Abstract
The aim of this study was to compare the efficacy and safety of treatment with Janus kinase inhibitors for alopecia areata, measured by change in Severity of Alopecia Tool (SALT) score. A systematic review following Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines was performed using Medline, EMBASE and Cochrane library. All studies investigating the efficacy of treatments for alopecia areata were included. Primary outcomes were the proportion of patients with alopecia areata achieving 30%, 50%, 75%, 90% and 100% improvement in SALT score after treatment with a Janus kinase inhibitor. A meta-analysis was performed including all randomized controlled trials investigating Janus kinase inhibitors. A total of 37 studies matched the inclusion criteria and were included. Meta-analysis was performed based on 5 randomized studies. Regarding patients with alopecia areata defined as ≥ 50% scalp hair loss, baricitinib 4 mg once daily demonstrated the highest efficacy. However, among patients with alopecia areata defined as a SALT score ≥ 50, oral deuruxolitinib 12 mg twice daily demonstrated the highest efficacy. Deuruxolitinib and baricitinib appear to be promising drugs for the treatment of alopecia areata. However, the response depends on the dosage of the drug. More randomized trials, with identical inclusion criteria and dose and duration of treatment, are required to confirm these findings.
Key words: alopecia areata, polygenic autoimmune disease, JAK inhibitors, biological
SIGNIFICANCE.
The available treatments for alopecia areata are of limited efficacy, and have a high risk of adverse effects and high recurrence rates, especially for patients with severe alopecia areata. Janus kinase inhibitors are among the newly developed systematic treatments with great potential for treatment of alopecia areata. The aim of this review was to investigate the efficacy and safety of these treatments, which is an urgent need to implement evidence-based practice guidelines, to enable the best possible patient care. The results of this study suggest that patients with alopecia areata unresponsive to traditional therapies may benefit from treatment with baricitinib or deuruxolitinib.
Alopecia areata (AA) is an autoimmune, dynamic disorder causing recurrent episodes of hair loss (1). It can affect any hair-bearing area and varies from patchy diffuse alopecia to universal loss of hair. The global incidence of AA varies from 0.57% to 3.8% in hospital-based studies, and, in general population studies the lifetime incidence of AA varies from 1.7% to 2.1% (2). It is speculated that the variation reflects differences in populations and types of studies (3–6). AA is predominantly seen in young people (6, 7). The development of AA has a strong genetic component (8). Atopic eczema, vitiligo and autoimmune disorders, such as systemic lupus erythematosus, are associated with AA, implying shared, but as-yet unidentified, pathogenic mechanisms (9, 10). The disease is dynamic, with alternating flares and remissions. The relapse rate of AA is 85%, and for those who have had the diagnosis for more than 20 years, it is practically 100%, suggesting progression over time (3, 11).
The selection of therapies for patients with AA is frequently based on the age of the patients, and the extent and impact of hair loss (12). There is a wide range of therapeutic options, from camouflage, to topical therapy (e.g. immunotherapy (diphenylcyclopropenone, and dinitrochlorobenzene), to intralesional corticosteroids, and systemic treatment (e.g. hydroxychloroquine). All of these are, however, off-label, and have shown only limited results (13). The extent of AA may be one of the predictors of psychological disorders among patients with severe hair loss (6). Discussing psychological comorbidities associated with AA, a 66–74% lifetime prevalence of psychiatric disorders has been reported in patients with AA, with a 38–39% lifetime prevalence of depression and a 39–62% prevalence of generalized anxiety disorder (6). An unmet need for therapy therefore exists in AA.
The histological response of AA involves cytotoxic CD8+NKG2D+ T adjoining the hair follicle site, which is promoted by increased interferon gamma (IFN-γ) and the common gamma chain (γc) cytokines interleukin (IL)-2, IL-7 and IL-15. Cytokines, such as IFN-γ and γc, bind to their receptors and activate Janus kinase mediated signalling, inducing phosphorylation of signal transducer and activation of transcription (STAT) molecules. Activation of Janus kinase receptors, specifically JAK 1/2 and JAK 1/3, and activation of transcription (STAT) led to further cellular immune response, which finally result in more IFN-γ and IL-15 production in hair follicles (14). JAK inhibitors may be a new treatment option for AA. These target several cytokines, including IL-2, IL-7, IL-15, IL-21 and IFN- γ, which all appear to be involved in the pathogenesis of AA (13). Furthermore, the JAK/STAT signalling pathway is involved in the hair cycle, and is reportedly upregulated in the catagen and telogen phases of the hair cycle, but suppressed in the anagen (15, 16). Several JAK inhibitors have been tested in AA, and the aim of this systematic review and meta-analysis is to investigate their efficacy and safety in patients with AA.
METHODS
This study was conducted based on a systematic literature search for studies using Medline (PubMed), EMBASE and Cochrane Library to identify JAK inhibitors drugs used in the treatment of AA (date of search April 2022). This was investigated by using the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (17). Two of the authors (FS and TM) independently searched the medical databases PubMed, EMBASE, and Cochrane library, using the following search terms: (alopecia areata) AND (tofacitinib) OR (ruxolitinib) OR (baricitinib) OR (ritlecitinib) OR (brepocitinib) OR (Ruxolitinib) OR (delgocitinib) OR (CTP-543) OR (JAK-STAT pathway) OR (JAK inhibitor). Furthermore, studies were identified by screening reference lists of key articles and review articles.
