Background:
Human epidermal growth factor receptor 2 (HER2) is a well-developed therapeutic target in breast and gastric cancer (GC). However, the impact of HER2 on survival and benefit from fluorouracil-based adjuvant chemotherapy remains unclear in patients with GC.
Materials and Methods:
This multicenter cohort study involved 5622 consecutive stage II/III GC patients. HER2 expression was assessed prospectively via immunohistochemistry (IHC). The staining intensity was graded on a scale of 0 to 3+. An IHC score of 2+or 3+was defined as high expression, and a score of 3+was defined as overexpression.
Results:
HER2 overexpression was independently associated with a lower 5-year overall survival (OS) in stage II [hazard ratio (HR), 2.10; 95% CI: 1.41–3.11], but not in stage III GC (HR, 1.00; 95% CI, 0.82–1.20). Further analysis revealed that stage II patients with high HER2 expression showed a poorer response to chemotherapy than stage II patients with low HER2 expression (P interaction=0.024). The HRs for 5-year OS were 0.51 (95% CI, 0.38–0.70) for stage II patients with low HER2 expression, 0.58 (95% CI, 0.51–0.66) for stage III patients with low HER2 expression, 1.13 (95% CI, 0.61–2.09) for stage II patients with high HER2 expression, and 0.47 (95% CI, 0.36–0.61) for stage III patients with high HER2 expression.
Conclusions:
Fluorouracil-based adjuvant chemotherapy is insufficient for stage II GC patients with high HER2 expression, indicating that prospective trials are required to validate alternative HER2-targeted adjuvant therapies in the individuals above.
Keywords: adjuvant chemotherapy, gastric cancer, HER2, immunohistochemistry
Introduction
Highlights
Human epidermal growth factor receptor 2 (HER2) overexpression was a predictor of poor outcomes in stage II gastric cancer.
Stage II patients with high expression of HER2 could not benefit from chemotherapy.
HER2 expression was not associated with survival in stage III patients.
HER2 expression was not associated with chemotherapy response in stage III patients.
Human epidermal growth factor receptor 2 (HER2) is a member of the epidermal growth factor receptor family of receptor tyrosine kinases. When activated, HER proteins homodimerize or heterodimerize and initiate intricate cellular signaling pathways leading to cellular proliferation, tumorigenesis, and tumor cell metastasis1,2. Based on the ToGA (treatment of HER2-positive advanced gastric or gastroesophageal junction cancer) study, HER2-targeted therapy plus chemotherapy is the standard first-line treatment for advanced HER2-positive gastric cancer (GC), and HER2 expression can be used to predict the response to HER2-targeted therapies3. Notably, one recent study highlighted the importance of HER2 as a predictive biomarker for the efficacy of immune therapy in patients with GC4.
However, the prognostic relevance of HER2 expression in resectable GC has not been sufficiently validated and remains controversial. Approximately half of the relevant clinical studies showed that high HER2 expression is a significant predictor of poor survival5–8, but other studies showed that HER2 is not a prognostic factor9–12. Furthermore, HER2 amplification is crucial in conferring broad-spectrum chemoresistance13,14, but a lack of consensus exists regarding HER2 status as a predictive biomarker of the response to adjuvant chemotherapy in patients with GC15. Current clinical studies have fully demonstrated the importance of HER2 as a therapeutic target in advanced GC. Therefore, determining the prognostic relevance of HER2 after surgery for GC is important not only to accurately inform patients about their prognosis but also to select the best potential adjuvant treatment for each patient to improve long-term survival. Owing to the complicated biological nature of malignancies, specific biomarkers exhibit completely distinct relationships with survival and chemotherapy response in patients with various pathological stages16,17. Currently, little is known concerning the influence of HER2 expression on survival in subgroups of patients stratified by cancer stage. As a minority of gastric tumors show HER2 overexpression, further study with a larger sample size is required to fully understand the correlation between HER2 expression and clinical outcome.
Therefore, this study aimed to assess the ability of HER2 expression to predict prognosis and adjuvant chemotherapy benefit in patients with stage II/III GC based on a large number of GC patients from three oncology centers. We performed a subgroup analysis according to cancer stage to clarify the prognostic value of HER2 expression and its association with the benefit of adjuvant chemotherapy in patients with different cancer stages.
Methods
Study design and setting
This study was an analysis of the Multidisciplinary Alliance of Gastric Integrative Studies (MAGIS) cohort data set, a multicenter cohort including three well-known centers across China (centers 1, 2, and 3). The three centers were high-volume hospitals with extensive experience in GC surgery and comprehensive treatment. Before the MAGIS cohort was established, each center maintained its clinical cohort of GC patients18–20. However, data fields, processing, and normalization needed to be standardized. To promote relevant research in GC, we compiled the data of the three distinct institutes using a consistent standard and then produced the MAGIS cohort in 2019. This research was funded by the National Key R&D Program of China. The cohort was developed and registered with the Chinese clinical trial registry (http://www.chictr.org.cn). All three centers’ cohorts contain various prospectively collected clinical data and follow-up information. The center 1 cohort (the Xijing Hospital of Digestive Diseases Gastric Cancer Cohort) included patients enrolled from 2008 to 201818,19,21,22, and the center 2 cohort (the China National Cancer Center Gastric Cancer Cohort)18,23 and the center 3 cohort (the Tianjin Cancer Hospital Gastric Cancer Cohort)20,24,25 included patients who were treated from 2001 to 2018. In brief, after undergoing gastrectomy surgery, all patients were followed up through telephone interviews or in person at an outpatient clinic every 3–6 months for 3 years and annually thereafter until death to ascertain their survivorship and adjuvant treatment information. The follow-up information was then compared with the clinical data captured from electronic medical records and annual reports from the national cancer registry to confirm their accuracy. Each participating center’s ethics committee approved the study protocol.
Inclusion and exclusion criteria
This work is reported in line with the REMARK (REporting recommendations for tumor MARKer prognostic studies) criteria, Supplemental Digital Content 1, http://links.lww.com/JS9/A281. The inclusion criteria were as follows: age 18 years or older, histologically confirmed gastric adenocarcinoma, pathologically negative resection margins (R0), pathologically proven tumor-node-metastasis (pTNM) stage II/III GC, and complete clinicopathological and follow-up data. The exclusion criteria were as follows: other types of malignancies in the stomach, history of gastrectomy or other malignant tumors, palliative surgical resection, pathologically proven TNM stage I GC, peritoneal dissemination or distant metastasis, neoadjuvant chemotherapy or radiotherapy before surgery, perioperative mortality (one month after surgery), missing values (the percentage of missing values for each covariate was lower than 7%), and unknown HER2 status. The flow diagram is presented in Figure 1A. Ultimately, a total of 5622 consecutive patients with stage II/III GC who underwent radical gastrectomy without preoperative therapy between May 2010 and September 2017 were included. The numbers of patients at medical centers 1–3 were 3304 (center 1: 1039 stage II and 2265 stage III), 1693 (center 2: 627 stage II and 1066 stage III), and 625 (center 3: 193 stage II and 432 stage III), respectively.
Figure 1.
