Abstract
NAD(P)H-dependent quinone oxidoreductase (NQO) is an essential enzyme in living organisms and cells protecting them from oxidative stress. NQO reduces coenzyme Q (CoQ) using NAD(P)H as an electron donor. In the present study, we searched for coenzyme Q10 reducing activity from fractions of gel filtration-fractionated rat liver homogenate. In addition to the large-molecular-weight fraction containing NQO, CoQ10 reducing activity was also detected in a low-molecular-weight fraction. Furthermore, dicumarol, a conventional inhibitor of NQO1 (DT diaphorase), did not inhibit the reduction but quercetin did, suggesting that the activity was not due to NQO1. After further purification, the NADH-dependent CoQ10-reducing compound was identified as riboflavin. Riboflavin is an active substituent of other flavin compounds such as FAD and FMN. These flavin compounds also reduced not only CoQ homologues but also vitamin K homologues in the presence of NADH. The mechanism was speculated to work as follows: NADH reduces flavin compounds to the corresponding reduced forms, and subsequently, the reduced flavin compounds immediately reduce bio-quinones. Furthermore, the flavin-NADH system reduces CoQ10 bound with saposin B, which is believed to function as a CoQ transfer protein in vivo. This flavin-dependent CoQ10 reduction, therefore, may function in aqueous phases such as the cell cytosol and bodily fluids.
Keywords: NAD(P)H quinone oxidoreductase (NQO), riboflavin, oxidative stress
Introduction
NAD(P)H-dependent quinone oxidoreductase (NQO, EC 1.6.99.2) is believed to be an essential reducing enzyme that reduces bio-quinones such as coenzyme Q (CoQ) and vitamin K (VK) using NADH or NADPH as a hydrogen donor. The reduced forms of these quinones play important roles as antioxidants and as reductants in blood coagulation. NQO has two isozymes, NQO1 (DT-diaphorase) and NQO2. These NQOs play important roles in metabolic detoxification,(1–3) suppressing cancer,(4–6) and protection from oxidative stress.(7–9) We have focused on the biological function of protection from oxidative stress. Marine organisms inhabiting shallow waters such as coral reefs are exposed to strong sunlight, including UVA and UVB, and higher concentrations of oxygen than those in other marine environments, resulting in strong oxidative stress. We previously reported that a marine bacterium, non-luminescent marine Vibrio spp. isolated from the posterior gut of the holothurian Thelenota ananas, which inhabits the shallow-water reef flat of the Great Barrier Reef, has increased CoQ8H2 during UVA (peak emission = 360 nm) irradiation.(10) We believe that CoQ8 reducing activities, including those of NQO, were improved against oxidative stress induced by UVA irradiation. In fact, we have observed that dichlorophenolindophenol (DCPIP) reducing activity, which is a conventional method for measuring NQO activity, is increased during irradiation (unpublished data).
The biological importance of the reduced form of CoQ10 (CoQ10H2) has been given much attention since its tocopherol-mediated peroxidation (TMP) mechanism was proposed by Bowry and Stocker.(11) Lipid peroxidation is proceeded by a lipid peroxyl radical-mediated chain reaction.(12) And α-tocopherol (α-Toc) donates its phenolic hydrogen to the lipid peroxyl radical, a mediator of the chain reaction, and eliminates it, resulting in inhibition of lipid peroxidation by suppressing the propagation of the chain reaction. The α-Toc becomes an α-Toc radical with the donation of a hydrogen, and the α-Toc radical scavenges another lipid peroxyl radical in vitro. However, in vivo, where the concentration of lipid peroxyl radicals is thought to be very low, it is postulated that the reaction between α-Toc radicals and lipid peroxyl radicals is rare. Under such conditions, unreacted α-Toc radicals accumulate in lipid-rich circumstances. Then α-Toc radicals absorb hydrogens from lipid molecules to form α-Toc and lipid radicals. Therefore, the chain propagation is mediated by α-Toc radicals instead of lipid peroxyl radicals. These processes are defined as the TMP and assumed to occur in regions of atherosclerosis. To prevent the TMP, the reduction of α-Toc radicals by other antioxidants is required, and reduced CoQ10 and ascorbic acid are antioxidants with this function. In fact, reduced CoQ10 suppresses lipid peroxidation in autooxidation of collected human plasma, and after reduced CoQ10 is eliminated, the rate of lipid peroxidation is accelerated although the α-Toc remains in the plasma.(13) This result suggests that reduced CoQ10 is required to prevent lipid peroxidation in human plasma, so maintaining reduced CoQ10 is biologically essential.
In healthy humans, plasma CoQ10 is maintained in a reduced form, and the proportion of oxidized to total CoQ10 (%CoQ10) is under 5.0%.(14,15) However, we have found that %CoQ10 values are elevated in oxidative stress-related diseases such as ischemic heart infarction-percutaneous transluminal coronary angioplasty,(14) severe chronic liver damage,(15) and amyotrophic lateral sclerosis.(16)
In this study, we searched for aqueous CoQ10 reducing activity that is different from NQO. Takahashi et al.(17) described that intact Hep G2 cells show extracellular CoQ10 reducing activity located on the plasma membrane. However, the active compound has not been identified. We conducted CoQ10 reduction by rat liver homogenate and its gel-filtrated fractions in the presence of NADH. CoQ10 reducing activity was observed in the homogenate and in high-molecular-weight fractions due to NQO as expected. To our surprise, we also observed high NADH-dependent CoQ10 reducing activity in low-molecular-weight fractions under 1,000 Da. The activity was not inhibited by dicumarol, which is a conventional inhibitor of NQO1 (DT-diaphorase), but it was inhibited by quercetin. We confirmed that the NADH-dependent CoQ10 reducing compound is riboflavin (RF) by using time-of-flight mass spectrometry (TOFMS), and further confirmed that the RF derivatives flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD) also showed NADH-dependent CoQ reducing activity. Furthermore, the combination of these RF derivatives and NADH reduced not only CoQ10 but also CoQ homologues (CoQ9 and 8) and VK homologues (VK1 and 2).