Inclusion and exclusion criteria
All studies were included if they were in English and investigated patients with AA, alopecia universalis (AU) or alopecia totalis (AT), who were treated with JAK inhibitors. Both topical and systemic treatments were included. There were no limits or restrictions regarding the type of study, publication date, sex, the severity of AA, age or country of origin. The inclusion criteria were: (i) human subjects; (ii) severity of AA measured by the Severity of Alopecia Tool (SALT) score before and/or after treatment; and/or change in SALT score measured in final as percentage change in SALT score. Studies were excluded if they did not measure the severity of AA by using the SALT score.
The primary outcome was the proportion of patients achieving 30%, 50%, 75%, 90% and 100% improvement in SALT score. The percentage change from baseline is calculated as follows:
Percentage change from baseline = (SALTbaseline – SALTfollowup)/SALTbaseline × 100%
Secondary outcome was the proportion of patients achieving a complete response, partial response, and no response. This was used only if 30%, 50%, 90% and 100% improvement in SALT scores were not directly provided. Complete response was defined in this study as hair regrowth ≥ 95%. Partial response was defined as hair regrowth of < 95% and > 0%, and no response defined as a 0% hair regrowth. n case of the included studies did not report the improvement in SALT score based on these definitions, the results were directly reported in this review. Other reported outcomes were any adverse events reported after treatment.
Data extraction
After the inclusion of all relevant studies, the same authors independently performed data extraction. Studies were screened according to title and abstract. All relevant studies were included, and duplicates were removed. The extraction data are listed in Tables SI and SII, including the name of the first author, year of publication, study type, medication and dosage, proportion and age of patients, proportion of male patients, duration of AA, SALT score at baseline, inclusion criteria regarding the severity of AA and prior treatments, the definition of control group and information about concurrent treatment was registered in Tables SI and SII. Outcomes included the proportion of patients (i) achieving 30%, 50%, 75%, 90% or 100% improvement in SALT score or (ii) achieving complete response, partial response or no response. Relapse rate and the proportion of patients experiencing adverse effects are registered in Tables SII and SIII, respectively.
Data regarding SALT improvement was extracted directly from the tables of the manuscripts of the relevant studies. If data were provided only by graphs, Engauge Digitizer Software (Engauge Digitizer (markummitchell.github.io), accessed 1 July 2021) was used to extract numerical data from the graphs.
Statistical analysis
Efficacy outcomes were based on the improvement in SALT score. When statistically appropriate (at least 2 randomized controlled trials studies provided usable data for relevant outcomes), meta-analyses were conducted. These were performed for the outcomes of 30%, 50%, 75%, 90% and 100% improvement in SALT score. Subgroup analyses were performed depending on the severity of AA and type of medication (oral or topical). The meta-analysis was conducted using random-effects models by using Review Manager Version 5.4 ((https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman) assessed on 10 April 2022). One outcome measure was used for meta-analyses: the risk difference (RD) for binary outcomes, both with a 95% confidence interval (95% CI). The RD of 0.2 means that the risk of an event in focus is 20% points more likely in the experimental group than in the control group.
RESULTS
The database search resulted in 1,875 non-duplicate studies (Fig. 1). The following JAK inhibitors have been tested in relation to their effect on AA; ruxolitinib (JAK1 and JAK2 inhibitor), tofacitinib (JAK1 and JAK3 inhibitor), baricitinib (JAK 1 and JAK2 inhibitor), ritlecitinib (JAK3 inhibitor), brepocitinib (JAK1 inhibitor), and delgocitinib (JAK1, JAK2 and JAK3 inhibitor). A total of 37 studies matched the inclusion criteria and were included in this study (18–54). Twenty-two studies originated from North America (18–22, 26, 27, 29, 31, 35, 37, 41, 42, 44–48, 50, 51, 53, 54), 11 studies from Asia (28, 32–34, 36, 38–40, 43, 49, 52), 3 studies from Australia (24, 25, 30) and 1 study from Europe (23). The included original articles were 7 randomized trials (19–22, 26, 27, 33), 5 non-randomized trials (25, 42, 44, 53, 54), 4 retrospective (28, 39, 43, 51) and 21 case-series (18, 23, 24, 29–32, 34–38, 40, 41, 45–50, 52). Four studies included only children with AA (24, 35, 36, 46), 5 studies both children and adults (28, 32, 37, 45, 47), and the rest included only adults (Table SI). The severity of AA was reported by 26 studies (Table SI). Twenty-four studies investigated oral tofacitinib, where the most common dose of the drug was 5 mg twice daily (18, 23, 24, 28–30, 32–36, 38–43, 45, 47, 49–53). Topical 2% tofacitinib once or twice daily was investigated in 3 studies (41, 44, 46). Sublingual tofacitinib 5 mg twice daily was investigated in 1 study (25). Oral CTP-543 (deuruxolitinib, a deuterated ruxolitinib), 4, 8, or 12 mg twice daily, was investigated in 1 study (19). Baricitinib, 1, 2 or 4 mg daily, was investigated in 2 studies (27, 20). Topical delgocitinib was investigated by 1 study, where the dose was 30 mg/g twice daily (21). Oral ritlecitinib (initially 200 mg once daily and subsequently 50 mg once daily) and brepocitinib (initially 60 mg once daily and subsequently 30 mg once daily) were investigated in 2 studies (22, 26).
