Abstract
Background
Double burden of malnutrition (DBM) is an emerging global public health problem. The United Nations member states adopted eradicating all forms of malnutrition as an integral component of the global agenda. However, there is evidence of a high burden of undernutrition among women and rising rates of overweight and obesity, especially in low and middle income countries (LMICs). Therefore, this study aimed to investigate the prevalence and associated factors of underweight, overweight, and obesity among women of reproductive age in LMICs.
Methods
Data for the study were drawn from a recent 52 Demographic and Health Surveys (DHS) conducted in LMICS. We included a sample of 1,099,187 women of reproductive age. A multilevel multinomial logistic regression model was used to identify factors associated with DBM. Adjusted relative risk ratio (RRR) with a 95% Confidence Interval (CI) was reported to show an association.
Results
The prevalence of underweight, overweight, and obesity in LMICs among women of reproductive age was 15.2% (95% CI: 15.1–15.3), 19.0% (95% CI: 18.9- 19.1), and 9.1% (95% CI: 9.0–9.2), respectively. This study found that women aged 24–34 years, aged ≥ 35 years, with primary, secondary, and above educational level, from wealthy households, using modern contraceptives, exposed to media (radio and television), and with high parity (more than one birth) were more likely to have overweight and obesity and less likely to have underweight. Moreover, the risk of having obesity (RRR = 0.59; 95% CI = 0.58–0.60 and overweight (RRR = 0.78; 95% CI = 0.77–0.79) were lower among rural women, while the risk of being underweight was (RRR = 1.13; 95% CI = 1.11–1.15) higher among rural women compared to urban women.
Conclusion
The prevalence of underweight, overweight, and obesity was high among women of reproductive age in LMICs. Underweight, overweight, and obesity are influenced by sociodemographic, socioeconomic, and behavioral-related factors. This study shows that, in order to achieve Sustainable Development Goal 2, a multifaceted intervention approach should be considered to prevent both forms of malnutrition in women of reproductive age. This can be achieved by raising awareness and promoting healthy behaviors such as healthy eating and physical activity, especially among educated women, women from wealthy households, and women exposed to the media.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-023-16045-4.
Keywords: Double burden of malnutrition, Low- and middle-income countries, Women of reproductive age
Background
The double burden of malnutrition (DBM) continues to be a major global public health problem. It is defined as the coexistence of both undernutrition and overnutrition in the same population across the life course [1, 2]. Globally, nearly one-third of the population suffered from at least one form of malnutrition [3]. The double burden of malnutrition is increasing globally, particularly in low and middle income countries (LMICs). Globally, obesity has doubled over the past 30 years, while obesity in LMICs has tripled over the past 20 years [4, 5].
Even though underweight among women has been a major public health concern in LMICs for several decades, due to population aging and increased prevalence of risk factors such as unhealthy diets, physical inactivity, and substance use such as alcohol consumption and cigarette smoking led to a significant shift in epidemiological trend from underweight to overweight and nutritional transitions [6–8]. Nutrition-related diseases and conditions such as nutritional deficiencies, obesity, hypertension, cardiovascular diseases, cancer, and diabetes mellitus are emerging at a faster rate in LMICs than in high-income countries [9]. Overweight/obesity is a major risk for non-communicable diseases (NCDs) morbidity and mortality such as cardiovascular diseases (CVDs), chronic kidney diseases, cancer, musculoskeletal disorders, type 2 diabetes mellitus, and respiratory problems [10–16]. Globally, NCDs are the leading causes of mortality and morbidity, and one of the major challenges of the 21st century [17]. Non-communicable diseases kill 41 million people annually, accounting for 71% of all deaths [18]. Eighty percent of NCD deaths occur in LMICs [19]. The World Health Organization (WHO) projects that by 2030, NCDs will overtake infectious, maternal, neonatal, and nutritional conditions as the leading cause of morbidity and mortality and that the most percentage increase in deaths from NCD will occur in LMICs [20]. Moreover, individuals with underweight are at a major risk of experiencing CVDs including stroke, heart attack, coronary artery disease, and infectious diseases [21].
The DBM is devastating and higher among women than men [4, 5]. It affects their health and the health of their offspring. Overweight/obesity among women is associated with increased pregnancy and childbirth related complications such as gestational diabetes, pre-eclampsia, gestational hypertension, postpartum hemorrhage, instrumental delivery, cesarean delivery, low birth weight, preterm birth, congenital malformation, large-for-gestational-age babies and perinatal death [22–28]. In addition, underweight women are more likely to have pregnancy and childbirth-related complications, such as low birth weight, small for gestational age, preterm birth, and neonatal mortality [22–24, 29, 30].
Although the global prevalence of underweight among women declined from 14.6% in 1995 to 9.7% in 2014, underweight in South Asia and central and East Africa remains unacceptably high and the rate of reduction in underweight is significantly different from country to country [31, 32]. In addition, the global prevalence of obesity among women increased from 6·4% to 14·9% over the past four decades [32]. The prevalence of overweight/obesity among women of reproductive age was 14.9% in Ethiopia, 57.4% in Uganda, 66.7% in Nigeria, 74.1% in Tanzania, 87% in South Africa, 32% in Bangladesh, and 63% in Maldives [33–36].
Studies have assessed factors associated with DBM including age [34, 36–42], educational status [37, 39–45], household wealth status [40, 44–49], breastfeeding [42], marital status [50, 51], place of residence [36, 37, 40, 41, 44], family size [52], types/frequency of diet consumption [51, 52], parity [53], using contraceptives [54, 55], mass media exposure (frequency of watching television, frequency of listing to the radio) [38, 43, 56, 57], and physical activity [51].
The United Nations Sustainable Development Goal 2 (SDG-2) aims to eradicate all forms of malnutrition by 2030 [58]. However, according to the NCD Risk Factors collaborators, there is a zero chance of this being achieved at the global level [59]. Evidence suggests that no country has reversed the rise in obesity at the national level [60]. Also, according to 29 Demographic and Health Survey data and 4 national Surveys, obesity declined only marginally in rural Benin and stabilized in urban areas, and the annual rate of increase in the prevalence of overweight among women of reproductive age in Mexico slowed [61]. Furthermore, previous studies conducted on the prevalence and associated factors of DBM in LMICS were mainly country-specific. Therefore, this study aimed to investigate the prevalence and factors associated with DBM among women of reproductive age in LMICs using nationally representative data. A comprehensive assessment of DBM and its associated factors in at risk populations is critical for developing policies and plans to end all forms of malnutrition and promote well-being by 2030.
Methods
Sources of data and sampling procedure
This study used the most recent Demographic Health Survey (DHS) data from 52 LMICs carried out from 2010 to 2021. The DHS is a nationally representative, cross-sectional survey conducted in LMICs that provides reliable data on women, men, and children. The DHS surveys uses uniform data collection procedures, sampling, questionnaires, and coding. This makes the results comparable across countries.
To assure the national representativeness, the survey used a two-stage cluster sampling technique. In the first stage, the selection of proportional clusters/enumeration areas was performed using each country’s most recent population and housing census as a sampling frame. In the second stage, a systematic selection of households from the newly created cluster was performed. A detailed description of the DHS sampling design and data collection procedures has been found in each country’s DHS report. A total of 1,464,481 women of reproductive age (15–49 years) were interviewed in 52 LMICs. For this study, a total of 1,099,187 non-pregnant women of reproductive age who had a body mass index (BMI) measurement were used for analysis (Fig. 1).
Fig. 1.

Flow chart for data extraction
Study variables and measurement
Outcome variable
Body mass index derived from women’s weight in kilograms divided by the square of her height in meters (kg/m2) was the dependent variable. The weight and height were measured using standard technique by trained field technicians. Electronic Seca scales with a digital screen were used to measure weight and a stadiometer were used measure height [62]. According to WHO cutoff points, BMI was divided into four categories as underweight if the BMI is < 18.5 kg/m2, normal if it is 18.5–24.9 kg/m2, overweight if it is 25–29.9 kg/m2 and obese if it is ≥ 30 kg/m2 [63].
Independent variables
Based on previous literatures [37, 38, 40, 41, 49, 53, 64, 65], several independent variables such as age, educational status of women, mass media access (frequency of watching television, frequency of listening to the radio, and frequency of reading newspaper/magazines), accessing health care, working status, birth order, terminated pregnancy, household wealth status, family size, sex of household head, marital status, parity, and contraceptive use were included as the individual level variables of the study. Whereas residence was considered as the community level variable in this study. Detailed coding and operation definitions of variables are presented in Supplementary Table 1.
Data processing and analyses
Datasets were appended together to explore the pooled prevalence of underweight, overweight, obesity, and its associated factors among women of reproductive age in LMICs. Data cleaning and statistical analysis were carried out using STATA version 16 [66]. All statistical analysis was performed based on sample weighting. Frequencies and percentages were used to describe the background characteristics of the study participants. To identify associated factors of underweight, overweight, and obesity we used the multilevel multinomial logistic regression since BMI (dependent variable) is a categorical variable with four categories and DHS data are hierarchical, i.e. individuals were nested within communities. Normal BMI was used as the reference group. In particular, four models were constructed; null model contains only the outcome variable and clusters to assess the random effects between clusters, model I contains individual-level variables only, model II includes a community-level variable only, and model III includes both individual and community-level variables. The best-fitted model was selected by using deviance to identify factors associated with DBM and the model with the least deviance was selected (model III).
