduce further neurologic injury13. Although patients with cervical spine fractures and severe SCI will surely benefit from surgical management14, surgeries may be delayed or not performed due to several reasons15. One important reason is that patients’ families may not consent to surgical treatment when other management options are available16. Therefore, knowing the precise instantaneous death risk and mortality rates in cervical spine fractures with severe SCI can offer valuable knowledge and evidence to surgeons and family members in making critical healthcare decisions.
Unlike traditional survival analyses, we aimed to use a hazard function to calculate the instantaneous death risk in such patients. Additionally, the mortality risk in these patients is relatively high in the first several months following injury and decreases over time. Therefore, the survival rates calculated at baseline may not be appropriate during the follow-up. Conditional survival (CS) only includes patients who have already survived for a certain period and is a more appropriate parameter in such situations17,18. However, CS is commonly used in cancer research19, and to the best of our knowledge, we are the first to propose the use of this concept in the interpretation of survival rates in patients with cervical spine fractures and severe SCI.
Nomograms are commonly used as prognostic devices to calculate the probability of a clinical event in medicine and cancer20. However, similar to traditional survival analyses, nomograms based on baseline parameters cannot accurately predict the survival rates of medium-term or long-term survivors.
Therefore, in this study, the hazard function was used to calculate the instantaneous death risk of such patients, and CS was used to estimate the precise survival rates of survivors during fixed periods. We also constructed a series of nomograms to predict the prognoses of these patients. In this study, we aimed to determine the instantaneous death risk and CS of patients with cervical fractures and severe SCI. Additionally, we developed conditional nomograms to account for the survival of different durations to accurately predict the survival rate and promote personalized medicine.
Methods
The study and the informed consent protocol were approved by the ethics committee of Xijing Hospital of Air Force Medical University (KY20212199-F-1). Our results are reported in line with STROCSS (strengthening the reporting of cohort, cross-sectional and case–control studies in surgery) 2021 criteria21, Supplemental Digital Content 1, http://links.lww.com/JS9/A211.
Patient data were obtained from Xijing Hospital, Xi’an Honghui Hospital, Tangdu Hospital, The Second Affiliated Hospital of Xi’an Jiaotong University, and Wenzhou Medical University Second Affiliated Hospital. These are locally affiliated tertiary care centers with extensive experience in treating patients with cervical fractures and severe SCI. Patients admitted to these hospitals with cervical fractures and severe SCI between 1 January 2012 and 30 May 2019, were included.
The inclusion criteria were the following: cervical fractures (confirmed using imaging) and severe SCI [American Spinal Injury Association impairment scale (ASIA) grade A or B]22. ASIA grade A is defined as the absence of all motor and sensory functions, and ASIA grade B is defined as patients having some sensory function but no motor function.
The following were the exclusion criteria: serious infections, blood dyscrasias, tumors, or congenital spinal deformities; serious cardiopulmonary diseases; death prior to hospital admission; ankylosing spondylitis, diffuse idiopathic skeletal hyperostosis, or other chronic spondylitis disorders; ASIA grades C, D, or E (ASIA grade C is defined as patients whose motor function is preserved below the neurological level, and more than half of key muscles below the neurological level have a muscle grade less than 3, while ASIA grade D is defined as patients whose motor function is preserved below the neurological level, and at least half of key muscles below the neurological level have a muscle grade of 3 or more. Patients with ASIA grade E may have normal motor and sensory examinations, but still may have abnormal reflexes or other neurologic phenomena); only thoracic or lumbar injuries; and brain injury.
The reason for the first two exclusion criteria is that these comorbidities may adversely affect the prognosis, which may result in an underestimation of the survival rate. Similarly, including patients who died before reaching the hospital can also result in an underestimation of the survival rate since most patients were likely directly killed by serious traffic accidents. Furthermore, the mortality in patients with cervical fractures and ankylosing spondylitis or diffuse idiopathic skeletal hyperostosis tends to be much higher than that in patients without these conditions23. Therefore, including such patients may also result in an underestimation of the general survival rate. Patients with brain injury were also excluded because brain injury may interfere with ASIA grading.
We collected data such as demographic characteristics, comorbidities, cervical spine fracture levels, dislocation status (yes or no), injury mechanism, ASIA grade, the surgery type and timing, and common complications. To avoid any possible bias, the baseline characteristics were compared between patients who were included and those who were lost to follow-up. There was no significant difference between the groups. The included patients were followed up from admission until death or 1 June 2022, whichever was later. Data regarding death were collected via phone calls, short messages, or household registration agencies in government departments.
Propensity score matching with 1 : 1 matching was used to form two groups of patients, which could reduce the bias between groups24. The propensity score was calculated using a logistic regression model with the following variables: age, injury factors, fracture level, comorbidities, surgical treatment, operation timing, and complications. The matching was performed using the nearest neighbor matching method with a caliper width of 0.20.
According to the ASIA scale, we conducted neurological examinations at the time of admission to evaluate the degree of SCI. We divided the fracture levels into two subgroups: upper cervical spine fractures (C1–C4) and lower cervical spine fractures (C5–C7). Injury mechanisms were divided into low-energy injuries (due to a fall on the ground or sports activities) and high-energy injuries (due to motor vehicle accidents, fall from height, or violence). Experienced spine surgeons decide on the surgery type through discussion and then perform the surgeries. The timing of surgery was defined as the duration between the injury and surgery. We divided the timing of surgery into early (<3 days), medium (3–7 days), and late (>7 days) surgery durations.
