ABSTRACT
Over the past decade, endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) has become an indispensable tool in the diagnostic armamentarium of the pulmonologist. As the expertise with EBUS-TBNA has evolved and several innovations have occurred, the indications for its use have expanded. However, several aspects of EBUS-TBNA are still not standardized. Hence, evidence-based guidelines are needed to optimize the diagnostic yield and safety of EBUS-TBNA. For this purpose, a working group of experts from India was constituted. A detailed and systematic search was performed to extract relevant literature pertaining to various aspects of EBUS-TBNA. The modified GRADE system was used for evaluating the level of evidence and assigning the strength of recommendations. The final recommendations were framed with the consensus of the working group after several rounds of online discussions and a two-day in-person meeting. These guidelines provide evidence-based recommendations encompassing indications of EBUS-TBNA, pre-procedure evaluation, sedation and anesthesia, technical and procedural aspects, sample processing, EBUS-TBNA in special situations, and training for EBUS-TBNA.
KEY WORDS: EBUS-TBNA, guidelines, recommendations
EXECUTIVE SUMMARY
Pre-Procedure Evaluation, Sedation, Anesthesia, and Oxygen Administration during EBUS-TBNA
ANTIPLATELET AND ANTICOAGULANT THERAPY AND PRE-PROCEDURE COAGULATION TESTING
Aspirin may be safely continued in patients undergoing EBUS-TBNA (3A)
In patients undergoing EBUS-TBNA, Clopidogrel may be continued peri-procedurally in patients in whom the risk of thrombosis outweighs the risk of bleeding (3A)
Anti-coagulants should be discontinued before EBUS-TBNA for the duration individually recommended for each agent. (UPP)
Among patients undergoing EBUS-TBNA, routine pre-procedure testing for coagulation function and platelet count is not recommended. (UPP)
Pre-procedure testing for coagulation function and platelet counts may be performed in patients at high-risk of bleeding (anticoagulant therapy, liver disease, chronic kidney disease, bleeding diathesis, or history of active bleeding). (UPP)
ANTIBIOTIC PROPHYLAXIS
Prophylactic antibiotics for EBUS-TBNA are not recommended. (2A)
SEDATION AND ANESTHESIA
It is suggested that sedation be administered for patients undergoing EBUS-TBNA. (UPP)
A pre-procedural assessment to anticipate difficult airway should be performed using an airway history and a focussed physical examination. (UPP)
EBUS-TBNA should be performed using either moderate sedation or deep sedation/general anesthesia. (1A)
EBUS-TBNA can be performed using either bronchoscopist-directed moderate sedation or anesthesiologist-directed deep sedation/general anesthesia. (2B)
While performing EBUS-TBNA, the depth of sedation should be objectively monitored by a designated member of the team. (UPP)
For all patients undergoing EBUS-TBNA under moderate sedation, non-invasive blood pressure, oxygen saturation, heart rate and respiratory rate should be monitored throughout the procedure. (UPP)
Among patients with suspected or known cardiac disease, electrocardiographic monitoring should be additionally done during the EBUS-TBNA procedure. (UPP)
For patients undergoing EBUS-TBNA under deep sedation/general anesthesia, standard ASA monitoring protocols should be followed as feasible. (UPP)
A combination of sedative agents is preferred over a single agent alone, when performing EBUS-TBNA under moderate sedation. (UPP)
The choice of sedative agent(s) can be determined by operator preference, availability, and monitoring facilities. (UPP)
Use of propofol for sedation should preferably be supervised by an anaesthesiologist. (UPP)
Supraglottic airway device is the preferred conduit when performing EBUS-TBNA under general anesthesia. (2A)
For patients undergoing EBUS-TBNA under moderate sedation, cricothyroid lignocaine injection may be preferred over spray-as-you-go technique for topical anesthesia. (1B)
While using the spray-as-you-go technique, 1% lignocaine should be preferred for topical anaesthesia. (1A)
OXYGEN ADMINISTRATION
It is suggested to use oxygen supplementation via common oxygen delivery devices (nasal prongs/nasopharyngeal catheter), along with pulse oximetry monitoring as a routine practice in patients undergoing EBUS-TBNA. (UPP)
II. Technical and Procedural Aspects of EBUS-TBNA
NEED FOR FLEXIBLE BRONCHOSCOPIC EXAMINATION BEFORE EBUS
Based on operator judgement, a flexible bronchoscopy for airway assessment may be performed prior to the EBUS procedure. (UPP)
ROUTE OF INSERTION
It is preferable to use the oral route for EBUS scope insertion. (1A)
When the oral route is not feasible, using the nasal route for EBUS scope insertion is acceptable if performed by experienced operators. (UPP)
SONOGRAPHIC NODAL CHARACTERISTICS AND ELASTOGRAPHY
Endosonographic lymph nodal characteristics do not preclude nodal aspiration for diagnosis of mediastinal adenopathy. (3A)
Elastography is not recommended to differentiate malignant from benign mediastinal lymph nodes except for research purposes. (2A)
EBUS elastography does not obviate the need for tissue sampling. (3A)
ENDOSCOPIC ULTRASOUND WITH BRONCHOSCOPE-GUIDED FINE NEEDLE ASPIRATION (EUS-B-FNA)
EBUS-TBNA is the preferred initial modality in most patients to sample accessible mediastinal lymph nodes. (1A)
In patients with inaccessible or difficult to access lymph nodes by EBUS-TBNA, addition of EUS-B-FNA to EBUS-TBNA is recommended for diagnosis of malignant nodal involvement and optimal staging in patients with lung cancer. (1A)
EUS-B-FNA is recommended in special situations such as lesions inaccessible by EBUS-TBNA, technical difficulty of EBUS-TBNA, mediastinal lymph node sampling in pediatric patients, intolerance of bronchoscopy, or airway compromise. (3A)
EUS-B-FNA should only be performed by experienced EBUS operators after dedicated training. (UPP)
NEEDLE CHARACTERISTICS
In patients undergoing EBUS-TBNA, we recommend that either 21G or 22G may be used. (1A)
The group did not make any recommendation for or against the use of 19G, 25G, ProCore and Franseen tip needles. The choice of the needle is based upon the operator’s discretion. (UPP)
In patients undergoing EBUS-TBNA, the use of a new needle is preferable. (3A)
USE OF SUCTION AND STYLET
EBUS-TBNA may be performed with or without the application of vacuum suction. (1A)
EBUS-TBNA may be performed with or without using a stylet. (1B)
Stylet maybe used for capillary effect if vacuum suction is not being applied. (UPP)
USE OF BALLOON OVER ULTRASOUND PROBE
Balloon should be routinely attached during EBUS-TBNA. Inflation of balloon prior to needle puncture may be performed as per operator requirement. (UPP)
NUMBER OF NEEDLE PASSES
At least 3 passes per lymph node sampled should be obtained for all patients during EBUS-TBNA. (UPP)
At least 3 passes per lymph node station sampled should be done for staging of NSCLC using EBUS-TBNA. (2A)
At least 4 passes are advised for diagnostic testing and molecular profiling in patients suspected of having lung cancer. (2A)
In patient suspected to have granulomatous mediastinal lymphadenopathy, additional samples should be sent for microbiological molecular analysis. (UPP)
NUMBER OF NEEDLE AGITATIONS
At least 10 agitations per pass should be performed for obtaining adequate samples. (UPP)
PREVENTION OF COMPLICATIONS
General principles of asepsis should be rigorously followed. (UPP)
Stylet and needle should be handled in a sterile manner especially when multiple passes are obtained. (UPP)
During introduction and removal of needle into and from channel, retraction of needle into sheath should be visually confirmed to prevent scope damage. Needle should be locked properly. (UPP)
In known asthmatics, bronchodilator administration immediately prior to the procedure may help prevent bronchospasm during the procedure. (UPP)
Sampling should be avoided from densely calcified lymph nodes to avoid inadvertent needle breakage. (UPP)
Excessive force should be avoided during needle insertion; in this situation, the puncture site should be slightly changed. (UPP)
III. Sample Processing and Rapid On-Site Evaluation
CELL BLOCK PREPARATION
Cell blocks should be made routinely in addition to direct slide smears while obtaining samples during EBUS-TBNA. (2A)
RAPID ON-SITE EVALUATION (ROSE)
EBUS-TBNA can be performed irrespective of availability of ROSE. (1A)
ROSE should be performed where available. (2A)
Pulmonologist adequately trained in cytopathology, may perform ROSE when ROSE by pathologist is not feasible. (3A)
IV. EBUS-TBNA in Special Situations
EBUS-TBNA FOR NON-NODAL PATHOLOGIES
Considering the high complication rates, it is preferable to avoid EBUS-guided aspiration of mediastinal bronchogenic cyst. (3B)
A diagnostic aspiration may be considered only when alternative modes of diagnosis are either not feasible or have been unsuccessful. (UPP)
EBUS-guided pericardial fluid aspiration should not be performed routinely. (UPP)
EBUS-TBNA may be used to evaluate intra-thoracic thyroid lesions, particularly in cases where percutaneous image-guided needle aspiration is risky and not feasible. (3B)
ENDOBRONCHIAL ULTRASOUND-GUIDED TRANSVASCULAR NEEDLE ASPIRATION (EBUS-TVNA) AND TRANSBRONCHIAL INTRAVASCULAR NEEDLE ASPIRATION (EBUS-TIVNA)
EBUS-TVNA and EBUS-TIVNA may be considered only when alternative modes of diagnosis are either not feasible or have been unsuccessful. (UPP)
EBUS-TVNA and EBUS-TIVNA should only be performed by an experienced operator for difficult-to-approach lesions after risk-benefit analysis on a case-to-case basis, and patients should be closely monitored for long-term complications. (3A)
Rapid on-site evaluation (ROSE) should be performed in all cases of EBUS-TVNA to minimize the number of needle passes. (UPP)
ENDOBRONCHIAL ULTRASOUND-GUIDED INTRA-NODAL FORCEPS BIOPSY (EBUS-IFB)
EBUS- IFB may be performed only by an experienced operator as an additional modality for patients with negative rapid-on-site evaluation (ROSE) or in situations when tissue biopsy is required for definitive diagnosis (e.g. lymphoma).(2B)
EBUS-IFB may also be considered in patients with prior non-diagnostic EBUS-TBNA. (UPP)
ENDOBRONCHIAL ULTRASOUND-GUIDED MEDIASTINAL CRYOBIOPSY
EBUS-guided mediastinal cryobiopsy should be performed by an experienced operator, in patients with negative rapid-on-site evaluation (ROSE), or prior non-diagnostic EBUS-TBNA. (UPP)
EBUS-TBNA AND EUS-B-FNA IN CHILDREN
EBUS-TBNA and EUS-B-FNA can be performed by experienced operators for sampling mediastinal lymph nodes in children. (3A)
V. TRAINING IN EBUS-TBNA
At least 40 procedures should be performed over a minimum of 2 years to overcome the initial learning curve and achieve acceptable yield in EBUS-TBNA. (2A)
For the training program in EBUS-TBNA, a virtual reality simulator may be incorporated into the traditional apprenticeship model, wherever feasible. (3B)
INTRODUCTION
The advent of the endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) has revolutionized the practice of respiratory medicine. Since its inception, this technique has rapidly become an indispensable tool as part of the bronchoscopist’s diagnostic armamentarium. As expertise with EBUS-TBNA evolved, indications of its use also expanded and several innovations occurred, especially related to needle sizes, visual optics, and accessories. However, several aspects of EBUS-TBNA are still not standardized. Since the diagnostic yield of EBUS-TBNA depends on several patient-related, equipment-related or operator-related factors, it is important to have uniform guidelines regarding the optimal performance of EBUS-TBNA. With this aim in mind, the Department of Pulmonary, Critical Care and Sleep Medicine at the All India Institute of Medical Sciences (AIIMS Delhi), New Delhi collaborated with the Indian Chest Society (ICS), and Indian Association for Bronchology (IAB) to form a working group to develop guidelines for EBUS-TBNA.
Objective of the guideline
The objective is to provide evidence-based information regarding various aspects of EBUS-TBNA, including the procedural and sampling techniques, anesthesia and sedation, sample preparation, EBUS-TBNA in special situations, and training requirements.
Scope of the guideline
This guideline was formulated following a detailed and systematic search to extract relevant literature pertaining to various aspects of EBUS-TBNA. Topics covered in the guideline include:
Indications, complications, and contraindications of EBUS-TBNA procedure
Sedation, premedication, and anesthesia for EBUS-TBNA
Technical aspects during the EBUS-TBNA procedure: including equipment-related and operator-related aspects
Specimen preparation methods
EBUS-TBNA in special situations
Training requirements for EBUS-TBNA.
Target audience of the guideline
This guideline is aimed to serve as a reference guide for respiratory physicians globally. The objective is to provide evidence-based information regarding all aspects of EBUS-TBNA, including the procedural and sampling techniques, anesthesia and sedation, sample preparation, EBUS in special situations, and training requirements.
METHODOLOGY
This guideline was formulated after detailed appraisal of the available evidence following extensive literature search. At the outset, relevant questions were formulated after several rounds of discussions among the working group. Systematic searches were performed in the electronic databases (PubMed, EmBase, and Cochrane) to identify potentially relevant studies. Existing guidelines or recommendations made by other major international associations in respiratory medicine were also reviewed in detail.
The literature search process began in July 2021 and continued up to November 2021. Monthly, e-mail alerts were sent to identify newly published literature. The search strategy included a combination of MeSH terms and free text terms and only included publications in English. All screened articles were categorized as (1) relevant, (2) possibly relevant, or (3) not relevant to the question being researched. All articles were categorized according to the topics listed above in the “Scope of the guidelines.” Initially, a detailed appraisal of the evidence was performed by at least five members of the working group, wherein studies were further selected based on relevance and a provisional grading of the recommendations was formulated. The modified GRADE system was used to classify the quality of available evidence as 1, 2, 3, or usual practice point (UPP: defined as important practical points lacking research evidence) [Table 1].[1] The strength of recommendation was made after considering factors such as: available volume of evidence, consistency in the evidence, applicability and generalizability to the target population, and practicality for implementation.
Table 1.
Quality of Evidence and Strength of Recommendations
| Quality of Evidence | Level |
|---|---|
| Evidence from >1 well-conducted RCT or meta-analysis of RCTs | 1 |
| Evidence from at least 1 moderate quality RCT, or well-designed trial without randomization, or cohort/case-control studies | 2 |
| Evidence from descriptive studies, reports of expert committees, or opinion of respected authorities based on clinical experience | 3 |
| Not backed by sufficient evidence, however, consensus reached by working group based on clinical experience and expertise | UPP |
|
| |
| Strength of Recommendations | Grade |
|
| |
| Strong recommendation to do (or not to) where the benefits clearly outweigh the risks (or vice versa) for most, if not all patients | A |
| Weak recommendation, where the benefits and risks are more closely balanced or are more uncertain | B |
Subsequently, the relevant articles were mailed to all group members for appraisal and inputs. Regular e-mail communications were continued, and the suggestions received were incorporated and the grading revised accordingly. For questions, where evidence was lacking, expert opinions were used to formulate consensus recommendations.
A two-day meeting was held in April 2022 at AIIMS, New Delhi wherein the expert committee members participated. Initially, the evidence pertaining to each question was presented in detail to the members separately in four parallel break-away groups moderated by a group chair and recorded by rapporteurs. All recommendations were formulated based on the level of evidence as well as the consensus opinion of the group members. After discussions and appropriate modifications, the recommendations were presented to the joint working group and finalized by consensus.
A draft guideline document was circulated to all group members and their suggestions were incorporated after online discussions. It was also proposed to revise and update the guidelines after five years.
SECTION I: INDICATIONS AND CONTRAINDICATIONS FOR EBUS-TBNA
What are the various indications and contraindications for EBUS-TBNA?
The mediastinum is the anatomical compartment that runs the length of the thoracic cavity between the pleural sacs. This compartment extends longitudinally from the thoracic inlet to the superior surface of the diaphragm and contains several structures including the heart, great vessels, trachea, esophagus, thymus, lymphatics, and nerves. The mediastinum is also clinically significant due to the variety of physical anomalies and pathologies that can occur in this region. Hence, various modalities have been devised to enable tissue sampling from mediastinal masses and lymph nodes.
Traditionally, bronchoscopy was performed to obtain transbronchial nodal aspirates without ultrasound guidance and this procedure is referred to as conventional transbronchial needle aspiration (c-TBNA). Endobronchial ultrasound (EBUS) is a bronchoscopic technique which enables ultrasonographic examination of the airway wall, mediastinal structures, and lungs. Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is now a widely accepted, minimally invasive procedure for tissue sampling of mediastinal lesions. The target lesions should be within the sonographic range of the trachea and the proximal bronchi. EBUS-TBNA has been found to have a superior diagnostic yield compared with c-TBNA for mediastinal lymphadenopathy of benign (77% vs 61%) and malignant (61% vs 55%) etiology.[2,3]
The use of the EBUS scope via the esophageal route has also been described and is referred to as transesophageal bronchoscopic ultrasound-guided fine needle aspiration (EUS-B-FNA).
The mediastinal lymph nodes have been anatomically demarcated into defined stations by the International Association for the Study of Lung Cancer [Figure 1].[4] The lymph node stations which are accessible by EBUS-TBNA include the upper and lower paratracheal stations (stations 2 and 4), subcarinal (station 7), hilar on either side (station 10), and interlobar (stations 11 and 12) on either side. The lymph node stations which are accessible by EUS-B-FNA include left paratracheal (stations 2L and 4L), prevertebral (station 3P), subcarinal (station 7), para-esophageal (station 8), and pulmonary ligament (station 9).The right lower paratracheal station (4R) is usually not accessible via the esophageal approach unless significantly enlarged. The subaortic (station 5) and para-aortic (station 6) lymph nodes are conventionally inaccessible to both approaches and may require a trans-vascular sampling approach.
Figure 1.
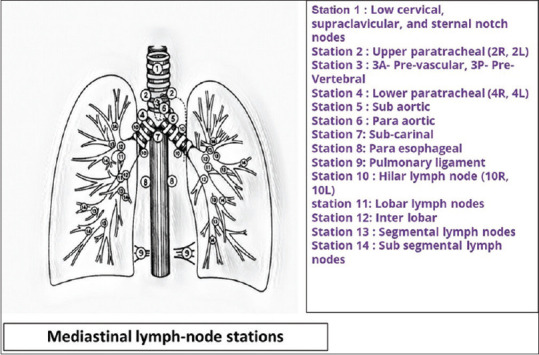
International Association for the Study of Lung Cancer (IASLC) nomenclature for mediastinal lymph node stations
Indications for EBUS-TBNA
The indications of EBUS-TBNA are summarized in Table 2. In general, EBUS-TBNA should be used for the sampling of mediastinal lesions within the sonographic range of the tracheo-bronchial tree when simpler and less invasive methods of diagnosis are not feasible. The evidence regarding the utility of EBUS-TBNA in various indications is discussed below.
Table 2.
Indications for EBUS-TBNA
| Category | Indications |
|---|---|
| Diagnostic (common) | Suspected Granulomatous lymphadenopathy Tuberculosis Sarcoidosis Diagnosis and Staging of Lung cancer (central masses and mediastinal lymphadenopathy) Diagnosis of Lymphoma Evaluation of mediastinal lymphadenopathy in patients with extra-thoracic malignancies |
| Diagnostic (uncommon) | Aspiration of thyroid lesions Sampling of left adrenal gland lesions Mediastinal lymphadenopathy in children |
| Therapeutic | Drainage of mediastinal cystic lesions Transbronchial needle injection (EBUS-TBNI) of chemotherapeutic agents |
| Special situations* | Aspiratio Intravascular (IVNA) and Trans-vascular sampling of mediastinal lesions (TVNA) Mediastinal pleural nodules/thickening Pre/para vertebral soft tissue lesions Pericardial effusion Intra-cardiac mass Visualization Pulmonary embolism |
*EBUS TBNA can be a consideration for these indications when other modalities are not feasible
MEDIASTINAL LYMPH NODE TUBERCULOSIS
Several studies have examined the diagnostic yield of EBUS-TBNA for tuberculosis. A meta-analysis by Li et al.,[5] including 14 studies (684 patients) with suspected tubercular mediastinal lymphadenopathy found a pooled diagnostic yield of EBUS-TBNA of 80% (95% CI,74%-86%). Another study by Dhasmana et al.,[6] wherein EBUS-TBNA samples were subjected for GeneXpert for detecting TB, reported a sensitivity of 72.6% (95% CI, 62%-81%) and a specificity of 96.3% (95% CI, 89.1%-99.1%) in culture positive patients. The combination of GeneXpert with cytology increased the sensitivity to 96.6%. In the Indian setting, a combination of GeneXpert and culture with phenotypic drug sensitivity testing on samples obtained using EBUS-TBNA has been shown to facilitate detection of drug-resistant tuberculosis.[7]
SARCOIDOSIS
A meta-analysis by Agarwal et al.[8] which included 15 studies with 553 patients reported a diagnostic yield of EBUS-TBNA for sarcoidosis ranging from 54% to 93% with a pooled diagnostic yield of 79% (95% CI; 71-86%). This is higher than the previously reported yield of cTBNA which was found to be 62% (95% CI; 52 – 71%).[9] Furthermore, von Bartheld et al.[10] demonstrated in a randomized controlled trial that EBUS-TBNA provides higher diagnostic yield for sarcoidosis compared with conventional bronchoscopic biopsies (80% vs 53%; P < 0.001). However, it has also been reported that adding transbronchial lung biopsy (TBLB) to EBUS-TBNA results in a significantly higher diagnostic yield than EBUS-TBNA alone.[11]
LUNG CANCER
A meta-analysis which included 5 studies with 532 patients of confirmed non-small cell lung cancer (NSCLC) reported no significant difference in outcomes between EBUS-TBNA and mediastinoscopy for staging of lung cancer.[12] In another meta-analysis of mediastinal staging comprising 1914 pre-operative lung cancer patients, the pooled sensitivities for EBUS-TBNA and mediastinoscopy were 0.84 (95% CI, 0.79–0.88) and 0.86 (95% CI, 0.82–0.90), respectively. There were fewer complications in the EBUS-TBNA arm.[13]
LYMPHOMA
EBUS-TBNA has proved less effective for diagnosing lymphoma as histopathological characterization of lymph nodal architecture is suboptimal. A meta-analysis of 14 studies with 425 patients of suspected lymphoma reported overall sensitivity of 66.2% (95% CI, 55%–75.8%) and specificity of 99.3% (95% CI, 98.2%–99.7%).The sensitivity and specificity were higher for diagnosing recurrent lymphoma than de novo lymphoma.[14]
OTHER INDICATIONS
For patients with extra-thoracic malignancies with metastasis to mediastinal lymph nodes, the diagnostic yield of EBUS-TBNA ranges from 78.8% to 79%.[15,16] Less common uses of EBUS include detection of intra-cardiac masses, pulmonary embolism, and pulmonary artery hydatid cysts; drainage of infected mediastinal cyst; transbronchial needle injection (TBNI) of chemotherapy; and measuring airway wall thickness in tracheobronchomalacia and chronic asthma.[17,18,19,20,21,22]
CONTRAINDICATIONS FOR EBUS-TBNA
Contraindications to EBUS-TBNA are similar to those for bronchoscopy, hence these are extrapolated from the existing flexible bronchoscopy guidelines.[23] Table 3 summarizes the absolute and relative contraindications for EBUS-TBNA.
Table 3.
Contraindications for EBUS-TBNA
| Absolute Contraindications | Relative Contraindications |
|---|---|
| Lack of informed consent Uncorrectable severe bleeding diathesis Unstable cardiac arrhythmias Recent MI (<4 weeks) or ongoing unstable angina Benign/malignant stenosis of upper airway (supra-glottic or glottic) | Refractory hypoxemia* (SpO2<90% or PaO2<60 mm Hg with ≥6 L oxygen) or respiratory failure. EUS-B-FNA may be considered as an alternative for accessible lesions Uncontrolled severe hypertension** Narrow airways relative to larger size of the scope (e.g., subglottic stenosis, central airway obstruction, children younger than 5 years). However, in these situations EUS-B-FNA may be considered for accessible lesions. |
*Procedure may be performed after a risk-benefit analysis, where there are skilled operators and anaesthesiologist backup available. **Severe asymptomatic HTN is defined as SBP >180 mm Hg and/or DBP >110 mm Hg. Patient may be taken up for procedure after optimization of blood pressure
Evidence summary
The pooled diagnostic yield of EBUS-TBNA for mediastinal lymph node tuberculosis is 80%. When GeneXpert is performed on EBUS-TBNA samples, the sensitivity is 72.6% (95% CI, 62%-81%) and the specificity is 96.3% (95% CI, 89.1%-99.1%) in culture positive patients. The combination of GeneXpert with cytology increases sensitivity to 96.6%.
The diagnostic yield of EBUS-TBNA for sarcoidosis ranges from 54 to 93% and is superior to c-TBNA and transbronchial lung biopsy alone. The addition of transbronchial lung biopsy and endobronchial biopsy to EBUS-TBNA increases overall diagnostic yield.
EBUS-TBNA has similar diagnostic performance as mediastinoscopy for mediastinal staging in lung cancer. However, EBUS-TBNA is a less invasive modality compared to mediastinoscopy.
EBUS-TBNA has a sensitivity of 66.2% and an excellent specificity of 99% for the diagnosis of lymphoma. The yield of EBUS-TBNA for the diagnosis and subtyping of lymphoma is better in cases of recurrent than de-novo disease.
EBUS-TBNA is an effective modality for differentiating between benign and malignant causes of mediastinal lymphadenopathy among patients with extra-thoracic malignancy.
