Abstract
The current Response Evaluation Criteria in Solid Tumors for measuring tumor response in osteosarcoma may be sub-optimal, as even responsive bone tumors may show limited change in tumor diameters. This limits the use of traditional imaging assessment tools. Therefore, discerning osteosarcoma response to therapy on magnetic resonance imaging before surgery is often difficult, and it is typically evaluated after surgery by assessing the amount of necrosis in resected surgical specimens. To address these challenges, sodium fluoride (Na18F) positron emission tomography/computed tomography (PET/CT) scans can be utilized to better image bone response to therapy, as, fluoride is avidly taken up by bone. Na18F Response Criteria in Solid Tumors (NAFCIST) has been developed as a novel method to evaluate treatment response using Na18F PET/CT. Current evidence supporting NAFCIST comes from a pilot study that evaluated alpha particle radium-223 in patients with osteosarcoma. In this review, practical guidance for utilizing NAFCIST in the context of bone tumors is illustrated to aid future studies.
Key words: osteosarcoma, PET/CT, precision oncology, sodium fluoride, bone tumors
Highlights
-
•
Response evaluation by conventional imaging in osteosarcoma is challenging as responding tumors may have limited shrinkage.
-
•
Na18F PET/CT scans offer an opportunity for better evaluation of treatment response given the fluoride avidity in bone.
-
•
NAFCIST have been developed and compare favorability to other response criteria.
Introduction
Osteosarcoma is an aggressive bone tumor with poor clinical outcomes in the relapsed/recurrent setting.1 Although relatively rare, it is the most frequent malignant type of bone tumors.2, 3, 4 Osteosarcoma commonly occurs at a bimodal age distribution in the distal femur, proximal tibia, or proximal humerus.2,3,5,6 Treatment for localized disease classically includes surgery, pre-operative, and possibly post-operative chemotherapy. Recurrence, however, occurs in many patients and dramatically compromises the overall survival.5 The disseminated disease commonly presents with lung metastases and in itself confers a feature of poor prognosis.7,8 Treatment options in the recurrent and metastatic settings are limited although many potential therapies are being explored in early-phase clinical trials.5,9,10 In this concept paper, we elaborate on a novel method that uses sodium fluoride scans to evaluate treatment response and activity of bone-targeted therapies specifically in the context of alpha particle radiopharmaceuticals in osteosarcoma.
Challenges In Radiological Diagnosis
