Abstract
Aims
The mechanisms underlying ageing-induced vascular remodelling remain unclear. This study investigates the role and underlying mechanisms of the cytoplasmic deacetylase sirtuin 2 (SIRT2) in ageing-induced vascular remodelling.
Methods and results
Transcriptome and quantitative real-time PCR data were used to analyse sirtuin expression. Young and old wild-type and Sirt2 knockout mice were used to explore vascular function and pathological remodelling. RNA-seq, histochemical staining, and biochemical assays were used to evaluate the effects of Sirt2 knockout on the vascular transcriptome and pathological remodelling and explore the underlying biochemical mechanisms. Among the sirtuins, SIRT2 had the highest levels in human and mouse aortas. Sirtuin 2 activity was reduced in aged aortas, and loss of SIRT2 accelerated vascular ageing. In old mice, SIRT2 deficiency aggravated ageing-induced arterial stiffness and constriction–relaxation dysfunction, accompanied by aortic remodelling (thickened vascular medial layers, breakage of elastin fibres, collagen deposition, and inflammation). Transcriptome and biochemical analyses revealed that the ageing-controlling protein p66Shc and metabolism of mitochondrial reactive oxygen species (mROS) contributed to SIRT2 function in vascular ageing. Sirtuin 2 repressed p66Shc activation and mROS production by deacetylating p66Shc at lysine 81. Elimination of reactive oxygen species by MnTBAP repressed the SIRT2 deficiency–mediated aggravation of vascular remodelling and dysfunction in angiotensin II–challenged and aged mice. The SIRT2 coexpression module in aortas was reduced with ageing across species and was a significant predictor of age-related aortic diseases in humans.
Conclusion
The deacetylase SIRT2 is a response to ageing that delays vascular ageing, and the cytoplasm–mitochondria axis (SIRT2–p66Shc–mROS) is important for vascular ageing. Therefore, SIRT2 may serve as a potential therapeutic target for vascular rejuvenation.
Keywords: Ageing, Arterial stiffness, Vascular remodelling, SIRT2, p66Shc, mROS
Structured Graphical Abstract
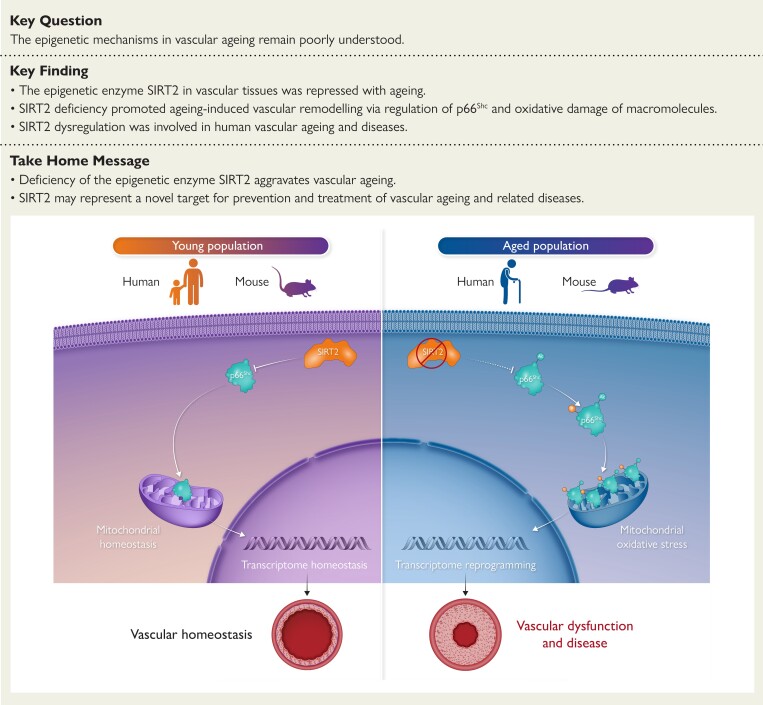
Structured Graphical Abstract.
The epigenetic regulator sirtuin 2 (SIRT2) governs a cytoplasm–mitochondria signal to repress vascular ageing. Ageing reduces SIRT2 protein levels and activity, which results in the hyperacetylation and activation of the adaptor protein p66Shc and production of mitochondrial reactive oxygen species (mROS), which subsequently reprogrammes the vascular transcriptome to aggravate ageing-induced vascular remodelling and diseases. The circulating SIRT2 may serve as a prognostic biomarker for ageing-related vascular diseases, and the cytoplasm–mitochondria axis SIRT2–p66Shc–mROS could be targeted for treating ageing-related vascular diseases.
See the editorial comment for this article ‘Sirtuin 2 in vascular ageing: the forsaken child?’, by Y.M. Puspitasari, https://doi.org/10.1093/eurheartj/ehad366.
Translational perspective.
Ageing promotes the likelihood of developing cardiovascular diseases, which increasingly contribute to global mortality, and vascular rejuvenation is geroprotective. However, the epigenetic mechanism underlying ageing-induced vascular remodelling remains unclear. The data obtained from humans and mice demonstrate that the cytoplasmic deacetylase sirtuin 2 (SIRT2) is a protective factor against vascular ageing, which relies on a novel cytoplasm–mitochondria signal (SIRT2–p66Shc–mROS axis). These findings suggest that SIRT2 activators can serve as potential drugs for delaying vascular ageing and ageing-induced angiopathies.
Introduction
Ageing increases the likelihood of developing cardiovascular diseases, increasing global mortality.1,2 In ageing individuals, arteries undergo pathological remodelling, resulting in age-related arterial diseases, including hypertension, atherosclerosis, arterial aneurysms, and coronary artery diseases.3,4 Improving vascular health via counteracting age-related signals promotes healthy ageing and extends lifespan.5 Therefore, vascular rejuvenation is geroprotective. However, the mechanisms underlying ageing-induced vascular remodelling remain unclear.
The impairment of intracellular metabolites, such as nicotinamide adenine dinucleotide (NAD+), is a cause of vascular ageing.6,7 Nicotinamide adenine dinucleotide is not only a coenzyme for redox reactions but also an essential cofactor for non-redox NAD+-dependent enzymes, including sirtuins, poly (ADP-ribose) polymerases, and CD38.8 Impairment of the endothelial NAD+ signalling network is a reversible cause of vascular ageing.6 Nicotinamide adenine dinucleotide–boosting therapies are beneficial in inhibiting ageing-induced vascular remodelling and other functional declines in rodents and humans.9–11 Members of the sirtuin (SIRT) family are NAD+-dependent deacetylases that regulate longevity and cardiovascular diseases.12–15 Sirtuin 2 (SIRT2) is not only predominantly localized in the cytoplasm but also translocated into the nucleus and mitochondria under stress.16–18 The epigenetic factor SIRT2 acts as a deacylase to reduce acetylation, benzoylation, lactylation, and crotonylation of histones and non-histone proteins.19,20 Studies during the past years have revealed the key roles of SIRT2 in extending lifespan and repressing ageing-induced functional decline in diverse organs.21–23 For instance, overexpression of SIRT2 delayed systemic ageing. It extended the lifespan of premature mice by inducing the checkpoint kinase BubR1,21 and SIRT2 activation also contributed to the reversion of reproductive ageing in mice.24 Besides, SIRT2 in the bone marrow delayed haematopoietic stem cell senescence and repressed the ageing-associated insulin resistance via an acetylation switch of the NLRP3 inflammasome.22,25 In the nervous system, SIRT2 promoted white matter oligodendrogenesis during development,26 and we found that restoring SIRT2 in oligodendrocyte progenitor cells promoted remyelination during ageing.27 However, few studies have attempted to understand SIRT2 in cardiovascular ageing. Our previous study demonstrated that SIRT2 regulated cardiac ageing by maintaining metabolic health via activating LKB1–AMPK signalling.23 Thus, it is reasonable to hypothesize that SIRT2 may participate in age-dependent vascular remodelling, but little direct evidence is currently available.
Mitochondrial reactive oxygen species (mROS) is pivotal to vascular remodelling and ageing.28,29 Mitochondrial reactive oxygen species–targeted drugs show therapeutic values for treating cardiovascular diseases.30,31 Cytoplasmic and nuclear factors can regulate mROS by targeting enzymes located in the mitochondria.32 However, the regulatory mechanism underlying cytoplasm/nucleus–mitochondria–mROS signalling in vascular ageing remains largely unknown.32 The mitochondrial adaptor protein p66Shc is a cytoplasm–mitochondria traveller that is phosphorylated and activated in the cytoplasm and translocated into the mitochondria to participate in the generation of hydrogen peroxide.14,33 Inhibition of p66Shc in mice can prolong the lifespan34 and reduce vascular ageing and related diseases such as myocardial ischaemia, stroke, and diabetic angiopathy.35–41 However, the upstream cytoplasmic regulator of the p66Shc–mROS axis is not fully understood in aged cardiovascular tissues.
In the present study, we identified a previously unrecognized role of the epigenetic regulator SIRT2 in age-dependent vascular ageing. Profiling of human and mouse aortas revealed that SIRT2 had the highest expression among the SIRT family. Sirtuin 2 protein levels and activity declined with ageing, and SIRT2 deficiency aggravated vascular dysfunction and remodelling in old mice. Transcriptome and biochemical analyses revealed that SIRT2 regulated vascular ageing partially by inhibiting the ageing-controlling protein p66Shc and its downstream mROS. In contrast, the reactive oxygen species (ROS) scavenger MnTBAP rescued vascular remodelling in aged mice. In humans, the SIRT2 coexpression module was correlated with ageing and had a predictive significance for age-related aortic diseases. These findings establish an unrecognized role for the deacetylase SIRT2 as a protector against vascular ageing and provide evidence that the cytoplasm–mitochondria axis SIRT2–p66Shc–mROS is critical for ageing-induced vascular remodelling.
Methods
Animals
C57BL/6 wild-type (WT) and Sirt2 knockout (Sirt2-KO) mice were used as described previously.23 The mice were kept in a pathogen-free facility, fed ad libitum, and maintained on a standard 12 h light/dark cycle. The responses of males and females to ageing-induced vascular remodelling are very different.42,43 To control the influence of sex, we chose to analyse male mice only. C57BL/6 strain mice are susceptible to cardiovascular ageing and diseases and their risk factors such as diet-induced obesity and type 2 diabetes.44–46 Thus, we used the C57BL/6 strain because this strain is the most widely used in studies of cardiovascular biology and diseases and the best-known of all inbred strains.47 All animal protocols were approved by the Laboratory Animal Ethics Committee of the Institute of Basic Medical Sciences (Chinese Academy of Medical Sciences) and West China Second University Hospital (Sichuan University).
To analyse the roles of Sirt2 in vascular ageing, male WT and Sirt2-KO mice were maintained for 24 months. For angiotensin II (Ang II)–induced vascular remodelling, the mice were subcutaneously challenged with Ang II (1.3 mg/kg/day; Sigma-Aldrich, A9525) for 28 days with minipumps.48–50 To eliminate ROS, the mice were intraperitoneally injected with MnTBAP (5 mg/kg/day; MedChemExpress, HY-126397) for 4 weeks.30 For MnTBAP treatment, MnTBAP was dissolved in saline (vehicle). Control mice were injected with an equal volume of saline solution only.
Statistical analysis
All values are expressed as mean ± standard deviation. Data normality was evaluated using the Shapiro–Wilk test. When reporting two groups with a normal distribution, we used a standard Student’s t-test. Otherwise, the non-parametric Mann–Whitney U test was applied. In the analysis of data from more than two groups, we used one-way ANOVA followed by the Bonferroni post hoc test when the assumptions satisfied a normal distribution. Otherwise, we used the non-parametric Kruskal–Wallis test followed by Dunn’s post hoc test to correct for multiple comparisons. Detailed methods for each analysis are described in the figure legends. All statistical analyses were performed using GraphPad Prism 9, and P-values < .05 were considered significant.
Detailed materials and methods, including the study subjects and patients, are described in Supplementary data online.
