Abstract
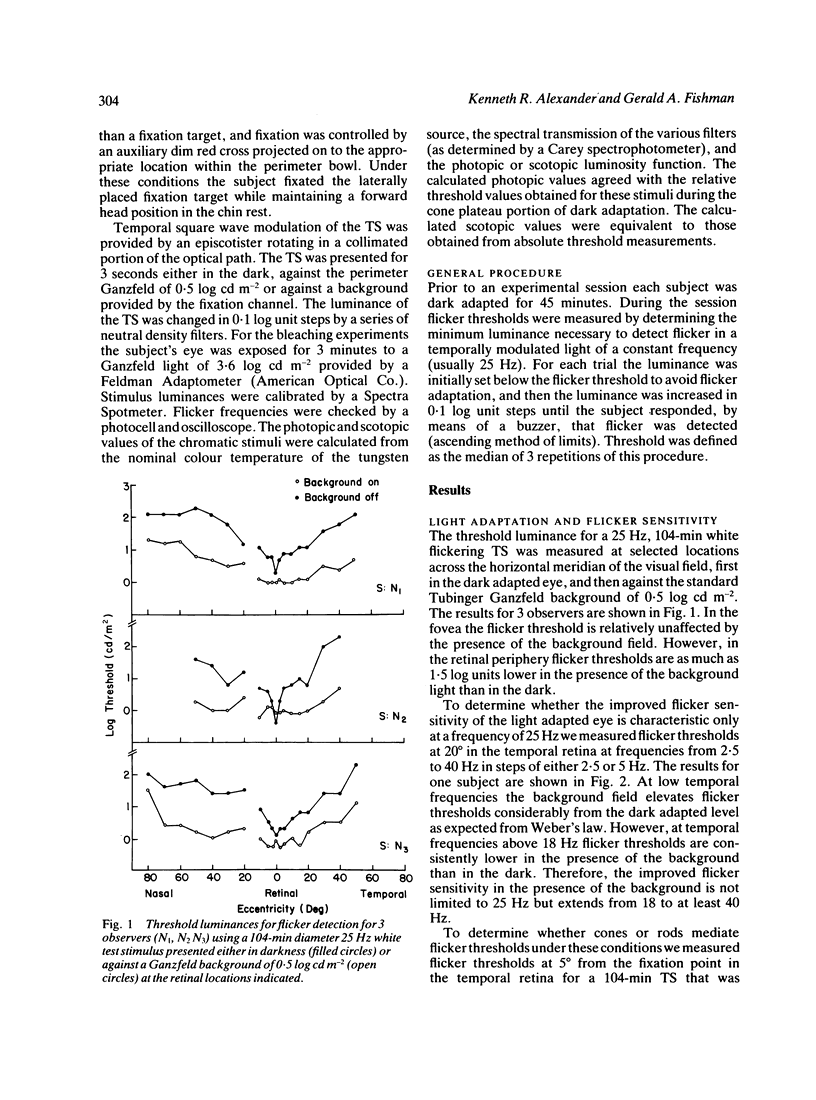
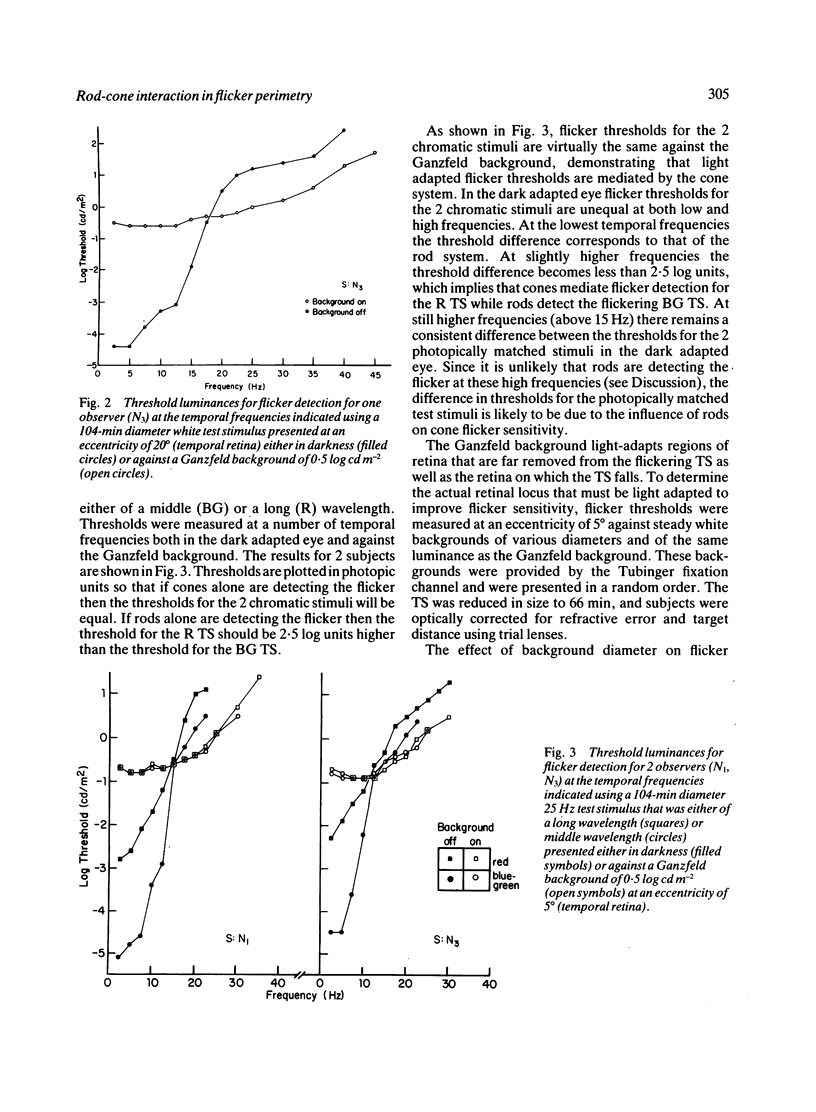
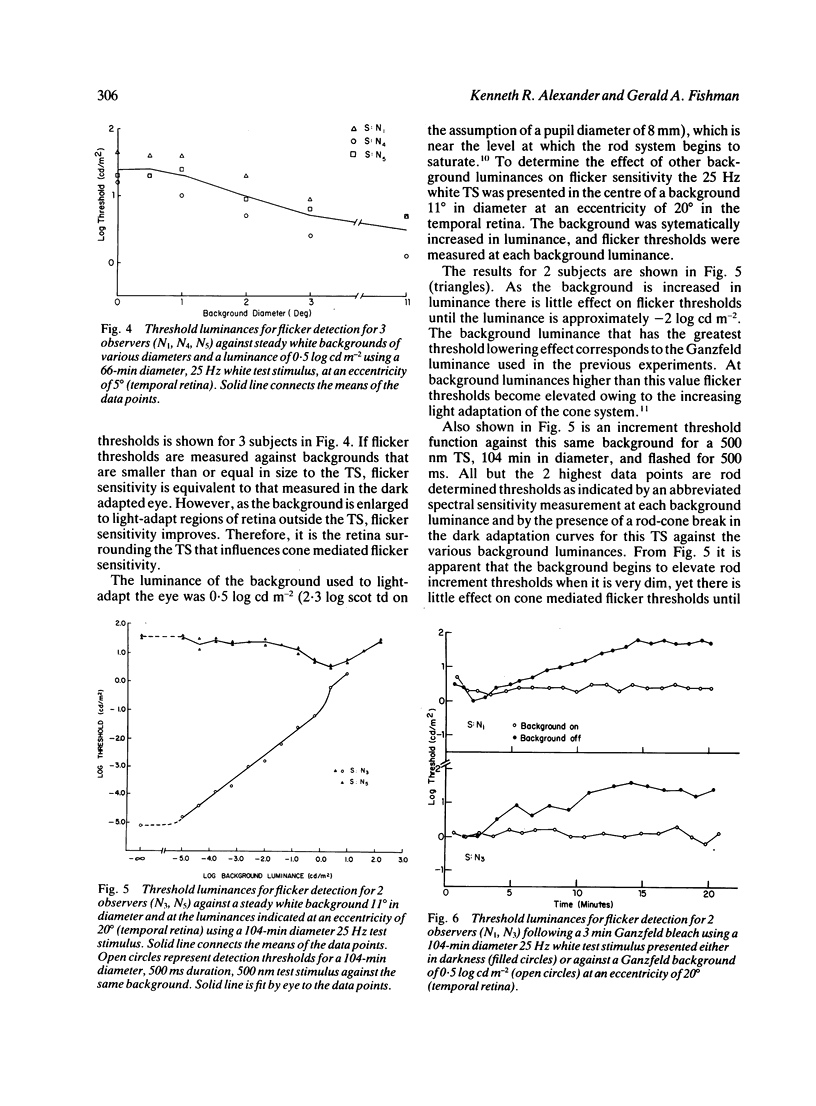
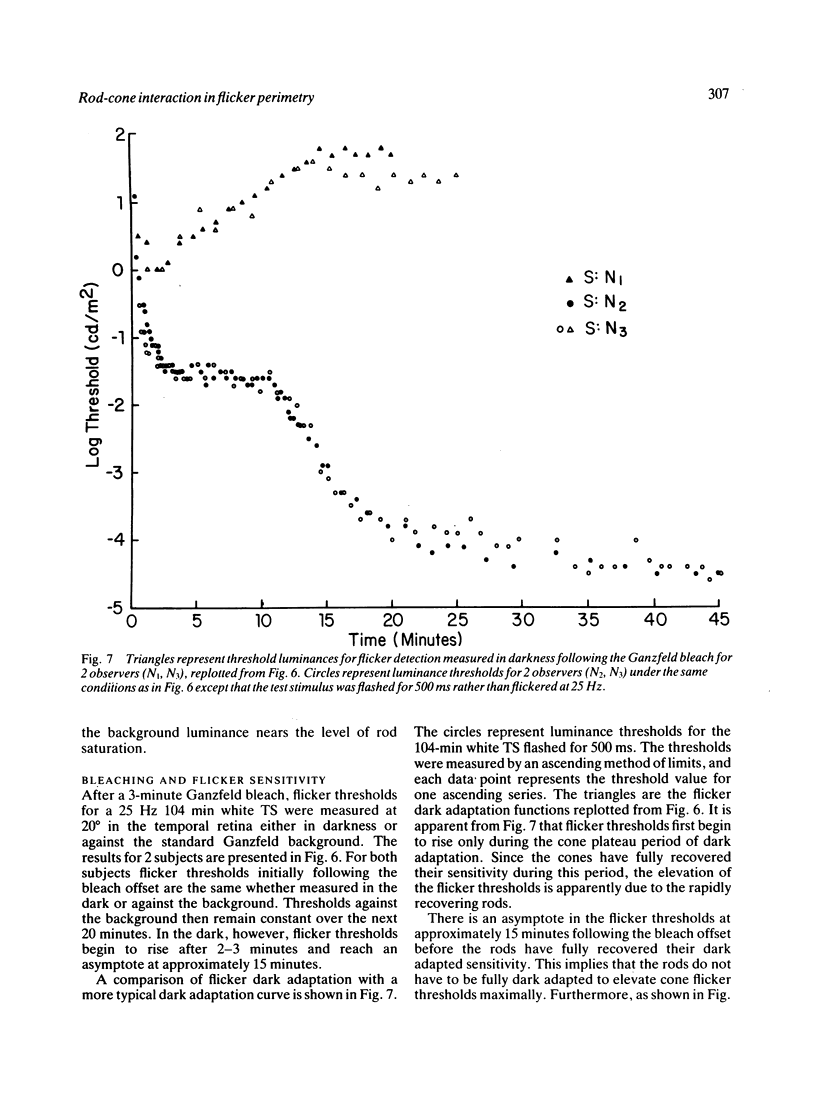
We have assessed the influence of the rod system on cone flicker sensitivity during flicker perimetry. For temporal frequencies above 18 Hz extrafoveal cone-mediated flicker thresholds for a white test stimulus are as much as 1.5 log units lower when measured against a large background light that saturates the rods than when measured in darkness. Following a Ganzfeld bleach extrafoveal cone flicker thresholds are at their minimum once the cones have recovered their sensitivity, but then thresholds rise as the rods begin to recover from the bleach. Our results indicate that the flicker sensitivity of the extrafoveal cone system at high temporal frequencies is influenced by the rods surrounding the flickering test