Response assessment has been historically challenging in osteosarcoma given the nature of the disease in the bone. For example, responsive tumors may show necrosis but still a limited change in tumor diameters is observed. This usually limits the use of traditional imaging assessment tools e.g. Response Evaluation Criteria in Solid Tumors (RECIST) that relies on dynamic changes in measurements.11,12 Traditionally, the response to therapy in patients with osteosarcoma has been evaluated with pathological analysis following surgical excision, which might not be feasible in metastatic or recurrent patients with multiple lesions.13 Functional qualitative imaging including [18F]2-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography (FDG–PET/CT), therefore, offers an opportunity for tracking tumor activity in lieu of a diameter-based assessment. Incorporating anatomical size changes and functional activity has the potential to enable better assessment of response to therapeutic options.14,15 FDG–PET/CT-based response criteria have been developed for use in solid tumors, namely PET Response Criteria in Solid tumors (PERCIST).16,17 PERCIST has been suggested in different studies to provide a potentially better assessment of the tumor response status.18, 19, 20, 21
Na18F PET/CT As A Potential Modality for Osteosarcoma
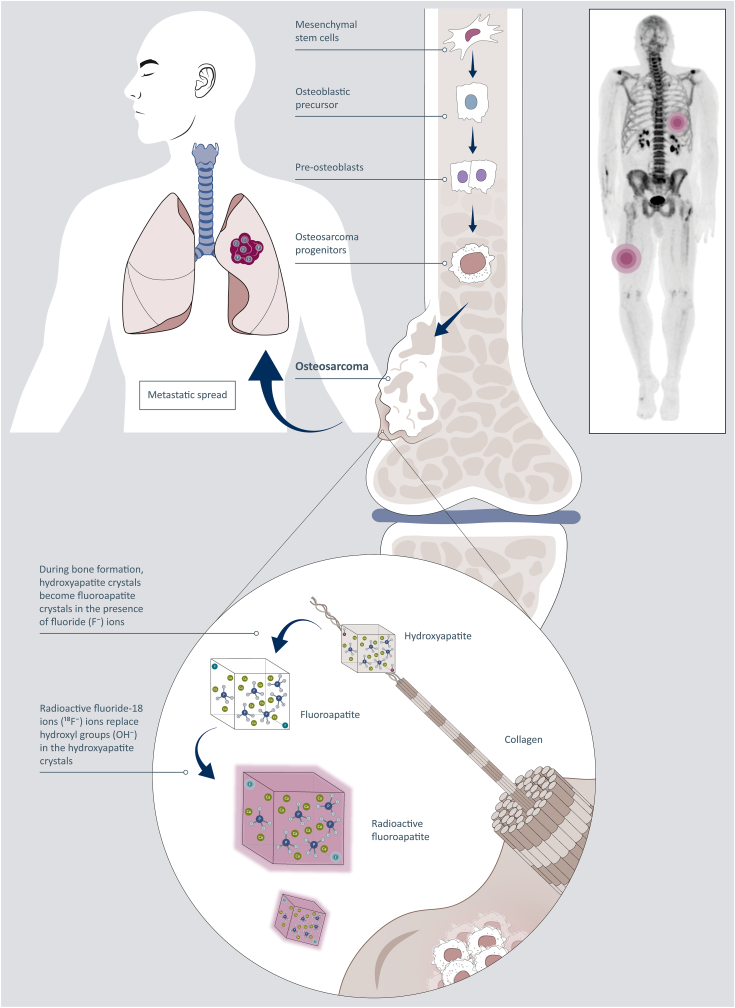
Another analog of FDG-based PET/CT is sodium fluoride (18F-NaF) PET/CT which uses sodium fluoride (18F-NaF) as the radioactive material.12,22 Na18F is an old radiopharmaceutical that was used in gamma camera–based scintigraphy before the introduction of technetium (Tc)-based compounds. It can be used for the assessment of bone metabolism, primarily in the evaluation of malignancy and metastatic disease of the bony skeleton. Na18F uptake is both a reflection of blood flow to the bones and of bone remodeling. A renewed interest in NaF-based PET imaging has evolved over the past few years given its high bone specificity.23 The radiotracer is injected intravenously and once diffused through capillaries feeding the bones where turnover is highest, the 18F is exchanged for a hydroxyl group in the bone mineral hydroxyapatite crystal form Ca10(PO4)6(OH)2, leading to the formation of the 18F-fluoroapatite crystal form Ca10(PO4)6F2 (Figure 1). The same phenomenon occurs in bone formation in soft tissues. This means that soft-tissue metastases of osteosarcoma become fluoride-ion (18F–) avid and can be detected and evaluated using NaF PET/CT. Comparative studies have suggested a better image and higher sensitivity of detection of bony lesions with NaF PET/CT compared to 99mTc-MDP whole-body scan, conventional CT and magnetic resonance imaging images, and FDG–PET/CT.23
Figure 1.
Schematic illustration of the role of sodium-18-fluoride (Na18F)-PET imaging in metastatic osteosarcoma. The fluoride ion (18F−) of Na18F is replacing one hydroxyl group (OH-) in the bone hydroxyapatite forming radioactive fluorapatite which can be imaged with a PET camera. Bone formation is present in soft-tissue metastases of osteosarcoma, and 18F-fluoroapatite in osteoblastic cells will be visualized using a PET camera. Thus Na18F-PET can be used for staging metastatic osteosarcoma because soft-tissue metastases become visible. PET, positron emission tomography.
