Abstract
Background and Objective
The molecular mechanisms that underpin platelet granule secretion remain poorly defined. Filamin A (FLNA) is an actin-crosslinking and signaling scaffold protein whose role in granule exocytosis has not been explored despite evidence that FLNA gene mutations confer platelet defects in humans.
Methods and Results
Using platelets from platelet-specific conditional Flna-knockout mice, we showed that the loss of FLNA confers a severe defect in alpha (α)- and dense (δ)-granule exocytosis, as measured based on the release of platelet factor 4 (aka CXCL4) and adenosine triphosphate (ATP), respectively. This defect was observed following activation of both immunoreceptor tyrosine-based activation motif (ITAM) signaling by collagen-related peptide (CRP) and G protein–coupled receptor (GPCR) signaling by thrombin and the thromboxane mimetic U46619. CRP–induced spikes in intracellular calcium [Ca2+]i were impaired in FLNA-null platelets relative to controls, confirming that FLNA regulates ITAM-driven proximal signaling. In contrast, GPCR-mediated spikes in [Ca2+]i in response to thrombin and U46619 were unaffected by FLNA. Normal platelet secretion requires complexing of the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins synaptosomal-associated protein 23 (SNAP23) and syntaxin-11 (STX11). We determined that FLNA coimmunoprecipitates with both SNAP23 and STX11 upon platelet stimulation.
Conclusion
FLNA regulates GPCR-driven platelet granule secretion and associates with SNAP23 and STX11 in an activation-dependent manner.
Keywords: blood platelets, cytoskeleton, exocytosis, filamins, G protein–coupled receptors, SNARE proteins
Essentials
-
•
Platelets are cells that secrete important molecules for blood clotting.
-
•
Filamin A (FLNA) is a protein that maintains the normal structure of platelets.
-
•
Mutant platelets with missing FLNA have defects in secretion.
-
•
FLNA associates with other proteins (called SNAREs) to control normal platelet function.
1. Introduction
Platelets are circulating blood cells that regulate hemostasis [1], thrombosis [1], inflammation [2], wound healing [3], and angiogenesis [4]. In their resting state, platelets circulate as minuscule disks that store a multitude of pro-hemostatic, pro-angiogenic, and pro-inflammatory molecules within their granules [4]. Upon activation, platelets exhibit physical expansion mediated by the actin cytoskeleton [5]. In activated platelets, there is a rapid rise in intracellular calcium ([Ca2+]i), which culminates in the release of bioactive molecules from 3 types of intracellular stores: dense (δ) granules, alpha (α) granules, and lysosomes [6]. A critical step in granule exocytosis is the tethering of the granule membrane with the platelet plasma membrane (PM) [7].
In activated platelets, the granule-PM union is mediated by a group of proteins termed soluble N-ethylmaleimide sensitive factor attachment protein receptors (SNAREs) [8]. SNAREs are present both on the granule surface (v-SNAREs) and the PM surface (t-SNAREs). The merger of v- and t-SNAREs forms a transbilayer that represents the fusion of the granule membrane and the PM, thus allowing secretion of granule contents [7]. Loss-of-function studies have identified multiple proteins that participate in granule-to-PM fusion in platelets, including synaptosomal-associated protein 23 (SNAP23) [9], syntaxin-11 (STX11) [10], RAB27B [11], munc18b (aka STXBP2) [12], and munc13-4 (aka UNC13D) [13]. Although these studies clearly support the notion that SNAREs play an integral role in platelet granule exocytosis, there is less clear-cut knowledge of the upstream mechanisms that regulate SNARE activity.
SNAREs are closely associated with the actin cytoskeleton [14]. The actin cytoskeleton forms the structural framework of platelets, is responsible for shape change during activation, and is therefore critical for regulation of platelet granule secretion. Woronowicz et al. [15] showed that disruption of the actin cytoskeleton with latrunculin A inhibited α-granule release. The study further demonstrated that the actin cytoskeleton associates with several SNAREs, including VAMP8, STX2, STX4, and SNAP23 [15]. In addition to interacting with SNAREs, the actin cytoskeleton binds and is regulated by numerous accessory proteins expressed in platelets, for example, gelsolin [16], cortactin [17], arp2/3 (aka ARPC3) [18], wave (aka WASF1) [19], and filamin A (FLNA) [20]. Given the close association between the actin cytoskeleton and SNAREs, it is of interest to probe the role of individual actin-binding proteins in the context of platelet granule secretion.
FLNA is a ubiquitously expressed 280-kDa dimeric protein that crosslinks actin filaments [21,22] and anchors the cytoskeleton to cell surface receptors [23]. By linking the cytoskeleton to the PM and by virtue of its numerous (>50) ligands, FLNA serves as a critical signaling scaffold protein [[24], [25], [26]]. Specifically, FLNA binds multiple proteins with documented roles in platelet function, including gpIb-IX-V (aka GP1A) [27], heterodimeric integrins αIIb and β3 (ITGA2B and ITGB3, respectively) [28], SYK [29], PACSIN2 [30], and STIM1 [31].
Considerable evidence from human studies indicates that mutations in the FLNA gene result in aberrant platelet function. For example, Berrou et al. [32] reported that patients with FLNA mutations exhibited an aberrant platelet response to collagen, as determined from studies of aggregation, secretion, and thrombus formation; they noted that different mutations could confer either a loss- or gain-of-function phenotype. In another human study, a 100-amino acid extension to the C terminus resulted in a hyperresponsive platelet phenotype [33]. These reports suggest that the integrity of the FLNA gene is critical for normal platelet function, although the precise mechanisms underpinning FLNA-related platelet signaling are unclear.
In the present study, we employed a platelet-specific conditional knockout mouse model to dissect the role of FLNA in platelet granule secretion. Data from a previous study that employed a comparable mouse model indicated that surface P-selectin expression, a marker for α-granule secretion, was defective in platelets lacking FLNA [29]. Here we report that both α- and δ-granule secretion are severely impaired in FLNA-null platelets, both in the immunoreceptor tyrosine-based activation motif (ITAM)-dependent glycoprotein VI (GPVI [aka GP6]-driven) and G protein–coupled receptor (GPCR)-dependent (thrombin [aka F2]-driven) signaling pathways. However, we note that GPCR-driven proximal signaling, based on increases in [Ca2+]i, is independent of FLNA. We also provide the first evidence of an association between FLNA and SNAREs in platelets, secondary to GPCR ligation.
