BACKGROUND AND OBJECTIVE:
Reported recurrence rates of chronic subdural hematoma treated by burr-hole surgery with postoperative drainage vary considerably in the literature. We performed a systematic review and meta-analysis to define the recurrence rate of burr-hole surgery with postoperative drainage.
METHODS:
PubMed and EMBASE were searched, and Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were followed. We used the Newcastle-Ottawa scale and Cochrane risk-of-bias tool for quality assessment of included studies and the random-effects model to calculate pooled incidence rates in R with the metaprop function if appropriate.
RESULTS:
The search yielded 2969 references; 709 were screened full text, and 189 met the inclusion criteria. In 174 studies (34 393 patients), the number of recurrences was reported as per patient and 15 studies (3078 hematomas) reported the number of recurrences per hematoma, for a pooled incidence of 11.2% (95% CI: 10.3-12.1; I2 = 87.7%) and 11.0% (95% CI: 8.6-13.4; I2 = 78.0%), respectively. The pooled incidence of 48 studies (15 298 patients) with the highest quality was 12.8% (95% CI 11.4-14.2; I2 = 86.1%). Treatment-related mortality (56 patients) has a pooled incidence of 0.7% (95% CI 0.0-1.4; I2 = 0.0%).
CONCLUSION:
The recurrence rate of chronic subdural hematoma treated by burr-hole surgery and postoperative drainage is 12.8%.
KEY WORDS: Hematoma, Subdural, Chronic, Trephining, Recurrence, Mortality
ABBREVIATIONS:
- aSDH
acute subdural hematoma
- cSDH
chronic subdural hematoma
- I2
I-squared statistic
- NA
not available
- PI
prediction interval.
Over the past 3 decades, the incidence of chronic subdural hematoma (cSDH) has nearly tripled for patients older than 80 years, and because of the aging population, it is expected to become the most common cranial neurosurgical condition among adults by 2030.1,2 Although burr-hole surgery is the first treatment of choice for symptomatic cSDH,3-5 this procedure inherently carries a risk of a recurrent hematoma. Reported recurrence rates vary considerably (0%-33%), probably because surgical strategies also vary widely, from 1 or 2 burr holes with or without postoperative drainage to subdural or epidural location of drains.6-8
In recent years, less invasive, nonsurgical options to treat cSDH have been explored, such as medical treatment (steroids or tranexamic acid) or embolization of the middle meningeal artery, but randomized controlled trials are still lacking or could not prove any benefit.9,10 To accurately calculate how many patients need to be included in such randomized controlled trials (RCTs), sample size calculations should be based on reliable outcome measures in the control group, treated according to modern standards.
As the use of postoperative drainage has been shown to be clearly superior to burr-hole surgery without postoperative drainage,5 we performed a systematic review and meta-analysis to define a reliable rate of recurrence after burr-hole surgery with postoperative drainage in patients with a cSDH.
METHODS
Search Strategy and Study Selection
The results of this systematic review are reported in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist.11 This review was not registered, and a protocol was not prepared. We searched Medline (ovid) and EMBASE (embase.com) on 4th June 2021 from inception, using terms on subdural hematoma and burr hole, to identify all studies from 1946 reporting on recurrent cSDH after burr-hole surgery (see Supplementary Table 1, http://links.lww.com/ONS/A922, entitled “Syntax search”).
Articles with less than 25 patients, certain publication types (letter to the editor, commentary, survey, narrative review, study protocol), or records containing other language than English, German, French, Spanish, or Dutch were excluded. Systematic reviews and meta-analyses were only taken into consideration for forward and backward snowballing to identify any additional relevant articles. Records without abstracts were automatically passed into the full-text screening phase.
Studies were eligible for inclusion if (1) patients were 18 years or older; (2) were diagnosed with a cSDH, acute-on-chronic subdural hematoma, or when a study described both cSDH and subdural hygroma; (3) treated with burr-hole surgery and postoperative drainage; and (4) the number of recurrences or recurrence rate was explicitly reported. In Figures 1-3, we present 3 images of a cSDH, acute-on-chronic subdural hematoma, and a subdural hygroma. Postoperative drainage had to be performed in a minimum of 95% of cases to be included. A definition of a cSDH recurrence was not mandatory for inclusion, but the study had to describe recurrences or reoperations. When a study consisted of multiple treatment groups with different strategies and the patient group of interest was distinguishable (burr-hole surgery with additional drainage), the data regarding the number of patients, recurrences, and postoperative drainage of that specific subgroup of patients were collected. In case a study described that patients did not receive a postoperative drain because of brain expansion during surgery, the study was included.
FIGURE 1.
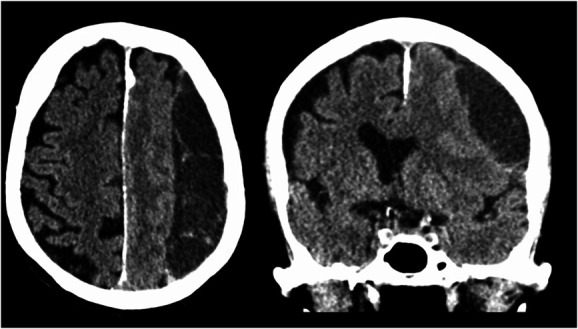
Computed tomography scan of a left-sided chronic subdural hematoma in an axial and coronal plane.
FIGURE 3.
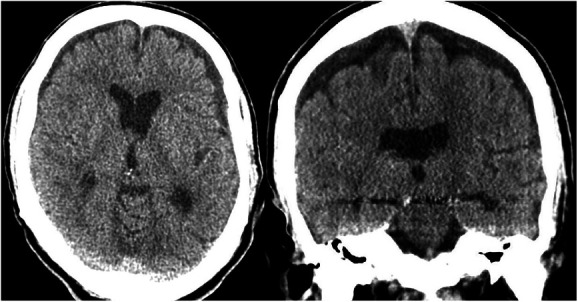
Computed tomography scan of a bilateral subdural hygroma in an axial and coronal plane.
FIGURE 2.
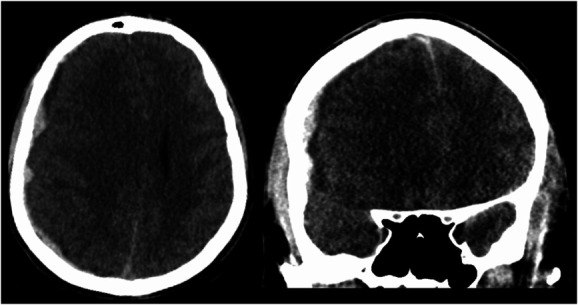
Computed tomography scan of a right-sided acute-on-chronic subdural hematoma in an axial and coronal plane.
Exclusion criteria were (1) medical treatment for cSDH or embolization of the middle meningeal artery before, or after, burr-hole surgery; (2) other type of surgery than burr-hole surgery; (3) enlarged and endoscopy-assisted burr-hole surgery; and (4) reported structural cause for cSDH, such as arachnoid cysts or vascular malformations.
Data Extraction
Two investigators (R.L. and M.F.) independently screened title and abstract to identify potential suitable records. Differences in judgment were discussed and resolved with mutual consent. Next, both investigators independently screened full texts, based on the inclusion and exclusion criteria and independently performed forward and backward snowballing of systematic reviews and meta-analyses to identify any additional relevant articles. Systematic reviews and meta-analyses were only taken into consideration for forward and backward snowballing to identify any additional relevant articles. A third rater (W.P.V.) adjudicated any discrepancies.
Data Collection
The following preoperative characteristics were extracted: the number of patients, mean or median age, sex, antithrombotic therapy, history of trauma, Glasgow Coma Scale (GCS) score, hematoma laterality, and total number of cSDHs (bilateral hematomas counted as 2 hematomas). Regarding operative treatment the following characteristics were collected: number of burr-holes made as per standard protocol per side, number of patients receiving irrigation, type of irrigation used, number of patients receiving a postoperative drain, type of drain, number of patients not receiving a drain because of brain expansion during surgery, and postsurgical treatment. Whenever the definition of a recurrent cSDH was stated, this was noted, as well as the number of recurrences, whether the recurrence was measured per patient or per hematoma, number of recurrences treated by surgery, and number of patients in whom the recurrence was detected by computed tomography scan, or clinical symptoms. Furthermore, data on frequency and causes of mortality directly related to cSDH treatment and the occurrence of re-recurrence were collected when possible.
Quality Assessment
The Newcastle-Ottawa scale for cohort studies, which is validated for assessing the quality of observational cohort studies, was used.12 According to the assigned number of stars, the following subdivision was made: 7–9, high quality; 4–6, high risk of bias; and 0–3, very high risk of bias. In case there was no nonexposed cohort in an included study, the maximum amount of stars to be assigned was 8. Thereby, the subdivision for these studies subsequently was 6–8, high quality; 3–5, high risk of bias; and 0–2, very high risk of bias. In addition, the Cochrane risk of bias for randomized controlled trials was used for studies in which a randomization method was used.13 Risk-of-bias judgment was determined by the overall result of the 6 domains: low risk of bias (low risk of bias for all domains), some concerns (some concerns in at least 1 domain for this result but not to be at high risk of bias for any domain), or high risk of bias (high risk of bias in at least 1 domain) accordingly. Each paper was graded and assigned a score by 2 authors [R.L. and D.V.]. A subgroup analysis was performed of the studies with the highest quality, meaning studies with Newcastle-Ottawa scale score 7–9 and score 6–8 in case of the absence of a nonexposed cohort and low risk of bias judgment determined on the Cochrane risk of bias for randomized controlled trials.
Statistical Analysis
RStudio (R: A language and environment for statistical computing. R Foundation for Statistical Computing. URL https://www.R-project.org/) with the “meta” and “metafor” package was used to perform single-arm meta-analysis including 95% CI. To assess whether effect sizes were consistent across the included studies, heterogeneity was quantified. A P-value ≤.1 for the χ2 test of heterogeneity was considered to suggest significant heterogeneity. In addition, the I-squared (I2) statistic was used, which describes the percentage of variation across studies that is due to heterogeneity rather than chance. I2 values of 25%, 50%, and 75% would, respectively, assign adjectives of low, moderate, and high categories of heterogeneity.14 In case of high heterogeneity, the random-effects model was used to correctly interpret the results.15 Metaprop function in R was used to determine single-arm prevalence, whenever possible. In addition, for single-arm prevalence of recurrence, a forest plot was created, and 95% prediction interval (PI) was calculated with the metaprop function in case of at least 10 included studies.16 The prediction interval helps in the clinical interpretation of the heterogeneity by estimating what true treatment effects can be expected in future settings.17
Single-arm prevalence analyses of a variable consisting of either 0% or 100% incidences in the included studies was determined by dividing the total number of cases in the included studies by the total number of cases of this variable.
We assessed the presence of bias using a funnel plot. The funnel plot was created with the pooled incidence and random-effects model by plotting the recurrence rate against the standard error.
RESULTS
Literature Search
The online search yielded a total of 4140 records. Screening the references of 33 systematic reviews yielded 15 additional articles. Duplicates were removed and yielded 2969 articles. After screening on title and abstract, 2260 records were excluded. The full texts of the remaining 709 articles were assessed for eligibility. Of the remaining full texts, 520 were excluded. In total, 189 articles reporting on 36 971 patients were selected for further analysis (Figure 4). Most studies were of a retrospective nature (n = 166) and otherwise randomized controlled trials (n = 23).
FIGURE 4.
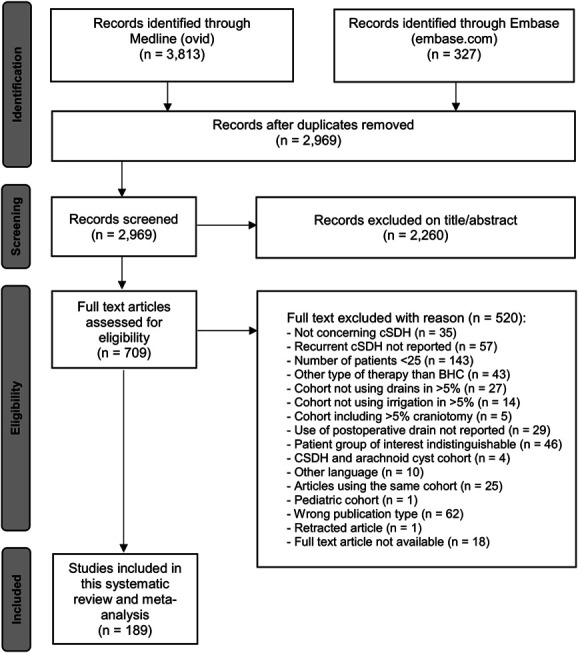
Flow chart diagram of literature search and selection.
Quality Assessment
The funnel plot shows that included studies do not seem to be symmetrically ranged around the pooled incidence of recurrence (Figure 5). Smaller studies seem to cause a more scattered array of recurrence rate. The risk-of-bias scores are shown in Table 1. Regarding the Newcastle-Ottawa scale and adjusted scale for cohort studies, the highest quality was observed in 50 (30.9%) studies, high risk of bias was observed in 104 (64.2%) studies, and very high risk in 8 (4.9%) studies.
FIGURE 5.
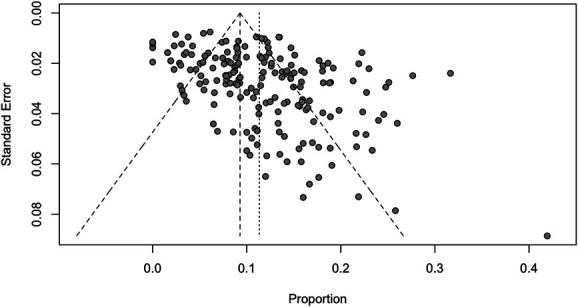
Funnel plot of recurrence rates reported in 189 included studies (36 971 patients) for evaluating bias. The plot shows that the included studies in this meta-analysis do not seem to be symmetrically ranged around the pooled incidence of recurrence, shown by the smaller dashed line. This could be due to the relatively high heterogeneity reported in this study.
TABLE 1.