Fig. 1.
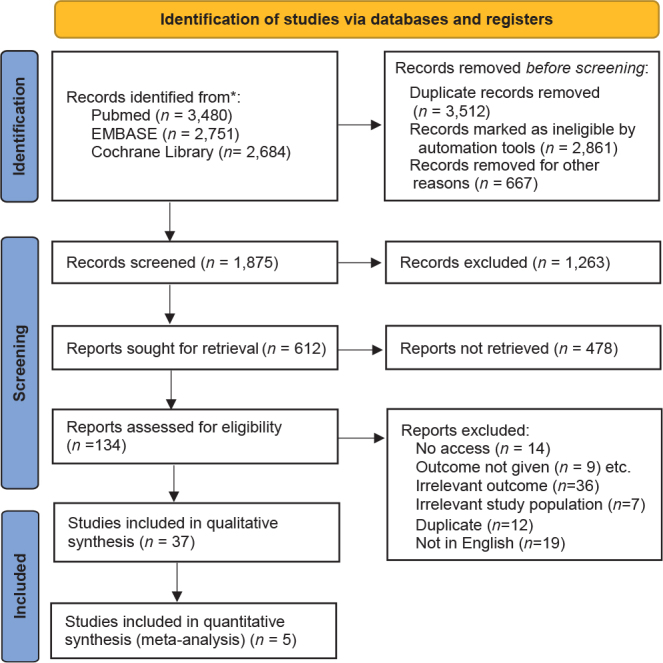
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Oral ruxolitinib was investigated in 3 studies with doses of 10–25 mg twice daily (33, 37, 54). Topical 0.6% ruxolitinib once daily was investigated in 1 study (48). In 1 study, the name of the JAK inhibitor drug was not mentioned (31). For further details on each study, see Tables SI and SII.
Meta-analysis
Primary outcomes: 30%, 50%, 75%, 90% and 100% improvement in SALT score. Five randomized studies provided data on improvement in SALT score and were included in the meta-analysis (22, 26, 27, 19, 20).
Alopecia areata defined as ≥ 50% scalp hair loss. With respect to SALT30 (30% improvement from baseline), 2 studies (n = 247), investigated the treatment with brepocitinib and ritlecitinib among patients with AA defined as ≥ 50% scalp hair loss. Both treatments showed similar results compared with the control groups. However, treatment with brepocitinib was reported to be superior compared with ritlecitinib (brepocitinib: RD 69%, 95% CI 44–93 vs ritlecitinib: RD 48%, 95% CI 33–63) (Fig. S1).
With respect to SALT50, 3 studies (n = 357) investigated the treatment with baricitinib, brepocitinib and ritlecitinib among patients with AA defined as ≥ 50% scalp hair loss. All treatments showed similar results compared with the control groups. However, treatment with baricitinib 4 mg once daily was superior to other treatments (baricitinib: RD 63%, 95% CI 44–82 vs brepocitinib: RD 47, 95% CI 32–62; and ritlecitinib: RD 33%, 95% CI 19–47). A dose-response relationship was observed for baricitinib (Fig. S2).
With respect to SALT75, 3 studies (n = 357) investigated the treatment with baricitinib, brepocitinib and ritlecitinib among patients with AA defined as ≥ 50% scalp hair loss. Almost all treatments showed similar results compared with the control groups (only 1 single study for ritlecitinib). Baricitinib 4 mg daily was superior compared with brepocitinib and ritlecitinib (baricitinib: RD 45%, 95% CI 25–65 vs brepocitinib: RD 38%, 95% CI 24–53 and ritlecitinib: RD 25%, 95% CI 12–38) (Fig. S3).
With respect to SALT90, 3 studies (n = 357) investigated the treatment with baricitinib, brepocitinib and ritlecitinib among patients with AA defined as ≥ 50% scalp hair loss. Almost all treatments showed results compared with the control groups (only 1 single study for brepocitinib and ritlecitinib). Baricitinib 4 mg once daily was superior compared with brepocitinib and ritlecitinib (baricitinib: RD 41%, 95% CI 22–60 vs brepocitinib: RD 32%, 95% CI 18–46 and ritlecitinib: RD 23%, 95% CI 10–36). A dose-response relationship was observed for baricitinib (Fig. S4).
With respect to SALT100, 3 studies (n = 357) investigated the treatment with baricitinib, brepocitinib and ritlecitinib among patients with AA defined as ≥ 50% scalp hair loss. The treatment with baricitinib 4 mg and brepocitinib (only 1 study regarding the latter) showed similar results compared with the control groups, with baricitinib being superior (baricitinib: RD 26%, 95% CI 1–43 vs brepocitinib: RD 13%, 95% CI 0.3–23). A dose-response relationship was observed for baricitinib (Fig. S5).
