Abstract
Background
Mitochondrial energetics are an important property of aging muscle, as generation of energy is pivotal to the execution of muscle contraction. However, its association with functional outcomes, including leg power and cardiorespiratory fitness, is largely understudied.
Methods
In the Study of Muscle, Mobility, and Aging, we collected vastus lateralis biopsies from older adults (n = 879, 70–94 years, 59.2% women). Maximal State 3 respiration (Max OXPHOS) was assessed in permeabilized fiber bundles by high-resolution respirometry. Capacity for maximal adenosine triphosphate production (ATPmax) was measured in vivo by 31P magnetic resonance spectroscopy. Leg extension power was measured with a Keiser press system, and VO2 peak was determined using a standardized cardiopulmonary exercise test. Gender-stratified multivariate linear regression models were adjusted for age, race, technician/site, adiposity, and physical activity with beta coefficients expressed per 1-SD increment in the independent variable.
Results
Max OXPHOS was associated with leg power for both women (β = 0.12 Watts/kg, p < .001) and men (β = 0.11 Watts/kg, p < .050). ATPmax was associated with leg power for men (β = 0.09 Watts/kg, p < .05) but was not significant for women (β = 0.03 Watts/kg, p = .11). Max OXPHOS and ATPmax were associated with VO2 peak in women and men (Max OXPHOS, β women = 1.03 mL/kg/min, β men = 1.32 mL/kg/min; ATPmax β women = 0.87 mL/kg/min, β men = 1.50 mL/kg/min; all p < .001).
Conclusions
Higher muscle mitochondrial energetics measures were associated with both better cardiorespiratory fitness and greater leg power in older adults. Muscle mitochondrial energetics explained a greater degree of variance in VO2 peak compared to leg power.
Keywords: Cardiorespiratory fitness, Mitochondria, Muscle, Power
Decreases in cardiorespiratory fitness and skeletal muscle contractile performance with aging contribute to loss of mobility in older adults (1,2). Loss of mobility is associated with an increased risk of disability and the need for care (3). Identification of therapeutic targets and development of pharmacological as well as nutritional and lifestyle interventions could prolong functional health with aging. To date, the majority of interventions have focused on exercise, which have proven to be effective at preventing age-related disability and declines in physical function (4–6). Despite these promising findings, the biological mechanisms that underlie age-related changes in muscle and physical function remain unclear.
Mitochondria have long been suggested as key drivers of many age-related phenotypes (7), particularly in energetically demanding tissues (8,9), and especially in skeletal muscle (10–15). Generation of adenosine triphosphate (ATP) by mitochondrial oxidative phosphorylation (OXPHOS) provides the key energy substrate for muscle contraction and locomotion. Cross-sectional studies of old and young individuals have found lower levels of skeletal muscle mitochondrial content, function, and efficiency with older ages (16–20). However, only a portion of these studies have examined whether mitochondrial function is associated with measures of physical performance. Over 2 decades ago, 31P magnetic resonance spectroscopy (31P MRS) was used to determine that the in vivo capacity for maximal adenosine triphosphate production (ATPmax) was lower in older (N = 40, 68.8 ± 5.9 years) than younger adults (N = 9, 38.8 ± 7.9 years) (12). Within the same cohort, VO2 peak was also lower in the older group, and higher ATPmax correlated with higher VO2 peak values among older adults with decreased muscle volume (13). More recently, in a small group of adults ranging from 24 to 91 years of age (N = 38), mitochondrial energetics were assessed in vitro by high-resolution muscle fibers respirometry in permeabilized muscle fibers (PMF) and in vivo by 31P MRS, and both measures had age-associated declines. Higher values of both muscle mitochondrial measures also correlated with higher VO2 peak (21). Taken together, this study, along with others, implies that skeletal muscle mitochondrial energetics play an important role for both cardiorespiratory fitness (15,21,22). However, fewer studies have examined the relationship between mitochondrial energetics and other aspects of muscle function, including muscle contractile performance (21,23,24). In summary, these studies collectively show that mitochondrial energetics decline with age, and emerging evidence indicates that this decline likely contributes to poorer muscle and physical function in older adults.
Despite initial evidence identifying mitochondrial energetics as an important biologic determinant of aging-related outcomes, an inherent challenge in interpreting these findings is the methodologic and statistical variability across studies. Indeed, many of these studies were small, and thus, they were generally under-powered. Other studies did not include large enough samples of older people to assess the contributions of skeletal muscle energetics in aging muscle performance or fitness. Prior work has also been hampered by lack of gender diversity and was therefore unable to assess relationships in women and men separately. Lastly, several studies did not account for adiposity and physical activity, which are important confounders shown to be strongly associated with declines in muscle mitochondrial energetics (25,26), strength, and endurance in aging skeletal muscle.
The Study of Muscle, Mobility and Aging (SOMMA) (27) was designed to extend these prior findings in a larger cohort and to better understand whether mitochondrial function is associated with muscle power or cardiorespiratory fitness while controlling for important confounders such as demographic and behavioral characteristics (gender, race, and physical activity). In SOMMA, we utilized 31P MRS, the gold standard measure of in vivo muscle mitochondrial energetics, to measure ATPmax is known to be affected by mitochondrial complex activities, including oxygen consumption, efficiency, and Ca2+ handling (12). We also assessed muscle mitochondrial energetics by high-resolution respirometry in PMF, a complementary approach that interrogates mitochondrial OXPHOS capacity at the myocellular level in vitro. This method enables the muscle sample to be assessed without limitations of physiological factors including supplies of substrates and oxygen saturation. Here, we test the hypothesis that higher in vitro and in vivo measures of skeletal muscle mitochondrial energetics are associated with greater leg muscle power generation and cardiorespiratory fitness in older women and men. Given that muscle power is determined primarily by individual muscle fiber contractile properties and neuromuscular function rather than oxidative capacity (28), we also hypothesized that muscle mitochondrial energetics may explain more of the variance in cardiorespiratory fitness than leg extension power.
Method
Study Cohort and Recruitment
Participants aged 70 and older were recruited for the SOMMA (https://www.sommastudy.com/) from April 2019 to December 2021 across 2 clinical sites; the University of Pittsburgh and Wake Forest University School of Medicine (27). Participants were eligible for this study if willing and able to complete a muscle tissue biopsy and MRS. For a list of abbreviated terminology used in this report, refer to Supplementary Table 1. Individuals who reported an inability to walk ¼ mile or climb a flight of stairs, an active malignancy, or advanced chronic disease (eg, severe heart or lung disease that would prevent walking ¼ mile, severe kidney disease on dialysis, Parkinson’s disease, dementia) were excluded as were those with medical contraindication to biopsy or MRS such as chronic anticoagulation or incompatible metal implants. In-person assessments ensured that all were able to walk 400 m at enrollment. All participants provided written informed consent, and the study was approved by the Western Institutional Review Board (20180764) for all participating sites.
