Graphical abstract
Keywords: Natural plants, Carrier-free nanoplatform, Extracellular vesicle, Charcoal nanocomponent, Nanoaggregates
Highlights
-
•
Carrier-free nanoplatforms with self-assembly from natural plants.
-
•
Nano self-assembly of pure small molecules for enhanced bioactivity.
-
•
Extracellular vesicles containing active substances from fresh plants.
-
•
Charcoal nanocomponents with carbon skeleton structure from charred plants.
-
•
Nanoaggregate particles with wide curative effects from decoction of plants formulae.
Abstract
Background
Natural plants as well as traditional Chinese medicine have made outstanding contributions to the health and reproduction of human beings and remain the basis and major resource for drug innovation. Carrier-free nanoplatforms completely self-assembled by pure molecules or therapeutic components have attracted increasing attention due to their advantages of improved pharmacodynamics/pharmacokinetics, reduced toxicity, and high drug loading. In recent years, carrier-free nanoplatforms produced by self-assembly from natural plants have contributed to progress in a variety of therapeutic modalities. Notably, these nanoplatforms based on the interactions of components from different natural plants improve efficiency and depress toxicity.
Aim of Review
In this review, different types of self-assembled nanoplatforms are first summarized, mainly including nanoassemblies of pure small molecules isolated from different plants, extracellular vesicles separated from fresh plants, charcoal nanocomponents obtained from charred plants, and nanoaggregates from plants formulae decoctions.
Key Scientific Concepts of Review: We mainly focus on composition, self-assembly mechanisms, biological activity and modes of action. Finally, a future perspective of existing challenges with respect to the clinical application of plant-based carrier-free nanoplatforms is discussed, which may be instructive to further develop effective carrier-free nanoplatforms from natural plants in the future.
Introduction
In the course of fighting against disease, mankind first apply natural plants to relieve pain [1]. Therefore, in the early history of various ancient civilizations, there is no lack of records of herbal medicines. In particular, working Chinese people have accumulated rich experience in the processes of long-term production practices and fighting against diseases and have gradually formed unique traditional Chinese medicine (TCM) [2]. Natural plants have made outstanding contributions to the health and reproduction of all mankind. Furthermore, natural ingredients from medicinal plants are characterized by abundant scaffold diversity and structural complexity, which have played important roles in drug discovery [3]. Among the 1,881 drugs approved by the U.S. Food and Drug Administration from 1981 to 2019, approximately 65 % were natural products or natural product-based drugs [4]. Some well-known drugs, including artemisinin, paclitaxel, camptothecin, Guanfu base A, and huperzine A, have all been discovered in natural plants. Recently, natural active molecules have been used for the treatment of coronavirus disease 2019 [5], [6]. Therefore, natural plants are receiving increasing attention in treating diseases. Discovery of natural lead compounds from plants has been, and will continue to be, the frontier of fundamental research of biological medicine and a promising path to drug research and development.
Nanodrug delivery and targeting systems have provided advanced opportunities in recent years and can significantly improve therapeutic efficacy for the treatment of human diseases [7], [8]. The drug molecules are loaded into nanocarriers and ultimately released to the diseased region after active or passive targeting. Thus, nanotechnology is widely used to deliver active ingredients from natural plants to improve their solubility and chemical stability and prolong their blood circulation time [9], [10]. However, the preparation of nanocarriers is too laborious for clinical translation, and most nanocarriers pose the risks of undesirable immune responses and carrier-related toxicity. Moreover, their low drug-loading capacity and premature drug leakage restrict their therapeutic efficacy. Fortunately, carrier-free nanoplatforms completely self-assembled from pure drugs or therapeutic components in the absence of exotic nontherapeutic carriers have been developed [11], [12], [13]. No additional nanocarriers are involved in the preparation, which can enhance drug-loading efficiency (even up to 100 %), avoid toxicity of nanocarriers, and facilitate large scalable production and clinical translation. Therefore, carrier-free nanoparticles have been developed as an emerging promising platform for treating diseases [14], [15], [16].
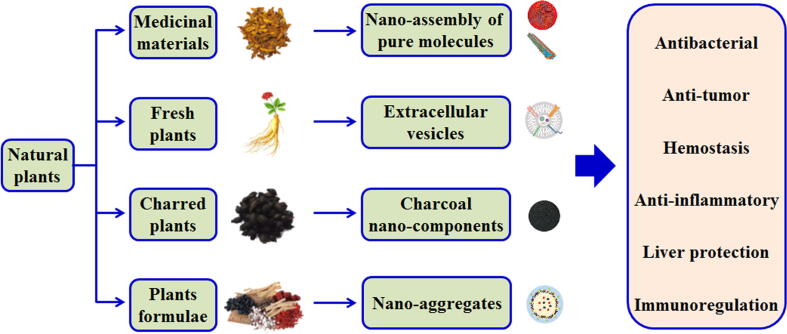
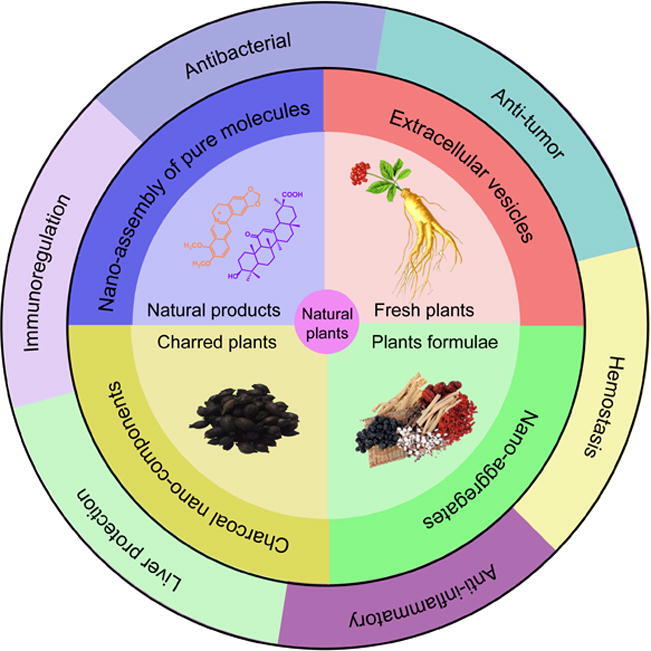
Natural medicinal plants are inclined to cure some certain diseases and can be a source for potential drugs, which are increasingly recognized by researchers and clinicians [1], [3], [4]. Thus, the emerging carrier-free nanoplatforms with self-assembly from natural plants over the past decade urgently need to be summarized and reviewed. In this review, an emphasis will be placed on four types of nanoplatforms (Fig. 1): (i) nanoassembly of pure small molecules isolated from different plants; (ii) extracellular vesicles separated from fresh plants; (iii) charcoal nanocomponents obtained from charred plants; and (iv) nanoaggregates from the decoction of plant formulae. We will focus on the sources of small molecules, the self-assembly mechanisms, the compositions, and the biological activities. Finally, we discuss the prospects and future challenges of carrier-free nanoplatforms from natural plants.
Fig. 1.
Carrier-free nanoplatforms from natural plants with enhanced bioactivity.
Carrier-free nanoplatforms of pure natural molecules
Plant alkaloid-based nano self-assembly
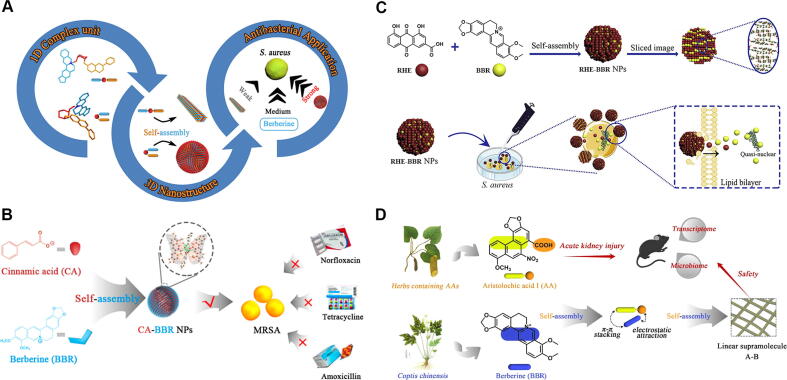
Natural alkaloids, which are widely distributed in plants, are a group of important bioactive ingredients containing basic nitrogen atoms that exhibit multiple biological activities [17], [18]. Berberine (BBR), a famous natural isoquinoline alkaloid, is isolated from Coptis chinensis and other Coptis species plants. BBR possesses remarkable antibacterial action and other pharmacological activities [19], [20]. Lei’s group [21] discovered that BBR could interact with baicalin (BA) to form nanoparticles (BA-BBR) by self-assembly, while wogonoside (WOG) interacted with BBR to form nanofibers (WOG-BBR) by self-assembly (Fig. 2A). BA and WOG are flavonoid glycosides which are the main active components from Scutellaria baicalensis. BA-BBR displayed significantly enhanced bacteriostatic activity, whereas WOG-BBR showed a weaker effect than BBR. This was because BA-BBR formation was governed by electrostatic and hydrophobic interactions to orient hydrophilic glucuronic acid toward the outside. Thus, BA-BBR were attached much easier onto bacteria and sustained release BBR in large quantities, thereby inducing the collapse of the bacterial population and the decrease in biofilm. As for WOG-BBR, they were difficult to attach onto bacteria owing to their hydrophobic properties. Furthermore, it was found [22] that BA-BBR nanoparticles could promote a synergistic effect on diarrhea-predominant irritable bowel syndrome mice compared with simple mixing. This synergistic effect might be related to brain-gut peptides, immune inflammation, and intestinal flora, which are important interrelated components of the microbiota–gut–brain axis. Cinnamic acid (CA) is a representative compound from Cinnamomum cassia [23]. CA could also directly self-assemble into nanoparticles with BBR by hydrogen bonds and π − π stacking interactions [24]. Compared with first-line antibiotics (norfloxacin, amoxicillin, and tetracycline), CA-BBR nanoparticles showed a better inhibitory effect on multidrug-resistant Staphylococcus aureus, owing to their preferential adherence to the surface of S. aureus leading to synergetic converging bacterial attack (Fig. 2B). Next, 3,4,5-methoxycinnamic acid (3,4,5-TCA), a derivative of CA, was isolated from Polygala tenuifolia as an effective antibacterial agent with a phenylpropanoid skeleton [25]. 3,4,5-TCA-BBR nanoparticles [26] were almost uniform spherical particles, and their size (diameter: 92.6 nm) was larger than that of CA-BBR (diameter: 66.0 nm).
Fig. 2.
(A) Self-assembly between berberine (BBR) and flavonoid glycosides (baicalin/wogonoside) for antibacterial application. Reproduced with permission from Ref. [21]. Copyright 2019, American Chemical Society; (B) Self-assembly between cinnamic acid (CA) and BBR for inhibiting multidrug-resistant Staphylococcus aureus. Reproduced with permission from Ref. [24]. Copyright 2020, American Chemical Society; (C) Self-assembly between rhein (RHE) and BBR for antibacterial activity. Reproduced with permission from Ref. [29]. Copyright 2020, Elsevier Publishing Group; (D) Self-assembly between aristolochic acid (AA) and BBR for neutralizing acute nephrotoxicity. Reproduced with permission from Ref. [31]. Copyright 2021, American Chemical Society.
Rheum palmatum is a medicinal plant with purgation effects. Its main active ingredient is rhein (RHE), which has an anthraquinone skeleton [27], [28]. Tian et al. [29] found that RHE could also interact with BBR to accomplish nano self-assembly (Fig. 2C). In spherical RHE-BBR particles, RHE served as a layered framework by hydrogen bonding interactions, and BBR was inserted into the backbone by electrostatic interactions. RHE-BBR nanoparticles had stronger inhibitory effects on S. aureus than single components. Aristolochic acid (AA), from Aristolochia debilis, has been shown to cause a variety of severe side effects, such as acute kidney injury, AA nephropathy, and liver cancer [30]. AA and BBR can assemble into linear heterogeneous supramolecules in aqueous solution [31]. The hydrophilic groups were inside and the hydrophobic groups were outside in the AA-BBR nanostructure, which might play an important role in blocking the toxic site of AA (Fig. 2D). In vivo assays showed that AA-BBR supramolecules significantly reduced AA-induced acute kidney injury compared with AA. This method offered a novel strategy to overcome the toxicity problems of medicinal plants containing AA. Hu’s group [32] further found that BBR alkylation (BBRA) could form nanodrugs with rhamnolipids (RHL) via self-assembly. Four types of BBRA-RHL nanoparticles with RHL shielding of positive charge and increasing hydrophilicity were successfully obtained. BBRA-RHL nanoparticles could penetrate the mucus barrier and effectively eradicate Helicobacter pylori biofilms to achieve antibacterial activity. Moreover, the authors discovered that BBRA nanoparticles exerted antitumor effects by activating mitochondrial apoptosis pathways [33]. Next, BBR was linked together with paclitaxel (PTX) by a disulfide bond to obtain a drug-drug conjugate (PTX-ss-BBR) [34]. This conjugate could be self-assembled to form nanoparticles through π-π stacking and hydrophobic interactions. PTX-ss-BBR nanoparticles exerted a synergistic antitumor effect due to BBR’s mitochondria-targeting delivery and GSH-responsible drug release, which dissipated mitochondria membrane potential and upregulated ROS levels to induce apoptosis of cancer cells. In conclusion, BBR provided a new template for small molecules to self-assemble into nanostructures to enhance bioactivity. The main driving force of the formation of self-assembly between BBR and small molecules in aqueous solution is the electrostatic interaction and π-π stacking. After the formation of one-dimensional structure, they continue to form three-dimensional nanostructures under the action of hydrogen bonding and π-π stacking. Thus, these small molecules should include carboxyl group which can interact with the quaternary ammonium ion of BBR. Besides, the aromatic structure of small molecules is also essential, which can interact with isoquinoline ring of BBR. However, whether small molecules can self-assemble with BBR needs further experimental verification.
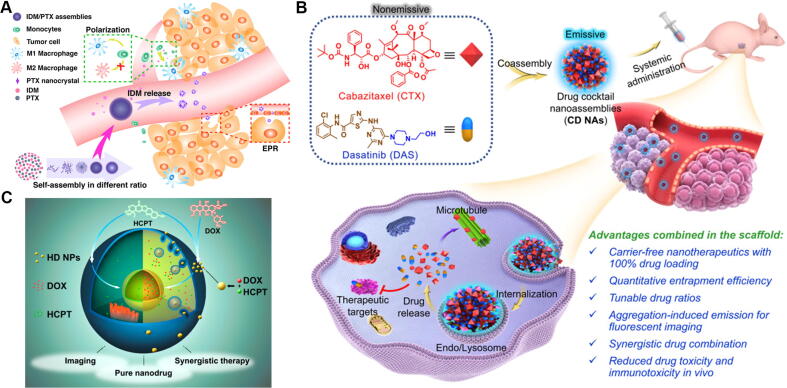
PTX, first separated from Taxus chinensis, is a widely applied chemotherapy agent with an extensive spectrum of antitumor activity [35], [36]. Pei et al. [37] synthesized a PTX dimer (PTX-S-PTX) via a mono thioether linker. PTX-S-PTX nanovesicles, formed by self-assembly, exhibited effective cytotoxicity and served as carriers to load fluorescent molecules for simultaneous bioimaging. Indomethacin (IDM) is a nonsteroidal anti-inflammatory drug that restrains the synthesis of prostaglandin E2 and increases the proinflammatory macrophage ratio and immune response to cancer cells [38], [39]. Zhang et al. [40] built carrier-free IDM-PTX nanocrystal self-assemblies (Fig. 3A). In the IDM-PTX nanostructure, PTX nanocrystals were cast with IDM, similar to a “brick cement” architecture. IDM-PTX assemblies showed enhanced synergetic antitumor effects based on chemotherapy and immunotherapy. Cabazitaxel (CTX) [41], a derivative of PTX, is a hydrophobic drug, while dasatinib (DAS) [42], a polytyrosine kinase inhibitor, is an amphiphile. Therefore, Chen et al. [43] assembled nanoparticles constructed from CTX and DAS (Fig. 3B). DAS-CTX nanoparticles showed enhanced synergetic antitumor efficiency and alleviated systemic toxicity. Meanwhile, DAS exhibited aggregation-induced emission that could be surveyed for bioimaging.
Fig. 3.
(A) Self-assembly between indomethacin (IDM) and paclitaxel (PTX) for synergetic antitumor effect. Reproduced with permission from Ref. [40]. Copyright 2019, American Chemical Society; (B) Schematic illustration of self-assembly between cabazitaxel (CTX) and dasatinib (DAS) for combination cancer therapy. Reproduced with permission from Ref. [43]. Copyright 2021, Ivyspring International Publisher; (C) Illustration of different intracellular drug accumulation of nano-assembly between 10-hydroxycamptothecin (HCPT) and doxorubicin (DOX). Reproduced with permission from Ref. [52]. Copyright 2015, American Chemical Society.
