Keywords: glomerular disease, glomerulonephritis, immunology, immunosuppression, lymphocytes, nephritis
Abstract
Significance Statement
Treatment of acute, crescentic glomerulonephritis (GN) consists of unspecific and potentially toxic immunosuppression. T cells are central in the pathogenesis of GN, and various checkpoint molecules control their activation. The immune checkpoint molecule B and T-lymphocyte attenuator (BTLA) has shown potential for restraining inflammation in other T-cell–mediated disease models. To investigate its role in GN in a murine model of crescentic nephritis, the authors induced nephrotoxic nephritis in BTLA-deficient mice and wild-type mice. They found that BTLA has a renoprotective role through suppression of local Th1-driven inflammation and expansion of T regulatory cells and that administration of an agonistic anti-BTLA antibody attenuated experimental GN. These findings suggest that antibody-based modulation of BTLA may represent a treatment strategy in human glomerular disease.
Background
Modulating T-lymphocytes represents a promising targeted therapeutic option for glomerulonephritis (GN) because these cells mediate damage in various experimental and human GN types. The immune checkpoint molecule B and T-lymphocyte attenuator (BTLA) has shown its potential to restrain inflammation in other T-cell–mediated disease models. Its role in GN, however, has not been investigated.
Methods
We induced nephrotoxic nephritis (NTN), a mouse model of crescentic GN, in Btla-deficient (BtlaKO) mice and wild-type littermate controls and assessed disease severity using functional and histologic parameters at different time points after disease induction. Immunologic changes were comprehensively evaluated by flow cytometry, RNA sequencing, and in vitro assays for dendritic cell and T-cell function. Transfer experiments into Rag1KO mice confirmed the observed in vitro findings. In addition, we evaluated the potential of an agonistic anti-BTLA antibody to treat NTN in vivo.
Results
The BtlaKO mice developed aggravated NTN, driven by an increase of infiltrating renal Th1 cells. Single-cell RNA sequencing showed increased renal T-cell activation and positive regulation of the immune response. Although BTLA-deficient regulatory T cells (Tregs) exhibited preserved suppressive function in vitro and in vivo, BtlaKO T effector cells evaded Treg suppression. Administration of an agonistic anti-BTLA antibody robustly attenuated NTN by suppressing nephritogenic T effector cells and promoting Treg expansion.
Conclusions
In a model of crescentic GN, BTLA signaling effectively restrained nephritogenic Th1 cells and promoted regulatory T cells. Suppression of T-cell–mediated inflammation by BTLA stimulation may prove relevant for a broad range of conditions involving acute GN.
Introduction
CD4+ T cells play a major role in immune-mediated diseases, including crescentic GN, in humans and rodent models.1,2 In a mouse model of crescentic GN (nephrotoxic nephritis [NTN]), both Tbet+ Th1 and RORyt+ Th17 orchestrate proinflammatory responses by recruiting neutrophils and macrophages and stimulating antibody production through B cells.3,4 On the other hand, Foxp3+ Tregs protect from tissue damage by suppressing T effector cells.5 Various mechanisms of Treg-mediated suppression have been identified, ranging from membrane-bound molecules, such as CD25, CD39, CD73, and programmed cell death ligand 1 (PD-L1), to soluble cytokines, such as IL-10 and TGF-β.6–9 In addition, expression of the chemokine receptors CXCR3 and CCR6 enables trafficking of Tregs into areas of Th1 or Th17 cell-mediated inflammation, respectively, thus directing the anti-inflammatory response.10,11 In the kidney, loss of this finely tuned equilibrium leads to glomerular inflammation and—ultimately—loss of function.
To ensure a specific T-cell activation and proliferation, many signals need to work in concert. First, the T-cell receptor (TCR) binds to its specific antigen, presented by antigen-presenting cells, such as dendritic cells (DC). This engagement alone, however, does not result in T-cell activation because additional stimulating secondary and tertiary signals are needed.12 Counteracting these activating signals are a variety of inhibitory checkpoint molecules, the most prominent ones being the cytotoxic T-lymphocyte associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1). Emerging evidence now suggests a central role of another checkpoint molecule, the B and T-lymphocyte attenuator (BTLA), in regulating the magnitude of T-cell activation.13,14 BTLA is an immunoglobulin domain superfamily protein, expressed predominantly on DCs, B cells, and T cells. It mediates anti-inflammatory signals by recruiting the phosphatases SHP1 and SHP2 to its immunoreceptor tyrosine-based inhibitory motifs on activation.15,16
Signaling through BTLA was initially found to reduce T-cell proliferation and cytokine production in vitro.17 Btla-knockout (BtlaKO) mice develop autoantibodies and autoimmune hepatitis-like disease at 7 months of age and show severely aggravated immune responses in several T-cell–based disease models, including experimental autoimmune encephalomyelitis, cardiac transplant rejection, airway inflammation, and LPS-induced endotoxic shock.18–22 Besides its ability to limit T-cell activation, BTLA also affects innate immunity by regulating γδ-T cells by inhibition of IL-17 and TNFα production.23 In addition, BTLA signaling was shown to influence Treg homeostasis and differentiation. Jones et al.24 demonstrated Treg induction by BTLA-expressing DEC205+CD8a+ DCs.
Furthermore, as a selective deletion of Btla on Foxp3+ Tregs resulted in a decrease in Treg numbers, a cell-intrinsic supportive function of BTLA in Tregs was postulated.25 Overexpression of BTLA was recently described to be beneficial in a murine model of kidney transplant rejection.26,27 The same study showed a reduced BTLA expression in T cells of patients with acute kidney transplant rejection, suggesting a role in human disease. Moreover, elevated BTLA expression on Th1, Th2, and Th17 cells was associated with active disease in patients with systemic lupus erythematosus.28
While these results suggest an important role of BTLA in controlling renal inflammation, its effect on the pathogenesis of GN and the therapeutic potential of enhancing BTLA signaling in GN has not been investigated. Considering that the center pillar of GN treatment still consists of rather unspecific and often toxic immunosuppression, we aimed to clarify the effect of BTLA signaling on GN formation and progression and evaluate BTLA as a targeted approach for future GN treatment.
Methods
Animals
BtlaKO mice were developed previously and are available at Jackson laboratories (Strain number 006355).19 OT-II mice (B6.Cg-Tg(TcraTcrb)425Cbn/J) are commercially available at Jackson laboratories (Strain number 004194). Rag1KO mice (B6.129S7-Rag-1tm1Mom) are commercially available at Jackson laboratories (Strain number 002216). All mice were kept on a C57BL/6 background and housed under specific pathogen-free conditions. Animal experiments were performed according to national and institutional animal care and ethical guidelines, and the LANUV NRW approved them.
Animal Experiments and Functional Studies
Groups of BtlaKO mice and littermate controls (referred to as WT) were analyzed between the ages of 8 weeks and 8 months. NTN was induced in 8- to 16-week-old male BtlaKO and WT controls by tail vein injection of 9 µL per gram body weight of nephrotoxic sheep serum (NTS, Probetex) on two consecutive days (total dosage 18 µL per gram body weight). Organs were harvested at indicated time points after cardiac perfusion with PBS. For in vivo treatment experiments, three administrations of 100 µg of anti-BTLA antibody (Clone 6A6, BioXcell) or isotype control (polyclonal Armenian hamster IgG, BioXcell) were administered by tail vain injections on days −1, 3, 7 (prophylactic study) or 3, 7, and 10 after NTN induction (total dosage 300 µg). For in vitro studies, splenocytes from male, 8–16-week-old BtlaKO, OT-II mice or WT mice were harvested. Urine samples were collected at indicated time points throughout NTN. Albuminuria was determined by standard ELISA (Bethyl Laboratories). Urinary creatinine was measured using a standard assay (Cayman Chemicals). BUN was measured using standard laboratory methods or colorimetric assay (Cayman Chemicals). For quantification of NTS-specific mouse IgG, high-binding microplates (Greiner Bio-One) were coated with NTS, diluted 1:10 in ELISA coating buffer (50 mM NaHCO3 in PBS, Carl Roth) at 4°C overnight. After washing with PBS +0.1% Tween20, nonspecific binding sites were blocked for 1 hour at room temperature using 1% bovine serum albumin (BSA, Merck) in PBS. Mouse serum samples were diluted 1:2000 (for IgG) or 1:500 (for IgG subtypes) in blocking buffer, and a serial dilution (1:2) was performed. After incubation at 4°C overnight, the wells were washed twice and subsequently incubated with biotinylated IgG (1:10.000, Jackson Immuno Research) or its subtype (1:5000) detection antibodies in the ELISA blocking buffer for 2 hours at RT. Afterward, the wells were washed twice and incubated with streptavidin-peroxidase in the ELISA blocking buffer (1:5000, Natutec) for 1 hour at RT. After washing the wells three times, anti-NTS antibodies were developed with σ-phenylene diamine dihydrochloride (Thermo Fisher Scientific) and H2O2 (Carl Roth) as substrates to detect immobilized anti-NTS antibodies. The reaction was stopped using 1 M H2SO4 (Carl Roth), and the absorbance was measured at 492 nm using a Tecan reader. Antibody titers were expressed as the dilution of plasma samples showing a positive result (optical density=0.2) in the ELISA assay.