SECTION II: PRE-PROCEDURE EVALUATION
Should antiplatelet and anticoagulant therapy be discontinued before EBUS-TBNA?
The evidence on cessation of antiplatelets and anti-coagulants before bronchoscopy is primarily extrapolated from the recommendations for gastro-duodenoscopy and colonoscopy by the European Society of Gastrointestinal Endoscopy (ESGE) guidelines[24] and the British Society of Gastroenterologists,[25] and the recommendations for peri-operative management of anti-thrombotic agents by the American College of Chest Physicians.[26] The British Thoracic Society[27] and the ICS/NCCP/IAB guidelines[23] for diagnostic flexible bronchoscopy in adults recommend discontinuing warfarin 5 days before, and direct oral anticoagulants (DOACs) 2 days before bronchoscopic biopsy (endobronchial or transbronchial).They also recommend that clopidogrel be discontinued 7 days before bronchoscopic biopsy (five days as per the ICS/NCCP/IAB guidelines) in patients with low-risk cardiac conditions. Low-dose aspirin can be safely continued and should be started if clopidogrel is being stopped. In patients with cardiac conditions with high risk of thrombosis (e.g., coronary stent in situ), a cardiology opinion should be sought regarding the discontinuation of clopidogrel, and procedural decisions should be taken after careful risk-benefit consideration. Similarly, in patients with high-risk cardiac conditions on anticoagulation, a cardiology opinion should be obtained, and low molecular weight heparin (LMWH) should be started 2 days after stopping warfarin. The last dose of LMWH should be administered 24 hours before the procedure.
Evidence for discontinuing antiplatelets and anti-coagulants prior to EBUS is sparse. In a prospective observational study of 42 patients without known coagulopathy and using dual antiplatelet therapy (clopidogrel and aspirin), no bleeding or hemoptysis was observed during 24 hours after EBUS-TBNA.[28] Another retrospective study found no significant acute or delayed bleeding complications after EBUS-TBNA or EUS-FNA in patients using clopidogrel compared to those who were not on clopidogrel.[29] Similarly, during a retrospective analysis of 12 EBUS-TBNA procedures performed[30] on patients taking clopidogrel, no significant bleeding complication was noted.
The optimum duration for which antiplatelets and anticoagulants should be discontinued is also unclear. Gil et al.[31] reported that the risk of bleeding complications was similar irrespective of whether the antiplatelets and anticoagulants were discontinued within or beyond 5 days of the EBUS-TBNA procedure. Herman et al.[32] systematically reviewed the risk of bleeding in patients undergoing pulmonary procedures while on antiplatelet or anticoagulants, and concluded that the safety of patients undergoing EBUS-TBNA, while receiving clopidogrel or dual antiplatelet therapy (DAPT) cannot be established due to inconsistent and inconclusive evidence from the available studies.
In another retrospective subgroup analysis of an EBUS-TBNA registry that included 148 patients on clopidogrel or prasugrel, a higher occurrence of minor procedural bleeding (25%) was observed in patients who discontinued clopidogrel <5 days prior to procedure compared to patients who had discontinued antiplatelet >5 days before the procedure (5.3%). Among the latter group, two patients experienced post-procedural non-ST elevation myocardial infarction (NSTEMI), and one patient died from complications of acute myocardial infarction.[33]
Swiatek et al.[34] did a retrospective cohort study on patients undergoing EBUS TBNA while taking clopidogrel (n = 13), aspirin (n = 103), both (n = 23) or none (n = 270) and found similar incidence of significant bleeding between all the groups.
Evidence summary
Aspirin use is not associated with increased risk of bleeding during EBUS-TBNA.
Use of clopidogrel is not associated with an increased risk of clinically significant bleeding during EBUS-TBNA.
Discontinuation of anticoagulants for a shorter duration than recommended for other indications (e.g., transbronchial lung biopsy) is not associated with an increased risk of clinically significant bleeding.
Recommendations
Aspirin may be safely continued in patients undergoing EBUS-TBNA (3A)
In patients undergoing EBUS-TBNA, clopidogrel may be continued peri-procedurally in patients in whom the risk of thrombosis outweighs the risk of bleeding (3A).
Anti-coagulants should be discontinued before EBUS-TBNA for the duration individually recommended for each agent. (UPP).
Should coagulation tests be performed prior to EBUS-TBNA?
Bleeding during EBUS-TBNA is uncommon and was encountered in 0.2% of the cases in the EBUS AQUIRE registry.[35] Studies of transbronchial lung biopsy in patients who do not have risk factors for bleeding did not show any correlation between the occurrence of bleeding and routine pre-procedure testing for coagulation function.[36,37] The British Thoracic Society[27] and the ICS/NCCP/IAB guidelines[23] for flexible bronchoscopy do not recommend routine pre-procedure testing of hemoglobin, platelet count, or coagulation parameters because these tests lack predictive value for bleeding risk. The guidelines[23,27] recommend that these tests be performed in patients at high risk of bleeding[37] such as use of anticoagulants, presence of liver disease, chronic kidney disease, history of bleeding diathesis in self or family, and ongoing active bleeding. When these tests are indicated, platelet count should be more than 20000/μL for performing bronchoalveolar lavage and more than 50000/μL for performing any bronchoscopic biopsy. Pre-procedure International Normalized Ratio (INR) should be less than 1.5.[23] There are no studies that have examined these cutoffs specifically on patients undergoing EBUS-TBNA.
Evidence summary
Among patients who do not have risk factors for bleeding, studies on transbronchial biopsy do not show any correlation between risk of bleeding and routine pre-procedure coagulation tests.
The risk of bleeding with EBUS-TBNA is minimal. Data on coagulation testing may be extrapolated from these studies on transbronchial lung biopsy.
Recommendations
Among patients undergoing EBUS-TBNA, routine pre-procedure testing for coagulation function and platelet count is not recommended.(UPP)
Pre-procedure testing for coagulation function and platelet counts may be performed in patients at high-risk of bleeding (anticoagulant therapy, liver disease, chronic kidney disease, bleeding diathesis, or history of active bleeding). (UPP)
Is there a role of antibiotic prophylaxis prior to EBUS-TBNA?
Post-bronchoscopy fever is a common complication and may occur after 10-20% procedures.[38,39] The fever is largely due to an increase in inflammatory cytokines and is not associated with bacteremia or new opacities on chest radiographs.[40] Guidelines do not recommend the routine use of antibiotics for flexible bronchoscopy.[23,27]
Takagi et al.[41] conducted a single-center RCT including 90 patients who underwent EBUS-TBNA, of which 43 patients received penicillin-based antibiotics and 47 did not. The primary outcome was fever over 5 days, and the secondary outcomes were occurrence of leukocytosis, CRP elevation, or any pulmonary or mediastinal infection needing antibiotics within 28 days. None of the outcomes differed significantly between the two groups. Similarly, a retrospective chart review of 96 patients who underwent EBUS-TBNA, including 30 who received post-procedure antibiotics, found no difference in incidence of post-procedure fever or other infectious complications between the two groups.[42]
The American Heart Association (AHA) guideline[43] on infective endocarditis (IE) does not recommend antibiotic prophylaxis for IE during bronchoscopy in patients with high-risk cardiac conditions, unless the procedure involves incision of the respiratory tract mucosa. As there is no data to support the use of antibiotics for infective endocarditis prophylaxis among patients undergoing EBUS-TBNA, no recommendation was made for the same.
Evidence summary
The use of prophylactic antibiotics for EBUS-TBNA is not associated with a significant reduction in the incidence of post-procedure fever or infectious complications.
Recommendation
Prophylactic antibiotics for EBUS-TBNA are not recommended (2A)
SECTION III: SEDATION AND ANESTHESIA DURING EBUS-TBNA
Should sedation be administered during EBUS-TBNA?
Sedation refers to the administration of drugs to induce a decreased level of consciousness in a patient during a medical procedure to facilitate patient comfort and obtain adequate diagnostic yield. Although flexible bronchoscopy (FB) is often performed without sedation, evidence suggests that the use of sedation shortens the duration of FB and increases the willingness of patients to return for a repeat procedure.[44]
We were unable to find studies which have directly compared the outcomes of EBUS-TBNA performed with or without sedation. However, EBUS-TBNA is usually a longer procedure than flexible bronchoscopy, and the EBUS scope has a larger diameter than the flexible bronchoscope. EBUS-TBNA also entails repeated contact with luminal surfaces. Hence, the use of sedation is reasonable to enhance patient comfort and minimize coughing, thereby improving the chances of adequate tissue sampling and optimizing diagnostic yield.
Since an inadvertent increase in depth of sedation may compromise the airway patency and/or breathing, a careful pre-procedural assessment is necessary before performing bronchoscopy under sedation. This should primarily be aimed toward identification of a difficult airway. The American Society of Anesthesiologists (ASA) suggests eliciting an airway history and a focused physical examination for this purpose.[45] A history of difficult intubation in the past, obesity, obstructive sleep apnea, and other conditions associated with difficult laryngoscopy or intubation (e.g. ankylosis, tonsillar hypertrophy, subglottic stenosis, etc.) should be sought. Physical findings which predict difficult airway include reduced mouth opening, overbite, long incisors, reduced range of motion of the head and neck, Mallampati score >2, and reduced thyromental distance (less than 60 mm or three finger breadths).
Evidence summary
There are no studies which compare the diagnostic performance of EBUS-TBNA with or without sedation.
Recommendation
We suggest that sedation be administered for patients undergoing EBUS-TBNA. (UPP)
A pre-procedural assessment to anticipate difficult airway should be performed using an airway history and a focussed physical examination. (UPP)
What should be the depth of sedation for EBUS-TBNA?
The depth of sedation ranges from minimal sedation to general anesthesia (GA). The ASA has defined the various depths of sedation based on patient responsiveness, need for an artificial airway, spontaneous respiration, and hemodynamic function.[46] Accordingly, during minimal sedation (anxiolysis) and moderate sedation (“conscious sedation”), the patient remains responsive to verbal stimuli, breathes spontaneously, and does not require an artificial airway. A deeply sedated patient may require repeated or painful stimulation to elicit response and may require an artificial airway. Further increase in the depth of sedation results in general anesthesia (GA), wherein the patient becomes unarousable to painful stimuli and needs an artificial airway. The likelihood of hemodynamic impairment increases proportionally with the depth of sedation. Hence, objective monitoring of the depth of sedation, hemodynamic status, and oxygenation is imperative.
We found ten studies (two randomized controlled trials and eight observational studies) which had compared the efficacy and safety of EBUS-TBNA performed at different depths of sedation. One observational study found no difference in the diagnostic yield and complication rate of EBUS-TBNA performed under either GA or deep sedation.[47] The remaining nine studies compared the use of either minimal or moderate sedation with deep sedation or GA. In a retrospective analysis, Yarmus et al. found that EBUS-TBNA under deep sedation provided a higher diagnostic yield than moderate sedation (80% versus 66%, P < 0.01) and allowed a greater number of lymph nodes to be sampled (2.2 versus 1.4 per patient).[48] However, four other observational studies found no difference in the diagnostic yield between procedures performed under moderate or deep sedation.[49,50,52] In one of these studies, deep sedation was associated with a higher incidence of hypotension and desaturation.[52] In a retrospective cohort study, the diagnostic yield and occurrence of major complications was similar between the moderate sedation and GA groups.[53] Conte et al. observed a comparable diagnostic accuracy and complication rate during EBUS-TBNA performed under minimal or deep sedation.[54]
Two randomized trials compared the diagnostic yield of EBUS-TBNA performed under moderate sedation or GA. Casal et al. found no difference in the diagnostic yield in the two groups (GA, 68.9% versus moderate sedation, 70.7%; P = 0.82). No major complications were reported; however, minor complications were higher with moderate sedation (29.6% versus 5.3%, P < 0.001).[55] Similarly, Fernandes et al. found no difference in the diagnostic yield, complication rate, patient satisfaction, or operator difficulty between moderate sedation or GA.[56]
The use of deep sedation and general anesthesia may necessitate an artificial airway or hemodynamic support, necessitating the presence of an anesthesiologist. The term “monitored anesthesia care” refers to the delivery of the anesthesia service for a medical procedure by a qualified provider.[57] In contrast, it has been shown that moderate sedation can be safely delivered by the bronchoscopist without compromising diagnostic yield.[58,59,60,61] A trained bronchoscopy nurse can also assist the bronchoscopist in administering sedative agents.[59,61] Studies which have compared bronchoscopist-directed moderate sedation and anesthesiologist-directed deep sedation have found comparable diagnostic accuracy and procedural safety.[50,52,54] Nonetheless, the expert panel opined that when a longer procedure is anticipated (e.g., staging EBUS), the use of deep sedation administered by an anesthesiologist may be desirable.
Evidence summary
EBUS-TBNA can be safely performed under moderate sedation.
There is no difference in the diagnostic yield, complication rate or patient comfort of EBUS-TBNA performed during moderate sedation or deep sedation/general anesthesia.
There is no difference in the diagnostic yield or procedural safety of EBUS-TBNA performed under moderate sedation directed by the bronchoscopist and under deep sedation/general anesthesia administered by anesthesiologist.
Recommendations
EBUS-TBNA should be performed using either moderate sedation or deep sedation/general anesthesia. (1A)
EBUS-TBNA can be performed using either bronchoscopist-directed moderate sedation or anesthesiologist-directed deep sedation/general anesthesia. (2B)
While performing EBUS-TBNA, the depth of sedation should be objectively monitored by a designated member of the team. (UPP)
For all patients undergoing EBUS-TBNA under moderate sedation, non-invasive blood pressure, oxygen saturation, heart rate and respiratory rate should be monitored throughout the procedure. (UPP)
Among patients with suspected or known cardiac disease, electrocardiographic monitoring should be additionally done during the EBUS-TBNA procedure.(UPP)
For patients undergoing EBUS-TBNA under deep sedation/general anesthesia, standard ASA monitoring protocols should be followed as feasible. (UPP)
What should be the preferred sedative agents for EBUS-TBNA?
The commonly used sedative agents include midazolam, propofol, ketamine, and dexmedetomidine. Opioid analgesics (e.g. fentanyl or remifentanil) are often co-administered with sedatives. However, remifentanil is currently not available in India. A few small randomized controlled trials (RCTs) have compared dexmedetomidine with other sedatives for EBUS-TBNA. A comparison between dexmedetomidine and propofol in 50 patients undergoing EBUS-TBNA found similar level of patient cooperation and diagnostic yield with both agents. Patients who received dexmedetomidine were more likely to perceive the procedure and less likely to return for a repeat procedure (41.1% versus 83.3%, P = 0.007).[62] Compared to remifentanil, dexmedetomidine was associated with fewer respiratory adverse events (apnea, bradypnea, or desaturation), but led to delayed postoperative recovery.[63]
Two RCTs have compared dexmedetomidine and midazolam. Kim et al. found no difference in the oxygen desaturation events but found a lower cough score with dexmedetomidine.[64] The second study randomized 197 patients to either dexmedetomidine infusion or midazolam bolus and found that patients who received dexmedetomidine were less likely to require rescue midazolam boluses but were more likely to develop hypotension and bradycardia.[65]
Only a few studies have examined the effect of combining sedatives during EBUS-TBNA. A combination of ketamine plus midazolam or ketamine plus propofol has been reported equally safe and provided similar procedural satisfaction for patients and bronchoscopists.[66] Observational studies have also compared the use of a single sedative with a combination of sedatives and found both approaches to be safe and effective.[67,68] The use of a combination of sedatives may reduce the dose requirement for individual drugs but may prolong recovery time.[67]
The working group noted that the use of propofol directed by bronchoscopists has not been adequately studied. Propofol may be associated with rapid onset of cardiorespiratory compromise and decrease in consciousness levels which may result in inadvertent general anesthesia.[69] Hence, the group felt that the supervision of an anesthesiologist may be preferable for the use of propofol during EBUS-TBNA.
Evidence summary
There is paucity of evidence to establish the superiority of any particular sedative agent (dexmedetomidine, propofol, midazolam or fentanyl) regarding diagnostic yield or procedural safety of EBUS-TBNA.
There is no difference in the diagnostic yield, complication rate or patient satisfaction between the use of individual or combination sedatives for EBUS-TBNA.
Combining sedatives for EBUS-TBNA may reduce the dose requirements for individual sedative but may prolong the recovery time.
Recommendation
A combination of sedative agents is preferred over a single agent alone, when performing EBUS-TBNA under moderate sedation. (UPP)
The choice of sedative agent(s) can be determined by operator preference, availability, and monitoring facilities. (UPP)
Use of propofol for sedation should preferably be supervised by an anaesthesiologist. (UPP)
Airway conduits for EBUS-TBNA procedures
Performing EBUS-TBNA under GA requires the placement of an artificial airway. These may be in the form of a supraglottic airway (SGA) device, an endotracheal (ET) tube or a rigid bronchoscope. The use of an SGA is theoretically more attractive than the alternative modalities because it allows for a complete endosonographic evaluation of the airway including easier access to the upper paratracheal lymph nodes. In contrast, upper paratracheal node sampling may be challenging via an ET tube or rigid bronchoscopy. Among the SGAs, the second-generation SGAs are preferable to first-generation devices for EBUS-TBNA as the former incorporate a gastric drain to prevent aspiration.
Piccioni et al. demonstrated that an SGA is easy to place and allows successful passage of the EBUS scope.[70] The only RCT which has compared airway conduits included 77 patients randomized into an SGA or rigid bronchoscopy group and found no difference in the EBUS procedure duration, time to recovery, or diagnostic yield in the two arms.[71]
Studies are lacking with respect to the optimal technique of ventilation during EBUS-TBNA, hence, no recommendation could be framed for the same. Therefore, the preferred technique of ventilation (apneic oxygenation, assisted or controlled ventilation, manual or high-frequency jet ventilation) depends on the anesthesiologist’s discretion.
Evidence summary
While performing EBUS-TBNA under GA, there is no difference in the diagnostic yield of the procedure performed via supraglottic airway or rigid bronchoscopy.
There is no published literature on the use of endotracheal tube as an airway conduit for EBUS-TBNA performed under GA.
Recommendations
Supraglottic airway device is the preferred conduit when performing EBUS-TBNA under general anesthesia. (2A)
How should topical anesthesia be administered during EBUS-TBNA?
When EBUS-TBNA is performed under moderate sedation, it is necessary to apply adequate topical anesthesia to the pharynx, vocal cords, and trachea to ensure patient comfort. Lignocaine is the most commonly used topical agent and may be delivered to the vocal cords via either the spray-as-you-go method (through the bronchoscope channel) or by injection through the cricothyroid membrane. In a meta-analysis of five RCTs involving patients undergoing FB, cricothyroid injection was associated with lesser cough, higher operator-rated procedural satisfaction and lower cumulative lignocaine dose compared with the spray-as-you-go technique.[72] Mittal et al. performed an RCT comparing cricothyroid injection with the spray-as-you-go technique for EBUS-TBNA under moderate sedation and found superior topical anesthesia with the cricothyroid injection.[73]
In order to determine the optimum concentration of lignocaine for topical anesthesia during EBUS-TBNA, Biswal et al. compared 1% and 2% lignocaine using the spray-as-you-go technique and found no difference in the operator-rated procedural satisfaction or patient comfort between the two groups. However, the use of 1% lignocaine resulted in a significantly lower cumulative lignocaine dose (178.5 mg versus 248.6 mg, P < 0.01).[74] The lignocaine spray can be delivered either directly through the bronchoscope channel or using a spray catheter passed through the channel. In a small RCT involving 40 patients, Lee et al.[75] demonstrated that the use of a spray catheter for delivery may reduce significant coughing episodes during EBUS-TBNA.
There are no studies which have examined the optimal method or dose of topical anesthesia of the pharynx during EBUS-TBNA. However, based on the evidence from flexible bronchoscopy studies, the use of 5 sprays of 10% lignocaine appears sufficient.[76]
Evidence summary
Among patients undergoing EBUS-TBNA under moderate sedation, topical anesthesia using cricothyroid injection provides superior operator-rated procedural satisfaction and results in lesser cough than the spray-as-you-go technique.
For the topical anesthesia of vocal cords using the spray-as-you-go technique during EBUS-TBNA, 1% lignocaine is equally efficacious as 2% lignocaine. The use of 1% lignocaine results in a lower cumulative lignocaine dosage administered.
Recommendations
For patients undergoing EBUS-TBNA under moderate sedation, cricothyroid lignocaine injection may be preferred over the spray-as-you-go technique for topical anesthesia. (1B)
While using the spray-as-you-go technique, 1% lignocaine should be preferred for topical anaesthesia. (1A)
SECTION IV: OXYGEN ADMINISTRATION DURING EBUS
Should oxygen be administered routinely while performing EBUS under sedation?
Oxygen saturation commonly decreases during flexible bronchoscopy, especially in patients with low forced expiratory volume in 1st second (FEV1) on spirometry or low peak expiratory flow rate (PEFR). However, oxygen desaturation can occur at any level of FEV1 and with any depth of sedation.[77,78,79] The British Thoracic Society[27] and the Indian Chest Society (ICS)/National College of Chest Physicians (NCCP)/Indian Association for Bronchology (IAB) guidelines[23] on flexible bronchoscopy in adults recommend that all patients should be monitored by continuous pulse oximetry during bronchoscopy and that oxygen should be administered using standard oxygen delivery devices, e.g. nasal or pharyngeal catheter, when desaturation is significant (i.e., SpO2 fall >4% or SpO2 <90%) and prolonged (>1 minute), to reduce the risk of hypoxemia-related complications.
Hypoxemia during bronchoscopy is multifactorial,[80] and also depends on the procedure performed (e.g., during broncho-alveolar lavage, SpO2 <90% may occur in 89% of cases).[81] A retrospective study of 261 patients undergoing EBUS-TBNA found that 27% of patients had desaturation to SpO2 <90% despite receiving supplemental oxygen during the procedure. Higher dose of sedation is associated with a greater likelihood of desaturation.[82]
There are no comparative studies of EBUS-TBNA performed with and without oxygen supplementation. In our practice however, EBUS-TBNA requires at least moderate sedation that may occasionally result in greater peri-procedural sustained desaturation (SpO2 <90% for more than 1 minute). The CHEST Quality Improvement Registry, Evaluation and Education (ACQUIRE registry) reported a 0.3% rate of sustained hypoxemia during EBUS-TBNA despite the use of oxygen supplementation in all cases.[35]
The basic principles of oxygen therapy remain applicable in the setting of EBUS-TBNA. For instance, in patients with obstructive airway disease with chronic hypercapnic respiratory failure, judicious use of oxygen is advised, to prevent worsening hypercapnia secondary to worsening V/Q mismatch.
Evidence summary
Available evidence from studies on flexible bronchoscopy suggest that hypoxemia occurs commonly during the procedure. Low baseline FEV1, baseline SpO2 <90%, and need for higher doses of sedation during the procedure, are risk factors for procedural desaturation.
As the EBUS scope is larger, and the procedure requires greater sedation, desaturation is likely to be a common occurrence.
Recommendation
We suggest use of oxygen supplementation via common oxygen delivery devices (nasal prongs/nasopharyngeal catheter), along with pulse oximetry monitoring as a routine practice in patients undergoing EBUS-TBNA (UPP)
Should standard oxygen or high flow nasal oxygen/nasal CPAP be used for oxygenation during EBUS-TBNA?
Although supplemental oxygen is used routinely during the EBUS-TBNA procedure, there is equipoise regarding the ideal method of oxygen delivery.
Various studies have compared high-flow nasal cannula (HFNC) with nasal prongs or nasal catheter for oxygen delivery during EBUS-TBNA. Among 40 patients who underwent EBUS-TBNA with supplemental oxygen using either HFNC or nasal prongs, the oxygen desaturation was significantly lesser in the HFNC group, but no difference was observed in patient comfort or willingness to return for a repeat procedure.[83] Similarly, another single-center RCT of HFNC versus nasal cannula oxygen found lesser desaturation and a higher nadir SpO2 in the HFNC arm. Further, the HFNC group exhibited lower patient discomfort.[84] Takakuwa et al. compared HFNC with conventional nasal cannula in 31 EBUS-TBNA procedures and found that the cumulative dose of midazolam used for sedation was higher in the HFNC arm. Despite this, the HFNC group had significantly lesser desaturation and higher value of nadir SpO2.[85]
Other studies have examined the performance of EBUS-TBNA with nasal continuous positive airway pressure (nCPAP) compared to oxygen supplementation and demonstrated that administering oxygen alone was associated with a greater decline in oxygen saturation as well as longer desaturation time.[86,87]
However, although HFNC and nCPAP use is associated with lower occurrence of procedural hypoxemia compared with supplemental oxygen using conventional nasal cannula or catheter, there is no evidence of reduction in major complications (e.g. need for invasive mechanical ventilation or ICU stay). Further, HFNC and nCPAP can also add to the cost and complexity of the procedure. It is also evident that the patients with high-risk factors for procedural desaturation in whom HFNC and nCPAP may be preferred, cannot be identified with the currently available evidence. Hence, no recommendation is being made for or against the use of these devices for oxygen delivery.
Evidence summary
The evidence for oxygen delivery using various devices during EBUS-TBNA is sparse.
The use of high-flow nasal oxygen (HFNO) or nasal continuous positive airway pressure (nCPAP) device may result in fewer desaturation events and higher nadir of SpO2 during EBUS-TBNA. However, the clinical significance of the same is uncertain.
Recommendation
No recommendations were made regarding the oxygenation modality for EBUS-TBNA performed under moderate sedation.
SECTION V: TECHNICAL AND PROCEDURAL ASPECTS OF EBUS-TBNA
Should flexible bronchoscopy be done prior to all EBUS procedures?