General Study Cohort Measurements
SOMMA baseline assessments were completed over the course of several days and included self-reported gender, race, ethnicity, health history, medications, diet, and physical activity. Height was measured on stadiometers and weight on digital scales. VO2 peak was measured with cardiopulmonary exercise testing (CPET). ATPmax and body composition were measured with MRS. Whole-body MR scans were processed with Dixon water-fat imaging using AMRA Researcher (AMRA Medical AB, Linköping, Sweden) to calculate abdominal subcutaneous and visceral adipose tissue (ASAT and VAT) volumes (29–31). ASAT volume includes the abdomen from the top of the femoral head to the top of the thoracic vertebra T9, and VAT volume includes the abdominal cavity, excluding the adipose outside the abdominal skeletal muscles and the cavity/posterior of the spine and back muscles. A percutaneous biopsy of the vastus lateralis was performed in the fasting and rested state. Community Healthy Activities Model Program for Seniors (CHAMPS) questionnaire (32,33) was used to calculate total energy expenditure per week (cal/wk) in all physical activities.
Skeletal Muscle Biopsy Collection and Processing
Percutaneous biopsies were collected from the middle region of the musculus vastus lateralis under local anesthesia using a Bergstrom canula with suction (34). Following this, the specimen was blotted dry of blood and interstitial fluid and dissected free of any connective tissue and intermuscular fat. Approximately 20 mg of the biopsy specimen was placed into ice-cold BIOPS media (10 mM Ca–EGTA buffer, 0.1 M free calcium, 20 mM imidazole, 20 mM taurine, 50 mM potassium 2-[N-morpholino]-ethanesulfonic acid, 0.5 mM dithiothreitol, 6.56 mM MgCl2, 5.77 mM ATP, and 15 mM phosphocreatine [PCr], pH 7.1) for respirometry, as previously described (22). Myofiber bundles of approximately 2–3 mg were teased apart using a pair of sharp tweezers and a small Petri dish containing ice-cold BIOPS media. After mechanical preparation, myofiber bundles were chemically permeabilized for 30 minutes with saponin (2 mL of 50 µg/mL saponin in ice-cold BIOPS solution) placed on ice and a rocker (25 rpm). Myofiber bundles were washed twice (10 minutes each) with ice-cold MiR05 media (0.5 mM ethylenediaminetetraacetic acid, 3 mM MgCl2·6H2O, 60 mM K-lactobionate, 20 mM taurine, 10 mM KH2PO4, 20 mM N-2-hydroxyethylpiperazine-Nʹ-2-ethanesulfonic acid, 110 mM sucrose, and 1 g/L bovine serum albumin, pH 7.1) on an orbital shaker (25 rpm). The second wash in MiR05 contained blebbistatin (25 μM), a myosin II ATPase inhibitor, that was used to inhibit muscle contraction. Fiber bundle wet weight was determined immediately after permeabilization using an analytical balance (Mettler Toledo, Columbus, OH).
Mitochondrial Respiration Protocol
A standardized substrate uncoupler inhibitor titration protocol was run in duplicate to assess the activity of mitochondrial electron transport system in permeabilized muscle fibers (PMF). Following weighing, the PMF bundles were then transferred to the respiration chambers of an Oxygraph 2K instrument (Oroboros Inc., Innsbruck, Austria). Assays were run at 37°C in MiR05 supplemented with blebbistatin (25 μM) while O2 concentration in respiratory chambers were maintained between 400 and 200 µM. Maximal complex I- and II-supported oxidative phosphorylation (Max OXPHOS, also known as State 3 respiration) was measured in the presence of pyruvate (5 mM), malate (2 mM), glutamate (10 mM), succinate (10 mM), and adenosine diphosphate (4.2 mM). Cytochrome c (10 µM) was used to test the integrity of the outer mitochondrial membrane and any sample showing a response greater than 15% were omitted from analysis. Steady-state O2 flux data were analyzed and normalized to fiber bundle wet weight using Datlab 7.4 software. Respiratory control ratio (ADP-stimulated respiration/non-ADP-stimulated respiration in the presence of pyruvate and malate) was 7.08 ± 3.26. The mean coefficient of variation for duplicates of Max OXPHOS measurement was 11.5% across both clinical sites.
31P MRS of the Quadriceps to Determine ATPmax
The rate of PCr recovery following an acute bout of knee extensor exercise was determined in vivo with 31P MRS to calculate maximal mitochondrial ATP production (ATPmax) (35). Participants were instructed to lie in a supine position, and the right vastus lateralis muscle was shifted as close to the isocenter as possible while the knees were supported to position the right knee joint in 20°–30° of flexion. A 12″ 31P/1H dual-tuned, surface RF coil (PulseTeq, Limited (Chobham, Woking, United Kingdom)) was centered to the right distal vastus lateralis muscle. The participant was trained, then did a first bout of repeated isometric knee extension (30 seconds) against the resistance of an ankle strap. A subsequent bout was adjusted for length of time of muscle contraction (18–36 seconds) based on the first bout to achieve adequate PCr breakdown without accumulation of lactate. A 3-Tesla MR magnet (Siemen’s Medical System—Prisma Health [Pittsburgh, PA] or Skyra [Wake Forest]) was used to collect the phosphorous spectra through the quadriceps. Levels of PCr, inorganic phosphate, phosphodiesters, and ATP peak areas were determined using standard 1-pulse experiments with a 100-millisecond block pulse acquiring 7500-Hz bandwidth into 2 048 points throughout the knee extension exercise. Determination of ATPmax was performed in jMRUI v6.0 using a value of 24.5 mM for resting PCr, and postexercise recovery PCr levels were used to calculate rates of mitochondrial ATP resynthesis as previously described (35,36). The mean coefficient of variation for duplicates of ATPmax measurement was 9.9% across both clinic sites.
CPET for VO2 Peak
A standardized CPET, using a modified Balke or manual protocol, was administered to participants to measure ventilatory gases, oxygen and carbon dioxide inhaled and exhaled during exercise (37). Two slow 5-minute walking tests were conducted before and after the maximal effort test to assess walking energetics at preferred walking speed and a slow fixed speed of 1.5 mph. Participants who were excluded from the maximal effort symptom-limited peak test had acute electrocardiogram (ECG) abnormalities, uncontrolled blood pressure or history of myocardial infarction, unstable angina or angioplasty in the preceding 6 months. Testing for VO2 peak began at the participant’s preferred walking speed with incremental rate (0.5 mph) and/or slope (2.5%) increased in 2-minute stages until respiratory exchange ratio, ratio between VCO2 and VO2, was ≥1.05 and self-reported Borg Rating of Perceived Exertion (38) was ≥17. Blood pressure, pulse oximetry, and ECG were monitored throughout exercise. VO2 peak was determined in the BREEZESUITE software as the highest 30-second average of VO2 (L/min) achieved. The data were manually reviewed to ensure the correct VO2 peak was selected for each participant.
Leg Power Measurement
Knee extensor leg power was assessed using a Keiser Air 420 exercise machine. The right leg was used unless contraindicated because of prior joint replacement or range of motion restrictions. Anyone with a recent (6 months) stroke, aneurysm, cerebral hemorrhage, or systolic blood pressure >180 or <90 mmHg was excluded from testing. If the examiner observed or the participant reported excessive pain or discomfort on a standard scale, testing was discontinued. Resistance to test power was based on determination of the 1 repetition maximum leg extensor strength. Power was tested at 40%, 50%, 60%, and 70% of the participant’s maximum (39).