Baseline Characteristics of the 189 Included Studies
| Author and year of publication | No.of patients | Mean/median age (SD/IQR/range) | Male sex (%) | No. of patients on antithrombotic medication (%) | No. of patients with a history of trauma (%) | No. of patients with a history of alcohol abuse (%) | No. of patients with unilateral cSDH (%) | No. of patients with bilateral cSDH (%) | Total no. of hematomas | No. of burr-holes performed | Irrigation method appliedb | Postoperative drain location | Postoperative drainage timec | Follow-up in months | No. of patients with recurrence (%) | Quality assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abboud et al,18 2018 | 201 | 72 (range 25-95) | 140 (70) | 84 (42) | 85 (42) | NA | 183 (91) | 18 (9) | 219 | 1 | Warm saline | Subdural | ≤48 h | 6 | 38 (18.9) | 5h |
| Abouzari et al,19 2007 | 84 | 56.5 (range 21-88) | 59 (70) | 0 (0) | 0 (0) | 0 (0) | 84 (100) | 0 (0) | 84 | 1 | Warm saline | NA | ≤48 h | 3 | 9 (10.7) | Highi |
| Adachi et al,20 2014 | 120 | 79 | 76 (63) | 47 (39) | NA | 23 (19) | 120 (100) | 0 (0) | 120 | 1 | Other | Subdural | ≤24 h | 6 | 11 (9.2) | 7h |
| Adrian et al,21 2017 | 60 | 69 | 41 (68) | 6 (10) | NA | 3 (5) | 59 (98) | 1 (2) | 61 | 1 | Warm saline | Subdural, subperiosteal | ≤24 h | 3 | 5 (5) | 6h |
| Ahmed et al,22 2011 | 25 | NA | NA | NA | NA | NA | NA | NA | NA | 1 | Saline | Subdural | ≤48 h | 1 | 4 (16) | Highi |
| Ak et al,23 2017 | 71 | NA | NA | NA | NA | NA | NA | NA | NA | 2 or 3 | NA | NA | NA | NA | 8 (11.3) | 2h |
| Amano et al,24 2020e | 323 | 80.0 (±8.6) and 75.5 (±11.0) | 209 (65) | 108 (33) | 222 (69) | NA | 209 (65) | 114 (35) | 437 | 1 | Other | Subdural | NA | 6 | 62 (16.5)f | 5h |
| Aung et al,25 1999 | 50 | 62 (range 54-72) | 29 (58) | NA | 50 (100) | NA | NA | NA | NA | 2 | Ringer solution/Hartmann solution | Subdural | ≤48 h | 3 | 0 (0) | 4h |
| Baechli et al,26 2004 | 354 | 68.3 (±17.0) (range 2-94) | 228 (64) | 144 (41) | 272 (77) | NA | 276 (78) | 78 (22) | 432 | 1 or 2 | NA | Subdural | ≤48 h | NA | 48 (13.6) | 5h |
| Bankole et al,27 2011 | 73 | NA | NA | 2 (3) | 30 (41) | 13 (18) | 51 (70) | 22 (30) | 95 | 1 or 2 | Saline | Subdural | ≤48 h | NA | 9 (12.5) | 5g |
| Bartek et al,28 2017 | 1254 | NA | NA | 555 (44) | NA | NA | 938 (75) | 316 (25) | 1570 | NA | NA | Subgaleal, subperiosteal | NA | 6 | 169 (13.5) | 6h |
| Bartley et al,29 2020 | 172 | 74.5 (±12) and 75.1 (±13) | 127 (74) | 80 (47) | NA | NA | 139 (81) | 33 (19) | 205 | 1 or 2 | Warm Ringer solution | Subdural | ≤24 h | 6 | 15 (8.7) | 5h |
| Bellut et al,30 2012 | 113 | 77 (±13) and 71 (±13) | 77 (68) | 62 (55) | NA | NA | 83 (73) | 30 (27) | 143 | 2 | Warm saline | Subdural, subperiosteal | ≤48 h | 3 | 18 (15.9) | 5h |
| Blaauw et al,31 2020 | 1029 | 73.5 (±11) | 773 (75) | 571 (55) | 571 (55) | NA | 772 (75) | 257 (25) | 1286 | 3 | 115 (11.2) | 6h | ||||
| Borger et al,32 2012 | 322 | 76 (±7.9) (range 65-94) | 162 (50) | 233 (72) | NA | 245 (76) | 77 (24) | 399 | 1 | Saline | Subdural | NA | 0.5 | 89 (22.3)f | 4h | |
| Carlisi et al,33 2017 | 35 | 81.3 (6.3) | 22 (63) | 22 (63) | 22 (63) | NA | 29 (83) | 6 (17) | 41 | NA | NA | NA | ≤48 h | NA | 3 (8.6) | 4h |
| Carslen et al,34 2011 | 206 | NA | NA | NA | NA | NA | NA | NA | NA | 1 | Ringer solution/Hartmann solution | Subdural, subgaleal | ≤24 h | 6 | 29 (14.1) | 5g |
| Castro-Rodriguez et al,35 2016 | 200 | 88.5 (±3.2) | 107 (54) | 71 (36) | 114 (57) | 3 (2) | 167 (84) | 33 (17) | 233 | 2 | Saline | Subdural | ≤72 h | 3 | 26 (13) | 6h |
| Certo et al,36 2019 | 30 | 77.1 (range 57-87) and 76.4 (range 61-91) | 19 (63) | NA | NA | NA | NA | NA | NA | 1 | Saline | Subdural | ≤72 h | 16 | 1 (3.3) | 4g |
| Chan et al,37 2017 | 149 | 74 (range 42-95) | 116 (78) | 46 (31) | NA | 13 (9) | 149 (100) | 0 (0) | 149 | 2 | NA | NA | ≤48 h | 6 | 13 (8.7) | 7g |
| Chang et al,38 2020 | 122 | 73.6 (±11.6) (range 36-95) | 83 (68) | 71 (58) | 73 (60) | 9 (7) | 79 (65) | 43 (35) | 165 | 6 | 14 (11.5) | 7h | ||||
| Chandran et al,39 2017 | 52 | 33.7 (range 18-40) | 32 (62) | 2 (4) | 48 (92) | 13 (25) | 52 (100) | 0 (0) | 52 | 1 | Other | NA | NA | 1 | 1 (1.9) | 2h |
| Chen et al,40 2020 | 171 | 66.0 (range 59-75) | 137 (80) | 22 (13) | 99 (58) | NA | 138 (81) | 33 (19) | 204 | 6 | 13 (6.4)f | Highi | ||||
| Cheng et al,41 2014 | 342 | 77.2 (±11.4) | 235 (69) | 51 (15) | 201 (59) | NA | 269 (79) | 73 (21) | 415 | NA | Saline | Subdural | ≤120 h | 3 | 41 (11.9) | 5h |
| Choi et al,42 2016 | 502 | 67 (±13) | 341 (68) | NA | 302 (60) | NA | NA | NA | NA | 1 or 2 | NA | NA | NA | NA | 37 (7.4) | 4h |
| Choi et al,43 2020 | 230 | 69.4 (±13.1) | 164 (71) | 36 (16) | NA | 34 (15)9 | 144 (63) | 86 (37) | 316 | NA | 49 (21.3) | 5h | ||||
| Chon et al,44 2012 | 420 | 67.3 (range 37-92) | 334 (80) | 151 (36) | 237 (56) | NA | 352 (84) | 68 (16) | 488 | 1 | NA | NA | ≤72 h | 3 | 92 (21.9) | 6h |
| Choudhury et al,45 1994 | 44 | 65 | 37 (84) | 4 (9) | 31 (70) | NA | 37 (84) | 7 (16) | 51 | 2 | Saline | Subdural | ≤72 h | 3 | 1 (2.3) | 5h |
| Dobran et al,46 2019 | 50 | 92.4 (range 90-100) | 32 (64) | 35 (70) | 27 (54) | 3 (6) | 34 (68) | 16 (32) | 66 | 6 | 7 (14) | 4h | ||||
| D'Oria et al,47 2020 | 210 | 66.5 (±6) and 67.2 (±7.1) | 115 (55) | 55 (26) | NA | 13 (6) | 210 (100) | 0 (0) | 210 | 1 or 2 | Saline | Subdural | ≤48 h | 30 | 31 (14.8) | 5h |
| Djientcheu et al,48 2011 | 195 | 55 (range 21-89) | 155 (79) | 3 (2) | 159 (82) | 25 (13) | 156 (80) | NA | NA | 1 or 2 | Saline | Subgaleal | ≤48 h | 9 | 6 (3.1) | 4h |
| Dran et al,49 2007 | 198 | 75 (±13) (range 33-98) | 142 (72) | 58 (29) | 150 (76) | 13 (7) | 169 (85) | 29 (15) | 227 | 1 | Saline | Subdural | ≤48 h | 17.5 | 16 (8) | 6h |
| Drapkin,50 1991 | 53 | Range 16-97 | 29 (55) | 5 (9) | 27 (51) | NA | 50 (94) | 3 (6) | 59 | 2 | Saline | Subdural | Other | NA | 10 (19) | 5h |
| Edem et al,51 2019 | 74 | 70 (range 24-96) | 57 (77) | 0 (0) | NA | NA | 74 (100) | 0 (0) | 74 | NA | NA | NA | NA | 6 | 16 (21.6) | 6g |
| Eggert et al,52 1984 | 100 | NA | NA | 19 (19) | NA | NA | NA | NA | NA | NA | NA | NA | NA | 17 | 11 (11) | 3h |
| Eppel et al,53 1999 | 50 | 67.3 and 70.3 (range 27-96) | 35 (70) | 9 (18) | 50 (100) | 8 (16) | 42 (84) | 8 (16) | 58 | 1 or 2 | Other | Subdural | ≤120 h | 28 | 8 (16) | 5h |
| Ernestus et al,54 1997 | 94 | NA | NA | NA | NA | NA | NA | NA | NA | NA | Saline | Subdural | ≤96 h | NA | 17 (18.1) | 4h |
| Erol et al,55 2005 | 35 | NA | NA | NA | NA | NA | 32 (91) | 3 (9) | 38 | 1 or 2 | Saline | Subdural | ≤48 h | 1 | 5 (14.3) | Highi |
| Flint et al,56 2017 | 659 | 76 (range 67-83) | 464 (70) | NA | NA | NA | NA | NA | NA | 1 | NA | Subdural | ≤48 h | 6 | 60 (9.1) | 6g |
| Flores et al,57 2017 | 220 | 59 | 167 (76) | NA | NA | NA | 220 (100) | 0 (0) | 220 | 2 | Saline | Subdural | ≤24 h | 12 | 13 (5.9) | 4h |
| Frati et al,58 2004 | 35 | 69.4 | 24 (69) | NA | 35 (100) | 0 (0) | 30 (86) | 5 (14) | 40 | 1 | Saline | Subdural | ≤72 h | 12 | 5 (14.3) | 4h |
| Fujisawa et al,59 2021 | 208 | 74 (IQR 66-83), 74 (IQR 66-81) | 153 (74) | 32 (15) | 130 (63) | 12 (5) | NA | NA | NA | 3-6 | 19 (9.1) | Highi | ||||
| Gabarros et al,60 2000 | 83 | 66.5 (range 17-86) | 59 (71) | 1 (1) | NA | NA | 78 (94) | 5 (6) | 88 | 2 | Saline | NA | ≤48 h | 12 | 10 (12) | 6g |
| Gelabert Gonzalez et al,61 2005 | 1000 | 72.7 (±11.4) | 628 (63) | 122 (12) | 617 (62) | 132 (13) | 903 (90) | 97 (10) | 1097 | NA | Saline | Subdural | ≤120 h | NA | 61 (6.1) | 4h |
| Gernsback et al,62 2016 | 215 | 66 | 155 (72) | 157 (73) | NA | NA | 195 (91) | 33 (15) | 261 | 1 or 2 | NA | Subdural | ≤24 h | NA | 16 (6.1)f | 3h |
| Gilsbach et al,63 1980 | 51 | 58.7 and 54.8 | 37 (73) | NA | NA | NA | 45 (88) | 6 (12) | 57 | 1 | Ringer solution/Hartmann solution | Subdural | NA | NA | 9 (17.7) | 4h |
| Glancz et al,64 2019 | 577 | 78 (IQR 98-85) | 394 (68) | 245 (42) | 361 (63) | NA | 386 (67) | 177 (31) | 740 | 1 or 2 | NA | Subdural, subgaleal | ≤72 h | 2 | 45 (7.8) | 7h |
| Gonugunta a Buxton,65 2001 | 184 | 68 and 72 | 86 (47) | 34 (18) | NA | NA | NA | NA | NA | 1 | Other | Subdural | NA | 6 (minimum) | 27 (14.7) | 4h |
| Goto et al,66 2015 | 414 | 77.3 | 279 (67) | 84 (20) | NA | NA | 323 (78) | 91 (22) | 505 | 6 | 37 (8.9) | 7h | ||||
| Gurelik et al,67 2007 | 42 | 58.4 | 28 (67) | NA | 7 (17) | 0 (0) | NA | NA | NA | 1 or 2 | Warm saline | Subdural | ≤48 h | 8 | 8 (19) | Highi |
| Hamilton et al,68 1993 | 29 | 64 | NA | NA | NA | NA | NA | NA | NA | 1 | NA | Subdural | NA | 4 | 3 (10.3) | 3g |
| Han et al,69 2009 | 180 | 62.2 (±14.3) | 131 (73) | NA | 180 (100) | NA | 155 (86) | 25 (14) | 205 | 1 or 2 | Warm saline | Subdural | ≤120 h | 1 (minimal) | 10 (5.6) | 5h |
| Hani et al,70 2019 | 361 | 72.6 (±11.0) + 74.8 (±11.0) | 244 (68) | 191 (53) | NA | NA | NA | NA | NA | 2 | NA | Subdural, subgaleal | ≤48 h | 6 | 83 (23) | 6h |
| Harders et al,71 1982 | 100 | 61.6 and 62.9 | 70 (70) | 19 (19) | 62 (62) | 7 (7) | 92 (92) | 8 (8) | 108 | 2 | Ringer solution/Hartmann's solution | Subdural | Other | 17 | 24 (24) | 3h |
| Hennig & Kloster,72 1999 | 90 | NA | NA | NA | NA | NA | 82 (91) | 8 (9) | 98 | 2 | Gentamicin-induced irrigation | Subdural | ≤48 h | NA | 7 (7.1)f | 3g |
| Heringer et al,73 2017 | 96 | NA | NA | NA | NA | NA | 74 (77) | 22 (23) | 96 | 1 or 2 | Saline | Subdural | NA | 7.6 | 15 (15.6) | 5g |
| Hirai et al,74 2021 | 320 | 77.3 (±10.9) | 228 (71) | 94 (29) | NA | NA | 274 (86) | 46 (14) | 366 | 3 | 37 (10.6)f | 8h | ||||
| Hori et al,75 2018 | 92 | 81.9 (±8.5) and 77.5 (±8.9) | 63 (68) | 25 (27) | 61 (66) | 1 (1) | 77 (84) | 15 (16) | 107 | NA | NA | Subdural | NA | 3 | 15 (16.3) | 6h |
| Hsieh et al,76 2016 | 75 | 71.9 (±12.5) | 53 (71) | 20 (27) | 38 (51) | 19 (25) | 52 (69) | 23 (31) | 98 | 1 | Saline | Subdural | NA | 8 (mean) | 7 (9.3) | 5h |
| Huang et al,77 2013 | 98 | 69.3 (±12.8) | 81 (83) | 15 (15) | 73 (74) | 14 (14) | 73 (74) | 25 (26) | 123 | 1 or 2 | Other | Subdural | NA | 10.6 (mean) | 14 (14.3) | 5h |
| Huang et al,78 2020 | 140 | 68.7 (±12.7) | 114 (81) | 91 (65) | NA | NA | NA | NA | 1 | NA | Subdural | ≤48 h | 3 | 12 (8.6) | 4h | |
| Iftikar et al,79 2016 | 34 | 66.7 (±14.0) | 28 (82) | NA | NA | NA | NA | NA | NA | NA | Saline | Subdural | ≤48 h | 1-10 (min-max) | 6 (17.6) | 6g |
| Ishfaq,80 2017 | 62 | 72 (range 55-85) | 41 (66) | NA | 33 (53) | NA | 52 (84) | 10 (16) | 72 | 1 or 2 | NA | Subdural, subgaleal | ≤96 h | NA | 7 (11.3) | Highi |
| Ishibashi et al,81 2011 | 34 | 79.1 (10) | 19 (56) | 5 (15) | NA | NA | 31 (91) | 3 (9) | 36 | 1 | Warm saline | Subdural | ≤48 h | NA | 1 (2.9) | Highi |
| Jang et al,82 2015 | 30 | 68 (range 58-78.5) | 21 (70) | 5 (17) | NA | 6 (20) | NA | NA | NA | 2 | Saline | Subdural | NA | 3 | 3 (10) | 9g |
| Jang et al,83 2020 | 291 | 71.8 (±11.8) | 208 (71) | 93 (32) | NA | 125 (43) | 175 (60) | 116 (40) | 407 | 1 or 2 | Saline | NA | ≤72 h | 2 | 29 (10) | 7h |
| Janowski & Kunert,84 2012 | 45 | 66 (range 24-86) | 30 (67) | NA | NA | NA | 42 (93) | 3 (7) | 48 | NA | NA | Subdural | ≤24 h | NA | 7 (15.6) | 5h |
| Jeong et al,85 2014 | 125 | 69.4 (±12.3) | 92 (74) | 35 (28) | 81 (65) | NA | 96 (77) | 29 (23) | 154 | NA | Warm saline | NA | NA | 3 | 8 (6.4) | 3h |
| Jukovic et al,86 2014 | 35 | NA | NA | NA | NA | NA | NA | NA | NA | NA | Warm saline | Subdural | ≤48 h | 6 | 0 (0) | 5g |
| Jung et al,87 2015 | 182 | 68.1 | 131 (72) | 46 (25) | 125 (69) | 65 (36) | 147 (81) | 35 (19) | 217 | 1 or 2 | Ringer solution/Hartmann solution | Subdural | ≤72 h | 12 | 25 (13.7) | 7h |
| Kale et al,88 2017 | 90 | 55.61 (±18.7) | 54 (60) | 12 (13) | 75 (83) | NA | 75 (83) | 15 (17) | 105 | 1 or 2 | Saline | Subdural | ≤96 h | 6 | 9 (8.6)f | 5h |
| Kaliaperumal et al,7 2012 | 50 | Range 17-91 | 33 (66) | 25 (50) | 29 (58) | NA | 42 (84) | 8 (16) | 58 | 2 | Warm saline | Subdural, subperiosteal | ≤48 h | 6 | 0 (0) | Highi |
| Kang et al,89 2007 | 302 | 236 (78) | NA | NA | NA | 267 (88) | 35 (12) | 337 | NA | NA | ≤48 h | 3 | 24 (7.9) | 4h | ||
| Kaminogo et al,90 1999 | 38 | 69.3 (±12.0) | 34 (89) | NA | 25 (66) | NA | 32 (84) | 6 (16) | 44 | 1 | Saline | Subdural | ≤24 h | 6 | 4 (9.1)f | 5h |