Conventional EBUS scopes have limited ability to perform a complete airway inspection because of a larger external diameter, poor visual quality, and limited manoeuvrability as compared to a flexible video bronchoscope;[88] hence, it is a good practice to perform flexible bronchoscopy prior to all EBUS procedures despite lack of direct evidence to support the same. No study has directly addressed the question as to whether performing flexible bronchoscopy routinely before EBUS-TBNA provides superior procedure-related outcomes. In a randomized trial of sixty-two subjects, bronchial segment visualization was superior while using a video EBUS scope with 10 degrees forward oblique view as compared to the conventional EBUS scope with 35 degrees forward oblique view. Additional flexible bronchoscopy for adequate airway assessment was required for five subjects in the conventional EBUS group compared to none in the video EBUS group. No differences were observed in sample adequacy and diagnostic yield (secondary outcomes).[89]
What are the characteristics of various EBUS scopes?
A brief description of specifications of the currently available convex-probe EBUS scopes (Olympus, Fujifilm, and Pentax) is tabulated in Table 4. The total length and working length are approximately 880 mm and 600 mm, respectively. The distal end outer diameter ranges from 6.6-7.4 mm, the working channel from 2-2.2 mm, direction of view 10-45 degrees forward oblique, and field of view from 80-120 degrees. The maximum up/down angulation ranges from 120 to 160/70 to 90 degrees.[90,91,92,93] The newer EBUS scopes have a straighter view, larger field of view, increased angulation, improved optics, and smaller external diameter.[91,94]
Table 4.
Characteristics of different EBUS scopes
| Parameter | Olympus- BF-UC 180F | Olympus- BF-UC 190F | Pentax EB-1970UK/EB19-J10U | Fujifilm E-530US |
|---|---|---|---|---|
| Working channel diameter | 2.2 mm | 2.2 mm | 2 mm | 2 mm |
| Working length | 600 mm | 600 mm | 600 mm | 610 mm |
| Field of view | 800 | 800 | 1000 | 1200 |
| Direction of view | 350 forward oblique | 200 forward oblique | 450 forward oblique | 100 forward oblique |
| Depth of field/observation range | 2-50 mm | 2-50 mm | 2-50 mm | 3-100 mm |
| Outer diameter (distal tip) | 6.9 mm | 6.6 mm | 7.4 mm | 6.7 mm |
| Outer diameter insertion tube | 6.2 mm | 6.3 mm | 6.3 mm | Flexible portion diam=6.3 mm |
| Max angulation up/down | 1200/900 | 1600/700 | 1200/900 | 1300/900 |
| Total length | 880 mm | 890 mm | 860 mm | 880 mm |
Evidence summary
There is insufficient evidence regarding the need for flexible bronchoscopy prior to all EBUS procedures
A video EBUS scope may improve the ability to perform an adequate airway inspection.
Recommendation
Based on operator judgement, a flexible bronchoscopy for airway assessment may be performed prior to the EBUS procedure. (UPP)
What should be the preferred route of insertion of EBUS scope?
Traditionally, the EBUS bronchoscope is inserted through the oral route due to its larger diameter and rigid distal end compared to the conventional adult flexible video bronchoscope. The rationale for using the nasal route relies on possible improved stability and protection from inadvertent scope bite.
Studies comparing the nasal with the oral route for inserting the EBUS scope are sparse. In the AQuIRE bronchoscopy registry, where 95% of procedures were EBUS, the most common insertion route was through a laryngeal mask airway (48.6%), followed by oral (33.1%), nasal (12.2%), endotracheal tube (6.6%), and tracheostomy tube (0.4%), although no comparisons were made between them.[49]
A few case reports have reported successful insertion of EBUS scope via nasal route when the oral route could not be used.[95] A retrospective analysis of 196 subjects undergoing linear probe EBUS procedures found that nasal insertion was possible only in 73.5% of procedures, whereas oral insertion was successful in all procedures.[96] There was no difference in procedure duration, complication rate, and yield of the specimen between the two groups. The only RCT comparing the nasal and oral routes of EBUS scope insertion was done by Beaudoin et al.,[97] on 220 subjects with the primary objective being patient comfort. Of all subjects, 27 (24.5%) had a failed nasal insertion while oral insertion succeeded in all. No difference was seen between the nasal and oral groups in terms of patient comfort, overall patient satisfaction, physician-reported subject comfort, procedure duration, doses of sedatives, diagnostic yield, and the number of nodal stations sampled. Three episodes of epistaxis (all minor) occurred in the nasal group while none occurred in the oral group. In another retrospective analysis of 120 patients, Han et al.[98] found that nasal insertion was successful in 86.7% while the oral route had to be used in the remaining subjects. The most common complications were epistaxis (24.2%) and local pain (20%). The previously published CHEST guidelines on EBUS made no recommendation on the preferred route of EBUS due to insufficient quality of evidence to support any one route over the other.[99]
Evidence summary
Both oral and nasal routes have been used for EBUS scope insertion. The nasal route has higher chances of failed insertion and a higher risk of epistaxis while procedure duration, patient comfort, and yield are similar as attained by oral scope insertion.
Recommendations
It is preferable to use the oral route for EBUS scope insertion. (1A)
When the oral route is not feasible, using the nasal route for EBUS scope insertion is acceptable if performed by experienced operators. (UPP)
What is the utility of sonographic nodal characteristics and elastography during EBUS?
EBUS allows the assessment of ultrasonographic features of the lymph nodes such as size, shape, margins, necrosis, and others. Sonographic lymph nodal characteristics during EBUS-TBNA were first reported by Fujiwara et al.[100] in 2010. Sonographic characteristics of lymph nodes have been evaluated to differentiate malignant from benign nodes. Criteria used for ultrasonographic characteristics are shown in Table 5.[101,102]
Table 5.
Criteria for ultrasonographic characteristics of lymph nodes
| Characteristic | Description |
|---|---|
| Size | Measured in two perpendicular axes. |
| Shape | Round (long axis/short axis <1.5) and oval (if long axis/short axis >1.5). Triangular shape was used in one study (Gogia et al., 2015) if three distinct arms could be seen by the operator.[101] |
| Margins | Distinct margins, described as when more than half of lymph node margins are distinctly visible |
| Echogenicity | Heterogenous or homogenous. |
| Coagulation necrosis sign (CNS) | Hypoechoic area with the absence of blood flow on doppler |
| Central hilar structure (CHS) | Defined as a linear flat hyperechoic area in the center of lymph node. |
| Calcification | Hyperechoic shadows with acoustic shadowing. |
| Conglomeration | Defined as loss of intervening tissue between two lymph nodes. |
| Central intranodal vessel | Well-defined, smooth hyperechoic wall that was >1 mm in diameter, located toward the center of the lymph node, and demonstrated blood flow on color doppler and used to evaluate the Color Power Doppler Index (CPDI) (Nakajima et al., 2012).[102] |
The pathophysiology behind these features is that in malignant etiology, the lymph node enlarges with increased vascularity but maintains its shape. There is a loss of central hilar structure and central necrosis due to rapid turnover and neovascularization in the periphery.
Lymph node size of more than 1 cm was associated with malignancy in numerous studies[103,104,105,106,107,108,109,110,111] but not in others.[100,112,113] The absence of a central hilar structure is associated with malignancy with variable sensitivity and specificity.[104,106,108,109,111,112,114,115]
The presence of distinct nodal margins was associated with malignancy in several studies[100,106,108,110,111,112]; however, a large study by Ayub et al.[104] found that distinct margins favored a benign pathology, while some other studies found this parameter to be non-contributory.[101,105,116]
Intranodal blood flow on color doppler was a less commonly studied feature and was found to suggest malignancy in some studies,[102,105,106] but not in others.[110,112,116]
Overall, the common features that favor malignancy are nodal size (short axis diameter >1 cm), heterogeneous echotexture, absence of central hilar structure, round shape, and presence of central/coagulation necrosis [Table 6].[117]
Table 6.
Commonly studied sonographic features that favor malignancy
| Lymph node characteristic | Sensitivity | Specificity |
|---|---|---|
| Size (>1 cm) | 63-100% | 5-81% |
| Round shape | 10-99% | 16-100% |
| Heterogenous echotexture | 10-99% | 17-100% |
| Absence of central hilar structure | 28-100% | 9-100% |
| Presence of central/coagulation necrosis | 8-91% | 66-100% |
A meta-analysis by Agrawal et al.[117] which included 4333 patients with 8204 lymph nodes analyzed from 29 studies, found that 5 lymph node characteristics (size >1 cm, distinct margins, round shape, absence of central hilar structure (CHS), and color power doppler index had good sensitivity (more than 0.7) for diagnosing malignant lymph nodes. The most sensitive feature for malignant etiology was the absence of CHS (sensitivity: 91%), while the least sensitive was the presence of coagulation necrosis (sensitivity: 38%). The most specific individual feature for malignancy was the presence of CNS (pooled specificity: 93%) followed by heterogeneous echogenicity (pooled specificity: 82%).
To date, no single sonographic lymph node parameter is available as a definite differentiating feature between benign and malignant nodes. This has led to the development of various composite scores. Schmid-Bindert et al.[108] found that a combination of size, shape, echogenicity, and margins had an odds ratio (OR) of 3.5 for malignancy. Shafiek et al.[109] added the absence of CHS to the score by Schmid-Bindert and demonstrated that a value of >5 in this new score had a sensitivity of 78% and specificity of 86% for malignant LNs, with an area under curve (AUC) of 0.85. The Canada Lymph Node Score (CLNS) included distinct margins, size >1 cm, absence of CHS, and presence of central necrosis as a 4-point score, with a score >2 suggestive of malignancy.[111] However, prospective validation of these scores is needed to determine their utility in EBUS sampling.
Literature regarding lymph node features of non-malignant etiologies is limited. Studies by Dhooria et al.[118] and Erol et al.[119] concluded that the absence of heterogeneous lymph nodes, absence of coagulation necrosis sign, and presence of conglomeration favor sarcoidosis over tuberculosis. Similarly, Agrawal et al.[112] and Imai et al.[120] found that homogeneous echotexture, presence of central hilar structure, and absence of coagulation necrosis sign may favor sarcoidosis over malignancy. A recent study by Madan et al.[121] reported that in tubercular endemic settings, the presence of coagulation necrosis sign, heterogeneous echotexture, and absent central intranodal vessel was more common in tuberculosis than malignancy, with the absence of lymph node conglomeration having the highest overall diagnostic accuracy for differentiation between the two etiologies.
Elastography
Elastography assesses tissue stiffness and the distribution of stiffness in the selected region and is measured in response to mechanical stress. Lymph nodes infiltrated by malignancy are stiffer and less deformable.[122] The estimation of stiffness can be qualitative or quantitative. Qualitatively, the most commonly described method of EBUS elastography is 3-color pattern classification by Izumo[122]: Type 1, predominantly non-blue (green, yellow, and red); Type 2, partially blue, partially non-blue (green, yellow, and red); and Type 3, predominantly blue (stiff). Other qualitative methods include a four or five color classification on similar principles.[123,124] However, with increasing groups, subjectivity tends to increase, and thus these classifications have not found universal application.
Quantitative methods include strain ratio (SR), stiff area ratio (SAR) or blue color proportion (BCP), and strain histogram methods. Strain ratio is the ratio of the absolute value of strain in region of interest to strain in the surrounding normal tissue. Various cut-offs have been described, ranging from 2.47[125] to 32,[123] but none have been standardized for either selecting the two areas or the cut-off value. Stiff area ratio or blue color proportion quantifies blue color (in percentage) in the lymph node using image analysis software and has also used different cut-offs in various studies ranging from 31.1%[126] to 41%.[127] The strain histogram method is one wherein the machine itself forms a histogram depending upon the color in the region of interest with a value ranging from 0 to 255, where 0 is coded for blue (least compressible), and 255 is coded for red (most compressible).[128,129]
A meta-analysis by Madan et al.[130] including 20 studies with over 1600 patients and 2712 nodes revealed pooled diagnostic sensitivity of EBUS elastography of 0.90 (95% CI, 0.84-0.94) and pooled specificity of EBUS elastography of 0.79 (95% CI, 0.73-0.84). There was no difference between the sensitivity and specificity of qualitative versus quantitative methods of elastography assessment. The AUC for elastography was 0.90 overall; and for the 3-color pattern (type 3 vs. type 1) was 0.91. The lowest AUC was for SR at 0.83 (0.80-0.86). A recent study suggested that elastography should be used as an additional feature for differentiating benign from malignant lymph nodes.[131] It must be noted that most studies enrolled predominantly suspected or proven malignancy patients.[129,132,133,134] Only one study evaluated elastography in a TB endemic setting.[135]
Only one randomized study has compared real-time EBUS-TBNA with or without elastography and found that the elastography group had fewer non-diagnostic samples, (21% vs. 2.3%, P = 0.001), and greater positive pathology results for malignant cells, (16% vs. 42%, P = 0.005).[128] However, apart from the small sample size, a major limitation of this study was the unusually low sensitivity, with a high percentage of insufficient samples in the EBUS group. Recently, studies have combined elastography with EBUS-B mode features and CT characteristics in an attempt to enhance diagnostic yield.[136,137] An important limitation of a majority of studies on elastography is that assessment has been done after the completion of procedure.
In addition, it is important to bear in mind that the elastography procedure has inherent limitations such as the amount of pressure applied while generating the images, the peri-nodal area selected for estimation of SR, and subjective interpretation. Strain ratio also showed significant intra-observer variability. The findings may also be affected by coughing or other movements as compared to a motionless field. Although various studies have found moderate[131,138] to excellent[113,124] inter-observer agreement for different elastography parameters, these drawbacks must be considered while interpreting EBUS elastography.[113]
Evidence summary
No single endosonographic mediastinal lymph node characteristic can reliably distinguish between malignant and benign etiologies
Based on pre-test probability, a combination of sonographic lymph node features may help in differentiating between various etiologies
Commonly studied features that favor malignancy are size (short axis diameter >1 cm), heterogenous echotexture, absence of central hilar structure, round shape, presence of central/coagulation necrosis.
To differentiate malignant from benign nodes, highest pooled sensitivity was found for the absence of central hilar structure (91%) and highest pooled specificity for the presence of central necrosis (93%).
In settings where malignancy is more prevalent, elastography may help differentiate malignant from benign lymph nodes with varying degree of sensitivity (64% to 100%) and specificity (65% to 92%). However, elastography data from tuberculosis endemic settings is sparse.
The most widely used elastography parameter is the Izumo 3-colour classification.
Various cut-offs have been used for quantitative elastography parameters to differentiate malignant nodes from benign, but none have been standardized.
Recommendations
Endosonographic lymph nodal characteristics do not preclude nodal aspiration for diagnosis of mediastinal adenopathy. (3A)
Elastography is not recommended to differentiate malignant from benign mediastinal lymph nodes except for research purposes. (2A)
EBUS elastography does not obviate the need for tissue sampling. (3A)
What are the indications and utility of bronchoscopic ultrasound-guided fine needle aspiration via esophageal route (EUS-B-FNA)?
Endoscopic ultrasound with bronchoscope-guided fine needle aspiration (EUS-B-FNA) is a procedure in which an EBUS scope is inserted through the esophageal route by a bronchoscopist. EUS-B-FNA permits access to the left-sided paratracheal nodes (station 2L and 4L), lower mediastinal lymph nodes (stations 8 and 9) and sub-diaphragmatic lymph nodes. It can also sample stations 5 and 6 via transvascular route and large 4R lymph nodes. EUS-FNA is the term used when a regular echoendoscope (EUS scope) is inserted into the esophagus for fine needle aspiration. EUS-B-FNA or EUS-FNA cannot sample lymph node stations 2R, 10R, 10L, 11R and 11L, which can be sampled by EBUS TBNA. Combined ultrasound-B-FNA (CUS-B-NA) is the combination of EBUS-TBNA and EUS-B-FNA performed by the same endobronchoscope as a single procedure. CUS-B-NA can reach virtually all mediastinal nodal stations except station 3 (pre vascular lymph nodes).[88,139,140,141,142,143]
In well-designed RCTs, the diagnostic yield of EUS-B-FNA and EBUS-TBNA were comparable (68%-87% versus 70%-91%, respectively).[144,145,146] The most common lymph nodes sampled were station 7 followed by 4L. When secondary outcomes were assessed, EUS-B-FNA required lower doses of sedation and topical anesthesia, had shorter duration of procedure (median, 15.3 min vs 11.3 min; P = 0.001), less frequent oxygen desaturations, lower cough score and higher operator satisfaction compared to EBUS-TBNA.[145] Similarly, Madan et al.[144] found lower operator-rated cough, higher operator-rated patient comfort and shorter procedure duration (secondary outcomes) in the EUS-B-FNA group. A recent study randomized 358 patients with suspected stage 1 and 2 Sarcoidosis into EUS-B-FNA (173) and EBUS-TBNA (185) groups and found no difference in granuloma detection rate (EUS-B-FNA 68% versus EBUS-TBNA 70%) and sensitivity (82% versus 78%).[146] Thus, EUS-B-FNA as compared to EBUS-TBNA may provide greater patient tolerance and operator comfort with a similar diagnostic yield when predominantly stations 7 and 4L are sampled. Other common reasons to perform EUS-B-FNA are inaccessibility by EBUS-TBNA,[147,148,149,150,151] technical difficulty of EBUS TBNA,[147,148,149,151] mediastinal lymph node sampling in pediatric patients,[152] poor respiratory condition,[150,151] and intolerance of bronchoscopy due to cough or dyspnea.[149,151]
Various observational studies have evaluated CUS-B-NA for staging of mediastinal lymph nodes in patients with suspected or known lung cancer and diagnosis of Sarcoidosis. For mediastinal lymph node staging, the sensitivity of CUS-B-NA is higher than EBUS-TBNA or EUS-B-FNA alone and ranges from 73 to 96%, 46 to 92%, and 45 to 95%, respectively.[147,149,153,154] A meta-analysis (4 studies and 465 subjects) reported the pooled sensitivity of CUS-B-NA and EBUS-TBNA as 91% and 80.3%, respectively (P = 0.004).[151] A RCT of 148 subjects assessed the impact of procedure sequence while performing CUS-B-NA for lung cancer staging and observed a significant gain in diagnostic accuracy (from 86.5% to 97.3%, P = 0.016) and sensitivity (from 60.0% to 92.0%, P = 0.008) if EBUS-TBNA was added to EUS-B-FNA, but not when EUS-B-FNA was added to EBUS-TBNA.[155] Similarly, in a meta-analysis of 13 studies (2395 subjects), the addition of EBUS-TBNA to EUS (EUS-FNA or EUS-B-FNA) increased sensitivity by 0·22 (95% CI; 0·16–0·29), while addition of EUS (EUS-FNA or EUS-B-FNA) to EBUS-TBNA increased sensitivity by 0·12 (0·08–0·18). There was no difference in the mean sensitivity and negative predictive value between studies that used an EBUS-scope (EUS-B-FNA) or a regular echoendoscope (EUS-FNA) to do endo-esophageal ultrasound. The mean sensitivity and negative predictive value of the combined approach were 0·86 (0·81–0·90) and 0·92 (0·89–0·93), respectively.[154] For diagnosis of sarcoidosis, the sensitivity of CUS-B-NA (91%) was greater than either EBUS-TBNA (76%) or EUS-B-FNA (70%).[156]
As per an official guideline, CUS-B-NA is preferred over either procedure alone for mediastinal nodal staging in suspected or proven NSCLC with suspicious nodes seen on imaging. If CUS-B-FNA is not available, then EBUS-TBNA alone is acceptable (Recommendation grade C, low-quality evidence).[141]
Evidence summary
EUS-B-FNA cannot sample lymph node stations 2R, 10R, 10L, 11R and 11L, which can be sampled by EBUS TBNA. EUS-B-FNA can sample stations 8,9 and subdiaphragmatic lymph nodes. It can also sample stations 5 and 6 via transvascular route and large 4R lymph node. CUS-B-NA can reach virtually all mediastinal nodal stations except station 3 (prevascular lymph nodes).
The diagnostic yield and sensitivity of EUS-B-FNA for diagnosis of mediastinal adenopathy ranges from 68-87% and 45-95% respectively.
Studies comparing EBUS-TBNA and EUS-B-FNA have predominantly sampled lymph node stations 7 and 4L with a similar diagnostic yield. However, EUS-B-FNA may have higher operator and patient comfort.
As compared to either procedure alone, combined EBUS TBNA and EUS-B-FNA (CUS-B-NA) increases the diagnostic yield and staging accuracy in patients with lung cancer and possibly sarcoidosis.
Recommendation
EBUS-TBNA is the preferred initial modality in most patients to sample accessible mediastinal lymph nodes. (1A)
In patients with inaccessible or difficult to access lymph nodes by EBUS-TBNA, addition of EUS-B-FNA to EBUS-TBNA (CUS-B-NA) is recommended for diagnosis of malignant nodal involvement and optimal staging in patients with lung cancer. (1A)
EUS-B-FNA is recommended in special situations such as lesions inaccessible by EBUS-TBNA, technical difficulty of EBUS-TBNA, mediastinal lymph node sampling in pediatric patients, intolerance of bronchoscopy or airway compromise. (3A)
EUS-B-FNA should only be performed by experienced EBUS operators after dedicated training. (UPP)
What is the ideal needle size for performing EBUS-TBNA?
Currently, there are various needle sizes commercially available for performing EBUS-TBNA, such as 19G, 21G, 22G, and 25G. There is significant heterogenicity in the studies involving EBUS needle sizes and results are often conflicting. The CHEST guideline on EBUS-TBNA published in 2016 gave a grade 1C recommendation for using a 21G or 22G needle as an acceptable option.[99]
Conventionally, 21G or 22G needles have been used in EBUS-TBNA. Giri et al. in their meta-analysis published in 2015[157] compared the 21-G and 22-G needles after including 5 studies and involving 1720 patients. No difference in diagnostic yield or sample adequacy was found between the two groups.
The 19G and 25G needles have been recently introduced with growing data regarding their use. In a recent meta-analysis,[158] the diagnostic sensitivities of 19G, 21G, 22G, and 25G needles in lymph nodes suspected for lung cancer were compared. Fourteen studies involving 1296 participants were included; of these, only one study provided data on the 25G needle, while results regarding 19G, 21G, and 22G needles were present in seven, nine, and twelve studies, respectively. A high diagnostic yield with overall sensitivity of 93%, 87%, and 85% was found for 19G, 21G and 22G needles, respectively. No significant difference in the diagnostic yield was found between the different needle sizes.
A large systematic review and meta-analysis published recently evaluated the effect of needle size on the diagnosis of sarcoidosis.[159] It included 65 studies (35 prospective, 30 retrospective), with a total of 4242 patients and found that the 19G needle had higher sensitivity compared with the 21G and 22G needle. However, only 5 studies involving 149 patients had data on the 19G needle and all these were observational in design.
The 19G needle provided high success rate in a retrospective single-operator single-center study when used in 15 patients suspected to have sarcoidosis.[160] Comparative prospective studies of 19G vs 21G/22G needles are limited and have provided conflicting results. A prospective study of 60 patients comparing 19G vs 21G did not find any difference in the overall diagnostic yield.[161] Another prospective study by Wolters et al.[162] compared 19G and 22G in 107 patients. A novel endpoint of sample weight corrected for blood content was used in this study. It was found that the amount of tissue and tumor cell count per slide was significantly higher while using the 19G needle. Pickering et al.[163] reported that the cell area in cell blocks obtained by 19G was significantly higher than that obtained by 21G. In a randomized trial comparing 19G with 22G, tissue core procurement was similar in both groups.[164] Furthermore, there was no significant difference in tumor cellularity, tumor surface area, quantity of DNA extracted, and successful next-generation sequencing.
25G needle has been hypothesized to be more flexible and thus useful for sampling difficult-to-access nodal sites with lesser contamination with blood. In a prospective observational study of 50 patients by Sood et al.,[165] no difference in sample adequacy or diagnostic yield was found when 22G and 25G needle were compared. Retrospective comparisons between 22G and 25G needles have demonstrated similar sample adequacy and diagnostic yield.[166,167] Gafoor et al. described 4 cases[168] where access to the 2R lymph node was not possible by the 22G needle. Here, 25G needles were successful at obtaining diagnostic samples from the 2R lymph node station.
In addition to the different needle sizes available, novel needle designs have also been introduced with purported advantages. The ProCore needle has a core-trap close to the needle tip. It receives the tissue sample while performing fine needle aspiration and is claimed to increase acquisition of core samples. Evidence on the yield and safety of ProCore needles is scarce. The only randomized controlled trial identified on ProCore needle[169] had randomized 100 patients in a 1:1 ratio to 22G ProCore and 22G standard needle (Vizishot, Olympus) arms and found no difference in sensitivity, sample adequacy, or safety between the two groups. Franseen tip needles have been used more commonly in gastroenterology practice during EUS with promising results and its use in EBUS is growing. A single center experience with 100 patients sampled with a Franseen tip EBUS needle (Acquire-Boston Scientific) showed encouraging results with acquisition of core biopsies in 87% of patients.[170]
Evidence summary
The various needle sizes currently available (19G, 21G, 22G, 25G) have comparable sample adequacy and diagnostic sensitivity.
There is insufficient evidence to recommend one needle size over the other.
Recommendations
In patients undergoing EBUS-TBNA, we recommend that either 21G or 22G may be used. (1A)
The group did not make any recommendation for or against the use of 19G, 25G, ProCore and Franseen tip needles. The choice of the needle is based upon the operator’s discretion. (UPP)
Can EBUS-TBNA needles be re-used?