The Intra-class Correlation Coefficient (ICC), and the Median Odds Ratio (MOR) were computed to assess the clustering effect/variability. The intra-class correlation was calculated for each of the models as ICC = the variance of each model/ (variance of each model + 3:29) [67] and the MOR was calculated as; MOR = exp(0.95√ cluster level variance) [68]. Proportional Change in Variance (PCV) was computed for models I, II, and III with respect to the variance in the empty model as PCV = (variance of the empty model—variance of the model with more terms (model I, II, or III) / variance of the empty model [68].
First, we fitted a bivariable multilevel multinomial logistic regression model for each independent variable to select variables for multivariable analysis, and variables with p-value ≤ 0.20 in the bivariable multilevel multinomial logistic regression analysis were included in multivariable analysis (Supplementary Table 2). Finally, results for the multivariable analysis have been presented as adjusted relative risk ratio (RRR), with their corresponding 95% confidence intervals (CI).
Results
Background characteristics of study participants
A total of 1,099,187 women in LMICs were included in the study. Of the total, 876,682 (79.8%) were from male-headed households, 554,009 (50.8) watched television at least once a week, 599,994 (54.6%) did not use contraceptives and 756,027 (68.8%) were currently in union. Nearly two-thirds (705,926, 64.2%) of study participants were rural residents, and 495,824 (45.1%) women had secondary education. The mean age of the participants was 30.0 ± 9.9 years (Table 1).
Table 1.
Background characteristics of women of reproductive age in LMICs, 2010–2021
| Variables | Frequency | Percent |
|---|---|---|
| Age | ||
| Mean ± SD | 30.3 ± 9.9 | |
| 15–24 | 370,336 | 33.7 |
| 25–34 | 332,638 | 30.3 |
| 35–49 | 396,213 | 36.0 |
| Educational status | ||
| Not educated | 247,490 | 22.5 |
| Primary | 212,586 | 19.3 |
| Secondary | 495,824 | 45.1 |
| Higher | 143,287 | 13.1 |
| Household wealth status | ||
| Poorest | 198,179 | 18.0 |
| Poorer | 215,199 | 19.6 |
| Middle | 224,562 | 20.4 |
| Richer | 231,009 | 21.0 |
| Richest | 230,238 | 21.0 |
| Marital status | ||
| Currently in union | 756,027 | 68.8 |
| Not currently in union | 343,160 | 31.2 |
| Working status | ||
| Not working | 287,813 | 54.5 |
| Working | 240,128 | 45.5 |
| Family size | ||
| ≤ 5 | 416,937 | 37.9 |
| 6–10 | 616,785 | 56.1 |
| > 10 | 65,465 | 6.0 |
| Frequency of reading newspaper or magazine | ||
| Not at all | 737,359 | 67.6 |
| Less than once a week | 193,452 | 17.7 |
| At least once a week | 158,576 | 14.5 |
| Almost every day | 1,426 | 0.2 |
| Frequency of watching television | ||
| Not at all | 337,284 | 30.9 |
| Less than once a week | 190,570 | 17.5 |
| At least once a week | 554,009 | 50.8 |
| Almost every day | 8,898 | 0.8 |
| Frequency of listening to radio | ||
| Not at all | 768,762 | 70.5 |
| Less than once a week | 139,771 | 12.8 |
| At least once a week | 175,588 | 16.1 |
| Almost every day | 6,748 | 0.6 |
| Sex of household head | ||
| Male | 876,682 | 79.8 |
| Female | 222,505 | 20.2 |
| Residence | ||
| Urban | 393,261 | 35.8 |
| Rural | 705,926 | 64.2 |
| Ever had terminated pregnancy | ||
| No | 951,168 | 86.6 |
| Yes | 147,989 | 13.4 |
| Contraceptive use | ||
| Not using | 599,994 | 54.6 |
| Use traditional method | 423,756 | 38.5 |
| Use modern method | 75,429 | 6.9 |
| Currently breastfeeding | ||
| No | 902,426 | 82.1 |
| Yes | 196,761 | 17.9 |
| Parity | ||
| Nulipara | 318,835 | 29.0 |
| Primiparous | 152,514 | 13.9 |
| Multiparous | 506,966 | 46.1 |
| Grand Multiparous | 120,872 | 11.0 |
| Accessing health care | ||
| Not big problem | 568,872 | 52.6 |
| Big problem | 512,150 | 44.4 |
Prevalence of underweight, overweight and obesity
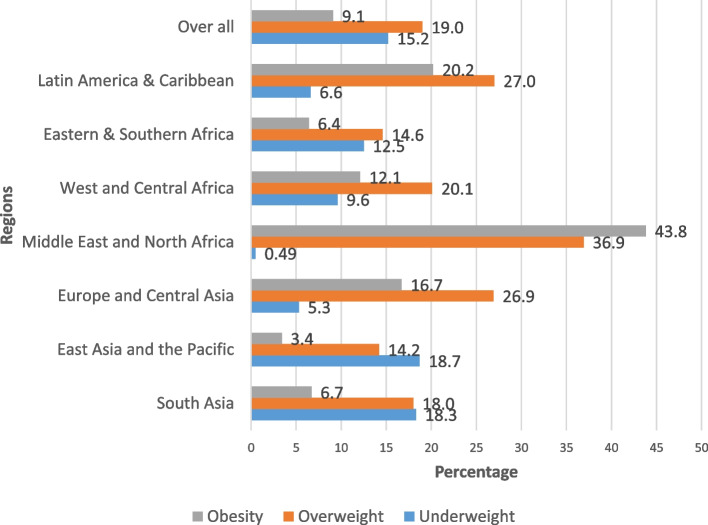
The pooled prevalence of underweight in LMICs among women of reproductive age was 15.2 (95% CI: 15.1–15.3), ranging from 0.2% in Egypt to 26.3% in Timor-Leste. The pooled prevalence of overweight in LMICs among women of reproductive age was 19.0 (95% CI: 18.9–19.1), ranging from 5.9% in Ethiopia to 37.8% in Jordan. The pooled prevalence of obesity in LMICs among women of reproductive age was 9.1 (95% CI: 9.0–9.2) (Fig. 2) and it ranged from 1.6% in Ethiopia to 47.8% in Egypt (Table 2).
Fig. 2.
Regional prevalence of underweight, overweight and obesity among women of reproductive age
Table 2.
Prevalence of underweight, overweight and obesity by countries among women of reproductive age
| World region | Countries | Survey year | Underweight Frequency (%) | Overweight Frequency (%) | Obesity Frequency (%) |
|---|---|---|---|---|---|
| South Asia | Maldives | 2016/17 | 713(10.5) | 2,031(30.0) | 1,320(19.5) |
| Nepal | 2016 | 1,057(17.2) | 1,04717.0) | 313(5.1) | |
| Bangladesh | 2017/18 | 2,207( 11.8) | 4,781(25.6) | 1,218 (6.5) | |
| India | 2019/21 | 124,181(18.6) | 117,188(17.6) | 43,215(6.5) | |
| Pakistan | 2017 | 336(8.7) | 1,172(30.3) | 831(21.4) | |
| Pooled prevalence | 2016–2021 | 128,494(18.3) | 126,219(18.0) | 46,897(6.7) | |
| East Asia and the Pacific | Cambodia | 2014 | 1,501(13.9) | 1,637(15.2 | 301(2.8) |
| Myanmar | 2014/15 | 1.880(15.4) | 2,329(19.1) | 678(5.7) | |
| Timor-Leste | 2016 | 3,102(26.3) | 969(8.2) | 194(1.6) | |
| Pooled prevalence | 2014–2016 | 6,483(18.7) | 4,935(14.2) | 1,173(3.4) | |
| Europe and Central Asia | Albania | 2017/18 | 460(4.4) | 3,006(28.8) | 1,733(16.6) |
| Armenia | 2015/16 | 207(3.6) | 1,712(30.0) | 858(15.0) | |
| Tajikistan | 2017 | 718(7.3) | 2,351(23.8) | 317(13.3) | |
| Kyrgyz Republic | 2012 | 540(7.2) | 1,790(23.8) | 897(11.9) | |
| Turkey | 2013 | 290(3.5) | 2,365(28.7) | 2,188(26.6) | |
| Pooled prevalence | 2012–2018 | 2,215(5.3) | 11,224(26.9) | 5,993(16.7) | |
| Middle East and North Africa | Egypt | 2014 | 46(0.2) | 7,106(36.5) | 9,305(47.8) |
| Jordan | 2017/18 | 80(1.2) | 2,433(37.8) | 2.020(31.4) | |
| Pooled prevalence | 2014–2018 | 126(0.49) | 9,539(36.9) | 11,325(43.8) | |
| West and Central Africa | Burkina Faso | 2010 | 192(15.5) | 612(7.9) | 293(3.8) |
| Benin | 2017/18 | 781(10.8) | 1,184(16.5) | 667(9.3) | |
| Central Democratic Congo | 2013/14 | 1,167(14.1) | 1,036(12.5) | 281(3.4) | |