Development of NaF PET/CT Response Criteria
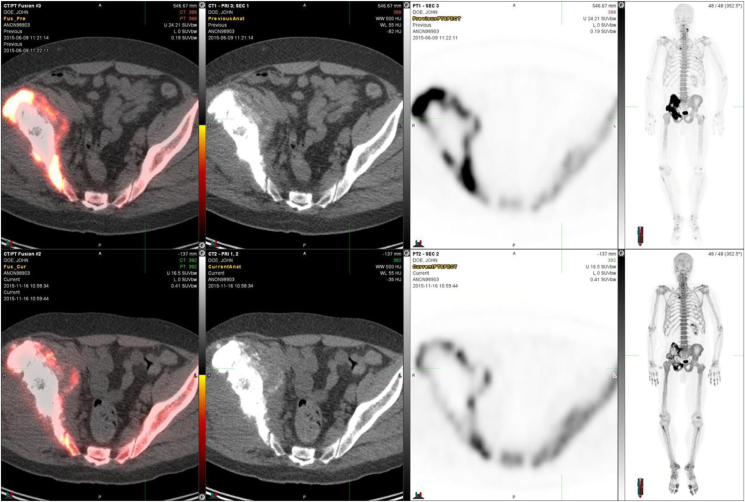
The first NaF PET/CT Response Criteria in Solid Tumors (NAFCIST) were proposed in 2019 to accommodate the need for a newer response assessment tool in osteosarcoma.24 The tool was developed as part of a phase I study of alpha particle therapy (radium-223) in advanced osteosarcoma that included 18 patients.24 In this study, NaF PET/CT identified osteosarcoma lesions in many sites that were not avid on FDG–PET/CT, indicating a potential role for NaF PET/CT in osteosarcoma's baseline evaluation of lesions and response to therapy. Moreover, NaF PET/CT could detect skeletal metastases of osteosarcoma better than other imaging methods.24 Post-treatment pathologic response was evaluated in a patient whose tumor showed extensive tumor necrosis after two doses. More importantly, the NaF PET/CT and FDG–PET/CT scans depicted the lesions more accurately than conventional scans. In fact, the two were often complementary, with NaF PET/CT showing more bony lesions than other modalities. Therefore, a preliminary NAFCIST has been developed similar to PERCIST based on data from patients with osteosarcoma treated in this phase I trial.12 An example is shown in Figure 2 which demonstrates a 63-year-old male patient who had metastatic fibroblastic osteosarcoma and received six cycles of 223RaCl2. The patient demonstrated a metabolic response visible on FDG–PET/CT. This patient had three target lesions measurable on bone scintigraphy and more than five indicator lesions visible on NaF PET/CT. Visual inspection of the bone scintigraphy revealed a reduction in the number and size of lesions in this patient. Quantitatively, this patient had a reduction in standardized uptake values (SUV) per NaF PET/CT (from 96.3 to 37.1 and 26.7) as measured after three cycles and six cycles of 223RaCl2. It is important to highlight that the fluoride ion and radium cation do not form a theragnostic pair similar to that of gallium-68 and lutenium-177 in another setting.25 However, calcium and radium could form a pair and therefore the fluoride ion here can act as a surrogate marker.
Figure 2.
A 63-year-old male diagnosed with metastatic fibroblastic osteosarcoma in the right pelvis with soft-tissue expansion. Quantitatively, NaF-PET-images demonstrated an SUV decrease (96.3 → 37.1 → 26.7) after three and six cycles of 223RaCl2, respectively. A cumulative activity of 50.3 MBq of 223Ra was administered. The upper row shows the left pelvic transaxially PET/CT-fusion image, CT image, PET image, and whole-body maximal intensity projection (MIP) image before any treatment, and the lower-row corresponding images after six cycles of 223RaCl2. CT, computed tomography; NaF, sodium fluoride; PET, positron emission tomography; SUV, standardized uptake values.
NAFCIST Criteria
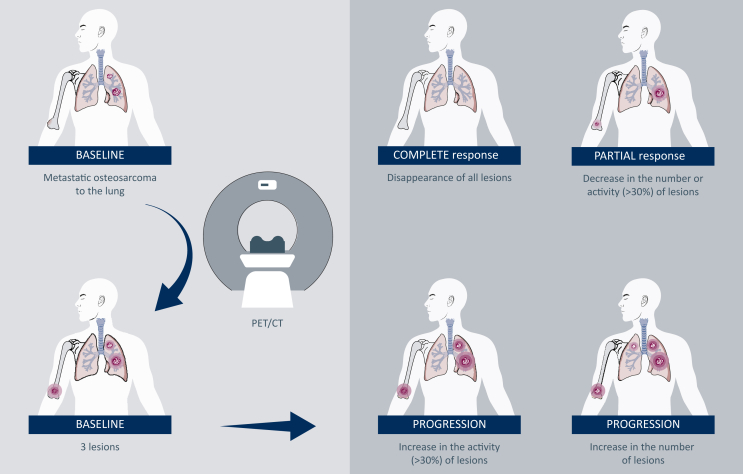
Based on the preliminary data presented in the previous study, NAFCIST criteria (Figure 3) were proposed for the evaluation of osteosarcoma using a 30% cut-off level to demonstrate response or progression which was based on changes in other parameters including biomarkers (Table 1).12 For example, NAFCIST correlated with changes in bone alkaline phosphatase level, a tumor marker in osteosarcoma.
