Summary
Background
Microsatellite stable (MSS) and RAS-mutant metastatic colorectal cancer (mCRC) patients are characterized by an immunosuppressive microenvironment and a low response rate to immunotherapy. Chemotherapy and anti-angiogenesis therapy have been reported to potentially promote immunotherapy response. This study aims to assess the preliminary anti-tumor activity and safety of sintilimab plus bevacizumab, oxaliplatin and capecitabine as a treatment option for patients with RAS-mutant MSS mCRC.
Methods
This study was an open-label, single-arm, phase II trial in China. Patients with unresectable, RAS-mutant and MSS metastatic colorectal adenocarcinoma received treatment by intravenous sintilimab (200 mg, day 1) plus bevacizumab (7.5 mg/kg, day 1), oxaliplatin (135 mg/m2, day 1) and oral capecitabine (1 g/m2, day 1–14) in each 21-day cycle. The primary endpoints included objective response rate (ORR) and adverse events. Biomarker analysis was performed to identify potential predictors of good response to treatment. This study is registered with ClinicalTrials.gov, number NCT04194359.
Findings
Between April 2021 and December 2021, 25 patients were enrolled. Two (8%) patients showed complete response (CR), 19 (76%) had partial response (PR) and 4 (16%) presented with stable disease. ORR reached 84% (95% CI, 63.9–95.5) and the disease control rate was 100% (95% CI, 86.3–100). The median progression-free survival (PFS) was 18.2 months for the full analysis set. The most common treatment-related adverse events (TRAEs) in all grades were anemia (21/25, 84%), neutropenia (20/25, 80%), and hand-foot syndrome (14/25, 56%). The most frequent grade 3 or 4 TRAEs were neutropenia (3/25, 12%) and increased alanine transaminase (2/25, 8%). No grade 5 adverse events occurred. In the exploration of biomarkers, 5 patients could be characterized as TTN/OBSCN “double-hit” after treatment, and the copy number variants burden was significantly decreased in tumor tissues after treatment compared with the baseline. Nanostring panel RNA sequencing analysis indicated a better tumor immune microenvironment cell infiltration in CR/PR patients compared with non-CR/PR patients as well as the PFS-long (≥12.5 months) group compared with the PFS-short group.
Interpretation
Combination treatment with sintilimab plus bevacizumab, oxaliplatin and capecitabine as first-line treatment demonstrated a promising antitumor activity and a manageable safety profile in RAS-mutant, MSS and unresectable mCRC. Exploratory biomarker assessment analysis showed that some RAS-mutant and MSS patients changed into “immune-hot” subtype after the treatment.
Funding
This study was supported by the Key R&D Program of Zhejiang Province (2021C03125 to Ying Yuan), the National Natural Science Foundation of China (81872481 to Ying Yuan, 82072624 to Kefeng Ding), the Fundamental Research Funds for the Central Universities (No. 226-2022-00009 to Kefeng Ding), and the Zhejiang Provincial Natural Science Foundation of China (No. LY22H160024 to Hanguang Hu).
Keywords: Microsatellite stable, RAS mutation, Metastatic colorectal cancer, Immune checkpoint inhibitors, PD-1
Research in context.
Evidence before this study
FOLFOX/FOLFIRI plus bevacizumab are the first-line option for patients with RAS-mutant, microsatellite stable, unresectable metastatic colorectal cancer. Immune checkpoint inhibitors are largely ineffective in microsatellite stable metastatic colorectal cancer. Preclinical researches suggested the combination of immunotherapy, standard first-line chemotherapy and antiangiogenic therapy have a synergistic effect to break the treatment predicament in those patients. We searched PubMed, with no language restrictions, for studies published between April 1, 2016, and April 1, 2023, on immune checkpoint inhibitors for unresectable metastatic colorectal cancer with RAS-mutant and microsatellite stable status, using the search terms (“RAS mutation” AND “microsatellite stable”) AND (“unresectable” OR “metastatic”) AND (“colorectal cancer” OR “colon cancer” OR “rectal cancer”) AND (“immunotherapy” OR “PD-1 blockade” OR “anti-PD-1” OR “immune checkpoint inhibitor”) AND (“antiangiogenesis” OR “bevacizumab”). We found report from a phase II NIVACOR trials evaluate the efficacy of the association between immune checkpoint agent (nivolumab) with a triplet chemotherapy (FOLFOXIRI) and anti-VEGF inhibitor (bevacizumab) in metastatic colorectal cancer RAS/BRAF mutated, regardless of microsatellite status.
Added value of this study
This open-label phase II study showed that sintilimab plus bevacizumab, oxaliplatin and capecitabine as first-line combination treatment demonstrated a promising antitumor activity and a manageable safety profile in RAS-mutant, microsatellite stable, unresectable metastatic colorectal cancer. And this treatment regime exerts certain advantages in both ORR and median PFS in RAS-mutant and MSS mCRC compared to similar studies.
Implications of all the available evidence
Our study provides further evidence of the clinical activity of immune checkpoint inhibitors combined with antiangiogenic therapy and chemotherapy as first-line combination treatment in RAS-mutant, microsatellite stable, unresectable metastatic colorectal cancer. Exploratory biomarker assessment analysis showed that some RAS-mutant and MSS patients changed into “immune-hot” subtype after the treatment.
Introduction
In to the Global Cancer Statistics 2020, colorectal cancer (CRC) remains the second most common cause of cancer-related mortality in the world.1 According to the latest Chinese Cancer Report, CRC was the fourth leading cause of cancer-related deaths in China.2 More specifically, both the incidence and mortality of CRC show a trend of growing year by year.2 Among people newly diagnosed with CRC, 20% have distant metastases.3,4 A poor 5-year survival rate of less than 20% was observed in metastatic colorectal cancer (mCRC).3,4
The standard of therapy for mCRC patients constitutes chemotherapy regimens based on fluorouracil, oxaliplatin and/or irinotecan combined with agents targeting angiogenesis (bevacizumab) or the epidermal growth factor receptor (cetuximab) based on the RAS and BRAF status.5,6 The proportion of RAS gene mutation in mCRC reaches 50–56%.7, 8, 9 In mCRC patients with RAS mutation, a bevacizumab regimen combined with chemotherapy is the standard first-line treatment.5,6 However, compared with the RAS wild-type mCRC, patients with RAS mutation have a poor prognosis and short survival time.10 It has also been shown that RAS mutant mCRC has an immunosuppressive microenvironment.11 Thus, improving the effectiveness of its first-line treatment has become a current treatment challenge.
In recent years, immune checkpoint inhibitors have pioneered new treatment regimens and dramatically improved the prognosis due to their long-term durable responses in several solid cancers including malignant melanoma and lung cancer. However, immune checkpoint inhibitors are largely ineffective in microsatellite stable (MSS) mCRC, and only a small set of mCRC with microsatellite instability-high (MSI-H) status shows effective responses.12,13 This may be related to the low expression of tumor-specific antigens, antigen presentation defect and overactivation of intrinsic immunosuppressive pathways in MSS mCRC cases.14,15 Remarkably, MSS patients comprise the majority (about 95%) of mCRC.12,16 Thus, new approaches to improve the response of immunotherapy are required for this subtype.