| Cote d’vore | 2011/12 | 319(7.6 | 790(18.8) | 291(6.9) | |
| Chad | 2014 | 1,860(18.9) | 885(9.0) | 239(2.4) | |
| Cameroon | 2018 | 380(6.1) | 1,478(23.6) | 843(13.4) | |
| Congo | 2011/12 | 705(13.6) | 866(16.7 | 626(12.1) | |
| Mauritania | 2019/21 | 524(7.7) | 1,837(26.9) | 1,832(26.8) | |
| Gabon | 2012 | 350(7.0) | 1,252(25.1) | 1,018(20.4) | |
| Ghana | 2014 | 265(6.1) | 1,081(24.8) | 661(15.2) | |
| Gambia | 2019/20 | 740(13.4) | 1,229(22.3) | 747(13.9) | |
| Guinea | 2018 | 455(9.4) | 873(17.3) | 412(8.5) | |
| Liberia | 2019/20 | 202(5.3) | 919(24.2) | 464(12.2) | |
| Mali | 2018 | 459(10.1) | 862(19.0) | 384(8.5) | |
| Nigeria | 2018 | 1,564(11.9) | 2,389(18.1) | 1,305(9.9) | |
| Niger | 2011 | 661(14.4) | 603(13.1) | 375(8.1) | |
| Sierra leone | 2019 | 476(6.7) | 1,397(19.7) | 565(8.0) | |
| Senegal | 2012 | 1,158(20.2) | 822(14.3) | 748(13.0) | |
| Togo | 2013/14 | 303(6.9) | 855(19.5) | 486(11.1) | |
| Pooled prevalence | 2010–2021 | 12,561(9.6) | 20,970(20.1) | 12,237(12.1) | |
| Eastern & Southern Africa | Burundi | 2016/17 | 1,482(18.7) | 491(6.2) | 167(1.7) |
| Ethiopia | 2016 | 3,093(22.0) | 835(5.9) | 223(1.6) | |
| Comoros | 2012 | 333(6.7) | 1,196(24.0) | 730(14.7) | |
| Kenya | 2014 | 1,181(8.8) | 3,033(22.7) | 1,363(10.2) | |
| Lesotho | 2014 | 135(4.2) | 809(25.2) | 626(19.5) | |
| Madagascar | 2021 | 1,627(18.3) | 985(11.1) | 244(2.7) | |
| Malawi | 2015/16 | 522(7.1) | 1,124(15.2) | 416(5.6) | |
| Mozambique | 2011 | 1,024(8.4) | 1,480(12.1) | 616(5.0) | |
| Namibia | 2013 | 552(13.8) | 734(18.4) | 530(13.2) | |
| Rwanda | 2019/20 | 399(5.8) | 1,410(20.6) | 397(5.8) | |
| South Africa | 2016 | 96(3.1) | 834(26.6) | 1,118(35.7) | |
| Tanzania | 2015 | 1,117(9.3) | 3,102(18.4) | 1,206(10.0) | |
| Uganda | 2016 | 463(8.6) | 896(16.6) | 383(7.1) | |
| Zimbabwe | 2015 | 545(6.00) | 2,032(22.4) | 1,141(12.6) | |
| Pooled prevalence | 2011–2021 | 12,569(12.5) | 18,961(14.6) | 9,160(6.4) | |
| Latin America & Caribbean | Dominican Republic | 2013 | 625(7.2) | 2,578(29.8) | 1,793(20.7) |
| Guatemala | 2014/15 | 688(2.8) | 7,720 (31.9) | 4,814(19.9) | |
| Haiti | 2016/17 | 954(10.5) | 1,865(20.6) | 1,017(11.2) | |
| Honduras | 2011/12 | 1,012(4.7) | 6,154(28.6) | 5,115(23.7) | |
| Pooled prevalence | 2011–2017 | 3,279(6.6) | 18,317(27.0) | 12,739(20.2) |
Multilevel analyses
Random parameter estimation and model selection
Table 3 shows the multilevel multinomial regression model of random effects estimates of DBM. Random effect analysis in the null model was used to test for clustering effects on DBM. The results showed a significant difference in DBM between clusters (ICC = 9.9%), which indicated that the clusters accounted for 9.9% of the variance in DBM. In Model III, variability in DBM between clusters was reduced (ICC, 7.3%). In the model I and model III, the explained variances were 16.7% and 27.8% respectively. This implied that a large amount of variances in DBM has been explained by model III. To identify factors associated with DBM, model III which contains both individual and community level variables was selected as the most suitable due to the least deviance. Therefore, the final interpretation of results (fixed results) was based on model III. Fixed effect results of model I and model 2 are presented in Supplementary Table 3.
Table 3.
The random effects of the multilevel multinomial logistic regression in assessing the factors associated with DBM
| Parameters | Null model | Model I | Model III | Model III |
|---|---|---|---|---|
| Community level variance | 0.36 | 0.30 | 0.34 | 0.26 |
| Intraclass Correlation Coefficient (ICC) | 0.099 | 0.083 | 0.094 | 0.073 |
| Proportional Change in Variance (PCV) | Reference | 0.167 | 0.056 | 0.278 |
| Median Odds Ratio (MOR) | 1.77 | 1.68 | 1.74 | 1.62 |
| Deviance (-2Log-likelihood) | 2,456,811.4 | 2,244,835.0 | 242,176.8 | 2,239,708.2 |
Null model contains only the outcome variable and cluster numbers, model I; a model that includes only individual-level variables, model II, a model that includes only community-level variables, and model III; a model that includes both individual and community-level variables
Factors associated with underweight, overweight and obesity (Fixed effects)
Table 4 presents the estimated adjusted relative risk ratios (RRR) with their 95% CIs from multi-level multinomial logit models on underweight, overweight, and obesity for women of reproductive age in LMICs. Women aged 25–34 years and ≥ 35 years were 1.94 (RRR = 1.94, 95% 1.90–1.97) and 2.69 (RRR = 2.69, 95% 2.64–2.75) times higher risks for having overweight compared to women aged 15–24 years, respectively. Similarly, women aged 25–34 years and ≥ 35 years were 2.48 (RRR = 2.48, 95% 2.41–2.55 and 4.18 (RRR = 4.18, 95% 4.05–4.31) times higher risks for obesity compared to women aged 15–24 years, respectively. Conversely, women in the age group 25–34 years had 36% (RRR = 0.64, 95% 0.63–0.66) and ≥ 35 years had 47% (RRR = 0.53, 95%CI; 0.52–0.52) lower risks for having underweight compared to women aged 15–24 years. Women who belonged to households of the poorer (RRR = 1.24; 95% CI = 1.22–1.27), middle (RRR = 1.48, 95% CI = 1.46–1.51), richer (RRR = 1.72, 95% CI = 1.68–1.75), and richest (RRR = 1.98; 95% CI = 1.94–2.02) wealth quintiles had a higher risk of experiencing the overweight compared to women from poorest households. Also, women who belonged to households of the poorer (RRR = 1.13; 95% CI = 1.10–1.17), middle (RRR = 1.41, 95% CI = 1.37–1.45), richer (RRR = 1.72, 95% CI = 1.67–1.77), and richest (RRR = 2.20; 95% CI = 2.14–2.27) wealth quintiles had significantly higher risks of experiencing the obesity relative to women from poorest households. While women from the richest, richer, middle, and poorer households had 43% (RRR = 0.57, 95%CI; 0.55–0.56), 33% (RRR = 0.67, 95%CI; 0.66–0.68), 26% (RRR = 0.74, 95%CI; 0.73–0.75), and 17% (RRR = 0.83, 95%CI; 0.81–0.84) times lower risk of having underweight compared to women from poorest households, respectively. Being rural dwellers was associated with a 1.13 (RRR = 1.13; 95% CI = 1.11–1.15) times higher risk of having underweight, but at decreased risk of overweight and obesity by 22% (RRR = 0.78; 95% CI = 0.77–0.79) and 41% (RRR = 0.59; 95% CI = 0.58–0.60) respectively compared to urban dwellers.
Table 4.