Since CoQ10 is lipophilic and RF is hydrophilic, CoQ10 should be water-solubilized to interact with RF. For this purpose, water-soluble saposin B (SapB) is useful, because SapB can bind CoQ10 and make it water-soluble.(18) We demonstrated that RF induced the NADH-dependent reduction of CoQ10 bound with SapB in water.
Materials and Methods
Chemicals
RF, FMN, FAD, and other chemicals were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Rat liver was purchased from COSMO-BIO CO. Ltd. (Tokyo, Japan).
Preparation of rat liver homogenate
Four grams of rat liver was homogenized using a polytetrafluoroethylene head pestle and a glass homogenizer by gradually adding 40 ml of phosphate-buffered saline (PBS). Then the crude homogenate was centrifuged at 3,000 rpm under 4°C for 10 min to remove insoluble precipitate. The supernatant was collected as a homogenate and stored at 4°C until use.
Fractionation of rat liver homogenate by gel filtration
A gel filtration column was previously prepared. Ten grams of polyacrylamide beads (Bio-Gel P-30; BIO-RAD, Hercules, CA) were suspended with 100 ml of 40 mM phosphate buffer (pH 7.4), and the swelled beads were loaded to an empty column (60 ml, polypropylene; Supelco, St. Louis, MO). Forty milliliters of the rat liver homogenate were poured gently into the column and retained. Elution was carried out with PBS, pH 7.4, and five fractions of 40 ml each were collected.
Then, fractions 3 and 4 containing low-molecular-weight compounds were concentrated by C18 solid phase extraction (SPE). A C18 SPE tube (DSC-C18, 2 g/12 ml; Supelco, Sigma-Aldrich Japan, Tokyo, Japan) was loaded with 20 ml of a fraction and subsequently washed with an equal volume of water. Elution was then carried out with 0.8% Triton X-100 containing PBS (5 ml).
Measurement of DCPIP reducing activity
DCPIP was dissolved in PBS containing 0.8% Triton X-100 (DCPIP concentration, 200 μM). An NADH (10 mM) solution in PBS with 0.8% Triton X-100 was also prepared. The DCPIP solution (500 μl), the NADH solution (200 μl), and the rat liver homogenate or fractions (300 μl) were quickly added into a plastic cuvette (final concentrations, DCPIP: 100 μM, NADH: 2.0 mM), and subsequently the change in absorbance at 613 nm was immediately measured with a spectrophotometer.
Measurement of CoQ and VK homologue reducing activity
CoQ or VK aqueous solution was prepared. Each CoQ homologue (CoQ10, 9, and 8) was suspended in PBS containing 0.8% Triton X-100, and the suspension was shaken vigorously for 5 min. Each supernatant was collected as a CoQ solution after centrifugation at 2,000 rpm for 10 min. Each vitamin K homologue (VK1 and VK2) was also similarly suspended in PBS and centrifuged. After removing the upper layer of undissolved VKs, the aqueous phase was taken as the VK solution. The concentration of each CoQ or VK homologue was adjusted to 400 μM by the addition of PBS containing 0.8% Triton X-100.
Next, reaction mixtures containing CoQ (200 μM or 100 μM) or VK (100 μM) homologues, NADH (2.0 mM), and the rat liver homogenate, or each fraction, or flavin compounds (5–20 μM) were prepared in PBS and incubated at 37°C. Changes in concentrations of CoQ or VK homologues were determined with an HPLC system equipped with an electrochemical detector (HPLC-ECD) as described below.
Isolation of CoQ10-SapB complexes from human urine
Purification of CoQ10-SapB complexes from human urine was performed using a modified method described by Jin et al.(18) Briefly, 500 ml of human urine collected from a healthy volunteer was reduced to a pH under 3.0 with the addition of 1.0 M phosphoric acid. Then, 20 g of silica gel was added to the urine to adsorb the complexes. After stirring well for 20 min to completely adsorb the complexes to the silica gel, the gel was collected by filtration. After washing the gel with approximately 20 ml of water adjusted to pH 2.0 with phosphoric acid, the complexes were eluted with 50 ml of phosphate buffer (40 mM, pH 8.0).
SapB analysis with polyacrylamide gel electrophoresis and western blotting
Urine and CoQ10-SapB complex elution (1 ml) were applied to C-18 columns (Empore Extraction Disk Cartridges, 4 mm/1 ml; CDS Analytical Inc., Oxford, PA) respectively, and the adsorbed SapB eluted with 60% acetonitrile solution (1 ml). After drying with nitrogen gas, these were redissolved in SDS-PAGE loading buffer (2% SDS, 10% glycerol, 50 mM Tris-HCl buffer pH 6.8, 6% 2-mercaptoethanol) (20 μl) and heated at 100°C for 3 min. Samples were separated by electrophoresis through a polyacrylamide gradient gel (5–20% acrylamide; ATTO, Tokyo, Japan). Proteins were transferred to a PVDF membrane and incubated with mouse anti-human SapB monoclonal antibodies for 45 min at room temperature. Anti-human SapB monoclonal antibodies were purified from mouse ascites. Proteins were visualized with HRP-conjugated secondary antibodies (Bio-Rad Japan, Tokyo, Japan).
HPLC analysis
To observe changes in concentrations of reduced and oxidized forms of CoQ homologues, including CoQ10, 9, and 8, a reverse phase HPLC-ECD was used. Methanol/2-propanol [70/30 (v/v)] containing NaClO4 (50 mM) was delivered at 1.0 ml/min as a mobile phase. A CAPCELL PAK C18 (5 μm, 250 mm × 4.6 mm; Shiseido, Tokyo, Japan) was used for separation. Simultaneous detection of the CoQ homologue oxidized form was carried out by a tandemly connected RC-10 (15 mm × 4.0 mm; Osaka-soda, Osaka, Japan) on the downstream of the separation column. The applied voltage of the ECD against an Ag/AgCl reference electrode was 600 mV.
