Abstract
AIM
To assess the efficacy versus the adverse effects of various concentrations of atropine in the prevention of myopia in Asian children.
METHODS
Databases (PubMed, EMBASE, the Cochrane Library and Web of science) were comprehensively searched from inception to April 2022. Types of studies included were randomized clinical trials (RCTs). The published languages were limited to English. Two researchers assessed the quality of included studies independently using Cochrane risk of bias tool based on the Cochrane Handbook for Systematic Reviews of Interventions. Funnel plots and Egger's test were used for detection of publication bias. Meta-analyses were conducted using STATA (version 15.0; StataCorp).
RESULTS
A total of 15 RCTs involving 2268 patients were included in the study. In the atropine group, spherical equivalent progressed at a significantly lower rate [weighted mean difference (WMD)=0.39, 95% confidence interval (CI): 0.23, 0.54] than in the control group. A WMD of 0.15 mm was associated with less axial elongation (95%CI -0.19, -0.10). Different doses showed statistically significant differences (P<0.05) and an improved effect could result from a higher concentration. Changes in photopic pupil size and mesopic pupil size in atropine group is 0.70 mm (95%CI: 0.33, 1.06) and 0.38 mm (95%CI: 0.22, 0.54) more than the control group. In the present Meta-analysis, no changes in accommodative amplitude (AA) were associated with atropine administration. Atropine administration increased the risk of adverse effects by 1.37 times.
CONCLUSION
Concentrations of less than 1% atropine are able to effectively retard diopter and axis growth of myopia in Asian children in a dose-dependent manner. Meanwhile, it caused pupil enlargement, but induced no change in the AA within this range. Further study is required to determine the dosage needed to achieve maximum efficacy and minimal side effects.
Keywords: atropine, myopia, Asian children, Meta-analysis
INTRODUCTION
Myopia, or nearsightedness, is one of the most common refractive errors. It occurs when the cornea or lens is too powerful (refractive myopia) or the eyeball is longer than normal (axial myopia), which causes distant objects to focus in front of rather than on the retina[1]. Eyeball development and eye axis elongation are usually the main concerns in myopia children, and the complications we focus on also primarily refer to axial myopia. Even though myopia can be corrected to improve the blurring of objects at a distance, a number of complications associated with myopia pose a serious threat to natural health, which include staphyloma, glaucoma, cataracts, choroidal neovascularization, retinal breaks and detachments[2]. In recent decades, myopia and its complications have become one of the major threats to human visual health. It was estimated that about 1406 million people have myopia worldwide in 2000, and by 2050 this number will reach about 4760 million[3]. The prevalence of myopia in adults ranged from 15% to 49% in the developed countries[4]. Meanwhile, there are racial and ethnic differences in the magnitude and prevalence of myopia, both of which are greater in Asian than in other parts of the world[1]. A distinct geographical distribution is also characterized by myopia, and its prevalence is higher in eastern and southern Asia[5], where people has been reported to be of Chinese origin and about 95% of which need glasses or contact lenses to ensure clear vision beyond the arm[6], myopia is also extremely common in Republic of Korea and Japan[7]–[8]. For the eye grows throughout childhood, myopia usually develops in school-age children and adolescents[9]. Longitudinal data show that the annual prevalence of myopia among school-age children may be as high as 20%-30%[10]. Myopia occurs in 20%-30% of children aged 6 to 7, and in as many as 84% of high school students in Taiwan, China and Singapore[11]. Children with myopia progress rapidly until early adulthood, when it slows down[11]. Therefore, the progress of myopia control in Asian children deserves attention.
In the current state of myopia treatment, options include progressive addition of executive bifocal spectacle lenses, peripheral defocusing lenses, contact lenses, orthokeratology, multifocal soft contact lenses, outdoor activities and pharmaceutical agents[12]. Various options for slowing the progression of myopia in children were evaluated in a 2011 Cochrane database review[1]. According to this review, antimuscarinic agents are likely to slow the progression of myopia and atropine is the most commonly used and studied antimuscarinic drug for treating myopia[13]. In recent years, atropine has been proved to the most effective drug in slowing the progression of myopia[14].
Several studies have been conducted on the topical effect of atropine on myopia progression since Bedrossian's early studies in the 1960s and 1970s, which includes retrospective ones, prospective ones, and randomized trials[15]. As a way for myopia control, Yen et al[16] conducted the first placebo-controlled randomized trial of 1% atropine in 1989, for the lack of data on axial length (AL) and side effects such as photophobia caused by high doses of atropine, many subjects were pushed out halfway through the study. The Shih et al's[17] study in 1999 and subsequent randomized clinical trials (RCTs) such as the “Atropine for the Treatment of Childhood Myopia 2” (ATOM2) study[18] and the “Low-concentration Atropine of Myopia Progression” (LAMP) study[19] have begun to investigate lower concentrations of atropine. Despite numerous studies demonstrating atropine's effectiveness in controlling myopia, it has not yet been approved by Food and Drug Administration (FDA), and its optimal dose is still being studied[20]. A previous Meta-analysis[21] of atropine validity found that it differed by race, with Asian populations proving more likely to benefit from atropine than the whites. The severity of adverse effects associated with atropine application, including photophobia, also vary among races[22]. There had been Meta-analysis[21]–[24] examining the effects of atropine on myopia control in all regions, but no studies have been conducted on the specific group of Asian children with a high prevalence of the condition. Apart from the side effects caused by high concentrations such as photosensitive and difficulty working at close range, other concerns involve the unknown effects of using atropine on pupils. Only one Meta-analysis[25] has examined that pupillary diameter (PD) and accommodative amplitude (AA) respond to atropine in a nonlinear manner, which incorporates observational studies.
Based on the latest RCTs, we conducted this systematic review and Meta-analysis, with the aim to investigate the efficacy of different doses and concentrations of atropine drops on the spherical equivalent (SE) and AL of Asian children, and how atropine affects PD and AA, indices that have seldom been discussed in previous Meta-analyses.
SUBJECTS AND METHODS
Eligibility Criteria
In order to qualify for inclusion in this review, studies must meet the following criteria: 1) only RCTs were included; 2) Asian subjects aged less than 18y diagnosed with myopia; 3) atropine concentration of less than 1% was administered in the included group; 4) myopia control measures other than atropine or placebo were used in the control group; 5) at least one outcome of interest was reported, including SE, AL, PD, AA and any side effects. Literature whose full text could not be obtained, complete data were not available and repeated publication were excluded. Evaluations were limited to English-language articles.
Literature Searches and Data Extraction
The literature was obtained from PubMed, the Cochrane Library, Embase, and Web of Science database from their inception to April 2022, using Medical Subject Headings and free words combined with “myopia” and “atropine”, we develop search formulas according to the “Population, Intervention, Comparison, Outcome and Study design (PICOS)” principles and adjust search strategies to specific databases as appropriate. Reference lists of identified articles were also searched for further relevant articles.