Camptothecin (CPT), isolated from Camptotheca acuminata, is a typical quinoline-based alkaloid. It has been widely proven to inhibit the DNA topoisomerase I enzyme and exhibit prominent antitumor activity against various cancers [44]. CPT could self-assemble into helical nanoribbons, whereas CPT derivatives (10-hydroxy CPT and carboxylic CPT) self-aggregated into flat nanoribbons and cylindric nanorods, respectively [45]. CPT-based self-assemblies with J-type patterns were stable in aqueous solution, which could avoid hydrolysis of CPT-based drugs to increase their antitumor activity. Zhou et al. [46] synthesized an aminated CPT prodrug (CPT-NH2), which could self-assemble into nanofibers with length of several micrometers and width of 100 nm. These CPT-NH2 nanofibers could rapidly enter cancer cells and efficiently release the active CPT to exhibit cytotoxicity. Next, amphipathic CPT derivatives were designed and synthesized, and these CPT derivatives could self-assemble into nanocapsules for drug self-delivery. A CPT-floxuridine conjugate amphiphile with a hydrolysable ester linkage was developed to prepare liposome-like nanocapsules (CFNs) [47]. CFNs showed high drug loading and no premature drug release owing to the highly stable codelivery without the need for any carrier. In vivo delivery of CFNs resulted in longer blood circulation, higher tumorous accumulation of drugs, and enhanced efficacy in murine tumor models. Pei’s group [48], [49] established a lactose-modified CPT amphiphile that self-assembles into a carrier-free supramolecular structure for targeted drug delivery. These CPT amphiphiles were synthesized with ROS- or GSH-responsive bonds to target the release of CPT in cancer cells. More importantly, the assembled supramolecular structure could load other chemotherapeutics or fluorescent molecules to exert synergistic therapy or enable theranostics. Curcuminoids are extracted from Curcuma longa with the traditional effect of activating blood circulation [50]. Xiao et al. [51] constructed carrier-free self-delivery systems based on curcuminoids and CPT derivatives (irinotecan and topotecan) for targeted cancer therapy. The surface charges of nanoparticles changed by approximately − 10 mV under neutral conditions to + 40 mV under acidic environments. Next, Liang’s group [52] used a simple “green” reprecipitation procedure to fabricate dual-drug nano self-assemblies. 10-Hydroxycamptothecin (HCPT) could interact with doxorubicin (DOX), a common clinical chemotherapy drug, by self-nanocrystallization (Fig. 3C). HCPT-DOX nanoparticles showed enhanced synergistic antitumor effects due to the increased accumulation of HCPT in the nucleus, while HCPT and DOX directly mixed and exhibited antagonistic effects. Further study [53] showed that HCPT-DOX nanoparticles improved the intracellular drug retention of DOX to as much as 2-fold in drug-resistant MCF-7R breast cancer cells to resist P-glycoprotein (P-gp) efflux and enhanced synergistic antiproliferation efficiency against drug-resistant cancer cells.
Natural triterpene-based nano self-assembly
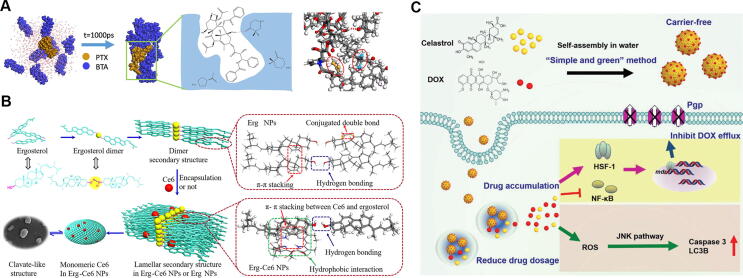
Natural triterpenoids consisting of six isoprene units are widely distributed in plants and have a range of useable biological effects [54]. Recent studies [55], [56] showed that triterpenoids could self-assemble into supramolecular structures with a variety of shapes, such as nanofibers, nanotubes, vesicles, and spheres. Cyclic triterpenes have rigid skeletons and many chiral carbon atoms, and the position and number of hydroxyl groups are also different. They can be assembled into organogels by π-π stacking, intermolecular van der Waals force, hydrogen bond, hydrophobicity, dipole and other non-covalent bond interactions. In the one-dimensional direction, long fibrous, ribbon-like, tubular or helical nanostructures are formed. These longer structures are then wound to form a three-dimensional network structure in space, and the organic solvent is captured in this spatial structure, which usually shows the assembly morphology of fiber, sheet, vesicle and so on. These phenomena could be applied to the areas of drug delivery to enhance drug stability, solubility, and bioactivity. Oleanolic acid (OA), which exists in Ligustrum lucidum, has remarkable antioxidant and antitumor activity [57], [58]. OA could self-assemble into nanospheres with sizes of < 200 nm, and the diameter increased slightly when loaded with other molecules [59], [60], [61]. When the OA carrier interacting with molecules became saturated, the excessive molecules coexisted in a physically mixed form with the nanospheres. Thus, OA nanosphere had a complete multilayer spherical shell nanostructure, which could be used as ideal drug delivery vehicle. Lupeol (Lup), betulinic acid (BTA), and betulinol (Bet) are lupine-type pentacyclic triterpenoids and are separated from Lupinus luteus [62]. Lup nanoparticles showed a nanospherical structure with interspersed nanofibers [59], [63]. BTA- and Bet-based nanoparticles showed nanofibers or slab-like shapes with sizes of greater than 500 nm, of which all surface charges were slightly negative [59], [60]. Furthermore, Wang et al. [64] constructed a supramolecular structure formed by BTA and PTX via hydrogen bonding and hydrophobic interactions (Fig. 4A). BTA-PTX nanoparticles exhibited biosafety and low toxicity and exhibited synergistic enhancement of antitumor efficacy. BTA induced Bcl-2 down-regulation or p53 up-regulation to block the cell cycle in the S phase, while PTX prompted microtubule polymerization to mediate the induction of apoptosis. Phytosterols such as stigmasterol (Sti), ergosterol (Erg), and β-sitosterol (BS) are important secondary metabolites in many medicinal plants [65]. Sti and Erg could form nanorods in aqueous solution, while BS nanoparticles showed irregular morphology [59], [63]. Compared with Sti- and BS-based nanoplatforms, Erg was the ideal carrier molecule to load the photosensitizer chlorin e6 (Ce6) [66]. The Erg-Ce6 nanodrugs (Fig. 4B) could increase reactive oxygen species (ROS) generation by promoting type I photoreactions for cancer therapy. Poricoic acid A is a tricyclic triterpenoid, and dehydrotrametenolic acid (DTA) and dehydrotumulosic acid are tetracyclic triterpenoids that are separated from Poria cocos. These triterpenoids could self-assemble into gel scaffolds, which showed excellent controlled gelation, sustained release, and good safety [63]. Yang et al. [63] assembled oral PTX-loaded DTA nanoparticles through hydrogen bonding. These nanoparticles could penetrate the gastrointestinal tract by hijacking the apical sodium-dependent bile transporter-based intestinal transport system, thereby treating disease through oral drug delivery. Liquidambaric acid (LA), separated from Liquidambar formosana, exhibits excellent anticancer activity [67]. Celastrol (CST), a friedelane-type pentacyclic triterpenoid [68], is isolated from Tripterygium wilfordii. Xiao et al. [69] assembled carrier-free nanoparticles with CST and DOX to overcome DOX resistance for synergistic combination chemotherapy (Fig. 4C). The spherical CST-DOX nanoparticles could significantly increase cellular DOX accumulation by restraining nuclear factor kappa-B to depress P-gp expression and then led to autophagy and apoptosis of multidrug-resistant cells via the ROS/JNK signaling pathway.
Fig. 4.
(A) Combination mode diagram and molecular simulation of interaction between betulinic acid (BTA) and paclitaxel (PTX). Reproduced with permission from Ref. [64]. Copyright 2020, Elsevier Publishing Group; (B) Schematic representation of self-assembly behavior of ergosterol (Erg) and molecular stacking models of co-assembled Erg-Ce6 nanoparticles. Reproduced with permission from Ref. [66]. Copyright 2019, American Chemical Society; (C) Schematic diagram of celastrol-doxorubicin nanoparticles reversing drug resistance in tumor cells. Reproduced with permission from Ref. [69]. Copyright 2018, The Royal Society of Chemistry.
Panax ginseng is used for reinforcement of vital energy in its traditional functions [70], [71]. Ginsenoside Ro (Ro), an active ingredient of ginseng, could increase the solubility of saikosaponin a (SSa), which is derived from Bupleurum chinense [72]. Ro preferentially formed nanovesicles with SSa. Moreover, some hydrophobic molecules without hydrophilic groups (such as quercetin and coumarin) were mainly inserted into the hydrophobic layer of the Ro vesicle, and molecules including both hydrophilic and hydrophobic fragments (such as BA and SSa) were located in the palisade layer of the Ro vesicle [73]. Next, interactions between other ginsenosides (Rb1 and Rg1) from ginseng with SSa were further explored [74]. Rg1 could form spherical micelles with SSa in aqueous medium. Compared with Rg1, a greater number of sugars in Rb1 established more binding sites with SSa. Thus, worm-like micelles were formed by Rb1 and SSa molecules. Platycodin, isolated from Platycodon grandiflorum, was forecasted to be an ideal carrier in pharmaceutical fields to increase the solubility of hydrophobic molecules [75]. The concentration-dependent structural variation of platycodin was observed, and the platycodin-based aggregate morphologies included spherical, elliptical, tubular, oblate, and necklace-like micelles and multilamellar and multicompartment vesicles.
Glycyrrhizic acid (GL) and glycyrrhetinic acid (GA) are found in Glycyrrhiza uralensis [76]. In terms of chemical structure, GL is an amphiphilic molecule with a hydrophilic glucuronic acid residue and hydrophobic triterpenoid aglycone (GA residue). Zhao et al. [77], [78] found that GL could form an injectable low-molecular-weight hydrogel with anisotropic nanoclusters in aqueous solution. The GL hydrogel could restrain the growth of Gram-positive S. aureus, while it showed no effect on Gram-negative Escherichia coli. Similar to other triterpene glycosides, GL could also form host–guest complexes by self-assembly, thereby being used as a carrier to improve the water-solubility of hydrophobic drugs. Molecular dynamics simulation [79] showed that GL aggregation occurred with PTX to form GL-PTX clusters at a ratio of 3:1 with a size of approximately 10 nm. Next, baicalein-loaded GL nanomicelles [80] were also prepared, which improved the solubility of baicalein in water by more than 4,500 times and generated a sustained-release effect of baicalein. GA is the aglycone of GL. Bag et al. [81] reported that GA could form nanosized spherical and flower-like nanostructures by self-assembly with fibrillar networks, yielding thermoreversible gels. Wu et al. [82] considered dipole–dipole interactions between sodium and carboxylates to be the main driving force for the formation of GA hydrogels. Furthermore, the GA hydrogel exhibited the properties of adsorption and sustainable release of some fluorescent dyes. Yang’s group [59] reported a nanomedicine-cum-carrier drug delivery system with GA and OA (Fig. 5A). GA-OA nanoparticles, established through noncovalent interactions, not only showed high drug loading and excellent stability but also exhibited a synergistic antitumor effect. In addition, PTX was loaded into GA-OA nanoparticles to further improve the antitumor effect. Compared with free drugs, GA-OA-PTX nanoparticles could reduce liver damage caused by chemotherapy drugs via upregulating key antioxidant pathways, enhance pharmacological activity, and improve the antitumor efficacy.
Fig. 5.
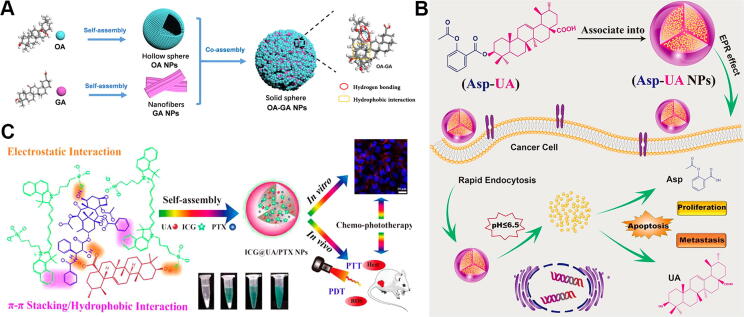
(A) Self-assembly between oleanolic acid (OA) and glycyrrhetinic acid (GA). Reproduced with permission from Ref. [59]. Copyright 2020, American Chemical Society; (B) Schematic illustration of formation of aspirin-ursolic acid (Asp-UA) nanoparticles and their inhibitory effect on tumor metastasis. Reproduced with permission from Ref. [86]. Copyright 2018, American Chemical Society; (C) Illustration of self-assembly between UA, paclitaxel (PTX), and indocyanine green (ICG) for synergistic antitumor effect. Reproduced with permission from Ref. [90]. Copyright 2017, American Chemical Society. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Ursolic acid (UA) is discovered in many natural plants, including Rosmarinus officinalis, Cornus officinalis, and Prunella vulgaris, which show a wide range of biological properties, such as antitumor, anti-inflammatory, and antidiabetic properties [83], [84]. Fan et al. [85] established a carrier-free nanodrug by the self-assembly of UA. UA nanoparticles could cause apoptosis in A549 cells by decreasing the expression of COX-2/VEGFR2/VEGFA and offer the potential for liver disease prevention. Additionally, the nanoparticles could also increase immunostimulatory activity to improve the activation of CD4 + T cells, which proved the potential of UA nanoparticles for immunotherapy. Next, UA interacted with aspirin (Asp), an anti-inflammatory drug, to assemble nanodrugs, which displayed outstanding physicochemical characteristics, such as a controlled release rate, suitable mean diameter, and good surface zeta potential [86]. Asp-UA nanoparticles inhibited tumor metastasis with low toxicity (Fig. 5B). Zhao et al. [87] developed multimodal nanoparticles with UA, indocyanine green (ICG), and lactobionic acid (LBA). UA-LBA-ICG nanoparticles not only exhibited anti-proliferative activities on HepG2 cells by increasing ROS, but also self-monitored nanodrugs targeting tumors by a by near-infrared (NIR) laser. Jiang et al. [88] developed a carrier-free dual-drug nanodelivery system with UA and DOX, which was further modified with a HER2 aptamer. These coassembled nanodrugs increased the tumor targeting of drugs and significantly inhibited tumor growth in HER2-overexpressing cancers. In addition to DOX, a fluorophore dye was also assembled into UA-DOX nanoparticles for cancer treatment and diagnosis [89]. PTX could also interact with UA to establish a “self-contained bioactive nanocarrier” system [60]. The hydrophobic interactions and hydrogen bonding played leading roles in the formation of UA-PTX nanoparticles. Reduction of hydrogen bonding sites and increased steric hindrance could influence drug loading capacity. Similarly, the fluorescent molecule ICG was also loaded in UA-PTX nanoparticles to establish a theranostic nanoplatform [90]. The developed nanodrugs showed efficient accumulation in the tumor sites of subcutaneous H22 cell xenograft mice through enhanced permeability and retention effects; this accumulation was then imaged by NIR fluorescence of ICG (Fig. 5C). Finally, UA-PTX-ICG nanoparticles synergistically suppressed tumor growth by photodynamic therapy, photothermal therapy and chemotherapy. Zhang et al. [91] constructed a “core–shell” carrier-free nanodrug with UA and epigallocatechin gallate, which was modified by EpCAM-aptamer for hepatocellular carcinoma treatment. The nanocomplex with low cytotoxicity and good biosafety could activate innate immunity and acquired immunity, leading to a synergistic therapeutic effect. Ou et al. [92] synthesized poly(ursolic acid) (PUA) by polycondensation of UA. PUA could self-assemble into nanoparticles with PTX. PUA-PTX nanoparticles enhanced tumor accumulation, prolonged blood circulation time, improved antitumor efficacy, and showed no obvious toxicity.
