Abstract
This study aimed to assess the potential health risks associated with consuming three commonly consumed medicinal herbs in Malawi: Azadirachta indica, Mondia whitei, and Moringa oleifera. The concentrations of five metal(loids) (As, Cd, Pb, Cr, and Co) were determined using inductively coupled plasma mass spectrometry, while their safety was assessed by comparing the measured values with the legislated maximum contaminant levels (MCL)and reported metal(loids) concentrations in other countries. The results indicated significant variations of metal(loids) concentrations amongst the studied medicinal herbs, with Azadirachta indica containing the highest mean As (0.078 ± 0.010 mg kg−1) and Cd (0.049 ± 0.05 mg kg−1) concentrations and Mondia whitei and Moringa oleifera contained the highest mean Co (1.01 ± 0.05 mg kg−1) and Cr (1.42 ± 1.18 mg kg−1) concentrations, respectively. However, the mean concentrations of As, Cd, Pb, Cr, and Co fell below the MCL set by World Health Organization (WHO), Alimentarius Commissions, and European Commission. The estimated daily intake (EDI) for each metal(loid) was less than 1, indicating that the studied medicinal herbs do not pose serious health risks to non-regular consumers. The study also emphasizes the importance of assessing the potential risks associated with consuming medicinal herbs contaminated with heavy metals or metalloids, as it can seriously threaten human health.
Keywords: Medicinal herbs, Azadirachta indica, Mondia whitei, Moringa oleifera, Toxic metal(loids)
Graphical Abstract
Highlights
-
•
Three medicinal herbs were analyzed, and detectable levels of As, Cd, Pb, Cr and Co were found in all samples.
-
•
Pb and Cr were the most abundant metal(loids) in the tested medicinal herbs, while Cd was the least abundant.
-
•
M. oleifera had the lowest mean concentration of As, and A. indica had the highest.
-
•
The concentrations of metal(loids) in the studied medicinal herbs were within acceptable limits for human consumption.
1. Introduction
Consumption of indigenous medicinal herbs is popular in Malawi with Azadirachta indica, Mondia whitei and Moringa oleifera being reported among the commonly consumed herbs [1], [2]. Medicinal herbs have been in use in Malawi for a long time, and have traditionally been prescribed by local herbalists for various ailments including malaria, asthma, hormonal dysfunctions, genital enlargement and labor stimulation [1], [3]. The popularity of medicinal herbs in Malawi is connected with their ease of access, therapeutic efficacy, relatively low cost [2], [3], [4] as well as the widespread public opinion that assumes medicinal herbs to have no side effects. For instance, roots and leaves of Kigelia africana have been reported to possess anticancer activity against breast, renal and melanoma cancers [5] with no side effects reported by traditional herbalists. The dichloromethane extracts from roots and leaves of K. africana have also been reported to inhibit growth of renal cell line with no side effects locally reported [1], [5]. However, the presence of arsenic in medicinal herbs is a concern because long-term exposure to elevated levels of arsenic can lead to health effects, such as skin lesions, cardiovascular disease, neurological effects, and an increased risk of certain types of cancers. Several studies of K. africana, leaves, barks and un-ripen K. africana fruit have reported carcinogenic and genotoxic effects in humans [6], [7]. Aqueous and organic extracts of K. africana have been reported to inhibit cell growth in humans [6], [7] which censoriously misses out in herbalist reports of effectiveness of medicinal herbs [7], [8]. Furthermore, Saini et al. [7] reported that ingestion of large amounts of unripen K. africana fruit is life threatening because of the elevated metal(loids) concentrations in the fruit.
Different plants genotypes also vary in meta(loid) concentration in various parts of the plants. For instance, some plants such as rice accumulate elevated arsenic compared to other cereals [7], [9], [10]. Furthermore, plant uptake and concentration of metal(loids) from soil also varies with geographical location. For instance, arsenic content in rice from certain parts of India is higher than that in Malawi. In reference to the cited variations, the World Health Organization (WHO) recommends that food as well as medicinal herbs should be assessed for safety, potency, and efficacy so that only safe medicinal herbs are made available to consumers. This requirement is necessary to protect consumers from health risks associated with ingestion of elevated toxic metal(loids) present in medicinal herbs [11], [12]. However, there are currently no established MCLs for As, Cd and Pb in medicinal herbs. Nevertheless, certain countries have established MCLs for As, Cd and Pb in food and these levels can be used as a general guide for As, Cd and Pb concentrations in medicinal herbs. For instance, the World Health Organization (WHO) established guideline values 0.20, 0.150 and 0.015 mg kg−1, for As, Cd and Pb respectively whereas the European commission established the maximum contaminant limits of 0.15, 0.20 and 0.020 mg kg−1 respectively. Despite the above cited restrictions, studies of these toxic metal(loids) in commonly ingested and widely marketed medicinal herbs in Malawi such as Azadirachta indica, Mondia whitei and Moringa oleifera from Malawi have not been reported in literature. It is against this background that this study was commissioned to (1) evaluate As, Cd, Pb, Cr and Co concentration in Azadirachta indica, Mondia whitei and Moringa oleifera, and (2) to assess potential human health risks associated with consumption of the herbs by calculating the cancer risk (CR) of ingesting each of the metal(loid). Azadirachta indica, Mondia whitei and Moringa oleifera were selected for this study because they are widely marketed in major supermarkets and street vending outlets and they are popularly ingested local medicinal herbs in Malawi [2], [3] and southern parts of Africa due to their wide range therapeutic effects [1], [13].
2. Materials and methods
2.1. Sampling
Twenty-four medicinal herb samples were sourced from supermarkets and vending outlets in Lilongwe, Malawi in 2018. Each medicinal herb sample was packed in air-tight polyethylene zipper bags. Medicinal herbs details were accurately coded on the zipper bags. The collected medicinal herbs were classed into 3 genotypes namely: Azadirachta indica (n = 24), Mondia whitei (n = 16) and Moringa oleifera (n = 20; Table 1).
Table 1.
Studied medicinal herbs showing Common names, scientific names and number of samples analyzed for each genotype.
| Common name | Scientific name | Number of samples |
|---|---|---|
| Gondolosi | Mondia whitei | 24 |
| Moringa | Moringa oleifera | 16 |
| Neem | Azadirachta indica | 20 |
2.2. Sample digestion
Each medicinal herb sample was oven-dried to constant weight at 80 °C for 72 h in the laboratory and then finely ball-milled into powder (> 5 µm) using a ball-milling equipment (MM400, Retsch, Germany). Exactly 0.20 mg of each powdered medicinal herb sample and certified reference material (CRM) for plant (ERM 200; n = 3), weighed using an analytical balance, was mixed with 2 mL of concentrated nitric acid (70 %; Fisher Scientific, UK) after which the samples were left overnight to digest. Exactly 3 mL of hydrogen peroxide (32 %; Fisher Scientific, UK) was added to each sample before being digested in a Mars 5 microwave oven (Matthews Inc., USA) as follows: (i) ramping up to 50 °C for 10 min, (ii) ramping up to 75 °C for 10 min, and (iii) holding at 95 °C for 30 min. The ramping time was 5 min in each case. The applied power in all steps was set at 1450 W. The sample digests were cooled to room temperature and the volume of each sample solution was made up to 15.0 mL with ultra-pure water and then centrifuged at 4500 rounds per minute for 12 min and then transferred in another in 15 mL vials in readiness for analytes determination.