Multivariable multi-level multinomial analysis of the factors associated with malnutrition in LMICs
| Variables | Underweight ARRR (95% CI) |
Overweight ARRR (95% CI) |
Obesity ARRR (95% CI) |
|---|---|---|---|
| Age | |||
| 15–24 | 1 | 1 | 1 |
| 25–34 | 0.64 (0.63–0.66)** | 1.94 (1.90–1.97) ** | 2.48 (2.41–2.55) ** |
| 35–49 | 0.53 (0.52–0.54)** | 2.69 (2.64–2.75) ** | 4.18 (4.05–4.31) ** |
| Educational status | |||
| Not educated | 1 | 1 | 1 |
| Primary | 0.74 (0.73–0.75)** | 1.33 (1.31–1.35) ** | 1.54 (1.50–1.57) ** |
| Secondary | 0.86 (0.85–0.88)** | 1.32 (1.31–1.35) ** | 1.48 (1.44–1.51) ** |
| Higher | 0.68 (0.66–0.69)** | 1.40 (1.37–1.44) ** | 1.39 (1.35–1.44) ** |
| Household wealth status | |||
| Poorest | 1 | 1 | 1 |
| Poorer | 0.83 (0.81–0.84) ** | 1.24 (1.22–1.27) ** | 1.13 (1.10–1.17) ** |
| Middle | 0.74 (0.73–0.75)** | 1.48 (1.46–1.51) ** | 1.41 (1.37–1.45) ** |
| Richer | 0.67 (0.66–0.68)** | 1.72 (1.68–1.75) ** | 1.72 (1.67–1.77) ** |
| Richest | 0.57 (0.55–0.59)** | 1.98 (1.94–2.02) ** | 2.20 (2.14–2.27) ** |
| Marital status | |||
| Not currently in union | 1 | 1 | 1 |
| Currently in union | 1.01 (0.97–1.03) | 1.04 (0.99–1.05) | 1.02 (0.98–1.04) |
| Family size | |||
| ≤ 5 | 1 | 1 | 1 |
| 6–10 | 1.12 (1.11–1.14)** | 0.97 (0.94–1.03) | 0.98 (0.96–1.02) |
| > 10 | 1.08 (1.05–1.11)** | 0.96 (0.93–1.01) | 1.02 (0.99–1.06) |
| Frequency of reading newspaper or magazine | |||
| Not at all | 1 | 1 | 1 |
| Less than once a week | 1.05 (1.00–1.08) | 1.00 (0.99–1.02) | 0.99 (0.97–1.01) |
| At least once a week | 1.03 (0.98–1.06) | 1.04 (1.02–1.06) * | 1.05 (0.99–1.08) |
| Almost every day | 0.98 (0.76–1.15) | 1.03 (0.88–1.21) | 1.13 (0.93–1.37) |
| Frequency of watching television | |||
| Not at all | 1 | 1 | 1 |
| Less than once a week | 1.02 (0.99–1.04) | 1.06 (0.96–1.08) | 1.04 (0.9–1.11) |
| At least once a week | 1.01 (0.99–1.03) | 1.52 (1.50–1.54) ** | 2.25 (2.20–2.30) ** |
| Almost every day | 0.78 (0.71–0.86)* | 1.63 (1.52–1.74) ** | 2.81 (2.59–3.05) ** |
| Frequency of listening to radio | |||
| Not at all | 1 | 1 | 1 |
| Less than once a week | 0.94 (0.91–1.01) | 1.01 (0.97–1.05) | 1.10 (0.97–1.19) |
| At least once a week | 0.58 (0.57–0.59)** | 1.34 (1.32–1.36) * | 1.77 (1.73–1.80) ** |
| Almost every day | 0.57 (0.52–0.63)** | 1.23 (1.14–1.32) * | 1.62 (1.48–1.77) ** |
| Sex of household head | |||
| Male | 1 | ||
| Female | 0.96 (0.95–1.09) | 1.03 (0.96–1.05) | 1.05 (0.99–1.10) |
| Residence | |||
| Urban | 1 | 1 | 1 |
| Rural | 1.13 (1.11–1.15)** | 0.78 (0.77–0.79) ** | 0.59 (0.58–0.60) ** |
| Contraceptive use | |||
| Not using | 1 | 1 | 1 |
| Use traditional method | 0.96 (0.93–1.02) | 1.09 (0.95–1.11)** | 0.97 (0.89–1.12)** |
| Use modern method | 0.92 (0.90–0.93)* | 1.00 (0.98–1.01) | 1.15 (1.12–1.17) ** |
| Currently breastfeeding | |||
| No | 1 | 1 | 1 |
| Yes | 0.97 (0.92–1.03) | 0.78 (0.77–0.79)** | 0.69 (0.68–0.71)** |
| Parity | |||
| Nulliparous | 1 | 1 | 1 |
| Primiparous | 0.61 (0.59–0.62)** | 1.75 (1.71–1.79) ** | 1.99 (1.93–2.06) ** |
| Multiparous | 0.64 (0.62–0.65) ** | 1.91 (1.87–1.95) ** | 2.47 (2.39–2.54) ** |
| Grand Multiparous | 0.67 (0.65–0.69) ** | 1.92 (1.87–1.97) ** | 3.16 (3.04–3.28) ** |
ARRR Adjusted relative risk ratio, CI Confidence interval
*P-value < 0.05, **P-value < 0.01
The risk of being overweight was higher among women with primary education (RRR = 1.33; 95% CI = 1.31–1.35), secondary education (RRR = 1.32, 95%CI = 1.31–1.35), and higher education (RRR = 1.40, 95%CI = 1.37–1.44) compared with non-educated women. The risk of obesity was higher among women with primary education (RRR = 1.54; 95% CI = 1.50–1.57), secondary education (RRR = 1.48, 95%CI = 1.44–1.51), and higher education (RRR = 1.39, 95%CI = 1.35–1.44) compared women without formal education. However, compared with women without formal education, women with primary education had 26% (RRR = 0.74; 95% CI = 0.73–0.75), secondary education had 14% (RRR = 0.86, 95%CI = 0.85–0.88) and higher education had 32% (RRR = 0.68, 95%CI = 0.66–0.69) lower risk of underweight. Respondents living with a family size of 6–10 had 12% (RRR = 1.12, 95%CI = 1.11–1.14) and > 10 had 8% (RRR = 1.08, 95%CI = 1.05–1.11) higher risk of underweight compared to women living with ≤ 5 family size.
The risk of being overweight was higher among primiparous (RRR = 1.75, 95%CI = 1.71–1.79), multiparous (RRR = 1.91, 95%CI = 1.87–1.95), grand multiparous women (RRR = 1.92, 95%CI = 1.87–1.97), women who watched television at least once a week (RRR = 1.52, 95%CI = 1.50–1.54), almost every day (RRR = 1.63, 95%CI = 1.52–1.74), women who listened to the radio at least once a week (RRR = 1.34, 95%CI = 1.32–1.36), and almost every day (RRR = 1.23, 95%CI = 1.14–1.32).
The risk of obesity was higher among primiparous (RRR = 1.99, 95%CI = 1.93–2.06), multiparous (RRR = 2.47, 95%CI = 2.39–2.54), grand multiparous (RRR = 3.16, 95%CI = 3.04–3.28), women who watched television at least once a week (RRR = 2.25, 95%CI = 2.20–2.30), and almost every day (RRR = 2.81, 95%CI = 2.59–3.05), women who listened to the radio at least once a week (RRR = 1.77, 95%CI = 1.73–1.80), and almost every day (RRR = 1.62, 95%CI = 1.48–1.77). Lactating mothers were less likely to be overweight (RRR = 0.78, 95%CI = 0.77–0.79) and obese (RRR = 0.69, 95%CI = 0.68–0.71) compared to non-lactating mothers. Moreover, the risk of underweight was less likely among primiparous (RRR = 0.61, 95%CI = 0.59–0.62), multiparous (RRR = 0.64, 95%CI = 0.62–0.64), grand multiparous (RRR = 0.67, 95%CI = 0.65–0.69), those using modern contraceptives (RRR = 0.92, 95%CI = 0.90–0.93), those watching television almost every day (RRR = 0.78, 95%CI = 0.71–0.81), those listening radio at least once a week (RRR = 0.58, 95%CI = 0.57–0.59), and almost every day (RRR = 0.57, 95%CI = 0.52–0.63)compared to counterparts (Table 4).
Discussion
This study examined the prevalence and associated factors of DBM indicators (underweight, overweight, and obesity) among women of reproductive age in LMICs using 52 nationally representative data. This study builds literature on sociodemographic, socioeconomic, obstetric, and behavioral factors associated with DBM among women of reproductive age in LMICs based on a nationally representative survey. The results indicated a substantial DBM among women of reproductive age in LMICs. We found that educational status, age, household wealth status, frequency of watching television, frequency of radio listening, parity, use of modern contraceptives, not lactating and urban dwelling were positively associated with overweight and obesity. Educational status, age, household wealth status, frequency of watching television, frequency of radio listening, parity, use of modern contraceptives, family size and urban dwelling were negatively associated with being underweight. The findings of this study will assist policymakers to identify the population groups at risk of DBM for better development of programs, which could in turn play a substantial role in reducing the burden of non-communicable diseases.
In the present study, the pooled prevalence of underweight is 15.2 (95% CI: 15.1, 15.3). Furthermore, we found that a high burden of being overweight (nearly one in five women) and obese (nearly one in 10 women) co-occurs with a high burden of being underweight in LMICs. Prevalence rates for underweight, overweight, and obesity show considerable variation across countries. Variations were found in other studies as well [69, 70]. This difference may actually be due to differences in physical activity levels, dietary habits and awareness of malnutrition in different countries. Previous studies indicated that the prevalence of obesity was more than 20% in 14 Latin American countries [70] and more than 30% in several countries in the Middle East and in North and southern Africa among women [59]. A previous study reported overweight and obesity in LMICs ranged from a low of 4.7% in the Democratic Republic of Korea to a high of 88.3% in Tonga [70]. The contribution of underweight, overweight, and obesity to the burden of disease and mortality have been well documented [71–73]. The existing evidence shows that underweight and obesity are among the top 10 leading risk factors for the global burden of disease. Decreased physical activity and changes in diet are among the main contributors to obesity [74, 75]. In addition to undernutrition, a profound shift in nutrition from the end of famine (pattern 3) to the consumption of more energy-dense diets (pattern 4) is a public health concern for most LMICs and requires urgent action. This shift from a traditional diet to a Western-style diet is a key factor contributing to the prevalence of obesity-related NCDs in LMICs [76–78]. In response to the rising prevalence of DBM, WHO has introduced a comprehensive strategy to stop the increase in obesity prevalence by 2025, with the potential to simultaneously reduce the risk of undernutrition and diet-related NCDs [79, 80]. This comprehensive strategy is designed to provide easy access to a healthy and nutritious diet that promotes a healthy weight. Specifically, the WHO proposed a roadmap so-called Double-Duty Actions (DDAs) to tackle the DBM. This road map includes programs, policies, and interventions that have the potential to simultaneously reduce the risk or burden of all forms of malnutrition [81]. Public health actions such as dietary/nutrition counseling, media outreach, nutrition labeling, issuing of dietary guidelines, and taxes on sugar-sweetened beverages are widely recommended as part of national strategies to combat overweight, obesity, and obesity-related NCDs [82]. However, such public health actions are not common in most LMICs [78]. In addition, interventions to address overweight/obesity should focus on women of reproductive age to increase their awareness of the impact of healthy foods such as vegetables, grains, and fruits, as well as television viewing and a sedentary lifestyle. Therefore, we strongly recommend DDAs activities such as adopting healthy dietary habits during adolescence and antenatal care nutritional counseling should be strengthened to reduce both forms of malnutrition among women of reproductive age.
