Abstract
We previously demonstrated that chimeric porcine parvovirus-like particles (PPV:VLP) carrying heterologous epitopes, when injected intraperitoneally into mice without adjuvant, activate strong CD4+ and CD8+ T-cell responses specific for the foreign epitopes. In the present study, we investigated the immunogenicity of PPV:VLP carrying a CD8+ T-cell epitope from the lymphocytic choriomeningitis virus (LCMV) administered by mucosal routes. Mice immunized intranasally with recombinant PPV:VLP, in the absence of adjuvant, developed high levels of PPV-specific immunoglobulin G (IgG) and/or IgA in their serum, as well as in mucosal sites such as the bronchoalveolar and intestinal fluids. Antibodies in sera from mice immunized parenterally or intranasally with PPV:VLP were strongly neutralizing in vitro. Intranasal immunization with PPV:VLP carrying the LCMV CD8+ T-cell epitope also elicited a strong peptide-specific cytotoxic-T-cell (CTL) response. In contrast, mice orally immunized with recombinant PPV:VLP did not develop any antibody or CTL responses. We also showed that mice primed with PPV:VLP are still able to develop strong CTL responses after subsequent immunization with chimeric PPV:VLP carrying a foreign CD8+ T-cell epitope. These results highlight the attractive potential of PPV:VLP as a safe, nonreplicating antigen carrier to stimulate systemic and mucosal immunity after nasal administration.
Mucosal surfaces are frequently the first site of contact between the host and environmental hazards such as infectious agents or carcinogens. Therefore, the mucosa-associated lymphoid tissues are particularly important for protection against diseases for which entry and pathogenesis involve the mucosal system (i.e., the respiratory, gastrointestinal, and genital tracts), such as salmonellosis, tuberculosis, and AIDS. The mucosal immune system contains defense mechanisms, including secretory immunoglobulin A (IgA) antibodies and cytotoxic-T-cell (CTL) responses (6, 11), against foreign aggressions. Therefore, antigen carrier vectors would have to elicit mucosal, as well as systemic, immune responses in order to develop fully efficient prophylactic or therapeutic vaccines against various pathogens such as, for instance, the human papillomaviruses, which cause the development of cervical cancer, or Helicobacter pylori, which is responsible for the development of gastric ulcer. However, vaccines administered by parenteral routes generally fail to stimulate mucosal immune responses. Therefore, it is necessary to develop efficient and safe antigen vectors which will be able to trigger systemic and mucosal immune responses when administered by mucosal routes.
Virus-like particle (VLP) vectors represent promising vaccine candidates. Indeed, they can stimulate immune responses (i) against the VLP proteins themselves, such as parvovirus-like particles (12, 14), human immunodeficiency virus type 1 (HIV-1) Gag particles (4), rubella virus VLP (20), and human papillomavirus-like particles (3), or (ii) against foreign peptides or proteins expressed by chimeric VLP, such as yeast retrotransposon Ty:VLP (7, 9) hepatitis B surface-antigen VLP (15), Semliki Forest virus VLP (23), or potyvirus-like particles (5).
We previously studied the immunogenicity of virus-like particles produced in insect cells by self-assembly of the recombinant VP2 capsid protein from porcine parvovirus (PPV:VLP) carrying various heterologous peptides corresponding to CD4+ or CD8+ T-cell epitopes. These PPV:VLP administered in mice by the intraperitoneal (i.p.) route, in the absence of adjuvant, were shown to induce a whole range of immune responses, including Th1 CD4+ T lymphocytes (10, 22) and protective antiviral cytotoxic CD8+ T lymphocytes (21). These results prompted us to investigate the immune responses induced by these pseudoparticles after mucosal administration. This study demonstrates that VLP prepared with a single PPV protein and administered by the intranasal route, in the absence of adjuvant, induced specific IgG and/or IgA antibodies in the serum and in bronchoalveolar lavages and intestinal fluids. Chimeric PPV:VLP carrying a lymphocytic choriomeningitis virus (LCMV) CD8+ CTL epitope inserted into the N terminus of PPV VP2 and administered by the intranasal (i.n.) route also stimulated an LCMV-specific CTL response showing the strong potential of this antigen carrier system in developing safe and efficient vaccines that are able to stimulate systemic and mucosal immune responses.
MATERIALS AND METHODS
Preparation of recombinant parvovirus-like particles.