The interaction between the upregulation of angiogenic signaling pathways and tumor immune suppression has been confirmed.17,18 Vascular endothelial growth factor (VEGF) reduced tumor-antigen presentation by suppressing the maturation of dendritic cells and upregulating the expression of programmed death-ligand 1 (PD-L1) on dendritic cells to suppress the function of CD8+ T cells.18,19 The upregulation of VEGF/vascular endothelial growth factor receptor (VEGFR) pathway also enhances the proliferation of regulatory T cells and induces the imbalance of M1 and M2 macrophages.18,20 Antiangiogenic therapy can reverse the immunosuppressive tumor microenvironment by normalizing blood vessels and secondarily induce T-cell infiltration and activation to promote immunotherapy in solid cancers including CRC.21
Preclinical research revealed that oxaliplatin could induce CT26 (murine colon cancer cell with MSS status and KRAS G12D mutation) immunogenic tumor cell death and increase CD8+ T cell infiltration at the tumor site.22, 23, 24 In vivo, 5-fluorouracil could eliminate secondarily enhanced T cell-dependent antitumor responses by myeloid-derived suppressor cells through increasing IFN-γ production by tumor-specific CD8+ T cells infiltrating the tumor.25 These data provide the rationale that chemotherapy could induce immunogenicity in tumors with an additive or synergistic benefit when combined with immune checkpoint blockers.26
Herein, we report the efficacy, safety and biomarker results of the phase II study investigating the combination of sintilimab plus bevacizumab, oxaliplatin and capecitabine as first-line therapy in patients with RAS-mutant, MSS, unresectable mCRC.
Methods
Study design and participants
The present study is an open-label, single-arm, phase II trial performed at the Second Affiliated Hospital of Zhejiang University School of Medicine in China to assess the antitumor activity and safety of sintilimab plus bevacizumab, oxaliplatin and capecitabine as first-line treatment in mCRC patients with RAS-mutant, MSS status. This study is registered with ClinicalTrials.gov, number NCT04194359. Eligible patients were aged 18–75 years with histologically confirmed metastatic colorectal adenocarcinoma and unresectable mCRC confirmed by a multidisciplinary team (MDT). They presented RAS mutation and BRAF wild-type status by gene sequencing and MSS, as confirmed by polymerase chain reaction (PCR) using a panel of six mononucleotide repeat markers (BAT-25, BAT-26, NR-21, NR-24, NR-27, and MONO-27). Inclusion and exclusion criteria are available in Supplementary Methods. The manuscript adheres to CONSORT reporting guidelines.
Procedures
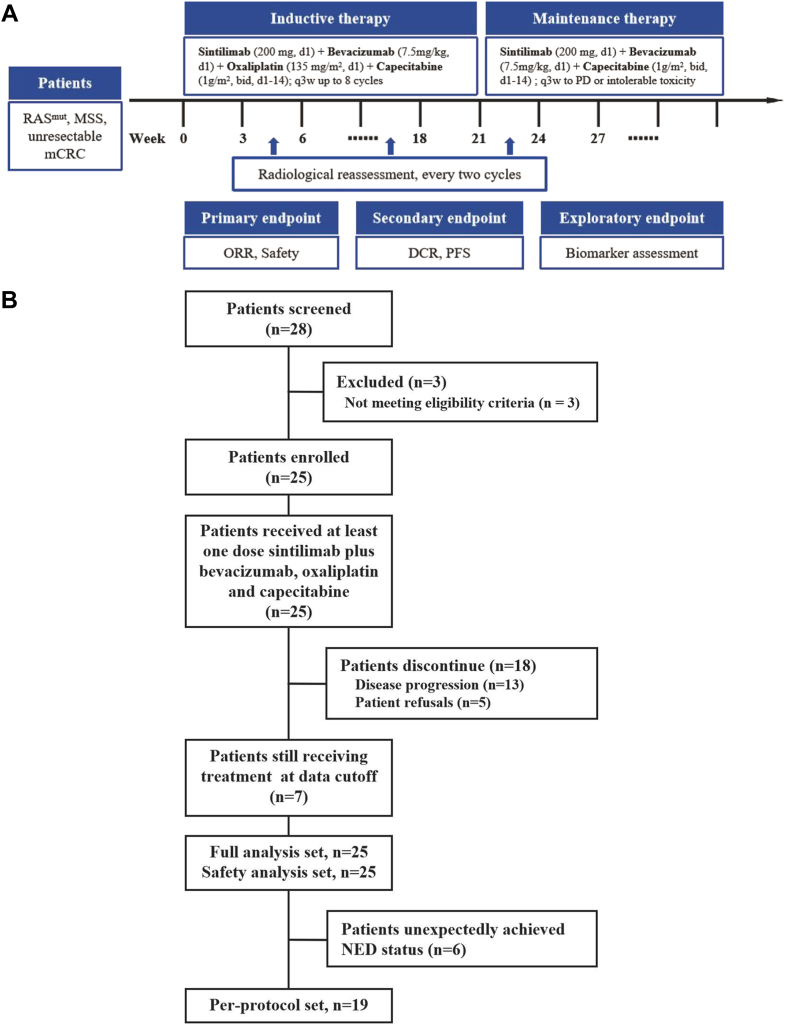
As shown in Fig. 1A, eligible patients received treatment with intravenous sintilimab (200 mg, day 1), bevacizumab (7.5 mg/kg, day 1), oxaliplatin (135 mg/m2, day 1) and oral capecitabine (1 g/m2, day 1–14) in each 21-day cycle. Up to 8 courses of inductive therapy would be given. Patients with objective response or stable disease (SD) would continue to receive sintilimab (200 mg, day 1) plus bevacizumab (7.5 mg/kg, day 1) and oral capecitabine (1 g/m2, day 1–14) in each 21-day cycle as maintenance therapy until the confirmation of disease progression, death, unacceptable toxicity, or withdrawal of consent. We assessed response to treatment every 2 cycles (6 weeks) using CT or MRI based on Response Evaluation Criteria in Solid Tumors (RECIST) (version 1.1) criteria. Adverse events were monitored and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0).
Fig. 1.
(A) Flowchart of therapeutic regimen. (B) Flow diagram of participants in the study.
Outcomes
The primary endpoints included objective response rate (ORR) according to RECIST version 1.1 and safety (adverse events and serious adverse events), and the secondary endpoints were disease control rate (DCR) and progression-free survival (PFS). ORR was defined as the proportion of patients with a best objective response of complete response (CR) or partial response (PR) according to RECIST criteria (version 1.1). DCR was defined as the proportion of patients with CR, PR, or SD according to RECIST criteria (version 1.1), while PFS was defined as the time from enrollment to the first documented disease progression according to RECIST version 1.1, or to death from any cause, whichever occurred first.