Morphometric Studies
For standard histological stainings and IHC, kidneys were perfused with PBS and fixed with 4% paraformaldehyde (PFA). Glomerular scarring and crescent formation were determined in a minimum of 25 glomeruli per mouse in 2-μm thick periodic acid–Schiff–stained kidney sections. The number of glomerular crescents was counted in a blinded manner. To analyze glomerulosclerosis, glomeruli were assigned a score from 0 to 4 depending on the extent of sclerosis in a blinded manner. In addition, the percentage of crescentic glomeruli was counted. For IHC, paraffin-embedded sections were rehydrated using a descending series of ethanol concentrations. Antigen retrieval was performed for 20 minutes in citrate buffer (pH 6) using a pressure cooker. Subsequently, tissues were blocked with peroxidase blocking buffer and an Avidin/Biotin Blocking Kit (SP-2001, Vector Laboratories), stained with antibodies directed against CD3 (dilution 1:100, Clone CD3-12, Bio-Rad) and MAC-2 (dilution 1:3000, Clone M3/38, Cedarlane), and developed using an HRP immunodetection Kit (Vectastain Elite ABC anti-mouse Kit) and DAB staining solution (DAB Substrate Kit, Vector Laboratories). At least 25 glomerular cross-sections and 20 tubulointerstitial high-power fields (magnification, ×400) per kidney section were analyzed in a blinded fashion and positive cells counted manually. For immunofluorescence staining, mouse kidneys were perfused with PBS, snap-frozen, and embedded in 22-oxacalcitriol compound (Tissue-Tek OCT compound, Sakura). Four-micrometer thick slides were fixed with 4% PFA and blocked using 5% normal donkey serum with 0.1% Triton-sodium azide. Subsequently, slides were incubated with primary antibodies against Podocin (rabbit anti-podocin antibody, P0372, Sigma-Aldrich) and Cy3-coupled donkey anti-sheep IgG (Jackson Immuno Research) over night at 4°C. To visualize anti-podocin antibody binding, slides were incubated with AF488-coupled donkey anti-rabbit antibody for 1 hour at room temperature (Jackson Immuno Research). Subsequently, slides were mounted with Prolong Gold and DAPI (ProLong Gold Antifade Reagent with DAPI, Invitrogen). IF was analyzed on a Zeiss Apotome 2 microscope.
Leukocyte Isolation
Spleens were harvested in PBS and passed through 70-μm nylon meshes. After lysis of erythrocytes with ammonium chloride, cells were washed and passed through 40-μm meshes. Cells were then washed again and transferred into a 96-well plate for antibody staining. Kidneys were minced and incubated in a digestion medium (RPMI 1640 medium containing 10% FCS, 1% penicillin/streptomycin, 250 µg/ml collagenase B, and 30 U/ml DNase) at 37°C for 45 minutes. Tissues were then dissociated using the gentleMACS dissociator (Miltenyi Biotec) to obtain a single-cell suspension. To further purify the immune cell fraction, Percoll gradient (37% Percoll; GE Healthcare) centrifugation was performed at 500g at room temperature for 20 minutes. The leukocyte layer was carefully aspirated and transferred to a 96-well plate for antibody staining.
Staining for Flow Cytometry
For blocking of unspecific antibody binding, cells were preincubated with anti-CD16/32 antibody for 5 minutes at 4°C. Subsequently, fluorochrome-labeled antibodies were added and incubated for 30 minutes at 4°C. The following antibodies were used for surface stainings: CD45 (30-F11), CD19 (1D3), and CD25 (PC61) purchased from BD Bioscience and CD3 (17A2), CD4 (GK1.5), ydTCR (GL3), CD8a (53–6.7), CD11c (N418), CD11b (M1/70), F4/80 (BM8), I-A/I-E (M5/114.15.2), XCR1 (ZET), BTLA (6A6), CXCR3 (CXCR3-173), CD62L (MEL-14), CD44 (IM7), Ly6C (HK1.4), and Ly6G (1A8) all purchased from Biolegend. For intracellular and intranuclear staining, samples were processed using a commercial intranuclear staining kit (Foxp3 Staining Kit; eBiosciences). Fluorochrome-labeled antibodies against IL-17 (TC11-18H10.1), IFNγ (XMG1.2), Tbet (4B10) (all Biolegend), Foxp3 (FJK-16s, eBiosciences), and RORγt (Q31-378, BD Biosciences) were used. For intracellular cytokine staining, cells were treated with PMA (50 ng/ml; Sigma-Aldrich) and ionomycin (1 μg/ml; Calbiochem-Merck) in the presence of Brefeldin A (10 μg/ml; Sigma-Aldrich) for 3 hours. Zombie Aqua staining (Biolegend) was used to exclude dead cells during flow cytometry and ensure viability of the cells after the stimulation procedure. Experiments were analyzed on a BD LSR Fortessa Cytometer (BD Bioscience).
DC:OT-2 Assay
DCs were isolated from spleens of BtlaKO and WT mice using magnetic-activated cell sorting according to the manufacturer’s protocol (CD11c MicroBeads UltraPure, Miltenyi Biotec). Subsequently, DCs were incubated with 10 µg/ml OVA 323-339 for 2 hours at 37°C in a 96-well plate to allow for specific antigen presentation. T helper cells were isolated from spleens of OT-2 mice using a CD4 T-cell isolation kit (CD 4 T-cell isolation kit, mouse, Miltenyi Biotec) and a magnetic separator and incubated with 0.2 µL/ml CFSE (CellTrace CFSE, Invitriogen) for 20 minutes at 37°C. Finally, a total of 100,000 OT-2 T helper cells were cocultured with 100.000 OVA-loaded BtlaKO DCs or WT DCs for 3 days. At day 3, cells were washed and stained for CD45 and CD4 and proliferation was measured using a BD LSR Fortessa Cytometer (BD Bioscience). In addition, cytokine ELISAs were performed from the supernatant using commercially available kits (Biolegend).
Treg Suppression Assay
Splenocytes from BtlaKO and WT mice were isolated and incubated with CD19 MicroBeads (Miltenyi Biotec) and sorted using a magnetic cell sorter (Miltenyi Biotec). CD19neg cells were then stained with a mix of fluorescently labeled antibodies and DAPI. Subsequently, cell sorting was performed on a FACS Aria III (BD Bioscience). T effector cells were gated as CD4+CD25−CD44−DAPI− and T regulatory cells as CD4+CD25+CD44−CTLA-4+ DAPI−. Next, purified T effector cells were incubated with 5-µM CellTrace Violet (Invitrogen) for 20 minutes at 37°C. After counting, 100,000 T effector cells were cocultured with Tregs at a 1:1 ratio and stimulated with anti-CD3/CD28 beads (Dynabeads, Gibco) following manufacturer’s protocol at 37°C in an RPMI 1640 medium containing 10% FCS and 1% penicillin/streptomycin. After 3 days, proliferation was measured on a LSR Fortessa Cytometer (BD Bioscience), and cytokine ELISAs were performed from the supernatant.
T-Cell Apoptosis Assay
One day before organ harvest, 96-well plates were coated with sheep IgG (Sigma-Aldrich) at 4°C and kept overnight. Splenocytes were isolated from BtlaKO mice and littermate controls 10 days after induction of NTN as described above. After lysis of erythrocytes, 5 × 105 splenocytes were resuspended in a T-cell medium (RPMI 1640 medium containing 10% FCS, 1% penicillin/streptomycin, 50 µM β-mercaptoethanol), transferred into the 96-well plate, and incubated at 37°C. After 24 hours, cells were washed and stained with fluorochrome-labeled antibodies against CD4 (GK1.5), CD25 (PC61), and CD127 (A7R34). Next, cells were stained for Annexin V and 7-AAD using the FITC Annexin V Apoptosis Detection Kit (Biolegend) following manufacturer’s protocol and analyzed on a LSR Fortessa Cytometer (BD Bioscience).
T-Cell Transfer Experiments
Splenic T cells from BtlaKO and WT mice were isolated and enriched for CD4+ T helper cells using a magnetic cell sorter and commercially available T helper cell isolation kits (Miltenyi Biotec). Subsequently, the cells were stained with fluorescently labeled antibodies sorted on a FACS Aria III (BD Bioscience). DAPI was used to control for viability. T effector cells were gated as CD4+CD25−CD44−DAPI− and T regulatory cells as CD4+CD25+CD44−CTLA-4+ DAPI−. Purified T effector cells were then labeled with CFSE (Invitrogen) following manufacturer’s protocol. 7.5 × 105 T effector cells and Tregs were then injected i.v. into Rag1KO mice. Three days after transfer, mice were killed and splenic T effector cell proliferation was measured on a Canto Cytometer.