2. Materials and Methods
2.1. Reagents
Antibodies against SNAP23 (cat. #111202), STX11 (cat. #110113), munc18-2 (cat. #116102), and RAB27B (cat. #168103) were purchased from Synaptic Systems. The antibody against munc13-4 (cat. #sc-271300) was obtained from Santa Cruz Biotechnology. Antibodies against VAMP8 (cat. #13060S) and glyceraldehyde 3-phosphate dehydrogenase (clone D16H11, cat. #5174S) were purchased from Cell Signaling Technologies. The anti-FLNA antibody, bovine serum albumin, apyrase, indomethacin, and heparin were obtained from Millipore Sigma. The RGDS peptide was obtained from Bio-Techne Canada. Thrombin and adenosine diphosphate (ADP) agonists were purchased from Chrono-Log. Thrombin receptor-activating peptide (TRAP-6) was obtained from Bachem. The thromboxane mimetic U46619 was purchased from Tocris Bioscience. True Blot horseradish peroxidase–conjugated anti-rabbit and anti-mouse secondary antibodies were obtained from Rockland Immunochemicals. A GPVI (aka GP6)-specific collagen-related peptide (CRP) was obtained from the Versiti Blood Research Institute Protein Chemistry Core Laboratory [34].
2.2. Mice
Conditional knockout mice were generated in which FLNA expression was deleted in the megakaryocyte/platelet cell lineage [35]. All parental strains were obtained from The Jackson Laboratories: STOCK Flnatm1.1Caw/J (Strain #010907), with the allele, simply called Flnafl hereafter (note that Flna is present on the X chromosome); and C57BL/6-Tg(Pf4-icre)Q3Rsko/J (strain #008535), with the transgene, simply called Pf4-cre hereafter. Both parental strains were backcrossed onto 129S1/SvImJ (strain #002448) at least 6 times for genetic background standardization. Then, female Flnafl/fl mice that also carried Pf4-cre/+ were bred with male Flnafl/Y mice. All experiments used FLNA-null platelets from Flnafl/fl, Pf4-cre/+ females and Flnafl/Y, Pf4-cre/+ males; littermate Flnafl/fl females and Flnafl/Y males were employed as controls.
2.3. Mouse platelet preparation
Mouse blood was drawn by retro-orbital plexus bleeding using heparinized capillary tubes and collected into acid citrate dextrose buffer. Whole blood was diluted with Tyrode's buffer (0.32 mM monosodium phosphate, 150 mM NaCl, 2.65 mM KCl, 10.5 mM HEPES, 12 mM NaHCO3, 2.1 mM MgCl2, and 5 mM glucose) and centrifuged at 200g for 7 minutes. Platelet-rich plasma was harvested and centrifuged at 800g for 10 minutes to isolate a platelet pellet, which was then gently resuspended in Tyrode's buffer. The platelet suspension was adjusted to a concentration of 2 × 108 platelets/mL and allowed to rest at room temperature for 20 minutes. Washed platelets were supplemented with 2 mM CaCl2 prior to use.
2.4. Human platelet preparation
Whole blood was drawn by venipuncture from healthy adult volunteers after informed consent and approval from the Clinical Research Ethics Board at the University of British Columbia in accordance with the Declaration of Helsinki. Whole blood was collected in acid citrate dextrose and centrifuged at 200g for 10 minutes, and platelet-rich plasma was carefully separated and spun at 800g for 10 minutes in the presence of 1 μM prostaglandin E1 (Millipore Sigma). The platelet pellet was washed by resuspension in Tyrode's buffer containing 0.35% (weight/volume) bovine serum albumin, 10 U heparin, and 1 μM prostaglandin E1. The platelets were allowed to rest at 37°C for 10 minutes prior to centrifugation at 800g for 10 minutes and resuspended in Tyrode's buffer containing 2 mM CaCl2. The platelets were allowed to rest for 45 minutes at 37°C prior to use.
2.5. Adenosine triphosphate (ATP) secretion assay
ATP secretion was quantified as a measure of platelet dense granule secretion, as described previously [36,37]. Briefly, 2 × 108 platelets/mL were activated with specific agonists for 6 minutes at 37°C; ATP release was measured based on luminescence using a luciferin/D-luciferase reagent (Chrono-Log).
2.6. Platelet factor 4 (PF4; aka CXCL4) enzyme-linked immunosorbent assay
To quantify PF4 (aka CXCL4) release as a measure of α-granule secretion, platelets (2 × 108/mL) were stimulated with specific agonists for 10 minutes at 37°C. The reactions were stopped by centrifugation at 12,000g. The supernatants (releasate fractions) were snap frozen and stored at −80°C until required. For determination of the total PF4 content, lysates of the pellets (cell fractions) were prepared by addition of 1% (volume/volume) Triton X-100 in Tyrode's buffer prior to snap freezing. The amount of PF4 in the supernatants and pellets was measured using Quantikine enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems) according to the manufacturer’s instructions.
2.7. Immunoprecipitation and Western blotting
To study protein-protein interactions by immunoprecipitation, resting and agonist-stimulated human platelets were solubilized in a lysis buffer (50 mM Tris, pH=7.4, 250mM NaCl, 5 mM EDTA, 50 mM NaF, 1 mM sodium orthovanadate, 1% NP-40, 0.02% sodium azide, and 1 mM PMSF, supplemented with protease and phosphatase inhibitors). Lysates were incubated overnight at 4°C with either an anti-FLNA or anti-SNAP23 antibody conjugated to either Protein A or G Dynabeads (Thermo Fisher Scientific). The beads were washed 3 times with lysis buffer prior to elution of bead-associated proteins into the sample buffer. For Western blotting, proteins were resolved using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane. The membranes were incubated with various primary antibodies, followed by incubation with horseradish peroxidase-conjugated secondary antibodies. For immunoprecipitation experiments, True Blot secondary antibodies were used. Immunoreactive bands were visualized using a chemiluminescence reagent (Cell Signaling Technologies) and a Bio-Rad ChemiDoc imaging system.
2.8. [Ca2+]i
Measurements of [Ca2+]i were performed as previously described [37]. Briefly, washed resting platelets (1 × 107/mL) were loaded with 2.5 μM of the calcium indicator dye Fluo-4/AM (Life Technologies) for 3 minutes in calcium-free Tyrode's buffer at 37°C. The platelets were then stimulated with specific agonists in the presence of 1 mM CaCl2 [37]. Changes in real-time fluorescence intensity were measured using the Becton-Dickinson Accuri C6 flow cytometer.
2.9. Transmission electron microscopy
Washed platelets from the control and platelet-specific conditional Flna-knockout mice were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH=7.4) for 30 minutes and then washed in 0.1 M phosphate buffer 3 times for 10 minutes. Next, the samples were incubated with 1% osmium tetroxide in 0.1 M phosphate buffer for 1 hour and washed in distilled water 3 times. The samples were stained en bloc with 2.5% aqueous uranyl acetate for 30 minutes and then passed through a series of increasing concentrations of ethanol (30%, 50%, 75%, 90%, and 100%) 3 times. Next, the samples were submerged in a series of ethanol-Epon resin mixtures at different concentrations (25%, 50%, 75%, and 100%) 3 times. The sample was embedded in a BEEM capsule and polymerized in a 60°C oven. Then, 70-nm–thin sections were cut using the Leica Ultracut 7 ultramicrotome and mounted on formvar-coated 200-mesh copper grids. The grids were stained for 10 minutes in 2% uranyl acetate and 5 minutes in Reynold lead citrate. The samples were viewed using the Tecnai Spirit transmission electron microscope, which operated at 120 kV. Images were acquired using a DVC1500M camera (AMT Imaging).
