Abstract
People often confuse smell loss with taste loss, so it is unclear how much gustatory function is reduced in patients self-reporting taste loss. Our pre-registered cross-sectional study design included an online survey in 12 languages with instructions for self-administering chemosensory tests with 10 household items. Between June 2020 and March 2021, 10,953 individuals participated. Of these, 5,225 self-reported a respiratory illness and were grouped based on their reported COVID test results: COVID-positive (COVID+, N = 3,356), COVID-negative (COVID−, N = 602), and COVID unknown for those waiting for a test result (COVID?, N = 1,267). The participants who reported no respiratory illness were grouped by symptoms: sudden smell/taste changes (STC, N = 4,445), other symptoms excluding smell or taste changes (OthS, N = 832), and no symptoms (NoS, N = 416). Taste, smell, and oral irritation intensities and self-assessed abilities were rated on visual analog scales. Compared to the NoS group, COVID+ was associated with a 21% reduction in taste (95% confidence interval (CI): 15–28%), 47% in smell (95% CI: 37–56%), and 17% in oral irritation (95% CI: 10–25%) intensity. There were medium to strong correlations between perceived intensities and self-reported abilities (r = 0.84 for smell, r = 0.68 for taste, and r = 0.37 for oral irritation). Our study demonstrates that COVID-19-positive individuals report taste dysfunction when self-tested with stimuli that have little to none olfactory components. Assessing the smell and taste intensity of household items is a promising, cost-effective screening tool that complements self-reports and may help to disentangle taste loss from smell loss. However, it does not replace standardized validated psychophysical tests.
Keywords: olfaction, gustation, chemesthesis, anosmia, ageusia, taste–smell confusion
Introduction
The SARS-CoV-2 virus had a major impact on all three chemical senses involved in flavor perception: smell, taste, and chemesthesis. While smell loss became known as the cardinal symptom of COVID-19 early in the pandemic and was studied extensively, the veracity of the associations between COVID-19 and taste loss has been called into question (Saniasiaya et al. 2021; Hannum et al. 2022). Unlike the relatively common loss of smell seen with viral infections of the upper respiratory tract (Seiden, 2004), pre-pandemic accounts indicate that post-viral taste loss is rare (Henkin et al. 1975; Pribitkin et al. 2003). Most patients who present to the clinic with “taste” complaints actually have a normal gustatory function, and systematic evaluation of the chemical senses indicates the presence of olfactory dysfunction (Pribitkin et al. 2003). Notably, less than 1% of 1,176 patients who presented to a specialized clinic reporting olfactory and/or gustatory dysfunction had actual taste loss, while 32% had smell loss (Pribitkin et al. 2003). Discrepancies between patients’ reports and results of direct chemosensory evaluations include the common semantic confusion between taste and smell or flavor (Boltong et al. 2011). Much of what the public describes colloquially as “taste” encompasses not only taste but also retronasal smell (Murphy et al. 1977; Murphy and Cain 1980) and chemesthesis (Duffy and Hayes 2020). Although rare, there have been cases of gustatory dysfunction following influenza-like illness documented by clinical testing (Henkin et al. 1975). However, because retronasal smell, taste, and chemesthesis abilities are not routinely assessed by clinicians and patients lack the ability to dissociate these senses from one another, it remains unclear to which extent taste and chemesthesis are affected when individuals reported the loss of smell, such as in the early days of the COVID-19 pandemic. While numerous survey-based studies, including our own (Parma et al. 2020; Gerkin et al. 2021), have suggested that COVID-19 affects taste function more than in other respiratory diseases or healthy individuals (see Saniasiaya et al. 2021; Hannum et al. 2022), studies that used various kinds of taste stimuli (versus self-report) have yielded conflicting results. In studies with self-administered home tests, where participants with COVID-19 prepared 4 solutions with prototypical tastants and identified the taste quality, taste dysfunction was seen in 42–72% of participants (Petrocelli et al. 2020; Vaira, Deiana, et al. 2020; Vaira, Lechien, et al. 2020; Vaira, Salzano, et al. 2020). Conversely, studies that used standardized taste tests, such as taste strips (Hintschich et al. 2020; Le Bon, Payen, et al. 2021; Le Bon, Pisarski, et al. 2021) or the Waterless Empirical Taste Test (Cao et al. 2022) suggested that the sense of taste was generally well preserved (Hintschich et al. 2020) or only mildly affected in individuals with acute COVID-19 infection (Haldrup et al. 2020; Le Bon, Payen, et al. 2021; Le Bon, Pisarski, et al. 2021), causing some authors to speculate that reports of taste loss with COVID-19 were probably due to taste/flavor confusion and a loss of retronasal smell (Hintschich et al. 2020). Notably, a recent systematic review and meta-analysis found that more studies have assessed taste function in individuals with COVID-19 using tastants dissolved in liquid solutions than in paper strips such as taste strips (Hannum et al. 2022). Additionally, results of the meta-analysis suggested that taste solutions worked better than taste strips (reduced heterogeneity) and supported findings from self-report survey-based studies that indicated a diminished sense of taste in individuals with COVID-19 (Hannum et al. 2022). Alternatively, clinic-based assessments with validated tools may have still missed true taste loss, if the dysfunction was transient and had resolved by the time testing occurred in a clinical setting.
The primary purpose of the present study was to determine to which extent chemosensory functions were affected by COVID-19 by using direct testing with unstandardized chemosensory stimuli in a large global sample. To this end, we designed a remote online chemosensory home test that independently captured the perceived intensity of taste, smell, and oral irritation of common household items in parallel with self-assessments of chemosensory ability using a previously published online survey (Parma et al. 2020; Gerkin et al. 2021; Ohla et al. 2022; Albayay et al. 2022; Weir et al. 2022).
Our pre-registered hypotheses (https://osf.io/6bfua) focused on all 3 chemosensory modalities across 6 groups that were formed based on reported respiratory illness and a positive COVID test result (COVID+), reported respiratory illness and negative COVID test result (COVID−), reported respiratory illness and waiting for a COVID test result (COVID?), did not report respiratory illness but smell and/or taste changes (STC), did not report respiratory illness but other symptoms except smell/taste changes (OthS), and did not report respiratory illness or any symptoms at all (NoS). Below, we draw attention to the taste-specific hypotheses that are the focus of this study. First, we hypothesized that individuals with COVID-19 would show greater impairment of all chemosensory modalities, including taste when measured with odorless gustatory stimuli (i.e. sucrose and salt), as compared to people with other respiratory illnesses or no symptoms. Specifically, we expected participants in the COVID-19+ group to rate household items as being less intense than those in the COVID− and NoSymptoms groups for all three chemosensory modalities. To substantiate this hypothesis, we further explored whether perceived intensities of chemosensory stimuli would be most reduced closer to the onset of COVID-19 rather than later on in the disease (>15 days post symptom onset), as worst chemosensory function had previously been observed during the first week of infection (Vaira, Lechien, et al. 2020).
Second, we hypothesized that the self-reported ability to taste, smell, and perceive oral irritation would be positively associated with the corresponding experienced intensity of household tastants, odorants, and stimuli irritating the oral mucosa, respectively. It is worth noting that we expected a stronger correlation between self-reported ability and perceived intensity for the sense of smell compared to the sense of taste, due to the well-known phenomenon of taste–smell confusion. Hence, we expected that the overall ratings for the self-reported ability to taste would be lower than the intensity of experienced odorless tastants, such as sugar and salt.