| Kanyi et al,91 2018 | 119 | 61.3 (range 19-94) | 95 (80) | 0 (0) | 65 (55) | 41 (34) | 95 (80) | 23 (19) | 141 | 2 | Saline | Subdural | ≤48 h | 0.5 | 6 (5) | 4h |
| Kareem & Adams,92 2018 | 36 | 79 (range 55-95) | 26 (72) | 24 (67) | 34 (94) | NA | 30 (83) | 6 (17) | 42 | 1 | Warm Ringer solution | Subperiosteal | ≤48 h | 3 | 4 (11) | 3h |
| Katayama et al,93 2018 | 88 | 75.8 (±9.5) | 67 (76) | 10 (11) | NA | NA | 77 (88) | 11 (13) | 99 | NA | Saline | Subdural | ≤24 h | 3 | 11 (12.5) | Highi |
| Khan et al,94 2019 | 60 | 62 (±13.7) (range 38-94) | 40 (67) | 0 (0) | NA | NA | 53 (88) | 7 (12) | 67 | 1 or 2 | Other | Subdural | NA | NA | 14 (23.3) | Highi |
| Kim et al,95 2011 | 259 | 63.7 (±16.9) | 191 (74) | NA | 167 (64) | 15 (6) | NA | NA | NA | 1 or 2 | NA | NA | ≤72 h | 6 | 23 (8.9) | 3g |
| Kim et al,96 2014 | 114 | 67.6 (±11.5) | 88 (77) | NA | 51 (45) | NA | 114 (100) | 0 (0) | 114 | NA | Saline | NA | ≤72 h | 3 | 28 (24.6) | 6g |
| Kim et al,97 2016 | 100 | 70.4 (±12.6) | 62 (62) | 36 (36) | 100 (100) | NA | NA | NA | NA | 1 | Saline | NA | ≤48 h | 6 | 26 (26) | 9g |
| Kim,98 2017 | 246 | 68.6 (±12.2) | 173 (70) | 59 (24) | 187 (76) | NA | 183 (74) | 63 (26) | 309 | 1 or 2 | NA | Subdural | NA | 6 | 31 (12.6) | 7h |
| Kiymaz et al,99 2007 | 29 | 62.7 (±3.2) | 24 (83) | NA | 18 (62) | 2 (7) | NA | NA | NA | 2 | Warm saline | Subdural | ≤96 h | NA | 2 (6.9) | 4g |
| Klein et al,100 2021 | 407 | 74.7 (±13.2) and 76.6 (±8.2) | 280 (69) | 209 (51) | NA | NA | 317 (78) | 90 (22) | 497 | 32.7 ± 16.1 | 72 (17.7) | 7h | ||||
| Kocaman & Yilmaz,101 2019 | 30 | 78 (range 64-92) | 20 (67) | NA | NA | NA | 30 (100) | 0 (0) | 30 | 2 | Warm saline | Subdural | NA | 6 | 5 (16.7) | 4h |
| Kotwica & Brzezinski,102 1991 | 131 | Range 18-82 | 101 (77) | 0 (0) | 93 (71) | 45 (34) | NA | NA | NA | 2 | Saline | Subdural | ≤48 h | 24 (minimum) | 3 (2.3) | 3h |
| Kristof et al,103 2008 | 75 | 73 | 38 (51) | NA | 48 (64) | NA | 58 (77) | 17 (23) | 92 | NA | Ringer solution/Hartmann solution | Subdural | ≤96 h | NA | 17 (22.7) | 6g |
| Krupp & Jans,104 1995 | 214 | 65 (±15) | 128 (60) | 13 (6) | 28 (13) | 15 (7) | 167 (78) | 45 (21) | 257 | 1 or 2 | Other | Subdural | ≤72 h | NA | 53 (25) | 5h |
| Kurabe et al,105 2010 | 182 | 77.3 (range 65-98) | 123 (68) | 38 (21) | NA | 40 (22) | 148 (81) | 34 (19) | 216 | 1 | NA | Subdural | ≤48 h | 3 | 14 (7.7) | 5h |
| Kuroki et al,106 2001 | 45 | 67.3 (range 43-88) | 32 (71) | NA | NA | NA | 40 (89) | 5 (11) | 50 | 1 | Saline | Subdural | ≤120 h | 44 | 5 (11.1) | 6h |
| Kutty & Johny,107 2014 | 70 | NA | 55 (79) | 25 (36) | NA | NA | 53 (76) | 17 (24) | 87 | 1 | Saline | Subdural | ≤72 h | 3 | 2 (2.9) | Highi |
| Kwon et al,108 2000 | 145 | 59.3 (range 23-89) | 104 (72) | NA | NA | NA | 115 (79) | 30 (21) | 175 | 1 | Saline | Subdural | ≤120 h | 6 | 6 (4.1) | 5h |
| Lee et al,109 2004 | 38 | 70 | 25 (66) | NA | NA | 6 (16) | NA | NA | NA | 2 | Saline | Subdural | ≤72 h | NA | 6 (16) | 6g |
| Lee et al,110 2009 | 32 | 65.3 (±12.1) | 25 (78) | NA | NA | NA | NA | NA | NA | 2 | Saline | NA | ≤120 h | NA | 7 (22) | 5g |
| Lee & Park,111 2014 | 114 | 77.9 | 81 (71) | 30 (26) | 70 (61) | 33 (29) | 86 (75) | 28 (25) | 142 | NA | Saline | Subdural | ≤72 h | 3 | 19 (16.7) | 5h |
| Lee et al,112 2018 | 131 | 68 (±17) | 85 (65) | 35 (27) | 71 (54) | NA | NA | NA | NA | 1 | Warm saline | Subdural | ≤72 h | 6 | 7 (5.3) | 5h |
| Lepic et al,113 2021 | 55 | 72.8 (±11.5) | 37 (67) | NA | NA | NA | 36 (65) | 19 (35) | 67 | 1 | 3 (5.5) | 3h | ||||
| Leung et al,114 2001 | 46 | NA | NA | NA | NA | NA | NA | NA | NA | NA | Saline | NA | NA | NA | 3 (6.5) | 6h |
| Liliang et al,115 2002 | 75 | 65.3 (range 16-92) | 63 (84) | NA | 53 (71) | 7 (9) | 59 (79) | 16 (21) | 91 | 1 or 2 | Saline | NA | ≤48 h | 30 | 3 (4) | 6h |
| Li et al,116 2017 | 115 | 68.3 (±5.1) | 76 (66) | 16 (14) | NA | NA | 103 (90) | 24 (21) | NA | 6 | 11 (9.6) | 6h | ||||
| Lin,117 2011 | 270 | 62.3 (±24.5) | 218 (81) | NA | NA | NA | NA | NA | NA | 1 | Saline | Subdural | ≤72 h | NA | 32 (11.9) | 6g |
| Liu et al,118 2010 | 398 | 58.1 (±18.1) | 338 (85) | 6 (2) | 275 (69) | 12 (3) | 304 (76) | 94 (24) | 492 | 1 | Saline | NA | ≤120 h | NA | 15 (3.8) | 3h |
| Liu et al,119 2019 | 328 | 65.1 (±13.8) (range 22-93) | 281 (86) | 17 (5) | 170 (52) | NA | 267 (81) | 61 (19) | 389 | 1 | Other | NA | ≤48 h | 6 | 8 (2.4) | 8h |
| Lo et al,120 2013 | 98 | 69.3 (±12.8) (range 29-93) | 81 (83) | 15 (15) | 73 (74) | 14 (14) | 73 (74) | 25 (26) | 123 | 1 or 2 | Saline | Subdural | NA | 3 | 14 (14.3) | 5h |
| Lu et al,121 2018 | 87 | 68.4 (±6.5) (range 55-82) | 71 (82) | NA | 72 (83) | NA | 87 (100) | 0 (0) | 87 | 2 | Warm saline | Subdural | NA | 6-12 | 4 (4.6) | 5h |
| Maldaner et al,122 2019 | 253 | 75 | 190 (75) | 106 (42) | NA | NA | 180 (71) | 73 (29) | 326 | 2 | NA | Subperiosteal | ≤48 h | 3 | 40 (15.8) | 7h |
| Markwalder,123 2000 | 32 | 61.4 | 22 (69) | NA | 26 (81) | NA | 27 (84) | 5 (16) | 37 | 2 | NA | Subdural | ≤48 h | 1.5 | 1 (3.1) | 3h |
| Martinez Perez et al,124 2020 | 90 | 76.4 (±11.2) | 71 (79) | 42 (47) | NA | NA | 78 (87) | 12 (13) | 102 | 2 | NA | Subdural | ≤48 h | 6 | 17 (18.9) | 8h |
| Mellergard & Wisten,125 1996 | 218 | 70.5 (range 11-93) | 155 (71) | 22 (10) | 135 (62) | 32 (15) | 193 (89) | 25 (11) | 243 | 1 | Saline | Subdural | ≤48 h | 1.5 | 30 (12.3)f | 4h |
| Mersha et al,126 2020 | 195 | 57.6 | 137 (70) | NA | 124 (64) | NA | 147 (75) | 48 (25) | 243 | 1 | Warm saline | Subdural | ≤24 h | 4 | 13 (6.6) | 6h |
| Mezue et al,127 2011 | 116 | NA | NA | 26 (22) | 91 (78) | NA | NA | NA | NA | 1 or 2 | NA | NA | NA | NA | 9 (7.8) | 3h |
| Miah et al,128 2020 | 60 | 73 (range 34-95) | 49 (82) | 31 (52) | 45 (75) | NA | 39 (65) | 21 (35) | 81 | 1 or 2 | Warm Ringer solution | Subdural | ≤48 h | 6 | 13 (22) | 7g |
| Miki et al,129 2019 | 277 | 78.6 (±11.1) | 181 (65) | 70 (25) | 193 (70) | NA | 234 (84) | 43 (16) | 320 | 1 | Other | Subdural | ≤48 h | 3 | 50 (18.1) | 7h |
| Missori et al,130 2000 | 31 | 38 (range 20-50) | 24 (77) | NA | 24 (77) | 2 (6) | 27 (87) | 4 (13) | 35 | 1 | Other | NA | ≤24 h | 2 | 2 (6) | 4h |
| Morales-Gomez et al,131 2020 | 155 | 65.9 (±16.6) (range 18-95) | 127 (82) | 8 (5) | 101 (65) | 14 (9) | 124 (80) | 31 (20) | 186 | 2 | 18 (11.6) | 5h | ||||
| Mori & Maeda,132 2001 | 500 | 67.3 (±15.3) and 71.3 (±14.2) | 359 (72) | 26 (5) | 286 (57) | 32 (6) | 412 (82) | 88 (18) | 588 | 2 | Saline | Subdural | ≤48 h | 3 | 49 (9.8) | 4h |
| Motoie et al,133 2018 | 787 | 79 (IQR 72-85) | 559 (71) | 199 (25) | NA | 195 (daily alcohol consumption)d (25) | 671 (85) | 116 (15) | 903 | NA | Other | Subdural | NA | NA | 96 (12.2) | 6h |
| Munoz-Bendix et al,134 2017 | 112 | NA | 63 (56) | 63 (56) | NA | NA | 88 (79) | 24 (21) | 136 | 1 | 25 (22.3) | 3h | ||||
| Nakagawa et al,135 2019 | 381 | NA | NA | NA | NA | NA | NA | NA | NA | 1 | Saline | Subdural | ≤24 h | 6 | 71 (18.6) | 4h |
| Nakaguchi et al,136 2001 | 106 | 67 | 82 (77) | 0 (0) | 63 (59) | NA | 86 (81) | 20 (19) | 126 | 1 | Saline | NA | ≤48 h | 3 | 21 (17)f | 5h |
| Nunta-Aree et al,137 2017 | 75 | 60.7 (66 median) | 79 (hematoma's) (NA) | 35 (47) | 31 (41) | 2 (3) | 48 (64) | 27 (36) | 102 | 2 | Other | NA | NA | 2 | 10 (13.3) | 4h |
| Okano et al,138 2014 | 448 | 71.1 (range 19-97) | 314 (70) | 18 (4) | 225 (50) | NA | 344 (77) | 104 (23) | 552 | 1 | Saline | Subdural | NA | 42 | 40 (8.9) | 7h |
| Oral et al,139 2015 | 78 | 68.1 and 66.1 (median) | 57 (73) | 9 (12) | 58 (74) | NA | 73 (94) | 5 (6) | 83 | 1 or 2 | Warm saline | Subdural, subgaleal | ≤72 h | 3 | 4 (5.1) | 5h |
| Penchet et al,140 1998 | 236 | 71.7 | 146 (62) | 57 (24) | 182 (77) | 18 (8) | 195 (83) | 41 (17) | 276 | 1 or 2 | Saline | NA | ≤48 h | 3 | 10 (4.2) | 3h |
| Piotrowski & Krombholz-Reindl,141 1996 | 200 | 67.5 | 150 (75) | 45 (23) | 109 (55) | 6 (3) | 163 (82) | 37 (19) | 237 | 1 | NA | Subdural | NA | 16 (8) | 3h | |
| Poulsen et al,142 2014 | 177 | NA | NA | NA | NA | NA | NA | NA | NA | 1 | Warm Ringer solution | Subdural, subperiosteal | NA | 3 | 28 (15.8) | Highi |
| Qian et al,143 2017 | 242 | 66.3 (±10.9) (range 36-93) | 148 (61) | 54 (22) | 145 (60) | 79 (33) | 242 (100) | 0 (0) | 242 | 1 | Warm Ringer solution | Subdural | ≤120 h | 6 | 39 (16.1) | 7h |
| Raghavan et al,144 2020 | 153 | 72.2 (±13.1) | 93 (61) | 41 (27) | 82 (54) | 26 (17) | 105 (69) | 48 (31) | 201 | 1 | NA | Subdural | NA | 1 | 24 (15.7) | 6g |
| Ram et al,145 1993 | 37 | 70.8 (±10) | 25 (68) | NA | NA | NA | 33 (89) | 4 (11) | 41 | 2 | Saline | Subdural | ≤48 h | 1 | 5 (13.5) | Highi |
| Regan et al,146 2015 | 61 | 72 (range 52-98) | 36 (59) | 29 (48) | 38 (62) | 9 (15) | NA | NA | 80 | 1 or 2 or 3 | Other | Subdural | NA | NA | 4 (6.6) | 5g |
| Ridwan et al,147 2019 | 197 | NA | NA | NA | NA | NA | NA | NA | NA | 1 or 2 | NA | Subdural | ≤72 h | 2 | 37 (18.8) | 6g |
| Rohde et al,148 2002 | 376 | 64 | 242 (64) | NA | NA | NA | NA | NA | NA | 1 | Ringer solution/Hartmann solution | Subdural | NA | NA | 119 (31.6) | 5h |
| Rovlias et al,149 2015 | 986 | 69 (range 29-96) | 650 (66) | 237 (24) | 503 (51) | 132 (13) | 907 (92) | 79 (8) | 1065 | 2 | Other | Subdural | Other | 3 | 117 (11.9) | 6h |
| Ryu et al,150 2018 | 187 | 67 (range 22-93) | 135 (72) | 76 (41) | 93 (50) | NA | 187 (100) | 0 (0) | 187 | 1 | Saline | Subdural | ≤48 h | 3 | 22 (11.8) | 6h |
| Sah & Rawal,151 2018 | 102 | NA | NA | NA | NA | NA | 80 (78) | 22 (22) | 124 | NA | NA | Subdural | NA | NA | 9 (8.8) | 5g |
| Santarius et al,5 2009 | 108 | 74.4 (46-94) | 83 (77) | 49 (45) | NA | NA | 87 (81) | 21 (19) | 129 | 2 | Warm Ringer solution | Subdural | ≤48 h | 6 | 10 (9) | Highi |
| Sarnvivad et al,152 2011 | 97 | 60.5 (±20.1) | 66 (68) | 21 (22) | NA | NA | 76 (78) | 21 (22) | 118 | 1 | Saline | NA | NA | NA | 15 (16) | 4g |
| Schoedel et al,153 2016 | 697 | 70.1 (range 1-97) | 461 (66) | 226 (32) | 317 (45) | NA | NA | NA | NA | NA | NA | Subdural | NA | NA | 155 (22.2) | 5g |
| Schwarz et al,154 2015 | 193 | 71.4 (±13.6) | 137 (71) | 87 (45) | 143 (74) | 9 (5) | 138 (72) | 55 (28) | 250 | NA | Other | Subdural | ≤24 h | 3 | 35 (18.1) | 8h |
| ShafiqAlam et al,155 2017 | 428 | NA | NA | NA | NA | NA | NA | NA | NA | NA | Saline | NA | NA | NA | 53 (12.4) | 5h |
| Shah et al,156 2014 | 25 | NA | 15 (60) | NA | NA | NA | NA | NA | NA | 1 | Warm saline | Subdural | ≤48 h | 6 | 3 (12) | Highi |
| Shen et al,157 2019 | 457 | 68.8 (range 23-92) | 376 (82) | 28 (6) | 235 (51) | NA | 311 (68) | 146 (32) | 603 | 1 | Saline | Subdural | ≤72 h | 3 | 69 (15.1) | 8h |
| Shim et al,158 2019 | 60 | 74.5 (range 67-90) | 44 (73) | 0 (0) | 30 (50) | NA | NA | NA | NA | 1 | Saline | Subdural | NA | NA | 8 (13.3) | 5g |
| Singh et al,159 2011 | 52 | 61.2 | 47 (90) | NA | NA | NA | 52 (100) | 0 (0) | 52 | 1 | Gentamicin-induced irrigation | Subdural | NA | 1 | 1 (2) | Highi |
| Singh et al,160 2014 | 100 | NA | NA | 24 (24) | 72 (72) | 34 (34) | 87 (87) | 13 (13) | NA | 1 or 2 | Saline | Subdural | ≤48 h | 6 | 9 (9) | Highi |
| Sjavik et al,161 2017 | 1260 | 73 (±11) and 74 (±13) and 74 (±13) | 878 (70) | 222 (18) | NA | NA | 1005 (80) | 217 (17) | 1439 | 1 | Gentamicin-induced irrigation | Subdural, subgaleal | ≤24 h | 6 | 169 (13.4) | 6h |
| Song et al,162 2014 | 97 | 70 (range 15-93) | 64 (66) | NA | 61 (63) | NA | 94 (97) | 3 (3) | 100 | 1 | Other | Subdural | ≤48 h | 3 | 16 (16.5) | 6h |
| Sousa et al,163 2013 | 778 | 64.3 (±15.9) (range 14-93) | 643 (83) | NA | 470 (60) | NA | 604 (78) | 174 (22) | 952 | 1 | Saline | Subdural | ≤48 h | 3 (minimum) | 42 (5.4) | 5h |
| Stanisic et al,164 2013 | 107 | 72.1 (±12.8) | 72 (67) | NA | NA | NA | 84 (79) | 23 (21) | 130 | 1 | Saline | Subdural | ≤24 h | 7 | 17 (15.9) | 7h |
| Sucu & Akar,165 2014 | 119 | 65.7 (±16.9) | NA | NA | NA | NA | 84 (71) | 35 (29) | 154 | 1 or 2 | NA | NA | ≤48 h | 1 | 4 (3.4) | 2h |
| Suzuki et al,166 1998 | 67 | NA | NA | NA | NA | NA | NA | NA | NA | 1 | Warm saline | Subdural | ≤72 h | NA | 2 (3) | 4g |