Statistical Analyses
Pearson’s correlation (r) coefficients and scatterplots were used to describe the bivariate relationships between the independent (muscle mitochondrial energetics) and outcome (leg power and VO2 peak) variables. In linear regression models, independent variables were standardized prior to analyses. We included an interaction term for gender and all main exposures to assess whether the associations differed for men and women. Outcome variables, leg power and VO2 peak, were standardized to each participant’s body weight. There were 4 models to test these associations, confounders were adjusted in each additive model, and results were stratified by gender. Model 1 adjusted for technician or site. Model 2 adjusted for Model 1 plus age and self-identified race. Model 3 adjusted for Model 2 plus height and adiposity (ASAT + VAT). Model 4 adjusts for all covariates in Model 3 with the addition of physical activity derived from CHAMPS questionnaire. Analyses were completed in SAS version 9.40 and R studio version 4.05 (SAS Institute, Cary, NC).
Analysis Sample
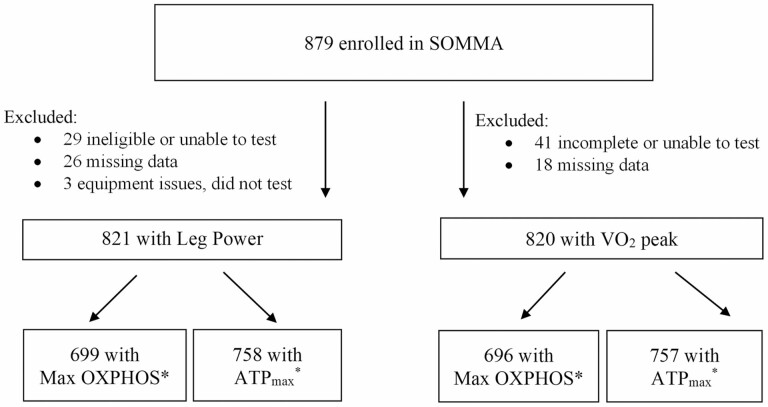
A total of 879 participants provided consent and completed baseline measurements across both clinical sites (Figure 1). Of the 879 participants with complete baseline measures: 821 participants also completed measures of peak leg power along with mitochondrial energetic measures. Eight hundred and twenty participants completed measures of VO2 peak and mitochondrial energetic measures. Participants with missing leg power or VO2 peak measures were primarily due to safety-related ineligibility or incomplete test results. Missing mitochondrial measures (Max OXPHOS or ATPmax) were due to missing data or data did not meet minimum quality control assessments (Figure 1). A characteristic comparison of those with and without missing data for the analytic cohort of Max OXPHOS and VO2 peak is in Supplementary Table 3. Differences observed in both physical activity and adiposity of these groups are likely driven by the higher number of women (74.3%) in the missing data group.
Figure 1.
SOMMA participants selected for cross-sectional analyses. *Participants missing mitochondrial respirometry measurements (Max OXPHOS) were primarily due to samples not meeting minimum quality data exclusion standards listed in Methods. Participants with missing MR measurements (ATPmax) were due to safety reasons preventing testing or the data were excluded if rate cannot be calculated due to acidic conditions. ATPmax = maximal adenosine triphosphate production; Max OXPHOS = maximal complex I- and II-supported oxidative phosphorylation; MR = magnetic resonance; SOMMA = Study of Muscle, Mobility and Aging.
Results
Participant Characteristics
The characteristics of the participants are reported by gender-specified tertiles of Max OXPHOS (Table 1) or ATPmax (Supplementary Table 2). We found no significant interaction between gender and any of the primary independent (Max OXPHOS and ATPmax) and dependent variables (leg power and VO2 peak) in our analyses. The gender interaction term for Max OXPHOS and outcomes were not significant (leg power, p = .94; VO2 peak, p = .34). This was also true for ATPmax (leg power, p = .90; VO2 peak, p = .33). This interaction model adjusted for age, site (ATPmax), or technician (Max OXPHOS). Older adults in the third tertile with the highest muscle mitochondrial energetics (Table 1 and Supplementary Table 2) tend to have higher physical activity, leg power, and VO2 peak (all p < .01) than those with lower mitochondrial energetic measures. Those in the highest tertile of Max OXPHOS also had lower body mass index (p < .01) than those with lower Max OXPHOS. On average, participants were overweight, had moderate activity levels, and had a wide range of leg power, cardiorespiratory fitness, and mitochondrial function. The mean ATPmax for women and men was 0.52 and 0.57 mM/s, and the mean Max OXPHOS for women and men was 55.03 and 65.71 pmol/(s * mg), respectively.
Table 1.
Baseline Characteristics of SOMMA Older Adults by Gender-Specified Tertiles of Max OXPHOS
| Tertiles of Max OXPHOS | ||||||
|---|---|---|---|---|---|---|
| Characteristics | First (N = 248) | Second (N = 249) | Third (N = 248) | Overall (N = 745) | p Value | |
| Max OXPHOS, pmol/(s * mg) | 42.45 ± 7.28 | 57.15 ± 6.28 | 79.56 ± 15.24 | 59.72 ± 18.46 | <.001 | |
| Women | 139 (56.0) | 140 (56.2) | 139 (56.0) | 418 (56.1) | .999 | |
| Age, years | 76.90 ± 5.36 | 76.45 ± 5.05 | 75.78 ± 4.55 | 76.38 ± 5.01 | .044 | |
| Site | Pittsburgh | 121 (48.8) | 110 (44.2) | 135 (54.4) | 366 (49.1) | .073 |
| Wake Forest | 127 (51.2) | 139 (55.8) | 113 (45.6) | 379 (50.9) | ||
| Non-Hispanic White | 192 (77.4) | 223 (89.6) | 223 (89.9) | 638 (85.6) | <.001 | |
| Height, m | 1.66 ± 0.10 | 1.66 ± 0.10 | 1.67 ± 0.10 | 1.66 ± 0.10 | .699 | |
| Weight, kg | 77.6 ± 15.1 | 76.8 ± 16.0 | 73.9 ± 15.0 | 76.1 ± 15.5 | .020 | |
| BMI, kg/m2 | 28.1 ± 4.5 | 27.8 ± 4.8 | 26.5 ± 4.2 | 27.5 ± 4.5 | .002 | |
| Physical activity*, cal/wk | 3 462.8 ± 3 368.6 | 3 753.9 ± 2 745.3 | 4 504.6 ± 3 030.0 | 3 906.9 ± 3 085.5 | .005 | |
| ASAT volume, L | 7.90 ± 3.21 | 7.60 ± 3.20 | 6.83 ± 2.88 | 7.44 ± 3.13 | <.001 | |
| VAT volume, L | 4.58 ± 2.32 | 4.36 ± 2.38 | 3.84 ± 2.27 | 4.26 ± 2.34 | .002 | |
| Total adiposity volume, L | 12.48 ± 4.46 | 11.96 ± 4.64 | 10.67 ± 4.32 | 11.70 ± 4.53 | <.001 | |
| Peak leg power, Watts/kg | 2.2 ± 0.9 | 2.4 ± 0.8 | 2.6 ± 0.8 | 2.4 ± 0.9 | <.001 | |
| VO2 peak, mL/kg/min | 18.69 ± 4.42 | 19.90 ± 4.15 | 22.81 ± 5.05 | 20.52 ± 4.87 | <.001 | |
| ATPmax, mM/s | 0.49 ± 0.12 | 0.53 ± 0.12 | 0.62 ± 0.18 | 0.55 ± 0.15 | <.001 | |
Notes: All characteristics reported as mean ± SD or N (%). ASAT = abdominal subcutaneous adipose tissue; ATPmax = maximal adenosine triphosphate production, in vivo; BMI = body mass index; Max OXPHOS = maximal complex I- and II-supported oxidative phosphorylation, in vitro; SOMMA = Study of Muscle, Mobility and Aging; VAT = visceral adipose tissue.
