Abstract
Recent advances in human immunodeficiency virus (HIV) diagnostics have improved the management of disease progression significantly, which have also boosted the efficacy of antiviral therapies. The detection of HIV at the earliest is very important. A highly recognized and effective virological biomarker for acute HIV infections is p24 antigen. This brief overview is based on advances of HIV diagnosis while focusing on the latest HIV testing technologies including HIV-specific antigens detecting assays of both anti-HIV antibodies and p24 antigen. In addition to other emerging molecular diagnostics for acute HIV infection, the utilization of p24 antigen has been summarized. Moreover, it has been explained how these immunoassays have reduced the window period for detection of HIV in the acute stage of infection.
Keywords: antibodies, core protein p24, diagnosis, HIV, immunoassay
Viral diseases are considered as a major public health concern worldwide. Either these viruses are blood-borne for example, hepatitis B virus (HBV), hepatitis C virus (HCV), and human immunodeficiency virus (HIV), or from other sources, for example, SARS-CoV-2, and monkeypox virus, but there is a need of rapid diagnosis, treatment, and prevention. However, HIV is a causative agent of acquired immunodeficiency syndrome (AIDS), and could be categorized by the appearance of opportunistic infections and a long period of clinical latency [1, 2, 3, 4, 5].
HIV replicate in T lymphocytes while AIDS is characterized by the loss of helper T cells that express the CD4+ surface proteins [6]. The CD4+ cells count (<200/mm3) could be used as a marker for disease severity and the risk of opportunistic infections [7]. In the early phase of HIV, the virus is detectable in the blood via cDNA, RNA, and p24 antigen. The presence of indeterminate HIV antibodies is usually detected in the acute stage of infection. Detection of the virus in the acute stage is of significant importance concerning various issues, for example, public health, clinical diagnosis, and early treatment [7]. Patients with acute stage of the disease are at the highest risk of secondary transmission as they contain high viral load [8].
Since the emergence of the HIV pandemic, the immunoassays for HIV detection have been continuously developed to detect the HIV-specific antibodies. The third-generation assays, in contrast to the first and second generation, detect immunoglobulin G (IgG) and immunoglobulin M (IgM) at notable advancement in HIV diagnosis [9, 10, 11]. HIV-specific antibodies are not produced during acute infection; therefore, it is a major limitation in immunoassays. Various attempts have been made for the detection of HIV during the acute phase of infection, for example, the detection of HIV-RNA that contribute significantly to HIV diagnosis [12, 13, 14, 15]. The RNA-based detection has some limitation related to effectiveness, is time-consuming, and needs expert personnel for operation and result interpretation; therefore, the fourth-generation immunoassay was developed for detection of acute infection to utilize the antigen–antibodies combination [16, 17]. The fourth-generation assay is less expensive, easy to perform, and is an automated assay. This assay simultaneously detects antibodies and a capture immunoassay for detection of the most abundant protein of HIV virions, that is, p24. This latest assay (ADVIA, Centaur HIV Ag/Ab) can differentiate p24 antigens and antibodies in the serum and therefore have reduced the window period. Among them, some of the assays can be used to detect HIV from serum, plasma, and even from whole blood samples in a very limited time, that is, 20 min. Some reports rated to fourth-generation immunoassays have claimed a sensitivity of 88% and above, of the latest assays which detect p24 antigen [18]. Other studies have reported more advanced fourth-generation immunoassays with the highest specificity and sensitivity. The accurate diagnosis of HIV is dependent on the analytical sensitivity of the test to p24 antigen and the efficiency to detect the diversity in HIV strains. The rapid evolution of HIV has resulted in the production of various subtypes and recombinant viruses [19, 20].
Currently, HIV-1 is categorized into various groups like M, O, N, and P. Among them, group M is the most prevalent group of HIV-1, which has been further classified into nine subtypes (A, B, C, D, F, G, H, J, and K) and more than 75 circulating recombinant forms (CRFs) and several unique recombinant forms (URFs) [19]. This extensive diversity of HIV poses challenges for reliable detection assays maintenance in routine diagnosis. Several cases of HIV strains have been reported to be escaping in fourth-generation assays [11, 21]. A recent study reported that HIV diversity impacted the sensitivity of fourth-generation assays in the early HIV infection [22]. Of note, highly active antiretroviral therapy (HAART) is the treatment regimen, particularly the cocktail of three or more anti-retroviral drugs. The combination of these drugs inhibits replication of HIV-1, and by action reduces the viral load, reducing the morbidity and mortality of patients, and boosts the immune system. It is noted that when HAART is interrupted, HIV-1 antigen persists and viral rebound may occur, which ultimately increases the level of CD4+ cells and p24 antigen [23].
The current review was aimed to present an overview based on advances in HIV diagnosis while focusing on the latest HIV testing technologies, including HIV-specific antigens and antibodies detecting assays. The review also focuses on p24 antigen assays and other emerging molecular diagnostics, and these immunoassays have reduced the window period for detection of HIV in the acute stage of infection.
HIV Ab detection assays
HIV-1 and HIV-2 have various available commercial diagnostic assays for screening blood, infection detection, and analysis of the disease continuation in infected individuals since 1986. These assays could be classified into four major groups: those used to detect anti-HIV antibodies, p24 antigen, viral nucleic acid (detection or quantification), and T-lymphocytes count estimation [24].
The most particular and common immunoassay utilized for HIV antigen-antibody detection is the enzyme-linked immunosorbent assay (ELISA). The technique has developed from tests of viral-lysate-based IgG to first-generation tests (window period, 40–50 days), second-generation tests (30–35 days) containing synthetic peptide antigens, and recombinant third-generation tests (20 days) which detect IgG, IgM, and fourth-generation HIV tests [24]. Certain limitations noted in the above-mentioned tests were, first-generation tests were less efficient in specificity compared with sensitivity, second-generation assays were noted to be excellent in regard to specificity and sensitivity but the maximum cross-reactivity of Abs was prominent with group M subtypes, and third-generation assays were used in detection of the early stages of infection. Moreover, these assays are still used in developing countries for detection of vaccine-induced Abs as well as infection acquired by HIV individuals [3].