2.3. Determination of metal(loids) using ICP-MS
The analytical measurements of As, Cd, Pb, Cr and Co in the digested medicinal herb samples were carried out with inductively coupled plasma mass spectroscopy (ICP-MS, Agilent 7900 series) in inorganic mode, after its calibration using the certified stock solutions and optimization according to the recommendation of the manufacturer. Three digestion blanks containing 1 % HNO3 in ultra-pure water (V/V), prepared from concentrated nitric acid (70 % HNO3)obtained from Mallinckrodt Chemicals, USA and CRM of algae material (ERM CD 200) were prepared and measured alongside each medicinal herb sample for quality control purposes. All metal(loid) concentrations were determined on a dry weight (dwt) basis. Rhodium (Rh), obtained from High Purity Standards, Charleston (USA), with a concentration of 0.010 mg kg−1, were used as an internal standard (ISTD) to correct instrument drift. Aqu Trace multi-element standard used in preparing calibration standards was obtained from ChemService (USA). The mass charge ratio (m/z) of As (75), Cd (110), Pb (208), Cr (52), Co (59) and Rh (103) were selected for analyzes. Concentrations of analytes were determined using a six-point external calibration curve of 0, 0.001, 0.005, 0.025, 0.050, and 0.100 mg kg−1. Samples were introduced into the instrument using a concentric nebulizer and a double pass spray chamber. The instrument was operated in normal multi-element gas mode.
2.4. Quality control: analyses of certified reference materials (CRM)
Accuracy of measuring As, Cd, Pb, Cr and Co concentrations in medicinal herbs was checked using certified reference material (CRM) ERM CD 200 (n = 6). Mean recoveries of As, Cd, Pb, Cr and Co were found between 94 % and 106 % (Table 2) which indicated a good agreement between certified and measured values. Paired t-tests revealed no significant differences between measured and certified values for As, Cd, Pb and Co (p > 0.05) indicating high accuracy and precision. However, certified values for Cr were not known for ERM CD 200.
Table 2.
Accuracy checks of As, Cd, Pb, Cr and Co measurements using certified reference material (CRM) ERM CD 200 showing certified and mean measured concentration (± SD) in mg kg−1 and recoveries obtained for each analyte in % (n = 6).
| Metal (loid) | Certified value | Mean measured value | Recovery, % |
|---|---|---|---|
| As | 0.50 ± 0.1 | 0.52 ± 0.04 | 104 |
| Cd | 0.50 ± 0.1 | 0.53 ± 0.5 | 106 |
| Pb | 1.0 ± 0.2 | 0.94 ± 0.5 | 94 |
| Cr | - | 0.48 ± 15 | - |
| Co | 50 ± 10 | 0.45 ± 5 | 90 |
Note: SD, standard deviations.
2.5. Exposure estimation and health risk assessment
2.5.1. General safety of Malawian medicinal herbs
The safety of consuming medicinal herbs was assessed by comparing the measured concentrations of As, Cd, Pb, Cr and Co with respective maximum contaminant levels (MCL) of each metal(loid) set by different regulatory authorities. These authorities include the European Commission, specifically Regulation (EC) No. 2073/2005 for leafy vegetables and herbs, as well as World Health Organization (WHO) and the Joint FAO/WHO Expert Committee Food Additive (JECFA) [14].
2.5.2. Exposure estimation
The estimated daily intake (EDI) of As, Cd, Pb, Cr and Co in milligrams per kilogram per day per person (mg kg−1 day−1 person−1) through the consumption of medicinal herbs was estimated using Eq. (1), which was adapted from the study conducted by Zeng et al. in 2015 [15].
| (1) |
In our calculations, the variable C represents the ‘average concentration’ of As, Cd, Pb, Cr and Co in medicinal herbs measured in mg kg−1, the variable IR represents the average daily ingestion rate of medicinal herbs expressed in kg day−1 person−1’ and bwt represents the estimated bodyweight of adult consumers in kg. For our calculations, we used an average daily IR of 10 g day−1 person−1 [16].
2.5.3. Cancer risk assessment
We calculated the cancer risk (CR) of ingesting As, Cd, Pb, Cr and Co through medicinal herb consumption employing Eq. (2), which was adapted from the study conducted by Zeng et al. [15].
| (2) |
In our calculations, the variable CR represents the probability of lifetime cancer risk, the variable CSF represents the cancer slope factor. For our calculations, we used the following CSF values: 1.5, 0.38, 0.0085, 1.5, and 0.5 given in mg kg−1 day−1 for As, Cd, Pb, Cr and Co, respectively, set by the US EPA [17], [18], [19], [20]. The Hazard Quotient (HQ), defined as “the ratio between EDI and oral reference dose (RFD)” (Eq. 3) and the Hazard Index (HI), defined as “the sum of HQ” (Eq. (4)) adapted from Zeng et al. [15], Munoz et al. [21] and Nolos et al. [18] were used to assess the human health risk of consuming the medicinal herbs, and CR that exceeded the maximum threshold value of 1.00 × 10−4 was deemed to expose consumers to elevated carcinogens levels [15], [18], [19].
| (3) |
| (4) |
In our calculations, the variable RFD represents the reference dose measured in μg/kg bwt * day) [17], [18], [19], the variable HQ represents the Hazard Quotient and the variable HI represents the Hazard Index. For our calculations, we used the following oral RFD values: 0.0003, 0.001, 0.0035, 0.03 0.001 given in mg kg−1 * day for As, Cd, Pb, Cr and Co, respectively, set by the US EPA [17], [20].
2.6. Statistics
Data analysis was performed using Minitab 19 statistical software and Microsoft Excel 2016 for Windows 10. Two-way analysis of variance (ANOVA) was obtained using general linear model and was used to evaluate significant differences among the studied variables in different sample matrices. Fisher tests were also used to decide level of significant at either p < 0.01 and < 0.001 probability levels.
3. Results and discussion
3.1. Evaluation of metalloids concentration in medicinal herbs
In this study, we investigated the concentration of As, Cd, Pb, Cr and Co in three commonly used medicinal herbs in Malawi: Moringa oleifera, Azadirachta indica, and Mondia whitei. All the medicinal herb samples analyzed in this study contained detectable levels of As, Cd, Pb, Cr and Co as shown in Table SM1. The concentrations of metal(loids) ranged from 0.039 to 0.085 mg kg-1 for As, 0.013 to 0.094 mg kg-1 for Cd, 0.836 to 1.384 mg kg-1 for Pb, 0.271 to 2.929 mg kg-1 for Cr, and 0.635 to 1.252 mg kg-1 for Co. The mean concentrations of metal(loids) decreased in the following order: Pb (1.064 mg kg-1) > Cr (1.063 mg kg-1) > Co (0.884 mg kg-1) > As (0.062 mg kg-1) > Cd (0.032 mg kg-1) (Fig. 1). This indicates that Pb and Cr were the most abundant metal(loids) while Cd was the least abundant metal(loid) among the tested medicinal herbs. These results indicate that medicinal herbs can serve as a potential source of exposure to various metal(loids) including As, Cd, Pb, Cr, and Co with concentrations that varied among the different herbs tested. These findings are consistent with previous studies that have reported high levels of Pb in medicinal plants [22]. However, no significant differences were observed between the mean Pb and Cr concentrations in the studied medicinal herbs (p > 0.05), indicating that their levels were comparable. Nonetheless, we have provided a detailed analyses of the results and discussion of each metal(loid), including their maximum contaminant levels (MCL), variation across the different herbs and regions, in the subsequent paragraphs.