Results
Sirtuin 2 expression and activity in vascular ageing in humans and mice
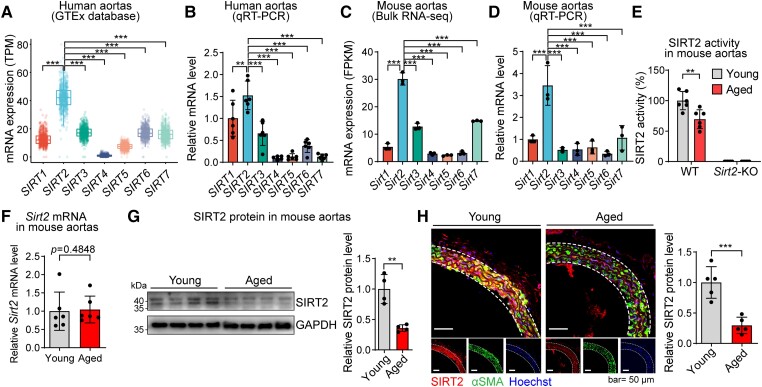
To test the potential roles of sirtuins in vascular ageing, we profiled the basal levels of SIRT1–SIRT7 mRNAs in human and mouse aortas. We analysed aortic transcriptome data from 607 human donors using the Genotype-Tissue Expression (GTEx) database. Interestingly, SIRT2 (but not the more popular SIRT1 or SIRT6) had the highest basal expression levels among the sirtuins in human aortas (Figure 1A). This finding was verified using quantitative real-time PCR (qRT-PCR) of human aortic samples (Figure 1B). Besides, Sirt2 also had the highest basal expression levels among sirtuins in mouse aortas, as evidenced by bulk RNA-seq and qRT-PCR analyses (Figure 1C and D). Thus, we tested the enzymatic activity of SIRT2 in the aortas of young and aged mice and observed that SIRT2 activity was reduced in the aortas of aged mice compared with that in young mice (Figure 1E). However, Sirt2 mRNA levels in the aorta were comparable between young and aged mice (Figure 1F). Western blot and immunofluorescence staining revealed that the protein levels of SIRT2 were reduced in aged aortas and vascular smooth muscle cells (VSMCs) (Figure 1G and H). In addition, we also found that the ageing-induced SIRT2 protein decline was partially due to the activation of the tyrosine kinase c-Src (see Supplementary data online, Figure S1).23,51 These findings suggested that SIRT2 protein levels and activity were reduced in the aortas of aged mice.
Figure 1.
Sirtuin 2 expression pattern in young and aged aortas. (A) Levels of SIRT1–SIRT7 mRNAs in human aortas analysed using the Genotype-Tissue Expression database (n = 607). (B) Quantitative real-time PCR analysis of SIRT1–SIRT7 mRNA levels in human aortas (n = 6). (C) The bulk RNA-seq analysis of the expression level of sirtuin members Sirt1–Sirt7 in mouse aortas (n = 3). (D) Quantitative real-time PCR analysis of sirtuin members Sirt1–Sirt7 in mouse aortas (n = 3). (E) Sirtuin 2 enzymatic activity was reduced in aged aortas (24 months old) compared with that in young aortas (4 months) of mice (n = 6). (F) mRNA level of Sirt2 in aortas from young and aged mice (n = 6). (G) Protein level of sirtuin 2 (both bands) in aortas from young and aged mice (n = 4). (H) Immunofluorescence staining revealed that the sirtuin 2 protein level was reduced in aged aortas compared with that in young aortas of mice. Representative images and quantitative results are shown (n = 5). αSMA, alpha-smooth muscle actin. Statistical analyses were performed using the Kruskal–Wallis test with Dunn’s post hoc test (A), one-way ANOVA with the Bonferroni post hoc test (B–D), unpaired Student’s t-test (E, G, H), and the Mann–Whitney U test (F). **P < .01, *** P < .001.
Sirtuin 2 deficiency promotes ageing-induced vascular remodelling
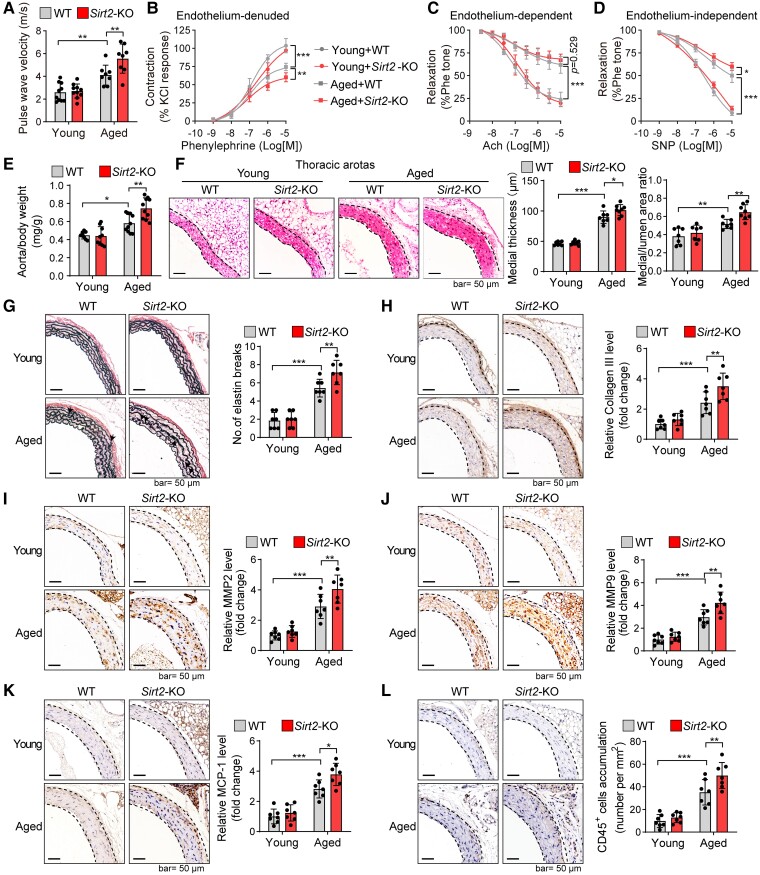
The decline in SIRT2 expression and activity in aged aortas prompted us to investigate its involvement in ageing-induced vascular dysfunction. Male Sirt2-KO mice and their WT littermates were maintained for 24 months to explore the potential role of SIRT2 in vascular ageing (see Supplementary data online, Figure S2A). As we reported previously, the body weight, heart rate, and blood pressure between aged Sirt2-KO and WT mice were comparable.23 To evaluate the effects of SIRT2 on vascular function, we measured pulse wave velocity (PWV), a measure of arterial stiffness.52 Compared with young mice, the aged WT mice showed significantly increased PWV values, which were further increased in aged Sirt2-KO mice (Figure 2A), indicating that SIRT2 deficiency aggravated the ageing-induced increase in arterial stiffness. To further test the effects of SIRT2 on arterial constriction–relaxation function, the aortas were dissociated for an ex vivo functional study.50 Phenylephrine-induced vascular constriction in endothelium-denuded aortas was impaired in aged mice and further deteriorated with SIRT2 deficiency (Figure 2B). Although endothelium-dependent relaxation was identical in aged WT and Sirt2-KO mice (Figure 2C), aged Sirt2-KO mice exhibited poorer endothelium-independent relaxation (Figure 2D). These findings suggest that SIRT2 loss aggravates ageing-induced vascular dysfunction, which may correlate with dysfunction of the middle layer but not the endothelium.
Figure 2.
Sirtuin 2 deficiency aggravates ageing-induced vascular dysfunction and remodelling. (A) Pulse wave velocity measurements in young and aged mice revealed that Sirt2 knockout promoted an ageing-induced increase in arterial stiffness (n = 8–10). (B–D) Ex vivo analysis of the vascular constriction–relaxation function of aortas from mice (n = 5). (B) Arterial vessel contractions mediated through phenylephrine. (C) Endothelium-dependent relaxation in response to acetylcholine (Ach). (D) Endothelium-independent relaxation in response to nitric oxide donor sodium nitroprusside (NO-donor SNP), an endothelium-independent agonist. (E) Sirt2 knockout increased aged mice’s aorta weight-to-body weight ratio (n = 8–10). The aorta weight/body weight ratio of young and aged wild-type and Sirt2 knockout mice was analysed. (F) Sirt2 knockout enhanced vascular remodelling in aged mice. Haematoxylin–eosin staining of the thoracic aortas was performed, and the medial thickness and the ratio of the media area to the vessel lumen were quantified (n = 7–8). (G) Elastin van Gieson staining of elastin fibres in thoracic aortas. Representative images and quantitative results are shown (n = 7). Arrows denote broken elastin fibres. (H–L) Immunohistochemical staining of collagen III (H), matrix metallopeptidase 2 (I), matrix metallopeptidase 9 (J), monocyte chemotactic protein-1 (K), and CD45 (L). Representative images and quantitative results are shown (n = 7). Statistical analyses were performed using one-way ANOVA with the Bonferroni post hoc test. *P < .05, **P < .01, ***P < .001.
Anatomical and histological analyses were performed to test whether the aggravation of vascular dysfunction in aged Sirt2-KO mice was coupled with vascular remodelling. Aged mice had an increased aorta weight-to-body weight (AW/BW) ratio compared with young WT mice. Significantly, Sirt2-KO promoted an ageing-induced increase in the AW/BW ratio (Figure 2E). We also performed haematoxylin–eosin (H&E) staining to determine whether the increased AW/BW ratio was associated with potential vascular remodelling. Compared with the thoracic and abdominal aortas of aged WT mice, aged Sirt2-KO mice exhibited an increased medial thickness and ratio of the media area to the vessel lumen (Figure 2F; see Supplementary data online, Figure S2B). The breakage of elastin fibres is a hallmark of increased arterial stiffness and vascular ageing.53 Thus, we performed elastin van Gieson (EVG) staining to monitor fibre breakage and found that SIRT2 deficiency promoted the breakage of elastin fibres in the aortas of aged mice (Figure 2G). We also observed that Sirt2-KO aggravated other vascular ageing features, such as collagen deposition (Figure 2H; see Supplementary data online, Figure S2C). Matrix metalloproteinases (MMPs, MMP2 and MMP9) are critical to vascular remodelling and ageing.54 We observed that Sirt2-KO promoted ageing-induced overexpression of MMP2 and MMP9 in mouse aortas (Figure 2I and J; see Supplementary data online, Figure S2D), suggesting that SIRT2 may regulate MMPs to participate in age-dependent vascular remodelling and stiffness.
Inflammation is another hallmark of vascular medial layer remodelling.55,56 Therefore, the inflammatory factors and immune cells in the aorta were examined. The chemokine monocyte chemotactic protein-1 (MCP-1) and total immune cells (CD45+) were enriched in aged aortas and were further elevated by Sirt2-KO (Figure 2K and L). In addition, immunostaining analysis demonstrated that Sirt2-KO promoted ageing-induced infiltration of dendritic cells and neutrophils into mouse aortas (see Supplementary data online, Figure S3A and B). Pro-inflammatory M1 macrophages promote vascular remodelling and disease.57,58 Significantly, Sirt2-KO also promoted macrophage infiltration and M1 polarization in aged aortas (see Supplementary data online, Figure S3C–F).
These findings demonstrate that decreased SIRT2 expression leads to increased age-induced vascular arterial stiffness and constriction–relaxation dysfunction, which may be partially due to vascular medial layer remodelling.
