Abstract
The study aimed to assess whether factors related to cognitive performance were associated with the development of dementia. Additionally, the study aimed to establish whether cognitive performance at baseline or change in cognition between baseline and follow-up (five-year period) had a stronger association with whether an individual would fulfill a dementia criterion at follow-up. The data was collected from 2002 to 2011. Logistic regression was applied to the AGES-Reykjavik Study epidemiological data. The analysis, which builds upon previous data analyses of the same dataset, included 1,491 participants between the ages of 66 and 90. All those included were considered to have normal cognition at baseline; 8.2% (n = 123) of them fulfilled a dementia criterion at follow-up five years later. The study's results indicated that being high on cognitive reserve factors reduced the risk of developing dementia. Compared to other known dementia risk factors, cognitive reserve factors (education level, participation in leisure activities, and self-reported health) were more likely than others to have an association with dementia. Additionally, the study's findings showed that cognitive performance at baseline, rather than change in cognition between baseline and follow-up five years later, had a stronger association with dementia at the follow-up assessment. Together, these findings support the notion that promoting high cognitive reserve throughout the lifespan and reaching high cognitive performance is important in reducing dementia risk.
Keywords: Cognitive aging, dementia, cognitive performance, cognitive change, cognitive reserve, AGES-Reykjavik Study
The World Health Organization’s Global status report on the public health response to dementia published in 2021 [1] roughly estimates that the global prevalence of dementia for those over 65 years is around 6.9 percent. According to a recently published article on the global prevalence of dementia, dementia cases are expected to increase from 57 million in 2019 to 153 million in 2050 [2]. Even though there is a growing body of evidence suggesting that dementia incidence is declining in certain parts of the world [3], significant increases in the number of people affected by dementia are to be expected by 2050 due to population aging and population growth [2].
Globally, the cost of dementia is very high, both in the economic and societal sense, and it is only expected to increase in the coming years [1]. Dementia dramatically impacts the lives of those living with the condition. At the same time, it also puts a heavy load on family members and other informal caregivers. Costs can be reduced, and the impact on society can be diminished by postponing the onset of dementia, even if only by a few years [1].
There is great value in predicting which individuals are more likely to develop dementia, allowing for early intervention. Many factors contribute to dementia risk and cognitive decline, and they could be used to make these predictions. Among those that have been identified are hypertension [4], diabetes [5], coronary artery disease [6], atrial fibrillation [7], depression [8], body composition [9], physical activity [10], mobility and strength [11], sleep [12], diet [13], smoking [8], alcohol consumption [8], level of education [14], multilingualism [15], participation in leisure activities [16] and self-reported health [17]. According to the 2020 report of the Lancet Commission on dementia prevention, intervention, and care, 12 modifiable risk factors have been identified that contribute to approximately 40% of dementia cases [18]. These factors are low education, little social contact, smoking, excessive alcohol consumption, air pollution, physical inactivity, depression, hearing impairment, diabetes, hypertension, obesity, and traumatic brain injury. Preventative measures for these risk factors are appropriate at all stages in life. It is never too early and never too late to mitigate dementia risk, although the impact on individual risk factors differs between different stages of life.
Cognitive performance, cognitive decline, and cognitive trajectories are concepts often examined when establishing the risk of developing dementia. Although many intuitively assume that cognitive decline and the risk of developing dementia go hand in hand, that is not necessarily the case. A few studies have presented evidence suggesting that factors previously associated with cognitive aging or cognitive decline are more likely to predict cognitive performance than cognitive decline [8, 19–21].
In a study that examined age trajectories of cognitive function over eight years, Zaninotto et al. [8] found that although several modifiable factors, such as poor physical function, physical inactivity, and smoking, were predictive of cognitive decline, more factors had an association with the baseline of cognitive function. Similarly, Ritchie et al. [19] found that when a wide variety of factors relating to genetics, health, physical fitness, and sociodemographic status were tested for an association with cognitive change, only a limited number significantly predicted changes in cognition. However, many factors contributed to explaining the variance in cognitive performance. This underscores that factors correlating with cognition do not necessarily predict cognitive decline. According to a recent meta-analysis examining the relationship between education and the rate of cognitive decline, the current body of evidence suggests that the association between the two is negligible even though education is considered a preventative factor for dementia [21]. Salthouse [20] goes as far as to state that based on the available literature; no recommendation can be made regarding factors that could affect cognitive decline.
Present study
In a recently published paper using the AGES-Reykjavik dataset, we confirmed that many known risk factors for dementia and cognitive aging listed above are related to cognitive performance among older adults [22]. Factors that were shown to have a relationship with cognitive performance were mobility, leisure activities, foreign language knowledge, education level, self-reported health, physical strength, coronary artery disease, alcohol consumption, and hypertension. These factors did, however, not have the same relationship with cognitive change over five years. Of the abovementioned variables, only mobility and physical strength were associated with cognitive change. The current study aimed to evaluate whether the factors related to cognitive performance would be associated with whether a person considered to have normal cognition at baseline would fulfill a dementia criterion at follow-up five years later. Additionally, the study aimed to ascertain whether cognitive performance at baseline or change in cognition between baseline and follow-up (five-year period) had a stronger association with whether an individual would fulfill a dementia criterion at follow-up.
Methods
Study population
The data for this study is from the Age Gene/Environment Susceptibility-Reykjavik Study (AGES-Reykjavik Study). The AGES-Reykjavik Study is a continuation of the Reykjavik Study, a study that the Icelandic Heart Association (IHA) initiated in 1967 that included individuals living in the Reykjavik area of Iceland that were born between the years 1907 and 1935. The AGES-Reykjavik Study aimed to examine risk factors for disease and disability in the older population. A subgroup of the Reykjavik Study cohort survivors was randomly selected and asked to participate further in the AGES-Reykjavik Study. The data was collected at two different time points, approximately five years apart for each participant, between 2002 and 2011. Altogether, 5.764 individuals participated in the first round of data collection for the AGES-Reykjavik Study, with 3.316 participating in the baseline and follow-up rounds of data collection. For a more detailed description of the study design, please refer to previously published material [22, 23]. Of the individuals who participated in the follow-up data collection, 16.2% (n = 536) fulfilled the study ‘s dementia criterion at baseline. Of those included in the data analysis (who did not fulfill the dementia criterion at the baseline measurement), 8.2% (n = 123) fulfilled the criterion at follow-up.