Fig. 1.
Variation of concentrations of As (a) and Cd (b) in Mondia whitei (Mondia; n = 24), Moringa oleifera (Moringa; n = 16) and Azadirachta indica (Neem; n = 20). Medicinal herbs that share common letters (A, B and C) are not significantly different from each other. The boxes represent first and third quartile range metalloid concentrations; the line across a box represents the median value; and whiskers represent minimum and maximum values.
3.2. Arsenic
In this study, the lowest mean concentration of As was found in Moringa oleifera (0.048 ± 0.008 mg kg−1), while the highest mean concentration of As was observed in Azadirachta indica (0.078 ± 0.010 mg kg−1) (Table SM1, Fig. 2a). These findings confirm that medicinal plants, including Moringa oleifera, Azadirachta indica, and Mondia whitei, have the ability to absorb arsenic from the environment, resulting in elevated levels of this element in the plants [22], [23], [24]. The mean concentration of As decreased in the order of Azadirachta indica > Mondia whitei > Moringa oleifera (Table SM1, Fig. 2a), indicating that Azadirachta indica accumulates higher As compared to the other two medicinal herbs. Furthermore, mean concentrations of As were also compared with those from other countries worldwide. The results indicated that mean As concentrations for Malawian Moringa in this study (Table SM1, Fig. 2a) were at least 2-fold lower than those reported in herbs from Bulgaria (range: 0.012–0.225 mg kg−1) [4] and Poland (range: 0.120 ± 0.310 mg kg−1) [4], [25], [26], [27], [28], [29]. Another study conducted by Ahmed et al. [30] found As concentrations ranging from 0.040 to 0.126 mg kg−1 in medicinal plants from Pakistan. A study conducted in India reported As concentrations ranging from 0.16 to 1.1 mg kg−1 in medicinal plants [31]. Similarly, in a study conducted by Vuong et al. [32], mean As concentrations of 0.343 mg kg−1, 1.628 mg kg−1, 0.182 mg kg−1 and 0.295 mg kg−1 in Artemisia vulgaris L., Plantago asiatica L., Eleusine indica (L.) and Phyllanthus urinaria L., respectively [26], [27], [28], [29], [32].
Fig. 2.
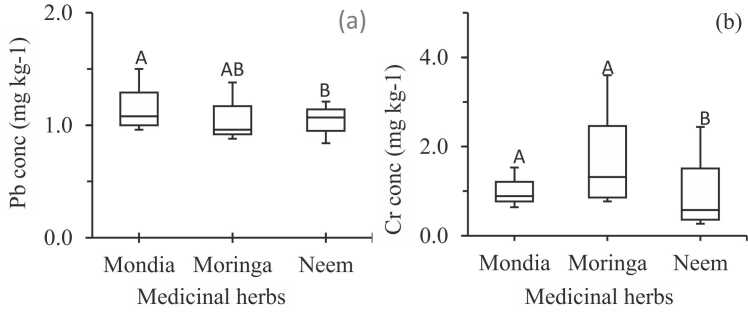
Variation of Pb (a) and Cr (b) concentrations in M. whitei (Mondia, n = 24), M. oleifera (Moringa; n = 16) and A. indica (Neem; n = 20). Medicinal herbs that share common letters (A–C) are not significantly different from each other. The boxes represent first and third quartile range metalloid concentrations; the line across a box represents the median value; and whiskers represent minimum and maximum values.
The concentrations of As observed in Malawian medicinal herbs in this study were compared to those reported in medicinal herbs from other African countries. For Moringa oleifera, the results revealed that the mean As concentration obtained in this study (range: 0.048–0.078 mg kg−1) fell within the range reported in Ghana (0.06–2.04 mg kg−1) [33], but it was significantly lower than that from Nigeria (range: 0.17–1.21 mg kg−1) [2], [27], [34], Ethiopia (range: 0.24–1.11 mg kg−1) [2], [35], [36] and Uganda (range: 0.29–0.99 mg kg−1) [2], [28]. It is important to note that the US Food and Drug Administration (FDA) has established a maximum contaminant level (MCL) of 3.00 mg kg−1 for As in herbs and spices [37]. The concentrations of As observed in the tested medicinal plants were significantly lower than the FDA’s MCL, indicating that they are safe for human consumption. The findings of the study suggest that medicinal plants have the ability to accumulate As from the environment, and the accumulation concentration varying among different species. However, further studies should be conducted to gain better understanding of the health implications of long-term exposure to low levels of As through the consumption of medicinal plants. This is particularly important considering that prolonged exposure to low levels of As can result in adverse health effects, including cancer and skin lesions [38], [39].
In addition, the mean As concentrations obtained in this study were compared to the maximum contaminant level (MCL) established by various regulatory institutions. The results indicated that the mean As concentrations in Malawian herbs (0.062 mg kg−1) analyzed in this study were at least three times lower than the MCLs set by World Health Organisation (WHO) and European Commission (EC) Regulation No. 2073/2005 for herbs (0.200 mg kg−1) [40], [41], as well as those set by China (0.200 mg kg−1), Thailand (4.00 mg kg−1) and Canada (5.00 mg kg−1). Therefore, the As concentration in the studied medicinal herbs fall within the acceptable limits for human consumption and pose a minimal threat to human health.
The low concentration of As in Malawian Moringa oleifera may be attributed to high soil-pH and organic matter content in soils of the Chikwawa districts, where Moringa oleifera was obtained. This is supported by the positive correlation between higher soil pH and organic matter content, and lower As concentration in Moringa oleifera leaves. Regarding Azadirachta indica, the results revealed that the mean As concentration obtained in this study (mean: 0.062 mg kg−1; range: 0.048–0.078 mg kg−1) was significantly lower than that found in Nigerian (mean: 1.16 mg kg−1; range 0.10–2.97 mg kg−1), Ethiopian (5.5 mg kg−1; range: 1.0–13.9 mg kg−1) and Tanzanian (mean 0.22 mg kg−1, range 0.03–0.44 mg kg−1) medicinal herbs.
The study revealed that the concentration of As in Malawian medicinal herbs was generally low and fell below the acceptable limits for human consumption. Among the herbs studied Moringa oleifera exhibited the lowest mean concentration of As, while Azadirachta indica had the highest. Furthermore, the concentration of As in Malawian Moringa oleifera was similar to that reported in other African countries but lower than the levels observed in Bulgaria and Poland.
Additionally, the study found that the concentration of As in Malawian medicinal herbs was significantly lower than the MCL established by the World Health Organization (WHO), the European Commission (EC), China, Thailand, and Canada. These findings offer valuable insights into the arsenic levels in the specified commonly consumed Malawian medicinal herbs, comparing them with those in other countries and regulatory thresholds. However, it is important to note that the study’s analyses were for the specified medicinal herbs, and therefore, the results may not be representative of all medicinal herbs cultivated in Malawi or other regions. This information can prove beneficial to herbalists and researchers seeking to reduce As levels in the studied medicinal herbs.
