Abstract
Background
This network meta-analysis aimed to compare the effects of bariatric surgery, novel glucose-lowering agents (SGLT2i, GLP1RA, DPP4i), and insulin for patients with type 2 diabetes mellitus (T2DM) and obesity.
Methods
Four databases were searched from inception to April 2023 to identify randomized controlled trials (RCTs) comparing bariatric surgery, SGLT2i, GLP1RA, DPP4i, insulin, and/or placebo/usual care among patients with T2DM and obesity in the achievement of HbA1c < 7.0 per cent within one year, and 12-month changes in HbA1c and body weight.
Results
A total of 376 eligible RCTs (149 824 patients) were analysed. Bariatric surgery had significantly higher rates of achieving HbA1c < 7.0 per cent than SGLT2i (RR = 2.46, 95 per cent c.i. = 1.28, 4.92), DPP4i (RR = 2.59, 95 per cent c.i. = 1.36, 5.13), insulin (RR = 2.27, 95 per cent c.i. = 1.18, 4.58) and placebo/usual care (RR = 4.02, 95 per cent c.i. = 2.13, 7.93), but had no statistically significant difference from GLP1RA (RR = 1.73, 95 per cent c.i. = 0.91, 3.44), regardless of oral (RR = 1.33, 95 per cent c.i. = 0.66, 2.79) or injectable (RR = 1.75, 95 per cent c.i. = 0.92, 3.45) administration. Significantly more GLP1RA patients achieved HbA1c < 7.0 per cent than other non-surgical treatments. Bariatric surgery had the greatest reductions in HbA1c (∼1 per cent more) and body weight (∼15 kg more) at 12 months. Among novel glucose-lowering medications, GLP1RA was associated with greater reductions in HbA1c than SGLT2i (−0.39 per cent, 95 per cent c.i. = −0.55, −0.22) and DPP4i (−0.51 per cent, 95 per cent c.i. = −0.64, −0.39) at 12 months, while GLP1RA (−1.74 kg, 95 per cent c.i. = −2.48, −1.01) and SGLT2i (−2.23 kg, 95 per cent c.i. = −3.07, −1.39) showed greater reductions in body weight than DPP4i at 12 months.
Conclusion
Bariatric surgery showed superiority in glycaemic control and weight management compared to non-surgical approaches. GLP1RA administered by oral or injectable form demonstrated reduced HbA1c and body weight at 12 months, and was preferable over other non-surgical treatments among patients with T2DM and obesity.
PROSPERO registration no
CRD42020201507
This network meta-analysis compared the effects of bariatric surgery, novel glucose-lowering medications, and conventional anti-diabetes treatments among 149 824 patients with diabetes and obesity from 376 randomized controlled trials. Bariatric surgery showed superiority in glycaemic control and weight management compared to all non-surgical approaches. GLP1RA demonstrated reduced HbA1c and body weight at 12 months, and was preferable over other non-surgical treatment options.
Introduction
The prevalence of type 2 diabetes mellitus (T2DM) and obesity is surging at an alarming rate globally and is projected to spread in the next few decades1,2. T2DM and obesity are closely intertwined, as excess weight is deemed a risk factor for T2DM3. Therefore, ideal anti-diabetic therapies should be those with weight-loss or, at least, weight-neutral effects.
Lifestyle modifications, such as medical nutrition therapies, education and physical activities, and anti-diabetes medications, are fundamental to treating T2DM and obesity. Systematic reviews and meta-analyses indicated that behaviour management is associated with modest HbA1c reductions, ranging from 0.3 to 2.0 per cent4,5. However, the results were mixed for weight outcomes, due to the variety of eating patterns, programme components, and patient compliance4. Among various anti-diabetes medications, insulin, sulfonylurea, and thiazolidinediones are associated with weight gain, while metformin induces modest weight loss6,7. In comparison, novel glucose-lowering agents, such as glucagon-like peptide-1 receptor agonists (GLP1RA), sodium-glucose cotransporter-2 inhibitors (SGLT2i), and dipeptidyl peptidase-4 inhibitors (DPP4i) not only have beneficial effects in glycaemic control, but also have weight-loss or weight-neutral effects6,8. In addition, novel glucose-lowering agents have protective effects in cardiovascular diseases and all-cause mortality9,10.
Bariatric surgery is a recommended therapy for patients with T2DM and obesity. According to the latest Standards of Medical Care in Diabetes released by the American Diabetes Association (ADA), bariatric surgery can be considered to treat T2DM for adult patients with BMI ≥ 30 kg/m2 (or ≥27.5 kg/m2 for Asians) who failed non-surgical methods11. Indeed, mounting evidence suggests that bariatric surgery has superior effects in T2DM remission and weight management, and can even lower cardiovascular morbidity and mortality12.
Comparative effects on improvements in metabolic syndromes and reductions in risks of comorbidities and mortality have been widely studied between bariatric surgery and traditional therapies12,13, or among different novel glucose-lowering agents9,14. While there are emerging studies exploring the comparative effectiveness of bariatric surgery and novel glucose-lowering agents among patients with T2DM15–18, pooled evidence from systematic reviews and network meta-analyses is not yet available. In light of the tremendous burden of T2DM and obesity, there is a continuing clinical need to establish evidence-based recommendations for both physicians and patients. Thus, the aim of this study was to systematically review the current evidence and report results of a network meta-analysis to explore and quantify the comparative effects of bariatric surgery, SGLT2i, GLP1RA, DPP4i, insulin, and placebo/usual care among patients with T2DM and obesity.
Methods
Protocol and registration
This study was conducted in accordance with the PRISMA extension statements for network meta-analyses of health care interventions19 and registered at PROSPERO (registration no.: CRD42020201507).
Eligibility criteria
Eligible participants were adult patients (age ≥18 years old) with T2DM and obesity. The mean BMI of the included patients should be ≥30 kg/m2 (or ≥27.5 kg/m2 for Asians). Patients who were under 18 years old, had a low BMI (<30 kg/m2; or <27.5 kg/m2 for Asians), were pregnant, or did not have a diagnosis of T2DM were excluded.