The construction, characterization, and purification of the chimeric and control recombinant PPV:VLP were previously described in detail (21, 22). The PPV VP2 gene was expressed either with the 118–132 peptide sequence [PPV:VLP-(LCMV)] or without this sequence (PPV:VLP) in a baculovirus vector system. After infection of Sf9 insect cells, the recombinant VLPs were purified by salt precipitation with 20% ammonium sulfate followed by dialysis. Characterization of PPV:VLP and PPV:VLP-(LCMV) obtained by CsCl sedimentation analysis and electron microscopy revealed properties identical to native PPV virions.
Mice and peptide.
Female BALB/c mice were purchased from Iffa Credo (L’Arbresle, France). The p118–132 synthetic peptide RPQASGVYMGNLTAQ corresponding to an H-2d-restricted CTL epitope from the LCMV nucleoprotein (1) was synthesized by Neosystem (Strasbourg, France).
Mice immunization.
Mice were immunized by intraperitoneal (i.p.), i.n., or oral routes with 10 μg of either recombinant control PPV:VLP or chimeric PPV:VLP carrying the p118–132 epitope. Unanesthetized mice received an i.n. administration of 20 μl of solution deposited in the nostrils. Orally immunized mice were gavaged through a rigid steel gavage tube containing 0.5 ml of solution. All immunizations were performed in saline in the absence of adjuvant.
Collection of samples.
First, mice were bled by retro-orbital plexus puncture to collect the sera. Then, to collect the feces, mice were individually spread over airy boxes on filter paper to avoid the urine contamination. After 1 h, their feces were collected in EDTA buffer containing 10 μg of trypsin inhibitor (Sigma, St. Louis, Mo.) per ml and 0.1% of sodium azide. After incubation for 20 min at 4°C, the tubes were centrifuged for 2 min, and 1 mM phenylmethylsulfonyl fluoride (Sigma) diluted in ethanol was added to each supernatant. Finally, mice were sacrificed, and the tracheas were cannulated. Bronchoalveolar lavage (BAL) fluids were recovered by two consecutive lavages with 0.4 ml of phosphate-buffered saline (PBS). BAL fluids were centrifuged at 4,000 × g for 5 min to remove cells. Sera, feces, and BAL fluids were stored at −20°C prior to antibody titration by enzyme-linked immunosorbent assay (ELISA).
Antibody assay.
At different times postimmunization, sera, BAL fluids, and fecal fluids were individually collected and tested for antibody responses by ELISA. Microtiter trays (Nunc, Roskilde, Denmark) were coated with 2 μg of PPV:VLP per ml in 50 mM (pH 9.6) carbonate buffer (Na2CO3 and NaHCO3) at 4°C overnight. After three washes in PBS (Seromed, Munich, Germany) containing 0.1% Tween 20, diluted sera or fluids were added to the wells and incubated for 1 h at 37°C. After three washes, the wells were treated with goat anti-mouse IgG- or IgA-peroxidase conjugates (Sigma) for 1 h at 37°C. After being washed, the substrate solution prepared with o-phenylenediamine (Sigma) and hydrogen peroxide was added to the plates. Optical densities (OD) were then read at 492 nm in an ELISA reader (Multiscan MS; Labsystem). Serum ELISA results are expressed as the log10 titer as calculated by linear regression analysis plotting dilution versus A492, with the titer being defined as the log10 of the highest dilution which gave twice the absorbance of negative control serum diluted 1/100. Results are given as the arithmetic mean ± the standard error of individual serum titers. Antibodies in BAL or fecal fluids are expressed as the OD × 1,000 obtained with, respectively, 1/8- or 1/6-diluted fluids.
In vitro protection assay.
The protection was determined by using an immunoperoxidase monolayer assay. Briefly, swine testis (ST) cells were cultured in 96-well plates at 10,000 cells/well. Mouse sera were serially diluted, starting at 1:10. A mixture of 30 μl of sera and PPV was incubated for 2 h at 37°C. After the reaction, 50 μl of the virus mixture was added to the ST cells and left for 90 min at 37°C. Then, the virus was removed and 200 μl of fresh medium was added to the cells and cultured for 5 days. For staining, cells were fixed with 99% ethanol for 45 min at room temperature. The plates were then incubated with 50 μl of anti-PPV rabbit serum diluted 1:400 in PBS–1% Tween 80–0.5 M NaCl for 20 min at 37°C, washed with PBS, and incubated with peroxidase-conjugated protein A (dilution, 1:200) for 20 min at 37°C. Finally, the color was developed by adding a 0.4% solution of amino-ethyl carbaxole for 30 min at room temperature. The plates were washed with PBS to remove the unbound precipitates. In most cases, the color could be observed visually. Neutralization titer was determined as the highest dilution at which no color was detected.