Later in the 1990s, companies made fourth-generation tests for HIV antigen–antibody detection in a combined slot. These techniques were based on the principles of ELISA and chemiluminescence tests. The test negative gap was narrowed by these tests into minimum time (2–18 days). The limitation associated with these tests included only a single result of detection of antigen–antibody interaction. These assays lack to evaluate generally and geographically circulating subtypes of HIVI/II. The Food and drug administration (FDA) approved the Abbot Architect method of these procedures in August 2010, with 99.94% sensitivity and 99.95% specificity of repeat testing in a study population [25]. Similarly, in 2020, the FDA approved G4 ELISA of antibody rapid test, the research team evaluated G4 ELISA which displayed 100% sensitivity for HIV-1 and 98.18% for HIV-2 while specificity was 100% [26]. According to a study, fourth-generation ELISA (Abbot Architect) tests are more specific than third generation. The study evaluated 7516 subjects for third-generation ELISA with 46% positive predictive value (PPV), and 70% PPV was noted for fourth generation (n = 7802) [27]. The frequently used fourth-generation assays are presented in Table 1.
Table 1.
Fourth-generation tests for HIV antigen-antibody
| Test | Manufacturer | Specificity (%) | References |
|---|---|---|---|
| ADVIA Centaur HIV Ag/Ab Combo | Siemens | 99.74 | [28] |
| AxSYM HIV Ag/Ab Combo | Abbott Laboratories | 99.9 | [29] |
| ARCHITECT HIV Combo | Abbott Laboratories | 99.4 | [30] |
| VIDAS HIV DUO Ultra | BioMerieux | 99.84 | [31] |
| Cobas Core HIV Combi EIA | Roche Diagnostics | 99.3 | [30] |
| Elecsys HIV Combi | Roche Diagnostics | 99.8 | [32] |
| HIV Combi Modular E170 | Roche Diagnostics | 99.7 | [30] |
| Enzygnost HIV Integral | Dade Behring | 99.6 | [30] |
| Genscreen Ag/Ab HIV Ultra | Bio Rad | 99.7 | [30] |
| Genscreen PlusHIV Ag/Ab | Bio Rad | 99.9 | [28] |
| Vironostika HIV Uniform II Ag/Ab | Organon Teknika | 97.21 | [30] |
| Liaison XL Murex HIV Ab/Ag | DiaSorin | 98.5 | [33] |
EIA, HIV, human immunodeficiency virus.
Confirmation of HIV by immunoblot assays
For the diagnosis of HIV, the serological testing of fourth-generation assay must be confirmed via secondary confirmatory method. The most frequently used secondary confirmatory method for HIV infection could be Western blots (WBs) or immunoblot assays (IBAs) using different HIV recombinant proteins, that is, p17, p24, p31, gp41, gp120, 124. In order to reduce the window time of WBs for primary screening (ELISA), WHO recommends recombinant immunoblot assay (RIBA), used as secondary confirmatory method for the detection of HIV or HCV in the early phase of infection, when the primary screening is imprecise or indeterminate. According to authors, IBAs has the highest positivity rate than WB assay. They compared two IBAs, INNO-LIA HIV 1/2, and Geenius HIV 1/2 with WB [34]. Another study from Wuhan China, also investigated HIV-1 11068 positive cases of two fourth-generation kits of ELISA on WB and RIBA (WANTAI, Biopharm, China). The indeterminate cases of RIBA were significantly less than WB [35]. In contrast, RIBA is mostly used for the detection of HCV detection than HIV [36, 37].
Fifth-generation HIV tests
In 2015, Bio-Rad developed BioPlex 2200 HIV Ag\Ab screening test method as diagnostic assay. This multiplex analysis method was called fifth-generation HIV detection assays, later approved by the FDA. The fifth-generation HIV immunoassay detects HIV-1 p24 Ag, HIV-1 Ab (groups M and O), and HIV-2 Ab and offers discrete single signal to cutoff (S/CO) ratio data. A positive screen is defined as S/CO ratio of 1 or more on any of the three components of the BioPlex assay, with all BioPlex positive screens being repeated before advancing to confirmatory testing. Like fourth-generation tests, it provides screening of both antigen–antibody but separately of both analytes. The BioPlex fifth-generation assay results were evaluated similarly for both sensitivity and specificity [27]. Similarly, LIAISON HIV Ag-Ab immunoassay has also proved excellent diagnostic sensitivity in the detection of HIV in the early phase of infection with the least of window timing (2–5 days). According to a previously conducted study, the diagnostic performance of LIAISON was evaluated for HBV, HCV, and HIV infection along with increasing S/CO for subsequent confirmation of HIV-1 via LIAISON. Among all HIV positive (n = 229) patients, 1.6% were reactive with 141 (62.1%) confirmed. The increasing ratio of S/CO to 4 automatically increases PPV without uncertainty in sensitivity [38].
Rapid HIV assays
Rapid HIV assays are card-based procedures that have mainly gone through first- and third-generation assays as a basic screening test. These tests are developed for whole blood, serum, and oral fluid. In 2012, FDA approved a newly developed point of care method named “OraQuick” for home testing, with effective testing capabilities [39]. This test is different from other conventional tests, and can be performed without any laborious assistance at home within 20 min. Similarly, Geenius semi-automated and differentiation assay for HIV-1 and HIV-2 is a rapid assay approved (FDA) and can only be used as supplemental technique in the fourth-generation algorithm, but not as a screening test. It has been reported that Geenius has 100% sensitivity and 96% specificity [38]. Presently, there is a combo of fourth- and fifth-generation rapid assays which is used for the detection of HIV antigen and antibodies but does not distinguish between antibodies of HIV-1 and HIV-2. It has been reported in a study that it has 100% of specificity 88.2% sensitivity [39].
