Abstract
In vertebrates, p53 participates in numerous biological processes including cell cycle regulation, apoptosis, differentiation, and oncogenic transformation. When insect SF-21 cells were infected with a recombinant of the baculovirus Autographa californica nuclear polyhedrosis virus (AcMNPV) overexpressing human p53, p53 formed a stable complex with the product of the AcMNPV orf92, a novel protein p33. The interaction between p53 and p33 was further confirmed by immunoprecipitation studies. When individually expressed in SF-21 cells, human p53 localized mainly in the nucleus whereas baculovirus p33 displayed diffuse cytoplasmic staining and punctuate nuclear staining. However, coexpression of p33 with p53 resulted in exclusive nuclear localization of p33. In both SF-21 and TN-368 cells, p53 expression induced typical features of apoptosis including nuclear condensation and fragmentation, oligonucleosomal ladder formation, cell surface blebbing, and apoptotic body formation. Coexpression of p53 with a baculovirus inhibitor of apoptosis, p35, OpIAP, or CpIAP, blocked apoptosis, whereas coexpression with p33 enhanced p53-mediated apoptosis approximately twofold. Expression of p53 in SF-21 cells stably expressing OpIAP inhibited cell growth in the presence or absence of p33. Thus, human p53 can influence both insect cell growth and death and baculovirus p33 can modulate the death-inducing effects of p53.
The p53 gene, a tumor suppressor gene which is mutated in more than 50% of human cancers, encodes a multifunctional protein which plays a pivotal role in the regulation of cell cycle progression and programmed cell death (reviewed in references 2, 17, 18, 25, 26, 30, 31, and 58). A number of factors affect the decision of cells to undergo cell cycle arrest or apoptosis. p53-mediated apoptosis prevails under conditions in which DNA is damaged, survival factors are limiting, or an activated oncogene forces the cell into a replicative cycle. Regulation by p53 may be exerted by direct protein signaling and/or by its ability to transcriptionally activate other genes.
p53, a sequence-specific transcriptional activator (reviewed in reference 53), has been shown to activate under physiological conditions a number of cellular genes which mediate its roles in cell cycle arrest and apoptosis. p21 is likely to be its main effector in inducing a G1 block (reviewed in reference 26), while a more recently identified target, 14-3-3ς, has been proposed to be responsible, at least in part, for its G2 arrest (22). The ability of p53 to induce apoptosis is more complex because in some cases the transactivation function of p53 has been shown to be required, while in others, a transactivation-independent function was demonstrated (reviewed in reference 26). p53 target genes which play roles in apoptosis include bax and IGF-BP3 (reviewed in references 17 and 26). Additionally, it was recently shown that a class of genes termed PIG genes, some of which function in reactive oxygen species metabolism, are specifically induced by p53 during the apoptotic process (43). In keeping with its function as a transcriptional activator, p53 has been shown to interact with several polypeptide components of the general transcription factors TFIID and TFIIH (17, 26) and also with the p300/CBP coactivators (3, 19, 32). There is evidence that p53 may interact with and regulate DNA repair and/or replication factors as well (26).
The transcription-independent role of p53 in apoptosis is not fully understood, but protein interactions with p53 may be involved. Perhaps the best-studied p53 interactor is the oncoprotein MDM2, which binds to the N terminus of p53 (9, 28, 39, 40, 42). MDM2 both inhibits the ability of p53 to activate transcription (39, 40, 51) and targets p53 for proteasome-mediated degradation (7, 21, 27). Some additional proteins which may functionally interact with p53 include the c-Abl nuclear tyrosine kinase (16), the Wilms’ tumor suppressor protein Wt1 (35), and the p33ING1 tumor suppressor (15).
Many DNA viruses encode p53 binding proteins that affect p53 function. The simian virus 40 large tumor antigen binds to the p53 central conserved region and represses its transactivation function (5, 13). The adenovirus 55-kDa protein E1B binds to the p53 activation domain in a region which overlaps the MDM2 interaction region (33) and causes p53 to repress transcription (60), while the adenovirus E4orf6 product interacts with p53 and abrogates TATA binding protein-associated factor binding (12). The interaction of the human papillomavirus E6 product with p53 leads to ubiquitin-mediated degradation of the p53 protein (24). It has also been reported that the hepatis B virus X protein (57) as well as Epstein-Barr virus-encoded EBNA-5 (49) and BZLF1 (61) can bind to p53. The fact that a number of viruses encode gene products that can interact with p53 suggests that alteration of p53 apoptotic function is a prerequisite for mounting a productive viral infection (reviewed in references 50 and 58).
Baculoviruses have two classes of genes which can block apoptosis induced during viral infection: p35-like genes and inhibitor of apoptosis (IAP) genes (reviewed in reference 11). p35 of Autographa californica nuclear polyhedrosis virus (AcMNPV) is a general, stoichiometric inhibitor of caspases, a family of cysteine proteases which are activated by proteolytic processing and are involved in the execution of cell death. AcMNPV infection of Spodoptera frugiperda SF-21 cells activates SF-caspase-1 (1, 37, 47). Whereas p35 blocks the activity of mature SF-caspase-1, some members of the IAP family (e.g., baculovirus OpIAP) block the processing and activation of this caspase (47). The mechanism by which baculoviruses activate caspases remains unclear, but it is known that baculovirus DNA replication and/or late viral gene expression are required for maximum levels of apoptosis (10, 29). Overexpression of ie-1, a baculovirus gene involved in both viral DNA replication and gene expression, is sufficient to induce apoptosis in SF-21 cells (44), but other factors are also likely to be involved.
In the present study, we have found that AcMNPV encodes a 33-kDa protein which interacts with the human p53 protein. This observation led us to investigate the activity of human p53 in insect cells and how p33 binding influences p53 activity. We provide evidence that p53 expression induces either cell growth arrest or apoptosis, depending on the presence of a member of the IAP family of apoptosis inhibitors in insect cells, and that baculovirus p33 enhances p53-mediated apoptosis in insect cells.
MATERIALS AND METHODS
Cells and media.
S. frugiperda IPLB-SF21 (SF-21) (52), hsOpIAPpacR (38), and Trichoplusia ni TN-368 (23) cells were maintained at 27°C in TC-100 growth medium (GIBCO BRL, Gaithersburg, Md.) supplemented with 10% fetal bovine serum and 0.26% tryptose broth (Difco Laboratories, Inc., Detroit, Mich.) as described previously (41).