Cytotoxic assay.
After immunization with PPV:VLP-(LCMV), mice were sacrificed and the spleens were surgically removed. Spleen cells were stimulated in vitro with 1 μM p118–132 peptide as previously described (18) in the presence of syngeneic irradiated naive spleen cells for 5 days. The cytotoxic activity of these effector cells at various effector/target ratios was tested on 51Cr-labeled P815 target cells pulsed with a 50 μM concentration of the p118–132 peptide as previously described (21). In these conditions, p118–132 peptide-coated H-2d-restricted P815 target cells were previously shown to be susceptible to the CTL response directed against p118–132 (1). The released radioactivity was measured in the supernatant. The percentage of specific lysis was calculated as follows: 100 × (experimental release − spontaneous release)/(maximum release − spontaneous release). The maximum release was generated by adding 1 M HCl to target cells, and spontaneous release was obtained with target cells incubated without effector cells.
RESULTS
Analysis of serum antibody responses induced by PPV:VLP administered by mucosal routes.
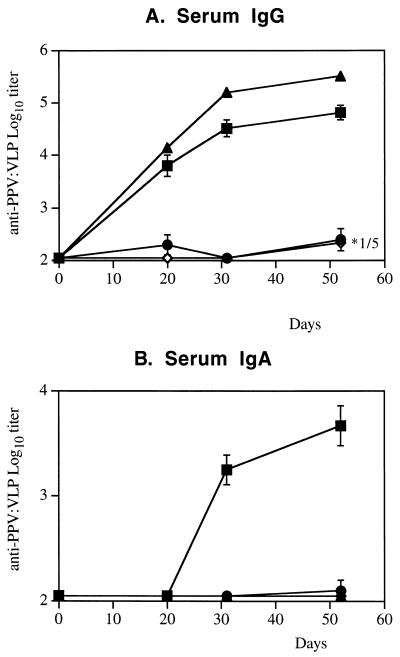
We previously demonstrated that chimeric PPV:VLP carrying a CD8+ epitope administered to mice by the i.p. route induced a strong CTL response specific for the heterologous epitope, as well as an antibody response directed against the parvovirus VP2 protein (21, 22). In the present study, we investigated the immune responses induced by these pseudoparticles administered by mucosal route. BALB/c mice were immunized by i.n., oral, or i.p. routes with 10 μg of PPV:VLP in the absence of adjuvant. The serum antibody responses were analyzed after one, two, or three injections of PPV:VLP particles. As illustrated in Fig. 1A, PPV:VLP administered i.n. to mice stimulated a strong PPV-specific serum IgG antibody response even after a single injection. The kinetics and the level of this antibody response were similar to the IgG response induced by i.p. immunization. Moreover, high levels of anti-PPV IgA antibodies were detected in the sera of mice immunized by the i.n. route, whereas no IgA response was found in the sera of i.p. or orally immunized mice (Fig. 1B). Mice immunized by the oral route did not develop a detectable IgG or IgA antibody response.
FIG. 1.
The i.n. administration of recombinant PPV:VLP induces serum antibody production. BALB/c mice (four or five per group) were immunized by different routes on days 0, 21, and 42 with 10 μg of PPV:VLP in the absence of adjuvant. Control mice were not immunized. Mice were bled at different times after injections, and individual sera were tested for PPV:VLP-specific IgG (A) or IgA (B) antibodies. Results are expressed as the arithmetic mean ± the standard error from four to five mice per group. In panel A, ∗1/5 means that only one of five mice produced IgG with a 3.22 log10 titer. Route of immunization: ■, i.n.; ●, oral; ▴, i.p.; ◊, none (control).
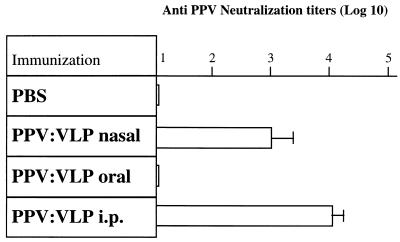
The sera of mice which had received three injections of PPV:VLP by various routes were tested at several dilutions in an in vitro PPV neutralization assay (Fig. 2). Mice immunized by the i.n. route developed high titers of PPV-neutralizing antibodies compared to control mice or mice immunized by the oral route. Similar high titers of neutralizing antibodies were also induced after the i.p. administration of PPV:VLP.
FIG. 2.