3.3. Cadmium
Cadmium concentrations in medicinal herbs ranged from 0.013 to 0.049 mg kg−1, with Azadirachta indica exhibiting the highest mean concentration of 0.049 ± 0.05 mg kg−1, which was at least twice as high as that in Moringa oleifera (0.027 mg kg−1) and Mondia whitei (p < 0.01; Table SM1, Fig. 2b). The mean concentration of Cd decreased in the order of Azadirachta indica > Moringa oleifera > Mondia whitei (Table SM1, Fig. 2b). The mean Cd concentrations obtained in this study are at least three times lower than those reported for Moringa oleifera in Thailand (mean: 0.150 mg kg−1; range: 0.020–0.182 mg kg−1) [42], Mondia whitei in Rwanda (mean: 0.080 mg kg−1; range = 0.050–0.090 mg kg−1) [43], and Azadirachta indica in Nigeria (mean: 0.350 mg kg−1; range: 0.120–0.540 mg kg−1) [4], [44].
The study also found that Azadirachta indica had the highest mean Cd concentration which was at least twice higher than that in Moringa oleifera and Mondia whitei (Table SM1, Fig. 2a) suggesting that different herbs accumulate varying levels of Cd. These results align with other studies that have reported higher levels of Cd in Azadirachta indica. For instance, a study conducted in India reported a mean Cd concentration of 0.06 mg kg−1 in Moringa oleifera and 0.29 mg kg−1 in Azadirachta indica [45]. Another study in Nigeria reported a mean Cd concentration of 0.16 mg kg−1 in Moringa oleifera and 0.44 mg kg−1 in Azadirachta indica) [46], which are considerably higher compared to the findings in this study. In comparison to other medicinal herbs, a study conducted by Dghaim et al. [47] reported mean Cd concentration ranging from 0.03 to 0.43 mg kg−1 (mean: 0.14 mg kg−1) in medicinal herbs commonly consumed in the United Arab Emirates while Sepehri et al. [48] found Cd concentrations ranging from 0.012 mg kg−1 to 0.0793 mg kg− 1 in medicinal plants consumed in Iran indicating that Cd concentrations in Malawian medicinal herbs are significantly lower than those reported in medicinal plants from other regions [12], [32], [49], [50], [51], [52].
In comparison to the World Health Organization (WHO) and European Commission MCL for Cd in herbs and spices, set at 0.3 mg kg-1 [39], [40], [41], [53], it was observed that all the Malawian medicinal herbs analyzed in this study had Cd concentrations below this limit. This observation suggests that the medicinal herbs analyzed in this study are safe for consumption in terms of Cd contamination. However, it is necessary to consistently check and monitor cadmium levels in medicinal herbs to ensure safety and prevent potential health risks.
3.4. Lead
The lowest and highest mean concentrations of Pb were observed as 0.89 mg kg−1 in Moringa oleifera and 1.39 mg kg-1 in Mondia whitei (Fig. 3a, Table SM1), indicating that the mean Pb concentration in the studied herbs decreased in the following order: Mondia whitei > Azadirachta indica > Moringa oleifera (Fig. 3a, Table SM1). The mean Pb concentration obtained in this study (1.07 mg kg−1) is at least 10 times significantly lower than the reported concentrations for Azadirachta indica in Pakistan (range: 9–15 mg kg−1) [12], and in Kolkata, India (mean = 16.53 mg kg-1), and Horse chestnut (mean: 2.00–8.20 mg kg−1; F = 9.4; p < 0.01) [12], [44]. However, the mean Pb concentrations obtained in this study were not significantly different from that reported for Chamomile blossom (p > 0.05; range: 0.44–0.85 mg kg−1) and peppermint leaves (p > 0.05; range: 0.46–1.11 mg kg-1) from Poland [54], [55].
Fig. 3.

Variation of Co concentrations in M. whitei (mondia; n = 24), M. oleifera (moringa; n = 16) and A. indica (neem; n = 20). Medicinal herbs that share common letters (A–C) are not significantly different from each other. The boxes represent first and third quartile range metalloid concentrations; the line across a box represents the median value; and whiskers represent minimum and maximum values.
Furthermore, mean Pb concentrations in all samples (1.07 mg kg−1; Fig. 3a, Table SM1) was 10 times lower than the MCL for Pb concentrations in herbs (10 mg kg−1) regulated by WHO [56]. These results suggest that the studied medicinal herbs have relatively low concentrations of Pb, which is an important finding considering the potential health risks associated with Pb exposure. The study provides significant information regarding the safety of these medicinal herbs which can serve as a guide for selecting and using them in traditional medicine. However, it is essential to acknowledge that the results may not apply universally to other geographic regions or populations due to potential variations in concentrations of Pb in medicinal herbs, influenced by factors such as the soil and water quality, climate, and agricultural practices. Moreover, the study did not investigate the potential health effects of consuming these herbs with low concentrations of Pb. Hence, additional research is required to assess the safety and effectiveness of utilizing these herbs in traditional medicine.
3.5. Chromium
The mean concentrations of Cr in Azadirachta indica, Mondia whitei and Moringa oleifera were determined to be 0.81 ± 0.61, 0.961 ± 0.23 and 1.42 ± 1.18 mg kg−1, respectively (Fig. 3b). The results indicated that M. oleifera had the highest mean Cr concentration, followed by M. whitei and A. indica (Table SM1, Fig. 3b). This suggests that different medicinal herbs possess varying levels of Cr, and certain herbs may be more susceptible to contamination than others. Notably, 58 % of the analyzed medicinal herb samples (14 out of the 24) exceeded the MCL of 2.00 mg kg-1 set by the World Health Organization (WHO) for Cr in herbs and vegetables [15], [57], as well as EC No. 1441/2007 [39], [53]. These findings indicate that a significant proportion of medicinal herbs sold in Malawi may raise safety concerns due to high levels of Cr contamination.
The increased Cr levels observed in certain medicinal herbs may be attributed to contamination during the harvesting and processing stages during plant production [57], emphasizing the need for proper hygiene and handling procedures to mitigate contamination risks. However, it is crucial to evaluate these results in conjunction with the estimated daily intake (EDI) of medicinal herbs by Malawian communities in order to comprehensively assess risks and implement appropriate interventions [15]. These findings underscore the potential health hazards associated with the consumption of contaminated medicinal herbs and emphasize the importance of considering EDI in evaluating risk levels [15].
3.6. Cobalt
The mean Co concentrations of cobalt in A. indica, M. oleifera and M. whitei were determined to be 0.81 mg kg-1 (range: 0.64–0.96 mg kg-1), 0.84 mg kg-1 (range: 0.65–1.06 mg kg-1), and 1.01 ± 0.05 mg kg−1; (range: 0.77–1.25 mg kg−1), respectively (Table SM1, Fig. 3). these findings indicate that Mondia whitei exhibited the highest mean Co concentration, followed by Moringa oleifera and Azadirachta indica (Table SM1, Fig. 3). The average Co concentrations obtained in this study was 0.88 mg kg−1, which is below the World Health Organization (WHO) guideline value of 1.0 mg kg−1 for Co concentration in food [56]. The study also demonstrated a decreasing Co concentration trend of Mondia whitei > Moringa oleifera > Azadirachta indica. These observations suggest that Malawian medicinal herbs currently available in supermarkets are generally safe for consumption in terms of Co content.