Plasmids.
phsEHp53 was constructed by replacing the ced9 open reading frame (ORF) within the BglII-EcoRI fragment of phsEpihisced-9VI+ (48) with a BglII-SmaI fragment containing the human p53 ORF. The human p53 insert was prepared by PCR using pEV55Hp53 as a template, a 5′ primer in the sense orientation (5′-CGAGATCTGAGGAGCCGCAGTCAGATC), and a 3′ primer in the antisense orientation (5′-TCCCCCGGGTCAGTCTGAGTCAGGCC). The PCR product was digested with BglII and SmaI before ligation. phsORF92F was constructed by inserting a BglII-SmaI-digested PCR product, containing the baculovirus orf92 (4), in place of the cat ORF within the BglII-EcoRI fragment of pHSP70PLVI+CAT (10). The primers used for PCR were 5′-GCGAGATCTATGATACCGCTGACGC (corresponding to the 5′ orf92 end) and 5′-TCCCCCGGGTTATTTGTCATCGTCGTCCTTGTAGTCTTGCAAATTTAAC (corresponding to the 3′ end of orf92), extended with the sequence DYKDDDDK, constituting a Flag tag. pXE2213 was used as the template and was constructed by cloning the 2,213-bp XhoI-EcoRI fragment containing the AcMNPV orf92 (4) into pBluescript II KS+ (Stratagene, La Jolla, Calif.). Plasmid phsFlagHisVI+ was constructed to facilitate the N-terminal tagging of proteins with a Flag epitope and a nickel binding site (His6 tag). to construct phsFlagHisVI+, Op-iap, the gene encoding OpIAP, was removed from a plasmid expressing Flag-Op-iap (54) and replaced with overlapping oligonucleotides coding for a His6 tag and containing sites of restriction endonucleases BglII, Bsu36I, XmaI, and NotI. The sequence of plasmid phsFlagHisVI+ in the Flag-His6 tag region was 1ATGAGCTCCCGAGACTACAAGGACGACGATGACAAACTCGATCGAGATTCCCGGCATCATCATCATCATCACAGATCTCCTGAGGCCCGGGCGGCCGC98, where the translation initiation codon is from nucleotides (nt) 1 to 3, followed by a Flag tag (nt 13 to 36), a His6 tag (nt 54 to 72), and a polylinker (nt 73 to 98). To construct phsFHORF92, PCR-amplified orf92 was digested with BglII and SmaI and inserted between the BglII and SmaI sites of phsFlagHisVI+. The 5′ primer for orf92 amplification was same as that used to construct phsORF92F; the 3′ primer was 5′-TCCCCCGGGTTATTGCAAATTTAAC. Plasmids expressing cat (pHSP70PLVI+CAT), p35 (pHSP35VI+), Op-iap (pHSOpIAPVI+), Cp-iap (pHSCpIAPVI+), Ac-iap (pHSAcIAPVI+), N-terminally hemagglutinin (HA).11His6-tagged cat (pHSP70VI+EpihisCAT) or Op-iap (pHSP70VI+EpihisOpIAP), and C-terminally Flag-tagged rpr (pHSP70VI+RPR-Flag) were previously described (10, 20, 54).
Coimmunoprecipitations and immunoblotting.
SF-21 cells (106) in 60-mm-diameter culture dishes were transiently transfected by using Lipofectin (41) and 4 μg of each plasmid expressing Flag- or HA.11 epitope (Epi)-tagged genes under Drosophila melanogaster hsp70 promoter (Phsp70) control. At 16 h after transfection, cells were mock or AcMNPV infected at a multiplicity of infection of 5 and heat shocked 6 h following infection for 30 min at 42°C as described previously (10). At 3 h after heat shock, cells were harvested, pelleted at 2,000 × g for 3 min, and lysed in 200 μl of 50 mM Tris-HCl (pH 8.0)–50 mM NaCl–0.5% Nonidet P-40 (NP-40)–1 mM dithiothreitol–1 mM phenylmethylsulfonyl fluoride (NP-40 lysis buffer). The lysate was centrifuged at 2,000 × g for 5 min, and 20 μl of the supernatant was reserved for Western blotting as a positive control of expression. Ten microliters of anti-Flag M2 affinity resin (Eastman Kodak Co., New Haven, Conn.) was incubated with the remainder of the supernatant for 3 h at 4°C with vigorous agitation. The resin was washed five times in 1 ml of NP-40 lysis buffer, and bound proteins were eluted with 0.1 M glycine-HCl (pH 3.5) as recommended by manufacturer. The lysate and eluted samples were subjected to electrophoresis through sodium dodecyl sulfate (SDS)–10% polyacrylamide gels, and proteins were transferred onto Hybond ECL enhanced chemiluminescence membranes (Amersham, Buckinghamshire, United Kingdom). Epi-tagged proteins were detected with mouse HA.11 (anti-Epi) monoclonal antibody and rabbit anti-mouse immunoglobulin G (IgG)-horseradish peroxidase conjugate (Amersham). Flag-tagged proteins were detected with mouse anti-Flag monoclonal antibody M2 (Eastman Kodak) and rabbit anti-mouse IgG-horseradish peroxidase conjugate. Immunoblots were visualized with the ECL Western blotting system.
Immunofluorescence.
SF-21 cells (0.5 × 106) were seeded on glass coverslips in 35-mm-diameter culture dishes and transfected with 1 μg of each plasmid expressing Flag- or Epi-tagged genes under Phsp70 control. Transfected cells were infected and then heat shocked as described above. At 3 h after heat shock, cells were fixed and treated as previously described (45). Flag- or Epi-tagged proteins were detected with mouse anti-Flag (M2) or mouse anti-Epi monoclonal antibodies and lissamine rhodamine-conjugated goat anti-mouse IgG-IgM antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; Sigma, St. Louis, Mo.). Immunofluorescent images and DAPI staining were visualized on a confocal microscope as described previously (20).
Apoptosis assays and internucleosomal DNA fragmentation.
SF-21 or TN-368 cells (0.8 × 106) were seeded in 60-mm-diameter culture dishes and transfected with 1 μg of the indicated plasmids. Cells were heat shocked 18 h after transfection and examined by light microscopy 12 or 24 h later. Phase-contrast photography of transfected cells was performed on an Olympus IX50 inverted microscope equipped with a PM-10AK camera. To determine the percentage of apoptosis (48), viable cells excluding trypan blue (10) were counted as described previously (44). For nucleosomal ladder preparation, cells were harvested at various times after heat shock, pelleted at 12,000 × g for 2 min, and resuspended in 20 mM Tris-HCl (pH 7.6)–10 mM EDTA–0.2% Triton X-100–200 μg of protease K per ml. After 12 to 18 h at room temperature, lysates were extracted four times with 1:1 (vol/vol) phenol-chloroform. DNA was precipitated with 2 volumes of ethanol and 0.1 volume of 5 M NaCl, treated with RNase A, and then subjected to electrophoresis through 1.2% agarose gels.