Studies should have reported at least two of the treatments among bariatric surgery, glucose-lowering agents, insulin, and placebo/usual care. Placebo/usual care was defined as any therapies other than bariatric surgery, novel glucose-lowering agents, and insulin; and it included but was not limited to metformin, sulfonylureas, glipizide, and lifestyle modifications. This was because any of these treatment effects were generally considered modest compared to bariatric surgery or novel glucose medications in terms of glucose and weight management, and this study did not focus on investigating the effects among different traditional oral anti-diabetic treatments and lifestlye modifications. Patients should be followed for at least 12 weeks (or 3 months) after the baseline. Studies with patients having non-operative treatments but with prior bariatric surgery were excluded. Studies with patients taking two or more novel glucose-lowering agents (for example, combination use of SGLT2i and DPP4i) were also excluded, because the effects of combination use of novel glucose-lowering agents were not the study objective. The primary outcome was the achievement of the HbA1c target (<7.0 per cent) within one year. Secondary outcomes were the changes in clinical parameters from baseline to 6 and 12 months. The clinical parameters included HbA1c, body weight, systolic blood pressure (SBP) and diastolic blood pressure (DBP). BMI was not included as a study outcome because body weight was already being measured as an outcome and including BMI would be redundant and may not provide additional information. The occurrence of severe hypoglycaemia and all-cause mortality within 1 year was also measured. Included studies should report at least one of the outcomes listed above.
The target study design was randomized controlled trials (RCTs). Non-randomized trials, observational studies, case reports/series, qualitative studies, review articles, and conference abstracts were excluded. Included studies were written in English without restrictions on the year of publication.
Information sources and search
Two investigators (T. Wu and M. S. H. Chung) conducted the study searching, screening, and selecting processes independently. Four electronic databases (PubMed, EMBASE, MEDLINE, and Cochrane Library) were searched from inception to 28 October 2020. An updated search was conducted in February 2022 to identify eligible papers published between October 2020 and February 2022. Another round of searching was conducted before publication in April 2023. The keywords for searching and filters used in each database are listed in Appendix S1. After removing duplicates, two investigators (T. Wu and M. S. H. Chung) screened the study titles and abstracts first, and then reviewed full texts to identify eligible studies. A senior co-author (C. K. H. Wong) was consulted to resolve discrepancies.
Data collection process and data items
Two investigators (T. Wu and M. S. H. Chung) extracted data independently from included studies into uniform Excel worksheets. Data items listed below were collected from each study: name of the first author, year of publication, location and dates of conducted studies, treatments, sample sizes, duration of follow-up, baseline characteristics of the included participants in each arm (age, sex, HbA1c, BMI, and duration of T2DM), and funding. For dichotomous outcomes (that is, achievement of HbA1c < 7.0 per cent, occurrence of severe hypoglycaemia, and death within 1 year), the total number of patients and the number of patients with the outcomes of interest were collected. For continuous outcomes (that is, changes in metabolic parameters at different time points), the number of patients, value changes in means from baseline, and standard deviations were extracted.
Geometry of the network
Network plots were drawn to present the geometry of the treatment network of comparisons across trials for each pre-specified outcome. The nodes in the network geometry represent different treatments (bariatric surgery, SGLT2i, GLP1RA, DPP4i, insulin, and placebo/usual care), while the edges represent the head-to-head comparisons between network nodes. The size of nodes and thickness of edges are associated with the numbers of included studies and the amount of relevant data, respectively.
Risk of bias within individual studies and across studies
The risk of bias and quality of individual RCTs included were assessed by the Cochrane risk bias tool20, which measures selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases. Robvis visualization Risk-of-Bias Tool for randomized trials (RoB2) (https://www.riskofbias.info) was used to generate the traffic light plot and the summary plot. Stata command ‘netfunnel’ was used to generate comparison-adjusted funnel plots and assess publication bias.
Summary measures and statistical analysis
Pooled relative risks (RRs) and mean differences (MDs) were estimated for dichotomous and continuous outcomes, respectively. For direct treatment comparisons, random-effects models and fixed-effect models (if the number of trials was no more than three) were adopted to generate the evidence in Stata using the ‘metan’ command21, which was used to perform meta-analysis of studies with two groups. If there was substantial statistical heterogeneity between trials (that is I2 > 50 per cent), a random-effects model was used if the results (RR or MD and 95 per cent c.i.) from individual trials largely fell on one side of the null line. In other cases, a narrative summary of each study was provided instead22.
Indirect and mixed comparisons were performed in R (V. 4.1.2) using rjags (V.4–12) and gemtc (V.1.0-1) packages. Random-effects network meta-analysis was conducted within a Bayesian framework using the Markov Chain Monte Carlo (MCMC) simulations. The binomial likelihood with log link was used to measure RR for dichotomous outcomes (for example, achievement of HbA1c < 7 per cent within 1 year), while normal likelihood with identity link was used to measure MD for continuous outcomes (for example, changes in HbA1c at 6 months). A four-chain model was run with an adaptation phase of 20 000 iterations followed by 50 000 model iterations. The convergence of network models derived from MCMC simulations was assessed by trace plots and Gelman–Rubin–Brooks plots. I2 was used (I2 > 50 per cent is considered significant heterogeneity) to assess the heterogeneity.
Transitivity assumption (similarity across studies for valid indirect comparison between treatments) was assessed by visual inspection of a table containing categorized study characteristics23, including age (<50 and ≥50 years old), HbA1c (<7.5 per cent and ≥7.5 per cent), BMI (<35 kg/m2 and ≥35 kg/m2), and duration of T2DM (<5 and ≥5 years) at baseline and percentage of women (<50 per cent and ≥50 per cent). The inconsistency between direct and indirect comparisons was assessed by node-splitting analysis. The results were presented in forest plots and/or league tables. In forest plots, prediction intervals24, which are the expected range of true range of true effects in future settings, were provided, whereas in league tables, indirect comparisons were presented in the inferior-left part and direct comparisons were displayed in the superior-right part. The rank of treatments was estimated based on the surface under the cumulative ranking curve (SUCRA), ranging from 0 to 100 per cent. A high SUCRA value indicates that there is a high possibility for the treatment to be in a top rank25.
Certainty of evidence
The certainty of evidence was assessed by using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach26. The certainty for each comparison was rated as high, moderate, low, or very low, taking into consideration study design, risk of bias, inconsistency, indirectness, imprecision, and others (publication bias, presence of large effects, dose–response gradient, and confounding). Imprecision was assessed by using a non-contextualized approach, in which the null effect was the threshold of interest27. An online tool, GRADEPro GDT (https://gdt.gradepro.org/), was used to grade the certainty of evidence.
Sensitivity analyses
Sensitivity analyses were conducted for the comparisons between GLP1RA (oral and subcutaneous delivery) and other treatments, to explore the impacts of the route of administration. Furthermore, achievements of the HbA1c target, and changes in HbA1c, and body weight, from baseline to 6 months and 12 months were measured and compared across treatments for patients with the following baseline characteristics: HbA1c ≥ 8.0 per cent and BMI ≥ 35 kg/m2. Studies with extreme results were removed when conducting sensitivity analyses.