An i.n. immunization with PPV:VLP induces neutralizing antibodies. BALB/c mice (four to five per group) were immunized by different routes on days 0, 21, and 42 with 10 μg of PPV:VLP in the absence of adjuvant. Control mice were not immunized. Mice were bled 10 days after the last injection, and individual sera were tested for neutralizing activities. Results are expressed as the arithmetic mean ± the standard error for four or five mice per group of log10 neutralization titers.
Intranasal immunization with PPV:VLP induces mucosal antibody responses.
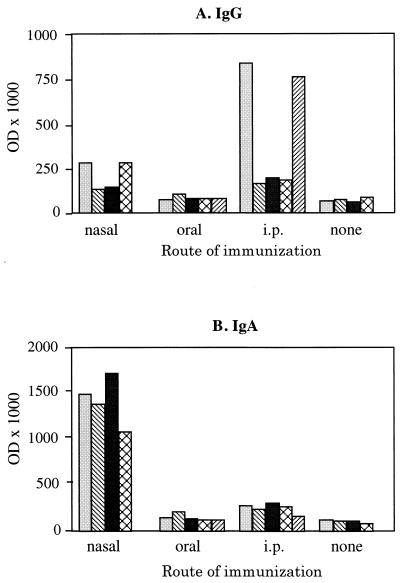
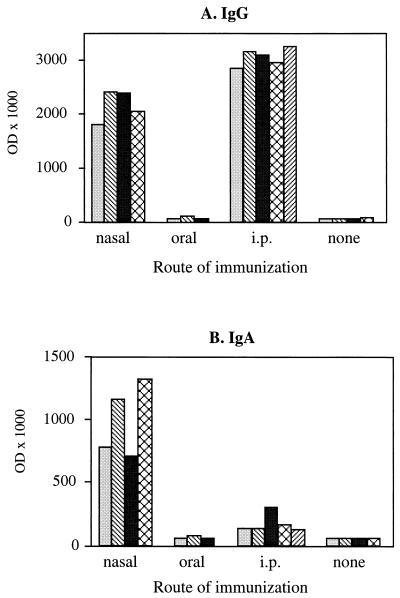
We next analyzed the capacity of PPV:VLP to stimulate the production of antibodies at the mucosal level. We first collected the feces of mice which had been immunized with PPV:VLP by different routes in the absence of adjuvant, and we then tested these samples for the presence of antibodies. The analysis of PPV:VLP-specific antibodies in these feces (Fig. 3A) showed that i.n. or orally immunized mice did not develop a significant anti-PPV IgG antibody production, even after three injections of the particles. After i.p. injection, only two mice developed a significant IgG response. However, all mice immunized i.n. with PPV:VLP produced high titers of PPV-specific IgA antibodies in their feces (Fig. 3B). In contrast, no PPV-specific IgA antibodies were detected in the feces of mice immunized by oral or i.p. injections. PPV:VLP administered i.n. to BALB/c mice also induced strong specific IgG and IgA antibody responses in the BAL fluids (Fig. 4). In contrast, the oral administration of PPV:VLP did not result in a detectable antibody synthesis in these fluids, whereas the i.p. route induced a strong IgG response in the absence of detectable IgA.
FIG. 3.
Antibody responses in the feces of mice mucosally immunized with recombinant PPV:VLP. BALB/c mice (four or five per group) were immunized by different routes on days 0, 21, and 42 with 10 μg of PPV:VLP in the absence of adjuvant. Control mice were not immunized. Mouse feces were harvested 10 days after the last injection and were individually tested for the presence of PPV:VLP-specific IgG (A) or IgA (B) antibodies. Results represent the OD × 1,000 at a 1:6 dilution of mouse feces extract. Each histogram represents an individual mouse.
FIG. 4.
Antibody produced in the bronchoalveolar fluids of mice mucosally immunized with recombinant PPV:VLP. BALB/c mice (three to five per group) were immunized by different routes on days 0, 21, 42, and 70 with 10 μg of PPV:VLP in the absence of adjuvant. Control mice were not immunized. Mouse BAL fluids were collected 9 days after the last injection and individually tested for the presence of PPV:VLP-specific IgG (A) or IgA (B) antibodies. Results represent the OD × 1,000 at a 1:8 dilution of mouse BAL fluid. Each histogram represents an individual mouse.
Induction of CTL responses by i.n. immunization with PPV:VLP carrying an LCMV CD8+ epitope.