2.10. Statistical analysis
For comparisons involving 2 groups, the Student’s t-test was used to determine the effect of genotype (control vs Flna knockout) on platelet secretion. For comparisons involving >2 groups, an analysis of variance (ANOVA) and Bonferroni multiple post-hoc comparison tests were used to determine statistical significance, which was set at P <.05.
3. Results
3.1. FLNA regulates platelet secretion
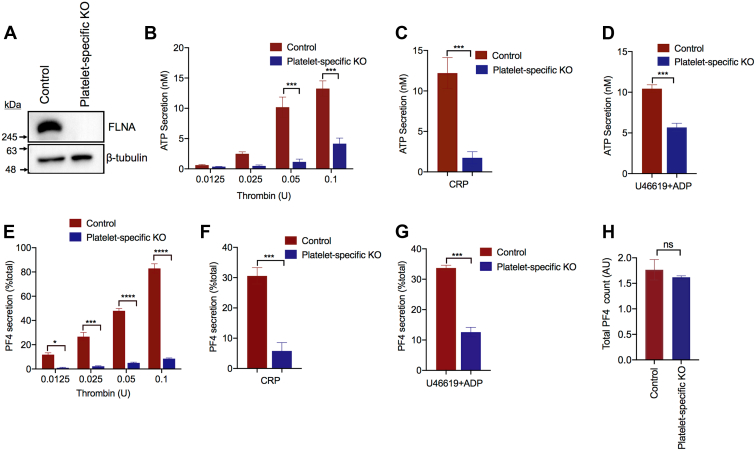
To gain a better understanding of how FLNA regulates platelet secretion, we used mice with a platelet-specific deletion of the Flna gene. The knockout of FLNA in the platelets was confirmed using Western blotting (Figure 1A). The platelet-specific nature of the Flna deletion was verified by blotting of kidney tissue lysates (Supplementary Fig. 3B). Consistent with previous reports, the conditional Flna knockout mice exhibited macrothrombocytopenia [29]. FLNA-null platelets exhibited a 2- to 10-fold reduction (P < .001) in δ-granule secretion, as measured based on ATP release (Figure 1B–D). This defect was evident upon stimulation by thrombin (Figure 1B), CRP (Figure 1C), and the thromboxane mimetic U46619 combined with ADP (Figure 1D). Similarly, α-granule secretion, as measured based on PF4 release, was significantly (P < .05) attenuated by the loss of FLNA expression (Figure 1E–G). However, no difference in ATP release was observed between control and FLNA-null platelets treated with the phorbol ester PMA (Supplementary Fig. 4). ATP secretion induced by CRP was also unaffected by the presence of the ADP scavenger apyrase (Supplementary Fig. 5). These data indicate that FLNA is a critical determinant of both α- and δ-granule release, downstream of multiple receptor-dependent signaling pathways. Importantly, there was no significant difference (P > .05) in the total PF4 content between the control and FLNA-null platelets (Figure 1H).
Figure 1.
Filamin A (FLNA) is required for platelet δ- and α-granule secretion. (A) Equal amounts of platelet lysate from control and platelet-specific conditional Flna-knockout (KO) mice were resolved by SDS-PAGE and immunoblotted to confirm the knockout of FLNA in the platelets. Beta-tubulin is shown as a loading control. (B–D) Platelets were isolated from whole blood of control mice (red bars) and platelet-specific Flna-KO mice (platelet-specific KO, blue bars). Washed platelets (2 × 108/mL) were stimulated at 37°C for 6 minutes with (B) thrombin, (C) collagen-related peptide (1 μg/mL), or (D) the thromboxane mimetic U46619 (1 μM) plus 10 μM ADP. Bar graphs represent the resultant ATP secretion, as measured based on luminescence using the Chronolume luciferase reagent. Data are presented as mean ± SEM (∗∗∗P < .001, based either on the Student’s t-test or analysis of variance and Bonferroni multiple comparison tests, as appropriate) and represent 4 independent experiments. (E–G) Bar graphs depict the release of platelet factor 4 (aka CXCL4) from control (red bars) platelets and FLNA-null (platelet-specific KO, blue bars) mice in response to (E) thrombin, (F) collagen-related peptide (1 μg/mL), or (G) U46619 (1 μM) plus 10 μM adenosine diphosphate. Data are presented as mean ± SEM (∗P < .05, ∗∗∗P < .001, ∗∗∗∗P < .0001, based either on the Student’s t-test or analysis of variance and Bonferroni multiple comparison tests, as appropriate) and represent 4 independent experiments. (H) The bar graph depicts the total platelet factor 4 content in control (red bars) platelets and FLNA-null (platelet-specific KO, blue bars) platelets based on enzyme-linked immunosorbent assay of platelet lysates. Data are presented as mean ± SEM (not significant, based on the Student’s t-test). ADP, adenosine diphosphate; CRP, collagen-related peptide; ns, not significant.
3.1.1. The relative number of α- and δ-granules is comparable between control and FLNA-null platelets
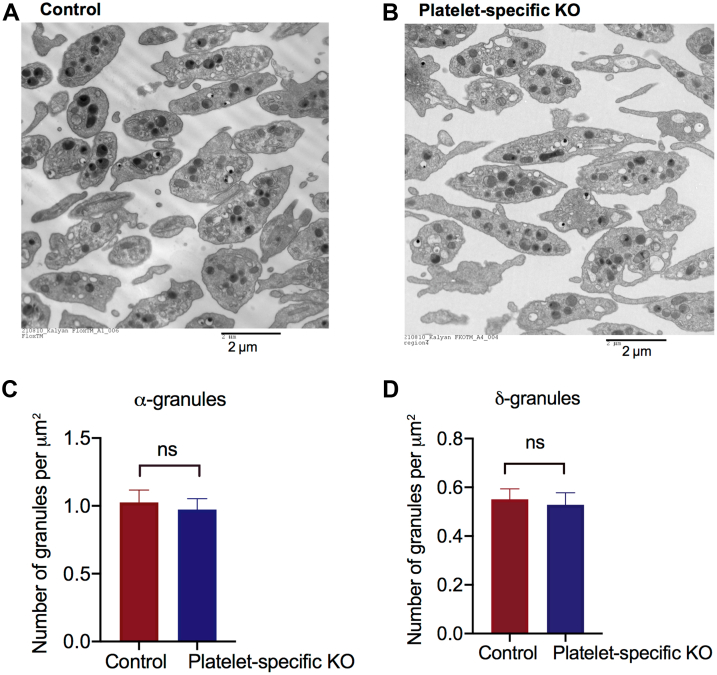
Because Flna platelet-specific knockout mice display macrothrombocytopenia [29], we examined the relative abundance of granules in the control and FLNA-null platelets at an ultrastructural level (Figure 2A, B). Using transmission electron microscopy, we counted the number of α- and δ-granules in the control and FLNA-null platelets and normalized these counts to the cell surface area to account for the increased size of FLNA-null platelets. The relative numbers of α- and δ-granules in the FLNA-null platelets were similar to those in the controls (P > .05) (Figure 2C, D), indicating that the secretion defect observed in the FLNA-null platelets was not attributable to reduced granule biogenesis by the FLNA-null megakaryocytes.
