Abstract
Over the past few decades, advances in immunological knowledge have led to the identification of novel immune checkpoints, reinvigorating cancer immunotherapy. Immunotherapy, represented by immune checkpoint inhibitors, has become the leader in the precision treatment of cancer, bringing a new dawn to the treatment of most cancer patients. Galectin-9 (LGALS9), a member of the galectin family, is a widely expressed protein involved in immune regulation and tumor pathogenesis, and affects the prognosis of various types of cancer. Galectin-9 regulates immune homeostasis and tumor cell survival through its interaction with its receptor Tim-3. In the review, based on a brief description of the signaling mechanisms and immunomodulatory activities of galectin-9 and Tim-3, we summarize the targeted expression patterns of galectin-9 in a variety of malignancies and the promising mechanisms of anti-galectin-9 therapy in stimulating anti-tumor immune responses.
Keywords: Galectin-9, Immune checkpoint inhibitors, Immunotherapy, Monoclonal antibody, Tim-3
Introduction
Galectins are an interesting family of β-galactoside-binding proteins that are widely present not only in animals but also in bacteria and fungi at varying levels. They are a highly conserved core sequence, defined by the evolutionarily conserved carbohydrate recognition domain (CRD)1. Their biological role was initially understood to be limited to recognition of endogenous (“self”) carbohydrate ligands during embryogenesis and early development, but it was later discovered that they also play a key role in tissue repair, adipogenesis, cancer development, and the regulation of immune homeostasis2,3. The galectins protein family has two typical characteristics: (1) sharing significant similarities in a conserved amino acid sequence; (2) a high affinity for β-galactoside sugars. Currently, 15 galectins have been identified in mammals and 11 are found in humans, acting both intracellularly and extracellularly. Galectins can be classified into three subtypes based on the protein structure: (1) proto-type, is a protein containing one CED, which usually form homodimers, including galectin-1, galectin-2, galectin-5, galectin-7, galectin-10, galectin-11, galectin-13, galectin-14, and galectin-15; (2) chimera-type, galectin-3, is the only chimeric galectin which contains a non-lectin domain linked to a CRD. It contains two domains, a C-terminal CRD and a non-carbohydrate binding N-terminal domain, which self-associate into an oligomer; (3) tandem-repeat-type, has two CRDs with different binding specificity, which are conjugated by ligating peptides, including galectin-4, galectin-6, galectin-8, galectin-9, and galectin-12 (Fig. 1)4,5. Galectins have cytoplasmic properties and can only be synthesized in the cytoplasm. It is not related to the traditional endoplasmic reticulum/Golgi trafficking mechanism and is secreted through non-classical pathways. Multiple pathways have been proposed to explain the mechanisms by which galectins leave the cell via the non-classical pathways, including the dependence of galectins secretion on its oligomerization, the use of direct translocation pathways for translocating across the plasma membrane, and the release of galectins in extracellular vesicles6. Additional studies support a model of galectins release either through exosomes or lysosomes7. Many galectins are located outside of the cell and participate in interactions with other extracellular proteins. This family is widely distributed in human tissues and has a wide range of biological functions.
Figure 1.
Galectin family in mammals. Fifteen galectins have been described in mammals, 11 of which have also been found in humans (located in the light-colored area at the top of the diagram). This figure shows the structural variation and classification of different subtypes of galectins.
Galectin-9 (Gal-9), also known as LGALS9, was first identified as a transmembrane urate transporter in 1997 from rat embryonic kidney8, an eosinophil attractant in T-lymphocytes9, and an auto-antigen in human Hodgkin’s lymphoma tissue10. Recently, it has gained much attention due to its therapeutic potential in numerous pathological disorders and strong immunomodulatory effects. Thus, the expression level of Gal-9 was involved in a variety of physiological functions, such as cell growth, differentiation, adhesion, communication, and cell death. In addition, aberrant expression of Gal-9 in solid tumors is associated with the tumor occurrence or metastasis11, 12, 13, 14. For instant, high expression level of Gal-9 in tumor cells promoted the colony formation of melanoma cells, whereas downregulation of Gal-9 in these cells correlated with cell proliferation without colony formation11.
Moreover, accumulating evidences have demonstrated that galectins can directly influence both innate and adaptive anticancer immunity by glycan receptors. It is worth nothing that Gal-9, unlike other galectin members, is essentially an immune system inhibitor: it promotes differentiation of regulatory T cells (Tregs), and reduces T-helper 17 (Th17) and Th1 cells, resulting in suppression of excessive immunity and inflammation15,16. Gal-9 specifically interacts with one of its receptors, T-cell immunoglobulin and mucin-domain containing-3 (Tim-3), and induces CD8+ T cell apoptosis17. Furthermore, Gal-9 stimulates IL-12 production by ligating Tim-3 to initiate adaptive immune responses15,18. It is possible that the Gal-9-Tim-3 complex may play critical role in different physiological conditions. However, it was shown that Gal-9 activity was incompletely eliminated by antagonistic Tim-3 antibodies in T-cell lines19. The role of Gal-9 in immunoregulation seems to be complex. At present, Gal-9/Tim-3 is an emerging immune checkpoint inhibitor (ICI) following PD-1/PD-L1. The use of ICIs stimulates the immune microenvironment and enhances the anticancer immune responses, thus improving the therapeutic effect of patients. Compared with radiotherapy and chemotherapy, immunotherapy has better anticancer effects and fewer side effects, and has expanded the options for patients with relapse-resistant drugs after first-line treatment20. However, primary and acquired resistance significantly reduce the success of ICIs, so there is an urgent need to develop new strategies to enable more patients to achieve long-term remission through cancer immunotherapy. We focus on an emerging biomarker, Gal-9, which, unlike other galectins, plays a role in both promoting and inhibiting tumor growth, depending on interactions with T cells, antigen-presenting cells, or receptors on tumor cells21.
In this review, we discuss the interaction mechanism of Gal-9/Tim-3, focus on the expression pattern of Gal-9 in different malignant tumors and the potential mechanism, and provide a theoretical basis for the therapeutic potential of Gal-9 in the immunotherapy of malignant tumors.
Molecular structure and distribution of galectin-9
Molecular structure of Gal-9
Gal-9 is encoded by the LGALS9 gene and located on human chromosome 17. It has a characteristic amino acid sequence and is a highly conserved sugar recognition domain consisting of approximately 130 amino acid sequences. Gal-9 belongs to tandem-repeat-type galectins which containing two separated homologous CRD domains, namely N-terminal CRD (N-CRD) and C-terminal CRD (C-CRD). It is an integral membrane protein that exists as two informs, a long form and a short form, which differ by an internal stretch of 32 amino acids22,23.
Cell distribution of Gal-9
In human cells, Gal-9 is primarily localized to the cytosol, which is based on antibodies targeting proteins from multiple genes. Hofbauer cells, Kupffer cells, and Paneth cells show single-cell type-specific enhancement, which is generally expressed in immune cells24, 25, 26. The expression of gal-9 is significantly enhanced in HMC-1, THP-1, U-937, MOLT-4, and REH cell lines, all cancer cell lines, mainly derived from myeloid and lymphoid (data from The Human Protein Atlas https://www.proteinatlas.org/).
Tissue distribution of gal-9
Gal-9 is expressed in almost all organ systems, and its protein and RNA expression levels varied. The protein and RNA encoded by the LGALS9 gene are highly expressed in bone marrow and lymphoid tissues. At the protein level, Gal-9 is highly expressed in the brain, lung, endocrine tissue, gastrointestinal tract, liver, gallbladder, kidney, urinary bladder, male tissues, female tissues, and muscle tissue. At the RNA level, it is expressed relatively high in the blood (data from The Human Protein Atlas https://www.proteinatlas.org/).
Physiological function of galectin-9
Many studies have shown that Gal-9 is involved in many physiological processes and is an indispensable key element in maintaining normal physiological functions of the body. Gal-9 plays an important role in growth and development27, angiogenesis28, central nervous system stability29, T-cell homeostasis30, maintenance of liver homeostasis24, regulation of lysosomes and autophagy, and maintenance of intestinal stability25. A growing body of evidence suggests that Gal-9 plays a variety of roles in maintaining and regulating immunity. The function of Gal-9 as an eosinophilic chemoattractant helps in the recruitment of eosinophils through T cells, trigging eosinophils activation31. Gal-9 is involved in Th17/Treg immunoregulation by mediating immunosuppression and differentiation32. The effect of Gal-9 on activated T cells is concentration-dependent. At higher concentrations, Gal-9 induces apoptosis of activated CD8+ and CD4+ T cells, but at lower concentrations, it increases the cytokine production by activated T cells. At higher concentrations, bivalent Gal-9 can be oligomerized, and therefore may aggregate more signaling proteins or induce binding with more ligands, which is important for apoptotic signaling33. Besides, Gal-9 inhibits B-cell signaling by binding to B-cell receptors34. Gal-9 promotes interactions between B cells and vascular endothelial cells while transmitting anergic signals to control B cells reactivity35. Maturation of dendritic cells (DCs) is a key step to initiate the immune response, and Gal-9 can promote the maturation of DCs through upregulated expression of co-stimulatory molecules such as CD40, CD54, CD80, etc., and HLA-DR. IL-1β and interferon (IFN)-γ also increase the expression of Gal-9 while promoting the maturation of DCs36. Recent evidence has shown that Gal-9 in the cytosol regulates the phagocytosis of DCs by regulating the plasma membrane structure. Gal-9 is an evolutionally conserved lectin required to maintain the structure and function of the cortical cytoskeleton in DCs, and Gal-9 plays an important role in modulating the function of human DCs. DCs lacking Gal-9 showed a significant decrease in cytokine secretion upon infection, suggesting a poor initiation of the immune response37.
Galectin-9-Tim-3 signaling
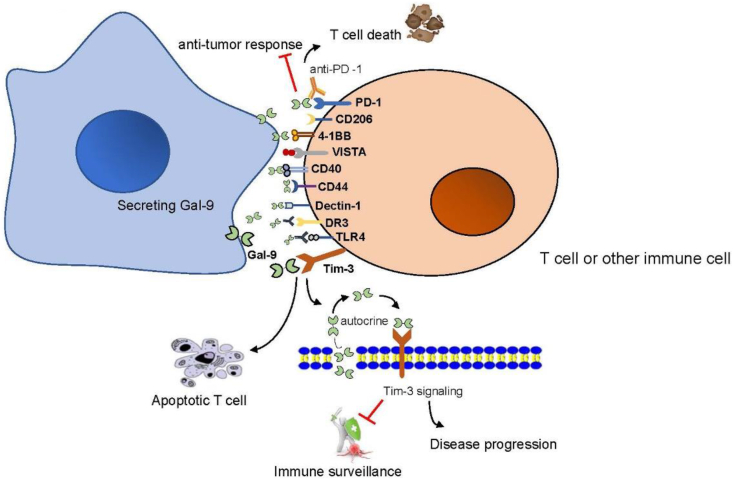
Gal-9 can interact with various extracellular matrix proteins and cell surface ligands as well as Tim-3 receptor. Table 1 lists the roles of Gal-9 and their binding partners in the regulation of immune response and tumor biology.
Table 1.