Alopecia areata defined as severe AA by SALT score ≥ 50. Two other studies (n = 1,782) investigated achievement of SALT50 based on treatment with deuruxolitinib and baricitinib among patients with severe AA defined as a SALT score ≥ 50. Both treatments showed almost similar results compared with the placebo groups (regarding deuruxolitinib: only 8 and 12 mg). However, treatment with deuruxolitinib 12 mg twice daily was superior compared with baricitinib (deuruxolitinib 12 mg: RD 48%, 95% CI 30–66 vs baricitinib 4 mg: RD 42%, 95% CI 35–49). A dose-response relationship was observed for both deuruxolitinib and baricitinib (Fig. S6).
Two studies (n = 1,782) investigated achievement of SALT75 based on treatment with deuruxolitinib and baricitinib among patients with severe AA defined as a SALT score ≥ 50. Both treatments showed similar results compared with the placebo group (regarding deuruxolitinib: only 8 and 12 mg). However, treatment with deuruxolitinib 12 mg twice daily was superior compared with baricitinib (deuruxolitinib 12 mg: RD 34%, 95% CI 16–51 vs baricitinib 4 mg: RD 31%, 95% CI 25–37). A dose-response relationship was observed for both deuruxolitinib and baricitinib (Fig. S7).
Two studies (n = 1,782) investigated achievement of SALT90 based on treatment with deuruxolitinib and baricitinib among patients with severe AA defined as SALT score ≥ 50. Both treatments showed similar results compared with the placebo groups. Treatment with deuruxolitinib 12 mg twice daily was superior compared with baricitinib (deuruxolitinib 12 mg: RD 33%, 95% CI 17–49 vs baricitinib 4 mg: RD 22%, 95% CI 17–28). A dose-response relationship was observed both for deuruxolitinib and baricitinib (Fig. S8).
Safety of JAK inhibitors for alopecia
The reported adverse events are shown in Table SIII. In relation to oral tofacitinib, the most common events included upper respiratory tract infection, urinary tract infection, nasopharyngitis, herpes zoster infection, conjunctivitis, increase in alanine transaminase (ALT), aspartate aminotransferase (AST), cholesterol, triglycerides and decrease in haemoglobin and low white blood cell count. Cutaneous events included acne, acneiform eruptions and folliculitis (Table SIII).
Regarding topical tofacitinib, only 1 study reported application site irritation as an adverse events in 11% (n = 11) (46). Sublingual tofacitinib was not associated with any adverse events (25). In relation to deuruxolitinib, the most common adverse events included nasopharyngitis, upper respiratory tract infection, cough, headache, nausea, diarrhoea, acne, folliculitis and an increase in blood creatine phosphokinase reported (19). Adverse effects in relation to baricitinib included upper respiratory infection, nasopharyngitis, urinary infection, herpes simplex infection, herpes zoster infection, increase in blood creatine kinase, headache, nausea, and acne (20, 27). In a single study investigating treatment with topical delgocitinib, 10% (n = 2) reported folliculitis as an adverse event (21). The adverse events of ritlecitinib and brepocitinib were similar, including upper respiratory tract infection, nasopharyngitis, headache, diarrhoea, acne, and folliculitis (26, 22). In relation to oral ruxolitinib, upper respiratory tract infection, urinary tract infection, herpes zoster infection, leukopaenia, weight gain, fatigue, acne and folliculitis were reported (33, 37, 54). No adverse effects were reported regarding treatment with topical ruxolitinib (48).
DISCUSSION
The results of this systematic review and meta-analysis indicate that treatment with baricitinib, 4 mg once daily, and deuruxolitinib, 12 mg twice daily, appear to be superior to other treatments independent of the chosen definition of AA, i.e. ≥ 50% scalp hair loss or SALT score ≥ 50%. These definitions may present the same amount of hair loss. Conservatively, in the current study 2 separate meta-analyses were conducted according to outcome measures used. Baricitinib proved more effective, when AA was defined as ≥ 50% scalp hair loss and deuruxolitinib proved more effective, when AA was defined by a SALT score ≥ 50%. Therefore, it seems that the inclusion criteria regarding disease severity may influence interpretation of the results. The studies also show that demographic factors, such as age, sex, duration of AA, duration of treatment and concurrent treatment, may influence the treatment results (Table SI). Differences therefore exist between the groups. With respect to baricitinib, the majority of patients were female and older, with a longer duration of AA compared with the patients in the comparative studies. In the baricitinib study, the participants were allowed to use minoxidil as concurrent treatment, and were treated for 36 weeks, whereas ritlecitinib and brepocitinib studies did not allow any concurrent treatment and the treatment period was 20–24 weeks.
The deuruxolitinib study included more women, who were younger with a shorter duration of AA compared with the other studies. Regarding the study investigating baricitinib (AA as SALT score ≥ 50%), which was compared with deuruxolitinib, almost half of the patients were previously treated with immunosuppressant and other specific treatments regarding AA (Table SI). No information about previous treatment was provided in the study investigating deuruxolitinib.