Sirtuin 2 deficiency reprogrammes the vascular transcriptome and aggravates mitochondrial oxidative stress
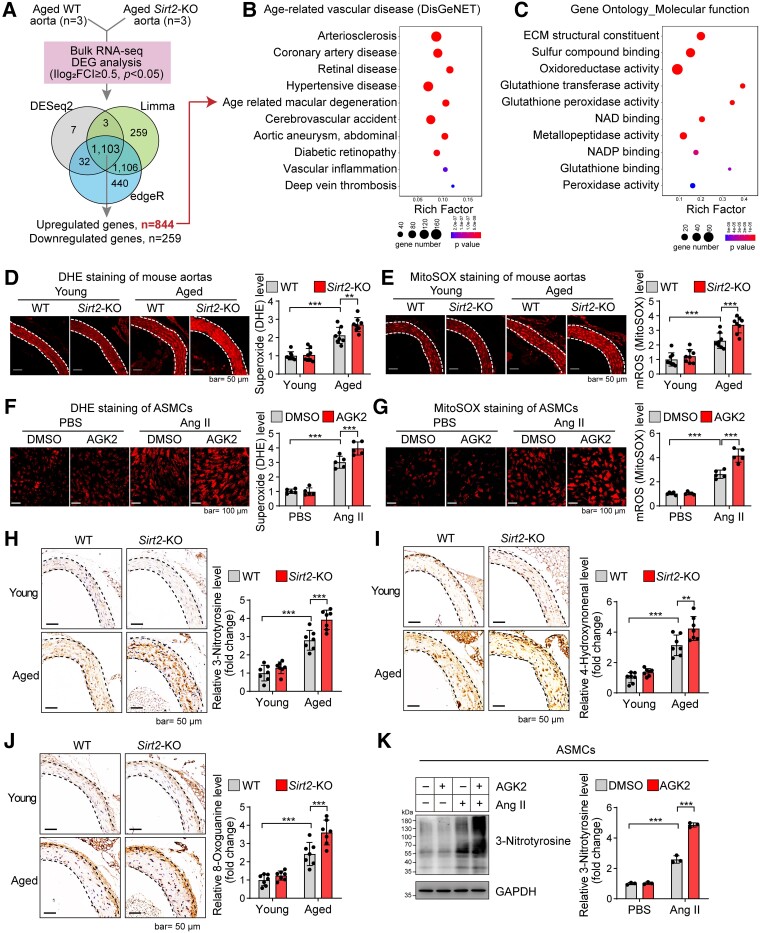
To further elucidate the mechanism underlying the role of SIRT2 in vascular ageing, the transcriptomes of aged aortas from WT and Sirt2-KO mice were analysed using bulk RNA-seq. Transcriptome analysis revealed that 844 genes were up-regulated and 259 were down-regulated following Sirt2-KO in aged aortas (Figure 3A). Sirtuin 2 deficiency in aged aortas led to the enrichment of gene sets involved in age-dependent arterial diseases, such as atherosclerosis and arterial aneurysms, suggesting that SIRT2 regulates a gene signature that participates in age-dependent arterial diseases (Figure 3B). However, the effects of SIRT2 on vascular remodelling and ageing may not result from cell death or proliferation, as evidenced by the transcriptome data and in vitro cell experiments (see Supplementary data online, Figure S4A–D).
Figure 3.
Sirtuin 2 regulates gene signatures associated with age-related arterial diseases and oxidative stress. (A) Bulk RNA-seq analysis of the transcriptome of aged aortas of wild-type and Sirt2 knockout mice (n = 3). Venn plot showing the overlapped genes among differentially expressed genes identified by three methods. A total of 844 genes were up-regulated, while 259 were down-regulated in aged Sirt2 knockout aortas (|log2 FC| ≥ 0.5, adjusted P < .05). (B) Sirt2 knockout led to the enrichment of genes involved in age-related arterial diseases. Human disease enrichment analysis (DisGeNET) was performed with the up-regulated genes using ToppGene Suite. (C) Sirt2 knockout led to the enrichment of genes involved in the oxidation–reduction process. Gene Ontology Molecular_function enrichment analysis was performed with the up-regulated genes using ToppGene Suite. (D, E) Sirt2 knockout increased ageing-induced oxidative stress in mouse aortas. Total cellular superoxide and mitochondrial reactive oxygen species were monitored by dihydroethidium and MitoSOX staining, respectively. Representative results and quantitative results of dihydroethidium (D) and MitoSOX staining (E) are shown (n = 8). (F, G) Sirtuin 2 inhibition promoted stress-induced oxidative stress in aortic smooth muscle cells. Aortic smooth muscle cells were treated with the sirtuin 2–specific inhibitor AGK2 (10 μM) with/without angiotensin II (1 μM) treatment for 24 h. Total cellular superoxide and mitochondrial reactive oxygen species were monitored by dihydroethidium and MitoSOX staining, respectively. Representative results and quantitative results of dihydroethidium (F) and MitoSOX staining (G) are shown (n = 5). (H–J) Sirt2 knockout increased ageing-induced oxidation of macromolecules in mouse aortas: (H) protein oxidation (3-nitrotyrosine); (I) lipid peroxidation (4-hydroxynonenal); and (J) DNA oxidation (8-oxoguanine). Representative results and quantitative results are shown (n = 7). (K) Sirtuin 2 inhibition promoted stress-induced protein oxidation in aortic smooth muscle cells. Aortic smooth muscle cells were treated with the sirtuin 2–specific inhibitor AGK2 (10 μM) with/without angiotensin II (1 μM) treatment for 24 h. Protein oxidation was analysed by western blot. Representative blots and quantitative results are shown (n = 3). Statistical analyses were performed using one-way ANOVA with the Bonferroni post hoc test (D–K). **P < .01, ***P < .001.
To further understand the transcriptome regulated by SIRT2 in aged aortas, enrichment analysis was performed to analyse the gene ontology (GO) molecular function gene signatures. Sirtuin 2 loss led to the enrichment of gene signatures involved in oxidative stress (Figure 3C), indicating that SIRT2 may regulate the oxidation–reduction system to affect age-related arterial diseases. To validate this finding, we measured total cellular superoxide and mROS levels using dihydroethidium (DHE) and MitoSOX staining. Sirt2 knockout increased ageing-induced up-regulation of total superoxide (DHE) and mROS (MitoSOX) in mouse aortas (Figure 3D and E). We also examined the effect of SIRT2 on oxidative stress in aortic smooth muscle cells (ASMCs). Oxidative stress was induced by treatment with Ang II, an ageing-induced effector peptide hormone of the renin–angiotensin system,59 and SIRT2 was inhibited by the specific inhibitor, AGK2.60 AGK2 treatment significantly aggravated the Ang II–induced increase in total superoxide and mROS levels (Figure 3F and G). To further evaluate oxidative stress in aortic tissues and cells, we monitored the oxidative damage to macromolecules by analysing protein oxidation [3-nitrotyrosine (3-NTR)], lipid peroxidation (4-hydroxynonenal), and DNA oxidation (8-oxoguanine). Ageing induced oxidative damage to macromolecules, such as proteins, lipids, and DNA in the aorta, further aggravated by Sirt2-KO (Figure 3H–J; see Supplementary data online, Figure S5). Sirtuin 2 inhibition also aggravated macromolecular damage (protein oxidation) induced by Ang II in ASMCs (Figure 3K). However, the effects of SIRT2 on oxidative stress may not be due to antioxidative activity because Sirt2-KO did not affect the enrichment of oxidative response signals and expression of antioxidants in aged aortas (see Supplementary data online, Figure S6).
Sirtuin 2 deacetylates and inhibits the adaptor protein p66Shc to target mitochondrial reactive oxygen species accumulation
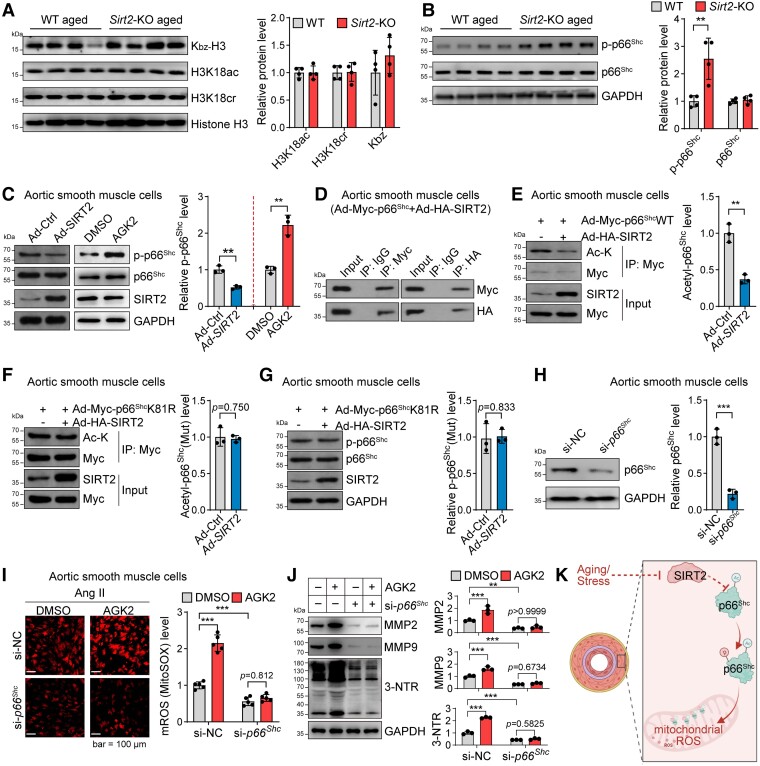
Because SIRT2 regulates lysine modifications of histone and non-histone proteins,18,23,61–65 we first tested whether SIRT2 regulates histone modifications to modulate the vascular transcriptome directly. Sirtuin 2 has been reported to regulate histone acetylation, crotonylation, and benzoylation under stress conditions.18,61–64 Therefore, we measured histone acetylation, crotonylation, and benzoylation in the aorta of aged WT and Sirt2-KO mice. However, SIRT2 deficiency did not affect H3K18ac, H3K18cr, or benzoylation in the aged aortas (Figure 4A). These findings suggested that SIRT2 indirectly regulates the vascular transcriptome. Thus, we considered the candidate targets that elevate ROS to reprogramme the transcriptome and are known regulators of ageing.
Figure 4.
Sirtuin 2 regulates oxidative stress by deacetylating p66Shc. (A) Sirtuin 2 effects on histone modification in aortas from aged mice. Representative western blot and quantitative results are shown (n = 4). (B) Sirtuin 2 deficiency increased p66Shc phosphorylation in the aortas of aged mice. Representative and quantitative results are shown (n = 4). (C) Sirtuin 2 repressed p66Shc phosphorylation in aortic smooth muscle cells. Aortic smooth muscle cells were infected with an adenovirus carrying sirtuin 2/control adenovirus or treated with the sirtuin 2 inhibitor AGK2 (10 μM)/DMSO for 24 h, followed by western blot analysis. Representative and quantitative results are shown (n = 3). (D) Sirtuin 2 interacted with p66Shc in aortic smooth muscle cells. Aortic smooth muscle cells were infected with an adenovirus carrying Myc-tagged p66Shc and HA-tagged sirtuin 2, followed by immunoprecipitation with Myc and HA antibodies and western blot analysis (n = 3). (E) Sirtuin 2 deacetylated p66Shc in aortic smooth muscle cells. Aortic smooth muscle cells were infected with Ad-Myc-p66Shc with Ad-SIRT2 or Ad-Ctrl, followed by immunoprecipitation with anti-Myc antibodies and western blot analysis of acetylated lysine (Ac-K) on p66Shc (n = 3). (F, G) Sirtuin 2 deacetylated p66Shc at lysine 81 (K81) to inhibit p66Shc phosphorylation in aortic smooth muscle cells. Aortic smooth muscle cells were infected with Myc-tagged K81R (lysine to arginine) mutant p66Shc (Ad-Myc-p66ShcK81R) with Ad-SIRT2 or Ad-Ctrl, followed by immunoprecipitation with anti-Myc antibodies and western blot analysis of acetylated lysine on p66Shc (F) (n = 3) and phosphorylation of p66Shc (G) (n = 3). (H) siRNA-mediated knockdown of p66Shc in aortic smooth muscle cells. The cells were transfected with si-NC or si-p66Shc for 48 h. Then, a western blot was performed to analyse protein expression (n = 3). (I) p66Shc contributed to sirtuin 2 function in regulating mitochondrial reactive oxygen species in aortic smooth muscle cells. Aortic smooth muscle cells were transfected with si-p66Shc or control si-NC for 24 h, followed by treatment with/without the sirtuin 2–specific inhibitor AGK2 (10 μM) and angiotensin II (1 μM) for another 24 h. Mitochondrial reactive oxygen species were monitored by MitoSOX staining. Representative and quantitative results are shown (n = 5). (J) p66Shc contributed to sirtuin 2 function in regulating protein oxidation and matrix metalloproteinase expression in aortic smooth muscle cells. The cells were treated as in (I), and protein expression was analysed by western blot. Representative blots and quantitative results are shown (n = 3). (K) Illustration showing that sirtuin 2 regulates p66Shc acetylation and phosphorylation to repress mitochondrial reactive oxygen species in vascular cells. Statistical analyses were performed using unpaired Student’s t-test (A–H) and one-way ANOVA with the Bonferroni post hoc test (I, J). **P < .01, ***P < .001.