Biomarker analysis
Baseline and/or post-treatment biopsy or operative specimens were obtained as required from patients for exploratory biomarker assessment. PD-L1 expression level, whole exome sequencing (WES), Nanostring panel RNA sequencing, Cell infiltration analysis, tumor immune microenvironment (TIME) signature analysis, Gene set enrichment and pathway analysis are available in Supplementary Methods.
Statistical analysis
According to previous studies, the ORR of doublet chemotherapy plus bevacizumab as the first line of treatment is approximately 55% (H0 = 55%) in CRC.27, 28, 29, 30 We expected that the regimen of sintilimab plus bevacizumab, oxaliplatin and capecitabine could increase the ORR from 55% to 80% (H1 = 80%), and a sample size of 22 patients would provide at least 81% power to detect this estimated improvement at a one-sided α level of 5%. Considering an approximate drop-out incidence of 10%, a total sample size of 25 patients was planned for this study.
The full analysis set (FAS) and safety analysis set (SAS), both of which comprised all eligible patients who received at least one dose of sintilimab plus bevacizumab, oxaliplatin and capecitabine, were used to conduct the efficacy and safety assessment, respectively. Patients that underwent surgical treatment were excluded from the per-protocol set (PPS). We used the Kaplan–Meier method for estimating PFS.
Statistical analyses were conducted using SAS software (V9.4, SAS Institute, Cary, NC, USA) and R (V4.0, R Foundation for Statistical Computing, https://www.R-project.org/). The log-rank test was used as primary analysis for comparison between metastasis subgroups. Differences between efficacy response subgroups were analyzed using the (nonparametric) Wilcoxon’ s rank-sum test (Mann–Whitney U-test), whereas differences between pre-treatment and post-treatment specimens were analyzed using Wilcoxon’ s signed-rank test. 95% confidence intervals (CIs) for the response rate were calculated using the Clopper-Pearson method. All reported P values are two sided and a P value < 0.05 were considered statistically significant.
Ethics statement
The trial was conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice, the Declaration of Helsinki, and applicable local regulations. The protocol was reviewed and approved by the Ethics Committee of the Second Affiliated Hospital of Zhejiang University School of Medicine (Approval Number: 2020-552), and all patients provided written informed consent before study enrolment.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors verified that this study was done according to the protocol and was attested for data accuracy and completeness. All authors had full access to all of the data in the study and accepted responsibility for the decision to submit the final manuscript for publication.
Results
Patients
This study enrolled 25 eligible patients between April 2021 and December 2021. A total of 25 patients were included in FAS and SAS (Fig. 1B). All patients were treated with at least one dose of protocol-specified treatment. Seven patients were still being treated with maintenance therapy at the data cut-off day (November 30, 2022), and the median follow-up duration was 16.5 months (ranging from 6.6 to 22.1 months, interquartile range (IQR) 14.7–18.7). Six (24%) patients underwent surgical treatment and unexpectedly achieved no evidence of disease (NED) status. Thus, 19 patients were included in PPS. One patient underwent palliative surgery of metastases after disease progression. One patient reached clinical CR (cCR) after treatment of sintilimab plus bevacizumab, oxaliplatin and capecitabine.
The baseline and disease characteristics of the enrolled patients are shown in Table 1. At baseline, the median age was 60 years (range 45–74, IQR 56–65). Most patients were male (18/25, 72%). The primary tumor site was right-sided colon in 36% (9/25) and left-sided colon and rectum in 64% (16/25) of patients. The Eastern Cooperative Oncology Group (ECOG) status was 0/1 in 100% of cases. The most frequent types of metastases were liver (15 patients, 60%), lymph node (10 patients, 40%), and lung (7 patients, 28%). KRAS codon 12 mutation and KRAS codon 13 mutation were detected in 56% (14/25) and 20% (5/25) patients, respectively. In a total of 20 patients with PD-L1 immunohistochemistry (IHC) results, the combined positive score (CPS) of 3 patients was >1. Among the 17 patients with tumor mutation burden (TMB) results, a median TMB of 5.23 mut/Mb (IQR 3.56–10.03) and TMB >10 mut/Mb was observed in 5 patients.
Table 1.
Baseline demographic and clinical characteristics.
| Characteristics | Patients (n = 25) |
|---|---|
| Age, years, median (IQR), n (%) | 60 (56–65) |
| <60 | 10 (40%) |
| ≥60 | 15 (60%) |
| Sex, n (%) | |
| Male | 18 (72%) |
| Female | 7 (28%) |
| ECOG performance status, n (%) | |
| 0 | 7 (28%) |
| 1 | 18 (72%) |
| Primary tumor location, n (%) | |
| Left colon and rectum | 16 (64%) |
| Right colon | 9 (36%) |
| Number of metastatic organs, n (%) | |
| 1 | 16 (64%) |
| ≥2 | 9 (36%) |
| Metastatic organa, n (%) | |
| Liver | 15 (60%) |
| Lung | 7 (28%) |
| Lymph node | 10 (40%) |
| Other | 3 (12%) |
| RAS mutation type, n (%) | |
| KRAS codon 12 | 14 (56%) |
| KRAS codon 13 | 5 (20%) |
| NRAS | 2 (8%) |
| Others | 4 (16%) |
| PD-L1 expression, CPS, n (%) | |
| CPS ≤1 | 17 (68%) |
| CPS >1 | 3 (12%) |
| Unknown | 5 (20%) |
| TMB (mut/Mb), median (IQR), n (%) | 5.23 (3.56–10.03) |
| TMB <5 | 8 (32%) |
| TMB ≥5, ≤10 | 4 (16%) |
| TMB >10 | 5 (20%) |
| Unknown | 8 (32%) |
Abbreviations: CPS, combined positive score; ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range; TMB, tumor mutation burden.
Multiple answers allowed.
Efficacy
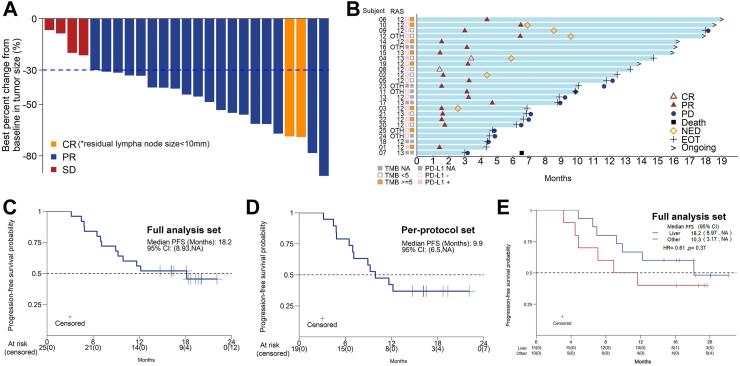
Among the 25 patients who were eligible and assessable for efficacy in the FAS, an objective response was recorded in 21 (84%) based on RECIST version 1.1, while the DCR was 100%. Patients with liver metastases (N = 15) presented a higher ORR (14/15, 93%) compared to the overall ORR (84%). Two (8%) patients showed cCR, including one confirmed to be pathological CR after surgical resection. Nineteen patients (76%) had PR and 4 patients (16%) had SD (Table 2). All 25 patients showed tumor shrinkage, with the best percent change in the target lesion diameter from baseline shown in Fig. 2A. The median PFS in the FAS according to Kaplan–Meier estimations was 18.2 months (95% CI, 8.93-NA) (Fig. 2C), and the median PFS in the PPS was 9.9 months (95% CI, 6.5-NA) (Fig. 2D). Median PFS was 18.2 months (95% CI 6.97–NA) in the patients with liver metastasis and 10.3 months (3.17–NA) in the patients with non-liver metastasis (HR 0.61, log-rank test P = 0.37, Fig. 2E). Seven patients were still receiving maintenance therapy at the data cut-off day (Fig. 2B). Of the 25 patients, 6 underwent surgical treatment and achieved NED status under the guidance of MDT, including 5 with unexpected conversion of liver metastases and 1 with retroperitoneal lymph node metastases (Supplemental Figure S1). One patient underwent palliative surgery of metastases after disease progression.