Finally, we explored the possibility that perceived smell, taste, and oral irritation would be differentially affected across individuals to assess whether some respondents may experience smell–taste confusion. For this, we used a data-driven approach to cluster individuals based on their “chemosensory profiles” using the data from self-reported chemosensory abilities, and the responses to the home test, using the data from perceived intensities of stimuli across smell, taste, and oral irritation.
Methods
Data were collected using a crowd-sourced, multilingual, online study with a multi-national reach: the GCCR Smell-&-Taste-Check. The tool, generally completed in less than 30 min, is comprised of two parts: a self-assessment (survey with up to 46 questions) and a home test (with up to 28 questions). The GCCR Smell-&-Taste-Check is available at the OSF project archive (https://osf.io/sp8eq). The data reported here were collected in 12 languages (Czech, Dutch, English, French, German, Italian, Japanese, Korean, Portuguese, Russian, Spanish, and Turkish) and included participants from 127 countries.
As part of the self-assessment participants were asked to rate their abilities to smell, taste, and perceive oral irritation in the same format as previously used in the Global Consortium for Chemosensory Research (GCCR) survey (Parma et al. 2020). As part of the home test, participants rated the intensity of the smell or taste of common household items (e.g. shampoo, sugar, coffee, etc.) as appropriate for the stimulus (i.e. shampoo was not rated for taste intensity), and the intensity of nasal and oral irritants (e.g. smelling vinegar, tasting mustard). Participants were provided with a list of 75 items that were selected to be culturally diverse. These included 42 odors (including 7 cosmetics/detergents, 22 spices, 13 fruits/vegetables, and 17 other items), 4 tastes (including sweet, sour, bitter, salty), and 8 oral and 4 nasal irritants (Supplementary Table 1). Although the majority of these items included mixtures of chemicals, sweet and salty were measured via table sugar and salt, respectively. Participants were instructed to select 10 items (one per category) available in their households. Self-reported chemosensory abilities and perceived chemosensory intensities of the household items were reported on a 101-point visual analog scale (0–100) anchored with “no sensation” and “very intense” (Parma et al. 2020). Additionally, we collected data on demographics, including age, gender, pregnancy (for women only), education, smoking, socio-economic status (SES), number of social contacts, current symptoms, COVID test status, and medical history.
The study was publicized via the GCCR website (https://gcchemosensr.org) and social and traditional media. All volunteers provided consent and confirmed that they were 18 or older. All participants completed both the self-assessment and the home test. They had the option to skip items in the home test. For example, participants could opt to smell only 2 out of 4 olfactory items by selecting items from the “spices” and “fruits” and omitting the “cosmetics” and “other” categories. Or they could omit an entire modality such as tasting the oral irritant. The latter would lead to incomplete data for a given variable and different sample sizes for the different sensory modalities. We chose this route to accommodate the reality of participants from diverse cultures and to increase compliance by increasing the chance that participants will find options available in their household. Accordingly, our analyses focused on the average ratings across categories (e.g. all olfactory stimuli) rather than the ratings of individual items. The institutional review board of the Faculty of Psychology & Sports Science at the Westfälische Wilhelms-Universität Münster approved the study (no. 2020-27-NB). Additional approval was obtained for the Russian test version by the Bioethics Committee at the A.N. Severtsov Institute of Ecology & Evolution RAS (no. 2020-41-NC). Consent was documented electronically within the online study interface.
Participants
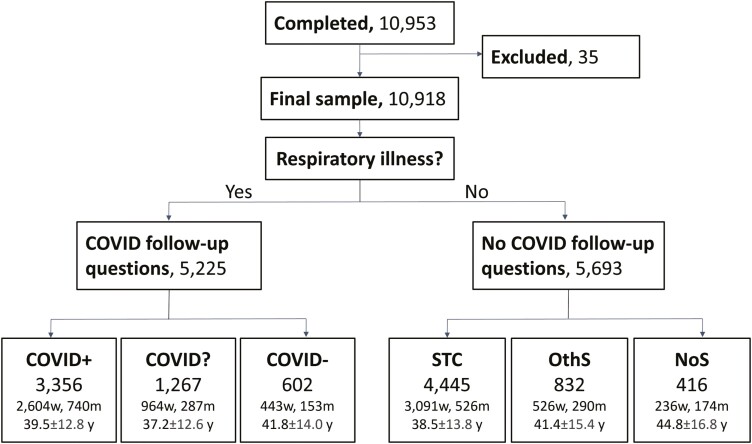
In total, 10,953 participants completed the survey between 2020 June 26, and 2021 March 23. Of these, 35 were excluded because they provided implausible data (no COVID diagnosis, but COVID onset date). Data from the remaining 10,918 participants are reported here, and their group age and gender information are summarized in Figure 1. Of these, 5,225 participants reported a respiratory illness and were subsequently asked about their COVID-19 status. A total of 3,356 reported a positive and 602 a negative COVID-19 test result or clinical diagnosis; these groups were classified as COVID-positive (COVID+) and COVID-negative (COVID−), respectively. Individuals with a respiratory illness who were awaiting test results were classified as COVID unknown (COVID?; N = 1,267). The remaining 5,693 participants reported no respiratory illness and were, as per survey design, not asked about COVID-19, but only about current symptoms. Based on the reported symptoms, we grouped these remaining participants into those reporting sudden smell/taste changes (STC, N = 4,445), other symptoms excluding smell or taste changes primary pre-registered hypothesis (OthS, N = 832), and no symptoms at all (NoS, N = 416). Notably, the design may have led to underreporting of COVID-19 by miscategorizing some respondents with COVID-19 as “healthy” if they reported no respiratory illness at the beginning of the survey. The design favors, however, a rigorous classification of individuals with a diagnosis of COVID-19 (positive and negative) and minimizes the risk of false positives. The demographics of the six groups are summarized in Supplementary Table 2.
Fig. 1.
Flow diagram showing the participants included in the study. Exclusions were based on implausible data. Participants were split based on their response (yes/no) to the question asking whether they have a respiratory illness. Only those who responded with “yes” were asked whether they had COVID-19. They were further split according to their report of a positive (COVID+) or negative (COVID−) diagnosis or if they were waiting for a test result and were suspected to have COVID-19 (COVID?). Those who responded “no” to the respiratory illness question were split by the symptoms reported: only smell and/or taste-related changes (STC), any other symptom but smell/taste changes (OthS), or no symptoms at all (NoS). Age ± SD in years (y); gender is reported as women (w) and men (m), remaining participants identified as “other” or did not share.
Data analyses
Data analyses were performed in R version 4.0.4 (R Core, 2021) using the tidyverse+ (Wickham et al. 2019), FactoMineR (Lê et al. 2008), cluster (Maechler et al. 2022), plotly (Sievert 2019), car (Fox and Weisberg 2019), and multcomp (Hothorn et al. 2008) packages. We computed separate smell, taste, and oral irritation composite scores based on the average of all available perceived intensity ratings by chemosensory modality because participants could opt to smell as many as four household items and taste 4 different foods. In contrast, they tasted and smelled only one irritating item to minimize the carryover of irritation. Missing ratings were not imputed leading to variable sample sizes for different analyses.