*Physical activity from Community Healthy Activities Model Program for Seniors questionnaire.
Bivariate Correlations of Age, Power, Fitness, and Muscle Mitochondrial Energetics
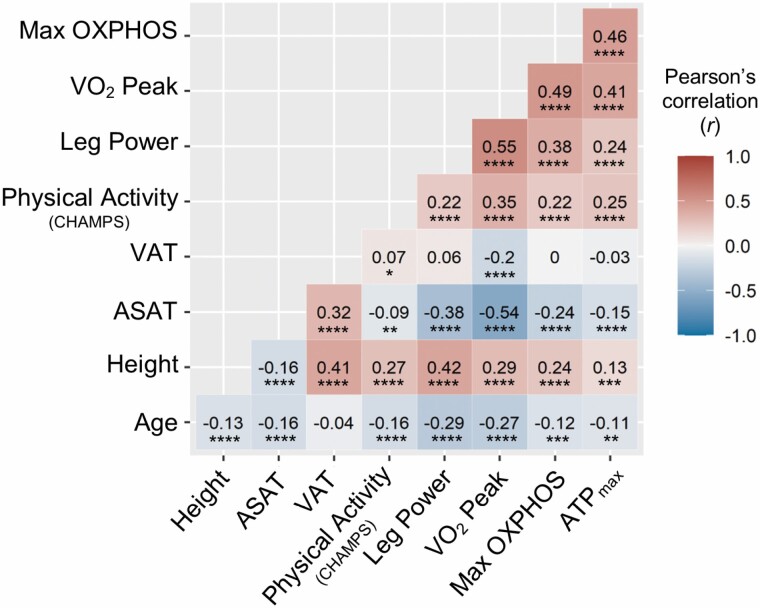
Older age was correlated with lower height, ASAT volume, physical activity, leg power, and VO2 peak (all p < .001). Older age also correlated with lower mitochondrial energetic measures, Max OXPHOS (r = −0.12, p < .001) and ATPmax (r = −0.11, p < .01; Figure 2). Adiposity, VAT (r = −0.2) and ASAT (r = −0.54), negatively correlated with VO2 peak (all p < .001), but only ASAT negatively correlated with leg power (r = −0.38, p < .001). ASAT also negatively correlated with both Max OXPHOS (r = −0.24) and ATPmax (r = −0.15, all p < .001). The in vitro Max OXPHOS and in vivo ATPmax measures of mitochondrial energetics positively correlated with one another (r = 0.46, p < .001). Higher muscle mitochondrial energetics, both Max OXPHOS and ATPmax, correlated with greater height and physical activity (r = 0.13–0.25, all p < .001) and with better functional outcomes; leg power and VO2 peak (r = 0.24–0.49, all p < 0.001), the highest correlation was between Max OXPHOS and VO2 peak.
Figure 2.
Bivariate correlation matrix of all continuous variables. Bivariate correlations of mitochondrial energetics (ASAT = abdominal subcutaneous adipose tissue; ATPmax = maximal adenosine triphosphate production; Max OXPHOS = maximal complex I- and II-supported oxidative phosphorylation; VAT = visceral adipose tissue), continuous covariates, and primary outcome variables. Pearson’s correlation (r) displayed for each pair of variables. Refer to Figure 1 flow chart for the number of participants for each bivariate correlation. A gradient color scheme was applied to reflect the numerical values where increasing degrees of red intensity is toward +1.0 correlation and decreasing shades of blue represent correlations toward −1.0. p Values for these correlations were *p < .05, **p < .01, ***p < .001, ****p < .0001. CHAMPS = Community Healthy Activities Model Program for Seniors.
Associations of Muscle Mitochondrial Energetics and Leg Power in Older Adults
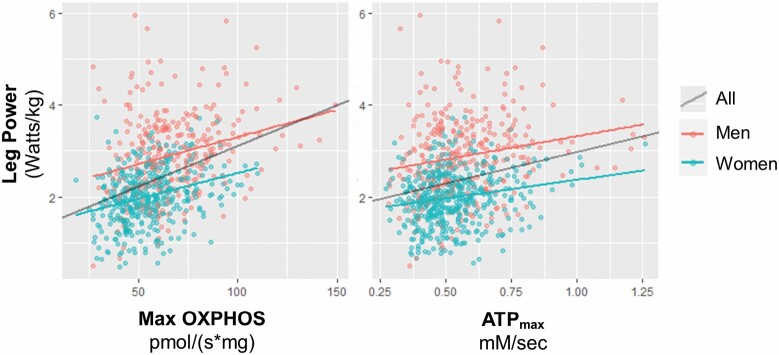
In linear regression models, higher Max OXPHOS was associated with greater leg power in all participants and when stratified by gender and after adjustment for covariates (Figure 3 and Table 2). The R2 fit for these models when stratified by gender ranged from 0.11 to 0.30, but when adjusted for gender, increased to 0.39–0.45, suggesting that gender explains a large portion of the variance in leg power. ATPmax was also associated with leg power for all SOMMA participants and for women and men separately in Model 1. The associations between ATPmax and leg power were attenuated, and these remained significant in fully adjusted Model 4 for all participants and for men but were no longer significant for women. The R2 of gender-stratified models for ATPmax and leg power ranged from 0.03 to 0.26 and for all participants was 0.06–0.45. The R2 for the model with only adjustment for site/technician was low (0.03–0.06) suggesting that ATPmax explains little of the variance in leg power. The partial R2 values for Model 4 with Max OXPHOS (Supplementary Table 4a) and ATPmax (Supplementary Table 4b) as exposures and leg power as the outcome show that Max OXPHOS, along with age, gender, and adiposity explain the most variance in leg power. We explored adjusting for body size (interaction of height and weight) in models not shown. Total adiposity, ASAT and VAT, was a better adjustment—congruent with previous studies showing that adiposity plays a role in muscle mitochondrial energetics (25,26).
Figure 3.
Associations of muscle mitochondrial energetics and leg power in older adults. Scatterplots with linear regression lines are unadjusted and variables nonstandardized. Maximal complex I- and II-supported oxidative phosphorylation or State 3 respiration (Max OXPHOS) and maximal adenosine triphosphate production (ATPmax) were standardized to SD = 1 for regression models shown in Table 2.
Table 2.