Two reviewers independently extracted all data, and disagreements were resolved through discussion and, if necessary, the involvement of a third author. A title and abstract review was conducted first, and duplicated and ineligible articles were discarded. Data extracted from selected articles are as follows: first author, study design, year of publication, country or areas, baseline characteristics of subjects, intervention measures, follow-up time and outcome indicators.
Quality Assessment and Statistical Analysis
Seven aspects of Cochrane Collaboration's tool were used to assess the quality of the included RCTs: random sequence generation, allocation concealment, masking of participants and researchers, masking of outcome assessment, incomplete outcome data, selective outcome reporting, and other bias. Each item was assessed as “low risk of bias”, “high risk of bias” or “unclear risk of bias”. Two researchers independently evaluated the literature quality and a discussion was conducted to resolve discrepancies.
Data analysis was performed using STATA (version15.0; Stata Corp). The I2 test was used to quantify heterogeneity, when I2 were statistically significant, a random effects model was used; otherwise, a fixed effects model was applied. Analyses of statistical sensitivity and if possible, subgroup analyses were conducted to identify possible sources of heterogeneity. Analyzing Funnel plots and Egger's test of all the included trials provided evidence of possible publication bias.
RESULTS
Characteristics of Included Studies
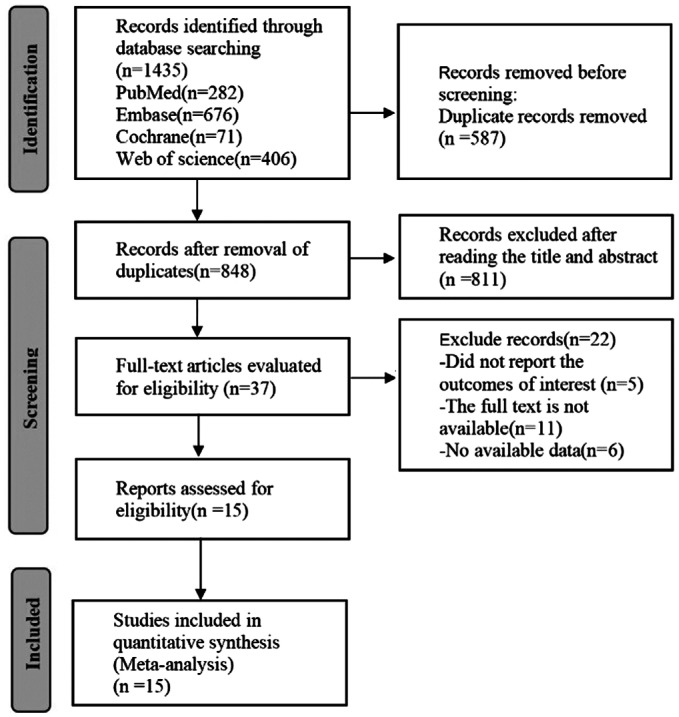
In total, 1435 scientific items were found. After removing duplicates, 848 eligible articles remained, 811 of which were excluded by reading the titles and abstracts, and finally 15 eligible articles[19],[26]–[39] were included after reading the full text (Figure 1).
Figure 1. PRISMA flow diagram of the study process.
PRISMA: Preferred reporting items for systematic review and Meta-analysis.
The total sample size of participants in 15 RCTs incorporated in our study was 2268, of whom 1404 received atropine treatment and 864 placebo or non-atropine treatment. There were 21 test groups applying different concentrations of atropine, including 14 groups using 0.01% atropine, 1 group using 0.02% atropine, 2 groups using 0.025% atropine, and 2 groups using 0.05% and 0.5% atropine each; there were 16 control groups using various types of glasses or placebo. According to the geographical location of the studies, 11 were in China (6 were conducted in mainland China, 4 in Hong Kong, 1 in Taiwan), 3 in Japan, and 1 in India, consequently, all participants were Asian (Table 1).
Table 1. The basic characteristics of the inclusion studies.
| Study | Study design | Year | Country | Sample size (male) |
Age (y)/mean (SD) |
Intervention |
Follow-up (mo) | Outcomes | |||
| Experimental group | Control group | Experimental group | Control group | Experimental group | Control group | ||||||
| Shih et al[26] | RCT | 2001 | Taiwan, China | 66 | 61 | NR | NR | 0.5% atropine+multi-focal lenses | Multi-focal lenses | 18 | F2 |
| Wang et al[27] | RCT | 2017 | China | 63 (36) | 63 (31) | 9.1 (1.4) | 8.7 (1.5) | 0.5% atropine | Placebo | 12 | F2 |
| Kinoshita et al[28] | RCT | 2018 | Japan | 20 (9) | 20 (10) | 10.87 (1.38) | 10.40 (1.86) | 0.01% atropine+OK | OK | 12 | F2 |
| Tan et al[29] | RCT | 2019 | Hong Kong, China | 30 (10) | 34 (15) | 9.09 (1.17) | 9.09 (1.11) | 0.01% atropine+OK | OK | 1 | F1; F2 |
| Yam et al[19] | RCT | 2019 | Hong Kong, China | 109 (54) | 111 (66) | 8.45 (1.81) | 8.42 (1.72) | 0.05% atropine | Placebo | 12 | F1; F2; F3; F4; F5; F6 |
| 108 (65) | 8.54 (1.71) | 0.025% atropine | |||||||||
| 110(63) | 8.23 (1.83) | 0.01% atropine | |||||||||
| Kinoshita et al[30] | RCT | 2020 | Japan | 38 (18) | 35 (18) | 10.33 (1.59) | 10.37 (1.65) | 0.01% atropine+OK | OK | 24 | F2 |
| Li et al[31] | RCT | 2020 | Hong Kong, China | 102 (53) | 93 (58) | 8.5 (1.69) | 8.5 (1.8) | 0.05% atropine | Placebo | 12 | F1; F2; F6 |
| 91 (60) | 8.5 (1.67) | 0.025% atropine | |||||||||
| 97 (53) | 8.4 (1.76) | 0.01% atropine | |||||||||
| Tan et al[32] | RCT | 2020 | Hong Kong, China | 36 | 36 | NR | NR | 0.01% atropine+OK | OK | 12 | F1; F2; F3; F4; F5; F6 |
| Wei et al[33] | RCT | 2020 | China | 110 (56) | 110 (61) | NR | NR | 0.01% atropine | Placebo | 12 | F1; F2; F3 |
| Zhao et al[34] | RCT | 2021 | China | 20 (10) | 20 (11) | 9.65 (1.53) | 9.7 (1.49) | 0.01% atropine+spectacles | Spectacles | 12 | F1; F2 |
| 20 (9) | 20 (10) | 10.9 (1.29) | 11.00 (1.17) | 0.01% atropine+OK | OK | ||||||
| Cui et al[35] | RCT | 2021 | China | 106 (55) | 89 (47) | 9.4 (1.7) | 9.3 (1.4) | 0.01% atropine | Spectacles | 24 | F1; F2; F4; F6 |
| 105 (55) | 9.6 (1.8) | 0.02% atropine | |||||||||
| Hao and Zhao[36] | RCT | 2021 | China | 21 (11) | 24 (13) | 10.10 (1.22) | 10.13 (1.19) | 0.01% atropine+OK | OK | 12 | F2 |
| Hieda et al[37] | RCT | 2021 | Japan | 84 (38) | 84 (36) | 8.99 (1.44) | 8.98 (1.50) | 0.01% atropine | Placebo | 24 | F1; F2; F3; F4; F5 |
| Saxena et al[38] | RCT | 2021 | India | 47 | 45 | 10.6 (2.2) | 10.8 (2.2) | 0.01% atropine | Placebo | 12 | F1; F2; F4; F5; F6 |
| Wang et al[39] | RCT | 2022 | China | 21 (13) | 19 (7) | 9.90 (1.58) | 9.89 (1.94) | 0.01% atropine+spectacles | Spectacles | 3 | F1;F2 |
F1: Spherical equivalent/logMAR; F2: Axial length; F3: Adverse events; F4: Photopic pupil size (mm); F5: Mesopic pupil size (mm); F6: Accommodation amplitude (D); OK: Orthokeratology; RCT: Randomized clinical trial; NR: Not report.