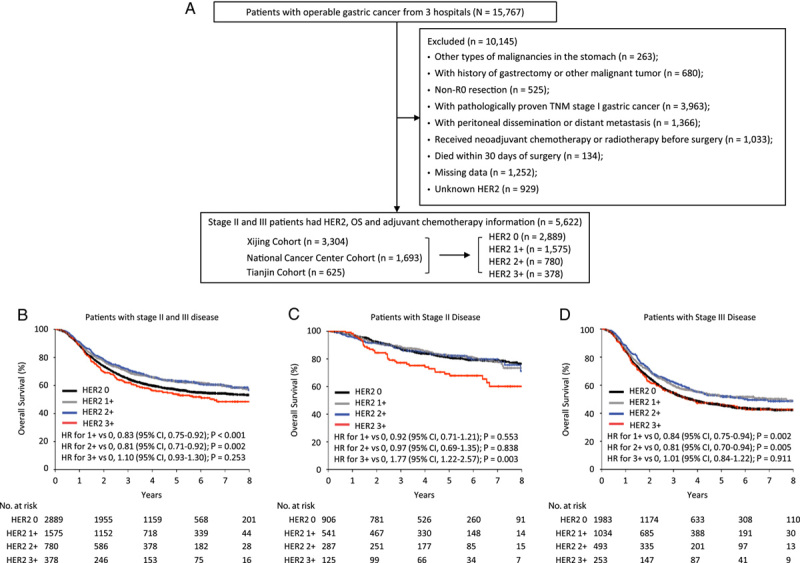
Study design and prognostic value of HER2 expression. (A) Study profile; (B–D) Kaplan–Meier estimates of overall survival according to HER2 expression in patients with stages II and III disease (B), patients with stage II disease (C), and patients with stage III disease (D). HER2, human epidermal growth factor receptor 2; HR, hazard ratio.
Data collection and processing
The clinicopathological characteristics of each patient were retrieved from the MAGIS cohort database and hospital information systems. Because the pTNM staging system changed during the study period, it was uniformly adjusted according to the 8th edition of the American Joint Committee on Cancer (AJCC) staging manual.
Therapeutic interventions and outcomes
In this study, standard fluorouracil-based adjuvant chemotherapy was recommended for patients. Adjuvant chemotherapy was defined as the administration of one or more cycles of chemotherapy after surgery. Among 4834 patients who received standard adjuvant chemotherapy, 741 (15.3%) received single-agent 5-fluorouracil, and 4093 (84.7%) received multiagent chemotherapy (a combination of 5-fluorouracil and cisplatin/oxaliplatin, doxorubicin, or paclitaxel/docetaxel).
The primary outcome was overall survival (OS), which was assessed from the date of gastrectomy to the date of death or the last follow-up. The secondary outcome was disease-free survival (DFS), which was defined as the time from the date of primary surgery until the first evidence of relapse, metastasis, or death from any cause, whichever occurred first, and was scored as an event. The last follow-up dates in this study were 9 October 2020, for center 1, 31 December 2018, for center 2, and 1 August 2020, for center 3.
Immunohistochemistry and in situ hybridization
Paraffin-embedded tissue sections were used for HER2 immunohistochemistry (IHC) analysis. HER2 staining was performed prospectively within one week after surgery. To minimize the possible effects of sample heterogeneity, we only performed the HER2 IHC test on surgical resection specimens. In addition, a small section of HER2-positive GC tissue sample was loaded as a positive control for each sample as a quality control measure. All tissue sections were automatically stained with a Bond-Max automatic stainer using primary antibodies against HER2/neu monoclonal antibodies (Clone 4B5; Roche Diagnostics). Fluorescence in situ hybridization (FISH) analysis was carried out in representative patients with HER2 IHC 2+from the MAGIS cohort according to the manufacturer’s protocol using the HER2 gene detection kit (FISH) (Guang Zhou LBP Medicine Science & Technology Co., Ltd). HER2 IHC expression and FISH amplification were evaluated and scored by experienced pathologists according to Hofmann’s criteria26,27. The IHC staining intensity was graded on a scale of 0 to 3+(representative images are shown in Fig. S1, Supplemental Digital Content 2, http://links.lww.com/JS9/A282). For the final scores, an IHC score of 0 was defined as a loss of expression, an IHC score of 0 or 1+was defined as low expression, an IHC score of 2+or 3+was defined as high expression, and an IHC score of 3+was defined as overexpression. Amplification of HER2 by FISH was defined as a HER2/CEP17 ratio of at least 2.0 (representative images are shown in Fig. S2, Supplemental Digital Content 2, http://links.lww.com/JS9/A282).
Statistical analysis
Baseline patient characteristics were described according to the HER2 expression status and were compared using one-way ANOVA for continuous variables and the χ 2 test for categorical variables. Bivariate logistic regression was used to identify risk factors for HER2 overexpression, and factors that were statistically significant with P less than 0.05 as well as pathologic T stage were included as covariates in multivariable models.
Survival outcomes were estimated using Kaplan–Meier curves and compared using a two-sided log-rank test. Univariate and multivariate hazard ratios (HRs) with 95% CIs were calculated using the Cox proportional hazards model. Interactions between HER2 status and treatment were evaluated using the Cox proportional hazards method in a 2×2 factorial design.
Subgroup analysis
To determine whether the effect of HER2 on survival and adjuvant chemotherapy response varied with different pathological stages and chemotherapy drugs, we established Cox models for each subgroup to estimate HRs.
Validation of the analysis
We adopted multiple statistical approaches to validate the robustness of our main results, including univariable and multivariable regression analyses, and stratification of cohorts. To minimize the influence of the difference in baseline clinicopathologic factors on the analysis of adjuvant chemotherapy benefits, we used propensity score matching to compare survival between the adjuvant chemotherapy and surgery-only groups.
A two-sided P value less than 0.05 was considered statistically significant. Statistical analyses were conducted using SPSS (version 26.0; SPSS Inc.) and R software (version 3.3.2; statistical software).
Results
HER2 expression and association with baseline characteristics
The IHC results obtained for HER2 expression in all 5622 patients were as follows: score 0, 2889 patients (51.4%); score 1+, 1575 patients (28.0%); score 2+, 780 patients (13.9%); and score 3+, 378 patients (6.7%). Importantly, the intensity of HER2 staining was in good agreement among the three different centers (Supplementary Fig. S1E, Supplemental Digital Content 2, http://links.lww.com/JS9/A282). The rates of HER2 score 2+were 13.6% (449/3304), 13.2% (224/1693), and 13.9% (87/625) in centers 1, 2, and 3, respectively. The rates of HER2 score 3+were 7.5% (247/3304), 5.5% (93/1693), and 6.1% (38/625) in centers 1, 2, and 3, respectively.
The demographic and clinicopathological characteristics of the study participants are summarized in Table 1. In univariate analysis, the frequency of HER2 overexpression was correlated with age, gender, primary tumor location, histological differentiation, and pathologic N stage (Table 2), and was more common in older patients, male patients, and those with tumors located in the gastroesophageal junction (GEJ), well or moderate histological differentiation, and advanced N stage. In multivariate analysis, HER2 overexpression status was significantly associated with histological differentiation and pathologic N stage. HER2 overexpression was less common in tumors with poor histological differentiation [odds ratio (OR), 0.27; 95% CI, 0.16–0.47; P<0.001] and more common in advanced N stage disease (OR, 2.11; 95% CI, 1.51–2.95; P<0.001) (Table 2).
Table 1.