The AGES-Reykjavik Study was approved by the Icelandic National Bioethics Committee (VSN 00–063) and by the Institutional Review Board of the U.S. National Institute on Aging, National Institutes of Health, with participants providing their written consent.
Research design and measures
Exposure variables
Eighteen exposure variables were chosen for the analysis and they are listed in Table 1. These variables had previously been identified as predictors of cognition in the literature. They had been used as exposure variables in a previous study of cognitive performance and cognitive change within the same dataset [22]. Sex and age were included in the models as control variables [24, 25].
Table 1.
Description of exposure variables
| Sex | Male, female |
| Age | Subject age in years at the first measurement |
| Mobility | Timed up and go test (TUG) measured in seconds [26]. A lower score represents better mobility |
| Leisure activities | Average of days per month engaged, reported for mental and social leisure activities (movies, lectures, church, crossword puzzles, board/card games, and computer games) |
| Foreign languages | Number of foreign languages spoken |
| Education | Education level completed: Primary school, secondary, college, university (Primary and secondary school were combined into one group) |
| Self-reported health | Self-estimation of general health: Excellent, very good, good, fair, poor (Two categories: 1 – poor and fair, 2 – good to excellent) |
| Physical strength | Maximum strength value in leg in Newtons |
| Smoking | Smoking status: Never smoked, previous smoker, current smoker |
| Coronary artery disease | Coronary artery disease diagnosis based on rose angina, MI ECG, and use of nitrates: Yes, possible case, no |
| Alcohol consumption | Grams of alcohol per week consumed |
| Depression | Geriatric Depression Scale score [27] |
| Diabetes | Diagnosed as diabetes by self-report, fasting glucose, or medication use: Yes, no |
| Hypertension | Hypertension, derived from physiological measurements (systolic blood pressure, diastolic blood pressure): Yes, pre-hypertension, no |
| Body Fat Percentage | Bioelectric Impedance (BIA): Percent body fat |
| ApoE carrier | Apolipoprotein E (ApoE) genotype positive carrier: Yes, no |
| Relative grey matter volume | Grey matter volume (ml) divided with intracranial volume (ICVa) |
| Relative white matter volume | White matter volume (ml) divided with intracranial volume (ICVa) |
The table is an adapted version of Table 1 from Valsdóttir et al. [22]
a The sum of grey matter volume, white matter volume, white matter lesion volume, and cerebral spinal fluid volume
Assessment of cognitive function and change in cognition
At two different points, five years apart, participants completed eight cognitive tests administered to assess their cognitive performance. The tests comprised three cognitive domains, a memory domain (two tests), a speed of processing domain (four tests), and an executive function domain (two tests). The memory domain included a modified version of the California Verbal Learning Test (CVLT) [28] and Digits Forward [29]. The speed of processing domain included Figure Comparison [30], Digit Symbol Substitution Test (Wechsler, 1955), and a modified Stroop Test, Part I and II [31]. The executive function domain included Digits Backwards [29] and a modified Stroop Test, Part III [32]. A general cognitive ability variable was created by combining the results from the eight cognitive tests [33]. The general cognitive ability score was based on z-scores of the raw scores for each cognitive test, averaged over all eight variables. Further description of these calculations can be found in previously published material [34]. The variable for change in general cognitive ability was calculated by subtracting the baseline measurement from a follow-up measurement collected five years later.
Dementia criterion
Participants were evaluated for mild cognitive impairment and dementia with a three-step process [23]. All participants underwent a cognitive screening based on the Mini-Mental State Examination [35] and the Digit Symbol Substitution Test [29]. A diagnostic battery of neuropsychological tests was administered to those with positive screening results, and if necessary, they were examined by a neurologist. Finally, a panel of a geriatrician, neurologist, neuropsychologist, and neuroradiologist decided on a consensus diagnosis in line with international guidelines.
This assessment was performed in the same manner at baseline and follow-up and by the same panel of professionals. Individuals considered by the panel to have mild cognitive impairment or dementia fulfilled the criterion. To improve readability, this criterion will be referred to as a dementia criterion in the following text. The assessment was performed for the purpose of this study and, therefore, is not equivalent to a clinical diagnosis of dementia.
Analytical sample
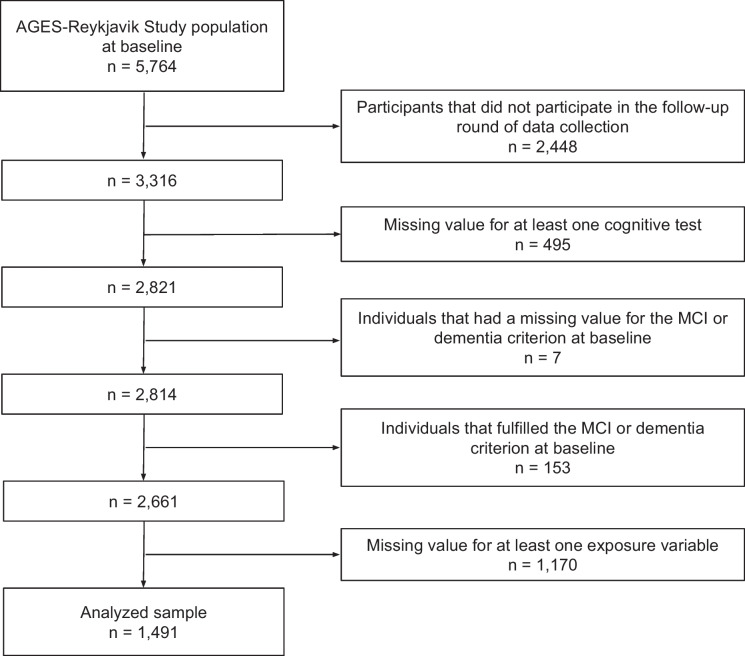
A flowchart of the participant selection is shown in Fig. 1. Participants that fulfilled the dementia criterion at baseline were excluded from the analysis. Therefore, all participants were considered to have normal cognition at baseline.