Risk of Bias Assessment
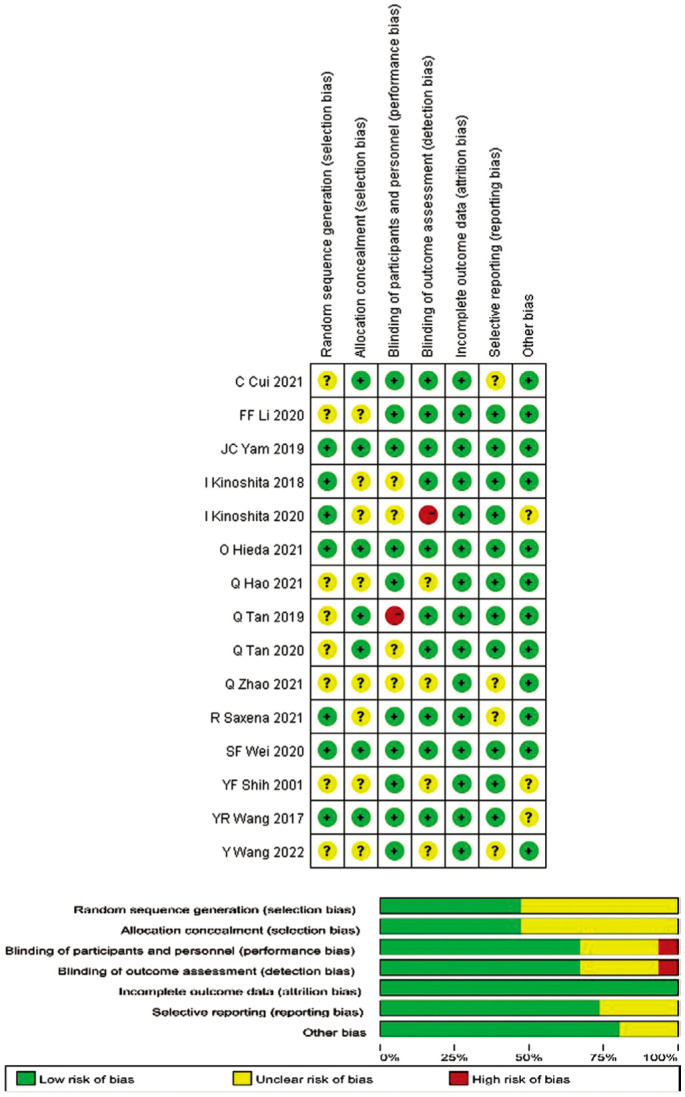
Figure 2 shows the results of the methodological quality evaluation based on the Cochran Handbook. Only three studies were assessed as “low risk of bias”, while the remaining articles had varying degrees of risk of bias. Although randomization was emphasized in all selected studies, the method of random assignment was not described in eight studies, so they were still evaluated as: “unclear risk of bias”.
Figure 2. Quality evaluation of included trials: risk of bias graph and risk of bias summary.
Meta-analysis Results
Change in spherical equivalent
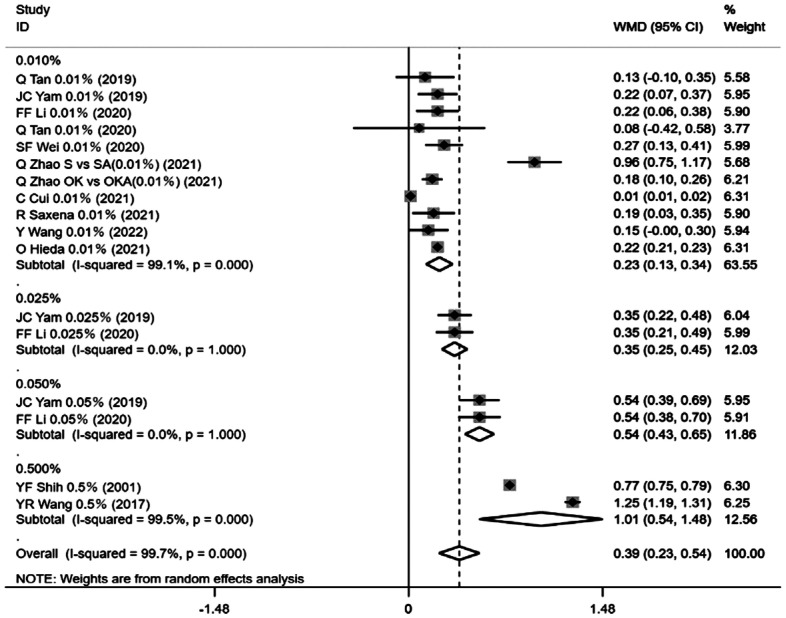
The amount of change in SE was reported in 12 of all the 15 articles that measured SE before and after drug administration. Change in SE at the longest follow-up time of each study was used as an outcome indicator, and the results of heterogeneity analysis (I2=99.7%, P=0.0001) led to the selection of a random effects model as the method of merging data. The effect of atropine group was superior to the control group in terms of change in SE in all cases, with a statistically significant difference [weighted mean difference (WMD)=0.39, 95% confidence interval (CI) (0.23, 0.54), P=0.0001].
Subgroup analysis based on atropine concentration indicated less heterogeneity between studies were found in the 0.025% group (I2=0, P=1.000) and the 0.05% group (I2=0, P=1.000), more heterogeneity in the 0.01% group (I2=99.1%, P=0.0001) and the 0.5% group (I2=99.5%, P=0.0001). Eleven studies of 0.01% atropine group [WMD=0.23, 95%CI (0.13, 0.34), P=0.0001], 2 studies of 0.025% atropine group [WMD=0.35, 95%CI (0.25, 0.45), P=0.0001], 2 studies of 0.05% atropine group [WMD=0.54, 95%CI (0.43, 0.65), P=0.0001], 2 studies of 0.5% atropine group [WMD=1.01, 95%CI (0.54, 1.48), P=0.0001] showed a smaller change in SE than the control group, all differences were statistically significant (Figure 3).