Clinical and demographic variables by HER2 status.
| Variable | All cohort (n=5622) | HER2 0 (n=2889) | HER2 1+ (n=1575) | HER2 2+ (n=780) | HER2 3+ (n=378) | P |
|---|---|---|---|---|---|---|
| Age (years) | 0.001 | |||||
| Mean±SD | 57.5±10.7 | 57.0±10.9 | 57.9±10.7 | 58.1±10.4 | 58.8±10.1 | |
| Sex | 0.003 | |||||
| Female | 1409 (25.1) | 776 (26.9) | 379 (24.1) | 181 (23.2) | 73 (19.3) | |
| Male | 4213 (74.9) | 2113 (73.1) | 1196 (75.9) | 599 (76.8) | 305 (80.7) | |
| Primary tumor location | <0.001 | |||||
| GEJ cancer | 1766 (31.4) | 830 (28.7) | 520 (33.0) | 266 (34.1) | 150 (39.7) | |
| Gastric cancer | 3856 (68.6) | 2059 (71.3) | 1055 (67.0) | 514 (65.9) | 228 (60.3) | |
| Histology | <0.001 | |||||
| High differentiation | 133 (2.4) | 56 (1.9) | 35 (2.2) | 25 (3.2) | 17 (4.5) | |
| Middle differentiation | 1062 (18.9) | 378 (13.1) | 281 (17.8) | 237 (30.4) | 166 (43.9) | |
| Poor differentiation | 4427 (78.8) | 2455 (85.0) | 1259 (80.0) | 518 (66.4) | 195 (51.6) | |
| Size (cm) | <0.001 | |||||
| Mean±SD | 5.3±2.6 | 5.5±2.8 | 5.0±2.4 | 5.1±2.2 | 5.3±2.1 | |
| Perineural invasion | 0.015 | |||||
| No | 1482 (26.4) | 748 (25.9) | 389 (24.7) | 239 (30.6) | 106 (28.0) | |
| Yes | 4140 (73.6) | 2141 (74.1) | 1186 (75.3) | 541 (69.4) | 272 (72.0) | |
| Lymphovascular invasion | 0.032 | |||||
| No | 2169 (38.6) | 1078 (37.3) | 616 (39.1) | 335 (42.9) | 140 (37.0) | |
| Yes | 3453 (61.4) | 1811 (62.7) | 959 (60.9) | 445 (57.1) | 238 (63.0) | |
| Examined lymph nodes | 0.076 | |||||
| ≥16 | 5275 (93.8) | 2690 (93.1) | 1482 (94.1) | 742 (95.1) | 361 (95.5) | |
| <16 | 347 (6.2) | 199 (6.9) | 93 (5.9) | 38 (4.9) | 17 (4.5) | |
| T stage | <0.001 | |||||
| T1 | 119 (2.1) | 49 (1.7) | 42 (2.7) | 21 (2.7) | 7 (1.9) | |
| T2 | 467 (8.3) | 216 (7.5) | 138 (8.8) | 69 (8.8) | 44 (11.6) | |
| T3 | 1814 (32.3) | 803 (27.8) | 578 (36.7) | 298 (38.2) | 135 (35.7) | |
| T4 | 3222 (57.3) | 1821 (63) | 817 (51.9) | 392 (50.3) | 192 (50.8) | |
| N stage | 0.020 | |||||
| N0 | 1046 (18.6) | 539 (18.7) | 285 (18.1) | 165 (21.2) | 57 (15.1) | |
| N1 | 1063 (18.9) | 557 (19.3) | 286 (18.2) | 142 (18.2) | 78 (20.6) | |
| N2 | 1401 (24.9) | 678 (23.5) | 422 (26.8) | 213 (27.3) | 88 (23.3) | |
| N3 | 2112 (37.6) | 1115 (38.6) | 582 (37.0) | 260 (33.3) | 155 (41.0) | |
| Pathologic stage | 0.020 | |||||
| Stage II | 1859 (33.1) | 906 (31.4) | 541 (34.3) | 287 (36.8) | 125 (33.1) | |
| Stage III | 3763 (66.9) | 1983 (68.6) | 1034 (65.7) | 493 (63.2) | 253 (66.9) | |
| Adjuvant chemotherapy | 0.554 | |||||
| No | 788 (14.0) | 387 (13.4) | 228 (14.5) | 118 (15.1) | 55 (14.6) | |
| Yes | 4834 (86.0) | 2502 (86.6) | 1347 (85.5) | 662 (84.9) | 323 (85.4) |
GEJ, gastroesophageal junction; HER2, human epidermal growth factor receptor 2.
Table 2.
Univariable and multivariable logistic regression analyses for predicting HER2 score 3+.
| Univariable analysis | Multivariable model | |||||
|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | P | OR | 95% CI | P |
| Age (per 1-year increase) | 1.01 | 1.00–1.02 | 0.015 | 1.00 | 0.99–1.01 | 0.696 |
| Female (vs. male) | 0.70 | 0.54–0.91 | 0.008 | 0.86 | 0.65–1.13 | 0.265 |
| Primary tumor location (vs. GEJ cancer) | 0.68 | 0.55–0.84 | <0.001 | 0.84 | 0.67–1.06 | 0.147 |
| Histological differentiation (vs. well) | <0.001 | <0.001 | ||||
| Moderate | 1.26 | 0.74–2.16 | 0.391 | 1.18 | 0.69–2.03 | 0.543 |
| Poor | 0.31 | 0.19–0.53 | <0.001 | 0.27 | 0.16–0.47 | <0.001 |
| Tumor size (per 1 cm increase) | 0.99 | 0.95–1.03 | 0.716 | |||
| Perineural invasion (vs. absent) | 0.91 | 0.72–1.15 | 0.442 | |||
| Lymphovascular invasion (vs. absent) | 1.07 | 0.86–1.33 | 0.523 | |||
| Examined lymph nodes (vs. ≥16) | 0.70 | 0.43–1.16 | 0.163 | |||
| T stage (vs. T1) | 0.019 | 0.164 | ||||
| T2 | 1.66 | 0.73–3.80 | 0.226 | 1.40 | 0.60–3.28 | 0.439 |
| T3 | 1.29 | 0.59–2.82 | 0.529 | 0.97 | 0.43–2.19 | 0.934 |
| T4 | 1.01 | 0.47–2.21 | 0.972 | 0.92 | 0.41–2.06 | 0.844 |
| N stage (vs. N0) | 0.169 | <0.001 | ||||
| N1 | 1.37 | 0.97–1.96 | 0.078 | 1.38 | 0.95–1.99 | 0.088 |
| N2 | 1.16 | 0.83–1.64 | 0.389 | 1.37 | 0.95–1.96 | 0.088 |
| N3 | 1.37 | 1.01–1.88 | 0.047 | 2.11 | 1.51–2.95 | <0.001 |
GEJ, gastroesophageal junction; HER2, human epidermal growth factor receptor 2; OR, odds ratio.
Impact of HER2 status on survival
To evaluate the prognostic capability of HER2 expression in patients with stage II/III GC, we used Kaplan–Meier survival analysis to compare OS according to HER2 status. In univariate analysis, HER2 status was associated with outcomes in the study cohort as a whole (P<0.001), and patients with HER2 overexpression appeared to have the worst prognosis (Fig. 1B; Supplementary Table S1, Supplemental Digital Content 3, http://links.lww.com/JS9/A283). Multivariate analysis controlling for potential confounders revealed no significant relationship between HER2 status and OS (P=0.182) (Supplementary Table S2, Supplemental Digital Content 3, http://links.lww.com/JS9/A283).
To evaluate our findings regarding the value of HER2 expression in prognosis prediction among patients at different disease stages, we divided the patients into stage II and stage III subgroups. In patients with stage II disease (n=1859), a HER2 score of 3+was associated with a lower 5-year OS rate, whereas all other expression levels were comparable (Fig. 1C; Supplementary Table S1, Supplemental Digital Content 3, http://links.lww.com/JS9/A283). Following multivariable adjustment, the HR for HER2 score 3+versus score 0 was 2.10 (95% CI, 1.41–3.11; P<0.001) (Supplementary Table S3, Supplemental Digital Content 3, http://links.lww.com/JS9/A283), indicating the potential clinical significance of HER2 for survival prediction in patients with stage II GC. Among patients with stage III disease (n=3763), patients with a HER2 score of 1 or 2+had a significantly better prognosis than those with a HER2 score of 0 or 3+in univariate analysis (Fig. 1D). Nevertheless, multivariate analysis indicated that HER2 expression was not associated with the outcome (P=0.395) (Supplementary Table S4, Supplemental Digital Content 3, http://links.lww.com/JS9/A283).