Manufacturers of EBUS needles recommend against the reuse of EBUS needles due to risks of device failure, cross contamination, and infection. Consequently, data regarding re-use of needles is very limited. Our literature search resulted in 3 relevant studies, all from a single center. In these studies, EBUS needles were re-used once if the patient could not afford a new EBUS needle, after informed consent and a thorough needle sterilization.
The first study was a retrospective analysis of 500 patients and did not find any difference between the adequacy of cytological samples or diagnostic yield with new versus re-used EBUS needles.[171] In another larger retrospective analysis of 1582 patients, the diagnostic yield was significantly lower in the re-used needle group.[172] In the last study, a retrospective analysis of 1816 subjects, the positivity rate was adversely affected by needle re-use, with an odds ratio of 0.62 (95% CI, 0.47-0.81) and P value of <0.001.[173]
Evidence summary
Based on retrospective observational studies, re-use of EBUS-TBNA needles was found to be safe. However, re-used needles provided lower diagnostic yield.
Recommendations
In patients undergoing EBUS-TBNA, the use of a new needle is preferable. (3A)
Should suction be routinely used while performing EBUS-TBNA?
The aspiration of mediastinal lymph node during EBUS-TBNA may be done with or without the application of suction. After puncturing the mediastinal lymph node, negative suction is applied using a syringe that enables variable negative suction pressure, up to about 20 ml. Alternatively, the procedure may be done without the application of suction by removing the stylet and jabbing the needle within the lymph node. By this method, the “capillary suction” enables the material to get aspirated into the needle.[174] The use of suction has not yet been standardized for EBUS. It may be hypothesized that application of suction may increase the yield by increasing the number of cells aspirated. On the other hand, vacuum suction may increase the bloodiness of the sample thereby compromising the sample quality. Hence, the optimum suction to be applied during EBUS TBNA is not clear.
Four randomized controlled trials have assessed the utility of suction in EBUS TBNA and found no difference between use of suction and no-suction in terms of adequacy and diagnostic yield. Casal et al.[174] compared the concordance rate of adequacy and diagnostic yield of samples between 0 ml and 10 ml suction in EBUS-TBNA in 115 patients. This study analyzed 192 lymph nodes and concluded that regardless of lymph node size, no differences in adequacy, diagnosis, or quality was found between samples obtained with or without suction.
A single-center, prospective, randomized, non-inferiority trial assessed whether no-suction, and 10 mL suction are inferior to 20 mL suction with respect to adequacy and diagnostic yield of EBUS-TBNA aspirates. The overall adequacy in the no-suction, 10 mL, and 20 mL suction was 90%, 83.49%, and 77.88%, respectively. No-suction and 10 mL suction were non-inferior to 20 mL suction in terms of sample adequacy. Similarly, the overall diagnostic yield was comparable among the three groups. The proportion of aspirates which were predominantly bloody was similar (no-suction—10.9%, 10 mL—13.8%, 20 mL—15.4%; P = 0.62).[175]
Another trial evaluated the role of suction and stylet during EBUS-TBNA using variable combinations, i.e., suction with stylet, suction without stylet, and stylet without suction. No significant differences were observed among the suction–stylet, suction–no stylet, and stylet–No-suction groups in specimen adequacy rate (87.1, 88.2, 85.9%, respectively) or diagnostic yield of malignancy (32.2, 31.8, 31.0%, respectively). However, the use of suction was associated with more tissue-core acquisition. This study also showed no difference in the proportion of bloody specimens obtained with or without suction.[176]
Similarly, other studies have shown no difference in the adequacy, diagnostic yield or sample quality between suction and no-suction EBUS-TBNA.[177,178] However, a retrospective study showed higher diagnostic accuracy using slow pull capillary suction compared to vacuum suction.[179] In contrast, another RCT found that applying suction increased the diagnostic yield of cellblocks (suction 56.1% vs no suction 10.5%, P < 0.001) but not of smears obtained during EBUS-TBNA of suspected malignant mediastinal nodes.[180]
Evidence summary
EBUS-TBNA has been performed with or without the application of vacuum suction. The adequacy of the “no-suction” procedure ranged from 79% to 92% and diagnostic yield ranged from 31% to 96%. The adequacy of the “suction” technique ranged from 77 to 96% and diagnostic yield ranged from 10.5 to 96%.
There was no difference in the quality of the samples obtained irrespective of the suction applied, however the tissue core acquisition is increased with the use of suction.
Regardless of various lymph node characteristics, there is no difference in the adequacy and diagnostic yield of EBUS-TBNA with or without application of vacuum suction.
Recommendation
EBUS-TBNA may be performed with or without the application of vacuum suction. (1A)
Should stylet be routinely used for EBUS-TBNA?
As a general practice during EBUS-TBNA, the aspiration needle enters the lymph node with its inner lumen occluded with a metal stylet, which is subsequently removed after the needle enters the targeted node. The purpose of this stylet is to prevent airway cells, bronchial wall debris, and blood from filling the inner lumen and degrading sample quality. However, evidence regarding the efficacy of the stylet for this purpose is yet unclear. Previously published guidelines on EBUS-TBNA make no recommendation for or against the usage of stylet during EBUS procedure.[99]
Two RCTs and one prospective cohort study have tried to evaluate whether addition of stylet translates into better sample adequacy or diagnostic yield.[176,181,182] However, no difference was observed in the above parameters between both the techniques (87% vs 82% for no stylet and stylet respectively, P = 0.37).[182] The procedural time was slightly longer (ranging from 14 seconds to 2 minutes), while using the stylet compared to without.[176,181]
Evidence summary
There was no significant difference in sample adequacy or diagnostic yield when EBUS-TBNA is done with or without the use of stylet.
Non-usage of the stylet decreased the procedural time by approximately 14 seconds to 2 minutes.
Recommendations
EBUS-TBNA may be performed with or without using a stylet. (1B)
Stylet maybe used for capillary effect if vacuum suction is not being applied. (UPP)
Should balloon be routinely used for EBUS-TBNA?
During EBUS, ultrasound images can be obtained either by direct apposition of the probe with the airway, or by attaching a balloon over the ultrasound probe and inflating it with saline.[183] The currently available EBUS scopes can be fitted with a disposable balloon over their distal ultrasound tips. Although the saline-filled balloon can enhance image acquisition,[184] it is unclear if that translates into a better diagnostic yield.
After extensive search, no relevant studies were found which directly compared performance of EBUS with or without balloon, and therefore no recommendations to this effect can be made.
However, from a practical perspective, balloons are commonly used when the target lymph nodes are in relatively challenging locations such as the left paratracheal station (4L). The working group opined that use of balloon may enhance image quality at certain lymph node stations, e.g., 4L. However, it is worth remembering that the balloons come at an additional, albeit small, and cost and are made of latex and thus cannot be used in patients with latex allergy.
Evidence summary
There is lack of evidence for the effect of using balloon on the sample adequacy or diagnostic yield of EBUS-TBNA.
Recommendation
Balloon should be routinely attached during EBUS-TBNA. Inflation of balloon prior to needle puncture may be performed as per operator requirement. (UPP)
What is the minimal number of needle passes required for obtaining adequate sample during EBUS-TBNA?
A needle “pass” implies a distinct entry and exit of the needle through the airway wall. In other words, it refers to the number of aspirations that are made during EBUS-TBNA from a sampling site. The number of needle passes per sampling site could have implications on outcome and the procedural efficiency. Furthermore, the number of needle passes performed for various indications could differ and no definite consensus on this has been developed yet.
Lee et al.[185] conducted a study on 102 patients with mediastinal lymphadenopathy with potentially operable non-small cell lung carcinoma (NSLC). Every target lymph nodal station was punctured 4 times (without ROSE) and the sample adequacy reached 100% after 3 passes. The sensitivity for differentiating malignant from benign lymph node station was 69.8%, 83.7%, 95.3%, and 95.3% for one, two, three, and four passes, respectively. Consequently, it was suggested that for diagnosing NSCLC, at least 3 passes per lymph node station are required.
Mutational analysis/molecular marker testing in advanced adenocarcinoma may require additional tissue quantity. Only one retrospective study has attempted to quantify the number of passes required to have adequate tissue for molecular testing along with ROSE.[186] Among 85 patients with either adenocarcinoma or NSCLC, 95.3% of specimens were found adequate for molecular profiling. A median of 4 passes (total either from one or two lymph node stations) were necessary to obtain this sample adequacy.
Only one study has analyzed the number of EBUS needle passes required for optimum diagnostic yield in a benign pathology (sarcoidosis). This study included 109 patients with stage I/II Sarcoidosis undergoing EBUS-TBNA and found that the cumulative yield through the first, second, third, fourth, fifth, and sixth passes from a target lymph node was 63%, 75%, 82%, 85%, 86%, and 88%, respectively.[187] Among 55 patients who were sampled from more than one nodal site, the cumulative yields of 2 passes per lesion for 2 lesions (total of 4 passes) and of 4 passes for single lesion were 86% and 84%, respectively (P = 1.00). Based on these results, it is likely that at least 4 passes per patient (for single or multiple lesions) are needed to diagnose sarcoidosis. With respect to TB, although no relevant study was found, the working group opined that EBUS specimens must be tested for TB using appropriate methods, e.g., cartridge-based nucleic acid amplification test (CBNAAT) and cultures.
Evidence summary
In patients with NSCLC, 3 passes per lymph node station results in a sample adequacy of nearly 100% with a sensitivity of 95.3% for diagnostic accuracy.
In patients with NSCLC, obtaining 4 passes results in an adequate sample for molecular profiling in 95.3% of procedures.
In patients with sarcoidosis, the cumulative yield with 4 passes per patient (4 from one lymph node station or 2 each from 2 lymph nodes) was 85%.
Recommendation
At least 3 passes per lymph node sampled should be obtained for all patients during EBUS-TBNA. (UPP)
At least 3 passes per lymph node station sampled should be done for staging of NSCLC using EBUS-TBNA. (2A)
At least 4 passes are advised for diagnostic testing and molecular profiling in patients suspected of having lung cancer (2A).
In patient suspected to have granulomatous mediastinal lymphadenopathy, additional samples should be sent for microbiological molecular analysis. (UPP)
How many needle agitations should be done inside the lymph node during EBUS-TBNA?
While performing EBUS-TBNA, the needle is used to puncture a lymph node under real-time ultrasonographic guidance. Needle agitations (also referred to as revolutions, excursions, or jabs) refer to subsequent back and forth movements of the needle inside the node. Agitations are performed to induce the transfer of the lymph node material into the needle lumen, assisted by capillary action alone, or by application of vacuum suction in addition to the capillary action. However, the number of agitations required for optimal diagnostic yield is uncertain. A comparison between 10 versus 20 needle revolutions in 133 sarcoidosis patients did not reveal any difference in diagnostic yield or specimen adequacy.[188] Furthermore, no difference was observed in the procedure duration, the proportion of subjects with grossly bloody specimens, or complications between the 2 groups.
Evidence summary
There is no difference in the diagnostic yield and specimen adequacy of EBUS-TBNA performed with either 10 or 20 agitations of the needle inside the lymph node in subjects with sarcoidosis.
Recommendation
At least 10 agitations per pass should be performed for obtaining adequate samples. (UPP)
What are the potential complications of EBUS-TBNA and how to minimize them?
EBUS-TBNA is generally a safe procedure. The data regarding complications of EBUS-TBNA are not just sparse but likely not to be a true reflection of the actual complication rate as complications are generally under-reported. The 2016 guidelines on technical aspects of EBUS-TBNA do not address the issue of procedural complications and how to minimize them.[99] Various complications have been reported during EBUS-TBNA, ranging from transient hypoxia to death. For the purpose of reporting, we have broadly classified all complication into infectious and non-infectious.[189,190,191] Table 7 summarizes the most common reported complications during EBUS-TBNA.
Table 7.
Common complications of EBUS-TBNA
| Infectious complications | Non-infectious complications |
|---|---|
| Pneumonia (0.19%) | Transient hypoxia (5-25%) |
| Mediastinitis (0.18%) | Bleeding (especially in patients on antiplatelets/anti-coagulation) (0.68%) |
| Mediastinal abscess formation | Pneumothorax (0.03%) |
| Sepsis (0.01%) | Assembly/needle malfunction (0.2-1.3%) |
A systematic review found that the rate of serious adverse events (defined as either fatal/causing permanent impairment/requiring hospital admission) is less than 0.05%.[189] An observational study by Çağlayan et al.[190] from Turkey reported the rate of serious adverse events to be 0.16%. Another review by Vaidya et al. found that although complication rates were less than 1.4% across all studies with common events being bleeding, infection, pneumothorax, and anesthesia-related complications.[192] Scope damage may also occur in up to 1.3% of cases.[192] Apart from the above, some rarer complications include bacterial pericarditis, pneumoperitoneum, hemothorax, hemotympanum and endobronchial polyp formation.[193,194,195,196,197]
Although no evidence is available to recommend specific interventions to minimize complications, the guidelines expert group suggested a few general measures as useful practice measures as summarized below.
Recommendations
General principles of asepsis should be rigorously followed. (UPP)
Stylet and needle should be handled in a sterile manner especially when multiple passes are obtained. (UPP)
During introduction and removal of needle into and from channel, retraction of needle into sheath should be visually confirmed to prevent scope damage. Needle should be locked properly. (UPP)
In known asthmatics, bronchodilator administration immediately prior to the procedure may help prevent bronchospasm during the procedure. (UPP)
Sampling should be avoided from densely calcified lymph nodes to avoid inadvertent needle breakage. (UPP)
Excessive force should be avoided during needle insertion; in this situation, the puncture site should be slightly changed. (UPP)
SECTION VI: SAMPLE PROCESSING AND RAPID ON-SITE EVALUATION
Should cell block be prepared in all cases of EBUS-TBNA?
Cell block preparation is a technique that involves processing of blood clots, sediments, or tissue fragments from cytological specimen into paraffin blocks so that they can be processed as histopathology specimens. Broadly, this technique involves transfer of EBUS aspirated to a specialized solution or formalin instead of glass slides. It brings out tissue architectural information and can be used for immunohistochemistry (IHC) and molecular studies.[198] Various methods of preparing cell block have been described, such as simple sedimentation, Agarose method, egg albumin method, tissue coagulum clot method, formalin fixed paraffin embedded technique, and various others.[198]
Rahman et al.[199] prospectively studied the diagnostic performance of cell block and cytology in 93 patients undergoing EBUS-TBNA and who had both cytology and cell block prepared. A definitive diagnosis by either method was made in 77 patients (83% overall yield), in 74 (79.6%) cases based on cytology alone, and in 68 (73%) based on cell blocks alone. For malignancies, the overall yield of EBUS-TBNA was 91% (Cytology: 89%, cell block: 82%). Using cell block, better tumor histology—76% (35/46), definite histological subtyping of NSCLC—32.6% (15/46) and immunohistochemistry (IHC) (82.5%) were feasible. All patients where IHC was done on cell block were judged to be adequate for mutational analysis. For suspected benign etiology, there was no additional benefit of cell blocks in tuberculosis, with a diagnostic yield of 81% and 56% for cytology and cell blocks, respectively. For sarcoidosis however, cell blocks provided 14.3% additional yield compared to cytology alone (78.6% vs 64.3%).[199]
Santos et al.[200] conducted a prospective study in 270 patients to determine the contribution of cell block by EBUS-TBNA on diagnostic yield in lung cancer. Cell blocks were prepared by air drying and clotting specimen on filter paper and placing them in 10% formalin. Among the total 697 EBUS-TBNA aspirates performed, 54 (7.7%) cell blocks provided additional information. Of the 189 non-diagnostic smears, cell blocks provided additional pathological information in 50 patients, thus increasing the yield of EBUS-TBNA from 72.9% to 80%. Cell blocks also made epithelial growth factor receptor (EGFR) mutation analysis possible in 39/64 patients whose TBNA samples showed metastatic adenocarcinoma (60.1%). Overall, cell blocks provided clinically significant information in 83 of the 270 patients (30.7%).[200]
In multiple other retrospective studies, it has been shown that cell blocks increase the diagnostic yield of EBUS-TBNA in sarcoidosis and are useful in lung cancer for histological subtyping, immunohistochemistry, and mutation analysis.[201,202,203,204] In a recent review, the authors suggest that cell blocks should be routinely prepared in addition to slide smears.[205]
Evidence summary
In lung cancer, cell blocks of EBUS-TBNA specimens have been shown to improve the identification of tumor histology and allow subtyping of NSCLC, IHC and mutation testing.
Cell blocks made from EBUS-TBNA specimen have been shown to increase the diagnostic yield in sarcoidosis.
Recommendation
Cell blocks should be made routinely in addition to direct slide smears while obtaining samples during EBUS-TBNA. (2A)
How should EBUS aspirate samples be prepared and processed?
The various methods by which samples obtained by EBUS could be processed include[205]:
Preparing slides from the material obtained.
Sample may be used for cellblock preparations.
Placing the samples in alcohol-based medium for processing automatically in liquid-based cytology machine.
Collection of clots in the needle for processing as histopathology specimen.
Slides from EBUS samples may be made by various methods (squash, pull apart, loop press) with an aim to achieve a monolayer of cells.[205] There is no proven advantage of one method over the other. EBUS samples are both air-dried and wet-fixed in 95% ethanol. The air drying prevents cell loss while the wet fixing preserves the shape and structure of cells.[205] The air-dried smears are stained by Romanowski stains such as Toluidine Blue for rapid on-site evaluation (ROSE) and used later for May-Grunwald-Giemsa staining. The ethanol-fixed smears are sent for Papanicolaou staining.
Specimens can also be used for cell block preparation in which sample is put into a separate vial containing either alcohol-based fixative or formalin. For cell blocks, various collecting media including saline, 10% formalin, Rosewell Park Memorial Institute (RPMI) media and cytolyt, among others have been described. When lymphoma and infections are suspected, RPMI media and normal saline are used to send samples for flow cytometry and microbiological studies.[206]
The clots which remain in needle lumen after the procedure can be sent in formalin for histopathology examination.
Liquid-based cytology (LBC) is also an option for EBUS sample preparation. It is optimal for viewing the cellular features as it reduces the air-drying artifact and obscures the background element, thus reducing the number of unsatisfactory smears. However, this technique is costly as the processing occurs in an automated LBC machine.
Rotolo et al.,[206] retrospectively, compared various techniques of EBUS specimen preparation from 199 patients (139 suspected neoplasm and 60 suspected granulomatous disease). The techniques compared were cytology slides (n = 42; 21%); cell-block (n = 25; 13%); core-tissue (n = 60; 30%); combination of cytology slides and core-tissue (n = 51; 26%); and combination of cytology slides and cell-block (n = 21; 10%). The authors found the diagnostic yield, accuracy, and area under the curve (AUC) of various methods to be as follows: a) cytology slides—81%, 80%, and 0.90; b) cell-block—48%, 33%, and 0.67; c) core-tissue—87%, 99%, and 0.96; d) cytology slides + core-tissue—80%, 100%, and 1.00; and e) cytology slides + cell-block— 86%, 100%, and 1.00, respectively.
Their results were significantly discordant from previously reported literature for cell blocks. The authors attributed this to lack of experience of their pathologist in cell block reporting, as the commonly used techniques at their institution were slide cytology and core tissue.[206] The authors concluded that the use of combination techniques improves the diagnostic performance but will be more resource consuming; and that the choice of specimen processing method should take into consideration the pathologist’s preference and expertise. No comparison of diagnostic accuracy in malignant versus benign etiology was provided. As discussed in the previous section, Rahman et al. compared cytology and cell block among 93 patients undergoing EBUS-TBNA and did not find cell block to increase diagnostic yield. However, among malignant cases, cell block provided better identification of tumor morphology and enabled histologic subtyping.[199]
Toth et al.[207] retrospectively compared cytology with histological core biopsy techniques in 177 consecutive patients who underwent EBUS-TBNA and found that diagnostic yield for histology was better than cytology for benign disorders. The combined yield of histology and cytology was higher than that of cytology alone. For malignant disorders, however, there was no difference between cytology, histology, or combination of both. Overall, combining histologic and cytologic results led to fewer missed diagnoses than by either method individually.
For microbiological investigations, EBUS-TBNA aspirate may be sent in sterile containers with normal saline. Material can be sent for bacterial, fungal, and mycobacterial cultures.[205] In countries with high burden of tuberculosis, it is important to submit the EBUS aspirate for Xpert Mtb/Rif assay. The Xpert assay has a sensitivity of more than 72% and specificity of more than 96% in EBUS aspirates from mediastinal nodes.[6] In a study from India aimed at studying the role of Xpert in differentiating TB from sarcoidosis among 147 patients who underwent EBUS-TBNA, Xpert MTB/RIF was positive in 26 (49.1%) and two (2.1%) patients with TB and sarcoidosis, respectively. The sensitivity and specificity of Xpert MTB/RIF for diagnosing TB was 49.1%, 97.9%, respectively, in EBUS samples.[208]
Overall, it appears that adding histology samples offers higher diagnostic accuracy than only cytological preparation of EBUS-TBNA. However, this is more resource consuming and the specimen processing method chosen should take into consideration the pathologist’s preference and expertise.
Does Rapid On-Site Evaluation improve the diagnostic performance of EBUS?
Rapid On-Site Evaluation (ROSE) is a real-time technique for examining cytological specimen and is undertaken along with sequential sampling.[209] ROSE requires a cytological microscope to be placed in the procedure room and is helpful by evaluating sample adequacy, ensuring triage of samples for ancillary studies and providing a provisional diagnosis.[209,210] The EBUS-TBNA specimen expressed on a slide are stained by rapid stains (Romanowsky stains for air-dried and rapid Papanicolaou stains for wet fixed slides) and are assessed by a cytopathologist on site.[210]
The utility of ROSE in enhancing the performance status of EBUS has been evaluated extensively in the past decade. Oki et al.[211] randomized 108 patients (55 in ROSE group and 53 in non-ROSE group) and found that the diagnostic yield of both two groups was similar. However, the non-ROSE group underwent more additional procedures and higher number of nodal punctures than the ROSE group. Trisolini et al.[212] studied the effect of ROSE on molecular analysis of lung cancer in 197 subjects and found that patients in the ROSE arm were less likely to have sample inadequacy for molecular testing (0 vs 6, P = .05) and were more likely to have the bronchoscopy terminated after a single biopsy site (58.9% vs 44.1%, P = .01).[212] In a randomized trial of 80 patients, who underwent conventional TBNA with or without ROSE, or EBUS-TBNA with or without ROSE (20 patients in each group), the yield for EBUS-TBNA with or without ROSE was similar (67% and 68%, respectively).[213]
Other prospective studies without no-ROSE comparator have also been conducted. Bharati et al.[214] prospectively evaluated the role of ROSE in 47 patients undergoing EBUS-TBNA in a TB endemic setting and reported that ROSE assessment matched the final diagnosis in 84.45% cases.[214] Caupena et al.[215] reported a concordance >95% between ROSE and final cytological diagnosis in patients with NSCLC. Various other retrospective studies have shown a high concordance between ROSE and final diagnosis but did not show a significant increase in diagnostic yield or accuracy.[216,217,218,219] The ROSE group, however, requires significantly lesser lesions to be examined and lesser aspirates per procedure.[219] In another retrospective analysis of 236 patients (122 in ROSE group and 114 in non-ROSE group), it was found that the pathologically suspicious specimens on cytology and non-diagnostic specimens on pathology were both significantly lower in the ROSE group than in the non-ROSE group (8.7% vs. 14.6%, P = 0.038; and 0.9% vs. 4.4%, P = 0.018, respectively).[220]
The feasibility of ROSE by the pulmonologist has also been studied. Hopkins et al.[221] prospectively compared ROSE by pathologist, medical scientist, and respiratory registrar (Medical scientist had previously performed ROSE on 50 procedures and respiratory registrars received four hour-long sessions of training from pathologist). The concordance of medical scientist was 90% while respiratory registrars had more than 70% concordance with the pathologist. Another retrospective analysis of ROSE by pulmonologist in 125 patients (after 1 month training by cytopathologist) found the sensitivity, specificity, and diagnostic accuracy of ROSE to be 88.5%, 83.0%, and 86.4%, respectively.[222]
A systematic review and meta-analysis that included 5 studies and 618 subjects found no significant difference in diagnostic yield of EBUS-TBNA with or without ROSE. However, the use of ROSE did result in significantly lower number of needle passes.[223]
Evidence summary
ROSE does not enhance the diagnostic yield of EBUS-TBNA
Performing ROSE led to a reduction in number of passes and lesser requirement of additional bronchoscopic procedures
ROSE performed by a trained pulmonologist had a concordance rate of approximately 70-88% to that of a pathologist.
Recommendation
EBUS-TBNA can be performed irrespective of availability of ROSE. (1A)
ROSE should be performed where available. (2A)
Pulmonologist adequately trained in cytopathology, may perform ROSE when ROSE by pathologist is not feasible. (3A)
SECTION VII: EBUS-TBNA IN SPECIAL SITUATIONS
This section deals with the newer advances and sampling approaches related to EBUS-TBNA. The expert committee opined that EBUS-TBNA in certain special situations should be done only by experienced intervention pulmonologists who have performed at least 100 EBUS-TBNA procedures and have been trained in the advanced procedures. It is preferable to have a thoracic surgery backup while undertaking these procedures.
What is the utility of EBUS-TBNA in Non-Nodal pathologies?
Apart from nodal pathology, EBUS has been used to evaluate bronchogenic cysts of the mediastinum, pericardial effusion, and thyroid gland lesions. EBUS has also been used to diagnose pulmonary embolism in certain situations.[224] However, published literature about the utility and safety of EBUS-TBNA in non-nodal pathologies is sparse.
