Abstract
Developmental periods such as gestation and adolescence have enhanced plasticity leaving the brain vulnerable to harmful effects from nicotine use. Proper brain maturation and circuit organization is critical for normal physiological and behavioral outcomes. Although cigarette smoking has declined in popularity, noncombustible nicotine products are readily used. The misperceived safety of these alternatives lead to widespread use among vulnerable populations such as pregnant women and adolescents. Nicotine exposure during these sensitive developmental windows is detrimental to cardiorespiratory function, learning and memory, executive function, and reward related circuitry. In this review, we will discuss clinical and preclinical evidence of the adverse alterations in the brain and behavior following nicotine exposure. Time-dependent nicotine-induced changes in reward related brain regions and drug reward behaviors will be discussed and highlight unique sensitivities within a developmental period. We will also review long lasting effects of developmental exposure persisting into adulthood, along with permanent epigenetic changes in the genome which can be passed to future generations. Taken together, it is critical to evaluate the consequences of nicotine exposure during these vulnerable developmental windows due to its direct impact on cognition, potential trajectories for other substance use, and implicated mechanisms for the neurobiology of substance use disorders.
Keywords: Adolescence, E-cigarettes, Epigenetics, Prenatal, Tobacco, Transgenerational transmission
1. Introduction
Nicotine, the primary psychoactive component of tobacco, activates nicotinic acetylcholine receptors (nAChRs), pentameric ligand-gated ion channels which are widely distributed throughout the brain [1,2]. These receptors appear early in development and are functionally active in the fetal brain to mediate many critical aspects of brain maturation [1–4]. Nicotine exposure during critical developmental periods disrupts neural development, and often has long-lasting effects on associated behaviors [3–7]. In this review, we aim to examine the brain regions highly impacted by nicotine exposure at vulnerable developmental stages. We will discuss implications from human studies of neurobiology and behavior following nicotine exposure. Animal studies will further elucidate specific modifications in brain regions and neural networks.
2. Prenatal nicotine exposure
A true evaluation of nicotine exposure during pregnancy is difficult to attain as the parameters of exposure vary widely. In the United States, self-reports of smoking during pregnancy in 2020 found 5.5 % of all births were to mothers who smoked at some point during the pregnancy with disparities noted by urbanization levels [8]. Extent of exposure is further complicated by use of noncombustible nicotine delivery systems such as e-cigarettes and nicotine replacement therapies (NRT), which are misperceived as “safe” alternatives. The marketed safety of e-cigarettes contributes to their use during pregnancy and is used at rates similar to standard combustible cigarettes [9]. Widespread use of either noncombustible delivery systems is of great concern since these are not completely safe alternatives and result in detrimental health outcomes in the mother and children [9,10].
Although non-nicotine tobacco smoke constituents have been reported to impact fetal brain development [11], nicotine alone is likely the major contributor to the harmful effects of maternal tobacco use on their offspring. Animal studies have shown that nicotine readily crosses the placental barrier to interact with receptors widely distributed throughout the fetus and richly expressed in the fetal brain [12,13]. These nAChRs are functional [14] and are upregulated by maternal nicotine intake [15]. The brain develops and organizes in an “inside out” pattern in which deep neural layers are formed before the outer superficial cortex, a process that is conserved between humans and rodents. Thus, the proper development of the brain and subsequent behavior is vulnerable to nicotine’s effects, as is described below (see Fig. 1).
Fig. 1.
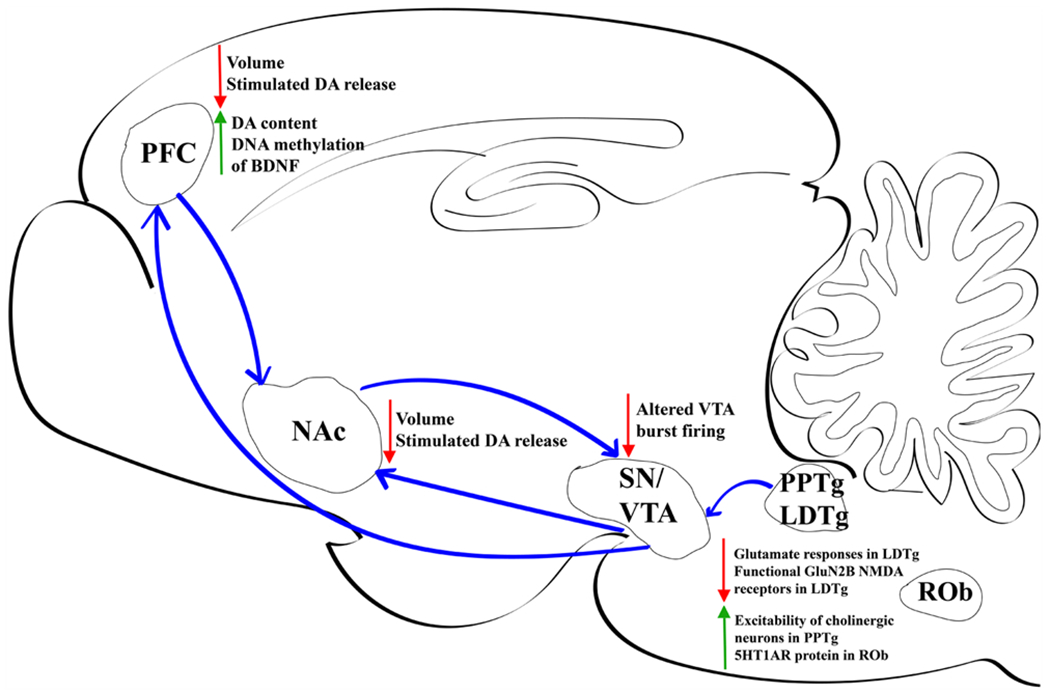
Major nicotine-induced alterations within rodent brain during prenatal period. PFC, prefrontal cortex. NAc, nucleus accumbens. SN, substantia nigra. VTA, ventral tegmental area. PPTg, pedunculopontine nucleus. LDTg, laterodorsal tegmental. ROb, raphe obscurus. DA, dopamine.
2.1. Insights from human studies
Maternal smoking during pregnancy is associated with lower birth weight, preterm birth, and smaller frontal lobe and cerebellar volumes in the brains of human infants [16]. Reduced brain volume, cerebral gray and white matter, and gyrification persists in children (aged 10) exposed to nicotine by maternal smoking throughout pregnancy when compared to non-exposed children [17]. Increased behavioral and emotional problems in prenatally exposed children (age 6–8) have been associated with decreased volumes of the caudate and nucleus accumbens (NAc) and thinning of the superior frontal and parietal cortices [18]. Prenatal nicotine exposure, reported as smoking more than one cigarette per day during the second trimester of pregnancy, is associated with orbitofrontal cortex thinning correlating to enhanced likelihood of drug experimentation during adolescence [19,20]. This effect has been linked to higher DNA methylation of brain-derived neurotrophic factor-6 exon, leading to overall long-term downregulated expression [19,21]. Prenatal exposure to maternal smoking also increased likelihood of smoking, number of drugs tried, and increased striatal volume, including caudate nucleus, putamen and NAc, in humans with a single nucleotide polymorphism within an untranslated region of a gene coding for the alpha6 nAChR subunit [22]. Behavioral problems, particularly attention deficit hyperactivity disorder (ADHD), are significantly increased in children of self-reported pregnant smokers or environmental tobacco smoke exposure [23,24]. Hyperactivity, impulsivity and oppositional behaviors in children whose mothers smoked were only seen in those with a specific dopamine (DA) transporter polymorphism [25]. Prenatal nicotine exposure, through smoking one pack of cigarettes or less, also induces impairments of language tasks in offspring, with a specific involvement of the DA D2 receptor gene [26]. Functional MRI studies have shown reduced responses to reward in the striatum of adolescents who were exposed prenatally to nicotine through maternal smoking of at least one cigarette a day, consistent with vulnerability of this reward circuit for substance dependence [27].
2.2. Hindbrain and midbrain
The hindbrain, consisting of the pons and medulla develops early in gestation and is susceptible to the harmful effects of prenatal nicotine exposure. Maternal smoking is a major cause of sudden infant death syndrome (SIDS) which has often been attributed to dysfunction in the brainstem [28–30]. The pons is a vital region of the brainstem that controls unconscious processes such as sleep and respiration [31]. Within the pons, the pedunculopontine tegmental nucleus (PPTg) is critical for response to arousal, sleep, attention and motivational stimuli. The PPTg has been heavily associated with SIDS due to its involvement in REM sleep and control of respiration and exhibits an overall excitatory effect of prenatal nicotine exposure [32,33]. The offspring of pregnant rat dams exposed to cigarette smoke from embryonic (E) day 14 until birth exhibit changes in the cholinergic neurons of this nucleus, rendering them more excitable [34]. Human studies evaluating brain tissue following SIDS in infants exposed to nicotine through maternal smoking have shown decreased nAChR binding in the nucleus pontis oralis (PoO), a critical component of the cholinergic ascending arousal system [35]. Altered nAChR binding was also seen in three rostral medullary sites containing serotonin (5-HT) neurons which have previously been shown to have abnormal indices of serotonin neurotransmission in SIDS infants [36]. Animal studies support an impact of prenatal nicotine exposure on serotonergic function. Prenatal nicotine exposure from the start of pregnancy in Rhesus macaque monkeys causes significant serotonergic hyperinnervation of brainstem, a hallmark of SIDS, that was reversed with Vitamin C cotreatment [37]. Prenatal/perinatal nicotine exposure via osmotic minipump in mice from E7-P8 results in decreased firing activity of raphe obscurus (ROb) neurons in neonates and increased expression of serotonin autoreceptors (5HT1ARs) [38]. In vitro culture of medullary raphé neurons demonstrates reduced chemosensitivity of 5-HT neurons and blunted nicotine-induced excitatory activity following perinatal (E7-P8) nicotine exposure [39]. The strength of these effects diminishes as neurons mature and may reflect features underlying SIDS. Thus, nAChRs appear to regulate brainstem 5-HT systems during a critical prenatal period, with resulting deficits in cardiorespiratory function following aberrant nicotine exposure.
Prenatal nicotine has also been shown to critically modulate dopamine (DA) function. Both the PPTg and laterodorsal tegmental (LDTg) nuclei send cholinergic projections to the midbrain ventral tegmental area (VTA), where they activate nAChRs on both DA and non-DA neurons [40]. Through this pathway, and direct projections from the LDTg to NAc [41], activation of the LDTg can stimulate DA release. Modulation of DA release through VTA-NAc terminals has been implicated in reward related behaviors and nicotine reinforcement [42]. As with other pontine nuclei, the LDTg has emerged as a highly plastic region susceptible to functional alterations during development [43]. Electrophysiological analysis of in vitro brain slices has shown that oral nicotine (300 μg/ml) exposure throughout gestation reduces nicotine-induced intracellular calcium and AMPA-mediated glutamate responses in LDTg neurons [44,45]. Further work has highlighted cell-type specific alterations in NMDA receptor-mediated signaling and function in LDTg neurons [46]. Specifically, prenatal nicotine exposure decreases functional GluN2B-containing synaptic NMDA receptors in cholinergic LDTg neurons, with a concurrent increased function in smaller, putative GABA neurons. Thus, prenatal nicotine exposure may result in reduced excitatory cholinergic modulatory tone in target brain regions, which may have important implications for downstream functions such as DA release and behaviors such as motivation, cognition and nicotine reinforcement. Indeed, we know prenatal nicotine exposure through osmotic minipump (3 mg/kg/day) in rats increases adolescent DA levels in the prefrontal cortex (PFC), striatal D3 receptor binding, and D2 receptor functional coupling in ventral striatum and pallidum [47]. Prenatal nicotine exposure throughout gestation (6 mg/kg/day) also alters burst firing of DA neurons [48], increases heterogeneity and complexity of neurons in subregions of the VTA [49,50], and decreases nicotine-induced striatal and cortical DA release [51,52]. DA-linked behavioral alterations include cocaine-induced locomotion in a sex-specific manner in adolescents [47], as well as cocaine self-administration [53]. All these alterations in signaling impact output to target regions and present possible mechanisms underlying enhanced risk for development of drug dependence later in life.