Figure 2.
The relative numbers of δ- and α-granules are comparable between control and filamin A (FLNA)-null platelets. (A, B) Representative transmission electron microscope micrographs of (A) control and (B) FLNA-null platelets. Bar = 2 μm. (C) The bar graph depicts the mean number of α-granules per squared micrometer of cell area in control (red bars) and FLNA-null (platelet-specific knockout, blue bars) platelets. Data are presented as mean ± SEM (not significant, based on the Student’s t-test) and represent the analysis of a minimum of 50 platelets. (D) The bar graph depicts the mean number of δ-granules per squared micrometer of cell area in control (red bars) and FLNA-null (platelet-specific knockout, blue bars) platelets. Data are presented as mean ± SEM (ns, not significant, based on the Student’s t-test) and represent the analysis of a minimum of 50 platelets. KO, knockout; ns, not significant.
3.2. ITAM-driven proximal signaling, but not GPCR-driven proximal signaling, is regulated by FLNA
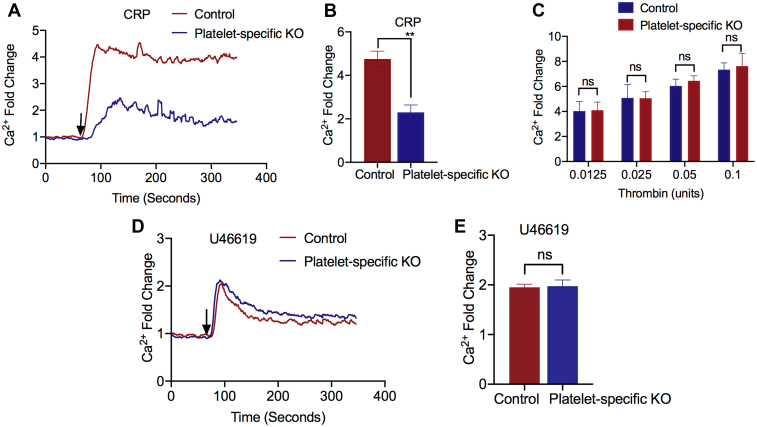
To determine the mechanism underpinning the secretion defect in the FLNA-null platelets, we evaluated the role of FLNA in the initial, post-activation platelet response, ie, proximal signaling. The normal increases in [Ca2+]i upon ligation of the GPVI (aka GP6) receptor with CRP were abrogated in the FLNA-null platelets (Figure 3A, B). In contrast, activation of GPCR-driven pathways did not reveal FLNA-dependent differences in [Ca2+]i. Similar increases in [Ca2+]i were observed following stimulation by thrombin (Figure 3C) or the thromboxane mimetic U46619 (Figure 3D, E). Taken together, these data indicate that GPCR-driven proximal signaling is independent of FLNA expression, consistent with previous reports that FLNA regulates ITAM-driven platelet signaling [29]. We, therefore, inferred that FLNA regulates granule secretion via downstream protein-protein interactions with the secretory protein machinery.
Figure 3.
The activation of G protein–coupled receptor-mediated proximal signaling in platelets is independent of filamin A (FLNA). (A) Washed platelets from control (red line) and Flna knockout (platelet-specific KO, blue line) mice were loaded with the calcium indicator dye Fluo-4/AM prior to activation with collagen-related peptide (CRP) in the presence of 1 mM CaCl2. Representative tracing illustrating the changes in [Ca2+]i is shown. (B) The bar graph depicts the maximal CRP–induced increase in [Ca2+]i relative to that at baseline in control (red bar) and FLNA-null (platelet-specific KO, blue bar) platelets. Data are presented as mean ± SEM (∗∗P < .01, based on the Student’s t-test) and represent a minimum of 4 independent experiments. (C) The bar graph depicts the maximal thrombin (aka F2)-induced increase in [Ca2+]i relative to that at baseline in control (red bar) and FLNA-null (platelet-specific KO, blue bar) platelets at the indicated doses and in the presence of 1 mM CaCl2. Data are presented as mean ± SEM (not significant, based on analysis of variance and Bonferroni multiple comparison tests) and represent a minimum of 4 independent experiments. (D) Representative tracings of U46619-induced [Ca2+]i flux in control (red line) and FLNA-null (platelet-specific KO, blue line) platelets. (E) The bar graph depicts the maximal U46619-induced increase in [Ca2+]i relative to that at baseline in control (red bar) and FLNA-null (platelet-specific KO, blue bar) platelets. Data are presented as mean ± SEM (not significant, based on the Student’s t-test) and represent a minimum of 4 independent experiments. CRP, collagen-related peptide; ns, not significant.