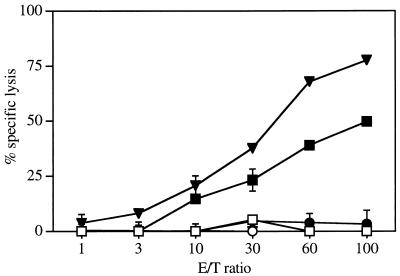
We next investigated the capacity of i.n. administration of PPV:VLP to stimulate cytotoxic T cells. A foreign peptide, p118–132, corresponding to an H-2d-restricted CD8+ CTL epitope from LCMV was inserted into the N terminus of PPV VP2 to prepare chimeric PPV:VLP-(LCMV). We previously demonstrated that these chimeric PPV:VLP-(LCMV) pseudoparticles injected into mice by the i.p. route in the absence of adjuvant induce a strong CTL response that protects mice against a lethal LCMV challenge (21).
Mice were immunized with 10 μg of PPV:VLP-(LCMV) by the i.n. or oral routes, in the absence of adjuvant, and immune splenocytes were stimulated in vitro with the p118–132 peptide. As shown in Fig. 5, immunization of mice by the oral route was unable to activate CTLs. In contrast, the i.n. administration of PPV:VLP-(LCMV) induced a strong peptide-specific CTL response. As expected, mice injected with control PPV:VLP did not develop any p118–132-specific CTL response, indicating that the observed CTL activity was specific for the inserted LCMV peptide (data not shown). Thus, these results show that the i.n. administration of recombinant PPV can stimulate splenic CD8+ cytotoxic T cells, although the response induced by the nasal route was slightly less efficient than the response induced by the i.p. route. However, in contrast to mice immunized i.p., animals which received administration of PPV:VLP (LCMV) i.n. were not protected against an intracerebral challenge with LCMV (data not shown).
FIG. 5.
The i.n. immunization with recombinant PPV:VLP expressing a CD8+ LCMV T-cell epitope induces a CTL response. BALB/c mice were immunized on days 0 and 21 by i.n. (square), oral (circle), or i.p. (triangle) routes with 10 μg of PPV:VLP-(LCMV) in the absence of adjuvant. After 10 days, spleen cells were stimulated in vitro with the p118–132 peptide in the presence of irradiated syngeneic spleen cells for 5 days. The cytotoxic activity of these effector cells was measured on 51Cr-labeled P815 target cells pulsed with the p118–132 peptide (solid symbols) or incubated with medium alone (open symbols). The data represent the mean percentages of the specific lysis values from duplicate samples.
Immunity to the PPV:VLP vector does not interfere with the capacity of chimeric PPV:VLP-(LCMV) to induce CTL responses against the heterologous epitope.
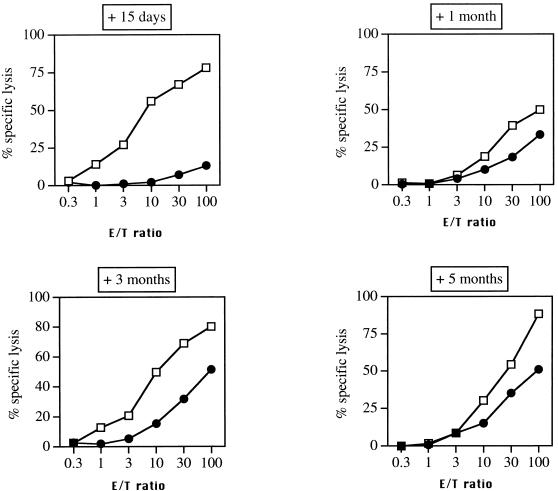
We than analyzed the effect of priming with PPV:VLP on the subsequent response to chimeric PPV:VLP carrying a CTL epitope. Mice received two i.p. injections of PPV:VLP at different times prior to i.p. immunization with the chimeric PPV:VLP-(LCMV). As shown in Fig. 6, unprimed mice developed a strong CTL response, whereas the CTL response of mice which had received the PPV:VLP 15 days prior to injection of the chimeric particles was almost totally suppressed. However, this inhibitory effect was transient, and mice primed 1, 3, or 5 months before immunization with chimeric particles developed good CTL responses against the LCMV epitope. Therefore, priming with PPV:VLP has little effect on the subsequent immune response against the CTL epitope carried by these pseudoparticles if the new immunization is performed at least 1 month after priming.
FIG. 6.