Studies from our laboratory and others have revealed that the adaptor protein p66Shc transcriptionally regulates genes involved in the oxidation–reduction process, thus affecting ageing-induced vascular remodelling and lifespan.33–36,39,40,66 Sirtuin 2 did not affect p66Shc total protein levels in the aortas of aged mice. However, we observed that SIRT2 deficiency up-regulated p66Shc phosphorylation (Figure 4B), which promotes p66Shc mitochondrial translocation and activation to generate mROS.33,34,66 In cultured ASMCs, overexpression of SIRT2 with an adenovirus repressed p66Shc phosphorylation (Figure 4C). In contrast, SIRT2 inhibition with AGK2 up-regulated p66Shc phosphorylation in ASMCs (Figure 4C). Further evidence revealed that SIRT2 directly inhibited p66Shc. Immunoprecipitation assays showed that SIRT2 bound to p66Shc in ASMCs (Figure 4D). In ASMCs, adenovirus-mediated SIRT2 overexpression reduced acetylation of WT p66Shc (Figure 4E). Therefore, we explored the acetylation site of p66Shc to determine whether SIRT2 regulated p66Shc via an acetylation-dependent mechanism. By searching a public database,67 we identified three conserved lysine residues in the CH2 domain at positions 7, 9, and 81 of p66Shc; only lysine 81 (K81) could be acetylated. Thus, we mutated p66Shc at K81 and observed that adenovirus-mediated SIRT2 overexpression did not reduce the acetylation level of the mutated p66Shc in ASMCs, suggesting that SIRT2 deacetylates p66Shc at K81 (Figure 4F). In addition, SIRT2 overexpression did not reduce the phosphorylation of mutated p66Shc, revealing that K81 acetylation of p66Shc determines its phosphorylation (Figure 4G). Thus, we identified K81 as the core target site for SIRT2 deacetylation of p66Shc, which accounts for SIRT2-mediated repression of p66Shc phosphorylation/activation. Collectively, SIRT2 inhibits the adaptor protein p66Shc via an acetylation–phosphorylation mechanism.
Since the ageing-controlling protein p66Shc is a core regulator of mitochondrial and cellular oxidative stress,33–36,40,66 we also tested whether p66Shc contributes to the SIRT2-mediated inhibition of oxidative stress by silencing p66Shc with siRNA (Figure 4H). In ASMCs, SIRT2 inhibition with AGK2 aggravated Ang II–induced accumulation of total superoxide and mROS, and these effects of AGK2 were blocked by p66Shc knockdown (Figure 4I; see Supplementary data online, Figure S7). In addition, p66Shc contributed to the role of SIRT2 in regulating protein oxidation and MMP2/9 expression in ASMCs (Figure 4J). Thus, p66Shc contributes to the role of SIRT2 in regulating oxidative stress and MMPs in vascular cells, which may explain the role of SIRT2 in vascular remodelling and ageing. These findings reveal that SIRT2 deficiency activates p66Shc to drive mitochondrial oxidative signalling, reprogramming the transcriptome of vascular cells to accelerate ageing-induced vascular remodelling (Figure 4K).
Reactive oxygen species partially contributes to sirtuin 2 function in vascular ageing
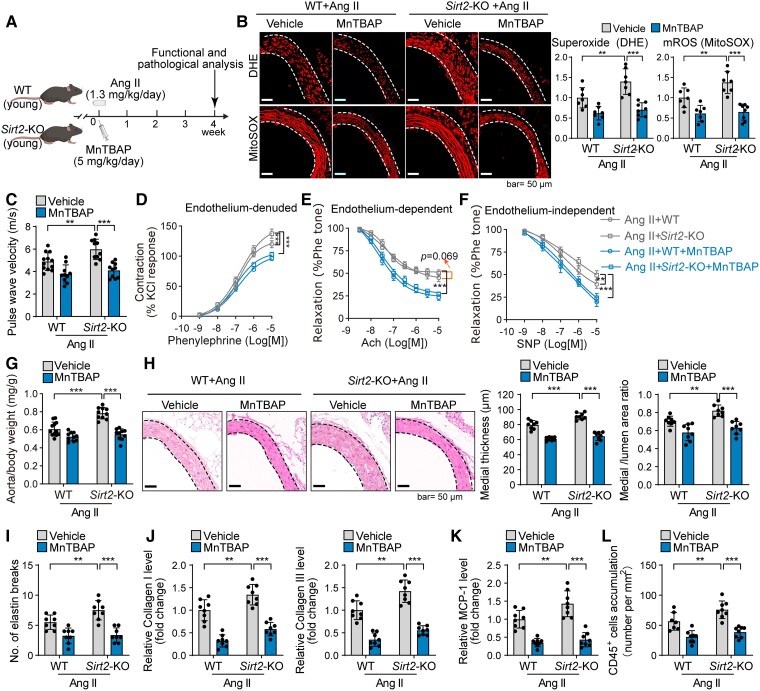
The overload of mROS is a fundamental mechanism underlying vascular remodelling and ageing-related vascular diseases.28,31,32 To test the role of ROS in SIRT2 deficiency–mediated aggravation of vascular remodelling, we first used a mouse vascular remodelling model induced by Ang II (Figure 5A). This ageing-related vasoconstricting peptide hormone promotes vascular remodelling.4,23,68 Sirtuin 2 deficiency promoted Ang II–induced accumulation of total superoxide and mROS, as well as oxidation of macromolecules (Figure 5B; see Supplementary data online, Figure S8A–C). Functional analysis demonstrated that SIRT2 loss promoted Ang II–induced vascular dysfunction, including increased vascular stiffness and constriction–relaxation dysfunction (Figure 5C–F). Sirtuin 2 loss also promoted Ang II–mediated vascular remodelling, including thickened vascular medial layers, breakage of elastin fibres, collagen I and III depositions, MMP2 and MMP9 overexpression, and inflammation (Figure 5G–L; see Supplementary data online, Figure S8D–H). Next, we examined the involvement of ROS in SIRT2 function during vascular ageing by eliminating ROS with MnTBAP (5 mg/kg/day), a known superoxide dismutase mimetic and superoxide scavenger.30,69 Notably, MnTBAP reduced the effects of Sirt2-KO on vascular ROS accumulation, macromolecule oxidation, vascular stiffness, constriction–relaxation dysfunction, remodelling, and inflammation in Ang II–infused mice (Figure 5B–L; see Supplementary data online, Figure S8A–H). Besides, the effects of Sirt2-KO on M1 macrophage polarization in the aorta were repressed by MnTBAP in Ang II–infused mice (see Supplementary data online, Figure S8H–K). In addition, ROS contributed to the inhibitory effects of SIRT2 on Ang II–induced overexpression of MMPs in ASMCs (see Supplementary data online, Figure S8L), revealing that the SIRT2–ROS axis in smooth muscle cells (SMCs) contributes to vascular remodelling.
Figure 5.
Sirtuin 2–reactive oxygen species axis contributes to vascular remodelling and dysfunction in angiotensin II–challenged mice. (A) Experimental design. Male young (4-month-old) wild-type and Sirt2 knockout mice were infused with angiotensin II (1.3 mg/kg/day) to replicate vascular ageing phenotypes. The mice were treated with/without the superoxide scavenger MnTBAP (5 mg/kg/day, i.p.) treatment for 4 weeks. (B) MnTBAP reduced reactive oxygen species in angiotensin II–infused wild-type and Sirt2 knockout mice. Young wild-type and Sirt2 knockout mice were infused with angiotensin II (1.3 mg/kg/day) and intraperitoneally treated with MnTBAP (5 mg/kg/day, daily) or saline (vehicle) for 4 weeks. Then, reactive oxygen species levels in mouse thoracic aortas were analysed by dihydroethidium (total superoxide) and MitoSOX (mitochondrial reactive oxygen species) staining (n = 7–8). (C) MnTBAP treatment reduced the angiotensin II–induced increase in pulse wave velocity (n = 10–12). (D–F) Ex vivo analysis of the vascular constriction–relaxation function of aortas from mice (n = 5). (D) Arterial vessel contractions mediated through phenylephrine. (E) Endothelium-dependent relaxation in response to acetylcholine. (F) Endothelium-independent relaxation in response to nitric oxide donor sodium nitroprusside. (G) MnTBAP treatment reduced the angiotensin II–induced increase in aorta weight (n = 10–12). (H) Representative and quantitative results showing MnTBAP treatment reduced angiotensin II–induced aortic remodelling (n = 8). (I) Elastin van Gieson staining of elastin fibres in thoracic aortas. Quantitative results are shown (n = 8). (J–L) Immunohistochemical staining of collagen I and III (J), monocyte chemotactic protein-1 (K), and CD45 (L). Quantitative results are shown (n = 7–8). Statistical analyses were performed using one-way ANOVA with the Bonferroni post hoc test. **P < .01, ***P < .001.
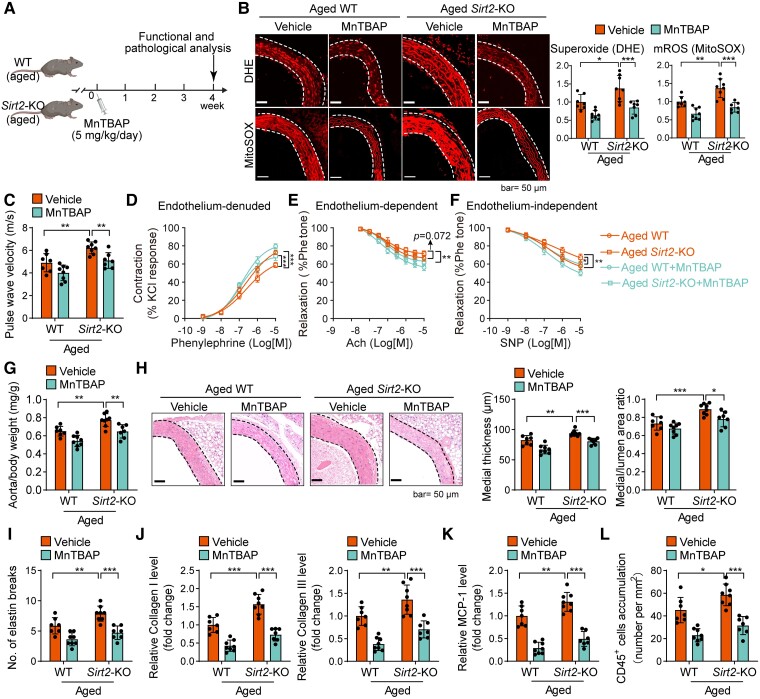
To further confirm whether ROS can serve as a target for treating established vascular remodelling and dysfunction in aged mice, we applied a therapeutic strategy by treating aged WT and Sirt2-KO mice with MnTBAP for 4 weeks (Figure 6A). MnTBAP therapy repressed the effects of SIRT2 deficiency on ROS accumulation and oxidation of macromolecules (proteins, lipids, and DNA) in aged mice (Figure 6B; see Supplementary data online, Figure S9A–C). MnTBAP treatment significantly inhibited vascular dysfunction in aged mice and blocked the effects of Sirt2-KO on ageing-induced vascular dysfunction, including increased vascular stiffness (Figure 6C) and constriction–relaxation dysfunction (Figure 6D–F). Additionally, we observed that MnTBAP treatment reduced vascular remodelling and blocked the effects of SIRT2 deficiency on the increase in aorta weight (Figure 6G) and medial remodelling, including thickened vascular medial layers, breakage of elastin fibres, collagen I and III depositions, MMP2 and MMP9 overexpression, and inflammation (MCP-1 overexpression and immune cell infiltration) in aged mice (Figure 6H–L; see Supplementary data online, Figure S9D–H). MnTBAP also repressed the effects of Sirt2-KO on M1 macrophage polarization in aged aortas (see Supplementary data online, Figure S9I and J). These findings demonstrate that ROS contributes to SIRT2 function in vascular remodelling and may serve as a target for treating established vascular ageing.
Figure 6.