Cell division assays.
hsOpIAPpacR cells (0.25 × 106) were seeded in 60-mm-diameter culture dishes and transfected with 0.5 μg of the appropriate plasmid. Cells were heat shocked 18 h after transfection and examined, as described above, over a 5-day period.
RESULTS
Association of human p53 and baculovirus p33 in insect cells.
Baculoviruses are widely used as vectors for expression of biologically active proteins, and the AcMNPV recombinants expressing mammalian p53 proteins were previously constructed for this purpose. In the course of this work, we observed that a 33-kDa polypeptide (p33) consistently copurified with either murine or human p53. To identify p33, a human p53 was expressed in SF-21 cells by using vEVHp53wt (14) and purified by immunoaffinity procedures (56). Copurified p33 was separated from p53 by SDS-polyacrylamide gel electrophoresis and sequenced by Edman degradation. The sequence of the amino-terminal 17 residues was identical to that of the predicted product of the AcMNPV orf92 (Fig. 1). Alignment of AcMNPV p33 with its homologs in Bombyx mori nuclear polyhedrosis virus (BmNPV) and O. pseudotsugata nuclear polyhedrosis virus (OpMNPV) shows that the OpMNPV homolog contains a 24-amino-acid insertion near the C terminus of the protein but otherwise is well conserved, with approximately 81% sequence identity between the OpMNPV homolog and the AcMNPV or BmNPV homolog (Fig. 1). Computer-based analysis of the orf92-encoded protein sequences revealed no significant sequence homology to any other proteins in the available databases.
FIG. 1.
Alignment of baculovirus p33 proteins. The N-terminal sequence of the purified p33 protein determined by Edman degradation is boxed. Identical amino acids are indicated by dots below the AcMNPV sequence. Gaps inserted to facilitate alignment are shown by dashes. AcMNPV, BmNPV, and OpMNPV sequences are from references 4, 36, and 46, respectively.
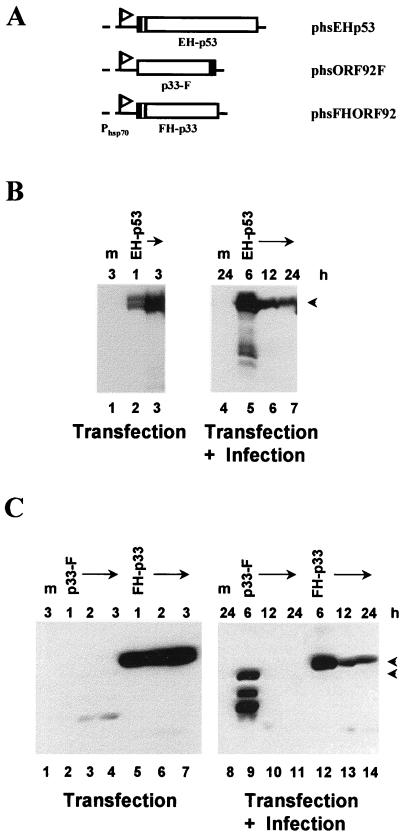
To confirm that p33 binds to p53, we performed immunoprecipitation studies. To facilitate detection of p53 and p33, Epi and His6 tags were fused to the p53 amino terminus (EH-p53) and a Flag tag was fused to the C terminus of the p33 protein (p33-F) or to the N-terminus along with a His6 tag (FH-p33) (Fig. 2A). These epitope-tagged versions of p53 and orf92 were transiently expressed in uninfected or in AcMNPV-infected SF-21 cells by using an insect heat shock promoter (Phsp70). Both mock- and AcMNPV-infected SF-21 cells transfected with plasmid phsEHp53-expressed EH-p53, which was detectable for at least 3 h following heat shock induction (Fig. 2B). The C- and N-terminally tagged forms of p33 expressed from phsORF92F and phsFHORF92, respectively, differed significantly in their levels of expression or stability. FH-p33 was easily detectable in both mock- and virus-infected insect cells from 1 to 3 h after heat shock and at various times after infection. In contrast, p33-F was detected only at 6 h after infection, and other, smaller products (possibly degradation products) were observed (Fig. 2C). Based on the expression data in Fig. 2, we selected 6 h after infection to heat shock transfected cells and 3 h after heat shock to examine the ability of p33 to interact with p53.
FIG. 2.
Construction and expression of epitope-tagged versions of human wild-type p53 and baculovirus p33. (A) Schematic representation of the plasmids encoding epitope-tagged p53 and p33. The p53 and p33 genes were placed under the transcriptional control of the D. melanogaster Phsp70, indicated by a flag. The Epi and Flag tags are denoted by the filled regions; a sequence encoding six histidines (checkered region) was included in phsEHp53 and phsFHORF92. The identity of each chimeric protein or plasmid is shown below or on the right. (B and C) Immunoblot analysis of the expression of Epi-tagged p53 (B) and Flag-tagged p33 (C). SF-21 cells were mock transfected (m) or transfected with a plasmid expressing the protein indicated above each lane. Cells were harvested after induction by heat shock as indicated above each lane in the left-hand panels. Equal amounts of cell lysates were analyzed by SDS-polyacrylamide gel electrophoresis followed by Western blotting with the mouse anti-Epi monoclonal antibody (B) or with the mouse anti-Flag monoclonal antibody M2 (C). Positions of full-length proteins are indicated by arrowheads on the right. To analyze the expression of tagged p53 and p33 during infection (right-hand panels), transfected SF-21 cells were infected 18 h after transfection and then heat shocked at 6, 12, or 24 h postinfection (as indicated above each lane). Cells were harvested 3 h after heat shock, and equal amounts of cell lysates were analyzed as described above.
