Abstract
All human immunodeficiency virus (HIV) isolates can grow readily in primary CD4+ T cells, but they can be distinguished by their ability to replicate in macrophages and established T-cell lines. The macrophage-tropic viruses are generally non-syncytium inducing (NSI), whereas the T-cell-line-tropic viruses are syncytium inducing (SI) in cultured cells. We now demonstrate that infection of CD4+ T cells by NSI and SI viruses shows a differential effect on production of β-chemokines and gamma interferon. Infection by NSI viruses increased production of MIP-1α, MIP-1β, and gamma interferon, whereas infection by SI viruses had no effect or decreased production of these cytokines. Production of RANTES was slightly increased during infection by both virus phenotypes. This differential effect of NSI and SI viruses was observed at the level of β-chemokine mRNA as well as at the level of protein expression. Infection by NSI viruses also increased CD4+ cell proliferation. These results may have relevance for a differential role of HIV strains in AIDS pathogenesis.
All isolates of human immunodeficiency virus (HIV) can infect primary CD4+ T cells, but they can show a great variability with respect to biological properties such as replication rate, cytotropism, and syncytium-inducing (SI) capability (20). The viral strains which infect macrophages are generally nonsyncytium inducing (NSI) and use the chemokine receptor CCR5 for entry. Viral isolates that infect transformed CD4+ T-cell lines are of the SI phenotype and use the chemokine receptor CXCR4 (1, 4). Most viruses isolated in the early stages of infection and during the asymptomatic phase appear to be of the NSI macrophage-tropic phenotype. The emergence of T-cell-line-tropic SI viruses later in the course of the infection generally correlates with CD4+ T-cell decline and the development of AIDS (7, 35, 36). The molecular basis of the NSI-SI switch involves modifications in the viral envelope protein which can change coreceptor usage from CCR5 to CXCR4 and probably to other additional coreceptors (1, 2, 4, 11, 20).
Certain members of the β-chemokine family such as MIP-1α, MIP-1β, RANTES, and MCP-2 have been shown to inhibit infection by NSI but not SI strains of HIV-1 (10, 14, 15, 25). These chemokines may produce their anti-HIV effect by blocking virus interaction with CCR5 or down-regulating expression of this receptor (1, 37). The clinical relevance of chemokines in the transmission and pathogenesis of HIV is still poorly understood. In addition to competing with HIV for shared coreceptors, chemokines may play a crucial role in the attraction of viral target cells to areas of infection and the generation of an antiviral response (12). Attempts to correlate levels of chemokine production in vitro with the clinical stage of infected individuals have produced variable results (3, 8, 24, 32, 38), which may be partially explained by the differences in the culture conditions and the cell populations examined.
HIV infection itself has also been suggested to be a major factor influencing immune response by altering the production of certain cytokines secreted by cells of the immune system (5, 13, 20, 29). In the present study, we have examined the influence of HIV infection on the production of β-chemokines and other cytokines. Our results show a differential effect of NSI and SI viruses on the production by CD4+ T cells of the β-chemokines MIP-1α and MIP-1β, as well as gamma interferon (IFN-γ). In addition, infection by NSI viruses enhanced CD4+ cell proliferation. The results could provide insight into the ability of certain viruses to be preferentially expressed and to establish infection in lymphoid tissue during primary infection. These effects could play an important role in the immunopathology of HIV infection.
MATERIALS AND METHODS
Viruses.
Four primary HIV-1 isolates (designated SV, NB, LSP, and EM) were obtained from the peripheral blood mononuclear cells (PBMC) of clinically healthy HIV-infected subjects by standard procedures (6). One primary isolate (KP) was recovered from a subject with AIDS. The biologic phenotype of the SV, NB, and LSP isolates was NSI as determined in cultured MT-2 cells (18); EM, KP, and the SF2 strain of HIV-1, isolated from a patient with candidiasis (21), were of the SI phenotype. EM, KP, and SF2 have a tropism for T-cell lines in vitro and are resistant to the inhibitory effects of the β-chemokines, MIP-1α, MIP-1β, and RANTES. The other isolates are chemokine sensitive (25). All viruses have been cultured only in PBMC. The 50% tissue culture infectious dose (TCID50) for each virus was determined in PBMC as described elsewhere (28). In brief, virus was diluted 10-fold and inoculated onto PBMC plated in 10 replicate wells. The extent of replication was measured every 3 days in the culture fluid by a p24 antigen enzyme-linked immunosorbent assay (ELISA). The final titer was calculated at the peak time of virus production (28).
Cells and cell culture medium.
For cell culture assays, PBMC were obtained from heparinized whole blood from HIV-seronegative donors (Irwin Memorial Blood Centers, San Francisco, Calif.) by Ficoll-Hypaque density gradient centrifugation (Sigma Chemical, St. Louis, Mo.) (6). CD4+ cells were isolated from the PBMC by using anti-CD4 antibody-coupled immunomagnetic beads (Dynal Inc., Lake Success, N.Y.) (26), resulting in more than 95% CD4+ CD3+ cells (<1% CD8+ cells) as assessed by flow cytometry (23). CD8+ cells were isolated with anti-CD8 antibody-coupled immunomagnetic beads from the PBMC after 3 days of stimulation with phytohemagglutinin (PHA; Sigma Chemical) (3 μg/ml) (26). Cell cultures were grown in RPMI 1640 medium (BioWhittaker, Walkersville, Md.) supplemented with 10% heat-inactivated (56°C, 30 min) fetal calf serum (FCS) (Gemini Bioproducts, Calabasas, Calif.), 100 U of recombinant human interleukin 2 (IL-2) (Glaxo Wellcome, Research Triangle Park, N.C.) per ml, 2 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin (Cell Culture Facility, University of California, San Francisco) per ml (complete medium).
Cultures of acutely infected peripheral blood cells.
PBMC and purified CD4+ cells were cultured in the RPMI 1640 complete medium in the presence of 3 μg of PHA per ml for 3 days at a cell density of 3 × 106/ml. The cells were then washed and incubated at 37°C for 30 min with Polybrene (Sigma; 2 μg/ml). Subsequently 10 TCID50 of the virus strains SV, NB, EM, and SF2 per 106 cells or a dilution of the LSP or KP viruses that yielded similar levels of reverse transcriptase (RT) activity (16) was used for infection.