Fig. 1.
Flowchart of the participant selection for the data analysis
Statistical analysis
Counts and percentages for discrete variables and means and standard deviations for continuous variables were displayed for all exposure variables.
A logistic multivariable regression model analyzed how the exposure variables were associated with whether a person would fulfill a dementia criterion at the follow-up assessment.
An additional logistic multivariable regression model was used to establish whether cognitive performance or change in cognitive performance over five years were more strongly associated with whether a person was likely to fulfill a dementia criterion at the follow-up measurement. The Akaike's information criterion (AIC) [36] was calculated for each model and used to ascertain which of the models was a better fit.
The Benjamini–Hochberg correction was used for all logistic regression models to control for false discovery rate caused by multiple comparisons [37].
Results
Descriptive statistics
Table 2 shows descriptive statistics for all exposure variables, means and standard deviations for continuous variables, and counts and percentages for discrete variables. Age and sex were included as control variables in each of the models.
Table 2.
Descriptive statistics for exposure variables (measured at baseline) showing means, standard deviations, counts and percentages
| All participants | Healthy cognition at follow-up | Fulfil dementia criterion at follow-up | ||||
|---|---|---|---|---|---|---|
| n = 1491 | n = 1368 | n = 123 | ||||
| M | SD | M | SD | M | SD | |
| Age, mean | 74.11 | 4.41 | 73.85 | 4.30 | 77.10 | 4.45 |
| Mobility | 11.23 | 2.51 | 11.11 | 2.36 | 12.46 | 3.60 |
| Leisure activity | 5.79 | 3.70 | 5.92 | 3.72 | 4.43 | 3.10 |
| Foreign languages | 2.17 | 1.50 | 2.23 | 1.50 | 1.50 | 1.22 |
| Physical strength | 347.08 | 115.03 | 349.13 | 115.98 | 324.28 | 101.54 |
| Alcohol consumption | 17.82 | 34.07 | 17.92 | 33.68 | 16.71 | 38.23 |
| Depression | 1.88 | 1.89 | 1.83 | 1.88 | 2.43 | 1.91 |
| Body fat percentage | 29.16 | 8.14 | 29.36 | 8.11 | 26.95 | 8.22 |
| Relative grey matter volume | 46.02 | 3.13 | 46.14 | 3.12 | 44.71 | 2.93 |
| Relative white matter volume | 26.19 | 1.75 | 26.26 | 1.71 | 25.34 | 1.96 |
| n (%) | n (%) | n (%) | ||||
| Male | 626 (42.0) | 566 (41.4) | 60 (48.8) | |||
| Education (Primary and secondary school as reference) | 1043 (70.0) | 933 (68.2) | 110 (89.4) | |||
| College | 263 (17.6) | 254 (18.6) | 9 (7.3) | |||
| University | 185 (12.4) | 181 (13.2) | 4 (3.3) | |||
| Self-reported health-good to excellent | 1168 (78.3) | 1091 (79.8) | 77 (62.6) | |||
| Smoking (Never as reference) | 659 (44.2) | 610 (44.6) | 49 (39.8) | |||
| Previously | 686 (46.0) | 627 (45.8) | 59 (48.0) | |||
| Current | 146 (9.8) | 131 (9.6) | 15 (12.2) | |||
| Coronary artery disease (No as reference) | 1152 (77.3) | 1066 (77.9) | 86 (69.9) | |||
| Possible case | 100 (6.7) | 91 (6.7) | 9 (7.3) | |||
| Yes | 239 (16.0) | 211 (15.4) | 28 (22.8) | |||
| Diabetes-diagnosed with | 136 (9.1) | 122 (8.9) | 14 (11.4) | |||
| Hypertension (No as reference) | 179 (12.0) | 170 (12.4) | 9 (7.3) | |||
| Pre-Hypertension | 604 (40.5) | 555 (40.6) | 49 (39.8) | |||
| Yes | 708 (47.5) | 643 (47.0) | 65 (52.8) | |||
| ApoE e4 carrier | 394 (26.4) | 345 (25.2) | 49 (39.8) | |||
Table 3 shows the means and standard deviations for all eight cognitive tests comprising the general cognitive ability variable at baseline and follow-up (including participants that fulfilled the dementia criterion at follow-up, n = 1,491). A paired t-test comparison was performed, comparing the baseline and follow-up measurements.
Table 3.
Descriptive statistics for cognitive test outcomes at baseline and follow-up with a paired t-test comparison
| Baseline | Follow-up | Comparison | |||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M (difference) | SD | p | |
| STROOP trial 1 (time in seconds) | 18.72 | 3.57 | 19.80 | 4.94 | 1.08 | 3.79 | < .001 |
| STROOP trial 2 (time in seconds) | 25.50 | 5.30 | 27.22 | 7.14 | 1.71 | 5.73 | < .001 |
| STROOP trial 3 (time in seconds) | 58.95 | 18.62 | 64.31 | 22.96 | 5.36 | 21.07 | < .001 |
| Figure comparison (total correct in 60 s) | 20.20 | 4.86 | 18.64 | 5.24 | – 1.56 | 3.67 | < .001 |
| Digits Backwards (total correct spans) | 4.97 | 1.71 | 4.68 | 1.65 | – 0.30 | 1.52 | < .001 |
| Digits Forward (total correct spans) | 7.79 | 1.97 | 7.65 | 1.81 | – 0.14 | 1.64 | < .001 |
| DSST (total correct cells) | 33.48 | 10.02 | 30.34 | 10.57 | – 3.15 | 5.56 | < .001 |
| CVLT (trial 1-4: number of unique target words) | 28.44 | 7.02 | 26.48 | 7.72 | – 1.96 | 6.07 | < .001 |
P-values that are displayed in bold have a significant p-value after a Benjamini–Hochberg correction [37]
Change in cognitive performance between baseline and follow-up was statistically significant for all eight cognitive tests.