It is worth noting, however, that this study only analyzed a limited number of medicinal herbs, and further investigations are required to evaluate the Co concentration in a broader range of medicinal herbs in Malawi. Additionally, while the Co concentration in the analyzed herbs remains below the recommended limit, it is crucial to consider the cumulative effect of consuming multiple herbs and other food sources with Co content. Nevertheless, the results highlight the necessity for continuous monitoring of Co concentration in medicinal herbs to ensure their safety for human consumption.
3.7. Human health risk assessment
3.7.1. Estimated daily intake (EDI)
The present study aimed to assess the potential human health risks associated with elevated levels of As, Cd, Pb, Cr and Co resulting from the consumption of medicinal herbs. The evaluation was based on a scenario involving the daily intake of 0.010 kg of medicinal herb by an individual with an average body weight of 58 kg [16], [58]. The estimated daily dietary intakes (EDI) of each metal or metalloid are provided in Table 3. Among the metals analyzed, Cd exhibited the lowest EDI value (3.45 × 10−6), while Pb showed the highest EDI value (1.24 × 10−4; Table 3), consistent with previous reports on metal(loid) concentrations in plant materials [59]. Comparisons of the EDI values among the medicinal herbs revealed that Azadirachta indica had lower EDI values for As, Cd, Pb, Cr and Co compared to Mondia whitei and Moringa olifera, indicating that the former may be a safer option. The overall EDI values for As (7.07 × 10−6), Cd (3.33 × 10−6), Pb (1.24 × 10−4), Cr (1.19 × 10−4) and Co (1.05 × 10−4) found in this study (Table 3) were below the recommended tolerable EDI values of 0.13, 0.06, 0.21, 0.20 and 0.30 for As, Cd, Pb, Cr and Co in herbs [59]. These results indicate limited health risk to consumers of medicinal herbs regarding to the studied metals and metalloids. The relatively low soil concentrations of As, Cd, Pb, Cr and Co reported in many regions of Malawi, compared to the reference countries, may contribute to the observed low EDI values [58].
Table 3.
Estimated daily intake (EDI), cancer risks (CR) and Hazard Quotient (HQ) for As, Cd, Pb, Cr and Co with estimated average of 0.010 kg day−1 consumption rate of Malawian medicinal herbs for a person with an average age of 58 years and body mass of 55 kg. (Cm, metalloid concentration (mg kg−1) in specified medicinal herbs).
| Sample | Metal (loid) | Cm | EDI | CR | HQ |
|---|---|---|---|---|---|
| M. whitei | As | 0.06 | 1.03 × 10−5 | 1.55 × 10−5 | 3.45 × 10−2 |
| Cd | 0.02 | 3.45 × 10−6 | 1.31 × 10−6 | 3.45 × 10−3 | |
| Pb | 1.125 | 1.94 × 10−4 | 1.65 × 10−6 | 5.54 × 10−2 | |
| Cr | 0.958 | 1.65 × 10−4 | 8.26 × 10−5 | 1.65 × 10−1 | |
| Co | 1.005 | 1.73 × 10−4 | 1.73 × 10−4 | 5.78 × 10−3 | |
| HI | 2.64 × 10−1 | ||||
| M. olifera | As | 0.048 | 4.1 × 10−6 | 6.21 × 10−6 | 1.38 × 10−2 |
| Cd | 0.027 | 2.3 × 10−6 | 8.84 × 10−7 | 2.33 × 10−3 | |
| Pb | 1.017 | 8.8 × 10−6 | 7.45 × 10−7 | 2.50 × 10−2 | |
| Cr | 1.421 | 1.2 × 10−4 | 6.13 × 10−5 | 1.23 × 10−1 | |
| Co | 0.837 | 7.2 × 10−5 | 7.32 × 10−6 | 2.41 × 10−3 | |
| HI | 1.66 × 10−1 | ||||
| A. indica | As | 0.078 | 6.7 × 10−6 | 1.01 × 10−5 | 2.24 × 10−2 |
| Cd | 0.049 | 4.2 × 10−6 | 1.61 × 10−6 | 4.22 × 10−3 | |
| Pb | 1.051 | 9.1 × 10−5 | 7.70 × 10−7 | 2.59 × 10−2 | |
| Cr | 0.814 | 7.0 × 10−5 | 3.51 × 10−5 | 7.02 × 10−2 | |
| Co | 0.810 | 7.0 × 10−5 | 6.98 × 10−5 | 2.33 × 10−3 | |
| HI | 1.25 × 10−1 | ||||
| All herbs | As | 0.062 | 7.07 × 10−6 | 1.06 × 10−5 | 2.36 × 10−2 |
| Cd | 0.032 | 3.33 × 10−6 | 1.27 × 10−6 | 3.33 × 10−3 | |
| Pb | 1.064 | 1.24 × 10−4 | 1.05 × 10−6 | 3.55 × 10−2 | |
| Cr | 1.064 | 1.19 × 10−4 | 5.96 × 10−5 | 1.19 × 10−1 | |
| Co | 0.884 | 1.05 × 10−4 | 1.05 × 10−4 | 3.50 × 10−3 | |
| HI | 2.0 × 10−1 |
The study aimed to assess the concentrations of As, Cd, Pb, Cr, and Co in medicinal herbs available in supermarkets in Malawi, as well as the associated human health risks resulting from the ingestion of these metalloids through consumption of these herbs. The findings revealed that Moringa oleifera, had the highest mean concentration of As, with 58 % of the samples exceeding the MCL established by World Health Organization (WHO) and Codex Alimentarius Commission [39]. The mean Cd concentrations in all herbs were below the MCL, however, 54 % of the samples had Cd concentrations surpassing the MCL set by the European Union (EU). The mean Pb concentrations were below the MCL defined by the WHO and Codex Alimentarius Commission, but 46 % of the samples contained Pb concentrations above the maximum residue limit (MRL) set by the EU. The mean Cr concentrations were below the MCL set by the WHO and Codex Alimentarius Commission, but 58 % of the samples had Cr concentrations surpassing the MCL. The mean Co concentrations were below the guideline value recommended by the WHO for Co concentration in food. Overall, the estimated daily intake (EDI) values for As, Cd, Pb, Cr, and Co were below the recommended tolerable values, indicating limited health risk to consumers of medicinal herbs in terms of these metal(loids).
The study provides essential insights into the concentrations of metalloids in medicinal herbs in Malawian supermarkets, which is particularly relevant given the traditional use of medicinal herbs in the country. The elevated concentrations of As, Cd, Pb, and Cr detected found in some samples are concerning due to the potential adverse health effects associated with chronic exposure to these metal(loids). The fact that a majority of the samples exceeded the MCLs for As and Cr as well as the MRLs for Cd and Pb, raises alarm and suggests that contamination during the harvesting and processing of these herbs is a significant issue.
It important to note that the EDI values reported in this study were calculated based on the assumption of consuming a daily dosage of 0.010 kg medicinal herbs by an individual with an average body weight of 58 kg. While these values indicate a limited health risk to consumers, it is crucial to consider the possibility of higher doses and potential chronic exposure over time. Furthermore, although the EDI values were found to be below the recommended tolerable values, it is essential to acknowledge that these values are not absolute, and there remains a potential for health risks, especially with prolonged exposure. This study emphasizes the necessity for enhanced regulation and monitoring of medicinal herbs in Malawian supermarkets to ensure their safety for consumption.