Library Preparation and Sequencing of Renal Immune
Kidneys’ single-cell suspension was enriched for CD45+ cells (Miltenyi Biotec) using magnetic cell separation following manufacturer’s protocol (MACS Separator, Miltenyi Biotec). Subsequently, cells were stained with antibodies against CD45 and sorted on an FACS Aria III (BD Bioscience). DAPI was used to exclude nonviable cells. DAPI− CD45+ cells in ×1 PBS containing 0.04% BSA (700–1200 nuclei/µl concentration) were checked for viability and processed with the Chromium Next GEM Single Cell 3ʹ Kit v3.1 with dual indices, aiming for a target of 10,000 cells/sample. Cells and the appropriate mastermix were loaded on a Chromium Next GEM Chip G and run on the Chromium Controller to generate Gel Bead-In-Emulsions (GEMs) according to manufacturer’s protocol. After incubation, the GEMs were broken and the pooled fractions were recovered. Silane magnetic beads were used to remove leftover biochemical reagents and primers from the post-GEM reaction mixture. Full-length, barcoded cDNA was then amplified by PCR to generate sufficient amounts for library construction. Library preparation was performed, including end repair, A-tailing, adaptor ligation, and PCR. The final libraries were quantified (Qubit) and validated (Tape Station) and pooled, and the pool was then quantified using the Peqlab KAPA Library Quantification Kit and the Applied Biosystems 7900HT Sequence Detection System. Libraries were sequenced on an Illumina NovaSeq 6000 sequencing instrument with 29+89 bp read length.
Bioinformatical Analysis of scRNAseq Data
Basic Analysis
Fastqs were generated using the command mkfastq of the 10× Genomics software cellranger (v6.1.2). The filtered gene count matrices of the four samples were generated using cellranger's count command and the mm10 reference genome (version preprocessed by cellranger). For the two WT and two BtlaKO samples, 3.5K, 5.4K, 6.4K, and 8.4K cells were recovered, respectively. Sequencing depths of the samples were approximately 64.9K, 60.3K, 52.2K, and 37.2K reads per cell in the same order. That resulted in a median number of genes per cell between 2K and 2.3K for each sample. All given numbers were rounded to the first decimal place. Raw data is made available on gene expression omnibus (GEO accession number: GSE223534).
Preprocessing and Integration
Downstream analysis was performed using the Seurat R package (version 4.1.1).29 Cells with a very small (<500 genes) or large (>5000 genes) transcriptome recovery were discarded to reduce noise and the number of possible doublets, respectively. Only cells with mitochondrial expression of <5% and ribosomal expression of <50% were kept to avoid working with low-quality cells in downstream steps. For normalization, we have used the standard log-normalization approach with the standard scaling factor (10e4). Samples were then integrated using the standard Seurat integration procedure.
Dimensionality Reduction and Clustering
We followed the standard Seurat workflow to perform principal component analysis, extracting the first 100 principal components. On the basis of the data generated by Seurat's Elbow plot function, we automatically determined the number of dimensions used for dimensionality reduction and clustering of all immune cells. In particular, we selected the dimension where the standard deviation starts to drop below 2; this automatic selection procedure was confirmed by visual inspection of the elbow plot. Subsequently, we have executed the UMAP algorithm and the FindNeighbors function with the aforementioned dimensions. Clustering was performed using the FindClusters function with a resolution of 0.1. Otherwise, we used standard parameters. The dimensionality reduction and clustering procedure for the T cells was performed similarly, but we used only the first 12 dimensions by visual inspection of the elbow plot because the complexity and variability within T-cell population drops significantly as expected compared with the first level of resolution (all immune cells). In this step, we used a clustering resolution of 0.6. Cluster 6 was identified as contamination with macrophages, probably because of the formation of doublets, and was discarded. The other cells were reclustered using the same workflow, this time with a clustering resolution of 0.5. For all downstream steps, we worked with the remaining 5412, 7361, 2939, and 4521 cells for the knockout and wild-type samples, respectively. At the T-cell level, we worked accordingly with 826, 1433, 366, and 609 cells in the same mentioned order.
Marker Identification and Cell-Type Annotation
Markers of the clusters at both clustering levels were computed with the Wilcoxon rank sum test implemented in Seurat using the FindAllMarkers function. The top 20 positive markers of each cluster were used to manually curate the cell types of the immune cells (the initial clustering level of all cells). The reference database used to determine the cell types is the single-cell RNASeq database PanglaoDB.30 We determined the identity of the resulting cluster subpopulations by visualizing the expression patterns of most basic markers (e.g., Cd4+, Cd8+, and some alpha, beta, gamma, and delta chain genes) across clusters to understand the approximate structure of T-cell subpopulations in our data set. Subsequently, we have computed the module scores of many known standard subpopulations of T cells for each cell in each cluster using the AddModuleScore function. By box plotting the distribution of the scores of each module for each cluster, we identified the subpopulations of the T cells by visually inspecting and comparing the module score distributions. To assign a cell type to a subpopulation, we require that the median score is at least higher than zero or that no other type is fitting better while the median being close to zero. In the case that two cell-type modules are fitting equally well into the same subcluster, we annotate the cluster with both unless we could biologically exclude one of them. For instance, in the case of clusters where almost all cells are Cd4+, we exclude the possibility of the cluster being a subpopulation of cytotoxic T cells. Cycling T cells were recognized using cell cycle genes integrated within Seurat and corresponding G2M and S scores. The markers of the various T-cell subpopulations were obtained from the Biocompare website (www.biocompare.com, date of access December 8, 2022).
Pseudobulk Differential Expression Analysis
For differential expression analysis between BtlaKO and wild-type samples, we used a pseudobulk analysis approach in each cluster separately at both clustering levels (at the immune cell level and the level of T-cell subpopulations); in short, we used the summarized expression of each gene across all cells for each cluster and sample. In this way, we got for each cluster a count matrix, with columns corresponding to the KO and WT samples. Subsequently, we used R/Bioconductor package DESeq2 (v1.36.0) to perform standard bulk RNA-Seq differential expression analysis. Furthermore, we used the R package fdrtool (v1.2.17) to compute readjusted P-values on the basis of the vector of test statistics generated by DESeq2.31,32
GO Enrichment Analysis
For GO enrichment analysis, we used gprofiler2 (v0.2.1) for up- and downregulated genes separately.33 All genes with adjusted P-value ≤0.05 and absolute log2FoldChange >0.25 were included in the GO enrichment analysis. Otherwise, we used standard parameters. To reduce GO terms and visualize them hierarchically with treemaps, we used the package rrvgo (v1.8.0), running the standard procedure on each cluster.34
Statistical Analysis
Results are expressed as mean ± SEM. Groups were compared by the Student t test or multicomparison one-way ANOVA; a P value of <0.05 was considered statistically significant. Bioinformatical analysis of scRNAseq data is detailed above.
Results
BTLA is Differentially Expressed in Renal T Cells during Homeostasis and NTN
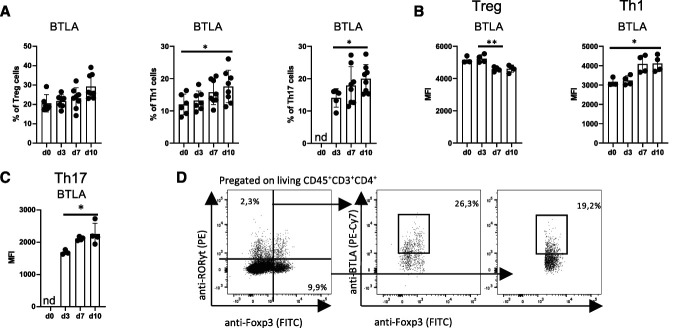
Previous work has confirmed expression of BTLA on all major lymphocyte populations and DCs.14 Its expression and regulation in renal leukocytes under homeostatic conditions and during glomerular inflammation, however, have not been studied. Our flow cytometry analysis revealed that under healthy conditions, around 20% of renal Foxp3+ Tregs and 10% of renal Tbet+ Th1 cells expressed BTLA, whereas no BTLA+ RORyt+ Th17 cells were detected (Figure 1A). In line, BTLA expression on the single-cell level was nearly two-fold higher in Tregs compared with Th1 cells (Figure 1B). Interestingly, NTN led to an increase in BTLA expression in both renal Th1 and Th17 cells but to a decrease in renal Treg cells (Figure 1, B–D). Systemically, this phenomenon was not observed because all three T helper subtypes in the spleen maintained or increased BTLA expression levels at later stages of NTN (Supplemental Figure 1, A and B). Of note, both Xcr1+ dendritic cells (cDC1) and B cells expressed the highest levels of BTLA both in the kidney and spleen, but did not show significant changes in expression levels over time (Supplemental Figure 1, A–D).