HER2 status and benefit from adjuvant chemotherapy
To evaluate whether HER2 expression is a predictive marker of the adjuvant chemotherapy response, we investigated the association between HER2 status and survival in patients receiving and not receiving adjuvant chemotherapy. Treatment with adjuvant chemotherapy was significantly associated with a higher OS rate in both stage II and stage III subgroup patients with low HER2 expression and in stage III subgroup patients with high HER2 expression (all P<0.001; Fig. 2A, B, D). Interestingly, patients in the stage II subgroup with high HER2 expression showed no survival benefit from adjuvant chemotherapy (Fig. 2C). Patients in the stage II subgroup with high HER2 expression had 5-year OS rates of 81.9% when treated solely with surgery and 77.5% when treated concurrently with chemotherapy (HR=1.13; 95% CI: 0.61–2.09; P=0.695). A test for the interaction between HER2 status and treatment also revealed that the benefit observed in HER2 low-expression patients was greater than that observed in HER2 high-expression patients in the stage II subgroup (P=0.024 for the interaction) but not in the stage III subgroup (P=0.177 for the interaction) (Fig. 2E). The trend of DFS was similar to that of OS. Treatment with adjuvant chemotherapy was significantly associated with a higher DFS rate in both the stage II and stage III subgroup patients with low HER2 expression, as well as in the stage III subgroup patients with high HER2 expression (all P<0.05; Figure S3A, S3B, and S3D, Supplemental Digital Content 2, http://links.lww.com/JS9/A282), but not in the stage II subgroup patients with high HER2 expression (P=0.674; Figure S3C, Supplemental Digital Content 2, http://links.lww.com/JS9/A282).
Figure 2.
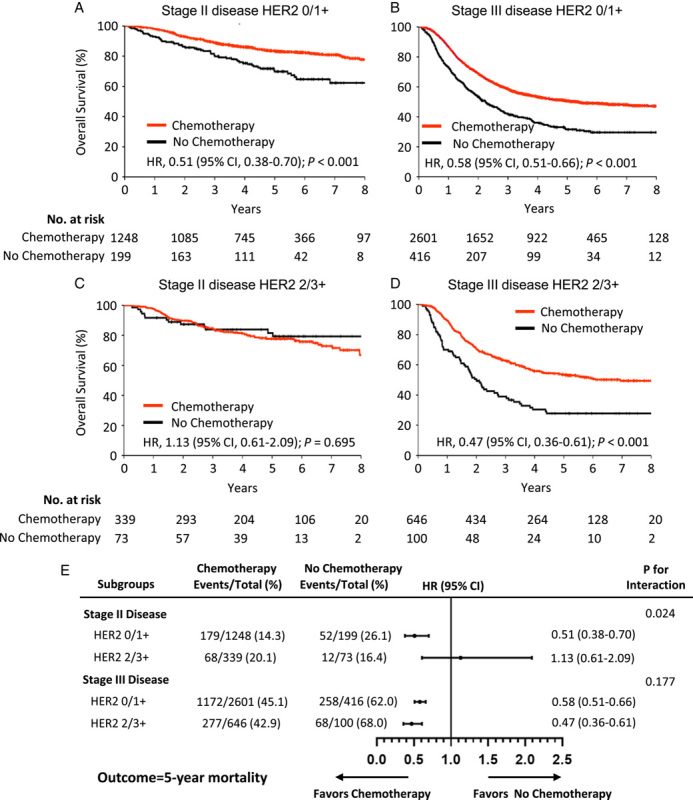
Relationship between HER2 status and benefit from adjuvant chemotherapy. (A) Stage II subgroup with low HER2 expression [immunohistochemistry (IHC) scores of 0/1+]; (B) stage III subgroup with low HER2 expression; (C) stage II subgroup with high HER2 expression (IHC scores of 2/3+); (D) stage III subgroup with high HER2 expression; (E) treatment interaction with HER2 expression for 5-year overall survival. HER2, human epidermal growth factor receptor 2; HR, hazard ratio.
Considering that the significant association between HER2 status and chemotherapy efficacy was confined to patients with stage II disease, we divided the patients into four further detailed subgroups based on HER2 expression (score 0 to 3+) and obtained consistent results (Fig. 3). To mitigate the effects of differences in baseline characteristics among patients received postoperative adjuvant chemotherapy and surgery only, we adjusted for confounding factors by propensity score matching. After matching, the baseline characteristics of the two groups of patients were similar (Table S5, 6, Supplemental Digital Content 3, http://links.lww.com/JS9/A283). The results revealed that adjuvant chemotherapy was still not associated with any improvement in overall survival in both HER2 IHC 2+and 3+cohorts (both P>0.05) (Fig. S4, Supplemental Digital Content 2, http://links.lww.com/JS9/A282).
Figure 3.
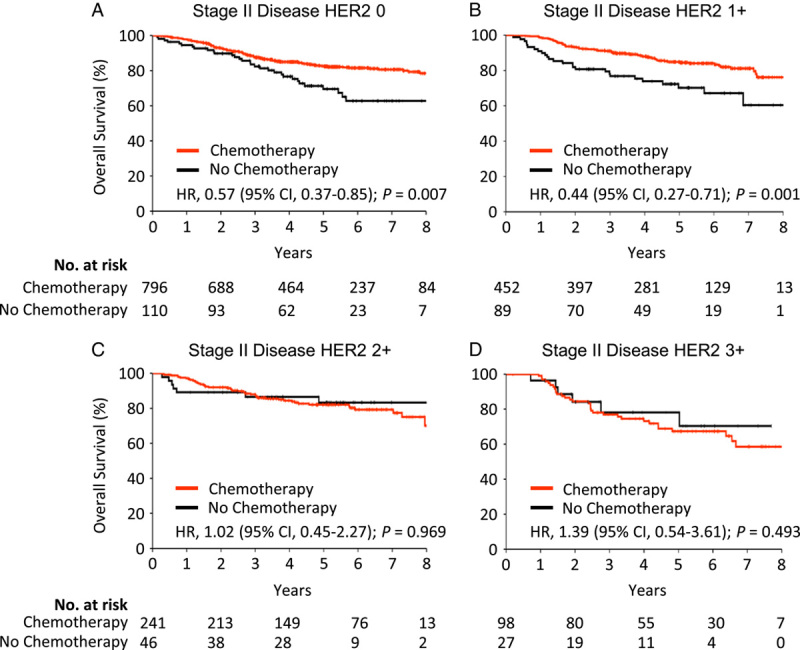
Relationship between HER2 status and benefit from adjuvant chemotherapy in the stage II subgroup. (A) Stage II subgroup with a HER2 expression score of 0; (B) stage II subgroup with a HER2 expression score of 1+; (C) stage II subgroup with a HER2 expression score of 2+; (D) stage II subgroup with a HER2 expression score of 3+. HER2, human epidermal growth factor receptor 2; HR, hazard ratio.