Logistic regression analysis
Associations based on exposure factors
A logistic regression analysis was used to assess whether exposure factors, previously identified as predictors of dementia risk, were significantly associated with whether participants would go on to fulfill a dementia criterion between the baseline assessment and the follow-up assessment. Table 4 shows the results of the analysis.
Table 4.
Logistic multivariable regression model showing how exposure variables are associated with the development of dementia
| 95% CI for Odds ratio | |||||||
|---|---|---|---|---|---|---|---|
| Exposure factors | B | SE(B) | Wald | Odds ratio | Lower | Higher | p |
| Male | 0.880 | 0.457 | 3.704 | 2.412 | 0.984 | 5.912 | .054 |
| Age | 0.134 | 0.027 | 25.290 | 1.143 | 1.085 | 1.204 | < .001 |
| Mobility | 0.063 | 0.036 | 3.097 | 1.065 | 0.993 | 1.143 | .078 |
| Leisure activities | – 0.087 | 0.033 | 7.063 | 0.916 | 0.859 | 0.977 | .008 |
| Foreign languages | – 0.174 | 0.101 | 2.968 | 0.841 | 0.690 | 1.024 | .085 |
| Education (Primary or secondary school education as reference) | |||||||
| College education | – 0.996 | 0.412 | 5.858 | 0.369 | 0.165 | 0.827 | .016 |
| University education | – 1.502 | 0.600 | 6.268 | 0.223 | 0.069 | 0.722 | .012 |
| Self-reported health | – 0.636 | 0.236 | 7.248 | 0.529 | 0.333 | 0.841 | .007 |
| Physical strength | 0.000 | 0.001 | 0.001 | 1.000 | 0.997 | 1.003 | .982 |
| Smoking (Never having smoked as reference) | |||||||
| Previously smoked | 0.084 | 0.233 | 0.128 | 1.087 | 0.688 | 1.717 | .720 |
| Current smoker | 0.516 | 0.357 | 2.091 | 1.676 | 0.832 | 3.374 | .148 |
| Coronary artery disease (No coronary artery disease as reference) | |||||||
| Possible case of coronary artery disease | 0.092 | 0.406 | 0.051 | 1.096 | 0.494 | 2.429 | .821 |
| Confirmed case of coronary artery disease | 0.166 | 0.265 | 0.389 | 1.180 | 0.701 | 1.986 | .533 |
| Alcohol consumption | 0.001 | 0.003 | 0.025 | 1.001 | 0.994 | 1.007 | .873 |
| Depression | 0.004 | 0.054 | 0.006 | 1.004 | 0.902 | 1.117 | .940 |
| Diabetes | 0.164 | 0.336 | 0.239 | 1.178 | 0.610 | 2.275 | .625 |
| Hypertension (No hypertension as reference) | |||||||
| Pre-hypertension | 0.700 | 0.413 | 2.874 | 2.014 | 0.896 | 4.526 | .090 |
| No hypertension | 0.625 | 0.410 | 2.332 | 1.869 | 0.838 | 4.171 | .127 |
| Body Fat Percentage | – 0.075 | 0.023 | 10.666 | 0.928 | 0.888 | 0.971 | .001 |
| ApoE e4 carrier | 0.823 | 0.217 | 14.315 | 2.277 | 1.487 | 3.487 | < .001 |
| Relative gray matter volume* | – 0.263 | 0.109 | 5.821 | 0.769 | 0.621 | 0.952 | .016 |
| Relative white matter volume* | – 0.320 | 0.108 | 8.819 | 0.726 | 0.588 | 0.897 | .003 |
| Nagelkerke R2 | .255 | ||||||
P-values that are displayed in bold have a significant p-value after a Benjamini–Hochberg correction [37]
* Per standard deviation
The model was statistically significant, (22) = 174.88, p < 0.001, indicating that the model could distinguish between participants who fulfilled a dementia criterion five years later and those who did not. To evaluate the discriminative power of the variables in the model, a receiver operating characteristic (ROC) analysis was performed. The area under the ROC curve (AUC-ROC) was 0.84 (95% CI = 0.80—0.87).
The model shows that those with more brain volume, a higher level of education, better self-reported health, more leisure activities, and a higher body fat percentage were less likely than others to fulfill the criterion. However, ApoE ε4 carriers were more likely to fulfill the dementia criterion than non-carriers.
Associations between dementia criterion and cognitive performance and change in cognitive performance
Three logistic regression analyses were used to assess the associations between the fulfillment of a dementia criterion at a follow-up assessment and three measurements related to cognition, cognitive performance at baseline, cognitive performance at follow-up, and the change in cognitive performance between the two measures. Table 5 shows the results of the analysis.
Table 5.