All analyses were performed using Stata version 13.1 (Stata Corp LP, College Station, TX, USA) and R version V.4.1.2. Two-tailed P-values <0.05 indicate statistical significance. Datasets used in network meta-analyses, and the Stata and R codes used to run the analyses are provided in the Supplemental materials (Appendix S2, S3).
Results
Study characteristics
Figure S1 depicts the flowchart of study identification, screening, and selection. A total of 27 073 unique papers were identified from four databases. Through title and abstract screening, 20 544 ineligible papers were excluded when applying inclusion and exclusion criteria. Then, 6143 papers were further discarded after reviewing full papers. Ten and seven eligible papers were added after the updated searches conducted in Feburary 2022 and April 2023, respectively. Finally, 403 eligible papers were included for systematic review, and 376 papers were included for meta-analysis. Among 149 824 patients included in the network meta-analysis, 566 underwent bariatric surgery; 35,942, 20,900, 39,566, 10,051, and 42 799 were treated with GLP1RA, SGLT2i, DPP4i, insulin, and placebo/usual care, respectively. Among patients assigned to the placebo group, most of them have at least tried lifestyle modifications or metformin.
Table S1 lists the characteristics of included studies and patients. The majority of the studies in the network meta-analysis were: published in 2011 and afterward (∼79 per cent); multinational studies (∼65 per cent); funded by industry (∼84 per cent); and had a follow-up period of less than 1 year (∼63 per cent). A total of 3622 (10.1 per cent) patients in the GLP1RA group were treated with oral GLP1RA.
Achievement of glycaemic targets
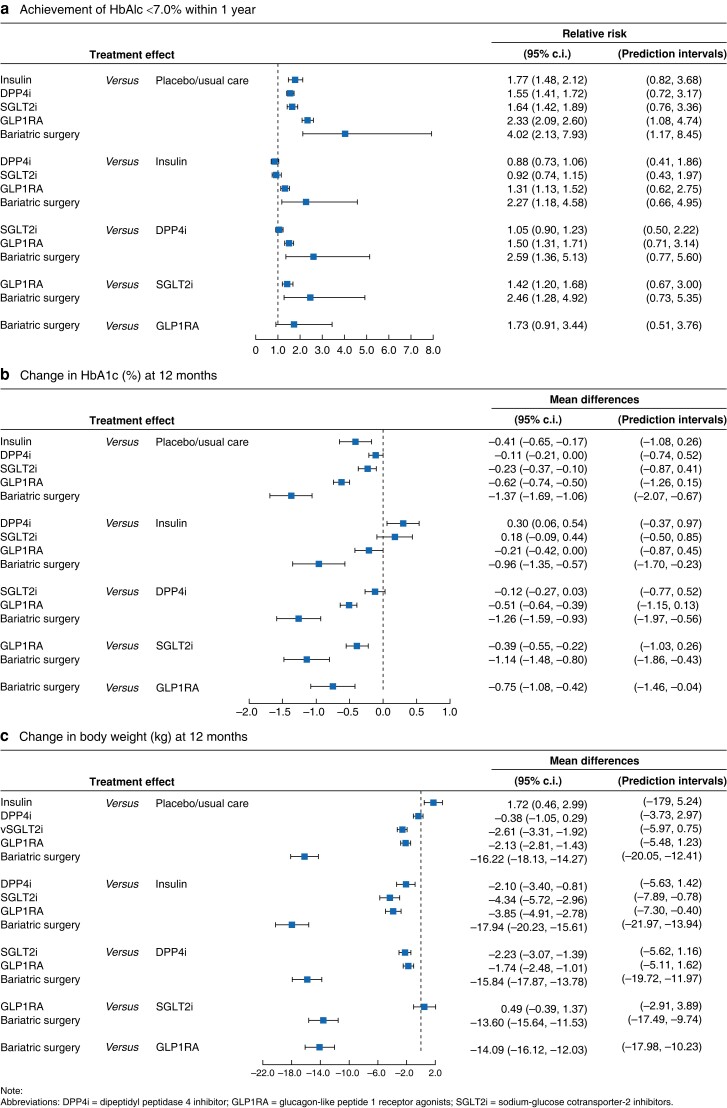
A total of 185 randomized trials including 100 686 patients measured the outcome of the achievement of HbA1c < 7.0 per cent. The most common treatments were placebo/usual care (134 trials, 24 687 patients), GLP1RA (77 trials, 27 326 patients), and DPP4i (81 trials, 25 908 patients). The mean baseline age (≥50 years old), HbA1c (≥7.5 per cent), and BMI (<35 kg/m2) of the patients appeared to be similar across most comparisons, except that 7 of 185 trials (3.8 per cent) had a mean age of <50 years, 5 (2.7 per cent) trials had baseline HbA1c < 7.5 per cent, and 6 (3.2 per cent) trials had patients with baseline BMI ≥ 35 kg/m2 (Table S2a). Therefore, the transitivity assumption was considered valid. The geometric distributions of the treatment arms for each outcome are displayed in Fig. 1. The network meta-analysis showed that more patients undergoing bariatric surgery achieved HbA1c < 7.0 per cent within 1 year than patients in SGLT2i (RR = 2.46, 95 per cent c.i.: 1.28, 4.92), DPP4i (RR = 2.59, 95 per cent c.i.: 1.36, 5.13), and placebo/usual care (RR = 4.02, 95 per cent c.i.: 2.13, 7.93) groups. GLP1RA was associated with an increased chance of achieving the glycaemic target compared to SGLT2i (RR = 1.42, 95 per cent c.i.: 1.20, 1.68), DPP4i (RR = 1.50, 95 per cent c.i.: 1.31, 1.71), and insulin (RR = 1.31, 95 per cent c.i.: 1.13, 1.52) (Table 1a and Fig. 2a). Statistically significant differences were not identified among SGLT2i, DPP4i, and insulin in achieving the glycaemic target. Results from direct and indirect comparisons did not show significant statistical differences.
Fig. 1.
Network maps. Achievement of HbA1c < 7.0 per cent within 1 year, and changes in HbA1c and body weight, at 12 months
Fig. 2.
