Abstract
Introduction:
The objective of this study was to assess incremental costs and benefits of a human papillomavirus (HPV) vaccination program expanded to include “mid-adults” (adults aged 27 through 45 years) in the United States.
Methods:
We adapted a previously published, dynamic mathematical model of HPV transmission and HPV-associated disease to estimate the incremental costs and benefits of a 9vHPV program for people aged 12 through 45 years compared to a 9vHPV program for females aged 12 through 26 years and males aged 12 through 21 years.
Results:
A 9vHPV program for females aged 12 through 26 years and males aged 12 through 21 years was estimated to cost < $10,000 quality-adjusted life year (QALY) gained, compared to no vaccination. Expanding the 9vHPV program to include mid-adults was estimated to cost $587,600 per additional QALY gained when including adults through age 30 years, and $653,300 per additional QALY gained when including adults through age 45 years. Results were most sensitive to assumptions about HPV incidence among mid-adults, current and historical vaccination coverage, vaccine price, and the impact of HPV diseases on quality of life.
Conclusions:
Mid-adult vaccination is much less cost-effective than the comparison strategy of routine vaccination for all adolescents at ages 11 to 12 years and catch-up vaccination for women through age 26 years and all men through age 21 years.
Keywords: Human papillomavirus, nonavalent HPV vaccine, cost-effectiveness, cost-utility, disease transmission models, vaccines
Introduction
In October 2018, the United States Food and Drug Administration (FDA) approved an application to expand the age indication for the 9-valent human papillomavirus (HPV) vaccine (9vHPV, Gardasil 9, Merck and Co., Inc., Whitehouse Station, NJ) from age 9 through 26 years to include persons aged 27 through 45 years [1]. In the United States, the Advisory Committee on Immunization Practices (ACIP) provides recommendations on use of vaccines in the civilian population [2,3]. Before the age indication for 9vHPV was expanded through age 45 years, ACIP had recommended routine HPV vaccination for adolescents at age 11 or 12 years with catch-up vaccination for females through age 26 years, for males through age 21 years, and for special populations (such as men who have sex with men, or MSM) through age 26 years, if not previously adequately vaccinated [4,5]. In response to a new FDA licensure and/or indication, ACIP reviews evidence, including health economic data, for consideration of vaccination recommendations. ACIP reviewed evidence related to the FDA expanded age indication for 9vHPV over four meetings in 2018 and 2019.
The purpose of this modeling study was to estimate the potential impact and cost-effectiveness of HPV vaccination for “mid-adults” (adults aged 27 through 45 years) in the United States. Results from this study, along with results from four other modeling studies, were presented to ACIP to inform recommendations for 9vHPV among adults through age 45 years [6,7]. In June 2019, ACIP recommended catch-up HPV vaccination for all persons through age 26 years; this change harmonized catch-up age recommendations across genders. ACIP did not recommend catch-up vaccination for all mid-adults aged 27 through 45 years, but instead recommended shared clinical decision-making regarding potential HPV vaccination for this age group [1].
Methods
Study questions addressed
We addressed the following study question: What is the cost-effectiveness of vaccinating people aged 12 through 45 years with 9vHPV (“mid-adult vaccination strategy”), compared with vaccinating females aged 12 through 26 years and males aged 12 through 21 years (“comparison strategy”)? We assessed costs and benefits of each HPV vaccination strategy over a 100-year time horizon, and all future costs and QALYs were discounted by 3% annually, in accordance with established standards for cost-effectiveness studies in the United States [8,9]. All costs were updated for inflation to third quarter 2018 US dollars using the Personal Consumption Expenditures price index (www.bea.gov).
We examined the costs and benefits of adding mid-adult vaccination to an existing program that has been vaccinating females through age 26 years and males through age 21 years during the previous eleven years, like the situation in the United States when mid-adult vaccination strategies were being considered by ACIP (i.e., prior to the June 2019 vote). Specifically, in the “comparison strategy” the cutoff (maximum) age of catch-up vaccination was set at age 26 years for women and 21 years for men in all 100 years of the vaccination program, whereas under the “mid-adult vaccination” strategy, the cutoff age of catch-up vaccination was set at 26 years for women and 21 years for men in years 1 through 11 of the vaccination program and was later increased to age 45 years in years 12 through 100 of the vaccination program. In addition to examining age 45 years as the cutoff age for catch-up vaccination in the mid-adult strategy, we also examined cutoff ages of 30, 35, and 40 years.
Cost-effectiveness ratios
We calculated the incremental cost per quality-adjusted life year (QALY) gained by mid-adult vaccination according to the following expression:
where ICER denotes incremental cost-effectiveness ratio, NetCost denotes vaccination costs minus medical costs averted by vaccination, Q denotes QALYs gained, and the subscripts “midadult” and “comparison” refer to the vaccination strategies we examined [8].
Perspective and scope of analysis
We used a health care sector perspective and included direct medical costs associated with HPV vaccination and HPV-associated health outcomes without regard to who incurs these costs. We included the following health outcomes caused by HPV when estimating the medical costs averted and QALYs saved by HPV vaccination: anogenital warts; cervical intraepithelial neoplasia (CIN); juvenile-onset and adult-onset recurrent respiratory papillomatosis (RRP); cancers in females (cervical, vaginal, vulvar, anal, and oropharyngeal); and cancers in males (anal, oropharyngeal, and penile).
Model overview
We applied a deterministic, dynamic mathematical model of 9vHPV vaccination; we describe this model briefly in this paper and provide details in the Technical Appendix and in previous applications of the model [10]. In this paper, the brief description focuses on how the model assesses reductions in health outcomes associated with HPV 16, as the model uses an analogous approach for the nine HPV vaccine types (6, 11, 16, 18, 31, 33, 45, 52, and 58).
The model is stratified by sex and by year of age from 8 to 99 years old. For each birth cohort over time, the model estimates the percentage of people who fall into each of the following four compartments: (1) Not vaccinated, never acquired HPV 16; (2) Vaccinated, never acquired HPV 16; (3) Not vaccinated, ever acquired HPV 16; or (4) Vaccinated, ever acquired HPV 16.