Cells were infected for 2 h at 37°C and then washed and plated at a density of 106/ml in duplicate in complete medium. The cultures were passaged every 2 to 3 days for 2 weeks. From day 7, the cell density was adjusted to 1 × 106 or 2 × 106 cells/ml at each passage. The medium was exchanged at each passage, and the collected fluid was centrifuged at 3,000 rpm (Sorvall RT6000) for 20 min to remove cellular debris and stored frozen at −70°C. CD14+ cells were not detected in the CD4+ T-cell cultures (<1% by fluorescence-activated cell sorting analyses), and the possible contribution of an adherent population was excluded by transferring the culture to new plates on days 7 and 10. Viral replication was monitored by measuring RT activity in the culture fluid (16).
Assays involving gp120 from NSI and SI viruses.
PHA-stimulated CD4+ cells were cultured for 2 weeks in the presence of gp120 from an NSI (gp120 Thai clade E) or an SI (SF2 clade B) strain of HIV at 10, 100, and 1,000 ng/ml (strains provided by Susan Barnett, Chiron Corp., Emeryville, Calif.). Cell culture and collection of the supernatants were performed as described above.
Measurement of β-chemokines in the culture supernatants.
The quantity of RANTES, MIP-1α, MIP-1β, IFN-γ, and IL-4 in duplicate culture fluids was determined with solid-phase ELISA kits from R&D Systems (Minneapolis, Minn.). The heat-inactivated FCS and recombinant human IL-2, used in cell culture, did not contain any antigenically cross-reactive RANTES, MIP-1α, or MIP-1β as determined by ELISA.
Detection and quantitation of mRNA for chemokines.
Total cellular RNA was isolated by suspension of CD4+ cells in Trizol (Gibco-BRL, Gaithersburg, Md.) followed by chloroform extraction and isopropanol precipitation. RNA was resuspended in nuclease-free water and quantitated by UV absorbance at 260 nm. RNA (3 μg) from each sample was used for detection and comparative analysis of chemokine-mRNA species by the RiboQuant Multi-Probe RNase protection assay system according to the manufacturer’s instructions (Pharmingen, San Diego, Calif.).
Flow cytometry.
Cells from the culture sample were incubated for 4 h at 37°C with Brefeldin A (Sigma; 1 μg/ml). Subsequently the cells were fixed and permeabilized with the FIX & PERM cell permeabilization kit according to the manufacturer’s instructions (Caltag Laboratories, South San Francisco, Calif.). This procedure gives the antibody access to intracellular structures and retains the morphological scatter characteristic of the intact cells. Monoclonal antibody p24 conjugated with fluorescein isothiocyanate (Coulter, Miami, Fla.) (used at 1 μg/106 cells) was provided by Alan Landay (Rush Presbyterian-St. Luke’s Medical Center, Chicago, Ill.). Monoclonal antibody for MIP-1α (mouse anti-human MIP-1α–phycoerythrin) was purchased from Pharmingen. Monoclonal antibodies to CD4, CD8, and CD14 were obtained from Becton Dickinson (San Jose, Calif.). To determine the percentage of p24+ cells, a total of 15,000 events/sample were analyzed. Dead cells and tissue debris were excluded according to forward and side scatter properties in order to analyze only the population with the morphology of live cells.
Proliferation by thymidine uptake.
Lymphocytes from uninfected and infected cultures on day 13 were resuspended in RPMI 1640 medium with 10% FCS and plated at 105 cells/well in 96-well plates. [3H]thymidine was added to the cultures, and 10 to 16 h later, the amount of radioactivity incorporated was determined in a scintillation counter by standard procedures.
Statistics.
Data were analyzed by the Wilcoxon rank-sum test and the Wilcoxon signed-rank test.
RESULTS
β-Chemokine production by uninfected PBMC and CD4+ and CD8+ cells.
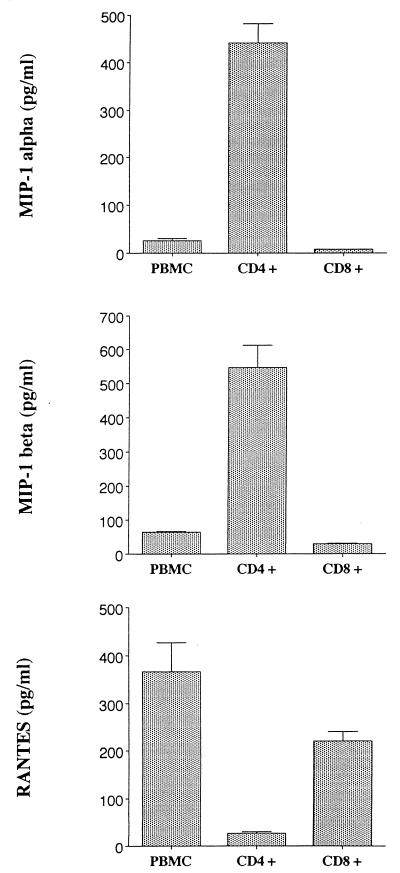
To evaluate the effect of HIV infection on the production of MIP-1α, MIP-1β, and RANTES, we first examined the pattern of chemokines produced by uninfected cultures of PBMC or CD4+ or CD8+ T cells previously stimulated with PHA. Culture supernatants from different time points were evaluated by ELISA for the levels of MIP-1α, MIP-1β, and RANTES. As noted before (24), no difference in β-chemokine production was noted immediately after mitogen stimulation. However, we were particularly interested in the pattern of chemokine production after several days of culture, since this period is when virus production is maximal after acute infection. By day 13 after PHA stimulation, CD4+ T cells showed a different pattern of chemokine production from those of CD8+ T cells and PBMC. The separated CD4+ T cells produced predominantly MIP-1α and MIP-1β (846 ± 518 and 976 ± 728 pg/ml, respectively), whereas CD8+ T cells and PBMC produced predominantly RANTES (455 ± 274 and 1,324 ± 621 pg/ml, respectively) (Fig. 1). The similarity in the pattern of chemokine production between PBMC and purified CD8+ T cells may be partially explained by CD8+ T cells being the predominant cell population in the PBMC culture as assessed on day 13 by fluorescence-activated cell sorting analyses (CD8+ CD4− cells, 78.5% ± 4.9%; CD4+ CD8−, 15% ± 1.4%). The potential role of CD8+ cells in suppressing MIP-1α and MIP-1β production in PBMC is presently being studied.