This study found that a positive relationship exists between the frequency of watching television overweight and obesity. This finding was consistent with previously published studies from Ghana [43], Tanzania [38], Bangladesh [56], and Myanmar [57] that showed women who watched television had a higher risk of being overweight and obese compared to those who did not watch television. This result is not surprising given that a positive association between spending a long time watching television and an increase in sitting time has been shown, which results in a reduced level of physical activity and reduced resting metabolism [56, 83, 84]. A study revealed that the effects of television watching extend beyond reduced levels of physical activity to increased calorie consumption and influence people to make unhealthy diet choices while watching as a result of advertising [85–87]. A Study conducted in Ghana and Kenya documented nearly half (48.3%) of all advertisements were for sugar-sweetened beverages [85]. Moreover, in LMICs, having a television is a proxy indicator for higher socioeconomic status, which in turn increases the consumption of energy-dense and junk foods [38].
This study showed that women from the richest, richer and middle households were more likely to be overweight and obese while less likely to be underweight compared to those who resided in the poorest households. Likewise, previous studies have consistently shown that women from wealthy households have a higher risk of being overweight and obese, and a lower risk of being underweight [40, 46–49]. In LMICs, unhealthy practices such as consuming more energy-dense diets and following a sedentary lifestyle vary by socioeconomic status. Furthermore, women living in the poorest households are less likely to have access to adequate and diverse diets, water, clothing, and good shelter. As a result, the poorest people are at high risk of developing from various communicable diseases due to macronutrient or micronutrient deficiency, poor hygiene, and sanitation [88, 89].
Global evidence shows that education is one of the most important media that influence the economy, attitude, health behaviors, and outcomes, including physical activity, diet, and body weight [90–92]. Thus, educated individuals have better health status, due to the improvement in socioeconomic status, health information, and health behaviors. However, this was not the case in this study, which suggests that women of childbearing age with primary, secondary, and tertiary education were more likely to be overweight and obese compared to women with no formal education. This finding is similar to previous studies conducted in LMICs [37, 40–43]. A possible explanation for this, in LMICs, higher educational attainment is associated with higher socioeconomic status and material resources which in turn women to more likely take Western diets, which are characterized by high protein and energy-dense foods, and use a vehicle for transport or practice more sedentary employment (for example, office work) [93]. However, in contrast to our study, a study from china found that women of reproductive age with secondary and higher education were less likely to be overweight and obese and not significantly associated with being underweight [39]. This inconsistency may be attributable to differences in socioeconomic, policy, and nutritional transition across population groups. The association between education and overweight/obesity is contextual, varies over time, and is closely related to the ongoing nutritional transition across countries [94, 95]. Furthermore, this disparity may also suggest that health policies in LMICs have traditionally focused on health problems related to infectious diseases and undernutrition, while interventions targeting rapid shifts in diet and epidemiology are likely underway. Therefore, we hypothesize that establishing a causal relationship between educational status and DBM indicators is difficult because of possible confounding with unobserved characteristics. Future researchers should address the causal relationship between education and obesity by conducting experimental or quasi-experimental studies.
The results in relation to age were mixed. While in general a significant positive association was observed between age and overweight and obesity, a negative association was observed with underweight. These findings are consistent with previous studies. A study by Amugsi DA et al., found that older age was positively associated with being overweight and obese in Ghana, Mozambique, Kenya, and Nigeria [42]. In the same study, older age was positively associated with being overweight in Democratic Republic of Congo. Moreover, older age was associated positively with overweight among women in Ethiopia, Tanzania, Maldives, Bangladesh, India, and China [34, 37–41]. Consistent with our findings, studies from Ethiopia, Nigeria, and India found that older age was negatively associated with being underweight [36, 41, 42]. In contrast, Amugsi DA et al., and Song J et al., reported that older women were more like to be underweight [39, 42]. The positive relationship found between older age and overweight and obesity may potentially be explained by several factors. First, advanced age is associated with parity and another associated factor of overweight and obesity. Evidence documented that women usually gain weight during pregnancy which could be associated with higher lifetime weight retention if weight loss does not occur post-partum [96–99]. As observed in this study, primiparous, multiparous, and grand multiparous women were more likely to be overweight and obese and less likely to be underweight as compared with nulliparous women. Second, body composition and hormonal changes that occur during aging may contribute to fat accumulation [100]. Third, the fact that adolescence is a time of rapid physical, psychosocial, and cognitive development increases the need for nutrients that may be linked to undernutrition. The relationship between age and underweight has been documented in previous studies. For example, a study in Ethiopia and India observed a significant negative association between age and underweight [36, 41].
Consistent with other studies in the LMIC region [36, 37, 40, 41], we found that compared with rural women, urban women were more at risk of being overweight and obese, while urban women were less likely to be underweight. Similarly, a study in china demonstrated that urban women were more likely to be overweight and obese [39]. Possible reasons for the association between urban dwellers and overweight and obesity may be that urban dwellers are more likely to consume processed, packaged and refrigerated foods, and to be physically inactive. Besides, women who resided in rural areas may be engaged in occupational physical activities such as agricultural occupations subject them to labor-intensive activities and therefore, unlikely to gain as much weight as urban women could be a possible explanation for the negative relationship between rural residents and overweight and obesity.
Furthermore, this study revealed that the risk of being underweight is lower among modern contraceptive users. Previous research from Nigeria and Myanmar has observed similar relationships [54, 55]. Also, we found the risk of obesity is higher among modern contraceptive users. This result is supported by a study among women of reproductive age in Ethiopia; it revealed that using combined oral contraceptives increased the occurrence of obesity [101]. Additionally, similar findings have been reported in studies conducted in Kenya, Myanmar and India [41, 101, 102]. The reason behind this association may be the consequence of might be hormonal effect of contraceptives (i.e. progesterone and estrogen) that contribute to weight gain. Progesterone increases appetite and estrogen facilitates lipid metabolism and fat accumulation which in turn increases obesity [103].
We found that risk of being overweight and obese was less likely among lactating mothers. Consistent with our findings, Amugsi DA et al. suggested that the risk of being overweight and obese in the five countries was less likely among lactating mothers [42]. Besides, health benefits of breastfeeding for both mothers and babies have been documented [104–109]. Breastfeeding provides a child with ideal nutrition, protects child from certain diseases, and supports growth and development [104, 105]. Breastfeeding can also help to reduce breast and ovarian cancer, type 2 diabetes, postpartum depression, and high blood pressure [106–109].
Strengths and limitations of study
This study has its own strengths and limitations. The main strength of this study is the use of large nationally representative samples with appropriate statistical modeling. The use of large nationally representative data and multilevel analysis helps to provide more robust estimates of observed associations as well as enhance the generalizability of the results. Although this study used a nationally representative dataset and appropriate model, the results should be interpreted in light of some limitations. First, this study used cross-sectional data, which does not provide itself to the establishment of a temporal relationship between the factors and outcome variables. Second, we are unable to incorporate important covariates such as physical activity, dietary intake, other comorbid conditions, and energy expenditure, as the DHS did not collect information on these variables. Third, although BMI is important WHO recommended indicator of nutritional status measurement, it cannot differentiate between body fat and lean body mass. Furthermore, this study did not examine DBM at the population and within households can be considered as a limitation of the study.
Conclusion
The prevalence of underweight, overweight, and obesity was high among women of reproductive age in LMICs. Educational status, age, household wealth status, frequency of watching television, frequency of listening to radio, parity, and using modern contraceptives were positively associated with overweight and obesity and negatively associated with being underweight. Moreover, being rural dweller and breastfeeding were negatively associated with obesity and overweight, and rural women had a higher risk of being underweight than urban women. This study shows that in order to achieve Sustainable Development Goal 2, multifaceted intervention approaches should be considered to prevent both forms of malnutrition in women of reproductive age. This can be achieved by raising awareness and promoting healthy behaviors such as healthy eating and physical activity, especially among educated women, women from wealthy households, and women exposed to the media.
Supplementary Information
Additional file 1: Supplementary Table 1. Coding strategy of variables used in analysis.
Additional file 2: Supplementary Table 2. Bivariable multi-level multinomial analysis of the factors associated with double burden of malnutrition in low and middle income countries.
Additional file 3: Supplementary Table 3. Fixed effects of multi-level multinomial analysis of individual and community level variables.
Acknowledgements
Authors would like to acknowledge Demographic Health and Survey (DHS) program managers, which granted us the permission to use DHS data for this study.