Statistical analyses
The instantaneous death risk was calculated using a hazard function curve with a fixed bandwidth kernel approach that incorporated boundary kernels25,26. The Kaplan–Meier (KM) method was used to evaluate the survival rates, and the log-rank test was used to test the statistical differences between subgroups. Cox regression models were used to determine the risk factors for mortality. Variables with P values less than 0.05 in the univariate Cox analysis were included in the multivariate Cox analysis. The multivariable Cox regression was fitted using stepwise backward selection based on Akaike’s Information Criterion27. Finally, factors with prognostic significance in the multivariate Cox regression analysis were used to build nomograms, which were developed using the rms package in R v4.1.2 (R Foundation for Statistical Computing, Vienna, Austria)28.
The 5-year CS was defined as the probability of survival for 5 years from the day that the patients had already survived for a period of time29. For example, the 5-year CS at 1 year refers to the survival rate for 5 years in patients who had already survived for 1 year. The details of the calculations have been provided previously30. Restricted cubic splines were used to find the relationship between the probability of death and age and to identify the cutoff limit for age.
The area under the receiver operating characteristic curve (AUC) and the area under the time-dependent receiver operating characteristic curve (time-dependent AUC) was calculated to assess discriminative ability. Generally, AUC greater than 0.7 indicated a reasonable estimation. Calibration plots were calculated to assess the calibrating ability using the bootstrapping method (1000 resampling bootstrap)31. Agreement between observed and predicted outcomes was evaluated using calibration plots, which incorporated grouped KM estimates and the continuous hazard regression function31.
Statistical analyses were conducted using STATA v14.2 (Stata Corp., College Station, Texas, USA), SPSS v22.0 (IBM Corporation, Armonk, New York, USA), and R v4.1.2 (R Foundation for Statistical Computing). Continuous variables are presented as mean±SD or median [interquartile range (IQR)] and were compared using the independent sample t test or the Mann–Whitney U test, as appropriate. Categorical variables are presented as frequencies (%) and compared using the χ 2 or Fisher’s exact tests. The statistical significance was set at P less than 0.05.
Results
Finally, we included 450 patients for further analysis after using propensity score matching. The overall mean age of the patients was 46.3±15.5 and 45.6±15.3 years in patients with ASIA grade A and grade B, respectively. Nearly 80% of the patients experienced high-energy injuries that resulted in cervical spine fractures (Table 1). Approximately 40% of patients had upper cervical spine (C1–C4) fractures. More than 80% of the patients underwent surgeries, and pneumonia was the commonest complication.
Table 1.
Baseline characteristics after propensity score matching.
| N (%) | |||
|---|---|---|---|
| Demographics | ASIA A (n=225) | ASIA B (n=225) | P |
| Age, years | |||
| Mean (SD) | 46.3 (15.5) | 45.6 (15.3) | 0.657 |
| Median (IQR) | 46 (33–55) | 45 (35.5–60) | 0.883 |
| Sex | 0.886 | ||
| Male | 197 (87.6) | 198 (88.0) | |
| Female | 28 (12.4) | 27 (12.0) | |
| Dislocation | 86 (38.2) | 79 (35.1) | 0.493 |
| Injury factors | 0.107 | ||
| High-energy injury | 192 (85.3) | 179 (79.6) | |
| Low-energy injury | 33 (14.7) | 46 (20.4) | |
| Fracture level | 0.126 | ||
| Upper cervical spine (C1–C4) | 86 (38.2) | 102 (45.3) | |
| Lower cervical spine (C5–C7) | 139 (61.8) | 123 (54.7) | |
| Comorbidity | |||
| Chest injury | 35 (15.6) | 49 (21.8) | 0.090 |
| Fractures in other parts | 37 (16.4) | 23 (10.2) | 0.052 |
| Hypertension | 8 (3.6) | 9 (4.0) | 0.805 |
| Diabetes | 7 (3.1) | 8 (3.6) | 0.793 |
| Surgical treatment | 0.209 | ||
| Yes | 200 (88.9) | 191 (84.9) | |
| No | 25 (11.1) | 34 (15.1) | |
| Operation timing | 0.357 | ||
| Nonsurgery | 25 (11.1) | 34 (15.1) | |
| Early term | 60 (26.7) | 57 (25.3) | |
| Medium term | 101 (44.9) | 87 (38.7) | |
| Later term | 39 (17.3) | 47 (20.9) | |
| Complications | |||
| Pneumonia | 55 (24.4) | 46 (20.4) | 0.309 |
| Respiratory failure | 9 (4.0) | 13 (5.8) | 0.382 |
| Deep veinous thrombosis | 1 (0.4) | 2 (0.9) | 0.562 |
| Decubitus | 3 (1.3) | 8 (3.6) | 0.127 |
| Urinary tract infection | 10 (4.4) | 6 (2.7) | 0.309 |
ASIA, American Spinal Injury Association impairment scale; IQR, interquartile range.
Instantaneous death risk and survival curve
The instantaneous death risk was the highest at the time of injury and rapidly decreased in the first 12 months after injury (Fig. 1A–G). In the first 12 months after the injury, the instantaneous death risk fluctuated within a relatively low-risk range. We also found that surgical treatments, especially early-term surgery, decreased the instantaneous death risk more quickly than nonsurgical treatments (Fig. 1C, E). Additionally, we observed that the instantaneous death risk increased significantly when patients developed pneumonia or respiratory failure (Fig. 1D, G).
Figure 1.