Another HPLC system was used for analysis of the CoQ10 reducing compound (RF). The mobile phase and a separation column were a 35% methanol aqueous solution containing ammonium acetate (100 mM) delivered at 0.8 ml/min and a CAPCELL PAK C18 (5 μm, 250 mm × 4.6 mm; Shiseido), respectively. Detection was carried out with a UV detector monitoring absorption at 220 nm.
LC/TOFMS analysis
To obtain mass-to-charge ratios (m/z) of an unknown NADH-dependent CoQ10 reducing compound, HPLC combined with TOFMS (LC/TOFMS, JMS-T100LC; JEOL Ltd., Tokyo, Japan) was used. Positive electrospray ionization (ESI) was performed at an ionization potential of 2,000 V. The optimized applied voltages to the ring lens, outer orifice, inner orifice, and ion guide were 20 V, 30 V, 20 V, and 750 V, respectively.
Results
Measuring DCPIP or CoQ10 reducing activity in rat liver fractions
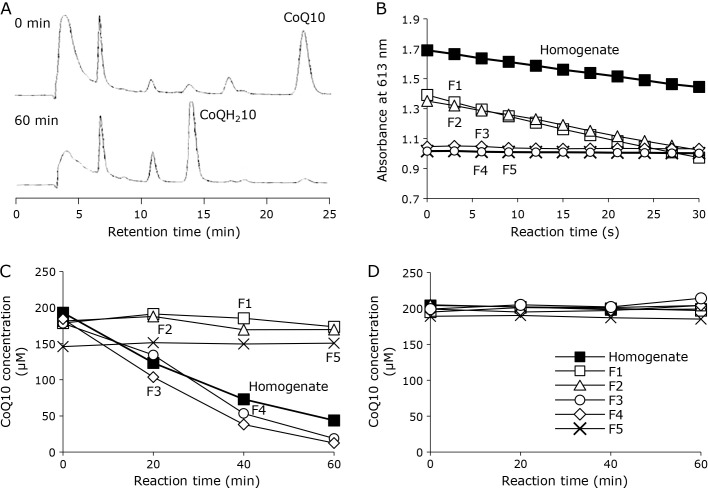
To examine the CoQ10 reducing activity in prepared rat liver homogenate, the homogenate was added to a CoQ10 (approximately 200 μM) suspension in PBS containing 0.8% Triton X-100. The CoQ10 was reduced to CoQH210 during a 60-min incubation in the presence of 2.0 mM NADH (Fig. 1A). Since CoQ10 reducing activity was confirmed, the homogenate was fractionated into five fractions by a gel filtration column loaded with acrylamide gel particles as described in the Materials and Methods. The five collected fractions were examined for DCPIP and CoQ10 reducing activities in the presence of NADH (2.0 mM), respectively (Fig. 1B and C), while the reductions did not occur in the absence of NADH (Fig. 1). The DCPIP and CoQ10 reduction rates of all fractions are summarized in Table 1. DCPIP reducing activity was observed in fractions 1 and 2, but not in fractions 3–5 (Fig. 1B). Considering that the DCPIP assay is a conventional NQO detection method, and the molecular weights of NQO1 and NQO2 are approximately 55 kDa and 52 kDa, respectively, the reducing activity in fractions 1 and 2 was thought to be from NQO. In contrast, the profile of CoQ10 reduction activity was different from that of DCPIP. Only slight CoQ10 reduction activity was observed in fractions 1 and 2, whereas the homogenate showed strong CoQ10 reducing activity; however, fractions 3 and 4, which contained no protein but had low-molecular-weight compounds (<1 kDa), showed CoQ10 reducing activity similar to the homogenate (Fig. 1C). These fractions did not show any reducing activity without NADH (Fig. 1D), so the reduction activity was dependent on NADH as with NQO. Furthermore, 2.0 mM NADH did not reduce CoQ10 without the homogenate fractions (data not shown). These results suggest that the CoQ10 reducing activity needs a low-molecular-weight compound found in fractions 3 and 4.
Fig. 1.
Reduction of CoQ10 and DCPIP with rat liver homogenate or isolated fractions. (A) HPLC chromatograms of reaction mixtures containing CoQ10 (200 μM), NADH (2.0 mM), and rat liver homogenate at 0 min (upper) and 60 min (lower) during incubation at 37°C. (B) Time course of DCPIP reduction with rat liver homogenate (■) or isolated fraction 1 (□), 2 (△), 3 (◇), 4 (○), and 5 (×). (C) Time course of CoQ10 reduction with rat liver homogenate (■) or isolated fraction 1 (□), 2 (△), 3 (◇), 4 (○), and 5 (×). (D) Time course of CoQ10 reduction in the absence of NADH with rat liver homogenate (■) or isolated fraction 1 (□), 2 (△), 3 (◇), 4 (○), and 5 (×).
Table 1.