Abbreviations
- CI
Confidence Interval
- DBM
Double Burden of Malnutrition
- DHS
Demographic and Health Survey
- RRR
Relative risk ratio
- LMICs
Low and Middle income Countries
Authors’ contributions
AZA: developed the concept, reviewed literature, carried out the statistical analysis, interpreted the results and prepared the manuscript. YY, AML, ZTT, MGW, GAT, TSA, ABT, DC and HGA: reviewed literature, involved in analysis, interpretation and prepared the manuscript. All the authors read, draft and approved the manuscript.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Availability of data and materials
The datasets used and/or analysed during the current study is available in a public, open access repository which is accessible online www.measuredhs.com.
Declarations
Ethics approval and consent to participate
Since this study used secondary data analysis of publically available survey data, ethical approval is not required. However, to use the data we requested DHS Program and we received an authentication letter from archive@dhsprogram.com.
Consent for publication
Not required.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization . Double burden of malnutrition. 2017. [Google Scholar]
- 2.Shrimpton R, Rokx C. The Double Burden of Malnutrition: A Review of Global Evidence. Health, Nutrition and Population Discussion Paper. Washington: World Bank; 2012. [Google Scholar]
- 3.World Health Organization. WHO accelerates work on nutrition targets with new commitments [Internet]. 2021. Available from: https://www.who.int/news/item/07-12-2021-who-accelerates-work-onnutrition-targets-with-new-commitments#:~:text=Today%2Cone%20third%20of%20all,8%20million%20deaths%20per%20year.
- 4.Minicuci N, Biritwum RB, Mensah G, Yawson AE, Naidoo N, Chatterji S, et al. Socio demographic and socioeconomic patterns of chronic non-communicable disease among the older adult population in Ghana. Glob Health Action. 2014;7:1–13. doi: 10.3402/gha.v7.21292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, et al. The global obesity pandemic: Shaped by global drivers and local environments. Lancet. 2011;378:804–14. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 6.Herbert K, et al. Prevalence of risk factors for non-communicable diseases in prison populations worldwide: a systematic review. The Lancet. 2012;379(9830):1975–1982. doi: 10.1016/S0140-6736(12)60319-5. [DOI] [PubMed] [Google Scholar]
- 7.Mamun AA, Finlay JE. Shifting of undernutrition to overnutrition and its determinants among women of reproductive ages in the 36 low to medium income countries. Obes Res Clin Pr. 2015;9:75–86. doi: 10.1016/j.orcp.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Kim BY, Nam H, Yoo JJ, Cho YY, Choi DH, Jung CH, et al. Association between alcohol consumption status and obesity-related comorbidities in men: data from the 2016 Korean community health survey. BMC Public Health. 2021;21(1):1–8. doi: 10.1186/s12889-021-10776-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vorster HH, Kruger A, Margetts BM. The nutrition transition in Africa: can it be steered into a more positive direction? Nutrients. 2011;3(4):429–441. doi: 10.3390/nu3040429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katzmarzyk PT, Reeder BA, Elliott S, Joffres MR, Pahwa P, Raine KD, et al. Body mass index and risk of cardiovascular disease, cancer and all-cause mortality. Can J Public Heal. 2012;103(2):147–151. doi: 10.1007/BF03404221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riquelme R, Rezende LFM, Guzmán-Habinger J, Chávez JL, Celis-Morales C, Ferreccio C, et al. Non-communicable diseases deaths attributable to high body mass index in Chile. Sci Rep. 2021;11(1):1–8. doi: 10.1038/s41598-021-94974-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felisbino-Mendes MS, Cousin E, Malta DC, Machado ÍE, Ribeiro ALP, Duncan BB, et al. The burden of non-communicable diseases attributable to high BMI in Brazil, 1990–2017: Findings from the Global Burden of Disease Study. Popul Health Metr. 2020;18(Suppl 1):1–13. doi: 10.1186/s12963-020-00219-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng M, Fleming T, Robinson M, Cristiana A, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the global burden of disease study 2013. The Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cefalu WT, Bray GA, Home PD, et al. Advances in the science, treatment, and prevention of the disease of obesity: reflections from a diabetes care editors’ expert forum. Diabetes Care. 2015;38:1567–1582. doi: 10.2337/dc15-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fruh SM. Obesity: risk factors, complications, and strategies for sustainable long-term weight management. J Am Assoc Nurse Pract. 2017;29:S3–14. doi: 10.1002/2327-6924.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Capodaglio P, Liuzzi A, Italiano I. Obesity: a disabling disease or a condition favoring disability? Eur J Phys Rehabil Med. 2013;49:395–398. [PubMed] [Google Scholar]
- 17.Boutayeb A, Boutayeb S. The burden of non communicable diseases in developing countries. Int J Equity Health. 2005;4(1):1–8. [DOI] [PMC free article] [PubMed]
- 18.Ding D, et al. The economic burden of physical inactivity: a global analysis of major non-communicable diseases. Lancet. 2016;388(10051):1311–1324. doi: 10.1016/S0140-6736(16)30383-X. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization . Noncommunicable diseases country profiles 2018. Geneva: World Health Organization; 2018. [Google Scholar]
- 20.WHO | Regional Office for Africa. Noncommunicable Diseases [Internet]. Available: https://www.afro.who.int/health-topics/noncommunicable-diseases. Accessed 19 Mar 2020.
- 21.Kamal SM, Islam A. Socio-economic correlates of malnutrition among married women in Bangladesh. Malays J Nutr. 2010;16:349–359. [PubMed] [Google Scholar]
- 22.Liu L, Ma Y, Wang N, Lin W, Liu Y, Wen D. Maternal body mass index and risk of neonatal adverse outcomes in China: A systematic review and meta-analysis. BMC Pregnancy Childbirth. 2019;19(1):1–12. doi: 10.1186/s12884-019-2249-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parker MG, Ouyang F, Pearson C, Gillman MW, Belfort MB, Hong X, et al. Prepregnancy body mass index and risk of preterm birth: Association heterogeneity by preterm subgroups. BMC Pregnancy Childbirth. 2014;14(1):1–10. doi: 10.1186/1471-2393-14-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vinturache A, McKeating A, Daly N, Sheehan S, Turner M. Maternal body mass index and the prevalence of spontaneous and elective preterm deliveries in an Irish obstetric population: A retrospective cohort study. BMJ Open. 2017;7(10):1–13. doi: 10.1136/bmjopen-2016-015258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchi J, Berg M, Dencker A, et al. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev. 2015;16:621–638. doi: 10.1111/obr.12288. [DOI] [PubMed] [Google Scholar]
- 26.Mrema D, Lie RT, Østbye T, Mahande MJ, Daltveit AK. The association between pre pregnancy body mass index and risk of preeclampsia: A registry based study from Tanzania. BMC Pregnancy Childbirth. 2018;18(1):1–8. doi: 10.1186/s12884-018-1687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doi L, Williams AJ, Marryat L, Frank J. Cohort study of high maternal body mass index and the risk of adverse pregnancy and delivery outcomes in Scotland. BMJ Open. 2020;10(2):1–9. doi: 10.1136/bmjopen-2018-026168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang S, Liu H, Li N, Dong W, Li W, Wang L, et al. Relationship between gestational body mass index change and the risk of gestational diabetes mellitus: a community-based retrospective study of 41,845 pregnant women. BMC Pregnancy Childbirth. 2022;22(1):1–10. doi: 10.1186/s12884-022-04672-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gul R, Iqbal S, Anwar Z, Ahdi SG, Ali SH, Pirzada S. Pre-pregnancy maternal BMI as predictor of neonatal birth weight. PLoS One. 2020;15(10 October):1–9. doi: 10.1371/journal.pone.0240748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He Z, Bishwajit G, Yaya S, Cheng Z, Zou D, Zhou Y. Prevalence of low birth weight and its association with maternal body weight status in selected countries in Africa: A cross-sectional study. BMJ Open. 2018;8(8):1–8. doi: 10.1136/bmjopen-2017-020410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matos UR, Mesenburg MA, Victora CG. Socioeconomic inequalities in the prevalence of underweight, overweight, and obesity among women aged 20–49 in low- and middle-income countries. Int J Obes (London) 2020;44(3):609–616. doi: 10.1038/s41366-019-0503-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.NCD Risk Factor Collaboration (NCD-RisC) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387(10026):1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ajayi IOO, Adebamowo C, Adami HO, Dalal S, Diamond MB, Bajunirwe F, et al. Urban-rural and geographic differences in overweight and obesity in four sub-Saharan African adult populations: a multi-country cross-sectional study. BMC Public Health. 2016;16(1):1–13. doi: 10.1186/s12889-016-3789-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hashan MR, Rabbi F, Haider SS, Gupta RD. Prevalence and associated factors of underweight, overweight and obesity among women of reproductive age group in the Maldives : Evidence from a nationally representative study. PLoS One. 2020;15(10):1–14. doi: 10.1371/journal.pone.0241621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khanam M, Osuagwu UL, Sanin KI, Haque MA, Rita RS, Agho KE, Ahmed T. Underweight, Overweight and Obesity among Reproductive Bangladeshi Women: A Nationwide Survey. Nutrients. 2021;13(12):4408. doi: 10.3390/nu13124408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mengesha Kassie A, Beletew Abate B, Wudu Kassaw M, Gebremeskel Aragie T. Prevalence of Underweight and Its Associated Factors among Reproductive Age Group Women in Ethiopia: Analysis of the 2016 Ethiopian Demographic and Health Survey Data. J Environ Public Health. 2020;2020. [DOI] [PMC free article] [PubMed]