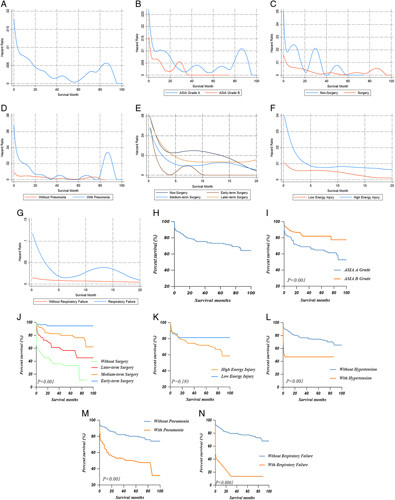
Hazard function curve for instantaneous death risk in cervical spine fracture patients with severe SCI (A) and stratified by ASIA grade (B), surgery (C), pneumonia (D), surgery timing (E), injury energy (F) and respiratory failure (G). Kaplan–Meier survival curves illustrating the overall survival (H) and stratified by ASIA grade (I), surgery timing (J), injury energy (K), hypertension (L), pneumonia (M), and respiratory failure (N). ASIA, American Spinal Injury Association impairment scale; SCI, spinal cord injury.
We found that patients with ASIA grade A and high-energy injuries were more likely to die (Fig. 1I, K). Additionally, the survival rates were improved by surgeries, especially with early-term surgical treatment (Fig. 1J). However, hypertension, pneumonia, and respiratory failure significantly decreased the survival of the patients with severe SCI (Fig. 1L–N).
We found that the optimal cutoff limit for age was 45 years (Fig. 2). Therefore, patients under 45 years of age had a low risk of death, while those older than 45 years had a high risk of death. We further conducted prognosis analysis to identify the prognoses in patients of different ages (Supplementary Figures 1, Supplemental Digital Content 2, http://links.lww.com/JS9/A212 and 2, Supplemental Digital Content 3, http://links.lww.com/JS9/A213). The prognosis was found to be worse in patients older than 45 years of age (P<0.001).
Figure 2.
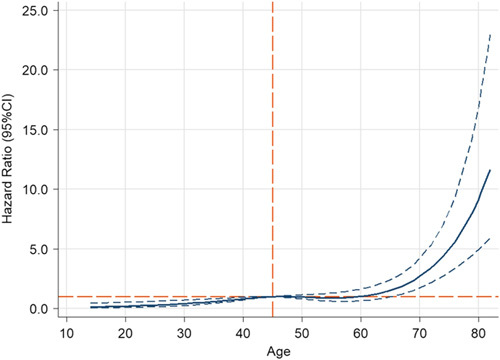
Cubic spline models showing the association between age and mortality risk. The long orange dashed transverse line represents the hazard ratio of 1. The long dashed orange vertical line represents the age of 45. The solid blue line and the long dashed blue line represent the estimated hazard ratio and its 95% CI.
5-year CS in patients with cervical spine fracture and severe SCI
We noted that the 5-year CS increased constantly from 73.3% at baseline to 88.0% after 2 years of survival (Table 2). We also found that the 5-year CS in patients with ASIA grade A increased from 64.9% at baseline to 87.1% after 2 years of survival, whereas that in patients with high-energy injuries increased from 71.8% at baseline to 85.4% after 2 years of survival. Patients who had respiratory insufficiency had the lowest 5-year survival rate at baseline (13.6%); however, their survival rate significantly increased after 2 years of survival (50.0%). Similarly, patients with pneumonia had a significantly low 5-year survival rate (50.4%); however, their 5-year survival rate gradually increased after surviving for a certain period. Additionally, the 5-year CS in different age groups was also tested. We found that the 5-year survival rate in patients under 45 years of age was relatively high (Supplementary Table 1, Supplemental Digital Content 4, http://links.lww.com/JS9/A214). However, the 5-year survival rates increased from 54.4 to 74.8% in patients older than 60 years after 2 years of survival.
Table 2.
Five-year conditional survival rates.
| Conditional 5-year relative survival (%) | |||||
|---|---|---|---|---|---|
| Variables | 5-Year relative survival at baseline (%) | At 3 months (%) | At 6 months (%) | At 1 year (%) | At 2 years (%) |
| Total | 73.3±2.2 | 82.5±2.1 | 83.2±2.1 | 84.5±2.3 | 88.0±2.7 |
| ASIA grade | |||||
| A grade | 64.9±3.4 | 76.0±3.5 | 76.3±3.7 | 77.1±3.9 | 87.1±3.6 |
| B grade | 82.1±2.7 | 87.5±2.4 | 88.8±2.3 | 87.5±3.9 | 89.5±3.9 |
| Injury mechanism | |||||
| High-energy injury | 71.8±2.5 | 81.0±2.3 | 81.2±2.4 | 82.6±2.7 | 85.4±3.3 |
| Low-energy injury | 81.5±4.5 | 90.6±3.7 | 94.6±3.0 | 94.6±3.0 | 100 |
| Surgery | |||||
| Yes | 79.8±2.2 | 85.7±2.0 | 85.8±2.2 | 86.4±2.3 | 92.4±2.1 |
| No | 31.5±6.4 | 51.6±9.0 | 51.6±9.0 | 64.0±9.9 | 68.8±10.1 |
| Pneumonia | |||||
| Yes | 50.4±5.1 | 66.9±5.6 | 70.6±5.6 | 75.3±6.5 | 84.8±6.3 |
| No | 80.2±2.3 | 86.4±2.1 | 87.2±2.1 | 87.8±2.2 | 88.9±2.9 |
| Respiratory insufficiency | |||||
| Yes | 13.6±7.3 | 33.3±15.7 | 33.3±15.7 | 42.9±18.7 | 50.0±20.4 |
| No | 77.4±2.1 | 84.7±2.0 | 85.5±2.0 | 86.4±2.3 | 89.9±2.6 |
ASIA, American Spinal Injury Association impairment scale
Cox regression analysis
At baseline, we found that age, ASIA grade A, nonsurgical treatment, pneumonia, and respiratory failure were risk factors for death (Table 3). However, in patients who had already survived for 12 months, we found that age, nonsurgical treatment, ASIA grade A, respiratory failure, and decubitus were risk factors for death (Table 3). Only early surgical treatment was a constant predictor of survival at baseline [hazard ratio (HR): 0.08, 95% CI: 0.03–0.20, P<0.001], 6 months (HR: 0.06, 95% CI: 0.02–0.25, P<0.001), and 12 months (HR: 0.07, 95% CI: 0.01–0.39, P=0.002).