3. Perinatal/early postnatal nicotine exposure
Smokers often try to quit shortly after becoming aware of their pregnancy [54,55], however tend to relapse by the third trimester or will often start again after birth [56,57]. Pregnant women are often prescribed noncombustible products and other nicotine replacement therapies such as transdermal nicotine patches and oral smokeless products (snus). Given that nicotine readily crosses the placenta and these products have shown detectable levels of nicotine in breast milk [58,59], the marketed safety of these products is of great concern. Although some argue e-cigarettes are overall safer than combustible cigarettes [60], exposure to nicotine at these vulnerable time points is still harmful for the mother and child, possibly leading to very similar health outcomes as traditional smoking [61,62]. Rodent models provide a reasonable analog to human development to further evaluate neurobiological effects of nicotine exposure at this vulnerable period. Rodents are born underdeveloped and complete neural development postnatally [63]. In rats, the initial 12 postnatal days parallel the third human trimester, marked by extensive neurogenesis, synaptogenesis, and circuit maturation [63–67]. Maturation of sensory circuits, thalamic synaptic contacts and formation are critical milestones during this developmental period vulnerable to disruption by nicotine exposure (see Fig. 2). Thus, the first two weeks of postnatal development in rodents serve as good models for the impact of nicotine on third trimester human gestation. Although maternal nicotine use during pregnancy can result in neurological risks to growth and development, there is increasing evidence that postnatal exposure to environmental tobacco smoke may also be neurotoxic [68]. Several studies have shown a relationship between childhood smoke exposure and ADHD and oppositional behavior [69–72]. Thus, even though humans are born more mature than rodents, they are still at risk for tobacco and nicotine exposure after birth.
Fig. 2.
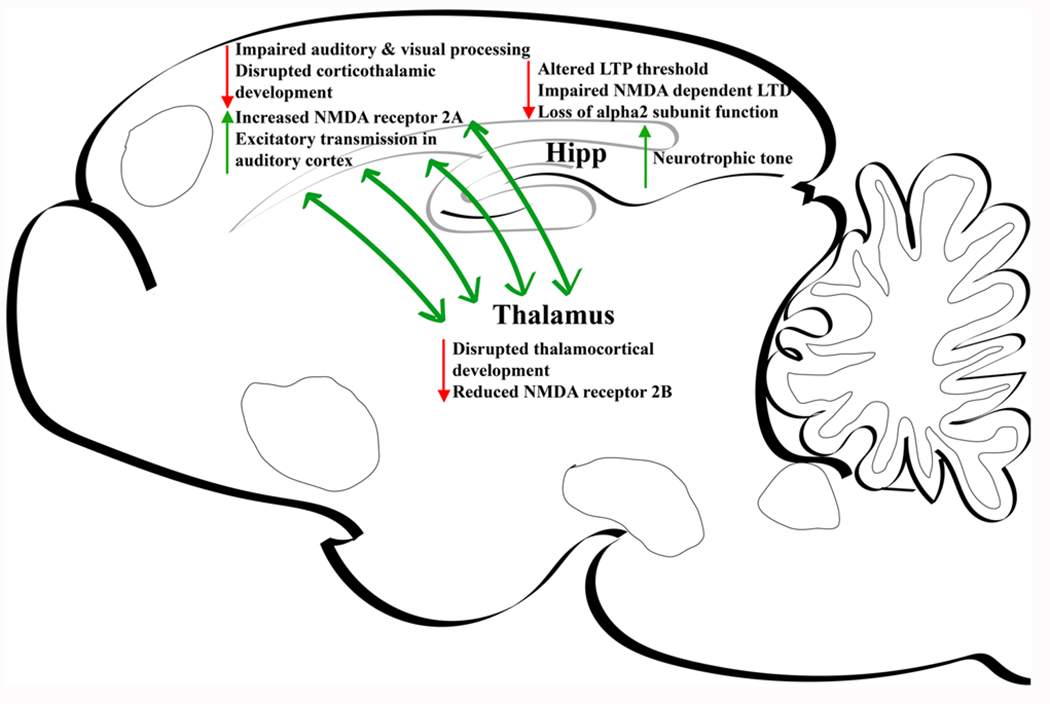
Major nicotine-induced alterations within rodent during postnatal/perinatal period. Hipp, hippocampus.
3.1. Hippocampus
The hippocampus, a crucial region for memory and learning, is heavily impacted by the neurotoxic effects of nicotine, resulting in persistent molecular changes and impairments [73]. Whereas significant hippocampal development occurs during the third trimester of pregnancy in humans, the equivalent developmental period in rodents is the first two postnatal weeks [74]. During the early postnatal period in rodents, the hippocampal cholinergic system undergoes a transient upregulation of nAChRs which regulate critical developmental events [75–77]. During early postnatal life, α7 * and β2 * nAChRs modulate GABAergic and glutamatergic transmission that underlie nicotine-elicited changes in network synchronization [78]. Immature hippocampus is characterized by giant depolarizing potentials (GDPs) that result from synergistic interaction of glutamate and GABA neurons, the latter being excitatory at this developmental stage due to an immature chloride gradient [4,79]. During early postnatal development nicotine increases GDPs via activation of GABA neurons [80]. Furthermore, both nicotine and endogenous acetylcholine increase synaptic efficacy in early postnatal glutamatergic synapses [81]. Later, α7 nAChRs have been shown to control the timing of GABAergic conversion from excitatory to inhibitory signaling which normally occurs during the second postnatal week [82]. Chronic early neonatal nicotine exposure in rats increases neurotrophic tone during this critical period of hippocampal development [83] and alters hippocampal morphology [84]. Mouse pups exposed to nicotine during the first two postnatal weeks also show substantial impairment of long-term hippocampal dependent spatial memory during adolescence [85]. This memory impairment may result from altered nicotinic modulation of long-term potentiation (LTP), with a loss of normal α2*nAChR function, and impaired NMDA receptor-dependent long-term depression (LTD) [85–87].
3.2. Sensory cortices
Sensory systems in rodents exhibit a critical period of early postnatal development when topographical projections from thalamus to cortex are refined by inputs from the external environment [88,89]. There is a transient appearance of α7 nAChRs in sensory cortices and thalamus that correspond to the timing of this critical period [90–92]. Cortical α7 nAChR expression is regulated by activity in these sensory pathways during this developmental window [93,94]. These α7 nAChRs are functionally active during initial stages of thalamocortical circuit formation and enhance the NMDA receptor-mediated component of excitatory postsynaptic potentials [95]. Manipulation of these nAChRs with chronic nicotine exposure during the second, but not the first or fourth, postnatal weeks disrupts synaptic development in the auditory cortex by altering NMDA receptor-mediated synaptic signaling [96]. Furthermore, chronic nicotine treatment from postnatal days 8–12 increases cortical NMDA receptor 2 A mRNA levels and reduces thalamic NMDA receptor 2B mRNA levels for up to 2 weeks, consistent with cortical glutamate hyperstimulation [97]. This brief chronic neonatal nicotine treatment eliminates normal nAChR signaling in adult auditory cortex and impairs performance on an auditory cue avoidance task without impacting basic auditory or motor functions [98]. nAChRs other than α7 have also been shown to regulate sensory developmental processes [99–101]. In this case, descending corticothalamic projections from layer VI cortical neurons, that critically regulate attention, have peak currents in the first postnatal month that are mediated by α5- and β2-containing heteromeric nAChRs [99]. Chronic postnatal nicotine treatment or elimination of β2 nAChR subunits impair performance on adult passive avoidance behavior and auditory discrimination tasks [100,101]. Taken together, these studies show that nicotine treatment during a critical period of sensory cortex development impairs cognitive tasks that use sensory cues, without impacting normal sensory function. These findings are consistent with clinical studies which have shown that children whose mothers smoked while pregnant are more likely to show long-term impairments in auditory processing tasks [102]. There are sex differences in the sensitivity of different sensory processes to developmental tobacco exposure, with adolescent females showing impairment in tasks requiring somatosensory or visual processing while males showing greater impairment in auditory tasks [103]. Possible sex differences in the impact of developmental nicotine on subsequent sensory cognitive processes have not been carefully evaluated in animal studies.
4. Adolescent nicotine exposure
Adolescence is a vulnerable developmental period marked by heightened neuroplasticity that is susceptible to drug-induced alterations. Exploratory drug use, of nicotine in particular, is standard behavior in adolescence despite known long term detrimental consequences to actively maturing brain regions and pathways [6,104,105]. Most nicotine users initiate smoking during adolescence and establish daily use before the age of 18 [106–108]. Recent national surveys have demonstrated a large increase in the use of electronic cigarettes (e-cigarettes) among school age students and a concurrent decline in combustible cigarette use [109–111]. In 2021 alone, an estimated 5.22 million high school students and 1.34 million middle school students reported ever using a tobacco product [112]. Teen users of both combustible cigarettes and e-cigarettes show signs of dependence and difficulty quitting [113–115]. Nicotine exposure during this highly plastic state induces long term changes in critical circuitry, maturation of monoamine systems, and behavior, including reward related behaviors (see Fig. 3) [4,6,7].
Fig. 3.
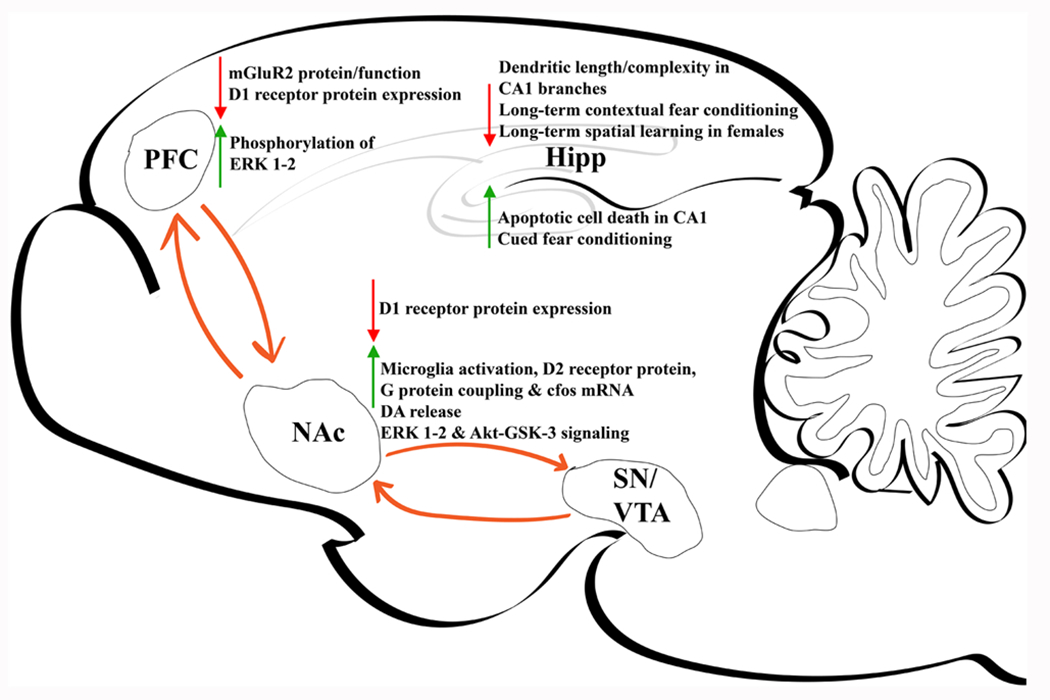
Major nicotine-induced alterations within rodent brain during adolescent period. PFC, prefrontal cortex. NAc, nucleus accumbens. SN, substantia nigra. VTA, ventral tegmental area. DA, dopamine.
4.1. Prefrontal cortex
The development of the PFC is critically important for the maturation of executive control, emotional regulation, and cognitive flexibility [116]. Increasing cognitive capacity during adolescence coincides with a decrease in cortical grey matter thickness, which results from experience-dependent strengthening of active synapses and concomitant loss of remaining connections [117–119]. As the brain matures in later adolescence there is decreased connectivity between subcortical regions and increased top-down regulatory control by the medial PFC, which results in diminished reactivity to emotional cues [120]. In 2008 deBry and Tiffany [121] proposed a Tobacco-Induced Neurotoxicity of Adolescent Cognitive Development (TINACD) theory that smoking during early adolescence leads to increased impulsivity and inattention resulting from changes in PFC function. Support for this theory has since been provided by human studies that show that early onset smoking contributes to long-lasting decreases in task-related attention and inhibitory control behaviors [122], as well as prefrontal attentional network function [123]. Consistent with the negative impact of adolescent nicotine on cognitive performance, recent clinical studies [124,125], including a longitudinal one [126], have shown that teen e-cigarette use results in lower academic achievement. There are no significant differences between the impact of using e-cigarettes and combustible cigarettes on school performance, suggesting an important role for nicotine.
Consistent with these clinical findings, cognitive performance on PFC-dependent tasks in animals is negatively influenced by adolescent nicotine exposure [127]. Nicotine treatment of adolescent rats, but not adults, results in increased impulsive behavior and decreased attention in adulthood [116]. Lasting synaptic changes underlie the attention deficits caused by nicotine exposure. In adolescent rats, nicotine exposure over ten consecutive days (P34–43) decreases inhibitory presynaptic mGluR2 protein expression and function, leading to diminished synaptic plasticity of the PFC and inability to filter out irrelevant stimuli [128]. Neuronal hyperactivity and decreases in D1 receptor expression, as well as increased phosphorylation of ERK 1–2 within the PFC in response to adolescent (P35–44) nicotine exposure has also been linked to mood and anxiety-like phenotypes in rats [129]. This is consistent with clinical observations of a bidirectional association of depression with teen smoking and vaping [130,131].