It also underscores the significance of consumer education regarding the potential risks associated with the consumption of these herbs and the importance of adopting safe handling and preparation practices.
3.7.2. Cancer risk
The study assessed the cancer risk (CR) associated with the exposure of As, Cd, Pb, Cr and Co through from the consumption of the three medicinal herbs. The calculated CR values for these metalloids were 1.06 × 10−5, 1.27 × 10−6, 1.05 × 10−6, 5.96 × 10−5, and 1.05 × 10−4, respectively (Table 3). It is worth noting CR values for below10−6 are considered negligible range, while values above 10−4 are deemed unacceptable. Values falling between 10−6 and 10−4 are within an acceptable range [19], [60], [61]. In this study, the CR value for Co in Mondia whitei exceeded the acceptable range, indicating a concerning risk of cancer associated with cobalt exposure through the consumption of Mondia whitei. On the other hand, the CR values for As, Cd, Pb and Cr were either within the acceptable range or negligible (Table 3) in all the three medicinal herbs, suggesting that these medicinal herbs are safe in terms of these metalloids. Overall, both the EDI and CR values obtained in this study indicate a low health risk of cancer for consumers of Malawian medicinal herbs.
The Hazard Quotient (HQ) was calculated for ingesting Mondia whitei (2.64 × 10−1), Moringa olifera (1.66 × 10−1) and Azadirachta indica (1.25 × 10−1) at a dosage of 0.010 kg day−1 (Table 3). The resulting HQ values were all below 1.0, which indicates that Malawian herbs are generally safe, as HQs values less than 1.0 are considered to pose insignificant health risks [62]. Among the metalloids, Cr contributed the highest HQ value (60 %) while Cd had the lowest contribution to HQ (2 %) (Table 3).
Based on the presented results, the cancer risk (CR) values for As, Cd, Pb, Cr and Co in the three medicinal herbs were predominantly within an acceptable range or negligible, indicating that these herbs are safe concerning these metalloids. However, the CR value for Co in Mondia whitei exceeded the acceptable range, suggesting a potential cancer risk associated with its consumption of this particular herb. These findings align with previous studies that have also highlighted the potential health risks associated with Co exposure [19], [60], [63]. The Hazard Quotient (HQ) values for these three medicinal herbs were consistently low and below the threshold of 1.0, indicating insignificant health risks for consumers. Among the metalloids, Cr had the highest contribution to the HQ, while Cd had the least contribution. These results are in line with prior studies that have reported low HQ values for medicinal herbs [62], [64].
4. Conclusions
This study analyzed the concentrations of As, Cd, Pb, Cr, and Co in medicinal herb samples from Malawi. All the metalloids were detectable in all the samples analyzed, with Pb and Cd being the most and least abundant metalloids, respectively. The mean As concentrations were within acceptable limits for human consumption and posed a minimal threat to human health. These results suggest that the consumption of Malawian medicinal herbs is mostly safe with respect to the studied metalloids, but caution should be exercised when consuming Mondia whitei due to its potential cancer risk associated with Co exposure. We recommend that regular monitoring of metal(loid) concentrations in medicinal herbs should be carried out to ensure their safety for human consumption.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was funded by the UK government through the Commonwealth Scholarship awarded to Angstone Thembachako Mlangeni, a commonwealth scholar (MWCS-2015-334).
Handling Editor: Dr. L.H. Lash
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.toxrep.2023.07.004.
Appendix A. Supplementary material
Supplementary material
Data Availability
Data will be made available on request.
References
- 1.Naidoo K.K., Coopoosamy R.M. A comparative analysis of two medicinal plants used to treat common skin conditions in South Africa. Afr. J. Pharm. Pharm. 2011;5:393–397. doi: 10.5897/AJPP11.048. [DOI] [Google Scholar]
- 2.Zamawe C., King C., Jennings H.M., Fottrell E. Associations between the use of herbal medicines and adverse pregnancy outcomes in rural Malawi: a secondary analysis of randomised controlled trial data. BMC Complement. Altern. Med. 2018;18:1–8. doi: 10.1186/s12906-018-2203-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.L.M. Robison, D. Sclar, T. Skaer, Medicinal Plant use by Traditional Healers in Malawi: Focus on Neem, Tephrosia, Moringa, Jatropha, Marula and Natal Mahogany, 2002.
- 4.Arpadjan S., Çelik G., Taşkesen S., Güçer Ş. Arsenic, cadmium and lead in medicinal herbs and their fractionation. Food Chem. Toxicol. 2008;46:2871–2875. doi: 10.1016/j.fct.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 5.Fouche G., Cragg G.M., Pillay P., Kolesnikova N., Maharaj V.J., Senabe J. In vitro anticancer screening of South African plants. J. Ethnopharmacol. 2008;119:455–461. doi: 10.1016/j.jep.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Agyare C., Dwobeng A.S., Agyepong N., Boakye Y.D., Mensah K.B., Patrick George Ayande A., Adarkwa-Yiadom M. Antimicrobial, antioxidant, and wound healing properties of Kigelia Africana. Adv. Pharm. Sci. 2013;2013:10. doi: 10.1155/2013/692613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saini S., Kaur H., Verma B., Ripudaman A., Singh S.K. Kigelia Africana (Lam.) benth. – an overview. Indian J. Nat. Prod. Resour. 2008;8:190–197. [Google Scholar]
- 8.Owolabi O.J., Omogbai E.K.I., Obasuyi O. Antifungal and antibacterial activities of the ethanolic and aqueous extract of Kigelia Africana (Bignoniaceae) stem bark. Afr. J. Biotechnol. 2007;6:1677–1680. doi: 10.5897/AJB2007.000-2244. [DOI] [Google Scholar]
- 9.Mlangeni A.T., Perez M., Raab A., Krupp E.M., Norton G.J., Feldmann J. Simultaneous stimulation of arsenic methylation and inhibition of cadmium bioaccumulation in rice grain using zero valent iron and alternate wetting and drying water management. Sci. Total Environ. 2020;711 doi: 10.1016/j.scitotenv.2019.134696. [DOI] [PubMed] [Google Scholar]
- 10.Norton G.J., Duan G., Dasgupta T., Islama M.R., Lei M., Zhu Y., Deacon C.M., Moran A.C., Islam S., Zhao F., et al. Environmental and genetic control of arsenic accumulation and speciation in rice grain: comparing a range of common cultivars grown in contaminated sites across Bangladesh, China, and India. Environ. Sci. Technol. 2009;43:8381–8386. doi: 10.1021/es901844q. [DOI] [PubMed] [Google Scholar]
- 11.A.A. Maitlo, W.B. Jatoi, A.F. Memon, A.H. Soomro, Assessment of Zinc, Lead, Chromium, and Cobalt in Commonly Consumed Herbal Medicines in Sindh, Pakistan, 2020. [DOI] [PubMed]
- 12.Kirmani, Determination of some toxic and essential trace metals in some medicinal and edible plants of Karachi City, J. Basic Appl. Sci., 2011, pp. 89–95. 〈 10.6000/1927-5129.2011.07.02.03〉. [DOI]
- 13.R.M. Coopoosamy, K.K. Naidoo, An Ethnobotanical Study of Medicinal Plants Used by Traditional Healers in Durban, South Africa, vol. 6, 2012, pp. 818–23. 〈 10.5897/AJPP11.700〉. [DOI]
- 14.Santos E.E., Lauria D.C., Porto C.L. Assessment of daily intake of trace elements due to consumption of foodstuffs by adult inhabitants of Rio de Janeiro City. Sci. Total Environ. 2004;327:69–79. doi: 10.1016/j.scitotenv.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Zeng F., Wei W., Li M., Huang R., Yang F., Duan Y. Heavy metal contamination in rice-producing soils of Hunan Province. China Potential Health Risks. 2015:15584–15593. doi: 10.3390/ijerph121215005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D. Kathyola, T. Dzowela, Anthropometric Measurements and Prevalence of Underweight, Overweight and Obesity in Adult Malawians: Nationwide Population Based NCD STEPS Survey, 2014. 〈 10.11604/pamj.2013.15.108.2622〉. [DOI] [PMC free article] [PubMed]
- 17.Ma L., Wang L., Jia Y., Yang Z. Arsenic speciation in locally grown rice grains from Hunan Province, China: spatial distribution and potential health risk. Sci. Total Environ. 2016;557–558:438–444. doi: 10.1016/j.scitotenv.2016.03.051. [DOI] [PubMed] [Google Scholar]
- 18.Nolos R.C., Agarin C.J.M., Domino M.Y.R., Bonifacio P.B., Chan E.B., Mascareñas D.R., Senoro D.B. Health risks due to metal concentrations in soil and vegetables from the six municipalities of the island province in the Philippines. Int. J. Environ. Res. Public Health. 2022:19. doi: 10.3390/ijerph19031587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finley B.L., Monnot A.D., Paustenbach D.J., Gaffney S.H. Derivation of a chronic oral reference dose for cobalt. Regul. Toxicol. Pharm. 1970;64:491–503. doi: 10.1016/j.yrtph.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 20.U.S. EPA Selenium and Compounds, CASRN 7782-49-2. Integr. Risk Inf. Syst. Chem. Assess. Summ., 1991. 〈https://cfpub.epa.gov/ncea/iris2/chemicalLanding.c〉.