Table 3.
Univariable and multivariable Cox regression results of the risk factors of mortality in baseline, ≥6-month and 12-month survivors.
| Baseline survivors | ≥6 months survivors | ≥12 months survivors | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | |
| Covariables | Univariable analysis | Multivariable analysis | Univariable analysis | Multivariable analysis | Univariable analysis | Multivariable analysis | ||||||
| Age | 1.05 (1.04–1.06) | <0.001 | 1.04 (1.02–1.05) | <0.001 | 1.03 (1.01–1.05) | 0.002 | 1.02 (1.00–1.04) | 0.045 | 1.04 (1.02–1.06) | 0.001 | 1.05 (1.02–1.07) | <0.001 |
| Sex | ||||||||||||
| Male | Ref | Ref | – | – | Ref | Ref | – | – | Ref | Ref | – | – |
| Female | 0.97 (0.54–1.72) | 0.908 | – | – | 0.76 (0.30–1.90) | 0.556 | – | – | 0.76 (0.27–2.13) | 0.607 | – | – |
| Cervical fracture site | ||||||||||||
| C1–C4 | Ref | Ref | – | – | Ref | Ref | – | – | Ref | Ref | – | – |
| C5–C7 | 0.93 (0.65–1.35) | 0.709 | – | – | 0.73 (0.43–1.24) | 0.246 | – | – | 1.62 (0.88–3.00) | 0.124 | – | – |
| Dislocation | 0.79 (0.54–1.13) | 0.198 | – | – | 0.74 (0.44–1.23) | 0.240 | – | – | 0.90 (0.50–1.62) | 0.719 | – | – |
| ASIA grade | ||||||||||||
| A grade | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| B grade | 0.42 (0.28–0.62) | 0.001 | 0.29 (0.19–0.43) | <0.001 | 0.43 (0.25–0.74) | 0.002 | 0.31 (0.17–0.56) | <0.001 | 0.30 (0.15–0.57) | <0.001 | 0.20 (0.09–0.44) | <0.001 |
| Injury mechanism | ||||||||||||
| High-energy injury | Ref | Ref | – | – | Ref | Ref | Ref | Ref | Ref | Ref | ||
| Low-energy injury | 0.59 (0.34–1.04) | 0.068 | – | – | 0.22 (0.07–0.70) | 0.011 | 0.28 (0.08–0.94) | 0.039 | 0.27 (0.10–0.90) | 0.033 | 0.48 (0.14–1.64) | 0.241 |
| Comorbidity | ||||||||||||
| Hypertension | 3.42 (1.73–6.77) | <0.001 | 1.66 (0.79–3.49) | 0.183 | 20.73 (0.01–82694.02) | 0.586 | – | – | 0.05 (0.00–574.32) | 0.527 | – | – |
| Diabetes | 0.72 (0.23–2.27) | 0.575 | – | – | 1.46 (0.46–4.66) | 0.527 | – | – | 0.05 (0.00–54.21) | 0.395 | – | – |
| Chest injury | 2.25 (1.26–4.00) | 0.006 | 1.53 (0.80–2.92) | 0.201 | 1.54 (0.78–3.05) | 0.211 | – | – | 0.86 (0.43–1.73) | 0.670 | – | – |
| Fractures in other parts | 4.89 (1.80–13.26) | 0.002 | 2.49 (0.87–7.18) | 0.091 | 26.17 (1.24–552.81) | 0.036 | – | 0.956 | 0.04 (0.00–1.18) | 0.062 | – | – |
| Complications | ||||||||||||
| Pneumonia | 3.52 (2.44–5.07) | <0.001 | 2.39 (1.63–3.52) | <0.001 | 3.18 (1.89–5.35) | <0.001 | 1.94 (1.12–3.36) | 0.018 | 2.52 (1.37–4.63) | 0.003 | 1.93 (0.95–3.92) | 0.069 |
| Respiratory failure | 8.14 (4.95–13.38) | <0.001 | 3.79 (1.93–7.47) | <0.001 | 5.94 (2.54–13.88) | <0.001 | 3.09 (1.04–9.13) | 0.042 | 5.47 (1.95–15.35) | 0.001 | 68.98 (15.66–303.77) | 0.006 |
| Deep veinous thrombosis | 1.72 (0.24–12.29) | 0.591 | – | – | – | 0.715 | – | – | – | 0.750 | – | – |
| Decubitus | 1.08 (0.34–3.40) | 0.899 | – | – | 2.40 (0.74–7.74) | 0.144 | – | – | 3.54 (1.07–11.64) | 0.038 | 5.34 (1.30–21.89) | 0.020 |
| Urinary tract infection | 1.25 (0.55–2.85) | 0.594 | – | – | 1.94 (0.77–4.88) | 0.159 | – | – | 2.49 (0.98–6.36) | 0.056 | – | – |
| Operation timing | ||||||||||||
| Nonsurgery | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Early term | 0.04 (0.17–0.10) | <0.001 | 0.08 (0.03–0.20) | <0.001 | 0.04 (0.01–0.14) | <0.001 | 0.06 (0.02–0.25) | <0.001 | 0.05 ( (0.01–0.21) | <0.001 | 0.07 (0.01–0.39) | 0.002 |
| Medium term | 0.21 (0.13–0.32) | <0.001 | 0.34 (0.19–0.61) | <0.001 | 0.29 (0.15–0.54) | <0.001 | 0.40 (0.18–0.91) | 0.028 | 0.47 (0.21–1.05) | 0.065 | 0.60 (0.20–1.80) | 0.362 |
| Later term | 0.48 (0.30–0.76) | 0.056 | 0.70 (0.39–1.26) | 0.231 | 0.61 (0.30–1.22) | 0.160 | 1.01 (0.42–2.43) | 0.975 | 0.95 (0.40–2.28) | 0.908 | 1.65 (0.51–5.30) | 0.401 |
ASIA, American Spinal Injury Association impairment scale; Ref, Reference.