Forest plots. a Achievement of HbA1c < 7.0 per cent within 1 year; b changes in HbA1c (%) at 12 months; c changes in body weight (kg) at 12 months
Changes in HbA1c and body weight
A total of 184 trials (99 864 patients) and 66 trials (47 610 patients) reported the changes in HbA1c at 6 and 12 months, respectively. For the measurement of HbA1c at 6 months, 10 (5.4 per cent) of 184 trials had baseline HbA1c < 7.5 per cent, 12 trials (6.5 per cent) had patients with baseline age <50 years old, and 13 trials (7.1 per cent) had patients with baseline BMI ≥ 35 kg/m2 (Table S2d). Similarly, only 4 (6.1 per cent), 8 (12.1 per cent), and 5 (7.6 per cent) out of 66 trials had patients with baseline age <50 years, HbA1c < 7.5 per cent, and BMI ≥ 35 kg/m2, respectively (Table S2b). The geometric distributions of the treatment arms for each outcome are displayed in Fig. 1 and Fig. S2. Table 1b and Table S3a indicate that statistically significantly greater reductions in HbA1c were observed in the bariatric surgery group than in all other groups at 6 and 12 months. These reductions were considered clinically significant, as 1 per cent or more reductions in HbA1c were achieved in patients with bariatric surgery than other patients at 6 or 12 months (Fig. 2b). Among novel glucose-lowering agents, GLP1RA was associated with a greater reduction in HbA1c than SGLT2i or DPP4i at both 6 and 12 months, while SGLT2i and DPP4i had similar effects in lowering HbA1c levels. The effects of SGLT2i, DPP4i, and insulin on HbA1c reductions were similar at 6 months, but insulin showed beneficial effects at 12 months when comparing with DPP4i (−0.30 per cent, 95 per cent c.i.: −0.54, −0.06). These results were consistent with those in the subgroup analysis, where patients were limited to those with baseline HbA1c ≥ 8.0 per cent—bariatric surgery had the greatest effects in lowering HbA1c levels, and patients with bariatric surgery experienced 0.9–1.6 per cent more reductions in HbA1c than any other treatment at 12 months (Table S4b2).
One hundred and forty-six trials (80 371 patients) and 58 trials (48 082 patients) measured the changes in body weight from baseline to 6 and 12 months, respectively. The included patients in most trials had baseline age (≥50 years old), HbA1c (≥7.5 per cent) and BMI (<35 kg/m2) (Table S2c and e). Bariatric surgery showed superior effects in weight reduction compared to any of the other treatments, with weight loss of 7.8–11.1 kg at 6 months and 13.6–17.9 kg at 12 months. GLP1RA and SGLT2i were comparable in weight management, and they had better outcomes than DPP4i at both 6 and 12 months. Weight gain was observed in patients treated with insulin compared to patients in all other groups at both 6 and 12 months (Table 1c, Fig. 2c, and Table S3b). Similarly, the subgroup analysis showed that patients with bariatric surgery and baseline Hba1c ≥ 8.0 per cent lost ∼15 kg more weight than any other treatment at 12 months (Table S4b3).
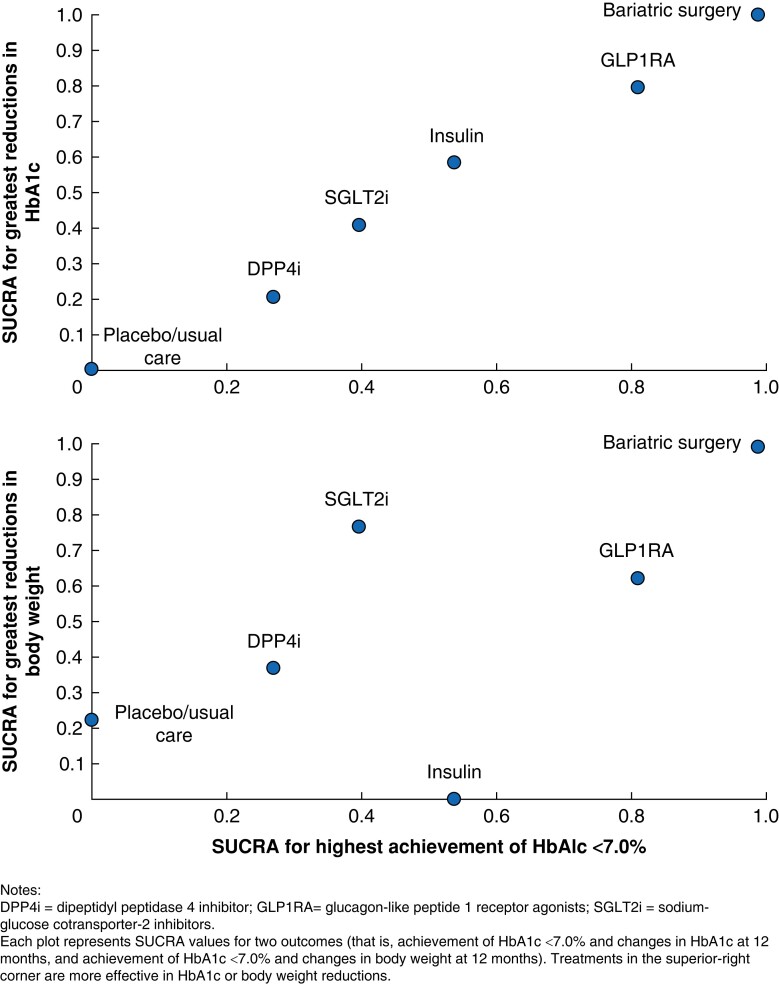
The SUCRA clustered ranking plot (Fig. 3) illustrated that bariatric surgery was the most efficacious treatment with the highest achievement rate of HbA1c < 7.0 per cent and the greatest reductions in HbA1c and body weight at 12 months. Among non-surgical treatments, GLP1RA showed preferable effects in glycaemic control and weight management. While insulin was associated with HbA1c improvement, it ranked last in weight loss. In contrast, placebo/usual care showed low efficacy in both weight and glycaemic control.
Fig. 3.
Clustered ranking plot of surface under cumulative ranking curves (SUCRA) of achievement of HbA1c < 7.0 and reductions in HbA1c and body weight at 12 months
Changes in blood pressure
Although the reductions in blood pressure of bariatric surgery patients were not statistically significantly different from other groups at 6 months, bariatric surgery was associated with greater reductions in SBP reductions than GLP1RA (−2.70 mmHg, 95 per cent c.i.: −4.78, −0.64), DPP4i (−4.53 mmHg, 95 per cent c.i.: −6.70, −2.43), insulin (−4.12 mmHg, 95 per cent c.i.: −6.40, −1.79), and placebo/usual care (−4.86 mmHg, 95 per cent c.i.: −6.86, −2.89) at 12 months (Table S3c, S3e). However, no statistically significant differences in blood pressure reductions were found between bariatric surgery and SGLT2i at both 6 and 12 months. Besides, SGLT2i patients were found to have more reductions in both SBP and DBP than GLP1RA and DPP4i, while GLP1RA had more reductions in SBP than DPP4i at 6 and 12 months (Table S3d, S3f).