Figure 3. Forest plot of the effect of <1% atropine on change in SE.
SE: Spherical equivalent.
Change in axial length
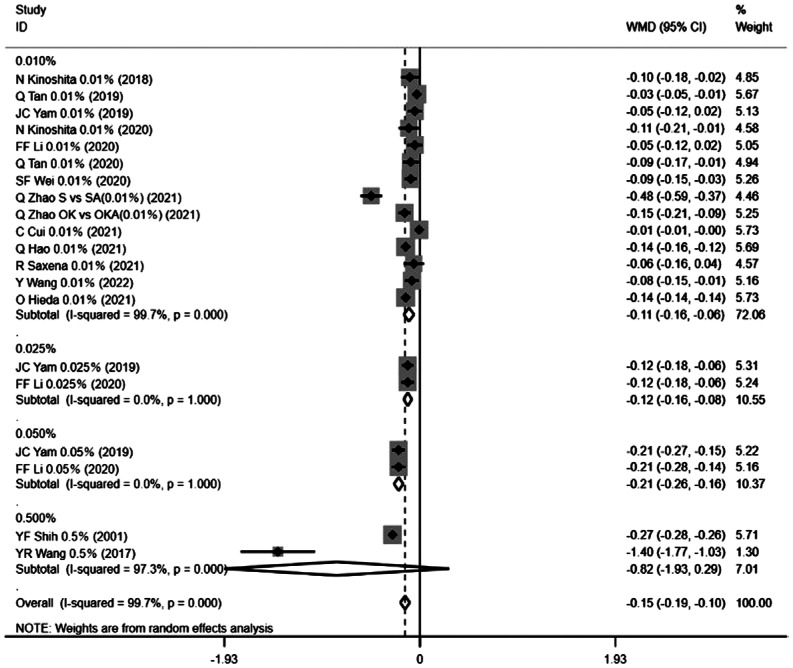
Changes in AL before and after atropine use were discussed in all eligible studies, as an indicator of outcome, AL was measured at baseline and at the longest follow-up. There was heterogeneity among the 15 studies (I2=99.7%, P=0.0001), and analysis using a random-effects model showed that the atropine group significantly delayed the axial elongation compared with the control group [WMD=-0.15, 95%CI (-0.19, -0.10), P=0.0001]. Subgroup analysis based on atropine concentration showed that 0.01% atropine [WMD=-0.11, 95%CI (-0.16, -0.06), P=0.0001], 0.025% atropine [WMD=-0.12, 95%CI (-0.16, -0.08), P=0.0001] and 0.05% atropine [WMD=-0.21, 95%CI (-0.26, -0.16), P=0.0001] test groups had statistically smaller axis changes than the control group. While the results of the 0.5% atropine group were not statistically significant [WMD=-0.82, 95%CI (-1.93, 0.29), P=0.147], the use of 0.5% atropine did not inhibit the growth of the AL in the current included study (Figure 4).
Figure 4. Forest plot of the effect of <1% atropine on change in axial length.
Change in pupillary diameter and accommodative amplitude
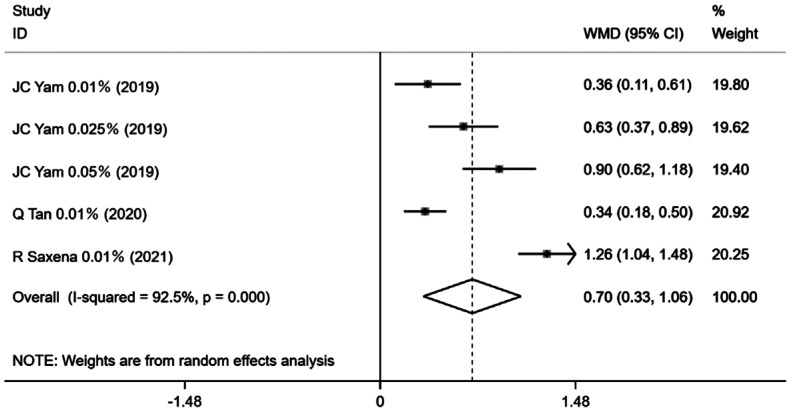
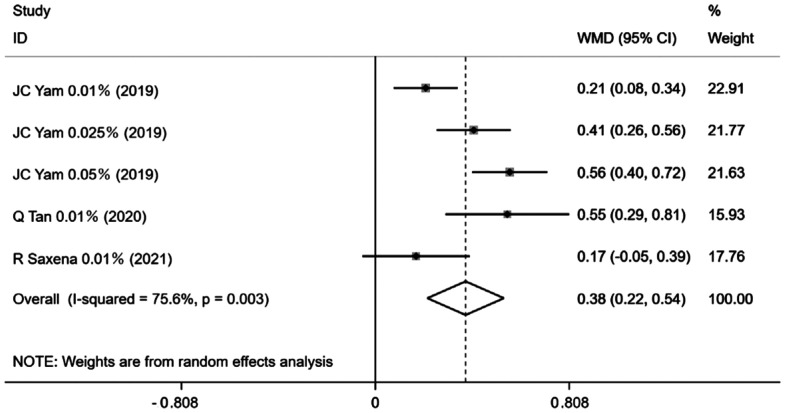
The data on changes in photopic pupil size was available in only 5 trial groups of the 15 studies. All dosed for 12mo, with the pooled estimates suggest that, in comparison with the control group, change in photopic pupil size was 0.70 [95%CI (0.33, 1.06), P=0.0001; Figure 5]. Similarly, changes in mesopic pupil size were also mentioned in 5 trial groups across all studies, with analysis demonstrating heterogeneity (I2=75.6%, P=0.003) and a pooled effect size [WMD=0.38, 95%CI (0.22, 0.54), P=0.0001; Figure 6].
Figure 5. Forest plot of the effect of <1% atropine on change in photopic pupil size.
Figure 6. Forest plot of the effect of <1% atropine on change in mesopic pupil size.