Conditional nomogram construction and validation
We constructed nomograms at baseline (Fig. 3A) and in 6-month (Fig. 3D) and 12-month survivors (Fig. 3G) according to the risk factors. For the first injury and follow-up, we first chose a suitable nomogram and then added the individual scores to obtain a total score. Finally, we used the total score to predict an individual’s 1-year, 3-year, and 5-year survival probabilities.
Figure 3.
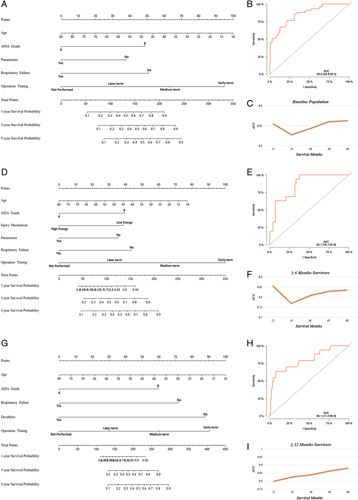
(A) A nomogram to predict 1-year, 3-year, and 5-year survival for baseline patients and validated by AUC (B) and time-dependent AUC (C) in the training cohort. (D) A nomogram to predict 1-year, 3-year, and 5-year survival for 6-month survivors and validated by AUC (E) and time-dependent AUC (F) in the training cohort. (G) A nomogram to predict 1-year, 3-year, and 5-year survival for 12-month survivors and validated by AUC (H) and time-dependent AUC (I) in the training cohort. AUC, area under the receiver operating characteristic curve; time-dependent AUC, under the time-dependent receiver operating characteristic curve.
We further investigated the performance of these nomograms. The AUC in the training cohort was 0.856 at baseline (Fig. 3B), 0.857 in 6-month survivors (Fig. 3E), and 0.801 in 12-month survivors (Fig. 3H). More importantly, the time-dependent AUC in survivors of various durations remained greater than 0.8 in the prediction of survival for 5 years (Fig. 3C, F, I).
The calibration curves of the nomograms that were applied to the training and validation cohorts are also shown (Fig. 4). The results revealed that the nomograms had a good agreement in predicting the survival probability between the predicted survival rates and observed survival rates in both the training and validation cohorts.
Figure 4.
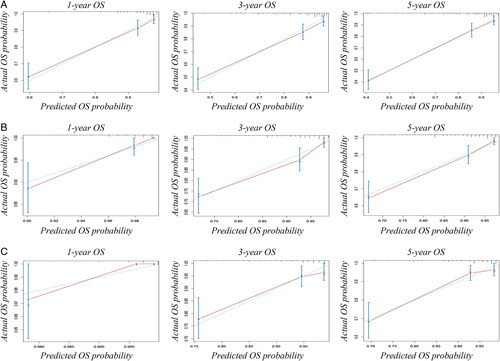
Calibration curves in the training and validation cohorts. (A) Calibration curves of baseline nomogram to predict 1-year, 3-year, and 5-year survival. (B) Calibration curves of 6-month survivors nomogram to predict 1-year, 3-year, and 5-year survival. (C) Calibration curves of 12-month survivors nomogram to predict 1-year, 3-year, and 5-year survival. OS, overall survival.
Discussion
In the present study, we analyzed the detailed survival data of patients with cervical spine fractures and severe SCI. The novelty and strengths of our study include the following: we estimated the instantaneous death risk in these patients for different periods, which adds to the knowledge and has not been reported previously; we estimated the CS rates, which help us understand the precise survival rate in medium-term and long-term survivors; we developed conditional nomograms for different survival periods to accurately predict the survival rates and push forwards toward personalized medicine strategies; we demonstrated favorable discrimination and predictive abilities of nomograms in predicting survival rates.
Currently, the commonest analysis model for time-to-event data includes KM survival function estimators and life tables32. Though these models can provide us with the probability of surviving to time x, we cannot know the instantaneous death risk/rate at time x 33. Therefore, in the present study, we used the hazard function to estimate the instantaneous death risk/rate at time x. The hazard function depicts the natural interpretation of death34 and how the risk changes over time in terms of the instantaneous death risk/rate in survivors. Additionally, the hazard function also helps determine the periods with the greatest risk of death35. Our findings revealed that the patients had the highest risk of death during the first 12 months following injury, and early surgery could help rapidly decrease this risk. Furthermore, our results also help determine the follow-up in these patients36,37.