Figure 1.
BTLA is differentially expressed in renal T cells during homeostasis and NTN. Flow cytometry analysis of renal BTLA expression on key T-cell subsets before (d0) and at three different time points after NTN induction (days 3, 7, and 10). Mean fluorescent intensity (MFI) is used as a parameter for BTLA expression on each individual cell. (A) Percentages of BTLA expression on indicated T-cell subsets. Around 20% of Foxp3+ Tregs express BTLA during homeostasis and throughout NTN. On the other hand, roughly 10% of Tbet+ Th1 cells express BTLA during homeostasis. After NTN induction, frequencies of BTLA+ Th1 cells increase. Similar upregulation is observed in RORyt+ Th17 cells. (B) While being high during homeostasis, BTLA expression decreases on a single-cell level in Tregs with disease progression. By contrast, BTLA expression increases in Tbet+ Th1 cells. (C) MFI of BTLA on RORyt+ Th17 cells increases throughout NTN. (D) Representative gating strategy of BTLA expression on Foxp3+ Tregs and RORyt+ Th17 at day 7 after NTN induction. Depicted is a representative experiment from a total of three experiments. *P < 0.05, **P < 0.01 (determined by one-way ANOVA).
BtlaKO Mice Show an Aggravated Course of NTN
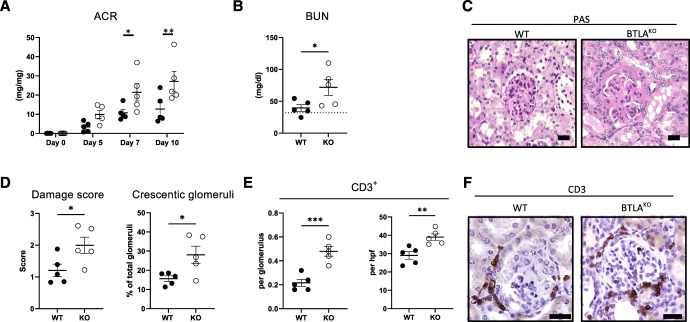
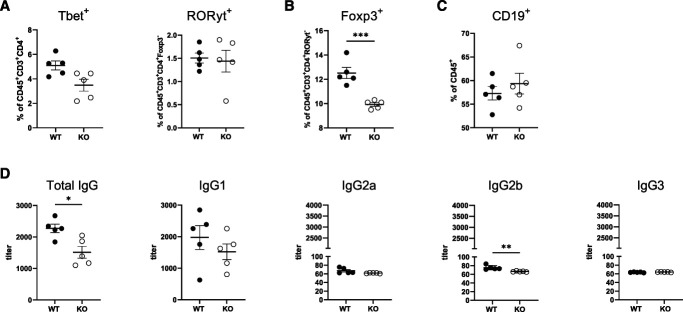
To investigate the role of BTLA signaling in kidney disease, BtlaKO mice were analyzed during homeostasis and glomerular inflammation using the NTN model. BtlaKO mice stayed fertile and healthy up to 8 months of age and did not show any spontaneous renal phenotype (Supplemental Figure 2, A–D). Nonetheless, we detected an increase in total splenocyte numbers and a significant increase in splenic CD4+ T helper cells (Supplemental Figure 2, E and F). Of note, none of the investigated T helper cell subpopulations differed between the groups (Supplemental Figure 2F). In line with these findings, cytokine production of isolated splenocytes was unchanged in BtlaKO mice, suggesting an expansion of naïve T helper subgroups and/or T follicular helper cells (Supplemental Figure 2G). Composition of renal immune cells was unaffected by the knockout under homeostatic conditions (data not shown). Next, BtlaKO mice and littermate controls were challenged with nephrotoxic sheep serum (NTS). Similar glomerular binding of sheep IgG was confirmed by IF (Supplemental Figure 3). Interestingly, BtlaKO mice showed increased proteinuria during NTN with the most significant differences in albumin-to-creatinine ratio (ACR) at days 7 and 10 after NTS injection (Figure 2A). Accordingly, blood urea nitrogen (BUN), histological damage, and number of interstitial and glomerular CD3+ T cells and glomerular macrophages were all significantly increased in BtlaKO mice 10 days after NTN induction (Figure 2, B–F, Supplemental Figures 4A and 5A). Of note, proteinuria peaked around day 14 of NTN and subsequently decreased, showing similar levels at day 21 between the groups (Supplemental Figure 6A). Histological damage 21 days after NTN did not show significant differences (Supplemental Figures 5B and 6B).
Figure 2.
Nephritic BtlaKO mice show an aggravated course of NTN. (A) Urine albumin-to-creatinine ratios (ACR) at indicated time points after NTN induction and (B) blood urea nitrogen (BUN) levels after 10 days of NTN are increased in BtlaKO mice compared with littermate controls. Dotted line shows mean BUN levels of healthy mice. (C) Representative kidney PAS stainings of wild-type and BtlaKO mice 10 days after NTN. (D) Nephritic BtlaKO mice show increased glomerular damage score and crescent formation as quantified in a blinded manner 10 days after NTS injection. (E) Quantification of glomerular and interstitial CD3+ cells in the kidneys of nephritic mice of indicated genotype using IHC. (F) Representative IHC staining of renal CD3+ cells. Depicted is one representative experiment (n=5 versus 5) from a total of three experiments. Scale bar 20 µm; *P < 0.05, **P < 0.01, ***P < 0.001 (determined by the Student t test).
BTLA Deficiency Results in Local Th1 Dysregulation
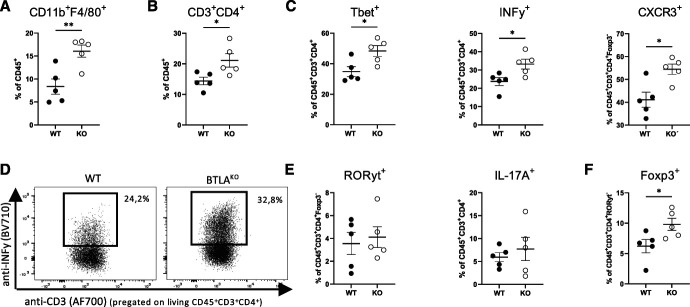
To characterize the renal immune response, we performed flow cytometry 10 days after NTN induction. Consistent with aggravated tissue damage, increased frequencies of renal F4/80+ macrophages were found in BtlaKO mice (Figure 3A). Furthermore, total T helper cell frequencies were significantly increased in kidneys of BtlaKO mice (Figure 3B). Analysis of the inflammatory T helper cell subsets revealed a specific increase in Tbet+, INFγ+, and CXCR3+ Th1 cells (Figure 3, C and D). RORyt+ Th17 cell frequencies and IL-17 production were unchanged between the groups, as were frequencies of NKT cells, CD8a+ cytotoxic T cells, and γδ-T cells (Figure 4E and Supplemental Figure 4, B and C). Similarly, Xcr1+ cDC1, CD11b+ cDC2, and monocytes did not differ between the groups (Supplement Figure 4D). Because the observed increase in Th1 immunity could be due to the reduced number of renal Treg, we next analyzed this T-cell subset in nephritic kidneys. Surprisingly, instead of a decrease, we found renal Treg frequencies to be significantly increased in BtlaKO mice (Figure 3F). Furthermore, especially CXCR3+ Tregs, designated for infiltration into areas of Th1 inflammation, were significantly increased, arguing against a defect in Treg migration (Supplemental Figure 4E).
Figure 3.
BTLA deficiency results in local Th1 dysregulation during NTN. Flow cytometry analysis shows a significant increase in (A) renal F4/80+ macrophages and (B) total T helper cells in BtlaKO mice. (C) Increased frequencies of Tbet+ Th1, INFy+ T helper cells, and CXCR3+ T helper cells in BtlaKO mice reveal a selective dysregulation of Th1 immunity. (D) Representative dot plots of renal INFy+ T helper cells. (E) Frequencies of renal RORyt+ Th17 cells and IL-17a+ T helper cells are unchanged. Data shown from one representative experiment (n=5 versus 5) from a total of four independent experiments conducted. *P < 0.05, **P < 0.01, ***P < 0.001 (determined by the Student t -test).