| Tagle et al,167 2003 | 100 | 77 (±13) | 77 (77) | 21 (21) | 43 (43) | NA | 84 (84) | 16 (16) | 116 | 1 or 2 | Other | NA | ≤72 h | 66 | 13 (13) | 2h |
| Takei et al,168 2021 | 277 | 82 (range 76-89) and 87 (range 76-92) | 211 (76) | 79 (29) | NA | NA | 247 (89) | 30 (11) | 307 | 3 | 35 (11.4)f | 8h | ||||
| Tahsim-Oglou et al,169 2012 | 247 | 75 (±13) and 77 (±8) | 165 (67) | NA | NA | NA | 193 (78) | 54 (22) | 281 | 2 | Warm Ringer solution | Subdural | ≤48 h | 1 | 62 (25.1) | 7h |
| Tailor et al,170 2017 | 123 | 75.6 | 88 (72) | 30 (24) | NA | NA | 97 (79) | 26 (21) | 149 | 1 or 2 | NA | Subdural | NA | 6 | 10 (8.1) | 4g |
| Tanikawa et al,171 2001 | 33 | 69.3 (±14.9) | NA | NA | NA | NA | NA | NA | NA | 2 | Saline | Subdural | ≤72 h | 6 | 4 (12.1) | Highi |
| Taussky et al,172 2008 | 76 | 69 (±12) (range 38-89) | 54 (71) | 53 (70) | NA | NA | 55 (72) | 21 (28) | 97 | 1 or 2 | Ringer solution/Hartmann solution | NA | ≤48 h | 1 | 13 (13.4)f | 5h |
| Thavara et al,173 2019 | 63 | 61.4 (±13.2) | 48 (76) | 9 (14) | 31 (49) | 6 (10) | 63 (100) | 0 (0) | 63 | 1 | Saline | Subdural | ≤24 h | NA | 1 (1.6) | 5g |
| Toi et al,174 2019 | 342 | 77 (65-80) and 78 (65-79) | 248 (73) | 57 (17) | 223 (65) | NA | 342 (100) | 0 (0) | 342 | 1 | Other | Subdural | Other | 3 | 39 (11.4) | Highi |
| Tomita et al,175 2018 | 102 | 77.2 (±10.7) | 66 (65) | 21 (21) | 76 (75) | 4 (4) | 89 (87) | 13 (13) | 115 | 1 | Saline | Subdural | NA | NA | 8 (7.8) | 4h |
| Tommiska et al,176 2019 | 71 | 78 (range 57- 93) | 50 (70) | 48 (68) | 62 (87) | NA | 55 (77) | 15 (21) | 85 | 1 | Warm Ringer solution | Subdural | ≤48 h | 6 | 4 (5.6) | 7g |
| Torihashi et al,177 2008 | 337 | 75.5 (range 28-96) | 228 (68) | 24 (7) | 81 (24) | NA | 268 (80) | 69 (20) | 406 | 1 | Saline | Subdural | NA | 1 | 61 (18.1) | 7h |
| Tosaka et al,178 2015 | 46 | NA | NA | NA | NA | NA | NA | NA | 46 | NA | Saline | Subdural | NA | 1.5 | 5 (10.9)f | 4g |
| Tsai et al,179 2010 | 129 | 71 (range 22-97) | 100 (78) | 11 (9) | 90 (70) | 6 (5) | 84 (65) | 45 (35) | 174 | 2 | Saline | Subdural | NA | NA | 12 (9.3) | 4h |
| Tugcu et al,180 2014 | 292 | 61.9 (±17.8) (range 1-96) | 200 (68) | 56 (19) | 160 (55) | 4 (1) | 210 (72) | 82 (28) | 374 | 2 | Warm saline | NA | ≤96 h | 1 | 43 (14.7) | 7h |
| Vasella et al,181 2018 | 28 | 70.4 (±16.1) | 24 (86) | NA | NA | NA | 19 (68) | 9 (32) | 37 | 2 | Warm saline | Subdural, subperiosteal | ≤48 h | 9 | 1 (3.6) | 2h |
| Wan et al,182 2017 | 31 | 73.6 (range 61-83) | 18 (58) | 1 (3) | 26 (84) | NA | 31 (100) | 0 (0) | NA | 1 | Saline | Subdural | ≤120 h | 3 | 13 (41.9) | 4g |
| Wang et al,183 2016 | 53 | 66.7 (±13.1) | 44 (83) | 0 (0) | 45 (85) | 8 (15) | 61 | 1 | Saline | Subdural | ≤72 h | 1 | 9 (17) | 5g | ||
| Wang et al,184 2017 | 45 | 67.3 (±12.9) | 38 (84) | 6 (13) | 26 (58) | 0 (0) | 45 (100) | 0 (0) | 45 | 1 | Saline | Subdural | Other | 3 | 5 (11.1) | 6g |
| Wang et al,185 2017 (1) | 88 | 65.5 (±7.8) | 53 (60) | NA | NA | NA | 88 (100) | 0 (0) | 88 | 1 | Warm saline | NA | ≤72 h | 12 | 6 (6.8) | 5g |
| Wang et al,186 2017 (2) | 57 | 64.6 | 52 (91) | 0 (0) | 47 (82) | NA | NA | NA | NA | 1 | Warm saline | Subdural | ≤72 h | 6 | 0 (0) | 6g |
| Wang et al,187 2020 | 653 | 72 (IQR 64-80) | 561 (86) | NA | NA | 234 (current drinking)d (36) | 504 (77) | 149 (23) | 802 | 3 | 96 (14.7) | 8h | ||||
| Weigel et al,188 2015 | 93 | 75.6 (±12.9) and 72.6 (±13) | NA | 0 (0) | NA | NA | 73 (78) | 20 (22) | 113 | 1 or 2 | Other | Subdural | ≤72 h | 3 | 13 (11.5)f | 5h |
| Weng et al,189 2019 | 190 | 68 (range 27-86) | 118 (62) | NA | 161 (85) | NA | 190 (100) | 0 (0) | 190 | 1 | Other | Subdural | NA | 6 | 17 (8.9) | 4h |
| Won et al,190 2021 | 176 | 75 (range 65-80) and 74.5 (range 62-80) | 126 (72) | 87 (49) | NA | NA | 120 (68) | 56 (32) | 232 | 3 | 40 (22.7) | Highi | ||||
| Wu et al,191 2020 | 331 | NA | 285 (86) | 22 (6) | 239 (72) | 95 (29) | 268 (81) | 63 (19) | 394 | 3 | 30 (9.1) | 6h | ||||
| Yadav et al,192 2016 | 140 | 53 (±22.1) (range 18-75) | 101 (72) | NA | 140 (100) | NA | 140 (100) | 0 (0) | 140 | 1 | Warm saline | Subgaleal | NA | 21 | 5 (3.6) | 6g |
| Yagnick et al,193 2019 | 60 | 64.3 | 51 (85) | 21 (35) | 20 (33) | NA | 42 (70) | 18 (30) | 78 | 2 | Other | Subdural | NA | 8 | 0 (0) | 5h |
| Yamada et al,194 2018 | 1080 | 72.1 (±14.3) (range 13-101) | 711 (66) | 124 (11) | 827 (77) | 5 (0) | 883 (82) | 197 (18) | 1227 | 1 | Other | Subdural | ≤48 h | NA | 119 (11) | 5h |
| Yamada & Notori,195 2020 | 193 | 78.8 (±0.8) and 78.2 (±9.8) and 79.2 (±8.7) | 160 (83) | 72 (37) | NA | 48 (25) | 85 (44) | 108 (56) | 232 | 1 | Other | Subdural | ≤24 h | 3 | 16 (6.9)f | Highi |
| Yamamoto et al,196 2003 | 105 | 71.4 (±9.6) and 71.4 (±10.5) | 73 (70) | 4 (4) | 78 (74) | 41 (39) | 82 (78) | 23 (22) | 128 | 1 or 2 | Warm saline | NA | ≤48 h | 6 | 11 (10.5) | 7h |
| Yan et al,197 2017 | 52 | 66.4 (±9.4) | 37 (71) | 12 (23) | NA | NA | 41 (79) | 11 (21) | 63 | 1 | Saline | Subdural | ≤72 h | 12 | 7 (13.7) | 4g |
| Yan et al,198 2018 | 231 | NA | 188 (81) | 0 (0) | 0 (0) | NA | 201 (87) | 30 (13) | 261 | NA | Other | NA | ≤48 h | 3 | 33 (14.3) | 5h |
| You et al,199 2018 | 226 | 65.1 (±13.4) | 184 (81) | 14 (6) | 161 (71) | NA | 160 (71) | 66 (29) | 292 | 1 or 2 | Saline | Subdural | ≤48 h | 12 | 34 (15) | 8h |
| Yu et al,200 2009 | 97 | 67 (range 14-93) | 82 (85) | NA | NA | 11 (11) | 74 (76) | 24 (25) | 121 | 1 | Saline | Subdural | Other | 3 | 8 (8.2) | 5h |
| Zakaraia et al,201 2008 | 40 | 59.7 | 29 (73) | 0 (0) | NA | NA | NA | NA | NA | 2 | Saline | Subdural | ≤72 h | 6 | 4 (10) | Highi |
| Zhang et al,202 2018 | 31 | 75.1 | 22 (71) | 13 (42) | 24 (77) | 9 (29) | 29 (94) | 2 (6) | 33 | 2 | Warm saline | Subdural | NA | 6 | 8 (25.1) | 6g |
| Zhang et al,203 2019 | 570 | 71 (IQR 62-79) | 422 (74) | 123 (22) | 380 (67) | 52 (9) | 333 (58) | 237 (42) | 807 | 1 or 2 | Gentamicin-induced irrigation | Subdural, subperiosteal | ≤48 h | 6 | 70 (12.3) | 7h |
| Zumofen et al,204 2009 | 147 | 71.5 (range 42-93) | 113 (77) | 41 (28) | 117 (80) | 12 (8) | 111 (76) | 36 (24) | 183 | 2 | Saline | Subperiosteal | ≤48 h | 3 | 22 (15) | 5h |
| Pooled incidence (95% CI, I2) | — | — | 71.9% (70.6-73.2; 84.3%) | 23.6% (21.3-26.0; 98.5%) | 64.2% (55.1-73.3; 99.8%) | 12.3% (10.4-14.1; 95.8%) | — | — | — | a | See Table 5 | See Table 6 | See Table 6 | — | See Results section | See Results section |
CSF, cerebrospinal fluid; cSDH, chronic subdural hematoma; I2, I-squared statistic; NA, not available.
A single burr-hole per side was made in 55.8%, a double burr-hole per side in 19.3% of cases, and in 24.9% it was reported that either a single burr-hole or a double burr- hole was used, and these could not be separated.
Labeled according to 6 categories we established: Saline, warm saline, Ringer solution/Hartmann solution, warm Ringer solution, Gentamicin-induced irrigation and other. Category “saline” was abbreviated, full name: saline/normal saline/isotonic saline/physiological saline. “other” included: unknown (described in included studies as: irrigation/washout/rinsing), normal saline or artificial CSF, artificial CSF, or irrigation with ARTCEREB.
Labeled according to 6 categories we established: ≤24 h, ≤48 h, ≤72 h, ≤96 h, ≤120 h, and other. “Other” included different drainage periods described in studies: 24–144 h, 192 ± 96 h, 48–144 h, 8 h until <5 mL per hour draining volume, and 24–216 h.
Excluded in calculating history of alcohol abuse proportion.
Only 71 patients with a bilateral cSDH underwent operation.
Study which calculates the recurrence rate as proportion of the total number of hematomas.
Quality assessment performed using the Newcastle-Ottawa scale.
Quality assessment performed using the adjusted Newcastle-Ottawa scale.
Quality assessment performed using the Cochrane risk-of-bias tool for randomized trials. High means high risk of bias.
Recurrence Rate and Mortality
Baseline characteristics of the 189 included studies reporting the number of recurrences are shown in Table 1. The pooled incidence of 174 studies with 34 393 patients reporting 4208 recurrences per number of patients was 11.2% (95% CI: 10.3-12.1; I2 = 87.7%; 95% PI: 0.0-22.0). In addition, the pooled incidence of 15 studies with 3078 hematomas reporting 370 recurrences per number of hematomas was 11.0% (95% CI: 8.6-13.4; I2 = 78.0%, 95% PI: 2.0-20.0). Forest plots are shown in the Supplementary Fig. 1, http://links.lww.com/ONS/A923 and Supplementary Fig. 2, http://links.lww.com/ONS/A924.
The pooled incidence of reported recurrence rates at 3 months (25 articles) was 12.7% (95% CI: 10.7-14.7; I2 = 86.1%) and 9.8% (95% CI: 7.6-11.9; I2 = 89.0%) at 6 months (21 articles). The pooled incidence of recurrence in patients treated by 1 burr-hole was 12.2% and in patients treated by 2 burr-holes was 12.8%.
Pooled mortality incidences are displayed in Table 2. Mortality related to cSDH treatment was seen in 56 patients with a pooled incidence of 0.7% (95% CI 0.0-1.4; I2 = 0.0%) (Table 3).
TABLE 2.
Pooled Incidence of Mortality per Time Period
| Mortality period | Number of studies | Number of patients | Pooled incidence (95% CI; I2) |
|---|---|---|---|
| In-hospital mortality | 39 | 188 | 1.3% (0.9-1.7; 78.9%) |
| 30-d mortality | 29 | 229 | 2.5% (1.7-3.2; 86.6%) |
| 3-mo mortality | 9 | 256 | 3.6% (1.9-5.3; 90.0%) |
| 6-mo mortality | 15 | 493 | 6.5% (3.6-9.4; 97.2%) |
| Mortality without time period indication | 45 | 199 | 2.0% (1.4-2.5; 73.6%) |
I2, I-squared statistic.
All calculated with random-effects model.
TABLE 3.
Studies Reporting Mortality Causes Related to cSDH Treatment
| Author and year | Mortality cause(s) related to cSDH treatment (number of cases) |
|---|---|
| Bartley et al,29 2020 | Intracerebral hematoma (n = 1), basal ganglion infarction (n = 1) |
| Bellut et al,30 2012 | Postoperative intraparenchymal hematoma (n = 1) |
| Carlisi et al,33 2017 | Postoperative (n = 1) |
| Chen et al,40 2020 | Recurrent cSDH (n = 2) |
| Choi et al,42 2016 | After taking anticoagulants (n = 3) |
| Djientcheu et al,48 2011 | Brain herniation (n = 1), inhalation pneumonia in comatose patients with delayed treatment (n = 3) |
| Eppel et al,53 1999 | Postoperative brain hemorrhage (n = 1) |
| Gabarros et al,60 2000 | Recurrent bleeding (n = 1) |
| Hennig et al,72 1999 | Infection (n = 1), rebleed after first operation (n = 1) |
| Kotwica et al,102 1991 | Large ischemic stroke of the hemisphere compressed by the hematoma (n = 1), purulent meningitis, followed by subdural empyema (n = 1) |
| Lepic et al,113 2021 | Sequence of rebleeding episodes and clinical worsening (n = 1) |
| Liu et al,119 2019 | Recurrence of cSDH for which consent for repeat surgery was not given (n = 1) |
| Mellergard et al,125 1996 | Subdural empyema after third recurrence operation (n = 1) |
| Mezue et al,127 2011 | Secondary infection (n = 1) |
| Missori et al,130 2000 | Acute subdural hemorrhage (n = 1) |
| Morales-Gomez et al,131 2020 | aSDH (n = 1), parenchymal hematoma (n = 1) |
| Penchet et al,140 1998 | Empyema (n = 1), aSDH (n = 1) |
| Piotrowski et al,141 1996 | Cerebral decompensation which were comatose before operation (n = 6) |
| Ridwan et al,147 2019 | Cerebrovascular accidents (n = 2) |
| Rohde et al,148 2002 | Intracerebral bleeding (n = 4) |
| Schoedel et al,153 2016 | Acute secondary hemorrhage (n = 5) |
| Shen et al,157 2019 | Spontaneous ventricular hemorrhage after operation (n = 1), postoperative acute epidural hemorrhage (n = 1) |
| Sucu et al,165 2014 | Subdural empyema (n = 1), aSDH (n = 1) |
| Suzuki et al,166 1998 | Extreme disturbance of consciousness did not improve postoperatively and pneumonia supervened (n = 1) |
| Tagle et al,167 2003 | Recurrence of cSDH (n = 1) |
| Tsai et al,179 2010 | aSDH (n = 2) |
| Zhang et al,203 2019 | Because of removal of the drain after which aSDH developed, the patient died 45 d later in the hospital because of pneumonia (n = 1) |
| Zumofen et al,204 2009 | Continued to bleed acutely from subdural membranes despite trepanation, refrained from second intervention (n = 2) |
aSDH, acute subdural hematoma; cSDH, chronic subdural hematoma.