Galectin-9 and its binding partners in the regulation of immune response and tumor biology.
| Ligand | Targeted cells | Biological function | Refs. |
|---|---|---|---|
| Tim-3 | Dendritic cells, monocytes | Maturation promotion and cytokine secretion | 36,38 |
| Tim-3 | T cells | Exhaustion or apoptosis | 32,39 |
| Tim-3 | Natural killer cells | Regulating cell function at the maternal–fetal interface in early pregnancy | 40 |
| PD-1 | T cells | Suppressing Gal-9/TIM-3-induced T cell apoptosis | 41 |
| Dectin-1 | Macrophages | Tolerogenic macrophage programming and adaptive immune suppression | 42 |
| CD206 | Macrophages | Driving angiogenesis and producing chemokines to support tumor growth | 43 |
| CD40 | T cells | Suppressing proliferation and inducing cell death | 44 |
| 4-1BB | T cells | Transducing signal and controlling functional activity | 45 |
| VISTA | T cells | Apoptosis | 46 |
| DR3 | Regulatory T cells | Facilitating the activity of DR3 with respect to promoting Treg function that limits inflammatory disease | 47 |
| CD44 | Regulatory T cells | Increasing cell stability and function, and enforcing cell differentiation and maintenance | 48 |
| TLR-4 | Microglia | Alleviating brain injury and promoting neuronal restoration | 49 |
Abbreviations: PD-1, programmed cell death protein 1; VISTA, V-domain Ig-containing suppressor of T cell activation; TLR-4, Toll-like receptor-4
Tim-3 is the most famous Gal-9 binding receptor, and it is the most closely related among the multiple receptors of Gal-9. A large number of data suggest that Tim-3 is an immune checkpoint receptor that promotes immune homeostasis by regulating innate and adaptive immunity, and negatively regulates T cell response by inducing Th1 cell apoptosis50. To date, many studies have investigated the interaction between Gal-9 and Tim-3. Under physiological conditions, the binding between Gal-9 and Tim-3 induces the exhaustion or apoptosis of effector T cells, which affects T cell tolerance, negatively regulates IFN-γ secretion, and induces apoptosis of Th1 and Th17 cells; hence plays an important role in the regulation of Th1/Th17 polarization32. When associated with the plasma membrane, the Tim-3-Gal-9 complex triggers downstream signaling that promotes cell renewal and forms an autocrine toop51. Tim-3-Gal-9 autocrine loop is activated in human acute myeloid leukemia cells through protein kinase C (PKC)/mTOR pathway, which induces high levels of Gal-9 secretion and the release of soluble Tim-3, leading to reduced immune surveillance and disease progression52. A growing body of evidence suggests that the interaction of Tim-3-Gal-9 has different phenotypes and functions during the progression of multiple tumors. The Tim-3-Gal-9 pathways contributes to the suppressive tumor microenvironment (TME) in human through Tregs promotion and TCR activation53. Both Gal-9 and Tim-3 can inhibit anti-cancer immune surveillance52. Gal-9 and Tim-3 are also expressed in some solid tumors, and tumor cells use these proteins to escape immune attack from the host54,55. The interaction of Tim-3-Gal-9 can induce apoptosis of CD4+ and CD8+ tumor-infiltrating lymphocytes (TILs) and inhibit T cells immune response. Blocking the Tim-3-Gal-9 pathway can restore the function of TILs. An anti-Tim-3 antibody blocks their interaction to increase the proliferation and cytokine production of Tim-3-positive T cells in humans and mice. Besides, an anti-Tim-3 antibody shows anticancer effects in a mouse tumor model (including restoration of their ability to express IFN-γ, IL-2, perforin, and granzyme B)56, 57, 58, 59. The latest study further highlights Gal-9 as a target for cancer immunotherapy. In growing tumors, IFN-β is produced by DCs and cancer cells, while IFN-γ is released by activated CD8+ T cells. The synergistic effect of IFN-β and IFN-γ upregulates the expression and secretion of Gal-9 in antigen-presenting cells (APC) and cancer cells, thereby inhibiting the anti-tumor response by inducing T cell death41 (Fig. 2). Currently, blocking the interaction of Tim-3-Gal-9 is considered to be a promising therapy in human malignancies.
Figure 2.
The binding partners of Gal-9. Gal-9 binds to multiple receptors on the cell surface. Binding of Gal-9 to Tim-3 induces T cell apoptosis under physiological conditions, and forms autocrine loop with Tim-3 under pathological conditions, leading to reduced immune surveillance and promoting disease progression. PD-1 binding to Gal-9 plays a key role in inhibiting anti-tumor response and promoting T cell death.
Galectin-9 expression in cancer
The expression of Gal-9 in cancer has been extensively studied, and the levels of Gal-9 in tumor cells or tumor tissues are different compared to normal controls. Thus far, in many types of malignancies, such as breast cancer54, human papilloma virus (HPV)-associated cervical carcinoma59, pancreatic carcinoma60, glioblastoma multiforme61, cutaneous T-cell lymphoma62, chronic lymphocytic leukemia63, tumor cells or tumor tissue contained high or increased levels of Gal-9 compared to normal para-cancerous tissues. On the other hand, in some malignancies, such as gastric cancer, colon cancer, esophageal carcinoma, melanoma, hepatocellular carcinoma, lung cancer, renal cell carcinoma, adrenal carcinoma, and prostate cancer cells, the Gal-9 expression has been observed to be down-regulated in corresponding healthy counterparts11,64, 65, 66, 67, 68. The expression level of Gal-9 in different tumors not only affects the occurrence and progression of tumors, but also affects their prognosis (Table 2).
Table 2.
Expression and role of galectin-9 in different types of cancer.
| Type of cancer | Expression of Gal-9 | Activity | Possible mechanism | Prognosis | Refs. |
|---|---|---|---|---|---|
| Gastric cancer | Down-regulated | Tumor suppressor | Inducing tumor cell apoptosis | Favorable | 71,72 |
| Colorectal cancer | Down-regulated | Tumor suppressor | Inducing tumor cell apoptosis and inhibiting proliferation, reducing tumor escape immune surveillance, and inducing autophagy | Favorable | 64,75, 76, 77 |
| Lung cancer | Down-regulated | Tumor suppressor | Suppressing tumor cell adhesion and invasion, and activating NK cells | Favorable | 78,80,81 |
| Hepatocellular carcinoma | Down-regulated | Tumor suppressor | Inducing tumor cell apoptosis, and inhibiting the anti-tumor immune response by inducing T cell senescence in Kupffer cells and other APCs | Favorable | 66,83,84 |
| Esophageal carcinoma | Down-regulated | Tumor suppressor | Inducing tumor cell apoptosis | Favorable | 68,85,86 |
| Melanoma | Down-regulated | Tumor suppressor | Inducing tumor cell apoptosis | Favorable | 11,94,95 |
| Breast cancer | Up-regulated | Tumor promotor | Inhibiting immune surveillance through the Tim-3-Gal-9 pathway, and inducing tumor cell aggregation to reduce metastasis | Favorable | 13,54,69 |
| Glioma | Up-regulated | Tumor promotor | Tumor immunosuppression or immune escape through the Tim-3-Gal-9 pathway | Unfavorable | 61,88 |
| Pancreatic carcinoma | Up-regulated | Tumor promotor | Reprogramming macrophages and exerting immunosuppressive effects | Unfavorable | 42,60,91 |
| Cervical carcinoma | Up-regulated | Tumor promotor | Tumor immune escape through activation of the Tim-3-Gal-9 pathway | Unfavorable | 59,93 |
| Lymphoma | Up-regulated | Tumor promotor | Reducing CD8+ T cell infiltration | Unfavorable | 62 |
| Leukemia | Up-regulated | Tumor promotor | Immune escape through the Tim-3-Gal-9 pathway | Unfavorable | 51,52,101 |
Double-edged sword role of Galectin-9 in tumors
Unlike other galectins, Gal-9 both promotes and inhibits tumor activity, showing a “double-edged sword” effect in malignant tumors. Expression of Gal-9 in tumors is not only related to tumor cell adhesion or metastasis, but also involved in the regulation of immune response through interaction with different receptors. Considering the importance of Gal-9 in cancer, targeting Gal-9 expression holds promise in cancer therapy. The following sections discuss the specific role of Gal-9 in various cancers (Table 2).
Galectin-9 and breast cancer
Interestingly, Gal-9 has been shown to have anti-metastatic potential in breast cancer, possibly because Gal-9 induces tumor cell aggregation and reduced adhesion of breast cancer cells to the extracellular matrix, thereby preventing metastasis and improving patients’ survival13,69. In a recent study, researchers found that breast tumor cells expressed levels of Gal-9 and Tim-3, especially Gal-9, compared with healthy breast tissues from the same patients, and that these proteins were co-localized. Furthermore, it was further found that breast cancer cells activated the Tim-3-Gal-9 pathway by expressing LPHN1 and its ligand FLRT3, and transferred Gal-9 to the cell surface to protect breast cancer cells against cytotoxic immune attack while inhibiting host anti-cancer immune surveillance54.
Galectin-9 and gastric cancer
Previous studies have shown that higher levels of Gal-9 expression were observed in gastric cancer patients without lympho-vascular invasion, lymph node metastasis, or distant metastasis, and Gal-9 expression is closely related to better survival rates of gastric cancer70. A meta-analysis involving 2093 patients with gastric cancer, including eight studies, also showed that low expression of Gal-9 was significantly associated with a poorer prognosis71. Down-regulation of Gal-9 mRNA levels was observed in gastric cancer tissues67. Further, an in vitro study showed that recombinant human Gal-9 (rh-Gal-9) suppresses gastric cancer cell lines proliferation by inducing apoptosis, regulating receptor tyrosine kinases (RTK) pathways and angiogenesis-related molecules, and altering miRNA expression profiles72. The Gal-9 promotor region could bind to peroxisome proliferator-activated receptor γ (PPARγ), and Gal-9 activity increased in gastric cancer cells with overexpression of PPARγ. PPARγ inhibited gastric cancer cell invasion, migration, and epithelial-mesenchymal transformation by up-regulation of Gal-973. Tim-3 was significantly expressed in TILs of patients with gastric cancer and was an independent prognostic factor of gastric cancer. Gal-9 was mainly expressed in gastric cancer cells74. The mechanism of Tim-3 in gastric cancer and its interaction with Gal-9 is not yet clear, and further studies are needed in the future.
Galectin-9 and colorectal cancer
Gal-9 expression in colon cancer tumor tissues was lower than that in para-cancerous tissues, and low levels of Gal-9 expression were positively correlated with a poor histological grade and lymph node metastasis of colon cancer (P < 0.05). Patients with high Gal-9 expression had a longer overall survival at long-term follow-up (P < 0.05). The infiltration rate of CD56+ natural killer (NK) cells in tissues with high Gal-9 expression was significantly increased. Both Gal-9 secreted by colon cancer cells and rh-Gal-9 increased the recruitment of NK cells by increasing the expression of Rho/ROCK1 signaling, an essential part of many cytoskeleton-dependent processes, suggesting that tumors could escape immune surveillance through a regulatory mechanism that reduced Gal-9 expression64. Another study showed that overexpression of Gal-9 promoted apoptosis and inhibited proliferation in colon cancer cells. MiR-455-5p was identified as the upstream regulatory microRNA (miRNA) of Gal-9 and demonstrated that miR-455-5p reduced Gal-9 expression by directly targeting its 3’-untranslated region75. In a mouse colon cancer model, most tumor-infiltrating CD8+ T cells expressed Tim-3, and Gal-9 secreted by tumor cells increased apoptosis of tumor-infiltrating CD8+ T cells. Anti-Tim-3 antibody blockaded the Tim-3-Gal-9 signaling, reduced CD8+ T cells apoptosis, and also inhibited tumor growth in mice55. Using pharmacological inhibitors induced mitochondrial dysfunction would not affect the ability of human colorectal cancer cells to secrete Gal-9, but could significantly reduce Gal-9 exocytosis and the presence of Gal-9 on the cell surface, thus reducing the Tim-3-Gal-9 mediated tumor immune escape, suggesting that targeted de-functionalization of mitochondria in malignant cells may be a novel strategy for anti-cancer immunotherapy76. Rh-Gal-9 has potent antitumor activity against refractory KRAS mutant colorectal cancer cells and induces fatal frustrated autophagy, depending on the increased basal autophagic flux, but has no effect on BRAF mutant77.