Previous studies investigating JAK inhibitors in relation to AA have reported different factors influencing the therapeutic effect. A previous meta-analysis that investigated tofacitinib, baricitinib and ruxolitinib concluded that demographic factors, such as age, sex, previous failure of systemic therapy and duration of AA, did not appear to influence the treatment response (55). However, the study was based only on low-evidence non-randomized studies (55). In contrast, a study of 90 patients treated with oral tofacitinib found a negative correlation between duration of AA/AT/AU and the latest percentage change in SALT score, but also a higher improvement in SALT score change among patients with AA vs those with AT/AU subtypes (51). Another meta-analysis investigating oral tofacitinib in patients with AA, found that the dose and duration of treatment were factors influencing the therapeutic effect. Treatment with tofacitinib > 5 mg twice daily for more than 6 months had a higher pooled good/complete response rate compared with treatment with a lower dose twice daily for less than 6 months (56). In the current study, a dose-response relationship was observed similar to these findings regarding treatment with baricitinib and deuruxolitinib.
Two randomized clinical studies were not included in the current meta-analysis (33, 21). One randomized clinical study investigated the efficacy of oral ruxolitinib 20 mg twice daily vs oral tofacitinib 5 mg twice daily, but was not included due to different inclusion criteria of AA severity (33). Both drugs demonstrated remarkable hair regrowth, with a mean change in SALT score of 93.8% in the ruxolitinib group and 95.2% in the tofacitinib group (33). No statistical difference between the drugs was observed regarding hair regrowth at the end of the 6-month treatment. However, the ruxolitinib group showed a shorter duration for initial hair regrowth (33). Another randomized clinical study that was not included in the meta-analysis was the only study investigating topical treatment, with delgocitinib in 20 patients with AA ≥ 30% scalp involvement (21). Half of the patients treated (n = 10) demonstrated improvement in SALT score, ranging from 0.4% to 69.1%, with 20% (n = 2) achieving > 50% improvement in SALT score (21).
The most common adverse effects due to oral JAK inhibitors were upper respiratory tract infections, urinary infections, headache, laboratory abnormality, and acne. These side-effects are similar to those reported in previous reviews of JAK inhibitors in patients with alopecia (55, 56). Laboratory abnormalities included cytopaenias, lipid abnormalities and an increase in blood creatine phosphokinase. No cases of reactivation of tuberculosis or new malignancies were reported. JAK inhibitors (except deuruxolitinib) have been used in patients with rheumatoid arthritis (RA) for a longer time, providing useful data on the safety of treatment with JAK inhibitors in patients with RA. In addition, a network study investigating the safety of baricitinib, tofacitinib and some other JAK inhibitors used for RA in randomized clinical trials, reported that the occurrence of serious adverse events did not differ significantly between the JAK inhibitors (57). Regarding baricitinib as monotherapy, an increased risk of serious adverse effects, such as thrombotic events, was reported in patients with RA (58). Another study investigating 5,671 patients treated with tofacitinib as monotherapy reported that 107 (1.8%) developed malignancies with lung cancer (n = 24), lymphoma (n = 10), and gastric cancer (n = 6) being the most common (55, 59). Other studies have reported reactivation of tuberculosis in relation to baricitinib (55, 60) and tofacitinib (55, 61). Despite serious adverse effects being less reported in AA studies, it is important to highlight that data regarding the safety of JAK inhibitors in relation to AA is still in its infancy.
The strengths of this study include a meta-analysis regarding the efficacy of drugs based on high-quality evidence (randomized studies) and subgroup analysis based on identical outcome measures. Regarding limitations, the current findings are based on only a few randomized studies with small sample sizes and demographic differences. In addition, the subtypes of AA, as well as dosage and duration of treatment, varied between the studies, potentially affecting the findings.
In conclusion, these results suggest that patients with AA are likely to benefit from treatment with JAK inhibitors, especially deuruxolitinib 12 mg twice daily or baricitinib 4 mg once daily. JAK inhibitors are associated with mild adverse effects in relation to AA. However, more randomized clinical trials, using identical inclusion criteria and outcome parameters, are needed in order to draw further conclusions regarding treatment with JAK inhibitors in patients with AA.
Supplementary Material
ACKNOWLEDGEMENTS
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Conflicts of interest disclosures
MASH reported receiving grants from Leo Foundation. GBEJ reported receiving grants from AbbVie, LEO Foundation, Afyx, InflaRx, Janssen-Cilag, Novartis, UCB, CSL Behring, Regeneron, Sanofi, Boehringer Ingelheim, Union Therapeutics, and Toosonix and personal fees from Coloplast, Chemocentryx, LEO Pharma, Incyte, Kymera, and VielaBio. KSI has been part of advisory boards and received personal fees from Astra Zeneca, Leo Pharma; Sanofi Genzymes and Eli Lilly. MASH, GBEJ and KSI declare that none of the mentioned conflicts of interest had any influence to the content of this manuscript.