Table 2.
Efficacy outcomes.
| All patients (N = 25) | Liver metastasis (N = 15) | Other metastasis (N = 10) | |
|---|---|---|---|
| Best overall response | |||
| Complete response (CR), n (%) | 2 (8%) | 0 | 2 (20%) |
| Partial response (PR), n (%) | 19 (76%) | 14 (93%) | 5 (50%) |
| Stable disease (SD), n (%) | 4 (16%) | 1 (7%) | 3 (30%) |
| Progressive disease (PD), n (%) | 0 | 0 | 0 |
| ORR, n (%, 95% CI) | 21 (84%, 63.9–95.5%) | 14 (93.3%, 68.1–99.8%) | 7 (70%, 34.8–93.3%) |
| DCR, n (%, 95% CI) | 25 (100%, 86.3–100%) | 15 (100%, 78.2–100%) | 10 (100%, 69.2–100%) |
Abbreviations: ORR, objective response rate; DCR, disease control rate.
Fig. 2.
(A) Waterfall plot of the best percent change in target lesion diameter from baseline (full analysis set, n = 25). (B) Swimmer plots of patients. (C) Kaplan–Meier curves of PFS for the full analysis set (n = 25). (D) Kaplan–Meier curves of PFS for the per-protocol set (n = 19). (E) Kaplan–Meier curves of PFS classified by metastatic organs for the full analysis set.
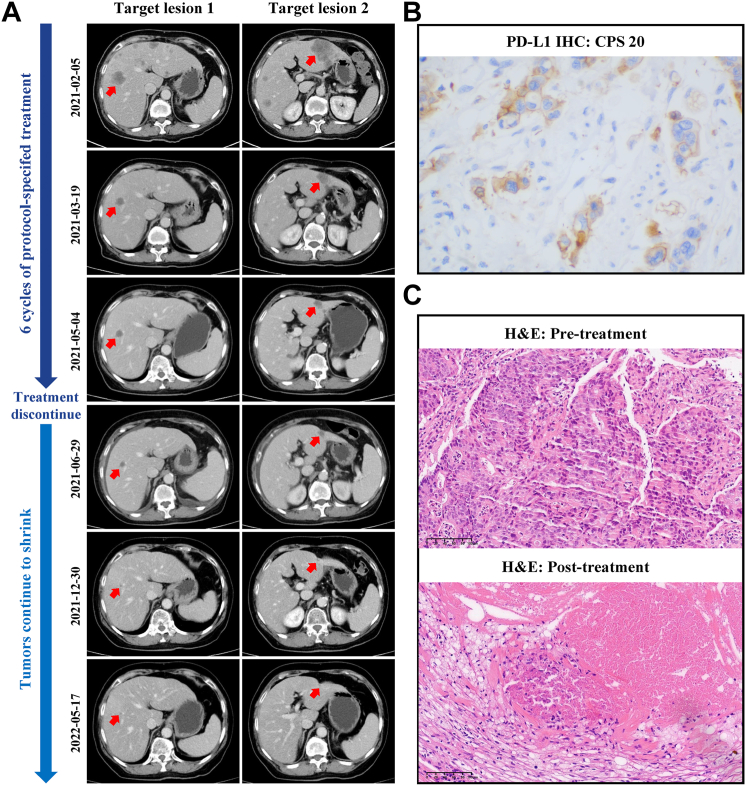
Moreover, patient No.1 discontinued treatment after receiving 6 cycles of protocol-specified treatment. However, until the latest follow-up date, target lesions of liver metastases continued to shrink (Fig. 3A). Notably, the CPS of PD-L1 of pretreatment specimens of this patient was 20 (Fig. 3B). As shown in Fig. 3C, the H&E staining of post-treatment specimen of patient No.4 achieved pathological CR.
Fig. 3.
Radiological and pathological response to protocol-specified treatment in representative patients. (A) Radiological response from patient No.1. (B) Representative PD-L1 IHC staining of pretreatment specimens of patient No.1. (C) H&E staining of post-treatment specimen of patient No.4 showing pathological CR compared to pre-treatment.
Safety
All twenty-five patients received at least one dose of protocol-specified treatment and were evaluated for safety. Treatment was generally well tolerated. The treatment-related adverse events (TRAEs) and immune-related adverse events were summarized in Table 3. At the data cut-off date (November 30, 2022), the most common TRAEs in all grades were anemia (21/25, 84%), neutropenia (20/25, 80%), nausea (14/25, 56%), hand-foot syndrome (14/25, 56%), leukocytopenia (14/25, 56%), and hypertension (14/25, 56%). The most frequent grade 3 or 4 TRAEs were neutropenia (3/25, 12%) and increased alanine transaminase (2/25, 8%). No grade 5 adverse events occurred during the study.
Table 3.
Treatment-emergent adverse events (TEAEs) since the initiation of protocol-specified treatment.