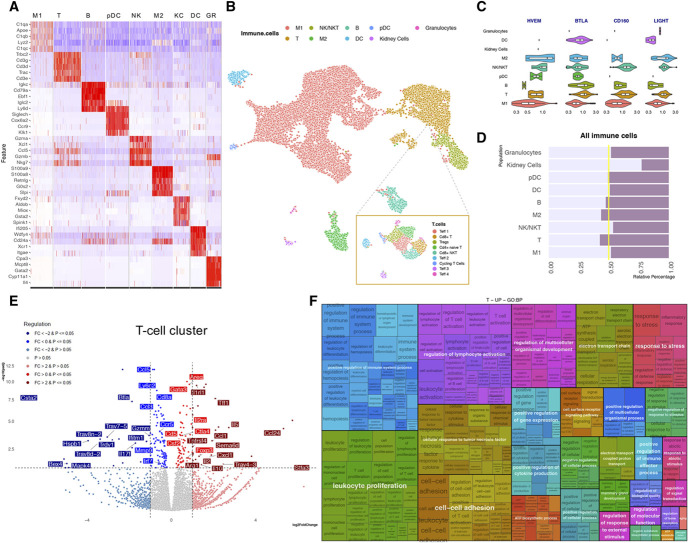
Figure 4.
scRNAseq reveals enhanced T-cell activation in nephritic BtlaKO mice. (A) Heatmap of sequenced cells showing the top 5 markers of each cell cluster. Markers were sorted by adjusted P value. Eight immune cell clusters (M1—macrophage cluster 1; T—Tcells; B—B cells; pDC—plasmacytoid dendritic cells; NKT—NK T cells; M2—macrophage cluster 2; GR—granulocytes) and one nonimmune cell cluster (kidney cells; KC) were identified. (B) UMAP plot of total cells sequenced and T-cell subclustering. (C) Relative abundance of the indicated transcripts (Hvem [Tnfrsf14], Btla, Cd160, Light [Tnfsf14]) in the identified renal cell clusters. BTLA is predominantly expressed in T cells, B cells, and DCs; its ligand HVEM in macrophages cluster M2 and NK/NKT cell cluster. (D) Relative percentage of the indicated clusters showing a relative increase in M2 and T-cell clusters in kidneys of BtlaKO mice. (E) Pseudobulk analysis showing significantly upregulated (red) and downregulated (blue) genes in the T-cell cluster. (F) Treemap displaying the positively enriched gene ontology (GO) terms in T cells between BtlaKO and WT mice samples. GO terms were identified using gprofiler2 with a significance cutoff of 0.05 (see Method section for more details). The size of rectangles in the treemap are proportional to the significance of the enrichment of the GO term (−log10 of adjusted P value).
Single-Cell RNA Sequencing Reveals Activation of T Cells in Nephritic BtlaKO Mice
To understand the transcriptional changes driven by the absence of BTLA, we performed single-cell RNA sequencing of renal immune cells 10 days after NTS injection (Supplemental Figure 7A). Immune cells were clustered according to their expression of known markers (Figure 4, A and B). In nephritic wild-type mice, Btla was predominantly expressed in T cells, B cells, and DCs, confirming our flow cytometry data (Figure 4C). In addition, Hvem (Tnfsfr14), Light (Tnfsf14), and Cd160, the other ligands of the BTLA/HVEM pathway, were expressed to a varying degree also on macrophages (Figure 4C).
Overall immune cell composition showed a relative expansion in the macrophage M2 and T-cell clusters in BtlaKO mice, corroborating our flow cytometry data (Figure 4D). Analysis of the T-cell cluster transcriptome revealed an upregulation of proinflammatory signals, such as Il1r, Ccl1, Csf2, and Il2, in BtlaKO mice as well as anti-inflammatory transcripts associated with Treg function, such as Foxp3, Ctla4, Il10, Il2ra, and Areg (Figure 4E). The overall increased inflammatory signal was reflected in the GO term over-representation analysis, showing enrichment of terms associated with positive regulation of lymphocyte activation and leukocyte proliferation (Figure 4F and Supplemental Figure 8).
Next, T-cell subclustering identified 9 clusters, four of which were proportionally expanded and subsequently identified as cycling T cells, T regulatory cells, and T effector cell clusters 1 and 3 (Supplemental Figure 7, B and C). On the basis of their transcriptional profile, cells in the T effector cluster 1 (Teff 1) were identified as Th1-like cells, whereas T effector cluster 3 (Teff 3) represented Th2-like cells. Whereas the Teff 1 cluster showed significantly increased transcription of proinflammatory markers, such as Il1rl1, Ccr7, Ccr10, and Cd93, the Teff 3 cluster showed decreased transcription of inflammatory and activation molecules, such as Cd74, Il13, and Mdk (Supplemental Figure 7, D and E). Of note, the Treg cluster exhibited increased transcription of markers associated with their suppressive function, namely Il10, Myb, Gzmc, Tgfbr, and Il2ra, a finding also reflected in the GO enrichment analysis (Supplemental Figure 7, F and G and Supplemental Figure 8).
Splenic Treg Frequencies Are Reduced in Nephritic BtlaKO Mice
To investigate whether the renal phenotype was caused by a local dysregulation or rather a systemic effect of BTLA deficiency, we analyzed splenocytes and the antigen-specific humoral response of BtlaKO mice and littermates at different time points throughout NTN. After 10 days of NTN, no significant changes were seen in splenic B cells, DCs (cDC1 and cDC2), macrophages, and monocytes (data not shown). Notably, frequencies of splenic proinflammatory T-cell subsets Tbet+ Th1 and RORyt+ Th17 did not differ between the groups either (Figure 5A). However, a significant reduction in Foxp3+ Treg frequencies in BtlaKO mice was observed (Figure 5B). To analyze the effect of BTLA deficiency on the autologous phase, serum antibody titers were measured on day 10 and day 21 of NTN. Although no significant differences were detected in anti-NTS-IgM and total anti-NTS-IgG after 10 days of NTN, we observed a significant reduction of total mouse anti-NTS-IgG at day 21 (Supplemental Figure 4F and Figure 5D). Especially IgG2b, the subclass predominantly associated with the autologous stage of NTN, was reduced (Figure 5D). The overall renal and systemic immunophenotype prevailed also at this later time point, showing significantly higher frequencies of renal macrophages and an increase—albeit not significant—of Th1 cells while systemic Treg frequencies were significantly reduced in BtlaKO mice (Supplemental Figure 6, C and D).
Figure 5.
Splenic Treg frequencies are reduced in nephritic BtlaKO mice. (A) Flow cytometry analysis of splenic T cells shows no differences in Tbet+ Th1 and RORyt+ Th17 cell frequencies after 10 days of NTN. (B) Splenic Treg frequencies are significantly decreased in BtlaKO mice compared with WT controls at day 10 of NTN. (C) Splenic B-cell frequencies 21 days after NTN induction do not differ between the groups. (D) Quantification of antigen-specific total serum IgG and indicated IgG subgroups after 21 days of NTN as determined by standard ELISA. * P < 0.05, **P < 0.01, ***P < 0.001 (determined by the Student t test).
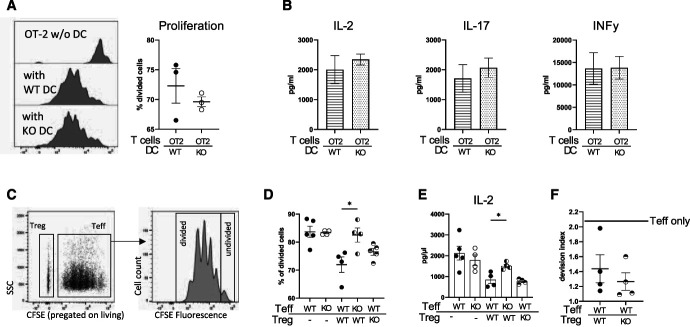
BTLA-Deficient T Effector Cells Evade Treg Suppression
To mechanistically clarify the observed dysregulation of T cells, we next analyzed DCs, T effector cells, and Tregs in more depth. In line with unchanged DC numbers in BtlaKO mice, antigen presentation and T-cell activation and skewing through BTLA-deficient DCs were comparable with wild-type DCs in vitro, demonstrating preserved antigen presentation properties (Figure 6, A and B). Next, Treg function was assessed using Treg suppression assays (Figure 6C). Proliferation of T effector cells was effectively suppressed by BtlaKO Tregs, comparable in magnitude with wild-type Tregs (Figure 6, C and D). In agreement with these findings, no significant differences in IL-2 concentrations in the supernatant of WT T effector:WT Treg and WT T effector:KO Treg cocultures were observed, arguing against a functional defect of BtlaKO Tregs (Figure 6E). Furthermore, survival of nephritogenic Tregs was not affected by BTLA depletion (Supplemental Figure 9). Confirming the intact suppressive capacity in vivo, cotransfer experiments in Rag1KO mice revealed similar suppression of T effector cell proliferation by BtlaKO Tregs and WT Tregs (Figure 6F). Finally, to test whether BTLA-deficient T effector cells evade Treg-mediated suppression, proliferation of BtlaKO T effector cells was assessed in coculture with WT Tregs. Surprisingly, BtlaKO T effector cells indeed exhibited increased proliferation and were more activated in vitro as evidenced by increased IL-2 concentrations in the supernatant, demonstrating that, while BtlaKO Tregs are functional and viable, BTLA-deficient T effector cells evade Treg-mediated suppression (Figure 6, D and E).