Nucleic acid-based HIV-detection
Since 1995, several nucleic acid-based detection tests have been established for the estimation of viral RNA (HIV) in blood plasma. Three main techniques used for the detection of HIV nucleic acid are reverse transcriptase polymerase chain reaction (RT-PCR), branched chain DNA (bDNA,) and nucleic acid sequence-based amplification (NASBA). The viral RNA (HIV-1) detection was achieved after amplification of products with labeled probe, which was finally detected by immunoassay or chemiluminesence technology [40]. Currently, real-time techniques (PCR-based HIV RNA assays) constantly detecting fluorescence emanated during every cycle of PCR [41]. HIV-1 RNA estimation is achieved during the first phase of PCR, as compared with conventional PCR or other technologies and provide more reliable results. Owing to new developed assays, the detection number has been improved much higher than old technologies (~50 copies/mL) [42]. According to a current study, a new combo of two detection techniques, including PCR (gene amplification), followed by ELISA (NAT-ELISA), has been developed by a group of scientists for the detection of HIV-1,2 and other blood-borne viruses (HBV & HCV), in their window timing. The WHO standard protocol for PCR amplified products were followed; 321bp of HIV-1, 291bp (HIV-2), 229bp of HBV, and 105bp of HCV. The limit of detection (LOD) 95% and LOD 97.7% of HIV-1, HIV-2 HBV, and HCV were 13 IU/mL, 6 IU/mL, 15 IU/mL and 11 IU/mL, and 15 IU/mL, 7 IU/mL, 17 IU/mL, and 12 IU/mL individually. The overall sensitivity and specificity of NAT-ELISA assay was noted at 97.4% and 99.4%, respectively. The newly developed assay (NAT-ELISA) suggests overcoming complications of previously used methods such as cost-effectiveness, handling, and compromising sensitivity for high performance [43].
Nano-technology in HIV diagnosis
Precise, accurate, and minute technologies are required to characterize, visualize, and manipulate molecular targets at the nanoscale [44]. Nanotechnology refers to the fabrication, design, characterization, and application of devices and materials whose size is 1–100 nm from at least one dimension. Nanotubes, nanofibers, nanosheets, nanorods, nanoplates, nanodots, nanowires, and nanobelts have been defined based on this description [24]. The rapid developments in nanoscience and nanotechnology have driven innovations in all fields of science, including biosensors, where cutting-edge research is carried out to develop the diagnosis of various diseases [45]. Nano-sized materials are opening new opportunities in the area of sensing by adding more value to existing sensing technologies [46]. They increase the loading of recognition elements on the platforms, signal amplification, speed of response, detection range, selectivity, interference, specificity, cost-effectiveness, and stability [47]. Substantially, multifarious nanomaterials play a key role in developing point of care testing and multi-modality sensing tools.
A number of direct and indirect methods have been developed for the detection and diagnosis of HIV [32]. These various approaches detect various biomarkers, including different types of HIV antibodies (HIV type 1 and HIV type 2 antibodies), viral p17 and p24 antigen, viral nucleic acid, CD4 T lymphocytes, and HIV-related enzymes [48]. Nanotechnology provides possibilities in the development of these bio-sensing assays by increasing sensitivity and specificity and detection at low viral concentrations [49, 50]. Through nanotechnology, devices with a high degree of specificity and sensitivity can be designed to allow the interaction of various cells and other components at the molecular level [51]. The nanoclusters exhibited different properties such as high surface-to-volume ratio, and optical and electrical properties that increase these biosensors' sensitivity and specificity [52]. Metal oxide and metallic nanoparticles, nanoclusters, quantum dots, and carbon nanostructures are valuable materials for developing a highly sensitive and specific biosensor for the diagnosis of HIV.
Harvey and colleagues designed and developed a nanobased approach for the rapid detection of serum-based HIV. This sensing technology eliminates preprocessing of the sample, such as purification of oligonucleotides and further amplification. In this approach, the protein interacts with the carbon nanotube surface and then hybridization occurs to detect the virus, yielding a photoluminescence response [53]. Similarly, the development of POC lateral flow assays (LFAs), termed as nano-tags that detect the HIV-1 DNA marker using gold nanoparticles and surface-enhanced Raman scattering. The LFAs are therefore highly accurate with a 100 times higher sensitivity than fluorescent or colorimetric detection method [54].
Gray and colleagues applied nanotechnology to design and develop a mobile antibody-based biochip sensor using smartphone mechanism. They reported that this biochip sensor would be precise and accurate in the diagnosis of anti-gp41 with the highest specificity and sensitivity (100%) and give the results within 1 min [55]. Other nano-based techniques that show high specificity and sensitivity in diagnosing HIV nucleic acid include a photoelectrochemical biosensor, amplified electrochemiluminescence biosensor, strand displacement amplification, and copolymer nanospheres.
Plasmonic nanoparticles-based approach for HIV diagnosis
Localized surface plasmon resonance (LSPR) is commonly not exhibited in the bulk materials but associated with nanosized materials [56]. Surface plasmon resonance (SPR)-based diagnosis leads to alter the refractive index quantity in the electromagnetic field [57]. This approach is reliable, sensitive, and rapid and offers hugely responsive sensing to various biomolecular reactions, including viruses. An LSPR approach has been exploited to detect HIV at point of care in resource limited laboratories. For the detection of various subtypes of HIV, a nano-plasmonic-based approach has been developed. This assay has the highest accuracy (98 ± 39 copies/mL) and reliability. Gold nanoparticle-based immunochemistry is done using a whole blood sample, and there is no need for sample preprocessing to capture the agent (virus). A plate reader, usually a microplate titer, is used to quantify the transmission/absorbance to measure the captured intact virus [58].
However, the nano-based techniques to screen the biomarker of interest should be selected at a proper timeframe to confirm that the marker of interest is present. As there is a specific range of timeframe for each marker that is, HIV nucleic acid (RNA) can be detected about 10 days after infection while the antibody testing should occur 3–5 weeks following the infection.