4.2. Nucleus accumbens
The NAc is a critical region for reward and reinforcement, rich in dopaminergic VTA terminals and a major site of nicotine-induced DA release. In vivo microdialysis has shown that subcutaneous nicotine administration across two weeks induces increased DA release in the NAc core and shell of adolescent male rats (P35–48) when compared to adults [132]. Elevated DA release specific to adolescence highlights a role of NAc in altered reward behavior. Adolescent rodents are more sensitive than adults to the acute rewarding effects of nicotine, as shown by conditioned place preference [133–138], with some studies demonstrating reward after a single pairing of drug and context [139,140]. Many studies have also shown that adolescent rats readily acquire intravenous nicotine self-administration and have higher drug intake than adults [137,141–144]. Clinical studies have shown that nicotine exposure during adolescence, but not adulthood, is associated with subsequent increases in substance use (i.e., “gateway hypothesis”) [6,7,105,145]. Brief pretreatment of early adolescent rats (postnatal days (P) 28–31) with a low dose of nicotine that models smoking initiation significantly increases self-administration of cocaine, ethanol, fentanyl, and methamphetamine when compared to adults [146–151]. This nicotine pretreatment increases microglial markers, D2 receptor protein and G protein coupling, and cfos mRNA expression in adolescent, but not adult, NAc [146,149]. NAc D2 receptors and CX3CL1 are a mechanistic interface for nicotine-induced microglial activation with resulting pruning of glutamate synapses and enhanced cocaine reinforcement in adolescents [149]. These studies highlight a novel glia driven mechanism in adolescent drug exposure which may be further investigated in additional nicotine use behaviors.
In addition to being an important hub for reward processing, the NAc shell is critically involved in regulating emotional processing [152,153]. As with the PFC, nicotine exposure during adolescence (P35–44) upregulates ERK 1/2 and Akt-GSK-3 signaling pathways within the NAc and downregulates D1R expression into adulthood [154]. These changes are accompanied by alterations in neuronal firing patterns within the NAc, and anxiety and depressive-like behavioral abnormalities. The ERK 1/2 pathway is critical for cellular processes such as proliferation, differentiation, and survival. It has also been identified as an important regulatory mechanism for signaling specificity [155]. DA signaling at D1 receptors is a specific neurochemical mechanism necessary for induction of persistent dendrite remodeling in adolescence and adulthood [116]. It should be noted that D1 receptor alterations are not observed in the NAc when nicotine is given earlier in adolescence but, rather, changes in D2 receptors are observed [149]. Thus, even within the period of adolescence there are differences in nicotine-induced changes in DA function. This is consistent with findings from animal studies of a unique sensitivity of the early adolescent period to nicotine effects on reward pathways as compared to late adolescence and adulthood [139,146,156]. This also provides a biological basis for the clinical observation that the age of first cigarette use is a critical determinant of tobacco dependence, with those who begin in their early teens having the greatest difficulty quitting [157,158].
4.3. Hippocampus
The hippocampus is not fully mature by adolescence and nicotine continues to produce unique, age-dependent effects. Whereas acute nicotine treatment promotes CA3–CA1 synaptic potentiation in adult mice, it does not in adolescents [159]. Acute nicotine is also less effective in adolescents than adults at enhancing context pre-exposure facilitation, a hippocampal-dependent cognitive task [160].
Chronic nicotine treatment of mice for the first two weeks of adolescence increases aptotic cell death in the hippocampal molecular layer and CA1 immediately following drug treatment [161]. Lasting morphological changes resulting from chronic nicotine treatment during adolescence are also seen in adult mice, with reduced dendritic length and complexity in apical CA1 branches [162]. Chronic nicotine exposure in adolescent rats enhances cued fear conditioning in adulthood, while equivalent exposure in adulthood does not impact task performance [163]. In contrast, chronic adolescent nicotine exposure impairs performance on two different tests of long-term contextual fear conditioning in mice [162,164], and long-term spatial learning in female rats only [165]. Sex differences in nicotine-induced changes are critical to consider given that puberty and gonadal maturation also impact hippocampal function [166]. The structure, cell composition, and function of the hippocampus mature differentially by sex after puberty [167,168]. Taken together, hippocampal dependent learning and memory is uniquely susceptible to the detrimental effects of nicotine that persist into adulthood. The differential effects of nicotine-induced learning and behavior during adolescence may be influenced by sex and should be a major consideration for further investigation.
5. Epigenetics and intergenerational transmission
It is critical to consider drug-induced epigenetic modifications during these vulnerable developmental periods. Epigenetic modifications describe structural or chemical changes to the genome that typically result in altered functional expression of genes [169]. Epigenetic changes can occur in response to a wide range of stimuli, including exposure to drugs of abuse, particularly nicotine [170–173]. These alterations can occur either after direct exposure, or be multigenerational in which germ cells were exposed, or transgenerational in which there is no direct exposure of the offspring [174]. Multigenerational and transgenerational inheritance suggests offspring of smokers may have altered brain and behavioral responses or predispositions before direct exposure to drugs themselves.
The prenatal period has marked epigenomic plasticity relevant for human brain development [175,176]. Meta-analysis of blood from human newborn infants born of mothers who smoked during pregnancy found differentially methylated CpGs associated with gene expression in pathways and processes critical to development [177,178]. Smoking-related CpG sites may play a more profound role in neurodevelopment and drive alterations from prenatal nicotine exposure. Alterations in DNA methylation patterns in response to prenatal nicotine through maternal smoking during pregnancy exist at birth and can persist into adolescence [179,180]. Of note, maternal smoking-related methylation sites in human adolescents have been associated with schizophrenia in adolescence and adulthood [179]. Indeed, altered DNA methylation patterns have been observed in human fetal cortex following in utero exposure and are implicated in reduced neuronal count independent of cell apoptotic processes [180]. Clinical studies suggest that prenatal nicotine exposure may have major developmental consequences across multiple offspring generations, as shown by studies in which grandmaternal smoking during their pregnancy increases the likelihood of early childhood asthma or a diagnosis of autism in their grandchildren [181,182].
Preclinical studies have also shown nicotine-induced alterations that persist across multiple generations of offspring. Mice offspring exposed to nicotine during gestation demonstrate increased DNA methylation patterns in the lungs and downregulation of mesenchymal peroxisome proliferator-activated receptor-γ (PPARγ) persisting into the third generation with parallel asthma-like phenotypes [183–185]. Germ cells are also impacted following perinatal nicotine exposure, with DNA methylation remodeled in offspring spermatozoa [186]. Mice exposed to nicotine during the perinatal period exhibit multiple neurochemical and behavioral alterations across F1 and F2 generations [187–189]. These include increased oral nicotine consumption, hyperactivity and risk-taking behaviors, altered corticostriatal nAChR and DA transporter function and methylome deficits, as well as BDNF functional deficits and hypothalamic-pituitary-adrenal axis dysregulation. These changes result from differential impacts on discrete epigenetic factors in a brain region-selective fashion and are modulated by the Chrna5 D397N polymorphism in adolescent mice [190].
Adolescent nicotine exposure also elicits epigenetic alterations that impact future behaviors. Nicotine exposure across 35 days during adolescence of rats (P25–59) of both sexes leads to learning and cognitive deficits in their offspring [191]. In another study of adolescent mice, chronic nicotine exposure via osmotic minipump and stress led to reduced nicotine sensitization in further generations, persisting into the third generation in female mice [171]. Evidence of indirect “transgenerational” transmission resulting from adolescent nicotine exposure is critical because this has major implications for neural vulnerabilities that would be present as early as conception. Recent efforts focused on maternal or paternal exposure to nicotine prior to conception have also shown that epigenetic changes resulting from nicotine exposure can occur beyond the perinatal period [192–194]. These findings indicate permanent epigenetic alterations induced by nicotine exposure and posit critical implications for future investigation of altered neurochemistry and behaviors.
Nicotine-induced epigenetic alterations persisting across multiple generations challenges the classic notion of a genetic “reset” during reproduction. Collective genetic and behavioral changes in offspring with and without direct exposure to nicotine (see Fig. 4) highlights novel developmental vulnerabilities. Thus, it is important to assess all critical windows of exposure for therapeutic targets and interventions.
Fig. 4.
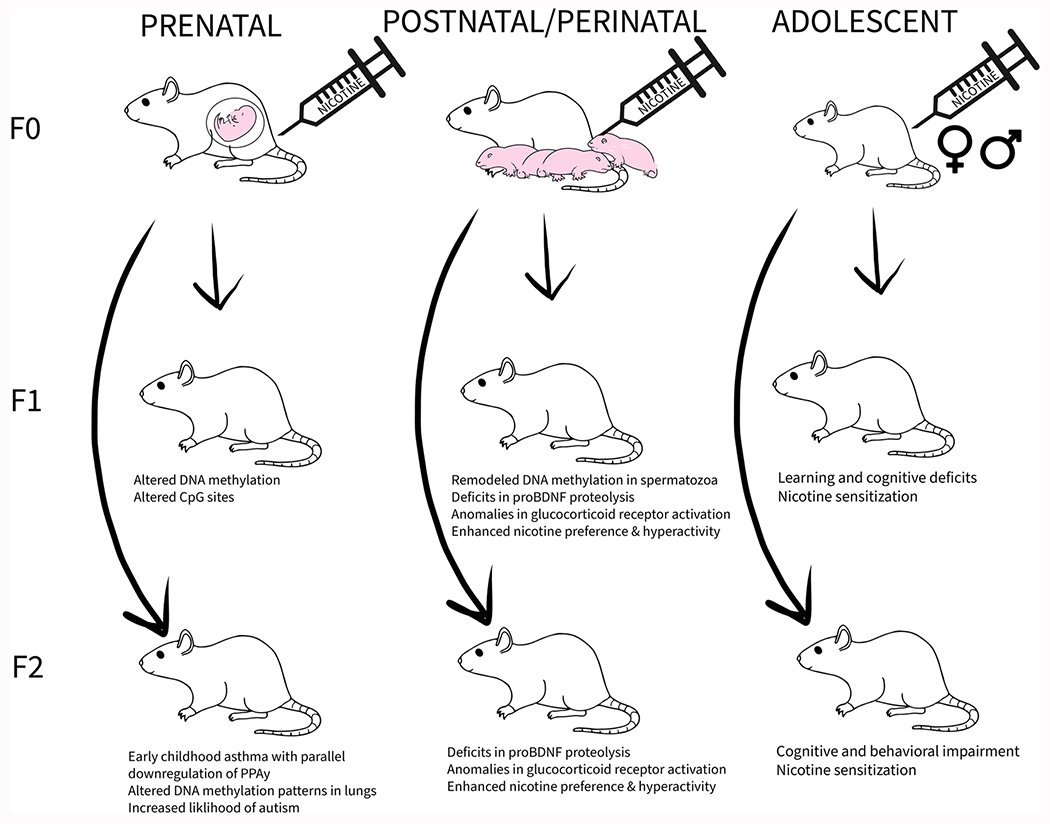
Multigenerational and transgenerational transmission of nicotine-induced effects. This diagram illustrates multigenerational transmission of effects following direct prenatal and perinatal nicotine exposure in F0 mothers. Transgenerational transmission effects, resulting without direct exposure to nicotine, are described in F2 generation from prenatal and perinatal exposure. Adolescent nicotine exposure in animals from both sexes elicits transgenerational effects in F1 and F2 offspring.
6. Conclusions
In this review, we present evidence from human and animal studies showing brain regions that are impacted by developmental nicotine exposure (see Table 1). Our review is not fully comprehensive since some areas involved in addiction, such as the medial habenula, have not been fully studied across developmental timepoints. There is some indication, however, that this area that mediates the aversive effects of nicotine responds differently in adolescents than adults [195]. Thus, there is more work to be done. However, the available data show that developmental periods of regional heightened plasticity are vulnerable to the harmful effects of nicotine [105,196,197]. Despite marketed safety of noncombustible products and e-cigarettes, exposure to nicotine alone can drive these negative effects on the brain and behavior. We know initial exposures have unique and persistent effects into adulthood [3,4,6,7,105,145]. Interestingly, a compounding effect of exposure can arise in which prenatal nicotine-induced alterations can further exacerbate effects of adolescent exposure with long term implications in reward related functions and behaviors [19–22, 103, 198]. Further, nicotine-induced epigenetic changes and transgenerational transmission highlight permanent changes that are critical to evaluate in future studies. Permanent and compounding effects of nicotine create a cycle leading to susceptibility for nicotine use and the dangerous potential for use of other controlled substances due to altered reward circuitry. Thus, it is critical to advance our understanding of mechanisms underlying developmental nicotine exposure, possible therapeutic interventions, and pursue policy changes to limit exposure.