Figure 3.
A proposal for NAFCIST for future studies. New lesions or >30% increase in NAFCIST (sum of five lesions) is a progression. For a response >30%, decrease in NAFCIST is required. NAFCIST, Na18F Response Criteria in Solid Tumors.
Table 1.
NAFCIST criteria
| Response category | Criteria |
|---|---|
| Complete metabolic response | Normalization of all lesions (target and non-target) to SUV less than the mean skeletal SUV and equal to the normal surrounding tissue SUV |
| Partial metabolic response | >30% decrease in SUV peak |
| Progressive metabolic disease | >30% decrease in SUV peak, >75% increase in total Na 18F uptake, or new lesions |
| Stable metabolic disease | Does not meet other criteria |
Adapted from Kairemo et al.12
NAFCIST, Na18F Response Criteria in Solid Tumors; SUV, standardized uptake values.
Interestingly, the study reported that NAFCIST but not PERCIST correlates with overall survival (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2023.101575). Moreover, the NAFCIST change correlated inversely with the cumulated activity, indicating that probably the administrated activity should be higher to obtain a response. There was no correlation between the administrated activity and PERCIST changes.
NAFCIST is maximally an SUV peak sum of five lesions. SUV peak is the average SUV value in 1 cm3 of the most active lesion. Only two lesions per organ are accepted. A typical example is two bone lesions, two lung lesions, and one soft-tissue lesion. During the follow-up, all these lesions may change, but in our practice so far this has not happened. The idea is to pick the five most active lesions and remember to limit the maximum to two lesions per organ.
NAFCIST criteria compare favorably with RECIST and PERCIST criteria in primary bone tumors (Table 2). In fact, NAFCIST could supplant PET/CT functional response by adding metabolic response criteria for bone-forming diseases. Therefore, NAFCIST may represent a more accurate method of categorizing osteosarcoma than RECIST, which mainly relies on unidimensional measurements of tumor lesions and the sum of diameters. At least currently, NAFCIST and PERCIST should be considered complementary to each other.
Table 2.
The new NAFCIST criteria versus RECIST and PERCIST in primary bone tumors
| RECIST | PERCIST | NAFCIST | |
|---|---|---|---|
| Characteristics | Anatomic response criteria for soft-tissue disease | Functional response criteria reflecting tumor glucose metabolism | Metabolic response criteria for bone-forming disease |
| Advantages | Commonly used | Response determination is possible regardless of the location | Response determination is possible regardless of the organ |
| Disadvantages | Limited to ‘measurable’ soft-tissue disease | Limited to FDG avid disease | Limited to NaF avid disease |
FDG, [18F]2-fluoro-2-deoxy-D-glucose; NaF, sodium fluoride; NAFCIST, Na18F Response Criteria in Solid Tumors; PERCIST, Positron Emission Tomography Response Criteria in Solid Tumors.
Limitations
There are several limitations to the current use of NAFCIST. First, data on NAFCIST originate from a single study that needs to be prospectively validated by a larger cooperative group study. Prospective studies with the aim of confirming preliminary data previously presented and comparing NAFCIST to other methods of assessment may be helpful. Second, Na18F scans can have a lot of false positives due to benign diseases which can be substantial in adult patients. Therefore, they may not be used in staging; but rather they may be used as baseline and follow up evaluation for radiopharmaceutical therapy to assess response.
Conclusions
In summary, Na18F PET could become an essential part of osteosarcoma management where Na18F PET and 18F-FDG–PET are complementary. NAFCIST outcomes were consistent with disease characteristics indicated by alkaline phosphatase levels and bone destruction. However, these are preliminary findings that are hypothesis generating. A large-scale, prospective analysis through a cooperative group trial is warranted for the validation of NAFCIST in osteosarcoma. Herein, we provide a framework for using NAFCIST to evaluate the activity of bone-targeted therapy for future studies.