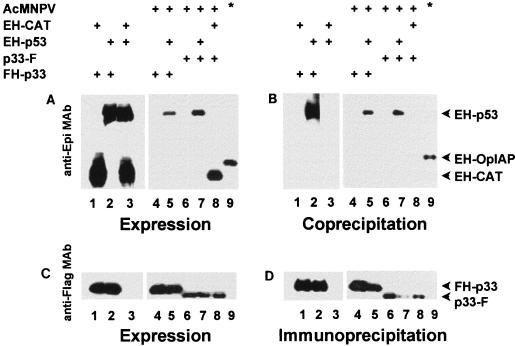
To determine if p53 interacts with p33, lysates of mock- or AcMNPV-infected SF-21 cells transfected with plasmids expressing Epi- or Flag-tagged proteins were immunoprecipitated with anti-Flag antibody resin. Coprecipitating Epi-tagged proteins were detected by Western blot analysis using an anti-Epi antibody (Fig. 3). An N-terminally Epi- and His6-tagged version of CAT (EH-CAT) served as a negative control. A C-terminally Flag-tagged version of Reaper (RPR-F) and an N-terminally Epi- and His6-tagged version of OpIAP (EH-OpIAP) served as a positive control for interaction (54). To ensure that EH-p53 did not bind to the anti-Flag resin, we used lysates of cells transfected with phsEHp53 and phsFlagHisVI+ (data not shown) or phsEHp53 and pHSP70VI+EpihisCAT (Fig. 3, lane 3). EH-p53 was not precipitated by the resin. All proteins were appropriately expressed. Both FH-p33 and p33-F interacted with EH-p53 (Fig. 3, lanes 5 and 7) but not with EH-CAT (Fig. 3, lane 8), confirming that baculovirus p33 proteins and human p53 form a complex in the context of both infected and uninfected SF-21 cells.
FIG. 3.
Baculovirus p33 forms a stable complex with human p53. SF-21 cells were transfected with plasmids expressing the indicated combinations of Flag- and Epi-tagged genes under Phsp70 control (indicated by plus signs). Transfected cells were further mock or AcMNPV infected (indicated by plus signs adjacent to AcMNPV) and heat shocked 6 h following infection. At 3 h after heat shock, aliquots of cell lysates were immunoprecipitated with anti-Flag monoclonal antibody (MAb) resin. Bound proteins were eluted with 0.1 M glycine-HCl (pH 3.5), separated by SDS-polyacrylamide gel electrophoresis, transferred onto a Hybond ECL membrane, and probed with the anti-Flag monoclonal antibody (D). To detect coprecipitating Epi-tagged proteins, the membrane was stripped and reprobed with the anti-Epi monoclonal antibody (B). Expression of the tagged proteins was confirmed by immunoblotting aliquots of the total-cell lysates with the anti-Flag (C) or anti-Epi (A) monoclonal antibody. The identities of visualized proteins are indicated on the right; antibodies used for Western blotting are shown on the left. Lanes 9 are indicated by an asterisk and show a positive control for coprecipitation: Flag-tagged Reaper and Epi-tagged OpIAP.
Subcellular localization of the p53 and p33 proteins in insect cells.
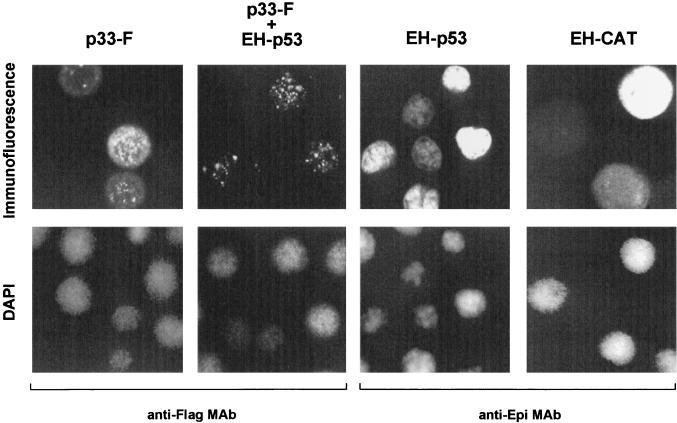
The ability of FH-p33 or p33-F to bind EH-p53 suggested that these proteins should localize to the same subcellular location when coexpressed during infection or transfection. To determine their subcellular locations, infected SF-21 cells were transfected with plasmids expressing p33-F in the presence or absence of EH-p53. In the absence of EH-p53, p33-F displayed a diffuse staining pattern in the cytoplasm and a punctate staining pattern in the nucleus. In the presence of EH-p53, p33-F was localized exclusively to the nucleus and displayed a punctate staining pattern (Fig. 4). The same staining patterns were observed in uninfected cells and in AcMNPV-infected SF-21 cells transfected with plasmids expressing FH-p33 alone or with EH-p53 (data not shown). EH-p53 was localized to the nuclei of both infected (Fig. 4) and transfected (data not shown) cells in the presence or absence of p33, and it appeared to be distributed more uniformly than p33. Cells expressing EH-CAT or DAPI staining served as controls for cytoplasmic or nuclear localization. Thus, p53 appears to facilitate or direct p33 to the nucleus, where p33 targets a limited number of subnuclear sites, possibly in association with a portion of the nuclearly localized p53 protein.
FIG. 4.
Subcellular localization of human wild-type p53 and baculovirus p33 expressed in AcMNPV-infected insect cells. SF-21 cells were transfected with plasmids expressing the indicated proteins. Transfected cells were further infected with AcMNPV and heat shocked after 6 h. At 3 h after heat shock, the cells were fixed in methanol and double stained with DAPI (bottom row) and antibody. EH-p53, EH-CAT, and p33-F were visualized with the mouse anti-Flag monoclonal antibody (MAb) M2 and the mouse anti-Epi monoclonal antibody, as indicated at the bottom) and then with lissamine rhodamine-conjugated goat anti-mouse IgG and IgM antibodies (top row). The same field of cells was examined with a confocal microscope for both DAPI and immunofluorescence.
p53 induces apoptosis in insect cells.
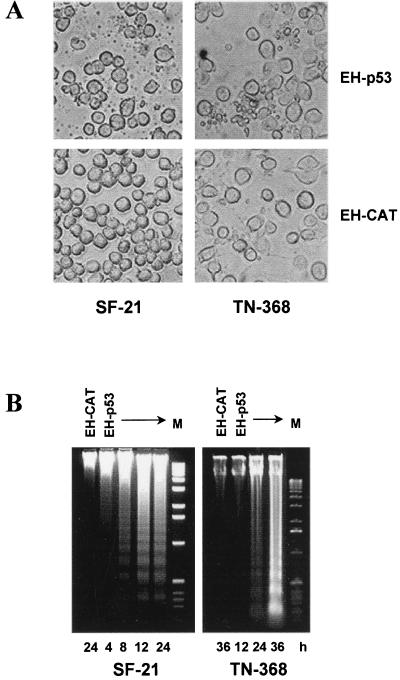
An insect equivalent of p53 has not been identified, and it was of interest to determine if human p53 exerts any of its biological effects in the context of insect cells. To test if p53 induces apoptosis in insect cells, SF-21 and TN-368 cells were transfected with phsEHp53 or with pHSP70VI+EpihisCAT expressing EH-CAT as a control. Cells expressing EH-p53 exhibited extensive membrane blebbing and apoptotic body formation (Fig. 5A, top row), but cells expressing EH-CAT had no signs of apoptotic activity (Fig. 5A, bottom row). Nuclear condensation and fragmentation were confirmed by DAPI staining in cells exhibiting membrane blebbing (data not shown). Expression of EH-p53, but not EH-CAT, also resulted in cleavage of cellular DNA into oligonucleosomal fragments as early as 8 h after heat shock in SF-21 cells and by 24 h after heat shock in TN-368 cells (Fig. 5B). Therefore, p53 induces in insect cell lines SF-21 and TN-368 cell death with the morphological and biochemical features of apoptosis.