One study reported that the in-hospital mortality rate in this patient population was 38%38, while another recent study showed that the 1-year mortality rate was 36.5%16. In our study, the mortality rate was relatively lower than these reported rates. One possible explanation may be that previous studies included only older patients. The mortality rate in older patients is significantly higher than that in younger people39. However, the cutoff limit for age while dividing the risk of patients has not been clarified. Therefore, we used restricted cubic splines to identify the optimal cutoff limit. Our results revealed that patients older than 45 years of age were at a high risk of death.
Systematic biases are very common in observational studies; however, these are often difficult to overcome40. In our present study, we found that surgical treatment was one of the most important factors associated with the prognosis. However, we assumed that this result was affected by selection bias because some patients were unable to undergo surgical treatment. These patients may have greater morbidity, thus resulting in a worse prognosis. Selection bias, which may be attributable to unmeasured variables as well as to a wide range of measured variables, indicates a lack of comparability between study groups. Therefore, we used a multivariate regression model to account for potential confounders in our study. A randomized prospective trial may minimize the effects of selection bias and demonstrate the real benefits of surgical treatment on the prognosis. However, such a trial is difficult to conduct because surgical treatments cannot be randomized. However, early surgical treatment helps improve the prognosis in general41, and our results support this point of view.
CS considers the years that a patient has already survived when estimating their survival probability29,30. However, this concept is mainly used in the field of cancer. To the best of our knowledge, this is the first study to introduce this concept in the field of severe SCI. Although the traditional survival rate is valuable for patients at baseline, it cannot provide precise survival probability during follow-up. As shown in our study, the 5-year survival rate increased constantly from 73.3% at baseline to 88.0% in 2-year survivors. Notably, in patients with ASIA grade A, the 5-year survival rate increased from 64.9% at baseline to 87.1% after 2 years of survival. Additionally, our results also indicated that the risk factors change with survival time. For example, old age is always a risk factor at baseline as well as at 6 and 12 months, while decubitus is not a risk factor at baseline and in 6-month survivors but is a risk factor in 12-month survivors. On the one hand, knowing the CS and changes in risk factors is critically important for surgeons during follow-up and counseling42,43. On the other hand, our results provide new information on how prognoses change over time, which can help doctors and patients observe positive changes in disease mortality.
Previous studies have highlighted that one limitation of nomograms is that they assume that the outcomes remain constant over the survival time20. However, the mortality and risk factors vary over the duration of survival44,45. Therefore, to remedy this limitation, we developed conditional nomograms to fit different survival periods. A suitable nomogram can be chosen to predict the survival rate of certain survivors more accurately. Additionally, for internal or external validation, the AUC of the nomograms indicated good prediction performance. Furthermore, the calibration plots also demonstrated a good correspondence between the predicted and observed survival rates. Additionally, due to differences in diagnosis, treatments, and prognosis between patients with cervical spine fractures with and without severe SCI, we intend to perform further studies to highlight the differences in the prognosis between these patients.
Our study has some limitations. First, our study was based on retrospective data; therefore, an inherent bias is inevitable. It is important to validate these nomograms in prospective studies. Second, our results were based on data from Chinese patients, and they may not be applicable to other population groups. Therefore, validations in different patient populations are warranted. Third, patients who died before admission were excluded, which may have resulted in an overestimation of the survival rates. Finally, there may be a potential bias due to nonresponses. Previous studies have indicated that nonresponders may be older and have greater morbidities, which might have resulted in selection bias and a lack of representation46,47. However, more recent studies have demonstrated that nonresponders did not significantly affect the risk and death estimates48–51.
Conclusion
We estimated the instantaneous death risk during various periods in patients with cervical spine fractures and severe SCI. We also presented the CS rates in these patients, which improved our understanding of the exact survival rates in medium-term and long-term survivors. Additionally, we constructed conditional nomograms that are suitable for survivors of different periods and can be used as accurate predictive tools for the risk of death. Such clinical tools can help surgeons and patients understand certain prognoses and improve their shared decision-making approaches. Finally, our results support our progress toward personalized medicine.
Ethical approval
The study has been approved by the ethics committee of Xijing Hospital of Fourth Military Medical University (KY20222235-C-1).
Sources of funding
There was no funding source for this study.
Author contribution
J.H.: conceptualization, methodology, software, data curation, visualization, investigation, and writing – original draft preparation; K.Y.: data curation, methodology, and software; C.W.: software, data curation, visualization, and investigation; Q.-C.T.: software, data curation, and visualization; H.B. and J.W.: software and validation; B.L. and Z.-X.W.: conceptualization, methodology, supervision, and writing – reviewing and editing.
Conflicts of interest disclosure
The authors declare no potential conflicts of interest.
Research registration unique identifying number (UIN)
Name of the registry: Research Registry.
Unique identifying number or registration ID: researchregistry7624.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.researchregistry.com/browse-theregistry#home/registrationdetails/620283ed2e9d2d001edb46f4/
Guarantor
Zi-Xiang Wu.
Data availability statement
Data are available upon reasonable request.
Acknowledgments
Not applicable.
Supplementary Material
Footnotes
J.H., K.Y., and C.W., the first three authors, contributed equally to this article.
B.L. and Z.X.W., the last two authors, contributed equally to this manuscript.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, http://www.journal-surgery.net.
Published online 31 March 2023
Contributor Information
Jinfeng Huang, Email: jinfenghuang_study@yeah.net.
Kang Yan, Email: yankang1029@163.com.
Chenyu Wu, Email: 929889362@qq.com.
Quan-Chang Tan, Email: sci_research@126.com.
Hao Bai, Email: fmmubai@126.com.
Jing Wang, Email: wongjing2014@163.com.