Associations of Muscle Mitochondrial Energetics and Leg Power in Older Adults
| Model 1 (site/technician) | Model 2 (Model 1 + age, race) | Model 3 (Model 2 + height, VAT, ASAT) | Model 4 (Model 3 + physical activity) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Group | β (95% CI) | Model fit (R2) | β | R2 | β | R2 | β | R2 | |
| Max OXPHOS | 395 | Women | 0.17 ****(0.11, 0.23) | 0.11 | 0.15 ****(0.10, 0.21) | 0.19 | 0.12 ****(0.06, 0.18) | 0.30 | 0.12 ***(0.06, 0.17) | 0.30 |
| 304 | Men | 0.20 ***(0.10, 0.30) | 0.11 | 0.16 **(0.06, 0.25) | 0.20 | 0.11 *(0.01, 0.20) | 0.28 | 0.11 *(0.01, 0.20) | 0.29 | |
| 699 | All | 0.31 ****(0.25, 0.37) | 0.16 | 0.17 ****(0.11, 0.22) | 0.39 | 0.13 ****(0.07, 0.19) | 0.45 | 0.13 ****(0.07, 0.18) | 0.45 | |
| ATPmax | 454 | Women | 0.10 ***(0.05, 0.16) | 0.03 | 0.08 **(0.03, 0.13) | 0.13 | 0.04 (-0.01, 0.03) | 0.26 | 0.03(-0.02, 0.08) | 0.26 |
| 304 | Men | 0.16 **(0.06, 0.26) | 0.04 | 0.11 *(0.01, 0.21) | 0.15 | 0.09 (-0.00, 0.18) | 0.26 | 0.09 *(0.00, 0.19) | 0.26 | |
| 758 | All | 0.20 ****(0.14, 0.26) | 0.06 | 0.10 ***(0.05, 0.15) | 0.37 | 0.07 ***(0.02, 0.11) | 0.45 | 0.07 ***(0.02, 0.11) | 0.45 | |
Notes: Maximal complex I- and II-supported oxidative phosphorylation or State 3 respiration (Max OXPHOS) and maximal adenosine triphosphate production (ATPmax) were standardized to SD = 1. For models with all participants, gender was adjusted for in Models 2, 3, and 4. p Values are as follows: *p < .05, **p < .01, ***p< .001, ****p < .0001. ASAT = abdominal subcutaneous adipose tissue; VAT = visceral adipose tissue.
Associations of Muscle Mitochondrial Energetics and Cardiorespiratory Fitness in Older Adults
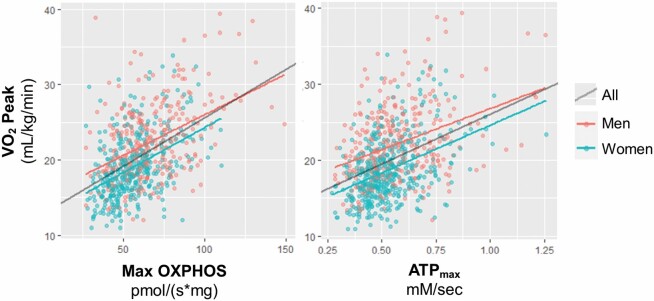
Higher mitochondrial energetic measures, both Max OXPHOS and ATPmax, were significantly associated with greater cardiorespiratory fitness (VO2 peak) in all participants and when stratified by women and men (Figure 4 and Table 3). Adjustment for covariates slightly attenuated these associations, but they remained highly significant in fully adjusted Model 4. Interestingly, both in vitro and in vivo measures of muscle mitochondrial energetics explained a moderate amount of the variance in VO2 peak. Along with age, race, height, adiposity, and physical activity, Model 4 for both Max OXPHOS and ATPmax explains more than half of the variance (R2 = 0.54–0.63) in the VO2 peak for SOMMA women and men. Further, the R2 in Model 1 where only site/technician was accounted for, ranged from 0.19 to 0.29, indicating that these mitochondrial energetic measures may encompass 20%–30% of the variance in cardiorespiratory fitness of SOMMA older adults. Partial R2 values for Model 4 with Max OXPHOS (Supplementary Table 4a) and ATPmax (Supplementary Table 4b) as exposures and VO2 peak as the outcome show that both Max OXPHOS or ATPmax, along with age, gender, and adiposity, explain the most variance in cardiorespiratory fitness (VO2 peak). The highest partial R2 (0.26–0.29) was ASAT in models where muscle mitochondrial energetics were the exposures and VO2 peak was the outcome in women.
Figure 4.
Associations of muscle mitochondrial energetics and cardiorespiratory fitness in older adults. Scatterplots with linear regression lines are unadjusted and variables nonstandardized. Maximal complex I- and II-supported oxidative phosphorylation or State 3 respiration (Max OXPHOS) and maximal adenosine triphosphate production (ATPmax) were standardized to SD = 1 for regression models shown in Table 3.
Table 3.
Associations of Muscle Mitochondrial Energetics and Cardiorespiratory Fitness in Older Adults
| Model 1 (site/technician) |
Model 2 (Model 1 + age, race) | Model 3 (Model 2 + height, VAT, ASAT) | Model 4 (Model 3 + physical activity) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Group | β (95% CI) | Model fit (R2) | β | R2 | β | R2 | β | R2 | |
| Max OXPHOS | 384 | Women | 1.65 ****(1.27, 2.04) | 0.26 | 1.48 ****(1.11, 1.85) | 0.35 | 1.13 ****(0.83, 1.42) | 0.62 | 1.03 ****(0.74, 1.32) | 0.63 |
| 312 | Men | 2.06 **** (1.57, 2.55) |
0.26 | 1.77 ****(1.31, 2.24) | 0.35 | 1.41 ****(0.98, 1.83) | 0.52 | 1.32 ****(0.90, 1.74) | 0.54 | |
| 696 | All | 2.24 ****(1.92, 2.55) | 0.29 | 1.69 ****(1.39, 2.00) | 0.42 | 1.31 ****(1.05, 1.57) | 0.61 | 1.22 ****(0.96, 1.47) | 0.63 | |
| ATPmax | 448 | Women | 1.68 ****(1.34, 2.02) | 0.19 | 1.47 ****(1.14, 1.80) | 0.27 | 1.03 ****(0.77, 1.30) | 0.56 | 0.87 ****(0.60, 1.14) | 0.58 |
| 309 | Men | 2.02 ****(1.52, 2.52) | 0.20 | 1.72 ****(1.24, 2.19) | 0.31 | 1.61 ****(1.20, 2.02) | 0.52 | 1.50 ****(1.09, 1.91) | 0.53 | |
| 757 | All | 2.03 ****(1.72, 2.33) | 0.20 | 1.58 ****(1.30, 1.86) | 0.37 | 1.30 ****(1.07, 1.53) | 0.59 | 1.17 ****(0.94, 1.40) | 0.61 | |
Notes: Maximal complex I- and II-supported oxidative phosphorylation or State 3 respiration (Max OXPHOS) and maximal adenosine triphosphate production (ATPmax) were standardized to SD = 1. For models with all participants, gender was adjusted for in Models 2, 3, and 4. p Values are as follows: *p < .05, **p < .01, ***p < .001, ****p < .0001. ASAT = abdominal subcutaneous adipose tissue; VAT = visceral adipose tissue.