| TEAEs, n (%) | Patients (N = 25) |
||||
|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Any grade | |
| Anemia | 19 (76%) | 2 (8%) | 0 | 0 | 21 (84%) |
| Neutropenia | 16 (64%) | 1 (4%) | 2 (8%) | 1 (4%) | 20 (80%) |
| Nausea | 11 (44%) | 3 (12%) | 0 | 0 | 14 (56%) |
| Hand-foot syndrome | 8 (32%) | 6 (24%) | 0 | 0 | 14 (56%) |
| Leukocytopenia | 8 (32%) | 6 (24%) | 0 | 0 | 14 (56%) |
| Aspartate transaminase increased | 7 (28%) | 5 (20%) | 1 (4%) | 0 | 13 (52%) |
| Lipase increased | 12 (48%) | 0 | 0 | 0 | 12 (48%) |
| Proteinuria | 10 (40%) | 0 | 0 | 0 | 10 (40%) |
| Thrombocytopenia | 7 (28%) | 3 (12%) | 0 | 0 | 10 (40%) |
| Vomiting | 9 (36%) | 0 | 0 | 0 | 9 (36%) |
| Hypothyroidism | 7 (28%) | 0 | 0 | 0 | 7 (28%) |
| Triglycerides increased | 6 (24%) | 1 (4%) | 0 | 0 | 7 (28%) |
| Fatigue | 6 (24%) | 1 (4%) | 0 | 0 | 7 (28%) |
| Blood bilirubin increased | 3 (12%) | 3 (12%) | 1 (4%) | 0 | 7 (28%) |
| Alanine transaminase increased | 3 (12%) | 2 (8%) | 2 (8%) | 0 | 7 (28%) |
| Peripheral neurotoxicity | 13 (52%) | 0 | 0 | 0 | 13 (52%) |
| Hoarseness | 5 (20%) | 0 | 0 | 0 | 5 (20%) |
| Rash | 2 (8%) | 1 (4%) | 1 (4%) | 0 | 4 (16%) |
| Thyroiditis | 3 (12%) | 0 | 0 | 0 | 3 (12%) |
| Diarrhea | 2 (8%) | 0 | 0 | 0 | 2 (8%) |
| Troponin increased | 2 (8%) | 0 | 0 | 0 | 2 (8%) |
| Fever | 0 | 2 (8%) | 0 | 0 | 2 (8%) |
| Alkaline phosphatase increased | 1 (4%) | 0 | 0 | 0 | 1 (4%) |
| Amylase increased | 0 | 1 (4%) | 0 | 0 | 1 (4%) |
| Hypertension | 12 (48%) | 2 (8%) | 0 | 0 | 14 (56%) |
Biomarkers
Our small-sample analysis did not identify any significant differences in the biomarkers of PD-L1 expression, RAS mutation type and TMB between the CR/PR and non-CR/PR patients.
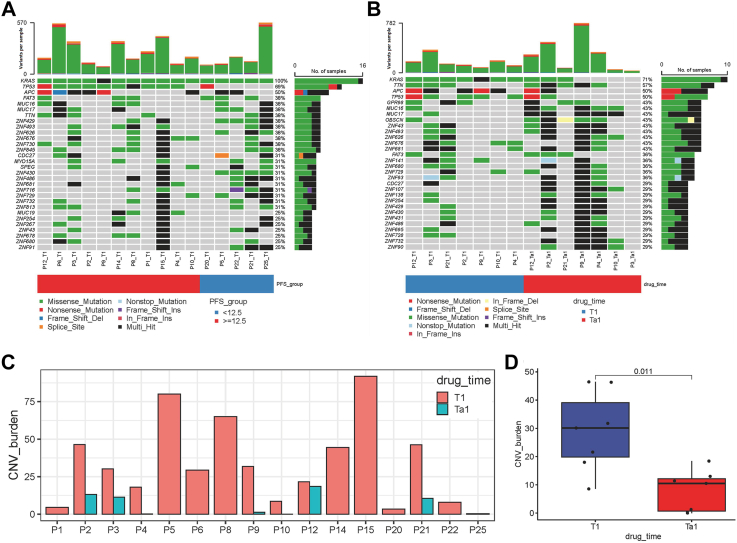
WES was carried out in 64% (16/25) of patients in this study. As shown in Fig. 4A, the most frequently altered genes at baseline (T1) were KRAS, TP53 and APC. In addition, TP53, ZNF430, ATM, and ZNF429 had significant differences in the frequency of genomic alterations between the PFS <12.5 and ≥ 12.5 months groups (Fig. 4A). When comparing the genomic alterations of 7 patients who underwent surgical treatment in the baseline (T1) with those after treatment (T1a), KRAS mutations were not detected in 4 patients, and 5 patients had TTN/OBSCN “double-hit” in tumor tissues after treatment (Fig. 4B). Interestingly, the CNV burden was significantly decreased in tumor tissues after treatment (T1a) compared with the baseline (T1) (Fig. 4C and D).
Fig. 4.
WES analysis. (A) Overall frequency of top 30 gene alterations at baseline (T1) between the PFS <12.5 (N = 5) and ≥12.5 months (N = 11) groups. (B) Frequency of genomic alterations of 7 patients underwent surgical treatment between the baseline (T1) and after treatment (T1a). (C–D) CNV burden of patients (N = 7) between the baseline (T1) and after treatment (T1a). Each point in the boxplot represents for one sample. Whiskers show the minimum and maximum of all the data. The horizontal line in the box represents the median, and the top and bottom of each box indicate the 25th and 75th percentile. The significance for differences between T1 and T1a was tested using Wilcoxon’ s rank-sum test. Statistical tests were two sided.
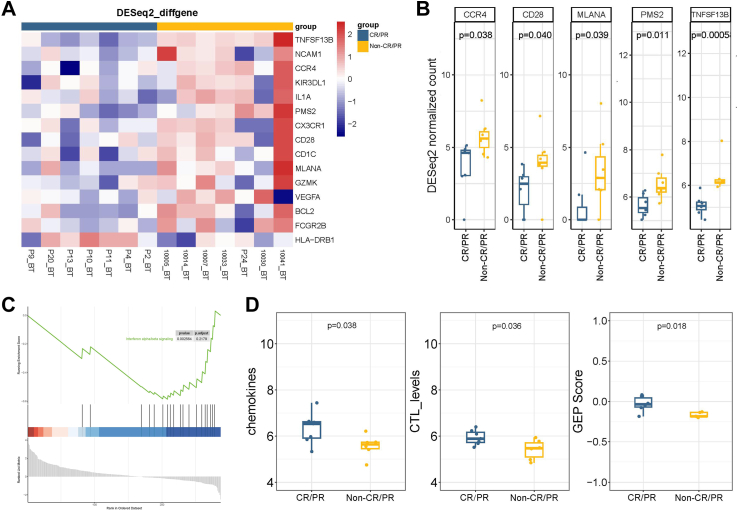
The transcriptional profiling of baseline tissue samples was performed to compare CR/PR and non-CR/PR patients to find possible pretreatment biomarkers that can be predictive of the therapy of sintilimab plus bevacizumab, oxaliplatin and capecitabine. When comparing CR/PR patients, biopsies in non-CR/PR patients revealed a higher transcriptional level of CCR4, CD28, MLANA, PMS2 and TNFSF13B (Fig. 5A and B). Gene set enrichment analysis (GSEA) was conducted to identify the biological function of the changed genes between the two groups, which revealed that genes were significantly enriched in “interferon alpha/beta signaling” (Fig. 5C). Meanwhile, different TIME cell infiltration scores of the baseline tumor specimen were analyzed. The scores of chemokines, CTL levels and gene expression profiling (GEP) were significantly higher in CR/PR patients (Fig. 5D). These results indicated that there was a better TIME cell infiltration in CR/PR patients compared with non-CR/PR patients, which needs to be confirmed by further research.
Fig. 5.