NADH-dependent reduction rate of DCPIP and CoQ10 with a rat liver homogenate and fraction 1–5
| Substrate | Reduction rate (μM/min) | |||||
|---|---|---|---|---|---|---|
| Homogenate | Fraction 1 | Fraction 2 | Fraction 3 | Fraction 4 | Fraction 5 | |
| DCPIP | 28.8 | 48.8 | 43.8 | — | — | — |
| CoQ10 | 2.95 | 0.30 | 0.90 | 4.05 | 3.25 | — |
Inhibition of CoQ10 reduction activity with dicumarol or quercetin
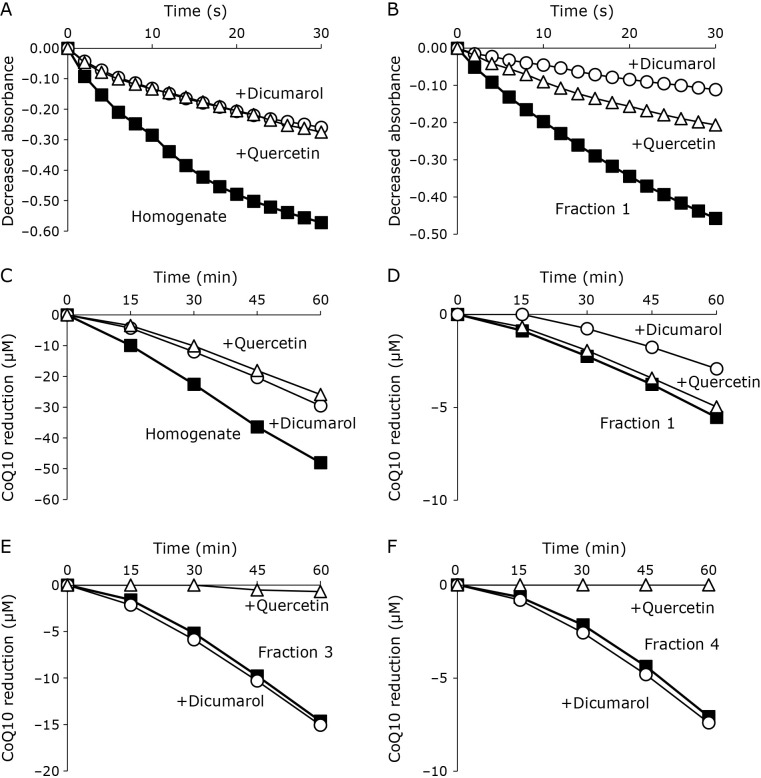
NQO is inhibited by flavonoids, and dicumarol and quercetin are established inhibitors of NQO1 and NQO2, respectively. Thus, we examined the inhibition of DCPIP and CoQ10 reduction by dicumarol and quercetin. DCPIP reductions in the homogenate (Fig. 2A), fraction 1 (Fig. 2B), and fraction 2 (data not shown) were inhibited by 100 μM dicumarol or 100 μM quercetin, indicating that NQO1 and NQO2 were eluted in fractions 1 and 2. Consistent with these results, CoQ10 reductions in the homogenate (Fig. 2C), fraction 1 (Fig. 2D), and fraction 2 (data not shown) were also inhibited by dicumarol or quercetin (100 μM each). In contrast, CoQ10 reductions in fraction 3 (Fig. 2E) and fraction 4 (Fig. 2F) were almost completely suppressed by 100 μM quercetin, whereas the addition of 100 μM dicumarol had no effect. These data clearly indicate that NADH-dependent CoQ10 reduction was induced by a low-molecular-weight compound other than NQO.
Fig. 2.
Inhibition of DCPIP and CoQ10 reducing activity with dicumarol and quercetin. DCPIP reduction with rat liver homogenate (A) and fraction 1 (B) and its inhibition with 100 μM of dicumarol (○) or quercetin (△). CoQ10 reduction with the rat liver homogenate (C), fraction 1 (D), fraction 3 (E), and fraction 4 (F), and its inhibition with 100 μM of dicumarol (○) or quercetin (△).
Identification of the low-molecular-weight CoQ reducing activity in rat liver homogenate
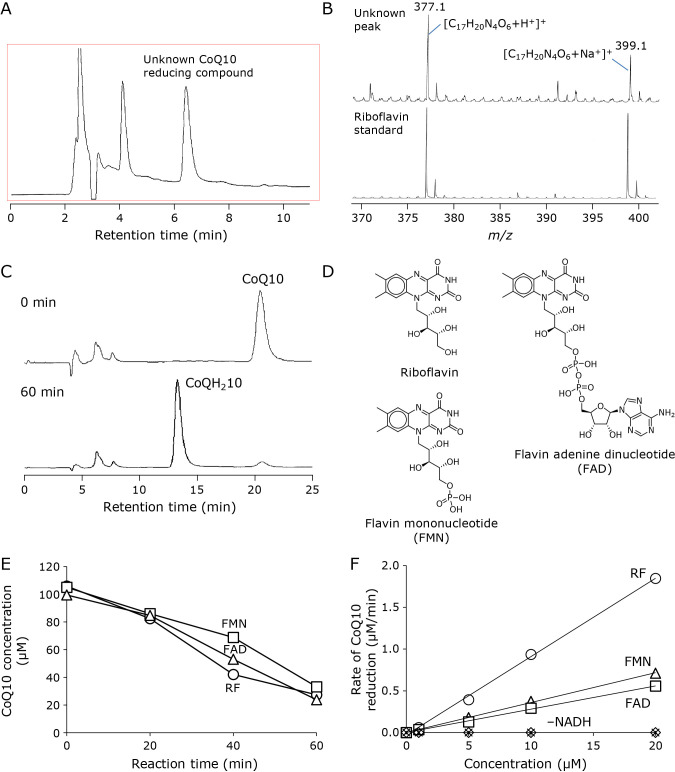
Since the CoQ10 reduction activity in fractions 3 and 4 was postulated to be a low-molecular-weight compound, the compound was concentrated using a C18 SPE tube and eluted with PBS containing 0.8% Triton X-100 as described in the Materials and Methods. The concentrated eluate was analyzed by an HPLC system equipped with a UV detector monitoring at 210 nm. Three major peaks were observed on the chromatogram (Fig. 3A). After collecting individual peaks, CoQ10 reducing activity in each peak was examined. A peak detected at 6.3–7.0 min of retention time showed CoQ10 reducing activity.
Fig. 3.
Identification of unknown CoQ10 reducing activity in rat liver homogenate. (A) HPLC chromatogram of a concentrated fraction containing the CoQ10 reducing compound. The reducing compound was detected at 6.4 min of retention time. (B) MS spectra of the CoQ10 reducing compound (upper) and RF standard (lower) as measured by optimized TOFMS with positive ESI. (C) HPLC chromatograms of a reaction mixture containing RF (10 μM), NADH (2.0 mM), and CoQ10 (70 μM) after 0 min (upper) and 60 min (lower) incubation at 37°C. (D) Chemical structures of RF, FMN, and FAD. (E) Time course of CoQ reduction induced by NADH (2.0 mM) and 10 μM of RF (○), FMN (□), and FAD (△) during incubation at 37°C. (F) Dependency of CoQ10 reduction on concentrations of RF compounds. Initial reduction rate was measured during CoQ10 (100 μM) reduction with RF (○), FMN (△), or FAD (□) in the presence of NADH (2.0 mM), and with RF (×), FMN (▲), or FAD (◇) in the absence of NADH.