The use of p24 assays in early detection
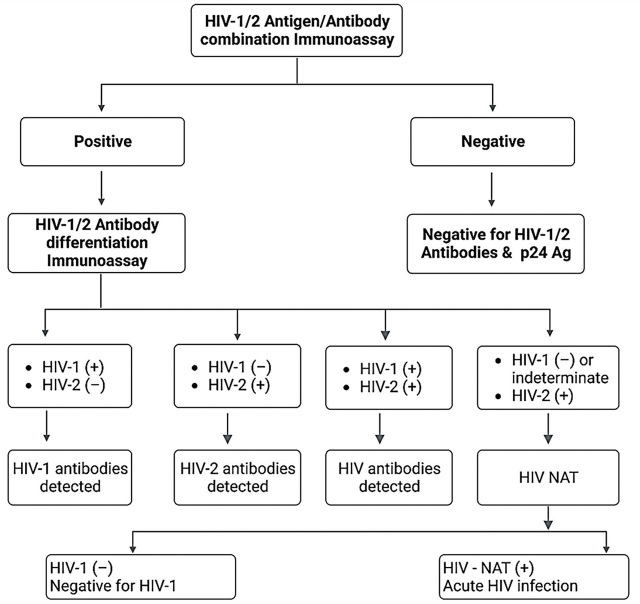
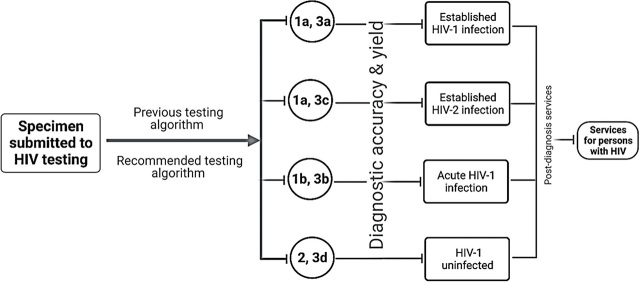
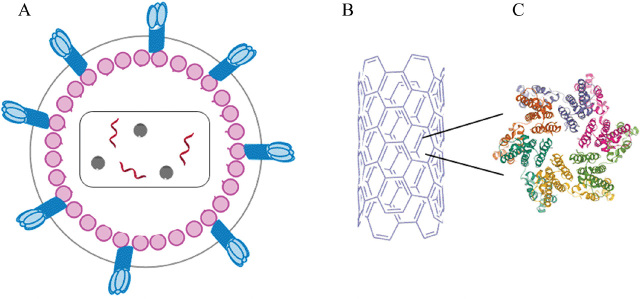
As discussed earlier, the period in which HIV is present in the body but the antibody response has not been mounted is known as acute HIV infection. Infection in the acute stage can only be detected via highly sensitive diagnostic assays that detect viral antigens [59, 60, 61]. The fourth-generation assays are recommended in early infection in adult individuals [62, 63]. The recommended laboratory HIV testing algorithm by the Center for Disease Control (CDC) along with the analytical framework for accurate detection of HIV infection is represented in Figures 1 and 2. In the earliest stage of infection, the HIV RNA is an important detectable viral biomarker and therefore the nucleic acid amplification test could be used for HIV detection [63]. The nucleic acid test is generally not approved as a quantitative diagnostic tool in several countries [64]. On the other hand, the p24 assays have been recommended for early HIV detection. The commercially available p24 antigen or antibodies-based assays have been reported to have higher sensitivities across the clades and recombinant forms. It has also been described that small sequence differences do alter the p24 tertiary structure and therefore the sensitivity of p24 antigen detection-based assay was not affected. Several emerging ultra-sensitive methods for detection of HIV p24 antigen are represented in Table 2. The FDA approved fourth-generation assays and standalone p24 assays have demonstrated increased sensitivity of next-generation p24 antigen detection platforms. The structure of HIV along with the p24 antigen is represented in Figure 3. Several studies have examined the properties of p24 antigen and its association with other biomarkers during HIV infection [65]. With the advanced research in virology, the next-generation technologies for intended resources limited setting could use the p24 antigen as the most effective virological target.
Figure 1.
Recommended laboratory HIV testing algorithm by CDC [66]. CDC, Center for Disease Control; HIV, human immunodeficiency virus.
Figure 2.
Recommended analytical framework by CDC for accurate HIV diagnosis. CDC, Center for Disease Control; HIV, human immunodeficiency virus.
Table 2.
Emerging ultra-sensitive methods for detection of HIV p24 antigen
| S. no. | Legend | Reported by |
|---|---|---|
| Nanoparticles/beads based | ||
| 1 | Gold† | [50] |
| 2 | Platinum-shell coated gold‡ | [67] |
| 3 | Carbon§ | [68] |
| 4 | Latex† | [69] |
| Enzyme based | ||
| 5 | Urease|| | [70] |
| 6 | Catalase|| | [71] |
| 7 | Horseradish peroxidase|| | [72] |
| 8 | Alkaline phosphatase|| | [73] |
| 9 | Glucose oxidase|| | [74] |
Signal enhancement method.
Signal enhancement and p24 nanobody.
Biosensor platform.
Signal enhancement and biosensor platform.
HIV, human immunodeficiency virus.
Figure 3.
Structure of HIV and p24 antigen. (A) A complete virion, (B) capsid fullerene cone superstructure, and (C) monomer unit [8]. HIV, human immunodeficiency virus.
Conclusion
The testing technologies and the new-generation assays for HIV screening have been developed rapidly. The earlier diagnosis of HIV is very important in several aspects; therefore, new algorithms need to be established. The biomarkers-based diagnosis has been the main detection method for HIV diagnosis. For rapid detection, high diagnostic accuracy and earlier detection of new technologies with the use of nanotechnology are needed.
Acknowledgments
The authors are thankful to Dr. Sher Ali Department of Food Engineering, School of Animal Science and Food Engineering, University of São Paulo, CEP13635-900, Pirassununga, SP, Brazil for language editing and assistance in writing the manuscript.
