Abstract
BACKGROUND:
A tubeless, on-body automated insulin delivery (AID) system (Omnipod 5 Automated Insulin Delivery System) demonstrated improved glycated hemoglobin A1c levels and increased time in range (70 mg/dL to 180 mg/dL) for both adults and children with type 1 diabetes in a 13-week multicenter, single-arm study.
OBJECTIVE:
To assess the cost-effectiveness of the tubeless AID system compared with standard of care (SoC) in the management of type 1 diabetes (T1D) in the United States.
METHODS:
Cost-effectiveness analyses were conducted from a US payer’s perspective, using the IQVIA Core Diabetes Model (version 9.5), with a time horizon of 60 years and an annual discount of 3.0% on both costs and effects. Simulated patients received either tubeless AID or SoC, the latter being defined as either continuous subcutaneous insulin infusion (86% of patients) or multiple daily injections. Two cohorts (children: <18 years; adults: ≥18 years) of patients with T1D and 2 thresholds for nonsevere hypoglycemia (nonsevere hypoglycemia event [NSHE] <54 mg/dL and <70 mg/dL) were considered. Baseline cohort characteristics and treatment effects of different risk factors for tubeless AID were sourced from the clinical trial. Utilities and cost of diabetes-related complications were obtained from published sources. Treatment costs were derived from US national database sources. Scenario analyses and probabilistic sensitivity analyses were performed to test the robustness of the results.
RESULTS:
Treating children with T1D with tubeless AID, considering an NSHE threshold of less than 54 mg/dL, brings incremental life-years (1.375) and quality-adjusted life-years (QALYs) (1.521) at an incremental cost of $15,099 compared with SoC, resulting in an incremental cost-effectiveness ratio of $9,927 per QALY gained. Similar results were obtained for adults with T1D assuming an NSHE threshold of less than 54 mg/dL (incremental cost-effectiveness ratio = $10,310 per QALY gained). Furthermore, tubeless AID is a dominant treatment option for children and adults with T1D assuming an NSHE threshold of less than 70 mg/dL compared with SoC. The probabilistic sensitivity analyses results showed that compared with SoC, in both children and adults with T1D, tubeless AID was cost-effective in more than 90% of simulations, assuming a willingness-to-pay threshold of $100,000 per QALY gained. The key drivers of the model were the cost of ketoacidosis, duration of treatment effect, threshold of NSHE, and definition of severe hypoglycemia.
CONCLUSIONS:
The current analyses suggest that the tubeless AID system can be considered a cost-effective treatment compared with SoC in people with T1D from a US payer’s perspective.
Plain language summary
The tubeless, automated insulin delivery system (AID) (Omnipod 5 Automated Insulin Delivery System, Insulet Corporation) is cleared for the treatment of individuals aged 2 years and older with type 1 diabetes. Clinical study data have shown that the use of this device is safe and results in improved glucose control (lower hemoglobin A1c and less hypoglycemia). The current study demonstrated that, as compared with standard of care (SoC), the tubeless system can be considered cost-effective and, in some scenarios, demonstrates cost savings and health gain in the United States.
Implications for managed care pharmacy
This first health-economic evaluation comparing tubeless AID vs SoC for the treatment of individuals with type 1 diabetes in the United States showed higher treatment costs but also higher quality-adjusted life-years associated with tubeless AID. Higher treatment costs were partially offset by much higher hypoglycemia and ketoacidosis costs in the SoC arm. A greater understanding of the cost-effectiveness of treatments in type 1 diabetes benefits both clinicians and policymakers.
Type 1 diabetes (T1D) mellitus, an autoimmune disease, is marked by loss of insulin-producing pancreatic β-cells, resulting in chronic hyperglycemia, serious complications, and death if untreated.1-3 As of 2019, 244,000 children and adolescents (<20 years) and 1.6 million adults (≥20 years) in the United States were estimated to be diagnosed with T1D and using insulin.4
T1D incurs substantial economic and societal burden, with $14.4B of T1D costs associated with direct medical costs and lost income.5 T1D also causes diabetes-related distress, associated with worsening of diabetes management,6 and includes symptoms such as feeling of powerlessness and negative social perceptions, negatively affecting quality of life (QoL) of both patients and their families/caregivers. Additionally, 55% of individuals with T1D reported having a fear of hypoglycemia,7 disrupting sleep quality, leisure time, work productivity, and decision-making.8-11 With estimates of the projected lifetime economic burden of T1D in the United States exceeding $800B,12 there is not only a clinical but also an economic impetus for providing a cost-effective (CE) treatment that will allow individuals with T1D to reach their glycemic goals, avoid diabetes-related complications, and improve QoL.
T1D is associated with lifelong daily, but constantly changing, insulin therapy, thereby necessitating frequent blood glucose (BG) monitoring to determine the dose of insulin needed to achieve euglycemia. Traditionally, treatment comprised multiple daily injections (MDIs) of insulin, with BG levels monitored via finger pricking and blood samples collected with a meter and test strip.13-15 Alternative treatment options such as wearable insulin pumps for continuous subcutaneous insulin infusion (CSII) and continuous glucose monitors (CGMs) for ongoing real-time assessment of glucose levels are associated with reduced burden and improved glycemic outcomes.16 However, many patients with T1D in the United States are still not able to meet recommended treatment goals despite increasing adoption of these devices: by a 2018 estimate, 83% and 79% of children and adults, respectively, did not meet the recommended hemoglobin A1c (A1c) goal by the American Diabetes Association (ADA).15,17 Additionally, the risk of hypoglycemia remains a limiting factor in the efficient management of T1D, as a modest overdelivery of insulin can lead to acute, life-threatening complications. Thus, there lies a need for better treatment alternatives that can provide glucose-lowering benefits while simultaneously reducing the risk of hypoglycemia.3,15,17
The Omnipod 5 Automated Insulin Delivery System (Insulet Corporation), an automated insulin delivery (AID) system, was cleared by the US Food and Drug Administration in January and August 2022 for people with T1D, aged 6 years and older and 2 years and older, respectively.18 Unlike other AID systems with tubed insulin pumps, Omnipod 5 has a novel tubeless design consisting of a handheld wireless controller and a disposable adhesive Pod.18 The latter contains an algorithm that communicates directly with a CGM (Dexcom G6) and automatically adjusts insulin delivery in response to current and predicted glucose values.19 In a recently published study comparing 13 weeks of the tubeless AID system with standard of care (SoC, either CSII or MDI) in children and adults with T1D, tubeless AID significantly improved A1c levels and time with sensor glucose in target range (70-180 mg/dL) for both age groups. Moreover, time spent in hypoglycemia (<70 mg/dL) significantly decreased in adults, whereas it remained the same as SoC in children.20 This same study reported that adults experienced improvement in diabetes distress, hypoglycemic confidence, and treatment satisfaction while using the tubeless system.21
Because of limited health care budgets, novel treatment interventions must demonstrate not only efficacy and safety but also cost-effectiveness. Thus, the current study aimed to assess the cost-effectiveness of tubeless AID vs SoC for the management of children and adults with T1D in the United States, using cost-utility analysis that allows both costs and impact on patient QoL to be valued.