Reactive oxygen species contributes to sirtuin 2 deficiency–mediated aggravation of vascular remodelling and dysfunction in aged mice. (A) Experimental design. Aged wild-type and Sirt2 knockout mice received MnTBAP (5 mg/kg/day, i.p.) or vehicle therapy for 4 weeks. (B) MnTBAP reduced reactive oxygen species in aged wild-type and Sirt2 knockout mice. Aged wild-type and Sirt2 knockout mice were intraperitoneally treated with MnTBAP (5 mg/kg/day, daily) or saline (vehicle) for 4 weeks. Then, reactive oxygen species levels in mouse thoracic aortas were analysed by dihydroethidium (total superoxide) and MitoSOX (mitochondrial reactive oxygen species) staining (n = 7–8). (C) MnTBAP therapy mitigated sirtuin 2 effects on pulse wave velocity in aged mice (n = 7–8). (D–F) Ex vivo analysis of mouse aortas’ vascular constriction–relaxation function (n = 5). Arterial vessel contractions mediated through phenylephrine (D), endothelium-dependent relaxation in response to acetylcholine (E), endothelium-independent relaxation in response to nitric oxide donor sodium nitroprusside (F), and an endothelium-independent agonist. (G) MnTBAP therapy reduced sirtuin 2 effects on aorta weight in aged mice (n = 7–8). (H) Representative and quantitative results showing MnTBAP therapy reduced sirtuin 2 effects on aortic remodelling in aged mice (n = 7–8). (I) Elastin van Gieson staining of elastin fibres in thoracic aortas. Quantitative results are shown (n = 7–8). (J–L) Immunohistochemical staining of collagen I and III (J), monocyte chemotactic protein-1 (K), and CD45 (L). Quantitative results are shown (n = 7–8). Statistical analyses were performed using one-way ANOVA with the Bonferroni post hoc test. *P < .05, **P < .01, ***P < .001.
Sirtuin 2 is associated with ageing-related vascular remodelling and related diseases in humans
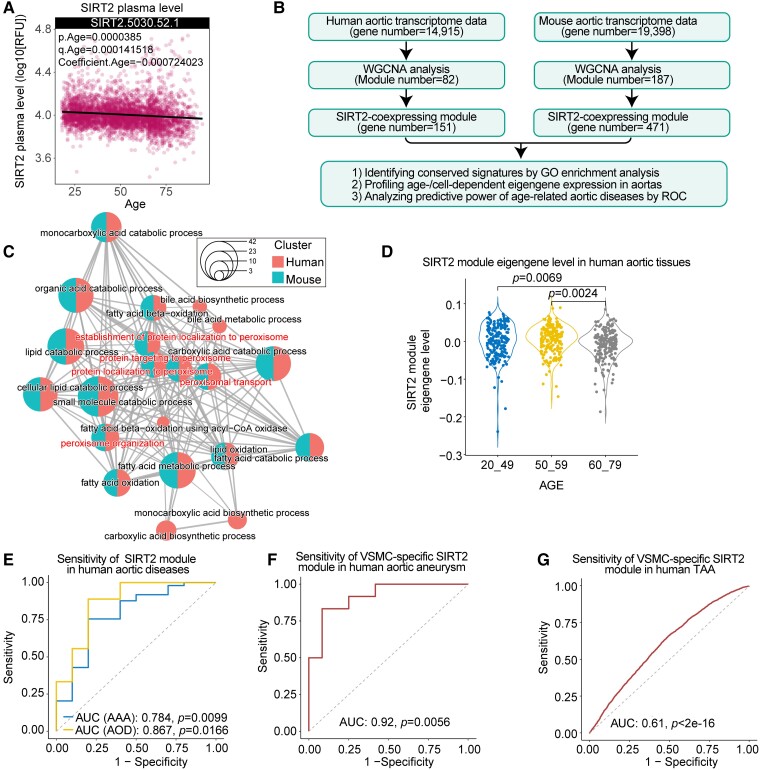
To explore the potential implications of SIRT2 in human vascular ageing, we examined plasma SIRT2 levels across the lifespan using a public proteome dataset,70 which included 2925 plasma proteins from 4263 young adults to nonagenarians (18–95 years old). The results showed that the plasma levels of SIRT2 were negatively correlated with age (Figure 7A),70 indicating that a decline in SIRT2 levels may be an important risk factor for ageing and related diseases. Since the Sirt2 mRNA level is not changed with ageing, we performed an alternative analysis of SIRT2-coexpressing genes in human and mouse aortas to test their potential roles in vascular biology and diseases.71 To this end, Weighted Gene Coexpression Network Analysis (WGCNA) was performed to identify the SIRT2 coexpression module using transcriptome data of 607 human aortic tissues and 10 mouse aortic tissues (Figure 7B; see Supplementary data online, Figure S10A and B).71,72 Then, GO signalling pathway enrichment analysis of the SIRT2 coexpression module was performed. The results revealed the enrichments of pathways involved in the oxidation–reduction system and mitochondrial metabolism in both the human and mouse SIRT2 coexpression modules (Figure 7C). Thus, the effects of SIRT2 on the mitochondrial oxidation–reduction system may be conserved for SIRT2 function in the vascular system, which is consistent with our findings in the aged mouse aorta (Figs 3–6). Next, we compared the expression levels of the SIRT2 coexpression module eigengene in humans and mice of various ages. We observed that the SIRT2 coexpression module eigengene was reduced in old (>60 years) human aortas compared with young aortas (Figure 7D).
Figure 7.
Sirtuin 2 in ageing-related arterial remodelling and diseases in humans. (A) Plasma sirtuin 2 level is decreased with ageing. Plasma sirtuin 2 level across the lifespan was analysed using a public proteome dataset,70 which involved plasma protein data from 4263 young adults to nonagenarians (18–95 years old). (B) Flowchart for the identification of the sirtuin 2 coexpression module in human and mouse aortas. (C) Network showing the gene ontology enrichment of the sirtuin 2 coexpression module in humans and mice. The dot size denotes the ratio of genes within the pathway, and the dot colour represents the species. (D) Comparison of the expression level of the sirtuin 2 coexpression module eigengene in human aortas across three age groups (Kruskal–Wallis test with Dunn’s post hoc test). (E–G) Receiver operating characteristic (ROC) curves of the sirtuin 2 module level show that the sirtuin 2 coexpression module is associated with age-related human aortic diseases. Area under the curve was calculated by receiver operating characteristic analysis. (E) Sirtuin 2 coexpression module exhibits a predictive significance for human abdominal aortic aneurysm and aortic occlusive disease. Gene expression data (GSE57691) of human abdominal aortic aneurysm and atherosclerosis (aortic occlusive disease) were used to analyse the level of the sirtuin 2 coexpression module, and subsequently, receiver operating characteristic analysis was performed. (F) Sirtuin 2 coexpression module in smooth muscle cells shows a predictive significance for aortic aneurysms. Gene expression data (GSE140947) of fresh human normal aortic media smooth muscle cells and aortic aneurysm media smooth muscle cells were subjected to evaluate the sirtuin 2 coexpression module, and subsequently, receiver operating characteristic analysis was performed. (G) Sirtuin 2 coexpression module in single smooth muscle cells exhibits a predictive significance for thoracic aortic aneurysms. scRNA-seq data of control and human thoracic aortic aneurysm tissues (GSE155468) were used to analyse the sirtuin 2 coexpression module level in smooth muscle cells, and then receiver operating characteristic analysis was performed at the single-cell level.
To examine the potential implications of the SIRT2 coexpression module in age-related aortic diseases, we applied the receiver operating characteristic (ROC) curve to analyse the predictive capabilities of the SIRT2 coexpression module in distinguishing aortic tissues from controls and patients with age-related aortic diseases. The results showed that the SIRT2 coexpression module exhibited a predictive significance for abdominal aortic aneurysm (AAA) and aortic occlusive disease (AOD), with an area under the curve (AUC) of 0.784 and 0.867, respectively (Figure 7E). These findings suggest that the SIRT2 coexpression module has a predictive significance for ageing and age-related aortic disease. Finally, we explored the expression pattern of the SIRT2 coexpression module eigengene in human aortic cells using public scRNA-seq data of human aortic tissues (GSE155468).73 Among the major types of vascular cells (SMCs, endothelial cells, lymphocytes, and myeloid cells), SMCs had the highest expression levels of SIRT2-coexpressing genes (see Supplementary data online, Figure S10C), which was matched with our findings in mice (Figure 1F). In addition, the level of SIRT2-coexpressing genes was reduced in SMCs from age-related thoracic aortic aneurysms (TAA) compared with control aortic samples (see Supplementary data online, Figure S10D). Thus, we performed ROC analysis using SMC gene expression data from the human control and aortic aneurysm (AA) samples. The results revealed that the SIRT2 coexpression module level in SMCs has a high predictive significance for AA, with an AUC of 0.92 at the patient level (Figure 7F). We also performed ROC analysis using scRNA-seq data of TAA. The analysis showed that the SIRT2 coexpression module level in SMCs has a predictive significance for TAA, with an AUC of 0.61 at the single-cell level (Figure 7G).
The integrative analysis of multi-omics data, including 4263 proteomes, 607 transcriptomes, and 52 882 single-cell transcriptomes, reveals that the role of cytoplasmic SIRT2 in human vascular ageing and age-related vascular diseases is related to its effects on VSMCs and the mitochondrial oxidation–reduction system. These findings are consistent with our findings in mice.
Discussion
In the present study, we identified a previously unrecognized role of the epigenetic factor SIRT2 in vascular ageing in humans and mice. Among the sirtuins, SIRT2 has the highest basal level in the aortas of humans and mice, and SIRT2 activity and protein levels are reduced with ageing. In old mice, SIRT2 deficiency aggravates vascular dysfunction, which correlates with mitochondrial oxidative stress, transcriptome reprogramming, and medial remodelling. Sirtuin 2 expression has also been correlated with ageing and related vascular diseases in humans. The epigenetic regulator SIRT2 governs mROS production by deacetylating and inhibiting p66Shc and regulates vascular transcriptome reprogramming to participate in ageing-induced vascular remodelling and arterial diseases (Structured Graphical Abstract).
Ageing is an independent risk factor for vascular dysfunction,3,74 but the underlying mechanisms remain largely unknown. Although the roles of sirtuins in vascular diseases have been analysed in young mice,40,75–79 their roles in vascular ageing remain unknown. The functions of sirtuins in individual tissues largely depend on their expression levels.16 Analysis of sirtuin expression patterns revealed that SIRT2 had the highest expression in human and mouse aortas. The nuclear members SIRT1/6/7 are also expressed in human and mouse aortas at considerable levels. Previous studies in vascular biology mainly focused on the roles of SIRT1 and SIRT6,16,41,80 and our previous studies showed that SIRT1 and SIRT6 regulated aortic diseases such as atherosclerosis and arterial aneurysms.81–83 Recently, SIRT7 was reported to protect the vascular endothelium.76 The mitochondrial sirtuins SIRT3 and SIRT5 are moderately expressed in the aortas, and some studies reported their roles in arterial thrombosis and hypertension.78,84 Notably, mitochondrial SIRT4 was lowly expressed in the aortas, consistent with its mild roles in vascular biology.85 All these studies were performed on young animals. The roles of endogenous sirtuins in vascular ageing remain unknown. Our evidence in old animals demonstrated that SIRT2 deficiency amplified ageing-induced vascular remodelling and that SIRT2 correlated with ageing and vascular disease in humans. Therefore, the epigenetic enzyme SIRT2 is critical for preventing ageing-induced vascular remodelling. Further studies are needed to evaluate the role of other sirtuins in vascular ageing and disease in older animals.