Bo Liao, Email: liaobo@fmmu.edu.cn.
Zi-Xiang Wu, Email: wuzixiang@fmmu.edu.cn.
References
- 1. Pearson AM, Martin BI, Lindsey M, et al. C2 vertebral fractures in the medicare population: incidence, outcomes, and costs. J Bone Joint Surg Am 2016;98:449–456. [DOI] [PubMed] [Google Scholar]
- 2. Schoenfeld AJ, Sielski B, Rivera KP, et al. Epidemiology of cervical spine fractures in the US military. Spine J 2012;12:777–783. [DOI] [PubMed] [Google Scholar]
- 3. DePasse JM, Durand W, Palumbo MA, et al. Sex- and sport-specific epidemiology of cervical spine injuries sustained during sporting activities. World Neurosurg 2019;122:E540–E545. [DOI] [PubMed] [Google Scholar]
- 4. Niemi-Nikkola V, Saijets N, Ylipoussu H, et al. Traumatic spinal injuries in Northern Finland. Spine (Phila Pa 1976) 2018;43:E45–E51. [DOI] [PubMed] [Google Scholar]
- 5. GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18:56–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fredø HL, Rizvi SA, Lied B, et al. The epidemiology of traumatic cervical spine fractures: a prospective population study from Norway. Scand J Trauma Resusc Emerg Med 2012;20:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leucht P, Fischer K, Muhr G, et al. Epidemiology of traumatic spine fractures. Injury 2009;40:166–172. [DOI] [PubMed] [Google Scholar]
- 8. Fredø HL, Bakken IJ, Lied B, et al. Incidence of traumatic cervical spine fractures in the Norwegian population: a national registry study. Scand J Trauma Resusc Emerg Med 2014;22:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oliver M, Inaba K, Tang A, et al. The changing epidemiology of spinal trauma: a 13-year review from a Level I trauma centre. Injury 2012;43:1296–1300. [DOI] [PubMed] [Google Scholar]
- 10. Javeed S, Dibble CF, Greenberg JK, et al. Upper limb nerve transfer surgery in patients with tetraplegia. JAMA Netw Open 2022;5:e2243890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Monje M. Spinal cord injury – healing from within. N Engl J Med 2021;384:182–184. [DOI] [PubMed] [Google Scholar]
- 12. Ushida T, Katayama Y, Hiasa Y, et al. Mirogabalin for central neuropathic pain after spinal cord injury: a randomized, double-blind, placebo-controlled, phase 3 study in Asia. Neurology 2023;100:e1193–e1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burke JF, Yue JK, Ngwenya LB, et al. Ultra-early (<12 hours) surgery correlates with higher rate of American Spinal Injury Association Impairment Scale conversion after cervical spinal cord injury. Neurosurgery 2019;85:199–203. [DOI] [PubMed] [Google Scholar]
- 14. Qiu Y, Chen Y, Xie Y, et al. Comparative analysis of the efficacy of early and late surgical intervention for acute spinal cord injury: a systematic review and meta-analysis based on 16 studies. Int J Surg 2021;94:106098. [DOI] [PubMed] [Google Scholar]
- 15. Oner C, Rajasekaran S, Chapman JR, et al. Spine trauma – what are the current controversies? J Orthop Trauma 2017;31(suppl 4):S1–S6. [DOI] [PubMed] [Google Scholar]
- 16. Zeitouni D, Catalino M, Kessler B, et al. 1-year mortality and surgery incidence in older US adults with cervical spine fracture. World Neurosurg 2020;141:e858–e863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hieke S, Kleber M, König C, et al. Conditional survival: a useful concept to provide information on how prognosis evolves over time. Clin Cancer Res 2015;21:1530–1536. [DOI] [PubMed] [Google Scholar]
- 18. Shack L, Bryant H, Lockwood G, et al. Conditional relative survival: a different perspective to measuring cancer outcomes. Cancer Epidemiol 2013;37:446–448. [DOI] [PubMed] [Google Scholar]
- 19. Eloranta S, Smedby KE, Dickman PW, et al. Cancer survival statistics for patients and healthcare professionals – a tutorial of real-world data analysis. J Intern Med 2021;289:12–28. [DOI] [PubMed] [Google Scholar]
- 20. Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol 2015;16:e173–e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mathew G, Agha R, Albrecht J, et al. STROCSS 2021: strengthening the reporting of cohort, cross-sectional and case–control studies in surgery. Int J Surg 2021;96:106165. [DOI] [PubMed] [Google Scholar]
- 22. Roberts TT, Leonard GR, Cepela DJ. Classifications in brief: American Spinal Injury Association (ASIA) Impairment Scale. Clin Orthop Relat Res 2017;475:1499–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Romero-Muñoz LM, Tipper G, Segura-Fragoso A, et al. Outcomes of spinal cord injury following cervical fracture in ankylosing spondylitis and diffuse idiopathic skeletal hyperostosis (DISH): a prospective cohort study. Neurocirugía 2022;33:275–283. [DOI] [PubMed] [Google Scholar]
- 24. Mo S, He Y, Zhu G, et al. A novel Peng’s test in reducing bile leakage after partial hepatectomy for hepatocellular carcinoma: from an animal study to a clinical cohort Propensity score matching comparative study. Int J Surg 2022;104:106748. [DOI] [PubMed] [Google Scholar]
- 25. Hess KR, Serachitopol DM, Brown BW. Hazard function estimators: a simulation study. Stat Med 1999;18:3075–3088. [DOI] [PubMed] [Google Scholar]