To assess the robustness of our findings, we classified the patients into internal and external data sets based on their affiliation with the participating centers (internal data set: center 1, nearly 60% of the patients were drawn from this center; external data set: pooling data from centers 2 and 3). We obtained similar results for the two independent data sets (Fig. 4). In both data sets, adjuvant chemotherapy was associated with improved survival in stage III patients, regardless of low or high HER2 expression (all P<0.05). The survival benefit of adjuvant chemotherapy persisted in stage II patients with low HER2 expression (both P<0.05) but not in stage II patients with high HER2 expression (P=0.616 and 0.481).
Figure 4.
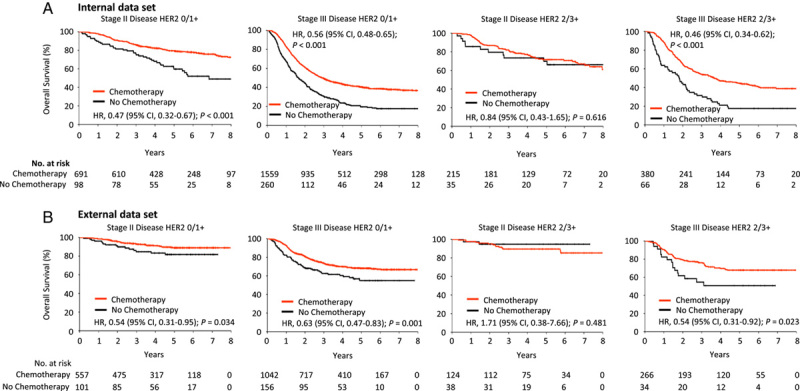
Relationship between HER2 expression and benefit from adjuvant chemotherapy in different cohorts. The association between HER2 expression and overall survival was investigated in the internal data set (A) and external data set (B). HER2, human epidermal growth factor receptor 2; HR, hazard ratio.
To assess the effect of HER2 amplification on our findings, we performed FISH on 130 representative patients with HER2 IHC 2+and identified 30/130 patients (23.1%) with amplification. This proportion of HER2 amplification is consistent with that in previous studies3,12. Of the 130 patients with HER2 scores 2+, 55 were stage II and 75 were stage III. Consistent with the results in the original cohort, adjuvant chemotherapy was significantly associated with a higher OS rate in patients with stage III disease (P=0.013), while patients with stage II disease showed no survival benefit from adjuvant chemotherapy (P=0.570) (Fig. S5A, S5D, Supplemental Digital Content 2, http://links.lww.com/JS9/A282). Then we performed a subgroup analysis of tumors with and without HER2 amplification. This analysis showed that the association between HER2 status and adjuvant chemotherapy effectiveness in patients with HER2 score 2+was unaffected by HER2 amplification (Fig. S5, Supplemental Digital Content 2, http://links.lww.com/JS9/A282).
To address the confounding effect of different chemotherapy regimens, we divided the patients into three groups based on the number of adjuvant chemotherapy drugs. In contrast to patients with low HER2 expression (Fig. 5A, B), those with high HER2 expression did not benefit from chemotherapy, even if they had received multiagent chemotherapy (Fig. 5C, D). Additionally, the association between the HER2 status and adjuvant chemotherapy effectiveness was unaffected by the depth of invasion of the primary tumor (T1/T2 vs. T3/T4), the number of metastatic lymph nodes (N0/N1 vs. N2/N3), and the primary tumor site (GEJ vs. gastric) (Supplementary Fig. S6–S8, Supplemental Digital Content 2, http://links.lww.com/JS9/A282).
Figure 5.
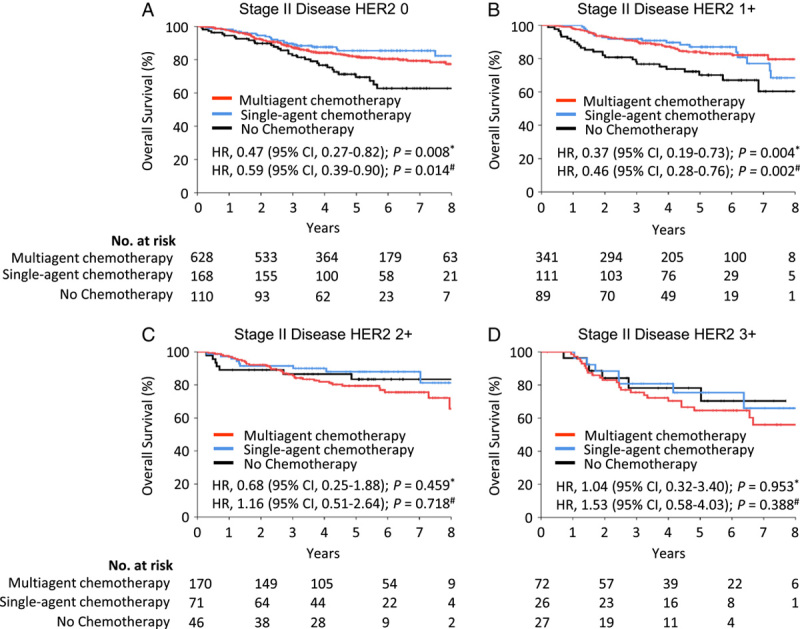
Relationship between the number of adjuvant chemotherapy drugs and overall survival in the stage II patients, stratified by HER2 expression. (A) Overall survival in stage II patients with a HER2 IHC score of 0; (B) overall survival in stage II patients with a HER2 immunohistochemistry (IHC) score of 1+; (C) overall survival in stage II patients with a HER2 IHC score of 2+; (D) overall survival in stage II patients with a HER2 IHC score of 3+. *Received single-agent chemotherapy versus no adjuvant chemotherapy; #Received multiagent chemotherapy versus no adjuvant chemotherapy. HER2, human epidermal growth factor receptor 2; HR, hazard ratio.
Discussion
In this study, we found that HER2 overexpression tumors were more often of advanced N stage and were well or moderately differentiated in terms of histology. Furthermore, we confirmed that HER2 overexpression tumors were linked with a worse outcome than HER2 loss-of-expression tumors in patients with stage II disease. This effect is independent of several well-established risk factors, including the primary tumor’s invasion depth and the number of metastatic lymph nodes. The most critical finding in the present study was that stage II patients with high HER2 expression did not benefit from fluorouracil-based adjuvant chemotherapy.
According to previous multicenter studies, the rate of HER2 IHC 3+in Chinese patients with GC ranged between 8 and 10%12,28. In our investigation, the rate of HER2 overexpression determined by IHC alone was 6.7%, slightly lower than the previously reported values. HER2 overexpression is more frequent in advanced GC than in early GC15,29. This slight discrepancy can be explained by the fact that, unlike many other studies, our cohort only included patients with resectable stage II/III disease.
In univariate analysis, we confirmed that HER2 overexpression was predominantly associated with older age8,11,30, male sex8,11,12,30, tumors with a GEJ location12,30, well-differentiated tumors or moderate tumor differentiation8,12,31,32, and regional lymph node metastasis8,31. Unlike previous studies, we used multivariate analysis to further assess the risk factors for HER2 overexpression. Multivariate analysis revealed that HER2 overexpression was independently associated with only histological differentiation and lymph node metastasis. In this study, patients with well-differentiated tumors were more likely to be elderly, male, and have GEJ cancers (Table S7, Supplemental Digital Content 3, http://links.lww.com/JS9/A283), which was in line with previous results10. As a result, HER2 status may be indirectly correlated with age, gender, and tumor location. More importantly, we discovered that the relationship between HER2 overexpression and lymph node metastasis was stronger in multivariate analysis than in univariate analysis. Accordingly, this result suggests that tumors overexpressing HER2 are more likely to spread through the lymphatic system.