Methods
MODELING APPROACH
IQVIA Core Diabetes Model (CDM) version 9.5 (http://www.core-diabetes.com/) was used to perform long-term projections of clinical and cost outcomes from the US third-party payer’s perspective. CDM, a proprietary, interactive computer simulation model, includes a series of interdependent Markov submodels that perform real-time simulations of the progression of diabetes-related complications and associated mortality. CDM and its validation studies have been previously described in literature.22-24
Projected outcomes included incidence of complications, rates of clinical events, per-patient costs, life-years (LYs), and quality-adjusted LYs (QALYs) gained over a lifelong time horizon (ie, 60 years). CE was defined as incremental cost-effectiveness ratio (ICER), that is, cost per additional unit of QALY gained for the intervention (tubeless AID) vs the comparator (SoC) in individuals with T1D. Net monetary benefit (NMB) results are also shown (NMB>0 = CE). A willingness-to-pay (WTP) threshold of $100,000 per QALY gained was assumed in the current analyses. Both costs and effects were discounted by 3.0% annually, as recommended for the United States.25 All prices were stated in US dollar (USD) price-level 2020 ex VAT and all analyses were run with 1,000 individuals for 1,000 iterations.
The guidelines to report cost-effectiveness modeling in diabetes developed by the Mount Hood Diabetes Challenge working group were followed.26
MODEL INPUTS
Population Inputs. Baseline characteristics incorporated in the model were obtained from a clinical trial of the tubeless AID system published by Brown et al.20 The study included people aged 6-70 years diagnosed with T1D for at least 6 months, with a point-of-care screening A1c less than 10.0% (86 mmol/mol), and without a history of severe hypoglycemia or diabetic ketoacidosis (DKA) in the past 6 months. In the current analyses, the study data were reanalyzed using revised age cohorts to clearly distinguish children (<18 years) from adults (≥18 years). At baseline, many participants were already achieving or near target glycemia with a mean baseline A1c close to ADA goals for both cohorts. Baseline characteristics available in the clinical trial were age, duration of diabetes, ethnicity, A1c, blood pressure, and body mass index. Those that were not reported were obtained from the placebo arm of the EASE 3 study, which was conducted in adults with T1D.27 In the children cohort, it was assumed that no comorbidities were present at study start. For values that were not collected in clinical trial, such as alcohol consumption and smoking, default values in IQVIA CDM were used. The applied baseline characteristics for the base-case population are shown in Supplementary Table 1 (567.7KB, pdf) , available in online article.
Besides the base-case population, scenario analyses were also performed on the following 2 subgroups of patients: (1) those who had an SoC time below range (TBR; <70 mg/dL) of greater than or equal to 4% (Supplementary Table 2 (567.7KB, pdf) ) and (2) those with A1c of greater than or equal to 8% at study start (Supplementary Table 3 (567.7KB, pdf) ).
Clinical Inputs. Treatment effects (Table 1) were obtained from the clinical trial for the revised age cohorts (data on file). For comparison, the efficacy results for the original age cohorts described in Brown et al20 are shown in Supplementary Table 4 (567.7KB, pdf) .
TABLE 1.
Treatment Effects
| Parameter (units) | Children (6-17.9 years) | Adults (≥18 years) | ||
|---|---|---|---|---|
| SoC | Tubeless AID | SoC | Tubeless AID | |
| Base case | ||||
| Change in baseline A1c (%), mean (SD) | 0.000 | -0.690 (0.640) | 0.000 | -0.360 (0.510) |
| NSHE <70 mg/dL (per 100 patient-years) event rate | 28,226 | 27,062 | 29,145 | 17,461 |
| NSHE <54 mg/dL (per 100 patient-years) event rate | 5,607 | 5,398 | 6,569 | 3,131 |
| SHE 1 (requiring nonmedical assistance) (per 100 patient-years) event rate | 18.0 | 3.6 | 29.5 | 7.2 |
| DKA (per 100 patient-years) event rate | 13.2 | 3.6 | 10.3 | 0.0 |
| TBR ≥4% | ||||
| Change in baseline A1c (%), mean (SD) | 0.000 | -0.430 (0.600) | 0.000 | -0.270 (0.410) |
| NSHE <70 mg/dL (per 100 patient-years) event rate | 71,113 | 47,812 | 59,438 | 29,360 |
| NSHE <54 mg/dL (per 100 patient-years) event rate | 21,384 | 12,894 | 15,867 | 5,529 |
| SHE 1 (requiring nonmedical assistance) (per 100 patient-years) event rate | 18.0 | 18.0 | 29.5 | 13.2 |
| DKA (per 100 patient-years) event rate | 13.2 | 0.0 | 10.3 | 0.0 |
| A1c ≥8% | ||||
| Change in baseline A1c (%), mean (SD) | 0.000 | -1.180 (0.600) | 0.000 | -0.840 (0.690) |
| NSHE <70 mg/dL (per 100 patient-years) event rate | 18,614 | 21,855 | 24,705 | 12,517 |
| NSHE <54 mg/dL (per 100 patient-years) event rate | 4,773 | 4,371 | 6,361 | 2,868 |
| SHE 1 (requiring nonmedical assistance) (per 100 patient-years) event rate | 18.0 | 0.0 | 29.5 | 0.0 |
| DKA (per 100 patient-years) event rate | 13.2 | 9.6 | 10.3 | 0.0 |
A1c = hemoglobin A1c; AID = automated insulin delivery; DKA = diabetes ketoacidosis; NSHE = severe hypoglycemic event; SHE = severe hypoglycemic event; SoC = standard of care; TBR = time below range.