Treatment Modalities
Pooled incidences and recurrence rates per irrigation method, type of postoperative drainage, and drainage time are shown in Tables 4-6, respectively. Ringer solution (also titled Hartmann solution) showed a recurrence rate of 21.4%, and irrigation fluid at room temperature (22 °C) showed a recurrence rate of 12.0%.
TABLE 4.
Pooled Incidence of Irrigation Method
| Irrigation method | No.of studies | No. of patients | Pooled incidence | Recurrence rate | Recurrence rate combined (95%CI; I2)b |
|---|---|---|---|---|---|
| Saline/normal saline/isotonic saline/physiological saline | 75 | 13.364 | 47.8% | 11.1% | 12.0% |
| Ringer solution/Hartmann solution | 9 | 1292 | 4.6% | 21.4% | |
| Gentamicin-induced irrigation method | 4 | 1972 | 7.0% | 12.5% | |
| Warm saline | 28 | 2893 | 10.4% | 9.7% | 11.5% |
| Warm Ringer solution | 8 | 1113 | 4.0% | 15.7% | |
| Othera | 28 | 7313 | 26.2% | Not determined | Not determined |
I2, I-squared statistic.
Other includes Hartmann solution with gentamicin, normal saline or artificial CSF, artificial CSF, irrigation with ARTCEREB and those describing only irrigation/washout/rinsing.
Saline/normal saline/isotonic saline/physiological saline and Ringer solution/Hartmann solution are combined to determine the recurrence rate, and warm saline and warm Ringer solution are combined to investigate the effect of normal vs warm irrigation method.
Calculated by dividing the number of patient per group by the total number of patients in which the irrigation method was described.
TABLE 6.
Pooled Incidence of Postoperative Drainage Time
| Drainage time in hoursa | No. of studies | No. of patients | Pooled incidence | Recurrence rate |
|---|---|---|---|---|
| ≤24 h | 17 | 4577 | 16.7% | 11.4% |
| ≤48 h | 57 | 12 619 | 46.0% | 11.2% |
| ≤72 h | 28 | 5500 | 20.0% | 13.3% |
| ≤96 h | 5 | 657 | 2.4% | 14.5% |
| ≤120 h | 11 | 2465 | 9.0% | 8.3% |
| Otherb | 6 | 1623 | 5.9% | Not determined |
When a drainage time range was given in a study, the maximum drainage time was accounted for and calculated with in the corresponding variable.
Other includes different drainage time periods described in studies: 24–144 h, 192 ± 96 h, 48–144 h, 8 h until <5 mL per hour draining volume, and 24–216 h.
Calculated by dividing the number of patients per group by the total number of patients in which postoperative drainage time was described.
TABLE 5.
Pooled Incidence and Recurrence Rate per Type of Postoperative Drain Location Used
| Location of postoperative drain | No. of studiesa | No. of patients | Pooled incidence | Recurrence rateb |
|---|---|---|---|---|
| Subdural | 139 | 24 965 | 80.9% | 12.8% |
| Subperiosteal | 7 | 780 | 2.5% | 13.6% |
| Subgaleal | 8 | 1596 | 5.2% | 9.9% |
| Other | 13 | 3536 | 11.4% | Not determined |
In 9 studies, more than 2 drain locations were reported and could be separated per location and was calculated additionally.
In 42 studies of which drain location was unknown, the first author was emailed to retrieve the drain location. Hereby, we determined 9 drain locations extra.
Calculated by dividing the number of patients per group by the total number of patients in which postoperative drain location was described.
“Other” includes studies reporting 2 or more drain locations which could not be separated.
Subgroup Analyses
We performed a subgroup analysis of the 50 studies with the highest quality regarding risk-of-bias judgment, of which 48 (15 298 patients) described the number of recurrences (2019 recurrences) as per the patient, and 2 studies (597 patients) described the recurrences (72 hematomas) as per the number of hematomas. Pooled incidence of recurrences were 12.8% (95% CI 11.4-14.2; I2 = 86.1%; 95% PI 2.0-25.0) and 12.0% (95% CI 9.4-14.7; I2 = 0.0%), respectively (see Supplementary Fig. 3 for forest plot, http://links.lww.com/ONS/A925). The pooled incidence of patients on antithrombotic medication, history of trauma, and history of alcohol abuse is 28.2% (95% CI 23.4-33.1; I2 = 98.2%), 64.4% (95% CI 55.5-73.3; I2 = 99%), and 15.3% (95% CI 10.8-19.8; I2 = 97.2%), respectively. Recurrence rates in high-quality studies per the irrigation method were as follows: saline/normal saline/isotonic saline/physiological saline, 13.8%, Ringer solution/Hartmann solution, 9.6%; gentamicin-induced irrigation method, 9.6% (determined in 1 study); warm saline, 10.7%; and warm Ringer solution of 10.0% (determined in 1 study). For the type of drainage method in high-quality studies, recurrence rate for a subdural drain was 13.6% and for subperiosteal drain was 15.8% (determined from 1 study), and a subgaleal drain was not used in high-quality studies. Regarding the duration of postoperative drainage times ≤24 hours, ≤48 hours, ≤72 hours, ≤96 hours, and ≤120 hours, the respective recurrence rates are 14.1%, 12.6%, 13.0%, 13.4% (determined in 1 study), and 13.6%.
Definitions of Recurrence Used
In 167 articles, a definition of a recurrent cSDH was stated. In 66 of these articles, reoperation was the sole definition of recurrence. In 57 articles, a combination of clinical, radiological factors and a reoperation was regarded as recurrence; in 28 articles, clinical and radiological factors defined recurrence. In 2 articles, solely clinical symptoms were regarded as a recurrence and in 14 articles only radiological features. We determined the recurrence rates per definition, which are 10.5%, 12.4%, 13.9%, 14.0%, and 9.4%, respectively. In 29 articles, a period of 3 months was used for the recurrence definition and in 24 articles a period of 6 months. In the subgroup of 50 studies with the highest quality, 23 articles used a combination of clinical, radiological factors and a reoperation as definition of recurrence. In 9 articles clinical and radiological factors determined a recurrence and in 4 articles only radiological factors. Furthermore, 17 of these high-quality studies used a period of 3 months and 7 used a period of 6 months. We analyzed the pooled incidences and recurrence rates of treatment modalities of the studies using a combination of clinical, radiological factors and a reoperation as recurrence definition (Supplementary Tables 2-4, http://links.lww.com/ONS/A926, http://links.lww.com/ONS/A927, and http://links.lww.com/ONS/A928).
DISCUSSION
This systematic review shows a pooled incidence of recurrence of 12.8% after burr-hole surgery and postoperative drainage in patients with a chronic subdural hematoma. Because this recurrence rate has been accurately determined, it can serve as an outcome measure for power calculations to determine sample sizes for future randomized clinical trials.
Most of the included studies were of a retrospective nature, the majority was judged to carry a high to very high risk of bias, and the RCTs were all considered to be of low quality. The overall pooled incidence is 11.2%. In our subgroup analysis of only those studies of the highest quality, the pooled incidence of recurrence was remarkably higher at 12.8%, which could infer that studies with poor quality systematically underestimate the recurrence rate. Therefore, the most reliable pooled incidence of recurrence is 12.8%. Our analyses of the pooled incidence of recurrence at 3 and 6 months shows that both periods do not accurately approach the overall recurrence rate of cSDH, possibly because of the low number of studies of which data could be derived from.
Recurrence of a cSDH leads to rehospitalization and reoperation, resulting in worse clinical outcome, loss of independency in these often very frail patients, and a significant increase in health care costs.205-208 Prevention of recurrences is therefore important, and various nonsurgical treatments are now being explored in randomized clinical trials, including medication (tranexamic acid).9,209 In a recent RCT, the use of dexamethasone vs placebo demonstrated that fewer recurrences occurred but that less favorable outcomes and more adverse events were noted in the dexamethasone group.10
This systematic review also provided information on recurrence rates depending on the method of irrigation used. Ringer solution (also titled Hartmann solution) showed a relatively high recurrence rate, as did irrigation fluid at room temperature (22 °C) when compared with irrigation at body temperature (37 °C), which is in line with a recent study.210 The pooled recurrence rate of subgaleal drainage was lower compared with subdural and subperiosteal drainage, although subgaleal drainage was used in only 8 studies, making it hard to draw firm conclusions. In the literature, recurrence rates after burr-hole surgery do not appear to be affected by drain location.211 However, 2 recent systematic reviews and meta-analysis showed that the insertion of a subperiosteal drain resulted in a significantly lower recurrence rate compared with a subdural drain but did not take into account a subgaleal drain.212,213 Furthermore, we researched whether patient characteristics or treatment modalities could be of influence on the high-quality recurrence rate by performing subgroup analyses. Only the irrigation method, saline/normal saline/isotonic saline/physiological saline, seems to be associated with recurrence because this rate is higher compared with the other determined rates in between treatment modalities. The pooled incidences of recurrence in the high-quality group could therefore be regarded as more stable and should be used as most reliable recurrence rates.
Limitations
A relatively high statistical heterogeneity was observed for nearly all calculated pooled incidences in the current systematic review and meta-analysis. This could be due to clinical heterogeneity because clinical diversity in studies, despite the strict inclusion and exclusion criteria, is inevitable. A difference in baseline characteristics of included studies was observed. Some studies for example applied certain age restrictions, and some only included unilateral hematomas or patients with antithrombotic therapy, and across studies, different treatment methods regarding number of burr-holes, type of irrigation, and location of the postoperative drain and different definitions of recurrence were used. Moreover, methodological heterogeneity also attributes to the statistical heterogeneity because retrospective and prospective studies and RCTs are included. When measuring prevalence of a phenomenon in diverse environments, it is expected that highly heterogeneous studies are assembled.214 Because all included studies present a recurrence rate and therefore essentially measure the same parameter, we think it is worth summarizing despite clinical and methodological heterogeneity. Furthermore, in the literature, a wide range of recurrence rates are used, and the current study is a first step to accurately provide one overall recurrence rate. A subgroup analysis of studies with the highest quality was performed and still showed high heterogeneity meaning that even in these studies clinical and methodological differences are present and cannot be avoided.
Prediction interval calculation of studies with the highest quality showed that the recurrence rate in 95% of future studies is expected to range from 2% to 25%. This wide prediction interval is likely caused by the high heterogeneity of studies because the prediction interval is a summary of the spread of underlying effects in the studies included in the random-effects meta-analysis.16 The created forest plots show a considerable in-between study difference regarding recurrence rate, even in the studies with the highest quality. Consequently, the calculated pooled recurrence rate should be interpreted with some caution.
Fortunately, randomized controlled trials are being increasingly conducted in neurosurgery. However, for power calculations to determine sample size, it is crucial to use accurate outcome measures. The current study provides the most accurate known recurrence rate for cSDH treated by burr-hole surgery and postoperative drainage. Therefore, we propose to use the recurrence rate calculated in this study for future sample size calculations. Furthermore, to compare the results of these clinical studies, consistency in terms and definitions is essential, whereas definitions for a recurrent cSDH vary considerably in the literature. We would like to recommend using the following standardized definition of a recurrent cSDH in future studies: A recurrent cSDH is defined as recurring or aggravated clinical symptoms, caused by a radiologically proven, ipsilateral reaccumulation of cSDH within 6 months of prior surgical treatment, for which additional treatment is necessary. It then still remains debatable whether a recurrence on the contralateral side is a recurrence or a newly diagnosed cSDH.
CONCLUSION
The overall recurrence rate of chronic subdural hematoma after burr-hole surgery and postoperative drainage is 12.8%. This rate should be interpreted with some caution because observed heterogeneity of included studies is high. A unified definition of cSDH recurrence after surgical treatment is advocated to ensure reliable comparison between future studies.
Supplementary Material
Footnotes
Supplemental digital content is available for this article at operativeneurosurgery-online.com.
Contributor Information
Merijn Foppen, Email: m.foppen@amsterdamumc.nl.
Kari-Anne Mariam Slot, Email: k.slot@amsterdamumc.nl.
William Peter Vandertop, Email: wp.vandertop@amsterdamumc.nl.
Dagmar Verbaan, Email: d.verbaan@amsterdamumc.nl.
Funding
This study did not receive any funding or financial support.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
SUPPLEMENTAL DIGITAL CONTENT
Supplemental Content Table 1. Syntax search.
Supplementary Fig 1. Forest plot and prediction interval of pooled incidence of recurrences per number of patients.
Supplementary Fig 2. Forest plot and prediction interval of pooled incidence of recurrences per number of hematomas.
Supplementary Fig 3. Forest plot and prediction interval of pooled incidence of recurrences of studies with the highest quality.
Supplemental Content Table 2. Pooled incidence and recurrence rate of irrigation methods in studies using a definition of clinical and radiological factors and a reoperation.
Supplemental Content Table 3. Pooled incidence and recurrence rate of postoperative drain location used in studies using a definition of clinical and radiological factors and a reoperation.
Supplemental Content Table 4. Pooled incidence and recurrence rate of postoperative drainage time used in studies using a definition of clinical and radiological factors and a reoperation.
COMMENTS
This systematic review and meta-analysis provide valuable insights into the recurrence rates of chronic subdural hematoma (cSDH) after burr-hole evacuation with postoperative drainage. While several reviews and meta-analyses have been published on this topic, this study stands out for its focus on recurrence rates and its unique analysis of the impact of irrigation solutions.
The review's findings suggest that the recurrence rate of cSDH after burr-hole evacuation with postoperative drainage is 12.8%, providing neurosurgeons with important information to inform their decision-making processes. Moreover, the study's examination of the effects of different irrigation solutions on recurrence rates provides additional insight into potential interventions that could improve patient outcomes.
Overall, this review underscores the importance of ongoing research into the management of cSDH and the need for clinicians to carefully consider the specific factors that may impact recurrence rates when making treatment decisions. The study's rigorous methodology and clear reporting of results make it a valuable contribution to the current literature on this topic.