REFERENCES
- 1.Solimani F, Meier K, Ghoreschi K. Emerging topical and systemic JAK inhibitors in dermatology. Front Immunol 2019; 10: 2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uzuncakmak TK, Engin B, Serdaroglu S, Tuzun Y. Demographic and clinical features of 1,641 patients with alopecia areata, alopecia totalis, and alopecia universalis: a single-center retrospective study. Skin Appendage Disord 2021; 7: 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang S, Yang J, Liu JB, Wang HY, Yang Q, Gao M, et al. The genetic epidemiology of alopecia areata in China. Br J Dermatol 2004; 151: 16–23. [DOI] [PubMed] [Google Scholar]
- 4.Guzmán-Sánchez DA, Villanueva-Quintero GD, Alfaro Alfaro N, McMichael A. A clinical study of alopecia areata in Mexico. Int J Dermatol 2007; 46: 1308–1310. [DOI] [PubMed] [Google Scholar]
- 5.Mirzoyev SA, Schrum AG, Davis MDP, Torgerson RR. Lifetime incidence risk of alopecia areata estimated at 2.1% by Rochester Epidemiology Project, 1990–2009. J Invest Dermatol 2014; 134: 1141–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villasante Fricke AC, Miteva M. Epidemiology and burden of alopecia areata: a systematic review. Clin Cosmet Investig Dermatol 2015; 24: 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilhar A, Etzioni A, Paus R. Alopecia areata. N Engl J Med 2012; 366: 1515–1525. [DOI] [PubMed] [Google Scholar]
- 8.Johnson R, Roh E, Saavedra A, Wolff K. Fitzpatrick’s colour atlas and synopsis of clinical dermatology. 8th edn. New York: McGrawHill Edu; 2017. [Google Scholar]
- 9.Rodriguez TA, Fernandes KE, Dresser KL, Duvic M. National Alopecia Areata Registry. Concordance rate of alopecia areata in identical twins supports both genetic and environmental factors. J Am Acad Dermatol 2010; 62: 525–527. [DOI] [PubMed] [Google Scholar]
- 10.Alkhalifah A, Alsantali A, Wang E, McElwee KJ, Shapiro J. Alopecia areata update: part II. Treatment. J Am Acad Dermatol 2010; 62: 191–202. [DOI] [PubMed] [Google Scholar]
- 11.Trüeb RM, Dutra H, Dias MFRG. A comment on JAK inhibitors for treatment of alopecia areata. Int J Trichology 2018; 10: 193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strazzulla LC, Wang EHC, Avila L, Lo Sicco K, Brinster N, Christiano AM, et al. Alopecia areata: an appraisal of new treatment approaches and overview of current therapies. J Am Acad Dermatol 2018; 78: 15–24. [DOI] [PubMed] [Google Scholar]
- 13.Zheng C, Tosti A. Alopecia areata: new treatment options including Janus kinase inhibitors. Dermatol Clin 2021; 39: 407–415. [DOI] [PubMed] [Google Scholar]
- 14.Xing L, Dai Z, Jabbari A, Cerise JE, Higgins CA, Gong W, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med 2014; 20: 1043–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harel S, Higgins CA, Cerise JE, Dai Z, Chen JC, Clynes R, et al. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci Adv 2015; 1: e1500973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Triyangkulsri K, Suchonwanit P. Role of janus kinase inhibitors in the treatment of alopecia areata. Drug Des Devel Ther 2018; 27: 2323–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009; 151: W65–W94. [DOI] [PubMed] [Google Scholar]
- 18.Benton S, Farah R, Freese R, Hordinsky M. Tofacitinib as a pragmatic treatment choice for alopecia areata: a retrospective review. Dermatol Ther 2022; 35: e15310. [DOI] [PubMed] [Google Scholar]
- 19.King B, Mesinkovska N, Mirmirani P, Bruce S, Kempers S, Guttman-Yassky E, et al. Phase 2 randomized, dose-ranging trial of CTP-543, a selective Janus Kinase inhibitor, in moderate-to-severe alopecia areata. J Am Acad Dermatol 2022; 87: 306–313. [DOI] [PubMed] [Google Scholar]
- 20.King B, Ohyama M, Kwon O, Zlotogorski A, Ko J, Mesinkovska NA, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med 2022; 386: 1687–1699. [DOI] [PubMed] [Google Scholar]
- 21.Mikhaylov D, Glickman JW, Del Duca E, Nia J, Hashim P, Singer GK, et al. A phase 2a randomized vehicle-controlled multi-center study of the safety and efficacy of delgocitinib in subjects with moderate-to-severe alopecia areata. Arch Dermatol Res 2022; Mar 1. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Guttman-Yassky E, Pavel AB, Diaz A, Zhang N, Del Duca E, Estrada Y, et al. Ritlecitinib and brepocitinib demonstrate significant improvement in scalp alopecia areata biomarkers. J Allergy Clin Immunol 2022; 149: 1318–1328. [DOI] [PubMed] [Google Scholar]