Logistic multivariable regression model showing how cognitive performance, and change in cognitive performance, are associated with the development of dementia
| 95% CI for Odds ratio | |||||||
|---|---|---|---|---|---|---|---|
| Exposure factors | B | SE(B) | Wald | Odds ratio | Lower | Higher | p |
| Model 1—baseline | |||||||
| Male | – 0.086 | 0.225 | 0.147 | 0.917 | 0.590 | 1.426 | .702 |
| Age | 0.073 | 0.024 | 9.279 | 1.076 | 1.026 | 1.128 | .002 |
| STROOP trial 1 (time in seconds)-baseline | 0.019 | 0.126 | 0.022 | 1.019 | 0.796 | 1.304 | .883 |
| STROOP trial 2 (time in seconds)—baseline | 0.024 | 0.118 | 0.042 | 1.024 | 0.813 | 1.290 | .838 |
| STROOP trial 3 (time in seconds)—baseline | – 0.103 | 0.096 | 1.162 | 0.902 | 0.747 | 1.088 | .281 |
| Figure comparison (total correct in 60 s)—baseline | – 0.181 | 0.161 | 1.271 | 0.834 | 0.609 | 1.143 | .260 |
| Digits Backwards (total correct spans)—baseline | – 0.265 | 0.133 | 3.997 | 0.767 | 0.591 | 0.995 | .046* |
| Digits Forward (total correct spans)—baseline | – 0.099 | 0.132 | 0.564 | 0.906 | 0.699 | 1.173 | .453 |
| DSST (total correct cells)—baseline | – 0.991 | 0.210 | 22.357 | 0.371 | 0.246 | 0.560 | < .001 |
| CVLT (trial 1—4: number of unique target words)—baseline | – 0.592 | 0.133 | 19.751 | 0.553 | 0.426 | 0.718 | < .001 |
| Nagelkerke R2 | .298 | ||||||
| AIC | 670.426 | ||||||
| Model 2—follow up | |||||||
| Male | 0.151 | 0.263 | 0.332 | 1.164 | 0.695 | 1.947 | .564 |
| Age | 0.021 | 0.029 | 0.505 | 1.021 | 0.964 | 1.080 | .477 |
| STROOP trial 1 (time in seconds)—follow-up | 0.049 | 0.140 | 0.121 | 1.050 | 0.798 | 1.382 | .728 |
| STROOP trial 2 (time in seconds)—follow-up | 0.016 | 0.134 | 0.013 | 1.016 | 0.780 | 1.322 | .908 |
| STROOP trial 3 (time in seconds)—follow-up | 0.024 | 0.104 | 0.051 | 1.024 | 0.835 | 1.256 | .821 |
| Figure comparison (total correct in 60 s)—follow-up | – 0.116 | 0.215 | 0.291 | 0.891 | 0.585 | 1.357 | .590 |
| Digits Backwards (total correct spans)—follow-up | – 0.351 | 0.161 | 4.769 | 0.704 | 0.513 | 0.965 | .029* |
| Digits Forward (total correct spans)—follow-up | – 0.367 | 0.151 | 5.868 | 0.693 | 0.515 | 0.932 | .015 |
| DSST (total correct cells)—follow-up | – 2.467 | 0.307 | 64.438 | 0.085 | 0.046 | 0.155 | < .001 |
| CVLT (trial 1—4: number of unique target words)—follow-up | – 1.128 | 0.167 | 45.887 | 0.324 | 0.234 | 0.449 | < .001 |
| Nagelkerke R2 | .545 | ||||||
| AIC | 465.366 | ||||||
| Model 3—change | |||||||
| Male | – 0.198 | 0.207 | 0.915 | 0.820 | 0.547 | 1.231 | .339 |
| Age | 0.139 | 0.022 | 38.443 | 1.149 | 1.100 | 1.201 | < .001 |
| STROOP trial 1 (time in seconds)—change | – 0.196 | 0.079 | 6.219 | 0.822 | 0.705 | 0.959 | .013 |
| STROOP trial 2 (time in seconds)—change | – 0.234 | 0.095 | 6.027 | 0.791 | 0.657 | 0.954 | .014 |
| STROOP trial 3 (time in seconds)—change | – 0.011 | 0.090 | 0.016 | 0.989 | 0.829 | 1.178 | .898 |
| Figure comparison (total correct in 60 s)—change | – 0.100 | 0.106 | 0.891 | 0.904 | 0.734 | 1.114 | .345 |
| Digits Backwards (total correct spans)—change | – 0.022 | 0.101 | 0.048 | 0.978 | 0.803 | 1.191 | .826 |
| Digits Forward (total correct spans)—change | – 0.045 | 0.107 | 0.178 | 0.956 | 0.776 | 1.178 | .673 |
| DSST (total correct cells)—change | – 0.523 | 0.105 | 24.950 | 0.593 | 0.483 | 0.728 | < .001 |
| CVLT (trial 1—4: number of unique target words)—change | – 0.246 | 0.101 | 5.921 | 0.782 | 0.641 | 0.953 | .015 |
| Nagelkerke R2 | .223 | ||||||
| AIC | 756.402 | ||||||
P-values that are displayed in bold have a significant p-value after a Benjamini–Hochberg correction [37]
* P-values marked with an asterisk are smaller than 0.05 but are considered non-significant after the Benjamini–Hochberg correction
The models for cognitive performance at baseline, (10) = 206.43, p < 0.001, cognitive performance at follow-up, (10) = 402.86, p < 0.001, and change in cognitive performance,(10) = 151.69, p < 0.001, were all statistically significant.
Predictably, the Nagelkerke R2 for the cognitive performance at follow-up was the highest of the three models. These results were to be expected since the dementia criterion was partly based on data collected at follow-up. In line with that, the model also had the lowest AIC value out of the three models (Table 5). Compared to the other models, cognitive performance at follow-up showed a stronger association with whether an individual would fulfill a dementia criterion during the follow-up assessment. More interestingly, based on the AIC values, the model for cognitive performance at baseline was better-fit than the model for change in cognitive performance (Table 5). These results mean that cognitive performance at baseline had a stronger association with whether an individual would fulfill a dementia criterion than change in cognitive performance between baseline and follow-up.
Discussion
This study analyzed which factors were associated with whether a person with normal cognition at baseline would fulfill a dementia criterion at follow-up five years later. Participating in more leisure activities, having a higher education level, and having better self-reported health, were associated with an individual being less likely to fulfill a dementia criterion at follow-up five years later. Having a lower body fat percentage, being an ApoE ε4 carrier, and having less brain volume, were associated with an individual being more likely to fulfill a dementia criterion at follow-up five years later.
It is well established that being an ApoE ε4 carrier is a strong risk factor for developing dementia [38]. It has also been shown that brain volume predicts dementia some years later [39]. The correlation between low body fat percentage and a higher likelihood of developing dementia is somewhat surprising since obesity is generally considered a risk factor for dementia [40]. However, longitudinal analyses suggest that although obesity is a risk factor for dementia, weight loss is common among those in the pre-clinical stages of dementia, which could explain the relationship between body composition and dementia risk in this dataset [41–43].
Cognitive reserve is a concept widely used in the cognitive aging literature. It refers to how the differential vulnerability of cognitive processes to brain aging can, to some extent, be explained by the adaptability of cognitive processes [44]. Proxy measures, usually ones that are easily quantifiable, are commonly used to measure cognitive reserve; these include education level, engagement with cognitively stimulating leisure activities, and occupational status [44–47]. It is generally accepted that having a high cognitive reserve predicts higher cognitive performance and lower dementia risk [47, 48].