To test the primary pre-registered hypothesis that participants with COVID-19 would exhibit a reduced perceived intensity to taste, smell, and irritating stimuli compared to participants without COVID-19 and healthy individuals, participants were grouped according to their COVID-19 status, and data were submitted to separate analyses of variance (ANOVAs). In these analyses, we compared 6 diagnostic groups (COVID+, COVID−, COVID?, STC, OthS, and NoS) on the perceived intensity of the household items and self-reported ability (without specific stimuli) to smell, taste, and perceive oral irritation. For the comparisons between ability and intensity for chemesthesis, we used oral irritation ratings as these were included in both the survey and the home test.
To test the secondary pre-registered hypothesis that self-reported abilities to taste, smell, and perceive oral irritation were positively associated with the respective perceived intensities of real-life stimuli, we computed Spearman correlation coefficients between the two measures for each sense separately. We then compared the correlation coefficients derived for each of the three chemosensory modalities using the cocor package in R (Diedenhofen and Musch, 2015) to test the hypothesis that the association between self-reported ability and perceived intensity for smell would be stronger than the association between self-reported ability and perceived intensity for taste and oral irritation.
Additionally, to test whether perceived chemosensory intensity was influenced by the time passed since the onset of COVID-19 suggesting that chemosensory function during the first week of infection was the poorest, we compared the intensity scores of COVID+ individuals who participated in this study within 0–7 days (N = 737), 8–14 days (N = 597), or more than 15 days (N = 1,831) since the onset of COVID-19 symptom using a one way ANOVA.
An exploratory multiple factor analysis (MFA) using FactoMineR was performed to test the rationale of our a priori diagnostic grouping shown in Figure 1.
Finally, we used Agglomerative Hierarchical Clustering (AHC) with Ward’s clustering method (Nielsen, 2016) on the self-reported ability and perceived intensity of taste, smell, and oral irritation from the entire sample. Our objective was to distinguish individuals based purely on their combined chemosensory responses, creating distinct “chemosensory profiles” that encompassed all available chemosensory data, regardless of diagnosis. The chemosensory abilities and perceived intensities of resulting clusters were compared by one-way ANOVA and Tukey’s HSD test. To explore the characteristics of the identified clusters, we analyzed the continuous demographic variables with one-way ANOVA and Tukey’s HSD test and categorical variables with the Chi-squared test. The alpha level for all analyses was set a priori at 0.05. Corrections for multiple comparisons were made as necessary. Effect sizes were computed for inferential tests. The data were assessed for the assumptions of the respective statistical tests employed. For details, please refer to the script used for analyses (https://github.com/GCCR/GCCR004).
Results
Reduced perceived intensity of taste, smell, and oral irritation stimuli in COVID-19
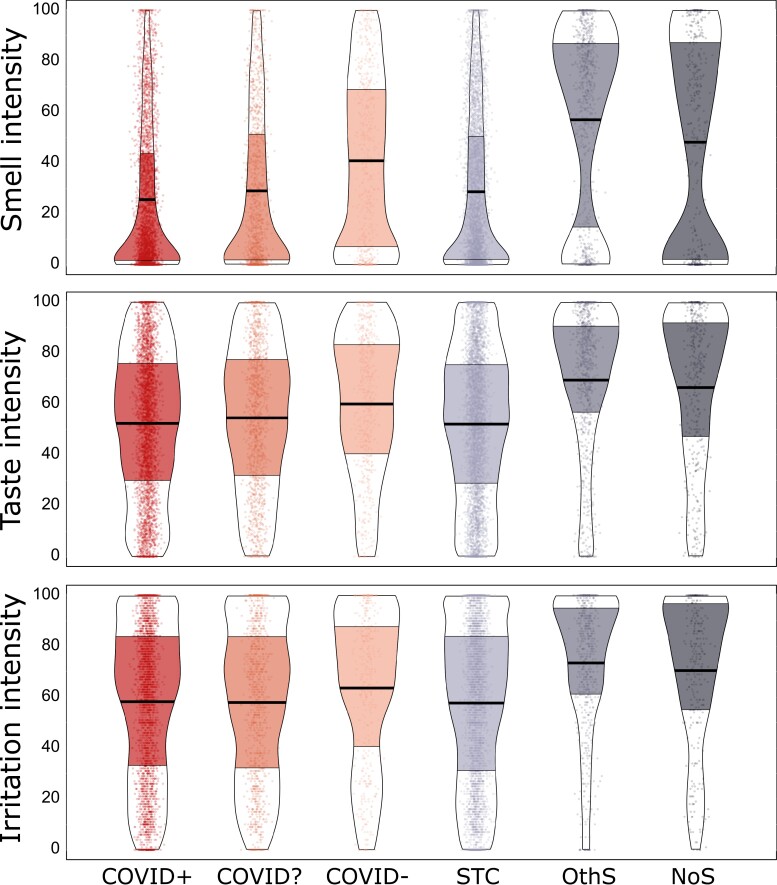
Groups reporting confirmed (COVID+) or suspected COVID-19 (COVID? and STC) rated the intensity of taste, smell, and oral irritation from foods and household items as being less intense than the COVID−, OthS and NoS groups (Group effect for smell: F(5, 10536) = 178.8, P < 0.001, = 0.08, taste: F(5, 10541) = 70.5, P < 0.001, = 0.03, and oral irritation: F(5, 8424) = 40.16, P < 0.001, = 0.02; Fig. 2). Compared to the NoS group, COVID+ exhibited a 21% reduction in taste intensity (95% CI: 15–28%), a 47% reduction in smell intensity (95% CI: 37–56%), and a 17% reduction in oral irritation intensity (95% CI: 10–25%; see Supplementary Table 3 for pairwise tests). To confirm reductions in retronasal smell did not drive reductions in ratings of taste intensity, we re-analyzed the data on perceived taste intensity by selecting stimuli that are purely gustatory in nature (i.e. salt and sugar) and excluding taste stimuli that also had an odor (i.e. lemon juice and coffee beans or tea leaves). The subset of data that included only odorless taste stimuli confirmed the results seen with data from all taste stimuli (F(5, 10386) = 57.2 P < 0.001, = 0.03; Supplementary Fig. 1).
Fig. 2.
Perceived intensity of taste, smell, and oral irritation when sampling food or household items for six groups of participants. Participants are grouped according to COVID diagnosis or symptoms (from left to right) into COVID-positive (COVID+; N = 3,275), unknown COVID status (COVID?; N = 1,224), and COVID-negative (COVID−; N = 579), those who reported sudden smell/taste changes (STC; N = 4,271), those with other symptoms excluding smell or taste changes (OthS; N = 802), and those with no symptoms (NoS; N = 396). They rated the perceived intensity of smell, taste, and oral irritating stimuli using a visual analog scale (0–100). Points represent individual subject data (jittered horizontally), the center horizontal bars depict the median, the shapes reflect the density of the distribution, and the colored areas show interquartile ranges. For a similar presentation of data for self-reported chemosensory ability, see Supplementary Fig. 3.