3.3. FLNA associates with SNAP23 and STX11 in TRAP-activated platelets
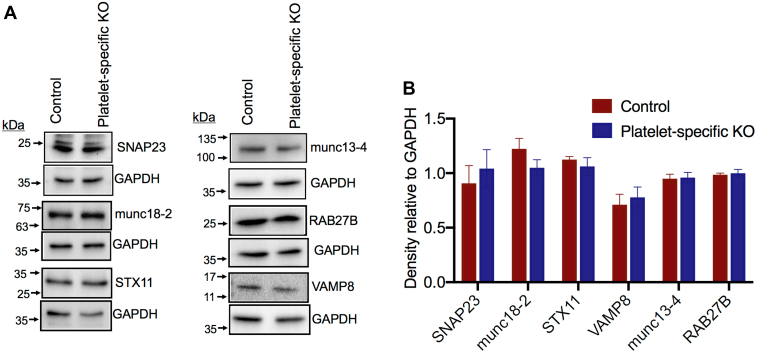
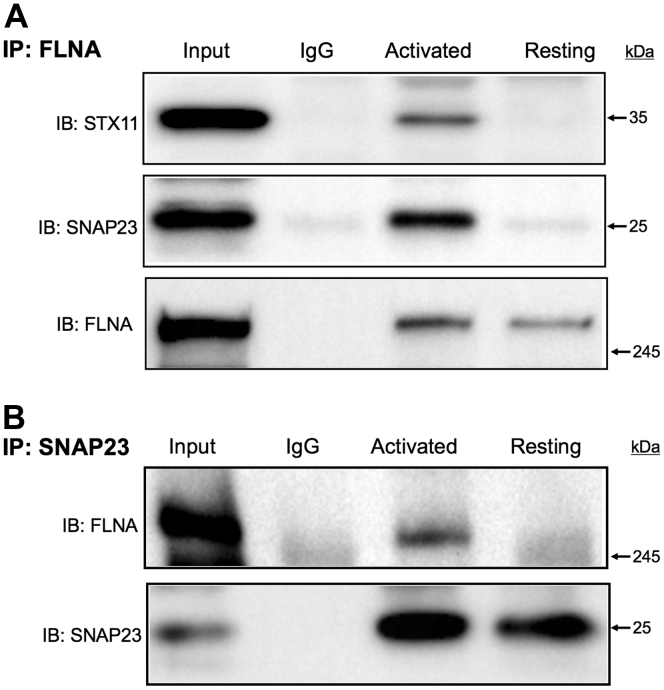
FLNA anchors numerous proteins to the cytoskeleton and, thus provides a scaffold for a large number of ligands [38]. Importantly, the platelet cytoskeleton interacts with SNAREs that directly mediate granule exocytosis [15]. To test the hypothesis that FLNA controls granule secretion by regulating SNARE function, we first compared, between the control and FLNA-null platelets, the overall expression of 6 proteins with defined roles in granule secretion: SNAP23, munc-18-2, STX11, VAMP8, munc-13-4, and RAB27B. The expression of these 6 proteins was similar in the control and FLNA-null platelets (Figure 4A, B). We then set out to determine how FLNA interacts with functionally relevant SNAREs. Published data indicate that normal granule secretion is contingent on the association between SNAP23 and STX11 [10]. Notably, co-immunoprecipitation experiments revealed that both SNAP23 and STX11 associate with FLNA upon thrombin receptor activating peptide (TRAP) stimulation (Figure 5A). We further confirmed the association between FLNA and SNAP23 using reciprocal co-immunoprecipitation (Figure 5B). This association was also observed in response to CRP and thrombin (Supplementary Fig. 1A, B). In addition, the association of SNAP23 and STX11 with FLNA was somewhat diminished in the presence of inhibitors (apyrase, indomethacin, and RGDS peptide) (Supplementary Fig. 2), suggesting that the FLNA-SNARE association is amplified by secondary signaling following receptor ligation. These data are consistent with the existence of a tripartite complex consisting of FLNA, SNAP23, and STX11, in activated platelets. This observation was further supported by a reduced association between SNAP23 and STX11 in the FLNA-null platelets (Supplementary Fig. 3A). Collectively, our findings are consistent with a model in which FLNA serves as a scaffold for a normal association between platelet granules and t-SNAREs (SNAP23 and STX11), thus allowing for normal platelet granule release (Figure 6).
Figure 4.
Relative expression of secretory proteins in control and filamin A (FLNA)-null platelets. (A) Equal amounts of lysates from resting control and FLNA-null platelets were resolved using SDS-PAGE. Western blots (representative of 3 independent experiments) were probed with antibodies against specific platelet secretory proteins, as indicated. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is shown as a loading control. (B) The bar graph depicts the densitometric analysis of the immunoblots in (A), representing protein expression in control (red bars) and FLNA-null (platelet-specific knockout, blue bars) platelets. Data are presented as mean ± SEM (not significant, based on the Student’s t-test). KO, knockout.
Figure 5.
Filamin A (FLNA) associates with synaptosomal-associated protein 23 (SNAP23) and syntaxin-11 (STX11) in human platelets upon ligation of the thrombin receptor. (A) Human platelets were activated with thrombin receptor (aka F2R)-activating peptide (10 μM) prior to solubilization with NP-40 lysis buffer. Co-immunoprecipitation (co-IP) was performed in resting and activated platelets by incubating lysates with an anti-FLNA antibody. Co-immunoprecipitates were resolved using SDS-PAGE and probed with antibodies against FLNA, STX11, and SNAP23. Co-IP data indicate an association between FLNA and SNAP23 upon thrombin receptor ligation. The negative control co-IP (IgG) is also shown. Blots are representative of 3 individual experiments, using blood from different donors. (B) Human platelets were activated with thrombin receptor-activating peptide (10 μM) prior to solubilization with NP-40 lysis buffer. Co-IP was performed in resting and activated platelets by incubating the lysates with an anti-SNAP23 antibody. Co-immunoprecipitates were resolved using SDS-PAGE and probed with antibodies against FLNA and SNAP23. The negative control co-IP (IgG) is also shown. Blots are representative of 3 individual experiments, using blood from different donors.
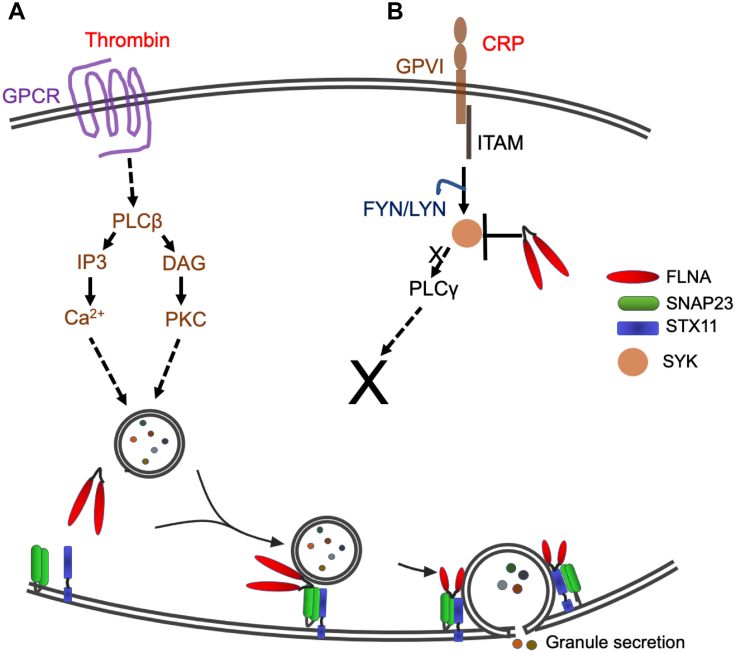
Figure 6.
Proposed model for regulation of platelet granule secretion by filamin A (FLNA). FLNA regulates platelet granule secretion in an agonist-dependent manner. (A) Ligation of the platelet G protein–coupled receptors (GPCR) by thrombin (aka F2) activates phospholipase C beta (aka PLCB1), which converts phosphatidylinositol 4,5-bisphosphate to inositol 1,4,5-trisphosphate and diacylglycerol (DAG). Inositol 1,4,5-trisphosphate promotes the release of intracellular Ca2+ ([Ca2+]i), and DAG activates protein kinase C epsilon (aka PRKCE). Both Ca2+ and DAG drive granule secretion. Notably, FLNA associates with SNAP23 and STX11 to participate in granule fusion with the plasma membrane thus mediating secretion. (B) Ligation of an immunoreceptor tyrosine-based activation motif-coupled (ITAM) receptor (GPVI, aka GP6) with collagen-related peptide (CRP) activates SYK via SRC family kinases (FYN and LYN). Loss of FLNA expression precludes the normal rise in [Ca2+]i, thus blocking platelet secretion at the second messenger level upstream.