FIG. 1.
PBMC or purified CD4+ cells were stimulated for 3 days with 3 μg of PHA per ml, washed, and subsequently passaged every 2 to 3 days by exchanging the entire amount of culture fluid with fresh culture medium. Purified CD8+ cells were obtained from PBMC stimulated with PHA for 3 days. On day 7 and thereafter, cells were replated at a density of 2 × 106/ml. The concentration of chemokine in the cell culture fluids was measured by ELISA in duplicate (Quantikine; R&D Systems). The results show the maximal level of chemokines produced (adjusted per 106 viable cells) as measured in cultured fluids on day 13. The data are representative of two to four different experiments.
The ability of CD4+ T cells to produce chemokines, particularly MIP-1α and MIP-1β, increased progressively during the course of the 2-week culture. Conversely, the ability of CD8+ cells and PBMC to produce MIP-1α and MIP-1β remained unchanged, whereas RANTES production increased over the 2-week period (data not shown).
Effect of infection with NSI and SI isolates of HIV on the production of MIP-1α, MIP-1β, and RANTES.
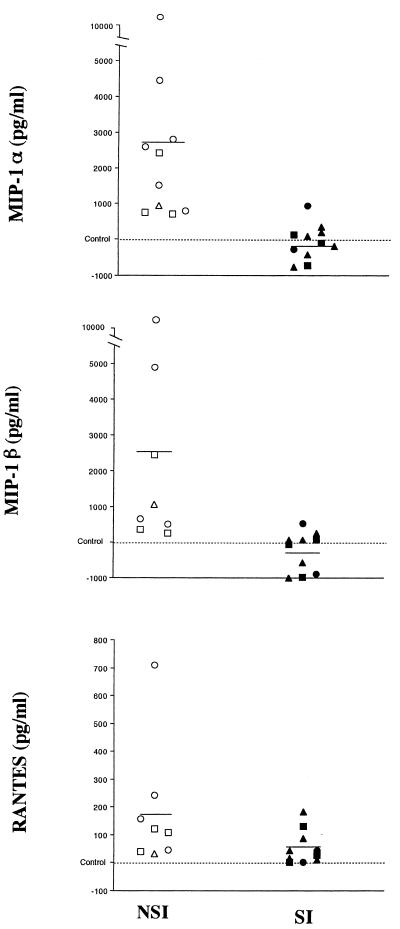
PHA-stimulated PBMC or CD4+ T cells from seronegative donors were acutely infected with a low input (10 TCID50) of NSI or SI HIV isolates. The infection of CD4+ cells influenced chemokine production depending on the viral phenotype used. Infection of the cells with NSI isolates led to a significant increase in MIP-1α and MIP-1β production compared to uninfected controls and cultures infected by SI viruses (P < 0.01); in contrast, infection with SI isolates generally gave a slight but not significant reduction in release of MIP-1α and MIP-1β compared to control uninfected cells (mean values: for MIP-1α, NSI, 3,620 pg/ml; SI, 400 pg/ml; control, 1,018 pg/ml; for MIP-1β, NSI, 3,088 pg/ml; SI, 325 pg/ml; control, 511 pg/ml) (Fig. 2). Both virus phenotypes increased RANTES production compared to control uninfected cultures (P < 0.01) although low levels were consistently detected (mean values: NSI, 205 pg/ml; SI, 75 pg/ml; control, 22 pg/ml) (Fig. 2). Infection of PBMC by either NSI or SI virus phenotypes did not produce any effect on the levels of production of these chemokines compared to the levels produced by control cultures (data not shown).
FIG. 2.
Relative variation in β-chemokine production by HIV-1-infected CD4+ T cells versus the uninfected controls. The differences among the levels of MIP-1α, MIP-1β, and RANTES produced by CD4+ T cells infected with several viral isolates (NSI, SV [○], NB [□], and LSP [▵]; SI, KP [▴], EM [■], and SF2 [•]) and their concurrent control cultures (uninfected cells from the same donors) are shown. Cells from seven different donors were used. Purified CD4+ T cells were stimulated for 3 days with 3 μg of PHA per ml, washed, and subsequently infected with various NSI and SI virus strains and cultured as described in Materials and Methods. Each symbol represents results with the virus in a separate experiment. The concentration of chemokines in the cell culture fluids was measured by ELISA in duplicate (Quantikine; R&D Systems). The levels of chemokines measured in culture fluids from day 13 are shown (one measurement from day 11 of cells plated at 106 on day 8 is included) in reference to the level in the control cultures. Control level ranges (picograms per milliliter): MIP-1α, 58 to 4,261; MIP-1β, 46 to 1,117; RANTES, undetectable to 38. All results are adjusted per 106 viable cells. Cell numbers (106) on day 13: control (n = 6), 1.2 ± 0.21; NSI (n = 8), 0.93 ± 0.24; SI (n = 9), 0.67 ± 0.17. Compared to control cultures, NSI viruses increased MIP-1α and MIP-1β production significantly (P < 0.01); both NSI and SI viruses increased RANTES production significantly (P < 0.01) (Wilcoxon signed-rank test).
For these studies, in order to exclude the possibility that the effect on chemokine production resulted from a variation in the number of viable cells, infected and uninfected cultures were plated at the same density (1 × 106 or 2 × 106 cells/ml) at each passage and the data were adjusted per 106 viable cells. Viability in the cultures of CD4+ T cells infected with NSI isolates, as assessed by trypan blue exclusion on days 11 and 12, was 83 to 87%, whereas viability in cultures infected with SI isolates was 70 to 77%. Control cultures showed 96 to 97% viable cells. Thus, infection by both types of virus isolates gave some reduction in the absolute number of cells compared to control cultures, but only NSI isolates produced an increase in the level of chemokine production measured per viable cell (Fig. 2).