To test the hypothesis that reductions in the perception of taste, smell, and oral irritation were related to acute COVID-19 infection, we compared the intensity ratings of individuals who completed the survey within the first 7 days (n = 737), 8–14 days (n = 597), and >15 days (n = 1,831) from the onset of their COVID-19 symptoms. Intensities differed between these groups for all three chemosensory modalities (smell: F(2,3099) = 524.9, P < 0.001, = 0.25, taste: F(2,3091)) = 153.0, P < 0.001, = 0.09, and irritation: F(2,2573) = 56.3, P < 0.001, = 0.04; Supplementary Fig. 2). Post hoc tests revealed the lowest smell, taste, and oral irritation intensities for those individuals who completed the survey within 7 days, higher intensities for those who completed the survey between days 8 and 14, and the highest intensities were found for those who participated 15 days or more after symptom onset. All pairwise comparisons were significant except for oral irritation which did not significantly differ between the first two time segments (Supplementary Table 4).
Strong links between chemosensory intensity perception and self-reported ability, but a subset of individuals show taste/smell confusion
We found medium to strong correlations between self-reported chemosensory ability and the rated intensity of the chemosensory stimuli for smell (r = 0.84), taste (r = 0.68), and oral irritation (r = 0.37; all P < 0.001; see Supplementary Fig. 4), with significantly stronger associations for smell than for the other two chemical senses (P < 0.0001).
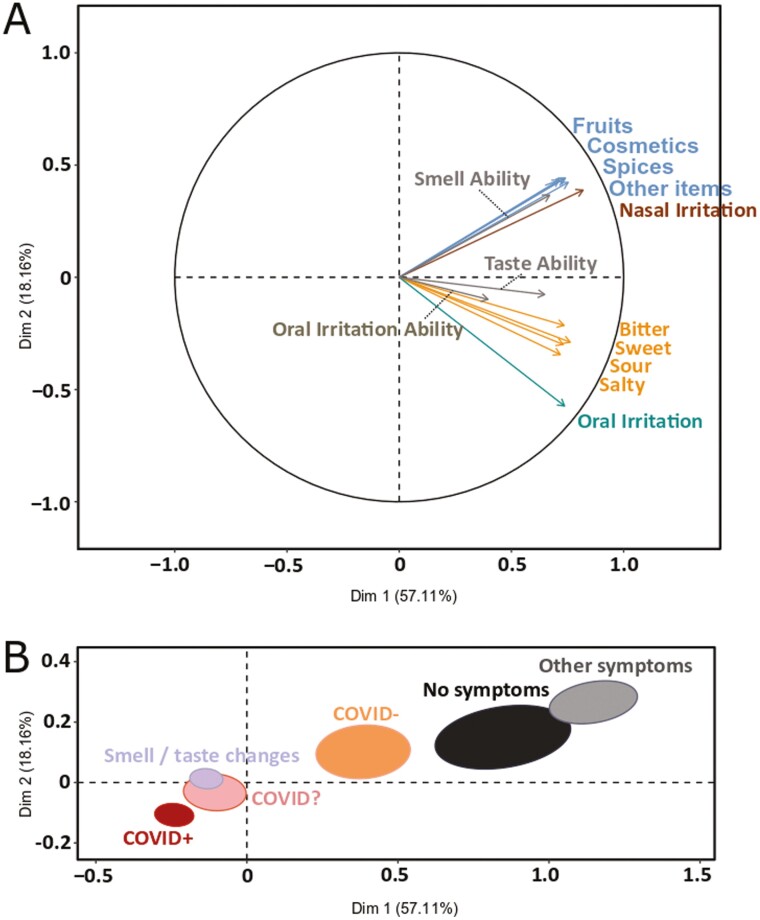
To corroborate the finding from the bivariate analyses, we also performed a MFA across all data, finding that self-assessment of chemosensory ability was associated with the respective ratings of chemosensory intensity (Fig. 3a). Regardless of the item selected, smell ratings were correlated across all categories (i.e. cosmetics, spices, fruits, and other). Similarly, all taste ratings correlated across categories (i.e. sweet, sour, salty, and bitter) as well as with ratings of stimuli eliciting oral irritation. The distribution of the a priori identified diagnostic groups suggests that Dimension 1 reflects a spectrum from respiratory illness to respiratory health and Dimension 2 reflects chemosensory function from low to high. The proximity and overlap of the confidence ellipses of the COVID+, COVID?, and STC groups suggest these may belong to one latent group that is characterized by substantial impairment of chemosensory function (Fig. 3b).
Fig. 3.
MFA on self-reported chemosensory abilities and perceived intensities. (a) Correlation circle, including all ratings. (b) Map of the six groups (COVID+, N=3,275; COVID−, N = 579; COVID?, N = 1,224; STC, N = 4,271; OthS, N = 802; NoS, N = 396) with 95% confidence ellipses. The groups are distributed based on the chemosensory profiles computed from the self-reported abilities and perceived intensities of taste, smell, and oral irritation.
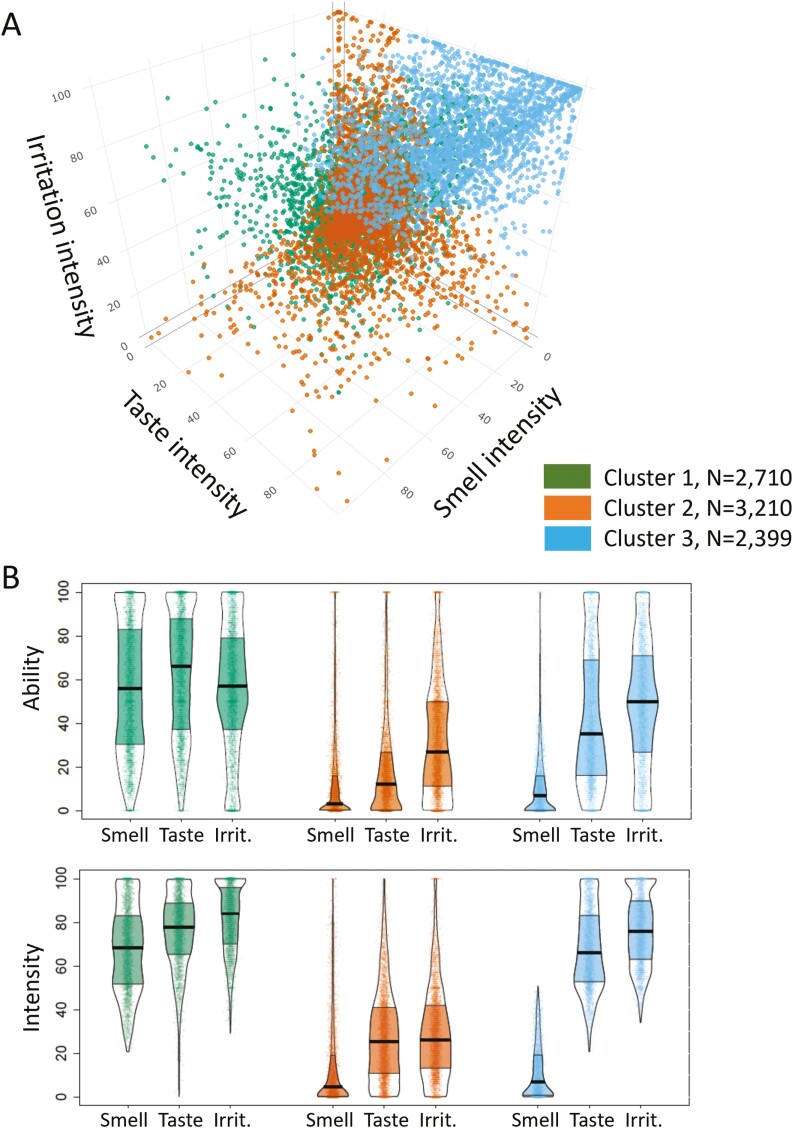
To explore the relative contribution of each chemosense to the integrity of the overall chemosensory function in a data-driven manner, we applied AHC to ratings of chemosensory ability and intensity regardless of diagnosis. As shown in Figure 4, three chemosensory cluster profiles were identified: the “minimally impaired” for all three senses, with good correspondence between ratings of intensity of food and household items and self-report ability to smell/taste/perceive oral irritation (Cluster 1), the “severely impaired” for all three senses, with good correspondence between intensity ratings and self-reported ability (Cluster 2) and the “severely smell impaired” who exhibited, good correspondence between intensity ratings and self-reported ability for smell but not for taste, and to some extent oral irritation (Cluster 3). Specifically, participants indicated that their self-reported ability was lower compared to the rated intensity of the same items, suggesting that they conflated their reduced ability to smell with a reduced ability to taste and perceive oral irritation. Altogether, these results suggest that these participants may be misappropriating a true reduction in their ability to smell with their ability to taste and perceive irritation, indicating that they may have possibly confounded taste and irritation with smell (Cluster 3). The demographics of all three clusters are summarized in Supplementary Table 4.