Then, the peak was analyzed by optimized LC/TOFMS with positive ESI to determine the molecular formula of the unknown compound. Two related ions were detected on the MS spectrum of the unknown peak, and their mass-to-charge ratios were determined as 377.1429 and 399.1282, respectively (Fig. 3B, upper). Both ions were thought to be H+ and Na+ adducts ion of the compound. From the m/z values, the formula of the compound was determined as C17H20N4O6 [Theoretical m/z value 377.1456 (+H+) and 399.1275 (+Na+)]. Since the molecular formula matched RF {7,8-dimethyl-10-[(2S,3S,4R)-2,3,4,5-tetrahydroxypentyl]benzo[g]pteridine-2,4-dione}, an authentic standard was also analyzed by LC/TOFMS. The chromatographic retention (data not shown) and MS spectrum (Fig. 3B lower) were identical with those of the compound. These results suggest that the NADH-dependent CoQ10 reducing activity in the low-molecular-weight fraction of rat liver homogenate was due to RF. The content of RF in rat liver has been determined as approximately 35 μg/g.(19–21) Considering the treated rat liver weight of 4.0 g and the volume of the prepared homogenate, the RF concentration of the homogenate was estimated to be approximately 9.3 μM.
Then, we conducted the NADH-dependent CoQ10 reduction catalyzed by authentic RF. RF (10 μM) reduced approximately 70 μM CoQ10 during a 60-min incubation in the presence of NADH (2.0 mM) (Fig. 3C); however, CoQ10 was not reduced in the absence of NADH or RF (Fig. 3F). One mole of RF reduced approximately 7 moles of CoQ10, suggesting that RF works as a catalyst in CoQ10 reduction. Furthermore, the CoQ10 reduction activities of two biologically essential derivatives of RF, FMN, and FAD (Fig. 3D), were also examined. Both FMN (10 μM) and FAD (10 μM) also catalyzed strong NADH-dependent CoQ10 reducing activity like RF did (Fig. 3E). Further, we examined dependency of CoQ10 reduction on the concentration of RF compounds. Initial reduction rate (0–30 min) increased with increasing concentrations of RF compounds in NADH (2.0 mM)-dependent CoQ10 (100 μM) reduction (Fig. 3F). Interestingly, RF showed the highest reduction activity among RF compounds. It is speculated to be due to the strong electron-withdrawing effect of phosphate groups in FMN and FAD molecules. However, the further investigation is needed to confirm the speculation. Nonetheless, it was suggested that intact RF may be biologically important as not only a substrate for FMN and FAD biosynthesis, but also a reductant of CoQ10 for protection from oxidative stress.
Reduction of CoQ homologues and VK by RF
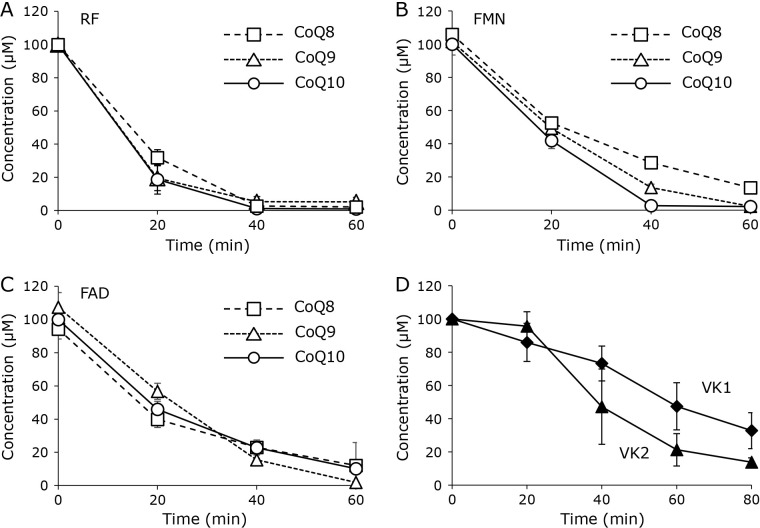
The substrate for reduction by the system of RF compounds and NADH was investigated. CoQ8 and 9, which are homologues of CoQ10 that have different isoprene side-chain lengths, were examined. A combination of 20 μM RF and 2.0 mM NADH reduced CoQ8 and CoQ9 suspended in a homogeneous solution similar to CoQ10 (Fig. 4A). FMN (Fig. 4B) and FAD (Fig. 4C) also reduced these CoQ homologues. We also examined the reduction of vitamin K (VK) homologues VK1 and VK2. Both VKs are also substrates for NQO, and the reduced forms are biologically active. RF (20 μM) with 2.0 mM NADH reduced VK1 and VK2; however, the reduction rates were smaller than those of the CoQ homologues (Fig. 4D). VKs have far lower oxidation potentials than CoQ homologues do. Therefore, simultaneous oxidation of VKs might have occurred during the reduction.
Fig. 4.
Reduction of CoQ and VK homologues by RF compounds (n = 3, plots are expressed as means ± SD). Reduction of CoQ homologues CoQ8 (□), CoQ9 (△), and CoQ10 (○) induced by 20 μM of RF (A), FMN (B), and FAD (C) in the presence of NADH (2.0 mM). (D) Reduction of VK homologues VK1 (◆) and VK2 (▲) during incubation in the presence of RF (20 μM) and NADH (2.0 mM).