Other natural molecule-based nano self-assembly
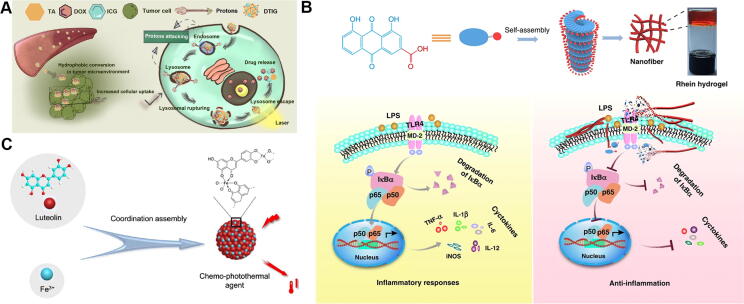
Wang et al. [93] prepared lollipop-like dual-drug-loaded nanoparticles (GPDs), which were developed based on the self-assembly of gossypol, polydopamine, and DOX via π–π stacking. Polydopamine filled the gaps between DOX and gossypol to form the super-compact long-circulating nanoparticles. Therefore, GPD might broaden the therapeutic window of drugs for various tumors, indicating great potential for the treatment of cancer. With the aid of electronic interactions, DOX could also self-assemble into a carrier-free nanotransformer (DTIG) [94] with tannic acid [95] and ICG. DTIG first transitioned to oversized hydrophobic particles in acidic lysosomes to escape from lysosomes and then rapidly returned to smaller hydrophilic particles to release drugs in the cytoplasm (Fig. 6A). This reversible hydrophilic-hydrophobic conversion and reassembly process showed enhanced antitumor efficacy and an obvious advantage for prognosis. Epigallocatechin gallate (EGCG), a condensed tannin isolated from green tea, has shown therapeutic effects against tumors [96]. The nanoplatform [97] combining EGCG and melittin, derived from the venom of Apis mellifera [98], induced ROS generation to offer a new strategy for cancer therapy. Emodin, a natural anthraquinone similar to RHE, could fabricate a kidney-specific nanocomplex with chitosan and metal ions by coordination-driven assembly, which manifested obvious attenuation of fibrotic progression in mice [99]. On the other hand, RHE could self-assemble into nanofiber network-based hydrogels without structural modifications or the use of any carriers [100]. The RHE hydrogels, with little cytotoxicity, accomplished dephosphorylation of IκBα, restrained nuclear translocation of p65 in the NF-κB cellular signaling pathway, and alleviated neuroinflammation with a long-lasting effect (Fig. 6B). Luteolin (Lut), a flavonoid separated from Lonicera japonica [101], possesses multiple pharmacological activities. Liu et al. [102] reported a coordination-driven assembly strategy to construct Lut-based nanoparticles in combination with ferric ions (Fe3+) (Fig. 6C). These Lut/Fe3+ nanoparticles not only notably enhanced Lut’s solubility but also broadened the absorption spectrum to the NIR region, which achieved efficient antitumor effects with chemotherapy and photothermal therapy. Quercetin, which exists in Sophora japonica, has a powerful antioxidant capacity [103]. Shang et al. [104] developed a quercetin-mediated self-assembly nanomedicine consisting of DOX and ferrous ions (Fe2+). This nanomedicine increased accumulation of DOX at tumor sites and exhibited effective synergistic antitumor effects on triple-negative breast cancer cells via the mitochondrial damage pathway. Quercetin with powerful antioxidant capacity could relieve the cardiotoxicity of DOX. All-trans retinoic acid (ATRA), an acyclic diterpenoid, has been clinically used for the treatment of acute promyelocytic leukemia [105]. Aggregation or deposition occurred after stirring ATRA in aqueous solution. Therefore, retinoic hydroxamic acid (RHA), a hydroxamic derivative of ATRA, was obtained, which contained a lipophilic hydrocarbon backbone and hydrophilic hydroxamic group. These functional groups could prompt RHA to form nanoparticles by self-assembly [106]. Thereafter, RHA nanoparticles showed potent antitumor effects against A-375 melanoma cells.
Fig. 6.
(A) Illustration of carrier-free nanoplatform DTIG for improving tumor drug delivery programmatically. Reproduced with permission from Ref. [94]. Copyright 2020, American Chemical Society; (B) Schematic depiction of neuroinflammatory prevention induced by rhein nano-assembly. Reproduced with permission from Ref. [100]. Copyright 2019, Springer Nature Limited; (C) Schematic illustration of self-assembly of luteolin and Fe3+ with proposed supramolecular structure. Reproduced with permission from Ref. [102]. Copyright 2020, Elsevier Publishing Group.
Extracellular vesicles from fresh plants
Similar to the exosomes secreted into the extracellular space by mammalian cells [107], fresh natural plant-derived extracellular vesicles (EVs) are membranous vesicles that contain a lipid bilayer as a skeleton and can load various proteins, miRNAs, polysaccharides, and other active substances [108], [109], [110]. EVs can serve as extracellular messengers to regulate cell–cell and interspecies communication. In recent years, EVs from fresh plants have been regarded as natural therapeutics against a variety of human diseases and nanoplatforms to efficiently deliver specific drugs [111], [112], [113].
Zingiber plant-derived EVs
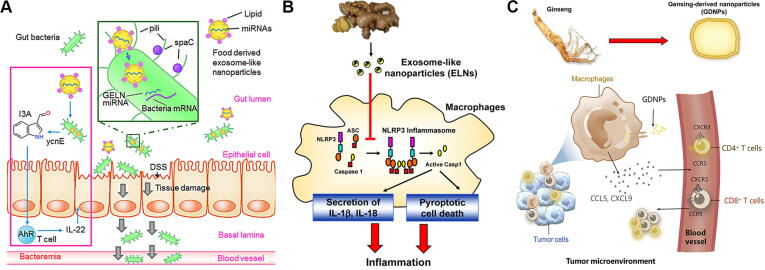
Ginger (Zingiber officinale) has been used to treat a diversity of health issues, such as colds, migraines, nausea, and gastrointestinal disorders [114]. Zhang et al. [115] isolated ginger-derived EVs (GEVs) and demonstrated their efficient colon targeting through oral administration. GEVs with a negative zeta potential and a size of 230 nm involved lipids, proteins, microRNAs, and some active compounds (such as 6-gingerol and 6-shogaol). GEVs could enhance anti-inflammatory cytokines (IL-10 and IL-22) and reduce proinflammatory cytokines (TNF-α, IL-6 and IL-1b), thus reducing acute colitis and preventing colitis-associated cancer. Next, GEVs contained microRNAs that targeted various genes in Lactobacillus rhamnosus [116] or showed anti-inflammatory effects in intestinal Caco-2 cells [117]. GEV-RNAs or indole-3-carboxaldehyde (I3A) were sufficient to induce the production of IL-22; thus, GEVs could ameliorate mouse colitis through IL-22-dependent mechanisms by reshaping the gut microbiota (Fig. 7A). Chen et al. [118] studied the inhibitory effects of GEVs against the activation of the nucleotide-binding domain and leucine-rich repeat-containing family pyrin domain-containing 3 (NLRP3) inflammasome in macrophages (Fig. 7B). The lipids in GEVs block the assembly of the NLRP3 inflammasome and restrain pathways downstream of inflammasome activation including caspase cleavage, secretion of IL-1β and IL-18, and pyroptotic cell death. Together, GEVs could be considered new potent agents to block NLRP3 inflammasome assembly and activation in disease settings. Zhuang’s group [119] found that oral GEVs could protect mice against alcohol-induced liver damage. GEVs participated in the activation of nuclear factor erythroid 2-related factor 2 (Nrf2), which resulted in the expression of some liver detoxifying/antioxidant genes and inhibited ROS production. The active ingredient in GEVs, shogaol was shown to play a role in the induction of Nrf2 in a TLR4/TRIF-based manner, which further contributed to liver protection. Moreover, the authors [120] proved that GEVs could be selectively recognized by the periodontal pathogen Porphyromonas gingivalis through interactions between phosphatidic acid (PA) in GEVs and hemin-binding protein 35 (HBP35) on the surface of P. gingivalis. In addition, the degree of unsaturation of PA played an important role in the GEV-mediated interaction with HBP35. The cargo molecules in GEVs interacted with multiple pathogenic factors in P. gingivalis simultaneously, and GEVs were deemed to prevent/treat chronic periodontitis.
Fig. 7.
(A) Ginger-derived extracellular vesicles regulating gut microbiota composition and host physiology, thereby enhancing gut barrier function to alleviate colitis. Reproduced with permission from Ref. [116]. Copyright 2018, Elsevier Inc.; (B) Ginger-derived extracellular vesicles inhibiting NLRP3 inflammasome activation. Reproduced with permission from Ref. [118]. Copyright 2019, American Chemical Society; (C) Ginseng-derived extracellular vesicles enhancing immune checkpoint antibody efficacy by reprogramming cold tumor microenvironment. Reproduced with permission from Ref. [140]. Copyright 2022, The American Society of Gene and Cell Therapy.
Next, GEVs were used as a delivery platform for DOX to treat colon cancer [121]. In addition to loading with DOX, GEVs were decorated with folic acid. The nanoparticles could be efficiently taken up by colon cancer cells, had an excellent pH-based DOX-release profile compared with DOX liposomes, and increased the inhibition of tumor growth compared with free DOX. Next, GEVs were used to deliver siRNA against CD98 (siRNA-CD98) to colon tissues [122]. SiRNA-CD98 reduced the expression of CD98, and this reduction played a crucial role in colitis and colitis-associated cancer. The siRNA-loaded GEVs were effectively targeted to the colon and reduced the expression of CD98, which offers great promise as an efficient siRNA-delivery vehicle. Therefore, GEVs are not just an attractive treatment strategy for human diseases but also potential lipids to deliver active molecules, siRNA, and even proteins to different types of cells [123]. Moreover, GEVs showed multiple benefits, such as low toxicity, tissue-specific targeting, and minimal hazardous effects on the environment.
Citrus plant-based EVs
Citrus plants, such as Citrus reticulata, Citrus aurantium, and Citrus grandis, are widely used as medicinal plants to regulate the flow of vital energy. Pocsfalvi et al. [124] isolated EVs from the fruit juice of C. aurantium, in which patellin-3-like, clathrin heavy chain, HSP70, 14–3-3 protein, G3PD and FBA6 were highly expressed. EVs with a size of 50–70 nm from Citrus limon [125], [126], [127] containing citrate, vitamin C, and miRNAs had a significant protective effect against oxidative stress on mesenchymal stromal cells. C. limon EVs could also inhibit cell proliferation through the TRAIL-mediated pathway in different tumor cell lines. Lei et al. [128] discovered an elegant three-way interaction between gut microbes, bile, and C. limon-derived EVs. Daily consumption of these EVs increased the percentage of probiotic Lactobacillus rhamnosus GG in the small intestine by enhancing bile resistance.
Xiao et al. [129] obtained EVs from grapefruit (Citrus paradisi), and they found that highly expressed miRNAs in EVs could potentially regulate the expression of inflammatory cytokines and cancer-related genes in vitro. Grapefruit EVs could be selectively taken up by intestinal macrophages [130] and then inhibit the production of IL-1β and TNF-α and increase the expression of heme oxygenase-1. Therefore, grapefruit EVs ameliorated dextran sulfate sodium-induced colitis in mice. Stanly et al. [131] demonstrated that grapefruit EVs specifically inhibited the proliferation of lung, skin and breast cancer cells. Grapefruit EVs reduced the expression of cyclins B1 and B2, intercellular cell adhesion molecule-1, cathepsins and cleaved PARP-1 to inhibit Akt and ERK signaling. Grapefruit EVs also served as nanovehicles to deliver chemotherapeutic agents, proteins, and short interfering RNAs to different types of cells [132], [133], [134], [135]. MiR-18a encapsulated in grapefruit EVs possessed an antimetastatic effect. The miR-18a-mediated macrophage IFNγ was necessary for the subsequent induction of IL-12, and then IL-12 activated natural killer T cells to inhibit colon tumour liver metastasis. Next, miR17 could also be carried by grapefruit EVs for rapid delivery to the mouse brain via intranasal administration. These EVs were selectively taken up by GL-26 tumor cells, and showed therapeutic efficacy in mouse brain tumors.
Vitis plant-derived EVs
Grape (Vitis vinifera) was used to relieve muscle pain and promote eruption in ancient China. EVs separated from grape contain proteins, lipids, and microRNAs [136]. Grape EVs can be taken up by intestinal macrophages and then induce the expression of the antioxidation gene heme oxygenase-1 and the anti-inflammatory cytokine IL-10 [137]. Thereafter, Wnt signaling is activated, which is critical for maintaining intestinal homeostasis. Grape EVs can also be taken up by intestinal stem cells by penetrating the intestinal mucus barrier [138]. These EVs caused significant induction of Lgr5hi intestinal stem cells via the Wnt/β-catenin pathway. Oral administration of grape EVs resulted in protection of mice from dextran sulfate sodium-mediated colitis through induction of intestinal stem cells.
Other plant-based EVs
Cao’s group [139], [140] isolated EVs from fresh roots of ginseng that contained lipids, nucleotides, proteins, and active molecules (such as ginsenoside Rg3). Ginseng EVs could promote polarization of M2 to M1-like macrophages through the Toll-like receptor-4/myeloid differentiation antigen 88 signaling pathway and then increase ROS production to lead to apoptosis of mouse melanoma cells. The authors also explored a combinatorial strategy with both ginseng EVs and PD-1 monoclonal antibody, which showed the ability to alter the cold tumor microenvironment to enhance immune checkpoint inhibitor antitumor treatment (Fig. 7C). Separated from Asparagus cochinchinensis, mushrooms, and broccoli, EVs inhibited the proliferation of hepatocellular carcinoma cells, alleviated liver damage in mice, and inhibited mouse colitis by activating dendritic cell AMP-activated protein kinase, respectively [141], [142], [143]. Perut et al. [144] isolated EVs from strawberry juice, which had a high content of anthocyanins, folic acid, flavonols, and vitamin C. Fragaria-derived EVs prevented oxidative stress in a dose-dependent manner in human mesenchymal stromal cells. Thus, Fragaria-derived EVs might be used in food with potential health-promoting activity. Tea flower-derived EVs contain large amounts of functional proteins, polyphenols, flavonoids, and lipids [145]. In vitro and in vivo experiments revealed that these EVs induced ROS amplification to trigger mitochondrial damage and arrest the cell cycle, resulting in anti-proliferation and anti-invasion activities. Next, cabbage- and cucumber-derived EVs could be used as alternative drug delivery vehicles to load lipophilic molecules to manifest biological activities [146], [147]. Furthermore, EVs can also be isolated from Dendropanax morbifera [148], coconut water [149], and sunflower [150]. D. morbifera-derived EVs were deemed to act as an anti-melanogenic agent to promote the development of natural cosmetics.
Charcoal nanocomponents from charred plants
Charred plants have a history of more than 2,000 years and are still widely used in clinical practice because of their special therapeutic effects. Charred plants are referred to as charcoal drugs in TCMs and possess extensive pharmacological effects, especially for hemostasis, and are formed by carbonization at similar high temperatures. In recent years, many scholars have separated charcoal nanocomponents (CNs) from charred plants [151], [152]. These CNs contain a carbon skeleton structure with a size of < 10 nm, which is similar to the structure and properties of carbon quantum dots [153] in nanomaterials.
CNs with hemostatic effects
The Cirsium plants Cirsium setosum and Cirsium japonicum are clinically used for cooling blood and hemostasis in traditional functions. Luo et al. [154] separated CNs from charred C. setosum, which had a nearly spherical shape with a diameter of 2.6 nm and luminescence and fluorescence emission properties. These CNs could activate the fibrinogen system and stimulate the extrinsic blood coagulation system. Next, CNs from charred C. japonicum with diameters of 2–11 nm showed remarkable inhibition of hemorrhage induced by mouse tail amputation or liver scratching [155]. Charred pollen of Typha angustifolia (CPTA) is a type of calcined drug that has been used to promote hemostasis for thousands of years. CPTA-CNs could increase fibrinogen to activate partial thromboplastin time [156]. Furthermore, transformation rules of flavonoids during the heating process of CPTA could be used to control the quality, and seven minutes was determined to be the optimal processing time with a heating temperature of 272 °C [157], [158]. Charred Juncus effusus (CJE) is the charcoal-processed product obtained by high-temperature heating of dried stem pith of J. effusus and has been used to treat hemorrhagic conditions. Cheng et al. [159] separated hydrophobic CJE-CNs from CJE. CJE-CNs not only showed outstanding hemostatic effects but also protected against hemorrhage-induced liver injury. Meanwhile, CJE-CNs reduced the serum levels of biochemical indicators of liver damage, such as total bilirubin, direct bilirubin, alanine amino transferase, aspartate aminotransferase, and alkaline phosphatase. Qu and Zhao’s group [160], [161], [162], [163], [164] studied a series of CNs for hemostasis from charred Phellodendron chinense, charred Schizonepeta tenuisfolia, charred spica of S. tenuisfolia, and charred Selaginella tamariscina. CNs derived from these charcoal plants presented similarities and differences in structural features, physicochemical properties and hemostatic bioactivity. All derived CNs showed remarkable hemostatic effects in tail amputation and liver scratch models, which increased fibrinogen and platelet contents and decreased prothrombin time by activating the fibrinogen system and stimulating the extrinsic blood coagulation system. Further studies [165], [166] showed that flavonoids were the main active ingredients in charcoal plant-derived CNs, and the key proteins related to hemostatic diseases might include SERPINC1, FVIII, FX, FII and FXII [167]. The hemostatic mechanism might reverse the imbalanced metabolites through alanine, aspartate and glutamate metabolism and the citrate cycle pathway [168]. These results provide new insights into the material basis of charred plants for hemostatic bioactivity.
CNs with anti-inflammatory and antioxidant activities
CNs exist in charred flowers of Lonicera japonica (CFLJ), ranging from 1.0 to 10.0 nm in diameter [169]. CFLJ-CNs alleviated lipopolysaccharide-induced inflammation by reducing the expression of tumor necrosis factor-α, IL-1β, and IL-6 and relieved the fever and hypothermia caused by inflammation. Similarly, charred mulberry silkworm cocoon-derived CNs exhibited remarkable anti-inflammatory activity, which was likely mediated by the inhibition of TNF-α and IL-6 [170]. Hu et al. [171] separated CNs from the charred root of Sophora flavescens (CRSF). CRSF-CNs showed anti-inflammatory and antioxidative effects, which could reduce the levels of NF-κB, TNF-α, IL-6, glutathione, superoxide dismutase, catalase, malondialdehyde, glutathione peroxidase, and inducible nitric oxide synthase to inhibit ethanol-induced acute gastric ulcers. Zhao et al. [172] found that CNs from charred roots of Paeonia lactiflora could increase superoxide dismutase levels and reduce malondialdehyde in a carbon tetrachloride-induced acute liver injury model. Therefore, the CNs could inhibit free radical-induced liver cell lipid peroxidation and scavenge oxygen free radicals to exhibit hepatoprotective effects.