Fig. 4.
Differences in self-reported abilities and perceived intensities for smell, taste, and oral irritation between 3 clusters obtained by the AHC on perceived intensities irrespective of reported diagnosis. (a) 3D plot on smell, taste, and oral irritation intensities. Dots represent individual subject data, clusters are color-coded. (b) Self-reported abilities and perceived intensities of foods and items of the three chemosensory modalities of smell, taste, and oral irritation for the three clusters. Points represent individual subject data (jittered horizontally), the center horizontal bars depict the medians, the shapes reflect the density of the distribution, and the colored areas show the interquartile range. Cluster 1 (green) was minimally impaired, while cluster 2 (orange) was severely impaired for all 3 chemical senses; cluster 3 (blue) showed severe loss of smell but not of taste or oral irritation.
Discussion
Taste and smell loss have been cardinal self-reported symptoms of infection, particularly with early variants of the SARS-CoV-2 virus (Coelho et al. 2022). With any smell loss coincident with a viral illness, patients often present with complaints of “taste loss” that manifest as changes in food flavor, which result from congestion causing an impaired (retronasal) smell. Very early in the pandemic, initial reports of impaired taste were widely assumed to reflect this sort of taste/smell/flavor confusion but growing evidence from patient reports suggests COVID-19 might also impact the sense of taste (unlike the typical cold). Here, we use a large cohort of 10,953 individuals to demonstrate that COVID-19 not only affects smell but also taste and chemesthesis, at least in individuals who became ill between June 2020 and March 2021. The finding of a reduced perceived taste intensity for pure gustatory stimuli like sugar and salt suggests that COVID-19-associated complaints of taste loss are real and not merely a result of taste–smell confusions, in a majority of individuals. A hierarchical cluster analysis corroborated this finding.
Findings from our study confirm and extend results from previous studies on the utility of assessing ratings of perceived intensity of actual chemosensory stimuli to evaluate chemosensory dysfunction (Iravani et al. 2020; Snitz et al. 2022). For smell, the intensity of household items was reduced by 47% in the COVID-19+ group compared to the presumably healthy NoSymptoms group. The remarkable consistency observed between our study and others (Iravani et al. 2020; Snitz et al. 2022) substantiates the notion that despite the considerable diversity in the chemical stimuli encountered in household items, including their intensity and composition, the present results offer valuable insights for classifying cohorts of patients.
Extending previous findings, we found that taste intensity and oral irritation intensity when tasting real food items were also markedly reduced by 21% and 17%, respectively, in COVID-19+ participants. Reductions in chemosensory intensity were most profound in those participating during the first two weeks of their COVID-19 illness, thus supporting the interpretation that there is a direct causal relationship between COVID-19 and the broad loss of chemosensation. Importantly, these results are in line with others (Iravani et al. 2020; Snitz et al. 2022) and suggest that, with appropriate instructions in place, self-testing—even when using simple household items—can be a valuable tool for monitoring changes in chemosensory function.
Significant progress has been made to understand the cellular and molecular mechanisms of COVID-19-associated smell loss. Findings from postmortem samples of respiratory and olfactory mucosae and whole olfactory bulbs of COVID-19 patients who died a few days after infection with SARS-Cov-2 confirmed previous inferences (Cooper et al. 2020; Khan et al. 2021) that the sustentacular cells, but not the olfactory sensory neurons, are the main target for SARS-CoV-2 (Cooper et al. 2020; Khan et al. 2021). These findings suggest that the sudden anosmia in COVID-19 is caused by olfactory sensory neurons lacking adequate support from sustentacular cells (Butowt et al. 2022; Finlay et al. 2022). Although less is known about the underlying mechanisms of impairment of taste and oral irritation in COVID-19, it is clear that SARS-CoV-2 infects the oral cavity (Huang et al. 2021). The virus is found in saliva, and human taste cells express essential mechanisms of entry of the virus into the host cell, including the angiotensin-converting enzyme 2 and the transmembrane protease serine 2 (TMPRSS2) (Sakaguchi et al. 2020; Doyle et al. 2021). Interestingly, it has been shown that patients’ self-reports of smell/taste loss are positively associated with salivary viral load (Huang et al. 2021). Furthermore, taste function could be affected by the high levels of the pro-inflammatory cytokines -TNF-α, IFN-γ, and IL-6 observed in the serum of COVID-19 patients, which can impair stem cell function, and hence, taste bud cell renewal (Doyle et al. 2021). Another complementary mechanism contributing to decreased taste perception in individuals with anosmia could be the abolition of the enhancement of taste by smell that is assumed to occur in healthy individuals (Ai and Han 2022; Hintschich et al. 2020).
Subjective ratings of olfactory function are considered by some to be unreliable and inaccurate because participants are prone to under- or over-reporting biases or may use response scales idiosyncratically. This should be considered a weakness of our study. However, subjective olfactory ratings may still be accurate reflections of ability when certain conditions are met. For example, olfactory ratings of people with severe hyposmia or anosmia have an accuracy rate of 70–80% (Lötsch and Hummel 2019) suggesting that individuals may be unaware of loss until formally examined (Landis et al. 2003), but those who do report chemosensory impairment have a high likelihood of this being a genuine symptom. That is, the relationship is asymmetrical: true loss may not be noticed until testing, but self-reports of loss are typically accurate. Furthermore, questions about smell or taste are frequently ambiguous; a classic example is asking respondents to comment on their sense of taste without specifying that the question is specific about basic taste qualities such as sweetness and not flavor (taste/smell confusion) (Rozin 1982; Gerkin et al. 2021; Weir et al. 2022). Furthermore, the scale associated with a question may be insufficiently granular (Cao et al. 2022). For example, drawing from simple surveys used to assess sino-nasal or oral symptoms prior to and during the early stages of the COVID-19 pandemic, “loss of taste and smell” was frequently presented as a single symptom to be reported on a binary (yes/no) question in clinical tests like the SNOT-22 (Kennedy et al. 2013). Questions about smell and taste experiences when using dichotomous questions versus visual analog scales, provide different information about an individual’s ability to experience smell, taste, and chemesthesis during and after COVID-19 or other respiratory illnesses (Gerkin et al. 2021). The lack of granularity from assessments that rely on identification rather than intensity, for example, “Do you taste anything?” followed by “Do you recognize the taste?” after tasting different foods, might also be the reason why recent home taste tests failed to be associated with standardized tests in the clinic (Mazzatenta 2022)—although this approach produced reliable results for olfactory function (Li et al. 2022).