Severe hypoglycaemia and all-cause mortality
One hundred and sixty-one trials reported the occurrence of severe hypoglycaemia in 75 486 patients, and 187 trials measured the occurrence of all-cause mortality among 99 377 patients. No patients in the bariatric surgery group died within 1 year of follow-up. The occurrence of severe hypoglycaemia and all-cause mortality within one year was rare and did not show statistically significant differences between groups, except that insulin was found to be associated with higher risks of severe hypoglycaemia than DPP4i (RR = 3.23, 95 per cent c.i.:1.30, 9.48) and placebo/usual care (RR = 2.62, 95 per cent c.i.: 1.07, 8.25) (Table S3g and S3h).
Risk of bias
The overall quality of the included studies was considered to be satisfactory. The major sources of risks were from blinding of participants and personnel (performance bias) and blinding of outcome assessment (detection bias) (Fig. S4). No global inconsistency was detected in the outcomes reported, while inconsistencies between direct and indirect comparisons were rarely detected, as reported in Table S3 and S4. The publication bias across the included studies was low, as the shapes of funnel plots are generally symmetrical (Fig. S3).
Certainty of evidence
The certainty of evidence is summarized in Table S5. In general, the estimates of this study were close to true effects for comparisons between bariatric surgery and placebo/usual care, between novel glucose-lowering agents and placebo/usual care, and between any two novel glucose-lowering agents. However, the quality of evidence between bariatric surgery and novel glucose-lowering agents was low to moderate, largely due to indirectness.
Sensitivity analysis
In the sensitivity analysis, six trials compared the patients with oral GLP1RA (3318 patients) with patients treated with other interventions in achieving HbA1c < 7.0 per cent28–33. As indicated in Table S4a1, bariatric surgery was not associated with increased RR of the achievement of HbA1c < 7.0 per cent compared with either injectable (RR = 1.75, 95 per cent c.i.: 0.92, 3.45) or oral GLP1RA (RR = 1.33, 95 per cent c.i.: 0.66, 2.79). However, patients with bariatric surgery experienced the greatest reductions in HbA1c and body weight at 12 months among all groups (Table S4aS2 and aS3). Injectable and oral GLP1RA showed similar effects in glycaemic control and weight management, and had superb effects in achieving HbA1c < 7.0 per cent within 1 year compared with SGLT2i, DPP4i, and conventional therapies. No statistically significant differences were detected between injectable GLP1RA and SGLT2i (0.62 kg, 95 per cent c.i.: −0.34, 1.59) or between oral GLP1RA and SGLT2i (0.13 kg, 95 per cent c.i.: −1.24, 1.50) at 12 months for changes in body weight at 12 months.
After limiting patients with baseline HbA1c ≥ 8.0 per cent, the results on the achievement of HbA1c < 7.0 per cent, and changes in HbA1c and body weight at 12 months were not changed greatly—bariatric surgery had superb effects compared with other treatments, followed by GLP1RA (in glycaemic control) or SGLT2i (in weight reductions) (Table S4b). Insufficient data were available to conduct network meta-analysis after limiting patients with baseline BMI over 35 kg/m2 when measuring the changes in HbA1c and body weight at 12 months (Table S4c).
Discussion
This network meta-analysis allowed indirect comparisons between bariatric surgery and novel glucose-lowering agents, and confirmed the findings from previous meta-analyses12,13 that bariatric surgery leads to greater reductions in HbA1c, body weight, and other metabolic parameters than non-surgical treatments. More importantly, what this analysis added to existing knowledge is that bariatric surgery has efficacious effects in glycaemic control and weight management compared to novel glucose-lowering agents. In summary, this network meta-analysis showed that bariatric surgery had superiority in achieving glycaemic targets compared with non-surgical treatments, including novel glucose-lowering agents and conventional therapy. Among non-surgical treatments, GLP1RA was a preferable option for patients with T2DM and obesity. Clinicians caring for patients seeking optimal treatment for their T2DM and severe obesity should discuss the relevant treatment options in the context of these findings.
For the achievement of the HbA1c target within one year, the results favoured bariatric surgery over all other treatments, and GLP1RA showed the greatest efficacy among non-surgical treatments. While SGLT2i and DPP4i had similar effects, placebo/usual care had modest effects in glycaemic control. However, statistically significant differences in the achievement of HbA1c < 7.0 per cent were not detected between bariatric surgery and GLP1RA. The insignificant results could be due to insufficient data—few existing RCTs compared bariatric surgery with GLP1RA. As for changes in metabolic parameters, bariatric surgery showed superior effects in improving HbA1c levels through 6–12 months, followed by GLP1RA, insulin or SGLT2i, DPP4i, and placebo/usual care. GLP1RA and SGLT2i had similar effects in weight management, whereas no statistically significant differences were found in body weight reductions between DPP4i and placebo/usual care groups. These results were in line with findings from existing (network) meta-analyses, which indicated that patients with GLP1RA achieved better glycaemic and/or weight outcomes than SGLT2i and DPP4i, while SGLT2i provided greater glycaemic and weight effects than DPP4i34–36. Different subclasses of GLP1RA promote weight loss through a similar mechanism—GLP1RA stimulates postprandial insulin secretion, reduces glucagon secretion, and delays gastric emptying, which in turn reduces appetite and food cravings, and introduces weight reduction37,38. However, the extent of weight loss may vary among different subclasses, among which semaglutide has greater weight loss effects than exenatide, dulaglutide or liraglutide38. This may be because semaglutide-associated weight loss is centrally mediated through activating the brain areas involved in appetite control and reward38,39. More significant reductions in blood pressure were observed in the SGLT2i group than in other treatments at 6 months, while bariatric surgery showed superiority in reducing SBP at 12 months. Echoing a previous meta-analysis, bariatric surgery did not significantly reduce the blood pressure compared with non-surgical methods13. The reductions in blood pressure among SGLT2i users might potentially explain the cardioprotective effects of SGLT2i40.
This study showed that the incidence of severe hypoglycaemia and all-cause mortality within one year was low among all groups, and statistically significant differences were rarely detected. Similar results can be found in published meta-analyses, which suggested that novel glucose-lowering agents were not associated with increased risks of severe hypoglycaemia41–43, and DPP4i had no effects on mortality44. Results from meta-analyses suggested that bariatric surgery had low perioperative mortality and was associated with lower long-term mortality than non-operated controls45–47. None of the patients with bariatric surgery died in RCTs included in this study, because none of the RCTs involving bariatric surgery were designed to report mortality as a primary outcome. Another network meta-analysis comprising 764 trials supported the protective effects of SGLT2i and GLP1RA on risks of mortality when used as adjunctive therapies to existing diabetes treatment48. One possible explanation for the discrepancies between the published results and ours was the heterogeneity in inclusion and exclusion criteria among various systematic reviews. Furthermore, it usually takes a long time to observe a fatal event. Due to cost and other considerations, most RCTs had a short follow-up period (most are less than 2 years in this review), and thus it is difficult to capture these outcomes within a short period.