- 21.Munoz O., Zamorano P., Garcia O., Bastías J.M. Arsenic, cadmium, mercury, sodium, and potassium concentrations in common foods and estimated daily intake of the population in Valdivia (Chile) using a total diet study. Food Chem. Toxicol. 2017:109. doi: 10.1016/j.fct.2017.03.027. [DOI] [PubMed] [Google Scholar]
- 22.Duruibe J.O., Ogwuegbu M.O.C., Egwurugwu J.N. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007;2:112–118. doi: 10.1016/j.proenv.2011.09.146. [DOI] [Google Scholar]
- 23.Bhattacharya P., Welch A.H., Stollenwerk K.G., McLaughlin M.J., Bundschuh J., Panaullah G. Arsenic in the environment: biology and chemistry. Sci. Total Environ. 2007;379:109–120. doi: 10.1016/j.scitotenv.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 24.Nriagu J.O., Bhattacharya P., Mukherjee A.B., Bundschuh J. In: Bhattacharya P., Mukherjee A.B., Bundschuh J., Zevenhoven R., Loeppert R.H., editors. Vol. 9. 2007. Chapter 1 arsenic in soil and groundwater: an overview; pp. 3–60. (Trace Metals and other Contaminants in the Environment). [Google Scholar]
- 25.Lozak A., Soltyk K., Ostapczuk P., Fijalek Z. Determination of selected trace elements in herbs and their infusions. Sci. Total Environ. 2002;289:33–40. doi: 10.1016/S0048-9697(01)01015-4. [DOI] [PubMed] [Google Scholar]
- 26.Kandic I., Milan Kragovic, Petrovic J., Momčilovic M., Stojmenovic M. 1 heavy metals content in selected medicinal plants produced and consumed in Serbia and their daily intake in herbal infusions. Toxics. 2023;11:1–19. doi: 10.3390/toxics11020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nkuba L., Mohammed N. Heavy metals and essential elements in selected medicinal plants commonly used for medicine in Tanzania. Chem. Sci. Int. J. 2017;19:1–11. doi: 10.9734/csji/2017/31963. [DOI] [Google Scholar]
- 28.Luo L., Wang B., Jiang J., Fitzgerald M., Huang Q., Yu Z., Li H., Zhang J., Wei J., Yang C., et al. Heavy metal contaminations in herbal medicines: determination, comprehensive risk assessments, and solutions. Front. Pharmacol. 2021;11:1–14. doi: 10.3389/fphar.2020.595335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quds T., Ahmed M., Shakeel S., Jalbani N., Mazhar F., Azhar I. Determination of the heavy metal contents of frequently used herbal products in Pakistan. Trop. J. Pharm. Res. 2021;20:377–382. doi: 10.4314/tjpr.v20i2.23. [DOI] [Google Scholar]
- 30.A M., J M., S F., U F., S A., A S., I M., K Z., H S. Heavy metals analysis, phytochemical, phytotoxic and anthelmintic investigations of crude methanolic extract, subsequent fractions and crude saponins from Polygonum Hydropiper L. BMC Complement. Altern. Med. 2014:14. doi: 10.1186/1472-6882-14-465. (no pagination) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.M. Ahammad, M. Islam, K. Towhid Osman, M.G. Kibria, M.J. Ahammad, M. Islam, K.T. Osman, Dynamics of Cadmium and Lead in Some Soils of Chittagong, Bangladesh Data·February 2013 CITATIONS 2 READS 125 Dynamics of Cadmium and Lead in Some Soils of Chittagong, Bangladesh, vol. 2.
- 32.Vuong T.X. Determining the content of toxic elements (Pb, Cd, and As) in herbal plants collected from different sites in Northern Vietnam. J. Vietnam. Environ. 2020;12:70–77. doi: 10.13141/jve.vol12.no2.pp70-77. [DOI] [Google Scholar]
- 33.Kwaansa-Ansah E.E., Nti S.O., Opoku F. Heavy metals concentration and human health risk assessment in seven commercial fish species from Asafo market, Ghana. Food Sci. Biotechnol. 2019;28:569–579. doi: 10.1007/s10068-018-0485-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fayiga A., Nwoke O. Metal (loid)s in farmland soils and strategies to reduce bioavailability. Open J. Environ. Biol. 2017;2:009–024. doi: 10.17352/ojeb.000003. [DOI] [Google Scholar]
- 35.Habibollahi M.H., Sharafi K., Omer A.K. Analysis of minerals and toxic elements in commonly consumed herbal medicines in Zahedan, Iran, and associated human health risk assessment. J. Food Prot. 2022;85:1797–1806. doi: 10.4315/JFP-22-173. [DOI] [PubMed] [Google Scholar]
- 36.Özden H. Assessment of toxic metals in commonly used herbs and spices in Turkey. İstanbul J. Pharm. 2021;51:392–397. doi: 10.26650/istanbuljpharm.2021.1000200. [DOI] [Google Scholar]
- 37.Arsenic F.D.A. Elemental Analysis Manual for Food and Related Product. 2012. Speciation in rice and rice products using high performance liquid chromatography-inductively coupled plasma-mass spectrometric determination; pp. 1–23. [Google Scholar]
- 38.Codex Committee on Food Additives and Contaminants (CCFAC) Working Document for Information and Use in Discussions Related to Contaminants and Toxins in the GSCTFF, Fifth Session, Hague, 2011.