Comparison of expression of immune-related genes and cell type scores between CR/PR (N = 7) and non-CR/PR patients (N = 7). (A–B) Expression of immune-related genes per pretreatment sample (BT) of response and non-response groups. (C) GSEA pathway enrichment analysis of different expressed genes between CR/PR and non-CR/PR patients. (D) Different cell type scores in per pretreatment sample of CR/PR and non-CR/PR patients. Each point in the boxplot represents for one sample. Whiskers show the minimum and maximum of all the data. The horizontal line in the box represents the median, and the top and bottom of each box indicate the 25th and 75th percentile. The significance for differences between CR/PR and non-CR/PR patients was tested using Wilcoxon’ s rank-sum test. Statistical tests were two sided.
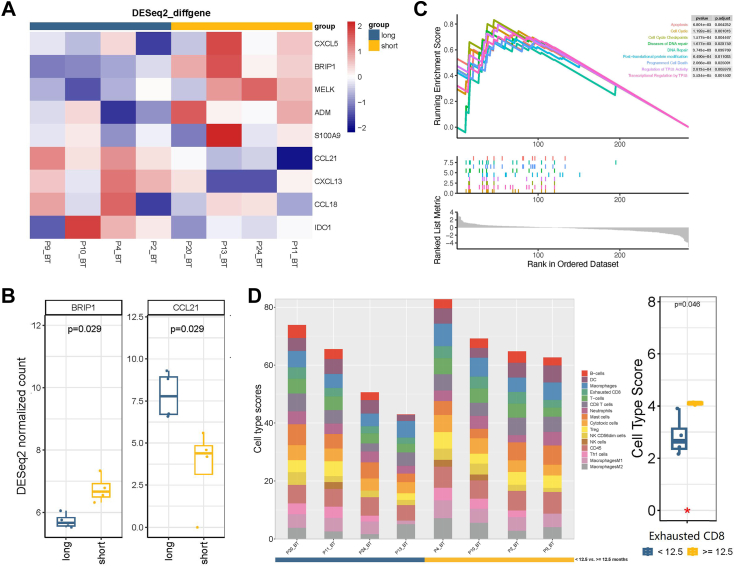
Transcriptional profiling results analysis and GSEA pathway enrichment analysis of baseline tissue samples were also performed between PFS-short (<12.5 months) and PFS-long (≥12.5 months) groups (Fig. 6A–C). Specifically, according to the cell type score, compared with the PFS-short group, the PFS-long group had a higher score of exhausted CD8+ T cells (Fig. 6D).
Fig. 6.
Comparison of expression of immune-related genes and cell type scores between PFS-short (<12.5 months, N = 4) and PFS-long (≥12.5 months, N = 4) groups. (A–B) Expression of immune-related genes per pretreatment sample (BT) PFS-short and PFS-long groups. (C) GSEA pathway enrichment analysis results of different expressed genes between PFS-short and PFS-long groups. (D) Cell type scores in per pretreatment sample of PFS-short and PFS-long groups. Each point in the boxplot represents for one sample. Whiskers show the minimum and maximum of all the data. The horizontal line in the box represents the median, and the top and bottom of each box indicate the 25th and 75th percentile. The significance for differences between PFS-short and PFS-long groups was tested using Wilcoxon’ s rank-sum test. Statistical tests were two sided.
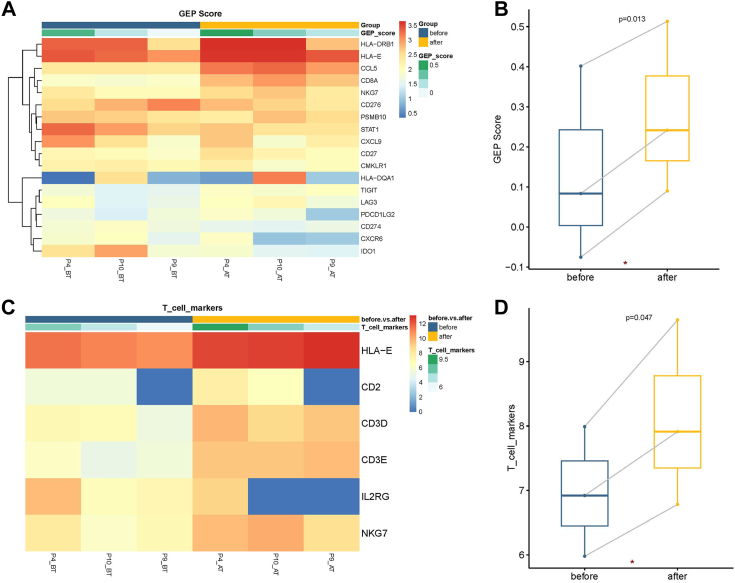
The TIME changes in pre-treatment and post-treatment were also analyzed in our study (Fig. 7). GEP score (Fig. 7A and B) and T cell markers (Fig. 7C and D) were evaluated in post treatment tissues (AT) compared to those in pretreatment tissues (BT).
Fig. 7.
TIME changes between lesions of pre-treatment and post-treatment in CR/PR patients (N = 3). (A–B) Pre-to-post-treatment changes of GEP score in CR/PR patients. (C–D) Pre-to-post-treatment changes of T cell markers in CR/PR patients. Each point in the boxplot represents for one sample. Whiskers show the minimum and maximum of all the data. The horizontal line in the box represents the median, and the top and bottom of each box indicate the 25th and 75th percentile. The significance for differences between pre-treatment and post-treatment samples was tested using Wilcoxon’ s signed-rank test. Statistical tests were two sided.
Discussion
This phase II study reached its primary endpoint and showed that the addition of sintilimab to first-line bevacizumab plus oxaliplatin and capecitabine might improve ORR and PFS, and these were also safe and tolerable in mCRC patients with MSS status and RAS mutation. Compared with the approximately 43–59% ORR and 8–9 months PFS of standard first-line doublet chemotherapy plus bevacizumab in RAS-mutant mCRC,27, 28, 29, 30 our trial reached an ORR value of 84% and PFS of 18.2 months in the FAS. Our results provided a desirable synthetic strategy that combined immunotherapy, targeted therapy, and chemotherapy in patients with MSS status and RAS-mutant mCRC.
Recently, a number of similar studies have yielded preliminary findings, such as CheckMate 9 × 8, AtezoTRIBE, NIVACOR Trial and so on. The CheckMate 9 × 8 evaluated nivolumab in combination with mFOLFOX6 plus bevacizumab for the treatment of first-line mCRC.31 Although the primary endpoint of PFS was not met, nivolumab combined with mFOLFOX6 plus bevacizumab achieved higher PFS rates after 12 months. Specifically, a marked benefit was observed with the addition of nivolumab to mFOLFOX6 plus bevacizumab in consensus molecular subtype (CMS) 3 patients (median PFS of 16.1 months vs 8.7 months) in exploratory subgroup analyses. Coincidentally, the mCRC patients with MSS status and KRAS mutation enrolled in our study were exactly CMS 3 type. In the proficient mismatch repair (pMMR) subgroup of the AtezoTRIBE trial, for patients allocated to the atezolizumab group (FOLFOXIRI + bevacizumab + atezolizumab), a median PFS of 12.9 months was reported compared with 11.4 months in the control group (FOLFOXIRI + bevacizumab). In addition, the ORR and DCR were 59% vs 64%, and 92% vs 93%, respectively.32 The NIVACOR trial assessed the efficacy and safety of nivolumab in combination with FOLFOXIRI and bevacizumab in the first-line setting in patients with RAS/BRAF-mutant mCRC. In the subgroup analysis of MSS patients, the ORR was 78.9%, DCR was 96.2%, and the median PFS was 9.8 months (95% CI 8.18–15.24).33 It could be seen that our treatment with sintilimab plus bevacizumab, oxaliplatin and capecitabine exerts certain advantages in both ORR and median PFS in RAS-mutant and MSS mCRC.