FIG. 5.
Expression of human p53 in insect cells induces typical apoptosis. (A) Light microscopy of SF-21 and TN-368 cells transfected with plasmids expressing EH-p53 or, as a negative control, EH-CAT. Photographs were taken at 12 h (SF-21) or 24 h (TN-368) after heat shock, using an Olympus IX50 microscope. (B) Insect cells were transfected with plasmids expressing the proteins indicated the lanes. Total DNA was harvested at various times after heat shock (indicated at the bottom) and analyzed by agarose gel electrophoresis. M, DNA molecular weight markers (1-kb DNA ladder).
The baculovirus p35 and IAPs block p53-induced apoptosis.
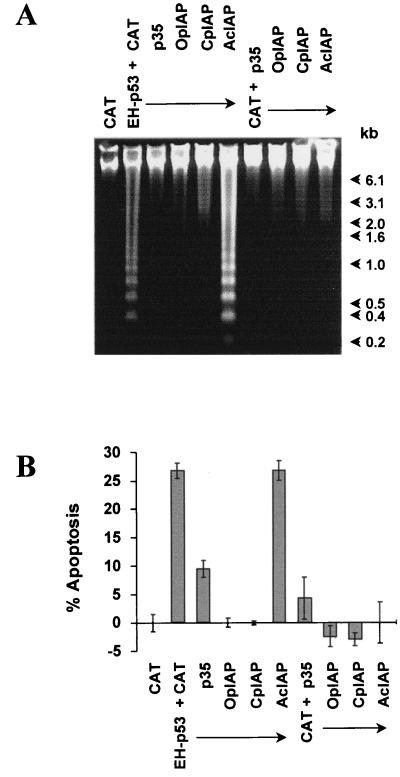
Baculoviral p35 and IAPs were coexpressed with EH-p53 to determine whether these proteins could block p53-induced apoptosis. Coexpression of p35, OpIAP, or CpIAP with EH-p53 resulted in a complete inhibition of p53-induced apoptosis (Fig. 6A). Both OpIAP and CpIAP completely restored viability of cells compared to the cells expressing CAT alone (Fig. 6B). AcIAP, also known as AcIAP1 (4, 11), was unable to block p53-induced apoptosis, consistent with previous observations that this baculoviral IAP is unable to block apoptosis in SF-21 cells (10). Cotransfection of phsEHp53 with a plasmid expressing p35 resulted in a threefold reduction in the percentage of apoptotic cells. However, the number of viable cells observed when p35 was cotransfected with CAT was less than that observed for the cells transfected with CAT alone, but no membrane blebbing was observed. This is consistent with previous observations that p35 may reduce cell growth in SF-21 cells (44). No protection was observed with a plasmid expressing AcIAP (Fig. 6). Thus, p35, OpIAP, and CpIAP, but not AcIAP, are able to block apoptosis initiated by p53 in transient expression assays.
FIG. 6.
Inhibition of p53-induced cell death in insect cells by antiapoptotic genes. SF-21 cells were cotransfected with plasmids expressing the proteins indicated at the top. Cellular DNA was harvested 24 h after heat shock and examined by electrophoresis in a 1.2% agarose gel (A). Positions of DNA molecular weight markers (1-kb DNA ladder) are indicated at the right. The percentage of cells undergoing apoptosis was determined by trypan blue exclusion 24 h after heat shock (B). A plasmid expressing CAT was used as a negative control and to balance plasmid DNA concentrations. The percentage of apoptotic cells was calculated relative to the CAT control, set at 0%, which was similar to the level for mock-transfected controls.
p53 blocks cell growth in the presence or absence of p33 in insect cells.
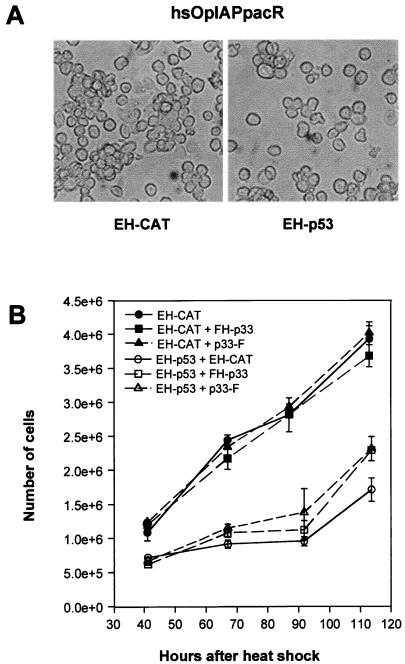
To determine if p53 is able to affect insect cell growth, we examined p53 expression in the SF-21-derived cell line hsOpIAPpacR, which stably expresses OpIAP and is partially resistant to apoptosis (38). hsOpIAPpacR cells were transfected with phsEHp53 or pHSP70VI+EpihisCAT, as a negative control, and heat shocked at 18 h after transfection. There was no evidence of unusual morphology, apoptosis, or necrotic cell death in these transfected cells (Fig. 7A and data not shown). Instead, there were twofold more viable EH-CAT-expressing cells than EH-p53-expressing cells at 50 h after heat shock and threefold more by 90 h (Fig. 7B). Thus, p53 blocks cell growth in insect cells in the presence of an antiapoptotic IAP. Expression of EH-p33 or p33-F alone in hsOpIAPpacR cells did not increase or reduce the rate of cell growth compared to the cells expressing the control EH-CAT (Fig. 7B). Coexpression of FH-p33 or p33-F with EH-p53 in this cell line had no influence on the ability of p53 to block cell growth.
FIG. 7.
Expression of human p53 in insect cells blocks cell growth. S. frugiperda hsOpIAPpacR cells stably expressing Op-iap were transfected with plasmids expressing the indicated proteins. Cells were photographed 72 h after heat shock, using an Olympus IX50 microscope (A). Viable cells were counted in the presence of trypan blue (B). A plasmid expressing EH-CAT was used as a negative control and to balance plasmid DNA concentrations.
Baculovirus p33 enhances p53-mediated apoptosis in insect cells.