A systemic review aimed at describing mediastinal cysts also evaluated a subgroup comprising 15 patients who underwent EBUS-TBNA for the bronchogenic cyst. Complications were reported in 3 (20%) patients and included cyst infection, rupture, and mediastinitis.[225] Similarly, another case series of three patients reported a high complication rate following cyst aspiration via EBUS-TBNA.[226] Transbronchial pericardial fluid aspiration has been reported; however, it is more risky than regular anterior pericardiocentesis and the only advantage it may offer is in specific settings when needed on priority while awaiting a formal anterior transthoracic ultrasound.[227] There are only a few case reports of utilizing EBUS-TBNA for pericardial effusion; however, the inherent risk of cardiac injury and pericardial infection preclude its routine use.
EBUS-TBNA has been found to be useful for evaluating thyroid lesions, particularly those with a complete intrathoracic location, wherein other diagnostic modalities are not feasible.[228] In a retrospective series of 12 cases of EBUS-TBNA used for thyroid lesion sampling using a 22G needle, no EBUS-related complications were reported.[229] A comparison of transdermal sampling and EBUS-TBNA of thyroid lesions with 19G and 21G needles found that the EBUS technique produced more cell block material with 19G needle; safety wise, both methods were comparable.[230]
Evidence summary
Cystic lesions of the mediastinum
EBUS-TBNA has been used for diagnosis and therapeutic aspiration of mediastinal bronchogenic cysts
The use of EBUS-TBNA for mediastinal cystic lesions has high complication rates
The common complications include infective mediastinitis, cyst infection, rupture, and pneumonia
Pericardial effusion
There is limited data on the utility of EBUS-guided pericardial fluid aspiration.
However, in a few selected reports, EBUS has been used for aspiration of difficult-to-access loculated pericardial effusion.
Thyroid lesions
EBUS- TBNA has been used as one of the modalities to evaluate thyroid lesions, particularly those with an intra-thoracic location, and demonstrated acceptable diagnostic yield.
Recommendations
Considering the high complication rates, it is preferable to avoid EBUS-guided aspiration of mediastinal bronchogenic cyst. (3B)
A diagnostic aspiration may be considered only when alternative modes of diagnosis are either not feasible or have been unsuccessful. (UPP)
EBUS-guided pericardial fluid aspiration should not be performed routinely. (UPP)
EBUS-TBNA may be used to evaluate intra-thoracic thyroid lesions, particularly in cases where percutaneous image-guided needle aspiration is risky and not feasible. (3B)
What is the safety and efficacy of Trans-vascular and Intravascular EBUS-TBNA?
Endobronchial ultrasound-guided trans-vascular needle aspiration (EBUS-TVNA) and transbronchial intravascular needle aspiration (EBUS-TIVNA) are done to diagnose difficult-to-approach lesions which cannot be sampled without passing through a blood vessel. Usually, nodal stations 5 and 6 require trans-vascular procedures. Intravascular lesions such as vascular tumors are sampled with the EBUS-TIVNA approach.
A systemic review of case reports describing the role of EBUS-TIVNA in diagnosing pulmonary arterial tumors such as sarcomas and tumor emboli demonstrated that EBUS-TIVNA in diagnosing intravascular tumors appears to be a feasible, safe, and minimally invasive procedure.
Multiple small retrospective studies and case series have described the use of trans-vascular needle aspiration for mediastinal lesion sampling. However, the decision to puncture a major blood vessel to obtain a diagnostic specimen should be made on a case-to-case basis after thorough consideration of the risks and benefits. Complications observed are hematoma and hemomediastinum.[231] This procedure has been done under GA or under conscious sedation using 22G or 21G EBUS needles. The vessels commonly traversed are pulmonary artery branches and aorta. EBUS-TVNA has a diagnostic accuracy varying from 71% to 100%.[232,233,234] Few case series from India have also reported a similar yield and diagnostic accuracy.[235,236,237]
Trans-vascular endosonographic-guided sampling is an important adjunct to conventional endoscopic techniques to sample intrathoracic lesions that are not accessible without vascular puncture. Larger prospective trials are warranted to explore its diagnostic potential. Trans-vascular biopsy should be undertaken only if other proven methods are not readily available or have higher potential morbidity.
Evidence summary
EBUS-TVNA has diagnostic accuracy varying from 71% - 100%.
EBUS-TVNA and EBUS-TIVNA have been performed only as a last resort procedure in settings with thoracic surgery backup.
The serious complications, although uncommon, were bleeding, hematoma, hemo-mediastinum, and pseudoaneurysm of the vessel.
Recommendations
EBUS-TVNA and EBUS-TIVNA may be considered only when alternative modes of diagnosis are either not feasible or have been unsuccessful. (UPP)
EBUS-TVNA and EBUS-TIVNA should only be performed by an experienced operator for difficult-to-approach lesions after risk-benefit analysis on a case-to-case basis, and patients should be closely monitored for long-term complications. (3A)
Rapid on-site evaluation (ROSE) should be performed in all cases of EBUS-TVNA to minimize the number of needle passes.(UPP)
What is the role of intra-nodal forceps biopsy during EBUS-TBNA?
The accuracy of tissue sampling and diagnostic yield of EBUS-TBNA has improved with the development of various needle sizes from 25G to 19G. However, in certain conditions like sarcoidosis and lymphoma, EBUS may be non-diagnostic; furthermore, in the current era of targeted therapy in lung cancer, more tissue sample is required for molecular marker testing and next-generation sequencing to guide further treatment. EBUS-guided intra-nodal forceps biopsy (EBUS-IFB) has been developed to address these deficiencies for better tissue acquisition. A recent meta-analysis revealed that the addition of EBUS-IFB to EBUS-TBNA improves the overall diagnostic yield of sampling intra-thoracic adenopathy compared to EBUS-TBNA alone. However, the complication rates of the combined approach are higher than with EBUS-TBNA alone but lower than transbronchial or surgical biopsies.[238] Among the 12 observational studies[239,240,241,242,243,244,245,246,247,248,249,250] that compared the diagnostic yield, safety, and efficacy of combined EBUS-IFB/EBUS-TBNA versus EBUS-TBNA alone, only one study found EBUS-FNA cytology to be more efficacious compared to EBUS-IFB.[247] In the remaining 11 studies, the overall diagnostic yield of a combined procedure was higher than EBUS-TBNA alone. Similarly, in a retrospective study of 12 patients, it was demonstrated that EBUS-IFB was diagnostic in all the four patients in whom EBUS-TBNA alone did not provide a diagnostic material.[240]
In another prospective analysis, it was found that adding EBUS-IFB increased the diagnostic sensitivity of EBUS-TBNA from 50.0 to 82.0%. EBUS-guided forceps biopsies measuring ≥3 mm enabled a specific diagnosis to be established more often than did forceps biopsies <3 mm (90.9% vs. 57.1%) suggesting larger forceps biopsy samples provide higher yield.[242] Similar results were seen in a study by Darwiche et al.[250] wherein addition of EBUS-IFB increased the diagnostic yield of EBUS-TBNA from 64 to 93% in benign conditions. In patients with a negative rapid-on-site evaluation (ROSE) during EBUS-TBNA, the use of EBUS-IFB has been shown to be safe and augments the diagnostic yield, especially in benign etiologies of mediastinal lymphadenopathy.[243]
Evidence summary
EBUS-IFB is a safe procedure when performed by experienced operators.
Combining EBUS-IFB and EBUS-TBNA for the evaluation of mediastinal lymphadenopathy improves the overall diagnostic yield, particularly in benign conditions.
Among the patients with negative rapid-on-site cytology, the use of EBUS-IFB improved the diagnostic yield of EBUS-TBNA.
Recommendations
EBUS- IFB may be performed only by an experienced operator as an additional modality for patients with negative rapid-on-site evaluation (ROSE) or in situations when tissue biopsy is required for definitive diagnosis (e.g. lymphoma).(2B)
EBUS-IFB may also be considered in patients with prior non-diagnostic EBUS-TBNA. (UPP)
What is the role of EBUS-guided mediastinal cryobiopsy during EBUS-TBNA?
EBUS-TBNA provides an excellent diagnostic yield for primary lung malignancies. However, the limited amount of tissue obtained may be insufficient to diagnose benign mediastinal diseases. In this context, endobronchial cryotherapy has been used as a diagnostic and therapeutic tool in bronchoscopy for providing larger specimens. It uses a cryoprobe which delivers liquid nitrogen or carbon dioxide and freezes the tissue. Through rapid freeze-thaw cycles, cryotherapy causes cell death and tissue necrosis and helps retrieve larger tissue for histopathological examination. EBUS-guided mediastinal cryobiopsy is a novel approach for sampling mediastinal lymph nodes using a special 1.1 mm cryo-probe.
A recent RCT by Zhang et al.[251] comparing the efficacy and safety of EBUS-TBNA with EBUS-guided mediastinal cryobiopsy demonstrated that the overall diagnostic yield for EBUS-TBNA was 79.9%, while it was 91.8% for transbronchial mediastinal cryobiopsy (P = 0.001). The diagnostic yields were similar for metastatic lymphadenopathy (94.1% vs. 95.6%, P = 0.58). However, cryobiopsy was more sensitive than EBUS-TBNA in uncommon tumors (91.7% vs. 25%, P = 0.001) and benign disorders (80.9% vs. 53.2%, P = 0.004), especially sarcoidosis. No clinically significant procedure-related complications were encountered. Few more case series, including one from India, have demonstrated the procedure to be safe and associated with high diagnostic yield when performed by experienced operators.[252,253]
Evidence summary
EBUS-guided mediastinal lymph node cryobiopsy is a safe procedure when performed by experienced operators.
The addition of EBUS-guided mediastinal cryobiopsy has been shown to improve the diagnostic yield of EBUS-TBNA, particularly in benign pathologies.
Recommendation
EBUS-guided mediastinal cryobiopsy should be performed by an experienced operator, in patients with negative rapid-on-site evaluation (ROSE), or prior non-diagnostic EBUS-TBNA. (UPP)
What is the utility of EBUS-TBNA and EUS-B-FNA in children?
EBUS-TBNA and EUS-B-FNA are established modalities for sampling mediastinal lymph nodes in adults. EBUS-TBNA has been used for diagnosing TB lymphadenitis in pediatric patients.[254] Performing EBUS-TBNA in the pediatric population with currently available EBUS bronchoscopes is technically challenging due to the smaller tracheal diameter; EUS-B-FNA is, therefore, may be a viable option for such patients.[255,256]
There is a paucity of prospective data regarding the safety and efficacy of EBUS-TBNA and EUS-B-FNA in the pediatric population. In retrospective studies, the diagnostic yield of EBUS-TBNA/EUS-B-FNA in children ranges from 37% to 100%.[152,257] A multi-centric Indian study reported that EBUS-TBNA and EUS-B-FNA had 79.1% sensitivity in pediatric subjects.[258] Although no major complications were noted, minor complications occurred in 8.9% of patients, including transient hypoxemia, tachycardia, hypotension, airway bleeding, and excessive coughing. A recent meta-analysis reported pooled diagnostic yield of 61% (95%CI, 43%-77%), while procedure-related complications were seen in 3.5% of cases. Transcutaneous mediastinal ultrasonography has been described for mediastinal lymphadenopathy in children, but it cannot visualize stations 8, 9, 11, 12, 13, and 14.[259]
Evidence summary
EBUS-TBNA and EUS-B-FNA are safe modalities for diagnostic evaluation of mediastinal lymphadenopathy in children
Recommendation
EBUS-TBNA and EUS-B-FNA can be performed by experienced operators for sampling mediastinal lymph nodes in children. (3A)
SECTION VIII: TRAINING IN EBUS-TBNA
What are the training or experience requirements for achieving competence in EBUS-TBNA?
With increasing demand for EBUS training among pulmonologists, structured training programs are gaining widespread acceptance in teaching institutions across the world. However, there is paucity of data regarding the minimum number of procedures required to achieve competence in EBUS. In a recent systematic review comprising 16 observational studies, the threshold numbers needed to attain proficiency in EBUS-TBNA and overcome the initial learning curve varied from 10 to 100, with a mean of 37-44 procedures.[260] Also, it was seen that acceptable EBUS-TBNA yield increases with increasing experience among trainees. Bellinger et al.[261] demonstrated that physicians who performed more than ten procedures per year achieved higher diagnostic yields compared to those who performed fewer than ten procedures annually (86 vs. 68%, P < 0.01). The yield of trainees also improved with every ten procedures (79%, 90%, and 95%, respectively) and that of attending physicians with experience (1–25 procedures: 78% yield, 26–50 procedures: 87% yield, and 50 + procedures: 90% yield). Among trainees, failure rates declined steadily. This study concluded, therefore, that a minimum of 20–25 procedures are needed to achieve acceptable levels of diagnostic yield. Due to lack of robust evidence for the number of procedures required to achieve optimum EBUS competence, the recommendations are primarily based on expert committee opinion.
Evidence summary
EBUS-TBNA yield improves with increasing experience amongst bronchoscopists and trainees.
The number needed to overcome the initial learning curve of EBUS varied from 10 to 100 procedures.
Recommendation
At least 40 procedures should be performed over a minimum of 2 years to overcome the initial learning curve and achieve acceptable yield in EBUS-TBNA. (2A)
Is there a role for simulation-based training of EBUS-TBNA?
Bronchoscopy training, especially with EBUS, has significantly evolved over the past decade due to improved accessibility to mediastinal structure sampling. The Indian flexible bronchoscopy guidelines have recommended that a structured program be implemented for basic flexible bronchoscopy training using modalities like virtual-reality-(VR) based simulators, anatomical models, lectures, or conventional apprenticeships, as available.[23] However, the impact of simulator-based EBUS training on real-life patient outcomes is still not clear. Presently, there is no consensus on the ideal teaching method for EBUS training.
Since EBUS-TBNA is very operator dependent and has a long initial learning curve, simulation-based training might help reduce the time for the initial learning curve, and an assessment tool could be used to assess the operator’s performance.
A recent systematic review of 8 observational studies and 2 RCTs evaluated the role of simulation-based training of EBUS-TBNA. In most studies that compared simulator-based training with traditional apprenticeship-based training, no differences between procedure time and successful biopsies were observed. It was concluded that the EBUS simulator and EBUS assessment tools could be objectively used to assess the training of an EBUS trainee, and the number of procedures needed to overcome the initial learning curve is approximately 40.[260]
However, in one retrospective observational study, simulation training was associated with higher occurrence of minor complications.[262] In a randomized comparison of virtual reality-based training with apprenticeship training, the VR trained novices’ attained a higher score than those who did apprenticeship.[263] This shows that both these methods are equivalent in improving the skill of trainees in EBUS; however, VR training is more effective with better scores as assessed by EBUS assessment tools. Another recently published study suggested that a multimodal simulation-based curriculum can improve EBUS skills and knowledge.[264]
Evidence summary
Virtual-reality simulator training is more effective than traditional apprenticeship training for EBUS-TBNA when assessed by EBUS assessment tools. However, data on patient-oriented outcomes are not available.
Recommendation
For the training program in EBUS-TBNA, a virtual reality simulator may be incorporated into the traditional apprenticeship model, wherever feasible. (3B)
CONCLUSIONS
The endobronchial ultrasound has become an invaluable diagnostic tool for the pulmonologist. Although the number of EBUS-TBNA procedures is rapidly increasing, substantial variability exists in its technique among the operators. This document is an evidence-based compilation and assessment of the available scientific literature related to EBUS-TBNA that covers various important aspects such as appropriate patient selection and pre-procedure preparation, sedation and monitoring, technical considerations during the procedure, and training requirements. Standard technical and procedural recommendations of EBUS-TBNA practices for respiratory physicians have been formulated.
Financial support and sponsorship
The activity was supported by an educational grant by the Indian Chest Society and the Indian Association for Bronchology.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE:An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen H, Lou L, Chen T, Zou Y, Wang B, Xu Z, et al. Comparison of transbronchial needle aspiration with and without ultrasound guidance for diagnosing benign lymph node adenopathy. Diagn Pathol. 2020;15:36. doi: 10.1186/s13000-020-00958-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Signorini F, Panozzi M, Proietti A, Alì G, Fanucchi O, Picchi A, et al. Conventional Transbronchial Needle Aspiration (cTBNA) and EBUS-Guided Transbronchial Needle Aspiration (EBUS-TBNA):A retrospective study on the comparison of the two methods for diagnostic adequacy in molecular analysis. J Mol Pathol. 2021;2:296–305. [Google Scholar]
- 4.Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P, et al. The IASLC lung cancer staging project:A proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4:568–77. doi: 10.1097/JTO.0b013e3181a0d82e. [DOI] [PubMed] [Google Scholar]
- 5.Li W, Zhang T, Chen Y, Liu C, Peng W. Diagnostic value of convex probe endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal tuberculous lymphadenitis:A systematic review and meta-analysis. Med Sci Monit. 2015;21:2064–72. doi: 10.12659/MSM.894526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhasmana DJ, Ross C, Bradley CJ, Connell DW, George PM, Singanayagam A, et al. Performance of Xpert MTB/RIF in the diagnosis of tuberculous mediastinal lymphadenopathy by endobronchial ultrasound. Ann Am Thorac Soc. 2014;11:392–6. doi: 10.1513/AnnalsATS.201308-250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chhajed PN, Vaidya PJ, Mandovra NP, Chavhan VB, Lele TT, Nair R, et al. EBUS-TBNA in the rapid microbiological diagnosis of drug-resistant mediastinal tuberculous lymphadenopathy. ERJ Open Res. 2019;5:00008–2019. doi: 10.1183/23120541.00008-2019. doi:10.1183/23120541.00008-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal R, Srinivasan A, Aggarwal AN, Gupta D. Efficacy and safety of convex probe EBUS-TBNA in sarcoidosis:A systematic review and meta-analysis. Respir Med. 2012;106:883–92. doi: 10.1016/j.rmed.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal R, Aggarwal AN, Gupta D. Efficacy and safety of conventional transbronchial needle aspiration in sarcoidosis:A systematic review and meta-analysis. Respir Care. 2013;58:683–93. doi: 10.4187/respcare.02101. [DOI] [PubMed] [Google Scholar]
- 10.von Bartheld MB, Dekkers OM, Szlubowski A, Eberhardt R, Herth FJ, in 't Veen JCCM, et al. Endosonography vs conventional bronchoscopy for the diagnosis of sarcoidosis:The GRANULOMA randomized clinical trial. JAMA. 2013;309:2457–64. doi: 10.1001/jama.2013.5823. [DOI] [PubMed] [Google Scholar]
- 11.Gupta D, Dadhwal DS, Agarwal R, Gupta N, Bal A, Aggarwal AN. Endobronchial ultrasound-guided transbronchial needle aspiration vs conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest. 2014;146:547–56. doi: 10.1378/chest.13-2339. [DOI] [PubMed] [Google Scholar]
- 12.Figueiredo VR, Cardoso PFG, Jacomelli M, Santos LM, Minata M, Terra RM. EBUS-TBNA versus surgical mediastinoscopy for mediastinal lymph node staging in potentially operable non-small cell lung cancer:A systematic review and meta-analysis. J Bras Pneumol. 2020;46:e20190221. doi: 10.36416/1806-3756/e20190221. [DOI] [PubMed] [Google Scholar]
- 13.Ge X, Guan W, Han F, Guo X, Jin Z. Comparison of endobronchial ultrasound-guided fine needle aspiration and video-assisted mediastinoscopy for mediastinal staging of lung cancer. Lung. 2015;193:757–66. doi: 10.1007/s00408-015-9761-3. [DOI] [PubMed] [Google Scholar]
- 14.Labarca G, Sierra-Ruiz M, Kheir F, Folch E, Majid A, Mehta HJ, et al. Diagnostic accuracy of endobronchial ultrasound transbronchial needle aspiration in lymphoma. A systematic review and meta-analysis. Ann Am Thorac Soc. 2019;16:1432–9. doi: 10.1513/AnnalsATS.201902-175OC. [DOI] [PubMed] [Google Scholar]
- 15.Dziedzic D, Peryt A, Szolkowska M, Langfort R, Orlowski T. Evaluation of the diagnostic utility of endobronchial ultrasound-guided transbronchial needle aspiration for metastatic mediastinal tumors. Endosc Ultrasound. 2016;5:173–7. doi: 10.4103/2303-9027.183973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nambirajan A, Longchar M, Madan K, Mallick SR, Kakkar A, Mathur S, et al. Endobronchial ultrasound-guided transbronchial needle aspiration cytology in patients with known or suspected extra-pulmonary malignancies:A cytopathology-based study. Cytopathology. 2019;30:82–90. doi: 10.1111/cyt.12656. [DOI] [PubMed] [Google Scholar]
- 17.Sanz-Santos J, Andreo F, García-Olivé I, Remón J, Monsó E. Diagnosis of acute pulmonary embolism by endobronchial ultrasound as an incidental finding. Respiration. 2011;81:150–1. doi: 10.1159/000319700. [DOI] [PubMed] [Google Scholar]
- 18.Cetinkaya E, Yılmaz A, Özgül A, Gençoğlu A, Günlüoğlu G. Left atrial mass demonstrated during endobronchial ultrasound session. Respiration. 2011;81:57–8. doi: 10.1159/000319701. [DOI] [PubMed] [Google Scholar]
- 19.Casal RF, Jimenez CA, Mehran RJ, Eapen GA, Ost D, Sarkiss M, et al. Infected mediastinal bronchogenic cyst successfully treated by endobronchial ultrasound-guided fine-needle aspiration. Ann Thorac Surg. 2010;90:e52–3. doi: 10.1016/j.athoracsur.2010.06.062. [DOI] [PubMed] [Google Scholar]
- 20.Miyazu Y, Miyazawa T, Kurimoto N, Iwamoto Y, Ishida A, Kanoh K, et al. Endobronchial ultrasonography in the diagnosis and treatment of relapsing polychondritis with tracheobronchial malacia. Chest. 2003;124:2393–5. doi: 10.1378/chest.124.6.2393. [DOI] [PubMed] [Google Scholar]
- 21.Soja J, Grzanka P, Sładek K, Okoń K, Ćmiel A, Mikoś M, et al. The use of endobronchial ultrasonography in assessment of bronchial wall remodeling in patients with asthma. Chest. 2009;136:797–804. doi: 10.1378/chest.08-2759. [DOI] [PubMed] [Google Scholar]
- 22.Khan F, Anker CJ, Garrison G, Kinsey CM. Endobronchial ultrasound–guided transbronchial needle injection for local control of recurrent non–small cell lung cancer. Ann Am Thorac Soc. 2015;12:101–4. doi: 10.1513/AnnalsATS.201408-358BC. [DOI] [PubMed] [Google Scholar]
- 23.Mohan A, Madan K, Hadda V, Tiwari P, Mittal S, Guleria R, et al. Guidelines for diagnostic flexible bronchoscopy in adults: Joint Indian Chest Society/National College of Chest Physicians (I)/Indian association for bronchology recommendations. Lung India. 2019;36((Suppl 2)):S37–89. doi: 10.4103/lungindia.lungindia_108_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boustière C, Veitch A, Vanbiervliet G, Bulois P, Deprez P, Laquiere A, et al. Endoscopy and antiplatelet agents. European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2011;43:445–61. doi: 10.1055/s-0030-1256317. [DOI] [PubMed] [Google Scholar]
- 25.Veitch AM, Vanbiervliet G, Gershlick AH, Boustiere C, Baglin TP, Smith LA, et al. Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants:British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Gut. 2016;65:374–89. doi: 10.1136/gutjnl-2015-311110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Douketis JD, Spyropoulos AC, Spencer FA, Mayr M, Jaffer AK, Eckman MH, et al. Perioperative management of antithrombotic therapy:Antithrombotic therapy and prevention of thrombosis, 9th ed:American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141((2 Suppl)):e326S–50S. doi: 10.1378/chest.11-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rand IAD, Blaikley J, Booton R, Chaudhuri N, Gupta V, Khalid S, et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults:Accredited by NICE. Thorax. 2013;68((Suppl 1)):i1–44. doi: 10.1136/thoraxjnl-2013-203618. [DOI] [PubMed] [Google Scholar]
- 28.Webb TN, Flenaugh E, Martin R, Parks C, Bechara RI. Effect of routine clopidogrel use on bleeding complications after endobronchial ultrasound-guided fine needle aspiration. J Bronchology Interv Pulmonol. 2019;26:10–4. doi: 10.1097/LBR.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 29.Meena N, Abouzgheib W, Patolia S, Rosenheck J, Boujaoude Z, Bartter T. EBUS-TBNA and EUS-FNA:Risk assessment for patients receiving clopidogrel. J Bronchology Interv Pulmonol. 2016;23:303–7. doi: 10.1097/LBR.0000000000000312. [DOI] [PubMed] [Google Scholar]
- 30.Stather DR, MacEachern P, Chee A, Tremblay A. Safety of endobronchial ultrasound-guided transbronchial needle aspiration for patients taking clopidogrel:A report of 12 consecutive cases. Respiration. 2012;83:330–4. doi: 10.1159/000335254. [DOI] [PubMed] [Google Scholar]
- 31.Gil HI, Ko RE, Lee K, Um SW, Kim H, Jeong BH. Effects of antithrombotic treatment on bleeding complications of EBUS-TBNA. Medicina (Kaunas) 2021;57:142. doi: 10.3390/medicina57020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herman DD, Thomson CC, Brosnhan S, Patel R, Trosini-Desert V, Bilaceroglu S, et al. Risk of bleeding in patients undergoing pulmonary procedures on antiplatelet or anticoagulants:A systematic review. Respir Med. 2019;153:76–84. doi: 10.1016/j.rmed.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 33.Rabin A, Krumme AA, Keyes C, Folch EE. A24 Epidemiology, Diagnosis, and Treatment in Pleural Disease and IP. American Thoracic Society; 2020. [[Last accessed on 2021 Dec 10]]. Risks associated with anti-platelet medication continuation among patients undergoing endobronchial ultrasound-guided transbronchial needle aspiration; p. A1110. (American Thoracic Society International Conference Abstracts) Available from: https://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A1110 . [Google Scholar]
- 34.Swiatek K, Guthrie R, Elliott J, Jordan K. Antiplatelet therapy in patients undergoing EBUS-TBNA:Risk vs benefit. Chest. 2016;150((4 Suppl)):1017A. [Google Scholar]
- 35.Eapen GA, Shah AM, Lei X, Jimenez CA, Morice RC, Yarmus L, et al. Complications, consequences, and practice patterns of endobronchial ultrasound-guided transbronchial needle aspiration. Chest. 2013;143:1044–53. doi: 10.1378/chest.12-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bjørtuft O, Brosstad F, Boe J. Bronchoscopy with transbronchial biopsies:Measurement of bleeding volume and evaluation of the predictive value of coagulation tests. Eur Respir J. 1998;12:1025–7. doi: 10.1183/09031936.98.12051025. [DOI] [PubMed] [Google Scholar]
- 37.Kozak EA, Brath LK. Do “screening”coagulation tests predict bleeding in patients undergoing fiberoptic bronchoscopy with biopsy? Chest. 1994;106:703–5. doi: 10.1378/chest.106.3.703. [DOI] [PubMed] [Google Scholar]
- 38.Moon KM, Choi CM, Ji W, Lee JS, Lee SW, Jo KW, et al. Clinical characteristics of and risk factors for fever after endobronchial ultrasound-guided transbronchial needle aspiration:A retrospective study involving 6336 patients. J Clin Med. 2020;9:152. doi: 10.3390/jcm9010152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim SY, Lee JW, Park YS, Lee CH, Lee SM, Yim JJ, et al. Incidence of fever following endobronchial ultrasound-guided transbronchial needle aspiration. Tuberc Respir Dis (Seoul) 2017;80:45–51. doi: 10.4046/trd.2017.80.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Facciolongo N, Patelli M, Gasparini S, Lazzari Agli L, Salio M, Simonassi C, et al. Incidence of complications in bronchoscopy. Multicentre prospective study of 20,986 bronchoscopies. Monaldi Arch Chest Dis. 2009;71:8–14. doi: 10.4081/monaldi.2009.370. [DOI] [PubMed] [Google Scholar]
- 41.Takagi H, Nagaoka T, Ando K, Tsutsumi T, Ichikawa M, Koyama R, et al. Efficacy of antibiotic prophylaxis after endobronchial ultrasound-guided transbronchial needle aspiration:A preliminary prospective study. J Pulm Respir Med. 2017;7:4. [Google Scholar]
- 42.Park SS, Lim HJ, Heo EY, Chung HS, Kim DK. C39 The Spy Who Loved Bronchoscopy: Advances in Interventional Pulmonary Procedures. American Thoracic Society; 2014. [[Last accessed on 2022 Jul 17]]. The role of antibiotics following endobronchial ultrasound-guided transbronchial needle aspiration; p. A4400. (American Thoracic Society International Conference Abstracts) Available from: https://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2014.189.1_MeetingAbstracts.A4400 . [Google Scholar]
- 43.Wilson W, Taubert KA, Gewitz M, Lockhart PB, Baddour LM, Levison M, et al. Prevention of infective endocarditis. Circulation. 2007;116:1736–54. doi: 10.1161/CIRCULATIONAHA.106.183095. [DOI] [PubMed] [Google Scholar]
- 44.Hong KS, Choi EY, Park DA, Park J. Safety and efficacy of the moderate sedation during flexible bronchoscopic procedure:A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2015;94:e1459. doi: 10.1097/MD.0000000000001459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Apfelbaum JL, Hagberg CA, Connis RT, Abdelmalak BB, Agarkar M, Dutton RP, et al. 2022 American Society of Anesthesiologists Practice Guidelines for management of the difficult airway*. Anesthesiology. 2022;136:31–81. doi: 10.1097/ALN.0000000000004002. [DOI] [PubMed] [Google Scholar]
- 46.Continuum of Depth of Sedation: Definition of General Anesthesia and Levels of Sedation/Analgesia. [[Last accessed on 2021 Oct 04]]. Available from: https://www.asahq.org/standards-and-guidelines/continuum-of-depth-of-sedation-definition-ofgeneral-anesthesia-and-levels-of-sedationanalgesia .