- 23.Esteves M, Lopes S, Azevedo F, Pedrosa A. Effectiveness of oral tofacitinib dose tapering in a case of alopecia areata universalis. Skin Appendage Disord 2021; 7: 36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jerjen R, Meah N, Trindade de Carvalho L, Wall D, Eisman S, Sinclair R, et al. Treatment of alopecia areata in pre-adolescent children with oral tofacitinib: A retrospective study. Pediatr Dermatol 2021; 38: 103–108. [DOI] [PubMed] [Google Scholar]
- 25.Lai VWY, Bokhari L, Sinclair R. Sublingual tofacitinib for alopecia areata: a roll-over pilot clinical trial and analysis of pharmacokinetics. Int J Dermatol 2021; 60: 1135–1139. [DOI] [PubMed] [Google Scholar]
- 26.King B, Guttman-Yassky E, Peeva E, Banerjee A, Sinclair R, Pavel AB, et al. A phase 2a randomized, placebo-controlled study to evaluate the efficacy and safety of the oral Janus kinase inhibitors ritlecitinib and brepocitinib in alopecia areata: 24-week results. J Am Acad Dermatol 2021; 85: 379–387. [DOI] [PubMed] [Google Scholar]
- 27.King B, Ko J, Forman S, Ohyama M, Mesinkovska N, Yu G, et al. Efficacy and safety of the oral Janus kinase inhibitor baricitinib in the treatment of adults with alopecia areata: phase 2 results from a randomized controlled study. J Am Acad Dermatol 2021; 85: 847–853. [DOI] [PubMed] [Google Scholar]
- 28.Dincer Rota D, Emeksiz MAC, Erdogan FG, Yildirim D. Experience with oral tofacitinib in severe alopecia areata with different clinical responses. J Cosmet Dermatol 2021; 20: 3026–3033. [DOI] [PubMed] [Google Scholar]
- 29.Wambier CG, Craiglow BG, King BA. Combination tofacitinib and oral minoxidil treatment for severe alopecia areata. J Am Acad Dermatol 2021; 85: 743–745. [DOI] [PubMed] [Google Scholar]
- 30.Kerkemeyer KLS, John JM, Sinclair R, Bhoyrul B. Response of alopecia areata of the beard to oral tofacitinib. J Am Acad Dermatol 2020; 82: 1228–1230. [DOI] [PubMed] [Google Scholar]
- 31.Yale K, Pourang A, Plikus MV, Mesinkovska NA. At the crossroads of 2 alopecias: androgenetic alopecia pattern of hair regrowth in patients with alopecia areata treated with oral Janus kinase inhibitors. JAAD Case Rep 2020; 6: 444–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akdogan N, Ersoy-Evans S, Doğan S, Atakan N. Experience with oral tofacitinib in two adolescents and seven adults with alopecia areata. Dermatol Ther 2019; 32: e13118. [DOI] [PubMed] [Google Scholar]
- 33.Almutairi N, Nour TM, Hussain NH. Janus kinase inhibitors for the treatment of severe alopecia areata: an open-label comparative study. Dermatology 2019; 235: 130–136. [DOI] [PubMed] [Google Scholar]
- 34.Chen YY, Lin SY, Chen YC, Yang CC, Lan CE. Low-dose tofacitinib for treating patients with severe alopecia areata: an efficient and cost-saving regimen. Eur J Dermatol 2019; 29: 667–669. [DOI] [PubMed] [Google Scholar]
- 35.Craiglow BG, King BA. Tofacitinib for the treatment of alopecia areata in preadolescent children. J Am Acad Dermatol 2019; 80: 568–570. [DOI] [PubMed] [Google Scholar]
- 36.Dai YX, Chen CC. Tofacitinib therapy for children with severe alopecia areata. J Am Acad Dermatol 2019; 80: 1164–1166. [DOI] [PubMed] [Google Scholar]
- 37.Liu LY, King BA. Ruxolitinib for the treatment of severe alopecia areata. J Am Acad Dermatol 2019; 80: 566–568. [DOI] [PubMed] [Google Scholar]
- 38.Serdaroğlu S, Engin B, Çelik U, Erkan E, Aşkın Ö, Oba Ç, et al. Clinical experiences on alopecia areata treatment with tofacitinib: a study of 63 patients. Dermatol Ther 2019; 32: e12844. [DOI] [PubMed] [Google Scholar]
- 39.Shin JW, Huh CH, Kim MW, Lee JS, Kwon O, Cho S, et al. Comparison of the treatment outcome of oral tofacitinib with other conventional therapies in refractory alopecia totalis and universalis: a retrospective study. Acta Derm Venereol 2019; 99: 41–46. [DOI] [PubMed] [Google Scholar]
- 40.Shivanna CB, Shenoy C, Priya RA. Tofacitinib (selective janus kinase inhibitor 1 and 3): a promising therapy for the treatment of alopecia areata: a case report of six patients. Int J Trichology 2018; 10: 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng MW, Kehl A, Worswick S, Goh C. Successful treatment of severe alopecia areata with oral or topical tofacitinib. J Drugs Dermatol 2018; 17: 800–803. [PubMed] [Google Scholar]