The current study's results indicate that being high on cognitive reserve (CR) factors reduced the risk of developing dementia, similar to evidence presented in other studies [49–51]. It should be noted that these studies all emphasize the importance of lifelong engagement with reserve-enhancing activities to mitigate the likelihood of developing dementia. Interestingly, when Dekhtyar et al. [49] compared ApoE ε4 carriers with high and low cognitive reserve, the results showed that those with higher cognitive reserve had a reduced risk of dementia. Similarly, Xu et al. [51] established that high lifespan CR is associated with reduced dementia risk, even among those with high brain pathologies.
Some studies have attempted to gain an understanding of the mechanisms underlying cognitive reserve. The findings of those studies suggest that CR's mediating role in the relationship between cognitive function and brain pathology is delaying the onset of cognitive decline rather than affecting the rate of decline [52, 53]. Those with higher CR generally have higher cognitive performance, delaying the onset of decline. However, once dementia symptom-related decline starts among those with high CR, it happens faster than in those with lower CR.
When these findings regarding cognitive reserve are considered alongside recently published work that used the same dataset to assess the association of known dementia risk factors with cognitive performance, change in cognitive performance, and brain pathology [22], some questions arise. What matters more regarding whether a person will eventually be diagnosed with dementia? Is it the cognitive performance a person has reached or the rate of decline that person experiences? To answer these questions, additional analyses were performed to establish whether cognitive performance or change in cognitive performance would be more strongly related to dementia. The findings showed that cognitive performance at baseline, rather than change in cognition between baseline and follow-up five years later, had a stronger association with dementia at the follow-up assessment. These findings further support the notion that promoting high cognitive reserve throughout the lifespan and reaching high cognitive performance is important in reducing dementia risk.
Interestingly, many factors measured at baseline that had an association with cognitive performance at follow-up (including mobility, physical strength, coronary artery disease, and hypertension) did not have an association with dementia at follow-up [22]. Compared to other known dementia risk factors, CR factors were more likely than others to have an association with dementia. Factors associated with cognitive performance without being associated with brain pathology were those most likely associated with dementia risk. That is probably because brain pathology explains those associations to a great extent. These results further confirm that cognitive reserve factors contribute to dementia risk independently of brain pathology.
Furthermore, risk factors linked to lifestyle diseases (such as hypertension, diabetes, and coronary artery disease) recorded at baseline correlate with brain volume measured five years later at follow-up [22]. Brain volume measured at baseline is also associated with dementia risk recorded at follow-up five years later. However, lifestyle diseases related to brain volume do not relate to dementia risk. It would have been interesting to study whether a relationship would have appeared between these lifestyle diseases and dementia risk if data had been collected for a second follow-up five years after the first follow-up measurement.
The results indicate that managing lifestyle diseases contributes to brain health over an extended period, eventually contributing to dementia risk. It is, however, also possible to contribute to cognitive aging by focusing on cognitive reserve factors through means that are not dependent on brain pathology. Based on this, we have two avenues available to influence cognitive aging that are independent of each other to some extent.
Strengths and limitations
Both strengths and limitations of the study relate to the dataset used for the analyses, as has previously been described [22]. Participants for the AGES-Reykjavik Study dataset were randomly selected from a previous research study, the Reykjavik Study, that included around 30,000 randomly selected individuals that were living in Reykjavik in 1967 [23]. This selection method increases the likelihood that the group of participants chosen for the study represented the population of Icelandic individuals born in the early 1900s rather well. The random selection ensured that individuals that were invited to participate in the study varied in their capabilities, including in the dataset both high and low-functioning individuals. Additionally, the AGES-Reykjavik dataset has a wide variety of information regarding various risk factors for cognitive aging, results from cognitive tests, and brain imaging data. Finally, the size of the dataset and the quality of the assessments performed on the participants give added weight to the study’s findings. The Icelandic population is demographically similar to the other Nordic countries in many ways [23, 54, 55]. People in these countries tend to have access to the same opportunities in life, for example when it comes to educational attainment [56], which adds to the generalizability of the findings.
Although the dataset strengthens the study in many ways, it also contributes to its limitations. Since the data was not collected with this study in mind, some information that might have been useful was not collected. Therefore, information that could have added to the study’s explanatory power, was unavailable. Part of the dataset is also based on self-reported information, which could be a source of inaccuracy. Information about sleep was, for example, collected by asking participants how many hours they slept on an average night. Another limitation is that around half of the participants were excluded from the final data analysis because of the statistical methods used. Given the number of exposure variables used in a single model, this was to be expected and was difficult to avoid. This may have introduced a selection bias since those who completed the data collection could be in better shape than those with missing data. Since the focus of this article is not on the specific scores participants got on the cognitive tests, this was not considered to have a significant effect on the study results.
Finally, even though the differences in performance on cognitive tests were statistically significant for all eight measurements, the mean changes were not always substantial and possibly not of clinical significance. As was pointed out by Salthouse [20], if there is no differential change in performance between measurements, it can prove challenging to use change in cognition to assess associations with other outcomes. That could explain why static measurements of cognitive performance had a stronger link to dementia risk than changes in cognition.
Future research
Based on the results of this study, it would be interesting to gain further insight into the dynamics between cognitive performance, cognitive decline, and dementia risk. A study design where measurements would be performed over a more extended period and taken more than twice during the study period could provide valuable information. Such a design could provide information about whether cognitive change would have a stronger association with dementia risk if the time between measurements were longer.
Another potential avenue for future research would be to design a study investigating the possible causal mechanisms behind the relationship between cognitive reserve factors and dementia risk. Understanding if and how cognitive reserve factors, such as education level and participation in leisure activities, directly contribute to dementia risk could help researchers and policymakers navigate which prevention strategies to focus on. Such a study could, however, prove difficult to perform since that would require young individuals to be randomized into a research group and then monitored for decades. A clever and novel study design that could overcome this hindrance could prove very useful.