Jason H. Huang
Temple, Texas, USA
REFERENCES
- 1.Rauhala M, Luoto TM, Huhtala H, et al. The incidence of chronic subdural hematomas from 1990 to 2015 in a defined Finnish population. J Neurosurg. 2019;132(4):1-11. [DOI] [PubMed] [Google Scholar]
- 2.Balser D, Farooq S, Mehmood T, Reyes M, Samadani U. Actual and projected incidence rates for chronic subdural hematomas in United States Veterans Administration and civilian populations. J Neurosurg. 2015;123(5):1209-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lega BC, Danish SF, Malhotra NR, Sonnad SS, Stein SC. Choosing the best operation for chronic subdural hematoma: a decision analysis. J Neurosurg. 2010;113(3):615-621. [DOI] [PubMed] [Google Scholar]
- 4.Yadav YR, Parihar V, Namdev H, Bajaj J. Chronic subdural hematoma. Asian J Neurosurg. 2016;11(04):330-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santarius T, Kirkpatrick PJ, Ganesan D, et al. Use of drains versus no drains after burr-hole evacuation of chronic subdural haematoma: a randomised controlled trial. Lancet. 2009;374(9695):1067-1073. [DOI] [PubMed] [Google Scholar]
- 6.Santarius T, Qureshi HU, Sivakumaran R, Kirkpatrick PJ, Kirollos RW, Hutchinson PJ. The role of external drains and peritoneal conduits in the treatment of recurrent chronic subdural hematoma. World Neurosurg. 2010;73(6):747-750. [DOI] [PubMed] [Google Scholar]
- 7.Kaliaperumal C, Khalil A, Fenton E, et al. A prospective randomised study to compare the utility and outcomes of subdural and subperiosteal drains for the treatment of chronic subdural haematoma. Acta Neurochir (Wien). 2012;154(11):2083-2089;discussion 2088-2089. [DOI] [PubMed] [Google Scholar]
- 8.Brennan PM, Kolias AG, Joannides AJ, et al. The management and outcome for patients with chronic subdural hematoma: a prospective, multicenter, observational cohort study in the United Kingdom. J Neurosurg. 2017;127(4):732-739. [DOI] [PubMed] [Google Scholar]
- 9.Edlmann E, Holl DC, Lingsma HF, et al. Systematic review of current randomised control trials in chronic subdural haematoma and proposal for an international collaborative approach. Acta Neurochir (Wien). 2020;162(4):763-776. [DOI] [PubMed] [Google Scholar]
- 10.Hutchinson PJ, Edlmann E, Bulters D, et al. Trial of dexamethasone for chronic subdural hematoma. N Engl J Med. 2020;383(27):2616-2627. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012. [DOI] [PubMed] [Google Scholar]
- 12.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; 2021. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed July 1, 2021. [Google Scholar]
- 13.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroll JB, Moustgaard R, Gotzsche PC. Dealing with substantial heterogeneity in cochrane reviews. Cross-sectional study. BMC Med Res Methodol. 2011;11(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deeks JJHJ, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.3. Cochrane. 2022. [Google Scholar]
- 17.IntHout J, Ioannidis JP, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6(7):e010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abboud T, Duhrsen L, Gibbert C, Westphal M, Martens T. Influence of antithrombotic agents on recurrence rate and clinical outcome in patients operated for chronic subdural hematoma. Neurocirugia (Astur). 2018;29(2):86-92. [DOI] [PubMed] [Google Scholar]
- 19.Abouzari M, Rashidi A, Rezaii J, et al. The role of postoperative patient posture in therecurrence of traumatic chronic subdural hematoma after burr-hole surgery. Neurosurgery. 2007;61(4):794-797;discussion 797. [DOI] [PubMed] [Google Scholar]
- 20.Adachi A, Higuchi Y, Fujikawa A, et al. Risk factors in chronic subdural hematoma: comparison of irrigation with artificial cerebrospinal fluid and normal saline in a cohort analysis. PLoS One. 2014;9(8):e103703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei Chih AN, Sii Hieng AW, A Rahman NA, Abdullah JM. Subperiosteal drainage versus subdural drainage in the management of chronic subdural hematoma (a comparative study). Malays J Med Sci. 2017;24(1):21-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed SAD, Agrawal D, Kale S, et al. A comparative study of treatment of chronic subdural hematoma—burr hole drainage versus continuous closed drainage. Indian J Neurotrauma. 2011;8(1):17-23. [Google Scholar]
- 23.Ak H, Gülşen İ, Yaycioǧlu S, et al. The effects of membranous abnormalities on mortality and morbidity in chronic subdural hematomas. J Neurol Sci. 2015;32(1):154-160. [Google Scholar]
- 24.Amano T, Matsuo S, Miyamatsu Y, Yamashita S, Nakamizo A. Impact of antithrombotic therapy on surgical treatment in patients with chronic subdural hematoma. J Clin Neurosci. 2020;74:55-60. [DOI] [PubMed] [Google Scholar]
- 25.Aung TH, Wong WK, Mo HP, Tsang CS. Management of chronic subdural haematoma: burr hole drainage, replacement with Hartmann's solution, and closed-system drainage. Hong Kong Med J. 1999;5(4):383-386. [PubMed] [Google Scholar]
- 26.Baechli H, Nordmann A, Bucher HC, Gratzl O. Demographics and prevalent risk factors of chronic subdural haematoma: results of a large single-center cohort study. Neurosurg Rev. 2004;27(4):263-266. [DOI] [PubMed] [Google Scholar]
- 27.Bankole OB, Yusuf AS, Kanu OO, Ukponmwan E, Nnadi MN, Arigbabu SO. Chronic subdural haematoma: clinical presentation, surgical treatment and outcome at the Lagos university teaching hospital. Afr J Neurol Sci. 2011;30(1):10-17. [Google Scholar]
- 28.Bartek J, Jr., Sjavik K, Stahl F, et al. Surgery for chronic subdural hematoma in nonagenarians: a Scandinavian population-based multicenter study. Acta Neurol Scand. 2017;136(5):516-520. [DOI] [PubMed] [Google Scholar]
- 29.Bartley A, Jakola AS, Tisell M. The influence of irrigation fluid temperature on recurrence in the evacuation of chronic subdural hematoma. Acta Neurochir. 2020;162(3):485-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellut D, Woernle CM, Burkhardt JK, Kockro RA, Bertalanffy H, Krayenbuhl N. Subdural drainage versus subperiosteal drainage in burr-hole trepanation for symptomatic chronic subdural hematomas. World Neurosurg. 2012;77(1):111-118. [DOI] [PubMed] [Google Scholar]
- 31.Blaauw J, Jacobs B, den Hertog HM, et al. Neurosurgical and perioperative management of chronic subdural hematoma. Front Neurol. 2020;11:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borger V, Vatter H, Oszvald A, Marquardt G, Seifert V, Guresir E. Chronic subdural haematoma in elderly patients: a retrospective analysis of 322 patients between the ages of 65-94 years. Acta Neurochir (Wien). 2012;154(9):1549-1554. [DOI] [PubMed] [Google Scholar]
- 33.Carlisi E, Feltroni L, Tinelli C, Verlotta M, Gaetani P, Dalla Toffola E. Postoperative rehabilitation for chronic subdural hematoma in the elderly. An observational study focusing on balance, ambulation and discharge destination. Eur J Phys Rehabil Med. 2017;53(1):91-97. [DOI] [PubMed] [Google Scholar]
- 34.Carlsen JG, Cortnum S, Sorensen JC. Recurrence of chronic subdural haematomata with and without post-operative drainage. Br J Neurosurg. 2011;25(3):388-390. [DOI] [PubMed] [Google Scholar]
- 35.Castro-Rodriguez C, Roman-Pena P, Aran-Echabe E, Gelabert-Gonzalez M. Chronic subdural haematomas in very elderly patients. Rev Esp Geriatr Gerontol. 2016;51(6):309-316. [DOI] [PubMed] [Google Scholar]
- 36.Certo F, Maione M, Altieri R, et al. Pros and cons of a minimally invasive percutaneous subdural drainage system for evacuation of chronic subdural hematoma under local anesthesia. Clin Neurol Neurosurg. 2019;187:105559. [DOI] [PubMed] [Google Scholar]
- 37.Chan DY, Woo PY, Mak CH, et al. Use of subdural drain for chronic subdural haematoma? A 4-year multi-centre observational study of 302 cases. J Clin Neurosci. 2017;36:27-30. [DOI] [PubMed] [Google Scholar]
- 38.Chang CL, Sim JL, Delgardo MW, Ruan DT, Connolly ES, Jr. Predicting chronic subdural hematoma resolution and time to resolution following surgical evacuation. Front Neurol. 2020;11:677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chandran RS, Nagar M, Sharmad MS, et al. Single parietal burr-hole craniostomy with irrigation and drainage for unilateral chronic subdural hematoma in young adults <40 years: a rationale behind the procedure. J Neurosci Rural Pract. 2017;08(03):389-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen S, Chen Z, Yang B, Xu T, Tu XK. Use of siphon irrigation during burr-hole craniostomy to evacuate chronic subdural hematoma: a retrospective cohort comparison study. Medicine. 2020;99(21):e20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng SY, Chang CK, Chen SJ, Lin JF, Tsai CC. Chronic subdural hematoma in elderly Taiwan patients: a retrospective analysis of 342 surgical cases. Int J Gerontol. 2014;8(1):37-41. [Google Scholar]
- 42.Choi JJ, Kim HS, Lee KC, Hur H, Jo YY. Prediction of in-hospital mortality and morbidity using high-sensitivity C-reactive protein after burr hole craniostomy. J Anesth. 2016;30(6):956-960. [DOI] [PubMed] [Google Scholar]
- 43.Choi J, Pyen J, Cho S, Kim J, Koo Y, Whang K. Influence of antithrombotic medication on the risk of chronic subdural hematoma recurrence after burr-hole surgery. J Korean Neurosurg Soc. 2020;63(4):513-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chon KH, Lee JM, Koh EJ, Choi HY. Independent predictors for recurrence of chronic subdural hematoma. Acta Neurochir (Wien). 2012;154(9):1541-1548. [DOI] [PubMed] [Google Scholar]
- 45.Choudhury AR. Avoidable factors that contribute to complications in the surgical treatment of chronic subdural haematoma. Acta Neurochir (Wien). 1994;129(1-2):15-19. [DOI] [PubMed] [Google Scholar]
- 46.Dobran M, Marini A, Nasi D, et al. Clinical outcome of patients over 90 years of age treated for chronic subdural hematoma. J Korean Neurosurg Soc. 2022;65(1):123-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D'Oria S, Dibenedetto M, Squillante E, et al. Chronic subdural hematomas: single versus double burr holes. J Neurosurg Sci. 2020;64(2):216-218. [DOI] [PubMed] [Google Scholar]
- 48.Djientcheu VP, Esiene A, Yamgoue T, Tchaleu B, Ze Minkande J. Surgical treatment and outcome of 195 cases of non acute subdural haematoma at the Yaoundé Central Hospital: the need for landmarked burr holes. Afr J Neurol Sci. 2011;30(2):21-27. [Google Scholar]
- 49.Dran G, Berthier F, Fontaine D, Rasenrarijao D, Paquis P. Effectiveness of adjuvant corticosteroid therapy for chronic subdural hematoma: a retrospective study of 198 cases. Neurochirurgie. 2007;53(6):477-482. [DOI] [PubMed] [Google Scholar]
- 50.Drapkin AJ. Chronic subdural hematoma: pathophysiological basis for treatment. Br J Neurosurg. 1991;5(5):467-473. [DOI] [PubMed] [Google Scholar]
- 51.Edem I, Moldovan ID, Turner A, Alkherayf F. A comparative study of chronic subdural hematoma burr hole craniostomy treatment: to irrigate or not to irrigate. Interdiscip Neurosurg. 2019;18:100492. [Google Scholar]
- 52.Eggert HR, Harders A, Weigel K, Gilsbach J. Rezidive nach Bohrlochtrepanation chronischer Subduralhämatome. Min - Minimally Invasive Neurosurg. 1984;27(05):141-143. [DOI] [PubMed] [Google Scholar]
- 53.Eppel MSI, Gorzer H, Ferraz-Leite H. Head injury and chronic subdural hematoma. Acta Chirurgica Austriaca. 1999;31(156):60-63. [Google Scholar]
- 54.Ernestus RI, Beldzinski P, Lanfermann H, Klug N. Chronic subdural hematoma: surgical treatment and outcome in 104 patients. Surg Neurol. 1997;48(3):220-225. [DOI] [PubMed] [Google Scholar]
- 55.Erol FS, Topsakal C, Faik Ozveren M, Kaplan M, Tiftikci MT. Irrigation vs. closed drainage in the treatment of chronic subdural hematoma. J Clin Neurosci. 2005;12(3):261-263. [DOI] [PubMed] [Google Scholar]
- 56.Flint AC, Chan SL, Rao VA, Efron AD, Kalani MA, Sheridan WF. Treatment of chronic subdural hematomas with subdural evacuating port system placement in the intensive care unit: evolution of practice and comparison with bur hole evacuation in the operating room. J Neurosurg. 2017;127(6):1443-1448. [DOI] [PubMed] [Google Scholar]
- 57.Flores G, Vicenty JC, Pastrana EA. Post-operative seizures after burr hole evacuation of chronic subdural hematomas: is prophylactic anti-epileptic medication needed? Acta Neurochir (Wien). 2017;159(11):2033-2036. [DOI] [PubMed] [Google Scholar]
- 58.Frati A, Salvati M, Mainiero F, et al. Inflammation markers and risk factors for recurrence in 35 patients with a posttraumatic chronic subdural hematoma: a prospective study. J Neurosurg. 2004;100(1):24-32. [DOI] [PubMed] [Google Scholar]
- 59.Fujisawa N, Oya S, Yoshida S, et al. A prospective randomized study on the preventive effect of Japanese herbal Kampo medicine Goreisan for recurrence of chronic subdural hematoma. Neurol Med Chir. 2020;61(1):12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gabarros A, Acebes JJ, Rodriguez R, et al. Results of surgical treatment in chronic subdural hematoma. Comparison between two technique: twist-drill and continuous closed drainage versus two burr holes and open external drainage. Neurocirugia. 2000;11(5):377-390. [Google Scholar]
- 61.Gelabert-Gonzalez M, Iglesias-Pais M, Garcia-Allut A, Martinez-Rumbo R. Chronic subdural haematoma: surgical treatment and outcome in 1000 cases. Clin Neurol Neurosurg. 2005;107(3):223-229. [DOI] [PubMed] [Google Scholar]
- 62.Gernsback J, Kolcun JP, Jagid J. To drain or two drains: recurrences in chronic subdural hematomas. World Neurosurg. 2016;95:447-450. [DOI] [PubMed] [Google Scholar]
- 63.Gilsbach J, Eggert HR, Harders A. External closed drainage treatment of chronic subdural hematomas after bore-hole trepanation. Unfallchirurgie. 1980;6(3):183-186. [DOI] [PubMed] [Google Scholar]
- 64.Glancz LJ, Poon MTC, Coulter IC, Hutchinson PJ, Kolias AG, Brennan PM. Does drain position and duration influence outcomes in patients undergoing burr-hole evacuation of chronic subdural hematoma? Lessons from a UK multicenter prospective cohort study. Neurosurgery. 2019;85(4):486-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gonugunta V, Buxton N. Warfarin and chronic subdural haematomas. Br J Neurosurg. 2001;15(6):514-517. [DOI] [PubMed] [Google Scholar]
- 66.Goto Y, Oka H, Hino A. Lumboperitoneal shunt surgery using valve implantation on the paravertebral spinal muscle. World Neurosurg. 2021;146:e1092–e1096. [DOI] [PubMed] [Google Scholar]
- 67.Gurelik M, Aslan A, Gurelik B, Ozum U, Karadag O, Kars HZ. A safe and effective method for treatment of chronic subdural haematoma. Can J Neurol Sci. 2007;34(1):84-87. [DOI] [PubMed] [Google Scholar]
- 68.Hamilton MG, Frizzell JB, Tranmer BI. Chronic subdural hematoma: the role for craniotomy reevaluated. Neurosurgery. 1993;33(1):67-72. [DOI] [PubMed] [Google Scholar]
- 69.Han HJ, Park CW, Kim EY, Yoo CJ, Kim YB, Kim WK. One vs. two burr hole craniostomy in surgical treatment of chronic subdural hematoma. J Korean Neurosurg Soc. 2009;46(2):87-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hani L, Vulcu S, Branca M, et al. Subdural versus subgaleal drainage for chronic subdural hematomas: a post hoc analysis of the TOSCAN trial. J Neurosurg. 2020;133(4):1147-1155. [DOI] [PubMed] [Google Scholar]
- 71.Harders A, Eggert HR, Weigel K. Behandlung des chronischen Subduralhämatoms mit externer geschlossener Drainage - Bericht über 100 konsekutive Fälle. Min - Minimally Invasive Neurosurg. 1982;25(05):147-152. [DOI] [PubMed] [Google Scholar]
- 72.Hennig R, Kloster R. Burr hole evacuation of chronic subdural haematomas followed by continuous inflow and outflow irrigation. Acta Neurochir. 1999;141(2):171-176. [DOI] [PubMed] [Google Scholar]
- 73.Heringer LC, Sousa UdO, Oliveira MF, et al. The number of burr holes and use of a drain do not interfere with surgical results of chronic subdural hematomas. Arq Neuropsiquiatr. 2017;75(11):809-812. [DOI] [PubMed] [Google Scholar]
- 74.Hirai S, Yagi K, Hara K, Kanda E, Matsubara S, Uno M. Postoperative recurrence of chronic subdural hematoma is more frequent in patients with blood type A. J Neurosurg. 2021;135(4):1203-1207. [DOI] [PubMed] [Google Scholar]
- 75.Hori YS, Ebisudani Y, Aoi M, Fukuhara T. Elevated serum fibrinogen degradation products on admission is a novel predictive factor for recurrence of chronic subdural hematoma. World Neurosurg. 2018;118:e753–e757. [DOI] [PubMed] [Google Scholar]