CNs with anti-gout effect
Pueraria lobata is commonly used for dispelling wind and removing heat in TCM. CNs from the charred root of P. lobata (CRPL) were nearly spherical with diameters of approximately 5 nm [173]. CRPL-CNs reduced blood uric acid levels in rat models by inhibiting the activity of xanthine oxidase and reduced the degree of swelling and pathological damage in gouty arthritis. Most interestingly, CRPL-CNs were able to increase the water solubility of BA [174]. Finally, charred fruit of Citrus aurantium-derived CNs could also relieve gouty arthritis induced by monosodium urate crystals, and lowered serum uric acid by restraining xanthine oxidase activity [175].
CNs with other bioactivities
Jiaosanxian (JSX) is a “blind” medicine in TCM and is obtained by the carbonization of three plants, including the fruit of Crataegus pinnatifida, the fruit of Hordeum vulgare, and Massa medicata fermentata. JSX-CNs were proven to regulate blood sugar levels [176]. Moreover, CNs from charred fruit of C. pinnatifida also exerted hypoglycemic effects and thus might act as disaccharidase inhibitors to restrain the bioactivities of sucrase and maltase [177]. Glycyrrhiza uralensis-based CNs with a spherical structure and size of 2–10 nm were synthesized by an environmentally-friendly-one-step pyrolysis process and possessed an explicit anti-GU effect [178]. Zhao et al. [179] synthesized environmentally-friendly fluorescent Forsythia-derived CNs (F-CNs) in a one-pot method. F-CNs showed natural anti-wood rot fungus activity against two fungi (G. trabeum and C. versicolor), which indicated thst F-CNs might be used as environmentally protective wood preservatives. Charred leaves of Artemisia argyi-derived CNs (CLAA-CNs) obtained by a smoking simulation method exhibited the antibacterial ability of Gram-negative bacteria by inhibiting cell wall activity and changing the enzymatic secondary structure [180]. However, CLAA-CNs showed no significant antibacterial function against Gram-positive bacteria. On the other hand, CLAA-CNs could strengthen anti-frostbite ability by reducing the concentrations of IL-1βk and TNF-α and decreasing the levels of blood glucose caused by frostbite [181]. Qu’s group [182] found that CNs from charred root of Zingiber officinale had remarkable analgesic effects in thermal and chemical stimulus tests, which were possibly mediated by 5-hydroxytryptamine levels and opioid-like mechanisms. They [183] also found that CNs from cigarette mainstream smoke exhibited anti-anxiety and sedative effects, which might be associated with the reduction in glutamate and dopamine and the increase in norepinephrine in the mouse brain and serum. Finally, GL-based and curcumin-based CNs were also prepared by high-temperature heating of GL and curcumin, respectively [184], [185]. These small molecule-based CNs could inhibit the propagation of some viruses, such as porcine reproductive and respiratory syndrome virus, enterovirus 71, pseudorabies virus, and epidemic diarrhea virus.
Nanoaggregates from plants formulae
Plant formulae are one of the most widely used dosage forms of TCMs in the clinic. A variety of complex chemical and physical changes often occur in the process of decoction. Recently, nanoaggregate particles (NAs) with nanometer sizes and different dispersed states with wide curative effects have been discovered in plant formula decoctions, which usually contain proteins, polysaccharides, and micromolecules [186], [187], [188].
Bai-Hu decoction (BHD), a traditional Chinese decoction used for the treatment of fever, contains three medical plants and one mineral, including the root of Anemarrhena asphodeloides, the root of Glycyrrhiza uralensis, Japonica rice, and gypsum. NAs from BHD with a size of 97 nm were formed through cooperation of the chemical constituents of these four herbs [189], [190]. Liquiritin from licorice and polysaccharides from Japonica rice could enhance the solubility of mangiferin and neomangiferin from A. asphodeloides, while inorganic ions, including Ca2+, Mg2+, and Zn2+ in gypsum, acted as zeta potential modifiers. BHD-NAs exhibited remarkable antipyretic effects by inhibiting the expression of IL-1β, TRPV4, NF-κB, and TNF-α. Ke and Rao’s group [191] isolated NAs from Ma-Xing-Shi-Gan decoction (MXSGD) and detected their inhibition of cell proliferation. MXSGD, which contains four herbs (Ephedra sinica, the seed of Prunus armeniaca, the root of Glycyrrhiza uralensis, and gypsum), has been used for clearing lung and relieving asthma for thousands of years in TCM. The formation of MXSGD-NAs was dependent on the interaction of amphiphilic molecules (ephedrine and pseudoephedrine), which could further interact with other molecules through ionic or hydrophobic interactions. Next, Ge-Gen-Qin-Lian decoction (GGQLD)-derived NAs [192] exhibited stronger hypoglycemic and antioxidant activities, which could promote the permeation of active monomers (e.g., BA) into cells. Naoluo Xintong Decoction (NXD), consisting of six herbs and one animal medicine, is a clinically proven prescription used to treat ischemic stroke. NXD-derived NAs with typical characteristics of 200 nm size were mainly composed of polysaccharides, proteins, and saponins [193]. They could improve nerve function, have brain-protective effects, reduce oxidative stress, and inhibit cell apoptosis.
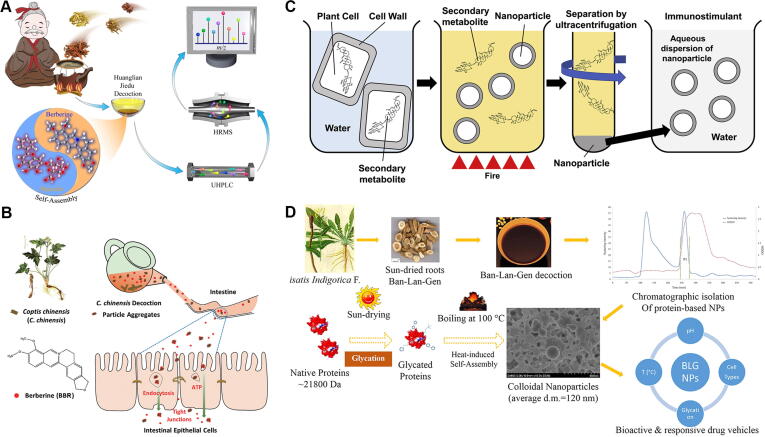
Huang-Lian-Jie-Du decoction (HLJDD) contains the root of C. chinensis, the bark of P. chinense, the root of S. baicalensis, and the fruit of Gardenia jasminoides in a proportion of 3:2:2:3 and is widely used for purging heat by removing toxins. HLJDD-NAs had 14 marker compounds, and BA and BBR were significantly higher in NAs due to self-assembly complexation (Fig. 8A) [194], [195], [196]. HLJDD-NAs showed neuroprotective effects in cobalt chloride-treated differentiated PC12 cells. Next, the NAs of licorice protein-BBR were obtained in the decocting process of C. chinensis and licorice [197], which could improve the antimicrobial activity of BBR. Moreover, NAs were also achieved by decocting the root of C. chinensis itself [198]. These NAs with sizes of 200–400 nm and negative charges were mainly composed of polysaccharides and small molecules, which promoted the absorption of BBR in the intestine (Fig. 8B). Meanwhile, the NAs could also dynamically regulate intestinal tissue permeability and the expression of tight junction proteins in intestinal epithelial cells by activating Peyer’s patch-associated immunity [199]. Similar to C. chinensis, NAs could also be obtained during the process of licorice decoction [200]. These NAs mainly contained licorice protein in contrast to C. chinensis. Licorice-derived NAs solubilized astragaloside IV by encapsulation and promoted the proliferation of normal hepatocytes. Iitsuka et al. [201] found that sugar-based NAs were also separated in boiling licorice water extracts (Fig. 8C). Phagocytosis of sugar-based NAs could lead to immunostimulant effects by increasing the expression of proteins and genes of inflammatory cytokines in macrophage cells.
Fig. 8.
(A) Self-assembled phytochemical complex with naturally-occurring modality in Huang-Lian-Jie-Du decoction. Reproduced with permission from Ref. [194]. Copyright 2021, Elsevier Publishing Group; (B) Illustration of promotion mechanisms of particle aggregates in Coptis chinensis decoction on absorption of berberine in small intestine. Reproduced with permission from Ref. [198]. Copyright 2020, The Royal Society of Chemistry; (C) Scheme of immune efficacy of herbal decoction based on nano-aggregate particles. Reproduced with permission from Ref. [201]. Copyright 2018, Elsevier Publishing Group; (D) Formation of nano-aggregate particles in boiling Isatis indigotica decoction. Reproduced with permission from Ref. [202]. Copyright 2017, Elsevier Publishing Group.
Zhou et al. [202] isolated protein-based NAs from a decoction of the root of Isatis indigotica. Two glycated proteins (BLGP1 and BLGP2) were identified in these NAs, and they could facilitate the growth of normal cells and inhibit that of carcinogenic cells (Fig. 8D). At the same time, six groups of NAs with sizes ranging from 57 to 300 nm were successfully isolated from the I. indigotica decoction [203]. All of the obtained NAs had a high contents of polysaccharides and high similarities in other compositions. However, four of these six NAs showed significant antiviral activity against influenza virus H1N1 and distinct cytotoxicity toward MDCK cells in vitro. Furthermore, protein-based NAs could also be obtained from decoction of the root of Pseudostellaria heterophylla [204], Phaseolus angularis [205], and the seed of P. armeniaca [206], which might be used to encapsulate hydrophobic drugs to improve their efficacy. Li et al. [207] obtained heat-stable decoctosomes from a decoction of the root of Rhodiola crenulata. These decoctosomes comprised lipids, chemical compounds, proteins, and sRNAs (e.g., HJT-sRNA-m7 and PGY-sRNA-6), which showed potent antifibrotic and anti-inflammatory effects. The herbal decoctosome provided an effective oral delivery route for nucleic acid therapy.
Conclusions and perspectives
Natural plants as well as TCMs are attracting increasing attention from researchers and clinical scientists due to their effective protection against illnesses with lower toxicity and higher synergistic potential. Despite the appearance of chemically synthesized drugs, natural molecules remain the basis and major resource for drug innovation. Currently, more than half of the drugs on the market are based on natural products and related molecules. Artemisinin, derived from Artemisia annua for the treatment of malaria, is a notable example. Furthermore, the China National Medical Products Administration approved the inclusion of Jinhua Qinggan granules, Lianhua Qingwen granules and capsules, and Xuebijing injection among new treatments for coronavirus disease 2019. Undoubtedly, natural plants or herbal medicines from TCMs are receiving unprecedented attention and are now reaching a new golden age.
Nanoassembly of pure small molecules isolated from natural plants into nanomedicine without any carriers may open an alternative avenue and offer a new perspective for medicinal plant research (Table 1). Natural pentacyclic triterpenoids are widely studied for self-assembly through the intermolecular forces of hydrogen bonds of carboxyl and hydroxyl groups, as well as the interactions of the triterpenoid skeleton. In addition, some natural alkaloids can be interacted with specific small molecules to assemble nanoplatform. BBR, the most studied, can self-assemble into nanoparticles with carboxyl-based natural molecules through electrostatic interactions and π − π interactions. EVs separated from fresh plants have also been used for therapeutic purposes for various diseases (Table 2). EVs can be obtained on a large scale from beneficial renewable plants and they may reduce cytotoxicity or any other negative side effect due to being evolved in plant cells. Furthermore, Plants-derived EVs have the potential as drug delivery nanocarriers which can be easily modified to target specific ligands. Charred plants are one kind of distinctive TCM that have been widely used to treat various bleeding syndromes in the clinic. CNs isolated from charred plants are widely studied and found to exhibit hemostatic, anti-inflammatory, antioxidant, and anti-gout effects, as well as other activities. In addition, decoction of plant formulae is another traditional clinical method of TCM. In particular, the combination spirit of TCM has been widely accepted in the clinic. Complex interactions always occur at the time of the water decoction process of plant formulae after dissolving active ingredients, and most significantly, they also generate NAs. The phenomenon of NAs in water decoction may be caused by direct noncovalent combination between the active ingredients or the primary metabolites, such as proteins, polysaccharides, and nucleic acids.
Table 1.
Nano-assembly of pure small molecules isolated from natural plants.
| Molecule 1 | Molecule 2 | Morphology | Interaction forces of self-assembly | Bioactivity | Research level | Refs. |
|---|---|---|---|---|---|---|
| Berberine | Baicalin | Nanosphere | Electrostatic and hydrophobic interactions | Antibacterial activity/ Treatment of diarrhea |
In vitro/ Zebrafish model/ Mice model |
[21], [22] |
| Berberine | Wogonoside | Nanofiber | Electrostatic and hydrophobic interactions | Antibacterial activity |
In vitro/ Zebrafish model |
[21] |
| Berberine | Cinnamic acid | Nanosphere | Hydrogen bond/ π-π stacking interaction |
Antibacterial activity |
In vitro/ Zebrafish model |
[24] |
| Berberine | 3,4,5-Methoxycinnamic acid | Nanosphere | Hydrogen bond/ π-π stacking interaction |
Antibacterial activity | In vitro | [26] |
| Berberine | Rhein | Nanosphere | π-π interaction/ Electrostatic interaction |
Antibacterial activity |
In vitro/ Zebrafish model |
[29] |
| Berberine | Aristolochic acid | Network nanofiber | Electrostatic interaction/ π-π interaction |
Neutralizing acute nephrotoxicity |
In vitro/ Zebrafish model/ Mice model |
[31] |
| Berberine alkylation | Rhamnolipid | Nanosphere | Electrostatic and hydrophobic interactions | Antibacterial activity |
In vitro/ Mice model |
[32] |
| Paclitaxel dimer | None | Nanovesicle | Hydrophobic interaction | Antitumor |
In vitro/ Mice model |
[37] |
| Paclitaxel | Indomethacin | Nanosphere | π-π stacking/ H-bonding/ Hydrophobic interaction |
Antitumor |
In vitro/ Mice model |
[40] |
| Cabazitaxel | Dasatinib | Nanosphere | π-π stacking/ hydrogen bonding/ Van der Waals interaction |
Antitumor |
In vitro/ Mice model |
[43] |
| Camptothecin/ Camptothecin derivative |
None | Helical nanoribbon/ Flat nanoribbon/ Cylindric nano-rod |
π-π interaction/ Hydrogen bond |
Antitumor |
In vitro/ Mice model |
[45], [46], [47], [48], [49] |
| Irinotecan/ Topotecan |
Curcuminoids | Nanosphere | Intermolecular non-covalent interactions | Antitumor |
In vitro/ Mice model |
[51] |
| 10-Hydroxycamptothecin | Doxorubicin | Nanosphere | π-π stacking/ Hydrophobic interactions | Antitumor drug resistance | In vitro | [52], [53] |
| Oleanolic acid | Paclitaxel | Nanosphere | Hydrogen bonding/ Hydrophobic interaction | Antitumor |
In vitro/ Mice model |
[59], [60] |
| Lupeol | None | Nanosphere with nanofiber interspersed | Hydrogen bonding/ Van der Waals force/ π-π stacking | Drug delivery vehicle |
In vitro/ Mice model |
[59], [63] |
| Betulinol | Ursolic acid | Nanosphere | Hydrogen bonding/ Van der Waals force/ π-π stacking | Drug delivery vehicle | In vitro | [59], [60] |
| Betulinic acid | Paclitaxel | Nanofiber | Hydrogen bond/ Hydrophobic interaction | Antitumor |
In vitro/ Mice model |
[64] |
| Ergosterol | Chlorin e6 | Clavate-shaped nanoparticle | π-π stacking/ Hydrophobic interaction |
Antitumor |
In vitro/ Mice model |
[66] |
| Dehydrotrametenolic acid | Paclitaxel | Nanosphere | Hydrogen bonding/ Van der Waals force/ π-π stacking | Antitumor |
In vitro/ Mice model |
[63] |
| Celastrol | Doxorubicin | Nanosphere | π-π stacking/ Electrostatic interaction | Antitumor drug resistance | In vitro | [69] |
| Ginsenoside Ro | Saikosaponin a | Nanosphere | – | – | Virtual prediction | [72], [73] |
| Glycyrrhizic acid | Paclitaxel/ Baicalein |
– | – | – | Virtual prediction/ Preparation and characterization |
[79], [80] |
| Glycyrrhetinic acid | None/ Oleanolic acid and/or Paclitaxel |
Nanosphere with fibrillar network | Hydrogen bonding/ Van der Waals force/ π-π stacking | Antitumor |
In vitro/ Mice model |
[59], [81], [82] |
| Ursolic acid | Aspirin | Nanosphere | Hydrogen bond/ Hydrophobic interaction | Tumor metastasis therapy |
In vitro/ Mice model |
[86] |
| Ursolic acid | Indocyanine green and lactobionic acid |
Nanosphere | π-π stacking/ Hydrophobic interaction/ Electrostatic interaction | Tumor theranostics |
In vitro/ Mice model |
[87] |
| Ursolic acid | Doxorubicin/ Paclitaxel |
Nanosphere | Electrostatic interaction/ π-π interaction/ Hydrophobic interaction |
Antitumor |
In vitro/ Mice model |
[88], [89], [90] |
| Doxorubicin | Indocyanine green and tannic acid | Nanosphere | π-π interaction/ Electronic interaction | Antitumor |
In vitro/ Mice model |
[94] |
| Rhein | None | Network nanofiber | π-π interaction/ Hydrogen bond/ Electrostatic interaction |
Treating neural inflammation |
In vitro | [100] |
-Not applicable.