We describe our model as “simplified” because it incorporates the following three simplifications not typically found in other published HPV models [11–13]. First, in our model, cervical cancer screening is assumed to occur but is not explicitly modeled. Second, we use a simplified approach to approximate HPV transmission dynamics, in which annual, age- and sex-specific probabilities of HPV acquisition are adjusted each year according to the cumulative reduction in HPV acquisition in the population. Third, we do not model natural history of HPV infection. Instead, incidence of HPV-associated health outcomes is assumed to remain constant over time in the absence of HPV vaccination. Vaccine-attributable reductions in age- and sex-specific incidence of HPV 16-associated health outcomes each year are approximated based on the vaccine-attributable, age- and sex-specific reductions in cumulative lifetime HPV 16 acquisition as of the given year.
We assumed that everyone vaccinated would complete a full vaccine series, with initiation and completion occurring in the same calendar year. We further assumed people vaccinated through age 14 years received a 2-dose series, while people initiating vaccination at age ≥ 15 years received a 3-dose series, as recommended by ACIP. This simplifying assumption of 100% vaccine series completion allows for easier interpretation of our results regarding the cost-effectiveness of mid-adult vaccination.
Parameter values
Assumptions regarding vaccine efficacy, cost per series, and coverage are summarized in Table 1. We assumed the annual probability of being vaccinated, among adults not previously vaccinated, was 2.6% and 1.9% for women and men, respectively, aged ≥ 19 years (up through the applicable recommended age). These uptake assumptions were selected based on the following rationale. Under a 2.6% annual probability of vaccination for women aged ≥ 19 years, average 3-dose coverage among females aged 19 through 26 years who were not vaccinated prior to age 19 years would be 11.0%, which is consistent with estimated vaccine uptake of 11.8% among females aged 19 through 26 years who were not vaccinated prior to age 19 years, as of 2014 [14]. The 1.9% annual probability of vaccination for men aged ≥ 19 years was selected such that the rate of vaccine uptake among adult men relative to male adolescents would be consistent with that of adult women relative to female adolescents in our model.
Table 1.
Vaccine-related model parameter values: HPV vaccine efficacy, cost, and coverage assumptions
| Vaccine characteristic | Lower bound | Base case | Upper bound |
|---|---|---|---|
|
| |||
| Efficacy and cost | |||
| Type-specific vaccine efficacy [27] | 85% | 95% | 100% |
| Cost of 3-dose series including administrationa | $530 | $714 | $742 |
| Annual probability of vaccination | |||
| Females, age 12 yearsb | 29.5% | 29.5% | 56.4% |
| Females, age 13–18 years | 7.7% | 12.9% | 14.3% |
| Females, age ≥ 19 years | 1.5% | 2.6% | 2.9% |
| Males, age 12 yearsb | 24.9% | 24.9% | 48.7% |
| Males, age 13–18 years | 1.7% | 9.7% | 14.2% |
| Males, age ≥ 19 years | 0.3% | 1.9% | 2.8% |
Vaccine duration of protection was assumed to be lifelong.
Annual vaccination probabilities were based on HPV vaccination coverage rates [14,28,29] as described in the Technical Appendix.
Vaccine cost per dose was assumed to be $144.18 (public cost) and $217.11 (private sector cost) based on CDC vaccine price list for adults (https://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/) as of December 16, 2018. Cost of administration per dose was assumed to be $8 public and $30 private [30]. The base case value of $714 per series was applied to adults aged ≥ 19 years and reflects a weighted average of the public and private costs, assuming the public price for 10% of recipients. For people aged < 19 years, we assumed a cost per 3-dose series (including administration) of $636 (range: $530 - $742), based on the average of the public price ($168.10) and the private price ($217.11) per dose (from the CDC vaccine price list for pediatric vaccines) and assuming the public price for 50% of recipients. Cost of a 2-dose series was assumed to be two-thirds that of a 3-dose series and was applied for those initiating vaccination prior to age 15 years.
For simplicity, vaccination at age 12 years in our model incorporates vaccination occurring through age 12 years. This simplification has no major effect on our results as we assume no HPV acquisition occurs prior to age 13 years.
For all parameters, the Technical Appendix provides a complete list of all values we applied in the model, along with documentation of references and assumptions. As many of these parameters have age- and sex-specific values, listing all parameter values in a single table in this manuscript is not practical. Instead, for illustrative purposes, Table 2 provides selected parameter values for a selected age group and sex: women aged 40 through 44 years.
Table 2.
Selected examples of parameter values used in model: Incidence, lifetime cost per case, and number of quality-adjusted life years (QALYs) lost per HPV-associated health outcome for women aged 40 through 44 yearsa
| Health outcome | Annual incidence per 100,000 in absence of vaccination program, base case value (range) | Lifetime cost per case, base case value (range) | Number of QALYs lost per case, base case value (range) |
|---|---|---|---|
|
| |||
| Anogenital warts | 108.0 (81.5–139.0) | $860 ($430–$2,790) | 0.024 (0.008–0.100) |
| CIN 1 | 190.0 (105.0–308.0) | $1,390 ($960–$1,810) | 0.007 (0.000–0.105) |
| CIN 2/3 | 100.0 (52.0–163.0) | $2,560 ($1,070–$4,150) | 0.010 (0.000–0.115) |
| Adult-onset RRP | 0.3 (<0.1–0.9) | $67,200 ($32,300–$433,700) | 0.47 (0.15–3.43) |
| Cervical cancer | 14.8 (14.4–15.1) | $72,800 ($43,500–$82,500) | 4.80 (4.16–6.61) |
| Anal cancer | 1.3 (1.2–1.4) | $93,600 ($53,000–$134,100) | 5.19 (3.64–7.95) |
| Vaginal cancer | 0.3 (0.2–0.3) | $116,500 ($30,300–$145,200) | 6.01 (3.86–9.46) |
| Vulvar cancer | 1.6 (1.5–1.8) | $51,400 ($26,400–$59,700) | 3.69 (2.43–6.34) |
| Oropharyngeal cancer | 0.9 (0.8–1.0) | $126,500 ($65,400–$146,900) | 7.07 (5.81–9.25) |
This table includes selected parameter values for women aged 40 through 44 years. See the Technical Appendix for a complete description of all model parameter values, ranges, and sources.
Costs are reported in third quarter 2018 U.S. dollars. CIN: cervical intraepithelial neoplasia. RRP: recurrent respiratory papillomatosis.