Figure 6.
BTLA-deficient T effector cells evade Treg suppression. (A) Proliferation profile of OT-2 T cells after 3 days of stimulation by OVA pulsed WT or BtlaKO DCs and quantification of proliferation as measured by the percentage of divided cells. No differences in T-cell proliferation were detected between the groups. (B) Concentration of indicated cytokines in the supernatant of DC:OT-2 cocultures measured by ELISA reveals unchanged antigen presentation properties. (C) Representative proliferation profile of BTLA-deficient T effector cells after 3 days of stimulation with CD3/CD28 beads in the presence of Tregs in a 1:1 ratio. (D) Quantification of proliferation measured by the percentage of divided cells shows a robust suppression of BTLA-deficient Tregs and an increased proliferation of BTLA-deficient T effector cells. (E) IL-2 concentrations in the supernatant of the indicated cocultures after 3 days of stimulation, measured using standard ELISA techniques. (F) Analysis of T effector cell proliferation 3 days after cotransfer of Tregs and T effector cells into Rag1KO mice. Horizontal line indicates T effector cell proliferation in the absence of Tregs. *P < 0.05, **P < 0.01 (determined by one-way ANOVA).
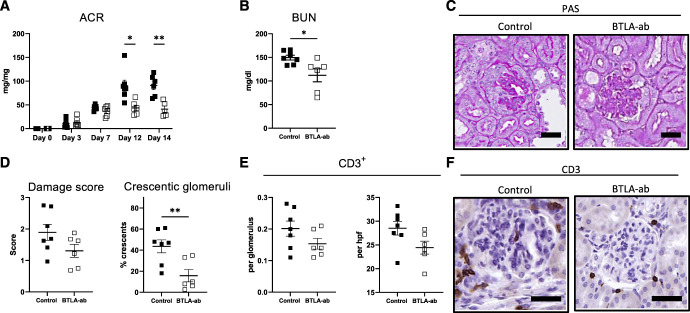
Administration of an Agonistic anti-BTLA Antibody Attenuates NTN
To test, whether BTLA activation might be used therapeutically, wild-type mice were challenged with NTS and treated with either a nondepleting agonistic anti-BTLA antibody (clone 6A6) or an isotype-control antibody, starting 3 days after NTN induction. At this time point, disease was already established as significant proteinuria and first signs of glomerular damage were observed (Supplemental Figure 5C and Figure 7A). Administration of the anti-BTLA antibody effectively attenuated kidney disease as shown by a significant decrease in ACR and BUN as well as reduction in histological damage (Figure 7, A–D and Supplemental Figure 5D). In addition, IHC stainings showed a nonstatistically significant reduction of glomerular and interstitial CD3+ T cells and intraglomerular macrophages (Figure 7, E and F and Supplemental Figure 10A). Glomerular C3 deposition was unaffected by the treatment (Supplemental Figure 10B). Interestingly, flow cytometry revealed reduced frequencies of renal F4/80+ macrophages in the treatment group while no difference was observed in DCs or B cells (Figure 8, A–C). Treatment resulted in significant reduction of proinflammatory Tbet+ Th1 cell frequencies while RORyt+ Th17 cell frequencies were not affected (Figure 8D). This effect was not observed in the spleen (Figure 8F). Surprisingly, both renal and systemic Foxp3+ Treg frequencies were increased in the treatment group, underscoring the subset-dependent effect of BTLA signaling (Figure 8, D–F). Percentages of living Tregs were similar between the groups both in the kidney and in the spleen arguing against an effect of BTLA stimulation on survival (Supplemental Figure 11, A and B). Furthermore, Neuropilin-1 and Helios expression on Tregs was similar between the groups, suggesting an expansion of thymus-derived Tregs (tTregs) rather than induction of peripheral Tregs (pTregs) (Figure 8G). Finally, no effect on splenic B-cell frequencies and antigen-specific antibody titers was observed in the treatment group, again suggesting T-cell modulation as the main effect of anti-BTLA antibody administration (Supplemental Figure 11, C–E). In an additional study, prophylactic administration of the antibody starting two days before NTN induction showed similar results, underscoring the anti-inflammatory effect of BTLA activation (Supplemental Figures 12 and 13).
Figure 7.
Administration of agonistic anti-BTLA antibody attenuates NTN. Administration of an agonistic anti-BTLA antibody attenuates NTN as shown by significantly reduced (A) albumin-to-creatinine ratios and (B) BUN after 14 days of NTN. (C) Representative renal PAS stainings of indicated groups after 14 days of NTN. (D) Mice treated with the agonistic antibody show reduced glomerular damage and crescent formation. (E) Reduced numbers of glomerular and interstitial CD3+ cells are observed in the treatment group compared with controls, detected with IHC. (F) Representative IHC stainings for CD3 of indicated groups (n=7 versus 6); scale bars represent 20 µm. *P < 0.05, **P < 0.01 (determined by the Student t test).
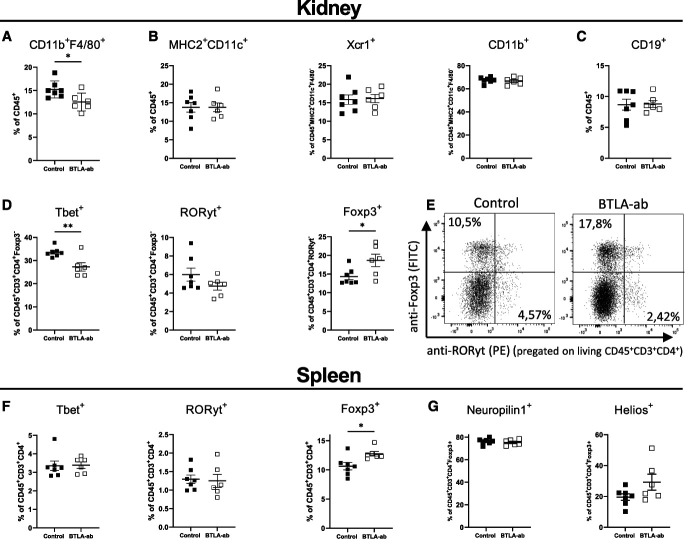
Figure 8.
Anti-BTLA antibody treatment suppresses nephritogenic Th1 cells and increases tTreg frequencies. Flow cytometry analysis shows a significant reduction of renal (A) macrophages in the treatment group while (B) dendritic cells (total MHC2+CD11c+, Xcr1+ cDC1 and CD11b+ cDC2) and (C) B-cell frequencies are unchanged. (D) Frequencies of renal T helper subsets, Tbet+ Th1, RORyt+ Th17 cells, and Foxp3+ Tregs. A significant decrease in Th1 cells and increase in Tregs underscore the potent anti-inflammatory effect of BTLA stimulation. (E) Gating and representative dot plots of renal Th17 cells and Tregs. (F) Analysis of splenic RORyt+ Th17 and Tbet+ Th1 reveals no differences between the groups while Foxp3+ Tregs are significantly increased in the treatment group. (G) Expression of Neuropilin-1 and Helios on splenic Tregs is not affected by BTLA stimulation. *P < 0.05, **P < 0.01 (determined by the Student t test).
Discussion
Because of their fundamental role during T-cell activation, modulation of checkpoint proteins emerges as a promising tool to treat a variety of immune-mediated diseases. In this study, we demonstrate a differential expression of BTLA on renal immune cells during GN. CD4+ Foxp3- T effector cells quickly upregulate BTLA upon NTN induction, whereas BTLA expression on CD4+ Foxp3+ Tregs, although high during homeostatic conditions, decreases throughout disease. These findings are in line with earlier in vitro data which suggested a T-cell subset-specific role of BTLA signaling.35 Because no spontaneous renal inflammation was observed, our findings point toward a dispensable role of BTLA for renal tissue homeostasis. This is in contrast to reported functions in the liver, where BTLA deficiency led to spontaneous autoimmune hepatitis.18 In addition, Stienne et al.25 showed a dysregulation of B cells and T follicular helper cells leading to increased IgA production in the gut of older mice carrying a specific BtlaKO in B cells or CD4+ T cells. Of note, both studies did not report a renal phenotype.