Galectin-9 and lung cancer
Gal-9 expression was detected in all pathological types of non-small cell lung cancer (NSCLC), and Gal-9 levels were only associated with Tim-3 levels on tumor cells, while Gal-9 was widely associated with other immune checkpoints (including PD-1, PD-L1, and Tim-3) on TILs. High Gal-9 expression on tumor cells suggested a possible longer survival in patients, while high Gal-9 expression on TILs might indicate early tumor relapse78. However, a recent study on small cell lung cancer (SCLC) has shown that low Gal-9 expression with TILs can predict early recurrence of patients with stage I-III SCLC. Patients with SCLC with high immune risk score showed lower Gal-9 expression, and Gal-9 expression was significantly associated with changes in the tumor-immune microenvironment and immune infiltration79. Limited studies have investigated the role of Gal-9 in lung cancer. Increased cytoplasmic Gal-9 expression in tumor cells may inhibit lung metastasis, which may be related to the suppressive effect of Gal-9 on adhesion and invasion of tumor cells80. Gal-9 induced macrophages to differentiate into plasmacytoid DC-like macrophages, which may enhance the activation of NK cells that prolonged the survival of lung cancer-bearing mice81. The interaction of Tim-3-Gal-9 not only enhances anticancer immunity but also suggests that the Tim-3-Gal-9 is the key mechanism of primary or secondary resistance to anti-PD-1 in metastatic NSCLC patients, which may become a new target for immunotherapy combinations82.
Galectin-9 and hepatocellular carcinoma
Gal-9 has also been shown to have an antitumor effect in hepatocellular carcinoma (HCC), and its main thrust is to bring apoptosis. Gal-9 induced apoptosis of HCC cells independently of Tim-3. miR-1246 was a miRNA associated with the antitumor effect of Gal-9 in HCC, which enhanced the apoptotic effect of Gal-9 through the miR-1246-DYRK1A-caspase-9 axis83. Low expression of Gal-9 and low CD8+ TILs count were significantly associated with poor prognosis in HCC patients84. In hepatitis B virus (HBV)-associated HCC microenvironment, TILs-derived IFN-γ stimulated the expression of Gal-9 on APC (especially Kupffer cells). Tim-3 positive T cells in HCC were co-localized with Gal-9 positive Kupffer cells. Tim-3-Gal-9 signaling pathway T cell senescence, and blocking this pathway could restore T cell effector function56. This means that Gal-9 behaves dichotomous behaviors in HCC. Further studies showed that IFN-γ significantly induced the up-regulation of Gal-9 expression in HCC cells, and Gal-9 overexpression inhibited tumor growth and metastasis in vitro and in vivo66.
Galectin-9 and esophageal carcinoma
Esophageal carcinoma includes two histological subtypes: esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). Rh-Gal-9 induced mitochondrial dysregulation in ESCC cells (considered to be an irreversible step in apoptosis), thereby mediating tumor cell apoptosis and inhibiting proliferation through JNK and p38 activation85. Similarly, Gal-9 showed antitumor effects in EAC cells by inducing apoptosis. The increased expression level of IL-8 in EAC cells treated with Gal-986. The elevated expression of IL-8 has been associated with a poor prognosis of esophageal cancer patients87. Thus, suggesting that Gal-9 resistance in EAC cells maybe attribute to IL-8 expression induced by Gal-9. Data from a Chinese study showed that low Gal-9 expression level was associated with a poor prognosis in ESCC patients68.
Galectin-9 and glioma
Gal-9 and Tim-3 were expressed at an increased level in glioma tissues and TILs, respectively, and were correlated with tumor malignancy. The interaction of Tim-3 on TILs with Gal-9 expressed by tumors induced dysfunction or exhaustion of TILs. Blockading the Tim-3-Gal-9 pathway might delay glioma progression and improve KPS socre88. A study involving 1,027 patients with glioma suggests that Gal-9 plays a key role in the malignant progression of glioblastoma multiforme (GBM), with the highest expression in GBM, especially in the mesenchymal GBM subtype. Patients with high Gal-9 expression had a significantly lower survival rate than those with low Gal-9 expression. Further analysis of immune function suggested that Gal-9 might exert tumor immunosuppression or immune escape by positively regulating M2 tumor-associated macrophages61. In the tumor microenvironment of glioma, the Tim-3-Gal-9 pathway is affected by isocitrate dehydrogenase (IDH) mutant. IDH mutant reduced the interaction rate between Tim-3 positive T cells and Gal-9 positive microglia/macrophages, suggesting that IDH mutant could convey resistance to immune checkpoint inhibition and may be one of the ways that patients benefit from immunotherapy89.
Galectin-9 and pancreatic carcinoma
Gal-9 was highly expressed in human pancreatic carcinoma, and patients’ blood immune cells also showed higher Gal-9 expression. The study showed that neoadjuvant chemotherapy significantly reduced Gal-9 expression on pancreatic carcinoma cells. Gal-9 serum levels were significantly lower for the long-term (>12 months) compared with short-term survivors. Gal-9 promoted the phenotypic and functional polarization of macrophages towards M2 phenotype, and significantly reduced the secretion of TNF-α and INF-γ by T cells, which might support the further growth of tumors60. Gal-9 binds to one of its receptors, Dectin 1 (which is highly expressed on macrophages in pancreatic ductal adenocarcinoma), leading to tolerogenic macrophage programming and adaptive immunosuppression. The blockade of Gal-9 was also found to cause tumor regression and prolong survival42. A study of the efficacy of chimeric antigen receptor (CAR) T cells in pancreatic ductal adenocarcinoma (PDA) showed that the combination of CAR T cells with a biological inhibitor of Gal-9 significantly enhanced CAR T cell cytotoxicity against PDA cells, suggesting an immunosuppressive effect of Gal-9.90 PDA-infiltrating γδ T cells (a subset of CD3+ CD4- CD8- lymphocytes) not only release IL-10 and IL-17, but also express Gal-9 and PD-L1, hence establishing a powerful immunosuppressive microenvironment that promotes the development and progression of PDA.91 However, exogenous rh-Gal-9 shows an opposite effect to endogenous Gal-9. Rh-Gal-9 may induce apoptosis through cytochrome release, which is associated with changes in miRNAs, thereby inhibiting tumor cell proliferation, suggesting that exogenous rh-Gal-9 may be a new therapeutic agent for pancreatic cancer92.
Galectin-9 and cervical carcinoma
Cervical carcinoma is known to be mainly caused by HPV infection. The latest study found that Gal-9 and Tim-3 were highly expressed in cervical carcinoma patients, and their levels were significantly higher in HPV-positive cervical carcinoma patients than in HPV-negative cervical carcinoma and cervical intraepithelial neoplasia (CIN) patients, and were associated with tumor malignancy and disease. It was further found that the activation of the Tim-3-Gal-9 pathway was involved in the immune escape of cervical carcinoma by promoting the secretion of TGF-β and IL-10 by Treg cells, thereby inhibiting the cytotoxic function of Th1 and CD8+ T cells59. Gal-9 and Tim-3 are overexpressed in cervical carcinoma tissues, and the biological effect is mediated through their epigenetics (hypo-methylation in the promoter regions). SUV39H1 (H3K9me3-specific histone methyltransferase) contributes to the changes of the methylation state of Gal-9 and Tim-3 by up-regulating the H3K9me3 (trimethylation of histone 3 lysine 9 at gene promoter region is an important epigenetic mechanism for silencing gene expression) level at the DNMT3A promoter region, and reverses the expression of Gal-9 and Tim-3, thus helping to improve the immune-imbalanced tumor microenvironment93.
Galectin-9 and melanoma
In malignant melanoma, Gal-9 has a tumor suppressor function, and the weakening to loss of Gal-9 is closely related to the progression of metastasis11. High Gal-9 expression is associated with better prognosis11,94. Gal-9 inhibits the invasion and metastasis of malignant melanoma by blocking adhesion to endothelium and extracellular matrix80. Subsequent studies have shown that Gal-9 has direct apoptotic activity toward melanoma cells95. In patients with metastatic malignant melanoma, Gal-9 binding to CD206 on M2 macrophages promotes angiogenesis and chemokine production, which supports tumor growth and leads to poor prognosis in patients, suggesting that targeting the interaction between this interaction may be a novel approach to improve the local antitumor effects of macrophages43. Accumulation of Gal-9 positive melanoma cells was observed in melanoma metastases ex vivo96. DNA methylation plays a central role in the pathogenesis and disease progression of melanoma97. Studies have shown a significant correlation between Gal-9 and Tim-3 methylation and mRNA expression and immune cell infiltration. High expression of Gal-9 and Tim-3 mRNA levels are significantly associated with increased overall survival, suggesting the feasibility of combining immune checkpoint molecules such as Gal-9 and Tim-3 as predictive biomarkers94.
Galectin-9 and lymphoma
Cutaneous T-cell lymphoma (CTCL) is a type of peripheral T-cell lymphoma. A study showed that CTCL tumor cells expressed Gal-9, while Gal-9 expression was almost undetectable in normal skin, and high Gal-9 expression was associated with reduced CD8+ T cell infiltration. Serum Gal-9 levels were increased in patients with advanced CTCL and correlated with disease severity markers, suggesting that Gal-9 expression in tumor cells might be related to disease progression in CTCL. On the other hand, exogenous administration of rh-Gal-9 could induce CTCL cell lines apoptosis by activating caspase-3 and caspase-9 independently of Tim-3, and inhibit tumor growth in tumor-bearing mice in vivo, and rh-Gal-9 combined with anti-Tim-3 blocking antibody had a synergistic effect on tumor inhibition in vivo62. The genetic status of Gal-9 may have an impact on cancer development. According to the COSMIC database, the mutant rate of Gal-9 in different cancers ranges from 0% to 2.5%, and the mutation frequency of Gal-9 is higher in diffuse large B-cell lymphoma (DLBCL) patients (3.2%), which may interfere with the interaction between Gal-9 and Tim-398. At present, there are few studies on Gal-9 in various subtypes of lymphoma, and the effect of Gal-9 on the pathogenesis and prognosis of various subtypes remains to be further studied.