In addition, the NICHE study of neoadjuvant immunotherapy for colon cancer is also noteworthy. Patients were treated with a single dose of ipilimumab 1 mg/kg and two doses of nivolumab 3 mg/kg and underwent surgery within 6 weeks. Thirty patients with pMMR tumors were evaluable for efficacy analyses. Pathologic response was observed in 9/30 (30%, 95% CI 14–46%) patients. The NICHE study was the first neoadjuvant immunotherapy study in colon cancer. And it suggests that pMMR colon cancer is not completely unresponsive to immunotherapy. For pMMR/MSS tumors, identifying potential biomarkers that distinguish responders from non-responders to immunotherapy in CRC is the focus of future research.
Interestingly, in our study, 24% (6/25) of patients underwent R0 surgical resection and unexpectedly achieved NED status. A pooled analysis of 11 studies showed 28.1% R0 resection rate of overall metastases in triplet chemotherapy (FOLFOXIRI) plus bevacizumab.34 Meanwhile, the R0 resection rate in a phase II study (BeTRI) of FOLFOXIRI plus bevacizumab as initial chemotherapy for patients with untreated mCRC was 22.7%.35 Another pooled analysis of 29 published trials including 3500 patients suggested that the resection rate of any metastatic site was 9.3% and the resection rate of only liver metastases was 18% for the FOLFIRI plus bevacizumab regimen.36 Our findings could also provide some references for additional insights into the application of sintilimab plus bevacizumab, oxaliplatin and capecitabine as a conversion regimen for patients with MSS and RAS-mutant mCRC.
In exploratory biomarker analysis, we used assays from DNA and RNA levels, as well as multi-dimensional analysis from efficacy response (CR/PR vs non-CR/PR), PFS-long and PFS-short, pre-treatment and post-treatment. It has been reported that CRC with TTN/OBSCN “double-hit” was an “immune-hot” subtype with potentially better immunotherapeutic efficacy and a predictor of favorable prognosis.37 In hepatobiliary cancers, Yang X et al. showed that copy number variants (CNV) could predict the efficacy of immune checkpoint inhibitor-based therapy, which was inversely associated with survival38; patients with lower CNV risk scores had longer overall survival and PFS than those with higher CNV risk scores. In our study, 5 of 7 patients featured TTN/OBSCN “double-hit” in tumor tissues after treatment. In addition, the CNV burden was significantly decreased after treatment with sintilimab plus bevacizumab, oxaliplatin and capecitabine. Our NanoString results indicated that there was a better TIME cell infiltration in CR/PR patients compared with non-CR/PR patients, as well as in post-treatment samples compared with baseline samples. To some extent, these RAS-mutant and MSS patients changed into an “immune-hot” subtype after treatment with a regimen of sintilimab plus bevacizumab, oxaliplatin and capecitabine. The specific mechanisms behind these findings deserve further exploration.
We acknowledge that this phase II trial has several limitations. First, it had a single-arm design without a control group, which may induce some selection bias. Second, this single-center trial had a limited sample size. Moreover, the absence of overall survival analysis made it uncertain whether this regimen confers long-term survival benefits. The efficacy and safety of sintilimab plus bevacizumab, oxaliplatin and capecitabine need to be further evaluated in a randomized controlled study using a larger sample size. We are currently launching a phase III, randomized, open-label, multicentric clinical trial (NCT05171660) to further analyze the effects, safety, and prognostic biomarkers of sintilimab plus bevacizumab, oxaliplatin and capecitabine as first-line treatment in RAS mutant, MSS mCRC patients when compared with that of bevacizumab plus oxaliplatin and capecitabine. This upcoming trial will recruit 494 patients from 20 centers randomly (1:1) disseminated into two groups.
In summary, sintilimab plus bevacizumab, oxaliplatin and capecitabine as first-line combination treatment demonstrated a high ORR, DCR and a manageable safety profile in RAS-mutant, MSS and unresectable mCRC assessed by MDT. This suggests that sintilimab plus bevacizumab, oxaliplatin and capecitabine is a promising combination strategy that is expected to provide more clinical benefits. Exploratory biomarker assessment analysis revealed that RAS-mutant and MSS patients changed into an “immune-hot” subtype after treatment with a regimen of sintilimab plus bevacizumab, oxaliplatin and capecitabine.
Contributors
Y Y, XF F and KF D were responsible for trial conception, design and protocol written. XF F, N Z, CH Z, J L, SS W, HG H, CX D, D L, YM S, D X, JW W, LF S, J W, ZH W, HF C, XJ L, NJ Y, Q X, M M recruited patients and collected data, N Z and D L did the statistical analysis. LH W, XF F and SS W evaluated images. N Z and CH Z wrote the initial manuscript and had full access to and verified all study data. Y Y, KF D, XF F and SZ Z substantively revised it. All authors critically reviewed drafts of the manuscript and approved the final manuscript.
Data sharing statement
The study protocol is provided in the supplementary appendix. The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of interests
The authors declare that they have no competing interests.
Acknowledgements
All the co-authors would like to thank the patients and their families, the study investigators, coordinators, and research staff. Sintilimab was provided by Innovent Biologics, Inc. WES and Nanostring panel RNA sequencing were assisted by Shanghai Tongshu Biotechnology Co., Ltd and Jiangsu Simcere Diagnostics Co., respectively.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102123.
Contributor Information
Kefeng Ding, Email: dingkefeng@zju.edu.cn.
Ying Yuan, Email: yuanying1999@zju.edu.cn.
Appendix A. Supplementary data
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Rongshou Zheng S.Z., Zeng H., Wang S., et al. Cancer incidence and mortality in China, 2016. J Natl Cancer Center. 2022;2(2022):1–9. doi: 10.1016/j.jncc.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Available from: 2021. https://seer.cancer.gov/statfacts/html/colorect.html Cancer stat facts: colorectal cancer. [cited January 28, 2021].