Extending findings in other T-cell–based disease models, we highlight a protective function of BTLA in the kidney during NTN.20,21,22 The aggravated disease in BtlaKO mice involved a dysregulation of T effector cells, which is in agreement with previous publications reporting a central role of BTLA for the control of T-cell activation.16
Intriguingly, renal Th1 immunity was selectively increased compared with wild-type controls. This finding extends on recently published in vitro data, showing a negative correlation of BTLA signaling and transcription of an INFγ response gene set.25 Mechanistically, we demonstrate that BTLA-deficient T effector cells evade Treg-mediated suppression in vitro, a finding consistent with previous observations by others.35 By contrast, BtlaKO Tregs retained their suppressive capacity in vitro and in vivo, exhibited similar survival, and did not show impaired renal trafficking. Furthermore, renal Tregs from BtlaKO mice showed increased transcription of suppressive molecules and cytokines, such as CTLA-4, MYB, and IL-10, suggesting a heightened state of activation. The increased recruitment of renal Tregs in nephritic BtlaKO mice and their transcriptional changes thus likely reflect their attempt to downregulate overactivated Th1 cells.
Whereas findings regarding the role of BTLA on T effector cells are largely unambiguous, conflicting data exist on its effect on Foxp3+ Tregs. Although early work did not show any effect of BTLA deletion on Tregs, recently, BTLA signaling was shown to activate transcription factors associated with Treg differentiation in vitro.25 By contrast, Albring et al. demonstrated a proliferative effect through BTLA activation on Tregs rather than de novo differentiation in vivo.35 Adding to the complexity, BTLA+ DCs were shown to induce pTregs in a model of EAE.24 Our own in vivo data suggest the promotion of thymus-derived Tregs using BTLA during renal inflammation, rather than induction of pTregs, as the number of Treg increased but expression of Neuropilin-1 and Helios—markers associated with pTreg induction—was unaffected by BTLA modulation.
Most importantly, our study shows a robust anti-inflammatory and renoprotective effect of therapeutic administration of the agonistic anti-BTLA antibody in vivo, complementing our findings in BtlaKO mice. In addition to increasing Treg frequencies systemically and in the kidney, anti-BTLA antibody treatment reduced T effector cell frequencies in nephritic kidneys and attenuated NTN, suggesting BTLA activation as a potential treatment option for glomerular inflammation in humans. Of note, no immunosuppressive effect on splenic T effector cells or B cells was observed, suggesting a rather specific and local immunosuppression in the kidney. We, thus, propose a two-fold suppressive effect in the kidney through directly inhibiting T effector cells as well as indirectly through stimulation and expansion of the Treg subpopulation. As nephritogenic Th1 cells express markedly higher amounts of BTLA as compared with other T-cell subsets, they seem to be especially susceptible to BTLA-mediated suppression.
Limiting our mechanistic findings is the inherent issue with whole-body knockout mice As our expression analysis using flow cytometry and single-cell RNA sequencing demonstrated a significant regulation of BTLA only on renal T cells, the observed phenotype is most likely attributable to T-cell dysregulation. However, both B cells and DCs express high levels of BTLA and might influence T-cell immunity. To address this issue, we applied different independent approaches to investigate DC and B-cell function. Because no differences in in vitro antigen presentation and T-cell activation by BTLA-deficient DCs was observed and frequencies of DCs were unaffected by BTLA knockout or in vivo stimulation, a dominant contribution of DCs to the observed phenotype seems unlikely. The same holds true for B cells because the observed decrease in immunoglobulin titers did not translate to less inflammation and antibody-mediated BTLA stimulation had no effect on NTS-specific antibody production. However, given the complex expression pattern of BTLA, cell-type–specific deletion may reveal the full complexity of this signaling pathway in the kidney.25 To this end, future studies will have to address the differential effect on T-cell subsets and B cells in different glomerular inflammation models in more detail.
Which patients might benefit from a BTLA-based therapy? Clinical data show a dysregulation of BTLA in patients with lupus erythematodes as well as ANCA-associated vasculitis.36,37 Furthermore, preclinical data from murine transplant models suggest that BTLA may prevent acute kidney rejection.26,27 Our own study now extends these findings and proposes antibody-based modulation of BTLA as a potential treatment strategy for acute GN.
Supplementary Material
Disclosures
S. Brähler reports Research Funding: AstraZeneca. A.M. Mandel reports Employer: BearingPoint GmbH and University Hospital of Cologne; Consultancy: BearingPoint GmbH; and Other Interests or Relationships: Else Kröner-Fresenius-Stiftung, Eva Luise und Horst Köhler Stiftung, Lothar-Bernd Zimmerhackl Prize (Sandoz). L. Völker reports Consultancy: Sanofi-Genzyme; AstraZeneca; Research Funding: Sanofi-Genzyme; and Honoraria: Sanofi-Genzyme. P. Brinkkoetter reports Research Funding: Sanofi; and Honoraria: Sanofi Genzyme, Alexion, Pfizer, Bristol Meyers Squibb, Bayer, Travere, Novartis, Astra Zeneca. T. Benzing reports Consultancy: Advisory activity for Otsuka in the field of cystic kidney disease and hyponatremia; and Honoraria: Otsuka, Novartis, Hexal, Amgen, Sanofi-Genzyme and Roche. All remaining authors have nothing to disclose.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) grant BR4917/3, the Center for Molecular Medicine Cologne, and the Advanced Cologne Clinician Scientist Program of the University of Cologne (to S. Brähler). J. Becker-Gotot was supported by the Deutsche Forschungsgemeinschaft under Germany's Excellence Strategy—EXC2151—390873048 by an intramural BONFOR group leader fellowship. D. Hawiger received funding from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (R01AI113903) and National Multiple Sclerosis Society (RG-1902-33632). B. Schermer was supported by the DFG (SCHE1562/7, CRU329). P. Brinkkötter declares research funding from the DFG BR-2955/8 and from the German Federal Ministry of Education and Research (STOP-FSGS 01GM1901E). C. Kurts received funding by the DFG (SFBTRR259 A01, SFB1192 A8, GRK2168 272482170, EXC2151 390873048). T. Benzing was supported by the DFG (BE221 and CRU329).
Acknowledgments
We thank Sachiko Kolar, Angelika Köser, Francesco Landini, the FACS and Imaging Core Unit of the Max Planck Institute for Aging as well as the Cologne Center for Genomics (CCG) for excellent technical support.
Author Contributions
Conceptualization: Thomas Benzing, Sebastian Brähler, Paul Brinkkötter, Paul Diefenhardt, Heike Göbel, Daniel Hawiger, Christian Kurts, Linus A. Völker.
Data curation: Janine Becker-Gotot, Marie Braumann, Paul Diefenhardt, Thomas Schömig, Claudio Sierra-Gonzalez, Bastian Trinsch.
Formal analysis: Ali T. Abdallah, Janine Becker-Gotot, Paul Diefenhardt.
Funding acquisition: Sebastian Brähler, Paul Brinkkötter.
Investigation: Sebastian Brähler, Paul Diefenhardt.
Methodology: Ali T. Abdallah, Paul Diefenhardt.
Project administration: Sebastian Brähler, Paul Diefenhardt.
Resources: Thomas Benzing, Sebastian Brähler, Paul Brinkkötter, Christian Kurts, Bernhard Schermer.
Supervision: Thomas Benzing, Sebastian Brähler.
Validation: Paul Diefenhardt, Lioba Ester, Amrei M. Mandel.
Visualization: Paul Diefenhardt.
Writing – original draft: Sebastian Brähler, Paul Diefenhardt.
Writing – review & editing: Ali T. Abdallah, Janine Becker-Gotot, Thomas Benzing, Sebastian Brähler, Paul Brinkkötter, Paul Diefenhardt, Lioba Ester, Daniel Hawiger, Christian Kurts, Amrei M. Mandel, Linus A. Völker.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/JSN/E467.
Supplemental Figure 1. Splenic and renal BTLA expression.
Supplemental Figure 2. BtlaKO mice show increased splenic T-cell frequencies but do not develop spontaneous nephritis.
Supplemental Figure 3. Glomerular deposition of nephrotoxic sheep IgG.
Supplemental Figure 4. Renal immunity and NTS-specific humoral response after 10 days of NTN.
Supplemental Figure 5. Representative images of PAS stainings of BtlaKO and antibody treatment studies.
Supplemental Figure 6. ACR and immunophenotyping of BtlaKO and WT mice after 21 days of NTN.
Supplemental Figure 7. scRNAseq analysis of renal T-cell subclusters.
Supplemental Figure 8. Top 20 GO terms upregulated in total T cells and Tregs.
Supplemental Figure 9. BTLA-deficient nephritogenic T cells show similar rates of apoptosis.
Supplemental Figure 10. Glomerular macrophage infiltration and C3 deposition in anti-BTLA antibody–treated mice.
Supplemental Figure 11. Treg and Th1 survival and humoral responses in anti-BTLA-antibody–treated mice.
Supplemental Figure 12. Prophylactic BTLA-antibody administration attenuates NTN.
Supplemental Figure 13. Flow cytometry analysis of prophylactic BTLA antibody study.