The comparator data on A1c and nonsevere hypoglycemia event (NSHE, not needing third-party assistance) rates came from the status before the trial (ie, baseline), implying that no treatment effect was applied in the SoC arm. Severe hypoglycemia events (SHEs) and DKA rates were obtained from the clinical trial for the active arm and the US T1D Exchange Registry15,28 for the SoC arm. Although SHEs are defined as needing third-party assistance, the trial data did not specify if this assistance was nonmedical or medical. Therefore, in the base case it was assumed that assistance is provided by a nonmedical third-party, which is cheaper and more conservative. As symptomatic NSHEs were not recorded in the trial, NSHEs were defined based on CGM measurements using 2 cut-off values: less than 54 mg/dL (level 2 hypoglycemia) and less than 70 mg/dL (level 1 hypoglycemia). Despite a frequent absence of symptoms at less than 70 mg/dL and no impact on QoL within the model, level 1 hypoglycemia is still regarded as clinically relevant as it can carry increased risk under some circumstances including driving. Level 2 hypoglycemia is more stringent and considered as the base case, as this is the threshold at which neuroglycopenic symptoms usually occur and requires immediate action to restore euglycemia.29
Changes in other physiological parameters such as blood pressure and serum lipid levels were not applied as these were not reported in the trial. Baseline values were assumed to follow the model’s default progression equations. It is worth noting that the evolution of blood pressure, cholesterol, body weight, and body mass index in children differs from that of adults and it was assumed that from the age of 18 years onward, the effects recorded in adults would apply rather than the ones measured in children.
The SoC was defined as either CSII (86% of patients) or MDI, as this was the observed baseline distribution within trial participants.
Cost Inputs. Only direct medical costs associated with pharmacy, management, and T1D complications were considered in the current analyses. Treatment-related costs comprised insulin, needles (where applicable), insulin pump, CGM, or self-monitoring of BG (SMBG). The unit costs of insulin were based on wholesale acquisition costs from the MediSpan PriceRx database.30
The cost of insulin varies for children and adults and is dependent on the average number of units of insulin needed per day in the clinical trial at baseline (SoC arm) or during the trial (tubeless AID arm) (Supplementary Table 5 (567.7KB, pdf) ).
The cost of tubeless AID was set at $51.95 per 3-day use period (Insulet communication). For the SoC arm, costs were determined according to the observed distribution of baseline therapy method in the clinical trial: CGM (97% and 98% of children and adults) or SMBG and CSII (88% and 84% of children and adults) or MDI. The total costs used per arm are shown in Table 2.
TABLE 2.
Total Costs (USD)
| Children (6-17.9 years) | Adults (≥18 years) | |||
|---|---|---|---|---|
| SoC | Tubeless AID | SoC | Tubeless AID | |
| Base case | ||||
| Basal insulin | 1,820 | 2,357 | 2,715 | 2,698 |
| Bolus insulin | 2,463 | 2,274 | 2,722 | 2,604 |
| SMBG | 64 | — | 29 | — |
| CGM | 5,212 | 5,309 | 5,185 | 5,309 |
| Needles | 47 | — | 74 | — |
| Insulin pump | 4,383 | — | 4,066 | — |
| Omnipod 5 | — | 6,325 | — | 6,325 |
| Annual total | 13,990 | 16,264 | 14,790 | 16,936 |
| TBR ≥4% | ||||
| Basal insulin | 1,588 | 1,697 | 2,800 | 2,628 |
| Bolus insulin | 2,003 | 2,027 | 3,064 | 2,958 |
| SMBG | — | — | — | — |
| CGM | 5,407 | 5,309 | 5,269 | 5,309 |
| Needles | 20 | — | 92 | — |
| Insulin pump | 4,720 | — | 3,838 | — |
| Omnipod 5 | — | 6,325 | — | 6,325 |
| Annual total | 13,738 | 15,357 | 15,063 | 17,219 |
| A1c ≥8% | ||||
| Basal insulin | 1,900 | 2,840 | 3,527 | 3,771 |
| Bolus insulin | 2,746 | 2,545 | 3,347 | 2,863 |
| SMBG | 121 | — | 198 | — |
| CGM | 5,039 | 5,309 | 4,685 | 5,309 |
| Needles | 47 | — | 114 | — |
| Insulin pump | 4,393 | — | 3,580 | — |
| Omnipod 5 | — | 6,325 | — | 6,325 |
| Annual total | 14,245 | 17,019 | 15,450 | 18,268 |
— = not applicable; A1c = hemoglobin A1c; AID = automated insulin delivery; CGM=continuous glucose monitoring; SMBG = self-blood glucose monitoring; SoC = standard of care; TBR = time below range; USD = US dollar.
The cost of complications, collected in 2020 via a targeted literature search, are shown in Supplementary Table 6 (567.7KB, pdf) . All costs were expressed in USD, and wherever necessary, the costs were inflated using the latest available Bureau of Labor Statistics consumer price index for medical care in 2020.31
Utility. Utility values, calculated for every patient in each simulation year, were used to estimate the average QALY. Utilities were rated on a scale of 0 to 1, with 0 denoting death (no QoL) and 1 denoting a healthy individual without complications. Disutilities due to illness have values ranging from −1 to 0, causing the QoL utility to either decrease or remain constant. The QALYs were assessed using the minimal Core Default Method, which involves taking the lowest state utility associated with existing comorbidities and adding event disutilities for any events that occur in that year, in order to create annual utility scores for each simulated patient.23
In the absence of trial-specific utilities, publicly available sources such as Peasgood et al32 and Beaudet et al33 were used (Supplementary Table 7 (567.7KB, pdf) ). A key driver of CE results in T1D is the disutility assigned to hypoglycemic events. In the current analyses, diminishing disutilities were applied to NSHE, implying that the higher the incidence of NSHE, the lower is the disutility applied per event as the patient becomes accustomed to having those events.34 This is a conservative approach vs applying a fixed disutility per NSHE.