Conclusion
The study's results indicated that being high on cognitive reserve factors, such as leisure activity participation, education level, and self-reported health, reduced the risk of developing dementia. Compared to other known dementia risk factors, cognitive reserve factors were more likely than others to be associated with dementia. Additionally, the results of this study suggest that what is more critical concerning whether a person will eventually be diagnosed with dementia is the cognitive performance that person has reached compared to the rate of cognitive decline that person eventually experiences. Together, these findings support the notion that promoting high cognitive reserve throughout the lifespan and reaching high cognitive performance is important in reducing dementia risk.
Funding
This work was supported by The Foundation of St. Josef’s Hospital in cooperation with The Icelandic Gerontological Research Center, National University Hospital of Iceland. The AGES-Reykjavik Study was supported by the National Institutes of Health (Intramural Research Programs of the National Institute of Aging and the National Eye Institute, ZIAEY00401), National Institutes of Health contract number N01-AG-1–2100, the Icelandic Heart Association, and the Icelandic Parliament.
Additional grants were provided by Landspítali – University Hospital Research Fund, the Icelandic Gerontological Society, the Council on Aging in Iceland, Helga Jónsdóttir and Sigurliði Kristjánsson Memorial Fund and the Sustainability Institute and Forum (SIF) at Reykjavik University.
Declarations
Competing interests
The authors declare that there is no conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Global status report on the public health response to dementia. World Health Organization, Geneva, 2021. Accessed: Jul. 26, 2022. [Online]. Available: https://www.who.int/publications-detail-redirect/9789240033245.
- 2.Nichols E, et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7(2):e105–e125. doi: 10.1016/S2468-2667(21)00249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolters FJ, et al. Twenty-seven-year time trends in dementia incidence in Europe and the United States: The Alzheimer Cohorts Consortium. Neurology. 2020;95(5):e519–e531. doi: 10.1212/WNL.0000000000010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker KA, Power MC, Gottesman RF. Defining the relationship between hypertension, cognitive decline, and dementia: A review. Curr Hypertens Rep. 2017;19(3):24. doi: 10.1007/s11906-017-0724-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feinkohl I, Price JF, Strachan MWJ, Frier BM. The impact of diabetes on cognitive decline: potential vascular, metabolic, and psychosocial risk factors. Alzheimer’s Res Therapy. 2015;7(1):46. doi: 10.1186/s13195-015-0130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abete P, et al. Cognitive impairment and cardiovascular diseases in the elderly. A heart–brain continuum hypothesis. Ageing Res Rev. 2014;18:41–52. doi: 10.1016/j.arr.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Nishtala A, et al. Atrial fibrillation and cognitive decline in the Framingham Heart Study. Heart Rhythm. 2018;15(2):166–172. doi: 10.1016/j.hrthm.2017.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaninotto P, Batty GD, Allerhand M, Deary IJ. Cognitive function trajectories and their determinants in older people: 8 years of follow-up in the English Longitudinal Study of Ageing. J Epidemiol Community Health. 2018;72(8):685–694. doi: 10.1136/jech-2017-210116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pugazhenthi S, Qin L, Reddy PH. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2017;1863(5):1037–1045. doi: 10.1016/j.bbadis.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham C, O’Sullivan R, Caserotti P, Tully MA. Consequences of physical inactivity in older adults: A systematic review of reviews and meta-analyses. Scand J Med Sci Sports. 2020;30(5):816–827. doi: 10.1111/sms.13616. [DOI] [PubMed] [Google Scholar]
- 11.Cooper R, et al. Objective measures of physical capability and subsequent health: a systematic review. Age Ageing. 2011;40(1):14–23. doi: 10.1093/ageing/afq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sindi S, et al. Sleep disturbances and dementia risk: A multicenter study. Alzheimer’s Dement. 2018;14(10):1235–1242. doi: 10.1016/j.jalz.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Román GC, Jackson RE, Gadhia R, Román AN, Reis J. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Revue Neurologique. 2019;175(10):724–741. doi: 10.1016/j.neurol.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Casanova R, Saldana S, Lutz MW, Plassman BL, Kuchibhatla M, Hayden KM. Investigating predictors of cognitive decline using machine learning. J Gerontol B Psychol Sci Soc Sci. 2020;75(4):733–742. doi: 10.1093/geronb/gby054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antoniou M. The advantages of bilingualism debate. Annu Rev Linguist. 2019;5(1):395–415. doi: 10.1146/annurev-linguistics-011718-011820. [DOI] [Google Scholar]
- 16.Cheng S-T. Cognitive reserve and the prevention of dementia: The role of physical and cognitive activities. Curr Psychiatry Rep. 2016;18(9):85. doi: 10.1007/s11920-016-0721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiss J, Puterman E, Prather AA, Ware EB, Rehkopf DH. A data-driven prospective study of dementia among older adults in the United States. PLOS ONE. 2020;15(10):e0239994. doi: 10.1371/journal.pone.0239994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livingston G, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritchie SJ, et al. Predictors of ageing-related decline across multiple cognitive functions. Intelligence. 2016;59:115–126. doi: 10.1016/j.intell.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salthouse TA. Correlates of cognitive change. J Exp Psychol Gen. 2014;143(3):1026–1048. doi: 10.1037/a0034847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seblova D, Berggren R, Lövdén M. Education and age-related decline in cognitive performance: Systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev. 2020;58:101005. doi: 10.1016/j.arr.2019.101005. [DOI] [PubMed] [Google Scholar]