Cancer incidence assumptions in the absence of HPV vaccination were based on data from CDC’s National Program of Cancer Registries (NPCR) and NCI’s Surveillance, Epidemiology, and End Results (SEER) program for 2006–2010, which cover approximately 94.8% of the U.S. population. As described in the Technical Appendix, multiple sources were used for the incidence of the other health outcomes [31–35], costs [31,36–41], and quality of life assumptions [42-46].
Sensitivity analyses
We examined how the cost-effectiveness of mid-adult HPV vaccination varied when modifying key model assumptions. We first conducted one-way sensitivity analyses to see how the cost-effectiveness of mid-adult HPV vaccination would change when varying the following seven sets of parameter values one at a time: annual age- and sex-specific probabilities of acquiring HPV in the absence of vaccination; cost of vaccination; vaccine efficacy; lifetime medical treatment cost per case of each HPV-associated health outcome; number of QALYs lost per case of each health outcome; incidence rates of the health outcomes in the absence of HPV vaccination; and percentages of the health outcomes attributable to vaccine-type HPV. For the HPV acquisition probabilities, we explored an alternate scenario in which we increased the acquisition probabilities for ages ≥ 30 years (briefly, rather than declining by about 75% from age 30 years through age 60 years as in the base case, the annual, type-specific probabilities of HPV acquisition were held constant from age 30 years through age 45 years and declined by about 25% from age 45 years through age 60 years; see Technical Appendix). For the other parameter sets noted above, we conducted one-way sensitivity analyses by varying each parameter set one at a time, to its lower bound and upper bound values, while holding all other parameters constant at their base case values.
We also conducted multi-way probabilistic sensitivity analyses to examine how the cost-effectiveness of mid-adult 9vHPV vaccination would change when the following four sets of parameter values were varied simultaneously: medical treatment costs per case of each HPV-associated health outcome, number of QALYs lost per case of each health outcome, incidence rates of the health outcomes in the absence of HPV vaccination, and percentages of the health outcomes attributable to vaccine-type HPV. Further, in each simulation, our probabilistic sensitivity analyses also allowed for the possibility that the model under- or over-estimated the number of health outcomes averted by vaccination by up to 25%. We summarized the results of the probabilistic sensitivity analyses by (1) calculating the 2.5th and 97.5th percentiles of the estimated cost per QALY gained by mid-adult vaccination across the 10,000 simulations and (2) constructing cost-effectiveness acceptability curves showing the percentage of the simulations in which the estimated cost per QALY gained by mid-adult vaccination fell below a given threshold ranging from $0 to $1,000,000.
Additional analyses: Exploratory study questions
We performed three additional analyses to examine how our results might change when (1) applying a different comparison strategy, (2) accounting for potential trends in HPV-associated cancers, and (3) examining a large-scale, one-year mid-adult vaccination program. In the first additional analysis, we used a comparison strategy of vaccinating people aged 12 through 26 years, which differed slightly from our main analysis in which the comparison strategy was vaccination of females aged 12 through 26 years and males aged 12 through 21 years.
Our model assumes that in the absence of HPV vaccination, incidence of HPV-associated cancers would be constant over time at pre-vaccine era incidence rates. In the second additional analysis, we examined an extreme scenario in which cancer incidence in the absence of HPV vaccination was assumed to follow recent trends. Specifically, we assumed that cancer incidence rates for all age groups would have the following annual percentage changes in all 100 years of our model: cervical: −1.6%; vulvar: 1.3%; vaginal: −0.6%; anal (women): 2.9%; oropharyngeal (women): 0.8%; anal (men): 2.1%; oropharyngeal (men): 2.7%, based on reported trends from 1999–2015 in the United States [15].
In the third additional analysis, we examined the cost-effectiveness of a one-year mid-adult vaccination strategy in which 50% of unvaccinated mid-adults (females aged 27 through 45 years and males aged 22 through 45 years) were vaccinated in a single year, with no mid-adult vaccination in subsequent years. We assumed this one-year program would impose no additional costs other than the cost of vaccine and administration as assumed for mid-adult vaccination in our main analyses. We also examine different coverage assumptions for this one-year program: a lower coverage scenario in which 2.6% and 1.9% of unvaccinated mid-adult females and males were vaccinated, respectively, and a higher coverage scenario in which 100% of all unvaccinated mid-adults were vaccinated.
Results
Health impact of vaccination
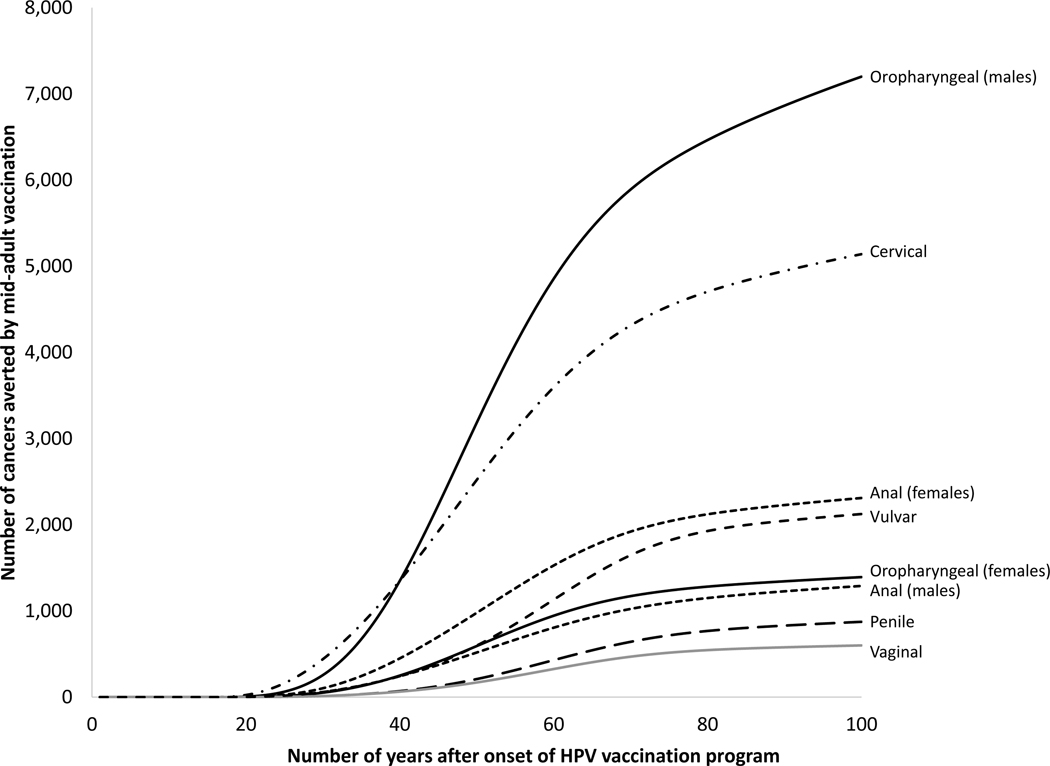
Under base case assumptions, the comparison strategy (vaccinating females aged 12 through 26 years and males aged 12 through 21 years) was estimated to avert almost 1.5 million HPV-associated cancers over 100 years compared to no vaccination, including about 1 million cancers in females and about 0.5 million cancers in males (Table 3). The mid-adult vaccination strategy, expanding vaccination through age 45 years, would increase the total number of cancers averted by an estimated 20,934 additional HPV-associated cancers over 100 years versus the comparison strategy, including about 12,000 cancers in females and 9,000 cancers in males. Oropharyngeal and cervical cancer accounted for most of the cancer cases averted by mid-adult vaccination (Figure 1).