ANALYTICAL APPROACH
Base-Case and Scenario Analyses. Base-case analyses examined the CE of the tubeless AID system vs SoC in 2 cohorts of patients with T1D (cohort 1: children aged <18 years and cohort 2: adults aged ≥18 years) with NSHEs defined at a threshold of less than 54 mg/dL. In addition, scenario analyses were conducted to evaluate how changes to key parameters in the modeling analyses impact the results of the base-case analyses.
The current study was a lifelong analysis with a time horizon of 60 years as the base case. Scenarios with shorter (5, 10, 20, and 40 years) and longer (80 years) time horizons were also investigated, as therapy options will not remain constant over these time frames.
The Pittsburg cardiovascular disease (CVD) risk equation was selected as the base case for children because the study on which this equation is based included children, whereas the Epidemiology of Diabetes Interventions and Complications (EDIC) trial included adults only and is used as the base case for the adult population. As a scenario, the EDIC trial and Pittsburg CVD risk equation was tested for children and adults, respectively.
As previously mentioned, a scenario analysis was performed using a less stringent definition on NSHE (<70 mg/dL) and on SHE in which it was assumed that all SHE needed medical attention. As DKA event rates in the SoC arm were taken from literature, and with the cost of DKA being an important driver within the model, a scenario analysis was conducted where the cost of DKA was assumed to be zero.
In the base case, the A1c progression over time according to the EDIC study was applied, meaning that an annual increase in A1c of 0.045% was assumed. This means that the initial difference in A1c between the 2 arms is maintained lifelong. Therefore, as a scenario, an alternative progression of A1c according to the Swedish National Diabetes Registry was applied. By doing so, A1c in the 2 arms converge, and the duration of the treatment effect is limited in time.
Analyses were conducted for the general study population, but also for subgroups of higher-risk patients with a starting A1c greater than or equal to 8% or a TBR of greater than or equal to 4%. Additionally, a scenario analysis was conducted reducing the impact on A1c by 50% and increasing the rate of NSHE (to the level of SoC during childhood, and by 50% in adults) seen with the tubeless AID system.
Lastly, in the CDM, the noncombined mortality was selected. This means that disease-specific mortality resulting from transition probabilities (eg, case fatalities for myocardial infarction, stroke, and end-stage renal disease) was combined with a non–disease-specific mortality taken from life tables.35
Probabilistic Sensitivity Analyses. Probabilistic sensitivity analyses (PSAs) were performed using Monte Carlo simulations together with a nonparametric bootstrapping approach to determine parameter uncertainty around CE outcomes. The parameters included in the PSAs were per-individual characteristics, treatment efficacy, utility, and cost of complications. Treatment effects were sampled based on the estimated SE detailed in Table 1. The utility data were varied according to the variability reported in SE values displayed in Supplementary Table 7 (567.7KB, pdf) . Sampling was done following the β distribution. For sampling individuals’ baseline characteristics, truncated normal distributions were used following the mean and SE/SD reported in Supplementary Table 1 (567.7KB, pdf) . Results were presented in the cost-effectiveness plane and as cost-effectiveness acceptability curves (CEACs).
COMPLIANCE WITH ETHICS GUIDELINES
This cost-effectiveness analysis, based on the CDM model, was used to simulate the long-term clinical and economic outcomes of the tubeless AID system and SoC based on existing literature findings and completed clinical trials. It does not involve any new studies on human participants or animals directly performed by any of the authors.
Results
COST-EFFECTIVENESS RESULTS
Base-Case Population Analyses. The base-case analysis showed that over a 60-year time horizon, in children with T1D, tubeless AID resulted in higher LYs (1.375) and QALYs (1.521) at an incremental cost of $15,099 compared with SoC, thereby generating an ICER of $9,927 per QALY gained. As this ICER is less than the WTP threshold of $100,000 per QALY gained, tubeless AID can be considered CE compared with SoC (Table 3). Similar results were obtained for adults with T1D (incremental LY = 1.022; incremental QALY = 1.112; ICER = $10,310 per QALY gained) (Table 3).
TABLE 3.
Cost-Effectiveness Results of Base-Case Analysis Comparing Tubeless AID vs SoC
| Parameters | NSHE <54 mg/dL | NSHE <70 mg/dL | |||
|---|---|---|---|---|---|
| Tubeless AID | SoC | Tubeless AID | SoC | ||
| Children | |||||
| LYs | 24.978 | 23.604 | 24.978 | 23.604 | |
| QALYs | 17.966 | 16.444 | 17.039 | 15.519 | |
| Total costs ($) | 499,539 | 484,440 | 553,141 | 555,624 | |
| Comparison: Tubeless AID vs SoC | |||||
| Incremental LY | 1.375 | 1.375 | |||
| Incremental QALY | 1.521 | 1.519 | |||
| Incremental cost ($) | 15,099 | -2,483 | |||
| ICER ($/QALY gained) | 9,927 | Dominant | |||
| NMB | 137,001 | 154,383 | |||
| Adults | |||||
| LYs | 20.321 | 19.299 | 20.321 | 19.299 | |
| QALYs | 13.807 | 12.695 | 13.807 | 12.695 | |
| Total costs ($) | 441,023 | 429,558 | 480,200 | 488,230 | |
| Comparison: Tubeless AID vs SoC | |||||
| Incremental LY | 1.022 | 1.022 | |||
| Incremental QALY | 1.112 | 1.123 | |||
| Incremental cost ($) | 11,465 | -8,029 | |||
| ICER ($/QALY gained) | 10,310 | Dominant | |||
| NMB | 99,375 | 120,329 | |||
All costs shown in US dollars.
AID = automated insulin delivery; ICER = incremental cost-effectiveness ratio; LY = life-year; NMB = net monetary benefit; NSHE = nonsevere hypoglycemia event; QALY = quality-adjusted life-year; SoC=standard of care.