Effect of prior priming with PPV:VLP on the immunogenicity of recombinant PPV:VLP expressing a viral CTL epitope. BALB/c mice were primed with PBS or with 10 μg of control PPV:VLP by i.p. injection on days 0 and 14. After 15 days or 1, 3, or 5 months, all mice were immunized twice i.p. with 10 μg of PPV:VLP(LCMV) at 3-week intervals. At 10 days after the last injection, spleen cells were stimulated in vitro with the p118–132 peptide in the presence of syngeneic spleen cells. The cytotoxic activity of these effector cells was measured on 51Cr-labeled P815 target cells pulsed with the p118–132 peptide. The data represent the mean percentages of the specific lysis values from duplicate samples. Symbols: ●, PPV:VLP; □, PBS.
DISCUSSION
In the present study, we demonstrate that recombinant PPV:VLP administered i.n. in the absence of adjuvant stimulates IgA and/or IgG antibody responses both in sera and in bronchoalveolar and intestinal mucosal sites. Moreover, this route of immunization also provides an efficient way to induce CTL responses.
Numerous studies have analyzed the capacity of various vectors to stimulate mucosal responses. However, only a few of these studies succeeded in inducing mucosal responses by using nonreplicative vectors. Indeed, for instance, HPV16-L1 VLP-specific serum and mucosal antibodies were induced in mice only if these VLP were delivered by the attenuated PhoPc strain of Salmonella typhimurium (16). Purified antigens or peptides usually require adjuvants, such as cholera toxin, to stimulate immune responses after i.n. or oral immunization. For instance, i.n. administration of VLP prepared by self-assembly of rotavirus structural proteins was shown to assure a full protection of mice against rotavirus challenge, but this strong efficiency required the addition of cholera toxin (17). Similarly, i.n. immunization with the HIV-1 gp120 protein or with ovalbumin peptides containing CTL epitopes require the coadministration of cholera toxin to induce CTL activity (19). However, cholera toxin is not likely to be approved for use as an adjuvant in vaccines due to its serious side effects. It should, however, be noted that a nontoxic mutant of heat-labile Escherichia coli enterotoxin was recently shown to act as an adjuvant after i.n. coimmunization with a peptide corresponding to a measles virus CTL epitope (18). This nontoxic mutant, LTK63, was also able to induce a protective immunity against H. pylori in mice immunized by intragastric administration of H. pylori antigens (13). It should, however, be mentioned that our study is still the first to demonstrate that a nonreplicative antigen carrier system can induce strong and neutralizing immune responses after mucosal immunization of conscious mice in the absence of any adjuvant. Indeed, Balmelli et al. (2) recently demonstrated that nasal immunization of mice with HPV16 VLP elicits neutralizing antibodies in mucosal secretions, whereas oral immunization, even in the presence of cholera toxin, did not stimulate antibody response. It should however be remarked that these results were obtained in anesthetized mice and that nasal immunization of conscious mice with HPV16 VLP was inefficient.
To our knowledge, this study was also the first demonstration that CTL responses can be induced by inert nonreplicative antigen administered i.n. without adjuvant. So far, we analyzed only systemic CTL responses and it remains to be determined whether PPV:VLP can also induce CTL responses at the mucosal level. Although a CTL response induced by i.n. immunization with chimeric PPV:VLP carrying an LCMV epitope did not confer protection against a lethal intracerebral challenge with the virus, these results open the possibility of stimulating both humoral and cellular responses by the mucosal administration of a safe vector. More-detailed studies will be necessary to determine whether the lack of protection observed after i.n. immunization is due to a lower CTL frequency or to other parameters such as CTL localization.
The strong immunogenicity of PPV:VLP could be related to its capacity to stimulate efficient T-helper-cell responses. Indeed, we previously showed that PPV:VLP is very efficiently presented by major histocompatibility complex (MHC) class II molecules. Using hybrid PPV:VLP carrying a CD4+ T-cell epitope, we showed that the ability of antigen-presenting cells (APC) to stimulate epitope-specific T-cell hybridomas was 100-fold more efficient with these PPV:VLPs than with the free peptide (10). In this study, we also demonstrated that these PPV:VLPs behave as a conventional exogenous antigen and are processed in endosomal-lysosomal acidic vesicles. The presentation of a foreign CD4+ T-cell epitope carried by PPV:VLP is sensitive to brefeldin A and cycloheximide, indicating that the antigenic peptides are loaded on nascent MHC class II molecules (10). This high efficiency of PPV:VLP presentation to T cells suggests that the parvovirus particle uptake may occur via an active mechanism, such as receptor-dependent endocytosis or phagocytosis. Alternatively, this efficient T-cell stimulation may be due to the activation of APC by these pseudoparticles, leading to an upregulation of MHC class II or costimulatory molecules. These hypotheses are currently under investigation.