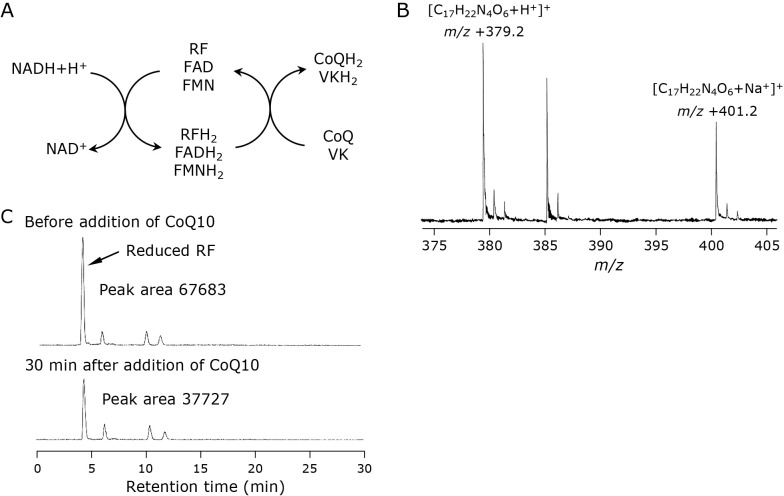
A mechanism for quinone reduction with RF and NADH
As shown above, the combination of RF derivatives and NADH reduces biological quinones such as CoQ and VK. It is believed that NQO plays a principal role in quinone reduction in vivo, and the mechanism has been characterized.(22–24) NAD(P)H reduces FAD, which is an active center of NQO, to FADH2, and the FADH2 subsequently reduces quinone to hydroquinone. The combination of RF compounds and NADH is thought to reduce bio-quinones, similar to NQO (Fig. 5A). The oxidation potentials of NADH, RF, VK3, and CoQ10 have been determined as −0.54 V,(25) −0.259 V,(26) 0.930 V,(27) and 0.770 V(27) vs a standard calomel electrode, respectively. Considering these values, electron transfer may spontaneously occur from NADH to CoQ10 or VKs via RF despite the absence of an enzyme. Since NADH cannot reduce CoQ10 directly, RF should play an important role in the reduction.
Fig. 5.
A plausible mechanism for the reduction of quinones such as CoQ and VK with a combination of NADH and RF compounds (A). MS spectrum of a peak speculated as a reduced RF (B). Changes in the peak before (upper) and 30 min after (lower) addition of CoQ10. Inserted numbers describe peak areas (C).
Considering the potential of RF as −0.259 V, the reduced RF should be extremely unstable. We examined the reduced RF from an NADH and RF mixture using LC/TOFMS. We detected a small peak of possible reduced RF, and simultaneously observed a proton adduct ion (m/z +379.1) and a sodium adduct ion (m/z +401.1) on its MS spectrum in positive ionization mode (Fig. 5B). The peak decreased by the addition of CoQ10 to the solution, suggesting that the reduced RF reacted with CoQ10 (Fig. 5C). However, we failed to isolate the reduced RF, indicating that the equilibrium of Eq. 1 is to the left. Thus, the reduced RF reduces bio-quinones to their reduced forms (Eq. 2), and RF derivatives work as catalysts in this mechanism.
| (1) |
| (2) |
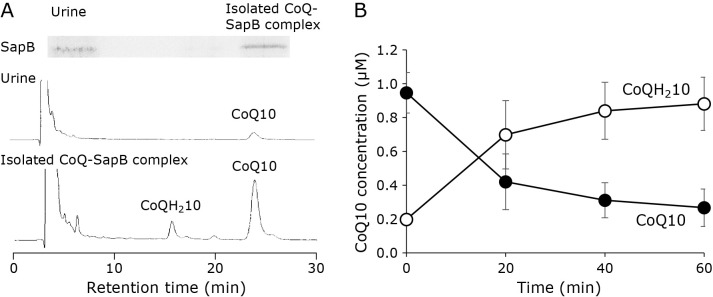
Reduction of CoQ10 associated with SapB in the aqueous phase
Our results indicated that CoQ homologues (CoQ8, 9, and 10) can be reduced with the RF-NADH system in a 0.8% Triton X-100 suspension. However, the interaction of CoQ with the RF-NADH system is generally difficult, because CoQ homologues are strongly hydrophobic, whereas both RF and NADH are hydrophilic. Therefore, for the reduction of CoQ homologues by this system, it is necessary for them to be stably distributed in the aqueous phase. Recently, we found that a water-soluble protein, SapB, binds CoQ10, and a complex of CoQ10 and SapB can stably exist in aqueous solution.(18) We also found that SapB and its precursor prosaposin (Psap) enable the stabilization of CoQ10 in the aqueous phase when bound to CoQ10 in vivo.(28) We therefore tested the reduction of CoQ10 bound to SapB by the RF-NADH system. The CoQ10-SapB complex was isolated from human urine, and the collected fraction of the complex was confirmed to contain both SapB and CoQ10 (Fig. 6A). The fraction was incubated with RF (1.0 μM) and NADH (1.0 mM), and CoQ10 bound to SapB was reduced to CoQ10H2, similar to that in a 0.8% Triton X-100 suspension (Fig. 6B). This suggests that CoQ10 reduction can occur in the aqueous phase if CoQ10 is bound to SapB.
Fig. 6.
RF and NADH system-induced reduction of CoQ10 bound to SapB. CoQ10-SapB complexes were collected from human urine. (A) Immuno-detection of SapB from human urine (upper left) and the collected complex (upper right). And HPLC chromatograms of human urine (middle) and the collected complex (lower). Electrochemical detection was carried out using a tandemly connected platinum catalyst column and an optimized ECD. (B) Time course of CoQ10 reduction in SapB complexes induced by RF (1.0 μM) and NADH (1.0 mM) at 37°C (n = 3, plots are expressed as means ± SD).
Discussion
Quinone reduction is essential in vivo. The reduced form of CoQ plays important roles not only as a simple antioxidant for scavenging free radicals, but also as a reductant of α-Toc radicals for anti-TMP activity. And reduced VK, another bio-quinone, is also necessary for blood coagulation.
In the present study, we focused on aqueous CoQ reducing activity. Generally, it is difficult for such reduction to occur, because CoQ is strongly hydrophobic whereas RF and its derivatives and NADH are water soluble. It is necessary to stabilize CoQ homologues in the aqueous phase for that reduction. We demonstrated that a flavin compound-NADH system can reduce CoQ10 bound with SapB isolated from human urine. We believe that this system serves as a CoQ10 reduction pathway along with NQO in aqueous environments, including the cytosol and bodily fluids.