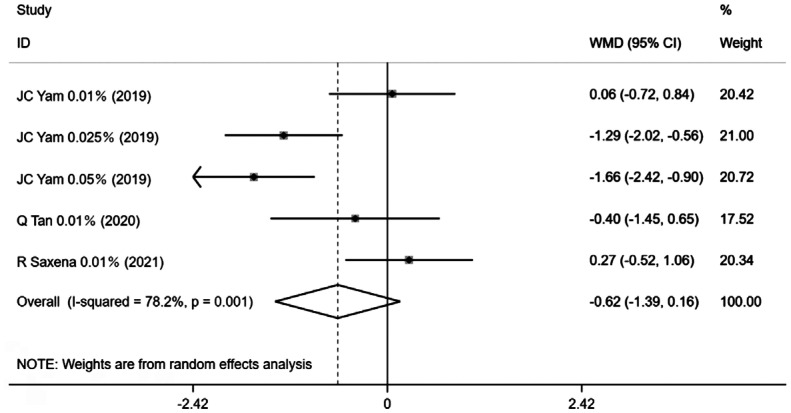
Five trial groups in 3 publications with 12mo of follow-up period measured change in AA at the maximum follow-up. The overall heterogeneity of all studies combined was 78.2% (P=0.001), consequently, a random-effects Meta-analysis was conducted and the results revealed that there were no statistically significant differences in change in AA between the two groups [WMD=-0.62, 95%CI (-1.39, 0.16), P=0.119; Figure 7]. Atropine administration did not cause AA changes in the current Meta-analysis.
Figure 7. Forest plot of the effect of <1% atropine on change in AA.
AA: Accommodative amplitude.
Side effects
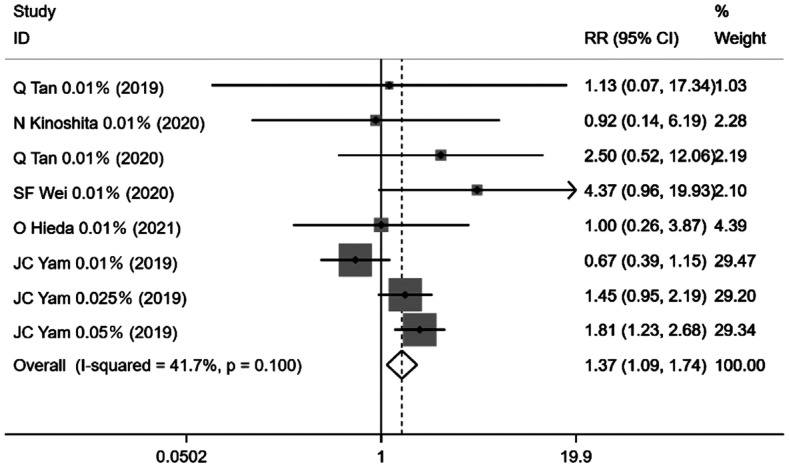
Eight groups of studies clearly reported the occurrence of adverse events, which included conjunctivitis, keratitis, allergy, light sensitivity, photophobia, near-distance vision impairment, and systemic problems during drug administration. Only the number of cases with adverse reactions was counted and statistically combined as a dichotomous categorical variable, with a pooled effect size with RR of 1.37 [95%CI (1.09, 1.74), P=0.008; Figure 8] which means the risk of adverse reactions with atropine was 1.37 times higher than no administration of atropine.
Figure 8. Forest plot of the effect of <1% atropine on side effects.
Sensitivity Analysis and Publication Bias
After removing each included study one by one from the Meta-analysis, a new Meta-analysis was conducted in order to observe the impact of a single study. When all studies were excluded, the combined effects of change in SE, change in AL, change in PD, change in AA and side effects did not change significantly, indicating the conclusion of this study was stable.
In order to evaluate publication bias in these studies, Funnel plots and Egger's test were conducted. The change in SE was asymmetrically distributed in the funnel plot, then P<0.05 was found by Egger's test, indicating possible publication bias. No publication bias was discovered for the remaining results.
DISCUSSION
Various English databases were screened using strict inclusion and exclusion criteria before selecting 15 published studies, all of them were RCTs. A total of 2268 study subjects were distributed among 21 trials and 16 control groups. These studies found that the shortest time required atropine was 3mo, and the longest was 2y.
According to the Meta-analysis of our study, atropine at concentrations less than 1% could notably slow down the SE growth and axial elongation in Asian children. Compared to the control, atropine group were significantly more likely to delay the progression of SE [WMD=0.39, 95%CI (0.23, 0.54)]. Included studies showed high heterogeneity, which remained in 0.01% and 0.5% atropine group after performing subgroup analyses based on concentration, and no heterogeneity was found in the 0.025% and 0.05% atropine groups, both were more effective than control groups [WMD=0.35, 95%CI (0.25, 0.45) and WMD=0.54, 95%CI (0.43, 0.65)]. And as predicted by the Meta-regression analyses, the effect was stronger at higher concentrations with statistically significant differences. With regard to the change in AL, atropine groups revealed 0.15 mm (95%CI: -0.19, -0.10) less increase in AL than the control groups, subgroup analysis served to reduce heterogeneity, whereas 0.5% atropine was found to be ineffective statistically, 0.01%, 0.025%, and 0.05% atropine groups showed 0.11 mm (95%CI: -0.16, -0.06), 0.12 mm (95%CI: -0.16, -0.08), and 0.21 mm (95%CI: -0.26, -0.16) less increase in AL than the control groups respectively. Statistically significant differences between doses of atropine were observed based on Meta-regression, with the higher tending to have greater influence. Heterogeneity may result from several factors such as included studies at a high risk of bias, varied duration of medication, differed control measures. Some studies combined other therapy such as orthokeratology with atropine, while the control groups used orthokeratology only, for the difference between them was only the presence of atropine, so studies with blank controls like this still included in our study, which may have been a source of heterogeneity. Based on sensitivity analysis, excluding selected studies one by one from the Meta-analysis did not significantly alter the results, which indicates that our findings are stable, credible and convincing.
It is confirmed by our Meta-analysis that atropine with a concentration of less than 1% is effective in slowing the progression of myopia in Asian children. Within this range, an atropine dose-dependent difference was observed in retarding the change in SE and eye axis, higher levels exhibit superior benefits in terms of SE and AL. Our results were consistent with earlier Meta-analysis[40] conducted in 2011, although the efficacy of 0.01% atropine was not evaluated in that study. A Meta-analysis[41] published in 2021 was partially concordant with our finding that various concentration of atropine showed statistically significant differences, however, it seems that low dosage atropine is more the prevalent dosage, probably owing to the fact that this analysis incorporates part of the Spanish results, whose geographical scope is not limited to the Asian. In 2021, a Meta-analysis with geographical scope unrestricted also reached the same conclusions as our study[42]. In another Meta-analysis[22] published in 2017, atropine displayed the same efficacy at various doses, but it included cohort studies to uncover the overall effects of different doses, thus bringing down the level of evidence. There was also a study[23] based on seven randomized controlled trials examining only the effectiveness of 0.01% atropine, concluding that it offer benefits in delaying eye axis elongation but play no valid roles on diopter values. Furthermore, according to a network Meta-analysis[14] published in 2016, pharmacological treatment, such as atropine, is most effective in slowing myopia progression without dose dependence. A subsequent network Meta-analysis[43] in 2022 concluded that dose did not affect ranking probability for efficacy, however, only three doses of 0.05%, 0.5% and 1% are discussed, with the most commonly noted 0.01% atropine excluded. A previous study[21] has illustrated that Asian children are more likely to experience atropine effects than Caucasians, and myopia ranks first among the causes of blindness in East Asian countries[11], our Meta-analysis for this specific population yielded different results from previous studies. Notably, in the present analysis, 0.5% atropine did not show a retarding effect on ocular axis growth, which is a significant departure from previous perceptions. As early as 1999, Shih et al[17] explored the function of 0.5%, 0.25%, and 0.1% atropine on myopia children aged 6 to 13y and found that the 0.5% was most effective. Later, a RCT by Shih et al[26] in 2001 further illustrated that inhibited eye elongation accounted for at least part of the role of 0.5% atropine in myopia progression. Similarly, both Huang et al[14] and Wang et al[27] illustrated 0.5% atropine was effective in stalling eye axis elongation. However, an interesting phenomenon was discovered for the first time in a Meta-analysis[42] that 0.5% atropine showed less efficacy in slowing myopia progression during the second year. In term of rebound, ATOM2 study[18] reported that in one-year washout, a similar rebound was seen in the 0.5% and 0.1% atropine groups, but much less in the 0.01% group. In our study, effects of 0.5% atropine on AL was only mentioned in 2 articles, with 18 and 12mo follow-up respectively. The small number of included trials and high heterogeneity may contribute to these differences. Another possible reason is that the validity of 0.5% atropine decreases with prolonged administration. Typically, a specific dose of atropine on myopia control was used for 1-2y before switching to another dose for ethical reasons, which increases the difficulty in long-term follow-up[42], so it is necessary to further investigate the efficacy of atropine over a longer period of time.