- 47.Cornelissen CG, Dapper J, Dreher M, Müller T. Endobronchial ultrasound-guided transbronchial needle aspiration under general anesthesia versus bronchoscopist-directed deep sedation:A retrospective analysis. Endosc Ultrasound. 2019;8:204–8. doi: 10.4103/eus.eus_65_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yarmus LB, Akulian JA, Gilbert C, Mathai SC, Sathiyamoorthy S, Sahetya S, et al. Comparison of moderate versus deep sedation for endobronchial ultrasound transbronchial needle aspiration. Ann Am Thorac Soc. 2013;10:121–6. doi: 10.1513/AnnalsATS.201209-074OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ost DE, Ernst A, Lei X, Feller-Kopman D, Eapen GA, Kovitz KL, et al. Diagnostic yield of endobronchial ultrasound-guided transbronchial needle aspiration. Chest. 2011;140:1557–66. doi: 10.1378/chest.10-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Öztaş S, Aka Aktürk Ü, Alpay LA, Meydan B, Ogün H, Taylan M, et al. A comparison of propofol-midazolam and midazolam alone for sedation in endobronchial ultrasound-guided transbronchial needle aspiration:A retrospective cohort study:EBUS under different types of sedation. Clin Respir J. 2017;11:935–41. doi: 10.1111/crj.12442. [DOI] [PubMed] [Google Scholar]
- 51.Franzen D, Schneiter D, Weder W, Kohler M. Impact of sedation technique on the diagnostic accuracy of endobronchial ultrasound-guided transbronchial needle aspiration. Endosc Ultrasound. 2017;6:257–64. doi: 10.4103/2303-9027.190925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boujaoude Z, Arya R, Shrivastava A, Pratter M, Abouzgheib W. Impact of moderate sedation versus monitored anesthesia care on outcomes and cost of endobronchial ultrasound transbronchial needle aspiration. Pulm Med. 2019;2019:4347852. doi: 10.1155/2019/4347852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ando K, Ohkuni Y, Fukazawa M, Abe M, Takeshi A, Kaneko N. Sedation with meperidine for endobronchial ultrasound-guided transbronchial needle aspiration. J Bronchol Interv Pulmonol. 2010;17:329–33. doi: 10.1097/LBR.0b013e3181f4196d. [DOI] [PubMed] [Google Scholar]
- 54.Conte SC, Spagnol G, Confalonieri M, Brizi B. Deep sedation versus minimal sedation during endobronchial ultrasound transbronchial needle aspiration. Monaldi Arch Chest Dis. 2018;88:967. doi: 10.4081/monaldi.2018.967. [DOI] [PubMed] [Google Scholar]
- 55.Casal RF, Lazarus DR, Kuhl K, Nogueras-González G, Perusich S, Green LK, et al. Randomized trial of endobronchial ultrasound–guided transbronchial needle aspiration under general anesthesia versus moderate sedation. Am J Respir Crit Care Med. 2015;191:796–803. doi: 10.1164/rccm.201409-1615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fernandes MGO, Santos VF, Martins N, Sucena MC, Passos MM, Marques MM, et al. Endobronchial ultrasound under moderate sedation versus general anesthesia. J Clin Med. 2018;7:421. doi: 10.3390/jcm7110421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghisi D, Fanelli A, Tosi M, Nuzzi M, Fanelli G. Monitored anesthesia care. Minerva Anestesiol. 2005;71:533–8. [PubMed] [Google Scholar]
- 58.Agostini L, Facciolongo N, Lusuardi M, Casalini E, Galeone C, Lasagni L, et al. Endobronchial ultrasound-guided transbronchial needle aspiration under conscious sedation with meperidine and midazolam. Monaldi Arch Chest Dis. 2017;87:768. doi: 10.4081/monaldi.2017.768. [DOI] [PubMed] [Google Scholar]
- 59.Chrissian AA, Bedi H. Bronchoscopist-directed continuous propofol infusion for targeting moderate sedation during endobronchial ultrasound bronchoscopy:A practical and effective protocol. J Bronchology Interv Pulmonol. 2015;22:226–36. doi: 10.1097/LBR.0000000000000187. [DOI] [PubMed] [Google Scholar]
- 60.Dhooria S, Sehgal IS, Gupta N, Aggarwal AN, Behera D, Agarwal R. Diagnostic yield and complications of EBUS-TBNA performed under bronchoscopist-directed conscious sedation:Single center experience of 1004 subjects. J Bronchology Interv Pulmonol. 2017;24:7–14. doi: 10.1097/LBR.0000000000000332. [DOI] [PubMed] [Google Scholar]
- 61.Khemasuwan D, Teerapuncharoen K, Griffin DC. Diagnostic yield and safety of bronchoscopist-directed moderate sedation with a bolus dose administration of propofol during endobronchial ultrasound bronchoscopy. J Bronchology Interv Pulmonol. 2018;25:181–8. doi: 10.1097/LBR.0000000000000462. [DOI] [PubMed] [Google Scholar]
- 62.Lin TY, Huang YC, Kuo CH, Chung FT, Lin YT, Wang TY, et al. Dexmedetomidine sedation for endobronchial ultrasound-guided transbronchial needle aspiration, a randomised controlled trial. ERJ Open Res. 2020;6:00064–2020. doi: 10.1183/23120541.00064-2020. doi:10.1183/23120541.00064-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.St-Pierre P, Tanoubi I, Verdonck O, Fortier LP, Richebé P, Côté I, et al. Dexmedetomidine versus remifentanil for monitored anesthesia care during endobronchial ultrasound-guided transbronchial needle aspiration:A randomized controlled trial. Anesth Analg. 2019;128:98–106. doi: 10.1213/ANE.0000000000003633. [DOI] [PubMed] [Google Scholar]
- 64.Kim J, Choi SM, Park YS, Lee CH, Lee SM, Yoo CG, et al. Dexmedetomidine versus midazolam for sedation during endobronchial ultrasound-guided transbronchial needle aspiration:A randomised controlled trial. Eur J Anaesthesiol. 2021;38:534–40. doi: 10.1097/EJA.0000000000001370. [DOI] [PubMed] [Google Scholar]
- 65.Kumari R, Jain K, Agarwal R, Dhooria S, Sehgal IS, Aggarwal AN. Fixed dexmedetomidine infusion versus fixed-dose midazolam bolus as primary sedative for maintaining intra-procedural sedation during endobronchial ultrasound-guided transbronchial needle aspiration:A double blind randomized controlled trial. Expert Rev Respir Med. 2021;15:1597–604. doi: 10.1080/17476348.2021.1918000. [DOI] [PubMed] [Google Scholar]
- 66.Dal T, Sazak H, Tunç M, Sahin S, Yılmaz A. A comparison of ketamine-midazolam and ketamine-propofol combinations used for sedation in the endobronchial ultrasound- guided transbronchial needle aspiration:A prospective, single- blind, randomized study. J Thorac Dis. 2014;6:742–51. doi: 10.3978/j.issn.2072-1439.2014.04.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sazak H, Tunc M, Alagoz A, Pehlivanolu P, Demirci NY, Alıcıİ O, et al. Assessment of perianesthesic data in subjects undergoing endobronchial ultrasound-guided transbronchial needle aspiration. Respir Care. 2015;60:567–76. doi: 10.4187/respcare.03547. [DOI] [PubMed] [Google Scholar]
- 68.Cases Viedma E, Andreo García F, Flandes Aldeyturriaga J, Reig Mezquida JP, Briones Gómez A, Vila Caral P, et al. Tolerance and safety of 5 models of sedation during endobronchial ultrasound. Arch Bronconeumol. 2016;52:5–11. doi: 10.1016/j.arbres.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 69.An Updated Report by the American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice Guidelines for Sedation and Analgesia by Non-Anesthesiologists. Anesthesiology. 2002;96:1004–17. doi: 10.1097/00000542-200204000-00031. [DOI] [PubMed] [Google Scholar]
- 70.Piccioni F, Codazzi D, Paleari MC, Previtali P, Delconte G, Fumagalli L, et al. Endosonographic evaluation of the mediastinum through the i-gel O 2 supraglottic airway device. Tumori. 2021;107:86–90. doi: 10.1177/0300891619871104. [DOI] [PubMed] [Google Scholar]
- 71.Anwar M, Fritze R, Base E, Wasserscheid T, Wolfram N, Koinig H, et al. Infraglottic versus supraglottic jet-ventilation for endobronchial ultrasound-guided transbronchial needle aspiration:A randomised controlled trial. Eur J Anaesthesiol. 2020;37:999–1007. doi: 10.1097/EJA.0000000000001220. [DOI] [PubMed] [Google Scholar]
- 72.Madan K, Iyer H, Arunachalam M, Mittal S, Tiwari P, Hadda V, et al. The cricothyroid versus the spray-as-you-go method for topical anesthesia during flexible bronchoscopy:A systematic review and meta-analysis of randomized controlled trials. Lung India. 2021;38:416–24. doi: 10.4103/lungindia.lungindia_937_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mittal S, Gupta N, Iyer H, Biswal S, Tiwari P, Hadda V, et al. The cricothyroid versus Spray-As-You-Go method for topical anesthesia during Endobronchial Ultrasound-guided Transbronchial Needle Aspiration (EBUS-TBNA):The CRISPEN randomized clinical trial. Lung India. 2021;38:223–8. doi: 10.4103/lungindia.lungindia_801_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Biswal S, Mittal S, Hadda V, Mohan A, Khilnani G, Pandey R, et al. 1% versus 2% lignocaine for airway anesthesia in endobronchial ultrasound-guided transbronchial needle aspiration:A pilot, double-blind, randomized controlled trial. Lung India. 2018;35:467–71. doi: 10.4103/lungindia.lungindia_148_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee HJ, Haas AR, Sterman DH, Solly R, Vachani A, Gillespie CT. Pilot randomized study comparing two techniques of airway anaesthesia during curvilinear probe endobronchial ultrasound bronchoscopy (CP-EBUS) Respirology. 2011;16:102–6. doi: 10.1111/j.1440-1843.2010.01861.x. [DOI] [PubMed] [Google Scholar]
- 76.Iyer H, Mishra M, Sindhwani G, Mittal S, Tiwari P, Hadda V, et al. Five versus 10 pharyngeal sprays of 10% lignocaine for topical anesthesia during flexible bronchoscopy:A multicenter, randomized controlled trial. J Bronchology Interv Pulmonol. 2022 doi: 10.1097/LBR.0000000000000869. doi:10.1097/LBR.0000000000000869. [DOI] [PubMed] [Google Scholar]
- 77.Jones AM, O'Driscoll R. Do all patients require supplemental oxygen during flexible bronchoscopy? Chest. 2001;119:1906–9. doi: 10.1378/chest.119.6.1906. [DOI] [PubMed] [Google Scholar]
- 78.Yildiz P, Özgül A, Yilmaz V. Changes in oxygen saturation in patients undergoing fiberoptic bronchoscopy. CHEST. 2002;121:1007–8. doi: 10.1378/chest.121.3.1007. [DOI] [PubMed] [Google Scholar]
- 79.Attaran D, Towhidi M, Toosi MAM. The relationship between peak expiratory flow rate before bronchoscopy and arterial oxygen desaturation during bronchoscopy. Acta Med Iran. 2008;46:95–8. [Google Scholar]
- 80.Markou NK, Kanakaki MC, Boutzouka E, Damianos A, Ikonomou A, Myriantheus P, et al. Fluctuations in gas exchange and cardiovascular parameters during flexible bronchoscopy. J Bronchol Interv Pulmonol. 1999;6:241–6. [Google Scholar]
- 81.Fang WF, Chen YC, Chung YH, Woon WT, Tseng CC, Chang HW, et al. Predictors of oxygen desaturation in patients undergoing diagnostic bronchoscopy. Chang Gung Med J. 2006;29:306–12. [PubMed] [Google Scholar]
- 82.Smyth R, Brennan V, Breen D. Determinants of intra-procedure hypoxia during EBUS for cancer. Lung Cancer. 2016;91:S15. [Google Scholar]
- 83.Irfan M, Ahmed M, Breen D. Assessment of high flow nasal cannula oxygenation in endobronchial ultrasound bronchoscopy:A randomized controlled trial. J Bronchology Interv Pulmonol. 2021;28:130–7. doi: 10.1097/LBR.0000000000000719. [DOI] [PubMed] [Google Scholar]
- 84.Yilmazel Ucar E, Araz Ö, Kerget B, Akgun M, Saglam L. Comparison of high-flow and conventional nasal cannula oxygen in patients undergoing endobronchial ultrasonography. Intern Med J. 2020;51:1935–9. doi: 10.1111/imj.15001. [DOI] [PubMed] [Google Scholar]
- 85.Takakuwa O, Oguri T, Asano T, Fukuda S, Kanemitsu Y, Uemura T, et al. Prevention of hypoxemia during endobronchial ultrasound-guided transbronchial needle aspiration:Usefulness of high-flow nasal cannula. Respir Investig. 2018;56:418–23. doi: 10.1016/j.resinv.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 86.Oral Tapan O, Genc S, Tertemiz KC, Ozgen Alpaydin A, Itil BO, Iyilikci Karaoglan L. The effect of Oxygen application with nCPAP for the prevention of desaturation during EBUS-TBNA. Int J Clin Pract. 2021;75:e14045. doi: 10.1111/ijcp.14045. [DOI] [PubMed] [Google Scholar]
- 87.Belenguer Muncharaz A, Lidón Mateu-Campos M, RecatalàMora M, Gil Tomás A, Llavador Ros G, Altaba Tena S, et al. Empleo de la presión positiva continua en la vía aerea en la sedación de la ecobroncoscopia. Rev Patol Respir. 2019;22:84–90. [Google Scholar]
- 88.Bonta PI, Crombag L, Annema JT. Linear endobronchial and endoesophageal ultrasound:A practice change in thoracic medicine. Curr Opin Pulm Med. 2016;22:281–8. doi: 10.1097/MCP.0000000000000269. [DOI] [PubMed] [Google Scholar]
- 89.Yarmus L, Akulian J, Ortiz R, Thompson R, Oakjones-Burgess K, Arias S, et al. A randomized controlled trial evaluating airway inspection effectiveness during endobronchial ultrasound bronchoscopy. J Thorac Dis. 2015;7:1825–32. doi: 10.3978/j.issn.2072-1439.2015.09.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.EBUS Bronchoscope (BF-UC180F) | Olympus America | Medical. [[Last accessed on 2021 Dec 28]]. Available from: https://medical.olympusamerica.com/products/bf-uc180f-ebus-bronchoscope .
- 91.Xiang Y, Zhang F, Akulian J, Yarmus L, Feller-Kopman D, Wang KP. EBUS-TBNA by a new Fuji EBUS scope. J Thorac Dis. 2013;5:36–9. doi: 10.3978/j.issn.2072-1439.2012.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Endobronchial Ultrasound (EBUS) | Fujifilm Healthcare. [[Last accessed on 2021 Dec 28]]. Available from: https://healthcaresolutions-us.fujifilm.com/endoscopy/pulmonary/endobronchial-ultrasound-ebus .
- 93.Endoscopic Ultrasound | PENTAX Medical (EMEA) [[Last accessed on 2021 Dec 28]]. Available from : https://www.pentaxmedical.com/pentax/en/95/1/EB19-J10U-Ultrasound-Video-Bronchoscope .