The base-case analysis also showed that the treatment costs were higher in patients using tubeless AID ($421,275 for children and $350,524 for adults) compared with SoC ($346,910 for children and $291,438 for adults). However, these extra treatment costs were partially offset by much higher adverse event costs (DKA and NSHE) in the SoC arm (Table 4). The cost of other complications is not so different between the 2 arms, as individuals in the tubeless AID arm live longer and have more time to generate costs despite the lower event rates. An exception is the cost of renal complications with much higher costs in the SoC arm due to a substantially higher incidence in end-stage renal disease.
TABLE 4.
Breakdown of Costs (per Average Patient; Base Case)
| Breakdown of costs (USD) | NSHE <54 mg/dL | NSHE <70 mg/dL | ||
|---|---|---|---|---|
| Tubeless AID | SoC | Tubeless AID | SoC | |
| Children | ||||
| Total cost | 499,539 | 484,440 | 553,141 | 555,624 |
| Treatment cost | 421,275 | 346,910 | 421,275 | 346,910 |
| Management | 3,375 | 3,167 | 3,375 | 3,167 |
| Cardiovascular disease | 27,401 | 24,653 | 27,401 | 24,653 |
| Renal disease | 19,171 | 24,495 | 19,171 | 24,495 |
| Ulcer/amputation/neuropathy | 2,691 | 2,871 | 2,691 | 2,871 |
| Eye disease | 8,984 | 10,594 | 8,984 | 10,594 |
| NSHE | 12,246 | 19,939 | 65,848 | 91,122 |
| SHE 1 (requiring nonmedical assistance) | 139 | 514 | 139 | 514 |
| SHE 2 (requiring medical assistance) | — | — | — | — |
| DKA | 4,258 | 51,297 | 4,258 | 51,297 |
| Adults | ||||
| Total cost | 441,023 | 429,558 | 480,200 | 488,230 |
| Treatment cost | 350,524 | 291,438 | 350,524 | 291,438 |
| Management | 2,846 | 2,698 | 2,846 | 2,698 |
| Cardiovascular disease | 38,645 | 37,534 | 38,645 | 37,534 |
| Renal disease | 28,472 | 28,522 | 28,472 | 28,522 |
| Ulcer/amputation/neuropathy | 5,243 | 5,154 | 5,243 | 5,154 |
| Eye disease | 6,604 | 7,100 | 6,604 | 7,100 |
| NSHE | 8,562 | 17,074 | 47,739 | 75,745 |
| SHE 1 (requiring nonmedical assistance) | 127 | 467 | 127 | 467 |
| SHE 2 (requiring medical assistance) | — | — | — | — |
| DKA | — | 39,571 | — | 39,571 |
AID = automated insulin delivery; DKA = diabetic ketoacidosis;
NSHE = nonsevere hypoglycemic event; SHE = severe hypoglycemic event; SoC = standard of care; USD = US dollar.
Supplementary Tables 8 and 9 (567.7KB, pdf) show the number of clinical events per 1,000 patient-years for patients treated with tubeless AID compared with those on SoC. It was observed that patients receiving SoC would have a higher risk of developing a majority of the clinical events, especially DKA and NSHE.
Scenario Analyses. Detailed results of different scenarios for children and adults with T1D (NSHE <54 mg/dL and NSHE <70 mg/dL) are shown in Supplementary Table 10 (567.7KB, pdf) and Supplementary Table 11 (567.7KB, pdf) , respectively.
The ICER improved strongly when applying the less stringent threshold of less than 70 mg/dL to define NSHE and tubeless AID became dominant (improved health outcomes and lower costs), stressing the importance of the definition of NSHE. Applying a different CVD risk equation had a negligible impact on the ICER. Applying another progression of A1c did change the ICER, doubling it in children and increasing it by approximately 50% in adults. Despite this, the ICERs remained well below the WTP threshold, and the tubeless AID can still be considered to be CE vs SoC. Testing higher-risk groups improved the ICER slightly. Reducing the time horizon of the analysis increased the ICER of tubeless AID in children with T1D, explained by the limited impact of tubeless AID on NSHE recorded in the study and a very low rate of complications in the first years after diagnosis of diabetes; however, CE was maintained for all time horizons. Assuming that all SHEs require medical attention reduced the ICER, whereas assuming no cost for a DKA episode and reducing the impact of tubeless AID on A1c and NSHE increased the ICER. However, the ICERs still remained below the WTP threshold.
PSAs. In the children and adult cohorts, the PSA showed that most of the observations fell in the northeast (68% and 64%) and southeast (30% and 30%) quadrants of the scatterplot for NSHE less than 54 mg/dL. The ICER scatterplot was further complemented with a CEAC that showed the probability of tubeless AID being CE (93% and 91%) as compared with SoC at the defined WTP threshold of $100,000 per QALY gained for both the cohorts, respectively (Figure 1A-D).
FIGURE 1.
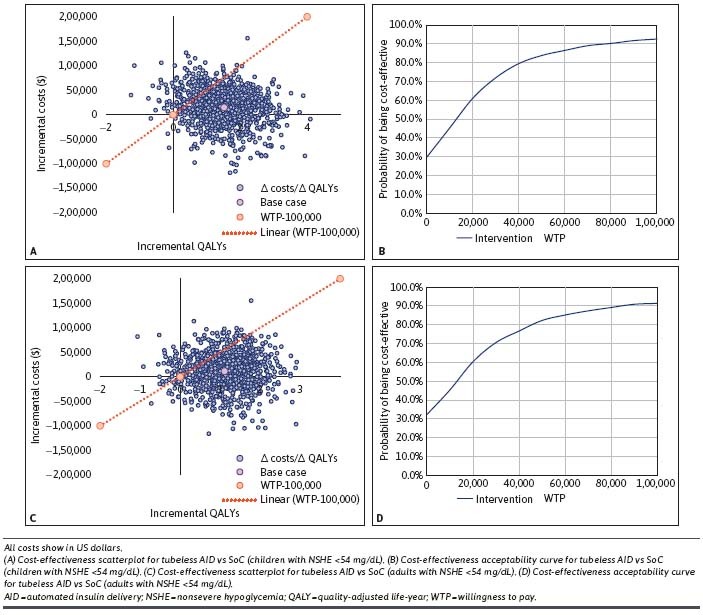
Cost-Effectiveness Scatterplots and Cost-Effectiveness Acceptability Curves (Base-Case Population Comparing Tubeless AID vs SoC, NSHE <54 mg/dL)
Similarly, in the children and adult cohorts, the PSA showed that most of the observations fell in the northeast (45% for both cohorts) and southeast (51% for both cohorts) quadrants of the scatterplot for NSHE less than 70 mg/dL. The CEAC demonstrated the likelihood of the tubeless AID system being CE (94% for both cohorts) as compared with SoC at the defined WTP threshold of $100,000 per QALY gained for both the cohorts, respectively (Supplementary Figure 1A-D (567.7KB, pdf) ).