Abbreviations:
- AIDS
acquired immunodeficiency syndrome
- bDNA
branched chain DNA
- CDC
Center for Disease Control
- CRFs
circulating recombinant forms
- ELISA
enzyme-linked immunosorbent assay
- FDA
food and drug administration
- HAART
highly active antiretroviral therapy
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- IBAs
immunoblot assays
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- LFAs
lateral flow assays
- LSPR
localized surface plasmon resonance
- NASBA
nucleic acid sequence-based amplification
- RIBA
recombinant immunoblot assay
- RT-PCR
reverse transcriptase polymerase chain reaction
- SPR
surface plasmon resonance
- URFs
unique recombinant forms
- WB
western blot
Footnotes
Author contributions. All authors made substantial contributions to the conception and design of the study and acquisition of data. MK and RM contributed substantially to data analysis. A, MD, AW, and MK drafted the manuscript, and SA, MS, and RM revised it critically for important intellectual content. All authors approve the final version submitted for publication and agree to be accountable for all aspects of the work and take responsibility for statements made in the published article.
Conflict of interest statement. The author has completed and submitted the International Committee of Medical Journal Editors Uniform Disclosure Form for Potential Conflicts of Interest. None of the authors disclose any conflict of interest.
Data sharing statement. The present review is based on the references cited. All data generated or analyzed during the present study are included in this published article and the citations herein. Further details, opinions, and interpretation are available from the corresponding author on reasonable request.
References
- [1].do Nascimento WM, Machiavelli A, Ferreira LGE, Cruz Silveira L, de Azevedo SSD, Bello G. et al. Gut microbiome profiles and associated metabolic pathways in HIV-infected treatment-naïve patients. Cells. 2021;10:385. doi: 10.3390/cells10020385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jain C, Sharma L, Advani U, Kumar M, Tak A, Jain M. A cross-sectional study of socio-demographic and clinical profile of HIV patients at ART plus centre, Sawai Man Singh Hospital, Jaipur, India. JID Health. 2021;4:321–6. [Google Scholar]
- [3].Seyoum E, Demissie M, Worku A, Mulu A, Abdissa A, Berhane Y. HIV, hepatitis B virus, and hepatitis C virus co-infection among HIV positives in antiretroviral treatment program in selected hospitals in Addis Ababa: a retrospective cross-sectional study. PLoS One. 2022;17:e0267230. doi: 10.1371/journal.pone.0267230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Abdullah, Ali S, Cançado FACQ, de Oliveira CAF. The emergence of monkeypox virus, new challenges to the healthcare settings in Pakistan. J Med Virol. 2023;95:e27899. doi: 10.1002/jmv.27899. [DOI] [PubMed] [Google Scholar]
- [5].Waris A, Atta UK, Ali M, Asmat A, Baset A. COVID-19 outbreak: current scenario of Pakistan. New Microbes New Infect. 2020;35:100681. doi: 10.1016/j.nmni.2020.100681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kek H, Laumaea A, Parise S, Poumbourios P, Hearps AC, Jaworowski A. Differential expression of HIV envelope epitopes on the surface of HIV-Infected macrophages and CD4+ T cells. Antiviral Res. 2021;191:105085. doi: 10.1016/j.antiviral.2021.105085. [DOI] [PubMed] [Google Scholar]
- [7].Malonga GA, Jary A, Leducq V, Moudiongui Mboungou Malanda D, Boumba ALM, Chicaud E. et al. Seroprevalence and molecular diversity of Human Herpesvirus 8 among people living with HIV in Brazzaville, Congo. Sci Rep. 2021;11:17442. doi: 10.1038/s41598-021-97070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gray ER, Bain R, Varsaneux O, Peeling RW, Stevens MM, McKendry RA. p24 revisited: a landscape review of antigen detection for early HIV diagnosis. AIDS. 2018;32:2089–102. doi: 10.1097/QAD.0000000000001982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Alexander TS. Human immunodeficiency virus diagnostic testing: 30 years of evolution. Clin Vaccine Immunol. 2016;23:249–53. doi: 10.1128/CVI.00053-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Alemayehu AE. Analysis of HCV coinfections among newly diagnosed HIV cases in Germany. Publication server of Robert Koch Institue; 2021. pp. 1–154. [Google Scholar]
- [11].Qiu X, Sokoll L, Duong Ly T, Coignard C, Eshleman SH, Mohr P. et al. An improved HIV antigen/antibody prototype assay for earlier detection of acute HIV infection. J Clin Virol. 2021;145:105022. doi: 10.1016/j.jcv.2021.105022. [DOI] [PubMed] [Google Scholar]
- [12].Adetunji AA, Adewumi MO, Michael OS, Fayemiwo SA, Ogunniyi A, Taiwo BO. Rapid HIV antigen–antibody assays and detection of acute HIV infection in Sub-Saharan Africa. Am J Trop Med Hyg. 2019;101:285–6. doi: 10.4269/ajtmh.19-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tavakoli A, Karbalaie Niya MH, Keshavarz M, Ghaffari H, Asoodeh A, Monavari SH. et al. Current diagnostic methods for HIV. Future Virol. 2017;12:141–55. [Google Scholar]
- [14].Ali M, Nadeem M, Numan M, Khalil AT, Maqbool K, Yousaf MZ. et al. Thirty years of HIV in Pakistan: a systematic review of prevalence and current scenario. Future Virol. 2017;12:609–23. [Google Scholar]
- [15].Haq I, Ullah R, Din M, Ahmad S, Anwar F, Ali M, Khan HU. Unrecognized HIV infection in asymptomatic volunteer blood donors at district Peshawar, Khyber Pakhtunkhwa, Pakistan. New Microbes New Infect. 2020;35:100685. doi: 10.1016/j.nmni.2020.100685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Barik SK, Mohanty K, Bisht D, Joshi B, Jena S, Tripathy SP. An overview of enzyme immunoassay: the test generation assay in HIV/AIDS testing. J AIDS Clin Res. 2018;9:1–5. [Google Scholar]