The differential effect on the production of MIP-1α and MIP-1β does not correlate with the extent of virus replication, the percentage of viable infected cells, or the viral envelope gp120.
The extent to which a strain of HIV-1 can spread among the cells inoculated in vitro can be reflected by the kinetics and level of virus replication. The different effects on chemokine production by NSI and SI isolates could be related to a differential ability of the virus to replicate and spread in the cultures of CD4+ T cells. We, therefore, quantified the level of virus replication by the RT assay and also by the percentage of infected cells detected by fluorescence analysis for the intracellular presence of the viral p24 core antigen.
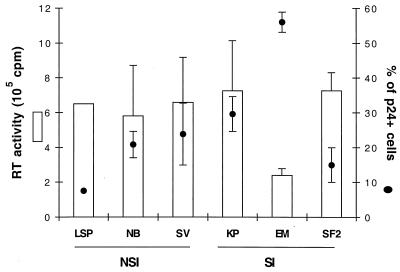
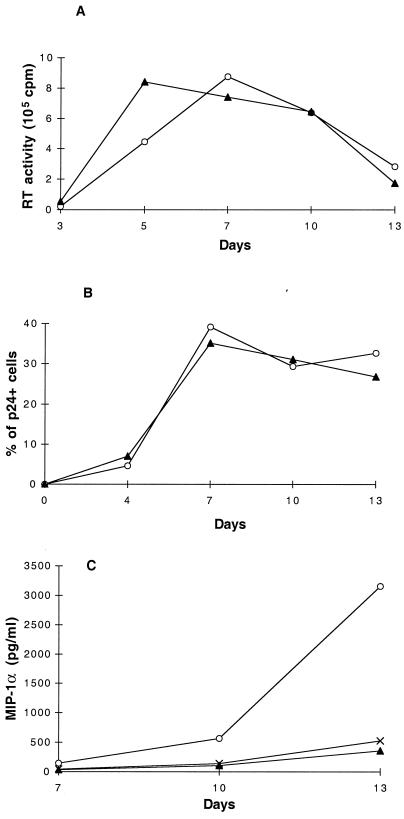
No substantial differences were detected in the levels of virus replication between NSI and SI virus isolates (Fig. 3 and 4A), with the exception of the SI isolate EM, which consistently gave a lower rate and lower level of virus production (Fig. 3). Notably, a similar percentage of infected cells (p24+) was detected between cultures infected with the NSI isolate SV and the SI isolate KP (Fig. 3 and 4B). The higher level of infected cells in cultures infected with the EM isolate may be related to its lower rate of replication, which could affect the extent of cell death and result in an accumulation of infected cells. In these studies, no direct correlation was observed between the kinetics of virus replication and spread and the level of chemokine production. The peak of virus replication occurred prior to the peak of β-chemokine production (Fig. 4C). Using dual staining techniques, we determined that the increased expression of MIP-1α and MIP-1β during NSI virus replication could be found in both p24-positive and p24-negative cells (data not shown).
FIG. 3.
Viral replication and percentages of HIV-infected cells in cultures of CD4+ T cells inoculated with different virus isolates. Purified CD4+ T cells were stimulated for 3 days with 3 μg of PHA per ml, washed, and subsequently infected with various NSI and SI virus strains and cultured as described in Materials and Methods. Viral replication was monitored by measuring the amount of RT activity per milliliter of culture fluid (16). Peak levels of RT activity during the course of the culture are shown. The percentage of productively infected CD4+ T cells was analyzed by flow cytometry for the intracellular presence of the viral core p24 antigen. As a positive control, the chronically infected cell line E line (17) was used and showed >97% p24+ cells. In the uninfected control, the p24 antigen was undetectable (<1%) (data not shown). Numbers of separate studies performed with the different virus isolates: (for RT) LSP, n = 1; NB, n = 3; SV, n = 6; KP, n = 7; EM, n = 3; SF2, n = 2; (for flow cytometry) LSP, n = 1; NB, n = 3; SV, n = 4; KP, n = 5; EM, n = 3; SF2, n = 3.
FIG. 4.
Kinetics of virus replication, number of infected cells, and production of MIP-1α in CD4+ T cells infected with NSI and SI virus isolates. Purified CD4+ T cells from the same donor were stimulated for 3 days with 3 μg of PHA per ml, washed, and subsequently infected with 10 TCID50 of the NSI virus isolate SV (○) or the SI virus isolate KP (▴) per 106 cells. After infection, the cultures were passaged every 2 to 3 days by exchanging the entire amount of culture fluid with fresh culture medium. On day 7 and thereafter, cells were replated at 106 cells/ml. Viral replication was monitored by measuring the amount of RT activity per milliliter of culture fluid (16) (A). The percentage of productively infected CD4+ T cells was determined by flow cytometry measuring the intracellular presence of the viral p24 antigen (B). As a positive control, the chronically infected E line was used, showing >97% p24+ cells. In the uninfected control, the p24 core antigen was undetectable (<1%) (data not shown). The concentration of MIP-1α per milliliter of cell culture fluid was measured by ELISA in duplicate (Quantikine; R & D Systems). Results with uninfected control cells are shown (×) (C). The results are representative of seven separate studies. Similar findings were made for MIP-1β production (data not shown).
To determine whether the gp120 from NSI and SI isolates was sufficient to reproduce the effect of the viruses on chemokine production, we cultured PHA-stimulated CD4+ T cells for 13 days in the presence of 10, 100, and 1,000 ng of gp120 from an NSI and an SI strain of HIV-1 (Thai E and SF2, respectively) per ml. No effect on the production of MIP-1α, MIP-1β, and RANTES compared to untreated, uninfected control cultures was detected (data not shown). In this regard, a concentration of 1,000 ng of gp120 per ml for HIV-2 has been previously reported to induce β-chemokine production in vitro (30).
Effect of HIV infection on β-chemokine mRNA expression.