stimulus. The rods reduce flicker sensitivity maximally in the dark adapted state, and their suppressive influence is minimised only by strong rod bleaches or by large backgrounds that saturate the rod system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Change of organization in the receptive fields of the cat's retina during dark adaptation. J Physiol. 1957 Aug 6;137(3):338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL C. J., RITTLER M. C. The diagnostic value of flicker perimetry in chronic simple glaucoma. Trans Am Acad Ophthalmol Otolaryngol. 1959 Jan-Feb;63(1):89–98. [PubMed] [Google Scholar]
- Goldberg S. H., Frumkes T. E., Nygaard R. W. Inhibitory influence of unstimulated rods in the human retina: evidence provided by examining cone flicker. Science. 1983 Jul 8;221(4606):180–182. doi: 10.1126/science.6857279. [DOI] [PubMed] [Google Scholar]
- Hood D. C. Suppression of the frog's cone system in the dark. Vision Res. 1972 May;12(5):889–907. doi: 10.1016/0042-6989(72)90013-2. [DOI] [PubMed] [Google Scholar]
- Kelly D. H. Adaptation effects on spatio-temporal sine-wave thresholds. Vision Res. 1972 Jan;12(1):89–101. doi: 10.1016/0042-6989(72)90139-3. [DOI] [PubMed] [Google Scholar]
- King-Smith P. E., Carden D. Luminance and opponent-color contributions to visual detection and adaptation and to temporal and spatial integration. J Opt Soc Am. 1976 Jul;66(7):709–717. doi: 10.1364/josa.66.000709. [DOI] [PubMed] [Google Scholar]
- MacLeod D. I. Rods cancel cones in flicker. Nature. 1972 Jan 21;235(5334):173–174. doi: 10.1038/235173a0. [DOI] [PubMed] [Google Scholar]
- Mason R. J., Snelgar R. S., Foster D. H., Heron J. R., Jones R. E. Abnormalities of chromatic and luminance critical flicker frequency in multiple sclerosis. Invest Ophthalmol Vis Sci. 1982 Aug;23(2):246–252. [PubMed] [Google Scholar]
- Massof R. W., Fleischman J. A., Fine S. L., Yoder F. Flicker fusion thresholds in Best macular dystrophy. Arch Ophthalmol. 1977 Jun;95(6):991–994. doi: 10.1001/archopht.1977.04450060077005. [DOI] [PubMed] [Google Scholar]
- Skottun B. C., Nordby K., Magnussen S. Photopic and scotopic flicker sensitivity of a rod monochromat. Invest Ophthalmol Vis Sci. 1981 Dec;21(6):877–879. [PubMed] [Google Scholar]
- Sokol S., Riggs L. A. Electrical and psychophysical responses of the human visual system to periodic variation of luminance. Invest Ophthalmol. 1971 Mar;10(3):171–180. [PubMed] [Google Scholar]
- Wolf E., Gaeta A. M., Geer S. E. Critical flicker frequencies in flicker perimetry. Standards and confidence limits. Arch Ophthalmol. 1968 Sep;80(3):347–351. doi: 10.1001/archopht.1968.00980050349010. [DOI] [PubMed] [Google Scholar]