RF is dietarily taken up as water-soluble vitamin B2 in the intestine by RF transporters, i.e., human riboflavin transporter (hRFVT)-1, -2, and -3 in humans,(29) and is widely distributed in vivo not only in cytosol, but also in bodily fluids. Cytosolic RF is enzymatically modified to FMN and FAD by RF kinase and FAD synthetase, respectively. FMN and FAD are then used as an active center and a cofactor in many enzymes.(30) In contrast, the concentration of non-modified RF is estimated to be more than 100 μM in hepatic cytosol, considering that the RF content in liver is approximately 35 μg/g. Therefore, the present study suggests that these cytosolic RF compounds may not only enzymatically but also chemically contribute to the preservation of intracellular quinone reduction homeostasis.
The concentrations of RF in human bodily fluids have been also investigated in plasma (3.1–10.4 nM),(31) milk (13.3–19.7 nM),(32) and spermoplasm (79.7–183.8 nM).(33) Among these fluids, spermoplasm clearly shows a significantly high RF content, suggesting major biological importance. We previously demonstrated that the CoQ10-SapB complex is present in human sperm.(18) It has been reported that the redox state of NAD+/NADH is significantly lower in seminal plasma of infertile men than in that of fertile men,(34) suggesting that NADH-dependent CoQ10 reduction might not work effectively in these fluids. In fact, there have been many reports indicating that CoQ10 administration improves male infertility, and that the mechanism is due to its antioxidant activity in addition to energy production.(35–38) Furthermore, oral uptake of reduced CoQ10 (ubiquinol 10) effectively improves sperm density, sperm motility, and sperm morphology.(39) Therefore, the non-enzymatic reduction of bio-quinones may play an important role in the maintenance of their redox status in vivo.
Abbreviations
- CoQ
coenzyme Q
- DCPIP
dichlorophenolindophenol
- ECD
electrochemical detector
- ESI
electrospray ionization
- FAD
flavin adenine dinucleotide
- FMN
flavin mononucleotide
- hRFVT
human riboflavin transporter
- NQO
NAD(P)H-dependent quinone oxidoreductase
- Psap
prosaposin
- RF
riboflavin
- SapB
saposin B
- SPE
solid phase extraction
- TMP
tocopherol-mediated peroxidation
- TOFMS
time-of-flight mass spectrometry
- VK
vitamin K
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Chesis PL, Levin DE, Smith MT, Ernster L, Ames BN. Mutagenicity of quinones: pathways of metabolic activation and detoxification. Proc Natl Acad Sci U S A 1984; 81: 1696–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaiswal AK, McBride OW, Adesnik M, Nebert DW. Human dioxin-inducible cytosolic NAD(P)H:menadione oxidoreductase. cDNA sequence and localization of gene to chromosome 16. J Biol Chem 1988; 263: 13572–13578. [PubMed] [Google Scholar]
- 3.den Braver-Sewradj SP, den Braver MW, Toorneman RM, et al. Reduction and Scavenging of Chemically Reactive Drug Metabolites by NAD(P)H:Quinone Oxidoreductase 1 and NRH:Quinone Oxidoreductase 2 and Variability in Hepatic Concentrations. Chem Res Toxicol 2018; 31: 116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keyes SR, Fracasso PM, Heimbrook DC, Rockwell S, Sligar SG, Sartorelli AC. Role of NADPH:cytochrome c reductase and DT-diaphorase in the biotransformation of mitomycin C1. Cancer Res 1984; 44 (12 Pt 1): 5638–5643. [PubMed] [Google Scholar]
- 5.Asher G, Lotem J, Cohen B, Sachs L, Shaul Y. Regulation of p53 stability and p53-dependent apoptosis by NADH quinone oxidoreductase 1. Proc Natl Acad Sci U S A 2001; 98: 1188–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garate M, Wong RP, Campos EI, Wang Y, Li G. NAD(P)H quinone oxidoreductase 1 inhibits the proteasomal degradation of the tumour suppressor p33(ING1b). EMBO Rep 2008; 9: 576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wefers H, Komai T, Talalay P, Sies H. Protection against reactive oxygen species by NAD(P)H: quinone reductase induced by the dietary antioxidant butylated hydroxyanisole (BHA). FEBS Lett 1984; 169: 63–66. [DOI] [PubMed] [Google Scholar]
- 8.Beyer RE, Segura-Aguilar J, Di Bernardo S, et al. The role of DT-diaphorase in the maintenance of the reduced antioxidant form of coenzyme Q in membrane systems. Proc Natl Acad Sci U S A 1996; 93: 2528–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegel D, Gustafson DL, Dehn DL, et al. NAD(P)H:quinone oxidoreductase 1: role as a superoxide scavenger. Mol Pharmacol 2004; 65: 1238–1247. [DOI] [PubMed] [Google Scholar]
- 10.Dunlap WC, Fujisawa A, Yamamoto Y. UV radiation increases the reduced coenzyme Q ratio in marine bacteria. Redox Rep 2002; 7: 320–323. [DOI] [PubMed] [Google Scholar]
- 11.Bowry VW, Stocker R. Tocopherol-mediated peroxidation. The prooxidant effect of vitamin E on the radical-initiated oxidation of human low-density lipoprotein. J Am Chem Soc 1993; 115: 6029–6044. [Google Scholar]
- 12.Yamamoto Y, Komuro E, Niki E. Antioxidant activity of ubiquinol in solution and phosphatidylcholine liposome. J Nutr Sci Vitaminol (Tokyo) 1990; 36: 505–511. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto Y, Kawamura M, Tatsuno K, et al. Formation of lipid hydroperoxides in the cupric ion-induced oxidation of plasma and low density lipoprotein. In: Davies KJA, ed. Oxidative Damage and Repair. New York: Pergamon Press, 1991; 287–291. [Google Scholar]