Galectin-9 and leukemia
Acute myeloid leukemia (AML) is derived from self-renewing leukemic stem cells (LSCs). Studies have confirmed that the serum Gal-9 was significantly elevated in human AML cells and a variety of preleukemic diseases, such as myelodysplastic syndromes (MDSs) and myeloproliferative neoplasms (MPNs), and myeloproliferative neoplasms (MPNs). Gal-9 and Tim-3 constitute an autocrine stimulatory loop for LSC self-renewal and human AML development, and Gal-9/Tim-3 autocrine signaling co-activates NF-κB and β-catenin signaling to facilitate expansion and transformation of malignant bone marrow clones51. Another study found that the Tim-3-Gal-9 autocrine loop was activated in AML cells through PKC/mTOR pathways52. Gal-9 is overexpressed in AML, especially when relapse after hematopoietic stem cell transplantation (HSCT), suggesting a poor prognosis. There is a strong positive correlation between Gal-9 and proteasome subunit beta type-8 (PSMB8), which plays a synergistic role in the progression of AML99. Drug resistance is a real barrier to chemotherapy in patients with many cancers, including AML, and has multiple forms, namely, multidrug resistance (MDR). The development of MDR in AML is related to changes in the Tim-3-Gal-9 signaling pathway100. Gal-9 and Tim-3 are significantly elevated in patients with chronic lymphocytic leukemia (CLL) and are closely associated with disease progression. Blocking the Tim-3/Gal-9 pathway weakens Treg cell’s function and promotes CLL cell clearance63,101. Gal-9 may produce immune dysfunction, characterized by accumulation of non-functional and exhausted CD8+ T cells102. Gal-9 is more expressed in advanced clinical stages of CLL patients103. These results suggest the importance of Gal-9 as a prognostic factor for leukemia and may be a promising molecular target for the treatment of leukemia, which may provide more combined therapy options.
According to the above discussion, Gal-9 plays a different role in different tumors, which can promote and inhibit cancer. In the role of promoting cancer, it mainly induces apoptosis of anti-tumor immune cells by binding to T cell surface receptors (especially Tim-3), which reduces anti-tumor immunity and promotes tumor growth. Gal-9 can directly induce tumor cell apoptosis, inhibit tumor cell migration, invasion, epithelial-mesenchymal transformation, and induce tumor cell cycle arrest. Gal-9 is involved in both immune escape and anti-tumor immune response. However, to date, it is not clear how changes in Gal-9 expression amplification in patients affect anti-tumor immune response. The correlation between the expression of Gal-9 and the characteristics of tumor-infiltrating immune cells needs more research (Fig. 3).
Figure 3.
Different expression of Gal-9 in various types of human cancers. Gal-9 expression can be abnormally up-regulated or down-regulated during tumor-genesis to promote or inhibit tumor progression. Red arrow: Gal-9 increases with tumor progression. Blue arrow: Gal-9 decrease during tumorigenesis.
Immune potential of galectin-9 in cancer therapy
As mentioned above, the expression and function of Gal-9 vary dramatically in different types of cancers. Detection of Gal-9 expression in cancer has guiding significance for treatment selection and prognosis. For example, Gal-9 plays an important role as a tumor suppressor in gastric cancer, colorectal cancer, lung cancer, hepatocellular carcinoma, esophageal carcinoma, and melanoma14,64,70,72,78,86. In this case, inducing tumor cells to express more Gal-9 may benefit tumor therapy, while targeting Gal-9 may lead to tumor progression and metastasis. At present, several studies have shown that exogenous administration of human recombinant Gal-9 can significantly inhibit the growth of such tumors, which is effective new preparation. On the other hand, we will focus on cancer with Gal-9 as a tumor promoter that may benefit from anti-Gal-9 targeted therapy.
By reviewing a large number of relevant studies, we concluded that the increased expression of Gal-9 in patients with glioma, pancreatic carcinoma, cervical carcinoma, ovarian carcinoma, leukemia, and lymphoma is often indicative of malignant tumor behavior and poor prognosis. Specifically, higher levels of Gal-9 have been associated with increased susceptibility of glioma patients to develop malignant brain tumors, and Gal-9 levels were negatively associated with overall survival. Gal-9 was highly expressed in serum, intra-tumoral, and peripheral blood monocytes of patients with pancreatic carcinoma, which was associated with a low survival rate after metastasis. Increased expression of Gal-9 in epithelial tissue predicted an adverse response to treatment of high-grade serous ovarian carcinoma. Leukemia patients with higher levels of Gal-9 showed treatment failure. Higher levels of Gal-9 on TILs predicted shorter relapse-free survival18,60,63,104,105. The use of anti-Gal-9 targeted therapy is likely to greatly improve clinical outcomes in these patients, and the combination of anti-Gal-9 therapy and ICIs may play a more powerful immunomodulatory role. These beneficiaries have the following important characteristics, suggesting that Gal-9 is an ideal tumor immunotherapy target. 1) Gal-9 is widely expressed in tumor cells, which is higher than that in adjacent tissues. Gal-9 can protect tumor cells against cytotoxic cell-dependent killing, and an increased level of Gal-9 can help tumor cells avoid phagocytosis and killing by the host immune system. 2) Gal-9 participates in tumor immune escape in such tumor cells by inducing apoptosis of Tim-3-positive T cells, and regulating T cell activity through the death receptor 3 signaling pathway, suggesting that targeting Gal-9 can block Gal-9-induced host immune surveillance escape. 3) The increased expression of Gal-9 in such tumors is often closely related to malignant proliferation. Gal-9 is involved in tumor progression, tumor angiogenesis, and other processes, contributing to the distant aggregation and adhesion of tumor cells. Therefore, targeting Gal-9 has significant therapeutic potential in inhibiting its malignant behavior. 4) Increased expression and secretion of Gal-9 drive the production of chemokines and inflammatory cytokines. Chemokines support tumor growth and poor prognosis, while inflammatory cytokines are involved in inhibiting TME formation and the Th2 inflammatory state that supports tumor growth. These characteristics suggest the reasons why this population may benefit from anti-Gal-9 targeted therapy21,42,43,54,106, 107, 108.
Gal-9 is a widely expressed protein in immune cells, and its expression is involved in the regulation of various normal physiological functions. So, how can anti-Gal-9 targeted therapy avoid the off-target effects of normal tissues to achieve precision therapy? First, the expression pattern of Gal-9 in malignant tumors is different from that of normal cells. Studies have shown that Gal-9 is expressed mainly inside T cells, and its secreted form is barely detected under physiological conditions30. Serum Gal-9 levels can be significantly increased due to increased production of cancer cells, and cellular or tissue damage109,110. Excessive circulating Gal-9 competes for the availability of receptors outside their cell vicinity, such as Tim-3, PD-1, CD44, Dectin-1, etc., which may trigger unwanted signals, such as immunosuppression and apoptosis107,110. Meanwhile, studies and public database analysis of The Human Protein Atlas found that Dectin-1 and CD44 were almost unexpressed or low expressed in normal tissues and organs, while significantly expressed in malignancies42,111,112. Anti-Gal-9 targeted therapy works by competitively blocking the abnormal binding signal between Gal-9 and its receptors in tumors and manipulating its secretory pathways to normalize Gal-9 levels. Second, Gal-9-Tim-3 in tumor biology is different from normal cells. In many hematopoietic lineage tumors and solid cancers, the interaction between Gal-9 and Tim-3 may play a role in regulating tumor cell expansion and survival. The secretion of Gal-9 and Tim-3 was not detected in normal hematopoietic stem cells but significantly increased in myeloid LSCs. The co-secretion of Gal-9 and Tim-3 promoted the expansion and transformation of malignant myeloid clones51. Third, Gal-9 contains two CRDs in the N- and C-terminal regions, with different activity characteristics. N-CRD is involved in the regulation of innate immune cells, and C-CRD is more effective in inducing T-cell apoptosis113. One study found that anti-Gal-9 specific antibodies targeting C-CRD significantly inhibited tumor growth and improved survival in tumors with high Gal-9 expression, and reduced differentiation of tumor-associated macrophages into protumor phenotypes. However, its binding to N-CRD was significantly lower than that of C-CRD, which also proved that it has no direct effect on monocyte differentiation, suggesting that anti-Gal-9 antibodies did not interfere with the regulation of innate immune function114. Finally, Gal-9, expressed in various normal tissues and organs, binds to cell surface receptors to elicit transient signals that participate in the regulation of T or B cell homeostasis in normal tissues. It also can be used to distinguish non-self from self by reading different glycan signals on pathogens and regulating host immune response, as well as facilitating timely shutdown of adaptive immunity to prevent autoimmune diseases115,116.
Synergistic treatment of anti-galectin-9/Tim-3 in the context of existing immune checkpoint blockade
Immune checkpoint blockade (ICB) therapy has recently emerged as a significant clinical breakthrough in the treatment of advanced malignancies117,118. Although a series of ICIs, such as PD-1 and CTLA-4 inhibitors, have proven successful in a range of malignancies, there are subsets of patients that do not respond to these agents due to the upregulation of adaptive and innate resistance mechanisms by the tumor and its surrounding microenvironment119. Thus, significant challenges remain, including understanding pathways of resistance, optimizing patient selection, improving the management of immune-related adverse events, and identifying rational therapeutic combinations120. As new immunotherapeutic strategies are developed, there is a need for rational implementation of novel immunotherapy combinations that target complementary mechanisms of immunotherapy resistance intrinsic to each patient and tumor type.
Gal-9 and Tim-3 do not have an exclusive ligand-receptor as they have multiple binding partners involved in different signaling pathways121. Gal-9 is highly positively correlated with PD-1/PD-L1 in glioma patients and can be used as a biomarker for PD-1/PD-L1 tumor therapy104. A recent study showed that Gal-9 is a PD-1-binding protein, and the binding of Gal-9 to PD-1 is mainly mediated by the CRD of Gal-9 and the n116-linked glycan of PD-1. The two T cell inhibitory receptors PD-1 and Tim-3 are co-expressed on exhausted T cell differentiation, and PD-1 interacts with Gal-9 and Tim-3 to attenuate Gal-9/Tim-3-induced apoptosis of PD-1+ Tim-3+ T cells in cancers41. In addition, PD-1 expression desensitizes T cells to cell death mediated by the interaction of Gal-9 with Tim-3, and Gal-9 expression and secretion is regulated by IFNs122. These evidences confirm that Gal-9 as an important regulator of tumor immune response that can be targeted for cancer immunotherapy. Combined blockade of Gal-9 and PD-1 synergistically protects and promotes T cell activation in orthotopic pancreatic ductal adenocarcinoma42, strongly suggesting that anti-Gal-9 therapy may also be used in conjunction with ICIs for cancer treatment. Furthermore, anti-Gal-9 therapy reduces the resistance and increases the sensitivity of PD-1 inhibitors in tumor immunotherapy. Tim-3 expression in CD8+ T cells and Gal-9 expression in myeloid-derived suppressor cells are involved in lung cancer drug resistance to nivolumab, a monoclonal antibody (mAb) targeting PD-1. Anti-Tim-3 blocking antibody interferes with the Gal-9/Tim-3 pathway, thereby reversing resistance to PD-1 in lung cancer patients82. Tim-3+ PD-1+ TILs show a significant decrease in IL-2, TNF, and IFN-γ production, blocking Gal-9 enhanced the expression of these cytokines, thereby increasing the therapeutic effect of PD-1123. The expression of PD-L1 and Gal-9 in triple-negative breast cancer is increased by nuclear activation of NF-κB after treatment with taxane and anthracyclines. Cytotoxic agents, which increase immune checkpoint expression in tumor cells may increase the efficacy of ICIs therapy124.