Table 3.
Base case estimates of health impact: Estimated number of HPV-associated cancers averted by vaccination over 100-year vaccination program
| Subgroup examined | Comparison strategy (vaccination of females aged 12–26 years, and males aged 12–21 years) | Mid-adult vaccination strategy (vaccination of females and males aged 12–45 years) | Incremental benefit of mid-adult vaccination |
|---|---|---|---|
|
| |||
| Cancers averted in females | 957,776 | 969,345 | 11,568 |
| Cancers averted in males | 489,776 | 499,142 | 9,366 |
| Total cancers averted | 1,447,552 | 1,468,486 | 20,934 |
The first column of results shows the number of cancers averted by the comparison scenario of routine 9vHPV vaccination of 12-year-old females and males with catch-up vaccination through age 26 years for females and 21 years for males (versus no vaccination). The second column of results shows the number of cancers averted when adding mid-adults to the vaccination program (routine 9vHPV vaccination of 12-year-old females and males with catch-up vaccination through age 45 years for females and males versus no vaccination). The third column of results shows the incremental benefits of mid-adult vaccination (vaccination through age 45 years) and was calculated as the number of cancers averted by the mid-adult vaccination scenario minus the number of cancers averted by the comparison scenario. These outcomes were not discounted.
Figure 1:
Estimated number of cancer cases averted by mid-adult vaccination through age 45 years: The cumulative number of cancer cases averted by vaccination of females and males aged 12–45 years vs. the comparison strategy (vaccination of females aged 12–26 years, and males aged 12–21 years), by cancer site (oropharyngeal, cervical, anal, vaginal and vulvar, and penile) over the 100-year vaccination program. The mid-adult vaccination strategy was assumed to be implemented in year 12 of the vaccine program (i.e., in the mid-adult vaccination strategy, the cutoff age of catch-up vaccination was set at 26 years for women and 21 years for men in years 1 through 11 of the vaccination program and was later increased to age 45 years in years 12 through 100). These outcomes were not discounted.
Vaccination costs, medical costs averted, and QALYs gained by vaccination
The comparison strategy was estimated to result in $44,306 million in vaccination costs, $33,786 million in medical costs averted, and a gain of 1.15 million QALYs (Table 4), versus no vaccination. The mid-adult vaccination strategy was estimated to increase vaccination costs to $57,662 million, to increase the averted medical costs to $34,307 million, and to increase the number of QALYs gained to 1.17 million.
Table 4.
Base case estimates of cost-effectiveness: Discounted incremental costs, incremental number of quality-adjusted life years (QALYs) gained, and cost-effectiveness (incremental cost per QALY gained) of 9vHPV vaccination strategies
| Item estimated | Comparison strategy (vaccination of females aged 12–26 years, and males aged 12–21 years) | Mid-adult vaccination strategy (vaccination of females and males aged 12–45 years) |
|---|---|---|
|
| ||
| Vaccination costs ($ millions) | 44,306 | 57,662 |
| Direct medical costs averted ($ millions) | 33,786 | 34,307 |
| Number of QALYs gained | 1,146,255 | 1,165,901 |
| Incremental cost ($ millions) | 10,520 | 12,835 |
| Incremental gain in QALYs | 1,146,255 | 19,645 |
| Incremental cost per QALY gained ($/QALY) | 9,200 | 653,300 |
QALY: quality-adjusted life year. All future costs and QALYs were discounted at 3% annually. For both columns of results, the first three rows show the vaccination costs, direct medical costs averted, and QALYs gained, compared to no vaccination. The cost per QALY gained by the comparison strategy was calculated versus no vaccination, and the incremental cost ($10,520 million) reflects vaccination costs ($44,306 million) minus averted direct medical costs ($33,786 million). The cost per QALY gained by the mid-adult vaccination strategy was calculated versus the comparison strategy, and the incremental cost ($12,835 million) reflects the additional vaccination costs ($57,662 million minus $44,306 million) minus the additional direct medical costs averted ($34,307 million minus $33,786 million). Incremental cost-effectiveness ratios have been rounded to the nearest $100.
Costs are reported in third quarter 2018 U.S. dollars. When updated to second quarter 2020 U.S. dollars, the cost effectiveness ratios shown in this table changed from $9,200 to $9,500 and from $653,300 to $678,700.
Cost-effectiveness of vaccination
The comparison strategy was estimated to cost $9,200 per QALY gained, compared to no vaccination (Table 4). Mid-adult vaccination was estimated to cost $653,300 per additional QALY gained, versus the comparison strategy. When we examined mid-adult vaccination by smaller age increments, the incremental cost per QALY gained increased as the upper age cutoff for mid-adult vaccination increased (Table 5). For example, expanding vaccination through age 30 years was estimated to cost $587,600 per QALY gained versus the comparison strategy, whereas expanding vaccination from age 40 years to age 45 years was estimated to cost $781,000 per QALY gained.