- 14.Yamamoto Y, Yamashita S. Plasma ubiquinone to ubiquinol ratio in patients with hepatitis, cirrhosis, and hepatoma, and in patients treated with percutaneous transluminal coronary reperfusion. Biofactors 1999; 9: 241–246. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto Y, Yamashita S, Fujisawa A, Kokura S, Yoshikawa T. Oxidative stress in patients with hepatitis, cirrhosis, and hepatoma evaluated by plasma antioxidants. Biochem Biophys Res Commun 1998; 247: 166–170. [DOI] [PubMed] [Google Scholar]
- 16.Nagase M, Yamamoto Y, Miyazaki Y, Yoshino H. Increased oxidative stress in patients with amyotrophic lateral sclerosis and the effect of edaravone administration. Redox Rep 2016; 21: 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi T, Mine Y, Okamoto T. Extracellular coenzyme Q10 (CoQ10) is reduced to ubiquinol-10 by intact Hep G2 cells independent of intracellular CoQ10 reduction. Arch Biochem Biophys 2019; 672: 108067. [DOI] [PubMed] [Google Scholar]
- 18.Jin G, Kubo H, Kashiba M, et al. Saposin B is a human coenzyme q10-binding/transfer protein. J Clin Biochem Nutr 2008; 42: 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray AZ, Greenstein LM, Sherman HC. Fluorometric studies of the riboflavin contents of muscle and liver. J Biol Chem 1946; 165: 91–94. [PubMed] [Google Scholar]
- 20.Fujiwara M, Kuriyama K. Effect of PCB (polychlorobiphenyls) on L-ascorbic acid, pyridoxal phosphate and riboflavin contents in various organs and on hepatic metabolism of L-ascorbic acid in the rat. Jpn J Pharmacol 1977; 27: 621–627. [DOI] [PubMed] [Google Scholar]
- 21.Shibata K, Shimizu A, Fukuwatari T. Vitamin B1 deficiency does not affect the liver concentrations of the other seven kinds of B-group vitamins in rats. Nutr Metab Insights 2013; 6: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li R, Bianchet MA, Talalay P, Amzel LM. The three-dimensional structure of NAD(P)H:quinone reductase, a flavoprotein involved in cancer chemoprotection and chemotherapy: mechanism of the two-electron reduction. Proc Natl Acad Sci U S A 1995; 92: 8846–8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavelier G, Amzel LM. Mechanism of NAD(P)H:quinone reductase: ab initio studies of reduced flavin. Proteins 2001; 43: 420–432. [PubMed] [Google Scholar]
- 24.Deller S, Macheroux P, Sollner S. Flavin-dependent quinone reductases. Cell Mol Life Sci 2008; 65: 141–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karyakin A, Karyakina E, Schuhmann W, Schmidt HL. Electropolymerized azines: part II. In a search of the best electrocatalyst of NADH oxidation. Electroanalysis 1999; 11: 553–557. [Google Scholar]
- 26.Malinauskas A, Ruzgas T, Gorton L. Tuning the redox potential of riboflavin by zirconium phosphate in carbon paste electrodes. Bioelectrochem Bioenerg 1999; 49: 21–27. [DOI] [PubMed] [Google Scholar]
- 27.Mukai K, Itoh S, Morimoto H. Stopped-flow kinetic study of vitamin E regeneration reaction with biological hydroquinones (reduced forms of ubiquinone, vitamin K, and tocopherolquinone) in solution. J Biol Chem 1992; 267: 22277–22281. [PubMed] [Google Scholar]
- 28.Jin G, Horinouchi R, Sagawa T, et al. Coenzyme Q10-binding/transfer protein saposin B also binds γ-tocopherol. J Clin Biochem Nutr 2008; 43: 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Subramanian VS, Ghosal A, Kapadia R, Nabokina SM, Said HM. Molecular mechanisms mediating the adaptive regulation of intestinal riboflavin uptake process. PLoS One 2015; 10: e0131698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lienhart WD, Gudipati V, Macheroux P. The human flavoproteome. Arch Biochem Biophys 2013; 535: 150–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hustad S, McKinley MC, McNulty H, et al. Riboflavin, flavin mononucleotide, and flavin adenine dinucleotide in human plasma and erythrocytes at baseline and after low-dose riboflavin supplementation. Clin Chem 2002; 48: 1571–1577. [PubMed] [Google Scholar]
- 32.Hampel D, Shahab-Ferdows S, Adair LS, et al. Thiamin and riboflavin in human milk: effects of lipid-based nutrient supplementation and stage of lactation on vitamer secretion and contributions to total vitamin content. PLoS One 2016; 11: e0149479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kodentsova VM, Vrzhesinskaya OA, Evdokimov VV. Riboflavin content in male spermoplasm. Bull Exp Biol Med 2003; 135: 258–260. [DOI] [PubMed] [Google Scholar]
- 34.Galimov SN, Gromenko JY, Bulygin KV, Galimov KS, Galimova EF, Sinelnikov MY. The level of secondary messengers and the redox state of NAD+/NADH are associated with sperm quality in infertility. J Reprod Immunol 2021; 148: 103383. [DOI] [PubMed] [Google Scholar]
- 35.Yao DF, Mills JN. Male infertility: lifestyle factors and holistic, complementary, and alternative therapies. Asian J Androl 2016; 18: 410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mancini A, Balercia G. Coenzyme Q10 in male infertility: physiopathology and therapy. Biofactors 2011; 37: 374–380. [DOI] [PubMed] [Google Scholar]
- 37.Balercia G, Mancini A, Paggi F, et al. Coenzyme Q10 and male infertility. J Endocrinol Invest 2009; 32: 626–632. [DOI] [PubMed] [Google Scholar]
- 38.Balercia G, Buldreghini E, Vignini A, et al. Coenzyme Q10 treatment in infertile men with idiopathic asthenozoospermia: a placebo-controlled, double-blind randomized trial. Fertil Steril 2009; 91: 1785–1792. [DOI] [PubMed] [Google Scholar]
- 39.Safarinejad MR, Safarinejad S, Shafiei N, Safarinejad S. Effects of the reduced form of coenzyme Q10 (ubiquinol) on semen parameters in men with idiopathic infertility: a double-blind, placebo controlled, randomized study. J Urol 2012; 188: 526–531. [DOI] [PubMed] [Google Scholar]