Galectin-9-Tim-3 targeting agents under development
There is increasing evidence that Gal-9-Tim-3 interaction plays a critical role in the context of cancer. In human AML, the secretion of Gal-9 and Tim-3 induces AML cells to evade immune surveillance and form an autocrine Gal-9-Tim-3 loop to drive the self-renewal of AML stem cells51. Gal-9 and Tim-3 expression levels were higher in the blood and bone marrow of AML patients who failed chemotherapy, and targeting the Gal-9-Tim-3 axis could be effectively combined with induction chemotherapy to increase the likelihood of complete remission in AML patients105. Furthermore, transgenic overexpression of Tim-3 has been shown to lead to increased frequency of CD11b+ Ly-6G+ myeloid suppressor cells, as well as a similar effect with Gal-9 overexpression, and loss of Tim-3 reversed this expansion and restored normal immune responses125. In breast cancer models, anti-Tim-3 antibody administration increased granzyme B expression in CD8+ T cells and elicited an immune-mediated response to chemotherapy. Anti-Tim-3 and anti-Gal-9 antibodies showed equal effectiveness in improving the ability to respond to paclitaxel chemotherapy126. Collectively, these data suggest that the Gal-9-Tim-3 interactions inhibit immune responses and facilitate tumor growth. Therefore, the development of small-molecule inhibitors targeting Gal-9-Tim-3 has therapeutic potential for cancers whose growth is potentially mutually regulated by the Gal-9-Tim-3 signaling.
In cancer, Tim-3 expression specifically marks the most dysfunctional or terminally exhausted subset of tumor-infiltrating CD8+ T cells. High expression of Tim-3 is associated with poor prognosis of solid malignancies and accumulated preclinical models suggest therapeutic benefits of Tim-3 blockade in regulating TME and limiting tumor growth, especially in combination with PD-1 blockade41,127,128. In addition to Tim-3+ CD8+ T cells, Tim-3+ Treg cells are also targeted by anti-Tim-3 antibodies. Tim-3+ Treg cells account for more than 50% of total Treg cells in malignant solid tumors, and their expression is correlated with tumor severity and progression. Anti-Tim-3 antibodies have been developed with high affinity and selectivity for Tim-3, specifically blocking the binding of Tim-3 to one or more its ligands, such as Gal-9, phosphatidylserine (PtdSer), carcinoembryonic antigen cell adhesion-related molecule 1 (CEACAM1), and high mobility group protein B1 (HMGB1). Antagonistic ligand-blocking anti-Tim-3 antibodies have the potential to eliminate T cell inhibition, activate antigen-specific T cells, and enhance anti-tumor immunity121,129,130.
To date, many human phase 1/2 clinical trials of Tim-3 antibodies have been initiated (Table 3), as well as ongoing clinical trials targeting Gal-9. The first trial data to be reported was TSR-022, a humanized anti-Tim-3 IgG4 antibody. TSR-022 monotherapy resulted in stable disease in 31 patients with advanced solid tumors and one partial response in a patient with leiomyosarcoma, with no dose-limiting toxicity observed131. This provides support for combination therapy with anti-PD-1 antibodies (such as TSR-042). The clinical data of TSR-022, in monotherapy or in combination with TSR-042 in patients who progressed after anti-PD-1 treatment indicated that the combination of TSR-022 and TSR-042 (500 mg) was generally well-tolerated in both NSCLC and melanoma patients, and clinical activities have been observed in combination therapy. Particularly, at high doses of TSR-022 (300 mg) with an objective response rate (ORR) of 15% (3/20) and stable disease of 40% (8/20)132. Eli Lilly and Company reported that their anti-Tim-3 antibody, LY3321367, was preliminarily well-tolerated and passed phase I clinical trials133. According to the latest trial data, LY3321367 monotherapy had an ORR of 0%, disease control rate (DCR) of 35%, and progression-free survival (PFS) of 1.9 months in anti-PD-1/L1 refractory NSCLC patients (n = 23), ORR of 7%, DCR of 50%, and PFS of 7.3 months in PD-1/L1 responders (n = 14). In patients treated in combination with PD-L1 antibody, LY300054, ORR was 4% and DCR was 42% (n = 91). Data from this study indicate that LY3321367 has an acceptable safety profile and modest antitumor activity, and further studies in large cancer populations with assessable tumor samples for biological evaluation are needed to determine its efficacy134. Novartis’ anti-Tim-3 antibody MBG453 in combination with spartalizumab, an anti-PD-1 antibody, also showed preliminary signs of antitumor activity and calculated a recommended phase II dose of 800 mg Q4W135. These data are consistent with data from preclinical models showing that the combination of anti-Tim-3 and anti-PD-1/PD-L1 is superior to monotherapy. Since December 2020, a Phase 1/2 clinical trial (NCT04666688) has been conducted to evaluate the safety and anti-tumor activity of LYT-200 (a mAb targeting Gal-9 protein) alone and in combination with chemotherapy or anti-PD-1 in patients with metastatic solid tumors (such as pancreatic cancer, cholangiocarcinoma, etc.). This is the first clinical trial to investigate targeting Gal-9 mAb, and we look forward to reporting the data from subsequent studies. Overall, existing human clinical trials data suggest that targeting Gal-9/Tim-3 and inhibiting Tim-3 can enhance the antitumor effect of PD-1 blockade. These results offer new insights into cancer patients who are unable to benefit from anti-PD-1 antibodies.
Table 3.
Anti-Tim-3 clinical trials.
| Reagent name (manufacturer) | ClinicalTrials.gov Identifier | Phase | Cancer type | Co-blockade | Primary outcome | Secondary outcome | Recruitment status |
|---|---|---|---|---|---|---|---|
| Sym023 (Symphogen A/S) | NCT03489343 | I | Solid tumors and lymphoma | Monotherapy | MTD; RP2D | Immunogenicity; ORR; SD; TTP | Completed |
| Sym023 (Symphogen A/S) | NCT03311412 | I | Solid tumors and lymphoma | Anti-PD-1 (Sym021) | Treatment emergent AEs meeting DLT criteria (safety and tolerability) | Immunogenicity; ORR; SD; TTP; maximum concentration | Recruiting |
| TSR-022 (Tesaro) | NCT03680508 | II | Locally advanced or metastatic liver cancer | Anti-PD-1 (TSR-042) | ORR | DOR; TTP; PFS; alpha-fetoprotein (AFP) response | Recruiting |
| TSR-022 (Tesaro) | NCT02817633 | I | Advanced solid tumors | Anti-PD-1 (TSR-042) | DLT; AEs; ORR | DOR; DCR; OS; t1/2 of TSR-022 in combination with TSR-042 | Recruiting |
| TSR-022 (Tesaro) | NCT04139902 | II | Melanoma | Anti-PD-1 (TSR-042) | MPR | AEs; relapse-free survival; OS; frequency of cancellations of surgery | Recruiting |
| LY3321367 (Eli Lilly and Company) | NCT03099109 | Ia/Ib | Advanced relapsed/refractory solid tumors | Anti-PD-1 (LY3300054) | Number of participants with DLTs | Cmax of LY3321367; Cmax of LY3321367 in combination with LY3300054; PFS; DOR; TTR; DCR | Active, no recruiting |
| RO7247669 (Hoffmann-La Roche) | NCT04785820 | II | Advanced or metastatic esophageal squamous cell carcinoma | Targets both Tim-3 and PD-1 | OS | AEs; DCR; PFS; serum concentrations of RO7247669; CD8+Tim-3+ | Recruiting |
| AZD7789 (AstraZeneca) | NCT04931654 | I/IIa | NSCLC | Targets both Tim-3 and PD-1 | AEs; DLT; preliminary anti-tumor activity of AZD7789 | ORR; DCR; DOR; OS; Cmax; immunogenicity; clearance; | Not yet recruiting |
| MBG453 (Novartis Pharmaceuticals) | NCT03961971 | I | Glioblastoma multiforme | Anti-PD-1 (spartalizumab) | Number of participants with serious AEs | PFS; OS; ORR | Recruiting |
| MBG453 (Novartis Pharmaceuticals) | NCT04823624 | II | Myelodysplastic syndromes | Monotherapy | ORR | OS; PFS; TTP; DOR | Not yet recruiting |
| MBG453 (Novartis Pharmaceuticals) | NCT02608268 | I-Ib/II | Advanced malignancies | Anti-PD-1 (PDR001) | Safety and tolerability; ORR; DLTs | BOR; presence and concentration of anti-MBG453 antibodies; Cmax; OS; DOR; PFS; | Active, not recruiting |
| MBG453 (Novartis Pharmaceuticals) | NCT03946670 | II | Myelodysplastic syndromes | Randomized; HMA (decitabine or azacitidine) | CR rate; PFS | OS; event free survival; leukemia-free survival; response rate; time to CR | Active, not recruiting |
| INCAGN02390 (Incyte Corporation) | NCT03652077 | I | Advanced malignancies | Monotherapy | Number of treatment-emergent adverse events; maximum tolerated dose of INCAGN02390 | ORR; DOR; DCR; PFS; immunogenicity; level of binding of INCAGN02390 to Tim-3 | Active, not recruiting |
| BMS-986258 (Bristol-Myers Squibb) | NCT03446040 | I-II | Advanced cancer | Anti-PD-1 (Nivolumab), human recombinant hyaluronidase | AEs; serious AEs; incidence of AEs leading to discontinuation or to death | ORR; PFS; Cmax; DOR; incidence of anti-drug antibody to BMS-986258 | Recruiting |
| RO7121661 (Hoffmann-La Roche) | NCT03708328 | I | Solid tumors, metastatic melanoma; NSCLC | Targets both Tim-3 and PD-1 | DLT; ORR; DCR; DOR; PFS | Cmax; total clearance; Volume of Distribution at Steady State; terminal half-life | Active, not recruiting |
| BGB-A425 (BeiGene) | NCT03744468 | I | Solid tumors | Anti-PD-1 (Tislelizumab) | Safety and tolerability (MTD, RP2D and MAD) | DOR; DCR; PFS; Cmax; terminal half-life; immunogenicity | Recruiting |
Abbreviations: MTD, maximum tolerated dose; RP2D, recommended phase 2 dose; OR, objective response rate; SD, stable disease; TTP, time to progression; DLT, dose-limiting toxicities; AE, adverse event; PFS, progression free survival; DOR, duration of response; DCR, disease control rate; MPR, major pathologic response; Cmax, maximum concentration; BOR, best overall response; NSCLC, non-small cell lung cancer; MAD, maximum administered dose.
Conclusions
Gal-9 has attracted much attention in recent years due to its immunomodulatory role in various cancers. Gal-9 is expressed in virtually all organ systems and regulates cellular signaling by binding to its receptor, namely Tim-3, PD-1, CD44, CD40, 4-1BB, DR3, VISTA, and TLR-4. Gal-9 is involved in many physiological functions, such as growth, differentiation and death, cell adhesion, communication, and immune regulation. At present, many studies have agreed that the expression of Gal-9 is related to human cancer. The expression of Gal-9 plays different roles in different solid tumors and hematological tumors. Gal-9 has been established as a reliable, sensitive, and easily measured non-invasive biomarker, which can be combined with other disease parameters to jointly define disease activity and severity under various pathological conditions and can also be used as a prognostic monitoring indicator. Subsequent studies will focus on the specific role of dynamic changes in Gal-9 levels in monitoring disease progression and facilitating individualized treatment decisions. How to normalize the level of Gal-9 should be considered in subsequent studies, possibly through competitive blocking, interference of secretion pathways, and traditional treatment strategies. There are currently no obvious inhibitors of Gal-9, and the development of novel Gal-9 inhibitors to reduce the risk of cancers associated with high levels of Gal-9 may have great therapeutic potential in improving the clinical outcomes of cancer patients.
The interaction between Gal-9 and Tim-3 plays a key role in tumor immunity, and blocking the Gal-9/Tim-3 pathway induces anti-tumor immune responses and inhibits tumor growth. Therefore, activating cell costimulatory molecules by combining anti-Gal-9 antibodies or anti-Tim-3 antibodies and other immune checkpoint inhibitors may be a new type of immunotherapy for cancer treatment in the near future. Novel immunotherapy is a latent approach with great clinical benefits. Meanwhile, it is necessary to explore the synergistic or antagonistic relationship between different immune checkpoints to provide more scientific and reasonable individualized treatment, to open up a new path for cancer treatment.