Discussion
Cardiorespiratory fitness and leg power are important for maintaining physical function and mobility in older adults. The principal finding of this study is that skeletal muscle mitochondrial energetics, along with gender, race, and adiposity, explain a significant portion of the variance in leg muscle power and cardiorespiratory fitness in a large group of older women and men. When we adjusted for the potential confounder, adiposity, it attenuated the beta coefficient of muscle mitochondrial energetics with both leg power and VO2 peak. Partial R2 values indicated that adiposity was a crucial adjustment in these models, especially for women. Whereas, adjusting for physical activity attenuated the beta coefficient for both measures of mitochondrial energetics with VO2 peak but not with leg power as the outcome. Consistent with our secondary hypotheses, our results indicate that 2 complementary measurements of mitochondrial energetics had higher associations with cardiorespiratory fitness (VO2 peak) than lower-extremity muscle function (leg power). Specifically, we found that in vitro mitochondrial energetics assessed in permeabilized muscle fibers (PMF) retained signification associations in fully adjusted models with both leg power and VO2 peak. In comparison, in vivo maximal ATP production (ATPmax) also had significant associations with VO2 peak but was not as robustly associated with leg power. Finally, our analyses in this large cohort of older adults, stratified by women and men, confirm and build on prior observations (21–23,26) that skeletal muscle mitochondrial energetics play a vital role in aging muscle and physical health.
Muscle Mitochondrial Energetics and Leg Extension Power in Older Adults
Peak muscle power, the maximum force production over a single muscle contraction or force per unit time, is an important predictor of functional limitations in older adults (2). The decline in muscle power output in older adults is likely due to a quantitative decline in muscle mass, impaired individual muscle fiber contractile properties, and reduced neuromuscular function (28). We find that muscle mitochondrial energetics, in particular Max OXPHOS, was associated with higher maximal leg extension power in older adults, even when the association analyses were stratified by gender. In contrast, although higher ATPmax was associated with higher leg power in all participants and in men but not women, the low R2 model fits indicated that ATPmax explains only a small portion of variance with leg power in these older adults. We note that these findings are in line with a report by Zane et al., describing an association between in vivo mitochondrial energetics and knee extensor strength in a group of older adults (n = 326, 52.7% women, age range: 24–97) (23).
Here, we found that Max OXPHOS is associated with leg power even though power generation predominantly relies on ATP generated from the PCr and anaerobic glycolysis systems, rather than oxidative phosphorylation. This raises the possibility that other aspects of mitochondrial function are playing a role in leg power generation. For example, muscle from healthy older men (n = 10, 70 ± 1 years) who had lower oxidative capacity compared with younger men (n = 10, 26 ± 2 years) also had higher rates of mitochondrial hydrogen peroxide emission (40), and hydrogen peroxide is a mediator of cellular oxidative stress which can impair calcium handling and myofilament function which would be detrimental to power generation (41). Lower oxidative capacity of muscle may also be associated with impaired Ca2+ handling, an important function of mitochondria that is key for triggering contraction in striated muscle (42). Another possibility is that the state of mitochondrial bioenergetics may be a general biomarker of muscle health and therefore correlates with other energy-generating pathways such as the PCr and anaerobic glycolysis systems. These speculations warrant further study. Our findings underly the multifaceted role of mitochondria in skeletal muscle health of older adults including power generation.
Muscle Mitochondrial Energetics and Cardiorespiratory Fitness in Older Adults
We found that skeletal muscle Max OXPHOS and ATPmax were robust predictors of VO2 peak, in line with other studies that have explored this relationship in smaller cohorts (12,19,43). Importantly, most of these association outcomes were not different across the genders, as our stratified analyses show. Many of these associations were also independent of age, race, body size, and physical activity. Covariates that significantly confounded this relationship included physical activity, height, and weight. While there is some evidence that VO2 peak declines more precipitously than muscle oxidative capacity with aging (44,45), these findings are consistent with the hypothesis that the loss of mitochondrial function contributes to the decline in VO2 peak with aging.
A strength of our study is the inclusion of both Max OXPHOS and ATPmax to show how they each relate to the same performance outcomes within a large group of older adults. It was surprising to us that ATPmax explained very little of the variance in leg power in these older adults. The in vitro assessment of Max OXPHOS was more strongly associated with leg power and VO2 peak than the in vivo assessment of ATPmax was with these outcomes. This could be due to differences in local substrate levels, and/or delivery, transport, and metabolism of substrates to fuel ATPmax in vivo that are standardized in the ex vivo respirometry assay. On the other hand, these differences could be due to features of the measurements themselves, such as variability or measurement error. For example, ATPmax assumes a constant value for resting PCr and/or ATP across all individuals. Without an actual measure of these metabolite levels, ATPmax is perhaps a more variable assessment of muscle mitochondrial energetics. We note that the mean muscle mitochondrial measures are similar to data we and others have published in the past for older adults (22,25,26).
An additional strength of this study was that these analyses were shown in the overall population as well as stratified by gender, lending insight into how adjusting for gender versus stratifying the analyses by gender result in different model fits (R2). We speculate that the model fits were lower for stratified analyses due to the lower numbers—loss in statistical power, but also, sexual dimorphism widens the range on variables, and this could reinforce the regression line fit and increase the R2 value. We further acknowledge that our study participants were older, predominantly (85.6%) non-Hispanic White older which limits our ability to generalize to other groups. A minor limitation to this cross-sectional study is that values were missing for some measures; however, we found no differences in covariates among participants from each analytic subgroup (ie, Max OXPHOS or ATPmax and leg power; Max OXPHOS or ATPmax and VO2 peak). Another limitation of our approach is that we cannot be certain whether covariates included in our model, including physical activity, are truly confounders or mediators of the association between mitochondrial energetics and leg power or VO2 peak. In future analyses, we will use longitudinal data to help disentangle these relationships.
In summary, these findings show that skeletal muscle mitochondrial energetics are associated with leg muscle power and cardiovascular fitness in aging, and this suggests that in vitro Max OXPHOS makes a greater contribution to fitness and strength than the in vivo ATPmax measure. Finally, our data also support the notion that mitochondrial energetics is more important for cardiorespiratory fitness than for power generation in older adults. Important future analyses would be to test the longitudinal outcomes of these relationships; to interrogate whether or not change in muscle mitochondrial energetics will predict change in cardiorespiratory fitness. Both VO2 peak and leg power are supported by multiple tissue–organ systems working in concert and represent an integrative systems physiology. Further understanding the tissue and organelle contributions to VO2 peak and leg power will allow development of countermeasures to mitigate decline with aging.
Supplementary Material
Acknowledgments
We acknowledge all the staff and investigators listed (27), and we thank all the SOMMA participants who enabled this research.
Contributor Information
Theresa Mau, San Francisco Coordinating Center, California Pacific Medical Center Research Institute, San Francisco, California, USA; Department of Epidemiology and Biostatistics, University of California, San Francisco, California, USA.
Li-Yung Lui, San Francisco Coordinating Center, California Pacific Medical Center Research Institute, San Francisco, California, USA; Department of Epidemiology and Biostatistics, University of California, San Francisco, California, USA.
Giovanna Distefano, AdventHealth, Translational Research Institute, Orlando, Florida, USA.
Philip A Kramer, Department of Internal Medicine-Gerontology and Geriatric Medicine, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA.
Sofhia V Ramos, AdventHealth, Translational Research Institute, Orlando, Florida, USA.
Frederico G S Toledo, Department of Medicine-Division of Endocrinology and Metabolism, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Adam J Santanasto, Department of Epidemiology, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Eric G Shankland, Department of Radiology, University of Washington, Seattle, Washington, USA.
David J Marcinek, Department of Radiology, University of Washington, Seattle, Washington, USA.