- 94.Patel P, Wada H, Hu HP, Hirohashi K, Kato T, Ujiie H, et al. First evaluation of the new thin convex probe endobronchial ultrasound scope:A human ex vivo lung study. Ann Thorac Surg. 2017;103:1158–64. doi: 10.1016/j.athoracsur.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 95.Mittal S, Madan K, Hadda V, Mohan A, Guleria R. Nasal route for endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA):An alternative modality in difficult oral bronchoscope insertion. Lung India. 2017;34:472–4. doi: 10.4103/lungindia.lungindia_2_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Beaudoin S, Ferland N, Martel S, Delage A. Feasibility of using the nasal route for linear endobronchial ultrasound. Lung. 2014;192:921–6. doi: 10.1007/s00408-014-9638-x. [DOI] [PubMed] [Google Scholar]
- 97.Beaudoin S, Martel S, Pelletier S, Lampron N, Simon M, Laberge F, et al. Randomized trial comparing patient comfort between the oral and nasal insertion routes for linear endobronchial ultrasound. J Bronchology Interv Pulmonol. 2016;23:39–45. doi: 10.1097/LBR.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 98.Han D, Kim H, Shin A, Kim J, Ahn J, Ha J. Feasibility and safety of using the nasal route for convex probe endobronchial ultrasound. Am J Crit Care Med. 2017;195:A4571. [Google Scholar]
- 99.Wahidi MM, Herth F, Yasufuku K, Shepherd RW, Yarmus L, Chawla M, et al. Technical aspects of endobronchial ultrasound-guided transbronchial needle aspiration. Chest. 2016;149:816–35. doi: 10.1378/chest.15-1216. [DOI] [PubMed] [Google Scholar]
- 100.Fujiwara T, Yasufuku K, Nakajima T, Chiyo M, Yoshida S, Suzuki M, et al. The utility of sonographic features during endobronchial ultrasound-guided transbronchial needle aspiration for lymph node staging in patients with lung cancer. Chest. 2010;138:641–7. doi: 10.1378/chest.09-2006. [DOI] [PubMed] [Google Scholar]
- 101.Gogia P, Insaf TZ, McNulty W, Boutou A, Nicholson AG, Zoumot Z, et al. Endobronchial ultrasound:Morphological predictors of benign disease. ERJ Open Res. 2016;2:00053–2015. doi: 10.1183/23120541.00053-2015. doi:10.1183/23120541.00053-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nakajima T, Anayama T, Shingyoji M, Kimura H, Yoshino I, Yasufuku K. Vascular Image patterns of lymph nodes for the prediction of metastatic disease during EBUS-TBNA for mediastinal staging of lung cancer. J Thorac Oncol. 2012;7:1009–14. doi: 10.1097/JTO.0b013e31824cbafa. [DOI] [PubMed] [Google Scholar]
- 103.Garcia-Olivé I, Monsó E, Andreo F, Sanz J, Castellà E, Llatjós M, et al. Sensitivity of linear endobronchial ultrasonography and guided transbronchial needle aspiration for the identification of nodal metastasis in lung cancer staging. Ultrasound Med Biol. 2009;35:1271–7. doi: 10.1016/j.ultrasmedbio.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 104.Ayub II, Mohan A, Madan K, Hadda V, Jain D, Khilnani GC, et al. Identification of specific EBUS sonographic characteristics for predicting benign mediastinal lymph nodes. Clin Respir J. 2018;12:681–90. doi: 10.1111/crj.12579. [DOI] [PubMed] [Google Scholar]
- 105.Wang L, Wu W, Hu Y, Teng J, Zhong R, Han B, et al. Sonographic features of endobronchial ultrasonography predict intrathoracic lymph node metastasis in lung cancer patients. Ann Thorac Surg. 2015;100:1203–9. doi: 10.1016/j.athoracsur.2015.04.143. [DOI] [PubMed] [Google Scholar]
- 106.Alici IO, Yılmaz Demirci N, Yılmaz A, Karakaya J, Özaydın E. The sonographic features of malignant mediastinal lymph nodes and a proposal for an algorithmic approach for sampling during endobronchial ultrasound:Which one to sample? Clin Respir J. 2016;10:606–13. doi: 10.1111/crj.12267. [DOI] [PubMed] [Google Scholar]
- 107.Wang Memoli JS, El-Bayoumi E, Pastis NJ, Tanner NT, Gomez M, Huggins JT, et al. Using endobronchial ultrasound features to predict lymph node metastasis in patients with lung cancer. Chest. 2011;140:1550–6. doi: 10.1378/chest.11-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schmid-Bindert G, Jiang H, Kähler G, Saur J, Henzler T, Wang H, et al. Predicting malignancy in mediastinal lymph nodes by endobronchial ultrasound:A new ultrasound scoring system:EBUS in mediastinal lymph nodes. Respirology. 2012;17:1190–8. doi: 10.1111/j.1440-1843.2012.02223.x. [DOI] [PubMed] [Google Scholar]
- 109.Shafiek H, Fiorentino F, Peralta AD, Serra E, Esteban B, Martinez R, et al. Real-time prediction of mediastinal lymph node malignancy by endobronchial ultrasound. Arch Bronconeumol. 2014;50:228–34. doi: 10.1016/j.arbres.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 110.Abedini A, Razavi F, Mehravaran H, Toutkaboni MP, Kashefizadeh A, Emami H, et al. Identificationof sonographic features for predicting benign versus malignant mediastinal or hilar lymph nodes using endobronchial ultrasound. Oman Med J. 2020;35:e112. doi: 10.5001/omj.2020.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hylton DA, Turner S, Kidane B, Spicer J, Xie F, Farrokhyar F, et al. The Canada lymph node score for prediction of malignancy in mediastinal lymph nodes during endobronchial ultrasound. J Thorac Cardiovasc Surg. 2020;159:2499–507.e3. doi: 10.1016/j.jtcvs.2019.10.205. [DOI] [PubMed] [Google Scholar]
- 112.Agrawal SP, Ish P, Goel AD, Gupta N, Chakrabarti S, Bhattacharya D, et al. Diagnostic utility of endobronchial ultrasound features in differentiating malignant and benign lymph nodes. Monaldi Arch Chest Dis. 2018;88:928. doi: 10.4081/monaldi.2018.928. [DOI] [PubMed] [Google Scholar]
- 113.Korrungruang P, Boonsarngsuk V. Diagnostic value of endobronchial ultrasound elastography for the differentiation of benign and malignant intrathoracic lymph nodes:EBUS elastography. Respirology. 2017;22:972–7. doi: 10.1111/resp.12979. [DOI] [PubMed] [Google Scholar]
- 114.Jhun BW, Um SW, Suh GY, Chung MP, Kim H, Kwon OJ, et al. Clinical value of endobronchial ultrasound findings for predicting nodal metastasis in patients with suspected lymphadenopathy:A prospective study. J Korean Med Sci. 2014;29:1632–8. doi: 10.3346/jkms.2014.29.12.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Evison M, Morris J, Martin J, Shah R, Barber PV, Booton R, et al. Nodal staging in lung cancer:A risk stratification model for lymph nodes classified as negative by EBUS-TBNA. J Thorac Oncol. 2015;10:126–33. doi: 10.1097/JTO.0000000000000348. [DOI] [PubMed] [Google Scholar]
- 116.Gu Y, Shi H, Su C, Chen X, Zhang S, Li W, et al. The role of endobronchial ultrasound elastography in the diagnosis of mediastinal and hilar lymph nodes. Oncotarget. 2017;8:89194–202. doi: 10.18632/oncotarget.19031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Agrawal S, Goel AD, Gupta N, Lohiya A, Gonuguntla HK. Diagnostic utility of endobronchial ultrasound (EBUS) features in differentiating malignant and benign lymph nodes-A systematic review and meta-analysis. Respir Med. 2020;171:106097. doi: 10.1016/j.rmed.2020.106097. doi: 10.1016/j.rmed.2020.106097. [DOI] [PubMed] [Google Scholar]
- 118.Dhooria S, Agarwal R, Aggarwal AN, Bal A, Gupta N, Gupta D. Differentiating tuberculosis from sarcoidosis by sonographic characteristics of lymph nodes on endobronchial ultrasonography:A study of 165 patients. J Thorac Cardiovasc Surg. 2014;148:662–7. doi: 10.1016/j.jtcvs.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 119.Erol S, Anar C, Erer OF, Biçmen C, Aydoğdu Z. The contribution of ultrasonographic characteristics of mediastinal lymph nodes on differential diagnosis of tuberculous lymphadenitis from sarcoidosis. Tanaffos. 2018;17:250–6. [PMC free article] [PubMed] [Google Scholar]
- 120.Imai N, Imaizumi K, Ando M, Shimokata T, Ogawa T, Ito S, et al. Echoic features of lymph nodes with sarcoidosis determined by endobronchial ultrasound. Intern Med. 2013;52:1473–8. doi: 10.2169/internalmedicine.52.9082. [DOI] [PubMed] [Google Scholar]
- 121.Madan K, Madan M, Mittal S, Tiwari P, Hadda V, Mohan A, et al. The utility of the ultrasonographic characteristics in differentiating between malignant and tuberculous mediastinal lymphadenopathy during EBUS-TBNA. J Bronchology Interv Pulmonol. 2023;30:47–53. doi: 10.1097/LBR.0000000000000850. [DOI] [PubMed] [Google Scholar]
- 122.Izumo T, Sasada S, Chavez C, Matsumoto Y, Tsuchida T. Endobronchial ultrasound elastography in the diagnosis of mediastinal and hilar lymph nodes. Jpn J Clin Oncol. 2014;44:956–62. doi: 10.1093/jjco/hyu105. [DOI] [PubMed] [Google Scholar]
- 123.He HY, Huang M, Zhu J, Ma H, Lyu XD. Endobronchial ultrasound elastography for diagnosing mediastinal and hilar lymph nodes. Chin Med J (Engl) 2015;128:2720–5. doi: 10.4103/0366-6999.167296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sun J, Zheng X, Mao X, Wang L, Xiong H, Herth FJF, et al. Endobronchial ultrasound elastography for evaluation of intrathoracic lymph nodes:A pilot study. Respiration. 2017;93:327–38. doi: 10.1159/000464253. [DOI] [PubMed] [Google Scholar]
- 125.Çağlayan B, İliaz S, Bulutay P, Armutlu A, Uzel I, Öztürk AB. The role of endobronchial ultrasonography elastography for predicting malignancy. Turk Gogus Kalp Damar Cerrahisi Derg. 2020;28:158–65. doi: 10.5606/tgkdc.dergisi.2020.18508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nakajima T, Inage T, Sata Y, Morimoto J, Tagawa T, Suzuki H, et al. Elastography for predicting and localizing nodal metastases during endobronchial ultrasound. Respiration. 2015;90:499–506. doi: 10.1159/000441798. [DOI] [PubMed] [Google Scholar]
- 127.Uchimura K, Yamasaki K, Sasada S, Hara S, Ikushima I, Chiba Y, et al. Quantitative analysis of endobronchial ultrasound elastography in computed tomography-negative mediastinal and hilar lymph nodes. Thorac Cancer. 2020;11:2590–9. doi: 10.1111/1759-7714.13579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hernández Roca M, Pérez Pallarés J, Prieto Merino D, Valdivia Salas M del M, García Solano J, Fernández Álvarez J, et al. Diagnostic value of elastography and endobronchial ultrasound in the study of hilar and mediastinal lymph nodes. J Bronchology Interv Pulmonol. 2019;26:184–92. doi: 10.1097/LBR.0000000000000550. [DOI] [PubMed] [Google Scholar]
- 129.Verhoeven RLJ, Trisolini R, Leoncini F, Candoli P, Bezzi M, Messi A, et al. Predictive value of endobronchial ultrasound strain elastography in mediastinal lymph node staging:The E-predict multicenter study results. Respiration. 2020;99:484–92. doi: 10.1159/000507592. [DOI] [PubMed] [Google Scholar]
- 130.Madan K, Madan M, Iyer H, Mittal S, Madan NK, Rathi V, et al. Utility of elastography for differentiating malignant and benign lymph nodes during EBUS-TBNA:A systematic review and meta-analysis. J Bronchology Interv Pulmonol. 2022;29:18–33. doi: 10.1097/LBR.0000000000000781. [DOI] [PubMed] [Google Scholar]
- 131.Gompelmann D, Kontogianni K, Sarmand N, Kaukel P, Krisam J, Eberhardt R, et al. Endobronchial ultrasound elastography for differentiating benign and malignant lymph nodes. Respiration. 2020;99:779–83. doi: 10.1159/000509297. [DOI] [PubMed] [Google Scholar]
- 132.Verhoeven RLJ, de Korte CL, van der Heijden EHFM. Optimal endobronchial ultrasound strain elastography assessment strategy:An explorative study. Respiration. 2019;97:337–47. doi: 10.1159/000494143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fujiwara T, Nakajima T, Inage T, Sata Y, Sakairi Y, Tamura H, et al. The combination of endobronchial elastography and sonographic findings during endobronchial ultrasound-guided transbronchial needle aspiration for predicting nodal metastasis. Thorac Cancer. 2019;10:2000–5. doi: 10.1111/1759-7714.13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ma H, An Z, Xia P, Cao J, Gao Q, Ren G, et al. Semi-quantitative analysis of EBUS elastography as a feasible approach in diagnosing mediastinal and hilar lymph nodes of lung cancer patients. Sci Rep. 2018;8:3571. doi: 10.1038/s41598-018-22006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gupta R, Joshi S, Bhatia A, Tayal N, Pandey P. Qualitative role of endobronchial elastography with endobronchial ultrasound in differentiating malignant and benign lesions:A retrospective single-center study from India. Egypt J Bronchol. 2019;13:630–5. [Google Scholar]
- 136.Zhi X, Chen J, Wang L, Xie F, Zheng X, Li Y, et al. Endobronchial ultrasound multimodal imaging for the diagnosis of intrathoracic lymph nodes. Respiration. 2021;100:898–908. doi: 10.1159/000515664. [DOI] [PubMed] [Google Scholar]
- 137.Fang S, Chang L, Chen F, Mao X, Gu W. Endobronchial ultrasound elastography combined with computed tomography in differentiating benign from malignant intrathoracic lymph nodes. Surg Innov. 2021;28:590–9. doi: 10.1177/1553350620978027. [DOI] [PubMed] [Google Scholar]
- 138.Fournier C, Dhalluin X, Wallyn F, Machuron F, Bouchindhomme B, Copin MC, et al. Performance of endobronchial ultrasound elastography in the differentiation of malignant and benign mediastinal lymph nodes:Results in real-life practice. J Bronchology Interv Pulmonol. 2019;26:193–8. doi: 10.1097/LBR.0000000000000551. [DOI] [PubMed] [Google Scholar]
- 139.Bugalho A, de Santis M, Szlubowski A, Rozman A, Eberhardt R. Trans-esophageal endobronchial ultrasound-guided needle aspiration (EUS-B-NA):A road map for the chest physician. Pulmonology. 2018;24:32–41. doi: 10.1016/j.rppnen.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 140.Mondoni M, D'Adda A, Terraneo S, Carlucci P, Radovanovic D, Marco FD, et al. Choose the best route:Ultrasound-guided transbronchial and transesophageal needle aspiration with echobronchoscope in the diagnosis of mediastinal and pulmonary lesions. Minerva Med. 2015;106:13–9. [PubMed] [Google Scholar]
- 141.Vilmann P, Clementsen P, Colella S, Siemsen M, De Leyn P, Dumonceau JM, et al. Combined endobronchial and esophageal endosonography for the diagnosis and staging of lung cancer:European Society of Gastrointestinal Endoscopy (ESGE) Guideline, in cooperation with the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS) Endoscopy. 2015;47:545–59. doi: 10.1055/s-0034-1392040. [DOI] [PubMed] [Google Scholar]
- 142.Hegde PVC, Goudie E, Liberman M. Endosonographic lymph node staging by combined endobronchial ultrasound (EBUS) and endoscopic ultrasound:Technique and technical tricks. Oper Tech Thorac Cardiovasc Surg. 2018;23:136–50. [Google Scholar]
- 143.Dietrich CF, Annema JT, Clementsen P, Cui XW, Maximilian M, Jenssen C. Ultrasound techniques in the evaluation of the mediastinum, part I:Endoscopic ultrasound (EUS), endobronchial ultrasound (EBUS) and transcutaneous mediastinal ultrasound (TMUS), introduction into ultrasound techniques. J Thorac Dis. 2015;7:E311–25. doi: 10.3978/j.issn.2072-1439.2015.09.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Madan K, Mittal S, Madan NK, Tiwari P, Jain D, Arava S, et al. EBUS-TBNA versus EUS-B-FNA for the evaluation of undiagnosed mediastinal lymphadenopathy:The TEAM randomized controlled trial. Clin Respir J. 2020;14:1076–82. doi: 10.1111/crj.13244. [DOI] [PubMed] [Google Scholar]
- 145.Oki M, Saka H, Ando M, Tsuboi R, Nakahata M, Oka S, et al. Transbronchial vs transesophageal needle aspiration using an ultrasound bronchoscope for the diagnosis of mediastinal lesions. Chest. 2015;147:1259–66. doi: 10.1378/chest.14-1283. [DOI] [PubMed] [Google Scholar]
- 146.Crombag LMM, Mooij-Kalverda K, Szlubowski A, Gnass M, Tournoy KG, Sun J, et al. EBUS versus EUS-B for diagnosing sarcoidosis:The International Sarcoidosis Assessment (ISA) randomized clinical trial. Respirology. 2022;27:152–60. doi: 10.1111/resp.14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Oki M, Saka H, Ando M, Kitagawa C, Kogure Y, Seki Y. Endoscopic ultrasound-guided fine needle aspiration and endobronchial ultrasound-guided transbronchial needle aspiration:Are two better than one in mediastinal staging of non–small cell lung cancer? J Thorac Cardiovasc Surg. 2014;148:1169–77. doi: 10.1016/j.jtcvs.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 148.Hwangbo B, Lee GK, Lee HS, Lim KY, Lee SH, Kim HY, et al. Transbronchial and transesophageal fine-needle aspiration using an ultrasound bronchoscope in mediastinal staging of potentially operable lung cancer. Chest. 2010;138:795–802. doi: 10.1378/chest.09-2100. [DOI] [PubMed] [Google Scholar]
- 149.Hwangbo B, Lee HS, Lee GK, Lim KY, Lee SH, Kim HY, et al. Transoesophageal needle aspiration using a convex probe ultrasonic bronchoscope. Respirology. 2009;14:843–9. doi: 10.1111/j.1440-1843.2009.01590.x. [DOI] [PubMed] [Google Scholar]
- 150.Araya T, Demura Y, Kasahara K, Matsuoka H, Yamamura K, Nishitsuji M, et al. Usefulness of transesophageal bronchoscopic ultrasound-guided fine-needle aspiration in the pathologic and molecular diagnosis of lung cancer lesions adjacent to the esophagus. J Bronchology Interv Pulmonol. 2013;20:121–6. doi: 10.1097/LBR.0b013e31829182a0. [DOI] [PubMed] [Google Scholar]
- 151.Dhooria S, Aggarwal AN, Gupta D, Behera D, Agarwal R. Utility and safety of endoscopic ultrasound with bronchoscope-guided fine-needle aspiration in mediastinal lymph node sampling:Systematic review and meta-analysis. Respir Care. 2015;60:1040–50. doi: 10.4187/respcare.03779. [DOI] [PubMed] [Google Scholar]
- 152.Madan K, Iyer H, Madan NK, Mittal S, Tiwari P, Hadda V, et al. Efficacy and safety of EBUS-TBNA and EUS-B-FNA in children:A systematic review and meta-analysis. Pediatr Pulmonol. 2021;56:23–33. doi: 10.1002/ppul.25124. [DOI] [PubMed] [Google Scholar]
- 153.Herth FJ, Krasnik M, Kahn N, Eberhardt R, Ernst A. Combined endoscopic-endobronchial ultrasound-guided fine-needle aspiration of mediastinal lymph nodes through a single bronchoscope in 150 patients with suspected lung cancer. Chest. 2010;138:790–4. doi: 10.1378/chest.09-2149. [DOI] [PubMed] [Google Scholar]
- 154.Korevaar DA, Crombag LM, Cohen JF, Spijker R, Bossuyt PM, Annema JT. Added value of combined endobronchial and oesophageal endosonography for mediastinal nodal staging in lung cancer:A systematic review and meta-analysis. Lancet Respir Med. 2016;4:960–8. doi: 10.1016/S2213-2600(16)30317-4. [DOI] [PubMed] [Google Scholar]
- 155.Kang HJ, Hwangbo B, Lee GK, Nam BH, Lee HS, Kim MS, et al. EBUS-centred versus EUS-centred mediastinal staging in lung cancer:A randomised controlled trial. Thorax. 2014;69:261–8. doi: 10.1136/thoraxjnl-2013-203881. [DOI] [PubMed] [Google Scholar]
- 156.Filarecka A, Gnass M, Wojtacha J, Soja J, Pankowski J, Czyżewski D, et al. Usefulness of combined endobronchial and endoscopic ultrasound-guided needle aspiration in the diagnosis of sarcoidosis:A prospective, multicenter trial. Pol Arch Intern Med. 2020;130:7–8. doi: 10.20452/pamw.15399. [DOI] [PubMed] [Google Scholar]
- 157.Giri S, Pathak R, Yarlagadda V, Karmacharya P, Aryal MR, Martin MG. Meta-analysis of 21- versus 22-G aspiration needle during endobronchial ultrasound-guided transbronchial needle aspiration. J Bronchology Interv Pulmonol. 2015;22:107–13. doi: 10.1097/LBR.0000000000000159. [DOI] [PubMed] [Google Scholar]
- 158.Yu Lee-Mateus A, Garcia-Saucedo JC, Abia-Trujillo D, Labarca G, Patel NM, Pascual JM, et al. Comparing diagnostic sensitivity of different needle sizes for lymph nodes suspected of lung cancer in endobronchial ultrasound transbronchial needle aspiration:Systematic review and meta-analysis. Clin Respir J. 2021;15:1328–36. doi: 10.1111/crj.13436. [DOI] [PubMed] [Google Scholar]
- 159.Kassirian S, Hinton SN, Iansavitchene A, Amjadi K, Chee A, Dhaliwal I, et al. Effect of needle size on diagnosis of sarcoidosis with endobronchial ultrasound-guided transbronchial needle aspiration systematic review and meta-analysis. Ann Am Thorac Soc. 2022;19:279–90. doi: 10.1513/AnnalsATS.202103-366OC. [DOI] [PubMed] [Google Scholar]
- 160.Balwan A. Endobronchial ultrasound-guided transbronchial needle aspiration using 19-G needle for sarcoidosis. J Bronchology Interv Pulmonol. 2018;25:260–3. doi: 10.1097/LBR.0000000000000502. [DOI] [PubMed] [Google Scholar]
- 161.Elmufdi FS, Peterson MK, Niccum D, Asche S, Ham K. Evaluating yield of 19 versus 21 G EBUS-TBNA needles:A prospective study. J Bronchology Interv Pulmonol. 2021;28:29–33. doi: 10.1097/LBR.0000000000000674. [DOI] [PubMed] [Google Scholar]
- 162.Wolters C, Darwiche K, Franzen D, Hager T, Bode-Lesnievska B, Kneuertz PJ, et al. A prospective, randomized trial for the comparison of 19-G and 22-G endobronchial ultrasound-guided transbronchial aspiration needles;introducing a novel end point of sample weight corrected for blood content. Clin Lung Cancer. 2019;20:e265–73. doi: 10.1016/j.cllc.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 163.Pickering EM, Holden VK, Heath JE, Verceles AC, Kalchiem-Dekel O, Sachdeva A. Tissue acquisition during EBUS-TBNA:Comparison of cell blocks obtained from a 19G versus 21G needle. J Bronchology Interv Pulmonol. 2019;26:237–44. doi: 10.1097/LBR.0000000000000562. [DOI] [PubMed] [Google Scholar]
- 164.Dooms C, Vander Borght S, Yserbyt J, Testelmans D, Wauters E, Nackaerts K, et al. A randomized clinical trial of flex 19G needles versus 22G needles for endobronchial ultrasonography in suspected lung cancer. Respiration. 2018;96:275–82. doi: 10.1159/000489473. [DOI] [PubMed] [Google Scholar]
- 165.Sood R, Alape D, Thakkar D, Shadchehr S, Acash G, Tronic BJ, et al. Comparison of sample adequacy and diagnostic yield of the 21-G and 25-G EBUS TBNA needles. J Bronchol Interv Pulmonol. 2022;29:34–8. doi: 10.1097/LBR.0000000000000753. [DOI] [PubMed] [Google Scholar]
- 166.Sakaguchi T, Inoue T, Miyazawa T, Mineshita M. Comparison of the 22-gauge and 25-gauge needles for endobronchial ultrasound-guided transbronchial needle aspiration. Respir Investig. 2021;59:235–9. doi: 10.1016/j.resinv.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 167.Di Felice C, Young B, Matta M. Comparison of specimen adequacy and diagnostic accuracy of a 25-gauge and 22-gauge needle in endobronchial ultrasound-guided transbronchial needle aspiration. J Thorac Dis. 2019;11:3643–9. doi: 10.21037/jtd.2019.04.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gafoor K, Belete H, Walczyszyn M, Zapata D, Husta B. Utility of a 25-versus 22-G EBUS needle in difficult-to-access 2R lymph nodes. J Bronchology Interv Pulmonol. 2018;25:e17–9. doi: 10.1097/LBR.0000000000000428. [DOI] [PubMed] [Google Scholar]
- 169.Dhooria S, Sehgal IS, Prasad KT, Muthu V, Gupta N, Bal A, et al. Diagnostic yield and safety of the ProCore versus the standard EBUS-TBNA needle in subjects with suspected sarcoidosis. Expert Rev Med Devices. 2021;18:211–6. doi: 10.1080/17434440.2021.1876560. [DOI] [PubMed] [Google Scholar]
- 170.Balwan A, Bixby B, Grotepas C, Witt BL, Iravani A, Ansari S, et al. Core needle biopsy with endobronchial ultrasonography:Single center experience with 100 cases. J Am Soc Cytopathol. 2020;9:249–53. doi: 10.1016/j.jasc.2020.04.009. [DOI] [PubMed] [Google Scholar]
- 171.Dhooria S, Sehgal IS, Gupta N, Ram B, Aggarwal AN, Behera D, et al. Yield of new versus reused endobronchial ultrasound-guided transbronchial needle aspiration needles:A retrospective analysis of 500 patients. Lung India. 2016;33:367–71. doi: 10.4103/0970-2113.184867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Prasad KT, Dhooria S, Sehgal IS, Muthu V, Ram B, Gupta N, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the economically disadvantaged:A retrospective analysis of 1582 individuals. Lung India. 2018;35:483–7. doi: 10.4103/lungindia.lungindia_176_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dhooria S, Sehgal IS, Gupta N, Prasad KT, Aggarwal AN, Agarwal R. Diagnostic utility and safety of endobronchial ultrasound-guided transbronchial needle aspiration in the elderly. J Bronchology Interv Pulmonol. 2020;27:22–9. doi: 10.1097/LBR.0000000000000605. [DOI] [PubMed] [Google Scholar]
- 174.Casal RF, Staerkel GA, Ost D, Almeida FA, Uzbeck MH, Eapen GA, et al. Randomized clinical trial of endobronchial ultrasound needle biopsy with and without aspiration. Chest. 2012;142:568–73. doi: 10.1378/chest.11-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mohan A, Iyer H, Madan K, Hadda V, Mittal S, Tiwari P, et al. A randomized comparison of sample adequacy and diagnostic yield of EBUS-TBNA using various suction pressures. Adv Respir Med. 2021;89:268–76. doi: 10.5603/ARM.a2021.0054. [DOI] [PubMed] [Google Scholar]
- 176.Lin X, Ye M, Li Y, Ren J, Lou Q, Li Y, et al. Randomized controlled trial to evaluate the utility of suction and inner-stylet of EBUS-TBNA for mediastinal and hilar lymphadenopathy. BMC Pulm Med. 2018;18:192. doi: 10.1186/s12890-018-0751-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kassirian S, Mitchell MA, McCormack DG, Zeman-Pocrnich C, Dhaliwal I. Rapid On-site Evaluation (ROSE) in capillary pull versus suction biopsy technique with endobronchial ultrasound-transbronchial needle aspiration (EBUS-TBNA) J Bronchology Interv Pulmonol. 2021;29:48–53. doi: 10.1097/LBR.0000000000000776. [DOI] [PubMed] [Google Scholar]