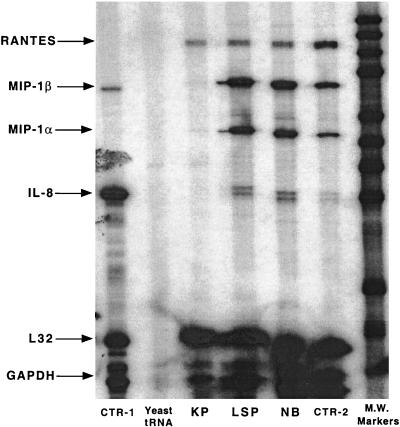
To investigate further the mechanism of the differential effects on β-chemokine production by NSI and SI viruses, we determined the levels of mRNA for chemokines in the infected and uninfected CD4+ cell cultures by an RNase protection assay. The levels of expression of the constitutive genes L32 and glyceraldehyde-3-phosphate dehydrogenase were included to allow quantitative comparisons among the different samples. As noted in Fig. 5, infection with the SI isolate KP abolished the expression of mRNA for MIP-1α and MIP-1β as well as IL-8. In contrast, infection with the NSI viruses LSP and NB resulted in an increased expression of mRNA for MIP-1α, MIP-1β, and IL-8 compared to the control levels. The mRNA level for RANTES was not affected by infection with either virus phenotype.
FIG. 5.
Effect of HIV infection on the level of chemokine mRNA production. Purified CD4+ T cells from the same donor were stimulated for 3 days with 3 μg of PHA per ml, washed, and subsequently infected with the SI virus isolate (KP) and the NSI isolates (LSP and NB). After infection, the cultures were passaged every 2 to 3 days by exchanging the entire amount of culture fluid with fresh culture medium. On day 8, cells were replated at 106 cells/ml. Total mRNA was isolated on day 11, and the level of mRNA for β-chemokines was detected by an RNase protection assay (3 μg of cellular RNA on total reaction) (RPA system; Pharmingen). L32 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as controls. CTR-1 is a positive control from Pharmingen; the CTR-2 control came from uninfected cells. The results were quantitated by densitometry with NIH Image version 1.61 (available from zippy.nimh.nih.gov). A linear range film exposure was used for quantitation. The film was overexposed to include the effect of infection on the level of IL-8 mRNA. The analysis shows differences between NSI and control mRNA of 2.5 to 4 times for the level of the MIP mRNAs and 6 times for the level of IL-8 mRNA. Differences between NSI and SI are greater than 10-fold for MIPs. IL-8 mRNA was not detected in SI-infected samples. M.W., molecular weight.
Differential effect of NSI and SI HIV strains on the production of IFN-γ.
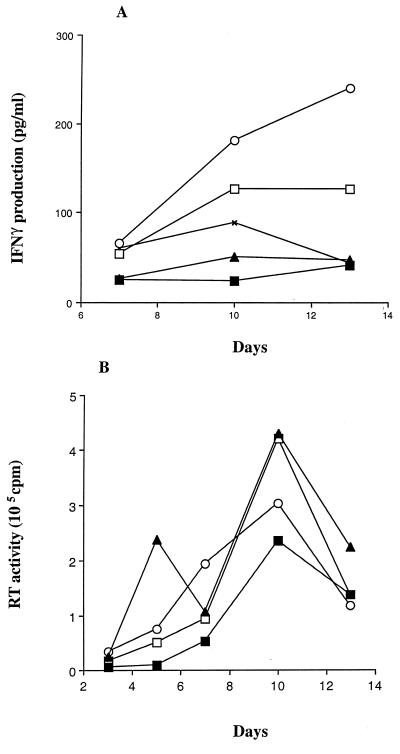
The release of the β-chemokines MIP-1α, MIP-1β, and RANTES has previously been associated with a type 1 immune response (34). We therefore considered the possible differential effect of NSI and SI isolates on the release of IFN-γ and IL-4, cytokines prototypical of a Th1 and a Th2 response, respectively (9). IL-4 was not detected in any of the culture fluids, including control culture fluids (<15 pg/ml). IFN-γ production was enhanced in CD4+ T cells infected with the NSI strains, whereas it was reduced in the cultures infected with the SI strains (Fig. 6A). This effect did not correlate directly with the level of virus replication (Fig. 6B).
FIG. 6.
Effect of HIV infection on the production of IFN-γ. Purified CD4+ T cells were stimulated for 3 days with 3 μg of PHA per ml, washed, and subsequently infected with 10 TCID50 of the NSI virus strains SV (□) and NB (○) and the SI strain EM (▴) per 106 cells or a dilution of KP (■), an SI virus, that yielded similar levels of RT activity. The uninfected control culture was monitored for the same period. After infection, the cultures were passaged every 2 to 3 days by exchanging the entire amount of culture fluid with fresh culture medium. On day 7 and thereafter, cells were replated at 2 × 106 cells/ml. The concentration of IFN-γ in the cell culture fluids was measured by ELISA in duplicate (Quantikine; R & D Systems), and the data were adjusted per 106 cells (A). Viral replication was measured as RT activity per milliliter of culture fluid (16) (B). The results are representative of two separate experiments.
T-cell proliferation.
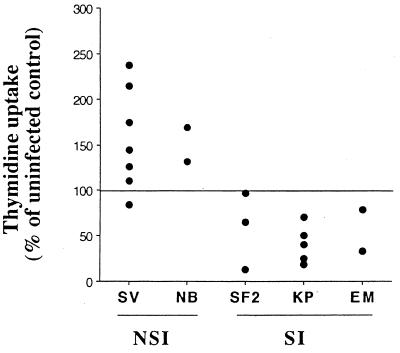
To examine the possibility that NSI HIV-1 isolates may cause a general stimulation of CD4+ T cells in addition to increasing production of certain cytokines, we measured the proliferation of CD4+ T cells infected by NSI or SI viruses. CD4+ T cells at 13 days after infection were plated in complete medium devoid of IL-2 in the presence of [3H]thymidine, and the amount of radioactivity incorporated was measured after 10 to 16 h. Day 13 was chosen since it corresponds to the time of maximal chemokine production (Fig. 2). The results showed that infection with the NSI isolates generally led to an increase in cell proliferation compared to the uninfected control, whereas infection with SI isolates resulted in a lower level of cell proliferation (Fig. 7).