Table 1.
Summary of brain and behavioral changes following nicotine exposure during prenatal, postnatal/perinatal, and adolescent developmental periods.
| Brain Region | Exposure Age | Nicotine delivery system | Behavioral/Brain outcome | Reference |
|---|---|---|---|---|
| Frontal Lobe |
Prenatal – Human
Prenatal - Mice Prenatal - Rats Adolescence - Rats |
Maternal cigarette smoking Oral exposure (200 μg/ml; GD14- P0) Osmotic minipump (3 mg/kg/day; GD4–18) Subcutaneous (0.4 mg/kg; P34–43) Subcutaneous (0.4 mg/kg; P35–44) |
Reduced frontal lobe, low birth weight, increased affective/behavioral problemsIncreased likelihood of substance use in adolescence; Thinning of orbitofrontal lobe, increased DNA methylation of BDNFInduced AD/HD- related behavioral changes; Decreased nicotine-stimulated release of DA in the prefrontal cortex (P56–63)Increased DA levels in the prefrontal cortex in adolescent rats (P32–33) Increased impulsive behavior, impaired measures of attention; Downregulated mGluR2 protein and decreases function in prefrontal cortex in adulthood (P78)Induced cognitive deficits, anxiety and depressive-like behaviors; Decreased D1 receptor expression and increased phosphorylation of ERK 1–2 within the PFC (P75) | 16, 1819–215247128129 |
| Sensory Cortices |
Prenatal – Human
Postnatal - Mice Postnatal - Rats |
Maternal cigarette smoking Oral exposure (200 μg/ml; gestation until P21) Oral exposure (200 μg/ml; gestation until P21) Subcutaneous (1 or 2 mg/ kg; P8–14) Subcutaneous (1 mg/kg; P8–12) Subcutaneous (0.7 mg/kg; P8-P12) |
Impaired auditory and visual processing in adolescenceImpaired auditory discrimination (P60)Hypersensitivity in passive avoidance paradigm through loss of functional α5 and β2 subunit in adult mice (>P90) Enhanced excitatory transmission in auditory cortex Increased cortical NMDA receptor 2 A and reduced thalamic NMDA receptor 2B mRNA levelsImpaired auditory learning through nAChR signaling dysfunction in adulthood (P60) | 102, 103101 10096 97 98 |
| Nucleus Accumbens |
Prenatal - Human
Prenatal - Rats Adolescence - Rats |
Maternal cigarette smoking Osmotic minipump (3 mg/kg/day) Subcutaneous (0.4 mg/kg; P35–44) Subcutaneous (0.4 mg/kg; P35–48) Intravenous (2 ×30 μg/kg/0.1 ml; P28–31) |
Reduced NAc volume Enhanced cocaine-induced locomotor activity in females; Increased D3 receptor and increased quinelorane-stimulated [35S]GTPγS binding in the ventral striatum of males (P32)Induced depressive and anxiogenic behavior; Upregulated ERK 1/2 and Akt-GSK-3 signaling pathways within the NAc, and downregulated D1R expression into adulthood (P75)Increased DA release in the NAc core and shell of adolescent male rats (P35–48) when compared to adult Enhanced nicotine-induced cocaine self-administration in rats (P32); Increased microglial activation, D2 receptor protein, G protein coupling, and cfos expression in the NAc | 1847154132146, 149 |
| Hippocampus |
Postnatal - Mice
Postnatal – Rats Adolescence – Mice Adolescence – Rats |
Osmotic minipump (approx. 21 mg/kg/day; P1-P15) Oral gastral intubation (6 mg/kg/day in milk; P1-P8) Oral exposure (50 ug/ml in 2 % saccharin; P30-P45) Osmotic minipump (approx. 12.6 mg/kg/day; P32-P45) Osmotic minipump (approx. 1 mg/kg/day; P28-P42) Osmotic minipump (approx. 3 mg/kg/day; P28-P42) Subcutaneous (0.7 mg/kg x 2 daily; P28-P43) |
Impaired hippocampal dependent spatial memory linked to altered LTP threshold, loss of normal α2 * nAChR function, and impaired NMDA receptor-dependent long term depression (LTD) (P28–46)Increased neurotrophic tone (BDNF, FGF-2, NT-3, and IGF-1 mRNA expression) (P8) Decreased neuronal area and increased packing density (P8)Increased apoptotic cell death in the hippocampal molecular layer and CA1Reduced dendritic length and complexity in apical CA1 branches in adulthood with impaired contextual fear conditioningEnhanced cued fear conditioningImpaired long-term contextual fear conditioningImpaired long-term spatial learning in female rats only | 85–878384161162163164165 |
| Ventral Tegmental Area | Prenatal - Rats | Gestational exposure (6 mg/kg/day starting GD3) | Altered burst firing patterns in a subset of DA neurons within VTA of adolescent rats (P42-P58) | 48 |
| Brainstem/Hindbrain |
Prenatal - Human Prenatal - Rhesus Monkey Prenatal – Mice Prenatal – Rats Prenatal/perinatal – Mice |
Maternal cigarette smoking Osmotic mini pump (10–20 cigarettes per day) Oral exposure throughout gestation (300 μg/ml nicotine) Cigarette smoke exposure (3 x 350 ml; E14 until birth) Osmotic minipump (60 mg/kg/day; E7-P8) |
Reduced nAChR binding within serotonin neurons in pontis oralis and raphe obscurus in SIDS infant brain stem tissue Increased serotonin and serotonin metabolite; reversed by Vitamin C co treatment Reduced nicotine-induced intracellular calcium and AMPA-mediated glutamate responses in LDTg neurons (P7–15)Decreased functional GluN2B-containing synaptic NMDA receptors in cholinergic LDTg neurons, with a concurrent increased function in smaller, putative GABA neurons (P11–15)Increased excitability of cell membrane from pedunculopontine nucleus neurons (P12–21)Decreased firing activity in raphe obscurus neurons (P3) and increased expression of serotonin autoreceptors (5HT1ARs) (P5) | 35 3744, 45463438 |
Acknowledgements
We would like to thank Michelle Ren for her help in preparing this manuscript.
Funding
This work was supported by the University of California Irvine (UCI) NIDA T32 training grant (T32DA050558) (EMC) and NIH Grant (R01, DA048899) (FL and SL).
Footnotes
The multifaceted activities of nervous and non-nervous neuronal nicotinic acetylcholine receptors in physiology and pathology. Eds: Dr Cecilia Gotti, Prof Francesco Clementi, Prof Michele ZOli.
CRediT authorship contribution statement
Emily M. Castro, M.S. Writing – Original Draft, Writing – Review & Editing, Visualization. Shahrdad Lotfipour, Ph.D. Writing – Review & Editing. Frances M. Leslie, Ph.D. Conceptualization, Writing – Original Draft, Writing – Review & Editing, Visualization, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
No data was used for the research described in the article.
References
- [1].Changeux J-P, Nicotine addiction and nicotinic receptors: lessons from genetically modified mice, Nat. Rev. Neurosci 11 (6) (2010) 389–401, 10.1038/nrn2849. [DOI] [PubMed] [Google Scholar]
- [2].Zoli M, Pistillo F, Gotti C, Diversity of native nicotinic receptor subtypes in mammalian brain, Neuropharmacology 96 (2015) 302–311, 10.1016/j.neuropharm.2014.11.003. [DOI] [PubMed] [Google Scholar]
- [3].Dwyer JB, Broide RS, Leslie FM, Nicotine and brain development, Birth Defects Res. Part C: Embryo Today.: Rev 84 (1) (2008) 30–44, 10.1002/bdrc.20118. [DOI] [PubMed] [Google Scholar]
- [4].Dwyer JB, McQuown SC, Leslie FM, The dynamic effects of nicotine on the developing brain, Pharmacol. Ther 122 (2) (2009) 125–139, 10.1016/j.pharmthera.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Smith AM, Dwoskin LP, Pauly JR, Early exposure to nicotine during critical periods of brain development: mechanisms and consequences, J. Pediatr. Biochem 1 (2) (2010) 125–141, 10.3233/JPB-2010-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Leslie FM, Unique, long-term effects of nicotine on adolescent brain, Pharmacol. Biochem. Behav 197 (2020), 173010, 10.1016/j.pbb.2020.173010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ren M, Lotfipour S, Leslie F, Unique effects of nicotine across the lifespan, Pharmacol., Biochem., Behav 214 (2022), 173343, 10.1016/j.pbb.2022.173343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Whittington JR, Simmons PM, Phillips AM, Gammill SK, Cen R, Magann EF, Cardenas VM, The use of electronic cigarettes in pregnancy: a review of the literature, Obstet. Gynecol. Surv 73 (9) (2018) 544–549, 10.1097/OGX.0000000000000595. [DOI] [PubMed] [Google Scholar]
- [9].Kim S, Oancea SC, Electronic cigarettes may not be a “safer alternative” of conventional cigarettes during pregnancy: evidence from the nationally representative PRAMS data, BMC Pregnancy Childbirth 20 (1) (2020) 557, 10.1186/sl2884-020-03247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Blanc J, Tosello B, Ekblad MO, Berlin I, Netter A, Nicotine replacement therapy during pregnancy and child health outcomes: a systematic review, Int. J. Environ. Res. Public Health 18 (8) (2021) 4004, 10.3390/ijerphl8084004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Slotkin TA, Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates, Toxicol. Appl. Pharmacol 198 (2) (2004) 132–151, 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- [12].Nekhayeva IA, Nanovskaya TN, Pentel PR, Keyler DE, Hankins GD, Ahmed MS, Effects of nicotine-specific antibodies, Nic311 and Nic-IgG, on the transfer of nicotine across the human placenta, Biochem. Pharmacol 70 (11) (2005) 1664–1672, 10.1016/j.bcp.2005.08.013. [DOI] [PubMed] [Google Scholar]
- [13].Keyler DE, Lesage MG, Dufek MB, Pentel PR, Changes in maternal and fetal nicotine distribution after maternal administration of monoclonal nicotine-specific antibody to rats, Int. Immunopharmacol 6 (11) (2006) 1665–1672, 10.1016/j.intimp.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gallardo KA, Leslie FM, Nicotine-stimulated release of [3H] norepinephrine from fetal rat locus coeruleus cells in culture, J. Neurochem 70 (2) (1998) 663–670, 10.1046/j.1471-4159.1998.70020663.x. [DOI] [PubMed] [Google Scholar]
- [15].Pentel PR, Keyler DE, Chen Y, LeSage MG, Dufek MB, Le C, Leslie FM, Vaccination against nicotine does not prevent nicotine-induced changes in fetal nicotinic receptor binding and c-fos mRNA expression in rats, Neurotoxicol. Teratol 28 (5) (2006) 589–596, 10.1016/j.ntt.2006.08.001. [DOI] [PubMed] [Google Scholar]
- [16].Ekblad M, Korkeila J, Parkkola R, Lapinleimu H, Haataja L, Lehtonen L, Maternal smoking during pregnancy and regional brain volumes in preterm infants, J. Pediatr 156 (2) (2010) 185–190, e1. [DOI] [PubMed] [Google Scholar]
- [17].Zou R, Boer OD, Felix JF, Muetzel RL, Franken IHA, Cecil CAM, El Marroun H, Association of maternal tobacco use during pregnancy with preadolescent brain morphology among offspring, JAMA Netw. Open 5 (8) (2022), e2224701, 10.1001/jamanetworkopen.2022.24701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].El Marroun H, Schmidt MN, Franken IHA, Jaddoe VWV, Hofman A, van der Lugt A, Verhulst FC, Tiemeier H, White T, Prenatal tobacco exposure and brain morphology: a prospective study in young children, Article 4, Neuropsychopharmacology 39 (4) (2014), 10.1038/npp.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lotfipour S, Ferguson E, Leonard G, Perron M, Pike B, Richer L, Séguin JR,Toro R, Veillette S, Pausova Z, Paus T, Orbitofrontal cortex and drug use during adolescence: role of prenatal exposure to maternal smoking and BDNF genotype, Arch. Gen. Psychiatry 66 (11) (2009) 1244–1252, 10.1001/archgenpsychiatry.2009.124. [DOI] [PubMed] [Google Scholar]
- [20].Lotfipour S, Ferguson E, Leonard G, Miettunen J, Perron M, Pike GB,Richer L, Séguin JR, Veillette S, Jarvelin MR, Moilanen I, Maki P,Nordstrom T, Puasova Z, Viejola J, Paus T, Maternal cigarette smoking during pregnancy predicts drug use via externalizing behavior in two community-based samples of adolescents, Addiction 109 (10) (2014) 1718–1729, 10.1111/add.12665. [DOI] [PubMed] [Google Scholar]
- [21].Toledo-Rodriguez M, Lotfipour S, Leonard G, Perron M, Richer L, Veillette S, Pausova Z, Paus T, Maternal smoking during pregnancy is associated with epigenetic modifications of the brain-derived neurotrophic factor-6 exon in adolescent offspring, Am. J. Med. Genet. Part B: Neuropsychiatr. Genet 153B (7) (2010) 1350–1354, 10.1002/ajmg.b.31109. [DOI] [PubMed] [Google Scholar]