Table 5.
Base case estimates of costs and benefits of 9vHPV vaccination strategies by age group
| Sex and ages vaccinated, in years | Vaccination costs ($ millions) | Medical costs averted ($ millions) | Number of QALYs gained | Incremental cost per QALY gained (versus preceding strategy)a |
|---|---|---|---|---|
|
| ||||
| Females 12–26 and males 12–21 | $44,306 | $33,786 | 1,146,255 | $9,200 |
| Females and males, 12–30 | $47,875 | $33,942 | 1,152,064 | $587,600 |
| Females and males, 12–35 | $50,873 | $34,062 | 1,156,713 | $619,000 |
| Females and males, 12–40 | $54,154 | $34,189 | 1,161,560 | $650,600 |
| Females and males, 12–45 | $57,662 | $34,307 | 1,165,901 | $781,000 |
For the comparison strategy shown in the top row (vaccination of females aged 12 through 26 years and males aged 12 through 21 years), incremental cost-effectiveness was calculated as compared to no vaccination. For all other strategies, incremental cost-effectiveness was calculated as compared to the preceding strategy (e.g., vaccination of females and males aged 12 through 45 years was compared to the strategy of vaccination of females and males aged 12 through 40 years). Incremental cost-effectiveness ratios (right-hand column) have been rounded to the nearest $100.
Costs are reported in third quarter 2018 U.S. dollars. When updated to second quarter 2020 U.S. dollars, the cost effectiveness ratios shown in this table changed from $9,200 to $9,500, from $587,600 to $610,500, from $619,000 to $643,000, from $650,600 to $675,900, and from $781,000 to $811,300.
Sensitivity analyses
In one-way sensitivity analyses, the incremental cost per QALY gained for the mid-adult vaccination strategy through age 45 years varied from $324,600 to $789,300 (Table 6), compared to $653,300 in the base case. Similarly, the cost per QALY gained by the mid-adult vaccination strategy through age 30 years varied from $370,900 to $715,000, compared to $587,600 in the base case. The cost-effectiveness of mid-adult vaccination was most favorable when applying the upper bound values for the number of QALYs lost per health outcome, in the lower coverage scenario, in the scenario of increased HPV incidence for ages ≥ 30 years, and when applying the lower vaccine price per series. The cost-effectiveness of mid-adult vaccination was least favorable when applying the lower bound values for the number of QALYs lost per health outcome and when applying the lower bound value for the percentage of each health outcome attributable to vaccine-type HPV.
Table 6.
Sensitivity analyses: Incremental cost per quality-adjusted life year (QALY) gained by mid-adult HPV vaccination through age 30 years or through age 45 years when varying one or more parameter values
| Parameter varied | Cost per QALY gained | |
|---|---|---|
|
| ||
| Vaccination through age 30 yearsa | Vaccination through age 45 yearsb | |
|
| ||
| Base case (no parameters varied) | $587,600 | $653,300 |
| One-way sensitivity analyses | ||
| More QALYs lost per health outcome | $370,900 | $438,500 |
| Lower coverage scenario | $397,400 | $531,600 |
| Increased HPV incidence for ages ≥ 30 yearsc | $402,800 | $324,600 |
| Lower vaccine price per series ($530) | $429,300 | $478,100 |
| Higher % of disease due to HPV vaccine types | $481,000 | $532,400 |
| Higher incidence rates of health outcomes | $542,300 | $610,800 |
| Higher medical cost per health outcome | $575,000 | $642,600 |
| Higher coverage scenario | $576,900 | $623,300 |
| Lower vaccine efficacy (85%) | $586,200 | $690,900 |
| Higher vaccine efficacy (100%) | $589,700 | $636,900 |
| Lower medical cost per health outcome | $600,300 | $665,800 |
| Higher vaccine price per series ($742) | $611,700 | $680,000 |
| Lower incidence rates of health outcomes | $626,200 | $692,800 |
| Lower % of disease due to HPV vaccine types | $707,900 | $789,400 |
| Fewer QALYs lost per health outcome | $715,000 | $780,300 |
| Multi-way sensitivity analyses | ||
| 2.5th and 97.5th percentiles of simulations | $360,800 to $926,600 | $413,300 to $1,026,300 |
“Vaccination through age 30 years” column shows the cost-effectiveness of a mid-adult vaccination program through age 30 years (vaccination of females and males aged 12 through 30 years, compared to vaccination of females aged 12 through 26 years and males aged 12 through 21 years).
“Vaccination through age 45 years” column shows the cost-effectiveness of a mid-adult vaccination program through age 45 years (vaccination of females and males aged 12 through 45 years, compared to vaccination of females aged 12 through 26 years and males aged 12 through 21 years).
In the base case, annual type-specific HPV acquisition probabilities in the absence of vaccination decline by about 75% from age 30 years through age 60 years. In the “Increased HPV incidence for ages ≥ 30 years” scenario, HPV acquisition probabilities in the absence of vaccination do not decline from age 30 years through age 45 years and decline by 25% from age 45 years through age 60 years. For example, in the base case, the annual probability of acquiring HPV 16 in the absence of vaccination is assumed to be 1.41% at age 30 years, 0.55% at age 40 years, 0.41% at age 50 years, and 0.34% at age 60 years. In the scenario of increased incidence for ages ≥ 30 years, the annual probability of acquiring HPV 16 in the absence of vaccination is assumed to be 1.41% at age 30 years, 1.41% at age 40 years, 1.29% at age 50 years, and 1.06% at age 60 years. See the Technical Appendix for more details.
The results shown in this table for the low and high coverage scenarios were obtained when varying the vaccine uptake rates for all age groups. The cost per QALY gained by vaccination through age 30 years and through age 45 years was $506,100 and $582,700, respectively, when applying lower uptake rates for mid-adults only and $601,600 and $663,900, respectively, when applying higher uptake rates for mid-adults only.