- 76.Hsieh CT, Su IC, Hsu SK, Huang CT, Lian FJ, Chang CJ. Chronic subdural hematoma: differencesbetween unilateral and bilateral occurrence. J Clin Neurosci. 2016;34:252-258. [DOI] [PubMed] [Google Scholar]
- 77.Huang YH, Yang KY, Lee TC, Liao CC. Bilateral chronic subdural hematoma: what is the clinicalsignificance? Int J Surg. 2013;11(7):544-548. [DOI] [PubMed] [Google Scholar]
- 78.Huang CJ, Liu X, Zhou XT, et al. Neuroendoscopy-assisted evacuation of chronic subdural hematoma with mixed CT density through a novel small bone flap. J Neurol Surg A Cent Eur Neurosurg. 2020;81(06):549-554. [DOI] [PubMed] [Google Scholar]
- 79.Iftikhar M, Rauf M, Malik A, Javed G, Siddiqui U. Comparison of irrigation versus no irrigation during burr hole evacuation of chronic subdural hematoma. J Neurol Surg A Cent Eur Neurosurg. 2016;77(05):416-421. [DOI] [PubMed] [Google Scholar]
- 80.Ishfaq A. Outcome in chronic subdural hematoma after subdural vs. subgaleal drain. J Coll Phys Surg Pak. 2017;27(7):419-422. [PubMed] [Google Scholar]
- 81.Ishibashi A, Yokokura Y, Adachi H. A comparative study of treatments for chronic subdural hematoma: burr hole drainage versus burr hole drainage with irrigation. Kurume Med J. 2011;58(1):35-39. [DOI] [PubMed] [Google Scholar]
- 82.Jang KM, Kwon JT, Hwang SN, Park YS, Nam TK. Comparison of the outcomes and recurrence with three surgical techniques for chronic subdural hematoma: single, double burr hole, and double burr hole drainage with irrigation. Korean J Neurotrauma. 2015;11(2):75-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jang KM, Choi HH, Mun HY, Nam TK, Park YS, Kwon JT. Critical depressed brain volume influences the recurrence of chronic subdural hematoma after surgical evacuation. Sci Rep. 2020;10(1):1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Janowski M, Kunert P. Intravenous fluid administration may improve post-operative course of patients with chronic subdural hematoma: a retrospective study. PLoS One. 2012;7(4):e35634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jeong SI, Kim SO, Won YS, Kwon YJ, Choi CS. Clinical analysis of risk factors for recurrence in patients with chronic subdural hematoma undergoing burr hole trephination. Korean J Neurotrauma. 2014;10(1):15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Juković M, Till V, Bačkalić T, Karan M, Petrić G. The use of the Karnofsky index in the assessment of clinical state in patients with chronic subdural hematoma: the first observation from Vojvodina. Med Glas. 2014;11(1):132-137. [PubMed] [Google Scholar]
- 87.Jung YG, Jung NY, Kim E. Independent predictors for recurrence of chronic subdural hematoma. J Korean Neurosurg Soc. 2015;57(4):266-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kale A, Öz İİ, Gun EG, Kalayci M, Gul S. Is the recurrence rate of chronic subdural hematomas dependent on the duration of drainage? Neurol Res. 2017;39(5):399-402. [DOI] [PubMed] [Google Scholar]
- 89.Kang MS, Koh HS, Kwon HJ, Choi SW, Kim SH, Youm JY. Factors influencing recurrent chronic subdural hematoma after surgery. J Korean Neurosurg Soc. 2007;41(1):11-15. [Google Scholar]
- 90.Kaminogo M, Moroki J, Ochi A, et al. Characteristics of symptomatic chronic subdural haematomas on high-field MRI. Neuroradiology. 1999;41(2):109-116. [DOI] [PubMed] [Google Scholar]
- 91.Kanyi JK, Ogada TV, Oloo MJ, Parker RK. Burr-hole craniostomy for chronic subdural hematomas by general surgeons in rural Kenya. World J Surg. 2018;42(1):40-45. [DOI] [PubMed] [Google Scholar]
- 92.Kareem H, Adams H. A closed system irrigation & drainage technique for surgical evacuation of chronic subdural haematomas. F1000Res. 2018;7:619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Katayama K, Matsuda N, Kakuta K, et al. The effect of Goreisan on the prevention of chronic subdural hematoma recurrence: multi-center randomized controlled study. J Neurotrauma. 2018;35(13):1537-1542. [DOI] [PubMed] [Google Scholar]
- 94.Khan HU, Atif K, Boghsani GT. Single versus double burr-hole drainage for chronic subdural hematoma: a study of relevant prognostic factors conducted in Pakistan. Pak J Med Sci. 2019;35(4):963-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim JH, Kang DS, Kim JH, Kong MH, Song KY. Chronic subdural hematoma treated by small or large craniotomy with membranectomy as the initial treatment. J Korean Neurosurg Soc. 2011;50(2):103-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim DH, Kim HS, Choi HJ, Han IH, Cho WH, Nam KH. Recurrence of the chronic subdural hematoma after burr-hole drainage with or without intraoperative saline irrigation. Korean J Neurotrauma. 2014;10(2):101-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim DI, Kim JH, Kang HI, Moon BG, Kim JS, Kim DR. Impact of time interval between trauma onset and burr hole surgery on recurrence of late subacute or chronic subdural hematoma. J Korean Neurosurg Soc. 2016;59(5):498-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim E. Embolization therapy for refractory hemorrhage in patients with chronic subdural hematomas. World Neurosurg. 2017;101:520-527. [DOI] [PubMed] [Google Scholar]
- 99.Kiymaz N, Yilmaz N, Mumcu C. Controversies in chronic subdural hematoma: continuous drainage versus one-time drainage. Med Sci Monit. 2007;13(5):cr240–cr243. [PubMed] [Google Scholar]
- 100.Klein J, Mauck L, Schackert G, Pinzer T. Do statins reduce the rate of revision surgery after chronic subdural hematoma drain? Acta Neurochir. 2021;163(7):1843-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kocaman U, Yilmaz H. Description of a modified technique (mini craniotomy-basal membranotomy) for chronic subdural hematoma surgery and evaluation of the contribution of basal membranotomy performed as part of this technique to cerebral expansion. World Neurosurg. 2019;122:e1002–e1006. [DOI] [PubMed] [Google Scholar]
- 102.Kotwica Z, Brzezinski J. Chronic subdural haematoma treated by burr holes and closed system drainage: personal experience in 131 patients. Br J Neurosurg. 1991;5(5):461-465. [DOI] [PubMed] [Google Scholar]
- 103.Kristof RA, Grimm JM, Stoffel-Wagner B. Cerebrospinal fluid leakage into the subdural space: possible influence on the pathogenesis and recurrence frequency of chronic subdural hematoma and subdural hygroma. J Neurosurg. 2008;108(2):275-280. [DOI] [PubMed] [Google Scholar]
- 104.Krupp WF, Jans PJ. Treatment of chronic subdural haematoma with burr-hole craniostomy and closed drainage. Br J Neurosurg. 1995;9(5):619-628. [DOI] [PubMed] [Google Scholar]
- 105.Kurabe S, Ozawa T, Watanabe T, Aiba T. Efficacy and safety of postoperative early mobilization for chronic subdural hematoma in elderly patients. Acta Neurochir. 2010;152(7):1171-1174. [DOI] [PubMed] [Google Scholar]
- 106.Kuroki T, Katsume M, Harada N, Yamazaki T, Aoki K, Takasu N. Strict closed-system drainage for treating chronic subdural haematoma. Acta Neurochir. 2001;143(10):1041-1044. [DOI] [PubMed] [Google Scholar]
- 107.Kutty SA, Johny M. Chronic subdural hematoma: a comparison of recurrence rates following burr-hole craniostomy with and without drains. Turk Neurosurg. 2014;24(4):494-497. [DOI] [PubMed] [Google Scholar]
- 108.Kwon TH, Park YK, Lim DJ, et al. Chronic subdural hematoma: evaluation of the clinical significance of postoperative drainage volume. J Neurosurg. 2000;93(5):796-799. [DOI] [PubMed] [Google Scholar]
- 109.Lee JY, Ebel H, Ernestus RI, Klug N. Various surgical treatments of chronic subdural hematoma and outcome in 172 patients: is membranectomy necessary? Surg Neurol. 2004;61(6):523-527;discussion 527-8. [DOI] [PubMed] [Google Scholar]
- 110.Lee JK, Choi JH, Kim CH, Lee HK, Moon JG. Chronic subdural hematomas: a comparative study of three types of operative procedures. J Korean Neurosurg Soc. 2009;46(3):210-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee J, Park JH. Clinical characteristics of bilateral versus unilateral chronic subdural hematoma. Korean J Neurotrauma. 2014;10(2):49-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee SH, Choi JI, Lim DJ, Ha SK, Kim SD, Kim SH. The potential of diffusion-weighted magnetic resonance imaging for predicting the outcomes of chronic subdural hematomas. J Korean Neurosurg Soc. 2018;61(1):97-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lepic M, Mandic-Rajcevic S, Pavlicevic G, Novakovic N, Rasulic L. Awake surgery in sitting position for chronic subdural hematoma. Acta Neurochir. 20212021;163(7):1857-1865. [DOI] [PubMed] [Google Scholar]
- 114.Leung GKK, Fan JKM, Tam MCH, Fan YW. Surgical complications of chronic subdural haematoma: a 5-year audit. Ann Coll Surg Hong Kong. 2001;5(3):99-103. [Google Scholar]
- 115.Liliang PC, Tsai YD, Liang CL, Lee TC, Chen HJ. Chronic subdural haematoma in young and extremely aged adults: a comparative study of two age groups. Injury. 2002;33(4):345-348. [DOI] [PubMed] [Google Scholar]
- 116.Li F, Hua C, Feng Y, Yuan H, Bie L. Correlation of vascular endothelial growth factor with magnetic resonance imaging in chronic subdural hematomas. J Neurol Sci. 2017;377:149-154. [DOI] [PubMed] [Google Scholar]
- 117.Lin X. Comparing twist-drill drainage with burr hole drainage for chronic subdural hematoma. Chin J Traumatol. 2011;14(3):170-173. [PubMed] [Google Scholar]
- 118.Liu Y, Xia JZ, Wu AH, Wang YJ. Burr-hole craniotomy treating chronic subdural hematoma: a report of 398 cases. Chin J Traumatol. 2010;13(5):265-269. [PubMed] [Google Scholar]
- 119.Liu LX, Cao XD, Ren YM, Zhou LX, Yang CH. Risk factors for recurrence of chronic subdural hematoma: a single center experience. World Neurosurg. 2019;132:e506–e513. [DOI] [PubMed] [Google Scholar]
- 120.Lo WL, Lee TC, Fang PS, Huang YH. Chronic subdural hematoma in patients under age 65 years: a comparative study of age cohort. Formos J Surg. 2013;46(1):10-14. [Google Scholar]
- 121.Lu W, Wang H, Wu T, Sheng X, Ding Z, Xu G. Burr-hole craniostomy with T-tube drainage as surgical treatment for chronic subdural hematoma. World Neurosurg. 2018;115:e756–e760. [DOI] [PubMed] [Google Scholar]
- 122.Maldaner N, Sosnova M, Sarnthein J, Bozinov O, Regli L, Stienen MN. Predicting functional impairment in patients with chronic subdural hematoma treated with burr hole trepanation: the FIT-score. Clin Neurol Neurosurg. 2019;182:142-147. [DOI] [PubMed] [Google Scholar]
- 123.Markwalder TM. The course of chronic subdural hematomas after burr-hole craniostomy with and without closed-system drainage. Neurosurg Clin N Am. 2000;11(3):541-546. [PubMed] [Google Scholar]
- 124.Martinez-Perez R, Tsimpas A, Rayo N, Cepeda S, Lagares A. Role of the patient comorbidity in the recurrence of chronic subdural hematomas. Neurosurg Rev. 2020;44(2):971-976. [DOI] [PubMed] [Google Scholar]
- 125.Mellergard P, Wisten O. Operations and re-operations for chronic subdural haematomas during a 25-year period in a well defined population. Acta Neurochir (Wien). 1996;138(6):708-713. [DOI] [PubMed] [Google Scholar]
- 126.Mersha A, Abat S, Temesgen T, Nebyou A. Outcome of chronic subdural hematoma treated with single burr hole under local anesthesia. Ethiop J Health Sci. 2020;30(1):101-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mezue WC, Ohaebgulam SC, Chikani MC, Erechukwu AU. Changing trends in chronic subdural haematoma in Nigeria. Afr J Med Med Sci. 2011;40(4):373-376. [PubMed] [Google Scholar]
- 128.Miah IP, Herklots M, Roks G, et al. Dexamethasone therapy in symptomatic chronic subdural hematoma (DECSA-R): a retrospective evaluation of initial corticosteroid therapy versus primary surgery. J Neurotrauma. 2020;37(2):366-372. [DOI] [PubMed] [Google Scholar]
- 129.Miki K, Abe H, Morishita T, et al. Double-crescent sign as a predictor of chronic subdural hematoma recurrence following burr-hole surgery. J Neurosurg. 2019;131(6):1905-1911. [DOI] [PubMed] [Google Scholar]
- 130.Missori P, Maraglino C, Tarantino R, et al. Chronic subdural haematomas in patients aged under 50. Clin Neurol Neurosurg. 2000;102(4):199-202. [DOI] [PubMed] [Google Scholar]
- 131.Morales-Gomez JA, Garcia-Estrada E, Garza-Baez A, Mercado-Flores M, de Leon AM. Subdural open drains as an effective and low-cost modality for the treatment of chronic subdural hematomas. Br J Neurosurg. Published online December 16, 2020. doi: 10.1080/02688697.2020.1858024. [DOI] [PubMed] [Google Scholar]
- 132.Mori K, Maeda M. Surgical treatment of chronic subdural hematoma in 500 consecutive cases: clinical characteristics, surgical outcome, complications, and recurrence rate. Neurol Med Chir. 2001;41(8):371-381. [DOI] [PubMed] [Google Scholar]
- 133.Motoie R, Karashima S, Otsuji R, et al. Recurrence in 787 patients with chronic subdural hematoma: retrospective cohort investigation of associated factors including direct oral anticoagulant use. World Neurosurg. 2018;118:e87–e91. [DOI] [PubMed] [Google Scholar]
- 134.Munoz-Bendix C, Pannewitz R, Remmel D, et al. Outcome following surgical treatment of chronic subdural hematoma in the oldest-old population. Neurosurg Rev. 2017;40(3):461-468. [DOI] [PubMed] [Google Scholar]
- 135.Nakagawa I, Park HS, Kotsugi M, et al. Enhanced hematoma membrane on DynaCT images during middle meningeal artery embolization for persistently recurrent chronic subdural hematoma. World Neurosurg. 2019;126:e473–e479. [DOI] [PubMed] [Google Scholar]
- 136.Nakaguchi H, Tanishima T, Yoshimasu N. Factors in the natural history of chronic subdural hematomas that influence their postoperative recurrence. J Neurosurg. 2001;95(2):256-262. [DOI] [PubMed] [Google Scholar]
- 137.Nunta-Aree S, Paruang T, Sitthinamsuwan B. Timing of brain expansion and recurrence after surgery of chronic subdural hematoma. J Med Assoc Thailand. 2017;100(4):s59–s64. [Google Scholar]
- 138.Okano A, Oya S, Fujisawa N, et al. Analysis of risk factors for chronic subdural haematoma recurrence after burr hole surgery: optimal management of patients on antiplatelet therapy. Br J Neurosurg. 2014;28(2):204-208. [DOI] [PubMed] [Google Scholar]
- 139.Oral S, Borklu RE, Kucuk A, Ulutabanca H, Selcuklu A. Comparison of subgaleal and subdural closed drainage system in the surgical treatment of chronic subdural hematoma. North. Clin Istanb. 2015;2(2):115-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Penchet G, Loiseau H, Castel JP. Chronic bilateral subdural hematomas. Neuro-Chirurgie. 1998;44(4):247-252. [PubMed] [Google Scholar]
- 141.Piotrowski WP, Krombholz-Reindl MA. Surgical outcome in chronic subdural hematoma. Unfallchirurgie. 1996;22(3):110-116. [PubMed] [Google Scholar]
- 142.Poulsen FR, Munthe S, Soe M, Halle B. Perindopril and residual chronic subdural hematoma volumes six weeks after burr hole surgery: a randomized trial. Clin Neurol Neurosurg. 2014;123:4-8. [DOI] [PubMed] [Google Scholar]
- 143.Qian Z, Yang D, Sun F, Sun Z. Risk factors for recurrence of chronic subdural hematoma after burr hole surgery: potential protective role of dexamethasone. Br J Neurosurg. 2017;31(1):84-88. [DOI] [PubMed] [Google Scholar]
- 144.Raghavan A, Smith G, Onyewadume L, et al. Morbidity and mortality after burr hole craniostomy versus craniotomy for chronic subdural hematoma evacuation: a single-center experience. World Neurosurg. 2020;134:e196–e203. [DOI] [PubMed] [Google Scholar]
- 145.Ram Z, Hadani M, Sahar A, Spiegelmann R. Continuous irrigation-drainage of the subdural space for the treatment of chronic subdural haematoma. A prospective clinical trial. Acta Neurochir. 1993;120(1-2):40-43. [DOI] [PubMed] [Google Scholar]
- 146.Regan JM, Worley E, Shelburne C, Pullarkat R, Watson JC. Burr hole washout versus craniotomy for chronic subdural hematoma: patient outcome and cost analysis. PLoS ONE. 2015;10(1):e0115085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ridwan S, Bohrer AM, Grote A, Simon M. Surgical treatment of chronic subdural hematoma: predicting recurrence and cure. World Neurosurg. 2019;128:e1010–e1023. [DOI] [PubMed] [Google Scholar]
- 148.Rohde V, Graf G, Hassler W. Complications of burr-hole craniostomy and closed-system drainage for chronic subdural hematomas: a retrospective analysis of 376 patients. Neurosurg Rev. 2002;25(1-2):89-94. [DOI] [PubMed] [Google Scholar]