FIG. 7.
Effect of HIV infection on T-cell proliferation. Lymphocytes from uninfected cultures and cultures infected with NSI virus isolates (SV and NB) or SI virus isolates (SF2, KP, and EM) were plated on day 13 in RPMI 1640 medium with 10% FCS at 105 cells/well in 96-well plates. [3H]thymidine (ICN Biomedicals, Costa Mesa, Calif.) was added to the cultures, and 10 to 16 h later, the amount of radioactivity incorporated was determined in a scintillation counter by standard procedures. Results of several separate experiments are presented and are statistically significant (NSI isolates versus control, P < 0.02; SI isolates versus control, P < 0.01; Wilcoxon signed-rank test).
DISCUSSION
A common finding during the asymptomatic stage of HIV infection is the presence of macrophage-tropic, NSI viruses in the blood of infected individuals (7, 35, 36). These viruses are often replaced by SI T-cell-line-tropic viruses as the infected individual progresses to disease (7, 20, 35, 36). What event(s) heralds the switch from the NSI to SI viruses that often precedes the drop in CD4+ T cells is not known but appears to be a compromise of the immune system, particularly CD8+ cell antiviral activity (19, 22). Recently, the β-chemokines, MIP-1α, MIP-1β, and RANTES, produced by several different cell types, have been found to inhibit infection of CD4+ cells by NSI but not by SI viruses (10). The role of these cytokines in HIV pathogenesis is not yet clear (24).
Although the approaches used in in vitro systems may not mimic directly the in vivo situation, the present studies were undertaken to determine whether infection of CD4+ cells by NSI versus SI viruses could influence β-chemokine production and thus play a role in the switch from the NSI to SI phenotype that occurs over time.
In the initial studies, it is noteworthy that we found a selective production of β-chemokines by immune cells after a 2-week period. Whereas uninfected CD4+ cells preferentially produced MIP-1α and MIP-1β, CD8+ cells selectively produced RANTES (Fig. 1). These results contrast with early findings showing no difference in β-chemokine production measured immediately after mitogen stimulation (24). These data are important for the observations made in subsequent studies with infected CD4+ cells. The results showed a differential effect of NSI and SI viruses on production of the β-chemokines MIP-1α and MIP-1β. At the peak of virus production, CD4+ cells infected by NSI virus strains had an increase in production of these β-chemokines, whereas cells infected by the SI viruses either showed no effect or had reduced production of these cytokines (Fig. 2). A slight increase in RANTES production (<200 pg/ml) was noted following infection by both NSI and SI viruses. This differential effect of NSI versus SI viruses on MIP-1α and MIP-1β production was not directly related to differences in level of viral replication, the number of virus-infected cells, or cell viability (Fig. 3 and 4). The observations support earlier findings in our laboratory with CD4+ lymphocytes (24) and by others using infection of macrophages with NSI strains (33). They differ somewhat from a recent study using a variety of NSI strains in cultured CD4+ cells (15). In those experiments, an increase in MIP-1α and MIP-1β was not consistently observed. These earlier results most likely reflect the lack of control for CD4+ cell viability and number (15). In further support of this differential effect of NSI viruses on β-chemokine production, the levels of mRNA for MIP-1α and MIP-1β were altered following virus infection (Fig. 5). We did not detect any effect on chemokine production by using the HIV-1 envelope gp120, whereas soluble HIV-2 gp120 has been reported elsewhere to enhance β-chemokine production in vitro (30). Whether the role of HIV-1 gp120 on chemokine production depends on the integrity of the virion and on the ability of cross-linking chemokine receptors (39) remains a possibility meriting further evaluation. Conceivably, the Tat protein that can increase transcription of the chemokine IL-8 and other cytokines (5, 31) plays a role in this phenomenon. This possibility is under further study.
Another unexpected observation was that production of the Th1 cytokine IFN-γ was increased by NSI viruses whereas the Th2 cytokine IL-4 was not detected by our assays in either infected or control cultures. IFN-γ, associated with a type 1 immune response, might preferentially elicit cell-mediated immune responses against HIV. The ability to induce immune activation may constitute a selective advantage for NSI HIV strains, rendering the host environment more receptive to the establishment of a productive infection. It would appear most likely that the increased production of the chemoattractant proteins by NSI viruses brings more inflammatory cells (containing target CD4+ T cells) to the site of HIV infection and thus encourages viral spread. In this sense, the NSI viruses profit from the effects of the chemokine production. Nevertheless, viral titers could eventually be lowered by the inhibitory activity of β-chemokines on HIV replication and by other suppressor factors produced by recruited CD8+ T cells (22). In any case, the NSI variants of HIV could modulate the host environment in favor of a chronic persistent infection. The subsequent appearance of SI strains, through a disruption in this complex balance between virus and host response, would lead to a faster progression of the disease.
The results of these experiments also indicated an increase in cell proliferation with NSI virus infection. It is known that HIV replicates most efficiently in activated cells (20, 27). Our findings suggest that NSI viruses play a role in activating cells after infection and thereby increase their ability to sustain replicating virus. Whether this activation is mediated directly by the virus or by cytokines released by the virus-infected cells remains to be determined.
In summary, the differential effect of NSI and SI viruses on CD4+ cell production of MIP-1α and MIP-1β and IFN-γ suggests that the virus subtype infecting an individual can influence the immunologic response. By inducing an inflammatory reaction, cells are brought to the site of HIV replication and can serve as further targets for the virus or as antiviral effector cells. Whereas we expected that the NSI viruses might decrease β-chemokine release and thus permit enhanced virus replication, the increased production of these cytokines and the induced proliferation of the target cells may in fact encourage viral spread to fresh CD4+ cells. With time, chemokine-resistant SI strains would emerge. Whether the induction by NSI viruses of IFN-γ which can enhance antiviral responses counters this apparent cell proliferation and increased number of CD4+ cells available for virus infection remains to be determined. Further studies should uncover how these different biological effects of NSI versus SI viruses might influence the antiviral activity of certain therapies and the pathogenic pathway of HIV infection.