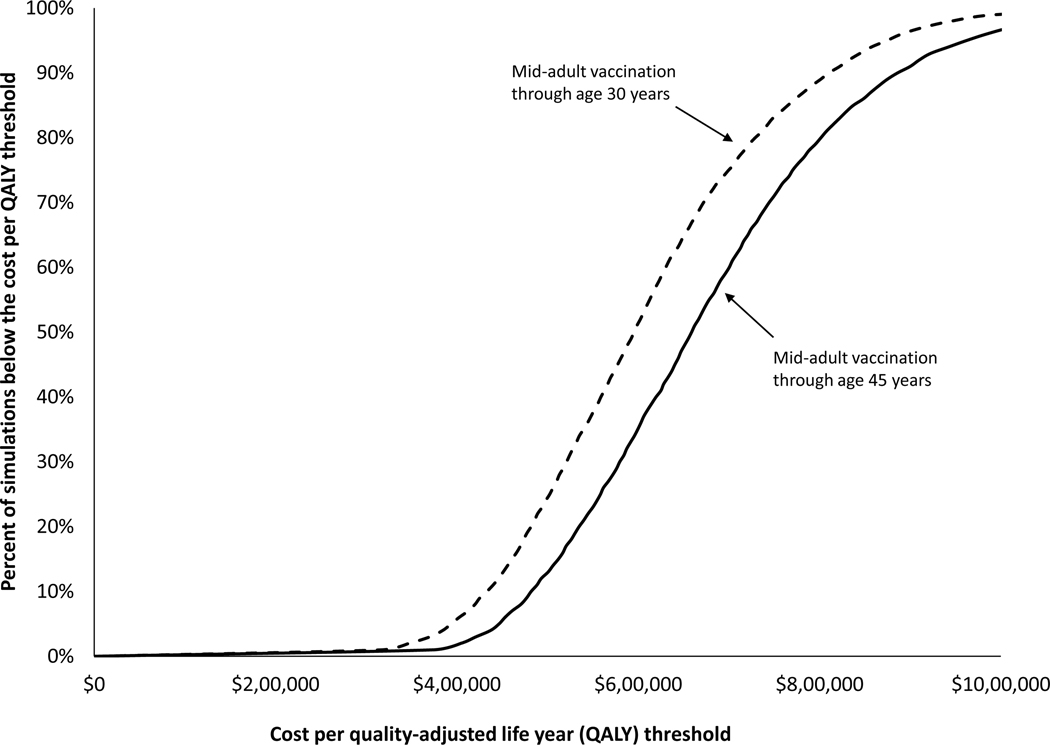
In multi-way sensitivity analyses, the cost per QALY gained by the mid-adult vaccination strategy through age 45 years ranged from $413,300 to $1,026,300 in 95% of the simulations (Table 6, Figure 2). The cost per QALY gained by the mid-adult vaccination strategy through age 30 years ranged from $360,800 to $926,600 in 95% of the simulations.
Figure 2 :
Cost-effectiveness acceptability curves: percentage of the simulations in which the incremental cost per QALY gained by mid-adult vaccination was at or below a given cost per QALY threshold. Results are shown for two mid-adult vaccination strategies: vaccination of females and males aged 12–30 years (dotted line) and aged 12–45 years (solid line), vs. the comparison strategy (vaccination of females aged 12–26 years, and males aged 12–21 years).
Additional analyses: Exploratory study questions
When we changed the comparison strategy to include catch-up vaccination through age 26 years for females and males (rather than through age 26 years for females and age 21 years for males), the cost per QALY gained by the mid-adult vaccination strategy through age 30 years (vs. the comparison strategy) was $563,500 (results not shown), compared to $587,600 (Table 5) in the main analysis. When we explored an extreme scenario of trends in HPV-associated cancer incidence throughout the 100-year model, the cost per QALY gained by mid-adult vaccination through age 45 years was $288,680 (results not shown), compared to $653,300 (Table 4) in the main analysis in which cancer incidence rates in the absence of vaccination were assumed to be constant over the 100-year model. When we examined a one-year mid-adult vaccination program, the cost per QALY gained by mid-adult vaccination through age 45 years was $348,200 at 50% coverage, $330,500 at 1.9% and 2.6% coverage for males and females, respectively, and $360,500 at 100% coverage (results not shown), compared to $653,300 in the main analysis of an ongoing mid-adult vaccination program under base case coverage assumptions. The Supplemental Appendix provides additional results.
Discussion
The cost-effectiveness of mid-adult vaccination is much less favorable than that of the comparison strategy of routine vaccination at ages 11 or 12 years with catch-up vaccination through age 26 years for women and age 21 years for men. A key theme across published HPV vaccine models in high-income countries is that cost-effectiveness of HPV vaccination usually becomes less favorable as age at vaccination increases beyond the teenage years [16]. This general theme was reflected in the health economic data presented to ACIP in June 2019, in which ours was one of the four out of five models reviewed in which the estimated cost per QALY gained by vaccinating adults through age 30 or 45 years exceeded $300,000. The highest cost per QALY estimates of the five models were from the HPV-ADVISE model, in which vaccination through age 30 and 45 years was estimated to cost about $830,000 and $1.5 million per QALY gained, respectively [11]. Results from these dynamic transmission models were consistent with a previous study based on a static model that found that adding HPV vaccination to existing cervical cancer screening (combined cytology and HPV DNA testing) cost about $100,000 to over $400,000 per QALY gained (in 2006 U.S. dollars) for 35- and 45-year-old women, depending on the frequency of screening [17].
Age- and sex- and HPV type-specific annual probabilities of acquiring HPV in the absence of the vaccination program are an important uncertainty in our model. If HPV incidence among adults aged ≥30 years, relative to those < 30 years, is greater than we assumed, then the cost per QALY gained by vaccination of adults through age 45 years could be lower than estimated in our base case analysis. For example, when we examined an alternate scenario in which we substantially increased the annual probabilities of HPV acquisition among those aged ≥ 30 years, the cost per QALY gained by extending vaccination through age 45 years was $324,600, compared to $653,300 when using our base case annual probabilities of HPV acquisition.