- 37.Tanwi TS, Chakrabarty S, Hasanuzzaman S. Double burden of malnutrition among ever-married women in Bangladesh: A pooled analysis. BMC Womens Health. 2019;19(1):2–9. doi: 10.1186/s12905-019-0725-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmed KY, Rwabilimbo AG, Abrha S, Page A, Arora A, Tadese F, et al. Factors associated with underweight, overweight, and obesity in reproductive age Tanzanian women. PLoS One. 2020;15(8):2004–11. doi: 10.1371/journal.pone.0237720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song J, Zhang J, Fawzi W, Huang Y. Double Burden of Malnutrition among Chinese. Nutrients. 2020;12(3102):1–12. doi: 10.3390/nu12103102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeshaw Y, Kebede SA, Liyew AM, Tesema GA, Agegnehu CD, Teshale AB, et al. Determinants of overweight/obesity among reproductive age group women in Ethiopia: Multilevel analysis of Ethiopian demographic and health survey. BMJ Open. 2020;10(3):1–7. doi: 10.1136/bmjopen-2019-034963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al Kibria GM, Swasey K, Hasan MZ, Sharmeen A, Day B. Prevalence and factors associated with underweight, overweight and obesity among women of reproductive age in India. Glob Heal Res Policy. 2019;4(1):1–12. doi: 10.1186/s41256-019-0117-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amugsi DA, Dimbuene ZT, Kyobutungi C. Correlates of the double burden of malnutrition among women: An analysis of cross sectional survey data from sub-Saharan Africa. BMJ Open. 2019;9(7):1–13. doi: 10.1136/bmjopen-2019-029545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doku DT, Neupane S. Double burden of malnutrition: increasing overweight and obesity and stall underweight trends among Ghanaian women. BMC Public Health. 2015;15(1):670. doi: 10.1186/s12889-015-2033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wariri O, Alhassan JAK, Mark G, Adesiyan O, Hanson L. Trends in obesity by socioeconomic status among non-pregnant women aged 15–49 y: A cross-sectional, multi-dimensional equity analysis of demographic and health surveys in 11 sub-Saharan Africa countries, 1994–2015. Int Health. 2021;13(5):436–445. doi: 10.1093/inthealth/ihaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ozodiegwu ID, Doctor HV, Quinn M, Mercer LD, Omoike OE, Mamudu HM. Is the positive association between middle-income and rich household wealth and adult sub-Saharan African women’s overweight status modified by the level of education attainment? A cross-sectional study of 22 countries. BMC Public Health. 2020;20(1):996. doi: 10.1186/s12889-020-08956-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neupane S, Prakash KC, Doku DT. Overweight and obesity among women: analysis of demographic and health survey data from 32 Sub-Saharan African Countries. BMC Public Health. 2016;16:30. doi: 10.1186/s12889-016-2698-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Biswas T, Garnett SP, Pervin S, Rawal LB. The prevalence of underweight, overweight and obesity in Bangladeshi adults: Data from a national survey. PLoS ONE. 2017;12(5):e0177395. doi: 10.1371/journal.pone.0177395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bishwajit G. Household wealth status and overweight and obesity among adult women in Bangladesh and Nepal. Obes Sci Pract. 2017;3(2):185–192. doi: 10.1002/osp4.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tesema AK, Liyew AM, Alem AZ, Yeshaw Y, Tesema GA, Teshale AB. Spatial distribution and determinants of undernutrition among reproductive age women of Ethiopia: A multilevel analysis. PLoS One. 2021;16(9):1–15. doi: 10.1371/journal.pone.0257664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seidu AA, Ahinkorah BO, Agbaglo E, Nyaaba AA. Overweight and obesity among women of reproductive age in Mali: What are the determinants? Int Health. 2021;13(5):428–435. doi: 10.1093/inthealth/ihaa094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mosha D, Paulo HA, Mwanyika-Sando M, Mboya IB, Madzorera I, Leyna GH, et al. Risk factors for overweight and obesity among women of reproductive age in Dar es Salaam. Tanzania BMC Nutr. 2021;7(1):1–10. doi: 10.1186/s40795-021-00445-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taklual W, Baye S, Mekie M, Andualem T. Double Burden of Malnutrition among Female Adolescent Students in Bahir Dar City, Amhara, Ethiopia. Biomed Res Int. 2020;2020. [DOI] [PMC free article] [PubMed]
- 53.Kushitor SB, Owusu L, Kushitor MK. The prevalence and correlates of the double burden of malnutrition among women in Ghana. PLoS One. 2020;15(12):1–12. doi: 10.1371/journal.pone.0244362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hong SA, Peltzer K, Lwin KT, Aung LS. The prevalence of underweight, overweight and obesity and their related socio-demographic and lifestyle factors among adult women in Myanmar, 2015–16. PLoS ONE. 2018;13(3):e0194454. doi: 10.1371/journal.pone.0194454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adebowale SA, Fagbamigbe FA, Bamgboye EA. Contraceptive use: implication for completed fertility, parity progression and maternal nutritional status in Nigeria. Afr J Reprod Health. 2011;15(4):60–67. [PubMed] [Google Scholar]
- 56.Ghose B. Frequency of TV viewing and prevalence of overweight and obesity among adult women in Bangladesh: a cross-sectional study. BMJ Open. 2017;7(1):e014399. doi: 10.1136/bmjopen-2016-014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Das Gupta R, Sajal IH, Hasan M, Sutradhar I, Haider MR, Sarker M. Frequency of television viewing and association with overweight and obesity among women of the reproductive age group in Myanmar: results from a nationwide cross-sectional survey. BMJ Open. 2019;9(3):e024680 [DOI] [PMC free article] [PubMed]
- 58.International Food Policy Research Institute (IFPRI). Global Nutrition Report . From Promise to Impact: Ending Malnutrition by 2030. Washington: IFPRI; 2016. p. 2016. [Google Scholar]
- 59.NCD Risk Factor Collab Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387:1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roberto CA, Swinburn B, Hawkes C. Huang TT-K, Costa SA, et al Patchy progress on obesity prevention: emerging examples, entrenched barriers, and new thinking. Lancet. 2015;385:2400–2409. doi: 10.1016/S0140-6736(14)61744-X. [DOI] [PubMed] [Google Scholar]
- 61.Jaacks LM, Slining MM, Popkin BM. Recent underweight and overweight trends by rural-urban residence among women in low- and middle-income countries. J Nutr. 2015;145(2):352–357. doi: 10.3945/jn.114.203562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.The DHS Program. DHS Methodology. http://dhsprogram.com/WhatWe-Do/Survey-Types/DHS-Methodology.cfm.
- 63.World Health Organisation . Obesity: preventing and managing global epidemic. Report of a WHO Expert Consultation. WHO Technical Report Series No 894. Geneva: WHO Technical Report Series No 894; 2000. [PubMed] [Google Scholar]
- 64.Uzêda JCO, Ribeiro-Silva RDC, Silva NDJ, Fiaccone RL, Malta DC, Ortelan N, et al. Factors associated with the double burden of malnutrition among adolescents, National Adolescent School-Based Health Survey (PENSE 2009 and 2015) PLoS ONE. 2019;14(6):1–11. doi: 10.1371/journal.pone.0218566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sunuwar DR, Singh DR, Pradhan PMS. Prevalence and factors associated with double and triple burden of malnutrition among mothers and children in Nepal: Evidence from 2016 Nepal demographic and health survey. BMC Public Health. 2020;20(1):1–11. doi: 10.1186/s12889-020-8356-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.StataCorp. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC, 2019.
- 67.Merlo J, Chaix B, Yang M, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: interpreting neighbourhood differences and the effect of neighbourhood characteristics on individual health. J Epidemiol Community Health. 2005;59:1. doi: 10.1136/jech.2004.028035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Merlo J, Chaix B, Ohlsson H, Beckman A, Johnell K, Hjerpe P, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: Using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006;60(4):290–297. doi: 10.1136/jech.2004.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hasan MM, Ahmed S, Soares Magalhaes RJ, Fatima Y, Biswas T, Mamun AA. Double burden of malnutrition among women of reproductive age in 55 low- and middle-income countries: progress achieved and opportunities for meeting the global target. Eur J Clin Nutr. 2022;76(2):277–287. doi: 10.1038/s41430-021-00945-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–81 [DOI] [PMC free article] [PubMed]
- 71.Abbafati C, Abbas KM, Abbasi-Kangevari M, Abd-Allah F, Abdelalim A, Abdollahi M, et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dai H, Alsalhe TA, Chalghaf N, Riccò M, Bragazzi NL, Wu J. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990–2017: An analysis of the Global Burden of Disease Study. PLoS Med. 2020;17(7):1–19. doi: 10.1371/journal.pmed.1003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wanjau MN, Aminde LN, Veerman JL. The avoidable disease burden associated with overweight and obesity in Kenya: A modelling study. eClinicalMedicine. 2022;50:101522. doi: 10.1016/j.eclinm.2022.101522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Swaminathan S, Hemalatha R, Pandey A, Kassebaum NJ, Laxmaiah A, Longvah T, et al. The burden of child and maternal malnutrition and trends in its indicators in the states of India: the Global Burden of Disease Study 1990–2017. Lancet Child Adolesc Heal. 2019;3(12):855–870. doi: 10.1016/S2352-4642(19)30273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Melaku YA, Wassie MM, Gill TK, Zhou SJ, Tessema GA. Burden of disease attributable to suboptimal diet, metabolic risks and low physical activity in Ethiopia and comparison with Eastern sub-Saharan African countries, 1990–2015: findings from the Global Burden of Disease Study 2015. BMC Public Health. 2018;18:1–20. doi: 10.1186/s12889-018-5438-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Popkin BM. Global nutrition dynamics: the world is shifting rapidly toward a diet linked with noncommunicable diseases. Am J Clin Nutr. 2006;84(2):289–298. doi: 10.1093/ajcn/84.1.289. [DOI] [PubMed] [Google Scholar]
- 77.Singh JE, Illner AK, Dokova K, Usheva N, Kostadinova T, Aleksandrova K. Mapping the global evidence on nutrition transition: a scoping review protocol. BMJ Open. 2020;10(6):e034730. doi: 10.1136/bmjopen-2019-034730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haggblade S, Duodu KG, Kabasa JD, Minnaar A, Ojijo NKO, Taylor JRN. Emerging Early Actions to Bend the Curve in Sub-Saharan Africa’s Nutrition Transition. Food Nutr Bull. 2016;37(2):219–241. doi: 10.1177/0379572116637723. [DOI] [PubMed] [Google Scholar]
- 79.WHO . World Health Organization. The double burden of malnutrition. Policy brief. Geneva: World Health Organization; 2017. [Google Scholar]
- 80.WHO (World Health Organ.) Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020. Geneva: WHO; 2013. [Google Scholar]
- 81.WHO Double Duty Actions for Nutrition. Available online: https://apps.who.int/iris/bitstream/handle/10665/255414/WHO-NMH-NHD-17.2-eng.pdf?ua=1.