Author contributions
Yan Lv, Xiao Ma, Yuxin Ma conceived and designed the study and also managed the collected data. All authors contributed to data collection and wrote the manuscript. Yuxin Du fully revised the manuscript, designed, and presented the figures and supported the work along with Jifeng Feng and Yan Lv.
Conflict of interests
The authors promise no potential conflicts of interest.
Funding
This work was supported by National Natural Science Foundation of China (No. 32000497 to Y. Du).
Data availability statement
Research data are not shared unless the corresponding authors agree.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Yuxin Du, Email: yuxindu0408@gmail.com.
Jifeng Feng, Email: jifengfeng2021@gmail.com.
References
- 1.Cooper D.N. Galectinomics: finding themes in complexity. Biochim Biophys Acta. 2002;1572(2–3):209–231. doi: 10.1016/s0304-4165(02)00310-0. [DOI] [PubMed] [Google Scholar]
- 2.Méndez-Huergo S.P., Blidner A.G., Rabinovich G.A. Galectins: emerging regulatory checkpoints linking tumor immunity and angiogenesis. Curr Opin Immunol. 2017;45:8–15. doi: 10.1016/j.coi.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Vasta G.R. Galectins in host-pathogen interactions: structural, functional and evolutionary aspects. Adv Exp Med Biol. 2020;1204:169–196. doi: 10.1007/978-981-15-1580-4_7. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad N., Gabius H.J., André S., et al. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J Biol Chem. 2004;279(12):10841–10847. doi: 10.1074/jbc.M312834200. [DOI] [PubMed] [Google Scholar]
- 5.Yang R.Y., Rabinovich G.A., Liu F.T. Galectins: structure, function and therapeutic potential. Expet Rev Mol Med. 2008;10:e17. doi: 10.1017/S1462399408000719. [DOI] [PubMed] [Google Scholar]
- 6.Popa S.J., Stewart S.E., Moreau K. Unconventional secretion of annexins and galectins. Semin Cell Dev Biol. 2018;83:42–50. doi: 10.1016/j.semcdb.2018.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bänfer S., Jacob R. Galectins in intra- and extracellular vesicles. Biomolecules. 2020;10(9):1232. doi: 10.3390/biom10091232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leal-Pinto E., Tao W., Rappaport J., Richardson M., Knorr B.A., Abramson R.G. Molecular cloning and functional reconstitution of a urate transporter/channel. J Biol Chem. 1997;272(1):617–625. doi: 10.1074/jbc.272.1.617. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto R., Matsumoto H., Seki M., et al. Human ecalectin, a variant of human galectin-9, is a novel eosinophil chemoattractant produced by T lymphocytes. J Biol Chem. 1998;273(27):16976–16984. doi: 10.1074/jbc.273.27.16976. [DOI] [PubMed] [Google Scholar]
- 10.Türeci O., Schmitt H., Fadle N., Pfreundschuh M., Sahin U. Molecular definition of a novel human galectin which is immunogenic in patients with Hodgkin's disease. J Biol Chem. 1997;272(10):6416–6422. doi: 10.1074/jbc.272.10.6416. [DOI] [PubMed] [Google Scholar]
- 11.Kageshita T., Kashio Y., Yamauchi A., et al. Possible role of galectin-9 in cell aggregation and apoptosis of human melanoma cell lines and its clinical significance. Int J Cancer. 2002;99(6):809–816. doi: 10.1002/ijc.10436. [DOI] [PubMed] [Google Scholar]
- 12.Kasamatsu A., Uzawa K., Nakashima D., et al. Galectin-9 as a regulator of cellular adhesion in human oral squamous cell carcinoma cell lines. Int J Mol Med. 2005;16(2):269–273. [PubMed] [Google Scholar]
- 13.Irie A., Yamauchi A., Kontani K., et al. Galectin-9 as a prognostic factor with antimetastatic potential in breast cancer. Clin Cancer Res. 2005;11(8):2962–2968. doi: 10.1158/1078-0432.CCR-04-0861. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Z.Y., Dong J.H., Chen Y.W., et al. Galectin-9 acts as a prognostic factor with antimetastatic potential in hepatocellular carcinoma. Asian Pac J Cancer Prev APJCP. 2012;13(6):2503–2509. doi: 10.7314/apjcp.2012.13.6.2503. [DOI] [PubMed] [Google Scholar]
- 15.Zhu C., Anderson A.C., Schubart A., et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6(12):1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 16.Seki M., Oomizu S., Sakata K.M., et al. Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin Immunol. 2008;127(1):78–88. doi: 10.1016/j.clim.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y., Song L., Sun J., et al. Expression of Galectin-9 and correlation with disease activity and vascular endothelial growth factor in rheumatoid arthritis. Clin Exp Rheumatol. 2020;38(4):654–661. [PubMed] [Google Scholar]
- 18.Labrie M., De Araujo L.O.F., Communal L., Mes-Masson A.M., St-Pierre Y. Tissue and plasma levels of galectins in patients with high grade serous ovarian carcinoma as new predictive biomarkers. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-13802-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bi S., Earl L.A., Jacobs L., Baum L.G. Structural features of galectin-9 and galectin-1 that determine distinct T cell death pathways. J Biol Chem. 2008;283(18):12248–12258. doi: 10.1074/jbc.M800523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen D.S., Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 21.Chou F.C., Chen H.Y., Kuo C.C., Sytwu H.K. Role of galectins in tumors and in clinical immunotherapy. Int J Mol Sci. 2018;19(2):430. doi: 10.3390/ijms19020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oda Y., Herrmann J., Gitt M.A., et al. Soluble lactose-binding lectin from rat intestine with two different carbohydrate-binding domains in the same peptide chain. J Biol Chem. 1993;268(8):5929–5939. [PubMed] [Google Scholar]
- 23.Barondes S.H., Cooper D.N., Gitt M.A., Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994;269(33):20807–20810. [PubMed] [Google Scholar]
- 24.Golden-Mason L., Rosen H.R. Galectin-9: diverse roles in hepatic immune homeostasis and inflammation. Hepatology. 2017;66(1):271–279. doi: 10.1002/hep.29106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sudhakar J.N., Lu H.H., Chiang H.Y., et al. Lumenal Galectin-9-Lamp2 interaction regulates lysosome and autophagy to prevent pathogenesis in the intestine and pancreas. Nat Commun. 2020;11(1):4286. doi: 10.1038/s41467-020-18102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang R., Hung M.C. The role of T-cell immunoglobulin mucin-3 and its ligand galectin-9 in antitumor immunity and cancer immunotherapy. Sci China Life Sci. 2017;60(10):1058–1064. doi: 10.1007/s11427-017-9176-7. [DOI] [PubMed] [Google Scholar]
- 27.John S., Mishra R. Galectin-9: from cell biology to complex disease dynamics. J Biosci. 2016;41(3):507–534. doi: 10.1007/s12038-016-9616-y. [DOI] [PubMed] [Google Scholar]
- 28.Aanhane E., Schulkens I.A., Heusschen R., et al. Different angioregulatory activity of monovalent galectin-9 isoforms. Angiogenesis. 2018;21(3):545–555. doi: 10.1007/s10456-018-9607-8. [DOI] [PubMed] [Google Scholar]
- 29.Chen H.L., Liao F., Lin T.N., Liu F.T. Galectins and neuroinflammation. Adv Neurobiol. 2014;9:517–542. doi: 10.1007/978-1-4939-1154-7_24. [DOI] [PubMed] [Google Scholar]
- 30.Chen H.Y., Wu Y.F., Chou F.C., et al. Intracellular galectin-9 enhances proximal TCR signaling and potentiates autoimmune diseases. J Immunol. 2020;204(5):1158–1172. doi: 10.4049/jimmunol.1901114. [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto R., Hirashima M., Kita H., Gleich G.J. Biological activities of ecalectin: a novel eosinophil-activating factor. J Immunol. 2002;168(4):1961–1967. doi: 10.4049/jimmunol.168.4.1961. [DOI] [PubMed] [Google Scholar]
- 32.Oomizu S., Arikawa T., Niki T., et al. Cell surface galectin-9 expressing Th cells regulate Th17 and Foxp3+ Treg development by galectin-9 secretion. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0048574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kashio Y., Nakamura K., Abedin M.J., et al. Galectin-9 induces apoptosis through the calcium-calpain-caspase-1 pathway. J Immunol. 2003;170(7):3631–3636. doi: 10.4049/jimmunol.170.7.3631. [DOI] [PubMed] [Google Scholar]
- 34.Cao A., Alluqmani N., Buhari F.H.M., et al. Galectin-9 binds IgM-BCR to regulate B cell signaling. Nat Commun. 2018;9(1):3288. doi: 10.1038/s41467-018-05771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chakraborty A., Staudinger C., King S.L., et al. Galectin-9 bridges human B cells to vascular endothelium while programming regulatory pathways. J Autoimmun. 2021;117 doi: 10.1016/j.jaut.2020.102575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dai S.Y., Nakagawa R., Itoh A., et al. Galectin-9 induces maturation of human monocyte-derived dendritic cells. J Immunol. 2005;175(5):2974–2981. doi: 10.4049/jimmunol.175.5.2974. [DOI] [PubMed] [Google Scholar]
- 37.Querol Cano L., Tagit O., Dolen Y., et al. Intracellular galectin-9 controls dendritic cell function by maintaining plasma membrane rigidity. iScience. 2019;22:240–255. doi: 10.1016/j.isci.2019.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson A.C., Anderson D.E., Bregoli L., et al. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318(5853):1141–1143. doi: 10.1126/science.1148536. [DOI] [PubMed] [Google Scholar]
- 39.Koguchi K., Anderson D.E., Yang L., O'Connor K.C., Kuchroo V.K., Hafler D.A. Dysregulated T cell expression of TIM3 in multiple sclerosis. J Exp Med. 2006;203(6):1413–1418. doi: 10.1084/jem.20060210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y.H., Zhou W.H., Tao Y., et al. The Galectin-9/Tim-3 pathway is involved in the regulation of NK cell function at the maternal-fetal interface in early pregnancy. Cell Mol Immunol. 2016;13(1):73–81. doi: 10.1038/cmi.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang R., Sun L., Li C.F., et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat Commun. 2021;12(1):832. doi: 10.1038/s41467-021-21099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daley D., Mani V.R., Mohan N., et al. Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance. Nat Med. 2017;23(5):556–567. doi: 10.1038/nm.4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Enninga E.A.L., Chatzopoulos K., Butterfield J.T., et al. CD206-positive myeloid cells bind galectin-9 and promote a tumor-supportive microenvironment. J Pathol. 2018;245(4):468–477. doi: 10.1002/path.5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaitaitis G.M., Wagner D.H., Jr. Galectin-9 controls CD40 signaling through a Tim-3 independent mechanism and redirects the cytokine profile of pathogenic T cells in autoimmunity. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0038708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Madireddi S., Eun S.Y., Lee S.W., et al. Galectin-9 controls the therapeutic activity of 4-1BB-targeting antibodies. J Exp Med. 2014;211(7):1433–1448. doi: 10.1084/jem.20132687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yasinska I.M., Meyer N.H., Schlichtner S., et al. Ligand-receptor interactions of galectin-9 and VISTA suppress human T lymphocyte cytotoxic activity. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.580557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Madireddi S., Eun S.Y., Mehta A.K., et al. Regulatory T cell-mediated suppression of inflammation induced by DR3 signaling is dependent on galectin-9. J Immunol. 2017;199(8):2721–2728. doi: 10.4049/jimmunol.1700575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu C., Thalhamer T., Franca R.F., et al. Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory T cells. Immunity. 2014;41(2):270–282. doi: 10.1016/j.immuni.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang T., Ma C., Wang T., et al. Galectin-9 promotes neuronal restoration via binding TLR-4 in a rat intracerebral hemorrhage model. NeuroMolecular Med. 2021;23(2):267–284. doi: 10.1007/s12017-020-08611-5. [DOI] [PubMed] [Google Scholar]