Table 2.
Nanoplatforms existed in fresh plants, charred plants, and plants formulae.
| Nanoplatform | Source | Bioactivity | Research level | Refs. |
|---|---|---|---|---|
| Extracellular vesicles | Zingiber officinale | Anti-inflammatory/ Treating colitis-based cancer/ Liver protection/ Antibacterial activity |
In vitro/ Mice model |
[115], [116], [117], [118], [119], [120] |
| Citrus aurantium | – | Isolation and characterization | [124] | |
| Citrus limon | Antioxidative effect/ Antitumor |
In vitro/ Mice model |
[125], [126], [127], [128] | |
| Citrus paradisi | Anti-inflammatory/ Antitumor |
In vitro/ Mice model |
[129], [130], [131], [132], [133], [134], [135] | |
| Vitis vinifera | Treating colitis |
In vitro/ Mice model |
[136], [138] | |
| Panax ginseng | Antitumor |
In vitro/ Mice model |
[139], [140] | |
| Asparagus cochinchinensis | Antitumor |
In vitro/ Mice model |
[141] | |
| Shiitake Mushroom | Liver protection |
In vitro/ Mice model |
[142] | |
| Broccoli | Treating colitis |
In vitro/ Mice model |
[143] | |
| Dendropanax morbifera | Anti-melanogenic activity |
In vitro/ Mice model |
[148] | |
| Charcoal nano-components | charred Cirsium setosum/ charred Cirsium japonicum/ charred Typha angustifolia/ charred Juncus effusus |
Hemostatic effect | In vitro | [154], [155], [156], [159] |
| charred Phellodendron chinense/ charred Schizonepeta tenuisfolia/ charred Selaginella tamariscina |
Hemostatic effect | In vitro | [160], [161], [162], [163], [164] | |
| charred Lonicera japonica/ charred Sophora flavescens/ charred Paeonia lactiflora |
Antiinflammatory/ Antioxidant activity |
In vitro/ Mice model |
[169], [170], [171], [172] | |
| charred Pueraria lobata/ charred Citrus aurantium |
Anti-gout effect |
In vitro/ Mice model |
[173], [175] | |
| Jiaosanxian/ charred Crataegus pinnatifida |
Hpyerglycemic activity |
In vitro/ Mice model |
[176], [177] | |
| charred Artemisia argyi | Antibacterial activity | In vitro | [180] | |
| charred Zingiber officinale | Analgesic effect |
In vitro/ Mice model |
[182] | |
| Nano-aggregates | Bai-Hu decoction | Antipyretic effect |
In vitro/ Mice model |
[189], [190] |
| Ma-Xing-Shi-Gan decoction | Inhibiting cell proliferation | In vitro | [191] | |
| Ge-Gen-Qin-Lian Decoction | Antioxidant activity | In vitro | [192] | |
| Huang-Lian-Jie-Du decoction | Neuroprotective Effect | In vitro | [194], [195], [196] |
-Not applicable.
Despite the therapeutic benefits of natural plants-derived carrier-free nanoplatforms, there are still some downsides. Carrier-free self-assembly of pure natural molecules have shown the potentiality for therapeutic activities. However, there are still lots of problems to be solved for their successful clinical application. The nanoassembly of other natural compounds from plants is still rare. The determination of whether a natural product can be self-assembled or coassembled still relies on test screening. Moreover, modulating the proportions of multiple compounds within one nanoplatform to improve the synergetic efficiency remains challenging. Finally, how to increase the active targeting capability of nanodrugs through surface modification is still an issue to be considered. Although EVs separated from fresh plants were discovered earlier, research on plant EVs is not in-depth enough, especially in the biomedical field, and most of them are in the laboratory research stage. Studies on fresh plant-based EVs need to be expanded further and are mainly focused on ginger at present. There are regional and seasonal differences in plant herbs, and whether EVs should be obtained in different regions and seasons needs systematic evaluation. Especially when plant-based EVs are used as therapeutic agents or drug carriers in the biomedical field, the safety, effectiveness, stability and quality standards of EVs need to be controlled. Moreover, many plant-based EVs are derived from fresh juice, and how to preserve extracted EVs is the limiting factor to determine suitability for large-scale processing. There is still a lack of research in this area, and some reports even include contrary aspects. The internal reasons for this necessitate further research. As for charred plants-derived CNs, there has not been a unified standard conclusion redarding the detailed mechanism of the pharmacological action in clinic application. It is only speculated that it may be related to the different structures and properties of CNs formed by different medicinal plants in different preparation processes, and further principles remain to be studied and discussed. Related studies on CNs must integrate multidisciplinary research ideas and explore more suitable research technologies and methods for CNs to decode charred plants. As for NAs from decoction of plants formulae, knowledge of the formation mechanism of NAs is not sufficiently mature enough. In particular, the energy changes and structural characteristics of self-precipitation in the process of decoction still need to be further explored. In addition, determination of whether NAs generated by water decoction have special pharmacological effects and clinical application value are also necessary directions of future research. The synergistic effect of NAs are the future research highlights.
In summary, carrier-free nanoplatforms with self-assembly from natural plants may be a promising alternative treatment strategy for human diseases. With more research on plant-based carrier-free nano self-assembly, we believe that there will be clinical trials and even medical products in the near future, which can be safely and reliably produced on a large scale.
CRediT authorship contribution statement
Zhongrui Li: Investigation, Conceptualization, Writing – original draft. Xiao Xu: Visualization, Writing – review & editing. Yun Wang: Writing – review & editing. Lingyi Kong: Conceptualization, Validation, Writing – original draft. Chao Han: Investigation, Conceptualization, Visualization, Writing – review & editing, Validation, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was co-supported by National Natural Science Foundation of China (82104359), the Fundamental Research Funds for the Central Universities (2632021ZD24), the China Postdoctoral Science Foundation (2021M691647), and the Open Project of State Key Laboratory of Natural Medicines (SKLNMKF202207).
Biographies

Zhongrui Li received her PhD in Medicinal Chemistry in 2019 from Zhengzhou University and continued her 2-year postdoctoral research at Nanjing Medical University. Her research interests include the discovery, structure, and bioactivity of natural products. She has authored 20 scientific publications.

Xiao Xu is a Ph.D. candidate at School of Traditional Chinese Pharmacy, China Pharmaceutical University. Her research focused on natural products-based nanodrug delivery system.

Yun Wang is a M.S. candidate at School of Traditional Chinese Pharmacy, China Pharmaceutical University. Her research focused on carrier-free nanoparticles with self-assembly from natural plants for enhanced bioactivity.

Ling Yi Kong received his PhD in Medicinal Chemistry in 1992 from Shenyang College of Pharmacy, and continued his two-year postdoctoral research at China Pharmaceutical University, where he has been working ever since. He was promoted to full professor (1997), dean of the School of Traditional Chinese Pharmacy (1997–2012), and vice-president since 2013. He was a visiting scholar to Meijo University, Japan (1998–1999), and a visiting professor to Kyushu University, Japan (2009). His research interest is the discovery of bioactive products from traditional Chinese medicines and other natural sources with potential application as medicines. He has authored some 300 scientific publications and seven monographs.

Chao Han received his PhD in Medicinal Chemistry in 2016 from China Pharmaceutical University, where he has been working ever since. He was promoted to assistant professor in 2019. His research interest is the discovery of bioactive products from traditional Chinese medicines and other natural sources with potential application as medicines. He has authored 30+ papers, 4 patents, and 2 monographs. He is reviewer and guest editor as well as editorial board member for multiple journals.
Footnotes
Peer review under responsibility of Cairo University.
Contributor Information
Lingyi Kong, Email: cpu_lykong@126.com.
Chao Han, Email: hanchao@cpu.edu.cn.
References
- 1.Li H., Wei W., Xu H. Drug discovery is an eternal challenge for the biomedical sciences. Acta Materia Medica. 2022;1:1–3. [Google Scholar]
- 2.Tang J.L., Liu B.Y., Ma K.W. Traditional Chinese medicine. Lancet. 2008;372:1938–1940. doi: 10.1016/S0140-6736(08)61354-9. [DOI] [PubMed] [Google Scholar]
- 3.Atanasov A.G., Zotchev S.B., Dirsch V.M. the International Natural Product Sciences Taskforce, Supuran CT. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov. 2021;20:200–216. doi: 10.1038/s41573-020-00114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman D.J., Cragg G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J Nat Prod. 2020;83:770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- 5.Ren W., Li P., Ma Y., Sun Q., Pu Q., Dong L., et al. Research progress of traditional Chinese medicine against COVID-19. Biomed Pharmacother. 2021;137 doi: 10.1016/j.biopha.2021.111310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren J.L., Zhang A.H., Wang X.J. Traditional Chinese medicine for COVID-19 treatment. Pharmacol Res. 2020;155 doi: 10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun T., Zhang Y.S., Pang B., Hyun D.C., Yang M., Xia Y. Engineered Nanoparticles for Drug Delivery in Cancer Therapy. Angew Chem Int Ed. 2014;53:12320–12364. doi: 10.1002/anie.201403036. [DOI] [PubMed] [Google Scholar]
- 8.Deng S., Gigliobianco M.R., Censi R., Martino P.D. Polymeric Nanocapsules as Nanotechnological Alternative for Drug Delivery System: Current Status. Challenges and Opportunities Nanomaterials. 2020;10:847. doi: 10.3390/nano10050847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patra J.K., Das G., Fraceto L.F., Campos E.V.R., Rodriguez-Torres M.P., Acosta-Torres L.S., et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 2018;16:71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiao L., Han M., Gao S., Shao X., Wang X., Sun L., et al. Research progress on nanotechnology for delivery of active ingredients from traditional Chinese medicines. J Mater Chem B. 2020;8:6333–6351. doi: 10.1039/d0tb01260b. [DOI] [PubMed] [Google Scholar]
- 11.Fu S., Li G., Zang W., Zhou X., Shi K., Zhai Y. Pure drug nano-assemblies: A facile carrier-free nanoplatform for efficient cancer therapy. Acta Pharm Sin B. 2022;12:92–106. doi: 10.1016/j.apsb.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang L., Zhao S., Fang F., Xu T., Lan M., Zhang J. Advances and perspectives in carrier-free nanodrugs for cancer chemo-monotherapy and combination therapy. Biomaterials. 2021;268 doi: 10.1016/j.biomaterials.2020.120557. [DOI] [PubMed] [Google Scholar]
- 13.Jiang S., Fu Y., Zhang X., Yu T., Lu B., Du J. Research Progress of Carrier-Free Antitumor Nanoparticles Based on Phytochemicals. Front Bioeng Biotech. 2021;9 doi: 10.3389/fbioe.2021.799806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karaosmanoglu S., Zhou M., Shi B., Zhang X., Williams G.R., Chen X. Carrier-free nanodrugs for safe and effective cancer treatment. J Control Release. 2021;329:805–832. doi: 10.1016/j.jconrel.2020.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Yang M.Y., Zhao R.R., Fang Y.F., Jiang J.L., Yuan X.T., Shao J.W. Carrier-free nanodrug: A novel strategy of cancer diagnosis and synergistic therapy. Int J Pharmaceut. 2019;570 doi: 10.1016/j.ijpharm.2019.118663. [DOI] [PubMed] [Google Scholar]
- 16.Mei H., Cai S., Huang D., Gao H., Cao H., He B. Carrier-free nanodrugs with efficient drug delivery and release for cancer therapy: From intrinsic physicochemical properties to external modification. Bioact Mater. 2022;8:220–240. doi: 10.1016/j.bioactmat.2021.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adejoke HT, Louis H, Amusan OO, Apebende. A review on classes, extraction, purification and pharmaceutical importance of plants alkaloid. J Med Chem Sci 2019;2:130-139.