A strength of our study lies in the clear and precise definition of the sensory experience that was being evaluated, such as distinguishing between taste and smell using specific examples of how taste and smell are different from flavor. In addition, we utilized continuous visual analog scales to measure both the ability and perceived intensity of various chemosensory stimuli, which we found to be highly correlated (0.84). This finding supports previous research suggesting self-reports of olfactory ability can be dependable and informative (Li et al. 2022). Moreover, our study provides novel evidence that taste can also be accurately assessed if it is distinctly defined, thereby reducing confusion with smell.
Another strength of our crowd-sourced study is the large sample size and sizable global recruitment spread as well as the combination of self-administered and self-assessments of smell, taste, and oral irritation intensity measures. Our data also include non-COVID control participants, unlike other studies using home tests that included only participants with COVID.
Because our questionnaire and home tests were administered in several countries and languages, we acknowledge that we cannot exclude biases or effects owing to language differences (Weir et al. 2022). However, we tried to minimize these effects through a standardized translation protocol (Brislin 1970; Moshontz et al. 2018). Also, our study population is a self-selected convenience sample that may be biased toward the inclusion of participants with an increased interest in smell and taste and/or their disturbances.
With the SARS-CoV-2 pandemic, the number of people affected by chemosensory impairment has been steadily increasing, and it will take years to reveal the extent of long-term and chronic chemosensory damage in the population. Notably, our study demonstrates that COVID-19-positive individuals report taste dysfunction when self-tested with stimuli that have minimal olfactory components.
Basic and translational research is needed to further our understanding of the underlying mechanisms of post-viral taste and smell impairment and recovery. From a clinical perspective, inexpensive taste and smell tests using household items—even if only collected at home—offer a quick, free, and safe remote-screening assessment for chemosensory dysfunction, which is not yet routinely performed. These features make self-assessments and home tests a practical and informative way to screen for large cohorts of patients with infectious diseases, like COVID-19, and for patients with limited or no access to medical care around the globe. Such approaches can support the clinical diagnosis with standardized psychophysical tests and the selection of appropriate treatment options as well as the monitoring of recovery of chemosensory function.
Supplementary Material
Acknowledgment
The authors wish to thank all study participants and all members of the Global Consortium for Chemosensory Research (GCCR) for their help with translations and survey design.
Contributor Information
Ha Nguyen, Monell Chemical Senses Center, Philadelphia, PA, USA.
Javier Albayay, Centro Interdipartimentale Mente/Cervello, Università degli Studi di Trento, Rovereto, Italy.
Richard Höchenberger, Inria, CEA, MIND, Université Paris-Saclay, Palaiseau, France.
Surabhi Bhutani, School of Exercise and Nutritional Sciences, San Diego State University, San Diego, CA, USA.
Sanne Boesveldt, Division of Human Nutrition and Health, Wageningen University, Wageningen, Netherlands.
Niko A Busch, Institute for Psychology, University of Münster, Münster, Germany.
Ilja Croijmans, Department of Language and Communication, Radboud University, Nijmegen, Netherlands.
Keiland W Cooper, Department of Neurobiology and Behavior, University of California Irvine, Irvine, CA, USA.
Jasper H B de Groot, Behavioural Science Institute, Radboud University, Nijmegen, Netherlands.
Michael C Farruggia, Department of Psychiatry, Yale University School of Medicine, New Haven, CT, USA.
Alexander W Fjaeldstad, Department of Otorhinolaryngology, Flavour Clinic, University Clinic for Flavour, Balance and Sleep, Gødstrup Regional Hospital, Herning, Denmark.
John E Hayes, Department of Food Science, The Pennsylvania State University, University Park, PA, USA.
Thomas Hummel, Department of Otorhinolaryngology, University of Dresden Medical School, Smell & Taste Clinic, Dresden, Germany.
Paule V Joseph, National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism, DIBCR, Section of Sensory Science and Metabolism, Bethesda, MD, USA.
Tatiana K Laktionova, Institute of Ecology and Evolution RAS, A N Severtsov, Moscow, Russia.
Thierry Thomas-Danguin, Research Center for Smell Taste and Feeding Behavior, INRAE CSGA, Dijon, France.
Maria G Veldhuizen, Department of Anatomy, Mersin Universitesi, Mersin, Turkey.
Vera V Voznessenskaya, Institute of Ecology and Evolution RAS, A N Severtsov, Moscow, Russia.
Valentina Parma, Monell Chemical Senses Center, Philadelphia, PA, USA.
M Yanina Pepino, Department of Food Science and Human Nutrition, Division of Nutritional Sciences, University of Illinois at Urbana-Champaign, Urbana, IL, USA; Department of Biomedical and Translational Sciences, Carle Illinois College of Medicine, University of Illinois at Urbana-Champaign, Urbana, IL, USA.
Kathrin Ohla, Department of Food Science, The Pennsylvania State University, University Park, PA, USA; Experimental Psychology Unit, Helmut-Schmidt-University/University of the Armed Forces Hamburg, Hamburg, Germany; Science & Research, dsm-firmenich, Satigny, Switzerland.
Funding
This work was supported, in part, by the National Science Foundation [grant number DGE-1839285 to C.K.]; the National Institute on Alcohol Abuse and Alcoholism [grant number Z01AA000135 to P.V.J.], the National Institute of Nursing Research [grant number 1ZIANR000035-01 to P.V.J.], the National Institute of Deafness and Other Communication Disorders [grant number U01DC019573 to J.E.H.], and the National Institute of Food and Agriculture via Hatch Act funds [grant number 698-921 to M.Y.P.; PEN04708 to J.E.H.].
Conflict of Interest
J.E.H. holds equity in Redolynt, LLC, which he co-founded in 2021. This company was granted an option to license technology covered by a provisional patent application filed jointly by the Pennsylvania State University, the University of Florida, and Arizona State University. This financial interest has been reviewed by the Conflict Interest Committee at Penn State and is subject to an active management plan. Redolynt has no direct involvement in the present work. K.O. is currently employed by dsm-firmenich; the company had no influence on the study design or interpretation of the results.
Author Contributions
All authors contributed to multiple aspects of the research, including conceptualization of various aspects of the design and analysis, data collection and interpretation and critical reviewing. H.N. and J.A. led the analytical efforts and are co-first authors; R.H. led the design of the online survey and implemented the online study; V.P., M.Y.P, and K.O. led the design of the survey, supervised all aspects of the project, led the drafting and revision of the manuscript and are co-last authors; all other authors are alphabetically listed to indicate equal contributions.
Data Availability
Data and code are available in the project GitHub repository (https://github.com/GCCR/GCCR004) with Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International Public License (CC BY-NC-ND 4.0; https://creativecommons.org/licenses/by-nc-nd/4.0/).