- 82.Frieden TR. A framework for public health action: the health impact pyramid. Am J Public Health. 2010;100(4):590–595. doi: 10.2105/AJPH.2009.185652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Healy GN, Wijndaele K, Dunstan DW, Shaw JE, Salmon J, Zimmet PZ, et al. Objectively measured sedentary time, physical activity, and metabolic risk: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab) Diabetes Care. 2008;31(2):369–371. doi: 10.2337/dc07-1795. [DOI] [PubMed] [Google Scholar]
- 84.Bickham DS, Blood EA, Walls CE, Shrier LA, Rich M. Characteristics of screen media use associated with higher BMI in young adolescents. Pediatrics. 2013;131(5):935–941. doi: 10.1542/peds.2012-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Green MA, Pradeilles R, Laar A, Osei-Kwasi H, Bricas N, Coleman N, et al. Investigating foods and beverages sold and advertised in deprived urban neighbourhoods in Ghana and Kenya: A cross-sectional study. BMJ Open. 2020;10(6):1–8. doi: 10.1136/bmjopen-2019-035680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ghavamzadeh S, Khalkhali HR, Alizadeh M. TV viewing, independent of physical activity and obesogenic foods, increases overweight and obesity in adolescents. J Health Popul Nutr. 2013;31:334–342. doi: 10.3329/jhpn.v31i3.16825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rosiek A, Maciejewska NF, Leksowski K, Rosiek-Kryszewska A, Leksowski Ł. Effect of Television on Obesity and Excess of Weight and Consequences of Health. Int J Environ Res Public Health. 2015;12(8):9408–9426. doi: 10.3390/ijerph120809408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alkerwi A, Vernier C, Sauvageot N, Crichton GE, Elias MF. Demographic and socioeconomic disparity in nutrition: application of a novel Correlated Component Regression approach. BMJ Open. 2015;5(5):e006814. doi: 10.1136/bmjopen-2014-006814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morseth MS, Grewal NK, Kaasa IS, Hatloy A, Barikmo I, Henjum S. Dietary diversity is related to socioeconomic status among adult Saharawi refugees living in Algeria. BMC Public Health. 2017;17(1):1–9. doi: 10.1186/s12889-017-4527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Viinikainen J, Bryson A, Böckerman P, Kari JT, Lehtimäki T, Raitakari O, et al. Does better education mitigate risky health behavior? A mendelian randomization study. Econ Hum Biol. 2022;46:0–2. doi: 10.1016/j.ehb.2022.101134. [DOI] [PubMed] [Google Scholar]
- 91.Gebretatyos H, Amanuel S, Ghirmai L, Gebreyohannes G, Tesfamariam EH. Effect of Health Education on Healthy Nutrition and Physical Activity among Female Teachers Aged 40–60 Years in Asmara, Eritrea: A Quasiexperimental Study. J Nutr Metab. 2020;2020. [DOI] [PMC free article] [PubMed]
- 92.Raghupathi V, Raghupathi W. The influence of education on health: An empirical assessment of OECD countries for the period 1995–2015. Arch Public Heal. 2020;78(1):1–18. doi: 10.1186/s13690-020-00402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kautzky-Willer A, Dorner T, Jensby A, et al. Women show a closer association between educational level and hypertension or diabetes mellitus than males: a secondary analysis from the Austrian HIS. BMC Public Health. 2012;12:392. doi: 10.1186/1471-2458-12-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liwin LK. Shifting educational gradients in body mass index trajectories of Indonesians: an age period cohort analysis. BMC Public Health. 2022;22(1):1–14. doi: 10.1186/s12889-022-13379-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baker DP, Smith WC, Muñoz IG, Jeon H, Fu T, Leon J, Salinas D, Horvatek R. The Population Education Transition Curve: Education Gradients Across Population Exposure to New Health Risks. Demography. 2017;54(5):1873–1895. doi: 10.1007/s13524-017-0608-9. [DOI] [PubMed] [Google Scholar]
- 96.Jayasinghe S, Herath MP, Beckett JM, Ahuja KDK, Street SJ, Byrne NM, et al. Gestational weight gain and postpartum weight retention in Tasmanian women: The Baby-bod Study. PLoS ONE. 2022;17(3):e0264744. doi: 10.1371/journal.pone.0264744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li W, Wang Y, Shen L, Song L, Li H, Liu B, et al. Association between parity and obesity patterns in a middle-aged and older Chinese population: a cross-sectional analysis in the Tongji-Dongfeng cohort study. Nutr Metab. 2016;13(1):1–8. [DOI] [PMC free article] [PubMed]
- 98.Kominiarek MA, Peaceman AM. Gestational weight gain. Am J Obstet Gynecol. 2017;217:642–651. doi: 10.1016/j.ajog.2017.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bhavadharini B, Anjana RM, Deepa M, Jayashree G, Nrutya S, Shobana M, et al. Gestational weight gain and pregnancy outcomes in relation to body mass index in Asian Indian women. Indian J Endocrinol Metab. 2017;21:588–593. doi: 10.4103/ijem.IJEM_557_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Han TS, Tajar A, Lean MEJ. Obesity and weight management in the elderly. Br Med Bull. 2011;97:169–196. doi: 10.1093/bmb/ldr002. [DOI] [PubMed] [Google Scholar]
- 101.Endalifer ML, Diress G, Addisu A, Linger B. The association between combined oral contraceptive use and overweight/obesity: a secondary data analysis of the 2016 Ethiopia Demographic and Health Survey. BMJ Open. 2020;10(12):e039229. doi: 10.1136/bmjopen-2020-039229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hong SA, Peltzer K, Lwin KT, et al. The prevalence of underweight, overweight and obesity and their related socio-demographic and lifestyle factors among adult women in Myanmar, 2015–16. PLoS ONE. 2018;13:e0194454. doi: 10.1371/journal.pone.0194454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mody SK, Han M. Obesity and contraception. Clin Obstet Gynecol. 2014;57:501–507. doi: 10.1097/GRF.0000000000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Krol KM, Grossmann T. Psychological effects of breastfeeding on children and mothers. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2018;61(8):977–985. doi: 10.1007/s00103-018-2769-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Frank NM, Lynch KF, Uusitalo U, Yang J, Lönnrot M, Virtanen SM, et al. The relationship between breastfeeding and reported respiratory and gastrointestinal infection rates in young children. BMC Pediatr. 2019;19(1):339. doi: 10.1186/s12887-019-1693-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chowdhury R, Sinha B, Sankar MJ, Taneja S, Bhandari N, Rollins N, Bahl R, Martines J. Breastfeeding and maternal health outcomes: a systematic review and meta-analysis. Acta Paediatr. 2015;104(467):96–113. doi: 10.1111/apa.13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Figueiredo B, Dias CC, Brandão S, Canário C, Nunes-Costa R. Breastfeeding and postpartum depression: State of the art review. J Pediatr (Rio J) 2013;89(4):332–8. doi: 10.1016/j.jped.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 108.Park S, Choi N-K. Breastfeeding and Maternal Hypertension. Am J Hypertens. 2018;31(5):615–621. doi: 10.1093/ajh/hpx219. [DOI] [PubMed] [Google Scholar]
- 109.Zhang BZ, Zhang HY, Liu HH, Li HJ, Wang JS. Breastfeeding and maternal hypertension and diabetes: a population-based cross-sectional study. Breastfeed Med. 2015;10(3):163–167. doi: 10.1089/bfm.2014.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table 1. Coding strategy of variables used in analysis.
Additional file 2: Supplementary Table 2. Bivariable multi-level multinomial analysis of the factors associated with double burden of malnutrition in low and middle income countries.
Additional file 3: Supplementary Table 3. Fixed effects of multi-level multinomial analysis of individual and community level variables.
Data Availability Statement
The datasets used and/or analysed during the current study is available in a public, open access repository which is accessible online www.measuredhs.com.