- 149.Rovlias A, Theodoropoulos S, Papoutsakis D. Chronic subdural hematoma: surgical management and outcome in 986 cases: a classification and regression tree approach. Surg Neurol Int. 2015;6(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ryu SM, Yeon JY, Kong DS, Hong SC. Risk of recurrent chronic subdural hematoma associated with early warfarin resumption: a matched cohort study. World Neurosurg. 2018;120:e855–e862. [DOI] [PubMed] [Google Scholar]
- 151.Sah S, Rawal D. Craniotomy does have its share in the management of chronic subdural hematoma. Indian J Neurotrauma. 2018;15(02/03):057-061. [Google Scholar]
- 152.Sarnvivad P, Chiewchanvechakul W, Chumnanvej S. Chronic subdural hematoma: drainage vs. no drainage. J Med Assoc Thailand. 2011;94(11):1352-1356. [PubMed] [Google Scholar]
- 153.Schoedel P, Bruendl E, Hochreiter A, et al. Restoration of functional integrity after evacuation of chronic subdural hematoma-an age-adjusted analysis of 697 patients. World Neurosurg. 2016;94:465-470. [DOI] [PubMed] [Google Scholar]
- 154.Schwarz F, Loos F, Dunisch P, et al. Risk factors for reoperation after initial burr hole trephination in chronic subdural hematomas. Clin Neurol Neurosurg. 2015;138:66-71. [DOI] [PubMed] [Google Scholar]
- 155.ShafiqAlam S, UdDin MA, Sheikh JI, et al. Chronic subdural hematoma-an experience of 432 cases in a tertiary care centre. JK Pract. 2017;22(1):20-24. [Google Scholar]
- 156.Shah S, Rehman L, Ahmed N, Chaudhry MA, Shabir A. Comparison of recurrence of chronic subdural haematoma after burr hole craniostomy with one time drainage and burr hole craniostomy with tube drainage. Pak J Med Health Sci. 2014;8(4):1027-1029. [Google Scholar]
- 157.Shen J, Yuan L, Ge R, et al. Clinical and radiological factors predicting recurrence of chronic subdural hematoma: a retrospective cohort study. Injury. 2019;50(10):1634-1640. [DOI] [PubMed] [Google Scholar]
- 158.Shim YW, Lee WH, Lee KS, Kim ST, Paeng SH, Pyo SY. Burr hole drainage versus small craniotomy of chronic subdural hematomas. Korean J Neurotrauma. 2019;15(2):110-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Singh SKSM, Sinha M, Singh V, et al. A randomized study of twist drill versus burr hole craniostomy for treatment of chronic subdural hematomas in 100 patients. Indian J Neurotrauma. 2011;8(2):83-88. [Google Scholar]
- 160.Suryanarayanan B, Choudhary A, Gupta L, Prasad A, Singh A, Singh S. A prospective randomized study of use of drain versus no drain after burr-hole evacuation of chronic subdural hematoma. Neurol India. 2014;62(2):169-174. [DOI] [PubMed] [Google Scholar]
- 161.Sjåvik K, Bartek J, Sagberg LM, et al. Assessment of drainage techniques for evacuation of chronic subdural hematoma: a consecutive population-based comparative cohort study. J Neurosurg. 2020;133(4):1113-1119. [DOI] [PubMed] [Google Scholar]
- 162.Song DH, Kim YS, Chun HJ, et al. The predicting factors for recurrence of chronic subdural hematoma treated with burr hole and drainage. Korean J Neurotrauma. 2014;10(2):41-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sousa EB, Brandao LF, Tavares CB, Borges IB, Neto NG, Kessler IM. Epidemiological characteristics of 778 patients who underwent surgical drainage of chronic subdural hematomas in Brasilia, Brazil. BMC Surg. 2013;13(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Stanisic M, Hald J, Rasmussen IA, et al. Volume and densities of chronic subdural haematoma obtained from CT imaging as predictors of postoperative recurrence: a prospective study of 107 operated patients. Acta Neurochir. 2013;155(2):323-333;discussion 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sucu HK, Akar O. Double-layer appearance after evacuation of a chronic subdural hematoma. Br J Neurosurg. 2014;28(1):93-97. [DOI] [PubMed] [Google Scholar]
- 166.Suzuki K, Sugita K, Akai T, Takahata T, Sonobe M, Takahashi S. Treatment of chronic subdural hematoma by closed-system drainage without irrigation. Surg Neurol. 1998;50(3):231-234. [DOI] [PubMed] [Google Scholar]
- 167.Tagle P, Mery F, Torrealba G, et al. Chronic subdural hematoma: a disease of elderly people. Rev Med Chile. 2003;131(2):177-182. [PubMed] [Google Scholar]
- 168.Takei J, Hirotsu T, Hatano K, et al. Modified computed tomography classification for chronic subdural hematoma features good interrater agreement: a single-center retrospective cohort study. World Neurosurg. 2021;151:e407–e417. [DOI] [PubMed] [Google Scholar]
- 169.Tahsim-Oglou Y, Beseoglu K, Hanggi D, Stummer W, Steiger HJ. Factors predicting recurrence of chronic subdural haematoma: the influence of intraoperative irrigation and low-molecular- weight heparin thromboprophylaxis. Acta Neurochir. 2012;154(6):1063-1068;discussion 1068. [DOI] [PubMed] [Google Scholar]
- 170.Tailor J, Fernando D, Sidhu Z, Foley R, Abeysinghe KD, Walsh DC. Clinical audit effectively bridges the evidence-practice gap in chronic subdural haematoma management. Acta Neurochir. 2017;159(4):627-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tanikawa M, Mase M, Yamada K, et al. Surgical treatment of chronic subdural hematoma based on intrahematomal membrane structure on MRI. Acta Neurochir. 2001;143(6):613-619;discussion 618-9. [DOI] [PubMed] [Google Scholar]
- 172.Taussky P, Fandino J, Landolt H. Number of burr holes as independent predictor of postoperative recurrence in chronic subdural haematoma. Br J Neurosurg. 2008;22(2):279-282. [DOI] [PubMed] [Google Scholar]
- 173.Thavara BD, Kidangan GS, Rajagopalawarrier B. Comparative study of single burr-hole craniostomy versus twist-drill craniostomy in patients with chronic subdural hematoma. Asian J Neurosurg. 2019;14(02):513-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Toi H, Fujii Y, Iwama T, et al. Determining if cerebrospinal fluid prevents recurrence of chronic subdural hematoma: a multi-center prospective randomized clinical trial. J Neurotrauma. 2019;36(4):559-564. [DOI] [PubMed] [Google Scholar]
- 175.Tomita Y, Yamada SM, Yamada S, Matsuno A. Subdural tension on the brain in patients with chronic subdural hematoma is related to hemiparesis but not to headache or recurrence. World Neurosurg. 2018;119:e518–e526. [DOI] [PubMed] [Google Scholar]
- 176.Tommiska P, Lonnrot K, Raj R, Luostarinen T, Kivisaari R. Transition of a clinical practice to use of subdural drains after burr hole evacuation of chronic subdural hematoma: the Helsinki experience. World Neurosurg. 2019;129:e614–e626. [DOI] [PubMed] [Google Scholar]
- 177.Torihashi K, Sadamasa N, Yoshida K, Narumi O, Chin M, Yamagata S. Independent predictors for recurrence of chronic subdural hematoma: a review of 343 consecutive surgical cases. Neurosurgery. 2008;63(6):1125-1129;discussion 1129. [DOI] [PubMed] [Google Scholar]
- 178.Tosaka M, Tsushima Y, Watanabe S, et al. Superficial subarachnoid cerebrospinal fluid space expansion after surgical drainage of chronic subdural hematoma. Acta Neurochir. 2015;157(7):1205-1214. [DOI] [PubMed] [Google Scholar]
- 179.Tsai TH, Lieu AS, Hwang SL, Huang TY, Hwang YF. A comparative study of the patients with bilateral or unilateral chronic subdural hematoma: precipitating factors and postoperative outcomes. J Trauma-Injury Infect Crit Care. 2010;68(3):571-575. [DOI] [PubMed] [Google Scholar]
- 180.Tanriverdi O, Baydin S, Hergunsel B, et al. Can recurrence of chronic subdural hematoma be predicted? A retrospective analysis of 292 cases. J Neurol Surg A Cent Eur Neurosurg. 2013;75(01):037-041. [DOI] [PubMed] [Google Scholar]
- 181.Vasella F, Akeret K, Smoll NR, et al. Improving the aesthetic outcome with burr hole cover placement in chronic subdural hematoma evacuation—a retrospective pilot study. Acta Neurochir. 2018;160(11):2129-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wan Y, Fei X, Jiang D, Chen H, Shi L, Wang Z. Clinical observation of treatment of chronic subdural hematoma with novel double needle minimally invasive aspiration technology. J Craniofac Surg. 2017;28(3):646-649. [DOI] [PubMed] [Google Scholar]
- 183.Wang QF, Cheng C, You C. A new modified twist drill craniostomy using a novel device to evacuate chronic subdural hematoma. Medicine. 2016;95(10):e3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang K, Chen D, Cao X, Gao L. A prospective comparative study of twist drill craniostomy versus burr hole craniostomy in patients with chronic subdural hematoma. Turk Neurosurg. 2017;27(1):60-65. [DOI] [PubMed] [Google Scholar]
- 185.Wang QP, Yuan Y, Guan JW, Jiang XB. A comparative study of irrigation versus no irrigation during burr hole craniostomy to treat chronic subdural hematoma. BMC Surg. 2017;17(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang W, Liu H, Yang J. Burr hole craniostomy irrigation with and without drainage during surgical treatment of chronic subdural hematoma: a retrospective study of 87 cases. Turk Neurosurg. 2017;31:31. [DOI] [PubMed] [Google Scholar]
- 187.Wang N, Hu J, Oppong-Gyebi A, et al. Elevated blood urea nitrogen is associated with recurrence of post-operative chronic subdural hematoma. BMC Neurol. 2020;20(1):411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Weigel R, Schlickum L, Weisser G, Krauss JK. Treatment concept of chronic subdural haematoma according to an algorithm using evidence-based medicine-derived key factors: a prospective controlled study. Br J Neurosurg. 2015;29(4):538-543. [DOI] [PubMed] [Google Scholar]
- 189.Weng W, Li H, Zhao X, et al. The depth of catheter in chronic subdural haematoma: does it matter? Brain Inj. 2019;33(6):717-722. [DOI] [PubMed] [Google Scholar]
- 190.Won SY, Dubinski D, Behmanesh B, et al. Supervised valsalva maneuver after burr hole evacuation of chronic subdural hematomas: a prospective cohort study. J Neurotrauma. 2021;38(7):911-917. [DOI] [PubMed] [Google Scholar]
- 191.Wu Q, Liu Q, Chen D, et al. Subdural drainage techniques for single burr-hole evacuation of chronic subdural hematoma: two drains frontal-occipital position versus one drain frontal position. Br J Neurosurg. 2020;35(3):324-328. [DOI] [PubMed] [Google Scholar]
- 192.Yadav YR, Parihar V, Chourasia ID, Bajaj J, Namdev H. The role of subgaleal suction drain placement in chronic subdural hematoma evacuation. Asian J Neurosurg. 2016;11(03):214-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yagnick NS, Moolchandani S, Sinha S, Mehta VS. Demonstration of brain expansion in cases of chronic SDH during admission leads to decreased rates of recurrence. Indian J Neurotrauma. 2019;16(01):63-66. [Google Scholar]
- 194.Yamada SM, Tomita Y, Murakami H, et al. Headache in patients with chronic subdural hematoma: analysis in 1080 patients. Neurosurg Rev. 2018;41(2):549-556. [DOI] [PubMed] [Google Scholar]
- 195.Yamada T, Natori Y. Prospective study on the efficacy of orally administered tranexamic acid and goreisan for the prevention of recurrence after chronic subdural hematoma burr hole surgery. World Neurosurg. 2020;134:e549–e553. [DOI] [PubMed] [Google Scholar]
- 196.Yamamoto H, Hirashima Y, Hamada H, Hayashi N, Origasa H, Endo S. Independent predictors of recurrence of chronic subdural hematoma: results of multivariate analysis performed using a logistic regression model. J Neurosurg. 2003;98(6):1217-1221. [DOI] [PubMed] [Google Scholar]
- 197.Yan K, Gao H, Zhou X, et al. A retrospective analysis of postoperative recurrence of septated chronic subdural haematoma: endoscopic surgery versus burr hole craniotomy. Neurol Res. 2017;39(9):803-812. [DOI] [PubMed] [Google Scholar]
- 198.Yan C, Yang MF, Huang YW. A reliable nomogram model to predict the recurrence of chronic subdural hematoma after burr hole surgery. World Neurosurg. 2018;118:e356–e366. [DOI] [PubMed] [Google Scholar]
- 199.You W, Zhu Y, Wang Y, et al. Prevalence of and risk factors for recurrence of chronic subdural hematoma. Acta Neurochir. 2018;160(5):893-899. [DOI] [PubMed] [Google Scholar]
- 200.Yu GJ, Han CZ, Zhang M, Zhuang HT, Jiang YG. Prolonged drainage reduces the recurrence of chronic subdural hematoma. Br J Neurosurg. 2009;23(6):606-611. [DOI] [PubMed] [Google Scholar]
- 201.Zakaraia AM, Adnan JS, Haspani MS, Naing NN, Abdullah JM. Outcome of 2 different types of operative techniques practiced for chronic subdural hematoma in Malaysia: an analysis. Surg Neurol. 2008;69(6):608-615;discussion 616. [DOI] [PubMed] [Google Scholar]
- 202.Zhang J, Liu X, Fan X, et al. The use of endoscopic-assisted burr-hole craniostomy for septated chronic subdural haematoma: a retrospective cohort comparison study. Brain Res. 2018;1678:245-253. [DOI] [PubMed] [Google Scholar]
- 203.Zhang JJY, Wang S, Foo ASC, et al. Outcomes of subdural versus subperiosteal drain after burr-hole evacuation of chronic subdural hematoma: a multicenter cohort study. World Neurosurg. 2019;131:e392–e401. [DOI] [PubMed] [Google Scholar]
- 204.Zumofen D, Regli L, Levivier M, Krayenbuhl N. Chronic subdural hematomas treated by burr hole trepanation and a subperiostal drainage system. Neurosurgery. 2009;64(6):1116-1122;discussion 1121-2. [DOI] [PubMed] [Google Scholar]
- 205.Leroy HA, Aboukais R, Reyns N, et al. Predictors of functional outcomes and recurrence of chronic subdural hematomas. J Clin Neurosci. 2015;22(12):1895-1900. [DOI] [PubMed] [Google Scholar]
- 206.Hashimoto T, Ohashi T, Watanabe D, et al. Usefulness of embolization of the middle meningeal artery for refractory chronic subdural hematomas. Surg Neurol Int. 2013;4(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tempaku A, Yamauchi S, Ikeda H, et al. Usefulness of interventional embolization of the middle meningeal artery for recurrent chronic subdural hematoma: five cases and a review of the literature. Interv Neuroradiol. 2015;21(3):366-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.de Araujo Silva DO, Matis GK, Kitamura MP, et al. Chronic subdural hematomas and the elderly: surgical results from a series of 125 cases: old “horses” are not to be shot. Surg Neurol Int. 2012;3(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Immenga S, Lodewijkx R, Roos Y, et al. Tranexamic acid to prevent operation in chronic subdural haematoma (TORCH): study protocol for a randomised placebo-controlled clinical trial. Trials. 2022;23(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bartley A, Bartek J, Jr., Jakola AS, et al. Effect of irrigation fluid temperature on recurrence in the evacuation of chronic subdural hematoma: a randomized clinical trial. JAMA Neurol. 2023;80(1):58-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Glancz LJ, Poon MTC, Hutchinson PJ, Kolias AG, Brennan PM. Drains result in greater reduction of subdural width and midline shift in burr hole evacuation of chronic subdural haematoma. Acta Neurochir. 2020;162(6):1455-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Greuter L, Hejrati N, Soleman J. Type of drain in chronic subdural hematoma-a systematic review and meta-analysis. Front Neurol. 2020;11:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ding H, Liu S, Quan X, Liao S, Liu L. Subperiosteal versus subdural drain after burr hole drainage for chronic subdural hematomas: a systematic review and meta-analysis. World Neurosurg. 2020;136:90-100. [DOI] [PubMed] [Google Scholar]
- 214.Imrey PB. Limitations of meta-analyses of studies with high heterogeneity. JAMA Netw Open. 2020;3(1):e1919325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Content Table 1. Syntax search.
Supplementary Fig 1. Forest plot and prediction interval of pooled incidence of recurrences per number of patients.
Supplementary Fig 2. Forest plot and prediction interval of pooled incidence of recurrences per number of hematomas.
Supplementary Fig 3. Forest plot and prediction interval of pooled incidence of recurrences of studies with the highest quality.
Supplemental Content Table 2. Pooled incidence and recurrence rate of irrigation methods in studies using a definition of clinical and radiological factors and a reoperation.
Supplemental Content Table 3. Pooled incidence and recurrence rate of postoperative drain location used in studies using a definition of clinical and radiological factors and a reoperation.
Supplemental Content Table 4. Pooled incidence and recurrence rate of postoperative drainage time used in studies using a definition of clinical and radiological factors and a reoperation.