- [22].Lotfipour S, Leonard G, Perron M, Pike B, Richer L, Séguin JR, Toro R,Veillette S, Pausova Z, Paus T, Prenatal exposure to maternal cigarette smoking interacts with a polymorphism in the α6 nicotinic acetylcholine receptor gene to influence drug use and striatum volume in adolescence, Mol. Psychiatry 15 (1) (2010) 6–8, 10.1038/mp.2009.63. [DOI] [PubMed] [Google Scholar]
- [23].Tiesler CM, Heinrich J, Prenatal nicotine exposure and child behavioural problems, Eur. Child Adolesc. Psychiatry 23 (10) (2014) 913–929, 10.1007/S00787-014-0615-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Minatoya M, Araki A, Itoh S, Yamazaki K, Kobayashi S, Miyashita C, Sasaki S, Kishi R, Prenatal tobacco exposure and ADHD symptoms at pre-school age: the Hokkaido Study on Environment and Children’s Health, Environ. Health Prev. Med 24 (1) (2019) 1–9, 10.1186/sl2199-019-0834-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Neuman RJ, Lobos E, Reich W, Henderson CA, Sun LW, Todd RD, Prenatal smoking exposure and dopaminergic genotypes interact to cause a severe ADHD subtype, Biol. Psychiatry 61 (12) (2007) 1320–1328, 10.1016/j.biopsych.2006.08.049. [DOI] [PubMed] [Google Scholar]
- [26].Eicher JD, Powers NR, Cho K, Miller LL, Mueller KL, Ring SM, Tomblin JB, Gruen JR, Associations of prenatal nicotine exposure and the dopamine related genes ANKK1 and DRD2 to verbal language, PloS One 8 (5) (2013), e63762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Müller KU, Mennigen E, Ripke S, et al. , Altered reward processing in adolescents with prenatal exposure to maternal cigarette smoking (for the IMAGEN Consortium), JAMA Psychiatry 70 (8) (2013) 847–856, 10.1001/jamapsychiatry.2013.44. [DOI] [PubMed] [Google Scholar]
- [28].Lavezzi AM, Ottaviani G, Matturri L, Adverse effects of prenatal tobacco smoke exposure on biological parameters of the developing brainstem, Neurobiol. Dis 20 (2) (2005) 601–607, 10.1016/j.nbd.2005.04.015. [DOI] [PubMed] [Google Scholar]
- [29].Duncan JR, Garland M, Myers MM, Fifer WP, Yang M, Kinney HC, Stark RI, Prenatal nicotine-exposure alters fetal autonomic activity and medullary neurotransmitter receptors: Implications for sudden infant death syndrome,J. Appl. Physiol 107 (5) (2009) 1579–1590, 10.1152/japplphysiol.91629.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bednarczuk N, Milner A, Greenough A, The role of maternal smoking in sudden fetal and infant death pathogenesis, Front. Neurol 11 (2020). ( 10.3389/fheur.2020.586068). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gupta D, Chapter 1—Neuroanatomy, in: Prabhakar H (Ed.), Essentials of Neuroanesthesia, Academic Press, 2017, pp. 3–40, 10.1016/B978-0-12-805299-0.00001-4. [DOI] [Google Scholar]
- [32].Kohyama J, Shimohira M, Itoh M, Fukumizu M, Iwakawa Y, Phasic muscle activity during REM sleep in infancy—normal maturation and contrastive abnormality in SIDS/ALTE and West syndrome, J. Sleep. Res 2 (4) (1993) 241–249, 10.llll/j.1365-2869.1993.tb00095.x. [DOI] [PubMed] [Google Scholar]
- [33].Kohyama J, Sudden infant death syndrome and the pedunculopontine tegmental nucleus, in: Sawaguchi T (Ed.), Sudden Infant Death Syndrome: From Pathophysiological Prospects, Springer, Japan, 2014, pp. 1–13, 10.1007/978-4-431-54315-2. [DOI] [Google Scholar]
- [34].Good CH, Bay KD, Buchanan RA, McKeon KA, Skinner RD, Garcia-Rill E, Prenatal exposure to cigarette smoke affects the physiology of pedunculopontine nucleus (PPN) neurons in development, Neurotoxicol. Teratol 28 (2) (2006) 210–219, 10.1016/j.ntt.2005.i2.006. [DOI] [PubMed] [Google Scholar]
- [35].Vivekanandarajah A, Nelson ME, Kinney HC, Elliott AJ, Folkerth RD,Tran H, Cotton J, Jacobs P, Minter M, McMillan K, Duncan JR, Broadbelt KG, Schissler K, Odendaal HJ, Angal J, Brink L, Burger EH, Coldrey JA, Dempers J, PASS Network, Nicotinic receptors in the brainstem ascending arousal system in SIDS with analysis of pre-natal exposures to maternal smoking and alcohol in high-risk populations of the safe passage study, Front. Neurol 12 (2021), 10.3389/fneur.2021.636668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Paterson DS, Hilaire G, Weese-Mayer DE, Medullary serotonin defects and respiratory dysfunction in sudden infant death syndrome, Respir. Physiol. Neurobiol 168 (1–2) (2009) 133–143, 10.1016/j.resp.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Slotkin TA, Seidler FJ, Spindel ER, Prenatal nicotine exposure in rhesus monkeys compromises development of brainstem and cardiac monoamine pathways involved in perinatal adaptation and sudden infant death syndrome: Amelioration by Vitamin C, Neurotoxicol. Teratol 33 (3) (2011) 431–434, 10.10l6/j.ntt.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cerpa VJ, Aylwin M, de la LO, Beltrán-Castillo S, Bravo EU, Llona IR, Richerson GA, Eugenín JL, The alteration of neonatal raphe neurons by prenatal–perinatal nicotine. meaning for sudden infant death syndrome, Am. J. Respir. Cell Mol. Biol 53 (4) (2015) 489–499, 10.1165/rcmb.2014-0329OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Avraam J, Wu Y, Richerson GB, Perinatal nicotine reduces chemosensitivity of medullary 5-HT neurons after maturation in culture, Neuroscience 446 (2020) 80–93, 10.1016/j.neuroscience.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Maskos U, The cholinergic mesopontine tegmentum is a relatively neglected nicotinic master modulator of the dopaminergic system: relevance to drugs of abuse and pathology, Br. J. Pharmacol 153 (S1) (2008) S438–S445, 10.1038/bjp.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Coimbra B, Soares-Cunha C, Vasconcelos NAP, Domingues AV, Borges S,Sousa N, Rodrigues AJ, Role of laterodorsal tegmentum projections to nucleus accumbens in reward-related behaviors, Nat. Commun 10 (1) (2019) 4138, 10.1038/s41467-019-11557-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Berry JN, Engle SE, McIntosh JM, Drenan RM, A6-containing nicotinic acetylcholine receptors in midbrain dopamine neurons are poised to govern dopamine-mediated behaviors and synaptic plasticity, Neuroscience 304 (2015) 161–175, 10.1016/j.neuroscience.2015.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kohlmeier KA, Polli FS, Plasticity in the brainstem: prenatal and postnatal experience can alter laterodorsal tegmental (LDT) structure and function, Front. Synaptic Neurosci (2020) 12, 10.3389/ihsyn.2020.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Christensen M, Nielsen M, Kohlmeier K, Electrophysiological changes in laterodorsal tegmental neurons associated with prenatal nicotine exposure: Implications for heightened susceptibility to addict to drugs of abuse, J. Dev. Orig. Health Dis 6 (3) (2015) 182–200, 10.1017/S204017441400049X. [DOI] [PubMed] [Google Scholar]
- [45].McNair LF, Kohlmeier KA, Prenatal nicotine is associated with reduced AMPA and NMDA receptor-mediated rises in calcium within the laterodorsal tegmentum: a pontine nucleus involved in addiction processes, J. Dev. Orig. Health Dis 6 (3) (2015) 225–241, 10.1017/S2040174414000439. [DOI] [PubMed] [Google Scholar]
- [46].Polli FS, Kohlmeier KA, Alterations in NMDAR-mediated signaling within the laterodorsal tegmental nucleus are associated with prenatal nicotine exposure, Neuropharmacology 158 (2019), 107744, 10.1016/j.neuropharm.2019.107744. [DOI] [PubMed] [Google Scholar]
- [47].Dwyer JB, Cardenas A, Franke RM, Chen Y, Bai Y, Belluzzi JD, Lotfipour S, Leslie FM, Prenatal nicotine sex-dependently alters adolescent dopamine system development, Transl. Psychiatry 9 (2019) 304, 10.1038/s41398-019-0640-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Dragomir A, Akay YM, Zhang D, Akay M, Ventral tegmental area dopamine neurons firing model reveals prenatal nicotine induced alterations, IEEE Trans. Neural Syst. Rehabil. Eng 25 (9) (2016) 1387–1396, 10.1109/TNSRE.2016.2636133. [DOI] [PubMed] [Google Scholar]
- [49].Zhang D, Dragomir A, Akay YM, Akay M, Nicotine exposure increases the complexity of dopamine neurons in the parainterfascicular nucleus (PIF) subregion of VTA, J. Neuroeng. Rehabil 11 (2014) 103, 10.1186/1743-0003-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kazemi T, Avci NG, Keller RF, Akay YM, Akay M, Investigating the influence of perinatal nicotine exposure on genetic profiles of neurons in the sub-regions of the VTA, Sci. Rep 10 (1) (2020), 10.1038/s41598-020-59248-0. Article 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Gold AB, Keller AB, Perry DC, Prenatal exposure of rats to nicotine causes persistent alterations of nicotinic cholinergic receptors, Brain Res 1250 (2009) 88–100, 10.1016/j.brainres.2008.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Alkam T, Mamiya T, Kimura N, Yoshida A, Kihara D, Tsunoda Y,Nabeshima T, Prenatal nicotine exposure decreases the release of dopamine in the medial frontal cortex and induces atomoxetine-responsive neurobehavioral deficits in mice, Psychopharmacology 234 (12) (2017) 1853–1869, 10.1007/S00213-017-4591-z. [DOI] [PubMed] [Google Scholar]
- [53].Franke RM, Park M, Belluzzi JD, Leslie FM, Prenatal nicotine exposure changes natural and drug-induced reinforcement in adolescent male rats, Eur. J. Neurosci 27 (11) (2008) 2952–2961, 10.l111/j.1460-9568.2008.06253.x. [DOI] [PubMed] [Google Scholar]
- [54].Solomon LJ, Quinn VP, Spontaneous quitting: self-initiated smoking cessation in early pregnancy, Nicotine Tob. Res 6 (Suppl_2) (2004) S203–S216, 10.1080/14622200410001669132. [DOI] [PubMed] [Google Scholar]
- [55].Heil SH, Herrmann ES, Badger GJ, Solomon LJ, Bernstein IM, Higgins ST, Examining the timing of changes in cigarette smoking upon learning of pregnancy, Prev. Med 68 (2014) 58–61, 10.1016/j.ypmed.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Harmer C, Memon A, Factors associated with smoking relapse in the postpartum period: an analysis of the child health surveillance system data in Southeast England, Nicotine Tob. Res.: Off. J. Soc. Res. Nicotine Tob 15 (5) (2013) 904–909, 10.1093/ntr/nts221. [DOI] [PubMed] [Google Scholar]
- [57].Cooper S, Orton S, Leonardi-Bee J, Brotherton E, Vanderbloemen L,Bowker K, Naughton F, Ussher M, Pickett KE, Sutton S, Coleman T, Smoking and quit attempts during pregnancy and postpartum: a longitudinal UK cohort, BMJ Open 7 (11) (2017), e018746, 10.1136/bmjopen-2017-018746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Drugs and Lactation Database (LactMed) [Internet] Bethesda (MD): National Library of Medicine (US); 2006-. Nicotine. [Updated 2020 Aug 17]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK501586/. [Google Scholar]
- [59].Nordenstam F, Lundell B, Edstedt Bonamy AK, Raaschou P, Wickström R, Snus users had high levels of nicotine, cotinine and 3-hydroxycotinine in their breastmilk, and the clearance was slower than in smoking mothers, in: Acta Paediatrica (Oslo, Norway: 1992), 108, 2019, pp. 1250–1255, 10.1111/apa.14602. [DOI] [PubMed] [Google Scholar]
- [60].Hajek P, Przulj D, Pesola F, Griffiths C, Walton R, McRobbie H, Coleman T, Lewis S, Whitemore R, Clark M, Ussher M, Sinclair L, Seager E, Cooper S,Bauld L, Naughton F, Sasieni P, Manyonda I, Myers Smith K, Electronic cigarettes versus nicotine patches for smoking cessation in pregnancy: a randomized controlled trial, Article 5, Nat. Med 28 (5) (2022), 10.1038/S41591-022-01808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wickström R, Effects of nicotine during pregnancy: human and experimental evidence, Curr. Neuropharmacol 5 (3) (2007) 213–222, 10.2174/157015907781695955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].McGrath-Morrow SA, Gorzkowski J, Groner JA, Rule AM, Wilson K, Tanski SE, Collaco JM, Klein JD, The effects of nicotine on development, Pediatrics 145 (3) (2020), e20191346, 10.1542/peds.2019-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sengupta P, The laboratory rat: relating its age with human’s, Int. J. Prev. Med 4 (6) (2013) 624–630. [PMC free article] [PubMed] [Google Scholar]