4. Discussion
The actin cytoskeleton plays a central role in modulating platelet secretion [14,15] and is connected to the PM by FLNA [21,22]. However, the role of FLNA in directing platelet granule secretion is unclear. The first study to show a secretion defect in FLNA-deficient platelets used a conditional GATA1-Cre mouse model; the secretion of α-granules was determined based on the surface levels of P-selectin [29]. Here, we report that FLNA is essential for efficient secretion of both platelet α- and δ-granules. Moreover, our data provide an insight into the specific roles of FLNA in transducing ITAM- and GPCR-driven signaling in platelets. Furthermore, we provide the first evidence of an association between FLNA and the SNARE proteins SNAP23 and STX11.
4.1. FLNA regulates GPVI-driven platelet secretion by regulating proximal signaling
Our data indicate that FLNA is required for platelet secretion in response to stimulation by thrombin, a thromboxane mimetic, and CRP (GPVI). The regulation of multiple signaling pathways by FLNA is consistent with its well-documented role as a major signaling hub and ability to control the function of multiple proteins, including SYK, gpIb-IX-V, and β3 integrins [[27], [28], [29],39]. The agonist-induced increase in [Ca2+]i is a critical proximal signaling event following receptor ligation [40]. Ligation of the platelet GPVI receptor by CRP activates the SRC family kinases FYN, LYN [41], and SYK [42], leading to phospholipase C-γ (PLC-γ, aka PLCG2)-driven cleavage of phosphatidylinositol 4,5-bisphosphate, forming inositol 1,4,5-trisphosphate, which releases cytosolic Ca2+ from internal stores [43]. Our finding that FLNA-null platelets fail to exhibit a rise in [Ca2+]i in response to CRP stimulation is consistent with published data reporting a scaffolding role of FLNA for SYK in the GPVI and C-type lectin signaling pathways [29]. Collectively, these data indicate that the secretion defect observed in the FLNA-null platelets is at least partly attributable to disrupted proximal signaling in response to GPVI stimulation.
4.2. GPCR-driven proximal signaling is independent of FLNA
Ligation of the thrombin or thromboxane receptor (aka TBXA2R)—both GPCRs—activates the Gq (aka GNAQ) signaling pathway, which activates phospholipase C-β (aka PLCB1), generating inositol 1,4,5-trisphosphate and diacylglycerol (DAG), thus potentiating platelet activation [44]. In contrast to our observations in CRP-stimulated platelets, we found that FLNA expression did not affect [Ca2+]i in response to either thrombin or the thromboxane mimetic U46619. Therefore, it can be inferred that GPCR-driven proximal signaling is independent of FLNA in platelets [29]. By extension, these data indicate that FLNA modulates GPCR-driven granule secretion downstream of [Ca2+]i increases. Furthermore, FLNA-null platelets express normal levels of other components of the granule secretory machinery, including SNAP23 [9], STX11 [10], munc13-4 [13], and Rab27B [11]. The defect in GPCR-driven secretion is consistent with the notion that FLNA plays a role in mediating SNARE activity during granule-PM fusion.
4.3. FLNA associates with SNAREs upon platelet activation
Transmembrane SNAREs (t-SNAREs) such as STX11 and SNAP23 associate with each other in platelets and play a crucial role in platelet secretion [9,10]. Familial hemophagocytic lymphohistiocytosis-4 is associated with lack of STX11 expression in platelets and a severe defect in both α- and δ-granule secretion [10]. In addition, STX11 associates with several other platelet SNAREs, including SNAP23 [10]. SNAP23 is another well-established regulator of platelet granule release [9,45,46]. The present study is the first to demonstrate an association between FLNA and functionally important SNAREs.
The actin cytoskeleton plays a critical role in the regulation of platelet secretion [14]. Notably, the actin cytoskeleton associates with both FLNA [22] and SNAREs [15]. Previous studies have suggested that F-actin (aka ACTB and ACTG1) associates with SNAP23 [15] and platelet granules [14,47,48]. Conceivably, by virtue of its function as an actin-crosslinking protein, FLNA is ideally positioned to mediate interactions among platelet granules, F-actin, SNAP23, and STX11 in activated platelets. This would also be consistent with the well-established role of FLNA as a scaffolding protein that integrates signals between the cytoskeleton and PM [26,49].
Interestingly, a mouse with a platelet-specific conditional deletion of Snap23 was recently documented as exhibiting macrothrombocytopenia and a defective response to agonist stimulation [9], which essentially phenocopies the mouse described here with a platelet-specific conditional deletion of Flna [29]. Given the striking similarity in the mouse and platelet phenotypes, it is tempting to speculate that FLNA and SNAP23 share a common function with regard to optimal platelet production, signal transduction, and granule secretion.
In summary, we report that FLNA plays a critical role in platelet granule secretion and associates with SNAP23 and STX11, specifically upon GPCR ligation. Given the clear impact of FLNA mutations on human platelet function [32,33,49,50], a better understanding of this complex signaling system will shed light on potential therapeutic targets for novel anti-platelet drugs. Further studies can probe the direct nature of protein-protein interactions among FLNA, SNAP23, and STX11 and, ultimately, determine how pharmacologic disruption of these interactions might be harnessed to optimize platelet function.
Acknowledgments
The authors thank Miki Fujita at the UBC BioImaging Facility (RRID: SCR_021304) for technical assistance with transmission electron microscopy of platelets and Takehide Murakami at the UBC Biomedical Research Centre for assistance with polymerase chain reaction (PCR) genotyping. Financial support for this project was provided by a Canadian Institutes of Health Research Operating Grant (MOP-142450), a John Evans Leaders Fund infrastructure grant from the Canada Foundation for Innovation, and a Michael Smith Foundation for Health Research Scholar Award (to H.K.) as well as a National Institutes of Health, National Heart, Lung, and Blood Institute R01 grant HL126743 (to H.F.). M.P. acknowledges support from a postdoctoral fellowship from The Arthritis Society of Canada.
Funding
Financial support for this project was provided by a Canadian Institutes of Health Research Operating Grant (MOP-142450), a John Evans Leaders Fund infrastructure grant from the Canada Foundation for Innovation, and a Michael Smith Foundation for Health Research Scholar Award (to H.K.) as well as a National Institutes of Health, National Heart, Lung, and Blood Institute R01 grant HL126743 (to H.F.). M.P. acknowledges support from a postdoctoral fellowship from The Arthritis Society of Canada.
Author contributions
K.G. designed the research, performed experiments, analyzed the data, and drafted and critically edited the manuscript. M.P. performed experiments and critically edited the manuscript. T.C.L., E.M.S., and H.F. designed the research and critically edited the manuscript. H.K. acquired the funding, designed the research, and drafted and critically edited the manuscript.
Relationship Disclosure
There are no competing interests to disclose.
Informed patient consent
All participants signed written informed consent.