Because assumptions about HPV acquisition by age are key drivers of our results, it is important to confirm these assumptions are consistent with available data. The base case HPV acquisition probabilities we applied are based on published models of the natural history of HPV infection and cervical cancer [18,19] and reflect the high incidence of HPV observed among adolescents and young adults. However, there is considerable range across studies in estimates of incidence rates of this common sexually transmitted infection [20–23]. Thus, our findings should be considered in light of the uncertainty in the age-specific HPV acquisition probabilities applied in our model. Further, the annual probabilities of HPV acquisition that we apply are based on genital acquisition. Our estimates of the impact of HPV vaccination on anal cancer and oropharyngeal cancer will therefore be biased, to the extent that the relative age distribution of genital infection differs from that of anal and oral infection. For example, if the average age of acquiring HPV is greater for anal infection than for genital infection, this difference would cause an underestimation of the impact of mid-adult vaccination on anal cancer in our model.
Mid-adult vaccination strategies are generally more cost-effective when the vaccine is assumed to provide protection against re-infection with previously-acquired but cleared HPV vaccine types [24–25]. An important limitation of our model is that it cannot specifically account for the possibility that vaccination could provide protection against re-infection. However, in the version of the model used for this study, we included a modification in the application of annual HPV acquisition probabilities that helps to mitigate the bias arising from not explicitly accounting for re-infection (see Technical Appendix section 1.2.1 for details).
Because our model uses a simple characterization of HPV transmission dynamics and does not explicitly model cervical cancer screening, our model is not well-suited for examining the potential contribution of mid-adult vaccination to cervical cancer elimination or HPV eradication efforts; more complex models are required [26]. However, our results do suggest that mid-adult vaccination could be relatively more efficient as a short-term, time-limited program rather than ongoing, a finding consistent with the HPV-ADVISE model [11]. With ongoing mid-adult vaccination, as persons vaccinated at younger ages become mid-adults, unvaccinated mid-adults would be expected to have a higher degree of protection from indirect (“herd”) effects of the comparison strategy (vaccination through age 26 years for women and age 21 years for men), making the cost-effectiveness of mid-adult vaccination less favorable over time [7].
Our analysis is subject to several limitations in addition to the three key simplifying features of our model noted in our description of the methods. For example, our model does not stratify beyond age and sex, and thus does not account for heterogeneity in risk behavior or for special populations such MSM who are at higher risk than other men for HPV infections and related diseases. Also, our model assumes lifelong duration of vaccine protection and is unable to examine scenarios of waning vaccine protection. Our model also assumes constant cancer incidence rates in the absence of vaccination. Our cost-effectiveness estimates were more favorable for HPV vaccination in general (including mid-adult vaccination) when we assumed recent trends in cancer incidence would continue.
Although our cost-effectiveness estimates are subject to considerable uncertainty, we consistently found that HPV vaccination of mid-adults is much less cost-effective than HPV vaccination of adolescents and young adults, across all scenarios we examined. Our results are generally consistent with those of most other models of the cost-effectiveness of HPV vaccination. Overall, the existing modeling evidence provides general support for the June 2019 ACIP recommendations, which call for catch-up HPV vaccination for all persons through age 26 years, but not for all mid-adults aged 27 through 45 years.
Supplementary Material
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- [1].Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human papillomavirus vaccination for adults: Updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2019;68(32):698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Smith JC, Hinman AR, Pickering LK. History and evolution of the Advisory Committee on Immunization Practices--United States, 1964–2014. MMWR Morb Mortal Wkly Rep. 2014;63(42):955–958. [PMC free article] [PubMed] [Google Scholar]
- [3].Markowitz LE, Gee J, Chesson H, Stokley S. Ten years of human papillomavirus vaccination in the United States. Acad Pediatr. 2018;18(2S):S3–S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014;63(RR-05):1–30. [PubMed] [Google Scholar]
- [5].Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination - updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65(49):1405–1408. [DOI] [PubMed] [Google Scholar]
- [6].O’Leary ST, Maldonado YA, Kimberlin DW. Update From the Advisory Committee on Immunization Practices. J Pediatric Infect Dis Soc. 2019;7(2):93–99. [DOI] [PubMed] [Google Scholar]
- [7].Chesson H. Overview of health economic models for HPV vaccination of mid-adults. Presented at the Advisory Committee on Immunization Practices meeting. Atlanta, GA: June 26, 2019. [Google Scholar]
- [8].Haddix AC, Teutsch SM, Corso PS. Prevention Effectiveness: A Guide to Decision Analysis and Economic Evaluation, 2nd edition. 2nd ed. New York: Oxford University Press; 2002. [Google Scholar]
- [9].Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG. Cost-Effectiveness in Health and Medicine: Second Edition. New York, NY: Oxford University Press; 2016. [Google Scholar]
- [10].Chesson HW, Meites E, Ekwueme DU, Saraiya M, Markowitz LE. Cost-effectiveness of nonavalent HPV vaccination among males aged 22 through 26 years in the United States. Vaccine. 2018;36(29):4362–4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Laprise JF, Chesson HW, Markowitz LE, et al. Effectiveness and cost-effectiveness of HPV vaccination through age 45 years in the United States. Ann Intern Med. 2019. doi: 10.7326/M19-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kim JJ, Goldie SJ. Cost effectiveness analysis of including boys in a human papillomavirus vaccination programme in the United States. BMJ. 2009;339:b3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Elbasha EH, Dasbach EJ. Impact of vaccinating boys and men against HPV in the United States. Vaccine. 2010;28(42):6858–6867. [DOI] [PubMed] [Google Scholar]
- [14].Williams WW, Lu PJ, O’Halloran A, et al. Surveillance of vaccination coverage among adult populations - United States, 2014. MMWR Surveill Summ. 2016;65(1):1–36. [DOI] [PubMed] [Google Scholar]