- 22.Valsdóttir V, et al. Cognition and brain health among older adults in Iceland: the AGES-Reykjavik study. Geroscience. 2022;44(6):2785–2800. doi: 10.1007/s11357-022-00642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris TB, et al. Age, gene/environment susceptibility–Reykjavik study: Multidisciplinary applied phenomics. Am J Epidemiol. 2007;165(9):1076–1087. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarrey AC, An Y, Kitner-Triolo MH, Ferrucci L, Resnick SM. Sex differences in cognitive trajectories in clinically normal older adults. Psychol Aging. 2016;31(2):166–175. doi: 10.1037/pag0000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh-Manoux A et al. Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. BMJ;344. 2012. 10.1136/bmj.d7622. [DOI] [PMC free article] [PubMed]
- 26.Podsiadlo D, Richardson S. The timed ‘Up & Go’: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 27.Yesavage JA, et al. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 28.Delis DC, Kramer JH, Kaplan E, Ober BA. California verbal learning test manual - Adult version (Research edition) New York: The Psychological Corporation; 1987. [Google Scholar]
- 29.Wechsler DW. WAIS-III: Wechsler adult intelligence scale. Manual. New York: Psychological Corporation; 1955. [Google Scholar]
- 30.Salthouse TA, Babcock RL. Decomposing adult age differences in working memory. Dev Psychol. 1991;27(5):763–776. doi: 10.1037/0012-1649.27.5.763. [DOI] [Google Scholar]
- 31.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18(6):643–662. doi: 10.1037/h0054651. [DOI] [Google Scholar]
- 32.Tabachnick BG, Fidell LS. Using multivariate statistics. 4. Boston: Allyn and Bacon; 2001. [Google Scholar]
- 33.Johnson W, te Nijenhuis J, Bouchard TJ. Still just 1 g: Consistent results from five test batteries. Intelligence. 2008;36(1):81–95. doi: 10.1016/j.intell.2007.06.001. [DOI] [Google Scholar]
- 34.Saczynski JS, et al. Cognitive impairment: An increasingly important complication of type 2 diabetes: The age, gene/environment susceptibility–Reykjavik study. Am J Epidemiol. 2008;168(10):1132–1139. doi: 10.1093/aje/kwn228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 36.Burnham KP, Anderson DR. Multimodel inference: Understanding AIC and BIC in model selection. Sociol Methods Res. 2004;33(2):261–304. doi: 10.1177/0049124104268644. [DOI] [Google Scholar]
- 37.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodological) 1995;57(1):289–300. [Google Scholar]
- 38.Scheltens P, et al. Alzheimer’s disease. Lancet. 2021;397(10284):1577–1590. doi: 10.1016/S0140-6736(20)32205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaser C, Franke K, Klöppel S, Koutsouleris N, Sauer H. BrainAGE in mild cognitive impaired patients: predicting the conversion to Alzheimer’s disease. PLoS One. 2013;8(6):e67346. doi: 10.1371/journal.pone.0067346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization. Risk reduction of cognitive decline and dementia: WHO guidelines. World Health Organization, Geneva, 2019. [Online]. Available: https://apps.who.int/iris/bitstream/handle/10665/312180/9789241550543-eng.pdf?sequence=1&isAllowed=y. Accessed 7 May 2021 [PubMed]
- 41.Kivimäki M, et al. Body mass index and risk of dementia: Analysis of individual-level data from 1.3 million individuals. Alzheimer’s Dement. 2018;14(5):601–609. doi: 10.1016/j.jalz.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedditizi E, Peters R, Beckett N. The risk of overweight/obesity in mid-life and late life for the development of dementia: a systematic review and meta-analysis of longitudinal studies. Age Ageing. 2016;45(1):14–21. doi: 10.1093/ageing/afv151. [DOI] [PubMed] [Google Scholar]
- 43.Singh-Manoux A, et al. Obesity trajectories and risk of dementia: 28 years of follow-up in the Whitehall II Study. Alzheimer’s Dement. 2018;14(2):178–186. doi: 10.1016/j.jalz.2017.06.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stern Y, et al. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer’s Dement. 2020;16(9):1305–1311. doi: 10.1016/j.jalz.2018.07.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Groot C, et al. Differential effects of cognitive reserve and brain reserve on cognition in Alzheimer disease. Neurology. 2018;90(2):e149–e156. doi: 10.1212/WNL.0000000000004802. [DOI] [PubMed] [Google Scholar]
- 46.Opdebeeck C, Martyr A, Clare L. Cognitive reserve and cognitive function in healthy older people: a meta-analysis. Aging Neuropsychol Cogn. 2016;23(1):40–60. doi: 10.1080/13825585.2015.1041450. [DOI] [PubMed] [Google Scholar]
- 47.Pettigrew C, Soldan A. Defining cognitive reserve and implications for cognitive aging. Curr Neurol Neurosci Rep. 2019;19(1):1–12. doi: 10.1007/s11910-019-0917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee DH et al. Effects of cognitive reserve in Alzheimer’s disease and cognitively unimpaired individuals. Front Aging Neurosci. 2022;13. 10.3389/fnagi.2021.784054. [DOI] [PMC free article] [PubMed]
- 49.Dekhtyar S, Marseglia A, Xu W, Darin-Mattsson A, Wang H-X, Fratiglioni L. Genetic risk of dementia mitigated by cognitive reserve: A cohort study. Ann Neurol. 2019;86(1):68–78. doi: 10.1002/ana.25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang H-X, MacDonald SWS, Dekhtyar S, Fratiglioni L. Association of lifelong exposure to cognitive reserve-enhancing factors with dementia risk: A community-based cohort study. PLOS Med. 2017;14(3):e1002251. doi: 10.1371/journal.pmed.1002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu H, et al. Association of lifespan cognitive reserve indicator with dementia risk in the presence of brain pathologies. JAMA Neurol. 2019;76(10):1184–1191. doi: 10.1001/jamaneurol.2019.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Formanek T, Kagstrom A, Winkler P, Cermakova P. Differences in cognitive performance and cognitive decline across European regions: a population-based prospective cohort study. Eur psychiatr. 2019;58:80–86. doi: 10.1016/j.eurpsy.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 53.Soldan A, et al. Cognitive reserve and long-term change in cognition in aging and preclinical Alzheimer’s disease. Neurobiol Aging. 2017;60:164–172. doi: 10.1016/j.neurobiolaging.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jørgensen TSH, et al. Ageing populations in the Nordic countries: Mortality and longevity from 1990 to 2014. Scand J Public Health. 2019;47(6):611–617. doi: 10.1177/1403494818780024. [DOI] [PubMed] [Google Scholar]
- 55.Knudsen AK, et al. Life expectancy and disease burden in the Nordic countries: results from the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Lancet Public Health. 2019;4(12):e658–e669. doi: 10.1016/S2468-2667(19)30224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jalovaara M, et al. Education, gender, and cohort fertility in the Nordic Countries. Eur J Population. 2019;35(3):563–586. doi: 10.1007/s10680-018-9492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]