Discussion
In this study, the use of tubeless AID was considered to be CE over a lifetime from a US third-party payer’s perspective. Additionally, when considering a less stringent NSHE threshold (<70 mg/dL), the tubeless AID became dominant as compared with SoC for both age groups. These findings are crucial to inform decision-making on this CE novel technology among individuals with T1D.
The PSA findings demonstrated that tubeless AID was CE in more than 90% of cases, in both cohorts and across both NSHE estimates. The average population included in the clinical trial had a very well-controlled A1c at baseline. Nevertheless, the results of the base-case population corroborated the findings for 2 high-risk subpopulations–A1c greater than or equal to 8% or TBR greater than or equal to 4%–and the conclusion remained unchanged in all additional scenarios.
NSHE is a major determinant of the incremental QALY estimated with tubeless AID. Because of impracticalities in accurately collecting patient-reported symptomatic NSHE over 13 weeks, an explicit measurement of these events was not possible. As a result, 2 surrogates were considered that relied on objective data collected from the CGM–“a measured glycemia of less than 70 mg/dL or less than 54 mg/dL lasting more than 15 minutes.” Using the less-than-70 mg/dL threshold, NSHE rates were extremely high, especially among children, possibly resulting in an overestimation of benefits, as some of these events might not have been symptomatic. On the other hand, CGM values less than 70 mg/dL is the default threshold for CGM alerts for patients and caregivers and thus result in real-time events, creating disease burden. In particularly hypoglycemicaverse populations, such as school-aged children, CGM alerts may be set higher at 80 mg/dL or more as many parents treat younger children prior to the CGM reaching 70 mg/dL, and thus, a threshold of 70 mg/dL may even underestimate NSHE burden.
The threshold (<54 mg/dL) used in the base case has also been used in another cost-effectiveness analysis,36 and values similar to our analyses have been reported. Notably, SoC data were based on only 2-week follow-up, a short time frame to record the frequency of these events, and thus, the rates of these events were possibly underestimated for this period. For the impact of NSHE on QoL, we applied the conservative diminishing approach. This and applying different NSHE thresholds mitigated the risk of overstating the impact of NSHE events.
Another important driver is the cost of a DKA event. The event rate is rather modest; however, the cost related to the event is important. It contributes strongly to the cost offset between the 2 arms, as these acute events are life-threatening and require an emergency department visit and hospitalization. DKA rates in the SoC and tubeless AID arm were taken from the T1D Exchange Registry study15 and a 13-week evaluation in the clinical trial, respectively, and are applied over the time horizon of the study. Nevertheless, a scenario analysis assuming no cost of DKA increased the ICER to approximately $40,000/QALY, which was still very much below the defined WTP threshold. It is reasonable to assume that the DKA event rate in the AID group is lower than the T1D Exchange rate. The presence of automated hyperglycemia minimization in the AID system reduces many cases of hyperglycemia from inadequate mealtime insulin coverage and likely raises the concern for failed insulin infusion as the cause of unexplained hyperglycemia. Anecdotally, this increased awareness has been observed to reduce DKA rates among AID users in clinical practice, however, further study is needed to confirm this trend.
In the base case, the 13-week A1c effects were assumed to be present lifelong, so no A1c progression convergence was performed. The Swedish National Diabetes Registry progression equation, used as an additional scenario, showed that the A1c effect would disappear after 5 years resulting in a 50% increase of the ICER; however, this did not change the CE conclusion.
SoC in our analysis was assumed to follow the distribution observed in the clinical trial at baseline (CSII in 86% and CGM in 97%-98% of patients), which affects the SoC cost as CSII and CGM have higher costs than MDI and SMBG. However, this distribution is reasonable, as it reflects relatively high use of technology, which is the direction supported by the ADA diabetes technology guideline.37
Considering the limited availability of literature on tubeless AID, the findings of the current study were compared against clinical and economic outcomes for other AID systems (Medtronic: 670 g, 770 g, 780 g, and Tandem Control-IQ) and insulin pump therapy vs MDI in children and adults with T1D. Use of AID or CSII was shown to be more CE, reliable, and accurate in controlling BG levels, extending life expectancy, and lowering the risk of diabetes-related complications in patients with T1D vs MDI.38-43 In addition, a systematic analysis of the clinical efficacy and cost-effectiveness of CSII for patients with T1D demonstrated that CSII provides better glycemic outcomes and fewer hypoglycemic episodes, in turn ensuring a better QoL.44 Similarly, in our study, besides being CE, tubeless AID treatment in children and adults with T1D led to greater LYs and a better QoL with fewer diabetes-related complications vs treatment with SoC.
LIMITATIONS
The results of our study are subject to some limitations. As there was no comparative arm in the pivotal trial,20 data from the 2-week conventional treatment run-in period were used, a time period supported as sufficient by international guidelines.45 However, with behavioral changes that sometimes accompany observation, limiting the comparison to the shorter observational period could have under- or over-estimated the effectiveness of tubeless AID. Scenario analyses in which impact on A1c and NSHE was reduced by 50% showed an increase in ICER; however, cost-effectiveness was maintained. Additionally, the trial period was only 13 weeks, whereas the model cycles were 1 year. It was assumed that A1c reduction from the first 13 weeks was still applicable at 52 weeks. This assumption was supported by 52-week follow-up data on the tubeless AID system, which showed sustained improvement in A1c over this time frame (mean A1c at same level after 3 and 12 months).46
Furthermore, projecting long-term treatment outcomes in children is challenging. Body weight and, as such, the dose (cost) of insulin will increase. Therefore, we assumed that at 18 years, the average dose of insulin and the treatment effect observed in adults were applied. However, there remains ambiguity regarding progress over time pertaining to physiological indicators. Given the recent speed of improved technologies for T1D over the past decade, it is likely that significantly improved systems will become available over the long term, which will build on these effects and further improve the glycemic and QoL benefits over existing systems.