Acknowledgements
VS was an Andrew Sabin Family Foundation Fellow at The University of Texas MD Anderson Cancer Center. VS acknowledges the support of The Jacquelyn A. Brady Fund. VS is supported by a US National Institutes of Health (NIH) grant (no. R01CA242845 and R01CA273168); MD Anderson Cancer Center Department of Investigational Cancer Therapeutics is supported by the Cancer Prevention and Research Institute of Texas (no. RP1100584), the Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy (no. 1U01 CA180964), NCATS (Center for Clinical and Translational Sciences) Grant (no. UL1 TR000371), and the MD Anderson Cancer Center Support Grant (no. P30 CA016672). AW and LB are supported by the Claudia von Schilling Foundation. The included figures were created with BioRender.com.
Disclosure
VS was affiliation with UT MD Anderson Cancer Center when this article was submitted. Currently, VS is affiliated with Sarah Cannon Research Institute, Nashville, TN. When this article was submitted VS reported research funding/grant support for clinical trials from AbbVie, Agensys, Inc., Alfasigma, Altum, Amgen, Bayer, BERG Health, Blueprint Medicines Corporation, Boston Biomedical, Inc., Boston Pharmaceuticals, Celgene Corporation, D3 Bio, Inc., Dragonfly Therapeutics, Inc., Exelixis, Fujifilm, GlaxoSmithKline, Idera Pharmaceuticals, Inc., Incyte Corporation, Inhibrx, Loxo Oncology, MedImmune, MultiVir, Inc., NanoCarrier, Co., National Comprehensive Cancer Network, NCI-CTEP, Northwest Biotherapeutics, Novartis, PharmaMar, Pfizer, Relay Therapeutics, Roche/Genentech, Takeda, Turning Point Therapeutics, UT MD Anderson Cancer Center, and Vegenics Pty Ltd.; travel support from ASCO, ESMO, Helsinn Healthcare, Incyte Corporation, Novartis, and PharmaMar; consultancy/advisory board participation for Helsinn Healthcare, Jazz Pharmaceuticals, Incyte Corporation, Loxo Oncology/Eli Lilly, MedImmune, Novartis, QED Therapeutics, Relay Therapeutics, Daiichi-Sankyo, and R-Pharm US; and other relationship with Medscape. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Kansara M., Teng M.W., Smyth M.J., Thomas D.M. Translational biology of osteosarcoma. Nat Rev Cancer. 2014;14:722–735. doi: 10.1038/nrc3838. [DOI] [PubMed] [Google Scholar]
- 2.Mirabello L., Troisi R.J., Savage S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the surveillance, epidemiology, and end results program. Cancer. 2009;115:1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mirabello L., Troisi R.J., Savage S.A. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer. 2009;125:229–234. doi: 10.1002/ijc.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arndt C.A., Rose P.S., Folpe A.L., Laack N.N. Common musculoskeletal tumors of childhood and adolescence. Mayo Clin Proc. 2012;87:475–487. doi: 10.1016/j.mayocp.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meltzer P.S., Helman L.J. New horizons in the treatment of osteosarcoma. N Engl J Med. 2021;385:2066–2076. doi: 10.1056/NEJMra2103423. [DOI] [PubMed] [Google Scholar]
- 6.Sadykova L.R., Ntekim A.I., Muyangwa-Semenova M., et al. Epidemiology and risk factors of osteosarcoma. Cancer Invest. 2020;38:259–269. doi: 10.1080/07357907.2020.1768401. [DOI] [PubMed] [Google Scholar]
- 7.Smeland S., Bielack S.S., Whelan J., et al. Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur J Cancer. 2019;109:36–50. doi: 10.1016/j.ejca.2018.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song K., Song J., Lin K., et al. Survival analysis of patients with metastatic osteosarcoma: a surveillance, epidemiology, and end results population-based study. Int Orthop. 2019;43:1983–1991. doi: 10.1007/s00264-019-04348-4. [DOI] [PubMed] [Google Scholar]
- 9.Ritter J., Bielack S.S. Osteosarcoma. Ann Oncol. 2010;21(suppl 7):vii320–vii325. doi: 10.1093/annonc/mdq276. [DOI] [PubMed] [Google Scholar]