References
- 1.Suárez-Fueyo A, Bradley SJ, Klatzmann D, Tsokos GC. T cells and autoimmune kidney disease. Nat Rev Nephrol. 2017;13:329–343. doi: 10.1038/nrneph.2017.34 [DOI] [PubMed] [Google Scholar]
- 2.Kurts C, Panzer U, Anders HJ, Rees AJ. The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol. 2013;13:738–753. doi: 10.1038/nri3523 [DOI] [PubMed] [Google Scholar]
- 3.Paust HJ Turner JE Steinmetz OM, et al. The IL-23/Th17 axis contributes to renal injury in experimental glomerulonephritis. J Am Soc Nephrol. 2009;20(5):969–979. doi: 10.1681/ASN.2008050556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tipping PG, Holdsworth SR. T cells in crescentic glomerulonephritis. J Am Soc Nephrol. 2006;17(5):1253–1263. doi: 10.1681/ASN.2005091013 [DOI] [PubMed] [Google Scholar]
- 5.Herrnstadt GR, Steinmetz OM. The role of Treg subtypes in glomerulonephritis. Cell Tissue Res. 2021;385:293–304. doi: 10.1007/s00441-020-03359-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostmann A Paust HJ Panzer U, et al. Regulatory T cell-derived IL-10 ameliorates crescentic GN. J Am Soc Nephrol. 2013;24(6):930–942. doi: 10.1681/ASN.2012070684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt A, Oberle N, Krammer PH. Molecular mechanisms of treg-mediated T cell suppression. Front Immunol. 2012;3:51. doi: 10.3389/fimmu.2012.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diefenhardt P Nosko A Kluger MA, et al. IL-10 receptor signaling empowers regulatory T cells to control Th17 responses and protect from GN. J Am Soc Nephrol. 2018;29(7):1825–1837. doi: 10.1681/ASN.2017091044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neumann K Ostmann A Breda PC, et al. The co-inhibitory molecule PD-L1 contributes to regulatory T cell-mediated protection in murine crescentic glomerulonephritis. Sci Rep. 2019;9:2038. doi: 10.1038/s41598-018-38432-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner JE Paust HJ Steinmetz OM, et al. CCR6 recruits regulatory T cells and Th17 cells to the kidney in glomerulonephritis. J Am Soc Nephrol. 2010;21(6):974–985. doi: 10.1681/ASN.2009070741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nosko A Kluger MA Diefenhardt P, et al. T-bet enhances regulatory T cell fitness and directs control of Th1 responses in crescentic GN. J Am Soc Nephrol. 2017;28(1):185–196. doi: 10.1681/ASN.2015070820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee H-G, Cho M-Z, Choi J-M. Bystander CD4+ T cells: crossroads between innate and adaptive immunity. Exp Mol Med. 2020;52:1255–1263. doi: 10.1038/s12276-020-00486-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrews LP, Yano H, Vignali DAA. Inhibitory receptors and ligands beyond PD-1, PD-L1 and CTLA-4: breakthroughs or backups. Nat Immunol. 2019;20:1425–1434. doi: 10.1038/s41590-019-0512-0 [DOI] [PubMed] [Google Scholar]
- 14.Ning Z, Liu K, Xiong H. Roles of BTLA in immunity and immune disorders. Front Immunol. 2021;12:654960. doi: 10.3389/fimmu.2021.654960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gavrieli M, Watanabe N, Loftin SK, Murphy TL, Murphy KM. Characterization of phosphotyrosine binding motifs in the cytoplasmic domain of B and T lymphocyte attenuator required for association with protein tyrosine phosphatases SHP-1 and SHP-2. Biochem Biophys Res Commun. 2003;312(4):1236–1243. doi: 10.1016/j.bbrc.2003.11.070 [DOI] [PubMed] [Google Scholar]
- 16.Sedy JR Gavrieli M Potter KG, et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol. 2005;6:90–98. doi: 10.1038/ni1144 [DOI] [PubMed] [Google Scholar]
- 17.Krieg C, Han P, Stone R, Goularte OD, Kaye J. Functional analysis of B and T lymphocyte attenuator engagement on CD4+ and CD8+ T cells. J Immunol. 2005;175(10):6420–6427. doi: 10.4049/jimmunol.175.10.6420 [DOI] [PubMed] [Google Scholar]
- 18.Oya Y Watanabe N Owada T, et al. Development of autoimmune hepatitis-like disease and production of autoantibodies to nuclear antigens in mice lacking B and T lymphocyte attenuator. Arthritis Rheum. 2008;58(8):2498–2510. doi: 10.1002/art.23674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe N Gavrieli M Sedy JR, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4:670–679. doi: 10.1038/ni944 [DOI] [PubMed] [Google Scholar]
- 20.Tao R Wang L Han R, et al. Differential effects of B and T lymphocyte attenuator and programmed death-1 on acceptance of partially versus fully MHC-mismatched cardiac allografts. J Immunol. 2005;175(9):5774–5782. doi: 10.4049/jimmunol.175.9.5774 [DOI] [PubMed] [Google Scholar]
- 21.Deppong C, Degnan JM, Murphy TL, Murphy KM, Green JM. B and T lymphocyte attenuator regulates T cell survival in the lung. J Immunol. 2008;181(5):2973–2979. doi: 10.4049/jimmunol.181.5.2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi Y Iwata A Suzuki K, et al. B and T lymphocyte attenuator inhibits LPS-induced endotoxic shock by suppressing Toll-like receptor 4 signaling in innate immune cells. Proc Natl Acad Sci U S A. 2013;110(13):5121–5126. doi: 10.1073/pnas.1222093110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bekiaris V, Šedý JR, Macauley MG, Rhode-Kurnow A, Ware CF. The inhibitory receptor BTLA controls γδ T cell homeostasis and inflammatory responses. Immunity 2013;39(6):1082–1094. doi: 10.1016/j.immuni.2013.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones A Bourque J Kuehm L, et al. Immunomodulatory functions of BTLA and HVEM govern induction of extrathymic regulatory T cells and tolerance by dendritic cells. Immunity 2016;45(5):1066–1077. doi: 10.1016/j.immuni.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stienne C Virgen-Slane R Elmén L, et al. Btla signaling in conventional and regulatory lymphocytes coordinately tempers humoral immunity in the intestinal mucosa. Cell Rep. 2022;38(12):110553. doi: 10.1016/j.celrep.2022.110553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H Wang Z Zhang J, et al. Combined immunotherapy with belatacept and BTLA overexpression attenuates acute rejection following kidney transplantation. Front Immunol. 2021;12:618737. doi: 10.3389/fimmu.2021.618737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J Zhang H Wang Z, et al. BTLA suppress acute rejection via regulating TCR downstream signals and cytokines production in kidney transplantation and prolonged allografts survival. Sci Rep. 2019;9:12154. doi: 10.1038/s41598-019-48520-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oster C Wilde B Specker C, et al. BTLA expression on Th1, Th2 and Th17 effector T-cells of patients with systemic lupus erythematosus is associated with active disease. Int J Mol Sci. 2019;20(18):4505. doi: 10.3390/ijms20184505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hao Y Hao S Andersen-Nissen E, et al. Integrated analysis of multimodal single-cell data. Cell 2021;184(13):3573–3587.e3529. doi: 10.1016/j.cell.2021.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franzén O, Gan LM, Björkegren JLM. PanglaoDB: a web server for exploration of mouse and human single-cell RNA sequencing data. Database (Oxford). 2019;2019:baz046. doi: 10.1093/database/baz046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strimmer K. fdrtool: a versatile R package for estimating local and tail area-based false discovery rates. Bioinformatics. 2008;24(12):1461–1462. doi: 10.1093/bioinformatics/btn209 [DOI] [PubMed] [Google Scholar]
- 32.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolberg L, Raudvere U, Kuzmin I, Vilo J, Peterson H. gprofiler2—an R package for gene list functional enrichment analysis and namespace conversion toolset g:Profiler. F1000Res 2020;9:ELIXIR-709. doi: 10.12688/f1000research.24956.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Supek F, Bošnjak M, Škunca N, Šmuc T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS One 2011;6:e21800. doi: 10.1371/journal.pone.0021800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Albring JC Sandau MM Rapaport AS, et al. Targeting of B and T lymphocyte associated (BTLA) prevents graft-versus-host disease without global immunosuppression. J Exp Med. 2010;207(12):2551–2559. doi: 10.1084/jem.20102017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiedemann A Lettau M Weißenberg SY, et al. BTLA expression and function are impaired on SLE B cells. Front Immunol. 2021;12:667991. doi: 10.3389/fimmu.2021.667991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Werner K Dolff S Dai Y, et al. The co-inhibitor BTLA is functional in ANCA-associated vasculitis and suppresses Th17 cells. Front Immunol. 2019;10:2843. doi: 10.3389/fimmu.2019.02843 [DOI] [PMC free article] [PubMed] [Google Scholar]