- [17].Sarcina L, Mangiatordi GF, Torricelli F, Bollella P, Gounani Z, Österbacka R. et al. Surface plasmon resonance assay for label-free and selective detection of HIV-1 p24 protein. Biosensors (Basel). 2021;11:180. doi: 10.3390/bios11060180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fitzgerald N, Cross M, O'Shea S, Fox J. Diagnosing acute HIV infection at point of care: a retrospective analysis of the sensitivity and specificity of a fourth-generation point-of-care test for detection of HIV core protein p24. Sex Transm Infect. 2017;93:100–1. doi: 10.1136/sextrans-2015-052491. [DOI] [PubMed] [Google Scholar]
- [19].Rashid A, Li K, Feng Y, Ahmad T, Getaneh Y, Yu Y. et al. HIV-1 genetic diversity a challenge for AIDS vaccine development: a retrospective bibliometric analysis. Hum Vaccin Immunother. 2022;18:2014733. doi: 10.1080/21645515.2021.2014733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Santos-Pereira A, Magalhães C, Araújo PMM, Osório N. Evolutionary genetics of Mycobacterium tuberculosis and HIV-1: “the tortoise and the hare”. Microorganisms. 2021;9:147. doi: 10.3390/microorganisms9010147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Qiu X, Sokoll L, Yip P, Elliott DJ, Dua R, Mohr P. et al. Comparative evaluation of three FDA-approved HIV Ag/Ab combination tests using a genetically diverse HIV panel and diagnostic specimens. J Clin Virol. 2017;92:62–8. doi: 10.1016/j.jcv.2017.05.005. [DOI] [PubMed] [Google Scholar]
- [22].Ly TD, Plantier JC, Leballais L, Gonzalo S, Lemée V, Laperche S. The variable sensitivity of HIV Ag/Ab combination assays in the detection of p24Ag according to genotype could compromise the diagnosis of early HIV infection. J Clin Virol. 2012;55:121–7. doi: 10.1016/j.jcv.2012.06.012. [DOI] [PubMed] [Google Scholar]
- [23].Duette G, Hiener B, Morgan H, Mazur FG, Mathivanan V, Horsburgh BA. et al. The HIV-1 proviral landscape reveals that Nef contributes to HIV-1 persistence in effector memory CD4+ T cells. J Clin Invest. 2022;132:e154422. doi: 10.1172/JCI154422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pai NP, Karellis A, Kim J, Peter T. Modern diagnostic technologies for HIV. Lancet HIV. 2020;7:e574–81. doi: 10.1016/S2352-3018(20)30190-9. [DOI] [PubMed] [Google Scholar]
- [25].Curtis KA, Rudolph DL, Pan Y, Delaney K, Anastos K, DeHovitz J. et al. Evaluation of the Abbott ARCHITECT HIV Ag/Ab combo assay for determining recent HIV-1 infection. PloS one. 2021;16:e0242641. doi: 10.1371/journal.pone.0242641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rossetti R, Smith T, Luo W, Masciotra S. Performance evaluation of the MedMira reveal G4 LAB S/P and POC HIV antibody rapid screening tests using plasma and whole blood specimens. J Clin Virol. 2020;127:104344. doi: 10.1016/j.jcv.2020.104344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Petersen J, Monteiro M, Dalal S, Jhala D. Reducing false-positive results with fourth-generation HIV testing at a veterans affairs medical center. Fed Pract. 2021;38:232–7. doi: 10.12788/fp.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pumarola T, Freeman J, Saxton E, Dillon P, Bal T, van Helden J. Performance evaluation of the ADVIA centaur(®) HIV Ag/Ab combo assay. J Virol Methods. 2010;170:16–20. doi: 10.1016/j.jviromet.2010.08.012. [DOI] [PubMed] [Google Scholar]
- [29].Ly TD, Laperche S, Brennan C, Vallari A, Ebel A, Hunt J. et al. Evaluation of the sensitivity and specificity of six HIV combined p24 antigen and antibody assays. J Virol Methods. 2004;122:185–94. doi: 10.1016/j.jviromet.2004.08.018. [DOI] [PubMed] [Google Scholar]
- [30].Ly TD, Ebel A, Faucher V, Fihman V, Laperche S. Could the new HIV combined p24 antigen and antibody assays replace p24 antigen specific assays? J Virol Methods. 2007;143:86–94. doi: 10.1016/j.jviromet.2007.02.013. [DOI] [PubMed] [Google Scholar]
- [31].Weber B, Gürtler L, Thorstensson R, Michl U, Mühlbacher A, Bürgisser P. et al. Multicenter evaluation of a new automated fourth-generation human immunodeficiency virus screening assay with a sensitive antigen detection module and high specificity. J Clin Microbiol. 2002;40:1938–46. doi: 10.1128/JCM.40.6.1938-1946.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Weber B, Orazi B, Raineri A, Thorstensson R, Bürgisser P, Mühlbacher A. et al. Multicenter evaluation of a new 4th generation HIV screening assay Elecsys HIV combi. Clin Lab. 2006;52:463–73. [PubMed] [Google Scholar]
- [33].Alonso R, López Roa P, Suárez M, Bouza E. New automated chemiluminescence immunoassay for simultaneous but separate detection of human immunodeficiency virus antigens and antibodies. J Clin Microbiol. 2014;52:1467–70. doi: 10.1128/JCM.03486-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mariaggi AA, Gardiennet E, Stefic K, Essat A, Cheret A, Goujard C. et al. Immunoblots may not be effective in confirming the recency of HIV-1 infection. J Virol Methods. 2021;290:114074. doi: 10.1016/j.jviromet.2021.114074. [DOI] [PubMed] [Google Scholar]
- [35].Liu P, Tang L, Kong WH, Zhu ZR, Xiao P, Wang X. et al. Anti-HIV-1 antibodies based confirmatory results in Wuhan, China, 2012–2018. PLoS One. 2020;15:e0238282. doi: 10.1371/journal.pone.0238282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hawthorne Hallen A, Samuelson A, Nordin M, Albert J, Bogdanovic G. Evaluation of bio-rad Geenius HIV-1 and-2 assay as a confirmatory assay for detection of HIV-1 and-2 antibodies. Clin Vaccine Immunol. 2014;21:1192–4. doi: 10.1128/CVI.00153-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Thai KTD, Götz H, Slingerland BCGC, Klaasse J, Schutten M, Geurtsvan Kessel CH. An analysis of the predictive value of the HIV Ag/Ab screening assay within the performance characteristics of the DiaSorin LIAISON XL for the detection of blood-borne viruses. J Clin Virol. 2018;102:95–100. doi: 10.1016/j.jcv.2018.02.018. [DOI] [PubMed] [Google Scholar]
- [38].Zeytinoğlu A, Kayın M, Altuğlu İ, Gökengin D, Sertöz R. Comparison of two anti-HIV confirmatory assays: recombinant HIV 1/2 line immunoassay and geenius HIV 1/2 confirmatory test. Mikrobiyol Bul. 2020;54:613–8. doi: 10.5578/mb.69786. [DOI] [PubMed] [Google Scholar]
- [39]. Cdc.gov. Types of HIV tests | testing | HIV basics | HIV/AIDS | CDC. [Internet]. 2020. [cited 2021 Dec 5] Available from: https://www.cdc.gov/hiv/basics/hiv-testing/test-types.html .