- 4.Biller L.H., Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. 2021;325(7):669–685. doi: 10.1001/jama.2021.0106. [DOI] [PubMed] [Google Scholar]
- 5.Benson A.B., Venook A.P., Al-Hawary M.M., et al. NCCN guidelines insights: colon cancer, version 2.2018. J Natl Compr Canc Netw. 2018;16(4):359–369. doi: 10.6004/jnccn.2018.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diagnosis, treatment guidelines for colorectal cancer working group C Chinese Society of Clinical Oncology (CSCO) diagnosis and treatment guidelines for colorectal cancer 2018 (English version) Chin J Cancer Res. 2019;31(1):117–134. doi: 10.21147/j.issn.1000-9604.2019.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox A.D., Fesik S.W., Kimmelman A.C., Luo J., Der C.J. Drugging the undruggable RAS: mission possible? Nat Rev Drug Discov. 2014;13(11):828–851. doi: 10.1038/nrd4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez-Medarde A., Santos E. Ras in cancer and developmental diseases. Genes Cancer. 2011;2(3):344–358. doi: 10.1177/1947601911411084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peeters M., Kafatos G., Taylor A., et al. Prevalence of RAS mutations and individual variation patterns among patients with metastatic colorectal cancer: a pooled analysis of randomised controlled trials. Eur J Cancer. 2015;51(13):1704–1713. doi: 10.1016/j.ejca.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Modest D.P., Ricard I., Heinemann V., et al. Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Ann Oncol. 2016;27(9):1746–1753. doi: 10.1093/annonc/mdw261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patelli G., Tosi F., Amatu A., et al. Strategies to tackle RAS-mutated metastatic colorectal cancer. ESMO Open. 2021;6(3) doi: 10.1016/j.esmoop.2021.100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le D.T., Uram J.N., Wang H., et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaz L.A., Jr., Shiu K.K., Kim T.W., et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022;23(5):659–670. doi: 10.1016/S1470-2045(22)00197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grasso C.S., Giannakis M., Wells D.K., et al. Genetic mechanisms of immune evasion in colorectal cancer. Cancer Discov. 2018;8(6):730–749. doi: 10.1158/2159-8290.CD-17-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaretsky J.M., Garcia-Diaz A., Shin D.S., et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375(9):819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eng C., Kim T.W., Bendell J., et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2019;20(6):849–861. doi: 10.1016/S1470-2045(19)30027-0. [DOI] [PubMed] [Google Scholar]
- 17.Motz G.T., Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol. 2011;11(10):702–711. doi: 10.1038/nri3064. [DOI] [PubMed] [Google Scholar]
- 18.Khan K.A., Kerbel R.S. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol. 2018;15(5):310–324. doi: 10.1038/nrclinonc.2018.9. [DOI] [PubMed] [Google Scholar]
- 19.Voron T., Colussi O., Marcheteau E., et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212(2):139–148. doi: 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terme M., Pernot S., Marcheteau E., et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 2013;73(2):539–549. doi: 10.1158/0008-5472.CAN-12-2325. [DOI] [PubMed] [Google Scholar]
- 21.Huang Y., Yuan J., Righi E., et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci U S A. 2012;109(43):17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Apetoh L., Ghiringhelli F., Tesniere A., et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 23.Tesniere A., Schlemmer F., Boige V., et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29(4):482–491. doi: 10.1038/onc.2009.356. [DOI] [PubMed] [Google Scholar]
- 24.Gou H.F., Huang J., Shi H.S., Chen X.C., Wang Y.S. Chemo-immunotherapy with oxaliplatin and interleukin-7 inhibits colon cancer metastasis in mice. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0085789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vincent J., Mignot G., Chalmin F., et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70(8):3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 26.Pfirschke C., Engblom C., Rickelt S., et al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity. 2016;44(2):343–354. doi: 10.1016/j.immuni.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tol J., Koopman M., Cats A., et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360(6):563–572. doi: 10.1056/NEJMoa0808268. [DOI] [PubMed] [Google Scholar]
- 28.Tang W., Ren L., Liu T., et al. Bevacizumab plus mFOLFOX6 versus mFOLFOX6 alone as first-line treatment for RAS mutant unresectable colorectal liver-limited metastases: the BECOME randomized controlled trial. J Clin Oncol. 2020;38(27):3175–3184. doi: 10.1200/JCO.20.00174. [DOI] [PubMed] [Google Scholar]
- 29.Stintzing S., Fischer von Weikersthal L., Decker T., et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer-subgroup analysis of patients with KRAS: mutated tumours in the randomised German AIO study KRK-0306. Ann Oncol. 2012;23(7):1693–1699. doi: 10.1093/annonc/mdr571. [DOI] [PubMed] [Google Scholar]
- 30.Diaz-Rubio E., Gomez-Espana A., Massuti B., et al. Role of Kras status in patients with metastatic colorectal cancer receiving first-line chemotherapy plus bevacizumab: a TTD group cooperative study. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0047345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lenz H.-J., Parikh A.R., Spigel D.R., et al. Nivolumab (NIVO) + 5-fluorouracil/leucovorin/oxaliplatin (mFOLFOX6)/bevacizumab (BEV) versus mFOLFOX6/BEV for first-line (1L) treatment of metastatic colorectal cancer (mCRC): phase 2 results from CheckMate 9X8. J Clin Oncol. 2022;40(4_suppl) 8. [Google Scholar]
- 32.Antoniotti C., Rossini D., Pietrantonio F., et al. Upfront FOLFOXIRI plus bevacizumab with or without atezolizumab in the treatment of patients with metastatic colorectal cancer (AtezoTRIBE): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2022;23(7):876–887. doi: 10.1016/S1470-2045(22)00274-1. [DOI] [PubMed] [Google Scholar]
- 33.Angela D., Iachetta F., Antonuzzo L., et al. Phase II study of nivolumab in combination with FOLFOXIRI/bevacizumab as first-line treatment in patients with advanced colorectal cancer RAS/BRAF mutated (mut): NIVACOR trial (GOIRC-03-2018) J Clin Oncol. 2022;40(16_suppl) 3509. [Google Scholar]
- 34.Tomasello G., Petrelli F., Ghidini M., Russo A., Passalacqua R., Barni S. FOLFOXIRI plus bevacizumab as conversion therapy for patients with initially unresectable metastatic colorectal cancer: a systematic review and pooled analysis. JAMA Oncol. 2017;3(7) doi: 10.1001/jamaoncol.2017.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shinozaki K., Yamada T., Nasu J., et al. A phase II study of FOLFOXIRI plus bevacizumab as initial chemotherapy for patients with untreated metastatic colorectal cancer: TRICC1414 (BeTRI) Int J Clin Oncol. 2021;26(2):399–408. doi: 10.1007/s10147-020-01811-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrelli F., Borgonovo K., Cabiddu M., et al. FOLFIRI-bevacizumab as first-line chemotherapy in 3500 patients with advanced colorectal cancer: a pooled analysis of 29 published trials. Clin Colorectal Cancer. 2013;12(3):145–151. doi: 10.1016/j.clcc.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Liu Z., Wang L., Guo C., et al. TTN/OBSCN 'Double-Hit' predicts favourable prognosis, 'immune-hot' subtype and potentially better immunotherapeutic efficacy in colorectal cancer. J Cell Mol Med. 2021;25(7):3239–3251. doi: 10.1111/jcmm.16393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang X., Hu Y., Yang K., et al. Cell-free DNA copy number variations predict efficacy of immune checkpoint inhibitor-based therapy in hepatobiliary cancers. J Immunother Cancer. 2021;9(5) doi: 10.1136/jitc-2020-001942. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.