Michael J Jurczak, Department of Medicine-Division of Endocrinology and Metabolism, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA; Center for Metabolism and Mitochondrial Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Ian Sipula, Department of Medicine-Division of Endocrinology and Metabolism, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA; Center for Metabolism and Mitochondrial Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Fiona M Bello, Department of Medicine-Division of Endocrinology and Metabolism, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA; Center for Metabolism and Mitochondrial Medicine, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, USA.
Kate A Duchowny, Social Environment and Health, Survey Research Center, Institute for Social Research, University of Michigan, Ann Arbor, Michigan, USA.
Anthony J A Molina, Department of Internal Medicine-Gerontology and Geriatric Medicine, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA; Department of Medicine-Division of Geriatrics, Gerontology, and Palliative Care, University of California San Diego School of Medicine, La Jolla, California, USA.
Lauren M Sparks, AdventHealth, Translational Research Institute, Orlando, Florida, USA.
Bret H Goodpaster, AdventHealth, Translational Research Institute, Orlando, Florida, USA.
Russell T Hepple, Department of Physical Therapy, University of Florida, Gainesville, Florida, USA.
Stephen B Kritchevsky, Department of Internal Medicine-Gerontology and Geriatric Medicine, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA.
Anne B Newman, Department of Epidemiology, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Peggy M Cawthon, San Francisco Coordinating Center, California Pacific Medical Center Research Institute, San Francisco, California, USA; Department of Epidemiology and Biostatistics, University of California, San Francisco, California, USA.
Steven R Cummings, San Francisco Coordinating Center, California Pacific Medical Center Research Institute, San Francisco, California, USA; Department of Epidemiology and Biostatistics, University of California, San Francisco, California, USA.
Paul M Coen, AdventHealth, Translational Research Institute, Orlando, Florida, USA.
Funding
The Study of Muscle, Mobility and Aging is funded by the National Institute on Aging (NIA) grant number R01AG059416. G.D. is supported by the American Diabetes Association (1-19-PDF-006). A.J.S. is supported by K01AG057726 and R01AG074956. K.A.D. is supported by K99AG066846. P.M.Co. is supported by R01AG060153 and R01AG060542. Study infrastructure support was funded in part by NIA Claude D. Pepper Older American Independence Centers at University of Pittsburgh (Pitt; P30AG024827), Wake Forest University School of Medicine (Wake; P30AG021332), the Clinical and Translational Science Institutes which is funded by the National Center for Advancing Translational Science at Wake (UL1TR001420) and Pitt (UL1TR001857), as well as the Pittsburgh Foundation at Pitt (MR2020 109502).
Conflict of Interest
S.R.C. and P.M.Ca. are consultants to Bioage Labs. All other authors declare no conflict of interest.
Author Contributions
Using Contributor Roles Taxonomy (CRediT) terms, many authors participated in multiple contributor roles. In brief, T.M. led the writing team and data meetings which culminated into codrafting the original manuscript with P.M.Co. L.-Y.L. led formal analyses and managed data curation. A.B.N., P.M.Ca., K.A.D., A.J.S., and P.A.K. provided the most critical edits that led to the improvement of the manuscript. G.D., S.V.R., D.J.M., E.G.S., M.J.J., A.J.A.M., and L.M.S., all contributed with either data validation, experimental designs, and/or resources. P.A.K., F.G.S.T., I.S., and F.M.B. all participated with key investigation contributions. S.R.C., P.M.Ca., A.B.N., S.B.K., R.T.H., and B.H.G. enabled the study with either funding acquisition, project administration, and/or conceptualization to the study.
References
- 1. Fiser WM, Hays NP, Rogers SC, et al. Energetics of walking in elderly people: factors related to gait speed. J Gerontol A Biol Sci Med Sci. 2010;65A(12):1332–1337. doi: 10.1093/gerona/glq137 [DOI] [PubMed] [Google Scholar]
- 2. Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev. 2012;40(1):4–12. doi: 10.1097/JES.0b013e31823b5f13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255–263. doi: 10.1093/gerona/59.3.m255 [DOI] [PubMed] [Google Scholar]
- 4. Pahor M, Blair SN, Espeland M, et al. ; LIFE Study Investigators. Effects of a physical activity intervention on measures of physical performance: results of the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61(11):1157–1165. doi: 10.1093/gerona/61.11.1157 [DOI] [PubMed] [Google Scholar]
- 5. Jack Rejeski W, Brubaker PH, Goff DC, et al. Translating weight loss and physical activity programs into the community to preserve mobility in older, obese adults in poor cardiovascular health. Arch Intern Med. 2011;171(10):880–886. doi: 10.1001/ARCHINTERNMED.2010.522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Santanasto AJ, Newman AB, Strotmeyer ES, Boudreau RM, Goodpaster BH, Glynn NW. Effects of changes in regional body composition on physical function in older adults: a pilot randomized controlled trial. J Nutr Health Aging. 2015;19(9):913–921. doi: 10.1007/s12603-015-0523-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bratic A, Larsson NG. The role of mitochondria in aging. J Clin Invest. 2013;123(3):951–957. doi: 10.1172/JCI64125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Muller-Hocker J. Cytochrome-c-oxidase deficient cardiomyocytes in the human heart: an age-related phenomenon. A histochemical ultracytochemical study. Am J Pathol. 1989;134(5):1167–1173. [PMC free article] [PubMed] [Google Scholar]
- 9. Ojaimi J, Masters CL, Opeskin K, McKelvie P, Byrne E. Mitochondrial respiratory chain activity in the human brain as a function of age. Mech Ageing Dev. 1999;111(1):39–47. doi: 10.1016/s0047-6374(99)00071-8 [DOI] [PubMed] [Google Scholar]
- 10. Trounce I, Byrne E, Marzuki S. Decline in skeletal muscle mitochondrial respiratory chain function: possible factor in ageing. Lancet. 1989;333(8639):637–639. doi: 10.1016/S0140-6736(89)92143-0 [DOI] [PubMed] [Google Scholar]
- 11. Boffoli D, Scacco SC, Vergari R, Solarino G, Santacroce G, Papa S. Decline with age of the respiratory chain activity in human skeletal muscle. BBA—Mol Basis Dis. 1994;1226(1):73–82. doi: 10.1016/0925-4439(94)90061-2 [DOI] [PubMed] [Google Scholar]
- 12. Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol. 2000;526(1):203–210. doi: 10.1111/j.1469-7793.2000.t01-1-00203.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Conley KE, Esselman PC, Jubrias SA, et al. Ageing, muscle properties and maximal O2 uptake rate in humans. J Physiol. 2000;526(pt 1):211–217. doi: 10.1111/j.1469-7793.2000.00211.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tonkonogi M, Fernström M, Walsh B, et al. Reduced oxidative power but unchanged antioxidative capacity in skeletal muscle from aged humans. Pflugers Arch Eur J Physiol. 2003;446(2):261–269. doi: 10.1007/S00424-003-1044-9 [DOI] [PubMed] [Google Scholar]