In the present study, we also showed that the oral administration of recombinant PPV:VLP failed to stimulate immune responses. This lack of immunogenicity may be due to the low dosage of particles used in this study, since large amounts of antigen are usually required to stimulate immune responses by the oral route due to antigen degradation in the stomach and intestine. In contrast, i.n. administration avoids the encounter of the antigen with the acidic and proteolytic environment of the stomach.
One surprising observation of this study is the fact that priming with PPV:VLP particles did not abolish the immunogenicity of chimeric particles carrying foreign epitopes if more than 15 days elapse between priming and immunization. Indeed, priming against most of vectors usually results in an inhibition of immune responses obtained after a boost immunization. This observation demonstrates that preexisting antibodies against the particles do not prevent their presentation by MHC class I molecules. The mechanisms by which PPV:VLPs are presented to CD8+ T cells have not yet been deciphered. In particular, it remains to be determined whether PPV:VLPs follow the classical route used by endogenously synthesized antigens, which is also accessible to exogenous antigens (8).
ACKNOWLEDGMENT
This work was carried out as a collaborative project between Institut Pasteur and Ingenasa in a Biotech project (EEC biotechnology BI04-CT96-024).
REFERENCES
- 1.Aichele P, Hengartner H, Zinkernagel R M, Schulz M. Antiviral cytotoxic T cell response induced by in vivo priming with a free synthetic peptide. J Exp Med. 1990;171:1815–1820. doi: 10.1084/jem.171.5.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balmelli C, Roden R, Potts A, Schiller J, de Grandi P, Nardelli-Haefliger D. Nasal immunization of mice with human papillomavirus type 16 virus-like particles elicits neutralizing antibodies in mucosal secretions. J Virol. 1998;72:8220–8229. doi: 10.1128/jvi.72.10.8220-8229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenstone H L, Nieland J D, de Visser K E, De Bruijn M L, Kirnbauer R, Roden R B, Lowy D R, Kast W M, Schiller J T. Chimeric papillomavirus virus-like particles elicit antitumor immunity against the E7 oncoprotein in an HPV16 tumor model. Proc Natl Acad Sci USA. 1998;95:1800–1805. doi: 10.1073/pnas.95.4.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffiths J C, Harris S J, Layton G T, Berrie E L, French T J, Burns N R, Adams S E, Kingsman A J. Hybrid human immunodeficiency virus Gag particles as an antigen carrier system: induction of cytotoxic T-cell and humoral immune responses by a Gag:V3 fusion. J Virol. 1993;67:3191–3198. doi: 10.1128/jvi.67.6.3191-3198.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jagadish M N, Hamilton R C, Fernandez C S, Schoofs P, Davern K M, Kalnins H, Ward C W, Nisbet I T. High level production of hybrid potyvirus-like particles carrying repetitive copies of foreign antigens in Escherichia coli. BioTechniques. 1993;11:1166–1170. doi: 10.1038/nbt1093-1166. [DOI] [PubMed] [Google Scholar]
- 6.Kilian M, Russel M W. Functions of mucosal immunoglobulins. In: Ogra P L, Mestecky J, Lamm M E, Strober W, McGhee J R, Bienenstock J, editors. Handbook of mucosal immunology. San Diego, Calif: Academic Press; 1994. pp. 127–143. [Google Scholar]
- 7.Kingsman A J, Burns N R, Layton G T, Adams S E. Yeast retrotransposon particles as antigen delivery systems. Ann N Y Acad Sci. 1995;754:202–213. doi: 10.1111/j.1749-6632.1995.tb44452.x. [DOI] [PubMed] [Google Scholar]
- 8.Kovacsovics-Bankowski M, Rock K L. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Eur J Immunol. 1995;267:243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 9.Layton G T, Harris S J, Gearing A J H, Hill-Perkins M, Cole J S, Griffiths J C, Burns N R, Kingsman A J, Adams S E. Induction of HIV-specific cytotoxic T lymphocytes in vivo with hybrid HIV-1 V3:Ty-virus-like particles. J Immunol. 1993;151:1097–1107. [PubMed] [Google Scholar]