Among non-surgical treatments, GLP1RA was the preferable option for patients with T2DM and obesity. Results from sensitivity analysis indicated that oral and injectable GLP1RA did not find statistically significant differences in achieving HbA1c < 7.0 per cent, and lowering HbA1c and body weight at 12 months. Compared with subcutaneous preparations, oral GLP1RA (that is, semaglutide) provides a non-injectable option and can improve acceptance and adherence among GLP1RA users with T2DM49,50. Available network meta-analysis also suggested that oral GLP1RA showed better efficacy in meeting glycaemic targets and reducing body weight than injectable GLP1RA among patients with T2DM inadequately controlled on basal insulin 51. Because direct comparisons between oral and injectable GLP1RA among patients with T2DM and obesity are rarely made, future RCTs are to be conducted for this target population.
This study has several strengths. First, to our knowledge, this study is the first network meta-analysis comparing and quantitatively summarizing the efficacy and safety outcomes among bariatric surgery, novel glucose-lowering agents, and conventional therapies. Second, RCTs, considered a gold standard method in clinical research, were the only eligible study design for inclusion. Other observational study designs, such as cohort studies and case-control studies, have built-in confounding issues and were thus not considered. Third, sensitivity analysis was conducted to explore whether the routes of administration affected the effects of GLP1RA.
Several limitations should be acknowledged. First, the number of patients in the bariatric surgery group (n = 566) is relatively small compared to the sample sizes of other groups, because only a few eligible RCTs compare bariatric surgery with non-surgical therapies52–62, such as novel glucose-lowering agents15. Thus, any extreme outcomes reported by studies with the bariatric surgery arm would drift the pooled results and lead to bias. Moreover, the comparisons between the bariatric surgery group and novel glucose-lowering agents were indirectly conducted, limiting the quality of evidence. In addition, the heterogeneity of the included studies may increase the uncertainty of the study outcomes. Second, it is acknowledged that not all patients in the non-surgical groups were eligible for bariatric surgery, because individual patient data were inaccessible and including treatment arms with a mean BMI ≥ 30 kg/m2 (or 27.5 kg/m2 for Asians) did not guarantee that all patients in those groups would have a BMI suitable for bariatric surgery. Even though an additional sensitivity analysis to include patients with BMI ≥ 35 kg/m2 was conducted, the statistically non-significant outcomes due to insufficient data advocates for the need for future clinical studies. Third, as this study focused on the metabolic values and safety outcomes recorded from baseline to up to 12 months, more evidence is needed on the long-term (>1 year) comparative outcomes. Lastly, this study did not evaluate the occurrence of mild to moderate treatment-related adverse events (such as back pain, nausea, vomiting, and urinary tract infection), and severe adverse events (such as anastomotic leak, venous thromboembolism, diabetic ketoacidosis, and amputation) among groups63,64.
Supplementary Material
Acknowledgements
The authors thank Professor Carel Le Roux for suggestions and Ms Sahar Sharif of HEHTA, University of Glasgow, for statistical assistance. The authors would also like to thank Miss Yihui Wei for study selection and data collection at the early phase of this study.
Tingting Wu and Carlos K. H. Wong are co-first authors of this paper, and John B. Dixon is the senior author.
Contributor Information
Tingting Wu, Department of Family Medicine and Primary Care, School of Clinical Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China.
Carlos K H Wong, Department of Family Medicine and Primary Care, School of Clinical Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China; Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China; Laboratory of Data Discovery for Health Limited (D24H), Hong Kong Science Park, New Territories, Hong Kong SAR, China.
David T W Lui, Division of Endocrinology and Metabolism, Department of Medicine, School of Clinical Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China.
Simon K H Wong, Division of Surgery, Chinese University of Hong Kong Medical Centre, Hong Kong SAR, China; Department of Surgery, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China.
Cindy L K Lam, Department of Family Medicine and Primary Care, School of Clinical Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China.
Matthew S H Chung, Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China.
David A McAllister, Public Health, School of Health and Wellbeing, University of Glasgow, Glasgow, UK.
Richard Welbourn, Department of Upper GI and Bariatric Surgery, Musgrove Park Hospital, Taunton, UK.
John B Dixon, Iverson Health Innovation Research Institute, Swinburne University of Technology, Melbourne, VIC, Australia.
Funding
This study was funded by the Health and Medical Research Fund Research Fellowship Scheme, Food and Health Bureau, Hong Kong SAR (Ref. No. #02160087). Carlos Wong is the guarantor. The funder/sponsor did not have any role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Health and Medical Research Fund Research Fellowship Scheme, Food and Health Bureau, Hong Kong SAR (Ref. No. #02160087), received by Carlos Wong.
Author contributions
Tingting Wu (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing—original draft), Carlos K. H. Wong (Conceptualization, Funding acquisition, Project administration, Resources, Software, Supervision, Writing—review & editing), David T. W. Lui (Writing—review & editing), Simon K. H. Wong (Writing—review & editing), Cindy L. K. Lam (Writing—review & editing), Matthew S. H. Chung (Investigation), David A. Mcallister (Methodology, Writing—review & editing), Richard Welbourn (Writing—review & editing), and John B. Dixon (Validation, Writing—review & editing).
Disclosures
C. K. H. Wong reports receipt of research funding from the EuroQoL Group Research Foundation, AstraZeneca, and Boehringer Ingelheim, unrelated to this work. J. B. Dixon reports personal fees from NHMRC, personal fees from Reshape, personal fees from Bariatric Advantage, personal fees from Novo Nordisk, personal fees from Nestle Health Science, personal fees from Johnson & Johnson, and personal fees from I-Nova, outside the submitted work. The authors declare no other conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
All data generated or analysed during this study are included in published articles listed in Table S1 and Appendix 2. This paper is not based on a previous communication to a society or meeting.
Ethical approval
The study protocol for this systematic review was developed in line with the PRISMA-NMA statement and registered in the PROSPERO database (http://www.crd.york.ac.uk/PROSPERO/ reference number CRD42020201507). No ethical approval was required.