- 15. Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci USA. 2005;102(15):5618–5623. doi: 10.1073/pnas.0501559102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tyrrell DJ, Bharadwaj MS, Van Horn CG, Kritchevsky SB, Nicklas BJ, Molina AJA. Respirometric profiling of muscle mitochondria and blood cells are associated with differences in gait speed among community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2015;70(11):1394–1399. doi: 10.1093/gerona/glu096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perry CGR, Kane DA, Lanza IR, Neufer PD. Methods for assessing mitochondrial function in diabetes. Diabetes. 2013;62(4):1041–1053. doi: 10.2337/db12-1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carnio S, LoVerso F, Baraibar MA, et al. Autophagy impairment in muscle induces neuromuscular junction degeneration and precocious aging. Cell Rep. 2014;8(5):1509–1521. doi: 10.1016/j.celrep.2014.07.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Broskey NT, Greggio C, Boss A, et al. Skeletal muscle mitochondria in the elderly: effects of physical fitness and exercise training. J Clin Endocrinol Metab. 2014;99(5):1852–1861. doi: 10.1210/jc.2013-3983 [DOI] [PubMed] [Google Scholar]
- 20. Amara CE, Shankland EG, Jubrias SA, Marcinek DJ, Kushmerick MJ, Conley KE. Mild mitochondrial uncoupling impacts cellular aging in human muscles in vivo. Proc Natl Acad Sci USA. 2007;104(3):1057–1062. doi: 10.1073/pnas.0610131104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gonzalez-Freire M, Scalzo P, D’Agostino J, et al. Skeletal muscle ex vivo mitochondrial respiration parallels decline in vivo oxidative capacity, cardiorespiratory fitness, and muscle strength: the Baltimore Longitudinal Study of Aging. Aging Cell. 2018;17(2):e12725. doi: 10.1111/acel.12725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coen PM, Jubrias SA, Distefano G, et al. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol A Biol Sci Med Sci. 2013;68(4):447–455. doi: 10.1093/gerona/gls196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zane AC, Reiter DA, Shardell M, et al. Muscle strength mediates the relationship between mitochondrial energetics and walking performance. Aging Cell. 2017;16(3):461–468. doi: 10.1111/acel.12568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tian Q, Mitchell BA, Zampino M, Fishbein KW, Spencer RG, Ferrucci L. Muscle mitochondrial energetics predicts mobility decline in well-functioning older adults: the Baltimore Longitudinal Study of Aging. Aging Cell. 2022;21(2) ( January):1–8. doi: 10.1111/acel.13552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Distefano G, Standley RA, Zhang X, et al. Physical activity unveils the relationship between mitochondrial energetics, muscle quality, and physical function in older adults. J Cachexia Sarcopenia Muscle. 2018;9(2):279–294. doi: 10.1002/jcsm.12272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grevendonk L, Connell NJ, McCrum C, et al. Impact of aging and exercise on skeletal muscle mitochondrial capacity, energy metabolism, and physical function. Nat Commun. 2021;12(1):1–17. doi: 10.1038/s41467-021-24956-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cummings SR, Newman AB, Coen PM, et al. The Study of Muscle, Mobility, and Aging (SOMMA). A unique cohort study about the cellular biology of aging and age-related loss of mobility. J Gerontol A Biol Sci Med Sci. Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reid KF, Pasha E, Doros G, et al. Longitudinal decline of lower extremity muscle power in healthy and mobility-limited older adults: influence of muscle mass, strength, composition, neuromuscular activation and single fiber contractile properties. Eur J Appl Physiol. 2014;114(1):29–39. doi: 10.1007/s00421-013-2728-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. West J, Romu T, Thorell S, et al. Precision of MRI-based body composition measurements of postmenopausal women. PLoS One. 2018;13(2):e0192495. doi: 10.1371/journal.pone.0192495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Karlsson A, Rosander J, Romu T, et al. Automatic and quantitative assessment of regional muscle volume by multi-atlas segmentation using whole-body water-fat MRI. J Magn Reson Imaging. 2015;41(6):1558–1569. doi: 10.1002/jmri.24726 [DOI] [PubMed] [Google Scholar]
- 31. Romu T, Borga M, Dahlqvist O. MANA—multi scale adaptive normalized averaging. Proc Int Symp Biomed Imaging. 2011:361–364. doi: 10.1109/ISBI.2011.5872424 [DOI] [Google Scholar]
- 32. Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33(7):1126–1141. doi: 10.1097/00005768-200107000-00010 [DOI] [PubMed] [Google Scholar]
- 33. Hekler EB, Buman MP, Haskell WL, et al. Reliability and validity of CHAMPS self-reported sedentary-to-vigorous intensity physical activity in older adults. J Phys Act Heal. 2012;9(2):225–236. doi: 10.1123/JPAH.9.2.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Evans WJ, Phinney SD, Young VR. Suction applied to a muscle biopsy maximizes sample size. Med Sci Sport Exerc. 1982;14(1):100. doi: 10.1249/00005768-198201000-00018 [DOI] [PubMed] [Google Scholar]
- 35. Blei ML, Conley KE, Kushmerick MJ. Separate measures of ATP utilization and recovery in human skeletal muscle. J Physiol. 1993;465(1):203–222. doi: 10.1113/jphysiol.1993.sp019673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Amara CE, Marcinek DJ, Shankland EG, Schenkman KA, Arakaki LSL, Conley KE. Mitochondrial function in vivo: spectroscopy provides window on cellular energetics. Methods. 2008;46(4):312–318. doi: 10.1016/j.ymeth.2008.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Balady GJ, Arena R, Sietsema K, et al. ; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology. Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122(2):191–225. doi: 10.1161/CIR.0b013e3181e52e69 [DOI] [PubMed] [Google Scholar]
- 38. Borg GAV. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. https://pubmed-ncbi-nlm-nih-gov.ucsf.idm.oclc.org/7154893/. Accessed June 30, 2022. [PubMed] [Google Scholar]
- 39. Winger ME, Caserotti P, Ward RE, et al. Jump power, leg press power, leg strength and grip strength differentially associated with physical performance: the Developmental Epidemiologic Cohort Study (DECOS). Exp Gerontol. 2021;145:111172. doi: 10.1016/j.exger.2020.111172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Holloway GP, Holwerda AM, Miotto PM, Dirks ML, Verdijk LB, van Loon LJC. Age-associated impairments in mitochondrial ADP sensitivity contribute to redox stress in senescent human skeletal muscle. Cell Rep. 2018;22(11):2837–2848. doi: 10.1016/j.celrep.2018.02.069 [DOI] [PubMed] [Google Scholar]
- 41. Powers SK, Ji LL, Kavazis AN, Jackson MJ. Reactive oxygen species: impact on skeletal muscle. Compr Physiol. 2011;1(2):941–969. doi: 10.1002/cphy.c100054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Olsson K, Cheng AJ, Al-Ameri M, et al. Impaired sarcoplasmic reticulum Ca2+ release is the major cause of fatigue-induced force loss in intact single fibres from human intercostal muscle. J Physiol. 2020;598(4):773–787. doi: 10.1113/JP279090 [DOI] [PubMed] [Google Scholar]
- 43. Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci. 2006;61(6):534–540. doi: 10.1093/gerona/61.6.534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang X, Kunz HE, Gries K, Hart CR, Polley EC, Lanza IR. Preserved skeletal muscle oxidative capacity in older adults despite decreased cardiorespiratory fitness with ageing. J Physiol. 2021;599(14):3581–3592. doi: 10.1113/JP281691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. DiStefano G, Standley RA, Dubé JJ, et al. Chronological age does not influence ex-vivo mitochondrial respiration and quality control in skeletal muscle. J Gerontol A Biol Sci Med Sci. 2017;72(4):535–542. doi: 10.1093/gerona/glw102 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.