HER2 is a major oncogenic driver across various malignancies, including breast cancer and GC33,34. Although it has been generally accepted that HER2 overexpression is a strong predictor of poor disease in breast cancer35,36, the relationship between HER2 expression and prognosis in GC remains controversial. In the whole cohort, our results indicated that HER2 status was not an independent prognostic factor9,10,12. Most consecutively admitted patients for GC surgery were prospectively tested for HER2. Therefore, the current study has the essential advantage of showing no evidence of selection bias among the participants. Interestingly, HER2 overexpression related to well histological differentiation was associated with a better prognosis12,31, whereas HER2 overexpression related to lymph node metastasis was associated with a worse prognosis8,31. Therefore, the relationship between HER2 status and survival is convoluted. Because the pathological stage is the most crucial factor affecting prognosis in patients with GC, we performed a subgroup analysis stratifying by pathological stage. To the best of our knowledge, few studies have focused specifically on the prognostic role of HER2 in GC patients with different cancer stages. We found that HER2 overexpression was associated with a more than two-fold increased risk of mortality and that this negative prognostic impact of HER2 was confined to stage II GC. For patients with stage III disease, although univariate analysis showed that patients with HER2 scores 1+and 2+had a better prognosis, multivariate regression analysis revealed that HER2 expression was not an independent prognostic factor. As shown in Table 1, the expression levels of HER2 were significantly associated with various clinicopathological factors, including age, sex, histological differentiation, tumor size, lymphovascular invasion, T stage, and N stage. Therefore, the significant association between HER2 expression and survival is an artifact of a complex interaction of multiple risk factors in univariate analysis of stage III disease. In multivariate analysis, the effects of other confounding factors were adjusted to reveal the true effect of HER2 expression on GC prognosis. Because patients with stage II GC have a considerably better prognosis than those with stage III GC, HER2 overexpression is more likely to drive malignant phenotypes in these patients. This finding is consistent with that of a previous study on colon cancer. Wu et al.37 reported a series of 2088 stage II/III colon cancer patients and found a negative prognostic impact of HER2 overexpression in only stage II patients without high-risk factors.
Notably, in patients with stage II GC, HER2 status was predictive of not only the prognosis of patients but also the efficacy of adjuvant chemotherapy. The OS benefit of adjuvant chemotherapy was lower in stage II patients with high HER2 expression than in those with low HER2 expression. According to data from a randomized clinical trial of postoperative chemoradiation versus surgery in patients with stages I–III GC (n=258), individuals with HER2-positive GC do not benefit from adjuvant chemoradiation compared to surgery alone6. Our findings extend those of the previous study, confirming that the detrimental effect of high HER2 expression on chemotherapy efficacy was observed only in patients with stage II GC, but not in patients with stage III disease. In contrast to our findings, data from another randomized clinical trial of postoperative adjuvant S-1 therapy versus surgery in patients with stage II/III GC (n=829) showed that HER2 had no impact on the efficacy of adjuvant chemotherapy11. As the minority of gastric tumors are HER2-positive, previous studies have the disadvantages of a relatively small number of HER2-positive patients and an insufficient number of patients for subgrouping analysis based on stage. Compared with previous studies, the number of patients in our study was the largest, allowing us to draw reliable conclusions.
The role of HER2 in drug resistance could partially explain why fluorouracil-based adjuvant chemotherapy was insufficient for stage II patients with high HER2 expression. Several studies have found that inhibition of HER2 using an anti-HER2 antibody causes a significant change in the drug sensitivity of gastric tumor cells13,38–40. Human gastric cancer stem cells engineered to downregulate HER2 could reduce drug resistance, including resistance to oxaliplatin and 5-fluorouracil13. In breast cancer, HER2 overexpression leads to rapid regrowth of tumor cells and multidrug resistance, including resistance to 5-fluorouracil14,41. This study suggests that the detrimental effect of high HER2 expression on the chemotherapy response was stronger in stage II patients than in stage III patients. Consistent results were obtained in both the internal data set and the external data set. The clonal evolution of cancer might explain this striking effect. Based on the concept of clonal evolution in cancer, tumors gain increased genetic heterogeneity and divergent subclonal evolution as the stage progresses42,43. The prognostic factors in patients with stage III GC are more complicated than those in patients with stage II GC, including extensive lymph node metastases and relatively high heterogeneity, which may attenuate the impact of HER2 expression on the chemotherapy response. This may explain the findings in our study.
HER2 is a promising therapeutic target for the development of novel drugs. Although HER2-targeted therapy has also been approved for the treatment of HER2-positive early breast cancer in an adjuvant setting, the applicability of HER2-targeted therapy to early-stage GC remains unclear. Only a few published phase II studies have explored the safety and efficacy of trastuzumab plus chemoradiotherapy/chemotherapy for patients with HER2-positive locally advanced esophagogastric/gastric adenocarcinoma in the perioperative setting44,45. They found that this combination therapy significantly improved the pathologic complete response rate. However, because of the lack of a control group, it is difficult to assess its effectiveness in terms of survival in these two studies. Recently, Hofheinz et al. reported the first trial showing significant improvement in tumor remission with the combination of a doublet HER2-targeted antibody (trastuzumab and pertuzumab) and preoperative chemotherapy compared with chemotherapy alone (35% vs. 12%) in HER2-positive locally advanced esophagogastric adenocarcinoma46. However, this study was limited by its relatively small sample size and short follow-up period. DFS and OS did not differ substantially between the two groups. Therefore, although anti-HER2 therapy improves the pathological remission rate of neoadjuvant therapy patients, whether it can improve long-term survival remains to be further studied. Importantly, there are no published prospective clinical trials evaluating the efficacy of HER2 inhibitors in the adjuvant setting. Additionally, recent studies have reported several more potent anti-HER2 treatments in metastatic GC patients, including trastuzumab deruxtecan47,48 and the combination of pembrolizumab and trastuzumab4,49. Thus, these findings, combined with ours, highlight the need for HER2-targeted adjuvant therapies in patients with stage II GC.
A key strength of this study is the use of a large multicenter sample of consecutive patients with a long follow-up time, which enabled us to obtain a well-characterized group of GC patients. In addition, HER2 expression testing was prospectively performed in all patients using a uniform detection method. Other strengths of the current study include the consistent statistical results in nearly all subgroups and the sensitivity analyses. The limitations of this study include the fact that many self-reported data on disease recurrence/distant metastases are more accurate. Therefore, in this study, we analyzed both DFS and OS to make the results more reliable. Generally, although all the patients enrolled in the study had received fluorouracil-based standard regimens for adjuvant chemotherapy, the chemotherapy regimens were not assigned; rather, they were usually presented by experienced physicians, limiting the power to attempt subgroup analyses on the effect of different chemotherapy regimens. Additionally, only patients with stage II/III gastric cancer were included in this study, we did not evaluate the effect of HER2 expression on the efficacy of palliative chemotherapy in patients with metastatic disease. Owing to this study’s real-world and nonrandomized nature, these results need to be validated in a prospective, more prominent, multicenter randomized trial.
Conclusions
In conclusion, the current study demonstrates that HER2 overexpression is a poor prognostic factor in patients with stage II GC but not in those with stage III GC. High HER2 expression identified a subgroup of patients with stage II GC who did not derive benefit from fluorouracil-based adjuvant chemotherapy. Therefore, further prospective studies are warranted to evaluate whether anti-HER2 therapy can enhance outcomes even further in patients with high HER2 expression. When adjuvant therapy is advised for individual patients, we advocate taking the HER2 status into account.