Footnotes
Funding informationCanadian Institutes of Health Research, Grant/Award Number: MOP-142450; John Evans Leaders Fund infrastructure grant, Canada Foundation for Innovation; H.F. reports grants from the National Institutes of Health, and the National Heart, Lung, and Blood Institute, Grant/Award Number: R01 HL126743; M.P. reports support from a postdoctoral fellowship from The Arthritis Society of Canada.
Handling Editor: Senis, Y
The online version contains supplementary material available at https://doi.org/10.1016/j.rpth.2022.100019
Supplementary material
References
- 1.Hou Y., Carrim N., Wang Y., Gallant R.C., Marshall A., Ni H. Platelets in hemostasis and thrombosis: novel mechanisms of fibrinogen-independent platelet aggregation and fibronectin-mediated protein wave of hemostasis. J Biomed Res. 2015;29:437–444. doi: 10.7555/JBR.29.20150121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zarbock A., Polanowska-Grabowska R.K., Ley K. Platelet-neutrophil-interactions: linking hemostasis and inflammation. Blood Rev. 2007;21:99–111. doi: 10.1016/j.blre.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Etulain J. Platelets in wound healing and regenerative medicine. Platelets. 2018;29:556–568. doi: 10.1080/09537104.2018.1430357. [DOI] [PubMed] [Google Scholar]
- 4.Golebiewska E.M., Poole A.W. Platelet secretion: from haemostasis to wound healing and beyond. Blood Rev. 2015;29:53–62. doi: 10.1016/j.blre.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandmann R., Koster S. Topographic cues reveal two distinct spreading mechanisms in blood platelets. Sci Rep. 2016;6:1–11. doi: 10.1038/srep22357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez E., Bermejo N., Berna-Erro A., Alonso N., Salido G.M., Redondo P.C., et al. Relationship between calcium mobilization and platelet alpha- and delta-granule secretion. A role for TRPC6 in thrombin-evoked delta-granule exocytosis. Arch Biochem Biophys. 2015;585:75–81. doi: 10.1016/j.abb.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Joshi S., Whiteheart S.W. The nuts and bolts of the platelet release reaction. Platelets. 2017;28:129–137. doi: 10.1080/09537104.2016.1240768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golebiewska E.M., Poole A.W. Secrets of platelet exocytosis-what do we really know about platelet secretion mechanisms? Br J Haematol. 2013;165:204–216. doi: 10.1111/bjh.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams C.M., Li Y., Brown E., Poole A.W. Platelet-specific deletion of SNAP23 ablates granule secretion, substantially inhibiting arterial and venous thrombosis in mice. Blood Adv. 2018;2:3627–3636. doi: 10.1182/bloodadvances.2018023291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye S., Karim Z.A., Al Hawas R., Pessin J.E., Filipovich A.H., Whiteheart S.W. Syntaxin-11, but not syntaxin-2 or syntaxin-4, is required for platelet secretion. Blood. 2012;120:2484–2492. doi: 10.1182/blood-2012-05-430603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tolmachova T., Abrink M., Futter C.E., Authi K.S., Seabra M.C. Rab27b regulates number and secretion of platelet dense granules. Proc Natl Acad Sci U S A. 2007;104:5872–5877. doi: 10.1073/pnas.0609879104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al Hawas R., Ren Q., Ye S., Karim Z.A., Filipovich A.H., Whiteheart S.W. Munc18b/STXBP2 is required for platelet secretion. Blood. 2012;120:2493–2500. doi: 10.1182/blood-2012-05-430629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren Q., Wimmer C., Chicka M.C., Ye S., Ren Y., Hughson F.M., Whiteheart S.W. Munc13-4 is a limiting factor in the pathway required for platelet granule release and hemostasis. Blood. 2010;116:869–877. doi: 10.1182/blood-2010-02-270934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flaumenhaft R., Dilks J.R., Rozenvayn N., Monahan-Earley R.A., Feng D., Dvorak A.M. The actin cytoskeleton differentially regulates platelet alpha-granule and dense-granule secretion. Blood. 2005;105:3879–3887. doi: 10.1182/blood-2004-04-1392. [DOI] [PubMed] [Google Scholar]
- 15.Woronowicz K., Dilks J.R., Rozenvayn N., Dowal L., Blair P.S., Peters C.G., et al. The platelet actin cytoskeleton associates with SNAREs and participates in alpha-granule secretion. Biochemistry. 2010;49:4533–4542. doi: 10.1021/bi100541t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barkalow K., Witke W., Kwiatkowski D.J., Hartwig J.H. Coordinated regulation of platelet actin filament barbed ends by gelsolin and capping protein. J Cell Biol. 1996;134:389–399. doi: 10.1083/jcb.134.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong N.H., Qi A., Weaver A.M. PI(3,5)P2 controls endosomal branched actin dynamics by regulating cortactin-actin interactions. J Cell Biol. 2015;210:753–769. doi: 10.1083/jcb.201412127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poulter N.S., Pollitt A.Y., Davies A., Malinova D., Nash G.B., Hannon M.J., et al. Platelet actin nodules are podosome-like structures dependent on Wiskott-Aldrich syndrome protein and ARP2/3 complex. Nat Commun. 2015;6:1–15. doi: 10.1038/ncomms8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oda A., Miki H., Wada I., Yamaguchi H., Yamazaki D., Suetsugu S., et al. WAVE/Scars in platelets. Blood. 2005;105:3141–3148. doi: 10.1182/blood-2003-04-1319. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura F., Osborn T.M., Hartemink C.A., Hartwig J.H., Stossel T.P. Structural basis of filamin A functions. J Cell Biol. 2007;179:1011–1025. doi: 10.1083/jcb.200707073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohta Y., Hartwig J.H., Stossel T.P. FilGAP, a Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodelling. Nat Cell Biol. 2006;8:803–814. doi: 10.1038/ncb1437. [DOI] [PubMed] [Google Scholar]
- 22.Popowicz G.M., Schleicher M., Noegel A.A., Holak T.A. Filamins: promiscuous organizers of the cytoskeleton. Trends Biochem Sci. 2006;31:411–419. doi: 10.1016/j.tibs.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Sharma C.P., Ezzell R.M., Arnaout M.A. Direct interaction of filamin (ABP-280) with the beta 2-integrin subunit CD18. J Immunol. 1995;154:3461–3470. [PubMed] [Google Scholar]
- 24.Stossel T.P., Condeelis J., Cooley L., Hartwig J.H., Noegel A., Schleicher M., et al. Filamins as integrators of cell mechanics and signalling. Nat Rev Mol Cell Biol. 2001;2:138–145. doi: 10.1038/35052082. [DOI] [PubMed] [Google Scholar]