- [64].Dobbing J, Undernutrition and the developing brain: the use of animal models to elucidate the human problem. Normal and abnormal development of brain and behaviour, Springer, Dordrecht, 1971, pp. 20–38. [Google Scholar]
- [65].Dobbing J, Sands J, Comparative aspects of the brain growth spurt, Early Hum. Dev 3 (1) (1979) 79–83. [DOI] [PubMed] [Google Scholar]
- [66].Tierney AL, Nelson CA, Brain development and the role of experience in the early years, Zero Three 30 (2) (2009) 9–13. [PMC free article] [PubMed] [Google Scholar]
- [67].Carter AM, Animal models of human pregnancy and placentation: alternatives to the mouse, Reprod. (Camb., Engl. ) 160 (6) (2020) R129–R143, 10.1530/REP-20-0354. [DOI] [PubMed] [Google Scholar]
- [68].Pagani LS, Environmental tobacco smoke exposure and brain development: the case of attention deficit/hyperactivity disorder, Neurosci. Biobehav. Rev 44 (2014) 195–205, 10.1016/j.neubiorev.2013.03.008. [DOI] [PubMed] [Google Scholar]
- [69].Fitzpatrick C, Barnett TA, Pagani LS, Parental bad habits breed bad behaviors in youth: exposure to gestational smoke and child impulsivity, Int. J. Psychophysiol 93 (1) (2014) 17–21. [DOI] [PubMed] [Google Scholar]
- [70].Pagani LS, Fitzpatrick C, Early childhood household smoke exposure predicts less task-oriented classroom behavior at age 10, Health Educ. Behav.: Off. Publ. Soc. Public Health Educ 43 (5) (2016) 584–591, 10.1177/1090198115614317. [DOI] [PubMed] [Google Scholar]
- [71].Pagani LS, Fitzpatrick C, Prospective associations between early long-term household tobacco smoke exposure and antisocial behaviour in later childhood, J. Epidemiol. Community Health 67 (7) (2013) 552–557, 10.1136/jech-2012-202191. [DOI] [PubMed] [Google Scholar]
- [72].Pagani LS, Nguyen AK, Fitzpatrick C, Prospective associations between early long-term household tobacco smoke exposure and subsequent indicators of metabolic risk at age 10, Nicotine Tob. Res.: Off. J. Soc. Res. Nicotine Tob 18 (5) (2016) 1250–1257, 10.1093/ntr/ntvl28. [DOI] [PubMed] [Google Scholar]
- [73].Zeid D, Kutlu MG, Gould TJ, Differential effects of nicotine exposure on the hippocampus across lifespan, Curr. Neuropharmacol 16 (4) (2018) 388–402, 10.2174/1570159X15666170714092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Seress L, Comparative anatomy of the hippocampal dentate gyrus in adult and developing rodents, non-human primates and humans, Prog. Brain Res 163 (2007) 23–798, 10.1016/S0079-6123(07)63002-7. [DOI] [PubMed] [Google Scholar]
- [75].Adams CE, Broide RS, Chen Y, Winzer-Serhan UH, Henderson TA, Leslie FM, Freedman R, Development of the α7 nicotinic cholinergic receptor in rat hippocampal formation, Dev. Brain Res 139 (2) (2002) 175–187, 10.1016/S0165-3806(02)00547-3. [DOI] [PubMed] [Google Scholar]
- [76].Son JH, Winzer-Serhan UH, Postnatal expression of α2 nicotinic acetylcholine receptor subunit mRNA in developing cortex and hippocampus, J. Chem. Neuroanat 32 (2–4) (2006) 179–190, 10.1016/j.jchemneu.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Baradaran R, Khoshdel-Sarkarizi H, Kargozar S, Hami J, Mohammadipour A, Sadr-Nabavi A, Peyvandi Karizbodagh M, Kheradmand H, Haghir H, Developmental regulation and lateralisation of the α7 and α4 subunits of nicotinic acetylcholine receptors in developing rat hippocampus, 75-78, Int. J. Dev. Neurosci. 80 (4) (2020) 303–318, 10.1002/jdn.10026. [DOI] [PubMed] [Google Scholar]
- [78].Le Magueresse C, Safiulina V, Changeux JP, Cherubini E, Nicotinic modulation of network and synaptic transmission in the immature hippocampus investigated with genetically modified mice, J. Physiol 576 (2) (2006) 533–546, 10.1113/jphysiol.2006.117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U,Saarma M, Kaila K, The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation, Nature 397 (6716) (1999) 251–255, 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- [80].Maggi L, Sher E, Cherubini E, Regulation of GABA release by nicotinic acetylcholine receptors in the neonatal rat hippocampus, J. Physiol 536 (1) (2001) 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Maggi L, Le Magueresse C, Changeux JP, Cherubini E, Nicotine activates immature “silent” connections in the developing hippocampus, Proc. Natl. Acad. Sci 100 (4) (2003) 2059–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Liu Z, Neff RA, Berg DK, Sequential interplay of nicotinic and GABAergic signaling guides neuronal development, science 314 (5805) (2006) 1610–1613. [DOI] [PubMed] [Google Scholar]
- [83].Son JH, Winzer-Serhan UH, Chronic neonatal nicotine exposure increases mRNA expression of neurotrophic factors in the postnatal rat hippocampus, Brain Res. 1278 (2009) 1–14. [DOI] [PubMed] [Google Scholar]
- [84].Huang LZ, Abbott LC, Winzer-Serhan UH, Effects of chronic neonatal nicotine exposure on nicotinic acetylcholine receptor binding, cell death and morphology in hippocampus and cerebellum, Neuroscience 146 (4) (2007) 1854–1868, 10.1016/j.neuroscience.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Nakauchi S, Malvaez M, Su H, Kleeman E, Dang R, Wood MA, Sumikawa K, Early postnatal nicotine exposure causes hippocampus-dependent memory impairments in adolescent mice: association with altered nicotinic cholinergic modulation of LTP, but not impaired LTP, Neurobiol. Learn. Mem 118 (2015)178–188, 10.1016/j.nlm.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Chen K, Nakauchi S, Su H, Tanimoto S, Sumikawa K, Early postnatal nicotine exposure disrupts the α2* nicotinic acetylcholine receptor-mediated control of oriens-lacunosum moleculare cells during adolescence in rats, Neuropharmacology 101 (2016) 57–67, 10.1016/j.neuropharm.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Nakauchi S, Su H, Trang I, Sumikawa K, Long-term effects of early postnatal nicotine exposure on cholinergic function in the mouse hippocampal CA1 region, Neurobiol. Learn. Mem 181 (2021), 107445, 10.1016/j.nlm.2021.107445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Hensch TK, Critical period regulation, Annu. Rev. Neurosci 27 (2004) 549–579, 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- [89].Cisneros-Franco JM, Voss P, Thomas ME, de Villers-Sidani E, Critical periods of brain development, in: Handbook of clinical neurology, Vol. 173, Elsevier, 2020, pp. 75–88. [DOI] [PubMed] [Google Scholar]
- [90].Fuchs JL, [125I] α-Bungarotoxin binding marks primary sensory areas of developing rat neocortex, Brain Res. 501 (2) (1989) 223–234, 10.1016/0006-8993(89)90640-9. [DOI] [PubMed] [Google Scholar]
- [91].Broide RS, O’connor LT, Smith MA, Smith JAM, Leslie FM, Developmental expression of α7 neuronal nicotinic receptor messenger RNA in rat sensory cortex and thalamus, Neuroscience 67 (1) (1995) 83–94, 10.1016/0306-4522(94)00623-D. [DOI] [PubMed] [Google Scholar]
- [92].Bina KG, Guzman P, Broide RS, Leslie FM, Smith MA, O’Dowd DK, Localization of α7 nicotinic receptor subunit mRNA and α-bungarotoxin binding sites in developing mouse somatosensory thalamocortical system, J. Comp. Neurol 363 (2) (1995) 321–332, 10.1002/cne.903630212. [DOI] [PubMed] [Google Scholar]
- [93].Broide RS, Robertson RT, Leslie FM, Regulation of α7 nicotinic acetylcholine receptors in the developing rat somatosensory cortex by thalamocortical afferents, J. Neurosci 16 (9) (1996) 2956–2971, 10.1523/JNEUROSCI.16-09-02956.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Bina KG, Park M, O’Dowd DK, Regulation of α7 nicotinic acetylcholine receptors in mouse somatosensory cortex following whisker removal at birth,J. Comp. Neurol 397 (1) (1998) 1–9, . [DOI] [PubMed] [Google Scholar]
- [95].Aramakis VB, Metherate R, Nicotine selectively enhances NMDA receptor-mediated synaptic transmission during postnatal development in sensory neocortex, J. Neurosci 18 (20) (1998) 8485–8495, 10.1523/JNEUROSCI.18-20-08485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Aramakis VB, Hsieh CY, Leslie FM, Metherate R, A critical period for nicotine-induced disruption of synaptic development in rat auditory cortex,J. Neurosci 20 (16) (2000) 6106–6116, 10.1523/JNEUROSCI.20-16-06106.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Hsieh CY, Leslie FM, Metherate R, Nicotine exposure during a postnatal critical period alters NR2A and NR2B mRNA expression in rat auditory forebrain, Dev. Brain Res 133 (1) (2002) 19–25, 10.1016/S0165-3806(01)00314-5. [DOI] [PubMed] [Google Scholar]
- [98].Liang K, Poytress BS, Chen Y, Leslie FM, Weinberger NM, Metherate R, Neonatal nicotine exposure impairs nicotinic enhancement of central auditory processing and auditory learning in adult rats, Eur. J. Neurosci 24 (3) (2006) 857–866, 10.1111/j.1460-9568.2006.04945.x. [DOI] [PubMed] [Google Scholar]
- [99].Kassam SM, Herman PM, Goodfellow NM, Alves NC, Lambe EK, Developmental excitation of corticothalamic neurons by nicotinic acetylcholine receptors, J. Neurosci 28 (35) (2008) 8756–8764, 10.1523/JNEUROSCI.2645-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Heath CJ, King SL, Gotti C, Marks MJ, Picciotto MR, Cortico-thalamic connectivity is vulnerable to nicotine exposure during early postnatal development through α4/β2/α5 nicotinic acetylcholine receptors, Neuropsychopharmacology 35 (12) (2010) 2324–2338, 10.1038/npp.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Horst NK, Heath CJ, Neugebauer NM, Kimchi EY, Laubach M, Picciotto MR, Impaired auditory discrimination learning following perinatal nicotine exposure or β2 nicotinic acetylcholine receptor subunit deletion, Behav. Brain Res 231 (1) (2012) 170–180, 10.1016/j.bbr.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Fried PA, Watkinson B, Gray R, Differential effects on cognitive functioning in 13-to 16-year-olds prenatally exposed to cigarettes and marihuana, Neurotoxicol. Teratol 25 (4) (2003) 427–436, 10.1016/S0892-0362(03)00029-1 [DOI] [PubMed] [Google Scholar]
- [103].Jacobsen LK, Slotkin TA, Mend WE, Frost SJ, Pugh KR, Gender-specific effects of prenatal and adolescent exposure to tobacco smoke on auditory and visual attention, Neuropsychopharmacology 32 (12) (2007) 2453–2464, 10.1038/sj.npp.1301398. [DOI] [PubMed] [Google Scholar]
- [104].Spear LP, Adolescent Neurodevelopment, J. Adolesc. Health.: Off. Publ. Soc. Adolesc. Med 52 (202) (2013) S7–13, 10.1016/j.jadohealth.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Yuan M, Cross SJ, Loughlin SE, Leslie FM, Nicotine and the adolescent brain, J. Physiol 593 (16) (2015) 3397–3412, 10.1113/JP270492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Miech R, Johnston L, O’Malley PM, Bachman JG, Patrick ME, Adolescent vaping and nicotine use in 2017-2018 - U.S. national estimates, N. Engl. J. Med 380 (2) (2019) 192–193, 10.1056/NEJMcl814130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Mendel JR, Berg CJ, Windle RC, Windle M, Predicting young adulthood smoking among adolescent smokers and nonsmokers, Am. J. Health Behav 36 (4) (2012) 542–554, 10.5993/AJHB.36.4.ll. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].U.S. Department of Health and Human Services. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2012. [accessed 2019 Feb 28]. [Google Scholar]