- 10.Lo-Man R, Rueda P, Sedlik C, Deriaud E, Casal I, Leclerc C. A recombinant virus-like particle system derived from parvovirus as an efficient antigen carrier to elicit a polarized Th1 immune response without adjuvant. Eur J Immunol. 1998;28:1401–1407. doi: 10.1002/(SICI)1521-4141(199804)28:04<1401::AID-IMMU1401>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 11.London S D. Cytotoxic lymphocytes in mucosal effector sites. In: Ogra P L, Mestecky J, Lamm M E, Strober W, McGhee J R, Bienenstock J, editors. Handbook of mucosal immunology. San Diego, Calif: Academic Press; 1994. pp. 325–336. [Google Scholar]
- 12.Lopez de Turiso J A, Cortés E, Martinez C, de Ybanez R R, Simarro I, Vela C, Casal J I. Recombinant vaccine for canine parvovirus in dogs. J Virol. 1992;66:2748–2753. doi: 10.1128/jvi.66.5.2748-2753.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchetti M, Rossi M, Giannelli V, Giuliani M M, Pizza M, Censini S, Covacci A, Massari P, Pagliaccia C, Manetti R, Telford J L, Douce G, Dougan G, Rappuoli R, Ghiara P. Protection against Helicobacter pylori infection in mice by intragastric vaccination with H. pylori antigens is achieved using a non-toxic mutant of E. coli heat-labile enterotoxin (LT) as adjuvant. Vaccine. 1998;16:33–37. doi: 10.1016/s0264-410x(97)00153-9. [DOI] [PubMed] [Google Scholar]
- 14.Martinez C, Dalsgaard K, de Turiso J A L, Cortés E, Vela C, Casal J I. Production of porcine parvovirus empty capsids with high immunogenic activity. Vaccine. 1992;10:684–690. doi: 10.1016/0264-410x(92)90090-7. [DOI] [PubMed] [Google Scholar]
- 15.Milich D R, Peterson D L, Zheng J, Hughes J L, Wirtz R, Schödel F. The hepatitis nucleocapsid as a vaccine carrier moiety. Ann N Y Acad Sci. 1995;754:187–201. doi: 10.1111/j.1749-6632.1995.tb44451.x. [DOI] [PubMed] [Google Scholar]
- 16.Nardelli-Haefliger D, Roden R B, Benyacoub J, Sahli R, Kraehenbuhl J P, Shiller J T, Lachat P, Potts A, De Grandi P. Human papillomavirus type 16 virus-like particles expressed in attenuated Salmonella typhimurium elicit mucosal and systemic neutralizing antibodies in mice. Infect Immun. 1997;65:3328–3336. doi: 10.1128/iai.65.8.3328-3336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Neal C M, Crawford S E, Estes M K, Conner M E. Rotavirus-like particles administered mucosally induce protective immunity. J Virol. 1997;71:8707–8717. doi: 10.1128/jvi.71.11.8707-8717.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Partidos C D, Pizza M, Rappuoli R, Steward M W. The adjuvant effect of a non-toxic mutant of heat-labile enterotoxin of Escherichia coli for the induction of measles virus-specific CTL responses after intranasal co-immunization with a synthetic peptide. Immunology. 1996;89:483–487. doi: 10.1046/j.1365-2567.1996.d01-790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porgador A, Staats H F, Faiola B, Gilboa E, Palker T J. Intranasal immunization with epitope peptides from HIV-1 or ovalbumin and the mucosal adjuvant cholera toxin induces peptide-specific CTLs and protection against tumor development in vivo. J Immunol. 1997;158:834–841. [PubMed] [Google Scholar]
- 20.Qiu Z, Ou D, Wu H, Hobman T C, Gillam S. Expression and characterization of virus-like particles containing rubella virus structural proteins. J Virol. 1994;68:4086–4091. doi: 10.1128/jvi.68.6.4086-4091.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sedlik C, Saron M F, Sarraseca J, Casal I, Leclerc C. Recombinant parvovirus-like particles as an antigen carrier: a novel nonreplicative exogenous antigen to elicit protective antiviral cytotoxic T cells. Proc Natl Acad Sci USA. 1997;94:7503–7508. doi: 10.1073/pnas.94.14.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sedlik C, Sarraseca J, Rueda P, Leclerc C, Casal I. Immunogenicity of poliovirus B and T cell epitopes presented by hybrid porcine parvovirus particles. J Gen Virol. 1995;76:2361–2368. doi: 10.1099/0022-1317-76-9-2361. [DOI] [PubMed] [Google Scholar]
- 23.Zhou X, Berglund P, Zhao H, Lilijeström P, Jondal M. Generation of cytotoxic and humoral immune responses by nonreplicative recombinant Semliki Forest virus. Proc Natl Acad Sci USA. 1995;92:3009–3013. doi: 10.1073/pnas.92.7.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]