Ethical approval
The Ethics Committee of the Xijing Hospital and the respective institutional ethics committees of the participating centers approved the study (KY20182088-F-1).
Sources of funding
This study was supported by the National Natural Science Foundation of China (82202837, 81972761), the National Key R&D Program of China (Grant Nos. 2016YFC1303200, 2017YFC0908300, 2019YFC1316304 and 2022YFC2505100), and Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-009A). All these study sponsors have no roles in the study design, in the collection, analysis, and interpretation of data. We gratefully acknowledge Chengyin Liu for editing the manuscript.
Author contribution
Y.N., Y.C., J.D., K.W., and H.L.: concept and design; X.G., L.Z., N.Z., and W.H.: drafting of the manuscript; G.J., Q.Z., Y.L., Z.L., L.S., H.L., K.W., J.D., Y.C., and Y.N.: critical revision of the manuscript for important intellectual content; X.G., L.Z., N.Z., and L.S.: statistical analysis; Y.N., K.W., X.G., and J.D.: funding; L.C., Z.L., and L.S.: administrative, technical, and material support; J.D., Y.C., and Y.N.: supervision. All authors were involved in the acquisition, analysis, or interpretation of data.
Conflicts of interest disclosure
The authors declare no conflicts of interest.
Research registration unique identifying number (UIN)
Name of the registry: Chinese Clinical Trial Registry chictr.org.cn
Unique identifying number or registration ID: ChiCTR1900026130.
Hyperlink to your specific registration (must be publicly accessible and will be checked): http://www.chictr.org.cn/showproj.aspx?proj=43603
Guarantor
Yongzhan Nie, Yingtai Chen, and Jingyu Deng.
Data availability statement
The data that support the findings of this study are available from the corresponding author, NYZ, upon reasonable request. The data are not publicly available since this could compromise the privacy of research participants.
Supplementary Material
Footnotes
Xianchun Gao, Lulu Zhao, Nannan Zhang, and Weili Han contributed equally to this article.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.journal-surgery.net.
Published online 12 April 2023
Contributor Information
Xianchun Gao, Email: gigaoxc@163.com.
Lulu Zhao, Email: lulu_2019@163.com.
Nannan Zhang, Email: natilen@163.com.
Weili Han, Email: hanweilibhxy@163.com.
Kun Liu, Email: 849137384@qq.com.
Junya Yan, Email: yjunya1471@126.com.
Ling Chen, Email: chenling_1976@163.com.
Yan Pan, Email: panpan@fmmu.edu.cn.
Renlong Li, Email: lirenlong@hotmail.com.
Wenjiao Li, Email: 1070289408@qq.com.
Haohao Zhang, Email: xiaodouhaohao@163.com.
Hongwei Li, Email: hongweili1102@163.com.
Shibo Wang, Email: 446995556@qq.com.
Xiaoliang Gao, Email: 1641782061@qq.com.
Penghui Niu, Email: m17853137288@163.com.
Wanqing Wang, Email: wangwq2021@126.com.
Gang Ji, Email: jigang@fmmu.edu.cn.
Qingchuan Zhao, Email: zhaoqc@fmmu.edu.cn.
Yuanyuan Lu, Email: luyuandreamer@aliyun.com.
Zengshan Li, Email: lizsh72@fmmu.edu.cn.
Lei Shang, Email: shanglei@fmmu.edu.cn.
Han Liang, Email: tjlianghan@126.com.
Kaichun Wu, Email: kaicwu@fmmu.edu.cn.
Jingyu Deng, Email: dengery@126.com.
Yingtai Chen, Email: yingtaichen@126.com.
Yongzhan Nie, Email: yongznie@fmmu.edu.cn.
References
- 1. Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001;2:127–137. [DOI] [PubMed] [Google Scholar]
- 2. Oh DY, Bang YJ. HER2-targeted therapies – a role beyond breast cancer. Nat Rev Clin Oncol 2020;17:33–48. [DOI] [PubMed] [Google Scholar]
- 3. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687–697. [DOI] [PubMed] [Google Scholar]
- 4. Janjigian YY, Kawazoe A, Yanez P, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature 2021;600:727–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu Z, Shi M, Li X, et al. HER2 copy number as predictor of disease-free survival in HER2-positive resectable gastric adenocarcinoma. J Cancer Res Clin Oncol 2021;147:1315–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gordon MA, Gundacker HM, Benedetti J, et al. Assessment of HER2 gene amplification in adenocarcinomas of the stomach or gastroesophageal junction in the INT-0116/SWOG9008 clinical trial. Ann Oncol 2013;24:1754–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 2011;29:4387–4393. [DOI] [PubMed] [Google Scholar]
- 8. Kim KC, Koh YW, Chang HM, et al. Evaluation of HER2 protein expression in gastric carcinomas: comparative analysis of 1,414 cases of whole-tissue sections and 595 cases of tissue microarrays. Ann Surg Oncol 2011;18:2833–2840. [DOI] [PubMed] [Google Scholar]
- 9. Ock CY, Kim TY, Lee KH, et al. Metabolic landscape of advanced gastric cancer according to HER2 and its prognostic implications. Gastric Cancer 2016;19:421–430. [DOI] [PubMed] [Google Scholar]
- 10. Aizawa M, Nagatsuma AK, Kitada K, et al. Evaluation of HER2-based biology in 1,006 cases of gastric cancer in a Japanese population. Gastric Cancer 2014;17:34–42. [DOI] [PubMed] [Google Scholar]
- 11. Terashima M, Kitada K, Ochiai A, et al. Impact of expression of human epidermal growth factor receptors EGFR and ERBB2 on survival in stage II/III gastric cancer. Clin Cancer Res 2012;18:5992–6000. [DOI] [PubMed] [Google Scholar]
- 12. Sheng WQ, Huang D, Ying JM, et al. HER2 status in gastric cancers: a retrospective analysis from four Chinese representative clinical centers and assessment of its prognostic significance. Ann Oncol 2013;24:2360–2364. [DOI] [PubMed] [Google Scholar]
- 13. Sun LF, Yang K, Wang YG, et al. The role of HER2 in self-renewal, invasion, and tumorigenicity of gastric cancer stem cells. Front Oncol 2020;10:1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Knuefermann C, Lu Y, Liu B, et al. HER2/PI-3K/Akt activation leads to a multidrug resistance in human breast adenocarcinoma cells. Oncogene 2003;22:3205–3212. [DOI] [PubMed] [Google Scholar]
- 15. Okines AF, Thompson LC, Cunningham D, et al. Effect of HER2 on prognosis and benefit from peri-operative chemotherapy in early oesophago-gastric adenocarcinoma in the MAGIC trial. Ann Oncol 2013;24:1253–1261. [DOI] [PubMed] [Google Scholar]
- 16. Cao Y, Liu H, Li H, et al. Association of O6-methylguanine-DNA methyltransferase protein expression with postoperative prognosis and adjuvant chemotherapeutic benefits among patients with stage II or III gastric cancer. JAMA Surg 2017;152:e173120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dalerba P, Sahoo D, Paik S, et al. CDX2 as a prognostic biomarker in stage II and stage III colon cancer. N Engl J Med 2016;374:211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao L, Han W, Yang X, et al. Exceeding 30 ELNs is strongly recommended for pT3-4N0 patients with gastric cancer: a multicenter study of survival, recurrence and prediction model. Cancer Sci 2021;112:3266–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gao X, Pan Y, Han W, et al. Association of systemic inflammation and body mass index with survival in patients with resectable gastric or gastroesophageal junction adenocarcinomas. Cancer Biol Med 2021;18:283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang N, Deng J, Wang W, et al. Negative lymph node count as an independent prognostic factor in stage III patients after curative gastrectomy: a retrospective cohort study based on a multicenter database. Int J Surg 2020;74:44–52. [DOI] [PubMed] [Google Scholar]