- 18.Bribi N. Pharmacological activity of alkaloids: a review. Asian J Bot. 2018;1:1–6. [Google Scholar]
- 19.Rauf A., Abu-Izneid T., Khalil A.A., Imran M., Shah Z.A., Emran T.B., et al. Berberine as a Potential Anticancer Agent: A Comprehensive Review. Molecules. 2021;26:7368. doi: 10.3390/molecules26237368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imenshahidi M., Hosseinzadeh H. Berberis Vulgaris and Berberine: An Update Review. Phytother Res. 2016;30:1745–1764. doi: 10.1002/ptr.5693. [DOI] [PubMed] [Google Scholar]
- 21.Li T., Wang P., Guo W., Huang X., Tian X., Wu G., et al. Natural Berberine-Based Chinese Herb Medicine Assembled Nanostructures with Modified Antibacterial Application. ACS Nano. 2019;13:6770–6781. doi: 10.1021/acsnano.9b01346. [DOI] [PubMed] [Google Scholar]
- 22.Li L., Cui H., Li T., Qi J., Chen H., Gao F., et al. Synergistic Effect of Berberine-Based Chinese Medicine Assembled Nanostructures on DiarrheaPredominant Irritable Bowel Syndrome In Vivo. Front Pharmacol. 2020;11:1210. doi: 10.3389/fphar.2020.01210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruwizhi N., Aderibigbe B.A. Cinnamic Acid Derivatives and Their Biological Efficacy. Int J Mol Sci. 2020;21:5712. doi: 10.3390/ijms21165712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang X., Wang P., Li T., Tian X., Guo W., Xu B., et al. Self-Assemblies Based on Traditional Medicine Berberine and Cinnamic Acid for Adhesion-Induced Inhibition Multidrug-Resistant Staphylococcus aureus. ACS Appl Mater Interfaces. 2020;12:227–237. doi: 10.1021/acsami.9b17722. [DOI] [PubMed] [Google Scholar]
- 25.Peperidou A., Pontiki E., Hadjipavlou-Litina D., Voulgari E., Avgoustakis K. Multifunctional Cinnamic Acid Derivatives. Molecules. 2017;22:1247. doi: 10.3390/molecules22081247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han N., Huang X., Tian X., Li T., Liu X., Li W., et al. Self-Assembled Nanoparticles of Natural Phytochemicals (Berberine and 3,4,5-Methoxycinnamic Acid) Originated from Traditional Chinese Medicine for Inhibiting Multidrug-Resistant Staphylococcus aureus. Curr Drug Deliv. 2021;18:914–921. doi: 10.2174/1567201817666201124121918. [DOI] [PubMed] [Google Scholar]
- 27.Wang H., Yang D., Li L., Yang S., Du G., Lu Y. Anti-inflammatory Effects and Mechanisms of Rhein, an Anthraquinone Compound, and Its Applications in Treating Arthritis: A Review. Nat Prod Bioprospect. 2020;10:445–452. doi: 10.1007/s13659-020-00272-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henamayee S., Banik K., Sailo B.L., Shabnam B., Harsha C., Srilakshmi S., et al. Therapeutic Emergence of Rhein as a Potential Anticancer Drug: A Review of Its Molecular Targets and Anticancer Properties. Molecules. 2020;25:2278. doi: 10.3390/molecules25102278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian X., Wang P., Li T., Huang X., Guo W., Yang Y., et al. Self-assembled natural phytochemicals for synergistically antibacterial application from the enlightenment of traditional Chinese medicine combination. Acta Pharm Sin B. 2020;10:1784–1795. doi: 10.1016/j.apsb.2019.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han J., Xian Z., Zhang Y., Liu J., Liang A. Systematic Overview of Aristolochic Acids: Nephrotoxicity, Carcinogenicity, and Underlying Mechanisms. Front Pharmacol. 2019;10:648. doi: 10.3389/fphar.2019.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang P., Guo W., Huang G., Zhen J., Li Y., Li T., et al. Berberine-Based Heterogeneous Linear Supramolecules Neutralized the Acute Nephrotoxicity of Aristolochic Acid by the Self-Assembly Strategy. ACS Appl Mater Interfaces. 2021;13:32729–32742. doi: 10.1021/acsami.1c06968. [DOI] [PubMed] [Google Scholar]
- 32.Shen Y., Zou Y., Chen X., Li P., Rao Y., Yang X., et al. Antibacterial self-assembled nanodrugs composed of berberine derivatives and rhamnolipids against Helicobacter pylori. J Control Release. 2020;328:575–586. doi: 10.1016/j.jconrel.2020.09.025. [DOI] [PubMed] [Google Scholar]
- 33.Song J., Lin C., Yang X., Xie Y., Hua P., Li H., et al. Mitochondrial targeting nanodrugs self-assembled from 9-O-octadecyl substituted berberine derivative for cancer treatment by inducing mitochondrial apoptosis pathways. J Control Release. 2019;294:27–42. doi: 10.1016/j.jconrel.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Cheng Y., Ji Y. Mitochondria-targeting nanomedicine self-assembled from GSH-responsive paclitaxel-ss-berberine conjugate for synergetic cancer treatment with enhanced cytotoxicity. J Control Release. 2020;318:38–49. doi: 10.1016/j.jconrel.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Zhu L., Chen L. Progress in research on paclitaxel and tumor immunotherapy. Cell Mol Biol Lett. 2019;24:40. doi: 10.1186/s11658-019-0164-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ezrahi S., Aserin A., Garti N. Basic principles of drug delivery systems – the case of paclitaxel. Adv Colloid Interfac. 2019;263:95–130. doi: 10.1016/j.cis.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Pei Q., Hu X., Zhou J., Liu S., Xie Z. Glutathione-responsive paclitaxel dimer nanovesicles with high drug content. Biomater Sci. 2017;5:1517–1521. doi: 10.1039/c7bm00052a. [DOI] [PubMed] [Google Scholar]
- 38.Dijkgraaf E.M., Moniek H., Bart T., Vogelpoel L.T.C., Renske G., Veena J., et al. Chemotherapy Alters Monocyte Differentiation to Favor Generation of Cancer-Supporting M2 Macrophages in the Tumor Microenvironment. Cancer Res. 2013;73:2480–2492. doi: 10.1158/0008-5472.CAN-12-3542. [DOI] [PubMed] [Google Scholar]
- 39.Totary-Jain H., Sionov R.V., Gallily R. Indomethacin Sensitizes Resistant Transformed Cells to Macrophage Cytotoxicity. Immunol Lett. 2016;176:1–7. doi: 10.1016/j.imlet.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang C., Long L., Xiong Y., Wang C., Peng C., Yuan Y., et al. Facile Engineering of Indomethacin-Induced Paclitaxel Nanocrystal Aggregates as Carrier-Free Nanomedicine with Improved Synergetic Antitumor Activity. ACS Appl Mater Interfaces. 2019;11:9872–9883. doi: 10.1021/acsami.8b22336. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y., Pan Y., Hu D., Peng J., Hao Y., Pan M., et al. Recent progress in nanoformulations of cabazitaxel. Biomed Mater. 2021;16 doi: 10.1088/1748-605X/abe396. [DOI] [PubMed] [Google Scholar]
- 42.Sun J., Russell C.C., Scarlett C.J., McCluskey A. Small molecule inhibitors in pancreatic cancer. RSC Med Chem. 2020;11:164–183. doi: 10.1039/c9md00447e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen X., Xie B., Huang L., Wan J., Wang Y., Shi X., et al. Quantitative self-assembly of pure drug cocktails as injectable nanomedicines for synergistic drug delivery and cancer therapy. Theranostics. 2021;11:5713–5727. doi: 10.7150/thno.55250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghanbari-Movahed M., Kaceli T., Mondal A., Farzaei M.H., Bishayee A. Recent Advances in Improved Anticancer Efficacies of Camptothecin Nano-Formulations: A Systematic Review. Biomedicines. 2021;9:480. doi: 10.3390/biomedicines9050480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qin S.Y., Peng M.Y., Rong L., Li B., Wang S.B., Cheng S.X., et al. Self-defensive nano-assemblies from camptothecin-based antitumor drugs. Regen Biomater. 2015;2:159–166. doi: 10.1093/rb/rbv011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou Z., Piao Y., Hao L., Wang G., Zhou Z., Shen Y. Acidity-responsive shell-sheddable camptothecin-based nanofibers for carrier-free cancer drug delivery. Nanoscale. 2019;11:15907–15916. doi: 10.1039/c9nr03872h. [DOI] [PubMed] [Google Scholar]
- 47.Liang X., Gao C., Cui L., Wang S., Wang J., Dai Z. Self-Assembly of an Amphiphilic Janus Camptothecin-Floxuridine Conjugate into Liposome-Like Nanocapsules for More Efficacious Combination Chemotherapy in Cancer. Adv Mater. 2017;29:1703135. doi: 10.1002/adma.201703135. [DOI] [PubMed] [Google Scholar]
- 48.Ma K., Shi J., Pei Y., Pei Z. A carrier-free supramolecular nanoprodrug based on lactosefunctionalized dimeric camptothecin via self-assembly in water for targeted and fluorescence imaging-guided chemo-photodynamic therapy. J Colloid Interf Sci. 2022;609:353–363. doi: 10.1016/j.jcis.2021.12.002. [DOI] [PubMed] [Google Scholar]
- 49.Hou C., Ma N., Shen Z., Chi G., Chao S., Pei Y., et al. A GSH-Responsive Nanoprodrug System Based on Self-Assembly of Lactose Modified Camptothecin for Targeted Drug Delivery and Combination Chemotherapy. Int J Nanomed. 2020;15:10417–10424. doi: 10.2147/IJN.S276470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Munekata P.E.S., Pateiro M., Zhang W., Dominguez R., Xing L., Fierro E.M., et al. Health benefits, extraction and development of functional foods with curcuminoids. J Funct Foods. 2021;79 [Google Scholar]
- 51.Xiao H., Guo Y., Liu H., Liu Y., Wang Y., Li C., et al. Structure-based design of charge-conversional drug self-delivery systems for better targeted cancer therapy. Biomaterials. 2020;232 doi: 10.1016/j.biomaterials.2019.119701. [DOI] [PubMed] [Google Scholar]
- 52.Chen F., Zhao Y., Pan Y., Xue X., Zhang X., Kumar A., et al. Synergistically Enhanced Therapeutic Effect of a Carrier-Free HCPT/DOX Nanodrug on Breast Cancer Cells through Improved Cellular Drug Accumulation. Mol Pharmaceutics. 2015;12:2237–2244. doi: 10.1021/mp500744m. [DOI] [PubMed] [Google Scholar]
- 53.Zhao Y., Chen F., Pan Y., Li Z., Xue X., Ma X., et al. Nanodrug Formed by Coassembly of Dual Anticancer Drugs to Inhibit Cancer Cell Drug Resistance. ACS Appl Mater Interfaces. 2015;7:19295–19305. doi: 10.1021/acsami.5b05347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biswas T., Dwivedi U.N. Plant triterpenoid saponins: biosynthesis, in vitro production, and pharmacological relevance. Protoplasma. 2019;256:1463–1486. doi: 10.1007/s00709-019-01411-0. [DOI] [PubMed] [Google Scholar]
- 55.Malík M., Velechovský J., Tlustos P. Natural pentacyclic triterpenoid acids potentially useful as biocompatible nanocarriers. Fitoterapia. 2021;151 doi: 10.1016/j.fitote.2021.104845. [DOI] [PubMed] [Google Scholar]
- 56.Bag B.G., Majumdar R. Self-assembly of Renewable Nano-sized Triterpenoids. Chem Rec. 2017;17:841–873. doi: 10.1002/tcr.201600123. [DOI] [PubMed] [Google Scholar]
- 57.Khwaza V., Oyedeji O.O., Aderibigbe B.A. Antiviral Activities of Oleanolic Acid and Its Analogues. Molecules. 2018;23:2300. doi: 10.3390/molecules23092300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gudoityte E., Arandarcikaite O., Mazeikiene I., Bendokas V., Liobikas J. Ursolic and Oleanolic Acids: Plant Metabolites with Neuroprotective Potential. Int J Mol Sci. 2021;22:4599. doi: 10.3390/ijms22094599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang J., Zhao H., Qiao W., Cheng J., Han Y., Yang X. Nanomedicine-Cum-Carrier by Co-Assembly of Natural Small Products for Synergistic Enhanced Antitumor with Tissues Protective Actions. ACS Appl Mater Interfaces. 2020;12:42537–42550. doi: 10.1021/acsami.0c12641. [DOI] [PubMed] [Google Scholar]
- 60.Wang J., Zhao H., Zhi K., Yang X. Exploration of the Natural Active Small-Molecule Drug-Loading Process and Highly Efficient Synergistic Antitumor Efficacy. ACS Appl Mater Interfaces. 2020;12:6827–6839. doi: 10.1021/acsami.9b18443. [DOI] [PubMed] [Google Scholar]
- 61.Bag B.G., Paul K. Vesicular and fibrillar Gels by Self-Assembly of nanosized oleanolic acid. Asian J Organic Chem. 2012;1:150–154. [Google Scholar]
- 62.Hordyjewska A., Ostapiuk A., Horecka A., Kurzepa J. Betulin and betulinic acid: triterpenoids derivatives with a powerful biological potential. Phytochem Rev. 2019;18:929–951. [Google Scholar]
- 63.Yang X., Ma C., Chen Z., Liu J., Liu F., Xie R., et al. Single small molecule-assembled nanoparticles mediate efficient oral drug delivery. Nano Res. 2019;12:2468–2476. doi: 10.1007/s12274-019-2470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang J., Qiao W., Zhao H., Yang X. Paclitaxel and betulonic acid synergistically enhance antitumor efficacy by forming co-assembled nanoparticles. Biochem Pharmacol. 2020;182 doi: 10.1016/j.bcp.2020.114232. [DOI] [PubMed] [Google Scholar]
- 65.O’Callaghan Y.O., McCarthy F.O., O’Brien N.M. Recent advances in Phytosterol Oxidation Products. Biochem Bioph Res Co. 2014;446:786–791. doi: 10.1016/j.bbrc.2014.01.148. [DOI] [PubMed] [Google Scholar]
- 66.Cheng J., Zhao H., Yao L., Li Y., Qi B., Wang J., et al. Simple and Multifunctional Natural Self-Assembled Sterols with Anticancer Activity-Mediated Supramolecular Photosensitizers for Enhanced Antitumor Photodynamic Therapy. ACS Appl Mater Interfaces. 2019;11:29498–29511. doi: 10.1021/acsami.9b07404. [DOI] [PubMed] [Google Scholar]
- 67.Yan R., Zhu H., Huang P., Yang P., Shen M., Pan Y., et al. Liquidambaric acid inhibits Wnt/β-catenin signaling and colon cancer via targeting TNF receptor-associated factor 2. Cell Rep. 2022;38 doi: 10.1016/j.celrep.2022.110319. [DOI] [PubMed] [Google Scholar]
- 68.Hou W., Liu B., Xu H. Celastrol: Progresses in structure-modifications, structure-activity relationships, pharmacology and toxicology. Eur J Med Chem. 2020;189 doi: 10.1016/j.ejmech.2020.112081. [DOI] [PubMed] [Google Scholar]
- 69.Xiao Y., Liu J., Guo M., Zhou H., Jin J., Liu J., et al. Synergistic combination chemotherapy using carrier-free celastrol and doxorubicin nanocrystals for overcoming drug resistance. Nanoscale. 2018;10:12639–12649. doi: 10.1039/c8nr02700e. [DOI] [PubMed] [Google Scholar]
- 70.Ratan Z.A., Haidere M.F., Hong Y.H., Park S.H., Lee J.O., Lee J., et al. Pharmacological potential of ginseng and its major component ginsenosides. J Ginseng Res. 2021;45:199–210. doi: 10.1016/j.jgr.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo M., Shao S., Wang D., Zhao D., Wang M. Recent progress in polysaccharides from Panax ginseng C. A Meyer Food Funct. 2021;12:494–518. doi: 10.1039/d0fo01896a. [DOI] [PubMed] [Google Scholar]
- 72.Dai X., Shi X., Wang Y., Qiao Y. Solubilization of saikosaponin a by ginsenoside Ro biosurfactant in aqueous solution: Mesoscopic simulation. J Colloid Interf Sci. 2012;384:73–80. doi: 10.1016/j.jcis.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 73.Dai X., Shi X., Ding H., Yin Q., Qiao Y. Dissipative Particle Dynamics Simulation of Ginsenoside Ro Vesicular Solubilization Systems. J Comput Theor Nanos. 2014;11:2046–2054. [Google Scholar]
- 74.Dai X., Shi X., Yin Q., Ding H., Qiao Y. Multiscale study on the interaction mechanism between ginsenoside biosurfactant and saikosaponin a. J Colloid Interf Sci. 2013;396:165–172. doi: 10.1016/j.jcis.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 75.Dai X., Ding H., Yin Q., Wan G., Shi X., Qiao Y. Dissipative particle dynamics study on self-assembled platycodin structures: The potential biocarriers for drug delivery. J Mol Graph Model. 2015;57:20–26. doi: 10.1016/j.jmgm.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 76.Selyutina O.Y., Polyakov N.E. Glycyrrhizic acid as a multifunctional drug carrier – From physicochemical properties to biomedical applications: A modern insight on the ancient drug. Int J Pharmaceut. 2019;559:271–279. doi: 10.1016/j.ijpharm.2019.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao X., Zhang H., Gao Y., Lin Y., Hu J. A Simple Injectable Moldable Hydrogel Assembled from Natural Glycyrrhizic Acid with Inherent Antibacterial Activity. ACS Appl Bio Mater. 2020;3:648–653. doi: 10.1021/acsabm.9b01007. [DOI] [PubMed] [Google Scholar]
- 78.Tucker I.M., Burley A., Petkova R.E., Hosking S.L., Penfold J., Thomas R.K., et al. Adsorption and self-assembly properties of the plant based biosurfactant. Glycyrrhizic acid J Colloid Interf Sci. 2021;598:444–454. doi: 10.1016/j.jcis.2021.03.101. [DOI] [PubMed] [Google Scholar]
- 79.Hussain M. Molecular Dynamics Simulations of Glycyrrhizic Acid Aggregates as Drug-Carriers for Paclitaxel. Curr Drug Deliv. 2019;16:618–627. doi: 10.2174/1567201816666190313155117. [DOI] [PubMed] [Google Scholar]
- 80.You G., Feng T., Zhang G., Chen M., Liu F., Sun L., et al. Preparation, optimization, characterization and in vitro release of baicalein-solubilizing glycyrrhizic acid nano-micelles. Int J Pharm. 2021;601 doi: 10.1016/j.ijpharm.2021.120546. [DOI] [PubMed] [Google Scholar]
- 81.Bag B.G., Majumdar R. Self-assembly of a renewable nano-sized triterpenoid 18β-glycyrrhetinic acid. RSC Adv. 2012;2:8623–8626. [Google Scholar]
- 82.Wu J., Lu J., Hu J., Gao Y., Ma Q., Ju Y. Self-assembly of sodium glycyrrhetinate into a hydrogel: characterisation and properties. RSC Adv. 2013;3:24906–24909. [Google Scholar]