- [40].Jagodzinski LL, Manak MM, Hack HR, Liu Y, Peel SA. Performance evaluation of a laboratory developed PCR test for quantitation of HIV-2 viral RNA. PLoS One. 2020;15:e0229424. doi: 10.1371/journal.pone.0229424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bakkour S, Deng X, Bacchetti P, Grebe E, Montalvo L, Worlock A. et al. Replicate aptima assay for quantifying residual plasma viremia in individuals on antiretroviral therapy. J Clin Microbiol. 2020;58:e01400–20. doi: 10.1128/JCM.01400-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Patel P, Raizes E, Broyles LN. Hunter's Tropical Medicine and Emerging Infectious Diseases. Tenth Edition. Elsevier; 2020. Human immunodeficiency virus infection; pp. 232–66. [Google Scholar]
- [43].Sharma P, Batheja G, Verma HN, Seth P. Development of a novel molecular assay based on conventional PCR liquid hybridization and ELISA (NAT-ELISA) for the detection of HIV, HCV, and HBV in blood donors. Indian J Med Microbiol. 2022;40:122–6. doi: 10.1016/j.ijmmb.2021.12.014. [DOI] [PubMed] [Google Scholar]
- [44].Naz F, Siddique YH. Nanotechnology: its application in treating neurodegenerative diseases. CNS Neurol Disord Drug Targets. 2021;20:34–53. doi: 10.2174/1871527319666200916121515. [DOI] [PubMed] [Google Scholar]
- [45].Farzin L, Shamsipur M, Samandari L, Sheibani S. HIV biosensors for early diagnosis of infection: the intertwine of nanotechnology with sensing strategies. Talanta. 2020;206:120201. doi: 10.1016/j.talanta.2019.120201. [DOI] [PubMed] [Google Scholar]
- [46].Hammou RA, Benhassou M, Ennaji MM. Emerging and Reemerging Viral Pathogens. Academic Press; 2020. Application of nanodiagnostics in viral infectious diseases; pp. 179–95. [Google Scholar]
- [47].Mozhgani SH, Kermani HA, Norouzi M, Arabi M, Soltani S. Nanotechnology based strategies for HIV-1 and HTLV-1 retroviruses gene detection. Heliyon. 2020;27:e04048. doi: 10.1016/j.heliyon.2020.e04048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Parekh BS, Ou CY, Fonjungo PN, Kalou MB, Rottinghaus E, Puren A. et al. Diagnosis of human immunodeficiency virus infection. Clin Microbiol Rev. 2018;32:e00064–18. doi: 10.1128/CMR.00064-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Dhal A, Kalyani T, Ghorai S, Sahu NK, Jana SK. Recent development of electrochemical immunosensor for the diagnosis of dengue virus NSI protein: a review. Sens Int. 2020;1:100030. [Google Scholar]
- [50].Tang S, Hewlett I. Nanoparticle-based immunoassays for sensitive and early detection of HIV-1 capsid (p24) antigen. J Infect Dis. 2010;201(Supplement_1):S59–64. doi: 10.1086/650386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Abbaszadeh S, Rashidipour M, Khosravi P, Shahryarhesami S, Ashrafi B, Kaviani M, Moradi Sarabi M. Biocompatibility, cytotoxicity, antimicrobial and epigenetic effects of novel chitosan-based quercetin nanohydrogel in human cancer cells. Int J Nanomedicine. 2020;15:5963–75. doi: 10.2147/IJN.S263013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zhang L, Mazouzi Y, Salmain M, Liedberg B, Boujday S. Antibodygold nanoparticle bioconjugates for biosensors: synthesis, characterization and selected applications. Biosens Bioelectron. 2020;165:112370. doi: 10.1016/j.bios.2020.112370. [DOI] [PubMed] [Google Scholar]
- [53].Kong H, Zhang W, Yao J, Li C, Lu R, Guo Z. et al. A RT-LAMP based hydrogen ion selective electrode sensing for effective detection HIV-1 RNA with high-sensitivity. Sens Actuators B Chem. 2020;329:129118. doi: 10.1016/j.snb.2020.129118. [DOI] [Google Scholar]
- [54].Yadav S, Senapati S, Desai D, Gahlaut S, Kulkarni S, Singh JP. Portable and sensitive Ag nanorods based SERS platform for rapid HIV-1 detection and tropism determination. Colloids Surf B Biointerfaces. 2021;198:111477. doi: 10.1016/j.colsurfb.2020.111477. [DOI] [PubMed] [Google Scholar]
- [55].Gray ER, Turbé V, Lawson VE, Page RH, Cook ZC, Ferns RB. et al. Ultra-rapid, sensitive and specific digital diagnosis of HIV with a dual-channel SAW biosensor in a pilot clinical study. NPJ Digit Med. 2018;1:35. doi: 10.1038/s41746-018-0041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tran VT, Nguyen H-Q, Kim Y-M, Ok G, Lee J. Photonic–plasmonic nanostructures for solar energy utilization and emerging biosensors. Nanomaterials (Basel). 2020;10:2248. doi: 10.3390/nano10112248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bonyar A. Label-free nucleic acid biosensing using nanomaterial-based localized surface plasmon resonance imaging: a review. ACS Appl Nano Mater. 2020;3:8506–21. [Google Scholar]