- [15].Van Dyne EA, Henley SJ, Saraiya M, Thomas CC, Markowitz LE, Benard VB. Trends in human papillomavirus-associated cancers - United States, 1999–2015. MMWR Morb Mortal Wkly Rep. 2018;67(33):918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Canfell K, Chesson H, Kulasingam SL, Berkhof J, Diaz M, Kim JJ. Modeling preventative strategies against human papillomavirus-related disease in developed countries. Vaccine. 2012;30 Suppl 5:F157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kim JJ, Ortendahl J, Goldie SJ. Cost-effectiveness of human papillomavirus vaccination and cervical cancer screening in women older than 30 years in the United States. Ann Intern Med. 2009;151(8):538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Myers ER, McCrory DC, Nanda K, Bastian L, Matchar DB. Mathematical model for the natural history of human papillomavirus infection and cervical carcinogenesis. Am J Epidemiol. 2000;151(12):1158–1171. [DOI] [PubMed] [Google Scholar]
- [19].Canfell K, Barnabas R, Patnick J, Beral V. The predicted effect of changes in cervical screening practice in the UK: results from a modelling study. Br J Cancer. 2004;91(3):530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Munoz N, Mendez F, Posso H, et al. Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. J Infect Dis. 2004;190(12):2077–2087. [DOI] [PubMed] [Google Scholar]
- [21].Apter D, Wheeler CM, Paavonen J, et al. Efficacy of human papillomavirus 16 and 18 (HPV-16/18) AS04-adjuvanted vaccine against cervical infection and precancer in young women: final event-driven analysis of the randomized, double-blind PATRICIA trial. Clin Vaccine Immunol. 2015;22(4):361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Winer RL, Hughes JP, Feng Q, Stern JE, Xi LF, Koutsky LA. Incident detection of high-risk human papillomavirus infections in a cohort of high-risk women aged 25–65 Years. J Infect Dis. 2016;214(5):665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157(3):218–226. [DOI] [PubMed] [Google Scholar]
- [24].Burger EA, Sy S, Nygard M, Kristiansen IS, Kim JJ. Too late to vaccinate? The incremental benefits and cost-effectiveness of a delayed catch-up program using the 4-valent human papillomavirus vaccine in Norway. J Infect Dis. 2015;211(2):206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Turner HC, Baussano I, Garnett GP. Vaccinating women previously exposed to human papillomavirus: a cost-effectiveness analysis of the bivalent vaccine. PLoS One. 2013;8(9):e75552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Simms KT, Steinberg J, Caruana M, et al. Impact of scaled up human papillomavirus vaccination and cervical screening and the potential for global elimination of cervical cancer in 181 countries, 2020–99: a modelling study. Lancet Oncol. 2019;20(3):394–407. [DOI] [PubMed] [Google Scholar]
- [27].Joura EA, Giuliano AR, Iversen O-E, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–723. [DOI] [PubMed] [Google Scholar]
- [28].Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(33):850–858. [DOI] [PubMed] [Google Scholar]
- [29].Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years--United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(29):784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Stoecker C, Hampton LM, Link-Gelles R, Messonnier ML, Zhou F, Moore MR. Cost-effectiveness of using 2 vs 3 primary doses of 13-valent pneumococcal conjugate vaccine. Pediatrics. 2013;132(2):e324–e332. [DOI] [PubMed] [Google Scholar]
- [31].Henk HJ, Insinga RP, Singhal PK, Darkow T. Incidence and costs of cervical intraepithelial neoplasia in a US commercially insured population. J Low Genit Tract Dis. 2010;14(1):29–36. [DOI] [PubMed] [Google Scholar]
- [32].Insinga RP, Glass AG, Rush BB. Diagnoses and outcomes in cervical cancer screening: A population-based study. Am J Obstet Gynecol. 2004;191(1):105–113. [DOI] [PubMed] [Google Scholar]
- [33].Hoy T, Singhal PK, Willey VJ, Insinga RP. Assessing incidence and economic burden of genital warts with data from a US commercially insured population. Curr Med Res Opin. 2009;25(10):2343–2351. [DOI] [PubMed] [Google Scholar]
- [34].Armstrong LR, Preston EJ, Reichert M, et al. Incidence and prevalence of recurrent respiratory papillomatosis among children in Atlanta and Seattle. Clin Infect Dis. 2000;31(1):107–109. [DOI] [PubMed] [Google Scholar]
- [35].Marsico M, Mehta V, Chastek B, Liaw KL, Derkay C. Estimating the incidence and prevalence of juvenile-onset recurrent respiratory papillomatosis in publicly and privately insured claims databases in the United States. Sex Transm Dis. 2014;41(5):300–305. [DOI] [PubMed] [Google Scholar]
- [36].Insinga RP, Glass AG, Rush BB. The health care costs of cervical human papillomavirus-related disease. Am J Obstet Gynecol. 2004;191(1):114–120. [DOI] [PubMed] [Google Scholar]
- [37].Deshmukh AA, Zhao H, Franzini L, et al. Total lifetime and cancer-related costs for elderly patients diagnosed with anal cancer in the United States. Am J Clin Oncol. 2018;41(2):121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lairson DR, Fu S, Chan W, Xu L, Shelal Z, Ramondetta L. Mean direct medical care costs associated with cervical cancer for commercially insured patients in Texas. Gynecol Oncol. 2017;145(1):108–113. [DOI] [PubMed] [Google Scholar]
- [39].Jacobson JJ, Epstein JB, Eichmiller FC, et al. The cost burden of oral, oral pharyngeal, and salivary gland cancers in three groups: commercial insurance, Medicare, and Medicaid. Head Neck Oncol. 2012;4:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lairson DR, Wu CF, Chan W, Dahlstrom KR, Tam S, Sturgis EM. Medical care cost of oropharyngeal cancer among Texas patients. Cancer Epidemiol Biomarkers Prev. 2017;26(9):1443–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fu S, Lairson DR, Chan W, Wu CF, Ramondetta L. Mean medical costs associated with vaginal and vulvar cancers for commercially insured patients in the United States and Texas. Gynecol Oncol. 2018;148(2):342–348. [DOI] [PubMed] [Google Scholar]
- [42].Drolet M, Brisson M, Maunsell E, et al. The psychosocial impact of an abnormal cervical smear result. Psychooncology. 2012;21(10):1071–1081. [DOI] [PubMed] [Google Scholar]
- [43].Drolet M, Brisson M, Maunsell E, et al. The impact of anogenital warts on health-related quality of life: a 6-month prospective study. Sex Transm Dis. 2011;38(10):949–956. [DOI] [PubMed] [Google Scholar]
- [44].Woodhall SC, Jit M, Soldan K, et al. The impact of genital warts: loss of quality of life and cost of treatment in eight sexual health clinics in the UK. Sex Transm Infect. 2011;87(6):458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jit M, Chapman R, Hughes O, Choi YH. Comparing bivalent and quadrivalent human papillomavirus vaccines: economic evaluation based on transmission model. BMJ. 2011;343:d5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.