- 21. Zheng G, Feng F, Guo M, et al. Harvest of at east 23 lymph nodes is indispensable for stage N3 gastric cancer patients. Ann Surg Oncol 2017;24:998–1002. [DOI] [PubMed] [Google Scholar]
- 22. Liu K, Feng F, Chen XZ, et al. Comparison between gastric and esophageal classification system among adenocarcinomas of esophagogastric junction according to AJCC 8th edition: a retrospective observational study from two high-volume institutions in China. Gastric Cancer 2019;22:506–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Niu P, Huang H, Zhao L, et al. Clinicopathological characteristics, survival outcomes, and genetic alterations of younger patients with gastric cancer: results from the China National Cancer Center and cBioPortal datasets. Cancer Med 2022;11:3057–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fang C, Wang W, Deng JY, et al. Proposal and validation of a modified staging system to improve the prognosis predictive performance of the 8th AJCC/UICC pTNM staging system for gastric adenocarcinoma: a multicenter study with external validation. Cancer Commun (Lond) 2018;38:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deng J, Yamashita H, Seto Y, et al. Increasing the number of examined lymph nodes is a prerequisite for improvement in the accurate evaluation of overall survival of node-negative gastric cancer patients. Ann Surg Oncol 2017;24:745–753. [DOI] [PubMed] [Google Scholar]
- 26. Guideline Recommendations for HER2 Detection in Gastric Cancer Group. Guidelines for HER2 detection in gastric cancer. Zhonghua Bing Li Xue Za Zhi 2011;40:553–557. [PubMed] [Google Scholar]
- 27. Hofmann M, Stoss O, Shi D, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology 2008;52:797–805. [DOI] [PubMed] [Google Scholar]
- 28. Huang D, Lu N, Fan Q, et al. HER2 status in gastric and gastroesophageal junction cancer assessed by local and central laboratories: Chinese results of the HER-EAGLE study. PLoS One 2013;8:e80290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pathmanathan N, Geng JS, Li W, et al. Human epidermal growth factor receptor 2 status of gastric cancer patients in Asia: results from a large, multicountry study. Asia Pac J Clin Oncol 2017;13:249–260. [DOI] [PubMed] [Google Scholar]
- 30. Kataoka Y, Okabe H, Yoshizawa A, et al. HER2 expression and its clinicopathological features in resectable gastric cancer. Gastric Cancer 2013;16:84–93. [DOI] [PubMed] [Google Scholar]
- 31. Nagatsuma AK, Aizawa M, Kuwata T, et al. Expression profiles of HER2, EGFR, MET and FGFR2 in a large cohort of patients with gastric adenocarcinoma. Gastric Cancer 2015;18:227–238. [DOI] [PubMed] [Google Scholar]
- 32. Begnami MD, Fukuda E, Fregnani JH, et al. Prognostic implications of altered human epidermal growth factor receptors (HERs) in gastric carcinomas: HER2 and HER3 are predictors of poor outcome. J Clin Oncol 2011;29:3030–3036. [DOI] [PubMed] [Google Scholar]
- 33. Goutsouliak K, Veeraraghavan J, Sethunath V, et al. Towards personalized treatment for early stage HER2-positive breast cancer. Nat Rev Clin Oncol 2020;17:233–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fassan M, Mastracci L, Grillo F, et al. Early HER2 dysregulation in gastric and oesophageal carcinogenesis. Histopathology 2012;61:769–776. [DOI] [PubMed] [Google Scholar]
- 35. Ronchi A, Pagliuca F, Zito Marino F, et al. Current and potential immunohistochemical biomarkers for prognosis and therapeutic stratification of breast carcinoma. Semin Cancer Biol 2021;72:114–122. [DOI] [PubMed] [Google Scholar]
- 36. Cooke T, Reeves J, Lanigan A, et al. HER2 as a prognostic and predictive marker for breast cancer. Ann Oncol 2001;12:S23–S28. [DOI] [PubMed] [Google Scholar]
- 37. Wu ZH, Hu HB, Deng YH. Association of HER2 expression with pathologic features and prognosis in stage II and III colon cancer. J Clin Oncol 2021;39:3524. [Google Scholar]
- 38. Sun Z, Yue L, Shen Z, et al. Downregulation of NPM expression by Her-2 reduces resistance of gastric cancer to oxaliplatin. Oncol Lett 2017;13:2377–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang X, Lu B, Dai C, et al. Caveolin-1 promotes chemoresistance of gastric cancer cells to cisplatin by activating WNT/beta–catenin pathway. Front Oncol 2020;10:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tomioka H, Mukohara T, Kataoka Y, et al. Inhibition of the mTOR/S6K signal is necessary to enhance fluorouracil-induced apoptosis in gastric cancer cells with HER2 amplification. Int J Oncol 2012;41:551–558. [DOI] [PubMed] [Google Scholar]
- 41. Pegram MD, Finn RS, Arzoo K, et al. The effect of HER-2/neu overexpression on chemotherapeutic drug sensitivity in human breast and ovarian cancer cells. Oncogene 1997;15:537–547. [DOI] [PubMed] [Google Scholar]
- 42. Greaves M, Maley CC. Clonal evolution in cancer. Nature 2012;481:306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yarchoan M, Johnson BA, III, Lutz ER, et al. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer 2017;17:569. [DOI] [PubMed] [Google Scholar]
- 44. Abali H, Yalcin S, Onal HC, et al. A phase II study of the combination of oxaliplatin, capecitabine, and trastuzumab and chemoradiotherapy in the adjuvant setting in operated patients with HER2-positive gastric or gastroesophageal junction cancer (TOXAG Study): a Turkish Oncology Group Study. Am J Clin Oncol 2021;44:301–307. [DOI] [PubMed] [Google Scholar]
- 45. Hofheinz RD, Hegewisch-Becker S, Kunzmann V, et al. Trastuzumab in combination with 5-fluorouracil, leucovorin, oxaliplatin and docetaxel as perioperative treatment for patients with human epidermal growth factor receptor 2-positive locally advanced esophagogastric adenocarcinoma: a phase II trial of the Arbeitsgemeinschaft Internistische Onkologie Gastric Cancer Study Group. Int J Cancer 2021;149:1322–1331. [DOI] [PubMed] [Google Scholar]
- 46. Hofheinz RD, Merx K, Haag GM, et al. FLOT versus FLOT/trastuzumab/pertuzumab perioperative therapy of human epidermal growth factor receptor 2-positive resectable esophagogastric adenocarcinoma: a randomized phase II trial of the AIO EGA Study Group. J Clin Oncol 2022;40:3750–3761. [DOI] [PubMed] [Google Scholar]
- 47. Shitara K, Bang YJ, Iwasa S, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med 2020;382:2419–2430. [DOI] [PubMed] [Google Scholar]
- 48. Yamaguchi K, Bang YJ, Iwasa S, et al. Trastuzumab deruxtecan in anti-human epidermal growth factor receptor 2 treatment-naive patients with human epidermal growth factor receptor 2-low gastric or gastroesophageal junction adenocarcinoma: exploratory cohort results in a phase II trial. J Clin Oncol 2023;41:816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Janjigian YY, Maron SB, Chatila WK, et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol 2020;21:821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, NYZ, upon reasonable request. The data are not publicly available since this could compromise the privacy of research participants.