- [109].Miech R, Johnston L, O’Malley PM, Bachman JG, Patrick ME, Trends in adolescent vaping, 2017–2019, N. Engl. J. Med 381 (15) (2019) 1490–1491, 10.1056/NEJMcl910739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Meza R, Jimenez-Mendoza E, Levy DT, Trends in tobacco use among adolescents by grade, sex, and race, 1991-2019, e2027465-e2027465, JAMA Netw. Open 3 (12) (2020), 10.1001/jamanetworkopen.2020.27465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Wang TW, Neff LJ, Park-Lee E, Ren C, Cullen KA, King BA, E-cigarette use among middle and high school students—United States, 2020, Morb. Mortal. Wkly. Rep 69 (37) (2020) 1310, 10.15585/mmwr.mm6937el. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Gentzke AS, Wang TW, Cornelius M, Park-Lee E, Ren C, Sawdey MD, Cullen KA, Loretan C, Jamal A, Homa DM, Tobacco product use and associated factors among middle and high school students – National Youth Tobacco Survey, United States, 2021, Morb. Mortal. Wkly. Rep, 71(SS- 5 (2022) 1–29, 10.15585/mmwr.ss7105al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Diemert LM, Bondy SJ, Brown KS, Manske S, Young adult smoking cessation: predictors of quit attempts and abstinence, Am. J. Public Health 103 (3) (2013) 449–453, 10.1016/j.addbeh.2018.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Case KR, Mantey DS, Creamer MR, Harrell MB, Kelder SH, Perry CL, E-cigarette-specific symptoms of nicotine dependence among Texas adolescents, Addict. Behav 84 (2018) 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Vogel EA, Prochaska JJ, Ramo DE, Andres J, Rubinstein ML, Adolescents’ E-cigarette use: increases in frequency, dependence, and nicotine exposure over 12 months, J. Adolesc. Health 64 (6) (2019) 770–775, 10.1016/j.jadohealth.2019.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Laviolette SR, Molecular and neuronal mechanisms underlying the effects of adolescent nicotine exposure on anxiety and mood disorders, Neuropharmacology 184 (2021), 108411, 10.1016/j.neuropharm.2020.108411. [DOI] [PubMed] [Google Scholar]
- [117].Gogtay N, Thompson PM, Mapping gray matter development: implications for typical development and vulnerability to psychopathology, Brain Cogn. 72 (1) (2010) 6–15, 10.1016/j.bandc.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Paus T, Growth of white matter in the adolescent brain: myelin or axon, Brain Cogn. 72 (1) (2010) 26–35, 10.1016/j.bandc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- [119].Tamnes CK, Østby Y, Fjell AM, Wesdye LT, Due-Tønnessen P, Walhovd KB, Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure, Cereb. Cortex 20 (3) (2010) 534–548, 10.1093/cercor/bhpll8. [DOI] [PubMed] [Google Scholar]
- [120].Casey BJ, Getz S, Galvan A, The adolescent brain, Dev. Rev 28 (1) (2008) 62–77, 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].DeBry SC, Tiffany ST, Tobacco-induced neurotoxicity of adolescent cognitive development (TINACD): a proposed model for the development of impulsivity in nicotine dependence, Nicotine Tob. Res 10 (1) (2008) 11–25, 10.1080/14622200701767811. [DOI] [PubMed] [Google Scholar]
- [122].Mashhoon Y, Betts J, Farmer SL, Lukas SE, Early onset tobacco cigarette smokers exhibit deficits in response inhibition and sustained attention, Drug Alcohol Depend. 184 (2018) 48–56, 10.1016/j.drugalcdep.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Musso F, Bettermann F, Vucurevic G, Stoeter P, Konrad A, Winterer G, Smoking impacts on prefrontal attentional network function in young adult brains, Psychopharmacology 191 (1) (2007) 159–169, 10.1007/S00213-006-0499-8. [DOI] [PubMed] [Google Scholar]
- [124].McCabe SE, West BT, Veliz P, Boyd CJ, E-cigarette use, cigarette smoking, dual use, and problem behaviors among US adolescents: results from a national survey (org/), J. Adolesc. Health 61 (2) (2017) 155–162, 10.1016/j.jadohealth.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Chadi N, Li G, Hadland SE, Adverse school outcomes and risky sexual health behaviors among high school students with e-cigarette and marijuana use, Subst. Use Misuse 56 (4) (2021) 517–521, 10.1080/10826084.2021.1883659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Deaifield CT, Chen-Sankey JC, McNeel TS, Bernat DH, Choi K, E-cigarette initiation predicts subsequent academic performance among youth: results from the PATH Study, Prev. Med 153 (2021), 106781, 10.1016/j.ypmed.2021.106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Counotte DS, Smit AB, Pattij T, Spijker S, Development of the motivational system during adolescence, and its sensitivity to disruption by nicotine, Dev. Cogn. Neurosci 1 (4) (2011) 430–443, 10.1016/j.dcn.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Counotte DS, Goriounova NA, Li KW, Loos M, van der Schors RC,Schetters D, Schoffelmeer ANM, Smit AB, Mansvelder HD, Pattij T,Spijker S, Lasting synaptic changes underlie attention deficits caused by nicotine exposure during adolescence, Article 4, Nat. Neurosci 14 (4) (2011), 10.1038/nn.2770. [DOI] [PubMed] [Google Scholar]
- [129].Jobson CLM, Renard J, Szkudlarek H, Rosen LG, Pereira B, Wright DJ,Rushlow W, Laviolette SR, Adolescent nicotine exposure induces dysregulation of mesocorticolimbic activity states and depressive and anxiety-like prefrontal cortical molecular phenotypes persisting into adulthood, Cereb. Cortex 29 (7) (2019) 3140–3153, 10.1093/cercor/bhyl79. [DOI] [PubMed] [Google Scholar]
- [130].Lechner WV, Janssen T, Kahler CW, Audrain-McGovern J, Leventhal AM, Bidirectional associations of electronic and combustible cigarette use onset patterns with depressive symptoms in adolescents, Prev. Med 96 (2017) 73–78, 10.1016/j.ypmed.2016.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Esmaeelzadeh S, Moraros J, Thorpe L, Bird Y, Examining the association and directionality between mental health disorders and substance use among adolescents and young adults in the US and Canada—a systematic review and meta-analysis, J. Clin. Med 7 (12) (2018) 543, 10.3390/jcm7120543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Corongiu S, Dessì C, Cadoni C, Adolescence versus adulthood: differences in basal mesolimbic and nigrostriatal dopamine transmission and response to drugs of abuse, Addict. Biol 25 (1) (2019) 1–11, 10.llll/adb.12721. [DOI] [PubMed] [Google Scholar]
- [133].Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP, Nicotine-induced conditioned place preference in adolescent and adult rats, Physiol. Behav 77 (1) (2002) 107–114, 10.1016/S0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- [134].Shram MJ, Funk D, Li Z, Lê AD, Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine, Psychopharmacology 186 (2) (2006) 201–208, 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- [135].Kota D, Martin BR, Robinson SE, Damaj MI, Nicotine dependence and reward differ between adolescent and adult male mice, J. Pharmacol. Exp. Ther 322 (1) (2007) 399–407, 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- [136].Torres OV, Tejeda HA, Natividad LA, O’Dell LE, Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development, Pharmacol. Biochem. Behav 90 (4) (2008) 658–663, 10.1016/j.pbb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Ahsan HM, de la Pena JBI, Botanas CJ, Kim HJ, Yu GY, Cheong JH, Conditioned place preference and self-administration induced by nicotine in adolescent and adult rats, Biomol. Ther 22 (5) (2014) 460, 10.4062/biomolther.2014.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Lenoir M, Starosciak AK, Ledon J, Booth C, Zakharova E, Wade D,Izenwasser S, Sex differences in conditioned nicotine reward are age-specific, Pharmacol. Biochem. Behav 132 (2015) 56–62, 10.1016/j.pbb.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Belluzzi JD, Lee AG, Oliff HS, Leslie FM, Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats, Psychopharmacology 174 (3) (2004) 389–395, 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- [140].Brielmaier JM, McDonald CG, Smith RF, Immediate and long-term behavioral effects of a single nicotine injection in adolescent and adult rats, Neurotoxicol. Teratol 29 (1) (2007) 74–80, 10.1016/j.ntt.2006.09.023. [DOI] [PubMed] [Google Scholar]
- [141].Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS, Adolescent-onset nicotine self-administration modeled in female rats, Psychopharmacology 169 (2) (2003) 141–149, 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- [142].Chen H, Matta SG, Sharp BM, Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug, Neuropsychopharmacology 32 (3) (2007) 700–709, 10.1038/sj.npp.1301135. [DOI] [PubMed] [Google Scholar]
- [143].Natividad LA, Torres OV, Friedman TC, O’Dell LE, Adolescence is a period of development characterized by short-and long-term vulnerability to the rewarding effects of nicotine and reduced sensitivity to the anorectic effects of this drug, Behav. Brain Res 257 (2013) 275–285, 10.1016/j.bbr.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Gellner CA, Belluzzi JD, Leslie FM, Self-administration of nicotine and cigarette smoke extract in adolescent and adult rats, Neuropharmacology 109 (2016) 247–253, 10.1016/j.neuropharm.2016.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Ren M, Lotfipour S, Nicotine gateway effects on adolescent substance use, West. J. Emerg. Med 20 (5) (2019) 696–709, 10.5811/westjem.2019.7.41661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Dao JM, McQuown SC, Loughlin SE, Belluzzi JD, Leslie FM, Nicotine alters limbic function in adolescent rat by a 5-HT1A receptor mechanism, Neuropsychopharmacology 36 (7) (2011) 1319–1331, 10.1038/npp.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].McQuown SC, Belluzzi JD, Leslie FM, Low dose nicotine treatment during early adolescence increases subsequent cocaine reward, Neurotoxicol. Teratol 29 (1) (2007) 66–73, 10.1016/j.ntt.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].McQuown SC, Dao JM, Belluzzi JD, Leslie FM, Age-dependent effects of low-dose nicotine treatment on cocaine-induced behavioral plasticity in rats, Psychopharmacology 207 (1) (2009) 143–152, 10.1007/s00213-009-1642-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Linker KE, Elabd MG, Tawadrous P, Cano M, Green KN, Wood MA, Leslie FM, Microglial activation increases cocaine self-administration following adolescent nicotine exposure, Nat. Commun 11 (1) (2020) 306, 10.1038/s41467-019-14173-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Cardenas A, Martinez M, Mejia AS, Lotfipour S, Early adolescent subchronic low-dose nicotine exposure increases subsequent cocaine and fentanyl self-administration in Sprague–Dawley rats, Behav. Pharmacol 32 (1) (2021) 86–91, 10.1097/FBP.0000000000000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Cardenas A, Lotfipour S, Age- and sex-dependent nicotine pretreatment effects on the enhancement of methamphetamine self-administration in Sprague-Dawley rats, Nicotine Tob. Res 24 (8) (2022) 1186–1192, 10.1093/ntr/ntab218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Chen YW, Rada PV, Bützler BP, Leibowitz SF, Hoebel BG, Corticotropinreleasing factor in the nucleus accumbens shell induces swim depression, anxiety, and anhedonia along with changes in local dopamine/acetylcholine balance, Neuroscience 206 (2012) 155–166, 10.1016/j.neuroscience.2011.12.009. [DOI] [PubMed] [Google Scholar]
- [153].Gabbay V, Ely BA, Li Q, Bangaru SD, Panzer AM, Alonso CM, Milham MP, Striatum-based circuitry of adolescent depression and anhedonia, J. Am. Acad. Child Adolesc. Psychiatry 52 (6) (2013) 628–641, 10.1016/j.jaac.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Hudson R, Green M, Wright DJ, Renard J, Jobson CEL, Jung T, Rushlow W, Laviolette SR, Adolescent nicotine induces depressive and anxiogenic effects through ERK 1-2 and Akt-GSK-3 pathways and neuronal dysregulation in the nucleus accumbens, Addict. Biol 26 (2) (2021), e12891, 10.1111/adb.12891. [DOI] [PubMed] [Google Scholar]
- [155].Wortzel I, Seger R, The ERK cascade: distinct functions within various subcellular organelles, Genes Cancer 2 (3) (2011) 195–209, 10.1177/1947601911407328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Adriani W, Macrì S, Pacifici R, Laviola G, Peculiar vulnerability to nicotine oral self-administration in mice during early adolescence, Neuropsychopharmacology 27 (2) (2002) 212–224, 10.1016/S0893-133X(02)00295-6. [DOI] [PubMed] [Google Scholar]
- [157].Cengelli S, O’Loughlin J, Lauzon B, Cornuz J, A systematic review of longitudinal population-based studies on the predictors of smoking cessation in adolescent and young adult smokers, Tob. Control 21 (3) (2012) 355–362, 10.1136/tc.2011.044149. [DOI] [PubMed] [Google Scholar]