We demonstrated that the SIRT2-coexpressing module is correlated with vascular ageing in humans and has a predictive significance for age-related aortic diseases, including atherosclerosis and arterial aneurysms. Ageing-related coronary artery diseases, such as acute myocardial infarction, are complications of atherosclerosis. Our previous study in a human cohort (n = 752) demonstrated that the functional genetic variants within the SIRT2 gene promoter were risk factors for acute myocardial infarction.86 Notably, we showed that circulating SIRT2 protein levels declined with ageing in humans.70 These findings revealed one possibility that loss of circulating SIRT2 may serve as a biomarker for biological ageing and a prognostic factor for ageing-related vascular diseases and complications. However, further studies are still needed to test whether circulating SIRT2 can regulate vascular cells to prevent vascular ageing in animals. If yes, it is worth testing the values of circulating SIRT2 in predicting vascular ageing and diseases, and further, the recombinant SIRT2 protein may be used to treat vascular ageing and related diseases. One such example is our current finding that circulating CCL17 predicted cardiac and vascular ageing in humans and mice, and recombinant anti-CCL17 antibodies delayed cardiac and vascular ageing in animals.49,50
Sirtuin 2 was considered an unwholesome factor in cancer biology for a long time.87,88 However, our and others’ studies have demonstrated the protective roles of SIRT2 in ageing-induced cardiac remodelling, insulin resistance, and remyelination.22,23,27 Therefore, SIRT2 may be a geroprotective deacetylase in ageing and age-related diseases, and SIRT2 activators may have potential health benefits. Overexpression of SIRT2 or treatment with nicotinamide mononucleotide (NMN, an NAD+ precursor) rejuvenated oligodendrocyte progenitor cells in old mice27 and extended the lifespan of progeria mice.21 Our findings in the present work suggested that SIRT2 deficiency was critical for aggravating vascular ageing in humans and mice. The activity of SIRT2 and the coexpression module were reduced in aged aortas in humans and mice. The activity of SIRT2 is controlled by the metabolite NAD+.15 Our previous study has demonstrated that NAD+ levels declined in aged tissues.27,89 The decline in NAD+ also contributed to the reduced SIRT2 activity. Activation of SIRT2 by supplying NAD+ may be a potential strategy for preventing or treating vascular ageing. Indeed, our recent studies revealed that activating SIRT2 with NMN (NAD+ donor) promoted remyelination during ageing.27 Besides, activating SIRT2 with NMN also delayed cardiac ageing in premature mice.21 Thus, activating intracellular SIRT2 with NAD+ donors or other specific SIRT2 activators may serve as a promising strategy for preventing vascular ageing and related vascular diseases in humans. Currently, many SIRT2 inhibitors have been identified or synthesized,60,87,88 but further studies screening SIRT2 activators are necessary to evaluate the application of SIRT2 as a therapeutic target for treating ageing-related diseases.
In aged mice, SIRT2 reprogrammes the transcriptome of the aorta. Cytoplasmic SIRT2 enters the nucleus to regulate histone acetylation, crotonylation, and benzoylation to regulate transcription directly.18,61,62 However, SIRT2 loss does not strongly affect H3K18ac, H3K18cr, or histone benzoylation in aged aortas. Therefore, SIRT2 may indirectly regulate vascular transcriptome reprogramming. Because the transcriptome data of aged mice revealed the reprogramming of oxidative pathways, we focused on oxidative stress, which induces macromolecular damage and serves as a signal regulator of the proteome, epigenome, and transcriptome.28,32,90 The cytoplasm–mitochondria traveller p66Shc regulates the transcriptome by increasing cellular mROS levels, subsequently affecting individual transcriptional events within the nuclei.33,66,91 Here, we observed that SIRT2 repressed the phosphorylation of p66Shc by deacetylating p66Shc at K81 to reduce mROS levels. p66Shc-driven mROS play a critical role in regulating the vascular transcriptome and phenotypes.34,37,40,66,91 Thus, SIRT2 loss induces the hyperacetylation/activation of p66Shc, resulting in ROS overload, transcriptome reprogramming, and subsequent ageing-induced vascular remodelling and diseases. Our findings highlight that the deacetylase SIRT2 acts as an orchestrator of the cytoplasm–mitochondria–nucleus axis to control the development of vascular remodelling and ageing.
Sirtuin 2 repressed p66Shc to reduce mROS generation. Our experiments in VSMCs suggested that either p66Shc silencing or MnTBAP treatment could block the inhibitory effects of SIRT2 deficiency on the expression of MMP2 and MMP9, which contribute to vascular remodelling and dysfunction.3,52,56 p66Shc hyperacetylation/hyperactivation and related oxidative damage and vascular remodelling (MMPs) were also observed in human AAA, an ageing-related aortic disease (see Supplementary data online, Figure S11). The superoxide scavenger MnTBAP also represses vascular remodelling in aged mice and partially represses the effects of SIRT2 deficiency. Thus, the p66Shc–ROS axis contributed to SIRT2 deficiency–induced MMPs in VSMCs, which may account for the aggravated vascular ageing observed in the aortas of Sirt2-KO mice. The cytoplasm–mitochondria axis SIRT2–p66Shc–mROS could be targeted for treating vascular ageing and diseases. However, the SIRT2–p66Shc–mROS axis is complex. MnTBAP only targets superoxide,92 the product of SIRT2 deficiency, rather than the SIRT2–p66Shc–mROS axis. In addition, as ROS in these animals were not found solely in SIRT2-expressed tissues/cells, the observed ROS-related findings may not be specific to SIRT2. For instance, oxidative stress affects endothelial cells and leads to age-related endothelial dysfunction,28 and the ROS scavenger might rescue endothelial function. Therefore, it would be interesting to study the role of ROS in SIRT2-expressed tissues and cells during vascular ageing.
This study had some limitations. We provided evidence that the p66Shc–mROS pathway partially contributed to the role of SIRT2 in vascular ageing, but our transcriptome analysis revealed that SIRT2 regulates multiple pathways in aged aortas. Sirtuin 2 also affects oxidative stress via multiple mechanisms.93,94 Thus, other mechanisms may contribute to the effects of SIRT2 on age-induced vascular dysfunction. Second, we used male mice to study SIRT2 function in vascular ageing; further studies in female animals are needed because the responses of males and females to ageing-induced vascular remodelling are very different.42,43 Finally, some clinical analyses were based on public datasets, and the lack of clinical information affected the statistical analysis. Thus, using cohorts with complete clinical character information for further analysis of SIRT2 function in human ageing and related vascular diseases would help strengthen the clinical conclusions of the present study.
Conclusions
In conclusion, our study demonstrates that SIRT2 is a repressor of vascular ageing, which may contribute to NAD+-mediated clinical benefits in preventing age-dependent vascular diseases. Therefore, SIRT2 is a potential therapeutic target for vascular and systemic rejuvenation.
Supplementary Material
Acknowledgements
The authors thank all members of the laboratory of Prof. De-Pei Liu for their technical support and constructive discussion. In addition, all authors thank Drs Ju-Ying Qian, Zhangwei Chen, and Ai-Jun Sun (Fudan University) for their assistance with this work. The authors thank Prof. Yong-Zhang Luo (Tsinghua University) for providing the mouse monoclonal anti-acetyl-p66ShcK81 antibody. Figure 4K was created using BioRender.com.
Contributor Information
Yang Zhang, Department of Biochemistry & Molecular Biology, State Key Laboratory of Common Mechanism Research for Major Diseases, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences & Peking Union Medical College, 5 Dong Dan San Tiao, Beijing 100005, China.
Xiaoman Wang, Department of Biochemistry & Molecular Biology, State Key Laboratory of Common Mechanism Research for Major Diseases, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences & Peking Union Medical College, 5 Dong Dan San Tiao, Beijing 100005, China.
Xun-Kai Li, Department of Biochemistry & Molecular Biology, State Key Laboratory of Common Mechanism Research for Major Diseases, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences & Peking Union Medical College, 5 Dong Dan San Tiao, Beijing 100005, China.
Shuang-Jie Lv, Department of Biochemistry & Molecular Biology, State Key Laboratory of Common Mechanism Research for Major Diseases, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences & Peking Union Medical College, 5 Dong Dan San Tiao, Beijing 100005, China.
He-Ping Wang, Department of Biochemistry & Molecular Biology, State Key Laboratory of Common Mechanism Research for Major Diseases, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences & Peking Union Medical College, 5 Dong Dan San Tiao, Beijing 100005, China.
Yang Liu, Key Laboratory of Birth Defects and Related Diseases of Women and Children of MOE, State Key Laboratory of Biotherapy, West China Second University Hospital, Sichuan University, No.17 People's South Road, Chengdu, Sichuan 610041, China; Division of Vascular Surgery, Department of General Surgery, and Laboratory of Cardiovascular Diseases, West China Hospital, Sichuan University, No.17 People's South Road, Chengdu, Sichuan 610041, China.
Jingyue Zhou, Key Laboratory of Birth Defects and Related Diseases of Women and Children of MOE, State Key Laboratory of Biotherapy, West China Second University Hospital, Sichuan University, No.17 People's South Road, Chengdu, Sichuan 610041, China; National Health Commission Key Laboratory of Chronobiology, Sichuan University, No.17 People's South Road, Chengdu, Sichuan 610041, China; Development and Related Diseases of Women and Children, Key Laboratory of Sichuan Province, West China Second University Hospital, Sichuan University, No.17 People's South Road, Chengdu, Sichuan 610041, China.
Hui Gong, Key Laboratory of Birth Defects and Related Diseases of Women and Children of MOE, State Key Laboratory of Biotherapy, West China Second University Hospital, Sichuan University, No.17 People's South Road, Chengdu, Sichuan 610041, China; National Clinical Research Center for Geriatrics, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, No.17 People's South Road, Chengdu, Sichuan 610041, China.
Xiao-Feng Chen, School of Basic Medical Sciences, Chengdu University of Traditional Chinese Medicine, 1166 Liutai Avenue, Chengdu, Sichuan 611137, China.
Si-Chong Ren, Department of Nephrology, First Affiliated Hospital of Chengdu Medical College, 783 Xindu Avenue, Chengdu, Sichuan 610500, China.
Huina Zhang, Beijing Institute of Heart Lung and Blood Vessel Diseases, Beijing Anzhen Hospital, Capital Medical University, No. 2 Anzhen Road, Beijing 10029, China.
Yuxiang Dai, Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai Institute of Cardiovascular Diseases, National Clinical Research Center for Interventional Medicine, 180 Fenglin Road, Shanghai 200032, China.
Hua Cai, Division of Molecular Medicine, Department of Anesthesiology, David Geffen School of Medicine at University of California Los Angeles, Los Angeles, CA 90095, USA; Division of Cardiology, Department of Medicine, David Geffen School of Medicine at University of California Los Angeles, Los Angeles, CA 90095, USA.
Bo Yan, Institute of Precision Medicine, Jining Medical University, 133 Hehua Road, Taibaihu New District, Jining, Shandong 272067, China.
Hou-Zao Chen, Department of Biochemistry & Molecular Biology, State Key Laboratory of Common Mechanism Research for Major Diseases, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences & Peking Union Medical College, 5 Dong Dan San Tiao, Beijing 100005, China; Medical Epigenetics Research Center, Chinese Academy of Medical Sciences, 5 Dong Dan San Tiao, Beijing 100005, China.
Xiaoqiang Tang, Key Laboratory of Birth Defects and Related Diseases of Women and Children of MOE, State Key Laboratory of Biotherapy, West China Second University Hospital, Sichuan University, No.17 People's South Road, Chengdu, Sichuan 610041, China; National Health Commission Key Laboratory of Chronobiology, Sichuan University, No.17 People's South Road, Chengdu, Sichuan 610041, China; Development and Related Diseases of Women and Children, Key Laboratory of Sichuan Province, West China Second University Hospital, Sichuan University, No.17 People's South Road, Chengdu, Sichuan 610041, China.
Supplementary data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
All authors declare no conflict of interest for this contribution.
Data Availability
All data are available in the manuscript or the supplementary materials.
Funding
This work was supported by grants from the National Key Research and Development Project of China (2020YFC2008003 and 2019YFA0801500), National Natural Science Foundation of China (81970426, 82030017, 82225007, and 82200437), Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CIFMS2021-I2M-1-050, CIFMS2021-I2M-1-008, CIFMS2022-I2M-2-002, and CIFM2022-RC180-03), and Shanghai Clinical Research Center for Interventional Medicine (19MC1910300).
Ethical Approval
All animal protocols were approved by the Laboratory Animal Ethics Committee of the Institute of Basic Medical Sciences (Chinese Academy of Medical Sciences) and West China Second University Hospital (Sichuan University). All protocols using human aortic samples were approved by the Ethical Committee of the Chinese Academy of Medical Sciences and Peking Union Medical College.
Pre-registered Clinical Trial Number
None supplied.