References
- Ai Y, Han P.. Neurocognitive mechanisms of odor-induced taste enhancement: a systematic review. Int J Gastron Food Sci. 2022:28:100535. doi: 10.1016/j.ijgfs.2022.100535 [DOI] [Google Scholar]
- Albayay J, Fontana L, Parma V, Zampini M.. Chemosensory dysfunction in long-term COVID-19 assessed by self-reported and direct psychophysical methods. Life 2022:12(10):1487. Article 10. doi: 10.3390/life12101487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boltong A, Keast RSJ, Aranda SK.. A matter of taste: making the distinction between taste and flavor is essential for improving management of dysgeusia. Support Care Cancer. 2011:19(4):441–442. doi: 10.1007/s00520-011-1085-0. [DOI] [PubMed] [Google Scholar]
- Brislin RW. Back-translation for cross-cultural research. J Cross-Cult Psychol. 1970:1(3):185–216. doi: 10.1177/135910457000100301. [DOI] [Google Scholar]
- Butowt R, Bilinska K, von Bartheld CS.. Olfactory dysfunction in COVID-19: new insights into the underlying mechanisms. Trends Neurosci. 2022:46(1):75–90. doi: 10.1016/j.tins.2022.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Yang A, D’Aloisio AA, Suarez L, Deming-Halverson S, Li C, Luo Z, Pinto JM, Werder EJ, Sandler DP, et al. Assessment of self-reported sense of smell, objective testing, and associated factors in middle-aged and older women. JAMA Otolaryngol–Head Neck Surg. 2022:148(5):408–417. doi: 10.1001/jamaoto.2022.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho DH, Reiter ER, French E, Costanzo RM.. Decreasing incidence of chemosensory changes by COVID-19 variant. Otolaryngol–Head Neck Surg. 2022:168(4):704–706. doi: 10.1177/01945998221097656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper KW, Brann DH, Farruggia MC, Bhutani S, Pellegrino R, Tsukahara T, Weinreb C, Joseph PV , Larson ED, Parma V, et al. COVID-19 and the chemical senses: supporting players take center stage. Neuron. 2020:107(2):219–233. doi: 10.1016/j.neuron.2020.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedenhofen B, Musch J.. cocor: a comprehensive solution for the statistical comparison of correlations. PLoS One. 2015:10(4):e0121945. doi: 10.1371/journal.pone.0121945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle ME, Appleton A, Liu Q-R, Yao Q, Mazucanti CH, Egan JM.. Human Type II taste cells express angiotensin-converting enzyme 2 and are infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Am J Pathol. 2021:191(9):1511–1519. doi: 10.1016/j.ajpath.2021.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy VB, Hayes JE.. Biological basis and functional assessment of oral sensation. In: Meiselman H, editor. Handbook of eating and drinking: interdisciplinary perspectives. Springer, Champ; 2020. p. 1–25. doi: 10.1007/978-3-319-75388-1_22-1 [DOI] [Google Scholar]
- Finlay JB, Brann DH, Abi Hachem R, Jang DW, Oliva AD, Ko T, Gupta R, Wellford SA, Moseman EA, Jang SS, et al. Persistent post–COVID-19 smell loss is associated with immune cell infiltration and altered gene expression in olfactory epithelium. Sci Transl Med. 2022:14(676):eadd0484. doi: 10.1126/scitranslmed.add0484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J, Weisberg S.. 2019. An R companion to applied regression. 3rd ed. Thousand Oaks (CA): Sage. https://socialsciences.mcmaster.ca/jfox/Books/Companion/. [Google Scholar]
- Gerkin RC, Ohla K, Veldhuizen MG, Joseph PV, Kelly CE, Bakke AJ, Steele KE, Farruggia MC, Pellegrino R, Pepino MY, et al. ; GCCR Group Author. Recent smell loss is the best predictor of COVID-19 among individuals with recent respiratory symptoms. Chem Senses. 2021:46:bjaa081. doi: 10.1093/chemse/bjaa081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldrup M, Johansen MI, Fjaeldstad AW.. Anosmia and ageusia as primary symptoms of COVID-19. Ugeskr Laeger. 2020:182(18):V04200205. [PubMed] [Google Scholar]
- Hannum ME, Koch RJ, Ramirez VA, Marks SS, Toskala AK, Herriman RD, Lin C, Joseph PV, Reed DR.. Taste loss as a distinct symptom of COVID-19: a systematic review and meta-analysis. Chem Senses. 2022:47:bjac001. doi: 10.1093/chemse/bjac001 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Henkin RI, Larson AL, Powell RD.. Hypogeusia, dysgeusia, hyposmia, and dysosmia following influenza-like infection. Ann Otol Rhinol Laryngol. 1975:84(5):672–682. doi: 10.1177/000348947508400519 [DOI] [PubMed] [Google Scholar]
- Hintschich CA, Wenzel JJ, Hummel T, Hankir MK, Kühnel T, Vielsmeier V, Bohr C.. Psychophysical tests reveal impaired olfaction but preserved gustation in COVID-19 patients. Int Forum Allergy Rhinol. 2020:10(9):1105–1107. doi: 10.1002/alr.22655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hothorn T, Bretz F, Westfall P.. Simultaneous inference in general parametric models. Biom J. 2008:50(3):346–363. doi: 10.1002/bimj.200810425 [DOI] [PubMed] [Google Scholar]
- Huang N, Pérez P, Kato T, Mikami Y, Okuda K, Gilmore RC, Conde CD, Gasmi B, Stein S, Beach M, et al. ; NIH COVID-19 Autopsy Consortium. SARS-CoV-2 infection of the oral cavity and saliva. Nat Med. 2021:27(5):892–903. doi: 10.1038/s41591-021-01296-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iravani B, Arshamian A, Ravia A, Mishor E, Snitz K, Shushan S, Roth Y, Perl O, Honigstein D, Weissgross R, et al. Relationship between odor intensity estimates and COVID-19 prevalence prediction in a Swedish population. Chem Senses. 2020:45(6):449–456. doi: 10.1093/chemse/bjaa034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy JL, Hubbard MA, Huyett P, Patrie JT, Borish L, Payne SC.. Sino-nasal Outcome Test (SNOT-22): a predictor of post-surgical improvement in patients with chronic sinusitis. Ann Allerg Asthma Immunol 2013:111(4):246–251.e2. doi: 10.1016/j.anai.2013.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Yoo S-J, Clijsters M, Backaert W, Vanstapel A, Speleman K, Lietaer C, Choi S, Hether TD, Marcelis L, et al. Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell. 2021:184(24):5932–5949.e15. doi: 10.1016/j.cell.2021.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis BN, Hummel T, Hugentobler M, Giger R, Lacroix JS.. Ratings of overall olfactory function. Chem Senses. 2003:28(8):691–694. doi: 10.1093/chemse/bjg061 [DOI] [PubMed] [Google Scholar]
- Lê S, Josse J, Husson F.. FactoMineR: an R package for multivariate analysis. J Stat Softw 2008:25:1–18. doi: 10.18637/jss.v025.i01 [DOI] [Google Scholar]