ACKNOWLEDGMENTS
This research was supported by a grant from the National Institutes of Health (AI30350). G.G. was the recipient of a fellowship from the Istituto Superiore di Sanita, Rome, Italy. Dan Mourich is the recipient of a fellowship from the California State Universitywide AIDS Research Program.
We thank Michael Luther, Glaxo Wellcome, for providing the ELISA kits used in these studies; Robert Balderas, Pharmingen, for the RNase protection assay kit; and Edward Barker and Carl Mackewicz for helpful comments. Ann Murai and Christine Beglinger are thanked for help in preparation of the manuscript.
REFERENCES
- 1.Berger E A. HIV entry and tropism: the chemokine receptor connection. AIDS. 1997;11:s3–s16. [PubMed] [Google Scholar]
- 2.Bjorndal A, Deng H, Jansson M, Fiore J R, Colognesi C, Karlsson A, Albert J, Scarlatti G, Littman D R, Fenyo E M. Coreceptor usage of primary human immunodeficiency virus type 1 isolates varies according to biological phenotype. J Virol. 1997;71:7478–7487. doi: 10.1128/jvi.71.10.7478-7487.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blazevic V, Heino M, Ranki A, Jussila T, Krohn K J E. RANTES, MIP and interleukin-16 in HIV infection. AIDS. 1996;10:1435–1436. doi: 10.1097/00002030-199610000-00021. [DOI] [PubMed] [Google Scholar]
- 4.Broder C C, Collman R G. Chemokine receptors and HIV. J Leukocyte Biol. 1997;62:20–29. doi: 10.1002/jlb.62.1.20. [DOI] [PubMed] [Google Scholar]
- 5.Buonaguro L, Barillari G, Chang H K, Bohan C A, Kao V, Morgan R, Gallo R C, Ensoli B. Effects of the human immunodeficiency virus type 1 tat protein on the expression of inflammatory cytokines. J Virol. 1992;66:7159–7167. doi: 10.1128/jvi.66.12.7159-7167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castro B A, Weiss C D, Wiviott L D, Levy J A. Optimal conditions for recovery of the human immunodeficiency virus from peripheral blood mononuclear cells. J Clin Microbiol. 1988;26:2371–2376. doi: 10.1128/jcm.26.11.2371-2376.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng-Mayer C, Seto D, Tateno M, Levy J A. Biologic features of HIV that correlate with virulence in the host. Science. 1988;240:80–82. doi: 10.1126/science.2832945. [DOI] [PubMed] [Google Scholar]
- 8.Clerici M, Balotta C, Trabattoni D, Papagno L, Ruzzante S, Rusconi S, Fusi M L, Colombo M C, Galli M. Chemokine production in HIV-seropositive long-term asymptomatic individuals. AIDS. 1996;10:1432–1433. doi: 10.1097/00002030-199610000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Clerici M, Shearer G M. The Th1-Th2 hypothesis of HIV infection: new insights. Immunol Today. 1994;15:575–581. doi: 10.1016/0167-5699(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 10.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1alpha, and MIP-1beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 11.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook D N, Beck M A, Coffman T M, Kirby S L, Sheridan J F, Prganell I B, Smithies O. Requirement of MIP-1α for an inflammatory response to viral infection. Science. 1995;269:1583–1585. doi: 10.1126/science.7667639. [DOI] [PubMed] [Google Scholar]
- 13.Gessani S, Borghi P, Fantuzzi L, Varano B, Conti L, Puddu P, Belardelli F. Induction of cytokines by HIV-1 and its gp120 protein in human peripheral blood monocyte/macrophages and modulation of cytokine response during differentiation. J Leukocyte Biol. 1997;62:49–53. doi: 10.1002/jlb.62.1.49. [DOI] [PubMed] [Google Scholar]
- 14.Gong W, Howard O M, Turpin J A, Grimm M C, Ueda H, Gray P W, Raport C J, Oppenheim J J, Wang J M. Monocyte chemotactic protein-2 activates CCR5 and blocks CD4/CCR5-mediated HIV-1 entry/replication. J Biol Chem. 1998;273:4289–4292. doi: 10.1074/jbc.273.8.4289. [DOI] [PubMed] [Google Scholar]
- 15.Greco G, Barker E, Levy J A. Differences in HIV replication in CD4+ lymphocytes are not related to β-chemokine production. AIDS Res Hum Retroviruses. 1998;14:1407–1411. doi: 10.1089/aid.1998.14.1407. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman A D, Banapour B, Levy J A. Characterization of the AIDS-associated retrovirus reverse transcriptase and optimal conditions for its detection in virions. Virology. 1985;147:326–335. doi: 10.1016/0042-6822(85)90135-7. [DOI] [PubMed] [Google Scholar]
- 17.Kaminsky L S, McHugh T, Stites D, Volberding P, Henle G, Henle W, Levy J A. High prevalence of antibodies to AIDS-associated retroviruses (ARV) in acquired immune deficiency syndrome and related conditions and not in other disease states. Proc Natl Acad Sci USA. 1985;82:5535–5539. doi: 10.1073/pnas.82.16.5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koot M, Vos A H V, Keet R P M, de Goede R E Y, Dercksen M W, Terpstra F G, Coutinho R A, Miedema F, Tersmette M. HIV-1 biological phenotype in long-term infected individuals evaluated with an MT-2 cocultivation assay. AIDS. 1992;6:49–54. doi: 10.1097/00002030-199201000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Landay A L, Mackewicz C, Levy J A. An activated CD8+ T cell phenotype correlates with anti-HIV activity and asymptomatic clinical status. Clin Immunol Immunopathol. 1993;69:106–116. doi: 10.1006/clin.1993.1157. [DOI] [PubMed] [Google Scholar]
- 20.Levy J A. HIV and the pathogenesis of AIDS. 2nd ed. Washington, D.C: American Society for Microbiology; 1998. [Google Scholar]