Conclusions
Our study showed that tubeless AID was either a CE or dominant treatment alternative compared with SoC for patients with T1D from the US third-party payer’s perspective. When the model assumptions were taken into consideration, tubeless AID showed more than 90% probability of being CE compared with SoC based on the WTP threshold of $100,000 per QALY gained. Sensitivity and scenario analyses further validated the robustness of the analyses.
ACKNOWLEDGMENTS
We thank Charu Pundir, Saurabh Trikha, Anshika Singhal, Koshu Mahajan, and Paranjoy Saharia of IQVIA for providing medical and editorial assistance with the manuscript. We thank Lauren M Huyett from Insulet Corporation for providing the clinical trial data for the revised age groups and for providing editorial assistance and feedback on the manuscript.
REFERENCES
- 1.World Health Organization. Diabetes. Accessed August 2022. https://www.who.int/news-room/fact-sheets/detail/diabetes
- 2.American Diabetes Association. The cost of diabetes. Accessed August 2022. https://diabetes.org/about-us/statistics/cost-diabetes
- 3.Mobasseri M, Shirmohammadi M, Amiri T, Vahed N, Hosseini Fard H, Ghojazadeh M. Prevalence and incidence of type 1 diabetes in the world: A systematic review and meta-analysis. Health Promot Perspect. 2020;10(2):98-115. doi:10.34172/hpp.2020.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. National diabetes statistics report. Estimates of diabetes and its burden in the United States. 2022. Accessed August 2022. https://www.cdc.gov/diabetes/data/statistics-report/index.html
- 5.Tao B, Pietropaolo M, Atkinson M, Schatz D, Taylor D. Estimating the cost of type 1 diabetes in the U.S.: A propensity score matching method. PLoS One. 2010;5(7):e11501. doi:10.1371/journal.pone.0011501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hessler DM, Fisher L, Polonsky WH, et al. Diabetes distress is linked with worsening diabetes management over time in adults with type 1 diabetes. Diabet Med. 2017;34(9):1228-34. doi:10.1111/dme.13381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahola AJ, Saraheimo M, Freese R, et al. Fear of hypoglycaemia and self-management in type 1 diabetes. J Clin Transl Endocrinol. 2016;4:13-8. doi:10.1016/j.jcte.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fulcher G, Singer J, Castañeda R, et al. The psychosocial and financial impact of non-severe hypoglycemic events on people with diabetes: Two international surveys. J Med Econ. 2014;17(10):751-61. doi:10.3111/13696998.2014.946992 [DOI] [PubMed] [Google Scholar]
- 9.Chatwin H, Broadley M, Hendrieckx C, et al. Unmet support needs relating to hypoglycaemia among adults with type 1 diabetes: Results of a multi-country web-based qualitative study. Diabet Med. 2022;39(1):e14727. doi:10.1111/dme.14727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freed S. Depression and diabetes: Mary de Groot – DIC Interview 2019. Accessed August 2022. https://www.diabetesincontrol.com/depression-and-diabetes-mary-de-groot-dic-interview/
- 11.Martyn-Nemeth P, Duffecy J, Fritschi C, Quinn L. Challenges imposed by hypoglycemia in adults with type 1 diabetes. Clin Nurs Res. 2019;28(8):947-67. doi:10.1177/1054773818774702 [DOI] [PubMed] [Google Scholar]
- 12.Sussman M, Benner J, Haller MJ, Rewers M, Griffiths R. Estimated lifetime economic burden of type 1 diabetes. Diabetes Technol Ther. 2020;22(2):121-30. doi:10.1089/dia.2019.0398 [DOI] [PubMed] [Google Scholar]
- 13.Hofer S, Meraner D, Koehle J. Insulin pump treatment in children and adolescents with type 1 diabetes. Minerva Pediatr. 2012;64(4):433-8. [PubMed] [Google Scholar]
- 14.Haviland N, Walsh J, Roberts R, Bailey TS. Update on clinical utility of continuous glucose monitoring in type 1 diabetes. Curr Diab Rep. 2016;16(11):115. doi:10.1007/s11892-016-0808-5 [DOI] [PubMed] [Google Scholar]
- 15.Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cleveland Clinic. Continuous glucose monitoring. Accessed August 2022. https://my.clevelandclinic.org/health/drugs/11444-glucose-continuons-glucose-monitoring#:~:text=No%2C%20CGM%20devices%20and%20insulin,based%20on%20instructions%20you%20give.