References
- 1. Roth GA, Forouzanfar MH, Moran AE, Barber R, Nguyen G, Feigin VL, et al. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med 2015;372:1333–1341. 10.1056/NEJMoa1406656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Camici GG, Liberale L. Aging: the next cardiovascular disease? Eur Heart J 2017;38:1621–1623. 10.1093/eurheartj/ehx239 [DOI] [PubMed] [Google Scholar]
- 3. Paneni F, Diaz Cañestro C, Libby P, Lüscher TF, Camici GG. The aging cardiovascular system: understanding it at the cellular and clinical levels. J Am Coll Cardiol 2017;69:1952–1967. 10.1016/j.jacc.2017.01.064 [DOI] [PubMed] [Google Scholar]
- 4. North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res 2012;110:1097–1108. 10.1161/CIRCRESAHA.111.246876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grunewald M, Kumar S, Sharife H, Volinsky E, Gileles-Hillel A, Licht T, et al. Counteracting age-related VEGF signaling insufficiency promotes healthy aging and extends life span. Science 2021;373:eabc8479. 10.1126/science.abc8479 [DOI] [PubMed] [Google Scholar]
- 6. Das A, Huang GX, Bonkowski MS, Longchamp A, Li C, Schultz MB, et al. Impairment of an endothelial NAD+-H2S signaling network is a reversible cause of vascular aging. Cell 2018;173:74–89.e20. 10.1016/j.cell.2018.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kane AE, Sinclair DA. Sirtuins and NAD+ in the development and treatment of metabolic and cardiovascular diseases. Circ Res 2018;123:868–885. 10.1161/CIRCRESAHA.118.312498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abdellatif M, Sedej S, Kroemer G. NAD+ metabolism in cardiac health, aging, and disease. Circulation 2021;144:1795–1817. 10.1161/CIRCULATIONAHA.121.056589 [DOI] [PubMed] [Google Scholar]
- 9. de Picciotto NE, Gano LB, Johnson LC, Martens CR, Sindler AL, Mills KF, et al. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell 2016;15:522–530. 10.1111/acel.12461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mills KF, Yoshida S, Stein LR, Grozio A, Kubota S, Sasaki Y, et al. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab 2016;24:795–806. 10.1016/j.cmet.2016.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yoshino M, Yoshino J, Kayser BD, Patti GJ, Franczyk MP, Mills KF, et al. Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science 2021;372:1224–1229. 10.1126/science.abe9985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Korotkov A, Seluanov A, Gorbunova V. Sirtuin 6: linking longevity with genome and epigenome stability. Trends Cell Biol 2021;31:994–1006. 10.1016/j.tcb.2021.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Winnik S, Auwerx J, Sinclair DA, Matter CM. Protective effects of sirtuins in cardiovascular diseases: from bench to bedside. Eur Heart J 2015;36:3404–3412. 10.1093/eurheartj/ehv290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Camici GG, Savarese G, Akhmedov A, Lüscher TF. Molecular mechanism of endothelial and vascular aging: implications for cardiovascular disease. Eur Heart J 2015;36:3392–3403. 10.1093/eurheartj/ehv587 [DOI] [PubMed] [Google Scholar]
- 15. Covarrubias AJ, Perrone R, Grozio A, Verdin E. NAD+ metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol 2021;22:119–141. 10.1038/s41580-020-00313-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grootaert MOJ, Bennett MR. Sirtuins in atherosclerosis: guardians of healthspan and therapeutic targets. Nat Rev Cardiol 2022;19:668–683. 10.1038/s41569-022-00685-x [DOI] [PubMed] [Google Scholar]
- 17. Liu G, Park S-H, Imbesi M, Nathan WJ, Zou X, Zhu Y, et al. Loss of NAD-dependent protein deacetylase sirtuin-2 alters mitochondrial protein acetylation and dysregulates mitophagy. Antioxid Redox Signal 2017;26:849–863. 10.1089/ars.2016.6662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eskandarian HA, Impens F, Nahori MA, Soubigou G, Coppée JY, Cossart P, et al. A role for SIRT2-dependent histone H3K18 deacetylation in bacterial infection. Science 2013;341:1238858. 10.1126/science.1238858 [DOI] [PubMed] [Google Scholar]
- 19. Sun X, Zhang Y, Chen X-F, Tang X. Acylations in cardiovascular biology and diseases, what’s beyond acetylation. EBioMedicine 2023;87:104418. 10.1016/j.ebiom.2022.104418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen X-F, Chen X, Tang X. Short-chain fatty acid, acylation and cardiovascular diseases. Clin Sci 2020;134:657–676. 10.1042/cs20200128 [DOI] [PubMed] [Google Scholar]
- 21. North BJ, Rosenberg MA, Jeganathan KB, Hafner AV, Michan S, Dai J, et al. SIRT2 induces the checkpoint kinase BubR1 to increase lifespan. EMBO J 2014;33:1438–1453. 10.15252/embj.201386907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. He M, Chiang HH, Luo H, Zheng Z, Qiao Q, Wang L, et al. An acetylation switch of the NLRP3 inflammasome regulates aging-associated chronic inflammation and insulin resistance. Cell Metab 2020;31:580–591.e5. 10.1016/j.cmet.2020.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tang X, Chen XF, Wang NY, Wang XM, Liang ST, Zheng W, et al. SIRT2 acts as a cardioprotective deacetylase in pathological cardiac hypertrophy. Circulation 2017;136:2051–2067. 10.1161/CIRCULATIONAHA.117.028728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bertoldo MJ, Listijono DR, Ho WJ, Riepsamen AH, Goss DM, Richani D, et al. NAD+ repletion rescues female fertility during reproductive aging. Cell Rep 2020;30:1670–1681.e7. 10.1016/j.celrep.2020.01.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luo H, Mu WC, Karki R, Chiang HH, Mohrin M, Shin JJ, et al. Mitochondrial stress-initiated aberrant activation of the NLRP3 inflammasome regulates the functional deterioration of hematopoietic stem cell aging. Cell Rep 2019;26:945–954.e4. 10.1016/j.celrep.2018.12.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jablonska B, Adams KL, Kratimenos P, Li Z, Strickland E, Haydar TF, et al. Sirt2 promotes white matter oligodendrogenesis during development and in models of neonatal hypoxia. Nat Commun 2022;13:4771. 10.1038/s41467-022-32462-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma XR, Zhu X, Xiao Y, Gu HM, Zheng SS, Li L, et al. Restoring nuclear entry of sirtuin 2 in oligodendrocyte progenitor cells promotes remyelination during ageing. Nat Commun 2022;13:1225. 10.1038/s41467-022-28844-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sies H, Belousov VV, Chandel NS, Davies MJ, Jones DP, Mann GE, et al. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat Rev Mol Cell Biol 2022;23:499–515. 10.1038/s41580-022-00456-z [DOI] [PubMed] [Google Scholar]
- 29. Münzel T, Gori T, Keaney JF Jr, Maack C, Daiber A. Pathophysiological role of oxidative stress in systolic and diastolic heart failure and its therapeutic implications. Eur Heart J 2015;36:2555–2564. 10.1093/eurheartj/ehv305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luo YX, Tang X, An XZ, Xie XM, Chen XF, Zhao X, et al. SIRT4 accelerates Ang II-induced pathological cardiac hypertrophy by inhibiting manganese superoxide dismutase activity. Eur Heart J 2017;38:1389–1398. 10.1093/eurheartj/ehw138 [DOI] [PubMed] [Google Scholar]
- 31. Tejero J, Shiva S, Gladwin MT. Sources of vascular nitric oxide and reactive oxygen species and their regulation. Physiol Rev 2019;99:311–379. 10.1152/physrev.00036.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Forman HJ, Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov 2021;20:689–709. 10.1038/s41573-021-00233-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mir HA, Ali R, Mushtaq U, Khanday FA. Structure-functional implications of longevity protein p66Shc in health and disease. Ageing Res Rev 2020;63:101139. 10.1016/j.arr.2020.101139 [DOI] [PubMed] [Google Scholar]
- 34. Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, et al. The p66Shc adaptor protein controls oxidative stress response and life span in mammals. Nature 1999;402:309–313. 10.1038/46311 [DOI] [PubMed] [Google Scholar]
- 35. Xiao Y, Xia J, Cheng J, Huang H, Zhou Y, Yang X, et al. Inhibition of S-adenosylhomocysteine hydrolase induces endothelial dysfunction via epigenetic regulation of p66Shc-mediated oxidative stress pathway. Circulation 2019;139:2260–2277. 10.1161/CIRCULATIONAHA.118.036336 [DOI] [PubMed] [Google Scholar]
- 36. Costantino S, Paneni F, Virdis A, Hussain S, Mohammed SA, Capretti G, et al. Interplay among H3K9-editing enzymes SUV39H1, JMJD2C and SRC-1 drives p66Shc transcription and vascular oxidative stress in obesity. Eur Heart J 2019;40:383–391. 10.1093/eurheartj/ehx615 [DOI] [PubMed] [Google Scholar]
- 37. Spescha RD, Klohs J, Semerano A, Giacalone G, Derungs RS, Reiner MF, et al. Post-ischaemic silencing of p66Shc reduces ischaemia/reperfusion brain injury and its expression correlates to clinical outcome in stroke. Eur Heart J 2015;36:1590–1600. 10.1093/eurheartj/ehv140 [DOI] [PubMed] [Google Scholar]
- 38. Francia P, delli Gatti C, Bachschmid M, Martin-Padura I, Savoia C, Migliaccio E, et al. Deletion of p66Shc gene protects against age-related endothelial dysfunction. Circulation 2004;110:2889–2895. 10.1161/01.Cir.0000147731.24444.4d [DOI] [PubMed] [Google Scholar]
- 39. Huang C, Ding T, Zhang Y, Li X, Sun X, Lv S, et al. The longevity protein p66Shc is required for neonatal heart regeneration. J Mol Cell Cardiol 2023;177:21–27. 10.1016/j.yjmcc.2023.02.004 [DOI] [PubMed] [Google Scholar]
- 40. Zhou S, Chen HZ, Wan YZ, Zhang QJ, Wei YS, Huang S, et al. Repression of p66Shc expression by SIRT1 contributes to the prevention of hyperglycemia-induced endothelial dysfunction. Circ Res 2011;109:639–648. 10.1161/CIRCRESAHA.111.243592 [DOI] [PubMed] [Google Scholar]
- 41. Mengozzi A, Costantino S, Paneni F, Duranti E, Nannipieri M, Mancini R, et al. Targeting SIRT1 rescues age- and obesity-induced microvascular dysfunction in ex vivo human vessels. Circ Res 2022;131:476–491. https://doi.org/101161CIRCRESAHA122320888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moreau KL. Modulatory influence of sex hormones on vascular aging. Am J Physiol Heart Circ Physiol 2019;316:H522–h526. 10.1152/ajpheart.00745.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhernakova DV, Sinha T, Andreu-Sánchez S, Prins JR, Kurilshikov A, Balder J-W, et al. Age-dependent sex differences in cardiometabolic risk factors. Nat Cardiovasc Res 2022;1:844–854. 10.1038/s44161-022-00131-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Guo X, Kassab GS. Variation of mechanical properties along the length of the aorta in C57bl/6 mice. Am J Physiol Heart Circ Physiol 2003;285:H2614–H2622. 10.1152/ajpheart.00567.2003 [DOI] [PubMed] [Google Scholar]
- 45. Dunn T. Spontaneous lesions of mice. In: Rilein WE and McCoy JR (eds.), Pathology of Laboratory Animals.: Charles C. Thomas, 1965, 303–329. [Google Scholar]
- 46. Ilyas I, Little PJ, Liu Z, Xu Y, Kamato D, Berk BC, et al. Mouse models of atherosclerosis in translational research. Trends Pharmacol Sci 2022;43:920–939. 10.1016/j.tips.2022.06.009 [DOI] [PubMed] [Google Scholar]
- 47. Riehle C, Bauersachs J. Small animal models of heart failure. Cardiovasc Res 2019;115:1838–1849. 10.1093/cvr/cvz161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tang X, Chen XF, Sun X, Xu P, Zhao X, Tong Y, et al. Short-chain enoyl-CoA hydratase mediates histone crotonylation and contributes to cardiac homeostasis. Circulation 2021;143:1066–1069. 10.1161/CIRCULATIONAHA.120.049438 [DOI] [PubMed] [Google Scholar]