- 178.Harris K, Maroun R, Attwood K, Chalhoub M. Comparison of cytologic accuracy of endobronchial ultrasound transbronchial needle aspiration using needle suction versus no suction. Endosc Ultrasound. 2015;4:115–9. doi: 10.4103/2303-9027.156737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.He X, Wu Y, Wang H, Yu G, Xu B, Jia N, et al. Slow-pull capillary technique versus suction technique in endobronchial ultrasound-guided transbronchial needle aspiration for diagnosing diseases involving hilar and mediastinal lymph node enlargement. Ther Adv Respir Dis. 2020;14:1753466620907037. doi: 10.1177/1753466620907037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chami HA, Abu Khouzam R, Makki M, Kahwaji S, Hochaimi N, Tamim H, et al. Randomized cross-over trial of endobronchial ultrasound transbronchial needle aspiration with or without suction in suspected malignant lymphadenopathy. J Bronchology Interv Pulmonol. 2021;29:131–9. doi: 10.1097/LBR.0000000000000801. [DOI] [PubMed] [Google Scholar]
- 181.Xu Y, Lin J, Jin Y, Wu X, Zheng H, Feng J. Is endobronchial ultrasound-guided transbronchial needle aspiration with a stylet necessary for lymph node screening in lung cancer patients? Braz J Med Biol Res. 2017;50:e6372. doi: 10.1590/1414-431X20176372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Scholten EL, Semaan R, Illei P, Mallow C, Arias S, Feller-Kopman D, et al. Stylet use does not improve diagnostic outcomes in endobronchial ultrasonographic transbronchial needle aspiration:A randomized clinical trial. Chest. 2017;151:636–42. doi: 10.1016/j.chest.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yasufuku K, Nakajima T, Fujiwara T, Chiyo M, Iyoda A, Yoshida S, et al. Role of endobronchial ultrasound-guided transbronchial needle aspiration in the management of lung cancer. Gen Thorac Cardiovasc Surg. 2008;56:268–76. doi: 10.1007/s11748-008-0249-4. [DOI] [PubMed] [Google Scholar]
- 184.Yasufuku K. Current clinical applications of endobronchial ultrasound. Expert Rev Respir Med. 2010;4:491–8. doi: 10.1586/ers.10.39. [DOI] [PubMed] [Google Scholar]
- 185.Lee HS, Lee GK, Lee HS, Kim MS, Lee JM, Kim HY, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer:How many aspirations per target lymph node station? Chest. 2008;134:368–74. doi: 10.1378/chest.07-2105. [DOI] [PubMed] [Google Scholar]
- 186.Yarmus L, Akulian J, Gilbert C, Feller-Kopman D, Lee HJ, Zarogoulidis P, et al. Optimizing endobronchial ultrasound for molecular analysis. How many passes are needed? Ann Am Thorac Soc. 2013;10:636–43. doi: 10.1513/AnnalsATS.201305-130OC. [DOI] [PubMed] [Google Scholar]
- 187.Oki M, Saka H, Ando M, Nakashima H, Shiraki A, Murakami Y, et al. How many passes are needed for endobronchial ultrasound-guided transbronchial needle aspiration for sarcoidosis?A prospective multicenter study. Respiration. 2018;95:251–7. doi: 10.1159/000485661. [DOI] [PubMed] [Google Scholar]
- 188.Dhooria S, Sehgal IS, Gupta N, Bal A, Prasad KT, Aggarwal AN, et al. A randomized trial evaluating the effect of 10 versus 20 revolutions inside the lymph node on the diagnostic yield of EBUS-TBNA in subjects with sarcoidosis. Respiration. 2018;96:464–71. doi: 10.1159/000490192. [DOI] [PubMed] [Google Scholar]
- 189.von Bartheld MB, van Breda A, Annema JT. Complication rate of endosonography (endobronchial and endoscopic ultrasound):A systematic review. Respiration. 2014;87:343–51. doi: 10.1159/000357066. [DOI] [PubMed] [Google Scholar]
- 190.Çağlayan B, Yılmaz A, Bilaçeroğlu S, Cömert SŞ, Demirci NY, Salepçi B. Complications of convex-probe endobronchial ultrasound-guided transbronchial needle aspiration:A multi-center retrospective study. Respir Care. 2016;61:243–8. doi: 10.4187/respcare.03838. [DOI] [PubMed] [Google Scholar]
- 191.Asano F, Aoe M, Ohsaki Y, Okada Y, Sasada S, Sato S, et al. Complications associated with endobronchial ultrasound-guided transbronchial needle aspiration:A nationwide survey by the Japan Society for Respiratory Endoscopy. Respir Res. 2013;14:50. doi: 10.1186/1465-9921-14-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vaidya PJ, Munavvar M, Leuppi JD, Mehta AC, Chhajed PN. Endobronchial ultrasound-guided transbronchial needle aspiration:Safe as it sounds. Respirology. 2017;22:1093–101. doi: 10.1111/resp.13094. [DOI] [PubMed] [Google Scholar]
- 193.Lee HY, Kim J, Jo YS, Park YS. Bacterial pericarditis as a fatal complication after endobronchial ultrasound-guided transbronchial needle aspiration. Eur J Cardiothorac Surg. 2015;48:630–2. doi: 10.1093/ejcts/ezu477. [DOI] [PubMed] [Google Scholar]
- 194.Muriana P, Carretta A, Ciriaco P, Rossetti F, Negri G. Isolated tension pneumoperitoneum following endobronchial ultrasound-guided transbronchial needle aspiration complicated by cardiac peri-arrest:A case report. Monaldi Arch Chest Dis. 2018;88:999. doi: 10.4081/monaldi.2018.999. [DOI] [PubMed] [Google Scholar]
- 195.Gupta R, Park HY, Kim H, Um SW. Endobronchial inflammatory polyp as a rare complication of endobronchial ultrasound-transbronchial needle aspiration. Interact Cardiovasc Thorac Surg. 2010;11:340–1. doi: 10.1510/icvts.2010.241661. [DOI] [PubMed] [Google Scholar]
- 196.Kwok M, Moran L, Ng T. Massive hemothorax from bronchial artery bleeding after endobronchial ultrasound transbronchial needle aspiration. R I Med J (2013) 2020;103:38–40. [PubMed] [Google Scholar]
- 197.Hussain H, Sehring M, McVay B. Hemotympanum post-bronchoscopy:An unusual complication! Respir Med Case Rep. 2020;31:101259. doi: 10.1016/j.rmcr.2020.101259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Krogerus L, Kholová I. Cell block in cytological diagnostics:Review of preparatory techniques. Acta Cytol. 2018;62:237–43. doi: 10.1159/000489769. [DOI] [PubMed] [Google Scholar]
- 199.Rahman KKM, Mohapatra PR, Panigrahi MK, Purkait S, Bhuniya S. Comparison of endobronchial ultrasound-guided transbronchial needle aspiration cytology versus cell blocks in adults with undiagnosed mediastinal lymphadenopathy. Lung India. 2021;38:425–30. doi: 10.4103/lungindia.lungindia_836_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sanz-Santos J, Serra P, Andreo F, Llatjós M, Castellà E, Monsó E. Contribution of cell blocks obtained through endobronchial ultrasound-guided transbronchial needle aspiration to the diagnosis of lung cancer. BMC Cancer. 2012;12:34. doi: 10.1186/1471-2407-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Erer OF, Erol S, Anar C, Aydoğdu Z, Özkan SA. Contribution of cell block obtained by endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of malignant diseases and sarcoidosis. Endosc Ultrasound. 2017;6:265–8. doi: 10.4103/2303-9027.180763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Alici IO, Demirci NY, Yılmaz A, Demirag F, Karakaya J. The combination of cytological smears and cell blocks on endobronchial ultrasound-guided transbronchial needle aspirates allows a higher diagnostic yield. Virchows Arch. 2013;462:323–7. doi: 10.1007/s00428-013-1374-8. [DOI] [PubMed] [Google Scholar]
- 203.Bharati V, Kumari N, Rao S, Sindhwani G, Chowdhury N. The value and limitations of cell blocks in endobronchial ultrasound-guided fine-needle aspiration cytology:Experience of a tertiary care center in North India. J Cytol. 2021;38:140–4. doi: 10.4103/JOC.JOC_210_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Demirci NY, Dikmen AU, Abdullayeva Z, Öztürk C. Contribution of cell blocks obtained through endobronchial ultrasound-guided transbronchial needle aspiration for the determination of lung cancer subtypes. Clin Respir J. 2018;12:1623–7. doi: 10.1111/crj.12719. [DOI] [PubMed] [Google Scholar]
- 205.Sehgal IS, Gupta N, Dhooria S, Aggarwal AN, Madan K, Jain D, et al. Processing and reporting of cytology specimens from mediastinal lymph nodes collected using endobronchial ultrasound-guided transbronchial needle aspiration:A state-of-the-art review. J Cytol. 2020;37:72–81. doi: 10.4103/JOC.JOC_100_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Rotolo N, Cattoni M, Crosta G, Nardecchia E, Poli A, Moretti F, et al. Comparison of multiple techniques for endobronchial ultrasound-transbronchial needle aspiration specimen preparation in a single institution experience. J Thorac Dis. 2017;9((Suppl 5)):S381–5. doi: 10.21037/jtd.2017.04.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Toth JW, Zubelevitskiy K, Strow JA, Kaifi JT, Kunselman AR, Reed MF. Specimen processing techniques for endobronchial ultrasound-guided transbronchial needle aspiration. Ann Thorac Surg. 2013;95:976–81. doi: 10.1016/j.athoracsur.2012.11.058. [DOI] [PubMed] [Google Scholar]
- 208.Dhooria S, Gupta N, Bal A, Sehgal IS, Aggarwal AN, Sethi S, et al. Role of Xpert MTB/RIF in differentiating tuberculosis from sarcoidosis in patients with mediastinal lymphadenopathy undergoing EBUS-TBNA:A study of 147 patients. Sarcoidosis Vasc Diffuse Lung Dis. 2016;33:258–66. [PubMed] [Google Scholar]
- 209.Li C, Xie W, Cao J, Feng J. Detailed procedure and clinical application overview of rapid on-site evaluation in diagnostic interventional pulmonology. J Res Med Sci. 2020;25:35. doi: 10.4103/jrms.JRMS_21_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Jain D, Allen TC, Aisner DL, Beasley MB, Cagle PT, Capelozzi VL, et al. Rapid on-site evaluation of endobronchial ultrasound-guided transbronchial needle aspirations for the diagnosis of lung cancer:A perspective from members of the pulmonary pathology society. Arch Pathol Lab Med. 2018;142:253–62. doi: 10.5858/arpa.2017-0114-SA. [DOI] [PubMed] [Google Scholar]
- 211.Oki M, Saka H, Kitagawa C, Kogure Y, Murata N, Adachi T, et al. Rapid on-site cytologic evaluation during endobronchial ultrasound-guided transbronchial needle aspiration for diagnosing lung cancer:A randomized study. Respiration. 2013;85:486–92. doi: 10.1159/000346987. [DOI] [PubMed] [Google Scholar]
- 212.Trisolini R, Cancellieri A, Tinelli C, de Biase D, Valentini I, Casadei G, et al. Randomized trial of endobronchial ultrasound-guided transbronchial needle aspiration with and without rapid on-site evaluation for lung cancer genotyping. Chest. 2015;148:1430–7. doi: 10.1378/chest.15-0583. [DOI] [PubMed] [Google Scholar]
- 213.Madan K, Dhungana A, Mohan A, Hadda V, Jain D, Arava S, et al. Conventional transbronchial needle aspiration versus endobronchial ultrasound-guided transbronchial needle aspiration, with or without rapid on-site evaluation, for the diagnosis of sarcoidosis:A randomized controlled trial. J Bronchology Interv Pulmonol. 2017;24:48–58. doi: 10.1097/LBR.0000000000000339. [DOI] [PubMed] [Google Scholar]
- 214.Bharati P, Deepak D, Kaushal M, Gupta P. Diagnostic utility of rapid on-site evaluation of endobronchial ultrasound-guided transbronchial needle aspiration samples:A study in a region of high tuberculosis burden. Cytopathology. 2021;32:428–35. doi: 10.1111/cyt.12975. [DOI] [PubMed] [Google Scholar]
- 215.Caupena C, Esteban L, Jaen A, Barreiro B, Albero R, Perez-Ochoa F, et al. Concordance between rapid on-site evaluation and final cytologic diagnosis in patients undergoing endobronchial ultrasound-guided transbronchial needle aspiration for non-small cell lung cancer staging. Am J Clin Pathol. 2020;153:190–7. doi: 10.1093/ajcp/aqz146. [DOI] [PubMed] [Google Scholar]
- 216.Gupta N, Klein M, Chau K, Vadalia B, Khutti S, Gimenez C, et al. Adequate at rapid on-site evaluation (ROSE), but inadequate on final cytologic diagnosis:Analysis of 606 cases of endobronchial ultrasound-guided trans bronchial needle aspirations (EBUS-TBNA) Diagn Cytopathol. 2019;47:367–73. doi: 10.1002/dc.24121. [DOI] [PubMed] [Google Scholar]
- 217.Gianella P, Soccal PM, Plojoux J, Frésard I, Pache JC, Perneger T, et al. Utility of rapid on-site cytologic evaluation during endobronchial ultrasound-guided transbronchial needle aspiration in malignant and nonmalignant disease. Acta Cytol. 2018;62:380–5. doi: 10.1159/000493334. [DOI] [PubMed] [Google Scholar]
- 218.Griffin AC, Schwartz LE, Baloch ZW. Utility of on-site evaluation of endobronchial ultrasound-guided transbronchial needle aspiration specimens. Cytojournal. 2011;8:20. doi: 10.4103/1742-6413.90081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Murakami Y, Oki M, Saka H, Kitagawa C, Kogure Y, Ryuge M, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of small cell lung cancer. Respir Investig. 2014;52:173–8. doi: 10.1016/j.resinv.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 220.Guo H, Liu S, Guo J, Li B, Li W, Lu Z, et al. Rapid on-site evaluation during endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis of hilar and mediastinal lymphadenopathy in patients with lung cancer. Cancer Lett. 2016;371:182–6. doi: 10.1016/j.canlet.2015.11.038. [DOI] [PubMed] [Google Scholar]
- 221.Hopkins E, Moffat D, Smith C, Wong M, Parkinson I, Nespolon W, et al. Accuracy of rapid on-site evaluation of endobronchial ultrasound guided transbronchial needle aspirates by respiratory registrars in training and medical scientists compared to specialist pathologists—an initial pilot study. J Thorac Dis. 2018;10:3922–7. doi: 10.21037/jtd.2018.06.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Umeda Y, Otsuka M, Nishikiori H, Ikeda K, Mori Y, Kobayashi T, et al. Feasibility of rapid on-site cytological evaluation of lung cancer by a trained pulmonologist during bronchoscopy examination. Cytopathology. 2019;30:628–33. doi: 10.1111/cyt.12771. [DOI] [PubMed] [Google Scholar]
- 223.Sehgal IS, Dhooria S, Aggarwal AN, Agarwal R. Impact of Rapid On-Site Cytological Evaluation (ROSE) on the diagnostic yield of transbronchial needle aspiration during mediastinal lymph node sampling:Systematic review and meta-analysis. Chest. 2018;153:929–38. doi: 10.1016/j.chest.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 224.Segraves JM, Daniels CE. Pulmonary embolus diagnosed by endobronchial ultrasound. Respir Med Case Rep. 2015;16:104–5. doi: 10.1016/j.rmcr.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Maturu VN, Dhooria S, Agarwal R. Efficacy and safety of transbronchial needle aspiration in diagnosis and treatment of mediastinal bronchogenic cysts. J Bronchology Interv Pulmonol. 2015;22:195–203. doi: 10.1097/LBR.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 226.Mohamed S, Patel AJ, Mehdi R, Menon A, Steyn R, Bishay E. Infected mediastinal cysts following endobronchial ultrasound guided biopsy-A case series. J Surg Case Rep. 2022;2022:rjac158. doi: 10.1093/jscr/rjac158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hohenforst-Schmidt W, Zarogoulidis P, Steinheimer M, Rupprecht H, Vogl T, Turner JF, et al. A New Endobronchial Ultrasound (EBUS) application for benign and malignant Pericardial Effusion (PE) Aspiration:Transbronchial Pericardial Effusion Aspiration (TPEA) with a Regular EBUS Transbronchial (TBNA) needle under apneic nasal jet-catheter ventilation. J Biomed. 2016;1:9–25. [Google Scholar]
- 228.Madan K, Mittal S, Hadda V, Jain D, Mohan A, Guleria R. Endobronchial ultrasound-guided transbronchial needle aspiration of thyroid:Report of two cases and systematic review of literature. Lung India. 2016;33:682–7. doi: 10.4103/0970-2113.192881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Casal RF, Phan MN, Keshava K, Garcia JM, Grosu H, Lazarus DR, et al. The use of endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of thyroid lesions. BMC Endocr Disord. 2014;14:88. doi: 10.1186/1472-6823-14-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zarogoulidis P, Huang H, Zhou J, Ning Y, Yang M, Wang J, et al. Thyroid cancer diagnosis with transdermal probe 22G U/S versus EBUS-convex probe TBNA-B 22G and 19G:Pros and cons. Expert Rev Med Devices. 2021;18:197–201. doi: 10.1080/17434440.2021.1880891. [DOI] [PubMed] [Google Scholar]
- 231.Harris K, Modi K, Kumar A, Dhillon SS. Endobronchial ultrasound-guided transbronchial needle aspiration of pulmonary artery tumors:A systematic review (with video) Endosc Ultrasound. 2015;4:191–7. doi: 10.4103/2303-9027.162996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Molina JC, Chaudry F, Menezes V, Ferraro P, Lafontaine E, Martin J, et al. Transvascular endosonographic-guided needle biopsy of intrathoracic lesions. J Thorac Cardiovasc Surg. 2020;159:2057–65. doi: 10.1016/j.jtcvs.2019.10.017. [DOI] [PubMed] [Google Scholar]
- 233.Kazakov J, Hegde P, Tahiri M, Thiffault V, Ferraro P, Liberman M. Endobronchial and endoscopic ultrasound-guided transvascular biopsy of mediastinal, hilar, and lung lesions. Ann Thorac Surg. 2017;103:951–5. doi: 10.1016/j.athoracsur.2016.08.111. [DOI] [PubMed] [Google Scholar]
- 234.Panchabhai TS, Machuzak MS, Sethi S, Vijhani P, Gildea TR, Mehta AC, et al. Endobronchial ultrasound–guided transvascular needle aspiration. J Bronchology Interv Pulmonol. 2015;22:306–11. doi: 10.1097/LBR.0000000000000227. [DOI] [PubMed] [Google Scholar]
- 235.Mehta RM, Biraris PR, Pattabhiraman V, Srinivasan A, Singla A, Kumar S, et al. Defining expanded areas in EBUS sampling:EBUS guided trans- and intra-pulmonary artery needle aspiration, with review of transvascular EBUS. Clin Respir J. 2018;12:1958–63. doi: 10.1111/crj.12764. [DOI] [PubMed] [Google Scholar]
- 236.Perathur AM, Joseph T, Nair SR, Varma U. “To do or not to do—That is the question”. Transvascular Needle Aspiration during EBUS (EBUS-TVNA) with review of the literature. Adv Respir Med. 2021;89:386–91. doi: 10.5603/ARM.a2021.0074. [DOI] [PubMed] [Google Scholar]
- 237.Goel MK, Kumar A, Maitra G, Verma RK, Garg N. A report of transaortic EBUS-FNA in a high-risk patient. J Bronchology Interv Pulmonol. 2021;28:e20–3. doi: 10.1097/LBR.0000000000000708. [DOI] [PubMed] [Google Scholar]
- 238.Agrawal A, Ghori U, Chaddha U, Murgu S. Combined EBUS-IFB and EBUS-TBNA vs EBUS-TBNA alone for intrathoracic adenopathy:A Meta-Analysis. Ann Thorac Surg. 2022;114:340–8. doi: 10.1016/j.athoracsur.2020.12.049. [DOI] [PubMed] [Google Scholar]
- 239.Bramley K, Pisani MA, Murphy TE, Araujo KL, Homer RJ, Puchalski JT. Endobronchial ultrasound-guided cautery-assisted transbronchial forceps biopsies:Safety and sensitivity relative to transbronchial needle aspiration. Ann Thorac Surg. 2016;101:1870–6. doi: 10.1016/j.athoracsur.2015.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Chen A, Chrissian A, Misselhorn D, Mayse M. Endobronchial ultrasound-guided transbronchial miniforceps biopsy of mediastinal and hilar lymph node stations. J Bronchology Interv Pulmonol. 2009;16:168–71. doi: 10.1097/LBR.0b013e3181af7a9f. [DOI] [PubMed] [Google Scholar]
- 241.Chrissian A, Misselhorn D, Chen A. Endobronchial-ultrasound guided miniforceps biopsy of mediastinal and hilar lesions. Ann Thorac Surg. 2011;92:284–8. doi: 10.1016/j.athoracsur.2011.03.069. [DOI] [PubMed] [Google Scholar]
- 242.Franke KJ, Bruckner C, Szyrach M, Ruhle KH, Nilius G, Theegarten D. The contribution of endobronchial ultrasound-guided forceps biopsy in the diagnostic workup of unexplained mediastinal and hilar lymphadenopathy. Lung. 2012;190:227–32. doi: 10.1007/s00408-011-9341-0. [DOI] [PubMed] [Google Scholar]
- 243.Mehta RM, Aurangabadbadwalla R, Singla A, Loknath C, Munavvar M. Endobronchial ultrasound-guided mediastinal lymph node forceps biopsy in patients with negative rapid-on-site-evaluation:A new step in the diagnostic algorithm. Clin Respir J. 2020;14:314–9. doi: 10.1111/crj.13133. [DOI] [PubMed] [Google Scholar]
- 244.Herth FJ, Morgan RK, Eberhardt R, Ernst A. Endobronchial ultrasound-guided miniforceps biopsy in the biopsy of subcarinal masses in patients with low likelihood of non-small cell lung cancer. Ann Thorac Surg. 2008;85:1874–8. doi: 10.1016/j.athoracsur.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 245.Herth FJ, Schuler H, Gompelmann D, Kahn N, Gasparini S, Ernst A, et al. Endobronchial ultrasound-guided lymph node biopsy with transbronchial needle forceps:A pilot study. Eur Respir J. 2012;39:373–7. doi: 10.1183/09031936.00033311. [DOI] [PubMed] [Google Scholar]
- 246.McLaughlin J, Liu C, Collins D, Webster K, Wang B, Mani H, et al. The safety and feasibility of endobronchial ultrasound bronchoscopy–guided intranodal forceps biopsies (EBUS-INF) Clin Pulm Med. 2020;27:113–7. [Google Scholar]
- 247.Wang JF, Baidoo C, Collins BT. Improved efficacy of endobronchial ultrasound-guided fine-needle aspiration biopsy in comparison to endobronchial ultrasound-guided miniforceps biopsy. Acta Cytol. 2014;58:125–30. doi: 10.1159/000357358. [DOI] [PubMed] [Google Scholar]
- 248.Radchenko CC, Cho PK, Kang L, Saettele TM. Performance of endobronchial-ultrasound guided miniforceps biopsy of targeted mediastinal and hilar lesions. Respir Med. 2019;158:92–6. doi: 10.1016/j.rmed.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 249.Ray AS, Li C, Murphy TE, Cai G, Araujo KLB, Bramley K, et al. Improved diagnostic yield and specimen quality with endobronchial ultrasound-guided forceps biopsies:A retrospective analysis. Ann Thorac Surg. 2020;109:894–901. doi: 10.1016/j.athoracsur.2019.08.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Darwiche K, Freitag L, Nair A, Neumann C, Karpf-Wissel R, Welter S, et al. Evaluation of a novel endobronchial ultrasound-guided lymph node forceps in enlarged mediastinal lymph nodes. Respiration. 2013;86:229–36. doi: 10.1159/000350867. [DOI] [PubMed] [Google Scholar]
- 251.Zhang J, Guo JR, Huang ZS, Fu WL, Wu XL, Wu N, et al. Transbronchial mediastinal cryobiopsy in the diagnosis of mediastinal lesions:A randomised trial. Eur Respir J. 2021;58:2100055. doi: 10.1183/13993003.00055-2021. [DOI] [PubMed] [Google Scholar]
- 252.Gonuguntla HK, Shah M, Gupta N, Agrawal S, Poletti V, Nacheli GC. Endobronchial ultrasound-guided transbronchial cryo-nodal biopsy:A novel approach for mediastinal lymph node sampling. Respirol Case Rep. 2021;9:e00808. doi: 10.1002/rcr2.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Genova C, Tagliabue E, Mora M, Aloè T, Dono M, Salvi S, et al. Potential application of cryobiopsy for histo-molecular characterization of mediastinal lymph nodes in patients with thoracic malignancies:A case presentation series and implications for future developments. BMC Pulm Med. 2022;22:5. doi: 10.1186/s12890-021-01814-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Madan K, Ayub II, Mohan A, Jain D, Guleria R, Kabra SK. Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) in mediastinal lymphadenopathy. Indian J Pediatr. 2015;82:378–80. doi: 10.1007/s12098-014-1665-3. [DOI] [PubMed] [Google Scholar]
- 255.Madan K, Garg P, Kabra SK, Mohan A, Guleria R. Transesophageal bronchoscopic ultrasound-guided fine-needle aspiration (EUS-B-FNA) in a 3-year-old child. J Bronchology Interv Pulmonol. 2015;22:347–50. doi: 10.1097/LBR.0000000000000169. [DOI] [PubMed] [Google Scholar]
- 256.Mittal S, Madan K, Mohan A. EBUS-TBNA in children:The road less travelled. Adv Respir Med. 2021;89:229–30. doi: 10.5603/ARM.a2021.0039. [DOI] [PubMed] [Google Scholar]
- 257.Mittal S, Bharati SJ, Kabra SK, Madan K. Paediatric endobronchial ultrasound-guided transbronchial needle aspiration:Anaesthetic and procedural considerations. Indian J Anaesth. 2018;62:150–1. doi: 10.4103/ija.IJA_514_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Dhooria S, Madan K, Pattabhiraman V, Sehgal IS, Mehta R, Vishwanath G, et al. A multicenter study on the utility and safety of EBUS-TBNA and EUS-B-FNA in children. Pediatr Pulmonol. 2016;51:1031–9. doi: 10.1002/ppul.23415. [DOI] [PubMed] [Google Scholar]
- 259.Madan K, Madan NK, Mittal S. Transcutaneous Mediastinal Ultrasonography (TMUS) Vs Endosonography (EBUS-TBNA and EUS-B-FNA) for evaluating intrathoracic lymphadenopathy in children. J Ultrasound Med. 2021;41:1301–2. doi: 10.1002/jum.15789. [DOI] [PubMed] [Google Scholar]
- 260.Sehgal IS, Dhooria S, Aggarwal AN, Agarwal R. Training and proficiency in endobronchial ultrasound-guided transbronchial needle aspiration:A systematic review. Respirology. 2017;22:1547–57. doi: 10.1111/resp.13121. [DOI] [PubMed] [Google Scholar]
- 261.Bellinger CR, Chatterjee AB, Adair N, Houle T, Khan I, Haponik E. Training in and experience with endobronchial ultrasound. Respiration. 2014;88:478–83. doi: 10.1159/000368366. [DOI] [PubMed] [Google Scholar]
- 262.Ong ASQ, Tan AH, Anantham D, Sharma K, Tan S, Lapperre TS, et al. Impact of simulation training on performance and outcomes of endobronchial ultrasound-guided transbronchial needle aspiration performed by trainees in a tertiary academic hospital. J Thorac Dis. 2018;10:5621–35. doi: 10.21037/jtd.2018.08.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Konge L, Clementsen PF, Ringsted C, Minddal V, Larsen KR, Annema JT. Simulator training for endobronchial ultrasound:A randomised controlled trial. Eur Respir J. 2015;46:1140–9. doi: 10.1183/13993003.02352-2015. [DOI] [PubMed] [Google Scholar]
- 264.Durairajan N, Venkat D, Soubani A, Jinjuvadia C, Mukadam Z, Lee SJ, et al. Impact of a multimodal simulation.based curriculum on endobronchial ultrasound skills. ATS Sch. 2022;3:258–69. doi: 10.34197/ats-scholar.2021-0046OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