- 42.Jabbari A, Sansaricq F, Cerise J, Chen JC, Bitterman A, Ulerio G, et al. An open-label pilot study to evaluate the efficacy of tofacitinib in moderate to severe patch-type alopecia areata, totalis, and universalis. J Invest Dermatol 2018; 138: 1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JS, Huh CH, Kwon O, Yoon HS, Cho S, Park HS. Nail involvement in patients with moderate-to-severe alopecia areata treated with oral tofacitinib. J Dermatolog Treat 2018; 29: 819–822. [DOI] [PubMed] [Google Scholar]
- 44.Liu LY, Craiglow BG, King BA. Tofacitinib 2% ointment, a topical Janus kinase inhibitor, for the treatment of alopecia areata: a pilot study of 10 patients. J Am Acad Dermatol 2018; 78: 403–404. [DOI] [PubMed] [Google Scholar]
- 45.Patel NU, Oussedik E, Grammenos A, Pichardo-Geisinger R. A Case report highlighting the effective treatment of alopecia universalis with tofacitinib in an adolescent and adult patient. J Cutan Med Surg 2018; 22: 439–442. [DOI] [PubMed] [Google Scholar]
- 46.Putterman E, Castelo-Soccio L. Topical 2% tofacitinib for children with alopecia areata, alopecia totalis, and alopecia universalis. J Am Acad Dermatol 2018; 78: 1207–1209. [DOI] [PubMed] [Google Scholar]
- 47.Castelo-Soccio L. Experience with oral tofacitinib in 8 adolescent patients with alopecia universalis. J Am Acad Dermatol 2017; 76: 754–755. [DOI] [PubMed] [Google Scholar]
- 48.Deeb M, Beach RA. A case of topical ruxolitinib treatment failure in alopecia areata. J Cutan Med Surg 2017; 21: 562–563. [DOI] [PubMed] [Google Scholar]
- 49.Erduran F, Adışen E, Aksakal AB. Excellent response to tofacitinib treatment in a patient with alopecia universalis. Acta Dermatovenerol Alp Pannonica Adriat 2017; 26: 47–49. [DOI] [PubMed] [Google Scholar]
- 50.Ibrahim O, Bayart CB, Hogan S, Piliang M, Bergfeld WF. Treatment of alopecia areata with tofacitinib. JAMA Dermatol 2017; 153: 600–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu LY, Craiglow BG, Dai F, King BA. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol 2017; 76: 22–28. [DOI] [PubMed] [Google Scholar]
- 52.Park HS, Kim MW, Lee JS, Yoon HS, Huh CH, Kwon O, et al. Oral tofacitinib monotherapy in Korean patients with refractory moderate-to-severe alopecia areata: a case series. J Am Acad Dermatol 2017; 77: 978–980. [DOI] [PubMed] [Google Scholar]
- 53.Kennedy Crispin M, Ko JM, Craiglow BG, Li S, Shankar G, Urban JR, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata JCI Insight 2016; 1: e89776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mackay-Wiggan J, Jabbari A, Nguyen N, Cerise JE, Clark C, Ulerio G, et al. Oral ruxolitinib induces hair regrowth in patients with moderate-to-severe alopecia areata. JCI Insight 2016; 1: e89790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Phan K, Sebaratnam DF. JAK inhibitors for alopecia areata: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol 2019; 33: 850–856. [DOI] [PubMed] [Google Scholar]
- 56.Guo L, Feng S, Sun B, Jiang X, Liu Y. Benefit and risk profile of tofacitinib for the treatment of alopecia areata: a systemic review and meta-analysis. J Eur Acad Dermatol Venereol 2020; 34: 192–201. [DOI] [PubMed] [Google Scholar]
- 57.Ho Lee Y, Gyu Song G. Comparative efficacy and safety of tofacitinib, baricitinib, upadacitinib, filgotinib and peficitinib as monotherapy for active rheumatoid arthritis. J Clin Pharm Ther 2020; 45: 674–681. [DOI] [PubMed] [Google Scholar]
- 58.Guidelli GM, Viapiana O, Luciano N, De Santis M, Boffini N, Quartuccio L, et al. Efficacy and safety of baricitinib in 446 patients with rheumatoid arthritis: a real-life multicentre study. Clin Exp Rheumatol 2021; 39: 868–873. [PubMed] [Google Scholar]
- 59.Curtis JR, Lee EB, Kaplan IV, Kwok K, Geier J, Benda B, et al. Tofacitinib, an oral Janus kinase inhibitor: analysis of malignancies across the rheumatoid arthritis clinical development programme. Ann Rheum Dis 2016; 75: 831–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Genovese MC, Kremer JM, Zamani O, Ludivico C, Krogulec M, Xie L, et al. Baricitinib, an oral Janus Kinase (JAK)1/JAK2 inhibitor, in patients with active rheumatoid arthritis (RA) and an inadequate response to TNF inhibitors: results of the phase 3 RA-beacon study. Ann Rheum Dis 2015; 74: 753–776. [Google Scholar]
- 61.Winthrop KL, Park SH, Gul A, Cardiel MH, Gomez-Reino JJ, Tanaka Y, et al. Tuberculosis and other opportunistic infections in tofacitinib-treated patients with rheumatoid arthritis. Ann Rheum Dis 2016; 75: 1133–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