Since many other viruses encode p53 binding proteins which abrogate the function of p53, we determined if p33 could affect p53-mediated apoptosis. Cotransfection of SF-21 cells with plasmids coexpressing either FH-p33 or p33-F with EH-p53 increased the number of apoptotic cells twofold compared to the cells expressing EH-p53 only (Fig. 8A). Expression of FH-p33 or p33-F alone did not induce apoptosis in SF-21 cells, as determined both by the number of apoptotic cells and the absence of oligonucleosomal ladder formation (Fig. 8).
FIG. 8.
Baculovirus p33 enhances the ability of p53 to induce apoptosis. SF-21 cells were cotransfected with plasmids expressing the indicated proteins. The percentage of cells undergoing apoptosis was determined by trypan blue exclusion 24 h after heat shock and calculated relative to the CAT control (A). Cellular DNA was harvested 24 h after heat shock and analyzed by agarose gel electrophoresis (B). Positions of DNA molecular weight markers are indicated at the right. A plasmid expressing EH-CAT was used as a negative control and to balance plasmid DNA concentrations.
DISCUSSION
While using AcMNPV as a gene expression vector, we unexpectedly discovered that human p53 forms a complex with the product of viral orf92, p33. This previously uncharacterized protein is conserved among sequenced baculoviruses but has no obvious sequence relationship to other known proteins in the databases. Because p33 was the only protein found associated with purified p53 and the complex was stable through a variety of purification procedures (data not shown), we considered this interaction to be potentially informative regarding p33 function and have investigated it further. Interaction with human p53 directs p33 to distinct subnuclear locations, indicating that this interaction occurs in vivo as well as in vitro. Since there are no known invertebrate homologs of p53, the question of the biological relevance of this interaction remains unclear. However, human p53 exhibits biological activity in insect cells which is remarkably similar to its known activities in mammalian cells, and it thus seems likely that p53-associated pathways are conserved in insect cells.
We have found that human p53 induces apoptosis in SF-21 and TN-368 cells and that it can also block cell growth in SF-21 cells which express the antiapoptotic baculovirus gene Op-iap. Human p53 is considered to be the “guardian of the genome” in mammals, based on its ability to regulate cell cycle progression from G1 to S and its ability to induce apoptosis in response to DNA damage (reviewed in references 26 and 31). The ability of insect cells to respond to p53 in such a similar manner as mammalian cells indicates the conservation of the basic pathways and suggests that insect cells have a p53 homolog or a functional equivalent. Coexpression of p33 with p53 accentuates the ability of p53 to induce apoptosis which may reflect a functional as well as physical interaction of p33 and p53.
It is not clear what role, if any, p33 stimulation of p53-induced apoptosis might have in baculovirus infection. Like many large DNA-containing viruses, AcMNPV is known to induce apoptosis during infection of some host cells. In the case of SF-21 cells, the induction of apoptosis is correlated with the activation of caspases (6, 8), and the activation of SF-caspase-1 (1) is specifically implicated in this process (47). Whereas p35 blocks active SF-caspase-1, OpIAP prevents the activation of this caspase during virus infection (47). OpIAP, in turn, is known to bind and inhibit a number of insect apoptotic inducers, including Reaper, HID, GRIM, and Doom (20, 54, 55), which have no known homologs in vertebrates. Thus the involvement of a p53-like protein during baculovirus infection remains unclear, but the possibility that IAPs also interact with p53-like proteins is not precluded. There is evidence to suggest that viral DNA replication may be involved in triggering the induction of apoptosis (10, 34), and this might effectively mimic DNA damage or unscheduled cell cycle progression, which, in turn, might elicit a p53-like apoptotic response. The role of p33 in accentuating p53-mediated apoptosis would then be related to viral alteration of cellular factors in preparation for viral DNA replication, which might predispose the cell to p53-mediated apoptosis. Although overexpression of the AcMNPV ie-1 gene is sufficient to cause apoptosis in SF-21 cells (44), the dynamics of this induction suggest that other factors or events are also involved.
We have attempted to mutate orf92 by insertion of the lacZ gene into this locus, but all attempts to isolate stable double-crossover recombinants have been unsuccessful (data not shown), suggesting that orf92 is an essential baculovirus gene. It may be possible to further explore the function of p33 by finding an insect protein which interacts with p33 by using an interaction-based screening system. If such a protein exists, it would be interesting to determine if it has p53-like properties. Although p53 homologs have been identified in numerous vertebrates, none have yet been found in invertebrates.
ACKNOWLEDGMENTS
We thank Somasekar Seshagiri, Jeanne McLachlin, and especially Elena A. Prikhod’ko for valuable suggestions.
This research was supported in part by Public Health Service grants CA58316 (to C.P.) and AI23719 (to L.K.M.).