- 25.Zhou A.X., Hartwig J.H., Akyurek L.M. Filamins in cell signaling, transcription and organ development. Trends Cell Biol. 2010;20:113–123. doi: 10.1016/j.tcb.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Zhou J., Kang X., An H., Lv Y., Liu X. The function and pathogenic mechanism of filamin A. Gene. 2021;784 doi: 10.1016/j.gene.2021.145575. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura F., Pudas R., Heikkinen O., Permi P., Kilpelainen I., Munday A.D., et al. The structure of the GPIb-filamin A complex. Blood. 2006;107:1925–1932. doi: 10.1182/blood-2005-10-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J., Das M., Yang J., Ithychanda S.S., Yakubenko V.P., Plow E.F., et al. Structural mechanism of integrin inactivation by filamin. Nat Struct Mol Biol. 2015;22:383–389. doi: 10.1038/nsmb.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Falet H., Pollitt A.Y., Begonja A.J., Weber S.E., Duerschmied D., Wagner D.D., et al. A novel interaction between FlnA and Syk regulates platelet ITAM-mediated receptor signaling and function. J Exp Med. 2010;207:1967–1979. doi: 10.1084/jem.20100222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Begonja A.J., Pluthero F.G., Suphamungmee W., Giannini S., Christensen H., Leung R., et al. FlnA binding to PACSIN2 F-BAR domain regulates membrane tubulation in megakaryocytes and platelets. Blood. 2015;126:80–88. doi: 10.1182/blood-2014-07-587600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez J.J., Albarran L., Jardin I., Sanchez-Collado J., Redondo P.C., Bermejo N., et al. Filamin A modulates store-operated Ca2+ entry by regulating STIM1 (stromal interaction molecule 1)-Orai1 association in human platelets. Arterioscler Thromb Vasc Biol. 2018;38:386–397. doi: 10.1161/ATVBAHA.117.310139. [DOI] [PubMed] [Google Scholar]
- 32.Berrou E., Adam F., Lebret M., Fergelot P., Kauskot A., Coupry I., et al. Heterogeneity of platelet functional alterations in patients with filamin A mutations. Arterioscler Thromb Vasc Biol. 2013;33:e11–e18. doi: 10.1161/ATVBAHA.112.300603. [DOI] [PubMed] [Google Scholar]
- 33.Berrou E., Adam F., Lebret M., Planche V., Fergelot P., Issertial O., et al. Gain-of-function mutation in filamin A potentiates platelet integrin αIIbβ3 activation. Arterioscler Thromb Vasc Biol. 2017;37:1087–1097. doi: 10.1161/ATVBAHA.117.309337. [DOI] [PubMed] [Google Scholar]
- 34.Eaton N., Subramaniam S., Schulte M.L., Drew C., Jakab D., Haberichter S.L., et al. Bleeding diathesis in mice lacking JAK2 in platelets. Blood Adv. 2021;5:2969–2981. doi: 10.1182/bloodadvances.2020003032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jurak Begonja A., Hoffmeister K.M., Hartwig J.H., Falet H. FlnA-null megakaryocytes prematurely release large and fragile platelets that circulate poorly. Blood. 2011;118:2285–2295. doi: 10.1182/blood-2011-04-348482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Golla K., Stavropoulos I., Shields D.C., Moran N. Peptides derived from cadherin juxtamembrane region inhibit platelet function. R Soc Open Sci. 2018;5 doi: 10.1098/rsos.172347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naik M.U., Patel P., Derstine R., Turaga R., Chen X., Golla K., et al. Ask1 regulates murine platelet granule secretion, thromboxane A2 generation, and thrombus formation. Blood. 2017;129:1197–1209. doi: 10.1182/blood-2016-07-729780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yue J., Huhn S., Shen Z. Complex roles of filamin-A mediated cytoskeleton network in cancer progression. Cell Biosci. 2013;3:1–12. doi: 10.1186/2045-3701-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cranmer S.L., Pikovski I., Mangin P., Thompson P.E., Domagala T., Frazzetto M., et al. Identification of a unique filamin A binding region within the cytoplasmic domain of glycoprotein Ibalpha. Biochem J. 2005;387:849–858. doi: 10.1042/BJ20041836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varga-Szabo D., Braun A., Nieswandt B. Calcium signaling in platelets. J Thromb Haemost. 2009;7:1057–1066. doi: 10.1111/j.1538-7836.2009.03455.x. [DOI] [PubMed] [Google Scholar]
- 41.Ezumi Y., Shindoh K., Tsuji M., Takayama H. Physical and functional association of the Src family kinases Fyn and Lyn with the collagen receptor glycoprotein VI-Fc receptor gamma chain complex on human platelets. J Exp Med. 1998;188:267–276. doi: 10.1084/jem.188.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poole A., Gibbins J.M., Turner M., Van Vugt M.J., Van de Winkel J.G., Saito T., et al. The Fc receptor γ-chain and the tyrosine kinase Syk are essential for activation of mouse platelets by collagen. EMBO J. 1997;16:2333–2341. doi: 10.1093/emboj/16.9.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pasquet J.M., Bobe R., Gross B., Gratacap M.P., Tomlinson M.G., Payrastre B., et al. A collagen-related peptide regulates phospholipase Cγ2 via phosphatidylinositol 3-kinase in human platelets. Biochem J. 1999;342:171–177. [PMC free article] [PubMed] [Google Scholar]
- 44.Offermanns S. Activation of platelet function through G protein-coupled receptors. Circ Res. 2006;99:1293–1304. doi: 10.1161/01.RES.0000251742.71301.16. [DOI] [PubMed] [Google Scholar]
- 45.Chen D., Lemons P.P., Schraw T., Whiteheart S.W. Molecular mechanisms of platelet exocytosis: role of SNAP-23 and syntaxin 2 and 4 in lysosome release. Blood. 2000;96:1782–1788. [PubMed] [Google Scholar]
- 46.Chen D., Bernstein A.M., Lemons P.P., Whiteheart S.W. Molecular mechanisms of platelet exocytosis: role of SNAP-23 and syntaxin 2 in dense core granule release. Blood. 2000;95:921–929. [PubMed] [Google Scholar]
- 47.Valentijn J.A., Valentijn K., Pastore L.M., Jamieson J.D. Actin coating of secretory granules during regulated exocytosis correlates with the release of rab3D. Proc Natl Acad Sci U S A. 2000;97:1091–1095. doi: 10.1073/pnas.97.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trifaro J.M., Gasman S., Gutierrez L.M. Cytoskeletal control of vesicle transport and exocytosis in chromaffin cells. Acta Physiol (Oxf) 2008;192:165–172. doi: 10.1111/j.1748-1716.2007.01808.x. [DOI] [PubMed] [Google Scholar]
- 49.Rosa J.P., Raslova H., Bryckaert M. Filamin A: key actor in platelet biology. Blood. 2019;134:1279–1288. doi: 10.1182/blood.2019000014. [DOI] [PubMed] [Google Scholar]
- 50.Saultier P., Vidal L., Canault M., Bernot D., Falaise C., Pouymayou C., et al. Macrothrombocytopenia and dense granule deficiency associated with FLI1 variants: ultrastructural and pathogenic features. Haematologica. 2017;102:1006–1016. doi: 10.3324/haematol.2016.153577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.