- 49. Zhang Y, Ye Y, Tang X, Wang H, Tanaka T, Tian R, et al. CCL17 Acts as a novel therapeutic target in pathological cardiac hypertrophy and heart failure. J Exp Med 2022;219:e20200418. 10.1084/jem.20200418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang Y, Tang X, Wang Z, Wang L, Chen Z, Qian J, et al. The chemokine CCL17 is a novel therapeutic target for cardiovascular aging. Signal Transduct Target Ther 2023;8:157. 10.1038/s41392-023-01363-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang Y, Yang J, Hong T, Chen X, Cui L. SIRT2: controversy and multiple roles in disease and physiology. Ageing Res Rev 2019;55:100961. 10.1016/j.arr.2019.100961 [DOI] [PubMed] [Google Scholar]
- 52. Boutouyrie P, Chowienczyk P, Humphrey JD, Mitchell GF. Arterial stiffness and cardiovascular risk in hypertension. Circ Res 2021;128:864–886. 10.1161/circresaha.121.318061 [DOI] [PubMed] [Google Scholar]
- 53. Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res 2018;123:849–867. 10.1161/CIRCRESAHA.118.311378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Donato AJ, Machin DR, Lesniewski LA. Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ Res 2018;123:825–848. 10.1161/CIRCRESAHA.118.312563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liberale L, Montecucco F, Tardif J-C, Libby P, Camici GG. Inflamm-ageing: the role of inflammation in age-dependent cardiovascular disease. Eur Heart J 2020;41:2974–2982. 10.1093/eurheartj/ehz961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liberale L, Badimon L, Montecucco F, Lüscher TF, Libby P, Camici GG. Inflammation, aging, and cardiovascular disease: JACC review topic of the week. J Am Coll Cardiol 2022;79:837–847. 10.1016/j.jacc.2021.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Puca AA, Carrizzo A, Spinelli C, Damato A, Ambrosio M, Villa F, et al. Single systemic transfer of a human gene associated with exceptional longevity halts the progression of atherosclerosis and inflammation in ApoE knockout mice through a CXCR4-mediated mechanism. Eur Heart J 2020;41:2487–2497. 10.1093/eurheartj/ehz459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mueller PA, Zhu L, Tavori H, Huynh K, Giunzioni I, Stafford JM, et al. Deletion of macrophage low-density lipoprotein receptor-related protein 1 (LRP1) accelerates atherosclerosis regression and increases C-C chemokine receptor type 7 (CCR7) expression in plaque macrophages. Circulation 2018;138:1850–1863. 10.1161/circulationaha.117.031702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Forrester SJ, Booz GW, Sigmund CD, Coffman TM, Kawai T, Rizzo V, et al. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol Rev 2018;98:1627–1738. 10.1152/physrev.00038.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, et al. Sirtuin 2 inhibitors rescue α-synuclein-mediated toxicity in models of Parkinson's disease. Science 2007;317:516–519. 10.1126/science.1143780 [DOI] [PubMed] [Google Scholar]
- 61. Huang H, Zhang D, Wang Y, Perez-Neut M, Han Z, Zheng YG, et al. Lysine benzoylation is a histone mark regulated by SIRT2. Nat Commun 2018;9:3374. 10.1038/s41467-018-05567-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bao X, Wang Y, Li X, Li X-M, Liu Z, Yang T, et al. Identification of ‘erasers’ for lysine crotonylated histone marks using a chemical proteomics approach. eLife 2014;3:e02999. 10.7554/eLife.02999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vaquero A, Scher MB, Lee DH, Sutton A, Cheng HL, Alt FW, et al. Sirt2 is a histone deacetylase with preference for histone H4 Lys 16 during mitosis. Genes Dev 2006;20:1256–1261. 10.1101/gad.1412706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Palomer E, Martín-Flores N, Jolly S, Pascual-Vargas P, Benvegnù S, Podpolny M, et al. Epigenetic repression of Wnt receptors in AD: a role for sirtuin2-induced H4K16ac deacetylation of Frizzled1 and Frizzled7 promoters. Mol Psychiatry 2022;27:3024–3033. 10.1038/s41380-022-01492-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Delaney K, Tan M, Zhu Z, Gao J, Dai L, Kim S, et al. Histone lysine methacrylation is a dynamic post-translational modification regulated by HAT1 and SIRT2. Cell Discov 2021;7:122. 10.1038/s41421-021-00344-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pei JF, Li XK, Li WQ, Gao Q, Zhang Y, Wang XM, et al. Diurnal oscillations of endogenous H2O2 sustained by p66Shc regulate circadian clocks. Nat Cell Biol 2019;21:1553–1564. 10.1038/s41556-019-0420-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hornbeck PV, Kornhauser JM, Latham V, Murray B, Nandhikonda V, Nord A, et al. 15 years of PhosphoSitePlus®: integrating post-translationally modified sites, disease variants and isoforms. Nucleic Acids Res 2019;47:D433–d441. 10.1093/nar/gky1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chi C, Fu H, Li YH, Zhang GY, Zeng FY, Ji QX, et al. Exerkine fibronectin type-III domain-containing protein 5/irisin-enriched extracellular vesicles delay vascular ageing by increasing SIRT6 stability. Eur Heart J 2022;43:4579–4595. 10.1093/eurheartj/ehac431 [DOI] [PubMed] [Google Scholar]
- 69. Archer SL, Marsboom G, Kim GH, Zhang HJ, Toth PT, Svensson EC, et al. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a basis for excessive cell proliferation and a new therapeutic target. Circulation 2010;121:2661–2671. 10.1161/circulationaha.109.916098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lehallier B, Gate D, Schaum N, Nanasi T, Lee SE, Yousef H, et al. Undulating changes in human plasma proteome profiles across the lifespan. Nat Med 2019;25:1843–1850. 10.1038/s41591-019-0673-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Koplev S, Seldin M, Sukhavasi K, Ermel R, Pang S, Zeng L, et al. A mechanistic framework for cardiometabolic and coronary artery diseases. Nat Cardiovasc Res 2022;1:85–100. 10.1038/s44161-021-00009-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Harel M, Ortenberg R, Varanasi SK, Mangalhara KC, Mardamshina M, Markovits E, et al. Proteomics of melanoma response to immunotherapy reveals mitochondrial dependence. Cell 2019;179:236–250.e18. 10.1016/j.cell.2019.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li Y, Ren P, Dawson A, Vasquez HG, Ageedi W, Zhang C, et al. Single-cell transcriptome analysis reveals dynamic cell populations and differential gene expression patterns in control and aneurysmal human aortic tissue. Circulation 2020;142:1374–1388. 10.1161/circulationaha.120.046528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Aging Biomarker Consortium; Bao H, Cao J, Chen M, Chen M, Chen W, Chen X, et al. Biomarkers of aging. Sci China Life Sci 2023; 66(5):893–1066. 10.1007/s11427-023-2305-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Grootaert MOJ, Finigan A, Figg NL, Uryga AK, Bennett MR. SIRT6 protects smooth muscle cells from senescence and reduces atherosclerosis. Circ Res 2021;128:474–491. 10.1161/CIRCRESAHA.120.318353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sun S, Qin W, Tang X, Meng Y, Hu W, Zhang S, et al. Vascular endothelium-targeted Sirt7 gene therapy rejuvenates blood vessels and extends life span in a Hutchinson-Gilford progeria model. Sci Adv 2020;6:eaay5556. 10.1126/sciadv.aay5556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Paulin R, Dromparis P, Sutendra G, Gurtu V, Zervopoulos S, Bowers L, et al. Sirtuin 3 deficiency is associated with inhibited mitochondrial function and pulmonary arterial hypertension in rodents and humans. Cell Metab 2014;20:827–839. 10.1016/j.cmet.2014.08.011 [DOI] [PubMed] [Google Scholar]
- 78. Liberale L, Akhmedov A, Vlachogiannis NI, Bonetti NR, Nageswaran V, Miranda MX, et al. Sirtuin 5 promotes arterial thrombosis by blunting the fibrinolytic system. Cardiovasc Res 2021;117:2275–2288. 10.1093/cvr/cvaa268 [DOI] [PubMed] [Google Scholar]
- 79. Zhang N, Zhang Y, Wu B, Wu S, You S, Lu S, et al. Deacetylation-dependent regulation of PARP1 by SIRT2 dictates ubiquitination of PARP1 in oxidative stress-induced vascular injury. Redox Biol 2021;47:102141. 10.1016/j.redox.2021.102141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ren S-C, Chen X, Gong H, Wang H, Wu C, Li P-H, et al. SIRT6 in vascular diseases, from bench to bedside. Aging Dis 2022;13:1015–1029. 10.14336/AD.2021.1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Zhang ZQ, Ren SC, Tan Y, Li ZZ, Tang X, Wang TT, et al. Epigenetic regulation of NKG2D ligands is involved in exacerbated atherosclerosis development in Sirt6 heterozygous mice. Sci Rep 2016;6:23912. 10.1038/srep23912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chen H-Z, Wang F, Gao P, Pei J-F, Liu Y, Xu T-T, et al. Age-associated sirtuin 1 reduction in vascular smooth muscle links vascular senescence and inflammation to abdominal aortic aneurysm. Circ Res 2016;119:1076–1088. 10.1161/circresaha.116.308895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wan YZ, Gao P, Zhou S, Zhang ZQ, Hao DL, Lian LS, et al. SIRT1-mediated epigenetic downregulation of plasminogen activator inhibitor-1 prevents vascular endothelial replicative senescence. Aging Cell 2014;13:890–899. 10.1111/acel.12247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Dikalova AE, Pandey A, Xiao L, Arslanbaeva L, Sidorova T, Lopez MG, et al. Mitochondrial deacetylase Sirt3 reduces vascular dysfunction and hypertension while Sirt3 depletion in essential hypertension is linked to vascular inflammation and oxidative stress. Circ Res 2020;126:439–452. 10.1161/CIRCRESAHA.119.315767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tang X, Chen XF, Chen HZ, Liu DP. Mitochondrial sirtuins in cardiometabolic diseases. Clin Sci 2017;131:2063–2078. 10.1042/CS20160685 [DOI] [PubMed] [Google Scholar]
- 86. Yang W, Gao F, Zhang P, Pang S, Cui Y, Liu L, et al. Functional genetic variants within the SIRT2 gene promoter in acute myocardial infarction. PLoS One 2017;12:e0176245. 10.1371/journal.pone.0176245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jing H, Hu J, He B, Negrón Abril YL, Stupinski J, Weiser K, et al. A SIRT2-selective inhibitor promotes c-Myc oncoprotein degradation and exhibits broad anticancer activity. Cancer Cell 2016;29:297–310. 10.1016/j.ccell.2016.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rumpf T, Schiedel M, Karaman B, Roessler C, North BJ, Lehotzky A, et al. Selective Sirt2 inhibition by ligand-induced rearrangement of the active site. Nat Commun 2015;6:6263. 10.1038/ncomms7263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gong H, Chen H, Xiao P, Huang N, Han X, Zhang J, et al. miR-146a impedes the anti-aging effect of AMPK via NAMPT suppression and NAD+/SIRT inactivation. Signal Transduct Target Ther 2022;7:66. 10.1038/s41392-022-00886-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol 2020;21:363–383. 10.1038/s41580-020-0230-3 [DOI] [PubMed] [Google Scholar]
- 91. Carlomosti F, D'Agostino M, Beji S, Torcinaro A, Rizzi R, Zaccagnini G, et al. Oxidative stress-induced miR-200c disrupts the regulatory loop among SIRT1, FOXO1, and eNOS. Antioxid Redox Signal 2017;27:328–344. 10.1089/ars.2016.6643 [DOI] [PubMed] [Google Scholar]
- 92. Melov S, Schneider JA, Day BJ, Hinerfeld D, Coskun P, Mirra SS, et al. A novel neurological phenotype in mice lacking mitochondrial manganese superoxide dismutase. Nat Genet 1998;18:159–163. 10.1038/ng0298-159 [DOI] [PubMed] [Google Scholar]
- 93. Zhang N, Zhang Y, Miao W, Shi C, Chen Z, Wu B, et al. An unexpected role for BAG3 in regulating PARP1 ubiquitination in oxidative stress-related endothelial damage. Redox Biol 2022;50:102238. 10.1016/j.redox.2022.102238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhao Y, Yang J, Liao W, Liu X, Zhang H, Wang S, et al. Cytosolic FoxO1 is essential for the induction of autophagy and tumour suppressor activity. Nat Cell Biol 2010;12:665–675. 10.1038/ncb2069 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the manuscript or the supplementary materials.