- 83.Khwaza V., Oyedeji O.O., Aderibigbe B.A. Ursolic Acid-Based Derivatives as Potential Anti-Cancer Agents: An Update. Int J Mol Sci. 2020;21:5920. doi: 10.3390/ijms21165920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mlala S., Oyedeji A.O., Gondwe M., Oyedeji O.O. Ursolic Acid and Its Derivatives as Bioactive Agents. Molecules. 2019;24:2751. doi: 10.3390/molecules24152751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fan L., Zhang B., Xu A., Shen Z., Guo Y., Zhao R., et al. Carrier-Free, Pure Nanodrug Formed by the Self-Assembly of an Anticancer Drug for Cancer Immune Therapy. Mol Pharmaceutics. 2018;15:2466–2478. doi: 10.1021/acs.molpharmaceut.8b00444. [DOI] [PubMed] [Google Scholar]
- 86.Li C., Lin J., Wu P., Zhao R., Zou J., Zhou M., et al. Small Molecule Nanodrug Assembled of Dual-Anticancer Drug Conjugate for Synergetic Cancer Metastasis Therapy. Bioconjugate Chem. 2018;29:3495–3502. doi: 10.1021/acs.bioconjchem.8b00657. [DOI] [PubMed] [Google Scholar]
- 87.Zhao R., Zheng G., Fan L., Shen Z., Jiang K., Guo Y., et al. Carrier-free nanodrug by co-assembly of chemotherapeutic agent and photosensitizer for cancer imaging and chemo-photo combination therapy. Acta Biomater. 2018;70:197–210. doi: 10.1016/j.actbio.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 88.Jiang K., Han L., Guo Y., Zheng G., Fan L., Shen Z., et al. A carrier-free dual-drug nanodelivery system functionalized with aptamer specific targeting HER2-overexpressing cancer cells. J Mater Chem B. 2017;5:9121–9129. doi: 10.1039/c7tb02562a. [DOI] [PubMed] [Google Scholar]
- 89.Bag B.G., Das S., Hasan S.N., Barai A.C. Nanoarchitectures by hierarchical self-assembly of ursolic acid: entrapment and release of fluorophores including anticancer drug doxorubicin. RSC Adv. 2017;7:18136–18143. [Google Scholar]
- 90.Guo Y., Jiang K., Shen Z., Zheng G., Fan L., Zhao R., et al. A Small Molecule Nanodrug by Self-Assembly of Dual Anticancer Drugs and Photosensitizer for Synergistic near-Infrared Cancer Theranostics. ACS Appl Mater Interfaces. 2017;9:43508–43519. doi: 10.1021/acsami.7b14755. [DOI] [PubMed] [Google Scholar]
- 91.Zhang B., Jiang J., Wu P., Zou J., Le J., Lin J., et al. A smart dual-drug nanosystem based on coassembly of plant and food-derived natural products for synergistic HCC immunotherapy. Acta Pharm Sin B. 2021;11:246–257. doi: 10.1016/j.apsb.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ou K., Xu X., Guan S., Zhang R., Zhang X., Kang Y., et al. Nanodrug Carrier Based on Poly(Ursolic Acid) with Self-Anticancer Activity against Colorectal Cancer. Adv Funct Mater. 2020;30:1907857. [Google Scholar]
- 93.Wang Y., Wu Y., Li K., Shen S., Liu Z., Wu D. Ultralong Circulating Lollipop-Like Nanoparticles Assembled with Gossypol, Doxorubicin, and Polydopamine via π–π Stacking for Synergistic Tumor Therapy. Adv Funct Mater. 2019;29:1805582. [Google Scholar]
- 94.Xiong H., Wang Z., Wang C., Yao J. Transforming Complexity to Simplicity: Protein-Like Nanotransformer for Improving Tumor Drug Delivery Programmatically. Nano Lett. 2020;20:1781–1790. doi: 10.1021/acs.nanolett.9b05008. [DOI] [PubMed] [Google Scholar]
- 95.Kaczmarek B. Tannic Acid with Antiviral and Antibacterial Activity as A Promising Component of Biomaterials—A Minireview. Materials. 2020;13:3224. doi: 10.3390/ma13143224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aggarwal V., Tuli H.S., Tania M., Srivastava S., Ritzer E.E., Pandey A., et al. Molecular mechanisms of action of epigallocatechin gallate in cancer: Recent trends and advancement. Semin Cancer Biol. 2022;80:256–275. doi: 10.1016/j.semcancer.2020.05.011. [DOI] [PubMed] [Google Scholar]
- 97.Qiao H., Fang D., Zhang L., Gu X., Lu Y., Sun M., et al. Nanostructured Peptidotoxins as Natural Pro-Oxidants Induced Cancer Cell Death via Amplification of Oxidative Stress. ACS Appl Mater Interfaces. 2018;10:4569–4581. doi: 10.1021/acsami.7b18809. [DOI] [PubMed] [Google Scholar]
- 98.Memariani M., Memariani M. Anti-fungal properties and mechanisms of melittin. Appl Microbiol Biotechnol. 2020;104:6513–6526. doi: 10.1007/s00253-020-10701-0. [DOI] [PubMed] [Google Scholar]
- 99.Qiao H., Sun M., Su Z., Xie Y., Chen M., Zong L., et al. Kidney-specific drug delivery system for renal fibrosis based on coordination-driven assembly of catechol-derived chitosan. Biomaterials. 2014;35:7157–7171. doi: 10.1016/j.biomaterials.2014.04.106. [DOI] [PubMed] [Google Scholar]
- 100.Zheng J., Fan R., Wu H., Yao H., Yan Y., Liu J., et al. Directed self-assembly of herbal small molecules into sustained release hydrogels for treating neural inflammation. Nat Commun. 2019;10:1604. doi: 10.1038/s41467-019-09601-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou Y., Ding B.Z., Lin Y.P., Wang H.B. MiR-34a, as a suppressor, enhance the susceptibility of gastric cancer cell to luteolin by directly targeting HK1. Gene. 2018;644:56–65. doi: 10.1016/j.gene.2017.10.046. [DOI] [PubMed] [Google Scholar]
- 102.Liu Y., Zhao L., Shen G., Chang R., Zhang Y., Yan X. Coordination self-assembly of natural flavonoids into robust nanoparticles for enhanced in vitro chemo and photothermal cancer therapy. Colloid Surface A. 2020;598 [Google Scholar]
- 103.Patel R.V., Mistry B.M., Shinde S.K., Syed R., Singh V., Shin H.S. Therapeutic potential of quercetin as a cardiovascular agent. Eur J Med Chem. 2018;155:889–904. doi: 10.1016/j.ejmech.2018.06.053. [DOI] [PubMed] [Google Scholar]
- 104.Shang L., Yang T., Yang C., Li Z., Kong L., Zhang Z. Metal ions-mediated self-assembly of nanomedicine for combinational therapy against triple-negative breast cancer. Chem Eng J. 2021;425 [Google Scholar]
- 105.Ni X., Hu G., Cai X. The success and the challenge of all-trans retinoic acid in the treatment of cancer. Crit Rev Food Sci Nutr. 2019;59:S71–S80. doi: 10.1080/10408398.2018.1509201. [DOI] [PubMed] [Google Scholar]
- 106.Liao H., Zhao S., Wang H., Liu Y., Zhang Y., Sun G. Self-Assembly Of Retinoid Nanoparticles For Melanoma Therapy. Int J Nanomed. 2019;14:7963–7973. doi: 10.2147/IJN.S196974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li P., Kaslan M., Lee S.H., Yao J., Gao Z. Progress in Exosome Isolation Techniques Theranostics. 2017;7:789–804. doi: 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cui Y., Gao J., He Y., Jiang L. Plant extracellular vesicles. Protoplasma. 2020;257:3–12. doi: 10.1007/s00709-019-01435-6. [DOI] [PubMed] [Google Scholar]
- 109.Rome S. Biological properties of plant-derived extracellular vesicles. Food Funct. 2019;10:529–538. doi: 10.1039/c8fo02295j. [DOI] [PubMed] [Google Scholar]
- 110.Kim J., Li S., Zhang S., Wang J. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian J Pharm Sci. 2022;17:53–69. doi: 10.1016/j.ajps.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dad H.A., Gu T.W., Zhu A.Q., Huang L.Q., Peng L.H. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol Ther. 2021;29:13–31. doi: 10.1016/j.ymthe.2020.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Karamanidou T., Tsouknidas A. Plant-Derived Extracellular Vesicles as Therapeutic Nanocarriers. Int J Mol Sci. 2022;23:191. doi: 10.3390/ijms23010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang Y., Wang J., Ma J., Zhou Y., Lu R. Focusing on Future Applications and Current Challenges of Plant Derived Extracellular Vesicles. Pharmaceuticals. 2022;15:708. doi: 10.3390/ph15060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mao Q.Q., Xu X.Y., Cao S.Y., Gan R.Y., Corke H., Beta T., et al. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe) Foods. 2019;8:185. doi: 10.3390/foods8060185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang M., Viennois E., Prasad M., Zhang Y., Wang L., Zhang Z., et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016;101:321–340. doi: 10.1016/j.biomaterials.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Teng Y., Ren Y., Sayed M., Hu X., Lei C., Kumar A., et al. Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host Microbe. 2018;24:637–652. doi: 10.1016/j.chom.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yin L., Yan L., Yu Q., Wang J., Liu C., Wang L., et al. Characterization of the MicroRNA Profile of Ginger Exosome-like Nanoparticles and Their Anti-Inflammatory Effects in Intestinal Caco-2 Cells. J Agric Food Chem. 2022;70:4725–4734. doi: 10.1021/acs.jafc.1c07306. [DOI] [PubMed] [Google Scholar]
- 118.Chen X., Zhou Y., Yu J. Exosome-like Nanoparticles from Ginger Rhizomes Inhibited NLRP3 Inflammasome Activation. Mol Pharmaceutics. 2019;16:2690–2699. doi: 10.1021/acs.molpharmaceut.9b00246. [DOI] [PubMed] [Google Scholar]
- 119.Zhuang X., Deng Z.B., Mu J., Zhang L., Yan J., Miller D., et al. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J Extracell Vesicles. 2015;4:28713. doi: 10.3402/jev.v4.28713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sundaram K., Miller D.P., Kumar A., Teng Y., Sayed M., Mu J., et al. Plant-Derived Exosomal Nanoparticles Inhibit Pathogenicity of Porphyromonas gingivalis. iScience. 2019;21:308–327. doi: 10.1016/j.isci.2019.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang M., Xiao B., Wang H., Han M.K., Zhang Z., Viennois E., et al. Edible Ginger-derived Nano-lipids Loaded with Doxorubicin as a Novel Drug-delivery Approach for Colon Cancer Therapy. Mol Ther. 2016;24:1783–1796. doi: 10.1038/mt.2016.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang M., Wang X., Han M.K., Collins J.F., Merlin D. Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis. Nanomedicine. 2017;12:1927–1943. doi: 10.2217/nnm-2017-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang M., Collins J.F., Merlin D. Do ginger-derived nanoparticles represent an attractive treatment strategy for inflammatory bowel diseases? Nanomedicine. 2016;11:3035–3037. doi: 10.2217/nnm-2016-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pocsfalvi G., Turiákb L., Ambrosone A., Gaudio P.D., Puska G., Fiume I., et al. Protein biocargo of citrus fruit-derived vesicles reveals heterogeneous transport and extracellular vesicle populations. J Plant Physiol. 2018;229:111–121. doi: 10.1016/j.jplph.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 125.Baldini N., Torreggiani E., Roncuzzi L., Perut F., Zini N., Avnet S. Exosome-like Nanovesicles Isolated from Citrus limon L. Exert Anti-oxidative Effect Curr Pharm Biotechno. 2018;19:877–885. doi: 10.2174/1389201019666181017115755. [DOI] [PubMed] [Google Scholar]
- 126.Raimondo S., Naselli F., Fontana S., Monteleone F., Dico A.L., Saieva L., et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget. 2015;6:19514–19527. doi: 10.18632/oncotarget.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yang M., Liu X., Luo Q., Xu L., Chen F. An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J Nanobiotechnol. 2020;18:100. doi: 10.1186/s12951-020-00656-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lei C, Teng Y, He L, Sayed M, Mu J, Xu F, et al. Lemon exosome-like nanoparticles enhance stress survival of gut bacteria by RNase P-mediated specific tRNA decay. iScience 2021;24:102511. [DOI] [PMC free article] [PubMed]
- 129.Xiao J., Feng S., Wang X., Long K., Luo Y., Wang Y., et al. Identification of exosome-like nanoparticle-derived microRNAs from 11 edible fruits and vegetables. PeerJ. 2018;6 doi: 10.7717/peerj.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang B., Zhuang X., Deng Z.B., Jiang H., Mu J., Wang Q., et al. Targeted Drug Delivery to Intestinal Macrophages by Bioactive Nanovesicles Released from Grapefruit. Mol Ther. 2014;22:522–534. doi: 10.1038/mt.2013.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stanly C., Alfieri M., Ambrosone A., Leone A., Fiume I., Pocsfalvi G. Grapefruit-Derived Micro and Nanovesicles Show Distinct Metabolome Profiles and Anticancer Activities in the A375 Human Melanoma Cell Line. Cells. 2020;9:2722. doi: 10.3390/cells9122722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang Q., Ren Y., Mu J., Egilmez N.K., Zhuang X., Deng Z., et al. Grapefruit-Derived Nanovectors Use an Activated Leukocyte Trafficking Pathway to Deliver Therapeutic Agents to Inflammatory Tumor Sites. Cancer Res. 2015;75:2520–2529. doi: 10.1158/0008-5472.CAN-14-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang Q., Zhuang X., Mu J., Deng Z.B., Jiang H., Zhang L., et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat Commun. 2013;4:1867. doi: 10.1038/ncomms2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Teng Y., Mu J., Hu X., Samykutty A., Zhuang X., Deng Z., et al. Grapefruit-derived nanovectors deliver miR-18a for treatment of liver metastasis of colon cancer by induction of M1 macrophages. Oncotarget. 2016;7:25683–25697. doi: 10.18632/oncotarget.8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhuang X., Teng Y., Samykutty A., Mu J., Deng Z., Zhang L., et al. Grapefruit-derived Nanovectors Delivering Therapeutic miR17 Through an Intranasal Route Inhibit Brain Tumor Progression. Mol Ther. 2016;24:96–105. doi: 10.1038/mt.2015.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pérez-Bermúdez P., Blesa J., Soriano J.M., Marcilla A. Extracellular vesicles in food: Experimental evidence of their secretion in grape fruits. Eur J Pharm Sci. 2017;98:40–50. doi: 10.1016/j.ejps.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 137.Mu J., Zhuang X., Wang Q., Jiang H., Deng Z.B., Wang B., et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol Nutr Food Res. 2014;58:1561–1573. doi: 10.1002/mnfr.201300729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ju S., Mu J., Dokland T., Zhuang X., Wang Q., Jiang H., et al. Grape Exosome-like Nanoparticles Induce Intestinal Stem Cells and Protect Mice From DSS-Induced Colitis. Mol Ther. 2013;21:1345–1357. doi: 10.1038/mt.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cao M., Yan H., Han X., Weng L., Wei Q., Sun X., et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J ImmunoTher Cancer. 2019;7:326. doi: 10.1186/s40425-019-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Han X., Wei Q., Lv Y., Weng L., Huang H., Wei Q., et al. Ginseng-derived nanoparticles potentiate immune checkpoint antibody efficacy by reprogramming the cold tumor microenvironment. Mol Ther. 2022;30:327–340. doi: 10.1016/j.ymthe.2021.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang L., He F., Gao L., Cong M., Sun J., Xu J., et al. Engineering Exosome-Like Nanovesicles Derived from Asparagus cochinchinensis Can Inhibit the Proliferation of Hepatocellular Carcinoma Cells with Better Safety Profile. Int J Nanomed. 2021;16:1575–1586. doi: 10.2147/IJN.S293067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu B., Lu Y., Chen X., Muthuraj P.G., Li X., Pattabiraman M., et al. Protective Role of Shiitake Mushroom-Derived Exosome-Like Nanoparticles in D-Galactosamine and Lipopolysaccharide-Induced Acute Liver Injury in Mice. Nutrients. 2020;12:477. doi: 10.3390/nu12020477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Deng Z., Rong Y., Teng Y., Mu J., Zhuang X., Tseng M., et al. Broccoli-Derived Nanoparticle Inhibits Mouse Colitis by Activating Dendritic Cell AMP-Activated Protein Kinase. Mol Ther. 2017;25:1641–1654. doi: 10.1016/j.ymthe.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Perut F., Roncuzzi L., Avnet S., Massa A., Zini N., Sabbadini S., et al. Strawberry-Derived Exosome-Like Nanoparticles Prevent Oxidative Stress in Human Mesenchymal Stromal Cells. Biomolecules. 2021;11:87. doi: 10.3390/biom11010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen Q., Li Q., Liang Y., Zu M., Chen N., Canup B.S.B., et al. Natural exosome-like nanovesicles from edible tea flowers suppress metastatic breast cancer via ROS generation and microbiota modulation. Acta Pharm Sin B. 2022;12:907–923. doi: 10.1016/j.apsb.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Abraham A.M., Wiemann S., Ambreen G., Zhou J., Engelhardt K., Brüßler J., et al. Cucumber-Derived Exosome-like Vesicles and PlantCrystals for Improved Dermal Drug Delivery. Pharmaceutics. 2022;14:476. doi: 10.3390/pharmaceutics14030476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.You J.Y., Kang S.J., Rhee W.J. Isolation of cabbage exosome-like nanovesicles and investigation of their biological activities in human cells. Bioact Mater. 2021;6:4321–4332. doi: 10.1016/j.bioactmat.2021.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee R., Ko H.J., Kim K., Sohn Y., Min S.Y., Kim J.A., et al. Anti-melanogenic effects of extracellular vesicles derived from plant leaves and stems in mouse melanoma cells and human healthy skin. J Extracell Vesicles. 2019;9:1703480. doi: 10.1080/20013078.2019.1703480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhao Z., Yu S., Li M., Gui X., Li P. Isolation of Exosome-Like Nanoparticles and Analysis of MicroRNAs Derived from Coconut Water Based on Small RNA High-Throughput Sequencing. J Agric Food Chem. 2018;66:2749–2757. doi: 10.1021/acs.jafc.7b05614. [DOI] [PubMed] [Google Scholar]