- 17.American Diabetes Association. Standards of medical care in diabetes-2019 abridged for primary care providers. Clin Diabetes. 2019;37(1):11-34. doi:10.2337/cd18-0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Insulet Corporation. Insulet announces FDA clearance of Omnipod®5 for children aged two years and older with type 1 diabetes 2022. Accessed November 2022. https://investors.insulet.com/news/news-details/2022/Insulet-Annonnces-FDA-Clearance-of-Omnipod-5-for-Children-Aged-Two-Years-and-Older-with-Type-1-Diabetes/default.aspx
- 19.Forlenza GP, Buckingham BA, Brown SA, et al. First outpatient evaluation of a tubeless automated insulin delivery system with customizable glucose targets in children and adults with type 1 diabetes. Diabetes Technol Ther. 2021;23(6):410-24. doi:10.1089/dia.2020.0546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown SA, Forlenza GP, Bode BW, et al. Multicenter trial of a tubeless, on-body automated insulin delivery system with customizable glycemic targets in pediatric and adult participants with type 1 diabetes. Diabetes Care. 2021;44(7): 1630-40. doi:10.2337/dc21-0172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polonsky WH, Hood KK, Levy CJ, et al. How introduction of automated insulin delivery systems may influence psychosocial outcomes in adults with type 1 diabetes: Findings from the first investigation with the Omnipod® 5 System. Diabetes Res Clin Pract. 2022;190:109998. doi:10.1016/j.diabres.2022.109998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McEwan P, Foos V, Palmer JL, Lamotte M, Lloyd A, Grant D. Validation of the IMS CORE Diabetes Model. Value Health. 2014;17(6):714-24. doi:10.1016/j.jval.2014.07.007 [DOI] [PubMed] [Google Scholar]
- 23.Palmer AJ, Roze S, Valentine WJ, et al. The CORE Diabetes Model: Projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decisionmaking. Curr Med Res Opin. 2004;20 Suppl 1:S5-26. doi:10.1185/030079904X1980 [DOI] [PubMed] [Google Scholar]
- 24.Palmer AJ, Roze S, Valentine WJ, et al. Validation of the CORE Diabetes Model against epidemiological and clinical studies. Curr Med Res Opin. 2004;20 Suppl 1:S27-40. doi:10.1185/030079904X2006 [DOI] [PubMed] [Google Scholar]
- 25.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093-103. doi:10.1001/jama.2016.12195 [DOI] [PubMed] [Google Scholar]
- 26.Palmer AJ, Si L, Tew M, et al. Computer modeling of diabetes and its transparency: A report on the eighth mount hood challenge. Value Health. 2018;21(6):724-31. doi:10.1016/j.jval.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenstock J, Marquard J, Laffel LM, et al. Empagliflozin as adjunctive to insulin therapy in type 1 diabetes: The EASE trials. Diabetes Care. 2018;41(12):2560-69. doi:10.2337/dc18-1749 [DOI] [PubMed] [Google Scholar]
- 28.Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: Updated data from the T1D exchange clinic registry. Diabetes Care. 2015;38(6):971-8. doi:10.2337/dc15-0078 [DOI] [PubMed] [Google Scholar]
- 29.Committee ADAPP. 6. Glycemic targets: Standards of medical care in diabetes–2022. Diabetes Care. 2022;45(Supplement_1):S83-96. doi:10.2337/dc22-S006 [DOI] [PubMed] [Google Scholar]
- 30.MediSpan PriceRx database. Accessed May 2021. https://pricerx.medispan.com/Refresh/Login.aspx
- 31.U.S. Bureau of Labor Statistics. Monthly labor review 2019. Accessed January 2020. https://www.bls.gov/opub/mlr/2019/
- 32.Peasgood T, Brennan A, Mansell P, Elliott J, Basarir H, Kruger J. The impact of diabetes-related complications on preference-based measures of health-related quality of life in adults with type I diabetes. Med Decis Making. 2016;36(8):1020-33. doi:10.1177/0272989X16658660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beaudet A, Palmer JL, Timlin L, et al. Cost-utility of exenatide once weekly compared with insulin glargine in patients with type 2 diabetes in the UK. J Med Econ. 2011;14(3):357-66. doi:10.3111/13696998.2011.579213 [DOI] [PubMed] [Google Scholar]
- 34.Lauridsen JT, Lønborg J, Gundgaard J, Jensen HH. Diminishing marginal disutility of hypoglycaemic events: Results from a time trade-off survey in five countries. Qual Life Res. 2014;23(9):2645-50. doi:10.1007/s11136-014-0712-x [DOI] [PubMed] [Google Scholar]
- 35.Institute for Health Metrics and Evaluation. GBD database 2019. Accessed May 2020. https://vizhub.healthdata.org/gbd-results/
- 36.Jendle J, Ericsson Å, Gundgaard J, Møller JB, Valentine WJ, Hunt B. Smart insulin pens are associated with improved clinical outcomes at lower cost versus standard-of-care treatment of type 1 diabetes in Sweden: A cost-effectiveness analysis . Diabetes Ther. 2021;12(1):373-88. doi:10.1007/s13300-020-00980-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Committee ADAPP. 7. Diabetes technology: Standards of medical care in diabetes–2022. Diabetes Care. 2021;45(Supplement_1):S97-112. doi:10.2337/dc22-S007 [DOI] [PubMed] [Google Scholar]
- 38.St Charles M, Lynch P, Graham C, Minshall ME. A. cost-effectiveness analysis of continuous subcutaneous insulin injection versus multiple daily injections in type 1 diabetes patients: A third-party US payer perspective. Value Health. 2009;12(5):674-86. doi:10.1111/j.1524-4733.2008.00478.x [DOI] [PubMed] [Google Scholar]
- 39.St Charles ME, Sadri H, Minshall ME, Tunis SL. Health economic comparison between continuous subcutaneous insulin infusion and multiple daily injections of insulin for the treatment of adult type 1 diabetes in Canada. Clin Ther. 2009;31(3):657-67. doi:10.1016/j.clinthera.2009.03.013 [DOI] [PubMed] [Google Scholar]
- 40.Doubova SV, Roze S, Ferreira-Hermosillo A, et al. Cost-effectiveness of the use of the continuous subcutaneous insulin infusion pump versus daily multiple injections in type 1 diabetes adult patients at the Mexican Institute of Social Security. Cost Eff Resour Alloc. 2019;17:19. doi:10.1186/s12962-019-0187-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saunders A, Messer LH, Forlenza GP. MiniMed 670G hybrid closed loop artificial pancreas system for the treatment of type 1 diabetes mellitus: Overview of its safety and efficacy. Expert Rev Med Devices. 2019;16(10):845-53. doi:10.1080/17434440.2019.1670639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petrovski G, Al Khalaf F, Campbell J, et al. One-year experience of hybrid closed-loop system in children and adolescents with type 1 diabetes previously treated with multiple daily injections: Drivers to successful outcomes. Acta Diabetol. 2021;58(2):207-13. doi:10.1007/s00592-020-01607-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381(18):1707-17. doi:10.1056/NEJMoa1907863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cummins E, Royle P, Snaith A, et al. Clinical effectiveness and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes: Systematic review and economic evaluation. Health Technol Assess. 2010;14(11):iii-iv, xi-xvi,, 1-181. doi:10.3310/hta14110 [DOI] [PubMed] [Google Scholar]
- 45.Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593-603. doi:10.2337/dci19-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Criego AB, Carlson AL, Forlenza GP, et al. 759-P: Glycemic outcomes over 15 months with the Omnipod 5 automated insulin delivery system. Diabetes. 2022;71(Supplement_1):759-P. doi:10.2337/db22-759-P [Google Scholar]