- [58].Inci F, Karaaslan MG, Mataji-Kojouri A, Shah PA, Saylan Y, Zeng Y. et al. Enhancing the nanoplasmonic signal by a nanoparticle sandwiching strategy to detect viruses. Appl Mater Today. 2020;20:100709. doi: 10.1016/j.apmt.2020.100709. [DOI] [Google Scholar]
- [59].Palmer S, Dijkstra M, Ket JC, Wahome EW, Walimbwa J, Gichuru E. et al. Acute and early HIV infection screening among men who have sex with men, a systematic review and meta-analysis. J Int AIDS Soc. 2020;23:e25590. doi: 10.1002/jia2.25590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Leyre L, Kroon E, Vandergeeten C, Sacdalan C, Colby DJ, Buranapraditkun S. et al. Abundant HIV-infected cells in blood and tissues are rapidly cleared upon ART initiation during acute HIV infection. Sci Transl Med. 2020;12:eaav3491. doi: 10.1126/scitranslmed.aav3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Peruski AH, Wesolowski LG, Delaney KP, Chavez PR, Owen SM, Granade TC. et al. Trends in HIV-2 diagnoses and use of the HIV-1/HIV-2 differentiation test – United States, 2010–2017. MMWR Morb Mortal Wkly Rep. 2020;69:63–6. doi: 10.15585/mmwr.mm6903a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Laracy J, Zucker J. The role of human immunodeficiency virus molecular diagnostics in ending the epidemic. Adv Mol Path. 2020;3:97–105. [Google Scholar]
- [63].Rinaldi F, Rolla S, Galli L, Poli A, Muccini C, Mastrangelo A. et al. Dynamics of HIV-1 POL antibodies after ART in chronic HIV-1 infection. New Microbiol. 2020;43:55–7. [PubMed] [Google Scholar]
- [64].Gay CL, Neo DT, Devanathan AS, Kuruc JD, McGee KS, Schmitz JL. et al. Efficacy, pharmacokinetics and neurocognitive performance of dual, NRTI-sparing antiretroviral therapy in acute HIV-infection. Aids. 2020;34:1923–31. doi: 10.1097/QAD.0000000000002652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Puthanakit T, Ananworanich J, Akapirat S, Pattanachaiwit S, Ubolyam S, Assawadarachai V. et al. Pattern and frequency of seroreactivity to routinely used serologic tests in early-treated infants with HIV. J Acquir Immune Defic Syndr. 2020;83:260–6. doi: 10.1097/QAI.0000000000002254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Branson BM, Owen SM, Wesolowski LG, Bennett B, Werner BG, Wroblewski KE, Pentella MA. Laboratory testing for the diagnosis of HIV infection: updated recommendations. 2014.
- [67].Loynachan CN, Thomas MR, Gray ER, Richards DA, Kim J, Miller BS. et al. Platinum nanocatalyst amplification: redefining the gold standard for lateral flow immunoassays with ultrabroad dynamic range. ACS Nano. 2018;12:279–88. doi: 10.1021/acsnano.7b06229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Parpia ZA, Elghanian R, Nabatiyan A, Hardie DR, Kelso DM. p24 antigen rapid test for diagnosis of acute pediatric HIV infection. J AIDS. 2010;55:413–9. doi: 10.1097/QAI.0b013e3181f1afbc. [DOI] [PubMed] [Google Scholar]
- [69].Nabatiyan A, Baumann MA, Parpia Z, Kelso D. A lateral flow-based ultra-sensitive p24 HIV assay utilizing fluorescent microparticles. J AIDS. 2010;53:55–61. doi: 10.1097/QAI.0b013e3181c4b9d5. [DOI] [PubMed] [Google Scholar]
- [70].Nandi S, Mondal A, Roberts A, Gandhi S. Biosensor platforms for rapid HIV detection. Adv Clin Chem. 2020;98:1–34. doi: 10.1016/bs.acc.2020.02.001. [DOI] [PubMed] [Google Scholar]
- [71].De La Rica R, Stevens MM. Plasmonic ELISA for the ultrasensitive detection of disease biomarkers with the naked eye. Nat Nanotechnol. :7821–4. doi: 10.1038/nnano.2012.186. 2012. [DOI] [PubMed] [Google Scholar]
- [72].Vallooran JJ, Handschin S, Pillai SM, Vetter BN, Rusch S, Beck HP, Mezzenga R. Lipidic cubic phases as a versatile platform for the rapid detection of biomarkers, viruses, bacteria, and parasites. Adv Funct Mater. 2016;26:181–90. [Google Scholar]
- [73].Cretich M, Daaboul GG, Sola L, Ünlü MS, Chiari M. Digital detection of biomarkers assisted by nanoparticles: application to diagnostics. Trends Biotechnol. 2015;33:343–51. doi: 10.1016/j.tibtech.2015.03.002. [DOI] [PubMed] [Google Scholar]
- [74].Ran P, Song J, Mo F, Wu J, Liu P, Fu Y. Nitrogen-doped graphene quantum dots coated with gold nanoparticles for electrochemiluminescent glucose detection using enzymatically generated hydrogen peroxide as a quencher. Microchimica Acta. 2019;186:1–7. doi: 10.1007/s00604-019-3397-6. [DOI] [PubMed] [Google Scholar]