- 21.Levy J A, Hoffman A D, Kramer S M, Landis J A, Shimabukuro J M, Oshiro L S. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984;225:840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- 22.Levy J A, Mackewicz C E, Barker E. Controlling HIV pathogenesis: the role of noncytotoxic anti-HIV activity of CD8+ cells. Immunol Today. 1996;17:217–224. doi: 10.1016/0167-5699(96)10011-6. [DOI] [PubMed] [Google Scholar]
- 23.Levy J A, Tobler L H, McHugh T M, Casavant C H, Stites D P. Long-term cultivation of T cell subsets from patients with acquired immune deficiency syndrome. Clin Immunol Immunopathol. 1985;35:328–336. doi: 10.1016/0090-1229(85)90093-5. [DOI] [PubMed] [Google Scholar]
- 24.Mackewicz C E, Barker E, Greco G, Reyes-Teran G, Levy J A. Do β-chemokines have clinical relevance in HIV infection? J Clin Invest. 1997;100:921–930. doi: 10.1172/JCI119608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackewicz C E, Barker E, Levy J A. Role of β-chemokines in suppressing HIV replication. Science. 1996;274:1393–1395. doi: 10.1126/science.274.5291.1393. [DOI] [PubMed] [Google Scholar]
- 26.Mackewicz C E, Ortega H W, Levy J A. CD8+ cell anti-HIV activity correlates with the clinical state of the infected individual. J Clin Invest. 1991;87:1462–1466. doi: 10.1172/JCI115153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Margolick J B, Volkman D J, Folks T M, Fauci A S. Amplification of HTLV-III/LAV infection by antigen-induced activation of T cells and direct suppression by virus of lymphocyte blastogenic responses. J Immunol. 1987;138:1719–1723. [PubMed] [Google Scholar]
- 28.McDougal J S, Cort S P, Kennedy M S, Cabridilla C D, Feorino P M, Francis D P, Hicks D, Kalyanaraman V S, Martin L S. Immunoassay for the detection and quantitation of infectious human retrovirus, lymphadenopathy-associated virus (LAV) J Immunol Methods. 1985;76:171–183. doi: 10.1016/0022-1759(85)90489-2. [DOI] [PubMed] [Google Scholar]
- 29.Merrill J E, Koyanagi Y, Chen I S Y. Interleukin-1 and tumor necrosis factor alpha can be induced from mononuclear phagocytes by human immunodeficiency virus type 1 binding to the CD4 receptor. J Virol. 1989;63:4404–4408. doi: 10.1128/jvi.63.10.4404-4408.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neoh L P, Akimoto H, Kaneko H, Hishikawa T, Hashimoto H, Hirose S, Kaneko Y, Yamamoto N, Sekigawa I. The production of beta-chemokines induced by HIV-2 envelope glycoprotein. AIDS. 1997;11:1062–1063. [PubMed] [Google Scholar]
- 31.Ott M, Emiliani S, Van Lint C, Herbein G, Lovett J, Chirmule N, McCloskey T W, Pahwa S, Verdin E. Immune hyperactivation of HIV-1 infected T cells mediated by tat and the CD28 pathway. Science. 1997;275:1481–1485. doi: 10.1126/science.275.5305.1481. [DOI] [PubMed] [Google Scholar]
- 32.Saha K, Bentsman G, Chess L, Volsky D J. Endogenous production of β-chemokines by CD4+, but not CD8+, T-cell clones correlates with the clinical state of human immunodeficiency virus type 1 (HIV-1)-infected individuals and may be responsible for blocking infection with non-syncytium-inducing HIV-1 in vitro. J Virol. 1998;72:876–881. doi: 10.1128/jvi.72.1.876-881.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidtmayerova H, Nottet H S L, Nuovo G, Raabe T, Flanagan C R, Dubrovsky L, Gendleman H E, Cerami A, Bukrinsky M, Sherry B. Human immunodeficiency virus type 1 infection alters chemokine beta peptide expression in human monocytes: implications for recruitment of leukocytes into brain and lymph nodes. Proc Natl Acad Sci USA. 1996;93:700–704. doi: 10.1073/pnas.93.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schrum S, Probst P, Fleischer B, Zipfel P F. Synthesis of the CC-chemokines MIP-1α, MIP-1β, and RANTES is associated with a type 1 immune response. J Immunol. 1996;157:3598–3604. [PubMed] [Google Scholar]
- 35.Tersmette M, de Goede R E Y, Al B J M, Winkel I N, Gruters R A, Cuypers H T, Huisman H G, Miedema F. Differential syncytium-inducing capacity of human immunodeficiency virus isolates: frequent detection of syncytium-inducing isolates in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. J Virol. 1988;62:2026–2032. doi: 10.1128/jvi.62.6.2026-2032.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tersmette M, Gruters R A, de Wolf F, de Goede R E Y, Lange J M A, Schellekens P T A, Goudsmit J, Huisman H G, Miedema F. Evidence for a role of virulent human immunodeficiency virus (HIV) variants in the pathogenesis of acquired immunodeficiency syndrome: studies on sequential HIV isolates. J Virol. 1989;63:2118–2125. doi: 10.1128/jvi.63.5.2118-2125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trkola A, Paxton W A, Monard S P, Hoxie J A, Siani M A, Thompson D A, Wu L, Mackay C R, Horuk R, Moore J P. Genetic subtype-independent inhibition of human immunodeficiency virus type 1 replication by CC and CXC chemokines. J Virol. 1998;72:396–404. doi: 10.1128/jvi.72.1.396-404.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ullum H, Cozzi Lepri A, Victor J, Aladdin H, Phillips A N, Gerstoft J, Skinhoj P, Pedersen B K. Induction of beta-chemokines in human immunodeficiency virus (HIV) infection: evidence that high levels of macrophage inflammatory protein 1-beta are associated with a decreased risk of HIV disease progression. J Infect Dis. 1998;177:331–336. doi: 10.1086/514192. [DOI] [PubMed] [Google Scholar]
- 39.Wong M, Fish E N. RANTES and MIP-1 alpha activate stats in T cells. J Biol Chem. 1998;273:309–314. doi: 10.1074/jbc.273.1.309. [DOI] [PubMed] [Google Scholar]