Reference
- 1. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono Cet al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BBet al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 2021;183:109119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eckel RH, Kahn SE, Ferrannini E, Goldfine AB, Nathan DM, Schwartz MWet al. Obesity and type 2 diabetes: what can be unified and what needs to be individualized? J Clin Endocrinol Metab 2011;96:1654–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Franz MJ, MacLeod J, Evert A, Brown C, Gradwell E, Handu Det al. Academy of nutrition and dietetics nutrition practice guideline for type 1 and type 2 diabetes in adults: systematic review of evidence for medical nutrition therapy effectiveness and recommendations for integration into the nutrition care process. J Acad Nutr Diet 2017;117:1659–1679 [DOI] [PubMed] [Google Scholar]
- 5. Chrvala CA, Sherr D, Lipman RD. Diabetes self-management education for adults with type 2 diabetes mellitus: a systematic review of the effect on glycemic control. Patient Educ Couns 2016;99:926–943 [DOI] [PubMed] [Google Scholar]
- 6. Van Gaal L, Scheen A. Weight management in type 2 diabetes: current and emerging approaches to treatment. Diabetes Care 2015;38:1161–1172 [DOI] [PubMed] [Google Scholar]
- 7. Malin SK, Kashyap SR. Effects of metformin on weight loss: potential mechanisms. Curr Opin Endocrinol Diabetes Obes 2014;21:323–329 [DOI] [PubMed] [Google Scholar]
- 8. Brown E, Wilding JPH, Barber TM, Alam U, Cuthbertson DJ. Weight loss variability with SGLT2 inhibitors and GLP-1 receptor agonists in type 2 diabetes mellitus and obesity: mechanistic possibilities. Obes Rev 2019;20:816–828 [DOI] [PubMed] [Google Scholar]
- 9. Alfayez OM, Al Yami MS, Alshibani M, Fallatah SB, Al Khushaym NM, Alsheikh Ret al. Network meta-analysis of nine large cardiovascular outcome trials of new antidiabetic drugs. Prim Care Diabetes 2019;13:204–211 [DOI] [PubMed] [Google Scholar]
- 10. Zheng SL, Roddick AJ, Aghar-Jaffar R, Shun-Shin MJ, Francis D, Oliver Net al. Association between use of sodium–glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA 2018;319:1580–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. American Diabetes Association . 8. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes–2021. Diabetes Care 2021;44:S100–SS10 [DOI] [PubMed] [Google Scholar]
- 12. Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003–2012. JAMA Surg 2014;149:275–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone Get al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ 2013;347:f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhuang XD, He X, Yang DY, Guo Y, He JG, Xiao HPet al. Comparative cardiovascular outcomes in the era of novel anti-diabetic agents: a comprehensive network meta-analysis of 166,371 participants from 170 randomized controlled trials. Cardiovasc Diabetol 2018;17:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liang Z, Wu Q, Chen B, Yu P, Zhao H, Ouyang X. Effect of laparoscopic Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus with hypertension: a randomized controlled trial. Diabetes Res Clin Pract 2013;101:50–56 [DOI] [PubMed] [Google Scholar]
- 16. Palikhe G, Gupta R, Behera BN, Sachdeva N, Gangadhar P, Bhansali A. Efficacy of laparoscopic sleeve gastrectomy and intensive medical management in obese patients with type 2 diabetes mellitus. Obes Surg 2014;24:529–535 [DOI] [PubMed] [Google Scholar]
- 17. Bhandari M, Mathur W, Kumar R, Mishra A, Bhandari M. Surgical and advanced medical therapy for the treatment of type 2 diabetes in class I obese patients: a short-term outcome. Obes Surg 2017;27:3267–3272 [DOI] [PubMed] [Google Scholar]
- 18. Yong W, Shibo W, Jingang L. Remission of insulin resistance in type 2 diabetic patients after gastric bypass surgery or exenatide therapy. Obes Surg 2012;22:1060–1067 [DOI] [PubMed] [Google Scholar]
- 19. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron Cet al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777–784 [DOI] [PubMed] [Google Scholar]
- 20. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman ADet al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bradburn MJ, Deeks JJ, Altman DG. Metan—an alternative meta-analysis command. Stata Tech Bull 1999;8 [Google Scholar]
- 22. Virgili G, Parravano M, Evans JR, Gordon I, Lucenteforte E. Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis. Cochrane Database Syst Rev 2018;10:CD007419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ho EK, Chen L, Simic M, Ashton-James CE, Comachio J, Wang DXMet al. Psychological interventions for chronic, non-specific low back pain: systematic review with network meta-analysis. BMJ 2022;376:e067718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. IntHout J, Ioannidis JP, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 2016;6:e010247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mbuagbaw L, Rochwerg B, Jaeschke R, Heels-Andsell D, Alhazzani W, Thabane Let al. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst Rev 2017;6:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek Jet al. GRADE Guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–394 [DOI] [PubMed] [Google Scholar]
- 27. Brignardello-Petersen R, El Alayli A, Husainat N, Kalot M, Shahid S, Aljabirii Yet al. Surgical management of patients with von Willebrand disease: summary of 2 systematic reviews of the literature. Blood Adv 2022;6:121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zinman B, Aroda VR, Buse JB, Cariou B, Harris SB, Hoff STet al. Efficacy, safety, and tolerability of oral semaglutide versus placebo added to insulin with or without metformin in patients with type 2 diabetes: the PIONEER 8 trial. Diabetes Care 2019;42:2262–2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aroda VR, Rosenstock J, Terauchi Y, Altuntas Y, Lalic NM, Morales Villegas ECet al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care 2019;42:1724–1732 [DOI] [PubMed] [Google Scholar]
- 30. Pieber TR, Bode B, Mertens A, Cho YM, Christiansen E, Hertz CLet al. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open-label, randomised, phase 3a trial. Lancet Diabetes Endocrinol 2019;7:528–539 [DOI] [PubMed] [Google Scholar]
- 31. Rosenstock J, Allison D, Birkenfeld AL, Blicher TM, Deenadayalan S, Jacobsen JBet al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 randomized clinical trial. JAMA 2019;321:1466–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rodbard HW, Rosenstock J, Canani LH, Deerochanawong C, Gumprecht J, Lindberg SOet al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care 2019;42:2272–2281 [DOI] [PubMed] [Google Scholar]
- 33. Davies M, Pieber TR, Hartoft-Nielsen ML, Hansen OKH, Jabbour S, Rosenstock J. Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes: a randomized clinical trial. JAMA 2017;318:1460–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Min SH, Yoon JH, Hahn S, Cho YM. Comparison between SGLT2 inhibitors and DPP4 inhibitors added to insulin therapy in type 2 diabetes: a systematic review with indirect comparison meta-analysis. Diabetes Metab Res Rev 2017;33. doi: 10.1002/dmrr.2818 [Epub 2016 Jun 8] [DOI] [PubMed] [Google Scholar]