Atropine is a non-selective antagonist that can be used for cycloplegia and mydriasis. Previous Meta-analyses rarely systematically examine the data for the two common adverse effects of atropine, namely increased PD and decreased AA. As these two factors appear to be the most responsible factors determining the initial dose of atropine in myopia management[18],[44]–[45], they were analyzed in the current Meta-analysis. Our study identified that the photopic pupil size of the atropine group was 0.70 mm larger than the control group, which was significantly more than 0.48 mm and 0.49 mm in the study of Chen and Yao[41] and Yam et al[19], while significantly less than 0.91 mm viewed in Chia et al's[46] study. For the mesopic pupil size, our results showed that the atropine group was 0.376 mm larger than the control group, which was significantly smaller than the differences of 0.49 mm from Chen and Yao[41] and 1.15 mm from Chia et al[46], but bigger than that of 0.23 mm from Yam et al[19]. In terms of photopic pupil size, we also remarked that the three concentrations of 0.01%, 0.025%, and 0.05% did not differ from each other, while for mesopic pupil, the impact of higher concentrations tended to be stronger. Meanwhile, it was surprising to notice that 12mo of atropine administration did not elicit any modifications in the children's AA. In general, those taking 0.50% atropine had photophobia or near work problems[17]–[18],[26],[45],[47]–[49], whereas these problems were infrequently encountered in patients with atropine concentrations less than 0.10%, which was aligned by the findings of a Meta-analysis[25] published in 2021. It was also revealed in the Meta-analysis that there have nonlinear dose-response relationships between atropine and PD or AA, the occurrence of side effects such as enlargement of PD increases steeply for atropine in concentrations less 0.10%, this may partially explain the consequences we observed in the change in mesopic pupil size. However, using 0.05% and 0.025% atropine produced no significant differences in side effects according to previous reports[19],[48], which is consistent with our findings in regard to photopic pupil size alterations. It is not uncommon for atropine to cause side effects[15], and the risk of adverse reactions in atropine users in our study was 1.37 times than that of non-users. Therefore, more data are needed to better understand and confirm adverse effects and its relationship to concentrations. The lack of sufficient data may be compounded by other factors such as the timing of measurement versus application. In most cases, atropine comes in a compounded form that is diluted before use, so stability and strength are critical, especially for lower concentrations, which has been mentioned in some reports[50]–[51]. The instability of atropine solution causes time-related side effects to peak for some time after instillation and diminish thereafter[25]. Furthermore, considerable heterogeneity among the included studies may have affected the power of our results.
As with any study, this one had some limitations. First, only English-language studies were included in this Meta-analysis, thus resulting in a bias. Second, despite strict inclusion and exclusion criteria, heterogeneity remained high after subgroup analyses. Still, this analysis showed stable and consistent based on the sensitivity analysis. Third, discontinuing atropine therapy resulted in a rebound effect and faster myopia progression in the trials[46],[52], and some very important aspects such as the relationship between efficacy and safety of atropine, dose and time had not been studied. Fourth, a variety of techniques have been used in different studies to measure indicators including SE, AL, AA and PD. However, as we compared changes in metrics to the control rather than absolute values, such influence is likely to be minimal.
In summary, atropine in concentrations less than 1% was effective in controlling the increase in refractive error and slowing the ocular axis elongation in Asian myopia children, with a statistically difference among concentrations; and atropine with a higher concentration had better efficacy. The use of atropine led to a 1.37-fold increased risk of side effects. After 12mo of administration, atropine with a concentration of less than 1% was potent in causing pupil enlargement in Asian children, with little influence on photopic pupil size for dosage, and a greater one on mesopic pupil size at higher concentrations, but failed to induce a significant change in the magnitude of accommodation. Therefore, a balance should be maintained between efficacy and side effects during dose selection. However, as the response of different ocular parameters to dose varies, there is an urgent need for randomized controlled studies with large samples and long-term follow-up to investigate the response of these indicators to dosage and time, so as to provide guideline and reference for subsequent individualized and safe dosing.
Acknowledgments
Conflicts of Interest: Wei XL, None; Wu T, None; Dang KR, None; Hu KK, None; Lu XT, None; Gong M, None; Du YR, None; Hui YN, None; Tian XM, None; Du HJ, None.