REFERENCES
- 1.Ahmad M, Srinivasula S M, Wang L, Litwack G, Fernandes-Alnemri T, Alnemri E S. Spodoptera frugiperda caspase-1, a novel insect death protease that cleaves the nuclear immunophilin FKBP46, is the target of the baculovirus antiapoptotic protein p35. J Biol Chem. 1997;272:1421–1424. doi: 10.1074/jbc.272.3.1421. [DOI] [PubMed] [Google Scholar]
- 2.Almog N, Rotter V. Involvement of p53 in cell differentiation and development. Biochim Biophys Acta. 1997;1333:F1–F27. doi: 10.1016/s0304-419x(97)00012-7. [DOI] [PubMed] [Google Scholar]
- 3.Avantaggiati M L, Ogryzko V, Gardner K, Giordano A, Levine A S, Kelly K. Recruitment of p300/CBP in p53-dependent signal pathways. Cell. 1997;89:1175–1184. doi: 10.1016/s0092-8674(00)80304-9. [DOI] [PubMed] [Google Scholar]
- 4.Ayres M D, Howard S C, Kuzio J, Ferber M L, Possee R D. The complete DNA sequence of Autographa californica nuclear polyhedrosis virus. Virology. 1994;202:586–605. doi: 10.1006/viro.1994.1380. [DOI] [PubMed] [Google Scholar]
- 5.Bargonetti J, Reynisdottir I, Friedman P N, Prives C. Site-specific binding of wild-type p53 to cellular DNA is inhibited by SV40 T antigen and mutant p53. Genes Dev. 1992;6:1886–1898. doi: 10.1101/gad.6.10.1886. [DOI] [PubMed] [Google Scholar]
- 6.Bertin J, Mendrysa S M, LaCount D J, Guar S, Krebs J F, Armstrong R C, Tomaselli K J, Friesen P D. Apoptotic suppression by baculovirus P35 involves cleavage by and inhibition of a virus-induced CED-3/ICE-like protease. J Virol. 1996;70:6251–6259. doi: 10.1128/jvi.70.9.6251-6259.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bottger A, Bottger V, Sparks A, Liu W L, Howard S F, Lane D P. Design of a synthetic Mdm2-binding mini protein that activates the p53 response in vivo. Curr Biol. 1997;7:860–869. doi: 10.1016/s0960-9822(06)00374-5. [DOI] [PubMed] [Google Scholar]
- 8.Bump N J, Hackett M, Hugunin M, Seshagiri S, Brady K, Chen P, Ferenz C, Franklin S, Ghayur T, Li P, Licari P, Markovich J, Shi L, Greenberg A, Miller L K, Wong W W. Inhibition of ICE family proteases by baculovirus antiapoptotic protein p35. Science. 1995;269:1885–1888. doi: 10.1126/science.7569933. [DOI] [PubMed] [Google Scholar]
- 9.Chen C-Y, Oliner J D, Zhan Q, Fornace A J, Jr, Vogelstein B, Kastan M B. Interactions between p53 and MDM2 in a mammalian cell cycle checkpoint pathway. Proc Natl Acad Sci USA. 1994;91:2684–2688. doi: 10.1073/pnas.91.7.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clem R J, Miller L K. Control of programmed cell death by the baculovirus genes p35 and iap. Mol Cell Biol. 1994;14:5212–5222. doi: 10.1128/mcb.14.8.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clem R J. Regulation of programmed cell death by baculoviruses. In: Miller L K, editor. The baculoviruses. New York, N.Y: Plenum Press; 1997. pp. 237–261. [Google Scholar]
- 12.Dobner T, Horikoshi N, Rubenwolf S, Shenk T. Blockage by adenovirus E4orf6 of transcriptional activation by the p53 tumor suppressor. Science. 1996;272:1470–1473. doi: 10.1126/science.272.5267.1470. [DOI] [PubMed] [Google Scholar]
- 13.Farmer G, Bargonetti J, Zhu H, Friedman P, Prywes R, Prives C. Wild type p53 activates transcription in vitro. Nature. 1992;358:83–86. doi: 10.1038/358083a0. [DOI] [PubMed] [Google Scholar]
- 14.Friedman P N, Kern S E, Vogelstein B, Prives C. Wild-type, but not mutant, human p53 proteins inhibit the replication activities of simian virus 40 large tumor antigen. Proc Natl Acad Sci USA. 1990;87:9275–9279. doi: 10.1073/pnas.87.23.9275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garkavtsev I, Grigorian I A, Ossovskaya V S, Chernov M V, Chumakov P M, Gudkov A V. The candidate tumor suppressor p33ING1 cooperates with p53 in cell growth control. Nature. 1998;391:295–298. doi: 10.1038/34675. [DOI] [PubMed] [Google Scholar]
- 16.Goga A, Liu X, Hambuch T M, Senechal K, Major E, Berk A J, Witte O N, Sawyers C L. p53-dependent growth suppression by the c-Abl nuclear tyrosine kinase. Oncogene. 1995;11:791–799. [PubMed] [Google Scholar]
- 17.Gottlieb T M, Oren M. p53 in growth control and neoplasia. Biochim Biophys Acta. 1996;1287:77–102. doi: 10.1016/0304-419x(95)00019-c. [DOI] [PubMed] [Google Scholar]
- 18.Greenblatt M S, Bennett W P, Hollstein M, Harris C C. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994;54:4855–4878. [PubMed] [Google Scholar]
- 19.Gu W, Shi X-L, Roeder R G. Synergistic activation of transcription by CBP and p53. Nature. 1997;387:819–823. doi: 10.1038/42972. [DOI] [PubMed] [Google Scholar]
- 20.Harvey A J, Bidwai A P, Miller L K. Doom, a product of the Drosophila mod (mdg4) gene, induces apoptosis and binds to baculovirus inhibitor-of-apoptosis proteins. Mol Cell Biol. 1997;17:2835–2843. doi: 10.1128/mcb.17.5.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 22.Hermeking H, Lengauer C, Polyak K, He T-C, Zhang L, Thiagalingam S, Kinzler K W, Vogelstein B. 14-3-3ς is a p53-regulated inhibitor of G2/M progression. Mol Cell. 1997;1:3–11. doi: 10.1016/s1097-2765(00)80002-7. [DOI] [PubMed] [Google Scholar]
- 23.Hink W F. Established insect cell line from the cabbage looper, Trichoplusia ni. Nature. 1970;226:466–467. doi: 10.1038/226466b0. [DOI] [PubMed] [Google Scholar]
- 24.Huibregtse J M, Scheffner J, Howley P M. Cloning and expression of the cDNA for E6-AP, a protein that mediates the interaction of the human papillomavirus E6 oncoprotein with p53. Mol Cell Biol. 1993;13:775–784. doi: 10.1128/mcb.13.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinzler K W, Vogelstein B. Life (and death) in a malignant tumor. Nature. 1996;379:19–20. doi: 10.1038/379019a0. [DOI] [PubMed] [Google Scholar]
- 26.Ko L J, Prives C. p53: puzzle and paradigm. Genes Dev. 1996;10:1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 27.Kubbutat M H, Jones S N, Vousden K H. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 28.Kussie P H, Gorina S, Marechal V, Elebaas B, Moreau J, Levine A J, Pavletich N P. Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science. 1996;274:948–953. doi: 10.1126/science.274.5289.948. [DOI] [PubMed] [Google Scholar]
- 29.LaCount D J, Friesen P D. Role of early and late replication events in induction of apoptosis by baculoviruses. J Virol. 1997;71:1530–1537. doi: 10.1128/jvi.71.2.1530-1537.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levine A J. The tumor suppressor genes. Annu Rev Biochem. 1993;62:623–651. doi: 10.1146/annurev.bi.62.070193.003203. [DOI] [PubMed] [Google Scholar]