- 10.Gill J., Gorlick R. Advancing therapy for osteosarcoma. Nat Rev Clin Oncol. 2021;18:609–624. doi: 10.1038/s41571-021-00519-8. [DOI] [PubMed] [Google Scholar]
- 11.Eisenhauer E.A., Therasse P., Bogaerts J., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Kairemo K., Rohren E.M., Anderson P.M., et al. Development of sodium fluoride PET response criteria for solid tumours (NAFCIST) in a clinical trial of radium-223 in osteosarcoma: from RECIST to PERCIST to NAFCIST. ESMO Open. 2019;4 doi: 10.1136/esmoopen-2018-000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosen G., Marcove R.C., Caparros B., Nirenberg A., Kosloff C., Huvos A.G. Primary osteogenic sarcoma: the rationale for preoperative chemotherapy and delayed surgery. Cancer. 1979;43:2163–2177. doi: 10.1002/1097-0142(197906)43:6<2163::aid-cncr2820430602>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 14.Beyer T., Townsend D.W., Brun T., et al. A combined PET/CT scanner for clinical oncology. J Nucl Med. 2000;41:1369–1379. [PubMed] [Google Scholar]
- 15.von Schulthess G.K., Steinert H.C., Hany T.F. Integrated PET/CT: current applications and future directions. Radiology. 2006;238:405–422. doi: 10.1148/radiol.2382041977. [DOI] [PubMed] [Google Scholar]
- 16.Wahl R.L., Jacene H., Kasamon Y., Lodge M.A. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(suppl 1):122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joo Hyun O., Lodge M.A., Wahl R.L. Practical PERCIST: a simplified guide to PET response criteria in solid tumors 1.0. Radiology. 2016;280:576–584. doi: 10.1148/radiol.2016142043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Min S.J., Jang H.J., Kim J.H. Comparison of the RECIST and PERCIST criteria in solid tumors: a pooled analysis and review. Oncotarget. 2016;7:27848–27854. doi: 10.18632/oncotarget.8425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joo Hyun O., Wahl R.L. PERCIST in perspective. Nucl Med Mol Imaging. 2018;52:1–4. doi: 10.1007/s13139-017-0507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riedl C.C., Pinker K., Ulaner G.A., et al. Comparison of FDG-PET/CT and contrast-enhanced CT for monitoring therapy response in patients with metastatic breast cancer. Eur J Nucl Med Mol Imaging. 2017;44:1428–1437. doi: 10.1007/s00259-017-3703-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shang J., Ling X., Zhang L., et al. Comparison of RECIST, EORTC criteria and PERCIST for evaluation of early response to chemotherapy in patients with non-small-cell lung cancer. Eur J Nucl Med Mol Imaging. 2016;43:1945–1953. doi: 10.1007/s00259-016-3420-7. [DOI] [PubMed] [Google Scholar]
- 22.Kairemo K., Roszik J., Anderson P., et al. 18F-sodium fluoride positron emission tomography (NaF-18-PET/CT) radiomic signatures to evaluate responses to alpha-particle Radium-223 dichloride therapy in osteosarcoma metastases. Curr Probl Cancer. 2021;45 doi: 10.1016/j.currproblcancer.2021.100797. [DOI] [PubMed] [Google Scholar]
- 23.Ahuja K., Sotoudeh H., Galgano S.J., et al. 18F-sodium fluoride PET: history, technical feasibility, mechanism of action, normal biodistribution, and diagnostic performance in bone metastasis detection compared with other imaging modalities. J Nucl Med Technol. 2020;48:9–16. doi: 10.2967/jnmt.119.234336. [DOI] [PubMed] [Google Scholar]
- 24.Subbiah V., Anderson P.M., Kairemo K., et al. Alpha particle radium 223 dichloride in high-risk osteosarcoma: a phase I dose escalation trial. Clin Cancer Res. 2019;25:3802–3810. doi: 10.1158/1078-0432.CCR-18-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee B.S., Kim M.H., Chu S.Y., et al. Improving theranostic gallium-68/Lutetium-177-labeled PSMA inhibitors with an albumin binder for prostate cancer. Mol Cancer Ther. 2021;20:2410–2419. doi: 10.1158/1535-7163.MCT-21-0251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.