REFERENCES
- 1.Walline JJ, Lindsley KB, Vedula SS, Cotter SA, Mutti DO, Ng SM, Twelker JD. Interventions to slow progression of myopia in children. Cochrane Database Syst Rev. 2020;1(1):CD004916. doi: 10.1002/14651858.CD004916.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russo A, Boldini A, Romano D, Mazza G, Bignotti S, Morescalchi F, Semeraro F. Myopia: mechanisms and strategies to slow down its progression. J Ophthalmol. 2022;2022:1004977. doi: 10.1155/2022/1004977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, Wong TY, Naduvilath TJ, Resnikoff S. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036–1042. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Pan CW, Ramamurthy D, Saw SM. Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol Opt. 2012;32(1):3–16. doi: 10.1111/j.1475-1313.2011.00884.x. [DOI] [PubMed] [Google Scholar]
- 5.Morgan IG, French AN, Ashby RS, Guo XX, Ding XH, He MG, Rose KA. The epidemics of myopia: aetiology and prevention. Prog Retin Eye Res. 2018;62:134–149. doi: 10.1016/j.preteyeres.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Spillmann L. Stopping the rise of myopia in Asia. Graefes Arch Clin Exp Ophthalmol. 2020;258(5):943–959. doi: 10.1007/s00417-019-04555-0. [DOI] [PubMed] [Google Scholar]
- 7.Jung SK, Lee JH, Kakizaki H, Jee D. Prevalence of myopia and its association with body stature and educational level in 19-year-old male conscripts in Seoul, South Korea. Invest Ophthalmol Vis Sci. 2012;53(9):5579–5583. doi: 10.1167/iovs.12-10106. [DOI] [PubMed] [Google Scholar]
- 8.Ding BY, Shih YF, Lin LLK, Hsiao CK, Wang IJ. Myopia among schoolchildren in East Asia and Singapore. Surv Ophthalmol. 2017;62(5):677–697. doi: 10.1016/j.survophthal.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Dolgin E. The myopia boom. Nature. 2015;519(7543):276–278. doi: 10.1038/519276a. [DOI] [PubMed] [Google Scholar]
- 10.Wang SK, Guo YF, Liao CM, Chen YX, Su GX, Zhang GH, Zhang L, He MG. Incidence of and factors associated with myopia and high myopia in Chinese children, based on refraction without cycloplegia. JAMA Ophthalmol. 2018;136(9):1017–1024. doi: 10.1001/jamaophthalmol.2018.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu PC, Huang HM, Yu HJ, Fang PC, Chen CT. Epidemiology of myopia. Asia Pac J Ophthalmol (Phila) 2016;5(6):386–393. doi: 10.1097/APO.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 12.Tran D, Heinrich C, Ali SF. The myopia epidemic: treatment options in the pediatric population. Int Ophthalmol Clin. 2021;62(1):231–240. doi: 10.1097/IIO.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 13.Pineles SL, Kraker RT, VanderVeen DK, Hutchinson AK, Galvin JA, Wilson LB, Lambert SR. Atropine for the prevention of myopia progression in children: a report by the American academy of ophthalmology. Ophthalmology. 2017;124(12):1857–1866. doi: 10.1016/j.ophtha.2017.05.032. [DOI] [PubMed] [Google Scholar]
- 14.Huang JH, Wen DZ, Wang QM, et al. Efficacy comparison of 16 interventions for myopia control in children: a network meta-analysis. Ophthalmology. 2016;123(4):697–708. doi: 10.1016/j.ophtha.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Kaiti R, Shyangbo R, Sharma IP. Role of atropine in the control of myopia progression- A review. Beyoglu Eye J. 2022;7(3):157–166. doi: 10.14744/bej.2022.07742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yen MY, Liu JH, Kao SC, Shiao CH. Comparison of the effect of atropine and cyclopentolate on myopia. Ann Ophthalmol. 1989;21(5):180–182,187. [PubMed] [Google Scholar]
- 17.Shih YF, Chen CH, Chou AC, Ho TC, Lin LL, Hung PT. Effects of different concentrations of atropine on controlling myopia in myopic children. J Ocul Pharmacol Ther. 1999;15(1):85–90. doi: 10.1089/jop.1999.15.85. [DOI] [PubMed] [Google Scholar]
- 18.Chia A, Chua WH, Cheung YB, Wong WL, Lingham A, Fong A, Tan D. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (atropine for the treatment of myopia 2) Ophthalmology. 2012;119(2):347–354. doi: 10.1016/j.ophtha.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 19.Yam JC, Jiang YN, Tang SM, Law AKP, Chan JJ, Wong E, Ko ST, Young AL, Tham CC, Chen LJ, Pang CP. Low-concentration atropine for myopia progression (LAMP) study: a randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology. 2019;126(1):113–124. doi: 10.1016/j.ophtha.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 20.Schittkowski M, Sturm V. Atropine for the prevention of progression in myopi—data, side effects, practical guidelines. Klin Monbl Augenheilkd. 2018;235(4):385–391. doi: 10.1055/s-0043-121982. [DOI] [PubMed] [Google Scholar]
- 21.Li SM, Wu SS, Kang MT, Liu Y, Jia SM, Li SY, Zhan SY, Liu LR, Li H, Chen W, Yang Z, Sun YY, Wang NL, Millodot M. Atropine slows myopia progression more in Asian than white children by meta-analysis. Optom Vis Sci. 2014;91(3):342–350. doi: 10.1097/OPX.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 22.Gong QW, Janowski M, Luo M, Wei H, Chen BJ, Yang GY, Liu LQ. Efficacy and adverse effects of atropine in childhood myopia: a meta-analysis. JAMA Ophthalmol. 2017;135(6):624–630. doi: 10.1001/jamaophthalmol.2017.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Y. Atropine 0.01% eye drops slow myopia progression: a systematic review and Meta-analysis. Int J Ophthalmol. 2019;12(8):1337–1343. doi: 10.18240/ijo.2019.08.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu AC, Stapleton F, Wei L, Wang WQ, Zhao BX, Watt K, Ji N, Lyu Y. Effect of low-dose atropine on myopia progression, pupil diameter and accommodative amplitude: low-dose atropine and myopia progression. Br J Ophthalmol. 2020;104(11):1535–1541. doi: 10.1136/bjophthalmol-2019-315440. [DOI] [PubMed] [Google Scholar]
- 25.Tran HDM, Sankaridurg P, Naduvilath T, Ha TTX, Tran TD, Jong M, Coroneo M, Tran YH. A meta-analysis assessing change in pupillary diameter, accommodative amplitude, and efficacy of atropine for myopia control. Asia Pac J Ophthalmol (phila) 2021;10(5):450–460. doi: 10.1097/APO.0000000000000414. [DOI] [PubMed] [Google Scholar]
- 26.Shih YF, Hsiao CK, Chen CJ, Chang CW, Hung PT, Lin LL. An intervention trial on efficacy of atropine and multi-focal glasses in controlling myopic progression. Acta Ophthalmol Scand. 2001;79(3):233–236. doi: 10.1034/j.1600-0420.2001.790304.x. [DOI] [PubMed] [Google Scholar]
- 27.Wang YR, Bian HL, Wang Q. Atropine 0.5% eyedrops for the treatment of children with low myopia. Medicine (Baltimore) 2017;96(27):e7371. doi: 10.1097/MD.0000000000007371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinoshita N, Konno Y, Hamada N, Kanda Y, Shimmura-Tomita M, Kakehashi A. Additive effects of orthokeratology and atropine 0.01% ophthalmic solution in slowing axial elongation in children with myopia: first year results. Jpn J Ophthalmol. 2018;62(5):544–553. doi: 10.1007/s10384-018-0608-3. [DOI] [PubMed] [Google Scholar]