- 26. Müller HG, Wang JL. Hazard rate estimation under random censoring with varying kernels and bandwidths. Biometrics 1994;50:61–76. [PubMed] [Google Scholar]
- 27. Moons KG, Altman DG, Reitsma JB, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 2015;162:W1–W73. [DOI] [PubMed] [Google Scholar]
- 28. Xu L, Peng ZW, Chen MS, et al. Prognostic nomogram for patients with unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization. J Hepatol 2015;63:122–130. [DOI] [PubMed] [Google Scholar]
- 29. Skuladottir H, Olsen JH. Conditional survival of patients with the four major histologic subgroups of lung cancer in Denmark. J Clin Oncol 2003;21:3035–3040. [DOI] [PubMed] [Google Scholar]
- 30. Hagens ERC, Feenstra ML, Eshuis WJ, et al. Conditional survival after neoadjuvant chemoradiotherapy and surgery for oesophageal cancer. Br J Surg 2020;107:1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 2010;21:128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prentice RL, Zhao S. Regression models and multivariate life tables. J Am Stat Assoc 2021;116:1330–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hess KR, Levin VA. Getting more out of survival data by using the hazard function. Clin Cancer Res 2014;20:1404–1409. [DOI] [PubMed] [Google Scholar]
- 34. Liu XR, Pawitan Y, Clements M. Parametric and penalized generalized survival models. Stat Methods Med Res 2018;27:1531–1546. [DOI] [PubMed] [Google Scholar]
- 35. Kölmel KF, Kulle B, Lippold A, et al. Survival probabilities and hazard functions of malignant melanoma in Germany 1972–1996, an analysis of 10433 patients. Evolution of gender differences and malignancy. Eur J Cancer 2002;38:1388–1394. [DOI] [PubMed] [Google Scholar]
- 36. Poo-Hwu WJ, Ariyan S, Lamb L, et al. Follow-up recommendations for patients with American Joint Committee on Cancer Stages I–III malignant melanoma. Cancer 1999;86:2252–2258. [PubMed] [Google Scholar]
- 37. Rogers GS, Kopf AW, Rigel DS, et al. Hazard-rate analysis in state I malignant melanoma. Arch Dermatol 1986;122:999–1002. [PubMed] [Google Scholar]
- 38. Daneshvar P, Roffey DM, Brikeet YA, et al. Spinal cord injuries related to cervical spine fractures in elderly patients: factors affecting mortality. Spine J 2013;13:862–866. [DOI] [PubMed] [Google Scholar]
- 39. Morita T, Takebayashi T, Irifune H, et al. Factors affecting survival of patients in the acute phase of upper cervical spine injuries. Arch Orthop Trauma Surg 2017;137:543–548. [DOI] [PubMed] [Google Scholar]
- 40. Grimes DA, Schulz KF. False alarms and pseudo-epidemics: the limitations of observational epidemiology. Obstet Gynecol 2012;120:920–927. [DOI] [PubMed] [Google Scholar]
- 41. Balas M, Prömmel P, Nguyen L, et al. Reality of accomplishing surgery within 24 hours for complete cervical spinal cord injury: clinical practices and safety. J Neurotrauma 2021;38:3011–3019. [DOI] [PubMed] [Google Scholar]
- 42. Palumbo C, Mistretta FA, Knipper S, et al. Conditional survival of patients with nonmetastatic renal cell carcinoma: how cancer-specific mortality changes after nephrectomy. J Natl Compr Canc Netw 2020;18:44–51. [DOI] [PubMed] [Google Scholar]
- 43. Han L, Dai W, Mo S, et al. Nomogram of conditional survival probability of long-term survival for metastatic colorectal cancer: a real-world data retrospective cohort study from SEER database. Int J Surg 2021;92:106013. [DOI] [PubMed] [Google Scholar]
- 44. Song K, Lin K, Guan H, et al. Conditional survival analysis for spinal chondrosarcoma patients after surgical resection. Spine (Phila Pa 1976) 2020;45:1110–1117. [DOI] [PubMed] [Google Scholar]
- 45. Ji T, Guo W, Yang R, et al. What are the conditional survival and functional outcomes after surgical treatment of 115 patients with sacral chordoma? Clin Orthop Relat Res 2017;475:620–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Veenstra MY, Friesema IH, Zwietering PJ, et al. Lower prevalence of heart disease but higher mortality risk during follow-up was found among nonrespondents to a cohort study. J Clin Epidemiol 2006;59:412–420. [DOI] [PubMed] [Google Scholar]
- 47. Eagan TM, Eide GE, Gulsvik A, et al. Nonresponse in a community cohort study: predictors and consequences for exposure-disease associations. J Clin Epidemiol 2002;55:775–781. [DOI] [PubMed] [Google Scholar]
- 48. Batty GD, Gale CR. Impact of resurvey non-response on the associations between baseline risk factors and cardiovascular disease mortality: prospective cohort study. J Epidemiol Community Health 2009;63:952–955. [DOI] [PubMed] [Google Scholar]
- 49. Rönmark EP, Ekerljung L, Lötvall J, et al. Large scale questionnaire survey on respiratory health in Sweden: effects of late- and non-response. Respir Med 2009;103:1807–1815. [DOI] [PubMed] [Google Scholar]
- 50. Knoll L, Felten MK, Ackermann D, et al. Non-response bias in a surveillance program for asbestos-related lung cancer. J Occup Health 2011;53:16–22. [DOI] [PubMed] [Google Scholar]
- 51. Gustavson K, Røysamb E, Borren I. Preventing bias from selective non-response in population-based survey studies: findings from a Monte Carlo simulation study. BMC Med Res Methodol 2019;19:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.