- Le Bon SD, Payen L, Prunier L, Steffens Y, Horoi M, Vaira LA, Hopkins C, Lechien JR, Saussez S.. Making scents of loss of taste in COVID-19: is self-reported loss of taste due to olfactory dysfunction? A prospective study using psychophysical testing. Int Forum Allerg Rhinol 2021:11(10):1504–1507. doi: 10.1002/alr.22815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bon SD, Pisarski N, Verbeke J, Prunier L, Cavelier G, Thill M-P, Rodriguez A, Dequanter D, Lechien JR, Le Bon O, et al. Psychophysical evaluation of chemosensory functions 5 weeks after olfactory loss due to COVID-19: a prospective cohort study on 72 patients. Eur Arch Otorhinolaryngol. 2021:278(1):101–108. doi: 10.1007/s00405-020-06267-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Stolper S, Draf J, Haehner A, Hummel T.. Smell, taste and trigeminal function: similarities and differences between results from home tests and examinations in the clinic. Rhinology. 2022:60(4):293–300. doi: 10.4193/Rhin21.430 [DOI] [PubMed] [Google Scholar]
- Lötsch J, Hummel T.. Clinical usefulness of self-rated olfactory performance: a data science-based assessment of 6000 patients. Chem Senses. 2019:44(6):357–364. doi: 10.1093/chemse/bjz029 [DOI] [PubMed] [Google Scholar]
- Maechler, M., Rousseeuw P, Struyf A, Hubert M, Hornik K.. cluster: Cluster Analysis Basics and Extensions. R package version 2.1.4 — For new features, see the ‘Changelog’ file (in the package source), 2022. https://CRAN.R-project.org/package=cluster
- Mazzatenta A. Physiological discrimination and correlation between olfactory and gustatory dysfunction in long-term COVID-19. Physiol Rep. 2022:10(22):e15486. doi: 10.14814/phy2.15486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshontz H, Campbell L, Ebersole CR, IJzerman H, Urry HL, Forscher PS, Grahe JE, McCarthy RJ, Musser ED, Antfolk J, et al. The psychological science accelerator: advancing psychology through a distributed collaborative network. Adv Meth Pract Psychol Sci. 2018:1(4):501–515. doi: 10.1177/2515245918797607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C, Cain WS.. Taste and olfaction: independence vs interaction. Physiol Behav 1980:24(3):601–605. doi: 10.1016/0031-9384(80)90257-7 [DOI] [PubMed] [Google Scholar]
- Murphy C, Cain WS, Bartoshuk LM.. Mutual action of taste and olfaction. Sensory Processes 1977:1(3):204–211. [PubMed] [Google Scholar]
- Nielsen, F. Introduction to HPC with MPI for data science. Springer Cham. 2016. https://link.springer.com/book/10.1007/978-3-319-21903-5 [Google Scholar]
- Ohla K, Veldhuizen MG, Green T, Hannum ME, Bakke AJ, Moein S, Tognetti A, Postma EM, Pellegrino R, Hwang L.-D, et al. A follow-up on quantitative and qualitatice olfactory dysfunction and other symptoms in patients recovering from COVID-19 smell loss. Rhinology 2022:60(3):207–217. doi: 10.4193/Rhin21.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma V, Ohla K, Veldhuizen MG, Niv MY, Kelly CE, Bakke AJ, Cooper KW, Bouysset C, Pirastu N, Dibattista M, et al. ; GCCR Group Author. More than smell-COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. Chem Senses. 2020:45(7):609–622. doi: 10.1093/chemse/bjaa041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrocelli M, Ruggiero F, Baietti AM, Pandolfi P, Salzano G, Salzano FA, Lechien JR, Saussez S, De Riu G, Vaira LA.. Remote psychophysical evaluation of olfactory and gustatory functions in early-stage coronavirus disease 2019 patients: the Bologna experience of 300 cases. J Laryngol Otol. 2020:134(7):571–576. doi: 10.1017/S0022215120001358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribitkin E, Rosenthal MD, Cowart BJ.. Prevalence and causes of severe taste loss in a chemosensory clinic population. Ann Otol Rhinol Laryngol. 2003:112(11):971–978. doi: 10.1177/000348940311201110 [DOI] [PubMed] [Google Scholar]
- Rozin P. “Taste-smell confusions” and the duality of the olfactory sense. Percept Psychophys. 1982:31(4):397–401. doi: 10.3758/BF03202667 [DOI] [PubMed] [Google Scholar]
- Sakaguchi W, Kubota N, Shimizu T, Saruta J, Fuchida S, Kawata A, Yamamoto Y, Sugimoto M, Yakeishi M, Tsukinoki K.. Existence of SARS-CoV-2 entry molecules in the oral cavity. Int J Mol Sci . 2020:21(17):6000. doi: 10.3390/ijms21176000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saniasiaya J, Islam MA, Abdullah B.. Prevalence and characteristics of taste disorders in cases of COVID-19: a meta-analysis of 29,349 patients. Otolaryngol--Head Neck Surg 2021:165(1):33–42. doi: 10.1177/0194599820981018 [DOI] [PubMed] [Google Scholar]
- Seiden AM. Postviral olfactory loss. Otolaryngol Clin North Am. 2004:37(6):1159–1166. doi: 10.1016/j.otc.2004.06.007 [DOI] [PubMed] [Google Scholar]
- Sievert, C. (2019). Interactive web-based data visualization with R, plotly, and shiny. https://plotly-r.com/
- Snitz K, Honigstein D, Weissgross R, Ravia A, Mishor E, Perl O, Karagach S, Medhanie A, Harel N, Shushan S, et al. An olfactory self-test effectively screens for COVID-19. Commun Med 2022:2(1):34. doi: 10.1038/s43856-022-00095-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira LA, Deiana G, Fois AG, Pirina P, Madeddu G, De Vito A, Babudieri S, Petrocelli M, Serra A, Bussu F, et al. Objective evaluation of anosmia and ageusia in COVID -19 patients: single-center experience on 72 cases. Head Neck 2020:42(6):1252–1258. doi: 10.1002/hed.26204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira LA, Lechien JR, Salzano G, Salzano FA, Maglitto F, Saussez S, De Riu G.. Gustatory dysfunction: a highly specific and smell-independent symptom of COVID-19. Indian J Otolaryngol Head Neck Surg. 2020. doi: 10.1007/s12070-020-02182-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira LA, Salzano G, Petrocelli M, Deiana G, Salzano FA, De Riu G.. Validation of a self-administered olfactory and gustatory test for the remotely evaluation of COVID-19 patients in home quarantine. Head Neck 2020:42(7):1570–1576. doi: 10.1002/hed.26228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir EM, Reed DR, Pepino MY, Veldhuizen MG, Hayes JE.. Massively collaborative crowdsourced research on COVID19 and the chemical senses: insights and outcomes. Food Qual Pref. 2022:97:104483. doi: 10.1016/j.foodqual.2021.104483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H, Averick M, Bryan J, Chang W, McGowan LD, François R, Grolemund G, Hayes A, Henry L, Hester J, et al. Welcome to the Tidyverse. J Open Source Softw. 2019:4(43):1686. doi: 10.21105/joss.01686 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and code are available in the project GitHub repository (https://github.com/GCCR/GCCR004) with Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International Public License (CC BY-NC-ND 4.0; https://creativecommons.org/licenses/by-nc-nd/4.0/).