- 31.Levine A J. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 32.Lill N L, Grossman S R, Ginsberg D, DeCaprio J, Livingston D M. Binding and modulation of p53 by p300/CBP coactivators. Nature. 1997;387:823–827. doi: 10.1038/42981. [DOI] [PubMed] [Google Scholar]
- 33.Lin J, Chen J, Elenbaas B, Levine A J. Several hydrophobic amino acids in the p53 amino-terminal domain are required for transcriptional activation, binding to mdm-2 and the adenovirus 5 E1B 55-kD protein. Genes Dev. 1994;8:1235–1246. doi: 10.1101/gad.8.10.1235. [DOI] [PubMed] [Google Scholar]
- 34.Lu A, Miller L K. The roles of eighteen baculovirus late expression factor genes in transcription and DNA replication. J Virol. 1995;69:975–982. doi: 10.1128/jvi.69.2.975-982.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maheswaran S, Englert C, Bennett P, Heinrich G, Haber D A. The Wt1 gene product stabilizes p53 and inhibits p53-mediated apoptosis. Genes Dev. 1995;9:2143–2156. doi: 10.1101/gad.9.17.2143. [DOI] [PubMed] [Google Scholar]
- 36.Majima K, Gomi S, Ohkawa T, Kamita S G, Maeda S. Complete nucleotide sequence of the 128,413 bp-long genome of the BmNPV: evolution and divergence of baculoviruses. 1996. GenBank accession no. L33180. [Google Scholar]
- 37.Manji G A, Hozak R R, LaCount D J, Friesen P D. Baculovirus inhibitor of apoptosis functions at or upstream of the apoptotic suppressor P35 to prevent programmed cell death. J Virol. 1997;71:4509–4516. doi: 10.1128/jvi.71.6.4509-4516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLachlin J R, Miller L K. Stable transformation of insect cells to coexpress a rapidly selectable marker gene and inhibitor of apoptosis. In Vitro Cell Dev Biol Anim. 1997;33:575–579. doi: 10.1007/s11626-997-0101-7. [DOI] [PubMed] [Google Scholar]
- 39.Momand J, Zambetti G P, Olson D C, George D, Levine A J. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 40.Oliner J D, Pietenpol J A, Thiagalingam S, Gyuris J, Kinzler K W, Vogelstein B. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature. 1993;362:857–860. doi: 10.1038/362857a0. [DOI] [PubMed] [Google Scholar]
- 41.O’Reilly D R, Miller L K, Luckow V A. Baculovirus expression vectors: a laboratory Manual. W. H. New York, N.Y: Freeman and Company; 1992. [Google Scholar]
- 42.Picksley S M, Vojtesek B, Sparks A, Lane D P. Immunochemical analysis of the interaction of p53 with MDM2; fine mapping of the MDM2 binding site on p53 using synthetic peptides. Oncogene. 1994;9:2523–2529. [PubMed] [Google Scholar]
- 43.Polyak K, Xia Y, Zweier J L, Kinzler K W, Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- 44.Prikhod’ko E A, Miller L K. Induction of apoptosis by baculovirus transactivator IE1. J Virol. 1996;70:7116–7124. doi: 10.1128/jvi.70.10.7116-7124.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prikhod’ko E A, Miller L K. Role of baculovirus IE2 and its RING finger in cell cycle arrest. J Virol. 1998;72:684–692. doi: 10.1128/jvi.72.1.684-692.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Russell R L Q, Rohrmann G F. A 25-kDa protein is associated with the envelopes of occluded baculovirus virions. Virology. 1993;195:532–540. doi: 10.1006/viro.1993.1404. [DOI] [PubMed] [Google Scholar]
- 47.Seshagiri S, Miller L K. Baculovirus inhibitors of apoptosis (IAPs) block activation of Sf-caspase-1. Proc Natl Acad Sci USA. 1997;94:13606–13611. doi: 10.1073/pnas.94.25.13606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seshagiri S, Miller L K. Caenorhabditis elegans CED-4 stimulates CED-3 processing and CED-3 induced apoptosis. Curr Biol. 1997;7:455–460. doi: 10.1016/s0960-9822(06)00216-8. [DOI] [PubMed] [Google Scholar]
- 49.Szekely L, Selivanova G, Magnusson K P, Klein G, Wiman K G. EBNA-5, an Epstein-Barr virus-encoded nuclear antigen, binds to the retinoblastoma and p53 proteins. Proc Natl Acad Sci USA. 1993;90:5455–5459. doi: 10.1073/pnas.90.12.5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teodoro J G, Branton P E. Regulation of apoptosis by viral gene products. J Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thut C J, Chen J L, Klemm R, Tjian R. P53 transcriptional activation mediated by coactivators TAFII40 and TAFII60. Science. 1995;267:100–104. doi: 10.1126/science.7809597. [DOI] [PubMed] [Google Scholar]
- 52.Vaughn J L, Goodwin R H, Tompkins G J, McCawley P. The establishment of two cell lines from the insect Spodoptera frugiperda (Lepidoptera: Noctuidae) In Vitro. 1977;13:213–217. doi: 10.1007/BF02615077. [DOI] [PubMed] [Google Scholar]
- 53.Vogelstein B, Kinzler K W. p53 function and dysfunction. Cell. 1992;70:523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- 54.Vucic D, Kaiser W J, Harvey A J, Miller L K. Inhibition of reaper-induced apoptosis by interaction with inhibitor of apoptosis proteins (IAPs) Proc Natl Acad USA. 1997;94:10183–10188. doi: 10.1073/pnas.94.19.10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vucic D, Kaiser W J, Miller L K. Inhibitor of apoptosis proteins physically interact with and block apoptosis induced by Drosophila proteins HID and GRIM. Mol Cell Biol. 1998;18:3300–3309. doi: 10.1128/mcb.18.6.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang E H, Friedman P N, Prives C. The murine p53 protein blocks replication of SV40 DNA in vitro by inhibiting the initiation functions of SV40 large T antigen. Cell. 1989;57:379–392. doi: 10.1016/0092-8674(89)90913-6. [DOI] [PubMed] [Google Scholar]
- 57.Wang X W, Forrester K, Yeh H, Feitelson M A, Gu J R, Harris C C. Hepatitis B virus X protein inhibits p53 sequence-specific DNA binding, transcriptional activity, and association with transcription factor ERCC3. Proc Natl Acad USA. 1994;91:2230–2234. doi: 10.1073/pnas.91.6.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.White E. Life, death, and the pursuit of apoptosis. Genes Dev. 1996;10:1–15. doi: 10.1101/gad.10.1.1. [DOI] [PubMed] [Google Scholar]
- 59.Wu X, Bayle J H, Olson D, Levine A J. The p53-mdm-2 autoregulatory feedback loop. Genes Dev. 1993;7:1126–1132. doi: 10.1101/gad.7.7a.1126. [DOI] [PubMed] [Google Scholar]
- 60.Yew P R, Liu X, Berk A J. Adenovirus E1B oncoprotein tethers a transcriptional repression domain to p53. Genes Dev. 1994;8:190–202. doi: 10.1101/gad.8.2.190. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Q, Gutsch D, Kenney S. Functional and physical interaction between p53 and BZLF1: implications for Epstein-Barr virus latency. Mol Cell Biol. 1994;14:1929–1938. doi: 10.1128/mcb.14.3.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]