- 29.Tan Q, Ng AL, Cheng GP, Woo VC, Cho P. Combined atropine with orthokeratology for myopia control: study design and preliminary results. Curr Eye Res. 2019;44(6):671–678. doi: 10.1080/02713683.2019.1568501. [DOI] [PubMed] [Google Scholar]
- 30.Kinoshita N, Konno Y, Hamada N, Kanda Y, Shimmura-Tomita M, Kaburaki T, Kakehashi A. Efficacy of combined orthokeratology and 0.01% atropine solution for slowing axial elongation in children with myopia: a 2-year randomised trial. Sci Rep. 2020;10(1):12750. doi: 10.1038/s41598-020-69710-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li FF, Kam KW, Zhang YZ, Tang SM, Young AL, Chen LJ, Tham CC, Pang CP, Yam JC. Differential effects on ocular biometrics by 0.05%, 0.025%, and 0.01% atropine: low-concentration atropine for myopia progression study. Ophthalmology. 2020;127(12):1603–1611. doi: 10.1016/j.ophtha.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Tan Q, Ng AL, Choy BN, Cheng GP, Woo VC, Cho P. One-year results of 0.01% atropine with orthokeratology (AOK) study: a randomised clinical trial. Ophthalmic Physiol Opt. 2020;40(5):557–566. doi: 10.1111/opo.12722. [DOI] [PubMed] [Google Scholar]
- 33.Wei SF, Li SM, An WZ, Du JL, Liang XT, Sun YY, Zhang DX, Tian JX, Wang NL. Safety and efficacy of low-dose atropine eyedrops for the treatment of myopia progression in Chinese children: a randomized clinical trial. JAMA Ophthalmol. 2020;138(11):1178–1184. doi: 10.1001/jamaophthalmol.2020.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Q, Hao Q. Clinical efficacy of 0.01% atropine in retarding the progression of myopia in children. Int Ophthalmol. 2021;41(3):1011–1017. doi: 10.1007/s10792-020-01658-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cui C, Li XJ, Lyu Y, Wei L, Zhao BX, Yu S, Rong JB, Bai YH, Fu AC. Safety and efficacy of 0.02% and 0.01% atropine on controlling myopia progression: a 2-year clinical trial. Sci Rep. 2021;11(1):22267. doi: 10.1038/s41598-021-01708-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hao Q, Zhao Q. Changes in subfoveal choroidal thickness in myopic children with 0.01% atropine, orthokeratology, or their combination. Int Ophthalmol. 2021;41(9):2963–2971. doi: 10.1007/s10792-021-01855-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hieda O, Hiraoka T, Fujikado T, et al. Efficacy and safety of 0.01% atropine for prevention of childhood myopia in a 2-year randomized placebo-controlled study. Jpn J Ophthalmol. 2021;65(3):315–325. doi: 10.1007/s10384-021-00822-y. [DOI] [PubMed] [Google Scholar]
- 38.Saxena R, Dhiman R, Gupta V, Kumar P, Matalia J, Roy L, Swaminathan M, Phuljhele S, Velpandian T, Sharma N. Atropine for the treatment of childhood myopia in India: multicentric randomized trial. Ophthalmology. 2021;128(9):1367–1369. doi: 10.1016/j.ophtha.2021.01.026. [DOI] [PubMed] [Google Scholar]
- 39.Wang YL, Zhu XX, Xuan Y, Wang M, Zhou XT, Qu XM. Short-term effects of atropine 0.01% on the structure and vasculature of the choroid and retina in myopic Chinese children. Ophthalmol Ther. 2022;11(2):833–856. doi: 10.1007/s40123-022-00476-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song YY, Wang H, Wang BS, Qi H, Rong ZX, Chen HZ. Atropine in ameliorating the progression of myopia in children with mild to moderate myopia: a meta-analysis of controlled clinical trials. J Ocul Pharmacol Ther. 2011;27(4):361–368. doi: 10.1089/jop.2011.0017. [DOI] [PubMed] [Google Scholar]
- 41.Chen CW, Yao JY. Efficacy and adverse effects of atropine for myopia control in children: a meta-analysis of randomised controlled trials. J Ophthalmol. 2021;2021:4274572. doi: 10.1155/2021/4274572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gan J, Li SM, Wu SS, Cao K, Ma D, He X, Hua ZY, Kang M, Wei S, Bai WL, Wang NL. Varying dose of atropine in slowing myopia progression in children over different follow-up periods by meta-analysis. Front Med (Lausanne) 2021;8:756398. doi: 10.3389/fmed.2021.756398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ha A, Kim SJ, Shim SR, Kim YK, Jung JH. Efficacy and safety of 8 atropine concentrations for myopia control in children. Ophthalmology. 2022;129(3):322–333. doi: 10.1016/j.ophtha.2021.10.016. [DOI] [PubMed] [Google Scholar]
- 44.Clark TY, Clark RA. Atropine 0.01% eyedrops significantly reduce the progression of childhood myopia. J Ocul Pharmacol Ther. 2015;31(9):541–545. doi: 10.1089/jop.2015.0043. [DOI] [PubMed] [Google Scholar]
- 45.Lee JJ, Fang PC, Yang IH, Chen CH, Lin PW, Lin SA, Kuo HK, Wu PC. Prevention of myopia progression with 0.05% atropine solution. J Ocul Pharmacol Ther. 2006;22(1):41–46. doi: 10.1089/jop.2006.22.41. [DOI] [PubMed] [Google Scholar]
- 46.Chia A, Chua WH, Wen L, Fong A, Goon YY, Tan D. Atropine for the treatment of childhood myopia: changes after stopping atropine 0.01%, 0.1% and 0.5% Am J Ophthalmol. 2014;157(2):451–457.e1. doi: 10.1016/j.ajo.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 47.Yi S, Huang YS, Yu SZ, Chen XJ, Yi H, Zeng XL. Therapeutic effect of atropine 1% in children with low myopia. J AAPOS. 2015;19(5):426–429. doi: 10.1016/j.jaapos.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 48.Fang PC, Chung MY, Yu HJ, Wu PC. Prevention of myopia onset with 0.025% atropine in premyopic children. J Ocul Pharmacol Ther. 2010;26(4):341–345. doi: 10.1089/jop.2009.0135. [DOI] [PubMed] [Google Scholar]
- 49.Loughman J, Flitcroft DI. The acceptability and visual impact of 0.01% atropine in a Caucasian population. Br J Ophthalmol. 2016;100(11):1525–1529. doi: 10.1136/bjophthalmol-2015-307861. [DOI] [PubMed] [Google Scholar]
- 50.Saito J, Imaizumi H, Yamatani A. Physical, chemical, and microbiological stability study of diluted atropine eye drops. J Pharm Health Care Sci. 2019;5:25. doi: 10.1186/s40780-019-0154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berton B, Chennell P, Yessaad M, Bouattour Y, Jouannet M, Wasiak M, Sautou V. Stability of ophthalmic atropine solutions for child myopia control. Pharmaceutics. 2020;12(8):781. doi: 10.3390/pharmaceutics12080781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tong L, Huang XL, Koh ALT, Zhang XE, Tan DTH, Chua WH. Atropine for the treatment of childhood myopia: effect on myopia progression after cessation of atropine. Ophthalmology. 2009;116(3):572–579. doi: 10.1016/j.ophtha.2008.10.020. [DOI] [PubMed] [Google Scholar]