- [158].Kendler KS, Myers J, Damaj MI, Chen X, Early smoking onset and risk for subsequent nicotine dependence: a monozygotic co-twin control study, Am. J. Psychiatry 170 (4) (2013) 408–413, 10.1176/appi.ajp.2012.12030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Stojanovic T, Velarde Gamez D, Schuld GJ, Bormann D, Cabatic M, Uhrin P, Lubec G, Monje FJ, Age-dependent and pathway-specific bimodal action of nicotine on synaptic plasticity in the hippocampus of mice lacking the miR-132/ 212 genes, Cells 11 (2) (2022) 261, 10.3390/cellsll020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Kutlu MG, Gould TJ, Effects of drugs of abuse on hippocampal plasticity and hippocampus-dependent learning and memory: contributions to development and maintenance of addiction, Learn. Mem. (Cold Spring Harb., N. Y.) 23 (10) (2016) 515–533, 10.1101/lm.042192.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Oliveira-da-Silva A, Vieira FB, Cristina-Rodrigues F, Filgueiras CC, Manhães AC, Abreu-Vilç Y, Increased apoptosis and reduced neuronal and glial densities in the hippocampus due to nicotine and ethanol exposure in adolescent mice, Int. J. Dev. Neurosci.: Off. J. Int. Soc. Dev. Neurosci 27 (6) (2009) 539–548, 10.1016/j.ijdevneu.2009.06.009. [DOI] [PubMed] [Google Scholar]
- [162].Holliday ED, Nucero P, Kutlu MG, Oliver C, Connelly KL, Gould TJ, Unterwald EM, Long-term effects of chronic nicotine on emotional and cognitive behaviors and hippocampus cell morphology in mice: comparisons of adult and adolescent nicotine exposure, Eur. J. Neurosci 44 (10) (2016) 2818–2828, 10.1111/ejn.13398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Smith L, Mcdonald C, Bergstrom H, Brielmaier J, Eppolito A, Wheeler T,Falco A, Smith R, Long-term changes in fear conditioning and anxiety-like behavior following nicotine exposure in adult versus adolescent rats, Pharmacol. Biochem. Behav 85 (1) (2006) 91–97, 10.1016/j.pbb.2006.07.014. [DOI] [PubMed] [Google Scholar]
- [164].Spaeth AM, Barnet RC, Hunt PS, Burk JA, Adolescent nicotine exposure disrupts context conditioning in adulthood in rats, Pharmacol., Biochem., Behav 96 (4) (2010) 501–506, 10.1016/j.pbb.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Mateos B, Borcel E, Loriga R, Luesu W, Bini V, Llorente R, Castelli MP, Viveros M-P, Adolescent exposure to nicotine and/or the cannabinoid agonist CP 55,940 induces gender-dependent long-lasting memory impairments and changes in brain nicotinic and CB(1) cannabinoid receptors, J. Psychopharmacol. (Oxf., Engl. ) 25 (12) (2011) 1676–1690, 10.1177/0269881110370503. [DOI] [PubMed] [Google Scholar]
- [166].Blakemore S, Burnett S, Dahl RE, The role of puberty in the developing adolescent brain, Hum. Brain Mapp 31 (6) (2010) 926–933, 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Benes FM, Turtle M, Khan Y, Farol P, Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood, Arch. Gen. Psychiatry 51 (6) (1994) 477–484, 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- [168].Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E, Neurogenesis in the adult is involved in the formation of trace memories, Nature 410 (6826) (2001) 372–376, 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- [169].Sweatt JD, Experience-dependent epigenetic modifications in the central nervous system, Biol. Psychiatry 65 (3) (2009) 191–197, 10.1016/j.biopsych.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Nielsen DA, Utrankar A, Reyes JA, Simons DD, Kosten TR, Epigenetics of drug abuse: predisposition or response, Pharmacogenomics 13 (10) (2012) 1149–1160, 10.2217/pgs.12.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Yohn NL, Caruso MJ, Blendy JA, Effects of nicotine and stress exposure across generations in C57BL/6 mice, Stress 22 (1) (2019) 142–150, 10.1080/10253890.2018.1532991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Jung Y, Hsieh LS, Lee AM, Zhou Z, Coman D, Heath CJ, Picciotto MR, An epigenetic mechanism mediates developmental nicotine effects on neuronal structure and behavior, Nat. Neurosci 19 (7) (2016) 905–914, 10.1038/nn.4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Goldberg LR, Gould TJ, Multigenerational and transgenerational effects of paternal exposure to drugs of abuse on behavioral and neural function, Eur. J. Neurosci 50 (3) (2019) 2453–2466, 10.1111/ejn.14060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Skinner MK, Environmental epigenetic transgenerational inheritance and somatic epigenetic mitotic stability, Epigenetics 6 (7) (2011) 838–842, 10.4161/epi.6.7.16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Spiers H, Hannon E, Schalkwyk LC, Smith R, Wong CCY, O’Donovan MC, Bray NJ, Mill J, Methylomic trajectories across human fetal brain development, Genome Res. 25 (3) (2015) 338–352, 10.1101/gr.180273.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Wheater ENW, Stoye DQ, Cox SR, Wardlaw JM, Drake AJ, Bastin ME, Boardman JP, DNA methylation and brain structure and function across the life course: a systematic review, Neurosci. Biobehav. Rev 113 (2020) 133–156, 10.1016/j.neubiorev.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Lee KWK, Richmond R, Hu P, French L, Shin J, Bourdon C, Reischl E,Waldenberger M, Zeilinger S, Gaunt T, McArdle W, Ring S, Woodward G, Bouchard L, Gaudet D, Smith GD, Relton C, Paus T, Pausova Z, Prenatal exposure to maternal cigarette smoking and DNA methylation: epigenome-wide association in a discovery sample of adolescents and replication in an independent cohort at birth through 17 years of age, Environ. Health Perspect 123 (2) (2015) 193–199, 10.1289/ehp.1408614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Joubert BR, Felix JF, Yousefi P, Bakulski KM, Just AC, Breton C, Reese SE,Markunas CA, Richmond RC, Xu C-J, Küpers LK, Oh SS, Hoyo C,Gruzieva O, Söderhäll C, Salas LA, Baïz N, Zhang H, Lepeule J, London SJ, DNA methylation in newborns and maternal smoking in pregnancy: genome-wide consortium meta-analysis, Am. J. Hum. Genet 98 (4) (2016) 680–696, 10.1016/j.ajhg.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Wiklund P, Karhunen V, Richmond RC, Parmar P, Rodriguez A, De Silva M,Wielscher M, Rezwan FI, Richardson TG, Veijola J, Herzig K-H, Holloway JW, Relton CL, Sebert S, Järvelin M-R, DNA methylation links prenatal smoking exposure to later life health outcomes in offspring, Clin. Epigenet 11 (1) (2019) 97, 10.1186/sl3148-019-0683-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Chatterton Z, Hartley BJ, Seok MH, Mendelev N, Chen S, Milekic M,Haghighi F, In utero exposure to maternal smoking is associated with DNA methylation alterations and reduced neuronal content in the developing fetal brain, Epigenetics Chromatin 10 (1) (2017) 1–11, 10.1186/sl3072-017-0111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Li YF, Langholz B, Salam MT, Gilliland FD, Maternal and grandmaternal smoking patterns are associated with early childhood asthma, Chest 127 (4) (2005) 1232–1241, 10.1016/S0012-3692(15)34472-X. [DOI] [PubMed] [Google Scholar]
- [182].Golding J, Ellis G, Gregory S, Birmingham K, Iles-Caven Y, Rai D, Pembrey M, Grand-maternal smoking in pregnancy and grandchild’s autistic traits and diagnosed autism, Sci. Rep 7 (1) (2017) 1–14, 10.1038/srep46179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Rehan VK, Liu J, Naeem E, Tian J, Sakurai R, Kwong K, Torday JS, Perinatal nicotine exposure induces asthma in second generation offspring, BMC Med. 10 (1) (2012) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Rehan VK, Liu J, Sakurai R, Torday JS, Perinatal nicotine-induced transgenerational asthma, Am. J. Physiol. -Lung Cell. Mol. Physiol 305 (7) (2013) L501–L507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Leslie FM, Multigenerational epigenetic effects of nicotine on lung function, BMC Med. 11 (1) (2013) 27, 10.1186/1741-7015-ll-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Altintaş A, Liu J, Fabre O, Chuang TD, Wang Y, Sakurai R, Rehan VK, Perinatal exposure to nicotine alters spermatozoal DNA methylation near genes controlling nicotine action, FASEB J.: Off. Publ. Fed. Am. Soc. Exp. Biol 35 (7) (2021), e21702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Buck JM, Sanders KN, Wageman CR, Knopik VS, Stitzel JA, O’Neill HC, Developmental nicotine exposure precipitates multigenerational maternal transmission of nicotine preference and ADHD-like behavioral, rhythmometric, neuropharmacological, and epigenetic anomalies in adolescent mice, Neuropharmacology 149 (2019) 66–82, 10.1096/Q.202100215R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Buck JM, O’Neill HC, Stitzel JA, Developmental nicotine exposure elicits multigenerational disequilibria in proBDNF proteolysis and glucocorticoid signaling in the frontal cortices, striata, and hippocampi of adolescent mice, Biochem. Pharmacol 168 (2019) 438–451, 10.1016/j.bcp.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Buck JM, O’Neill HC, Stitzel JA, Developmental nicotine exposure engenders intergenerational downregulation and aberrant posttranslational modification of cardinal epigenetic factors in the frontal cortices, striata, and hippocampi of adolescent mice, Epigenetics Chromatin 13 (1) (2020) 1–15, 10.1186/S13072-020-00332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Buck JM, O’Neill HC, Stitzel JA, The intergenerational transmission of developmental nicotine exposure-induced neurodevelopmental disorder-like phenotypes is modulated by the Chrna5 D397N polymorphism in adolescent mice, Behav. Genet 51 (6) (2021) 665–684, 10.1007/sl0519-021-10071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Renaud SM, Fountain SB, Transgenerational effects of adolescent nicotine exposure in rats: Evidence for cognitive deficits in adult female offspring, Neurotoxicol. Teratol 56 (2016) 47–54, 10.1016/j.ntt.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Dai J, Wang Z, Xu W, Zhang M, Zhu Z, Zhao X, Qiao Z, Paternal nicotine exposure defines different behavior in subsequent generation via hyper-methylation of mmu-miR-15b, Sci. Rep 7 (1) (2017) 1–15, 10.1038/S41598-017-07920-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Goldberg LR, Zeid D, Kutlu MG, Cole RD, Lallai V, Sebastian A, Gould TJ, Paternal nicotine enhances fear memory, reduces nicotine administration, and alters hippocampal genetic and neural function in offspring, Addict. Biol 26 (1) (2021), el2859, 10.1111/adb.12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].McCarthy DM, Bhide PG, Heritable consequences of paternal nicotine exposure: from phenomena to mechanisms, Biol. Reprod 105 (3) (2021)632–643, 10.1093/biolre/ioab116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Lee H, Kang M, Chung J-M, Noh J, Repeated nicotine exposure in adolescent rats: reduction of medial habenular activity and augmentation of nicotine preference, Physiol. Behav (2014) 138, 10.1016/j.physbeh.2014.11.034. [DOI] [PubMed] [Google Scholar]
- [196].Mishra A, Patni P, Hegde S, Aleya L, Tewari D, Neuroplasticity and environment: a pharmacotherapeutic approach toward preclinical and clinical understanding, Curr. Opin. Environ. Sci. Health 19 (2021), 100210, 10.1016/j.coesh.2020.09.004. [DOI] [Google Scholar]
- [197].Bernheim A, Halfon O, Boutrel B, Controversies about the enhanced vulnerability of the adolescent brain to develop addiction, Front. Pharmacol 4 (2013) 118, 10.3389/fphar.2013.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Abreu-Villaça Y, Seidler FJ, Tate CA, Cousins MM, Slotkin TA, Prenatal nicotine exposure alters the response to nicotine administration in adolescence: effects on cholinergic systems during exposure and withdrawal, Neuropsychopharmacology 29 (5) (2004) 879–890, 10.1038/sj.npp.1300401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.