- 150.Regente M., Corti-Monzón G., Maldonado A.M., Pinedo M., Jorrín J., Canal L. Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS Lett. 2009;583:3363–3366. doi: 10.1016/j.febslet.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 151.Liu G., Zheng H., Jiang Z., Zhao J., Wang Z., Pan B., et al. Formation and Physicochemical Characteristics of Nano Biochar: Insight into Chemical and Colloidal Stability. Environ Sci Technol. 2018;52:10369–10379. doi: 10.1021/acs.est.8b01481. [DOI] [PubMed] [Google Scholar]
- 152.Li D., Xu K., Zhao W., Liu M., Feng R., Li D., et al. Chinese Medicinal Herb-Derived Carbon Dots for Common Diseases: Efficacies and Potential Mechanisms. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.815479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mishra V., Patil A., Thakur S., Kesharwani P. Carbon dots: emerging theranostic nanoarchitectures. Drug Discov Today. 2018;23:1219–1232. doi: 10.1016/j.drudis.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 154.Luo J., Zhang M., Cheng J., Wu S., Xiong W., Kong H., et al. Hemostatic effect of novel carbon dots derived from Cirsium setosum Carbonisata. RSC Adv. 2018;8:37707–37714. doi: 10.1039/c8ra06340k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang Y., Kong H., Liu X., Luo J., Xiong W., Zhu Y., et al. Novel Carbon Dots Derived from Cirsii Japonici Herba Carbonisata and Their Haemostatic Effect. J Biomed Nanotechnol. 2018;14:1635–1644. doi: 10.1166/jbn.2018.2613. [DOI] [PubMed] [Google Scholar]
- 156.Yan X., Zhao Y., Luo J., Xiong W., Liu X., Cheng J., et al. Hemostatic bioactivity of novel Pollen Typhae Carbonisata-derived carbon quantum dots. J Nanobiotechnol. 2017;15:60. doi: 10.1186/s12951-017-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gao M., Bao B., Cao Y., Shan M., Cheng F., Jiang M., et al. Chemical Property Changes and Thermal Analysis during the Carbonizing Process of the Pollen Grains of Typha. Molecules. 2019;24:128. doi: 10.3390/molecules24010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gao M., Lan J., Bao B., Yao W., Cao Y., Shan M., et al. Effects of carbonized process on quality control, chemical composition and pharmacology of Typhae Pollen: A review. J Ethnopharmacol. 2021;270 doi: 10.1016/j.jep.2020.113774. [DOI] [PubMed] [Google Scholar]
- 159.Cheng J., Zhang M., Sun Z., Lu F., Xiong W., Luo J., et al. Hemostatic and hepatoprotective bioactivity of Junci Medulla Carbonisata-derived Carbon Dots. Nanomedicine. 2019;14:431–446. doi: 10.2217/nnm-2018-0285. [DOI] [PubMed] [Google Scholar]
- 160.Liu X., Wang Y., Yan X., Zhang M., Zhang Y., Cheng J., et al. Novel Phellodendri Cortex (Huang Bo)-derived carbon dots and their hemostatic effect. Nanomedicine. 2018;13:391–405. doi: 10.2217/nnm-2017-0297. [DOI] [PubMed] [Google Scholar]
- 161.Sun Z., Lu F., Cheng J., Zhang M., Zhang Y., Xiong W., et al. Haemostatic bioactivity of novel Schizonepetae Spica Carbonisata-derived carbon dots via platelet counts elevation. Artif Cell Nanomed B. 2018;46:S308–S317. doi: 10.1080/21691401.2018.1492419. [DOI] [PubMed] [Google Scholar]
- 162.Zhang M., Zhao Y., Cheng J., Liu X., Wang Y., Yan X., et al. Novel carbon dots derived from Schizonepetae Herba Carbonisata and investigation of their haemostatic efficacy. Artif Cell Nanomed B. 2018;46:1562–1571. doi: 10.1080/21691401.2017.1379015. [DOI] [PubMed] [Google Scholar]
- 163.Zhao Y., Zhang Y., Kong H., Zhang M., Cheng J., Luo J., et al. Haemostatic Nanoparticles-Derived Bioactivity of from Selaginella tamariscina Carbonisata. Molecules. 2020;25:446. doi: 10.3390/molecules25030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang M., Cheng J., Hu J., Luo J., Zhang Y., Lu F., et al. Green Phellodendri Chinensis Cortex-based carbon dots for ameliorating imiquimod-induced psoriasis-like inflammation in mice. J Nanobiotechnol. 2021;19:105. doi: 10.1186/s12951-021-00847-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chen Y., Yu H., Wu H., Pan Y., Wang K., Liu L., et al. Tracing novel hemostatic compounds from heating products of total flavonoids in Flos Sophorae by spectrum–effect relationships and column chromatography. J Sep Sci. 2015;38:1691–1699. doi: 10.1002/jssc.201500100. [DOI] [PubMed] [Google Scholar]
- 166.Chen Y., Yu H., Wu H., Pan Y., Wang K., Liu L., et al. A Novel Reduplicate Strategy for Tracing Hemostatic Compounds from Heating Products of the Flavonoid Extract in Platycladi cacumen by Spectrum-Effect Relationships and Column Chromatography. Molecules. 2015;20:16970–16986. doi: 10.3390/molecules200916970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li S., Xue X., Yang X., Zhou S., Wang S., Meng J. A Network Pharmacology Approach Used to Estimate the Active Ingredients of Moutan Cortex Charcoal and the Potential Targets in Hemorrhagic Diseases. Biol Pharm Bull. 2019;42:432–441. doi: 10.1248/bpb.b18-00756. [DOI] [PubMed] [Google Scholar]
- 168.Cheng P., Xue X., Su J., Lu M., Wang S., Meng J. 1H NMR-based metabonomic revealed protective effect of Moutan Cortex charcoal on blood-heat and hemorrhage rats. J Pharm Biomed Anal. 2019;169:151–158. doi: 10.1016/j.jpba.2019.02.044. [DOI] [PubMed] [Google Scholar]
- 169.Wu J., Zhang M., Cheng J., Zhang Y., Luo J., Liu Y., et al. Effect of Lonicerae japonicae Flos Carbonisata-Derived Carbon Dots on Rat Models of Fever and Hypothermia Induced by Lipopolysaccharide. Int J Nanomed. 2020;15:4139–4149. doi: 10.2147/IJN.S248467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang X., Zhang Y., Kong H., Cheng J., Zhang M., Sun Z., et al. Novel mulberry silkworm cocoon-derived carbon dots and their anti-inflammatory properties. Artif Cell Nanomed B. 2020;48:68–76. doi: 10.1080/21691401.2019.1699810. [DOI] [PubMed] [Google Scholar]
- 171.Hu J., Luo J., Zhang M., Wu J., Zhang Y., Kong H., et al. Carbonisata-Based Carbon Dots Against Ethanol Induced Acute Gastric Ulcer in Rats: Anti-Inflammatory and Antioxidant Activities. Int J Nanomed. 2021;16:2461–2475. doi: 10.2147/IJN.S289515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhao Y., Zhang Y., Kong H., Zhang M., Cheng J., Wu J., et al. Carbon Dots from Paeoniae Radix Alba Carbonisata: Hepatoprotective Effect. Int J Nanomed. 2020;15:9049–9059. doi: 10.2147/IJN.S281976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang X., Zhang Y., Zhang M., Kong H., Wang S., Cheng J., et al. Novel Carbon Dots Derived from Puerariae lobatae Radix and Their Anti-Gout Effects. Molecules. 2019;24:4152. doi: 10.3390/molecules24224152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Luo J., Kong H., Zhang M., Cheng J., Sun Z., Xiong W., et al. Novel Carbon Dots-Derived from Radix Puerariae Carbonisata Significantly Improve the Solubility and Bioavailability of Baicalin. J Biomed Nanotechnol. 2019;15:151–161. doi: 10.1166/jbn.2019.2675. [DOI] [PubMed] [Google Scholar]
- 175.Wang S., Zhang Y., Kong H., Zhang M., Cheng J., Wang X., et al. Antihyperuricemic and anti-gouty arthritis activities of Aurantii fructus immaturus carbonisata-derived carbon dots. Nanomedicine. 2019;14:2925–2939. doi: 10.2217/nnm-2019-0255. [DOI] [PubMed] [Google Scholar]
- 176.Sun Z., Lu F., Cheng J., Zhang M., Zhu Y., Zhang Y., et al. Hypoglycemic Bioactivity of Novel Eco-Friendly Carbon Dots Derived from Traditional Chinese Medicine. J Biomed Nanotechnol. 2018;14:2146–2155. doi: 10.1166/jbn.2018.2653. [DOI] [PubMed] [Google Scholar]
- 177.Lu F., Zhang Y., Cheng J., Zhang M., Luo J., Qu H., et al. Maltase and sucrase inhibitory activities and hypoglycemic effects of carbon dots derived from charred Fructus crataegi. Mater Res Express. 2019;6 [Google Scholar]
- 178.Liu Y., Zhang M., Cheng J., Zhang Y., Kong H., Zhao Y., et al. Novel Carbon Dots Derived from Glycyrrhizae Radix et Rhizoma and Their Anti-Gastric Ulcer Effect. Molecules. 2021;26:1512. doi: 10.3390/molecules26061512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhao X., Wang L., Ren S., Hu Z., Wang Y. One-pot synthesis of Forsythia@carbon quantum dots with natural antiwood rot fungus activity. Mater Design. 2021;206 [Google Scholar]
- 180.Wang H., Zhang M., Ma Y., Wang B., Shao M., Huang H., et al. Selective inactivation of Gram-negative bacteria by carbon dots derived from natural biomass-Artemisia argyi leaves. J Mater Chem B. 2020;8:2666–2672. doi: 10.1039/c9tb02735a. [DOI] [PubMed] [Google Scholar]
- 181.Kong H., Zhao Y., Zhu Y., Xiong W., Luo J., Cheng J., et al. Carbon dots from Artemisiae Argyi Folium Carbonisata: strengthening the anti-frostbite ability. Artif Cell Nanomed B. 2020;49:11–19. doi: 10.1080/21691401.2020.1862134. [DOI] [PubMed] [Google Scholar]
- 182.Zhang M., Cheng J., Zhang Y., Kong H., Wang S., Luo J., et al. Green synthesis of Zingiberis rhizoma-based carbon dots attenuates chemical and thermal stimulus pain in mice. Nanomedicine. 2020;15:851–869. doi: 10.2217/nnm-2019-0369. [DOI] [PubMed] [Google Scholar]
- 183.Zhao Y., Lu F., Zhang Y., Zhang M., Zhao Y., Luo J., et al. Water-Soluble Carbon Dots in Cigarette Mainstream Smoke: Their Properties and the Behavioural, Neuroendocrinological, and Neurotransmitter Changes They Induce in Mice. Int J Nanomed. 2021;16:2203–2217. doi: 10.2147/IJN.S291670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tong T., Hu H., Zhou J., Deng S., Zhang X., Tang W., et al. Glycyrrhizic-Acid-Based Carbon Dots with High Antiviral Activity by Multisite Inhibition Mechanisms. Small. 2020;16:1906206. doi: 10.1002/smll.201906206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lin C.J., Chang L., Chu H.W., Lin H.J., Chang P.C., Wang R.Y.L., et al. High Amplification of the Antiviral Activity of Curcumin through Transformation into Carbon Quantum Dots. Small. 2019;15:1902641. doi: 10.1002/smll.201902641. [DOI] [PubMed] [Google Scholar]
- 186.Gao Y., Dong Y., Guo Q., Wang H., Feng M., Yan Z., et al. Study on Supramolecules in Traditional Chinese Medicine Decoction. Molecules. 2022;27:3268. doi: 10.3390/molecules27103268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhao Q., Luan X., Zheng M., Tian X.H., Zhao J., Zhang W.D., et al. Synergistic Mechanisms of Constituents in Herbal Extracts during Intestinal Absorption: Focus on Natural Occurring Nanoparticles. Pharmaceutics. 2020;12:128. doi: 10.3390/pharmaceutics12020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Liu Q., Wen J., Peng Z., Liu F., Tong X. Review of the powder and decoction formulae in Traditional Chinese Medicine based on pharmacologically active substances and clinical evidence. J Tradit Chin Med. 2015;35:355–360. doi: 10.1016/s0254-6272(15)30110-2. [DOI] [PubMed] [Google Scholar]
- 189.Ping Y., Li Y., Lü S., Sun Y., Zhang W., Wu J., et al. A study of nanometre aggregates formation mechanism and antipyretic effect in Bai-Hu-Tang, an ancient Chinese herbal decoction. Biomed Pharmacother. 2020;124 doi: 10.1016/j.biopha.2020.109826. [DOI] [PubMed] [Google Scholar]
- 190.Lü S., Su H., Sun S., Guo Y., Liu T., Ping Y., et al. Isolation and characterization of nanometre aggregates from a Bai-Hu-Tang decoction and their antipyretic effect. Sci Rep. 2018;8:12209. doi: 10.1038/s41598-018-30690-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhou J., Gao G., Chu Q., Wang H., Rao P., Ke L. Chromatographic isolation of nanoparticles from Ma-Xing-Shi-Gan-Tang decoction and their characterization. J Ethnopharmacol. 2014;151:1116–1123. doi: 10.1016/j.jep.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 192.Lin D., Du Q., Wang H., Gao G., Zhou J., Ke L., et al. Antidiabetic Micro-/Nanoaggregates from Ge-Gen-Qin-Lian-Tang Decoction Increase Absorption of Baicalin and Cellular Antioxidant Activity In Vitro. BioMed Res Int. 2017;2017:9217912. doi: 10.1155/2017/9217912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhao G., Hong L., Liu M., Jiang H., Peng D., He L., et al. Isolation and Characterization of Natural Nanoparticles in Naoluo Xintong Decoction and Their Brain Protection Research. Molecules. 2022;27:1511. doi: 10.3390/molecules27051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chen M., Wang P., Li T., Li L., Li J., Bai H., et al. Comprehensive analysis of Huanglian Jiedu decoction: Revealing the presence of a self-assembled phytochemical complex in its naturally-occurring precipitate. J Pharm Biomed Anal. 2021;195 doi: 10.1016/j.jpba.2020.113820. [DOI] [PubMed] [Google Scholar]
- 195.Zhang C., Zhao R., Yan W., Wang H., Jia M., Zhu N., et al. Compositions, Formation Mechanism, and Neuroprotective Effect of Compound Precipitation from the Traditional Chinese Prescription Huang-Lian-Jie-Du-Tang. Molecules. 2016;21:1094. doi: 10.3390/molecules21081094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang H., Li T., Xiang H., Zhang X., Fang K., Wu G., et al. Origin and Formation Mechanism Investigation of Compound Precipitation from the Traditional Chinese Prescription Huang-Lian-Jie-Du-Tang by Isothermal Titration Calorimetry. Molecules. 2017;22:1456. doi: 10.3390/molecules22091456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Li W., Wang Z., Liu X., Han N., Li T., Lei H., et al. Based on weak bond chemistry, the interaction mechanism between glycyrrhiza protein and berberine in water decocting process of Rhizoma Coptidis and Liquorice was investigated. Acta Pharm Sin. 2021;56:2119–2126. [Google Scholar]
- 198.Wu J., Yang Y., Yuan X., Xu H., Chen Q., Ren R., et al. Role of particle aggregates in herbal medicine decoction showing they are not useless: considering Coptis chinensis decoction as an example. Food Funct. 2020;11:10480–10492. doi: 10.1039/d0fo02179b. [DOI] [PubMed] [Google Scholar]
- 199.Zhang Q., Yang Y., Ren R., Chen Q., Wu J., Zheng Y., et al. Self-assembled aggregations in Coptidis Rhizoma decoction dynamically regulate intestinal tissue permeability through Peyer’s patch-associated immunity. Chin Herb Med. 2021;13:370–380. doi: 10.1016/j.chmed.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhou J., Zhang J., Gao G., Wang H., He X., Chen T., et al. Boiling Licorice Produces Self-Assembled Protein Nanoparticles: A Novel Source of Bioactive Nanomaterials. J Agric Food Chem. 2019;67:9354–9361. doi: 10.1021/acs.jafc.9b03208. [DOI] [PubMed] [Google Scholar]
- 201.Iitsuka H., Koizumi K., Inujima A., Suzaki M., Mizuno Y., Takeshita Y., et al. Discovery of a sugar-based nanoparticle universally existing in boiling herbal water extracts and their immunostimulant effect. Biochem Biophys Rep. 2018;16:62–68. doi: 10.1016/j.bbrep.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhou J., Liu J., Lin D., Gao G., Wang H., Guo J., et al. Boiling-induced nanoparticles and their constitutive proteins from Isatis indigotica Fort. root decoction: Purification and identification. J Tradit Complement Med. 2017;7:178–187. doi: 10.1016/j.jtcme.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gao G, He C, Wang H, Guo J, Ke L, Zhou J, et al. Polysaccharide Nanoparticles from Isatis indigotica Fort. Root Decoction: Diversity, Cytotoxicity, and Antiviral Activity. Nanomaterials 2022;12:30. [DOI] [PMC free article] [PubMed]
- 204.Weng Q., Cai X., Zhang F., Wang S. Fabrication of self-assembled Radix Pseudostellariae protein nanoparticles and the entrapment of curcumin. Food Chem. 2019;274:796–802. doi: 10.1016/j.foodchem.2018.09.059. [DOI] [PubMed] [Google Scholar]
- 205.Meng G.T., Ching K.M., Ma C.Y. Thermal aggregation of globulin from an indigenous Chinese legume, Phaseolus angularis (red bean) Food Chem. 2002;79:93–103. [Google Scholar]
- 206.Lin D., Lin W., Gao G., Zhou J., Chen T., Ke L., et al. Purification and characterization of the major protein isolated from Semen Armeniacae Amarum and the properties of its thermally induced nanoparticles. Int J Biol Macromol. 2020;159:850–858. doi: 10.1016/j.ijbiomac.2020.05.070. [DOI] [PubMed] [Google Scholar]
- 207.Li X., Liang Z., Du J., Wang Z., Mei S., Li Z., et al. Herbal decoctosome is a novel form of medicine. Sci China Life Sci. 2019;62:333–348. doi: 10.1007/s11427-018-9508-0. [DOI] [PubMed] [Google Scholar]