- 39.Codex Alimentarius Commission Joint FAO/WHO Food Standards Programme CODEX Committee on Contaminants in Foods Fifth Session Working Document for Information and Use in Discussions Related to Contaminants and Toxins in the GSCTFF (Prepared by Japan and the Netherlands). (2011)., 2013.
- 40.The European Commission Commission Regulation (EU) 2015/1006 of 25 June 2015: amending regulation (EC) No 1881/2006 as regards maximum levels of inorganic arsenic in foodstuffs, Off. J. Eur. Union, vol. 2015, 2015, pp. 1993–5.
- 41.The Commission of The European Communities COMMISSION REGULATION (EC) No 1881/2006 of 19 december 2006 setting maximum levels for certain contaminants in foodstuffs (text with EEA relevance), Off. J. Eur. Union, 2006, pp. 5–24.
- 42.Limmatvapirat C., Limmatvapirat S., Charoenteeraboon J., Wessapan C., Kumsum A., Jenwithayaamornwech S., Luangthuwapranit P. Comparison of eleven heavy metals in Moringa oleifera Lam. products. Indian J. Pharm. Sci. 2015;77:485–490. doi: 10.4103/0250-474X.164782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janvier H., Muhizi T., Ndayambaje J.B., Akenga T.A. Nutritional value assessment of umufumba: a Rwandan wild edible plant mondia Whytei (Hook. F) Food Sci. Nutr. 2019;7:86–95. doi: 10.1002/fsn3.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akan J.C., Inuwa L.B., Chellube Z.M., Lawan B. Heavy metals in leaf, stem bark of neem tree (Azadirachta Indica) and roadside dust in Maiduguri Metropolis, Borno State, Nigeria. Environ. Pollut. 2012:2. doi: 10.5539/ep.v2n1p88. [DOI] [Google Scholar]
- 45.Chakraborti D., Singh S.K., Rahman M.M., Dutta R.N., Mukherjee S.C., Pati S., Kar P.B. Groundwater arsenic contamination in the Ganga River basin: a future health danger. Int. J. Environ. Res. Public Health. 2018;15:1–19. doi: 10.3390/ijerph15020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olatunji O., Ogunkunle C., Fatoba P. Heavy metals in African rice (Oryza Glaberrima) and their possible health risk: a review. Toxicol. Environ. Chem. 2019;101:811–825. [Google Scholar]
- 47.Dghaim R., Khatib S., Al, Rasool H., Khan M.A. Determination of heavy metals concentration in traditional.2015.Pdf. J. Environ. Public Health. 2015;2015:1–6. doi: 10.1155/2015/973878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sepehri M., Zokaei M., Zarei A., Jafari F. Determination of the concentration of heavy metals in medicinal plants and assessment of the risk to health. Amaz. Investig. 2018;7:335–343. [Google Scholar]
- 49.Bakar M.A., Chandra Bhattacherjy S. Assessment of heavy metals concentration in some selected medicinal plants collected from BCSIR, chittagong cultivation area in Bangladesh. Hamdard Med. 2012;55:26–32. [Google Scholar]
- 50.Wan Y., Camara A.Y., Yu Y., Wang Q., Guo T., Zhu L., Li H. Cadmium dynamics in soil pore water and uptake by rice: influences of soil-applied selenite with different water managements. Environ. Pollut. 2018;240:523–533. doi: 10.1016/j.envpol.2018.04.044. [DOI] [PubMed] [Google Scholar]
- 51.Mitra A., Chatterjee S., Gupta D.K. Arsenic contamination in the environment: the issues and solutions. Arsen. Contam. Environ. Issues Solut. 2017:1–218. doi: 10.1007/978-3-319-54356-7. [DOI] [Google Scholar]
- 52.Mitra A., Chatterjee S., Moogouei R., Gupta D.K. Arsenic accumulation in rice and probable mitigation approaches: a review. Agronomy. 2017;7:1–22. doi: 10.3390/agronomy7040067. [DOI] [Google Scholar]
- 53.Codex Alimentarius Commission Joint FAO/WHO Food Standards Programme Codex Committee on Contaminants in Foods: Working Document for Information and Use in Discussions Related to Contaminants and Toxins in the GSCTFF; Fifth Session. The Hague, Netherlands, 21–25 March 2011, Dioxi, 2011.
- 54.J. Mirosławski, A. Paukszto, Determination of the Cadmium, Chromium, Nickel, and Lead Ions Relays in Selected Polish Medicinal Plants and Their Infusion, 2018, pp. 147–51. 〈 10.1007/s12011-017-1072-5〉. [DOI] [PMC free article] [PubMed]
- 55.M. Haban, M. Habanova, P. Otepka, N. Lukac, M. Haban, M. Habanova, P. Otepka, N. Lukac, Concentration of Heavy Metals in Various Children’s Herbal Tea Types and Their Correlations, 2008, 1234. 〈 10.1080/03601230802174755〉. [DOI] [PubMed]
- 56.World Health Organization WHO Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues, vol. 1999, 2007.
- 57.Meseret M., Ketema G., Kassahun H. Health risk assessment and determination of some heavy metals in commonly consumed traditional herbal preparations in Northeast Ethiopia. J. Chem. 2020:2020. doi: 10.1155/2020/8883837. [DOI] [Google Scholar]
- 58.Mlangeni A.T., Lancaster S.T., Raab A., Krupp E.M., Norton G.J., Feldmann J. Impact of soil-type, soil-PH, and soil-metal (loids) on grain-As and Cd accumulation in malawian rice grown in three regions of Malawi. Environ. Adv. 2022;7 doi: 10.1016/j.envadv.2021.100145. [DOI] [Google Scholar]
- 59.Atique-Ullah A.K.M., Maksud M.A., Khan S.R., Lutfa L.N., Quraishi S.B. Dietary intake of heavy metals from eight highly consumed species of cultured fish and possible human health risk implications in Bangladesh. Toxicol. Rep. 2017;4:574–579. doi: 10.1016/j.toxrep.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.ATSDR Public Health Statement: Cobalt, Atlanta, USA, 2004.
- 61.Yan X., Zhang F., Zeng C., Zhang M., Devkota L.P., Yao T. Relationship between heavy metal concentrations in soils and grasses of roadside farmland in Nepal. Int. J. Environ. Res. Public Health. 2012;9:3209–3226. doi: 10.3390/ijerph9093209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roberta F., Maria J., Souza D.O., Silva E., Paula D., Martins C., Carolina A., Paulelli C., Barbosa F., Lemos B. Arsenic speciation in brazilian rice grains organically and traditionally cultivated: is there any difference in arsenic content ? FRIN. 2016;89:169–176. doi: 10.1016/j.foodres.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 63.Lai Y., Xi B., He L., Lin M., Cao L., Wu Y., Mou S., He S. Cadmium uptake by and translocation within rice (Oryza Sativa L.) seedlings as affected by iron plaque and Fe2O3. Pak. J. Bot. 2012;44:1557–1561. [Google Scholar]
- 64.Cicero N., Gervasi T., Durazzo A., Lucarini M., Macr A., Nava V., Giarratana F., Tardugno R., Vadal R., Santini A. Mineral and microbiological analysis of spices and aromatic herbs. Foods. 2022;11:1–12. doi: 10.3390/foods11040548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.