- 35. Inoue H, Tamaki Y, Kashihara Y, Muraki S, Kakara M, Hirota Tet al. Efficacy of DPP-4 inhibitors, GLP-1 analogues, and SGLT2 inhibitors as add-ons to metformin monotherapy in T2DM patients: a model-based meta-analysis. Br J Clin Pharmacol 2019;85:393–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shi Q, Wang Y, Hao Q, Vandvik PO, Guyatt G, Li Jet al. Pharmacotherapy for adults with overweight and obesity: a systematic review and network meta-analysis of randomised controlled trials. Lancet 2022;399:259–269 [DOI] [PubMed] [Google Scholar]
- 37. Latini R, Staszewsky L. Semaglutide and effective weight control. Lancet 2021;397:942–943 [DOI] [PubMed] [Google Scholar]
- 38. Lingvay I, Hansen T, Macura S, Marre M, Nauck MA, de la Rosa Ret al. Superior weight loss with once-weekly semaglutide versus other glucagon-like peptide-1 receptor agonists is independent of gastrointestinal adverse events. BMJ Open Diabetes Res Care 2020;8:e001706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gabery S, Salinas CG, Paulsen SJ, Ahnfelt-Ronne J, Alanentalo T, Baquero AFet al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight 2020;5:e133429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ni L, Yuan C, Chen G, Zhang C, Wu X. SGLT2i: beyond the glucose-lowering effect. Cardiovasc Diabetol 2020;19:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arnott C, Li Q, Kang A, Neuen BL, Bompoint S, Lam CSPet al. Sodium–glucose cotransporter 2 inhibition for the prevention of cardiovascular events in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J Am Heart Assoc 2020;9:e014908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss Det al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. The Lancet Diabetes & Endocrinology 2019;7:776–785 [DOI] [PubMed] [Google Scholar]
- 43. Cheng D, Fei Y, Liu Y, Li J, Chen Y, Wang Xet al. Efficacy and safety of dipeptidyl peptidase-4 inhibitors in type 2 diabetes mellitus patients with moderate to severe renal impairment: a systematic review and meta-analysis. PLoS One 2014;9:e111543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sinha B, Ghosal S. Meta-analyses of the effects of DPP-4 inhibitors, SGLT2 inhibitors and GLP1 receptor analogues on cardiovascular death, myocardial infarction, stroke and hospitalization for heart failure. Diabetes Res Clin Pract 2019;150:8–16 [DOI] [PubMed] [Google Scholar]
- 45. Robertson AGN, Wiggins T, Robertson FP, Huppler L, Doleman B, Harrison EMet al. Perioperative mortality in bariatric surgery: meta-analysis. Br J Surg 2021;108:892–897 [DOI] [PubMed] [Google Scholar]
- 46. Cardoso L, Rodrigues D, Gomes L, Carrilho F. Short- and long-term mortality after bariatric surgery: a systematic review and meta-analysis. Diabetes Obes Metab 2017;19:1223–1232 [DOI] [PubMed] [Google Scholar]
- 47. Syn NL, Cummings DE, Wang LZ, Lin DJ, Zhao JJ, Loh Met al. Association of metabolic–bariatric surgery with long-term survival in adults with and without diabetes: a one-stage meta-analysis of matched cohort and prospective controlled studies with 174772 participants. Lancet 2021;397:1830–1841 [DOI] [PubMed] [Google Scholar]
- 48. Palmer SC, Tendal B, Mustafa RA, Vandvik PO, Li S, Hao Qet al. Sodium–glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ 2021;372:m4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gallwitz B, Giorgino F. Clinical perspectives on the use of subcutaneous and oral formulations of semaglutide. Front Endocrinol (Lausanne) 2021;12:645507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hansen BB, Nuhoho S, Ali SN, Dang-Tan T, Valentine WJ, Malkin SJPet al. Oral semaglutide versus injectable glucagon-like peptide-1 receptor agonists: a cost of control analysis. J Med Econ 2020;23:650–658 [DOI] [PubMed] [Google Scholar]
- 51. Chubb B, Gupta P, Gupta J, Nuhoho S, Kallenbach K, Orme M. Once-daily oral semaglutide versus injectable GLP-1 RAs in people with type 2 diabetes inadequately controlled on basal insulin: systematic review and network meta-analysis. Diabetes Ther 2021;12:1325–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Courcoulas AP, Gallagher JW, Neiberg RH, Eagleton EB, DeLany JP, Lang Wet al. Bariatric surgery vs. lifestyle intervention for diabetes treatment: five year outcomes from a randomized trial. J Clin Endocrinol Metabol 2020;105:866–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni Get al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015;386:964–973 [DOI] [PubMed] [Google Scholar]
- 54. Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SAet al. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med 2017;376:641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Parikh M, Chung M, Sheth S, McMacken M, Zahra T, Saunders JKet al. Randomized pilot trial of bariatric surgery versus intensive medical weight management on diabetes remission in type 2 diabetic patients who do NOT meet NIH criteria for surgery and the role of soluble RAGE as a novel biomarker of success. Ann Surg 2014;260:617–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Azevedo FR, Santoro S, Correa-Giannella ML, Toyoshima MT, Giannella-Neto D, Calderaro Det al. A prospective randomized controlled trial of the metabolic effects of sleeve gastrectomy with transit bipartition. Obes Surg 2018;28:3012–3019 [DOI] [PubMed] [Google Scholar]
- 57. Courcoulas AP, Goodpaster BH, Eagleton JK, Belle SH, Kalarchian MA, Lang Wet al. Surgical vs medical treatments for type 2 diabetes mellitus: a randomized clinical trial. JAMA Surg 2014;149:707–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Courcoulas AP, Belle SH, Neiberg RH, Pierson SK, Eagleton JK, Kalarchian MAet al. Three-year outcomes of bariatric surgery vs lifestyle intervention for type 2 diabetes mellitus treatment: a randomized clinical trial. JAMA Surg 2015;150:931–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi Let al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366:1577–1585 [DOI] [PubMed] [Google Scholar]
- 60. Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SDet al. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med 2014;370:2002–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CEet al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366:1567–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Panosian J, Ding SA, Wewalka M, Simonson DC, Goebel-Fabbri A, Foster Ket al. Physical activity in obese type 2 diabetes after gastric bypass or medical management. Am J Med 2017;130:83–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chang SH, Freeman NLB, Lee JA, Stoll CRT, Calhoun AJ, Eagon JCet al. Early major complications after bariatric surgery in the USA, 2003–2014: a systematic review and meta-analysis. Obes Rev 2018;19:529–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ueda P, Svanstrom H, Melbye M, Eliasson B, Svensson AM, Franzen Set al. Sodium glucose cotransporter 2 inhibitors and risk of serious adverse events: nationwide register based cohort study. BMJ 2018;363:k4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in published articles listed in Table S1 and Appendix 2. This paper is not based on a previous communication to a society or meeting.