- 50.Hastings W.D., Anderson D.E., Kassam N., et al. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur J Immunol. 2009;39(9):2492–2501. doi: 10.1002/eji.200939274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kikushige Y., Miyamoto T., Yuda J., et al. A TIM-3/Gal-9 autocrine stimulatory loop drives self-renewal of human myeloid leukemia stem cells and leukemic progression. Cell Stem Cell. 2015;17(3):341–352. doi: 10.1016/j.stem.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 52.Gonçalves Silva I., Yasinska I.M., Sakhnevych S.S., et al. The tim-3-galectin-9 secretory pathway is involved in the immune escape of human acute myeloid leukemia cells. EBioMedicine. 2017;22:44–57. doi: 10.1016/j.ebiom.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yan J., Zhang Y., Zhang J.P., Liang J., Li L., Zheng L. Tim-3 expression defines regulatory T cells in human tumors. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0058006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yasinska I.M., Sakhnevych S.S., Pavlova L., et al. The Tim-3-galectin-9 pathway and its regulatory mechanisms in human breast cancer. Front Immunol. 2019;10:1594. doi: 10.3389/fimmu.2019.01594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kang C.W., Dutta A., Chang L.Y., et al. Apoptosis of tumor infiltrating effector TIM-3+CD8+ T cells in colon cancer. Sci Rep. 2015;5 doi: 10.1038/srep15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li H., Wu K., Tao K., et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2012;56(4):1342–1351. doi: 10.1002/hep.25777. [DOI] [PubMed] [Google Scholar]
- 57.Nagahara K., Arikawa T., Oomizu S., et al. Galectin-9 increases Tim-3+ dendritic cells and CD8+ T cells and enhances antitumor immunity via galectin-9-Tim-3 interactions. J Immunol. 2008;181(11):7660–7669. doi: 10.4049/jimmunol.181.11.7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klibi J., Niki T., Riedel A., et al. Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cells. Blood. 2009;113(9):1957–1966. doi: 10.1182/blood-2008-02-142596. [DOI] [PubMed] [Google Scholar]
- 59.Chen Z., Dong D., Zhu Y., Pang N., Ding J. The role of Tim-3/Galectin-9 pathway in T-cell function and prognosis of patients with human papilloma virus-associated cervical carcinoma. FASEB J. 2021;35(3) doi: 10.1096/fj.202000528RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seifert A.M., Reiche C., Heiduk M., et al. Detection of pancreatic ductal adenocarcinoma with galectin-9 serum levels. Oncogene. 2020;39(15):3102–3113. doi: 10.1038/s41388-020-1186-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yuan F., Ming H., Wang Y., et al. Molecular and clinical characterization of Galectin-9 in glioma through 1,027 samples. J Cell Physiol. 2020;235(5):4326–4334. doi: 10.1002/jcp.29309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakajima R., Miyagaki T., Kamijo H., et al. Possible therapeutic applicability of galectin-9 in cutaneous T-cell lymphoma. J Dermatol Sci. 2019;96(3):134–142. doi: 10.1016/j.jdermsci.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 63.Wdowiak K., Gallego-Colon E., Francuz T., et al. Increased serum levels of Galectin-9 in patients with chronic lymphocytic leukemia. Oncol Lett. 2019;17(1):1019–1029. doi: 10.3892/ol.2018.9656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y., Sun J., Ma C., et al. Reduced expression of galectin-9 contributes to a poor outcome in colon cancer by inhibiting NK cell chemotaxis partially through the Rho/ROCK1 signaling pathway. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0152599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lahm H., André S., Hoeflich A., et al. Comprehensive galectin fingerprinting in a panel of 61 human tumor cell lines by RT-PCR and its implications for diagnostic and therapeutic procedures. J Cancer Res Clin Oncol. 2001;127(6):375–386. doi: 10.1007/s004320000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen S., Pu J., Bai J., et al. EZH2 promotes hepatocellular carcinoma progression through modulating miR-22/galectin-9 axis. J Exp Clin Cancer Res. 2018;37(1):3. doi: 10.1186/s13046-017-0670-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang J., Zhu L., Cai Y., Suo J., Jin J. Role of downregulation of galectin-9 in the tumorigenesis of gastric cancer. Int J Oncol. 2014;45(3):1313–1320. doi: 10.3892/ijo.2014.2494. [DOI] [PubMed] [Google Scholar]
- 68.Hou N., Ma J., Li W., Zhao L., Gao Q., Mai L. T-cell immunoglobulin and mucin domain-containing protein-3 and galectin-9 protein expression: potential prognostic significance in esophageal squamous cell carcinoma for Chinese patients. Oncol Lett. 2017;14(6):8007–8013. doi: 10.3892/ol.2017.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamauchi A., Kontani K., Kihara M., Nishi N., Yokomise H., Hirashima M. Galectin-9, a novel prognostic factor with antimetastatic potential in breast cancer. Breast J. 2006;12(5 Suppl 2):S196–S200. doi: 10.1111/j.1075-122X.2006.00334.x. [DOI] [PubMed] [Google Scholar]
- 70.Jiang J., Jin M.S., Kong F., et al. Decreased galectin-9 and increased Tim-3 expression are related to poor prognosis in gastric cancer. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0081799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Long B., Yu Z., Zhou H., et al. Clinical characteristics and prognostic significance of galectins for patients with gastric cancer: a meta-analysis. Int J Surg. 2018;56:242–249. doi: 10.1016/j.ijsu.2018.06.033. [DOI] [PubMed] [Google Scholar]
- 72.Takano J., Morishita A., Fujihara S., et al. Galectin-9 suppresses the proliferation of gastric cancer cells in vitro. Oncol Rep. 2016;35(2):851–860. doi: 10.3892/or.2015.4452. [DOI] [PubMed] [Google Scholar]
- 73.Cho S.J., Kook M.C., Lee J.H., et al. Peroxisome proliferator-activated receptor γ upregulates galectin-9 and predicts prognosis in intestinal-type gastric cancer. Int J Cancer. 2015;136(4):810–820. doi: 10.1002/ijc.29056. [DOI] [PubMed] [Google Scholar]
- 74.Shen P., Yue R., Tang J., et al. Preferential Tim-3 expression on Treg and CD8(+) T cells, supported by tumor-associated macrophages, is associated with worse prognosis in gastric cancer. Am J Transl Res. 2016;8(8):3419–3428. [PMC free article] [PubMed] [Google Scholar]
- 75.Yang Q., Hou C., Huang D., et al. miR-455-5p functions as a potential oncogene by targeting galectin-9 in colon cancer. Oncol Lett. 2017;13(3):1958–1964. doi: 10.3892/ol.2017.5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sakhnevych S.S., Yasinska I.M., Fasler-Kan E., Sumbayev V.V. Mitochondrial defunctionalization supresses Tim-3-galectin-9 secretory pathway in human colorectal cancer cells and thus can possibly affect tumor immune escape. Front Pharmacol. 2019;10:342. doi: 10.3389/fphar.2019.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wiersma V.R., de Bruyn M., Wei Y., et al. The epithelial polarity regulator LGALS9/galectin-9 induces fatal frustrated autophagy in KRAS mutant colon carcinoma that depends on elevated basal autophagic flux. Autophagy. 2015;11(8):1373–1388. doi: 10.1080/15548627.2015.1063767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.He Y., Jia K., Dziadziuszko R., et al. Galectin-9 in non-small cell lung cancer. Lung Cancer. 2019;136:80–85. doi: 10.1016/j.lungcan.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 79.Chen P., Zhang L., Zhang W., et al. Galectin-9-based immune risk score model helps to predict relapse in stage I-III small cell lung cancer. J Immunother Cancer. 2020;8(2) doi: 10.1136/jitc-2020-001391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nobumoto A., Nagahara K., Oomizu S., et al. Galectin-9 suppresses tumor metastasis by blocking adhesion to endothelium and extracellular matrices. Glycobiology. 2008;18(9):735–744. doi: 10.1093/glycob/cwn062. [DOI] [PubMed] [Google Scholar]
- 81.Kadowaki T., Arikawa T., Shinonaga R., et al. Galectin-9 signaling prolongs survival in murine lung-cancer by inducing macrophages to differentiate into plasmacytoid dendritic cell-like macrophages. Clin Immunol. 2012;142(3):296–307. doi: 10.1016/j.clim.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 82.Limagne E., Richard C., Thibaudin M., et al. Tim-3/galectin-9 pathway and mMDSC control primary and secondary resistances to PD-1 blockade in lung cancer patients. OncoImmunology. 2019;8(4) doi: 10.1080/2162402X.2018.1564505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fujita K., Iwama H., Sakamoto T., et al. Galectin-9 suppresses the growth of hepatocellular carcinoma via apoptosis in vitro and in vivo. Int J Oncol. 2015;46(6):2419–2430. doi: 10.3892/ijo.2015.2941. [DOI] [PubMed] [Google Scholar]
- 84.Sideras K., Biermann K., Verheij J., et al. PD-L1, Galectin-9 and CD8(+) tumor-infiltrating lymphocytes are associated with survival in hepatocellular carcinoma. OncoImmunology. 2017;6(2) doi: 10.1080/2162402X.2016.1273309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chiyo T., Fujita K., Iwama H., et al. Galectin-9 induces mitochondria-mediated apoptosis of esophageal cancer in vitro and in vivo in a xenograft mouse model. Int J Mol Sci. 2019;20(11):2634. doi: 10.3390/ijms20112634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Akashi E., Fujihara S., Morishita A., et al. Effects of galectin-9 on apoptosis, cell cycle and autophagy in human esophageal adenocarcinoma cells. Oncol Rep. 2017;38(1):506–514. doi: 10.3892/or.2017.5689. [DOI] [PubMed] [Google Scholar]
- 87.Ogura M., Takeuchi H., Kawakubo H., et al. Clinical significance of CXCL-8/CXCR-2 network in esophageal squamous cell carcinoma. Surgery. 2013;154(3):512–520. doi: 10.1016/j.surg.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 88.Liu Z., Han H., He X., et al. Expression of the galectin-9-Tim-3 pathway in glioma tissues is associated with the clinical manifestations of glioma. Oncol Lett. 2016;11(3):1829–1834. doi: 10.3892/ol.2016.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sørensen M.D., Nielsen O., Reifenberger G., Kristensen B.W. The presence of TIM-3 positive cells in WHO grade III and IV astrocytic gliomas correlates with isocitrate dehydrogenase mutation status. Brain Pathol. 2021;31(3) doi: 10.1111/bpa.12921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yazdanifar M., Zhou R., Grover P., et al. Overcoming immunological resistance enhances the efficacy of A novel anti-tMUC1-CAR T cell treatment against pancreatic ductal adenocarcinoma. Cells. 2019;8(9):1070. doi: 10.3390/cells8091070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mondragón L., Kroemer G., Galluzzi L. Immunosuppressive γδ T cells foster pancreatic carcinogenesis. OncoImmunology. 2016;5(11) doi: 10.1080/2162402X.2016.1237328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Okura R., Fujihara S., Iwama H., et al. MicroRNA profiles during galectin-9-induced apoptosis of pancreatic cancer cells. Oncol Lett. 2018;15(1):407–414. doi: 10.3892/ol.2017.7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang L., Tian S., Zhao M., et al. SUV39H1-DNMT3A-mediated epigenetic regulation of Tim-3 and galectin-9 in the cervical cancer. Cancer Cell Int. 2020;20:325. doi: 10.1186/s12935-020-01380-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Holderried T.A.W., de Vos L., Bawden E.G., et al. Molecular and immune correlates of TIM-3 (HAVCR2) and galectin 9 (LGALS9) mRNA expression and DNA methylation in melanoma. Clin Epigenet. 2019;11(1):161. doi: 10.1186/s13148-019-0752-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wiersma V.R., de Bruyn M., van Ginkel R.J., et al. The glycan-binding protein galectin-9 has direct apoptotic activity toward melanoma cells. J Invest Dermatol. 2012;132(9):2302–2305. doi: 10.1038/jid.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cristiani C.M., Turdo A., Ventura V., et al. Accumulation of circulating CCR7(+) natural killer cells marks melanoma evolution and reveals a CCL19-dependent metastatic pathway. Cancer Immunol Res. 2019;7(5):841–852. doi: 10.1158/2326-6066.CIR-18-0651. [DOI] [PubMed] [Google Scholar]
- 97.Micevic G., Theodosakis N., Bosenberg M. Aberrant DNA methylation in melanoma: biomarker and therapeutic opportunities. Clin Epigenet. 2017;9:34. doi: 10.1186/s13148-017-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang T., Ren T., Song Z., et al. Genetic mutations of Tim-3 ligand and exhausted Tim-3(+) CD8(+) T cells and survival in diffuse large B cell lymphoma. J Immunol Res. 2020;2020 doi: 10.1155/2020/6968595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang Y., Xue S., Hao Q., Liu F., Huang W., Wang J. Galectin-9 and PSMB8 overexpression predict unfavorable prognosis in patients with AML. J Cancer. 2021;12(14):4257–4263. doi: 10.7150/jca.53686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kocibalova Z., Guzyova M., Borovska I., et al. Development of multidrug resistance in acute myeloid leukemia is associated with alterations of the LPHN1/GAL-9/TIM-3 signaling pathway. Cancers. 2021;13(14):3629. doi: 10.3390/cancers13143629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pang N., Alimu X., Chen R., et al. Activated Galectin-9/Tim3 promotes Treg and suppresses Th1 effector function in chronic lymphocytic leukemia. FASEB J. 2021;35(7) doi: 10.1096/fj.202100013R. [DOI] [PubMed] [Google Scholar]
- 102.Gajewski T.F., Schreiber H., Fu Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Taghiloo S., Allahmoradi E., Ebadi R., et al. Upregulation of galectin-9 and PD-L1 immune checkpoints molecules in patients with chronic lymphocytic leukemia. Asian Pac J Cancer Prev APJCP. 2017;18(8):2269–2274. doi: 10.22034/APJCP.2017.18.8.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liang T., Wang X., Wang F., Feng E., You G. Galectin-9: a predictive biomarker negatively regulating immune response in glioma patients. World Neurosurg. 2019;132:e455–e462. doi: 10.1016/j.wneu.2019.08.117. [DOI] [PubMed] [Google Scholar]
- 105.Dama P., Tang M., Fulton N., Kline J., Liu H. Gal9/Tim-3 expression level is higher in AML patients who fail chemotherapy. J Immunother Cancer. 2019;7(1):175. doi: 10.1186/s40425-019-0611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baloche V., Rivière J., Tran T.B.T., et al. Serial transplantation unmasks galectin-9 contribution to tumor immune escape in the MB49 murine model. Sci Rep. 2021;11(1):5227. doi: 10.1038/s41598-021-84270-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moar P., Tandon R. Galectin-9 as a biomarker of disease severity. Cell Immunol. 2021;361 doi: 10.1016/j.cellimm.2021.104287. [DOI] [PubMed] [Google Scholar]
- 108.Krautter F., Recio C., Hussain M.T., et al. Characterisation of endogenous Galectin-1 and-9 expression in monocyte and macrophage subsets under resting and inflammatory conditions. Biomed Pharmacother. 2020;130 doi: 10.1016/j.biopha.2020.110595. [DOI] [PubMed] [Google Scholar]
- 109.Liu F.T., Rabinovich G.A. Galectins: regulators of acute and chronic inflammation. Ann N Y Acad Sci. 2010;1183:158–182. doi: 10.1111/j.1749-6632.2009.05131.x. [DOI] [PubMed] [Google Scholar]
- 110.Steichen A.L., Simonson T.J., Salmon S.L., Metzger D.W., Mishra B.B., Sharma J. Alarmin function of galectin-9 in murine respiratory tularemia. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0123573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hassn Mesrati M., Syafruddin S.E., Mohtar M.A., Syahir A. CD44: a multifunctional mediator of cancer progression. Biomolecules. 2021;11(12):1850. doi: 10.3390/biom11121850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jang J.H., Kim D.H., Lim J.M., et al. Breast cancer cell-derived soluble CD44 promotes tumor progression by triggering macrophage IL1β production. Cancer Res. 2020;80(6):1342–1356. doi: 10.1158/0008-5472.CAN-19-2288. [DOI] [PubMed] [Google Scholar]
- 113.Li Y., Feng J., Geng S., et al. The N- and C-terminal carbohydrate recognition domains of galectin-9 contribute differently to its multiple functions in innate immunity and adaptive immunity. Mol Immunol. 2011;48(4):670–677. doi: 10.1016/j.molimm.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 114.Bertino P., Premeaux T.A., Fujita T., et al. Targeting the C-terminus of galectin-9 induces mesothelioma apoptosis and M2 macrophage depletion. OncoImmunology. 2019;8(8) doi: 10.1080/2162402X.2019.1601482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vasta G.R., Feng C., González-Montalbán N., et al. Functions of galectins as 'self/non-self'-recognition and effector factors. Pathog Dis. 2017;75(5):ftx046. doi: 10.1093/femspd/ftx046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Giovannone N., Smith L.K., Treanor B., Dimitroff C.J. Galectin-glycan interactions as regulators of B cell immunity. Front Immunol. 2018;9:2839. doi: 10.3389/fimmu.2018.02839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vuong J.T., Stein-Merlob A.F., Nayeri A., Sallam T., Neilan T.G., Yang E.H. Immune checkpoint therapies and atherosclerosis: mechanisms and clinical implications: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(6):577–593. doi: 10.1016/j.jacc.2021.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shang Q., Dong Y., Su Y., Leslie F., Sun M., Wang F. Local scaffold-assisted delivery of immunotherapeutic agents for improved cancer immunotherapy. Adv Drug Deliv Rev. 2022;185 doi: 10.1016/j.addr.2022.114308. [DOI] [PubMed] [Google Scholar]
- 119.Ferreira M.N., Choe J.H. Guiding immunotherapy combinations: who gets what? Adv Drug Deliv Rev. 2021;178 doi: 10.1016/j.addr.2021.113962. [DOI] [PubMed] [Google Scholar]
- 120.Sharma P., Siddiqui B.A., Anandhan S., et al. The next decade of immune checkpoint therapy. Cancer Discov. 2021;11(4):838–857. doi: 10.1158/2159-8290.CD-20-1680. [DOI] [PubMed] [Google Scholar]
- 121.Wolf Y., Anderson A.C., Kuchroo V.K. TIM3 comes of age as an inhibitory receptor. Nat Rev Immunol. 2020;20(3):173–185. doi: 10.1038/s41577-019-0224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Derosiers N., Aguilar W., DeGaramo D.A., Posey A.D., Jr. Sweet immune checkpoint targets to enhance T cell therapy. J Immunol. 2022;208(2):278–285. doi: 10.4049/jimmunol.2100706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sakuishi K., Apetoh L., Sullivan J.M., Blazar B.R., Kuchroo V.K., Anderson A.C. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207(10):2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yoon H.K., Kim T.H., Park S., et al. Effect of anthracycline and taxane on the expression of programmed cell death ligand-1 and galectin-9 in triple-negative breast cancer. Pathol Res Pract. 2018;214(10):1626–1631. doi: 10.1016/j.prp.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 125.Dardalhon V., Anderson A.C., Karman J., et al. Tim-3/galectin-9 pathway: regulation of Th1 immunity through promotion of CD11b+Ly-6G+ myeloid cells. J Immunol. 2010;185(3):1383–1392. doi: 10.4049/jimmunol.0903275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.de Mingo Pulido Á., Gardner A., Hiebler S., et al. TIM-3 regulates CD103(+) dendritic cell function and response to chemotherapy in breast cancer. Cancer Cell. 2018;33(1):60–74. doi: 10.1016/j.ccell.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fourcade J., Sun Z., Benallaoua M., et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207(10):2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhou Q., Munger M.E., Veenstra R.G., et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117(17):4501–4510. doi: 10.1182/blood-2010-10-310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Acharya N., Sabatos-Peyton C., Anderson A.C. Tim-3 finds its place in the cancer immunotherapy landscape. J Immunother Cancer. 2020;8(1) doi: 10.1136/jitc-2020-000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang D., Jiang F., Zaynagetdinov R., et al. Identification and characterization of M6903, an antagonistic anti-TIM-3 monoclonal antibody. OncoImmunology. 2020;9(1) doi: 10.1080/2162402X.2020.1744921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Weiss G.J., Luke J.J., Falchook G., et al. A phase 1 study of TSR-022, an anti-TIM-3 monoclonal antibody, in patients (pts) with advanced solid tumors. J ImmunTherapy Cancer. 2017 Paper presented at: [Google Scholar]
- 132.Davar D., Boasberg P., Eroglu Z., Falchook G., Gainor J., Hamilton E. A phase 1 study of TSR-022, an anti-TIM-3 monoclonal antibody, in combination with TSR-042 (anti-PD-1) in patients with colorectal cancer and post-PD-1 NSCLC and melanoma. J Immunother Cancer. 2018;6(Suppl 1):115O121. [Google Scholar]
- 133.Harding J.J., Patnaik A., Moreno V., et al. A phase Ia/Ib study of an anti-TIM-3 antibody (LY3321367) monotherapy or in combination with an anti-PD-L1 antibody (LY3300054):interim safety, efficacy, and pharmacokinetic findings in advanced cancers. J Clin Oncol. 2019;37(8_suppl):12. [Google Scholar]
- 134.Harding J.J., Moreno V., Bang Y.J., et al. Blocking TIM-3 in treatment-refractory advanced solid tumors: a phase Ia/b study of LY3321367 with or without an anti-PD-L1 antibody. Clin Cancer Res. 2021;27(8):2168–2178. doi: 10.1158/1078-0432.CCR-20-4405. [DOI] [PubMed] [Google Scholar]
- 135.Curigliano G., Gelderblom H., Mach N., et al. Phase I/Ib clinical trial of sabatolimab, an anti-TIM-3 antibody, alone and in combination with spartalizumab, an anti-PD-1 antibody, in advanced solid tumors. Clin Cancer Res. 2021;27(13):3620–3629. doi: 10.1158/1078-0432.CCR-20-4746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared unless the corresponding authors agree.